Abstract
This Perspective is intended to raise questions about the conventional interpretation of protein folding. According to the conventional interpretation, developed over many decades, a protein population can visit a vast number of conformations under unfolding conditions, but a single dominant native population emerges under folding conditions. Accordingly, folding comes with a substantial loss of conformational entropy. How is this price paid? The conventional answer is that favorable interactions between and among the side chains can compensate for entropy loss, and moreover, these interactions are responsible for the structural particulars of the native conformation. Challenging this interpretation, the Perspective introduces a proposal that high energy (i.e., unfavorable) excluding interactions winnow the accessible population substantially under physical–chemical conditions that favor folding. Both steric clash and unsatisfied hydrogen bond donors and acceptors are classified as excluding interactions, so called because conformers with such disfavored interactions will be largely excluded from the thermodynamic population. Both excluding interactions and solvent factors that induce compactness are somewhat nonspecific, yet together they promote substantial chain organization. Moreover, proteins are built on a backbone scaffold consisting of α‐helices and strands of β‐sheet, where the number of hydrogen bond donors and acceptors is exactly balanced. These repetitive secondary structural elements are the only two conformers that can be both completely hydrogen‐bond satisfied and extended indefinitely without encountering a steric clash. Consequently, the number of fundamental folds is limited to no more than ~10,000 for a protein domain. Once excluding interactions are taken into account, the issue of “frustration” is largely eliminated and the Levinthal paradox is resolved. Putting the “bottom line” at the top: it is likely that hydrogen‐bond satisfaction represents a largely under‐appreciated parameter in protein folding models.
Keywords: backbone‐based model, conformational entropy, excluding interactions, hydrogen‐bond satisfaction, protein folding, steric clash, Levinthal paradox
1. HISTORICAL BACKGROUND
Current ideas about protein structure formation already emerged with the advent of solved structures: complicated, well‐packed, macromolecular assemblies, with abundant intramolecular interactions (Figure 1). Further analysis showed that folded proteins have packing densities similar to those of small organic solids, 2 an ostensible consequence of the energetically optimal constellation of interactions between and among residue side chains. This text‐book perspective anchors a plausible intuition that the constellation of weak interactions, evident in the folded structure, is responsible for selecting that structure from the presumably vast unfolded population. Although refined many times over the years, this underlying–and usually unspoken–intuition persists to this day: a multitude of protein‐specific attractive interactions is responsible for selecting and stabilizing the native fold. 3 This view has led to an axiomatic conviction that at root, protein folding is essentially a many‐parameter energy minimization problem, which can be captured by an appropriate forcefield, schematically:
| (1) |
In early equilibrium folding studies, small proteins like ribonuclease and lysozyme were observed to fold in an “all‐or‐none” manner, where a plot of some structure‐disrupting factor (e.g., temperature or denaturing solvent) vs. the folded fraction of the population results in a sigmoidal (i.e., highly cooperative) curve. 4 At the curve's midpoint, half the population is folded, half is unfolded, with a negligible population of partially folded intermediates. With only two populated states, the folding process can be represented as a chemical equilibrium U(nfolded)⇌ N(ative) with equilibrium constant Keq = [N]/[U], for which the free energy difference between the folded and unfolded populations is given by
| (2) |
(R is the gas constant; T is the absolute temperature). ΔG'conformational has been measured for hundreds of proteins, and typical values fall within a narrow range between −5 to −15 kcal/mol, 5 the equivalent of a few water: water hydrogen bonds at most. When monitored using optical probes, the folding of such two‐state proteins usually follows first order kinetics, consistent with an ordinary chemical reaction where U and N are separated by a barrier and intermediates on the folding pathway are sequential. With good reason, these early folding studies concluded that proteins fold along preferred pathways.
FIGURE 1.
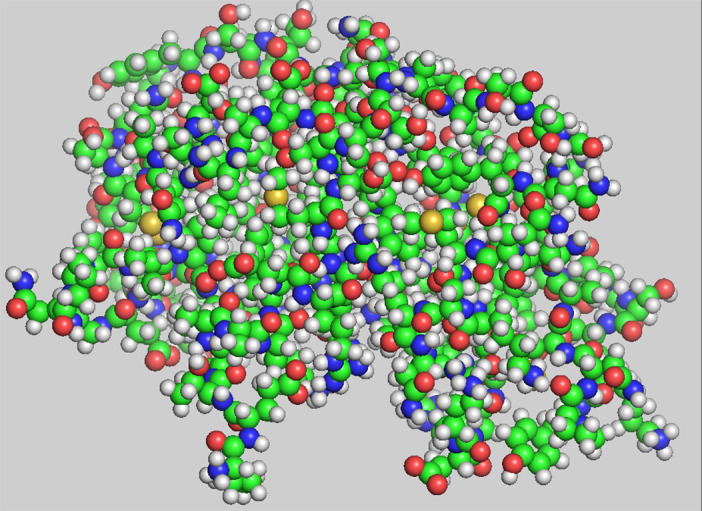
All‐atom representation of ribonuclease using CPK colors. Drawn with PyMol 1
This view was called into question when, in 1988, Roder et al. 6 and Udgaonker and Baldwin 7 observed that folding kinetics are multiphasic when measured by hydrogen exchange protection factors. The method can report the folding status of individual residues at successive time slices, providing a more fine‐grained picture than an optical probe. 8 , 9
Multiphasic kinetics prompted a re‐evaluation: do proteins fold by a unique pathway or by jmultiple pathways? In an insightful review, Baldwin characterized these competing views ‐ preferred pathways vs. multiple pathways ‐ as the classical view vs. the new view. 10 However, in either case, the underlying assumption remains: interactions responsible for overcoming conformational entropy persist in the final state and can therefore be detected by analyzing the X‐ray elucidated structure. This seeing is revealing assumption has motivated a number of approaches that emphasize attractive interactions, such as contact energies, 11 knowledge‐based potentials, 12 Gō models, 13 lattice models, etc.
1.1. Seeing is deceiving
Questioning the seeing is revealing view, it is proposed instead that substantial chain organization results from elimination of disfavored interactions–excluding interactions. Excluding interactions exclude high energy (i.e., disfavored) interactions, distilling the population and thereby enriching the fraction of native conformers at the expense of nonviable subpopulations. By definition, excluded subpopulations are not visible in the final structure and therefore are not captured in contact energies, knowledge‐based potentials, Gō models, lattice models, and the like, which are all based on attractive interactions. Yet, together with the drive toward chain compaction, excluding interactions can induce substantial chain organization.
Two main excluding interactions are considered here: (i) sterics and (ii) hydrogen bond disruption. Steric clash is well understood 14 : a stiff repulsive force keeps nonbonded atoms from approaching closer than van der Waals radii. Contrary to early simplifying assumptions, 15 systemic steric clash extends beyond immediate chain neighbors. 16 For example, an α‐helix cannot be followed by a β‐strand without an intervening turn or loop; otherwise the chain would encounter an i‐(i + 3) backbone: backbone steric clash. 17 , 18 Notably, a backbone: backbone clash is sequence independent, and it rarefies possible constructs substantially by eliminating chimeric mixtures of α‐helices and β‐strands.
