Abstract
Objective:
To (1) examine the potentiality of using the robot PARO to mediate care provided by the family and (2) identify problems when utilizing PARO in the home context.
Methods:
Family members of 7 households were asked to use PARO for at least three times per week, over 1 to 3 months. Research data, including standardized assessments, interviews, and observations, were collected at initial and subsequent monthly visits. Collected data were analyzed through descriptive statistics and inductive thematic content analysis.
Results:
Out of the seven participants, five responded positively to PARO, thereby achieving their goals of improving activity engagement, relaxation, a respite from supervision, and improved mood. A positive initial interaction with PARO showed continued interest to it. Participants were observed to communicate with caregivers and relate to PARO.
Discussion:
The application of PARO at home is possibly influenced by the persons’ initial level of interest toward PARO. It is crucial to perform careful observation and assessment before deciding to use PARO within the home context to support the life of older persons with dementia.
Keywords: family caregiver, Japan, person-centered care, seal robot, user acceptance
Introduction
As of 2017, Japan reached the world’s highest number of older adults. 1 These individuals often wish to live the rest of their lives in environments familiar to them, even as they may develop conditions requiring long-term care services such as dementia, needing long-term care from family members and health care practitioners (HCPs). However, fatigue and stress among persons caring for older persons with dementia resulting in domestic abuse have become a major problem in Japan. 2 Moreover, there is an expected shortage of HCPs making the provision of long-term care services a challenge. 3 “Hands-on care” alone may not be adequate to meet the demand, hence adapting “care by device” can be a viable strategy to compensate for the labor shortage.
As the Japanese government prioritizes the development and practical use of robots, it has become one of the major industries in Japan, flourishing in the field of medicine and nursing. 4 Most studies concerning the practical use of robots in health care have focused on the Japanese-made baby seal robot “PARO,” a neurological therapeutic medical device certified by the US Food and Drug Administration. 5
PARO is used in the medical and welfare fields in various developed countries and regions in the United States, Canada, Europe, Asia, and Oceania. PARO is effective in improving quality of life, enjoyment, emotional expression, social interaction, and reducing the usage of neuropsychiatric medication for stress and anxiety.6–9 Thus, PARO is recommended to people with mild to moderate agitation brought about by dementia who attend programs within health facilities.10,11
Despite the numerous studies, most of these were obtained from health care facilities administered by trained HCPs. The only research conducted within one’s natural environment did not look into the effects of using PARO solely at home as it was a combination of daytime PARO use in a day-care center and home. 11 As PARO can potentially serve as a medium in improving the drive of individuals caring for persons with dementia, it is of benefit to consider PARO use in diminishing stress among family members while contributing to the extension of community living among older persons with dementia, thereby becoming one of the solutions to providing high-quality home care. 12
To use PARO at home, the family must be able to operate it easily and effectively. Person-Centered Care (PCC) is an approach to dementia care that can be applied to support the person and their family. As this approach is not exclusive to HCPs, adapting the PCC approach to improve the quality of care at home is a possibility for both family members and HCPs. 13 PCC’s core assumption is that personhood can be maintained by meeting 5 fundamental psychosocial needs namely comfort, identity, attachment, occupation, and inclusion. Introducing PARO can potentially meet the five needs of PCC. 14 In utilizing PARO ethically, the thoughts of older persons with dementia must be considered regardless of the changes in behaviors observed.
This study aimed to explore the potential of using the robot “PARO” to support family caregivers in caring for older persons with dementia. The aims of this research were (1) to examine the potentiality of a PARO-mediated care provided by the family and (2) to identify the problems when utilizing PARO in the home context.
Methods
Design
An exploratory study was employed to investigate the possibilities of utilizing robots as part of home care. Because of the scarcity of existing research on utilizing PARO at home and facilitated by family members, an exploratory design was employed in this research as it is more flexible, which is necessary to gain new insights that can help further define the problem and plan future studies. 15
Tools and materials
PARO (Figure 1), a baby seal-shaped robot (ninth generation, about 57 cm, about 2.5 kg) developed at the National Institute of Advanced Industrial Science and Technology (Japan), was used in this study. Guided by the notions behind animal-assisted therapy, PARO was developed to facilitate the users’ psychological, physical, and social wellbeing. 16 PARO is capable of making an animal-like cry, moves its head and legs, and blinks. With artificial intelligence, it can remember the name of a person and endears itself to its owner with cute gestures and cries. High safety standards through antibacterial processed fur and magnetic shielding function enable PARO to be used in intensive care settings. 17
Figure 1.

