Abstract
Objectives:
We aim to evaluate the benefits and harms of intervertebral disc therapies (IDTs) in people with non-specific chronic low back pain (NScLBP).
Methods:
We conducted a systematic review and meta-analysis of randomized trials of IDTs versus placebo interventions, active comparators or usual care. EMBASE, MEDLINE, CENTRAL and CINHAL databases and conference abstracts were searched from inception to June 2020. Two independent investigators extracted data. The primary outcome was LBP intensity at short term (1 week–3 months), intermediate term (3–6 months) and long term (after 6 months).
Results:
Of 18 eligible trials (among 1396 citations), five assessed glucocorticoids (GCs) IDTs and were included in a quantitative synthesis; 13 assessed other products including etanercept (n = 2), tocilizumab (n = 1), methylene blue (n = 2), ozone (n = 2), chymopapaine (n = 1), glycerol (n = 1), stem cells (n = 1), platelet-rich plasma (n = 1) and recombinant human growth and differentiation factor-5 (n = 2), and were included in a narrative synthesis. Standardized mean differences (95% CI) for GC IDTs for LBP intensity and activity limitations were −1.33 (−2.34; −0.32) and −0.76 (−1.85; 0.34) at short term, −2.22 (−5.34; 0.90) and −1.60 (−3.51; 0.32) at intermediate term and −1.11 (−2.91; 0.70) and −0.63 (−1.68; 0.42) at long term, respectively. Odds ratios (95% CI) for serious and minor adverse events with GC IDTs were 1.09 (0.25; 4.65) and 0.97 (0.49; 1.91).
Conclusion:
GC IDTs are associated with a reduction in LBP intensity at short term in people with NScLBP. Positive effects are not sustained. IDTs have no effect on activity limitations. Our conclusions are limited by high heterogeneity and a limited methodological quality across studies.
Registration
PROSPERO: CRD42019106336.
Keywords: intervertebral disc, intradiscal therapy, low back pain, systematic review
Introduction
Low back pain (LBP) is a symptom defined as pain between the last ribs and the gluteal area. It is the primary cause of years lived with disability worldwide during the past three decades for both sexes combined. 1 If LBP duration exceeds 12 weeks, it is considered chronic LBP (cLBP). 2 When no underlying condition (i.e. infection, tumor or inflammation) is found, cLBP is considered non-specific (NScLBP).3,4 NScLBP can be related to various plausible anatomical nociceptive sources, including the intervertebral disc (ID).5,6
An increasing number of trials assessed the benefits and harms of ID therapies (IDTs) in people with NScLBP supposedly originating from the ID. IDT could be defined as an injection of a drug or a medical device directly into the ID, under fluoroscopic guidance. The effects of IDTs are assumed to rely on three mechanisms: (1) because of limited blood flow, delivering a drug directly into the ID could be more efficient than systemic treatments; 7 (2) during ID degeneration, pro-inflammatory soluble mediators are locally released, 8 so intradiscal injection of a drug targeting inflammation could combat biochemical adverse factors; 9 and (3) during ID degeneration, changes in biomechanical properties of the ID occur, so intradiscal injection of devices could combat biomechanical adverse factors. 7
The effects of IDTs depend on the nature of the drug or device injected. Four main mechanisms of action have been described: (1) a reduction of local inflammation with IDT of glucorticoids (GCs), 10 anti-tumor necrosis factor-α (TNF-α), 11 anti-interleukin-6 (IL-6), 12 and methylene blue);13–15 (2) a removal of ID herniation with IDT of collagenase, 16 chymopapaine 17 or ethanol gel); 18 (3) a stimulation of ID healing with IDT of platelet-rich plasma (PRP) 19 or stem cells; 16 and (4) a restoration of the ID biomechanical properties with IDT of an intradiscal device 16 or ozone. 20
Despite growing experimental and clinical data, the benefits and harms of IDTs for people with NScLBP remain debated. We aimed to review evidence on the benefits and harms of IDTs in people with NScLBP.
Materials and methods
Our study was registered with the International Prospective Register of Systematic Reviews (PROSPERO: CRD42019106336). We made no changes to the protocol or outcomes. All outcomes prespecified in the protocol are reported in the manuscript. This review was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist (Appendix 1). 21
Data sources
We searched for articles in EMBASE, MEDLINE, CENTRAL and CINHAL databases from inception to 13 July 2018. Our search was updated on 11 June 2020. The search strategy combined controlled vocabulary and free word text based on the synonyms of “intradiscal” and “low back pain” (Appendices 2 and 3). We limited our search to studies of humans and adults, without language restrictions. Study included were randomized controlled trial (RCT) and quasi-RCT defined as trial with a prospective identification of participants but using inadequate randomization approaches. Other meta-analyses and systematic reviews, cohort studies, case reports, case series, cross-sectional studies and studies assessing effectiveness on radicular pain as the primary outcome were excluded. We also hand-searched the references lists of selected trials identified from electronic searches and proceedings of physical and rehabilitation medicine, rheumatology and radiology from French and international conferences, and ClinicalTrials.gov.
Study selection and outcomes
The two first authors (board-certified rheumatologists) independently reviewed titles and abstracts, then full-text articles to assess eligibility. We included RCTs of adults (range 18 years and older) with NScLBP who received IDT versus a comparator. In the absence of a consensual definition of IDT, we defined IDT as an injection of a drug, biological product, gas or device using a needle inserted into the ID. We defined the comparator as (1) placebo (i.e. sham procedure or insertion of a needle into the ID with or without intradiscal injection of contrast, saline, anesthetic or supposedly inactive agent), (2) active intradiscal comparator (i.e. intradiscal injection of the same product but at a different dosage or intradiscal injection of a different supposedly active agent), (3) other non-intradiscal spinal injection therapies (i.e. epidural, intradural, foraminal or facet joint injections of GCs), or (4) usual care (i.e. unstandardized non-pharmacological and/or pharmacological treatment prescribed at the discretion of the treating physician). We did not consider as an IDT or a comparator radiofrequency denervation, intradiscal electro-thermal or laser therapies, lumbar surgery or any other lumbar procedures requiring general anesthesia, because no drug or medical device was injected into the ID during these interventions. Efficacy outcomes were patient-centered relevant core outcomes: 22 LBP intensity and LBP-specific activity limitations. Safety outcomes were immediate and post-IDT minor and serious adverse events as classified by the WHO-UMC system. 23
Data extraction
The two first authors independently extracted data on study characteristics, design, population, interventions, outcomes and funding sources by using a standardized extraction form (Appendix 4). Corresponding authors were contacted to collect missing data. For individual studies, the psycho-social risk factors of the population were assessed with available demographic and socio-professional information by two independent investigators, who were blinded to the other characteristics of the study. Psycho-social risk was defined as the risk of persistent activity limitations or work participation restriction at 12 months after the intervention according to the expert. Psycho-social risk factors were rated as low, moderate, high or unclear. The quality of the studies was assessed with JADAD scale, which evaluates randomization process, blinding, withdrawals and dropouts. The total score ranges from 0 (low-quality study) to 5 (high-quality study). A JADAD score ⩾4 was considered good quality. 24 Discrepancies were resolved by a consensus process between the two investigators and a third investigator in case of unresolved discrepancies. As requested by peer reviewers, we added assessments of the overall risk of bias a posteriori, using the Cochrane Risk of Bias tool. 25 Because there is low correlation between assessments of risk of bias using the Cochrane Risk of Bias tool and assessments of quality using the JADAD scale, we decided to keep the JADAD scoring also. 26 Studies of low quality were not excluded because we wanted to comprehensively report all currently available evidence addressing the research question. Rather than excluding studies of low quality, we decided to rate the quality of evidence as high, moderate, low, and very low, indicating a gradient of confidence in estimates of treatment effect, 27 so that the readers can fully interpret the results presented.
