ABSTRACT
This study aimed to examine the effects of Lactobacillus plantarum, a lactic acid bacteria strain isolated from kimchi, on the development of low-grade inflammation and type 2 diabetes mellitus (T2DM) exacerbated by chronic stress. C57BL/6 mice were fed either a high-fat diet (HFD) and randomized into an HFD group or a group that was fed an HFD and subjected to chronic cold exposure-related stress (HFDS), or mice were fed a normal diet (ND) and randomized into an ND group or a group that was fed an ND and subjected to chronic cold exposure-related stress (NDS). Lactobacillus plantarum LRCC5310 (108, 1010 CFU) and LRCC5314 (108, 1010 CFU) as well as L. gasseri BNR17 (108 CFU), as a positive control, were administered orally twice every day to all the mice for 12 weeks. The expression of Glut4 and adiponectin, main glucose transporter-related genes, was upregulated in the LRCC5310- and LRCC5314-treated groups. Levels of serum proinflammatory cytokines (tumor necrosis factor-α [TNF-α], interleukin-6 [IL-6]) and of mRNAs of proinflammatory genes (Tnf-α, Il-6, Ccl2, leptin) were elevated in HFDS mice. The expression of proinflammatory genes was downregulated in LRCC5310- and LRCC5314-treated groups; this was not the case for Tnf-α expression in HFDS mice. Levels of serum corticosterone and mRNA levels of stress-related genes (Npy, Y2r) were decreased in lactic acid bacteria (LAB)-fed groups, with only LRCC5314 downregulating Npy expression in HFDS mice. These results suggest that the LAB strains can normalize the expression of metabolic genes, inhibit inflammatory responses, and suppress stress in HFDS mice.
KEYWORDS: type 2 diabetes, lactic acid bacteria, insulin resistance, inflammation, stress, gut-brain axis, high-fat diet
INTRODUCTION
Type 2 diabetes mellitus (T2DM) is the most prevalent metabolic disorder worldwide because of considerable changes in modern lifestyles, including a high-fat diet and chronic stress (1). According to World Health Organization statistics, approximately 422 million people have diabetes, and 1.6 million deaths are directly associated with diabetes each year. One complication of T2DM is insulin resistance, a condition in which insulin sensitivity is reduced due to obesity (2) via inhibition of insulin-mediated absorption of glucose in peripheral tissues (3). Obesity is implicated in various diseases; excessive visceral adiposity is associated with “metabolic syndrome” characterized by insulin resistance, hyperglycemia, dyslipidemia, and hypertension (4). These disorders promote the development of T2DM and cardiovascular diseases, causing morbidity and mortality (5). Although drugs, diet, and surgical treatment can help manage and control obesity, they cause side effects. Therefore, there is an urgent need for drugs and dietary supplements with proven safety and efficacy to control obesity.
It has recently been found that psychological stress plays a role in the pathogenesis of T2DM (6). Obesity has been found to increase in parallel with stress, which is linked to the pathogenesis of many diseases (7). Some people lose weight, while others gain weight in response to chronic stress. This is because chronic stress not only increases β-adrenergic activation, a fat-burning mechanism, but also increases the intake of sugar- and fat-rich foods (8). Neuropeptide Y (NPY) is produced in the central amygdala, the arcuate hypothalamic nucleus (9), and the visceral adipose tissue (10). Activation of the hypothalamic-pituitary-adrenal axis triggers cortisol secretion and binding of NPY to the neuropeptide Y receptor 2 (Y2R) in adipocytes, which leads to adipocyte differentiation (11).
T2DM is considered an inflammatory disease (12–14). Obesity causes chronic, low-grade inflammation, which differs from normal inflammation in that there are no typical signs of inflammation, but it is also similar in that it involves the same inflammatory mediators and signaling pathways (15). Adipose tissue is a common site of inflammation in T2DM, with T and B lymphocytes in the adipose tissue being activated upon infiltration of macrophages into adipose tissue (16). These immune cells play an important role in promoting inflammation in various tissues by producing cytokines, such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and IL-1β (17). Although leptin is an adipokine that was originally identified as a major factor in food intake and weight control, surprisingly, increased inflammatory cytokine production in adipose tissue increases leptin expression in adipocytes, and synergism between the cytokines and leptin promotes inflammation (18, 19). Thus, activation of the immune system and chronic low-grade inflammation in the abdominal adipose tissue may be associated with obesity (20–22). Inflammatory cytokine antagonists capable of suppressing excessive inflammatory responses can inhibit disease development and progression, but they also have side effects that can abolish host defense against infection (23).
Lactic acid bacteria (LAB) are Gram-positive bacteria present in probiotics that can restore the balance of the gut microbiota (24, 25). As an imbalance in intestinal microbial flora has been identified in T2DM patients (26), LAB have been proposed as a safe and beneficial treatment for T2DM (27, 28). Lactobacillus gasseri and L. plantarum are LAB that have shown efficacy in regulating body weight and blood glucose in animal models of obesity and diabetes (29–32). With the routes of communication between the brain and microbiota being unraveled, the gut microbiota has emerged as an important component affecting all neuroimmune endocrine pathways (33). Microorganisms play a key role in the control of stress and the gut-brain axis under stress conditions (34). As described earlier, obesity, T2DM, inflammation, and stress are closely linked. Therefore, this study aimed to examine the effect of L. plantarum, a probiotic strain isolated from kimchi, on the development of obesity and T2DM exacerbated by chronic stress. This is the first study to confirm the efficacy of LAB in an animal model that combines chronic stress and a high-fat diet (HFD).
RESULTS
L. plantarum reduced weight gain and fasting blood glucose levels in cold-stress-exposed mice.
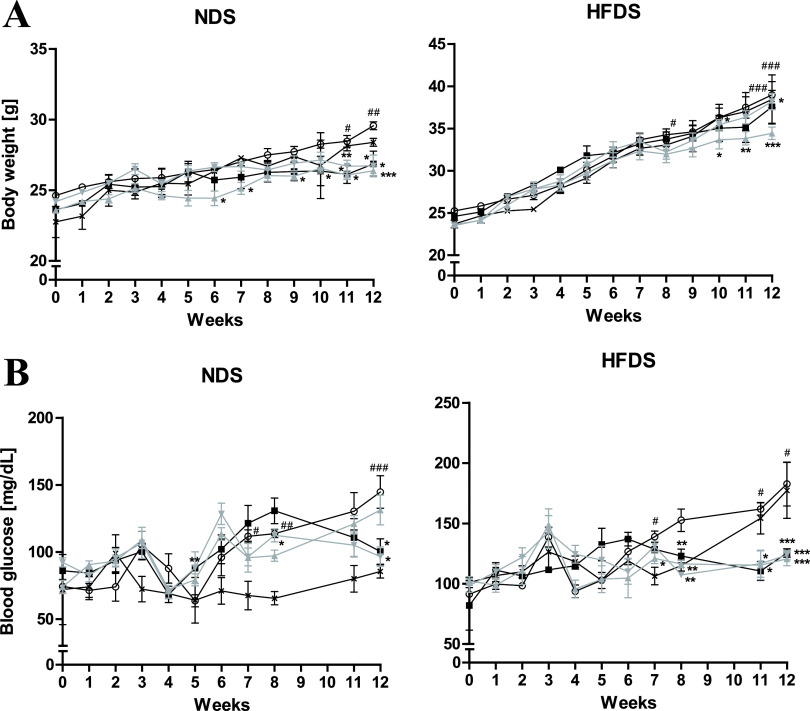
Studies have demonstrated the effect of BNR17 (Lactobacillus gasseri) on a mouse model of T2DM (28). Our study evaluated the antidiabetic efficiency of other LAB, such as Lactobacillus plantarum (LRCC5310 and LRCC5314), on chronic stress induced by cold exposure and an HFD in a mouse model (Fig. 1); the BNR17 group was used as a positive control at a concentration of 108 CFU. Chronic cold stress and an HFD induced an increase in body weight and fasting blood glucose levels compared to a normal diet (ND) and HFD (Fig. 2). Administration of LAB to the ND group induced a lower body weight gain than that observed in the untreated ND group and the ND group exposed to chronic cold exposure-related stress (NDS) (Fig. 2A). Mice exposed to chronic cold exposure-related stress and fed an HFD (HFDS) showed a significant difference in body weight at 8, 11, and 12 weeks compared with HFD-fed mice (Fig. 2A). Although not in a dose-dependent manner (LRCC5310 and LRCC5314 1010 CFU results are not shown), both LRCC5310- and LRCC5314-treated groups showed a decrease in weight gain compared to the HFDS group (Fig. 2A). Administration of BNR17 and LRCC5314 to the NDS group dramatically decreased the fasting blood glucose levels from 8 weeks to 12 weeks to levels comparable with those observed in the untreated ND mice at 12 weeks (Fig. 2B). In the HFDS group, oral LAB administration considerably lowered fasting blood glucose levels compared to untreated HFDS and HFD groups. This difference in fasting blood glucose levels between the untreated and LAB-treated HFDS groups was significant at 8 weeks (Fig. 2B). From these results, it can be inferred that oral administration of LAB can prevent an increase in blood glucose levels caused by obesity in this cold-stress-induced, HFD-fed (HFDS) mouse model that mimics T2DM.
