Abstract
Objective:
Recent cannabis exposure has been associated with lower rates of neurocognitive impairment in people with HIV (PWH). Cannabis’ anti-inflammatory properties may underlie this relationship by reducing chronic neuroinflammation in PWH. This study examined relations between cannabis use and inflammatory biomarkers in cerebrospinal fluid (CSF) and plasma, and cognitive correlates of these biomarkers within a community-based sample of PWH.
Methods:
263 individuals were categorized into four groups: HIV− non-cannabis users (n = 65), HIV+ non-cannabis users (n = 105), HIV+ moderate cannabis users (n = 62), and HIV+ daily cannabis users (n = 31). Differences in pro-inflammatory biomarkers (IL-6, MCP-1/CCL2, IP-10/CXCL10, sCD14, sTNFR-II, TNF-α) by study group were determined by Kruskal-Wallis tests. Multivariable linear regressions examined relationships between biomarkers and seven cognitive domains, adjusting for age, sex/gender, race/ethnicity, education, current CD4 count, and current tobacco use.
Results:
HIV+ daily cannabis users showed lower MCP-1 and IP-10 levels in CSF compared to HIV+ non-cannabis users (p = 0.015; p = 0.039), and were similar to HIV− non-cannabis users. Plasma biomarkers showed no differences by cannabis use. Among PWH, lower CSF MCP-1 and lower CSF IP-10 were associated with better learning performance (p = 0.016; p = 0.036).
Conclusions:
Current daily cannabis use was associated with lower levels of pro-inflammatory chemokines implicated in HIV pathogenesis and these chemokines were linked to the cognitive domain of learning which is commonly impaired in PWH. Cannabinoid-related reductions of MCP-1 and IP-10, if confirmed, suggest a role for medicinal cannabis in the mitigation of persistent inflammation and cognitive impacts of HIV.
Keywords: neuroinflammation, cognition, neurocognitive impairment, marijuana, cannabinoids, NeuroAIDS, HIV/AIDS, cerebrospinal fluid
Introduction
HIV disease is associated with elevated immune system activation and persistent inflammation in the central nervous system (CNS) (Hong & Banks, 2015; Letendre, 2011). Neuroinflammatory responses persist despite virally suppressive antiretroviral therapy (ART) (Vera et al., 2016), and are likely major contributors to the pathogenesis of HIV-associated neurocognitive impairment (Gannon, Khan, & Kolson, 2011; Saylor et al., 2016). Although the prevalence of HIV-associated dementia has markedly decreased in the ART era, milder forms of neurocognitive impairment remain common, with a prevalence of 20-50% among people with HIV (PWH) (Iudicello et al., 2019; Saloner & Cysique, 2017). HIV-associated neurocognitive impairment has been associated with increased risk of deficits in real-world function including medication adherence, employment, automobile driving, and quality of life (Casaletto, Weber, Iudicello, & Woods, 2017). Interventions that target neuroinflammation underlying these cognitive difficulties are lacking currently, but burgeoning evidence suggests there are potential therapeutic benefits of cannabis (Manuzak et al., 2018; Rizzo et al., 2018).
Cannabis use is common among PWH in the United States, with 23–56% reporting use in the past year (Pacek, Towe, Hobkirk, Nash, & Goodwin, 2018). PWH also frequently report using cannabis for medicinal purposes (25–35%) (Fogarty et al., 2007) primarily for pain relief, alleviation of anxiety and depression, and appetite stimulation (Woolridge et al., 2005). Randomized clinical trials of cannabis and cannabinoids show moderate evidence of clinical benefit for HIV-associated symptoms such as appetite/weight loss and sensory neuropathy (Ellis et al., 2009; National Academies of Sciences & Medicine, 2017; Whiting et al., 2015). Given high rates of use among PWH, growing state-based legalization in the U.S., and increased marketing of cannabis-based products, there is growing interest in examining the influence of cannabis on neurocognitive function in this population.
The current literature base examining the relationship of cannabis use and neurocognitive function in HIV shows inconsistent findings. Recent work from our group indicates that cannabis use is associated with lower rates of global neurocognitive impairment and higher performance in verbal fluency and learning domains selectively among PWH (Watson et al., 2020). Light cannabis-using PWH have also shown higher verbal fluency compared to HIV− light cannabis users (Thames, Mahmood, Burggren, Karimian, & Kuhn, 2016). Conversely, adverse effects of daily cannabis use on cognitive domains such as delayed recall/memory have also been observed (Cristiani, Pukay-Martin, & Bornstein, 2004), and several studies have shown comparable cognitive performance between PWH cannabis users and non-users (Chang, Cloak, Yakupov, & Ernst, 2006; Thames et al., 2017b; Wang, Liang, Ernst, Oishi, & Chang, 2020). Such variable findings suggest that characteristics of cannabis usage such as frequency and quantity of use, as well as other contextual and cohort factors, likely moderate its effects on neurocognitive performance (Gonzalez, Pacheco-Colon, Duperrouzel, & Hawes, 2017).
Cannabis’ anti-inflammatory properties may underlie the sometimes observed beneficial effect of cannabis exposure on neurocognitive function in HIV. Cannabis aside, inflammatory biomarker levels remain elevated in PWH and contribute to CNS injury even when HIV RNA levels are suppressed (Neuhaus et al., 2010; Wada et al., 2015). A key process in chronic HIV-associated neuroinflammation involves activated T-cell and monocyte migration to the brain. Subsequent interactions of infiltrating immune cells with astrocytes and microglia result in secretion of neurotoxic cytokines and chemokines (Hong & Banks, 2015; Ramesh, MacLean, & Philipp, 2013), and these pro-inflammatory factors in cerebrospinal fluid (CSF) and plasma have been related to worse neurocognitive performance in PWH (Burdo et al., 2013; Burlacu et al., 2020; Cohen et al., 2011; Imp et al., 2017; Kamat et al., 2012; Yuan et al., 2013).
Pre-clinical and human endocannabinoid system studies show that cannabinoids may mediate immunomodulatory actions that disrupt pro-inflammatory processes in HIV (Chen, Gao, Gao, Su, & Wu, 2017; Rom & Persidsky, 2013). Recent evidence has demonstrated associations between current cannabis use and reduced systemic inflammation among PWH, as indexed by lower levels of activated and inflammatory monocyte frequencies, and in plasma, lower levels of macrophage inflammatory protein (MIP)1α, interferon-gamma-inducible protein-10 (IP-10), also referred to as C-X-C motif chemokine ligand 10 (CXCL10), and tumor necrosis factor alpha (TNF-α) (Castro et al., 2019; Keen & Turner, 2015; Manuzak et al., 2018; Rizzo et al., 2018). In contrast, higher plasma levels of soluble cluster of differentiation 14 (sCD14) have been observed in cannabis-using compared to non-cannabis-using PWH (Castro et al., 2019), and many inflammatory plasma biomarkers (20 out of 21) have shown no differences by cannabis use among PWH (Manuzak et al., 2018). Higher levels of plasma interleukin-1β have also been observed among daily cannabis using PWH and HIV− individuals compared to non-users, with no differences in many inflammatory plasma biomarkers (23 out of 24) by cannabis use after controlling for multiple comparisons (Krsak et al., 2020). Thus, the prior literature is highly mixed, showing current cannabis use is associated with lower and higher levels of some plasma inflammatory biomarkers, and many peripheral markers show no evidence of cannabis-related modulation. No studies to date have examined the intersection of cannabis exposure, CNS inflammation, and cognition among PWH.
The goal of this study was to determine the relationship between cannabis use and HIV-associated inflammation, and the potential downstream association between inflammation and cognitive function. In study aim 1, we investigated the effects of no cannabis use, moderate cannabis use, and daily cannabis use on CSF and plasma biomarkers in PWH, with an HIV− non-cannabis-using control group. We hypothesized that cannabis use would be associated with lower levels of some inflammatory biomarkers among PWH, such as IP-10 and TNF-α, with others showing no differences by cannabis use, given the limited and mixed existing evidence. Post-hoc analyses investigated whether specific parameters of cannabis use over the past six months (cannabis quantity, cannabis recency, or cannabis frequency) correlated with CSF and plasma biomarkers.
