Abstract
In this review, we focus on the potential role of the γ-aminobutyric acidergic (GABAergic) system in age-related episodic memory impairments in humans, with a particular focus on Alzheimer’s disease (AD). Well-established animal models have shown that GABA plays a central role in regulating and synchronizing neuronal signaling in the hippocampus, a brain area critical for episodic memory that undergoes early and significant morphologic and functional changes in the course of AD. Neuroimaging research in humans has documented hyperactivity in the hippocampus and losses of resting state functional connectivity in the Default Mode Network, a network that itself prominently includes the hippocampus—presaging episodic memory decline in individuals at-risk for AD. Apolipoprotein ε4, the highest genetic risk factor for AD, is associated with GABAergic dysfunction in animal models, and episodic memory impairments in humans. In combination, these findings suggest that GABA may be the linchpin in a complex system of factors that eventually leads to the principal clinical hallmark of AD: episodic memory loss. Here, we will review the current state of literature supporting this hypothesis. First, we will focus on the molecular and cellular basis of the GABAergic system and its role in memory and cognition. Next, we report the evidence of GABA dysregulations in AD and normal aging, both in animal models and human studies. Finally, we outline a model of GABAergic dysfunction based on the results of functional neuroimaging studies in humans, which have shown hippocampal hyperactivity to episodic memory tasks concurrent with and even preceding AD diagnosis, along with factors that may modulate this association.
Keywords: γ-aminobutyric acid, Aging, Alzheimer’s disease, Episodic memory, Hippocampus
1. Introduction
Dementia is a progressive and chronic syndrome in which there is a loss of cognitive functions—usually accompanied or preceded by a deterioration of emotional control and social behavior—that leads to disability and dependency [1]. Alzheimer’s disease (AD), among the most severe and burdensome medical conditions worldwide, is the most common cause of dementia, accounting for 43.5–62.8% in developed countries, followed by vascular dementia, which represents 14.5–30.9% of dementia cases [2-4].
There is consensus that AD pathology starts with a preclinical or prodromal phase, which is thought to begin 20 or more years before clinical symptoms become apparent, but during which time individuals may self-report the worsening of cognitive functions [5,6]. This preclinical phase is followed by a transitional state preceding dementia known as mild cognitive impairment (MCI), which is characterized by objective impairment of cognitive function but relatively spared independence in activities of daily living [7,8]. It is estimated that 10–20% of individuals over the age of 65 are diagnosed with MCI, and the annual conversion rate to dementia is 5–20% [9,10]. After MCI, dementia onset is characterized by an exacerbation of cognitive and behavioral symptoms, and the loss of independence.
Episodic memory impairment is widely recognized as the hallmark symptom of AD [11], and its assessment is one of the principal clinical tools used to determine the stage of AD, with memory recognition tasks frequently employed to diagnose amnesic patterns of neuropsychological alterations [12-14]. Changes to other cognitive domains—e.g., language, executive and visuospatial functions—may be also present depending on the AD subtype and the progression of the disease.
Pathologically, at the microscopic level AD is characterized by the formation of extracellular senile plaques due to the deposition of amyloid β (Aβ) peptides, and the accumulation of hyperphosphorylated tau in neurofibrillary tangles [15-17]. Recent work suggests that tau and Aβ are interconnected, and together trigger the neurodegeneration in the brain that ultimately results in AD [18-20]. At the macroscopic level, AD is characterized by the presence of cortical and subcortical atrophy, particularly in hippocampal and medial temporal lobe regions which are highly vulnerable to the progression of AD pathology [21-24]. There is also an accumulation of cerebrovascular lesions [25-27]—infarcts, cerebral microbleeds, white matter hyperintensities, among others—exacerbated by sustained exposure to vascular risk factors such as hypertension and diabetes, which cause damage to the small vessels of the brain and accelerate Aβ and tau pathology [28-30].
Despite decades of research investigating β-amyloid as the trigger for a cascade of neuro-pathophysiological events that cause AD, the relationship between Aβ and cognition still remains weak: 20–30% of adults meet research criteria for preclinical AD based on levels of extracellular Aβ plaque deposition assessed by Pittsburgh Compound B (PiB) positron emission tomography (PET), yet are asymptomatic and cognitively normal on standard neuropsychological tests [31]. Moreover, the recent failures of several high profile late-stage clinical trials targeting Aβ highlights the urgent need to explore new mechanisms that could be involved in AD pathogenesis [32-34]. While both the cholinergic and glutamatergic systems have received considerable interest, the role of the γ-aminobutyric acidergic (GABAergic) system in human studies of aging and AD has garnered far less attention [35-37].
Animal models, however, have provided strong evidence for a GABAergic mechanism of memory impairment. According to these models, GABA plays a central role in regulating, synchronizing and preventing excess neuronal signaling in the hippocampus [38,39], a brain area critical for episodic memory [40-42] that undergoes early and significant morphologic and functional changes in AD [43]. Moreover, studies in AD animal models have revealed that early losses of GABAergic interneurons result in hippocampal hyperactivity [44-46]. This process may be mediated or exacerbated by the presence of apolipoprotein ε4 (APOE ε4), the highest genetic risk factor for AD [46]. On the other hand, neuroimaging studies in patients with AD and MCI have reported hyperactivity in the hippocampus [47] and losses of resting state functional connectivity in the Default Mode Network (DMN), a network that itself prominently includes the hippocampus [48]—presaging atrophy and episodic memory decline in individuals at-risk for AD [49,50]. Hence, neural network hyperexcitability is now recognized as part of the spectrum of changes that occur in AD, and it is possible that GABA loss may be the trigger. Interestingly, from a clinical point of view, AD and MCI patients present an increased risk of developing seizures—one of the most common manifestations of aberrant neural activity—reinforcing the evidence of GABAergic dysfunction and neural disturbances during the dementia course [51,52].
In this review, we focus on recent research on GABAergic dysregulation, which we argue may be an early biomarker of AD-related episodic memory decline. As several groups have thoroughly reviewed the animal work surrounding this topic [53-61], the primarily focus here will be on human work [37,62,63]. We will describe the evidence for a GABAergic mechanism in age- and AD-related cognitive decline, first by outlining the molecular and cellular basis of the GABAergic system and its role in memory and cognition. Next, we report the evidence of GABA dysregulations in AD and normal aging, both in animal and human studies. Finally, we will review functional neuroimaging studies in humans that have shown hippocampal hyperactivity to episodic memory tasks concurrent with and preceding AD diagnosis, along with factors that may modulate this association.
2. Role of the GABAergic system in cognition and behavior
2.1. The GABAergic system
Since its discovery in 1950 [64], GABA has been considered to be the principal inhibitory neurotransmitter in the mammalian brain, playing a crucial role synchronizing the activity of human cortical networks [39]. About 10% of cells in the brain are GABAergic, and their receptors make up almost half of all receptors in the human brain, being one of the most ubiquitous neurotransmitter systems [53,65,66] and participating in a wide range of physiological and behavioral processes, including learning and memory [60,67,68].
GABA is synthesized by the decarboxylation of glutamate via the enzyme glutamic acid decarboxylase (GAD), and GABA-transaminase is responsible for its elimination and metabolism [69,70]. After synthesis, GABA is stored in synaptic vesicles by a vesicular GABA transporter [71]. In the brain, GABA neurotransmission is primarily mediated by ionotropic (GABAA) and metabotropic (GABAB) receptors, which both can be found in presynaptic and postsynaptic terminals [65,72].
GABAA is the most common type of GABA receptor in the human brain. GABAA receptors are pentameric transmembrane receptors permeable to Cl−, and formed by an α-1 to 6, β-1 to 4 and γ-1 to 4 subunits—a, δ-, ε-, π- or Θ- subunits may substitute the γ subunit [72,73]. This confers a wide spectrum of possible GABAA configurations, although most GABAA receptors are composed of two α-, two β- and one γ-subunits [74]. Of note, GABAA subunit configuration may vary depending on the brain area assayed, and in some neurological conditions [62,75]. Interestingly, 25% of GABAA receptors in the hippocampus contain an α5 subunit, as compared to 7–8% in the rest of the brain [62,75]. Pharmacologically, GABAA transmission may be modulated by full agonists (e.g., muscimol), positive allosteric modulators (e.g., benzodiazepines, binding at the interface between α- and γ-subunits [76]), competitive antagonists (e.g., bicuculline), non-competitive antagonists (e.g., picrotoxin) and negative allosteric modulators (inverse agonists) [77]. On the other hand, GABAB receptors are G-protein coupled heterodimer receptors consisting of a GABAB1 and a GABAB2 subunit [60]. The principal agonist and antagonist substances of GABAB receptors are baclofen and saclofen, respectively [60]. Of import to the current review, immunohistochemical studies have reported a high expression of GABAB receptors in the hippocampus [78,79].
At the cellular level, in the neocortex most GABAergic neurons are interneurons. Interneurons are phenotypically diverse, and can be classified depending on their cytoarchitecture (e.g., basket, chandelier, stellate, neurogliaform cells, among other configurations), their molecular properties (parvalbumin (PV)-, calretinin-, calbindin-, somatostatin (SOM)-expressing, among others), the region that they innervate (local or long projecting interneurons), as well as the GABA synthesizing enzymes (GAD65- and GAD67-positive cells) [65,66,80-82]. This confers to GABAergic cells a considerable morphological and physiological diversity, which may explain in part the wide spectrum of functions in which the GABA system is implicated [83].
GABAergic inhibition may be classified as either phasic or tonic [37, 84]. Phasic inhibition is produced by the release of GABA from the presynaptic membrane to the synaptic cleft, hyperpolarizing the postsynaptic neuron via opening the GABAA receptor ion-gated channels—the inhibitory post synaptic potential [84]. The principal characteristic of phasic inhibition is the short gap of time in which postsynaptic receptors are exposed to GABA, principally due to the rapid diffusion of GABA to the extrasynaptic location [85]. Phasic inhibition generates synchronized activity in different populations of neurons [81, 86,87]. Tonic inhibition, on the other hand, is produced by the sustained activation of GABA receptors at the extrasynaptic location after the release of GABA to the synaptic cleft, reducing the likelihood that a neuron firing over time and preventing neural network hyperactivity [84]. Interestingly, synaptic plasticity in the hippocampus may be regulated by tonic inhibition, which is thought to be principally mediated by GABAA receptors containing the α5 subunit [88,89].
2.2. GABA in behavior and cognition
GABA is widely distributed in the brain and its receptors present a high diversity of conformations. As such, the GABAergic system has been associated with a wide range of behavioral and cognitive functions including the regulation of vigilance, anxiety, learned fear and memory [59,60,90,91]. Furthermore, GABA signaling has long been considered as a potential underlying mechanism in a number of diseases, including schizophrenia, anxiety disorders, depression, bipolar disorder, autism, and others [90,92-97].
The role of GABA in long term memory formation has been widely studied using animal models. Over two decades ago, the spatiotemporal activity of hippocampal GABAergic interneurons was found to be critical for the regulation of neural network activity involved in memory [67]. The GABAergic system in the hippocampus is responsible for maintaining the excitatory/inhibitory (E/I) balance and synchronizing the activity of several populations of pyramidal neurons within the hippocampus, as well as between remote regions of the brain [98]. GABAA mediated phasic inhibition from GABAergic interneurons has been shown to coordinate the neural activity between the hippocampus and the entorhinal cortex by generating and maintaining theta (4–12 Hz) and gamma (30–100 Hz) frequency oscillations, allowing for successful memory encoding and retrieval [99,100]. By contrast, GABAB mediated tonic inhibition is believed to terminate this coordinated activity, regulating its duration [101]. Moreover, GABAB activation may be implicated in the transition from theta and gamma oscillations, a process which may be related to the consolidation of memories [102,103].
It is also well known that GABA is involved in learned fear. Administration of GABAA agonists either before or after fear conditioning disrupts the acquisition of fear memories, while the administration of GABAA antagonists facilitates them [58,91]. These results indicate that GABAergic system is involved in both the formation and consolidation of conditioned fear stimuli. For this reason, GABAA modulation has been proposed as a target for intervention in the treatment of psychiatric conditions including posttraumatic stress disorder and social phobia disorder [104,105].
GABA activity may also be related to other cognitive processes, including working memory and thought suppression. Rao et al., (2000) showed that administration of bicuculline into the dorsolateral prefrontal cortex (DLPFC) of rhesus monkeys impairs working memory [106]. Likewise, other studies reported that DLPFC interneurons display activity specific for a working memory task in monkeys [107,108]. In humans, microarray postmortem studies showed downregulated genes encoding GABA-related proteins in the DLPFC of patients diagnosed with schizophrenia [108,109], who have been shown to have impairments in working memory as well as deficits in other cognitive functions [110-112]. Likewise, Schmitz et al., (2017) recently showed that GABA plays a key role in regulating a fronto-hippocampal circuit which enables the voluntary control over intrusive thoughts [113].
Together, these studies suggest that GABA plays a key role in many aspects of both normal and abnormal cognitive function [92]. These findings have motivated the study of GABAergic disturbances in aging, especially in the context of AD. In the next section, we review the evidence for alterations of GABAergic function in the aging brain, from animal to human studies.
3. Evidence of GABA dysregulations in Alzheimer’s disease and aging
3.1. Studies in animals
Changes in the GABAergic system in AD and normal aging have been widely studied using animal models. However, discordant results have made it challenging to disentangle the precise role of GABA in age-related cognitive decline. Several previous articles have thoroughly reviewed GABA dysregulations in animal models [53-61]. In this section, therefore, we will highlight and summarize the most important conclusions from these earlier reviews.
Pathological markers of AD are thought to be associated with altered GABA signaling. Traditionally, GABAergic neurons were considered to be more resistant to Aβ pathology as compared to cholinergic or glutamatergic neurons [114]. More recent in vitro experiments, however, have reported that Aβ neurotoxicity impairs GABAergic neuron activity and weakens inhibitory postsynaptic potentials by downregulating postsynaptic GABAA receptors [115,116]. Similarly, TgCRND8 mice, which exhibit early Aβ deposition, show a loss of GABAergic neurons starting at 6 months [115]. In line with these results, APP/PS1 mice present a 50–60% reduction in the number of GABAergic interneurons coexpressing SOM and NPY at 6 months, preceding pyramidal cell loss, which suggests that GABAergic dysfunction may be an early sign of AD-related pathology [44]. SOM-positive cells are the principal population of interneurons innervating the dendritic arborization of pyramidal cells and may be involved in functions such as dendritic plasticity, synchronization of rhythmic activity, and the formation of new spatial memories [44,83,117,118]. On the other hand, APP/PS1 mice display other alterations in GABAergic function, including a significant reduction of GABAB receptors in the hippocampus and dentate gyrus starting at 6 months and increasing with age [119,120]. Regarding tau pathology, JNPL3(P301L) mice, which expresses human tau at twice endogenous levels, show a significant reduction in the number of GAD-, SOM- and PV-positive cells in the hippocampus [45]. Furthermore, tau markers co-localize with these populations of interneurons, suggesting that tau may be promoting a loss of GABA neurotransmission in the hippocampus [45,46].
Although animal models show that GABAergic dysfunction parallels AD-related pathology, it is less clear whether increasing GABA neurotransmission may be beneficial to prevent cognitive impairment in these models. For instance, administration of the GABAA receptor agonist muscimol in young Long Evans rats was shown to impair, not enhance, retrieval of learned spatial information in the Morris water maze task [121]. It is also well known that the administration of benzodiazepines produces anterograde amnesia and learning deficits in animal models [59,122]. Moreover, young male mice (3 months) that received chronic administration of methyl ß-carboline-3carboxylate (β-CCM)—a negative allosteric modulator, or inhibitor of the GABAA receptor—made fewer errors in the retention phase of a T-maze task as compared to controls or animals treated with benzodiazepines, suggesting that β-CCM improves, rather than hinders, spatial learning and memory [59,123]. These findings indicate that increasing GABA activity—in young animals at least—may be disadvantageous, resulting in negative cognitive outcomes.