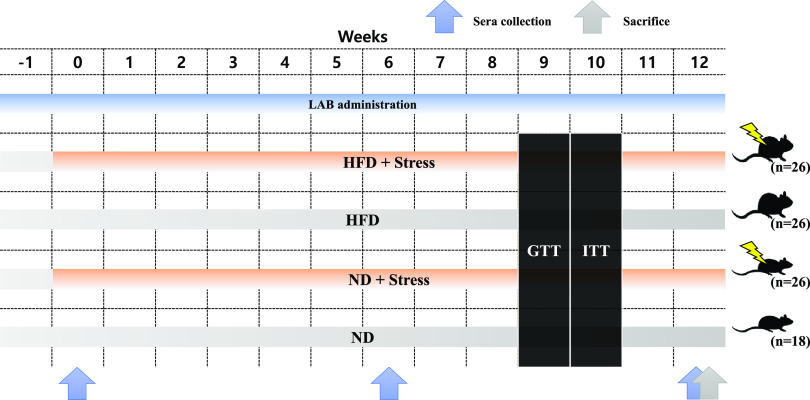
FIG 1.
Schematic of diet, stress, and LAB administration in mice. Male C57BL/6 mice were fed a ND or HFD with or without LAB. Their sera were collected at 0, 6, and 12 weeks, and the mice were sacrificed at 12 weeks. ND, normal diet; HFD, high-fat diet; GTT, glucose tolerance test; ITT, insulin tolerance test.
FIG 2.
Effect of cold stress and Lactobacilli on body weight and fasting blood glucose levels for 12 weeks. Body weight (A) and fasting blood glucose levels (B) of chronic stress-induced, high-fat diet-fed mice treated with LAB. Values represent the mean ± SEM (standard error of the mean); *P < 0.05, **P < 0.01, and ***P < 0.001 for comparisons between NDS or HFDS and the respective LAB administration groups; #P < 0.05, ##P < 0.01, and ###P < 0.001 for comparisons between ND or HFD and NDS or HFDS groups, respectively (Student’s t test). Symbols: ×, ND (n = 3) or HFD (n = 3); ○, NDS (n = 5) or HFDS (n = 5); ■, BNR17 (108, n = 5); ▴, LRCC5310 (108, n = 5); ▾, LRCC5314 (108, n = 5).
LAB improved host glucose and insulin sensitivity in the cold-stress-induced mouse model.
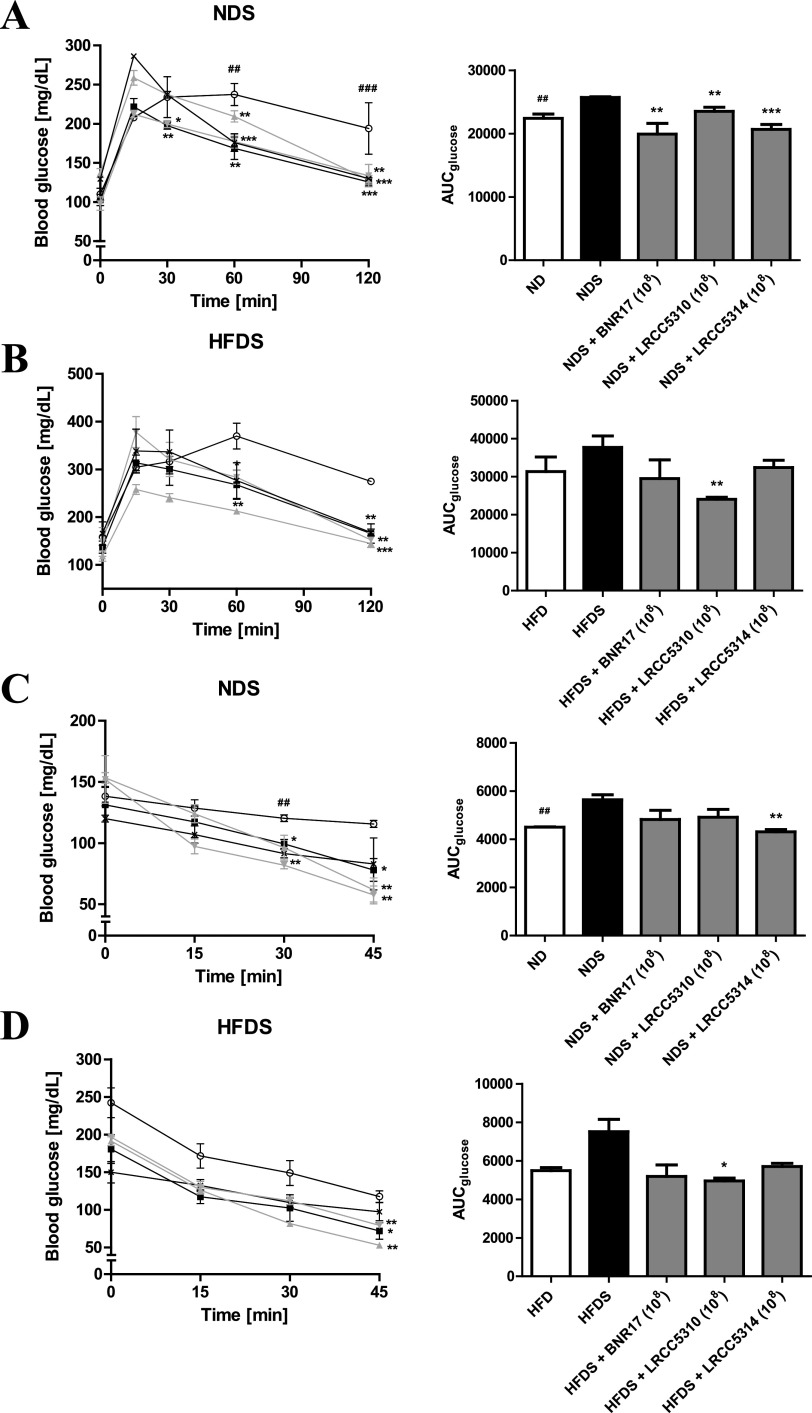
Intraperitoneal glucose tolerance tests (IPGTTs) and intraperitoneal insulin tolerance tests (IPITTs) were used to examine the effects of LAB on glucose homeostasis in our chronic cold-stress-induced, HFD-fed mouse model. In the IPGTT performed on the NDS group, after LAB administration, the levels of fasting blood glucose were significantly lower than levels observed in the untreated NDS group at 60 and 120 min; the area under the curve for glucose (AUCglucose) values in all LAB-treated NDS mice were lower than those observed in the untreated NDS mice (Fig. 3A). Although the fasting blood glucose levels in the HFDS group showed a tendency to decrease at 60 and 120 min after glucose injection, similar to the NDS group, only HFDS mice treated with LRCC5310 showed a significant decrease in AUCglucose (Fig. 3B). In the IPITT, administration of LAB showed significant decreases in blood glucose levels at 30 and 45 min in the NDS group and at 45 min in the HFDS group (Fig. 3C). Administration of LRCC5314 and LRCC5310 significantly decreased the AUCglucose values in the NDS and HFDS groups, respectively, compared with untreated mice in these groups (Fig. 3D).
