Abstract
Psoriasis is a chronic inflammatory disease involving the skin. Both genetic and environmental factors play a pathogenic role in psoriasis and contribute to the severity of the disease. Psoriasis, in fact, has been associated with different comorbidities such as diabetes, metabolic syndrome, gastrointestinal or kidney diseases, cardiovascular disease (CVD), and cerebrovascular diseases (CeVD). Indeed, life expectancy in severe psoriasis is reduced by up to 5 years due to CVD and CeVD. Moreover, patients with severe psoriasis have a higher prevalence of traditional cardiovascular (CV) risk factors, including dyslipidemia, diabetes, smoking, and hypertension. Further, systemic inflammation is associated with oxidative stress increase and induces endothelial damage and atherosclerosis progression. Different miRNA have been already described in psoriasis, both in the skin tissues and in the blood flow, to play a role in the progression of disease. In this review, we will summarize and discuss the most important miRNAs that play a role in psoriasis and are also linked to CVD.
Keywords: psoriasis, microRNAs, cardiovascular diseases
Introduction
Psoriasis is a complex and chronic inflammatory disease affecting 2–3% of the world’s population (1). The etiology of psoriasis is complex, and still debated, as the disorder is caused by the interaction among multiple genes, the immune system and environmental factors (2, 3).
From a dermatological point of view, psoriasis is characterized by hyperproliferative epidermis and cutaneous lymphocytic infiltration that can also involve joints causing a form of arthritis, known as psoriatic arthritis (PsA) (3).
A prominent role is played by the increased risk in these patients of major adverse cardiovascular events: this evidence has received increasing attention in the last decades (4, 5). This finding cannot be explained only on the basis of high prevalence of traditional cardiovascular risk factors seen in psoriatic patients (6); additional and independent factors such as the systemic inflammation associated with psoriasis, the presence of psoriasis-related comorbidities (7, 8) and the atherogenic side-effects of systemic therapies for psoriasis have also been reported (9).
Atherosclerosis and inflammation in psoriatic patients
Ample research work has demonstrated that inflammation is a key driver of atherosclerosis.
Indeed, the risk of cardiovascular events is not sufficiently indexed by cholesterol and other traditional risk factors. High-sensitivity C-reactive protein (hsCRP) and interleukin-6 (IL6) levels, despite significant reductions in low-density lipoprotein cholesterol (LDL-C) obtainable with maximal medical therapy, are associated with 'residual inflammatory risk'. Control of inflammation has increasingly become a viable pharmacologic target for primary and secondary prevention of atherosclerotic disease (10).
The inflammatory process seen in psoriasis shares many features with atherosclerosis including T helper 1-mediated inflammation, alterations in angiogenesis, and endothelial dysfunction that may link the pathogenesis of psoriasis with the development of atherosclerosis and cardiovascular disease (CVD) (11).
The typical histological features of the psoriatic plaque with dermal inflammation and leucocyte infiltration are similar to those of the atherosclerotic plaque. The theory of 'two plaques for one syndrome' has been hypothesized given the similarities in the molecular mechanisms and pro-inflammatory cytokine profile found in psoriatic lesions and those of atherosclerotic ones, with a comparable inflammatory infiltrate of T cells, macrophages and monocytes (9, 12).
Both atherosclerosis and psoriasis are characterized by immune system activation, involving T helper-1, T helper-17, regulatory T cells and inflammatory cytokines such as INFG, IL2, TNF and IL17 (13). These mediators play a key role in the development of psoriatic plaques, stimulating angiogenesis and keratinocyte proliferation and promote insulin resistance, metabolic abnormalities and endothelial dysfunction, which contribute to atherosclerotic plaque formation and progression (14, 15). In support of this theory, large epidemiologic studies have found increased rates of cardiovascular mortality, myocardial infarction (MI) (4, 5), atrial fibrillation and stroke (16) among patients with both mild and severe psoriasis. Recent comprehensive meta-analysis and cohort studies have confirmed that the association is statistically significant (16). For a definitive evaluation of the association, more long-term prospective studies will be needed.
Psoriatic patients, therefore, appear to have a shorter life expectancy, estimated as approximately 5 years (17). These subjects have increased prevalence of traditional cardiovascular risk factors such as diabetes, hypertension, dyslipidemia, tobacco use and obesity (7, 8). Psoriasis, as already pointed out and mainly if severe, appears to be an independent risk factor for atherosclerotic CVD, as the risk persists even after adjusting for the traditional risk factors, probably due to the role that systemic inflammation plays in determining premature atherosclerosis in these patients (4, 5).
The impact of psoriasis duration on aging and cardiovascular risk
Estimation of cardiovascular risk based on the traditional score system has limitations in peculiar clinical contexts. These scores are known to underperform because traditional cardiovascular risk factors do not fully explain the increased cardiovascular risk in patients with psoriasis. Consequently, cardiovascular risk is often underestimated in these individuals (18).
Although the relative risk of major adverse clinical events is greater for young patients with severe psoriasis vs old ones with mild disease, the greater population prevalence of mild psoriasis translates into a greater population-attributable risk of mild psoriasis for both MI and stroke. These findings emphasize that all patients with psoriasis, rather than only young patients with severe psoriasis, should be educated regarding the increased risk of CVD.
In addition, the risk of MI in psoriatic patients aged between 30 and 40 years vs tose between 50 and 60 years with a long history of psoriasis was compared (13). Attributable risk and excess risk calculated based on the adjusted relative risk of MI were much higher in the older group being roughly 70-fold higher.
Patients with psoriasis were more likely to have coronary artery disease (CAD) as visualized on coronary angiogram. This association was still evident after adjusting for established cardiovascular risk factors, leading the authors to conclude that psoriasis is independently associated with the presence of CAD (19). Finally, a strict correlation between the duration of psoriasis and the extension of CAD exists.
microRNA involved in psoriasis and CVD
miRNAs are short non-coding RNAs that inhibit translation and/or induce degradation of target messenger RNAs (mRNAs) (20).
The following miRNAs (summarized in Table 1) are the most important miRNAs modulated in psoriasis that are also involved in CVD.
Table 1.
miRNAs modulated in psoriasis and CVD.
| miRNAs/ROS source or disease | Tissue/organ | Species | Modulation | Targets | References |
|---|---|---|---|---|---|
| miR-200c | |||||
| Psoriasis | LS vs NL, LS vs HS, plasma | Human | Upregulated | Unknown | (21) |
| H2O2 | ECs | Human | Upregulated | eNOS; SIRT1; FOXO1; ZEB1 | (23, 24) |
| Atherosclerosis | Carotid plaques, plasma | Human | Upregulated | eNOS; SIRT1; FOXO1; ZEB1 | (25) |
| Familial hypercholesterolemia | Plasma | Human | Upregulated | ZEB1 | (35) |
| miR-200a | |||||
| Psoriasis | LS vs NL, plasma | Human | Upregulated | Unknown | (21) |
| Psoriasis | T cells | Human | Upregulated | Unknown | (22) |
| miR-200b, miR-141, miR-429 | |||||
| Psoriasis | LS vs NL | Human | Upregulated | Unknown | (21) |
| miR-33a | |||||
| Psoriasis | LS vs HS, plasma | Human | Upregulated | Unknown | (29) |
| Atherosclerosis | Atherosclerotic plaque | Mouse | Upregulated | ABCGA1; ABCG1 | (32) |
| miR-33a/b | |||||
| Atherosclerosis | Carotid plaque, plasma | Human | Upregulated | ABCA1; ABCG1; ZEB1 | (33, 25) |
| Familial hypercholesterolemia | Plasma | Human | Upregulated | ZEB1 | (34) |
| miR-133a | |||||
| Psoriasis | LS vs NL | Human | Upregulated | IGFR1 | (36) |
| MI | Heart | Human, mouse | Downregulated | Unknown | (37, 38) |
| AMI, unstable angina | Serum | Human | Upregulated | Unknown | (39) |
| Cardiac hypertrophy | Heart | Human, rat | Downregulated | RHOA; CDC42; NELFA/WHSC2 | (40) |
| Atherosclerosis | Atherosclerotic plaque, plasma | Human | Downregulated | MMP9 | (43) |
| Thoracic aortic aneurysm | Aortas | Human | Downregulated | Unknown | (45) |
| miR-135b | |||||
| Psoriasis | LS vs NS | Human | Upregulated | Unknown | (46) |
| MI | Cardiomyocytes | Mouse | Downregulated | CASP1 | (47) |
| Atherosclerosis | Serum of CAD | Human | Upregulated | MEF2C | (48) |
| Cardiac hypertrophy | Cardiomyocytes | Mouse | Downregulated | CACNA1C | (49) |
| miR-21 | |||||
| Psoriasis | LS vs HS | Human | Upregulated | Unknown | (50) |
| H2O2 | VSMC | Rat | Upregulated | PDCD4 | (56) |
| Atherosclerosis | Plaque | Human | Upregulated | SOD2; SPRY2 | (59) |
| Atherosclerosis | Heart/Plasma | Human | Upregulated | RECK, PDCD4 | (60) |
| Cardiac hypertrophy | Heart | Mouse | Upregulated | Unknown | (61) |
| HF | Serum | Human | Upregulated | Unknown | (63) |
| miR-22 | |||||
| Psoriasis | Blood | Human | Downregulated | Unknown | (64) |
| Psoriasis | Plasma of PsA vs ankylosing spondylitis | Human | Downregulated | Unknown | (65) |
| Cardiac hypertrophy | Cardiomyocytes | Mouse | Upregulated | SIRT1, HDAC4 | (67, 68) |
| IR | Cardiomyocytes | Rat | Downregulated | PUMA | (72) |
| Atherosclerosis | Femoral arteries | Human | Downregulated | Unknown | (73) |
| AMI | Plasma | Human | Downregulated | Unknown | (74) |
| miRNAs/ROS source or disease | Tissue/organ | Species | Modulation | Targets | References |
| miR-369 |
|||||
| Psoriasis | Serum, LS vs HS | Human | Upregulated | Unknown | (64, 75) |
| HF | Plasma of AMI with HF vs MI without HF | Human | Upregulated | Unknown | (78) |
| miR-378a | |||||
| Psoriasis | LS vs NL | Human | Upregulated | Unknown | (36) |
| Psoriasis | Keratynocites of LS vs NL and HS | Human | Upregulated | Unknown | (80) |
| Atherosclerosis | VSMCs | Human | Downregulated | IGF1; TLR8 | (86) |
| Atherosclerosis | Plaques | Mouse | Downregulated | ABCG1; SIRPA | (87, 88) |
| Cardiac hypertophy | Heart | Mouse | Downregulated | MAPK1; KSR1; GRB2; IGF1R | (89) |
| miR-378a* | |||||
| Psoriasis | Keratinocytes of LS vs NL | Human | Upregulated | VEGF | (80, 81) |
| miR-9 | |||||
| Psoriasis | LS vs NL | Human | Downregulated | Unknown | (36) |
| Deep vein thrombosis | Venous tissue | Mouse | Downregulated | NFKB1 | (92) |
| MS | PBMCs | Human | Upregulated | ABCA1 | (93) |
| ACS | PBMCs | Human, mouse | Downregulated | SDC2; OLR1 | (94, 95) |
| MI | Cardiomyocytes | Mouse | Upregulated | FSTL1 | (97) |
| Cardiac hypertophy | Heart | Mouse | Downregulated | Myocardin | (96) |
| miR-375 | |||||
| Psoriasis | LS vs NL | Human | Downregulated | IGF1R; JAK2 | (36, 100, 101) |
| HF | Blood | Human | Upregulated | Unknown | (102) |
| MI | Cardiomyocytes | Mouse | Upregulated | PDK1 | (103) |
| miR-340 | |||||
| Psoriasis | T cells | Mouse | Downregulated | IL17A | (108) |
| Cardiac hypertrophy | Cardiomyocytes | Mouse | Upregulated | Dystrophin | (111) |
| miR-19a | |||||
| Psoriasis | LS vs HS, hair roots | Mouse, human | Upregulated | TNF | (114, 116) |
| Atherosclerosis | Plasma, atherosclerotic plaque | Human | Upregulated | HBP1 | (117) |
| Cardiac hypertrophy | Cardiomyocytes | Mouse | Downregulated | ATROGIN1, MURF1, PDE5A | (118) |
| miR-424 | |||||
| Psoriasis | LS vs HS | Human | Downregulated | MEK1, CYCLIN E1 | (120) |
| Psoriasis | Hair shaft | Human | Upregulated | Unknown | (120) |
| Atherosclerosis | Blood | Mouse, human | Downregulated | APOC3 | (121) |
| IR | Heart | Mouse | Upregulated | CRISPLD2 | (122) |
| miR-146a | |||||
| Psoriasis | LS vs NL and vs HS | Human | Upregulated | FERMT1, NUMB | (123, 124) |
| Psoriasis | PBMCs | Human | Upregulated | Unknown | (126) |
| Atherosclerosis | Valvular tissues | Human | Upregulated | TLR4, IRAK1 | (127) |
| Atherosclerosis | Monocyte/macrophages | Mouse | Downregulated | MCSF, RELB | (128) |
| HF | Heart | Human | Upregulated | SUMO1 | (131) |
| miR-146b | |||||
| Psoriasis | LS vs NL and vs HS | Human | Upregulated | FERMT1, NUMB | (124) |
| Atherosclerosis | Aortic plaque | Mouse | Upregulated | BAG1, MMP16 | (129) |
| miR-143 | |||||
| Psoriasis | PBMCs | Human | Upregulated | SLCA26A4 | (132) |
| miR-145 | |||||
| Psoriasis | LS vs HS | Human | Downregulated | MLK3 | (133) |
| Atherosclerosis | Plasma of unstable CAD | Human | Upregulated | Unknown | (135) |
| Atherosclerosis | Carotid: symptomatic vs asymptomatic | Human | Upregulated | Unknown | (136) |
| Atherosclerosis | Aortic plaque | Mouse | Downregulated | KLF4 | (137) |
| Atherosclerosis | Carotid plaque | Human | Downregulated | OPG, KLF5 | (139) |
| MI | Plasma | Human | Upregulated | Unknown | (141) |
| Let7-a | |||||
| Psoriasis | T cells | Human | Downregulated | STAT3 | (145) |
| Hypertrophy | Heart | Mouse | Upregulated | CALM | (147) |
| MI | Heart | Pig | Downregulated | TGFBR3 | (148) |
| MI | Plasma | Human, pig | Downregulated | Unknown | (148) |
| Let7-b | |||||
| Psoriasis | Keratinocytes | Mouse | Downregulated | IL6 | (143) |
| Psoriasis | Plasma of PsA | Human | Downregulated | Unknown | (144) |
| Atherosclerosis | Carotid plaques: symptomatic vs asymptomatic | Human | Downregulated | Unknown | (146) |
| Atherosclerosis | Diabetic plaque | Human | Downregulated | Unknown | (146) |
| Atherosclerosis | Diabetic aorta | Mouse | Downregulated | Unknown | (146) |
miR-200 family
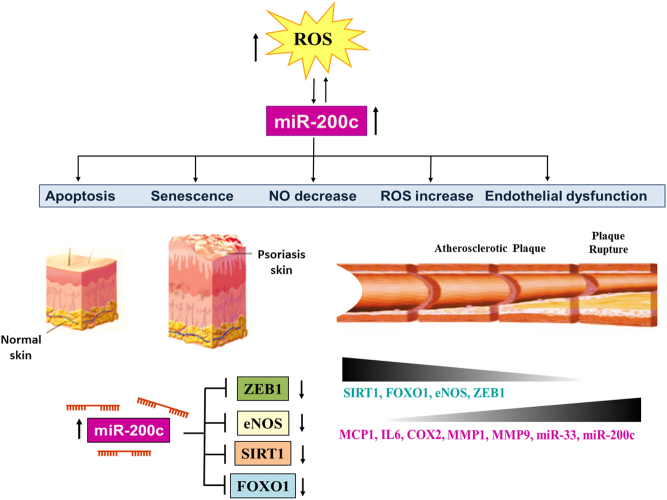
miR-200 family is composed of five members (i.e. miR200a, miR-200b, miR-200c, miR-429 and miR-141). This miRNA family has been shown to be induced in lesional skin (LS) of psoriatic patients compared to non-lesional (NL) one (21). Further, miR-200c is the only one also induced compared to healthy skin subjects (HS). The entire miRNA family was assayed in the bloodstream of psoriatic patients, and miR-200c and miR-200a were upregulated significantly compared to healthy subjects. The correlation analysis with psoriasis area severity index (PASI) and the duration of disease showed that only miR-200c expression levels positively correlated with both, indicating that miR-200c could be considered as an inflammatory biomarker for this disease (21). Moreover, miR-200c correlated with different determinants of cardiovascular risk, such as relative wall thickness (RWT), left ventricular (LV) mass, and E/e’ parameter, a marker of diastolic dysfunction (21). Circulating miR-200a correlated only with LV mass and augmentation index, a marker of stiffness, although not significantly (P = 0.06).
