Keywords: diabetes, glucose, prevention, translational
Abstract
Global prevalence of type 2 diabetes (T2D) is rising and may affect 700 million people by 2045. Totum-63 is a polyphenol-rich natural composition developed to reduce the risk of T2D. We first investigated the effects of Totum-63 supplementation in high-fat diet (HFD)-fed mice for up to 16 wk and thereafter assessed its safety and efficacy (2.5 g or 5 g per day) in 14 overweight men [mean age 51.5 yr, body mass index (BMI) 27.6 kg·m-2] for 4 wk. In HFD-fed mice, Totum-63 reduced body weight and fat mass gain, whereas lean mass was unchanged. Moreover, fecal energy excretion was higher in Totum-63-supplemented mice, suggesting a reduction of calorie absorption in the digestive tract. In the gut, metagenomic analyses of fecal microbiota revealed a partial restoration of HFD-induced microbial imbalance, as shown by principal coordinate analysis of microbiota composition. HFD-induced increase in HOMA-IR score was delayed in supplemented mice, and insulin response to an oral glucose tolerance test was significantly reduced, suggesting that Totum-63 may prevent HFD-related impairments in glucose homeostasis. Interestingly, these improvements could be linked to restored insulin signaling in subcutaneous adipose tissue and soleus muscle. In the liver, HFD-induced steatosis was reduced by 40% (as shown by triglyceride content). In the subsequent study in men, Totum-63 (5 g·day-1) improved glucose and insulin responses to a high-carbohydrate breakfast test (84% kcal carbohydrates). It was well tolerated, with no clinically significant adverse events reported. Collectively, these data suggest that Totum-63 could improve glucose homeostasis in both HFD-fed mice and overweight individuals, presumably through a multitargeted action on different metabolic organs.
NEW & NOTEWORTHY Totum-63 is a novel polyphenol-rich natural composition developed to reduce the risk of T2D. Totum-63 showed beneficial effects on glucose homeostasis in HFD-fed mice, presumably through a multitargeted action on different metabolic organs. Totum-63 was well tolerated in humans and improved postprandial glucose and insulin responses to a high-carbohydrate breakfast test.
INTRODUCTION
Global prevalence of diabetes is reaching epidemic proportions. In 2019, 463 million people were diagnosed with diabetes and this number may reach 700 million by 2045 (1). In parallel, over 1 billion people worldwide suffer from prediabetes, a nonpathological stage characterized by increased fasting blood glucose, postprandial glucose, and/or elevated hemoglobin A1c (2). Prediabetes is a risk factor for type 2 diabetes (T2D), as 5%–10% typically progress to T2D annually (3) and 70% eventually develop T2D over their life course (4). Prediabetes is a key stage for therapeutic intervention, for apparition of T2D can be prevented with lifestyle or pharmaceutical interventions (5).
T2D develops in a complex manner that results from a multitude of dysfunctional pathways rooted in a large panel of organs (6). Insulin resistance, one the hallmarks of T2D, manifests itself in major metabolic organs such as skeletal muscle, adipose tissue, or liver causing decrease of glucose uptake and increased glucose output. In the intestine, T2D is associated with microbial dysbiosis, an imbalance between bacteria species associated with healthy and unhealthy phenotypes (7), that could play a role in the development of the disease (8). The multiplicity of the pathophysiological mechanisms causing T2D makes traditional pharmaceutical interventions targeting one single pathway generally insufficient; it has therefore become common for patients to be treated with multiple drug classes (9, 10). In this context, therapeutic strategies simultaneously targeting several mechanisms rooted in different organs may constitute the most efficient option for patients. Interestingly, bioactive compounds naturally present in plants, such as polyphenols, may offer such qualities. Indeed, in vitro and in vivo data from recent studies suggest potential benefits of polyphenols in reducing hepatic glucose output, enhancing muscle glucose uptake, restoring insulin signaling, and altering microbiome (11), which could result in pleiotropic beneficial effects (12). A sensible selection of extracts pooling several of these bioactive molecules could therefore allow a multitargeted action on the mechanisms involved in the pathogenesis of complex metabolic diseases such as T2D, offering an excellent tool to prevent them, as already suggested by other authors (13) and international health institutions (14, 15). Data from epidemiological retrospective studies comfort this assumption, as many have shown a relationship between consumption of polyphenol-rich plant-based food and a lower incidence of metabolic pathologies (16–18).
On the basis of large in vitro and in vivo experimental screenings, Totum-63, a naturally polyphenol-rich natural composition based on 5 plant extracts designed to reduce T2D risk factors, has been developed. It contains a mixture of extracts from olive leaf (Olea europaea), bilberry (Vaccinium myrtillus), artichoke (Cynara scolymus), chrysanthellum (Chrysanthellum indicum subsp. afroamericanum B.L. Turner), and black pepper (Piper nigrum).
The aim of this study was to investigate the effects of Totum-63 in a murine model of diet-induced obesity and insulin resistance. We hypothesized that Totum-63 would prevent the development of obesity-associated impairments in various organs. Moreover, an exploratory clinical trial was conducted in 14 overweight men to assess the safety and tolerability of Totum-63, along with the effect of the supplementation on glucose and insulin response to a high-carbohydrate breakfast test.
METHODS
Characterization of Totum-63
Totum-63 is a patented blend of five plant extracts designed to act in combination to target the risk factors of developing T2D. The mixture contains extracts from olive leaf (Olea europaea), bilberry (Vaccinium myrtillus), artichoke (Cynara scolymus), chrysanthellum (Chrysanthellum indicum subsp. afroamericanum B.L. Turner), and black pepper (Piper nigrum). The chemical characterization of the batch used in this study was performed by Folin–Ciocalteu method for total polyphenol estimation, by AOAC Method 2017 for total fiber estimation and by HPLC-UV/visible/MS for quantification of all other potential compounds of interest (Table 1).
Table 1.
Chemical characterization of whole Totum-63 powder
| Compound Type | Extract Content (g/100 g Dry Weight) | Mean Daily Intake*(mg/kg body weight/day) |
|---|---|---|
| Total polyphenols** | 14.36 | 387.7 |
| Total anthocyanins | 0.81 | 21.9 |
| Monocaffeoylquinic acids | 1.18 | 31.9 |
| Chlorogenic acid | 0.85 | 23.0 |
| Other monocaffeoylquinic acids | 0.33 | 8.9 |
| Dicaffeoylquinic acids | 0.98 | 26.5 |
| Cynarine | 0.24 | 6.5 |
| Other dicaffeoylquinic acids | 0.74 | 20.0 |
| Caffeic acid | 0.01 | 0.3 |
| Oleuropein | 3.72 | 100.4 |
| Oleuropein isomers | 0.20 | 5.4 |
| Hydroxytyrosol | 0.04 | 1.1 |
| Luteolin | 0.01 | 0.3 |
| Luteolin-7-O-glucoside | 0.38 | 10.3 |
| Luteolin-7-O-glucuronide | 0.38 | 10.3 |
| Apigenin | 0.01 | 0.3 |
| Apigenin-7-O-glucoside | 0.01 | 0.3 |
| Apigenin-7-O-glucuronide | 0.25 | 6.8 |
| Apigenin 6-C-glucoside-8-C-arabinoside (Schaftoside) | 0.06 | 1.6 |
| Apigenin 6,8-C-diglucoside (Vicenin 2) | 0.06 | 1.6 |
| Eriodictyol | <0.01 | <0.3 |
| Eriodictyol-7-O-glucoside | 0.11 | 3.0 |
| Okanin-4-O-glucoside (Marein) | 0.05 | 1.4 |
| Isookanin-7-O-glucoside (Flavanomarein) | 0.05 | 1.4 |
| Maritimetin-6-O-glucoside (Maritimein) | 0.08 | 2.2 |
| Saponins | ||
| Chrysanthellin A | 0.01 | 0.3 |
| Chrysanthellin B | 0.27 | 7.3 |
| Alkaloid | ||
| Piperine | 0.004 | 0.1 |
| Fiber | ||
| Soluble Fiber | 13.7 | 369.9 |
| Insoluble Fiber | 3.3 | 89.1 |
*Mean daily intake was calculated based on an average dose of 2.7 g of extract/kg body weight/day given orally to the mice. **in gallic acid equivalent.
Animals
All animal procedures were approved by the local ethics committee (C2E2A, Auvergne, France). Male C57BL6JRj mice aged 6 wk were used for this study (Janvier Labs, France). All mice were housed at the INRAE Animal Facility Center (INRAE Theix, France) under standard 12-h light and 12-h dark cycle. Upon arrival, all mice were fed a grain-based low-fat diet (ND, A03, Safe Diets, France) for 1 wk of acclimatization until the age of 7 wk. Thereafter, mice were placed either on ND, purified ingredient-based high-fat diet (HFD: 60% kcal from fat, 260HF, Safe Diets, France) or on the same HFD supplemented with Totum-63 (HFD-T63, 2.7% wt/wt manufactured by Safe diets, France) for up to 16 wk. Food and water were supplied ad libitum.
Body Weight, Body Composition, and Energy Intake
Mice were weighed every week. Body composition was assessed at the end of the 16th wk by MRI (Echo Medical System). Fresh food was distributed every 2 to 3 days, and food intake was recorded. Energy density of the diets was 2.83 kcal/g (ND) and 5.28 kcal/g (HFD and HFD-T63).
Indirect Calorimetry
Mice from groups HFD (n = 8) and HFD-T63 (n = 9) were put in calorimetric cages (TSE System PhenoMaster/LabMaster, Germany) during the 16th wk of experiment. Energy expenditure (EE) was calculated using Weir equation (19) out of VO2 and VCO2 measurements for each cage sampled every 5 min. Respiratory exchange ratio (RER) was calculated as VCO2/VO2. Mice were allowed to adapt to individual calorimetric cages (22°C) for 24 h before monitoring data for 24 h. EE data were analyzed with an ANCOVA regression model, using body weight as a covariate.
Fecal Energy Analysis
Feces were collected in the cage bedding between the 11th and the 14th wk of experiment. Fecal energy density was determined by bomb calorimetry (IKA C200, Germany). Apparent energy assimilation efficiency (AEAE) was calculated as fecal energy density × average daily feces production/average daily energy intake over collection period (20).
Fecal Microbiota Analysis
Fresh feces were harvested before euthanization of the animal and immediately frozen into liquid nitrogen. Composition of microbiota was assessed by 16S rDNA taxonomical metasequencing approach (21, 22). Rarefied Chao1 richness index was calculated following the formula defined in Chao (23). Shannon diversity index was calculated following the formula defined in Shannon C.E. (24). Taxa present in average in all samples at a threshold > 0.5% or present in at least 10% of samples at a threshold > 0.5% are individually represented. In other cases, taxa are grouped and labeled «other» in bar plots. Principal coordinate analysis (PCoA) was plotted using dissimilarity Bray–Curtis matrix based on relative abundances.