FIG 3.
IPGTT and IPITT of cold-stress-induced, high-fat diet-fed mice treated with LAB for 12 weeks. IPGTTs of NDS groups (A) and HFDS groups (B) were conducted at 9 weeks, and IPITTs of NDS groups (C) and HFDS groups (D) were conducted at 10 weeks. Values represent the mean ± SEM; *P < 0.05, **P < 0.01, and ***P < 0.001 for comparisons between NDS or HFDS and respective LAB administration groups; #P < 0.05, ##P < 0.01, and ###P < 0.001 for comparisons between ND or HFD and NDS or HFDS groups, respectively (Student’s t test). Symbols: ×, ND (n = 3) or HFD (n = 3); ○, NDS (n = 5) or HFDS (n = 5); ■, BNR17 (108, n = 5); ▴, LRCC5310 (108, n = 5); ▾, LRCC5314 (108, n = 5).
L. plantarum improved host glucose homeostasis by regulating serum insulin levels.
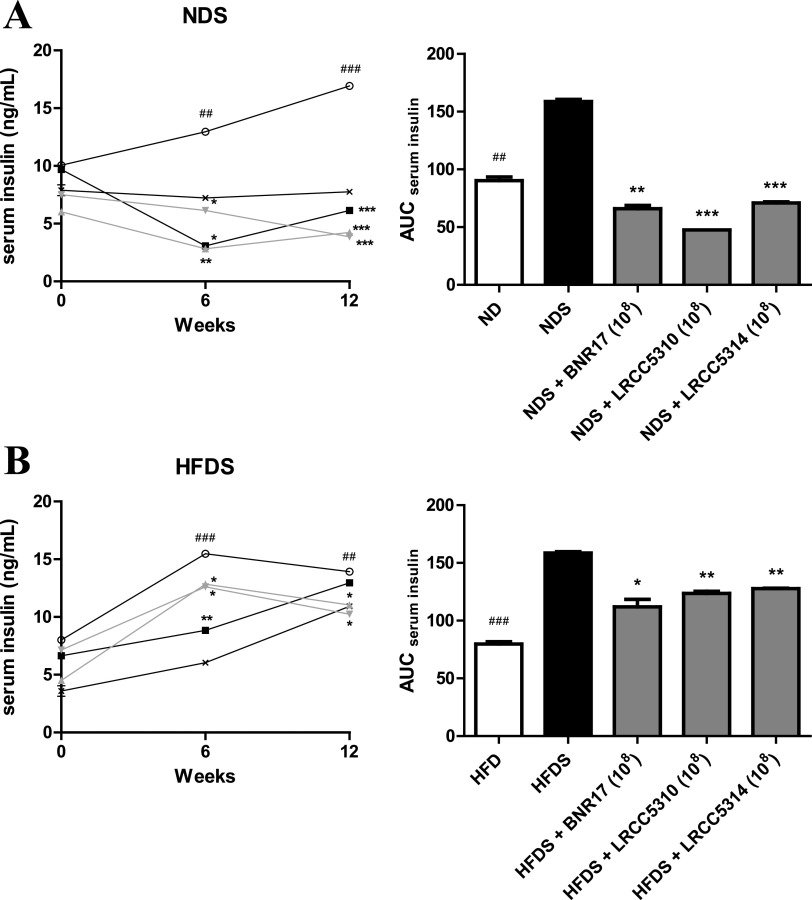
Based on the results showing that LAB improved host glucose and insulin sensitivity in our chronic stress-induced mouse model (Fig. 3), we measured serum insulin levels to investigate the link between the improved glucose homeostatic effect of LAB and serum insulin levels. To measure the serum insulin concentrations over time, sera were collected from all mice at 0, 6, and 12 weeks. A significant decrease was observed in area under the curve for serum insulin (AUCserum insulin) values after LAB administration to the NDS group (Fig. 4A). Except in BNR7-treated HDFS mice at 12 weeks, serum insulin concentrations and AUCserum insulin had significantly decreased after administration of LRCC5310 and LRCC5314 to HDFS mice compared to those values observed in untreated mice (Fig. 4B). These results indicate that supplementation with LAB can improve glucose homeostasis by regulating serum insulin levels in chronic stress-exposed, HFD-fed mice.
FIG 4.
Serum insulin levels of cold-stress-induced, high-fat diet-fed mice treated with LAB. Serum collection from NDS groups (A) and HFDS groups (B) was performed at 0, 6, and 12 weeks. Values represent the mean ± SEM; *P < 0.05, **P < 0.01, and ***P < 0.001 for comparisons between NDS or HFDS and respective LAB administration groups; #P < 0.05, ##P < 0.01, and ###P < 0.001 for comparisons between ND or HFD groups and NDS or HFDS groups, respectively (Student’s t test). Symbols: ×, ND (n = 3) or HFD (n = 3); ○, NDS (n = 5) or HFDS (n = 5); ■, BNR17 (108, n = 5); ▴, LRCC5310 (108, n = 5); ▾, LRCC5314 (108, n = 5).
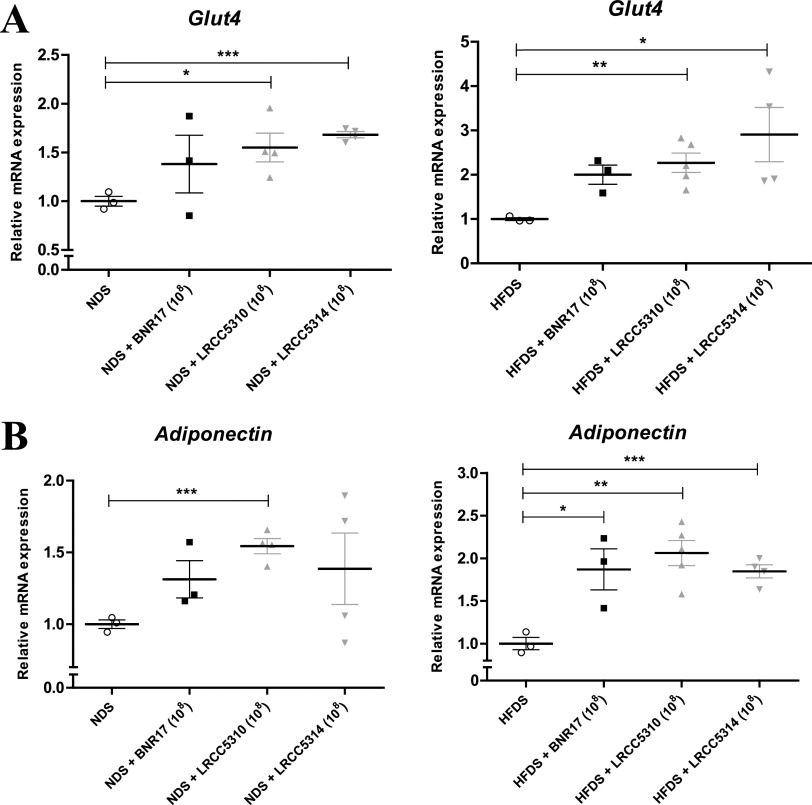
L. plantarum regulated glucose homeostasis by upregulating expression of Glut4 and adiponectin.
After confirming that long-term LAB administration regulated serum insulin levels in our experimental mouse model (Fig. 4), we further analyzed the expression of insulin metabolism- and glucose metabolism-related genes Glut4, the insulin-regulated glucose transporter, and adiponectin in epididymal white adipose tissue of all groups of mice by real-time PCR. Administration of LRCC5310 and LRCC5314, but not BNR17, upregulated Glut4 expression in the adipose tissue of NDS and HFDS mice compared to untreated mice (Fig. 5A). A similar upregulation of adiponectin expression was observed after the administration of all LAB to HFDS mice compared with untreated HFDS mice (Fig. 5B). Only LRCC5310 treatment upregulated adiponectin expression in NDS mice compared to untreated NDS mice (Fig. 5B).
FIG 5.
