Abstract
Self-healing injectable hydrogel biomaterials uniquely enable precise therapeutic deposition and deployment at specific bodily locations through versatile and minimally invasive processes that can preserve cargo integrity and cell viability. Despite the distinct advantages that injectable hydrogels offer in tissue engineering and therapeutic delivery, exceptionally few have been created using components naturally present in the cellular niche. In this work, we introduce a shear-thinning hydrogel based on guest-host complexation of gelatin. As a biocompatible, biodegradable, and non-immunogenic biopolymer derived from the most abundant extracellular matrix protein (collagen), gelatin offers great utility as the structural component of biomaterials. Taking advantage of reversible guest-host interactions between β-cyclodextrin (CD) and adamantane (AD) on modified gelatins, we report the first strategy to afford a self-healing material based solely on a functionalized extracellular matrix (ECM) protein. By varying the initial material formulation, hydrogels were synthesized with variable moduli and shear-thinability across a broad range. Gels were demonstrated to exhibit shear-thinning and self-healing properties, supporting protection of clinically relevant stem-cell-derived cardiomyocytes during injection. These materials are expected to expand clinical opportunities in cell delivery for in vivo tissue regeneration.
Keywords: hydrogel, shear-thinning, injectable, cell delivery, guest-host complexation
1. Introduction:
Hydrogels are comprised of hydrophilic macromolecular polymeric networks of natural, synthetic, or mixed origin that retain a high water content while preserving their 3D structural integrity 1. Over the past many decades, these materials have been extensively employed in a variety of biomedical applications including for the delivery of therapeutic agents 2-5; to support 3D cell culture and dynamically direct cell fate 6-10; as scaffolds for tissue engineering and regenerative medicine 11-14; and as components in biomedical devices, bioadhesives, and biosealants 15-17. This growing list of applications stems directly from the materials’ ability to be formulated in manners that are biocompatible, support nutrient diffusion, and offer tunable physiochemistry that mimics critical aspects of the native extracellular matrix (ECM) 18. In addition to the base polymer composition, the crosslinking type (physical vs. chemical) and density, and overall network biodegradability can be chosen directly to dictate the mechanical, structural, and biological properties of the resultant hydrogels 1,18.
Though hydrogels have gained tremendous interest as uniquely powerful materials in biomedicine, on-going challenges hamper their clinical use. Though pre-formed hydrogels can be customized to support cell transplantation and engraftment, invasive surgical procedures required for physical placement within patients increase risks of infection, complications, pain, patient discomfort, and medical care cost 19. Injectable hydrogel biomaterials offer distinct advantages for many applications, readily taking the shape of irregular cavities while providing a minimally invasive module of delivery. Since these systems can be introduced to specific bodily locations via syringe/catheter injection, they can be precisely placed in vivo without the need for complicated surgeries. Furthermore, therapeutic agents (e.g., proteins, drugs, cells) can be readily mixed into the precursor solution, affording a route for simple and uniform incorporation prior to administration in a potentially cargo-protective manner. As such, injectable hydrogels are of increasing interest in the field of tissue engineering and therapeutic delivery 20-22.
One common approach for engineering an injectable hydrogel is to design liquid precursors that undergo chemical crosslinking and gel in situ during/after injection. Though this approach has proven beneficial in many systems, there are potential drawbacks in requiring an external stimulus to initiate gelation or a multi-barrel syringe setup to mix reactants together during injection. Another important variable in this process is sol-gel transition time; a rapid crosslinking of the hydrogel may result in catheter/syringe obstruction prior to the deployment of the hydrogel, whereas a lagging crosslinking process may lead to material dissipation upon injection prior to setting. Moreover, release of unreacted precursors outside of the target zone due to slow gelation raises the risk of emboli formation and off-site cytotoxicity 20-22.
To overcome the challenges of in situ crosslinking systems, hydrogels with the ability to flow under application of shear stress (i.e., shear-thinning) and reform upon stress removal (i.e., self-healing) have gained popularity. Optimal systems undergo a reversible sol-to-gel transition in response to shear forces introduced during injection and subsequently exhibit rapid self-healing. Such injectable biomaterials have been achieved through physical self-assembly promoted through numerous noncovalent chemical interactions (e.g., hydrogen bonds, electrostatic interactions, hydrophobic interactions). While each individual bond is weak, cumulatively they can result in formation of materials with high structural integrity 21,23.
Though self-healing injectable biomaterials offer promise in many areas, one particularly powerful application has been towards promoting cardiac regeneration following myocardial infarction (MI) 24. In MI, occlusion of a coronary artery prevents oxygenated blood from reaching its intended destination in the heart. Tissue located downstream to the blockage becomes ischemic, with prolonged lack of oxygen resulting in irreversible necrosis of the heart muscle. As one of the least regenerative organs in the body, loss of heart tissue results in fibrotic scar formation that can cause significant deficit of contractile force and ultimately heart failure 25. With cardiovascular diseases being a leading cause of death and the number one healthcare cost globally, significant effort has been put into developing techniques to promote cardiac regeneration 26,27. Researchers have sought after therapeutic strategies dedicated to improving myocardial regeneration and restoration of function including direct injection of cardiomyocytes (CM) 28, stem cells 29, biomolecules 30,31, and chemokines 32. Though demonstrating some positive effect, these techniques suffer from significant impediments such as nonspecific delivery, low cell survival, and insignificant engraftment rate in the desired targeted region 33. Efforts of tissue engineering researchers have focused on overcoming these drawbacks by applying injectable biomaterials designed to protect the therapeutic cargo from degradation, improve targeted engraftment, and increase cell survival during and post injection 33.
A variety of self-healing hydrogel biomaterials have now been reported, including those to support enhanced cell delivery/engraftment in the treatment of MI. One such strategy utilized zwitterionic polycarboxybetaine-based microgels that self-assemble through charge-charge interactions around cells with tunable moduli 34. Other approaches have exploited the physical electrostatic crosslinking of alginate with divalent cations (e.g., Ca2+) and have demonstrated enhanced cell viability during in-gel injection compared to that in phosphate-buffered saline 35,36. A shear-thinning, engineered protein-based hydrogel with cell-adhesive domains has been reported to improve induced pluripotent stem cells derived endothelial cells viability during the injection process by protecting the cells from mechanical forces within the needle 37. In one particularly powerful demonstration involving hydrogel derived from ECM-presented glycosaminoglycans, injectable hyaluronic acid-based materials formed through guest-host interactions were demonstrated support endothelial progenitor delivery, enhance cell engraftment, and improve ischemic myocardium in rat and sheep MI models 38,39.