PARO.
Participants
The participants of this research are older persons with dementia living with their families. Inclusion criteria include older persons (over the age of 65 years) diagnosed with dementia by a physician, are expected to benefit from using PARO (upon evaluation of a licensed HCP and subsequent goal setting), and whose family member can operate PARO. Exclusion criteria include individuals with other comorbid conditions severely affecting the use of and interaction with PARO (such as individuals with severe motor impairment or blindness), who demonstrate a repulsive reaction to PARO, and those residing alone or with no family members.
Participants were recruited by handing advertisement brochures to dementia support groups within the Tokyo metropolitan area and Long-Term Care Insurance case managers’ gatherings. Interested individuals contacted the principal investigator (PI) to schedule an initial home visit with both the PI and an HCP. The PI explained the content of the research and the benefits of using PARO; PARO was then presented to the older persons with dementia for 30–60 min to examine for any repulsive responses such as being sad, anxious, or agitated as these can indicate their refusal to participate. Once eligibility screening was done, informed consent was obtained from the older persons with dementia and their family.
Data collection
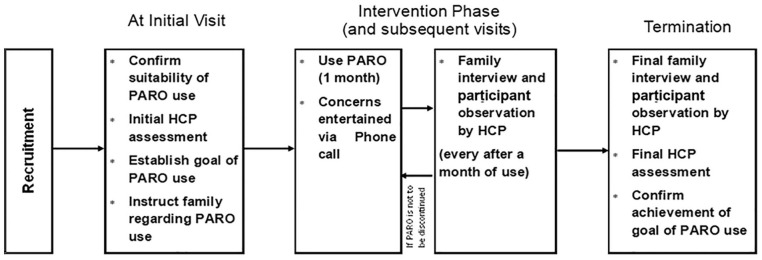
During the first visit, the participant’s caregiver provides a goal while being cognizant of the benefits of PARO identified in existing literature.6–9 The goal served as a motivation for the family to encourage PARO use but did not influence the type of intervention to be delivered at home. After determining the goal, the PI instructed the family regarding the set-up (placing PARO on an easily visible location and without startling the participant), operation, maintenance, and timing of PARO use (at least three times per week). Duration of the intervention per day spans from 15 to 180 min, depending on the individual’s motivation to interact with PARO. While participants are free to interact with PARO in any manner they want, the PI suggested petting, grooming, greeting, and hugging PARO as common activities done with PARO. In addition, the caregivers were encouraged to provide verbal and gestural prompts to encourage participant’s interaction with PARO. Examples of such include, “PARO has arrived,” “PARO is saying hello,” and “PARO is looking at you.” In addition, the caregivers were asked to allocate time for them to talk with the participant regarding PARO and other related topics. The caregiver can contact the PI at any time if they encounter any problem. Every month, the HCP conducted home visits. The intervention lasted for 1 to 3 months. The process of data collection was graphically shown in Figure 2.
Figure 2.
Process of data collection.
Standardized assessments
A series of standardized assessments were conducted at the start and the end of PARO intervention. Tests were as follow:
Mini-Mental State Examination-Japanese (MMSE-J), a Japanese version of the screening test wherein a grade lower than 23/30 may indicate dementia.18,19 The MMSE-J has a high criteria-related validity with a sensitivity of 0.80 and a specificity of 0.94. 19
Nishimura’s Activity of Daily Living Scale (N-ADL), a simple Japanese test that evaluates the level of ADL independence by observing the behavior of an older person with suspected dementia wherein a full score of 50 indicates normal. 20 The N-ADL, when compared with the Japanese standardized tool Hasegawa Dementia Scale, revealed a correlation coefficient of 0.709. 20
Dementia Behavior Disturbance (DBD) Scale, a 28 item, 5-point scale totaling 112 points used to observe the behavior of people with dementia. 21 In this study, the Japanese version of DBD was used. 22 The DBD Japanese version has a very good test–retest reliability, internal consistency (coefficient of 0.95), and inter-rater reliability. 22
Zarit Burden Interview (ZBI), an assessment tool that objectively evaluates the feeling of burden experienced by caregivers of people with dementia and other conditions requiring assistance. 23 In this research, we used the Japanese version standardized by Arai and colleagues. 24 The Japanese version exhibited high test–retest reliability (0.76), internal consistency (0.93), and high correlation with the Caregiver Epidemiologic Studies Depression scale (0.50). 24
Family interview
A semi-structured interview was conducted every visit. Key questions for jumpstarting the conversation are as follows:
How was the participant’s reaction to PARO?
