Abstract
The hypoxic tumor microenvironment is characterized by disordered vasculature and rapid proliferation of tumors, resulting from tumor invasion, progression and metastasis. The hypoxic conditions restrict efficiency of tumor therapies, such as chemotherapy, radiotherapy, phototherapy and immunotherapy, leading to serious results of tumor recurrence and high mortality. Recently, research has concentrated on developing functional nanomaterials to treat hypoxic tumors. In this review, we categorize such nanomaterials into (i) nanomaterials that elevate oxygen levels in tumors for enhanced oxygen-dependent tumor therapy and (ii) nanomaterials with diminished oxygen dependence for hypoxic tumor therapy. To elevate oxygen levels in tumors, oxygen-carrying nanomaterials, oxygen-generating nanomaterials and oxygen-economizing nanomaterials can be used. To diminish oxygen dependence of nanomaterials for hypoxic tumor therapy, therapeutic gas-generating nanomaterials and radical-generating nanomaterials can be used. The biocompatibility and therapeutic efficacy of these nanomaterials are discussed.
Keywords: nanomaterials, hypoxic tumor, tumor therapy, oxygen
INTRODUCTION
Hypoxia is a characteristic of most tumors [1,2]. Tumor hypoxia results from consumption of large amounts of oxygen by tumor cells for rapid proliferation, while the tumor vasculature is malformed and abnormal, limiting the adequate supply of oxygen [3,4]. In addition, the high interstitial pressure limits oxygen diffusion into deep tumor layers, leading to extreme hypoxia in deep tumors [5,6]. Compared with oxygenated healthy tissues, hypoxic tumors often display, among other things, high levels of reactive oxygen species (ROS), low pH and altered metabolism [7]. Hypoxia generally leads to intratumoral heterogeneity and inhibition of innate and adaptive immune responses, which promotes the probability of tumor metastasis [8,9]. Furthermore, it is recognized that some tumor cells can survive in hypoxic conditions [10], and even worse, the hypoxia-tolerant tumor cells are more resistant to traditional tumor therapies including radiotherapy [11,12], chemotherapy [13] and photodynamic therapy (PDT) [14,15]. Recently, several trials have been performed aiming to improve the hypoxic microenvironment, e.g. through use of inspiratory hyperoxia. Unfortunately, it is difficult to apply this technique to clinical therapy because of the severe structural malformation of microvessels in tumors and the side effects of hyperbaric oxygen therapy [16]. Thus, it is critically important to develop reliable methods for treatment of hypoxic tumors.
Nanomaterials have developed rapidly over the past decade, opening up new areas in biomedical applications including bioimaging, targeted drug delivery and tumor therapy [17–20]. As a result of the enhanced permeation and retention (EPR) effect, nanomaterials have prior accumulation in tumor tissues [21]. Also, nanomaterials are equipped to carry and deliver drugs (small molecules, proteins, DNA, etc.) via surface attachment, encapsulation and entrapping [22–24]. Nanomaterials serving as drug delivery systems have many advantages: (i) nanomaterials are conducive to change the pharmacokinetics of drugs and increase delivery efficiency [25]; (ii) nanomaterials possess high drug encapsulation capacity and controllable drug delivery in the circulation process to avoid drug leakage [26]; (iii) nanomaterials can be modified easily and further enhance multifunctionality, solubility and stability of nanomedicine, to make it favorable for in vivo administration [27].
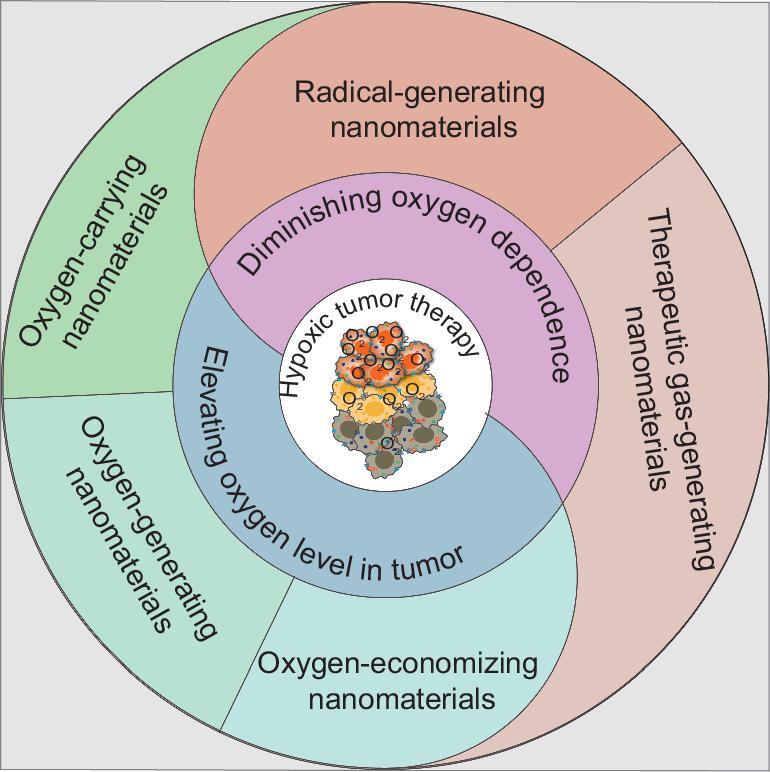
Here, we summarize recent studies on nanomaterials in hypoxic tumor therapy (Fig. 1). It is recognized that the hypoxic tumor microenvironment is attributed to the larger consumption of oxygen than the supply of oxygen in tumor tissue. Thus, elevating oxygen levels in tumor tissues would be the most direct way to treat hypoxic tumors. Diminishing oxygen dependence for hypoxic tumor therapy has also been postulated as a therapeutic option in recent years, e.g. by taking advantage of penetration of therapeutic gas or generation of toxic substances in situ in hypoxic tumor. In this review, two main aspects are discussed regarding use of nanomaterials to treat hypoxic tumors: (i) elevating oxygen level at tumor sites with use of nanomaterials including oxygen-carrying nanomaterials, oxygen-generating nanomaterials and oxygen-economizing nanomaterials, and (ii) diminishing oxygen dependence of nanomaterials by use of therapeutic gas-generating nanomaterials and radical-generating nanomaterials (Table 1). Also, potential challenges, including biocompatibility, and future prospects for nanomaterials to treat hypoxic tumors are discussed.
Figure 1.
Strategies for treatment of hypoxic tumor with nanomaterials.
Table 1.
Summary of nanomaterials for treatment of hypoxic tumor.
| Strategies | Nanomaterials for treatment of hypoxic tumor | Examples | Key references |
|---|---|---|---|
| Elevating oxygen level in tumor | Oxygen-carrying nanomaterials | Oxygen nanobubbles Hb-containing oxygen-carrying nanomaterials | [35–40] [45–54] |
| PFC-containing oxygen-carrying nanomaterials | [59–67] | ||
| Oxygen-generating nanomaterials | H2O2-based oxygen-generating nanomaterials | [69–75,84–87,89–91,95–102] | |
| H2O-based oxygen-generating nanomaterials | [103–106] | ||
| Oxygen-economizing nanomaterials | Respiration-inhibiting oxygen-economizing nanomaterials | [109–112,114–116] | |
| Diminishing oxygen dependence | Therapeutic gas-generating nanomaterials | NO-generating nanomaterials·OH-generating nanomaterials | [126,127][138–143] |
| Radical-generating nanomaterials | ·R-generating nanomaterials | [146–148] |
USE OF NANOMATERIALS TO ELEVATE OXYGEN LEVELS IN HYPOXIC TUMOR THERAPY
Many strategies to treat tumors are oxygen-dependent, including chemotherapy [28], radiotherapy [29,30], PDT [14] as well as immunotherapy [9,31]. Through various mechanisms, the low oxygen concentration in tumor tissues limits the efficacy of these therapies. To solve these problems, approaches have been made to modulate the concentration of oxygen in tumors. Both oxygen-carrying nanomaterials and oxygen-generating nanomaterials provide oxygen to relieve the hypoxic microenvironment and enhance the therapeutic effects of oxygen-dependent therapies. In addition, oxygen content can be temporarily improved by inhibiting respiration to economize oxygen in tumor cells.
Oxygen-carrying nanomaterials for hypoxic tumor therapy
Nanobubbles consisting of a stabilizing monolayered shell and gas core have the potential to carry gas molecules (e.g. oxygen) [32,33]. Oxygen nanobubbles with the ability to carry oxygen have been explored in delivery of oxygen for tumor oxygenation and enhancement of tumor therapy [34,35]. Such oxygen nanobubbles were constructed with an oxygen core and nanolayered shell including lipid, polymer, dextran and gas vesicles [36,37]. Drugs can be loaded within the nanobubbles either by encapsulation in the core or by coating the outer shell in a covalent or non-covalent method according to the hydrophobic or hydrophilic properties of the drugs. Oxygen nanobubbles have been used to suppress expression of hypoxic inducible factor-1α (HIF-1α) to enhance the therapeutic effects of radiotherapy and chemotherapy when mediated with different treatments [38,39]. Furthermore, dependent on external stimuli, oxygen may responsive-release in tumors to avoid premature oxygen release and reduce side effects. Song et al. used acetylated dextran, a pH-responsive polymer, to serve as the shell of oxygen nanobubbles, thus enabling release of oxygen to alleviate tumor hypoxia in the tumor microenvironment [34]. Although progress has been made with oxygen nanobubbles to enhance hypoxic tumor therapy, there remain problems with stability and storage of oxygen nanobubbles to avoid premature oxygen leakage [40].
Hemoglobin (Hb), an abundant natural metalloprotein in red blood cells (RBCs), is mainly used to transport and deliver oxygen in mammals. It is constructed of four globular polypeptide submits (α1, β1, α2, β2), each of which is composed of a structure with a porphyrin ring. The center of the porphyrin ring contains a ferrous ion (Fe2+), which is responsible for oxygen transport of Hb and each Fe2+ binds one oxygen molecule [41]. As an oxygen transport protein, Hb has drawn the interest of researchers towards blood substitutes. However, the free Hb cannot be directly used to transport oxygen because it can be easily broken into dimers with a short circulation time, high organ toxicity and strong oxygen affinity to obstruct oxygen release [42,43]. As a consequence, modification or encapsulation of Hb to prevent its breakage is necessary for effective oxygen transport in vivo.
