Abstract
In 2013, the WHO Strategic Advisory Group of Experts on Immunization (SAGE) requested WHO to develop a process and a plan to move the maternal immunization agenda forward in support of an increased alignment of data safety evidence, public health needs, and regulatory processes. A key challenge identified was the continued need for harmonization of maternal adverse event following immunization (AEFI) research and surveillance efforts within developing and developed country contexts. We conducted a systematic review as a preliminary step in the development of standardized AEFI definitions for use in maternal and neonatal clinical trials, post-licensure surveillance, and other vaccine studies. We documented the current extent and nature of variability in AEFI definitions and adverse event reporting among 74 maternal immunization studies, which reported a total of 240 different types of adverse events. Forty-nine studies provided explicit AEFI case definitions describing 35 separate types of AEFIs. We identified variability in how AEFIs were determined to be present, in how AEFI definitions were applied, and in the ways that AEFIs were reported. Definitions for key maternal/neonatal AEFIs differed on four discrete attributes: overall level of detail, physiological and temporal boundaries and cut-offs, severity strata, and standards used. Our findings suggest that investigators may proactively address these inconsistencies through comprehensive and consistent reporting of AEFI definitions and outcomes in future publications. In addition, efforts to develop standardized AEFI definitions should generate definitions of sufficient detail and consistency of language to avoid the ambiguities we identified in reviewed articles, while remaining practically applicable given the constraints of low-resource contexts such as limited diagnostic capacity and high patient throughput.
Keywords: Adverse events, Pregnancy, Vaccine safety, Maternal immunization, Case definitionsm, AEFI
1. Introduction
Since 1990, the world has experienced a dramatic decrease in early childhood mortality. In 2013, the global under-five mortality rate (U5MR) was 46 deaths per 1000 live births, nearly half the rate U5MR of 90 deaths per 1000 live births in 1990 [1]. However, the rate of this reduction in under-five mortality is still insufficient to reach the Millennium Development Goals’ target of a two-thirds reduction of 1990 mortality levels by the year 2015 [2,3].
Compared to under-five mortality, declines in newborn mortality have been much slower to materialize. As of 2012, nearly 40% of all under-five child deaths occur in the neonatal period, i.e., babies in their first 28 days of life [4]. Additionally, in developing countries, nearly half of all mothers and newborns fail to receive skilled care during and immediately after birth. The World Health Organization (WHO) estimates that up to two-thirds of newborn deaths can be prevented if known, effective health measures are provided at birth and during the first week of life [4].
A potential strategy to address this global health need is the immunization of pregnant women to prevent diseases in their newborn children. Trans-placental transfer of antibodies has been demonstrated in several studies, and may confer protection against influenza during a newborn’s first months of life [5]. This strategy is buoyed by the success of the global Maternal Neonatal Tetanus Elimination Initiative and recent vaccine studies demonstrating that immunization of pregnant women decreases newborn influenza [6,7]. However, there are challenges to introducing immunization programs in antenatal care in resource-poor settings, requiring careful consideration of existing regulatory processes and expansion of the evidence base to take into account local public health needs to inform maternal immunization programs and policy [8,9]. The WHO/PATH Maternal Influenza Immunization Project aims to address some of these challenges – specifically with respect to vaccine distribution, logistics, and potentially vaccine hesitancy and uptake – by promoting the integration of immunization into antenatal care platforms in low- and middle-income countries [10].
There are limitations to vaccine safety data in pregnant women as pregnant women are seldom included in clinical trials [11]. Most safety information comes from observational studies and analysis of post-licensure surveillance systems, such as those for influenza vaccines [12]. In 2014, the WHO Global Advisory Committee on Vaccine Safety (GACVS) reviewed inactivated influenza vaccine safety in pregnancy and found no safety signals [13], and three recent systematic reviews of influenza vaccine safety in pregnancy have also been reassuring [14–16]. Nevertheless, the absence of global standard definitions for maternal immunization adverse events hinders comparisons of safety data across studies and geographic regions. For these reasons, the WHO and Brighton Collaboration are developing standardized adverse event definitions and reporting practices for use in clinical trials in pregnant women and other post-licensure vaccine safety monitoring.
The objective of this systematic review is to determine the extent and nature of variability in AEFI definitions and adverse event reporting among maternal immunization studies. The review aims to characterize the heterogeneity of AEFI definitions and reporting methods, which will directly inform ongoing vaccine safety standardization efforts for the purposes of clinical trial design as well as vaccine pharmacovigilance after licensing. These efforts will enhance collection, reporting, and comparison of clinical and post-marketing surveillance safety data—advancing our collective understanding of vaccine safety in pregnancy, and contributing to the harmonization of vaccine pharmacovigilance.
2. Methods
2.1. Eligibility criteria and assessment
2.1.1. Types of studies
We included randomized controlled trials and observational studies that define one or more AEFIs for the purpose of safety monitoring. We also included reviews of maternal immunization studies; reviews were thought to potentially contain abstracted information on AEFI definitions that may not have existed, either at all or at the same level of detail, in the source publications. Maternal immunization reviews were therefore included to ensure that this content was not overlooked. We did not include unpublished studies.
2.1.2. Types of participants
Studies chosen for review included pregnant women of all ages. Studies that did not explicitly include pregnant women, either exclusively or as part of an at-risk demographic, were excluded.
2.1.3. Types of interventions
Eligible interventions included all vaccines evaluated in pregnant women.
2.1.4. Types of comparisons
We included studies making any relevant comparisons of vaccines against a control, such as placebo, unexposed or untreated group, or alternate vaccine formulation.
2.1.5. Types of outcome measures
Acceptable outcome measures included intervention efficacy, effectiveness, or safety. Specifically, studies that did not evaluate vaccine safety as a primary outcome were included if maternal, childbirth, or neonatal safety or adverse event data were reported.
2.1.6. Other selection criteria
Study setting had no impact on inclusion. We included studies conducted in any country or region, in rural, urban, or mixed contexts, and in any participant setting such as in-hospital or incommunity. Five studies published in languages other than English were considered for inclusion, but none were included in the final review due to lack of translation capacity. There was no constraint on date of publication.
2.2. Search strategy
We conducted a comprehensive and systematic search of published literature potentially containing data on maternal and neonatal adverse events following maternal immunization (Fig. 1).
Fig. 1.
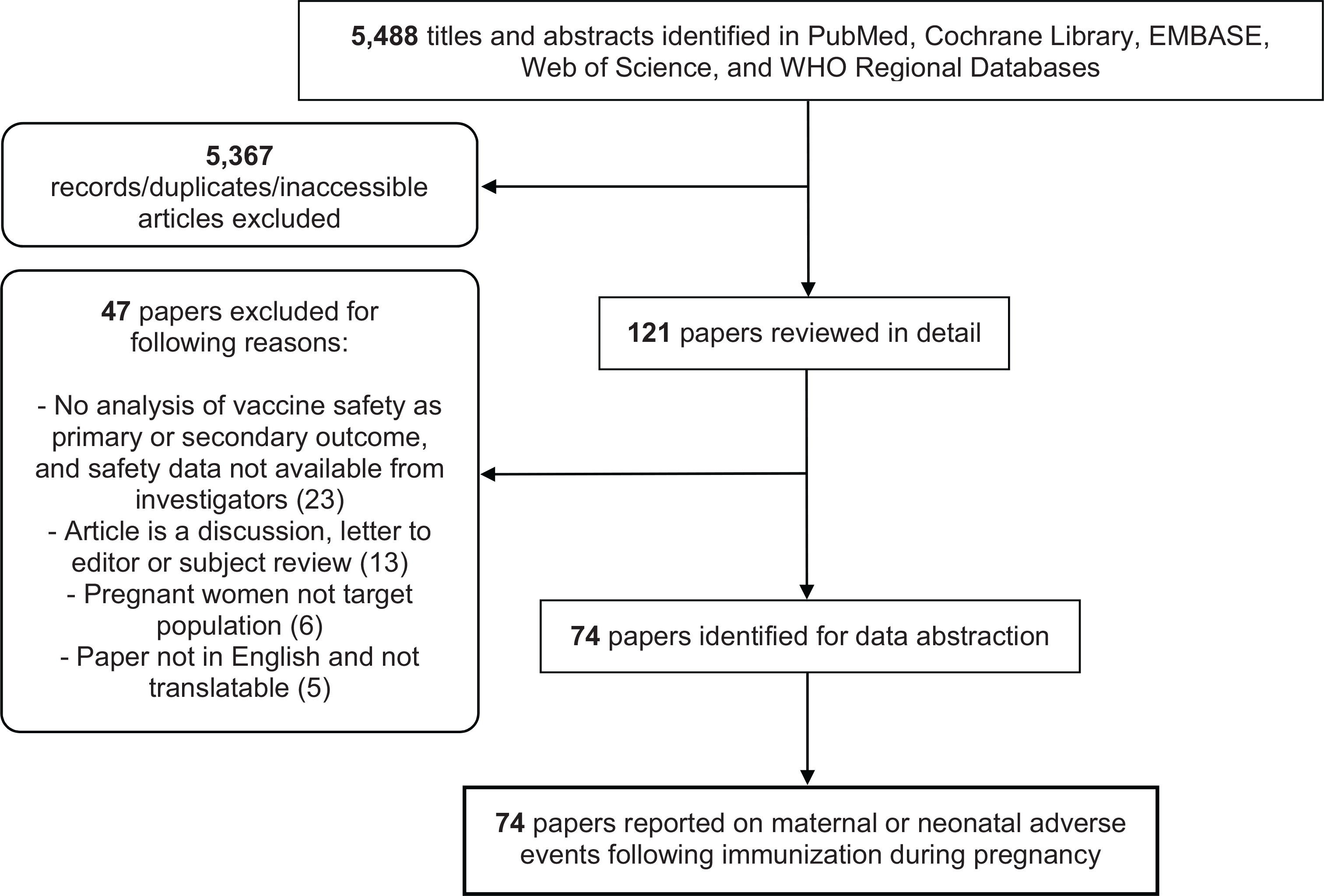
Systematic review workflow.