How do you feel about PARO as a caregiver?
Do you have any other comments about PARO from your experience of using PARO?
When these key questions did not facilitate any comments, more specific questions were asked such as “What kind of behavior did you see in your participant when using PARO?” The family’s comments were recorded through an audio recorder, which was later transcribed.
Participant observation
During monthly visits, the HCP unobtrusively observed and documented the participant’s behaviors, specifically the manner of interaction between the participant with PARO and the caregiver. 25 Observation spanned for approximately 1 h per visit wherein behaviors were noted at random intervals. To understand the documented behaviors, two Advanced Dementia Care Mappers reviewed and classified the behaviors according to the Dementia Care Mapping (DCM) evaluation framework, a mapping method used to record the behavior of dementia methodically. 25 They categorized the behaviors from the 23 pre-defined Behavior Category Code (BCC), details of which can be read in the manual published by the Bradford Dementia Group. 26 The definition of PCC was considered for determining whether a participant’s response was either positive or negative.
Moreover, the interest displayed by the participants toward PARO was documented monthly and subsequently plotted to a timeline graph in accord to a constructed five-level grading scale:
Completely ignore or reject PARO;
Even if presented and encouraged, hardly interacts with PARO;
If presented and encouraged, interact with PARO but without volitional movement;
If presented with PARO, touch voluntarily;
Requests for PARO use and touches PARO voluntarily.
Data analysis
Quantitative data were analyzed via computation of central tendencies pre-post-intervention to provide a descriptive picture of the participants’ state.
An inductive thematic content analysis was employed to analyze the interviews. Thematic content analysis is sufficient for exploratory research to identify key points from the participant’s account. 27 First, the PI assigned codes to the participant’s transcribed statements. Then, commonalities among these codes were synthesized into categories and, subsequently, into themes. Three experienced HCPs collectively reviewed these codes and categories to clarify any uncertainty in every step.
Ethics approval and consent to participate
The experiment protocol for involving humans was in accordance with the Declaration of Helsinki and the guidelines of Tokyo Metropolitan University. This study was approved by the Tokyo Metropolitan University (Approval code: HINO-159), which has authority over the author. The research team provided verbal and written explanations and obtained consent from participants and families. In particular, it communicated to the families, both verbally and written, that their privacy is protected, PARO does not have to be encouraged when participants are in poor health, and that even after consenting, participation can be stopped at any time if the participant indicates refusal.
Results
Out of 10 families who expressed intent to participate, seven families were included in this research. Reasons for exclusion are (1) volunteer does not have dementia, (2) family unwilling to support the entirety of the intervention, and (3) volunteer has an existing medical condition preventing full participation in the intervention.
The participants’ profiles and the result of standardized assessments are shown in Table 1. Participants were 6 women and 1 man with a mean age of 87.29 (SD = 7.04) years. Family caregivers include 4 sons, 2 daughters, and 1 daughter-in-law. During pre-intervention, mean participants’ scores were as follows: MMSE-J was 12.57 (SD = 6.08), N-ADL was 27.86 (SD = 16.22), DBD was 22.17 (SD = 11), and ZBI score was 26.85 (SD = 10.32). At post-intervention, mean participants’ scores (excluding missing values) were as follows: MMSE-J of 13 (SD = 2.12), N-ADL of 27.17 (SD = 10.76), DBD of 20.17 (SD = 8.42), and ZBI of 20.83 (SD = 11.55). None of the participants was diagnosed with any new long-term medical conditions, nor did their existing medicines changed throughout the intervention period. However, participant 2 fell ill and was no longer visited and re-evaluated as the family member was no longer willing to entertain the PI and HCP for personal reasons undisclosed. In addition, the MMSE-J of participant 6 was not taken due to feeling ill in the middle of the re-evaluation.
Table 1.
