Abstract
Objective The objective of this study is to describe the clinical presentation, tumor characteristics, natural history, and treatment patterns of sinonasal osteosarcoma.
Methods Fourteen patients who had been treated for osteosarcoma of the nasal cavity and paranasal sinuses at a tertiary care center were reviewed. In addition, a systematic review of the literature for osteosarcoma of the sinonasal cavity was performed.
Results In a systematic review, including 14 patients from the authors' institution, 53 total studies including 88 patients were assessed. Median follow-up was 18 months (interquartile range: 8–39 months). The most common presenting symptoms were facial mass or swelling (34%), and nasal obstruction (30%). The most common paranasal sinus involved by tumor was the maxillary sinus (64%), followed by the ethmoid sinuses (52%). The orbit (33%), dura (13%) and infratemporal fossa (10%) were the most common sites of local invasion. The majority of patients underwent surgery followed by adjuvant therapy (52.4%). Increasing age was associated with decreased overall survival rate (unit risk ratio [95% confidence interval (CI)] = 1.02 [1.003–1.043]; p = 0.0216) and T4 disease was associated with decreased disease-specific survival rate (hazard ratio [HR] = 2.87; p = 0.0495). The 2- and 5-year overall survival rates were 68 and 40%, respectively, while 2- and 5-year disease-specific survival rates were 71% and 44%, respectively.
Conclusion Sinonasal osteosarcomas are uncommon tumors and can pose a significant therapeutic challenge. Increasing age and T4 disease are associated with worse prognosis. This disease usually warrants consultation by a multidisciplinary team and consideration of multimodality therapy.
Keywords: osteogenic sarcoma, osteosarcoma, nasal cavity, paranasal sinuses, sinonasal, EEA, expanded endonasal approach
Introduction
Osteosarcoma, also called osteogenic sarcoma, originates from primitive bone-forming mesenchymal cells. It is the most common primary bone malignancy with an approximate incidence in the United States of 1:100,000 patients per year 1 and affects patients in a bimodal age distribution. 2 3 It usually affects the metaphyseal growth plates of the long bones, 2 but 6 to 13% of osteosarcomas are craniofacial. 4 In the head in neck, osteosarcoma most commonly arises in areas of mastication, namely, the mandible and maxilla, 1 and rarely involves the sphenoid and ethmoid bones. 5 6 The exact incidence of osteosarcoma is difficult to determine because the reporting of the sinonasal site in head and neck osteosarcoma is often reported in umbrella categories such as “extragnathic” or “skull/facial bones.”
Current management paradigms for sinonasal osteosarcoma have been extrapolated from experience with osteosarcoma in the rest of the body tailored to the anatomic considerations of the head and neck. In other parts of the body, treatment is primarily surgical, along with frequent use of neoadjuvant and/or adjuvant chemotherapy. 4 Radiation therapy (RT) does not typically play a major role in long-bone tumors as wide surgical margin is frequently obtainable, such as above the knee amputation for lower leg osteosarcomas. In addition, osteosarcomas are traditionally felt to be radioresistant tumors, requiring more than 60 Gy to be effective. 7 However, when these tumors occur in the sinonasal cavity, anatomic constraints including close proximity to the skull base and orbits make obtaining widely clear surgical margins difficult. With a significant proportion of this disease population consisting of young people, the prospect of highly morbid or disfiguring surgery may be daunting. Thus, primary chemoradiotherapy or surgery followed by adjuvant RT may be more commonly utilized in the sinonasal region, especially in patients where wide surgical margins are not possible. 8
Specific attention and guidance for osteosarcomas of the nasal cavity and paranasal sinuses is warranted as the close proximity to critical organs and neurovascular structures may potentially affect management paradigms. Prior studies reporting sinonasal osteosarcomas have largely consisted of case reports or case series focusing on specific scenarios such RT-induced skull base osteosarcomas. 9 10 11 Herein, we report 14 new cases of sinonasal osteosarcoma from our institution and a further 74 cases gathered in a systematic review from the literature. Given the paucity of data on sinonasal osteosarcoma, our aim is to describe the presentation, treatment patterns, and outcomes of this entity. In addition, we perform a meta-analysis of survival outcomes for this rare pathologic entity.
Methods
Institutional Retrospective Review
After gaining approval from our Institutional Review Board (IRB: 18–001238), International Classification of Diseases codes were used to query a retrospective institutional database for patients who presented to Mayo Clinic with sinonasal osteosarcoma from 1997 to 2018. This search yielded 14 patients and a retrospective chart review was subsequently performed. Endpoints captured included patient demographics and symptoms at presentation; tumor characteristics; treatment characteristics including surgical approach, use of neoadjuvant or adjuvant therapy, margin status, tumor recurrence, salvage therapy, and disease status at last follow-up.
Systematic Review: Data Sources and Search Strategies
A comprehensive search of several databases from each database's inception to October 2, 2018, English language was conducted. The databases included Ovid MEDLINE(R) and E-pub Ahead of Print, In-Process and Other Nonindexed Citations, and Daily, Ovid EMBASE, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, and Scopus. The search strategy was designed and conducted by an experienced librarian with input from the study's principle investigator. Controlled vocabulary supplemented with keywords was used to search for sinonasal ostesosarcomas.
Systematic Review: Study Selection
Study protocol was designed in accordance with the preferred reporting items of systematic reviews and meta-analysis (PRISMA) statement ( Fig. 1 ). Articles were identified by the research librarian and duplicates were removed. Study investigators also conducted a supplemental manual search using PubMed and the bibliographies of included articles. After 303 articles were screened by title and abstract by (C.M.L. and N.R.G.), 74 articles were selected to undergo full-text review. Articles were excluded if they were nonhuman studies, lacked relevant clinical information, or did not examine the pathology or anatomic site of interest ( Fig. 1 ). If articles or tumors were listed as involving maxilla but were primarily oral cavity tumors with minimal maxillary sinus involvement upon review of article images, they were excluded. When a single institution reported more than one paper with the same patient cohort, the most recent and comprehensive report was selected. After applying selection criteria, 53 articles were included in the systematic review. From these articles, the total patient cohort found in the literature was 74 patients. Risk of bias in case series were evaluated using the 20-item quality appraisal checklist developed by the Institute of Health Economics (IHE). 12 The items on the checklist document domains including study objective, design, population, intervention and cointervention, outcome measures, statistical analysis, results, and conclusion to assist the critical reviewer in assessing case series studies.
Fig. 1.