These data indicated that miR-200c increases in plaques and plasma of psoriatic patients and could be involved in the establishment of inflammation and CVD in psoriasis.
In another study, it has been shown that the upregulation of miR-200a in CD4+ T cells may induce immune dysfunction through Th17/Treg cells and relevant cytokines (i.e. IL17 and IL23) levels in psoriasis vulgaris patients and positively correlates with PASI (22).
miR-200c was upregulated in endothelial cells (ECs) upon oxidative stress exposure and is responsible for apoptosis, senescence (23), nitric oxide (NO) decrease and reactive oxygen species (ROS) increase, and all features associated with endothelial dysfunction (24). In particular, miR-200c targets zinc finger E-box binding homoeobox 1 (ZEB1) protein, inducing apoptosis and senescence of ECs (23) and disrupts the autoregulatory loop existing among Sirtuin1 (SIRT1), endothelial nitric oxide synthase (eNOS) and forkhead boxO1 (FOXO1), directly targeting all of them, causing NO decrease and ROS increase (24).
It is well known that endothelial dysfunction plays a key role in unstable plaques genesis. In keeping with this, miR-200c was increased in carotid plaques of atherosclerotic patients, and it is higher in unstable plaques vs stable plaques (25).
miR-200c was found to be elevated in the plasma of patients with atherosclerosis, although the circulating levels did not discriminate between stable or unstable plaques. Interestingly, the plasma levels were decreased after carotid endarterectomy (CEA), and after 1 month, the levels of miR-200c were increased in patients with unstable plaques, whereas 1 month after CEA miR-200c levels were still low in patients with stable plaques, indicating that increased levels of miR-200c are most probably associated with endothelial dysfunction and inflammation (25).
Interestingly, miR-200c levels positively correlated with biomarkers of plaque instability such as cyclooxygenase-2 (COX2), IL6, monocyte chemoattractant protein-1 (MCP1), metalloproteinase (MMP) 1, MMP9 and negatively correlated with stability biomarkers (i.e. ZEB1, eNOS, FOXO1 and eNOS).
Interestingly, miR-200c positively correlated with two important miRNAs, miR-33a/b (see following paragraph), which have been linked to atherosclerosis progression (26) and are key players of cholesterol homeostasis, since they are key regulators of high-density lipoprotein (HDL) cholesterol synthesis (27).
In conclusion, the miR-200 family is increased in psoriasis skin, and among its members, miR-200c plasma levels correlate with cardiovascular risk and inflammatory biomarkers (Fig. 1).
Figure 1.
miR-200c in psoriasis and CVD. miR-200c is upregulated by reactive oxygen species (ROS) and is responsible for apoptosis, senescence, endothelial dysfunction, ROS increase and nitric oxide decrease, all features associated with atherosclerosis. Indeed, miR-200c increases in carotid plaques and plasma of atherosclerotic patients vs healthy subjects and positively correlates with plaque instability biomarkers (i.e. MCP1, IL6, COX2, MMP1, MMP9 and miR-33) and negatively with stability biomarkers (i.e. SIRT1, FOXO1, eNOS and ZEB1). ROS modulation, endothelial dysfunction, cardiac remodeling and inflammation are also associated with psoriasis. Iin keeping with this, miR-200c is increased in LS vs NL and vs HS, and in plasma of psoriatic patients. miR-200c correlates with PASI and the duration of disease and with determinants of CVD (i.e. LV mass, E/e’ and RWT) in psoriatic patients.
miR-33a/b
miR-33a and miR-33b share the same seed sequence and are embedded in introns of sterol regulatory element-binding protein (SREBP)2 and SREBP1 genes, respectively (28). SREBP genes are important transcription factors that regulate lipid metabolism, and their transcriptional upregulation induces miR-33a and miR-33b expression (28).
miR-33a was found to be higher in plasma of patients with plaque psoriasis compared to controls and correlated positively with plasma insulin and the homeostatic model assessment (HOMA)-insulin resistance index value. Moreover, psoriasis patients displayed greater carotid intima-media thickness (IMT) than controls (29).
miR-33 are known to directly target the cholesterol transporter ATP-binding cassette transporter A1 (ABCA1) and ATP-binding cassette transporter G1 (ABCG1), proteins implicated in the efflux of cholesterol from macrophages to generate HDL (27). miR-33a/b also regulate fatty acid oxidation and insulin signaling (30, 31).
Given their role in cholesterol and lipid metabolism, different studies evaluated miR-33 inhibition in animal studies. miR-33 inhibition has been shown to raise plasma HDL-cholesterol and to protect from atherosclerosis in mice (32). miR-33 inhibition in non-human primates upregulates the hepatic expression of ABCA1, increasing plasma HDL-cholesterol levels and suppressing the plasma levels of very-low-density lipoprotein (VLDL)-associated triglycerides (33).
Interestingly, as aforementioned, miR-33a and miR-33b were found to be increased in carotid atherosclerotic plaques, suggesting their role in advanced atherosclerosis in humans (26).
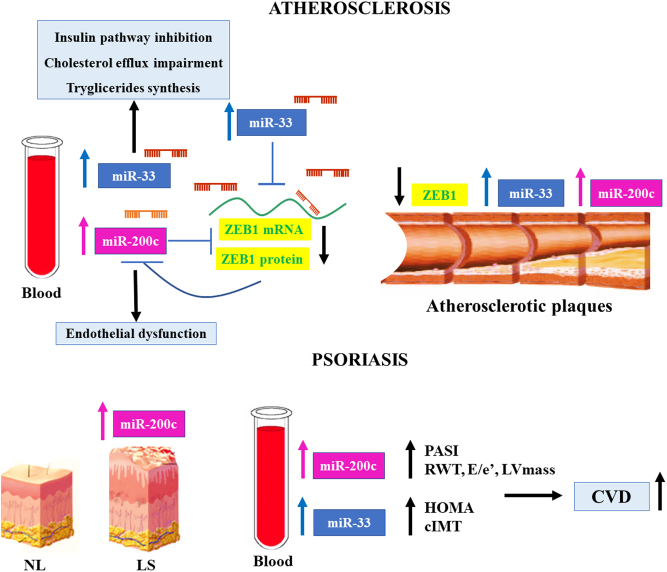
In keeping with this, miR-33a/b were also found to be upregulated in plasma of children with familial hypercholesterolemia, a disease that causes atherosclerosis in the pediatric age. miR-33a/b positively correlated with total cholesterol, LDL-cholesterol, apolipoprotein B, glycemia and CRP (34). Moreover, these miRNAs correlated also with miR-200c plasma levels, and the extracellular release of miR-200c was mediated by miR-33 since the overexpression of miR-33a/b in vitro in different cells (i.e. ECs, hepatic cells and embryonic kidney cells) was mediated by a ZEB1-decrease mechanism (35).
The latter is a transcriptional inhibitor of miR-200c; hence, ZEB1-decrease causes an increase of both intracellular and extracellular miR-200c expression levels (35).
A positive correlation between miR-200c and miR-33a/b was also found in the plasma and plaques of atherosclerotic patients as mentioned previously (25) (Fig. 2).
Figure 2.
miR-200c and miR-33 interplay in psoriasis and CVD. miR-200c and miR-33 positively correlate in plasma and plaques of atherosclerotic patients. A molecular mechanism among miR-33a/b and miR-200c increase does exist. Indeed, the overexpression of miR-33a/b in vitro in different cells (i.e. ECs, hepatic cells and embryonic kidney cells) causes the intracellular and extracellular increase of miR-200c via a ZEB1-decrease mechanism. ZEB1, in fact, is a direct target of both miR-33 and miR-200c and a transcriptional inhibitor of miR-200c. Hence, a ZEB1-decrease causes upregulation of both intracellular and extracellular miR-200c expression levels. An increase of miR-200c in skin plaques and plasma of psoriatic patients was also observed, and it positively correlates with PASI index and determinants of CVD risk (RWT, E/e’ and LV mass). Interestingly, miR-33a was found to be upregulated in the plasma of psoriatic patients and positively correlates with HOMA-insulin resistance index and cIMT. Therefore, a possible link in psoriasis could also exist among miR-33 and miR-200c, contributing to the increase of CVD in these patients.
Thus, a complex interplay among miRNAs is most probably the basis of metabolic dysfunction and inflammation and oxidative stress increase in psoriatic patients.
miR-133a
miR-133a was found to be downregulated in LS of psoriatic patients compared to NS (36). miR-133a expression levels increased after treatment with biologics.
Notably, miR-133a is muscle-specific miRNA, and it is involved in cardiac development and pathophysiology.
Interestingly, miR-133a is downregulated in both the infarcted area and the border zone both in humans, as well as in experimental animals (37, 38).
On the other hand, miR-133a levels in serum increased significantly in patients with acute myocardial infarction (AMI) or with unstable angina pectoris, and circulation increase of miR-133a positively correlates with the infarcted area (39).
Indeed, miR-133a decrease is involved in cardiac hypertrophy, and its overexpression inhibits hypertrophy (40). Moreover, miR-133a increase plays a major role in preventing cardiac fibrosis in chronic heart failure (HF) (41), and its increase in adult cardiac progenitor cells (CPCs) improves cardiac function, decreasing fibrosis and increasing cardiomyocyte proliferation and vascularization (42).
miR133a was found to be downregulated in plasma and in atherosclerotic plaques, and its decrease in vascular smooth muscle cells (VSMCs) results in the inhibition of proliferation and in the induction of apoptosis via MMP9 (43).
miR-133a has been shown to inhibit the osteogenic differentiation of VSMCs; therefore, its decrease induces arterial calcification (44).
Finally, miR-133a is also decreased in thoracic aortic aneurysms (TAA), and its decrease negatively correlates with aortic diameters (45).
Thus, miR-133a decrease in psoriasis could be involved in the onset of different CVD.
miR-135b
miR-135b was upregulated in LS compared to NS (46), and after treatment with biologics, miR-135b was downregulated, significantly returning to those levels detected in NS (36).
After treatment with biologics, miR-135b levels were associated with both PASI improvement and local inflammatory response decrease (36).
Interestingly, in MI, miR-135b is downregulated, and its overexpression attenuates pyroptosis, a caspase-1-dependent proinflammatory programmed cell death. Thus, miR-135b plays a protective role in MI (47).
Moreover, miR-135b was found to be upregulated in sera of atherosclerotic subjects with CAD compared to healthy control subjects. Interestingly, miR-135b directly targets myocyte enhancer factor 2C (MEF2C), which plays an important role in promoting ECs and VSMCs proliferation and migration (48).
Further, miR-135b is decreased in cardiac hypertrophy, and its overexpression is attenuated in an animal model of hypertrophy by targeting L-type calcium channel CACNA1C, a protein involved in cardiac hypertrophy signaling (49).
miR-135b upregulation in psoriasis resembles the modulation in atherosclerosis, whereas it is modulated in CVD differently.
miR-21
miR-21 was significantly upregulated in LS of patients with psoriasis compared to healthy skin (50), and narrow-band UV phototherapy treatment decreased its expression in skin biopsies derived from psoriatic patients (51). Moreover, a specific single nucleotide polymorphism in 3'UTR of integrin alpha-M (ITGAM) is associated with a binding site improvement for miR-21 that can manifest an aberrant function of innate immune cells, resulting in higher risk insurgence of psoriasis in women (52). Other data suggest that miR-21 upregulation could increase the inflammatory process, by suppressing the apoptosis pathway in dermal T-cells of psoriatic patients (53). A recent study has shown that the interaction between miR-21 and lncRNA maternally expressed gene3 (MEG3) can influence the apoptotic axis in human psoriatic keratinocytes (54).
miR-21 inhibition was able to reduce psoriatic phenotype in a mouse model of psoriasis (55).
miR-21 has been widely studied in CVD. miR-21 was shown to be upregulated by oxidative stress in rat VSMCs. Its upregulation was found to be protective from apoptosis since programmed cell death 4 (PDCD4) is a direct target of miR-21 (56). PDCD4, in fact, is a pro-apoptotic protein that inhibits the activity of the transcription factor AP-1 (57). The latter is a signaling molecule, which determines cell fate based on extracellular stimuli, including ROS.
