Abstract
Objectives
D-dimer elevations, suggesting a pro-thrombotic state and coagulopathy, predict adverse outcomes in coronavirus disease 2019 (COVID-19). However, the clinical significance of other coagulation markers, particularly the international normalized ratio (INR), is not well established. We conducted a systematic review and meta-analysis of the INR in COVID-19.
Methods
A literature search was conducted in PubMed, Web of Science and Scopus, between January 2020 and February 2021, for studies reporting INR values, measures of COVID-19 severity, and mortality (PROSPERO registration number: CRD42021241468).
Results
Thirty-eight studies in 7440 COVID-19 patients with low disease severity or survivor status during follow up (50 % males, mean age 57 years) and 2331 with high severity or non-survivor status (60 % males, mean age 69 years) were identified. The INR was significantly prolonged in patients with severe disease or non-survivor status than in patients with mild disease or survivor status (standard mean difference, SMD, 0.60; 95 % confidence interval, CI 0.42 to 0.77; p < 0.001). There was extreme between-study heterogeneity (I2 = 90.2 %; p < 0.001). Sensitivity analysis, performed by sequentially removing each study and re-assessing the pooled estimates, showed that the magnitude and direction of the effect size was not modified. The Begg's and Egger's t-tests did not show publication bias. In meta-regression, the SMD of the INR was significantly associated with C-reactive protein (p = 0.048) and D-dimer (p = 0.001).
Conclusions
Prolonged INR values were significantly associated with COVID-19 severity and mortality. Both INR prolongation and D-dimer elevations can be useful in diagnosing COVID-19-associated coagulopathy and predicting clinical outcomes.
Keywords: International normalized ratio, Coagulopathy, COVID-19 severity, Mortality
1. Introduction
Coronavirus disease 2019 (COVID-19) is frequently characterized by the presence of significant coagulopathy, particularly in the setting of a systemic release of pro-inflammatory and pro-oxidant cytokines and multi-organ compromise [1]. The COVID-19-associated coagulopathy typically involves the combined activation of coagulation, immune, and complement pathways and endothelial dysfunction [2,3]. This process results in the formation of thrombi both in the large vessels and in the microvasculature of the lungs and other organs, resembling disseminated intravascular coagulation (DIC) [2,4]. In terms of specific coagulation parameters, the most frequently observed alteration in patients with COVID-19 involves the elevation in the concentrations of D-dimer, one of the major fibrin degradation products that is released during the cleavage of crosslinked fibrin by plasmin and indicates the presence of recent or ongoing DIC and fibrinolysis [5]. Notably, D-dimer elevations in COVID-19 patients are also associated with severe forms of the disease and higher mortality [6]. While other coagulation markers are routinely tested in hospitalized COVID-19 patients, their exact pathophysiological role and clinical significance in this population are not well established. In particular, the pro-thrombin time (PT) and the international normalized ratio (INR), calculated by dividing the PT of an individual patient by that of a laboratory standard, are measured to assess both the extrinsic and the common coagulation pathways and can theoretically assist in the diagnosis of COVID-19-associated coagulopathy as well as the evaluation of the synthetic function of the liver [7]. However, the magnitude of the prolongation of the INR in COVID-19 patients with severe disease and coagulopathy is considered to be less prominent, and possibly less clinically significant, when compared to the D-dimer [8]. Pending further studies addressing this issue, and in the absence of a comprehensive critical appraisal of the evidence regarding the pathophysiological and prognostic role of the INR in COVID-19, we conducted a systematic review and meta-analysis of published studies reporting INR values, measures of COVID-19 severity and mortality. We hypothesized that COVID-19 patients with severe forms of the disease and/or not surviving during follow-up had a prolonged PT, and hence INR, when compared to patients with less severe forms of the disease or favorable outcomes, further supporting the presence of significant coagulopathy and a systemic pro-thrombotic state in the former. A meta-regression analysis was also conducted to identify associations between the INR effect size and several pre-defined biologically and clinically plausible parameters.
2. Materials and methods
2.1. Search strategy and study selection
A systematic literature search, using the terms “international normalized ratio” or “INR” and “coronavirus disease 19” or “COVID-19”, was conducted in the electronic databases of PubMed, Web of Science, and Scopus, from January 2020 to February 2021, to identify peer-reviewed studies reporting the INR in COVID-19 patients (PROSPERO registration number: CRD42021241468). The references of the retrieved articles were also reviewed to identify additional studies. Inclusion criteria were as follows: reporting continuous INR values in COVID-19 patients, investigating COVID-19 patients with different degrees of disease severity or survival status, adult patients, English language, ≥10 participants, and full-text available. Abstracts were independently screened by two investigators (AZ and PP). If relevant, the full articles were reviewed. The quality of individual studies was assessed using the Newcastle-Ottawa scale, with a score ≥6 indicating high quality [9].
2.2. Statistical analysis
Standardized mean differences (SMDs) and 95 % confidence intervals (CIs) were calculated to build forest plots of continuous data and evaluate differences in the values of INR between COVID-19 patients with low vs. high disease severity or survivor vs. non-survivor status. If necessary, the mean and standard deviation values were extrapolated from the corresponding median and interquartile range (IQR) values [10]. The Q-statistic was used to test the between-study heterogeneity of the SMD (significance level set at p < 0.10). Inconsistency across studies was evaluated using the I2 statistic: I2<25 %, no heterogeneity; I2 between 25 % and 50 %, moderate heterogeneity; I2 between 50 % and 75 %, large heterogeneity; and I2>75 %, extreme heterogeneity [11,12]. A random-effects model was used to calculate the pooled SMD and corresponding 95 % CIs in the presence of significant heterogeneity. Sensitivity analyses were conducted to evaluate the impact of individual studies on the overall effect size with the leave-one-out method [13]. The presence of publication bias was assessed with the Begg's adjusted rank correlation test and the Egger's regression asymmetry test [14,15]. The “trim-and-fill” procedure by Duval and Tweedie was also used to assess publication bias. This method recalculates a pooled SMD by incorporating the hypothetical missing studies as though they existed, to augment the observed data so that the funnel plot is more symmetric [16]. To explore possible contributors to the between-study variance, we investigated in meta-regression analysis the associations between the SMD and the following parameters: age, gender, study endpoint, study design (retrospective or prospective), geographical area where the study was conducted, liver function (aspartate aminotransferase, AST, alanine aminotransferase, ALT, albumin), coagulation markers (D-dimer, activated partial thromboplastin time, aPTT, fibrinogen), renal function (serum creatinine, urea), myocardial damage (troponin), tissue damage and sepsis (creatine kinase, CK, lactate dehydrogenase, LDH, procalcitonin), inflammation (C-reactive protein, CRP, white blood cell count, WBC, neutrophils, lymphocytes), glucose, diabetes, hypertension and cardiovascular disease. A p-value <0.05 was considered statistically significant. Analyses were performed using Stata 14 (STATA Corp., College Station, TX, USA). Our study was fully compliant with the PRISMA statement [17].