Towards designing a shear-thinning biomaterial to further improve cell viability and engraftment following injection, as well as provide a suitable environment for the long-term culture of a variety of cells, we took inspiration from the native ECM, most abundantly comprised of collagen protein 40,41. Though we expected a collagen-based material to provide appropriate physiochemical cues to support long term cell culture, collagen in its natural form is highly viscous and difficult to handle. Moreover, intact collagen has been reported to induce immunogenic response in some patients 42. Therefore, we hypothesized that utilizing gelatin, a biocompatible and non-immunogenetic protein originating from denatured collagen with potentially tunable and easy-to-use properties, would promote cell survival when used as an injectable scaffold.
Gelatin is a protein cured from collagen that has been reported to have biocompatible, biodegradable, non-immunogenic, and cell-integration properties 43. Despite these advantages, gelatin is a liquid at body temperature, and therefore cannot be used as a stable scaffold in vivo; chemical modifications are required to provide stability to its structure and enhance its mechanical properties while avoiding rapid degradation upon exposure to host-tissue 43. Many chemical and enzymatic modifications have been utilized to alter gelatin’s mechanical properties [e.g., glutaraldehyde, carbodiimide (EDAC), transglutaminase, methacrylation] 44-48. Though these approaches can yield stable materials that have been beneficial for cell culture and drug delivery, there has yet to be developed a method that affords a stimuli-free, by-product free, single-barrel injection of an in situ self-healing gelatin.
In this study, we sought to develop a self-healing and injectable gelatin-based platform for minimally invasive therapeutic delivery. Towards this, we functionalized gelatin with complementary association domains whose physical interaction could be disrupted with mild shear force and would rapidly reform upon force removal. We sought to employ guest-host chemistry based on physical hydrophobic bonding between a cyclic cup-shaped host (i.e., β-cyclodextrin) and a complimentary guest molecule (i.e., adamantane). These moieties were conjugated onto carboxylic acid moieties pendant to the gelatin biopolymer backbone using EDAC/NHS chemistry; rapid association and dissociation of the guest-host bonds upon application of low- and high-shear respectively resulted in the formation of a shear-thinning injectable gelatin-based platform that can potentially be used as a minimally invasive drug and cell delivery module (Figure 1).
Figure 1.
Rapid association and dissociation of guest-host complementary bonds. (a) Transient hydrophobic bond formation occurs between the host moiety (β-Cyclodetrin, CD) and its guest molecule (adamantane, AD). (b) Gelatins that have been covalently modified with CD and AD react to form a physically crosslinked hydrogel that exhibits both shear thinability and rapid self-healing.
2. Materials and Methods:
2.1. Materials
Gelatin type A (porcine skin, ~275 bloom, 40-50 kDa), β-cyclodextrin (CD), 1-adamantylamine hydrochloride (AD), p-Toluenesulfonyl Chloride (TsCl), o-Phthaldialdehyde (OPA), and 1,6-Hexanediamine (HDA) were purchased from Acros Organics (Fair Lawn, NJ). Sodium hydroxide (NaOH), acetone, acetonitrile, glycine, sodium tetraborate, ethanol, and anhydrous diethyl ether were purchased from Fischer Scientific (Fair Lawn, NJ). Ammonium chloride was purchased from JT Baker (Phillipsburg, NJ). Anhydrous dimethyl sulfoxide (DMSO), and di-tert-butyl dicarbonate (Boc) were purchased from Alfa Aesar (Tewksbury, MA). N-hydroxysuccinimide (NHS), 2-mercaptoethanol, and N,N-dimethyl formamide (DMF) were purchased from Sigma-Aldrich (St. Louis, MO). 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDAC) was purchased from AK Scientific (Union City, CA). d6-DMSO was purchased from Cambridge isotope laboratories (Andover, MA). Hydrochloric acid (HCl, 12M) was purchased from Avantoc (Center Valley, PA). All chemicals were used as received unless stated otherwise.
UV analysis was performed with SpectraMax M5 UV-VIS spectrophotometer (Molecular Devices, San Jose, CA). 1H NMR spectra performed in DMSO-d6 using Avance 300 MHz instrument (Bruker, Billerica, MA). Freeze drying of the polymer derivatives occurred by means of FreeZone 2.5plus lyophilizer (Labconco, Kansas City, MO). Rheology experiments performed on Physica MCR301 Rheometer (Anton Paar, Graz, Austria). Cell viability analysis was performed on a NC-200 NucleoCounter®.
2.2. Synthesis of Boc-Protected Adamantane-Modified Gelatin (Gel-AD-Boc)
Adamantane-modification of gelatin was performed by adaptation of a previously reported synthesis describing conjugation of 2-aminoethyl methacrylate to carboxylic acid side chains of gelatin 49. Briefly, the synthetic route involves Boc-protection of gelatin’s primary amines, NHS-activation of the Boc-protected gelatin, amide formation through reaction with AD, and final Boc deprotection (Supplementary Figures S1-S2). Here, Gelatin A (5 g, 1.46 mmol amine of lysine and hydroxylysine 50, 4.23 mmol carboxylic acids 51, 1 equiv) was dissolved in 50 mL anhydrous DMSO at 55 °C under nitrogen atmosphere and reflux conditions. After obtaining a homogeneous solution, Boc (635.1 mg, 2.91 mmol, 2 equiv) was added and the reaction mixture was stirred for 80 min. Molar excess of Boc was considered with respect to free amino groups of gelatin based on a content of 0.291 mmol free amine groups per gram of gelatin type A 50. EDAC (991 mg, 6.38 mmol, 1.5 equiv) was added and the solution was stirred for 5 minutes. NHS (979.5 mg, 8.51 mmol, 2 equiv) was included in the reaction mixture followed by stirring for 45 minutes. 1-Adamantylamine (AD; 835.7 mg, 5.53 mmol, 1.3 equiv) was added and the reaction occurred overnight (20 hrs) while stirring at 55 °C under nitrogen atmosphere with reflux conditions. Molar excesses of EDAC, NHS, and AD were considered in respect to carboxylic acid side chains based on a content of 0.851 mmol carboxylic acid groups per gram gelatin type A 51. Mixture was precipitated in a tenfold excess (500 mL) of cold acetone and filtered onto a glass filter (M). The residue was dissolved in minimal double-distilled water (DDW) at 50 °C and dialyzed (molecular weight cut-off: 3500 Da) against DDW for 48 hrs at 42 °C. Dialyzed product was frozen and lyophilized for 48 hrs and a light-yellow spongy material was obtained (1.75 g, denoted Gel-AD-Boc).