Interestingly, miR-21 is upregulated by shear stress and also protects ECs from apoptosis, increasing eNOS and NO production (58).
miR-21 upregulation also occurs in atherosclerotic plaques, decreasing the function of superoxide dismutase-2 (SOD2) antioxidant enzyme and targeting a negative regulator of the branching morphogenesis, sprouty homolog 2 (SPRY2). This, in turn, leads to the activation of ERK/MAP kinase provoking ROS increase and angiogenic progenitor cell (APC) migratory defects (59).
Moreover, miR-21 expression is increased in myocardial tissue and plasma of patients with aortic stenosis (60), in a mouse model of cardiac hypertrophy induced by aortic banding, and in neonatal rat cardiomyocytes after stimulation with hypertrophic agents (61); miR-21 upregulation in cardiac hypertrophic growth caused SPRY2 downregulation, enhancing the formation of various cellular protrusions and gap junction remodeling, enhancing conduction velocity (62).
miR-21 circulating levels have been also used as excellent biomarkers for the diagnosis of heart dysfunction (63).
In conclusion, miR-21 increase in psoriasis could be a possible link to CVDs such as atherosclerosis and cardiac hypertrophy.
miR-22
miR-22 circulating expression levels were significantly downregulated in the blood of patients affected by psoriasis vulgaris compared to healthy subjects (64). In addition, plasma levels of miR-22 were evaluated in PsA and ankylosing spondylitis, two chronic inflammatory rheumatic disorders. miR-22 circulating expression was found to be markedly lower in PsA subjects than in ankylosing spondylitis patients (65).
Psoriasis is widely regarded as a classical autoimmune disease. Interestingly, it has been shown that miR-22 levels are upregulated in the spleen and spinal cords of mice that developed autoimmune encephalomyelitis, and its high expression in inflammatory Th17 cells could protect mice from autoimmunity (66).
miR-22 also plays a pivotal role in CVD. miR-22 is a pro-hypertrophic miRNA, and in fact, miR-22 levels were increased in cardiomyocyte hypertrophy and in different cardiac hypertrophy mice models (67, 68). Moreover, cardiac-specific deletion of miR-22 inhibits stress-induced cardiac hypertrophy and remodeling (68, 69), whereas cardiac-specific overexpression of miR-22 elicited cardiac dilation and HF (70).
Moreover, pharmacological inhibition of miR-22 promoted cardiac functional recovery after MI by eliciting cardiac autophagy (71).
Ischemia-reperfusion (IR) in rat cardiomyocytes induced down-modulation of miR-22 and an increase of p53-upregulated-modulator-of-apoptosis (PUMA), thus increasing apoptosis (72).
Moreover, miR-22 expression is decreased in human femoral arteries with atherosclerotic plaques compared to healthy arteries (73). miR-22 plays a regulatory role in VSMC phenotype switching and vascular neointima lesion formation, by regulating multiple target genes (i.e. HDAC4, methyl-CpG binding protein 2 and ecotropic viral integration site-1). Overexpression of miR-22 can reverse the process of VSMC phenotype switching in the injured arteries and prevent postangioplasty restenosis, supporting a potential role for miR-22 and its target genes in a variety of proliferative vascular diseases (73).
Further, miR-22 circulating levels were significantly decreased in patients affected by AMI compared to controls, suggesting that it could be considered as a promising diagnostic biomarker (74).
All the previously described studies underlined a decrease in psoriasis of miR-22 that is likely associated with atherosclerotic plaques, whereas in cardiac tissues, the miR-22 decrease has a protective function, and its increase induces cardiac dilation and failure.
miR-369
miR-369 expression levels were found to be increased in serum samples and skin tissues deriving from psoriatic patients compared to healthy subjects, and a positive correlation existed between skin miR-369 levels and PASI (64, 75).
miR-369 has emerged as a key regulator of inflammatory response in dendritic cells since it decreases LPS-induced NO production, by directly targeting iNOS expression and simultaneously inhibiting nuclear translocation of NFKB (76). In keeping with this, miR-369 overexpression in these cells significantly decreased cytokine production (i.e. TNF, IL6, IL12, IL1A and IL1B) in response to LPS, and some anti-inflammatory cytokines, such as IL-10 and IL-1RA, were indirectly increased (76).
In line with its anti-inflammatory role, miR-369 overexpression is able to suppress cardiomyocyte apoptosis and inflammation pathways triggered by hypoxia (77).
Furthermore, plasma of patients with HF, following AMI, exhibited higher miR-369 expression levels, compared to subjects affected by MI without HF (78).
These observations suggest that miR-369 upregulation in psoriasis and CVD seems to play a protective role against the deleterious effect inflammatory pathways elicited in these diseases.
miR-378a/378a*
miRNA-378a, known also as miR-378 or miR-422b, originates from the first intron of the peroxisome proliferator-activated receptor gamma coactivator 1 beta (PPARGC1B) gene encoding PGC1B, a transcriptional regulator of oxidative energy metabolism. The complementary strand named miR-378a* is also miRNA active (79).
High levels of miR-378a were detected in the LS compared to NL. Further, biological treatment can restore its levels of expression to those in NS (36). Moreover, miR-378a was found to be upregulated in keratinocytes from LS of psoriasis compared to paired NL samples andalso vs healthy keratinocytes (80).
miR-378a* was also found to be upregulated in LS keratinocytes compared to NL keratinocytes (80).
Interestingly, miR-378a* induces vascular endothelial growth factor expression (VEGF) competing for the same binding site with miR-125, which, in fact, is downregulated in psoriatic plaque (81).
A growing body of studies suggests that miR-378a/378a*, encoded within PGC1B, plays a mediatory role in lipid pathway metabolism (82).
miR-378a has been shown to play a protective role against hyperlipidemia. It has been shown that miR-378/378* adipose tissue-specific transgenic mice counteract genetic and high-fat diet (HFD)-induced obesity, through the expansion of brown fat (BAT) (83), which is known to reduce plasma triglyceride and cholesterol levels (84).
On the other hand, knockout mice for miR-378a/378a* are also resistant to HFD-induced obesity and show a mitochondrial fatty acid metabolism and oxidative capacity increase in insulin-target tissues (e.g. liver, muscles, and adipose tissues). This occurs since miR-378 and miR-378a* counterbalance the metabolic actions of PGC1B (85).
miR-378a also plays a major role in atherosclerosis. In VSMCs of human atherosclerotic plaques, a positive correlation between miR-378a expression, PGC-1α levels and the media were found and were decreased compared to healthy subjects (86).
miR-378a induction is regulated by transcription factors such as nuclear respiratory factor 1 (NFR1) via interaction with PGC1A. Thus PGC1A decrease determines miR-378a suppression. Interestingly, miR-378a overexpression plays an anti-atherogenic role in inhibiting free fatty acid-induced VSMC proliferation, migration and inflammation directly targeting insulin growth factor 1 (IGF1) and toll-like receptor 8 (TLR8), which are two genes aberrantly upregulated in atherosclerotic vessels (86).
On the other hand, miR-378a has been shown to directly target ABCG1, a transporter cassette implicated in macrophage reverse cholesterol efflux to generate HDL (87). In line with previous studies, miR-378a was found to be decreased in atherosclerotic plaques in low‐density lipoprotein receptor-deficient mice (ApoE−/−) vs WT aortas (88).
miR-378a targets directly signal regulatory protein α (SIRPA), a negative modulator of macrophage phagocytosis and inflammation. Thus, miR-378a levels decrease in ApoE−/− mice atherosclerotic plaques, promotes inflammation, increases macrophage phagocytosis, and increases macrophage reverse cholesterol efflux.
According to a recent study, miR-378a has antihypertrophic activity in the heart, playing an important role in the regulation of myocardial remodeling (89).
Thus, miR-378a dysregulation in psoriasis could modulate cardiometabolic diseases associated with this disease.
miR-9
miR-9 was found to be downregulated in the LS compared to NL in psoriasis (36).
miR-9 plays a major role in the modulation of inflammatory pathways. In fact, it is not only induced by an NFKB1-dependent pathway in human monocytes (90), but it also targets NFKB1 (91), and thus a negative feedback loop exists between miR-9 and NFKB1.
Interestingly, in a peripheral artery disease rat model, it was found that deep vein thrombosis miR-9 decreased and NFKB1 increased, eliciting inflammation. Moreover, its overexpression alleviates inflammation and thrombosis in rats (92).
In metabolic syndrome, miR-9 was found to be upregulated in peripheral blood mononuclear cells (PBMCs) causing the decrease of its target ABCA1, diminishing cholesterol efflux and HDL production (93).
miR-9 was found to be decreased in PBMCs isolated from patients with the acute coronary syndrome (ACS) compared to healthy subjects and in aortic lysates of HFD-fed ApoE−/− mice compared to normal diet-fed ApoE−/− mice. miR-9 overexpression in HFD ApoE−/− mice reduced inflammation and atherosclerotic plaques, acting as a negative regulator of SDC2-dependent FAK/ERK signaling pathway activated in these mice (94).
In keeping with this, it was found that miR-9 can decrease atherosclerosis in an ACS mouse model, via oxidized low-density lipoprotein (lectin-like) receptor 1 direct targeting that inhibits p38MAPK, thus promoting vascular remodeling (95).
miR-9 was also found to be downregulated in rat hypertrophic cardiomyocytes, and its overexpression counteracts hypertrophy in a mouse cardiac hypertrophy model since myocardin is a direct target of miR-9 (96).
In contrast, inhibition of miR-9 in mice prevented cardiac remodeling following MI through the upregulation of its target follistatin-like 1 (FSTL1) that is known to protect cardiomyocytes from different pathologic injuries including MI (97).
Interestingly, miR-9 also plays an angiogenic role. ECs exposed to high glucose (HG) showed a decrease in miR-9 and an increase in chemokine receptor-4 (CXCR4). miR-9 overexpression in HG-treated cells increases cell proliferation, tubule formation, decreases apoptosis and inflammation targeting CXCR4 (98).
Thus, miR-9 is deeply involved in inflammatory and metabolic regulation, and its downregulation is also associated with different CVD.
miR-375
miR-375 was found to be downregulated in the LS of psoriasis patients compared to NL. Further, the biological treatment restores its levels of expression (36).
IGF receptor 1, which is overexpressed in psoriatic epidermis, regulates the keratinocyte proliferation, apoptosis, and differentiation (99) and has been shown to be a direct target of miR-375 (100).
miR-375 also targets Janus kinase 2 (JAK2) (101), which plays a pivotal role in both inflammatory and anti-inflammatory signaling in keratinocytes.
Patients with HF have high levels of miR-375 (102). In addition, it has been shown that the inhibition of miR-375 decreases the inflammatory response after MI in a rodent model (103), and further, after MI, IL10 inhibits miR-375 improving murine EPC's survival and function, promoting cardiac neovascularization and attenuating ischemic injury (104).
Thus, miR-375 decrease in psoriasis seems to play a protective role against inflammation and CVD.
miR-340
miR-340 is located in the intronic region of the ring finger protein 130 (RNF130) gene and is regulated by promoter hypermethylation (105).
miR-340 regulates the function and differentiation of immune cells and influences the progression of multiple autoimmune diseases, such as experimental immune encephalomyelitis (EAE), an animal model of brain inflammation and psoriasis (106).
Indeed, miR-340 inhibits the differentiation of Th2 cells through the downregulation of genes involved in the Th2 pathway, including IL4 (106).
Furthermore, miR-340 directly targets IL17A, a positive regulator of Th17 differentiation cells (107).
It was found that the expression of miR-340 was significantly decreased in mice treated with imiquimod (IMQ), the most widely accepted psoriasis animal model, with consequent upregulation of IL17A and development of psoriasis. Thus, miR-340 negatively regulates the IL17A expression and alleviates the severity of the disorder in the IMQ-induced psoriasis mouse model, giving an important role for Th17 cells in psoriasis development (108).
For these reasons, miR-340 has been suggested as a therapeutic target for the treatment of psoriasis and other IL17A-mediated autoimmune diseases (108).
Moreover, miR-340 has an important role in CVDs, such as eccentric hypertrophy, HF and cardiomyopathy. In particular, miR-340 upregulation is induced by cardiotrophin-1 (CT1) stimulation, a member of the IL6 family which initiates cardiac hypertrophy through the gp130/LIF receptor signaling pathway, which has a pivotal role in eccentric hypertrophy (109, 110)
miR-340 results in a downstream factor of gp130/LIF receptor pathway, and knockdown of miR-340 decreases the signal of CT1 and the hypertrophic response. At the same time, miR-340 is necessary for the induction and mediation of eccentric hypertrophy (111, 112).
Dystrophin is a structural protein present in the subsarcolemmal layer of cardiomyocytes and protects cells from stress during contraction and relaxation, and thus dystrophin deficiency results in the disruption of the sarcolemmal membrane and disorganization of the cytoskeleton (113).
It was found that dystrophin mRNA is a direct target of miR-340. miR-340 was found to be upregulated in failing heart caused by volume overload that in turns lead to alteration of cardiomyocytes sarcolemmal integrity and decompensation (111, 113)
Therefore, heart volume overload causes CT1 release which subsequently induces miR-340 upregulation that inhibits dystrophin, exacerbating eccentric hypertrophy and HF (111).
All the aforementioned studies show that alterations of miR-340 expression play a major role in inflammatory diseases and in cardiac pathogenesis.
miR-19a
miR-19a is a member of the miR-19 family together with miR-19b1 and miR-19b2, and they all share the same seed sequence. This family is located in the miR-17–92 polycistronic cluster that encodes miRNAs belonging to the miR-17, miR-18, miR-19 and miR-92 families.
