Abstract
In females, ovarian estradiol (E2) exerts both negative and positive feedback regulation on the neural circuits governing reproductive hormone secretion, but the cellular and molecular mechanisms underlying this remain poorly understood. In rodents, estrogen receptor α–expressing kisspeptin neurons in the hypothalamic anteroventral periventricular region (AVPV) are prime candidates to mediate E2 positive feedback induction of preovulatory gonadotropin-releasing hormone and luteinizing hormone (LH) surges. E2 stimulates AVPV Kiss1 expression, but the full extent of estrogen effects in these neurons is unknown; whether E2 stimulates or inhibits other genes in AVPV Kiss1 cells has not been determined. Indeed, understanding of the function(s) of AVPV kisspeptin cells is limited, in part, by minimal knowledge of their overall molecular phenotype, as only a few genes are currently known to be co-expressed in AVPV Kiss1 cells. To provide a more detailed profiling of co-expressed genes in AVPV Kiss1 cells, including receptors and other signaling factors, and test how these genes respond to E2, we selectively isolated actively translated mRNAs from AVPV Kiss1 cells of female mice and performed RNA sequencing (RNA-seq). This identified >13 000 mRNAs co-expressed in AVPV Kiss1 cells, including multiple receptor and ligand transcripts positively or negatively regulated by E2. We also performed RNAscope to validate co-expression of several transcripts identified by RNA-seq, including Pdyn (prodynorphin), Penk (proenkephalin), Vgf (VGF), and Cartpt (CART), in female AVPV Kiss1 cells. Given the important role of AVPV kisspeptin cells in positive feedback, E2 effects on identified genes may relate to the LH surge mechanism and/or other physiological processes involving these cells.
Keywords: kisspeptin, Kiss1, dynorphin, CART, VGF, mRNA, estrogen, estradiol, RP3V, reproduction, RNA-seq
A fundamental tenet of reproductive axis regulation is sex steroid feedback. In females, sex steroids secreted from the ovaries act in the brain to exert both negative and positive regulation on the neural circuits that govern reproductive hormone secretion. In female rodents, estradiol (E2) and progesterone (P4) act during diestrus and estrous to dampen and control the frequency and amplitude of pulsatile gonadotropin-releasing hormone (GnRH) secretion from forebrain neurons (negative feedback), thereby governing the downstream pulsatile secretion of luteinizing hormone (LH) from the pituitary (1-3). Conversely, on proestrus, higher levels of circulating E2 act in concert with P4 to stimulate a large “surge” secretion of GnRH (positive feedback), which itself stimulates the LH surge that drives ovulation (4-6). Despite these known effects, the cellular and molecular mechanisms by which sex steroids act in the brain to inhibit or stimulate GnRH secretion still remain poorly understood.
Because GnRH neurons lack estrogen receptor α (ERα), which mediates E2 positive and negative feedback, other “upstream” ERα-expressing cells have been proposed as neural loci of E2 feedback actions. Kisspeptin neurons are prime candidates to directly receive E2 signals and mediate ERα-induced effects on GnRH secretion (7). Encoded by Kiss1, kisspeptin is a potent activator of GnRH neurons and downstream LH secretion (8-14), and mutations in Kiss1 or Kiss1r (the kisspeptin receptor) induce striking reproductive hormone deficits and infertility (15-17). In the rodent hypothalamus, kisspeptin neurons are detected primarily in 2 regions, the continuum in the anterior hypothalamus comprised of the anteroventral periventricular nucleus (AVPV) and neighboring periventricular nucleus (PeN) (for simplicity, referred to here as the AVPV; sometimes also called the RP3V) and the arcuate nucleus (ARC) (11,18,19). Both the AVPV and ARC kisspeptin populations express sex steroid receptors, including ERα (20,21), and directly stimulate GnRH neurons, with AVPV kisspeptin neurons projecting to GnRH soma in the preoptic area (POA) and ARC kisspeptin neurons projecting to GnRH neurons’ distal projections near the median eminence (22,23).
Although ARC and AVPV Kiss1 cells both express ERα, E2 regulates the Kiss1 gene oppositely in the 2 regions, inhibiting Kiss1 expression in the ARC but stimulating Kiss1 expression in the AVPV (19,20,24,25). Correspondingly, in the absence of circulating E2 (eg, after gonadectomy), Kiss1 messenger RNA (mRNA) levels increase in the ARC but decrease in the AVPV (19,20,24,26). The differential effects of E2 on ARC and AVPV Kiss1 neurons likely relate to sex steroid feedback control of GnRH secretion. The ARC region is implicated in sex steroid negative feedback on GnRH pulse secretion (27-30), and ARC Kiss1 neurons likely participate in this process (31). Indeed, evidence now suggests that E2-responsive ARC Kiss1 neurons, which also coexpress the neuropeptides neurokinin B and dynorphin, are key components of the GnRH pulse generator circuitry (32-37). In contrast, Kiss1 neurons in the AVPV mediate E2 positive feedback induction of the sexually dimorphic GnRH surge that only occurs in females (5,38,39). Supporting this, Kiss1 levels in the AVPV are greater in females than males (19,40,41), and AVPV kisspeptin neurons in females but not males exhibit cellular activation during LH surges (21,39).
Although ERα is expressed in AVPV Kiss1 neurons, the extent and nature of estrogen effects in these neurons is not fully known. Besides stimulating Kiss1 expression, E2 may also have important stimulatory or inhibitory effects on other genes in AVPV Kiss1 cells, but this has not yet been determined. Given the important role of AVPV kisspeptin neurons in E2 positive feedback (5,42-44), E2 effects on other genes in these neurons may affect the LH surge mechanism, although other unknown physiological processes that AVPV kisspeptin neurons might participate in may also be affected. In fact, our understanding of the various function(s) of AVPV kisspeptin neurons is currently limited, in part, by not knowing the extensive molecular phenotype of these neurons. Because the AVPV region also contains many nonkisspeptin cell populations, phenotyping the kisspeptin neurons has thus far been restricted to histological double-label analyses. Consequently, only a small handful of genes (or their protein products) besides ERα are currently known to be co-expressed in AVPV kisspeptin cells, including galanin (Gal), tyrosine hydroxylase (TH), met-enkephalin (encoded by the proenkephalin gene, Penk), vasopressin receptor 1a (Avpr1a), and P4 receptor (Pgr) (45-49). In some case, this has proven useful for further elucidating the function and regulation of these kisspeptin cells, for example, identifying the important roles of P4 and vasopressin in the activation of AVPV kisspeptin neurons during the LH surge (44,45). Yet, the functional significance of other known co-expressed factors, like TH (46) or galanin, is still unknown.
A more detailed and extensive knowledge of the co-expressed genes in AVPV kisspeptin neurons, including steroid and peptide receptors, and signaling factors, and how these genes respond to different regulatory signals, like E2, is needed to fully understand the regulation and function(s) of these neurons. To address this gap in knowledge, the present study leveraged the recently developed RiboTag technique (50-52) to selectively isolate actively translated mRNAs from just the Kiss1 cells of the AVPV. The RiboTag approach has an added advantage of analyzing only mRNAs that are associated with polyribosomes and actively being translated into proteins (the “active translatome”), regardless of their transcriptional status. Combining this Ribotag isolation methodology with RNA sequencing (RNA-seq), we were able to (1) identify >13 300 mRNAs that are significantly co-expressed and actively being translated in AVPV Kiss1 neurons and (2) quantitatively compare the expression levels of these Kiss1 neuron-specific mRNAs in females under different E2 conditions to determine how estrogens alter the molecular profile of AVPV kisspeptin neurons.
Methods
Animals
To identify which gene transcripts are co-expressed and actively translated specifically in AVPV Kiss1 neurons, we used the Ribotag mouse model (50-52). Ribotag (Rpl22HA+) mice have loxP sites flanking exon 4 of the ribosomal protein L22 (RPL22) gene, followed by an identical coding sequence for exon 4 that has 3 hemagglutinin (HA) epitope sequences prior to the gene’s STOP codon (51,52). When Ribotag mice are crossed with a Cre mouse line, Cre-mediated recombination results in the expression of HA tags on ribosomes in cells expressing Cre. HA-tagged ribosomes can be isolated by immunoprecipitation and the accompanying RNA obtained, permitting isolation of actively translated mRNAs specifically from Cre expressing cells. Here, we crossed the Rpl22HA+ mice with a well-validated Kiss1Cre line developed by Elias (53) to obtain Kiss1Cre+/Rpl22HA+/+ progeny to be used for experimentation (n = 28). In these Kiss1Cre+/Rpl22HA+/+ mice, recombination of the RPL22 gene only occurs in Kiss1 cells, and hence, the HA-tagged ribosomal protein will be incorporated into the ribosomes of only Kiss1 cells. As an internal control for the specificity of the Ribotag-mediated isolation of mRNAs, we used a small cohort of Kiss1Cre−/Rpl22HA+/+ control mice (n = 8), which lack the HA tag and therefore should not permit HA pull-down of transcripts. All Kiss1Cre/Rpl22HA+/+ mice were genotyped by polymerase chain reaction (PCR) of tail DNA (referred to hence as Kiss1Cre+/Ribotag or Kiss1Cre−/Ribotag mice); these mice were also genotyped for unwanted germline recombination, and such mice were excluded from the study. In the final experiment, adult (10-12 weeks of age) female C57BL6 mice (n = 3) were used for histological validation of the RNA-seq expression data via in situ hybridization (ISH) analysis. All mice were housed 2 to 3 mice/cage in a 12:12 light:dark cycle (lights off at 6 pm), with ad libitum access to food and water. All animal procedures were approved by the local IACUC committees at the University of California, San Diego (Kiss1Cre/Ribotag mice) or Albany Medical College (C57BL6 mice).
Hormone Treatment and Tissue Collection
Expression of the Kiss1 gene in the AVPV is well-known to be stimulated by E2. Therefore, at 8 weeks of age, all female Kiss1Cre/Ribotag mice (Kiss1Cre+/Rpl22HA+/+ and Kiss1Cre−/Rpl22HA+/+ controls) were ovariectomized (OVX) under isoflurane anesthesia and briefly exposed to high E2 for 4 days, via a subcutaneous silastic capsule, to stimulate the AVPV Kiss1 gene. This dose of E2 has previously been shown in mice by our lab and others to elevate serum E2 levels, increase AVPV Kiss1 expression, and properly suppress morning LH levels for E2 negative feedback (18,20,25,40,47,54,55). We theorized that this brief high E2 pre-treatment would ensure strong Cre expression to fully drive recombination and incorporation of the HA-tagged ribosomes in AVPV Kiss1 cells similarly in all animals. After this 4-day pretreatment, all E2 silastic capsules were removed, and the mice (all already OVX) were given 1 week to allow any residual circulating E2 to be depleted. Following this E2 washout period, some mice were implanted with another similar high E2 silastic capsule to serve as the E2 group (n = 12 Kiss1Cre+; n = 4 Kiss1Cre− controls), while the remaining females received no additional hormone treatment to serve as the OVX (no E2) group (n = 16 Kiss1Cre+; n = 4 Kiss1Cre− controls). Five days later, all mice were sacrificed midday between 11 am and 2 pm, and blood and brains were collected. Blood serum collected at sacrifice was assayed to confirm elevated basal LH levels in the OVX group (due to lack of E2 negative feedback) and low LH levels in the E2 group (due to proper E2 negative feedback provided by the implant). Serum LH was measured in singlet via a sensitive mouse LH RIA (lower detection limit: 0.04 ng/mL; average reportable range: 0.04-75 ng/mL) performed by the University of Virginia’s Ligand Assay and Analysis Core. As expected, OVX females without E2 implants had elevated mean LH levels, whereas E2-treated OVX females showed reduced mean LH levels (3.04 ± 0.22 ng/mL vs 0.22 ± 0.04 ng/mL, respectively; P < 0.05).
Brains from Kiss1Cre/Ribotag females were immediately collected on dry ice and stored at −80oC. The anterior hypothalamic-POA region, including the AVPV, was microdissected and 2 consecutive 400 um thick coronal slices spanning the entire AVPV region were micropunched (2 mm diameter) per animal, as in previous studies (40). Micropunched brain samples were pooled within E2 and OVX treatments (n = 4 mice/pooled sample) to allow for sufficient yield of isolated mRNA using the Ribotag immunoprecipitation method. There were a total of 4 pooled Cre+ OVX samples, 3 pooled Cre+ E2 treatment samples, and 1 pooled sample each from OVX and E2 Cre− controls. Pooled micropunched AVPV samples were stored in 1.7 mL Eppendorf tubes at −80oC until immunoprecipitation was performed.
