Abstract
Purpose
This study aimed to determine the proportion of gastric cancer attributable to Helicobactor pylori in the Korean population. Infection with H. pylori has been recognized as the most significant risk factor for gastric cancer. In Korea, gastric cancer is the most common cancer that accounted for 13.3% of all cancers in 2016. In particular, men are most commonly diagnosed with gastric cancer; the age-standardized incidence rate in men is 49.6 per 100,000, which is more than twice the incidence in women.
Materials and Methods
The population attributable fraction (PAF) was calculated as a function of the relative risk (RR) of gastric cancer associated with H. pylori infections. To estimate PAF of gastric cancer due to H. pylori, the prevalence of H. pylori infections was extrapolated for the year of 1990 and a pooled RR was obtained by conducting a meta-analysis of studies recently published in Korea.
Results
The estimated prevalence of H. pylori was 76.4% in men and 71.9% in women. The RRs (95% confidence interval) pooled from case-control studies using a random effects model was 1.69 (1.29–2.22) for overall gastric cancer and 2.17 (1.04–4.55) for non-cardia gastric cancer. Using the RR for overall gastric cancer, the estimated PAFs due to H. pylori were 34.5% in men and 33.2% in women.
Conclusion
The occurrence of gastric cancer in Koreans may be affected by other risk factors in addition to H. pylori infection, which may contribute to increasing baseline risk for gastric cancer.
Keywords: Stomach neoplasms, Helicobacter pylori, Population attributable fraction, Meta-analysis, Korea
Introduction
Gastric cancer is one of the leading malignancies worldwide with varying incidence and mortality rates across geographic regions [1]. The highest incidence rates of gastric cancer are consistently being reported in East Asia [1]. In Korea, gastric cancer is the most commonly diagnosed cancer; in 2016, gastric cancer approximately 13% of all cancers. In particular, gastric cancer ranks the top incident cancer among men in Korea, with an age-standardized incidence rate of 49.6 per 100,000, which is twice the incidence rate in women. Although the incidence rates of gastric cancer gradually declined in Korea between 1999 and 2016, it nevertheless remains high [2].
Several previous studies have suggested that an increased risk of gastric cancer is associated with lifestyle behaviors, such as cigarette smoking, intensive alcohol consumption, high salt intake, consumption of processed meat, and low intake of fruits [3]. Furthermore, infection with Helicobacter pylori, which is categorized as a class I carcinogen, is the main risk factor for gastric cancer [4]. Individuals with a H. pylori infection have a 3 times higher risk of developing non-cardia gastric cancer than individuals who are not infected with H. pylori [5]. The high endemicity of H. pylori infection in Korea and the high prevalence of approximately 50%–60% [6] may reflect the high incidence rate of gastric cancer in Korea. However, the inflammatory response to an infection in a host and the virulence of the infection vary between individuals. Additionally, environmental exposures may also contribute to the increase in the risk of gastric cancers [1], while the widespread use of antibiotics for treating H. pylori infections may be associated with a decrease in the risk of the same cancers [7].
The population attributable fraction (PAF) is a measure to quantify the contribution of a certain risk factor to the burden of disease on a population level. It is calculated using the exposed proportion of the population (e.g., exposure prevalence of H. pylori) and the relative risk (RR) of disease (e.g., gastric cancer) due to the risk factor [8]. Thus, the PAF may be used to estimate the extent to which H. pylori infections contribute to the burden of gastric cancer on a population level. To develop public health policy and implement cancer control measures that are well-suited for a given population, the PAF is an important measure of quantifying the proportion of the gastric cancer burden that could be prevented if proper interventions were implemented to control H. pylori infections.
Given the changing trend of gastric cancer along with the changes in lifestyle behaviors, there is a need to re-evaluate the contribution of H. pylori to gastric cancer in the Korean population. Therefore, the purpose of this study was to estimate the PAF using recently published studies and to re-evaluate the risk of developing gastric cancer attributable to H. pylori infections among Koreans.
Materials and Methods
1. Estimation of the population attributable fraction
The PAF of gastric cancer due to H. pylori infection was calculated using Levin’s formula [9,10] which is the direct method to calculate the ratio of cases resulting from H. pylori infection to the total number of cases. The prevalence of H. pylori infections and RR for gastric cancer in Korean population were used in the formula. A counterfactual exposure was defined as no H. pylori infection.
