Abstract
Objectives
An increasing reliance on telemedicine for older adults with cognitive impairment requires a better understanding of the barriers and facilitators for this unique patient population.
Design
The study team queried PubMed, Embase, the Cochrane Library, PsycINFO, CINAHL, Scopus, and ClinicalTrials.gov on May 1, 2020 for studies in English published from January 2010 to May 2020.
Setting and Participants
We conducted a systematic review of articles investigating the use of telemedicine among older adults with Alzheimer’s disease and related dementia (ADRD) or mild cognitive impairment (MCI) that focused on the patient and care partner perspectives.
Methods
Telemedicine encounter purpose, technological requirements, and findings regarding sensory needs were extracted. The Cochrane Collaboration’s Risk of Bias Tool was applied for quality assessment.
Results
The search yielded 3551 abstracts, from which 90 articles were reviewed and 17 were included. The purpose of telemedicine encounters included routine care, cognitive assessment, and telerehabilitation. All studies reported successful implementation of telemedicine, supported by patient and care partner satisfaction, similar results on cognitive assessment and diagnosis compared to in-person visits, and improvement in outcome measures following rehabilitation. 16 studies relied upon staff and care partners to navigate technologies. Six studies reported participants reporting difficulty hearing the provider during the telemedicine visits. Five studies excluded participants with visual or hearing impairment due to the potential difficulty of using telemedicine technology. No studies reported technological adaptations to account for sensory impairment.
Conclusions and Implications
Telemedicine is well-received among patients and care partners, but successful delivery incorporates support staff and the care partners to navigate technologies. The exclusion of older adults with sensory impairment, especially given that it is highly prevalent, in developing telemedicine systems may further exacerbate access to care in this population. Adapting technologies for sensory needs is critical to the advancement of accessible dementia care through telemedicine.
Keywords: telemedicine, telehealth, dementia
Brief summary:
Successful delivery of telemedicine incorporates support staff and care partners to navigate technologies. Adapting technologies for sensory needs is critical to the advancement of accessible dementia care through telemedicine.
Introduction
Telemedicine has emerged as a rapidly expanding model of care for patients, including persons living with dementia and their care partners,1 especially in the setting of disrupted healthcare norms of the COVID-19 pandemic.2 Telemedicine via synchronous videoconferencing lends itself well to the clinical interview that is of primary importance in providing routine care for older adults with Alzheimer’s disease and related dementias (ADRD) and allows clinicians a glimpse into the patient’s home environment3 while maintaining physical distancing to protect this high-risk group.4 The success of this model of care, however, is dependent on a patient’s reliable access to technology in addition to the ability to see, hear, and understand the clinician. 2,5 Telemedicine delivery in the home requires a smartphone, tablet, or computer, along with a stable internet connection and the ability to troubleshoot technological issues.2 From the user perspective, telemedicine requires patients to have adequate hearing and vision, along with motor and cognitive abilities to connect with providers and understand their treatment plan.5 These requirements pose a challenge for older adults with ADRD, who may have different needs when interacting with telemedicine systems due to age-related changes in sensory and cognitive abilities.5,6
Age-related hearing loss is highly prevalent, with a prevalence up to 90% in persons with dementia.7–9 However, age-related hearing loss often goes untreated. Hearing loss can complicate providers’ assessment of cognitive function,10,11 and these difficulties may be compounded in telemedicine encounters when patients are limited in their ability to optimize audio and visual quality in a novel situation.5 Telemedicine encounters that do not adequately account for hearing loss may risk inaccurate understanding of instructions and limit the patient or care partner’s ability to enact their treatment plan. In addition, cognitive impairment is a risk factor for non-use of technology among older adults.12 Visual impairment is also highly prevalent, with more than 30% of persons with dementia estimated to have vision impairment,13 and global population estimates for moderate/severe vision impairment at 2.1 million.14 Furthermore, 11.3% of older adults 80 years or older have dual sensory impairment, with significantly increased risk for dementia.15 Thus, age-related changes in sensory and cognitive abilities must be taken into account when delivering telemedicine.
Successful delivery of telemedicine also depends on the technological proficiencies of the care partner and the ability of the patient with ADRD to interact with the provider remotely. This added burden of technological proficiency that telemedicine requires may further limit the successful uptake of telemedicine as a modality for routine care among older adults with ADRD. Without the necessary adaptations to account for technological and sensory needs in this population, older adults with ADRD may be quickly left behind as telemedicine becomes increasingly relied upon in the future. Furthermore, accounting for technological and sensory needs in this population presents an opportunity to extend access to dementia care and reduce the burden of transportation, financial costs, and travel time for individuals and care partners.