For the triple-label ISH co-expression experiment, ovary-intact adult C57Bl6 mice (n = 3) were used. For these C57Bl6 females, vaginal lavage and cytology were performed daily to track estrous cycle stage, and once in diestrus, female mice were sacrificed at midday (12-1 pm), and their brains collected onto dry ice and stored at −80°C. Frozen brains from these diestrus C57Bl6 females were sectioned on a cryostat into 20 um coronal sections, with sections encompassing the AVPV region and corresponding to approximately 0.01 mm anterior to bregma. These AVPV-containing brain sections were mounted onto SuperFrost Plus slides (Fisher Scientific) and stored at −80oC until the triple-label ISH assay.
Immunoprecipitation and RNA Extraction
Immunoprecipitation was performed on pooled Kiss1Cre/Ribotag AVPV samples following the recent protocols used by Sanz and colleagues (51,52) with a few modifications to optimize the procedure for AVPV Kiss1 cells. Pooled tissue samples were homogenized in a buffer solution at 3% weight by volume (homogenization buffer solution: 72% H2O, 9.6% NP-40, 9.6% 2M KCl, 3.2% 1.5M Tris-pH 7.4, 1.2% 1M MgCl2, 2% cyclohexamide 5 mg/mL, 1% protease inhibitors, 1% heparin 100 mg/mL, 0.5% RNAsin, and 0.1% dithiothreitol). Samples were then centrifuged for 10 min (10K) at 4oC and 10% of the sample lysate was transferred to a new tube, serving as the input (IN) sample. This Input sample contains all cell types found in the AVPV region, including but not restricted to Kiss1 neurons. An amount of 350 uL of lysis buffer (Qiagen Kit #74034) was added to the Input sample, vortexed, flash frozen, and stored at −80oC. Biolegend Purified anti-HA, 11 Epitope Tag Antibody (#901501; 0.25 uL of antibody/100 uL lysate) was added to the remaining lysate and incubated at 4oC for 2 h on a sample rotator. After the 2-h incubation, the sample was transferred to a tube of washed magnetic beads (Pierce Protein A/G #88803; 25 uL beads/100 uL sample). Magnetic beads were washed with 800 uL of the homogenization buffer solution as previously described prior to adding the sample to the beads. The antibody/bead sample was incubated for 1 h at 4oC on a sample rotator. After 1 h, the sample tubes were placed on a magnetic rack, the lysate was removed, and 800 uL of a high salt buffer (53.4% H2O, 30% 2M KCl, 10% NP-40, 3.3% 1.5M Tris-pH 7.4, 1.2% 1M MgCl2, 2% cyclohexamide 5 mg/mL, and 0.05% dithiothreitol) was added to wash the beads. The samples were then incubated for 10 min at 4°C on a sample rotator before the beads were washed again using the high salt buffer. This procedure was repeated 3 times, after which 350 uL of lysis buffer (Qiagen Kit #74034) was immediately added to each sample and vortexed for 30 sec. The samples were then placed in the magnetic rack and the lysate (termed the “IP sample”) was removed and stored at −80oC until RNA purification. The IP samples contain only RNA that was associated with HA-tagged ribosomes (ie, RNA specific to Kiss1 neurons in Cre+ mice). RNA was extracted from both the IN and IP samples using the Qiagen RNeasy Plus Micro Kit (#74034) per the kit instructions and then stored in aliquots at −80°C until reverse transcription PCR (RT-PCR) or RNA-seq.
RT-PCR Confirmation of Kiss1 Cell-specific mRNAs
To validate that the Kiss1Cre/Ribotag method effectively isolated mRNA from AVPV Kiss1 neurons and was also selective (ie, did not isolate mRNA from other non-Kiss1 cells in the micropunched tissue), we performed RT-PCR for 2 genes known to be co-expressed in AVPV Kiss1 cells (Kiss1 and TH) and a gene known to not be co-expressed in Kiss1 neurons (GnRH, which is expressed in some scattered cells residing in the greater AVPV/POA region). RNA (10 ng) from the input and IP samples was used to make complimentary DNA (cDNA) using the iScript cDNA Synthesis Kit (Bio-rad #1708891), per kit instructions. The cDNA was then used to test for the presence of Kiss1, TH, and GnRH in each sample, using RNA-specific primers for each transcript (Kiss1 Forward: CTGTGTCGCCACCTATGGGG; Kiss1 Reverse: GGCCTCTACAATCCACCTGC; TH Forward: CCCCCACCTGGAGTACTTTG; TH Reverse: ATCACGGGCAGACAGTAGACC; GnRH Forward: ATGGCCGGCATTCTACTGC; GnRH Reverse: CTGCTGGGTATAAAAACGC). cDNA (1 uL) was added to Jumpstart RedTaq mix (Sigma #P0982), primers, and H2O, and RT-PCR was run with the following conditions: 94 × 15’ (94 × 30”, 57 × 30”, 72 × 60”), repeat × 30 times; 72 × 5’, and 4 × 5’, with the GnRH PCR run with an annealing temperature of 52oC.
RNA-seq of Cre+ Samples and Bioinformatic Analysis
RNA from the Kiss1Cre+/Ribotag IP samples of the E2 and OVX groups was prepared and sequenced by the Genomics Center at University of California San Diego’s Institute for Genomic Medicine, which has extensive experience running RNA-seq. The quality of all RNA samples was determined using the Agilent High Sensitivity RNA ScreenTape System. Only samples with an RNA integrity number > 8.0 were used for library preparation. Unstranded mRNA library kits from Illumina that used polyA enrichment were used to create the library. RNA-seq was performed using Illumina’s HiSeq4000 platform, using the SR75 run type. Quality control analysis (Fastqc v0.11.8) was performed on the raw RNA-seq data, followed by read trimming (Trimmomatic 0.38), alignment (STAR v2.6.0a), and quantification (RSEMv1.3.0) of reads using GRChm38.p6/mm10 and Mus_musculus.GRCm38.98.gtf.
All RNA-seq analysis and statistics were completed by the Center for Computational Biology & Bioinformatics at University of California at San Diego. The per-gene/per-sample count data from the quality control and count preparation was used in conjunction with the per-sample metadata RNA-seq data, to integrate and annotate these inputs in preparation for data exploration and preprocessing using the edgeR (56) Bioconductor (57) package written in R (57). This analysis was limited to known protein coding genes. The annotated data were used with same edgeR Bioconductor packages written in R and limma (58) to explore and preprocess these data in preparation for differential expression testing. This data exploration file was used to identify transcripts produced by AVPV Kiss1 cells. Gene transcripts with counts per million (CPM) greater than 1 for at least 3 samples (the smallest group size in this experiment) were retained for further analysis, while all others were discarded. The data set is deposited to the Gene Expression Omnibus data repository (59). Using the same limma and edgeR Bioconductor packages written in R, we then used the voom technique (60) to test for differential expression of identified transcripts between the E2 and OVX (no E2) groups. The overall and differential expression data were then used with the WebGestalt (61) and R package to test for the presence of certain genes and differential expression of genes between E2 and OVX in annotated functions, pathways, and diseases. We also used the PathView (62) tool render and visualize Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway maps (63) by differential expression findings, with very low expressing transcripts (mean log CPM <1) in both E2 and OVX groups excluded from differential expression and KEGG pathway analyses. The KEGG database links gene catalogs from completely sequenced genomes to higher-level systemic cell and organismal functions and displays them in >500 KEGG pathway maps (https://www.genome.jp/kegg/pathway.html) representing current knowledge of the molecular interaction, reaction, and relation networks for different biological categories (metabolism, cellular processes, organismal systems, etc). The top KEGG pathways represented in our AVPV Kiss1 cell RNA-seq data set were determined, and for differential gene expression and pathway analyses comparing E2 and OVX conditions, statistical significance was set at P < 0.05.
Validation of Gene Co-expression Using Triple-Label In Situ Hybridization
To validate that several highly expressed genes identified in our Kiss1Cre/Ribotag RNA-seq experiment are in fact co-expressed in AVPV Kiss1 neurons, we performed 2 triple-label ISH assays using RNAscope. The first assay measured co-expression of Penk (proenkephalin) and Pdyn (prodynorphin) in AVPV Kiss1 neurons, 2 genes previously reported to be expressed in the AVPV region of rats (64). The second assay studied co-expression of Cartpt [cocaine- and amphetamine-regulated transcript prepropeptide (CART)] and Vgf (VGF nerve growth factor inducible), 2 genes highly expressed in the RNA-seq data that are also known to be expressed in the AVPV region (65,66) but have not previously been examined specifically in AVPV Kiss1 cells. We used the RNAscope fluorescent Multiplex kit with the following RNAscope catalogue riboprobes: Mm-Kiss1-C1—Mus musculus KiSS-1 metastasis-suppressor (Kiss1) mRNA, Mm-Pdyn-C2—Mus musculus prodynorphin (Pdyn) mRNA, Mm-Penk-C3–Mus musculus preproenkephalin (Penk) mRNA, Mm-Vgf-C2–Mus musculus VGF nerve growth factor inducible (Vgf) mRNA, and Mm-Cartpt-C3–Mus musculus CART prepropeptide (Cartpt) transcript variant 2 mRNA. The manufacturer’s recommended ISH protocol was followed. Briefly, brain tissue slices containing the AVPV were fixed in 4% paraformaldehyde at 4oC for 15 min. The AVPV tissue was then dehydrated in 50%, 70%, and 100% ethanol washes for 5 to 10 min, a hydrophobic barrier was created around the tissue, and 5 drops of Protease IV was added to each tissue section. The tissue was incubated for 30 min and the Protease IV was washed off twice with 1× phosphate-buffered saline solution. Excess liquid was removed and a premixed riboprobe solution (115.4 uL Kiss1, 2.3ul Penk, and 2.3ul Pdyn per slide) was added to each slide. The probe hybridized to the tissue for 2 hours at 40oC. The slides were then washed in 1× wash buffer (supplied with the RNAscope kit) twice for 2 min each. There were then 4 amplification steps alternating between 30 and 15 min per step. Between each amplification step, slides were washed twice in 1× wash buffer for 2 min. On the last amplification step, the Alt-C color module was used such that the fluorophores were Atto 550 for Kiss1, Atto 647 for Pdyn or Vgf, and Alexa 488 for Penk or Cartpt. Upon completion of the fourth amplification step and a final wash in 1× wash buffer, 4 drops of 4′,6-diamidino-2-phenylindole were added to each slide, which were incubated for 30 sec; excess DAPI was removed, and then the slides were immediately cover-slipped using ProLong Gold Antifade Mountant. Slides were stored at 4oC in the dark.
Images of mRNA fluorescent staining were obtained for the middle AVPV/ PeN region of each female using a Zeiss LSM 880 at 40× (oil) magnification. Images were analyzed using ZEISS Zen Blue software. For each female, the number of identifiable Kiss1-expressing neurons in the AVPV were counted, with a minimum of 90 Kiss1-expressing cells counted per female (all in diestrus stage). The number of Penk- and Pdyn-expressing cells or Cartpt- and Vgf-expressing cells that were co-localized with Kiss1-expressing cells was also counted. Using the RNAscope manufacturer’s counting criteria as a guideline, an AVPV cell was considered to express Kiss1 (or the other 2 genes) if there were ≥15 clustered dots, which represent individual mRNA copies for that specific gene. The percentage of AVPV Kiss1 neurons co-expressing Penk and/or Pdyn, or Cartpt- and/or Vgf, was determined for each female and then mean co-expression levels calculated.
Results
Validation of Ribotag Immunoprecipitation and Specificity of AVPV Kiss1 Cell Isolation
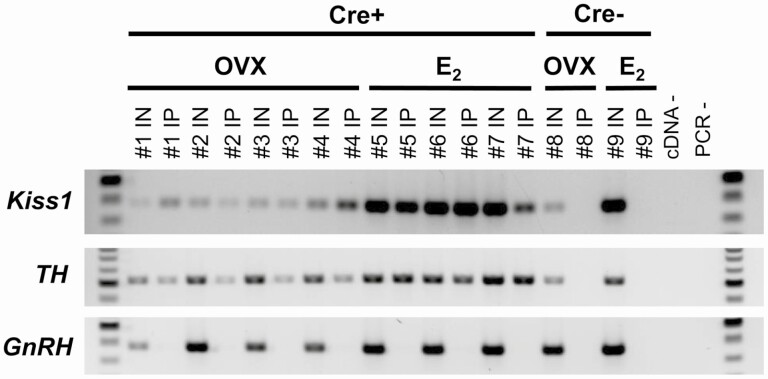
To confirm successful and selective immunoprecipitation pulldown of mRNA from AVPV Kiss1 cells, RT-PCR was performed using cDNA from both the IN samples (mRNA from all cells in the micropunched AVPV region) and IP samples (mRNA isolated from only Kiss1 cell ribosomes). As expected, both the IN and IP samples from Kiss1Cre+/Ribotag females expressed Kiss1 (Fig. 1), indicating successful isolation of mRNA from AVPV cells that produce Kiss1. Given prior histological reports of TH mRNA (and protein) co-expression in AVPV Kiss1 neurons (47,67,68), the TH gene was used as another positive control; as expected, TH was expressed in both the Kiss1Cre+ IN and IP samples (Fig. 1). To test whether mRNA isolation was specific to AVPV Kiss1 cells, RT-PCR was performed for GnRH, which is known to be expressed in the greater AVPV/POA region but not in Kiss1 cells. GnRH was detected in the IN sample of all Kiss1Cre+ mice, signifying its presence in the micropunches as expected but was not detected in any of the corresponding IP samples of the same mice, indicating that the mRNA isolation and pulldown procedure was specific to just the Kiss1 cells (Fig. 1). In further support of the pulldown specificity, none of the IP samples from Kiss1Cre− control mice, which lack HA-tagged ribosomes, expressed Kiss1, TH, or GnRH (Fig. 1), even though all 3 mRNAs were expressed in the IN samples of these Cre-control mice (Fig. 1). Collectively, these findings support that the isolation procedure selectively targeted mRNA from Kiss1 cells, but not other AVPV cells.
