Abstract
Depressive symptoms are more prevalent in persons with HIV (PWH) than HIV‐uninfected individuals. In HIV‐uninfected individuals, depression has been associated with atrophy in the hippocampus and other brain regions. In the present study, we investigated the impact of depression on brain structure in PWH. One hundred PWH participated in a cross‐sectional study (56.6 ± 6.4 yrs, range 41–70 yrs, 24 females, 63 African Americans). The Beck's Depression Inventory‐II (BDI‐II) was used to assess depressive symptoms. Structural MRI images were collected. Both the voxel‐based morphometry (VBM) technique and a region of interest (ROI) based approach were used to examine the relationship between hippocampal gray matter volume (GMv) and depressive symptoms. The impact of HIV CD4 nadir and antidepressants was also investigated. Both VBM and ROI approaches revealed that higher BDI‐II scores (implicating more severe depressive symptoms) were associated with loss of hippocampal GMv, especially in the right hippocampus and the right entorhinal cortex. Low CD4 nadir predicted additional hippocampal volume loss independent of depressive symptoms. Taking antidepressants did not have a detectable effect on hippocampal volume. In summary, having more depressive symptoms is associated with smaller hippocampal volume in PWH, and a history of severe immunosuppression (i.e., low CD4 nadir) correlates with additional hippocampal volume reduction. However, the impact of depression on hippocampal volume may be independent of HIV‐disease severity such as low CD4 nadir.
Keywords: atrophy, CD4, depression, hippocampus, HIV
In people with HIV infection, having more depressive symptoms is associated with smaller hippocampal volume. A history of severe immunosuppression (i.e., low CD4 nadir) correlates with additional hippocampal volume reduction. However, the impact of depression on hippocampal volume may be independent of HIV‐disease severity such as low CD4 nadir.
1. INTRODUCTION
Major depressive disorder (MDD) is a leading cause of disability worldwide (Ferrari et al., 2013). Theories of depression have proposed important roles of the frontal, limbic, and subcortical networks in MDD (Disner, Beevers, Haigh, & Beck, 2011; Jacobs, van Praag, & Gage, 2000; Price & Duman, 2020), including the amygdala, anterior cingulate cortex (ACC), medial temporal lobe (MTL) (especially the hippocampus), prefrontal cortex (PFC), striatum, and thalamus. Different modalities of brain imaging studies have provided converging evidence supporting the involvement of these brain regions and circuits in depression (Gray, Müller, Eickhoff, & Fox, 2020). Structural MRI is a standard procedure to detect and assess brain atrophy in both clinical practice and basic research. Using structural MRI techniques, numerous studies have shown that depression is associated with atrophy in the ACC, MTL, PFC, striatum, and thalamus (Bora, Fornito, Pantelis, & Yücel, 2012; Kempton et al., 2011; Koolschijn, van Haren, Lensvelt‐Mulders, Hulshoff Pol, & Kahn, 2009; Schmaal et al., 2016; Schmaal et al., 2017; Zhao et al., 2014). In particular, hippocampal volume loss has been the most frequently replicated brain imaging observation associated with depression (Arnone et al., 2016; Bora et al., 2012; Campbell, Marriott, Nahmias, & MacQueen, 2004; Kempton et al., 2011; Schmaal et al., 2016; Videbech & Ravnkilde, 2004).
In persons with HIV (PWH), the prevalence of lifetime MDD is approximately two to three times higher than in the general population (Rezaei et al., 2019). A recent comprehensive review estimates a prevalence of 24% for current MDD and 42% for lifetime MDD in PWH (Rubin & Maki, 2019). The high prevalence of depression is likely due to a combination of both psychosocial (e.g., early life stress, socioeconomical status, etc.) and biological factors (e.g., neurotoxicity due to HIV disease and antiretroviral treatment, chronic neuroinflammation, etc.) (Arseniou, Arvaniti, & Samakouri, 2014). Depression in PWH is linked to worse outcomes, including higher mortality rate (Ickovics et al., 2001; Pence et al., 2018), antiretroviral (ARV) nonadherence/virological failure (Gokhale et al., 2019), reduced quality of life (Rooney et al., 2019), cognitive impairment (Rubin & Maki, 2019) and steeper cognitive decline (Paolillo et al., 2020; Rubin & Maki, 2019). The most commonly affected cognitive domains are similar to those impacted by depression in the general population (Rock, Roiser, Riedel, & Blackwell, 2014), including executive function, learning and memory, processing speed, and motor function (Rubin & Maki, 2019). Several brain‐imaging studies have provided evidence supporting the involvement of the frontal, limbic, and subcortical regions in PWH with depression. Diffusion tensor imaging reveals increased fractional anisotropy (FA) in white matter in the frontal, temporal, cingulate, thalamus, striatum, and ventral tegmental area in PWH with depression (Smith et al., 2008; Stubbe‐Drger et al., 2012). Using a region‐of‐interest (ROI) based approach, three studies investigated the relationship between depression and the volume of several subcortical regions in PWH. Of the three studies, two reported null finding: one study reported no significant correlation between depressive symptoms and the volume of caudate or nucleus accumbens (Paul et al., 2005); the other study found no difference in basal ganglia volume between PWH with depression versus PWH without depression (Davison et al., 1997). In the third study, Kallianpur and colleagues investigated the relationship between depression and seven subcortical regions, including the caudate, hippocampus, nucleus accumbens, and other subcortical regions (Kallianpur et al., 2016). They found marginal negative correlations between depressive symptoms and the volume of the hippocampus (p = .053) and caudate (p = .046), but not the nucleus accumbens or any other regions (Kallianpur et al., 2016). Resting state functional connectivity technique revealed altered functional connectivity (FC) between regions within the frontal, limbic, and subcortical networks, especially the ACC and amygdala, in both chronic (McIntosh et al., 2018) and acute infection patients (Philippi et al., 2020). Data from positron emission tomography (PET) scan with ((11)C)DASB—a radiopharmaceutical for 5‐hydroxytryptamine transporter (5‐HTT)—suggests that synaptic serotonin levels are altered in PWH with depression (Hammoud et al., 2010). The association between altered serotonin levels and depression in PWH is further supported by the data from SIV‐infected rhesus macaques (Shah et al., 2019). However, overall brain imaging studies of depression in PWH have been limited and many questions remain to be addressed.
In this study, we aimed to investigate the relationship between depression and gray matter volume (GMv) in adults with HIV, using the voxel‐based morphometry (VBM) technique as well as an ROI‐based approach. The impact of antidepressants on hippocampal volume was investigated. In addition, we examined whether HIV clinical factors (i.e., current and nadir CD4 counts) affected brain structure by interacting with depression.