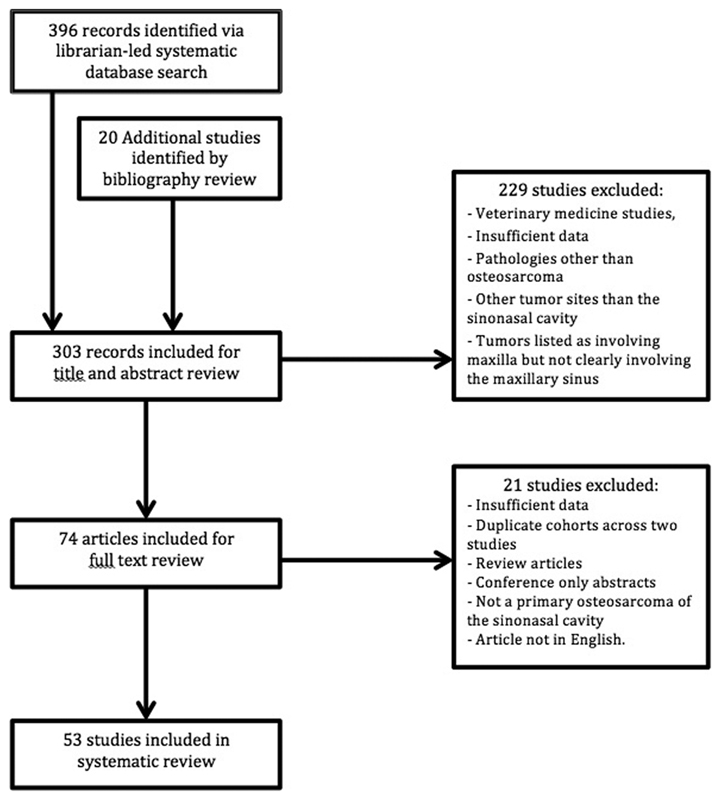
The preferred reporting items of systematic reviews and meta-analysis (PRISMA) flow diagram.
Systematic Review: Data Collection
Individual patient data were entered into a database including age, sex, symptoms at presentation, predisposing factors, histologic diagnosis, grade, treatment modality, and disease status at last follow-up. Survival outcomes originate from date of diagnosis. A patient was assigned into one of two American Joint Committee on Cancer (AJCC) tumor (T) stage groupings, T1 to T3 or T4, if the patient's data included information regarding the presence of tumor invasion of local structures. T stage was based on primary T stage as defined in the soft tissue sarcoma of the head and neck portion of the AJCC Cancer Staging Manual, 8th edition.
Statistical Analysis
Descriptive statistics were generated for patients included in the study including the institutional patient data and the patient data from the literature. Continuous features were summarized with mean, standard deviation, median, and range; categorical features were summarized with frequency count and percentage. Sample sizes for features with missing data are reported. Estimated rates of overall survival (OS) and disease specific survival (DSS) were calculated using the Kaplan–Meier method. For univariate and multivariate analysis, the Cox proportional hazards model was used. Routine statistical tests were performed using JMP Pro, Version 13.0.0 (SAS institute, Inc., Cary, North Carolina, United States)
Results
Single Institution Clinical Data
Fourteen patients (seven men; median age, 39 years; range: 17–77 years) with pathologically confirmed sinonasal osteosarcoma were identified from our single institution clinical data ( Table 1 ). The median follow-up time was 34 months (range: 5–155 months). The most common presenting symptom was nasal obstruction (8, 57%), followed in equal frequencies by epistaxis (4, 29%), V2 parasthesia/hypesthesia (4, 29%), facial swelling (4, 29%), and facial pain (4, 29%). Three (21%) patients had a remote history of craniofacial RT and experienced a postradiation latency osteosarcoma occurrence of 5, 17, and 24 years respectively. Histologic analysis of pathologic osteosarcoma specimens most frequently revealed chondroblastic (4, 29%), and fibroblastic (4, 29%), followed by osteoblastic (3, 21%) subtypes. Tumors were more commonly high grade (8, 57%). Additional details are listed in Table 1 .
Table 1. Institutional clinical data, fourteen patients with sinonasal osteosarcoma.
| Case | Sex | Age (y) | Symptoms | Prior RT | Post-RT latency (y) | Location | Histology | Grade | AJCC T-stage | Invasion | Treatment | Margin status | Status at last follow-up | Follow-up (mo) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 17 | Ep, NO, V1 V2, HA | N | – | M, S | O | H | T4 | D, Np, IFT, PP, Tb | NAj C, 1°CRT | – | DWD | 136 |
| 2 | F | 19 | NO | N | – | E | ND | L | T4 | La | 1° S | Neg | NED | 155 |
| 3 | M | 35 | FS, OS, FP | N | – | NC | C | H | T2 | – | NAj C, 1° S, Aj C | Neg | NED | 56 |
| 4 | M | 17 | Ep | Y | 17 | NC | O | H | T1 | – | 1° S + C | Neg | NED | 9 |
| 5 | F | 68 | NO | N | – | NC, E, S | C | H | T4 | D, ACF | 1° S; Aj RT | Neg | NED | 24 |
| 6 | M | 39 | NO, V2, FP | N | – | NC, E | F,O | L | T2 | – | 1°C | Neg | DWD | 68 |
| 7 | M | 39 | Ep, NO, Ea | N | – | NC, M, E, S | ND | H | T4 | La, O | P C | – | DWD | 4 |
| 8 | M | 77 | V2 | N | – | M | F | L | T4 | S | 1° S | Neg | DWD | 24 |
| 9 | F | 61 | FS | N | – | NC | C | L | T2 | – | 1° S | Neg | NED | 120 |
| 10 | M | 30 | V2, OS, FP | N | – | M, E, N | C | H | T4 | La, IFT, PP | NAj C, 1° S | – | DoC | 7 |
| 11 | F | 52 | NO | N | – | M, E, S, N | F | L | T4 | La, IFT, PP, C | P CRT | – | DWD | 44 |
| 12 | F | 74 | NO, Ea, FP | N | – | F, E, N | F | L | T4 | La | 1° S; Aj RT | Neg | DoC | 32 |
| 13 | F | 64 | Ep, NO, FS | Y | 5 | M, F, E, N | ND | H | T1 | – | 1° S | Neg | AWD | 5 |
| 14 | M | 24 | FS | Y | 24 | M, F, E | ND | H | T4 | La | NAj C, 1° S, Aj C | – | DWD | 49 |
Abbreviation: AJCC, American Joint Committee on Cancer; F, female; M, male; N, no; ND, not described; Neg, negative; T, tumor; Y, yes.
Symptoms: Ep, epistaxis; NO, nasal obstruction; V1:V1, anesthesia; V2:V2, anesthesia; HA, headache; FS, facial swelling; OS, oral swelling; FP, facial pain; Ea, epiphoria.
Location: M, maxillary sinus; E, ethmoid sinus; S, sphenoid sinus; F, frontal sinus; NC, nasal cavity.
Histology: O, osteoblastic; F, fibroblastic; C, chondroblastic.
Grade: H, high; L, low.
Invasion: D, dura; ACF, anterior cranial fossa; Or, orbit; S, skin; La, lamina papyracea; Np, nasopharynx; ITF, infratemporal fossa; PP, pterygoid plates; Tb, temporal bone; C, clivus.
Status at last follow-up: NED, no evidence of disease; AWD, alive with disease; DWD, dead with disease; DoC, dead of other cause.