2.3. Synthesis of Boc-Protected β-Cyclodextrin-Modified Gelatin (Gel-CD-Boc)
Synthesis of 6-(6-aminohexyl)amino-6-deoxy-β-cyclodextrin (CD-HDA)
6-(6-aminohexyl)amino-6-deoxy-β-cyclodextrin (CD-HDA) synthesis was performed by adaptation of previously reported similar syntheses 52-54 and shown in Supplementary Figure S3. A round-bottom flask was charged with CD (1 equiv, 20 g, 17.6 mmol) and DDW (150 mL), and cooled on ice to 0 °C for 1 hour. NaOH (3.1 equiv, 2.2 g, 55 mmol) was dissolved in 6.67 mL of DDW and added dropwise. By the end of the addition of NaOH, the solution turned completely homogeneous and clear. The suspension was stirred for 30 minutes at room temperature. TsCl (1.25 equiv, 4.2 g, 22 mmol) was dissolved in minimal acetonitrile (10 mL) and added dropwise to the CD suspension. Immediate precipitation was observed upon TsCl addition. The suspension was stirred at room temperature for two hrs. pH was adjusted to 8-8.5 by addition of approximately 10 g of solid ammonium chloride (NH4Cl) and suspension was cooled at 4 °C overnight. Reaction precipitate was collected by glass filter (M funnel) using vacuum pump and rinsed with DDW (400 mL), washed by acetone (300 mL), and dried under vacuum to fford the 6-o-monotosyl-6-deoxy-β-cyclodextrin intermediate [5.09 g, 3.948 mmol, 22.4% (molar)] as a white powder. 1H NMR (DMSO-d6), δ = 7.76 (d, 2H), 7.44 (d, 2H), 5.89-5.56 (m, 14H), 4.84 (s, 5H), 4.78 (s, 2H), 4.12-4.55 (m, 9H), 3.8-3.42 (m, 28H), 3.41-3.06 (m, overlaps with H2O), 2.42 (s, 3H) (Supplementary Figure S4).
A round-bottom flask was charged with 6-o-monotosyl-6-deoxy-β-cyclodextrin (5 gr, 3.878 mmol, 1 equiv,) and 1,6-hexanediamine (HDA; 20 gr, 172.1 mmol, 45 equiv) and dissolved in DMF (25 mL) under nitrogen conditions with a reflux condensing setup and a temperature-controlled silicon oil bath (80 °C). Reaction was carried out overnight (20 hrs) prior to room temperature cooling for one hour. Product was precipitated using cold acetone (500 mL, 4 °C) and collected under vacuum through a glass filter (M funnel). Precipitate was re-dissolved in DMF (25 mL) and then reprecipitated with acetone (500 mL), a process repeated 3 times. Precipitate was washed by cold diethyl ether (200 mL, 4 °C) twice and filtered by vacuum on M glass funnel as a pale-yellow slurry. Product was dried under vacuum for two hrs to yield species CD-HDA [3.06 gr, 2.48 mmol, 63.9% (molar)] as a light brown powder. 1H NMR (DMSO-d6), δ = 5.71 (br s, 14H), 4.83 (br s, 7H), 4.46 (br s, 6H), 3.79-3.5 (m, 28H), 3.5-3.17 (m, overlaps with H2O), 1.6-1.08 (m, 12H), (Supplementary Figure S5).
Gelatin Modification with CD-HDA
Synthesis of Boc-protected β-cyclodextrin-modified gelatin was performed by adaptation of the Gel-AD-Boc synthesis, with the single change of addition of 1.3 equiv CD-HDA instead of 1.3 equiv 1-adamantylamine. The synthetic routes are shown in Supplementary Figures S1 and S6. A light-brown powder was recovered (Gel-CD-Boc, 3.524 g product per 5 g of gelatin).
2.4. Synthesis of Adamantane- and β-Cyclodextrin-Modified Gelatins (Gel-AD and Gel-CD)
Boc deprotection was performed by adaptation of previously reported procedure 49, and shown in Supplementary Figures S2 and S6. Gel-AD-Boc and Gel-CD-Boc (100 mg mL−1) were each dissolved individually in DDW to which 0.5 v/v% of an aqueous HCl solution (12M) was added. The quantity of protective groups removed from modified gelatin was determined indirectly by evaluating the difference between the amine content in gelatin prior to and after Boc deprotection. Amine concentration was determined spectrometrically using o-phthaldialdehyde (OPA). The reaction between primary amines and OPA resulted in the formation of isoindol chromophores possessing a maximum absorbance λmax at 340 nm. The deprotection reaction proceeded over several days at 4 °C, and amine concentration was quantified relative to a calibration curve based on glycine standards (Supplementary Figure S7). Solution A was prepared by dissolving OPA in ethanol (5 mg in 2.5 mL) and diluting the mixture to 15 mL using DDW. 30 mL of borate buffer pH=10 were prepared separately by mixing 13 mL of NaOH 0.1M solution and 17 mL of Sodium Tetraborate solution (0.05 M in DDW). Solution B was prepared by adding 15 μL 2-mercapto-ethanol to previously prepared 30 mL borate buffer. The two solutions were then combined to prepare a 40 mL stock solution made of 10 mL solution A and 30 mL solution B. Samples at concentration of 100 mg mL−1 were diluted to 1 mg mL−1 with DDW prior to measurement. 100 μL of natural or modified gelatin samples (1 mg mL−1) at different time points throughout the deprotection reaction were added to a 96-well microplate. 200 μL stock solution was added to each well (300 μL total volume in each well) and absorbance at λmax = 340 nm was measured two minutes after addition of stock solution to the samples. Blank (i.e., mixture with water instead of gelatin) measurements were then subtracted from the sample measurements. Similar measurements were performed with glycine (0.03 – 0.5 mM) standards to obtain a calibration curve (Supplementary Figure S7). Calculation of the amount of free amine groups remaining after the modification enabled determination of the deprotection degree of the Boc-protected gelatins. Once the absorbance measurement reached the measurement of the unmodified gelatin, the samples were dialyzed for 48 hrs (molecular weight cut-off: 3500 Da) at 42 °C against DDW, frozen, and lyophilized prior to further use. Final products were denoted Gel-AD and Gel-CD.