Data synthesis
We conducted meta-analyses using a random-effect model with an inverse variance method for studies showing sufficient homogeneity in terms of design and comparator by using RevMan 5.3. Statistical heterogeneity was measured with the Cochran chi-square test and I2 statistic. 28 Outcomes were analyzed at three timepoints: (1) short term (1 week–<3 months), intermediate term (3–⩽6 months) and long term (>6 months) by using the most consistently reported duration data within each category. For continuous outcomes, scores were converted to means (standard deviations 19 ), as recommended by the Cochrane collaboration, 25 and pooled as standardized mean differences (SMDs) with 95% confidence intervals (CIs). Effects were considered null with SMD <0.2, weak with SMD 0.2–0.5, moderate with SMD 0.5–0.8 and large with SMD >0.8. 29 For dichotomous outcomes, we expressed the results for individual trials as odds ratios (ORs). For multiple arm studies, we combined relevant experimental groups and relevant comparator groups to avoid arbitrary omission or double counting of participants. Studies of low quality were included in the systematic review and meta-analysis. Only additional sensitivity analyses excluded poor-quality (JADAD score <3) and outlier studies. Publication bias was not assessed because the number of eligible trials was inadequate to draft a funnel plot. The strength of each body of evidence was summarized as high, moderate, low or very low according to the quality, consistency and precision of aggregated studies.27,30 When a quantitative synthesis was not appropriate because of high heterogeneity or a too small number of studies, we provided a narrative synthesis.
Results
Studies
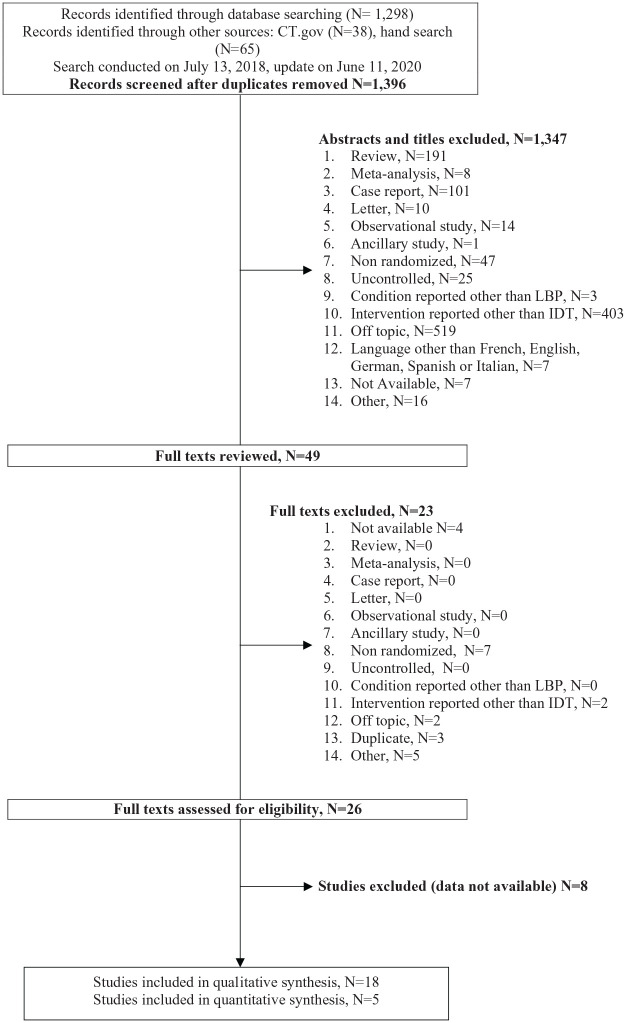
Our search yielded 1396 relevant references: 1347 were excluded on the basis of titles and abstracts and 23 after full-text review. Among the 26 remaining articles, eight had no available data and were excluded (Figure 1). Eighteen trials were included. Two trials included more than 100 participants, with sample sizes ranging from 15 to 135 participants.31,32 Ten trials assessed IDTs targeting local inflammation: five GCs,31–35 two anti-TNF-α,36,37 one anti-IL-612 and two methylene blue.13,15 Four trials assessed IDTs aiming at promoting disc healing: two recombinant human growth and differentiation factor-(rhGDF-5),38,39 one PRP 40 and one stem cells. 41 Two trials assessed IDTs targeting disc protrusion: one chymopapain 42 and one glycerol. 43 Two trials assessed IDTs aimed at restoring disc height by using ozone.44,45 Comparators were placebo in 16/18 (88.88%) trials: one comparator was a sham procedure with intramuscular injection of anesthetic 41 and 15 comparators were intradiscal injections of anesthetic,12,15,34,36,43 saline,13,31,33,35,37 contrast alone,32,40 excipients38,39 or distilled water. 42 Comparators were an active intradiscal comparator in 1/18 (5.55%) trials (ozone at a different dosages) 44 and usual care in 1/18 (5.55%). 45 No trial used another type of spinal injection as a comparator. Characteristics of the studies are in Table 1. Only 6/18 (33.33%) studies had a JADAD score ⩾4.13,15,31,32,34,40 Concerns were raised regarding the randomization and blinding methods, and 6/18 (33.33%) studies did not provide sufficient information regarding withdrawals and dropouts (Appendices 5, 6). All the 18 studies were considered for analyses, regardless of their overall quality. As requested by peer reviewers, the overall risk of bias was also summarized a posteriori using the Cochrane Risk of Bias tool (Appendix 7).
Figure 1.
Flow diagram.
Table 1.
Study characteristics.
| Title | Author (Country) | Year | Jadad score | Modic 1 only | Participants age (years) * | LBP duration (years) * | Psychosocial risk | LBP Intensity (/100) * | IDTG size | IDT | CG size | Comparator | Outcomes | Timepoints |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intradiscal injection therapy for degenerative chronic discogenic low back pain with end plate Modic changes | Cao et al.
31
(China) |
2011 | 4 | No | 42.3 | NR | B | 68 | 80 | 3 ml diprospan or 1 ml diprospan + 2 ml songmeile | 40 | 3 ml intradiscal saline | VAS ODI |
3 months 6 months |
| Implication of two different doses of intradiscal ozone-oxygen injection upon the pain alleviation in patients with low back pain: A randomized, single-blind study | Elawamy et al. 44 (Egypt) | 2018 | 3 | NR | 40.2 | NR | A | 82 | 30 | 10 ml (40 µg/ml) ozone | 30 | 10 ml (30 µg/ml) intradiscal ozone | VAS ODI |
1 month 6 months |
| The use of intradiscal steroid therapy for lumbar spinal discogenic pain: a randomized controlled trial | Khot et al.