LAB modulated mRNA expression of obesity and diabetes-related genes (Glut4 [A] and adiponectin [B]) in white adipose tissue. Cold-stress-induced, high-fat diet-fed mice, NDS groups, and HFDS groups were treated with LAB for 12 weeks, and the epididymal white adipose tissue was removed at 12 weeks. mRNA expression was measured by real-time PCR using Gapdh as an internal reference gene. Values represent the mean ± SEM; *P < 0.05, **P < 0.01, and ***P < 0.001 (Student’s t test).
L. plantarum suppressed proinflammatory activity in chronic cold-stress-induced type 2 diabetic mice.
To confirm that administration of LAB alleviates insulin resistance by regulating the inflammatory response, we examined the secretion of proinflammatory factors and related gene expression levels. TNF-α and IL-6 are major cytokines secreted by proinflammatory M1 macrophages in obesity that activate both T helper 1 and T helper 17 cells (35). C-C motif chemokine ligand 2 (CCL2) is a chemokine that promotes macrophage recruitment and infiltration into adipose tissue. We measured the systemic production of TNF-α and IL-6 by measuring their levels in sera collected at 0, 6, and 12 weeks. Both TNF-α and IL-6 levels in serum were significantly increased in NDS and HFDS mice compared with levels observed in ND and HFD mice, respectively (Fig. 6A and B). Administration of all LAB decreased the serum TNF-α levels by over 2-fold compared with the untreated NDS group at 12 weeks (Fig. 6A). Administration of LRCC5310 and LRCC5314, but not BNR17, decreased serum TNF-α levels in HFDS mice compared with untreated HFDS mice at 12 weeks (Fig. 6A). Although there was a significant decrease in serum IL-6 levels after the administration of LRCC5310 and LRCC5314 to NDS and HFDS mice compared with untreated NDS and HFDS mice, the decrease in IL-6 levels was not significant after LRCC5310 administration to NDS mice compared to untreated NDS mice (Fig. 6B). Next, we measured expression of Tnf-α, Il-6, and Ccl2 in epididymal white adipose tissue collected at 12 weeks. LRCC5314 treatment downregulated Il-6 expression in NDS and HFDS mice compared with untreated NDS and HFDS mice (Fig. 6C). Unlike the significant downregulation of Ccl2 expression by BNR17 treatment in the HFDS mice, administration of LRCC5310 and LRCC5314 resulted in downregulation of Ccl2 expression in all stress-induced mice (Fig. 6D). All mice treated with LAB showed downregulation of leptin expression compared to untreated mice; however, this downregulation was not significant for BNR17-treated mice in the HFDS group (Fig. 6E). Tnf-α expression was significantly downregulated in the white adipose tissue of LRCC5310- and LRCC5314-treated mice, but Tnf-α expression was not significantly downregulated in BNR17-treated mice in the NDS group compared to expression in untreated NDS mice; this downregulation was not significant in any of the LAB-treated HFDS mice (Fig. 6F). These results indicate that LAB administration under stress-induced and HFD-fed conditions can regulate proinflammatory cytokines, chemokines, such as Ccl2, and adipokines, such as leptin, to attenuate inflammation caused by obesity.
FIG 6.
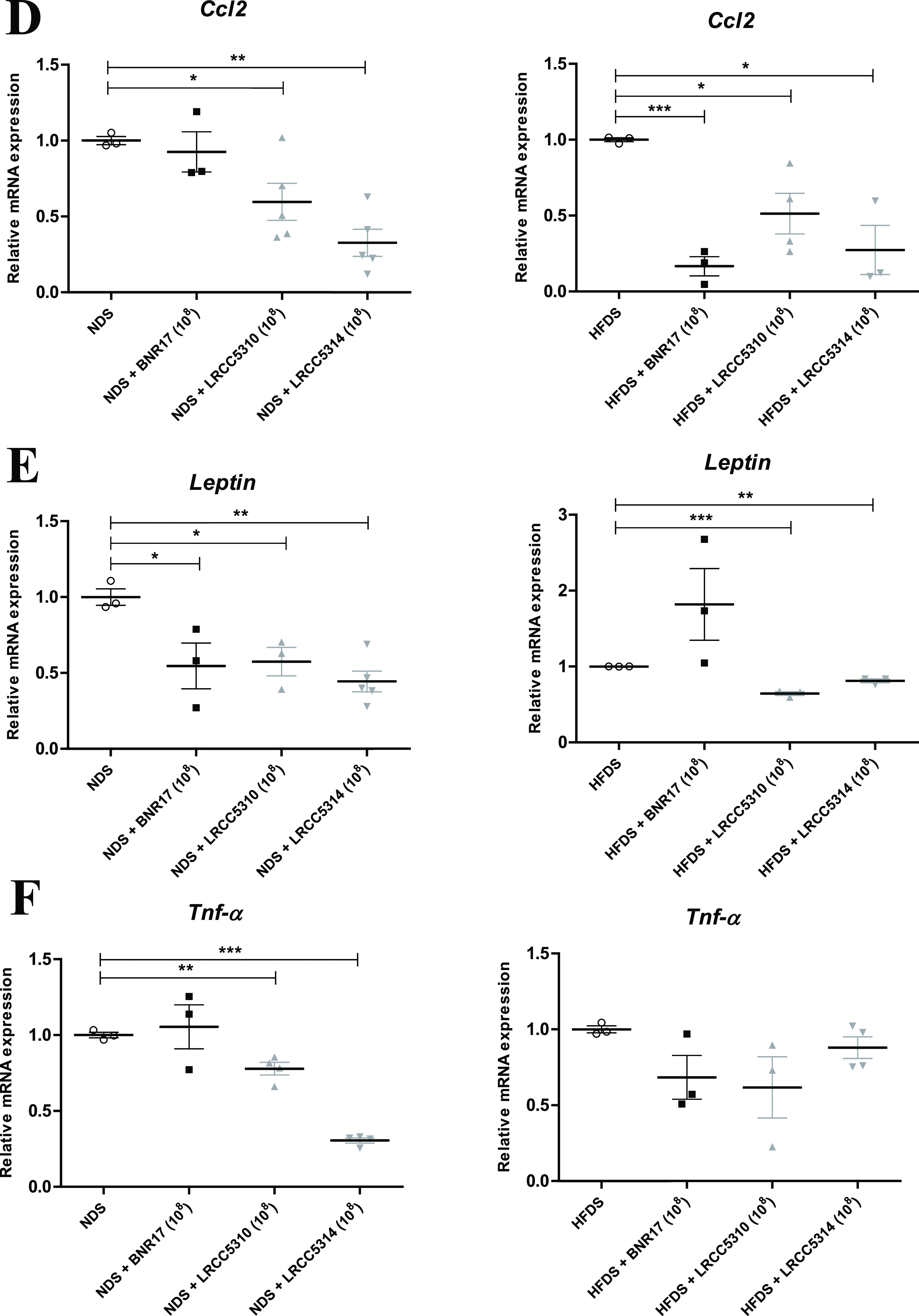
(Continued)
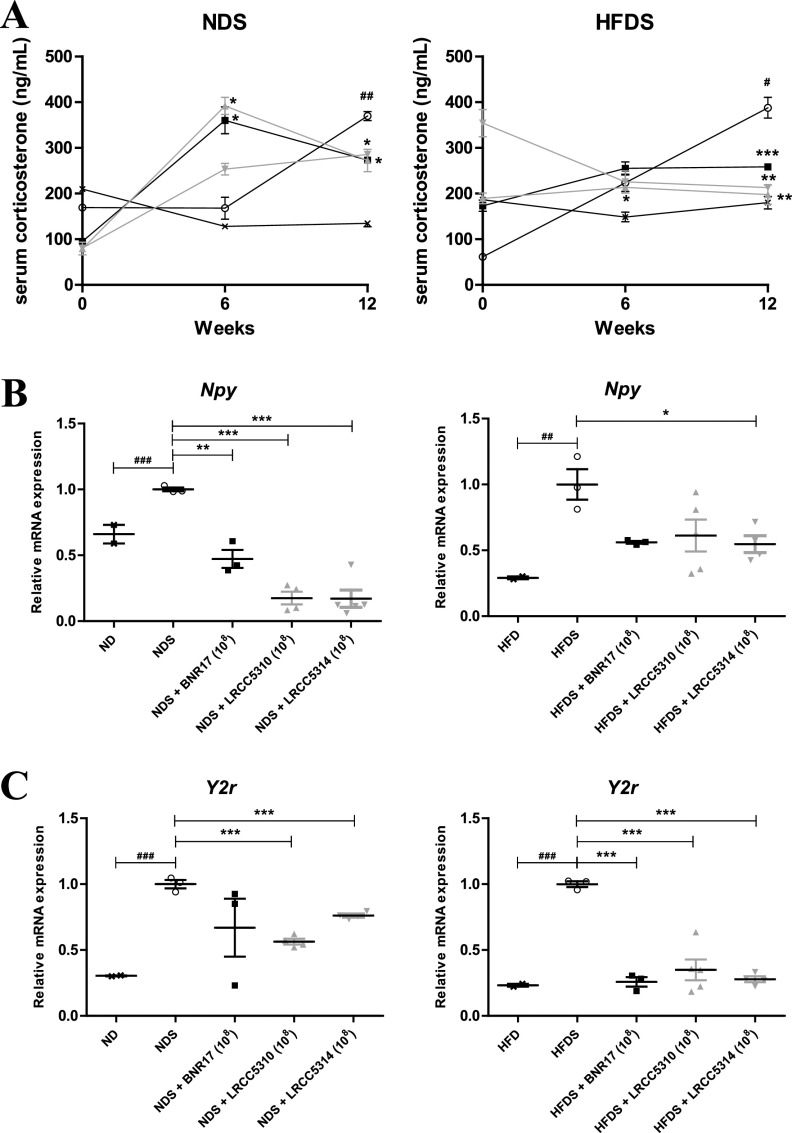
LAB attenuated stress-related factors in obesity and diabetes in chronic cold-stress-induced type 2 diabetic mice.