2.5. Rheological Analysis of Gelatin Hydrogels
Assessing Shear Thinability
The hydrogels were formed on the rheometer plate by mixing varying volume ratios of Gel-CD in DDW (400 mg mL−1) with Gel-AD in DDW (400 mg mL−1) between parallel plates (8 mm diameter). An overall volume of 40 μL was used to form hydrogels with 0.5 mm thickness. Silicon oil was used to seal the sample and prevent sample drying during analysis. The storage and loss moduli (G’ and G”, respectively) were recorded at constant angular frequency (10 rad s−1) with cyclic strain varying from low and high strain (10% and 400%, respectively) at room temperature. Angular frequency, low, and high strain were determined according to angular frequency sweep and strain amplitude sweep, respectively, below the limit of the linear viscoelastic region.
Oscillatory Time Sweeps of Gelatin Hydrogels varying in Gel-AD:Gel-CD ratios
G’ and G” of hydrogels were measured in hydrogels formed with varying ratios of Gel-AD:Gel-CD (5:1 to 1:5) and a constant combined total mass concentration (400 mg mL−1 in DDW). Measurements were conducted 30 min following sample mixing on the rheometer plate and spanned over 5 minutes at an angular frequency of 10 rad s−1 and oscillatory strain of 10%. Two hydrogels of each formation were measured, and an average and standard deviation of the results over time was determined.
2.6. Stem-Cell Derived Cardiomyocyte Injection and Viability Assays
WTC11 cell line of induced pluripotent stem-cell-derived-cardiomyocytes (iPSC-CMs) were gifted (Charles Murry laboratory, University of Washington, Pathology) on Day 18 of directed differentiation and immediately used for injection. To evaluate cell protection during injection under needle flow, iPSC-CMs were suspended and encapsulated (4 x 106 cells mL−1) within pro-survival cocktail solutions through gentle mixing of Gel-AD and Gel-CD (2:1, 400 mg mL−1 total). Pro-survival cocktail was prepared in RPMI media as previously reported 55 and contained 50% (v/v) growth factor-reduced Matrigel™, benzyloxycarbonyl-Val-Ala-Asp(O-methyl)-fluoromethyl ketone) (100 μM), Bcl-XL BH4 (50 nM, cell-permeant TAT peptide), cyclosporine A (200 nM), IGF-1 (100 ng mL−1), and pinacidil (50 μM). The gelatin-cell constructs and control suspensions, both in pro-survival cocktail, were carefully transferred to 1 mL syringes and injected at 100 μL s−1 through a 28-G needle. Cells were tested for viability using a NC-200 NucleoCounter® for live/dead cell counts and imaged. Final cell populations were assayed twice with n = 5 samples.
3. Results:
3.1. Chemical Modification of Gelatin
3.1.1. Synthesis of Modified Gelatin Derivatives
The present study aims to develop novel shear-thinning gelatin derivatives that mix to form an injectable gel that can be used for therapeutic cargo delivery. Gelatin contains many chemical functionalities in the side chains of the different amino acids which can be exploited for bioconjugation. Amine functionalities are present in the side chains of lysine and hydroxylysine; carboxylic acids in glutamic and aspartic acid; and hydroxyl functionalities in serine, threonine, and hydroxylysine 56. While most reported gelatin-modification strategies exploit gelatin’s primary amines as their reactive anchor to introduce non-natural chemical functionalities 57, we elected to use the carboxylic acid moieties as they are more abundant (7.7% -COOH relative to 3.3% -NH2, based on amino acid compositional make-up) 56. In order to introduce β-cyclodextrin (CD) as a pendant moiety on the gelatin backbone, CD was modified to contain a primary amine by addition of a single 1,6-hexanediamine (HDA) chain to the cyclic CD carbohydrate. This was achieved by initial mono-tosylation of CD, according to previously reported synthesis, under basic aqueous conditions 52-54. Chemical structure of product was verified using 1H NMR (Supplementary Figure S4) prior to overnight reaction with excess HDA in anhydrous DMF under dry N2 conditions at 80 °C. 1H NMR was used to confirm the structure of the product CD-HDA (Supplementary Figure S5). The modification of carboxylic acid functionalities present in the glutamate and aspartate side chains of the gelatin was carried out using conventional carbodiimide coupling in anhydrous DMSO under dry N2 conditions at 55°C overnight. First, the primary amine side chains of gelatin were protected using di-tert-butyl dicarbonate (Boc) in order to prevent crosslinking between activated carboxylic acid side chains with primary amine side chains. Then, 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDAC) was introduced, followed by addition of N-Hydroxysuccinimide (NHS) to stabilize the activated carboxylic acid groups (Supplementary Figure S1). Using a primary amine as a nucleophile (either 1-adamantylamine or CD-HDA), a nucleophilic substitution was realized, and a stable amide bond was formed. As a result, Adamantane (AD) or CD were chemically grafted onto the gelatin backbone (Supplementary Figures S2 and S6).
3.1.2. Deprotection of Primary Amines of Modified Gelatin
The quantity of incorporated Boc amino-protective groups in gelatin derivatives was determined by evaluating the difference between the amine content in modified compared to unmodified gelatin. As a result of primary amine protection with Boc, less amines in gelatin are available for reaction with OPA resulting in lower absorbance values at 340 nm. Amine deprotection was achieved through extended mild acid treatment (0.5% v/v of 12M HCl) and evaluated spectrometrically using OPA. As deprotection progressed over time, more primary amines become available to react with OPA, which results in higher absorbance values at 340 nm. Full Boc deprotection was achieved after ~2 weeks of mild acid treatment for all samples (Supplementary Figure S8). Importantly, unmodified gelatin that has been acid-treated does not show an increase in amine content compared to untreated gelatin over the course of the treatment, indicating that no acid-catalyzed gelatin backbone degradation occurs. Moreover, untreated samples showed no significant increase in primary amine content, confirming stability of Boc protection and justifying acid addition for deprotection. A calibration curve of amine concentration (mM) to absorbance was constructed using glycine standards of increasing concentrations (Supplementary Figure S7). From this linear correlation, we determined that our unmodified gelatin contained 0.291 mmol primary amines per gram. This finding is in line with previous primary amine measurements of gelatin type A that reported 0.284 – 0.288 mmol primary amines per gram of gelatin50.