Less well appreciated is the fact that a hydrogen bond donor or acceptor lacking a partner would be disfavored by ~ + 5 kcal/mol, 19 , 20 , 21 rivaling the entire free energy difference between the folded and unfolded states. 5 Of course, this penalty assumes that configurations exist in which essentially all hydrogen bond donors or acceptors can be hydrogen‐bond satisfied, either by solvent or by intramolecular partners. Over the years, many publications – including our own 22 – have reported finding unsatisfied polar groups in X‐ray structures, but these are a likely artifact of refinement strategies, which typically lack an explicit hydrogen bond potential. 23
A case in point involves ultra‐high resolution crystal structures, which nevertheless have an abundance of unsatisfied hydrogen bond donors/acceptors as well as numerous hard sphere clashes (Figure 2). For this Perspective, 18,383 residues in 110 proteins with resolution ≤1 Å were analyzed, finding that an unlikely 9.2% of the residues had backbone polar groups without hydrogen‐bond partners from either solvent or other protein atoms.
FIGURE 2.
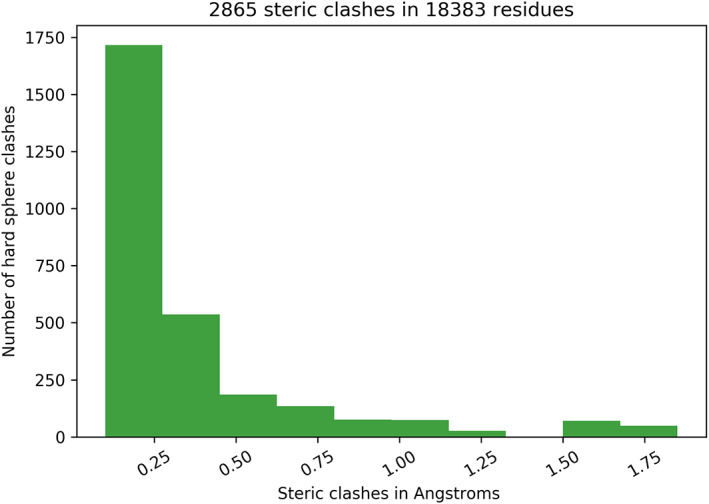
van der Waals radii: (C(sp3) = 1.64 Å, C(sp2) = 1.5 Å, O(sp2) = 1.35 Å, N(sp2) = 1.35 Å, H = 1.0 Å)
Hard sphere clashes were assessed using conservative van der Waals radii, 24 further scaled by 0.95. The histogram is limited to the 2,865 clashes having van der Waals overlaps exceeding 0.01 Å and excluding alli‐i + 3 clashes, that is, clashes between atoms separated by fewer than four contiguous covalent bonds. Such clashes occur frequently in proteins, and they are usually treated as a special case in forcefields; here, they are omitted.
1.2. A backbone‐based model of folding
An earlier Perspective introduced the hypothesis that the backbone is primarily–but certainly not entirely–responsible for determining the fold, as can be understood once hydrogen bond satisfaction is taken into account 25 ; see also the framework model of Kim and Baldwin. 26
Hydrogen bond satisfaction is a potent organizer in protein folding. In detail, many hydrogen bond donors/acceptors are removed from solvent access when a protein folds. These groups must be satisfied by intermolecular hydrogen‐bond partners in the folded structure. Why? If a hydrogen bond donor/acceptor is hydrogen‐bond satisfied by solvent when unfolded but unsatisfied when folded, the U ⇌ N equilibrium would be shifted far to the left, an inescapable thermodynamic consequence. 20 Moreover, there are only two completely extensible hydrogen‐bond‐satisfying conformers: α‐helices and β‐strands 14 (Figure 3). Of thermodynamic necessity, all proteins are built on backbone scaffolds of these two isodirectional, hydrogen‐bonded elements (with the occasional exception of small, metal‐binding polypeptides). This conclusion is easily confirmed upon analysis or visualization of structures in the Protein Data Bank. 28
FIGURE 3.
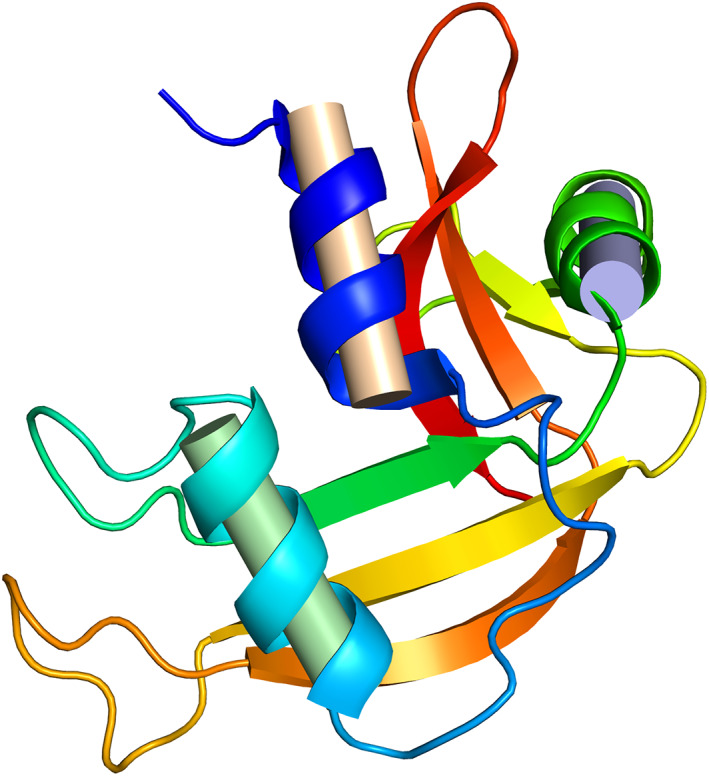
Ribbon diagram of ribonuclease, emphasizing the α‐helices (spirals) and β‐sheet (arrows). 27 Proteins are built on backbone scaffolds of these two isodirectional, hydrogen‐bonded building blocks, and they are the implicit reason why these popular representations are so illustrative. Figure courtesy of Loren Williams. Drawn with Pymol 1
Furthermore, the number of distinct backbone scaffolds is no more than ~10,000 for a protein domain, 29 , 30 not some incomprehensibly large number as is often assumed. Taking hen egg lysozyme (129 residues) as a template, a typical domain might have ~10 scaffold elements. In general, with 10 segments of either α‐helix or β‐strand, there are 2**10 possible scaffolds multiplied by any complexity introduced by interconnecting turns and loops. In proteins, these interconnections are typically short and conformationally restrictive, as shown in the histogram (Figure 4). 32
FIGURE 4.
