Significance
Predicting the effects of anthropogenic nutrient enrichment on plant communities is critical for managing implications for biodiversity and ecosystem services. Plant functional types that fix atmospheric nitrogen (e.g., legumes) may be at particular risk of nutrient-driven global decline, yet global-scale evidence is lacking. Using an experiment in 45 grasslands across six continents, we showed that legume cover, richness, and biomass declined substantially with nitrogen additions. Although legumes benefited from phosphorus, potassium, and other nutrients, these nutrients did not ameliorate nitrogen-induced legume decline. Given global trends in anthropogenic nutrient enrichment, our results indicate the potential for global decline in grassland legumes, with likely consequences for biodiversity, food webs, soil health, and genetic improvement of protein-rich plant species for food production.
Keywords: eutrophication, N deposition, legumes, Fabaceae, Nutrient Network
Abstract
Anthropogenic nutrient enrichment is driving global biodiversity decline and modifying ecosystem functions. Theory suggests that plant functional types that fix atmospheric nitrogen have a competitive advantage in nitrogen-poor soils, but lose this advantage with increasing nitrogen supply. By contrast, the addition of phosphorus, potassium, and other nutrients may benefit such species in low-nutrient environments by enhancing their nitrogen-fixing capacity. We present a global-scale experiment confirming these predictions for nitrogen-fixing legumes (Fabaceae) across 45 grasslands on six continents. Nitrogen addition reduced legume cover, richness, and biomass, particularly in nitrogen-poor soils, while cover of non–nitrogen-fixing plants increased. The addition of phosphorous, potassium, and other nutrients enhanced legume abundance, but did not mitigate the negative effects of nitrogen addition. Increasing nitrogen supply thus has the potential to decrease the diversity and abundance of grassland legumes worldwide regardless of the availability of other nutrients, with consequences for biodiversity, food webs, ecosystem resilience, and genetic improvement of protein-rich agricultural plant species.
Anthropogenic enrichment of nitrogen (N), phosphorus (P), and other nutrients from fertilizers and fossil fuel combustion is transforming natural ecosystems worldwide (1–5), leading to increased terrestrial plant productivity (6, 7) and loss of biodiversity (8, 9). Resource competition theory proposes that the capacity of species to persist at low levels of a limiting resource is a key mechanism underpinning competitive success. Consequently, plant functional types with specialized nutrient acquisition strategies are expected to have a competitive advantage in nutrient-limited environments but also to be especially vulnerable to nutrient enrichment (10–13).
Legumes (Fabaceae) are one of the largest families of flowering plants, contributing over 650 genera and 19,000 taxa to global plant diversity (14). This diversity is important for biodiversity conservation and for genetic improvement of protein-rich crops and forage species for sustainable livestock production (15–17). Furthermore, the ability to fix atmospheric N2 is one of the most important plant functional traits for influencing ecosystem processes, conferring N-fixing legumes with a disproportionately important role in ecosystem functioning (18, 19). For example, litter produced by legumes is nitrogen-rich and more easily decomposed by soil microorganisms, leading to flow on effects to higher trophic levels, including increased complexity of food webs and resistance of soil biophysical and chemical properties to ecosystem disturbance (20). As the success of legumes often arises from this capacity for symbiotic fixation of atmospheric N2 in N-limited environments (21, 22), atmospheric N-deposition and other pathways of anthropogenic N supply are expected to drastically reduce their competitive advantage in plant communities (1, 5, 11, 23). This is especially the case for obligate-N-fixers that cannot down-regulate N-fixation (24, 25) and hence at higher soil N are disadvantaged by the high energetic cost of N-fixation (26).
While concerns about global nutrient enrichment are focused on impacts of N on biodiversity and ecosystem productivity (1, 2, 27), changes in P and potassium (K) cycles (3, 4) or altered concentrations of other nutrients, can also influence the abundance and diversity of legumes in accordance with resource competition theory (10–13). Owing to the physiological demands of N-fixation, N-fixing legumes often have higher requirements for P, K, and other nutrients [e.g., molybdenum (Mo), iron (Fe), and calcium (Ca)] than non–N-fixing plants (28–31), and increases in these nutrients can favor N-fixing over non–N-fixing species, particularly in nutrient poor soils (21, 22). However, added nutrients may have synergistic effects (6, 32), leading to uncertainties in the expected net effect of P addition on the abundance of N-fixing legumes (26). For example, the phosphatases required for P acquisition from soils are rich in N; N addition may increase phosphatase investment, conferring legumes a superior phosphorus acquisition capacity in P- and N-limited environments (25, 29). Conversely, multiple nutrient addition is expected to allow nonlegumes to compete more effectively with legume species. Resulting light limitation may suppress legume growth and reduce the survival and establishment of new legume individuals (8, 9), especially of those legumes that are unable to reduce the costs of N fixation through down-regulation (10, 11, 15, 33–35).
Despite these theoretical predictions, empirical evidence for the individual and interactive effects of changes in nutrient availability on legumes in natural ecosystems is limited (29, 36–39). Some experimental studies have shown decreased legume abundance with N addition and increased with P addition, but these studies are typically conducted at a single site and show both positive and negative interactive effects among nutrients (e.g., refs. 37, 40, and 41). Furthermore, minimal evidence is available regarding the influence of K or micronutrient enrichment on legume responses (29), and the underlying mechanisms of legume responses to nutrient addition, such as soil and climatic conditions, have not been investigated at global scales (but see ref. 26 for forest ecosystems).
Using data from the Nutrient Network global collaborative experiment [https://nutnet.org/ (42)], we measured the cover, richness, and biomass responses of N-fixing legumes (hereafter legumes) to standardized experimental nutrient additions in 45 grasslands across six continents (SI Appendix, Fig. S1 and Table S1). Grasslands are a globally significant biome, covering more than one-third of the Earth’s ice-free land surface, accounting for a third of terrestrial net primary production (43), and supporting the livelihoods of more than 1.3 billion people. They are subject to chronic atmospheric nitrogen deposition due to fossil fuel combustion and are likely candidates for direct nitrogen fertilization (44). While N emissions in many regions of Europe have declined leading to plateaus or reductions in deposition (45), deposition in other world grasslands, such as the Mongolian Steppe, have increased in recent decades (e.g., ref. 46). Experimental sites included temperate and anthropic grasslands that spanned a broad range of geographical locations and ecological conditions, although were mostly from temperate latitudes (39) (SI Appendix, Table S1 and Fig. S1; see Methods for details).
Three nutrients (N, P, K+) were applied in factorial combinations, resulting in eight treatments enabling evaluation of the interactive effects of N, P, and K addition (6, 8) on legumes. Over 3 to 6 y, 10 g⋅m−2 N, P, and K were added annually to their respective treatment plots at the beginning of each site’s growing season; other nutrients in the K+ treatment [sulfur (S), magnesium (Mg), and micronutrients] were applied only in the first year to avoid toxicity (42). These nutrient levels were selected to ensure they were high enough to reduce nutrient limitation at a wide diversity of sites. They are at the higher end of the range for agricultural fertilizer application rates globally (5), and higher than atmospheric nutrient deposition rates (1, 3, 41, 43). In particular, our N-addition rate was about three times maximum current N-deposition rates in European grasslands and more generally across the globe (1, 47, 48).