33
(UK) |
2004 | 2 | NR | 42.8 | NR | B | 32 | 46 | 1 ml (40 mg) methylprednisolone acetate | 52 | 1 ml intradiscal saline | VAS ODI |
12 months |
| Intradiscal glycerol or bupivacaine in treating low back pain | Kotilainen et al.
43
(Finland) |
1997 | 1 | NR | 47.0 | 8.0 | Unclear | 58 | 9 | 1 ml 50% glycerol | 6 | 2 ml intradiscal 0.5% bupivacaine | VAS ODI |
0.5 month |
| Intradiscal glucocorticoid injection for patients with chronic low back pain associated with active discopathy: a randomized trial | Nguyen et al.
32
(France) |
2017 | 5 | Yes | 46.0 | 6.3 | C | 69 | 67 | 1 ml (25 mg) prednisolone acetate + 1 ml contrast | 68 | 1 ml intradiscal contrast | NRS QBPDS |
1 month 3 months 6 months |
| Therapeutic effect of medical ozone on lumbar disc herniation | Niu et al.
45
(China) |
2018 | 1 | NR | 48.0 | NR | Unclear | 87 | 60 | Ozone - 20 µg/ml - 40 µg/ml - 60 µg/ml |
20 | Usual care | VAS | 6 months |
| Intervertebral disc repair by allogeneic mesenchymal bone marrow cells: a randomized controlled trial | Noriega et al.
41
(Spain) |
2017 | 3 | NR | 38.0 | NR | Unclear | 65 | 12 | 25 × 10*6 allogenic MSCs in 2 ml saline | 12 | 2 ml intramuscular 1% mepivacaine | VAS ODI |
1 week 3 months 6 months |
| A randomized placebo-controlled trial of intradiscal methylene blue injection for treating chronic discogenic low back pain | Peng et al.
15
(China) |
2010 | 4 | NR | 41.7 | 3.4 | B | 70 | 36 | 1 ml (10 mg) methylene blue + 1 ml 2% lidocaine | 36 | 1 ml intradiscal 2% lidocaine | NRS ODI |
6 months |
| Single intradiscal administration of the tumor necrosis factor-alpha inhibitor, etanercept, for patients with discogenic low back pain | Sainoh et al.
36
(Japan) |
2016 | 2 | NR | 61.3 | NR | B | 82 | 38 | 10 mg etanercept + 2 ml 0.5% bupivacaine | 39 | 2 ml intradiscal 0.5% bupivacaine | NRS ODI |
1 month |
| Single intradiscal injection of the interleukin-6 receptor antibody tocilizumab provides short-term relief of discogenic low back pain; prospective comparative cohort study | Sainoh et al.
12
(Japan) |
2015 | 2 | NR | 60.2 | NR | Unclear | 84 | 30 | 40 mg tocilizumab + 1–2 ml 0.5% bupivacaine | 30 | 2 ml intradiscal 0.5% bupivacaine | NRS ODI |
1 month |
| Lumbar intradiskal platelet-rich plasma (prp) injections: a prospective, double-blind, randomized controlled study | Tuakli-Wosornu et al.
40
(USA) |
2016 | 5 | NR | 42.6 | NR | Unclear | 47 | 29 | 1–2 ml PRP | 18 | 1–2 ml intradiscal contrast | NRS | 1 month |
| A double-blind, placebo-controlled, dose-response pilot study evaluating intradiscal etanercept in patients with chronic discogenic low back pain or lumbosacral radiculopathy | Cohen et al.
37
(USA) |
2007 | 3 | NR | 39.3 | 5.3 | C | 43 | 30 | Etanercept - 0.1 mg - 0.25 mg - 0.5 mg - 0.75 mg - 1.0 mg - 1.5 mg |
6 | 0.5 ml intradiscal saline | VAS ODI |
1 month |
| Étude en double aveugle du traitement de la lombosciatique discale par chimionucléolyse | Feldman et al.
42
(France) |
1986 | 3 | No | 42.5 | 1.1 | B | 35 | 20 | 2 ml (400 UI) chymopapain | 19 | 2 ml intradiscal distilled water | VAS | 1 month 3 months |
| Diagnosis of discogenic low back pain in patients with probable symptoms but negative discography | Yu et al.
35
(China) |
2012 | 3 | NR | 44.9 | 2.1 | Unclear | 68 | 23 | 5 mg dexamethasone + contrast | 22 | Intradiscal saline + contrast | VAS ODI |
1 month 3 months 6 months |
| A clinical trial to evaluate the safety, tolerability and preliminary effectiveness of single administration intradiscal rhgdf-5 for the treatment of early stage lumbar disc degeneration | DePuy
38
(Korea) |
2014 | 3 | NR | 42.1 | NR | Unclear | NR | 22 | 1 mg rhGDF-5 | 9 | Intradiscal excipients: trehalose, glycine and hydrogen chloride | VAS ODI |
12 months |
| A multicenter, randomized, double-blind, placebo controlled, clinical trial to evaluate the safety, tolerability and preliminary effectiveness of 2 doses of intradiscal rhGDF-5 (single administration) for the treatment of early stage lumbar disc degeneration | DePuy
39
(USA) |
2014 | 3 | NR | 42.2 | NR | Unclear | NR | 14 | rhGDF-5 - 1 mg - 2 mg |
10 | Intradiscal excipients: trehalose, glycine and hydrogen chloride | VAS ODI |
12 months |
| Intradiscal glucocorticoids injection in chronic low back pain with active discopathy: a randomized controlled study | Tavares et al.
34
(France) |
2017 | 4 | Yes | 50.0 | 1.9 | C | 64 | 24 | 2 ml (50 mg) prednisolone acetate | 26 | 2 ml intradiscal 2% lidocaine | VAS ODI |
1 month 3 months 6 months |
| A multicenter randomized controlled trial on the efficacy of intradiscal methylene blue injection for chronic discogenic low back pain : the IMBI study | Kallewaard et al.
13
(Netherlands) |
2019 | 5 | No | 41.9 | 9.4 | Unclear | 66 | 40 | 1 ml (10 mg) methylene blue + 0.5 ml 2% lidocaine + 0.5 ml contrast | 41 | 1 ml intradiscal saline + 0.5 ml 2% lidocaine + 0.5 ml contrast |
NRS ODI |
6 weeks 3 months 6 months |
Baseline values.
Psychosocial risk: A, weak; B, moderate; C, high.
CG, comparator group; IDTG, intradiscal therapy group; LBP, low back pain; MSCs, mesenchymal stem cells; NR, not reported; NRS, numeric rating scale; ODI, Oswestry disability index ; PRP, platelet-rich plasma; QBPDI, Quebec back pain disability index; VAS, visual analogue scale.