3.2. Formation of Gelatin Guest-Host Hydrogels with Tunable Initial Moduli
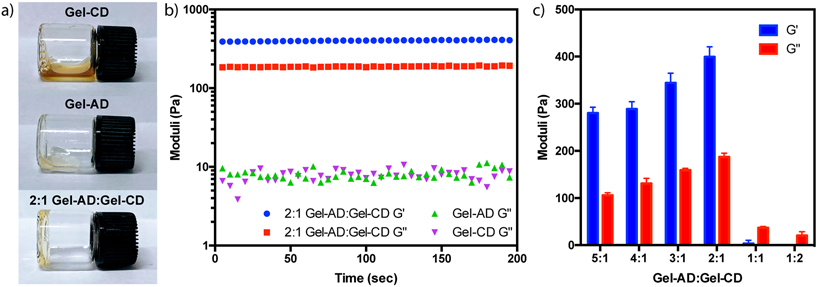
Though solutions of Gel-CD and Gel-AD were free-flowing liquids at room temperature, we anticipated that mixtures of Gel-CD with Gel-AD would result in soft hydrogel biomaterials formed through guest-host complexation based on physical hydrophobic bonding between CD and AD. Initial tests involved tube-inversion assays, with Gel-CD and Gel-AD mixed at varying ratios while keeping the total gelatin content within a sample constant. Reaction mixtures containing excess Gel-AD yielded stable hydrogels, remaining bound in its solid form to the bottom of scintillation vials when subjected to gravitational forces while inverted (Figure 2a).
Figure 2.
Tunable gelatin hydrogel formation through guest-host complexation. (a) Inversion tests of Gel-AD, Gel-CD, and 2:1 Gel-AD:Gel-CD (each mixture totaling 400 mg mL−1). (b) Oscillatory rheological time sweeps of Gel-AD, Gel-CD, and 2:1 Gel-AD:Gel-CD with G’ and G” measured at 10% strain, and an angular velocity of 10 rad sec−1. (c) Storage and loss moduli for gels formed with varying Gel-AD:Gel-CD ratios. Error bars indicate standard deviation of measurements about the mean for experimental duplicates.
To further characterize the viscoelastic properties of the formed gels, we performed rheological oscillatory time sweep experiments of gels of varying composition. Storage (G’) and loss (G”) moduli were determined for mixtures of Gel-AD:Gel-CD (spanning ratios 5:1 to 1:5). The concentration of each individual solution was 400 mg mL−1 regardless of the ratio of AD to CD, such that the any change in material properties could be directly attributed to changes in guest-host complexation. Analysis demonstrated that gels formed with 2:1 Gel-AD:Gel-CD yielded a solid-like material with G’ ~ 400 Pa and G” ~ 10 Pa (Figure 2b). As the Gel-AD:Gel-CD ratio increases, a reduction in storage modulus was observed, with both the 4:1 and 5:1 samples yielding solid materials with similar properties (Figure 2c). Interestingly, mixtures with excess Gel-CD relative to Gel-AD did not result in gel formation, as indicated by behavior of G”>G’ or an absence of storage modulus. These results indicate that stable hydrogel formation is dependent on the quantity of guest species compared to host moieties present in the gel. The ability to tune the mechanical properties of the gel, by controlling the ratio of host to guest and therefore controlling the physical crosslinking density, is an important feature that may allow a formation of a hydrogel with the desired mechanical properties suitable to the relevant application.
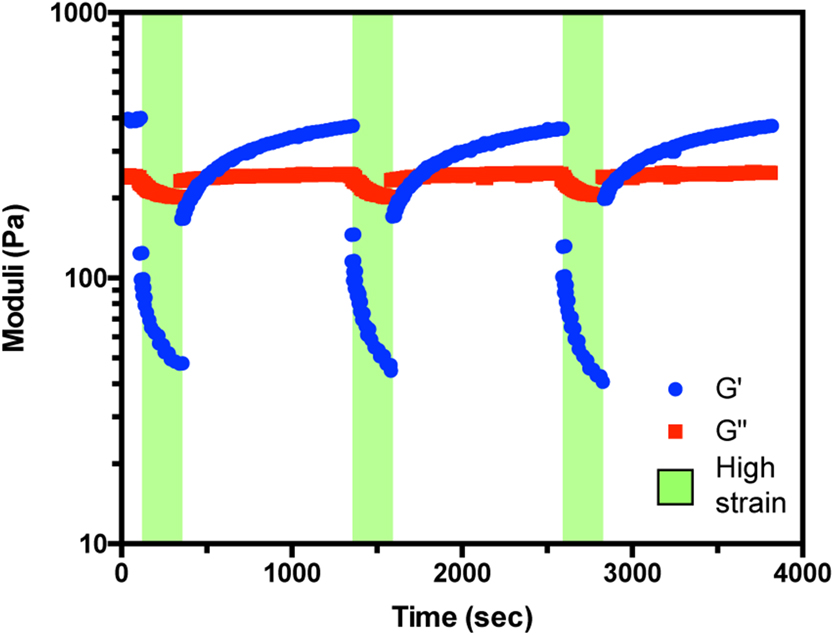
3.3. Shear-Thinability and Self-Healing of Gelatin Hydrogels
Having demonstrated that guest-host assembly of functionalized gelatins resulted in stable hydrogel formation, we sought to assess their responsiveness to high shear and their ability to undergo self-healing (Figure 3). Towards this, hydrogels of varying AD:CD ratios (5:1 to 2:1) were subjected to cycles of high oscillatory strain (400%) followed by low oscillatory strain (10%). At low shear, solid-like materials persisted as previously discussed (Figure 2). Under high strain, the loss moduli overtook the storage moduli in all samples, indicating a near-instantaneous disassembly for the preformed network and a gel-to-sol transition. Though not explicitly measured in these such rheological studies, such experimental findings correlate well with gel injectability 21.
Figure 3.
Shear thinning behavior of guest-host gelatin hydrogels. Rheology measurements of G’ (storage modulus; blue circles) and G” (loss modulus; red squares) at cycles of high oscillatory strain (400%, green background) followed by low oscillatory strain (10%, white background) of hydrogel composed of 2:1 Gel-AD:Gel-CD (400 mg mL−1). Data collected at 10 rad sec−1.
