Abstract
Recently, many of the studies have illustrated that the new pandemic SARS-CoV-2 can affect Central Nervous System through the olfactory bulb. In addition to investigating anosmia or hyposmia induced by this virus, a quantitative analysis was needed to clarify the taste and smell disorder of the new coronavirus. The four basic taste quality with five concentrations for sweet, sour, bitter, and salty were administered to 75 subjects divided into three groups: COVID-19 patients with taste disorder, COVID-19 patients without taste disorder, and control group.
The results indicated the increment of sweet (2.68 ± 0.14), sour (3.34 ± 0.12) and bitter (3.39 ± 0.2) thresholds in COVID-19 patients with taste disorder in comparison with patients without taste disorder that the threshold were: 2 ± 0.16, 2.11 ± 0.2 and 2.55 ± 0.5 for sweet, sour, and bitter respectively. On the other hand, the patients inversely showed a significant decrease in the salty taste threshold (0.51 ± 0.03) compared to COVID-19 positive control groups (1.11 ± 0.11).
Additionally, despite taste disorder in almost all of the patients with smell deficiency, only 30% of cases with taste disorder reported smell deficiency. It may be concluded that some of the taste disorders in patients with COVID-19 disorder could be associated with taste receptors dysfunction or the spread of infection to the cranial nerves responsible for the conduction of tastes sensation.
Keywords: COVID-19, cranial nerve, dysgeusia, gustatory test, salt taste hypersensitivity
Introduction
After the widespread of the new SARS-CoV-12 around the world, many studies were conducted to investigate its severe respiratory syndrome. However, several studies focused on the effect of this virus on other organs such as cardiovascular systems and neural systems [1,2]. The studies demonstrated that the new coronavirus could affect the central nervous system through the olfactory bulb or blood circulation. Furthermore, in addition to anosmia or hyposmia induction, as well as taste disorders, the virus may cause headache, eye-ache, earache, dizziness and hallucination [3]. According to the report of Moein et al., the publications included case reports or self-report surveys among different countries, and researchers proved the loss of smell and taste as a predictor of COVID-19 and presented the first validated quantitative olfactory testing of the sense of smell [4]. Owing to taste problems in many patients with smell loss, as well as the interaction between smell and taste sensations, it appeared that a quantitative taste test was necessary to determine whether COVID-19 could damage taste afferent or not [5]. Accordingly, in this study, we attempted to quantitatively measure the threshold of four basic tastes, namely sweet, salty, sour and bitter in patients suffered from COVID-19 and to assay the correlation of this sense with other disorders such as pains and some autonomic disorders in order to better identify the neurological effect of the virus. We searched for the validation of the hypothesis of measuring taste and smell as an easy and effective method to identify the disease and improve the medical decision-making.
Materials and methods
The current study was approved by the ethics committee of Baqiyatallah University of Medical Sciences with the code of: IR.MBSU.1399.096.The patients were invited to participate and kindly requested to fill up the satisfaction letters.
Subjects and setting
A total of 75 subjects, between 35 and 65 years old (mean age 47 years) (42 men and 33 women), were included in this study. They were divided into three groups: a) COVID-19 patients with taste disorder (n = 35), b) COVID-19 patients without taste disorder (COVID-19 positive control group, n = 20), and c) a healthy control group (n = 20). The COVID-19 infections were by PCR test.
Taste and smell evaluation
For the quantitative evaluation of taste disorder, we used a special taste kit, which was inspired from the report of Ji-sun Kim and et al., in 2019 with a brief modification [5]. The four basic taste quality with five concentrations for sweet, sour, bitter and salty were used. The taste substances included sucrose (sweet), NaCl (salty), tartaric acid (sour) and caffeine HCl (bitter) (Table 1). In this study, we added three lower concentrations of salty quality to the table: the 1/2, 1/5 and 1/10 of the lowest concentration. These three concentrations were indicated with an asterisk in Table 1.
Table 1.
Taste qualities and concentration series for the taste solution test
| Taste quality | No.1 | No.2 | No.3 | No.4 | No.5 |
|---|---|---|---|---|---|
| Sweet (Sucrose) | 0.003 | 0.025 | 0.1 | 0.2 | 0.8 |
| Sour (Tartaric acid) | 0.003 | 0.0125 | 0.05 | 0.1 | 0.2 |
| Salty (NaCl) | 0.0002 0.0001∗ 0.00004∗ 0.00002∗ |
0.002 | 0.02 | 0.04 | 0.08 |
| Bitter (Caffeine) | 0.00001 | 0.0002 | 0.001 | 0.005 | 0.04 |
The values are expressed as g/ml. The lower concentrations than 0.0002 g/ml of NaCl were presented with ∗ in the table.
