Abstract
Objective.
Child maltreatment is among the strongest predictors of posttraumatic stress disorder (PTSD). However, less than 40% of children who have been maltreated are ever diagnosed with PTSD, suggesting that exposure to child maltreatment alone is insufficient to explain this risk. This study examined whether epigenetic age acceleration, a stress-sensitive biomarker derived from DNA methylation, explains variation in PTSD diagnostic status subsequent to child maltreatment.
Method.
Children and adolescents (N = 70; 65.7% female), 8-15 years of age (M = 12.00, SD = 2.37) and exposed to substantiated child maltreatment within the twelve months prior to study entry, were enrolled. Participants provided epithelial cheek cells via buccal swab for genotyping and quantification of epigenetic age acceleration within a case-control design. PTSD diagnostic status was determined using the Child PTSD Symptoms Scale according to the DSM-IV-TR algorithm.
Results.
Epigenetic age acceleration predicted current PTSD status, revealing an effect size magnitude in the moderate range, OR = 2.35, 95% CI: 1.22 - 4.51, after adjusting for sample demographics, polygenic risk for PTSD, and lifetime exposure to other childhood adversities. Supplemental analyses demonstrated that epigenetic age acceleration was related to a greater severity of PTSD arousal symptoms (r = .29, p = .015). There were no differential effects for child maltreatment subtype on epigenetic age acceleration or PTSD status.
Conclusions.
The biological embedding of child maltreatment may explain variation in PTSD diagnostic status and serve as a novel approach for informing selective prevention or precision-based therapeutics for those at risk for PTSD.
Keywords: Child maltreatment, epigenetic age acceleration, posttraumatic stress disorder
Child maltreatment is an act of commission or omission on the part of a caregiver that results in harm or risk for harm toward a child, including acts of physical abuse, sexual abuse, and neglect (World Health Organization, 2014). Rates of child maltreatment in the U.S. are among the highest in industrialized nations (Gilbert et al., 2012) with annual incidences approaching one million children (Sedlak et al., 2010; U.S. Department of Health and Human Services, 2019) and an overall prevalence ranging from 12.5%-34.7% of the entire pediatric population (Kim et al., 2017; Wildeman et al., 2014). Of all the traumas and adversities children are exposed to, child maltreatment is among the strongest predictors of posttraumatic stress disorder (PTSD; McLaughlin et al., 2017), a debilitating psychiatric condition affecting 4.7%-5.0% of the general pediatric population (McLaughlin et al., 2013; Merikangas et al., 2010) but up to 14.2%-37.5% of those children exposed to maltreatment (Alisic et al., 2014; Scott et al., 2010; Widom, 1999). This increased prevalence is noteworthy from a public health perspective, as PTSD can have a severe and chronic course (Green et al., 2010; Santiago et al., 2013) that results in significantly higher medical costs (Bonomi et al., 2008) even when compared to more prevalent psychiatric disorders (Ivanova et al., 2011; Marciniak et al., 2005).
However, many children fail to meet PTSD diagnostic criteria following exposure to child maltreatment (Copeland et al., 2007), suggesting that child maltreatment alone is insufficient to explain the increased risk for PTSD. Accounting for this variation, conceptual models of the biological embedding of early life adversity (Lupien et al., 2009; Shonkoff et al., 2012) suggest that the effects of child maltreatment can alter long-term genomic control of broad mental health abilities, such as attention, mood, memory, and threat sensitivity (Juster et al., 2011), that increase the risk for PTSD and extend its course across developmental stages. Epigenetic modifications such as DNA methylation, the addition of a methyl group to cytosines often in cytosine-phosphate-guanine (CpG) dinucleotides (Adams, 1995; Aristizabal et al., 2019), are a candidate mechanism explaining how an adverse environment such as child maltreatment affects gene expression in a way that alters the presentation of downstream psychiatric phenotypes (McGowan & Roth, 2015; Szyf & Bick, 2013). Animal models of child maltreatment suggest a lasting impact on genomic function, where changes in DNA methylation and gene expression have been observed over time (Liu et al., 1997; Roth et al., 2009; Weaver et al., 2004). Translational research in humans is emerging, where a history of child maltreatment has predicted greater variation in DNA methylation at several stress-mediating sites, including SLC6A4 (Beach et al., 2011; Vijayendran et al., 2012), NR3C1 (McGowan et al., 2009; Romens et al., 2015; Turecki & Meaney, 2016), and FKBP5 (Mehta et al., 2013). These results indicate that child maltreatment may lead to sustained changes in epigenetic regulation of gene expression specifically in regions related to PTSD (Mehta et al., 2011; Yehuda et al., 2009). However, variation in complex traits such as PTSD will not be successfully informed by the examination of epigenetic marks at only one or several genomic regions. Rather, as with genetic variation, variation in DNA methylation subsequent to child maltreatment is most likely captured by an analysis that extends across many sites in the genome.
Epigenetic age (Horvath, 2013), a stress-sensitive biomarker derived from DNA methylation at 353 CpG sites across the genome, has emerged as a single, cross-tissue index of cellular aging with added explanatory power over chronological age when examining the risk for multiple domains of morbidity and mortality (Chen et al., 2016; Horvath et al., 2012; Marioni et al., 2015). Recent evidence suggests that exposure to early life adversity accelerates epigenetic aging as early as childhood (Jovanovic et al., 2017) with such acceleration continuing well into adulthood (Zannas et al., 2015). These results highlight how an adverse environment, such as one involving child maltreatment, can become embedded biologically to increase the risk for PTSD currently and over time. However, no study has examined whether accelerations in epigenetic age following exposure to child maltreatment explains variation in those who currently meet PTSD diagnostic criteria. Establishing epigenetic age acceleration as a biomarker of PTSD risk following child maltreatment has the potential to advance etiological models of PTSD as well as spur novel, precision-based interventions aimed at preventing or treating PTSD across the lifespan (Yehuda et al., 2013).
The current study tested whether accelerations in epigenetic age explain variation in current PTSD status following exposure to substantiated child maltreatment. Established risk factors for PTSD, including sample demographics (Brewin et al., 2000), polygenic risk (Duncan et al., 2018), and lifetime exposure to childhood adversities (Shenk et al., 2016), were included in the model to adjust risk estimates for epigenetic age acceleration and PTSD status. Supplemental analyses were conducted to identify whether epigenetic age acceleration was differentially related to PTSD symptom clusters, namely those most likely affected by changes in stress mediation. Finally, differences in epigenetic age acceleration and PTSD status were examined across child maltreatment subtypes to determine whether one or more subtypes were associated with accelerations in epigenetic age compared to other subtypes. The results of this study aim to advance efforts of examining epigenetic age acceleration as a potential biomarker explaining variation in PTSD status among those in the child maltreatment population, providing translational value for interventions designed to prevent or treat the occurrence and course of PTSD.