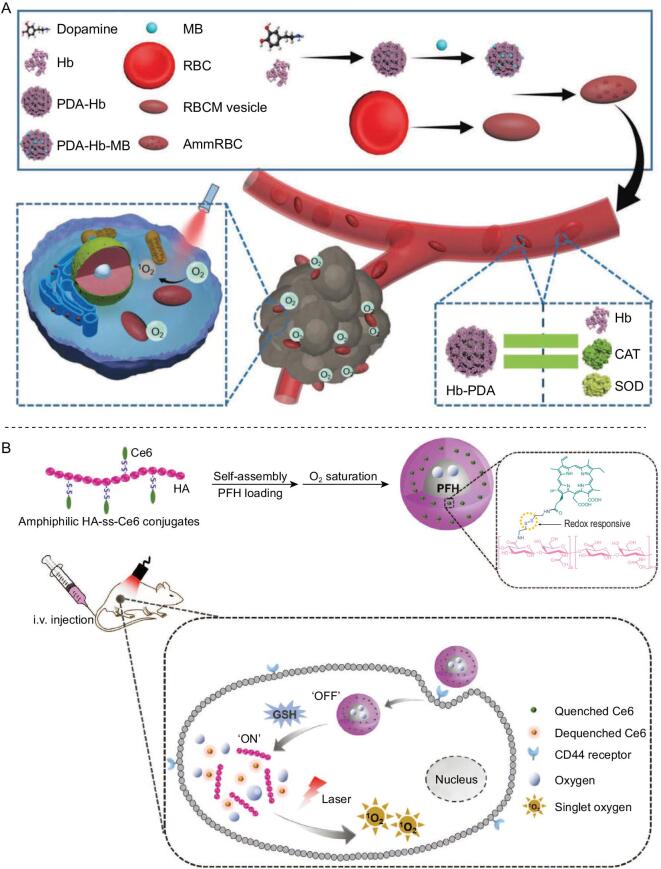
As the source of Hb, each RBC approximately contains 270 million Hb molecules [44]. Thus, RBCs have been directly used to transport oxygen for alleviation of hypoxia. Oxygen can be released from Hb in RBCs and diffuses to the surroundings, where it is then rapidly consumed by tumor cells closed to capillaries. When RBCs were used to carry a photosensitizer, generation of ROS was enhanced by adjacent Hb-carried oxygen [45]. As tumor vessels are abnormal and they leak, it is difficult for RBCs of larger size to infiltrate into deep tumors. Because of the EPR effects of nanomaterials, researchers have hybridized Hb with proteins or polymers at the nanoscale to enhance penetration and accumulation of Hb in tumor tissues [46,47]. Nanomaterials containing human serum albumin and Hb (C@HOPC) were prepared via a protein hybridization approach as oxygen nanocarriers for amplified PDT. C@HOPC showed gradual release of oxygen in hypoxic solution for many minutes [46]. In addition, encapsulation of Hb in poly lactic-co-glycolic acid (PLGA) or liposomes constructed as nanoscale oxygen carriers, have been used to reverse hypoxia-induced chemoresistance when combined with chemotherapeutic drugs [28,48]. However, Hb is very susceptible to oxide, resulting in a partial loss of oxygen-transporting capacity and even the generation of toxic substances during circulation in vivo. These side effects were exacerbated when Hb-based nanoparticles encountered the pathological abundant oxidizing hydrogen peroxide (H2O2) in the tumor environment [49]. To reduce oxidative damage to Hb, RBCs possess their own antioxidation system including glutathione (GSH) and antioxidant enzymes, such as catalase (CAT) and superoxide dismutase (SOD) [50,51]. To protect Hb from oxidation, work has been done to simulate construction of RBCs with an anti-oxidative system. In 1998, SOD and CAT were used to crosslink Hb with crosslinker glutaraldehyde to prevent Hb from disassociation and oxidation [52]. Other alternative antioxidant enzymes, such as rubrerythrin, have been used to protect Hb against high oxidative level [53]. Although these methods protect Hb from generating toxic substances, the crosslinker glutaraldehyde is a biocide, and is toxic to organisms, thus limiting the biomedical applications of crosslinked Hb. Considering this problem, Zhang's group designed a man-made RBC with enzyme-mimicking polydopamine, which could simulate the functions of CAT and SOD. The man-made RBC prevented Hb from oxidative destruction during blood circulation and in the tumor tissues. When methylene blue (MB) was absorbed in the man-made RBC, oxygen which was carried by the protected Hb relieved tumor hypoxia, and generation of ROS by MB enhanced tumor PDT under light irradiation (Fig. 2A). This method, based on nanomaterials, has potential in application of isolated Hb from blood, so as to address the shortage of packed RBCs in clinics [54].
Figure 2.
Oxygen-carrying nanomaterials to enhance tumor therapy. (A) Aggressive man-made RBCs containing polydopamine which functions like CAT and SOD in RBCs to protect Hb from oxidant damage during the circulation for hypoxia-resistant PDT. Reproduced with permission from [54]. Copyright 2018, Wiley-VCH. (B) Design and functions of redox-responsive and oxygen-delivering tumor-targeted photosensitizer to enhance the efficacy of tumor PDT. Reproduced with permission from [64]. Copyright 2019, Wiley-VCH.
Hb has good biocompatibility, but its oxygen-transporting efficiency is limited by its binding sites as each Hb can reversibly bind and release only four oxygen molecules. Benefiting from extremely low polarizability of fluorine, perfluorocarbon (PFC) possesses great gas solubility. Liquid PFC, as a nearly ideal and gas-like fluid, can easily dissolve a substance with similar low cohesive force, such as oxygen, carbon dioxide (CO2), nitrogen (N2), nitric oxide (NO), etc. According to clinical data, 1.5 times more Hb is required than PFC to carry the same content of oxygen [55]. In clinic, PFC emulsions (e.g. Fluosol-DA-20%) have been approved for intravenous use by the United States Food and Drug Administration (FDA) as an artificial blood substitute [56]. Because of its biocompatibility, PFC compounds have also been used as contrast agents for magnetic resonance imaging (MRI) and ultrasound (US) to diagnose diseases [57,58], and its oxygen-carrying capacity means that application of PFC to relieve tumor hypoxia has been considered in recent years [59]. Lipid-coated perfluorohexane and photosensitizer can improve the efficacy of PDT. The cytotoxicity of the nanomaterials with NIR irradiation (about 87%) to CT26 in hypoxia was much higher than that of lipid-coated photosensitizer (about 7%) in vitro. Although PFC can carry large amounts of oxygen, its super-hydrophobic structure makes it hard to modify, limiting its development in tumor therapy. Even so, many researchers have considered remolding PFC to carry oxygen to tumor tissues. Firstly, with their good loading ability, hollow nanoparticles including hollow Bi2Se3 and hollow mesoporous silica nanoparticles were used to load PFC. Hollow Bi2Se3 nanoparticles were able to simultaneously absorb X-ray irradiation and near-infrared ray (NIR) light. PFC saturated with oxygen in hollow Bi2Se3 can increase oxygen concentrations sharply in hypoxic solutions within about 2 minutes. The released oxygen could overcome hypoxia-associated RT-resistance of tumor and alleviate DNA damage in the RT process [60]. Hollow mesoporous silica nanoparticles were not only loaded with PFC to oxygenate tumor, but also loaded with photosensitizer (Ce6) or sonosensitizers (IR780) to generate ROS under NIR light or ultrasound [61,62]. PFC can also self-assemble with proteins or polymers (e.g. human serum albumin, hyaluronic acid (HA) and PLGA) to fabricate nanoparticles for enhancement of tumor therapy [63]. For example, redox-responsive amphiphilic HA-ss-Ce6 conjugates were self-assembled with PFC to construct tumor-targeted nanoparticles. The nanoparticles would accumulate in tumor sites through the EPR effect and enter the tumor cells via HA-related tumor overexpression receptor CD44 mediated endocytosis. Then the nanoparticles dissociate in the tumor redox microenvironment accompanied by fluorescence recovery and delivery of PFC with oxygen, thus enhancing generation of ROS for tumor therapy (Fig. 2B) [64]. Moreover, RBC membranes could be introduced to emulsify PFC to construct artificial RBCs for oxygen transportation [65]. In addition, artificial natural killer cells have been designed with a PFC core to carry oxygen for alleviating the hypoxic microenvironment. The artificial natural killer cells with the carried oxygen could selectively kill tumor cells and polarize macrophages by generation of H2O2 under glucose oxidase [66]. The encapsulation or emulsification of PFC could gradually deliver oxygen in tumor tissues, and the oxygen could also be rapidly released under the above-mentioned NIR light and ultrasound treatment, demonstrating that controllable release of oxygen could be realized in tumor therapy [55,67]. Thus, the therapeutic effects of oxygen-dependent tumor therapy methods could be enhanced by PFC-associated nanomaterials through transport of oxygen.
Compared with Hb, PFC can carry more oxygen at equal concentration. The release of oxygen from PFC is realized by simple diffusion via the oxygen concentration gradient, while the release of oxygen from Hb is related to Hb oxy-deoxy conformational change. Thus, the efficiency of oxygen release from PFC may be higher than that of Hb. Oxygen-carrying nanomaterials have been used to increase oxygen concentration in tumor tissues and alleviate tumor hypoxia. However, it is difficult for the oxygen-carrying nanomaterials to thoroughly relieve the hypoxia because they would be subject to great losses in the blood circulation, with consideration of potential oxygen toxicity to organs if the initially carried oxygen is too high.
Oxygen-generating nanomaterials for hypoxic tumor therapy
The concentration of oxygen in tumor tissues can also be increased by oxygen generated in situ in tumor sites. This would avoid any potential side effects of oxygen toxicity in the circulation. It has been recognized that H2O2 is overexpressed in tumor tissues because of abnormal metabolic processes. The catalytic decomposition of H2O2 in tumor sites is regional, accompanied by generation of oxygen which could freely diffuse for hypoxic tumor therapy. Nanomaterials that have been used to decompose H2O2 are mainly based on CAT and CAT-like nanozymes.
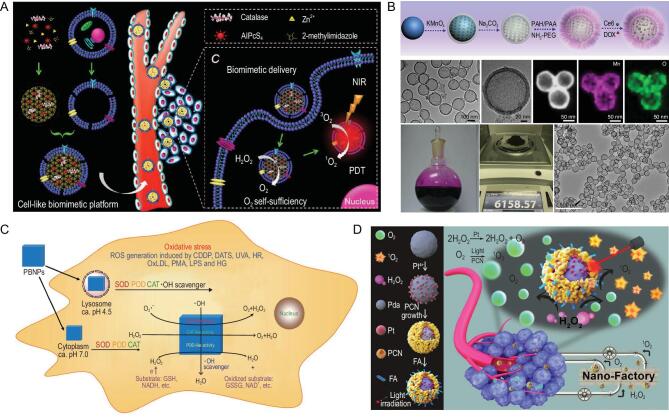
Natural CAT super-efficiently catalyzes decomposition of H2O2 to generate oxygen. However, instability of CAT in vivo in the presence of some physiological proteases and poor half-life, limit the applications of CAT [68]. To overcome these shortcomings, research has focused on the renovation of CAT. Zhang's group loaded CAT into the pores of metal-organic frameworks (MOFs), which preserved the CAT from degradation [69,70]. With the generation of oxygen in tumor sites, the CAT-loaded MOFs encapsulated other drugs such as AlPcS4 to enhance PDT (Fig. 3A) and DOX to enhance chemotherapy. Polymers could be fabricated to encapsulate CAT with covalent conjugation. HA and fluorinated polyethyleneimine have both been used to load CAT and then accumulated in tumor tissues to generate oxygen for enhanced PDT or sonodynamic therapy (SDT) [71–73]. A CAT-loaded hydrogel was used to generate oxygen in tumor to overcome the immunosuppressive tumor microenvironment [74]. It was recognized that overcoming hypoxia would be helpful to enhance tumor radiotherapy. Tumor radiotherapy has been enhanced by CAT-loaded tantalum oxide (TaOX) nanoshells via two pathways: (i) generation of oxygen by decomposition of H2O2 to overcome hypoxia; (ii) concentration of radiation energy by high-Z element tantalum within the tumor to enhance radiation-induced DNA damage [75]. Natural CAT has high specificity and activity, but it has poor stability and is susceptible to the environment, especially complicated physiologic environments. Moreover, large-scale extraction or preparation of natural CAT is costly.
Figure 3.
H2O2-based oxygen-generating nanomaterials for hypoxic tumor therapy. (A) CAT-loaded metal organic framework with cell-like biomimetic membrane for tumor-targeted PDT. Reproduced with permission from [70]. Copyright 2016, Wiley-VCH. (B) Synthesis of dual-drug loading H-MnO2-PEG nanoparticles and TEM images of H-MnO2-PEG. Reproduced with permission from [84]. Copyright 2017, Springer Nature. (C) Prussian blue nanoparticles with the ability of mimicking three antioxidant enzymes including CAT-like nanozymes to decompose H2O2 into oxygen. Reproduced with permission from [88]. Copyright 2016, American Chemical Society. (D) Preparation and functions of core-shell nanofactory for converting H2O2 from tumors into oxygen to enhance PDT. Reproduced with permission from [95]. Copyright 2018, Wiley-VCH.