Sources included all published maternal immunization studies conducted to date (randomized controlled trials and observational studies), identified via searches of PubMed, EMBASE, Web of Science, and the Cochrane Database. The search strategy used for this review was derived from prior work by Bonhoeffer et al including a systematic review of vaccine safety data reporting [17]. Publications citing key papers that evaluated or attempted to establish immunization study reporting standards (e.g. Bonhoeffer et al. [17]) were also included in the search process. Supplementary Table 1 details our maternal AEFI search strategy on PubMed and EMBASE; Fig. 1 indicates the total number of results from all searches.
2.3. Screening and data extraction
The initial screening was conducted by one reviewer; a two-reviewer system was employed throughout the remainder of the review workflow. We imported search results into Endnote X5, and one reviewer (T.R.F.) screened titles and abstracts for eligibility. We discarded articles if their titles and abstracts clearly bore no relevance to this review. We retrieved full texts of eligible studies, and discarded inaccessible studies (six articles published prior to 2000, were inaccessible). Consensus and/or discussion with a second reviewer (D. N.) resolved uncertainty during the screening process with regard to inclusion/exclusion of studies. We recorded the rationale for study exclusion as part of the screening process.
The objective of our review was to determine the variance in AEFI definitions across all maternal immunization literature irrespective of study design, rigor, outcome, or potential bias. Therefore, a methodological study quality assessment (e.g., a Grading of Recommendations Assessment, Development and Evaluation (GRADE) analysis) was not required for the purposes of this review. Studies included in this review were neither assessed for, nor ranked on the basis of, limitations in design or possible bias.
We abstracted data from all research studies and publications meeting the inclusion criteria into an Excel workbook (Supplemental Table 2). We compiled additional data required to fully characterize AEFI definitions used into a set of queries. We contacted study investigators as necessary to request study protocols and/or to address omitted or incomplete data on adverse event definitions. We tracked communication with study investigators over the course of the review to ensure complete follow-up.
2.4. AEFI definitions and reported outcomes
We abstracted detailed information specific to AEFI case definitions into a comprehensive AEFI definition table (Supplemental Table 3). This table includes all additional unpublished information regarding AEFI definitions obtained via communication with study authors. Additionally, we abstracted detailed information specific to monitored and/or reported adverse event outcomes into a reported outcomes table (Supplemental Table 4). This table includes all reported or monitored maternal, fetal, and neonatal outcomes, irrespective of whether any study provided a case definition or classification code for the outcome.
Prior to performing analyses, we removed duplicate case definitions and definitions with minor (i.e., typographical and/or non-semantically significant, such as “fetal death” versus “death, foetal”) variations. However, we separately listed AEFIs that share similar definitions but are assigned different names. For example, “stillbirth” and “late fetal death” are separately listed despite both being defined as “fetal death occurring at or after 20 weeks gestation.” Similarly, we separately listed definitions for a single AEFI that share similar criteria (e.g., birth weight) but otherwise demonstrate semantically significant differences (for example, “birth after 20 weeks” versus “birth at or after 20 weeks”). Such AEFI names and definitions are considered “unique” for the purposes of our review.
2.5. Analysis
Data analysis was specifically tailored to provide the WHO and Brighton Collaboration a comprehensive overview of the heterogeneity among AEFI definitions and reporting methods in the maternal immunization literature
We performed the following analyses:
Ranking of reported outcomes by frequency, using a series of generalized terms (e.g., miscarriage, preterm birth, stillbirth, etc.) to categorize each type of definition;
Enumeration of studies providing AEFI definitions and/or information regarding classification systems used;
Classification of AEFIs by individual affected (mother, fetus, or neonate);
Ranking of AEFIs by frequency of definition and/or monitoring;
Assessment of where in selected papers AEFIs are typically defined and/or reported on;
Characterization of inconsistency in AEFI names and definitions, in terms of overall detail and terminology, thresholds and cut-offs, severity strata, and standards used; and
Assessment of differences in studies’ definitions of what constitutes an adverse event.
3. Results
3.1. Study selection and characteristics
Out of a total of 5488 titles identified from electronic searches, 121 titles were selected as potentially relevant. All 121 papers were assessed for eligibility; following assessment, 47 papers were excluded with reason, and the remaining 74 papers were included in the review (Fig. 1). Of the 74 selected publications, ten were previously published reviews.
Half of the selected studies (37) focused on influenza vaccine, followed by yellow fever vaccines (5 studies), tetanus, diphtheria and acellular pertussis (Tdap) vaccine (4), and rubella vaccines (4) (Table 1). Other vaccines investigated included tetanus toxoid and vaccines against pertussis, diphtheria, varicella, group B streptococcus, hepatitis, human immunodeficiency virus (HIV), human papillomavirus (HPV), pneumococcal, cholera, cytomegalovirus, herpes simplex, meningococcal, polio, rabies, and respiratory syncytial virus. Five papers (of which three were reviews) examined vaccines against more than one pathogen.
Table 1.
Summary characteristics of included studies.
| Number of papers | |
|---|---|
| Selected publications by type | |
| Study type | |
| Retrospective cohort | 30 |
| Prospective cohort | 24 |
| Review | 10 |
| RCT | 10 |
| Cross-sectional | 3 |
| Case-control | 2 |
| Before/after | 1 |
| Selected publications by location | |
| Continent | |
| North America | 35 |
| Europe | 15 |
| South America | 7 |
| Asia | 6 |
| Africa | 2 |
| Australia | 0 |
| Multiple | 9 |
| Selected publications by vaccine | |
| Vaccine | |
| Influenza | 37 |
| Yellow fever | 5 |
| Tdap | 4 |
| Rubella | 4 |
| Varicella | 2 |
| Group B streptococcus | 2 |
| Hepatitis | 2 |
| HIV | 2 |
| Pneumococcal | 2 |
| HPV | 1 |
| Cholera | 1 |
| Cytomegalovirus | 1 |
| Herpes simplex | 1 |
| Meningococcal | 1 |
| Polio | 1 |
| Rabies | 1 |
| Respiratory syncytial virus | 1 |
| TT | 1 |
| Multiple | 5 |
The majority of studies were conducted in North America (35) or Europe (15) (Table 1). Of note, only two studies and two reviews were exclusively conducted in (or, in the case of reviews, provided AEFI definitions for studies exclusively conducted in) lower- or lower-middle-income countries [18–21]. Table 2 details these and other key characteristics of each study.
Table 2.
Characteristics of selected studies.
| Study authorand year [reference] | Continent | Study design | Type of vaccine | Primary study outcome | Safety-related outcome (if different from primary outcome) |
|---|---|---|---|---|---|
| Abzug, 2013 [28] | North America | Prospective cohort | Influenza | Safety and immunogenicity of H1N1 vaccine in HIV infected pregnant women | |
| Adedinsewo, 2013 [29] | North America | Retrospective cohort | Influenza | Maternal vaccination impact on prematurity and SGA | |
| Auffret, 2013 [30] | Europe | Prospective cohort | Influenza | Adverse event and vaccine safety of influenza vaccine in pregnant women | |
| Baker, 1988 [31] | North America | Prospective cohort | GBS | Antibody level in immunized pregnancies and in newborns | Maternal adverse effects |
| Baker, 2003 [32] | North America | RCT | GBS | Safety and immunogenicity in pregnant women | Maternal adverse effects |
| Bednarczyk, 2012† [12] | Multiple | Review | Influenza | Safety of influenza immunization for fetus and neonate | |
| Black, 2004 [33] | North America | Retrospective cohort | Influenza | Impact of influenza vaccination on pregnant women and risk of illness and safety in newborns | |
| Cantu, 2013 [34] | North America | Retrospective cohort | Influenza | Association of influenza vaccination with increased risk of adverse pregnancy outcomes | |
| Cavalcanti, 2007 [35] | South America | Before/after | Yellow fever | Effect of yellow fever vaccine on newborn malformation rates | |
| Chambers, 2013 [36] | North America | Prospective cohort | Influenza | Risk and safety of H1N1 vaccines in women exposed during pregnancy | |
| Chavant, 2013 [37] | Europe | Prospective cohort | Influenza | Safety of a/H1N1 vaccination during pregnancy | |
| Christian, 2011 [38] | North America | Prospective cohort | Influenza | Inflammatory response to vaccination in pregnant women | |