Participants
Participants’ mean (SD) age was 45.2 (6.4) years, disease duration 4.2 (2.9) years and LBP intensity 63.8/100 (16.9); 2/18 (11.11%) studies exclusively included patients with Modic one changes.32,34 Information regarding psycho-social risk factors of participants was provided in 9/18 (50.0%) reports: risk factors were considered low in one study, 44 moderate in five studies15,31,33,36,42 and high in three studies.32,34,37
Effectiveness
Glucocorticoids
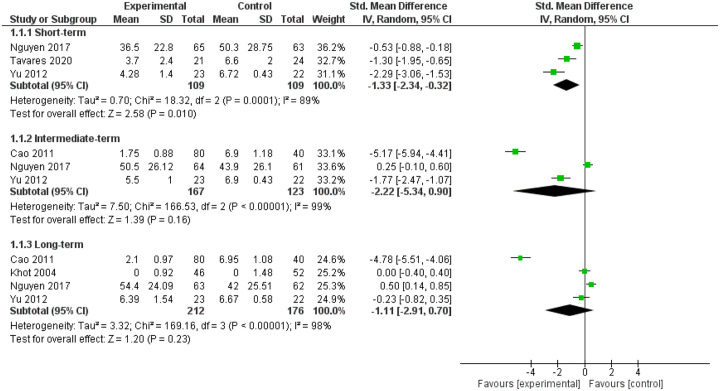
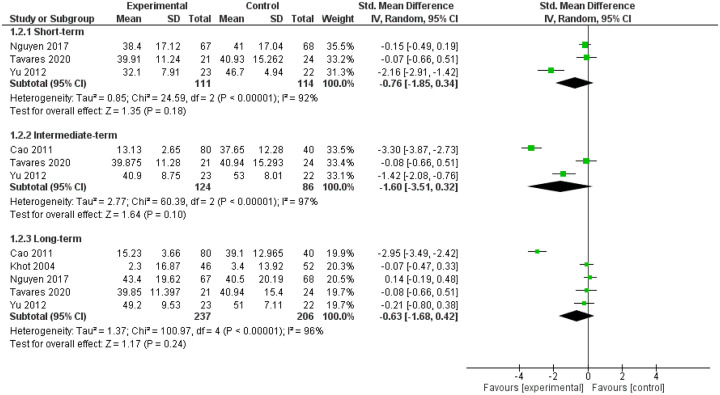
Five trials (n = 436 participants)31–35 compared GC IDTs (n = 235) with placebo [i.e. intradiscal saline (n = 114),31,33,35 intradiscal contrast alone (n = 63) 32 and intradiscal anesthetic (n = 24) 34 ] at short, intermediate and long term. The studies showed reduced LBP intensity favoring GC IDTs at short term [SMD (95% CI): −1.33 (−2.34;−0.32), I2 = 89%, moderate strength of evidence (MOE)] but not intermediate term [SMD (95% CI): −2.22 (−5.34; 0.90), I2 = 99%, low strength of evidence (LOE)] or long term [SMD (95% CI): −1.11 (−2.91; 0.70), I2 = 98%, MOE] (Figure 2). We found no significant reduction in LBP-specific activity limitations at short term [SMD (95% CI): −0.76 (−1.85; 0.34), I2 = 92%, LOE], intermediate term [SMD (95% CI): −1.60 (−3.51; 0.32), I2 = 97%, LOE] or long term [SMD (95% CI): −0.63 (−1.68; 0.42), I2 = 96%, MOE] (Figure 3). On sensitivity analysis, we confirmed no reduction in LBP intensity at intermediate term [SMD (95% CI): −0.74 (−2.72; 1.25), I2 = 96%] and long term [SMD (95% CI): 0.17 (−0.54; 0.88), I2 = 77%] and found no reduction in LBP-specific activity limitations at intermediate term [SMD (95% CI): −0.74 (−2.05; 0.57), I2 = 89%] and long term [SMD (95% CI): 0.03 (−0.23; 0.29), I2 = 0%] (Table 2; Appendices 8a and 8b).
Figure 2.
Forest plot for pain, comparing intervertebral disc therapies (IDTs) of corticosteroid versus placebo.
Figure 3.
Forest plot for activity limitations, comparing intervertebral disc therapies (IDTs) of corticosteroids versus placebo.
Table 2.
Effects on low back pain, activity limitations and adverse events of glucocorticoid intervertebral disc therapies versus placebo.
| Authors | Glucocorticoid | Comparator | N | Pain intensity (SMD) | Activity limitations (SMD) | Major AE (OR) | Minor AE (OR) |
|---|---|---|---|---|---|---|---|
| Cao et al. 31 | Diprospan 3 ml Diprospan 1 ml + Songmeile 2 ml |
Saline IDT | 120 | Intermediate term: −5.17 (−5.94; −4.41) Long term: −4.78 (−5.51; −4.06) |
Intermediate term: −3.30 (−3.83; −2.73) Long term: −2.65 (−3.49; −2.42) |
NR | NR |
| Khot et al. 33 | Methylprednisolone acetate 40 mg in 1ml | Saline IDT | 98 | Long term: 0.00 (−0.40; 0.40) | Long term: −0.07 (−0.47; 0.33) | NR | NR |
| Nguyen et al. 32 | Prednisolone acetate 25 mg in 1 ml + 1 ml of contrast | Contrast IDT | 135 | Short term: −0.53 (−0.88; −0.18) Intermediate term: 0.25 (−0.10; 0.60) Long term: 0.50 (0.14; 0.85) |
Short term: −0.15 (−0.49; 0.19) Long term: 0.14 (−0.19; 0.48) |
3.09 (0.12; 77.21) | 0.97 (0.49; 1.91) |
| Tavares et al. 34 | Prednisolone acetate 50 mg (2 ml) | Lidocain IDT | 50 | Short term: −1.30 (−1.95; −0.65) | Short term: −0.0 (−0.66; 0.51) Intermediate term: −0.08 (−0.66; 0.51) Long term: −0.08 (−0.66; 0.51) |
0.83 (0.16; 4.24) | 0 |
| Yu et al. 35 | Dexamethasone 5 mg + contrast | Saline + contrast IDT | 45 | Short term: −2.29 (−3.06; −1.53) Intermediate term: −1.77 (−2.47; −1.07) Long term: −0.23 (−0.82; 0.35) |
Short term: −0.76 (−1.85; 0.34) Intermediate term: −1.42 (−2.08; −0.76) Long term: −0.21 (−0.80; 0.38) |
NR | NR |
AE, adverse events; IDT, intervertebral disc therapies; NR, not reported; OR, odds ratio; SMD, standardized mean differences.
Etanercept
Two trials (n = 96)36,37 compared etanercept IDT (n = 60) with placebo [i.e. intradiscal saline (n = 6) and intradiscal anesthetic (n = 30)] at short term. The SMD (95% CI) was 0.03 (−1.08; 1.15; I2 = 79%, LOE) for LBP intensity and 0.26 (−0.78; 1.30; I2 = 76%, LOE) for LBP-specific activity limitations (Table 3, appendices 9a and 9b).
Table 3.
Effects on low back pain, activity limitations and adverse events of biological intervertebral disc therapies versus placebo.
| Authors | Intervention | Comparator | N | Pain intensity (SMD) at short term | Activity limitations (SMD) at short term | Major AE (OR) | Minor AE (OR) |
|---|---|---|---|---|---|---|---|
| Cohen et al. 37 | Etanercept | Saline IDT | 36 | 0.67 (−0.23; 1.56) | 0.86 (−0.04; 1.77) | NR | NR |
| Sainoh et al. 36 | Etanercept | Bupivacaine IDT | 77 | −0.48 (−0.99; 0.04) | −0.21 (−0.72; 0.30) | 0 | 0 |
| Sainoh et al. 12 | Tocillizumab | Bupivacaine IDT | 60 | −0.71 (−1.23; −0.18) | −0.97 (−1.50; −0.43) | 3.10 (0.12; 79.23) | 0 |
AE, adverse events; IDT, intervertebral disc therapies; NR, not reported; OR, odds ratio; SMD, standardized mean differences.