Besides natural enzymes, in 2013, nanomaterials with enzyme-like characteristics were defined as ‘nanozymes’ [76]. Development of these nanozymes has been rapid with the deeper understanding of nanotechnology and catalytic science. By mimicking or engineering the natural enzymes, nanozymes offer alternatives in many areas with advantages such as low cost, easy large-scale production, high stability and tunable activity. Among nanozymes, CAT-like nanozymes consisting of Prussian Blue (PB) or metals or metal oxides can efficiently catalyze decomposition of H2O2 into water and oxygen [77]. Next, we summarize that CAT-like nanozymes generate oxygen to alleviate tumor hypoxia for enhancement of tumor therapy.
Manganese ions play important roles in a variety of biological processes. The active sites of many enzymes contain manganese, such as manganese SOD, oxygen-evolving complex of photosystem II and CAT. In the last decade, the design and application of manganese oxidation catalysts have attracted keen interest in catalysis of H2O2 to generate oxygen. The form of nanomaterials containing manganese can be diversified with different constituents and structures. Manganese dioxide (MnO2) is the type most commonly modified for generation of oxygen [78]. Research has been done on hollow MnO2 nanoparticles, MnO2 nanodots and MnO2 nanosheets for decomposing H2O2 into oxygen for tumor therapy [79–83]. Not only could the hollow MnO2 catalyze the decomposition of H2O2 to alleviate the hypoxic condition, but it could also serve as a carrier to load high quantities of drugs with precisely controlled release because of its acid-responsive properties. The manganese ions released could be used in MRI to integrate diagnosis and treatment. The hollow MnO2 is uniform, with the potential for large-scale production for clinical use (Fig. 3B) [84]. The good performance of MnO2 means it is often combined with other materials to enhance the synergistic effects. MnO2 nanosheets can be anchored with calcinated TiO2-coated upconversion nanoparticles (UCNPs) to generate oxygen for circulated amplification of generation of ROS with 980 nm laser irradiation in deep hypoxic tumor tissue [85]. Metal ions and their complex compounds are often used as dopants to modify Mn-based nanomaterials. Mesoporous copper/manganese silicate nanospheres have been fabricated to enhance generation of ROS and GSH-activated Fenton reaction [86]. Manganese ferrite and ceria nanoparticle-doped mesoporous silica nanoparticles have been developed to scavenge ROS and produce oxygen for the polarization of macrophages [87]. Although many Mn-based nanoparticles have been developed, the synthesis of the nanoparticles is still at the lab, limiting their industrial translation. Furthermore, the high concentration of Mn2+ that would be released via the biodegradation of Mn-based nanoparticles may be toxic to cells and tissues. Therefore, sufficient data about their biocompatibility should be systemically investigated before further clinic translation.
PB, developed as a dye in the areas of paints and ink, is also used in medicine to detoxify patients from metal or elements poisoning. It has been approved as an antidote for thallotoxicosis by the FDA, illustrating its good biocompatibility for biomedical applications. Zhang's group first discovered that PB possessed multienzyme-like activity including CAT, peroxidase (POD) and SOD in 2016 (Fig. 3C) [88]. Since then, PB with CAT-like activity has been considered for oxygen generation in tumor therapy on the basis of excellent biologic security. PB nanoparticles were used as a core and coated with mesoporous organosilicon, which was loaded with photosensitizer [89,90]. H2O2 freely diffused into the inner PB, and was catalyzed into oxygen for the outer-loaded photosensitizer generating ROS to kill tumors. As a result of the high drug loading capability, hollow structure nanoparticles have attracted a lot of research interest. Hollow PB nanoparticles efficiently catalyzed H2O2 to generate oxygen, as well as loading drugs such as glucose oxidase for a cascade reaction to enhance starvation therapy [91]. Furthermore, hollow PB nanoparticles can generate heat energy under NIR light, offering an opportunity to combine photothermal therapy with enhanced starvation therapy. Although PB has good biocompatibility and has been used to catalyze the decomposition of H2O2 to generate oxygen, its catalytic activity is not high in weakly acidic tumor environments.
Platinum (Pt)-based nanomaterials have drawn interest in applications of tumor diagnosis and treatment because of their near-infrared spectral absorption property and good biocompatibility. Pt nanoparticles possess both CAT-like and POD-like activity dependent on temperature and pH [92]. Many works have used Pt with diverse forms to generate oxygen for tumor therapy [93,94]. Zhang's group designed a hybrid core-shell nanoplatform, containing polydopamine cores, Pt nanoparticle interlay and porphyrin-based MOF shells, to ameliorate a hypoxic microenvironment and enhance generation of lethal ROS [95]. Thereby, the nanoplatform reduced invasion and metastasis of tumors (Fig. 3D). Liu's group designed a nanozyme based on Pt with dual enzyme-like activities (PtFe@Fe3O4) [96]. Under acidic conditions, the nanozyme exhibited both CAT-like activities and POD-like activities. PtFe@Fe3O4 could effectively overcome hypoxia and inhibit pancreatic cell growth. In addition to the dual enzyme activities with CAT-like and POD-like activities, Pt nanoparticles could also be combined with Au nanoparticles to construct CAT-like and glucose oxidase-like nanozyme [97]. The nanozyme shell with porphyrin-based MOF could be used for enhancement of synergistic tumor therapy with prevention of tumor metastasis and recurrence. Apart from Pt nanoparticles, a Pt complex, cis, trans, cis-[Pt(N3)2(OH)2(NH3)2], generated oxygen to alleviate hypoxia under light irradiation [98].
Although these natural enzymes and nanozymes are able to catalyze H2O2 to generate oxygen in tumor sites for relieving tumor hypoxia, the amount of endogenous H2O2 in tumor tissues is restricted and it is difficult to generate abundant oxygen for efficient tumor therapy [99–101]. Liu's group developed a strategy to separately deliver H2O2 and CAT to tumor sites by liposomes. This strategy generated enough oxygen without the limitations of H2O2, which could not only enhance the therapeutic effects of radiotherapy but also help to reverse an immunosuppressive tumor microenvironment to favor antitumor immunity [102]. Although this strategy is novel and helps resolve the insufficient H2O2 at the tumor site, direct delivery of H2O2 in the blood circulation could lead to toxicity to organs because of its strong oxidizing property. Considering these problems, some researchers attempted to decompose H2O which is abundant at tumor sites in replacement of H2O2 for oxygen generation. In recent years, keen interest has been paid to water-splitting materials because of their potential applications in energy and environmental areas. Our group wondered about expanding the application of water-splitting materials to biomedical areas in vivo. Some natural materials (e.g. thylakoid) could be used to achieve efficient oxygen generation. Under 660 nm laser irradiation, the thylakoid membrane participated in the photosynthetic electron-transfer reaction to generate oxygen. Thylakoid membrane-coated synthetic nanoparticles normalized the hypoxic microenvironment and inhibited anaerobic respiration for hypoxic tumor treatment (Fig. 4A) [103]. However, extraction and preservation of thylakoid are complicated and the efficiency of oxygen generation is influenced by the physiologic environment. It is recognized that carbon nitride (C3N4, a water-splitting material) without the metal elements has potential for biomedical applications. Unfortunately, the absorption light of pure C3N4 is in the ultraviolet and visible range, which limits its biomedical applications because of low penetration depth with potential side effects of skin damage. Whereas the carbon dots were decorated into C3N4, the nanocomposite possessed enhanced red light absorption which could decompose H2O for oxygen generation [104]. With the addition of photosensitizer, the decorated C3N4 could overcome hypoxia and enhance the efficiency of PDT (Fig. 4B). Iron-doped C3N4 under two-photon irradiation has also been used to generate oxygen for tumor PDT [105]. Although C3N4 can be decorated and the absorption can be red-shifted, the modification is complex and time-consuming. Unlike C3N4, tungsten nitride (WN) with metallic property which can split water at a wavelength of 765 nm, is a promising material for oxygen production in vivo. WN has directly been used to provide oxygen via water-splitting for tumor oxygenation to treat tumor [106]. Water-splitting materials are often used for generating clean energy in the field of energy and environment, although the biocompatibility of these materials should be deliberated before clinical trial. Furthermore, high light energy is needed for these materials to split water into oxygen in vivo, which may lead to localized burning at the light irradiation site.
Figure 4.
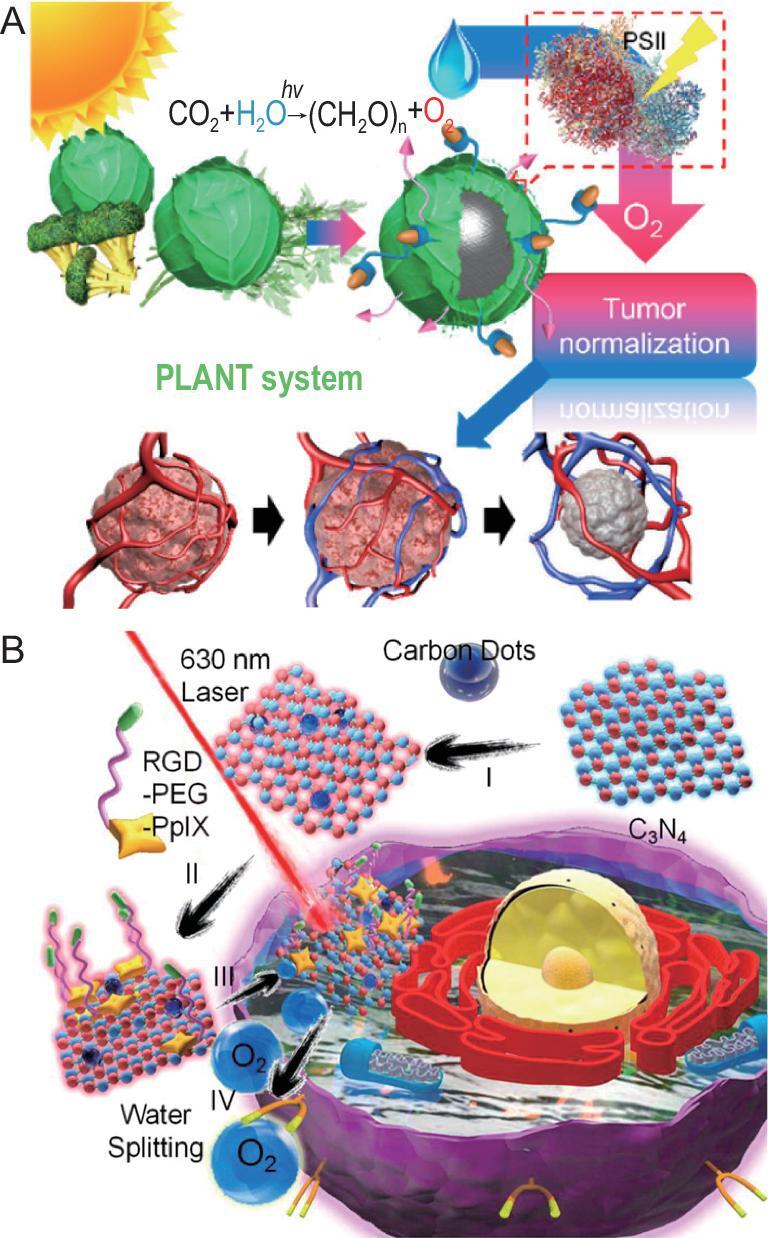
H2O-based oxygen-generating nanomaterials for hypoxic tumor therapy. (A) Tumor normalization by oxygen generation with thylakoid membrane-coated nanoparticles. Reproduced with permission from [103]. Copyright 2018, American Chemical Society. (B) Light-driven water splitting of nanocomposite for oxygen-generated sensitized PDT. Reproduced with permission from [104]. Copyright 2016, American Chemical Society.