Methods
Sample
The sample (N = 70) was generated using an epigenetic case-control association design examining variation in epigenetic age acceleration and PTSD diagnostic status for children exposed to substantiated maltreatment. Children between 8-15 years of age (M = 12.00, SD = 2.37) with a substantiated designation of child maltreatment in the twelve months prior to study entry were recruited from Child Protective Services (CPS) located in an urban, Mid-western county using a consecutive referral process. Fifty percent of the sample was exposed to child physical abuse, 41.4% was exposed to child sexual abuse, and 8.6% was exposed to child neglect. The majority of the sample was female (65.7%) and lived in a single-caregiver household (70.0%). Median family income was $10,000-$19,999. Racial demography was 50% African-American, 35.7% Caucasian, 10.0% Multi-racial, 2.9% Hispanic, and 1.4% Other.
Procedures
All procedures were approved by the local institutional review board prior to data collection. Inclusion criteria were: 1) a substantiated record of child maltreatment occurring within the twelve months prior to study entry, 2) a willingness to provide biospecimens via buccal swabs, 3) fluency in English, 4) a parent or caregiver, defined as the primary, custodial adult who has had legal, permanent custody for at least six months, who is willing to participate and who was not the perpetrator of abuse, 5) placement in a stable caregiving environment for at least six months, meaning the child resided in the same home with an adult that had legal rights to supervise and care for that child for the prior six months, and 6) those children who were currently taking psychotropic medication were required to have taken that medication for two months prior to study entry without dose adjustment during that time, as common classes of medications used to treat acute psychiatric distress in the child maltreatment population, such as antidepressant medications, require this time to identify the proper medicine and achieve therapeutic dose. Caregivers accompanied their child to the study visit to provide written informed consent and parental permission. Child assent was also obtained from each participant. A single study assessment was conducted to collect all data reported in the current study. Caregivers provided all demographic information pertaining to child age, race, sex, and family income. Children provided biospecimens for quantifying DNA methylation and reported on lifetime exposure to childhood adversity and PTSD symptoms. Families were compensated $40 for their participation.
Measures
Lifetime Exposure to Child Adversity.
The Comprehensive Trauma Interview-Screen (Horowitz, 1998) is a 22-item, self-report measure assessing lifetime exposure (1 = Yes, 0 = No) to a variety of adversities common in childhood, including exposure to natural disasters, motor vehicle accidents, serious medical procedures and hospitalizations, violence in the community, witnessing domestic violence, bullying, use of substances in the home, and child maltreatment. The CTI-Screen has been used in prior child maltreatment research (Barnes et al., 2009; Shenk et al., 2016) with moderate to substantial reliability in individual recall of adverse events across successive interviews (κ = .51-76) and good predictive validity with independent measures of PTSD symptoms. A composite index of the total number of childhood adversities endorsed other than child maltreatment, as the sample was selected based on a known history of exposure to child maltreatment, was calculated and used in subsequent statistical analysis to adjust PTSD risk estimates for exposure to other types of childhood adversity.
Epigenetic Age Acceleration.
The Infinium Methylation EPIC Beadchip (EPIC array, Illumina, San Diego CA, USA) was used to characterize variation in DNA methylation. This microarray-based platform quantifies DNA methylation with single nucleotide resolution at more than 850,000 CpG and non-CpG sites. First, DNA was extracted from buccal epithelial cells and 1μg of DNA was treated with sodium bisulfite using the Zymo EZ-96 DNA Methylation Kit™ (Zymo Research, Orange, CA, USA). Second, 200ng of bisulfite-treated DNA was amplified, fragmented, and hybridized on the EPIC array according to manufacturer protocols. Raw intensity values were directly loaded into R for quality control and normalization using the Minfi package (Bioconductor). Internal Illumina controls were used to assess the quality of staining, extension, hybridization, bisulfite conversion, and specificity. Signals from methylated (M) and unmethylated (U) bead types were calculated as = M/(U + M), resulting in beta values ranging from 0 (unmethylated) to 1 (methylated) and corresponding to 0 and 100% DNA methylation. All technical batch effects are accounted for using an empirical Bayes method (Johnson et al., 2007). Between samples differences in cell types composition were estimated using the method described by Smith et al (2015).
Epigenetic age was calculated using Horvath’s method (Horvath, 2013), a multivariate predictor based on the DNA methylation of 353 clock CpGs. Briefly, Horvath’s method used elastic net regression to identify and select CpGs that best predicted chronological age in 8,000 samples encompassing 51 tissue and cell-types. Epigenetic age acceleration estimates for this study were generated by submitting beta values on DNA methylation from the 850k EPIC Beadchip to the Horvath epigenetic clock calculator (https://dnamage.genetics.ucla.edu/home). This calculator produces estimates of epigenetic age acceleration by regressing each individual’s epigenetic age on his or her chronological age (see Figure 1 for the relation between epigenetic age and chronological age in this sample). Residuals from this regression model reflect the unique variation in epigenetic aging, after accounting for the effects of chronological age, that are then used in subsequent statistical modeling as estimates of epigenetic age acceleration. Positive values of epigenetic age acceleration indicate faster aging, where negative values indicate slower aging.
Figure 1.