NPY-Y2r ligand-receptor binding leads to obesity through adipocyte differentiation by increasing production of the stress-related hormone corticosterone (11). Hence, we measured serum corticosterone levels at 0, 6, and 12 weeks and mRNA levels of Npy and Y2r in epididymal white adipose tissue collected at 12 weeks to determine whether long-term administration of LAB could attenuate stress levels. We confirmed that serum corticosterone levels were dramatically increased in the stress-induced mice compared to levels in the nonstress-induced mice (Fig. 7A). At 12 weeks, LAB administration significantly reduced the serum corticosterone levels in all stress-induced groups compared to the respective untreated group (Fig. 7A). We confirmed that Npy and Y2r mRNA levels in the stress-induced groups were significantly increased compared with levels in the respective untreated groups (Fig. 7B and C). Npy expression was significantly downregulated in LRCC5314-treated mice compared to expression in untreated HFDS mice (Fig. 7B). In the NDS group, administration of all the LAB downregulated Npy expression compared to untreated mice (Fig. 7B). Over 2-fold (significant) downregulation of Y2r expression was observed after administration of LRCC5310 and LRCC5314 to NDS and HFDS mice compared with expression levels observed in untreated mice in both groups (Fig. 7C). Further, BNR17 treatment downregulated Y2r expression in HFDS mice compared to untreated mice (Fig. 7C). These results confirm the successful establishment of the mouse model of chronic cold stress and demonstrate that all three LAB strains could modulate the levels of the stress hormone, Npy, and Y2r to suppress the proliferation of adipocytes.
FIG 7.
Serum corticosterone levels (A) and mRNA expression of stress-related genes Npy (B) and Y2r (C) in white adipose tissue of cold-stress-induced, high-fat diet-fed mice treated with LAB. Serum collection from NDS groups and HFDS groups was performed at 0, 6, and 12 weeks, and the epididymal white adipose tissue was removed at 12 weeks. mRNA levels were measured by real-time PCR using Gapdh as an internal reference gene. Values represent the mean ± SEM; *P < 0.05, **P < 0.01, and ***P < 0.001 for comparisons between NDS or HFDS groups and respective LAB administration groups; #P < 0.05, ##P < 0.01, and ###P < 0.001 for comparisons between ND or HFD and NDS or HFDS groups, respectively (Student’s t test). ×, ND (n = 3) or HFD (n = 3); ○, NDS (n = 5) or HFDS (n = 5); ■, BNR17 (108, n = 5); ▴, LRCC5310 (108, n = 5); ▾, LRCC5314 (108, n = 5).
DISCUSSION
The current study is the first to demonstrate that LAB strains, Lactobacillus gasseri isolated from human breast milk and L. plantarum isolated from fermented vegetables such as kimchi, could attenuate insulin resistance and stress in a chronic cold-stress-induced, HFD-fed mouse model. For this study, we adopted two stress methods to perform a prestudy with an animal model of stress and obesity. First, we performed the forced swim stress method (36). Contrary to expectations, we observed that the HFDS mice showed lower body weight gain and fasting blood glucose levels than the HFD mice. In addition, IPGTTs and IPITTs revealed that insulin resistance was reduced in the HFDS mice (data not shown). However, it does not mean that the forced swim stress method is antidiabetic in mice fed an HFD. The second stress method was the cold exposure stress method used in this study. In a study using this stress method (11), researchers had observed that direct release of NPY, a sympathetic neurotransmitter, from adipocytes resulted in obesity in an HFD-fed animal model exposed to cold-related stress. Cold exposure stress activates sympathetic nerves to release NPY rather than norepinephrine, a hormone that activates fat burning, from mouse sympathetic nerves and increases the levels of NPY and Y2R produced in adipocytes. As a result, NPY-Y2R binding is increased, leading to increased proliferation and differentiation of adipocytes due to cold exposure stress. Thus, we verified that we could use this mouse model combining stress and an HFD to mimic T2DM and demonstrated the antidiabetic effect of LAB in this model (Fig. 2 to 4).
Studies have confirmed the probiotic properties and safety of Lactobacillus gasseri and L. plantarum (37–39). However, probiotic potential and safety will have to be shown for LRCC5310 and LRCC5314, the L. plantarum strains used in this study. As BNR17 was found to exert antidiabetic effects in a db/db mouse model (29), we used one concentration (1 × 108 CFU) of the L. gasseri BNR17 strain as a positive control to compare with two concentrations (1 × 108 and 1 × 1010 CFU) of LRCC5310 and LRCC5314 in this study.
Here, we focused on the reduction in body weight and fasting blood glucose levels by oral LAB administration in the mouse model generated by inducing chronic cold stress for 12 weeks. We found that both body weight and fasting blood glucose levels were effectively reduced at 12 weeks by LAB administration (Fig. 2). Our results are consistent with findings of previous studies that showed the antiobesity effects of L. plantarum (40, 41). As we observed a decrease in fasting blood glucose levels, we administered the IPGTT and IPITT, tests that are universally used to diagnose diabetes, to the mice and measured serum insulin concentrations (Fig. 3 and 4). In agreement with another study (42), administration of LAB attenuated insulin resistance in HFD-fed mice, but not in ND-fed mice (data not shown). To determine whether long-term administration of LAB has antidiabetic effects besides attenuating insulin resistance, we measured the expression levels of glucose transporter type 4 (Glut4) and adiponectin in epididymal adipose tissue of mice. Both GLUT4 and adiponectin are associated with improved insulin action (43, 44). The upregulation of Glut4 and adiponectin expression provides additional evidence for the antidiabetic efficacy of the LAB used in this study (Fig. 5). Thus, long-term administration of LAB was able to restore the expression of genes related to glucose metabolism that are downregulated due to obesity (45).
