Abstract
Collagen type II is a promising material to repair cartilage defects since it is a major component of articular cartilage and plays a key role in chondrocyte function. This study investigated the chondrogenic differentiation of bone marrow-derived mesenchymal stem cells (MSCs) embedded within a 3:1 collagen type I to II blend (Col I/II) hydrogel or an all collagen type I (Col I) hydrogel. Glycosaminoglycan (GAG) production in Col I/II hydrogels was statistically higher than in Col I hydrogels or pellet culture, and these results suggested that adding collagen type II promoted GAG production. Col I/II hydrogels had statistically lower alkaline phosphatase (AP) activity than pellets cultured in a chondrogenic medium. The ability of MSCs encapsulated in Col I/II hydrogels to repair cartilage defects was investigated by creating two cartilage defects in the femurs of rabbits. After 13 weeks, histochemical staining suggested that Col I/II blend hydrogels provided favorable conditions for cartilage repair. Histological scoring revealed a statistically higher cartilage repair score for the Col I/II hydrogels compared to either the Col I hydrogels or empty defect controls. Results from this study suggest that there is clinical value in the cartilage repair capabilities of our Col I/II hydrogel with encapsulated MSCs.
Keywords: extracellular matrix; fibrillogenesis; osteoarthritis, rabbit model; chondrogenic differentiation
Graphical Abstract
INTRODUCTION
Osteoarthritis, a disease defined by the loss of articular cartilage due to wear and degradation, causes pain and stiffness in the joints of over 30 million adults in the United States.1 Surgical procedures used to repair damaged articular cartilage are common in the United States, and their incidence rate grows at 5% annually.2 Articular cartilage lacks the inherent ability for self-repair due to the fact that it is both avascular and contains a low density of chondrocytes.3 The avascular nature of articular cartilage hinders the native wound healing process due to limited delivery of nutrients and progenitor cells to damaged tissue. Although there is no cure for osteoarthritis, there are many surgical treatment options for focal cartilage defects, including osteochondral grafts, autologous chondrocyte implantation, and marrow stimulation.4,5 However, these invasive options incur long rehabilitation times and usually promote the regrowth of fibrocartilage, which has mechanical properties inferior to native, hyaline cartilage.6 Therefore, we take a tissue engineering approach to cartilage regeneration to restore the damaged tissue.
Tissue engineering seeks to repair damaged tissues by introducing an optimized combination of cells, scaffold, and bioactive factors that can be transplanted into a patient.7 An ideal scaffold material should provide instructions to cells, which are either encapsulated in the material or recruited to the area, in order to maintain cell viability and regulate cell function.8 Hydrogels are a popular choice for tissue-engineered scaffolds due to ease of fabrication and cell incorporation, the ability to fit constructs to variously shaped defects, and facile modification to confer biomimetic properties.9 Although hydrogels are initially softer than cartilage, a multitude of studies have shown that the mechanical properties increase with time during implantation and that signals that induce hyaline cartilage formation also result in mechanical properties that match those of native cartilage.9 Among the different options, collagen hydrogels are attractive options for tissue-engineered scaffolds since collagen is found in many different tissues throughout the body and is also biocompatible.10 In addition, collagen fibrillogenesis, the spontaneous self-assembly of collagen monomers into fibrils and then larger collagen fibers, occurs at physiological conditions.11
Although collagen type II is the main component found in native cartilage tissue, collagen type I, which is abundantly available and already included in FDA-approved tissue engineering constructs, continues to be the most utilized type of collagen in tissue engineered scaffolds. The α2β1 integrin receptor in the cell membrane of bone marrow cells interacts with collagen type I, an important component of bone, to induce osteoblastic differentiation.12 MSCs encapsulated within collagen I hydrogels have been successfully differentiated into bone and used to repair bone in animal models.13 On the other hand, many studies have used collagen I hydrogels for cartilage engineering. For example, to repair cartilage defects in humans, early studies encapsulated autologous chondrocytes within hydrogel scaffolds created using collagen type I from different species.14,15 In another early study, porcine collagen type I hydrogel scaffolds with autologous bone marrow-derived MSCs were used in human osteoarthritic knees to repair cartilage defects more effectively than a cell-free scaffold.16 Furthermore, autologous bone marrow-derived MSCs were isolated, embedded within calf skin collagen type I, and used to repair cartilage defects in the weight-bearing region of a rabbit.17 Better integration with surrounding tissue and surface splitting, fibrillation, and thinning are some limitations of collagen type I hydrogels used for articular cartilage repair that will need to be addressed as areas for improvement.17
Despite promising results from early studies investigating the differentiation of MSCs into chondrocytes in collagen type I-based hydrogels, studies have also shown that collagen type II hydrogels promote the differentiation of embedded MSCs to chondrocytes more efficiently than collagen type I hydrogels.18,19 Although collagen type I is found in fibrocartilage tissue such as the intervertebral disc and meniscus, articular cartilage contains little to no collagen type I.20,21 In contrast, collagen type II makes up 90–95% of the extracellular matrix (ECM) collagen produced by chondrocytes.22 Despite some arthritogenic properties,23 collagen type II is organized in a macromolecular structure that chondrocytes sense, and these cell-matrix interactions help to maintain chondrocyte phenotype.24
Compared to collagen type I, collagen type II exhibits poor mechanical properties when forming a physically crosslinked hydrogel.25 In a study comparing fibrillogenesis of collagen type I and collagen type II, collagen hydrogels formed routinely in gels made of collagen type I but not when made of collagen type II.26 The same study also saw that the smaller fibers of collagen type II, as compared to collagen type I, were associated with more charged and hydrophobic residues that took part in more intramolecular interactions with fewer residues available for intermolecular interactions. The lack of intermolecular fibril interactions26 and slower fibrillation process27 are likely the reasons for lack of collagen type II gel formation.
Although a few papers have investigated collagen type II scaffolds,19,28 the lack of robust hydrogels and inferior mechanical properties, as noted previously, have resulted in strategies, such as crosslinking scaffolds, using a recombinant collagen type II, or creating composite scaffolds, that address these concerns. Chemical crosslinkers, such as 3-(3-dimethylaminopropyl)carbodiimide (EDC) and glutaraldehyde, have been utilized to modify scaffolds containing collagen type II29–37 but are not cytocompatible and cannot be used to encapsulate cells. Recombinant collagen type II has also been investigated as a scaffold, with encapsulated cells, for cartilage repair,28,38,39 but it is expensive and difficult to scale.40 Previous studies have combined collagen type II with a number of scaffold materials including silk fibroin,41 poly(ethylene glycol) (PEG),42,43 glycosaminoglycans (GAGs),30,44 alginate,18,45 and chitosan.34,46 Including collagen type II in composites increased ECM production specific to cartilage, regulated chondrocyte proliferation, and induced cartilage repair.36,42,43,46–49 Blends of collagen type I and II have been studied without cells in vivo,50 and with encapsulated chondrocytes in vitro and in vivo.47,51 To date, our study is the first to encapsulate mesenchymal stem cells (MSCs) in a collagen type I and II hydrogel and investigate them in an in vivo model of cartilage defect repair.
A number of different cell types, including chondrocytes and different varieties of stem cells, have been encapsulated in scaffolds for cartilage tissue engineering.7 Chondrocytes in healthy cartilage are responsible for both producing and maintaining the ECM through a homeostatic balance of cartilage synthesis and degradation. However, chondrocytes are difficult to harvest due to the fact that a biopsy removes very few viable cells and donor sites often become unhealthy after cartilage removal.52 Further expansion of biopsied chondrocytes in vitro is required due to the low yield of harvested cells, but the chondrocytes often dedifferentiate.53 Bone marrow-derived mesenchymal stems cells (MSCs) are a promising cell source for engineered articular cartilage due to their ease of isolation and ability to differentiate into chondrocytes under certain environmental conditions.54,55
Our lab previously developed and characterized hydrogel scaffolds made of a 3:1 collagen type I to collagen type II ratio (this formulation is hereafter referred to as Col I/II gels) to harness the biological activity of collagen type II and the superior gelation of collagen type I.56 Hydrogels were prepared with collagen type I:type II ratios of 1:0, 3:1, 1:1, 1:3, and 0:1, and we demonstrated that the addition of collagen type II alters the amount of total collagen incorporated in the hydrogel, network structure, and storage modulus. From the five different ratio blends created, the 3:1 blend formed robust hydrogels with superior mechanical properties compared to the other blends investigated.56 Building on the prior Col I/II blend characterization studies, the goal of the current study was to evaluate the in vitro chondrogenic differentiation potential of bone marrow-derived MSCs embedded within Col I/II gels and the ability of Col I/II gels with encapsulated cells to repair cartilage defects in vivo. We used autologous MSCs to eliminate the possibility of an immune response following implantation. Of the many animal models that have been developed to assess the efficacy of tissue engineered cartilage scaffolds, a rabbit model was chosen for preliminary investigation due to the fact that rabbits reach skeletal maturity in 9 months and have a 3 mm critical sized defect.57 The Col I/II hydrogel increased cartilage matrix GAG production and decreased unwanted phenotypes in vitro. In a rabbit model, the Col I/II hydrogel promoted integration with surrounding tissue and provided favorable conditions for cartilage repair. Results from this study suggest that there is a clinical value in the placement of a Col I/II hydrogel with encapsulated MSCs into a cartilage defect to aid in cartilage repair.
MATERIALS AND METHODS
Reagents.
Unless otherwise specified, all materials used were purchased from Sigma-Aldrich (St. Louis, MO).
Bone Marrow Collection.
All animal experiments were performed using protocols approved by the Purdue Animal Care and Use Committee (PACUC). Bone marrow was collected from both femurs and humeri of skeletally mature, male New Zealand White rabbits (Covance, Princeton, NJ). The rabbits were 9 months of age prior to bone marrow collection and weighed 3.6 ± 0.2 kg. The rabbits were anesthetized by intramuscular injection with a mixture of ketamine, xylazine, and butorphanol (35 mg/kg, 5 mg/kg, and 0.01 mg/kg respectively) and were maintained on isoflurane and oxygen with a mask. Bone marrow was collected from four sites including both the left and right proximal femurs and proximal humeri. The bone marrow extraction sites were clipped and scrubbed with chlorhexidine using standard techniques. Bone marrow was aspirated using an 18-gauge needle that was percutaneously inserted into the intertrochanteric fossa of the femur and the greater tubercle of the humerus. After the needle penetrated through the bone into the medullary cavity, the bone marrow was aspirated.
Stem Cell Isolation and Culture.
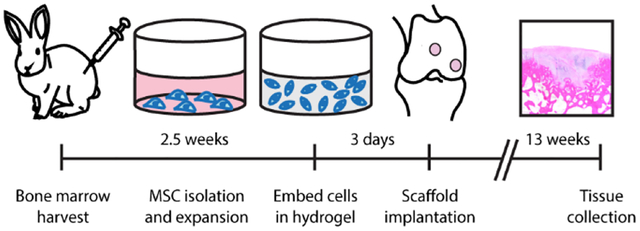
The marrow from each rabbit was pooled by rabbit, centrifuged for 10 minutes at 500g and resuspended in maintenance medium (low-glucose Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (Lonza, Walkersville, MD) and 1% penicillin-streptomycin). Autoclaved water was added to lyse the red blood cells. The marrow was gently mixed for 30 seconds before adding additional maintenance medium, and the suspension was centrifuged for 10 minutes at 500g. After the pellet was resuspended, the cells were counted, plated on 100-mm plates at a density of 107 cells per plate, and incubated at 37°C with 5% CO2. The first medium change was performed after four days of culture following a phosphate buffered saline (1x PBS) wash step, and subsequent medium changes were every three days. The cells were subcultured after 2.5 weeks upon reaching 70% - 80% confluency (Figure 1A).
Figure 1. Overview of rabbit study.