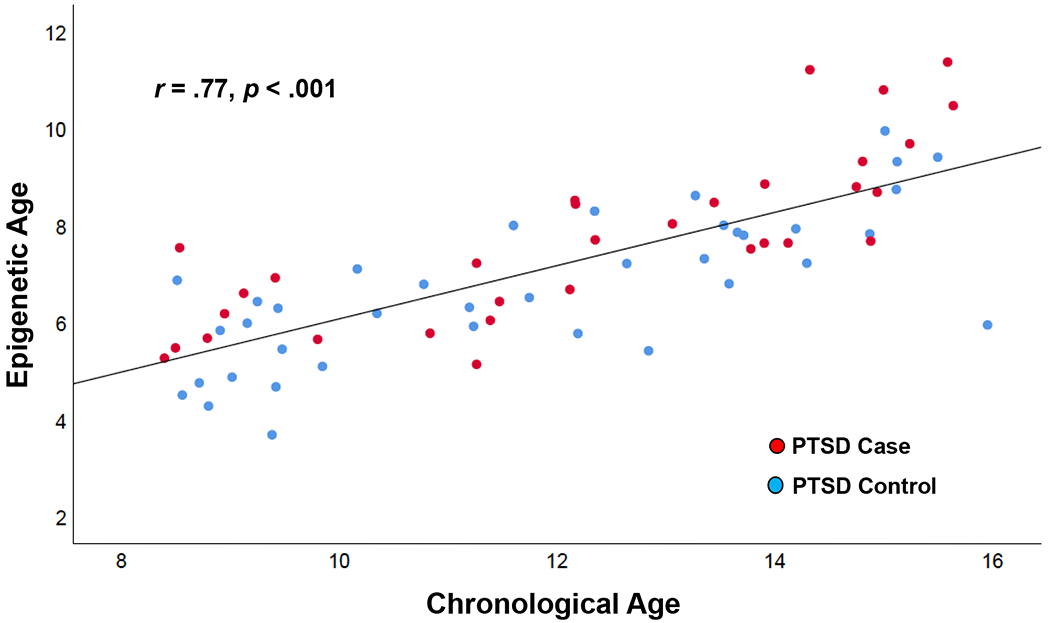
Correlation between Child Chronological Age and Raw Epigenetic Age Estimates according to PTSD Status. Lines represent linear fit and 95% confidence interval.
Polygenic Risk Scores for PTSD.
Prior population-level, genome-wide association studies of the PTSD phenotype have identified a number of single nucleotide polymorphisms (SNPs) which, when combined in a polygenic score, account for a small but statistically significant portion of variance in PTSD case status (Duncan et al., 2018). The Global Screening Array (GSA v1.0) was used to assess genome-wide genetic variation in buccal-derived genomic DNA from all participants and derive polygenic risk scores (PRS) using the summary statistics provided by Duncan et al (2018). The p-value threshold for the SNPs included in the PTSD PRS was set to p < .001, as this threshold provided the largest improvement in Nagelkerke R2 in preliminary models of PTSD diagnostic status (e.g. Euesden et al., 2015). To ensure the PTSD PRS obtained with this sample was not unduly biased by population stratification, the first five principal components used to estimate racial ancestry were entered as predictors in a multiple regression model with PTSD PRS as the outcome. Residuals from this model, which reflect the genetic risk for PTSD after accounting for population stratification, were then included in all subsequent statistical analyses.
PTSD Diagnosis.
The Child PTSD Symptoms Scale (CPSS; Foa et al., 2001) is a 24-item self-report measure of PTSD symptom severity and corresponding functional impairment for children 8-18 years of age exposed to a traumatic event. The CPSS contains 17 items that assess PTSD symptoms according to DSM-IV-TR diagnostic criteria, where items are rated on a 0 to 3 severity scale resulting in scores that range from 0 to 51. Internal consistency of the CPSS PTSD symptoms items in the current study was α = .91. Prior research has shown CPSS self-reported symptoms scores have good diagnostic accuracy in predicting PTSD status (You et al., 2017) and that generating PTSD diagnoses from CPSS symptom scores has good agreement (74.5%) with PTSD diagnoses generated from gold-standard psychiatric interviews, such as the Schedule of Affective Disorders and Schizophrenia for School-Aged Children (Gillihan et al., 2013). To be identified as a case in the current study, participants had to endorse 1 or more re-experiencing symptoms, 3 or more avoidance symptoms, and 2 or more arousal symptoms in the past month, consistent with establishing a PTSD diagnosis in prior CPSS research. An additional criterion was imposed for this study where PTSD cases were required to endorse at least 1 area of functional impairment, such as schoolwork, peer relationships, and engagement in pleasurable activities, along with the above symptom severity criteria. This additional criterion was imposed so that PTSD cases in this study most closely adhered to the PTSD diagnostic criteria specified in the DSM-IV-TR.
Data Screening and Analytic Strategy
Child sex (Female = 1, Male = 0), African-American ancestry (1 = Yes, 0 = No), family income, the total number of lifetime exposures to child adversities other than child maltreatment, and buccal cell count, which is an estimate of the quantity and type of cells collected from each swab, were entered into statistical modeling as covariates. Item-level missingness on the CPSS (n = 2 items or 0.17% of all CPSS items administered) was addressed using median, whole integer replacement based on all available, sample-wide observed responses for the respective CPSS items. The association between epigenetic age acceleration and PTSD diagnostic status was analyzed using logistic regression to assess the significance of risk and to report the effect size magnitude of this relation using the odds ratio (OR), consistent with genetic and epigenetic case-control association studies (Clarke et al., 2011). Supplemental analyses were then conducted to determine whether epigenetic age acceleration was related to specific PTSD symptom clusters and whether epigenetic age acceleration and PTSD diagnostic status varied by child maltreatment subtype.
Results
Epigenetic Age Acceleration and PTSD
The average total PTSD symptom severity in this sample was 14.77 (SD = 11.14). Mean severity for PTSD symptom clusters was 4.49 (SD = 3.70) for re-experiencing, 5.20 (SD = 4.80) for avoidance behavior, and 5.07 (SD = 3.92) for arousal. There was a total of 45.7% of PTSD cases and 54.3% of non-PTSD controls. Table 1 provides the results of a zero-order correlation matrix among all variables included in the logistic regression model along with PTSD symptom severity scores and PTSD diagnostic status. Overall, the logistic regression model provided a good fit to the observed data, Hosmer and Lemeshow test: χ2(8) = 8.92, p = .349, with a Nagelkerke R2 = .42. Individual parameter results of the logistic regression model demonstrated that accelerations in epigenetic age significantly predicted PTSD diagnostic status after adjusting for demographic variables, polygenic risk for PTSD, and lifetime exposure to childhood adversities other than child maltreatment, OR = 2.35 (95% CI: 1.22 - 4.51). The odds ratio indicates that a one unit increase in epigenetic age acceleration was associated with a 2.35 increase in the odds of having a PTSD diagnosis in this sample (see Table 2 for full model results). Figure 2 displays the relation between epigenetic age acceleration and the predicted probability of a PTSD diagnosis according to PTSD diagnostic status using model output.
Table 1.