In study aim 2, to determine the functional relevance of any anti-inflammatory cannabis effects observed, we first examined cognitive performance in PWH by cannabis use group, and next, examined relationships between inflammatory biomarker levels that were related to cannabis use in study aim 1, and cognitive performance in seven domains. We hypothesized that for biomarkers associated with cannabis use, lower levels of pro-inflammatory markers would relate to better performance in some cognitive domains among PWH, such as in verbal fluency and learning, based on previous findings from our group.
Methods
Participants and Design.
The sample included 263 community-dwelling adults enrolled in NIH-funded studies at UC San Diego’s HIV Neurobehavioral Research Program (HNRP). Study design and cohort selection have been described in detail previously (Heaton et al., 2011). Study visits took place between August 2001 and January 2018. All study procedures were approved by the UC San Diego Institutional Review Board. Participants provided written, informed consent.
Inclusion criteria for the current analyses were data present for a) detailed self-report of cannabis and other substance use; b) drug urine toxicology; c) inflammatory biomarkers; and d) neurocognitive and neuromedical assessments. Exclusion criteria for the parent studies included history of non-HIV-related neurological, medical, or psychiatric disorders that affect brain function (e.g., epilepsy, stroke, schizophrenia), learning disabilities, or a dementia diagnosis. Exclusion criteria for the current analyses were a) positive urine toxicology for addictive substances other than cannabis; b) report of any substance use disorder, including alcohol use disorder, in the past year other than cannabis; and c) reported use of any of the following in the past year: cocaine, methamphetamine, amphetamine, other stimulants, heroin, other opioids, sedatives, anti-anxiety drug abuse, hallucinogens, PCP, ketamine, or inhalants.
Cannabis Use.
Cannabis use was characterized by self-report and Δ9-tetrahydrocannabinol (THC) positive urine toxicology. Frequency, quantity, and recency of cannabis use was assessed via a modified timeline follow-back (TLFB) interview (Robinson, Sobell, Sobell, & Leo, 2014). This modified TLFB captures age of first use and average quantities and frequencies of use during participant-identified periods of cannabis use. To define recent cannabis use patterns among participants, TLFB estimates were used to obtain an estimate of days since last use, as well as total days used, total grams used, and average grams per day of use, over the past six months.
Three cannabis use groups were defined for this study: non-cannabis users, moderate cannabis users, and daily cannabis users. When we initially examined cannabis use frequency within our cohort, this three-group categorization appeared to best characterize the natural distribution of cannabis use patterns. Non-cannabis users reported either no use of cannabis over their lifetime or no use in the past five years and had THC negative urine toxicology. Moderate cannabis users reported use of cannabis within the past month, with an average pattern of weekly use over the past six month (ranging from a minimum of three days of use over six months to a maximum of three days of use per week over six months), and could have positive or negative THC urine toxicology (given that participants with less frequent cannabis use could likely have a positive or negative THC screen on their testing day). Daily cannabis users reported a pattern of daily use over the past six months, and had THC positive urine toxicology.
Inflammatory Biomarkers in CSF and Plasma.
Blood was drawn via venipuncture through the antecubital vein into an EDTA vacutainer. Plasma was centrifuged at 1,800 relative centrifugal force for 8 minutes at room temperature and aliquoted for storage at −80°C until the time of assay. CSF was collected via lumbar puncture using a non-traumatic spinal needle and aseptic technique. CSF was centrifuged at low speed to separate cells; both supernatants and cells were aliquoted and stored at −80°C and were not thawed until the time of assay. Six pro-inflammatory biomarkers in plasma and CSF were measured using commercially available immunoassays and run according to the manufactures' protocol: Interleukin-6 (IL-6); monocyte chemoattractant protein-1 (MCP-1), also referred to as chemokine (C-C motif) ligand 2 (CCL2); IP-10; sCD14; soluble tumor necrosis factor receptor type II (sTNFR-II) and TNF-α. Biomarker precision was ensured by a) assaying all specimens in duplicate; b) repeating assays of specimens with coefficients of variation greater than 20%; c) repeating 10% of all assays to assess operator and batch consistency; and d) regularly assessing batch effects.
Neurocognitive Testing.
Participants completed a standardized battery of well-validated neuropsychological tests designed to assess global cognition and seven domains: verbal fluency, executive function, attention/working memory, processing speed, learning, delayed recall/memory, and motor skills. Details about all tests included in this battery are published elsewhere (Carey et al., 2004). Raw test scores were transformed into normally-distributed T-scores which are demographically adjusted for age, years of formal education, sex/gender, and race based on normative samples of HIV− participants (Heaton, Miller, Taylor, & Grant, 2004; Norman et al., 2011). Cognitive domain summary T-scores were generated by averaging T-scores across tests within a cognitive domain.
Neuromedical, Psychiatric, and Other Substance Use Assessment.
Participants underwent a comprehensive neuromedical assessment. HIV infection was established by enzyme-linked immunosorbent assay with Western blot confirmation. Routine clinical chemistry panels, complete blood counts, rapid plasma reagin, hepatitis C virus antibody, and CD4+ T cells (flow cytometry) were performed. HIV viral load in plasma and CSF were measured using reverse transcriptase-polymerase chain reaction (Amplicor, Roche Diagnostics, Indianapolis, IN), with a lower limit of quantitation (LLQ) of 50 copies/ml. HIV viral load was dichotomized as detectable vs. undetectable at the LLQ of 50 copies/ml. Detailed medical and antiretroviral usage history was captured via a structured, clinician-administered questionnaire. Current depression symptoms were assessed by the Beck Depression Inventory (BDI-II) (Beck, Steer, & Brown, 1996). Current tobacco use was assessed by a modified TLFB interview (Robinson et al., 2014), and defined by use (yes/no) in the past six months. Height and weight were used to calculate body mass index (BMI).
Statistical Analyses.
Participants were categorized into four study groups based on HIV status and cannabis use: HIV− non-cannabis users, HIV+ non-cannabis users, HIV+ moderate cannabis users, and HIV+ daily cannabis users. Demographic, psychiatric, and HIV disease variables were compared across the four HIV/Cannabis groups using analysis of variance (ANOVA) or Kruskal-Wallis tests for continuous variables and χ2 or Fisher’s exact tests for categorical variables. Pair-wise comparisons were conducted to follow-up on significant omnibus results using Tukey’s Honest Significant Difference (HSD) tests or Wilcoxon tests for continuous outcomes and χ2 or Fisher’s exact tests for categorical outcomes, with false discovery rate (FDR) adjustment. We also compared cannabis use characteristics between the HIV+ moderate and daily user groups (age of first use; over the past six months: total days used, total grams used, and average grams per day of use; days since last use; and THC positive urine toxicology).
For study aim 1, Kruskal-Wallis tests were used to compare levels of CSF and plasma biomarkers between the four HIV/Cannabis groups. P values were adjusted using FDR correction for multiple comparisons, and p values < 0.05 were considered statistically significant. Biomarkers were adjusted for batch effects and log10 transformed (except for sCD14) to improve fit and limit the influence of outliers. Additionally, non-parametric tests which are robust to outliers were employed for study aim 1. Biomarker data was further examined for outliers, defined as any log-transformed biomarker values outside the 3 SD range. Sensitivity analyses were conducted on a subset of participants, with all outliers identified by this criteria removed. Criteria for covariate inclusion in our study aim 1 models were characteristics that differed by cannabis use between the three HIV+ groups; all characteristics assessed showed comparable levels across HIV+ groups, and thus no covariates were included in study aim 1 models. Additional sensitivity analyses were conducted to examine whether including current tobacco use as a covariate influenced the relationship between cannabis use group and inflammatory biomarkers. In post hoc analyses, Spearman’s rho correlations examined whether specific parameters of cannabis use over the past six months (total grams of cannabis used, days since last cannabis use, or total days of cannabis use) correlated with inflammatory biomarkers.
