SUMMARY
Protein homeostasis, or “proteostasis”, is indispensable for a balanced, healthy environment within the cell. However, when natural proteostasis mechanisms are overwhelmed from excessive loads of dysregulated proteins, their accumulation can lead to disease initiation and progression. Fortunately, the induced degradation of such disease-causing proteins by heterobifunctional molecules, i.e., PROteolysis TArgeting Chimeras (PROTACs), is emerging as a potential therapeutic modality. In the two decades since the PROTAC concept was proposed, several additional Targeted Protein Degradation (TPD) strategies have also been explored to target previously undruggable proteins, such as transcription factors. In this review, we will discuss the progress and evolution of the TPD field, the breadth of the proteins targeted by PROTACs and the biological effects of their degradation.
eTOC Blurb
Targeted protein degradation (TPD) by PROTACs allows drug hunters to eliminate disease-causing proteins from the cellular environment. In this review, Samarasinghe et al. discuss the beginning, the evolution and the current status of the TPD field.
Graphical Abstract

INTRODUCTION
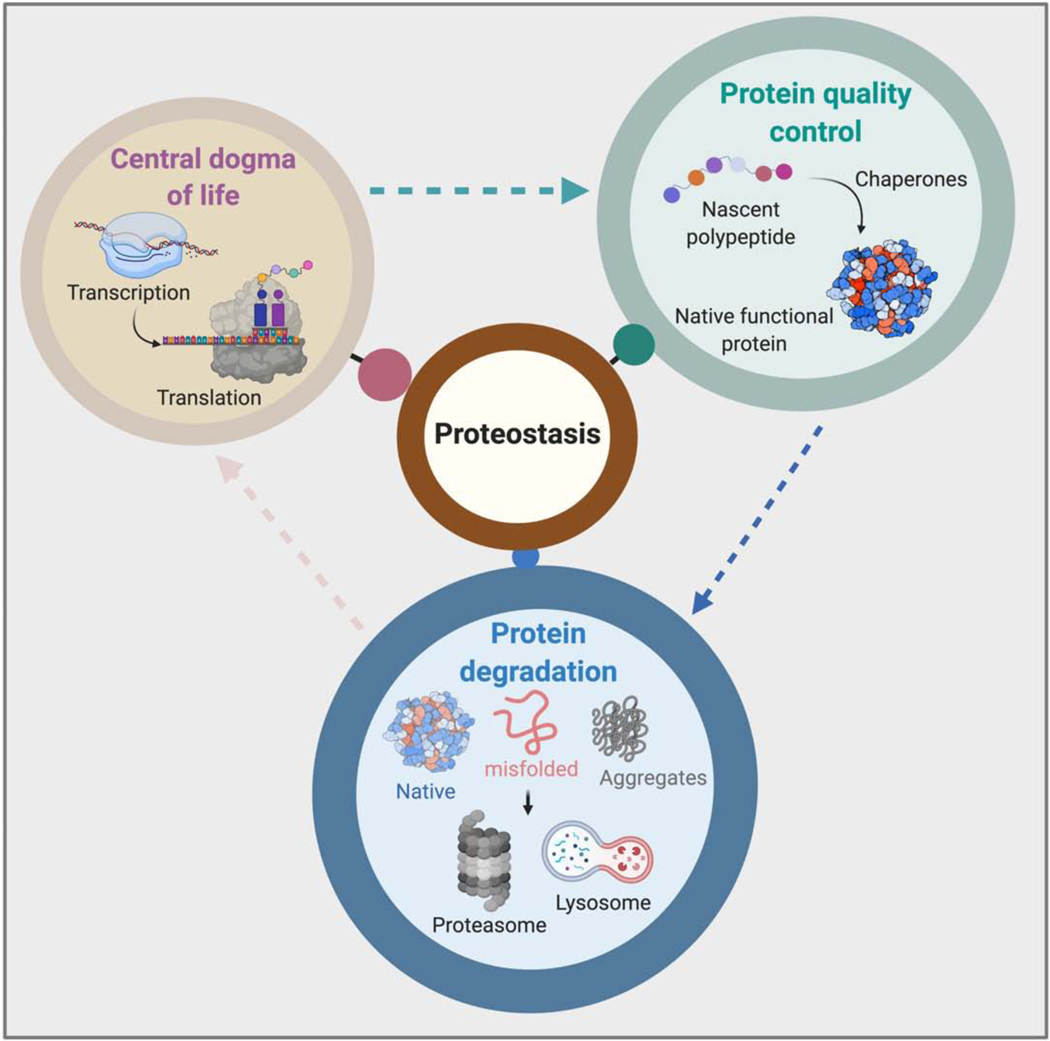
The cell is a complex system with coordinated biological processes carried out by over 20,000 individual proteins. In order to maintain the balance among these numerous biological processes, a handful of essential mechanisms for protein homeostasis (“proteostasis”) have coevolved along with the translational machinery that generates the proteome (Balch et al., 1977). Once synthesized, linear protein sequences fold into their ultimate ternary/quaternary structures that are required for their dedicated biological roles (Waudby et al., 2019). Concordantly, correct protein folding, biological activity and protein life-time are tightly interconnected and controlled by one or several protein quality control mechanisms within both the cytosol and the nucleus (Luo et al., 2016, Hutt and Balch, 2013).
Chaperones are an important class of quality control proteins whose role is to ensure proteins are correctly folded and transported to the appropriate destination. For example, Heat shock protein (HSP) 70, HSP 90 and other chaperones are key contributors to cellular protein folding machinery (Buck et al., 2007). The presence of these HSPs in a broad array of organisms from single celled organisms to complex higher order organisms like humans, highlights their importance in protein homeostasis (Figure 1). The structural integrity, activity and half-life of a given protein are sensitive to subtle changes in environmental conditions such as pH, temperature, and ionic strength (Martins de Oliveira et al., 2018). Moreover, when these conditions deviate from their norm, proteins tend to misfold or even form aggregates, creating harmful or even lethal consequences. It is also known that in the absence of chaperone activity, newly synthesized subunit proteins are not incorporated into functional quaternary structures (Sweeney et al., 2017). The next layer of proteostasis is the protein degradation machinery (Bainton, 1981, Doherty and McMahon, 2009, Takeshige et al., 1992) which comes into play when proteins are not properly folded, are aggregated or their functions are no longer needed in cellular processes. Bacteria and Archaea have evolved ATP-dependent AAA+ proteases to respond to such cellular stresses, while cells belonging to higher order Eukarya have dedicated protein removal systems, namely the ubiquitin proteasome system (UPS) and the multiple vesicular pathways that ultimately fuse with the lysosome causing the hydrolysis of misfolded or large aggregated protein cargo (Tyedmers et al., 2010). Undeniably, the chaperones and protein removal mechanisms play a paramount role in keeping the proteome in a balanced state -- overexpression or malfunction of a component in the proteostasis system can be deleterious to the cell (Lehtonen et al., 2019).
Figure 1. Components of proteostasis.
Protein synthesis, protein folding and clearance are key components of the proteostasis network.
TOWARDS TARGETING THE “UNDRUGGABLE” PROTEOME
The human genome encodes over 20,000 proteins, yet only a few classes of proteins are currently pharmaceutically vulnerable, i.e., those possessing enzymatic activity or whose biological function can be modulated upon small molecule binding (Fabbro et al., 2015). Computational or high throughput ligand discovery approaches are at the frontier of developing small molecule ligands for such proteins kinases, ligand-gated ion channels, trans-membrane proteins (e.g. GPCRs), nuclear hormone receptors, proteases and several other types of proteins that have been successfully drugged (Crews, 2010).
Overexpression, amplification, and mutations are common genetic aberrations associated with oncogenic proteins. For instance, HER2 overexpression is found in breast and gastro-esophageal cancer, while EGFR and BRAF mutations are more frequently associated with non-small cell lung cancer (NSCLC) and melanoma, respectively (Dhillon et al., 2007, Ménard et al., 2000). Although targeting these classical oncogenes has significantly improved short-term clinical outcomes, resistance mechanisms often develop, resulting in poor overall survival (Lovly and Shaw, 2014). These resistance mechanisms often emerge because therapeutically targeted oncogenic proteins, such as EGFR, are often found upstream of a given signaling pathway that drives oncogenesis. Targeting an upstream node in an oncogenic signaling pathway permits more flexibility for the rapidly proliferating cells to bypass the medicinally-neutralized oncogene and reroute the signaling events to maintain proliferative and survival capacity (Halaoui and McCaffrey, 2015). Additionally, point mutations, deletions and exon skipping are also identified as common drug resistance mechanisms (Lønning and Knappskog, 2013). Although targeting upstream oncoproteins is a current attractive therapeutic strategy, the direct targeting of downstream oncogenic effectors, such as transcription factors could successfully address resistance mechanisms that arise during tumorigenesis. Transcription factors carry out their biological activities via protein-protein interactions, thereby facilitating the recruitment of other effectors to perform distinct functions (Arkin et al., 2014). Thus, such proteins either have highly disordered regions within the quaternary structure or have larger flat surface areas that facilitate protein-protein interactions making them hard-to-drug targets by small molecules (Sammak and Zinzalla, 2015): hence these proteins were historically considered “undruggable”. Among them, some RAS family members, MYC, NF-κB and brachyury (TBXTA) are long considered hot targets in cancer biology (Dang et al., 2017).
Early chemotherapy strategies were largely based on non-specific inhibitors that interfere with physiological processes, such as DNA replication and cell division, which significantly affected normal cells in addition to cancer cells and resulted in severe toxicities in patients (Sara et al., 2018, Nurgali et al., 2018). Over the years, different targeted modalities have emerged and shown promising results in the clinic; however, the majority of such therapies were still based on small molecule inhibitors, antagonists or neutralizing antibodies (Baudino, 2015) that mediate their inhibitory activities only when they physically block either a catalytic pocket of an enzyme or a ligand binding site of proteins (Redman et al., 2015). Further as previously mentioned, they are prone to induce resistance mechanisms. This has been addressed with limited success by the development of new chemical scaffolds of the inhibitors to which the resistance had emerged (Engel et al., 2016). However, to better address limitations of these target occupancy-based pharmacological agents, new paradigms such as targeted protein degradation (TPD) have emerged and recently gained a significant attention in the drug development space (Sakamoto et al., 2001). By harnessing the quality control proteolytic machinery of the cells, TPD effects the removal of targeted proteins from the entire cellular system, requiring only a target binder – and transient one at that—to carry out its intended role. Moreover, unlike inhibitor-based strategies, TPD also permits removal of historically hard-to-drug protein targets as well. In the next section, we discuss TPD technologies such as PROteolysis TArgeting Chimeras (PROTACs) and their applications to target a variety of proteins, with a focus on transcription factors.
DEVELOPMENT OF PROTEOLYSIS TARGETING CHIMERAS (PROTACS)
Chemical biologists have long manipulated intracellular processes to answer questions in basic cell biology. From these explorations new technologies have been developed that are now being used to address limitations of existing therapeutic interventions. For example, targeted protein degradation, developed over the past two decades, co-opts the cellular ubiquitin-proteasomal pathway to induce the elimination of selective proteins. As a therapeutic modality, TPD has several advantages, e.g., it does not rely on an occupancy-driven pharmacology (Salami and Crews, 2017). Since the firstTPD proof-of-concept study in 2001, many studies have showcased the versatility, efficacy, and transformative advantage of TPD.
In order for a protein to be degraded by the proteasome, it must first be post-translationally modified by ubiquitin conjugation. Ubiquitination of substrate proteins is carried out by an energy consuming multistep process (Williams et al., 2019). Although the first two steps in this process, in which the ubiquitin is activated by an E1 enzyme and then transferred on to an E2 enzyme, are essential, the ultimate transfer of the activated ubiquitin onto the substrate protein with the aid of an E3 ligase enzyme is the crucial step in determining substrate specificity (Iconomou and Saunders, 2016). There are over 600 E3 ligase family members responsible for substrate specificity in the ubiquitin-proteasomal pathway. Because this broad substrate specificity is also the rate limiting step of the ubiquitination cycle, E3 ligases have been of the interest for the development of PROTACs. PROTAC molecules are composed of two protein recruiting elements covalently attached via a chemical linker. By design, one end of the PROTAC molecule interacts with the protein of interest (POI) and the other binds to a given E3 ligase. Thus, when the PROTAC molecule facilitates the molecular interaction between the E3 ligase and POI, thereby inducing polyubiquitination of the latter, which directs it to the proteasome for destruction (Figure 2A) (Lai and Crews, 2017).
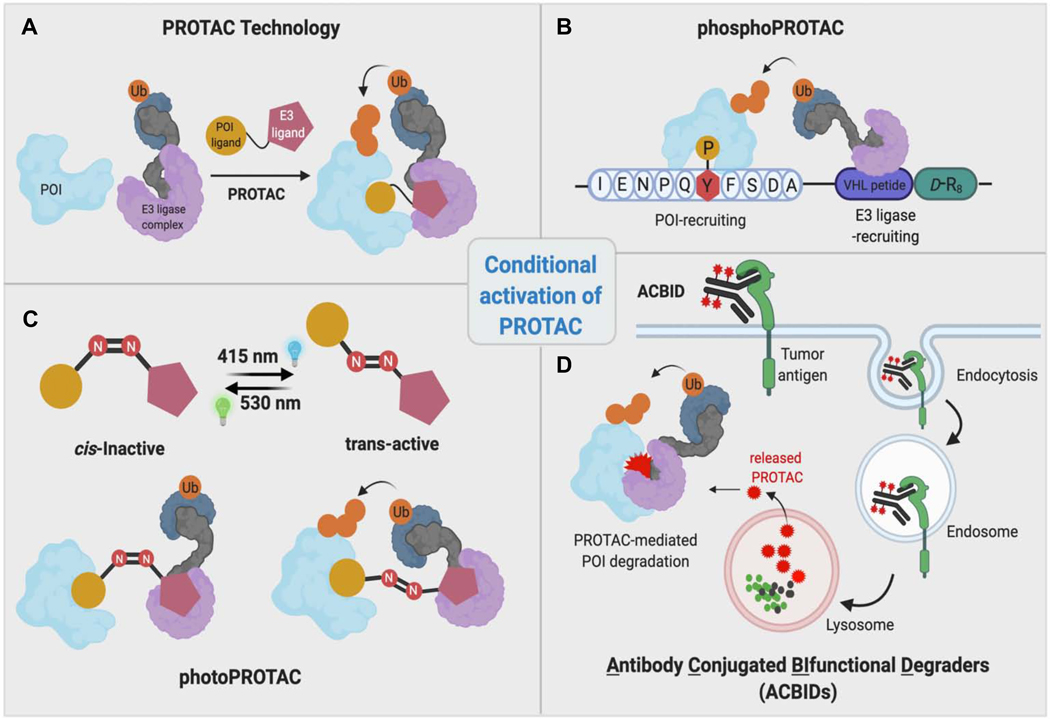
Figure 2. Conditional activation of PROTACs.
General PROTAC technology and other complementary strategies to control its activity. PhosphoPROTACs bind to the POI only under conditions where the tyrosine residue of the POI-recruiting peptide is phosphorylated upon activation of intracellular signaling. PhotoPROTACs are activated by cellular irradiation at a particular wavelength. ACBIDs release active PROTACs only upon entry into tumor cells via an antibody-specific tumor antigen.
In 2001, a PROTAC was designed to recruit methionine aminopeptidase-2 (MetAP-2; the POI) to the F-box protein SCF-ß-TRCP (the E3 ligase of interest) (Sakamoto et al., 2001). PROTAC-1 demonstrated the feasibility of the technology to induce target protein degradation upon recruitment of the E3 ligase to the proximity of the POI. The phosphopeptide architecture of PROTAC-1 hinted that TPD by this type of PROTAC could also be used to achieve POI degradation in a conditional manner--the E3 ligase recruiting moiety in PROTAC-1 is derived from a I𝝹B𝝰 degron where it only recruits the E3 ligase when this degron phosphorylated. Later in 2013, Hines et al. designed a cell-permeable “phosphoPROTAC” whereby these heterobifunctional molecules were designed such that POI binding by its ligand occurred following phosphorylation of the latter within the cell (Hines et al., 2013). More specifically, phosphoPROTACs are activated upon PROTAC phosphorylation in response to extracellular signals, which then facilitate POI binding to the PROTAC and recruitment to the von Hippel Lindau (VHL) E3 ligase (Figure 2B). Thus, phosphoPROTAC-mediated TPD is conditional, depending on the activation state of a given signaling pathway. Inspired by these initial efforts and photopharmacology, recently we showed that PROTAC activity can be controlled in a spatiotemporal manner by introducing a photoswitchable linker into the PROTAC design (Pfaff et al., 2019). In the development of photoswitchable PROTACs (“photoPROTACs”), we have incorporated a bistable ortho-terafluoroazobenzene moiety in linker (Figure 2C). The azo-cis-isomer derived from this photoswitchable moiety is highly stable compared to the parent azo-cis-isomer in which tetrafluoro substitutions on the phenyl ring are lacking (Knie et al., 2014). Therefore, the inactive cis-photoPROTAC is shown to be stable inside cells and only activated when the appropriate wavelength is applied -- converting the cis-PROTAC linker to the trans-isomer. Due to the bistable nature of ortho-terafluoroazobenzene moiety, continuous irradiation is not required compared to other approaches developed (Reynders et al., 2020, Jin et al., 2020). Furthermore, photoPROTACs provide reversible activation/inactivation of PROTAC activity compared to irreversible photocaged PROTACs (Kounde et al., 2020, Liu et al., 2020, Naro et al., 2020, Xue et al., 2019). In the future, these conditional activation strategies will serve as the basis for tissue- and tumor-specific PROTAC activation when coupled with efficient in vivo irradiation technologies.