Interestingly, IMQ-treated skin in mice differentially regulates the expression of the miR-17-92 cluster: miR-17 and miR-19 families are upregulated, while miR-92 is downregulated. However, overexpression or deletion of this cluster in keratinocytes or T cells does not have a relevant influence in IMQ-induced psoriasis, suggesting different functions and roles of miRNA families in T cell and keratinocytes (114).
Since miR-19a directly targets TNF (115), its expression levels were assayed in psoriasis to determine whether it could be a biomarker of this inflammatory disease.
It was found that miR-19a was upregulated in hair roots of psoriasis patients compared to healthy subjects. Moreover, miR-19a levels were negatively correlated with the duration of symptom onset in psoriasis patients (116).
Interestingly, miR-19a was found to be upregulated in sera of patients with coronary atherosclerosis vs healthy controls. miR-19a was shown to promote vascular inflammation and foam cell formation. In ApoE−/− mice fed with HFD, inhibition of miR-19a decreased atherosclerotic plaques and lipids load (117).
The miR-19 family has been shown to regulate cardiac hypertrophy. It was shown that the miR-19 family has a pro-hypertrophic role in rat neonatal cardiomyocytes since it directly targets the anti-hypertrophic proteins ATROGIN1 and MURF-1 (118).
On the contrary, another study showed that in a mouse model of cardiac hypertrophy, miR-19a/b expression was reduced in hypertrophic hearts. In keeping with this, miR-19a/b transgenic mice prevented cardiac hypertrophy and cardiac progression in response to angiotensin II via direct targeting of cardiac phosphodiesterase 5 (PDE5A) that regulates cardiac tone and vascular function (119).
miR-19a modulation is involved in psoriasis and in CVDs although discrepancies on its role are present in the literature.
miR-424
miR-424(322)/-503 are mammal-specific members of the extended miR-15/107 miRNA family, present on chromosome X.
miR-424 was found to be markedly decreased in psoriasis skin, although no significant difference in the sera of psoriasis patients vs healthy controls was found. miR-424 targets mitogen-activated protein kinase 1 (MEK1) and CYCLIN E1 that indeed were increased in psoriatic skin, leading to cell proliferation (120).
Hair shaft miR-424 levels, on the contrary, were significantly higher in psoriasis patients vs normal subjects. miR-424 levels do not correlate with other clinical psoriasis markers, disease duration or body surface area (120).
Interestingly, miR-424 was found to be decreased in the peripheral blood of atherosclerotic patients and in rat models of atherosclerosis (121).
miR‐424 targets apolipoprotein C3 (APOC3) gene directly, whose decrease causes NFKB1 signaling pathway repression. Thus, miR-424 upregulation or APOC3 silencing suppressed inflammation, proliferation, migration and induced aortic smooth muscle cells apoptosis.
In keeping with this, miR-424 upregulation decreases atherosclerosis progression, blocking APOC3‐mediated NFKB1 signaling pathway, whereas miR-424 downregulation accelerates atherosclerosis progression (121).
miR-424 upregulation was reported in cardiac IR injury in mice. In particular, IR in heart tissue and hypoxia/reoxygenation-injury in rat cardiomyocytes lead to miR-424 upregulation and repression of its direct target cysteine-rich secretory protein LCCL domain-containing 2 (CRISPLD2) (122). Decrease in CRISPLD2 expression triggers cardiac pyroptosis, associated with cytokine increase and activation of pro-inflammatory immune mediators, that finally leads to ischemic heart disease (122).
miR-424 is modulated in psoriasis and in CVDs, and its levels control proliferation and inflammatory pathways.
miR-146
The human miR-146 family is composed of two members, miR146a and miR146b, that share almost identical sequences and seed regions, located on different chromosomes.
miR-146a was found to be upregulated in psoriatic plaque epidermis vs healthy skin (123). It was observed that both miR-146a and miR-146b were upregulated in LS of psoriatic patients vs NL and vs HS (124). In that study, it was demonstrated that miR-146a targets and inhibits the proliferation marker fermitin family member 1 (FERMT1) that is also involved in the linkage of the actin cytoskeleton to the extracellular matrix, thus in the formation of a normal skin structure (125). Consequently, in human primary keratinocytes, overexpression of miR-146a inhibited cell proliferation.
miR-146a was found to be increased in PBMCs of psoriasis patients compared to healthy controls and correlated with PASI, suggesting that it could potentially act as a biomarker (126).
miR-146a has been found to be upregulated in valvular tissue from patients with atherosclerosis compared to controls (127), whereas in ApoE−/− mice, miR-146a was found to be decreased in both monocyte/macrophages and miR-146a delivery in ApoE−/−Ldlr(−/−) and Ldlr−/− mice decreased atherosclerosis reducing monocyte/macrophage activation in the absence of plasma lipid reduction (128).
This occurs since miR-146a acts as a negative regulator of NFKB 1 signaling (128).
Interestingly, miR-146b also was found to be upregulated in the aortic plaques of ApoE−/− mice and in response to inflammatory cytokines. It was found that miR-146b repressed proliferation and migration of VSMCs by downregulating BCL2-associated athanogene 1 (BAG1) and matrix metalloproteinase (MMP)16, respectively (129).
Interestingly, in transgenic mice overexpressing TNF in the heart, miR-146a levels were increased in cardiac ventricular tissue and also in a human cardiomyocyte cell line exposed to TNF (130).
miR-146a targets Fos and thus the Fos-containing transcription factor complex the activator protein 1 (AP1), which transcribes MMP9. Therefore, miR-146a increase downregulates the AP1-MMP9 pathway, whose activation is correlated to HF and myocardial dysfunction. Therefore, miR-146a has been proposed as a therapeutic tool for treating CVD associated with enhanced inflammation in the heart (130).
In a recent study, miR-146a was found to be upregulated in human hearts in HF patients compared to nonfailing ones. A negative correlation was found with small ubiquitin-like modifier 1 (SUMO1) mRNA that was identified as a target of miR-146a. SUMO1 is a positive regulator of the sarcoplasmic reticulum Ca2+-ATPase pump (SERCA2A). SERCA2A, in fact, undergoes SUMOylation that confers stability and increased activity to SERCA2A, restoring cardiac function in both mouse and swine HF models (131).
Thus, miR-146 is involved in psoriasis and CVD, where it plays anti-inflammatory roles in atherosclerosis but detrimental roles in HF.
miR-143/145
miR143/145 cluster is composed of two co-transcribed miRNAs.
It was shown that miR-143 upregulated in PBMCs of patients with psoriasis compared to healthy subjects, and significantly correlated with PASI (132). Interestingly, miR-143 decreased after treatment in psoriatic patients, suggesting that miR-143 could serve as an early biomarker for psoriasis severity and treatment response (132).
Indeed, miR-145 is also downregulated in psoriatic LS vs healthy skin and inhibits proliferation and chemokine secretion in keratinocytes (133).
miR-145 was significantly reduced in plasma of CAD patients who received state-of-the-art pharmacological treatment compared to healthy subjects (134).
In another study, circulating miR-145 was induced in unstable CAD compared with healthy controls. miR-145 was not modulated in stable CAD (135) and was also upregulated in patients with symptomatic vs asymptomatic carotid atherosclerosis (136).
miR-145a levels were found to be decreased in the aortas of ApoE −/− fed with western diets compared to controls and in human carotid atherosclerotic plaques compared to segments without plaques (137, 138).
Since miR-145 is highly expressed in VSMC, a VSMC-specific overexpression was used to determine its role in ApoE−/− mice fed with a western diet to induce atherosclerosis. miR-145a overexpression reduced plaque size, necrotic core and inflammation, whereas fibrous cap and collagen content increased, all features associated with plaque stability. It was found that miR-145a promotes VSMC differentiation toward the contractile phenotype via a mechanism that involves downregulation of its target Kruppel-like factor 4 and myocardin increase (137, 138).
In keeping with this, overexpression of miR-145 in the aorta of ApoE−/− mice reduced plaque size and decreased proliferation and apoptosis by direct targeting of osteoprotegerin (OPG) and KLF5 that are known to induce the NFKB1 signaling pathway, therefore reducing inflammation, as well (139).
Indeed, it was found that KLF2 induces the transcriptional upregulation of the miR-143/145 cluster. Extracellular vesicles secreted by KLF2-transduced ECs or shear-stress-stimulated ECs are enriched in miR-143/145 and are able to control gene expression in co-cultured VSMCs. Interestingly, extracellular vesicles derived from KLF2-expressing ECs reduced atherosclerotic lesion formation in ApoE/− mice (140).
Moreover, circulating miR-145 was found to be upregulated in MI patients, and miR-145 levels correlated with infarct sizes estimated by troponin T release (141).
miR-143 was shown to be the most abundant miRNA released in exosomes by rat cardiomyocytes cultured in ischemic conditions. The exosomes released were found to improve angiogenesis in matrigel plug assays. Finally, intramyocardial delivery of ischemic exosomes induced neovascularization following MI (142).
miR-143-145 modulation in psoriasis could be the basis of atherosclerosis and CVD in these patients.
Let-7 a/b
Let-7a and b belong to let-7 family of miRNA composed of nine isoforms (i.e. let-7a-1, 7a-2, 7a-3, 7b, 7c, 7d, 7e, 7f-1, 7f-2, 7g, 7i, and miR-98) encoded by 12 different genomic loci.
Interestingly, in the mice model of psoriasis, let-7b was also found to be downregulated in keratinocytes. In such a model, let-7b overexpression led to the decrease of its target IL6, promoting keratinocytes differentiation which resulted in improvement of psoriasis (143).
Plasma-derived extracellular vesicles were isolated and sequenced in PsA compared to cutaneous psoriasis only. It was found that let-7b extracellular levels were significantly decreased in PsA patients compared to psoriatic patients, suggesting that this miRNA could act as a promising biomarker for arthritis development (144).
Interestingly, let-7a also was found to be lower in T cells of psoriatic patients vs control and negatively correlated with signal transducer and activator of transcription 3 (STAT3) expression; further, let-7a overexpression decreased IFNG in T cells (145).
In human carotid plaque tissues, let-7b was downregulated in symptomatic compared to asymptomatic atherosclerotic patients. Diabetes is a condition that is considered to accelerate the atherosclerosis process, and let-7b decreased levels were also confirmed in diabetic plaque tissues compared to non-diabetic tissues (146). Interestingly, such decreased levels were also confirmed in the aortic tissue from diabetic ApoE−/− mice models compared to non-diabetic mice (146).
Interestingly, let-7a was decreased in Ang II-induced cardiac hypertrophy in vitro and in vivo, and its overexpression attenuated hypertrophy in mice (147).
let-7a downregulated the expression of its target Ccalmodulin (CALM) protein that together with Ca2+ induces the transcription of the hypertrophic genes (147).
let-7a was found to be downregulated in the myocardium of pigs after MI and also in the plasma of humans and pigs after MI (148). In this study, a let-7a overexpression was shown to be cardiac protective via inhibition of TGFBR3-p38 MAPK signaling pathways that regulate cardiomyocyte apoptosis (148).
The aforementioned papers showed that let-7a/b decrease seems to play a detrimental role in psoriasis and in eliciting CVD.
miRNA-based treatment of CVD
Given their importance in CVD, different miRNAs are currently used in preclinical studies and some of them could eventually be included in clinical trials.
This miRNA-based strategy holds good promise and has been demonstrated to be effective for hepatitis C therapy, where the feasibility of using miRNAs as a therapeutic tool is confirmed by the fact that a phase 2a clinical trial adopting anti-miR-122 has been completed successfully (149). This trial showed that the locked nucleic acid (LNA)-anti-miRNA is safe, not toxic and well-tolerated.
Different miRNAs modulations have been used in preclinical studies to counteract CVD insurgence by systemic delivery.
As mentioned earlier, miR-33 inhibition by anti-miRNA oligonucleotide that targets both miR-33a and miR-33b in non-human primates raises plasma HDL and lowers VLDL triglyceride levels and is a promising strategy for the treatment of dyslipidemias that increase CVD (33).
Moreover, systemic delivery of miR-9 mimics was shown to reduce isoproterenol-induced cardiac hypertrophy in mice and improve cardiac function (150); the systemic delivery of an LNA-oligonucleotide targeting the entire miR-15 family, which is upregulated in different forms of heart disease in both murine and porcine cardiac tissue, reduced infarct size and inhibited cardiac remodeling in the injured heart (151). Interestingly, the injected animals showed no evidence of LNA-associated toxicity or histopathologic abnormalities in the heart, liver, or kidneys.
miRNAs inhibition by antagomirs and sponges was successfully used in mice; in a cardiovascular setting, the in vivo inhibition of miR-133 by infusion of an antagomir or a sponge caused marked and sustained cardiac hypertrophy in mice, associated with a re-induction of fetal gene expression (40).
A drawback of the systemic delivery of miRNA therapy is that it targets predominantly the liver, although it has also been largely utilized to target the heart, and different strategies have been adopted to modulate miRNAs in a specific tissue or organ. Specific delivery is one of the most important problems due to the lack of clinical trials with miRNA-based technology (152).
To circumvent this problem, the delivery of miRNA mimics or anti-miRNA in a cardiac-specific or tissue-specific manner could be performed using serotype-specific adeno-associated virus 9 (AAV9) genetically modified to express a given miRNA mimic or antimir under a cardiac-specific or other tissue-specific promoters (153). AAV9 has been successfully used to deliver miRNAs to cardiac tissue, an additional advantage is that AAVs preferably infect nondividing cells, such as cardiomyocytes. Although many preclinical and phase I clinical studies have provided encouraging results regarding the safety of AAVs in humans, the clinical use of AAVs is still a challenge since they can induce the host immune response, and the AAV DNA can randomly integrate into the host genome. Although it occurs at a low rate, it was reported to occur.
Another possibility is minicircle; nonviral vectors that lack both the origin of replication and the antibiotic selection marker, carrying only short bacterial sequences, can be used to overexpress a miRNA. Their small size confers greater transfection efficiency, and the lack of bacterial backbone creates less immunogenicity and a longer time of expression.
Minicircles carrying an miR-210 expression cassette were injected intramyocardially in adult mice that underwent coronary ligation in order to demonstrate that miR-210 overexpression was able to improve cardiac function by upregulating angiogenesis and inhibited apoptosis (154).