Treatment: NAj, neoadjuvant; Aj, adjuvant; 1°, primary; C, chemotherapy; S, surgery; RT, radiation therapy; P, palliative.
Imaging from a representative case is seen with coronal computed tomography of the paranasal sinuses demonstrating dense calcification inferiorly in an opacified and mildly expanded left maxillary sinus ( Fig. 2A ). An intraoperative view of the left maxillary sinus with Caldwell–Luc approach shows a view of the tumor in situ ( Fig. 2B ) and removed from the left maxillary sinus as the surgical specimen ( Fig. 2C ).
Fig. 2.
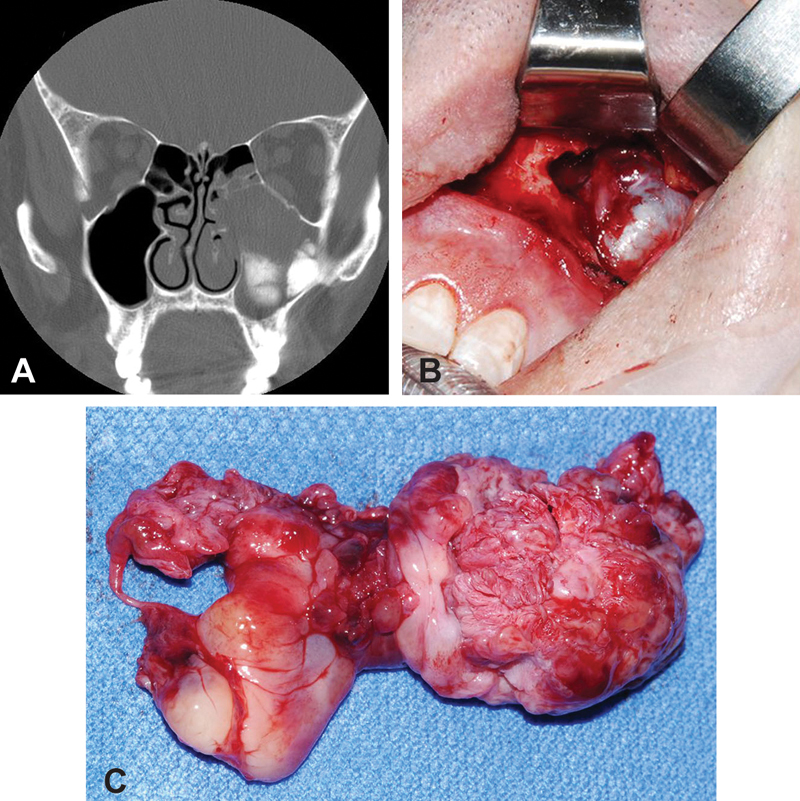
( A ) Coronal computed tomography of the paranasal sinuses demonstrates dense calcification inferiorly in an opacified and mildly expanded left maxillary sinus. ( B ) Intraoperative view of the left maxillary sinus with Caldwell–Luc approach and view of the tumor in situ ( C ) Specimen of osteosarcoma of the left maxillary sinus.
Systematic Review Data
In a systematic review, 53 total studies 7 11 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 capturing 75 patients were assessed ( Supplementary Tale S1 ; available online only). When combined with the 14 patients from our institution, a total of 88 patients were included for assessment. This total cohort of 88 patients is thus represented in the data in Tables 2 3 4 5 . Quality scores for included case series were tabulated and are available in Supplementary Table S2 (available online only). Median follow-up was 18 months (interquartile range 8–39 months; Table 2 ). A histogram of the included patient ages demonstrates a bimodal age distribution with peaks in the second and sixth decades of life ( Supplementary Fig. S1 ; available online only).
Table 2. Presenting characteristics of patients with sinonasal osteosarcoma included in the systematic review.
| Demographics | Mean (SD) | Patients n = 88 (%) |
|---|---|---|
| Age (y) | 38.5 (21.7) | |
| Sex | ||
| Male | 37/78 (47.4) | |
| Female | 41/78 (52.5) | |
| Presenting symptoms | ||
| Facial mass/swelling | 17/51 (33.3) | |
| Nasal obstruction | 16/51 (31.3) | |
| Headache | 12/51 (23.5) | |
| Epistaxis | 10/51 (19.6) | |
| Oral mass/swelling | 8/51 (15.7) | |
| Facial pain | 8/51 (15.7) | |
| Proptosis | 7/51 (13.7) | |
| Epiphoria | 6/51 (11.8) | |
| V2 hypesthesia/parasthesias | 6/51 (11.8) | |
| Past history | ||
| History of prior radiation therapy | 24/69 (34.7) | |
| Dose (Gy) | 57.2 (7.7) | |
| Duration prior to osteosarcoma (y) | 12.9 (8.4) | |
| Paget's disease | 1/40 (2.5) | |
| Retinoblastoma | 5/48 (7.2) | |
| Location of osteosarcoma involvement a | ||
| Maxillary sinus | 46/72 (63.8) | |
| Ethmoid sinus | 39/75 (52.0) | |
| Nasal cavity | 34/74 (45.9) | |
| Sphenoid sinus | 19/68 (27.9) | |
| Frontal sinus | 13/66 (19.7) | |
| Pathologic analysis | ||
| Histologic type b | ||
| Osteoblastic | 40/53 (75.4) | |
| Chondroblastic | 17/53 (31.5) | |
| Fibroblastic | 11/53 (20.4) | |
| Grade | ||
| High grade | 21/34 (61.7) | |
| Low grade | 13/34 (38.2) | |
| AJCC T stage | ||
| T1–T3 | 29/71 (40.8) | |
| T4 c | 42/71 (59.1) | |
| Invasion of orbit | 23/71 (32.4) | |
| Invasion of dura | 13/71(18.3) | |
| Invasion of pterygoid muscles | 8/71 (11.4) | |
| Invasion of infratemporal fossa | 7/71(10.0) | |
| Invasion of nasopharynx | 5/71 (7.1) | |
| Invasion of clivus | 4/71 (5.7) | |
| Invasion of skin | 3/71 (4.2) | |
Abbreviation: AJCC, American Joint Committee on Cancer; SD, standard deviation; T, tumor.
Tumor may involve multiple sites.
A portion of tumors contained multiple histologic types.
Several tumors had invasion into numerous structures.