Participants’ profile and the result of standardized assessments.
| Participant 1 | Participant 2 | Participant 3 | Participant 4 | Participant 5 | Participant 6 | Participant 7 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age/Sex | 86/F | 82/F | 97/F | 79/F | 97/M | 85/F | 85/F | |||||||
| Diagnosis and care level* | Alzheimer’s Dementia (level 1) | Alzheimer’s Dementia (level 1) | Alzheimer’s Dementia (level 4) | Alzheimer’s Dementia (level 3) | Alzheimer’s Dementia (level 4) | Dementia (level 4) | Alzheimer’s Dementia (level 1) | |||||||
| Caregiver (age) | Daughter-in-law (55) | Eldest daughter (55) | Eldest son (62) | Eldest daughter (54) | Eldest son (54) | Husband (88) and eldest son (62) | Eldest son (61) | |||||||
| Standardized score: | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post |
| MMSE-J | 16 | 17 | 21 | NT | 4 | 10 | 13 | 5 | 15 | 19 | 5 | NT | 14 | 14 |
| N-ADL | 42 | 36 | 46 | NT | 2 | 15 | 41 | 40 | 14 | 14 | 25 | 27 | 25 | 31 |
| DBD | 23 | 23 | 17 | NT | 12 | 20 | 35 | 35 | 19 | 14 | 12 | 11 | 40 | 18 |
| ZBI | 14 | 13 | 29 | NT | 27 | 28 | 40 | 35 | 33 | 30 | 12 | 7 | 33 | 12 |
| Goal (achievement) | • Activity Engagement (Not achieved) | • Feel Relaxed • Respite from Supervision (Achieved) |
• Activity Engagement • Improve Mood (Achieved) |
• Feel Relaxed • Activity Engagement • Respite from Supervision (Not Achieved) |
• Activity Engagement • Feel Relaxed (Achieved) |
• Activity Engagement • Improve Mood (Achieved) |
• Feel Relaxed • Improve Mood (Achieved) |
|||||||
DBD, Dementia Behavior Disturbance; MMSE-J, Mini-Mental State Examination-Japanese; N-ADL, Nishimura’s Activity of Daily Living Scale; NT, not tested; ZBI, Zarit Burden Interview.
Care level: Japan’s classification system wherein level 5 is the most severe.
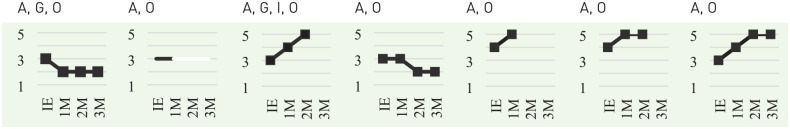
In observing the participants’ interaction with PARO (Table 2), communicating with others (BCC: articulation) and relating to PARO (BCC: objects) were common to all participants. In addition, during the interview, family caregivers of two participants (1 and 3) reported that the use of PARO reminded the participant of their experience in caring for a child or their pet (BCC: going back). The caregiver of participant 3 also reported that the participant showed an increased level of curiosity by asking questions (BCC: intellectual), such as “where do seals live.”
Table 2.
Results of observation.
| Participant 1 | Participant 2 | Participant 3 | Participant 4 | Participant 5 | Participant 6 | Participant 7 | |
|---|---|---|---|---|---|---|---|
| BCC* | A, G, O | A, O | A, G, I, O | A, O | A, O | A, O | A, O |
| Change in Interest$,‡ |
|
||||||
BCC, Behavior Category Code.
BCC: A (articulation) = interaction with others, G (going back) = reminiscence and life review, I (intellectual) = use of intellectual abilities, O (objects) = displaying attachment to or relating to inanimate objects.
Change in interest: 5 = requests for and touch PARO voluntarily, 4 = touch PARO voluntarily if it is presented, 3 = interact with PARO if encouraged by others, 2 = hardly interacts with PARO even if encouraged, 1 = completely ignore and rejects PARO.
Scored at initial visit and subsequent monthly visits.