2. METHODS
2.1. Participants
One hundred and four PWH from the Washington, DC area were recruited for this study. All participants completed a brief phone screening and an hour‐long in‐person screening to determine eligibility (41–70 years old, at least 8 years of education, not claustrophobic, safe for MRI scan, no drug use in at least 3 months, no history of serious brain injury or psychotic disorders). During the in‐person screening visit and prior to enrollment, a written consent form, approved by the Georgetown University's Institutional Review Board, was obtained from each participant. Eligible participants completed (a) an extensive, seven‐domain, neuropsychological test battery (Blackstone et al., 2012; Hassanzadeh‐Behbahani et al., 2020); (b) a MRI scanning session; (c) a hospital visit for measurement of vital signs and blood counts of CD4, CD8, and HIV viral load; (d) self‐report measures of lifetime substance use, depression, mania, HIV history (including self‐reported lifetime nadir CD4 count), and current medications (including date of last use); and (e) an examination‐day urine drug screen. Previous studies have shown that self‐reported nadir CD4 is reliable and strongly correlated with available medical records (Buisker, Dufour, & Myers, 2015; Ellis et al., 2011). Two participants were excluded due to MRI abnormalities (McCune‐Albright Syndrome [n = 1], suspected benign tumor [n = 1]). The Beck's Depression Inventory (BDI‐II) total score was used to determine which participants had current depression (total BDI‐II ≥10, n = 30). The cut‐off score of 10 has been used by several other studies with clinical patient populations (Low & Hubley, 2006; Moullec, Plourde, Lavoie, Suarthana, & Bacon, 2015) and was chosen in the present study as previous studies suggest that hippocampal volume reduction may emerge early on, that is, while depressive symptoms are still rather mild (Kallianpur et al., 2016). In addition, we conducted a sensitivity analysis using a cut‐off score of 14 and obtained similar results. Two BDI‐II outliers (total BDI‐II > 3 SDs above the mean) were excluded, leaving a total sample of 100. Three participants were not able to provide a sufficient blood sample during the visit, so current CD4, CD8, and HIV viral load counts are based on 97 participants (current depression n = 29, without current depression n = 68). One participant (without current depression) did not remember his nadir CD4, so self‐report nadir CD4 group differences were calculated using data from 99 participants. One participant in the currently depressed group was not prescribed combination antiretroviral therapy (cART) medication and was left out of the cART adherence analysis (for a complete breakdown of ARV medications used by the participants, see Figure S1). As in our previous study (Hassanzadeh‐Behbahani et al., 2020), HAND status was determined for each participant based on performance on cognitive testing and the number of impairments reported on the Activities of Daily Living questionnaire. In order to determine the potential impact of antidepressant use on the relationship between depression and GMv, a list of generic and brand name antidepressants (57 total, Table S1) was crosschecked against each participant's self‐reported current medications.
2.2. Neuropsychological testing
All participants underwent a comprehensive neuropsychological assessment comprising 12 standardized tests that assessed seven neurocognitive domains, including speeded information processing, verbal fluency, learning, memory, executive function, working memory, and motor abilities (Table S2). Additionally, participants completed the Lawton and Brody Activities of Daily Living questionnaire (1969) in which they self‐reported any declines on everyday tasks (e.g., managing finances, managing medications, etc.). A global deficit score (GDS) was computed for each participant to determine neurocognitive impairment based on a previously published algorithm (Blackstone et al., 2012; Carey et al., 2004), with a higher GDS indicating a worse global neurocognitive function. The GDS and the daily function were then used for HAND diagnosis using the standard Frascati guideline (Antinori et al., 2007).
2.3. MRI acquisition
MR images were obtained at the Georgetown University Medical Center using a 3‐Tesla Siemens (Erlangen, Germany) Magnetom Trio with a 12‐channel head coil (n = 58) or a 3‐Tesla Siemens Prisma‐Fit scanner with a 20‐channel head coil (n = 42). The same MR collection parameters were used on both scanners to acquire high‐resolution T1‐weighted images (Siemens 3D‐MPRAGE): 1 mm3 isotropic resolution, TR/TE = 1900/2.52 ms, flip angle = 9°, 160 contiguous 1 mm sagittal slices, FoV = 256 mm (256 × 256 matrix). The effect of different scanners was modeled as a nuisance categorical variable for all statistical tests and additional analyses without the nuisance categorical variable did not change the determination of significance for any reported results. MRI, blood samples, and other data were collected at the same visit on the same day.
2.4. MRI preprocessing and data analysis
SPM12 (https://www.fil.ion.ucl.ac.uk/spm/) and the Computational Anatomy Toolbox (CAT12, version 12.5) (www.neuro.uni-jena.de/cat/) were used for preprocessing and analyzing MRI data using the default pipelines and parameters. Briefly, the pipeline for processing structural MR images in CAT12 includes bias‐field correction, spatial‐adaptive Non‐Local Means denoising, and simultaneous skull‐stripping (including adaptive maximum a posteriori estimation) and tissue type segmentation with partial volume estimation. VBM was performed to analyze group differences in GMv between subjects with and without current depression. In all MRI analyses, age, education (self‐reported number of years), sex at birth, race (grouped as African‐American or non‐African‐American), total intracranial volume (TIV), lifetime depression (grouped as greater than or less than 1 year), and MR scanner (see Section 2.3) were included as covariates of no interest. Similar results were observed when lifetime depression was simply coded as yes or no (regardless of duration). One participant was biracial (African American/Hispanic) and was coded as African American in this analysis. Voxelwise clusters that showed a significant difference in GMv (FWE cluster‐level correction p < .05, voxelwise threshold p < .001) were extracted for follow‐up analyses with antidepressant use, blood test results, and self‐reported nadir CD4.
Given that the hippocampus and other regions in the medial temporal lobe (MTL) have a complex shape and anatomical variability that complicates voxelwise alignment between subjects, high resolution subject‐specific parcellations of six MTL subregions (bilateral anterior hippocampus, posterior hippocampus, and entorhinal cortex) were obtained using the Automatic Segmentation of Hippocampal Subfields (ASHS) software package (https://www.nitrc.org/projects/ashs) (Yushkevich et al., 2015). The GMv of the six ROIs was extracted for additional between‐group comparison.
2.5. Data analysis
All statistical analyses other than VBM analyses were conducted in SPSS 25.0 (Chicago, IL) and included nuisance covariates for age, education, race, and sex. For analyses that included GMv, TIV and scanner were also included as nuisance variables. Two‐sample t‐tests were used to identify group differences in continuous variables (age, education, disease duration, and Total BDI‐II); whereas, chi‐square tests were used to test for group differences in categorical demographic variables (proportion male, African‐American, with detectable viral load, adhering to cART regimen within 2 days prior to visit, using anti‐depressants, with a history of alcohol, tobacco, and marijuana use). A significance threshold of p < .05 was used for all analyses of group differences. For non‐normally distributed demographic counts (current and nadir CD4), Wilcoxon rank sum tests were used to compare distributions between groups. Separate ANCOVAs modeled with linear effects were run to investigate the potential effect of education and lifetime depression as well as the impact of antidepressant use on the relationship between current depression and ROI GMv. Linear ANCOVAs were also used to test for differences in GMv within each of the six MTL subregions. Correction for multiple comparisons of the six MTL subregions output was performed using Benjamini and Hochberg FDR‐correction as implemented by the online SDM software library calculator (https://www.sdmproject.com/utilities/?show=FDR). Partial Pearson correlations were used to examine the relationship between current and nadir CD4 and ROI GMv. A Fisher's z‐test was conducted using an online calculator based on Eid et al.'s work to compare the correlation of nadir CD4 with right hippocampal GMv between groups (https://www.psychometrica.de/correlation.html#fisher). SPSS's Binary Logistic Regression model was used to calculate the odds ratios for having a detectable viral load due to current depression. A moderation analysis was run using SPSS PROCESS v3.4 and examined effect of nadir CD4 on the relationship between GMv and Total BDI‐II. For data visualization (Figures 1, 3, and 4, Figures S4 and S5), unstandardized residualized values for ROI GMv, MTL subregion GMvs, Total BDI‐II, and current and nadir CD4 were calculated after accounting for effects of nuisance covariates using SPSS's linear regression model without mean centering.