For study aim 2, we first examined cognitive performance across HIV+ non-cannabis users, HIV+ moderate cannabis users, and HIV+ daily cannabis users. Next, multivariable linear regressions examined relationships between any biomarkers showing lower levels with cannabis use in study aim 1 and cognitive domain T-scores among PWH. Given that T-scores adjust for demographic factors that can influence cognitive performance: age, years of formal education, sex/gender, and race/ethnicity, we did not include any further demographic covariates in our models. We included current CD4+ T cell count and current tobacco use as covariates. Effect sizes for regression analyses were presented as estimated regression coefficients (b). P values for the association of a biomarker with each cognitive domain were adjusted using FDR correction and p values < 0.05 were considered statistically significant. Given that a portion of the HIV+ cohort was not virally suppressed, sensitivity analyses were conducted on a subset of PWH with undetectable plasma HIV RNA.
Results
The study cohort of 263 community-dwelling adults included 198 PWH and 65 HIV−people, and ranged in age from 18 to 70 years old (M = 42.3, SD = 11.1). Four study groups included the HIV− non-cannabis users (n = 65) comparison group, and PWH categorized into three groups based on cannabis use: HIV+ non-cannabis users (n = 105), HIV+ moderate cannabis users (n = 62), and HIV+ daily cannabis users (n = 31).
Sample characteristics by HIV/Cannabis group are presented in Table 1. There were no significant differences in age, years of formal education, current tobacco use, nor hepatitis C virus across study groups. While there was a greater proportion of men, greater proportion of non-White people of color, higher depression symptoms, and lower BMI in the three HIV+ groups compared to the HIV− group, these characteristics did not differ among the HIV+ cannabis use subgroups. Thus, these characteristics were not included as covariates in study aim 1 models. Furthermore, no HIV disease characteristics (e.g. duration of HIV disease, current CD4+ T cell count, HIV RNA in plasma and CSF) differed across HIV+ cannabis use subgroups, and were not included as covariates in study aim 1 models.
Table 1.
Sample characteristics (N=263)
| Characteristic | HIV− Non-Cannabis Users |
HIV+ Non-Cannabis Users |
HIV+ Moderate Cannabis Users |
HIV+ Daily Cannabis Users |
p-value |
|---|---|---|---|---|---|
| n = 65 | n = 105 | n = 62 | n = 31 | ||
| Demographics | |||||
| Age (years) | 44.9 (11.7) | 40.2 (10.9) | 43.3 (10.8) | 42.0 (10.5) | ns |
| Education (years) | 13.6 (2.4) | 13.6 (2.9) | 13.7 (2.3) | 13.0 (2.9) | ns |
| Sex/gender (% men)1 | 67.7% | 81.9% | 95.2% | 87.1% | 0.004 |
| Race/ethnicity1 | 0.008 | ||||
| White | 66.2% | 41.0% | 58.1% | 38.7% | |
| Black | 15.4% | 22.9% | 19.4% | 38.7% | |
| Latino | 18.5% | 30.5% | 14.5% | 19.4% | |
| Asian | 0% | 1.9% | 1.6% | 3.2% | |
| Other | 0% | 3.8% | 6.5% | 0% | |
| HIV Disease | |||||
| Duration of HIV (years) | --- | 7.2 (1.8–15.8) | 9.4 (2.2–16.1) | 6.3 (3.6–14.6) | ns |
| Nadir CD4+ T cells | --- | 227 (89–385) | 175 (40–318) | 203 (48–388) | ns |
| Current CD4+ T cells | --- | 479 (323–687) | 505 (294–681) | 506 (378–761) | ns |
| ART status (% on) | --- | 66.7% | 80.7% | 74.2% | ns |
| Total ART duration (years) | --- | 4.9 (5.4) | 5.1 (4.7) | 4.6 (4.2) | ns |
| HIV RNA in plasma (% undetectable) | --- | 71.4% | 77.1% | 60.9% | ns |
| HIV RNA in CSF (% undetectable) | --- | 90.0% | 92.0% | 95.5% | ns |
| Psychiatric, Substance use, Medical | |||||
| Depression (BDI-II score)1 | 1.5 (0–7.3) | 10 (4–16) | 8.5 (4–16.8) | 12 (4–22) | <0.001 |
| Current tobacco use2 | 23.1% | 26.7% | 35.5% | 35.5% | ns |
| BMI | 30.0 (6.9) | 27.0 (4.9) | 26.5 (4.5) | 25.7 (5.1) | <0.001 |
| Hepatitis C Virus | 12.3% | 8.7% | 11.7% | 10.0% | ns |
Data are presented as Mean(SD), Median (IQR), or %
Only four characteristics varied between the four groups: sex/gender, race/ethnicity, depression symptoms, and BMI. All three HIV+ groups had a higher proportion of men and people of color, higher depression symptoms, and lower BMI compared to the HIV− group but these variables did not differ by cannabis use between HIV+ groups
Use in the past six months
Abbreviations: ns = non-significant (p ≥ 0.05); HIV = human immunodeficiency virus; ART = antiretroviral therapy; BDI-II = Beck Depression Inventory-2nd Edition; BMI = Body Mass Index
Self-reported cannabis use characteristics were compared between HIV+ moderate and daily users are presented in Table 2. While age of first use was comparable between the two groups, over the past six months, daily users reported higher total days used, higher total grams used, and higher grams per day of use compared to moderate users (p < 0.001, p < 0.001, p = 0.04). Daily users reported a median of 0.5 grams per day of use (IQR = 0.2–1.1), while moderate users reported a median of 0.3 grams per day of use (IQR = 0.2–0.8). Per inclusion criteria for the daily users, 100% had THC positive urine toxicology, while 47.5% of moderate users had THC positive urine toxicology (p < 0.001).
Table 2.
Cannabis use characteristics of HIV+ moderate and daily users
| Characteristic | Moderate Cannabis Users |
Daily Cannabis Users |
p-value |
|---|---|---|---|
| n=62 | n=31 | ||
| Age of first use | 16.8 (4.9) | 15.4 (4.0) | ns |
| Total days used1 | 18 (6–37) | 180 (171–180) | <0.001 |
| Total grams used1 | 6 (2–19) | 90 (36–165) | <0.001 |
| Average grams per day1,2 | 0.3 (0.2–0.8) | 0.5 (0.2–1.1) | 0.04 |
| Days since last use | 3 (1.0–9.5) | 1 (0.5–1.0) | <0.001 |
| THC+ urine toxicology | 47.5% | 100.0% | <0.001 |
Data are presented as Mean (SD) or Median (IQR)
Over the past six months
Per day of cannabis use
Abbreviations: ns = non-significant (p ≥ 0.05); HIV = human immunodeficiency virus; THC = Δ9-tetrahydrocannabinol
CSF Biomarkers: Lower MCP-1 and IP-10 in Daily Cannabis Users
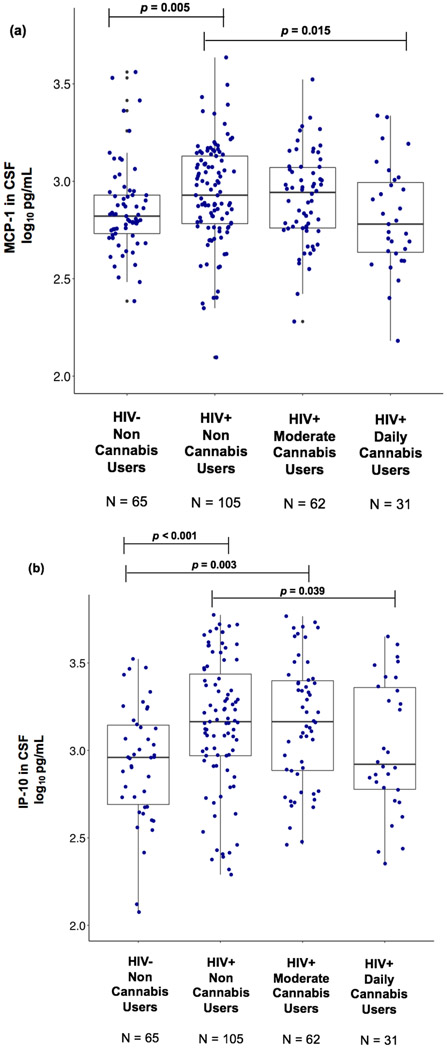
Kruskal-Wallis tests revealed a significant omnibus difference across HIV/Cannabis groups in MCP-1 and IP-10 levels in CSF (p = 0.027; p = 0.001) with FDR adjustment. Follow-up pairwise comparisons showed that MCP-1 and IP-10 levels in CSF were significantly lower in HIV+ daily cannabis users compared to HIV+ non-cannabis users (p = 0.015; p = 0.039; Figures 1a and 1b). Furthermore, CSF MCP-1 was higher in HIV+ non-cannabis users compared to the HIV− non-cannabis users (p = 0.005; Figure 1a). CSF IP-10 was higher in HIV+ non-cannabis and moderate cannabis users compared to the HIV− non-cannabis users (p < 0.001, p = 0.003; Figure 1b). No differences were observed in IL-6, sCD14, sTNFR-II, and TNF-α levels in CSF across HIV/Cannabis groups.
