ABSTRACT
Dengue viruses (DENV) and Zika virus (ZIKV) are related mosquito-borne flaviviruses with similar disease manifestations, vector ecologies, and geographic ranges. The ability to differentiate these viruses serologically is vital due to the teratogenic nature of ZIKV and the potential confounding of preexisting cross-reactive anti-DENV antibodies. Here, we illustrate the kinetics of the IgM neutralizing antibody (NAb) response using longitudinal samples ranging from acute ZIKV infection to late convalescence from individuals with evidence of prior DENV infection. By serially depleting antibody isotypes prior to the neutralization assay, we determined that IgM contributes predominantly to ZIKV neutralization and is less cross-reactive than the IgG NAb. The IgM NAb peaked around 14 days (95% confidence interval [95% CI], 13 to 15) and had a median duration of 257 days (95% CI, 133 to 427). These results demonstrate the persistence of IgM NAb after ZIKV infection and imply its potential role in diagnosis, vaccine evaluation, serosurveillance, and research on flavivirus-host interactions.
KEYWORDS: Zika virus, dengue virus, IgM neutralization, serology diagnostics
INTRODUCTION
After the introduction of Zika virus (ZIKV) to the Western Hemisphere in 2015 and the large epidemic throughout Central and South America, the virus was linked to several thousand cases of birth defects, miscarriages, and stillbirths in several countries (1–6). Given the potential severity of ZIKV infections in pregnant women, definitive differentiation of ZIKV from closely related flaviviruses like dengue viruses (DENVs) is crucial. However, this is difficult in individuals with previous DENV exposure, which induces long-lasting broadly cross-reactive antibodies (Abs) (7–10). Coupled with the expansion of multiple vaccination trials for DENV and ZIKV, as well as a recently licensed tetravalent DENV vaccine (11), highly specific serodiagnostic assays are essential, particularly in regions where the viruses are known to cocirculate (12, 13).
Definitive diagnosis of ZIKV depends on the identification of either ZIKV RNA or ZIKV-specific Abs. Virus-binding IgM in bodily fluids can indicate a recent infection, as IgM usually develops quickly but with limited duration after infection, followed by long-lasting IgG. However, IgM induced by some flaviviruses, including ZIKV, can also be long lasting (>12 months) (14–19). Previous studies in humans and nonhuman primates indicate similar kinetics of ZIKV-binding IgM following ZIKV infection with or without prior DENV exposure (18, 20). IgM in primary DENV infection appears within 5 days of symptom onset and peaks at around 2 weeks before declining to undetectable levels approximately 6 months later (21). In contrast, highly cross-reactive IgG makes up the bulk of the humoral immune response after secondary DENV (sequential DENV/DENV) infections and can be detected within a few days of acute infection, with a marked increase during early convalescence (22–24). Similarly to secondary DENV infection, sequential DENV/ZIKV infections also resulted in significantly earlier and higher IgG levels than did naive DENV and primary ZIKV infections (18, 19). This indicated that the anamnestic cross-reactive DENV and ZIKV Abs may largely be IgG.
While previous studies focused on the kinetics and specificities of the virus-binding IgM and IgG in ZIKV and DENV infections as determined by enzyme-linked immunosorbent assay (ELISA), this study reports our findings on the kinetics and specificities of virus-neutralizing Abs (NAbs), especially IgM NAb, in ZIKV infections. As NAbs are typically more specific than virus-binding Abs, virus neutralization assays have been used for confirmatory diagnosis among flaviviruses. NAb measurement is also critical in evaluating flaviviral vaccines, as its level usually correlates with protection (25–28). However, the NAbs arising from most sequential DENV/ZIKV infections tend to have highly cross-reactive NAb to the prior infecting virus (DENV), compromising this assay’s accuracy in confirmatory diagnosis when only one serum sample may be available for testing. In sequential DENV/DENV or DENV/ZIKV infections, ELISA measurement of IgM and IgG to both viruses from paired (acute and convalescent) samples are required for more accurate diagnosis of the recent infection, but paired samples collected at the correct times are rare, as most patients are likely to seek medical help after the acute infection phase.
Previously, we studied the effect of IgG depletion from clinical samples prior to the neutralization assay to aid in differential diagnosis of DENVs and ZIKV (29). Cross-reactive NAb could be significantly reduced in 15% to 76% of samples, depending on the timing of serum collection, simply by removing IgG. In several instances, cross-reactivity was completely ablated after IgG depletion. Several questions arose from that study, including these: Is IgM the major isotype with neutralization activity after the removal of IgG? How long does ZIKV-neutralizing IgM last after acute infection? When does ZIKV-neutralizing IgM peak? What are the levels of cross-reactive neutralizing IgM in individuals with previous DENV exposure? Here, we investigate the kinetics and specificities of NAbs from longitudinal plasma samples (n = 118) from 17 sequential DENV/ZIKV infection subjects using specific Ab isotype depletions prior to implementing a chimeric reporter virus-based microfocus reduction neutralization test (R-mFRNT) against ZIKV and two DENVs.
MATERIALS AND METHODS
Viruses and cells.
Vero cells were grown and maintained in Dulbecco modified Eagle medium (DMEM) supplemented with penicillin-streptomycin, l-glutamine, and fetal bovine serum (FBS). Live reporter chimeric viruses, R-WN/ZIKV, R-WN/DENV1, and R-WN/DENV2, expressing the premembrane (prM) and envelope (E) proteins of ZIKV PRVABC59, DENV1 16007, or DENV2 16681 and a ZsGreen reporter in a West Nile virus (WNV) replication vector were generated at the Division of Vector-Borne Diseases, Centers for Disease Control and Prevention (DVBD-CDC) (30, 31). Stocks of DENV1 strains 16007 and West Pac74 were obtained from the Reference Collection at DVBD-CDC and grown in Vero cells.
Diagnostic specimens.
We obtained ethics approval to use human specimens previously collected in the Recipient Epidemiology and Donor Evaluation Study III (REDS-III) US Zika Natural History Study (CDC IRB number 7088). Plasma samples from blood donors in Puerto Rico were screened with ZIKV nucleic acid tests (NATs) and serology tests from April to December 2016, coinciding with the ZIKV epidemic in the region (18, 32). During that year, 39,717 cases of ZIKV infections were reported to the Puerto Rico Department of Health. In contrast, only 198 cases of DENV infection were reported, 91 of which were confirmed cases by reverse transcription (RT)-PCR, while 107 cases were suspected. Most of these DENV cases were reported in the early part of 2016, and DENV cases were historically low compared to data from previous years. No cases of DENV/ZIKV coinfections were identified in 2016. While the existence of a DENV/ZIKV coinfection cannot be ruled out, the epidemiological data suggest that DENV circulation was low and a coinfection was not likely during that time.
