Mutations in MYD88 (MYD88MUT) are present in approximately 93%–97% of patients with Waldenström macroglobulinemia (WM), nearly all of which correspond to the c.978T>C transversion resulting in a p.Leu265Pro (L265P) substitution at the protein level.1,2 MYD88MUT helps support the diagnosis of WM and differentiate from other IgM-secreting B-cell malignancies, such as marginal zone lymphoma and IgM myeloma, where it is absent or rarely expressed.2 The presence of MYD88MUT is also associated with a better prognosis, lower risk of histological transformation, and predicts response to the BTK inhibitor ibrutinib in WM patients.3–7 These findings prompted the World Health Organization, National Comprehensive Cancer Network, and WM Workshop guidelines to recommend MYD88MUT testing for all suspected WM cases.8
Despite the importance of MYD88MUT, a uniform means for identifying them is currently lacking. The original studies that established the incidence of MYD88L265P used an allele-specific polymerase chain reaction (AS-PCR) with CD19-selected bone marrow (BM) aspirates to maximize sensitivity.2 However, presorting B-cells before AS-PCR is not feasible for most clinical laboratories; hence, unselected BM aspirates are routinely used for the clinical detection of MYD88L265P.9 Additional testing with Sanger sequencing is recommended in patients with wild-type (WT) MYD88 by AS-PCR to evaluate for rare non-L265P MYD88MUT.9 Targeted next-generation sequencing (NGS) has emerged as an alternative to AS-PCR to identify MYD88L265P, but the sensitivity of NGS for MYD88L265P detection in WM patients is unknown. This prompted us to compare the results for MYD88L265P detection by NGS against AS-PCR in 414 consecutive WM patients who had both assays performed synchronously.
We used CD19-selected BM aspirate to detect MYD88L265P by quantitative AS-PCR, followed by Sanger sequencing to evaluate for non-L265P MYD88MUT in patients with MYD88WT by AS-PCR as previously described.2,6,7 Qualitative AS-PCR (lower limit of detection ~1%) for MYD88L265P was also performed on unselected BM aspirate in the Molecular Diagnostics Laboratory, Brigham & Women’s Hospital (Boston, MA). The findings for MYD88L265P were compared against a clinically validated and targeted NGS assay (Rapid Heme Panel) using unselected BM aspirate from the same patients.10 The NGS assay has an average coverage of 1500X with <5% of the amplicons with 50X coverage, and reproducibly could detect single nucleotide variants at allele frequencies of ≥5%.10 The median coverage of the MYD88 amplicon was 1521X (range 305–3707X). Calculations were performed with R (R Foundation for Statistical Computing, Vienna, Austria). The Dana-Farber/Harvard Cancer Center IRB approved this study, and all patients provided written consent for the use of their samples.
Clinical characteristics at the time of MYD88MUT testing are shown in Supplemental Digital Table 1, http://links.lww.com/HS/A180. Using AS-PCR with CD19-selected BM samples, 391 patients (94.4%) had MYD88L265P identified. Sanger sequencing of the 23 patients with MYD88WT by AS-PCR revealed that one patient had a dinucleotide substitution that resulted in MYD88L265P, while another had a MYD88S243N mutation. Overall, the prevalence of MYD88MUT in this cohort was 393/414 (95%), and did not differ between treatment-naïve and previously treated WM patients (94% versus 96%, respectively; P = 0.37).
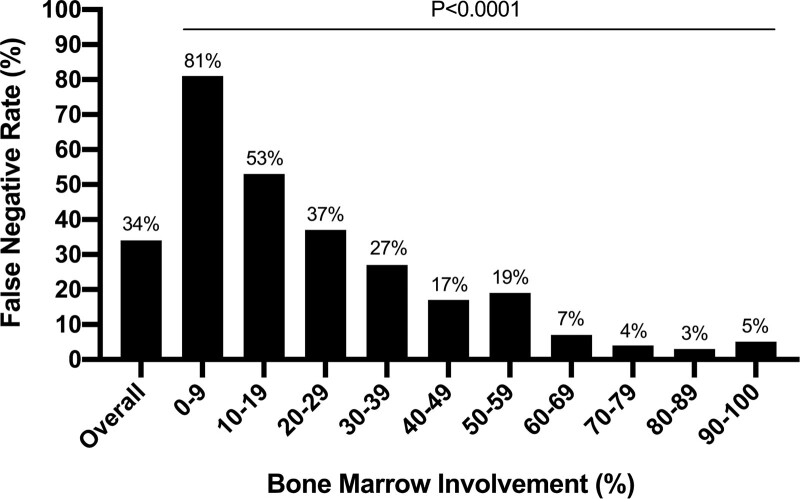
We compared the results for MYD88L265P by NGS with unselected BM aspirate against AS-PCR with CD19-selected BM. Among the 391 patients with MYD88L265P identified by AS-PCR, only 259 patients (66%) had MYD88L265P identified by the NGS method; the median variant allele fraction for MYD88L265P was 5.95% (range 0.5%–86.5%). No patient had MYD88L265P identified by NGS that AS-PCR did not also identify. The test performance statistics comparing the 2 methods are summarized in Table 1. We then evaluated factors that impacted the sensitivity of MYD88L265P detection by NGS. Modeling the false negative results by age, sex, hemoglobin level, platelet count, serum IgM level, BM involvement, prior treatment status, and MYD88 amplicon coverage revealed only BM involvement represented a significant predictor (P < 0.0001). Accordingly, the false negative rate for MYD88L265P by NGS increased significantly with decreasing BM involvement (Figure 1).