Zero-order Correlations among Parameters in Logistic Regression Model and PTSD Outcomes
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|
| 1. Child Sex | - | ||||||||
| 2. African-American Ancestry | .06 | - | |||||||
| 3. Family Income | −.09 | −.33** | - | ||||||
| 4. Lifetime Adversity | −.03 | −.40*** | .04 | - | |||||
| 5. Buccal Cell Count | .45*** | −.01 | .22 | .07 | - | ||||
| 6. PTSD Polygenic Risk Score | .16 | −.00 | .14 | .03 | .02 | - | |||
| 7. Epigenetic Age Acceleration | .16 | −.23 | .17 | .08 | .12 | .07 | - | ||
| 8. PTSD Symptom Severity | .19 | −.25* | .15 | .62*** | .11 | .14 | .24* | - | |
| 9. PTSD Diagnostic Status | −.00 | −.23 | .24* | .40*** | .00 | .14 | .32** | .74*** | - |
Note. ‘Sex’ was dummy coded as Female = 1, Male = 0; ‘African-American Ancestry’ was dummy coded as Yes = 1, No = 0; ‘Lifetime Trauma and Adversity” is a composite index of the total number of lifetime traumas and adversities endorsed other than child maltreatment; PTSD = posttraumatic stress disorder.
= p ≤ .001;
p ≤ .01;
= p ≤ .05.
Table 2.
Logistic Regression Model Results
| Parameter | b | SE | Wald | p | OR | 95% CI |
|---|---|---|---|---|---|---|
| Constant | 1.32 | 5.05 | 0.07 | .794 | ||
| Child Sex | 0.18 | 0.72 | 0.06 | .801 | 1.20 | 0.29 - 4.91 |
| African-American Ancestry | 0.70 | 0.75 | 0.89 | .347 | 2.02 | 0.47 - 8.73 |
| Family Income | 0.35 | 0.17 | 4.21 | .040 | 1.42 | 1.02 - 1.98 |
| Lifetime Adversity | 0.47 | 0.15 | 9.49 | .002 | 1.59 | 1.18 - 2.14 |
| Buccal Cell Count | −0.07 | 0.07 | 1.06 | .303 | 0.93 | 0.81 - 1.07 |
| PTSD Polygenic Risk Score | 0.23 | 0.33 | 0.47 | .491 | 1.26 | 0.66 - 2.41 |
| Epigenetic Age Acceleration | 0.85 | 0.33 | 6.52 | .011 | 2.35 | 1.22 - 4.51 |
Note. ‘Sex’ was dummy coded as Female = 1, Male = 0; ‘African-American Ancestry’ was dummy coded as Yes = 1, No = 0; ‘Lifetime Trauma and Adversity” is a composite index of the total number of lifetime traumas and adversities endorsed other than child maltreatment; PTSD = posttraumatic stress disorder; SE = standard error for the beta value; OR = odds ratio; 95% CI = 95% confidence interval for the odds ratio.
Figure 2.
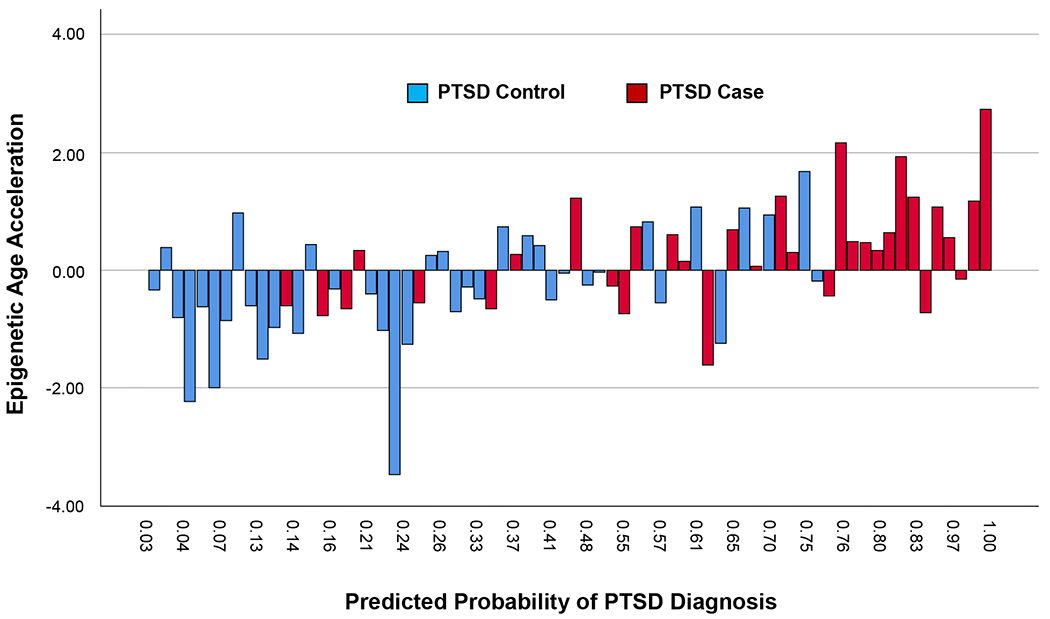
Predicted Probabilities of a DSM-IV-TR Diagnosis of Posttraumatic Stress Disorder (PTSD) Arranged According to Epigenetic Age Acceleration Estimates and Actual PTSD Diagnostic Status. Predicted probabilities (N = 70) were obtained for each child in the sample from the logistic regression model results presented in Table 2. These probabilities are arranged according to observed epigenetic age acceleration estimates and PTSD diagnostic status.
Subsequent tests were conducted to assess the robustness of the results generated from logistic regression model. One, to promote future replication efforts, a simple logistic regression model was performed to generate an unadjusted risk estimate of the relation between epigenetic age acceleration and PTSD diagnostic status. The resulting unadjusted estimate was OR = 2.08, p = .011, 95% CI: 1.18-3.65. Two, a sensitivity analysis was performed where covariates that did not significantly predict PTSD status in the primary logistic regression were removed from the model prior to re-assessing the risk for PTSD based on accelerations in epigenetic age. Results from this empirically-derived and parsimonious model demonstrated that epigenetic age acceleration continued to predict PTSD diagnostic status, OR = 2.10, p = .014, 95% CI: 1.16-3.80, after adjusting this estimate for family income and exposure to lifetime adversities other than child maltreatment.
Supplemental analyses were performed to determine whether epigenetic age acceleration was more closely associated with one or more PTSD symptom clusters. Bivariate correlations among epigenetic age acceleration and each of the three DSM-IV-TR PTSD symptom clusters, re-experiencing, avoidance behavior, and arousal, showed a significant and moderate effect size relation between epigenetic age acceleration and PTSD arousal symptoms, r = .29, p = .015. There were no differences across child maltreatment subtypes with respect to epigenetic age acceleration, F(2,67) = .907, p = .409, or PTSD diagnostic status, χ2(2) = .236, p = .889. Cumulative exposure to lifetime childhood adversity other than child maltreatment was unrelated to epigenetic age acceleration (r = .08, p = .523).