Table 3. Treatment patterns and outcomes for patients with sinonasal osteosarcoma included in the systematic review.
| Treatment characteristics | Median (interquartile range) | Patients n = 88 (%) |
|---|---|---|
| Neoadjuvant therapy | 12/82 (14.6) | |
| Primary surgery alone | 23/82 (28.0) | |
| Primary surgery followed by adjuvant therapy | 43/82 (52.4) | |
| Primary radiation and/or chemotherapy | 16/82 (19.5) | |
| Outcomes | ||
| Status at last follow-up | ||
| No evidence of disease | 33/78 (42.3) | |
| Alive with disease | 10/78 (12.8) | |
| Dead of disease | 31/78 (39.7) | |
| Dead of other cause | 4/78 (5.1) | |
| Follow up duration (mo) | 18 (8–39) | |
| Pattern of failure | ||
| Local Recurrence | 17/18 (94.4) | |
| Regional recurrence | 1/18 (5.5) | |
| Distant metastases | 8/18 (44.4) | |
| Overall survival | ||
| 2 years | (68.1) | |
| 5 years | (40.3) | |
| Disease specific survival | ||
| 2 years | (71.3) | |
| 5 years | (43.9) | |
Table 4. Univariate analysis of prognostic factors for overall survival and disease specific survival for patients included in the systematic review.
| Overall survival | Disease specific survival | |||||
|---|---|---|---|---|---|---|
| Unit risk ratio (95% CI) |
HR | p -Value | Unit risk ratio (95% CI) |
HR | p -Value | |
| Age (y) | 1.02 (1.003–1.043) | – | 0.0216 | 1.015 (0.994–1.037) | – | 0.148 |
| Male sex | – | 2.07 | 0.0753 | – | 3.506 | 0.0611 |
| AJCC T4 vs. T1–T3 stages | – | 2.81 | 0.0253 * | – | 3.566 | 0.0100 |
| Primary therapy | ||||||
| Surgery | – | 0.49 | 0.0808 | – | 0.481 | 0.084 |
| No surgery | – | – | ||||
| Adjuvant therapy | ||||||
| Yes | – | 0.6078 | 0.1726 | – | 0.694 | 0.351 |
| No | – | – | ||||
| Histologic subtype | ||||||
| Osteoblastic vs. chondroblastic | – | 1.88 | 0.436 | – | 8.04 | 0.066 |
| Fibroblastic vs. chondroblastic | – | 4.79 | 0.067 | – | 3.78 | 0.216 |
| Multiple vs. chondroblastic | – | 3.25 | 0.178 | – | 6.52 | 0.096 |
Abbreviations: CI, confidence interval; HZ, hazard ratio.
Table 5. Multivariate analysis of prognostic factors for overall survival and disease specific survival for patients included in the systematic review.
| Overall survival | Disease specific survival | |||||
|---|---|---|---|---|---|---|
| Factor | Unit risk ratio (95% CI) |
HR | p -Value | Unit risk ratio (95% CI) |
HR | p -Value |
| Increasing Age | 1.030 (1.009–1.053) | – | 0.0052 | 1.022 (0.998–1.046) | – | 0.0683 |
| T4 vs. T1–T3 | – | 2.18 | 0.1075 | – | 2.87 | 0.0495 |
| Male Sex | – | 1.80 | 0.1913 | – | 1.75 | 0.265 |
Abbreviations: CI, confidence interval; HZ, hazard ratio.
Presenting characteristics of all sinonasal osteosarcoma patients are summarized in Table 2 . The most common presenting symptoms were facial mass or swelling (34%), nasal obstruction (30%) and headache (26%), epistaxis (20%), oral mass (17%), facial pain (17%), and proptosis (15%). Thirty-four percent of patients (24/69) had a history of prior craniofacial RT. The most common indications for prior craniofacial RT include retinoblastoma (6/24), nasopharyngeal carcinoma (4/24), and rhabdomyosarcoma (3/24). The mean dose of radiation patients received was 57.2 Gy and the mean time between prior radiation and presenting with osteosarcoma was 12.9 years. Also 2.5% of patients (1/40) had a history of Paget's disease and 7.2% (5/48) had a history of retinoblastoma. Cases where a presenting symptom or tumor characteristic was not described in a manuscript were noted. The total number of cases that reported a presenting characteristic was communicated by their inclusion as the denominator in the patient column ( Table 2 ).
Tumor characteristics in patients with sinonasal osteosarcoma are also summarized in Table 2 . The most common paranasal subsite involved by tumor was the maxillary sinus (63.8%), followed by the ethmoid sinuses (52%), nasal cavity (46%), sphenoid sinus (28%), and frontal sinus (20%). Osteoblastic osteosarcoma (76%) was the most common histologic subtype followed by chondroblastic (32%) and fibroblastic (20%) osteosarcoma. High-grade disease (63%) was more common than low grade (37%). The majority of patients (59.1%) presented with T4 disease versus T1 to T3 disease (40.8%). The orbit (32.4%), dura (18.3%), and infratemporal fossa (10.0%) were the most common sites of local tumor invasion.
Treatment patterns for patients with sinonasal osteosarcoma are summarized in Table 3 . The vast majority of patients received their primary therapy as surgery (80.5%), with 23 of 82 (28.0%) patients undergoing primary surgery alone and 43 of 82 (52.4%) patients undergoing primary surgery followed by adjuvant therapy. A smaller proportion underwent primary chemotherapy or RT (19%). Patterns of treatment failure were described in 18 patients. Of these 18 patients, 17 patients (94.4%) had local recurrence, 1 patient (5.5%) had regional recurrence, and 8 patients (44.4%) had distant metastasis.
At last follow-up, 42.3% of patients were alive with no disease, 12.8% were alive with active disease, 39.7% had died of their disease, and 5.1% were dead of other cause ( Table 3 ). Two- and 5-year OS rates were 68 and 45%, respectively, while 2- and 5-year DSS rates were 71.3 and 43.9%, respectively. A Kaplan–Meier estimate of overall survival rate for sinonasal osteosarcoma is shown in Fig. 3 . Statistically significant univariate discriminators of decreased OS included increasing age (unit risk ratio [95% confidence interval (CI)] = 1.02 [1.003–1.043]) and T4 disease (hazard ratio [HR] = 2.81; p = 0.0253; Table 4 ). Only age retained statistical significance in the multivariate analysis for OS ( Table 5 ). In the univariate analysis of DSS, only T4 disease (HR = 3.566; p = 0.0100) was statistically significant ( Table 4 ). T4 disease retained statistical significance in the multivariate analysis for DSS (HR = 2.87; p = 0.0495; Table 5 ).
Fig. 3.
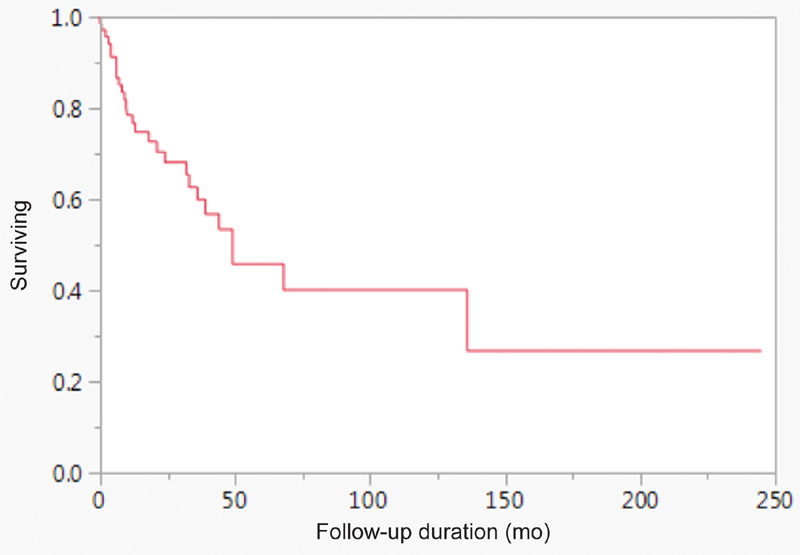
Kaplan–Meier estimate of overall survival for patients with sinonasal osteosarcoma included in the systematic review.