Tocilizumab
One trial (n = 60) 12 compared tocilizumab IDT (n = 30) with placebo [i.e. intradiscal saline (n = 30)] at short term. The SMD (95% CI) was −0.71 (−1.23; −0.18) for LBP intensity and −0.97 (−1.50; −0.43) for LBP-specific activity limitations, favoring tocilizumab IDT (Table 3, appendix 10a and 10b).
Methylene blue
Two trials (n = 152)13,15 compared methylene blue IDT (n = 76) with placebo [i.e. intradiscal anesthetic (n = 36) or intradiscal anesthetic and isotonic saline (n = 41)] at short, intermediate and long term. They revealed no statistically significant difference between the two groups for LBP intensity at short term [SMD (95% CI): −0.18 (−0.62; 0.25)], intermediate term [SMD (95% CI): −0.12 (−0.56; 0.32)] or long term [SMD (95% CI): −1.32 (−3.75; 1.11)] or for LBP-specific activity limitations at short term [SMD (95% CI): −0.28 (−0.72; 0.16)], intermediate term [SMD (95% CI): −0.08 (−0.51; 0.36)] or long term [SMD (95% CI): −1.64 (−4.63; 1.35)] (Table 4, appendices 11a and 11b).
Table 4.
Effects on low back pain, activity limitations and adverse events of other intervertebral disc therapies versus placebo.
| Authors | Intervention | Comparator | N | Pain intensity (SMD) | Activity limitations (SMD) | Major AE (OR) | Minor AE (OR) |
|---|---|---|---|---|---|---|---|
| NCT0112400639; NCT0118233738 | rhGDF5 | Excipient IDT | 55 | Long term : −0.07 (−0.76; 0.62) | Long term: −0.01 (−0.58; 0.55) | 1.48 (0.30; 7.35) | 1.56 (0.37; 6.68) |
| Elawamy et al.
44
; Niu et al. 45 |
Ozone | Ozone IDT (other dosage) Usual care |
60 80 |
Short term: 0.20 (−0.51; 0.91) Long term: 0.30 (−2.21; 0.91) Long term: −0.62 (−2.43; 1.20) |
Short term: 0.10 (−0.19; 0.39) Long term: −0.04 (−0.19; 0.11) |
NR NR |
NR NR |
| Kotilainen et al. 43 | Glycerol | Anesthetic IDT | 11 | Short term: −0.03 (−1.56; 1.50) | Short term: 0.19 (−1.34; 1.73) | NR | NR |
| Tuakli et al. 40 | PRP | Contrast IDT | 47 | Short term: −0.27 (−0.86; 0.32) | Short term: −0.05 (−0.64; 0.53) | NR | NR |
| Noriega et al. 41 | Stems cells | IM anesthetic | 72 | Short term: 0.68 (−0.15; 1.51) Intermediate term: −0.10 (−0.90; 0.70) Long term: −0.37 (−1.37; 0.44) |
Short term: 0.41 (−0.40; 1.22) Intermediate term: −0.49 (−1.31; 0.32) Long term: −0.44 (−1.25; 0.37) |
0 | 0.17 (0.03; 0.98) |
| Peng et al. 15 | Methylen blue | Anesthetic IDT | 71 | Long term: −2.57 (−3.51; 1.93) | Long term: −3.18 (−3.89; 2.47) | NR | NR |
| Kallewaard et al. 13 | Methylen blue | Saline + lidocaine + constrast IDT | 81 | Short term: −0.18 (−0.62; 0.25) Intermediate term: −0.12 (−0.56; 0.32) Long term: −1.32 (−3.37; 1.11) |
Short term: −0.28 (−0.72; 0.16) Intermediate term: −0.08 (−0.51; 0.36) Long term: −0.13 (−0.57; 0.32) |
5.39 (0.25; 115.86) | NC |
| Feldman et al. 42 | Chymopapaïn | Distilled water IDT | 38 | Short term: ↘ 55% versus
26% Intermediate term: ↘ 65% versus 42% |
short term : ↘ 36 % versus 19 % | 0.43 (0.12; 1.59) | 0.28 (0.01; 7.44) |
AE, adverse events; IDT, intervertebral disc therapies; IM, intramuscular; NR, Not Reported; OR, odds ratio; SMD, standardized mean differences.
Ozone
Two trials (n = 140)44,45 assessed the effectiveness of ozone IDT. Elawamy et al. 44 (n = 60) compared two different doses of ozone IDT [40 µg/ml (n = 30) versus 30 µg/ml (n = 30)]. The trials showed no statistically significant difference between the two groups for LBP intensity at short term [SMD (95% CI): −0.14 (−0.64; 0.37)] or long term [SMD (95% CI): 0.30 (−2.21; 0.81)] or for LBP-specific activity limitations at short term [SMD (95% CI): 0.17 (−0.34; 0.68)] or long term [SMD (95% CI): −0.14 (−0.64; 0.37)] (Table 4, Appendices 12a and 12b). Niu et al. 45 (n = 80 participants) compared ozone IDT (n = 60) with usual care (n = 20). The SMD (95% CI) was −1.32 (−1.87; −0.77) for LBP intensity at long term (Table 4, Appendix 13).
Chymopapaine
One trial (n = 39) 42 compared chymopapaine IDT (n = 20) with placebo (i.e. intradiscal distilled water [n = 19]). The authors reported a reduction in LBP intensity of 55% and 65% at short and intermediate term in the experimental group versus 26% and 42% in the comparator group and a reduction of LBP-specific activity limitations of 36% at short term in the experimental group versus 19% in the comparator group (Table 4).
Glycerol
One trial (n = 11) 43 compared glycerol IDT (n = 9) with placebo [i.e. intradiscal anesthetic (n = 2)] at short term. The SMD (95% CI) was −0.03 (−1.56; 1.50) for LBP intensity and 0.19 (−1.34; 1.73) for LBP-specific activity limitations (Table 4, Appendices 14a and 14b).
Stem cells
One trial (n = 24) 41 compared stem-cell IDT (n = 12) with placebo [i.e. intramuscular anesthetic (n = 12)] at short, intermediate and long term. The SMDs (95% CI) were 0.68 (−0.15; 1.51), −0.10 (−0.90; 0.70) and −0.37 (−1.17; 0.44) for LBP intensity and 0.41 (−0.40; 1.22), −0.49 (−1.31; 0.32) and −0.44 (−1.25; 0.37), respectively, for LBP-specific activity limitations (Table 4, Appendices 15a and 15b).
Platelet-rich plasma
One trial (n = 47) 40 compared PRP IDT (n = 29) with placebo [i.e. intradiscal contrast alone (n = 18)] at short term. The SMD (95% CI) was −0.27 (−0.86; 0.32) for LBP intensity and −0.05 (−0.64; 0.53) for LBP-specific activity limitations (Table 4, Appendices 16a and b).