Discussion
This is the first study to demonstrate that epigenetic age acceleration predicts current PTSD status following recent exposure to substantiated child maltreatment. These results are consistent with prior research (Mehta et al., 2013) and predictions from biological models (Juster et al., 2011; Lupien et al., 2009; Shonkoff et al., 2012) positing that exposure to early adverse environments, particularly child maltreatment, alters biological stress-mediating systems related to PTSD. Such findings may offer important insights into which children exposed to maltreatment may meet PTSD criteria and which may not. There are several design features of this study that strengthen inferences about the relation between epigenetic aging and PTSD status. One, all participants had been exposed to substantiated child maltreatment in the twelve months prior to study entry. Using substantiated cases of child maltreatment follows official government procedures for determining whether child maltreatment occurred (U.S. Department of Health and Human Services, 2019). Thus, these results may represent the biological changes and subsequent risk for PTSD experienced by those members of the child maltreatment population who have been exposed to substantiated maltreatment. Two, PTSD symptoms and corresponding functional impairment were measured using a well-established and validated instrument that was used to generate PTSD diagnoses according to the prevailing diagnostic algorithm at the time the study began. Finally, established risks of PTSD, such as child and family demographics, genetic risks for PTSD, as well as lifetime exposure to childhood adversities other than child maltreatment, were included in models so that the unique association between epigenetic age acceleration and PTSD status could be determined.
Going beyond broad associations, the current study added specificity by showing the relation between epigenetic age acceleration and PTSD status was driven by a greater severity of symptoms in the arousal cluster domain, including such symptoms as hypervigilance, irritability, exaggerated startle responses, and difficulties sleeping. These supplemental analyses offer support for how changes in stress-mediating systems can affect downstream phenotypes in a theoretically-consistent manner as well as insight on how to advance future translational research on ways to prevent or treat PTSD in the child maltreatment population. Specifically, from a behavioral intervention perspective, the acquisition and use of relaxation skills, such as breathing retraining, and emotion regulation strategies, such as behavioral activation, following exposure to child maltreatment could be an efficient and effective way for targeting changes in DNA methylation while also preventing or reducing the severity of corresponding arousal symptoms, thereby reducing the risk for PTSD. Prior research has shown changes in DNA methylation as a result of behavioral intervention that corresponded with improvements in clinical endpoints (Yehuda et al., 2013).
In this same vein, future research examining mediational processes that lead to and sustain accelerations in epigenetic age in the child maltreatment population over time can also provide an opportunity to identify novel targets for PTSD intervention. Neuroendocrine disruption is a hallmark feature of early life adversity (Gunnar & Vazquez, 2001; McEwen et al., 2016) and one specific profile involves elevated and prolonged neuroendocrine activation in the initial years following child maltreatment that is followed by an attenuation of cortisol secretion over time (Trickett et al., 2010). Prolonged exposure to elevated concentrations of cortisol can alter DNA methylation and the expression of glucocorticoid responsive genes (Lee et al., 2010; Thomassin et al., 2001). Similarly, treatment with the glucocorticoid agonist, dexamethasone, produced changes in DNA methylation at multiple CpG sites used to calculate epigenetic age acceleration (Zannas et al., 2015). This remodeling of DNA methylation in response to prolonged neuroendocrine activation, particularly in glucocorticoid-sensitive genomic regions included in the epigenetic age index, can explain how exposure to early life adversity may accelerate epigenetic age and explain variation in PTSD status and course. Testing empirically whether neuroendocrine disruption mediates the acceleration of epigenetic age in long-term, prospective cohort research with the child maltreatment population has the potential to identify those who may be at risk for a persistent course of PTSD and how this course may be prevented or even treated across the lifespan.
An important consideration of these results is the use of the DSM-IV-TR diagnostic algorithm to establish cases of PTSD, an algorithm that is no longer in use. Data collection for the current study began at a time when DSM-IV-TR was the prevailing diagnostic model, which is why PTSD symptoms were measured according to DSM-IV-TR criteria and not the current DSM-5 (American Psychiatric Association, 2013). Research on the differences between PTSD measured in DSM-IV-TR and DSM-5 is ongoing, although existing research suggests that PTSD prevalence estimates between DSM-IV-TR and DSM-5 are similar (Elhai et al., 2012) with slight reductions in DSM-5 PTSD prevalence largely due to the removal of the unexpected or accidental death of a family member or friend due to natural causes as a traumatic event (Kilpatrick et al., 2013). Many measures of PTSD, including the CPSS (Foa et al., 2018), have now been revised to validly measure PTSD according to the new DSM-5 criteria. Future research can therefore assess and potentially replicate the current findings by examining epigenetic age acceleration in the child maltreatment population using these updated assessment instruments.
Future research in this area could also provide important information regarding protective factors that may prevent the accelerated epigenetic aging that can occur in a portion of the child maltreatment population. While there is literature to support factors that promote resilience in response to child maltreatment, including individual factors (e.g. higher cognitive ability, effective self-regulation, etc.) and supportive family and community relationships (Heller et al., 1999), there is currently little evidence to demonstrate whether or which of these might affect DNA methylation and epigenetic aging directly. Identifying specific factors that may impact epigenetic aging in response to trauma, and thereby decrease the likelihood of developing PTSD, would allow for early intervention to focus on bolstering those factors when child maltreatment has been disclosed and substantiated. Additionally, given that previous research has suggested that epigenetic aging following early life adversity continues to progress into adulthood (Zannas et al., 2015), future studies could examine the rate of epigenetic aging following a childhood diagnosis of PTSD and subsequent trauma intervention. Information regarding therapies that prevent or slow the development of accelerated epigenetic aging and its associated costs would be highly valuable.
There are several limitations to the current study that also warrant consideration. One, the sample is small and drawn from a segment of the child maltreatment population where known instances of child maltreatment were investigated and substantiated. Thus, results may not generalize to the broader child maltreatment population or measuring child maltreatment in other ways. Two, this case-control study, where the entire sample was exposed to substantiated child maltreatment, is unable to determine whether child maltreatment was the cause of variation in DNA methylation and epigenetic age acceleration. Prior research has found that self-reported child maltreatment was associated with changes in DNA methylation at glucocorticoid sensitive genomic sites related to PTSD (Mehta et al., 2013) while other studies have failed to find a relation between self-reported child maltreatment and accelerations in epigenetic aging (Zannas et al., 2015). Future research should continue to evaluate the role child maltreatment plays in accelerating epigenetic aging after accounting for exposure to additional childhood adversities and across different measurement strategies, namely substantiated cases versus self-report methods, as these different measurement strategies can produce highly discordant results when measuring child maltreatment and its effects (Baldwin et al., 2019). Ideally, research incorporating both measurement strategies holds the greatest potential to establish what effect child maltreatment may have on epigenetic aging and how best to detect this effect. Three, this study is cross-sectional and therefore it remains unknown whether accelerations in epigenetic aging in childhood can predict PTSD status in adulthood or a chronic course of this disorder. Four, the number of families who refused to participate in this study due to the inclusion criterion of being willing to provide biospecimens was not recorded, thus it is not possible to state whether or not this criterion created a selection bias in the current sample. Imposing such a criterion has historically and disproportionately affected participation by individuals of non-European descent. However, this concern is mitigated in part by the racial demography of the current sample, where members from diverse minority groups constituted the majority of the sample. Finally, this study focused on child maltreatment that occurred in the past year and did not examine maltreatment during sensitive or critical periods of development (Marini et al., 2020). Exposure to maltreatment during such periods, such as prior to age four, may exert long-lasting effects on biological stress processes that explain the development and course of PTSD and related disorders (Lupien et al., 2009).