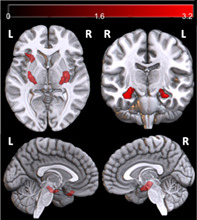
FIGURE 1.

Lower gray matter volume in participants with current depression. (a) Voxelwise locations of greater gray matter volume in nondepressed than currently depressed subjects, uncorrected p < .001, at least 300 contiguous voxels. Only the right hippocampal cluster (3,289 mm3) survived cluster‐level significance correction for multiple comparisons (FWE p = .038). (b) Residualized values for total BDI‐II and GMv of the right hippocampal cluster in Figure 1a. The scatter plot is for illustration purpose only
FIGURE 3.
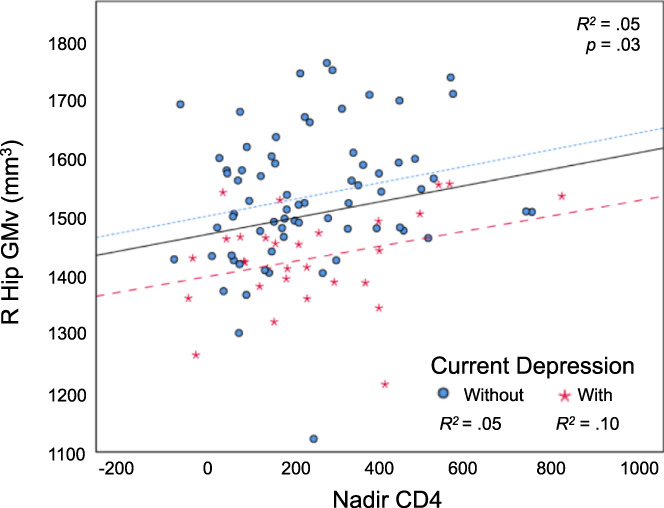
Nadir CD4 is correlated with right hippocampal gray matter volume. There was no significant difference between groups in correlation with nadir CD4 (with current depression: (r[22] = .44, n = 30, p = .03); without current depression: (r[61] = .27, n = 69, p = .03; z = 0.86, p = .20). Residualized values for nadir CD4 and GMv. R Hip GMv, the GMv of the right hippocampal cluster in Figure 1a. Fitted lines: all participants (black solid line), participants with current depression (red loosely dashed line), and participants without current depression (blue densely dashed line)
FIGURE 4.
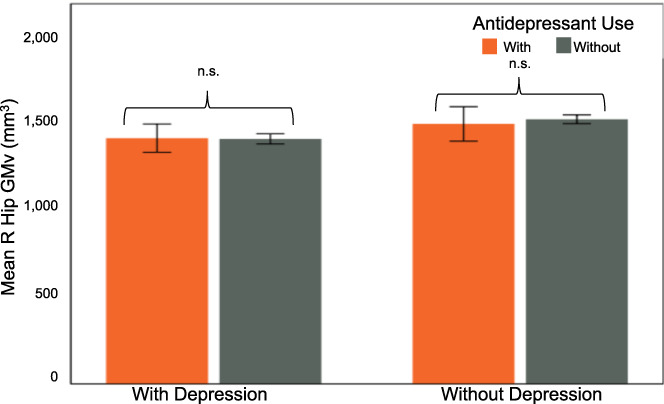
No difference in right hippocampal gray matter volume within groups based on antidepressant use. Residualized values for right hippocampal ROI gray matter volume. With current depression: F(1,22) = 0.43, p = .52. Without current depression: F(1,62) = 0.89, p = .35. Error bars show 95% CI
3. RESULTS
3.1. Demographics
No significant difference was observed between groups (with depression vs. without depression) for most demographics (age, sex, race) or any clinical (nadir CD4, estimated HIV disease duration, current CD4, having a detectable HIV viral load) factor. Additionally, there were no significant group differences in the proportion of participants adhering to their cART regimen or taking antidepressants. There was a significant difference in education between groups — those with current depression averaged 1.4 fewer years of education than those without current depression (p = .02). However, we conducted additional analysis to confirm that education did not affect the conclusion of the study (see below).
There was no significant difference in neurocognitive performance between the two groups (see Table S2 for a complete list), nor in the lifetime use of tobacco, alcohol, or marijuana (Table 1).
TABLE 1.
Demographics and clinical factors
| With depression | Without depression | n | p | |
|---|---|---|---|---|
| n = 30 | n = 70 | |||
| M (SD) | M (SD) | |||
| Age (yrs) | 56.6 (6.4) | 56.6 (6.4) | 100 | .98 |
| Education (yrs) | 13.3 (2.4) | 14.7 (3.1) | 100 | .02* |
| Male (%) | 73.3 | 77.1 | 100 | .68 |
| African American (%) | 66.7 | 61.4 | 100 | .62 |
| Nadir CD4 (cells/μL) a | 185.5 (2–850) | 200 (2–800) | 99 | .74 |
| HIV disease duration (yrs) | 28.1 (9.0) | 25.1 (9.3) | 100 | .13 |
| Current CD4 (cells/μL) a | 595 (167–1,093) | 732.5 (31–1,798) | 97 | .12 |
| Detectable VL (%) | 27.6 | 14.7 | 97 | .14 |
| cART adherence (%) | 96.6 | 97.1 | 99 | .88 |
| Using antideps (%) | 33.3 | 20.0 | 100 | .15 |
| Alcohol use (%) | 96.7 | 95.7 | 100 | .82 |
| Tobacco use (%) | 60.0 | 52.9 | 100 | .51 |
| Marijuana use (%) | 63.3 | 54.3 | 100 | .40 |
| With HAND (%) | 20.0 | 25.7 | 100 | .54 |
| Total BDI‐II | 14.4 (4.1) | 4.1 (2.7) | 100 | <.001** |
Note: Age = at last birthday; Education = full time years completed; Male = sex at birth; African American = participants who self‐identify as any part African American; Nadir CD4 = self‐reported lifetime nadir; Disease Duration = years since self‐reported year of first positive test; Current CD4 = obtained during visit; Detectable VL = plasma viral load (VL) of <20 copies/mL was considered undetectable; Alcohol/Tobacco/Marijuana Use = self‐reported use of the substance more than five times in one's lifetime; With HAND = participants diagnosed with HIV‐associated Neurocognitive Disorders; cART adherence = self‐reported use of combination antiretroviral therapy within 2 days prior to visit. p values are uncorrected.
Median and range.
p < .05.
p < .01.