2. Exposure prevalence of H. pylori
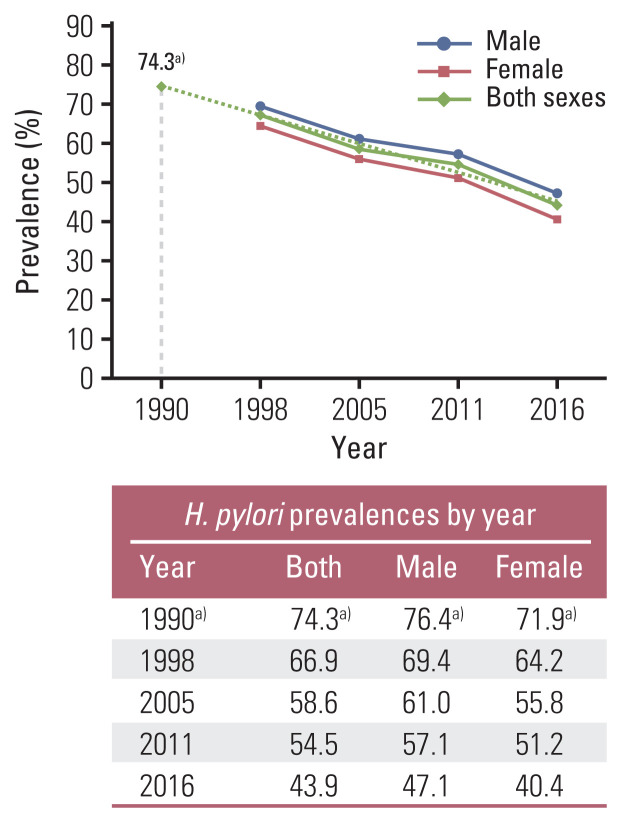
Estimates for the prevalence of H. pylori infections were have been adapted from a report by Lim et al. [11], which was conducted at a different time point. Using these data, which were reported in 1998, 2005, 2011, and 2016, prevalence estimates of 76.4% for men and 71.9% for women in 1990 were extrapolated (Fig. 1). The H. pylori prevalence could be used subsequently to evaluate the PAFs of gastric cancer 25 years later (1990–2016).
Fig. 1.
Back extrapolation to estimate the Helicobacter pylori prevalence in 1990 among Koreans. a)Estimates extrapolated backward using the reported H. pylori prevalences in 1998, 2005, 2011, and 2016.
3. Risk estimate of gastric cancer
A meta-analysis was conducted to evaluate the overall combined risk of gastric cancer from H. pylori infection and determine the PAFs among the Korean population. Instead of applying the RR for gastric cancer, the odds ratio (OR) pooled from the studies conducted in Korea was applied in the PAF formula. A meta-analysis was performed to obtain the pooled OR after updating a systematic review [12] to collect risk estimates from recently published studies up to 2019. The analysis included the published Korean studies written in English or Korean to examine H. pylori infection as a risk factor with regard to gastric cancer risk. The search keywords that were used in the PubMed (http://www.ncbi.nlm.nih.gov/pubmed/) and Koreamed (http://www.koreamed.org/SearchBasic.php) databases included ‘Helicobacter pylori,’ ‘gastric cancer,’ ‘stomach cancer,’ and ‘Korea.’ Eligible articles were limited to observational studies conducted in Korea that had age- and sex-matched control groups. Of the 4,513 full-text publications retrieved from searching databases until December 31, 2019, 11 eligible articles were eventually included in the meta-analysis (S1 Fig.). Data including study period, study population and sample size, cancer types by tumor site, and risk estimates of cancer by infection status were extracted from 11 studies [13–23] including hospital-based case-cohort and case-control studies. The cases were divided into overall, non-cardia, and cardia forms of gastric cancer based on the tumor site. Additionally, a sensitivity analysis was conducted to assess whether the selective studies dealt with adjustment for risk factors other than H. pylori infection, such as cigarette smoking and alcohol drinking (Table 1).
Table 1.
Summary table of information extracted from the selected 11 observational studies, Korea, 1997–2019
| No. | Author (year) | Study period | Type and source of study subjects | No. of cases | No. of controls | Category of infection | OR (95% CI) | Potential risk factors of gastric cancer |
|---|---|---|---|---|---|---|---|---|
| 1 | Kim et al. (1997) [13] | 1994 | Hospital-based (Hallym University Hospital) | 160 | 160 | Negative Positive |
1.00 OG: 1.39 (0.89–2.17) |
No consideration of risk factors other than H. pylori infection in the analysis |
| 2 | Chang et al. (2001) [14] | 1997–1998 | Hospital-based (Hallym University Hospital and Hanyang University Hospital) | 136 | 136 | Negative Positive |
