Vaccines based on the spike glycoprotein of SARS-CoV-2 are being rolled out globally to control transmission and limit morbidity and mortality due to COVID-19. Current evidence indicates strong immunogenicity and high short-term efficacy for BNT162b2 (Pfizer–BioNTech) and ChAdOx1 nCoV-19 (Oxford–AstraZeneca).1, 2, 3 Both vaccines are delivered through a prime-boost strategy, and many countries, including the UK, have used dose intervals longer than 3–4 weeks, expecting to maximise first-dose coverage and immunogenicity. With continued high global incidence, and potential for more transmissible SARS-CoV-2 variants, data on longer-term vaccine efficacy and antibody dynamics in infection-naive individuals are essential for clarifying the need for further booster doses.
To identify early indications of waning antibody levels to the spike protein (S-antibody) after complete two-dose vaccination, we did a cross-sectional analysis of fully vaccinated adults (aged ≥18 years) who submitted capillary blood samples for Virus Watch, a longitudinal community cohort study in England and Wales.4 The study received ethical approval from the Hampstead NHS Health Research Authority Ethics Committee (20/HRA/2320). Sera were tested using Elecsys Anti-SARS-CoV-2 S and N electro-chemiluminescent immunoassays (Roche Diagnostics, Basel, Switzerland); the S assay targets total antibodies to the S1 subunit of the spike protein (range 0·4–25 000 units per mL [U/mL]), whereas the N assay targets total antibodies to the full-length nucleocapsid protein, which we took as a proxy for previous SARS-CoV-2 infection (specificity 99·8% [99·3–100]).5 Serological results were linked with demographic and clinical information collected at enrolment and with weekly self-reported vaccination status.
605 adults submitted a valid sample on June 14–15, 2021. 321 (53%) of 605 participants were women, and the median age was 63 years (IQR 58–67). Of 605 participants, 186 (31%) were categorised as clinically vulnerable, 117 (19%) as clinically extremely vulnerable, and 302 (50%) as not clinically vulnerable (additional participant characteristics and definitions of clinical vulnerability are available in the appendix). Participants contributed a single sample, taken 14–154 days after their second vaccine dose (median 42 days [IQR 30–53]). 197 (33%) of 605 samples were from BNT162b2 vaccinees and 405 (67%) samples were from ChAdOx1 vaccinees; vaccine type was missing for three (<1%) participants. The median interval between first and second doses was 77 days (IQR 70–78).
Participants with previous infection (N-seropositive; n=47) had a median S-antibody level of 9091 U/mL (IQR 3143 to 16 135), with 2·5-fold lower median levels for ChAdOx1 (median 5179 [IQR 2432·5 to 9513·5]) than BNT162b2 (median 13 025 [9091 to ≥25 000]). N-seronegative individuals had seven-fold lower average S-antibody levels than N-seropositive individuals (median 1257 U/mL [616 to 3526]) and six-fold lower median levels were seen after ChAdOx1 (median 864 [IQR 481 to 1395]) compared to BNT162b2 (median 5311 [3133 to 8829]) within this infection-naive group.
We examined the distribution of S-antibody levels for confirmed N-seronegative samples 14–20 days, 21–41 days, 42–55 days, 56–69 days, and 70 days or more after second vaccination to infer the general trend in antibody levels with time, stratified by vaccine type, with p values derived from non-parametric tests for trend. We excluded two individuals with shorter dose intervals of 21–28 days (and assumed those missing first dose date had a longer dose interval) as this has been demonstrated (in part, through preliminary data) to be less immunogenic than longer intervals for both ChAdOx1 and BNT162b2,6, 7 giving a total of 552 individuals included in the analysis.