3. Results
3.1. Literature search and study selection
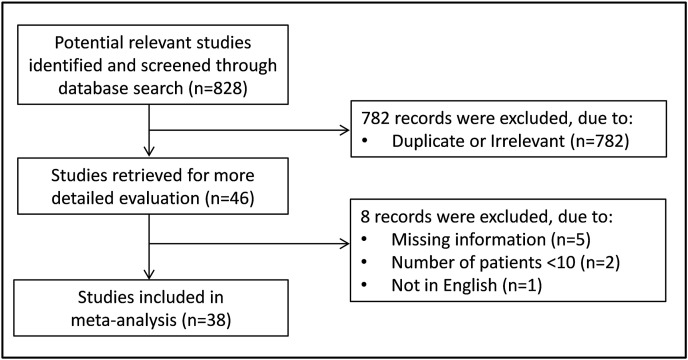
A flow chart of the screening process is described in Fig. 1 . A total of 828 studies were initially identified. From them, 782 were excluded after the first screening because they were either duplicates or irrelevant. After a full-text review of the remaining 46 studies, 8 were further excluded because they failed to meet the inclusion criteria. Thus, 38 studies were included in the final meta-analysis (Table 1 ) [[18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55]]. A total of 9771 COVID-19 patients were investigated, 7440 (50 % males, mean age 57 years) with low disease severity or survivor status and 2331 (60 % males, mean age 69 years) with high severity or non-survivor status during follow-up.
Fig. 1.
Flow chart of study selection.
Table 1.
Characteristics of the studies included in the meta-analysis.
| Mild disease or survivor | Severe disease or non-survivor | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
First Author, Country [Ref] |
Study design | Endpoint | NOS (stars) | n | Age (Years) | Gender (M/F) | INR (Mean ± SD) | n | Age (Years) | Gender (M/F) | INR (Mean ± SD) |
| Aladağ N et al., Turkey [18] |
R | Survival | 7 | 35 | 68 | 22/13 | 1.17 ± 0.22 | 15 | 68 | 6/9 | 1.14 ± 0.24 |
| Altschul DJ et al., USA [19] |
R | Survival | 7 | 1733 | 63 | 771/962 | 1.10 ± 0.15 | 621 | 73 | 327/294 | 1.15 ± 0.15 |
| Bao C et al., China [20] |
P | Disease severity | 5 | 129 | NR | NR | 1.07 ± 0.09 | 49 | NR | NR | 1.23 ± 0.17 |
| Bastug A et al., Turkey [21] |
R | ICU transfer | 7 | 145 | 43 | 81/64 | 1.15 ± 0.39 | 46 | 71 | 26/20 | 1.17 ± 0.41 |
| Bocci MG et al., Italy [22] |
P | Survival | 5 | 23 | 57 | 17/6 | 1.07 ± 0.11 | 17 | 77 | 12/5 | 1.12 ± 0.13 |
| Bonetti G et al., Italy [23] |
R | Survival | 7 | 74 | 62 | 51/23 | 1.05 ± 0.07 | 70 | 78 | 45/25 | 1.14 ± 0.19 |
| Carlino MV et al., Italy [24] |
NR | ICU transfer | 5 | 18 | 47 | 8/10 | 1.07 ± 0.05 | 10 | 73 | 8/2 | 1.12 ± 0.25 |
| Cheng B et al., China [25] |
R | Disease progression | 7 | 205 | 49 | 71/134 | 0.98 ± 0.09 | 251 | 60 | 140/111 | 1.00 ± 0.09 |
| Cheng L et al., China [26] |
R | Survival | 6 | 53 | 54 | 29/24 | 1.08 ± 0.15 | 36 | 69 | 20/16 | 1.12 ± 0.21 |
| Dong Y et al., China [27] |
R | Disease severity | 7 | 94 | 40 | 34/60 | 1.01 ± 0.07 | 53 | 60 | 29/24 | 1.03 ± 0.08 |
| Gong J et al., China [28] |
R | Disease severity | 5 | 161 | 45 | 89/72 | 1.03 ± 0.07 | 28 | 64 | 12/16 | 1.07 ± 0.07 |
| Gue YX et al., UK [29] |
R | Disease severity | 5 | 171 | 67 | 84/87 | 1.03 ± 0.07 | 145 | 81 | 104/41 | 1.10 ± 0.15 |
| Hou W et al., China [30] |
R | Disease progression | 7 | 84 | 47 | 34/50 | 1.13 ± 0.07 | 17 | 72 | 10/7 | 1.10 ± 0.15 |
| Jin X et al., China [31] |
R | Disease severity | 7 | 105 | NR | NR | 0.96 ± 0.10 | 42 | NR | NR | 1.21 ± 0.24 |
| Kayina CA et al., India [32] |
P | Disease severity | 6 | 215 | 50 | 146/69 | 1.17 ± 0.17 | 20 | 51 | 14/6 | 1.44 ± 0.32 |
| Ke C et al., China [33] |
R | Survival | 7 | 148 | 60 | 83/65 | 1.72 ± 0.72 | 46 | 70 | 32/14 | 1.39 ± 0.70 |
| Kong M et al., China [34] |
R | Disease severity | 7 | 123 | 53 | 59/64 | 1.00 ± 0.15 | 87 | 68 | 45/42 | 1.00 ± 0.15 |
| Lei P et al., China [35] |
R | Disease severity | 5 | 50 | 65 | 22/28 | 1.04 ± 0.07 | 65 | 69 | 36/29 | 1.07 ± 0.11 |
| Linli Z et al., China [36] |
R | Survival | 7 | 142 | 56 | 90/52 | 1.08 ± 0.12 | 50 | 68 | 34/16 | 1.19 ± 0.13 |
| Liu J et al., China [37] |
R | Disease severity | 5 | 27 | 43 | 8/19 | 1.00 ± 0.10 | 13 | 60 | 7/6 | 1.00 ± 0.10 |
| Lorente L et al., Spain [38] |
R | Disease severity | 7 | 118 | 64 | 53/65 | 1.19 ± 0.18 | 25 | 71 | 7/18 | 1.27 ± 0.19 |
| Luo HC et al., China [39] |
R | Survival | 6 | 73 | 62 | 39/34 | 1.03 ± 0.07 | 12 | 67 | 9/3 | 1.15 ± 0.08 |
| Mertoglu C et al., Turkey [40] |
R | ICU transfer | 7 | 532 | 48 | 306/226 | 1.13 ± 0.10 | 23 | 59 | 13/10 | 1.19 ± 0.19 |