3.2. Voxel‐based morphometry
Uncorrected VBM results revealed decreased GMv in bilateral hippocampus and left orbitofrontal cortex among those with current depression compared to those without (Figure 1a, Table S3). The right hippocampal cluster survived correction for multiple comparisons (clusterwise FWE p < .05) and GMv was extracted from this cluster for subsequent statistical analyses of demographic and clinical factors. Figure 1b shows a scatterplot of GMv of the right hippocampal cluster against the BDI‐II score (after controlling for age and other factors, see Methods). Within the subset of PWH with current depression, there was a marginal correlation between GMv of the right hippocampal cluster and the BDI‐II score (p = .054). The two BDI‐II‐based outliers (see Section 2.1) were removed for this analysis; however, including them did not change the determination of significance (Table S3). Additionally, education was not significantly correlated with GMv of the right hippocampal cluster (F[1,92] = 0.001, p = .98). For the GMv of the right hippocampal cluster, the difference between groups was not due to a history of lifetime depression (F[1,90] = .20, p = .66; Figure S2), and VBM produced similar results after removing history of lifetime depression as a covariate (data not shown).
3.3. MTL subregion gray matter volume
We analyzed GMv in six MTL subregions: bilateral anterior hippocampus, posterior hippocampus, and entorhinal cortex (ERC) (Figure S3). Only right ERC contained greater GMv in nondepressed compared to depressed subjects (F(1,93) = 11.76, p(unc.) = .001, p(FDR) = .006); though, right anterior hippocampus showed a similar trend (F(1,93) = 5.65, p(unc.) = .02, p(FDR) = .06, Figure 2). Left anterior hippocampus (r(93) = −.24, p(unc.) = .02, p(FDR) = .03) and right posterior hippocampus (r(93) = −.25, p(unc.) = .02, p(FDR) = .03) GMv each correlated with Total BDI‐II (Table S4) but did not show significant depression‐group differences (left anterior F(1,93) = 3.18, p(unc.) = .08; right posterior F(1,93) = 2.82, p(unc.) = .10).
FIGURE 2.

Lower gray matter volume in right ERC among participants with current depression. Gray matter volume (residualized values) in MTL subregions between currently depressed and not currently depressed participants. Right ERC had significantly less GMv for those with current depression. **FDR‐corrected p = .006. *uncorrected p = .02. Error bars show 95% CI
3.4. Nadir CD4
Nadir CD4 correlated with the right hippocampal cluster's GMv (Figure 3) (r[91] = .23, n = 100, p = .03; Figure 3) but not with total BDI‐II (p = .43; Figure S4) and did not have a moderating effect on the relationship between total BDI‐II and right hippocampal GMv (model summary: F(9,89) = 18.3, p < .001, R 2 = .65; interaction of total BDI‐II and nadir CD4 on right hippocampal GMv: ΔR 2 = .003, b = −0.01, t(89) = −0.92, p = .36).
3.5. Current CD4
There was no observed correlation between current CD4 and GMv of the right hippocampal cluster or total BDI‐II (Figure S5).
3.6. Detectable viral load
Most participants had an undetectable viral load (79/97 or 81.4%), and there was no significant group difference in observing a detectable viral load (with depression = 27.6%, without depression = 14.7%, X 2[1,97] = 2.23, p = .14). Including depression in a binary logistic regression model did not increase prediction of a detectable viral load compared to an intercept‐only model (overall model fit: X 2[5] = 3.68, p = .60; depression as a predictor: b = 0.66, Wald's X 2[1] = 1.44, p = 0.23, OR = 1.94 [95% CI: 0.66, 5.73]).
3.7. Impact of antidepressants
Twenty‐four participants were using antidepressants at the time of their visit (10 with vs. 14 without current depression). There was no significant interaction between the effects of current depression and antidepressant use on the GMv of the right hippocampal cluster (F[1,90] = 0.38, p = .54; Figure 4).
4. DISCUSSION
We examined the relationship between gray matter volume (GMv) and depression in adults with HIV using structural MRI techniques. These middle‐ to advanced‐aged, predominantly African American PWH participants (41–70 y.o.) were relatively healthy—the majority of them were on stable cART and had undetectable viral load (Table 1). Our results suggest that current depression is associated with loss of hippocampal GMv in PWH, and the association is not affected by whether or not they are currently taking an antidepressant medication. The effect might be stronger for the right hippocampus. In addition, low CD4 nadir is associated with further GMv reduction in the hippocampus, but there was no interaction between CD4 nadir and depression—suggesting the two factors may be independently associated with hippocampal GMv.
The hippocampus is a critical region affected in MDD and gray matter loss in hippocampus is frequently reported in depression studies within the general population (Arnone et al., 2016; Bora et al., 2012; Campbell et al., 2004; Kempton et al., 2011; Schmaal et al., 2016; Videbech & Ravnkilde, 2004), including in individuals with a first episode of major depression (Cole, Costafreda, McGuffin, & Fu, 2011). By contrast, the association between depression and hippocampal atrophy in PWH has not been well established. To the best of our knowledge, only one published study has reported a marginal effect of depression on hippocampal GMv in PWH (Kallianpur et al., 2016). In that study, a region‐of‐interest (ROI) based approach was used to examine the relationship between depression and GMv of the hippocampus and six other subcortical ROIs. A linear regression analysis (controlling for TIV) revealed a negative correlation between hippocampal GMv and total BDI‐II scores (β = −0.32, p = .053), suggesting a potential link between hippocampal atrophy and depression in PWH. By contrast, in the present study, using a voxelwise approach and a more stringent statistical threshold to control for false positives (FWE‐corrected at a cluster level), we provided further evidence supporting the association between depression and hippocampal GMv in PWH. However, only the right hippocampal cluster survived the correction for multiple comparisons (FWE at a cluster‐level), suggesting that the effect might be stronger in the right hippocampus, which is consistent with the findings of meta‐analysis studies within the general population (Arnone et al., 2016; Cole et al., 2011; Du et al., 2012; Santos, Bezerra, Carvalho, & Brainer‐Lima, 2018; Videbech & Ravnkilde, 2004). Moreover, using the ASHS software that is capable of segmenting hippocampal subfields, we investigated the relationship between depression and GMv of six hippocampal subfields (including bilateral entorhinal cortex, anterior hippocampus, and posterior hippocampus). Results suggest a strong effect in the right entorhinal cortex (and, to a lesser degree, the right anterior hippocampus) (Figure 2 and Table S3)—furthering support for a stronger association with depression in the right rather than the left hippocampus (Malykhin, Carter, Seres, & Coupland, 2010; Sawyer, Corsentino, Sachs‐Ericsson, & Steffens, 2012). The greater loss of GMv in the entorhinal cortex and the anterior hippocampus may reflect the functional specialization of these subfields (i.e., emotion and the anterior hippocampus; Poppenk, Evensmoen, Moscovitch, & Nadel, 2013) and may contribute to the high prevalence of learning/encoding impairment (compared to memory impairment) in PWH in the era of cART (Heaton et al., 2011).
Theories of depression have proposed a critical role of the hippocampus in depression (Disner et al., 2011; Jacobs et al., 2000; Price & Duman, 2020). Hippocampal volume has been shown to be predictive of response to antidepressants (Colle et al., 2018) and electroconvulsive therapy (Joshi et al., 2016), with smaller hippocampal volumes predicting a lower response rate. Based on the neuropil theory of depression, the loss of hippocampal volume in depression is mainly driven by a reduction in neuropil (Czéh & Lucassen, 2007), as shown in both stress‐based animal models (Lucassen et al., 2014) and human postmortem studies (Stockmeier et al., 2004) (which also revealed a decrease in pyramidal neuron soma size). Elevated levels of glucocorticoids as well as decreased levels of brain derived neurotrophic factor (BDNF) and other neurotropic factors are key factors responsible for the reduction in neuropil (Castrén, Võikar, & Rantamäki, 2007; Czéh & Lucassen, 2007). These neural mechanisms likely also underlie the loss of hippocampal volume in PWH with depression in the present study, at least partially. HIV‐disease can add additional injury to these complex systems, including HIV and ARV neurotoxicity, persisting chronic neuroinflammation, past and current immunosuppression, and common comorbidities in PWH (such as substance abuse). A combination of these factors can result in a loss of hippocampal volume (Jernigan et al., 2005; Kuhn et al., 2017; Pfefferbaum et al., 2014) and may contribute to the high prevalence of depression in PWH (Arseniou et al., 2014).