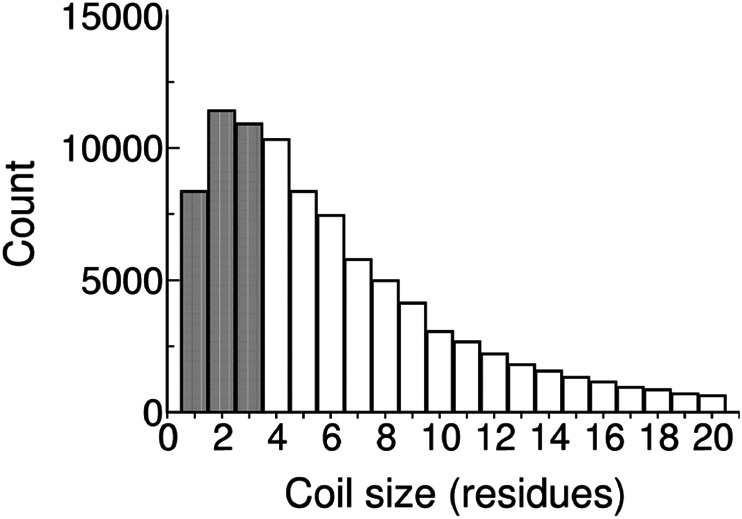
Histogram of all non‐α‐helix, non‐β‐sheet fragment lengths from the coil library 31
This limitation on the number of available scaffolds for a protein domain is imposed by the necessity of satisfying backbone hydrogen bonds without violating excluded volume and, apart from glycine and proline, is sequence independent. The remaining chain organization is then contributed by the sequence, where residue side chains do, of course, play the determinative role in selecting from available scaffolds. 33
1.3. Statistical thermodynamics of protein folding
The observation of multiphasic folding kinetics motivated a quest for a theory of protein folding grounded in authentic statistical thermodynamics. An important condition for a suitable theory arises from the realization that the number of protein sequences has continued to increase exponentially while the number of distinct structures has increased only linearly and is approaching a plateau. 34 Accordingly, the theory, by its nature, should give rise to a limited number of distinct folds. Energy Landscape Theory (ELT) is such a theory. 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 The theory seeks to quantify the balance between favorable potential energy vs. unfavorable conformational entropy by considering all possible positions and conformations of interacting atoms in the population, weighted by their corresponding energy levels. Taking this free energy surface into account, the goal is to map folding dynamics as the population negotiates routes from U to N along multiple pathways.
ELT is based on the theory of spin glasses. 44 Spin glasses are frustrated systems, so called because all favorable pairwise interactions cannot be satisfied simultaneously. Consequently, a spin glass system has a multiplicity of stable ground states, similar by analogy to the way different sequences of the 20 amino acids can engender a diversity of stable native folds. The folding process is represented pictorially as a funnel, where a population of folding proteins progresses down a multiplicity of pathways, with each molecule in the population negotiating its own route from the funnel's mouth to its spout.
Dating back to Anfinsen's early folding experiments, 45 there has been a lingering question about how individual molecules avoid meta‐stable traps en route from U to N. Another way of posing this question is to ask why a single native fold prevails instead of multiple alternative native folds. In spin glass theory, the term for this issue is “frustration,” and in ELT the solution to the conundrum is called the “principle of minimal frustration”. 46 That is, evolution has selected sequences which avoid kinetic traps as they progress down their respective folding funnels. A funneled landscape is explicitly sequence‐dependent, and every unique sequence is necessarily associated with its own particular folding funnel, even closely related sequences such as homologs. 25
In the alternative backbone‐based model, frustration is not important because, with the exception of proline and glycine, backbone scaffolds are sequence‐independent. Persisting segments are expected to emerge only in the form of hydrogen‐bond‐satisfied modules such as foldons, 47 , 48 super‐secondary structure, 49 or essentially complete scaffold formation. 50 Prior to forming such modules, the population would be essentially unfolded, dominated by chains with indistinct microscopic trajectories and with most polar groups hydrogen bonded to solvent molecules.
The backbone‐based model of folding is consistent with the observed emergence of largely intact structures in the folding transition state because a myriad of conceivable, partially‐folded conformers would be winnowed from the population unless they are hydrogen‐bond satisfied. In detail, when folding is modeled as an ordinary chemical reaction, U ⇌ I ‡ ⇌ N, the transition‐state species I‡, situated at the top of the highest free‐energy barrier, is not detectable. Here, ϕ‐value analysis is the method of choice for characterizing the extent to which structure has emerged in the transition state. 51 , 52 When ϕ‐analysis was first introduced, it was expected that ϕ‐values would be either 0 or 1, corresponding to no interaction or complete interaction in I‡. In practice, such values are rare, and for understandable reasons: Sanchez and Kiefhaber observed that with few exceptions, ϕf, the ϕ‐value in the folding direction (U → N), is ~0.3, giving “a picture of transition states as distorted native states for the major part of a protein or for large substructures.” 53 Similarly, Daggett and Fersht reported that:
"The transition state for unfolding/folding is, almost without exception, highly structured. It is an ensemble of related structures that have some or much of the secondary structure intact and disrupted packing interactions." 54
Further, structure space and sequence space are separable in the backbone‐based model: of course, it is important to emphasize that the sequence does play a determinative role in selecting a specific scaffold from the repertoire of accessible scaffolds. However, this repertoire is pre‐determined by the limited number of ways in which interacting α‐helices and strands of β‐sheet can form viable assemblies, given the constraints imposed by excluded volume, hydrogen‐bond satisfaction, and exposure of hydrophobic groups. 25 The inherently restrictive nature of such constraints explains why only a small number of super‐secondary structure motifs 49 is observed in folded proteins. (A super‐secondary structure motif is a composite of several contiguous elements of repetitive secondary structure: αα, ββ, and βΑΒ.) Implicitly, if natural backbone scaffolds are restricted to a limited sequence‐independent repertoire, then evolution can only modify these fundamental folds by varying the sequence, not by inventing additional de novo folds.
The recognition that structure space and sequence space are separable makes a telling difference in understanding the origins of protein structure. Toward this end, Banavar and colleagues have mounted an ongoing effort to capture this distinction in a physics‐based approach. 55 , 56 , 57 Remarkably, that effort has now culminated in a demonstration that the building blocks of proteins can be captured entirely from first principles, with no adjustable parameters, and no reference to sequence information or chemical particulars. 58
2. A FEW RECENT SUCCESSES
There have been a number of recent successes in predicting protein folding. To name just four: David Baker's Rosetta, 59 Marks and Sander's use of evolutionary sequence co‐variation, 60 Evans & Senior's use of artificial intelligence 61 and David Shaw's Anton simulations. 62 The first three achieved proven success in blind protein structure prediction contests, 63 and although their methods differ, all are rooted in pattern recognition, confirming that patterns exist. Notably, none of these three approaches are based on a statistical thermodynamic theory of folding. Anton simulations, the fourth method, is discussed in the next section.