Recently, several Antibody Conjugated BIfunctional Degraders (ACBIDs) have been developed to achieve tumor selectivity (Figure 2D). These trifunctional macromolecules consist of PROTACs covalently conjugated to a tumor specific antibody via a cleavable linker. Since ACBIDs are significantly larger than PROTACs, they only enter cells via a surface tumor-antigen-mediated endocytosis. Thus, ACBIDs have been shown to induce PROTAC-mediated degradation of several target proteins selectively in cancer cells (Maneiro et al., 2020, Dragovich et al., 2020).
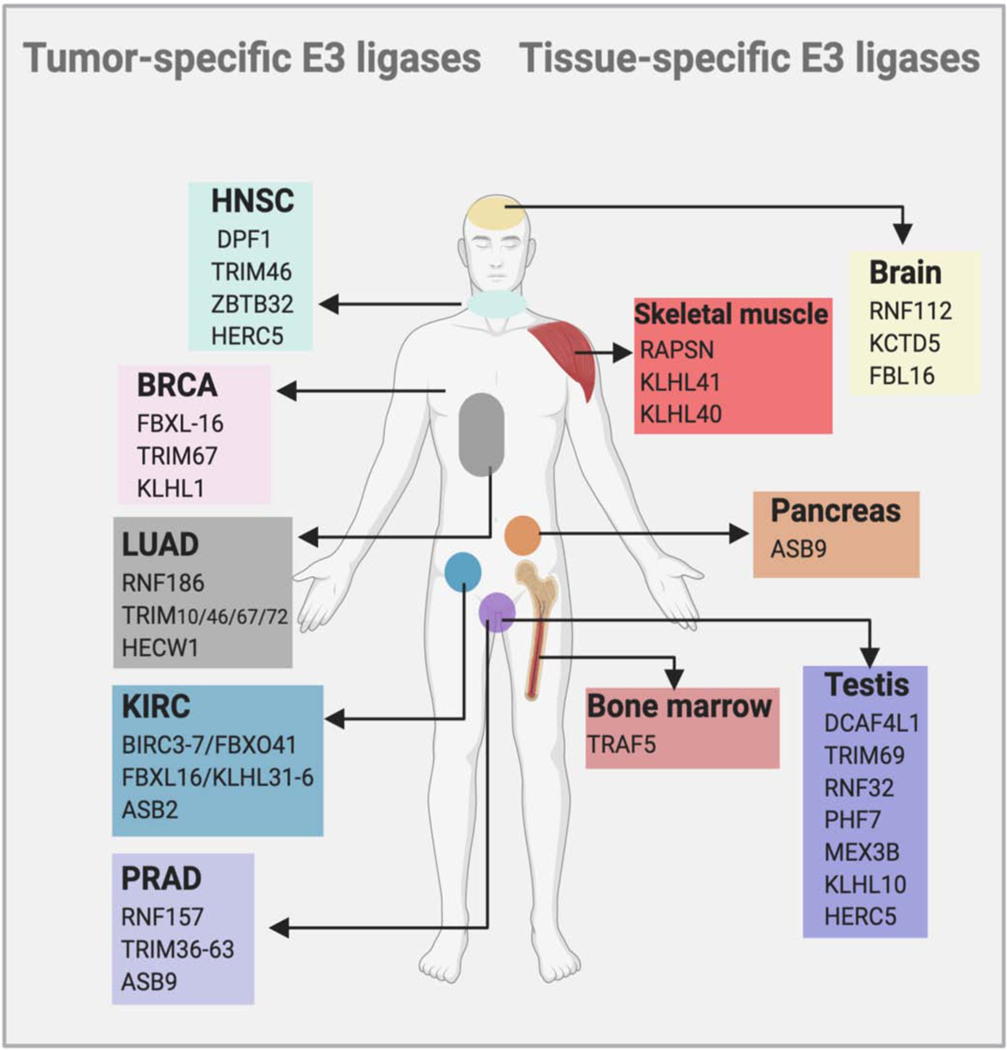
Another interesting approach that can be used to achieve tumor-selective PROTAC activity is the exploitation of tissue-specific and tumor-specific E3 ligases. Recently, Schapira et al. and Khan at al. collectively suggested that several tumor-specific and tissue-specific E3 ligases can be exploited for the development of selective-PROTACs (Figure 3). Although different E3 ligases such as VHL, Mouse Double Minute 2 homologue (MDM2), Cereblon (CRBN), DCAF15/16, RNF4/114 and KEAP1 can be used to design successful PROTACs, strategies to identify ligands for tumor-specific E3 ligases is of prime importance (Nalawansha and Crews, 2020). One excellent example of the benefits of tissue-selective TPD is the recent development of BCL-xL targeting PROTACs. Generally, BCL-xL inhibitors are associated with severe platelet toxicities (Debrincat et al., 2015). However, when these inhibitors were used as ligands in the design of BCL-xL-targeting PROTACs, platelet toxicities are largely mitigated: given the very low VHL and CRBN expression in platelets, PROTACs based on these E3 ligases did not induce degradation of BCL-xL in platelets but induced robust degradation in tumor cells and inhibited tumor growth in patient-derived xenograft models (He et al., 2020c, Zhang et al., 2019, He et al., 2020b). Thus, the identification of E3 ligases that are specific to a certain tissue or a tumor type will expand the selective use of PROTACs by reducing on-target toxicity in the clinic. While identification and characterization of such E3 ligases is straightforward thanks to publicly available RNA-seq data, development of selective, small molecule binders for them may still remain a challenge.
Figure 3. Tumor and tissue- specific E3 ligases.
Compared to small molecule inhibitors, PROTACs permit tumor or tissue selective target engagement and subsequent degradation of the target. Numerous E3 ligases are enriched in certain tissues or tumors, suggesting use of such E3 ligases in PROTAC design will accomplish tumor or tissue selective degradation (Khan et al., 2020, Schapira et al., 2019).
PROTEIN CLASSES TARGETED BY INDUCED DEGRADATION
During the first decade of TPD, only a handful of proteins were targeted for PROTAC-mediated degradation. Although initial peptide based-PROTACs successfully proved proximity-dependent protein degradation, their potencies were largely restricted by their limited cell permeability issues. However, in 2008, the first all small molecule-based PROTAC was successfully developed by recruiting the E3 ligase MDM2, using nutlin-3a as the E3 ligase ligand (Schneekloth et al., 2008). Since MDM2 is the negative regulator of tumor suppressor p53, hijacking this E3 ligase showed the additional benefit of the stabilization of p53 with the downregulation of POI (Hines et al., 2019). Since the development of a VHL- binding small molecule ligand in 2012, the TPD field expanded exponentially (Buckley et al., 2012) with the successful targeting of a large number of disease-relevant proteins for PROTAC-mediated degradation (Figure 4).
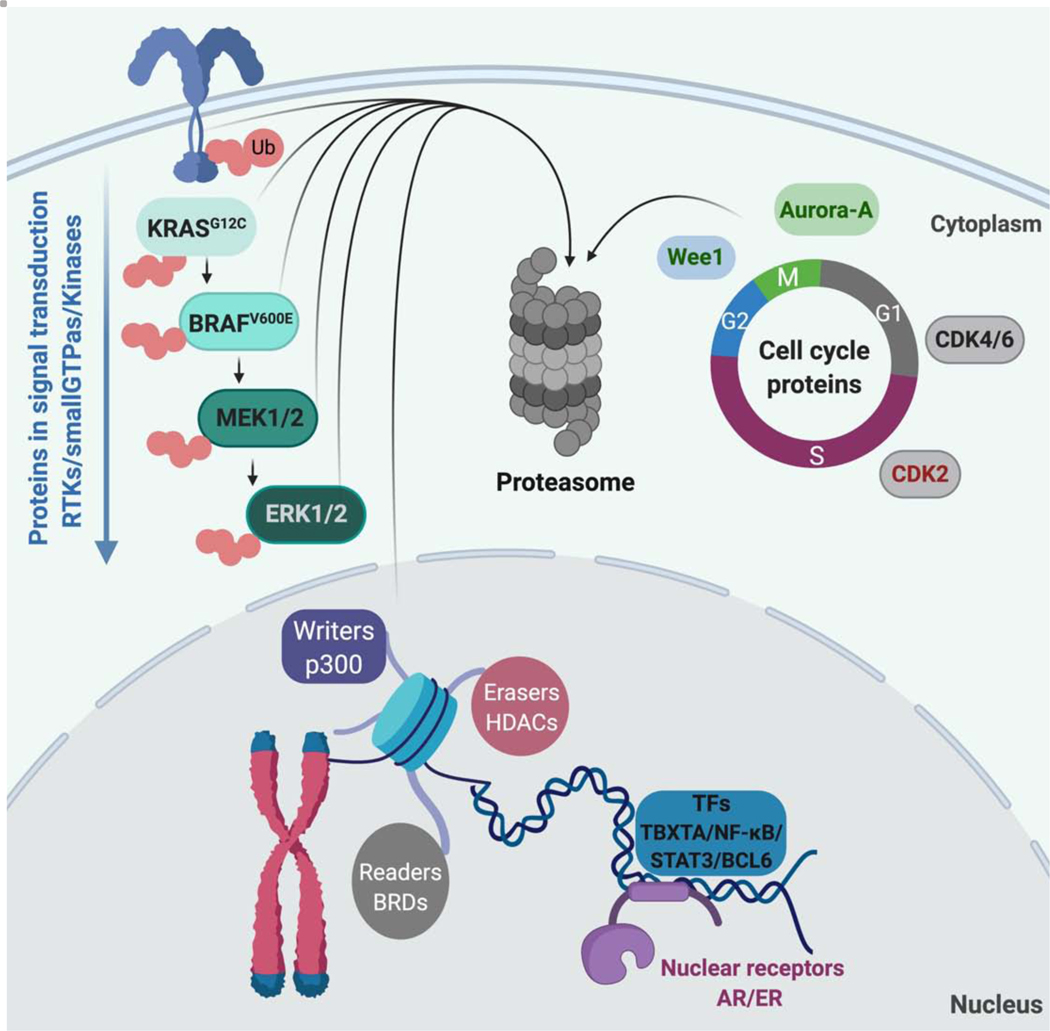
Figure 4. Protein classes targeted by induced degradation.
Receptors, kinases, epigenetic proteins and several transcription factors have been successfully degraded by induced proximity-based approaches.
Proteins in Signal Transduction Receptors
EGFR-
The epidermal growth factor receptor (EGFR) is a primary signal transducer that physically interacts with extracellular signal molecules such as EGF (Wee and Wang, 2017). Upon binding to EGF, EGFR activates a number of signaling pathways that regulate both physiological and pathological processes. EGFR overexpression and point mutations are common in cancers including NSCLC (Morgillo et al., 2016). PROTACs have the potential to target proteins in many intracellular locations, and accordingly our lab has shown that PROTACs can access transmembrane proteins like EGFR. In one study, a competitive inhibitor of EGFR, lapatinib, was used as the warhead for recruiting EGFR and the previously-mentioned VHL ligand used as the E3 ligase-recruiting moiety (Burslem et al., 2018a). Furthermore, a PROTAC developed using a second-generation inhibitor, afatinib, induced degradation of the gefitinib-resistant mutant-EGFR (L858R/T790M). Recently, Zhang et al. synthesized third- and fourth-generation EGFR inhibitors with pyrido[3.4-d]pyrimidine scaffold. Using these inhibitors, they have designed PROTACs that induce EGFR degradation in HCC827 cells with low nanomolar potency and induced apoptosis and cell cycle arrest (Zhang et al., 2020a). In addition, several other EGFR-targeting PROTACs have also been developed recently (Cheng et al., 2020, Zhang et al., 2020b, He et al., 2020a, Zhao et al., 2020).
HER2 and c-Met-
HER2 is a member of the EGFR family and is an attractive therapeutic target in multiple solid tumors, including breast cancer. HER2 overexpression in cancer leads to heterodimerization with other EGFR members and autophosphorylation that induces downstream proliferative signaling pathways associated oncogenesis (Ménard et al., 2000). A lapatinib-based PROTAC developed by Burslem et al., induced HER2 degradation in addition to EGFR, although it is worth noting that subtle changes in the linker length of lapatinib-based PROTACs significantly improved its preference for EGFR over HER2 (Burslem et al., 2018a). HER2-degrading PROTACs were more efficacious in terms of their antiproliferative effect compared to inhibition of HER2 in HER2-dependent cancer cells.
c-Met is another receptor tyrosine kinase involved in downstream RAS/RAF/MEK and PI3K/AKT signaling. Foretinib, a c-Met inhibitor, was used in PROTAC design by Burslem et al. to test whether the c-Met degradation is advantageous compared to inhibition of its kinase activity. A foretinib-based, VHL-recruiting PROTAC could induce c-Met degradation and inhibition of downstream signaling compared to its inactive control PROTAC (Burslem et al., 2018a). Further, near complete c-Met degradation was achieved within 6 h: thus, the PROTAC-mediated TPD provides temporal benefit over genetic knock down approaches, which take a relatively longer time to work. Furthermore, these studies support the fact that targeted RTK degradation yields greater and more sustained reduction in downstream signaling compared to just the inhibition of kinase activity
Alpha 1A-adrenergic receptor (α1A-AR)-
The alpha 1A-adrenergic receptor (α1A-AR) is a G-protein coupled receptor (GPCR) implicated in prostate cancer (Kojima et al., 2006). α1A-AR is known to promote cancer cell proliferation via activation of channels that pump Ca2+ into prostate cells. Since, Ca2+ influx is critical for prostate cancer cell proliferation, it has been suggested that α1A-AR inhibition could be a potential strategy to inhibit prostate cancer cell growth (Kojima et al., 2006). The first GPCR-PROTAC was developed by coupling the α1A-AR antagonist, prazosin, to the CRBN recruiting ligand, pomalidomide. Li et al. showed that this PROTAC successfully target α1A-AR and could induces its degradation in a prostate cancer cell line at micromolar concentrations (Li et al., 2020a). However, despite the α1A-AR targeting PROTAC having low potency this study demonstrated it could induce antiproliferative effects both in vitro. More importantly, PROTAC-mediated α1A-AR degradation resulted a significant tumor regression in PC-3 based mouse xenograft models. In addition, other receptors such as FLT3 and SLC have also been successfully targeted by the PROTAC technology (Burslem et al., 2018b, Bensimon et al., 2020). These early receptor-targeting chimeric degraders engaged with substrate receptors and E3 ligase machinery intracellularly. However, recent technologies such as lysosome targeting chimeras (LYTACs) and antibody-based targeting chimeras (AbTACs) trigger receptor degradation by engaging with it extracellularly (Banik et al., 2020, Cotton et al., 2021). Since development of antibodies against receptor of interest is more selective and straight forward, these recent developments will significantly expand the targeted degradation space for cell surface receptors with biologic-based degraders.
Membrane-associated Proteins
GTPases
KRAS-
Considering the advantages TPD over conventional inhibition, multiple proteins have been targeted for proteasomal degradation by converting readily available small molecule inhibitors into PROTACs. RAS proteins had long been considered undruggable proteins until the development of a series of covalent inhibitors that bind KRASG12C (Ostrem et al., 2013, Hallin et al., 2020, Canon et al., 2019). Using a PROTAC constructed from a second generation KRASG12C inhibitor (MRTX849) and a VHL ligand, Bond et al., have shown that oncogenic KRASG12C can be degraded via TPD (Bond et al., 2020). KRASG12C degradation was seen in both homozygous and heterozygous KRASG12C cell lines at low micromolar PROTAC concentrations. This study demonstrated the feasibility of endogenous KRAS degradation, a potential avenue to pursue as a therapeutic option in KRAS-driven cancers.