Oxygen-economizing nanomaterials for hypoxic tumor therapy
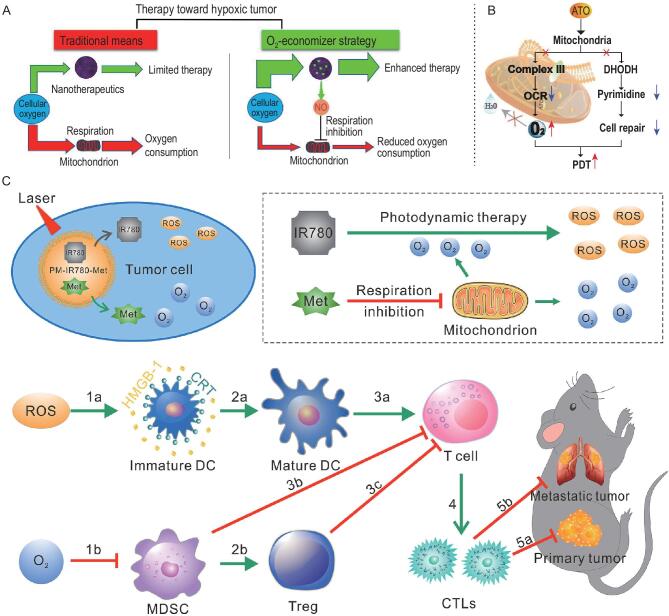
Respiration is the main mechanism that consumes oxygen in living cells [107]. Even though aerobic glycolysis is the major way for tumor cells to acquire energy, mitochondrial respiration still plays an important role in progression of tumors [108]. There has been research interest in the strategy of inhibiting respiration to economize oxygen for enhanced tumor therapy. Feng's group put forward the concept of an ‘oxygen-economizer’ to inhibit cell respiration and reduce oxygen consumption by relieving tumor hypoxia (Fig. 5A). The respiration was inhibited by the in situ generated NO in PLGA nanoparticles loaded with NO donor and Ce6, which could spare endogenous oxygen and further overcome the hypoxia barrier to enhance PDT [109]. Similarly, another group developed a method to reduce oxygen consumption by inhibiting mitochondria-associated oxidative phosphorylation with the oxygen-regulator atovaquone (ATO) (Fig. 5B). The ATO was self-assembled with PLGA-polyethylene glycol (PLGA-PEG) and photosensitizer verteporfin (VER) to form nanoparticles, which exhibited powerful antitumor PDT effects both in vitro and in vivo under laser irradiation [110]. ATO could also be encapsulated with indocyanine green derivatives in gelatin, which is sensitive for matrix metallopeptidase 2 (MMP-2) enzyme. This size-shrinkable gelatin-based vehicle stays intact before accumulating in tumor tissues, then the particle is transiently ruptured in the presence of MMP2 which is overexpressed in tumor tissues. This behavior has helped nanoparticles to enter deeply located, hypoxic regions to enhance tumor therapy [111]. Except for nanocarriers, ATO and a photosensitizer of Ce6-based self-delivery nanomedicine were designed via π−π stacking and hydrophobic interaction (ASCN). ACSN was carrier-free with a high drug loading rate and low excipient-induced systemic toxicity. The solubility and stability of ATO and Ce6 were dramatically improved by ACSN, which strengthened the cellular internalization and intratumoral permeability. The use of nanomedicine ACSN has been observed to result in tumor hypoxia relief and tumor growth inhibition [112]. As a potential alternative to ATO, metformin, commonly used as a first-line treatment for type II diabetes, can directly inhibit the activity of complex I in the mitochondrial electron transport chain. Thus, metformin was used to inhibit cell mitochondrial respiration and economize oxygen for tumor oxygenation [113]. Hydrophilic metformin and modified hydrophobic Ce6 were co-encapsulated in liposomes to modulate tumor hypoxia and enhance tumor PDT when the tumors were exposed to 660 nm laser light [114]. It was reported that metformin co-loaded with IR780 in amphipathic poly(ϵ-caprolactone)-poly(ethylene glycol)(PEG-PCL) was used to defeat relapsed and refractory malignancies by the synergistic effects of tumor oxygenation and PDT/PTT [115]. Furthermore, when the carriers were changed to longer circulation lifetime carriers of platelet membranes (PM), the alleviation of tumor hypoxia by inhibition of mitochondrial respiration with metformin could weaken the myeloid derived suppressor cell (MDSC)-regulated immunosuppressive pathway, and the oxygen-boosted PDT could trigger the immunogenic cell death (ICD)-based pathway (Fig. 5C). Finally, the activated immune system inhibited tumor metastasis [116]. These results showed that inhibiting mitochondrial respiration effectively oxygenated tumor and confirmed good therapeutic effects for tumor inhibition when combined with other therapeutics. Further ways to modulate cell metabolism and economize oxygen by reducing oxygen consumption in tumors should be explored. A novel strategy to supplement oxygen by reducing oxygen consumption, as well as many misgivings including the efficiency of hypoxia alleviation and the side effects of inhibiting mitochondria respiration, is emerging.
Figure 5.
Oxygen-economizing nanomaterials for hypoxic tumor therapy. (A) Traditional therapeutic means and oxygen-economizer strategy for tumor treatments. Reproduced with permission from [109]. Copyright 2019, American Chemical Society. (B) Mechanism of enhancement of PDT by decreasing the cell oxygen consumption rate and improving the oxygen content by ATO. Reproduced with permission from [111]. Copyright 2019, Wiley-VCH. (C) Mechanism of enhancement of PDT and inhibiting mitochondrial respiration by IR780 and metformin to evoke immune response and inhibit primary tumor progression and tumor metastasis [116]. Reproduced with permission. Copyright 2020, Elsevier.
DIMINISHING OXYGEN DEPENDENCE OF NANOMATERIALS FOR HYPOXIC TUMOR THERAPY
Oxygen supply at tumor sites could improve oxygen-dependent tumor therapy; however, the increased oxygen at tumor sites is restive. If the increase in oxygen is insufficient, the promotion of oxygen-dependent tumor therapy is limited; on the contrary, the increased oxygen in the host may lead to side effects. For instance, it is recognized that hyperbaric oxygen therapy may cause diseases of hyperoxic seizures or barotrauma sickness [117]. Therefore, oxygen-independent tumor therapies are urgently required to treat hypoxic tumors.
Therapeutic gas-generating nanomaterials for hypoxic tumor therapy
Some gaseous molecules play an essential role in physiological modulation. Gas therapy, a promising therapeutic strategy, is under development for treatment of inflammation-associated diseases. Among these, distinguished therapeutic effects of NO on cardiovascular diseases generated a Nobel Prize in Physiology or Medicine in 1998. Gaseous molecules can freely diffuse into deep tumor tissue. As a consequence, gas therapy has potential for treatment of hypoxic tumors because of it great penetrability. As gas is apt to aimlessly diffuse throughout the whole body, approaches are required for its delivery in a controllable concentration and specific accumulation in tumor tissues to reduce any potential systemic side effects. To date, gas therapy treatments have mainly involved inhalation of gas, oral administration or gas prodrugs. However, both the inhalation and oral administration of gas are impeded by non-specific delivery and off-target toxicities. Strategies using gas prodrugs or nanomedicines offer a potential solution for gas-controllable delivery in biomedical applications.
NO, a messenger molecule, has essential roles in several physiological functions [118,119], including the immune response, angiogenesis and cardiovascular homeostasis. However, NO is a concentration-dependent ‘double-edged sword’ in tumor therapy. At high concentration (>1 mM), NO directly kills tumor cells for tumor inhibition. At relatively low concentration (1 μM−1 mM), NO not only inhibits expression of P-glycoprotein and multidrug resistance-associated proteins [120], but also relieves tumor hypoxia, which offers opportunities for synergistic tumor therapy [121]. The mechanisms of NO to relieve hypoxic tumor include modulating blood vessel relaxation to increase blood flow, accelerating the metabolism of intracellular GSH [122] and decreasing tumor oxygen consumption rate to oxygenate tumor [123]. The great effects of NO on alleviation of hypoxia are pushing strategies to achieve targeted delivery and controllable release of NO in tumor tissues in hypoxic microenvironments to be developed for subsequent synergistic therapy [124]. Sortino's group has done lots of work in this area involving NO release in nanoassemblies [125]. To treat hypoxic tumor, Shi's group designed an X-ray-activated synergistic NO gas/radiotherapy system (PEG-USMSs-SNO) via engineering UCNPs with S-nitrosothiol (R-SNO)-grafted mesoporous silica. NO was specifically released after the breakage of S-N bond under X-ray radiation, then the released NO could stimulate hypoxic tumor radiosensitization and further improve the radiotherapeutic effects against hypoxic tumors (Fig. 6A) [126]. Besides its sensitization functions, NO has been reported to treat deeply hypoxic tumor because of its free diffusing functions. An NO generator was designed by oxidation of l-arginine, which was incorporated into a porphyrinic metal-organic framework (l-Arg@PCN). The l-Arg@PCN was subsequently coated with homologous targeting tumor cell membrane to accumulate in tumor tissues. Local generation of ROS by PCN under NIR irradiation led to the converse, l-Arg into NO. By combining PDT and gas therapy, an increase in oxidative/nitrification stress and inhibition of growth and proliferation capability of tumor cells were realized [127]. Gaseous molecules regulate some physiological processes, but the regulatory effects are transient and limited. Combining gas therapy with other therapeutic approaches can yield better therapeutic performance.
Figure 6.
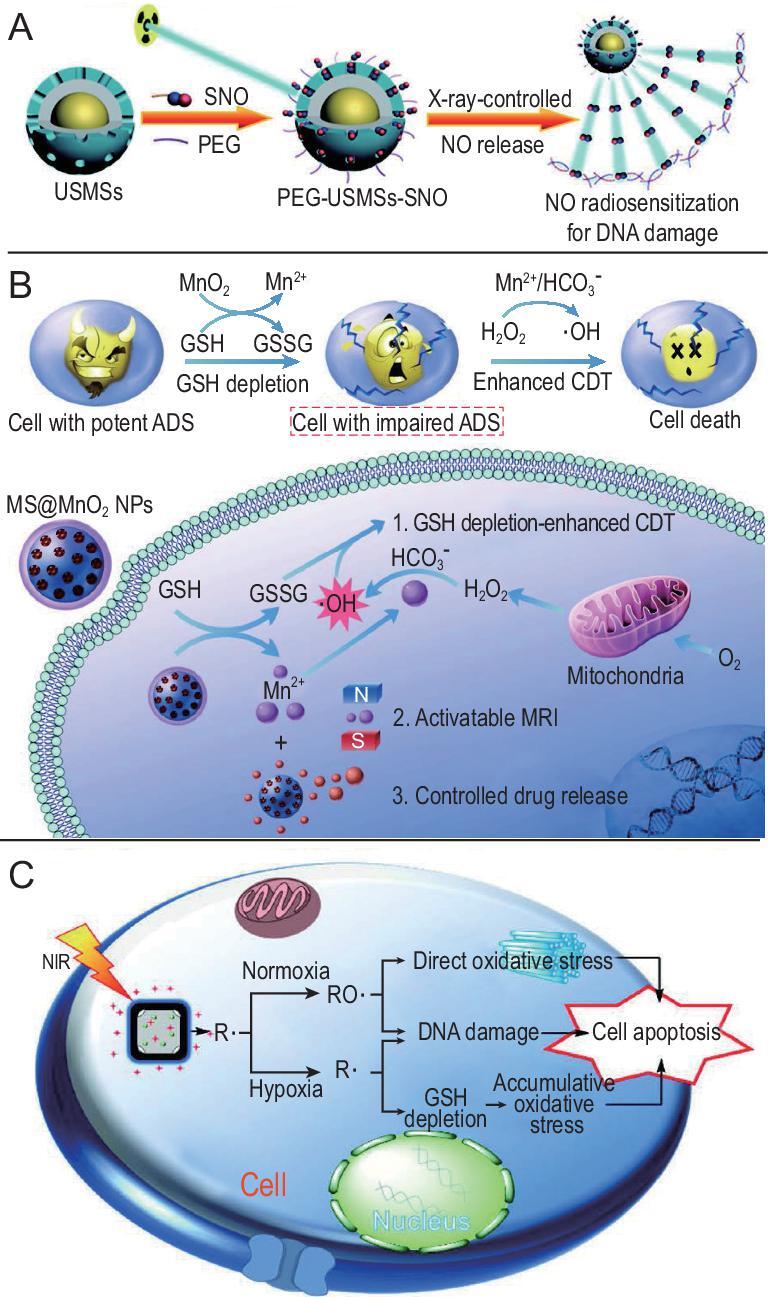
Diminishing oxygen dependence of nanomaterials for hypoxic tumor therapy. (A) X-ray controlled NO release to sensitize radiotherapy for DNA damage. Reproduced with permission from [126]. Copyright 2015, Wiley-VCH. (B) The mechanism of MnO2 as a smart chemodynamic agent for enhanced CDT of tumors. Reproduced with permission from [141]. Copyright 2017, Wiley-VCH. (C) Therapeutic mechanism of the free radicals under different oxygen tensions. Reproduced with permission from [146]. Copyright 2017, Wiley-VCH.