Selected individuals with previous DENV exposure were confirmed by positive DENV IgG ELISA at index, and only individuals with an acute ZIKV infection at index confirmed by a positive ZIKV NAT were included. To capture the initial time of ZIKV IgM seroconversion, only subjects that showed seroconversion after index were selected for the study. Among the entire 53 subjects enrolled in the Zika Natural History Study, 17 individuals who had had longitudinal samples collected (5 to 7 samples per individual; total of 118 samples), ranging from index to a median of 362 days (minimum of 163 to maximum of 399 days) postindex (PI), met our criteria to be included.
Specific Ab isotype depletion.
IgG was depleted from plasma samples diluted 1:10 in BA1 buffer (Hanks M-199 salts without glutamine, 0.05 M Tris, pH 7.6, 1% bovine serum albumin, 2 mM L-glutamine, 0.35 g/liter sodium bicarbonate, 100 U/ml penicillin, 100 mg/liter streptomycin, 1 mg/liter Fungizone) by adsorption with protein G-Sepharose fast flow beads (GE Healthcare, Chicago, IL) overnight at 4°C (29). One of every 12 sample pairs (before and after depletion) was randomly selected to confirm the efficiency of IgG depletion by using a quantitative human IgG ELISA kit (Innovative Research, Inc., Novi, MI). For dual IgG/IgM (IgG/M) or IgG/IgA (IgG/A) depletion, IgG-depleted samples were further adsorbed by goat anti-human IgM or goat anti-human IgA conjugated to agarose (200 to 300 μg/sample) (Millipore Sigma, Burlington MA). The percentage of IgM or IgA depletion was determined using a quantitative human IgM or IgA ELISA kit (Innovative Research, Inc., Novi, MI).
Live R-mFRNT.
Live reporter chimeric viruses, described above, were used in the reporter virus-based microneutralization test, R-mFRNT (25). These chimeric reporter viruses express strong reporter signals in Vero cells quickly, permitting a fast and high-throughput assay for all three viruses for each sample simultaneously. Briefly, nondepleted (diluted 1:20) and depleted (diluted 1:10) samples were heat inactivated at 56°C for 30 min. Further 2-fold dilution series of the heat-inactivated specimens were mixed with equal volumes of R-WN/DENV1, R-WN/DENV2, or R-WN/ZIKV diluted in medium containing 8% naive human serum (providing standardized labile serum factor) and incubated for 1 h at 37°C and 5% CO2. Plasma/virus mixtures (30 μl/well containing 100 to 200 focus forming units [FFU] of reporter virus) were transferred in triplicate to confluent Vero cells in 96-well, black-wall plates with clear flat bottoms (Greiner). Virus was adsorbed for 90 min at 37°C and 5% CO2 before the addition of Gibco FluorBrite DMEM (ThermoFisher, Franklin, MA) without FBS (150 μl/well). After a 24- to 28-h incubation at 37°C and 5% CO2, the plates were live imaged for automatic measurement of fluorescent FFU using a Celigo image cytometer (Nexcelom, Lawrence, MA). The input reporter virus was back titrated by 2-fold serial dilutions in six replicates for each experiment. The virus neutralization curves were generated by a 4-parameter nonlinear regression dose response to calculate the effective concentration for 90% neutralization (EC90), using GraphPad Prism software version 6.
PRNT.
A traditional plaque reduction neutralization test (PRNT) was performed using DENV1 strains 16007 and West Pac74 in 6-well Vero cell plates, as previously described (29). The PRNT results were also determined by virus neutralization curve using GraphPad as described above to measure EC90 (PRNT90).
Statistical analysis.
The NAb kinetic curves were modeled with a spline using a B-spline basis and generalized estimating equations (GEE) to account for within-sample correlated data over time. The peak titers and 95% confidence intervals (95% CIs) were calculated from the spline GEE model using bootstrapping and estimating maximum fitted values from each iteration. The two-tailed paired t test or two-way analysis of variance (ANOVA) with Tukey’s multiple comparison was used to compare the NAb titers against two strains of DENV1 in PRNT and the titers before and after Ab isotype serial depletions. ZIKV-neutralizing IgM seroreversion was estimated using a lognormal survival model, with the day of initial NAT detectability imputed based on ZIKV RNA doubling time during the viremia ramp-up phase in macaque plasma samples (32). The survival, geepack, and emmeans packages were utilized in R software (version 4.0.1).
RESULTS
To better understand the ZIKV NAb response, we tested longitudinal samples from 17 ZIKV-infected individuals with previous DENV exposure in neutralization assays before and after IgG depletion. Nondepleted and depleted samples were tested for NAbs to DENV1, DENV2, and ZIKV. Only DENV1 and DENV2 were tested to measure cross-reactive NAbs, since all four DENV serotypes are highly cross-reactive to each other, and this cross-reactivity can be demonstrated by testing two or more DENV serotypes. Changes in endpoint titers of NAbs to all three viruses were compared before and after IgG depletion to evaluate the levels of ZIKV-specific NAb in these individuals. In this study, the percentage of IgG depletion averaged 99.6% (±0.43% standard deviation [SD]) among the tested samples.