Previous studies investigating the use of telemedicine for older adults with ADRD have largely focused on reducing the burden and stress of care partners, with relatively little focus on the patient experience.1 A closer examination of the unique issues regarding technological feasibility and sensory needs from a patient perspective may inform future efforts to support older adults with ADRD engaging in telemedicine. Given the growing population of older adults with dementia who face difficulty in navigating the health care system,16 improving telemedicine systems to adapt to the needs of this population is imperative. The objective of this study was to perform a systematic review to understand the state of the literature on synchronous in-home or clinic video-based telemedicine visits for older adults with ADRD or mild cognitive impairment (MCI), and characterize technological barriers and facilitators in providing care that is responsive to their needs, including sensory related.
Methods
Literature Search Strategy
The study team queried PubMed via PubMed.gov, Embase via Embase.com, the Cochrane Library via Wiley, PsycINFO and CINAHL via EBSCOhost, and Scopus via Elsevier, and ClinicalTrials.gov on May 1, 2020 for studies in English published from January 2010 to May 2020. We chose 2010 as the starting point to understand how telemedicine technology has evolved in the past 10 years. This review adhered to the Primary Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (Appendix 1).17 The team performed a pilot search in PubMed to identify key articles to build a search strategy that included relevant terminology. An informationist (CLP) trained in performing database searches for systematic reviews developed the search strategy of relevant terms that included both controlled vocabulary, where appropriate, and keyword terms for the concepts of ADRD or MCI and telemedicine. The complete search strategy is provided in Appendix 2. The initial search yielded 6414 studies, of which 2863 duplicates were removed, resulting in 3551 unique studies (Appendix 3).
Study Selection
Two reviewers (SJK and CP) independently screened the title and abstract for each article according to pre-defined criteria. Studies were included if they met the following criteria: 1) study population is focused on older adults with ADRD or MCI 2) intervention is a video-based synchronous telemedicine visit delivered at home or outside the home, 3) interventions and outcomes that involve direct participation by the older adult with ADRD or MCI, rather than the care partner alone, 4) published from 2010 to 2020, 5) written in English, and 6) full-text available. Studies were excluded if they met the following criteria: 1) study not written in English, 2) full-text unavailable, 3) wrong study design (review, not peer-reviewed), 4) wrong population (persons without MCI/ADRD, care partners are primary study population), 5) wrong intervention (mobile health apps, asynchronous telemedicine, primary focus on care partner support). Conference proceedings, abstract submissions, and graduate theses were excluded. All study desgns were considered as long as the manuscript included original data. Each title/abstract reviewed was categorized as “include for full-text review”, “maybe”, or “exclude.” Relevant systematic reviews or literature reviews were also included for the purpose of identifying additional articles. Discrepancies were resolved by discussion between both reviewers and adjudication by an independent third reviewer (CLN). Following title and abstract screening, the remaining studies underwent full-text screening. Two reviewers (SJK and CP) completed the full text-screen using a hierarchical method of exclusion with discrepancies resolved by a third reviewer (CLN). Lastly, the reference lists of the included articles and systematic/literature reviews were examined to identify additional articles for inclusion.
Data Extraction and Quality Assessment
All study data were extracted using a standardized data collection form, which included the following: design (pilot study, randomization), study location, intervention location, sample size, recruitment method, participant inclusion/exclusion criteria, encounter purpose, intervention content, outcomes, staff/care partner requirements, technological requirements/issues, participant sensory impairment, and demographic characteristics. Significant methodological heterogeneity precluded a meta-analysis of the included studies. Qualitative summary focused on intervention characteristics, technological requirements, and participant level characteristics.
The Cochrane Collaboration’s Risk of Bias tool was used for quality assessment as shown in Appendix 4. Criteria included random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias.18 Two reviewers (SJK and CP) independently assessed each of the included studies, rating them as ‘high’, ‘low’, or ‘unclear’ risk of bias for each criterion. Discrepancies in quality assessment were resolved by a third reviewer (CLN). For each domain, the support for judgment is detailed in Appendix 5. The full-text manuscripts for each study were used in summarizing the support for judgment. Among other domains included in the Cochrane Risk of Bias Tool, ‘blinding of participants and personnel’ as well as ‘other sources of bias’ were not included in this review. Blinding of participants and personnel was not possible given the nature of telemedicine interventions, and was therefore excluded from the assessment. All studies reported insufficient information to assess other sources of bias, so this domain was also excluded from Appendix 4.