| Mori S et al., Japan [41] |
R | Disease severity | 5 | 23 | 69 | 13/10 | 1.08 ± 0.11 | 22 | 58 | 21/1 | 1.22 ± 0.20 |
| Ponziani FR et al., Italy [42] |
R | ICU transfer | 7 | 438 | 63 | 263/175 | 1.04 ± 0.07 | 77 | 70 | 60/17 | 1.06 ± 0.04 |
| Pourabdollah Toutkaboni et al., Iran [43] |
R | Survival | 5 | 456 | 55 | 282/174 | 1.19 ± 0.18 | 89 | 64 | 68/21 | 1.24 ± 0.15 |
| Sadeghi A et al., China [44] |
R | ICU transfer | 7 | 159 | 57 | 66/93 | 1.28 ± 0.51 | 55 | 62 | 30/25 | 1.60 ± 0.95 |
| Sayad B et al., Iran [45] |
NR | Survival | 5 | 35 | 64 | 21/14 | 1.40 ± 0.47 | 39 | 67 | 23/16 | 1.50 ± 0.72 |
| Shahriarirad R et al., Iran [46] |
R | Disease severity | 6 | 102 | NR | 64/38 | 1.28 ± 0.15 | 11 | NR | 7/4 | 1.85 ± 0.49 |
| Sun JT et al., China [47] |
P | Disease severity | 7 | 49 | 50 | 26/23 | 0.97 ± 0.06 | 50 | 71 | 34/16 | 1.07 ± 0.15 |
| Tsibouris P et al., Greece [48] |
R | Survival | 7 | 45 | NR | NR | 1.15 ± 0.10 | 16 | NR | NR | 1.31 ± 0.29 |
| Wang C et al., China [49] |
R | Disease severity | 6 | 35 | 38 | 17/18 | 1.16 ± 0.15 | 10 | 43 | 6/4 | 1.24 ± 0.32 |
| Wang JH et al., China [50] |
R | Survival | 7 | 1074 | 61 | 502/572 | 1.04 ± 0.08 | 61 | 74 | 43/18 | 1.22 ± 0.15 |
| Xue G et al., China [51] |
NR | Disease severity | 7 | 56 | 61 | 30/26 | 0.98 ± 0.07 | 58 | 64 | 34/24 | 1.01 ± 0.12 |
| Zhang Yaf et al., China [52] |
R | Disease severity | 7 | 84 | 44 | 29/55 | 1.15 ± 0.09 | 31 | 65 | 20/11 | 1.21 ± 0.13 |
| Zhang Yan et al., China [53] |
R | Disease progression | 6 | 54 | 65 | 28/26 | 1.01 ± 0.07 | 17 | 68 | 10/7 | 1.09 ± 0.07 |
| Zhou C et al., China [54] |
R | Disease severity | 7 | 95 | 35 | 38/57 | 0.98 ± 0.07 | 28 | 40 | 17/11 | 0.99 ± 0.07 |
| Zou Y et al., China [55] |
R | Disease severity | 5 | 277 | 50 | 138/139 | 1.01 ± 0.06 | 26 | 65 | 20/6 | 1.06 ± 0.10 |
Abbreviations: ICU, intensive care unit; NOS, Newcastle-Ottawa quality assessment scale for case-control studies; NR, not reported; P, prospective; R, retrospective.
Thirty studies were conducted in Asia [18,20,21,[25], [26], [27], [28],[30], [31], [32], [33], [34], [35], [36], [37],[39], [40], [41],[43], [44], [45], [46], [47],[49], [50], [51], [52], [53], [54], [55]], seven in Europe [[22], [23], [24],29,38,42,48], and one in America [19]. Thirty-one studies had a retrospective design [18,19,21,23,[25], [26], [27], [28], [29], [30], [31],[33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44],46,[48], [49], [50],[52], [53], [54], [55]], four were prospective [20,22,32,47], whilst the remaining three did not describe the study design [24,45,51]. Sixteen studies investigated disease severity based on current clinical guidelines [20,27,28,31,34,35,37,38,41,46,47,49,51,52,54,55], three on disease progression [25,30,52], and five on ICU transfer [21,24,40,42,44], whereas the remaining 14 studies investigated survival [18,19,22,23,26,29,32,33,36,39,43,45,48,50]. In all studies, the reported INR was measured within the first 24–48 h from admission.
3.2. Meta-analysis
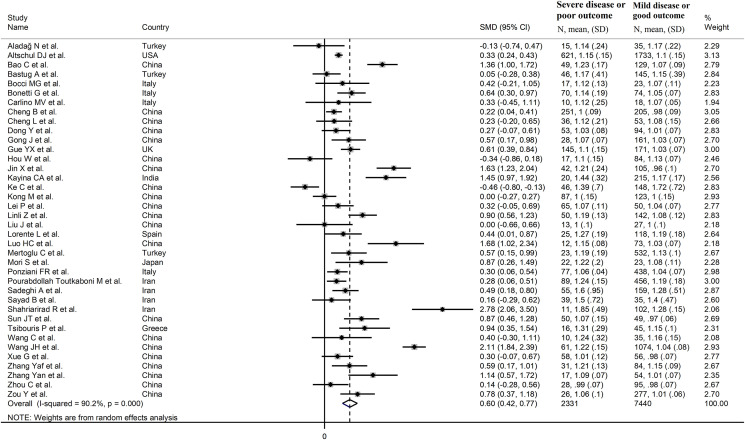
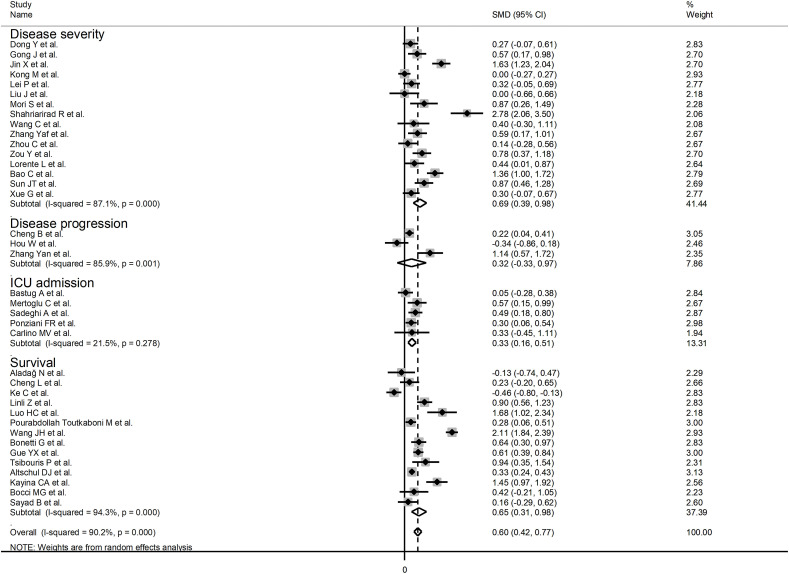
The overall SMD of the INR between COVID-19 patients with low vs. high severity or survivor vs. non-survivor status is described in Fig. 2 . In three studies, patients with high severity or non-survivor status had a lower INR when compared to those with low severity or survivor status (mean difference range, -0.13 to -0.46) [18,30,33]. However, only one study reported a statistically significant difference [33]. There were no differences in two studies (mean difference 0.00) [34,37]. In the remaining studies, the INR was lower in patients with low severity or survivor status (mean difference range, 0.05 to 2.78), with a statistically significant difference reported in 23 studies [19,20,23,25,28,29,31,32,36,[38], [39], [40], [41], [42], [43], [44],[46], [47], [48],50,52,53,55].