| Conlin, 2013 [39] | North America | Retrospective cohort | Influenza | Safety of H1N1 vaccine in Pregnant US military women | |
| Cottin, 2013† [40] | Multiple | Review | Yellow fever | Adverse events from yellow fever vaccine | |
| da Silva, 2011 [41] | South America | Prospective cohort | Rubella | Safety of Rubella vaccine during pregnancy | |
| Dana, 2009 [42] | Multiple | Prospective cohort | HPV | Safety of HPV vaccine during pregnancy-pregnancy outcomes and birth defects | |
| De Vries, 2014 [43] | Europe | Prospective cohort | Influenza | Adverse events of adjuvanted a/H1N1 vaccination during pregnancy | |
| Ergenoglu, 2012 [44] | Europe | Prospective cohort | Rubella | Safety of Rubella vaccine during pregnancy | |
| Harjulehto-Mervaala, 1994 [45] | Europe | Prospective cohort | OPV | Fetal development and perinatal outcome after OPV vaccination during pregnancy | |
| Hashim, 2012* [18] | Africa | Cross-sectional | Cholera | Birth outcomes between exposed and unexposed pregnancies | |
| Heikkinen, 2012 [24] | Multiple | Prospective cohort | Influenza | Influenza vaccine safety in pregnant women and neonates | |
| Huang, 2013 [46] | Asia | Prospective cohort | Rabies | Safety of post-exposure prophylaxis during pregnancy | |
| Kallen, 2012 [47] | Europe | Retrospective cohort (registry data) | Influenza | Pregnancy outcomes post H1N1 vaccination | |
| Kharbanda, 2012 [48] | North America | Prospective cohort | Influenza | Adverse effects from trivalent or monovalent influenza vaccination during pregnancy | |
| Kharbanda, 2013 [49] | North America | Retrospective cohort | Influenza | Adverse events between exposed and unexposed pregnant women, specifically, preterm and small for gestational age births | |
| Launay, 2012 [50] | Europe | Prospective cohort | Influenza | Consequences of maternal vaccination on pregnancy outcomes and maternal seroprotection at delivery | |
| Lehmann, 2003*† [19] | Asia | Review | Pneumococcal | Pneumococcal vaccine safety review | |
| Lin, 2012 [51] | Asia | Retrospective cohort | Influenza | Adverse events after AdimFlu-S vaccination in pregnant women | |
| Lin, 2013 [52] | Asia | Prospective cohort | Influenza | Immune response of the three vaccine viral strains | Incidence of pre-specified adverse events and all serious/non-serious adverse events |
| Louik, 2013 [53] | North America | Prospective cohort | Influenza | Safety of H1N1 vaccine during pregnancy | |
| Ludvigsson, 2013 [54] | Europe | Retrospective cohort | Influenza | Adverse pregnancy outcomes from influenza H1N1 vaccination | |
| Mackenzie, 2012 [55] | Europe | Prospective cohort | Influenza | Adverse events and pregnancy outcome post H1N1 vaccination | |
| Makris, 2012† [56] | Multiple | Review | Multiple | Safety of various maternal vaccines | |
| Moro, 2011 [57] | North America | Retrospective cohort | Influenza (monovalent) | Adverse events of monovalent influenza vaccination during pregnancy | |
| Moro, 2011 [58] | North America | Retrospective cohort | Influenza (trivalent) | Adverse events of influenza vaccine between exposed and unexposed pregnancies | |
| Moro, 2012† [59] | Multiple | Review | Influenza | Safety of influenza vaccines on pregnant women and neonates with emphasis on a/H1N1 monovalent vaccine | |
| Moro, 2013 [60] | North America | Retrospective cohort | Influenza | Maternal and infant outcomes for vaccinated pregnant women | |
| Moro, 2014 [61] | North America | Retrospective cohort | Hepatitis | Vaccine maternal adverse effects | |
| Munoz, 2001 [7] | North America | RCT | Multiple | Safety and immunogenicity of PSV in pregnant women | |
| Munoz, 2003 [62] | North America | RCT | RSV | Safety and immunogenicity of RSV vaccine during pregnancy | |
| Munoz, 2005 [63] | North America | Retrospective cohort | Influenza | Safety of influenza vaccination during pregnancy | |
| Munoz, 2014 [64] | North America | RCT | Tdap | Safety of Tdap vaccine during pregnancy | Infant response to DTaP vaccine |
| Naleway, 2014† [65] | North America | Review | Influenza | Safety of influenza vaccination during pregnancy | |
| Nishioka, 1998 [23] | South America | Case control | Yellow fever | Effect of yellow fever vaccination on spontaneous abortion | |
| Nordin, 2013 [66] | North America | Retrospective cohort | Influenza | Adverse Events after first trimester influenza vaccination | |
| Nordin, 2014 [67] | North America | Retrospective cohort | Influenza | Impact of influenza vaccine on preterm and SGA | |
| Omon, 2011 [68] | Europe | Prospective cohort | Influenza | Safety of non-adjuvanted H1N1 vaccine on pregnant women | |
| Oppermann, 2012 [69] | Europe | Prospective cohort | Influenza | H1N1 vaccine safety in pregnancy | |
| Orenstein, 2012*† [20] | Africa | Review | Multiple | Develop estimates of maternal and neonatal background morbidity and mortality | |
| Pardon, 2011 [70] | South America | Prospective cohort | Rubella | Fetal adverse events after rubella vaccination in pregnant women | |
| Pass, 2009 [71] | North America | RCT | CMV | CMV infection | Vaccine adverse effects and birth outcomes |
| Pasternak, 2012 [22] | Europe | Retrospective cohort (registry data) | Influenza | a/H1N1 vaccination association with major birth defects, preterm birth and fetal growth restriction | |
| Pasternak, 2012 [72] | Europe | Retrospective cohort (registry data) | Influenza | Risk of fetal death and spontaneous abortion from vaccination against a/H1N1 | |
| Pitisuttithum, 2011 [73] | Asia | RCT | HIV | Adverse events related and unrelated to pregnancy | |
| Quiambao, 2007* [21] | Asia | RCT | Pneumococcal | Immunogenicity and antibody transfer after pneumococcal vaccination | |
| Santosham, 2001 [74] | North America | RCT | Multiple | Safety and immunogenicity of Hib vaccines verses pneumococcal | |
| Sato, 2011 [75] | South America | Prospective cohort | Rubella | Fetal adverse events after rubella vaccination in pregnant women | Congenital rubella infection in newborns after exposure to vaccine |
| Shakib, 2013 [76] | North America | Retrospective cohort | Tdap | Safety of Tdap vaccine during pregnancy | |
| Sheffield, 2011 [77] | North America | Prospective cohort | Hepatitis | Feasibility and immunogenicity of an accelerated hepatitis B vaccine schedule in high-risk pregnant women | Maternal adverse effects |
| Sheffield, 2012 [78] | North America | Retrospective cohort | Influenza | First trimester influenza vaccination on neonatal outcomes | |
| Sheffield, 2013† [79] | Multiple | Review | Multiple | Standardized vital signs and laboratory assessments during maternal vaccine trials | |
| Silveira, 1995 [80] | South America | Case control | TT | Safety outcomes of TT in newborns | |
| Suzano, 2006 [81] | South America | Retrospective cohort | Yellow fever | Safety of yellow fever during pregnancy | |
| Talbot, 2010 [82] | North America | Cross-sectional | Tdap | Safety of Tdap less than 2 year after previous tetanus vaccination | |
| Tavares, 2011 [83] | Europe | Prospective cohort | Influenza | Safety outcomes in exposed and unexposed women | |
| Tavares, 2013† [84] | Multiple | Review | Herpes simplex | Risk of spontaneous abortion following HSV vaccination | |
| Thomas, 2012† [85] | Multiple | Review | Yellow fever | Adverse events from yellow fever vaccine in vulnerable populations | |
| Toback, 2012 [86] | North America | Retrospective cohort | Influenza | Safety of LAIV during pregnancy | |
| Tsai, 2010 [87] | Europe | Retrospective cohort | Influenza | Pregnancy outcomes in exposed and unexposed women | |
| Wilson, 2008 [88] | North America | Retrospective cohort (registry data) | Varicella | Outcomes after inadvertent exposure to Varicella vaccine during pregnancy | |
| Wise, 2000 [89] | North America | Retrospective cohort | Varicella | Adverse events from varicella vaccine | |
| Wright, 1999 [90] | North America | RCT | HIV | Safety of rgp120 during pregnancy | |
| Zheteyeva, 2012 [91] | North America | Retrospective cohort | Tdap | Safety of Tdap in pregnant women | |
| Zheteyeva, 2013 [92] | North America | Retrospective cohort | Meningococcal | Safety of meningococcal vaccine in pregnancy | |
Study conducted in lower- or lower-middle-income country.
Systematic review.
3.2. Reported adverse event outcomes
A total of 240 adverse events were reported among all selected publications (Supplemental Table 4). A significant proportion of these described typical pregnancy complications and a variety of congenital abnormalities. The specificity of adverse event reporting varied widely, with some events (e.g., “abnormal pregnancy,” “local reaction”) being vaguely defined or encompassing a range of adverse events. However, all reported AEFI definitions fell naturally within one of a series of AE categories specific to the AE target (mother, fetus, or neonate) (Table 4).
Table 4.
List of unique adverse event definitions provided by selected studies. Only adverse events explicitly defined by study authors (i.e., those studies defining AEFIs beyond simply referring to the coding/classification scheme used) are shown. Definitions with an asterisk are those used in studies conducted in lower-middle-income countries (LMICs).