Kinases
FAK-
Many proteins mediate multiple functions independent of their catalytic roles in biological processes. For example, scaffolding domains in kinases can interact with other effector proteins to initiate independent signaling events (Rauch et al., 2011). Focal adhesion kinase (Fak) is one such protein known to facilitate kinase-independent, scaffolding roles via interacting with multiple proteins at the plasma membrane (Fan et al., 2013). Moreover, Fak overexpression is found in both primary and metastatic cancers (Sulzmaier et al., 2014). In 2018, Cromm et al. developed a Fak-targeting PROTAC using defactinib, a clinical-stage Fak inhibitor, and the VHL ligand (Cromm et al., 2018). Using this Fak-targeting PROTAC, Cromm et al. successfully demonstrated the Fak degradation in both PC3 and MDA-MB-231 cancer cell lines. PROTAC-mediated Fak degradation significantly downregulated Fak autophosphorylation and downstream signaling, including Akt and paxillin phosphorylation. Furthermore, PROTAC treatment could inhibit cancer cell migration and invasion, a process that cancer cells acquire during cancer metastasis, far more effectively than defactinib. This study highlights two major advantages of TPD over inhibition: 1) PROTAC- mediated Fak degradation could achieve significantly higher downregulation of FAK levels and downstream signaling over the inhibitor; and 2) PROTAC-mediated elimination of the targeted protein will lead to the removal of all associated biological functions such as scaffolding roles, which is not achievable with small molecule inhibitors.
Bruton’s Tyrosine Kinase (BTK)-
Bruton’s tyrosine kinase transduces B-cell receptor signaling by binding to a protein complex that includes PLC𝜸2 (Rickert, 2013, Wang et al., 2019a) and its hyperactivity is implicated in chronic lymphocytic leukemia (CLL) (Woyach et al., 2014). Increased BTK activation leads to increased levels of Ca2+ influx, which ultimately activates oncogenic signaling pathways such as MAPK, which, in turn, activates key oncogenic drivers such as NF-𝝹B, NFAT, and MYC (Pontoriero et al., 2019, Duyao et al., 1990). Several successful covalent and non-covalent BTK-PROTACs have been developed recently. Covalent PROTACs based on bosutinib or a derivative of ibrutinib showed potent BTK degradation when coupled with CRBN ligands (Huang et al., 2018, Buhimschi et al., 2018). Unlike bosutinib-based PROTACs, a PROTAC based on ibrutinib (MT-802) developed by Buhimschi et al. could also degrade BTK-C481S, an ibrutinib-resistant mutant frequently encountered in the clinic in CLL patients who had received ibrutinib. Mutation of the ibrutinib-reactive cysteine residue abolishes the ability of the inhibitor to bind covalently, thereby significantly reducing its effectiveness (Ahn et al., 2017). Despite this, MT-802 can nevertheless degrade BTK C481S resistant mutant kinase activity, whereas ibrutinib could only inhibit WT BTK activity (Buhimschi et al., 2018). Although ibrutinib and VHL-based reversible-PROTACs were unable to induce BTK degradation in the study by Buhimschi et al, another study showed that an irreversible-PROTAC based on same warheads could induce degradation of BTK (Xue et al., 2020, Buhimschi et al., 2018). These studies demonstrate that the combination of POI ligand and the E3 ligase ligand are crucial in developing successful degrader molecules. It is also important to highlight that the ligand-linker attachment site is crucial as Buhimschi et al. data indicated that different attachment sites with the same linker resulted in opposite degradation profiles. One important advantage of development of PROTACs using existing inhibitor molecules or their derivatives is improved selectivity compared to the inhibitor itself (Bondeson et al., 2018, Huang et al., 2018). A similar scenario was observed with reversible-BTK-PROTACs, and with further optimizations and improved PK properties these comparatively more target-specific degrader molecules will reduce the toxicities and resistance associated with ibrutinib.
p38-
The p38 family of MAPKs mediates intracellular stress sensing and consists of four known isoforms (i.e., p38𝝰, P38𝝱, p38𝜸 and p38δ (Yang et al., 2016). In addition to stress signaling, p38 kinase activity is implicated in enhanced cell survival, migration and proliferation as well as has been implicated in numerous cancers (Koul et al., 2013). Interestingly, different p38 isoforms can have opposing roles that depend on the context: for instance, p38𝝰 is considered to have both tumor suppressor roles and tumor-promoting activities (Han and Sun, 2007, Igea and Nebreda, 2015). Although numerous p38 inhibitors have been developed, it has been challenging to make them isoform-selective. To address potential isoform selectively, Smith, et al. explored diffential presentation of p38MAPK isoforms to VHL for potential PROTAC-mediated selective isoform degradation. Starting with the same pMAPK inhibitor (i.e., foretinib) and the same E3 ligase ligand, (Smith et al., 2019) generated two PROTACs that differed in their linker attachment sites in the VHL ligand. The resulting PROTACs displayed significantly different ability to induce the degradation of p38𝝰 and p38δ. When the p38 recruiting ligand, foretinib attached to one end of the VHL ligand via an amide bond, it could only degrade p38𝝰. However, foretinib attached to the other end of the VHL ligand via the phenyl group -- with a single atom difference in the linker length - displayed less activity towards p38𝝰 but it could significantly induce p38δ degradation. This study suggests that different forms of ternary complexes might determine isoform selectivity, although the orientation of recruited VHL ligand owing to different vector points of linker attachment still has to be considered as an important factor to achieve selective degradation. Therefore, while E3 ligase exploration is one option to develop selective degrader molecules, it is also advisable to employ E3 ligase ligands with multiple vector points and linker optimization strategies in order to induce potential protein:protein interactions between the target protein and E3 ligase complex. Different linker classes, designing principles and the dependency of PROTAC activity are discussed in greater detail elsewhere (Troup et al., 2020). Furthermore, recently a Rosetta-based modeling strategy was developed to predict productive ternary complex formation by a given PROTAC structure. Thus, this strategy will allow chemists to narrow down the number of PROTACs for a given target to be synthesized and evaluated (Zaidman et al., 2020, Bond and Crews, 2021).
ALK-
Anaplastic lymphoma kinase (ALK) drives tumorigenesis in multiple cancers, including NSCLC, renal cell carcinoma, and lymphoma (Holla et al., 2017). Gene amplification, point mutations in the ALK kinase domain and chromosomal translocations are all well-studied genetic abnormalities found in association with its oncogenic properties (Holla et al., 2017). There are several FDA-approved ALK such as ceritinib and brigatinib (Friboulet et al., 2014). However, like most inhibitors, ceritinib administration induces resistant mechanisms (e.g. mutations F1174L and R1275Q; fusion proteins- EML4-ALK and NPM-ALK) in patients (Mehlman et al., 2019). Several groups have developed successful ALK PROTACs using ceritinib as the ALK-recruiting element and VHL or CRBN ligands (Powell et al., 2018, Zhang et al., 2018). CRBN-based ALK PROTACs, TL13–12, TL13–112, MS4077 and MS40778 induced degradation of inhibitor insensitive ALK-fusion proteins and downregulated ALK signaling with antiproliferative effects (Powell et al., 2018). However, CRBN-based ALK PROTACs also induced degradation of other kinases, such as Fak and Aurora A and RSK. In additional studies, two VHL-recruiting ALK PROTAC were shown to induce ALK degradation and inhibit cancer cell growth (Kang et al., 2018) (Sun et al., 2020).
Since conventional inhibitors rely on occupancy-driven pharmacology, point mutations in the target protein can alter inhibitor affinities thereby compromising the anticipated clinical outcome. Similarly, gene amplification or protein overexpression requires increased inhibitor concentrations to reach expected clinical benefit, but which then can lead to adverse reactions in patients. Conversely, the catalytic nature of PROTACs allows enhanced degradation of the targeted protein thereby offering the potential for lower doses for PROTAC-based drugs relative to conventional inhibitors.
Other Kinases-
Several PROTACs targeting BCR-ABL have been developed based on dasatinib and bosutinib coupled to CRBN or VHL or IAP- recruiting ligands (Lai et al., 2016, Shimokawa et al., 2017, Burslem et al., 2019). One key feature observed in these PROTAC studies was E3 ligase-dependent selectivity towards POI degradation, where a CRBN-recruiting, dasatinib-based PROTAC degraded both c-ABL and BCR-ABL, but a VHL-recruiting dasatinib-based PROTAC could only degrade c-ABL. In addition, an IAP-recruiting dasatinib-based PROTAC induced the degradation of BCR-ABL and showed a sustained antiproliferative effects compared to the inhibitor (Shibata et al., 2018). Other kinases shown to be degradable by a PROTAC include Casein kinase 2 (CK2), which results in Akt signaling downregulation and p53 activation (Chen et al., 2018). In addition, a TANK-binding kinase 1 (TBK1)-targeting PROTAC showcased a similar selectivity towards TBK1 over another closely related IKK family member, IKKe (Crew et al., 2018). Moreover, several other kinases including but not limited to MEK1/2, ERK1/2, SGK3, BRAFV600E and PI3K have been successfully degraded via PROTACs (Hu et al., 2020, Tovell et al., 2019, Lebraud et al., 2016, Li et al., 2018a, Posternak et al., 2020, Alabi et al., 2020, Hines et al., 2013).
Proteins in Cell Cycle and Apoptosis
CDKs and Wee1-.
Two critical components regulate cell cycle progression, namely, a collection of kinases that allow cells to progress from one stage to the next by phosphorylating cell cycle-associated proteins (John et al., 2001) and cell cycle “check point” proteins that validate the completion of crucial events in each stage (Visconti et al., 2016). Given their importance in cell proliferation, these cell cycle components are attractive targets for cancer drugs. For example, AZD1775 inhibits Wee1 kinase, a G2/M cell cycle check point protein, thereby permitting cell cycle transition into mitosis thus leading to cancer cell apoptosis induction (Bi et al., 2019). However, like many inhibitors, AZD1775 has associated toxicities when used at doses required to obtain a clinical benefit (Cuneo et al., 2019). Also, these inhibitors affect not only Wee1 kinase activity but also other off-target kinases such as PLK1 (Wright et al., 2017). Since PROTACs have been shown to achieve selective target degradation using promiscuous kinase inhibitors, Li et al developed a selective CRBN-recruiting PROTAC to target Wee1 (Li et al., 2020b). As anticipated, a Wee1 PROTAC induces Wee1 degradation at low nanomolar concentrations while sparing PLK1. Interestingly, PROTAC-mediated Wee1 degradation induced DNA double stranded breaks and apoptosis at 10-fold lower concentrations than does inhibition alone by AZD1775. Furthermore, the Wee1 PROTAC displayed a potent antiproliferative effect that synergized with DNA repair enzyme inhibitors. Similarly, other proteins implicated in the cell cycle and apoptosis have been successfully targeted by PROTAC technology. In particular, use of promiscuous CDK inhibitors in PROTAC design enabled selective degradation of CDK2, CDK4, and CDK6 (Zhao and Burgess, 2019, Jiang et al., 2019, Zhou et al., 2020).
Aurora-A-
Aurora kinases are another subset of proteins that control cell cycle progression. Aurora-A overexpression is more common in NSCLC and associated with resistance to chemotherapy and radiotherapy (Kuang et al., 2017). Aurora-A is constitutively expressed in mitotically active cells where its expression is elevated during G2 and mitosis phases (Nikonova et al., 2013). In addition to its catalytic activity, Aurora-A engages with other proteins to mediate kinase-independent functions such as stabilization of oncogenic MYC proteins by physically interacting with them (Li et al., 2018b). Adhikari et al. recently developed an Aurora-A PROTAC by conjugating alisertib, an Aurora-A recruiting element to a CRBN-recruiting ligand (Adhikari et al., 2020) and successfully demonstrated that targeted Aurora-A degradation induced significant S-phase cell cycle arrest. This phenotype was not observed with Aurora-A inhibition, highlighting the fact that PROTAC-mediated downregulation led to the discovery of a new, non-catalytic role of Aurora-A during DNA replication.
BCL-2-
Targeting antiapoptotic proteins in cancer therapy has long been an attractive strategy. BCL-2 is a such protein, implicated in number of cancers including lymphoma and lung cancers (Schuetz et al., 2012). Using BCL-2 and MCL-1 inhibitors, Wang et al. showed that the induction of apoptosis through BCL-2 degradation by PROTACs is a viable option to target different cancer types (Wang et al., 2019b). However, since other BCL-2 family members are poorly characterized, the selectivity of BCL-2 targeting PROTACs towards the oncogenic BCL-2-isoform needs to be further evaluated.
Proteins in Epigenetic Regulation
While genetic aberrations are a hallmark of cancer, many cancer cells also modify epigenetic markers to promote cell proliferation and metastases (Bell and Gilan, 2020). Epigenetic modifications such as acetylation and methylation are key players in control of gene expression: while acetylation of histone tails results in more loosely compact chromosomes, removal of such modifications promotes less accessible and more compact DNA packaging (Portela and Esteller, 2010). Not surprisingly, histone acetyl transferases, deacetylases (HDACs) and histone-acetyl readers such as BRD2/3/4 are highly dysregulated in a range of cancer types (Zhao and Shilatifard, 2019).
The epigenetic reader protein BRD4 directly regulates expression patterns of an array of oncogenic proteins such as MYC, BCL-2 and RUNX2 (Baker et al., 2015). A number of studies targeted BRD4 and related BET domain-containing proteins for induced degradation using PROTACs. Raina et al. demonstrated an in vivo active, PROTAC ARV-771, that induces degradation of BRD4,2 and 3 with 1 nM DC50 potencies (Raina et al., 2016). As anticipated, ARV-771 decreased transcript levels of both full-length AR and AR-V7 leading to a strong anti-tumor response in enzalutamide-resistant mouse xenograft models of prostate cancer. Furthermore, as seen in cell-based studies, ARV-771 produced superior apoptotic effects than a BET inhibitor. Prior to this study, a CRBN-based BRD4-PROTAC, ARV-825 had highlighted the advantages of TPD over inhibition (Lu et al., 2015). In that study, Lu et al. showed that, unlike BRD4 inhibition that causes compensatory upregulation of BRD4, ARV-825 induced significant BRD4 removal resulting in superior anti-cancer properties with enhanced c-Myc suppression and subsequent cell proliferation inhibition and apoptosis induction in Burkitt’s lymphoma cells. In addition to these studies, other promising BRD-targeting PROTACs have also been developed (Winter et al., 2015, Gadd et al., 2017).
Similar to acetyl reading proteins, histone deacetylases have also been targeted for proteasomal degradation. Using PROTAC based TPD approach, HDAC1, 2, 3 pan-degraders, HDAC 3-specific degraders and HDAC6 degraders have been successfully developed (An et al., 2019, Smalley et al., 2020, Xiao et al., 2020). Other proteins that regulate gene expression epigenetically, such as p300, PCAF/GCN5, SMACA2/4, Sirtuin 2 and EZH2 have also been degraded using PROTACs, showcasing the successful application of TPD in different protein classes as well as its ability to induce degradation of protein complexes (Bassi et al., 2018, Kargbo, 2020, Schiedel et al., 2018, Hsu et al., 2020, Vannam et al.). Outside of these epigenetic regulators, Verheul et al. proposed an array of key epigenetic targets that are amenable to PROTAC-mediated degradation (J Verheul et al., 2020). They proposed key gene expression regulators of fetal hemoglobin, such as histone demethylase LSD1 and nucleosome remodeling and deacetylase (NuRD) silencing complex, as a potential therapeutic approach to mitigate beta-thalassemia and sickle cell disease.
Proteins Involved in Transcription
Nuclear Hormone Receptors
Unlike conventional transcription factors, nuclear hormone receptors contain ligand binding domains (LBD) and thereby can relay extracellular signals directly to the nucleus and regulate gene expression (Mangelsdorf et al., 1995). There are over 500 members in the nuclear receptor super family and they are sub-divided into four classes. Steroid receptors such as androgen receptors (AR) and estrogen receptors (ER) are included in class I and they are widely implicated in human cancer. Since these transcription factors (TFs) have a ligand binding domain, different pharmacotherapeutic strategies have been developed. However, the corollary resistance mechanisms like AR overexpression and splice variants with LBD truncation are often associated with poor treatment outcome (Antonarakis et al., 2014). Therefore, a number of PROTACs targeting AR and ER have been developed since 2003, and continued efforts have improved their druglike properties to progress them to the clinic.