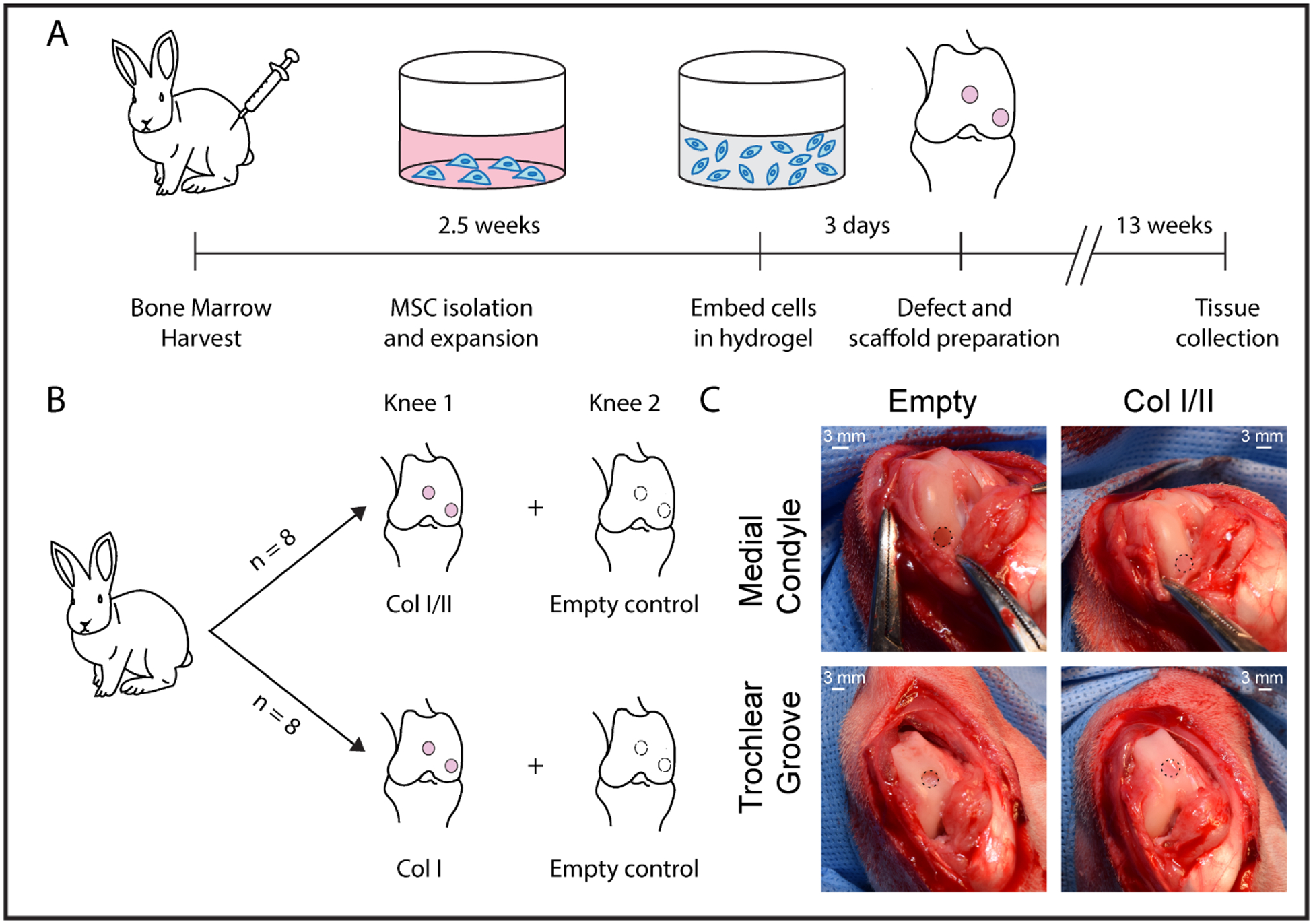
(A) In vivo workflow timeline and (B) experimental design. (C) Defect preparation and scaffold implantation. Hydrogel scaffolds, which are surrounded by a dotted line, were press fit into defects that were drilled into the femoral trochlear groove and medial condyle.
Collagen Scaffold Preparation.
An 11 mg/mL stock solution of collagen type II from lyophilized chicken sternum (Sigma-Aldrich, Saint Louis, MO) was prepared in 20 mM acetic acid. Upon sterile filtration, the concentration of the collagen type II stock solution was measured using a bicinchoninic acid (BCA) assay (Pierce, Rockford, IL) following the manufacturer’s protocol. The stock solution of collagen type II was then diluted to 8 mg/mL in 20 mM acetic acid prior to use. The collagen type I and II blend hydrogels were prepared using a modified protocol from Vazquez et al.56 The stock solution of collagen type II was combined with acid-solubilized collagen type I from rat tail (Corning, Corning, NY). The pH of the solutions was raised to 7.4 with the addition of 10x PBS, 1 M NaOH, and 1x PBS and diluted to a final concentration of 4 mg/mL total collagen. The gels were prepared with a 3:1 collagen type I to collagen type II ratio (Col I/II) or all collagen type I (Col I). Passage 3 cells were resuspended in collagen pre-polymerization solutions at a cell density of 5 × 106 cells/mL and a final volume of 50 μL. The hydrogels were allowed to polymerize at 37°C for 2 hours before the addition of medium. After polymerization, chondrogenic medium with or without added growth factor was added to the scaffolds for in vitro analysis. Defined chondrogenic medium was formulated with high-glucose DMEM supplemented with 1% ITS+Premix (BD Biosciences, San Jose, CA), 1% penicillin-streptomycin, 1 mM sodium pyruvate, 50 μM proline, 4 mM L-glutamine, 50 μg/mL ascorbic acid, 100 nM dexamethasone, and 10 ng/mL transforming growth factor-β3 (TGF-β3) (Peprotech, Rocky Hill, NJ). Defined chondrogenic medium without added growth factor was formulated the same way as the chondrogenic medium but lacked TGF-β3. Treatments grown in chondrogenic medium without added TGF-β3 growth factor are labeled with (-TGF) after the name of the treatment. For in vitro analysis, cell-hydrogel constructs were cultured for up to 4 weeks with 3 medium changes each week. MSCs grown in pellet culture served as a comparison in the study. To form the pellets, MSCs were centrifuged, washed, pelleted at a density of 2.5 × 105 cells/pellet, and cultured in a high-throughput culture system in a conical-bottom plate.58 Both hydrogels and pellets were maintained in free-floating conditions. For the in vivo study, autologous MSCs were encapsulated into the collagen scaffolds, as previously discussed, and were cultured for 3 days in maintenance medium prior to surgical implantation (Figure 1A). The medium was changed the day before implantation. After 3 days of culture, the cell-hydrogel constructs were press fit into the cartilage defects and filled the space without gaps or excess.
Papain Digestion.
The pellets were rinsed with PBS and digested with 125 μg/mL of activated papain solution in a papain digestion buffer (5 mM ethylenediaminetetraacetic acid (EDTA), 5 mM L-cysteine (Alfa Aesar, Ward Hill, MA), and 100 mM NaH2PO4) at 60°C for 24 hours.59 Cell-hydrogel constructs were lyophilized prior to digestion with papain solution. The digested product was then freeze-dried and resuspended in autoclaved water.
DNA Quantification.
DNA was measured using Hoechst dye as previously described.60 After combining the digested pellet or cell-hydrogel construct with the Hoechst dye solution, the fluorescence of the solution was read at a 340 nm excitation wavelength and a 465 nm emission wavelength. A standard curve was created using calf thymus DNA.
GAG Quantification.
GAG content was measured using a dimethyl methylene blue (DMMB) assay in which 20 μL of the digested pellets or cell-hydrogel constructs were combined with 30 μL of water and 250 μL DMMB dye solution (n = 5 or 6 for cell-gel scaffolds and pellets). The absorbance of the solution was read at 525 nm. A standard curve was created using chondroitin sulfate from shark cartilage (Seikagaku, Tokyo, Japan). The GAG values measured were normalized to the amount of DNA measured per sample.
AP Activity.
The medium was collected from both the pellets and cell-hydrogel constructs (n = 8 or 9 for Col I hydrogels, Col I/II hydrogels, and pellets with TGF-β3 (Pel) and n = 3 for pellets without TGF-β3 (Pel (-TGF))) after each week following a previously described protocol and using proper background controls.59 AP activity was measured by incubating 50 μL of the collected medium with 50 μL of p-nitrophenylphosphate substrate solution (1 mM MgCl2, 10 mg/mL p-nitrophenylphosphate, and 0.1 M glycine) for 2 hours at 37°C. A standard curve was created using p-nitrophenol in chondrogenic medium.
Gene Expression.
Both the pellets and the gels were washed with PBS then homogenized in lysis buffer and β-mercaptoethanol using a syringe needle. For each sample (n=4), three cell-hydrogel constructs or pellets were combined for the Col I, Col I/II, and pellet with TGF-β3 (Pel) treatments. A total of 5 pellets cultured without TGF-β3 (Pel (-TGF)) were combined for use as qRT-PCR samples (n=4). The NucleoSpin RNA kit from Macherey-Nagel (Bethlehem, PA) was used to isolate RNA. A High Capacity cDNA Reverse Transcription kit from Applied Biosystems (Foster City, CA) was used to synthesize complementary DNA from the isolated RNA. Relative expression levels were measured using qRT-PCR with the primer sequences (Table 1S) for collagen type I, II, and X, aggrecan, Sox9, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Integrated DNA Technologies, Skokie, IL). The samples were heated for 10 minutes at 95°C followed by 40 cycles for 15 seconds at 95°C, 15 seconds at 55 or 60°C, and 40 seconds at 68°C. All values were normalized to GAPDH levels with an average Ct value of 20.7 cycles and a standard deviation of 1.1 cycles. The differences in gene expression were calculated relative to negative controls using the ΔΔCt method.61
Surgical Implantation.
The rabbits were randomly assigned, as seen in Figure 1B, into two groups to receive a Col I scaffold (n=8) or a Col I/II scaffold (n=8) with autologous MSCs. Each rabbit received treatment in one knee (treatments were placed in both the medial condyle and trochlear groove), and one control knee was left empty. The knee that was chosen as the treatment knee was randomized so that half of the rabbits received the treatment in the right knee and the other half received the treatment in the left knee. Rabbits were induced with a mixture of ketamine, xylazine, and butorphanol (35 mg/kg, 5 mg/kg, and 0.01 mg/kg IM, respectively) prior to intubation with isoflurane and oxygen. The joint was entered, using sterile surgical techniques and a medial parapatellar approach, and two 3.2-mm diameter and 2-mm deep defects (Figure 1C) in the femur were created with a Hall Power Pro5100M surgical drill (ConMed, Utica, NY). One defect was placed in the medial condyle (weight-bearing region) during maximum flexion of the stifle, and the other was placed in the trochlear groove (the non-weight bearing region) 1 cm proximal to the origin of the cranial cruciate ligament. Within one knee, the same treatment was placed in the medial condyle and trochlear groove defect. The defects were rinsed with saline prior to treatment, and the control defects were rinsed with saline and left empty. Cell-hydrogel composites were washed with 1x PBS and press fit into both defects in one knee, and the defects in the other knee were left empty as a control. The surgical incision was closed in 3 separate layers using standard suturing techniques. All rabbits were given a subcutaneous injection of buprenorphine SR (ZooPharm, Windsor, CO) at 0.1 mg/kg. The rabbits were permitted time to recover before moving back to their cages and were allowed to move freely post operation. The rabbits were euthanized after 13 weeks with an overdose of barbiturate following guidelines from the American Veterinary Medical Association Panel on Euthanasia.
Defect Evaluation.
Following euthanasia, the stifle joints were evaluated grossly for joint capsule inflammation and defect healing. After isolation of the distal femur, the defects were fixed in 10% neutral buffered formalin, decalcified in a 0.5 M EDTA solution, and embedded in paraffin. Radiographs confirmed total decalcification. Sagittal sections (4 μm thick) from the center of the defect were stained with hematoxylin and eosin (HE) or safranin-O/fast green (SOFG). HE and SOFG slides were evaluated under a light microscope. A semi-quantitative histochemical scoring system adapted from O’Driscoll et al. (Table 2S) was used to evaluate tissue repair in 9 different categories with a total possible tissue repair score that ranged between 0 to 24 points.62 All slides were examined and scored by two blinded observers, including one board-certified veterinary pathologist (A.C.). Independent histochemical scores by observers were averaged.
Statistical Analysis.
All data were expressed as the mean ± standard deviation. An alpha level of 0.05 was selected for statistical significance. Unpaired t-tests were performed for normalized GAG content values between the same treatment at different timepoints for pellets without TGF-β3 (Pel (-TGF)) and the Col I hydrogels. Welch’s t-tests were performed for normalized GAG content values between the same treatment at different timepoints for pellets with TGF-β3 (Pel) and the Col I/II hydrogels. One-way analysis of variance (ANOVA) and Tukey’s post hoc test were performed to analyze normalized GAG content at a single timepoint (p < 0.05). A mixed-effect model and Tukey’s post hoc test were performed to analyze AP activity (p < 0.05) at a single timepoint. A one-way ANOVA and Tukey’s post hoc test were performed to analyze AP Activity of a treatment over time (p < 0.05). Single factor ANOVA and Tukey’s post hoc tests were for gene expression analysis (sox9, aggrecan, collagen type I, and collagen type X) and histology scoring analysis. An ANOVA and Games Howell’s post hoc test were performed for gene expression analysis of collagen type II.