Discussion
Sinonasal osteosarcoma is an exceedingly rare entity. To date, no study as investigated survival in this patient cohort and factors that may affect this. Our systematic review and meta-analysis of the published literature captured 88 patients including 14 previously unreported patients from our own institution. The population in this review demonstrates a 2-year and 5-year OS rates of 68 and 40%, respectively, and 2-year and 5-year DSS rates were 71.3 and 43.9%, respectively. Increasing age was a predictor of worse OS (unit risk ratio [95% CI] = 1.03 [1.009–1.053]; p = 0.0052) and T4 stage was a predictor of worse DSS (HR = 2.87; p = 0.0495).
Patterns of Disease and Presentation
Our study showed a similar equal gender distribution 31 and mean age in the 30's 18 in a bimodal distribution which is consistent with previous studies 62 of osteosarcoma of the general head and neck region. In addition, the patients, in this review, have risk factors consistent with other reports including history of retinoblastoma, 64 Paget's disease 65 66 and a significant proportion of patients with a previous history of radiation. 67 68 69 The most common presenting symptoms are that of localized mass effect including facial swelling and nasal obstruction. 26 68 In particular, the very nonspecific symptoms of epistaxis and headache commonly occurred in this cohort. These presenting symptoms should alert the treating physician to maintain a high index of suspicion for a sinonasal mass, such as osteosarcoma. Consistent with some 70 reports but not all, 68 our review found osteoblastic osteosarcoma to be the most common histologic variant. The majority of patients presented at high stage, like previous studies of head and neck osteosarcoma. 31
Survival Outcomes
The 5-year OS rate in our patients was 40.3% and the 5-year DSS rate was 43.9%. No large-scale analysis of survival of osteosarcoma in the sinonasal cavity has been performed. The best comparison for data is in relevant literature of osteosarcoma of the general head and neck region. Reports of survival outcomes in osteosarcoma of the head and neck are wide, ranging from a review reporting a 37% 5-year OS rate 1 to a population based analysis of the national cancer database reporting a 5-year DSS rate of 59.7%. 7 Other studies of head and neck osteosarcoma report survival ranges from 35 to 55%. 1 7 31 41 71 The 5-year OS rate in our study is 40.3%. In contrast to sites like the mandible, tumors of the sinonasal cavity are less visible, may produce more vague symptoms, and may present later. Moreover, they may not be as accessible to complete negative-margin surgical resection, thus having the potential for reduced survival.
Prognostic Factors
Previous studies have identified age, histology, stage, grade, and tumor size as prognostic factors 1 7 27 32 72 73 in osteosarcoma of the general head and neck region. Based on these findings, we hypothesized that likewise, age, sex, local invasion, and AJCC stage would be prognostic factors in our data. The AJCC classification in this study was limited to only two groupings, T1 to T3 and T4. This is because differentiating T1 to T3 staging is based on size and most reports in the literature don't report an exact size. In addition, most cases were reported prior to the current edition of AJCC. However, because T4 is reserved for local invasion, a factor that was reported in the majority of cases, distinction of T1 to T3 versus T4 was able to be included in this study. As the risk of regional spread of osteosarcoma is generally rare, the majority of included studies did not include the status of regional spread at presentation. Therefore, full staging including n stage was not available for the majority of included cases. On univariate analysis in the meta-analysis data, increasing age and invasion of local structures was found to be associated with decreased OS rate. However, on multivariate analysis, only increasing age carried over to show statistical significance ( p = 0.0052). This confirms increasing age as a prognostic variable for sinonasal osteosarcoma.
Management
Given the limited number of cases of sinonasal osteosarcoma, no consensus has been established on the ideal treatment modality for this entity. Practitioners generally agree that negative margin surgery remains the mainstay for treatment as previous studies have shown that surgical treatment in the head and neck portends a better prognosis. 7 31 In this study, 82% of patients received primary surgery. A total of 57% patients received chemotherapy, half of these in a neoadjuvant protocol, and 29% received RT. The use of surgical therapy improved OS rate; however, this did not reach statistical significance ( p = 0.0808). Margin and nodal status were not included in the vast majority of the cases included in the literature and thus was not included in our analysis, though it has been shown to be an important prognostic factor in OS of other cancers of the head and neck. 5 74 Local recurrence was by far the most common pattern of failure, seen in 94% of patients who have recurrence. This is consistent with prior literature 41 and suggests difficulty in achieving wide surgical margins. Treatment failure in the form of distant metastases was less common but still prevalent in 44.4% of patients, while recurrence in the form of regional metastasis was seen in just one patient (5.5%). Given the high rate of local failure, the literature 41 suggests that there may be a role for adjuvant therapy in the management of this entity.
In general, primary bone malignancies are known to be radioresistant tumors, and there is a historically limited role for RT. 72 However, several patients in this review have been treated with RT, either in the primary treatment or adjuvant setting. Certainly, more exact forms of therapy may hold promise for better delivery of RT and may be an option of last resort in the patient with unresectable disease. This review has found that other kinds of radiation have been tried including neutron therapy, 54 and endoscopic resection therapy followed by Cyberknife RT. 10 Further investigation would need to be done into these other modalities to define their role in this disease.
Strengths and Limitations
Strengths of our study include its systematic design and meta-analysis of survival data. As with any systematic review, the quality of the resultant review is limited by the quality of the publications available in the literature, which limited some conclusions. In particular, the lack of margin status explicitly reported in included studies, along with the lack of regional lymph node staging limited our ability to include these factors in the univariate or multivariate analyses. Moreover, our analysis is limited by the rarity of this disease entity and relatively few published reports in the literature, the heterogeneity of reported treatment patterns, incomplete reporting of patient characteristics, and the variability of reported follow-up timeframes. As with any retrospective study, inherent limitations include the potential for inaccurate recording of patient or tumor-specific information, selection bias, and confounding of variables.
Conclusion
Sinonasal osteosarcoma is a rare malignancy that presents insidiously. Our systematic review shows that sinonasal osteosarcoma presents and behaves similarly to head and neck osteosarcomas of other sites. The 5-year OS and DSS rates are 40 and 43.9%, respectively. Increasing age is a predictor of worse OS rate ( p = 0.0052) and T4 stage is a predictor of worse DSS rate ( p = 0.0495). Further rigorous multi-institutional prospective studies would help to better characterize this disease entity.