RhGDF-5
Two trials (n = 55 participants)38,39 compared rhGDF-5 IDT (n = 36) with placebo [i.e. intradiscal excipients (trehalose, glycine and HCl) (n = 19)] at long term. The SMD (95% CI) was −0.07 (−0.76; 0.62) I2 = 33%, LOE, for LBP intensity and −0.01 (−0.58; 0.55) I2 = 0%, LOE, for LBP-specific activity limitations (Table 4, Appendices 17a and b).
Harms
Overall, 9/17 (52.9%) studies (ozone, PRP, glycerol, methylene blue, 3/5 GC and 1/3 biologics studies) did not report safety outcomes. For GC IDT, two studies32,34 involving 180 participants reported 8/180 serious adverse events [OR 1.09 (95% CI 0.25; 4.65)] and 69/180 minor adverse events [0.97 (0.49; 1.91)] (Tables 2, 3, 4, Appendix 18). For biologics IDT, two studies12,36 involving 120 participants reported 1/120 serious adverse event [OR 3.10 (95% CI 0.12; 79.23)] and 0/120 minor adverse events. For chymopapaine IDT, 42 the authors reported 1/38 serious adverse event [OR 0.43 (95% CI 0.12; 1.59)] and 1/38 minor adverse events [0.28 (0.01; 4.44)]. For stem cells IDT, 41 authors reported 1/24 minor adverse event [OR 0.17 (95% CI 0.03; 0.98)] and no serious adverse event. Finally, for rhGDF-5 IDT, two studies38,39 involving 55 participants reported 1/55 serious adverse event [OR 1.60 (95% CI 0.34; 7.57)] and 1/55 minor adverse event [1.48 (0.37; 5.98)].
Discussion
We found GC IDTs associated with reduced LBP intensity at short term in people with NScLBP. Positive effects were not sustained. We found no effect on activity limitations. However, it is difficult to conclude that GC IDTs were significantly associated with reduced LBP intensity at short term based on a meta-analysis of three studies with only 218 participants. For other IDTs, evidence is limited because of the small number of studies.
After a local injection of GCs, systemic effects persist for 21 days but are not observed beyond then, 46 which may explain why the positive effects of GC IDTs are not sustained. We observed no effect of GC IDTs on LBP-specific activity limitations, despite some discrepancies (no effect in the Nguyen et al. 32 and Tavares et al. 34 trials and important effects favoring GC IDTs in the Cao et al. 31 and Yu et al. 35 trials), with consistent results on sensitivity analyses. However, activity limitations and participation restrictions are complex dimensions of human functioning and unlikely to improve after an IDT as a stand-alone intervention.
For biologics IDT, Sainoh et al.12,36 reported moderate positive effects of tocilizumab IDT at short term and a similar trend for etanercept IDT. Conversely, Cohen et al. did not find a positive effect of etanercept IDT at short term. 37 Some clinical and methodological differences may explain these results. The Cohen et al. study was a small multi-arm (n = 6) trial, comparing different doses of etanercept, from 0.1 to 1.5 mg. Experimental data suggest that effective doses of etanercept range from 10 to 20 mg, 47 closer to those used in the Sainoh et al. study. Niu et al. 45 reported a large effect of ozone IDT [SMD −1.32 (95% CI −1.87; −0.77)] versus usual care at long term. However, these findings have not been replicated by independent groups and the overall risk of bias was high. Peng et al. 15 reported large effects with methylene blue IDT at long term for both pain and activity limitation. The overall risk of bias was low, but the sample size was small. 15 In addition, the evolution of pain in the comparator group was unusual. 15 These results were not replicated in the Kallewaard et al. 13 study. We included the effects of other IDTs in a narrative synthesis. Overall, we found no clear effects of these IDTs for both LBP intensity and activity limitation. No studies raised serious safety concerns, but adverse events were rarely reported.
Our review has limitations. First, we found heterogeneity across studies regarding interventions and comparators. After grouping interventions according to the therapeutic intradiscal agent used, groups were small and a quantitative synthesis was possible only for GC IDTs versus placebo. As the number of studies suitable for meta-analyses is low, it could be of interest to perform some aspects of qualitative systematic review for the other studies to glean directions for future studies. Among studies assessing the same intradiscal agent, we found heterogeneity for doses and injected volumes. Most of the comparators were placebo interventions, but the nature of the agent injected, doses and volumes varied across studies. The rationale for using these placebo interventions was poor, and a specific negative effect cannot be ruled out. This situation may explain the unusual evolution reported in the comparator group of some studies such as symptom stagnancy or even worsening.34,35 We also found heterogeneity for populations. Participants had various levels of psycho-social risk factors. In addition, clinical and/or imaging findings consistent with a plausible anatomical nociceptive source (i.e. ID) were rarely reported. Second, the overall methodological quality of the included studies was limited. Concerns were raised regarding the randomization and blinding processes: among the five studies assessing GC IDTs, four had an unclear blinding process31,33–35 and two had an unclear randomization process.31,33 The statistical heterogeneity found in our meta-analysis could be a result of both clinical and methodological diversities. Finally, some IDTs were designed to target radicular pain rather than LBP, but we assessed only LBP. Furthermore, we purposely excluded radiofrequency denervation, electro-thermal and laser therapies because no drug or device was injected into the ID during these interventions. However, to our knowledge, there is no consensus about what an IDT is.
In summary, limited evidence suggests that GC IDTs are associated with a reduction in LBP intensity at short term in people with NScLBP. For other IDTs, we found no clear effects. Because positive effects of GC IDTs are not sustained, studies aiming at assessing the effect of intradiscal therapy using anti-inflammatory molecules with a longer lasting effect such as mesenchymal stem cells or PRP are currently ongoing (e.g. NCT03737461 and NCT03712527, respectively).
Supplemental Material
Supplemental material, sj-pdf-1-tab-10.1177_1759720X211028001 for Intervertebral disc therapies for non-specific chronic low back pain: a systematic review and meta-analysis by Camille Daste, Stéphanie Laclau, Margaux Boisson, François Segretin, Antoine Feydy, Marie-Martine Lefèvre-Colau, François Rannou and Christelle Nguyen in Therapeutic Advances in Musculoskeletal Disease
Acknowledgments
The authors thank Catherine Weill from the Bibliothèque Inter-Universitaire de Santé who kindly contributed to the writing of the literature search equations for this review and Laura Smales for professional copyediting.
Footnotes
Contributorship: Conception and design of the study. CD, AF, MMLC, FR, CN.
Drafting of the original protocol. CD, MMLC, FR, CN.
Acquisition of data. CD, SL, MB, FS, CN.
Coordination of the study. CN.
Design of the statistical analysis plan. CD, CN.
Data analysis and interpretation. CD, SL, CN.
Drafting of the present manuscript. CD, SL, CN.
Final approval. CD, SL, MB, FS, AF, MMLC, FR, CN.