RESULTS
Cells in collagen blend hydrogels are viable and contract gels over time
Given that the Col I/II gels have not been used with cells before, we assessed their cytocompatibility and interactions with cells. MSCs encapsulated within Col I/II gels at two different cell densities (1 × 106 or 5 × 105 cells/mL) were cultured in maintenance medium for three days. Cells were found throughout the volume of the gel (Figure 1S) and had a high viability (>85%) (Fig. 2S). Cell interactions with the matrix were evaluated by examining contraction of the collagen gels. Cells were cultured in chondrogenic medium with or without TGF-β3 at the higher cell density used throughout the rest of the experiments (5 × 106 cells/mL), and the degree of contraction over time was assessed by measuring the projected cross-sectional area of the hydrogel-cell constructs from images (Figure 3S). At day 3, a Kruskal-Wallis with Dunn’s multiple comparisons test revealed no statistical difference in percentage of the original area between the Col I and Col I/II hydrogels cultured in chondrogenic medium with and without TGF-β3 (Figure 4S). In addition, after 28 days in culture there was no statistical difference in contraction of the hydrogels regardless of medium or hydrogel composition. At all other timepoints, the hydrogels cultured with TGF-β3 contracted more quickly than hydrogels cultured without TGF-β3, regardless of hydrogel.
Collagen blend hydrogels increased GAG production
The GAG content normalized to DNA content in each scaffold with encapsulated MSCs or pellet was analyzed after a 14- and 28-day culture period with the results shown in Figures 2A and 2B, respectively. Corresponding averaged GAG content normalized to DNA content, averaged GAG content, averaged DNA content, and averaged dry weight values at 14 and 28 days are shown in Table 3S and Table 4S, respectively. Col I/II and Col I hydrogels cultured without TGF-β3 were also analyzed after a 14- and 28-day culture period (Table 3S and Table 4S). There was no statistical difference in normalized GAG content when comparing the Col I/II and Col I hydrogels when they were cultured in medium with added TGF-β3 for 14 days. At 14 days there was a statistically greater (p = 0.04) amount of normalized GAG in the Col I hydrogels when compared to pellets with TGF-β3 (Pel). Normalized GAG content increased between days 14 and 28 in all treatments except for pellets cultured without TGF-β3 (Pel (-TGF)). After 28 days in culture, there was a statistically greater amount of normalized GAG in the Col I/II hydrogels compared to the Col I hydrogels, pellets with TGF-β3 (Pel), and pellets without TGF-β3 (Pel (-TGF)). After 28 days in culture, there was no statistical difference in normalized GAG content when comparing Col I hydrogels, Col I/II hydrogels cultured without TGF-β3 (Col I/II (-TGF)), Col I hydrogels cultured without TGF-β3 (Col I (-TGF)), and pellets with TGF-β3 (Pel) (Table 4S).
Figure 2. Collagen blend hydrogel increased GAG production when cultured with TGF-β and did not promote an increase in AP activity.
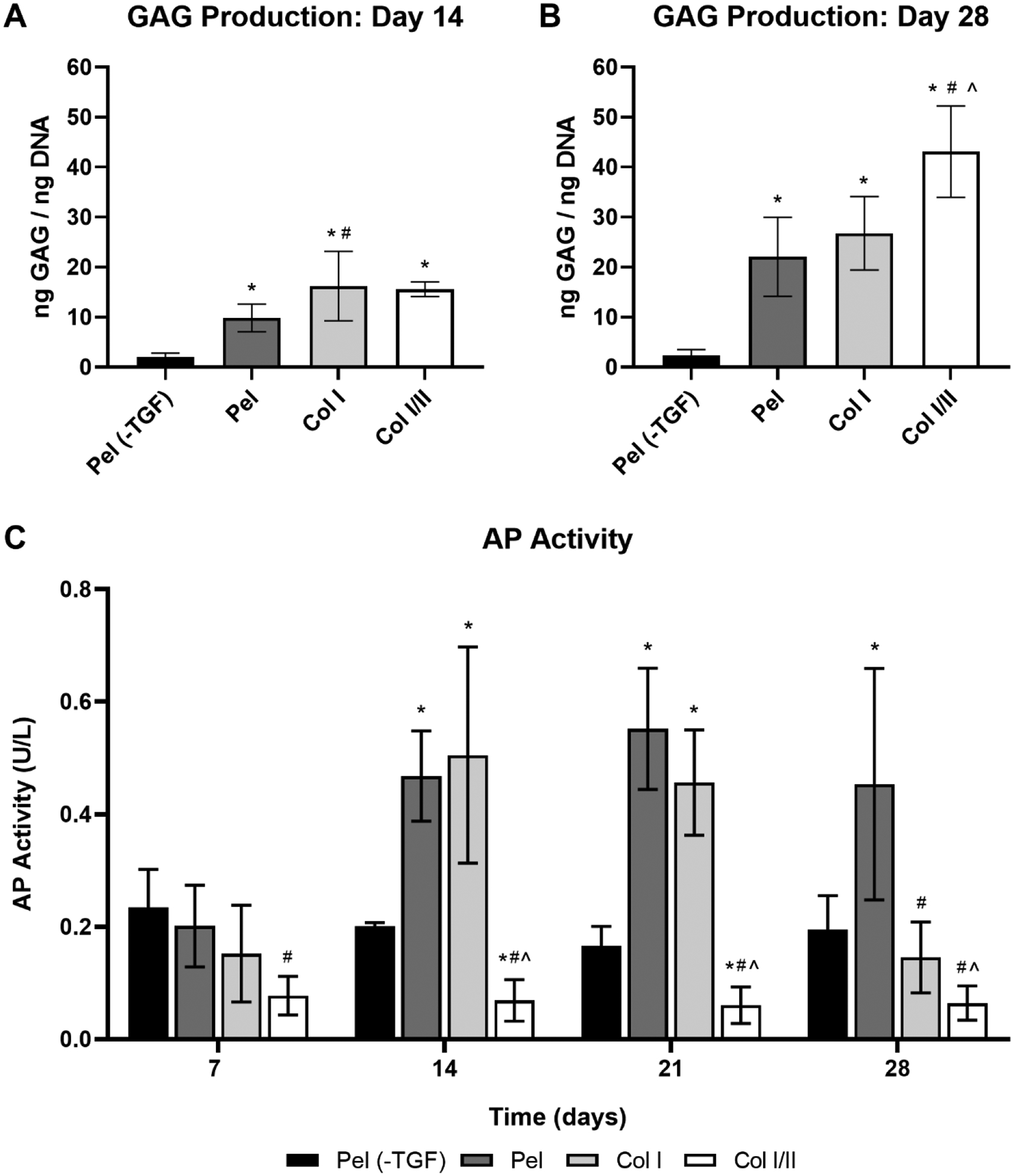
GAG/DNA ratio of the cell-hydrogel constructs and pellets after a (A) 14-day or (B) 28-day culture period. Values are expressed as mean ± SD (n = 5 or 6 for cell-gel scaffolds and pellets). A one-way ANOVA and Tukey’s post hoc test were performed within a timepoint (p < 0.05).* indicates a statistical difference in GAG production from pellets without TGF-β3 (Pel (-TGF)) within a timepoint, # indicates a statistical difference in GAG production from the pellets with TGF-β3 (Pel) within a timepoint, and ^ indicates a statistical difference in GAG production from the collagen type I hydrogels (Col I) within a timepoint. (C) AP activity of rabbit MSCs over time in removed culture medium at 7, 14, 21, or 28 days (n = 8 or 9 for Col I hydrogels, Col I/II hydrogels, and pellets with TGF-β3 (Pel) and n = 3 for pellets without TGF-β3 (Pel (-TGF))). A mixed-effect model and Tukey’s post hoc test were performed (p < 0.05). * indicates a statistical difference in AP activity from pellets without TGF-β3 (Pel (-TGF)) within a timepoint, # indicates a statistical difference in AP activity from the pellets with TGF-β3 (Pel) within a timepoint, and ^ indicates a statistical difference in AP activity from the collagen type I hydrogels (Col I) within a timepoint.
Collagen blend hydrogels did not promote an increase in AP activity
Medium aliquots were taken at 7, 14, 21, and 28 days and analyzed for AP activity. The Col I hydrogel and pellet with added TGF-β3 (Pel) each increased over time until a peak at about 14 days and 21 days, respectively (Figure 2C). A similar peak in AP activity was not seen in the Col I/II hydrogels or pellet without added TGF-β3 (Pel (-TGF)), and there was no significant increase in AP activity over time. Compared to the Col I/II hydrogel, the pellet with added TGF-β3 (Pel) had significantly higher AP activity at each time point, the Col I hydrogel had significantly higher AP activity at days 7, 14, and 21, and the pellet without added TGF-β3 (Pel (-TGF)) had significantly higher AP activity at days 14 and 21.
Sox9 gene expression was upregulated in the Col I/II hydrogels compared to Col I gels
Gene expression levels were measured using qRT-PCR in cells cultured in Col I/II hydrogels, Col I hydrogels, pellets with TGF-β3 (Pel), and pellets without TGF-β3 (Pel (-TGF)) after 14 days as seen in Figure 3. There was a statistically higher collagen type II gene expression in the pellets cultured with TGF-β3 (Pel) compared to Col I/II hydrogels, Col I hydrogels, and pellets without TGF-β3 (Pel (-TGF)). The collagen type II expression levels of both Col I/II hydrogels and Col I hydrogels were statistically higher than those of pellets cultured without TGF-β3 (Pel (-TGF)). Relative sox9 gene expression was upregulated in the Col I/II hydrogels and pellets cultured with TGF-β3 (Pel) when compared to either the Col I hydrogels or pellets cultured without TGF-β3 (Pel (-TGF)). The Col I/II hydrogels and pellets cultured with TGF-β3 (Pel) had statistically higher aggrecan and collagen type I gene expression levels than the pellets cultured without TGF-β3 (Pel (-TGF)). Finally, collagen type X gene expression was statistically upregulated in both Col I/II hydrogels and Col I hydrogels when compared to the pellets cultured without TGF-β3 (Pel (-TGF)).
Figure 3. Relative gene expression of chondrogenic and collagen genes at 2 weeks.