CD4 nadir is the lowest ever lymphocyte CD4 count and a strong predictor of neurocognitive impairment in PWH in the cART era (Ellis et al., 2011; Valcour et al., 2006). The impact of low CD4 nadir is generally referred to as an important “legacy event” effect. That is, the depth of immune suppression associated with low CD4 nadir may have caused irreversible neural injury that persists years later. We and others have demonstrated that low CD4 nadir is associated with cortical thinning as well as cortical and subcortical volume reduction (Cohen et al., 2010; Guha et al., 2016; Hassanzadeh‐Behbahani et al., 2020; Hua et al., 2013; Mac Duffie et al., 2018; Sanford et al., 2017), including in the hippocampus (Cohen et al., 2010; Gongvatana et al., 2014). In the present study, we also found a correlation between CD4 nadir and GMv of the right hippocampal cluster in both PWH with depression and PWH without depression. Critically, there is no interaction between CD4 nadir and depression in regards to hippocampal volume, suggesting that depression and low CD4 nadir may independently (and additively) affect the hippocampus and corresponding cognitive functions in PWH (Rubin & Maki, 2019).
Antidepressant medication is the current standard of treatment for MDD. By targeting important signaling pathways that are affected in MDD, antidepressant medication has shown to be effective in treating MDD (compared to a placebo) in many clinical trials. In addition, antidepressant treatment is shown to result in an increase in hippocampal volume in two studies – one in the anterior hippocampus (Arnone et al., 2013) and the other in the posterior hippocampus (Schermuly, Wolf, Lieb, Stoeter, & Fellgiebel, 2011), although other studies did not find such an effect (for a review, see Enneking, Leehr, Dannlowski, & Redlich, 2020). In the present study, taking antidepressants did not have a significant effect on hippocampal volume, in line with many other studies (Enneking et al., 2020). The lack of group difference in taking antidepressants could be explained by greater mood‐stabilizing effects of antidepressants in the without current depression group and the relatively short timeframe for depressive symptoms (2 weeks prior to completion) that the BDI‐II captures. In addition, another probable explanation is that the magnitude of benefits from antidepressants depends on the severity of baseline symptoms, with a greater benefit in patients with more severe depression versus a negligible to nonexistent benefit for those with mild or moderate symptoms (Fournier et al., 2010). Accordingly, in the present study, symptoms were rather mild in the majority of PWH in the depressed group (i.e., total BDI‐II≥10). Furthermore, as a cross‐sectional study, the present study was not designed to directly investigate the impact of antidepressants on hippocampal volume.
There are several limitations of this study. First, this is a cross‐sectional study without control participants and without longitudinal data, thus the actual relationship (i.e., causation) between depression and hippocampal volume remains to be investigated—as does the three‐way relationship among depression, hippocampal volume, and HIV‐disease. Future longitudinal studies with patients and controls are necessary to tackle this important question (ideally with both acute and chronic infection patients). Second, all participants in this study have chronic HIV‐disease (with a mean duration of 26 years). Therefore, it remains to be examined whether the association between depression and hippocampal atrophy is present or emerges in PWH with acute infection and if so, whether hippocampal volume reduction during acute infection is predictive of severity of future symptoms or response to antidepressants. This is an important question as depression is prevalent and affects known mood pathways in PWH with acute infection (Philippi et al., 2020) and hippocampal volume reduction is present in individuals with a first episode of major depression (Cole et al., 2011). Third, there are known gender differences in depression, including in neural mechanisms (Labonté et al., 2017) and prevalence (with a higher prevalence in females than in males in both the general population [Salk, Hyde, & Abramson, 2017] and in PWH [Jain et al., 2020]). In the present study, sex at birth was always included as a covariate, but we did not have a sufficient number of female participants to vigorously investigate the potential difference in gender. Fourth, in the present study, the definition of current depression is solely based on the BDI‐II total score. While the BDI‐II is a widely used depressive symptom inventory, and has been shown to correlate with MDD severity (Steer, Brown, Beck, & Sanderson, 2001), it is not explicitly designed as a diagnostic tool. Ideally, our study would be confirmed with a clinical diagnosis of major depressive disorder, which was not available or feasible in the present study. Therefore, the results in the present study should be interpreted with caution: our cutoff for depression was chosen to include those with mild depressive symptoms, as even mild symptoms of depression may be related to brain changes and the brain changes may make PWH susceptible to future worse depressive symptoms and potentially cognitive impairment. It remains to be tested whether greater HIV disease severity or a more severe presentation of depression would evidence a smaller hippocampus and/or more widespread hippocampal atrophy (i.e., in the posterior hippocampus). Fifth, even though the BDI‐II only assesses the depressive symptoms in the past 2 weeks, the symptoms might reflect a long‐lasting and chronic mood disturbance, as supported by the findings of hippocampal volume reduction in individuals with a first episode of major depression (Cole et al., 2011). However, a longitudinal study is needed to directly answer this question.
5. CONCLUSIONS
Current depression, regardless of antidepressant use, was associated with smaller hippocampal gray matter volume in PWH. Low CD4 nadir contributed to additional volume reduction in the hippocampus independent of depression.
CONFLICT OF INTEREST
The authors declare no competing financial interests.
DATA ANALYSIS
Statistical analysis conducted by Margarita Bronshteyn, Fan Nils Yang, Kyle F. Shattuck, Georgetown University Medical Center.
Supporting information
Appendix S1: Supporting Information
ACKNOWLEDGMENTS
We wish to thank all participants for their time and participation, Shiva Hassanzadeh‐Behbahani for help with data analysis, and the assistance for patient care from the Georgetown University Clinical Research Unit (GU‐CRU), which has been supported by Grant # UL1TR000101 (previously UL1RR031975) through the Clinical and Translational Science Awards Program (CTSA). The study is supported by award 1R01MH108466 (X.J.) from the National Institutes of Health.
Bronshteyn M, Yang FN, Shattuck KF, et al. Depression is associated with hippocampal volume loss in adults with HIV. Hum Brain Mapp. 2021;42:3750–3759. 10.1002/hbm.25451
Fan Nils Yang and Kyle F. Shattuck contributed equally to this work.
Funding information National Institutes of Health, Grant/Award Number: 1R01MH108466; Georgetown University Clinical Research Unit, Grant/Award Number: UL1TR000101
DATA AVAILABILITY STATEMENT
To protect patients’ privacy, deidentified data are available upon reasonable request.