Figure 1.
RT-PCR verification of successful isolation of mRNA specifically from AVPV Kiss1 cells in adult Kiss1Cre+/Ribotag female mice. The number above each lane denotes the pooled sample number (n = 4 mice/pooled sample). Input (IN) samples contain mRNA from all cell types, including Kiss1 cells, in the micropunched tissue of the greater AVPV region (prior to the selective HA immunoprecipitation step). IP samples contain transcripts selectively isolated with the HA immunoprecipitation process, which is dependent on Cre and should only occur in Kiss1 cells of Cre+ mice. Thus, the IP lanes for Cre+ samples (IP#1 to IP#7) signify mRNA identified in AVPV Kiss1 cells, including Kiss1 and Th as predicted. By contrast, GnRH mRNA is present in all IN samples as expected but not in the IP of any Cre+ samples (IP#1 to IP#7), supporting that the pulldown is selective to mRNA from Kiss1 cells. As an additional validation, Cre− controls, which do not have HA-tagged ribosomes regardless of hormone condition, do not contain mRNA in their IP samples (IP#8 and IP#9), even though Kiss1, Th, and GnRH are all expressed in the Cre− IN samples (IN#8, IN#9) as expected. cDNA and PCR negative controls are located on the right of the gel, and 100 bp ladder is shown on both sides of the gel.
RNA-Seq Quality Control and Gene Transcript Expression in AVPV Kiss1 Cells
The RNA integrity value of all Kiss1Cre+ IP samples was 9.4 or greater, indicating RNA quality sufficient for sequencing via RNA seq. These samples all exceeded the minimum desired sequencing parameters in the number of total reads (≥20 million), number of aligned reads (≥10 million), and fraction of uniquely aligned reads (≥80%), providing confidence in the quality of the RNA. The mean per-position Phred scores were high (above 30) at all positions, further indicating high sequence quality. Levels of overrepresented sequences and Adapter Content were low, suggesting that the library prep was also of high quality.
RNA-seq of the Kiss1Cre+/Ribotag samples indicated that Kiss1 cells in the AVPV of adult females produce approximately 13 300 different mRNA transcripts, including Kiss1 and genes previously known to co-expressed with AVPV kisspeptin, like TH, Gal, Pgr, and Esr1 (Fig. 2). We first determined what genes were most highly expressed in AVPV Kiss1 cells, regardless of hormonal status. Secretogranin II (Scg2), a member of the secretogranin family of neuroendocrine secretory proteins, was the transcript with the highest expression. Other very highly expressed transcripts included several genes related to intracellular signaling, such as Gprasp1, Gna, Atp1a3, Calm1, and Ywhag, genes important for protein synthesis and regulation, including Cpe, Hspa8, Hsp90ab1, Eef1a1, Ubb, and Ubc, genes involved in essential cellular processes, like Gaa, Pkm, Kif1a, and Ckb, and genes involved with secretion and synapses, like App, Syp, and Rtn1. The 50 transcripts with the highest overall mean expression (ie, the mean expression of all OVX and E2 samples) in AVPV Kiss1 cells are listed in Table 1.
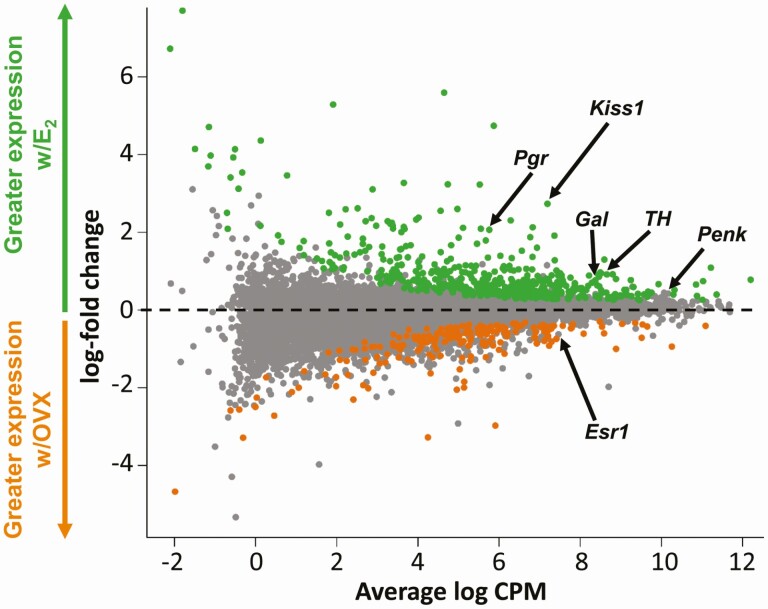
Figure 2.
A glimma plot demonstrating the expression of >13 300 transcripts selectively isolated from adult female AVPV Kiss1 cells and subjected to RNA-seq. Each dot represents a single gene transcript. The x axis represents the overall mean expression levels of the transcripts, with higher x axis values indicating higher mRNA expression. The y axis represents the expression difference between females treated with E2vs OVX females, with greater positive values indicating higher mRNA levels in E2 females and greater negative values signifying higher mRNA levels in OVX females. Green dots are gene transcripts that are expressed significantly more (P < 0.05) in the E2vs OVX condition, grey dots are transcripts that are expressed similarly between OVX and E2 conditions, and orange dots are transcripts that are expressed significantly more (P < 0.05) in the OVX vs E2 condition. Several genes previously identified in AVPV Kiss1 cells using traditional histological assays are noted by arrows.
Table 1.
The top 50 genes with the greatest expression in female AVPV Kiss1 cells
| ENSEMBL ID | ENTREZ ID | Gene | Total mean expression | Mean E2 expression | Mean OVX expression | Adjusted P-value |
|---|---|---|---|---|---|---|
| ENSMUSG00000050711 | 20 254 | Scg2 | 12.20 | 12.64 | 11.87 | < 0.01 |
| ENSMUSG00000027523 | 14 683 | Gnas | 11.69 | 11.66 | 11.71 | 0.76 |
| ENSMUSG00000015656 | 15 481 | Hspa8 | 11.67 | 11.75 | 11.61 | 0.26 |
| ENSMUSG00000025579 | 14 387 | Gaa | 11.66 | 11.70 | 11.63 | 0.61 |
| ENSMUSG00000019505 | 22 187 | Ubb | 11.47 | 11.51 | 11.44 | 0.77 |
| ENSMUSG00000040907 | 23 2975 | Atp1a3 | 11.44 | 11.51 | 11.40 | 0.32 |
| ENSMUSG00000037742 | 13 627 | Eef1a1 | 11.41 | 11.54 | 11.31 | 0.06 |
| ENSMUSG00000043388 | 243 339 | Tmem130 | 11.40 | 11.48 | 11.35 | 0.34 |
| ENSMUSG00000022892 | 11 820 | App | 11.36 | 11.59 | 11.19 | < 0.01 |
| ENSMUSG00000025151 | 94 275 | Maged1 | 11.22 | 11.84 | 10.75 | < 0.01 |
| ENSMUSG00000023944 | 15 516 | Hsp90ab1 | 11.21 | 11.24 | 11.18 | 0.58 |
| ENSMUSG00000043384 | 67 298 | Gprasp1 | 11.10 | 11.22 | 11.02 | 0.25 |
| ENSMUSG00000006930 | 15 114 | Hap1 | 11.10 | 11.19 | 11.04 | 0.19 |
| ENSMUSG00000055430 | 58 243 | Nap1l5 | 11.09 | 10.86 | 11.26 | 0.04 |
| ENSMUSG00000008348 | 22 190 | Ubc | 11.05 | 11.03 | 11.05 | 0.90 |
| ENSMUSG00000026223 | 64 294 | Itm2c | 11.03 | 11.45 | 10.71 | < 0.01 |
| ENSMUSG00000004207 | 19 156 | Psap | 11.01 | 11.16 | 10.90 | 0.02 |
| ENSMUSG00000021270 | 15 519 | Hsp90aa1 | 10.94 | 10.95 | 10.93 | 0.89 |
| ENSMUSG00000019923 | 52 696 | Zwint | 10.93 | 10.93 | 10.94 | 0.99 |
| ENSMUSG00000006651 | 11 803 | Aplp1 | 10.93 | 10.95 | 10.91 | 0.83 |
| ENSMUSG00000032294 | 18 746 | Pkm | 10.89 | 11.03 | 10.79 | 0.06 |
| ENSMUSG00000058297 | 94 214 | Spock2 | 10.88 | 11.08 | 10.72 | < 0.01 |
| ENSMUSG00000001175 | 12 313 | Calm1 | 10.85 | 11.23 | 10.57 | < 0.01 |
| ENSMUSG00000030695 | 11 674 | Aldoa | 10.77 | 10.90 | 10.68 | 0.14 |
| ENSMUSG00000020315 | 20 742 | Sptbn1 | 10.76 | 10.84 | 10.70 | 0.84 |
| ENSMUSG00000002265 | 18 616 | Peg3 | 10.75 | 10.72 | 10.78 | 0.82 |
| ENSMUSG00000070802 | 43 4128 | Pnmal2 | 10.73 | 10.65 | 10.80 | 0.27 |
| ENSMUSG00000023004 | 22 143 | Tuba1b | 10.65 | 10.64 | 10.66 | 0.95 |
| ENSMUSG00000039278 | 30 052 | Pcsk1n | 10.62 | 10.54 | 10.69 | 0.51 |
| ENSMUSG00000072235 | 22 142 | Tuba1a | 10.60 | 10.57 | 10.62 | 0.75 |
| ENSMUSG00000014602 | 16 560 | Kif1a | 10.60 | 10.65 | 10.55 | 0.51 |
| ENSMUSG00000062825 | 11 465 | Actg1 | 10.60 | 10.65 | 10.55 | 0.43 |
| ENSMUSG00000019986 | 52 906 | Ahi1 | 10.59 | 10.63 | 10.56 | 0.68 |
| ENSMUSG00000033585 | 17 984 | Ndn | 10.56 | 10.39 | 10.69 | 0.19 |
| ENSMUSG00000051391 | 22 628 | Ywhag | 10.56 | 10.67 | 10.48 | 0.10 |
| ENSMUSG00000031144 | 20 977 | Syp | 10.51 | 10.64 | 10.42 | 0.14 |
| ENSMUSG00000034994 | 13 629 | Eef2 | 10.48 | 10.60 | 10.39 | 0.06 |
| ENSMUSG00000041607 | 17 196 | Mbp | 10.44 | 10.22 | 10.61 | 0.40 |
| ENSMUSG00000057738 | 20 740 | Sptan1 | 10.44 | 10.47 | 10.42 | 0.93 |
| ENSMUSG00000037852 | 12 876 | Cpe | 10.44 | 10.43 | 10.44 | 0.93 |
| ENSMUSG00000018451 | 103 712 | 6330403K07Rik | 10.38 | 10.30 | 10.44 | 0.46 |
| ENSMUSG00000066357 | 83 669 | Wdr6 | 10.38 | 10.39 | 10.37 | 0.88 |
| ENSMUSG00000021087 | 104 001 | Rtn1 | 10.34 | 10.43 | 10.28 | 0.25 |
| ENSMUSG00000026204 | 19 275 | Ptprn | 10.32 | 10.61 | 10.10 | < 0.01 |
| ENSMUSG00000025855 | 19 085 | Prkar1b | 10.30 | 10.40 | 10.22 | 0.188 |
| ENSMUSG00000001270 | 12 709 | Ckb | 10.29 | 10.52 | 10.12 | 0.01 |
| ENSMUSG00000026576 | 11 931 | Atp1b1 | 10.27 | 10.39 | 10.18 | 0.09 |
| ENSMUSG00000025203 | 20 250 | Scd2 | 10.26 | 10.12 | 10.37 | 0.29 |
| ENSMUSG00000074657 | 16 572 | Kif5a | 10.26 | 10.35 | 10.19 | 0.28 |
| ENSMUSG00000036699 | 72 693 | Zcchc12 | 10.25 | 9.71 | 10.66 | < 0.01 |
Transcripts are listed in descending order of total mean expression (mean log CPM, regardless of hormone condition). P < 0.05 signifies significant differential expression between e2 and ovx conditions, denoted in bold type.