Fig. 2.
Forest plot of studies reporting INR values in patients with COVID-19.
The pooled results confirmed that the INR values were statistically significantly prolonged in patients with severe disease or non-survivor status (SMD = 0.60, 95 % CI 0.42 to 0.77, p < 0.001) (Fig. 2). Extreme heterogeneity between studies was observed (I2 = 90.2 %, p < 0.001). The INR values remained statistically significantly prolonged in patients with severe disease or non-survivor status (SMD = 0.55, 95 % CI 0.39 to 0.72, p < 0.001; I2 = 84.8 %, p < 0.001) after excluding two relatively large studies that accounted for nearly 36 % of the overall sample size [19,50].
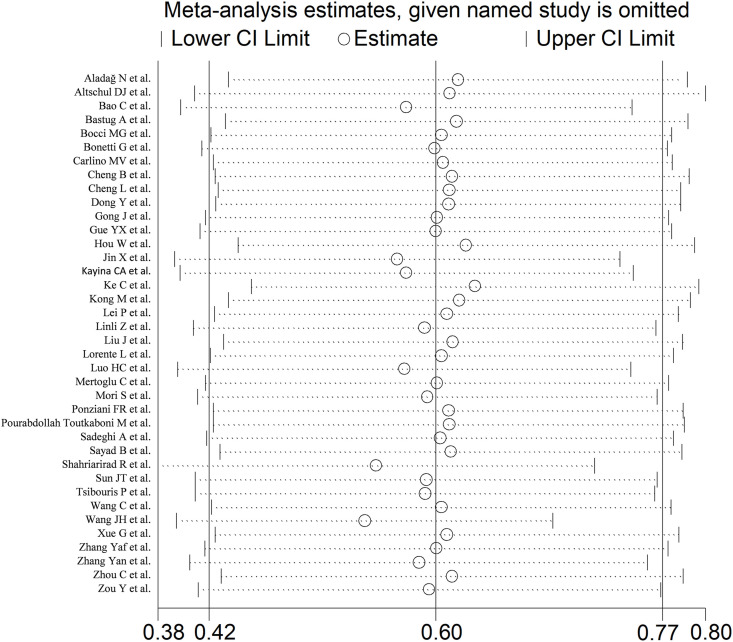
Sensitivity analysis, performed by sequentially removing individual studies and re-assessing the pooled estimates, showed that the magnitude and the direction of the effect size were not substantially modified (effect size range, between 0.54 and 0.63) (Fig. 3 ).
Fig. 3.
Sensitivity analysis of the association between INR and COVID-19. The influence of individual studies on the overall standardized mean difference (SMD) is shown. The middle vertical axis indicates the overall SMD, and the two vertical axes indicate the 95 % confidence intervals (CIs). The hollow circles represent the pooled SMD when the remaining study is omitted from the meta-analysis. The two ends of each broken line represent the 95 % CIs.
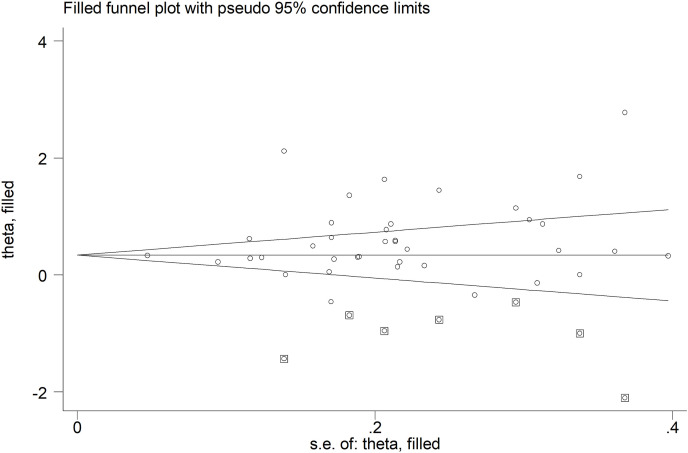
No publication bias was detected with the Begg's (p = 0.15) or the Egger's (p = 0.12) t-tests. However, the trim-and-fill method identified seven potential missing studies to add to the left side of the funnel plot to ensure symmetry (Fig. 4 ). The adjusted SMD, albeit attenuated, remained significant (SMD = 0.35, 95 % CI 0.14 to 0.56, p = 0.001).
Fig. 4.
Funnel plot of studies investigating low vs. high severity or survivor vs. non-survivor status after trimming and filling. Dummy studies and genuine studies are represented by enclosed circles and free circles, respectively.
3.3. Meta-regression
Both CRP (t = 2.07, p = 0.048) and D-dimer (t = 3.72, p = 0.001) were statistically significantly and positively associated with the pooled SMD. A non-significant trend was also observed with troponin (t = 2.17, p = 0.06) (Table 2 ). By contrast, no statistically significant correlations were observed between the SMD and age (t = -1.3, p = 0.18), gender (t = 0.26, p = 0.80), WBC (t = -0.06, p = 0.96), neutrophils (t = 1.01, p = 0.32), lymphocytes (t = 1.61, p = 0.12), procalcitonin (t = 1.81, p = 0.10), AST (t = -0.27, p = 0.79), ALT (t = -1.19, p = 0.24), albumin (t = 0.08, p = 0.94), LDH (t = 1.20, p = 0.24), CK (t = 1.44, p = 0.18), creatinine (t = 0.13, p = 0.90), urea (t = 0.16, p = 0.88), glucose (t = 1.02, p = 0.33), aPTT (t = -0.11, p = 0.91), fibrinogen (t = -0.69, p = 0.50), diabetes (t = -0.33, p = 0.74), hypertension (t = -0.01, p = 0.99) and cardiovascular disease (t = 0.08, p = 0.94) (Table 2).
Table 2.