REFERENCES
- Antinori, A. , Arendt, G. , Becker, J. T. , Brew, B. , Byrd, D. A. , Cherner, M. , … Wojna, V. E. (2007). Updated research nosology for HIV‐associated neurocognitive disorders. Neurology, 69, 1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone, D. , Job, D. , Selvaraj, S. , Abe, O. , Amico, F. , Cheng, Y. , … McIntosh, A. M. (2016). Computational meta‐analysis of statistical parametric maps in major depression. Human Brain Mapping, 37, 1393–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone, D. , McKie, S. , Elliott, R. , Juhasz, G. , Thomas, E. J. , Downey, D. , … Anderson, I. M. (2013). State‐dependent changes in hippocampal grey matter in depression. Molecular Psychiatry, 18, 1265–1272. [DOI] [PubMed] [Google Scholar]
- Arseniou, S. , Arvaniti, A. , & Samakouri, M. (2014). HIV infection and depression. Psychiatry and Clinical Neurosciences, 68, 96–109. [DOI] [PubMed] [Google Scholar]
- Blackstone, K. , Moore, D. J. , Franklin, D. R. , Clifford, D. B. , Collier, A. C. , Marra, C. M. , … Heaton, R. K. (2012). Defining neurocognitive impairment in HIV: Deficit scores versus clinical ratings. The Clinical Neuropsychologist, 26, 894–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora, E. , Fornito, A. , Pantelis, C. , & Yücel, M. (2012). Gray matter abnormalities in major depressive disorder: A meta‐analysis of voxel based morphometry studies. Journal of Affective Disorders, 138, 9–18. [DOI] [PubMed] [Google Scholar]
- Buisker, T. R. , Dufour, M.‐S. K. , & Myers, J. J. (2015). Recall of nadir CD4 cell count and most recent HIV viral load among HIV‐infected, socially marginalized adults. AIDS and Behavior, 19, 2108–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, S. , Marriott, M. , Nahmias, C. , & MacQueen, G. M. (2004). Lower hippocampal volume in patients suffering from depression: A meta‐analysis. The American Journal of Psychiatry, 161, 598–607. [DOI] [PubMed] [Google Scholar]
- Carey, C. L., Woods, S. P., Gonzalez, R., Conover, E., Marcotte, T. D., Grant, I., & Heaton, R. K. HNRC Group (2004): Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. Journal of Clinical and Experimental Neuropsychology 26, 307–319. [DOI] [PubMed] [Google Scholar]
- Castrén, E. , Võikar, V. , & Rantamäki, T. (2007). Role of neurotrophic factors in depression. Current Opinion in Pharmacology, 7, 18–21. [DOI] [PubMed] [Google Scholar]
- Cohen, R. A. , Harezlak, J. , Schifitto, G. , Hana, G. , Clark, U. , Gongvatana, A. , … Navia, B. (2010). Effects of nadir CD4 count and duration of human immunodeficiency virus infection on brain volumes in the highly active antiretroviral therapy era. Journal of Neurovirology, 16, 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, J. , Costafreda, S. G. , McGuffin, P. , & Fu, C. H. Y. (2011). Hippocampal atrophy in first episode depression: A meta‐analysis of magnetic resonance imaging studies. Journal of Affective Disorders, 134, 483–487. [DOI] [PubMed] [Google Scholar]
- Colle, R. , Dupong, I. , Colliot, O. , Deflesselle, E. , Hardy, P. , Falissard, B. , … Corruble, E. (2018). Smaller hippocampal volumes predict lower antidepressant response/remission rates in depressed patients: A meta‐analysis. World Journal of Biological Psychiatry, 19, 360–367. [DOI] [PubMed] [Google Scholar]
- Conover, E. , Marcotte, T. D. , Grant, I. , Heaton, R. K. , & HNRC Group (2004). Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. Journal of Clinical and Experimental Neuropsychology, 26, 307–319. [DOI] [PubMed] [Google Scholar]
- Czéh, B. , & Lucassen, P. J. (2007). What causes the hippocampal volume decrease in depression? Are neurogenesis, glial changes and apoptosis implicated? European Archives of Psychiatry and Clinical Neuroscience, 257, 250–260. [DOI] [PubMed] [Google Scholar]
- Davison, S. E. , Aylward, E. H. , McArthur, J. C. , Selnes, O. A. , Lyketsos, C. , Barta, P. E. , & Pearlson, G. D. (1997). A quantitative MRI study of the basal ganglia in depression in HIV seropositive men. Journal of Neuro‐AIDS, 1, 29–41. [DOI] [PubMed] [Google Scholar]
- Disner, S. G. , Beevers, C. G. , Haigh, E. A. P. , & Beck, A. T. (2011). Neural mechanisms of the cognitive model of depression. Nature Reviews. Neuroscience, 12, 467–477. [DOI] [PubMed] [Google Scholar]
- Du, M.‐Y. , Wu, Q.‐Z. , Yue, Q. , Li, J. , Liao, Y. , Kuang, W.‐H. , … Gong, Q.‐Y. (2012). Voxelwise meta‐analysis of gray matter reduction in major depressive disorder. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 36, 11–16. [DOI] [PubMed] [Google Scholar]
- Ellis, R. J. , Badiee, J. , Vaida, F. , Letendre, S. , Heaton, R. K. , Clifford, D. , … CHARTER Group . (2011). CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS (London, England), 25, 1747–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enneking, V. , Leehr, E. J. , Dannlowski, U. , & Redlich, R. (2020). Brain structural effects of treatments for depression and biomarkers of response: A systematic review of neuroimaging studies. Psychological Medicine, 50, 187–209. [DOI] [PubMed] [Google Scholar]
- Ferrari, A. J. , Charlson, F. J. , Norman, R. E. , Patten, S. B. , Freedman, G. , Murray, C. J. L. , … Whiteford, H. A. (2013). Burden of depressive disorders by country, sex, age, and year: Findings from the global burden of disease study 2010. PLoS Medicine, 10, e1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier, J. C. , DeRubeis, R. J. , Hollon, S. D. , Dimidjian, S. , Amsterdam, J. D. , Shelton, R. C. , & Fawcett, J. (2010). Antidepressant drug effects and depression severity: A patient‐level meta‐analysis. JAMA, 303, 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokhale, R. H. , Weiser, J. , Sullivan, P. S. , Luo, Q. , Shu, F. , & Bradley, H. (2019). Depression prevalence, antidepressant treatment status, and association with sustained HIV viral suppression among adults living with HIV in care in the United States, 2009–2014. AIDS and Behavior, 23, 3452–3459. [DOI] [PubMed] [Google Scholar]