The RNA-seq analysis revealed genes for many receptors, neuropeptides, and neurotransmitter synthesis that are co-expressed in female AVPV Kiss1 cells. Along with confirming the few previously known co-expressed ligands (Gal, Th, and Penk), we identified a number of new ligand transcripts in AVPV Kiss1 cells (Table 2). For example, we found that AVPV Kiss1 cells, like ARC Kiss1 cells, express abundant levels of Pdyn under both E2 and OVX conditions, although significantly higher in OVX (Table 2). Vgf, Pnoc, and Cartpt were some additional newly-described ligand transcripts (Table 2). Genes relating to gamma-aminobutyric acid (GABA) synthesis or transport, like Vgat, Gad1, and Gad2 were also expressed at very high levels, with the glutamate-related gene Vglut2 also present at moderate levels. Along with confirming known co-expressed receptors ERα (Esr1), Pgr, vasopressin receptor 1a (Avpr1a), and prolactin receptor (Prlr), AVPV Kiss1 cells were also found to express a number of other receptors important for neuroendocrine or neural function, such as thyroid hormone receptor (Thra), CRH receptor (Crhr1), glucocorticoid receptor (Nr3c1), androgen receptor (AR), kappa opioid receptor (Oprl1), and multiple variants of the neuropeptide Y receptor (Npy1r, Npy2r, Npy4r, Npy5r) (Table 2). Interestingly, while Esr1 was very highly expressed, Esr2 (estrogen receptor beta) was only present at very low levels (Table 2).
Table 2.
Genes related to a number of ligands (top) and receptors (bottom) expressed to different degrees in AVPV Kiss1 cells
| LIGANDSENSEMBL ID | ENTREZ ID | Gene | Overall Mean expression |
Mean E2 expression | Mean OVX expression | Log FC | Adjusted p value |
|---|---|---|---|---|---|---|---|
| ENSMUSG00000045573 | 18 619 | Penk | 10.164 | 10.405 | 9.984 | -0.422 | 0.057 |
| ENSMUSG00000037428 | 381 677 | Vgf | 9.928 | 10.307 | 9.644 | -0.664 | 0.001 |
| ENSMUSG00000026787 | 14 417 | Gad2 | 9.757 | 10.01 | 9.566 | -0.445 | 0.003 |
| ENSMUSG00000070880 | 14 415 | Gad1 | 9.460 | 9.519 | 9.417 | 0.102 | 0.351 |
| ENSMUSG00000037771 | 22 348 | Vgat | 8.640 | 8.673 | 8.716 | -0.076 | 0.655 |
| ENSMUSG00000000214 | 21 823 | Th | 8.655 | 9.177 | 8.263 | -0.915 | <0.001 |
| ENSMUSG00000116158 | 280 287 | Kiss1 | 7.192 | 8.759 | 6.016 | -2.734 | <0.001 |
| ENSMUSG00000024907 | 14 419 | Gal | 8.287 | 8.686 | 7.987 | -0.701 | 0.020 |
| ENSMUSG00000021647 | 27 220 | Cartpt | 7.372 | 8.104 | 6.823 | 1.278 | <0.001 |
| ENSMUSG00000029361 | 18 125 | Nos1 | 7.314 | 7.538 | 7.145 | -0.393 | 0.010 |
| ENSMUSG00000027400 | 18 610 | Pdyn | 7.207 | 6.824 | 7.494 | 0.669 | 0.022 |
| ENSMUSG00000045731 | 18 155 | Pnoc | 6.652 | 6.970 | 6.414 | -0.556 | 0.025 |
| ENSMUSG00000031980 | 11 606 | Agt | 6.311 | 5.922 | 6.603 | 0.687 | 0.059 |
| ENSMUSG00000030500 | 140 919 | Vglut2 | 5.762 | 5.840 | 5.704 | -0.140 | 0.537 |
| ENSMUSG00000005892 | 22 044 | Trh | 5.285 | 5.220 | 5.333 | 0.106 | 0.838 |
| ENSMUSG00000049796 | 12 918 | Crh | 5.075 | 5.023 | 5.115 | 0.086 | 0.905 |
| ENSMUSG00000061762 | 21 333 | Tac1 | 4.554 | 4.313 | 4.735 | 0.432 | 0.229 |
| ENSMUSG00000024256 | 11 516 | Adcyap1 | 4.147 | 4.459 | 3.913 | -0.557 | 0.152 |
| ENSMUSG00000029236 | 56 183 | Nmu | 4.132 | 4.145 | 4.121 | -0.032 | 0.926 |
| ENSMUSG00000032532 | 12 424 | Cck | 3.495 | 4.005 | 3.112 | -0.902 | 0.157 |
| RECEPTORSENSEMBL ID | ENTREZ ID | Gene | OverallMean Expression | Mean E 2 expression | Mean OVXexpression | Log FC | Adjusted P value |
| ENSMUSG00000058756 | 21 833 | Thra | 9.075 | 8.895 | 9.210 | 0.314 | 0.143 |
| ENSMUSG00000029778 | 11 517 | Pacapr1 | 8.478 | 8.358 | 8.569 | 0.211 | 0.163 |
| ENSMUSG00000019768 | 13 982 | Esr1 | 7.884 | 7.672 | 8.044 | 0.372 | 0.105 |
| ENSMUSG00000027584 | 18 389 | Oprl1 | 7.176 | 7.086 | 7.244 | 0.157 | 0.303 |
| ENSMUSG00000024431 | 14 815 | Nr3c1 | 6.542 | 6.296 | 6.726 | 0.430 | 0.056 |
| ENSMUSG00000005268 | 19 116 | Prlr | 6.476 | 6.883 | 6.171 | -0.714 | 0.011 |
| ENSMUSG00000044288 | 12 801 | Cnr1 | 6.408 | 6.092 | 6.645 | 0.554 | 0.023 |
| ENSMUSG00000046532 | 11 835 | Ar | 6.279 | 6.394 | 6.192 | -0.205 | 0.366 |
| ENSMUSG00000039059 | 99 296 | Hrh3 | 6.180 | 6.350 | 6.052 | -0.301 | 0.243 |
| ENSMUSG00000025905 | 18 387 | Oprk1 | 6.022 | 5.874 | 6.133 | 0.254 | 0.215 |
| ENSMUSG00000028004 | 18 167 | Npy2r | 5.908 | 4.206 | 7.185 | 2.976 | <0.001 |
| ENSMUSG00000031870 | 18 667 | Pgr | 5.759 | 6.936 | 4.875 | -2.064 | <0.001 |
| ENSMUSG00000021779 | 21 834 | Thrb | 4.223 | 4.296 | 4.169 | -0.132 | 0.655 |
| ENSMUSG00000055737 | 146 00 | Ghr | 4.046 | 4.068 | 4.030 | -0.044 | 0.895 |
| ENSMUSG00000036437 | 18 166 | Npy1r | 4.031 | 4.360 | 3.785 | -0.595 | 0.117 |
| ENSMUSG00000030043 | 21 336 | Tacr1 | 3.847 | 3.731 | 3.934 | 0.201 | 0.485 |
| ENSMUSG00000044014 | 18 168 | Npy5r | 3.524 | 2.967 | 3.942 | 0.964 | 0.029 |
| ENSMUSG00000047259 | 17 202 | Mc4r | 3.363 | 3.537 | 3.232 | -.0318 | 0.496 |
| ENSMUSG00000018634 | 12 921 | Crhr1 | 3.067 | 2.796 | 3.270 | 0.480 | 0.289 |
| ENSMUSG00000011171 | 22 355 | Vipr2 | 3.032 | 2.912 | 3.122 | 0.208 | 0.640 |
| ENSMUSG00000020123 | 54 140 | Avpr1a | 1.919 | 1.440 | 2.277 | 0.845 | 0.136 |
| ENSMUSG00000021055 | 13 983 | Esr2 | 1.379 | 1.358 | 1.395 | 0.033 | 0.971 |
| ENSMUSG00000048337 | 19 065 | Npy4r | 0.130 | 2.620 | -1.740 | -4.360 | < 0.001 |
Transcripts are listed in descending order of overall mean expression (mean logCPM from all samples, regardless of hormonal condition). P-values < 0.05 denote significantly different expression between OVX and E2 conditions.
Differential Expression of Gene Transcripts in AVPV Kiss1 Cells
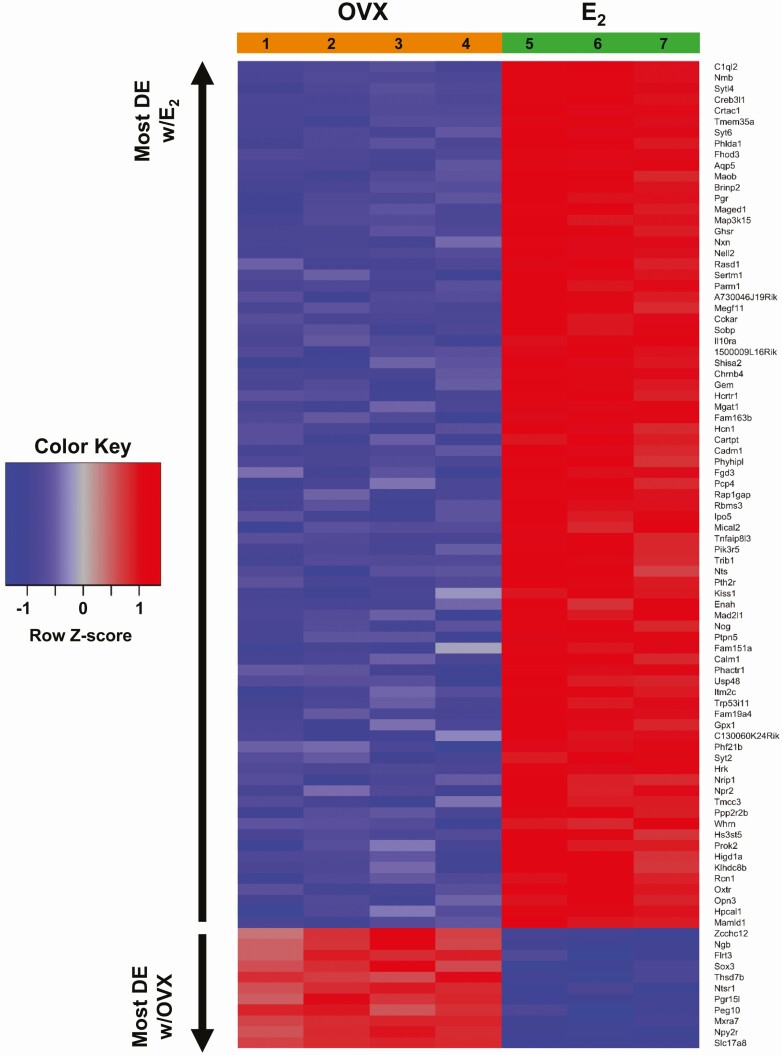
Kiss1 cells in the AVPV are regulated by E2, with higher AVPV Kiss1 expression when E2 is elevated and lower Kiss1 expression in OVX (no E2) females. One of our goals was to identify what other specific transcripts are significantly increased or decreased in AVPV Kiss1 cells under conditions of elevated E2vs absent E2. Of the >13 300 transcripts in AVPV Kiss1 cells, 683 gene transcripts were significantly differentially expressed based on E2 status, with 484 transcripts, including Kiss1, being significantly higher in the E2 condition (Fig. 2, green dots; P < 0.05) and 199 transcripts being significantly higher in the OVX condition (Fig. 2, orange dots; P < 0.05). The remaining >12 600 genes did not show significantly altered expression levels with or without E2 (Fig. 2, gray dots). Fig. 3 depicts a heat map showing the expressions levels of the top 90 genes with the greatest differential expression between E2 and OVX conditions; interestingly, the vast majority (~90%) of these top 90 differentially expressed genes, including Kiss1, were higher in the E2 condition.
Figure 3.
A heat map depicting the top 90 (based on t value) AVPV Kiss1 cell transcripts that are most differentially expressed (DE) between E2 and OVX conditions. Of these 90 gene transcripts, 79 were more highly expressed in E2-treated females, whereas 11 were expressed more in the OVX condition. Row Z-scores indicate the relationship between the transcript expression level for that sample and mean expression level of that transcript, with red representing high gene expression and blue representing low gene expression. The individual sample numbers, 4 for the OVX condition and 3 for E2 condition, are provided at the top of the heat map underneath the appropriate hormonal treatment.