Radical-generating nanomaterials for hypoxic tumor therapy
Free radicals exist within living organisms to maintain homeostasis. Almost all free radicals are highly reactive because of their unpaired valence electrons. They play important roles in cell metabolism in the normal level, but at abnormally high levels they can randomly destruct cellular ingredients (lipid membrane, proteins and DNA) and cause cell death. Unlike type II PDT (singlet oxygen generation based on energy transfer from excited photosensitizers to oxygen molecules) generating singlet oxygen dependent on oxygen and only initiated in a well-oxygenated environment, free radicals such as hydroxyl radicals (·OH) and carbon-centered radicals (R·) can be produced in the absence of oxygen [128]. Because of their high reactivity and oxygen-independent generation ability, free radicals have potential hypoxic tumor therapy.
In contrast to the type II PDT used in most existing PDT systems, type I PDT can transfer energy from photosensitizers to other substrates (e.g. H2O) and produce respective free radicals. Many studies have reported that type I PDT has great efficiency, even under hypoxic conditions [129,130]. Titanium dioxide (TiO2) is widely used in nanomaterials, with high photocatalytic activity and good biocompatibility. When TiO2 nanomaterials absorb suitable photon energy (generally ultraviolet), the photogenerated holes can oxidize the surrounding H2O to form ·OH [131]. A series of studies on the killing effects of TiO2 on different tumor cells was carried out in vitro under ultraviolet light irradiation. Subsequent animal experiments demonstrated the good antitumor effect of TiO2, with the skin at tumor sites cut open for irradiation because of the limits of penetration of ultraviolet [132]. Attempts were made to prolong the activated wavelength of TiO2 for better antitumor effects in vivo. In one instance, a carbon-nanodot-decorated TiO2 nanotube composite was developed as carbon-nanodot can transfer light from 650 nm to 325–425 nm, which prolonged the activated wavelength to produce ·OH [133]. UCNP (e.g. NaYF4: Yb3+, Tm3+)-decorated TiO2 have been developed via near-infrared-to-ultraviolet optical conversion for near-infrared light triggering photochemical reaction of TiO2 [134]. Hydrogenated TiO2 demonstrated efficient optical absorption of near-infrared light, which is beneficial for direct excitation of TiO2 with near-infrared irradiation [135]. However, there are still some challenges to clinical applications of TiO2, such as the efficiency of type II PDT and the biocompatibility.
Some metal irons (e.g. Fe2+, Mn2+) can transform H2O2 into ·OH without the help of oxygen and external energy input, according to Fenton or Fenton-like reactions [136]. Because ·OH is able to oxidize most organic molecules including lipids, proteins and DNA at high rates, Fenton or Fenton-like reactions can be used to kill tumor cells independently of oxygen [137]. Bu's group first suggested the concept of chemodynamic therapy (CDT), which used Fenton or Fenton-like reactions to generate ·OH and induce cell death independent of oxygen concentration and without the need for external energy input by laser irradiation [138]. PEGylated single-atom Fe-containing nanocatalysts have been reported that can effectively generate ·OH in an acidic tumor microenvironment [139,140]. Chen's group designed a self-reinforcing CDT nanoagent based on MnO2, which depleted intracellular reductive glutathione, and the generated Mn2+ induced a Fenton-like reaction to destroy tumor cells [141]. Specifically, in the presence of bicarbonate (HCO3−), MnO2 reacted with glutathione to yield glutathione disulfide and Mn2+, which could trigger ·OH production from H2O2 with the help of HCO3− (Fig. 6B). A porous MIL-100(Fe) layer was coated on Fe3O4 nanoparticles, followed by attachment of upconversion nanoparticles (Fe3O4@MIL-100(Fe)-UCNPs, or FMU) for hypoxic tumor therapy. UCNPs could transfer NIR to UV/vis light, and Fe3O4@MIL-100(Fe) absorbed UV/vis light to generate ·OH independent of tumor oxygen. Based on a photo-Fenton reaction, FMU could produce more ·OH for hypoxic tumor therapy [142]. Similar to the above mentioned problem of the limited content of endogenous H2O2, the amounts of endogenous H2O2 also limit the catalytic efficacy of the Fenton reaction. Zhang's group developed an ATP-responsive Fenton nanosystem by autocatalyzing Fe3+ into Fe2+ as well as the generation of H2O2 under glucose oxidase to produce ·OH. The nanosystem generated adequate H2O2 by glucose oxidase catalyzing glucose and then induced remarkable production of ·OH by the Fenton reaction [100]. Vitamin C-loaded mesoporous magnetic nanocubes (MMNCs) with ROS self-generation and self-enhancement also have been used for hypoxic tumor therapy. Vitamin C served as an original source for H2O2 generation and MMNCs possessed Fenton reagent-like activity for ROS self-enhancement to treat hypoxic tumor [143]. Our group developed a Fenton-like bioreactor based on engineered facultative anaerobes for tumor therapy. Facultative anaerobes choose to travel to the tumor site because of the hypoxic tumor microenvironment, which could be used to transport nanomedicine to tumor regions. The bacteria overexpressed respiratory chain enzyme II to increase H2O2 generation, which was beneficial to realize a self-supplied therapeutic Fenton-like reaction [144].
Other than ·OH, R· are generated in the absence of oxygen and high toxicity to cells. Thermally decomposable azo initiators are commonly used in generation of R·, and are always applied in free radical polymerization and biological systems to produce oxidative stress [145]. Generation of radicals by decomposing these initiators is thermally dependent, and thus is limited under physiological conditions. A heater is required to speed up production of free radicals for tumor therapy. Almost simultaneously, Xia's group and Zhang's group designed similar systems with initiator loaded-gold nanocages, which were filled with a phase-change material to realize the controllable production of free radicals for tumor therapy [146,147]. Gold nanocages, photothermal conversion agents with fine biocompatibility, were used both as heat source and initiator carrier. The radical source came from the same initiator, 2,2′-azobis[2-(2-imidazolin-2-yl)propane] dihydrochloride (AIPH). Under NIR irradiation, the produced heat by gold nanocages not only caused decomposition of AIPH to generate R·, but also released the blocked R· with the phase transition of the copolymer. This free radical-based therapy had good antitumor efficacy regardless of whether oxygen was sufficient or not (Fig. 6B). To expand the multifunctional or theranostic applications in hypoxic tumor therapy, it is essential to monitor R· release in real time. A smart free-radical system was developed to monitor production of free radicals in real time. Fluorescent dyes and quenchers were linked with the initiators, which could homolytically cleave in the presence of free radicals. After homolytic cleavage of the initiators, the fluorescent dyes and quenchers were separated and recovered because of the R· production [148]. Although promising, this approach to generate R· for hypoxic tumor therapy requires improvement in some respects before clinical application, such as the potential cytotoxicity from unsatisfactory degradability of inorganic photothermal agents, low initiator loading capacity and limited generation efficiency of R· for hypoxic tumor therapy.
CONCLUSION AND PERSPECTIVES
Recent studies of different strategies to treat hypoxic tumors based on nanomaterials were categorized as (i) elevating oxygen level via nanomaterials in tumor tissue for enhancement of oxygen-dependent tumor therapies and (ii) therapies with diminishing oxygen dependence of nanomaterials. Tumor hypoxia could be relieved by supplying oxygen in tumors through use of oxygen-carrying nanomaterials, oxygen-generating nanomaterials or oxygen-economizing nanomaterials, which sensitized or enhanced therapeutic effects of oxygen-dependent tumor therapy. Because of the complexity of hypoxic tumors, much research has also focused on strategies with diminished oxygen dependence of nanomaterials for hypoxic tumor therapy.
Although lots of exciting results have been put forward over the past decade, challenges remain for clinical applications of nanomaterials for treatment of hypoxic tumors, including biocompatibility and validity of nanomaterials. One of the most critical concerns regarding nanomaterials is the biocompatibility, particularly in nanomaterials containing heavy metals, and nanomaterials with good biocompatibility should be preferentially selected. Similar to implantable nanomaterials, biocompatibility can be improved by surface modification, including chemical modification and physical modification. In addition, because of the dynamic changeable hypoxic conditions, it can be hard to continually alleviate hypoxia in tumor therapy through use of oxygen-carrying nanomaterials or oxygen-generating nanomaterials. Premature release of oxygen from oxygen-carrying materials can occur in the blood circulation, which may lead to cytotoxicity to other host organs. Thus, there is a need to develop nanomaterials subject to controllable release of oxygen rather than simply being oxygen carriers. Fortunately, there have been reports of controllable release of drug from nanomaterials via exogenous or endogenous stimulation, so these approaches may be applicable to controllable release of oxygen from oxygen-carrying nanomaterials. The water-splitting nanomaterials used to generate oxygen also face several problems including low efficiency, poor dispersion and unknown biocompatibility in vivo. Development of water-splitting nanomaterials seems to have hit a ‘bottleneck’, but we believe the related problems will be solved in future. Furthermore, therapies based on nanomaterials with diminished oxygen dependence could directly inhibit growth of tumors, with the potential to avoid the concerns regarding oxygen-related nanomaterials. But specific killing of tumor cells by those nanomaterials with diminished oxygen dependence requires further attention. Targeting ability of such materials may be realized by modifying materials with suitable targeting groups or targeted cell membrane. Furthermore, many nanomaterials used to treat hypoxic tumors are dependent on laser irradiation, but the penetration depth of the used laser in the body is insufficient. Thus, strategies are required for treatment of hypoxic tumors based on nanomaterials without limitation in penetration depth of laser.
There has been much development in immunotherapies in recent years, but the therapeutic effects of immunotherapy can also be limited by hypoxic conditions, e.g. expression of HIF-1α in the tumor microenvironment. Nanomaterials designed to modulate hypoxia and improve tumor immune microenvironment such as immune cells may be explored in future. In all, it is believed that nanomaterials are increasingly important in the field of hypoxic tumor therapy as a result of their great drug delivery, good tumor accumulation and various functions.
Contributor Information
Mei-Zhen Zou, The Institute for Advanced Studies, Key Laboratory of Biomedical Polymers of Ministry of Education & Department of Chemistry, Wuhan University, Wuhan 430072, China.
Wen-Long Liu, School of Chemistry and Materials Science, South-Central University for Nationalities, Wuhan 430074, China.
Han-Shi Chen, The Institute for Advanced Studies, Key Laboratory of Biomedical Polymers of Ministry of Education & Department of Chemistry, Wuhan University, Wuhan 430072, China.
Xue-Feng Bai, The Institute for Advanced Studies, Key Laboratory of Biomedical Polymers of Ministry of Education & Department of Chemistry, Wuhan University, Wuhan 430072, China.