3. SIMULATIONS
Folding simulations can be classified into two distinct types. Type 1 simulations test whether the parameters are sufficient to predict an experimental outcome. Anton simulations 62 mentioned above are of this type. Type 2 deliberately biases the answer toward the experimental outcome to observe how that outcome emerges. Often, a Gō model 13 is used for type 2 simulations. To our knowledge, neither type penalizes conformers in which hydrogen bond donors/acceptors are completely unsatisfied by either intramolecular partners or solvent.
Returning to Anton simulations, in a breakthrough contribution, Shaw and co‐workers reported 0.1–1.0‐millisecond simulations that can fold small proteins to their native structures successfully and reversibly. 62 These highly successful Anton simulations, like many others, represented hydrogen bonds by fixed point charges, a representation that does not lend itself to an effective strategy for penalizing unsatisfied polar groups. Long ago, Hagler and Lifson argued that geometry is preferred to energy in representing hydrogen bonds, and for purposes of recognizing unsatisfied polar groups, that may well be the case today. 64
However, as Sosnick et al. observed, in comparison with experimental data these simulations “exhibit excessive intramolecular H‐bonding even for the most expanded conformations.” 65 In other words, the simulations captured native folds despite failing to capture some presumably relevant details of the experimentally observed pathway. Even so, Lindorff‐Larsen et al. find that, “In most cases, folding follows a single dominant route in which elements of the native structure appear in an order highly correlated with their propensity to form in the unfolded state.” 62
Similarly, GDR analyzed hydrogen bonding in a 1‐millisecond simulation of BPTI, 66 using data kindly provided by David Shaw. This unpublished analysis was undertaken for a 2013 seminar presentation at D.E. Shaw Research. The simulation, 66 comprising 4*10 11 2.5‐femtosecond time steps, was initiated with folded, solvated BPTI, which “transitioned reversibly among a small number of structurally distinct long‐lived states” while still maintaining the overall native topology throughout. Analyzing the last 1,000 structures, polar groups left unsatisfied by either solvent or intramolecular partners usually ranged within an interval between 5 and 25 residues, with occasional larger spikes. The implausibly large number of unsatisfied groups notwithstanding, the overall native topology remained intact because these groups were infrequently situated within scaffold elements of secondary structure (Figure 5).
FIGURE 5.
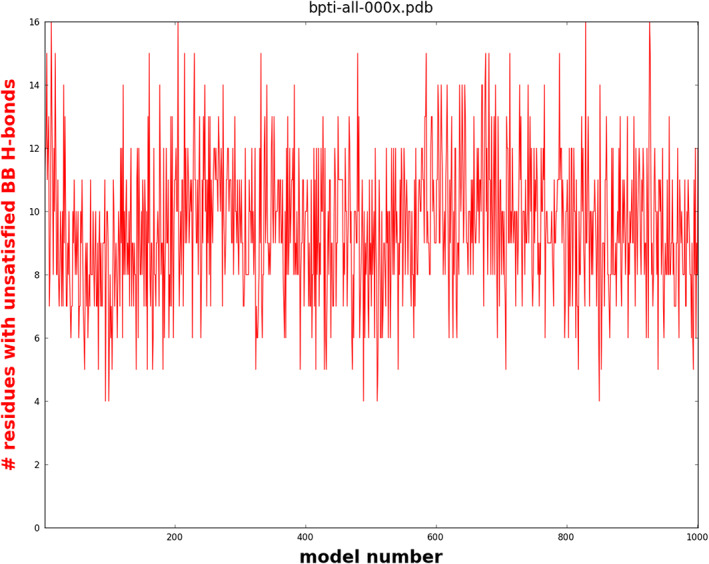
Polar groups with unsatisfied hydrogen bonds in the last 1,000 structures range between 5 and 25, with occasional larger spikes
3.1. Molten globules and foldons
There are two main types of molten globule intermediates: wet 67 and dry. 68 Wet molten globule intermediates have partially formed hydrogen‐bonded scaffolds 69 ; the remaining chain is presumably solvent‐accessible. Dry molten globule intermediates are an alternative form of the native fold that has expanded from a close‐packed (locked) to a loose‐packed (unlocked) state, where liquid‐like van der Waals interactions persist and water does not yet enter the core. 50 Neumaier and Kiefhaber characterized the unlocked state in villin headpiece subdomain, showing that “rather than being expanded, the unlocked state represents an alternatively packed, compact state, demonstrating that native proteins can exist in several compact folded states…” 70 Neither type of molten globule has been characterized sufficiently to ascertain whether it can harbor unsatisfied polar groups, an unlikely condition for reasons given above.
Foldons are small cooperative units that are stabilized by intramolecular hydrogen bonds, which can be detected by hydrogen exchange, 47 , 48 , 71 and they span a broad range of stabilities. The least stable foldons form and dissipate rapidly while the residual chain remains unfolded and presumably solvent‐accessible. Foldons are expected to be hydrogen‐bond satisfied; if not, the hydrogen exchange method could not have detected them. Englander has shown that foldon assembly is all‐or‐none, consistent with the premise that intermediates are strongly disfavored because, inescapably, some hydrogen bond donors/acceptors would be left unsatisfied, shielded from solvent hydrogen bonds and unable to realize compensating intramolecular hydrogen bonds.
3.2. Mind the gap
Proteins fold according to the intrinsic laws of physics and chemistry, whereas models and simulations can be conditioned by the expectations of investigators. Often, a conceptual gap separates one from the other.
A clear, although extreme, example is illustrated by earlier mathematical “proofs” that the protein folding problem is NP‐complete (i.e., loosely speaking, there is no known way to guarantee that the problem can be solved in a realistic time interval). The approach involved constructing a model of protein folding and then proving that the model is NP‐complete. Typically, the underlying model was elegant but overly generalized, and therefore misleading.
A corresponding conceptual gap between theory and experiment is at issue when assessing whether proteins fold by preferred pathways or parallel pathways – the classical view or the new view. 10 Indeed, these contrasting views of thermodynamic populations were already articulated long before they were associated with protein folding. The following is from the introduction to Statistical Mechanics by Fowler and Guggenheim published in 1939:
"We will have to decide whether the assembly, when left to itself in the way already specified, tends to settle down mainly into one or other of a small preferred group of stationary states, whose properties are or control the equilibrium properties of the assembly; or whether it shows no such discrimination, but wanders apparently or effectively at random over the whole range of stationary states made accessible by the general conditions of the problem." 72
That's the classical view vs. the new view in a paragraph.
The computer models used to substantiate theory can be analyzed in atomic detail, but experiment‐based data in solution are not accessible at an equivalent resolution. Interpretation of experimental folding data is particularly problematic for the wealth of well‐studied two‐state proteins because the route from U to N cannot be inferred solely from knowledge of the end states, and interpretation must resort to kinetic analysis. These obstacles complicate efforts to understand whether or not the theory models experimental reality.