- Gongvatana, A. , Correia, S. , Dunsiger, S. , Gauthier, L. , Devlin, K. N. , Ross, S. , … Cohen, R. A. (2014). Plasma cytokine levels are related to brain volumes in HIV‐infected individuals. Journal of Neuroimmune Pharmacology, 9, 740–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray, J. P. , Müller, V. I. , Eickhoff, S. B. , & Fox, P. T. (2020). Multimodal abnormalities of brain structure and function in major depressive disorder: A meta‐analysis of neuroimaging studies. The American Journal of Psychiatry, 177, 422–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha, A. , Brier, M. R. , Ortega, M. , Westerhaus, E. , Nelson, B. , & Ances, B. M. (2016). Topographies of cortical and subcortical volume loss in HIV and aging in the cART era. Journal of Acquired Immune Deficiency Syndromes 1999, 73, 374–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammoud, D. A. , Endres, C. J. , Hammond, E. , Uzuner, O. , Brown, A. , Nath, A. , … Pomper, M. G. (2010). Imaging serotonergic transmission with [11C]DASB‐PET in depressed and non‐depressed patients infected with HIV. NeuroImage, 49, 2588–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassanzadeh‐Behbahani, S. , Shattuck, K. F. , Bronshteyn, M. , Dawson, M. , Diaz, M. , Kumar, P. , … Jiang, X. (2020). Low CD4 nadir linked to widespread cortical thinning in adults living with HIV. NeuroImage: Clinical, 25, 102155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton, R. K. , Franklin, D. R. , Ellis, R. J. , McCutchan, J. A. , Letendre, S. L. , Leblanc, S. , … CHARTER Group, HNRC Group . (2011). HIV‐associated neurocognitive disorders before and during the era of combination antiretroviral therapy: Differences in rates, nature, and predictors. Journal of Neurovirology, 17, 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua, X. , Boyle, C. P. , Harezlak, J. , Tate, D. F. , Yiannoutsos, C. T. , Cohen, R. , … HIV Neuroimaging Consortium . (2013). Disrupted cerebral metabolite levels and lower nadir CD4 + counts are linked to brain volume deficits in 210 HIV‐infected patients on stable treatment. NeuroImage: Clinical, 3, 132–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ickovics, J. R. , Hamburger, M. E. , Vlahov, D. , Schoenbaum, E. E. , Schuman, P. , Boland, R. J. , … HIV Epidemiology Research Study Group . (2001). Mortality, CD4 cell count decline, and depressive symptoms among HIV‐seropositive women: Longitudinal analysis from the HIV epidemiology research study. JAMA, 285, 1466–1474. [DOI] [PubMed] [Google Scholar]
- Jacobs, B. L. , van Praag, H. , & Gage, F. H. (2000). Adult brain neurogenesis and psychiatry: A novel theory of depression. Molecular Psychiatry, 5, 262–269. [DOI] [PubMed] [Google Scholar]
- Jain, M. K. , Li, X. , Adams‐Huet, B. , Tiruneh, Y. M. , Luque, A. E. , Duarte, P. , … Nijhawan, A. E. (2020). The risk of depression among racially diverse people living with HIV: The impact of HIV viral suppression. AIDS Care, 1–9. [DOI] [PubMed] [Google Scholar]
- Jernigan, T. L. , Gamst, A. C. , Archibald, S. L. , Fennema‐Notestine, C. , Mindt, M. R. , Marcotte, T. D. , … Grant, I. (2005). Effects of methamphetamine dependence and HIV infection on cerebral morphology. The American Journal of Psychiatry, 162, 1461–1472. [DOI] [PubMed] [Google Scholar]
- Joshi, S. H. , Espinoza, R. T. , Pirnia, T. , Shi, J. , Wang, Y. , Ayers, B. , … Narr, K. L. (2016). Structural plasticity of the hippocampus and amygdala induced by electroconvulsive therapy in major depression. Biological Psychiatry, 79, 282–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallianpur, K. J. , Sakoda, M. , Gangcuangco, L. M. A. , Ndhlovu, L. C. , Umaki, T. , Chow, D. , … Shikuma, C. M. (2016). Frailty characteristics in chronic HIV patients are markers of white matter atrophy independently of age and depressive symptoms: A pilot study. Open Medical Journal, 3, 138–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempton, M. J. , Salvador, Z. , Munafò, M. R. , Geddes, J. R. , Simmons, A. , Frangou, S. , & Williams, S. C. R. (2011). Structural neuroimaging studies in major depressive disorder. Meta‐analysis and comparison with bipolar disorder. Archives of General Psychiatry, 68, 675–690. [DOI] [PubMed] [Google Scholar]
- Koolschijn, P. C. M. P. , van Haren, N. E. M. , Lensvelt‐Mulders, G. J. L. M. , Hulshoff Pol, H. E. , & Kahn, R. S. (2009). Brain volume abnormalities in major depressive disorder: A meta‐analysis of magnetic resonance imaging studies. Human Brain Mapping, 30, 3719–3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn, T. , Schonfeld, D. , Sayegh, P. , Arentoft, A. , Jones, J. D. , Hinkin, C. H. , … Thames, A. D. (2017). The effects of HIV and aging on subcortical shape alterations: A 3D morphometric study. Human Brain Mapping, 38, 1025–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labonté, B. , Engmann, O. , Purushothaman, I. , Menard, C. , Wang, J. , Tan, C. , … Nestler, E. J. (2017). Sex‐specific transcriptional signatures in human depression. Nature Medicine, 23, 1102–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low, G. D. , & Hubley, A. M. (2006). Screening for depression after cardiac events using the Beck depression inventory‐II and the geriatric depression scale. Social Indicators Research, 82, 527. [Google Scholar]
- Lucassen, P. J. , Pruessner, J. , Sousa, N. , Almeida, O. F. X. , Van Dam, A. M. , Rajkowska, G. , … Czéh, B. (2014). Neuropathology of stress. Acta Neuropathologica (Berl), 127, 109–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Duffie, K. E. , Brown, G. G. , Mc Kenna, B. S. , Liu, T. T. , Meloy, M. J. , Tawa, B. , … TMARC Group . (2018). Effects of HIV infection, methamphetamine dependence and age on cortical thickness, area and volume. NeuroImage: Clinical, 20, 1044–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malykhin, N. V. , Carter, R. , Seres, P. , & Coupland, N. J. (2010). Structural changes in the hippocampus in major depressive disorder: Contributions of disease and treatment. Journal of Psychiatry & Neuroscience—JPN, 35, 337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh, R. C. , Paul, R. , Ndhlovu, L. C. , Hidalgo, M. , Lobo, J. D. , Walker, M. , … Kallianpur, K. J. (2018). Resting‐state connectivity and spontaneous activity of ventromedial prefrontal cortex predict depressive symptomology and peripheral inflammation in HIV. Journal of Neurovirology, 24, 616–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moullec, G. , Plourde, A. , Lavoie, K. L. , Suarthana, E. , & Bacon, S. L. (2015). Beck depression inventory II: Determination and comparison of its diagnostic accuracy in cardiac outpatients. European Journal of Preventive Cardiology, 22, 665–672. [DOI] [PubMed] [Google Scholar]
- Paolillo, E. W. , Pasipanodya, E. C. , Moore, R. C. , Pence, B. W. , Atkinson, J. H. , Grelotti, D. J. , … Moore, D. J. (2020). Cumulative burden of depression and neurocognitive decline among persons with HIV: A longitudinal study. Journal of Acquired Immune Deficiency Syndromes 1999, 84, 304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul, R. H. , Brickman, A. M. , Navia, B. , Hinkin, C. , Malloy, P. F. , Jefferson, A. L. , … Flanigan, T. P. (2005). Apathy is associated with volume of the nucleus accumbens in patients infected with HIV. The Journal of Neuropsychiatry and Clinical Neurosciences, 17, 167–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pence, B. W. , Mills, J. C. , Bengtson, A. M. , Gaynes, B. N. , Breger, T. L. , Cook, R. L. , … Mugavero, M. J. (2018). Association of increased chronicity of depression with HIV appointment attendance, treatment failure, and mortality among HIV‐infected adults in the United States. JAMA Psychiatry, 75, 379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum, A. , Rogosa, D. A. , Rosenbloom, M. J. , Chu, W. , Sassoon, S. A. , Kemper, C. A. , … Sullivan, E. V. (2014). Accelerated aging of selective brain structures in human immunodeficiency virus infection: A controlled, longitudinal magnetic resonance imaging study. Neurobiology of Aging, 35, 1755–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippi, C. L. , Reyna, L. , Nedderman, L. , Chan, P. , Samboju, V. , Chang, K. , … SEARCH 010/RV254 and RV304/SEARCH 013 study teams . (2020). Resting‐state neural signatures of depressive symptoms in acute HIV. Journal of Neurovirology, 26, 226–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppenk, J. , Evensmoen, H. R. , Moscovitch, M. , & Nadel, L. (2013). Long‐axis specialization of the human hippocampus. Trends in Cognitive Sciences, 17, 230–240. [DOI] [PubMed] [Google Scholar]