1.00 OG: 1.82 (1.10–3.00) |
Adjusted for age, sex, marital status, socioeconomic status; No consideration of smoking and alcohol drinking status |
| 3 | Kim et al. (2005) [15] | 1997–1998 | Hospital-based (Seoul National University Hospital and Asan Medical Center) | 295 | 295 | Negative Positive |
1.00 OG: 1.71 (1.13–2.58) |
Adjusted for age, sex, a past medical history of gastritis or gastric ulcer, educational level; No significant difference in smoking and alcohol status between cases and controls |
| 4 | Shin et al. (2005) [16] | 1993–1999 | Korean Multi-center Cancer Cohort (4 urban or rural areas in Korea including 19 sites) | 86 | 344 | Negative Positive |
1.00 OG: 1.06 (0.80–1.40) NG: 1.07 (0.77–1.49) CG: 0.88 (0.38–2.28) |
Adjusted for education, alcohol consumption, and cumulative doses of smoking (pack-years) |
| 5 | Gwack et al. (2006) [17] | 1993–2002 | Korean Multi-center Cancer Cohort | 100 | 400 | Negative Positive |
1.00 OG: 0.96 (0.68–1.36) |
Adjusted for years of education, smoking and alcohol drinking status |
| 6 | Cho et al. (2010) [18] | 2003–2007 | Hospital based (National Cancer Center) | 2,819 | 562 | Negative Positive |
1.00 OG: 3.13 (2.46–3.97) NG: 3.17 (2.48–4.04) CG: 2.98 (2.16–4.02) |
Adjusted for age, sex, family history of gastric cancer, residence during childhood, education, socioeconomic status during childhood, drink water, smoking and alcohol consumption |
| 7 | Chung et al. (2012) [19] | 2004–2010 | Hospital based (Seoul National University Hospital Healthcare System Gangnam Center) | 277 | 1,108 | Negative Positive |
1.00 OG: 1.16 (0.77–1.76) |
Adjusted for age, sex, family history of gastric cancer, atrophic gastritis and intestinal metaplasia, current smoking, alcohol consumption |
| 8 | Kim et al. (2012) [20] | 2003–2011 | Hospital-based (Seoul National University Bundang Hospital) | 829 | 270 | Negative Positive |
1.00 OG: 2.49 (1.78–3.49) NG: 3.06 (1.90–4.93) CG: 2.62 (0.90–7.65) |
Adjusted for smoking and alcohol drinking status |
| 9 | Gong et al. (2014) [21] | 2000–2010 | Hospital-based (Asan Medical Center) | 327 | 327 | Negative Positive |
1.00 OG: 2.93 (1.88–4.59) |
Adjusted for current smoking and alcohol drinking status (multivariate conditional logistic regression) |
| 10 | Eom et al. (2016) [22] | 1997–2013 | Hospital-based (Chungbuk National University Hospital, Eulgi University Hospital, Asan Medical Center) | 846 | 846 | Negative Positive |
1.00 OG: 1.43 (1.12–1.81) |
Adjusted for education, cumulative smoking amount, alcohol intake amount, body mass index |
| 11 | Baek et al. (2020) [23] | 2006–2017 | Hospital-based (Seoul National University Bundang Hospital) | 1,124 | 1,463 | Negative Positive |
1.00 OG: 2.06 (1.20–4.50) |
Adjusted for age, sex, smoking and alcohol drinking status |
CG, cardia gastric cancer; CI, confidence interval; NG, non-cardia gastric cancer; OG, overall gastric cancer; OR, odds ratio.