Table 1.
Test Performance of Targeted Next-generation Sequencing and Allele-specific PCR for MYD88L265P in WM Patients
| AS-PCR | NGS | ||
|---|---|---|---|
| CD19-selected BM | Unselected BM | Unselected BM | |
| True positive, no | 391 | 377 | 259 |
| True negative, no | 24 | 24 | 24 |
| False positive, no | 0 | 0 | 0 |
| False negative, no | 0 | 14 | 132 |
| Concordance (κ), % | Ref. | 97 (0.76) | 68 (0.19) |
| Sensitivity (95% CI), % | Ref. | 96 (94–98) | 66 (61–71) |
| Specificity (95% CI), % | Ref. | 100 (83–100) | 100 (83–100) |
| PPV (95% CI), % | Ref. | 100 (99–100) | 100 (98–100) |
| NPV (95% CI), % | Ref. | 63 (46–78) | 15 (10–22) |
Results from both AS-PCR and targeted NGS with unselected bone marrow aspirate samples were compared against AS-PCR with CD19-selected bone marrow aspirate samples for MYD88L265P.
AS-PCR = allele-specific polymerase chain reaction; BM = bone marrow; CI = confidence interval; NGS = next-generation sequencing; NPV = negative predictive value; PPV = positive predictive value.
Figure 1.
Impact of bone marrow involvement on false negative rates for MYD88L265P with targeted next-generation sequencing. AS-PCR with CD19-selected bone marrow aspirate samples for MYD88L265P was used as the reference assay for this analysis. AS-PCR = allele-specific polymerase chain reaction.
To examine the relative importance of AS-PCR versus B-cell selection on NGS sensitivity for MYD88L265P, we compared the AS-PCR results with both CD19-selected and unselected BM for all patients (Table 1). Among the 391 patients with MYD88L265P identified by AS-PCR with CD19-selected BM, 377 (96%) had MYD88L265P identified by AS-PCR when using unselected BM. This corresponds to a 45% increase in sensitivity with AS-PCR over NGS with unselected BM aspirate (96% versus 66%, respectively). These findings demonstrate that sequencing by NGS, rather than B-cell selection, accounts for most of the false negative results generated by NGS versus AS-PCR.
Overall, the findings demonstrate the use of unselected BM aspirate and NGS fails to detect MYD88L265P in one-third of WM patients. We also show the false negative rate with NGS correlates with BM involvement, although false negative results still occurred with all levels of BM involvement. Sampling bias due to patchy BM involvement or hemodilution (ie, the admixture of peripheral blood during BM aspiration) of tumor samples below the NGS threshold may account for this observation.11 Our findings should herald efforts to optimize MYD88MUT testing in WM patients. Although tumor enrichment with B-cell selection can improve MYD88L265P detection,12 it is not feasible for most clinical laboratories. We show the use of AS-PCR for MYD88L265P with unselected BM aspirate which produces a 45% increase in sensitivity over NGS, and may be sensitive enough to overcome the adverse impact of low BM involvement and/or hemodilution. Likewise, increased depth of NGS coverage could be considered to improve sensitivity, as NGS is ideally suited to simultaneously evaluate the TIR exon coding region of MYD88 for both L265P and non-L265P mutations. Evaluating alternative sensitive approaches adoptable to clinical practice to identify MYD88MUT in WM patients also warrants consideration. The use of digital droplet PCR has shown high concordance with AS-PCR for the detection of MYD88L265P.13,14 Moreover, the detection of MYD88L265P in cell-free DNA is feasible in WM patients and may represent a novel tissue for molecular testing.15–18 These efforts to optimize molecular testing are collectively important to ensure the accurate identification of MYD88MUT, which impact diagnosis, prognosis, and treatment selection in WM patients.9