Many recent reports feature pictures of folding funnels, conceptual illustrations that are not based on an experimentally‐derived energy surface. An exception is the work of Barrick and colleagues, who constructed overlapping subsets of the seven ankyrin repeats of the Drosophila Notch receptor and measured their stabilities. 73 From these data, they assembled a complete equilibrium free energy landscape (Figure 5 of their paper). Notably, the landscape “shows an early free energy barrier and suggests preferred low‐energy routes for folding.73”
To identify the origin of preferred folding routes, Tripp and Barrick redesigned the ankyrin energy landscape by adding stabilizing C‐terminal consensus repeats to the five natural N‐terminal repeats. 74 The folding pathway was successfully re‐routed and once again followed “the lowest channel through the energy landscape.”
Does the flux always define preferred folding pathways, or can preferred pathways be abolished? To answer this question, Barrick and Aksel analyzed repeat proteins built from identical consensus repeats, again assembling a detailed energy landscape from the experimental results. 75 As expected, parallel folding pathways were detected. Quoting the authors,
"This finding of parallel pathways differs from results from kinetic studies of repeat‐proteins composed of sequence‐variable repeats, where modest repeat‐to‐repeat energy variation coalesces folding into a single, dominant channel. Thus, for globular proteins, which have much higher variation in local structure and topology, parallel pathways are expected to be the exception rather than the rule." 75
Technical obstacles impede a detailed quantitative comparison between these experimental energy surfaces and folding routes from landscape theory. Qualitatively though, experiment and theory seem to differ: the experiments are consistent with folding along preferred pathways (the classical view), while the theory emphasizes folding along multiple (parallel) pathways (the new view). Nevertheless, a caveat remains: assembly of these experimental energy surfaces was made possible by manipulating individual units in ankyrin repeats.
In general, how should multiphasic folding kinetics 6 , 7 be interpreted if other proteins, like ankyrin, “coalesce folding into a single, dominant channel?” In fact, this would be the expected outcome for either stepwise assembly of foldon units 47 or hierarchic self‐assembly. 76 , 77 , 78 , 79 In such models, marginally stable modules interact, resulting in larger modules which, in turn, further interact in an iterative, step‐wise cascade that ultimately coalesces into the native state.
A timely experimental study of Bhatia et al. 80 may reconcile the conflicting views about folding pathway uniqueness. These authors state that “although evidence supporting the existence of more than one folding/unfolding pathway continues to grow, there is little evidence for a large multitude of pathways as envisaged by energy landscape theory.” Implicit in this study is the related question of whether multiple folding pathways converge prior to N or instead remain discrete throughout the entire trajectory from U to N, as is often depicted in folding funnel diagrams.
Bhatia et al. 80 analyzed the folding of MNEI (a single‐chain construct that interconnects a monellin heterodimer) using time‐resolved fluorescence decay as assessed by four assiduously positioned FRET pairs in four different MNEI variants. Their analysis also encompassed a large body of pervious work.
Importantly, experimental detection of multiple pathways is typically identified solely by separable kinetic curves, but here kinetics events are mapped onto structural events along the four parallel pathways. MNEI secondary structure comprises a 17‐residue α‐helix and a 5‐stranded β‐sheet. Using kinetics to follow structure formation, Bhatia et al. 80 found that the most likely pathway‐averaged sequence of events was (1) helix formation, (2) core consolidation, (3) β‐sheet formation, and (4) overall compaction of the end‐to‐end distance. Notably, these authors observed that “parts of the protein that are closer in the primary sequence acquire structure before parts separated by longer sequence”, consistent with an earlier report showing a strong correlation between folding rates and contact order in simple, two‐state proteins. 81
Based on their data, Bhatia et al. 80 proposed a “phenomenological model”, in which the major folding route “involves sequential formation of local short‐range contacts and then nonlocal long‐range contacts,” as anticipated in earlier hierarchic models of protein organization and folding. 76 , 79 , 82
A hierarchic model is a bottom up model that converges when substructures of persisting stability (relative to kT) are formed, as described above. Importantly, all four parallel folding paths are found to converge prior to formation of the native state. This converged state is suggestive of a dry molten globule intermediate, 50 and it is tempting to speculate that its formation may correspond to the transition state in classical studies. If so, earlier events, detected by fast kinetics and classified as discrete pathways, may evade detection using classical approaches. This possibility would reconcile apparent conflicts about the uniqueness of the folding pathway. As the authors note, “the nature of the barriers that dictate the relative fluxes of molecules on the parallel pathways is yet to be understood.” Clearly, more work and further clarification will surely follow.
3.3. Origins of specificity
Backbone hydrogen bonding is a substantial source of folding specificity. In comparison, conformational entropy always favors the unfolded state nonspecifically, while hydrophobic burial always favors the folded state, again nonspecifically. Only hydrogen bonding switches from favoring intramolecular interactions to favoring solvent interactions when shifting from folding conditions to unfolding conditions.
Furthermore, under folding conditions, unsatisfied polar groups are of high energy and would therefore contribute negligibly to the thermodynamic population (see above), yet conferring specificity, as described in the following quote from von Hippel and Berg that refers to nucleic acid specificity 83 :
"These are not large numbers, and it is important to recognize that much more favorable free energy is likely to be lost per mispaired position than is gained per proper recognition event. This follows because a mispositioned base pair can result in the total loss of at least one hydrogen‐bonding interaction; i.e., a protein hydrogen bond donor will end up "facing" a nucleic acid donor, or an acceptor will be "buried" facing an acceptor. In either case at least one hydrogen bond that was broken in removing the protein and nucleic acid donor (or acceptor) groups from contact with the solvent is not replaced, and an unfavorable contribution of as much as +5 kcal/mol may be added to the binding free energy unless the protein‐DNA complex can adjust its overall conformation somewhat to minimize this problem. This phenomenon illustrates the principle that generally applies to recognition interactions that are based on hydrogen‐bond donor‐acceptor complementarity in water; i.e., correct donor‐acceptor interactions may not add much to the stability of the complex, but incorrect hydrogen‐bond complementarities are markedly destabilizing. Thus, differential specificity of this type is largely attributable to the unfavorable effects of incorrect contacts."
Protein folding studies tend to conflate factors that stabilize the folded state with factors that select for the specific conformation of that state, a questionable assumption. 84 The reason ribonuclease remains stable at temperature T1 instead of a higher temperature, T2, differs from the reason it adopts a specific fold. Typically, mutations that destabilize proteins may shift the U ⇌ N equilibrium toward U, but a population of N remains. Matthews and numerous co‐workers have deposited hundreds of variant T4 lysozyme structures and, despite differing stabilization energies, they all adopt the T4 lysozyme fold. 85 By way of a macroscopic analogy, a house can be stabilized against “denaturation” from a storm by installing cross‐beams and support columns, but the specific layout of the rooms would remain unaltered.