Conflict of interest statement: AF, MMLC, FR and CN are authors of the following article “Nguyen C, Boutron I, Baron G, Sanchez K, Palazzo C, Benchimol R, Paris G, James-Belin É, Lefèvre-Colau MM, Beaudreuil J, Laredo JD, Béra-Louville A, Cotten A, Drapé JL, Feydy A, Ravaud P, Rannou F, Poiraudeau S. Intradiscal Glucocorticoid Injection for Patients With Chronic Low Back Pain Associated With Active Discopathy: A Randomized Trial. Ann Intern Med. 2017 Apr18; 166(8):547–556,” which reported the positive effect of an intradiscal injection of glucocorticoids on pain at short term in patients with chronic low back pain and active discopathy.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Christelle Nguyen  https://orcid.org/0000-0001-7141-3230
https://orcid.org/0000-0001-7141-3230
Data availability statement to your paper: Data will be available upon request by contacting Associate Professor Christelle Nguyen (christelle.nguyen2@aphp.fr).
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Camille Daste, Université de Paris, Faculté de Santé, UFR de Médecine de l’Université de Paris, Paris, France; AP-HP.Centre-Université de Paris, Service de Rééducation et de Réadaptation de l’Appareil Locomoteur et des Pathologies du Rachis, Hôpital Cochin, Paris, France; INSERM UMR-S 1153, Centre de Recherche Épidémiologie et Statistique, Sorbonne Paris Cité, ECaMO Team, Paris, France.
Stéphanie Laclau, AP-HP.Centre-Université de Paris, Service de Rééducation et de Réadaptation de l’Appareil Locomoteur et des Pathologies du Rachis, Hôpital Cochin, Paris, France.
Margaux Boisson, AP-HP.Centre-Université de Paris, Service de Rééducation et de Réadaptation de l’Appareil Locomoteur et des Pathologies du Rachis, Hôpital Cochin, Paris, France.
François Segretin, AP-HP.Centre-Université de Paris, Service de Rééducation et de Réadaptation de l’Appareil Locomoteur et des Pathologies du Rachis, Hôpital Cochin, Paris, France.
Antoine Feydy, Université de Paris, Faculté de Santé, UFR de Médecine de l’Université de Paris, Paris, France; INSERM UMR-S 1153, Centre de Recherche Épidémiologie et Statistique, Sorbonne Paris Cité, ECaMO Team, Paris, France; AP-HP.Centre-Université de Paris, Service de Radiologie B, Hôpital Cochin, Paris, France.
Marie-Martine Lefèvre-Colau, Université de Paris, Faculté de Santé, UFR de Médecine de l’Université de Paris, Paris, France; AP-HP.Centre-Université de Paris, Service de Rééducation et de Réadaptation de l’Appareil Locomoteur et des Pathologies du Rachis, Hôpital Cochin, Paris, France; INSERM UMR-S 1153, Centre de Recherche Épidémiologie et Statistique, Sorbonne Paris Cité, ECaMO Team, Paris, France; Institut Fédératif de Recherche sur le Handicap, Paris, France.
François Rannou, Université de Paris, Faculté de Santé, UFR de Médecine de l’Université de Paris, Paris, France; AP-HP.Centre-Université de Paris, Service de Rééducation et de Réadaptation de l’Appareil Locomoteur et des Pathologies du Rachis, Hôpital Cochin, Paris, France; INSERM UMR-S 1124, Toxicité Environnementale, Cibles Thérapeutiques, Signalisation Cellulaire et Biomarqueurs (T3S), Campus Saint-Germain-des-Prés, Paris, France.
Christelle Nguyen, Rééducation et Réadaptation de l’Appareil Locomoteur et des Pathologies du Rachis, AP-HP.Centre-Université de Paris, Hôpital Cochin, 27, Rue du Faubourg Saint-Jacques, Paris, 75014, France; Université de Paris, Faculté de Santé, UFR de Médecine de l’Université de Paris, Paris, France; INSERM UMR-S 1124, Toxicité Environnementale, Cibles Thérapeutiques, Signalisation Cellulaire et Biomarqueurs (T3S), Campus Saint-Germain-des-Prés, Paris, France.
References
- 1. GBD 2017. Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet 2018; 392: 1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dionne CE, Dunn KM, Croft PR, et al. A consensus approach toward the standardization of back pain definitions for use in prevalence studies. Spine 2008; 33: 95–103. [DOI] [PubMed] [Google Scholar]
- 3. Haute Autorité de Santé. [Lombalgie chronique de l’adulte et chirugie, recommandation de bonne pratique]. 2015. [Google Scholar]
- 4. Chou R, Qaseem A, Snow V, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med 2007; 147: 478–491. [DOI] [PubMed] [Google Scholar]
- 5. Smeets RJ, Wade D, Hidding A, et al. The association of physical deconditioning and chronic low back pain: a hypothesis-oriented systematic review. Disabil Rehabil 2006; 28: 673–693. [DOI] [PubMed] [Google Scholar]
- 6. Nguyen C, Lefèvre-Colau MM, Kennedy DJ, et al. Low back pain. Lancet 2018; 392: 2547. [DOI] [PubMed] [Google Scholar]
- 7. Humzah MD, Soames RW. Human intervertebral disc: structure and function. Anat Rec 1988; 220: 337–356. [DOI] [PubMed] [Google Scholar]
- 8. Johnson ZI, Schoepflin ZR, Choi H, et al. Disc in flames: roles of TNF-α and IL-1β in intervertebral disc degeneration. Eur Cell Mate 2015; 30: 104–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burke JG, Watson RWG, McCormack D, et al. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J Bone Joint Surg Br 2002; 84: 196–201. [DOI] [PubMed] [Google Scholar]
- 10. Buttermann GR. The effect of spinal steroid injections for degenerative disc disease. Spine J 2004; 4: 495–505. [DOI] [PubMed] [Google Scholar]
- 11. Karppinen J, Korhonen T, Malmivaara A, et al. Tumor necrosis factor-α monoclonal antibody, infliximab, used to manage severe sciatica. Spine (Phila Pa 1976) 2003; 28: 750–753. [PubMed] [Google Scholar]
- 12. Sainoh T, Orita S, Miyagi M, et al. Single intradiscal injection of the interleukin-6 receptor antibody tocilizumab provides short-term relief of discogenic low back pain; prospective comparative cohort study. J Orthop Sci 2016; 21: 2–6. [DOI] [PubMed] [Google Scholar]
- 13. Kallewaard JW, Wintraecken VM, Geurts JW, et al. A multicenter randomized controlled trial on the efficacy of intradiscal methylene blue injection for chronic discogenic low back pain: the IMBI study. Pain 2019; 160: 945–953. [DOI] [PubMed] [Google Scholar]
- 14. Peng B, Zhang Y, Hou S, et al. Intradiscal methylene blue injection for the treatment of chronic discogenic low back pain. Eur Spine J 2007; 16: 33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Peng B, Pang X, Wu Y, et al. A randomized placebo-controlled trial of intradiscal methylene blue injection for the treatment of chronic discogenic low back pain. Pain 2010; 149: 124–129. [DOI] [PubMed] [Google Scholar]
- 16. Knezevic NN, Mandalia S, Raasch J, et al. Treatment of chronic low back pain – new approaches on the horizon. J Pain Res 2017; 10: 1111–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jenner JR, Buttle DJ, Dixon AK. Mechanism of action of intradiscal chymopapain in the treatment of sciatica: a clinical, biochemical, and radiological study. Ann Rheum Dis 1986; 45: 441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Volpentesta G, De Rose M, Bosco D, et al. Lumbar percutaneous intradiscal injection of radiopaque gelified ethanol (Discogel) in patients with low back and radicular pain. J Pain Relief 2014: 145. [Google Scholar]
- 19. Charneux L, Demoulin C, Vanderthomment M, et al. [Platelet-rich plasma (PRP) and disc lesions: a review of the literature]. Neurochirurgie 2017; 63: 473–477. [DOI] [PubMed] [Google Scholar]
- 20. Giurazza F, Guarnieri G, Murphy KJ, et al. Intradiscal O2O3: Rationale, injection technique, short- and long-term outcomes for the treatment of low back pain due to disc herniation. Can Assoc Radiol J 2017; 68: 171–177. [DOI] [PubMed] [Google Scholar]
- 21. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009; 6: e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chiarotto A, Boers M, Deyo RA, et al. Core outcome measurement instruments for clinical trials in nonspecific low back pain. Pain 2018; 159: 481–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. World Health Organization. Quality assurance and safety of medicines team. (2002). Safety of medicines: a guide to detecting and reporting adverse drug reactions: why health professionals need to take action. Geneva: World Health Organization, 2002. [Google Scholar]
- 24. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17: 1–12. [DOI] [PubMed] [Google Scholar]
- 25. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration, www.handbook.cochrane.org (2011, accessed 13 July 2018).