Fasting Glycemia, Insulinemia, and HOMA-IR Score
After a 6-h fasting period, glycemia was assessed out of a drop of whole blood from the tail of the mice with a glucometer (Stat Strip Xpress, Nova Biomedical, UK) and ∼30 µL of blood was collected from the tail in an EDTA-treated capillary (Microvette CB300, Sarstedt, Germany) before being centrifuged for 10 min at 2,000 g. Plasma was collected and frozen at –80°C until analysis. Plasma insulin level was estimated using mouse ultrasensitive insulin ELISA kit provided by Alpco (Alpco Diagnostics). HOMA-IR score was calculated from insulin and glucose levels according to Matthews et al. (25).
Oral Glucose Tolerance Test
Oral glucose tolerance test (OGTT) was performed at the end of the 16th wk of experiment. Blood glucose was monitored in 6-h fasted mice for 2 h following an oral gavage of a glucose solution (2.3 g/kg lean mass). Glycemia was recorded before (0), 30, 60, 90, and 120 min after glucose gavage. Insulin levels were assessed by ELISA in plasma at 0, 30, and 120 min after gavage. Circulating glucose and insulin levels were measured as described above. Total and baseline-adjusted glucose and insulin areas under the curve (AUC) were determined using the trapezoidal method.
Insulin Stimulation before Euthanasia
To assess tissue-specific insulin signaling in the metabolic organs, neutral protamine Hagedorn insulin (human NPH insulin, 0.75 U/kg lean mass) or an equivalent volume of NaCl 0.9% was injected intraperitoneally 10 min before euthanization in mice after 10 wk of experiment. Out of 10 to 12 mice in each group, 5 mice received an injection of vehicle and five to seven mice received an injection of insulin. Liver, gastrocnemius muscle, soleus muscle, and inguinal and epididymal fat pads were carefully dissected and snap frozen in liquid nitrogen.
Tissue Lysis and Western Blot
A piece of 50 mg of frozen tissue was added to 20 volumes of NP-40 buffer (Tris-HCl 50 mM, NaCl 150 mM, NaF 1 mM, Na3VO4 1 mM, Nonidet P-40 1%, sodium deoxycholate 0.25%, pH 7.4) supplemented with freshly added protease inhibitor cocktail (P8340, Sigma-Aldrich) and phosphatase inhibitor tablets (Thermo Fisher Scientific). The tissues were homogenized on ice using a glass potter before samples were centrifuged at 14,000 g for 10 min (+4°C), and the supernatant was collected. The protein content of the supernatant was determined using a commercial DC protein assay (Bio-Rad), and all samples were subsequently diluted to a standard concentration with 2× Laemmli buffer. Blotting was performed as described by Ennequin et al. (26), with the following specificities: Membranes were incubated overnight at 4°C with primary antibodies Akt (pan C67E7), p-Akt thr308 (C31E5E), p-Akt ser473 (D9E), HSL, and p-HSL ser563 all at concentration of 1:1,000, purchased from Cell Signaling. After incubation, the membranes were washed with TTBS and exposed to appropriate dilutions of antispecies horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. Membranes were then washed 3 times in TTBS before being exposed to an enhanced chemiluminescent solution (Clarity Western ECL; Bio-Rad) for 1 min. Membranes were exposed using a Bio-Rad ChemiDoc system, and band densities were determined using image analysis software (Image Lab V6.0, Bio-Rad). Stain Free (Bio-Rad) blot image was used as total protein loading control, and data were normalized accordingly (27).
Quantitative Real-Time PCR
Total RNA was extracted from snap-frozen liver samples using TRIzol (Thermo Fisher Scientific). cDNA was synthesized from 2 μg of RNA with high-capacity cDNA transcription kit (Applied Biosystems, Life Technologies). PCR amplification was carried out using the CFX Bio-Rad system with TaqMan probe sets (Thermo Fisher Scientific), and the ΔΔCt method was used to quantify mRNA levels. Gene expression was normalized using B2m as a housekeeping gene. Data are represented using the Rq which is normalized to the control group or Rq = 2−ΔΔCt [ΔCt = Ct (target)-Ct (B2m); ΔΔCt = ΔCt (sample)-ΔCt (control)].
Liver Triglycerides
Hepatic triglycerides (TGs) were assessed using a TG quantification kit (Abcam) following the assay instructions.
Hematoxylin-Eosin Staining
Tissues were fixed for 48 h at 4°C in paraformaldehyde and kept in ethanol 70% for 2 to 3 h before automated tissue processing. They were then embedded in paraffin, and sections were cut at 4 µm with a microtome before being stained with hematoxylin-eosin (H&E). Sections were briefly dewaxed and rehydrated in successive xylene and ethanol baths and stained in Gill II hematoxylin for 4 min and in 0.25% eosin Y bath for 45 s. Finally, sections were dehydrated with ethanol and xylene and slides were mounted with Eukitt medium.
Oil Red O Staining
Livers were frozen in OCT medium after euthanasia. Tissues were cut at 10 µm with a cryostat and fixed in cold 10% formalin for 5 min. Slides were stained for 8 min in oil red O placed in an oven at 60°C. Gill II hematoxylin was used to counterstain nuclei.
Exploratory Clinical Trial
Fourteen male overweight subjects (age: 51.5 ± 6.2 yr; height: 172 ± 3.8 cm; weight: 82 ± 6.6 kg; BMI: 27.6 ± 1.9 kg·m-2) were enrolled in an interventional, single arm with sequential design, single-center, prospective, pilot tolerance study (registered on clinicaltrial.gov under NCT02790489). This clinical study has been accepted by the French National Agency for Medicines and Health Products Safety and the South-East VI Ethical Committee under number 2015-A01864-45. The study was conducted in accordance with the ethical principles set forth in the Declaration of Helsinki, 1964, as amended in Edinburgh in October 2000 and Somerset West, South Africa, 1996, and in conformance with good clinical practice. Each participant personally and freely gave his informed consent before being enrolled in the study. Principal inclusion criteria were 45- to 65-yr-old male subjects, with BMI between 25 and 30 kg·m-2, stable weight, physical activity level, and eating habits for 3 mo before the beginning of the study. Principal exclusion criteria were antidiabetic treatment, lipid-regulating or antidyslipidemia medication, or any treatment that could interfere with the evaluation of the study endpoints, subjects consuming dietary supplements or food products supplemented with phytosterols, beta-glucans, konjac, and/or cinnamon, subject with extreme eating habits, and subject with unstable blood pressure ≥ 160/95 mmHg. Seventeen subjects were screened and 15 included in the study. One subject was withdrawn from the study due to a Wolff–Parkinson–White syndrome occurring during the study. The experimental design used intra-individual comparison in the same group of subjects. Each included subject took part to two periods of 4 wk during which two different doses of Totum-63 were taken daily (period 1, V1 to V2: 2.5 g per day and period 2, V3 to V4: 5 g per day) as we wanted to investigate tolerability of the lower-dose regimen before increasing to the assumed efficacious dose. Totum-63 was administered via 625 mg caps that subjects ingested three times a day (1 or 2 in the morning, 1 or 2 at noon, and 2 or 4 in the evening, depending on the dose). Between these two periods, a 2-wk washout period (V2 to V3) was observed. Fasted (12 h) blood and urine samples were taken, an electrocardiogram recorded, and the subject's heart rate, blood pressure, body weight, and waist size were measured during visits 0, 2, 3, and 4 (V0, V2, V3, and V4). Finally, 2 high-carbohydrate breakfast tolerance tests (84% kcal from carbohydrates) were carried out during visits 3 and 4 (V3 and V4). Before the tolerance test (t = −15'), the subjects were catheterized, and a blood sample was collected to measure the main study endpoints. Five minutes later (t = −30'), the subjects drank 250 mL of water (V3) or the daily dose of Totum-63 (5 g) with 250 mL of water (V4), before eating a high-carbohydrate breakfast tolerance tests in 12 min (t = 0'). Blood glucose and insulin kinetics were determined from eight blood samples taken before and after eating breakfast at time −10' (prechallenge), −5' (prechallenge), +15', +30', +45', +60', +90', and +120'.
Statistical Analysis
Prism V.7.0 and 8.0 (GraphPad Software) and SAS/STAT software V.9.4 (SAS Institute) were used to run statistic tests and draw figures.
For microbiota analyses, the following statistic plan was applied: Each taxon (expressed in relative abundance at the phylum, family, and genus levels) and rarefied Chao/Shannon indices were analyzed using a one-way (treatment) analysis of variance (ANOVA) statistical model with the MIXED SAS procedure. For the analysis of the relative abundances of taxa, a Benjamini and Hochberg procedure was applied to control the false discovery rate (FDR) due to multiple hypothesis tests on all taxa. For the analysis of Shannon and rarefied Chao1 indexes, a Dunnett correction was applied for the pairwise comparisons.
For the clinical trial, the following plan was applied: Raw results of the two oral carbohydrate tolerance tests carried out at V3 and V4 were subjected to a two-way (subject and treatment) controlled repeated-measures analysis over time using ANOVA. A post hoc contrast test was used versus time 0. Paired t tests were performed between V4 and V3 for parameters calculated out of insulin and glucose values. On all biological safety parameters, an ANOVA (two ways: subject and visit) associated with post hoc calculation of probability based on MLS means was used to compare all the results and in particular V2, V3, and V4 to V0 and V4 to V3.
For all other analyses, the following statistic plan was applied: A Shapiro–Wilk normality test was used to determine whether the data are consistent with a Gaussian distribution. If data were not distributed according to the normal distribution, a Kruskal–Wallis nonparametric test was used followed by Dunn test for post hoc comparison. When normal distribution was assumed, measures were subjected to one-way or two-way ANOVA with Tukey’s or Bonferroni’s test for multiple comparisons, respectively. In the case of a measurement repeated over time, differences between groups and time points were tested using a repeated-measure two-way ANOVA followed by Sidak’s post hoc test for multiple comparisons. If a piece of data was missing making it impossible to run a repeated-measures 2-way ANOVA, a mixed-effects analysis was used instead.
Values are presented as the means ± SE unless specified otherwise. The differences were considered statistically significant at P < 0.05.