Androgen receptor-
Considering the significance of AR overexpression in prostate cancer progression, multiple PROTACs have been developed to induce its degradation. AR was one of the early protein targets degraded by PROTAC technology, which at that time was used mostly to explore the possibilities of protein degradation by hijacking E3 ligases as an academic exercise (Sakamoto et al., 2003). Interestingly, the first all small molecule-based PROTAC was also developed to target AR by combining non-steroidal androgen receptor ligand to nutlin3a as a MDM2-recruiting ligand (Schneekloth et al., 2008). In a subsequent study, Gonzalez et al. and others have developed another VHL binding peptide- and dihydroxytestosterone-based PROTACs to degrade AR (Rodriguez-Gonzalez et al., 2008, Tang et al., 2009, Schneekloth et al., 2004). Although these early AR PROTACs showed poor potencies, several subsequent AR-targeting PROTACs were developed with half-maximal degradation concentrations (DC50) in the nanomolar range. In 2018, Salami et al. developed a novel class of AR PROTAC (ARCC-4) by appending the AR antagonist, enzalutamide, to a small molecule VHL ligand (Salami et al., 2018). Using ARCC-4, AR was successfully degraded in castration-resistant prostate cancer cell lines VCap and LNCaP with single digit nanomolar DC50. Also, ARCC-4 could induce degradation of multiple, clinically relevant AR mutants and displayed superior antiproliferative effects compared to enzalutamide. In early studies, the use of the natural AR agonist, dihydrotestosterone, in the PROTAC design was not as effective as the use of AR antagonists, such as enzalutamide. Although no structural data is available to support, a possible explanation for this difference is that when agonist-based PROTACs binds to AR, it exposes DBD of AR and facilitate DNA binding in way that can impede induced proximity dependent secondary ubiquitination event, whereas antagonist-based PROTAC might facilitate a completely different conformational rearrangement in a way that it facilitates efficient ubiquitination due to the potential exposure of lysine residues.
An AR PROTAC is one of the first two PROTACs that have progressed to the clinic. ARV-110 is being evaluated in Phase I clinical trials in patients with metastatic, castration-resistant prostate cancer (Petrylak et al., 2020). Based on the recent update, patients did not show severe adverse effects except mild nausea and diarrhea. Out of 12 patients treated with ARV-110, two patients had significantly lower PSA levels and a partial response. However, consistent with the preclinical data, several patients with ARV-110-insensitive mutants, such as L702H point mutation or AR-V7 splice variant did not respond well (NCT03888612). Therefore, when ARV-110 progresses to Phase II trials, selection criteria for the patient population should be carefully considered to achieve maximum overall clinical benefit.
More recently, another AR PROTAC (MTX-23) has been developed to induce the degradation of the commonly encountered splice variant, AR-V7, which lacks the ligand binding domain (Lee et al., 2020). Using a previously known DBD binding ligand, Lee et al. developed a VHL-recruiting PROTAC that can induce the degradation of both full-length AR and splice variant AR-V7. MTX-34 displayed antiproliferative effects in multiple cell lines that are resistant to second-line anti-androgen therapy. Furthermore, this PROTAC induced tumor regression in mice. Alternatively, Raina et al. have shown that their BRD4 targeting PROTAC, ARV-771, also can induce significant downregulation of full-length AR and splice variant AR-V7 through the degradation of BRD4 (Raina et al., 2016).
Estrogen receptors-
Breast cancer is the most common cancer type in women, and estrogen receptor-α (ERα) positive cases are the most prevalent (Duffy, 2006). ERα overexpression plays a critical role in the breast cancer cell proliferation and metastasis (Lillo Osuna et al., 2019). ER is a ligand-dependent transcription factor, which when bound by its natural ligand, estradiol, induces gene expression. Given its importance in breast cancer, induced ERα downregulation is an attractive potential treatment modality. Over 15 different ER-targeting PROTACs have been developed using natural ER agonists, peptides, selective estrogen receptor degraders and modulators as ER-recruiting ligands. Recently, an interesting approach to achieve tumor selective delivery of ER PROTAC has been reported (Dragovich et al., 2020), in which PROTACs targeting ERα were covalently conjugated to a HER2 specific antibody, and used to induce ERα degradation to a similar extent as the unconjugated PROTACs. Recently, raloxifene-based ER PROTACs were developed that recruit CRBN or VHL, and in a series of linker composition optimizations, Roberts et al. designed a more drug-like ERα degrader with half-maximum target degradation at 1 nM (Roberts et al., 2020).
ER-targeting PROTAC, ARV-471, has progressed into Phase I clinical trials and a recent clinical update on ARV-471 indicated a good safety profile and antitumor activity in patients with ER+/HER2- breast cancer (NCT04072952) (Flanagan et al., 2019). Intriguingly, evaluation of patient tumor biopsies demonstrated up to 90% ER degradation. Further ARV-471 dose escalation and combination therapy with the CDK4/6 inhibitor, palbociclib, are expected to be evaluated in the near future.
Other TFs
STAT3-
Extracellular cytokines and growth hormones activate Janus kinases (JAKs), which in turn, activate a family of TFs known as “signal transducers and activators of transcription” (STATs) including STAT3 (Johnston and Grandis, 2011). Constitutive STAT3 activation has been implicated in several human cancers. Activated STAT3 binds as a dimer to upstream consensus DNA sequences of its target genes. Since the Src-homology 2 (SH2) domain in STAT3 is essential for its dimerization, small molecule inhibitors that bind to the SH2 domain have been used in the clinic (Fagard et al., 2013). However, their lack of selectivity for STAT3 over other STAT family members, and the acquired resistance mechanisms, such as SH2-independent activation of STAT3, have resulted in poor their clinical success. Fortunately, these limitations associated with an inhibitor-based approach have been successfully addressed by converting a STAT3 inhibitor into a bifunctional protein degrader. By recruiting CRBN to the vicinity of the TF, SD-36 induced selective STAT3 degradation without significantly affecting the levels of other STAT isoforms (Bai et al., 2019). Selective STAT3 degradation resulted in a 1000-fold greater effect than just STAT3 inhibition on downstream gene expression. SD-36 displayed excellent in vivo activity in xenograft tumor models suggesting a potential application to circumvent limitations associated with traditional therapeutic approaches. This study demonstrates that transcription factors (beyond steroid receptors) are also amenable to degradation by PROTAC technology, which holds promise for targeting the many transcription factors known to be associated with disease.
Beta catenin-
Beta-catenin is a classical oncogenic transcription factor whose increased stability or hyperactivation contribute to tumorigenesis in multiple cancer types. In addition, mutations within beta-catenin itself also have been identified as oncogenic drivers (Kim and Jeong, 2019). Although a number of small molecule modulators have been described in the literature, only a few have inhibited beta-catenin-mediated transcription. Hwang et al. identified a beta-catenin inhibitor using a cell-based, high throughput reporter assay; interestingly, this “inhibitor “mediates its anti-tumor activity by actually inducing proteasomal degradation of beta-catenin (Hwang et al., 2016). Alternatively, stapled peptides have been developed to inhibit beta catenin interactions with T-cell factor (TCF) transcription factors. Although these peptides could disrupt targeted beta-catenin interactions, they did not show in vivo activity (Cui et al., 2018). Recently, beta-catenin-targeting PROTACs were developed by covalently attaching beta-catenin-binding stapled peptides to VHL-recruiting peptide (Liao et al., 2020). Despite their clinical limitations as peptide-based PROTACs, they nonetheless displayed a long-lasting degradation of beta-catenin and strong inhibition of Wnt/beta-catenin signaling. In future, it will be worthwhile to identify and investigate small molecules that can bind to beta-catenin for their applicability within PROTAC chemical space to create degraders with more drug-like properties and increased efficiencies.
BCL6-
B-cell lymphoma- 6 (BCL-6) is a member of the BTB and zinc finger transcription factor family. BCL-6 overexpression is associated with multiple cancers, including glioblastoma, breast cancer and B-cell lymphoma, wherein it promotes cancer cell motility and proliferation (Cardenas et al., 2017). Small molecule-based BCL-6 degraders have been identified serendipitously in a structure-based drug design campaign, and Kerres et al. identified a sub-class of BTB domain-binding molecules (BI-3802) that can induce BCL-6 degradation (Kerres et al., 2017). Even though the mechanism of action of this small molecule-mediated target degradation was not fully understood at that time, it has been suggested that the DBD of BCL-6 is required for the observed degradation by the small molecule. Chemical knockdown of BCL-6 using BI-3802 showed antiproliferative activity in diffuse large B cell lymphoma (DLBCL) cells. A follow-up study to unravel the mechanism of action of BI-3802 found that BI-3802 induces BCL-6 oligomerization, with subsequent E3 ligase recruitment and degradation (Słabicki et al., 2020). Interestingly, in a separate study, although a CRBN-recruiting BCL-6 PROTAC could successfully induce BCL-6 degradation, it failed to show antiproliferative effects in DLBCL cells (McCoull et al., 2018).
ALTERNATIVE STRATEGIES TO CONTROL PROTEIN LEVELS: GENE EXPRESSION REGULATION
Transcriptional
CRISPR-based strategies-
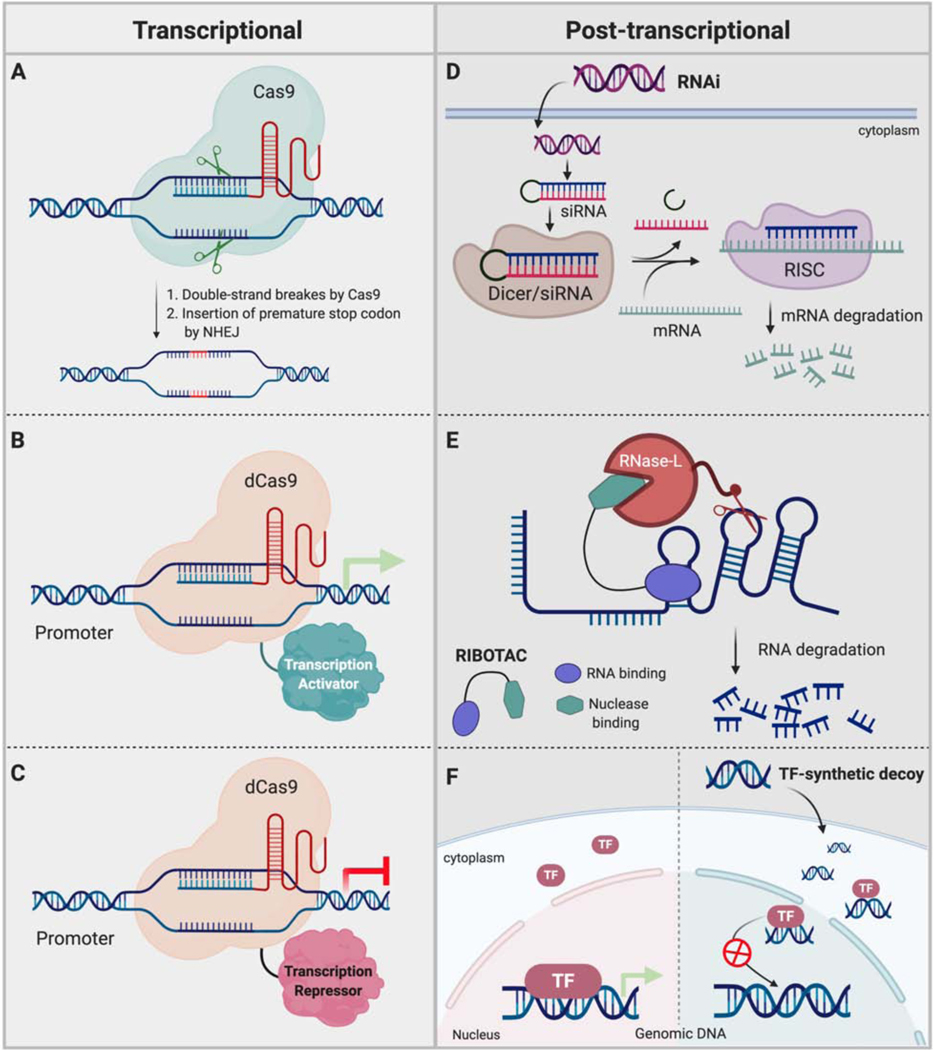
As an alternative to direct protein knockdown strategies, regulating gene expression at the DNA level is also becoming popular due to the recent advances in gene editing technologies (Figure 5A). For example, zinc finger domains that recognize three nucleotides can be fuse to recognize longer DNA stretches and coupled with endonucleases such as FokI have led to a variety of gene editing tools (Carroll, 2011). Similarly, another technology has been developed using “transcriptional activator-like effector nucleases”, or TALENs, by fusing FokI to an array of TALE proteins (Ousterout and Gersbach, 2016). One TALE motif recognizes a single nucleotide and thereby an array of these proteins can achieve sequence specificity for intended double strand breaks. “Clustered regulatory interspaced short palindromic repeats” (CRISPR) has also been successfully adopted to achieve genome engineering. The CRISPR system includes Cas9 nuclease and a guide RNA that simultaneously binds to Cas9 nuclease and to the targeted DNA sequence (Moon et al., 2019). Once bound to the target sequence, Cas nuclease can introduce double strand breaks. Thus, using these technologies, numerous manipulations such as introducing mutations, insertions, deletions, knocking out an entire gene and precise base editing has been done to control protein levels/function. However, these genetic manipulations are not reversible and sometimes it can be inherited.
Figure 5. Alternative strategies to control protein levels – regulation of gene expression.
Techniques such as CRISPR-mediated gene editing and transcriptional regulation have been widely used to control target proteins level at genetic/transcriptional level. RNA interference, RIBOTAC and synthetic DNA-decoys have also been used to regulate protein levels.
In addition to genetic manipulations, CRISPR technology also has been widely used to control gene expression by utilizing catalytically dead Cas proteins (dCas9) and gRNAs that target specific genomic loci (McCarty et al., 2020): it has been shown that just Cas proteins recruitment to the transcription start site of a targeted gene using a matching gRNA is sufficient to block gene expression. Furthermore, Cas proteins can be engineered by fusing them to transcriptional repressor domains such as KRAB (Figure 5B). (Yeo et al., 2018). In a similar manner, recruitment of activators via Cas:gRNA induced robust target gene expression (Figure 5C). (Konermann et al., 2015). Alternatively, Cas protein has been fused to epigenetic modifiers or readers to accomplish intended transcriptional regulation in a loci dependent manner (Pulecio et al., 2017).
Post-transcriptional
RNA interference-
Unlike TPD and genome engineering technologies, RNA interference (RNAi) mediates downregulation of proteins at the post-transcriptional mRNA level (Setten et al., 2019). Inspired by natural RNAi processes where cellular machinery induces degradation of foreign mRNA via small interfering RNAs (siRNA) or micro RNAs (miRNA), synthetic siRNA drugs have been developed to treat multiple diseases including various forms of cancer. Since siRNA therapeutics depend on Watson-Crick base pairing to induce target mRNA degradation, the level of any target protein including proteins considered as undruggable can be regulated simply by selecting the complementary sequences in the siRNA design (Figure 5D). Several siRNA drugs have progressed to the clinic as potential treatments for multiple cancer types. DCR-MYC and siG12D-LODER targeting oncogenic MYC and KRASG12D,respectively, are being evaluated in patients with hepatocellular carcinoma and advanced pancreatic cancer (Tolcher et al., 2015). However, compared to PROTACs, RNAi approaches require more time to achieve the intended knockdown of the targeted protein.
RIBOTAC-
Recently, RIBOnuclease TArgeting Chimeras (RIBOTAC) were developed by conjugating a small molecule that binds to oncogenic microRNAs (miR-21 and miR-96) to a small molecule ligand that recruits ribonuclease L. Costales et al. successfully demonstrated that the microRNA-21 degradation by RIBOTAC could inhibit breast cancer metastasis by causing the upregulation of tumor suppressor proteins such as PDCD4 or downregulating pro-apoptotic FOXO1 (Figure 5E). These studies explore not only RNA small molecule binders but also a strategy to induce selective degradation of disease relevant RNAs (Costales et al., 2020).
TF decoys (TFD)-
Unlike other gene silencing strategies, transcription factor decoys are double-stranded short DNA sequences based on a given TF consensus binding DNA sequence (Figure 5F). Once introduced, TFD act on the targeted TF and binds it to interfere or reduce the availability of free TFs for promoter binding (Hecker and Wagner, 2017). Thus, TFD can either downregulate or upregulate gene expression depending on the role of the targeted TF. Using this technology, various undruggable TFs have been successfully targeted, demonstrated that TFD could serve as a potential therapeutic strategy for multiple indications, including cancer. Different forms of TFD that target STAT3 have been evaluated in multiple cancer cell lines. For instance, a cyclic-STAT3-decoy and a STAT3-decoy with two binding sequences have been shown to reduce STAT3-regulated gene expression in bladder cancer cells and SW480 colon cancer cells (Lee et al., 2018). TFD targeting of STAT3 has been evaluated in the clinic and showed good safety profiles and downregulated targeted gene expression along with inhibiting tumor growth. In addition to STAT3, other TFs such as NF-κB, E2F, CREB and NFAT have been successfully targeted by TFD, with NF-κB TFD has also been evaluated in the clinic for multiple indications (Wang et al., 2018).