Univariate meta-regression analysis between effect size and possible contributors to heterogeneity.
| Parameter | N | T | p |
|---|---|---|---|
| Age | 34 | -1.38 | 0.18 |
| Gender | 35 | 0.06 | 0.95 |
| White blood cell count | 31 | -0.06 | 0.95 |
| Neutrophils | 26 | 1.01 | 0.32 |
| Lymphocytes | 33 | 1.61 | 0.12 |
| C-reactive protein | 28 | 2.07 | 0.048 |
| Procalcitonin | 13 | 1.81 | 0.10 |
| Aspartate aminotransferase | 27 | -0.27 | 0.79 |
| Alanine aminotransferase | 29 | -1.19 | 0.24 |
| Albumin | 23 | 0.08 | 0.94 |
| D-Dimer | 26 | 3.72 | 0.001 |
| Troponin | 11 | 2.17 | 0.06 |
| Creatine kinase | 13 | 1.44 | 0.18 |
| Creatinine | 25 | 0.13 | 0.90 |
| Urea | 22 | 0.16 | 0.88 |
| Lactate dehydrogenase | 22 | 1.20 | 0.24 |
| Glucose | 11 | 1.02 | 0.33 |
| Activated partial thromboplastin time | 24 | -0.11 | 0.91 |
| Fibrinogen | 21 | -0.69 | 0.50 |
| Diabetes | 22 | -0.33 | 0.74 |
| Cardiovascular disease | 19 | 0.08 | 0.94 |
| Hypertension | 18 | -0.32 | 0.75 |
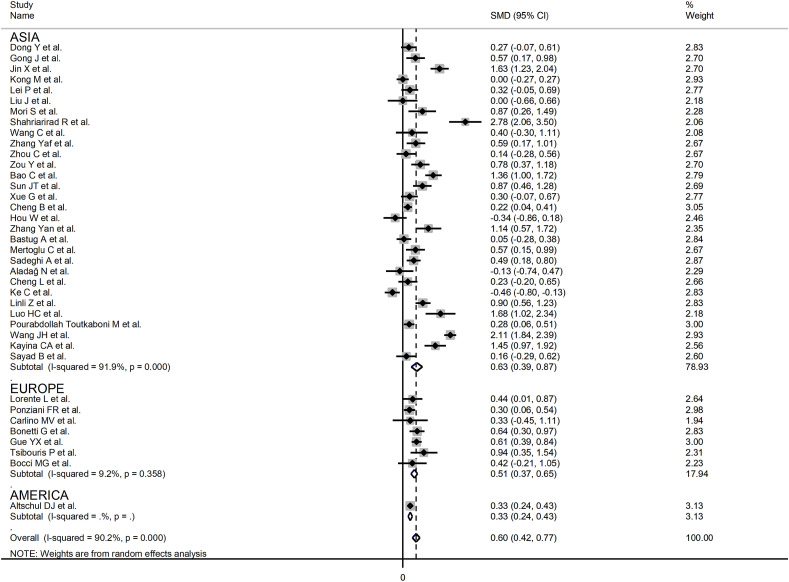
In sub-group analysis, the pooled SMD value in retrospective studies (SMD = 0.57, 95 % CI 0.38 to 0.76, p < 0.001; I2 = 91.0, p < 0.001) was not statistically significantly lower than that observed in prospective studies (SMD = 1.07, 95 % CI 0.66 to 1.48, p < 0.001; I2 = 68.8, p = 0.02; t = 1.33, p = 0.19). The pooled SMD value in studies evaluating disease severity based on clinical guidelines (SMD = 0.69, 95 % CI 0.39 to 0.98, p < 0.001; I2 = 87.1, p < 0.001) or survival (SMD = 0.65, 95 % CI 0.31 to 0.98, p < 0.001; I2 = 94.3, p < 0.001) was not statistically significantly higher than that observed in studies assessing disease progression (SMD = 0.32, 95 % CI -0.33 to 0.97, p = 0.33; I2 = 85.9, p = 0.001) or ICU admission (SMD = 0.33, 95 % CI 0.16 to 0.51; p < 0.001, I2 = 21.5, p = 0.28; t = -0.24, p = 0.81) (Fig. 5 ). Similarly, the pooled SMD value in European studies (SMD = 0.51, 95 % CI 0.37 to 0.65, p < 0.001; I2 = 9.2 %, p = 0.36) was not statistically significantly lower than that observed in Asian studies (SMD = 0.63, 95 % CI 0.39 to 0.87, p < 0.001; I2 = 91.9 %, p < 0.001; t = -0.56, p = 0.58) (Fig. 6 ). A relatively lower heterogeneity was observed in European studies (I2 = 9.2 %) and in those investigating ICU admission (I2 = 21.5 %).
Fig. 5.
Forest plot of studies reporting INR values in patients with COVID-19 according to disease severity or survival status. The diamond represents the point estimate and confidence intervals after combining and averaging the individual studies. The vertical line through the vertical points of the diamond represents the point estimate of the averaged studies.
Fig. 6.
Forest plot of studies reporting INR values in patients with COVID-19 according to the geographic area where the study was conducted. The diamond represents the point estimate and confidence intervals after combining and averaging the individual studies. The vertical line through the vertical points of the diamond represents the point estimate of the averaged studies.
4. Discussion
In our systematic review and meta-analysis, COVID-19 patients with severe forms of the disease or those who did not survive during follow-up had significantly prolonged INR values within 24–48 h from admission when compared to patients with milder disease or favorable outcomes. The magnitude of the observed SMD value, 0.60, indicates the presence of a biologically and clinically relevant difference between the groups [56]. Although the between-group heterogeneity was extreme the sequential omission of individual studies did not substantially influence the overall SMD value. Furthermore, there was no evidence of publication bias. The results of the meta-regression analysis suggest that the magnitude of the reported alterations in the INR in severe COVID-19 patients are significantly associated with the D-dimer, an established marker of coagulopathy and poor prognosis in this patient group, and the CRP, reflecting a close interplay between COVID-19-associated coagulopathy and systemic pro-inflammatory state [1,2]. Whilst the lack of significant associations with markers of liver injury (AST and ALT) or synthetic capacity (albumin) suggests that the observed alterations of the INR primarily reflect a state of coagulopathy, additional studies are required to investigate the relationship between the INR and liver dysfunction in COVID-19.
COVID-19-associated coagulopathy is characterized by some unique features that are at least in part driven by the direct interaction between the causative agent, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and endothelial cells [3]. As a result, the endothelial von Willebrand factor (VWF) and angiopoietin 2 are released into the circulation with consequent platelet activation and aggregation and stimulation of pro-inflammatory pathways [57,58]. While this phenomenon, and the related thrombus formation, is initially localized in the lungs, the further systemic activation of the coagulation system with thrombosis in multiple organs characterizes the clinical progress of patients with severe COVID-19 [2]. This initial phase of hypercoagulable state, which can be identified using viscoelastic tests [59], is followed by one of consumptive coagulopathy. The latter, paralleled by a shift from pulmonary compromise to multi-organ dysfunction, is characterized by a prolonged PT or INR, in addition to thrombocytopenia and D-dimer elevations [2]. However, while the PT and, consequently, the INR have been thought to be relatively maintained in the early stages of COVID-19, the results of our meta-analysis indicate the presence of significant elevations in these parameters within the first 24–48 of hospitalization, suggesting the presence of consumptive coagulopathy. This observation is supported by recent recommendations for the diagnosis of COVID-19-associated coagulopathy that include the presence of a prolonged PT (>1s) or a prolonged INR (>1.2), in addition to thrombocytopenia (platelet count <150 × 109/L), D-dimer elevations (>2 times the upper limit of normal), and presence of clinically overt thrombotic manifestations [2]. However, the mean INR value in patients with severe disease or non-survivor status was >1.2 in only 14 of the 38 identified studies in our meta-analysis [20,[31], [32], [33],38,41,[43], [44], [45], [46],[48], [49], [50],52], suggesting that further studies are required to determine the most accurate cut-off value for diagnostic and prognostic purposes.