Several studies have focused on the modulation of the GABAA α5 receptor, which is indeed a promising therapeutic target due to its high expression in the hippocampus and its well-established role in memory. Nonetheless, most studies testing the effect of inverse agonists of the GABAA α5 receptor, which have effects opposite to the agonists of that receptor, have been conducted in young animals [123], and when these drugs were tested in aged animals, no beneficial effects on memory were observed [124]. This discrepancy may be partially explained by the fact that GABA neurotransmission changes substantially across the lifespan, from an excitatory to an inhibitory neurotransmitter [65]. Interestingly, GABAA α5 positive allosteric modulators show the opposite pattern: Koh et al. (2013) found that a novel positive allosteric modulator of the GABAA α5 receptor improved memory performance in aged male Long Evans rats (24–26 months), while having no effect in young (6 month old) rats [124]. The authors proposed that these results may be explained by the excess of activation that occurs with age in the hippocampus, especially in the CA3 area [124,125]. In line with this hypothesis, GABAA α5 receptors are especially involved in tonic inhibition, and their selective reduction has been shown to produce network hyperactivity in the hippocampus [126]. Similarly, novel benzodiazepine-like ligands, which act on GABAA α receptors, have been shown to reverse working memory deficits in aged (21–22 month old) C57BL/6 mice [127].
Regarding the modulation of GABAB receptors, the administration of the GABA agonist baclofen impairs learning of spatial information in wild-type animals [128], while the administration of CGP35348—a GABAB receptor antagonist—improves it [129]. In line with these results, Bañuelos et al. reported that the medial prefrontal cortex of aged (22 month) male Fischer rats showed a lower expression of GABAB R1a-b and R2, and a higher expression of the GABA synthesizing GAD67 than did younger (6 month) animals. Further, systemic administration of CGP35348 improved working memory in the aged group, while it had a detrimental effect in the young group [130]. Likewise, the GABAB receptors antagonist SGS742 improved memory performance in a two-way active avoidance task, an eight-arm radial maze, and a Morris water maze task in rodents and in non-human primates [131-133]. By contrast, Beas and colleagues(2017) reported that a lower expression of the GABAB1 receptor in the medial prefrontal cortex of aged rats was associated with a better performance on a set-shifting task, suggesting a role for GABAB1 receptors in cognitive flexibility [134]. Paradoxically, intra-cerebral administration of baclofen has been shown to improve cognitive flexibility in aged rats [134]. Hence, modulation of GABAB receptor may have different consequences depending on the studied brain area and the assessed cognitive function, such that increasing GABAB neurotransmission may improve cognitive flexibility but impair working memory.
Altogether, recent research suggests that GABAergic dysfunction may be an early sign of AD pathology in animal models. Nonetheless, the picture is complex, and it is not clear how to modulate GABA neurotransmission to prevent age-related cognitive impairment. The age of the animal may be a critical variable: while decreasing GABAergic function in early life appears to facilitate memory, downregulating this system in mid to late life appears to have the opposite effect, leading to cognitive impairment. Moreover, modulation of GABAA and GABAB receptors may have different effects on cognition. Other variables that may mediate the relationship between GABA and cognitive performance are the brain area in which the GABAergic dysfunction is produced and the subpopulation of interneurons [135], among other parameters.
3.2. Studies in humans
3.2.1. Postmortem studies
Despite numerous neuropathological studies investigating alterations of GABA levels and function in postmortem samples of AD patients [37,63], no clear consensus has been reached [37], potentially due to heterogeneity of the brain regions assessed, differences in the post-mortem delay, differing sample sizes, and the control over confounding variables (e.g., sex, medication use, cause of death, etc.) [63]. Several investigations of the hippocampus [136,137], as well as other limbic areas such as the cingulate cortex [138,139] and amygdala [137, 139] have shown reduced GABA levels in the brains of AD patients. However, of note, the hippocampus is one of the regions that showed the most discordant results, and several reports did not confirm GABA downregulation in this area, although these studies had small sample sizes [138,139]. Similarly, reduced GABA levels in AD have been shown to occur in the temporal [138,140-143], frontal [136,138,141] and parietal [138,140,141] cortices. By contrast, the caudate, putamen and the globus pallidus were found to be spared [136-138,143]. These results suggest that GABA reduction may be present in brain regions that are more susceptible to AD neurodegeneration, including cortical and limbic regions, while subcortical structures may be spared.
Other changes in the GABAergic system reported in postmortem studies include reductions in GAD and GABA receptors. GAD-65, which is principally expressed in neurons at the synapse, is thought to be reduced in the hippocampus and temporal cortex of AD patients [144]. Likewise, a downregulation of GAD has been found in vascular and mixed dementia [145]. As summarized by Govindpani et al., (2017) differences in GABAA receptors in AD arise out of both the subunit composition of GABAA receptors, and the anatomical localization [37]. In the hippocampus, several authors have reported reduced concentration or expression of both GABAA α1 [146,147] and GABAA α5 [147, 148] subunits in AD individuals [37]. GABAA α1 and GABAA α5 receptor concentrations have been reported to be reduced in the temporal and entorhinal cortex, respectively [149,150]. Finally, Rissman et al. found a reduction in mRNA immunoreactivity of GABAA α1 (20–25% reduction) and α5 (32–35% reduction) receptors in both patients with MCI and probable dementia, which suggests that hippocampal GABAA receptor abnormalities may appear in the initial stages of AD [148]. However, although interesting, these results should be interpreted with caution because these reductions in mRNA may result only in small differences in the protein levels [147].
Taken together, although postmortem studies may present limitations due to the presence of noise in GABA levels produced by the agonal state [141], the majority of studies have reported GABA alterations in the AD brain, although the magnitude of these changes and the affected brain areas are still a subject of debate. Future research in these areas—for example, postmortem studies of patients diagnosed with MCI or studies that aim to correlate Braak staging with GABA levels—are critically needed to achieve consensus regarding GABAergic changes that occur in AD.
3.2.2. Cerebrospinal fluid studies
Several groups have analyzed GABA concentration in the cerebrospinal fluid (CSF) of AD patients. While a number have reported lower CSF GABA levels for AD patients as compared to matched controls [151-155], others found no significant associations [156-158], and one group reported increased GABA levels in AD patients [159]. A recent meta-analysis by Manyevitch et al. using data from 12 studies (including 182 AD patients and 176 controls) revealed a 26% reduction of CSF GABA levels for AD-group relative to the control group (P-value=0.01). However, high heterogeneity between the studies included was also noted [160]. GABA downregulation has also been reported in patients with Biswanger’s disease [161]—a subcortical type of vascular dementia—and Parkinson’s disease [153], suggesting that GABA changes in the CSF may be not specific to AD.
As in the post-mortem studies, the current literature in this topic is also characterized by methodological diversity and limitations. First, many studies did not match AD and control groups by age or sex, and, as reported by Bareggi et al. (1982), GABA declines with age in the CSF [162]. Moreover, several studies included AD patients at different stages of the disease. Finally, most studies did not adjust their results for the use of benzodiazepines or other central nervous system medications that may impact GABA levels in the CSF.
Hence, more research is needed, particularly in light of the fact that CSF biomarkers (hyperphosphorylated tau, Aβ42, Aβ42/40 ratio, among others) are routinely collected and used in clinical practice [163]. Replications in larger cohort studies in which it will be easier to control for potential confounders and make use of additional clinical information, associating GABA and other biomarkers of AD-related pathology in the CSF, and longitudinal studies including patients with MCI, will help to establish how GABA levels are affected in and by AD.
3.2.3. Magnetic resonance spectroscopy studies
Brain metabolites and biochemical processes can be measured in vivo via magnetic resonance spectroscopy (MRS). MRS classifies molecules according to their resonance and spectral patterns in a specific location of the brain, which is decided a priori [164]. GABA levels may be corrected by the resonance of other macromolecules. Without this correction, the GABA signal includes the resonance of other metabolites, and introduces bias in MRS experiments [164-166].
MRS has been extensively used to characterize the role of GABA and other metabolites in psychiatric conditions such as schizophrenia [92, 167] and more recently substance use disorder [168-172]. During the last 5 years, several authors have measured the GABA levels in healthy elderly subjects or patients diagnosed with AD or MCI. These studies have confirmed that GABA levels decline with age in frontal [173,174], parietal [173,174], and occipital [174,175] regions, as well as in the anterior cingulate cortex and right hippocampus [176]. Of note, sex may be one of the parameters that modulates this decline, as females have been shown to present an increased GABA age-related decline in the frontal lobes [173].
Table 1 summarizes the characteristics of those studies published to date which have evaluated both GABA levels via MRS, and either cognitive performance or cognitive status in individuals starting in midlife. Several authors reported positive correlations between GABA levels and cognitive performance, especially executive functions [174,177,178]. On the other hand, Bai et al. reported lower GABA concentration in the medial parietal cortex of AD patients as compared to matched controls, while no differences were found in the frontal cortex [179]. Porges et al. found an association between global cognitive performance (evaluated using the MoCA screening test) and GABA in the medial frontal cortex, while finding no significant results regarding the medial parietal cortex [180]. Similarly, individuals diagnosed with MCI showed lower GABA levels in the anterior and posterior cingulate cortex [177, 181], and also had a positive PiB PET, which suggests that MCI subjects had cognitive impairment caused by AD pathology. While episodic memory has most clearly been linked to hippocampal function, this area represents a particularly challenging area to image using MRS, which may explain the dearth of published studies assessing hippocampal GABA in older adults. However, pilot data from our lab, in which we measured GABA in the right hippocampus using a Mescher–Garwood point-resolved spectroscopy (MEGA-PRESS) sequence in a small (N = 20) sample of male and female healthy adults aged 50–71, found that sex moderated the relationship between hippocampal GABA and episodic memory, such that women with lower GABA concentration (measured as the peak ratio of GABA+, which reflects the fact that the GABA signal additionally contains macromolecules and homocarnosine [166], to creatine) showed worse memory performance than both women with higher GABA concentration, and men, regardless of GABA concentration, suggesting that the effect of GABA in cognition may be modulated by sex. Moreover, this interaction was independent of hippocampal volume.
Table 1.
MRS studies measuring GABA in aged individuals and/or patients with AD.
| Author first (Year) | Groups | Average Age (Range or ±SD) Sex (%) |
Brain areas | Cognitive Testing | Principal findings |
|---|---|---|---|---|---|
| Bai et al. (2014) [179] | 15 Controls 15 AD patients |
Controls: 66.3 (±4.6); ♀, 57.3% AD: 65.7 (±8.5); ♀, 60.0% |
mPLmFL | Global cognition: MMSE | AD patients presented lower GABA+ levels in the mPL No correlation between MMSE and GABA+ signal in the mPL or in the mFL |
| Hermansetal. (2018) [174] | 30 Young participants 29 Healthy older adults |
Young group: 23.2 (±4.3); ♀, 53.3% Elderly group: 67.5 (±3.9); ♀, 55.2% |
LSM PreSMA RIFC StriatumOL | Proactive and reactive inhibition: Stop signal task | GABA+ levels were on average lower in older adults Older adults with lower GABA in the preSMA were slower at stopping (worse inhibition function) |
| Huang et al. (2017) [176] | 17 Young adults 15 Healthy older adults 21 MCI 17 AD |
Young group: 24.4 (±2.6); ♀, 58.8% Elderly group: 61.9 (±6.3); ♀, 60.0% MCI: 64.6 (±7.7); ♀, 61.9% AD: 65.4 (±8.4); ♀, 52.9% |
ACCrH | N/A | Decreased GABA levels at the rH location for healthy older adults compared to younger adults No difference in GABA levels between healthy older adults and patients with MCI or AD |
| Jiménez-Balado et al. (2021) [277] [Under Review] | 20 Healthy older adults | 61 (±6.7); ♀,55.0% | rH | Episodic Memory: Directed Forgetting | Females with lower GABA levels presented a lower performance in episodic memory |
| Marenco et al. (2018) [178] | 229 Healthy volunteers | 30 (18-54); ♀, 55.5% | dACC |
Verbal memory Working memory: N-back task Visual memory Processing speed Executive function: Wisconsin Card Sorting Test (WCST) Digit span |
GABA+ was inversely correlated with age GABA levels mediated the relationship between age and WCST decline |
| Oeltzschner et al. (2019) [177] | 13 Controls 13 MCI |
Controls: 63.6 (±7.8); ♀, 53.8% MCI: 69.6 (±7.7); ♀, 23.1% |
ACCPCC |
Global cognition: MMSE Executive function: Fluency letter Memory: California verbal learning test; Brief visuoespatial memory test |
MCI subjects presented lower GABA levels in the ACC and PCC No association between GABA levels and cognitive performance in neuropsychological tests |
| Porges et al. (2017) [180] | 94 Healthy older adults | 73.1 (±9.9); ♀, 57.4% | mFLmPL | Global cognition: MOCA | GABA was inversely correlated with age at both mFC and mPC locations. Reduced frontal, but not posterior, GABA concentration was associated with lower MoCA scores |
| Riese et al. (2014) [181] | 21 Controls 15 Amnesic MCI |
Controls: 70.5 (±4.0); ♀, 33.3% MCI: 74.2 (±9.6); ♀, 26.6% |
PCC | CERAD-Plus test battery: MMSE; letter fluency; verbal learning, recall and recognition; figure copy and recall; Boston naming test; TMT A and B; category, and letter fluency; RAVLT; Visual Paired Associates test from the WMS-R; short version of the Stroop | GABA was not correlated with age GABA positively correlated with CERAD word learning score Amnesic MCI patients presented lower GABA levels in the PCC |
| Simmonite et al. (2019) [175] | 17 Young participants 18 Healthy older adults |
Young group: 20.7 (±1.4); ♀, 52.6% Elderly group: 76.5 (±8.7); ♀, 60.0% |
OL |
Perceptual processing: Contour detection; Digit-Symbol coding; Pattern comparison; Cambridge face perception test upright; Cambridge face perception test inverted; Dot speed and Dot coherence. Executive function:COWAT; TMT A and B. Memory: Cambridge Face memory test |
Older participants presented lower GABA+ levels in the OL Positive correlation between GABA performance in COWAT, trail making test B, Cambridge face perception test upright and dot coherence |
| Thielen et al. (2019) [272] | 14 Controls 17 Diabetic participants |
Controls:55.0(±8); ♀, 7.1% Diabetics: 55.1 (±6); ♀, 17.6% |
mPFCPrecuneus | Episodic memory: face profession encoding task (inside scanner) | Higher mPFC GABA levels in diabetic participants Negative correlation between GABA in mPFC and memory |
| Van Bussel et al. (2016) [273] | 21 Control high cognition 20 Diabetic high cognition 18 Controllow cognition Diabetic-low cognition |
High cognitive function: 62.7(±6.6); ♀, 43.9% Low cognitive function:61.1(±9.7); ♀, 43.6% |
OL |
Verbal memory: Verbal word learning Executive function: Stroop test Verbal fluency |
Higher GABA levels in diabetic participants Positive correlation between GABA and HbA1c Higher GABA in participants with increased HbA1c and lower cognition |
Notes: ACC, anterior cingulate cortex; AD, Alzheimer’s disease; COWAT, Controlled oral word association test; dACC, dorsal anterior cingulated cortex; HbA1c, Hemoglobin A1C; LSM, left sensorimotor cortex; MCI, mild cognitive impairment; mFL, medial frontal lobe; MMSE, Mini-Mental State Examination; MOCA, Montreal Cognitive Assessment; mPFC, medial prefrontal cortex; mPL, medial parietal lobe; OL, occipital lobe; PL, parietal lobe; PCC, posterior cingulate cortex; preSMA, bilateral pre supplementary area; rH, right hippocampus; RAVLT, Rey Auditory Verbal Learning Test; RIFC, right inferior frontal cortex; TMT, Trail Making Test; WCST, Wisconsin Card Sorting Test; WMS-R, Wechsler Memory Scale revised version.