A sex-stratified meta-analysis was performed using risk estimates. As the heterogeneity assessment revealed no significant differences between the studies, the pooled OR and 95% confidence intervals (CIs) were estimated based on the random effects model. Heterogeneity was defined as a Higgins’ I2 value > 50% [24]. Subgroup analyses were conducted for different tumor sites of gastric cancer. A p-value of < 0.05 was considered statistically significant. Stata ver. 14.0 (StataCorp., College Station, TX) was used to perform the meta-analysis to obtain pooled ORs and to assess publication bias.
4. Sensitivity analysis and CIs
A sensitivity analysis was conducted to compare the different values of PAF that resulted from using exposure prevalence rates in different time points (1990, 1998, 2005, 2011, and 2016). To assess the uncertainty of the risk estimates or prevalence rates, the delta method was used to estimate the variance of the PAF and 95% CIs.
Results
To estimate the prevalence of H. pylori infection in the year 1990, the levels of H. pylori prevalence reported in the selective years among Koreans were used and extrapolated backwards. The estimated prevalence in 1990 was 74.3% in both sexes (76.4% in men and 71.9% in women) (Fig. 1).
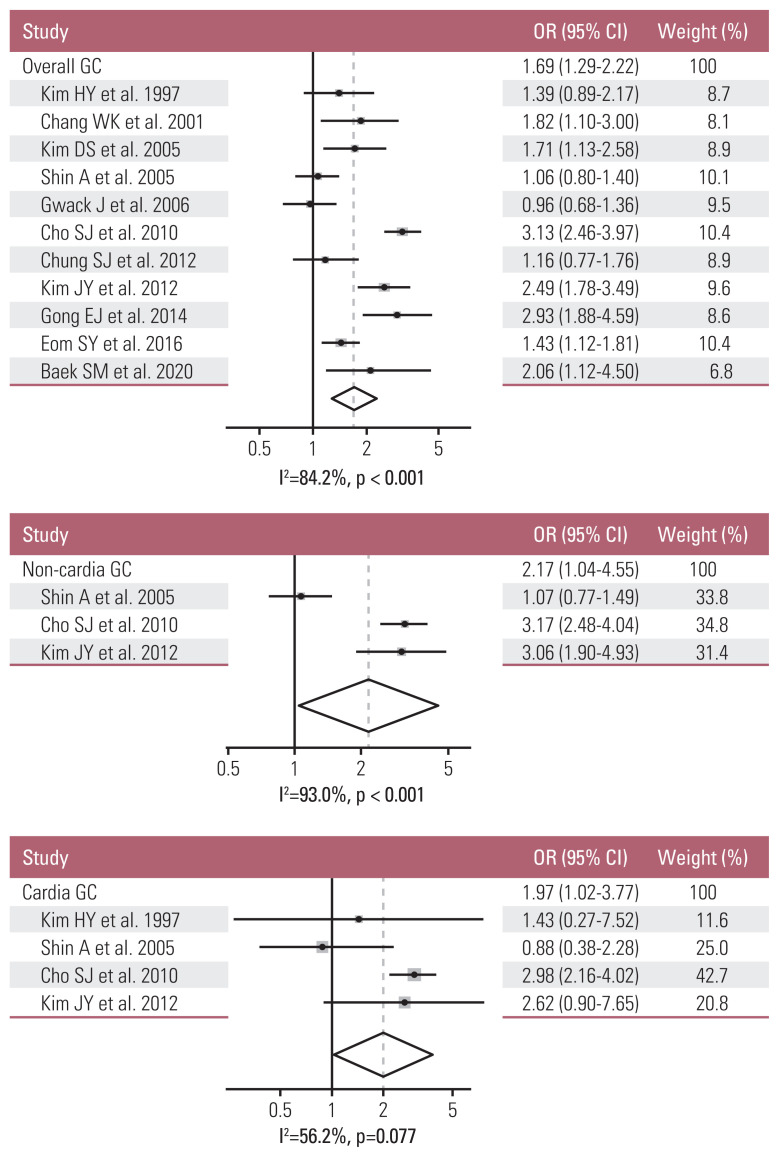
Among 11 eligible studies, the studies were separately selected for meta-analyses to obtain the pooled ORs by tumor site of gastric cancer: 11 studies were used for overall gastric cancers, three studies for non-cardia gastric cancers, and four studies for cardia gastric cancers. In a meta-analysis, the pooled OR (95% CIs) for overall gastric cancers was 1.69 (1.29–2.22) (I2=84.2%, p < 0.001). After stratifying the data by tumor site, the pooled ORs (95% CIs) for non-cardia and cardia gastric cancers were 2.17 (1.04–4.55) (I2=93.0%, p < 0.001) and 1.97 (1.02–3.77) (I2=56.2%, p=0.077), respectively. The pooled OR for non-cardia gastric cancers was higher than that for overall gastric cancer and statistically significant (Fig. 2).
Fig. 2.
Summary effect size of Helicobacter pylori infection on overall, non-cardia, and cardia gastric cancer (GC) among Koreans: a meta-analysis [13–23]. CI, confidence interval; OR, odds ratio.
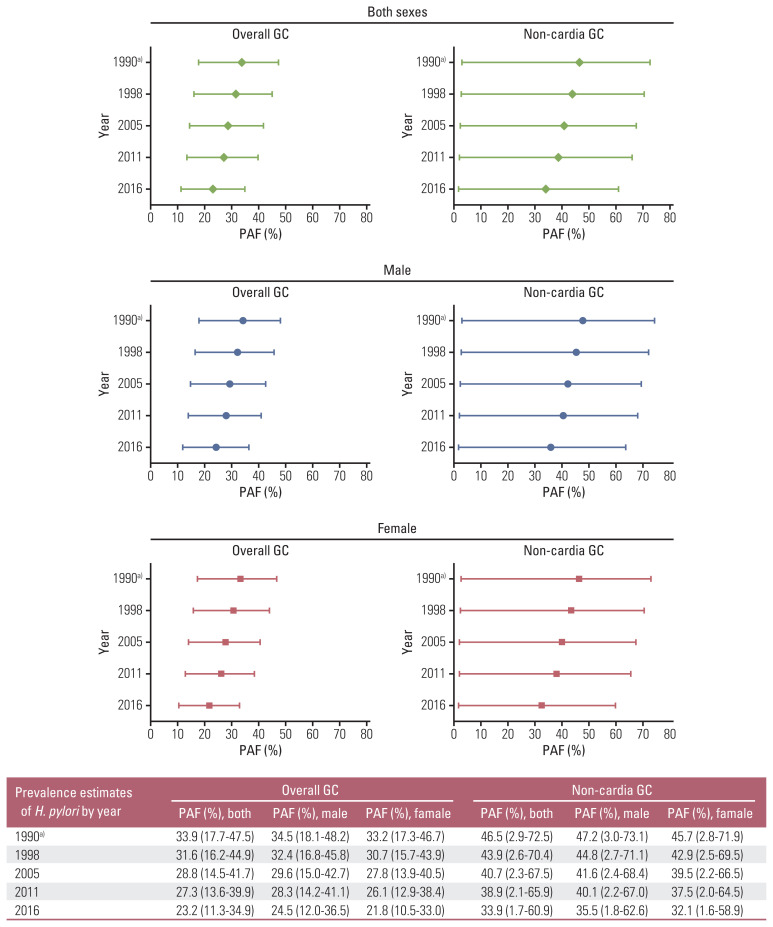
To obtain the PAF estimates, the pooled ORs for overall and non-cardia gastric cancers were separately applied in the Levin’s formula along with the exposure prevalence of H. pylori infection in 1990 obtained from the back extrapolation (Fig. 3). Using the exposure prevalence from 1990, the PAF estimates for overall gastric cancer were 34.5% for men and 33.2% for women. For non-cardia gastric cancer, the PAF estimates were 47.2% for men and 45.7% for women. Using the exposure prevalence from 2005, the PAF estimates for overall gastric cancer were 29.6% for men and 27.8% for women. For non-cardia gastric cancer, the PAF estimates were 41.6% for men and 39.5% for women. When the exposure prevalence from 2016 was used, the PAF estimates were 24.5% in men and 21.8% in women for overall gastric cancers, and 35.5% in men and 32.1% in women for non-cardia gastric cancers. When more recent exposure prevalence rates of H. pylori were applied, decreasing PAF estimates and smaller ranges of 95% CIs were observed in both sexes. No major differences in the PAF estimates were observed between the sexes (Fig. 3).