Table 2 compares the expression levels between OVX and E2 conditions for a number of relevant ligands (or their synthesizing proteins) and receptors found in AVPV Kiss1 cells. As expected, differential expression analysis revealed that the known E2-responsive gene Pgr was, like Kiss1, significantly higher in E2vs OVX (no E2) females (Figs. 2 and 3; Table 2). Several other ligand or receptor genes, such as TH, Gal, Gad2, Prlr, and Npy4r, also exhibited significantly greater expression levels with E2 treatment compared to OVX (P < 0.05), whereas fewer genes, like Npy2r, Npy5r, Pdyn, and Cnr1, were expressed higher in OVX than E2 conditions (P < 0.05; Fig. 3; Table 2). Many other ligand and receptor genes, like Esr1, Penk, Gad1, Vgat, and Vglut2 were expressed at similar levels between OVX and E2 conditions (Table 2).
Biological Pathways Regulated by Gene Transcripts Expressed in AVPV Kiss1 Neurons
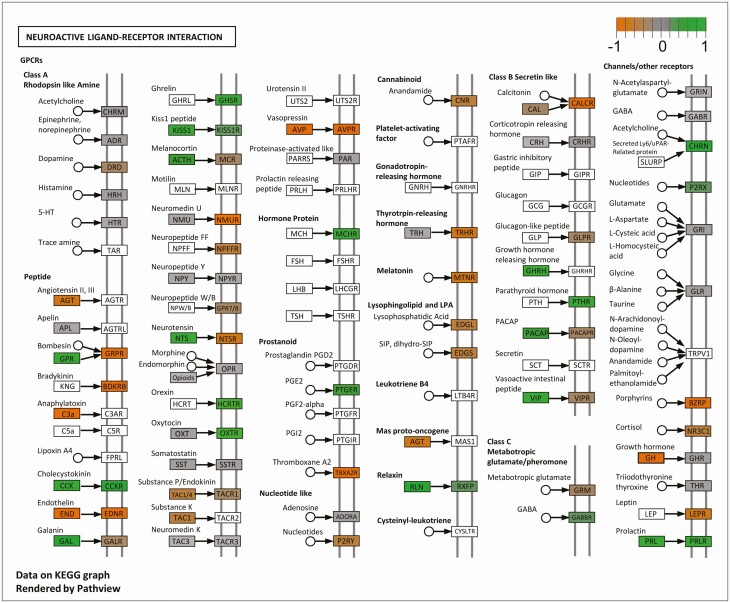
KEGG pathway analysis (63) was performed to determine how RNA transcripts expressed by AVPV Kiss1 cells cluster within specific known biological pathways and whether the expression of these clustered transcripts tends to activate or inhibit these biological processes. Of the top 10 KEGG pathways with the lowest false discovery rate (pGFdr) represented by AVPV Kiss1 cell RNA transcripts, regardless of hormone status, there were several signaling pathways, such as the mitogen-activated protein kinase and calcium signaling pathways, as well as pathways involved in amphetamine addiction and alcoholism (Table 3). Comparing specifically between the E2 and OVX (no E2) groups, there were only 3 KEGG pathways that were significantly different between the 2 hormone conditions (P < 0.05 for each), of which the neuroactive ligand-receptor interaction (NLRI) pathway contained the most genes and was the most significant (based on the lowest false discovery rate and the lowest P-value; Table 3). This particular KEGG pathway, shown in Figure 4, includes many neuropeptides and receptors known to regulate neural and endocrine function and/or reproduction. The specific gene groups (containing 1 or more genes) in the NLRI pathway found to be significantly expressed in AVPV Kiss1 cells are depicted as nonwhite boxes in Figure 4 (ie, all gray, green, and orange boxes); gene groups in the NLRI pathway that were not expressed in our data set are depicted as white boxes. Overall, our AVPV Kiss1 cell data set had 86 gene groups—comprising 175 individual gene transcripts—that were expressed in this NLRI pathway.
Table 3.
KEGG biological pathways represented by gene transcripts produced by AVPV Kiss1 cells
| Pathway name | ID | Pathway genes expressed in AVPV Kiss1 cells (n) | Differentially expressed genes (n) | False discovery rate | P-value | Status |
|---|---|---|---|---|---|---|
| Top 10 KEGG pathways (regardless of hormonal status) | ||||||
| Mitogen-activated protein kinase signaling pathway | 04010 | 219 | 0.00022 | |||
| Amphetamine addiction | 05031 | 60 | 0.00037 | |||
| Regulation of actin cytoskeleton | 04810 | 167 | 0.00063 | |||
| Calcium signaling pathway | 04020 | 143 | 0.00063 | |||
| Alcoholism | 05034 | 104 | 0.00088 | |||
| Basal cell carcinoma | 05217 | 42 | 0.00092 | |||
| Herpes simplex infection | 05168 | 127 | 0.00108 | |||
| Cytokine-cytokine receptor interaction | 04060 | 86 | 0.00108 | |||
| Neurotrophin signaling pathway | 04722 | 111 | 0.00899 | |||
| RNA transport | 03013 | 143 | 0.01451 | |||
| KEGG pathways that differ significantly between E2 and OVX (no E2) conditions | ||||||
| Neuroactive ligand-receptor interaction | 04080 | 175 | 32 | 0.00022 | <0.001 | Activated |
| Amphetamine addiction | 05031 | 60 | 12 | 0.00574 | <0.05 | Activated |
| Jak-STAT signaling pathway | 04630 | 89 | 13 | 0.01656 | <0.05 | Inhibited |
The top 10 biological pathways (identified and sorted by the lowest false discovery rate), represented by transcripts expressed by AVPV Kiss1 cells, regardless of hormonal status. The only 3 KEGG pathways that differed significantly (P < 0.05) between E2 and OVX females are listed at the bottom, with the neuroactive ligand-receptor interaction pathway containing the most differentially expressed transcripts. The status column refers to an estimated overall effect of all the identified genes, relative to the E2 condition (ie, “activated” or “inhibited” more in the E2 group).
Figure 4.
The neuroactive ligand-receptor interaction (NLRI) KEGG pathway and gene groups that are significantly expressed by AVPV Kiss1 cells in this pathway. Grey shaded boxes are genes or gene groups that are expressed but not significantly different between OVX and E2 females. Green shaded boxes are genes or gene groups that are expressed at significantly greater levels (P < 0.05) in E2 than OVX females, whereas orange boxes depict greater expression (P < 0.05) in OVX females. White boxes are genes in this NLRI pathway that were not expressed in our Kiss1-Ribotag RNA-seq dataset.
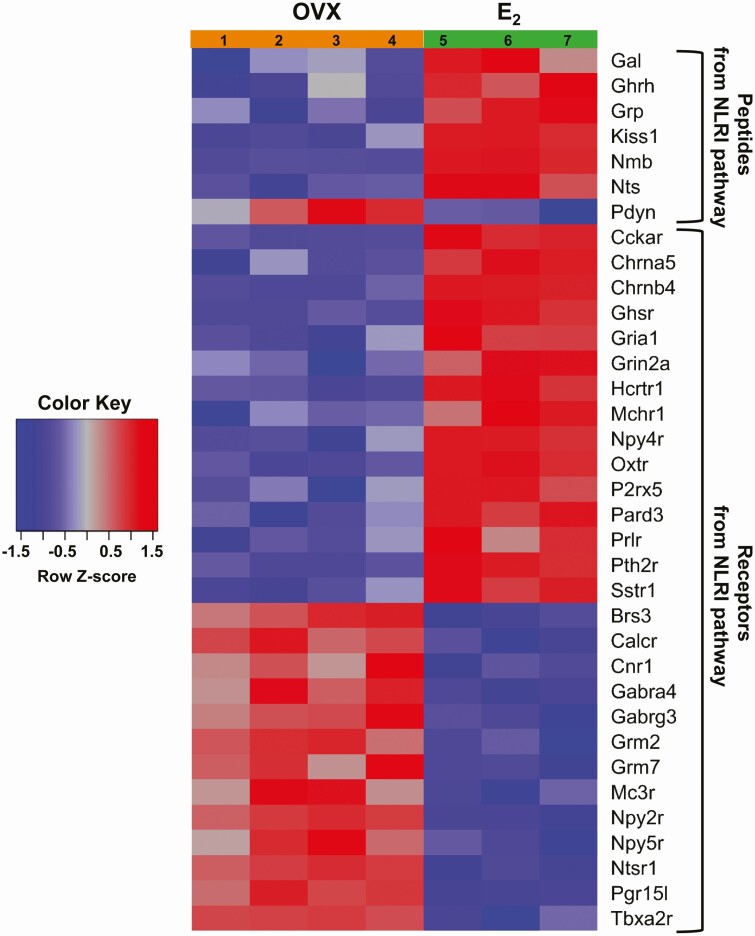
Gene groups in the NLRI pathway that were differentially expressed (P < 0.05) between E2 and OVX (no E2) conditions are depicted in Figure 4 as green or orange boxes, respectively. Of the 175 individual transcripts in the NLRI pathway found to be expressed in AVPV Kiss1 cells, only 32 transcripts (6 for peptides and 26 for receptors), were differentially expressed between E2 and OVX conditions, and these are shown in a heat map in Figure 5. Interestingly, 5 of the 6 differentially expressed peptide transcripts, including Kiss1, were more highly expressed with E2 treatment, whereas Pdyn was the only protein ligand in the NLRI pathway whose RNA was expressed at higher levels in the OVX condition (Fig. 5). Of the 26 receptors in the NLRI pathway that were differentially expressed between E2 and OVX, approximately half [such as cholecystokinin receptor A (Cckar), NPY receptor 4 (Npy4r), and prolactin receptor (Prlr)] had higher expression with E2 while the other half [including NPY receptor 2 (Npy2r), calcitonin receptor (Calcr), and some GABA receptor subunits (Garbra3, Gabra4)] had greater expression in the OVX condition (Fig. 5).
Figure 5.
Heat map of the 32 individual AVPV Kiss1 cell transcripts in the NLRI KEGG pathway (Fig. 4) that are significantly differentially expressed (P < 0.05) between E2 and OVX conditions. A total of 6 peptide transcripts and 26 receptor transcripts from AVPV Kiss1 cells were differentially expressed in the NLRI pathway and are listed alphabetically in the heat map. Row Z-scores indicate the relationship between the transcript expression level for that sample and mean expression level of that transcript, with red denoting high expression and blue denoting low expression. The individual sample numbers are provided at the top of the heat map underneath the appropriate hormonal treatment.
Validation of RNA-seq data using triple label ISH
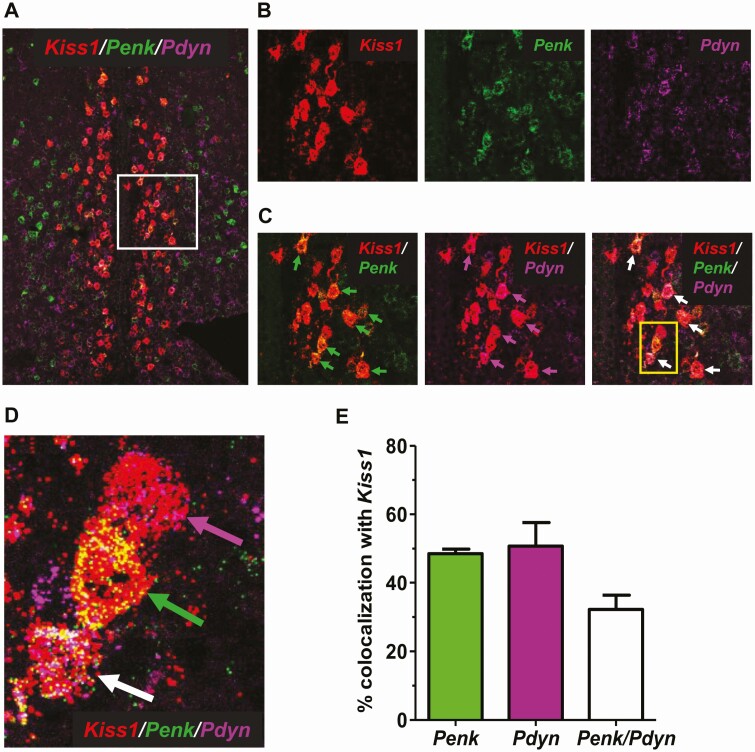
Prior studies relied on co-labeling histology experiments to identify genes (or their protein products) co-expressed in AVPV Kiss1 cells; to date, such studies have demonstrated about a dozen such co-expressed genes or proteins (21,45-49,69,70). Our present RNA-seq data identified thousands of mRNA transcripts expressed in female AVPV Kiss1 cells, including many for signaling factors or their receptors. To use a second method to confirm that transcripts identified in our Kiss1Cre+/Ribotag experiment are in fact expressed in the AVPV cells that actively express Kiss1 in adulthood, we performed several ISH assays in AVPV sections collected from adult diestrus females. In the first assay, in addition to Kiss1 expression, we examined Penk because it was very highly expressed in our RNA-seq data (Table 2) and met-enkephalin, a protein products of Penk, was previously reported to be moderately (~33%) co-expressed with kisspeptin protein in the AVPV (assessed with IHC) (48). We also examined Pdyn because it was highly expressed in our RNA-seq data and was the only neuropeptide ligand differentially expressed at higher levels in the OVX group (Table 2, Fig. 5), which may have interesting functional implications. In addition, Pdyn is known to be co-expressed in ARC Kiss1 cells but has not yet been shown to be expressed in other Kiss1 populations. In a triple-label ISH assay, we found that Kiss1, Penk, and Pdyn are all highly expressed in the AVPV region of diestrus females and show considerable co-expression with each other (Fig. 6). More than 50% of AVPV Kiss1 cells also co-express Penk (Fig. 6C and 6E), and similarly, ~50% of AVPV Kiss1 cells co-express Pdyn (Fig. 6C and 6E). Moreover, there were many identified AVPV cells where all 3 genes were co-expressed (Fig. 6C and 6D); specifically, ~32% of AVPV Kiss1 cells co-expressed both Penk and Pdyn (Fig. 6E) in diestrus females.