Acknowledgments
The authors acknowledge Larry Prokop, MLS, for his assistance in comprehensively searching several databases for the systematic review. The authors would like to acknowledge the Mayo Clinic Center for Clinical and Translational Science (CCaTS) for assistance with statistical analysis. This publication was supported by Grant Number UL1 TR002377 from the National Center for Advancing Translational Sciences (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Health.
Funding Statement
Funding Internal departmental funding was utilized without commercial sponsorship or support.
Footnotes
Conflict of Interest None declared.
Supplementary Material
References
- 1.Kassir R R, Rassekh C H, Kinsella J B, Segas J, Carrau R L, Hokanson J A. Osteosarcoma of the head and neck: meta-analysis of nonrandomized studies. Laryngoscope. 1997;107(01):56–61. doi: 10.1097/00005537-199701000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Ottaviani G, Jaffe N. New York, NY: Springer-Verlag; 2009. The epidemiology of osteosarcoma; pp. 3–13. [DOI] [PubMed] [Google Scholar]
- 3.Noone A, Howlader N, Krapcho M. National Cancer Institute; Bethesda, MD: 2018. SEER Cancer Statistics Review, 1975–2015. [Google Scholar]
- 4.Goorin A M, Abelson H T, Frei E., III Osteosarcoma: fifteen years later. N Engl J Med. 1985;313(26):1637–1643. doi: 10.1056/NEJM198512263132605. [DOI] [PubMed] [Google Scholar]
- 5.Wanebo H J, Koness R J, MacFarlane J K. Head and neck sarcoma: report of the Head and Neck Sarcoma Registry. Head Neck. 1992;14(01):1–7. doi: 10.1002/hed.2880140102. [DOI] [PubMed] [Google Scholar]
- 6.Vege D S, Borges A M, Aggrawal K, Balasubramaniam G, Parikh D M, Bhaser B. Osteosarcoma of the craniofacial bones. A clinico-pathological study. J Craniomaxillofac Surg. 1991;19(02):90–93. doi: 10.1016/s1010-5182(05)80614-6. [DOI] [PubMed] [Google Scholar]
- 7.Oda D, Bavisotto L M, Schmidt R A. Head and neck osteosarcoma at the University of Washington. Head Neck. 1997;19(06):513–523. doi: 10.1002/(sici)1097-0347(199709)19:6<513::aid-hed9>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 8.Ackland D, Robinson D, Lee P VS, Dimitroulis G. Design and clinical outcome of a novel 3D-printed prosthetic joint replacement for the human temporomandibular joint. Clin Biomech (Bristol, Avon) 2018;56:52–60. doi: 10.1016/j.clinbiomech.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Hauff S J, Leuin S, Nation J, Korn B S, Levy M, DeConde A.Osteosarcoma of the skull base in the pediatric population: A case report and literature reviewJournal of Neurological Surgery, Part B: Skull Base Conference: 26th Annual Meeting North American Skull Base Society Scottsdale, AZ, United States Conference Publication.201677
- 10.Yamada S M, Ishii Y, Yamada S, Kuribayashi S, Kumita S, Matsuno A. Advanced therapeutic strategy for radiation-induced osteosarcoma in the skull base: a case report and review. Radiat Oncol. 2012;7:136. doi: 10.1186/1748-717X-7-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Echchikhi Y, Loughlimi H, Touil A, Kebdani T, Benjaafar N. Radiation-induced osteosarcoma of the skull base after radiation therapy in a patient with nasopharyngeal carcinoma: a case report and review of the literature. J Med Case Reports. 2016;10(01):334. doi: 10.1186/s13256-016-1112-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo B, Moga C, Harstall C, Schopflocher D. A principal component analysis is conducted for a case series quality appraisal checklist. J Clin Epidemiol. 2016;69:199–20700. doi: 10.1016/j.jclinepi.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Ahrari A, Labib M, Gravel D, Macdonald K I. Primary osteosarcoma of the skull base treated with endoscopic endonasal approach: A case report and literature review. J Neurol Surg Rep. 2015;76(02):e270–e274. doi: 10.1055/s-0035-1564606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yip C-C, Kersten R C, McCulley T J, Ballard E T, Kulwin D R. Osteogenic sarcoma after orbital radiation rhabdomyosarcoma. Ophthalmology. 2003;110(10):1996–1999. doi: 10.1016/S0161-6420(03)00478-0. [DOI] [PubMed] [Google Scholar]
- 15.Alzahrani M, Robier A, Pointreau Y, Bakhos D. A rare case of radiation-induced osteosarcoma of the ethmoid sinus. Case Rep Otolaryngol. 2011;2011:786202. doi: 10.1155/2011/786202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amaral M B, Buchholz I, Freire-Maia B. Advanced osteosarcoma of the maxilla: a case report. Med Oral Patol Oral Cir Bucal. 2008;13(08):E492–E495. [PubMed] [Google Scholar]
- 17.Avitia S, Osborne R F. Radiation-induced osteosarcoma of the maxillary sinus. Ear Nose Throat J. 2007;86(06):329–330. [PubMed] [Google Scholar]
- 18.Barosa J, Ribeiro J, Afonso L, Fernandes J, Monteiro E. Head and neck sarcoma: analysis of 29 cases. Eur Ann Otorhinolaryngol Head Neck Dis. 2014;131(02):83–86. doi: 10.1016/j.anorl.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Bone R C, Biller H F, Harris B L. Osteogenic sarcoma of the frontal sinus. Ann Otol Rhinol Laryngol. 1973;82(02):162–165. doi: 10.1177/000348947308200212. [DOI] [PubMed] [Google Scholar]
- 20.Bradley C, McClymont L G, Reid R. Osteogenic sarcoma of the ethmoid sinus. J Laryngol Otol. 1988;102(12):1176–1178. doi: 10.1017/s0022215100107649. [DOI] [PubMed] [Google Scholar]
- 21.Bundgaard T, Frost-Jensen V, Buhl L. Sarcomatous change in a previously benign osteofibroma in the maxillary sinus. Arch Otorhinolaryngol. 1988;245(01):22–24. doi: 10.1007/BF00463543. [DOI] [PubMed] [Google Scholar]
- 22.Chaudhari C S, Kumavat P V, Khare M S, Ughade P A, Kshirsagar G R. Primary osteogenic sarcoma of fronto ethmoidal sinuses in a child: with unusual site and age group. Bombay Hosp J. 2015;57(02):217–220. [Google Scholar]