Fig. 3.
Population attributable fraction (PAF, %) for gastric cancer (GC) due to Helicobacter pylori infection in Koreans. a)Estimates extrapolated backward using the reported H. pylori prevalences in 1998, 2005, 2011, and 2016.
When using nine selective studies adjusted for smoking and drinking status, the pooled OR for overall gastric cancer was 1.72 (1.55–1.92) (I2=87.2%, p < 0.001) that was higher than the OR of 1.69 using 11 studies. Along with the higher OR, the level of PAFs were also higher showing narrow CIs (S2 Table).
Discussion
In this study, we report that H. pylori infections account for approximately 35% of overall gastric cancer cases and more than 45% of non-cardia gastric cancer cases in the Korean population. Using studies conducted in Korea, the risk estimate for gastric cancer was pooled and used to obtain PAF estimates using the exposure prevalence of H. pylori at different time points. For both sexes, the PAF due to H. pylori decreased as the prevalence of H. pylori decreased over time. Our results indicate that H. pylori is more strongly associated with non-cardia and early gastric cancer than with overall gastric cancer; higher PAF point estimates of 46.5% and 49.3% for non-cardia and early gastric cancer, respectively, were obtained in both sexes (S3 Table). The estimated level of PAF for early gastric cancer may reflect the Korean situation where the number of patients diagnosed with early gastric cancer has increased due to the increase in cancer screening by gastrointestinal endoscopy [25]. A further study after collecting more studies that focus on early gastric cancer among Koreans is required to attain the supportive results overcome the issue of limited number of studies and heterogeneity.
The PAF estimates of H. pylori infection can help determine whether gastric cancer may be prevented if H. pylori were eliminated. In this meta-analysis, the RR for disease (gastric cancer) and exposure prevalence of the risk factor of interest (H. pylori) were combined to estimate the total impact on the population. As cancer is a rare disease to occur in public, the RR may be substituted for the pooled OR obtained from a meta-analysis of related observational studies. The prevalence of certain risk factors plays a more important role in the estimation of Levin’s PAF than the RR of a disease. In this study, we decided to apply the pooled risk estimate using observational studies conducted in Korea due to a lack of prospective studies following H. pylori–infected patients prior to the occurrence of gastric cancer. Using the same strategies as in a previous study conducted a decade ago [26], we attempted to select recent eligible studies published until 2019. Although a larger number and wider range of studies were used in our analysis than in the study from 2010 [26], the pooled risk and PAF estimates were similar (OR, 1.69; PAF, approximately 35%).
In Asia countries, the estimated PAF of H. pylori regarding gastric cancer mortality is known to be higher than that in Europe or America because of the high prevalence of H. pylori infection in Asia [27]. The previously mentioned study [26] reported a PAF of 78%–80% for non-cardia gastric cancer using a RR of 5.9 adopted from a pooled analysis of cohort studies from the Helicobacter and Cancer Collaborative Group [28], while in our study, we reported a PAF of approximately 45% for non-cardia gastric cancer using a pooled OR of 2.17. Compared to neighboring countries of China or Japan, a PAF for non-cardia gastric cancer among Koreans was in the middle of those for Chinese and Japanese reporting approximately 37% and 65%, respectively [29,30]. Especially for gastric cancer mortality in Asian men, the estimated PAF due to H. pylori were 82%, 76%, and 63% in Japan, Korea, and China, respectively [27]. Due to socioeconomic improvement as well as antibiotic treatment in all three Asian countries mentioned, prevalence of H. pylori infection has declined along with H. pylori eradication and it showed a potential that leads to lessen the burden of chronic disease related to H. pylori [27,29,30]. If the PAF of the previous study had been properly interpreted and represented the real situation in Korea, it would have resulted in more eminent declines in the incidence of gastric cancer as the prevalence of H. pylori infections in Koreans has decreased. According to several studies, the decline in the prevalence of H. pylori infections has resulted in a decrease in the incidence of gastric cancer [1]. Furthermore, the beneficial effect of H. pylori eradication has been demonstrated in many studies reporting significantly lower risk of non-cardia gastric cancer in the absence of H. pylori [31,32]. As H. pylori treatment has been recommended to prevent gastric cancer in various long-term Korean studies, its effectiveness has already been confirmed [33–35]. However, the incidence of gastric cancer in Korea has not declined dramatically despite a downward trend in the prevalence of H. pylori infections has been observed in Korea. These findings highlight that underlying risk factors for gastric cancer, in addition to H. pylori infection, may exist in the Korean population. Hence, there is a need to interpret the PAF estimates in this study in light of other potential risk factors that are distinctive for Koreans.