We used a standardized protocol (6, 42) to annually measure cover, richness, and biomass of legumes, forbs, and grasses in 1-m2 permanent plots (Methods), starting in the year prior to the first nutrient application (Yinitial). Across all years and sites, we recorded 170 species of N-fixing grassland legumes, comprising 50 genera (SI Appendix, Table S2). The most species-rich genera were Trifolium (25 spp.), Astragalus (12 spp.), Vicia (11 spp.), and Lupinus (11 spp.). Vicia sativa, Trifolium repens, and Vicia hirsuta were the most frequent species across our sites (9.1%, 5.1%, and 4.9% of total occurrences, respectively). Each site contained one to eight legume species (Methods and SI Appendix, Table S1). Most legume species were perennials (∼60%), including 10 woody or shrub species (∼6% of species). On average, ∼3% and 4% of total live cover comprised annual and perennial legumes, respectively.
We present results of nutrient addition for the third and the last available sampling year (years 3 to 6) after starting nutrient application in each site [noting sites started applying experimental treatments in different calendar years and ran for different lengths of time (SI Appendix, Table S1)]. To measure the relative impact of N, P, and K+ addition on legumes, we calculated the log ratio (LR) of legume abundance and richness in the third or last year in each plot versus the initial (pretreatment) value [LR = ln (Yfinal/Yinitial)]. We used the pretreatment legume abundance in the LR instead of control plots (49) to control for initial legume abundance and spatial variability among plots (8, 50). We also calculated measures of legume colonization and extinction in each plot, and evaluated the effect of initial soil nutrient concentrations, community structure, and climatic conditions as contingencies for nutrient addition effects (see Methods for details). We analyzed the data using linear mixed-effects models (51–53), with nutrient treatments (i.e., N, P, K+, and their interactions) as fixed effects, and blocks nested within sites as random effects. Confidence intervals for model parameters were bootstrapped as a conservative method for hypothesis testing (51, 52) (see Methods for details).
Results and Discussion
Effects of Nutrient Addition on Legumes.
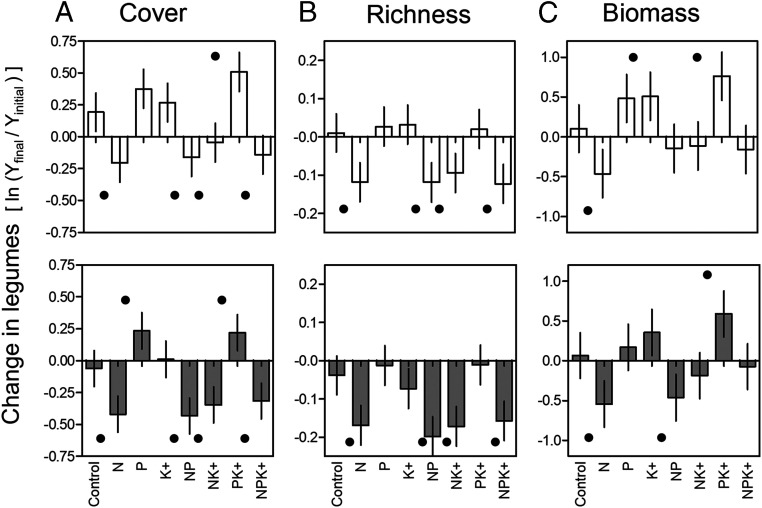
We expected N addition to reduce the competitive advantage of legumes, resulting in a decline in legume relative abundance and richness (11–13, 23, 30, 33, 54). This prediction was strongly supported (Fig. 1 and SI Appendix, Figs. S2 and S3 and Tables S3–S5), with an average 32% decline in legume cover (LRN = −0.397) after only 3 y of chronic N addition (SI Appendix, Tables S3 and S4). Nitrogen addition did not significantly reduce nonleguminous forb cover (LRN = −0.086; SI Appendix, Table S6A), and increased grass cover (LRN = 0.129; SI Appendix, Table S6B), showing that the negative effect of N addition was specific to legumes. Nitrogen also significantly reduced legume species richness by 12% and biomass by 43% (LRN = −0.129 and LRN= −0.569, respectively; Fig. 1 and SI Appendix, Figs. S3 and S4 and Tables S3–S5).
Fig. 1.
Change in legume cover (A), richness (B), and biomass (C) for the third year (top row) and last (third to sixth) year after initiation of the experiment (bottom row). Changes were expressed as response ratios, the natural logarithm of the relative change from initial values (Methods); positive and negative values indicate increases and decreases, respectively. Bars represent means ± SEMs, and dots (•) indicate treatment means that were statistically different from the controls. No response ratio in control plots were statistically different from zero, indicating that controls remained the same on average over time. Note the different y-axis ranges. Cover and richness data were available for 45 sites and biomass data for 26 sites.
The clear declines in legume cover, biomass, and richness with N addition occurred despite the potential for some N-fixing legumes to down-regulate N fixation (24), further supporting our predictions. Down-regulation is proposed to underpin the “tropical paradox,” enabling high species richness and abundance of legumes in late successional tropical rainforests with high soil N (25). Our result indicates that in temperate grasslands around the world, 1) the high cost of fixation in obligate N-fixing legumes, and/or 2) inferior competitive growth strategies in facultative N-fixing legumes compared with other herbaceous species, outweighed any potential amelioration of these costs through down-regulation of N fixation. Nevertheless, it is possible that down-regulation reduced the magnitude of the negative N-addition effect. This contrasting result in grasslands compared with tropical forests highlights a need for further evaluation of the importance of down-regulation in grasslands, and potential drivers of differences from tropical forests. For example, vertebrate herbivores, nutrient leaching, or grass–legume dynamics, could maintain ongoing or fluctuating N deficits in grasslands, that reduce evolutionary pressures for legumes to down-regulate (refs. 55 and 56; see ref. 57 for evolutionary persistence of fixation). Further investigation could be achieved by directly assessing nodulation, N fixation, and rhizobial biomass in experimental plots (35), and more broadly by better global documentation of which legume species are facultative and which are obligate N fixers.
We predicted that P and K+ addition would increase legume abundance, particularly in the absence of N addition (26, 28, 30, 31, 37, 39, 58), despite the potential for concurrent increases in nonlegume competitors. Accordingly, we found that P addition (without N) significantly increased the cover of legumes by an average of 34% by the last experimental year (the average 20% increase after 3 y was not significant; Fig. 1). By contrast, K+ addition alone did not significantly increase legume cover (Fig. 1 and SI Appendix, Tables S3 and S4 and Figs. S2–S4).
Together, P and K+ addition increased the cover of legumes by an average of 37% after 3 y (32% by the last experimental year; Fig. 1 and SI Appendix, Tables S3–S5), even though P also enhanced grass cover (but not non–N-fixing forbs, LRP grass = 0.13; SI Appendix, Table S6). The increase in legume cover with P and K+ was additive [P × K interaction and LR(K + P) vs. KP contrast included zero; SI Appendix, Fig. S2 and Tables S3–S5], suggesting that in the absence of N addition, K and/or other added nutrients became limiting to N-fixers when P requirements were met (29, 59).