RESULTS
Animal Study
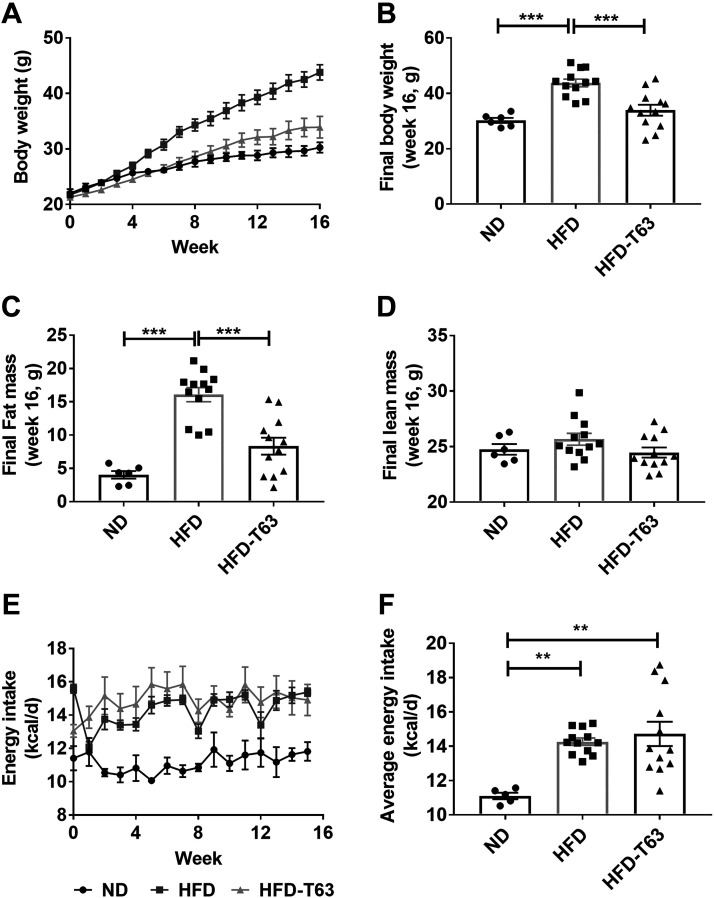
Totum-63 prevents excessive fat mass and body weight gain, independently of energy intake.
Body weight evolution over 16 wk is presented in Fig. 1A. As expected, mice fed a HFD displayed higher final body weight, compared to ND. Interestingly, supplementation with Totum-63 significantly reduced body weight gain, compared to HFD (Fig. 1B). This effect was mainly explained by a lower fat mass gain in HFD-T63, compared to HFD (Fig. 1C), as lean mass was not significantly different between groups (Fig. 1D). No effect of the supplementation on average energy intake was observed, compared to HFD (Fig. 1F), suggesting that food intake may not be involved in the effects of Totum-63 on body weight and fat mass.
Figure 1.
Body weight, energy intake, and body composition in mice euthanized after 16 wk. A: evolution of body weight. B: final (week 16) body weight. Fat mass (C) and lean mass (D) assessed by MRI at week 16. E: evolution of energy intake over 16 wk. F: average overall energy intake. One-way ANOVA followed by Tukey’s post hoc test, **P < 0.01; ***P < 0.001. Values as means ± SE. n = 6–12 animals for all experiments.
Totum-63 does not alter energy expenditure and decreases energy assimilation in the intestine.
Total 24-h EE was measured during the 16th wk of experiment in HFD and HFD-T63 mice in calorimetric cages. A power outage occurred during the calorimetric cage experiment resulting in a loss of data and a low number of replicates (n = 3) in group ND. Data for this group are presented as a dotted line and were not included in the statistical analysis. ANCOVA-adjusted EE revealed a 3.22 kcal/day difference between HFD and HFD-T63 that did not reach statistical significance (P = 0.4799, Fig. 2A). Complete ANCOVA report is available in Supplemental Fig. S6 (all Supplemental material is available at https://doi.org/10.6084/m9.figshare.13359482). Similarly, no difference was observed in total EE (Fig. 2B) and 24-h average RER (Fig. 2C). Fecal energy was measured by bomb calorimetry in dried feces collected in the cages. Because mice in group ND were fed a grain-based diet (as opposed to purified ingredient-based diet in HFD and HFD-T63), the comparison of feces with groups HFD and HFD-T63 would be irrelevant. Thus, this group was not included in the statistical analysis and is presented in the figure as a dotted line. Totum-63-supplemented mice showed higher fecal energy density (Fig. 2D). Similarly, the daily amount of feces excreted was higher in supplemented mice (Fig. 2E), and consequently, AEAE was significantly reduced in Totum-63-supplemented animals (Fig. 2F), suggesting lower energy assimilation in the digestive tract.
Figure 2.
Calorimetric measurements and apparent energy assimilation efficiency in mice euthanized after 16 wk. Mice were acclimatized in calorimetric cages for 24 h; then, respiratory measurements were monitored for another 24 h. Measurements in calorimetric cages were performed mice during week 16. A: ANCOVA regression fitting curves of EE using body weight as a covariant. ANCOVA-bodyweight-adjusted EE revealed no significant difference (P = 0.4799) between HFD and HFD-T63. Complete ANCOVA report is available in Supplemental Fig. S6. B: 24-h total energy expenditure (EE). C: respiratory exchange ratio (RER). D: fecal energy was measured by bomb calorimetry out of feces collected in the cages in the animals between week 11 and week 14. E: 24-h feces production (after drying). F: apparent energy assimilation efficiency (AEAE). Unpaired two-tailed t test or Welsh’s unpaired two-tailed t test or Mann–Whitney test. *P < 0.05. Values as means ± SE. n = 8 or 9 animals for indirect calorimetric experiments and n = 12 animals for fecal energy measurements for groups HFD and HFD-T63. ND group is shown as a dotted line in the graphs and was not included in the statistical analysis. HFD, high-fat diet.
Totum-63 improves HFD-induced gut microbial imbalance.
Fresh feces were collected in mice after 16 wk of experiment. HFD induced a decrease in richness (Chao) and diversity (Shannon) indexes, compared to ND (P < 0.001, Fig. 3, A and B). Interestingly, Totum-63 supplementation was able to partially restore diversity index, compared to HFD (P < 0.05, Fig. 3B). Similarly, principal coordinate analysis (PCoA) of relative abundances at phylum, family, and genus levels revealed two main clusters for mice in groups ND and HFD. Mice belonging in group HFD-T63 present intermediate taxonomic profiles, between these two clusters (Fig. 3, C–E). Complete characterization of microbiota at phylum, family, and genus levels was performed in frozen feces (Fig. 4). At phylum level, HFD mice displayed higher relative abundance of Firmicutes and lower abundance of Bacteroidetes, compared to ND (Fig. 4A). Interestingly, mice in HFD-T63 group displayed an intermediate profile between ND and HFD, with significant decrease in relative abundance of Firmicutes and increase in Bacteroidetes (Table 2). The inversion of Bacteroidetes/Firmicutes ratio, at the phylum level (HFD vs. ND), was mainly explained at family level by a lower level of Porphyromonadaceae (a Bacteroidetes family) to the benefit of Lachnospiraceae (a Firmicutes family) (Fig. 4B and Table 3). Finally, at genus level, Totum-63 supplementation significantly increased relative abundance of Barnesiella (Porphyromonadaceae), Ruminococcus2 (Lachnospiraceae), Butyricicoccus (Ruminococcaceae), and Parasutterella (Sutterellaceae), whereas the relative abundance of Pseudoflavonifractor and Anaerotruncus (Ruminococcaceae), and Dorea and Clostridium_XIVb (Lachnospiraceae) was decreased compared with HFD (Fig. 4C and Table 4). Taken together, these data suggest that Totum-63 supplementation may have partially restored HFD-induced gut microbial imbalance.
Figure 3.
Richness, diversity, and PCoA of fecal microbiota in mice euthanized after 16 wk. A: rarefied Chao index (index of richness). B: Shannon diversity index. One-way ANOVA with MIXED SAS procedure followed by Dunnett’s t test for multiple comparisons, *P < 0.05; ***P < 0.001. Values as means ± SE. n = 5–12 animals for all measurements. C, D, and E: principal coordinate analysis (PCoA) using dissimilarity Bray–Curtis matrix based on relative abundances at phylum, family, and genus levels, with confidence ellipse. Mice in group HFD-T63 display an intermediate profile between ND and HFD. n = 5–12 animals for all measurements. HFD, high-fat diet.
Figure 4.
Relative abundance of fecal bacteria in mice euthanized after 16 wk at phylum, family, and genus levels. A: phylum level. Significant differences between groups are presented in Table 2. B: family level. Significant differences between groups are presented in Table 3. C: genus level. Significant differences between groups are presented in Table 4. Values as mean. n = 6–11 animals for all measurements.
Table 2.
Fecal microbiota: statistically significant differences of relative abundance at phylum level
| Phylum | Means ± SE and adj. P value |
|
|---|---|---|
| HFD-T63 vs. HFD | HFD-T63 vs. ND | |
| Bacteroidetes | 24.76 ± 2.69% vs.14.19 ± 2.45% | 24.76 ± 2.69% vs. 52.23 ± 3.80% |
| adj. P = 0.0272 | adj. P < 0.0001 | |
| Firmicutes | 57.22 ± 2.70% vs. 73.41 ± 2.46% | 57.22 ± 2.70% vs. 39.20 ± 3.81% |
| adj. P = 0.0012 | adj. P = 0.0021 | |
| Proteobacteria | NS | 5.86 ± 0.55% vs. 2.55% ± 0.77% |
| adj. P = 0.0033 | ||
Expressed as % of relative abundance and adjusted P values. Values as means ± SE. n = 6–11 animals for all measurements. HFD, high-fat diet; NS, not significantly different.
Table 3.
Fecal microbiota: statistically significant differences of relative abundance at family level
| Phylum | Family | Means ± SE and adj. P value |
|
|---|---|---|---|
| HFD-T63 vs. HFD | HFD-T63 vs. ND | ||
| Bacteroidetes | Bacteroidaceae | NS | 0.97 ± 0.42% vs. 6.06 ± 0.59% |
| adj. P < 0.0001 | |||
| Porphyromonadaceae | 15.21 ± 1.62% vs. 6.40 ± 1.48% | 15.21 ± 1.62% vs. 34.89 ± 2.29% | |
| adj. P = 0.0056 | adj. P < 0.0001 | ||
| Prevotellaceae | NS | 0.83 ± 0.71% vs. 5.72 ± 1.01% | |
| adj. P = 0.0031 | |||
| Firmicutes | Lachnospiraceae | 16.50 ± 1.99% vs. 25.40 ± 1.82% | NS |
| adj. P = 0.0167 | |||
| Ruminococcaceae | NS | 16.28 ± 1.57% vs. 5.02 ± 2.22% | |
| adj. P = 0.0027 | |||
| Streptococcaceae | NS | 0.92 ± 0.16% vs. 0.04 ± 0.23% | |
| adj. P = 0.0210 | |||
| Proteobacteria | Sutterellaceae | 1.55 ± 0.17% vs. 0.33 ± 0.16% | NS |
| adj. P = 0.0004 | |||
Expressed as % of relative abundance and adjusted P values. Values as means ± SE. N = 6–11 animals for all measurements. HFD, high-fat diet; NS, not significantly different.
Table 4.