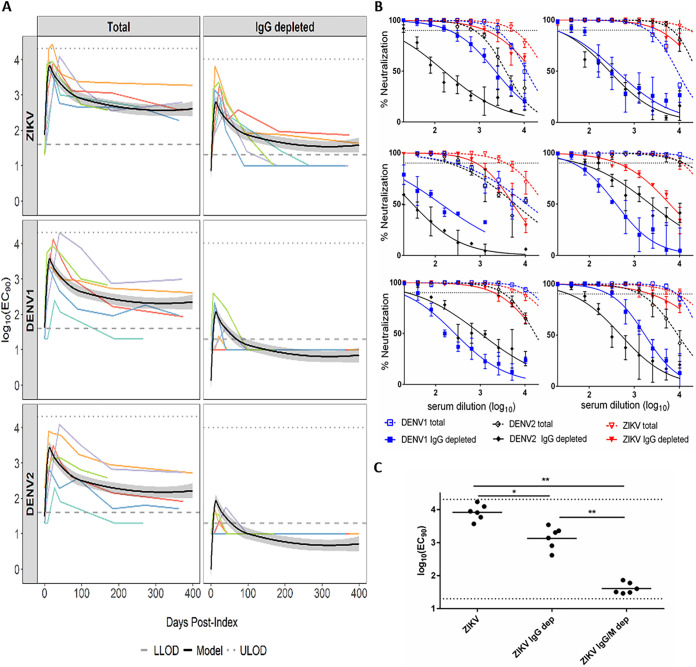
Two distinct patterns of virus-specific neutralization emerged. Six individuals (group 1) displayed cross-DENV NAb titers that were mostly ablated after IgG depletion (Fig. 1A). At index, only two of these individuals (patients 3 and 33) had detectable total NAb to DENV1 or DENV2, and one (patient 33) retained very low IgG-depleted NAb to DENV1 (Table 1). Plasma NAT results showed ZIKV RNA levels ranging from 2.08 to 6.10 log10 copies/ml at index. Analysis of longitudinal samples revealed cross-reactive total NAbs to all three viruses, with NAb to ZIKV equivalent to or slightly higher (<4-fold higher) than NAbs to DENVs among five of these individuals at all postindex time points (Fig. 1A, left). After IgG depletion, cross-NAbs to both DENVs were undetectable in 25 of the 34 samples collected postindex from all individuals, while ZIKV NAb was sustained at higher levels (>4-fold) than those to DENVs in most samples (Fig. 1A, right). The 9 samples (from five subjects) with detectable cross-reactive NAbs after IgG depletion were all collected within 42 days PI. All individuals retained IgG-depleted, ZIKV-specific NAbs for at least 97 to 399 days PI. Importantly, for those 5 subjects with residual DENV NAbs after IgG depletion, the titers were much lower (ranging from 7.4- to 173-fold lower) than that of ZIKV NAb. Spline modeling was used to establish the average NAb kinetic curves and estimate the peak in IgG-depleted ZIKV NAb titers at 14 days PI (95% CI, 13 to 15 days) (Fig. 1A). The virus neutralization curves plotted with the peak ZIKV NAb samples from these 6 individuals showed that the curves of DENV1 and DENV2 NAbs declined dramatically (shift to left) after IgG depletion, while all curves of ZIKV NAb largely remained high at all sample dilution points (Fig. 1B). Upon dual IgG/M depletion (confirmed with 99.8% ± 0.2% [mean ± SD] IgM depletion efficiency), ZIKV NAb from these peak day (ranging from days 10 to 40 PI) samples was reduced to minimal levels, indicating that IgM contributes to the anti-ZIKV NAb response. Interestingly, the average titer reductions were greater after IgG/M depletion (201-fold reduction from total NAb and 34-fold from IgG-depleted NAb) than after IgG depletion (6-fold reduction), indicating that IgM was the larger contributor to ZIKV-specific neutralization in these individuals (Fig. 1C).
FIG 1.
Individuals with highly ZIKV-specific neutralizing IgM. (A) NAbs of longitudinal plasma samples from 6 individuals in group 1. Lower limits of detection (LLOD) of IgG-depleted NAb (1.3 log10) and total NAb (1.6 log10) of the assay are represented by dashed lines. Upper limits of detection (ULOD) of IgG-depleted NAb (4.01 log10) and total NAb (4.31 log10) are represented by dotted lines. Bold black line represents estimated average NAb kinetic curves from the spline model with 95% confidence bounds (shaded regions). Colored lines represent longitudinal NAb titers of each individual. (B) Neutralization curves for 6 individual plasma samples at the time point with peak ZIKV NAb. Dotted line represents 90% neutralization (EC90). (C) Samples from the assay whose results are shown in panel B were further depleted of IgM (IgG/M dep) before R-mFRNT for comparison of the geometric mean NAb titers among total, IgG-depleted, and IgG/M-depleted outcomes. LLOD (1.3 log10) and ULOD (4.31 log10) are represented by dotted lines. Significance was measured by two-tailed paired t test (*, P < 0.05; **, P < 0.0001).
TABLE 1.
Serology assay results for index samples from individuals with low levels of cross-reactive neutralizing IgM
| Patient | R-mFRNT90 titer (log10 EC90) of: |
ELISA resultc |
Plasma NAT (log10 RNA copies/ml)d | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total NAb toa: |
IgG-depleted NAb tob: |
|||||||||
| DENV1 | DENV2 | ZIKV | DENV1 | DENV2 | ZIKV | ZIKV IgM | ZIKV IgG | DENV IgG | ||
| 3 | 1.89 | 2.29 | <1.60 | <1.30 | <1.30 | <1.30 | − | e | + | 5.15 |
| 13 | <1.60 | <1.60 | <1.60 | <1.30 | <1.30 | <1.30 | − | − | + | 2.08 |
| 15 | <1.60 | <1.60 | <1.60 | <1.30 | <1.30 | <1.30 | − | − | + | 3.21 |
| 22 | <1.60 | <1.60 | <1.60 | <1.30 | <1.30 | <1.30 | − | − | + | 2.84 |
| 28 | <1.60 | <1.60 | <1.60 | <1.30 | <1.30 | <1.30 | − | − | + | 4.15 |
| 33 | 2.16 | 1.65 | <1.60 | 1.38 | <1.30 | <1.30 | − | − | + | 6.10 |
R-mFRNT90 titer of total NAb titer in plasma sample neutralizing 90% of input virus. All samples were tested in triplicate.
R-mFRNT90 titer of IgG-depleted NAb titer in plasma sample neutralizing 90% of input virus. All samples were tested in triplicate.
IgM and IgG ELISA results are shown as positive (+), negative (−), or equivocal (e).
RNA copies/ml in plasma measured by quantitative RT-PCR (NAT).
The second pattern (group 2) included 11 individuals displaying a highly cross-reactive NAb to either DENV1 or DENV1/DENV2 that could not be sufficiently resolved by IgG depletion (Fig. 2). Similarly to group 1, all index samples from this group were negative for ZIKV NAb and for ZIKV IgM or IgG by ELISA (Table 2). Their ZIKV viral RNA loads were also within a range similar to that in group 1. However, unlike group 1, in which only 30% showed DENV NAbs at index, all subjects in group 2 had total NAbs to at least one DENV during ZIKV acute infection. Upon IgG depletion, the DENV NAb titers dropped below the detectable level in all but one subject (the single positive subject had a close-to-detectable-level titer to DENV2) at index (Table 2). For follow-up samples, total NAbs to either of the DENVs were similar to or higher than NAb to ZIKV in 98% (66/67) of samples. The levels of cross-DENV total NAbs were significantly higher (>4-fold) in more than half of these samples (38 samples from nine subjects). However, only 14 of them (36.8%) remained significantly higher after IgG depletion, while 18 samples (47.3%) became higher in NAb to ZIKV than to DENVs. Although IgG depletion increased ZIKV specificity in most samples of this group, the modeled average NAb kinetics of ZIKV and DENVs remained similar (Fig. 2). Consistent with those individuals in group 1, the spline model fitted to data from group 2 also had a peak in ZIKV IgG-depleted NAb titer at 14 days PI (95% CI, 13 to 15 days) (Fig. 2).