| AEFI target (mother, neonate, fetus) | AEFI type | AEFI type, detail | AEFI definition, description, or classification reference used [references] |
|---|---|---|---|
| Mother | Local | Local reaction | Pain, redness, or swelling [82] Redness, swelling, induration or pain confirmed by comparison to a 2 cm circle (≥circle records as positive) [21] |
| Systemic | Anaphylaxis | Physician assessment or Brighton definition [57,58] Sudden onset (<3d after vaccination) and rapid progression of symptoms involving multiple systems organ classes: dermatologic, cardiovascular, and respiratory (per Brighton collaboration definition) [40] |
|
| Bell’s palsy | Brighton definition [57,58] | ||
| Fever | “Feeling feverish” and/or temperature measured to be >100.4°F [82] Temperature ≥ 38 °C [51,52] |
||
| Guillain-Barre syndrome | Physician assessment or Brighton definition [57,58] | ||
| Influenza-like illness | Oral temperature > 37.8 °C with at least one influenza-like symptom (cough, sore throat, rhinorrhea, nasal obstruction) [50] | ||
| Mild adverse event | Headache, fever, or myalgia [81] | ||
| Pregnancy-related | Abnormal pregnancy | Ectopic pregnancy/spontaneous abortion/stillborn delivery [87] | |
| Maternal death | Death from direct or indirect obstetric causes during pregnancy or <42 days after pregnancy termination [20]* | ||
| Normal pregnancy | Normal, live-born delivery [87] | ||
| Pre-eclampsia | Pregnancies with an ICD-9-CM-coded diagnosis of preeclampsia (642.4×–642.7×) occurring during pregnancy [39] | ||
| Pregnancy complications | Any or none, based on self-report [75] | ||
| Preterm labor | Pregnancies with an ICD-9-CM-coded diagnosis of threatened premature labor (hereafter referred to as premature labor; 644.0×) or early (spontaneous) onset of delivery occurring during pregnancy with initial diagnosis of either premature labor or premature delivery at least 1 day after pandemic H1N1 or seasonal influenza immunization [39] | ||
| Severe acute maternal morbidity | Direct or indirect obstetric complications that threaten the woman’s survival but do not lead to her death [20]* | ||
| Fetus | In utero | Elective abortion | Induced termination of a pregnancy due to personal choice or medical reasons prior to 20 weeks post-conception day (or 22 weeks of gestation) [83] |
| Fetal death | Spontaneous abortion and stillbirth combined [42] Nonviable conceptus in pregnancies after more than 20 weeks amenorrhea [72] |
||
| Induced abortion | Therapeutic or elective abortion [87] | ||
| Intrauterine fetal death | Fetal death with unknown gestation time [57,58] | ||
| Late fetal death | Fetal death occurring ≥20 w gestation [88] | ||
| Miscarriage/spontaneous abortion | Abortion occurring between start of week 7 and end of week 22 of gestation [22] Any non-deliberate interruption of an intrauterine pregnancy before the 28th week of gestation (since the last menstrual period) in which the fetus is dead when expelled (WHO 1970)[23] Delivery between 14 w and 21 w + 6 days gestation [50] Delivery prior to 20 weeks [34] Fetal death occurring <20 weeks gestation [57–59,61,91,92] Intrauterine death of a fetus under 500 g or of gestational age under 22 weeks’ amenorrhea [37] Intrauterine death of fetus at <24 w gestation [83] Loss before 22 weeks gestation [24] Pregnancy loss before week 22 [43] Pregnancy termination within 20 weeks of conception [18]* Spontaneous loss of conceptus before 20 weeks amenorrhea [42] Spontaneous pregnancy loss at <20 gestational weeks [36] Termination of a pregnancy without human interference prior to 20 weeks post-conception day (or 22 weeks of gestation) [84] Loss of a fetus <500 g or prior to 22 weeks gestation [75] |
||
| Stillbirth | Death at birth [20]* Delivery of a non-viable fetus at or after 20 weeks [34] Delivery of a non-viable fetus at or after 22 completed weeks [72] Fetal death occurring >20 weeks gestation [57,58] Fetal death of ≥500 g [57–60] Fetal demise ≥20 w gestation [91,92] Fetus born after 20 weeks gestation without pulse [18]* Intrauterine death of a fetus over 500 g or of gestational age more than 22 weeks’ amenorrhea [37] Intrauterine death of fetus at ≥24 w gestation [83] Loss of a fetus ≥500 g or at 22 weeks gestation or later [75] Pregnancy loss after week 22 [43] |
||
| Neonate | Congenital abnormality | Gross malformation | Physical defect present in baby at birth, including any abnormality visible on a naked baby (e.g. cleft lip or palate, Down syndrome, spina bifida, limb defects, etc.) [43] |
| “Various” | Classified using CDC and Prevention Metropolitan Atlanta Congenital Defects Program guidelines. All structural–morphological, chromosomal or genetic anomalies were included in this definition, regardless of whether the fetus was delivered dead or alive, and included birth defects identified by prenatal ultrasound, amniocentesis or examination of the products of conception after elective or spontaneous abortion [83] | ||
| Perinatal | Early neonatal death | Death of a liveborn infant within the first week of life [20]* | |
| Low Apgar score | Score < 7 at 5 min [50,54] | ||
| Low birth weight | Birth weight < 2500 g [22,54,75,84] Birth weight < 2500 g measured by study team within 7 days of life or extracted from official birth records [20]* |
||
| Postterm birth | Birth 42 weeks or greater [43] | ||
| Preterm birth | Birth ≤ 36 completed weeks [54] Birth at <37 weeks gestation [75,84] Birth between 22 w and 36 w + 6 days gestation [50] Birth between 32 and 36 weeks [43] Clinical estimate of gestational age at birth of less than 37 completed weeks [29] Estimated gestational age <37 completed weeks as determined by ultrasound, last menstrual period, or validated exam within 7 days of life [20]* Live birth <37 weeks gestation [91,92] |
||
| Small for gestational age | <10th percentile for sex and gestational age in live born infants using standard US growth charts for full and preterm infants (NCHS 2000 growth curves or Lubchenko) [36] <10th percentile of gestational age-specific birth weight within cohort [54,66] Brenner’s standard for fetal growth less than the 10th percentile [34] Children with measurements of weight, length, and head circumference <2 SD than reference values (calculated from full-term infant measurements of combined reference cohorts) [45] Lowest 10th percentile of birth weight for each gestational week [29] Measurements <2 SD from expected weight [47] |
||
| Very low birth weight | Birth weight under 1500 g [42] | ||
| Very preterm birth | Birth between 22 and 31 weeks [43] Birth less than 32 weeks gestation [42] |
||
| Postnatal | Atypical infant behavior | Deviation from normal feeding, crying, defecating, urinating, sleeping, and growing behavior as defined by mother [18]* | |
| Infant death | Live births dying later in infancy [18]* | ||
| Late neonatal death | Death of a liveborn infant between 1 and 4 weeks of life [20]* | ||
| Recurring illness | Illness lasting more than two weeks or occurring twice or more often [18]* | ||
Of the 240 identified adverse events, 100 were specific to the mother, 21 to the fetus, and 119 to the neonate. Seventy-seven of the 119 neonate-specific adverse events were categorized as congenital malformations or abnormalities. Table 3 lists the ten adverse events most frequently reported.
Table 3.
Top ten adverse events most frequently reported in selected studies.
| Adverse event | Number of times reported |
|---|---|
| Miscarriage/spontaneous abortion | 31 |
| Preterm birth | 31 |
| Stillbirth | 25 |
| Fever, maternal | 19 |
| Pre-eclampsia | 14 |
| Site pain | 12 |
| Low birth weight | 12 |
| Elective abortion | 11 |
| Respiratory distress | 11 |
| Small for gestational age | 11 |
3.3. Provision of AEFI definitions or classifications
Of the 74 selected studies, 49 provided one or more case definitions describing a total of 35 AEFIs. Across all studies, a total of 77 “unique” AEFI case definitions (excluding typographical or non-semantically significant variations) were found. Of the 77 unique case definitions, 11 definitions came from studies conducted in low- and lower-middle-income countries. The defined adverse events ranged in specificity, though most tended toward describing a single phenomenon (e.g., pre-eclampsia) rather than a group of related phenomena (e.g., “local reaction”). The most frequently defined AEFIs (stillbirth, miscarriage, preterm birth) were also the most often reported adverse events (Table 3). However, definitions were notably few in number for commonly expected or minor AEFIs such as site pain or fever. Table 4 lists all unique AEFI names and definitions, grouped by individual affected.
A substantial number of included studies (25) did not provide any AEFI definitions, but were included due to the reporting of AEFIs during pregnancy and the newborn period. Many of these studies cited the use of one of a number of adverse event classification systems in lieu of providing definitions (Supplemental Table 3); specific codes for some AEs were provided (Supplemental Table 4). Of note, most of these studies were retrospective analyses drawing data from registries and/or patient records.
3.4. Consistency of AEFI reporting
Of those studies providing AEFI definitions, most (particularly those examining maternal/fetal/neonatal AEFIs as the primary outcome) defined key AEFIs in Section 2 and subsequently reported on each in Section 3 (Supplemental Table 3). In total, 52 studies adhered to this reporting standard; of these studies, 38 reported on AEFIs as a primary outcome.
Studies that monitored or assessed a wide variety of adverse events, such as congenital abnormalities, typically specified a classification system used to detect or categorize adverse events (Supplemental Table 4). Classification systems in typical use included the International Classification of Diseases revision 9 (ICD-9) and the Medical Dictionary for Regulatory Activities (MedDRA). These studies often provided an exhaustive list of detected events in Section 3 without specific definitions.
3.5. Variability in adverse event definitions
Adverse event definitions were found to vary substantially between studies, as shown in Table 4. Specifically, definitions for key maternal/neonatal AEFIs differed on four attributes: overall level of detail, physiological and temporal boundaries and cut-offs, severity strata, and standards used.
3.5.1. Level of detail
Definitions for key AEFIs varied widely in terms of terminology and level of detail. For example, Pasternak et al., 2012 defines miscarriage/spontaneous abortion as “abortion occurring between” certain weeks of gestation [22]; in contrast, Nishioka et al. [23] cite the WHO 1970 definition, which defines the same event as “any non-deliberate interruption of an intrauterine pregnancy before the 28th week of gestation in which the fetus is dead when expelled”. Other definitions use similar, but not identical, terminology, such as “pregnancy loss,” “fetal death,” and “intrauterine death.”