Fecal microbiota: statistically significant differences of relative abundance at genus level
| Phylum | Family | Genus | Means ± SE and adj. P value |
||
|---|---|---|---|---|---|
| HFD-T63 vs. HFD | HFD-T63 vs ND | ||||
| Actinobacteria | Coriobacteriaceae | Enterorhabdus | NS | 2,13 ± 0,24% vs. 0.49 ± 0.34% | |
| adj. P = 0,0041 | |||||
| Bacteroidetes | Bacteroidaceae | Bacteroides | NS | 0.97 ± 0.42% vs. 6.06 0.59% | |
| adj. P < 0.0001 | |||||
| Porphyromonadaceae | Barnesiella | 9.36 ± 1.10% vs. 3.12 ± 1.00% | 9.36 ± 1.10% vs. 16.55 ± 1.55%) | ||
| adj. P = 0.0029 | adj. P < 0.0055 | ||||
| Prevotellaceae | Prevotella | NS | 0.04 ± 0.14% vs. 3.14 ± 0.20% | ||
| adj. P = 0.0001 | |||||
| Firmicutes | Lachnospiraceae | Clostridium_XIVa | NS | 1.02 ± 0.23% vs. 2.45 ± 1.82% | |
| adj. P = 0.0069 | |||||
| Clostridium_XIVb | 0.29 ± 0.08% vs. 0.58 ± 1.82% | NS | |||
| adj. P = 0.0432 | |||||
| Dorea | 0.50 ± 0.77% vs. 4.09 ± 0.70% | NS | |||
| adj. P = 0.0128 | |||||
| Ruminococcus2 | 0.73 ± 0.07% vs. 0.07 ± 0.06% | 0.73 ± 0.07% vs. 0.14 ± 0.10% | |||
| adj. P < 0.0001 | adj. P = 0.0008 | ||||
| Ruminococcaceae | Anaerotruncus | 0.01 ± 0.02% vs. 0.13 ± 0.02% | NS | ||
| adj. P = 0.0029 | |||||
| Butyricicoccus | 0.09 ± 0.02% vs. 0.01 ± 0.02% | NS | |||
| adj. P = 0.0161 | |||||
| Pseudoflavonifractor | 0.23 ± 0.07% vs. 0.55 ± 0.06% | NS | |||
| adj. P = 0.0128 | |||||
| Ruminococcus | NS | 0.00 ± 0.04% vs. 0.26 ± 0.05% | |||
| adj. P = 0.0030 | |||||
| Streptococcaceae | Streptococcus | NS | 0.92 ± 0.16% vs. 0.04 ± 0.23% | ||
| adj. P = 0.0224 | |||||
| Proteobacteria | Sutterellaceae | Parasutterella | 1.55 ± 0.17% vs. 0.33 ± 0.16% | NS | |
| adj. P = 0.0004 | |||||
Expressed as % of relative abundance and adjusted p values. Values as means ± SE. N = 6–11 animals for all measurements. HFD, high-fat diet; NS, not significantly different.
Totum-63 improves whole body glucose homeostasis.
Fasting glycemia, insulinemia, and subsequent HOMA-IR index were monitored over 16 wk of supplementation. Exposure to HFD resulted in a slight but significant increase in fasting glycemia from week 8 to week 16, compared with ND. Interestingly, fasting glycemia was significantly reduced in Totum-63-supplemented mice at weeks 2, 4, and 8, but this difference was no longer significant at weeks 12 and 16 (Fig. 5A). After 16 wk, fasting insulin levels were increased in group HFD, compared with ND and HFD-T63 groups (Fig. 5B). Subsequently, HOMA-IR was increased in HFD, compared with ND. Supplementation with Totum-63 was able to reduce HOMA-IR, compared with HFD (Fig. 5C). Fasting glucose and insulin curves in Totum-63-supplemented mice appear to be delayed by ∼8 wk, compared with HFD (glucose and insulin values of HFD at week 16 are very close to those of HFD-T63 at week 8), suggesting that Totum-63 may have delayed the onset of HFD-induced hyperinsulinemia.
Figure 5.
Glucose homeostasis in mice euthanized after 16 wk. A: fasting glycemia was measured in all mice after a 5-h fast at week 2, week 4, week 8, week 12, and week 16. B: plasma fasting insulin was measured in the same conditions at week 4, week 8, week 12, and week 16. C: HOMA-IR score was calculated out of insulin and glycemia values. OGTT: fasted mice received an oral gavage of 2.3 g/kg lean mass glucose during the 16th wk of experiment. D: evolution of glycemia following OGTT. E: total glucose AUC following OGTT. F: baseline-adjusted glycemia following OGTT. G: baseline-adjusted glucose AUC following OGTT. H: total insulin response following OGTT. I: total insulin AUC following OGTT. J: baseline-adjusted insulin following OGTT. K: baseline-adjusted insulin AUC following OGTT. Repeated-measures two-way ANOVA followed by Sidak’s post hoc test, different from HFD-T63: $P < 0.05; $$P < 0.01; $$$P < 0.001. Different from ND: ^^^P < 0.001. Kruskal–Wallis test followed by Dunn’s post hoc test, *P < 0.05; **P < 0.01. Values as means ± SE. n = 6–12 animals for all measurements. AUC, areas under the curve; OGTT, oral glucose tolerance test.
OGTT was performed in all mice during the 16th wk of experiment; glucose and insulin levels were monitored for 2 h. Total glucose response to the oral challenge is presented in Fig. 5D. Total glucose AUC was significantly increased in HFD-fed mice, compared to ND (Fig. 5E). Supplemented mice tended to display lower total glucose AUC versus HFD, although the difference did not reach statistical significance threshold (P = 0.066, Fig. 5E). Since glycemia was different at baseline, we calculated the baseline-adjusted glucose response and corresponding AUC (Fig. 5, F and G). No differences were observed between groups, suggesting that the higher total glucose response in HFD was mainly due to the difference at baseline. Total insulin response following OGTT reveals significantly higher insulin levels in HFD at 30 min, compared to ND and HFD-T63 (Fig. 5H). Totum-63-supplemented animals had similar insulin response to that of ND. Total insulin AUC following an OGTT was also significantly increased in HFD-fed mice, compared to ND. Totum-63 supplementation significantly reduced total insulin AUC compared with HFD (Fig. 5I). Here again, since basal insulin levels were different among groups, we calculated baseline-adjusted insulin response and AUC. The difference at 30 min between HFD versus ND and HFD-T63 was still significant (P < 0.001, Fig. 5J); however, baseline-adjusted insulin AUC was only significantly different between HFD and ND (P < 0.05, Fig. 5K).
Collectively, these results suggest that HFD-driven homeostasis impairments in this model are mainly characterized by mild fasting hyperglycemia and more severe fasting hyperinsulinemia. Totum-63 supplementation appears to have delayed the apparition of HFD-induced insulin resistance.
Totum-63 restores insulin sensitivity in soleus muscle and inguinal adipose tissue.
Based on the previous results obtained in the 16-wk study animals, and in an attempt to focus on the earlier effects of Totum-63 in improving glucose homeostasis, insulin signaling was assessed in the liver, gastrocnemius, soleus, inguinal fat, and epididymal fat of mice supplemented or not with Totum-63 for 10 wk, before the apparent decompensation on glucose homeostasis visible in the 16-wk study. Evolution of fasting glycemia, insulin, HOMA-IR score, and glucose and insulin responses to an OGTT were also monitored in these mice and were similar to mice belonging to the 16-wk study. All data are available in Supplemental Fig. S2.
Insulin-stimulated phosphorylation state of Akt on its 2 activated residues (Ser473 and Thr308) in these organs was compared with vehicle condition. In the liver, the gastrocnemius muscle and, in the epididymal adipose tissue, two-way ANOVA revealed no significant interaction, suggesting that all three groups responded in a similar fashion to vehicle and insulin stimulation, in these organs (Fig. 6, A, B, C, D, J, and K). In the soleus muscle, the two-way ANOVA interaction for pAkt Thr308/Akt ratio came close to significance threshold (P = 0.063). Post hoc test for multiple comparisons was run and revealed a significant difference between vehicle and insulin conditions only for groups ND and HFD-T63. In group HFD, Akt phosphorylation ratio on Thr308 residue in response to insulin was blunted compared with vehicle condition, suggesting a dysfunction of insulin pathway. Interestingly, in soleus muscle, Totum-63 supplementation was able to restore insulin-stimulated Akt phosphorylation on Thr308 residue (Fig. 6F). In inguinal adipose tissue, insulin-stimulated Akt phosphorylation ratio (ser473) was increased compared to vehicle condition in group HFD-T63 only. In group HFD (and ND), no significant effect of insulin was observed, compared with vehicle condition (Fig. 6G). Besides, in this tissue, phosphorylation ratio of HSL, a protein which phosphorylation is inhibited by insulin, was assessed in insulin versus vehicle condition. Insulin failed to significantly inhibit HSL phosphorylation in group HFD, suggesting here again a dysfunctional insulin pathway. Interestingly, supplementation with Totum-63 was able to restore the effect of insulin (Fig. 6I). In summary, Totum-63 supplementation may restore insulin signaling when it is found to be impaired by exposure to a HFD, specifically, in this study, in inguinal adipose tissue and, to a lesser extent, in soleus muscle (statistical trend only).
Figure 6.
Insulin signaling in the liver, gastrocnemius, and soleus muscle, inguinal and epididymal adipose tissue, with representative blots in mice euthanized after 10 wk. Vehicle (n = 5/group) versus insulin (n = 5 or 7/group)-stimulated phosphorylation ratios of two Akt residues (ser473 and thr308) were assessed by Western blot in liver (A and B), gastrocnemius muscle (C and D), soleus muscle (E and F), inguinal adipose tissue (G and H), and epididymal adipose tissue (J and K). Two-way ANOVA interaction showed a statistical trend in Fig. 5. F: (soleus, pAkt thr308/Akt ratio, P = 0.063) and Bonferroni’s post hoc test was run to compare vehicle vs. insulin-stimulated Akt phosphorylation within groups. G: in inguinal adipose tissue, Akt phosphorylation ratio on ser473 residue showed significant 2-way ANOVA interaction (P = 0.027). Bonferroni’s post hoc test was run to compare vehicle vs. insulin-stimulated Akt phosphorylation within groups. I: vehicle vs. insulin-stimulated HSL phosphorylation ratio in inguinal adipose tissue. L: vehicle vs. insulin-stimulated HSL phosphorylation ratio in epididymal adipose tissue. Two-way ANOVA followed by Bonferroni’s post hoc test, *P < 0.05; **P < 0.01; ***P < 0.001. NS: Not significantly different. Values as means ± SE. n = 4–7 animals for all measurements.
Totum-63 improves HFD-induced liver steatosis.
After 16 wk of experiment, histological analysis with hematoxylin-eosin and oil red O staining revealed lower lipid accumulation in Totum-63 supplemented mice, compared with HFD (Fig. 7A). TG content quantification confirmed histological analysis with mice in group HFD showing a significant increase in liver triglycerides, compared with ND. Interestingly, supplementation with Totum-63 improved this parameter (vs. HFD, –40%, P < 0.05, Fig. 7B). We assessed the expression of several genes related to fatty acid (FA) metabolism, in the liver. In line with the above-mentioned results on liver steatosis markers, the expression of Cidec, a gene coding for lipid droplet-associated proteins, was significantly increased in group HFD, compared with ND (P < 0.001). Its expression was significantly reduced in Totum-63-supplemented mice, compared with HFD (P < 0.05, Fig. 7C). No other significant differences were found between HFD and HFD-T63 in any of the gene expressions assessed.
Figure 7.