Upon cessation of high strain conditions, an elastic solid-like behavior occurred (G’>G”), and a gradual recovery to the initial storage modulus was observed. The t1/2 constant of recovery was ~5 min, corresponding to the time required for gelatin chain diffusion and AD/CD complexation to occur. Similar self-healing mechanical characteristics were observed regardless of the number of cycles performed. These results indicate that the materials are capable of recovery following high-shear events such as injection, that will potentially allow for retention of the matrix with the intercalated therapeutic cargo at the desired target site.
3.4. Gelatin Materials Support Cell Injection
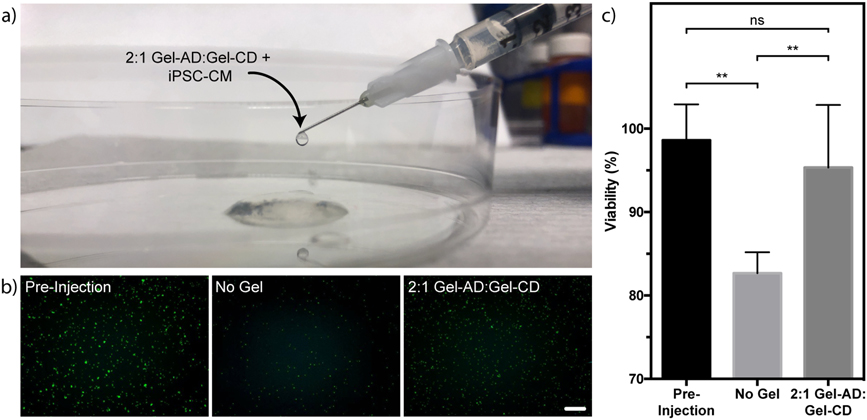
Encouraged by the shear-responsiveness of the gelatin-based hydrogel materials, we sought to directly assess their injectability. Mixtures of Gel-AD and Gel-CD were prepared in 1 mL syringes outfitted with 28 G needles (0.184 mm inner diameter). We anticipated that the shear forces associated with manual extrusion would induce the same reversible gel-to-sol transition that was observed rheologically. Indeed, gel mixtures were readily injectable, transitioning back to a solid form shortly after injection (100 μL s−1) (Figure 4a).
Figure 4.
Guest-host gelatin hydrogel protect cells during injection. (a) 2:1 Gel-AD:Gel-CD mixtures containing encapsulated iPSC-CMs are injectable. (b-c) Cell injected in gelatin materials exhibit higher survival and viability than those injected in free suspension in pro-survival cocktail alone. (b) Live cells are stained in green for each experimental condition. (c) Quantification of iPSC-CM viability. Error bars correspond to ±1 standard deviation about the mean for n = 5 experimental replicates (**p < 0.01, unpaired two-tailed t test; ns indicates non-significance). Scale bar = 100 μm.
Finally, we tested the ability of the Gel-AD/Gel-CD materials to support cell delivery. Knowing that direct injection of stem cell-derived cardiomyocytes into the heart muscle following MI has proven to be an effective therapy in non-human primate models 28, even in spite of poor engraftment rates and cell survival through the injection process (<20%), we tested the ability of Gel-AD/Gel-CD materials to deliver and protect iPSC-CMs during injection under needle flow. iPSC-CMs were suspended in pro-survival cocktail, a collection of growth factors previously optimized to maximize cell engraftment 55, and injected through a 28-G needle at 100 μL s−1 as a free cell suspension or in the 2:1 Gel-AD:Gel-CD mixture. Following injection, iPSC-CMs in the 2:1 Gel-AD:Gel-CD mixture were determined to be 95 ± 7% viable, a value that was statistically indistinguishable with the iPSC-CM population suspended in pro-survival cocktail prior to injection (98 ± 4%). Free cell suspension of iPSC-CMs exhibited a statistically significant loss in viability immediately following injection (83 ± 3% viable), attributed to cell-damaging forces during injection that shear-thinning materials are known to help dissipate. We anticipate that the protective nature of the shear-thinning guest-host gelatin gels could have significant clinical implications in cardiac regeneration and beyond.
4. Discussion:
In this work, we have added gelatin-based materials to the growing arsenal of shear-thinning biomaterials. Derived from the most abundant ECM protein, these materials offer unique advantages with respect to biocompatibility, biodegradability, and non-immunogenicity, as well the ability to support 3D cell culture and function. Hydrogel precursors (Gel-AD and Gel-CD) are synthesized through simple, straight-forward, and scalable chemistries, and can be lyophilized and stably stored until the point of use. Through altered molar ratios and total biopolymer content, materials with different mechanical properties can be accessed. Self-healing materials are assembled through non-covalent guest-host interactions that have been previously applied to stabilize networks based on synthetic polymers and proteoglycans, and have found utility both in vitro and in vivo. Though not explicitly assessed as part of this study, given prior results with other base materials 38,39, the guest-host chemistry itself is not expected to elicit an immune response should our gelatin-based system be applied in vivo.
In addition to exhibiting shear-thinability and self-healing as engineered, the gelatin guest-host hydrogels were demonstrated as injectable through needles of clinically relevant sizes and at a constant rate. Cells encapsulated in gels were protected from membrane-disrupting shear forces present during injection, exhibiting higher viability in gels compared to cells injected directly as a suspension. Given challenges with cell engraftment and poor viability associated with current delivery protocols, the introduced materials are likely to further cell transplantation as a viable therapy for variety of debilitating diseases. Given our promising findings with stem-cell derived cardiomyocytes, coupled with recent cell therapy successes in non-human primate models 28, we are excited at the prospect of using these materials to aid in the treatment of MI.
5. Conclusion:
In this work, we have demonstrated that the incorporation of CD and AD moieties onto the gelatin backbone enables facile generation of physically crosslinked hydrogel biomaterials with tunable mechanical properties. Since CD and AD guest-host complexation is rapid and reversible, this chemistry enables unique materials to be generated that are injectable and exhibit repeatable self-healing. In studies involving induced pluripotent stem-cell derived cardiomyocytes, we have demonstrated material utility in supporting cell protection during injection in a clinically relevant cell type. As the first self-healing hydrogel platform to be based solely on ECM proteins, as well as derived from the most abundant protein in the human body, these materials are well positioned for a variety of applications in cell/therapeutic delivery and tissue engineering.