A similar pattern was evident for legume biomass, with P and K+ addition on average across nutrient treatments leading to significant increases in legume biomass after 3 y (25%, LRP = 0.22 and 29%, LRK = 0.25 respectively; SI Appendix, Table S5), although the effect of P was not significant for the final year analysis (43% for K+ addition; LRK = 0.36; Fig. 1 and SI Appendix, Table S5). We did not detect significant changes in legume richness due to P or K+ addition (Fig. 1 and SI Appendix, Tables S3–S5).
Given the opposing effects of N versus P or K+ addition on legumes, effects of adding N in combination with P or K+ are difficult to predict, and empirical evidence is conflicting (10, 21, 22, 26, 36, 37). We hypothesized that legumes would decrease with high levels of N addition even with concurrent addition of P and K+, because the P and K+ required for N fixation confers less advantage to legumes when N is not limiting (26, 28, 37). Consistent with this hypothesis, we found that, despite the benefits of P addition, its combination with N resulted in a net reduction of legume cover and biomass that was nearly as large as the effect of N addition alone (LRNxP = −0.24, Fig. 1 and SI Appendix, Figs. S2 and S3 and Tables S3–S5). This indicates that the benefits of P were largely overridden by the suppressive effects of N (although this effect was significant only for legume cover). Similarly, statistical contrasts indicated that N addition obscured the positive effects of combined P and K+ addition on legumes (contrast 6 to 7 in SI Appendix, Fig. S4 and Table S5), again as would be expected where N-fixing capacity is no longer advantageous (29) (see also ref. 6). While N, P, and K+ have previously been recognized as influencing the abundance of N-fixing legumes (10, 11, 37, 60), our study describes N, P and K+ interactions in grassland ecosystems at a global scale.
Mechanisms Underpinning Legume Responses.
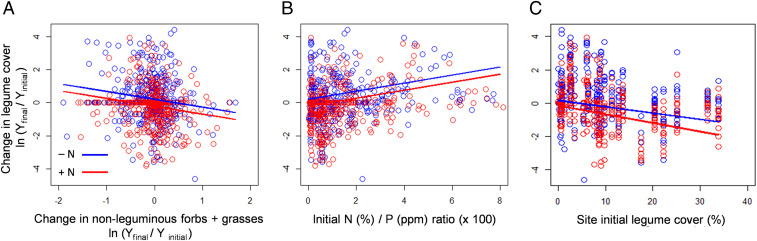
We expected N addition to suppress legumes by reducing their competitive advantage over co-occurring, non–N-fixing forbs and grasses (10–13, 33, 37). Supporting this prediction, plot-scale mixed-effects models showed that cover of nonlegume forbs plus grasses significantly increased with N addition (see above; SI Appendix, Table S6C), and this increase was significantly associated with the reduction in legume cover (Fig. 2A and SI Appendix, Table S7). Furthermore, through increases in cover and biomass (6, 8, 9), N addition reduced photosynthetically active radiation (PAR) at the soil surface (SI Appendix, Fig. S5 and Table S8), consistent with previous evidence that light reduction impairs legume performance (35, 55) (see also ref. 9). Taken together, these results are consistent with previous evidence (10, 11, 37, 60) suggesting nutrient-related increases in nonlegume forbs and grasses may affect legumes through increased competition, particularly for light.
Fig. 2.
Community drivers of the change in legume cover after three years of N addition. The relative change in legume cover (natural log of response ratio) was explained by nitrogen addition (red lines versus blue lines for plots without N) and by the relative change in cover of nonleguminous forbs + grasses (LRF+G) (A), the initial soil N:P ratio (B), and the initial cover of legumes of the site (C). Nitrogen (A and B) and N addition × legume cover (C) persisted as significant drivers (P < 0.05) after stepwise model reduction. Blue symbols indicate plots without N addition, and red symbols indicate plots with N addition. Results for the last year at all sites were qualitatively similar (SI Appendix, Tables S7 and S10–S12).
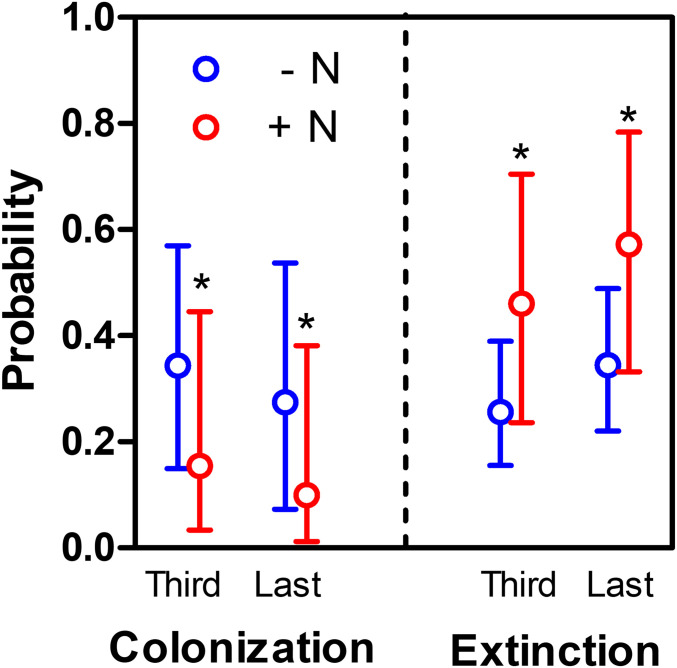
We also asked whether the strong negative effect of N on legumes arose through a reduction in legume establishment (e.g., due to diminished seed accumulation and/or microsite availability), or reduction in growth and survival of mature individuals (61, 62). We found evidence for both mechanisms: By the third year, N addition had significantly reduced legume species colonization rates from 34 to 15% and increased local legume species extinction from 25 to 46% (Fig. 3 and SI Appendix, Table S9). The proportion of annual richness was only slightly higher for legumes (mean = 0.46 ± 0.39), than forbs (0.39 ± 0.26) or grasses (0.36 ± 0.25), suggesting that a higher proportion of species with annual life history seems unlikely to solely explain observed negative N-addition impact on legumes. Indeed, the trends were consistent across both perennial and annual species of legume: Extinction of perennial legume species increased from 21 to 35%, and of annual legume species from 45 to 72%. Similarly, colonization of perennial legume species decreased from 5.5 to 2.7% and from 1 to <1% for annuals (SI Appendix, Table S9).
Fig. 3.
Colonization and extinction probabilities for legume species in plots without (blue symbols) and with (red symbols) N addition for the third and last experimental year. Symbols are estimates ± bootstrapped 95% confidence intervals. Asterisks indicate that the logit of the confidence interval for the N effect did not include zero in the reduced model (SI Appendix, Table S9). Colonization was assessed in plots where legumes were initially absent but later present, and extinction in plots where legumes were initially present but disappeared by the third or last year of the experiment. Estimates are based on 468 to 726 plots in 32–42 sites. See SI Appendix, Table S9 for further details and for colonization and extinction of annual and perennial legume species.