This study also provides important context for interpreting the iNNOVATE trial that evaluated ibrutinib plus rituximab in WM patients. Compared to ibrutinib monotherapy in the pivotal trial,6,7 ibrutinib plus rituximab induced a higher major response rate (73% versus 0%) and median PFS (>4 versus 0.4 yrs) in MYD88WT patients.19,20 However, the iNNOVATE trial used an NGS assay (mean coverage >500X) with either unselected BM aspirate or formalin-fixed paraffin-embedded specimens to identify MYD88MUT,19 whereas the pivotal ibrutinib trial utilized AS-PCR and Sanger sequencing with CD19-selected BM aspirate.7,21 Given the high false negative rate with NGS, our findings suggest the iNNOVATE trial results may be confounded by the inclusion of patients with MYD88MUT in the MYD88WT cohort. Indeed, the prevalence of MYD88WT was at least two-fold higher in iNNOVATE than the established prevalence in WM (16% versus 3%–7%), suggesting the presence of misclassified MYD88WT patients.1,2,9 Preclinical studies have also demonstrated MYD88WT tumors have intrinsic ibrutinib resistance due to NF-kB activating mutations downstream of BTK.22–24 It is therefore unlikely the addition of rituximab accounts for the high activity of ibrutinib reported with combination therapy, particularly since rituximab monotherapy only induced a major response rate of 22% and median PFS of 2 years in MYD88WT patients.19,20 Nevertheless, our findings highlight the importance of using highly sensitive approaches when investigating MYD88MUT as a treatment biomarker. Such an approach is also critical given MYD88WT WM patients typically have low BM tumor burden,3 which adversely impacts the sensitivity of molecular testing. Our findings may also be important for molecular diagnostic testing in other B-cell malignancies, such as ABC DLBCL (30%–40%), primary Central Nervous System lymphoma (60%–80%), marginal zone lymphoma (5%–10%), and chronic lymphocytic leukemia (5%–10%), wherein MYD88MUT is frequently observed.
In summary, our data show that targeted NGS frequently yields false negative results for MYD88L265P in WM patients. Given the importance of MYD88MUT status in the management of WM, our findings highlight the importance for standardized testing methods for MYD88MUT in WM patients, as well as other diseases impacted by this mutation.
Acknowledgments
The authors would like to thank the Seigel Family Fund for WM Research, Orszag Family Fund for WM Research, Peter S. Bing, MD, International Waldenström’s Macroglobulinemia Foundation, Leukemia & Lymphoma Society (Grant: R6507-18), Kerry Robertson Fund for WM.
Disclosures
SPT, JJC, GY, and ZRH have received research funding and/or consulting fees from Pharmacyclics Inc., Janssen Pharmaceuticals Inc., the manufacturer of ibrutinib. SPT has received research funding from Bristol Myers Squibb, X4 Pharmaceuticals, and Beigene. JJC received research funding and/or consulting fees from Abbvie, Beigene, Kymera, and TG Therapeutics.
Sources of funding
SPT, ZRH, and GY are supported by an NIH SPORE in Multiple Myeloma (Grant: 2P50CA100707-16A1).
Supplementary Material
Footnotes
Supplemental digital content is available for this article.
References
- 1.Treon SP, Xu L, Yang G, et al. MYD88 L265P somatic mutation in Waldenström’s macroglobulinemia. N Engl J Med. 2012;367:826–833. [DOI] [PubMed] [Google Scholar]
- 2.Xu L, Hunter ZR, Yang G, et al. MYD88 L265P in Waldenström Macroglobulinemia, immunoglobulin M monoclonal gammopathy, and other B-cell lymphoproliferative disorders using conventional and quantitative allele-specific polymerase chain reaction. Blood. 2013;121:2051–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Treon SP, Cao Y, Xu L, et al. Somatic mutations in MYD88 and CXCR4 are determinants of clinical presentation and overall survival in Waldenstrom macroglobulinemia. Blood. 2014;123:2791–2796. [DOI] [PubMed] [Google Scholar]
- 4.Treon SP, Gustine J, Xu L, et al. MYD88 wild-type Waldenstrom macroglobulinaemia: differential diagnosis, risk of histological transformation, and overall survival. Br J Haematol. 2018;180:374–380. [DOI] [PubMed] [Google Scholar]
- 5.Zanwar S, Abeykoon JP, Durot E, et al. Impact of MYD88L265P mutation status on histological transformation of Waldenström macroglobulinemia. Am J Hematol. 2020;95:274–281. [DOI] [PubMed] [Google Scholar]
- 6.Treon SP, Tripsas CK, Meid K, et al. Ibrutinib in previously treated Waldenström’s macroglobulinemia. N Engl J Med. 2015;372:1430–1440. [DOI] [PubMed] [Google Scholar]