4.1. Limitations of the study
The extreme between-study heterogeneity in our analyses represents a significant limitation that curtails the generalizability of the results. It is possible that other, unreported, factors might have contributed to the observed heterogeneity. On the contrary, we did not observe publication bias, and the overall effect size was not influenced in sensitivity analysis. Another limitation is related to the fact that none of the selected studies reported serial INR measurements during hospitalization, or their association with severity or clinical outcomes. This warrants further research as a study conducted in the early phases of the COVID-19 pandemic has shown the presence of significant differences in the temporal patterns of both PT and D-dimer, based on measurements performed on day 1, 4, 7, 10, and 14 after admission, between survivors and non-survivors. In particular, non-survivors had significantly longer PT values and higher D-dimer concentrations at day 1, 10 and 14 [60].
5. Conclusions
Our systematic review and meta-analysis with meta-regression has shown that prolonged INR values, indicating the presence of systemic coagulopathy, are significantly associated with severe disease and increased mortality in patients with COVID-19. Additional studies are required to determine whether single or serial INR measurements, with or without D-dimer and other clinical and demographic characteristics, can further enhance early risk stratification and management strategies, and indicate the presence of liver dysfunction in this group.
Financial disclosure
This research was supported by a Visiting Professorship awarded to Professor Arduino A Mangoni by the University of Sassari, Italy.
The author contribution
Study Design: Angelo Zinellu, Panagiotis Paliogiannis, Ciriaco Carru, Arduino A Mangoni.
Data Collection: Angelo Zinellu, Panagiotis Paliogiannis.
Statistical Analysis: Angelo Zinellu, Panagiotis Paliogiannis.
Data Interpretation: Angelo Zinellu, Panagiotis Paliogiannis, Ciriaco Carru, Arduino A Mangoni.
Manuscript Preparation: Arduino A Mangoni.
Literature Search: Angelo Zinellu, Panagiotis Paliogiannis.
Funds Collection: n/a.
Declaration of competing interest
The authors declare no conflict of interests.
References
- 1.Fajgenbaum D.C., June C.H. Cytokine storm. N Engl J Med. 2020;383:2255–2273. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iba T., Warkentin T.E., Thachil J., Levi M., Levy J.H. Proposal of the definition for COVID-19-associated coagulopathy. J Clin Med. 2021:10. doi: 10.3390/jcm10020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giordo R., Paliogiannis P., Mangoni A.A., Pintus G. SARS-CoV-2 and endothelial cell interaction in COVID-19: molecular perspectives. Vasc Biol. 2021;3:R15–R23. doi: 10.1530/VB-20-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asakura H., Ogawa H. COVID-19-associated coagulopathy and disseminated intravascular coagulation. Int J Hematol. 2021;113:45–57. doi: 10.1007/s12185-020-03029-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weitz J.I., Fredenburgh J.C., Eikelboom J.W. A test in context: D-dimer. J Am Coll Cardiol. 2017;70:2411–2420. doi: 10.1016/j.jacc.2017.09.024. [DOI] [PubMed] [Google Scholar]
- 6.Paliogiannis P., Mangoni A.A., Dettori P., Nasrallah G.K., Pintus G., Zinellu A. D-dimer concentrations and COVID-19 severity: a systematic review and meta-analysis. Front Public Health. 2020;8:432. doi: 10.3389/fpubh.2020.00432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirsh J., Poller L. The international normalized ratio. A guide to understanding and correcting its problems. Arch Intern Med. 1994;154:282–288. doi: 10.1001/archinte.154.3.282. [DOI] [PubMed] [Google Scholar]
- 8.Iba T., Levy J.H., Levi M., Thachil J. Coagulopathy in COVID-19. J Thromb Haemostasis. 2020;18:2103–2109. doi: 10.1111/jth.14975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wells G.A., Shea B., O'Connell D., Peterson J., Welch V., Losos M., Tugwell P. 2013. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses the Ottawa hospital research Institute.http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [cited 2020 20/11/2020]. Available from: [Google Scholar]
- 10.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bowden J., Tierney J.F., Copas A.J., Burdett S. Quantifying, displaying and accounting for heterogeneity in the meta-analysis of RCTs using standard and generalised Q statistics. BMC Med Res Methodol. 2011;11:41. doi: 10.1186/1471-2288-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 13.Tobias A. Assessing the influence of a single study in the meta-analysis estimate. Stata Techn Bull. 1999;47:15–17. https://econpapers.repec.org/article/tsjstbull/y_3a1999_3av_3a8_3ai_3a47_3asbe26.htm Available from: [Google Scholar]
- 14.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 15.Sterne J.A., Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 16.Duval S., Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 17.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gotzsche P.C., Ioannidis J.P., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aladağ N., Atabey R.D. The role of concomitant cardiovascular diseases and cardiac biomarkers for predicting mortality in critical COVID-19 patients. Acta Cardiol. 2021 Apr;76(2):132–139. doi: 10.1080/00015385.2020.1810914. [DOI] [PubMed] [Google Scholar]
- 19.Altschul D.J., Unda S.R., Benton J., de la Garza Ramos R., Cezayirli P., Mehler M., Eskandar E.N. A novel severity score to predict inpatient mortality in COVID-19 patients. Sci Rep. 2020;10 doi: 10.1038/s41598-020-73962-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bao C., Tao X., Cui W., Yi B., Pan T., Young K.H., Qian W. SARS-CoV-2 induced thrombocytopenia as an important biomarker significantly correlated with abnormal coagulation function, increased intravascular blood clot risk and mortality in COVID-19 patients. Exp Hematol Oncol. 2020;9:16. doi: 10.1186/s40164-020-00172-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bastug A., Bodur H., Erdogan S., Gokcinar D., Kazancioglu S., Kosovali B.D., Ozbay B.O., Gok G., Turan I.O., Yilmaz G., Gonen C.C., Yilmaz F.M. Clinical and laboratory features of COVID-19: predictors of severe prognosis. Int Immunopharm. 2020;88:106950. doi: 10.1016/j.intimp.2020.106950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bocci M.G., Maviglia R., Consalvo L.M., Grieco D.L., Montini L., Mercurio G., Nardi G., Pisapia L., Cutuli S.L., Biasucci D.G., Gori C., Rosenkranz R., De Candia E., Carelli S., Natalini D., Antonelli M., Franceschi F. Thromboelastography clot strength profiles and effect of systemic anticoagulation in COVID-19 acute respiratory distress syndrome: a prospective, observational study. Eur Rev Med Pharmacol Sci. 2020;24:12466–12479. doi: 10.26355/eurrev_202012_24043. [DOI] [PubMed] [Google Scholar]