Potential Contingencies Influencing Nutrient Responses.
We expected legume responses to nutrient addition to vary among sites because of differing background rates of edaphic resource availability (10, 12, 13). With respect to N addition, we predicted greater legume decline in soils that were initially more favorable for legumes, including soils with lower initial N (11, 13, 55) or with higher P, K, or micronutrient concentrations (26, 38, 39, 55, 58). We predicted this because 1) potential for loss is limited if initial legume abundance is low, 2) nonfixing competitors would be more responsive to N addition at low N, resulting in greater rate of increase in competition for light (6), and 3) sites with initially higher N (or lower K or micronutrients) may already support a higher proportion of more competitive, facultative legumes that are less likely to decline with N addition (24, 25).
Analysis of site-scale data indeed showed that N addition led to greater legume decline at sites with lower initial soil N (SI Appendix, Fig. S6 and Table S10A), lower N:Pratio (Fig. 2B and SI Appendix, Table S7), or higher initial soil Fe (an element that is particularly critical for the legume symbiosis) (39, 58, 59, 63) (SI Appendix, Fig. S6 and Table S10A), supporting our hypothesis that N addition has the greatest impact on legumes in soils more favorable for legumes (64). We did not detect a significant relationship between initial soil N or Fe and initial legume abundance, inconsistent with our first hypothesized mechanism (1) for this response, i.e., that it resulted from initially low legume abundance in high N or low Fe sites. Our second hypothesized mechanism (2) was supported, with significantly greater increases in nonfixing competitors with N addition at lower initial soil N [N addition: 0.09 (0.05;0.13); Soil N × N addition: −0.15 (−0.25; −0.03)]. We could not test our third hypothesized mechanism (3), that greater legume decline at sites with lower initial soil N was explained by a lower proportion of more competitive, facultative legumes in sites with initially low soil N or Fe, because we lack data on nodulation and fixation rates (35, 36). Further investigation is thus needed to test whether facultative legumes increase in or colonize N-enriched sites that were initially low in N (24, 25).
Unexpectedly, N addition was more detrimental to legume cover in sites with lower initial soil K (i.e., in soils we expected to be already limiting for N fixation; SI Appendix, Table S10A). The reason for this is unclear but could involve correlations of soil K with other soil variables that impact legume cover (e.g., soil pH, r = 0.64, SI Appendix, Fig. S7). We detected no significant effects of initial soil P on legume response to N addition.
With respect to addition of P or K+, we expected a greater benefit to legumes when initial soil concentrations of P or K+ were lower (suggesting potentially greater P or K+ limitation), and when initial N was lower, suggesting greater activation of N fixation by legumes (21). We did not detect these effects directly (SI Appendix, Tables S11 and S12), but we did detect an increasing benefit of K+ addition with increasing initial soil P and decreasing initial soil K+ (SI Appendix, Table S12), i.e., in conditions that are otherwise more favorable for N fixation. This trend is consistent with our experimental result that legume response to addition of K+ was evident when combined with P addition.
Other potential contingencies influencing legume responses to nutrient addition include climate and initial community composition factors. We expected legume responses to nutrient addition to be stronger in mesic environments where moisture limitation does not constrain the benefits of N-rich leaves for photosynthesis (21) (but see also refs. 65–67), and in warmer environments with lower energetic constraints on N-fixation (26, 66). We did not detect any significant effects of mean annual precipitation but found that temperature significantly predicted N (although not P or K+) effects on legumes (SI Appendix, Tables S10–S12). In particular, N addition was more detrimental to legumes at sites with higher mean annual temperatures (SI Appendix, Table S10), providing further support for the hypothesis that N addition is more detrimental in environments more favorable for N-fixation (26, 55).
With respect to initial community composition, we predicted that legume response to nutrient addition may be constrained by 1) the ambient site-level cover and richness of legumes, and 2) availability of N-responsive, nonlegume competitors such as many nonnative grassland species (68, 69). Consistent with our first prediction (1), plot and site-scale regressions indicated that N addition was more detrimental to legumes at sites with higher initial legume cover and richness (Fig. 2C and SI Appendix, Table S9B), likely due to density dependent limits to decline at low initial cover values (11). For unknown reasons, control plots also declined in proportional legume cover over the experimental period, especially in sites with initially higher legume cover (Fig. 2C), but the decrease was 30% greater with N addition (SI Appendix, Table S7). Consistent with our second prediction (2), N addition was more detrimental to legumes in sites with higher initial nonnative plant species cover, potentially indicating greater scope for suppression by N-responsive competitors (68, 69). Our assessment of effects of N, P, and K+ addition across 45 grasslands thus emphasizes the importance of soil nutrients and plant community composition in combination with macroclimatic conditions as predictors of legume responses.
Conclusions
Our data from 45 standardized experiments in grasslands from six continents provide broad-scale evidence that the individual and interactive effects of nutrient addition on legumes correspond with predictions from resource competition theory (10, 26, 54). First, grassland legumes declined substantially with N addition at 10 g N⋅m−2⋅y−1 over at least 3 y. This finding demonstrates the generality of this response in temperate grasslands, which until now has only been documented in a range of isolated experiments and model predictions (11, 37, 40, 70). The reduction likely resulted from reduced competitive advantage of legumes over non–N-fixing plants, potentially mediated by light availability. Second, grassland legumes benefited from P and K+ addition in the absence of N addition, generalizing emerging localized evidence to global scales (29, 37, 41). Third, addition of P and/or K+ at rates exceeding current global deposition rates did little to ameliorate N-induced legume decline, providing global support for overriding impacts of high levels of N enrichment (11, 37). These results complement and support broader conclusions regarding impacts of N and P enrichment on grasslands, including increased grassland productivity (6, 71), reduced plant richness (8, 9), and changes in the spectrum of plant functional traits (72).
Anthropogenic activities are increasing N supplies through atmospheric deposition and direct fertilization, thereby transforming ecosystems across the globe (1–5). While further work is needed to investigate the role of elemental supply rates on changes in obligate and facultative legume relative abundance (24, 25, 35), our results highlight the potential for substantial impact of anthropogenic N enrichment on the abundance and diversity of plants with specialized N-acquisition strategies. In the context of atmospheric N deposition, we acknowledge our experiment applied N at approximately three times estimated annual maximum global N-deposition rates (1, 47), so likely overestimates the effects of N deposition over our experimental timeframe. On the other hand, global N deposition is expected to occur over decades to centuries, while our results were observed after only 3 to 6 y of nutrient addition. Earlier work (73) provides evidence for ongoing loss of legumes with long-term N addition across a wide a range of rates (0 to 20 g⋅m−2⋅y−1), so it is conceivable that our N-addition treatments could elicit broadly comparable responses to the cumulative effects of global N deposition. Nevertheless, further work is needed to understand implications of interactions between rates and duration of nutrient applications to better predict impacts of atmospheric N deposition.