FIG 2.
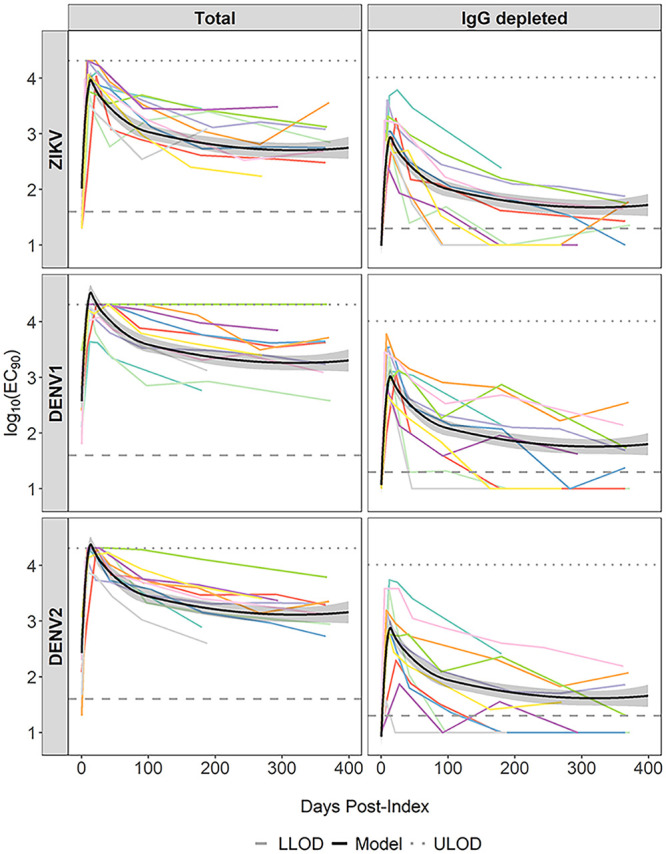
Individuals with cross-reactive neutralizing IgM to DENV1 and DENV2. NAbs of longitudinal plasma samples from 11 individuals in group 2. Lower limits of detection (LLOD) of IgM NAb (1.3 log10) and total NAb (1.6 log10) are represented by dashed lines. Upper limits of detection (ULOD) of IgM NAb (4.01 log10) and total NAb (4.31 log10) are represented by dotted lines. Bold black lines represent average NAb kinetic curves from the spline model with 95% confidence bounds (shaded regions). Colored lines represent longitudinal NAb titers of each individual.
TABLE 2.
Serology assay results for index samples from individuals with high levels of cross-reactive neutralizing IgM
| Patient | R-mFRNT90 titer (log10 EC90) of: |
ELISA resultc |
Plasma NAT (log10 RNA copies/ml)d | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total NAb toa: |
IgG-depleted NAb tob: |
|||||||||
| DENV1 | DENV2 | ZIKV | DENV1 | DENV2 | ZIKV | ZIKV IgM | ZIKV IgG | DENV IgG | ||
| 1 | 2.10 | 2.52 | <1.60 | <1.30 | <1.30 | <1.30 | − | − | + | 3.96 |
| 4 | 1.98 | 2.35 | <1.60 | <1.30 | <1.30 | <1.30 | − | − | + | 4.26 |
| 11 | 2.80 | 2.35 | <1.60 | <1.30 | <1.30 | <1.30 | − | − | + | 3.23 |
| 17 | 2.44 | 2.08 | <1.60 | <1.30 | <1.30 | <1.30 | − | − | + | 1.89 |
| 18 | 2.46 | <1.60 | <1.60 | <1.30 | <1.30 | <1.30 | − | − | + | 3.64 |
| 19 | 2.38 | <1.60 | <1.60 | <1.30 | <1.30 | <1.30 | − | − | + | 6.52 |
| 20 | 3.48 | 2.73 | <1.60 | <1.30 | <1.30 | <1.30 | − | − | + | 1.89 |
| 23 | 1.80 | 2.30 | <1.60 | <1.30 | 1.51 | <1.30 | − | − | + | 6.51 |
| 25 | 2.46 | 1.67 | <1.60 | <1.30 | <1.30 | <1.30 | − | − | + | 4.60 |
| 32 | 2.97 | 2.53 | <1.60 | <1.30 | <1.30 | <1.30 | − | − | + | 2.22 |
| 47 | 2.86 | 3.07 | <1.60 | <1.30 | <1.30 | <1.30 | − | − | + | 1.91 |
R-mFRNT90 titer of total NAb in plasma sample that inhibited at least 90% of input virus. All samples were tested in triplicate.
R-mFRNT90 titer of IgG-depleted NAb in plasma sample that inhibited at least 90% of input virus. All samples were tested in triplicate.
IgM and IgG ELISA results are shown as positive (+), negative (−), or equivocal (e).
RNA copies/ml in plasma measured by quantitative RT-PCR (NAT).