Results
The initial search identified 3551 unique abstracts (PubMed 907, EMBASE 1947, Cochrane 527, PsycINFO 739, CINAHL Plus 755, Scopus 1498, Clinicaltrials.gov 41), from which 3461 were excluded in the initial title and abstract screen. Of the remaining 90 articles that underwent full-text review, 17 studies met inclusion criteria (Appendix 3).19
Appendix 6 summarizes key characteristics of the of the studies included in this review, which include 7 randomized pilot studies, 9 non-randomized pilot studies, and 1 randomized controlled trial (RCT).20 8 studies conducted telemedicine visits with participants at remote or satellite clinics connecting via video-conference with providers at hospitals or academic centers (e.g., university affiliated hospital, university affiliated clinic, or university research facilities).21–28 In 4 studies, both the participant and provider were located at hospitals or academic centers.29–32 A smaller number of studies conducted telemedicine visits in the home setting20,33,34 or at adult day care centers and nursing homes.35,36 Included studies were published between 2011–2020, with 8 studies published in the US,22,24,26–28,32–34 followed by Canada,21,25,29 Australia,20,31 Italy,30,36 Portugal,35 and South Korea.23 5 studies randomized participants to in-person visit vs. telemedicine,20,25,29,31,36 while 3 studies randomized the order in which participants underwent both in-person and telemedicine visits.26,32,33 The majority of studies recruited participants through outpatient clinics,20,26,27,30,33,34,36 health/clinic/study registries,23,32,35 or community-based organizations,20,29 while others required a referral or consultation request from a geriatrician21 or primary care provider.22,24,25,28,31 Sample sizes ranged from 6 to 427 with a median of 94.23,29 Among studies reporting study duration, studies lasted on average 23 months. Among studies reporting intervention duration, the intervention period lasted on average 3 months (Appendix 7).
Mini-Mental State Examination (MMSE) was the most commonly used cognitive screening tool.20–22,30,32
Intervention Characteristics
In the majority of studies, the purpose of the telemedicine encounter was to provide routine care for participants with cognitive impairment (Appendix 6). Components of routine care included administering cognitive screening, medication review and initiation, follow up to therapy, assessing changes in symptoms, and providing care partner support and education.21–25,27,28,31,34 A minority of studies performed cognitive screening21,24,31 and measurement of vitals21,24,27 as part of an in-person check-in prior to the telemedicine visit.
Five studies administered cognitive screening via telemedicine where the primary objective was to assess the reliability or acceptability of these measures as an alternative to in-person assessment.26,30,32,33,35 The MMSE was the most commonly administered cognitive assessment via telemedicine.26,30,32
Three studies provided rehabilitation services through telemedicine focused on cognitive exercises and addressing care challenges with care partners.20,29,36
Outcome measures – feasibility, acceptability, reliability
Feasibility of telemedicine was a focus of 14 out of 17 studies, which reported feasibility of the telemedicine intervention (Appendix 7). Fourteen of 14 studies reporting feasibility found that the telemedicine intervention was feasible (Appendix 7). The most commonly used feasibility measure was participant satisfaction used in 7 studies.21,22,24,25,27,34,36 Acceptability was measured in 7 of 17 studies with all 7 studies reporting telemedicine was acceptable.24,25,29,30,32,34,35 Two of 7 studies administered acceptability surveys to participants,30,32 while 4 of 7 used participant satisfaction to measure acceptability,24,25,34,35 and 1 of 7 compared improvement in occupational performance measures after telerehabilitation vs. rehabilitation in-person.29 Four studies of 17 studies reported reliability measures by comparing cognitive assessment scores administered in-person vs. via telemedicine, with all 4 reporting telemedicine administration was reliable.26,30,33,35
Outcome measures – routine care and diagnosis
Among 9 studies focused on routine care, assessments via telemedicine allowed providers to diagnose cognitive impairment in participants in 6 studies.22,24,25,27,28,31 None of the included studies explicitly discussed misdiagnoses due to inability to examine a patient. In one study that employed telemedicine vs. in-person assessment for the purpose of diagnosis, the difference in agreement between the telemedicine group and in-person group was 1%, suggesting no substantial difference in diagnosis between the methods of assessment.31
Outcome measures – cognitive assessments
Among 5 studies administering cognitive assessments, all studies reported administering these measures via telemedicine was a reliable or acceptable alternative to in-person assessment with similar results on cognitive assessment comparing telemedicine to in-person visits.26,30,32,33,35