Figure 6.
Triple-label ISH analysis of Kiss1, Penk, and Pdyn coexpression in the female mouse AVPV. (A) A representative image of cells in the AVPV of a diestrus female, with Kiss1 (red), Penk (green), and Pdyn (purple) expression depicted. (B and C) Higher magnification images of the white boxed area in (A), showing good Kiss1 coexpression with Penk and/or Pdyn in the AVPV. (D) Higher magnification image of the 3 cells from the yellow box in C, showing co-expression of Kiss1/Penk (green arrow), Kiss1/Pdyn (purple arrow), or Kiss1/Penk/Pdyn (white arrow) in the AVPV. (E) Mean + SE percentage coexpression between Kiss1, Penk, and Pdyn in AVPV cells (n = 3 diestrus females).
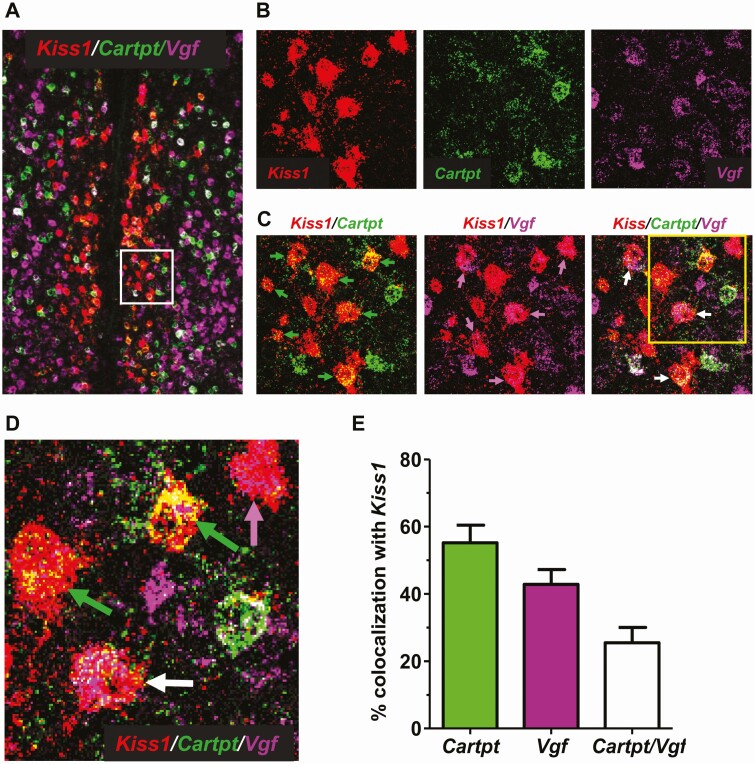
In another ISH assay, we validated 2 additional candidate genes, Cartpt and Vgf, that were highly expressed in the RNA-seq data (Table 2). Both Cartpt (CART) and Vgf (VGF nerve growth factor inducible) are known to be expressed in the AVPV region but have not been examined specifically in Kiss1 cells. Confirming our RNA-seq results, we found that both Cartpt and Vgf show significant co-expression with Kiss1 in the AVPV region of diestrus females (Fig. 7). Approximately 55% of AVPV Kiss1 cells co-express Cartpt (Fig. 7C and 7E), while ~40% of AVPV Kiss1 cells co-express Vgf (Fig. 7C and 7E). In these diestrus females, ~25% of identified AVPV Kiss1 cells co-expressed both Cartpt and Vgf (Fig. 7D and 7E).
Figure 7.
Triple-label ISH analysis of Kiss1, Cartpt, and Vgf co-expression in the AVPV of adult female diestrus mice. (A) A representative image of AVPV from a diestrus female, with Kiss1 (red), Cartpt (green), and Vgf (purple) expression depicted. (B and C) Higher magnification images of the white boxed area in (A), showing Kiss1 co-expression with Cartpt and/or Vgf. (D) Higher magnification image of cells from the yellow box in (C), showing co-expression of Kiss1/Cartpt (green arrow), Kiss1/Vgf (purple arrow), or Kiss1/Cartpt/Vgf (white arrow). (E) Mean + SE percentage coexpression between Kiss1, Cartpt, and Vgf in AVPV cells (n = 3 diestrus females).
Discussion
Kisspeptin neurons in the AVPV region are important components of the LH surge mechanism that triggers ovulation in females (5,42,71). Prior studies demonstrated that E2 upregulates Kiss1 gene expression in the AVPV (19,20) and permits circadian activation of these neurons in synchrony with the LH surge (21,39). Yet, how these AVPV Kiss1 neurons are regulated, positively or negatively, by other hormones or upstream neurons in relation to the surge process is still not completely characterized, nor have other possible co-transmitters in these Kiss1 neurons been fully determined. In addition, whether or not AVPV Kiss1 neurons participate in other reproductive and/or nonreproductive processes still remains unclear, in part because the phenotype of these cells is incompletely understood. To begin to address these outstanding issues, the present study asked several key questions, including (1) what other genes are actively co-expressed in AVPV Kiss1 cells of adult females? (2) Are there other co-expressed genes in these AVPV cells besides Kiss1 that are also under E2 regulation, and, if so, are these other genes stimulated or inhibited by E2? and (3) Are there other specific ligands or receptors present in AVPV Kiss1 cells that could lend insight into possible functions or regulation of these cells? The heterogenous nature of the AVPV region has restricted prior investigations to double label ISH or IHC studies. While useful, such histology approaches are costly and time consuming for assessing many genes and typically rely on studying targeted candidates one by one. We overcame this limitation here by using the Ribotag technique to selectively isolate all actively translated mRNA from just AVPV Kiss1 neurons, permitting identification of thousands of co-expressed gene transcripts and identifying estrogen effects on both known and novel genes that may be critical for AVPV Kiss1 neuron function.
Our goal was to isolate mRNA transcripts from Kiss1 neurons to identify on a large scale which other transcripts are co-expressed in these cells, at what levels these transcripts are expressed (high, medium, low), whether E2 exposure alters such expression levels, and, if so, in which direction (stimulatory, inhibitory). Before running the RNA-seq, we first used 4 complimentary assessments to validate that our Ribotag method properly isolated Kiss1 cell-specific transcripts. First, we performed PCR to confirm that Kiss1 mRNA was in fact present in the IP of Cre+ samples. Second, we used PCR to similarly confirm that Th, a gene previously shown with double ISH and IHC (for protein) to be highly coexpressed with AVPV Kiss1, was also expressed in the IP of Cre+ samples (Fig. 1). Third, we confirmed that GnRH, which is present in the greater AVPV/POA area but not in Kiss1 neurons, was expressed in the IN of all samples but not in the IP of any Cre+ samples (Fig. 1). This supports that the pulldown is selective and not isolating mRNA from non-Kiss1 cells (ie, GnRH cells) that are present in the IN. Fourth, we performed PCR on IN and IP samples from Cre− controls that do not have HA-tagged ribosomes in Kiss1 cells (or any other cells). The Cre− samples all expressed Kiss1, Th, and GnRH in their IN, as expected, but did not express any of these genes in their IP (Fig. 1), confirming that the pulldown method relies on Cre+ expression and does not appear to nonselectively isolate mRNA. Collectively, these validation steps showed that our Ribotag procedure isolated several mRNAs known to be in Kiss1 neurons, did not isolate mRNA known to be absent in Kiss1 neurons, and did not nonselectively isolate mRNA if Cre+ was absent. This gives confidence that the mRNAs identified with the subsequent RNA-seq are likely from AVPV Kiss1 neurons. Further supporting this, our RNA-seq data confirmed expression of the dozen or so known genes (or their protein products) previously reported to be co-expressed to varying degrees in AVPV Kiss1 cells, including Th (46, 47), Gal (48), Penk (48), Esr1 (20, 21), Esr2 (20, 69), Pgr (45), Avpr1a (49), Crh1 (70), Prlr (72), and Mc4R (53). Finally, as discussed more in the following text, triple-label ISH (Figs. 6 and 7) confirmed that Pdyn, Cartpt, and Vgf, genes newly identified in our RNA-seq data, were indeed co-expressed with Kiss1 mRNA in the AVPV of female mice.
The RNA-seq identified approximately 13 300 genes that were significantly expressed in AVPV Kiss1 neurons. Not surprisingly, many of the highest-expressing genes were for standard cell maintenance, intracellular signaling, protein synthesis and regulation, and neural signaling/secretion. This was echoed in some of the top KEGG biological pathways implicated by the gene transcripts, which contain genes important for critical cellular processes of neurons such as cellular organization, axonal growth, and organelle transfer (regulation of actin cytoskeleton), intracellular signal transduction (mitogen-activated protein kinase signaling pathway, calcium signaling pathway), neural firing, neurotransmitter signaling, and intercell interaction (cytokine-cytokine receptor interaction, calcium signaling pathway, amphetamine addiction), neural cell differentiation and survival (neurotrophin signaling pathway), and protein synthesis and regulation (RNA transport). Thus, many of the genes represented in some of these top pathways are likely found in many neurons, including but not limited to AVPV Kiss1 neurons. Interestingly, transcripts found in AVPV Kiss1 cells were highly represented in 2 of the 5 substance-dependence KEGG pathways, amphetamine addiction and alcoholism, perhaps because both of these KEGG pathways heavily involve dopamine and glutamate signaling (along with dynorphin), which AVPV Kiss1 neurons are known to co-express but whose function in these neurons—reproductive or other—is still unknown. The KEGG pathway that was most differentially expressed between E2 and OVX was the NLRI pathway, which contains Kiss1 and many other ligands and receptors involved in neural communication and regulation. This KEGG pathway revealed a number of newly identified co-expressed factors, including some that were E2 sensitive, which may either be co-released by AVPV kisspeptin neurons or underlie the regulation of these neurons by afferent circuits. The amphetamine addiction KEGG pathway, which has a number of neural signaling factors represented like dopamine, glutamate, and dynorphin, was also differentially expressed between E2 and OVX females.
Our overall mean expression analysis, along with the differentially expressed KEGG analysis, identified a number of neuropeptide ligands, or in the case of neurotransmitters, related synthesis or transport proteins, as well as receptors that are likely expressed in AVPV Kiss1 cells. Some of these co-expressed ligands and receptors were previously identified with double-label ISH or IHC assays, as previously noted. Along with those known genes, our RNA-seq data identified, for the first time, additional ligands and receptors of interest present in AVPV Kiss1 cells, including genes for neuropeptides (dynorphin, VGF nerve growth factor, CART, and nociceptin/orphanin FQ), neurotransmitters (GABA and glutamate), hormone receptors (thyroid hormone receptor, glucocorticoid receptor, and androgen receptor), and neuropeptide/neurotransmitter receptors (kappa opioid receptor, NPY receptor subtypes, and GABA receptors). These findings identify new signaling factors that may be co-released along with kisspeptin. Actions of these secreted factors could relate to the known role of AVPV kisspeptin cells in governing GnRH neurons during the preovulatory LH surge (E2 positive feedback) or yet-to-be identified functions of AVPV kisspeptin cells in other process.