- 23.Bonetti G., Manelli F., Patroni A., Bettinardi A., Borrelli G., Fiordalisi G., Marino A., Menolfi A., Saggini S., Volpi R., Anesi A., Lippi G. Laboratory predictors of death from coronavirus disease 2019 (COVID-19) in the area of Valcamonica, Italy. Clin Chem Lab Med. 2020;58:1100–1105. doi: 10.1515/cclm-2020-0459. [DOI] [PubMed] [Google Scholar]
- 24.Carlino M.V., Valenti N., Cesaro F., Costanzo A., Cristiano G., Guarino M., Sforza A. Predictors of Intensive Care Unit admission in patients with coronavirus disease 2019 (COVID-19) Monaldi Arch Chest Dis. 2020:90. doi: 10.4081/monaldi.2020.1410. [DOI] [PubMed] [Google Scholar]
- 25.Cheng B., Hu J., Zuo X., Chen J., Li X., Chen Y., Yang G., Shi X., Deng A. Predictors of progression from moderate to severe coronavirus disease 2019: a retrospective cohort. Clin Microbiol Infect. 2020;26:1400–1405. doi: 10.1016/j.cmi.2020.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng L., Yang J.Z., Bai W.H., Li Z.Y., Sun L.F., Yan J.J., Zhou C.L., Tang B.P. Prognostic value of serum amyloid A in patients with COVID-19. Infection. 2020;48:715–722. doi: 10.1007/s15010-020-01468-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong Y., Zhou H., Li M., Zhang Z., Guo W., Yu T., Gui Y., Wang Q., Zhao L., Luo S., Fan H., Hu D. A novel simple scoring model for predicting severity of patients with SARS-CoV-2 infection. Transbound Emerg Dis. 2020;67:2823–2829. doi: 10.1111/tbed.13651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gong J., Ou J., Qiu X., Jie Y., Chen Y., Yuan L., Cao J., Tan M., Xu W., Zheng F., Shi Y., Hu B. A tool for early prediction of severe coronavirus disease 2019 (COVID-19): a multicenter study using the risk Nomogram in wuhan and Guangdong, China. Clin Infect Dis. 2020;71:833–840. doi: 10.1093/cid/ciaa443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gue Y.X., Tennyson M., Gao J., Ren S., Kanji R., Gorog D.A. Development of a novel risk score to predict mortality in patients admitted to hospital with COVID-19. Sci Rep. 2020;10 doi: 10.1038/s41598-020-78505-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hou W., Zhang W., Jin R., Liang L., Xu B., Hu Z. Risk factors for disease progression in hospitalized patients with COVID-19: a retrospective cohort study. Infect Dis (Lond) 2020;52:498–505. doi: 10.1080/23744235.2020.1759817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin X., Duan Y., Bao T., Gu J., Chen Y., Li Y., Mao S., Chen Y., Xie W. The values of coagulation function in COVID-19 patients. PloS One. 2020;15 doi: 10.1371/journal.pone.0241329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kayina C.A., Haritha D., Soni L., Behera S., Nair P.R., Gouri M., Girish K., Deeparaj L., Maitra S., Anand R.K., Ray B.R., Baidya D.K., Subramaniam R. Epidemiological & clinical characteristics & early outcome of COVID-19 patients in a tertiary care teaching hospital in India: a preliminary analysis. Indian J Med Res. 2020;152:100–104. doi: 10.4103/ijmr.IJMR_2890_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ke C., Yu C., Yue D., Zeng X., Hu Z., Yang C. Clinical characteristics of confirmed and clinically diagnosed patients with 2019 novel coronavirus pneumonia: a single-center, retrospective, case-control study. Med Clin (Barc) 2020;155:327–334. doi: 10.1016/j.medcli.2020.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kong M., Zhang H., Cao X., Mao X., Lu Z. Higher level of neutrophil-to-lymphocyte is associated with severe COVID-19. Epidemiol Infect. 2020;148:e139. doi: 10.1017/S0950268820001557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lei P., Zhang L., Han P., Zheng C., Tong Q., Shang H., Yang F., Hu Y., Li X., Song Y. Liver injury in patients with COVID-19: clinical profiles, CT findings, the correlation of the severity with liver injury. Hepatol Int. 2020;14:733–742. doi: 10.1007/s12072-020-10087-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Linli Z., Chen Y., Tian G., Guo S., Fei Y. Identifying and quantifying robust risk factors for mortality in critically ill patients with COVID-19 using quantile regression. Am J Emerg Med. 2021 Jul;45:345–351. doi: 10.1016/j.ajem.2020.08.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu J., Li S., Liu J., Liang B., Wang X., Wang H., Li W., Tong Q., Yi J., Zhao L., Xiong L., Guo C., Tian J., Luo J., Yao J., Pang R., Shen H., Peng C., Liu T., Zhang Q., Wu J., Xu L., Lu S., Wang B., Weng Z., Han C., Zhu H., Zhou R., Zhou H., Chen X., Ye P., Zhu B., Wang L., Zhou W., He S., He Y., Jie S., Wei P., Zhang J., Lu Y., Wang W., Zhang L., Li L., Zhou F., Wang J., Dittmer U., Lu M., Hu Y., Yang D., Zheng X. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020 May;55:102763. doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lorente L., Martin M.M., Argueso M., Sole-Violan J., Perez A., Marcos Y.R.J.A., Ramos-Gomez L., Lopez S., Franco A., Gonzalez-Rivero A.F., Martin M., Gonzalez V., Alcoba-Florez J., Rodriguez M.A., Riano-Ruiz M., Guillermo O.C.J., Gonzalez L., Cantera T., Ortiz-Lopez R., Ojeda N., Rodriguez-Perez A., Dominguez C., Jimenez A. Association between red blood cell distribution width and mortality of COVID-19 patients. Anaesth Crit Care Pain Med. 2020 doi: 10.1016/j.accpm.2020.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luo H.C., You C.Y., Lu S.W., Fu Y.Q. Characteristics of coagulation alteration in patients with COVID-19. Ann Hematol. 2021;100:45–52. doi: 10.1007/s00277-020-04305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mertoglu C., Huyut M.T., Arslan Y., Ceylan Y., Coban T.A. How do routine laboratory tests change in coronavirus disease 2019? Scand J Clin Lab Invest. 2021;81:24–33. doi: 10.1080/00365513.2020.1855470. [DOI] [PubMed] [Google Scholar]