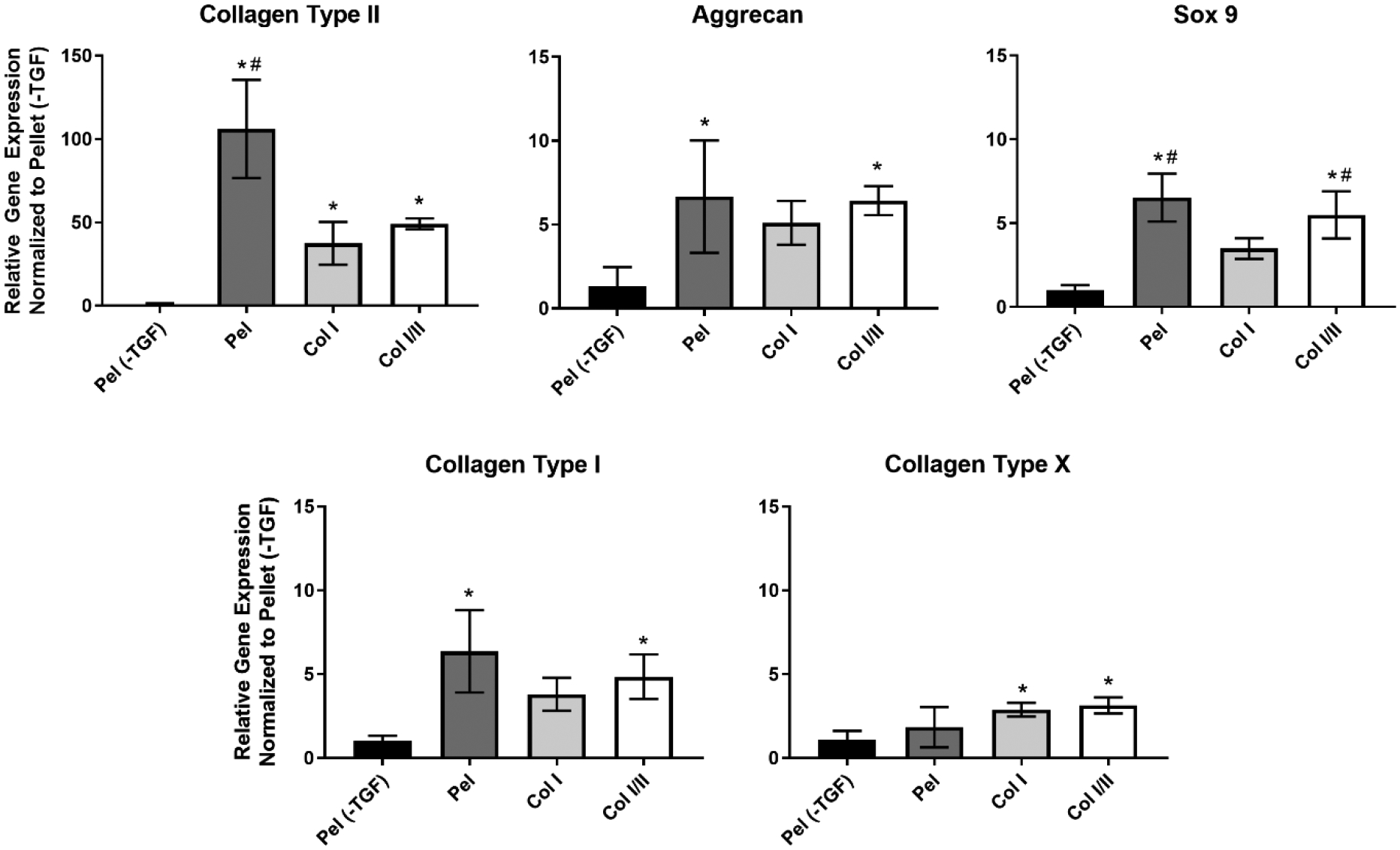
Relative gene expression of sox9, the master transcription factor involved in chondrogenesis, was upregulated in the Col I/II hydrogels and pellets with TGF-β3 (Pel) compared to either the Col I hydrogels or pellets without TGF-β3 (Pel (-TGF)). An ANOVA and Tukey’s post hoc tests were performed (p < 0.05) for gene expression analysis of sox9, aggrecan, collagen type I, and collagen type X (n=4). An ANOVA and a Games Howell post hoc test were performed (p < 0.05) for gene expression analysis of collagen type II. The * indicates statistical difference from the pellets without TGF-β3 (Pel (-TGF)), and the # indicates statistical difference from the Col I hydrogels.
Histological staining revealed that the repair tissue from Col I/II hydrogels with autologous MSCs matched surrounding articular cartilage
At 13 weeks post-operation, macroscopic examination during defect excision revealed no signs of joint capsule inflammation or degeneration around the defect. Osteoarthritis was not identified. After necropsy, the knees of the rabbits were dissected and photographed (Figures 5S – 8S) for macroscopic scoring (Table 5S) using categories adapted from Van den Borne et al.63 In addition, the scores were totaled to give an overall macroscopic score. As shown in Figure 9S, there were no statistical differences between the three treatments investigated in any of the macroscopic scoring categories (degree of defect repair, integration to border zone, and macroscopic appearance) for defects either in the trochlear groove or medial condyle. In addition, there were no statistical differences between the three treatments investigated when comparing overall repair assessment value for defects either in the trochlear groove or medial condyle.
Upon excision, tissues were processed, sectioned, and stained with H&E and SOFG. Histological analysis revealed differences between both the treatment groups and empty controls. Empty defects in both the trochlear groove and medial condyle lacked smooth surfaces and cellular morphologies expected in native cartilage tissue (Figure 4). Empty defects, which were devoid of collagen hydrogel, had a more fibrotic phenotype compared with the hydrogel-treated defects. When stained with SOFG, repair tissue in the empty defects in both regions were nearly void of any proteoglycan staining (Figure 5).
Figure 4. Col I/II hydrogel promoted cartilage tissue repair in vivo.
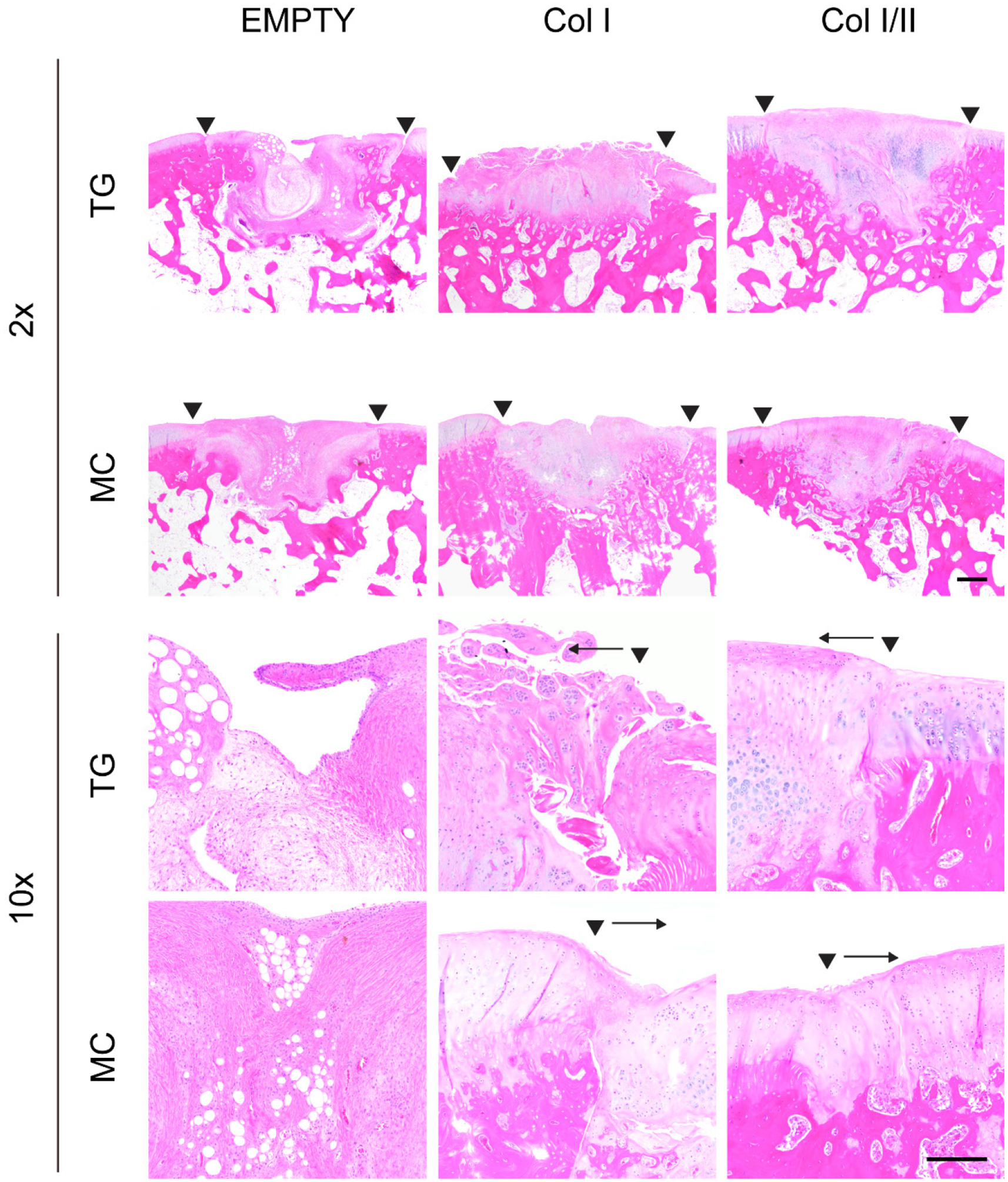
HE staining of repaired cartilage left empty or filled with either a Col I or Col I/II hydrogel in the trochlear groove (TG) or the medial condyle (MC) at two different magnifications (2x with a scale bar of 500 μm and 10x with a 200 μm scale bar). Staining revealed that the cellular morphology in the Col I/II hydrogels matched that of articular cartilage tissue surrounding the repair tissue. The two arrowheads indicate the edges of the repair tissue. A single arrowhead with an arrow indicates the edge of the repair tissue and the direction of the repair tissue, respectively.
Figure 5. Col I/II hydrogel promoted cartilage matrix production in vivo.
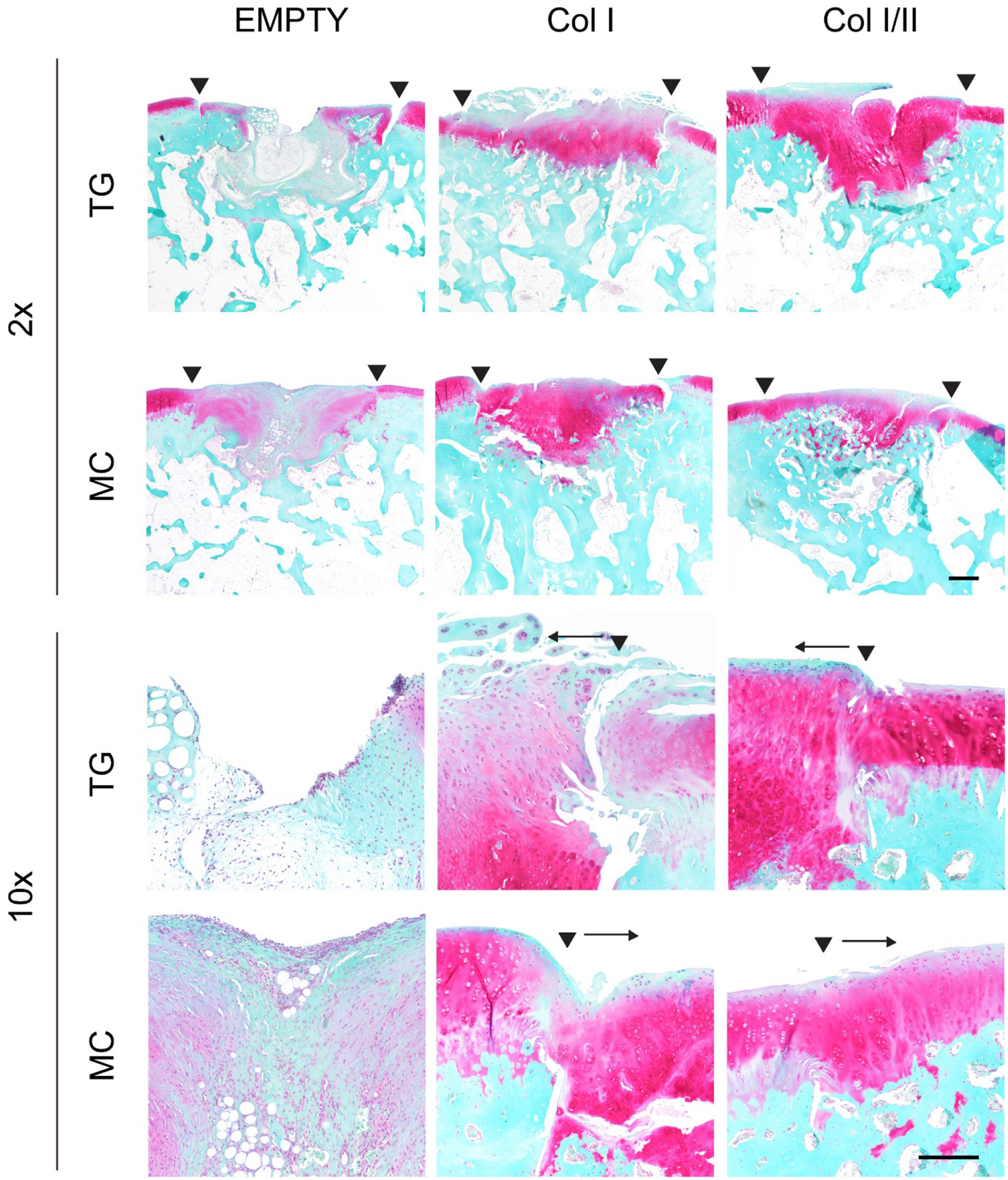
SOFG staining of repaired cartilage left empty or filled with either a Col I or Col I/II hydrogel in the trochlear groove (TG) or the medial condyle (MC) at two different magnifications (2x with a scale bar of 500 μm and 10x with a 200 μm scale bar). The Col I/II hydrogels had dark pink staining, which indicated the presence of proteoglycans. The two arrowheads indicate the edges of the repair tissue. A single arrowhead with an arrow indicates the edge of the repair tissue and the direction of the repair tissue, respectively.