Outcome measures – telerehabilitation
Among 3 studies using telemedicine for rehabilitation, all studies reported improvement in outcome measures after completing the intervention via telemedicine.20,29,36 Outcomes included improvement in Canadian Occupational Performance Measure,29 increase in MMSE score and episodic memory,36 and improvement in the caregiving mastery index.20
Outcome measures – satisfaction rates
Thirteen out of 17 studies reported participant satisfaction with telemedicine, with favorable satisfaction ratings but overall low response rates (Appendix 8). Studies did not use a uniform scoring system for satisfaction questionnaires. One study reported 65% of patients and 91% of care partners responding to satisfaction surveys preferred to see the specialist via telemedicine than in person.24 Another study reported that among patients and care partners responding to surveys, 100% of patients and 100% of care parnters preferred not driving for their care.27 In this study, the estimated mean (SD) round trip distance saved in miles was 67.1 (39.7), and the estimated mean round trip on the road saved in minutes was 74.5 (43.2).27 In addition, another study reporting miles traveled for clinic vists found that the mean (SD) miles traveled was 48.8 (38.70) in the in-person group compared to 2.9 (7.24) in the telemedicine group.34
Technological Requirements
Technological requirements and the role of support staff and care partners varied across participant telemedicine locations (Appendix 9). Among telemedicine visits conducted at home,20,33,34 all included a pre-visit orientation with study staff to install software, conduct test runs, and troubleshoot technological difficulties through a home visit20 or phone conversation.33,34 In addition, 2 studies provided additional, real-time support during the telemedicine visit via telephone in case participants or providers experienced technological difficulties.20,34 Studies conducting telemedicine visits in adult day care centers and nursing homes incorporated support staff who assisted the patient in performing the physical exam under the guidance of the provider.35,36
Eleven studies required care partner participation during telemedicine visits (Appendix 9). For studies conducting telemedicine visits in participant homes, care partners played a key role in facilitating the telemedicine encounter.20,33,34 One study reported care partners were instructed on how to modify the home environment to minimize distractions and maximize sound quality.33
For in-home telemedicine, other participant requirements included having a computer, tablet, or phone with internet access capable of operating the telemedicine software.20,33,34 In cases where participants did not own the necessary equipment, study staff loaned tablets with the telemedicine software installed. One study reported that lack of access to computers and broadband in addition to limited experience with computers were among reasons for participants declining telemedicine.34 For studies conducted outside the home, all necessary equipment were provided by the healthcare facility (Appendix 9).
Among studies reporting technological difficulties, connection and audio issues were most commonly reported. Connectivity issues included images freezing, delay in sound, echoing, and static noises.21,24,25,29,30 Six of 17 studies, reported audio difficulties, with participants reporting difficulty hearing the provider during the telemedicine visits.21,24,25,27,29,35 One study reported visits being terminated due to hearing difficulties, and that problems with rural internet connectivity prevented delivery of in-home telemedicine.27 The visual quality of telemedicine visits was reported as an issue in 4 of 17 studies.24,25,29,35 Three out of 17 studies reported exclusion of participants due to technologic reasons [(n=1 of 210),31 (n=63 of 222),34 (n=2 dyads of 33 dyads)33]. One out of these three studies reported the specific reasons, which included not having a home computer (n=46 out of 222), not being comfortable with computers (n=12 out of 222), and computers being too old or lacking broadband service (n=5 out of 222).34
Participant Characteristics
Five studies excluded participants with visual or hearing impairment due to the potential difficulty of using telemedicine technology in this population (Appendix 10).22,26,30,32,33 These studies did not include details on how they defined visual or hearing impairment. 5 of 17 studies did not include any language on the potential role of sensory impairment in delivering telemedicine.20,23,29,31,34 Among studies that did not exclude participants on the basis of sensory impairment, 5 of 17 studies reported communication challenges due to hearing loss.21,24,25,27,35 No studies reported adapting telemedicine equipment with headsets or amplification devices to mitigate communication challenges due to sensory impairment. Two of 17 studies reported challenges due to visual impairment.24,35 No studies adapted for visual impairment.