Fan Gao, The Institute for Advanced Studies, Key Laboratory of Biomedical Polymers of Ministry of Education & Department of Chemistry, Wuhan University, Wuhan 430072, China.
Jing-Jie Ye, The Institute for Advanced Studies, Key Laboratory of Biomedical Polymers of Ministry of Education & Department of Chemistry, Wuhan University, Wuhan 430072, China.
Han Cheng, The Institute for Advanced Studies, Key Laboratory of Biomedical Polymers of Ministry of Education & Department of Chemistry, Wuhan University, Wuhan 430072, China.
Xian-Zheng Zhang, The Institute for Advanced Studies, Key Laboratory of Biomedical Polymers of Ministry of Education & Department of Chemistry, Wuhan University, Wuhan 430072, China.
FUNDING
This work was supported by the National Natural Science Foundation of China (51833007, 51988102, 51690152 and 51573142).
Conflict of interest statement. None declared.
REFERENCES
- 1. Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer 2004; 4: 437–47. [DOI] [PubMed] [Google Scholar]
- 2. Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer 2011; 11: 393–410. [DOI] [PubMed] [Google Scholar]
- 3. Rankin EB, Giaccia AJ. Hypoxic control of metastasis. Science 2016; 352: 175–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Petrova V, Annicchiarico-Petruzzelli M, Melino Get al. The hypoxic tumour microenvironment. Oncogenesis 2018; 7: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jahanban-Esfahlan R, de la Guardia M, Ahmadi Det al. Modulating tumor hypoxia by nanomedicine for effective cancer therapy. J Cell Physiol 2018; 233: 2019–31. [DOI] [PubMed] [Google Scholar]
- 6. Bennewith KL, Dedhar S. Targeting hypoxic tumour cells to overcome metastasis. BMC Cancer 2011; 11: 504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sharma A, Arambula JF, Koo Set al. Hypoxia-targeted drug delivery. Chem Soc Rev 2019; 48: 771–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kumar V, Gabrilovich DI. Hypoxia-inducible factors in regulation of immune responses in tumour microenvironment. Immunology 2014; 143: 512–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Palazón A, Aragonés J, Morales-Kastresana Aet al. Molecular pathways: hypoxia response in immune cells fighting or promoting cancer. Clin Cancer Res 2012; 18: 1207–13. [DOI] [PubMed] [Google Scholar]
- 10. Masson N, Ratcliffe PJ. Hypoxia signaling pathways in cancer metabolism: the importance of co-selecting interconnected physiological pathways. Cancer Metab 2014; 2: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rofstad EK, Sundfør K, Lyng Het al. Hypoxia-induced treatment failure in advanced squamous cell carcinoma of the uterine cervix is primarily due to hypoxia-induced radiation resistance rather than hypoxia-induced metastasis. Br J Cancer 2000; 83: 354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Höckel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst 2001; 93: 266–76. [DOI] [PubMed] [Google Scholar]
- 13. Shannon AM, Bouchier-Hayes DJ, Condron CMet al. Tumour hypoxia, chemotherapeutic resistance and hypoxia-related therapies. Cancer Treat Rev 2003; 29: 297–307. [DOI] [PubMed] [Google Scholar]
- 14. Lu J, Yang L, Zhang Wet al. Photodynamic therapy for hypoxic solid tumors via Mn-MOF as a photosensitizer. Chem Commun 2019; 55: 10792–5. [DOI] [PubMed] [Google Scholar]
- 15. Liu Y, Liu Y, Bu Wet al. Hypoxia induced by upconversion-based photodynamic therapy: towards highly effective synergistic bioreductive therapy in tumors. Angew Chem Int Ed 2015; 54: 8105–9. [DOI] [PubMed] [Google Scholar]
- 16. Liu Y, Jiang Y, Zhang Met al. Modulating hypoxia via nanomaterials chemistry for efficient treatment of solid tumors. Acc Chem Res 2018; 51: 2502–11. [DOI] [PubMed] [Google Scholar]
- 17. Fan W, Yung B, Huang Pet al. Nanotechnology for multimodal synergistic cancer therapy. Chem Rev 2017; 117: 13566–638. [DOI] [PubMed] [Google Scholar]
- 18. Cheon J, Chan W, Zuhorn I. The Future of nanotechnology: cross-disciplined progress to improve health and medicine. Acc Chem Res 2019; 52: 2405. [DOI] [PubMed] [Google Scholar]
- 19. Mostafavi E, Soltantabar P, Webster TJ. Nanotechnology and picotechnology: a new arena for translational medicine. In: Yang L, Bhaduri SB, Webster TJ (eds). Biomaterials in Translational Medicine. Oxford: Elsevier, 2019, 191–212. [Google Scholar]
- 20. Wang J, Chen HJ, Hang Tet al. Physical activation of innate immunity by spiky particles. Nat Nanotechnol 2018; 13: 1078–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Montenegro JM, Grazu V, Sukhanova Aet al. Controlled antibody/(bio-) conjugation of inorganic nanoparticles for targeted delivery. Adv Drug Deliv Rev 2013; 65: 677–88. [DOI] [PubMed] [Google Scholar]
- 22. Molinaro R, Corbo C, Martinez JOet al. Biomimetic proteolipid vesicles for targeting inflamed tissues. Nat Mater 2016; 15: 1037–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brannon-Peppas L, Blanchette JO. Nanoparticle and targeted systems for cancer therapy. Adv Drug Deliv Rev 2004; 56: 1649–59. [DOI] [PubMed] [Google Scholar]
- 24. Sun W, Ji W, Hall JMet al. Self-assembled DNA nanoclews for the efficient delivery of CRISPR-Cas9 for genome editing. Angew Chem Int Ed 2015; 54: 12029–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cheng ZL, Zaki Al, Hui JZet al. Multifunctional nanoparticles: cost versus benefit of adding targeting and imaging capabilities. Science 2012; 338: 903–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liang R, Wei M, Evans DGet al. Inorganic nanomaterials for bioimaging, targeted drug delivery and therapeutics. Chem Commun 2014; 50: 14071–81. [DOI] [PubMed] [Google Scholar]
- 27. Guo ST, Huang L. Nanoparticles containing insoluble drug for cancer therapy. Biotechnol Adv 2014; 32: 778–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tian H, Luo Z, Liu Let al. Cancer cell membrane-biomimetic oxygen nanocarrier for breaking hypoxia-induced chemoresistance. Adv Funct Mater 2017; 27: 1703197. [Google Scholar]
- 29. Yang Y, Chen M, Wang Bet al. NIR-II driven plasmon-enhanced catalysis for timely supply of oxygen to overcome hypoxia induced radiotherapy tolerance. Angew Chem Int Ed 2019; 58:15069–75. [DOI] [PubMed] [Google Scholar]
- 30. Li J, Shang W, Li Yet al. Advanced nanomaterials targeting hypoxia to enhance radiotherapy. Int J Nanomed 2018; 13: 5925–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ruf M, Moch H, Schraml P. PD-L1 expression is regulated by hypoxia inducible factor in clear cell renal cell carcinoma. Int J Cancer 2016; 139: 396–403. [DOI] [PubMed] [Google Scholar]
- 32. Khan MS, Hwang J, Lee Ket al. Anti-tumor drug-loaded oxygen nanobubbles for the degradation of HIF-1α and the upregulation of reactive oxygen species in tumor cells. Cancers 2019; 11: 1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Iijima M, Gombodorj N, Tachibana Yet al. Development of single nanometer-sized ultrafine oxygen bubbles to overcome the hypoxia-induced resistance to radiation therapy via the suppression of hypoxia-inducible factor-1. Int J Oncol 2018; 52: 679–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Song R, Peng S, Lin Qet al. pH-responsive oxygen nanobubbles for spontaneous oxygen delivery in hypoxic tumors. Langmuir 2019; 35: 10166–72. [DOI] [PubMed] [Google Scholar]
- 35. Bhandari PN, Cui Y, Elzey BDet al. Oxygen nanobubbles revert hypoxia by methylation programming. Sci Rep 2017; 7: 9268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Song R, Hu D, Chung HYet al. Lipid-polymer bilaminar oxygen nanobubbles for enhanced photodynamic therapy of cancer. ACS Appl Mater & Inter 2018; 10: 36805–13. [DOI] [PubMed] [Google Scholar]
- 37. Owen J, McEwan C, Nesbitt Het al. Reducing tumour hypoxia via oral administration of oxygen nanobubbles. PLoS One 2016; 11: e0168088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Song L, Wang G, Hou Xet al. Biogenic nanobubbles for effective oxygen delivery and enhanced photodynamic therapy of cancer. Acta Biomater 2020.108: 313–25. [DOI] [PubMed] [Google Scholar]
- 39. Kheir JN, Scharp LA, Borden MAet al. Oxygen gas-filled microparticles provide intravenous oxygen delivery. Sci Transl Med 2012; 4: 140ra88. [DOI] [PubMed] [Google Scholar]
- 40. Cavalli R, Soster M, Argenziano M. Nanobubbles: a promising efficient tool for therapeutic delivery. Ther Deliv 2016; 7: 117–38. [DOI] [PubMed] [Google Scholar]
- 41. Jensen FB. The dual roles of red blood cells in tissue oxygen delivery: oxygen carriers and regulators of local blood flow. J Exp Biol 2009; 212: 3387–93. [DOI] [PubMed] [Google Scholar]
- 42. Gaudard A, Varlet-Marie E, Bressolle Fet al. Drugs for increasing oxygen transport and their potential use in doping. Sports Med 2003; 33: 187–212. [DOI] [PubMed] [Google Scholar]
- 43. Tsuchida E, Sou K, Nakagawa Aet al. Artificial oxygen carriers, hemoglobin vesicles and albumin−hemes, based on bioconjugate chemistry. Bioconjugate Chem 2009; 20: 1419–40. [DOI] [PubMed] [Google Scholar]
- 44. Grimshaw K, Sahler J, Spinelli SLet al. New frontiers in transfusion biology: identification and significance of mediators of morbidity and mortality in stored red blood cells. Transfusion 2011; 51: 874–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tang W, Zhen Z, Wang Met al. Red blood cell-facilitated photodynamic therapy for cancer treatment. Adv Funct Mater 2016; 26: 1757–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen Z, Liu L, Liang Ret al. Bioinspired hybrid protein oxygen nanocarrier amplified photodynamic therapy for eliciting anti-tumor immunity and abscopal effect. ACS Nano 2018; 12: 8633–45. [DOI] [PubMed] [Google Scholar]
- 47. Jiang L, Bai H, Liu Let al. Luminescent, oxygen-supplying, hemoglobin-linked conjugated polymer nanoparticles for photodynamic therapy. Angew Chem Int Ed 2019; 58: 10660–5. [DOI] [PubMed] [Google Scholar]
- 48. Yang J, Li W, Luo Let al. Hypoxic tumor therapy by hemoglobin-mediated drug delivery and reversal of hypoxia-induced chemoresistance. Biomaterials 2018; 182: 145–56. [DOI] [PubMed] [Google Scholar]
- 49. Jia Q, Ge J, Liu Wet al. A magnetofluorescent carbon dot assembly as an acidic H2O2-driven oxygenerator to regulate tumor hypoxia for simultaneous bimodal imaging and enhanced photodynamic therapy. Adv Mater 2018; 30: 1706090. [DOI] [PubMed] [Google Scholar]