The cartilage defects filled with the Col I hydrogel contained rounded cells in lacunar spaces with regions of eosinophilic matrix that matched surrounding articular cartilage (Figure 4). Spaces between the repair tissue and native cartilage on one or more edge were common in the defects filled with Col I hydrogel, and this finding indicated poor integration to adjacent tissue. Chondrocyte clustering was seen especially in areas on the edge of defects that were filled with the Col I hydrogel. In some defects filled with the Col I hydrogel, the superficial region had an irregular surface and areas with increased eosin in collagen fibers. In addition, some of the Col I hydrogel repair tissue contained bone fragments, which were adjacent to cartilage and did not integrate with the repair tissue. When stained with SOFG, the dark pink to red matrix staining seen in most of these defects was similar to native tissue, but there was a more fibrous superficial zone that lacked matrix staining (Figure 5).
The cellular morphology and proteoglycan staining for the Col I/II hydrogels matched that of the adjacent native articular cartilage and included chondrocytes in lacunar spaces (Figure 4). The defects in the medial condyle region had smooth and intact surfaces although some contained fissures. Compared to hypocellular regions in the defects filled with Col I hydrogels or left empty, the defects filled with the Col I/II hydrogels had more normal cellularity similar to the surrounding native tissue. However, some chondrocyte clusters were seen on the edge of the repair tissue. There were no major spaces lacking ECM between the surrounding cartilage and the Col I/II repair tissue. In the superficial zone, there were more flattened cells within a more organized ECM as seen in the surrounding native cartilage, but the defect area in the superficial zone also contained fibrous tissue. There were larger lacunar spaces in the deeper repair tissue of the trochlear groove defects filled with Col I/II hydrogels. When stained with SOFG, the repair tissue in both the trochlear groove and medial condyle regions that were filled with a Col I/II hydrogel demonstrated proteoglycan staining (Figure 5).
When comparing the healing between the trochlear groove and medial condyle, SOFG staining showed that defects treated with either hydrogel resulted in cartilage that was more similar to native tissue in the medial condyle compared to the trochlear groove (Figure 5). In the medial condyle defects filled with Col I hydrogels, the dark pink to red matrix staining seen in most of these defects was similar to native tissue, but there was a more fibrous superficial zone that lacked matrix staining. Similar matrix staining was seen in the trochlear groove defects filled with the Col I hydrogel, but only the repair tissue adjacent to the bone had staining for proteoglycans. The repair tissue in the trochlear groove region that was filled with a Col I/II hydrogel demonstrated proteoglycan staining throughout; however, the medial condyle defects that were filled with the Col I/II hydrogel had matrix staining that was similar to native tissue closer to the bone but less matrix staining in the superficial zone.
Col I/II hydrogels repaired cartilage defects significantly better than Col I hydrogels or empty defects
In Figure 6, semiquantitative histological evaluation revealed that there was a statistically higher overall cartilage repair score for the Col I/II hydrogels compared to the Col I hydrogels and the empty defect controls in both the medial condyle (weight-bearing region) and the trochlear groove (non-weight bearing region). More specifically, the hypocellularity score for the Col I/II hydrogels, in the trochlear groove defects, was statistically higher than the repair scores of the Col I hydrogels and the empty defect controls (Figure 10S). Trochlear groove defects had statistically higher repair scores in the Col I/II hydrogels compared to the empty defect controls in the categories of matrix staining, chondrocyte clustering, and bonding to adjacent cartilage. In defects created in the medial condyle, there were statistically higher repair scores in the Col I/II hydrogels compared to the empty defects in matrix staining, hypocellularity, and adjacent cartilage (Figure 11S). For all other scoring categories, there were no statistical differences between the three treatments of interest in a given defect location. The cartilage repair scores for empty defects was statistically higher in the medial condyle than the trochlear groove for the cellular morphology, matrix staining, and thickness categories. In contrast, the cartilage repair scores for the Col I/II treatment were statistically higher in the trochlear groove than the medial condyle for the cellular morphology, thickness, and chondrocyte clustering categories.
Figure 6. The Col I/II hydrogel repaired cartilage defects significantly better than either Col I or empty defects.
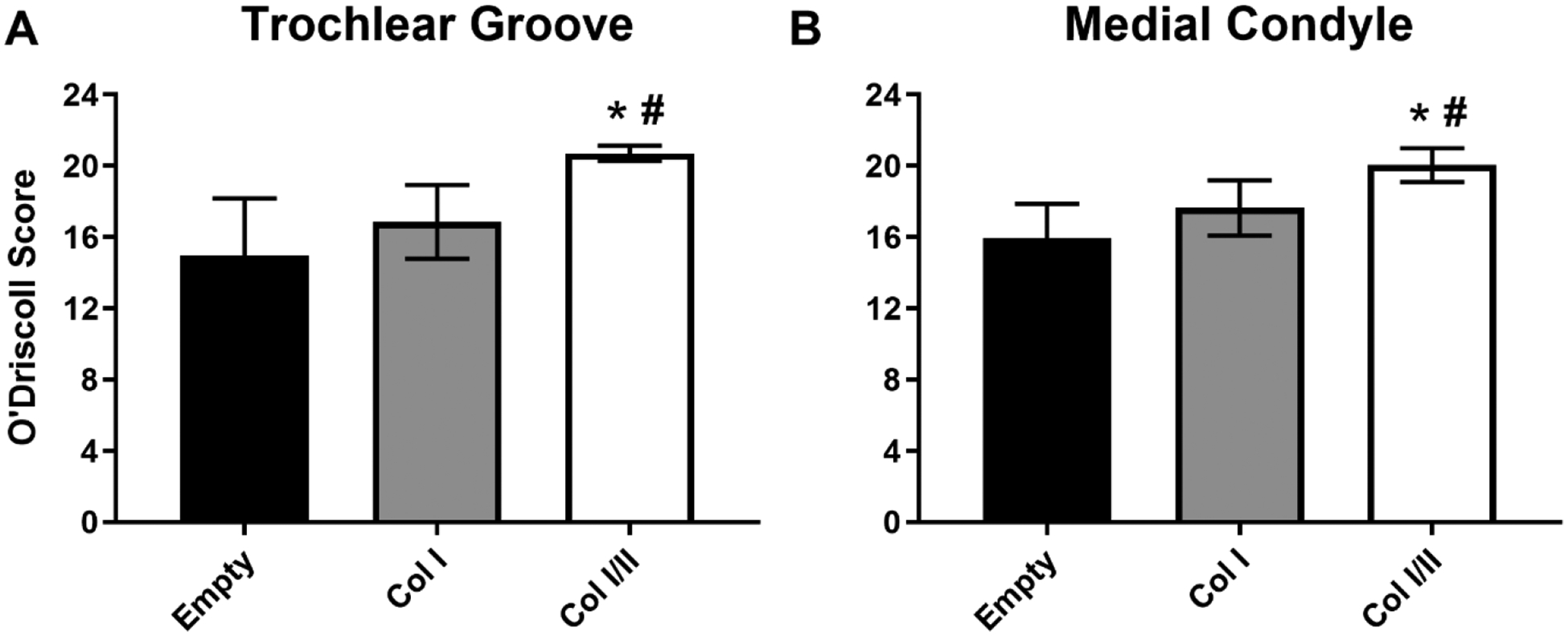
Histological evaluation using the O’Driscoll scoring criteria to assess cartilage repair within defects in the trochlear groove and medial condyle. The Col I/II hydrogel (n=8) repaired cartilage defects significantly better than either Col I (n=8) or empty defects (n=16). An ANOVA and Tukey’s post hoc test were performed (p < 0.05). * indicates statistical difference from the empty defect within a defect location, and # indicates statistical difference from the Col I hydrogel within a defect location.
DISCUSSION
Despite current advances in treatment options for patients suffering from osteoarthritis and other cartilage diseases, we lack a way to repair and regenerate articular cartilage. Collagen hydrogels have been widely utilized as a scaffold material for use in cartilage tissue engineering applications. Collagen type I, which is found in numerous places throughout the body including bone and scar tissue, continues to be the most utilized type of collagen in tissue engineered cartilage scaffolds even though it has also been shown to promote bone formation.64 Previous studies have shown that when cultured on collagen type I hydrogels, osteoblasts maintain their phenotype and MSCs are able to undergo osteogenesis.12,65 Furthermore, a recent study, which found that chondrocytes cultured in collagen type I hydrogels expressed significantly higher expression of dedifferentiation markers than alginate hydrogels or composite alginate and collagen type I hydrogels, underscores that collagen type I alone may not be the best choice for cartilage tissue engineering.66
On the other hand, collagen type II is found in native cartilage tissue and is a promising addition to a biomaterial to promote cartilage repair. For example, the addition of collagen type II to an alginate scaffold induced mesenchymal stem cells differentiation into a chondrogenic lineage in the presence of TGF-β1 and dexamethasone.18 A few papers have investigated collagen type II scaffolds19,28 despite the lack of robust hydrogels and inferior mechanical properties.25,27,56 Lazarini et al. created collagen type II hydrogels with encapsulated adipose-derived stem cells (ASCs), but extremely high concentrations of collagen type II make this a costly option.28 Lu et al. also created a collagen type II hydrogel with encapsulated ASCs and compared to a collagen type I hydrogel; however, this study was not able to decouple differences in the mechanical properties between the two formulations.19 Building on the known benefits of collagen type II in MSC differentiation and maintenance of chondrocyte differentiation, our lab previously characterized the network structure and mechanical properties of blended hydrogel scaffolds made of a 3:1 collagen type I to collagen type II ratio with the goal of developing a self-assembling hydrogel containing collagen II for cartilage tissue engineering.56 Our current study is the first to encapsulate mesenchymal stem cells (MSCs) in a collagen type I and II hydrogel and investigate both in vitro chondrogenic differentiation and in vivo cartilage repair potential.
The addition of collagen type II to a composite scaffold has been used in several previous studies as a cue to enhance chondrogenic potential of a tissue engineered scaffold.18,47,67–70 In addition, collagen type I and II blend hydrogels with embedded chondrocytes were able to maintain a rounded morphology and secrete ECM specific to cartilage.47 Our hydrogels contained encapsulated MSCs that we expected to secrete cartilage-specific ECM over the culture period. When cultured in chondrogenic medium for 28 days, there was a statistical increase in the GAG production in the Col I/II hydrogels compared to Col I hydrogels or pellet culture (Figure 2B). The increase in normalized GAG levels for the Col I/II hydrogels suggested that the addition of collagen type II promoted GAG production when cultured with TGF-β3. Results also suggest that the hydrogels, both Col I/II and Col I, themselves have a chondrogenic effect on the MSCs since there was no statistical difference in normalized GAG content when comparing Col I/II hydrogels cultured without TGF-β3 (Col I/II (-TGF)), Col I hydrogels cultured without TGF-β3 (Col I (-TGF)), and pellets with TGF-β3 (Pel) after 28 days in culture (Table 4S). The chondrogenic effects of collagen type II were not observed in a study where GAG content increased with increased collagen type I concentration in collagen type I and II hydrogels with encapsulated chondrocytes; however, it should be noted that the increased collagen type I concentration also increased the stiffness of the gels.47 Our results are consistent with previous studies that establish the superiority of collagen type II for promoting chondrogenesis of MSCs compared to collagen type I.18,45,71 For example, in alginate and collagen type II hydrogels with embedded MSCs, GAG production was upregulated when compared to alginate and collagen type I hydrogels with embedded MSCs.18 At each time point of our current study, the Col I/II hydrogels had statistically lower AP activity than the pellets cultured in chondrogenic medium (Figure 2C). There was also statistically lower AP activity in the Col I/II hydrogels compared to the Col I hydrogels at days 7,14, and 21. These results suggested that the addition of collagen II suppressed AP activity. An increase in AP activity is undesirable for MSCs embedded within a tissue engineered cartilage scaffold since AP is a hypertrophic marker and indicates early bone formation. In agreement with our study, a previous study found that collagen type II added to the medium enhanced GAG synthesis but did not increase alkaline phosphatase activity of mesenchymal progenitor cells.72
There are several variables that affect the collagen hydrogel fabrication process, and we had to choose whether to keep the concentration of collagen prior to polymerization constant as opposed to the mechanical properties of our hydrogels post-polymerization (there is a ~3-fold difference in the average G’ values of the Col I/II hydrogels (76.43 Pa) and Col I hydrogels (310.10 Pa) before cells are added (Figure 12S)). Because we chose to keep the former variable constant, we performed tests to ensure that our results could be attributed to the addition of collagen type II and were not due to changes in mechanical properties. To do so, we created an all collagen type I hydrogel that had mechanical properties that matched those of our Col I/II hydrogel (Table 6S and Figure 12S). There was a statistically lower amount of GAG produced in the Col I hydrogel and the collagen type I mechanically matched (MM) hydrogel compared to the Col I/II hydrogel (Figure 13SA). When compared to the Col I/II hydrogel, there was a statistical increase in AP activity at all time points for the MM hydrogel (Figure 13SB). From these data, we conclude that the addition of collagen type II promoted GAG production independent of mechanical properties.