Obesity-induced chronic inflammation, characterized by macrophage infiltration and proinflammatory cytokine expression, especially in white adipose tissue, contributes to the exacerbation of systemic insulin resistance and T2DM. High TNF-α levels have been found in T2DM patients (46), and increased production of TNF-α in adipose tissues is related to obesity-associated insulin resistance that leads to the development of T2DM (47). IL-6 is another proinflammatory cytokine that induces the development of T2DM by causing inflammation through its effects on immune cell proliferation, differentiation, migration, and apoptosis (48). CCL2 is the chemokine that recruits monocytes, memory T cells, and dendritic cells to sites of inflammation produced by either injury or infection (49). In patients with T2DM, CCL2 mRNA levels are elevated compared with levels observed in nondiabetic subjects (50). Leptin has recently emerged as a key link between metabolic responses and inflammation (18). Therefore, in this study, we measured the concentrations and expression levels of all these inflammatory factors and the adipokine leptin in serum and white adipose tissue to determine whether the administration of LAB could alleviate insulin resistance by controlling the inflammatory response. Administration of LAB for 12 weeks reduced the secretion and expression of proinflammatory cytokines TNF-α and IL-6 and the chemokine CCL2, which is related to low-grade inflammation, and reduced the induction of macrophage differentiation and infiltration. Although downregulation of Tnf-α expression in adipose tissue was insignificant, downregulation of Ccl2 expression was significant in the HFDS mice compared with untreated mice. In particular, the LRCC5310- and LRCC5314-treated groups showed a decrease in leptin expression in HFDS mice compared to the untreated HFDS mice. Thus, LAB administration appears to regulate the inflammatory response by reducing adipokine production (Fig. 6). After verifying that the stress method used in this study exacerbated diabetes, we investigated whether exposing the mice to chronic stress could change the stress marker levels in the serum and adipose tissue. The stress hormone cortisol is the primary endogenous adrenal steroid in mammals, including humans, whereas corticosterone is the primary adrenal corticosteroid in rodents (51). We found that long-term administration of LAB regulated the expression of Npy and Y2r mRNAs in adipose tissue (Fig. 7). These results suggest that LAB could also be used to prevent the development of T2DM.
Thus, we demonstrated that the LAB strains used in this study can help normalize the expression of metabolic genes, inhibit inflammatory responses, and suppress stress in obesity and insulin resistance. Further studies are required to determine the effects of LAB on macrophage infiltration, inflammation, and differentiation and the permeability of intestine epithelial cells (IECs).
MATERIALS AND METHODS
Animals and ethics statement.
Ninety-six male C57BL/6 mice, 9 weeks old and weighing between 22 and 24 g (Orient Bio, Inc., Republic of Korea), were randomized by weight and housed in polypropylene cages in a specific pathogen-free environment (52). All mice were maintained on a standard 12-h light/12-h dark cycle (lights on at 07:00 and lights off at 19:00 h) in a temperature-controlled environment (21 ± 3°C) and at a humidity of 50 ± 10% with access to purified water and chow ad libitum. All mice used in the experiment were euthanized with CO2. All procedures were conducted according to the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the protocol was approved by the Chung-Ang University Institutional Animal Care and Use Committee of the Laboratory Animal Research Center (IACUC number 2020-00010).
Experimental groups.
The mice were acclimated to their environment for 1 week before the start of the experiment. The animals were randomly divided into four groups according to exposure to chronic cold stress and the type of diet provided: ND-fed mice (ND, n = 18), mice fed an ND and subjected to chronic cold stress (NDS, n = 26), HFD-fed mice (HFD, n = 26), and mice fed an HFD and subjected to chronic cold stress (HFDS, n = 26) (Fig. 1). Lactobacillus plantarum was administered to all the mice in the ND, NDS, HFD, and HFDS groups in the following forms: LRCC5310 (108, 1010 CFU) and LRCC5314 (108, 1010 CFU); L. gasseri BNR17 (108 CFU) was administered as positive control. The HFD (D12492) was purchased from Research Diets (Research Diet, Inc., USA); the proportion of fat was ∼60% in the HFD and 4.5% in the ND (53, 54).
Lactic acid bacteria preparation.
BNR17 (Fatburn Plus, APYLD, Republic of Korea) consisted of 1.1 × 1010/g of viable, lyophilized bacteria; LRCC5314 and LRCC5310 consisted of 1 × 1011/g of viable, lyophilized bacteria. LAB samples for administration to mice were prepared by suspending the lyophilized bacterial powder in 200 μl of phosphate-buffered saline (PBS) to obtain the required concentration. These suspensions were prepared fresh daily and orally administered twice every day. Control groups were administered only 200 μl of PBS.
Chronic cold stress induction and measurement of body weight and blood glucose levels.
We applied cold stress by following a previously described method (11). Briefly, we placed the mice from the cold stress groups in 0.5-cm-deep ice-cold water at 4°C for 1 h every day for 12 weeks. The mice not subjected to cold stress were placed in 0.5-cm-deep water at room temperature. All stress induction was performed between 17:00 and 18:00 h. We returned the stressed mice to their cages with free access to food and water; they did not show any abnormal symptoms of grooming or eating. All 96 mice were fasted for 12 h (22:00 to 10:00 h). Body weight and blood glucose levels were measured at the same time (10:00 h) of the day once every week; the duration of the experiment was 12 weeks.
Glucose modulation by LAB in chronic stress-induced, high fat diet-fed mice.
Nine weeks after commencing the HFD and cold exposure stress, the mice were fasted for 12 h (55) and injected intraperitoneally with 10% d-glucose (Sigma-Aldrich, St. Louis, MO, USA) solution at a dose of 1 g/kg of body weight for an IPGTT (56). Blood samples were collected from the tail vein at 0, 15, 30, 60, and 120 min after glucose administration, and blood glucose levels were determined using a glucometer (Accu-Chek Active, Roche, Castle Hill, New South Wales, Australia). One week later, the mice were fasted for 4 h from 10:00 h onwards and were injected intraperitoneally with insulin (SAFC Biosciences, Inc., Lenexa, KS, USA) at a dose of 0.75 U/kg of body weight. Blood samples were obtained from the tail vein at 0, 15, 30, and 45 min after insulin injection for determination of glucose levels (57). Cold exposure was performed after the glucose and insulin tolerance tests on the same day.
Serum collection and quantification of cytokines, insulin, and corticosterone by enzyme-linked immunosorbent assay.
Blood samples in a 12-h fasting state were obtained from the submandibular vein. The blood samples were centrifuged at 2,600 × g for 20 min; the separated serum was preserved at −80°C to measure the parameters. Serum concentrations of the proinflammatory cytokines TNF-α and IL-6 (all from BD Bioscience, San Diego, CA, USA) were measured by sandwich enzyme-linked immunosorbent assay (ELISA). Briefly, 96-well plates were coated by overnight incubation with 50 μl of antigen-specific antibodies (2 μg/ml TNF-α, 3 μg/ml IL-6) at 4°C. Plates were subsequently blocked with 1% bovine serum albumin for 1.5 h at room temperature. Diluted serum samples were added to the wells and incubated overnight at 4°C, and biotinylated antibodies specific against the cytokines and avidin-alkaline phosphatase were then added. The reaction was developed by incubating with p-nitrophenyl phosphate for 30 min. Finally, the optical density of each well was measured at 405 nm using a microplate reader (Emax, Molecular Devices). Serum insulin and corticosterone levels were analyzed using an ultrasensitive mouse insulin ELISA kit (90080, Crystal Chem, Downers Grove, IL) and a corticosterone ELISA kit (ADI-900-097, Enzo Biochem, Farmingdale, NY), respectively.
Total RNA extraction and real-time PCR analysis.
Total RNA was extracted from 100 mg of epididymal white adipose tissue using TRIzol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The RNA was reverse transcribed to cDNA at 42°C over 1 h in a 25-μl cocktail containing 5× reverse transcriptase (RT) buffer, 10 mM deoxynucleoside triphosphates (dNTPs; 200 U), Maloney murine leukemia virus reverse transcriptase (MMLV-RT), and 100 pmol of oligo-dT primer. The concentration of cDNA was estimated, followed by quantitative real-time PCR (RT-PCR) with 2× iQTM SYBR green supermix (Bio-Rad, Hercules, CA, USA) to determine the mRNA levels of the target genes. Amplification was performed using a CFX Connect real-time PCR detection system (Bio-Rad) under the following conditions: 95°C for 2 min, 40 cycles at 95°C for 30 s, and 60°C for 20 s. To confirm PCR specificity, the PCR products were subjected to melting curve analysis. The comparative threshold method was used to calculate the relative amounts of mRNA in the experimental samples compared to those in control samples. Gene expression was normalized to the expression levels of Gapdh. Primer sequences for the target mouse genes are listed in Table 1.
TABLE 1.