Quality Assessment
Among the domains assessed using the Cochrane Risk of Bias Tool, the ‘incomplete outcome data’ domain had the greatest number of studies rated as “high risk” of bias (Appendix 4). Fourteen out of 17 studies had at least one domain with high risk of bias,21,22,33–36,23–28,30,32 while 2 out of 17 studies had low risk of bias across all assessed domains.20,31
Discussion
The purpose of this review was to understand the current state of the literature on the barriers and facilitators related to telemedicine use among older adults with cognitive impairment and their care partners with an emphasis on the technological requirements and accommodations for sensory needs, specifically hearing loss and visual impairment. Although substantial heterogeneity existed in the location of telemedicine encounters, participant characteristics, and measurement of outcomes, all studies demonstrated successful use of telemedicine for the purpose of routine care, cognitive assessment, or rehabilitation in older adults with ADRD or MCI. Significant barriers to telemedicine in this population included meeting technological requirements and adapting for sensory needs. Technological barriers included both the lack of equipment and the older adult’s ability to manipulate technologies independently. To address this difficulty, the majority of studies relied on support staff or the care partner as facilitators to set up the necessary equipment and troubleshoot technological issues. Sensory needs, such as hearing and vision-related communication difficulties, were highlighted in multiple studies as a barrier to successful uptake of telemedicine. However, strategies to mitigate these challenges were lacking. Further research is needed to develop solutions for sensory-related communication challenges in telemedicine encounters. Lastly, most telemedicine encounters occurred between healthcare facilities (provider at hospital connected to patients at remote clinics), likely due to difficulties in reimbursement for telemedicine visits. As in-home delivery of telemedicine has significantly increased due to the COVID-19 pandemic, there will be a growing need for continued research on improving in-home delivery of telemedicine, which will become a more routine part of clinical care.2,37
To facilitate the successful uptake of telemedicine technology among older adults with ADRD or MCI in the home, prior training and provision of equipment are key. In studies conducting telemedicine visits in the home, equipment set-up, orientation, and test-run prior to the telemedicine encounter helped familiarize users to the telemedicine environment. Individuals with dementia have progressive difficulty processing and responding to stimuli and stress may be caused by changes in routine and the environment.38,39 Care partners can play a key role in optimizing the home environment for telemedicine by minimizing clutter and background noise and simplifying tasks for the person with dementia. Orientation and training prior to the encounter can help set clear expectations for the care partner and reduce the novelty of the situation for the person with dementia. Patients and care partners can also provide valuable ideas for the development of new functions and services in telemedicine platforms to ensure the developments are user driven. Additional keys for success include loaning equipment such as tablets with software, arranging a backup plan through a phone conversation in case there are connection difficulties, and providing real-time assistance via telephone or a telemedicine platform that supports 3-way visits. For patients with hearing loss, the development of captioned services on telemedicine platforms can help to maximize communication accessibility, and captioning that uses automatic speech recognition is available through multiple videoconferencing platforms.40 Providers can aim to keep visits short35 and have a mechanism in place to deliver materials for the patient and care partner to review. For patients with visual impairment, electronic magnification and text to speech technology on smartphones or tablets may be used. Additionally, providers and care partners can modify the environment by improving lighting and reducing glare on screens.
Successful implementation of telemedicine systems for older adults requires consideration of user needs and their interaction with technology (Figure 1).5 One potential area for development in accommodating the needs of older adults is adapting technologies that account for sensory impairments. Potential adaptations for hearing loss may include integrating headsets, speakers, or personal sound amplifiers in the telemedicine system that patients and care partners can adjust according to their hearing needs as well as the default use of closed captions via automatic speech recognition.41–43 Additionally, support staff may benefit from specialized training in communicating with older adults with cognitive and sensory impairment. For visual impairment, support staff and providers can improve communication by using verbal descriptions without relying solely on gestures and facial experessions. Including a human factors engineer in the team can also be helpful in facilitating the adaptation of telemedicine technology.44 Given that hearing loss affects up to 90% older adults with ADRD and vision impairment affects more than 30% of persons with dementia,7–9,13 adapting telemedicine technologies to account for sensory changes has the potential to significantly improve the quality of telemedicine for these individuals. Increased satisfaction during telemedicine encounters, in turn, has the potential to better equip older adults to understand and enact their treatment plans. The exclusion of older adults with sensory impairment, particularly given that it is highly prevalent, in developing telemedicine systems limits the ability to optimize communication for these individuals and may further exacerbate access to care in this population. As in-home delivery of telemedicine continues to become a part of routine medical care, adapting technologies for sensory needs not only presents an opportunity to improve care for older adults with ADRD, but also improve care for older adults more generally experiencing sensory impairment.
Figure 1.