TRANSCRIPTION FACTOR TARGETING CHIMERAS (TRAFTACS)
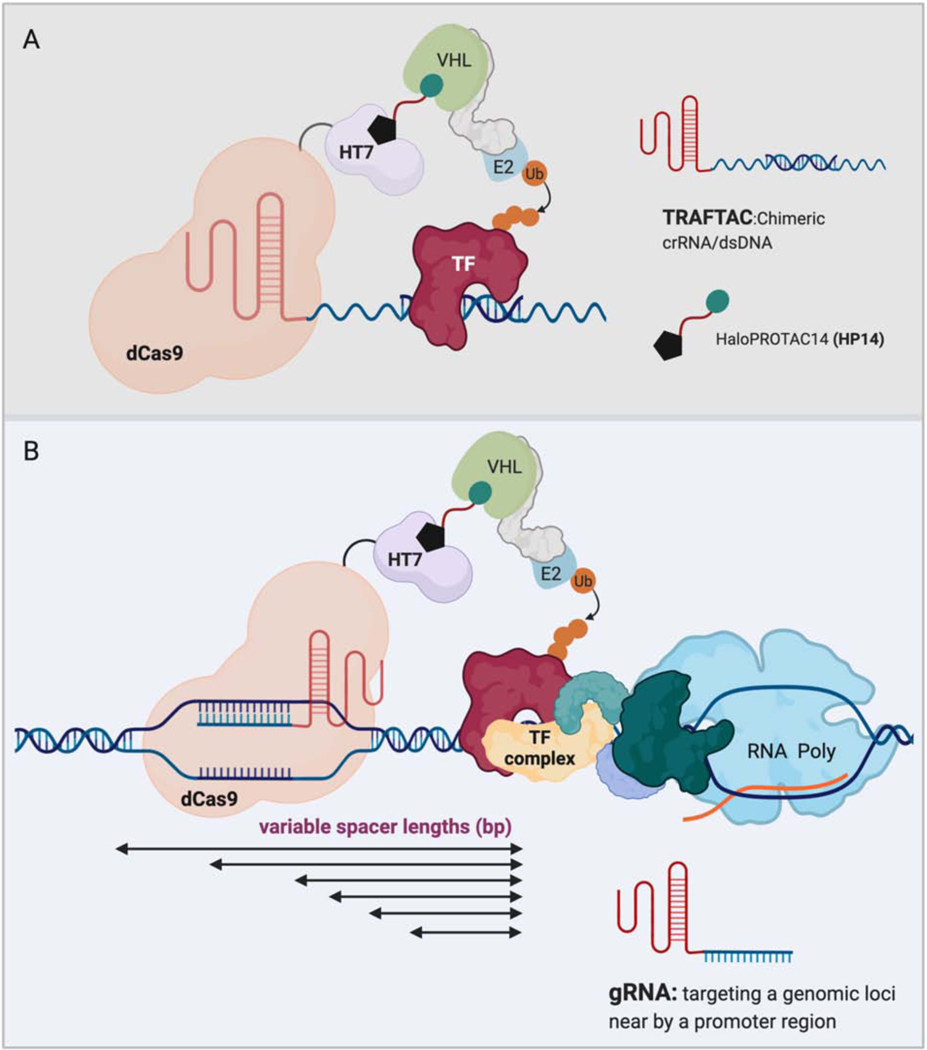
While a handful of TFs have been targeted based on event-driven pharmacology, the remaining TFs have been targeted mostly by indirect or direct -occupancy-driven pharmacology. In considering the use of TPD to reduce TF expression, there are two significant hurdles. First, because they chiefly function via interacting with other biological entities, TFs lack abundant potential binding pockets for small molecule ligands. Additionally, even though PROTAC-based TPD has the potential to target undruggable proteins by utilizing a ligand that binds to POI, finding a ligand is still challenging and involves time consuming discovery efforts. Transcription Factor Targeting Chimeras or TRAFTACs is a new concept that our lab recently developed to induce selective degradation of TFs (Samarasinghe et al., 2021). TRAFTAC involves the dimerization of a TF of interest and a E3 ligase recruiting intermediate fusion protein, dCas9-HaloTag, via a chimeric oligonucleotide comprised of the TF’s consensus DNA binding sequence and CRISPR RNA (crRNA) respectively. Therefore, in the presence of a HaloPROTAC, VHL-E3 ligase is also recruited to the vicinity of the TF of interest to induce its degradation by the proteasome. In a recent preprint version of this study from our lab, this technology has been successfully utilized to degrade two disease causing TFs, NF-κB and brachyury.
The two TRAFTACs, which are composed of a crRNA and either a κB sequence or brachyury DNA binding sequence, were introduced into cells that overexpress dCas9-HaloTag7. Upon introduction, the NF-κB targeting TRAFTAC induced significant degradation of NF-κB and downregulated NF-κB reporter gene expression in a HaloPROTAC-dependent manner. Similarly, the brachyury-targeting TRAFTAC could also induce degradation of both ectopically expressed brachyury-GFP and endogenous brachyury in the presence of HaloPROTAC14 (Figure 6A). Optimization of the spacer DNA length between the TF-binding sequence and crRNA sequence will allow TRAFTACs to target various DNA binding proteins. Thus, TRAFTACs is the first study to demonstrate a generalizable-degradation platform for proteins without available small molecule ligand binders.
Figure 6. Applications of transcription factor targeting chimeras (TRAFTACs).
A) Chimeric TRAFTACs induce the proximity between a transcription factor of interest and an E3-ligase complex in the presence of HP14. Subsequent ubiquitination results in the degradation of TF by the proteasome.
B) Conversion of TRAFTACs to a standard gRNA that targets a neighboring site of the promoter region could recruit an E3 ligase to the proximity of promoter-bound TF and induce subsequent degradation of TF by the proteasome. Spacer DNA between dCas9 binding site and TF-binding site can be adjusted using different gRNAs to achieve optimal/productive proximity between the recruited E3-ligase and the TF.
CONCLUSION AND PERSPECTIVES
Identification of biological processes and their functional effectors are invaluable not only to understand how our bodies function at the molecular level but also for the development of new therapeutic modalities. Induced protein degradation by heterobifunctional PROTACs is designed to work by co-opting the natural cellular ubiquitination process. PROTACs are composed of two ligands that bind to the POI and a E3 ligase, tethered together. Subsequent recruitment of these two entities in proximity induces ubiquitination of the POI and directs it to the cellular proteolytic machinery. In this way, PROTAC technology has been widely applied against various disease-causing proteins with different biological roles and has demonstrated multiple advantages over conventional inhibitory strategies. Since PROTAC is an event-driven modality, when a non-covalent POI ligand is used in PROTAC design it can induce the degradation of the POI in a sub-stoichiometric manner. Thus, administration of relatively low doses might provide prolonged effects on the target protein compared to small molecule inhibition. Also, application of TPD in oncology might overcome resistance mechanisms, such as overexpression of oncogenic proteins, and potentially reduce adverse effects compared to conventional inhibitor drugs due to the reported higher specificity of PRPOTACs. Furthermore, recent advances in the TPD field indicate that exploitation of tissue-specific E3 ligases (e.g., KLHL41 in skeletal muscle, RNF112 in brain tissues, TRIM69 in testis, ASB9 in pancreas) or tumor-specific E3 ligases (e.g., TRIM50 and FBXO2) will significantly improve tumor-selective mode of PROTACs (Schapira et al., 2019, Khan et al., 2020).
PROTACs, by definition, are capable of eradicating target proteins from the cellular environment. Unlike conventional inhibition, induced disposal of proteins not only interferes with catalytic activity of POI but also eliminates any other associated scaffolding roles upon which cancer cells rely. Since simultaneous, ligand-dependent engagement of PROTAC with the target protein and the E3 ligase is the first step involved in a successful PROTAC mechanism, the targeting of proteins without well-defined catalytic pockets such as TFs has been difficult. Only a few TFs, principally nuclear receptors, have been targeted by PROTACs due to the limited availability and difficulty in developing small molecule ligands for TFs, leaving. a large portion of TFs still remaining to be targeted by direct methods. Recently, we reported a generalizable strategy for targeted degradation of TFs:TRAFTACs -- chimeric oligonucleotides comprised of double-stranded DNA and crRNA -- were designed by harnessing the DNA binding ability of TFs and a cellular E3 ligase machinery. Due the flexibility of the proposed TRAFTAC strategy, TFs with known DNA binding sequences can be targeted for proteasomal degradation by incorporating those DNA sequences into TRAFTAC design. Thus, this method provides a general way to directly target previously undrugged TFs. Further, simple modification to this strategy has the potential to induce the selective degradation of only the promoter-bound pool of a TF to achieve specific downregulation of an individual gene (Figure 6B). This could be achieved by using a traditional gRNA that targets a specific locus neighboring the TF-binding promoter site of the targeted gene. Introduction of the dCas9-HaloTag7 fusion protein and a HaloPROTAC will recruit the E3 ligase complex to the vicinity of the promoter-bound TF to induce its ubiquitination and rapid degradation by the proteasome. Thus, direct targeting of TFs by TRAFTAC-like strategies may have profound effect towards cancer cells compared to the targeting of upstream signaling effector proteins or indirect strategies. Furthermore, future advancements to such direct technologies will ultimately provide more efficient and safe therapeutic avenues to explore different disease conditions where TF functions are dysregulated. Overall, two decades of TPD have shown us a remarkable progress towards precision targeting of disease-causing proteins for proteasomal degradation, and have proven that this technology has a great promise in the drug discovery world.
SIGNIFICANCE
Intracellular disease-causing proteins targeted by current treatment modalities are just a small population compared to the number of proteins that are yet to be drugged. A large portion of the proteome performs non-catalytic scaffolding roles in biological pathways that are crucial to the initiation and progression of certain diseases. Due the lack of well-defined active sites within them, such proteins long have been considered undruggable. However, recent advancements in the medicinal chemistry and chemical biology fields have contributed significantly to change the traditional view of such proteins. Since 2012, more than a decade after the PROTAC technology was introduced, the TPD field has started to rapidly evolve to successfully target numerous disease relevant proteins. The continued progress in the field has led to the development of direct and generalizable strategies such as TRAFTACs to target hard to drug transcription factors for proteasomal degradation. Thus, TPD holds a great promise to overcome obstacles that are inherently linked with troublesome proteins and for the future of medicines.
ACKNOWLEDGEMENTS
C.M.C. is funded by the NIH (R35CA197589) and is supported by an American Cancer Research Professorship. We thank Dr. John Hines and Dr. Dhanusha A. Nalawansha for editing, insightful discussions and comments. (Figures were created with BioRender.com)
Footnotes
DECLARATION OF INTEREST
C.M.C is founder, shareholder, and consultant to Arvinas, Inc. and Halda, LLC, which support research in his laboratory.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- ADHIKARI B, BOZILOVIC J, DIEBOLD M, SCHWARZ JD, HOFSTETTER J, SCHRÖDER M, WANIOR M, NARAIN A, VOGT M, DUDVARSKI STANKOVIC N, BALUAPURI A, SCHÖNEMANN L, EING L, BHANDARE P, KUSTER B, SCHLOSSER A, HEINZLMEIR S, SOTRIFFER C, KNAPP S & WOLF E. 2020. PROTAC-mediated degradation reveals a non-catalytic function of AURORA-A kinase. Nature Chemical Biology, 16, 1179–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AHN IE, UNDERBAYEV C, ALBITAR A, HERMAN SEM, TIAN X, MARIC I, ARTHUR DC, WAKE L, PITTALUGA S, YUAN CM, STETLER-STEVENSON M, SOTO S, VALDEZ J, NIERMAN P, LOTTER J, XI L, RAFFELD M, FAROOQUI M, ALBITAR M & WIESTNER A. 2017. Clonal evolution leading to ibrutinib resistance in chronic lymphocytic leukemia. Blood, 129, 1469–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALABI S, JAIME-FIGUEROA S, YAO Z, GAO Y, HINES J, SAMARASINGHE KTG, VOGT L, ROSEN N & CREWS CM 2020. Mutant-selective Degradation by BRAF-targeting PROTACs. bioRxiv, 2020.08.10.245159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AN Z, LV W, SU S, WU W & RAO Y. 2019. Developing potent PROTACs tools for selective degradation of HDAC6 protein. Protein & Cell, 10, 606–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANTONARAKIS ES, LU C, WANG H, LUBER B, NAKAZAWA M, ROESER JC, CHEN Y, MOHAMMAD TA, CHEN Y, FEDOR HL, LOTAN TL, ZHENG Q, DE MARZO AM, ISAACS JT, ISAACS WB, NADAL R, PALLER CJ, DENMEADE SR, CARDUCCI MA, EISENBERGER MA & LUO J. 2014. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med, 371, 1028–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARKIN MR, TANG Y & WELLS JA 2014. Small-molecule inhibitors of protein-protein interactions: progressing toward the reality. Chem Biol, 21, 1102–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAI L, ZHOU H, XU R, ZHAO Y, CHINNASWAMY K, MCEACHERN D, CHEN J, YANG C-Y, LIU Z, WANG M, LIU L, JIANG H, WEN B, KUMAR P, MEAGHER JL, SUN D, STUCKEY JA & WANG S. 2019. A Potent and Selective Small-Molecule Degrader of STAT3 Achieves Complete Tumor Regression In Vivo. Cancer Cell, 36, 498–511.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAINTON DF 1981. The discovery of lysosomes. Journal of Cell Biology, 91, 66s–76s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAKER EK, TAYLOR S, GUPTE A, SHARP PP, WALIA M, WALSH NC, ZANNETTINO AC, CHALK AM, BURNS CJ & WALKLEY CR 2015. BET inhibitors induce apoptosis through a MYC independent mechanism and synergise with CDK inhibitors to kill osteosarcoma cells. Scientific reports, 5, 10120–10120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALCH WE, MAGRUM LJ, FOX GE, WOLFE RS & WOESE CR 1977. An ancient divergence among the bacteria. Journal of Molecular Evolution, 9, 305–311. [DOI] [PubMed] [Google Scholar]
- BANIK SM, PEDRAM K, WISNOVSKY S, AHN G, RILEY NM & BERTOZZI CR 2020. Lysosome-targeting chimaeras for degradation of extracellular proteins. Nature, 584, 291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BASSI ZI, FILLMORE MC, MIAH AH, CHAPMAN TD, MALLER C, ROBERTS EJ, DAVIS LC, LEWIS DE, GALWEY NW, WADDINGTON KE, PARRAVICINI V, MACMILLAN-JONES AL, GONGORA C, HUMPHREYS PG, CHURCHER I, PRINJHA RK & TOUGH DF 2018. Modulating PCAF/GCN5 Immune Cell Function through a PROTAC Approach. ACS Chemical Biology, 13, 2862–2867. [DOI] [PubMed] [Google Scholar]
- BAUDINO TA 2015. Targeted Cancer Therapy: The Next Generation of Cancer Treatment. Curr Drug Discov Technol, 12, 3–20. [DOI] [PubMed] [Google Scholar]
- BELL CC & GILAN O. 2020. Principles and mechanisms of non-genetic resistance in cancer. British Journal of Cancer, 122, 465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENSIMON A, PIZZAGALLI MD, KARTNIG F, DVORAK V, ESSLETZBICHLER P, WINTER GE & SUPERTI-FURGA G. 2020. Targeted Degradation of SLC Transporters Reveals Amenability of Multi-Pass Transmembrane Proteins to Ligand-Induced Proteolysis. Cell Chemical Biology, 27, 728–739.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BI S, WEI Q, ZHAO Z, CHEN L, WANG C & XIE S. 2019. Wee1 Inhibitor AZD1775 Effectively Inhibits the Malignant Phenotypes of Esophageal Squamous Cell Carcinoma In Vitro and In Vivo. Frontiers in Pharmacology, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOND MJ, CHU L, NALAWANSHA DA, LI K & CREWS CM 2020. Targeted Degradation of Oncogenic KRASG12C by VHL-Recruiting PROTACs. ACS Central Science, 6, 1367–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOND MJ & CREWS CM 2021. Proteolysis targeting chimeras (PROTACs) come of age: entering the third decade of targeted protein degradation. RSC Chemical Biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BONDESON DP, SMITH BE, BURSLEM GM, BUHIMSCHI AD, HINES J, JAIME-FIGUEROA S, WANG J, HAMMAN BD, ISHCHENKO A & CREWS CM 2018. Lessons in PROTAC Design from Selective Degradation with a Promiscuous Warhead. Cell Chem Biol, 25, 78–87.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUCK TM, WRIGHT CM & BRODSKY JL 2007. The activities and function of molecular chaperones in the endoplasmic reticulum. Seminars in cell & developmental biology, 18, 751–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUCKLEY DL, VAN MOLLE I, GAREISS PC, TAE HS, MICHEL J, NOBLIN DJ, JORGENSEN WL, CIULLI A & CREWS CM 2012. Targeting the von Hippel-Lindau E3 ubiquitin ligase using small molecules to disrupt the VHL/HIF-1α interaction. Journal of the American Chemical Society, 134, 4465–4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUHIMSCHI AD, ARMSTRONG HA, TOURE M, JAIME-FIGUEROA S, CHEN TL, LEHMAN AM, WOYACH JA, JOHNSON AJ, BYRD JC & CREWS CM 2018. Targeting the C481S Ibrutinib-Resistance Mutation in Bruton’s Tyrosine Kinase Using PROTAC-Mediated Degradation. Biochemistry, 57, 3564–3575. [DOI] [PubMed] [Google Scholar]