Sequences of primers used for quantitative real-time PCR
| Gene name | Forward (5′ to 3′) | Reverse (5′ to 3′) | Product size (bp) | Accession no. |
|---|---|---|---|---|
| Gapdh | TGCACCACCAACTGCTTAGC | GGCATGGACTGTGGTCATGAG | 87 | NM_001289726.1 |
| Glut4 | CTTGGCTCCCTTCAGTTTG | TGCCTTGTGGGATGGAAT | 130 | AB008453.1 |
| Adiponectin | TCCTGGAGAGAAGGGAGAGAAAG | CCCTTCAGCTCCTGTCATTCC | 88 | NM_009605 |
| Tnf-α | GTGGTGCCAGCCGATGGGTT | CTGCCCGGACTCCGCAAAGTC | 300 | NM_013693 |
| Il-6 | ACAAAGCCAGAGTCCTTCAGAGAGA | GGCATAACGCACTAGGTTTGCCG | 238 | NM_031168 |
| Ccl2 | TTT TGT CAC CAA GCT CAA GAG A | ATT AAG GCA TCA CAG TCC GAG T | 279 | NM_011333.3 |
| Leptin | GTGTCGGTTCCTGTGGCTTT | TGGTCTTGATGAGGGTTTTGG | 95 | NM_008493.3 |
| Npy | GTGGATCTCTTCTCTCACAGAGG | GCCCAAACACACGAGCAGAG | 143 | NM_023456 |
| Y2r | TTGGCAACTCCCTGGTAATC | TTTCCACTCTCCCATCAAGG | 155 | NM_008731.3 |
Statistical analysis.
Statistical significance was determined by using GraphPad Prism (GraphPad Software, La Jolla, CA, USA). Results are shown as mean ± standard error of the mean (SEM), and statistical analysis was performed by using Student’s t test (between two groups). The area under the curve values for glucose (AUCglucose) and serum insulin (AUCserum insulin) were determined using the trapezoidal rule. A P value of <0.05 was considered statistically significant; P values of <0.05, <0.01, and <0.001 are indicated by asterisks (*) and pound (#) signs in the figures.
ACKNOWLEDGMENTS
This work was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through the Agricultural Microbiome R&D Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (91804-4).
We declare no conflict of interest.
Contributor Information
Kwang Woo Hwang, Email: khwang@cau.ac.kr.
Nancy E. Freitag, University of Illinois at Chicago
REFERENCES
- 1.Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, Cavan D, Shaw JE, Makaroff LE. 2017. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract 128:40–50. 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 2.Lebovitz HE. 2001. Insulin resistance: definition and consequences. Exp Clin Endocrinol Diabetes 109:S135–S148. 10.1055/s-2001-18576. [DOI] [PubMed] [Google Scholar]
- 3.Hardy OT, Czech MP, Corvera S. 2012. What causes the insulin resistance underlying obesity? Curr Opin Endocrinol Diabetes Obes 19:81–87. 10.1097/MED.0b013e3283514e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC. Jr., International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity. 2009. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120:1640–1645. 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 5.Yaribeygi H, Farrokhi FR, Butler AE, Sahebkar A. 2019. Insulin resistance: review of the underlying molecular mechanisms. J Cell Physiol 234:8152–8161. 10.1002/jcp.27603. [DOI] [PubMed] [Google Scholar]
- 6.Hackett RA, Steptoe A. 2017. Type 2 diabetes mellitus and psychological stress - a modifiable risk factor. Nat Rev Endocrinol 13:547–560. 10.1038/nrendo.2017.64. [DOI] [PubMed] [Google Scholar]
- 7.Mikurube H, Kaneko M, Murata C, Komaki Y, Ishikawa N, Higashiyama R, Fukasawa K, Watanabe T. 2005. Association of change in the type of job with prevalence of components of the metabolic syndrome-special reference to job stress. Nihon Koshu Eisei Zasshi 52:987–993. [PubMed] [Google Scholar]
- 8.Dallman MF, Pecoraro N, Akana SF, La Fleur SE, Gomez F, Houshyar H, Bell ME, Bhatnagar S, Laugero KD, Manalo S. 2003. Chronic stress and obesity: a new view of "comfort food". Proc Natl Acad Sci U S A 100:11696–11701. 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stanley BG, Magdalin W, Seirafi A, Nguyen MM, Leibowitz SF. 1992. Evidence for neuropeptide Y mediation of eating produced by food deprivation and for a variant of the Y1 receptor mediating this peptide's effect. Peptides 13:581–587. 10.1016/0196-9781(92)90093-i. [DOI] [PubMed] [Google Scholar]
- 10.Yang K, Guan H, Arany E, Hill DJ, Cao X. 2008. Neuropeptide Y is produced in visceral adipose tissue and promotes proliferation of adipocyte precursor cells via the Y1 receptor. FASEB J 22:2452–2464. 10.1096/fj.07-100735. [DOI] [PubMed] [Google Scholar]
- 11.Kuo LE, Kitlinska JB, Tilan JU, Li L, Baker SB, Johnson MD, Lee EW, Burnett MS, Fricke ST, Kvetnansky R, Herzog H, Zukowska Z. 2007. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat Med 13:803–811. 10.1038/nm1611. [DOI] [PubMed] [Google Scholar]
- 12.Donath MY, Shoelson SE. 2011. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 11:98–107. 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 13.Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. 2014. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract 105:141–150. 10.1016/j.diabres.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Kohlgruber A, Lynch L. 2015. Adipose tissue inflammation in the pathogenesis of type 2 diabetes. Curr Diab Rep 15:92. 10.1007/s11892-015-0670-x. [DOI] [PubMed] [Google Scholar]
- 15.Castro AM, Macedo-de la Concha LE, Pantoja-Meléndez CA. 2017. Low-grade inflammation and its relation to obesity and chronic degenerative diseases. Rev Med Hosp Gen (Mex) 80:101–105. 10.1016/j.hgmx.2016.06.011. [DOI] [Google Scholar]
- 16.Xia C, Rao X, Zhong J. 2017. Role of T lymphocytes in type 2 diabetes and diabetes-associated inflammation. J Diabetes Res 2017:6494795. 10.1155/2017/6494795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hotamisligil GS. 2006. Inflammation and metabolic disorders. Nature 444:860–867. 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 18.Iikuni N, Lam QL, Lu L, Matarese G, La Cava A. 2008. Leptin and inflammation. Curr Immunol Rev 4:70–79. 10.2174/157339508784325046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landman RE, Puder JJ, Xiao E, Freda PU, Ferin M, Wardlaw SL. 2003. Endotoxin stimulates leptin in the human and nonhuman primate. J Clin Endocrinol Metab 88:1285–1291. 10.1210/jc.2002-021393. [DOI] [PubMed] [Google Scholar]
- 20.de Luca C, Olefsky JM. 2008. Inflammation and insulin resistance. FEBS Lett 582:97–105. 10.1016/j.febslet.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chawla A, Nguyen KD, Goh YP. 2011. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol 11:738–749. 10.1038/nri3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ouchi N, Parker JL, Lugus JJ, Walsh K. 2011. Adipokines in inflammation and metabolic disease. Nat Rev Immunol 11:85–97. 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rider P, Carmi Y, Cohen I. 2016. Biologics for targeting inflammatory cytokines, clinical uses, and limitations. Int J Cell Biol 2016:9259646. 10.1155/2016/9259646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanz Y, Nadal I, Sanchez E. 2007. Probiotics as drugs against human gastrointestinal infections. Recent Pat Antiinfect Drug Discov 2:148–156. 10.2174/157489107780832596. [DOI] [PubMed] [Google Scholar]
- 25.Collins MD, Gibson GR. 1999. Probiotics, prebiotics, and synbiotics: approaches for modulating the microbial ecology of the gut. Am J Clin Nutr 69:1052S–1057S. 10.1093/ajcn/69.5.1052s. [DOI] [PubMed] [Google Scholar]