Many strategies have been developed to ameliorate the uptake of nucleic acids into tissues and cells.
In order to facilitate the lipid bilayer crossing of the target cell membrane, the oligonucleotide needs to be packaged into liposomes or nanoparticles (155).
A class of liposomes, termed 'lipidoid', has been developed, showing high levels of specific silencing of endogenous gene transcripts (156). Preliminary studies with lipidoids tested in animal models (mice, rats and non-human primates) met the safety and specificity standards.
Finally, nanotechnology offers an attractive drug delivery system, with comparable efficacy and less toxicity compared to other vehicles (157).
Since in psoriasis, the predominant tissue involved is the skin, the topical delivery of miRNA mimics or anti-miRNA is easier to achieve, although topically applied miRNA-based therapeutics show difficulties in penetrating the stratum corneum barrier of the skin. To circumvent this problem, the following approaches have been used:
(i) Ultra-deformable liposomes (UDLs) are liposomes with augmented skin penetration capability: ethosomes and transfersomes (158); (ii) surfactant-ethanol-cholesterol-osomes (SECosomes) comprising a cationic lipid, a helper lipid, cholesterol, a single-chain surfactant and ethanol that help to penetrate the skin barrier (159); (iii) finally, cell-penetrating peptides (CPPs) that bind nucleic acids and facilitate their entry through the skin could be used to deliver miRNA (160).
Nowadays, no trials on specific miRNAs are currently ongoing in psoriasis. This is probably due to the fact that miRNA modulation and regulation during psoriasis treatment have only been sparsely studied.
Further studies on miRNA involved in psoriasis and their possible involvement in CVD associated with this disease are needed in order to generate an individualized miRNA-based treatment of psoriasis.
Conclusions
Nowadays, miRNAs have been linked to many pathological conditions, and it is now clear that molecular circuits exist among miRNAs. Thus, the modulation of miRNAs caused by a disease in a specific tissue region or in a different tissue could either systemically or non-systemically induce a deleterious effect that could result in causing successive evident comorbidities associated with that disease.
In this review, we summarized those miRNAs that have been associated with psoriasis either in the skin and or in the blood flow that are known to play a role in CVD, and hence, could contribute to the establishment of secondary effects of psoriasis that shorten life expectancy.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Funding
This study was partly supported by the Italian Ministry of Health RF-02362708 grant to AM; by AFM Telethon 22522 grant to AM; by Idi-Farmaceutici s.r.l. grant IDI FAR19MG to AM; SG-12358253 grant to MD.
References
- 1.Boehncke W-H, Schön MP. Psoriasis. Lancet 2015. 386 983–994. ( 10.1016/S0140-6736(1461909-7) [DOI] [PubMed] [Google Scholar]
- 2.Mak RKH, Hundhausen C, Nestle FO. Progress in understanding the immunopathogenesis of psoriasis. Actas Dermo-Sifiliograficas 2009. 100(Supplement 2) 2–13. ( 10.1016/s0001-7310(09)73372-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ries M, Deeg KH, Heininger U. Demonstration of perivascular echogenicities in congenital cytomegalovirus infection by colour Doppler imaging. European Journal of Pediatrics 1990. 150 34–36. ( 10.1007/BF01959476) [DOI] [PubMed] [Google Scholar]
- 4.Armstrong EJ, Harskamp CT, Armstrong AW. Psoriasis and major adverse cardiovascular events: a systematic review and meta-analysis of observational studies. Journal of the American Heart Association 2013. 2 e000062. ( 10.1161/JAHA.113.000062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller IM, Ellervik C, Yazdanyar S, Jemec GBE. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. Journal of the American Academy of Dermatology 2013. 69 1014–1024. ( 10.1016/j.jaad.2013.06.053) [DOI] [PubMed] [Google Scholar]
- 6.Puig L.Cardiometabolic comorbidities in psoriasis and psoriatic arthritis. International Journal of Molecular Sciences 2017. 19(1) 58. ( 10.3390/ijms19010058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and hypertension: a systematic review and meta-analysis of observational studies. Journal of Hypertension 2013. 31 433–442; discussion 442–443. ( 10.1097/HJH.0b013e32835bcce1) [DOI] [PubMed] [Google Scholar]
- 8.Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and the risk of diabetes mellitus: a systematic review and meta-analysis. Journal of the American Medical Association Dermatology 2013. 149 84–91. ( 10.1001/2013.jamadermatol.406) [DOI] [PubMed] [Google Scholar]
- 9.Masson W, Lobo M, Molinero G. Psoriasis and cardiovascular risk: a comprehensive review. Advances in Therapy 2020. 37 2017–2033. ( 10.1007/s12325-020-01346-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aday AW, Ridker PM. Targeting residual inflammatory risk: a shifting paradigm for atherosclerotic disease. Frontiers in Cardiovascular Medicine 2019. 6 16. ( 10.3389/fcvm.2019.00016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nestle FO, Kaplan DH, Barker J. Psoriasis. New England Journal of Medicine 2009. 361 496–509. ( 10.1056/NEJMra0804595) [DOI] [PubMed] [Google Scholar]
- 12.Flammer AJ, Ruschitzka F. Psoriasis and atherosclerosis: two plaques, one syndrome? European Heart Journal 2012. 33 1989–1991. ( 10.1093/eurheartj/ehr425) [DOI] [PubMed] [Google Scholar]
- 13.Armstrong AW, Voyles SV, Armstrong EJ, Fuller EN, Rutledge JC. A tale of two plaques: convergent mechanisms of T-cell-mediated inflammation in psoriasis and atherosclerosis. Experimental Dermatology 2011. 20 544–549. ( 10.1111/j.1600-0625.2011.01308.x) [DOI] [PubMed] [Google Scholar]
- 14.Alexandroff AB, Pauriah M, Lang CC, Struthers AD, Armstrong DJ. Atherosclerosis as a systemic feature of psoriasis. Clinical and Experimental Dermatology 2011. 36 451–452. ( 10.1111/j.1365-2230.2010.03893.x) [DOI] [PubMed] [Google Scholar]
- 15.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature 2011. 473 317–325. ( 10.1038/nature10146) [DOI] [PubMed] [Google Scholar]
- 16.Ahlehoff O, Gislason GH, Jørgensen CH, Lindhardsen J, Charlot M, Olesen JB, Abildstrøm SZ, Skov L, Torp-Pedersen C, Hansen PR. Psoriasis and risk of atrial fibrillation and ischaemic stroke: a Danish Nationwide Cohort Study. European Heart Journal 2012. 33 2054–2064. ( 10.1093/eurheartj/ehr285) [DOI] [PubMed] [Google Scholar]
- 17.Torres T, Sales R, Vasconcelos C, Martins da Silva B, Selores M. Framingham Risk Score underestimates cardiovascular disease risk in severe psoriatic patients: implications in cardiovascular risk factors management and primary prevention of cardiovascular disease. Journal of Dermatology 2013. 40 923–926. ( 10.1111/1346-8138.12267) [DOI] [PubMed] [Google Scholar]
- 18.Eder L, Chandran V, Gladman DD. The Framingham risk score underestimates the extent of subclinical atherosclerosis in patients with psoriatic disease. Annals of the Rheumatic Diseases 2014. 73 1990–1996. ( 10.1136/annrheumdis-2013-203433) [DOI] [PubMed] [Google Scholar]
- 19.Armstrong AW, Harskamp CT, Ledo L, Rogers JH, Armstrong EJ. Coronary artery disease in patients with psoriasis referred for coronary angiography. American Journal of Cardiology 2012. 109 976–980. ( 10.1016/j.amjcard.2011.11.025) [DOI] [PubMed] [Google Scholar]
- 20.O’Brien J, Hayder H, Zayed Y, Peng C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Frontiers in Endocrinology 2018. 9 402. ( 10.3389/fendo.2018.00402) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magenta A, D’Agostino M, Sileno S, Di Vito L, Uras C, Abeni D, Martino F, Barillà F, Madonna S, Albanesi C, et al. The oxidative stress-induced mir-200c is upregulated in psoriasis and correlates with disease severity and determinants of cardiovascular risk. Oxidative Medicine and Cellular Longevity 2019. 2019 8061901. ( 10.1155/2019/8061901) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang XY, Chen XY, Li J, Zhang HY, Liu J, Sun LD. MiR-200a expression in CD4+ T cells correlates with the expression of Th17/Treg cells and relevant cytokines in psoriasis vulgaris: A case control study. Biomedicine and Pharmacotherapy 2017. 93 1158–1164. ( 10.1016/j.biopha.2017.06.055) [DOI] [PubMed] [Google Scholar]
- 23.Magenta A, Cencioni C, Fasanaro P, Zaccagnini G, Greco S, Sarra-Ferraris G, Antonini A, Martelli F, Capogrossi MC. miR-200c is upregulated by oxidative stress and induces endothelial cell apoptosis and senescence via ZEB1 inhibition. Cell Death and Differentiation 2011. 18 1628–1639. ( 10.1038/cdd.2011.42) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carlomosti F, D’Agostino M, Beji S, Torcinaro A, Rizzi R, Zaccagnini G, Maimone B, Di Stefano V, De Santa F, Cordisco S, et al. Oxidative stress-induced mir-200c disrupts the regulatory loop among SIRT1, FOXO1, and eNOS. Antioxidants and Redox Signaling 2017. 27 328–344. ( 10.1089/ars.2016.6643) [DOI] [PubMed] [Google Scholar]
- 25.Magenta A, Sileno S, D’Agostino M, Persiani F, Beji S, Paolini A, Camilli D, Platone A, Capogrossi MC, Furgiuele S. Atherosclerotic plaque instability in carotid arteries: miR-200c as a promising biomarker. Clinical Science 2018. 132 2423–2436. ( 10.1042/CS20180684) [DOI] [PubMed] [Google Scholar]
- 26.Karunakaran D, Thrush AB, Nguyen MA, Richards L, Geoffrion M, Singaravelu R, Ramphos E, Shangari P, Ouimet M, Pezacki JP, et al. Macrophage mitochondrial energy status regulates cholesterol efflux and is enhanced by anti-miR33 in atherosclerosis. Circulation Research 2015. 117 266–278. ( 10.1161/CIRCRESAHA.117.305624). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernández-Hernando C, Moore KJ. MicroRNA modulation of cholesterol homeostasis. Arteriosclerosis, Thrombosis, and Vascular Biology 2011. 31 2378–2382. ( 10.1161/ATVBAHA.111.226688) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramírez CM, Goedeke L, Rotllan N, Yoon JH, Cirera-Salinas D, Mattison JA, Suárez Y, de Cabo R, Gorospe M, Fernández-Hernando C. MicroRNA 33 regulates glucose metabolism. Molecular and Cellular Biology 2013. 33 2891–2902. ( 10.1128/MCB.00016-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.García-Rodríguez S, Arias-Santiago S, Orgaz-Molina J, Magro-Checa C, Valenzuela I, Navarro P, Naranjo-Sintes R, Sancho J, Zubiaur M. Abnormal levels of expression of plasma microRNA-33 in patients with psoriasis. Actas Dermo-Sifiliograficas 2014. 105 497–503. ( 10.1016/j.ad.2013.11.010) [DOI] [PubMed] [Google Scholar]
- 30.Marquart TJ, Allen RM, Ory DS, Baldán A. miR-33 links SREBP-2 induction to repression of sterol transporters. PNAS 2010. 107 12228–12232. ( 10.1073/pnas.1005191107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Najafi-Shoushtari SH, Kristo F, Li Y, Shioda T, Cohen DE, Gerszten RE, Näär AM. MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science 2010. 328 1566–1569. ( 10.1126/science.1189123). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rayner KJ, Sheedy FJ, Esau CC, Hussain FN, Temel RE, Parathath S, van Gils JM, Rayner AJ, Chang AN, Suarez Y, et al. Antagonism of miR-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. Journal of Clinical Investigation 2011. 121 2921–2931. ( 10.1172/JCI57275). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rayner KJ, Esau CC, Hussain FN, McDaniel AL, Marshall SM, van Gils JM, Ray TD, Sheedy FJ, Goedeke L, Liu X, et al. Inhibition of miR-33a/b in non-human primates raises plasma HDL and lowers VLDL triglycerides. Nature 2011. 478 404–407 ( 10.1038/nature10486). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martino F, Carlomosti F, Avitabile D, Persico L, Picozza M, Barillà F, Arca M, Montali A, Martino E, Zanoni C, et al. Circulating miR-33a and miR-33b are up-regulated in familial hypercholesterolaemia in paediatric age. Clinical Science 2015. 129 963–972. ( 10.1042/CS20150235) [DOI] [PubMed] [Google Scholar]
- 35.D’Agostino M, Martino F, Sileno S, Barillà F, Beji S, Marchetti L, Gangi FM, Persico L, Picozza M, Montali A, et al. Circulating miR-200c is up-regulated in paediatric patients with familial hypercholesterolaemia and correlates with miR-33a/b levels: implication of a ZEB1-dependent mechanism. Clinical Science 2017. 131 2397–2408 ( 10.1042/CS20171121). [DOI] [PubMed] [Google Scholar]