The novel identification of co-expressed receptors in AVPV kisspeptin cells could help identify how these cells are regulated by endocrine or neural factors. Like with the co-expressed ligands, the role of these expressed receptors could relate to the E2-induced LH surge mechanism or to other reproductive or nonreproductive processes that are currently unknown. Interestingly, and perhaps not surprisingly, many of the newly identified ligands and receptors have been linked in one way or another to GnRH and LH secretion and/or reproduction, although whether any of those roles are due to these proteins specifically in AVPV kisspeptin cells is not yet known. For other gene transcripts not already linked to reproduction, future studies can test whether they are related to the LH surge or other reproductive processes or, conversely, have nonreproductive functions, as is the case with ARC kisspeptin cells, which have also recently been implicated in temperature control and metabolism/energy balance (73-79). Relatedly, it is also important to stress that the function of identified receptors may or may not be to regulate the Kiss1 gene (or kisspeptin protein) specifically, but rather may regulate other genes or processes in these cells. For example, AR signaling is known to have no effect on AVPV Kiss1 gene expression, suggesting AR’s role may be to affect other genes or processes in AVPV Kiss1 cells. Ideally, the present data can be used as a spring board for future hypothesis testing of possible functional roles of AVPV kisspeptin neurons.
Many prior studies demonstrated that AVPV Kiss1 expression is stimulated by E2 and reduced in no E2 (OVX) or low E2 conditions. A present goal was to identify other transcripts in AVPV Kiss1 cells that are significantly increased or decreased by elevated E2. This could help identify additional players in the LH surge mechanism of which AVPV kisspeptin cells are integral. A prior large-scale expression study identified a number of genes in the entire AVPV/POA region as a whole that change expression with E2status, but it was not known in which specific cells, including Kiss1 cells, most changes were occurring (80). Here, we identified nearly 700 gene transcripts that appear to be differentially expressed in AVPV Kiss1 cells based on E2 status, with almost 500 transcripts being stimulated by E2, similar to the Kiss1 gene, and ~200 transcripts being reduced by E2 (Figs. 2, 3, and 5; Tables 1 and 2). Note that it is currently unknown which of these gene effects are due to E2 action directly in kisspeptin cells vs indirectly via other upstream neurons (or secondary effects). Supporting the possibility of some direct E2 regulation, AVPV Kiss1 cells highly express Esr1 (Table 2) (20, 21). Esr2 was also expressed, although only at very low levels, which matches previous ISH findings (20) of much lower co-expression of Esr2 than Esr1 with Kiss1. Still, some of the differential expression of genes in our data set may possibly be indirect due to E2 actions elsewhere. Regardless, it is likely that translational changes observed under the 2 E2 conditions could ultimately relate to E2-regulated processes, including most obviously the E2-driven LH surge and perhaps other yet-to-be identified behaviors or physiological events modulated by estrogens.
The genes identified in this study give insight into possible functions and regulation of AVPV kisspeptin cells. We have tried to be conservative in our analyses, and the present RNA-seq data ultimately requires additional confirmation from other methods to provide converging evidence for Kiss1 cell coexpression. To this end, we performed several triple-label ISH assays to validate the finding of 4 highly expressed genes, Pdyn, Penk, Cartpt, and Vgf, in the RNA-seq data set. None of these 4 genes had previously been reported to be co-expressed with Kiss1 in the AVPV, although 1 of Penk’s protein products, met-enkephalin, had been reported to be expressed with ~1/3 of AVPV kisspeptin neurons in colchicine-treated mice (measured with IHC (48)). Pdyn is known to be co-expressed with ARC kisspeptin neurons but not yet shown for other kisspeptin populations (34,55,81-84). Supporting our RNA-seq findings, the RNAscope assays found that a large number—approximately 40% to 55%—of AVPV Kiss1 cells express either Pdyn, Penk, Cartpt, or Vgf. The percentage of Penk co-labeling with Kiss1 was slightly higher than in the prior met-enkephalin/kisspeptin IHC study (~50% vs ~33%), although this could be due to differences in techniques, RNA vs protein levels, or estrogen status (diestrus females in our study vs mixed cycle stage in the IHC study). Pdyn and dynorphin protein had previously been reported in the greater AVPV region of rats (64,85), but this is the first demonstration of Pdyn (or Cartpt and Vgf) specifically in AVPV Kiss1 cells. The functional role of dynorphin, or any of the other 3 co-expressed neuropeptides for that matter, in these Kiss1 cells is unknown, although it is interesting that Pdyn levels in AVPV Kiss1 cells increase in the absence of E2, similar to Pdyn expression in the ARC region where it is also co-expressed with Kiss1 (55,81,83). Given that our ISH was in diestrus females, it is possible Pdyn/Kiss1 co-expression levels may be even higher than observed (50%) in other conditions where E2 levels are lower or absent, such as OVX or reproductive senescence. Likewise, the degree of Kiss1 co-expression with Cartpt and Vgf, both higher with E2 in our RNA-seq data, could change depending on sex steroid status.
Future studies can use the present RNA-seq findings to guide mechanistic interrogations of AVPV kisspeptin cell function and regulation. While our present ISH data validates the RNA-seq data for Pdyn, Penk, Cartpt, and Vgf genes, for thoroughness, additional co-labeling assays are needed in future studies to confirm co-expression of other genes of interest identified in this study. For such validation, it will be important to consider beforehand factors which may decrease or increase a given gene’s expression, such as sex steroid levels and female cycle stage, age, sex, circadian time of day, and metabolic status. For example, in our RNA-seq data, Npy4r was present at low to moderate levels under elevated E2 but virtually absent in OVX samples, whereas Npy2r was expressed at much higher levels under OVX than E2 conditions (Table 2). Indeed, our present data are limited to adult female mice with either elevated or absent circulating E2. Whether similar genes are co-expressed in males or at different physiological states remains to be determined, as does assessment of gene expression at different circadian times, given that AVPV kisspeptin cells are under control of the suprachiasmatic nucleus clock (21,39). As a final consideration for future IHC validation studies, our RNA-seq measured actively translated mRNAs, which are likely to correlate with protein levels; yet, it is possible in some cases that posttranslational modifications could lead to differences between the mRNA levels identified here and protein levels.
There are several considerations when interpreting the present data. The first is that we isolated mRNA from Kiss1 neurons across the entire AVPV-PeN continuum. Currently, these Kiss1 cells are thought of as one population that extends from the AVPV into the PeN, and there have yet to be reports of differential co-expression (or functional differences) between AVPV and PeN Kiss1 neurons. Still, we do not know if specific RNA-seq genes are co-expressed with Kiss1 in just the AVPV nucleus, just the PeN nucleus, or both; future histological studies are needed to assess this. The second consideration is that the present method used Kiss1Cre-mediated induction of the HA ribosome tag, which remains present thereafter, regardless of subsequent Kiss1 gene expression levels. In female mice, the Kiss1 gene first turns on in the AVPV ~2 weeks after birth (47), increasing thereafter to reach adult levels by ~ 4 weeks old (86). Thus, at the time of brain collection in adulthood, some AVPV cells with HA-tagged ribosomes may no longer express Kiss1 at all or may not currently be expressing the Kiss1 gene at that moment. Thus, the RNA-seq–identified transcripts require further histological co-expression validation to see if these genes co-express with AVPV cells actively expressing Kiss1, as was done in our Kiss1/Pdyn/Penk and Kiss1/Cartpt/Vgf triple-label assays. Finally, although unlikely, we cannot exclude the small possibility that polyribosomes in axons projecting from arcuate Kiss1 neurons to the AVPV region may have been captured in the AVPV tissue punches. As with the other caveats, this can be addressed with double-label validation assays. Ultimately, we view the present work as a starting point and valuable resource to the field for testing new hypotheses of AVPV kisspeptin cell regulation and functions, but we emphasize that such studies should first validate and confirm the co-expression of identified genes of interest.
Acknowledgements
The authors thank Adriana Esparza, Paige Steffen, and Ruby Parra for general lab assistance and Chanond Nasamran of the CCBB for assistance with RNA-seq data analysis.
Financial Support: This research was supported by National Institutes of Health (NIH) grants R01 HD090161, R01 HD100580, and P50 HD012303 to ASK and by NIH R00 HD092894 to SBZS. The University of Virginia Ligand Assay Core is supported by NIH R24HD102061. The Center for Computational Biology & Bioinformatics is funded through the Altman Clinical and Translational Research Institute (ACTRI) grant UL1TR001442. The UCSD IGM Genomics Center received support from NIH SIG grant S10 OD026929.
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
Some data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author by reasonable request or are publicly available in the Gene Expression Omnibus data repository noted in the references.
References
- 1. Toranzo D, Dupont E, Simard J, et al. . Regulation of pro-gonadotropin-releasing hormone gene expression by sex steroids in the brain of male and female rats. Mol Endocrinol. 1989;3(11):1748-1756. [DOI] [PubMed] [Google Scholar]
- 2. Kalra PS, Kalra SP. Modulation of hypothalamic luteinizing hormone-releasing hormone levels by intracranial and subcutaneous implants of gonadal steroids in castrated rats: effects of androgen and estrogen antagonists. Endocrinology. 1980;106(1):390-397. [DOI] [PubMed] [Google Scholar]
- 3. Herbison AE. A simple model of estrous cycle negative and positive feedback regulation of GnRH secretion. Front Neuroendocrinol. 2020;57:100837. [DOI] [PubMed] [Google Scholar]
- 4. Herbison AE. Control of puberty onset and fertility by gonadotropin-releasing hormone neurons. Nat Rev Endocrinol. 2016;12(8):452-466. [DOI] [PubMed] [Google Scholar]
- 5. Herbison AE. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V). Brain Res Rev. 2008;57(2):277-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Christian CA, Moenter SM. The neurobiology of preovulatory and estradiol-induced gonadotropin-releasing hormone surges. Endocr Rev. 2010;31(4):544-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kauffman AS, Clifton DK, Steiner RA. Emerging ideas about kisspeptin- GPR54 signaling in the neuroendocrine regulation of reproduction. Trends Neurosci. 2007;30(10):504-511. [DOI] [PubMed] [Google Scholar]
- 8. Dhillo WS, Chaudhri OB, Patterson M, et al. . Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrinol Metab. 2005;90(12):6609-6615. [DOI] [PubMed] [Google Scholar]
- 9. Navarro VM, Castellano JM, Fernández-Fernández R, et al. . Characterization of the potent luteinizing hormone-releasing activity of KiSS-1 peptide, the natural ligand of GPR54. Endocrinology. 2005;146(1):156-163. [DOI] [PubMed] [Google Scholar]
- 10. Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci U S A. 2005;102(6):2129-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gottsch ML, Cunningham MJ, Smith JT, et al. . A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145(9):4073-4077. [DOI] [PubMed] [Google Scholar]
- 12. Messager S, Chatzidaki EE, Ma D, et al. . Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci U S A. 2005;102(5):1761-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. d’Anglemont de Tassigny X, Fagg LA, Carlton MB, Colledge WH. Kisspeptin can stimulate gonadotropin-releasing hormone (GnRH) release by a direct action at GnRH nerve terminals. Endocrinology. 2008;149:3926-3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Han SK, Gottsch ML, Lee KJ, et al. . Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25(49):11349-11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100(19):10972-10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lapatto R, Pallais JC, Zhang D, et al. . Kiss1-/- mice exhibit more variable hypogonadism than Gpr54-/- mice. Endocrinology. 2007;148(10):4927-4936. [DOI] [PubMed] [Google Scholar]
- 17. Seminara SB, Messager S, Chatzidaki EE, et al. . The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614-1627. [DOI] [PubMed] [Google Scholar]
- 18. Smith JT, Dungan HM, Stoll EA, et al. . Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005;146(7):2976-2984. [DOI] [PubMed] [Google Scholar]
- 19. Kauffman AS, Gottsch ML, Roa J, et al. . Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology. 2007;148(4):1774-1783. [DOI] [PubMed] [Google Scholar]
- 20. Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146(9):3686-3692. [DOI] [PubMed] [Google Scholar]
- 21. Poling MC, Luo EY, Kauffman AS. Sex differences in steroid receptor coexpression and circadian-timed activation of kisspeptin and RFRP-3 neurons may contribute to the sexually dimorphic basis of the LH surge. Endocrinology. 2017;158(10):3565-3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yip SH, Campos P, Liu X, Porteous R, Herbison AE. Innervation of GnRH neuron distal projections and activation by kisspeptin in a new GnRH-Cre rat model. Endocrinology 2021;162(1):bqaa186. [DOI] [PubMed] [Google Scholar]