In contrast, DNA biochemists make a distinction between specificity and stability. Base‐paired specificity in double stranded DNA is due primarily to hydrogen‐bonded complementarity, whereas the larger contribution to overall stability comes from base‐stacking, with the favorable interaction free energy being enthalpic and dependent on the transition state dipoles of these heterocyclic (N‐containing) rings. 86
Summarizing, hydrogen‐bonding is a substantial source of specificity for both proteins and DNA. Proteins are built on scaffolds of the two hydrogen‐bonded elements, α‐helices and β‐strands, and strand complementarity in DNA is realized via hydrogen‐bonding. Unsatisfied hydrogen bond donors/acceptors are highly destabilizing, and they serve to concentrate native interactions by eliminating the otherwise abundant population of disfavored conformers. Three decades ago, the Richardson laboratory coined the term “negative design saying”:
"In designing (or predicting) a protein structure, it is not sufficient to show that the given sequence is compatible with a particular structure; we must also ensure that it is less compatible with alternative structures." 87
This concept played a critical role in early protein design efforts 87 , 88 and has guided the field ever since. In effect, hydrogen bond satisfaction 20 , 25 is nature's implementation of negative design.
Finally, assessing the free energy of a protein hydrogen bond is controversial. 89 For this Perspective, the cost of a completely unsatisfied polar group has been taken at +5 kcal/mol. Estimates taken from the literature range from +3 to +6 kcal/mol. 19 , 21 , 90 However, even using a low value of +3 kcal/mol, a few unsatisfied hydrogen bond donors or acceptors would still rival the typical entire free energy difference between the folded and unfold forms under folding conditions. Here, it is important to emphasize that these estimates refer to the energetic penalty paid by a polar group that lacks a hydrogen‐bonded partner, such as a broken hydrogen bond in the gas phase. 19
3.4. The Levinthal paradox
The much‐discussed Levinthal paradox was actually a back‐of‐the‐envelope conundrum demonstrating that proteins do not fold by randomly searching ϕ,ψ‐space. 91 Zwanzig et al. have shown that a suitably biased search can resolve this issue satisfactorily. 92 Moreover, if secondary structure is taken as the reference point rather than a random polypeptide chain, there is no “paradox,” as shown by Finkelstein. 93 A similar but even stronger conclusion holds if the cooperative formation of foldons, super‐secondary structure and scaffold elements are taken as the reference.
3.5. The bottom line
This Perspective seeks to reframe the protein folding problem by emphasizing the importance of excluding interactions, hydrogen bond satisfaction in particular. Although excluding interactions are nonspecific, they can induce highly specific chain organization. These under‐appreciated parameters could make a transformative difference if incorporated into models and simulations.
AUTHOR CONTRIBUTIONS
George D. Rose: Conceptualization; formal analysis; funding acquisition; investigation; methodology; project administration; validation; writing‐original draft; writing‐review & editing.
ACKNOWLEDGMENTS
Ι am indebted to Thomas Kiefhaber, Jayanth Banavar, and Tatjana Škrbić for discussion, to Gary Pielak, Peter von Hippel and Loren Williams for discussion and editorial suggestions, to Doug Barrick, Sandhya Bhatia, David Shaw and Jayant Udgaonkar for critical reading of sections that describe their work, and to the National Science Foundation for support.
Rose GD. Protein folding ‐ seeing is deceiving. Protein Science. 2021;30:1606–1616. 10.1002/pro.4096
Funding information National Science Foundation
REFERENCES
- 1. DeLano W. The PyMOL molecular graphics system. San Carlos, CA: DeLano Scientific LLC, 2003. [Google Scholar]
- 2. Richards FM. Areas, volumes, packing, and protein structure. Ann Rev Biophys Bioeng. 1977;6:151–176. [DOI] [PubMed] [Google Scholar]
- 3. Rose GD. What is life? Part II. Proteins. 2019;87:174–175. [DOI] [PubMed] [Google Scholar]
- 4. Ginsburg A, Carroll WR. Some specific ion effects on the conformation and thermal stability of ribonuclease. Biochemistry. 1965;4:2159–2174. [Google Scholar]
- 5. Sosnick TR, Barrick D. The folding of single domain proteins–have we reached a consensus? Curr Opin Struct Biol. 2011;21:12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Roder H, Elove GA, Englander SW. Structural characterization of folding intermediates in cytochrome c by H‐exchange labelling and proton NMR. Nature. 1988;335:700–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Udgaonkar JB, Baldwin RL. NMR evidence for an early framework intermediate on the folding pathway of ribonuclease a. Nature. 1988;335:694–699. [DOI] [PubMed] [Google Scholar]
- 8. Hu W, Walters BT, Kan ZY, et al. Stepwise protein folding at near amino acid resolution by hydrogen exchange and mass spectrometry. Proc Natl Acad Sci U S A. 2013;110:7684–7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kan ZY, Walters BT, Mayne L, Englander SW. Protein hydrogen exchange at residue resolution by proteolytic fragmentation mass spectrometry analysis. Proc Natl Acad Sci U S A. 2013;110:16438–16443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baldwin RL. The nature of protein folding pathways: The classical versus the new view. J Biomol NMR. 1995;5:103–109. [DOI] [PubMed] [Google Scholar]
- 11. Miyazawa S, Jernigan RL. A new substitution matrix for protein sequence searches based on contact frequencies in protein structures. Protein Eng. 1993;6:267–278. [DOI] [PubMed] [Google Scholar]
- 12. Sippl MJ. Knowledge‐based potentials for proteins. Curr Opin Struct Biol. 1995;5:229–235. [DOI] [PubMed] [Google Scholar]
- 13. Gō N. The consistency principle in protein structure and pathways of folding. Adv Biophys. 1984;18:149–164. [DOI] [PubMed] [Google Scholar]
- 14. Ramachandran GN, Sasisekharan V. Conformation of polypeptides and proteins. Adv Prot Chem. 1968;23:283–438. [DOI] [PubMed] [Google Scholar]
- 15. Flory PJ. Statistical Mechanics of Chain Molecules. New York: Wiley, 1969. [Google Scholar]
- 16. Pappu RV, Srinivasan R, Rose GD. The Flory isolated‐pair hypothesis is not valid for polypeptide chains: Implications for protein folding. Proc Natl Acad Sci U S A. 2000;97:12565–12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fitzkee NC, Rose GD. Steric restrictions in protein folding: An alpha‐helix cannot be followed by a contiguous beta‐strand. Protein Sci. 2004;13:633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fitzkee NC, Rose GD. Sterics and solvation winnow accessible conformational space for unfolded proteins. J Mol Biol. 2005;353:873–887. [DOI] [PubMed] [Google Scholar]