- 36.Chicharro P, Rodríguez-Jiménez P, Llamas-Velasco M, Montes N, Sanz-García A, Cibrian D, Vara A, Gómez MJ, Jiménez-Fernández M, Martínez-Fleta P, et al. Expression of miR-135b in psoriatic skin and its association with disease improvement. Cells 2020. 9 (7) 1603. ( 10.3390/cells9071603) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boštjančič E, Brandner T, Zidar N, Glavač D, Štajer D. Down-regulation of miR-133a/b in patients with myocardial infarction correlates with the presence of ventricular fibrillation. Biomedicine and Pharmacotherapy 2018. 99 65–71. ( 10.1016/j.biopha.2018.01.019) [DOI] [PubMed] [Google Scholar]
- 38.Bostjancic E, Zidar N, Stajer D, Glavac D. MicroRNAs miR-1, miR-133a, miR-133b and miR-208 are dysregulated in human myocardial infarction. Cardiology 2010. 115 163–169. ( 10.1159/000268088) [DOI] [PubMed] [Google Scholar]
- 39.Kuwabara Y, Ono K, Horie T, Nishi H, Nagao K, Kinoshita M, Watanabe S, Baba O, Kojima Y, Shizuta S, et al. Increased microRNA-1 and microRNA-133a levels in serum of patients with cardiovascular disease indicate myocardial damage. Circulation: Cardiovascular Genetics 2011. 4 446–454. ( 10.1161/CIRCGENETICS.110.958975) [DOI] [PubMed] [Google Scholar]
- 40.Carè A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, Bang ML, Segnalini P, Gu Y, Dalton ND, et al. MicroRNA-133 controls cardiac hypertrophy. Nature Medicine 2007. 13 613–618. ( 10.1038/nm1582) [DOI] [PubMed] [Google Scholar]
- 41.Sang HQ, Jiang ZM, Zhao QP, Xin F. MicroRNA-133a improves the cardiac function and fibrosis through inhibiting Akt in heart failure rats. Biomedicine and Pharmacotherapy 2015. 71 185–189. ( 10.1016/j.biopha.2015.02.030) [DOI] [PubMed] [Google Scholar]
- 42.Izarra A, Moscoso I, Levent E, Cañón S, Cerrada I, Díez-Juan A, Blanca V, Núñez-Gil IJ, Valiente I, Ruíz-Sauri A, et al. miR-133a enhances the protective capacity of cardiac progenitors cells after myocardial infarction. Stem Cell Reports 2014. 3 1029–1042. ( 10.1016/j.stemcr.2014.10.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi L, Yu C, Tian X, Ma C, Wang L, Xia D, Cui C, Chen X, Jiang T, Gu Y, et al. Effect of microRNA-133a-3p/matrix metalloproteinase-9 axis on the growth of atherosclerotic vascular smooth muscle cells. Experimental and Therapeutic Medicine 2019. 18 4356–4362. ( 10.3892/etm.2019.8070) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liao XB, Zhang ZY, Yuan K, Liu Y, Feng X, Cui RR, Hu YR, Yuan ZS, Gu L, Li SJ, et al. MiR-133a modulates osteogenic differentiation of vascular smooth muscle cells. Endocrinology 2013. 154 3344–3352. ( 10.1210/en.2012-2236) [DOI] [PubMed] [Google Scholar]
- 45.Jones JA, Stroud RE, O’Quinn EC, Black LE, Barth JL, Elefteriades JA, Bavaria JE, Gorman JH, Gorman RC, Spinale FG, et al. Selective microRNA suppression in human thoracic aneurysms: relationship of miR-29a to aortic size and proteolytic induction. Circulation: Cardiovascular Genetics 2011. 4 605–613. ( 10.1161/CIRCGENETICS.111.960419) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joyce CE, Zhou X, Xia J, Ryan C, Thrash B, Menter A, Zhang W, Bowcock AM. Deep sequencing of small RNAs from human skin reveals major alterations in the psoriasis miRNAome. Human Molecular Genetics 2011. 20 4025–4040. ( 10.1093/hmg/ddr331) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li A, Yu Y, Ding X, Qin Y, Jiang Y, Wang X, Liu G, Chen X, Yue E, Sun X, et al. MiR-135b protects cardiomyocytes from infarction through restraining the NLRP3/caspase-1/IL-1β pathway. International Journal of Cardiology 2020. 307 137–145. ( 10.1016/j.ijcard.2019.09.055) [DOI] [PubMed] [Google Scholar]
- 48.Xu Z, Han Y, Liu J, Jiang F, Hu H, Wang Y, Liu Q, Gong Y, Li X. MiR-135b-5p and MiR-499a-3p promote cell proliferation and migration in atherosclerosis by directly targeting MEF2C. Scientific Reports [Internet] 2015. 5 12276. ( 10.1038/srep12276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chu Q, Li A, Chen X, Qin Y, Sun X, Li Y, Yue E, Wang C, Ding X, Yan Y, et al. Overexpression of miR-135b attenuates pathological cardiac hypertrophy by targeting CACNA1C. International Journal of Cardiology 2018. 269 235–241. ( 10.1016/j.ijcard.2018.07.016) [DOI] [PubMed] [Google Scholar]
- 50.Sonkoly E, Wei T, Janson PCJ, Sääf A, Lundeberg L, Tengvall-Linder M, Norstedt G, Alenius H, Homey B, Scheynius A, et al. MicroRNAs: novel regulators involved in the pathogenesis of psoriasis? PLoS ONE 2007. 2 e610. ( 10.1371/journal.pone.0000610) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gu X, Nylander E, Coates PJ, Nylander K. Effect of narrow-band ultraviolet B phototherapy on p63 and microRNA (miR-21 and miR-125b) expression in psoriatic epidermis. Acta Dermato-Venereologica 2011. 91 392–397. ( 10.2340/00015555-1086) [DOI] [PubMed] [Google Scholar]
- 52.Hruska P, Kuruczova D, Vasku V, Bienertova-Vasku J. MiR-21 binding site SNP within ITGAM associated with psoriasis susceptibility in women. PLoS ONE 2019. 14 e0218323. ( 10.1371/journal.pone.0218323) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meisgen F, Xu N, Wei T, Janson PC, Obad S, Broom O, Nagy N, Kauppinen S, Kemény L, Ståhle M, et al. MiR-21 is up-regulated in psoriasis and suppresses T cell apoptosis. Experimental Dermatology 2012. 21 312–314. ( 10.1111/j.1600-0625.2012.01462.x). [DOI] [PubMed] [Google Scholar]
- 54.Jia HY, Zhang K, Lu WJ, Xu GW, Zhang JF, Tang ZL. LncRNA MEG3 influences the proliferation and apoptosis of psoriasis epidermal cells by targeting miR-21/caspase-8. BMC Molecular and Cell Biology 2019. 20 46. ( 10.1186/s12860-019-0229-9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guinea-Viniegra J, Jiménez M, Schonthaler HB, Navarro R, Delgado Y, Concha-Garzón MJ, Tschachler E, Obad S, Daudén E, Wagner EF. Targeting miR-21 to treat psoriasis. Science Translational Medicine 2014. 6 225re1. ( 10.1126/scitranslmed.3008089) [DOI] [PubMed] [Google Scholar]
- 56.Lin Y, Liu X, Cheng Y, Yang J, Huo Y, Zhang C. Involvement of microRNAs in hydrogen peroxide-mediated gene regulation and cellular injury response in vascular smooth muscle cells. Journal of Biological Chemistry 2009. 284 7903–7913. ( 10.1074/jbc.M806920200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang HS, Knies JL, Stark C, Colburn NH. Pdcd4 suppresses tumor phenotype in JB6 cells by inhibiting AP-1 transactivation. Oncogene 2003. 22 3712–3720. ( 10.1038/sj.onc.1206433) [DOI] [PubMed] [Google Scholar]
- 58.Weber M, Baker MB, Moore JP, Searles CD. MiR-21 is induced in endothelial cells by shear stress and modulates apoptosis and eNOS activity. Biochemical and Biophysical Research Communications 2010. 393 643–648. ( 10.1016/j.bbrc.2010.02.045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fleissner F, Jazbutyte V, Fiedler J, Gupta SK, Yin X, Xu Q, Galuppo P, Kneitz S, Mayr M, Ertl G, et al. Short communication: asymmetric dimethylarginine impairs angiogenic progenitor cell function in patients with coronary artery disease through a microRNA-21-dependent mechanism. Circulation Research 2010. 107 138–143. ( 10.1161/CIRCRESAHA.110.216770) [DOI] [PubMed] [Google Scholar]
- 60.Villar AV, García R, Merino D, Llano M, Cobo M, Montalvo C, Martín-Durán R, Hurlé MA, Nistal JF. Myocardial and circulating levels of microRNA-21 reflect left ventricular fibrosis in aortic stenosis patients. International Journal of Cardiology 2013. 167 2875–2881. ( 10.1016/j.ijcard.2012.07.021) [DOI] [PubMed] [Google Scholar]
- 61.Cheng Y, Ji R, Yue J, Yang J, Liu X, Chen H, Dean DB, Zhang C. MicroRNAs are aberrantly expressed in hypertrophic heart: do they play a role in cardiac hypertrophy? American Journal of Pathology 2007. 170 1831–1840. ( 10.2353/ajpath.2007.061170) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sayed D, Rane S, Lypowy J, He M, Chen IY, Vashistha H, Yan L, Malhotra A, Vatner D, Abdellatif M. MicroRNA-21 targets Sprouty2 and promotes cellular outgrowths. Molecular Biology of the Cell 2008. 19 3272–3282. ( 10.1091/mbc.e08-02-0159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang J, Xing Q, Zhou X, Li J, Li Y, Zhang L, Zhou Q, Tang B. Circulating miRNA21 is a promising biomarker for heart failure. Molecular Medicine Reports 2017. 16 7766–7774. ( 10.3892/mmr.2017.7575) [DOI] [PubMed] [Google Scholar]
- 64.Alatas ET, Kara M, Dogan G, Akın Belli A. Blood microRNA expressions in patients with mild to moderate psoriasis and the relationship between microRNAs and psoriasis activity. Anais Brasileiros de Dermatologia 2020. 95 702–707. ( 10.1016/j.abd.2020.07.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Perez-Sanchez C, Font-Ugalde P, Ruiz-Limon P, Lopez-Pedrera C, Castro-Villegas MC, Abalos-Aguilera MC, Barbarroja N, Arias-de la Rosa I, Lopez-Montilla MD, Escudero-Contreras A, et al. Circulating microRNAs as potential biomarkers of disease activity and structural damage in ankylosing spondylitis patients. Human Molecular Genetics 2018. 27 875–890. ( 10.1093/hmg/ddy008). [DOI] [PubMed] [Google Scholar]
- 66.Wang L, Qiu R, Zhang Z, Han Z, Yao C, Hou G, Dai D, Jin W, Tang Y, Yu X, et al. The microRNA miR-22 represses Th17 cell pathogenicity by targeting PTEN-regulated pathways. ImmunoHorizons 2020. 4 308–318. ( 10.4049/immunohorizons.2000008) [DOI] [PubMed] [Google Scholar]
- 67.Tu Y, Wan L, Zhao D, Bu L, Dong D, Yin Z, Cheng Z, Shen B. In vitro and in vivo direct monitoring of miRNA-22 expression in isoproterenol-induced cardiac hypertrophy by bioluminescence imaging. European Journal of Nuclear Medicine and Molecular Imaging 2014. 41 972–984. ( 10.1007/s00259-013-2596-3) [DOI] [PubMed] [Google Scholar]
- 68.Huang ZP, Chen J, Seok HY, Zhang Z, Kataoka M, Hu X, Wang DZ. MicroRNA-22 regulates cardiac hypertrophy and remodeling in response to stress. Circulation Research 2013. 112 1234–1243. ( 10.1161/CIRCRESAHA.112.300682) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gurha P, Abreu-Goodger C, Wang T, Ramirez MO, Drumond AL, van Dongen S, Chen Y, Bartonicek N, Enright AJ, Lee B, et al. Targeted deletion of microRNA-22 promotes stress-induced cardiac dilation and contractile dysfunction. Circulation 2012. 125 2751–2761. ( 10.1161/CIRCULATIONAHA.111.044354) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gurha P, Wang T, Larimore AH, Sassi Y, Abreu-Goodger C, Ramirez MO, Reddy AK, Engelhardt S, Taffet GE, Wehrens XH, et al. microRNA-22 promotes heart failure through coordinate suppression of PPAR/ERR-nuclear hormone receptor transcription. PLoS ONE 2013. 8 e75882. ( 10.1371/journal.pone.0075882) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gupta SK, Foinquinos A, Thum S, Remke J, Zimmer K, Bauters C, de Groote P, Boon RA, de Windt LJ, Preissl S, et al. Preclinical development of a microRNA-based therapy for elderly patients with myocardial infarction. Journal of the American College of Cardiology 2016. 68 1557–1571. ( 10.1016/j.jacc.2016.07.739) [DOI] [PubMed] [Google Scholar]
- 72.Jiao H, Chen R, Jiang Z, Zhang L, Wang H. miR-22 protect PC12 from ischemia/reperfusion-induced injury by targeting p53 upregulated modulator of apoptosis (PUMA). Bioengineered 2020. 11 209–218. ( 10.1080/21655979.2020.1729321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang F, Chen Q, He S, Yang M, Maguire EM, An W, Afzal TA, Luong LA, Zhang L, Xiao Q. miR-22 is a novel mediator of vascular smooth muscle cell phenotypic modulation and neointima formation. Circulation 2018. 137 1824–1841. ( 10.1161/CIRCULATIONAHA.117.027799) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Y, Chang W, Zhang Y, Zhang L, Ding H, Qi H, Xue S, Yu H, Hu L, Liu D, et al. Circulating miR-22-5p and miR-122-5p are promising novel biomarkers for diagnosis of acute myocardial infarction. Journal of Cellular Physiology 2019. 234 4778–4786. ( 10.1002/jcp.27274) [DOI] [PubMed] [Google Scholar]
- 75.Guo S, Zhang W, Wei C, Wang L, Zhu G, Shi Q, Li S, Ge R, Li K, Gao L, et al. Serum and skin levels of miR-369-3p in patients with psoriasis and their correlation with disease severity. European Journal of Dermatology 2013. 23 608–613. ( 10.1684/ejd.2013.2148) [DOI] [PubMed] [Google Scholar]