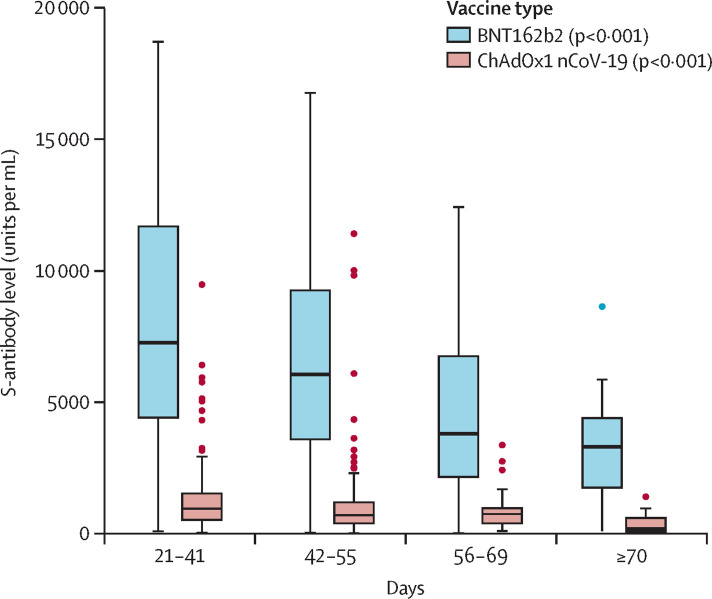
A significant trend of declining S-antibody levels was seen with time for both ChAdOx1 (p<0·001) and BNT162b2 (p<0·001; figure ; appendix), with levels reducing by about five-fold for ChAdOx1, and by about two-fold for BNT162b2, between 21–41 days and 70 days or more after the second dose. This trend remained consistent when results were stratified by sex, age, and clinical vulnerability (appendix). For BNT162b2, S-antibody levels reduced from a median of 7506 U/mL (IQR 4925–11 950) at 21–41 days, to 3320 U/mL (1566–4433) at 70 or more days. For ChAdOx1, S-antibody levels reduced from a median of 1201 U/mL (IQR 609–1865) at 0–20 days to 190 U/mL (67–644) at 70 or more days.
Figure.
Levels of antibody against the spike glycoprotein of SARS-CoV-2 (S-antibody) at defined timepoints after second dose of vaccination (with extended dose intervals) in individuals with no previous infection, stratified by vaccine type
p values derived from non-parametric tests for trend for each vaccine subgroup are given in parenthesis in the key.
Across both vaccine types, women had higher initial S-antibody levels than men at 21–42 days after complete vaccination; also ending with higher levels at 70 days or more (appendix). Similarly, those aged 18–64 years had higher levels at 21–42 days compared to those aged 65 years and older, with correspondingly higher levels at 70 or more days (appendix).
For BNT162b2 vaccinees, some disparity was noted by clinical vulnerability status in peak antibody levels at 21–41 days, although this pattern was not observed with ChAdOx1 (appendix). At 70 days or more, the pattern of disparities was different, with higher antibody levels in vulnerable groups for BNT162b2 and the reverse for ChAdOx1. These data suggest substantial underlying heterogeneity within clinical vulnerability groupings and are also limited by small numbers in the clinically extremely vulnerable strata. However, the trend for declining S-antibody levels with time remains consistent, and the low levels in clinically vulnerable ChAdOx1 vaccinees at 70 days or more might be cause for concern.
Our data suggest waning of S-antibody levels in infection-naive individuals over a 3–10-week period after a second dose of either ChAdOx1 or BNT162b2. These data are consistent with the decline in Spike-antibody and neutralising antibody levels observed after infection, although memory B-cell populations appear to be maintained.8, 9 As such, the clinical implications of waning antibody levels post-vaccination are not yet clear, and it remains crucial to establish S-antibody thresholds associated with protection against clinical outcomes.
Although trends were consistent after stratification by key variables that are likely to affect the immune response, there might be residual confounding due to age and dosing interval as small numbers precluded more precise strata. These findings are also limited by the cross-sectional nature of the data. This analysis should be repeated with a larger number of participants to allow better adjustment for potential confounding, and with longitudinal follow-up of antibody dynamics in individuals over 6–12 months to establish plateau levels, or time to seroreversion.
Higher antibody levels are possibly associated with greater protection against variants that can partially evade immunity, which could explain the observed higher efficacy (partly preliminary) of BNT162b2 compared to ChAdOx1 against the Delta variant (B.1.617.2).10, 11 Disparity in peak antibody levels between vaccine types, and to a lesser extent between population groups, might therefore be important if antibody levels in some groups drop below (as yet undefined) thresholds of protection earlier than in others. There is, however, accumulating evidence suggesting the importance of T-cell-mediated immunity, particularly in individuals with weak or absent antibody responses,12 so it is possible that T-cell responses compensate to some extent as antibody responses wane.