- 41.Mori S., Ai T., Otomo Y. Characteristics, laboratories, and prognosis of severe COVID-19 in the Tokyo metropolitan area: a retrospective case series. PloS One. 2020;15 doi: 10.1371/journal.pone.0239644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ponziani F.R., Del Zompo F., Nesci A., Santopaolo F., Ianiro G., Pompili M., Gasbarrini A. Gemelli against C-g. Liver involvement is not associated with mortality: results from a large cohort of SARS-CoV-2-positive patients. Aliment Pharmacol Ther. 2020;52:1060–1068. doi: 10.1111/apt.15996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pourabdollah Toutkaboni M., Askari E., Khalili N., Tabarsi P., Jamaati H., Velayati A.A., Dorudinia A., Rezaei M., Nadji S.A., Mohamadnia A., Khalili N. Demographics, laboratory parameters and outcomes of 1061 patients with coronavirus disease 2019: a report from Tehran, Iran. New Microbes New Infect. 2020 Nov;38:100777. doi: 10.1016/j.nmni.2020.100777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sadeghi A., Eslami P., Dooghaie Moghadam A., Pirsalehi A., Shojaee S., Vahidi M., Soheili A., Ghanimat F., Keshmiri Y., Abdi S., Zali M.R. COVID-19 and ICU admission associated predictive factors in Iranian patients. Caspian J Intern Med. 2020;11:512–519. doi: 10.22088/cjim.11.0.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sayad B., Rahimi Z. Blood coagulation parameters in patients with severe COVID-19 from Kermanshah Province, Islamic Republic of Iran. East Mediterr Health J. 2020;26:999–1004. doi: 10.26719/emhj.20.105. [DOI] [PubMed] [Google Scholar]
- 46.Shahriarirad R., Khodamoradi Z., Erfani A., Hosseinpour H., Ranjbar K., Emami Y., Mirahmadizadeh A., Lotfi M., Shirazi Yeganeh B., Dorrani Nejad A., Hemmati A., Ebrahimi M., Moghadami M. Epidemiological and clinical features of 2019 novel coronavirus diseases (COVID-19) in the South of Iran. BMC Infect Dis. 2020;20:427. doi: 10.1186/s12879-020-05128-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun J.T., Chen Z., Nie P., Ge H., Shen L., Yang F., Qu X.L., Ying X.Y., Zhou Y., Wang W., Zhang M., Pu J. Lipid Profile features and their associations with disease severity and mortality in patients with COVID-19. Front Cardiovasc Med. 2020;7:584987. doi: 10.3389/fcvm.2020.584987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsibouris P., Ekmektzoglou K., Agorogianni A., Kalantzis C., Theofanopoulou A., Toumbelis K., Petrogiannopoulos L., Poutakidis C., Goggaki S., Braimakis I., Vlachou E., Pouliakis A., Apostolopoulos P. Gastrointestinal involvement in COVID-19 patients: a retrospective study from a Greek COVID-19 referral hospital. Ann Gastroenterol. 2020;33:465–472. doi: 10.20524/aog.2020.0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang C., Deng R., Gou L., Fu Z., Zhang X., Shao F., Wang G., Fu W., Xiao J., Ding X., Li T., Xiao X., Li C. Preliminary study to identify severe from moderate cases of COVID-19 using combined hematology parameters. Ann Transl Med. 2020;8:593. doi: 10.21037/atm-20-3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang J.H., Chen R.D., Yang H.K., Zeng L.C., Chen H., Hou Y.Y. Inflammation-associated factors for predicting in-hospital mortality in patients with COVID-19. J Med Virol. 2021 May;93(5):2908–2917. doi: 10.1002/jmv.26771. [DOI] [PubMed] [Google Scholar]
- 51.Xue G., Gan X., Wu Z., Xie D., Xiong Y., Hua L., Zhou B., Zhou N., Xiang J., Li J. Novel serological biomarkers for inflammation in predicting disease severity in patients with COVID-19. Int Immunopharm. 2020;89:107065. doi: 10.1016/j.intimp.2020.107065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y., He L., Chen H., Lu S., Xiong Y., Liu J., Zheng Y., Wang S., Liu L. Manifestations of blood coagulation and its relation to clinical outcomes in severe COVID-19 patients: retrospective analysis. Int J Lab Hematol. 2020;42:766–772. doi: 10.1111/ijlh.13273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Y., Zheng L., Liu L., Zhao M., Xiao J., Zhao Q. Liver impairment in COVID-19 patients: a retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. 2020;40:2095–2103. doi: 10.1111/liv.14455. [DOI] [PubMed] [Google Scholar]
- 54.Zhou C., Huang Z., Tan W., Li X., Yin W., Xiao Y., Tao Z., Geng S., Hu Y. Predictive factors of severe coronavirus disease 2019 in previously healthy young adults: a single-center, retrospective study. Respir Res. 2020;21:157. doi: 10.1186/s12931-020-01412-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zou Y., Guo H., Zhang Y., Zhang Z., Liu Y., Wang J., Lu H., Qian Z. Analysis of coagulation parameters in patients with COVID-19 in Shanghai, China. Biosci Trends. 2020;14:285–289. doi: 10.5582/bst.2020.03086. [DOI] [PubMed] [Google Scholar]
- 56.Cohen J. second ed. Erlbaum; Hillsdale, NJ, USA: 1988. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- 57.Ward S.E., Curley G.F., Lavin M., Fogarty H., Karampini E., McEvoy N.L., Clarke J., Boylan M., Alalqam R., Worrall A.P., Kelly C., de Barra E., Glavey S., Ni Cheallaigh C., Bergin C., Martin-Loeches I., Townsend L., Mallon P.W., O'Sullivan J.M., O'Donnell J.S., Irish C.-V.S.I. Von Willebrand factor propeptide in severe coronavirus disease 2019 (COVID-19): evidence of acute and sustained endothelial cell activation. Br J Haematol. 2021;192:714–719. doi: 10.1111/bjh.17273. [DOI] [PubMed] [Google Scholar]
- 58.Pine A.B., Meizlish M.L., Goshua G., Chang C.H., Zhang H., Bishai J., Bahel P., Patel A., Gbyli R., Kwan J.M., Won C.H., Price C., Dela Cruz C.S., Halene S., van Dijk D., Hwa J., Lee A.I., Chun H.J. Circulating markers of angiogenesis and endotheliopathy in COVID-19. Pulm Circ. 2020;10 doi: 10.1177/2045894020966547. 2045894020966547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spiezia L., Campello E., Cola M., Poletto F., Cerruti L., Poretto A., Simion C., Cattelan A., Vettor R., Simioni P. More severe hypercoagulable state in acute COVID-19 pneumonia as compared with other pneumonia. Mayo Clin Proc Innov Qual Outcomes. 2020;4:696–702. doi: 10.1016/j.mayocpiqo.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemostasis. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]