- BURSLEM GM, SCHULTZ AR, BONDESON DP, EIDE CA, SAVAGE STEVENS SL, DRUKER BJ & CREWS CM 2019. Targeting BCR-ABL1 in Chronic Myeloid Leukemia by PROTAC-Mediated Targeted Protein Degradation. Cancer Research, 79, 4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURSLEM GM, SMITH BE, LAI AC, JAIME-FIGUEROA S, MCQUAID DC, BONDESON DP, TOURE M, DONG H, QIAN Y, WANG J, CREW AP, HINES J & CREWS CM 2018a. The Advantages of Targeted Protein Degradation Over Inhibition: An RTK Case Study. Cell chemical biology, 25, 67–77.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURSLEM GM, SONG J, CHEN X, HINES J & CREWS CM 2018b. Enhancing Antiproliferative Activity and Selectivity of a FLT-3 Inhibitor by Proteolysis Targeting Chimera Conversion. J Am Chem Soc, 140, 16428–16432. [DOI] [PubMed] [Google Scholar]
- CANON J, REX K, SAIKI AY, MOHR C, COOKE K, BAGAL D, GAIDA K, HOLT T, KNUTSON CG, KOPPADA N, LANMAN BA, WERNER J, RAPAPORT AS, SAN MIGUEL T, ORTIZ R, OSGOOD T, SUN JR, ZHU X, MCCARTER JD, VOLAK LP, HOUK BE, FAKIH MG, O’NEIL BH, PRICE TJ, FALCHOOK GS, DESAI J, KUO J, GOVINDAN R, HONG DS, OUYANG W, HENARY H, ARVEDSON T, CEE VJ & LIPFORD JR 2019. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature, 575, 217–223. [DOI] [PubMed] [Google Scholar]
- CARDENAS MG, OSWALD E, YU W, XUE F, MACKERELL AD JR. & MELNICK AM 2017. The Expanding Role of the BCL6 Oncoprotein as a Cancer Therapeutic Target. Clin Cancer Res, 23, 885–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARROLL D. 2011. Genome engineering with zinc-finger nucleases. Genetics, 188, 773–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN H, CHEN F, LIU N, WANG X & GOU S. 2018. Chemically induced degradation of CK2 by proteolysis targeting chimeras based on a ubiquitin-proteasome pathway. Bioorg Chem, 81, 536–544. [DOI] [PubMed] [Google Scholar]
- CHENG M, YU X, LU K, XIE L, WANG L, MENG F, HAN X, CHEN X, LIU J, XIONG Y & JIN J. 2020. Discovery of Potent and Selective Epidermal Growth Factor Receptor (EGFR) Bifunctional Small-Molecule Degraders. Journal of Medicinal Chemistry, 63, 1216–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COSTALES MG, AIKAWA H, LI Y, CHILDS-DISNEY JL, ABEGG D, HOCH DG, PRADEEP VELAGAPUDI S, NAKAI Y, KHAN T, WANG KW, YILDIRIM I, ADIBEKIAN A, WANG ET & DISNEY MD 2020. Small-molecule targeted recruitment of a nuclease to cleave an oncogenic RNA in a mouse model of metastatic cancer. Proceedings of the National Academy of Sciences, 117, 2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COTTON AD, NGUYEN DP, GRAMESPACHER JA, SEIPLE IB & WELLS JA 2021. Development of Antibody-Based PROTACs for the Degradation of the Cell-Surface Immune Checkpoint Protein PD-L1. Journal of the American Chemical Society. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CREW AP, RAINA K, DONG H, QIAN Y, WANG J, VIGIL D, SEREBRENIK YV, HAMMAN BD, MORGAN A, FERRARO C, SIU K, NEKLESA TK, WINKLER JD, COLEMAN KG & CREWS CM 2018. Identification and Characterization of Von Hippel-Lindau-Recruiting Proteolysis Targeting Chimeras (PROTACs) of TANK-Binding Kinase 1. Journal of Medicinal Chemistry, 61, 583–598. [DOI] [PubMed] [Google Scholar]
- CREWS CM 2010. Targeting the undruggable proteome: the small molecules of my dreams. Chemistry & biology, 17, 551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROMM PM, SAMARASINGHE KTG, HINES J & CREWS CM 2018. Addressing Kinase-Independent Functions of Fak via PROTAC-Mediated Degradation. Journal of the American Chemical Society, 140, 17019–17026. [DOI] [PubMed] [Google Scholar]
- CUI C, ZHOU X, ZHANG W, QU Y & KE X. 2018. Is β-Catenin a Druggable Target for Cancer Therapy? Trends Biochem Sci, 43, 623–634. [DOI] [PubMed] [Google Scholar]
- CUNEO KC, MORGAN MA, SAHAI V, SCHIPPER MJ, PARSELS LA, PARSELS JD, DEVASIA T, AL-HAWARAY M, CHO CS, NATHAN H, MAYBAUM J, ZALUPSKI MM & LAWRENCE TS 2019. Dose Escalation Trial of the Wee1 Inhibitor Adavosertib (AZD1775) in Combination With Gemcitabine and Radiation for Patients With Locally Advanced Pancreatic Cancer. J Clin Oncol, 37, 2643–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANG CV, REDDY EP, SHOKAT KM & SOUCEK L. 2017. Drugging the ‘undruggable’ cancer targets. Nature Reviews Cancer, 17, 502–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEBRINCAT MA, PLEINES I, LEBOIS M, LANE RM, HOLMES ML, CORBIN J, VANDENBERG CJ, ALEXANDER WS, NG AP, STRASSER A, BOUILLET P, SOLA-VISNER M, KILE BT & JOSEFSSON EC 2015. BCL-2 is dispensable for thrombopoiesis and platelet survival. Cell Death Dis, 6, e1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DHILLON AS, HAGAN S, RATH O & KOLCH W. 2007. MAP kinase signalling pathways in cancer. Oncogene, 26, 3279–3290. [DOI] [PubMed] [Google Scholar]
- DOHERTY GJ & MCMAHON HT 2009. Mechanisms of Endocytosis. Annual Review of Biochemistry, 78, 857–902. [DOI] [PubMed] [Google Scholar]
- DRAGOVICH PS, ADHIKARI P, BLAKE RA, BLAQUIERE N, CHEN J, CHENG Y-X, DEN BESTEN W, HAN J, HARTMAN SJ, HE J, HE M, REI INGALLA E, KAMATH AV, KLEINHEINZ T, LAI T, LEIPOLD DD, LI CS, LIU Q, LU J, LU Y, MENG F, MENG L, NG C, PENG K, LEWIS PHILLIPS G, PILLOW TH, ROWNTREE RK, SADOWSKY JD, SAMPATH D, STABEN L, STABEN ST, WAI J, WAN K, WANG X, WEI B, WERTZ IE, XIN J, XU K, YAO H, ZANG R, ZHANG D, ZHOU H & ZHAO Y. 2020. Antibody-mediated delivery of chimeric protein degraders which target estrogen receptor alpha (ERα). Bioorganic & Medicinal Chemistry Letters, 30, 126907. [DOI] [PubMed] [Google Scholar]
- DUFFY MJ 2006. Estrogen receptors: role in breast cancer. Crit Rev Clin Lab Sci, 43, 325–47. [DOI] [PubMed] [Google Scholar]
- DUYAO MP, BUCKLER AJ & SONENSHEIN GE 1990. Interaction of an NF-kappa B-like factor with a site upstream of the c-myc promoter. Proceedings of the National Academy of Sciences of the United States of America, 87, 4727–4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENGEL J, LATEGAHN J & RAUH D. 2016. Hope and Disappointment: Covalent Inhibitors to Overcome Drug Resistance in Non-Small Cell Lung Cancer. ACS Medicinal Chemistry Letters, 7, 2–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FABBRO D, COWAN-JACOB SW & MOEBITZ H. 2015. Ten things you should know about protein kinases: IUPHAR Review 14. British journal of pharmacology, 172, 2675–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAGARD R, METELEV V, SOUISSI I & BARAN-MARSZAK F. 2013. STAT3 inhibitors for cancer therapy: Have all roads been explored? JAK-STAT, 2, e22882–e22882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAN H, ZHAO X, SUN S, LUO M & GUAN JL 2013. Function of focal adhesion kinase scaffolding to mediate endophilin A2 phosphorylation promotes epithelial-mesenchymal transition and mammary cancer stem cell activities in vivo. J Biol Chem, 288, 3322–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLANAGAN JJ, QIAN Y, GOUGH SM, ANDREOLI M, BOOKBINDER M, CADELINA G, BRADLEY J, ROUSSEAU E, WILLARD R, PIZZANO J, CREWS CM, CREW AP, TAYLOR I & HOUSTON J. 2019. Abstract P5–04-18: ARV-471, an oral estrogen receptor PROTAC degrader for breast cancer. Cancer Research, 79, P5–04-18. [Google Scholar]
- FRIBOULET L, LI N, KATAYAMA R, LEE CC, GAINOR JF, CRYSTAL AS, MICHELLYS PY, AWAD MM, YANAGITANI N, KIM S, PFERDEKAMPER AC, LI J, KASIBHATLA S, SUN F, SUN X, HUA S, MCNAMARA P, MAHMOOD S, LOCKERMAN EL, FUJITA N, NISHIO M, HARRIS JL, SHAW AT & ENGELMAN JA 2014. The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Discov, 4, 662–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GADD MS, TESTA A, LUCAS X, CHAN K-H, CHEN W, LAMONT DJ, ZENGERLE M & CIULLI A. 2017. Structural basis of PROTAC cooperative recognition for selective protein degradation. Nature Chemical Biology, 13, 514–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALAOUI R & MCCAFFREY L. 2015. Rewiring cell polarity signaling in cancer. Oncogene, 34, 939–50. [DOI] [PubMed] [Google Scholar]
- HALLIN J, ENGSTROM LD, HARGIS L, CALINISAN A, ARANDA R, BRIERE DM, SUDHAKAR N, BOWCUT V, BAER BR, BALLARD JA, BURKARD MR, FELL JB, FISCHER JP, VIGERS GP, XUE Y, GATTO S, FERNANDEZ-BANET J, PAVLICEK A, VELASTAGUI K, CHAO RC, BARTON J, PIEROBON M, BALDELLI E, PATRICOIN EF, CASSIDY DP, MARX MA, RYBKIN II, JOHNSON ML, OU S-HI, LITO P, PAPADOPOULOS KP, JÄNNE PA, OLSON P & CHRISTENSEN JG 2020. The KRAS<sup>G12C</sup> Inhibitor MRTX849 Provides Insight toward Therapeutic Susceptibility of KRAS-Mutant Cancers in Mouse Models and Patients. Cancer Discovery, 10, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAN J & SUN P. 2007. The pathways to tumor suppression via route p38. Trends Biochem Sci, 32, 364–71. [DOI] [PubMed] [Google Scholar]
- HE K, ZHANG Z, WANG W, ZHENG X, WANG X & ZHANG X. 2020a. Discovery and biological evaluation of proteolysis targeting chimeras (PROTACs) as an EGFR degraders based on osimertinib and lenalidomide. Bioorg Med Chem Lett, 30, 127167. [DOI] [PubMed] [Google Scholar]
- HE Y, KOCH R, BUDAMAGUNTA V, ZHANG P, ZHANG X, KHAN S, THUMMURI D, ORTIZ YT, ZHANG X, LV D, WIEGAND JS, LI W, PALMER AC, ZHENG G, WEINSTOCK DM & ZHOU D. 2020b. DT2216-a Bcl-xL-specific degrader is highly active against Bcl-xL-dependent T cell lymphomas. J Hematol Oncol, 13, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HE Y, ZHANG X, CHANG J, KIM HN, ZHANG P, WANG Y, KHAN S, LIU X, ZHANG X, LV D, SONG L, LI W, THUMMURI D, YUAN Y, WIEGAND JS, ORTIZ YT, BUDAMAGUNTA V, ELISSEEFF JH, CAMPISI J, ALMEIDA M, ZHENG G & ZHOU D. 2020c. Using proteolysis-targeting chimera technology to reduce navitoclax platelet toxicity and improve its senolytic activity. Nat Commun, 11, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HECKER M & WAGNER AH 2017. Transcription factor decoy technology: A therapeutic update. Biochemical Pharmacology, 144, 29–34. [DOI] [PubMed] [Google Scholar]
- HINES J, GOUGH JD, CORSON TW & CREWS CM 2013. Posttranslational protein knockdown coupled to receptor tyrosine kinase activation with phosphoPROTACs. Proceedings of the National Academy of Sciences, 110, 8942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HINES J, LARTIGUE S, DONG H, QIAN Y & CREWS CM 2019. MDM2-Recruiting PROTAC Offers Superior, Synergistic Antiproliferative Activity via Simultaneous Degradation of BRD4 and Stabilization of p53. Cancer Res, 79, 251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLA VR, ELAMIN YY, BAILEY AM, JOHNSON AM, LITZENBURGER BC, KHOTSKAYA YB, SANCHEZ NS, ZENG J, SHUFEAN MA, SHAW KR, MENDELSOHN J, MILLS GB, MERIC-BERNSTAM F & SIMON GR 2017. ALK: a tyrosine kinase target for cancer therapy. Cold Spring Harbor molecular case studies, 3, a001115–a001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HSU JH-R, RASMUSSON T, ROBINSON J, PACHL F, READ J, KAWATKAR S, O’ DONOVAN DH, BAGAL S, CODE E, RAWLINS P, ARGYROU A, TOMLINSON R, GAO N, ZHU X, CHIARPARIN E, JACQUES K, SHEN M, WOODS H, BEDNARSKI E, WILSON DM, DREW L, CASTALDI MP, FAWELL S & BLOECHER A. 2020. EED-Targeted PROTACs Degrade EED, EZH2, and SUZ12 in the PRC2 Complex. Cell Chemical Biology, 27, 41–46.e17. [DOI] [PubMed] [Google Scholar]
- HU J, WEI J, YIM H, WANG L, XIE L, JIN MS, KABIR M, QIN L, CHEN X, LIU J & JIN J. 2020. Potent and Selective Mitogen-Activated Protein Kinase Kinase 1/2 (MEK1/2) Heterobifunctional Small-molecule Degraders. Journal of Medicinal Chemistry, 63, 15883–15905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUANG HT, DOBROVOLSKY D, PAULK J, YANG G, WEISBERG EL, DOCTOR ZM, BUCKLEY DL, CHO JH, KO E, JANG J, SHI K, CHOI HG, GRIFFIN JD, LI Y, TREON SP, FISCHER ES, BRADNER JE, TAN L & GRAY NS 2018. A Chemoproteomic Approach to Query the Degradable Kinome Using a Multi-kinase Degrader. Cell Chem Biol, 25, 88–99.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTT DM & BALCH WE 2013. Expanding proteostasis by membrane trafficking networks. Cold Spring Harb Perspect Biol, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HWANG SY, DENG X, BYUN S, LEE C, LEE SJ, SUH H, ZHANG J, KANG Q, ZHANG T, WESTOVER KD, MANDINOVA A & LEE SW 2016. Direct Targeting of β-Catenin by a Small Molecule Stimulates Proteasomal Degradation and Suppresses Oncogenic Wnt/β-Catenin Signaling. Cell Rep, 16, 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICONOMOU M & SAUNDERS DN 2016. Systematic approaches to identify E3 ligase substrates. The Biochemical journal, 473, 4083–4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IGEA A & NEBREDA AR 2015. The Stress Kinase p38α as a Target for Cancer Therapy. Cancer Research, 75, 3997. [DOI] [PubMed] [Google Scholar]
- J VERHEUL TC, TRINH VT, VÁZQUEZ O & PHILIPSEN S. 2020. Targeted Protein Degradation as a Promising Tool for Epigenetic Upregulation of Fetal Hemoglobin. ChemMedChem, 15, 2436–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIANG B, WANG ES, DONOVAN KA, LIANG Y, FISCHER ES, ZHANG T & GRAY NS 2019. Development of Dual and Selective Degraders of Cyclin-Dependent Kinases 4 and 6. Angewandte Chemie International Edition, 58, 6321–6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIN Y-H, LU M-C, WANG Y, SHAN W-X, WANG X-Y, YOU Q-D & JIANG Z-Y 2020. Azo-PROTAC: Novel Light-Controlled Small-Molecule Tool for Protein Knockdown. Journal of Medicinal Chemistry, 63, 4644–4654. [DOI] [PubMed] [Google Scholar]