Liver lipid metabolism in mice euthanized after 16 wk. A: representative histological liver images, hematoxylin-eosin staining (×10), and oil red O (×20). B: liver triglyceride content. Kruskal–Wallis test followed by Dunn’s post hoc test. *P < 0.05; **P < 0.01. C: expression of genes associated with FA oxidation, metabolism, storage, and uptake. One-way ANOVA followed by Tukey’s post hoc test or Kruskal–Wallis test followed by Dunn’s post hoc test. Different from HFD-T63: $P < 0.05; different from ND: ^P < 0.05; ^^P < 0.01. Values as means ± SE. n = 4–12 animals for all measurements. ANOVA, analysis of variance; HFD, high-fat diet.
Taken together, these data obtained in mice fed a HFD suggest that Totum-63 may improve body composition and glucose homeostasis. Various organs were involved in the beneficial effects of Totum-63; notably, we showed that insulin sensitivity was restored in inguinal adipose tissue and in soleus muscle, whereas liver steatosis was improved in supplemented animals. Moreover, in the gut, Totum-63 may have partially restored HFD-induced microbial imbalance. The effects of Totum-63 were then assessed in humans in an exploratory clinical trial.
Clinical Trial
In humans, Totum-63 is well tolerated and improves glucose and insulin response to a carbohydrate tolerance test.
The clinical trial included 14 male subjects (age: 51.5 ± 6.2 yr, BMI: 27.6 ± 1.9 kg·m-2); all baseline characteristics are provided in Table 5. Study design is summarized in Fig. 8A. An oral carbohydrate tolerance test was performed in each subject before (V3) and after (V4) the highest tested dose (5 g·day-1). Glycemia following carbohydrate ingestion was significantly reduced (T30 and T45 min) after supplementation, compared to before supplementation (Fig. 7B). Consequently, glucose peak (Cmax) was reduced after supplementation (Fig. 7C) and total glucose AUC tended to decrease, though not significantly (P = 0.08, Fig. 7D). Insulin response following the test was also reduced (Fig. 7E), and both insulin AUC and insulin peak (Cmax) were significantly reduced after 4 wk of supplementation with Totum-63 (Fig. 7, F and G). Finally, an insulin sensitivity index was calculated after glucose and insulin AUC and showed a significant decrease after supplementation (Fig. 7H). None of the adverse events reported during the study in some patients (headache, cold, low back pain, flatulence, etc.) was considered serious and related to Totum-63 supplementation. Similarly, the evolution of safety biological parameters and vital signs was considered stable and nonclinically relevant, by the investigators (Table 5).
Table 5.
General measurements and circulating safety parameters
|
V0 |
V2 |
V3 |
V4 |
|||||
|---|---|---|---|---|---|---|---|---|
| Variable | Mean | Std Dev | Mean | Std Dev | Mean | Std Dev | Mean | Std Dev |
| Biometry | ||||||||
| Weight, kg | 81.5 | 6.47 | 81.3 | 6.86 | 81.6 | 6.84 | 81.6 | 6.69 |
| Waist size, cm | 97.9 | 6.15 | 97.9 | 7.34 | 97.1 | 6.94 | 97.4 | 7.33 |
| BMI, kg·m–² | 27.6 | 1.85 | 27.5 | 1.97 | 27.5 | 2.05 | 27.6 | 1.99 |
| Vital parameters | ||||||||
| Systolic blood pressure, mmHg | 136 | 16.3 | 133 | 11.5 | 135 | 15.3 | 134 | 15.2 |
| Diastolic blood pressure, mmHg | 85.9 | 10.1 | 84.6 | 11.5 | 83.3 | 7.56 | 83.9 | 9.76 |
| Heat rate, beats/min | 63.2 | 13.8 | 62.9 | 13.7 | 64.0 | 11.5 | 62.8 | 12.9 |
| O2 saturation, % | 97.3 | 1.73 | 98.7 | 1.65 | 98.0 | 1.47 | 98.1 | 1.38 |
| PR interval, ms | 168 | 19.3 | 168 | 24.1 | 169 | 20.9 | 171 | 21.4 |
| QRS complex, ms | 88.1 | 5.40 | 89.4 | 6.01 | 90.6 | 6.45 | 88.5 | 5.61 |
| QT interval, ms | 399 | 39.1 | 400 | 28.9 | 405 | 34.5 | 395 | 26.6 |
| QTc interval, ms | 385 | 29.7 | 388 | 24.6 | 398 | 19.2 | 383 | 26.9 |
| QRS axis, ms | 26.1 | 29.6 | 24.1 | 27.4 | 26.9 | 29.5 | 24.2 | 29.3 |
| Blood analysis | ||||||||
| ALAT, UI/L | 40.9 | 13.4 | 38.2 | 13.2 | 36.6 | 14.5 | 39.0 | 10.8 |
| ALC. PHOSP, UI/L | 61.0 | 20.0 | 60.8 | 19.0 | 60.7 | 19.0 | 59.5 | 18.1 |
| ASAT, UI/L | 23.8 | 5.52 | 23.6 | 3.84 | 24.0 | 5.41 | 24.9 | 3.47 |
| Bilirubin, µmol·L–1 | 9.21 | 2.72 | 9.79 | 2.83 | 8.69 | 2.84 | 10.2 | 2.38 |
| Cholesterol HDL, mmol. mmol·L–1 | 1.43 | 0.40 | 1.48 | 0.46 | 1.41 | 0.38 | 1.41 | 0.36 |
| Cholesterol TOTAL, mmol·L–1 | 4.83 | 0.85 | 4.56 | 0.87 | 4.74 | 0.78 | 4.43aa | 0.81 |
| Cholesterol LDL, mmol·L–1 | 2.81 | 0.85 | 2.60a | 0.77 | 2.69 | 0.78 | 2.49aa,c | 0.71 |
| Creatinine, µmol·L–1 | 75.8 | 7.92 | 79.4 | 8.27 | 81.3aa | 9.99 | 76.7c | 9.02 |
| Fructosamine, µmol·L–1 | 216 | 13.9 | 220 | 11.1 | 214 | 15.5 | 205aa,cc | 15.5 |
| GGT, mUI/L | 47.3 | 31.9 | 45.8 | 34.1 | 44.1 | 34.1 | 51.7c | 35.2 |
| Glucose, mmol·L–1 | 4.71 | 0.41 | 4.83 | 0.50 | 4.59 | 0.38 | 4.82c | 0.56 |
| Insulin, mUI·L–1 | 8.08 | 6.51 | 9.76 | 9.08 | 9.90 | 10.2 | 10.9 | 8.35 |
| Oxidized LDL, % | 39.6 | 12.5 | 37.8 | 14.6 | 35.4 | 11.2a | 33.8 | 11.4 |
| Triglyceride, mmol·L–1 | 1.31 | 0.57 | 1.07a | 0.59 | 1.40 | 0.55 | 1.21 | 0.58 |
| Urea, mmol·L–1 | 5.74 | 1.40 | 5.98 | 1.43 | 6.31a | 1.74 | 6.19 | 1.42 |
| Urine analysis | ||||||||
| Urine Creatinine, µmol·L–1 | 17.6 | 6.57 | 16.5 | 6.67 | 17.6 | 6.45 | 17.5 | 4.87 |
Significant differences are shown in bold in the table. Two-way ANOVA (subject and visit) associated with post hoc calculation of probability based on MLS means. Different from V0: aP < 0.05; aaP < 0.01. Different from V3: cP < 0.05 mL; P < 0.01. No change was considered clinically relevant by the investigators. ALAT: alanine aminotransferase; ALC. PHOSP, alkaline phosphatase; ASAT, aspartate aminotransferase; BMI, body mass index; DIASTO BP, diastolic blood pressure; GGT, gamma-glutamyltranspeptidase; H. RATE, heart rate; QTc INTERVAL, corrected QT interval; SYSTO BP, systolic blood pressure. Values as means ± SD. n = 14 subjects for all measurements.
Figure 8.
Response to a carbohydrate challenge in the clinical study. A: schematic of the study design. Subjects were supplemented with the lower dose of Totum-63 (2.5 g·day−1) for 4 wk and then with the higher dose (5 g·day−1) for another 4 wk. A 2-wk washout period was observed between the 2 periods of supplementation. Carbohydrate tolerance test was performed before (V3) and after (V4) the highest dose distributed. The diagrams present the glucose and insulin response to a carbohydrate tolerance test before (V3) and after the second dose of supplementation (V4). Both doses were tested for 4 wk, and a 2-wk washout period was observed in between. B: evolution of circulating glucose before and after carbohydrate challenges. C: glucose AUC. D: glucose peak (Cmax). E: evolution of circulating insulin before and after carbohydrate challenges. F: insulin AUC. G: insulin peak (Cmax). H: insulin sensitivity index defined as AUCglucose × AUCinsulin. Repeated-measures ANOVA and Student’s t test for paired samples, *P < 0.05; **P < 0.01. Values as means ± SE. n = 14 subjects for all measurements. AUC, areas under the curve.
DISCUSSION
Totum-63 combines five plant extracts selected to act synergistically on different organs involved in the complex pathophysiology of T2D development. Its chemical characterization has revealed a high polyphenol content (>14% dry weight). Plant-derived polyphenols are considered promising strategies to tackle lifestyle-related pathologies such as metabolic syndrome and T2D (13, 28). Several of the identified compounds present in Totum-63 (notably: anthocyanins, oleuropein and its derivatives, chlorogenic acid, luteolin, caffeic acid, etc.) have already been studied for their effects on metabolic diseases (29–34).
This report describes the first exploratory results about the effects of Totum-63 obtained in HFD-fed mice and overweight individuals. In HFD-fed mice, we showed that chronic supplementation with Totum-63 incorporated in the food prevented diet-induced excessive weight and fat mass gain. The analysis of energy balance revealed similar energy intake but lower energy assimilation efficiency in supplemented mice, compared with HFD. HFD-induced detrimental effects on glucose homeostasis were partially prevented in supplemented mice; notably, the increase in the insulin resistance index HOMA-IR was markedly delayed. Restoration of insulin-stimulated Akt activation in inguinal fat and in soleus muscle might have contributed to the effect on glucose control. Finally, HFD-induced microbial imbalance and liver steatosis were improved following Totum-63 supplementation. Consistent with its chemical composition, rich in various bioactive compounds, our results suggest that several organs were affected by Totum-63 supplementation, although it remains to be addressed whether an additive effect of supplementation to that of body composition improvement exists to explain the beneficial effects on other tissues. Consecutive to the promising results obtained during these preclinical experiments, Totum-63 was patented, and an exploratory clinical trial was conducted in 14 male subjects to confirm the innocuity of the supplementation in humans and assess for the first time its effect on the response to a carbohydrate challenge. Totum-63 was well tolerated by subjects and was considered safe by the investigators. Interestingly, the glucose and insulin response to the carbohydrate challenge was improved after supplementation with 5 g·day-1.