- 19. Mitchell JBO, Price SL. The nature of the N ‐ H 0 = C hydrogen bond: An intermolecular perturbation theory study of the formamide/formaldehyde complex. J Comput Chem. 1990;11:1217–1233. [Google Scholar]
- 20. Fleming PJ, Rose GD. Do all backbone polar groups in proteins form hydrogen bonds? Protein Sci. 2005;14:1911–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pace CN, Fu H, Fryar KL, et al. Contribution of hydrogen bonds to protein stability. Protein Sci. 2014;23:652–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stickle DF, Presta LG, Dill KA, Rose GD. Hydrogen bonding in globular proteins. J Mol Biol. 1992;226:1143–1159. [DOI] [PubMed] [Google Scholar]
- 23. Panasik N Jr, Fleming PJ, Rose GD. Hydrogen‐bonded turns in proteins: The case for a recount. Protein Sci. 2005;14:2910–2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Srinivasan R, Rose GD. LINUS: A hierarchic procedure to predict the fold of a protein. Proteins. 1995;22:81–99. [DOI] [PubMed] [Google Scholar]
- 25. Rose GD, Fleming PJ, Banavar JR, Maritan A. A backbone‐based theory of protein folding. Proc Natl Acad Sci U S A. 2006;103:16623–16633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim PS, Baldwin RL. Intermediates in the folding reactions of small proteins. Annu Rev Biochem. 1990;59:631–660. [DOI] [PubMed] [Google Scholar]
- 27. Richardson JS. Early ribbon drawings of proteins. Nat Struct Biol. 2000;7:624–625. [DOI] [PubMed] [Google Scholar]
- 28. Berman HM, Westbrook J, Feng Z, et al. The Protein Data Bank. Nucleic Acids Res. 2000;28:235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chothia C. One thousand families for the molecular biologist. Nature. 1992;357:543–544. [DOI] [PubMed] [Google Scholar]
- 30. Przytycka T, Aurora R, Rose GD. A protein taxonomy based on secondary structure. Nat Struct Biol. 1999;6:672–682. [DOI] [PubMed] [Google Scholar]
- 31. Fitzkee NC, Fleming PJ, Rose GD. The protein coil library: A structural database of nonhelix, nonstrand fragments derived from the PDB. Proteins. 2005;58:852–854. [DOI] [PubMed] [Google Scholar]
- 32. Street TO , Fitzkee NC, Perskie LL, Rose GD. Physical‐chemical determinants of turn conformations in globular proteins. Protein Sci. 2007;16:1720–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rose GD. Ramachandran maps for side chains in globular proteins. Proteins. 2019;87:357–364. [DOI] [PubMed] [Google Scholar]
- 34. Koonin EV, Wolf YI, Karev GP. The structure of the protein universe and genome evolution. Nature. 2002;420:218–223. [DOI] [PubMed] [Google Scholar]
- 35. Shakhnovich E, Farztdinov G, Gutin AM, Karplus M. Protein folding bottlenecks: A lattice Monte Carlo simulation. Phys Rev Lett. 1991;67:1665–1668. [DOI] [PubMed] [Google Scholar]
- 36. Leopold PE, Montal M, Onuchic JN. Protein folding funnels: A kinetic approach to the sequence‐structure relationship. Proc Natl Acad Sci U S A. 1992;89:8721–8725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Camacho CJ, Thirumalai D. Kinetics and thermodynamics of folding in model proteins. Proc Natl Acad Sci U S A. 1993;90:6369–6372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Abkevich VI, Gutin AM, Shakhnovich EI. Specific nucleus as the transition state for protein folding: Evidence from the lattice model. Biochemistry. 1994;33:10026–10036. [DOI] [PubMed] [Google Scholar]
- 39. Sali A, Shakhnovich E, Karplus M. How does a protein fold? Nature. 1994;369:248–251. [DOI] [PubMed] [Google Scholar]
- 40. Bryngelson JD, Onuchic JN, Socci ND, Wolynes PG. Funnels, pathways, and the energy landscape of protein folding: A synthesis. Proteins. 1995;21:167–195. [DOI] [PubMed] [Google Scholar]
- 41. Dill KA, Bromberg S, Yue K, et al. Principles of protein folding–a perspective from simple exact models. Protein Sci. 1995;4:561–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wolynes PG, Onuchic JN, Thirumalai D. Navigating the folding routes. Science. 1995;267:1619–1620. [DOI] [PubMed] [Google Scholar]
- 43. Dill KA, Chan HS. From Levinthal to pathways to funnels. Nat Struct Biol. 1997;4:10–19. [DOI] [PubMed] [Google Scholar]
- 44. Rokhsar DS, Anderson PW, Stein DL. Self‐organization in prebiological systems: Simulations of a model for the origin of genetic information. J Mol Evol. 1986;23:119–126. [DOI] [PubMed] [Google Scholar]
- 45. Anfinsen CB. Principles that govern the folding of protein chains. Science. 1973;181:223–230. [DOI] [PubMed] [Google Scholar]
- 46. Ferreiro DU, Komives EA, Wolynes PG. Frustration in biomolecules. Q Rev Biophys. 2014;47:285–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Maity H, Maity M, Krishna MM, Mayne L, Englander SW. Protein folding: The stepwise assembly of foldon units. Proc Natl Acad Sci U S A. 2005;102:4741–4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Englander SW, Mayne L. The case for defined protein folding pathways. Proc Natl Acad Sci U S A. 2017;114:8253–8258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Levitt M, Chothia C. Structural patterns in globular proteins. Nature. 1976;261:552–558. [DOI] [PubMed] [Google Scholar]
- 50. Baldwin RL, Frieden C, Rose GD. Dry molten globule intermediates and the mechanism of protein unfolding. Proteins. 2010;78:2725–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Matthews CR. Pathways of protein folding. Annu Rev Biochem. 1993;62:653–683. [DOI] [PubMed] [Google Scholar]
- 52. Itzhaki LS, Otzen DE, Fersht AR. The structure of the transition state for folding of chymotrypsin inhibitor 2 analysed by protein engineering methods: Evidence for a nucleation‐condensation mechanism for protein folding. J Mol Biol. 1995;254:260–288. [DOI] [PubMed] [Google Scholar]
- 53. Sanchez IE, Kiefhaber T. Origin of unusual phi‐values in protein folding: Evidence against specific nucleation sites. J Mol Biol. 2003;334:1077–1085. [DOI] [PubMed] [Google Scholar]
- 54. Daggett V, Fersht A. The present view of the mechanism of protein folding. Nat Rev Mol Cell Biol. 2003;4:497–502. [DOI] [PubMed] [Google Scholar]
- 55. Banavar JR, Hoang TX, Maritan A, Seno F, Trovato A. Unified perspective on proteins: A physics approach. Phys Rev E Stat Nonlin Soft Matter Phys. 2004;70:041905. [DOI] [PubMed] [Google Scholar]