- 7.Treon SP, Meid K, Gustine J, et al. Long-term follow-up of ibrutinib monotherapy in symptomatic, previously treated patients with Waldenström macroglobulinemia. J Clin Oncol. 2021;39:565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castillo JJ, Advani RH, Branagan AR, et al. Consensus treatment recommendations from the tenth International Workshop for Waldenström macroglobulinaemia. Lancet Haematol. 2020;7:e827–e837. [DOI] [PubMed] [Google Scholar]
- 9.Treon SP, Xu L, Guerrera ML, et al. Genomic landscape of Waldenström macroglobulinemia and its impact on treatment strategies. J Clin Oncol. 2020;38:1198–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kluk MJ, Lindsley RC, Aster JC, et al. Validation and implementation of a custom next-generation sequencing clinical assay for hematologic malignancies. J Mol Diagn. 2016;18:507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Astle JM, Xu ML, Friedman T, et al. Limitations of poor bone marrow aspirations (for an accurate diagnosis) despite the multimodal analytical era: a longitudinal retrospective study. Am J Hematol. 2017;92:E600–E602. [DOI] [PubMed] [Google Scholar]
- 12.Gustine J, Meid K, Xu L, et al. To select or not to select? The role of B-cell selection in determining the MYD88 mutation status in Waldenström macroglobulinaemia. Br J Haematol. 2017;176:822–824. [DOI] [PubMed] [Google Scholar]
- 13.Drandi D, Genuardi E, Dogliotti I, et al. Highly sensitive MYD88L265P mutation detection by droplet digital polymerase chain reaction in Waldenström macroglobulinemia. Haematologica. 2018;103:1029–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lo Schirico M, Ferrante M, Dogliotti I, et al. Droplet digital PCR assay for MYD88 L265P: clinical applications in Waldenström macroglobulinemia. HemaSphere. 2020;4:e324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bagratuni T, Ntanasis-Stathopoulos I, Gavriatopoulou M, et al. Detection of MYD88 and CXCR4 mutations in cell-free DNA of patients with IgM monoclonal gammopathies. Leukemia. 2018;32:2617–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ntanasis-Stathopoulos I, Bagratuni T, Gavriatopoulou M, et al. Cell-free DNA analysis for the detection of MYD88 and CXCR4 mutations in IgM monoclonal gammopathies; an update with clinicopathological correlations. Am J Hematol. 2020;95:E148–E150. [DOI] [PubMed] [Google Scholar]
- 17.Wu YY, Jia MN, Cai H, et al. Detection of the MYD88L265P and CXCR4S338X mutations by cell-free DNA in Waldenström macroglobulinemia. Ann Hematol. 2020;99:1763–1769. [DOI] [PubMed] [Google Scholar]
- 18.Demos MG, Hunter ZR, Xu L, et al. Cell-free DNA analysis for detection of MYD88L265P and CXCR4S338X mutations in Waldenström macroglobulinemia. Am J Hematol. 2021;96:E250–E253. [DOI] [PubMed] [Google Scholar]
- 19.Dimopoulos MA, Tedeschi A, Trotman J, et al. ; iNNOVATE Study Group and the European Consortium for Waldenström’s Macroglobulinemia. Phase 3 trial of ibrutinib plus rituximab in Waldenström’s macroglobulinemia. N Engl J Med. 2018;378:2399–2410. [DOI] [PubMed] [Google Scholar]
- 20.Buske C, Tedeschi A, Trotman J, et al. Five-year follow-up of ibrutinib plus rituximab vs placebo plus rituximab for Waldenstrom’s macroglobulinemia: final analysis from the randomized phase 3 iNNOVATETM Study. Blood. 2020;136(suppl 1):24–26.32430494 [Google Scholar]
- 21.Treon SP, Xu L, Hunter Z. MYD88 mutations and response to ibrutinib in Waldenström’s macroglobulinemia. N Engl J Med. 2015;373:584–586. [DOI] [PubMed] [Google Scholar]
- 22.Yang G, Zhou Y, Liu X, et al. A mutation in MYD88 (L265P) supports the survival of lymphoplasmacytic cells by activation of Bruton tyrosine kinase in Waldenström macroglobulinemia. Blood. 2013;122:1222–1232. [DOI] [PubMed] [Google Scholar]
- 23.Yang G, Buhrlage SJ, Tan L, et al. HCK is a survival determinant transactivated by mutated MYD88, and a direct target of ibrutinib. Blood. 2016;127:3237–3252. [DOI] [PubMed] [Google Scholar]
- 24.Hunter ZR, Xu L, Tsakmaklis N, et al. Insights into the genomic landscape of MYD88 wild-type Waldenström macroglobulinemia. Blood Adv. 2018;2:2937–2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.