Several leads for some potential mechanisms have been investigated. To explain the preventive effects of Totum-63 on body weight and fat mass accumulation in HFD mice, we analyzed different components of the energy balance: energy intake, energy assimilation, and total EE (35). No differences were observed in energy intake. Energy assimilation appeared lower in Totum-63 supplemented mice as both fecal energy density and fecal production were increased, leading to reduced AEAE. This result suggests that digestive efficiency may be reduced in supplemented mice causing less energy to be absorbed in the intestine. The contribution of this mechanism in total body weight loss remains to be clarified. The present data suggest that, if we considered that the 1% difference in AEAE was constant throughout the study, the energy lost in the feces would represent 387 J per day and 43,349 J over 16 wk (10.4 kcal). Depending on the form of storage of these excess calories, 10.4 kcal would virtually convert to anywhere between 1.1 and 2.6 g of body weight. The final average difference between groups being 9.9 g, AEAE would then explain 11% to 26% of the difference, making nutrient malabsorption the most significant contributor of body weight difference between groups, in this experimental setup. However, this calculation must be interpreted with caution because the design of the present work does not allow for an accurate assessment of the magnitude of the different components of energy balance.
Interestingly, several marketed drugs based on the inhibition of calorie absorption have successfully been used over the past decades to treat obesity and associated pathologies such as T2D (36–38). An overview of current literature reveals that at least four ingredients used in Totum-63 have demonstrated inhibitory effects on digestive enzymes (39–42). Furthermore, at molecular level, several polyphenols found in Totum-63 have shown inhibitory effects on diverse digestive enzymes. Among them, anthocyanins present in bilberry extract have shown in rats a potent capacity to inhibit several glycosidases (43). Chlorogenic acid might be involved in α-amylase inhibition (44), though perhaps only weakly, according to a contradicting study (45). Oleuropein has also shown inhibitory activity on enzymes involved in both carbohydrate and lipid metabolism (46). Hydroxytyrosol, a derivative of oleuropein, was also proved to be a strong alpha-glucosidase inhibitor (47). Luteolin and its derivative luteolin 7-o-glucoside have showed a strong inhibition of alpha-glucosidase and α-amylase (48). Although it remains difficult in our study to discriminate the compounds responsible for a given effect, we can speculate, based on the existing work, that the inhibition of digestive enzymes may have played a role in the higher energy excretion in the feces observed following Totum-63 supplementation. EE, another major component of energy balance, was assessed in calorimetric cages in mice after 16 wk of experiment, at a time when supplemented animals exhibited dramatic body weight reduction, compared to group HFD (–31%). Total 24-h EE was not different between groups, suggesting that increased EE was not participating to the reduction of body weight, at that moment. ANCOVA of EE using body weight as a covariate confirmed the absence of statistically significant difference (P = 0.4799), though the regression curve of supplemented mice appeared higher than that of HFD (3.22 kcal/day). Though inconclusive, it should be kept in mind that, albeit modest and not significant when measured over 24 h, this tiny shift upward could result in larger effects over 16 wk. Moreover, we cannot rule out that EE had not contributed more to body weight reduction earlier in the study. This is supported by the modest increases in the expression of genes associated with thermogenic capacity (Ucp1, Dio2, and Prdm16) and mitochondrial biogenesis (Tfam and Nrf1) observed in BAT, following Totum-63 supplementation (Supplemental Fig. S3). Here again, the literature suggests that some compounds present in Totum-63, notably chlorogenic acid, oleuropein, and its derivative hydroxytyrosol, may be inducers of thermogenesis-associated genes, putatively through sympathetic stimulation (49–51).
Taken together, AEAE and EE were insufficient to explain the entire difference in body weight between HFD and HFD-T63. However, their relative contribution should not be overinterpreted because several requirements for a correct estimation of individual energy balance are not met in this work, such as the consideration of nondigestible energy, urine losses, or the fact that EE and AEAE were not measured over the same period. Moreover, we cannot rule out that those have not been higher at a different time in the study, which would make them greater contributors to the effects on body weight. Based on the literature, other potential mechanisms may also have participated in the reduction of body weight, such as the contribution of anaerobic microbial metabolism, consistent with the alteration of microbiota consecutive to Totum-63 supplementation, which can account for a very significant amount of total metabolism (52). Secondly, it is possible that the tiny upward shift of the EE regression curve measured over 24 h could add up to eventually result in a larger change in body weight over 16 wk. Indeed, other teams have already suggested that energy balance differences in the small range (2% to 5%) can result in significant long-term alterations (53), although they may be hard to highlight in traditional laboratory setup conditions. It is possible that these daily small differences could add up to eventually result in a larger change in body weight over 16 wk.
The second finding of this work was the improvement of HFD-induced impaired glucose homeostasis. Mice in group HFD displayed mild fasting hyperglycemia and more severe fasting hyperinsulinemia that resemble prediabetes development, when pancreas produces higher amounts of insulin to regulate glucose levels, to compensate the onset of insulin resistance in peripheral organs (3). The insulin response pattern to glucose increase seemed similar between HFD and HFD-T63 except it occurred much later in HFD-T63 (glucose and insulin values of HFD at week 16 were very close to those of HFD-T63 at week 8), suggesting that Totum-63 may have delayed the onset of fasting and OGTT-triggered hyperinsulinemia, a marker of insulin resistance. Interestingly, this feature is consistent with the effects of certain well-known insulin-sensitizing drugs (pioglitazone and rosiglitazone) that have demonstrated similar pattern in previous studies using HFD-fed mice. The improvement of insulin sensitivity was characterized in these studies by a dramatic reduction of insulin levels (fasting and in response to an OGTT) concomitant with insignificant effects on glucose levels (54, 55). Similarly, in a clinical study in women with polycystic ovary syndrome displaying insulin resistance, metformin treatment resulted in improvement of insulin sensitivity characterized by lower insulin levels and no change in glycemia (56).
The analysis of insulin signaling pathway in five different tissues involved in glucose uptake has shown that whenever it was impaired by HFD (in soleus muscle and inguinal adipose tissue, in this study), Totum-63 could restore insulin-stimulated Akt phosphorylation, suggesting an improvement of insulin sensitivity in supplemented mice. Interestingly, skeletal muscle is thought to be the tissue that accounts for most of the whole-body glucose uptake (57, 58). Our present data may suggest that oxidative-type muscle insulin signaling was more affected by HFD than that of mixed-type muscles, such as gastrocnemius, making the restoring effect of supplementation more visible in oxidative muscles. Adipose tissue, although probably not the greatest contributor to systemic glucose uptake, still plays a central role in whole-body insulin sensitivity via various mechanisms including secretion of free fatty acids which in turn can impair other organs’ function (59). Insulin is a major regulator of FFA secretion via inhibition of HSL (60). Noteworthily, we showed in this study that the inhibition of HSL phosphorylation by insulin was restored in inguinal fat consecutive to Totum-63 supplementation, making this mechanism a potential contributor to the systemic improvement in glucose control. A direct effect on the organs is plausible as well, since beneficial effects of polyphenols in enhancing glucose uptake have been reported in C2C12 muscle cells and 3T3-F442A preadipocytes (61, 62).
In the liver, Totum-63 supplementation resulted in a reduction of TG content and apparent improvement of steatosis, a powerful inducer of hepatic insulin resistance (63). To investigate the potential mechanisms involved, we hypothesized that compounds present in Totum-63 could alter the expression of genes associated with FA metabolism. Indeed, previous studies have reported that polyphenols could prevent fatty liver disease through inhibition of de novo lipogenesis (via AMPK–Srebp1-c pathway), stimulation of FA oxidation (again possibly through activation of AMPK), and by reducing inflammation (64, 65). Unexpectedly, we hardly found any effect of the supplementation on the expression of some of the major genes involved in FA metabolism, oxidation, storage, or uptake. Only Cidec gene expression was increased in group HFD compared to ND and decreased in supplemented mice, compared with HFD. Cidec is mainly expressed in adipose tissue but is also found in steatotic livers, where it is associated with lipid droplets (66). Cidec repression may therefore merely reflect the improvement of hepatic steatosis. This is supported by the fact that the expression of other genes associated with FA oxidation, metabolism, storage, and uptake were unaltered in supplemented mice. Similarly, AMPK activation was not different between groups and no significant effect of Totum-63 on inflammatory balance was observed (Supplemental Fig. S4). Taken together, these results suggest that the improvement of liver steatosis may be the consequence of reduced lipid flux entering the liver, rather than that of an action on lipid metabolism target genes. This assumption should however be taken with caution as our experimental setup might not have been optimal to highlight a potential effect on liver lipid metabolism. Indeed, the animals were fasted for 6 h before euthanasia and liver collection, making it likely for several of the active compounds in Totum-63 to be less present in the organisms and consequently less active on putative targets. Moreover, the fasting period itself is known to be a strong inhibitor of Srebp1-c gene expression and its target genes (67). Further experiments using different methodological conditions will be conducted to investigate the potential effects of Totum-63 on liver lipid metabolism.
Finally, the analyses of fecal microbiota suggest an improvement of HFD-induced microbial imbalance. Our results suggest that Totum-63 supplemented mice display an intermediate profile between groups HFD and ND, as shown by Shannon diversity index and PCoA clusters of relative abundances at phylum, family, and genus levels. At the phylum level, HFD caused an inversion of Bacteroidetes/Firmicutes ratio, compared to ND, consistent with previous work from other teams (68). This inversion was mainly explained at family level by lower relative abundance of Porphyromonadaceae (a Bacteroidetes family) to the benefit of Lachnospiraceae (a Firmicutes family). This dysbiosis was partially restored by Totum-63 supplementation, making it a putative contributor to the effects to prevent obesity and its associated diseases. Similarly, at the genus level, significant differences between HFD and HFD-T63 were found regarding the relative abundance of 10 bacteria. Interestingly, the relative abundance of Parasutterella and Barnesiella (two bacteria possibly linked with improvements in gut disorders associated with a high-fat diet) was increased in HFD-T63 group compared with HFD (69). Microbiota disturbances have recently been associated with obesity and development of insulin resistance (8) by exerting detrimental effects on metabolism via impaired production of short-chain fatty acid (SCFA) or development of metabolic endotoxemia (70). Metabolic endotoxemia is characterized by a low-grade inflammatory state that promotes insulin resistance in the tissues, due to increased absorption of bacterial fragments such as lipopolysaccharides (LPS) in the intestine (70). SCFA production results from hydrolysis and the fermentation of dietary polysaccharides by gut flora. They are thought to be able to enter the systemic circulation and induce benefits in insulin-sensitive organs, promoting systemic glucose control (71). Growing evidence seems to support that these mechanisms play a major role in the development of metabolic syndrome and other obesity-related complications (72–74), leading us to speculate that the improvement of microbial imbalance might have participated in the beneficial effects of Totum-63 on weight control and glucose homeostasis. However, it remains to be clarified whether these changes are a cause or a consequence of the restoration of a leaner phenotype.