3.5.2. Boundaries and cut-offs
Criteria for many defined AEFIs stipulate that a temporal or physiological threshold must be met or exceeded, such as fever being defined as temperature at or above 38 degrees Celsius. Here, study definitions revealed both stark and nuanced differences that may result in unexpected disagreements on AEFI classification across studies. This is best demonstrated by examining included studies’ gestational age cut-offs for miscarriage versus stillbirth. These cut-offs are defined using language that appears fairly homogenous (Table 4); however, upon close examination, substantial variations in gestational age thresholds become evident (Fig. 2a and b). One key issue is the fact that classification of miscarriage versus stillbirth will depend on whether the stated cut-off is inclusive or exclusive of (i.e., greater than versus greater than/equal to) the given gestational week. Such nuances in detail, both regarding these cut-offs as well as other factors (for example, some definitions also establish different criteria for when gestation actually begins) may introduce further uncertainty into the classification of fetal death.
Fig. 2.
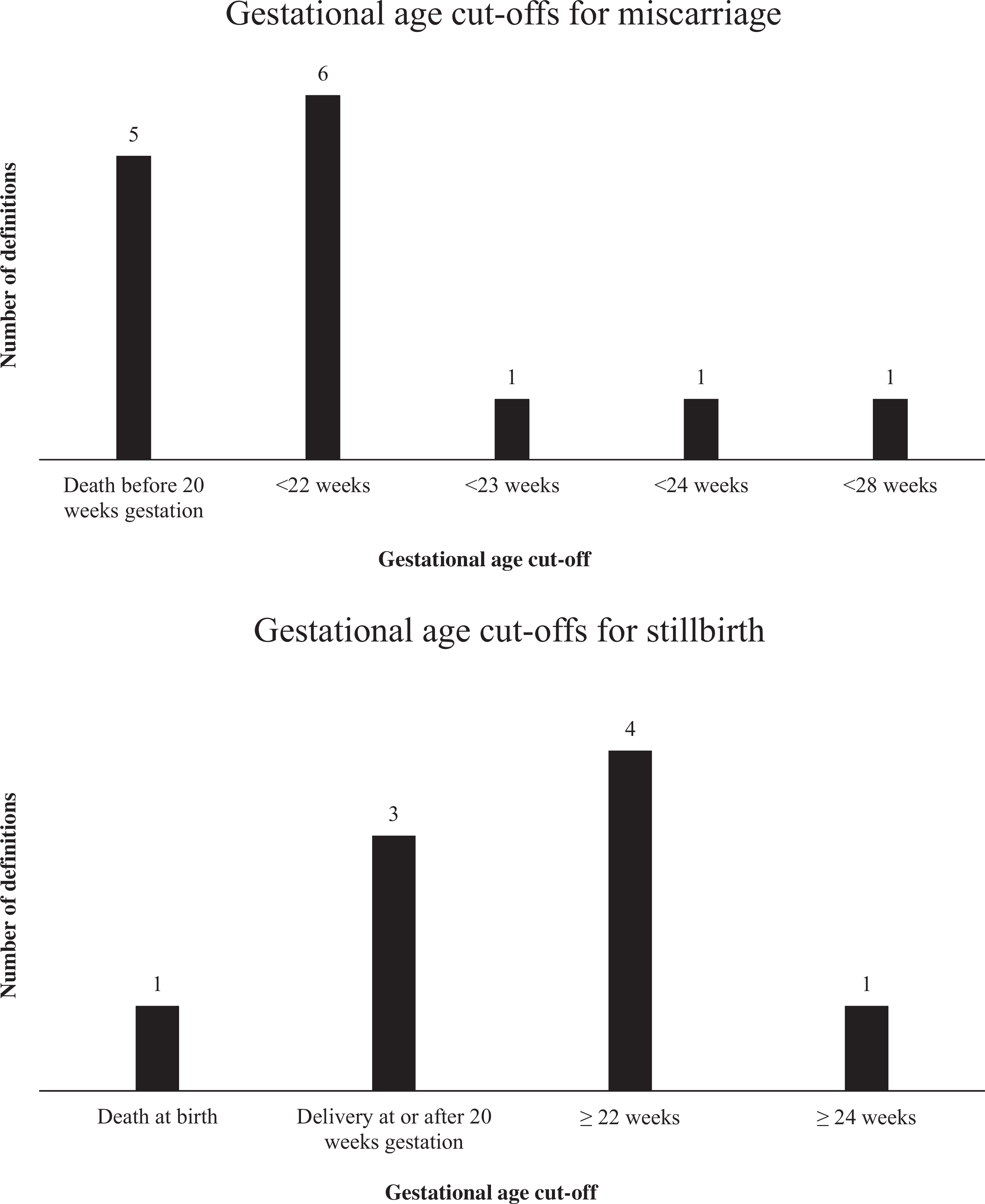
Gestational age cut-offs for (a) miscarriage/spontaneous abortion and (b) stillbirth.
In addition, some stillbirth and miscarriage definitions also included fetal mass criteria. This may raise the question of how to classify a fetal death should it (for example) meet gestational age, but not mass threshold, for a given definition.
3.5.3. Severity strata
Eighteen papers in our review specified adverse event strata. For example, the Heikkinen et al., 2012 definition of stillbirth differentiated between “early fetal loss” as fetal death before 22 weeks gestation and “late fetal loss” as death between 22 and 28 weeks [24]. Other papers provided strata for preterm birth, low birth weight, and other AEFIs; these definitions varied on criteria, thresholds, and number of strata for a given AEFI (Supplemental Table 2).
3.5.4. Standards used
Small for gestational age definitions exhibited variation in the use of fetal measurement standards. For example, Chambers et al. [36] and Cantu et al. [34] utilize Brenner and Lubchenco curves, respectively.
3.6. Defining an “Adverse Event”
In addition to the variability in definition provision and AEFI reporting, studies demonstrated substantial variation in what comprises an “adverse event”. Twenty-one studies offered explicit definitions of the term “adverse event” (Table 5). Among these definitions, six distinct variants of the term “adverse event” were described, e.g., “adverse obstetric event,” “adverse event of special interest,” etc. These definitions range broadly in specificity and, among definitions describing the same term, demonstrate little agreement even relative to that exhibited by more specific AEFI definitions. Table 5 summarizes all definitions provided for the term “adverse event” and variants used in selected studies.
Table 5.
List of definitions of the term “adverse event” and variations, as detailed in selected studies.
| Term | Definition [reference] |
|---|---|
| Adverse event | Any undesired, noxious or pathological change in participants as indicated by physical signs, symptoms, and/or laboratory changes that occurred following administration of one of the vaccines, whether or not considered vaccine-related (includes intercurrent illnesses or injuries and unexpected exacerbations of pre-existing conditions) [73] Any untoward medical occurrence in a patient or clinical investigation subject administered a pharmaceutical product regardless of its causal relationship to the study treatment. An AE can therefore be “any unfavorable and unintended sign(s), symptoms(s) or condition temporally related with the use of the investigational product” [77] |
| Adverse obstetric event | New, prespecified, medically attended pregnancy-related comorbidities or pregnancy complications [48] |
| Medically significant adverse event | Requiring two or more visits to a physician for the same condition or that resulted in hospitalization or an ER visit [73] |
| Adverse event of special interest | Any event considered as worthy of closer follow-up as described in recommendations for the Pharmacovigilance Plan following the administration of H1N1 pandemic vaccines [83] |
| Medically-attended adverse event | Event leading to an otherwise unscheduled visit to or from medical personnel for any reason, including visits to an accident and emergency department [83] |
| Neonatal adverse event | Visits with prespecified ICD-9 codes that occur from birth through 30 days old [49] |
| Serious adverse event | Defined per FDA guidelines [71] Any untoward medical occurrence that: resulted in death, was life-threatening, required hospitalization or prolongation of existing hospitalization, resulted in disability/incapacity, or was a congenital anomaly/birth defect in the child of a study participant [78] Reports containing information that the AE resulted in death, hospitalization, prolongation of hospitalization, life-threatening illness, persistent or significant disability, or congenital anomalies. Definition modified to exclude reports on hospitalizations for delivery unless they required prolonged stay in a hospital due to delivery complications or postpartum conditions [90] AEs resulting in or prolonging hospital admission, is life-threatening, fatal or resulting in significant or persisting disability [55] AEs including death, hospitalization, and cause for hospitalization and permanently disabling conditions [64] Any adverse experience any time after vaccination that resulted in any of the following outcomes: death, a life-threatening adverse experience, inpatient hospitalization or prolongation of existing hospitalization or a persistent or significant disability/incapacity, a congenital anomaly, or an important medical event that, based upon medical judgment, may have required medical or surgical intervention to prevent one of those outcomes [82] Deaths, life-threatening events, hospitalizations, persistent or significant disabilities, congenital anomalies, and other events of medical importance (FDA regulations) [89] Any event leading to hospitalization, prolonging hospitalization, entailing permanent handicap or disability, and life-threatening or fatal condition (per French public health code) [37] VAERS Report classified as serious if resulting in death, life-threatening illness, hospitalization or prolongation of hospitalization, permanent disability, or congenital anomaly [57–60] |
4. Discussion
Our study revealed that three major forms of adverse variability currently exist among published maternal immunization studies, including variability in: AEFI definitions, AEFI reporting, and defining what constitutes an adverse event. Each of these can be mitigated by the future adoption of standardized definitions, which should be of sufficient detail and consistency of language to avoid ambiguity. Specifically, definitions should be consistent in defining key terms, diagnostic methodology, cut-offs, thresholds, severity strata, and physiological or other evaluative criteria. The high frequency with which we found certain AEFIs to be defined and reported (stillbirth, miscarriage, preterm birth, etc.) may also inform the priorities of standardization efforts.
Importantly, investigators may proactively work to eliminate between-study variability through clear and consistent reporting of AEFI definitions and outcomes in future publications. We observed that nearly all papers in our review that examined AEFIs as a primary study outcome provided definitions for each AEFI in Section 2. In addition, these investigators took care in Section 3 to report on each AEFI previously defined, whether or not they were detected. Although significant heterogeneity was still evident across the AEFI definitions provided in these papers, the consistency of this definition-and-reporting approach helped considerably to minimize confusion in reporting of outcomes, compared to studies that provided no definitions (or that only provided definitions parenthetically in Section 3).