Figure 1. HIV+ daily cannabis users display lower levels of MCP-1 and IP-10 in CSF compared to HIV+ non-cannabis users.
Abbreviations: MCP-1 = monocyte chemoattractant protein-1; IP-10 = interferon-gamma-inducible protein-10; CSF = cerebrospinal fluid; HIV = human immunodeficiency virus
In panel (a), there was significant omnibus difference across the four HIV/Cannabis groups in the level of MCP-1 in CSF (p = 0.027). MCP-1 in CSF was significantly lower in HIV+ daily cannabis users compared to HIV+ non-cannabis users (p = 0.015). MCP-1 in CSF was significantly higher in HIV+ non-cannabis users compared to the HIV− non-cannabis users (p = 0.005).
In panel (b), there was significant omnibus difference across the four HIV/Cannabis groups in the level of IP-10 in CSF (p = 0.001). IP-10 in CSF was significantly lower in HIV+ daily cannabis users compared to HIV+ non-cannabis users (p = 0.039). IP-10 in CSF was significantly higher in HIV+ non-cannabis and moderate cannabis users compared to the HIV− non-cannabis users (p < 0.001, p = 0.003).
Plasma Biomarkers: No Differences by Cannabis Use
Plasma biomarkers IL-6, MCP-1, sCD14, sTNFR-II, and TNF-α showed no significant omnibus differences across HIV/Cannabis groups. There was a significant omnibus difference across HIV/Cannabis groups in plasma IP-10 levels (p < 0.001) with FDR adjustment. Follow-up pairwise comparisons showed that plasma IP-10 was elevated in all three HIV+ groups: HIV+ non-cannabis users (p < 0.001), HIV+ moderate cannabis users (p < 0.001), and HIV+ daily cannabis users (p = 0.042), compared to the HIV− group.
In sensitivity analyses with biomarker outliers removed, CSF and plasma findings did not differ from whole sample analyses. In sensitivity analyses adjusting for current tobacco use, CSF and plasma findings also did not differ from whole sample analyses, and no independent effects of tobacco use on CSF or plasma biomarker levels were observed.
Cannabis Use Parameters and Biomarker Correlations
Total grams of cannabis used over the past six months did not correlate significantly with any CSF or plasma biomarkers (ps > 0.05). Days since last cannabis use also did not correlate significantly with any CSF or plasma biomarkers (ps > 0.05). Two trend-level correlations were observed. Total grams of cannabis used trended towards a small to medium negative correlation with CSF IP-10 (r = −0.19, p = 0.083), indicating that more grams consumed was weakly linked to lower IP-10 in CSF. Days since last cannabis use trended towards a small to medium positive correlation with CSF MCP-1 (r = 0.18, p = 0.082), indicating more recent cannabis use was weakly linked to lower MCP-1 in CSF. Total days of cannabis use over the past six months had medium and significant negative correlations with CSF MCP-1 (r = –0.23, p = 0.026) and CSF IP-10 (r = –0.27, p = 0.011), indicating that greater days of cannabis use predicted lower levels of MCP-1 and IP-10 in CSF.
Cognitive Performance and CSF MCP-1 and IP-10
Across the HIV+ groups, HIV+ daily cannabis users had similar global cognitive performance with a trend of better performance (M = 48.8, SD = 6.0) compared to HIV+ moderate cannabis users (M = 46.4, SD = 6.5) and HIV+ non-cannabis users (M = 46.9, SD = 6.8) (p = 0.26). An analogous pattern was observed in the domains of: verbal fluency, attention/working memory, processing speed, learning, and motor skills, with a slightly and non-significantly higher performance among HIV+ daily cannabis users. Among all PWH, a negative association was found between MCP-1 in CSF and learning performance (b = −6.3, p = 0.016; Table 3), indicating that lower MCP-1 is related to better learning, while adjusting for current tobacco use and current CD4 count. A negative association was also detected between IP-10 in CSF and learning (b = −2.3, p = 0.036; Table 3) among PWH, indicating that lower IP-10 was associated with better learning performance with the same covariate and FDR adjustment. CSF MCP-1 and IP-10 did not relate significantly to any other cognitive domains among PWH.
Table 3.
Relationship of CSF biomarkers and cognitive domains among people with HIV
| MCP-1 | IP-10 | |||
|---|---|---|---|---|
| Cognitive Domain | beta (SE) | p-value1 | beta (SE) | p-value1 |
| Verbal Fluency | −2.1 (2.6) | ns | −1.2 (1.3) | ns |
| Executive Function | −2.4 (2.8) | ns | −1.2 (1.5) | ns |
| Attention/Working Memory | −3.8 (2.6) | ns | 0.1 (1.3) | ns |
| Processing Speed | −3.1 (2.7) | ns | −0.3 (1.4) | ns |
| Learning | −6.3 (2.6) | 0.016 | −2.3 (1.4) | 0.036 |
| Delayed Recall/Memory | −3.9 (2.6) | ns | −1.9 (1.4) | ns |
| Motor | −2.3 (3.0) | ns | −0.2 (1.6) | ns |
Cognitive T-scores are adjusted for age, sex/gender, race, and years of formal education, and models adjust for current tobacco use and current CD4+ T cell count
p-values were adjusted using false discovery rate (FDR) correction for multiple comparisons
Abbreviations: ns = non-significant (p ≥ 0.05); CSF = cerebrospinal fluid; HIV = human immunodeficiency virus; MCP-1 = monocyte chemoattractant protein-1; IP-10 = interferon-gamma-inducible protein-10
In sensitivity analyses with the PWH sample restricted to those with undetectable plasma HIV RNA viral load, CSF and plasma biomarker and cognitive domain findings (study aims 1 and 2) did not differ from whole sample analyses.