- 76.Scalavino V, Liso M, Cavalcanti E, Gigante I, Lippolis A, Mastronardi M, Chieppa M, Serino G. miR-369-3p modulates inducible nitric oxide synthase and is involved in regulation of chronic inflammatory response. Scientific Reports 2020. 10 15942. ( 10.1038/s41598-020-72991-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang J, Chen X, Huang W. MicroRNA-369 attenuates hypoxia-induced cardiomyocyte apoptosis and inflammation via targeting TRPV3. Brazilian Journal of Medical and Biological Research = Revista Brasileira de Pesquisas Medicas e Biologicas 2021. 54 e10550. ( 10.1590/1414-431X202010550) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liang J, Bai S, Su L, Li C, Wu J, Xia Z, Xu D. A subset of circulating microRNAs is expressed differently in patients with myocardial infarction. Molecular Medicine Reports 2015. 12 243–247. ( 10.3892/mmr.2015.3422) [DOI] [PubMed] [Google Scholar]
- 79.Krist B, Florczyk U, Pietraszek-Gremplewicz K, Józkowicz A, Dulak J. The role of mir-378a in metabolism, angiogenesis, and muscle biology. International Journal of Endocrinology 2015. 2015 281756. ( 10.1155/2015/281756) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Srivastava A, Meisgen F, Pasquali L, Munkhammar S, Xia P, Ståhle M, Landén NX, Pivarcsi A, Sonkoly E. Next-generation sequencing identifies the keratinocyte-specific miRNA signature of psoriasis. Journal of Investigative Dermatology 2019. 139 2547, .e12–2550.e12. ( 10.1016/j.jid.2019.05.019) [DOI] [PubMed] [Google Scholar]
- 81.Masalha M, Sidi Y, Avni D. The contribution of feedback loops between miRNAs, cytokines and growth factors to the pathogenesis of psoriasis. Experimental Dermatology 2018. 27 603–610. ( 10.1111/exd.13520) [DOI] [PubMed] [Google Scholar]
- 82.Gerin I, Bommer GT, McCoin CS, Sousa KM, Krishnan V, MacDougald OA. Roles for miRNA-378/378* in adipocyte gene expression and lipogenesis. American Journal of Physiology. Endocrinology and Metabolism 2010. 299 E198–E206. ( 10.1152/ajpendo.00179.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pan D, Mao C, Quattrochi B, Friedline RH, Zhu LJ, Jung DY, Kim JK, Lewis B, Wang YX. MicroRNA-378 controls classical brown fat expansion to counteract obesity. Nature Communications 2014. 5 4725. ( 10.1038/ncomms5725) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Berbée JFP, Boon MR, Khedoe PPSJ, Bartelt A, Schlein C, Worthmann A, Kooijman S, Hoeke G, Mol IM, John C, et al. Brown fat activation reduces hypercholesterolaemia and protects from atherosclerosis development. Nature Communications 2015. 6 6356. ( 10.1038/ncomms7356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Carrer M, Liu N, Grueter CE, Williams AH, Frisard MI, Hulver MW, Bassel-Duby R, Olson EN. Control of mitochondrial metabolism and systemic energy homeostasis by microRNAs 378 and 378*. PNAS 2012. 109 15330–15335. ( 10.1073/pnas.1207605109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chong H, Wei Z, Na M, Sun G, Zheng S, Zhu X, Xue Y, Zhou Q, Guo S, Xu J, et al. The PGC-1α/NRF1/miR-378a axis protects vascular smooth muscle cells from FFA-induced proliferation, migration and inflammation in atherosclerosis. Atherosclerosis 2020. 297 136–145. ( 10.1016/j.atherosclerosis.2020.02.001) [DOI] [PubMed] [Google Scholar]
- 87.Wang D, Yan X, Xia M, Yang Y, Li D, Li X, Song F, Ling W. Coenzyme Q10 promotes macrophage cholesterol efflux by regulation of the activator protein-1/miR-378/ATP-binding cassette transporter G1-signaling pathway. Arteriosclerosis, Thrombosis, and Vascular Biology 2014. 34 1860–1870. ( 10.1161/ATVBAHA.113.302879) [DOI] [PubMed] [Google Scholar]
- 88.Chen W, Li X, Wang J, Song N, Zhu A, Jia L. miR-378a modulates macrophage phagocytosis and differentiation through targeting CD47-SIRPα axis in atherosclerosis. Scandinavian Journal of Immunology 2019. 90 e12766. ( 10.1111/sji.12766) [DOI] [PubMed] [Google Scholar]
- 89.Ganesan J, Ramanujam D, Sassi Y, Ahles A, Jentzsch C, Werfel S, Leierseder S, Loyer X, Giacca M, Zentilin L, et al. MiR-378 controls cardiac hypertrophy by combined repression of mitogen-activated protein kinase pathway factors. Circulation 2013. 127 2097–2106. ( 10.1161/CIRCULATIONAHA.112.000882) [DOI] [PubMed] [Google Scholar]
- 90.Bazzoni F, Rossato M, Fabbri M, Gaudiosi D, Mirolo M, Mori L, Tamassia N, Mantovani A, Cassatella MA, Locati M. Induction and regulatory function of miR-9 in human monocytes and neutrophils exposed to proinflammatory signals. PNAS 2009. 106 5282–5287. ( 10.1073/pnas.0810909106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guo LM, Pu Y, Han Z, Liu T, Li YX, Liu M, Li X, Tang H. MicroRNA-9 inhibits ovarian cancer cell growth through regulation of NF-kappaB1. FEBS Journal 2009. 276 5537–5546. ( 10.1111/j.1742-4658.2009.07237.x) [DOI] [PubMed] [Google Scholar]
- 92.Ou M, Zhang Y, Cui S, Zhao S, Tu J. Upregulated MiR-9-5p protects Against inflammatory response in rats with deep vein thrombosis via inhibition of NF-κB p50. Inflammation 2019. 42 1925–1938. ( 10.1007/s10753-019-01031-z) [DOI] [PubMed] [Google Scholar]
- 93.D’Amore S, Härdfeldt J, Cariello M, Graziano G, Copetti M, Di Tullio G, Piglionica M, Scialpi N, Sabbà C, Palasciano G, et al. Identification of miR-9-5p as direct regulator of ABCA1 and HDL-driven reverse cholesterol transport in circulating CD14+ cells of patients with metabolic syndrome. Cardiovascular Research 2018. 114 1154–1164. ( 10.1093/cvr/cvy077) [DOI] [PubMed] [Google Scholar]
- 94.Zhang R, Song B, Hong X, Shen Z, Sui L, Wang S. microRNA-9 inhibits vulnerable plaque formation and vascular remodeling via suppression of the SDC2-dependent FAK/ERK signaling pathway in mice With atherosclerosis. Frontiers in Physiology 2020. 11 804. ( 10.3389/fphys.2020.00804) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yu DR, Wang T, Huang J, Fang XY, Fan HF, Yi GH, Liu Q, Zhang Y, Zeng XZ, Liu QB. MicroRNA-9 overexpression suppresses vulnerable atherosclerotic plaque and enhances vascular remodeling through negative regulation of the p38MAPK pathway via OLR1 in acute coronary syndrome. Journal of Cellular Biochemistry 2020. 121 49–62. ( 10.1002/jcb.27830) [DOI] [PubMed] [Google Scholar]
- 96.Wang K, Long B, Zhou J, Li PF. miR-9 and NFATc3 regulate myocardin in cardiac hypertrophy. Journal of Biological Chemistry 2010. 285 11903–11912. ( 10.1074/jbc.M109.098004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xiao Y, Zhang Y, Chen Y, Li J, Zhang Z, Sun Y, Shen H, Zhao Z, Huang Z, Zhang W, et al. Inhibition of MicroRNA-9-5p protects against cardiac remodeling following myocardial infarction in mice. Human Gene Therapy 2019. 30 286–301. ( 10.1089/hum.2018.059) [DOI] [PubMed] [Google Scholar]
- 98.Yi J, Gao ZF. MicroRNA-9-5p promotes angiogenesis but inhibits apoptosis and inflammation of high glucose-induced injury in human umbilical vascular endothelial cells by targeting CXCR4. International Journal of Biological Macromolecules 2019. 130 1–9. ( 10.1016/j.ijbiomac.2019.02.003) [DOI] [PubMed] [Google Scholar]
- 99.Krane JF, Gottlieb AB, Carter DM, Krueger JG. The insulin-like growth factor I receptor is overexpressed in psoriatic epidermis, but is differentially regulated from the epidermal growth factor receptor. Journal of Experimental Medicine 1992. 175 1081–1090. ( 10.1084/jem.175.4.1081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ye XM, Zhu HY, Bai WD, Wang T, Wang L, Chen Y, Yang AG, Jia LT. Epigenetic silencing of miR-375 induces trastuzumab resistance in HER2-positive breast cancer by targeting IGF1R. BMC Cancer 2014. 14 134. ( 10.1186/1471-2407-14-134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen B, Guo S, Yu Z, Feng Y, Hui L. Downregulation of microRNA-375, combined with upregulation of its target gene Janus kinase 2, predicts unfavorable prognosis in patients with gastric cancer. International Journal of Clinical and Experimental Pathology 2017. 10 11106–11113. [PMC free article] [PubMed] [Google Scholar]
- 102.Akat KM, Moore-McGriff D, Morozov P, Brown M, Gogakos T, Correa Da Rosa J, Mihailovic A, Sauer M, Ji R, Ramarathnam A, et al. Comparative RNA-sequencing analysis of myocardial and circulating small RNAs in human heart failure and their utility as biomarkers. PNAS 2014. 111 11151–11156. ( 10.1073/pnas.1401724111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Garikipati VNS, Verma SK, Jolardarashi D, Cheng Z, Ibetti J, Cimini M, Tang Y, Khan M, Yue Y, Benedict C, et al. Therapeutic inhibition of miR-375 attenuates post-myocardial infarction inflammatory response and left ventricular dysfunction via PDK-1-AKT signalling axis. Cardiovascular Research 2017. 113 938–949. ( 10.1093/cvr/cvx052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Garikipati VNS, Krishnamurthy P, Verma SK, Khan M, Abramova T, Mackie AR, Qin G, Benedict C, Nickoloff E, Johnson J, et al. Negative regulation of miR-375 by interleukin-10 enhances bone marrow-derived progenitor cell-mediated myocardial repair and function after myocardial infarction. Stem Cells 2015. 33 3519–3529. ( 10.1002/stem.2121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 2003. 421 744–748. ( 10.1038/nature01355). [DOI] [PubMed] [Google Scholar]
- 106.Hou T, Liao J, Zhang C, Sun C, Li X, Wang G. Elevated expression of miR-146, miR-139 and miR-340 involved in regulating Th1/Th2 balance with acute exposure of fine particulate matter in mice. International Immunopharmacology 2018. 54 68–77. ( 10.1016/j.intimp.2017.10.003) [DOI] [PubMed] [Google Scholar]
- 107.Honardoost MA, Naghavian R, Ahmadinejad F, Hosseini A, Ghaedi K. Integrative computational mRNA-miRNA interaction analyses of the autoimmune-deregulated miRNAs and well-known Th17 differentiation regulators: an attempt to discover new potential miRNAs involved in Th17 differentiation. Gene 2015. 572 153–162. ( 10.1016/j.gene.2015.08.043) [DOI] [PubMed] [Google Scholar]
- 108.Bian J, Liu R, Fan T, Liao L, Wang S, Geng W, Wang T, Shi W, Ruan Q. miR-340 alleviates psoriasis in mice through direct targeting of IL-17A. Journal of Immunology 2018. 201 1412–1420. ( 10.4049/jimmunol.1800189) [DOI] [PubMed] [Google Scholar]
- 109.Calabrò P, Limongelli G, Riegler L, Maddaloni V, Palmieri R, Golia E, Roselli T, Masarone D, Pacileo G, Golino P, et al. Novel insights into the role of cardiotrophin-1 in cardiovascular diseases. Journal of Molecular and Cellular Cardiology 2009. 46 142–148. ( 10.1016/j.yjmcc.2008.11.002) [DOI] [PubMed] [Google Scholar]
- 110.Bristow MR, Long CS. Cardiotrophin-1 in heart failure. Circulation 2002. 106 1430–1432. ( 10.1161/01.cir.0000034024.61382.42) [DOI] [PubMed] [Google Scholar]
- 111.Zhou J, Gao J, Zhang X, Liu Y, Gu S, Zhang X, An X, Yan J, Xin Y, Su P. microRNA-340-5p functions downstream of Cardiotrophin-1 to regulate cardiac eccentric hypertrophy and heart failure via target gene dystrophin. International Heart Journal 2015. 56 454–458. ( 10.1536/ihj.14-386) [DOI] [PubMed] [Google Scholar]
- 112.Wollert KC, Taga T, Saito M, Narazaki M, Kishimoto T, Glembotski CC, Vernallis AB, Heath JK, Pennica D, Wood WI, et al. Cardiotrophin-1 activates a distinct form of cardiac muscle cell hypertrophy. Assembly of sarcomeric units in series VIA gp130/leukemia inhibitory factor receptor-dependent pathways. Journal of Biological Chemistry 1996. 271 9535–9545. ( 10.1074/jbc.271.16.9535) [DOI] [PubMed] [Google Scholar]
- 113.Rybakova IN, Patel JR, Ervasti JM. The dystrophin complex forms a mechanically strong link between the sarcolemma and costameric actin. Journal of Cell Biology 2000. 150 1209–1214. ( 10.1083/jcb.150.5.1209) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wu D, Bi X, Qu L, Han L, Yin C, Deng J, Dong Z, Mi QS, Zhou L. miRNA miR-17-92 cluster is differentially regulated in the imiqumod-treated skin but is not required for imiqumod-induced psoriasis-like dermatitis in mice. Experimental Dermatology 2017. 26 82–84. ( 10.1111/exd.13186) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu M, Wang Z, Yang S, Zhang W, He S, Hu C, Zhu H, Quan L, Bai J, Xu N. TNF-α is a novel target of miR-19a. International Journal of Oncology 2011. 38 1013–1022. ( 10.3892/ijo.2011.924) [DOI] [PubMed] [Google Scholar]
- 116.Hirao H, Jinnin M, Ichihara A, Fujisawa A, Makino K, Kajihara I, Sakai K, Fukushima S, Inoue Y, Ihn H. Detection of hair root miR-19a as a novel diagnostic marker for psoriasis. European Journal of Dermatology 2013. 23 807–811. ( 10.1684/ejd.2013.2190) [DOI] [PubMed] [Google Scholar]
- 117.Chen H, Li X, Liu S, Gu L, Zhou X. MircroRNA-19a promotes vascular inflammation and foam cell formation by targeting HBP-1 in atherogenesis. Scientific Reports 2017. 7 12089. ( 10.1038/s41598-017-12167-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Song DW, Ryu JY, Kim JO, Kwon EJ, Kim DH. The miR-19a/b family positively regulates cardiomyocyte hypertrophy by targeting atrogin-1 and MuRF-1. Biochemical Journal 2014. 457 151–162. ( 10.1042/BJ20130833) [DOI] [PubMed] [Google Scholar]