Finally, our results suggest that legume decline is likely in the face of significant N enrichment regardless of changes in global P or K cycles. Owing to their disproportionate effects on ecosystem functions, widespread decline in grassland legumes is likely not only to contribute directly to global loss of plant diversity, but to affect nutritional quality for other trophic levels (19, 74), and to reduce food web complexity and ecosystem resilience (18–20). Furthermore, loss of legume genetic resources will reduce the potential for new or genetically improved protein-rich crop and pasture species important for food production (15–17). Reduced N fixation through loss of legumes could result in quantifiable impacts on the global N cycle that could potentially mitigate anthropogenic N deposition. However, even if N2 fixation ceased in all grasslands globally, this would represent only 5 to 10% of anthropogenic N deposition (1, 75), falling well-short of the estimated 45 to 75% reduction needed to restore the global N cycle below planetary boundaries (7, 27).
Methods
Site Selection.
Our experiment was replicated at 45 sites participating in the global Nutrient Network experiment (42). Sites included natural and anthropogenic grasslands from six continents, most in temperate climate zones (SI Appendix, Fig. S1 and Table S1). We included all Nutrient Network sites with N-fixing legumes in their regional composition (i.e., the entire spatial and temporal span for each site) and that had applied nutrient treatments for at least 3 y (see below). In sites with longer time spans (>7 y), we used data from years 0 to 6 only (as only 12 sites, all from the United States, had >7 y of data). N-fixer status of legume species was reported by each site principal investigator, based on local literature. Sites ranged in latitude from 54° N to 37° S (SI Appendix, Fig. S1), differing in initial degree of native versus nonnative species dominance (69) and spanning a broad spectrum of climates and soils. Climatic conditions ranged from 252 to 1,898 mm mean annual precipitation and 0.3 to 22.1 °C mean annual temperature, and pretreatment soils varied from 0.018 to 1.182% N and 9.25 to 227.62 mg⋅kg−1 P (SI Appendix, Table S1).
Experimental Treatments.
Each site established eight 5 × 5-m plots per block to accommodate a single replicate of eight treatments per block, with sites mostly containing three blocks (SI Appendix, Table S1). All sites had the same treatments, involving factorial combinations of nitrogen, phosphorus, and potassium addition and an untreated control. Nutrients were added annually as follows: 10 g N⋅m−2⋅y−1 as timed-release urea [(NH2)2CO], 10 g P⋅m−2⋅y−1 as triple-super phosphate [Ca(H2PO4)2], 10 g K⋅m−2⋅y−1 as potassium sulfate (K2SO4). Plots receiving potassium also received a once-off addition of other macro- and micronutrients in the first year: 100 g⋅m−2 of a mix containing Fe (15%), S (14%), Mg (1.5%), Mn (2.5%), Cu (1%), Zn (1%), B (0.2%), and Mo (0.05%). Nitrogen addition was approximately three times the maximum year 2000 estimated annual global N-deposition rate (1, 47), whereas P and K were added at substantially higher rates than currently occur (3, 76). We note, however, that our experimental timeframes of 3 to 6 y were short compared with decades to centuries of expected nutrient deposition. Furthermore, our N addition rate was also less than those used in other studies to evaluate N limitation of N fixation [e.g., 20 or 40 g⋅m−2⋅y−1 (35)].
Response Variables.
Plant cover and biomass were measured annually, beginning 1 y before treatments were applied. Within each treatment plot, the cover of each vascular plant species was estimated to the nearest 1% in a permanent 1 × 1-m subplot during the season of peak biomass at each location. Sites with two biomass peaks were measured at each peak, and data averaged between sampling dates in each year. For each plot in each year, we calculated the cumulative cover for species belonging to four key functional groups: N-fixing legumes, other forbs, graminoids (grasses + sedges). Adjacent to the 1 × 1-m cover subplot, all aboveground biomass was clipped in two 1 × 0.1-m strips (0.2 m2), dried at 60 °C and weighed to the nearest 0.01 g. Biomass was sorted to the functional group level in 30 of the 45 sites.
Legume abundance and diversity were expressed as cover, richness and biomass. For each response in each plot, we calculated ln-ratio (LR) of the current over the initial values in each plot (LR = ln [(Yfinal+1)/(Yinitial +1)]), where Yinitial and Yfinal are the initial and final values, and final values are either the third or last year of the study. We added 1 to the numerator and denominator because zeros make LR incalculable, although they can indicate an ecologically meaningful outcome (i.e., initial absence or total loss of legumes in the plot). We used current year/initial legume values as LR ratios (49) because this allowed us to study legume changes after accounting for variation in initial legume abundance and local conditions (8, 50), and to evaluate the effects of (standardized) initial conditions. We also checked for successional trends by evaluating changes in control plots in the LR analysis. In these plots, cover, richness, and biomass did not differ from initial values, suggesting that there were no perceptible successional changes. We also analyzed absolute cover richness and biomass, with initial values as covariables, with similar results as described under Statistical Analyses.
Site- and Plot-Level Covariates.
We used community, soil, and climatic covariates to investigate controls on N-fixing legume responses to nutrient additions at the site and plot level. In some cases, we calculated the ln-ratio of the covariate, using initial and final values as above (e.g., cover of grasses: LRG, see below).
Plant community covariates.
A range of plant community-level variables were calculated from the plant cover data for the third or last year of the experiment in each plot (plot-level predictors) or across all plots (site-level predictors). The latter were measured either at the beginning of the experiment or averaged across all plots in a site. At the plot level, we considered initial legume cover (percentage) and legume richness (number of species), initial total nonnative cover (percentage), initial live biomass (grams per square meter), and third or last year abundance of grasses and forbs (percentage). At the site level, we calculated initial mean legume cover (percentage), legume richness (number of species), total live plant biomass (grams per square meter), and mean native and nonnative plant cover (percentage).
PAR (micromoles of photons per square meter per second) was measured annually in the 1-m2 cover-sampling subplots, in cloud-free conditions between 10 AM and 2 PM during peak biomass (8, 42). One above-canopy and two perpendicular ground level measurements were made in each plot using a 1-m light ceptometer. Proportion of PAR was calculated as the ratio of the average ground level and the incident above-canopy PAR readings.
Soil covariates.
We measured soil chemical properties at the plot level at the beginning of the experiment in 37 of the 45 sites (SI Appendix, Table S1). In each plot, two 2.5-cm diameter × 10-cm depth soil cores, free of litter and vegetation, were collected, combined, homogenized, air-dried, and shipped to a single laboratory (A&L Laboratories) for analysis using standard methods. In particular, we measured soil pH, N:P ratio, total N (percentage), extractable P (parts per million), extractable K (parts per million), calcium (Ca, parts per million), magnesium (Mg, parts per million), sulfur (S, parts per million), sodium (Na, parts per million), manganese (Mn, parts per million), iron (Fe, parts per million), copper (Cu, parts per million), and boron (B, parts per million) (see https://nutnet.org/exp_protocol; see ref. 42 for further details).
Climatic covariates.
Mean annual precipitation (MAP) (in millimeters), mean annual temperature (MAT) (in degrees Celsius), and aridity index (AI) were derived from WorldClim (https://www.worldclim.org) based on site location (42).
Statistical Analyses.