Discussion
Study findings support our hypotheses that frequent cannabis exposure may reduce neuroinflammation in PWH, with possible downstream benefits for cognition. Daily cannabis use was associated with lower levels of pro-inflammatory chemokines MCP-1 and IP-10 in CSF, factors critical to immune cell migration and HIV pathogenesis. In contrast, cannabis use was not associated with inflammatory biomarker levels in plasma. HIV+ daily cannabis users showed similar cognitive performances with a trend toward higher scores in global cognition and several cognitive domains including learning compared to HIV+ moderate users and HIV+ non-cannabis users. Lower CSF levels of MCP-1 and IP-10 were related to better cognitive performance in the domain of learning, which is commonly impaired in HIV disease. When recent cannabis use parameters were examined, cannabis quantity and cannabis recency did not correlate significantly with any inflammatory biomarkers, but greater total days of cannabis use, or cannabis frequency, significantly predicted lower levels of MCP-1 and IP-10 in CSF. Findings indicate that regular, daily cannabis use may be important for reduced CNS inflammation in HIV. Importantly, daily cannabis users in this cohort reported a median of half a gram of cannabis use per day, and 75% of daily users reported less than 1.1 grams of cannabis use per day, suggesting that frequent, but not heavy cannabis use generally characterized this group. Therefore, inference about the anti-inflammatory benefit of cannabis in PWH cannot be extrapolated to more high-dose, heavy use of cannabis. Furthermore, given our study design is retrospective and cross-sectional, we cannot clarify actual cause-effect relationships regarding the inflammation modulatory effects of cannabis, which would require longitudinal study design.
Our study findings are novel in detection of lower pro-inflammatory chemokines with cannabis exposure specifically in CSF, and converge with recent research showing anti-inflammatory activity of cannabis in PWH on activated immune cells and systemic inflammation (Manuzak et al., 2018; Rizzo et al., 2018). Lower CSF MCP-1 and IP-10 with daily cannabis use and no differences by cannabis use for CSF biomarkers IL-6, sCD14, sTNFR-II, and TNF-α, suggest that MCP-1 and IP-10 may be specifically sensitive to cannabis-related modulation, and play a mechanistic role in any downstream cognitive benefit or physical symptom relief observed with regular cannabis use among PWH. Both IP-10 and MCP-1 are considered important contributors to neuroinflammation in HIV infection and reductions in their levels could have beneficial downstream effects for PWH. IP-10 is a major chemo-attractant for T-cells and activated monocytes, and in excess, leads to neurotoxic pro-inflammatory cytokine production and neuronal apoptosis, while MCP-1 mediates trafficking of infected macrophages across the blood brain barrier (Asensio et al., 2001; de Almeida et al., 2005; Pulliam et al., 2011; Simmons et al., 2013). Thus, our findings suggest that cannabis use may lead to reduced CNS-infiltration of T cells and monocytes. In a small pilot study, our group recently reported that cannabis recency (days since last use) was associated with lower levels of CSF interleukin-16 (IL-16) and C reactive protein (CRP) (Ellis et al., 2020b). These findings are complementary with the current study in suggesting that sustained reductions in neuroinflammation related to cannabis likely require ongoing exposure, although we only observed a small to medium and non-significant relationship between cannabis recency and CSF MCP-1 in this cohort. The current study’s findings suggest that the parameter of cannabis frequency is slightly more predictive of inflammatory biomarker levels compared to cannabis quantity and cannabis recency. An additional benefit of cannabis, potentially linked to its anti-inflammatory effects, is stabilization of the blood brain barrier, which we demonstrated in a separate report showing that more frequent use of cannabis in the past month was associated with higher blood brain barrier integrity in PWH (Ellis et al., 2020a). We extend this work in the current study, showing that lower MCP-1 and lower IP-10 relate to better learning performance, a cognitive domain which frequently shows mild deficits in PWH in the ART era (Heaton et al., 2010). Elevated MCP-1 and IP-10 in CSF have previously been observed in PWH with HIV-associated neurocognitive disorder (Mehla, Bivalkar-Mehla, Nagarkatti, & Chauhan, 2012; Yuan et al., 2013).
Taken together, findings are consistent with the notion that cannabinoids may modulate inflammatory processes in PWH, specifically in the CNS, and suggest a link between lower CNS inflammation and better neurocognitive function. Our finding that HIV+ daily cannabis users showed slightly and non-significantly better performance in several cognitive domains compared to HIV+ non-users reflects a similar pattern to a recent study (Wang et al., 2020), suggesting a signal of a neuroprotective effect which warrants further investigation of regular cannabis use among PWH. Our sensitivity analyses indicate that the relationships observed between cannabis, inflammatory biomarkers, and cognition are relevant to PWH with both detectable and undetectable plasma HIV RNA, and study findings were maintained when current tobacco use was adjusted for.
In plasma, we observed elevated IP-10 in all three HIV+ groups compared to the HIV− group, but we did not detect lower levels of plasma IP-10 nor TNF-α with cannabis use as previous studies found (Keen & Turner, 2015; Rizzo et al., 2018). Our null findings for the relationship between cannabis use and plasma biomarker levels are congruous with several other studies in which the vast majority of peripheral inflammatory and immune activation markers show no differences by cannabis use (Castro et al., 2019; Krsak et al., 2020; Manuzak et al., 2018). Although plasma levels of chemokines such as MCP-1 are generally reflective of inflammatory states and found to be elevated in patients with neurodegenerative diseases (Lee et al., 2018), chemokine levels in the CNS may more specifically indicate their role in microglial activation and proliferation and chemotaxis of blood monocytes to the CNS (Weiss, Downie, Lyman, & Berman, 1998). Thus, it is important to recognize the biological implications of differing chemokine levels found in CSF and plasma hence, differential cannabis effects. There may be several reasons why we observed distinct cannabis effects in CSF and plasma. Cannabinoids are highly lipid soluble, so their biological effects may be amplified and prolonged in CSF as compared to blood (Hložek et al., 2017; Huestis & Smith, 2007). Furthermore, immune responses between CNS and peripheral blood are typically compartmentalized and the dynamics of chemokine production, degradation, and removal in each may differ. Numerous HIV studies have shown that levels of neurotoxic chemokines such as MCP-1 and IP-10 are much higher in CSF than in plasma, suggesting that there are distinct production and metabolism processes for these chemokines in CSF compared to plasma (de Almeida et al., 2005; Yuan et al., 2015). Although we cannot determine the exact cellular sources of MCP-1 in CSF in our study, MCP-1 causes activation and proliferation of microglia and neurotoxicity, highlighting its role in neuroinflammation and brain outcomes (Hinojosa, Garcia-Bueno, Leza, & Madrigal, 2011; Yang et al., 2011). Lastly, CSF biomarkers may be more robust indicators of changes relevant to CNS function and neurocognitive outcomes compared to peripheral blood markers.
Mechanistically, our findings are consistent with the current preclinical literature showing cannabis-related modulation of pro-inflammatory processes in HIV via the endocannabinoid system (Costiniuk & Jenabian, 2019). While cannabinoid type 1 (CB1) receptors are the principal type found in the CNS and account for the psychoactive effects of ligands such as THC, cannabinoid type 2 (CB2) receptors also are expressed in the CNS by microglia and astrocytes (Bisogno & Di Marzo, 2010; Van Sickle et al., 2005). Preclinical models show activation of CB1 and CB2 receptors can induce apoptosis of activated T-cells and macrophages (Persidsky et al., 2015), downregulate pro-inflammatory cytokine and chemokine production (Nagarkatti, Pandey, Rieder, Hegde, & Nagarkatti, 2009), and inhibit HIV-associated synapse loss and neural injury (Kim, Shin, & Thayer, 2011; Ramirez et al., 2013). Both natural and synthetic cannabinoids have demonstrated neuroprotective effects after various types of CNS insults, and in particular under conditions of high inflammation (Bilkei-Gorzo et al., 2017; Chen et al., 2017). In vitro, THC treatment has been shown to suppress a number of pro-inflammatory factors including TNF-α, IL-6, and IL-8, and decrease NF-κB secretion in human osteosarcoma cells (Yang, Li, Han, Jia, & Ding, 2015) as well as monocyte-derived interleukin IL-1ß production and astrocyte secretion of MCP-1 and IL-6 from a human coculture system (Rizzo et al., 2019). In sum, there is substantial evidence that cannabinoids display beneficial effects on chronic inflammatory responses in HIV infection.