An important caveat is that reduced GABA concentration in MRS studies may be reflective of either a reduction in GABAergic neurons, or by GABAergic dysfunction (reduced GABA synthesis and neurotransmission) [179]. Future multimodal imaging approaches to complement MRS information, such as the use of PET techniques, may help to disentangle and specify the source of the reduced GABA signal.
4. Mechanisms linking GABA dysfunction and memory impairment in AD
The previous section focused on work investigating changes in the GABAergic system and cognitive impairment in aging. Here, we review the growing body of literature showing age- and AD-related neural hyperactivity during tasks that assess episodic memory.
The excessive neuronal activity which, in aging animal models, has been shown to reflect GABAergic dysfunction, is also present in aging humans. Numerous task-based functional MRI studies have identified clear neural disturbances in humans at risk for AD, in the early stages of AD, and in MCI, including excessive brain activity in the hippocampal and medial temporal lobe regions [182]. Notably, neuronal dysfunction precedes structural atrophy in AD, and includes greater activation in the hippocampus [183-185]. Further, MCI patients show greater activation of the hippocampal formation during episodic memory tasks as compared to both healthy older adults and patients with AD, suggesting that hippocampal hyperactivity may be specific to the MCI stage [182, 186,187]. Moreover, MCI patients who showed greater task-related medial temporal lobe activity presented with a higher risk of clinical decline after a two year follow-up [188]. Similarly, increased hippocampal hyperactivation has been shown to correlate with cortical thinning in AD-signature regions in both cognitive healthy and MCI patients, suggesting that hippocampal hyperactivity is associated with other hallmarks of AD [189,190]. In line with these results, animal studies have demonstrated that both levels of Aβ and tau pathology are increased by neural activity [191]. This result has been replicated in humans, such that PiB distribution of Aβ accumulation overlaps with increased network activity in AD patients [192,193]. Hence, hippocampal hyperactivity may be contributing to trigger the hallmark AD-related pathology in the early stages of AD, rather than being just a correlate.
Traditionally, hyperactivity was thought to reflect a mechanism that compensates for memory deficits [188,194]. For example, Dickerson et al. proposed that it may reflect the need to recruit additional neural resources from the hippocampus or other brain regions to compensate for AD pathology [182]. In line with this hypothesis, efficiency in encoding information is positively correlated with hippocampal activity in MCI patients [187]. Yassa et al., (2010) exploring mnemonic control using a pattern separation task, found that hippocampal hyperactivity is specifically localized at the CA3/dentate gyrus subregion [185], in line with animal studies [125]. Interestingly, the activation in the CA3/dentate gyrus subregion was inversely correlated to participants’ performance, suggesting that hippocampal hyperactivity is a marker of network impairment [185,47,195]. It is thus possible that age-related reductions in GABA levels in these critical hippocampal regions [196] may cause the pathological hyperactivity and disturbances in neural network connectivity that have now been widely reported, and which have been shown to correlate with episodic memory impairment [47, 185].
From a clinical point of view, seizures and epileptiform activity are likely one of the most common manifestations of aberrant neural activity. Interestingly, AD patients present a 10 fold increased risk of developing seizures [197], with prevalence rates in AD of 10–22% according to epidemiological data [198]. Moreover, Vossel et al., (2013) reported that MCI patients with seizures presented with symptoms of cognitive decline 6.8 years earlier than did MCI patients without seizures [199]. Interestingly, a number of studies of levetiracetam (Keppra), an anti-epileptic drug that is thought to indirectly enhance the function of GABA and target hippocampal hyperexcitability [200], have reported reduced brain activity and improved cognitive functioning across a variety of species [46], including several different mouse models of AD [201,202], aged mice [203-205], and in humans with AD [206,207]. Two studies by Bakker et al., (2015) exploring levetiracetam in amnesic MCI patients found that it reduced hippocampal hyperactivity (as indicated by decreased fMRI measured BOLD activation) and mitigated memory impairments [208,209]. These studies suggest that seizures in AD may be a product of neural hyperactivity due to GABAergic dysfunction [210].
On the other hand, neural disturbances in AD are not limited to the hippocampus. For instance, AD patients show increased activation in different regions of frontal and temporal cortices when performing different memory tasks [50,211]. Additionally, the DMN, a large-scale network composed of functionally connected hubs in the brain that are active when the brain is “at rest” (i.e., not completing a particular task) and deactivate under cognitive load, has been implicated as a potential biomarker of AD. Patients with AD show reduced DMN functional connectivity and task-related deactivation, especially in the hippocampus [183,212,213]. Similarly, these changes in the DMN are also observed in early in the course of AD, including in asymptomatic patients with amyloid deposits [193], and they predict the conversion from MCI to dementia [214,215].
It is possible that GABA reduction, and the subsequent E/I imbalance that ensues, may be involved in these network disturbances in addition to those frequently reported in the hippocampus [216]. In line with this hypothesis, the majority of brain energy consumption during rest is attributable to neuronal firing and glutamate and GABA recycling [217]. Kapogiannis et al. reported that GABA concentration in the posteromedial and posterior cingulated/precuneus cortex were associated with greater DMN deactivation [218]. Likewise, higher GABA levels measured via MRS are associated with increased deactivation in different nodes of the DMN during a working memory task, while Glutamate played the opposite role, having a less significant role in brain activity [219,220].
Together, the studies reviewed provide compelling evidence for the hypothesis that GABAergic dysfunction underscores the patterns of neural network disturbances that are associated with the episodic memory deficits traditionally seen in AD and its early course. However, as reported in the previous section, GABA research is characterized by a wide diversity of results, and no study to date has directly tested this hypothesis in humans. In addition, the association between GABA and cognitive decline may be modulated by numerous factors. In the next section, we will discuss three such potential variables: the presence of APOE ε4, female sex, and vascular risk factors.
4.1. Contribution of apolipoprotein ε4 polymorphism
The APOE ε4 polymorphism is considered the strongest genetic risk factor for late onset AD. Prevalence of APOE ε4 in the general population is estimated to be ~15%, depending on ethnicity, while up to 50% of AD cases present this polymorphism [221-223]. Individuals with heterozygotic and homozygotic-ε4 present a 3- and 15-fold risk of developing AD, respectively [221,224]. Moreover, 91% of APOE ε4 homozygotes will develop AD in the course of their lives [225].
Previous articles have reviewed the relationship between APOE ε4 and neural network hyperactivity [46]. Briefly, in animal models, APOE ε4 knock-in mice show an age-dependent decrease in hilar GABAergic interneurons, which play a crucial role synchronizing neuronal activity in the hippocampus [226]. This decrease in GABAergic interneurons observed in APOE ε4 animals might be a consequence of increased tau phosphorylation and levels of APOE neurotoxic fragments [227]. Moreover, reduction of GABAergic interneurons results in downregulated hippocampal neurogenesis, which might be restored by potentiating GABAergic neurotransmission [227]. As proposed by Najm et al., (2019) early interneuron loss may lead to impaired phasic and tonic inhibition which ultimately results in those neural network disturbances observed in APOE ε4 and AD animal models [46,228,229]. However, importantly, Nuriel et al. found that hippocampal hyperactivity in APOE ε4 knock-in mice was produced by a lack of background inhibition, especially in the entorhinal cortex, caused by a reduced responsiveness of pyramidal neurons, suggesting that functional alterations in glutamatergic system may also participate in the E/I imbalance [216]. Interestingly, the memory loss in human APOE knock-in mice can be rescued via the deletion of APOE ε4 in GABAergic interneurons, confirming the link between APOE ε4 and GABAergic interneuron loss. Similarly, Tong et al. showed that normal memory function might be restored in APOE ε4 knock-in mice either by transplantation of inhibitory interneuron progenitor cells or by increasing GABAergic neurotransmission via pentobarbital administration during middle adulthood [230,231].
In humans, APOE ε4 carriers present a 5-fold risk of developing temporal lobe epilepsy [232]. Moreover, hippocampal hyperactivation has been consistently associated with APOE ε4 polymorphism. For example, during a memory task, asymptomatic ε4 carriers exhibit increased activation in the frontal lobes and hippocampus as compared to ε4 non-carriers [233,234]. Likewise, Bookheimer et al. found that APOE ε4 carriers had higher hippocampal activity than did homozygous APOE ε3 carriers when recalling unrelated pairs of words [235]. In this study, the degree of hippocampal activity correlated with cognitive decline after a 2 year follow-up. Interestingly, these disturbances in neural activity observed in APOE ε4 carriers may appear many years before the appearance of clinical symptoms. For instance, two manuscripts reported that increased hippocampal activity during the encoding phase of an episodic memory task was evident in 20–35 year old APOE ε4 carriers [236,237]. Dennis et al., who followed a sample of young adults (~23 years) longitudinally, reported reduced functional connectivity in the medial temporal lobe in the ε4-carrying participants [237]. These results support the idea that neural network disturbances may be an early marker of APOE ε4-related cognitive impairment, although no study to date has directly tested whether the GABAergic system mediates this relationship. Indeed, to our knowledge, only one study to date has measured both GABA in vivo and APOE polymorphism, and in this study, no differences in GABA concentration in the brain region measured (the posterior cingulated cortex) was found between APOE ε4 carriers and non-carriers [181]. Finally, other alterations in brain activity observed in AD patients are also exacerbated by APOE ε4 polymorphism. For instance, subjects with AD having the APOE ε4 polymorphism present lower DMN deactivation than do AD patients without APOE ε4 [238,239].
In summary, APOE ε4 carriers show both an early loss of inhibitory interneurons as well as hippocampal hyperactivity, starting before prodromal AD. Increasing GABAergic neurotransmission may help to halt cognitive decline according to animal models, although no study has yet evaluated this possibility in humans. Human studies have, on the other hand, revealed episodic memory related hippocampal hyperactivity in APOE ε4 carriers. Hence, future studies that assess both GABA and functional brain activity in the hippocampus as a function of APOE ε4 status will be of great interest.
4.2. Contribution of female sex
It is well-established that the prevalence of AD is greater in women than in men, potentially due to the fact that age is the highest risk factor for AD, and women typically live longer [240,241]. Mielke reported that although the lifetime risk of developing AD is higher for women than men, the overall incidence is not higher for women relative to men [242]. However, others consider female sex to be one of the principal risk factors for developing AD, behind only age and APOE ε4 carrier status [221,243]. Carroll et al., (2010) for example, showed that hippocampal Aβ pathology was greater, and hippocampal-dependent cognitive performance as measured using a spontaneous alternation behavior task was worse, in female relative to male 3xTg-AD mice, but that neonatal hormone manipulations could alter these effects [244]. Whereas 3xTg-AD male mice who received flutamide, an androgen receptor antagonist that blocks testosterone, showed an increase in Aβ accumulation, 3xTg-AD female mice who were defeminized using transient testosterone treatment showed a decrease in Aβ accumulation. In line with this transgenic animal work showing that sexually dimorphic characteristics may arise from differences in the prenatal hormone milieu, Luo et al., (2020) recently reported that older women who were part of an opposite-sex twin pair (F–M) were shown to have a lower risk of dementia than were women who were part of a same-sex (F–F) pair [245]. These results suggest that the organizational effects of sex steroids in early (prenatal) development may have a profound effect cognitive impairment in old age, particularly for females. Indeed, female patients diagnosed with MCI present steeper declines in cognitive function than males [246]. Similarly, neuropathological studies have reported that women present an increased AD-related pathology, especially neurofibrillary tangles [247]. Moreover, female sex is also known to modulate other risk factors of AD, such as the effect of APOE ε4 on the conference of AD and symptomology. The risk for AD is significantly greater for, and the likelihood of occurrence markedly earlier in APOE ε4 carrying women relative to APOE ε4carrying men [221,248]. Further, female APOE ε4 carriers are more likely to progress from MCI to dementia than male carriers [249]. In line with these results, the consequences of APOE ε4 on brain connectivity may be modulated by sex, such that female APOE ε4 carriers have been shown to have significantly reduced DMN connectivity relative to male carriers [250]. However, Corona-Long et al. found no sex-related differences in task induced hippocampal hyperactivity in amnesic MCI patients, although it is unknown whether these differences may appear in the preclinical stages of AD [251].
Differences between men and women have been reported in the few studies to date that have investigated the relationship between GABAergic function and sex in humans. In animal models, female Tg2576 mice showed increased GABA levels in the hippocampus at 12 months relative to males [252], but also showed a steeper rate decline in GABA and GABA/glutamate ratio between 12 and 18 months as compared to males [252,253]. Similarly, Leung et al. (2012) reported an exacerbated age-related loss of hilar GABAergic interneurons in female APOE ε4 knock-in mice [254]. Pathological studies in humans have shown lower expression of GABAA α1,α2, α5, β3 in healthy older females in the superior temporal gyrus [255]. Regarding in vivo studies, Gao et al. found that females present a stronger negative correlation between GABA levels and age than males in the frontal region, in line with animal studies [173]. As mentioned previously, in human pilot data from our lab, older women presented higher hippocampal GABA levels than did men, although GABA concentration in females was inversely correlated with episodic memory performance.
Altogether, these results suggest a potential sexual dimorphism in the GABAergic system in aging. Several mechanisms may contribute to these sex-related differences. Estrogen has been shown to increase spontaneous GABA release [256]. Moreover, GABA levels may vary across the menstrual cycle, suggesting that the GABAergic system may be modulated by endocrine system in females [257]. Changes in the female hormonal system during the lifespan may also affect GABAergic system, as GABA levels decrease after menopause [258]. Importantly, estrogen deficiency has been associated with an increased risk for dementia, and replacement therapy may be useful to reduce this risk [259]. On the other hand, the effect of gonadal hormones in the GABAergic system is considered a relevant neurobiological mechanism in depression in women [260] and, in turn, depression is both a well-established risk factor for cognitive impairment, and has a higher prevalence rate in women [261,262].
Altogether, these findings suggest that sex may be an important modulating factor in the relationship between GABA disturbances and age-related cognitive decline. Hormonal levels and depression may account for these differences, and both factors should be considered in future research.
4.3. Contribution of vascular risk factors
Vascular cognitive impairment (VCI) is the second leading cause of dementia [2,3]. However, AD and VCI tend to coexist, and mixed forms of dementia are often under-diagnosed [29]. Beyond stroke, VCI is produced by the accumulation of subclinical cerebrovascular lesions on brain parenchyma, which are a consequence of microvascular disease due to the continuous exposure to vascular risk factors [263]. Interestingly, AD-related pathology correlates with cerebrovascular lesions, especially in APOE ε4 carriers [264]. Moreover, homozygous APOE ε4 carriers present a 3-fold risk of displaying white matter hyperintensities, the principal pathological hallmark of microvascular disease. Finally, both AD and VCI may share common risk factors such as hypertension and diabetes [28,30].
However, little research has investigated the association between vascular risk factors and GABA disturbances [265]. Interestingly, the presence of early-life white matter hyperintensities predicts the incidence of late-onset epilepsy, suggesting a link between cerebrovascular disease and neural network disturbances [266]. Moreover, hyperhomocysteinemia, a shared risk factor for AD and cerebrovascular disease, is known to reduce GABAA mediated neurotransmission—as homocysteine antagonizes GABAA receptors [267]. This effect of homocysteine on GABAA receptors may produce several changes at the cellular level that lead to an increase in matrix metalloproteinases and, thus, to an increase in blood brain barrier (BBB) permeability [267]. Moreover, experimental BBB disruption has been shown to downregulate GABA related genes and increase excitatory synaptogenesis [268-271]. Finally, several reports have confirmed reduced GABA levels in postmortem and CSF samples of patients with VCI [145,161].