In addition to H. pylori infections, host factors, environmental co-factors, and interaction between those factors may contribute to gastric cancer progression [36]. Potential risk factors specific to the Korean population may further explain the low PAF of gastric cancer due to H. pylori infections. First, dietary habits in Korea that originate from the past may increase the baseline risk for gastric cancer among Koreans. As refrigerators did not exist in the past, traditional methods of preserving foods, such as salting and seasoning, were used for several generations in Korea. Furthermore, a preference for salty and spicy foods, such as foods preserved or pickled by salting, seasoned vegetables, and kimchi, is common among Koreans who consume rice as a staple food. Moreover, diverse sauces fermented using salt (e.g., condiments or seasonings including soy sauce, bean paste, red pepper paste) are commonly used in Korean recipes to enhance a pungent taste. These dietary habits are associated with the formation of N-nitrosamines which may be carcinogenic [37]. The positive association between routine salt intake and gastric cancer has been reported in different studies [38,39]. In addition to consuming foods preserved by salting, there is strong evidence that alcohol consumption increases the risk for gastric cancer [3]. Moreover, alcohol consumption is associated with other factors, such as smoking, dietary behaviors, education level, and occupation [40], that may affect the risk for gastric cancer. In a previous meta-analysis of prospective cohorts from China, Japan, and Korea, smoking was associated with a 33% increased risk for non-cardia gastric cancer [41]. In the same study, the multiplex serology of H. pylori as a potential effect modifier was also examined: a positive interaction was found only among patients with a specific seromarker (HP0305/HP1564) which increased the risk of gastric cancer in current smokers [41]. Among environmental exposures etiologically associated with gastric cancer, the effect of several potential risk factors on cancer progression may vary by sex. In this study, the estimated PAF was similar for both sexes after considering of lifestyle behaviors, such as smoking or alcohol drinking. Since most studies used in the analysis did not use specific categorization of each potential risk factor, we could not demonstrate a clear difference between sexes in the impact of H. pylori infections on the risk of gastric cancer. The finding that the range of CIs gets narrower as adjusting for other risk factors, may imply the potential focusing on H. pylori infection solely (S2 Table). However, we did not report this result in depth in this study because the status of smoking and alcohol drinking were not identically adjusted in all nine studies.
The limitation of our study is that the population selected from each literature used in the meta-analysis is not perfectly generalize the total population of Korea. The study population included in the analysis was not intentionally selected for prospectively observing H. pylori infection patients; therefore, heterogeneity (I2 > 50%) may be observed between studies. Especially for non-cardia gastric cancer, our result of forest plot showed a high heterogeneity (I2=93.0%) that implies the characteristics of each study population from three different studies were too different to be in same classification of non-cardia cases. It leads to another limitation of lack of homogeneity of study population along with representativeness of a Korean population. As only hospital-based case-control studies were available in Korea, such study designs may reduce the representativeness of the study sample in contrast to population-based studies. Therefore, in the absence of national prospective studies regarding the issue of H. pylori infection, we attempted to estimate and evaluate the PAF of H. pylori to the best of our ability using Korean studies only, in which the health conditions at baseline related to H. pylori infections could not be controlled sufficiently. Therefore, our PAF estimates were lower compared to PAF estimates obtained in other analyses that used RR from prospective studies following H. pylori infected patients.
In conclusion, our results indicate that fraction of gastric cancer attributable to H. pylori infections is relatively low in Korea where the incidence of gastric cancer is high. The low PAF estimates for H. pylori infections may imply that additional underlying risk factors for gastric cancer may exist in the Korean population. Our findings help to better understand the impact of H. pylori infections on the risk of developing gastric cancer in Korea where H. pylori infection is highly endemic. Moreover, our results emphasize the need to examine the impact of additional risk factors among Koreans in order to reduce the burden of gastric cancer in Korea. Further studies are needed to understand the sex-based differences in the potential contribution of H. pylori infections to gastric cancer.
Acknowledgments
We would like to thank Dr. Sohee Park for her lecture on the concept of population attributable fraction to evaluate the disease burden of interest. We also thank Editage (www.editage.co.kr) for English language editing. This work was supported by a research grant from the National Cancer Center of Korea (NCC-1710141 and NCC-2010200).
Footnotes
Author Contributions
Conceived and designed the analysis: Park Y, Ki M.
Collected the data: Park Y, Ki M.
Contributed data or analysis tools: Park Y, Ki M.
Performed the analysis: Park Y, Ki M.
Wrote the paper: Park Y, Ki M.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.
Electronic Supplementary Material
Supplementary materials are available at the Cancer Research and Treatment website (https://www.e-crt.org).