- 23. Yip SH, Boehm U, Herbison AE, Campbell RE. Conditional viral tract tracing delineates the projections of the distinct kisspeptin neuron populations to gonadotropin-releasing hormone (GnRH) neurons in the mouse. Endocrinology. 2015;156(7):2582-2594. [DOI] [PubMed] [Google Scholar]
- 24. Poling MC, Kauffman AS. Organizational and activational effects of sex steroids on kisspeptin neuron development. Front Neuroendocrinol. 2013;34(1):3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim J, Semaan SJ, Clifton DK, Steiner RA, Dhamija S, Kauffman AS. Regulation of Kiss1 expression by sex steroids in the amygdala of the rat and mouse. Endocrinology. 2011;152(5):2020-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kauffman AS. Gonadal and nongonadal regulation of sex differences in hypothalamic Kiss1 neurones. J Neuroendocrinol. 2010;22(7):682-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ferin M, Carmel PW, Zimmerman EA, Warren M, Perez R, Vande Wiele RL. Location of intrahypothalamic estrogen-responsive sites influencing LH secretion in the female Rhesus monkey. Endocrinology. 1974;95(4):1059-1068. [DOI] [PubMed] [Google Scholar]
- 28. Scott CJ, Kuehl DE, Ferreira SA, Jackson GL. Hypothalamic sites of action for testosterone, dihydrotestosterone, and estrogen in the regulation of luteinizing hormone secretion in male sheep. Endocrinology. 1997;138(9):3686-3694. [DOI] [PubMed] [Google Scholar]
- 29. Smith ER, Davidson JM. Location of feedback receptors: effects of intracranially implanted steroids on plasma LH and LRF response. Endocrinology. 1974;95(6):1566-1573. [DOI] [PubMed] [Google Scholar]
- 30. Yeo SH, Herbison AE. Estrogen-negative feedback and estrous cyclicity are critically dependent upon estrogen receptor-α expression in the arcuate nucleus of adult female mice. Endocrinology. 2014;155(8):2986-2995. [DOI] [PubMed] [Google Scholar]
- 31. Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev. 2009;30(6):713-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Herbison AE. The gonadotropin-releasing hormone pulse generator. Endocrinology. 2018;159(11):3723-3736. [DOI] [PubMed] [Google Scholar]
- 33. Plant TM. The neurobiological mechanism underlying hypothalamic GnRH pulse generation: the role of kisspeptin neurons in the arcuate nucleus. F1000Res 2019;8:F1000 Faculty Rev-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wakabayashi Y, Yamamura T, Sakamoto K, Mori Y, Okamura H. Electrophysiological and morphological evidence for synchronized GnRH pulse generator activity among kisspeptin/neurokinin B/dynorphin A (KNDy) neurons in goats. J Reprod Dev. 2013;59(1):40-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Qiu J, Nestor CC, Zhang C, et al. . High-frequency stimulation-induced peptide release synchronizes arcuate kisspeptin neurons and excites GnRH neurons. eLife. 2016;5:e16246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Han SY, Kane G, Cheong I, Herbison AE. Characterization of GnRH pulse generator activity in male mice using GCaMP fiber photometry. Endocrinology. 2019;160(3):557-567. [DOI] [PubMed] [Google Scholar]
- 37. McQuillan HJ, Han SY, Cheong I, Herbison AE. GnRH pulse generator activity across the estrous cycle of female mice. Endocrinology. 2019;160(6):1480-1491. [DOI] [PubMed] [Google Scholar]
- 38. Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci. 2006;26(25):6687-6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Robertson JL, Clifton DK, de la Iglesia HO, Steiner RA, Kauffman AS. Circadian regulation of Kiss1 neurons: implications for timing the preovulatory gonadotropin-releasing hormone/luteinizing hormone surge. Endocrinology. 2009;150(8):3664-3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Semaan SJ, Dhamija S, Kim J, Ku EC, Kauffman AS. Assessment of epigenetic contributions to sexually-dimorphic Kiss1 expression in the anteroventral periventricular nucleus of mice. Endocrinology. 2012;153(4):1875-1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Homma T, Sakakibara M, Yamada S, et al. . Significance of neonatal testicular sex steroids to defeminize anteroventral periventricular kisspeptin neurons and the GnRH/LH surge system in male rats. Biol Reprod. 2009;81(6):1216-1225. [DOI] [PubMed] [Google Scholar]
- 42. Khan AR, Kauffman AS. The role of kisspeptin and RFamide-related peptide-3 neurones in the circadian-timed preovulatory luteinising hormone surge. J Neuroendocrinol. 2012;24(1):131-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Piet R, Kalil B, McLennan T, Porteous R, Czieselsky K, Herbison AE. Dominant neuropeptide cotransmission in kisspeptin-GABA regulation of GnRH neuron firing driving ovulation. J Neurosci. 2018;38(28):6310-6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Piet R, Fraissenon A, Boehm U, Herbison AE. Estrogen permits vasopressin signaling in preoptic kisspeptin neurons in the female mouse. J Neurosci. 2015;35(17):6881-6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stephens SB, Tolson KP, Rouse ML Jr, et al. . Absent progesterone signaling in kisspeptin neurons disrupts the LH surge and impairs fertility in female mice. Endocrinology. 2015;156(9):3091-3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stephens SBZ, Rouse ML, Tolson KP, et al. . Effects of selective deletion of tyrosine hydroxylase from kisspeptin cells on puberty and reproduction in male and female mice. eNeuro 2017;4(3):ENEURO.0150-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Semaan SJ, Murray EK, Poling MC, Dhamija S, Forger NG, Kauffman AS. BAX-dependent and BAX-independent regulation of Kiss1 neuron development in mice. Endocrinology. 2010;151(12):5807-5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Porteous R, Petersen SL, Yeo SH, et al. . Kisspeptin neurons co-express met-enkephalin and galanin in the rostral periventricular region of the female mouse hypothalamus. J Comp Neurol. 2011;519(17):3456-3469. [DOI] [PubMed] [Google Scholar]
- 49. Williams WP 3rd, Jarjisian SG, Mikkelsen JD, Kriegsfeld LJ. Circadian control of kisspeptin and a gated GnRH response mediate the preovulatory luteinizing hormone surge. Endocrinology. 2011;152(2):595-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sanz E, Evanoff R, Quintana A, et al. . RiboTag analysis of actively translated mRNAs in Sertoli and Leydig cells in vivo. PloS One. 2013;8(6):e66179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sanz E, Yang L, Su T, Morris DR, McKnight GS, Amieux PS. Cell-type-specific isolation of ribosome-associated mRNA from complex tissues. Proc Natl Acad Sci U S A. 2009;106(33):13939-13944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sanz E, Bean JC, Carey DP, Quintana A, McKnight GS. RiboTag: ribosomal tagging strategy to analyze cell-type-specific mRNA expression in vivo. Curr Protoc Neurosci. 2019;88(1):e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cravo RM, Margatho LO, Osborne-Lawrence S, et al. . Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience. 2011;173:37-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Stephens SB, Chahal N, Munaganuru N, Parra RA, Kauffman AS. Estrogen stimulation of Kiss1 expression in the medial amygdala involves estrogen receptor-α but not estrogen receptor-β. Endocrinology. 2016;157(10):4021-4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gottsch ML, Navarro VM, Zhao Z, et al. . Regulation of Kiss1 and dynorphin gene expression in the murine brain by classical and nonclassical estrogen receptor pathways. J Neurosci. 2009;29(29):9390-9395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Huber W, Carey VJ, Gentleman R, et al. . Orchestrating high-throughput genomic analysis with Bioconductor. Nat Methods. 2015;12(2):115-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Team RC. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2016. https://www.r-project.org/ [Google Scholar]
- 58. Ritchie ME, Phipson B, Wu D, et al. . limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gene Expression Omnibus. Gene expression omnibus data repository.https://www.ncbi.nlm.nih.gov/geo/. Uploaded March 3, 2021.
- 60. Law CW, Chen Y, Shi W, Smyth GK. Voom: precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 2014;15(2):R29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wang J, Vasaikar S, Shi Z, Greer M, Zhang B. WebGestalt 2017: a more comprehensive, powerful, flexible and interactive gene set enrichment analysis toolkit. Nucleic Acids Res. 2017;45(W1):W130-W137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Luo W, Brouwer C. Pathview: an R/Bioconductor package for pathway-based data integration and visualization. Bioinformatics. 2013;29(14):1830-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kanehisa M, Goto S, Kawashima S, Okuno Y, Hattori M. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004;32(Database issue):D277-D280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Simerly RB. Prodynorphin and proenkephalin gene expression in the anteroventral periventricular nucleus of the rat: sexual differentiation and hormonal regulation. Mol Cell Neurosci. 1991;2(6):473-484. [DOI] [PubMed] [Google Scholar]
- 65. Snyder SE, Salton SR. Expression of VGF mRNA in the adult rat central nervous system. J Comp Neurol. 1998;394(1):91-105. [PubMed] [Google Scholar]
- 66. Valera AG, Cavalcante JC, Elias CF, Felício LF. Cocaine- and amphetamine-regulated transcript is overexpressed in the anteroventral periventricular nucleus of pregnant rats. J Neuroendocrinol. 2006;18(9):711-714. [DOI] [PubMed] [Google Scholar]
- 67. Semaan SJ, Kauffman AS. Sexual differentiation and development of forebrain reproductive circuits. Curr Opin Neurobiol. 2010;20(4):424-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Clarkson J, Herbison AE. Dual phenotype kisspeptin-dopamine neurones of the rostral periventricular area of the third ventricle project to gonadotrophin-releasing hormone neurones. J Neuroendocrinol. 2011;23(4):293-301. [DOI] [PubMed] [Google Scholar]
- 69. Kanaya M, Higo S, Ozawa H. Neurochemical characterization of neurons expressing estrogen receptor beta in the hypothalamic nuclei of rats using in situ hybridization and immunofluorescence. Int J Mol Sci 2019;21(1):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Takumi K, Iijima N, Higo S, Ozawa H. Immunohistochemical analysis of the colocalization of corticotropin-releasing hormone receptor and glucocorticoid receptor in kisspeptin neurons in the hypothalamus of female rats. Neurosci Lett. 2012;531(1):40-45. [DOI] [PubMed] [Google Scholar]
- 71. Dror T, Franks J, Kauffman AS. Analysis of multiple positive feedback paradigms demonstrates a complete absence of LH surges and GnRH activation in mice lacking kisspeptin signaling. Biol Reprod. 2013;88(6):146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Brown RSE, Khant Aung Z, Phillipps HR, et al. . Acute suppression of LH secretion by prolactin in female mice is mediated by kisspeptin neurons in the arcuate nucleus. Endocrinology. 2019;160(5):1323-1332. [DOI] [PubMed] [Google Scholar]
- 73. Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, et al. . Arcuate kisspeptin/neurokinin B/dynorphin (KNDy) neurons mediate the estrogen suppression of gonadotropin secretion and body weight. Endocrinology. 2012;153(6):2800-2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rance NE, Dacks PA, Mittelman-Smith MA, Romanovsky AA, Krajewski-Hall SJ. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neuroendocrinol. 2013;34(3):211-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rønnekleiv OK, Qiu J, Kelly MJ. Arcuate kisspeptin neurons coordinate reproductive activities with metabolism. Semin Reprod Med. 2019;37(3):131-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Qiu J, Rivera HM, Bosch MA, et al. . Estrogenic-dependent glutamatergic neurotransmission from kisspeptin neurons governs feeding circuits in females. eLife 2018;7:e35656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Nestor CC, Qiu J, Padilla SL, et al. . Optogenetic stimulation of arcuate nucleus Kiss1 neurons reveals a steroid-dependent glutamatergic input to POMC and AgRP neurons in male mice. Mol Endocrinol. 2016;30(6):630-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Krull AA, Larsen SA, Clifton DK, Neal-Perry G, Steiner RA. A comprehensive method to quantify adaptations by male and female mice with hot flashes induced by the neurokinin B receptor agonist senktide. Endocrinology. 2017;158(10):3259-3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Oakley AE, Steiner RA, Chavkin C, Clifton DK, Ferrara LK, Reed SD. κ agonists as a novel therapy for menopausal hot flashes. Menopause. 2015;22(12):1328-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Vastagh C, Liposits Z. Impact of proestrus on gene expression in the medial preoptic area of mice. Front Cell Neurosci. 2017;11:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29(38):11859-11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lehman MN, Hileman SM, Goodman RL. Neuroanatomy of the kisspeptin signaling system in mammals: comparative and developmental aspects. Adv Exp Med Biol. 2013;784:27-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Rometo AM, Rance NE. Changes in prodynorphin gene expression and neuronal morphology in the hypothalamus of postmenopausal women. J Neuroendocrinol. 2008;20(12):1376-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Goodman RL, Lehman MN, Smith JT, et al. . Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148(12):5752-5760. [DOI] [PubMed] [Google Scholar]
- 85. Simerly RB, Swanson LW. The distribution of neurotransmitter-specific cells and fibers in the anteroventral periventricular nucleus: implications for the control of gonadotropin secretion in the rat. Brain Res. 1987;400(1):11-34. [DOI] [PubMed] [Google Scholar]
- 86. Semaan SJ, Kauffman AS. Daily successive changes in reproductive gene expression and neuronal activation in the brains of pubertal female mice. Mol Cell Endocrinol. 2015;401:84-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author by reasonable request or are publicly available in the Gene Expression Omnibus data repository noted in the references.