- 119.Senzaki H, Smith CJ, Juang GJ, Isoda T, Mayer SP, Ohler A, Paolocci N, Tomaselli GF, Hare JM, Kass DA. Cardiac phosphodiesterase 5 (cGMP-specific) modulates beta-adrenergic signaling in vivo and is down-regulated in heart failure. FASEB Journal 2001. 15 1718–1726. ( 10.1096/fj.00-0538com) [DOI] [PubMed] [Google Scholar]
- 120.Ichihara A, Jinnin M, Yamane K, Fujisawa A, Sakai K, Masuguchi S, Fukushima S, Maruo K, Ihn H. microRNA-mediated keratinocyte hyperproliferation in psoriasis vulgaris. British Journal of Dermatology 2011. 165 1003–1010. ( 10.1111/j.1365-2133.2011.10497.x) [DOI] [PubMed] [Google Scholar]
- 121.Li C, Zhang M, Dai Y, Xu Z. MicroRNA-424-5p regulates aortic smooth muscle cell function in atherosclerosis by blocking APOC3-mediated nuclear factor-κB signalling pathway. Experimental Physiology 2020. 105 1035–1049. ( 10.1113/EP088088) [DOI] [PubMed] [Google Scholar]
- 122.Lou Y, Wang S, Qu J, Zheng J, Jiang W, Lin Z, Zhang S. miR-424 promotes cardiac ischemia/reperfusion injury by direct targeting of CRISPLD2 and regulating cardiomyocyte pyroptosis. International Journal of Clinical and Experimental Pathology 2018. 11 3222–3235. [PMC free article] [PubMed] [Google Scholar]
- 123.Løvendorf MB, Mitsui H, Zibert JR, Røpke MA, Hafner M, Dyring-Andersen B, Bonefeld CM, Krueger JG, Skov L. Laser capture microdissection followed by next-generation sequencing identifies disease-related microRNAs in psoriatic skin that reflect systemic microRNA changes in psoriasis. Experimental Dermatology 2015. 24 187–193. ( 10.1111/exd.12604) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hermann H, Runnel T, Aab A, Baurecht H, Rodriguez E, Magilnick N, Urgard E, Šahmatova L, Prans E, Maslovskaja J, et al. miR-146b Probably Assists miRNA-146a in the Suppression of keratinocyte Proliferation and inflammatory Responses in Psoriasis. Journal of Investigative Dermatology 2017. 137 1945–1954. ( 10.1016/j.jid.2017.05.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Duperret EK, Ridky TW. Kindler syndrome in mice and men. Cancer Biology and Therapy 2014. 15 1113–1116. ( 10.4161/cbt.29482) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yang Z, Zeng B, Tang X, Wang H, Wang C, Yan Z, Huang P, Pan Y, Xu B. MicroRNA-146a and miR-99a are potential biomarkers for disease activity and clinical efficacy assessment in psoriasis patients treated with traditional Chinese medicine. Journal of Ethnopharmacology 2016. 194 727–732. ( 10.1016/j.jep.2016.08.028) [DOI] [PubMed] [Google Scholar]
- 127.Petrkova J, Borucka J, Kalab M, Klevcova P, Michalek J, Taborsky M, Petrek M. Increased expression of mir-146a in valvular tissue from patients with aortic valve stenosis. Frontiers in Cardiovascular Medicine 2019. 6 86. ( 10.3389/fcvm.2019.00086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li K, Ching D, Luk FS, Raffai RL. Apolipoprotein E enhances microRNA-146a in monocytes and macrophages to suppress nuclear factor-κB-driven inflammation and atherosclerosis. Circulation Research 2015. 117 e1–e11. ( 10.1161/CIRCRESAHA.117.305844) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sun D, Xiang G, Wang J, Li Y, Mei S, Ding H, Yan J. miRNA 146b-5p protects against atherosclerosis by inhibiting vascular smooth muscle cell proliferation and migration. Epigenomics 2020. 12 2189–2204. ( 10.2217/epi-2020-0155) [DOI] [PubMed] [Google Scholar]
- 130.Palomer X, Capdevila-Busquets E, Botteri G, Davidson MM, Rodríguez C, Martínez-González J, Vidal F, Barroso E, Chan TO, Feldman AM, et al. miR-146a targets Fos expression in human cardiac cells. Disease Models and Mechanisms 2015. 8 1081–1091. ( 10.1242/dmm.020768) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Oh JG, Watanabe S, Lee A, Gorski PA, Lee P, Jeong D, Liang L, Liang Y, Baccarini A, Sahoo S, et al. miR-146a suppresses SUMO1 expression and induces cardiac dysfunction in maladaptive hypertrophy. Circulation Research 2018. 123 673–685. ( 10.1161/CIRCRESAHA.118.312751) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Løvendorf MB, Zibert JR, Gyldenløve M, Røpke MA, Skov L. MicroRNA-223 and miR-143 are important systemic biomarkers for disease activity in psoriasis. Journal of Dermatological Science 2014. 75 133–139. ( 10.1016/j.jdermsci.2014.05.005) [DOI] [PubMed] [Google Scholar]
- 133.Yan JJ, Qiao M, Li RH, Zhao XT, Wang XY, Sun Q. Downregulation of miR-145-5p contributes to hyperproliferation of keratinocytes and skin inflammation in psoriasis. British Journal of Dermatology 2019. 180 365–372. ( 10.1111/bjd.17256) [DOI] [PubMed] [Google Scholar]
- 134.Fichtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, Liebetrau C, Weber M, Hamm CW, Röxe T, Müller-Ardogan M, et al. Circulating microRNAs in patients with coronary artery disease. Circulation Research 2010. 107 677–684. ( 10.1161/CIRCRESAHA.109.215566) [DOI] [PubMed] [Google Scholar]
- 135.D’Alessandra Y, Carena MC, Spazzafumo L, Martinelli F, Bassetti B, Devanna P, Rubino M, Marenzi G, Colombo GI, Achilli F, et al. Diagnostic potential of plasmatic microRNA signatures in stable and unstable angina. PLoS ONE 2013. 8 e80345. ( 10.1371/journal.pone.0080345) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cipollone F, Felicioni L, Sarzani R, Ucchino S, Spigonardo F, Mandolini C, Malatesta S, Bucci M, Mammarella C, Santovito D, et al. A unique microRNA signature associated with plaque instability in humans. Stroke 2011. 42 2556–2563. ( 10.1161/STROKEAHA.110.597575) [DOI] [PubMed] [Google Scholar]
- 137.Lovren F, Pan Y, Quan A, Singh KK, Shukla PC, Gupta N, Steer BM, Ingram AJ, Gupta M, Al-Omran M, et al. MicroRNA-145 targeted therapy reduces atherosclerosis. Circulation 2012. 126(Supplement 1) S81–S90. ( 10.1161/CIRCULATIONAHA.111.084186) [DOI] [PubMed] [Google Scholar]
- 138.Spinale FG, Koval CN, Deschamps AM, Stroud RE, Ikonomidis JS. Dynamic changes in matrix metalloprotienase activity within the human myocardial interstitium during myocardial arrest and reperfusion. Circulation 2008. 118(Supplement 14) S16–S23. ( 10.1161/CIRCULATIONAHA.108.786640) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.He M, Wu N, Leong MC, Zhang W, Ye Z, Li R, Huang J, Zhang Z, Li L, Yao X, et al. miR-145 improves metabolic inflammatory disease through multiple pathways. Journal of Molecular Cell Biology 2020. 12 152–162. ( 10.1093/jmcb/mjz015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hergenreider E, Heydt S, Tréguer K, Boettger T, Horrevoets AJG, Zeiher AM, Scheffer MP, Frangakis AS, Yin X, Mayr M, et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nature Cell Biology 2012. 14 249–256. ( 10.1038/ncb2441) [DOI] [PubMed] [Google Scholar]
- 141.Meder B, Keller A, Vogel B, Haas J, Sedaghat-Hamedani F, Kayvanpour E, Just S, Borries A, Rudloff J, Leidinger P, et al. MicroRNA signatures in total peripheral blood as novel biomarkers for acute myocardial infarction. Basic Research in Cardiology 2011. 106 13–23. ( 10.1007/s00395-010-0123-2) [DOI] [PubMed] [Google Scholar]
- 142.Ribeiro-Rodrigues TM, Laundos TL, Pereira-Carvalho R, Batista-Almeida D, Pereira R, Coelho-Santos V, Silva AP, Fernandes R, Zuzarte M, Enguita FJ, et al. Exosomes secreted by cardiomyocytes subjected to ischaemia promote cardiac angiogenesis. Cardiovascular Research 2017. 113 1338–1350. ( 10.1093/cvr/cvx118) [DOI] [PubMed] [Google Scholar]
- 143.Wu Y, Liu L, Bian C, Diao Q, Nisar MF, Jiang X, Bartsch JW, Zhong M, Hu X, Zhong JL. MicroRNA let-7b inhibits keratinocyte differentiation by targeting IL-6 mediated ERK signaling in psoriasis. Cell Communication and Signaling 2018. 16 58. ( 10.1186/s12964-018-0271-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pasquali L, Svedbom A, Srivastava A, Rosén E, Lindqvist U, Ståhle M, Pivarcsi A, Sonkoly E. Circulating microRNAs in extracellular vesicles as potential biomarkers for psoriatic arthritis in patients with psoriasis. Journal of the European Academy of Dermatology and Venereology 2020. 34 1248–1256. ( 10.1111/jdv.16203) [DOI] [PubMed] [Google Scholar]
- 145.Hu XP, Xie Q, Chen CF, Zhang W, Yu B. Let-7a inhibits T-cell proliferation and IFN-γ secretion by down-regulating STAT3 expression in patients with psoriasis. Cellular Physiology and Biochemistry 2017. 42 115–12. ( 10.1159/000477120) [DOI] [PubMed] [Google Scholar]
- 146.Brennan E, Wang B, McClelland A, Mohan M, Marai M, Beuscart O, Derouiche S, Gray S, Pickering R, Tikellis C, et al. Protective effect of let-7 mirna family in regulating inflammation in diabetes-associated atherosclerosis. Diabetes 2017. 66 2266–2277. ( 10.2337/db16-1405) [DOI] [PubMed] [Google Scholar]
- 147.Zhou X, Sun F, Luo S, Zhao W, Yang T, Zhang G, Gao M, Lu R, Shu Y, Mu W, et al. Let-7a is an antihypertrophic regulator in the heart via targeting calmodulin. International Journal of Biological Sciences 2017. 13 22–31. ( 10.7150/ijbs.16298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chen CY, Choong OK, Liu LW, Cheng YC, Li SC, Yen CYT, Wu MR, Chiang MH, Tsang TJ, Wu YW, et al. MicroRNA let-7-TGFBR3 signalling regulates cardiomyocyte apoptosis after infarction. EBiomedicine 2019. 46 236–247. ( 10.1016/j.ebiom.2019.08.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, van der Meer AJ, Patick AK, Chen A, Zhou Y, et al. Treatment of HCV infection by targeting microRNA. New England Journal of Medicine 2013. 368 1685–1694. ( 10.1056/NEJMoa1209026) [DOI] [PubMed] [Google Scholar]
- 150.Elton TS, Khan M, Terentyev D. MicroRNAs in cardiovascular disease. F1000 Medicine Reports 2011. 3 10. ( 10.3410/M3-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hullinger TG, Montgomery RL, Seto AG, Dickinson BA, Semus HM, Lynch JM, Dalby CM, Robinson K, Stack C, Latimer PA, et al. Inhibition of miR-15 protects against cardiac ischemic injury. Circulation Research 2012. 110 71–81. ( 10.1161/CIRCRESAHA.111.244442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hulot JS, Masurkar N. miRNA-Based Therapeutics for heart failure: why not? Journal of the American College of Cardiology 2020. 75 1801–1803. ( 10.1016/j.jacc.2020.03.003) [DOI] [PubMed] [Google Scholar]
- 153.Borel F, Kay MA, Mueller C. Recombinant AAV as a platform for translating the therapeutic potential of RNA interference. Molecular Therapy 2014. 22 692–701. ( 10.1038/mt.2013.285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hu S, Huang M, Li Z, Jia F, Ghosh Z, Lijkwan MA, Fasanaro P, Sun N, Wang X, Martelli F, et al. MicroRNA-210 as a novel therapy for treatment of ischemic heart disease. Circulation 2010. 122 (Supplement 11) S124–S131. ( 10.1161/CIRCULATIONAHA.109.928424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Fenske DB, Chonn A, Cullis PR. Liposomal nanomedicines: an emerging field. Toxicologic Pathology 2008. 36 21–29. ( 10.1177/0192623307310960) [DOI] [PubMed] [Google Scholar]
- 156.Akinc A, Zumbuehl A, Goldberg M, Leshchiner ES, Busini V, Hossain N, Bacallado SA, Nguyen DN, Fuller J, Alvarez R, et al. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nature Biotechnology 2008. 26 561–569. ( 10.1038/nbt1402) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bisht S, Feldmann G, Koorstra JB, Mullendore M, Alvarez H, Karikari C, Rudek MA, Lee CK, Maitra A, Maitra A. In vivo characterization of a polymeric nanoparticle platform with potential oral drug delivery capabilities. Molecular Cancer Therapeutics 2008. 7 3878–3888. ( 10.1158/1535-7163.MCT-08-0476) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kumar A, Pathak K, Bali V. Ultra-adaptable nanovesicular systems: a carrier for systemic delivery of therapeutic agents. Drug Discovery Today 2012. 17 1233–1241. ( 10.1016/j.drudis.2012.06.013) [DOI] [PubMed] [Google Scholar]
- 159.Geusens B, Van Gele M, Braat S, De Smedt SC, Stuart MCA, Prow TW, Sanchez W, Roberts MS, Sanders NN, Lambert J. Flexible nanosomes (SECosomes) enable efficient siRNA delivery in cultured primary skin cells and in the viable epidermis of ex vivo human skin. Advanced Functional Materials 2010. 20 4077–4090. ( 10.1002/adfm.201000484) [DOI] [Google Scholar]
- 160.Menegatti S, Zakrewsky M, Kumar S, De Oliveira JS, Muraski JA, Mitragotri S. De novo design of skin-penetrating peptides for enhanced transdermal delivery of peptide drugs. Advanced Healthcare Materials 2016. 5 602–609. ( 10.1002/adhm.201500634) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a