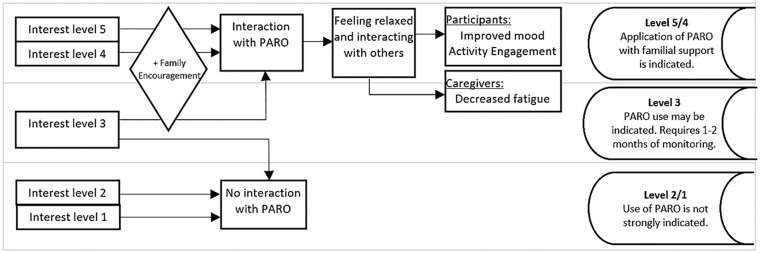
Regarding the change in the level of interest toward PARO (Table 2), the participants that maintained a high level of interest or increased a positive shift tended to talk with PARO, touch voluntarily or with minimal encouragement, lean toward PARO, and gaze at PARO with a smile and a relaxed facial expression. Deriving information from the participants’ interests, Figure 3 illustrates the applicability of utilizing PARO.
Figure 3.
Application of PARO use indicated by the level of the participant’s interest.
Discontinuation of the use of PARO was on the first month for two families, the second month for another two families, and on the third month for three families. Reasons for discontinuation were poor physical condition of the participant (4), loss of interest in PARO (2), and the family becoming too busy (1).
Most caregivers reported that participants displayed a positive reaction and behavior toward PARO and others. The caregiver of participant 2 recounted how PARO was able to take her mother’s attention, thereby keeping her mother from being exposed to possible accidents. S/he shared,
Before, my mother could not sit still and wait while I cooked breakfast. She would walk around the kitchen while being oblivious to the hot stove and pans. I could not focus on cooking. I need to keep an eye on my mother, where she was, what she was touching. So, I placed PARO on the dining table. What surprised me was that my mother was able to engage with PARO. She talks to PARO saying, “What a good boy, cute boy,” ‘Yes, I hear you, dear “Where do you want to go?,” and she gently patted PARO.
Another caregiver shared that having PARO as participant 7’s companion allowed the participant to be more accepting of other people. He reminisced,
My mom hated welcoming community care aides into our home even if she can’t even cook meals without someone’s assistance. So, despite being busy with our business, I had to come home to cook for her. But, since PARO’s arrival, she would always keep PARO next to her. This somehow also made her kinder to strangers . . . The other day, she thanked the care aides and wished them safety as they left. I am glad that we gave PARO a try.
The caregivers themselves also received direct and indirect benefits from interacting with PARO. Participant 2’s caregiver reflected on her understanding of dementia as she shared the following,
My mother asked, “Is this child a seal?” So, I responded to her with another question, “Do you know where this child (PARO) came from?” My mother answered, “I wonder maybe somewhere cold?” That made me think, maybe my mother understands more than I give her credit for.”
A summary of the interview comments from caregivers is found in Table 3.
Table 3.
Family caregiver’s comments regarding PARO use.
| Categories | Comments | Labels | |
|---|---|---|---|
| Participant’s reaction to PARO | |||
| After 1 month | Positive reaction | • “Talking PARO is like talking to a child” • “S/he sat up and reached to pet PARO” |
14 |
| Improved behavior | • “S/he stopped wandering around and stayed
seated” • “S/he became more accepting of care aids’ assistance” |
4 | |
| After 2 months | Positive reaction | • “S/he appears to love PARO very much” • “S/he often asks for PARO’s whereabouts” |
4 |
| Improved behavior | • “S/he talked more frequently. The conversation became gentler” | 4 | |
| Negative reaction | • “S/he said I should play with PARO instead” | 1 | |
| After 3 months | Positive reaction | • “S/he is always petting PARO” | 3 |
| Negative reaction | • “S/he Appears to not like PARO” | 3 | |
| Family’s experience with PARO | |||
| After 1 month | Felt soothed | • PARO was so cute • PARO is good enough to call it a pet |
4 |
| Increased interaction with participant | • We talked about our old pet • We played a trivia game about seals |
1 | |
| Maintenance and Function | • PARO doesn’t charge sometimes • PARO was heavier than I thought |
2 | |
| Benefits for caregiver | • PARO gave me time to complete chores | 1 | |
| Reduced feeling of guilt | • I can leave the participant without feeling like neglecting her | 1 | |
| After 2 months | Felt soothed | • I like PARO more than the participant | 4 |
| Increased interaction with participant | • Reminded me of how my mother used to be | 1 | |
| Benefits for caregiver | • I felt like I was being useful | 2 | |
| Reduced feeling of guilt | • The amount of care remains the same, but my feeling of guilt is less | 1 | |
| Not applicable for use | • The participant just isn’t interested in PARO | 1 | |
| After 3 months | Felt soothed | • I felt soothed by PARO. It may also have a positive effect on the participant | 2 |
| Benefits for caregiver | • I felt like I was useful to the participant | 1 | |
| Not applicable for use | • The participant did not use PARO. Maybe I did not facilitate it correctly | 1 |
All reported numbers of labels are cumulative.