Hence, GABA downregulation and microvascular disease may be linked by common shared risk factors and BBB dysfunction. However, the relationship is complex, and MRS studies have shown increased, rather than decreased, GABA levels in the occipital and medial prefrontal cortex of diabetic patients as compared to controls (Table 1), as well as negative correlations between GABA+ levels and cognition, such that higher GABA levels in these regions were associated with worse cognitive function in diabetic patients [272,273]. However, the balance between glutamate and GABA may be affected in diabetes due to the alteration in brain glucose metabolism, which results in the reduction of brain glycogen levels [274]. Interestingly, Sickmann et al. observed an increase in GABA levels after inhibiting glycogen in Type 2 diabetic rats, confirming the role of glycogen in maintaining the E/I balance [275]. Hence, glucose homeostasis and diabetes may be relevant factors to consider in future research in this topic.
5. Conclusion
Alzheimer’s disease is among the most severe and burdensome medical conditions worldwide. Despite decades of research, there are still no treatments available to either slow or halt its progression. In the current review, we explored evidence for GABAergic dysfunction as a harbinger for neural abnormalities and subsequent episodic memory impairments that characterize AD. According to the current literature, GABA levels show a decrease over the normal course of aging. This decline has been shown to be more pronounced in patients with AD and MCI, specifically in the cingulate cortex [177,181] and medial parietal lobe [179]. However, it is unknown whether these alterations predict the incidence of dementia when observed in healthy older adults. Neural network disturbances have also been reported to begin in the preclinical and mild stages of AD [189]. While animal models provide strong evidence that dysregulated GABAergic signaling, potentially moderated APOE ε4, causes this hippocampal hyperactivity and subsequent memory failures in aged-animals, this mechanism has not been directly tested in humans, either cross-sectionally or longitudinally. Future multimodal imaging approaches that combine MRS, PET, and fMRI techniques in humans at risk for developing AD or who have already begun the clinical course may help to clarify the interplay between GABAergic alterations, Aβ and tau accumulation, neural network hyperexcitation and memory loss, and help to determine the sequence of pathological events that trigger AD.
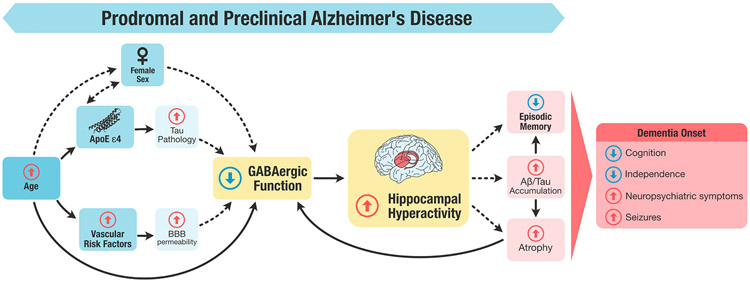
A schematic representation of our working model is illustrated in Fig. 1. We hypothesize that the effect of age on GABAergic levels may be modulated by female sex, APOE ε4 and cerebrovascular disease, which impair GABAergic function either independently or in interaction. These factors may decrease GABA levels by damaging interneurons or impairing GABAergic function (reduced GABA turnover, downregulation of GABAA receptors or GAD, among other mechanisms) resulting in circuit hyperexcitability in the hippocampus. Sustained exposition to hippocampal hyperactivity may lead to episodic memory loss, Aβ/tau accumulation, and atrophy, culminating in an increased likelihood of incident dementia.
Fig. 1.
Figure representing the role of the GABAergic system in memory impairment during the prodromal or preclinical stages of AD. GABAergic dysfunction results from a combination of factors working independently, and in interaction, including an increase in age, female sex, the presence of APOE ε4 polymorphism, and vascular risk factors. The reduction of GABA levels precipitates hippocampal hyperactivity, which in turn contributes to episodic memory impairments that are concomitant with or precede the incidence of dementia. Dashed lines represent those relationships that need further research to be confirmed. Note: Aβ, amyloid β; APOE ε4, apolipoprotein ε4 polymorphism; BBB, Blood brain barrier.
Therefore, according to our hypothesis, GABAergic dysfunction might precede both the clinical symptoms of dementia, and tau and Aβ accumulation, playing a pivotal role between risk factors and episodic memory impairment. Further study of the GABAergic system in aging may help to achieve a better understanding of AD and age-related cognitive decline. Moreover, GABA may represent a potential pharmacological target as suggested by previous clinical trials reporting positive benefits of levetiracetam on cognitive decline [206,209]. Importantly, previous research highlighted that hippocampal hyperactivity is specific to MCI and the preclinical stages of AD [182,189], and thus the therapeutic window to target GABA in pharmacological interventions may be in those stages preceding dementia (e.g., in individuals with subjective cognitive complaints). Considering that, due to the increase in life expectancy, there will be ~80 million dementia cases by 2040 [276], future work aimed at solving these questions is urgently need, and will help to reduce the devastating impact of dementia on both individuals, and society at large.
Acknowledgments
This research was supported in part by National Institute of Health, National Institute on Aging Grant R00AG055684 to TSE. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the NIH/NIA.
Footnotes
Conflicts of interest
Authors have nothing to disclose.
References
- [1].World Health Organization, Dementia, 2019. https://www.who.int/news-room/fact-sheets/detail/dementia (Accessed July 2020).
- [2].Goodman RA, Lochner KA, Thambisetty M, Wingo TS, Posner SF, Ling SM, Prevalence of dementia subtypes in United States Medicare fee-for-service beneficiaries, 2011-2013, Alzheimer Dement. 13 (1) (2017) 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lobo A, Launer LJ, Fratiglioni L, Andersen K, Di Carlo A, Breteler MM, Copeland JR, Dartigues JF, Jagger C, Martinez-Lage J, Soininen H, Hofman A, Prevalence of dementia and major subtypes in Europe: a collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group, Neurology 54 (11 Suppl 5) (2000) S4–S9. [PubMed] [Google Scholar]
- [4].Zhang Y, Xu Y, Nie H, Lei T, Wu Y, Zhang L, Zhang M, Prevalence of dementia and major dementia subtypes in the Chinese populations: a meta-analysis of dementia prevalence surveys, 1980-2010, J. Clin. Neurosci 19 (10) (2012) 1333–1337. [DOI] [PubMed] [Google Scholar]
- [5].Jessen F, Wiese B, Bachmann C, Eifflaender-Gorfer S, Haller F, Kölsch H, Luck T, Mösch E, van den Bussche H, Wagner M, Wollny A, Zimmermann T, Pentzek M, Riedel-Heller SG, Romberg HP, Weyerer S, Kaduszkiewicz H, Maier W, Bickel H, Prediction of dementia by subjective memory impairment: effects of severity and temporal association with cognitive impairment, Arch. Gen. Psychiatry 67 (4) (2010) 414–422. [DOI] [PubMed] [Google Scholar]
- [6].Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salvado O, Szoeke C, Macaulay SL, Martins R, Maruff P, Ames D, Rowe CC, Masters CL, Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study, Lancet Neurol. 12 (4) (2013) 357–367. [DOI] [PubMed] [Google Scholar]
- [7].Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E, Mild cognitive impairment: clinical characterization and outcome, Arch. Neurol 56 (3) (1999) 303–308. [DOI] [PubMed] [Google Scholar]
- [8].Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, Backman L, Albert M, Almkvist O, Arai H, Basun H, Blennow K, de Leon M, DeCarli C, Erkinjuntti T, Giacobini E, Graff C, Hardy J, Jack C, Jorm A, Ritchie K, van Duijn C, Visser P, Petersen RC, Mild cognitive impairment–beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment, J. Intern. Med 256 (3) (2004) 240–246. [DOI] [PubMed] [Google Scholar]
- [9].Petersen RC, Mild Cognitive Impairment, Continuum 22 (2016) 404–418 (2 Dementia). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Petersen RC, Clinical practice. Mild cognitive impairment, N. Engl. J. Med 364 (23) (2011) 2227–2234. [DOI] [PubMed] [Google Scholar]
- [11].Jahn H, Memory loss in Alzheimer’s disease, Dialogues Clin. Neurosci 15 (4) (2013) 445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lindeboom J, Weinstein H, Neuropsychology of cognitive ageing, minimal cognitive impairment, Alzheimer’s disease, and vascular cognitive impairment, Eur. J. Pharm 490 (1–3) (2004) 83–86. [DOI] [PubMed] [Google Scholar]
- [13].Schoemaker D, Gauthier S, Pruessner JC, Recollection and familiarity in aging individuals with mild cognitive impairment and Alzheimer’s disease: a literature review, Neuropsychol. Rev 24 (3) (2014) 313–331. [DOI] [PubMed] [Google Scholar]
- [14].Koen JD, Yonelinas AP, The effects of healthy aging, amnestic mild cognitive impairment, and Alzheimer’s disease on recollection and familiarity: a metaanalytic review, Neuropsychol. Rev 24 (3) (2014) 332–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dos Santos Picanco LC, Ozela PF, de M de Brito Brito Fatima, Pinheiro AA, Padilha EC, Braga FS, de Paula da Silva CHT, Dos Santos CBR, Rosa JMC, da Silva Hage-Melim LI, Alzheimer’s Disease: a review from the pathophysiology to diagnosis, new perspectives for pharmacological treatment, Curr. Med Chem 25 (26) (2018) 3141–3159. [DOI] [PubMed] [Google Scholar]
- [16].Chen HC, Kodell RL, Cheng KF, Chen JJ, Assessment of performance of survival prediction models for cancer prognosis, BMC Med. Res. Methodol 12 (2012) 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E, Alzheimer’s disease, Lancet 377 (9770) (2011) 1019–1031. [DOI] [PubMed] [Google Scholar]
- [18].van der Kant R, Goldstein LSB, Ossenkoppele R, Amyloid-β-independent regulators of tau pathology in Alzheimer disease, Nat. Rev. Neurosci 21 (1) (2020) 21–35. [DOI] [PubMed] [Google Scholar]
- [19].Ciccone L, Shi C, di Lorenzo D, Van Baelen AC, Tonali N, The positive side of the Alzheimer’s disease amyloid cross-interactions: the case of the Aβ 1-42 peptide with Tau, TTR, CysC, and ApoA1, Molecules 25 (10) (2020) 2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bloom GS, Amyloid-β and tau: the trigger and bullet in Alzheimer disease pathogenesis, JAMA Neurol. 71 (4) (2014) 505–508. [DOI] [PubMed] [Google Scholar]
- [21].Poulakis K, Pereira JB, Mecocci P, Vellas B, Tsolaki M, Kłoszewska I, Soininen H, Lovestone S, Simmons A, Wahlund LO, Westman E, Heterogeneous patterns of brain atrophy in Alzheimer’s disease, Neurobiol. Aging 65 (2018) 98–108. [DOI] [PubMed] [Google Scholar]
- [22].Fox NC, Cousens S, Scahill R, Harvey RJ, Rossor MN, Using serial registered brain magnetic resonance imaging to measure disease progression in Alzheimer disease: power calculations and estimates of sample size to detect treatment effects, Arch. Neurol 57 (3) (2000) 339–344. [DOI] [PubMed] [Google Scholar]
- [23].Fox NC, Freeborough PA, Rossor MN, Visualisation and quantification of rates of atrophy in Alzheimer’s disease, Lancet (Lond., Engl. ) 348 (9020) (1996) 94–97. [DOI] [PubMed] [Google Scholar]
- [24].Scheltens P, Launer LJ, Barkhof F, Weinstein HC, van Gool WA, Visual assessment of medial temporal lobe atrophy on magnetic resonance imaging: interobserver reliability, J. Neurol 242 (9) (1995) 557–560. [DOI] [PubMed] [Google Scholar]
- [25].Lo RY, Jagust WJ, Vascular burden and Alzheimer disease pathologic progression, Neurology 79 (13) (2012) 1349–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ramirez J, Berezuk C, McNeely AA, Scott CJ, Gao F, Black SE, Visible Virchow-Robin spaces on magnetic resonance imaging of Alzheimer’s disease patients and normal elderly from the Sunnybrook Dementia Study, J. Alzheimer Dis 43 (2) (2015) 415–424. [DOI] [PubMed] [Google Scholar]
- [27].Klohs J, An integrated view on vascular dysfunction in Alzheimer’s disease, Neurodegener. Dis 19 (2019) 109–127. [DOI] [PubMed] [Google Scholar]
- [28].Faraco G, Iadecola C, Hypertension: a harbinger of stroke and dementia, Hypertension 62 (5) (2013) 810–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Schneider JA, Arvanitakis Z, Bang W, Bennett DA, Mixed brain pathologies account for most dementia cases in community-dwelling older persons, Neurology 69 (24) (2007) 2197–2204. [DOI] [PubMed] [Google Scholar]
- [30].Diniz Pereira J, Gomes Fraga V, Morais Santos AL, Carvalho MDG, Caramelli P, Braga Gomes K, Alzheimer’s disease and type 2 diabetes mellitus: a systematic review of proteomic studies, J. Neurochem 9 (10) (2020), 10.1111/jnc.15166. [DOI] [PubMed] [Google Scholar]
- [31].Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, Ziolko SK, James JA, Snitz BE, Houck PR, Bi W, Cohen AD, Lopresti BJ, DeKosky ST, Halligan EM, Klunk WE, Frequent amyloid deposition without significant cognitive impairment among the elderly, Arch. Neurol 65 (11) (2008) 1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Salloway S, Honigberg LA, Cho W, Ward M, Friesenhahn M, Brunstein F, Quartino A, Clayton D, Mortensen D, Bittner T, Ho C, Rabe C, Schauer SP, Wildsmith KR, Fuji RN, Suliman S, Reiman EM, Chen K, Paul R, Amyloid positron emission tomography and cerebrospinal fluid results from a crenezumab anti-amyloid-beta antibody double-blind, placebo-controlled, randomized phase II study in mild-to-moderate Alzheimer’s disease (BLAZE), Alzheimer Res. Ther 10 (1) (2018) 018–0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Salloway SP, Sperling R, Fox NC, Sabbagh MN, Honig LS, Porsteinsson AP, Rofael H, Ketter N, Wang D, Liu E, Carr S, Black RS, Brashear HR, Long-term follow up of patients with mild-to-moderate Alzheimer’s disease treated with Bapineuzumab in a Phase III, open-label, extension study, J. Alzheimer Dis 64 (3) (2018) 689–707. [DOI] [PubMed] [Google Scholar]
- [34].Ostrowitzki S, Lasser RA, Dorflinger E, Scheltens P, Barkhof F, Nikolcheva T, Ashford E, Retout S, Hofmann C, Delmar P, Klein G, Andjelkovic M, Dubois B, Boada M, Blennow K, Santarelli L, Fontoura P, A phase III randomized trial of gantenerumab in prodromal Alzheimer’s disease, Alzheimer Res. Ther 9 (1) (2017) 017–0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Li Y, Sun H, Chen Z, Xu H, Bu G, Zheng H, Implications of GABAergic neurotransmission in Alzheimer’s disease, Front. Aging Neurosci 8 (31) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Reagh Z, Yassa M, Selective vulnerabilities and biomarkers in neurocognitive aging, FlOOOResearch 6 (2017) 491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Govindpani K, Calvo-Flores Guzmán B, Vinnakota C, Waldvogel HJ, Faull RL, Kwakowsky A, Towards a better understanding of GABAergic remodeling in Alzheimer’s disease, Int. J. Mol. Sci 18 (8) (2017) 1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Faingold CL, Gehlbach G, Caspary DM, On the role of GABA as an inhibitory neurotransmitter in inferior colliculus neurons: iontophoretic studies, Brain Res. 500 (1–2) (1989) 302–312. [DOI] [PubMed] [Google Scholar]