All analyses were conducted using R, version R-3.4.0 (77). We used mixed-effect models (51, 53) to evaluate the effect of nutrient treatments and community or soil covariates on N-fixing legume LR-cover, LR-richness, and LR-biomass. Models included nutrient addition and covariates as fixed effects and block within sites as nested random effects (see below). In all cases, we analyzed data from the third and last measured year (3, 4, 5, or 6) separately. We included experiment duration (i.e., years under treatment) as a covariate but it was never significant, so we do not report it. Initial cover of N-fixing legumes was significantly positively correlated with legume biomass and richness (Spearman correlation: r = 0.88 and r = 0.60, respectively), as were their respective LRs (r = 0.58, r = 0.28). Given that we included sites based on legume presence at the site level, not all plots within a site had initial legume cover.
To evaluate possible bias originating from these differences, we tested the same models considering only those plots that initially contained legumes. Results did not differ qualitatively across these subsets of the data, and we present results of models fitted with the larger dataset. Similarly, to avoid excessive zeros and error originating from spatial patchiness (e.g., because cover and biomass were necessarily sampled in different parts of each experimental plot), N-fixing legume biomass was analyzed only in the 26 sites that reported biomass data for at least 2 y. Moreover, to indicate whether N-fixing legume responses differed from other elements of the vegetation, we tested the response of non–N-fixing forbs, graminoids, and forbs + graminoids (LRF, LRG, and LRF+G) using an identical approach.
We also analyzed results using the absolute (non–ln-transformed) cover, richness, or biomass values. As with LR, we analyzed these data using the factorial design or as eight independent treatments. We used initial cover, richness, or biomass as covariates. Covariates were always conserved and concurred with LR in accounting for initial values in each plot. Untransformed and LR analysis led to similar conclusions. We opted for the LR approach in the main text because LR presents the relative change independently of site values, accounts for potential successional trends, and is easily comparable with other studies. In addition, LR has better statistical properties and is superior for our experimental structure (49). Untransformed data are presented in SI Appendix, Table S4.
To evaluate legume responses to different combinations of nutrient additions, we performed planned contrasts based on a priori questions (78). Contrasts are described in SI Appendix, Fig. S4. Estimable functions were determined on the basis of the planned contrast for the fixed effects while conserving the structure of the model with respect to random effects (78, 79). In addition, to estimate the effect of nutrient addition on legume colonization (plots where legumes were initially absent but later present) or local extinction (plots where legumes were initially present but became extinct by the end of the experiment), we used generalized linear mixed effect models (53), with a binomial error distribution and a logit link function, including blocks within sites as the random structure. Colonization was measured as the number of new legume species divided by the final number of legumes in the plot, whereas extinction was measured as the number of legume species lost in the period divided by the initial legume richness (modified from ref. 80). We replicated this for all legumes, and for perennial and annual/biennial legume species separately.
To evaluate plot-level drivers of N-fixing legume response to treatments, we tested the effect of changes in community variables with treatments (i.e., LRPAR, LRbiomass, and LRF+G), initial N-fixing legume abundance at the site level and soil N:P ratio for each plot. In particular, we evaluated whether competition and relative community change in each plot (i.e., relative change in the cover of forbs and grasses or change in biomass as proxies for competition intensity) affected legumes, including also quadratic terms (i.e., LRF+G + LR2F+G). Model selection was performed through stepwise elimination [e.g., function stepAIC in “mass” package (81)], with further eliminations using AIC criteria (53). In all these cases, to test the significance of parameter estimates, we generated confidence intervals using semiparametric bootstrapping with 9,999 randomizations (52). These results were consistent with approximate P values, but more conservative; as a reference, we also present approximate degrees of freedom based on Satterthwaite approximation (82).
To evaluate contingencies of nutrient effects on legume cover at the site level, we first calculated the main effects of N, P, and K+ in each site as the N addition treatment × Site, P addition × Site, and K addition × Site interactions in the full model, and then used initial soil, climatic or community variables at the site level as predictors of these changes. We utilized multiple regression using step-wise elimination [e.g., function stepAIC in the “mass” package (81)]. We checked for collinearity through variance inflation factor (v.i.f) criteria; v.i.f. were lower than 2.8. Residuals were approximately homoscedastic and normally distributed. We repeated this analysis for the third year and last year data from the experiment.
Supplementary Material
Acknowledgments
This work was generated using data from the Nutrient Network (https://nutnet.org/) experiment, funded at the site scale by individual researchers. Coordination and data management were supported by funding to E.T.B. and E.W.S. from the NSF Research Coordination Network (NSF-DEB-1042132) and Long-Term Ecological Research (NSF-DEB-1234162 to Cedar Creek Long-Term Ecological Research) programs, and the Institute on the Environment (DG-0001-13). We also thank the Minnesota Supercomputer Institute for hosting project data and the Institute of the Environment for hosting Network meetings. P.M.T. was supported by an Argentine Research Council fellowship (Consejo Nacional de Investigaciones Científicas y Técnicas) and the Australian Endeavour Programme.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2023718118/-/DCSupplemental.
Data Availability
Plant, PAR, climate, and soil nitrogen data have been deposited in the Environmental Data Initiative (EDI) repository (https://portal.edirepository.org/nis/mapbrowse?packageid=edi.838.1) (83). Source data are provided with this paper.