Discussion
Potential effectiveness of PARO activities
Five participants reacted positively to PARO and achieved their goals. This suggests that the benefits of PARO in the context of a facility may be transferred into the home context. The result of this research supports the previously reported efficacy of PARO in terms of improving activity engagement, relaxation, a respite from supervision, and improved mood.6–12,28–30 Furthermore, based on the characteristics of the positive results, it is possible to infer that using PARO may be beneficial to people who are interested in PARO, regardless of the severity of dementia.
PARO exhibits the potential to meet the needs enumerated by the PCC philosophy. 25 The use of PARO within the home context provided a sense of comfort by serving as a companion when facing unfamiliar individuals such as care aides. Comfort is a need that is primarily derived from a person’s kindness and consideration. However, PARO, through its inherent characteristics, can augment this need. Moreover, similar to the findings of Hung and associates, viewing PARO as a companion fostered a sense of attachment. Moreover, PARO has allowed the participants time to interact with their family members, providing a sense of connection with their family thereby fulfilling the need for inclusion within the household by inducing a sense of belongingness. In addition, through this interaction with family members, they get to talk and reminisce past life events, allowing them to revisit the building blocks of their identity. Finally, as the participants interact with both PARO and their family, they get to fulfill the need for occupation as they engage in individual activities such as grooming PARO, thereby playing a role as a caretaker of PARO. Moreover, PARO serves as a catalyst to engage in activities with shared meaning with their family members such as playing trivia quizzes and reminiscing past events. Meeting the five needs as identified in the PCC approach is said to improve the well-being of people with dementia. 25 Despite the minute to no change in terms of the standardized tests, the effect of utilizing PARO positively impacted the participants’ social and emotional health. Using PARO in the home context is beneficial to the caregivers as well as decreases caregiver fatigue and improves communication between the caregiver and the participant. Thus, effective use of PARO at home can be valid as a tool for care provision.
Factors identified for PARO use
From an environmental perspective, the use of PARO increased interaction with the participant, thereby obtaining healing and fun, creating a constructive cycle of wanting to interact again with PARO. However, PARO may not be appropriate for individuals who do not exhibit any sign of interest. As these responses were observed from the time of the first introduction or during the first month, it suggests the possibility of being able to predict the appropriateness of using PARO at the time of the first meeting. It is not reasonable to expect a positive result from PARO use with a family caregiver when PARO is rejected from the onset.
However, for participants who only interacted with PARO when encouraged (scored 3) at the initial visit, the level of interest may increase (participants 3 and 7) or decrease (participants 1 and 4). Hence, there should be a month trial period to confirm the participant’s level of interest as the potential for effective PARO use became apparent after 2 months of use.
In summary, this investigation suggested that when PARO is used in-home care, individuals who displayed an active interest in PARO from the beginning would voluntarily increase the time spent with PARO, leading to a decrease in behavioral and psychological symptoms of dementia. Results indicated that when an object such as PARO is introduced, the older person with dementia naturally becomes calm and proactively makes time to spend with it. Hence, PARO can be considered an effective support tool for family care. However, the study suggested that using PARO at home wherein an older person with dementia did not demonstrate any interest toward it may yield limited results.
Limitations
A limitation of this study is that the reason for discontinuation was not explored further. Future research should understand underlying reasons for discontinuation to design family-mediated intervention that caters to the family’s needs as well. It should be noted that the seven participants were recruited through a public notice, so the participating families had positive attitudes as caregivers, and the result should be interpreted in consideration of it. Accordingly, the benefits observed may not apply to all situations. In the future, using a larger number of participants with a randomized sampling method will be beneficial to investigate ways of providing more effective and practical support. In addition, it will be helpful to explore ways of combining home-based and facility services.