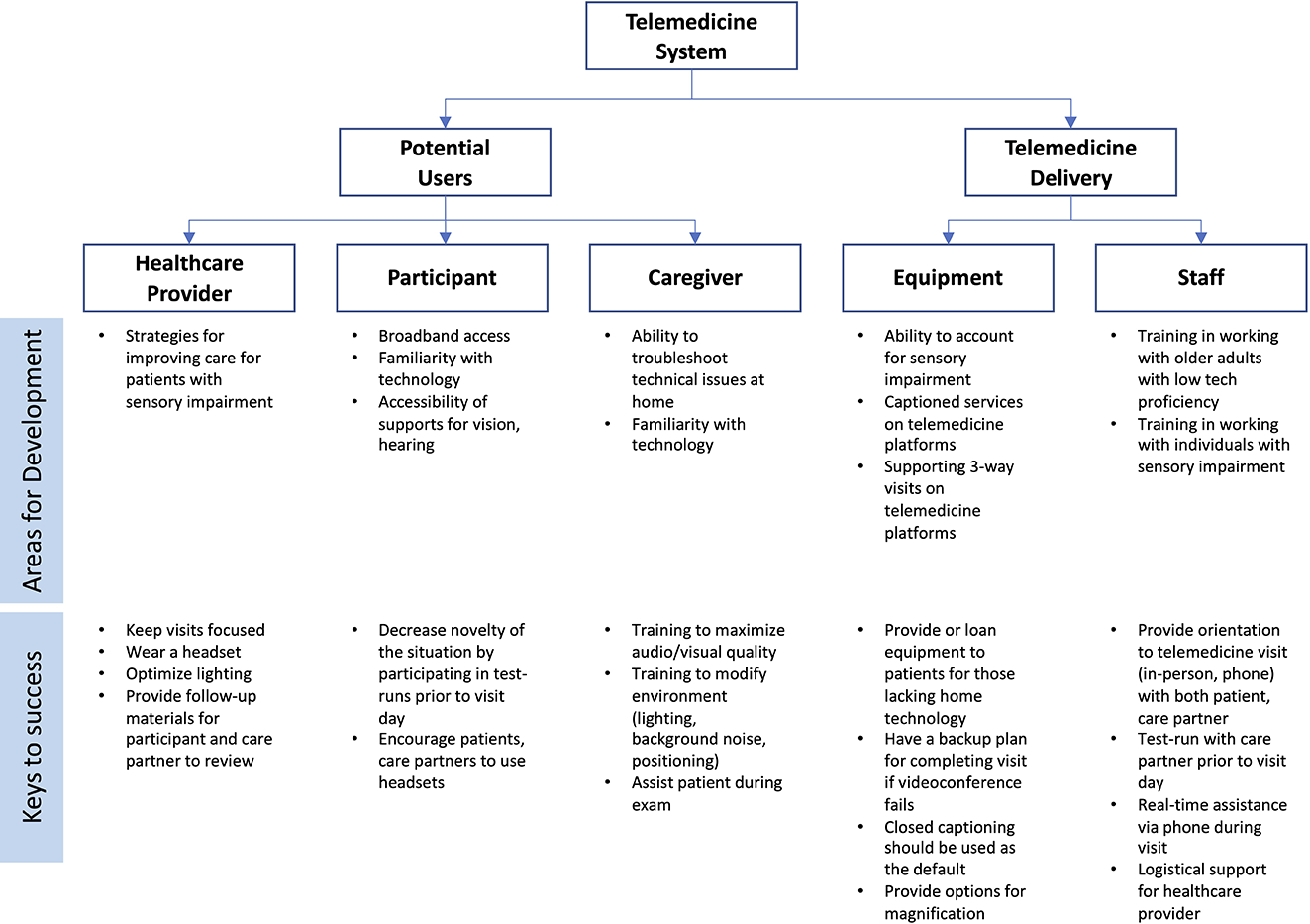
Factors to consider for successful implementation of telemedicine in older adults with dementia
Improving telemedicine in the near-term also has the benefit of reducing the burden of transportation and trips outside of the home for persons with dementia and their care partners. However, recent literature reporting the impact of physical distancing during the COVID-19 pandemic have highlighted increased stress for care partners and discontinuation of regular cognitive and physical therapies for persons with dementia.45 Another study found that long periods of physical isolation seem to exacerbate neuropsychiatric symptoms for older adults living with dementia.46 In-home delivery of telemedicine also reduces the concern for exposure and spread of COVID-19. The conveniences of telemedicine may help alleviate these immediate difficulties and reduce care partner stress in part reducing barriers to seeking care by eliminating travel to medical appointments and other in-person therapies.
Several recent studies have also demonstrated the importance and benefits of telemedicine during the COVID-19 pandemic.47 One study found that telemedicine by video conference was associated with improved resilience and wellbeing to both participants with neurocognitive disorders and care partners at home, highlighting the potential role of telemedicine as an acceptable approach to dementia care.47 Another study found that television-based telehealth support demonstrated potential for cognitive stimulation by providing access to COVID-19 information, recreational activities, and memory exercises.48 Benefits of telemedicine have been reported in dementia subtypes, including frontotemporal dementia, where telemedicine demonstrated validity as a triage tool to increase practice outreach and efficiency.49 Although telemedicine has demonstrated its many benefits during the current crisis, long-term considerations to address barriers to telemedicine care deserve equal attention in its potential to improve the accessibility of care for patients with dementia and their care partners.