Our study has several limitations. First, cross-sectional analyses of an observational cohort cannot establish cause-effect relationships. Second, we lacked an HIV− cannabis user group to compare cannabis effects on inflammatory markers by HIV status. While an HIV− cannabis-using group would allow for a more balanced design, our HIV− non-cannabis user group did allow us to observe differences in inflammatory markers between this control group and the three HIV+ cannabis use groups. Third, the majority of participants in this study were men and we were underpowered to examine sex/gender differences or similarities in cannabis-related modulation of inflammatory markers. Animal models have shown that endogenous sex hormones and synthetic steroid hormones influence physiological response to cannabinoids, and modulate drug sensitivity (Struik, Sanna, & Fattore, 2018), and a recent study among PWH suggests a few inflammatory biomarker levels differ by sex/gender (Rubin et al., 2019). Future work should include a larger cohort of women with HIV and examine whether relationships between cannabis, neuroinflammation, and cognition are similar or vary by sex/gender or by specific hormone levels. Fourth, our method of defining cannabis use groups combined self-report of frequency and quantity of use and urine toxicology. While this method is more comprehensive than most previous studies in PWH, which rely solely on self-report, self-report of drug use remains prone to inaccuracy due to possibility of recall bias and/or social desirability bias. Previous studies have more collected more detailed frequency of use data with the number of times of cannabis use per day to characterize heavier user groups (Bolla, Brown, Eldreth, Tate, & Cadet, 2002; Thames et al., 2016) while our study only characterized daily users. Further, two recent studies used a more precise method to categorize levels of cannabis exposure with direct measurement of cannabis metabolites in plasma; this data is not available for our sample (Manuzak et al., 2018; Rizzo et al., 2018). Additionally, we did not collect information concerning some potentially important aspects of cannabis use such as: recreational vs. medicinal use, cannabinoid composition (e.g. THC/CBD ratio), nor strength of cannabis strain. Such contextual variables may moderate the anti-inflammatory effects of cannabis observed in this study. Fifth, our study lacks data on anxiety disorders, post-traumatic stress disorder (PTSD), and numerous forms of social adversity that PWH commonly face in the U.S. and are detrimental to neurocognition (Rubin et al., 2017; Thames et al., 2017a; Watson et al., 2019). Lastly, our study did not collect standardized data on dietary factors nor regular physical exercise which can influence chemokine expression via pro-inflammatory or anti-inflammatory effects (Montoya et al., 2019). Future cannabis research in PWH should undertake careful assessment of cannabis use, anxiety and trauma-related disorders, social adversity, and dietary and physical exercise variables, as these factors should be considered for their influence on both inflammatory processes and neurocognitive outcomes.
Future studies in PWH are needed to investigate potential distinct effects of specific cannabinoids, and adult medicinal use, on brain structure and function. These relations among PWH can be clarified in longitudinal studies following designs of recent medicinal cannabis, neuroimaging, and cognition studies in a general clinical population (Gruber et al., 2018). Any neuroprotective effects of cannabis products on cognition are likely limited to specific cannabis/cannabinoid use parameters and individual characteristics of users (disease comorbidities, pharmacokinetic factors). In the general population, studies of heavy recreational cannabis use and cannabis dependence show CB1 receptor downregulation, which may be a mechanism for drug tolerance, lead to CB2 receptor desensitization on immune cells, and disrupt endocannabinoid system homeostasis (Hirvonen et al., 2012; Rotter et al., 2013). Thus, determination of harmful levels of cannabis use in relation to quantity and addictive potential must be considered in PWH.
Our work demonstrated some reduced inflammation in CSF, but not plasma, among HIV+ daily cannabis users who reported a median of half a gram of cannabis use per day. Of clinical relevance, lower levels of chemokines MCP-1 and IP-10 in CSF were associated with better cognitive performance in the domain of learning among PWH. Our findings point to the need for more targeted mechanistic studies of cannabis use and cognition specifically in PWH. In the context of HIV-associated chronic immune system activation and neuroinflammation, cannabis-based therapeutics may have a role in reducing inflammation and risk for downstream neurocognitive impairment.
Acknowledgements
This work was supported by the National Institute of Health (NIH), including the National Institute of Mental Health (NIMH) (grant numbers P30MH062512, N01MH22005, HHSN271201000036C, HHSN271201000030C); and the National Institute on Drug Abuse (NIDA) (grant numbers P50DA026306, P01DA12065).
CWW and LMC were supported by NIDA (grant number T32DA031098). SH was supported by the National Institute on Aging (NIA) (grant number AG063328). RE was supported by NIA (grant number R01AG048650-01A1). JI was supported by NIDA (grant numbers K23DA037793, R01DA047879)
Footnotes
The authors declare no conflicts of interest.
References
- Asensio VC, Maier J, Milner R, Boztug K, Kincaid C, Moulard M, … Fox HS (2001). Interferon-independent, human immunodeficiency virus type 1 gp120-mediated induction of CXCL10/IP-10 gene expression by astrocytes in vivo and in vitro. Journal of virology, 75(15), 7067–7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, & Brown GK (1996). Beck depression inventory-II. San Antonio, 78(2), 490–498. [Google Scholar]
- Bilkei-Gorzo A, Albayram O, Draffehn A, Michel K, Piyanova A, Oppenheimer H, … Imbeault S (2017). A chronic low dose of Δ 9-tetrahydrocannabinol (THC) restores cognitive function in old mice. Nature Medicine, 23(6), 782. [DOI] [PubMed] [Google Scholar]
- Bisogno T, & Di Marzo V (2010). Cannabinoid receptors and endocannabinoids: role in neuroinflammatory and neurodegenerative disorders. CNS & Neurological Disorders-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders), 9(5), 564–573. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Brown K, Eldreth D, Tate K, & Cadet J (2002). Dose-related neurocognitive effects of marijuana use. Neurology, 59(9), 1337–1343. [DOI] [PubMed] [Google Scholar]
- Burdo TH, Weiffenbach A, Woods SP, Letendre S, Ellis RJ, & Williams KC (2013). Elevated sCD163 in plasma but not cerebrospinal fluid is a marker of neurocognitive impairment in HIV infection. AIDS (London, England), 27(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlacu R, Umlauf A, Marcotte T, Soontornniyomkij B, Diaconu C, Bulacu-Talnariu A, … Ene L (2020). Plasma CXCL10 correlates with HAND in HIV-infected women. J Neurovirol, 26(1), 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, & Heaton RK (2004). Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol, 26(3), 307–319. [DOI] [PubMed] [Google Scholar]
- Casaletto KB, Weber E, Iudicello JE, & Woods SP (2017). Real-world impact of HIV-associated neurocognitive impairment Changes in the Brain (pp. 211–245): Springer. [Google Scholar]
- Castro F. d. O., Silva JM, Dorneles GP, Barros J, Ribeiro CB, Noronha I, … Pereira AJCS (2019). Distinct inflammatory profiles in HIV-infected individuals under ART using cannabis, cocaine or cannabis plus cocaine. AIDS (London, England). [DOI] [PubMed] [Google Scholar]
- Chang L, Cloak C, Yakupov R, & Ernst T (2006). Combined and independent effects of chronic marijuana use and HIV on brain metabolites. Journal of Neuroimmune Pharmacology, 1(1), 65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D-J, Gao M, Gao F-F, Su Q-X, & Wu J (2017). Brain cannabinoid receptor 2: expression, function and modulation. Acta Pharmacologica Sinica, 38(3), 312–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RA, de la Monte S, Gongvatana A, Ombao H, Gonzalez B, Devlin KN, … Tashima KT (2011). Plasma cytokine concentrations associated with HIV/hepatitis C coinfection are related to attention, executive and psychomotor functioning. Journal of neuroimmunology, 233(1-2), 204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costiniuk CT, & Jenabian M-A (2019). Cannabinoids and inflammation: implications for people living with HIV. Aids, 33(15), 2273–2288. [DOI] [PubMed] [Google Scholar]