References
- 1.Fowler D., et al., The global nitrogen cycle in the twenty-first century. Philos. Trans. R. Soc. Lond. B Biol. Sci. 368, 20130164 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dentener F., et al., Nitrogen and sulfur deposition on regional and global scales: A multimodel evaluation. Global Biogeochem. Cycles 9, 235–252 (1995). [Google Scholar]
- 3.Peñuelas J., et al., Human-induced nitrogen-phosphorus imbalances alter natural and managed ecosystems across the globe. Nat. Commun. 4, 153–226 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Sardans J., Peñuelas J., Potassium: A neglected nutrient in global change. Glob. Ecol. Biogeogr. 24, 261–275 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Potter P., Ramankutty N., Bennett E. M., Donner S. D., Characterizing the spatial patterns of global fertilizer application and manure production. Earth Interact. 14, 1–22 (2010). [Google Scholar]
- 6.Fay P. A., et al., Grassland productivity limited by multiple nutrients. Nat. Plants 1, 15080 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Rockström J., et al., A safe operating environment for humanity. Nature 461, 472–475 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Borer E. T., et al., Herbivores and nutrients control grassland plant diversity via light limitation. Nature 508, 517–520 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Harpole W. S., et al., Addition of multiple limiting resources reduces grassland diversity. Nature 537, 93–96 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Brauer V. S., Stomp M., Huisman J., The nutrient-load hypothesis: Patterns of resource limitation and community structure driven by competition for nutrients and light. Am. Nat. 179, 721–740 (2012). [DOI] [PubMed] [Google Scholar]
- 11.Suding K. N., et al., Functional- and abundance-based mechanisms explain diversity loss due to N fertilization. Proc. Natl. Acad. Sci. U.S.A. 102, 4387–4392 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tilman D., Resources: A graphical-mechanistic approach to competition and predation. Am. Nat. 116, 362–393 (1980). [Google Scholar]
- 13.Tilman D., Resource Competition and Community Structure (Princeton University Press, 1982). [PubMed] [Google Scholar]
- 14.Roskov Y., Zarucchi J., Novoselova M., Bisby F., Eds., “ILDIS world database of legumes” in Species 2000 and ITIS Catalogue of Life, 2019 Annual Checklist (Naturalis, Leiden, The Netherlands, 2019). [Google Scholar]
- 15.Adams M. A., Buchmann N., Sprent J., Buckley T. N., Turnbull T. L., Crops, nitrogen, water: Are legumes friend, foe, or misunderstood ally? Trends Plant Sci. 23, 539–550 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Graham P. H., Vance C. P., Legumes: Importance and constraints to greater use. Plant Physiol. 131, 872–877 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foyer C. H., et al., Neglecting legumes has compromised human health and sustainable food production. Nat. Plants 2, 16112 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Spehn E. M., et al., Ecosystem effects of biodiversity manipulations in European grasslands. Ecol. Monogr. 75, 37–63 (2005). [Google Scholar]
- 19.Hooper D. U. U., et al., Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol. Monogr. 75, 3–35 (2005). [Google Scholar]
- 20.Gao D., Wang X., Fu S., Zhao J., Legume plants enhance the resistance of soil to ecosystem disturbance. Front Plant Sci 8, 1295 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKey D., Legumes and nitrogen: The evolutionary ecology of a nitrogen-demanding lifestyle. Adv. Legum. Syst. 5 Nitrogen Factor 5, 211–228 (1994). [Google Scholar]
- 22.Vitousek P. M., Porder S., Houlton B. Z., Chadwick O. A., Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen-phosphorus interactions. Ecol. Appl. 20, 5–15 (2010). [DOI] [PubMed] [Google Scholar]
- 23.Storkey J., et al., Grassland biodiversity bounces back from long-term nitrogen addition. Nature 528, 401–404 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Menge D. N. L., Wolf A. A., Funk J. L., Diversity of nitrogen fixation strategies in Mediterranean legumes. Nat. Plants 1, 15064 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Hedin L. O., Brookshire E. N. J., Menge D. N. L., Barron A. R., The nitrogen paradox in tropical forest ecosystems. Annu. Rev. Ecol. Evol. Syst. 40, 613–635 (2009). [Google Scholar]
- 26.Houlton B. Z., Wang Y.-P. P., Vitousek P. M., Field C. B., A unifying framework for dinitrogen fixation in the terrestrial biosphere. Nature 454, 327–330 (2008). [DOI] [PubMed] [Google Scholar]
- 27.Steffen W., et al., Planetary boundaries: Guiding human development on a changing planet. Science 347, 1259855 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Augusto L., Delerue F., Gallet-Budynek A., Achat D. L., Global assessment of limitation to symbiotic nitrogen fixation by phosphorus availability in terrestrial ecosystems using a meta-analysis approach. Global Biogeochem. Cycles 27, 804–815 (2013). [Google Scholar]
- 29.Divito G. A., Sadras V. O., How do phosphorus, potassium and sulphur affect plant growth and biological nitrogen fixation in crop and pasture legumes? A meta-analysis. Field Crops Res. 156, 161–171 (2014). [Google Scholar]
- 30.Vitousek P. M., et al., Towards an ecological understanding of biological nitrogen fixation. Biogeochemistry 57, 1–45 (2002). [Google Scholar]
- 31.Vance C. P., Symbiotic nitrogen fixation and phosphorus acquisition. Plant nutrition in a world of declining renewable resources. Plant Physiol. 127, 390–397 (2001). [PMC free article] [PubMed] [Google Scholar]
- 32.Harpole W. S., et al., Nutrient co-limitation of primary producer communities. Ecol. Lett. 14, 852–862 (2011). [DOI] [PubMed] [Google Scholar]
- 33.Hautier Y., Niklaus P. A., Hector A., Competition for light causes plant biodiversity loss after eutrophication.Science 324, 636–638 (2009). [DOI] [PubMed] [Google Scholar]
- 34.Pennings S. C., et al., Do individual plant species show predictable responses to nitrogen addition across multiple experiments? Oikos 3, 547–555 (2005). [Google Scholar]
- 35.Taylor B. N., Menge D. N. L., Light regulates tropical symbiotic nitrogen fixation more strongly than soil nitrogen. Nat. Plants 4, 655–661 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Vitousek P. M., Menge D. N. L. L., Reed S. C., Cleveland C. C., Biological nitrogen fixation: Rates, patterns and ecological controls in terrestrial ecosystems. Philos. Trans. R. Soc. Lond. B Biol. Sci. 368, 2013–2019 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ren F., et al., Phosphorus does not alleviate the negative effect of nitrogen enrichment on legume performance in an alpine grassland. J. Plant Ecol. 10, 822–830 (2016). [Google Scholar]
- 38.Hungate B. A., et al., CO2 elicits long-term decline in nitrogen fixation. Science 304, 1291–1291 (2004). [DOI] [PubMed] [Google Scholar]
- 39.O’Hara G. W., Nutritional constraints on root nodule bacteria affecting symbiotic nitrogen fixation: A review. Aust. J. Exp. Agric. 41, 417–433 (2001). [Google Scholar]
- 40.Smith V. H., Effects of nitrogen–phosphorus supply ratios on nitrogen-fixation in agricultural and pastoral ecosystems. Biogeochemistry 18, 19–35 (1992). [Google Scholar]
- 41.Thorpe A. S., Perakis S., Catricala C., Kaye T. N., Nutrient limitation of native and invasive N2-fixing plants in northwest prairies. PLoS One 8, e84593 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borer E. T., et al., Finding generality in ecology: A model for globally distributed experiments. Methods Ecol. Evol. 5, 65–73 (2014). [Google Scholar]
- 43.Chapin F. S. III, Matson P. A., Mooney H. A., Principles of Terrestrial Ecosystem Ecology (Springer, 2011). [Google Scholar]
- 44.Du Y., et al., Nitrogen deposition increases global grassland N2O emission rates steeply: A meta-analysis. Catena 199, 105105 (2021). [Google Scholar]
- 45.Fagerli H., et al., “Transboundary particulate matter, photo-oxidants, acidifying and eutrophying components” (Joint MSC-W and CCC and CEIP Report, Stockholm University, Norwegian Meteorological Institute, 2019).