- 23.Chennupati S K, Norris R, Dunham B, Kazahaya K. Osteosarcoma of the skull base: case report and review of literature. Int J Pediatr Otorhinolaryngol. 2008;72(01):115–119. doi: 10.1016/j.ijporl.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 24.Donaldson M E, Geist J R, Daley T D. Osteosarcoma of the jaws in children. Int J Paediatr Dent. 2004;14(01):54–60. doi: 10.1111/j.1365-263x.2004.00519.x. [DOI] [PubMed] [Google Scholar]
- 25.Epley K D, Lasky J B, Karesh J W. Osteosarcoma of the orbit associated with Paget disease. Ophthal Plast Reconstr Surg. 1998;14(01):62–66. doi: 10.1097/00002341-199801000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Erdur Z B, Gözen E D, Inan H C. Osteosarcoma of the ethmoid sinus. J Craniofac Surg. 2018;29(05):e487–e488. doi: 10.1097/SCS.0000000000004502. [DOI] [PubMed] [Google Scholar]
- 27.Gadwal S R, Gannon F H, Fanburg-Smith J C, Becoskie E M, Thompson L D. Primary osteosarcoma of the head and neck in pediatric patients: a clinicopathologic study of 22 cases with a review of the literature. Cancer. 2001;91(03):598–605. [PubMed] [Google Scholar]
- 28.Galera-Ruiz H, Sanchez-Calzado J A, Rios-Martin J J, DeMingo-Fernandez E J, Muñoz Borge F. Sinonasal radiation-associated osteosarcoma after combined therapy for rhabdomyosarcoma of the nose. Auris Nasus Larynx. 2001;28(03):261–264. doi: 10.1016/s0385-8146(01)00056-6. [DOI] [PubMed] [Google Scholar]
- 29.Ginapolous H, Khan F R, Nickson J J. A case of osteogenic sarcoma of maxillary antrum cure by radiation therapy. J Radiol Electrol Med Nucl. 1975;56(05):429–431. [PubMed] [Google Scholar]
- 30.Gonzalez M E, Raghavan P, Cho B, Muttikkal T J, Rehm P K. Primary osteogenic osteosarcoma of the ethmoid sinus in an adolescent: case report. J Radiol Case Rep. 2016;10(02):1–9. doi: 10.3941/jrcr.v10i2.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta D, Vishwakarma S K.Osteogenic sarcoma of the frontal sinus Ann Otol Rhinol Laryngol 199099(6, Pt 1):489–490. [DOI] [PubMed] [Google Scholar]
- 32.Ha P K, Eisele D W, Frassica F J, Zahurak M L, McCarthy E F. Osteosarcoma of the head and neck: a review of the Johns Hopkins experience. Laryngoscope. 1999;109(06):964–969. doi: 10.1097/00005537-199906000-00023. [DOI] [PubMed] [Google Scholar]
- 33.Harris H H, Muller D J, Greenberg S D. Primary osteogenic sarcoma of the maxillary sinus. Report of two cases. Laryngoscope. 1963;73:429–445. doi: 10.1288/00005537-196304000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Ito T, Ozaki Y, Sato K, Oikawa M, Nakamura H. Radiation-induced osteosarcomas after treatment for frontal gliomas: a report of 2 cases. Neuro-oncol. 2010;27(02):103–109. doi: 10.1007/s10014-010-0267-7. [DOI] [PubMed] [Google Scholar]
- 35.Kachhara R, Nair S, Sandhyamani S, Bhattacharya R N. Primary osteogenic sarcoma involving sella-sphenoid sinus--case report. Neurol Med Chir (Tokyo) 1999;39(07):534–538. doi: 10.2176/nmc.39.534. [DOI] [PubMed] [Google Scholar]
- 36.Khosla A, Goulatia R K, Tickoo S C. Osteosarcoma of ethmoid sinus in an infant. Indian Pediatr. 1993;30(02):270–273. [PubMed] [Google Scholar]
- 37.Kohanawa R, Tabuchi K, Okubo H, Nagata M, Hara A. Primary osteogenic sarcoma of the ethmoid sinus: a case report. Auris Nasus Larynx. 2005;32(04):411–413. doi: 10.1016/j.anl.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 38.Lee J A, Choi S Y, Kang H J. Treatment outcome of osteosarcoma after bilateral retinoblastoma: a retrospective study of eight cases. Br J Ophthalmol. 2014;98(10):1355–1359. doi: 10.1136/bjophthalmol-2014-305116. [DOI] [PubMed] [Google Scholar]
- 39.Lee K B, Ang E S, Tan K C. Reconstructive challenges in the management of a rare case of sphenoid osteosarcoma--a case report. Singapore Med J. 2001;42(12):586–589. [PubMed] [Google Scholar]
- 40.Malalis J F, Lee J M, Jay W M. Primary osteosarcoma of the skull base in a pregnant patient. Neuroophthalmology. 2013;37(01):38–40. doi: 10.3109/01658107.2012.753914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mark R J, Sercarz J A, Tran L, Dodd L G, Selch M, Calcaterra T C. Osteogenic sarcoma of the head and neck. The UCLA experience. Arch Otolaryngol Head Neck Surg. 1991;117(07):761–766. doi: 10.1001/archotol.1991.01870190073015. [DOI] [PubMed] [Google Scholar]
- 42.Mathkour M, Garces J, Beard B, Bartholomew A, Sulaiman O AR, Ware M L. Primary high-grade osteosarcoma of the clivus: a case report and literature review. World Neurosurg. 2016;89:7.3E11–7.3E15. doi: 10.1016/j.wneu.2016.01.054. [DOI] [PubMed] [Google Scholar]
- 43.Oakley G M, Costa D J, Mitchell R B, Sotelo C. Osteosarcoma of the skull base in a 15-year-old boy. Ear Nose Throat J. 2011;90(10):479–480. doi: 10.1177/014556131109001006. [DOI] [PubMed] [Google Scholar]
- 44.Okinaka Y, Takahashi M. Osteosarcoma of the maxilla: report of a case and review of the literature concerning metastasis. J Oral Maxillofac Surg. 1997;55(10):1177–1181. doi: 10.1016/s0278-2391(97)90304-9. [DOI] [PubMed] [Google Scholar]
- 45.Papsidero M J, Baker S R. Osteogenic sarcoma of the ethmoid sinus. Otolaryngol Head Neck Surg. 1982;90(04):510–512. doi: 10.1177/019459988209000429. [DOI] [PubMed] [Google Scholar]
- 46.Park H-R, Min S K, Cho H D. Osteosarcoma of the ethmoid sinus. Skeletal Radiol. 2004;33(05):291–294. doi: 10.1007/s00256-003-0742-x. [DOI] [PubMed] [Google Scholar]
- 47.Park J U, Lee J H, Kim K H. Osteosarcoma of left maxillary sinus: a case report. J Oral Maxillofac Surg. 2014;72(09):e164. [Google Scholar]
- 48.Park Y-K, Ryu K N, Park H-R, Kim D-W. Low-grade osteosarcoma of the maxillary sinus. Skeletal Radiol. 2003;32(03):161–164. doi: 10.1007/s00256-002-0577-x. [DOI] [PubMed] [Google Scholar]