- Cristiani SA, Pukay-Martin ND, & Bornstein RA (2004). Marijuana use and cognitive function in HIV-infected people. The Journal of neuropsychiatry and clinical neurosciences, 16(3), 330–335. [DOI] [PubMed] [Google Scholar]
- de Almeida SM, Letendre S, Zimmerman J, Lazzaretto D, McCutchan A, & Ellis R (2005). Dynamics of monocyte chemoattractant protein type one (MCP-1) and HIV viral load in human cerebrospinal fluid and plasma. Journal of neuroimmunology, 169(1-2), 144–152. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Peterson S, Cherner M, Morgan E, Schrier R, Tang B, … Iudicello J (2020a). Beneficial Effects of Cannabis on Blood–Brain Barrier Function in Human Immunodeficiency Virus. Clinical Infectious Diseases. doi: 10.1093/cid/ciaa437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RJ, Peterson SN, Li Y, Schrier R, Iudicello J, Letendre S, … Cherner M (2020b). Recent cannabis use in HIV is associated with reduced inflammatory markers in CSF and blood. Neurology Neuroimmunology and Neuroinflammation, 7(5). doi: 10.1212/NXI.0000000000000809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RJ, Toperoff W, Vaida F, Van Den Brande G, Gonzales J, Gouaux B, … Atkinson JH (2009). Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology, 34(3), 672–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty A, Rawstorne P, Prestage G, Crawford J, Grierson J, & Kippax S (2007). Marijuana as therapy for people living with HIV/AIDS: social and health aspects. AIDS Care, 19(2), 295–301. [DOI] [PubMed] [Google Scholar]
- Gannon P, Khan MZ, & Kolson DL (2011). Current understanding of HIV-associated neurocognitive disorders pathogenesis. Current opinion in neurology, 24(3), 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R, Pacheco-Colon I, Duperrouzel JC, & Hawes SW (2017). Does Cannabis Use Cause Declines in Neuropsychological Functioning? A Review of Longitudinal Studies. J Int Neuropsychol Soc, 23(9-10), 893–902. doi: 10.1017/S1355617717000789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Sagar KA, Dahlgren MK, Gonenc A, Smith RT, Lambros AM, … Lukas SE (2018). The grass might be greener: medical marijuana patients exhibit altered brain activity and improved executive function after 3 months of treatment. Frontiers in pharmacology, 8, 983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F, … Atkinson JH (2010). HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy CHARTER Study. Neurology, 75(23), 2087–2096. doi: 10.1212/WNL.0b013e318200d727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, … Group, H. (2011). HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol, 17(1), 3–16. doi: 10.1007/s13365-010-0006-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, & Grant I (2004). Revised comprehensive norms for an expanded Halstead-Reitan Battery: Demographically adjusted neuropsychological norms for African American and Caucasian adults. Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- Hinojosa AE, Garcia-Bueno B, Leza JC, & Madrigal JL (2011). CCL2/MCP-1 modulation of microglial activation and proliferation. Journal of neuroinflammation, 8(1), 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirvonen J, Goodwin R, Li C-T, Terry G, Zoghbi S, Morse C, … Innis R (2012). Reversible and regionally selective downregulation of brain cannabinoid CB 1 receptors in chronic daily cannabis smokers. Molecular psychiatry, 17(6), 642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hložek T, Uttl L, Kadeřábek L, Balíková M, Lhotková E, Horsley RR, … Tylš F (2017). Pharmacokinetic and behavioural profile of THC, CBD, and THC+ CBD combination after pulmonary, oral, and subcutaneous administration in rats and confirmation of conversion in vivo of CBD to THC. European Neuropsychopharmacology, 27(12), 1223–1237. [DOI] [PubMed] [Google Scholar]
- Hong S, & Banks WA (2015). Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain Behav Immun, 45, 1–12. doi: 10.1016/j.bbi.2014.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huestis MA, & Smith ML (2007). Human cannabinoid pharmacokinetics and interpretation of cannabinoid concentrations in biological fluids and tissues Marijuana and the Cannabinoids (pp. 205–235): Springer. [Google Scholar]
- Imp BM, Rubin LH, Tien PC, Plankey MW, Golub ET, French AL, & Valcour VG (2017). Monocyte activation is associated with worse cognitive performance in HIV-infected women with virologic suppression. The Journal of infectious diseases, 215(1), 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iudicello JE, Hussain M, Watson C, Morgan E, Heaton RK, Stern R, & Alosco M (2019). HIV-associated neurocognitive disorders. Oxford Handbook of Adult Cognitive Disorders, 29–60. [Google Scholar]
- Kamat A, Lyons JL, Misra V, Uno H, Morgello S, Singer EJ, & Gabuzda D (2012). Monocyte activation markers in cerebrospinal fluid associated with impaired neurocognitive testing in advanced HIV infection. J Acquir Immune Defic Syndr, 60(3), 234–243. doi: 10.1097/QAI.0b013e318256f3bc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen L, & Turner AD (2015). Differential effects of self-reported lifetime marijuana use on interleukin-1 alpha and tumor necrosis factor in African American adults. Journal of behavioral medicine, 38(3), 527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Shin AH, & Thayer SA (2011). Activation of cannabinoid type 2 receptors inhibits HIV-1 envelope glycoprotein gp120-induced synapse loss. Molecular pharmacology, 80(3), 357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krsak M, Wada NI, Plankey MW, Kinney GL, Epeldegui M, Okafor CN, … Erlandson KM (2020). Self-Reported Cannabis Use and Markers of Inflammation in Men Who Have Sex With Men With and Without HIV. Cannabis and cannabinoid research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W-J, Liao Y-C, Wang Y-F, Lin I-F, Wang S-J, & Fuh J-L (2018). Plasma MCP-1 and cognitive decline in patients with Alzheimer’s disease and mild cognitive impairment: a two-year follow-up study. Scientific reports, 8(1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letendre S (2011). Central nervous system complications in HIV disease: HIV-associated neurocognitive disorder. Top Antivir Med, 19(4), 137. [PMC free article] [PubMed] [Google Scholar]
- Manuzak JA, Gott TM, Kirkwood JS, Coronado E, Hensley-McBain T, Miller C, … Martin JN (2018). Heavy cannabis use associated with reduction in activated and inflammatory immune cell frequencies in antiretroviral therapy–treated Human Immunodeficiency Virus–infected individuals. Clinical Infectious Diseases, 66(12), 1872–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehla R, Bivalkar-Mehla S, Nagarkatti M, & Chauhan A (2012). Programming of neurotoxic cofactor CXCL-10 in HIV-1-associated dementia: abrogation of CXCL-10-induced neuro-glial toxicity in vitro by PKC activator. Journal of neuroinflammation, 9(1), 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya JL, Jankowski CM, O’Brien KK, Webel AR, Oursler KK, Henry BL, … Erlandson KM (2019). Evidence-informed practical recommendations for increasing physical activity among persons living with HIV. AIDS (London, England), 33(6), 931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarkatti P, Pandey R, Rieder SA, Hegde VL, & Nagarkatti M (2009). Cannabinoids as novel anti-inflammatory drugs. Future medicinal chemistry, 1(7), 1333–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences, E., & Medicine. (2017). The health effects of cannabis and cannabinoids: The current state of evidence and recommendations for research: National Academies Press. [PubMed] [Google Scholar]