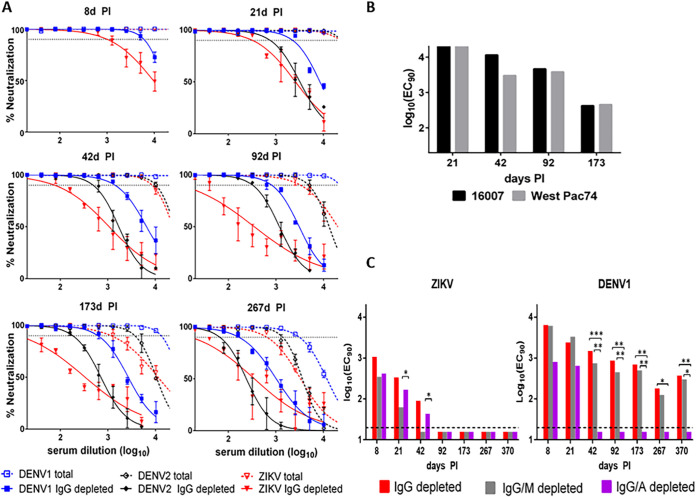
One individual (patient 19) in group 2 displayed IgG-depleted NAb titers that were more than 4-fold higher for DENV1 than for DENV2 or ZIKV throughout all convalescent time points (6 points are shown for examples in Fig. 3A). Using a DENV1 monoclonal Ab, some research demonstrated that different strains of DENV1, particularly the 16007 strain (used in our R-WN/DENV1 construct), may be more effectively neutralized in vitro than others due to a specific amino acid variation in the envelope (E) protein (E-204R in DENV1 16007) compared to strain WestPac74 (E-204K) (33). We tested some of the samples from subject 19 in a traditional PRNT with sequence-verified DENV1 strains 16007 and WestPac74 for a side-by-side comparison. No significant differences (three samples had less than a 2-fold difference, and one had a 3.8-fold difference) were noticed in NAb titers against these two virus strains (Fig. 3B), and titers to both were significantly higher than those to ZIKV. To further determine the NAb isotypes that may contribute to potential DENV1 anamnestic neutralization, samples from this individual were serially depleted of IgG/M or IgG/A (confirmed with 100% IgM and IgA depletion efficiency). Using a 2-way ANOVA with Tukey’s multiple-comparison test, ZIKV NAb titers did not significantly decrease at any time point following IgG/A depletion compared to those samples depleted of only IgG (Fig. 3C). On the other hand, on days 21 and 42, ZIKV NAb titers decreased significantly following depletion of IgG/M compared to depletion of only IgG. These results indicate that IgA had no significant impact on ZIKV neutralization, while IgM NAb was prominent during early convalescence after ZIKV infection, as observed in group 1 (Fig. 1C). Interestingly, DENV1 NAb titers following depletion of IgG/A were only detected up to day 21 and diminished after 42 days PI, whereas the titers after IgG/M depletion were not significantly lower than after IgG depletion at any time point. These results indicated that in addition to IgG, IgA contributed dominantly to the DENV1 neutralization in this individual (Fig. 3C).
FIG 3.
A subject with extremely high anamnestic DENV1 NAbs attributed mainly to IgA. (A) Neutralization curves with and without IgG depletion for plasma samples from individual 19 with higher NAb to DENV1 than to ZIKV at each time point. Dotted lines represent 90% neutralization. (B) Comparison of PRNT90 titers using DENV1 16007 and West Pac74 for 4 samples from the subject. No significant difference in NAb titers was measured by a two-tailed paired t test, indicating that the higher DENV1 titer was not attributable to the use of the DENV1 16007 strain in R-WN/DENV1. (C) NAb was tested in the R-mFRNT with DENV1 and ZIKV after IgG depletion, IgG/M depletion, and IgG/A depletion of plasma samples from the same individual. Asterisks indicate significant differences by ANOVA with Tukey’s multiple-comparison test (*, P < 0.05; **, P < 0.001; ***, P < 0.0001).
An estimation of the time to loss of ZIKV-neutralizing IgM (seroreversion) was calculated using a parametric lognormal survival model and samples from all 17 individuals (Fig. 4). As the index sample could be from any day of acute infection, we further calibrated the starting time for all individuals to the initial day of NAT detectability for the model (18). While neutralizing IgM to ZIKV after a sequential DENV/ZIKV infection peaked around 14 days PI for individuals in both groups, it persisted for more than 1 year in some of the subjects. Among all subjects in this study, 11 had a last sample collected beyond 1 year PI and 8 of them still retained detectable ZIKV IgM NAbs. The estimated median duration of ZIKV-neutralizing IgM was about 257 days (95% CI, 133 to 427 days) after initial NAT detectability (Fig. 4). Although the small subject numbers and various sample collection durations resulted in a wide range of neutralizing IgM durations with a broad CI in the model, the median duration of ZIKV-neutralizing IgM was similar to that of virus-binding IgM by ELISA (18).
FIG 4.
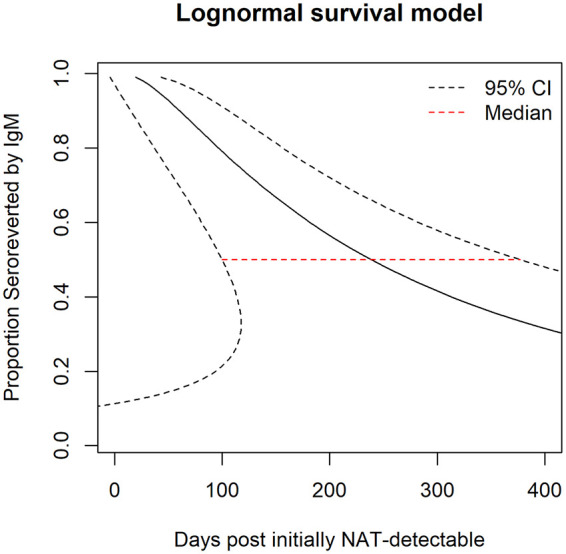
Persistence of anti-ZIKV neutralizing IgM. The seroreversion curve with 95% CI was calculated by parametric lognormal survival model fitted with data of all 17 individuals in the study. The day of initial NAT detectability was imputed for each individual using viral RNA loads at index and a previously determined ZIKV RNA doubling time in plasma (32). The median line indicates loss of detectable neutralizing IgM among 50% of individuals.
DISCUSSION
While the kinetics of virus-binding IgM and IgG in ZIKV infection have been characterized previously (15, 18–20), this study presents the kinetics and specificities of ZIKV-neutralizing Abs, including total and IgM NAbs, after sequential DENV/ZIKV infections in humans. To our knowledge, this is the first study to indicate that IgM elicited by ZIKV could be the most dominant isotype contributing to virus neutralization and that it is less cross-reactive to DENV than total NAbs that contain a robust amount of anamnestic IgG due to prior DENV exposure. Although IgG was the most abundant isotype in human blood and understandably contributed significantly to virus neutralization, depletion of IgM from samples showed that IgM most likely is the isotype exhibiting the dominant ZIKV-specific neutralizing activity during its peak presentation. Consistent with prior reports on ZIKV-binding IgM, in this limited sample set, we showed that neutralizing IgM was detectable for months or even more than 1 year PI, even when employing a very stringent neutralization assay at a 90% cutoff for seroconversion determination. In fact, the overall kinetics of ZIKV-neutralizing IgM, including its initial appearance, peak time, and duration, largely agreed with those of virus-binding IgM by ELISA (15, 18).
Although the in vitro results showed strong neutralization activity of ZIKV IgM, its capacity for protection in vivo requires further studies. In a mouse model, long-lived antigen-induced IgM plasma cells have been found to persist in the spleen and contribute to protection against lethal influenza virus challenge even in the absence of T-cell and memory B-cell activation (34, 35). Passive transfer of IgM against West Nile virus (WNV) is also shown to prolong the survival time of μMT mice after lethal challenge (35). The long-lived antigen-induced IgM plasma cells, unlike long-lived IgG plasma cells, were able to develop and somatically mutate in the absence of germinal centers but may not undergo extensive antigen selection (34). The strong and prolonged IgM NAb after ZIKV infection may be due to these long-lived plasma cells secreting IgM. Persistent ZIKV infection has been demonstrated by prolonged ZIKV RNA in human placenta and semen, suggesting that some reproductive tissues may serve as reservoirs for ZIKV (36–39). Such persistent infection may also contribute to the prolonged IgM response.