- [39].McCormick DA, GABA as an inhibitory neurotransmitter in human cerebral cortex, J. Neurophysiol 62 (5) (1989) 1018–1027. [DOI] [PubMed] [Google Scholar]
- [40].Tulving E, Markowitsch HJ, Episodic and declarative memory: role of the hippocampus, Hippocampus 8 (3) (1998) 198–204. [DOI] [PubMed] [Google Scholar]
- [41].Nyberg L, McIntosh AR, Houle S, Nilsson LG, Tulving E, Activation of medial temporal structures during episodic memory retrieval, Nature 380 (6576) (1996) 715–717. [DOI] [PubMed] [Google Scholar]
- [42].Schacter DL, Alpert NM, Savage CR, Rauch SL, Albert MS, Conscious recollection and the human hippocampal formation: evidence from positron emission tomography, Proc. Natl. Acad. Sci. USA 93 (1) (1996) 321–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Walhovd KB, Wesdye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve DN, Fischl B, Dale AM, Fjell AM, Consistent neuroanatomical age-related volume differences across multiple samples, Neurobiol. Aging 32 (5) (2011) 916–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ramos B, Baglietto-Vargas D, del Rio JC, Moreno-Gonzalez I, Santa-Maria C, Jimenez S, Caballero C, Lopez-Tellez JF, Khan ZU, Ruano D, Gutierrez A, Vitorica J, Early neuropathology of somatostatin/NPY GABAergic cells in the hippocampus of a PS1xAPP transgenic model of Alzheimer’s disease, Neurobiol. Aging 27 (11) (2006) 1658–1672. [DOI] [PubMed] [Google Scholar]
- [45].Levenga J, Krishnamurthy P, Rajamohamedsait H, Wong H, Franke TF, Cain P, Sigurdsson EM, Hoeffer CA, Tau pathology induces loss of GABAergic interneurons leading to altered synaptic plasticity and behavioral impairments, Acta Neuropathol. Commun 1 (34) (2013) 2051–5960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Najm R, Jones EA, Huang Y, Apolipoprotein E4, inhibitory network dysfunction, and Alzheimer’s disease, Mol. Neurodegener 14 (1) (2019) 019–0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Yassa MA, Stark CE, Pattern separation in the hippocampus, Trends Neurosci. 34 (10) (2011) 515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL, A default mode of brain function, Proc. Natl. Acad. Sci. U. S. A 98 (2) (2001) 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Nyberg L, Andersson M, Lundquist A, Salami A, Wåhlin A, Frontal contribution to hippocampal hyperactivity during memory encoding in aging, Front. Mol. Neurosci 12(2019), 229–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Sperling RA, Dickerson BC, Pihlajamaki M, Vannini P, LaViolette PS, Vitolo OV, Hedden T, Becker JA, Rentz DM, Selkoe DJ, Johnson KA, Functional alterations in memory networks in early Alzheimer’s disease, Neuromol. Med 12 (1) (2010) 27–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Pandis D, Scarmeas N, Seizures in Alzheimer disease: clinical and epidemiological data, Epilepsy Curr. 12 (5) (2012) 184–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Baker J, Libretto T, Henley W, Zeman A, The prevalence and clinical features of epileptic seizures in a memory clinic population, Seizure 71 (2019) 83–92. [DOI] [PubMed] [Google Scholar]
- [53].Villette V, Dutar P, GABAergic microcircuits in Alzheimer’s disease models, Curr. Alzheimer Res 14 (1) (2017) 30–39. [DOI] [PubMed] [Google Scholar]
- [54].Ambrad Giovannetti E, Fuhrmann M, Unsupervised excitation: GABAergic dysfunctions in Alzheimer’s disease, Brain Res. 15 (2019) 216–226. [DOI] [PubMed] [Google Scholar]
- [55].McQuail JA, Frazier CJ, Bizon JL, Molecular aspects of age-related cognitive decline: the role of GABA signaling, Trends Mol. Med 21 (7) (2015) 450–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Rissman RA, De Bias AL, Armstrong DM, GABA(A) receptors in aging and Alzheimer’s disease, J. Neurochem 103 (4) (2007) 1285–1292. [DOI] [PubMed] [Google Scholar]
- [57].Rozycka A, Liguz-Lecznar M, The space where aging acts: focus on the GABAergic synapse, Aging Cell 16 (4) (2017) 634–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Gasbarri A, Pompili A, 3 – The role of GABA in memory processes, in: Meneses A (Ed.), Identification of Neural Markers Accompanying Memory, Elsevier, San Diego, 2014, pp. 47–62. [Google Scholar]
- [59].Chapouthier G, Venault P, GABA-A receptor complex and memory processes, Curr. Top. Med. Chem 2 (8) (2002) 841–851. [DOI] [PubMed] [Google Scholar]
- [60].Heaney CF, Kinney JW, Role of GABA(B) receptors in learning and memory and neurological disorders, Neurosci. Biobehav. Rev 63 (2016) 1–28. [DOI] [PubMed] [Google Scholar]
- [61].Xu Y, Zhao M, Han Y, Zhang H, GABAergic inhibitory interneuron deficits in Alzheimer’s disease: implications for treatment, Front Neurosci. 14 (660) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Rissman RA, Mobley WC, Implications for treatment: GABAA receptors in aging, Down syndrome and Alzheimer’s disease, J. Neurochem 117 (4) (2011) 613–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Lanctot KL, Herrmann N, Mazzotta P, Khan LR, Ingber N, GABAergic function in Alzheimer’s disease: evidence for dysfunction and potential as a therapeutic target for the treatment of behavioural and psychological symptoms of dementia, Can. J. Psychiatry 49 (7) (2004) 439–453. [DOI] [PubMed] [Google Scholar]
- [64].Awapara J, Landua AJ, Fuerst R, Seale B, Free gamma-aminobutyric acid in brain, J. Biol. Chem 187 (1) (1950) 35–39. [PubMed] [Google Scholar]
- [65].Owens DF, Kriegstein AR, Is there more to GABA than synaptic inhibition? Nat. Rev. Neurosci 3 (9) (2002) 715–727. [DOI] [PubMed] [Google Scholar]
- [66].Klausberger T, Somogyi P, Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations, Science 321 (5885) (2008) 53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Paulsen O, Moser EI, A model of hippocampal memory encoding and retrieval: GABAergic control of synaptic plasticity, Trends Neurosci. 21 (7) (1998) 273–278. [DOI] [PubMed] [Google Scholar]
- [68].Izquierdo I, Medina JH, GABAA receptor modulation of memory: the role of endogenous benzodiazepines, Trends Pharm. Sci 12 (7) (1991) 260–265. [DOI] [PubMed] [Google Scholar]
- [69].Sherif FM, GABA-transaminase in brain and blood platelets: basic and clinical aspects, Prog. Neuropsychopharmacol. Biol. Psychiatry 18 (8) (1994) 1219–1233. [DOI] [PubMed] [Google Scholar]
- [70].Bloom FE, Iversen LL, Localizing 3H-GABA in nerve terminals of rat cerebral cortex by electron microscopic autoradiography, Nature 229 (5287) (1971) 628–630. [DOI] [PubMed] [Google Scholar]
- [71].Fon EA, Edwards RH, Molecular mechanisms of neurotransmitter release, Muscle Nerve 24 (5) (2001) 581–601. [DOI] [PubMed] [Google Scholar]
- [72].Chebib M, Johnston GA, The ‘ABC’ of GABA receptors: a brief review, Clin. Exp. Pharm. Physiol 26 (11) (1999) 937–940. [DOI] [PubMed] [Google Scholar]
- [73].McKernan RM, Whiting PJ, Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci. 19 (4) (1996) 139–143. [DOI] [PubMed] [Google Scholar]
- [74].Korpi ER, Gründer G, Liiddens H, Drug interactions at GABA(A) receptors, Prog. Neurobiol 67 (2) (2002) 113–159. [DOI] [PubMed] [Google Scholar]
- [75].Sieghart W, Sperk G, Subunit composition, distribution and function of GABA(A) receptor subtypes, Curr. Top. Med Chem 2 (8) (2002) 795–816. [DOI] [PubMed] [Google Scholar]
- [76].Sigel E, Mapping of the benzodiazepine recognition site on GABA(A) receptors, Curr. Top. Med Chem 2 (8) (2002) 833–839. [DOI] [PubMed] [Google Scholar]
- [77].Johnston GA, GABAA receptor pharmacology, Pharm. Ther 69 (3) (1996) 173–198. [DOI] [PubMed] [Google Scholar]
- [78].Chu DC, Penney JB Jr., Young AB, Cortical GABAB and GABAA receptors in Alzheimer’s disease: a quantitative autoradiographic study, Neurology 37 (9) (1987) 1454–1459. [DOI] [PubMed] [Google Scholar]
- [79].López-Bendito G, Shigemoto R, Kulik A, Vida I, Fairén A, Luján R, Distribution of metabotropic GABA receptor subunits GABAB1a/b and GABAB2 in the rat hippocampus during prenatal and postnatal development, Hippocampus 14 (7) (2004) 836–848. [DOI] [PubMed] [Google Scholar]
- [80].Hendry SH, Schwark HD, Jones EG, Yan J, Numbers and proportions of GABA-immunoreactive neurons in different areas of monkey cerebral cortex, J. Neurosci 7 (5) (1987) 1503–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Somogyi P, Klausberger T, Defined types of cortical interneurone structure space and spike timing in the hippocampus, J. Physiol 562 (Pt 1) (2005) 9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Jinno S, Structural organization of long-range GABAergic projection system of the hippocampus, Front. Neuroanat 3 (13) (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Maccaferri G, Lacaille JC, Interneuron diversity series: Hippocampal interneuron classifications–making things as simple as possible, not simpler, Trends Neurosci. 26 (10) (2003) 564–571. [DOI] [PubMed] [Google Scholar]
- [84].Farrant M, Nusser Z, Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors, Nat. Rev. Neurosci 6 (3) (2005) 215–229. [DOI] [PubMed] [Google Scholar]
- [85].Mozrzymas JW, Dynamism of GABA(A) receptor activation shapes the "personality" of inhibitory synapses, Neuropharmacology 47 (7) (2004) 945–960. [DOI] [PubMed] [Google Scholar]
- [86].Whittington MA, Traub RD, Interneuron diversity series: inhibitory interneurons and network oscillations in vitro, Trends Neurosci. 26 (12) (2003) 676–682. [DOI] [PubMed] [Google Scholar]
- [87].Buzsáki G, Chrobak JJ, Temporal structure in spatially organized neuronal ensembles: a role for interneuronal networks, Curr. Opin. Neurobiol 5 (4) (1995) 504–510. [DOI] [PubMed] [Google Scholar]
- [88].Dembitskaya Y, Wu YW, Semyanov A, Tonic GABA(A) conductance favors spike-timing-dependent over theta-burst-induced long-term potentiation in the Hippocampus, J. Neurosci 40 (22) (2020) 4266–4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Zarnowska ED, Keist R, Rudolph U, Pearce RA, GABAA receptor alpha5 subunits contribute to GABAA,slow synaptic inhibition in mouse hippocampus, J. Neurophysiol 101 (3) (2009) 1179–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Nuss P, Anxiety disorders and GABA neurotransmission: a disturbance of modulation, Neuropsychiatr. Dis. Treat 11 (2015) 165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Makkar SR, Zhang SQ, Cranney J, Behavioral and neural analysis of GABA in the acquisition, consolidation, reconsolidation, and extinction of fear memory, Neuropsychopharmacology 35 (8) (2010) 1625–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Egerton A, Modi nos G, Ferrera D, McGuire P, Neuroimaging studies of GABA in schizophrenia: a systematic review with meta-analysis, Transl. Psychiatry 7 (6) (2017), ell47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Luscher B, Shen Q, Sahir N, The GABAergic deficit hypothesis of major depressive disorder, Mol. Psychiatry 16 (4) (2011) 383–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Coghlan S, Horder J, Inkster B, Mendez MA, Murphy DG, Nutt DJ, GABA system dysfunction in autism and related disorders: from synapse to symptoms, Neurosci. Biobehav. Rev 36 (9) (2012) 2044–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Guidotti A, Auta J, Chen Y, Davis JM, Dong E, Gavin DP, Grayson DR, Matrisciano F, Pinna G, Satta R, Sharma RP, Tremolizzo L, Tueting P, Epigenetic GABAergic targets in schizophrenia and bipolar disorder, Neuropharmacology 60 (7–8) (2011) 1007–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Möhler H, Role of GABAA receptors in cognition, Biochem Soc. Trans 37 (Pt 6) (2009) 1328–1333. [DOI] [PubMed] [Google Scholar]
- [97].Engin E, Benham RS, Rudolph U, An emerging circuit pharmacology of GABA (A) receptors, Trends Pharmacol. Sci 39 (8) (2018) 710–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Lucas EK, Clem RL, GABAergic interneurons: the orchestra or the conductor in fear learning and memory? Brain Res. Bull 141 (2018) 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P, Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons, Nature 378 (6552) (1995) 75–78. [DOI] [PubMed] [Google Scholar]
- [100].Nakazono T, Jun H, Blurton-Jones M, Green KN, Igarashi KM, Gamma oscillations in the entorhinal-hippocampal circuit underlying memory and dementia, Neurosci. Res 129 (2018) 40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Mann EO, Kohl MM, Paulsen O, Distinct roles of GABA(A) and GABA(B) receptors in balancing and terminating persistent cortical activity, J. Neurosci 29 (23) (2009) 7513–7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Hollnagel JO, Maslarova A, Haq RU, Heinemann U, GABAB receptor dependent modulation of sharp wave-ripple complexes in the rat hippocampus in vitro, Neurosci. Lett 574 (2014) 15–20. [DOI] [PubMed] [Google Scholar]
- [103].Semyanov A, Walker MC, Kullmann DM, GABA uptake regulates cortical excitability via cell type-specific tonic inhibition, Nat. Neurosci 6 (5) (2003) 484–490. [DOI] [PubMed] [Google Scholar]
- [104].Ehlers A, Clark DM, A cognitive model of posttraumatic stress disorder, Behav. Res. Ther 38 (4) (2000) 319–345. [DOI] [PubMed] [Google Scholar]
- [105].Hackmann A, Clark DM, McManus F, Recurrent images and early memories in social phobia, Behav. Res Ther 38 (6) (2000) 601–610. [DOI] [PubMed] [Google Scholar]
- [106].Rao SG, Williams GV, Goldman-Rakic PS, Destruction and creation of spatial tuning by disinhibition: GABA(A) blockade of prefrontal cortical neurons engaged by working memory, J. Neurosci 20 (1) (2000) 485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Wilson FA, O’Scalaidhe SP, Goldman-Rakic PS, Functional synergism between putative gamma-aminobutyrate-containing neurons and pyramidal neurons in prefrontal cortex, Proc. Natl. Acad. Sci. U. S. A 91 (9) (1994) 4009–4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Lewis DA, Volk DW, Hashimoto T, Selective alterations in prefrontal cortical GABA neurotransmission in schizophrenia: a novel target for the treatment of working memory dysfunction, Psychopharmacology 174 (1) (2004) 143–150. [DOI] [PubMed] [Google Scholar]