- JOHN PC, MEWS M & MOORE R. 2001. Cyclin/Cdk complexes: their involvement in cell cycle progression and mitotic division. Protoplasma, 216, 119–42. [DOI] [PubMed] [Google Scholar]
- JOHNSTON PA & GRANDIS JR 2011. STAT3 signaling: anticancer strategies and challenges. Molecular interventions, 11, 18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANG CH, LEE DH, LEE CO, DU HA J, PARK CH & HWANG JY 2018. Induced protein degradation of anaplastic lymphoma kinase (ALK) by proteolysis targeting chimera (PROTAC). Biochemical and Biophysical Research Communications, 505, 542–547. [DOI] [PubMed] [Google Scholar]
- KARGBO RB 2020. SMARCA2/4 PROTAC for Targeted Protein Degradation and Cancer Therapy. ACS Medicinal Chemistry Letters, 11, 1797–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KERRES N, STEURER S, SCHLAGER S, BADER G, BERGER H, CALIGIURI M, DANK C, ENGEN JR, ETTMAYER P, FISCHERAUER B, FLOTZINGER G, GERLACH D, GERSTBERGER T, GMASCHITZ T, GREB P, HAN B, HEYES E, IACOB RE, KESSLER D, KÖLLE H, LAMARRE L, LANCIA DR, LUCAS S, MAYER M, MAYR K, MISCHERIKOW N, MÜCK K, PEINSIPP C, PETERMANN O, REISER U, RUDOLPH D, RUMPEL K, SALOMON C, SCHARN D, SCHNITZER R, SCHRENK A, SCHWEIFER N, THOMPSON D, TRAXLER E, VARECKA R, VOSS T, WEISS-PUXBAUM A, WINKLER S, ZHENG X, ZOEPHEL A, KRAUT N, MCCONNELL D, PEARSON M & KOEGL M. 2017. Chemically Induced Degradation of the Oncogenic Transcription Factor BCL6. Cell Rep, 20, 2860–2875. [DOI] [PubMed] [Google Scholar]
- KHAN S, HE Y, ZHANG X, YUAN Y, PU S, KONG Q, ZHENG G & ZHOU D. 2020. PROteolysis TArgeting Chimeras (PROTACs) as emerging anticancer therapeutics. Oncogene, 39, 4909–4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM S & JEONG S. 2019. Mutation Hotspots in the β-Catenin Gene: Lessons from the Human Cancer Genome Databases. Molecules and cells, 42, 8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNIE C, UTECHT M, ZHAO F, KULLA H, KOVALENKO S, BROUWER AM, SAALFRANK P, HECHT S & BLÉGER D. 2014. ortho-Fluoroazobenzenes: Visible Light Switches with Very Long-Lived Z Isomers. Chemistry – A European Journal, 20, 16492–16501. [DOI] [PubMed] [Google Scholar]
- KOJIMA Y, SASAKI S, SHINOURA H, HAYASHI Y, TSUJIMOTO G & KOHRI K. 2006. Quantification of alpha1-adrenoceptor subtypes by real-time RT-PCR and correlation with age and prostate volume in benign prostatic hyperplasia patients. Prostate, 66, 761–7. [DOI] [PubMed] [Google Scholar]
- KONERMANN S, BRIGHAM MD, TREVINO AE, JOUNG J, ABUDAYYEH OO, BARCENA C, HSU PD, HABIB N, GOOTENBERG JS, NISHIMASU H, NUREKI O & ZHANG F. 2015. Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature, 517, 583–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOUL HK, PAL M & KOUL S. 2013. Role of p38 MAP Kinase Signal Transduction in Solid Tumors. Genes & cancer, 4, 342–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOUNDE CS, SHCHEPINOVA MM, SAUNDERS CN, MUELBAIER M, RACKHAM MD, HARLING JD & TATE EW 2020. A caged E3 ligase ligand for PROTAC-mediated protein degradation with light. Chemical Communications, 56, 5532–5535. [DOI] [PubMed] [Google Scholar]
- KUANG P, CHEN Z, WANG J, LIU Z, WANG J, GAO J & SHEN L. 2017. Characterization of Aurora A and Its Impact on the Effect of Cisplatin-Based Chemotherapy in Patients with Non–Small Cell Lung Cancer. Translational Oncology, 10, 367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAI AC & CREWS CM 2017. Induced protein degradation: an emerging drug discovery paradigm. Nature Reviews Drug Discovery, 16, 101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAI AC, TOURE M, HELLERSCHMIED D, SALAMI J, JAIME-FIGUEROA S, KO E, HINES J & CREWS CM 2016. Modular PROTAC Design for the Degradation of Oncogenic BCR-ABL. Angew Chem Int Ed Engl, 55, 807–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEBRAUD H, WRIGHT DJ, JOHNSON CN & HEIGHTMAN TD 2016. Protein Degradation by In-Cell Self-Assembly of Proteolysis Targeting Chimeras. ACS Cent Sci, 2, 927–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE GT, NAGAYA N, DESANTIS J, MADURA K, SABAAWY HE, KIM W-J, VAZ RJ, CRUCIANI G & KIM IY 2020. Effects of MTX-23, a Novel PROTAC of Androgen Receptor Splice Variant-7 and Androgen Receptor, on CRPC resistant to Second-Line Antiandrogen Therapy. Molecular Cancer Therapeutics, molcanther.0417.2020. [DOI] [PubMed] [Google Scholar]
- LEE LYW, MOHAMMAD S, STARKEY T & LEE S-M 2018. STAT3 cyclic oligonucleotide decoy-a new therapeutic avenue for NSCLC? Translational lung cancer research, 7, S381–S384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEHTONEN Š, SONNINEN TM, WOJCIECHOWSKI S, GOLDSTEINS G & KOISTINAHO J. 2019. Dysfunction of Cellular Proteostasis in Parkinson’s Disease. Front Neurosci, 13, 457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI W, GAO C, ZHAO L, YUAN Z, CHEN Y & JIANG Y. 2018a. Phthalimide conjugations for the degradation of oncogenic PI3K. Eur J Med Chem, 151, 237–247. [DOI] [PubMed] [Google Scholar]
- LI Y, LI X, PU J, YANG Q, GUAN H, JI M, SHI B, CHEN M & HOU P. 2018b. c-Myc Is a Major Determinant for Antitumor Activity of Aurora A Kinase Inhibitor MLN8237 in Thyroid Cancer. Thyroid, 28, 1642–1654. [DOI] [PubMed] [Google Scholar]
- LI Z, LIN Y, SONG H, QIN X, YU Z, ZHANG Z, DONG G, LI X, SHI X, DU L, ZHAO W & LI M. 2020a. First small-molecule PROTACs for G protein-coupled receptors: inducing α1A-adrenergic receptor degradation. Acta Pharmaceutica Sinica B, 10, 1669–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI Z, PINCH BJ, OLSON CM, DONOVAN KA, NOWAK RP, MILLS CE, SCOTT DA, DOCTOR ZM, ELEUTERI NA, CHUNG M, SORGER PK, FISCHER ES & GRAY NS 2020b. Development and Characterization of a Wee1 Kinase Degrader. Cell Chem Biol, 27, 57–65.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIAO H, LI X, ZHAO, WANG Y, WANG X, WU Y, ZHOU X, FU W, LIU, HU H-G & CHEN Y-G 2020. A PROTAC peptide induces durable β-catenin degradation and suppresses Wnt-dependent intestinal cancer. Cell Discovery, 6, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILLO OSUNA MA, GARCIA-LOPEZ J, EL AYACHI I, FATIMA I, KHALID AB, KUMPATI J, SLAYDEN AV, SEAGROVES TN, MIRANDA-CARBONI GA & KRUM SA 2019. Activation of Estrogen Receptor Alpha by Decitabine Inhibits Osteosarcoma Growth and Metastasis. Cancer Res, 79, 1054–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU J, CHEN H, MA L, HE Z, WANG D, LIU Y, LIN Q, ZHANG T, GRAY N, KANISKAN HÜ, JIN J & WEI W 2020. Light-induced control of protein destruction by opto-PROTAC. Science Advances, 6, eaay5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LØNNING PE & KNAPPSKOG S. 2013. Mapping genetic alterations causing chemoresistance in cancer: identifying the roads by tracking the drivers. Oncogene, 32, 5315–5330. [DOI] [PubMed] [Google Scholar]
- LOVLY CM & SHAW AT 2014. Molecular pathways: resistance to kinase inhibitors and implications for therapeutic strategies. Clinical cancer research : an official journal of the American Association for Cancer Research, 20, 2249–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LU J, QIAN Y, ALTIERI M, DONG H, WANG J, RAINA K, HINES J, WINKLER JD, CREW AP, COLEMAN K & CREWS CM 2015. Hijacking the E3 Ubiquitin Ligase Cereblon to Efficiently Target BRD4. Chem Biol, 22, 755–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUO M, ZHAO X, SONG Y, CHENG H & ZHOU R. 2016. Nuclear autophagy: An evolutionarily conserved mechanism of nuclear degradation in the cytoplasm. Autophagy, 12, 1973–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANEIRO MA, FORTE N, SHCHEPINOVA MM, KOUNDE CS, CHUDASAMA V, BAKER JR & TATE EW 2020. Antibody–PROTAC Conjugates Enable HER2-Dependent Targeted Protein Degradation of BRD4. ACS Chemical Biology, 15, 1306–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANGELSDORF DJ, THUMMEL C, BEATO M, HERRLICH P, SCHÜTZ G, UMESONO K, BLUMBERG B, KASTNER P, MARK M, CHAMBON P & EVANS RM 1995. The nuclear receptor superfamily: the second decade. Cell, 83, 835–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTINS DE OLIVEIRA V, GODOI CONTESSOTO VD, BRUNO DA SILVA F, ZAGO CAETANO DL, JURADO DE CARVALHO S & PEREIRA LEITE VB 2018. Effects of pH and Salt Concentration on Stability of a Protein G Variant Using Coarse-Grained Models. Biophysical Journal, 114, 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCARTY NS, GRAHAM AE, STUDENÁ L & LEDESMA-AMARO R. 2020. Multiplexed CRISPR technologies for gene editing and transcriptional regulation. Nature Communications, 11, 1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCOULL W, CHEUNG T, ANDERSON E, BARTON P, BURGESS J, BYTH K, CAO Q, CASTALDI MP, CHEN H, CHIARPARIN E, CARBAJO RJ, CODE E, COWAN S, DAVEY PR, FERGUSON AD, FILLERY S, FULLER NO, GAO N, HARGREAVES D, HOWARD MR, HU J, KAWATKAR A, KEMMITT PD, LEO E, MOLINA DM, O’CONNELL N, PETTERUTI P, RASMUSSON T, RAUBO P, RAWLINS PB, RICCHIUTO P, ROBB GR, SCHENONE M, WARING MJ, ZINDA M, FAWELL S & WILSON DM 2018. Development of a Novel B-Cell Lymphoma 6 (BCL6) PROTAC To Provide Insight into Small Molecule Targeting of BCL6. ACS Chemical Biology, 13, 3131–3141. [DOI] [PubMed] [Google Scholar]
- MEHLMAN C, CHAABANE N, LACAVE R, KERROU K, RUPPERT AM, CADRANEL J & FALLET V. 2019. Ceritinib ALK T1151R Resistance Mutation in Lung Cancer With Initial Response to Brigatinib. Journal of Thoracic Oncology, 14, e95–e96. [DOI] [PubMed] [Google Scholar]
- MÉNARD S, TAGLIABUE E, CAMPIGLIO M & PUPA SM 2000. Role of HER2 gene overexpression in breast carcinoma. J Cell Physiol, 182, 150–62. [DOI] [PubMed] [Google Scholar]
- MOON SB, KIM DY, KO J-H & KIM Y-S 2019. Recent advances in the CRISPR genome editing tool set. Experimental & Molecular Medicine, 51, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORGILLO F, DELLA CORTE CM, FASANO M & CIARDIELLO F. 2016. Mechanisms of resistance to EGFR-targeted drugs: lung cancer. ESMO Open, 1, e000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NALAWANSHA DA & CREWS CM 2020. PROTACs: An Emerging Therapeutic Modality in Precision Medicine. Cell Chemical Biology, 27, 998–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NARO Y, DARRAH K & DEITERS A. 2020. Optical Control of Small Molecule-Induced Protein Degradation. Journal of the American Chemical Society, 142, 2193–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIKONOVA AS, ASTSATUROV I, SEREBRIISKII IG, DUNBRACK RL & GOLEMIS EA 2013. Aurora A kinase (AURKA) in normal and pathological cell division. Cellular and Molecular Life Sciences, 70, 661–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NURGALI K, JAGOE RT & ABALO R. 2018. Editorial: Adverse Effects of Cancer Chemotherapy: Anything New to Improve Tolerance and Reduce Sequelae? Frontiers in pharmacology, 9, 245–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSTREM JM, PETERS U, SOS ML, WELLS JA & SHOKAT KM 2013. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature, 503, 548–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OUSTEROUT DG & GERSBACH CA 2016. The Development of TALE Nucleases for Biotechnology. Methods in molecular biology (Clifton, N.J.), 1338, 27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETRYLAK DP, GAO X, VOGELZANG NJ, GARFIELD MH, TAYLOR I, DOUGAN MOORE M, PECK RA & BURRIS HA 2020. First-in-human phase I study of ARV-110, an androgen receptor (AR) PROTAC degrader in patients (pts) with metastatic castrate-resistant prostate cancer (mCRPC) following enzalutamide (ENZ) and/or abiraterone (ABI). Journal of Clinical Oncology, 38, 3500–3500. [Google Scholar]
- PFAFF P, SAMARASINGHE KTG, CREWS CM & CARREIRA EM 2019. Reversible Spatiotemporal Control of Induced Protein Degradation by Bistable PhotoPROTACs. ACS Central Science, 5, 1682–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PONTORIERO M, FIUME G, VECCHIO E, DE LAURENTIIS A, ALBANO F, IACCINO E, MIMMI S, PISANO A, AGOSTI V, GIOVANNONE E, ALTOBELLI A, CAIAZZA C, MALLARDO M, SCALA G & QUINTO I. 2019. Activation of NF-κB in B cell receptor signaling through Bruton’s tyrosine kinase-dependent phosphorylation of IκB-α. J Mol Med (Berl), 97, 675–690. [DOI] [PubMed] [Google Scholar]
- PORTELA A & ESTELLER M. 2010. Epigenetic modifications and human disease. Nature Biotechnology, 28, 1057–1068. [DOI] [PubMed] [Google Scholar]
- POSTERNAK G, TANG X, MAISONNEUVE P, JIN T, LAVOIE H, DAOU S, ORLICKY S, GOULLET DE RUGY T, CALDWELL L, CHAN K, AMAN A, PRAKESCH M, PODA G, MADER P, WONG C, MAIER S, KITAYGORODSKY J, LARSEN B, COLWILL K, YIN Z, CECCARELLI DF, BATEY RA, TAIPALE M, KURINOV I, UEHLING D, WRANA J, DUROCHER D, GINGRAS A-C, AL-AWAR R, THERRIEN M & SICHERI F. 2020. Functional characterization of a PROTAC directed against BRAF mutant V600E. Nature Chemical Biology, 16, 1170–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POWELL CE, GAO Y, TAN L, DONOVAN KA, NOWAK RP, LOEHR A, BAHCALL M, FISCHER ES, JÄNNE PA, GEORGE RE & GRAY NS 2018. Chemically Induced Degradation of Anaplastic Lymphoma Kinase (ALK). J Med Chem, 61, 4249–4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PULECIO J, VERMA N, MEJÍA-RAMÍREZ E, HUANGFU D & RAYA A. 2017. CRISPR/Cas9-Based Engineering of the Epigenome. Cell Stem Cell, 21, 431–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAINA K, LU J, QIAN Y, ALTIERI M, GORDON D, ROSSI AM, WANG J, CHEN X, DONG H, SIU K, WINKLER JD, CREW AP, CREWS CM & COLEMAN KG 2016. PROTAC-induced BET protein degradation as a therapy for castration-resistant prostate cancer. Proc Natl Acad Sci U S A, 113, 7124–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAUCH J, VOLINSKY N, ROMANO D & KOLCH W. 2011. The secret life of kinases: functions beyond catalysis. Cell Communication and Signaling, 9, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REDMAN JM, HILL EM, ALDEGHAITHER D & WEINER LM 2015. Mechanisms of action of therapeutic antibodies for cancer. Molecular immunology, 67, 28–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNDERS M, MATSUURA BS, BÉROUTI M, SIMONESCHI D, MARZIO A, PAGANO M & TRAUNER D. 2020. PHOTACs enable optical control of protein degradation. Science Advances, 6, eaay5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICKERT RC 2013. New insights into pre-BCR and BCR signalling with relevance to B cell malignancies. Nature Reviews Immunology, 13, 578–591. [DOI] [PubMed] [Google Scholar]