We therefore recommend that case definitions for all monitored AEFIs in maternal immunization studies be explicitly provided in Section 2 of the study report or an online supplement, with the incidence of each defined adverse event reported on in Section 3 or an online supplement. If a classification system is used, it should be cited in Section 2. To further reduce ambiguity in comparing data, we recommend that classification codes be provided in the results tables for each detected adverse event. These recommendations are summarized in Table 6.
Table 6.
Summary of recommendations for future efforts in standardization of AEFI definitions.
| Recommendation 1. Case definitions should be of sufficient detail and consistency of language to avoid ambiguity with respect to: | - Cut-offs/thresholds defining related AEs - Severity strata - Physiological or other evaluative criteria - Diagnostic methodology - Definition of key terms (e.g., gestational age as weeks of amenorrhea or as weeks post-conception) |
| Recommendation 2. Effort should be made to encourage the following qualities: | - Consistent reporting in Section 2 of AE definitions used or specific classification schemes/codes (e.g., ICD-9 codes) in all studies - Consistent reporting in results of all AEs defined |
| Recommendation 3. Explore the use of frequency analysis to inform standardization efforts where multiple case definitions for a given AE are available | |
| Recommendation 4. Prioritize appropriate standardization for future studies over “back-compatibility” with definitions in previous studies, given the substantial variability in existing definitions | |
Special attention must be paid to AEFI definitions used in studies conducted in low- and low-middle-income countries. It may be reasoned that AEFI definitions need to take into account factors such as the relative lack of diagnostic capacity, high patient load, etc. that may be present in some low-resource settings, which may imply a preference for less-specific AEFI definitions compared to other contexts. An example would be a requirement to confirm preterm birth via early-pregnancy ultrasound in a setting with limited access to sophisticated diagnostic equipment.
Due to the very small number of studies conducted in low- and low-middle-income (LMIC) countries in our review, we are limited in our ability to assess meaningful differences in AEFI definitions in these contexts. Some of the unique AEFI definitions provided in our LMIC studies appear to reflect less interest in the specifics of an adverse event, and more interest in the sensitive detection of any abnormal event (e.g., “atypical infant behavior” and “severe acute maternal morbidity,” two catch-all definitions unique to LMIC studies). We do not, however, recommend that rigor in AEFI definitions should be relaxed for studies in LMIC contexts. Rather, considerations specific to low-resource countries should be given careful thought in the process of developing standard AEFI definitions.
Our study has additional limitations. We searched for articles via four major literature databases, but we did not hand-search individual journals or gray literature. Maternal immunization studies that reported no safety outcomes may nevertheless have monitored for AEFIs using established case definitions; however, these studies were not included in our review. Finally, six older (pre-2000) articles were excluded due to inability to access the full papers, and five non-English candidate articles were not included in the final review due to lack of translation capacity (Fig. 1).
Efforts to standardize maternal immunization AEFI definitions are ongoing, with active efforts underway by WHO, the Brighton Collaboration, and other contributors [25–27]. Our review has provided a more complete picture of the landscape of maternal AEFI definitions currently in use, to help inform how best to approach standardization. One of the principal standardization efforts, the Global Alignment of Immunization Safety Assessment in Pregnancy (GAIA), is a WHO/Brighton Collaboration joint project aiming to provide standards and tools to establish a globally shared understanding of outcomes and approaches to monitoring them, with specific focus on LMIC needs and requirements.
Future standardized AEFI definitions developed through such initiatives will help ensure consistency of data and facilitate data comparisons and pooling; however, they should be accompanied by consistency of reporting. We believe that this is achievable, and that improving adverse event reporting practices is a first step that should be taken without hesitation. In view of a swiftly expanding global maternal immunization agenda, we have no reason to delay.
Supplementary Material
Acknowledgements
T. Roice Fulton (the corresponding author) had access to all of the data in the study, was responsible for study design, data abstraction and manuscript writing, and assumes full responsibility for data integrity and accuracy of analysis. Saad B. Omer had final responsibility for the decision to submit for publication. Contributors: Divya Narayanan was responsible for data abstraction and manuscript writing. Saad B. Omer, Justin Ortiz, Philipp Lambach, and Jan Bonhoeffer were involved in study design, data interpretation, and manuscript writing. All authors have approved the final version for submission.
Funding
This study was supported by a grant from the World Health Organization Initiative for Vaccine Research, award no. 61338.
Footnotes
Conflict of interest statement
None.
Disclaimer
The findings and conclusions in this report are those of the authors. The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the decisions or policies of the World Health Organization.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vaccine.2015.08.043.
References
- [1].WHO. WHO fact sheet no. 178: children: reducing mortality; 2014. Available at: (http://www.who.int/mediacentre/factsheets/fs178/en/) (accessed 20 April 2015).
- [2].WHO. Millennium development goal 4: reduce child mortality; 2015. Available at: (accessed 20 April 2015).
- [3].United Nations. Millennium declaration; 2000. Available at: (http://www.un.org/millennium/declaration/ares552e.htm) (accessed August 1 2015).
- [4].WHO. WHO fact sheet no. 333: newborns: reducing mortality; 2012. Available at: (http://www.who.int/mediacentre/factsheets/fs333/en/) (accessed 20 April 2015).
- [5].Jackson LA, Patel SM, Swamy GK, et al. Immunogenicity of an inactivated monovalent 2009 H1N1 influenza vaccine in pregnant women. J Infect Dis 2011;204:854–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Madhi SA, Cutland CL, Kuwanda L, et al. Influenza vaccination of pregnant women and protection of their infants. N Engl J Med 2014;371:918–31. [DOI] [PubMed] [Google Scholar]
- [7].Munoz FM, Bond NH, Maccato M, et al. Safety and immunogenicity of tetanus diphtheria and acellular pertussis (Tdap) immunization during pregnancy in mothers and infants: a randomized clinical trial. JAMA: J Am Med Assoc 2014;311:1760–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ortiz JR, Englund JA, Neuzil KM. Influenza vaccine for pregnant women in resource-constrained countries: a review of the evidence to inform policy decisions. Vaccine 2011;29:4439–52. [DOI] [PubMed] [Google Scholar]
- [9].Meeting of the Strategic Advisory Group of Experts on immunization, April 2013—conclusions and recommendations (Geneva: ). World Health Organization Weekly Epidemiological Record: 17 May 2013, vol. 88, 20 (pp. 201–216). Available at http://www.who.int/wer/2013/wer8820/en/ (accessed August 1 2015). [Google Scholar]
- [10].Maternal Influenza Immunization Project. Maternal influenza immunization project: building an immunization platform in conjunction with antenatal care in low and middle-income, countries; 2014. Available at:(http://www.who.int/immunization/research/development/influenzamaternalimmunization/en/} (accessed 22 October)
- [11].Gruber MF. Global and national initiatives to facilitate studies of vaccines in pregnant women. Clin Infect Dis 2014;59(Suppl. 7):S395–9(an official publication of the Infectious Diseases Society of America).
- [12].Bednarczyk RA, Adjaye-Gbewonyo D, Omer SB. Safety of influenza immunization during pregnancy for the fetus and the neonate. Am J Obstet Gynecol 2012;207:S38–46. [DOI] [PubMed] [Google Scholar]
- [13].Keller-Stanislawski B, Englund JA, Kang G, et al. Safety of immunization during pregnancy: a review of the evidence of selected inactivated and live attenuated vaccines. Vaccine 2014;32:7057–64. [DOI] [PubMed] [Google Scholar]
- [14].Fell DB, Dodds L, MacDonald NE, Allen VM, McNeil S. Influenza vaccination and fetal and neonatal outcomes. Expert Rev Vaccines 2013;12:1417–30. [DOI] [PubMed] [Google Scholar]
- [15].Bratton KN,Wardle MT, Orenstein WA, Omer SB. Maternal influenza immunization and birth outcomes of stillbirt and spontaneous abortion: a systematic review and meta-analysis.Clin Infect Dis 2015;60:e11–9(an official publication of the Infectious Diseases Society of America).
- [16].McMillan M, Porritt K, Kralik D, Costi L, Marshall H. Influenza vaccination during pregnancy: a systematic review of fetal death, spontaneous abortion, and congenital malformation safety outcomes. Vaccine 2015;33:2108–17. [DOI] [PubMed] [Google Scholar]
- [17].Bonhoeffer J, Zumbrunn B, Heininger U. Reporting of vaccine safety data in publications: systematic review. Pharmacoepidemiol Drug Saf 2005;14: 101–6. [DOI] [PubMed] [Google Scholar]
- [18].Hashim R, Khatib AM, Enwere G, et al. Safety of the recombinant cholera toxin B subunit, killed whole-cell (rBS-WC) oral cholera vaccine in pregnancy. PLoS Neglect Trop Dis 2012;6:e1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lehmann D, Pomat WS, Riley ID, Alpers MP. Studies of maternal immunisation with pneumococcal polysaccharide vaccine in Papua New Guinea. Vaccine 2003;21:3446–50. [DOI] [PubMed] [Google Scholar]
- [20].Orenstein LAV, Orenstein EW, Teguete I, et al. Background rates of adverse pregnancy outcomes for assessing the safety of maternal vaccine trials in sub-Saharan Africa. PLoS ONE 2012;7(10):e46638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Quiambao BP, Nohynek HM, Kayhty H, et al. Immunogenicity and reactogenicity of 23-valent pneumococcal polysaccharide vaccine among pregnant Filipino women and placental transfer of antibodies. Vaccine 2007;25:4470–7. [DOI] [PubMed] [Google Scholar]
- [22].Pasternak B, Svanstrom H, Molgaard-Nielsen D, et al. Risk of adverse fetal outcomes following administration of a pandemic influenza A(H1N1) vaccine during pregnancy. JAMA: J Am Med Assoc 2012;308:165–74. [DOI] [PubMed] [Google Scholar]
- [23].Nishioka Sde A, Nunes-Araujo FR, Pires WP, Silva FA, Costa HL. Yellow fever vaccination during pregnancy and spontaneous abortion: a case-control study. Trop Med Int Health: TM & IH 1998;3:29–33. [DOI] [PubMed] [Google Scholar]
- [24].Heikkinen T, Young J, van Beek E, et al. Safety of MF59-adjuvanted A/H1N1 influenza vaccine in pregnancy: a comparative cohort study. Am J Obstet Gynecol 2012;207:177 e1–e1778. [DOI] [PubMed] [Google Scholar]
- [25].WHO.WHO meeting to develop Brighton collaboration definitions of key terms used for monitoring the safety of immunization in pregnancy in mothers and newborn, children; 2014. Available at: (http://www.who.int/immunization/research/meetings_workshops/maternal_immunization_aefi_july14/en/} (accessed 20 April 2015).