In the context of recent advice in support of booster vaccinations from the UK's Joint Committee on Vaccination and Immunisation,13 and given the potentially rapid S-antibody decline suggested by these data, heterologous regimens, which preliminary data suggest elicit stronger antibody and T-cell responses,14, 15 might provide more durable immunity and greater protection against emerging variants. However, the ultimate effect of different dose intervals and various heterologous combinations on clinical outcomes remain important unanswered questions. Principally, the ethical basis for universal booster dose deployment in high-income settings should be carefully considered in the context of widening global vaccine inequities. Data on disparities in peak antibody levels and rates of decline might therefore inform targeted and equitable booster deployment.
ACH serves on the UK New and Emerging Respiratory Virus Threats Advisory Group. All other authors declare no competing interests. The research costs for the study have been supported by the Medical Research Council Grant awarded to University College London. The study also received US$15 000 of Facebook advertising credit to support a pilot social media recruitment campaign on Aug 18, 2020. Virus Watch received funding via the UK Government Department of Health and Social Care's Vaccine Evaluation Programme to provide monthly Thriva antibody tests to adult participants. This work was supported by the Wellcome Trust through a Wellcome Clinical Research Career Development Fellowship to RWA. Author contributions and members of the Virus Watch Collaborative are listed in the appendix.
Supplementary Material
References
- 1.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;13:373. doi: 10.1136/bmj.n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayward A, Fragaszy E, Kovar J, et al. Risk factors, symptom reporting, healthcare-seeking behaviour and adherence to public health guidance: protocol for Virus Watch, a prospective community cohort study. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-048042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The National SARS-CoV-2 Serology Assay Evaluation Group Performance characteristics of five immunoassays for SARS-CoV-2: a head-to-head benchmark comparison. Lancet Infect Dis. 2020;20:1390–1400. doi: 10.1016/S1473-3099(20)30634-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voysey M, Costa Clemens SA, Madhi SA, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397:881–891. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parry H, Tut G, Faustini S. BNT162b2 vaccination in people over 80 years of age induces strong humoral immune responses with cross neutralisation of P.1 Brazilian variant. SSRN. 2021 doi: 10.2139/ssrn.3816840. published online March 31. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wheatley AK, Juno JA, Wang JJ, et al. Evolution of immune responses to SARS-CoV-2 in mild-moderate COVID-19. Nat Commun. 2021;12:1–11. doi: 10.1038/s41467-021-21444-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaebler C, Wang Z, Lorenzi JCC, et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021;591:639–644. doi: 10.1038/s41586-021-03207-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernal JL, Andrews N, Gower C, et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 variant. medRxiv. 2021 doi: 10.1056/NEJMc2113090. published online May 24 (preprint). [DOI] [PubMed] [Google Scholar]
- 11.Sheikh A, McMenamin J, Taylor B, Robertson C, on behalf of Public Health Scotland and the EAVE II Collaborators SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397:2461–2462. doi: 10.1016/S0140-6736(21)01358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wyllie D, Jones HE, Mulchandani R, et al. SARS-CoV-2 responsive T cell numbers and anti-Spike IgG levels are both associated with protection from COVID-19: a prospective cohort study in keyworkers. medRxiv. 2020 doi: 10.1101/2020.11.02.20222778. published online Nov 4. (preprint). [DOI] [Google Scholar]
- 13.Joint Committee on Vaccination and Immunisation. Department of Health and Social Care JCVI interim advice on a potential coronavirus (COVID-19) booster vaccine programme for winter 2021 to 2022. June 30, 2021. https://www.gov.uk/government/publications/jcvi-interim-advice-on-a-potential-coronavirus-covid-19-booster-vaccine-programme-for-winter-2021-to-2022
- 14.Hillus D, Schwarz T, Tober-lau P, et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1-nCoV19 and BNT162b2: a prospective cohort study. medRxiv. 2021 doi: 10.1101/2021.05.19.21257334. published online June 2. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barros-Martins J, Ramos GM, Dopfer-Jablonka A, et al. Humoral and cellular immune response against SARS-CoV-2 variants following heterologous and homologous ChAdOx1 nCoV-19 / BNT162b2 vaccination. medRxiv. 2021 doi: 10.1101/2021.06.01.21258172. published online June 3. (preprint). [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.