References
- 1.Balakrishnan M, George R, Sharma A, Graham DY. Changing trends in stomach cancer throughout the world. Curr Gastroenterol Rep. 2017;19:36. doi: 10.1007/s11894-017-0575-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jung KW, Won YJ, Kong HJ, Lee ES. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2016. Cancer Res Treat. 2019;51:417–30. doi: 10.4143/crt.2019.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Cancer Research Fund International. Diet, nutrition, physical activity and stomach cancer [Internet] London: World Cancer Research Fund International; c2016. [cited 2019 Dec 20]. Available from: https://www.wcrf.org/sites/default/files/Stomach-Cancer-2016-Report.pdf. [Google Scholar]
- 4.IARC Working Group on the Evaluation of Carcinogenic Risk to Humans. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 61 [Internet] Lyon: International Agency for Research on Cancer; 1994. Schistosomes, liver flukes and Helicobacter pylori. [cited 2019 Dec 20]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK487782/ [PMC free article] [PubMed] [Google Scholar]
- 5.Ko KP. Epidemiology of gastric cancer in Korea. J Korean Med Assoc. 2019;62:398–406. [Google Scholar]
- 6.Lim SH, Kwon JW, Kim N, Kim GH, Kang JM, Park MJ, et al. Prevalence and risk factors of Helicobacter pylori infection in Korea: nationwide multicenter study over 13 years. BMC Gastroenterol. 2013;13:104. doi: 10.1186/1471-230X-13-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim N, Kim JJ, Choe YH, Kim HS, Kim JI, Chung IS, et al. Diagnosis and treatment guidelines for Helicobacter pylori infection in Korea. Korean J Gastroenterol. 2009;54:269–78. doi: 10.4166/kjg.2009.54.5.269. [DOI] [PubMed] [Google Scholar]
- 8.Brooks-Pollock E, Danon L. Defining the population attributable fraction for infectious diseases. Int J Epidemiol. 2017;46:976–82. doi: 10.1093/ije/dyx055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanley JA. A heuristic approach to the formulas for population attributable fraction. J Epidemiol Community Health. 2001;55:508–14. doi: 10.1136/jech.55.7.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levin ML. The occurrence of lung cancer in man. Acta Unio Int Contra Cancrum. 1953;9:531–41. [PubMed] [Google Scholar]
- 11.Lim SH, Kim N, Kwon JW, Kim SE, Baik GH, Lee JY, et al. Trends in the seroprevalence of Helicobacter pylori infection and its putative eradication rate over 18 years in Korea: a cross-sectional nationwide multicenter study. PLoS One. 2018;13:e0204762. doi: 10.1371/journal.pone.0204762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bae JM, Kim EH. Helicobacter pylori infection and risk of gastric cancer in Korea: a quantitative systematic review. J Prev Med Public Health. 2016;49:197–204. doi: 10.3961/jpmph.16.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim HY, Cho BD, Chang WK, Kim DJ, Kim YB, Park CK, et al. Helicobacter pylori infection and the risk of gastric cancer among the Korean population. J Gastroenterol Hepatol. 1997;12:100–3. doi: 10.1111/j.1440-1746.1997.tb00391.x. [DOI] [PubMed] [Google Scholar]
- 14.Chang WK, Kim HY, Kim DJ, Lee J, Park CK, Yoo JY, et al. Association between Helicobacter pylori infection and the risk of gastric cancer in the Korean population: prospective case-controlled study. J Gastroenterol. 2001;36:816–22. doi: 10.1007/s005350170003. [DOI] [PubMed] [Google Scholar]
- 15.Kim DS, Lee MS, Kim YS, Kim DH, Bae JM, Shin MH, et al. Effect modification by vitamin C on the relation between gastric cancer and Helicobacter pylori. Eur J Epidemiol. 2005;20:67–71. doi: 10.1007/s10654-004-1027-y. [DOI] [PubMed] [Google Scholar]
- 16.Shin A, Shin HR, Kang D, Park SK, Kim CS, Yoo KY. A nested case-control study of the association of Helicobacter pylori infection with gastric adenocarcinoma in Korea. Br J Cancer. 2005;92:1273–5. doi: 10.1038/sj.bjc.6602467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gwack J, Shin A, Kim CS, Ko KP, Kim Y, Jun JK, et al. CagA-producing Helicobacter pylori and increased risk of gastric cancer: a nested case-control study in Korea. Br J Cancer. 2006;95:639–41. doi: 10.1038/sj.bjc.6603309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho SJ, Choi IJ, Kim CG, Lee JY, Kook MC, Seong MW, et al. Helicobacter pylori seropositivity is associated with gastric cancer regardless of tumor subtype in Korea. Gut Liver. 2010;4:466–74. doi: 10.5009/gnl.2010.4.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung SJ, Park MJ, Kang SJ, Kang HY, Chung GE, Kim SG, et al. Effect of annual endoscopic screening on clinicopathologic characteristics and treatment modality of gastric cancer in a high-incidence region of Korea. Int J Cancer. 2012;131:2376–84. doi: 10.1002/ijc.27501. [DOI] [PubMed] [Google Scholar]
- 20.Kim JY, Lee HS, Kim N, Shin CM, Lee SH, Park YS, et al. Prevalence and clinicopathologic characteristics of gastric cardia cancer in South Korea. Helicobacter. 2012;17:358–68. doi: 10.1111/j.1523-5378.2012.00958.x. [DOI] [PubMed] [Google Scholar]