According to the reference, the researchers scaled the concentrations of each taste from 1 to 5 to quantify the thresholds2. The different solutions were filled in sterilized microtubes and swabbed on the patients’ tongues with a cotton-tipped applicator. The test began with the different flavoured solutions placed in front of the tongue from low to high concentration. The patients washed their mouths with water between each concentration. The flavours were administered in the order of sweet, sour, salty, and bitter, respectively. The sour, salty, and bitter solutions were positioned on the right lateral, left lateral, and back of the tongue, respectively. A demographic questionnaire was designed to assess other clinical symptoms.
Statistical analysis
The statistical analysis was performed by using SPSS version 24 software, both one-way ANOVA and chi-square test as a case-control analysis done for evaluation and comparison of thresholds of four taste quality in different groups and also Pierson’s chi-square with exact Fisher’s test was performed for assessment of taste threshold relation to other clinical symptoms. In this analysis, Cramer’s value as effect size was evaluated. The Linear Discriminant Analysis (LDA) has been done for demonstration of the power of prediction of COVID-19 by different kinds of taste thresholds.
Results
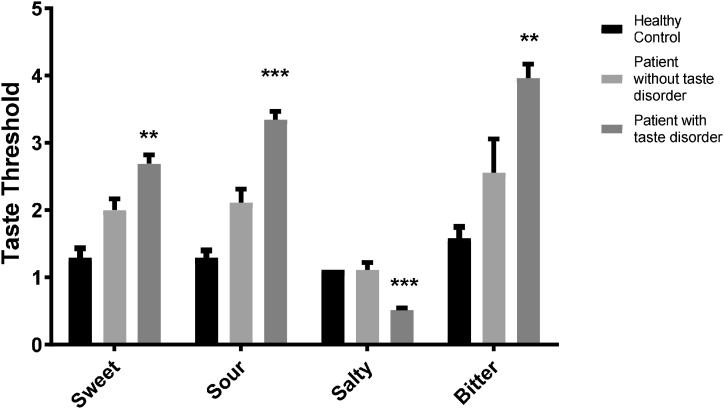
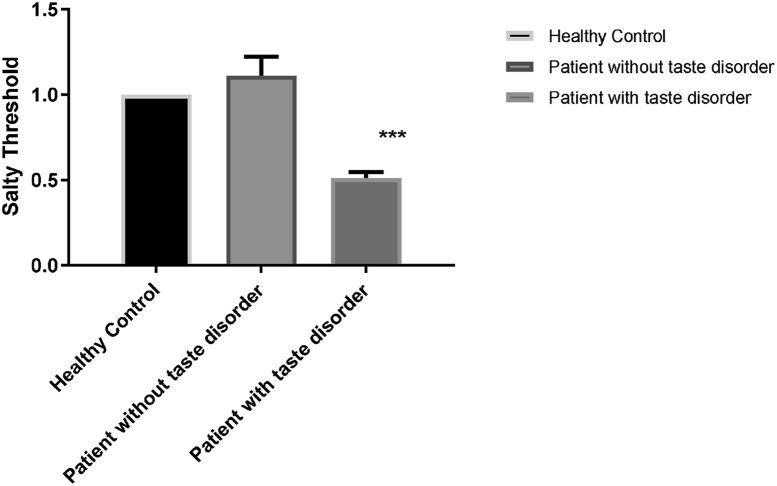
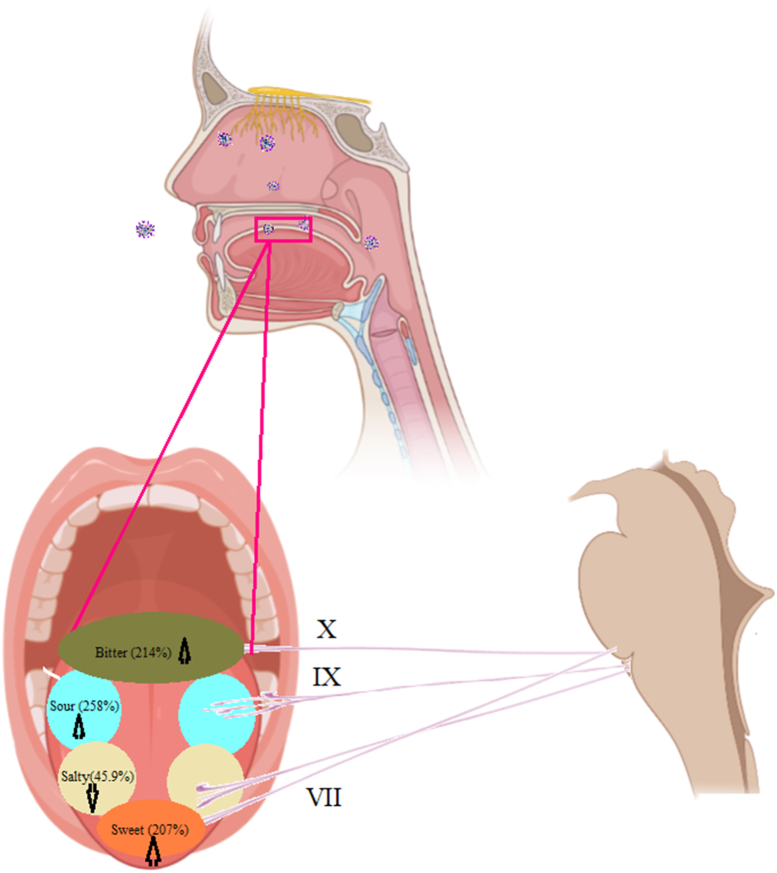
The results revealed an increase in the threshold for sweet, sour, and bitter tastes in the COVID-19 patients with taste disorders. The mean and standard error of the mean (SEM) for sweet, sour, and bitter tastes were 2.68 ± 0.14, 3.34 ± 0.12, and 3.39 ± 0.2 in COVID-19 with taste disorder and 2 ± 0.16, 2.11 ± 0.2 and 2.55 ± 0.5 in COVID-19 patients without taste disorder and 1.29 ± 0.14, 1.29 ± 0.11 and 1.58 ± 0.17 in healthy control group respectively. There was a significant decrease in the threshold for the salty taste (0.51 ± 0.03) in patients with taste disorder compared to the healthy control (1 ± 0) and the COVID-19 positive control groups (1.11 ± 0.11) (p < 0.001 and p < 0.01) (Fig. 1, Fig. 2). Fig. 3 has schematically represented the percentage of changes in taste quality threshold in COVID-19 patients with taste disorder.
Fig. 1.
Evaluation of four taste quality thresholds. The significant increment of thresholds for sweet, sour, and bitter tastes was shown in patients with COVID-19 and taste disorders. The salty taste indicated hypersensitivity in these patients. ∗∗ indicated P < 0.01 and ∗∗∗P < 0.001.
Fig. 2.
Illustration of hypersensitivity to salty taste. The cases with COVID-19 infection and taste deficiency showed hypersensitivity to NaCl concentrations lower than 0.0002 g/ml (0.0001, 0.00004 and 0.00002 g/ml). The number 1 in the Y-axis of the figure referred to 0.0002 g/ml of NaCl.∗∗∗ indicted to P < 0.0001.
Fig. 3.
Schematic representation of taste quality threshold changes in patients with taste disorder. The numbers indicated the percentage of increase or decrease of thresholds.
As Table 1 presents, owing to the high sensitivity of patients to salty taste, we followed the experiment with lower concentrations as 1/2, 1/5 and 1/10 of the 0.0002 g/ml. Fig. 2 illustrated the sensitivity of the patient to the lower concentration of salty taste and indicated a reduction in the threshold for salty taste quality towards lower concentrations than 0.0002 g/ml, as shown in Table 1 (1/2,1/5 and 1/10 of 0.0002 g/ml).
Table 2 indicates the clinical symptoms in patients, including headache (87.1%), dry mouth (54.83%), dry eyes (25.8%), loss of smell (38.7%), earache (29%), eye pain (25.8%), and dizziness (22.5%).
Table 2.