- 46.Liu X. J., et al., Environmental impacts of nitrogen emissions in China and the role of policies in emission reduction: Reactive nitrogen issues in China. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 378, 20190324 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dentener F., et al., Nitrogen and sulfur deposition on regional and global scales: A multimodel evaluation. Global Biogeochem. Cycles 20, GB4003 (2006). [Google Scholar]
- 48.Stevens C. J., et al., Nitrogen deposition threatens species richness of grasslands across Europe. Environ. Pollut. 158, 2940–2945 (2010). [DOI] [PubMed] [Google Scholar]
- 49.Hedges L. V., Gurevitch J., Curtis P. S., The meta-analysis of response ratios in experimental ecology. Ecology 80, 1150–1156 (1999). [Google Scholar]
- 50.Durlak J. A., How to select, calculate, and interpret effect sizes. J. Pediatr. Psychol. 34, 917–928 (2009). [DOI] [PubMed] [Google Scholar]
- 51.Pinheiro J. C., Bates D. M., Mixed-Effects Models in S and S-PLUS (Springer, New York, 2000). [Google Scholar]
- 52.Bates D. M., Maechler M., Bolker B., Walker S., Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015). [Google Scholar]
- 53.Zuur A. F., et al., Mixed Effects Models and Extensions in Ecology with R (Statistics for Biology and Health, Springer, 2009). [Google Scholar]
- 54.Xia J., Wan S., Global response patterns of terrestrial plant species to nitrogen addition. New Phytol. 179, 428–439 (2008). [DOI] [PubMed] [Google Scholar]
- 55.Vitousek P. M., Field C. B., Ecosystem constraints to symbiotic nitrogen fixers: A simple model and its implications. Biogeochemistry 46, 179–202 (1999). [Google Scholar]
- 56.Herben T., et al., Long-term time series of legume cycles in a semi-natural montane grassland: Evidence for nitrogen-driven grass dynamics? Funct. Ecol. 31, 1430–1440 (2017). [Google Scholar]
- 57.Menge D. N. L. L., Levin S. A., Hedin L. O., Evolutionary tradeoffs can select against nitrogen fixation and thereby maintain nitrogen limitation. Proc. Natl. Acad. Sci. U.S.A. 105, 1573–1578 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zahran H. H., Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol. Mol. Biol. Rev. 63, 968–689 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bonilla I., Bolaños L., “Mineral nutrition for legume-rhizobia symbiosis: B, Ca, N, P, S, K, Fe, Mo, Co, and Ni: A review” in Organic Farming, Pest Control and Remediation of Soil Pollutants, Lichtfouse E., Ed. (Springer, 2013), pp. 253–274. [Google Scholar]
- 60.Pinto P. de T., Litchman E., Interactive effects of N:P ratios and light on nitrogen-fixer abundance. Oikos 119, 567–575 (2010). [Google Scholar]
- 61.Tilman D., Niche tradeoffs, neutrality, and community structure: A stochastic theory of resource competition, invasion, and community assembly. Proc. Natl. Acad. Sci. U.S.A. 101, 10854–10861 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seabloom E. W., et al., Competition, seed limitation, disturbance, and reestablishment of California native annual forbs. Ecol. Appl. 13, 575–592 (2003). [Google Scholar]
- 63.Mitran T., et al., “Role of soil phosphorus on legume production” in Legumes for Soil Health and Sustainable Management, Meena R., Das A., Yadav G., and Lal R., Eds. (Springer, Singapore, 2018), pp. 487–510. [Google Scholar]
- 64.Güsewell S., N:P ratios in terrestrial plants: Variation and functional significance. New Phytol. 164, 243–266 (2004). [DOI] [PubMed] [Google Scholar]
- 65.Liao W., Menge D. N. L., Lichstein J. W., Ángeles-Pérez G., Global climate change will increase the abundance of symbiotic nitrogen-fixing trees in much of North America. Glob. Change Biol. 23, 4777–4787 (2017). [DOI] [PubMed] [Google Scholar]
- 66.Moles A. T., et al., Which is a better predictor of plant traits: Temperature or precipitation? J. Veg. Sci. 25, 1167–1180 (2014). [Google Scholar]
- 67.Pellegrini A. F. A., Staver A. C., Hedin L. O., Charles-Dominique T., Tourgee A., Aridity, not fire, favors nitrogen-fixing plants across tropical savanna and forest biomes. Ecology 97, 2177–2183 (2016). [DOI] [PubMed] [Google Scholar]
- 68.Flores-Moreno H., et al., Climate modifies response of non-native and native species richness to nutrient enrichment. Philos. Trans. R. Soc. Lond. B Biol. Sci. 371, 20150273 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seabloom E. W., et al., Plant species’ origin predicts dominance and response to nutrient enrichment and herbivores in global grasslands. Nat. Commun. 6, 7710 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Y., Nitrogen enrichment weakens ecosystem stability through decreased species asynchrony and population stability in a temperate grassland. Global Chang. Biol. 22, 1445–1455 (2016). [DOI] [PubMed] [Google Scholar]
- 71.Grace J. B., et al., Integrative modelling reveals mechanisms linking productivity and plant species richness. Nature 529, 390–393 (2016). [DOI] [PubMed] [Google Scholar]
- 72.Firn J., et al., Leaf nutrients, not specific leaf area, are consistent indicators of elevated nutrient inputs. Nat. Ecol. Evol. 3, 400–406 (2019). [DOI] [PubMed] [Google Scholar]
- 73.Seabloom E. W., Borer E. T., Tilman D., Grassland ecosystem recovery after soil disturbance depends on nutrient supply rate. Ecol. Lett. 23, 1756–1765 (2020). [DOI] [PubMed] [Google Scholar]
- 74.Duffy J. E., et al., The functional role of biodiversity in ecosystems: Incorporating trophic complexity. Ecol. Lett. 10, 522–538 (2007). [DOI] [PubMed] [Google Scholar]
- 75.Soussana J.-F., Tallec T., Can we understand and predict the regulation of biological N2 fixation in grassland ecosystems? Nutr. Cycl. Agroecosyst. 88, 197–213 (2010). [Google Scholar]
- 76.Vet R., et al., A global assessment of precipitation chemistry and deposition of sulfur, nitrogen, sea salt, base cations, organic acids, acidity and pH, and phosphorus. Atmos. Environ. 93, 3–100 (2014). [Google Scholar]
- 77.R Core Team , R, Version R-3.4.0 (R Foundation for Statistical Computing, 2017).
- 78.McLean R. A., Sanders W. L., Stroup W. W., A unified approach to mixed linear models. Am. Stat. 45, 54–64 (1991). [Google Scholar]
- 79.Voss D. T., Resolving the mixed model controversy. Am. Stat. 53, 352–356 (1999). [Google Scholar]
- 80.Anderson K. J., Temporal patterns in rates of community change during succession. Am. Nat. 169, 780–793 (2007). [DOI] [PubMed] [Google Scholar]
- 81.Venables W. N., Ripley B. D., Modern Applied Statistics with S (Springer, ed. 4, 2013). [Google Scholar]
- 82.Kuznetsova A., Brockhoff P. B., Christensen R. H. B., lmerTest package: Tests in linear mixed effects models. J. Stat. Softw. 82, 1–26 (2017). [Google Scholar]
- 83.Tognetti P. M., et al., Data from Negative Effects of Nitrogen Override Positive Effects of Phosphorus On Grassland Legumes Worldwide. Environmental Data Initiative. https://portal.edirepository.org/nis/mapbrowse?packageid=edi.838.1. Deposited 21 May 2021. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Plant, PAR, climate, and soil nitrogen data have been deposited in the Environmental Data Initiative (EDI) repository (https://portal.edirepository.org/nis/mapbrowse?packageid=edi.838.1) (83). Source data are provided with this paper.