Totum-63 was tested chronically in humans in an exploratory clinical trial, at two doses for 4 wk each. The product showed good tolerability: No adverse event related to Totum-63 administration was observed, and safety and vital parameters remained stable. Interestingly, the response to an oral carbohydrate tolerance test (glucose peak, insulin AUC and peak, insulin sensitivity index) was improved after 4 wk of supplementation (5 g·day-1), making Totum-63 a promising candidate for glucose management in humans. The results of this preliminary trial remain however to be confirmed in a double-blinded, randomized, placebo-controlled trial and should not be overinterpreted. Further clinical studies will assess the effects of the supplementation in target population (overweight, glucose-intolerant individuals).
In conclusion, these very first data obtained in mice show that Totum-63 supplementation led to improved body composition and glucose metabolism. We also report, in the translational study in humans, postprandial glucometabolic improvements, making Totum-63 a promising novel natural ingredient to prevent the progression of T2D epidemics and paving the way for phase II clinical investigations in target population.
GRANTS
The EE ANCOVA done for this work was provided by the NIDDK Mouse Metabolic Phenotyping Centers (MMPC, www.mmpc.org) using their Energy Expenditure Analysis page http://www.mmpc.org/shared/regression.aspx and supported by Grants DK076169 and DK115255.
DISCLOSURES
One or more authors are employed by Valbiotis. Valbiotis has designed and patented Totum-63. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
V.C., Y.F.O., F.L.J., M.B., C. Dubray, C. Dualé, N.M., T.M., N.B., M.C., S.L.P., and P.S. conceived and designed research; V.C., F.L.J., D.R., A.V., C.M., G.P., C. Dubray, C. Dualé, S.B., N.M., G.M., V.S., G.D., and F.D. performed experiments; V.C., F.L.J., M.B., A.V., C.M., G.P., C. Dubray, C. Dualé, N.M., and P.S. analyzed data; V.C., Y.F.O., M.B., C. Dubray, C. Dualé, G.P., N.M., B.G., M.C., S.L.P., and P.S. interpreted results of experiments; V.C., F.L.J., M.B., and D.R. prepared figures; V.C. drafted manuscript; V.C., Y.F.O., F.L.J., D.R., M.B., C.D., G.P., N.B., B.G., and P.S. edited and revised manuscript; V.C., Y.F.O., F.L.J., D.R., M.B., A.V., C.M., G.P., C. Dubray, C. Dualé, S.B., N.M., G.M., V.S., F.D., B.G., T.M., N.B., M.C., S.L.P., and P.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Mehdi Djelloul-Mazouz, Julien Hermet, and Philippe Denis from INRAE Unité de Nutrition Humaine (UNH, UMR 1019) and CRNH Auvergne (France) for the care and the attention to the animals. We are grateful to Isabelle Constant (INRAE, UMR 1213) for assistance in using the bomb calorimeter. We also thank Alice Besson, Allison Teixeira, Geoffrey Delcros, Lucas Bascoulergue, Lucy Garraud, and of course Monique Etienne for excellent technical skills while running the experiments. Many thanks to Odd-Erik Johansen (Nestlé Health Science) for scientific assistance and priceless advices in the process of writing and formatting this article. Finally, we thank people from the cellular images center (CICS, Clermont-Ferrand University Hospital) for the paraffine processing of our tissues and everyone at Biofortis (Merieux Nutrisciences, France) for the analysis of microbiome and help in interpreting the results.
REFERENCES
- 1.Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R; IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract 157: 107843–102019, 2019. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care 40: S11–S24, 2017.doi: 10.2337/dc17-S005. [DOI] [PubMed] [Google Scholar]
- 3.Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a high-risk state for diabetes development. Lancet 379: 2279–2290, 2012. doi: 10.1016/S0140-6736(12)60283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tuso P. Prediabetes and lifestyle modification: time to prevent a preventable disease. Perm J 18: 88–93, 2014. doi: 10.7812/TPP/14-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346: 393–403, 2002. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeFronzo RA. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 58: 773–795, 2009. doi: 10.2337/db09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tilg H, Moschen AR. Microbiota and diabetes: an evolving relationship. Gut 63: 1513–1521, 2014. doi: 10.1136/gutjnl-2014-306928. [DOI] [PubMed] [Google Scholar]
- 8.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin B, Ferrières J, Tanti J-F, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56: 1761–1772, 2007. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 9.Cho YK, Lee J, Kim HS, Park J-Y, Jung CH, Lee WJ. Clinical efficacy of quadruple oral therapy for type 2 diabetes in real-world practice: a retrospective observational study. Diabetes Ther 11: 2029–2039, 2020. doi: 10.1007/s13300-020-00881-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ku EJ, Lee D-H, Jeon HJ, Oh TK. Effectiveness and safety of empagliflozin-based quadruple therapy compared with insulin glargine-based therapy in patients with inadequately controlled type 2 diabetes: An observational study in clinical practice. Diabetes Obes Metab 21: 173–177, 2019. doi: 10.1111/dom.13476. [DOI] [PubMed] [Google Scholar]
- 11.Kim Y, Keogh JB, Clifton PM. Polyphenols and glycemic control. Nutrients 8: 17, 2016. doi: 10.3390/nu80100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villegas-Aguilar M del C, Fernández-Ochoa Á, Cádiz-Gurrea M de la L, Pimentel-Moral S, Lozano-Sánchez J, Arráez-Román D, Segura-Carretero A. Pleiotropic biological effects of dietary phenolic compounds and their metabolites on energy metabolism, inflammation and aging. Molecules 25: 596, 2020. doi: 10.3390/molecules25030596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graf BL, Raskin I, Cefalu WT, Ribnicky DM. Plant-derived therapeutics for the treatment of metabolic syndrome. Curr Opin Investig Drugs 11: 1107–1115, 2010. [PMC free article] [PubMed] [Google Scholar]
- 14.Hasler CM, Brown AC; American Dietetic Association. Position of the American Dietetic Association: functional foods. J Am Diet Assoc 109: 735–746, 2009. doi: 10.1016/j.jada.2009.02.023. [DOI] [PubMed] [Google Scholar]
- 15.Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen M-R, Tokgozoglu L, Wiklund O; ESC Scientific Document Group. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 41: 111–188, 2020. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 16.Arts ICW, Hollman PCH. Polyphenols and disease risk in epidemiologic studies. Am J Clin Nutr 81: 317S–325S, 2005. doi: 10.1093/ajcn/81.1.317S. [DOI] [PubMed] [Google Scholar]
- 17.Chiva-Blanch G, Badimon L. Effects of polyphenol intake on metabolic syndrome: current evidences from human trials. Oxid Med Cell Longev 2017: 5812401, 2017. doi: 10.1155/2017/5812401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hollman PCH, Geelen A, Kromhout D. Dietary flavonol intake may lower stroke risk in men and women. J Nutr 140: 600–604, 2010. doi: 10.3945/jn.109.116632. [DOI] [PubMed] [Google Scholar]
- 19.Weir JB, de V. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 109: 1–9, 1949. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moir L, Bentley L, Cox RD. Comprehensive energy balance measurements in mice. Curr Protoc Mouse Biol 6: 211–222, 2016. doi: 10.1002/cpmo.13. [DOI] [PubMed] [Google Scholar]
- 21.Ondov BD, Bergman NH, Phillippy AM. Interactive metagenomic visualization in a web browser. BMC Bioinformatics 12: 385, 2011. doi: 10.1186/1471-2105-12-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75: 7537–7541, 2009. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chao A. Nonparametric estimation of the number of classes in a population. Scand J Stat 11: 265–270, 1984. https://www.jstor.org/stable/4615964. [Google Scholar]
- 24.Shannon CE. A mathematical theory of communication. Bell Syst Tech J 27: 379–423, 1948. doi: 10.1002/j.1538-7305.1948.tb01338.x. [DOI] [Google Scholar]
- 25.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419, 1985. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 26.Ennequin G, Boisseau N, Caillaud K, Chavanelle V, Gerbaix M, Metz L, Etienne M, Walrand S, Masgrau A, Guillet C, Courteix D, Niu A, Li Y-P, Capel F, Sirvent P. Exercise training and return to a well-balanced diet activate the neuregulin 1/ErbB pathway in skeletal muscle of obese rats. J Physiol 593: 2665–2677, 2015. doi: 10.1113/JP270026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilda JE, Gomes AV. Western blotting using in-gel protein labeling as a normalization control: stain-free technology. Methods Mol Biol 1295: 381–391, 2015. doi: 10.1007/978-1-4939-2550-6_27. [DOI] [PubMed] [Google Scholar]
- 28.Guasch-Ferré M, Merino J, Sun Q, Fitó M, Salas-Salvadó J. Dietary polyphenols, mediterranean diet, prediabetes, and type 2 diabetes: a narrative review of the evidence. Oxid Med Cell Longev 2017: e6723931, 2017. doi: 10.1155/2017/6723931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bulotta S, Celano M, Lepore SM, Montalcini T, Pujia A, Russo D. Beneficial effects of the olive oil phenolic components oleuropein and hydroxytyrosol: focus on protection against cardiovascular and metabolic diseases. J Transl Med 12: 219, 2014. doi: 10.1186/s12967-014-0219-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahamad J, Toufeeq I, Khan MA, Ameen MSM, Anwer ET, Uthirapathy S, Mir SR, Ahmad J. Oleuropein: A natural antioxidant molecule in the treatment of metabolic syndrome. Phytother Res 33: 3112–3128, 2019. doi: 10.1002/ptr.6511. [DOI] [PubMed] [Google Scholar]
- 31.Tajik N, Tajik M, Mack I, Enck P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: a comprehensive review of the literature. Eur J Nutr 56: 2215–2244, 2017. doi: 10.1007/s00394-017-1379-1. [DOI] [PubMed] [Google Scholar]
- 32.Ambasta RK, Gupta R, Kumar D, Bhattacharya S, Sarkar A, Kumar P. Can luteolin be a therapeutic molecule for both colon cancer and diabetes? Brief Funct Genomics 18: 230–239, 2018. doi: 10.1093/bfgp/ely036. [DOI] [PubMed] [Google Scholar]
- 33.Dewanjee S, Chakraborty P, Mukherjee B, De FV. Plant-based antidiabetic nanoformulations: the emerging paradigm for effective therapy. Int J Mol Sci 21: 2217, 2020. doi: 10.3390/ijms21062217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pittalà V, Salerno L, Romeo G, Acquaviva R, Di Giacomo C, Sorrenti V. Therapeutic potential of caffeic acid phenethyl ester (cape) in diabetes. Curr Med Chem 25: 4827–4836, 2018. doi: 10.2174/0929867324666161118120908. [DOI] [PubMed] [Google Scholar]