Conclusions
Out of seven, five participants showed active interest and interaction from the first meeting. For these participants, PARO demonstrated efficacy. PARO may not be an ideal intervention for people who exhibit little interest at the time of introduction. This study found that the characteristics of individuals who could potentially benefit from PARO are distinguished not according to the severity of dementia but by one’s level of interest in PARO. In the context of family care, PARO is expected to elicit an active engagement with itself leading to the reduction of behavioral and psychological symptoms of dementia and provides respite to caregivers.
Acknowledgments
We would like to express our sincere gratitude to Chiyomi Yatsu, Daryl Patrick Gamboa Yao and Chihiro Sasaki for their valuable contribution to this research.
Footnotes
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: TS, who invented PARO, was not involved in tasks directly influencing the clinical data collection and analysis. All other authors declare no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was conducted with a 2012–2016, 2019–2021 fiscal years Basic Scientific Research grant from the Ministry of Education, Culture, Sports, Science, and Technology (B) [grant IDs: 24300202; 19H04504]. The funding parties did not influence the conduct of this research.
ORCID iD: Kaoru Inoue  https://orcid.org/0000-0001-6776-5487
https://orcid.org/0000-0001-6776-5487
Availability of data and materials: The datasets generated and/or analyzed during the current study are not publicly available to protect the participants’ right to privacy and confidentiality but are available from the corresponding author on reasonable request.
Contributor Information
Kaoru Inoue, Graduate School of Human Health Sciences, Tokyo Metropolitan University, 7-2-10 Higashiogu, Arakawa-ku, Tokyo 116-8551, Japan.
Kazuyoshi Wada, Graduate School of Systems Design, Tokyo Metropolitan University, Tokyo, Japan.
Takanori Shibata, Human Informatics and Interaction Research Institute, National Institute of Advanced Industrial Science and Technology (AIST), Tsukuba, Japan.
References
- 1. Global Note. Sekai no koureikaritsu (koureisha jinjou hiritsu) kunibetsu ranking suii, https://www.globalnote.jp/post-3770.html (2020, accessed 15 April 2020). (in Japanese)
- 2. Ministry of Health, Labor and Welfare. Heisei 28-nendo “koureishagyakutai no boushi, koureisha no yougosha ni taisuru shientou ni kansuru houritsu” ni motozuku taiou joukyoutou ni kansuru chousa kekka, https://www.mhlw.go.jp/stf/houdou/0000196989.html (2015, accessed 10 April 2020). (in Japanese)
- 3. Ministry of Health, Labor and Welfare. 2025 nen ni muketa kaigoujinzai ni kakaru jukyuusuikei (kakuteichi) ni tsuite, https://www.mhlw.go.jp/file/04-Houdouhappyou-12004000-Shakaiengokyoku-Shakai-Fukushikibanka/270624houdou.pdf_2.pdf (2015, accessed 11 August 2020). (in Japanese)
- 4. Robotto kakumei jitsugen kaigi. Robotto shinsenbyaku youyaku, http://www.kantei.go.jp/jp/singi/robot/pdf/senryaku_youyaku.pdf (2015, accessed 11 August 2020).
- 5. Shibata T. Development and spread of therapeutic medical robot, PARO: innovation of non-pharmacological therapy for dementia and mental health. J Inf Process Manag 2017; 60: 217–228. (in Japanese) [Google Scholar]
- 6. Kang HS, Makimoto K, Konno R, et al. Review of outcome measures in PARO robot intervention studies for dementia care. Geriatr Nurs 2020; 41: 207–214. [DOI] [PubMed] [Google Scholar]
- 7. Moyle W, Cooke M, Beattie E, et al. Exploring the effect of companion robots on emotional expression in older adults with dementia: a pilot randomized controlled trial. J Gerontol Nurs 2013; 39: 46–53. [DOI] [PubMed] [Google Scholar]
- 8. Petersen S, Houston S, Qin H, et al. The utilization of robotic pets in dementia care. J Alzheimers Dis 2017; 55: 569–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Robinson H, Macdonald B, Kerse N, et al. The psychosocial effects of a companion robot: a randomized controlled trial. J Am Med Dir Assoc 2013; 14: 661–667. [DOI] [PubMed] [Google Scholar]