The neutralizing IgM appeared to be more ZIKV specific than neutralizing IgG in sequential DENV/ZIKV infections; however, the specificity varied widely among subjects. Our previous study with a different set of subject samples (single time point) showed that the majority of subjects exhibited highly ZIKV-specific neutralizing IgM that was discernible from cross-reactive DENV (25). Only 6 of 17 subjects in this study were confirmed as having a recent ZIKV infection by IgM NAb when the models evaluated were fitted with data from all time points. All 11 individuals with indiscernible IgG-depleted NAb (group 2) had high DENV total NAbs at index during the acute ZIKV infection (before ZIKV seroconversion). We suspect that subjects in group 2 may have had more recent or multiple DENV exposures prior to the latest ZIKV infection, resulting in stronger anamnestic cross-reactive NAb response to DENVs. Index samples were only tested for the presence of ZIKV RNA, since transmission of DENV in Puerto Rico during 2016 was at record low levels (40). While DENV/ZIKV coinfections cannot be ruled out, their occurrence in these individuals is highly unlikely.
Upon IgG depletion, IgA was found to contribute to the high DENV1 NAb in one individual for 370 days PI. IgA is present in the urine, saliva, and serum of dengue patients, with a higher prevalence in patients with a sequential DENV/DENV infection, and may correlate with the severity of disease (41–43). Interestingly, this individual reported symptoms of fever, rash, joint pain, muscle aches, red eyes, and headache at time points 8 and 21 days PI. While the fever receded after 21 days PI, other symptoms persisted, including rash, red eyes, and headache, up to 92 days PI. Although interesting, this observation was only from a single subject and warrants further investigation into the potential role of IgA in DENV neutralization. Since we did not test other samples after IgG/A depletion, we cannot rule out that IgA may also play a role in the cross-NAb response to DENV in persons with recent ZIKV infections.
Preexisting DENV cross-reactive Abs with weak virus-neutralizing activity can enhance heterologous DENV infection through Ab-dependent enhancement (ADE) (44, 45). Emerging evidence from a human cohort study showed that prior DENV infection did not appear to enhance ZIKV infection or illness severity, but ZIKV infection (with or without prior DENV exposure) resulted in an increased risk of disease enhancement by subsequent DENV2 infection (46, 47). Interestingly, the preexisting DENV and/or ZIKV Ab may be more protective against certain serotypes of DENV (e.g., DENV1) while resulting in ADE and/or disease enhancement by other serotypes (e.g., DENV2). Moreover, the quantity and quality of preexisting Ab can determine the outcomes of disease protection or enhancement for some serotypes of DENV (e.g., DENV3). As human immunological interactions between primary and secondary DENV or ZIKV infection are highly complicated, more comprehensive characterizations of the Ab responses after sequential DENV/DENV or DENV/ZIKV infection will inform better diagnosis, vaccine evaluation, and therapeutics for DENV and/or ZIKV.
Our results demonstrate the importance of sustained neutralizing IgM in the humoral immune response against flaviviral diseases. While most individuals with previous DENV exposure displayed a cross-reactive NAb response, it is important to note that some individuals displayed a highly ZIKV-specific neutralizing IgM response. Determining and understanding Ab isotypes may help to differentiate these closely related viruses in recent infection. Additionally, the importance of ZIKV-specific IgM NAb should be assessed in studies evaluating correlates of protection from illness. This study demonstrated a significant IgM NAb response after sequential DENV/ZIKV infection and implies potential roles of IgM NAb in diagnosis, vaccine evaluation, serosurveillance, and virus-host interaction research.
Affinity purification of antibody isotypes, such as protein G-agarose for IgG, peptide M-agarose for IgM, and lectin-agarose for IgA, is widely employed and would preferably have been used for our study. However, to offset the significant yield loss, those purification procedures require much larger sample volumes than what was available. Regrettably, due to sample volume limitation and the multiple virus assays required, we were not able to conduct the study with purified antibody isotype. To conserve sample yield and minimally dilute sample volume to permit testing against all three viruses before and after antibody isotype separation, we chose the antibody isotype depletion method for the study. An investigation using purified antibody isotype would be ideal to further confirm our conclusion. It should also be noted that due to the limited number of individuals included in this study, further investigation with more sample sets from individuals experiencing sequential DENV/ZIKV is important to verify our observations reported in this study. In addition, with DENV cases raised again in areas of endemicity after the ZIKV epidemics waned, investigation of NAb profiles in subjects with sequential ZIKV/DENV or DENV/ZIKV/DENV exposures is also warranted.
ACKNOWLEDGMENT
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of CDC.
Contributor Information
Claire Y.-H. Huang, Email: yxh0@cdc.gov.
Yi-Wei Tang, Cepheid.