- 50. Jia Y, Duan L, Li J. Hemoglobin-based nanoarchitectonic assemblies as oxygen carriers. Adv Mater 2016; 28: 1312–8. [DOI] [PubMed] [Google Scholar]
- 51. Sun K, Zhang Y, D’Alessandro Aet al. Sphingosine-1-phosphate promotes erythrocyte glycolysis and oxygen release for adaptation to high-altitude hypoxia. Nat Commun 2016; 7: 12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. D’Agnillo F, Chang TMS. Polyhemoglobin-superoxide dismutase-catalase as a blood substitute with antioxidant properties. Nat Biotechnol 1998; 16: 667–71. [DOI] [PubMed] [Google Scholar]
- 53. Hathazi D, Mot AC, Vaida Aet al. Oxidative protection of hemoglobin and hemerythrin by cross-linking with a nonheme iron peroxidase: potentially improved oxygen carriers for use in blood substitutes. Biomacromolecules 2014; 15: 1920–7. [DOI] [PubMed] [Google Scholar]
- 54. Liu WL, Liu T, Zou MZet al. Aggressive man-made red blood cells for hypoxia-resistant photodynamic therapy. Adv Mater 2018; 30: 1802006. [DOI] [PubMed] [Google Scholar]
- 55. Riess JG. Understanding the fundamentals of perfluorocarbons and perfluorocarbon emulsions relevant to in vivo oxygen delivery. Artif Cells Blood Substitutes Biotechnol 2005; 33: 47–63. [DOI] [PubMed] [Google Scholar]
- 56. Spiess BD. Perfluorocarbon emulsions as a promising technology: a review of tissue and vascular gas dynamics. J Appl Physiol 2009; 106: 1444–52. [DOI] [PubMed] [Google Scholar]
- 57. Wu L, Wen X, Wang Xet al. Local intratracheal delivery of perfluorocarbon nanoparticles to lung cancer demonstrated with magnetic resonance multimodal imaging. Theranostics 2018; 8: 563–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ahrens ET, Helfer BM, O’Hanlon CFet al. Clinical cell therapy imaging using a perfluorocarbon tracer and fluorine-19 MRI. Magn Reson Med 2014; 72: 1696–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cheng Y, Cheng H, Jiang Cet al. Perfluorocarbon nanoparticles enhance reactive oxygen levels and tumour growth inhibition in photodynamic therapy. Nat Commun 2015; 6: 8785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Song G, Liang C, Yi Xet al. Perfluorocarbon-loaded hollow Bi2Se3 nanoparticles for timely supply of oxygen under near-infrared light to enhance the radiotherapy of cancer. Adv Mater 2016; 28: 2716–23. [DOI] [PubMed] [Google Scholar]
- 61. Yu Z, Zhou P, Pan Wet al. A biomimetic nanoreactor for synergistic chemiexcited photodynamic therapy and starvation therapy against tumor metastasis. Nat Commun 2018; 9: 5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chen J, Luo H, Liu Yet al. Oxygen-self-produced nanoplatform for relieving hypoxia and breaking resistance to sonodynamic treatment of pancreatic cancer. ACS Nano 2017; 11: 12849–62. [DOI] [PubMed] [Google Scholar]
- 63. Gao M, Liang C, Song Xet al. Erythrocyte-membrane-enveloped perfluorocarbon as nanoscale artificial red blood cells to relieve tumor hypoxia and enhance cancer radiotherapy. Adv Mater 2017; 27: 1701429. [DOI] [PubMed] [Google Scholar]
- 64. Hu D, Zhong L, Wang Met al. Perfluorocarbon-loaded and redox-activatable photosensitizing agent with oxygen supply for enhancement of fluorescence/photoacoustic imaging guided tumor photodynamic therapy. Adv Funct Mater 2019; 29: 1806199. [Google Scholar]
- 65. Zhuang J, Ying M, Spiekermann Ket al. Biomimetic nanoemulsions for oxygen delivery in vivo. Adv Mater 2018; 30: 1804693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zou MZ, Liu WL, Gao Fet al. Artificial natural killer cells for specific tumor inhibition and renegade macrophage re-education. Adv Mater 2019; 31: 1904495. [DOI] [PubMed] [Google Scholar]
- 67. Song X, Feng L, Liang Cet al. Ultrasound triggered tumor oxygenation with oxygen-shuttle nanoperfluorocarbon to overcome hypoxia-associated resistance in cancer therapies. Nano Lett 2016; 16: 6145–53. [DOI] [PubMed] [Google Scholar]
- 68. Gu Z, Biswas A, Zhao Met al. Tailoring nanocarriers for intracellular protein delivery. Chem Soc Rev 2011; 40: 3638–55. [DOI] [PubMed] [Google Scholar]
- 69. Zou MZ, Liu WL, Li CXet al. A multifunctional biomimetic nanoplatform for relieving hypoxia to enhance chemotherapy and inhibit the PD-1/PD-L1 axis. Small 2018; 14: e1801120. [DOI] [PubMed] [Google Scholar]
- 70. Cheng H, Zhu JY, Li SYet al. An O2 self-sufficient biomimetic nanoplatform for highly specific and efficient photodynamic therapy. Adv Funct Mater 2016; 26: 7847–60. [Google Scholar]
- 71. Phua SZF, Yang G, Lim WQet al. Catalase-integrated hyaluronic acid as nanocarriers for enhanced photodynamic therapy in solid tumor. ACS Nano 2019; 13: 4742–51. [DOI] [PubMed] [Google Scholar]
- 72. Li G, Yuan S, Deng Det al. Fluorinated polyethylenimine to enable transmucosal delivery of photosensitizer-conjugated catalase for photodynamic therapy of orthotopic bladder tumors postintravesical instillation. Adv Funct Mater 2019; 29: 1901932. [Google Scholar]
- 73. Li G, Wang S, Deng Det al. Fluorinated chitosan to enhance transmucosal delivery of sonosensitizer-conjugated catalase for sonodynamic bladder cancer treatment post-intravesical instillation. ACS Nano 2020; 14: 1586–99. [DOI] [PubMed] [Google Scholar]
- 74. Meng Z, Zhou X, Xu Jet al. Light-triggered in situ gelation to enable robust photodynamic-immunotherapy by repeated stimulations. Adv Mater 2019; 31: 1900927. [DOI] [PubMed] [Google Scholar]
- 75. Song G, Chen Y, Liang Cet al. Catalase-loaded TaOx nanoshells as bio-nanoreactors combining high-Z element and enzyme delivery for enhancing radiotherapy. Adv Mater 2016; 28: 7143–8. [DOI] [PubMed] [Google Scholar]
- 76. Wei H, Wang EK. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes. Chem Soc Rev 2013; 42: 6060–93. [DOI] [PubMed] [Google Scholar]
- 77. Liang MM, Yan XY. Nanozymes: from new concepts, mechanisms, and standards to applications. Acc Chem Res 2019; 52: 2190–200. [DOI] [PubMed] [Google Scholar]
- 78. Prasad P, Gordijo CR, Abbasi AZet al. Multifunctional albumin–MnO2 nanoparticles modulate solid tumor microenvironment by attenuating hypoxia, acidosis, vascular endothelial growth factor and enhance radiation response. ACS Nano 2014; 8: 3202–12. [DOI] [PubMed] [Google Scholar]
- 79. Zhang W, Li S, Liu Xet al. Oxygen-generating MnO2 nanodots-anchored versatile nanoplatform for combined chemo-photodynamic therapy in hypoxic cancer. Adv Funct Mater 2018; 28: 1706375. [Google Scholar]
- 80. Gordijo CR, Abbasi AZ, Amini MAet al. Design of hybrid MnO2-polymer-lipid nanoparticles with tunable oxygen generation rates and tumor accumulation for cancer treatment. Adv Funct Mater 2015; 25: 1858–72. [Google Scholar]
- 81. Yang X, Yang Y, Gao Fet al. Biomimetic hybrid nanozymes with self-supplied H+ and accelerated O2 generation for enhanced starvation and photodynamic therapy against hypoxic tumors. Nano Lett 2019; 19: 4334–42. [DOI] [PubMed] [Google Scholar]
- 82. Zhang X, Xi Z, Machuki JOet al. Gold cube-in-cube based oxygen nanogenerator: a theranostic nanoplatform for modulating tumor microenvironment for precise chemo-phototherapy and multimodal imaging. ACS Nano 2019; 13: 5306–25. [DOI] [PubMed] [Google Scholar]
- 83. Yu M, Duan X, Cai Yet al. Multifunctional nanoregulator reshapes immune microenvironment and enhances immune memory for tumor immunotherapy. Adv Sci 2019; 6: 1900037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Yang G, Xu L, Chao Yet al. Hollow MnO2 as a tumor-microenvironment-responsive biodegradable nano-platform for combination therapy favoring antitumor immune responses. Nat Commun 2017; 8: 902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zhang C, Chen WH, Liu LHet al. An O2 self-supplementing and reactive-oxygen-species-circulating amplified nanoplatform via H2O/H2O2 splitting for tumor imaging and photodynamic therapy. Adv Funct Mater 2017; 27: 1700626. [Google Scholar]
- 86. Liu C, Wang D, Zhang Set al. Biodegradable biomimic copper/manganese silicate nanospheres for chemodynamic/photodynamic synergistic therapy with simultaneous glutathione depletion and hypoxia relief. ACS Nano 2019; 13: 4267–77. [DOI] [PubMed] [Google Scholar]
- 87. Kim J, Kim HY, Song SYet al. Synergistic oxygen generation and reactive oxygen species scavenging by manganese ferrite/ceria co-decorated nanoparticles for rheumatoid arthritis treatment. ACS Nano 2019; 13: 3206–17. [DOI] [PubMed] [Google Scholar]
- 88. Zhang W, Hu S, Yin JJet al. Prussian blue nanoparticles as multienzyme mimetics and reactive oxygen species scavengers. J Am Chem Soc 2016; 138: 5860–5. [DOI] [PubMed] [Google Scholar]
- 89. Yang ZL, Tian W, Wang Qet al. Oxygen-evolving mesoporous organosilica coated prussian blue nanoplatform for highly efficient photodynamic therapy of tumors. Adv Sci 2018; 5: 1700847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hu JJ, Chen Y, Li ZHet al. Augment of oxidative damage with enhanced photodynamic process and MTH1 inhibition for tumor therapy. Nano Lett 2019; 19: 5568–76. [DOI] [PubMed] [Google Scholar]
- 91. Zhou J, Li M, Hou Yet al. Engineering of a nanosized biocatalyst for combined tumor starvation and low-temperature photothermal therapy. ACS Nano 2018; 12: 2858–72. [DOI] [PubMed] [Google Scholar]
- 92. Fan J, Yin JJ, Ning Bet al. Direct evidence for catalase and peroxidase activities of ferritin-platinum nanoparticles. Biomaterials 2011; 32: 1611–8. [DOI] [PubMed] [Google Scholar]
- 93. Liang S, Deng X, Chang Yet al. Intelligent hollow Pt-CuS janus architecture for synergistic catalysis-enhanced sonodynamic and photothermal cancer therapy. Nano Lett 2019; 19: 4134–45. [DOI] [PubMed] [Google Scholar]
- 94. Wei J, Li J, Sun Det al. A novel theranostic nanoplatform based on Pd@Pt-PEG-Ce6 for enhanced photodynamic therapy by modulating tumor hypoxia microenvironment. Adv Funct Mater 2018; 28: 1706310. [Google Scholar]
- 95. Wang XS, Zeng JY, Zhang MKet al. A versatile Pt-based core-shell nanoplatform as a nanofactory for enhanced tumor therapy. Adv Funct Mater 2018; 28: 1801783. [Google Scholar]
- 96. Li S, Shang L, Xu Bet al. A nanozyme with photo-enhanced dual enzyme-like activities for deep pancreatic cancer therapy. Angew Chem Int Ed 2019; 58: 12624–31. [DOI] [PubMed] [Google Scholar]