- 10. Jones C, Moyle W, Murfield J, et al. Does cognitive impairment and agitation in dementia influence intervention effectiveness? Findings from a cluster-randomized-controlled-trial with the therapeutic robot, PARO. J Am Med Dir Assoc 2018; 19: 623–626. [DOI] [PubMed] [Google Scholar]
- 11. Liang A, Piroth I, Robinson H, et al. A pilot randomized trial of a companion robot for people with dementia living in the community. J Am Med Dir Assoc 2017; 18: 871–878. [DOI] [PubMed] [Google Scholar]
- 12. Hung L, Liu C, Woldum E, et al. The benefits of and barriers to using a social robot PARO in care settings: a scoping review. BMC Geriatr 2019; 19: 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kitwood T. Dementia reconsidered: the person comes first. London: Open University Press, 1997. [Google Scholar]
- 14. Hung L, Gregorio M, Mann J, et al. Exploring the perceptions of people with dementia about the social robot PARO in a hospital setting. Dementia 2021; 20: 485–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Swedberg R. Exploratory research. In: Elman C, Gerring J, Mahoney J. (eds) The production of knowledge: enhancing progress in social science. Cambridge: Cambridge University Press, 2020, pp. 17–41. [Google Scholar]
- 16. Shibata T, Wada K. Robot therapy: a new approach for mental healthcare of the elderly—a mini-review. Gerontology 2011; 57: 378–386. [DOI] [PubMed] [Google Scholar]
- 17. National Institute of Advanced Industrial Science and Technology. Seal-type therapeutic robot Paro. http://paro.jp/?page_id=326 (accessed 15 March 2020).
- 18. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–198. [DOI] [PubMed] [Google Scholar]
- 19. Sugishita M, Hemmi I, Takeuchi T. Reexamination of the validity and reliability of the Japanese version of the Mini-Mental State Examination (MMSE-J). Jpn J Cogn Neurosci 2016; 18: 168–183. (in Japanese) [Google Scholar]
- 20. Kobayashi T, Hariguchi S, Nishimura K, et al. A new clinical scale for rating of mental states and activities of daily living of the elderly (NM scale and N-ADL). Jpn J Clin Psychiatry 1988; 17: 1653–1668. (in Japanese) [Google Scholar]
- 21. Baumgarten M, Becker R, Gauthier S. Validity and reliability of the Dementia Behavior Disturbance Scale. J Am Geriatr Soc 1990; 38: 221–226. [DOI] [PubMed] [Google Scholar]
- 22. Mizoguchi T, Iijima S, Eto F, et al. Reliability and validity of Japanese version of the Dementia Behavior Disturbance Scale. Jpn J Geriatr 1993; 30: 835–840. (in Japanese) [DOI] [PubMed] [Google Scholar]
- 23. Zarit SH, Zarit JM. The memory and behavior problems checklist and the burden interview. University Park, PA: Pennsylvania State University, Gerontology Center, 1990. [Google Scholar]
- 24. Arai Y, Kudo K, Hosokawa T, et al. Reliability and validity of the Japanese version of the Zarit Caregiver Burden Interview. Psychiatry Clin Neurosci 1997; 51: 281–287. [DOI] [PubMed] [Google Scholar]
- 25. Brooker D, Surr C. Dementia care mapping: principles and practice. Bradford: University of Bradford, 2005. [Google Scholar]
- 26. Bradford Dementia Group. DCM 8 user’s manual. Badford: University of Bradford, 2005. [Google Scholar]
- 27. Green J, Thorogood N. Analysing qualitative data. In: Thorogood N, Green JM. (eds) Qualitative methods for health research. London: SAGE, 2004, pp. 173–200. [Google Scholar]
- 28. Joranson N, Pedersen I, Rokstad AMM, et al. Effects on symptoms of agitation and depression in persons with dementia participating in robot-assisted activity: a cluster-randomized controlled trial. J Am Med Dir Assoc 2015; 16: 867–873. [DOI] [PubMed] [Google Scholar]
- 29. Moyle W, Jones CJ, Murfield JE, et al. Use of a robotic seal as a therapeutic tool to improve dementia symptoms: a cluster-randomized controlled trial. J Am Med Dir Assoc 2017; 18: 766–773. [DOI] [PubMed] [Google Scholar]
- 30. Moyle W, Jones C, Murfield J, et al. Effect of a robotic seal on the motor activity and sleep patterns of older people with dementia, as measured by wearable technology: a cluster-randomised controlled trial. Maturitas 2018; 110: 10–17. [DOI] [PubMed] [Google Scholar]