- [109].Mirnics K, Middleton FA, Marquez A, Lewis DA, Levitt P, Molecular characterization of schizophrenia viewed by microarray analysis of gene expression in prefrontal cortex, Neuron 28 (1) (2000) 53–67. [DOI] [PubMed] [Google Scholar]
- [110].Lett TA, Voineskos AN, Kennedy JL, Levine B, Daskalakis ZJ, Treating working memory deficits in schizophrenia: a review of the neurobiology, Biol. Psychiatry 75 (5) (2014) 361–370. [DOI] [PubMed] [Google Scholar]
- [111].Eich TS, Nee DE, Insel C, Malapani C, Smith EE, Neural correlates of impaired cognitive control over working memory in schizophrenia, Biol. Psychiatry 76 (2) (2014) 146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Van Snellenberg JX, Girgis RR, Horga G, van de Giessen E, Slifstein M, Ojeil N, Weinstein JJ, Moore H, Lieberman JA, Shohamy D, Smith EE, Abi-Dargham A, Mechanisms of working memory impairment in Schizophrenia, Biol. Psychiatry 80 (8) (2016) 617–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Schmitz TW, Correia MM, Ferreira CS, Prescot AP, Anderson MC, Hippocampal GABA enables inhibitory control over unwanted thoughts, Nat. Commun 8 (1) (2017) 017–00956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Pike CJ, Cotman CW, Cultured GABA-immunoreactive neurons are resistant to toxicity induced by beta-amyloid, Neuroscience 56 (2) (1993) 269–274. [DOI] [PubMed] [Google Scholar]
- [115].Krantic S, Isorce N, Mechawar N, Davoli MA, Vignault E, Albuquerque M, Chabot JG, Moyse E, Chauvin JP, Aubert I, McLaurin J, Quirion R, Hippocampal GABAergic neurons are susceptible to amyloid-β toxicity in vitro and are decreased in number in the Alzheimer’s disease TgCRND8 mouse model, J. Alzheimer Dis 29 (2) (2012) 293–308. [DOI] [PubMed] [Google Scholar]
- [116].Ulrich D, Amyloid-β impairs synaptic inhibition via GABA(A) receptor endocytosis, J. Neurosci 35 (24) (2015) 9205–9210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Harney SC, Jones MV, Pre- and postsynaptic properties of somatic and dendritic inhibition in dentate gyrus, Neuropharmacology 43 (4) (2002) 584–594. [DOI] [PubMed] [Google Scholar]
- [118].Baraban SC, Tallent MK, Interneuron Diversity series: Interneuronal neuropeptides–endogenous regulators of neuronal excitability, Trends Neurosci. 27 (3) (2004) 135–142. [DOI] [PubMed] [Google Scholar]
- [119].Martín-Belmonte A, Aguado C, Alfaro-Ruíz R, Moreno-Martínez AE, de la Ossa L, Martínez-Hernández J, Buisson A, Früh S, Bettler B, Shigemoto R, Fukazawa Y, Luján R, Reduction in the neuronal surface of post and presynaptic GABA(B) receptors in the hippocampus in a mouse model of Alzheimer’s disease, Brain Pathol. 30 (3) (2020) 554–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Martín-Belmonte A, Aguado C, Alfaro-Ruíz R, Moreno-Martínez AE, de la Ossa L, Martínez-Hernández J, Buisson A, Shigemoto R, Fukazawa Y, Luján R, Density of GABAB receptors is reduced in granule cells of the hippocampus in a mouse model of Alzheimer’s disease, Int. J. Mol. Sci 21 (7) (2020) 2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Moser MB, Moser EI, Distributed encoding and retrieval of spatial memory in the hippocampus, J. Neurosci 18 (18) (1998) 7535–7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Thiébot MH, Some evidence for amnesic-like effects of benzodiazepines in animals, Neurosci. Biobehav. Rev 9 (1) (1985) 95–100. [DOI] [PubMed] [Google Scholar]
- [123].Raffalli-Sebille MJ, Chapouthier G, Venault P, Dodd RH, Methyl betacarboline-3-carboxylate enhances performance in a multiple-trial learning task in mice, Pharm. Biochem Behav 35 (2) (1990) 281–284. [DOI] [PubMed] [Google Scholar]
- [124].Koh MT, Rosenzweig-Lipson S, Gallagher M, Selective GABA(A) α5 positive allosteric modulators improve cognitive function in aged rats with memory impairment, Neuropharmacology 64 (1) (2013) 145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Wilson IA, Ikonen S, Gallagher M, Eichenbaum H, Tanila H, Age-associated alterations of hippocampal place cells are subregion specific, J. Neurosci 25 (29) (2005) 6877–6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Glykys J, Mody I, Hippocampal network hyperactivity after selective reduction of tonic inhibition in GABA A receptor alpha5 subunit-deficient mice, J. Neurophysiol 95 (5) (2006) 2796–2807. [DOI] [PubMed] [Google Scholar]
- [127].Prevot TD, Li G, Vidojevic A, Misquitta KA, Fee C, Santrac A, Knutson DE, Stephen MR, Kodali R, Zahn NM, Arnold LA, Scholze P, Fisher JL, Marković BD, Banasr M, Cook JM, Savic M, Sibille E, Novel benzodiazepine-like ligands with various anxiolytic, antidepressant, or pro-cognitive profiles, Mol. Neuropsychiatry 5 (2) (2019) 84–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Deng PY, Xiao Z, Yang C, Rojanathammanee L, Grisanti L, Watt J, Geiger JD, Liu R, Porter JE, Lei S, GABA(B) receptor activation inhibits neuronal excitability and spatial learning in the entorhinal cortex by activating TREK-2 K+ channels, Neuron 63 (2) (2009) 230–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Serrats J, Cunningham MO, Davies CH, GABA(B) receptor modulation-to B or not to be B a pro-cognitive medicine? Curr. Opin. Pharm 35 (2017) 125–132. [DOI] [PubMed] [Google Scholar]
- [130].Bañuelos C, Beas BS, McQuail JA, Gilbert RJ, Frazier CJ, Setlow B, Bizon JL, Prefrontal cortical GABAergic dysfunction contributes to age-related working memory impairment, J. Neurosci 34 (10) (2014) 3457–3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Getova D, Bowery NG, The modulatory effects of high affinity GABA(B) receptor antagonists in an active avoidance learning paradigm in rats, Psychopharmacology 137 (4) (1998) 369–373. [DOI] [PubMed] [Google Scholar]
- [132].Getova DP, Bowery NG, Effects of high-affinity GABAB receptor antagonists on active and passive avoidance responding in rodents with gammahydroxybutyrolactone-induced absence syndrome, Psychopharmacology 157 (1) (2001) 89–95. [DOI] [PubMed] [Google Scholar]
- [133].Froesd W, Gallagher M, Jenkins H, Madrid A, Melcher T, Teichman S, Mondadori CG, Pearlman R, SGS742: the first GABA(B) receptor antagonist in clinical trials, Biochem. Pharm 68 (8) (2004) 1479–1487. [DOI] [PubMed] [Google Scholar]
- [134].Beas BS, McQuail JA, Ban Uelos C, Setlow B, Bizon JL, Prefrontal cortical GABAergic signaling and impaired behavioral flexibility in aged F344 rats, Neuroscience 345 (2017) 274–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Andrews-Zwilling Y, Gillespie AK, Kravitz AV, Nelson AB, Devidze N, Lo I, Yoon SY, Bien-Ly N, Ring K, Zwilling D, Potter GB, Rubenstein JL, Kreitzer AC, Huang Y, Hilar GABAergic interneuron activity controls spatial learning and memory retrieval, PloS One 7 (7) (2012), e40555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Perry TL, Yong VW, Bergeron C, Hansen S, Jones K, Amino acids, glutathione, and glutathione transferase activity in the brains of patients with Alzheimer’s disease, Ann. Neurol 21 (4) (1987) 331–336. [DOI] [PubMed] [Google Scholar]
- [137].Rossor MN, Garrett NJ, Johnson AL, Mountjoy CQ, Roth M, Iversen LL, A post-mortem study of the cholinergic and GABA systems in senile dementia, Brain: a J. Neurol 105 (Pt 2) (1982) 313–330. [DOI] [PubMed] [Google Scholar]
- [138].Ellison DW, Beal MF, Mazurek MF, Bird ED, Martin JB, A postmortem study of amino acid neurotransmitters in Alzheimer’s disease, Ann. Neurol 20 (5) (1986) 616–621. [DOI] [PubMed] [Google Scholar]
- [139].Sasaki H, Muramoto O, Kanazawa I, Arai H, Kosaka K, Iizuka R, Regional distribution of amino acid transmitters in postmortem brains of presenile and senile dementia of Alzheimer type, Ann. Neurol 19 (3) (1986) 263–269. [DOI] [PubMed] [Google Scholar]
- [140].Mohanakrishnan P, Fowler AH, Vonsattel JP, Husain MM, Jolles PR, Liem P, Komoroski RA, An in vitro 1H nuclear magnetic resonance study of the temporoparietal cortex of Alzheimer brains, Exp. Brain Res 102 (3) (1995) 503–510. [DOI] [PubMed] [Google Scholar]
- [141].Lowe SL, Francis PT, Procter AW, Palmer AM, Davison AN, Bowen DM, Gamma-aminobutyric acid concentration in brain tissue at two stages of Alzheimer’s disease, Brain J. Neurol 111 (Pt 4) (1988) 785–799. [DOI] [PubMed] [Google Scholar]
- [142].Arai H, Kobayashi K, Ichimiya Y, Kosaka K, Iizuka R, A preliminary study of free amino acids in the postmortem temporal cortex from Alzheimer-type dementia patients, Neurobiol. Aging 5 (4) (1984) 319–321. [DOI] [PubMed] [Google Scholar]
- [143].Seidl R, Cairns N, Singewald N, Kaehler ST, Lubec G, Differences between GABA levels in Alzheimer’s disease and Down syndrome with Alzheimer-like neuropathology, Naunyn Schmiedebergs Arch. Pharm 363 (2) (2001) 139–145. [DOI] [PubMed] [Google Scholar]
- [144].Schwab C, Yu S, Wong W, McGeer EG, McGeer PL, GAD65, GAD67, and GABAT immunostaining in human brain and apparent GAD65 loss in Alzheimer’s disease, J. Alzheimer Dis 33 (4) (2013) 1073–1088. [DOI] [PubMed] [Google Scholar]
- [145].Perry EK, Tomlinson BE, Blessed G, Bergmann K, Gibson PH, Perry RH, Correlation of cholinergic abnormalities with senile plaques and mental test scores in senile dementia, Br. Med J 2 (6150) (1978) 1457–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Mizukami K, Ikonomovic MD, Grayson DR, Sheffield R, Armstrong DM, Immunohistochemical study of GABAA receptor alpha1 subunit in the hippocampal formation of aged brains with Alzheimer-related neuropathologic changes, Brain Res. 799 (1) (1998) 148–155. [DOI] [PubMed] [Google Scholar]
- [147].Rissman RA, Mishizen-Eberz AJ, Carter TL, Wolfe BB, De Bias AL, Miralles CP, Ikonomovic MD, Armstrong DM, Biochemical analysis of GABA(A) receptor subunits alpha 1, alpha 5, beta 1, beta 2 in the hippocampus of patients with Alzheimer’s disease neuropathology, Neuroscience 120 (3) (2003) 695–704. [DOI] [PubMed] [Google Scholar]
- [148].Rissman RA, Bennett DA, Armstrong DM, Subregional analysis of GABA(A) receptor subunit mRNAs in the hippocampus of older persons with and without cognitive impairment, J. Chem. Neuroanat 28 (1–2) (2004) 17–25. [DOI] [PubMed] [Google Scholar]
- [149].Howell O, Atack JR, Dewar D, McKernan RM, Sur C, Density and pharmacology of alphas subunit-containing GABA(A) receptors are preserved in hippocampus of Alzheimer’s disease patients, Neuroscience 98 (4) (2000) 669–675. [DOI] [PubMed] [Google Scholar]
- [150].Limon A, Reyes-Ruiz JM, Miledi R, Loss of functional GABA(A) receptors in the Alzheimer diseased brain, Proc. Natl. Acad. Sci. U. S. A 109 (25) (2012) 10071–10076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Mohr E, Bruno G, Foster N, Gillespie M, Cox C, Hare TA, Tamminga C, Fedio P, Chase TN, GABA-agonist therapy for Alzheimer’s disease, Clin. Neuropharmacol 9 (3) (1986) 257–263. [DOI] [PubMed] [Google Scholar]
- [152].Zimmer R, Teelken AW, Trieling WB, Weber W, Weihmayr T, Lauter H, Gamma-aminobutyric acid and homovanillic acid concentration in the CSF of patients with senile dementia of Alzheimer’s type, Arch. Neurol 41 (6) (1984) 602–604. [DOI] [PubMed] [Google Scholar]
- [153].Manyam NV, Katz L, Hare TA, Gerber JC 3rd, Grossman MH, Levels of gamma-aminobutyric acid in cerebrospinal fluid in various neurologic disorders, Arch. Neurol 37 (6) (1980) 352–355. [DOI] [PubMed] [Google Scholar]
- [154].Enna SJ, Stern LZ, Wastek GJ, Yamamura HI, Cerebrospinal fluid gamma-aminobutyric acid variations in neurological disorders, Arch. Neurol 34 (11) (1977) 683–685. [DOI] [PubMed] [Google Scholar]
- [155].Kuroda H, Gamma-aminobutyric acid (GABA) in cerebrospinal fluid, Acta Med. Okayama 37 (3) (1983) 167–177. [DOI] [PubMed] [Google Scholar]
- [156].Jiménez-Jiménez FJ, Molina JA, Gómez P, Vargas C, de Bustos F, Benito-León J, Tallón-Barranco A, Ortí-Pareja M, Gasalla T, Arenas J, Neurotransmitter amino acids in cerebrospinal fluid of patients with Alzheimer’s disease, J. Neural Transm 105 (2–3) (1998) 269–277. [DOI] [PubMed] [Google Scholar]
- [157].Oishi M, Mochizuki Y, Yoshihashi H, Takasu T, Nakano E, Laboratory examinations correlated with severity of dementia, Ann. Clin. Lab Sci 26 (4) (1996) 340–345. [PubMed] [Google Scholar]
- [158].Tosca P, Canevari L, Di Paolo E, Ferrari R, Verzé S, Zerbi F, Dagani F, Glutamate and GABA levels in CSF from patients affected by dementia and olivoponto-cerebellar atrophy, Acta Neurol. Scand 85 (6) (1992) 430–435. [DOI] [PubMed] [Google Scholar]
- [159].Samakashvili S, Ibáñez C, Simó C, Gil-Bea FJ, Winblad B, Cedazo-Mínguez A, Cifuentes A, Analysis of chiral amino acids in cerebrospinal fluid samples linked to different stages of Alzheimer disease, Electrophoresis 32 (19) (2011) 2757–2764. [DOI] [PubMed] [Google Scholar]
- [160].Manyevitch R, Protas M, Scarpiello S, Deliso M, Bass B, Nanajian A, Chang M, Thompson SM, Khoury N, Gonnella R, Trotz M, Moore DB, Harms E, Perry G, Clunes L, Ortiz A, Friedrich JO, Murray IVJ, Evaluation of metabolic and synaptic dysfunction hypotheses of Alzheimer’s disease (AD): a meta-analysis of CSF markers, Curr. Alzheimer Res 15 (2) (2018) 164–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Tohgi H, Abe T, Takahashi S, Kimura M, A selective reduction of excitatory amino acids in cerebrospinal fluid of patients with Alzheimer type dementia compared with vascular dementia of the Binswanger type, Neurosci. Lett 141 (1) (1992) 5–8. [DOI] [PubMed] [Google Scholar]
- [162].Bareggi SR, Franceschi M, Bonini L, Zecca L, Smirne S, Decreased CSF concentrations of homovanillic acid and gamma-aminobutyric acid in Alzheimer’s disease. Age- or disease-related modifications? Arch. Neurol 39 (11) (1982) 709–712. [DOI] [PubMed] [Google Scholar]
- [163].Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R, NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease, Alzheimer Dement. 14 (4) (2018) 535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Ende G, Proton magnetic resonance spectroscopy: relevance of glutamate and GABA to neuropsychology, Neuropsychol. Rev 25 (3) (2015) 315–325. [DOI] [PubMed] [Google Scholar]
- [165].Harris AD, Puts NA, Barker PB, Edden RA, Spectral-editing measurements of GABA in the human brain with and without macromolecule suppression, Magn. Reson. Med 74 (6) (2015) 1523–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Rothman DL, Behar KL, Prichard JW, Petroff OA, Homocarnosine and the measurement of neuronal pH in patients with epilepsy, Magn. Reson. Med 38 (6) (1997) 924–929. [DOI] [PubMed] [Google Scholar]