Gene expression levels for cartilage-specific and collagen-related genes were measured using qRT-PCR in cells cultured in Col I/II hydrogels, Col I hydrogels, pellets with TGF-β3 (Pel), and pellets without TGF-β3 (Pel (-TGF)) after 14 days (Figure 3). There was an upregulation in two cartilage-specific genes, aggrecan and sox9, in the Col I/II gels compared to either pellets without TGF-β3 (Pel (-TGF)) or both the pellets without TGF-β3 (Pel (-TGF)) and Col I hydrogels, respectively. Sox9 is considered the master transcription factor involved in chondrogenesis since it is essential for both mesenchymal condensation and hypertrophy inhibition.73 In addition, a number of different extracellular matrix genes found in proliferating chondrocytes, including collagen type II, collagen type IX, collagen type XI, and aggrecan, are activated by sox9.74 Thus, the upregulation seen in sox9 was expected to correspond with an upregulation in collagen type II and a downregulation in collagen type X. An upregulation in collagen type II was seen, but there was no statistical difference in relative gene expression between the Col I/II and Col I hydrogels. Future proteomics experiments could shed insight into this discrepancy since gene and protein expression levels do not always correlate. Despite the upregulation in sox9, collagen type I expression in the Col I/II hydrogels and pellets with TGF-β3 (Pel) had statistically higher gene expression levels than the pellets without TGF-β3 (Pel (-TGF)) and a slight upregulation in collagen type X was seen in the Col I/II and Col I hydrogels. However, the low alkaline phosphatase activity for the Col I/II hydrogels, as well as previous studies indicating that collagen type X is a component of normal articular cartilage,75 suggested that the cells may not become hypertrophic. Overall, our results were consistent with those seen in alginate and collagen type II blend hydrogels with embedded MSCs where a number of chondrogenic genes and collagen type II synthesis were upregulated compared to alginate and collagen type I composite hydrogels with embedded MSCs or stem cells in monolayer.18
Our average modified O’Driscoll scores in both defect locations (Figure 6) were consistent with average scores seen in other studies where cartilage defect repair was investigated while using scaffolds including collagen type II in a rabbit model. A modified O’Driscoll score of approximately 20 was seen in studies investigating dried collagen type I and II scaffolds combined with microfracture and chemically crosslinked polyethylene glycol and collagen type II scaffolds with implanted autologous chondrocytes.43,50 Histological and immunohistochemical observations revealed that the Col I/II hydrogels, compared to Col I hydrogels and empty defects, provided a conducive environment for integration with surrounding tissue and allowed for favorable conditions in the process of cartilage repair (Figures 4 and 5). When the HE sections were analyzed, the empty defects contained a more fibrocartilage-like repair tissue with signs of fibrosis. A previous study demonstrated that the regenerated tissue that forms in empty cartilage defects is more fibrotic and deteriorates compared to defects with collagen matrices, and this finding is consistent with our observations.76 In addition, the superficial region of the trochlear groove defects filled with the Col I hydrogel did not contain a smooth surface representative of native cartilage but instead had a fibrillated surface and fibrotic areas. The mixture of cartilage-like tissue and fibrotic areas suggested that the repair tissue in the defects filled with Col I scaffolds formed a more fibrocartilage-like tissue, which is known to be less durable than hyaline cartilage and only produces short-term repair. In the medial condyle defects filled with Col I hydrogels, the repair tissue did not completely fill the defect and had unorganized areas that contained both cartilage and bone tissue. These areas of bone tissue were poorly integrated with surrounding cartilage and are believed to be remnants from defect drilling.
Lack of integration with surrounding tissue has been a reason for many failed attempts to repair a cartilage defect with a tissue engineered construct.77 Nonetheless, the combination of a collagen type I and II hydrogel promoted cell integration with surrounding tissue, including both the surrounding cartilage and underlying bone, and provided favorable conditions for cartilage repair. A similar degree of integration was seen in a study that encapsulated chondrocytes in collagen type I and II scaffolds that were used to repair cartilage defects in a rabbit model.51
Previous studies have shown that cartilage defects in a rabbit model almost completely repaired after 12–13 weeks.78,79 The improved healing may be due to the younger age of the rabbits, 2- to 3-month-old rabbits78 or 5- to 6-month-old rabbits,79 as compared to this study, which contained 9-month-old rabbits. In addition, improved healing may be due to the smaller defect size, 3 mm,79 compared to this study, which contained 3.2 mm defects. Spontaneous healing of defects has been described in young rabbits and small cartilage defects.80 Results from this study show the value in utilizing biomimetic materials to influence the differentiation of bone-marrow derived MSCs into chondrocytes.
A previous in vivo study that implanted a combination of collagen type II, fibrin sealant, and adipose-derived stem cells into rabbit knees observed the presence of chondrocyte-like cells throughout the tissue with increasing concentration in the deep zone, and the authors thus concluded that the defects were in an advanced healing stage.28 Our data are in accordance with this study as we have shown that MSCs encapsulated within our Col I/II scaffold can either differentiate to a chondrocyte phenotype or recruit chondrocytes from the surrounding tissue to populate the defect site. Larger lacunar spaces in the deeper repair tissue and flattened cells in the superficial zone of the trochlear groove defects filled with Col I/II hydrogels showed that cells within the repair tissue are in different stages of development.
Including collagen type II in our hydrogel increased the amount of cartilage-specific ECM molecules produced by cells in the tissue. Although there was an increase in collagen type II staining for the Col I/II scaffold (Figure 14S), it is difficult to interpret these results since collagen type II was added prior to polymerization. Picrosirius red was used to stain sections, which were imaged using polarized light microscopy to analyze collagen organization (Figure 15S). Although slight differences between treatments were observed, little to no collagen fiber organization, as seen in native tissue, was observed. However, other important markers of cartilage matrix, like proteoglycan staining in the SOFG slides, were more intense in the Col I/II hydrogels than both the Col I and empty defects. It should be noted that SOFG staining suggested that cartilage defects filled with Col I/II hydrogels were more similar to native tissue in the medial condyle compared to the trochlear groove, and that these results are consistent with literature that found mechanical stimulation aids in regeneration.81 In addition, increases in SOFG proteoglycan staining adjacent to the cells and in the surrounding matrix in the defects filled with Col I/II scaffolds supported the results from the in vitro GAG experiments. Taken together, these results suggested that the addition of collagen type II to a hydrogel with encapsulated MSCs is advantageous to promote cartilage matrix production.
CONCLUSION
The Col I/II construct used in this study induced and maintained the differentiation of MSCs into chondrocytes in vitro, and the cellular morphology and proteoglycan staining for the Col I/II hydrogels matched that of articular cartilage tissue surrounding the repair tissue in vivo. There was a greater degree of cartilage repair for the Col I/II hydrogels compared to the Col I hydrogels and the empty defect controls in both the medial condyle and the trochlear groove. Results from this study suggest that there is clinical value in the cartilage repair capabilities of our Col I/II hydrogel with encapsulated MSCs. However, a longer evaluation time frame must be investigated using a larger animal in future studies.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Purdue Davidson School of Chemical Engineering, National Institutes of Health [NIAMS R21AR065644 and R01AR065398], Indiana Clinical and Translational Sciences [NIH TL1TR001107 to CEK], Purdue Office of Research and Partnerships, and Purdue Office of the Provost. This publication was further supported by the Indiana Clinical and Translational Sciences Institute, funded in part by grant #UL1 TR001108 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award. The authors thank Victor Bernal-Crespo at the Purdue Histology Research Laboratory for performing all histology and immunohistochemistry. The authors also wish to thank Robyn McCain, Christa Crain, Kris Kazmierczak, Shery Park, and Cheryl Anderson of the Purdue Pre-Clinical Research Laboratory for their assistance with the rabbit study.
Footnotes
DISCLOSURE
The authors declare no competing financial interests.