REFERENCES
- 1.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI. 2013. The global distribution and burden of dengue. Nature 496:504–507. 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fauci AS, Morens DM. 2016. Zika virus in the Americas—yet another arbovirus threat. N Engl J Med 374:601–604. 10.1056/NEJMp1600297. [DOI] [PubMed] [Google Scholar]
- 3.Heymann DL, Hodgson A, Sall AA, Freedman DO, Staples JE, Althabe F, Baruah K, Mahmud G, Kandun N, Vasconcelos PF, Bino S, Menon KU. 2016. Zika virus and microcephaly: why is this situation a PHEIC? Lancet 387:719–721. 10.1016/S0140-6736(16)00320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lazear HM, Diamond MS. 2016. Zika virus: new clinical syndromes and its emergence in the Western Hemisphere. J Virol 90:4864–4875. 10.1128/JVI.00252-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Musso D, Gubler DJ. 2016. Zika virus. Clin Microbiol Rev 29:487–524. 10.1128/CMR.00072-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petersen LR, Jamieson DJ, Powers AM, Honein MA. 2016. Zika virus. N Engl J Med 374:1552–1563. 10.1056/NEJMra1602113. [DOI] [PubMed] [Google Scholar]
- 7.Crill WD, Hughes HR, Delorey MJ, Chang GJ. 2009. Humoral immune responses of dengue fever patients using epitope-specific serotype-2 virus-like particle antigens. PLoS One 4:e4991. 10.1371/journal.pone.0004991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, Stanfield SM, Duffy MR. 2008. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 14:1232–1239. 10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Speer SD, Pierson TC. 2016. Diagnostics for Zika virus on the horizon. Science 353:750–751. 10.1126/science.aah6187. [DOI] [PubMed] [Google Scholar]
- 10.Wahala WM, Silva AM. 2011. The human antibody response to dengue virus infection. Viruses 3:2374–2395. 10.3390/v3122374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guy B, Noriega F, Ochiai RL, L’Azou M, Delore V, Skipetrova A, Verdier F, Coudeville L, Savarino S, Jackson N. 2017. A recombinant live attenuated tetravalent vaccine for the prevention of dengue. Expert Rev Vaccines 16:1–13. 10.1080/14760584.2017.1335201. [DOI] [PubMed] [Google Scholar]
- 12.Redoni M, Yacoub S, Rivino L, Giacobbe DR, Luzzati R, Di Bella S. 2020. DENGUE: status of current and under-development vaccines. Rev Med Virol 30:e2101. 10.1002/rmv.2101. [DOI] [PubMed] [Google Scholar]
- 13.Simmons G, Stone M, Busch MP. 2018. Arbovirus diagnostics: from bad to worse due to expanding dengue virus vaccination and Zika virus epidemics. Clin Infect Dis 66:1181–1183. 10.1093/cid/cix972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chien YW, Liu ZH, Tseng FC, Ho TC, Guo HR, Ko NY, Ko WC, Perng GC. 2018. Prolonged persistence of IgM against dengue virus detected by commonly used commercial assays. BMC Infect Dis 18:156. 10.1186/s12879-018-3058-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffin I, Martin SW, Fischer M, Chambers TV, Kosoy O, Falise A, Ponomareva O, Gillis LD, Blackmore C, Jean R. 2019. Zika virus IgM detection and neutralizing antibody profiles 12–19 months after illness onset. Emerg Infect Dis 25:299–303. 10.3201/eid2502.181286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roehrig JT, Nash D, Maldin B, Labowitz A, Martin DA, Lanciotti RS, Campbell GL. 2003. Persistence of virus-reactive serum immunoglobulin M antibody in confirmed West Nile virus encephalitis cases. Emerg Infect Dis 9:376–379. 10.3201/eid0903.020531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stiasny K, Aberle JH, Chmelik V, Karrer U, Holzmann H, Heinz FX. 2012. Quantitative determination of IgM antibodies reduces the pitfalls in the serodiagnosis of tick-borne encephalitis. J Clin Virol 54:115–120. 10.1016/j.jcv.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 18.Stone M, Bakkour S, Lanteri MC, Brambilla D, Simmons G, Bruhn R, Kaidarova Z, Lee TH, Orlando Alsina J, Williamson PC, Galel SA, Pate LL, Linnen JM, Kleinman S, Busch MP, NHLBI Recipient Epidemiology Donor Evaluation Study REDS-III Program. 2020. Zika virus RNA and IgM persistence in blood compartments and body fluids: a prospective observational study. Lancet Infect Dis 20:1446–1456. 10.1016/S1473-3099(19)30708-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tonnerre P, Melgaco JG, Torres-Cornejo A, Pinto MA, Yue C, Blumel J, de Sousa PSF, de Mello VDM, Moran J, de Filippis AMB, Wolski D, Grifoni A, Sette A, Barouch DH, Hoogeveen RC, Baylis SA, Lauer GM, Lewis-Ximenez LL. 2020. Evolution of the innate and adaptive immune response in women with acute Zika virus infection. Nat Microbiol 5:76–83. 10.1038/s41564-019-0618-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCracken MK, Gromowski GD, Friberg HL, Lin X, Abbink P, De La Barrera R, Eckles KH, Garver LS, Boyd M, Jetton D, Barouch DH, Wise MC, Lewis BS, Currier JR, Modjarrad K, Milazzo M, Liu M, Mullins AB, Putnak JR, Michael NL, Jarman RG, Thomas SJ. 2017. Impact of prior flavivirus immunity on Zika virus infection in rhesus macaques. PLoS Pathog 13:e1006487. 10.1371/journal.ppat.1006487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prince HE, Matud JL. 2011. Estimation of dengue virus IgM persistence using regression analysis. Clin Vaccine Immunol 18:2183–2185. 10.1128/CVI.05425-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chanama S, Anantapreecha S, A-nuegoonpipat A, Sa-gnasang A, Kurane I, Sawanpanyalert P. 2004. Analysis of specific IgM responses in secondary dengue virus infections: levels and positive rates in comparison with primary infections. J Clin Virol 31:185–189. 10.1016/j.jcv.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Innis BL, Nisalak A, Nimmannitya S, Kusalerdchariya S, Chongswasdi V, Suntayakorn S, Puttisri P, Hoke CH. 1989. An enzyme-linked immunosorbent assay to characterize dengue infections where dengue and Japanese encephalitis co-circulate. Am J Trop Med Hyg 40:418–427. 10.4269/ajtmh.1989.40.418. [DOI] [PubMed] [Google Scholar]
- 24.Mongkolsapaya J, Dejnirattisai W, Xu XN, Vasanawathana S, Tangthawornchaikul N, Chairunsri A, Sawasdivorn S, Duangchinda T, Dong T, Rowland-Jones S, Yenchitsomanus PT, McMichael A, Malasit P, Screaton G. 2003. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat Med 9:921–927. 10.1038/nm887. [DOI] [PubMed] [Google Scholar]
- 25.Baldwin WR, Livengood JA, Giebler HA, Stovall JL, Boroughs KL, Sonnberg S, Bohning KJ, Dietrich EA, Ong YT, Danh HK, Patel HK, Huang CY, Dean HJ. 2018. Purified inactivated Zika vaccine candidates afford protection against lethal challenge in mice. Sci Rep 8:16509. 10.1038/s41598-018-34735-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hombach J, Solomon T, Kurane I, Jacobson J, Wood D. 2005. Report on a WHO consultation on immunological endpoints for evaluation of new Japanese encephalitis vaccines, WHO, Geneva, 2–3 September, 2004. Vaccine 23:5205–5211. 10.1016/j.vaccine.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Kreil TR, Burger I, Bachmann M, Fraiss S, Eibl MM. 1997. Antibodies protect mice against challenge with tick-borne encephalitis virus (TBEV)-infected macrophages. Clin Exp Immunol 110:358–361. 10.1046/j.1365-2249.1997.4311446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Gessel Y, Klade CS, Putnak R, Formica A, Krasaesub S, Spruth M, Cena B, Tungtaeng A, Gettayacamin M, Dewasthaly S. 2011. Correlation of protection against Japanese encephalitis virus and JE vaccine (IXIARO((R))) induced neutralizing antibody titers. Vaccine 29:5925–5931. 10.1016/j.vaccine.2011.06.062. [DOI] [PubMed] [Google Scholar]
- 29.Calvert AE, Boroughs KL, Laven J, Stovall JL, Luy BE, Kosoy OI, Huang CY. 2018. Incorporation of IgG depletion in a neutralization assay facilitates differential diagnosis of Zika and dengue in secondary flavivirus infection cases. J Clin Microbiol 56:e00234-18. 10.1128/JCM.00234-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kinney (Huang) CY. 28April2020. Chimeric West Nile/Zika viruses and method of use. US patent 10,632,185 B2.