Limitations and future research
There are several limitations in the current literature on telemedicine for older adults with ADRD. The majority of studies implemented telemedicine between healthcare facilities rather than in the home. Given that the technological and personnel requirements for conducting telemedicine visits are fundamentally different at a healthcare facility vs. in-home, further research is needed to better characterize technological barriers for older adults with cognitive impairment in the home. Additionally, this review included a focus on barrier and facilitiators to telemedicine with an emphasis on technological requirements as well as sensory needs, specifically hearing and vision. This review did not consider other potential individual-level barriers to telemedicine that can be experienced by persons with dementia, such as apraxia.
Regarding cognitive assessment, MMSE was the most commonly used instrument among the included studies. Given that individuals who appear to lack clinically significant cognitive deficits assessed by the MMSE may demonstrates deficits when assessed using other cognitive tests such as the Montreal Cognitive Assessment (MoCA) or Saint Louis University Mental Status (SLUMS),50 cognitive assessment to identify participants with MCI for inclusion may have been inadequate when limited to the MMSE.
An additional limitation is that among studies reporting race/ethnicity, education level, or socioeconomic position, participants were predominantly self-identified as white and were educated individuals from higher socioeconomic backgrounds familiar with using technology.22,28–30 No studies accounted for how factors that are known drivers of technology acceptance among older adults, such as eHealth literacy,51 technology self-efficacy and preferences and prior experiences,5,52–54 may influence the feasibility and acceptability of telemedicine. Given that minority and low-income adults have lower rates of high-tech device use,55–57 further research is needed to understand the unique barriers, needs, and facilitators to telemedicine in these populations and help ensure that strategies to implement telemedicine are responsive to the needs and preferences of diverse groups with varied preferences and experience with technology. An individualized user functional assessment may be used prior to the telemedicine encounter to better understand the specific sensory and technological needs of the patient and care partner. Using this knowledge of patient-specific barriers to care, members of the interdisciplinary care team may reach out in advance to address these specific barriers. This patient-specific plan might involve orienting care partners to telemedicine, providing necessary equipment or captioned services as outlined in Figure 1.
Lastly, given that response rates to satisfaction surveys were low as seen in Appendix 5, findings on high satisfaction with telemedicine among participants and care partners warrant careful consideration. A potential reason for non-response may have been lower satisfaction with telemedicine. Potential reasons for lower satisfaction may include the lack of in-person interaction with a provider which may be motivating and therapeutic. While telemedicine may reduce the need for travel, it also reduces social interactions outside of the home, which may have beneficial effects for the patient and care partner. In addition, patients may be concerned about the potential for missed diagnoses due to the inability to examine a patient in-person. Further studies may consider efforts to increase response rates to satisfaction surveys by administering questionnaires within the telemedicine encounter to understand further barriers to telemedicine use as well as targeting evaluations from non-responders or individuals who were unsatisfied to characterize the spectrum of patient and care partner experiences.
Conclusions and Implications
This systematic review characterizes the barriers and facilitators in providing care through telemedicine for older adults with cognitive impairment. Telemedicine is well-received among patients and care partners with high satisfaction rates. Successful in-home delivery of telemedicine relies upon support staff and the care partner to navigate technologies. Notably, technological adaptations for sensory needs among older adults is lacking. Technological adaptations, particularly those that respond to the unique needs of users, are critical to the advancement of accessible dementia care through telemedicine.58
Funding sources:
This work was supported by the National Institute on Aging (NIA)/National Institutes of Health (NIH) (K23 AG059900) (CLN), (R33 DC015062) (CLN), NIA/NIH (R01AG057725) (ESO), and NIDCD Diversity Supplement (R33 DC015062) (CP).
Conflicts of Interest: CLN reported receipt of grants from the National Institute on Aging (NIA) and the National Institute on Deafness and Other Communication Disorders (NIDCD). CLN reported serving as a volunteer board member for the nonprofits, Access HEARS and the Hearing Loss Association of America.
ESO reported receipt of grants from the National Institute on Aging (NIA).
CP reported receipt of grants from the National Institute on Deafness and Other Communication Disorders (NIDCD) Diversity Supplement.
Appendix 1.