REFERENCES
- (1).Cisternas MG; Murphy L; Sacks JJ; Solomon DH; Pasta DJ; Helmick CG Alternative Methods for Defining Osteoarthritis and the Impact on Estimating Prevalence in a US Population-Based Survey. Arthritis Care Res. 2016, 68 (5), 574–580. 10.1002/acr.22721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).McCormick F; Harris JD; Abrams GD; Frank R; Gupta A; Hussey K; Wilson H; Bach B; Cole B Trends in the Surgical Treatment of Articular Cartilage Lesions in the United States: An Analysis of a Large Private-Payer Database over a Period of 8 Years. J. Arthrosc. Relat. Surg 2014, 30 (2), 222–226. 10.1016/j.arthro.2013.11.001. [DOI] [PubMed] [Google Scholar]
- (3).Huey DJ; Hu JC; Athanasiou KA Unlike Bone, Cartilage Regeneration Remains Elusive. Science 2012, 338 (6109), 917–921. 10.1126/science.1222454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).de l’Escalopier N; Anract P; Biau D Surgical Treatments for Osteoarthritis. Ann. Phys. Rehabil. Med 2016, 59 (3), 227–233. 10.1016/j.rehab.2016.04.003. [DOI] [PubMed] [Google Scholar]
- (5).Madry H; Grün UW; Knutsen G Cartilage Repair and Joint Preservation. Dtsch. Aerzteblatt Online 2011, 108 (40), 669–677. 10.3238/arztebl.2011.0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Alford JW; Cole BJ Cartilage Restoration, Part 1: Basic Science, Historical Perspective, Patient Evaluation, and Treatment Options. Am. J. Sports Med 2005, 33 (2), 295–306. 10.1177/0363546504273510. [DOI] [PubMed] [Google Scholar]
- (7).Matsiko A; Levingstone TJ; O’Brien FJ Advanced Strategies for Articular Cartilage Defect Repair. Materials (Basel). 2013, 6 (2), 637–668. 10.3390/ma6020637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Seliktar D Designing Cell-Compatible Hydrogels for Biomedical Applications. Science 2012, 336 (6085), 1124–1128. 10.1126/science.1214804. [DOI] [PubMed] [Google Scholar]
- (9).Vilela CA; Correia C; Oliveira JM; Sousa RA; Espregueira-Mendes J; Reis RL Cartilage Repair Using Hydrogels : A Critical Review of in Vivo Experimental Designs. ACS Biomater. Sci. Eng 2015, 1, 726–739. 10.1021/acsbiomaterials.5b00245. [DOI] [PubMed] [Google Scholar]
- (10).Spiller KL; Maher SA; Lowman AM Hydrogels for the Repair of Articular Cartilage Defects. Tissue Eng. Part B Rev 2011, 17 (4), 281–299. 10.1089/ten.teb.2011.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Zhu S; Yuan Q; Yin T; You J; Gu Z; Xiong S; Hu Y Self-Assembly of Collagen-Based Biomaterials: Preparation, Characterizations and Biomedical Applications. J. Mater. Chem . B 2018, 6 (18), 2650–2676. 10.1039/c7tb02999c. [DOI] [PubMed] [Google Scholar]
- (12).Mizuno M; Fujisawa R; Kuboki Y Type I Collagen-Induced Osteoblastic Differentiation of Bone-Marrow Cells Mediated by Collagen-α2β1 Integrin Interaction. J. Cell. Physiol 2000, 184, 207–213. . [DOI] [PubMed] [Google Scholar]
- (13).Bai X; Gao M; Syed S; Zhuang J; Xu X; Zhang X Bioactive Hydrogels for Bone Regeneration. Bioact. Mater 2018, 3 (4), 401–417. 10.1016/j.bioactmat.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Ochi M; Uchio Y; Kawasaki K; Wakitani S; Iwasa J Transplantation of Cartilage-like Tissue Made by Tissue Engineering in the Treatment of Cartilage Defects of the Knee. J. Bone Jt. Surg., Br. Vol 2002, 84 (4), 571–578. 10.1302/0301-620X.84B4.0840571. [DOI] [PubMed] [Google Scholar]
- (15).Andereya S; Maus U; Gavenis K; Muller-Rath R; Miltner O; Mumme T; Schneider U First Clinical Experiences with a Novel 3D-Collagen Gel (CaRes(R)) for the Treatment of Focal Cartilage Defects in the Knee. Z Orthrop Ihre Grenzgev 2006, 144, 272–280. 10.1055/s-2006-933445. [DOI] [PubMed] [Google Scholar]
- (16).Wakitani S; Imoto K; Yamamoto T; Saito M; Murata N; Yoneda M Human Autologous Culture Expanded Bone Marrow-Mesenchymal Cell Transplantation for Repair of Cartilage Defects in Osteoarthritic Knees. Osteoarthr. Cartil 2002, 10 (3), 199–206. 10.1053/joca.2001.0504. [DOI] [PubMed] [Google Scholar]
- (17).Wakitani S; Goto T; Pineda SJ; Young RG; Mansour JM; Caplan AI; Goldberg VM Mesenchymal Cell-Based Repair of Large, Full-Thickness Defects of Articular Cartilage. J. Bone Jt. Surg 1994, 76-A No. 4, 579–592. 10.2106/00004623-199404000-00013. [DOI] [PubMed] [Google Scholar]
- (18).Bosnakovski D; Mizuno M; Kim G; Takagi S; Okumura M; Fujinaga T Chondrogenic Differentiation of Bovine Bone Marrow Mesenchymal Stem Cells (MSCs) in Different Hydrogels: Influence of Collagen Type II Extracellular Matrix on MSC Chondrogenesis. Biotechnol. Bioeng 2006, 93 (6), 1152–1163. 10.1002/bit.20828. [DOI] [PubMed] [Google Scholar]
- (19).Lu Z; Doulabi BZ; Huang C; Bank RA; Helder MN Collagen Type II Enhances Chondrogenesis in Adipose Tissue-Derived Stem Cells by Affecting Cell Shape. Tissue Eng. Part A 2010, 16 (1), 81–90. 10.1089/ten.TEA.2009.0222. [DOI] [PubMed] [Google Scholar]
- (20).Eyre DR; Muir H Quantitative Analysis of Types I and II Collagens in Human Intervertebral Discs at Various Ages. Biochim. Biophys. Acta, Protein Struct 1977, 492, 29–42. 10.1016/0005-2795(77)90211-2. [DOI] [PubMed] [Google Scholar]
- (21).Fox AJS; Bedi A; Rodeo SA The Basic Science of Human Knee Menisci. Sport. Heal. A Multidiscip. Approach 2012, 4 (4), 340–351. 10.1177/1941738111429419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Fox AJS; Bedi A; Rodeo SA The Basic Science of Articular Cartilage: Structure, Composition, and Function. Sports Health 2009, 1 (6), 461–468. 10.1177/1941738109350438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Bajtner E; Nandakumar KS; Engström A; Holmdahl R Chronic Development of Collagen-Induced Arthritis Is Associated with Arthritogenic Antibodies against Specific Epitopes on Type II Collagen. Arthritis Res. Ther 2005, 7 (5), R1148–57. 10.1186/ar1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Farjanel J; Schürmann G; Bruckner P Contacts with Fibrils Containing Collagen I, but Not Collagens II, IX, and XI, Can Destabilize the Cartilage Phenotype of Chondrocytes. Osteoarthr. Cartil 2001, 9 (SUPPL. A), S55–S63. 10.1053/joca.2001.0445. [DOI] [PubMed] [Google Scholar]
- (25).Calderon L; Collin E; Velasco-Bayon D; Murphy M; O’Halloran D; Pandit A Type II Collagen-Hyaluronan Hydrogel--A Step towards a Scaffold for Intervertebral Disc Tissue Engineering. Eur. Cell. Mater 2010, 20, 134–148. 10.22203/ecm.v020a12. [DOI] [PubMed] [Google Scholar]
- (26).Birk DE; Silver FH Collagen Fibrillogenesis in Vitro: Comparison of Types I, II, and III. Arch. Biochem. Biophys 1984, 235 (1), 178–185. 10.1016/0003-9861(84)90266-2. [DOI] [PubMed] [Google Scholar]
- (27).Dong M; Xu S; Bünger MH; Birkedal H; Besenbacher F Temporal Assembly of Collagen Type II Studied by Atomic Force Microscopy. Adv. Eng. Mater 2007, 9 (12), 1129–1133. 10.1002/adem.200700220. [DOI] [Google Scholar]
- (28).Lazarini M; Bordeaux-Rego P; Giardini-Rosa R; Duarte ASS; Baratti MO; Zorzi AR; de Miranda JB; Lenz Cesar C; Luzo Â; Olalla Saad ST Natural Type II Collagen Hydrogel, Fibrin Sealant, and Adipose-Derived Stem Cells as a Promising Combination for Articular Cartilage Repair. Cartilage 2017, 8 (4), 439–443. 10.1177/1947603516675914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Nehrer S; Breinan HA; Ramappa A; Young G; Shortkroff S; Louie LK; Sledge CB; Yannas IV; Spector M Matrix Collagen Type and Pore Size Influence Behaviour of Seeded Canine Chondrocytes. Biomaterials 1997, 18 (11), 769–776. 10.1016/S0142-9612(97)00001-X. [DOI] [PubMed] [Google Scholar]
- (30).Levingstone TJ; Matsiko A; Dickson GR; O’Brien FJ; Gleeson JP A Biomimetic Multi-Layered Collagen-Based Scaffold for Osteochondral Repair. Acta Biomater 2014, 10 (5), 1996–2004. 10.1016/j.actbio.2014.01.005. [DOI] [PubMed] [Google Scholar]
- (31).Buma P; Pieper JS; Van Tienen T; Van Susante JLC; Van Der Kraan PM; Veerkamp JH; Van Den Berg WB; Veth RPH; Van Kuppevelt TH Cross-Linked Type I and Type II Collagenous Matrices for the Repair of Full-Thickness Articular Cartilage Defects - A Study in Rabbits. Biomaterials 2003, 24 (19), 3255–3263. 10.1016/S0142-9612(03)00143-1. [DOI] [PubMed] [Google Scholar]
- (32).Lee CR; Grodzinsky AJ; Hsu HP; Spector M Effects of a Cultured Autologous Chondrocyte-Seeded Type II Collagen Scaffold on the Healing of a Chondral Defect in a Canine Model. J. Orthop. Res 2003, 21 (2), 272–281. 10.1016/S0736-0266(02)00153-5. [DOI] [PubMed] [Google Scholar]
- (33).Pieper JS; Van Der Kraan PM; Hafmans T; Kamp J; Buma P; Van Susante JLC; Van Den Berg WB; Veerkamp JH; Van Kuppevelt TH Crosslinked Type II Collagen Matrices: Preparation, Characterization, and Potential for Cartilage Engineering. Biomaterials 2002, 23 (15), 3183–3192. 10.1016/S0142-9612(02)00067-4. [DOI] [PubMed] [Google Scholar]
- (34).Choi B; Kim S; Lin B; Wu BM; Lee M Cartilaginous Extracellular Matrix-Modified Chitosan Hydrogels for Cartilage Tissue Engineering. ACS Appl. Mater. Interfaces 2014, 6 (22), 20110–20121. 10.1021/am505723k. [DOI] [PubMed] [Google Scholar]
- (35).Yang K; Sun J; Wei D; Yuan L; Yang J; Guo L; Fan H; Zhang X Photo-Crosslinked Mono-Component Type II Collagen Hydrogel as a Matrix to Induce Chondrogenic Differentiation of Bone Marrow Mesenchymal Stem Cells. J. Mater. Chem. B 2017, 5 (44), 8707–8718. 10.1039/c7tb02348k. [DOI] [PubMed] [Google Scholar]
- (36).Vickers SM; Squitieri LEES; Spector M Effects of Cross-Linking Type II Collagen-GAG Scaffolds on Chondrogenesis in Vitro: Dynamic Pore Reduction Promotes Cartilage Formation. Tissue Eng 2006, 12 (5), 1345–1355. 10.1089/ten.2006.12.1345. [DOI] [PubMed] [Google Scholar]
- (37).Nong L; Zhou D; Zheng C; Jiang Y-Q; Xu N-W; Zhao G-Y; Wei H; Zhou S-Y; Han H; Han L The Effect of Different Cross-Linking Conditions of EDC / NHS on Type II Collagen Scaffolds : An in Vitro Evaluation. Cell Tissue Bank. 2019, 20, 557–568. 10.1007/s10561-019-09790-7. [DOI] [PubMed] [Google Scholar]
- (38).Pulkkinen HJ; Tiitu V; Valonen P; Jurvelin JS; Rieppo L; Toyras J; Silvast TS; Lammi MJ; Kiviranta I Repair of Osteochondral Defects with Recombinant Human Type II Collagen Gel and Autologous Chondrocytes in Rabbit. Osteoarthr. Cartil 2013, 21 (3), 481–490. 10.1016/j.joca.2012.12.004. [DOI] [PubMed] [Google Scholar]
- (39).Muhonen V; Narcisi R; Nystedt J; Korhonen M; van Osch GJVM; Kiviranta I Recombinant Human Type II Collagen Hydrogel Provides a Xeno-Free 3D Micro-Environment for Chondrogenesis of Human Bone Marrow-Derived Mesenchymal Stromal Cells. J. Tissue Eng. Regen. Med 2017, 11 (3), 843–854. 10.1002/term.1983. [DOI] [PubMed] [Google Scholar]
- (40).Báez J; Olsen D; Polarek JW Recombinant Microbial Systems for the Production of Human Collagen and Gelatin. Appl. Microbiol. Biotechnol 2005, 69 (3), 245–252. 10.1007/s00253-005-0180-x. [DOI] [PubMed] [Google Scholar]
- (41).Lin X; Gao L; Li R; Cheng W; Zhang C; Zhang X Mechanical Property and Biocompatibility of Silk Fibroin – Collagen Type II Composite Membrane. Mater. Sci. Eng. C 2019, 110018. 10.1016/j.msec.2019.110018. [DOI] [PubMed] [Google Scholar]
- (42).Kontturi LS; Järvinen E; Muhonen V; Collin EC; Pandit AS; Kiviranta I; Yliperttula M; Urtti A An Injectable, in Situ Forming Type II Collagen/Hyaluronic Acid Hydrogel Vehicle for Chondrocyte Delivery in Cartilage Tissue Engineering. Drug Deliv. Transl. Res 2014, 4 (2), 149–158. 10.1007/s13346-013-0188-1. [DOI] [PubMed] [Google Scholar]