- 31.Kinney (Huang) CY. 13August2020. Chimeric reporter West Nile/dengue viruses and their use. US patent 2020/0255479 A1.
- 32.Williamson PC, Biggerstaff BJ, Simmons G, Stone M, Winkelman V, Latoni G, Alsina J, Bakkour S, Newman C, Pate LL, Galel SA, Kleinman S, Busch MP. 2020. Evolving viral and serological stages of Zika virus RNA-positive blood donors and estimation of incidence of infection during the 2016 Puerto Rican Zika epidemic: an observational cohort study. Lancet Infect Dis 20:1437–1445. 10.1016/S1473-3099(19)30706-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dowd KA, DeMaso CR, Pierson TC. 2015. Genotypic differences in dengue virus neutralization are explained by a single amino acid mutation that modulates virus breathing. mBio 6:e01559-15. 10.1128/mBio.01559-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bohannon C, Powers R, Satyabhama L, Cui A, Tipton C, Michaeli M, Skountzou I, Mittler RS, Kleinstein SH, Mehr R, Lee FE, Sanz I, Jacob J. 2016. Long-lived antigen-induced IgM plasma cells demonstrate somatic mutations and contribute to long-term protection. Nat Commun 7:11826. 10.1038/ncomms11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diamond MS, Shrestha B, Marri A, Mahan D, Engle M. 2003. B cells and antibody play critical roles in the immediate defense of disseminated infection by West Nile encephalitis virus. J Virol 77:2578–2586. 10.1128/jvi.77.4.2578-2586.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDonald EM, Duggal NK, Delorey MJ, Oksanish J, Ritter JM, Brault AC. 2019. Duration of seminal Zika viral RNA shedding in immunocompetent mice inoculated with Asian and African genotype viruses. Virology 535:1–10. 10.1016/j.virol.2019.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McDonald EM, Duggal NK, Ritter JM, Brault AC. 2018. Infection of epididymal epithelial cells and leukocytes drives seminal shedding of Zika virus in a mouse model. PLoS Negl Trop Dis 12:e0006691. 10.1371/journal.pntd.0006691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mead PS, Duggal NK, Hook SA, Delorey M, Fischer M, Olzenak McGuire D, Becksted H, Max RJ, Anishchenko M, Schwartz AM, Tzeng WP, Nelson CA, McDonald EM, Brooks JT, Brault AC, Hinckley AF. 2018. Zika virus shedding in semen of symptomatic infected men. N Engl J Med 378:1377–1385. 10.1056/NEJMoa1711038. [DOI] [PubMed] [Google Scholar]
- 39.Perez-Padilla J, Paz-Bailey G, Meaney-Delman D, Doyle K, Gary J, Rodriguez DM, Bhatnagar J, Perez-Rodriguez NM, Montalvo S, Alvarado L, Sharp TM. 2019. Persistent Zika virus infection associated with early fetal demise: a case report. Open J Obstet Gynecol 9:698–706. 10.4236/ojog.2019.95069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharp TM, Quandelacy TM, Adams LE, Aponte JT, Lozier MJ, Ryff K, Flores M, Rivera A, Santiago GA, Munoz-Jordan JL, Alvarado LI, Rivera-Amill V, Garcia-Negron M, Waterman SH, Paz-Bailey G, Johansson MA, Rivera-Garcia B. 2020. Epidemiologic and spatiotemporal trends of Zika virus disease during the 2016 epidemic in Puerto Rico. PLoS Negl Trop Dis 14:e0008532. 10.1371/journal.pntd.0008532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koraka P, Suharti C, Setiati TE, Mairuhu AT, Van Gorp E, Hack CE, Juffrie M, Sutaryo J, Van Der Meer GM, Groen J, Osterhaus AD. 2001. Kinetics of dengue virus-specific serum immunoglobulin classes and subclasses correlate with clinical outcome of infection. J Clin Microbiol 39:4332–4338. 10.1128/JCM.39.12.4332-4338.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vazquez S, Lozano C, Perez AB, Castellanos Y, Ruiz D, Calzada N, Guzman MG. 2014. Dengue specific immunoglobulins M, A, and E in primary and secondary dengue 4 infected Salvadorian children. J Med Virol 86:1576–1583. 10.1002/jmv.23833. [DOI] [PubMed] [Google Scholar]
- 43.Vazquez S, Perez AB, Ruiz D, Rodriguez R, Pupo M, Calzada N, Gonzalez L, Gonzalez D, Castro O, Serrano T, Guzman MG. 2005. Serological markers during dengue 3 primary and secondary infections. J Clin Virol 33:132–137. 10.1016/j.jcv.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 44.Halstead SB. 1979. In vivo enhancement of dengue virus infection in rhesus monkeys by passively transferred antibody. J Infect Dis 140:527–533. 10.1093/infdis/140.4.527. [DOI] [PubMed] [Google Scholar]
- 45.Katzelnick LC, Gresh L, Halloran ME, Mercado JC, Kuan G, Gordon A, Balmaseda A, Harris E. 2017. Antibody-dependent enhancement of severe dengue disease in humans. Science 358:929–932. 10.1126/science.aan6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Katzelnick LC, Bos S, Harris E. 2020. Protective and enhancing interactions among dengue viruses 1–4 and Zika virus. Curr Opin Virol 43:59–70. 10.1016/j.coviro.2020.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Katzelnick LC, Narvaez C, Arguello S, Lopez Mercado B, Collado D, Ampie O, Elizondo D, Miranda T, Bustos Carillo F, Mercado JC, Latta K, Schiller A, Segovia-Chumbez B, Ojeda S, Sanchez N, Plazaola M, Coloma J, Halloran ME, Premkumar L, Gordon A, Narvaez F, de Silva AM, Kuan G, Balmaseda A, Harris E. 2020. Zika virus infection enhances future risk of severe dengue disease. Science 369:1123–1128. 10.1126/science.abb6143. [DOI] [PMC free article] [PubMed] [Google Scholar]