- [26].Munoz FM, Weisman LE, Read JS, et al. Assessment of safety in newborns of mothers participating in clinical trials of vaccines administered during pregnancy. Clin Infect Dis 2014;59(Suppl7):S415–27(an official publication of the Infectious Diseases Society of America).
- [27].GAIA. Global alignment of immunization safety assessment in pregnancy; 2014.Available at: (https://brightoncollaboration.org/public/what-we-do/Projects/Gaia.html} (accessed 20 April 2015).
- [28].Abzug MJ, Nachman SA, Muresan P, et al. Safety and immunogenicity of 2009 pH1N1 vaccination in HIV-infected pregnant women. Clin Infect Dis 2013;56:1488–97(an official publication of the Infectious Diseases Society of America).
- [29].Adedinsewo DA, Noory L, Bednarczyk RA, et al. Impact of maternal characteristics on the effect of maternal influenza vaccination on fetal outcomes. Vaccine 2013;31:5827–33. [DOI] [PubMed] [Google Scholar]
- [30].Auffret M, Bene J, Gautier S, Moreau-Crepeaux S, Caron J. Pharmacovigilance monitoring of a cohort of pregnant women vaccinated against influenza A(H1N1)variant virus in the Nord-Pas de Calais region of northern France. Eur J Obst Gynecol Reprod Biol 2013;170:114–8. [DOI] [PubMed] [Google Scholar]
- [31].Baker CJ, Rench MA, Edwards MS, Carpenter RJ, Hays BM, Kasper DL. Immunization of pregnant women with a polysaccharide vaccine of group B streptococcus. N Engl J Med 1988;319:1180–5. [DOI] [PubMed] [Google Scholar]
- [32].Baker CJ, Rench MA, McInnes P. Immunization of pregnant women with group B streptococcal type III capsular polysaccharide-tetanus toxoid conjugate vaccine. Vaccine 2003;21:3468–72. [DOI] [PubMed] [Google Scholar]
- [33].Black SB, Shinefield HR, France EK, Fireman BH, Platt ST, Shay D. Effectiveness of influenza vaccine during pregnancy in preventing hospitalizations and out patient visits for respiratory illness in pregnant women and their infants. Am J Perinatol 2004;21:333–9. [DOI] [PubMed] [Google Scholar]
- [34].Cantu J, Biggio J, Jauk V, Wetta L, Andrews W, Tita A. Selective uptake of influenza vaccine and pregnancy outcomes J Maternal-fetal Neonat Med 2013;26:1207–11(the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet).
- [35].Cavalcanti DP, Salomao MA, Lopez-Camelo J, Pessoto MA. Early exposure to yellow fever vaccine during pregnancy. Trop Med Int Health: TM & IH 2007;12:833–7. [DOI] [PubMed] [Google Scholar]
- [36].Chambers CD, Johnson D, Xu R, et al. Risks and safety of pandemic H1N1 influenza vaccine in pregnancy: birth defects, spontaneous abortion, preterm delivery, and small for gestational age infants. Vaccine 2013:31:5026–32. [DOI] [PubMed] [Google Scholar]
- [37].Chavant F, Ingrand I, Jonville-Bera AP, et al. The PREGVAXGRIP study: a cohort study to assess foetal and neonatal consequences of in utero exposure to vaccination against A(H1N1)v2009 influenza. Drug Saf 2013;36:455–65(an international journal of medical toxicology and drug experience).
- [38].Christian LM, Iams JD, Porter K, Glaser R. Inflammatory responses to trivalent influenza virus vaccine among pregnant women. Vaccine 2011;29:8982–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Conlin AM, Bukowinski AT, Sevick CJ, DeScisciolo C, Crum-Cianflone NF. Safety of the pandemic H1N1 influenza vaccine among pregnant U.S. military women and their newborns. Obstet Gynecol 2013;121:511–8. [DOI] [PubMed] [Google Scholar]
- [40].Cottin P, Niedrig M, Domingo C. Safety profile of the yellow fever vaccine Stamaril(registered trademark): a 17-year review. Expert Rev Vaccines 2013:12:1351–68. [DOI] [PubMed] [Google Scholar]
- [41].da Silva e Sa GR, Camacho LA, Stavola MS, Lemos XR, Basilio de Oliveira CA, Siqueira MM. Pregnancy outcomes following rubella vaccination: a prospective study in the state of Rio de Janeiro, Brazil, 2001–2002. J lnfect Dis 2011;204(Suppl2):S722–8. [DOI] [PubMed] [Google Scholar]
- [42].Dana A, Buchanan KM, Goss MA, et al. Pregnancy outcomes from the pregnancy registry of a human papillomavirus type 6/11/16/18 vaccine. Obstet Gynecol 2009:114:1170–8. [DOI] [PubMed] [Google Scholar]
- [43].De Vries L, van Hunsel F, Cuppers-Maarschalkerweerd B, van Puijenbroek E, van Grootheest K. Adjuvanted A/H1N1 (2009) influenza vaccination during pregnancy: description of a prospective cohort and spontaneously reported pregnancy-related adverse reactions in the Netherlands. Birth Defects Res, A-Clin Mol Teratol 2014;100(10):731–8. [DOI] [PubMed] [Google Scholar]
- [44].Ergenoglu AM, Yeniel AO, Yildirim N, Kazandi M, Akercan F, Sagol S. Rubella vaccination during the preconception period or in pregnancy and perinatal and fetal outcomes. Turkish J Pediatr 2012:54:230–3. [PubMed] [Google Scholar]
- [45].Harjulehto-Mervaala T, Aro T, Hiilesmaa VK, Hovi T, Saxen H, Saxen L. Oral polio vaccination during pregnancy: lack of impact on fetal development and perinatal outcome Clin Infect Dis 1994;18:414–20(an official publication of the Infectious Diseases Society of America).
- [46].Huang G, Liu H, Cao Q, Liu B, Pan H, Fu C. Safety of post-exposure rabies prophylaxis during pregnancy: a follow-up study from Guangzhou, China. Hum Vaccines lmmunother 2013:9:177–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kallen B, Olausson PO. Vaccination against H1N1 influenza with Pandemrix((R)) during pregnancy and delivery outcome: a Swedish register study. BJOG 2012:119:1583–90(an international journal of obstetrics and gynaecology).
- [48].Kharbanda EO, Vazquez-Benitez G, Lipkind H, Naleway A, Lee G, Nordin JD. Inactivated influenza vaccine during pregnancy and risks for adverse obstetric events. Obstet Gynecol 2013;122:659–67. [DOI] [PubMed] [Google Scholar]
- [49].Kharbanda EO, Vazquez-Benitez G, Shi WX, et al. Assessing the safety of influenza immunization during pregnancy: the vaccine safety datalink. Am JObstet Gynecol 2012;207:S47–51. [DOI] [PubMed] [Google Scholar]
- [50].Launay O, Krivine A, Charlier C, et al. Low rate of pandemic A/H1N1 2009 influenza infection and lack of severe complication of vaccination in pregnant women: a prospective cohort study. PLoS ONE 2012;7:e52303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lin SY, Wu ET, Lin CH, Shyu MK, Lee CN. The safety and immunogenicity of trivalent inactivated influenza vaccination: a study of maternal-cord blood pairs in Taiwan. PLoS ONE 2013;8(6):e62983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Lin TH, Lin SY, Lin CH, et al. AdimFlu-S((R)) influenza A (H1N1) vaccine during pregnancy: the Taiwanese Pharmacovigilance Survey. Vaccine 2012:30:2671–5. [DOI] [PubMed] [Google Scholar]
- [53].Louik C, Ahrens K, Kerr S, et al. Risks and safety of pandemic H1N1 influenza vaccine in pregnancy: exposure prevalence, preterm delivery, and specific birth defects. Vaccine 2013;31:5033–40. [DOI] [PubMed] [Google Scholar]
- [54].Ludvigsson JF, Zugna D, Cnattingius S, et al. Influenza H1N1 vaccination and adverse pregnancy outcome. Eur J Epidemiol 2013;28:579–88. [DOI] [PubMed] [Google Scholar]
- [55].Mackenzie IS, MacDonal TM, Shakir S, et al. Influenza H1N1 (swine flu) vaccination: a safety surveillance feasibility study using self-reporting of serious adverse events and pregnancy outcomes. Br J Clin Pharmacol 2012;73:801–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Makris MC, Polyzos KA, Mavros MN, Athanasiou S, Rafailidis PI, Falagas ME. Safety of hepatitis B, pneumococcal polysaccharide and meningococcal polysaccharide vaccines in pregnancy: a systematic review. Drug Saf 2012:35:1–14(an international journal of medical toxicology and drug experience).