- 56. Hoang TX, Trovato A, Seno F, Banavar JR, Maritan A. Geometry and symmetry presculpt the free‐energy landscape of proteins. Proc Natl Acad Sci U S A. 2004;101:7960–7964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Banavar JR, Maritan A. Physics of proteins. Annu Rev Biophys Biomol Struct. 2007;36:261–280. [DOI] [PubMed] [Google Scholar]
- 58. Škrbić T, Maritan A, Giacometti A, Rose GD, Banavar JR. Building blocks of protein structures – Physics meets biology. Phys Rev E. 2021. [DOI] [PubMed] [Google Scholar]
- 59. Das R, Baker D. Macromolecular modeling with rosetta. Annu Rev Biochem. 2008;77:363–382. [DOI] [PubMed] [Google Scholar]
- 60. Marks DS, Colwell LJ, Sheridan R, et al. Protein 3D structure computed from evolutionary sequence variation. PLoS One. 2011;6:e28766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Senior AW, Evans R, Jumper J, et al. Improved protein structure prediction using potentials from deep learning. Nature. 2020;577:706–710. [DOI] [PubMed] [Google Scholar]
- 62. Lindorff‐Larsen K, Piana S, Dror RO, Shaw DE. How fast‐folding proteins fold. Science. 2011;334:517–520. [DOI] [PubMed] [Google Scholar]
- 63. Kryshtafovych A, Schwede T, Topf M, Fidelis K, Moult J. Critical assessment of methods of protein structure prediction (CASP)‐round XIII. Proteins. 2019;87:1011–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hagler AT, Lifson S. Energy functions for peptides and proteins. The amide hydrogen bond and calculation of amide crystal properties. J Am Chem Soc. 1974;96:5327–5335. [DOI] [PubMed] [Google Scholar]
- 65. Skinner JJ, Yu W, Gichana EK, et al. Benchmarking all‐atom simulations using hydrogen exchange. Proc Natl Acad Sci U S A. 2014;111:15975–15980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shaw DE, Maragakis P, Lindorff‐Larsen K, et al. Atomic‐level characterization of the structural dynamics of proteins. Science. 2010;330:341–346. [DOI] [PubMed] [Google Scholar]
- 67. Ptitsyn OB. Molten globule and protein folding. Adv Protein Chem. 1995;47:83–229. [DOI] [PubMed] [Google Scholar]
- 68. Jha SK, Udgaonkar JB. Direct evidence for a dry molten globule intermediate during the unfolding of a small protein. Proc Natl Acad Sci U S A. 2009;106:12289–12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hughson FM, Wright PE, Baldwin RL. Structural characterization of a partly folded apomyoglobin intermediate. Science. 1990;249:1544–1548. [DOI] [PubMed] [Google Scholar]
- 70. Neumaier S, Kiefhaber T. Redefining the dry molten globule state of proteins. J Mol Biol. 2014;426:2520–2528. [DOI] [PubMed] [Google Scholar]
- 71. Rumbley J, Hoang L, Mayne L, Englander SW. An amino acid code for protein folding. Proc Natl Acad Sci U S A. 2001;98:105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Fowler RH, Guggeneim EA. Statistical thermodynamics. London: Cambridge University Press, 1939. [Google Scholar]
- 73. Mello CC, Barrick D. An experimentally determined protein folding energy landscape. Proc Natl Acad Sci U S A. 2004;101:14102–14107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tripp KW, Barrick D. Rerouting the folding pathway of the notch ankyrin domain by reshaping the energy landscape. J Am Chem Soc. 2008;130:5681–5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Aksel T, Barrick D. Direct observation of parallel folding pathways revealed using a symmetric repeat protein system. Biophys J. 2014;107:220–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rose GD. Hierarchic organization of domains in globular proteins. J Mol Biol. 1979;134:447–470. [DOI] [PubMed] [Google Scholar]
- 77. Baldwin RL, Rose GD. Is protein folding hierarchic? I. local structure and peptide folding. Trends Biochem Sci. 1999;24:26–33. [DOI] [PubMed] [Google Scholar]
- 78. Baldwin RL, Rose GD. Is protein folding hierarchic? II. Folding intermediates and transition states. Trends Biochem Sci. 1999;24:77–83. [DOI] [PubMed] [Google Scholar]
- 79. Ozkan SB, Wu GA, Chodera JD, Dill KA. Protein folding by zipping and assembly. Proc Natl Acad Sci U S A. 2007;104:11987–11992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bhatia S, Krishnamoorthy G, Udgaonkar JB. Mapping distinct sequences of structure formation differentiating multiple folding pathways of a small protein. J Am Chem Soc. 2021;143:1447–1457. [DOI] [PubMed] [Google Scholar]
- 81. Gong H, Isom DG, Srinivasan R, Rose GD. Local secondary structure content predicts folding rates for simple, two‐state proteins. J Mol Biol. 2003;327:1149–1154. [DOI] [PubMed] [Google Scholar]
- 82. Crippen GM. The tree structural organization of proteins. J Mol Biol. 1978;126:315–332. [DOI] [PubMed] [Google Scholar]
- 83. von Hippel PH, Berg OG. On the specificity of DNA‐protein interactions. Proc Natl Acad Sci U S A. 1986;83:1608–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lattman EE, Rose GD. Protein folding – what's the question? Proc Natl Acad Sci U S A. 1993;90:439–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Matthews BW, Remington SJ. The three dimensional structure of the lysozyme from bacteriophage T4. Proc Natl Acad Sci U S A. 1974;71:4178–4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Devoe H, Tinoco I Jr. The stability of helical polynucleotides: Base contributions. J Mol Biol. 1962;4:500–517. [DOI] [PubMed] [Google Scholar]
- 87. Hecht MH, Richardson JS, Richardson DC, Ogden RC. De novo design, expression, and characterization of Felix: A four‐helix bundle protein of native‐like sequence. Science. 1990;249:884–891. [DOI] [PubMed] [Google Scholar]
- 88. Regan L, DeGrado WF. Characterization of a helical protein designed from first principles. Science. 1988;241:976–978. [DOI] [PubMed] [Google Scholar]
- 89. Bolen DW, Rose GD. Structure and energetics of the hydrogen‐bonded backbone in protein folding. Annu Rev Biochem. 2008;77:339–362. [DOI] [PubMed] [Google Scholar]
- 90. Tronrud DE, Holden HM, Matthews BW. Structures of two thermolysin‐inhibitor complexes that differ by a single hydrogen bond. Science. 1987;235:571–574. [DOI] [PubMed] [Google Scholar]
- 91. Levinthal C. How to fold graciously. In: Debrunner P, JCM T, Münck E, editors. Mössbauer spectroscopy in biological Sytems. Urbana: University of Illinois Press, 1969; p. 22–24. [Google Scholar]
- 92. Zwanzig R, Szabo A, Bagchi B. Levinthal's paradox. Proc Natl Acad Sci U S A. 1992;89:20–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Finkelstein AV, Garbuzynskiy SO. Reduction of the search space for the folding of proteins at the level of formation and assembly of secondary structures: A new view on the solution of Levinthal's paradox. ChemPhysChem. 2015;16:3375–3378. [DOI] [PubMed] [Google Scholar]