- [167].Rowland LM, Krause BW, Wijtenburg SA, McMahon RP, Chiappelli J, Nugent KL, Nisonger SJ, Korenic SA, Kochunov P, Hong LE, Medial frontal GABA is lower in older schizophrenia: a MEGA-PRESS with macromolecule suppression study, Mol. Psychiatry 21 (2) (2016) 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Prisciandaro JJ, Schacht JP, Prescot AP, Brenner HM, Renshaw PF, Brown TR, Anton RF, Intraindividual changes in brain GABA, glutamate, and glutamine during monitored abstinence from alcohol in treatment-naïve individuals with alcohol use disorder, Addict. Biol 25 (6) (2020) 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Prisciandaro JJ, Schacht JP, Prescot AP, Brenner HM, Renshaw PF, Brown TR, Anton RF, Evidence for a unique association between fronto-cortical glycine levels and recent heavy drinking in treatment naïve individuals with alcohol use disorder, Neurosci. Lett 706 (2019) 207–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Prisciandaro JJ, Schacht JP, Prescot AP, Renshaw PF, Brown TR, Anton RF, Brain glutamate, GABA and glutamine levels and associations with recent drinking in treatment-naïve individuals with alcohol use disorder versus light drinkers, Alcohol Clin. Exp. Res 43 (2) (2019) 221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Prisciandaro JJ, Schacht JP, Prescot AP, Renshaw PF, Brown TR, Anton RF, Associations between recent heavy drinking and dorsal anterior cingulate N-acetylaspartate and glutamate concentrations in non-treatmentseeking individuals with alcohol dependence, Alcohol Clin. Exp. Res 40 (3) (2016) 491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Prisciandaro JJ, Tolliver BK, Prescot AP, Brenner HM, Renshaw PF, Brown TR, Anton RF, Unique prefrontal GABA and glutamate disturbances in cooccurring bipolar disorder and alcohol dependence, Transl. Psychiatry 7 (7) (2017) el 163–el 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Gao F, Edden RA, Li M, Puts NA, Wang G, Liu C, Zhao B, Wang H, Bai X, Zhao C, Wang X, Barker PB, Edited magnetic resonance spectroscopy detects an age-related decline in brain GABA levels, NeuroImage 78 (2013) 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Hermans L, Leunissen I, Pauwels L, Cuypers K, Peeters R, Puts NAJ, Edden RAE, Swinnen SP, Brain GABA levels are associated with inhibitory control deficits in older adults, J. Neurosci 38 (36) (2018) 7844–7851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Simmonite M, Carp J, Foerster BR, Ossher L, Petrou M, Weissman DH, Polk TA, Age-related declines in occipital GABA are associated with reduced fluid processing ability, Acad. Radio 26 (8) (2019) 1053–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Huang D, Liu D, Yin J, Qian T, Shrestha S, Ni H, Glutamate-glutamine and GABA in brain of normal aged and patients with cognitive impairment, Eur. Radiol 27 (7) (2017) 2698–2705. [DOI] [PubMed] [Google Scholar]
- [177].Oeltzschner G, Wijtenburg SA, Mikkelsen M, Edden RAE, Barker PB, Joo JH, Leoutsakos JS, Rowland LM, Workman CI, Smith GS, Neurometabolites and associations with cognitive deficits in mild cognitive impairment: a magnetic resonance spectroscopy study at 7 Tesla, Neurobiol. Aging 73 (2019) 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Marenco S, Meyer C, van der Veen JW, Zhang Y, Kelly R, Shen J, Weinberger DR, Dickinson D, Berman KF, Role of gamma-amino-butyric acid in the dorsal anterior cingulate in age-associated changes in cognition, Neuropsychopharmacology 43 (11) (2018) 2285–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Bai X, Edden RA, Gao F, Wang G, Wu L, Zhao B, Wang M, Chan Q, Chen W, Barker PB, Decreased γ-aminobutyric acid levels in the parietal region of patients with Alzheimer’s disease, J. Magn. Reson Imaging 41 (5) (2015) 1326–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Porges EC, Woods AJ, Edden RA, Puts NA, Harris AD, Chen H, Garcia AM, Seider TR, Lamb DG, Williamson JB, Cohen RA, Frontal gamma-aminobutyric acid concentrations are associated with cognitive performance in older adults, Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2 (1) (2017) 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Riese F, Gietl A, Zölch N, Henning A, O’Gorman R, Kälin AM, Leh SE, Buck A, Warnock G, Edden RA, Luechinger R, Hock C, Kollias S, Michels L, Posterior cingulate γ-aminobutyric acid and glutamate/glutamine are reduced in amnestic mild cognitive impairment and are unrelated to amyloid deposition and apolipoprotein E genotype, Neurobiol. Aging 36 (1) (2015) 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Dickerson BC, Salat DH, Greve DN, Chua EF, Rand-Giovannetti E, Rentz DM, Bertram L, Mullin K, Tanzi RE, Blacker D, Albert MS, Sperling RA, Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD, Neurology 65 (3) (2005) 404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Celone KA, Calhoun VD, Dickerson BC, Atri A, Chua EF, Miller SL, DePeau K, Rentz DM, Selkoe DJ, Blacker D, Albert MS, Sperling RA, Alterations in memory networks in mild cognitive impairment and Alzheimer’s disease: an independent component analysis, J. Neurosci 26 (40) (2006) 10222–10231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Miller SL, Fenstermacher E, Bates J, Blacker D, Sperling RA, Dickerson BC, Hippocampal activation in adults with mild cognitive impairment predicts subsequent cognitive decline, J. Neurol., Neurosurg., Psychiatry 79 (6) (2008) 630–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Yassa MA, Stark SM, Bakker A, Albert MS, Gallagher M, Stark CE, Highresolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic mild cognitive impairment, Neuroimage 51 (3) (2010) 1242–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Hämäläinen A, Pihlajamäki M, Tanila H, Hänninen T, Niskanen E, Tervo S, Karjalainen PA, Vanninen RL, Soininen H, Increased fMRI responses during encoding in mild cognitive impairment, Neurobiol. Aging 28 (12) (2007) 1889–1903. [DOI] [PubMed] [Google Scholar]
- [187].Kircher TT, Weis S, Freymann K, Erb M, Jessen F, Grodd W, Heun R, Leube DT, Hippocampal activation in patients with mild cognitive impairment is necessary for successful memory encoding, J. Neurol. Neurosurg., Psychiatry 78 (8) (2007) 812–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Dickerson BC, Salat DH, Bates JF, Atiya M, Killiany RJ, Greve DN, Dale AM, Stern CE, Blacker D, Albert MS, Sperling RA, Medial temporal lobe function and structure in mild cognitive impairment, Ann. Neurol 56 (1) (2004) 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Putcha D, Brickhouse M, O’Keefe K, Sullivan C, Rentz D, Marshall G, Dickerson B, Sperling R, Hippocampal hyperactivation associated with cortical thinning in Alzheimer’s disease signature regions in non-demented elderly adults, J. Neurosci 31 (48) (2011) 17680–17688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, Grodstein F, Wright CI, Blacker D, Rosas HD, Sperling RA, Atri A, Growdon JH, Hyman BT, Morris JC, Fischl B, Buckner RL, The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals, Cereb. Cortex 19 (3) (2009) 497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Wu JW, Hussaini SA, Bastille IM, Rodriguez GA, Mrejeru A, Rilett K, Sanders DW, Cook C, Fu H, Boonen RA, Herman M, Nahmani E, Emrani S, Figueroa YH, Diamond MI, Clelland CL, Wray S, Duff KE, Neuronal activity enhances tau propagation and tau pathology in vivo, Nat. Neurosci 19 (8) (2016) 1085–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews-Hanna JR, Sperling RA, Johnson KA, Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease, J. Neurosci 29 (6) (2009) 1860–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Sperling RA, Laviolette PS, O’Keefe K, O’Brien J, Rentz DM, Pihlajamäki M, Marshall G, Hyman BT, Selkoe DJ, Hedden T, Buckner RL, Becker JA, Johnson KA, Amyloid deposition is associated with impaired default network function in older persons without dementia, Neuron 63 (2) (2009) 178–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Palop JJ, Mucke L, Network abnormalities and interneuron dysfunction in Alzheimer disease, Nat. Rev. Neurosci 17 (12) (2016) 777–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Rolls ET, The mechanisms for pattern completion and pattern separation in the hippocampus, Front Syst. Neurosci 7 (74) (2013) 00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Bakker A, Kirwan CB, Miller M, Stark CE, Pattern separation in the human hippocampal CA3 and dentate gyrus, Science 319 (5870) (2008) 1640–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Beagle AJ, Darwish SM, Ranasinghe KG, La AL, Karageorgiou E, Vossel KA, Relative incidence of seizures and myoclonus in Alzheimer’s disease, dementia with lewy bodies, and frontotemporal dementia, J. Alzheimer’S. Dis.: JAD 60 (1) (2017) 211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Friedman D, Honig LS, Scarmeas N, Seizures and epilepsy in Alzheimer’s disease, CNS Neurosci. Ther 18 (4) (2012) 285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Vossel KA, Beagle AJ, Rabinovici GD, Shu H, Lee SE, Naasan G, Hegde M, Cornes SB, Henry ML, Nelson AB, Seeley WW, Geschwind MD, Gorno-Tempini ML, Shih T, Kirsch HE, Garcia PA, Miller BL, Mucke L, Seizures and epileptiform activity in the early stages of Alzheimer disease, JAMA Neurol. 70 (9) (2013) 1158–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Macdonald RL, Kelly KM, Antiepileptic drug mechanisms of action, Epilepsia 36 (2) (1995), 10.1111/j.1528-1157.1995.tb05996.x. [DOI] [PubMed] [Google Scholar]
- [201].Sanchez PE, Zhu L, Verret L, Vossel KA, Orr AG, Cirrito JR, Devidze N, Ho K, Yu GQ, Palop JJ, Mucke L, Levetiracetam suppresses neuronal network dysfunction and reverses synaptic and cognitive deficits in an Alzheimer’s disease model, Proc. Natl. Acad. Sci 109 (42) (2012) E2895–E2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Shi JQ, Wang BR, Tian YY, Xu J, Gao L, Zhao SL, Jiang T, Xie HG, Zhang YD, Anti epileptics topiramate and levetiracetam alleviate behavioral deficits and reduce neuropathology in APPswe/PS1dE9 transgenic mice, CNS Neurosci. Ther 19 (11) (2013) 871–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Koh MT, Haberman RP, Foti S, McCown TJ, Gallagher M, Treatment strategies targeting excess hippocampal activity benefit aged rats with cognitive impairment, Neuropsychopharmacology 35 (4) (2010) 1016–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Haberman RP, Branch A, Gallagher M, Targeting neural hyperactivity as a treatment to stem progression of late-onset Alzheimer’s disease, Neurotherapeutics 14 (3) (2017) 662–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Devi L, Ohno M, Effects of levetiracetam, an antiepileptic drug, on memory impairments associated with aging and Alzheimer’s disease in mice, Neurobiol. Learn Mem 102 (2013) 7–11. [DOI] [PubMed] [Google Scholar]
- [206].Cumbo E, Ligori LD, Levetiracetam, lamotrigine, and phenobarbital in patients with epileptic seizures and Alzheimer’s disease, Epilepsy Behav. 17 (4) (2010) 461–466. [DOI] [PubMed] [Google Scholar]
- [207].Schoenberg MR, Rum RS, Osborn KE, Werz MA, A randomized, double-blind, placebo-controlled crossover study of the effects of levetiracetam on cognition, mood, and balance in healthy older adults, Epilepsia 58 (9) (2017) 1566–1574. [DOI] [PubMed] [Google Scholar]
- [208].Bakker A, Albert MS, Krauss G, Speck CL, Gallagher M, Response of the medial temporal lobe network in amnestic mild cognitive impairment to therapeutic intervention assessed by fMRI and memory task performance, NeuroImage Clin. 7 (2015) 688–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Bakker A, Krauss GL, Albert MS, Speck CL, Jones LR, Stark CE, Yassa MA, Bassett SS, Shelton AL, Gallagher M, Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment, Neuron 74 (3) (2012) 467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].DiFrancesco JC, Tremolizzo L, Polonia V, Giussani G, Bianchi E, Franchi C, Nobili A, Appollonio I, Beghi E, Ferrarese C, Adult-onset epilepsy in presymptomatic alzheimer’s disease: a retrospective study, J. Alzheimer Dis 60 (4) (2017) 1267–1274. [DOI] [PubMed] [Google Scholar]
- [211].Schwindt GC, Black SE, Functional imaging studies of episodic memory in Alzheimer’s disease: a quantitative meta-analysis, NeuroImage 45 (1) (2009) 181–190. [DOI] [PubMed] [Google Scholar]
- [212].Wang L, Zang Y, He Y, Liang M, Zhang X, Tian L, Wu T, Jiang T, Li K, Changes in hippocampal connectivity in the early stages of Alzheimer’s disease: evidence from resting state fMRI, NeuroImage 31 (2) (2006) 496–504. [DOI] [PubMed] [Google Scholar]
- [213].Hafkemeijer A, van der Grond J, Rombouts SA, Imaging the default mode network in aging and dementia, Biochim. Biophys. Acta 3 (2012) 431–441. [DOI] [PubMed] [Google Scholar]
- [214].Petrella JR, Sheldon FC, Prince SE, Calhoun VD, Doraiswamy PM, Default mode network connectivity in stable vs progressive mild cognitive impairment, Neurology 76 (6) (2011) 511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Bai F, Zhang Z, Yu H, Shi Y, Yuan Y, Zhu W, Zhang X, Qian Y, Default-mode network activity distinguishes amnestic type mild cognitive impairment from healthy aging: a combined structural and resting-state functional MRI study, Neurosci. Lett 438 (1) (2008) 111–115. [DOI] [PubMed] [Google Scholar]
- [216].Nuriel T, Angulo SL, Khan U, Ashok A, Chen Q, Figueroa HY, Emrani S, Liu L, Herman M, Barrett G, Savage V, Buitrago L, Cepeda-Prado E, Fung C, Goldberg E, Gross SS, Hussaini SA, Moreno H, Small SA, Duff KE, Neuronal hyperactivity due to loss of inhibitory tone in APOE4 mice lacking Alzheimer’s disease-like pathology, Nat. Commun 8 (1) (2017), 1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Shulman RG, Rothman DL, Behar KL, Hyder F, Energetic basis of brain activity: implications for neuroimaging, Trends Neurosci. 27 (8) (2004) 489–495. [DOI] [PubMed] [Google Scholar]
- [218].Kapogiannis D, Reiter DA, Willette AA, Mattson MP, Posteromedial cortex glutamate and GABA predict intrinsic functional connectivity of the default mode network, Neuroimage 64 (2013) 112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Hu Y, Chen X, Gu H, Yang Y, Resting-state glutamate and GABA concentrations predict task-induced deactivation in the default mode network, J. Neurosci 33 (47) (2013) 18566–18573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Chen X, Fan X, Hu Y, Zuo C, Whitfield-Gabrieli S, Holt D, Gong Q, Yang Y, Pizzagalli DA, Du F, Ongur D, Regional GABA concentrations modulate internetwork resting-state functional connectivity, Cereb. Cortex 29 (4) (2019) 1607–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM, Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium, JAMA 278 (16) (1997) 1349–1356. [PubMed] [Google Scholar]