Despite these limitations, the results of this study have implications for etiological models of PTSD in the child maltreatment population where epigenetic age acceleration added unique explanatory power in determining who met current PTSD status and who did not. With future replication, epigenetic age acceleration could be used as a biomarker for identifying those children who are at greatest risk for PTSD while offering important information for interventions aiming to prevent or treat PTSD.
Acknowledgements:
Research reported in this manuscript was supported by the National Institutes of Health under Award Numbers KL2TR000078, R01AG059682, and P50HD089922. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Adams RL (1995). Eukaryotic DNA methyltransferases--Structure and function. Bioessays, 17(2), 139–145. doi: 10.1002/bies.950170209 [DOI] [PubMed] [Google Scholar]
- Alisic E, Zalta AK, van Wesel F, Larsen SE, Hafstad GS, Hassanpour K, & Smid GE (2014). Rates of post-traumatic stress disorder in trauma-exposed children and adolescents: Meta-analysis. The British Journal of Psychiatry, 204(5), 335–340. doi: 10.1192/bjp.bp.113.131227 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and Statisical Manual of Mental Disorders (5th ed.). Washington, D.C.: American Psychiatric Press. [Google Scholar]
- Aristizabal MJ, Anreiter I, Halldorsdottir T, Odgers CL, McDade TW, Goldenberg A, … O’Donnell KJ (2019). Biological embedding of experience: A primer on epigenetics. Proc Natl Acad Sci U S A. doi: 10.1073/pnas.1820838116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin JR, Reuben A, Newbury JB, & Danese A (2019). Agreement Between Prospective and Retrospective Measures of Childhood Maltreatment: A Systematic Review and Meta-analysis. JAMA Psychiatry. doi: 10.1001/jamapsychiatry.2019.0097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes JE, Noll JG, Putnam FW, & Trickett PK (2009). Sexual and physical revictimization among victims of severe childhood sexual abuse. Child Abuse & Neglect, 33(7), 412–420. doi: 10.1016/j.chiabu.2008.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach SR, Brody GH, Todorov AA, Gunter TD, & Philibert RA (2011). Methylation at 5HTT mediates the impact of child sex abuse on women’s antisocial behavior: An examination of the Iowa adoptee sample. Psychosomatic Medicine, 73(1), 83–87. doi: 10.1097/PSY.0b013e3181fdd074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonomi AE, Anderson ML, Rivara FP, Cannon EA, Fishman PA, Carrell D, … Thompson RS (2008). Health care utilization and costs associated with childhood abuse. Journal of General Internal Medicine, 23(3), 294–299. doi: 10.1007/s11606-008-0516-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewin CR, Andrews B, & Valentine JD (2000). Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. Journal of Consulting and Clinical Psychology, 68(5), 748–766. [DOI] [PubMed] [Google Scholar]
- Chen BH, Marioni RE, Colicino E, Peters MJ, Ward-Caviness CK, Tsai PC, … Horvath S (2016). DNA methylation-based measures of biological age: meta-analysis predicting time to death. Aging (Albany NY), 8(9), 1844–1865. doi: 10.18632/aging.101020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke GM, Anderson CA, Pettersson FH, Cardon LR, Morris AP, & Zondervan KT (2011). Basic statistical analysis in genetic case-control studies. Nat Protoc, 6(2), 121–133. doi: 10.1038/nprot.2010.182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland WE, Keeler G, Angold A, & Costello EJ (2007). Traumatic events and posttraumatic stress in childhood. Archives of General Psychiatry, 64(5), 577–584. doi: 10.1001/archpsyc.64.5.577 [DOI] [PubMed] [Google Scholar]
- Duncan LE, Ratanatharathorn A, Aiello AE, Almli LM, Amstadter AB, Ashley-Koch AE, … Koenen KC (2018). Largest GWAS of PTSD (N=20 070) yields genetic overlap with schizophrenia and sex differences in heritability. Mol Psychiatry, 23(3), 666–673. doi: 10.1038/mp.2017.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhai JD, Miller ME, Ford JD, Biehn TL, Palmieri PA, & Frueh BC (2012). Posttraumatic stress disorder in DSM-5: Estimates of prevalence and symptom structure in a nonclinical sample of college students. Journal of Anxiety Disorders, 26(1), 58–64. doi: 10.1016/j.janxdis.2011.08.013 [DOI] [PubMed] [Google Scholar]
- Euesden J, Lewis CM, & O’Reilly PF (2015). PRSice: Polygenic Risk Score software. Bioinformatics, 31(9), 1466–1468. doi: 10.1093/bioinformatics/btu848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa EB, Asnaani A, Zang Y, Capaldi S, & Yeh R (2018). Psychometrics of the Child PTSD Symptom Scale for DSM-5 for Trauma-Exposed Children and Adolescents. J Clin Child Adolesc Psychol, 47(1), 38–46. doi: 10.1080/15374416.2017.1350962 [DOI] [PubMed] [Google Scholar]
- Foa EB, Johnson KM, Feeny NC, & Treadwell KRH (2001). The Child PTSD Symptom Scale: A preliminary examination of its psychometric properties. Journal of Clinical Child Psychology, 30(3), 376–384. [DOI] [PubMed] [Google Scholar]
- Gilbert R, Fluke J, O’Donnell M, Gonzalez-Izquierdo A, Brownell M, Gulliver P, … Sidebotham P (2012). Child maltreatment: Variation in trends and policies in six developed countries. Lancet, 379(9817), 758–772. doi: 10.1016/s0140-6736(11)61087-8 [DOI] [PubMed] [Google Scholar]
- Gillihan SJ, Aderka IM, Conklin PH, Capaldi S, & Foa EB (2013). The Child PTSD Symptom Scale: Psychometric properties in female adolescent sexual assault survivors. Psychological Assessment, 25(1), 23–31. doi: 10.1037/a0029553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, & Kessler RC (2010). Childhood adversities and adult psychiatric disorders in the National Comorbidity Survey Replication I: Associations with first onset of DSM-IV disorders. Archives of General Psychiatry, 67(2), 113–123. doi: 10.1001/archgenpsychiatry.2009.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, & Vazquez DM (2001). Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Development and Psychopathology, 13(3), 515–538. [DOI] [PubMed] [Google Scholar]