Demonstration of percentage of some disorders in patients with taste deficiency
| Dry mouth | Dry eyes | Dizziness | Headache | Earache | Eye ache | Smell deficiency | |
|---|---|---|---|---|---|---|---|
| Patient with taste disorder | 54.83% | 25.80% | 22.58% | 87.10% | 29% | 25.8% | 30% |
| Patient without taste disorder | 77.77% | 33.33% | 33.33% | 55.55% | 11.1% | 55.5% | 0% |
| Control | 0% | 0% | 0% | 0% | 0% | 0% | 0% |
Moreover, Pearson’s chi-square demonstrated significant relationships between thresholds for sweet, sour, and bitter tastes and disorders such as dry mouth, earache, headache, and loss of smell. The values of Cramér’s V test indicated a strong association between these variables (Table 3).
Table 3.
The relation between tastes thresholds and some disorders according to Pearson’s chi-square analysis. The significant value indicated with an asterisk
| Dry mouth |
Dry eyes |
Dizziness |
Headache |
Earache |
Eye ache |
Smell Qualitative |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cramer’s V | P value | Cramer’s V | P Value | Cramer’s V | P Value | Cramer’s V | P value | Cramer’s V | P value | Cramer’s V | P value | Cramer’s V | P value | |
| Sweet | 0.478 | 0.007∗ | 0.251 | 0.326 | 0.251 | 0.326 | 0.524 | 0.002∗ | 0.327 | 0.117 | 0.225 | 0.577 | 0.552 | 0.001∗ |
| Salty | 0.218 | 0.113 | 0.218 | 0.235 | 0.160 | 0.235 | 0.004 | 0.979 | –0.097 | 0.471 | –0.239 | 0.136 | 0.331 | 0.015∗ |
| Sour | 0.505 | 0.009∗ | 0.294 | 0.313 | 0.294 | 0.313 | 0.572 | 0.001∗ | 0.436 | 0.033∗ | 0.258 | 0.629 | 0.494 | 0.01∗ |
| Bitter | 0.486 | 0.014∗ | 0.239 | 0.553 | 0.235 | 0.553 | 0.553 | 0.002∗ | 0.415 | 0.05∗ | 0.271 | 0.581 | 0.388 | 0.088∗ |
The Cramer's Value interpreted as a measure of the strength of an association between two variables and the coefficient ranges from 0 to 1
The Linear Discriminate Analysis (LDA) was the other statistical analysis to survey the predictive role of taste examination for COVID-19 patients. This test indicted 89.3% correction of the quantitative taste examination for distinguishing patients from healthy persons. In this regard, the coefficients or weighs of each taste quality were presented in the first column, which represents the best coefficient for sour taste (0.92). The sweet taste with 0.74 and bitter with 0.41 coefficients subsequently was in the later degree. Because of a decrease in the salty taste threshold instead of an increase in these patients, the coefficient of salt variable reached –0.015 in the function. The Lambda number indicated the value of the LDA classifier (Table 4).
Table 4.
The results of Linear Discriminate Analysis indicted to 89.3% correction of quantitative taste examination for distinguishing of patients from healthy, and the coefficients or weighs of each taste quality are presented in the first column. The Lambda number indicated to the value of the LDA classifier
| Discriminant Function Coefficients | Wilks’ Lambda | Sig. | correctly classified |
|---|---|---|---|
| Sour 0.929 | 0.410 | 0.000 | 89.3% |
| Sweet 0.740 | |||
| Bitter 0.415 | |||
| Salty –0.015 |
Discussion
The quantitative and subjective assessment of the four taste qualities showed an increase in the threshold of sweet, sour, and bitter qualities in COVID-19 patients. But, the threshold for salty taste was considerably reduced.
The epidemiological studies showed that 41%–62% of COVID-19 patients were diagnosed with smell and taste disorders so that some studies suggested these disorders could be COVID-19 related [6,7]. Most of the data reported that taste pathogenesis in COVID-19 is based on self-reporting through questionnaires or phone interviews. They reported gustatory dysfunctions as ageusia or hypogeusia with prevalence ranging from 5.6% to 62.7% [[6], [7], [8]]. According to a study, 48–49% of COVID-19 patients experienced impairment of two or more taste qualities, and 60% of those who reported a taste loss also described a decrease in their perception of at least one specific taste quality [8].
There is a directly proportional connection between olfactory and gustatory, and the pathology of these two is closely related to each other [9,10]. There are many reasons for gustatory pathology, including upper airway infection, destruction of papillae or taste buds, poor oral health, and post-viral cranial nerve damage [9,11]. In COVID-19 patients with taste and smell disorder, the lack of nasal congestion or discharge could be attributed to sensorineural damage [7].