- 35.Grobe JL. Comprehensive assessments of energy balance in mice. Methods Mol Biol 1614: 123–146, 2017. doi: 10.1007/978-1-4939-7030-8_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heck AM, Yanovski JA, Calis KA. Orlistat, a new lipase inhibitor for the management of obesity. Pharmacotherapy 20: 270–279, 2000. doi: 10.1592/phco.20.4.270.34882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kopelman P, Groot G. D H, Rissanen A, Rossner S, Toubro S, Palmer R, Hallam R, Bryson A, Hickling RI. Weight loss, hba1c reduction, and tolerability of cetilistat in a randomized, placebo-controlled phase 2 trial in obese diabetics: comparison with orlistat (xenical). Obesity (Silver Spring) 18: 108–115, 2010. doi: 10.1038/oby.2009.155. [DOI] [PubMed] [Google Scholar]
- 38.Padwal R. Cetilistat, a new lipase inhibitor for the treatment of obesity. Curr Opin Investig Drugs 9: 414–421, 2008. [PubMed] [Google Scholar]
- 39.Adisakwattana S, Ruengsamran T, Kampa P, Sompong W. In vitro inhibitory effects of plant-based foods and their combinations on intestinal α-glucosidase and pancreatic α-amylase. BMC Complement Altern Med 12: 110, 2012. doi: 10.1186/1472-6882-12-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ben Salem M, Ben Abdallah Kolsi R, Dhouibi R, Ksouda K, Charfi S, Yaich M, Hammami S, Sahnoun Z, Zeghal KM, Jamoussi K, Affes H. Protective effects of Cynara scolymus leaves extract on metabolic disorders and oxidative stress in alloxan-diabetic rats. BMC Complement Altern Med 17: 328, 2017. doi: 10.1186/s12906-017-1835-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Komaki E, Yamaguchi S, Maru I, Kinoshita M, Kakehi K, Ohta Y, Tsukada Y. Identification of anti-α-amylase components from olive leaf extracts. Food Sci Technol Res 9: 35–39, 2003. doi: 10.3136/fstr.9.35. [DOI] [Google Scholar]
- 42.McDougall GJ, Kulkarni NN, Stewart D. Current developments on the inhibitory effects of berry polyphenols on digestive enzymes. Biofactors 34: 73–80, 2008. doi: 10.1002/biof.5520340108. [DOI] [PubMed] [Google Scholar]
- 43.Li J, Wu T, Li N, Wang X, Chen G, Lyu X. Bilberry anthocyanin extract promotes intestinal barrier function and inhibits digestive enzyme activity by regulating the gut microbiota in aging rats. Food Funct 10: 333–343, 2019. doi: 10.1039/c8fo01962b. [DOI] [PubMed] [Google Scholar]
- 44.Zheng Y, Yang W, Sun W, Chen S, Liu D, Kong X, Tian J, Ye X. Inhibition of porcine pancreatic α-amylase activity by chlorogenic acid. J Funct Food 64: 103587, 2020. doi: 10.1016/j.jff.2019.103587. [DOI] [Google Scholar]
- 45.Nyambe-Silavwe H, Williamson G. Chlorogenic and phenolic acids are only very weak inhibitors of human salivary α-amylase and rat intestinal maltase activities. Food Res Int 113: 452–455, 2018. doi: 10.1016/j.foodres.2018.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Polzonetti V, Natalini P, Vincenzetti S, Vita A, Pucciarelli S. Chapter 148—Modulatory effect of oleuropein on digestive enzymes. In: Olives and Olive Oil in Health and Disease Prevention, edited by Preedy VR, Watson RR.. San Diego, CA: Academic Press, p. 1327. –1333. doi: 10.1016/B978-0-12-374420-3.00148-0. [DOI] [Google Scholar]
- 47.Hadrich F, Bouallagui Z, Junkyu H, Isoda H, Sayadi S. The α-glucosidase and α-amylase enzyme inhibitory of hydroxytyrosol and oleuropein. J Oleo Sci 64: 835–843, 2015. doi: 10.5650/jos.ess15026. [DOI] [PubMed] [Google Scholar]
- 48.Kim J-S, Kwon C, Son K. Inhibition of alpha-glucosidase and amylase by luteolin, a flavonoid. Biosci Biotechnol Biochem 64: 2458–2461, 2000. doi: 10.1271/bbb.64.2458. [DOI] [PubMed] [Google Scholar]
- 49.Han X, Zhang Y, Guo J, You Y, Zhan J, Huang W. Chlorogenic acid stimulates the thermogenesis of brown adipocytes by promoting the uptake of glucose and the function of mitochondria. J Food Sci 84: 3815–3824, 2019. doi: 10.1111/1750-3841.14838. [DOI] [PubMed] [Google Scholar]
- 50.Hao J, Shen W, Yu G, Jia H, Li X, Feng Z, Wang Y, Weber P, Wertz K, Sharman E, Liu J. Hydroxytyrosol promotes mitochondrial biogenesis and mitochondrial function in 3T3-L1 adipocytes. J Nutr Biochem 21: 634–644, 2010. doi: 10.1016/j.jnutbio.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 51.Oi-Kano Y, Kawada T, Watanabe T, Koyama F, Watanabe K, Senbongi R, Iwai K. Oleuropein, a phenolic compound in extra virgin olive oil, increases uncoupling protein 1 content in brown adipose tissue and enhances noradrenaline and adrenaline secretions in rats. J Nutr Sci Vitaminol (Tokyo) 54: 363–370, 2008. doi: 10.3177/jnsv.54.363. [DOI] [PubMed] [Google Scholar]
- 52.Riedl RA, Atkinson SN, Burnett CML, Grobe JL, Kirby JR. The gut microbiome, energy homeostasis, and implications for hypertension. Curr Hypertens Rep 19: 27, 2017. doi: 10.1007/s11906-017-0721-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Even PC, Nadkarni NA. Indirect calorimetry in laboratory mice and rats: principles, practical considerations, interpretation and perspectives. Am J Physiol Regul Integr Comp Physiol 303: R459–R476, 2012. [Erratum in Am J Physiol Regul Integr Comp Physiol 309: R1460, 2015] doi: 10.1152/ajpregu.00137.2012. [DOI] [PubMed] [Google Scholar]
- 54.Kus V, Flachs P, Kuda O, Bardova K, Janovska P, Svobodova M, Jilkova ZM, Rossmeisl M, Wang-Sattler R, Yu Z, Illig T, Kopecky J. Unmasking differential effects of rosiglitazone and pioglitazone in the combination treatment with n-3 fatty acids in mice fed a high-fat diet. PLoS One 6: e27126, 2011. doi: 10.1371/journal.pone.0027126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rossmeisl M, Medrikova D, van Schothorst EM, Pavlisova J, Kuda O, Hensler M, Bardova K, Flachs P, Stankova B, Vecka M, Tvrzicka E, Zak A, Keijer J, Kopecky J. Omega-3 phospholipids from fish suppress hepatic steatosis by integrated inhibition of biosynthetic pathways in dietary obese mice. Biochim Biophys Acta 1841: 267–278, 2014. doi: 10.1016/j.bbalip.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 56.Sharma N, Siriesha Lugani Y, Kaur A, Ahuja VK. Effect of metformin on insulin levels, blood sugar, and body mass index in polycystic ovarian syndrome cases. J Family Med Prim Care 8: 2691–2695, 2019. doi: 10.4103/jfmpc.jfmpc_490_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 30: 1000–1007, 1981. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- 58.Thiebaud D, Jacot E, DeFronzo RA, Maeder E, Jequier E, Felber JP. The effect of graded doses of insulin on total glucose uptake, glucose oxidation, and glucose storage in man. Diabetes 31: 957–963, 1982. doi: 10.2337/diacare.31.11.957. [DOI] [PubMed] [Google Scholar]
- 59.Smith U, Kahn BB. Adipose tissue regulates insulin sensitivity: role of adipogenesis, de novo lipogenesis and novel lipids. J Intern Med 280: 465–475, 2016. doi: 10.1111/joim.12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kraemer FB, Shen W-J. Hormone-sensitive lipase control of intracellular tri-(di-)acylglycerol and cholesteryl ester hydrolysis. J Lipid Res 43: 1585–1594, 2002. doi: 10.1194/jlr.r200009-jlr200. [DOI] [PubMed] [Google Scholar]
- 61.Nachar A, Eid HM, Vinqvist-Tymchuk M, Vuong T, Kalt W, Matar C, Haddad PS. Phenolic compounds isolated from fermented blueberry juice decrease hepatocellular glucose output and enhance muscle glucose uptake in cultured murine and human cells. BMC Complement Altern Med 17: 138, 2017. doi: 10.1186/s12906-017-1650-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Torabi S, DiMarco NM. Original research: polyphenols extracted from grape powder induce lipogenesis and glucose uptake during differentiation of murine preadipocytes. Exp Biol Med (Maywood) 241: 1776–1785, 2016. doi: 10.1177/1535370216645213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perry RJ, Samuel VT, Petersen KF, Shulman GI. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature 510: 84–91, 2014. doi: 10.1038/nature13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li S, Tan HY, Wang N, Cheung F, Hong M, Feng Y. The potential and action mechanism of polyphenols in the treatment of liver diseases. Oxid Med Cell Longev 2018: 8394818, 2018. doi: 10.1155/2018/8394818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rodriguez-Ramiro I, Vauzour D, Minihane AM. Polyphenols and non-alcoholic fatty liver disease: impact and mechanisms. Proc Nutr Soc 75: 47–60, 2016. doi: 10.1017/S0029665115004218. [DOI] [PubMed] [Google Scholar]
- 66.Langhi C, Baldán Á. CIDEC/Fsp27 is regulated by PPARα and plays a critical role in fasting- and diet-induced hepatosteatosis. Hepatology 61: 1227–1238, 2015. doi: 10.1002/hep.27607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Horton JD, Bashmakov Y, Shimomura I, Shimano H. Regulation of sterol regulatory element binding proteins in livers of fasted and refed mice. Proc Natl Acad Sci USA 95: 5987–5992, 1998. doi: 10.1073/pnas.95.11.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Clarke SF, Murphy EF, Nilaweera K, Ross PR, Shanahan F, O'Toole PW, Cotter PD. The gut microbiota and its relationship to diet and obesity. Gut Microbes 3: 186–202, 2012. doi: 10.4161/gmic.20168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lacroix S, Pechereau F, Leblanc N, Boubertakh B, Houde A, Martin C, Flamand N, Silvestri C, Raymond F, Marzo VD, Veilleux A. Rapid and concomitant gut microbiota and endocannabinoidome response to diet-induced obesity in mice. mSystems 4: e00407–e00419, 2019. doi: 10.1128/mSystems.00407-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Festi D, Schiumerini R, Eusebi LH, Marasco G, Taddia M, Colecchia A. Gut microbiota and metabolic syndrome. World J Gastroenterol 20: 16079–16094, 2014. doi: 10.3748/wjg.v20.i43.16079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol 11: 577–591, 2015. doi: 10.1038/nrendo.2015.128. [DOI] [PubMed] [Google Scholar]
- 72.Boulangé CL, Neves AL, Chilloux J, Nicholson JK, Dumas M-E. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med 8: 42, 2016. doi: 10.1186/s13073-016-0303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boutagy NE, McMillan RP, Frisard MI, Hulver MW. Metabolic endotoxemia with obesity: is it real and is it relevant? Biochimie 124: 11–20, 2016. doi: 10.1016/j.biochi.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Burcelin R. Gut microbiota and immune crosstalk in metabolic disease. Biol Aujourdhui 211: 1–18, 2017. doi: 10.1051/jbio/2017008. [DOI] [PubMed] [Google Scholar]