- (43).Funayama A; Niki Y; Matsumoto H; Maeno S; Yatabe T; Morioka H; Yanagimoto S; Taguchi T; Tanaka J; Toyama Y Repair of Full-Thickness Articular Cartilage Defects Using Injectable Type II Collagen Gel Embedded with Cultured Chondrocytes in a Rabbit Model. J. Orthop. Sci 2008, 13 (3), 225–232. 10.1007/s00776-008-1220-z. [DOI] [PubMed] [Google Scholar]
- (44).Lee CR; Grodzinsky AJ; Spector M The Effects of Cross-Linking of Collagen-Glycosaminoglycan Scaffolds on Compressive Stiffness, Chondrocyte-Mediated Contraction, Proliferation and Biosynthesis. Biomaterials 2001, 22 (23), 3145–3154. 10.1016/S0142-9612(01)00067-9. [DOI] [PubMed] [Google Scholar]
- (45).Almeida HV; Sathy BN; Dudurych I; Buckley CT; O’Brien FJ; Kelly DJ Anisotropic Shape-Memory Alginate Scaffolds Functionalized with Either Type I or Type II Collagen for Cartilage Tissue Engineering. Tissue Eng. Part A 2017, 23 (1–2), 55–68. 10.1089/ten.tea.2016.0055. [DOI] [PubMed] [Google Scholar]
- (46).Ragetly G; Griffon DJ; Chung YS The Effect of Type II Collagen Coating of Chitosan Fibrous Scaffolds on Mesenchymal Stem Cell Adhesion and Chondrogenesis. Acta Biomater 2010, 6 (10), 3988–3997. 10.1016/j.actbio.2010.05.016. [DOI] [PubMed] [Google Scholar]
- (47).Yuan L; Li B; Yang J; Ni Y; Teng Y; Guo L; Fan H; Fan Y; Zhang X Effects of Composition and Mechanical Property of Injectable Collagen I/II Composite Hydrogels on Chondrocyte Behaviors. Tissue Eng. Part A 2016, 22 (11–12), 899–906. 10.1089/ten.tea.2015.0513. [DOI] [PubMed] [Google Scholar]
- (48).Mueller SM; Shortkroff S; Schneider TO; Breinan HA; Yannas IV; Spector M Meniscus Cells Seeded in Type I and Type II Collagen-GAG Matrices in Vitro. Biomaterials 1999, 20, 701–709. 10.1016/S0142-9612(98)00189-6. [DOI] [PubMed] [Google Scholar]
- (49).Lee CR; Grodzinsky AJ; Spector M Biosynthetic Response of Passaged Chondrocytes in a Type II Collagen Scaffold to Mechanical Compression. J. Biomed. Mater. Res. - Part A 2003, 64A (3), 560–569. 10.1002/jbm.a.10443. [DOI] [PubMed] [Google Scholar]
- (50).Enea D; Guerra D; Roggiani J; Cecconi S; Manzotti S; Quaglino D; Pasquali-Ronchetti I; Gigante A Mixed Type I and Type II Collagen Scaffold for Cartilage Repair: Ultrastructural Study of Synovial Membrane Response and Healing Potential versus Microfractures (A Pilot Study). Int. J. Immunopathol. Pharmacol 2013, 26 (4), 917–930. 10.1177/039463201302600410. [DOI] [PubMed] [Google Scholar]
- (51).Wang KH; Wan R; Chiu LH; Tsai YH; Fang CL; Bowley JF; Chen KC; Shih HN; Lai WFT Effects of Collagen Matrix and Bioreactor Cultivation on Cartilage Regeneration of a Full-Thickness Critical-Size Knee Joint Cartilage Defects with Subchondral Bone Damage in a Rabbit Model. PLoS One 2018, 13 (5), e0196779. 10.1371/journal.pone.0196779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Steinert AF; Ghivizzani SC; Rethwilm A; Tuan RS; Evans CH; Nöth U Major Biological Obstacles for Persistent Cell-Based Regeneration of Articular Cartilage. Arthritis Res. Ther 2007, 9 (3), 213. 10.1186/ar2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Dell’Accio F; De Bari C; Luyten FP Molecular Markers Predictive of the Capacity of Expanded Human Articular Chondrocytes to Form Stable Cartilage in Vivo. Arthritis Rheum 2001, 44 (7), 1608–1619. . [DOI] [PubMed] [Google Scholar]
- (54).Makris EA; Gomoll AH; Malizos KN; Hu JC; Athanasiou KA Repair and Tissue Engineering Techniques for Articular Cartilage. Nat Rev Rheumatol 2015, 11(1), 21–34. https://dx.doi.org/10.1038%2Fnrrheum.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Pittenger MF; Mackay AM; Beck SC; Jaiswal RK; Douglas R; Mosca JD; Moorman MA; Simonetti DW; Craig S; Marshak DR Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284 (5411), 143–147. 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- (56).Vázquez-Portalatín N; Kilmer CE; Panitch A; Liu JC Characterization of Collagen Type I and II Blended Hydrogels for Articular Cartilage Tissue Engineering. Biomacromolecules 2016, 17 (10), 3145–3152. 10.1021/acs.biomac.6b00684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Ahern BJ; Parvizi J; Boston R; Schaer TP Preclinical Animal Models in Single Site Cartilage Defect Testing: A Systematic Review. Osteoarthr. Cartil 2009, 17 (6), 705–713. 10.1016/j.joca.2008.11.008. [DOI] [PubMed] [Google Scholar]
- (58).Penick KJ; Solchaga LA; Welter JF High-Throughput Aggregate Culture System to Assess the Chondrogenic Potential of Mesenchymal Stem Cells. Biotechniques 2005, 39 (5), 687–690. 10.2144/000112009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Renner JN; Kim Y; Liu JC Bone Morphogenetic Protein-Derived Peptide Promotes Chondrogenic Differentiation of Human Mesenchymal Stem Cells. Tissue Eng. Part A 2012, 18 (23–24), 2581–2589. 10.1089/ten.tea.2011.0400. [DOI] [PubMed] [Google Scholar]
- (60).Kim YJ; Sah RLY; Doong JYH; Grodzinsky AJ Fluorometric Assay of DNA in Cartilage Explants Using Hoechst 33258. Anal. Biochem 1988, 174 (1), 168–176. 10.1016/0003-2697(88)90532-5. [DOI] [PubMed] [Google Scholar]
- (61).Bookout AL; Cummins CL; Mangelsdorf DJ; Pesola JM; Kramer MF High-Throughput Real-Time Quantitative Reverse Transcription PCR. In Current Protocols in Molecular Biology; John Wiley & Sons; New York, 2006; Vol. 831, pp 15.8.1–15.8.28. 10.1002/0471142727.mb1508s73. [DOI] [PubMed] [Google Scholar]
- (62).O’Driscoll SW; Keeley FW; Salter RB Durability of Regenerated Articular Cartilage Produced by Free Autogenous Periosteal Grafts in Major Full-Thickness Defects in Joint Surfaces under the Influence of Continuous Passive Motion. A Follow-up Report at One Year. J. Bone Jt. surgery 1988, 70 (4), 595–606. 10.2106/00004623-198870040-00017. [DOI] [PubMed] [Google Scholar]
- (63).van den Borne MPJ; Raijmakers NJH; Vanlauwe J; Victor J; de Jong SN; Bellemans J; Saris DBF International Cartilage Repair Society (ICRS) and Oswestry Macroscopic Cartilage Evaluation Scores Validated for Use in Autologous Chondrocyte Implantation (ACI) and Microfracture. Osteoarthr. Cartil 2007, 15 (12), 1397–1402. 10.1016/j.joca.2007.05.005. [DOI] [PubMed] [Google Scholar]
- (64).Antoine EE; Vlachos PP; Rylander MN Review of Collagen I Hydrogels for Bioengineered Tissue Microenvironments: Characterization of Mechanics, Structure, and Transport. Tissue Eng. Part B Rev 2014, 20 (6), 683–696. 10.1089/ten.teb.2014.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Lynch MP; Stein JL; Stein GS; Lian JB The Influence of Type I Collagen on the Development and Mainteance of the Osteoblast Phenotype in Primary and Passaged Rat Calvarial Osteoblast: Modification of Expression of Genes Supporting Cell Growth, Adhesion, and Extracellular Matrix Mineralization. Exp. Cell Res 1995, 216, 35–45. 10.1006/excr.1995.1005. [DOI] [PubMed] [Google Scholar]
- (66).Jin G; Kim H Efficacy of Collagen and Alginate Hydrogels for the Prevention of Rat Chondrocyte Dedifferentiation. J. Tissue Eng 2018, 9, 2041731418802438. 10.1177/2041731418802438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Rutgers M; Saris DB; Vonk LA; van Rijen MH; Akrum V; Langeveld D; van Boxtel A; Dhert WJ; Creemers LB Effect of Collagen Type I or Type II on Chondrogenesis by Cultured Human Articular Chondrocytes. Tissue Eng. Part A 2013, 19 (1–2), 59–65. 10.1089/ten.tea.2011.0416. [DOI] [PubMed] [Google Scholar]
- (68).Freyria A-M; Ronzière M-C; Cortial D; Galois L; Hartmann D; Herbage D; Mallein-Gerin F Comparative Phenotypic Analysis of Articular Chondrocytes Cultured within Type I or Type II Collagen Scaffolds. Tissue Eng. Part A 2009, 15 (6), 1233–1245. 10.1089/ten.tea.2008.0114. [DOI] [PubMed] [Google Scholar]
- (69).Nehrer S; Breinan HH; Ashkar S; Shortkroff S; Minas T; Sledge CB; Yannas IV; Spector M Characteristics of Articular Chondrocytes Seeded in Collagen Matrices in Vitro. Tissue Eng 1998, 4 (2), 175–183. 10.1089/ten.1998.4.175. [DOI] [Google Scholar]
- (70).Nehrer S; Breinan HA; Ramappa A; Shortkroff S; Young G; Minas T; Sledge CB; Yannas IV; Spector M Canine Chondrocytes Seeded in Type I and Type II Collagen Implants Investigated in Vitro. J. Biomed. Mater. Res 1997, 38 (2), 95–104. . [DOI] [PubMed] [Google Scholar]
- (71).Tamaddon M; Burrows M; Ferreira SA; Dazzi F; Apperley JF; Bradshaw A; Brand DD; Czernuszka J; Gentleman E Monomeric, Porous Type II Collagen Scaffolds Promote Chondrogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells in Vitro. Sci. Rep 2017, 7, 43519. 10.1038/srep43519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Chen CW; Tsai YH; Deng WP; Shih SN; Fang CL; Burch JG; Chen WH; Lai WF Type I and II Collagen Regulation of Chondrogenic Differentiation by Mesenchymal Progenitor Cells. J. Orthop. Res 2005, 23 (2), 446–453. 10.1016/j.orthres.2004.09.002. [DOI] [PubMed] [Google Scholar]
- (73).Giuliani N; Lisignoli G; Magnani M; Racano C; Bolzoni M; Dalla Palma B; Spolzino A; Manferdini C; Abati C; Toscani D; Facchini A; Aversa F New Insights into Osteogenic and Chondrogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells and Their Potential Clinical Applications for Bone Regeneration in Pediatric Orthopaedics. Stem Cells Int 2013, 2013, 312501. 10.1155/2013/312501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Jo A; Denduluri S; Zhang B; Wang Z; Yin L; Yan Z; Kang R; Shi LL; Mok J; Lee MJ; Haydon RC The Versatile Functions of Sox9 in Development, Stem Cells, and Human Diseases. Genes Dis 2014, 1 (2), 149–161. 10.1016/j.gendis.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Rucklidge GJ; Milne G; Robins SP Collagen Type X : A Component of the Surface of Normal Human, Pig, and Rat Articular Cartilage. 1996, 302 (224), 297–302. 10.1006/bbrc.1996.1024. [DOI] [PubMed] [Google Scholar]
- (76).Frenkel SR; Toolan B; Menche D; Pitman MI; Pachence JM Chondrocyte Transplantation Using a Collagen Bilayer Matrix for Cartilage Repair. J. Bone Joint Surg. Br 1997, 79 (5), 831–836. 10.1302/0301-620X.79B5.7278. [DOI] [PubMed] [Google Scholar]
- (77).Khan IM; Gilbert SJ; Singhrao SK; Duance VC; Archer CW Cartilage Integration: Evaluation of the Reasons for Failure of Integration during Cartilage Repair. A Review. Eur. Cells Mater 2008, 16, 26–39. 10.22203/eCM.v016a04. [DOI] [PubMed] [Google Scholar]
- (78).Deng J; She R; Huang W; Dong Z; Mo G; Liu B A Silk Fibroin/Chitosan Scaffold in Combination with Bone Marrow-Derived Mesenchymal Stem Cells to Repair Cartilage Defects in the Rabbit Knee. J. Mater. Sci. Mater. Med 2013, 24 (8), 2037–2046. 10.1007/s10856-013-4944-z. [DOI] [PubMed] [Google Scholar]
- (79).Yin H; Wang Y; Sun X; Cui G; Sun Z; Chen P; Xu Y; Yuan X; Meng H; Xu W; Wang A; Guo Q; Lu S; Peng J Functional Tissue-Engineered Microtissue Derived from Cartilage Extracellular Matrix for Articular Cartilage Regeneration. Acta Biomater 2018, 77, 127–141. 10.1016/j.actbio.2018.07.031. [DOI] [PubMed] [Google Scholar]
- (80).Wei X; Gao J; Messner K Maturation-Dependent Repair of Untreated Osteochondral Defects in the Rabbit Knee Joint. J. Biomed. Mater. Res 1997, 34 (1), 63–72. [DOI] [PubMed] [Google Scholar]
- (81).Salinas EY; Hu JC; Athanasious K A Guide for Using Mechanical Stimulation to Enhance Tissue-Engineered Articular Cartilage Properties. Tissue Eng. Part B Rev 2018, 24 (5), 345–358. 10.1089/ten.teb.2018.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