Structurally, though, gustatory cells are located in the sensory ganglion of the facial, glossopharyngeal, and vagus nerves. They serve the taste buds located on two-thirds of the anterior tongue, baseline tongue, pharynx, and larynx, respectively [9,12].
This study demonstrates the correlation between changes in taste thresholds and some neurologic symptoms (headache, earache, and dry mouth). It may indicate that many neurological abnormalities in COVID-19 typically observed in other common respiratory virus infections (influenza) may relate to the nervous system’s post-infectious inflammatory conditions or the neurotropic property of the pathogen. However, COVID-19 is more deleterious than other common viral respiratory disorders [13].
The damage to the taste buds caused by the novel coronavirus is not yet reported. Still, it may be considered due to the considerable increases in different taste thresholds. The significant expression of ACE-2 receptors in the tongue described by single-cell RNA-sequencing studies may suggest this hypothesis [7].
CT, MRI, and neurologic examination are the complementary methods that suggested a better understanding of the taste disorder mechanism [14].
Furthermore, the salty taste threshold decrease was notable, which was different from the other study, which reported the salty taste impairment as other taste quality in COVID-19 patients [8]. According to the literature, postnasal drip, dry mouth or dehydration, gastroesophageal reflux disease, infection, oral thrush, burning sensation in the mouth, hormonal imbalances, and medication side effects are possible causes of salty taste symptoms [15]. Because sodium deficiency can cause detrimental health issues, salty taste is crucial for detecting sodium ions, which play essential roles in osmotic balance, water homeostasis, pH regulation, and nerve activity. Moreover, the imbalances of angiotensin II, a suppressor of the salt taste sensitivity, and aldosterone, a slow enhancer, may play a role in this hypersensitivity. These vital hormones may exist in peripheral taste organs and contribute to salt intake and ingestive behaviour [16]. Based on the investigations, the relationship between salt taste sensitivity threshold (STST), blood pressure, and sodium intake [17] may be considered in COVID-19 patients.
Other studies addressed the regulatory role of salt taste sensitivity in sodium homeostasis [18]. It is suggested that (angiotensin II and aldosterone), sodium concentration of plasma and saliva, and amiloride-sensitive receptors facilitate the mechanism of this hypersensitivity19. Considering that changes in sodium hemostasis and sodium regulator hormone imbalance have been reported in COVID-19 infection [19,20].
Moreover, the components of saliva could explain the differences in salt perception among patients. It is reported that higher amounts of endopeptidases in saliva liquid could increase salt sensitivity. The researchers suggest that the saliva enzymes cloud produce salt-enhancing peptides and increase the sodium entrance through modifying sodium channels [21].
Conclusion
In conclusion, our results are consistent with other investigations indicating that taste modality is a good predictor for the COVID-19 virus infection. Additionally, the hypersensitivity to salt may exist in COVID-19 infection and increase the threshold for sweet, sour, and bitter tastes. The taste bud damage or cranial nerve disorder are the possibilities of increased threshold and the hypersensitivity to salty taste more likely related to changes in sodium hemostasis or saliva components. For a better understanding, more investigations are needed.
Transparency declaration
The authors have no conflicts of interest to declare for this study.
This paper was supported by Baqiyatallah University of Medical Sciences Research Council.
Ethical approval
The current study was approved by the ethics committee of Baqiyatallah University of Medical Sciences with the code of: IR.MBSU.1399.096.The patients were invited to participate and kindly requested to fill up the satisfaction letters.
Authors’ contributions
The research idea was proposed with the FB and MMS. The sections of theoretical framework and methodology were completed with ZS, FB and TM. The literature review and secondary data analysis were conducted with HA and MN. The method and analysis were completed with MMA, TM, HA. All authors read and approved the study.
Acknowledgements
The authors wish to thank all members of the research team and others who facilitated this study. We kindly acknowledge the guidance and advice from the Clinical Research Development Units of Baqiyatallah Hospital.