Supplementary Material
Acknowledgements:
The authors recognize and thank Dr. C. Murry (University of Washington) for providing iPSC-CMs and for helpful discussion involving cardiac cell transplantation. This work was supported by a University of Washington Faculty Startup Grant (C.A.D.), a Fulbright Scholarship (A.M.S.), and a University of Washington Bioengineering Cardiovascular Training Grant (National Institutes of Health Institutional National Research Service Award, T32EB001650, awarded to M.O.B.).
References:
- 1.Hoffman AS Hydrogels for biomedical applications. Adv. Drug Deliv. Rev 64, 18–23 (2012). [DOI] [PubMed] [Google Scholar]
- 2.Hoare TR & Kohane DS Hydrogels in drug delivery: Progress and challenges. Polymer (Guildf). 49, 1993–2007 (2008). [Google Scholar]
- 3.Youngblood RL, Truong NF, Segura T & Shea LD It’s All in the Delivery: Designing Hydrogels for Cell and Non-viral Gene Therapies. Molecular Therapy 26, 2087–2106 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badeau BA, Comerford MP, Arakawa CK, Shadish JA & DeForest CA Engineered modular biomaterial logic gates for environmentally triggered therapeutic delivery. Nat. Chem. 10, 251–258 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Badeau BA & DeForest CA Programming Stimuli-Responsive Behavior into Biomaterials. Annu. Rev. Biomed. Eng 21, 241–265 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Healy KE, Rezania A & Stile RA Designing biomaterials to direct biological responses. Ann. N. Y. Acad. Sci 875, 24–35 (1999). [DOI] [PubMed] [Google Scholar]
- 7.Tibbitt MW & Anseth KS Dynamic Microenvironments: The Fourth Dimension. Sci. Transl. Med 4, (2012). [DOI] [PubMed] [Google Scholar]
- 8.DeForest CA & Anseth KS Advances in Bioactive Hydrogels to Probe and Direct Cell Fate. Annu. Rev. Chem. Biomol. Eng 3, 421–444 (2012). [DOI] [PubMed] [Google Scholar]
- 9.Caliari SR & Burdick JA A practical guide to hydrogels for cell culture. Nat. Methods 13, 405–414 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shadish JA, Benuska GM & DeForest CA Bioactive site-specifically modified proteins for 4D patterning of gel biomaterials. Nat. Mater. 18, 1005–1014 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drury JL & Mooney DJ Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials 24, 4337–4351 (2003). [DOI] [PubMed] [Google Scholar]
- 12.Khademhosseini A & Langer R Microengineered hydrogels for tissue engineering. Biomaterials 28, 5087–5092 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Ifkovits JL & Burdick JA Review: Photopolymerizable and degradable biomaterials for tissue engineering applications. Tissue Eng. 13, 2369–2385 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Nicodemus GD & Bryant SJ Cell Encapsulation in Biodegradable Hydrogels for Tissue Engineering Applications. Tissue Eng. Part B. Rev 14, 149–165 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caló E & Khutoryanskiy VV Biomedical applications of hydrogels: A review of patents and commercial products. European Polymer Journal 65, 252–267 (2015). [Google Scholar]
- 16.Ghobril C & Grinstaff MW The chemistry and engineering of polymeric hydrogel adhesives for wound closure: a tutorial. Chem. Soc. Rev 44, 1820–35 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Annabi N, Yue K, Tamayol A & Khademhosseini A Elastic sealants for surgical applications. Eur. J. Pharm. Biopharm 95, 27–39 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seliktar D Designing Cell-Compatible Hydrogels for Biomedical Applications. Science 336, 1124–1128 (2012). [DOI] [PubMed] [Google Scholar]
- 19.Doenst T, Diab M, Sponholz C, Bauer M & Färber G The opportunities and limitations of minimally invasive cardiac surgery. Deutsches Arzteblatt International 114, 777–784 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thambi T, Li Y & Lee DS Injectable hydrogels for sustained release of therapeutic agents. J. Control. Release 267, 57–66 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Guvendiren M, Lu HD & Burdick JA Shear-thinning hydrogels for biomedical applications. Soft Matter 8, 260–272 (2012). [Google Scholar]
- 22.Lee JH Injectable hydrogels delivering therapeutic agents for disease treatment and tissue engineering. Biomater. Res 22, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang S Emerging biological materials through molecular self-assembly. Biotechnology Advances 20, 321–339 (2002). [DOI] [PubMed] [Google Scholar]
- 24.Laflamme MA & Murry CE Heart regeneration. Nature 473, 326–335 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerbin KA & Murry CE The winding road to regenerating the human heart. Cardiovasc. Pathol 24, 133–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bertero A & Murry CE Hallmarks of cardiac regeneration. Nat. Rev. Cardiol 15, 579–580 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vadakke-Madathil S & Chaudhry HW Cardiac regeneration time to revisit nature. Circ. Res 123, 24–26 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Chong JJH, Yang X, Don CW, Minami E, Liu Y-W, Weyers JJ, Mahoney WM, Van Biber B, Cook SM, Palpant NJ, Gantz JA, Fugate JA, Muskheli V, Gough GM, Vogel KW, Astley CA, Hotchkiss CE, Baldessari A, Pabon L, Reinecke H, Gill EA, Nelson V, Kiem H-P, Laflamme MA & Murry CE Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 510, 273–277 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amado LC, Saliaris AP, Schuleri KH, St. John M, Xie JS, Cattaneo S, Durand DJ, Fitton T, Kuang JQ, Stewart G, Lehrke S, Baumgartner WW, Martin BJ, Heldman AW & Hare JM Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc. Natl. Acad. Sci. U. S. A 102, 11474–11479 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rebouças J. de S., Santos-Magalhães NS & Formiga FR Cardiac regeneration using growth factors: Advances and challenges. Arquivos Brasileiros de Cardiologia 107, 271–275 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korf-Klingebiel M, Reboll MR, Klede S, Brod T, Pich A, Polten F, Napp LC, Bauersachs J, Ganser A, Brinkmann E, Reimann I, Kempf T, Niessen HW, Mizrahi J, Schönfeld HJ, Iglesias A, Bobadilla M, Wang Y & Wollert KC Myeloid-derived growth factor (C19orf10) mediates cardiac repair following myocardial infarction. Nat. Med. 21, 140–149 (2015). [DOI] [PubMed] [Google Scholar]