- 26.Furet JP, Kong LC, Tap J, Poitou C, Basdevant A, Bouillot JL, Mariat D, Corthier G, Dore J, Henegar C, Rizkalla S, Clement K. 2010. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes 59:3049–3057. 10.2337/db10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gomes AC, Bueno AA, de Souza RG, Mota JF. 2014. Gut microbiota, probiotics and diabetes. Nutr J 13:60. 10.1186/1475-2891-13-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yao K, Zeng L, He Q, Wang W, Lei J, Zou X. 2017. Effect of probiotics on glucose and lipid metabolism in type 2 diabetes mellitus: a meta-analysis of 12 randomized controlled trials. Med Sci Monit 23:3044–3053. 10.12659/MSM.902600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yun SI, Park HO, Kang JH. 2009. Effect of Lactobacillus gasseri BNR17 on blood glucose levels and body weight in a mouse model of type 2 diabetes. J Appl Microbiol 107:1681–1686. 10.1111/j.1365-2672.2009.04350.x. [DOI] [PubMed] [Google Scholar]
- 30.Kang JH, Yun SI, Park MH, Park JH, Jeong SY, Park HO. 2013. Anti-obesity effect of Lactobacillus gasseri BNR17 in high-sucrose diet-induced obese mice. PLoS One 8:e54617. 10.1371/journal.pone.0054617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X, Wang N, Yin B, Fang D, Jiang T, Fang S, Zhao J, Zhang H, Wang G, Chen W. 2016. Effects of Lactobacillus plantarum CCFM0236 on hyperglycaemia and insulin resistance in high-fat and streptozotocin-induced type 2 diabetic mice. J Appl Microbiol 121:1727–1736. 10.1111/jam.13276. [DOI] [PubMed] [Google Scholar]
- 32.Sakai T, Taki T, Nakamoto A, Shuto E, Tsutsumi R, Toshimitsu T, Makino S, Ikegami S. 2013. Lactobacillus plantarum OLL2712 regulates glucose metabolism in C57BL/6 mice fed a high-fat diet. J Nutr Sci Vitaminol (Tokyo) 59:144–147. 10.3177/jnsv.59.144. [DOI] [PubMed] [Google Scholar]
- 33.Carabotti M, Scirocco A, Maselli MA, Severi C. 2015. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol 28:203–209. [PMC free article] [PubMed] [Google Scholar]
- 34.Foster JA, Rinaman L, Cryan JF. 2017. Stress & the gut-brain axis: regulation by the microbiome. Neurobiol Stress 7:124–136. 10.1016/j.ynstr.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kern L, Mittenbuhler MJ, Vesting AJ, Ostermann AL, Wunderlich CM, Wunderlich FT. 2018. Obesity-induced TNFα and IL-6 signaling: the missing link between obesity and inflammation-driven liver and colorectal cancers. Cancers (Basel) 11:24. 10.3390/cancers11010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Can A, Dao DT, Arad M, Terrillion CE, Piantadosi SC, Gould TD. 2012. The mouse forced swim test. J Vis Exp 10.3791/3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodrigues da Cunha L, Fortes Ferreira CL, Durmaz E, Goh YJ, Sanozky-Dawes R, Klaenhammer T. 2012. Characterization of Lactobacillus gasseri isolates from a breast-fed infant. Gut Microbes 3:15–24. 10.4161/gmic.19489. [DOI] [PubMed] [Google Scholar]
- 38.Belicova A, Mikulasova M, Dusinsky R. 2013. Probiotic potential and safety properties of Lactobacillus plantarum from Slovak Bryndza cheese. Biomed Res Int 2013:760298. 10.1155/2013/760298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jung JH, Kim SJ, Lee JY, Yoon SR, You SY, Kim SH. 2019. Multifunctional properties of Lactobacillus plantarum strains WiKim83 and WiKim87 as a starter culture for fermented food. Food Sci Nutr 7:2505–2516. 10.1002/fsn3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu CC, Weng WL, Lai WL, Tsai HP, Liu WH, Lee MH, Tsai YC. 2015. Effect of Lactobacillus plantarum strain K21 on high-fat diet-fed obese mice. Evid Based Complement Alternat Med 2015:391767. 10.1155/2015/391767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi WJ, Dong HJ, Jeong HU, Ryu DW, Song SM, Kim YR, Jung HH, Kim TH, Kim YH. 2020. Lactobacillus plantarum LMT1-48 exerts anti-obesity effect in high-fat diet-induced obese mice by regulating expression of lipogenic genes. Sci Rep 10:869. 10.1038/s41598-020-57615-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee E, Jung SR, Lee SY, Lee NK, Paik HD, Lim SI. 2018. Lactobacillus plantarum strain Ln4 attenuates diet-induced obesity, insulin resistance, and changes in hepatic mRNA levels associated with glucose and lipid metabolism. Nutrients 10:643. 10.3390/nu10050643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang S, Czech MP. 2007. The GLUT4 glucose transporter. Cell Metab 5:237–252. 10.1016/j.cmet.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 44.Hecker PA, O'Shea KM, Galvao TF, Brown BH, Stanley WC. 2011. Role of adiponectin in the development of high fat diet-induced metabolic abnormalities in mice. Horm Metab Res 43:100–105. 10.1055/s-0030-1269898. [DOI] [PubMed] [Google Scholar]
- 45.Meng X, Qian Y, Jiang L-S, Kang J-M, Chen Y, Wang J, Liu S-K, Che Z-M, Zhao X. 2016. Effects of Lactobacillus plantarum SCS2 on blood glucose level in hyperglycemia mice model. Appl Biol Chem 59:143–150. 10.1007/s13765-015-0135-6. [DOI] [Google Scholar]
- 46.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. 1995. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest 95:2409–2415. 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akash MSH, Rehman K, Liaqat A. 2018. Tumor necrosis factor-alpha: role in development of insulin resistance and pathogenesis of type 2 diabetes mellitus. J Cell Biochem 119:105–110. 10.1002/jcb.26174. [DOI] [PubMed] [Google Scholar]
- 48.Akbari M, Hassan-Zadeh V. 2018. IL-6 signalling pathways and the development of type 2 diabetes. Inflammopharmacology 26:685–698. 10.1007/s10787-018-0458-0. [DOI] [PubMed] [Google Scholar]
- 49.Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. 1994. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci U S A 91:3652–3656. 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chacon MR, Fernandez-Real JM, Richart C, Megia A, Gomez JM, Miranda M, Caubet E, Pastor R, Masdevall C, Vilarrasa N, Ricard W, Vendrell J. 2007. Monocyte chemoattractant protein-1 in obesity and type 2 diabetes. Insulin sensitivity study. Obesity (Silver Spring) 15:664–672. 10.1038/oby.2007.578. [DOI] [PubMed] [Google Scholar]
- 51.Raff H. 2016. CORT, Cort, B, corticosterone, and now cortistatin: enough already! Endocrinology 157:3307–3308. 10.1210/en.2016-1500. [DOI] [PubMed] [Google Scholar]
- 52.Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. 1988. Diet-induced type II diabetes in C57BL/6J mice. Diabetes 37:1163–1167. 10.2337/diab.37.9.1163. [DOI] [PubMed] [Google Scholar]
- 53.Heydemann A. 2016. An overview of murine high fat diet as a model for type 2 diabetes mellitus. J Diabetes Res 2016:2902351. 10.1155/2016/2902351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang CY, Liao JK. 2012. A mouse model of diet-induced obesity and insulin resistance. Methods Mol Biol 821:421–433. 10.1007/978-1-61779-430-8_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jensen TL, Kiersgaard MK, Sorensen DB, Mikkelsen LF. 2013. Fasting of mice: a review. Lab Anim 47:225–240. 10.1177/0023677213501659. [DOI] [PubMed] [Google Scholar]
- 56.Andrikopoulos S, Blair AR, Deluca N, Fam BC, Proietto J. 2008. Evaluating the glucose tolerance test in mice. Am J Physiol Endocrinol Metab 295:E1323–E1332. 10.1152/ajpendo.90617.2008. [DOI] [PubMed] [Google Scholar]
- 57.Nagy C, Einwallner E. 2018. Study of in vivo glucose metabolism in high-fat diet-fed mice using oral glucose tolerance test (OGTT) and insulin tolerance test (ITT). J Vis Exp 10.3791/56672. [DOI] [PMC free article] [PubMed] [Google Scholar]