- 26. Hartling L, Ospina M, Liang Y, et al. Risk of bias versus quality assessment of randomised controlled trials: cross sectional study. BMJ 2009; 339: b4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ 2004; 328: 1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chou R, Hashimoto R, Friedly J, et al. Pain management injection therapies for low back pain. Rockville, MD: Agency for Healthcare Research and Quality, 2015. [PubMed] [Google Scholar]
- 30. van Tulder M, Furlan A, Bombardier C, et al. Updated method guidelines for systematic reviews in the Cochrane Collaboration Back Review Group. Spine 2003; 28: 1290–1299. [DOI] [PubMed] [Google Scholar]
- 31. Cao P, Jiang L, Zhuang C, et al. Intradiscal injection therapy for degenerative chronic discogenic low back pain with end plate Modic changes. Spine J 2011; 11: 100–106. [DOI] [PubMed] [Google Scholar]
- 32. Nguyen C, Boutron I, Baron G, et al. Intradiscal glucocorticoid injection for patients with chronic low back pain associated with active discopathy: a randomized trial. Ann Intern Med 2017; 166: 547–556. [DOI] [PubMed] [Google Scholar]
- 33. Khot A, Bowditch M, Powell J, et al. The use of intradiscal steroid therapy for lumbar spinal discogenic pain: a randomized controlled trial. Spine 2004; 29: 833–836; discussion 837. [DOI] [PubMed] [Google Scholar]
- 34. Tavares I, Thomas E, Cyteval C, et al. Intradiscal glucocorticoids injection in chronic low back pain with active discopathy: a randomized controlled study. Ann Phys Rehabil Med. Epub ahead of print 27 August 2020. DOI: 10.1016/j.rehab.2020.05.003. [DOI] [PubMed] [Google Scholar]
- 35. Yu Y, Liu W, Song D, et al. Diagnosis of discogenic low back pain in patients with probable symptoms but negative discography. Arch Orthop Trauma Surg 2012; 132: 627–632. [DOI] [PubMed] [Google Scholar]
- 36. Sainoh T, Orita S, Miyagi M, et al. Single intradiscal administration of the tumor necrosis factor-alpha inhibitor, etanercept, for patients with discogenic low back pain. Pain Med 2016; 17: 40–45. [DOI] [PubMed] [Google Scholar]
- 37. Cohen SP, Wenzell D, Hurley RW, et al. A double-blind, placebo-controlled, dose-response pilot study evaluating intradiscal etanercept in patients with chronic discogenic low back pain or lumbosacral radiculopathy. Anesthesiology 2007; 107: 99–105. [DOI] [PubMed] [Google Scholar]
- 38. DePuy. A clinical trial to evaluate the safety, tolerability and preliminary effectiveness of single administration intradiscal rhGDF-5 for the treatment of early stage lumbar disc degeneration https://www.clinicaltrials.gov/ct2/show/NCT01182337 (accessed 13 July 2018).
- 39. DePuy. A multicenter, randomized, double-blind, placebo controlled, clinical trial to evaluate the safety, tolerability and preliminary effectiveness of 2 doses of intradiscal rhGDF-5 (Single Administration) for the treatment of early stage lumbar disc degeneration, https://www.clinicaltrials.gov/ct2/show/NCT01124006 (accessed 13 July 2018).
- 40. Tuakli-Wosornu YA, Terry A, Boachie-Adjei K, et al. Lumbar intradiskal Platelet-Rich Plasma (PRP) injections: a prospective, double-blind, randomized controlled study. PM R 2016; 8: 1–10; quiz 10. [DOI] [PubMed] [Google Scholar]
- 41. Noriega DC, Ardura F, Hernandez-Ramajo R, et al. Intervertebral disc repair by allogeneic mesenchymal bone marrow cells: a randomized controlled trial. Transplantation 2017; 101: 1945–1951. [DOI] [PubMed] [Google Scholar]
- 42. Feldman J, Menkes CJ, Pallardy G, et al. [Double-blind study of the treatment of disc lumbosciatica by chemonucleolysis]. Rev Rhum Mal Osteoartic 1986; 53: 147–152. [PubMed] [Google Scholar]
- 43. Kotilainen E, Muittari P, Kirvela O. Intradiscal glycerol or bupivacaine in the treatment of low back pain. Acta Neurochir (Wien) 1997; 139: 541–545. [DOI] [PubMed] [Google Scholar]
- 44. Elawamy A, Kamel EZ, Hassanien M, et al. Implication of two different doses of intradiscal ozone-oxygen injection upon the pain alleviation in patients with low back pain: a randomized, single-blind study. Pain Physician 2018; 21: E25–E31. [PubMed] [Google Scholar]
- 45. Niu T, Lv C, Yi G, et al. Therapeutic effect of medical ozone on lumbar disc herniation. Med Sci Monit 2018; 24: 1962–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Younes M, Neffati F, Touzi M, et al. Systemic effects of epidural and intra-articular glucocorticoid injections in diabetic and non-diabetic patients. Joint Bone Spine 2007; 74: 472–476. [DOI] [PubMed] [Google Scholar]
- 47. Horii M, Orita S, Nagata M, et al. Direct application of the tumor necrosis factor-alpha inhibitor, etanercept, into a punctured intervertebral disc decreases calcitonin gene-related peptide expression in rat dorsal root ganglion neurons. Spine 2011; 36: E80–E85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tab-10.1177_1759720X211028001 for Intervertebral disc therapies for non-specific chronic low back pain: a systematic review and meta-analysis by Camille Daste, Stéphanie Laclau, Margaux Boisson, François Segretin, Antoine Feydy, Marie-Martine Lefèvre-Colau, François Rannou and Christelle Nguyen in Therapeutic Advances in Musculoskeletal Disease