- ROBERTS BL, MA Z-X, GAO A, LEISTEN ED, YIN D, XU W & TANG W. 2020. Two-Stage Strategy for Development of Proteolysis Targeting Chimeras and its Application for Estrogen Receptor Degraders. ACS Chemical Biology, 15, 1487–1496. [DOI] [PubMed] [Google Scholar]
- RODRIGUEZ-GONZALEZ A, CYRUS K, SALCIUS M, KIM K, CREWS CM, DESHAIES RJ & SAKAMOTO KM 2008. Targeting steroid hormone receptors for ubiquitination and degradation in breast and prostate cancer. Oncogene, 27, 7201–7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAKAMOTO KM, KIM KB, KUMAGAI A, MERCURIO F, CREWS CM & DESHAIES RJ 2001. Protacs: Chimeric molecules that target proteins to the Skp1–Cullin–F box complex for ubiquitination and degradation. Proceedings of the National Academy of Sciences, 98, 8554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAKAMOTO KM, KIM KB, VERMA R, RANSICK A, STEIN B, CREWS CM & DESHAIES RJ 2003. Development of Protacs to target cancer-promoting proteins for ubiquitination and degradation. Mol Cell Proteomics, 2, 1350–8. [DOI] [PubMed] [Google Scholar]
- SALAMI J, ALABI S, WILLARD RR, VITALE NJ, WANG J, DONG H, JIN M, MCDONNELL DP, CREW AP, NEKLESA TK & CREWS CM 2018. Androgen receptor degradation by the proteolysis-targeting chimera ARCC-4 outperforms enzalutamide in cellular models of prostate cancer drug resistance. Communications biology, 1, 100–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALAMI J & CREWS CM 2017. Waste disposal—An attractive strategy for cancer therapy. Science, 355, 1163. [DOI] [PubMed] [Google Scholar]
- SAMARASINGHE KTG, JAIME-FIGUEROA S, BURGESS M, NALAWANSHA DA, DAI K, HU Z, BEBENEK A, HOLLEY SA & CREWS CM 2021. Targeted degradation of transcription factors by TRAFTACs: TRAnscription Factor TArgeting Chimeras. Cell Chemical Biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMMAK S & ZINZALLA G. 2015. Targeting protein-protein interactions (PPIs) of transcription factors: Challenges of intrinsically disordered proteins (IDPs) and regions (IDRs). Prog Biophys Mol Biol, 119, 41–6. [DOI] [PubMed] [Google Scholar]
- SARA JD, KAUR J, KHODADADI R, REHMAN M, LOBO R, CHAKRABARTI S, HERRMANN J, LERMAN A & GROTHEY A. 2018. 5-fluorouracil and cardiotoxicity: a review. Ther Adv Med Oncol, 10, 1758835918780140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAPIRA M, CALABRESE MF, BULLOCK AN & CREWS CM 2019. Targeted protein degradation: expanding the toolbox. Nat Rev Drug Discov, 18, 949–963. [DOI] [PubMed] [Google Scholar]
- SCHIEDEL M, HERP D, HAMMELMANN S, SWYTER S, LEHOTZKY A, ROBAA D, OLÁH J, OVÁDI J, SIPPL W & JUNG M. 2018. Chemically Induced Degradation of Sirtuin 2 (Sirt2) by a Proteolysis Targeting Chimera (PROTAC) Based on Sirtuin Rearranging Ligands (SirReals). Journal of Medicinal Chemistry, 61, 482–491. [DOI] [PubMed] [Google Scholar]
- SCHNEEKLOTH AR, PUCHEAULT M, TAE HS & CREWS CM 2008. Targeted intracellular protein degradation induced by a small molecule: En route to chemical proteomics. Bioorg Med Chem Lett, 18, 5904–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHNEEKLOTH JS, FONSECA FN, KOLDOBSKIY M, MANDAL A, DESHAIES R, SAKAMOTO K & CREWS CM 2004. Chemical Genetic Control of Protein Levels: Selective in Vivo Targeted Degradation. Journal of the American Chemical Society, 126, 3748–3754. [DOI] [PubMed] [Google Scholar]
- SCHUETZ JM, JOHNSON NA, MORIN RD, SCOTT DW, TAN K, BEN-NIERAH S, BOYLE M, SLACK GW, MARRA MA, CONNORS JM, BROOKS-WILSON AR & GASCOYNE RD 2012. BCL2 mutations in diffuse large B-cell lymphoma. Leukemia, 26, 1383–1390. [DOI] [PubMed] [Google Scholar]
- SETTEN RL, ROSSI JJ & HAN S-P 2019. The current state and future directions of RNAi-based therapeutics. Nature Reviews Drug Discovery, 18, 421–446. [DOI] [PubMed] [Google Scholar]
- SHIBATA N, SHIMOKAWA K, NAGAI K, OHOKA N, HATTORI T, MIYAMOTO N, UJIKAWA O, SAMESHIMA T, NARA H, CHO N & NAITO M. 2018. Pharmacological difference between degrader and inhibitor against oncogenic BCR-ABL kinase. Scientific Reports, 8, 13549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIMOKAWA K, SHIBATA N, SAMESHIMA T, MIYAMOTO N, UJIKAWA O, NARA H, OHOKA N, HATTORI T, CHO N & NAITO M. 2017. Targeting the Allosteric Site of Oncoprotein BCR-ABL as an Alternative Strategy for Effective Target Protein Degradation. ACS Med Chem Lett, 8, 1042–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SŁABICKI M, YOON H, KOEPPEL J, NITSCH L, ROY BURMAN SS, DI GENUA C, DONOVAN KA, SPERLING AS, HUNKELER M, TSAI JM, SHARMA R, GUIRGUIS A, ZOU C, CHUDASAMA P, GASSER JA, MILLER PG, SCHOLL C, FRÖHLING S, NOWAK RP, FISCHER ES & EBERT BL 2020. Small-molecule-induced polymerization triggers degradation of BCL6. Nature, 588, 164–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMALLEY JP, ADAMS GE, MILLARD CJ, SONG Y, NORRIS JKS, SCHWABE JWR, COWLEY SM & HODGKINSON JT. 2020. PROTAC-mediated degradation of class I histone deacetylase enzymes in corepressor complexes. Chemical Communications, 56, 4476–4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH BE, WANG SL, JAIME-FIGUEROA S, HARBIN A, WANG J, HAMMAN BD & CREWS CM 2019. Differential PROTAC substrate specificity dictated by orientation of recruited E3 ligase. Nature Communications, 10, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SULZMAIER FJ, JEAN C & SCHLAEPFER DD 2014. FAK in cancer: mechanistic findings and clinical applications. Nature Reviews Cancer, 14, 598–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUN N, REN C, KONG Y, ZHONG H, CHEN J, LI Y, ZHANG J, ZHOU Y, QIU X, LIN H, SONG X, YANG X & JIANG B. 2020. Development of a Brigatinib degrader (SIAIS117) as a potential treatment for ALK positive cancer resistance. European Journal of Medicinal Chemistry, 193, 112190. [DOI] [PubMed] [Google Scholar]
- SWEENEY P, PARK H, BAUMANN M, DUNLOP J, FRYDMAN J, KOPITO R, MCCAMPBELL A, LEBLANC G, VENKATESWARAN A, NURMI A & HODGSON R. 2017. Protein misfolding in neurodegenerative diseases: implications and strategies. Translational Neurodegeneration, 6, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKESHIGE K, BABA M, TSUBOI S, NODA T & OHSUMI Y. 1992. Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction. Journal of Cell Biology, 119, 301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TANG YQ, HAN BM, YAO XQ, HONG Y, WANG Y, ZHAO FJ, YU SQ, SUN XW & XIA SJ 2009. Chimeric molecules facilitate the degradation of androgen receptors and repress the growth of LNCaP cells. Asian J Androl, 11, 119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOLCHER AW, PAPADOPOULOS KP, PATNAIK A, RASCO DW, MARTINEZ D, WOOD DL, FIELMAN B, SHARMA M, JANISCH LA, BROWN BD, BHARGAVA P & RATAIN MJ 2015. Safety and activity of DCR-MYC, a first-in-class Dicer-substrate small interfering RNA (DsiRNA) targeting MYC, in a phase I study in patients with advanced solid tumors. Journal of Clinical Oncology, 33, 11006–11006. [Google Scholar]
- TOVELL H, TESTA A, ZHOU H, SHPIRO N, CRAFTER C, CIULLI A & ALESSI DR 2019. Design and Characterization of SGK3-PROTAC1, an Isoform Specific SGK3 Kinase PROTAC Degrader. ACS Chem Biol, 14, 2024–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TROUP RI, FALLAN C & BAUD MGJ 2020. Current strategies for the design of PROTAC linkers: a critical review. Exploration of Targeted Anti-tumor Therapy, 1, 273–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TYEDMERS J, MOGK A & BUKAU B. 2010. Cellular strategies for controlling protein aggregation. Nature Reviews Molecular Cell Biology, 11, 777–788. [DOI] [PubMed] [Google Scholar]
- VANNAM R, SAYILGAN J, OJEDA S, KARAKYRIAKOU B, HU E, KREUZER J, MORRIS R, HERRERA LOPEZ XI, RAI S, HAAS W, LAWRENCE M & OTT CJ Targeted degradation of the enhancer lysine acetyltransferases CBP and p300. Cell Chemical Biology. [DOI] [PubMed] [Google Scholar]
- VISCONTI R, DELLA MONICA R & GRIECO D. 2016. Cell cycle checkpoint in cancer: a therapeutically targetable double-edged sword. Journal of Experimental & Clinical Cancer Research, 35, 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG Q, PECHERSKY Y, SAGAWA S, PAN AC & SHAW DE 2019a. Structural mechanism for Bruton’s tyrosine kinase activation at the cell membrane. Proceedings of the National Academy of Sciences, 116, 9390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG Z, HE N, GUO Z, NIU C, SONG T, GUO Y, CAO K, WANG A, ZHU J, ZHANG X & ZHANG Z. 2019b. Proteolysis Targeting Chimeras for the Selective Degradation of Mcl-1/Bcl-2 Derived from Nonselective Target Binding Ligands. Journal of Medicinal Chemistry, 62, 8152–8163. [DOI] [PubMed] [Google Scholar]
- WANG Z, POTOYAN DA & WOLYNES PG 2018. Modeling the therapeutic efficacy of NFκB synthetic decoy oligodeoxynucleotides (ODNs). BMC Systems Biology, 12, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAUDBY CA, DOBSON CM & CHRISTODOULOU J. 2019. Nature and Regulation of Protein Folding on the Ribosome. Trends in Biochemical Sciences, 44, 914–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEE P & WANG Z. 2017. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers, 9, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS KM, QIE S, ATKISON JH, SALAZAR-ARANGO S, ALAN DIEHL J & OLSEN SK 2019. Structural insights into E1 recognition and the ubiquitin-conjugating activity of the E2 enzyme Cdc34. Nature Communications, 10, 3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINTER GE, BUCKLEY DL, PAULK J, ROBERTS JM, SOUZA A, DHE-PAGANON S & BRADNER JE 2015. Phthalimide conjugation as a strategy for in vivo target protein degradation. Science, 348, 1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOYACH JA, BOJNIK E, RUPPERT AS, STEFANOVSKI MR, GOETTL VM, SMUCKER KA, SMITH LL, DUBOVSKY JA, TOWNS WH, MACMURRAY J, HARRINGTON BK, DAVIS ME, GOBESSI S, LAURENTI L, CHANG BY, BUGGY JJ, EFREMOV DG, BYRD JC & JOHNSON AJ 2014. Bruton’s tyrosine kinase (BTK) function is important to the development and expansion of chronic lymphocytic leukemia (CLL). Blood, 123, 1207–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WRIGHT G, GOLUBEVA V, REMSING RIX LL, BERNDT N, LUO Y, WARD GA, GRAY JE, SCHONBRUNN E, LAWRENCE HR, MONTEIRO ANA & RIX U. 2017. Dual Targeting of WEE1 and PLK1 by AZD1775 Elicits Single Agent Cellular Anticancer Activity. ACS Chem Biol, 12, 1883–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XIAO Y, WANG J, ZHAO LY, CHEN X, ZHENG G, ZHANG X & LIAO D. 2020. Discovery of histone deacetylase 3 (HDAC3)-specific PROTACs. Chemical Communications, 56, 9866–9869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XUE G, CHEN J, LIU L, ZHOU D, ZUO Y, FU T & PAN Z. 2020. Protein degradation through covalent inhibitor-based PROTACs. Chemical Communications, 56, 1521–1524. [DOI] [PubMed] [Google Scholar]
- XUE G, WANG K, ZHOU D, ZHONG H & PAN Z. 2019. Light-Induced Protein Degradation with Photocaged PROTACs. Journal of the American Chemical Society, 141, 18370–18374. [DOI] [PubMed] [Google Scholar]
- YANG C, CAO P, GAO Y, WU M, LIN Y, TIAN Y & YUAN W. 2016. Differential expression of p38 MAPK α, β, γ, δ isoforms in nucleus pulposus modulates macrophage polarization in intervertebral disc degeneration. Scientific Reports, 6, 22182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YEO NC, CHAVEZ A, LANCE-BYRNE A, CHAN Y, MENN D, MILANOVA D, KUO CC, GUO X, SHARMA S, TUNG A, CECCHI RJ, TUTTLE M, PRADHAN S, LIM ET, DAVIDSOHN N, EBRAHIMKHANI MR, COLLINS JJ, LEWIS NE, KIANI S & CHURCH GM. 2018. An enhanced CRISPR repressor for targeted mammalian gene regulation. Nat Methods, 15, 611–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAIDMAN D, PRILUSKY J & LONDON N. 2020. PRosettaC: Rosetta Based Modeling of PROTAC Mediated Ternary Complexes. Journal of Chemical Information and Modeling, 60, 4894–4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG C, HAN XR, YANG X, JIANG B, LIU J, XIONG Y & JIN J. 2018. Proteolysis Targeting Chimeras (PROTACs) of Anaplastic Lymphoma Kinase (ALK). Eur J Med Chem, 151, 304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG H, ZHAO HY, XI XX, LIU YJ, XIN M, MAO S, ZHANG JJ, LU AX & ZHANG SQ 2020a. Discovery of potent epidermal growth factor receptor (EGFR) degraders by proteolysis targeting chimera (PROTAC). Eur J Med Chem, 189, 112061. [DOI] [PubMed] [Google Scholar]
- ZHANG X, THUMMURI D, HE Y, LIU X, ZHANG P, ZHOU D & ZHENG G. 2019. Utilizing PROTAC technology to address the on-target platelet toxicity associated with inhibition of BCL-X(L). Chem Commun (Camb), 55, 14765–14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG X, XU F, TONG L, ZHANG T, XIE H, LU X, REN X & DING K. 2020b. Design and synthesis of selective degraders of EGFRL858R/T790M mutant. European Journal of Medicinal Chemistry, 192, 112199. [DOI] [PubMed] [Google Scholar]
- ZHAO B & BURGESS K. 2019. PROTACs suppression of CDK4/6, crucial kinases for cell cycle regulation in cancer. Chemical Communications, 55, 2704–2707. [DOI] [PubMed] [Google Scholar]
- ZHAO H-Y, YANG X-Y, LEI H, XI X-X, LU S-M, ZHANG J-J, XIN M & ZHANG S-Q 2020. Discovery of potent small molecule PROTACs targeting mutant EGFR. European Journal of Medicinal Chemistry, 208, 112781. [DOI] [PubMed] [Google Scholar]
- ZHAO Z & SHILATIFARD A. 2019. Epigenetic modifications of histones in cancer. Genome Biology, 20, 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOU F, CHEN L, CAO C, YU J, LUO X, ZHOU P, ZHAO L, DU W, CHENG J, XIE Y & CHEN Y. 2020. Development of selective mono or dual PROTAC degrader probe of CDK isoforms. Eur J Med Chem, 187, 111952. [DOI] [PubMed] [Google Scholar]