- [57].Moro PL, Broder K, Zheteyeva Y, et al. Adverse events following administration to pregnant women of influenza A (H1N1)2009 monovalent vaccine reported to the vaccine adverse event reporting system. Am J Obstet Gynecol 2011;205:473 e1–e4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Moro PL, Broder K, Zheteyeva Y, et al. Adverse events in pregnant women following administration of trivalent inactivated influenza vaccine and live attenuated influenza vaccine in the vaccine adverse event reporting system, 1990–2009. Am J Obstet Gynecol 2011;204:146 e1–e1467. [DOI] [PubMed] [Google Scholar]
- [59].Moro PL, Museru OI, Broder K, et al. Safety of influenza A (H1N1) 2009 live attenuated monovalent vaccin in pregnant women. Obstet Gynecol 2013;122:1271–8. [DOI] [PubMed] [Google Scholar]
- [60].Moro PL, Museru OI, Niu M, Lewis P, Broder K. Reports to the vaccine adverse event reporting system after hepatitis A and hepatitis AB vaccines in pregnant women. Am J Obstet Gynecol 2014;210:561 e1–e5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Moro PL, Tepper NK, Grohskopf LA, Vellozzi C, Broder K. Safety of seasonal influenza and influenza A (H1N1) 2009 monovalent vaccines in pregnancy. Expert Rev Vaccines 2012;11:911–21. [DOI] [PubMed] [Google Scholar]
- [62].Munoz FM, Englund JA, Cheesman CC, et al. Maternal immunization with pneumococcal polysaccharide vaccine in the third trimester of gestation. Vaccine 2001;20:826–37. [DOI] [PubMed] [Google Scholar]
- [63].Munoz FM, Greisinger AJ, Wehmanen OA, et al. Safety of influenza vaccination during pregnancy. Am J Obstet Gynecol 2005;192:1098–106. [DOI] [PubMed] [Google Scholar]
- [64].Munoz FM, Piedra PA, Glezen WP. Safety and immunogenicity of respiratory syncytial virus purified fusion protein-2 vaccine in pregnant women. Vaccine 2003;21:3465–7. [DOI] [PubMed] [Google Scholar]
- [65].Naleway AL, Irving SA, Henninger ML, et al. Safety of influenza vaccination during pregnancy: a review of subsequent maternal obstetric events and findings from two recent cohort studies. Vaccine 2014;32:3122–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Nordin JD, Kharbanda EO, Benitez GV, et al. Maternal safety of trivalent inactivated influenza vaccine in pregnant women. Obstet Gynecol 2013;121:519–25. [DOI] [PubMed] [Google Scholar]
- [67].Nordin JD, Kharbanda EO, Vazquez Benitez G, Lipkind H, Vellozzi C, Destefano F. Maternal influenza vaccine and risks for preterm or small for gestational age birth. J Pediatr 2014;164:1051–700. [DOI] [PubMed] [Google Scholar]
- [68].Omon E, Damase-Michel C, Hurault-Delarue C, et al. Non-adjuvanted 2009 influenza A (H1N1)v vaccine in pregnant women: the results of a French prospective descriptive study. Vaccine 2011;29:9649–54. [DOI] [PubMed] [Google Scholar]
- [69].Oppermann M, Fritzsche J, Weber-Schoendorfer C, et al. A(H1N1)v2009:a controlled observational prospective cohort study on vaccine safety in pregnancy. Vaccine 2012;30:4445–52. [DOI] [PubMed] [Google Scholar]
- [70].Pardon F, Vilarino M, Barbero P, et al. Rubella vaccination of unknowingly pregnant women during 2006 mass campaign in Argentina. J Infect Dis 2011;204(Suppl2):S745–7. [DOI] [PubMed] [Google Scholar]
- [71].Pass RF, Zhang C, Evans A, et al. Vaccine prevention of maternal cytomegalovirus infection. N Engl J Med 2009;360:1191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Pasternak B, Svanstrom H, Molgaard-Nielsen D, et al. Vaccination against pandemic A/H1N1 2009 influenza in pregnancy and risk of fetal death: cohort study in Denmark. BMJ (Clin Res Ed.) 2012;344:e2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Pitisuttithum P, Rerks-Ngarm S, Bussaratid V, et al. Safety and reactogenicity of canarypox ALVAC-HIV (vCP1521) and HIV-1 gp120 AIDSVAX B/E vaccination in an efficacy trial in Thailand. PLoS ONE 2011;6:e27837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Santosham M, Englund JA, McInnes P, et al. Safety and antibody persistence following Haemophilus influenzae type b conjugate or pneumococcal polysaccharide vaccines given before pregnancy in women of childbearing age and their infants. Pediatr Infect Dis J 2001;20:931–40. [DOI] [PubMed] [Google Scholar]
- [75].Sato HK, Sanajotta AT, Moraes JC, et al. Rubella vaccination of unknowingly pregnant women: the Sao Paulo experience, 2001. J Infect Dis 2011;204(Suppl2):S737–44. [DOI] [PubMed] [Google Scholar]
- [76].Shakib JH, Korgenski K, Sheng X, Varner MW, Pavia AT, Byington CL. Tetanus, diphtheria, acellular pertussis vaccine during pregnancy: pregnancy and infant health outcomes. J Pediatr 2013;163:1422–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Sheffield JS, Greer LG, Rogers VL, et al. Effect of influenza vaccination in the first trimester of pregnancy. Obstet Gynecol 2012;120:532–7. [DOI] [PubMed] [Google Scholar]
- [78].Sheffield JS, Hickman A, Tang J, et al. Efficacy of an accelerated hepatitis B vaccination program during pregnancy. Obstet Gynecol 2011;117:1130–5. [DOI] [PubMed] [Google Scholar]
- [79].Sheffield JS, Munoz FM, Beigi RH, et al. Research on vaccines during pregnancy: reference values for vital signs and laboratory assessments. Vaccine 2013;31:4264–73. [DOI] [PubMed] [Google Scholar]
- [80].Silveira CM, Caceres VM, Dutra MG, Lopes-Camelo J, Castilla EE. Safety of tetanus toxoid in pregnant women: a hospital-based case-control study of congenital anomalies. Bull World Health Org 1995;73:605–8. [PMC free article] [PubMed] [Google Scholar]
- [81].Suzano CE, Amaral E, Sato HK, Papaiordanou PM. The effects of yellow fever immunization (17DD) inadvertently used in early pregnancy during a mass campaign in Brazil. Vaccine 2006;24:1421–6. [DOI] [PubMed] [Google Scholar]
- [82].Talbot EA, Brown KH, Kirkland KB, Baughman AL, Halperin SA, Broder KR. The safety of immunizing with tetanus-diphtheria-acellular pertussis vaccine (Tdap) less than 2 years following previous tetanus vaccination: experience during a mass vaccination campaign of healthcare personnel during a respiratory illness outbreak. Vaccine 2010;28:8001–7. [DOI] [PubMed] [Google Scholar]
- [83].Tavares F, Cheuvart B, Heineman T, Arellano F, Dubin G. Meta-analysis of pregnancy outcomes in pooled randomized trials on a prophylactic adjuvanted glycoprotein D subunit herpes simplex virus vaccine. Vaccine 2013;31:1759–64. [DOI] [PubMed] [Google Scholar]
- [84].Tavares F, Nazareth I, Monegal JS, Kolte I, Verstraeten T, Bauchau V. Pregnancy and safety outcomes in women vaccinated with an AS03-adjuvanted split virion H1N1 (2009) pandemic influenza vaccine during pregnancy: a prospective cohort study. Vaccine 2011;29:6358–65. [DOI] [PubMed] [Google Scholar]
- [85].Thomas RE, Lorenzetti DL, Spragins W, Jackson D, Williamson T. The safety of yellow fever vaccine 17D or 17DD in children, pregnant women, HIV+ individuals, and older persons: systematic review. Am J Trop Med Hygiene 2012;86:359–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Toback SL, Beigi R, Tennis P, Sifakis F, Calingaert B, Ambrose CS. Maternal outcomes among pregnant women receiving live attenuated influenza vaccine. Influenza Other Respir Viruses 2012;6:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Tsai T, Kyaw MH, Novicki D, Nacci P, Rai S, Clemens R. Exposure to MF59-adjuvanted influenza vaccines during pregnancy—a retrospective analysis. Vaccine 2010;28:1877–80. [DOI] [PubMed] [Google Scholar]
- [88].Wilson E, Goss MA, Marin M, et al. Varicella vaccine exposure during pregnancy: data from 10 years of the pregnancy registry. J Infect Dis 2008;197(Suppl2):S178–84. [DOI] [PubMed] [Google Scholar]
- [89].Wise RP, Salive ME, Braun MM, et al. Postlicensure safety surveillance for varicella vaccine. JAMA: J Am Med Assoc 2000;284:1271–9. [DOI] [PubMed] [Google Scholar]
- [90].Wright P, Lambert JS, Gorse GJ, et al. Immunization with envelope MN rgp120 vaccine in human immunodeficiency virus-infected pregnant women. J Infect Dis 1999;180:1080–8. [DOI] [PubMed] [Google Scholar]
- [91].Zheteyeva Y, Moro PL, Yue X, Broder K. Safety of meningococcal polysaccharide-protein conjugate vaccine in pregnancy: a review of the vaccine adverse event reporting system. Am J Obstet Gynecol 2013;208, 478 e1–6. [DOI] [PubMed] [Google Scholar]
- [92].Zheteyeva YA, Moro PL, Tepper NK, et al. Adverse event reports after tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccines in pregnant women. Am J Obstet Gynecol 2012;207, 59 e1–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