- 49.Santos-Silva A R, Ribeiro A CP, Furuse C F. Maxillary osteosarcoma in a young patient undergoing postorthodontic treatment follow-up: the importance of ongoing oral examinations. Am J Orthod Dentofacial Orthop. 2011;139(06):845–848. doi: 10.1016/j.ajodo.2009.09.024. [DOI] [PubMed] [Google Scholar]
- 50.Sargi Z, Cova D, Vernon S E.Pathology quiz case. Sinonasal osteosarcoma Arch Otolaryngol Head Neck Surg 2008134121343–1344., 1344 [DOI] [PubMed] [Google Scholar]
- 51.Shahid H, Baharudin A, Halim A S, Biswal B M, Jihan W S. An extensive sinonasal osteosarcoma mimicking chondrosarcoma. Med J Malaysia. 2007;62(02):171–172. [PubMed] [Google Scholar]
- 52.Sheikh S, Pallagatti S, Aggarwal A, Gupta D, Puri N, Mittal A. Osteosarcoma of maxilla: a case report. J Clin Exp Dent. 2010;2(03):177–120. [Google Scholar]
- 53.Smith L M, Donaldson S S, Egbert P R, Link M P, Bagshaw M A. Aggressive management of second primary tumors in survivors of hereditary retinoblastoma. Int J Radiat Oncol Biol Phys. 1989;17(03):499–505. doi: 10.1016/0360-3016(89)90100-4. [DOI] [PubMed] [Google Scholar]
- 54.Smoron G L, Lennox A J, McGee J L. Osteogenic sarcoma of the maxilla: neutron therapy for unresectable disease. Sarcoma. 1999;3(02):141–144. doi: 10.1080/13577149977785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stavrakas M, Nixon I, Andi K. Head and neck sarcomas: clinical and histopathological presentation, treatment modalities, and outcomes. J Laryngol Otol. 2016;130(09):850–859. doi: 10.1017/S0022215116008604. [DOI] [PubMed] [Google Scholar]
- 56.Tseng C-C, Lin C-Z, Li W-Y.Pathology quiz case 2. Postirradiation sinonasal osteosarcoma Arch Otolaryngol Head Neck Surg 200513102173, 175–176 [DOI] [PubMed] [Google Scholar]
- 57.Uysal K M, Koyuncuoğlu M, Akman F. A rare tumor of craniofacial bones in children: a pediatric chondroblastic osteosarcoma case with diagnostic and therapeutic problems. Pediatr Hematol Oncol. 2001;18(02):147–152. doi: 10.1080/088800101300002991. [DOI] [PubMed] [Google Scholar]
- 58.Vlychou M, Ostlere S J, Kerr R, Athanasou N A. Low-grade osteosarcoma of the ethmoid sinus. Skeletal Radiol. 2007;36(05):459–462. doi: 10.1007/s00256-006-0231-0. [DOI] [PubMed] [Google Scholar]
- 59.Wei-wei L, Qiu-liang W, Guo-hao W, Zhi-hua C, Zong-yuan Z. Clinicopathologic features, treatment, and prognosis of postirradiation osteosarcoma in patients with nasopharyngeal cancer. Laryngoscope. 2005;115(09):1574–1579. doi: 10.1097/01.mlg.0000173166.48440.e4. [DOI] [PubMed] [Google Scholar]
- 60.Wolfowitz B L. Osteosarcoma and chondrosarcoma of the maxilla. J Laryngol Otol. 1973;87(04):409–416. doi: 10.1017/s0022215100077069. [DOI] [PubMed] [Google Scholar]
- 61.Yamada S M, Ishii Y, Yamada S. Skull base osteosarcoma presenting with cerebrospinal fluid leakage after CyberKnife treatment: a case report. J Med Case Reports. 2013;7:116. doi: 10.1186/1752-1947-7-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamaguchi S, Nagasawa H, Suzuki T. Sarcomas of the oral and maxillofacial region: a review of 32 cases in 25 years. Clin Oral Investig. 2004;8(02):52–55. doi: 10.1007/s00784-003-0233-4. [DOI] [PubMed] [Google Scholar]
- 63.Patel A J, Rao V Y, Fox B D. Radiation-induced osteosarcomas of the calvarium and skull base. Cancer. 2011;117(10):2120–2126. doi: 10.1002/cncr.25734. [DOI] [PubMed] [Google Scholar]
- 64.Friend S H, Bernards R, Rogelj S.A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma Nature 1986323(6089):643–646. [DOI] [PubMed] [Google Scholar]
- 65.Hansen M F, Nellissery M J, Bhatia P. Common mechanisms of osteosarcoma and Paget's disease. J Bone Miner Res. 1999;14 02:39–44. doi: 10.1002/jbmr.5650140209. [DOI] [PubMed] [Google Scholar]
- 66.Hansen M F, Seton M, Merchant A. Osteosarcoma in Paget's disease of bone. J Bone Miner Res. 2006;21 02:58–63. doi: 10.1359/jbmr.06s211. [DOI] [PubMed] [Google Scholar]
- 67.Robinson E, Neugut A I, Wylie P. Clinical aspects of postirradiation sarcomas. J Natl Cancer Inst. 1988;80(04):233–240. doi: 10.1093/jnci/80.4.233. [DOI] [PubMed] [Google Scholar]
- 68.Cahan W G, Woodard H Q, Higinbotham N L, Stewart F W, Coley B L. Sarcoma arising in irradiated bone; report of 11 cases. Cancer. 1948;1(01):3–29. doi: 10.1002/1097-0142(194805)1:1<3::aid-cncr2820010103>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 69.Arlen M, Higinbotham N L, Huvos A G, Marcove R C, Miller T, Shah I C. Radiation-induced sarcoma of bone. Cancer. 1971;28(05):1087–1099. doi: 10.1002/1097-0142(1971)28:5<1087::aid-cncr2820280502>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 70.Bertoni F, Dallera P, Bacchini P, Marchetti C, Campobassi A. The Istituto Rizzoli-Beretta experience with osteosarcoma of the jaw. Cancer. 1991;68(07):1555–1563. doi: 10.1002/1097-0142(19911001)68:7<1555::aid-cncr2820680717>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 71.Caron A S, Hajdu S I, Strong E W. Osteogenic sarcoma of the facial and cranial bones. A review of forty-three cases. Am J Surg. 1971;122(06):719–725. doi: 10.1016/0002-9610(71)90434-x. [DOI] [PubMed] [Google Scholar]
- 72.Smith R B, Apostolakis L W, Karnell L H. National cancer data base report on osteosarcoma of the head and neck. Cancer. 2003;98(08):1670–1680. doi: 10.1002/cncr.11716. [DOI] [PubMed] [Google Scholar]
- 73.Laskar S, Basu A, Muckaden M A. Osteosarcoma of the head and neck region: lessons learned from a single-institution experience of 50 patients. Head Neck. 2008;30(08):1020–1026. doi: 10.1002/hed.20820. [DOI] [PubMed] [Google Scholar]
- 74.Bernier J, Cooper J S, Pajak T F. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501) Head Neck. 2005;27(10):843–850. doi: 10.1002/hed.20279. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