- Heller SS, Larrieu JA, D’Imperio R, & Boris NW (1999). Research on resilience to child maltreatment: Empirical considerations. Child Abuse & Neglect, 23(4), 321–338. doi: 10.1016/S0145-2134(99)00007-1 [DOI] [PubMed] [Google Scholar]
- Horowitz L (1998). The relationship of child sexual abuse to revictimization: Mediating variables and developmental processes. Unpublished manuscript. Catholic University of America. Washington, D.C. [Google Scholar]
- Horvath S (2013). DNA methylation age of human tissues and cell types. Genome Biol, 14(10), R115. doi: 10.1186/gb-2013-14-10-r115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S, Zhang Y, Langfelder P, Kahn RS, Boks MP, van Eijk K, … Ophoff RA (2012). Aging effects on DNA methylation modules in human brain and blood tissue. Genome Biol, 13(10), R97. doi: 10.1371/journal.pgen.1002629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova JI, Birnbaum HG, Chen L, Duhig AM, Dayoub EJ, Kantor ES, … Phillips GA (2011). Cost of post-traumatic stress disorder vs major depressive disorder among patients covered by medicaid or private insurance. American Journal of Managed Care, 17(8), e314–323. [PubMed] [Google Scholar]
- Johnson WE, Li C, Rabinovic A, Houseman EA, Accomando WP, Koestler DC, … Kelsey KT (2007). Adjusting batch effects in microarray expression data using empirical Bayes methods DNA methylation arrays as surrogate measures of cell mixture distribution. Biostatistics, 8(1), 118–127. doi: 10.1093/biostatistics/kxj037 [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Vance LA, Cross D, Knight AK, Kilaru V, Michopoulos V, … Smith AK (2017). Exposure to Violence Accelerates Epigenetic Aging in Children. Sci Rep, 7(1), 8962. doi: 10.1038/s41598-017-09235-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juster RP, Bizik G, Picard M, Arsenault-Lapierre G, Sindi S, Trepanier L, … Lupien SJ (2011). A transdisciplinary perspective of chronic stress in relation to psychopathology throughout life span development. Development and Psychopathology, 23(3), 725–776. doi: 10.1017/s0954579411000289 [DOI] [PubMed] [Google Scholar]
- Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, & Friedman MJ (2013). National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. Journal of Traumatic Stress, 26(5), 537–547. doi: 10.1002/jts.21848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Wildeman C, Jonson-Reid M, & Drake B (2017). Lifetime Prevalence of Investigating Child Maltreatment Among US Children. Am J Public Health, 107(2), 274–280. doi: 10.2105/ajph.2016.303545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RS, Tamashiro KL, Yang X, Purcell RH, Harvey A, Willour VL, … Potash JB (2010). Chronic corticosterone exposure increases expression and decreases deoxyribonucleic acid methylation of Fkbp5 in mice. Endocrinology, 151(9), 4332–4343. doi: 10.1210/en.2010-0225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, … Meaney MJ (1997). Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science, 277(5332), 1659–1662. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, & Heim C (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews: Neuroscience, 10(6), 434–445. doi: 10.1038/nrn2639 [DOI] [PubMed] [Google Scholar]
- Marciniak MD, Lage MJ, Dunayevich E, Russell JM, Bowman L, Landbloom RP, & Levine LR (2005). The cost of treating anxiety: The medical and demographic correlates that impact total medical costs. Depression & Anxiety, 21(4), 178–184. doi: 10.1002/da.20074 [DOI] [PubMed] [Google Scholar]
- Marini S, Davis KA, Soare TW, Zhu Y, Suderman MJ, Simpkin AJ, … Dunn EC (2020). Adversity exposure during sensitive periods predicts accelerated epigenetic aging in children. Psychoneuroendocrinology, 113, 104484. doi: 10.1016/j.psyneuen.2019.104484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marioni RE, Shah S, McRae AF, Chen BH, Colicino E, Harris SE, … Deary IJ (2015). DNA methylation age of blood predicts all-cause mortality in later life. Genome Biol, 16, 25. doi: 10.1186/s13059-015-0584-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Nasca C, & Gray JD (2016). Stress Effects on Neuronal Structure: Hippocampus, Amygdala, and Prefrontal Cortex. Neuropsychopharmacology, 41(1), 3–23. doi: 10.1038/npp.2015.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PO, & Roth TL (2015). Epigenetic pathways through which experiences become linked with biology. Development and Psychopathology, 27(2), 637–648. doi: 10.1017/s0954579415000206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonte B, Szyf M, … Meaney MJ (2009). Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci, 12(3), 342–348. doi: 10.1038/nn.2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Koenen KC, Bromet EJ, Karam EG, Liu H, Petukhova M, … Kessler RC (2017). Childhood adversities and post-traumatic stress disorder: evidence for stress sensitisation in the World Mental Health Surveys. Br J Psychiatry, 211(5), 280–288. doi: 10.1192/bjp.bp.116.197640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Koenen KC, Hill ED, Petukhova M, Sampson NA, Zaslavsky AM, & Kessler RC (2013). Trauma exposure and posttraumatic stress disorder in a national sample of adolescents. J Am Acad Child Adolesc Psychiatry, 52(8), 815–830.e814. doi: 10.1016/j.jaac.2013.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta D, Gonik M, Klengel T, Rex-Haffner M, Menke A, Rubel J, … Binder EB (2011). Using polymorphisms in FKBP5 to define biologically distinct subtypes of posttraumatic stress disorder: Evidence from endocrine and gene expression studies. Archives of General Psychiatry. doi: 10.1001/archgenpsychiatry.2011.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta D, Klengel T, Conneely KN, Smith AK, Altmann A, Pace TW, … Binder EB (2013). Childhood maltreatment is associated with distinct genomic and epigenetic profiles in posttraumatic stress disorder. Proc Natl Acad Sci U S A, 110(20), 8302–8307. doi: 10.1073/pnas.1217750110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, … Swendsen J. (2010). Lifetime prevalence of mental disorders in U.S. adolescents: Results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A). Journal of the American Academy of Child & Adolescent Psychiatry, 49(10), 980–989. doi: 10.1016/j.jaac.2010.