- Price, R. B. , & Duman, R. (2020). Neuroplasticity in cognitive and psychological mechanisms of depression: An integrative model. Molecular Psychiatry, 25, 530–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaei, S. , Ahmadi, S. , Rahmati, J. , Hosseinifard, H. , Dehnad, A. , Aryankhesal, A. , … Ghashghaee, A. (2019). Global prevalence of depression in HIV/AIDS: A systematic review and meta‐analysis. BMJ Supportive & Palliative Care, 9, 404–412. [DOI] [PubMed] [Google Scholar]
- Rock, P. L. , Roiser, J. P. , Riedel, W. J. , & Blackwell, A. D. (2014). Cognitive impairment in depression: A systematic review and meta‐analysis. Psychological Medicine, 44, 2029–2040. [DOI] [PubMed] [Google Scholar]
- Rooney, A. S. , Moore, R. C. , Paolillo, E. W. , Gouaux, B. , Umlauf, A. , Letendre, S. L. , … HIV Neurobehavioral Research Program . (2019). Depression and aging with HIV: Associations with health‐related quality of life and positive psychological factors. Journal of Affective Disorders, 251, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin, L. H. , & Maki, P. M. (2019). HIV, depression, and cognitive impairment in the era of effective antiretroviral therapy. Current HIV/AIDS Reports, 16, 82–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salk, R. H. , Hyde, J. S. , & Abramson, L. Y. (2017). Gender differences in depression in representative national samples: Meta‐analyses of diagnoses and symptoms. Psychological Bulletin, 143, 783–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford, R. , Fernandez Cruz, A. L. , Scott, S. C. , Mayo, N. E. , Fellows, L. K. , Ances, B. M. , & Collins, D. L. (2017). Regionally specific brain volumetric and cortical thickness changes in HIV‐infected patients in the HAART era. Journal of Acquired Immune Deficiency Syndromes 1999, 74, 563–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos, M. A. O. , Bezerra, L. S. , Carvalho, A. R. M. R. , & Brainer‐Lima, A. M. (2018). Global hippocampal atrophy in major depressive disorder: A meta‐analysis of magnetic resonance imaging studies. Trends in Psychiatry and Psychotherapy, 40, 369–378. [DOI] [PubMed] [Google Scholar]
- Sawyer, K. , Corsentino, E. , Sachs‐Ericsson, N. , & Steffens, D. C. (2012). Depression, hippocampal volume changes, and cognitive decline in a clinical sample of older depressed outpatients and non‐depressed controls. Aging & Mental Health, 16, 753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schermuly, I. , Wolf, D. , Lieb, K. , Stoeter, P. , & Fellgiebel, A. (2011). State dependent posterior hippocampal volume increases in patients with major depressive disorder. Journal of Affective Disorders, 135, 405–409. [DOI] [PubMed] [Google Scholar]
- Schmaal, L. , Hibar, D. P. , Sämann, P. G. , Hall, G. B. , Baune, B. T. , Jahanshad, N. , … Veltman, D. J. (2017). Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA major depressive disorder working group. Molecular Psychiatry, 22, 900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal, L. , Veltman, D. J. , van Erp, T. G. M. , Sämann, P. G. , Frodl, T. , Jahanshad, N. , … Hibar, D. P. (2016). Subcortical brain alterations in major depressive disorder: Findings from the ENIGMA major depressive disorder working group. Molecular Psychiatry, 21, 806–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah, S. , Sinharay, S. , Matsuda, K. , Schreiber‐Stainthorp, W. , Muthusamy, S. , Lee, D. , … Hammoud, D. A. (2019). Potential mechanism for HIV‐associated depression: Upregulation of serotonin transporters in SIV‐infected macaques detected by 11C‐DASB PET. Frontiers in Psychiatry, 10, 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, C. A. , Stebbins, G. T. , Bartt, R. E. , Kessler, H. A. , Adeyemi, O. M. , Martin, E. , … Moseley, M. E. (2008). White matter anisotropy and depression symptoms in patients with HIV. The Journal of Neuropsychiatry and Clinical Neurosciences, 20, 458–465. [DOI] [PubMed] [Google Scholar]
- Steer, R. A. , Brown, G. K. , Beck, A. T. , & Sanderson, W. C. (2001). Mean Beck depression inventory‐II scores by severity of major depressive episode. Psychological Reports, 88, 1075–1076. [DOI] [PubMed] [Google Scholar]
- Stockmeier, C. A. , Mahajan, G. J. , Konick, L. C. , Overholser, J. C. , Jurjus, G. J. , Meltzer, H. Y. , … Rajkowska, G. (2004). Cellular changes in the postmortem hippocampus in major depression. Biological Psychiatry, 56, 640–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbe‐Drger, B. , Deppe, M. , Mohammadi, S. , Keller, S. S. , Kugel, H. , Gregor, N. , … German Competence Network HIV/AIDS . (2012). Early microstructural white matter changes in patients with HIV: A diffusion tensor imaging study. BMC Neurology, 12, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour, V. , Yee, P. , Williams, A. E. , Shiramizu, B. , Watters, M. , Selnes, O. , … Sacktor, N. (2006). Lowest ever CD4 lymphocyte count (CD4 nadir) as a predictor of current cognitive and neurological status in human immunodeficiency virus type 1 infection—The Hawaii Aging with HIV Cohort. Journal of Neurovirology, 12, 387–391. [DOI] [PubMed] [Google Scholar]
- Videbech, P. , & Ravnkilde, B. (2004). Hippocampal volume and depression: A meta‐analysis of MRI studies. The American Journal of Psychiatry, 161, 1957–1966. [DOI] [PubMed] [Google Scholar]
- Yushkevich, P. A. , Pluta, J. B. , Wang, H. , Xie, L. , Ding, S.‐L. , Gertje, E. C. , … Wolk, D. A. (2015). Automated volumetry and regional thickness analysis of hippocampal subfields and medial temporal cortical structures in mild cognitive impairment. Human Brain Mapping, 36, 258–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Y.‐J. , Du, M.‐Y. , Huang, X.‐Q. , Lui, S. , Chen, Z.‐Q. , Liu, J. , … Gong, Q.‐Y. (2014). Brain grey matter abnormalities in medication‐free patients with major depressive disorder: A meta‐analysis. Psychological Medicine, 44, 2927–2937. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information
Data Availability Statement
To protect patients’ privacy, deidentified data are available upon reasonable request.