- [222].Ward A, Crean S, Mercaldi CJ, Collins JM, Boyd D, Cook MN, Arrighi HM, Prevalence of apolipoprotein E4 genotype and homozygotes (APOE e4/4) among patients diagnosed with Alzheimer’s disease: a systematic review and meta-analysis, Neuroepidemiology 38 (1) (2012) 1–17. [DOI] [PubMed] [Google Scholar]
- [223].Zannis VI, Kardassis D, Zanni EE, Genetic mutations affecting human lipoproteins, their receptors, and their enzymes, Adv. Hum. Genet 21 (1993) 145–319. [DOI] [PubMed] [Google Scholar]
- [224].Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE, Systematic metaanalyses of Alzheimer disease genetic association studies: the AlzGene database, Nat. Genet 39 (1) (2007) 17–23. [DOI] [PubMed] [Google Scholar]
- [225].Liu CC, Kanekiyo T, Xu H, Bu G, Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy, Nat. Rev. Neurol 9 (2) (2013) 106–118, 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Andrews-Zwilling Y, Bien-Ly N, Xu Q, Li G, Bernardo A, Yoon SY, Zwilling D, Yan TX, Chen L, Huang Y, Apolipoprotein E4 causes age- and Tau-dependent impairment of GABAergic interneurons, leading to learning and memory deficits in mice, J. Neurosci 30 (41) (2010) 13707–13717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Li G, Bien-Ly N, Andrews-Zwilling Y, Xu Q, Bernardo A, Ring K, Halabisky B, Deng C, Mahley RW, Huang Y, GABAergic interneuron dysfunction impairs hippocampal neurogenesis in adult apolipoprotein E4 knockin mice, Cell Stem Cell 5 (6) (2009) 634–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Busche MA, Eichhoff G, Adelsberger H, Abramowski D, Wiederhold KH, Haass C, Staufenbiel M, Konnerth A, Garaschuk O, Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer’s disease, Science 321 (5896) (2008) 1686–1689. [DOI] [PubMed] [Google Scholar]
- [229].Davis KE, Fox S, Gigg J, Increased hippocampal excitability in the 3xTgAD mouse model for Alzheimer’s disease in vivo, PloS One 9 (3) (2014), e91203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Tong LM, Djukic B, Arnold C, Gillespie AK, Yoon SY, Wang MM, Zhang O, Knoferle J, Rubenstein JL, Alvarez-Buylla A, Huang Y, Inhibitory interneuron progenitor transplantation restores normal learning and memory in ApoE4 knock-in mice without or with Aβ accumulation, J. Neurosci 34 (29) (2014) 9506–9515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Tong LM, Yoon SY, Andrews-Z willing Y, Yang A, Lin V, Lei H, Huang Y, Enhancing GABA signaling during middle adulthood prevents age-dependent GABAergic interneuron decline and learning and memory deficits in ApoE4 mice, J. Neurosci 36 (7) (2016) 2316–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Kauffman MA, Consalvo D, Moron DG, Lereis VP, Kochen S, ApoE epsilon4 genotype and the age at onset of temporal lobe epilepsy: a case-control study and meta-analysis, Epilepsy Res. 90 (3) (2010) 234–239. [DOI] [PubMed] [Google Scholar]
- [233].Bassett SS, Yousem DM, Cristinzio C, Kusevic I, Yassa MA, Caffo BS, Zeger SL, Familial risk for Alzheimer’s disease alters fMRI activation patterns, Brain J. Neurol 129 (Pt 5) (2006) 1229–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Bondi MW, Houston WS, Eyler LT, Brown GG, fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease, Neurology 64 (3) (2005) 501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, Small GW, Patterns of brain activation in people at risk for Alzheimer’s disease, New Engl. J. Med 343 (7) (2000) 450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Filippini N, Macintosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE, Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele, Proc. Natl. Acad. Sci. U. S. A 106 (17) (2009) 7209–7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Dennis NA, Browndyke JN, Stokes J, Need A, Burke JR, Welsh-Bohmer KA, Cabeza R, Temporal lobe functional activity and connectivity in young adult APOE varepsilon4 carriers, Alzheimer Dement. 6 (4) (2010) 303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Persson J, Lind J, Larsson A, Ingvar M, Sleegers K, Van Broeckhoven C, Adolfsson R, Nilsson LG, Nyberg L, Altered deactivation in individuals with genetic risk for Alzheimer’s disease, Neuropsychologia 46 (6) (2008) 1679–1687. [DOI] [PubMed] [Google Scholar]
- [239].Pihlajamaki M, Sperling RA, Functional MRI assessment of task-induced deactivation of the default mode network in Alzheimer’s disease and at-risk older individuals, Behav. Neurol 21 (1) (2009) 77–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Dubai DB, Sex difference in Alzheimer’s disease: an updated, balanced and emerging perspective on differing vulnerabilities, Handb. Clin. Neurol 175 (2020) 261–273. [DOI] [PubMed] [Google Scholar]
- [241].Riedel BC, Thompson PM, Brinton RD, Age, APOE and sex: triad of risk of Alzheimer’s disease, J. Steroid Biochem Mol. Biol 160 (2016) 134–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Mielke MM, Sex and gender differences in Alzheimer’s disease dementia, Psychiatr 35 (11) (2018) 14–17. [PMC free article] [PubMed] [Google Scholar]
- [243].Pike CJ, Sex and the development of Alzheimer’s disease, J. Neurosci. Res 95 (1–2) (2017) 671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Carroll JC, Rosario ER, Kreimer S, Villamagna A, Gentzschein E, Stanczyk FZ, Pike CJ, Sex differences in β-amyloid accumulation in 3xTg-AD mice: role of neonatal sex steroid hormone exposure, Brain Res. 17 (2010) 233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Luo J, Beam CR, Karlsson IK, Pike CJ, Reynolds CA, Gatz M, Dementia risk in women higher in same-sex than opposite-sex twins, Alzheimer Dement. 12 (1) (2020) el 2049–el 2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Lin KA, Choudhury KR, Rathakrishnan BG, Marks DM, Petrella JR, Doraiswamy PM, Marked gender differences in progression of mild cognitive impairment over 8 years, Alzheimer’S. Dement.: J. Alzheimer’S. Assoc 1 (2) (2015) 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Barnes LL, Wilson RS, Bienias JL, Schneider JA, Evans DA, Bennett DA, Sex differences in the clinical manifestations of Alzheimer disease pathology, Arch. Gen. Psychiatry 62 (6) (2005) 685–691. [DOI] [PubMed] [Google Scholar]
- [248].Payami H, Montee KR, Kaye JA, Bird TD, Yu CE, Wijsman EM, Schellenberg GD, Alzheimer’s disease, apolipoprotein E4, and gender, JAMA 271 (17) (1994) 1316–1317. [PubMed] [Google Scholar]
- [249].Altmann A, Tian L, Henderson VW, Greicius MD, Sex modifies the APOErelated risk of developing Alzheimer disease, Ann. Neurol 75 (4) (2014) 563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Damoiseaux JS, Seeley WW, Zhou J, Shirer WR, Coppola G, Karydas A, Rosen HJ, Miller BL, Kramer JH, Greicius MD, Alzheimer’s Disease Neuroimaging I, Gender modulates the APOE ε4 effect in healthy older adults: convergent evidence from functional brain connectivity and spinal fluid tau levels, J. Neurosci 32 (24) (2012) 8254–8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Corona-Long CA, Tran TT, Chang E, Speck CL, Gallagher M, Bakker A, Comparison of male and female patients with amnestic mild cognitive impairment: Hippocampal hyperactivity and pattern separation memory performance, Alzheimer Dement. Diagn. Assess. Dis. Monit 12 (1) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Alia A, Roßner S, Gender, GABAergic dysfunction and AD, Aging 10 (12) (2018. 26) 3636–3637, 10.18632/aging.101672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Roy U, Stute L, Höfling C, Hardage-Rübsamen M, Matysik J, Roβner S, Alia A, Sex- and age-specific modulation of brain GABA levels in a mouse model of Alzheimer’s disease, Neurobiol. Aging 62 (2018) 168–179. [DOI] [PubMed] [Google Scholar]
- [254].Leung L, Andrews-Zwilling Y, Yoon SY, Jain S, Ring K, Dai J, Wang MM, Tong L, Walker D, Huang Y, Apolipoprotein E4 causes age- and sex-dependent impairments of hilar GABAergic interneurons and learning and memory deficits in mice, PloS One 7 (12) (2012), e53569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Pandya M, Palpagama TH, Turner C, Waldvogel HJ, Faull RL, Kwakowsky A, Sex- and age-related changes in GABA signaling components in the human cortex, Biol. Sex. Differ 10 (1) (2019) 018–0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Herbison AE, Heavens RP, Dyer RG, Oestrogen modulation of excitatory A1 noradrenergic input to rat medial preoptic gamma aminobutyric acid neurones demonstrated by microdialysis, Neuroendocrinology 52 (2) (1990) 161–168. [DOI] [PubMed] [Google Scholar]
- [257].Epperson CN, Haga K, Mason GF, Sellers E, Gueorguieva R, Zhang W, Weiss E, Rothman DL, Krystal JH, Cortical gamma-aminobutyric acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorder: a proton magnetic resonance spectroscopy study, Arch. Gen. Psychiatry 59 (9) (2002) 851–858. [DOI] [PubMed] [Google Scholar]
- [258].Wang D, Wang X, Luo MT, Wang H, Li YH, Gamma-aminobutyric acid levels in the anterior cingulate cortex of perimenopausal women with depression: a magnetic resonance spectroscopy study, Front Neurosci. 13 (785) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Paganini-Hill A, Henderson VW, Estrogen deficiency and risk of Alzheimer’s disease in women, Am. J. Epidemiol 140 (3) (1994) 256–261. [DOI] [PubMed] [Google Scholar]
- [260].Flores-Ramos M, Salinas M, Carvajal-Lohr A, Rodríguez-Bores L, [The role of gamma-aminobutyric acid in female depression], Gac. Med Mex 153 (4) (2017) 486–495. [DOI] [PubMed] [Google Scholar]
- [261].Kessler RC, Epidemiology of women and depression, J. Affect. Disord 74 (1) (2003) 5–13. [DOI] [PubMed] [Google Scholar]
- [262].Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D, Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis, Arch. Gen. Psychiatry 63 (5) (2006) 530–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Pantoni L, Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges, Lancet Neurol. 9 (7) (2010) 689–701. [DOI] [PubMed] [Google Scholar]
- [264].Kester MI, Goos JD, Teunissen CE, Benedictus MR, Bouwman FH, Wattjes MP, Barkhof F, Scheltens P, van der Flier WM, Associations between cerebral small-vessel disease and Alzheimer disease pathology as measured by cerebrospinal fluid biomarkers, JAMA Neurol. 71 (7) (2014) 855–862. [DOI] [PubMed] [Google Scholar]
- [265].Gacsályi I, Móricz K, Gigler G, Megyeri K, Machado P, Antoni FA, Persistent therapeutic effect of a novel α5-GABA(A) receptor antagonist in rodent preclinical models of vascular cognitive impairment, Eur. J. Pharm 834 (2018) 118–125. [DOI] [PubMed] [Google Scholar]
- [266].Johnson EL, Krauss GL, Lee AK, Schneider ALC, Kucharska-Newton AM, Huang J, Jack CR Jr., Gottesman RF, Association between white matter hyperintensities, cortical volumes, and late-onset epilepsy, Neurology 92 (9) (2019) e988–e995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Lominadze D, Tyagi N, Sen U, Ovechkin A, Tyagi SC, Homocysteine alters cerebral microvascular integrity and causes remodeling by antagonizing GABA-A receptor, Mol. Cell Biochem 371 (1–2) (2012) 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Ivens S, Kaufer D, Flores LP, Bechmann I, Zumsteg D, Tomkins O, Seiffert E, Heinemann U, Friedman A, TGF-beta receptor-mediated albumin uptake into astrocytes is involved in neocortical epileptogenesis, Brain J. Neurol 130 (Pt 2) (2007) 535–547. [DOI] [PubMed] [Google Scholar]
- [269].David Y, Cacheaux LP, Ivens S, Lapilover E, Heinemann U, Kaufer D, Friedman A, Astrocytic dysfunction in epileptogenesis: consequence of altered potassium and glutamate homeostasis? J. Neurosci 29 (34) (2009) 10588–10599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Weissberg I, Wood L, Kamintsky L, Vazquez O, Milikovsky DZ, Alexander A, Oppenheim H, Ardizzone C, Becker A, Frigerio F, Vezzani A, Buckwalter MS, Huguenard JR, Friedman A, Kaufer D, Albumin induces excitatory synaptogenesis through astrocytic TGF-β/ALK5 signaling in a model of acquired epilepsy following blood-brain barrier dysfunction, Neurobiol. Dis 78 (2015) 115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [271].Bar-Klein G, Lublinsky S, Kamintsky L, Noyman I, Veksler R, Dalipaj H, Senatorov VV Jr., Swissa E, Rosenbach D, Elazary N, Milikovsky DZ, Milk N, Kassirer M, Rosman Y, Serlin Y, Eisenkraft A, Chassidim Y, Parmet Y, Kaufer D, Friedman A, Imaging blood-brain barrier dysfunction as a biomarker for epileptogenesis, Brain J. Neurol 140 (6) (2017) 1692–1705. [DOI] [PubMed] [Google Scholar]
- [272].Thielen JW, Gancheva S, Hong D, Rohani Rankouhi S, Chen B, Apostolopoulou M, Anadol-Schmitz E, Roden M, Norris DG, Tendolkar I, Higher GABA concentration in the medial prefrontal cortex of Type 2 diabetes patients is associated with episodic memory dysfunction, Hum. Brain Mapp 40 (14) (2019) 4287–4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].van Bussel FC, Backes WH, Hofrnan PA, Puts NA, Edden RA, van Boxtel MP, Schram MT, Stehouwer CD, Wildberger JE, Jansen JF, Increased GABA concentrations in type 2 diabetes mellitus are related to lower cognitive functioning, Medicine 95 (36) (2016), e4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [274].Sickmann HM, Waagepetersen HS, Schousboe A, Benie AJ, Bouman SD, Obesity and type 2 diabetes in rats are associated with altered brain glycogen and amino-acid homeostasis, J. Cereb. Blood Flow Metab 30 (8) (2010) 1527–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [275].Sickmann HM, Waagepetersen HS, Schousboe A, Benie AJ, Bouman SD, Brain glycogen and its role in supporting glutamate and GABA homeostasis in a type 2 diabetes rat model, Neurochem. Int 60 (3) (2012) 267–275. [DOI] [PubMed] [Google Scholar]
- [276].Rizzi L, Rosset I, Roriz-Cruz M, Global epidemiology of dementia: Alzheimer’s and vascular types, BioMed. Res. Int 2014 (10) (2014) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [277].Jiménez-Balado J, Igwe K, Klem L, Buyukturkoglu K, Liu C, Guo J, Brickman AM, Irima A, Eich TS, Reduced hippocampal GABA is associated with poorer episodic memory in healthy older women: A pilot study, 2021. (Under Review). [DOI] [PMC free article] [PubMed] [Google Scholar]