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romens SE, McDonald J, Svaren J, & Pollak SD (2015). Associations between early life stress and gene methylation in children. Child Development, 86(1), 303–309. doi: 10.1111/cdev.12270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Lubin FD, Funk AJ, & Sweatt JD (2009). Lasting epigenetic influence of early-life adversity on the BDNF gene. Biological Psychiatry, 65(9), 760–769. doi: 10.1016/j.biopsych.2008.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago PN, Ursano RJ, Gray CL, Pynoos RS, Spiegel D, Lewis-Fernandez R, … Fullerton CS (2013). A systematic review of PTSD prevalence and trajectories in DSM-5 defined trauma exposed populations: Intentional and non-intentional traumatic events. PLoS One, 8(4), e59236. doi: 10.1371/journal.pone.0059236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott KM, Smith DR, & Ellis PM (2010). Prospectively ascertained child maltreatment and its association with DSM-IV mental disorders in young adults. Archives of General Psychiatry, 67(7), 712–719. doi: 10.1001/archgenpsychiatry.2010.71 [DOI] [PubMed] [Google Scholar]
- Sedlak AJ, Mettenburg J, Basena M, Petta I, McPherson K, & Greene A (2010). Fourth National Incidence Study of Child Abuse and Neglect (NIS-4): Report to Congress. Washington, DC: US Dept. of Health and Human Services, Administration for Children and Families, Administration on Children, Youth and Families, National Center on Child Abuse and Neglect. [Google Scholar]
- Shenk CE, Noll JG, Griffin AM, Allen EK, Lee SE, Lewkovich KL, & Allen B (2016). Psychometric Evaluation of the Comprehensive Trauma Interview PTSD Symptoms Scale Following Exposure to Child Maltreatment. Child Maltreat, 21(4), 343–352. doi: 10.1177/1077559516669253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff JP, Garner AS, Committee on Psychosocial Aspects of, C., Family, H., Committee on Early Childhood, A., Dependent, C., … Behavioral, P. (2012). The lifelong effects of early childhood adversity and toxic stress. Pediatrics, 129(1), e232–246. doi: 10.1542/peds.2011-2663 [DOI] [PubMed] [Google Scholar]
- Smith AK, Kilaru V, Klengel T, Mercer KB, Bradley B, Conneely KN, … Binder EB (2015). DNA extracted from saliva for methylation studies of psychiatric traits: evidence tissue specificity and relatedness to brain. Am J Med Genet B Neuropsychiatr Genet, 168b(1), 36–44. doi: 10.1002/ajmg.b.32278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyf M, & Bick J (2013). DNA methylation: A mechanism for embedding early life experiences in the genome. Child Development, 84(1), 49–57. doi: 10.1111/j.1467-8624.2012.01793.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomassin H, Flavin M, Espinas ML, & Grange T (2001). Glucocorticoid-induced DNA demethylation and gene memory during development. Embo j, 20(8), 1974–1983. doi: 10.1093/emboj/20.8.1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trickett PK, Noll JG, Susman EJ, Shenk CE, & Putnam FW (2010). Attenuation of cortisol across development for victims of sexual abuse. Development and Psychopathology, 22(1), 165–175. doi: 10.1017/s0954579409990332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turecki G, & Meaney MJ (2016). Effects of the Social Environment and Stress on Glucocorticoid Receptor Gene Methylation: A Systematic Review. Biol Psychiatry, 79(2), 87–96. doi: 10.1016/j.biopsych.2014.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services, Administration for Children and Families, Administration on Children, Youth, and Families, Children’s Bureau. (2019). Child Maltreatment 2017 Retrieved from https://www.acf.hhs.gov/cb/research-data-technology/statistics-research/child-maltreatment.
- Vijayendran M, Beach SR, Plume JM, Brody GH, & Philibert RA (2012). Effects of genotype and child abuse on DNA methylation and gene expression at the serotonin transporter. Front Psychiatry, 3, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, … Meaney MJ (2004). Epigenetic programming by maternal behavior. Nature Neuroscience, 7(8), 847–854. doi: 10.1038/nn1276 [DOI] [PubMed] [Google Scholar]
- Widom CS (1999). Posttraumatic stress disorder in abused and neglected children grown up. American Journal of Psychiatry, 156(8), 1223–1229. [DOI] [PubMed] [Google Scholar]
- Wildeman C, Emanuel N, Leventhal JM, Putnam-Hornstein E, Waldfogel J, & Lee H (2014). The prevalence of confirmed maltreatment among US children, 2004 to 2011. JAMA Pediatrics, 168(8), 706–713. doi: 10.1001/jamapediatrics.2014.410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2014, September 15–18, 2014). Investing in Children: The European Child and Adolescent Health Strategy 2015-2020 and the European Child Maltreatment Prevntion Action Plan 2015–2020. Paper presented at the Regional Committee for Europe, 64th Session, Copenhagen, Denmark. [Google Scholar]
- Yehuda R, Cai G, Golier JA, Sarapas C, Galea S, Ising M, … Buxbaum JD (2009). Gene expression patterns associated with posttraumatic stress disorder following exposure to the World Trade Center attacks. Biological Psychiatry, 66(7), 708–711. doi: 10.1016/j.biopsych.2009.02.034 [DOI] [PubMed] [Google Scholar]
- Yehuda R, Daskalakis NP, Desarnaud F, Makotkine I, Lehrner A, Koch E, … Bierer LM (2013). Epigenetic Biomarkers as Predictors and Correlates of Symptom Improvement Following Psychotherapy in Combat Veterans with PTSD. Frontiers in Psychiatry, 4. doi: 10.3389/fpsyt.2013.00118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You DS, Youngstrom EA, Feeny NC, Youngstrom JK, & Findling RL (2017). Comparing the diagnostic accuracy of five instruments for detecting posttraumatic stress disorder in youth. Journal of Clinical Child and Adolescent Psychology, 46(4), 511–522. doi: 10.1080/15374416.2015.1030754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannas AS, Arloth J, Carrillo-Roa T, Iurato S, Roh S, Ressler KJ, … Mehta D (2015). Lifetime stress accelerates epigenetic aging in an urban, African American cohort: relevance of glucocorticoid signaling. Genome Biol, 16, 266. doi: 10.1186/s13059-015-0828-5 [DOI] [PMC free article] [PubMed] [Google Scholar]