- 21.Gong EJ, Ahn JY, Jung HY, Lim H, Choi KS, Lee JH, et al. Risk factors and clinical outcomes of gastric cancer identified by screening endoscopy: a case-control study. J Gastroenterol Hepatol. 2014;29:301–9. doi: 10.1111/jgh.12387. [DOI] [PubMed] [Google Scholar]
- 22.Eom SY, Hong SM, Yim DH, Kwon HJ, Kim DH, Yun HY, et al. Additive interactions between PRKAA1 polymorphisms and Helicobacter pylori CagA infection associated with gastric cancer risk in Koreans. Cancer Med. 2016;5:3236–335. doi: 10.1002/cam4.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baek SM, Kim N, Kwon YJ, Lee HS, Kim HY, Lee J, et al. Role of serum pepsinogen II and Helicobacter pylori status in the detection of diffuse-type early gastric cancer in young individuals in South Korea. Gut Liver. 2020;14:439–49. doi: 10.5009/gnl19091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 25.Lee SH, Kim MC, Jeon SW, Lee KN, Park JJ, Hong SJ. Risk factors and clinical outcomes of non-curative resection in patients with early gastric cancer treated with endoscopic submucosal dissection: a retrospective multicenter study in Korea. Clin Endosc. 2020;53:196–205. doi: 10.5946/ce.2019.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shin A, Park S, Shin HR, Park EH, Park SK, Oh JK, et al. Population attributable fraction of infection-related cancers in Korea. Ann Oncol. 2011;22:1435–42. doi: 10.1093/annonc/mdq592. [DOI] [PubMed] [Google Scholar]
- 27.Ko KP, Shin A, Cho S, Park SK, Yoo KY. Environmental contributions to gastrointestinal and liver cancer in the Asia-Pacific region. J Gastroenterol Hepatol. 2018;33:111–20. doi: 10.1111/jgh.14005. [DOI] [PubMed] [Google Scholar]
- 28.Helicobacter and Cancer Collaborative Group. Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut. 2001;49:347–53. doi: 10.1136/gut.49.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang J, Chen Y, Shi J, Song C, Zhang J, Wang K. Population attributable burden of Helicobacter pylori-related gastric cancer, coronary heart disease, and ischemic stroke in China. Eur J Clin Microbiol Infect Dis. 2017;36:199–212. doi: 10.1007/s10096-016-2810-x. [DOI] [PubMed] [Google Scholar]
- 30.Shikata K, Doi Y, Yonemoto K, Arima H, Ninomiya T, Kubo M, et al. Population-based prospective study of the combined influence of cigarette smoking and Helicobacter pylori infection on gastric cancer incidence: the Hisayama Study. Am J Epidemiol. 2008;168:1409–15. doi: 10.1093/aje/kwn276. [DOI] [PubMed] [Google Scholar]
- 31.Kim N. Chemoprevention of gastric cancer by Helicobacter pylori eradication and its underlying mechanism. J Gastroenterol Hepatol. 2019;34:1287–95. doi: 10.1111/jgh.14646. [DOI] [PubMed] [Google Scholar]
- 32.Oh S, Kim N, Kwon JW, Shin CM, Choi YJ, Lee DH, et al. Effect of Helicobacter pylori eradication and ABO genotype on gastric cancer development. Helicobacter. 2016;21:596–605. doi: 10.1111/hel.12317. [DOI] [PubMed] [Google Scholar]
- 33.Choi JM, Kim SG, Choi J, Park JY, Oh S, Yang HJ, et al. Effects of Helicobacter pylori eradication for metachronous gastric cancer prevention: a randomized controlled trial. Gastrointest Endosc. 2018;88:475–85. doi: 10.1016/j.gie.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 34.Choi IJ, Kook MC, Kim YI, Cho SJ, Lee JY, Kim CG, et al. Helicobacter pylori therapy for the prevention of metachronous gastric cancer. N Engl J Med. 2018;378:1085–95. doi: 10.1056/NEJMoa1708423. [DOI] [PubMed] [Google Scholar]
- 35.Malfertheiner P. Helicobacter pylori treatment for gastric cancer prevention. N Engl J Med. 2018;378:1154–6. doi: 10.1056/NEJMe1800147. [DOI] [PubMed] [Google Scholar]
- 36.Fock KM, Ang TL. Epidemiology of Helicobacter pylori infection and gastric cancer in Asia. J Gastroenterol Hepatol. 2010;25:479–86. doi: 10.1111/j.1440-1746.2009.06188.x. [DOI] [PubMed] [Google Scholar]
- 37.Jagerstad M, Skog K. Genotoxicity of heat-processed foods. Mutat Res. 2005;574:156–72. doi: 10.1016/j.mrfmmm.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 38.D’Elia L, Rossi G, Ippolito R, Cappuccio FP, Strazzullo P. Habitual salt intake and risk of gastric cancer: a meta-analysis of prospective studies. Clin Nutr. 2012;31:489–98. doi: 10.1016/j.clnu.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 39.Kim J, Park S, Nam BH. Gastric cancer and salt preference: a population-based cohort study in Korea. Am J Clin Nutr. 2010;91:1289–93. doi: 10.3945/ajcn.2009.28732. [DOI] [PubMed] [Google Scholar]
- 40.Yang S, Lee J, Choi IJ, Kim YW, Ryu KW, Sung J, et al. Effects of alcohol consumption, ALDH2 rs671 polymorphism, and Helicobacter pylori infection on the gastric cancer risk in a Korean population. Oncotarget. 2017;8:6630–41. doi: 10.18632/oncotarget.14250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Butt J, Varga MG, Wang T, Tsugane S, Shimazu T, Zheng W, et al. Smoking, Helicobacter pylori serology, and gastric cancer risk in prospective studies from China, Japan, and Korea. Cancer Prev Res (Phila) 2019;12:667–74. doi: 10.1158/1940-6207.CAPR-19-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.