- 32.Dobaczewski M & Frangogiannis NG Chemokines in myocardial infarction: translating basic research into clinical medicine. Future Cardiol. 4, 347–51 (2008). [DOI] [PubMed] [Google Scholar]
- 33.Roche ET, Hastings CL, Lewin SA, Shvartsman DE, Brudno Y, Vasilyev NV, O’Brien FJ, Walsh CJ, Duffy GP & Mooney DJ Comparison of biomaterial delivery vehicles for improving acute retention of stem cells in the infarcted heart. Biomaterials 35, 6850–6858 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sinclair A, O’Kelly MB, Bai T, Hung HC, Jain P & Jiang S Self-Healing Zwitterionic Microgels as a Versatile Platform for Malleable Cell Constructs and Injectable Therapies. Adv. Mater. 30, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aguado BA, Mulyasasmita W, Su J, Lampe KJ & Heilshorn SC Improving viability of stem cells during syringe needle flow through the design of hydrogel cell carriers. Tissue Eng. Part A 18, 806–15 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruvinov E & Cohen S Alginate biomaterial for the treatment of myocardial infarction: Progress, translational strategies, and clinical outlook: From ocean algae to patient bedside. Adv. Drug Deliv. Rev 96, 54–76 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Wong Po Foo CTS, Lee JS, Mulyasasmita W, Parisi-Amon A & Heilshorn SC Two-component protein-engineered physical hydrogels for cell encapsulation. Proc. Natl. Acad. Sci. U. S. A 106, 22067–22072 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gaffey AC, Chen MH, Venkataraman CM, Trubelja A, Rodell CB, Dinh PV, Hung G, MacArthur JW, Soopan RV, Burdick JA & Atluri P Injectable shear-thinning hydrogels used to deliver endothelial progenitor cells, enhance cell engraftment, and improve ischemic myocardium. J. Thorac. Cardiovasc. Surg 150, 1268–76 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodell CB, Lee ME, Wang H, Takebayashi S, Takayama T, Kawamura T, Arkles JS, Dusaj NN, Dorsey SM, Witschey WRT, Pilla JJ, Gorman JH, Wenk JF, Burdick JA & Gorman RC Injectable Shear-Thinning Hydrogels for Minimally Invasive Delivery to Infarcted Myocardium to Limit Left Ventricular Remodeling. Circ. Cardiovasc. Interv 9, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Engler AJ, Carag-Krieger C, Johnson CP, Raab M, Tang H-Y, Speicher DW, Sanger JW, Sanger JM & Discher DE Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J. Cell Sci 121, 3794–802 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lockhart M, Wirrig E, Phelps A & Wessels A Extracellular matrix and heart development. Birth Defects Res. Part A Clin. Mol. Teratol 91, 535–550 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lynn AK, Yannas IV & Bonfield W Antigenicity and immunogenicity of collagen. J. Biomed. Mater. Res. B. Appl. Biomater 71, 343–54 (2004). [DOI] [PubMed] [Google Scholar]
- 43.Nikkhah M, Akbari M, Paul A, Memic A, Dolatshahi-Pirouz A & Khademhosseini A in Biomaterials from Nature for Advanced Devices and Therapies 37–62 (Wiley Blackwell, 2016). doi: 10.1002/9781119126218.ch3 [DOI] [Google Scholar]
- 44.Kuijpers AJ, Engbers GHM, Krijgsveld J, Zaat SAJ, Dankert J & Feijen J Cross-linking and characterisation of gelatin matrices for biomedical applications. J. Biomater. Sci. Polym. Ed 46 520–230 (2000). [DOI] [PubMed] [Google Scholar]
- 45.Sung HW, Huang DM, Chang WH, Huang RN & Hsu JC Evaluation of gelatin hydrogel crosslinked with various crosslinking agents as bioadhesives: in vitro study. J. Biomed. Mater. Res 46, 520–30 (1999). [DOI] [PubMed] [Google Scholar]
- 46.Nikkhah M, Eshak N, Zorlutuna P, Annabi N, Castello M, Kim K, Dolatshahi-Pirouz A, Edalat F, Bae H, Yang Y & Khademhosseini A Directed endothelial cell morphogenesis in micropatterned gelatin methacrylate hydrogels. Biomaterials 33, 9009–18 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nichol JW, Koshy ST, Bae H, Hwang CM, Yamanlar S & Khademhosseini A Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 31, 5536–5544 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Benton JA, DeForest CA, Vivekanandan V & Anseth KS Photocrosslinking of gelatin macromers to synthesize porous hydrogels that promote valvular interstitial cell function. Tissue Eng. - Part A 15, (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Nieuwenhove I, Stubbe B, Graulus G-J, Van Vlierberghe S & Dubruel P Protein functionalization revised: N-tert-butoxycarbonylation as an elegant tool to circumvent protein crosslinking. Macromol. Rapid Commun 35, 1351–5 (2014). [DOI] [PubMed] [Google Scholar]
- 50.Kale R & Bajaj A Ultraviolet spectrophotometric method for determination of gelatin crosslinking in the presence of amino groups. J. Young Pharm 2, 90–4 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mark HF & Kroschwitz JI Encyclopedia of Polymer Science and Engineering. (John Wiley & Sons, Inc., 1987). [Google Scholar]
- 52.Fetter RC, Salek JS, Sikorski CT, Kumaravel G & Lin FT Cooperative binding by aggregated mono-6-(Alkylamino)- β-cyclodextrins. J. Am. Chem. Soc 112, 3860–3868 (1990). [Google Scholar]
- 53.Kaya E & Mathias LJ Synthesis and characterization of physical crosslinking systems based on cyclodextrin inclusion/host-guest complexation. J. Polym. Sci. Part A Polym. Chem 48, 581–592 (2010). [Google Scholar]
- 54.Rodell CB, Kaminski AL & Burdick JA Rational design of network properties in guest-host assembled and shear-thinning hyaluronic acid hydrogels. Biomacromolecules 14, 4125–4134 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O’Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J & Murry CE Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat. Biotechnol 25, 1015–24 (2007). [DOI] [PubMed] [Google Scholar]
- 56.Mark JE Polymer Data Handbook. (Oxford University Press, 2009). [Google Scholar]
- 57.Van Hoorick J, Tytgat L, Dobos A, Ottevaere H, Van Erps J, Thienpont H, Ovsianikov A, Dubruel P & Van Vlierberghe S (Photo-)crosslinkable gelatin derivatives for biofabrication applications. Acta Biomater. (2019). doi: 10.1016/j.actbio.2019.07.035 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.