References
- 1.Bhatla A., Mayer M.M., Adusumalli S., Hyman M.C., Oh E., Tierney A. COVID-19 and cardiac arrhythmias. Heart Rhythm. 2020;17(9):1439–1444. doi: 10.1016/j.hrthm.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iadecola C., Anrather J., Kamel H. Effects of COVID-19 on the nervous system. Cell. 2020;183(1):16–27 e1. doi: 10.1016/j.cell.2020.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mirza J., Ganguly A., Ostrovskaya A., Tusher A., Viswanathan R. Command suicidal hallucination as initial presentation of coronavirus disease 2019 (COVID-19): a case report. Psychosomatics. 2020;61(5):561–564. doi: 10.1016/j.psym.2020.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moein S.T., Hashemian S.M., Mansourafshar B., Khorram-Tousi A., Tabarsi P., Doty R.L. Smell dysfunction: a biomarker for COVID-19. Int Forum Allergy Rhinol. 2020;10(8):944–950. doi: 10.1002/alr.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim J.S., Kim D.H., Jeon E.J., Kim B.G., Yu J., Shin H.I. Taste test using an edible taste film kit: a randomised controlled trial. BMJ Open. 2019;9(9) doi: 10.1136/bmjopen-2019-029077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agyeman A.A., Chin K.L., Landersdorfer C.B., Liew D., Ofori-Asenso R. Smell and taste dysfunction in patients with COVID-19: a systematic review and meta-analysis. Mayo Clin Proc. 2020;95(8):1621–1631. doi: 10.1016/j.mayocp.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mastrangelo A., Bonato M., Cinque P. Smell and taste disorders in COVID-19: from pathogenesis to clinical features and outcomes. Neurosci Lett. 2021;748:135694. doi: 10.1016/j.neulet.2021.135694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parma V., Ohla K., Veldhuizen M.G., Niv M.Y., Kelly C.E., Bakke A.J. More than smell—COVID-19 is associated with severe impairment of smell, taste, and chemesthesis. Chem Sense. 2020;45(7):609–622. doi: 10.1093/chemse/bjaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Enache R., Sarafoleanu D. Taste and smell disorders. Rom J Rhinol. 2012;2(7) [Google Scholar]
- 10.Palheta Neto F.X., Targino M.N., Peixoto V.S., Alcântara F.B., Jesus CCd, Araújo DCd. Anormalidades sensoriais: olfato e paladar. Arq Int Otorrinolaringol (Impresso) 2011;15(3):350–358. [Google Scholar]
- 11.Rawson N.E., Huang L. Symposium overview: impact of oronasal inflammation on taste and smell. Ann N Y Acad Sci. 2009;1170:581–584. doi: 10.1111/j.1749-6632.2009.04489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimmelman C. Van De Ware TR, Staecker H Otolaryngology Basic science and clinical review Thieme Medical Publishers; New York: 2006. Basic science of the oral cavity and gustation; pp. 627–633. [Google Scholar]
- 13.Marshall M. How COVID-19 can damage the brain. Nature. 2020;585(7825):342–343. doi: 10.1038/d41586-020-02599-5. [DOI] [PubMed] [Google Scholar]
- 14.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vroegop A.V., Eeckels A.-S., Van Rompaey V., Vanden Abeele D., Schiappoli M., Alobid I. COVID-19 and olfactory dysfunction-an ENT perspective to the current COVID-19 pandemic. B-ENT. 2020;16(1):81–85. [Google Scholar]
- 16.Baig A.M., Khaleeq A., Ali U., Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11(7):995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 17.Martinelli J., Conde S.R., Araujo A.R., Marcadenti A. Association between salt taste sensitivity threshold and blood pressure in healthy individuals: a cross-sectional study. Sao Paulo Med J. 2020;138(1):4–10. doi: 10.1590/1516-3180.2019.0166.R1.02102019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shigemura N. Angiotensin II and taste sensitivity. Jpn Dental Sci Rev. 2015;51(2):51–58. [Google Scholar]
- 19.Lippi G., South A.M., Henry B.M. Electrolyte imbalances in patients with severe coronavirus disease 2019 (COVID-19) Ann Clin Biochem. 2020;57(3):262–265. doi: 10.1177/0004563220922255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarvazad H., Cahngaripour S.H., Eskandari Roozbahani N., Izadi B. Evaluation of electrolyte status of sodium, potassium and magnesium, and fasting blood sugar at the initial admission of individuals with COVID-19 without underlying disease in Golestan Hospital. Kermanshah New Microbe New Infect. 2020;38:100807. doi: 10.1016/j.nmni.2020.100807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stolle T., Grondinger F., Dunkel A., Meng C., Medard G., Kuster B. Salivary proteome patterns affecting human salt taste sensitivity. J Agric Food Chem. 2017;65(42):9275–9286. doi: 10.1021/acs.jafc.7b03862. [DOI] [PubMed] [Google Scholar]