- 97. Liu C, Xing J, Akakuru OUet al. Nanozymes-engineered metal–organic frameworks for catalytic cascades-enhanced synergistic cancer therapy. Nano Lett 2019; 19: 5674–82. [DOI] [PubMed] [Google Scholar]
- 98. Xu S, Zhu X, Zhang Cet al. Oxygen and Pt(II) self-generating conjugate for synergistic photo-chemo therapy of hypoxic tumor. Nat Commun 2018; 9: 2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Chen Q, Chen J, Yang Zet al. Nanoparticle-enhanced radiotherapy to trigger robust cancer immunotherapy. Adv Mater 2019; 31: 1802228. [DOI] [PubMed] [Google Scholar]
- 100. Zhang L, Wan SS, Li CXet al. An adenosine triphosphate-responsive autocatalytic Fenton nanoparticle for tumor ablation with self-supplied H2O2 and acceleration of Fe(iii)/Fe(ii) conversion. Nano Lett 2018; 18: 7609–18. [DOI] [PubMed] [Google Scholar]
- 101. Huo MF, Wang LY, Chen Yet al. Tumor-selective catalytic nanomedicine by nanocatalyst delivery. Nat Commun 2017; 8: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Song X, Xu J, Liang Cet al. Self-supplied tumor oxygenation through separated liposomal delivery of H2O2 and catalase for enhanced radio-immunotherapy of cancer. Nano Lett 2018; 18: 6360–8. [DOI] [PubMed] [Google Scholar]
- 103. Zheng DW, Li B, Xu Let al. Normalizing tumor microenvironment based on photosynthetic abiotic/biotic nanoparticles. ACS Nano 2018; 12: 6218–27. [DOI] [PubMed] [Google Scholar]
- 104. Zheng DW, Li B, Li CXet al. Carbon-dot-decorated carbon nitride nanoparticles for enhanced photodynamic therapy against hypoxic tumor via water splitting. ACS Nano 2016; 10: 8715–22. [DOI] [PubMed] [Google Scholar]
- 105. Li RQ, Zhang C, Xie BRet al. A two-photon excited O2-evolving nanocomposite for efficient photodynamic therapy against hypoxic tumor. Biomaterials 2019; 194: 84–93. [DOI] [PubMed] [Google Scholar]
- 106. Wang SB, Zhang C, Liu XHet al. A Tungsten nitride-based O2 self-sufficient nanoplatform for enhanced photodynamic therapy against hypoxic tumors. Adv Ther 2019; 2: 1900012. [Google Scholar]
- 107. Denko NC Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat Rev Cancer 2008; 8: 705–13. [DOI] [PubMed] [Google Scholar]
- 108. Heiden MGV, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009; 324: 1029–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Yu W, Liu T, Zhang Met al. O2 economizer for inhibiting cell respiration to combat the hypoxia obstacle in tumor treatments. ACS Nano 2019; 13: 1784–94. [DOI] [PubMed] [Google Scholar]
- 110. Fan YT, Zhou TJ, Cui PFet al. Modulation of intracellular oxygen pressure by dual-drug nanoparticles to enhance photodynamic therapy. Adv Funct Mater 2019; 29: 1806708. [Google Scholar]
- 111. Xia D, Xu P, Luo Xet al. Overcoming hypoxia by multistage nanoparticle delivery system to inhibit mitochondrial respiration for photodynamic therapy. Adv Funct Mater 2019; 29: 1807294. [Google Scholar]
- 112. Zhao LP, Zheng RR, Chen HQet al. Self-delivery nanomedicine for O2-economized photodynamic tumor therapy. Nano Lett. 2020; 20: 2062–71. [DOI] [PubMed] [Google Scholar]
- 113. Zannella VE, Dal Pra A, Muaddi Het al. Reprogramming metabolism with metformin improves tumor oxygenation and radiotherapy response. Clin Cancer Res 2013; 19: 6741–50. [DOI] [PubMed] [Google Scholar]
- 114. Song X, Feng L, Liang Cet al. Liposomes co-loaded with metformin and chlorin e6 modulate tumor hypoxia during enhanced photodynamic therapy. Nano Res 2017; 10: 1200–12. [Google Scholar]
- 115. Yang ZY, Wang JF, Liu Set al. Defeating relapsed and refractory malignancies through a nano-enabled mitochondria-mediated respiratory inhibition and damage pathway. Biomaterials 2020; 229: 119580. [DOI] [PubMed] [Google Scholar]
- 116. Mai XL, Zhang YW, Fan HJet al. Integration of immunogenic activation and immunosuppressive reversion using mitochondrial-respiration-inhibited platelet-mimicking nanoparticles. Biomaterials 2020; 232:119699. [DOI] [PubMed] [Google Scholar]
- 117. Weaver LK, Hopkins RO, Chen KJet al. Hyperbaric oxygen for acute carbon monoxide poisoning. N Engl J Med 2002; 347: 1057–67. [DOI] [PubMed] [Google Scholar]
- 118. Moncada S, Erusalimsky JD. Opinion-Does nitric oxide modulate mitochondrial energy generation and apoptosis? Nat Rev Mol Cell Biol 2002; 3: 214–20. [DOI] [PubMed] [Google Scholar]
- 119. Bogdan C. Nitric oxide synthase in innate and adaptive immunity: an update. Trends Immunol 2015; 36: 161–78. [DOI] [PubMed] [Google Scholar]
- 120. Guo R, Tian Y, Wang Yet al. Near-infrared laser-triggered nitric oxide nanogenerators for the reversal of multidrug resistance in cancer. Adv Funct Mater 2017; 27: 1606398. [Google Scholar]
- 121. Jin Z, Wen Y, Hu Yet al. MRI-guided and ultrasound-triggered release of NO by advanced nanomedicine. Nanoscale 2017; 9: 3637–45. [DOI] [PubMed] [Google Scholar]
- 122. Deng Y, Jia F, Chen Set al. Nitric oxide as an all-rounder for enhanced photodynamic therapy: hypoxia relief, glutathione depletion and reactive nitrogen species generation. Biomaterials 2018; 187: 55–65. [DOI] [PubMed] [Google Scholar]
- 123. Riganti C, Miraglia E, Viarisio Det al. Nitric oxide reverts the resistance to doxorubicin in human colon cancer cells by inhibiting the drug efflux. Cancer Res 2005; 65: 516–25. [PubMed] [Google Scholar]
- 124. Jordan BF, Sonveaux P, Feron Oet al. Nitric oxide-mediated increase in tumor blood flow and oxygenation of tumors implanted in muscles stimulated by electric pulses. Int J Radiat Oncol Biol Phys 2003; 55: 1066–73. [DOI] [PubMed] [Google Scholar]
- 125. Sortino S. Light-controlled nitric oxide delivering molecular assemblies. Chem Soc Rev 2010; 39: 2903–13. [DOI] [PubMed] [Google Scholar]
- 126. Fan W, Bu W, Zhang Zet al. X-ray radiation-controlled NO-release for on-demand depth-independent hypoxic radiosensitization. Angew Chem Int Ed 2015; 54: 14026–30. [DOI] [PubMed] [Google Scholar]
- 127. Wan SS, Zeng JY, Cheng Het al. ROS-induced NO generation for gas therapy and sensitizing photodynamic therapy of tumor. Biomaterials 2018; 185: 51–62. [DOI] [PubMed] [Google Scholar]
- 128. Lv Z, Wei H, Li Qet al. Achieving efficient photodynamic therapy under both normoxia and hypoxia using cyclometalated Ru(ii) photosensitizer through type I photochemical process. Chem Sci 2018; 9: 502–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Li X, Zheng BD, Peng XHet al. Phthalocyanines as medicinal photosensitizers: developments in the last five years. Coord Chem Rev 2019; 379: 147–60. [Google Scholar]
- 130. Gilson RC, Black KCL, Lane DDet al. Hybrid TiO2–ruthenium nano-photosensitizer synergistically produces reactive oxygen species in both hypoxic and normoxic conditions. Angew Chem Int Ed 2017; 56: 10717–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Lan Y, Lu Y, Ren Z. Mini review on photocatalysis of titanium dioxide nanoparticles and their solar applications. Nano Energy 2013; 2: 1031–45. [Google Scholar]
- 132. Cai R, Kubota Y, Shuin Yet al. Induction of cytotoxicity by photoexcited TiO2 particles. Cancer Res 1992; 52: 2346–8. [PubMed] [Google Scholar]
- 133. Wang M, Hou Z, Al Kheraif AAet al. Mini review of TiO2-based multifunctional nanocomposites for near-infrared light-responsive phototherapy. Adv Healthc Mater 2018; 7: 1800351. [DOI] [PubMed] [Google Scholar]
- 134. Tang YN, Di WH, Zhai XSet al. NIR-responsive photocatalytic activity and mechanism of NaYF4:Yb,Tm@TiO2 core-shell nanoparticles. ACS Catal 2013; 3: 405–12. [Google Scholar]
- 135. Chen X, Liu L, Yu PYet al. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011; 331: 746–50. [DOI] [PubMed] [Google Scholar]
- 136. Zhang C, Bu W, Ni Det al. Synthesis of iron nanometallic glasses and their application in cancer therapy by a localized Fenton reaction. Angew Chem Int Ed 2016; 55: 2101–6. [DOI] [PubMed] [Google Scholar]
- 137. Hu P, Wu T, Fan Wet al. Near infrared-assisted Fenton reaction for tumor-specific and mitochondrial DNA-targeted photochemotherapy. Biomaterials 2017; 141: 86–95. [DOI] [PubMed] [Google Scholar]
- 138. Zhang C, Bu W, Ni Det al. Synthesis of iron nanometallic glasses and their application in cancer therapy by a localized Fenton reaction. Angew Chem Int Ed 2016; 55: 2101–6. [DOI] [PubMed] [Google Scholar]
- 139. Huo M, Wang L, Wang Yet al. Nanocatalytic tumor therapy by single-atom catalysts. ACS Nano 2019; 13: 2643–53. [DOI] [PubMed] [Google Scholar]
- 140. Shen Z, Liu T, Li Yet al. Fenton-reaction-acceleratable magnetic nanoparticles for ferroptosis therapy of orthotopic brain tumors. ACS Nano 2018; 12: 11355–65. [DOI] [PubMed] [Google Scholar]
- 141. Lin LS, Song J, Song Let al. Simultaneous Fenton-like ion delivery and glutathione depletion by MnO2-based nanoagent to enhance chemodynamic therapy. Angew Chem Int Ed 2018; 57: 4902–6. [DOI] [PubMed] [Google Scholar]
- 142. Wang X, Xu J, Yang Det al. Fe3O4@MIL-100(Fe)-UCNPs heterojunction photosensitizer: rational design and application in near infrared light mediated hypoxic tumor therapy. Chem Eng J 2018; 354: 1141–52. [Google Scholar]
- 143. Li JH, Liu Y, Li XNet al. ROS self-generation and hypoxia self-enhanced biodegradable magnetic nanotheranostics for targeted tumor therapy. Nanoscale Horiz 2020; 5: 350–8. [Google Scholar]
- 144. Fan JX, Peng MY, Wang Het al. Engineered bacterial bioreactor for tumor therapy via Fenton-like reaction with localized H2O2 generation. Adv Mater 2019; 31: 1808278. [DOI] [PubMed] [Google Scholar]
- 145. Liao W, Ning Z, Chen Let al. Intracellular antioxidant detoxifying effects of diosmetin on 2,2-azobis(2-amidinopropane) dihydrochloride (aaph)-induced oxidative stress through inhibition of reactive oxygen species generation. J Agric Food Chem 2014; 62: 8648–54. [DOI] [PubMed] [Google Scholar]
- 146. Wang XQ, Gao F, Zhang XZ. Initiator-loaded gold nanocages as a light-induced free-radical generator for cancer therapy. Angew Chem Int Ed 2017; 56: 9029–33. [DOI] [PubMed] [Google Scholar]
- 147. Shen S, Zhu C, Huo Det al. A hybrid nanomaterial for the controlled generation of free radicals and oxidative destruction of hypoxic cancer cells. Angew Chem Int Ed 2017; 56: 8801–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Wang XQ, Peng MY, Li CXet al. Real-time imaging of free radicals for mitochondria-targeting hypoxic tumor therapy. Nano Lett 2018; 18: 6804–11. [DOI] [PubMed] [Google Scholar]