- Neuhaus J, Jacobs DR Jr., Baker JV, Calmy A, Duprez D, La Rosa A, … Neaton JD (2010). Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis, 201(12), 1788–1795. doi: 10.1086/652749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman MA, Moore DJ, Taylor M, Franklin D Jr, Cysique L, Ake C, … Group, H. (2011). Demographically corrected norms for African Americans and Caucasians on the hopkins verbal learning test–revised, brief visuospatial memory test–revised, stroop color and word test, and wisconsin card sorting test 64-card version. J Clin Exp Neuropsychol, 33(7), 793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacek LR, Towe SL, Hobkirk AL, Nash D, & Goodwin RD (2018). Frequency of Cannabis use and medical Cannabis Use Among Persons Living With HIV in the United States: findings from a nationally representative sample. AIDS Education and Prevention, 30(2), 169–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persidsky Y, Fan S, Dykstra H, Reichenbach NL, Rom S, & Ramirez SH (2015). Activation of Cannabinoid Type Two Receptors (CB2) Diminish Inflammatory Responses in Macrophages and Brain Endothelium. J Neuroimmune Pharmacol, 10(2), 302–308. doi: 10.1007/s11481-015-9591-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulliam L, Rempel H, Sun B, Abadjian L, Calosing C, & Meyerhoff DJ (2011). A peripheral monocyte interferon phenotype in HIV infection correlates with a decrease in magnetic resonance spectroscopy metabolite concentrations. AIDS (London, England), 25(14), 1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh G, MacLean AG, & Philipp MT (2013). Cytokines and chemokines at the crossroads of neuroinflammation, neurodegeneration, and neuropathic pain. Mediators of inflammation, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez SH, Reichenbach NL, Fan S, Rom S, Merkel SF, Wang X, … Persidsky Y (2013). Attenuation of HIV-1 replication in macrophages by cannabinoid receptor 2 agonists. Journal of leukocyte biology, 93(5), 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo MD, Crawford RB, Bach A, Sermet S, Amalfitano A, & Kaminski NE (2019). Δ9-Tetrahydrocannabinol Suppresses Monocyte-Mediated Astrocyte Production of Monocyte Chemoattractant Protein 1 and Interleukin-6 in a Toll-Like Receptor 7–Stimulated Human Coculture. Journal of Pharmacology and Experimental Therapeutics, 371(1), 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo MD, Crawford RB, Henriquez JE, Aldhamen YA, Gulick P, Amalfitano A, & Kaminski NE (2018). HIV-infected cannabis users have lower circulating CD16+ monocytes and IFN-γ-inducible protein 10 levels compared with nonusing HIV patients. Aids, 32(4), 419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SM, Sobell LC, Sobell MB, & Leo GI (2014). Reliability of the Timeline Followback for cocaine, cannabis, and cigarette use. Psychology of addictive behaviors, 28(1), 154. [DOI] [PubMed] [Google Scholar]
- Rom S, & Persidsky Y (2013). Cannabinoid receptor 2: potential role in immunomodulation and neuroinflammation. Journal of Neuroimmune Pharmacology, 8(3), 608–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotter A, Bayerlein K, Hansbauer M, Weiland J, Sperling W, Kornhuber J, & Biermann T (2013). CB1 and CB2 receptor expression and promoter methylation in patients with cannabis dependence. European addiction research, 19(1), 13–20. [DOI] [PubMed] [Google Scholar]
- Rubin LH, Cook JA, Springer G, Weber KM, Cohen MH, Martin EM, … Milam J (2017). Perceived and post-traumatic stress are associated with decreased learning, memory, and fluency in HIV-infected women. AIDS (London, England), 31(17), 2393–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Neigh GN, Sundermann EE, Xu Y, Scully EP, & Maki PM (2019). Sex differences in neurocognitive function in adults with HIV: patterns, predictors, and mechanisms. Current psychiatry reports, 21(10), 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saloner R, & Cysique LA (2017). HIV-associated neurocognitive disorders: a global perspective. Journal of the International Neuropsychological Society, 23(9-10), 860–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, … McArthur JC (2016). HIV-associated neurocognitive disorder--pathogenesis and prospects for treatment. Nat Rev Neurol, 12(4), 234–248. doi: 10.1038/nrneurol.2016.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons RP, Scully EP, Groden EE, BENEDICT KF, Chang JJ, Lane K, … Altfeld M (2013). HIV-1 infection induces strong production of IP-10 through TLR7/9-dependent pathways. AIDS (London, England), 27(16), 2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struik D, Sanna F, & Fattore L (2018). The modulating role of sex and anabolic-androgenic steroid hormones in cannabinoid sensitivity. Frontiers in behavioral neuroscience, 12, 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thames AD, Kuhn TP, Mahmood Z, Bilder RM, Williamson TJ, Singer EJ, & Arentoft A (2017a). Effects of social adversity and HIV on subcortical shape and neurocognitive function. Brain Imaging and Behavior, 1–13. doi: 10.1007/s11682-017-9676-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thames AD, Kuhn TP, Williamson TJ, Jones JD, Mahmood Z, & Hammond A (2017b). Marijuana effects on changes in brain structure and cognitive function among HIV+ and HIV− adults. Drug Alcohol Depend, 170, 120–127. doi: 10.1016/j.drugalcdep.2016.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thames AD, Mahmood Z, Burggren AC, Karimian A, & Kuhn TP (2016). Combined effects of HIV and marijuana use on neurocognitive functioning and immune status. AIDS Care, 28(5), 628–632. doi: 10.1080/09540121.2015.1124983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, … Sharkey KA (2005). Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science, 310(5746), 329–332. doi: 10.1126/science.1115740 [DOI] [PubMed] [Google Scholar]
- Vera JH, Guo Q, Cole JH, Boasso A, Greathead L, Kelleher P, … Gunn RN (2016). Neuroinflammation in treated HIV-positive individuals: a TSPO PET study. Neurology, 86(15), 1425–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada NI, Jacobson LP, Margolick JB, Breen EC, Macatangay B, Penugonda S, … Bream JH (2015). The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS, 29(4), 463–471. doi: 10.1097/QAD.0000000000000545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Liang H, Ernst T, Oishi K, & Chang L (2020). Microstructural Brain Abnormalities in HIV+ Individuals with or without Chronic Marijuana Use. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CW-M, Paolillo EW, Morgan EE, Umlauf A, Sundermann EE, Ellis RJ, … Grant I (2020). Cannabis Exposure is Associated With a Lower Likelihood of Neurocognitive Impairment in People Living With HIV. JAIDS Journal of Acquired Immune Deficiency Syndromes, 83(1), 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CW-M, Sundermann EE, Hussain MA, Umlauf A, Thames AD, Moore RC, … Moore DJ (2019). Effects of trauma, economic hardship, and stress on neurocognition and everyday function in HIV. Health Psychology, 38(1), 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JM, Downie SA, Lyman WD, & Berman JW (1998). Astrocyte-derived monocyte-chemoattractant protein-1 directs the transmigration of leukocytes across a model of the human blood-brain barrier. The Journal of Immunology, 161(12), 6896–6903. [PubMed] [Google Scholar]
- Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, … Ryder S (2015). Cannabinoids for medical use: a systematic review and meta-analysis. Jama, 313(24), 2456–2473. [DOI] [PubMed] [Google Scholar]
- Woolridge E, Barton S, Samuel J, Osorio J, Dougherty A, & Holdcroft A (2005). Cannabis use in HIV for pain and other medical symptoms. Journal of pain and symptom management, 29(4), 358–367. [DOI] [PubMed] [Google Scholar]
- Yang G, Meng Y, Li W, Yong Y, Fan Z, Ding H, … Ke ZJ (2011). Neuronal MCP-1 mediates microglia recruitment and neurodegeneration induced by the mild impairment of oxidative metabolism. Brain pathology, 21(3), 279–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Li F-F, Han Y-C, Jia B, & Ding Y (2015). Cannabinoid receptor CB2 is involved in tetrahydrocannabinol-induced anti-inflammation against lipopolysaccharide in MG-63 cells. Mediators of inflammation, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Liu A, Qiao L, Sheng B, Xu M, Li W, & Chen D (2015). The relationship of CSF and plasma cytokine levels in HIV infected patients with neurocognitive impairment. BioMed research international, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Qiao L, Wei F, Yin J, Liu L, Ji Y, … Chen D (2013). Cytokines in CSF correlate with HIV-associated neurocognitive disorders in the post-HAART era in China. J Neurovirol, 19(2), 144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]