PRISMA Checklist
| Section/topic | # | Checklist item | Reported on page # |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both. | Title page |
| ABSTRACT | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number. | 1–2 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. | 3–5 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS). | 4 |
| METHODS | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide registration information including registration number. | NA |
| Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale. | 5–6 |
| Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched. | 5 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated. | Appendix 2 |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis). | 5–6 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators. | 6–7 |
| Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made. | 6 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis. | 7 |
| Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means). | 7 |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis. | NA |
| Section/topic | # | Checklist item | Reported on page # |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, selective reporting within studies). | 7 |
| Additional analyses | 16 | Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, metaregression), if done, indicating which were pre-specified. | NA |
| RESULTS | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram. | 7, Appendix 3 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and provide the citations. | 7–9 |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12). | 12–13, Appendix 4,5 |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and confidence intervals, ideally with a forest plot. | 9–10 |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency. | NA |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15). | NA |
| Additional analysis | 23 | Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, metaregression [see Item 16]). | NA |
| DISCUSSION | |||
| Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., healthcare providers, users, and policy makers). | 13–14 |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g., risk of bias), and at review-level (e.g., incomplete retrieval of identified research, reporting bias). | 17–18 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research. | 19 |
| FUNDING | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review. | Title page |
From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097. doi:10.1371/journal.pmed1000097
Appendix 2. Search strategy
PubMed Search Strategy
#1 (“Alzheimer Disease”[Mesh] OR “cognitive dysfunction”[mesh:noexp] OR “dementia”[mesh:noexp] OR “dementia, multi-infarct”[mesh] OR “dementia, vascular”[mesh:noexp] OR “frontotemporal dementia”[mesh:noexp] OR “frontotemporal lobar degeneration”[mesh:noexp] OR “memory disorders”[mesh:noexp] OR “pick disease of the brain”[mesh] OR “ADRD”[tw] OR “amentia”[tw] OR “amentias “[tw] OR “amnestic”[tw] OR “amnestics”[tw] OR “amnesia”[tw] OR “amnesias”[tw] OR “cognitive decline”[tw] OR “cognitive defect”[tw] OR “cognitive defects” [tw] OR “cognition disorder”[tw] OR “cognition disorders”[tw] OR “cognitive disorder” [tw] OR “cognitive disorders”[tw] OR “cognitive dysfunction”[tw] OR “cognitive dysfunctions”[tw] OR “cognitive impairment”[tw] OR “cognitive impairments”[tw] OR “ cognitive retention” [tw] OR “ dementia” [tw] OR “ dementias” [tw] OR “ demention” [tw] OR “dementions”[tw] OR “loss of memory”[tw] OR “MCI”[tw] OR “memory clinic”[tw] OR “memory clinics”[tw] OR “memory deficit”[tw] OR “memory deficits”[tw] OR “memory disorder”[tw] OR “memory disorders”[tw] OR “memory disorders” [tw] OR “memory loss”[tw] OR “mild cognitive impairment”[tw] OR “pick disease”[tw] OR “pick s disease”[tw] OR “picks disease”[tw] OR Alzheimer*[tiab])
#2 (“telemedicine”[mesh:noexp] OR “telerehabilitation”[mesh] OR “remote consultation”[mesh] OR “videoconferencing”[mesh] OR “distance counseling”[tw] OR “e health”[tw] OR “ehealth”[tw] OR “internet based”[tw] OR “m health”[tw] OR “mHealth”[tw] OR “mobile health”[tw] OR “mobile visit”[tw] OR “mobile visits”[tw] OR “online meeting”[tw] OR “online meetings”[tw] OR “online consult”[tw] OR “online consults”[tw] OR “online consultation”[tw] OR “online consultations”[tw] OR “online therapy”[tw] OR “online therapies”[tw] OR “online visit”[tw] OR “online visits”[tw] OR “online appointment”[tw] OR “online appointments”[tw] OR “remote appointment”[tw] OR “remote appointments”[tw] OR “remote assessment”[tw] OR “remote assessments”[tw] OR “remote consult”[tw] OR “remote consultation”[tw] OR “remote consultations”[tw] OR “remote consults”[tw] OR “remote management”[tw] OR “tele consultation”[tw] OR “tele consultations”[tw] OR “tele health”[tw] OR “tele medicine”[tw] OR “tele neurology”[tw] OR “teleconsultation”[tw] OR “teleconsultations”[tw] OR “telehealth”[tw] OR “telemedicine”[tw] OR “teleneurology”[tw] OR “telerehab”[tw] OR “telerehabilitation”[tw] OR “tele rehabilitation”[tw] OR “tele therapy”[tw] OR “tele therapies”[tw] OR “teletherapy”[tw] OR “teletherapies”[tw] OR “videoconferencing” [tw] OR “videoconference”[tw] OR “video conference”[tw] OR “video conferencing”[tw] OR “videoconferences”[tw] OR “video conferences”[tw] OR “video consult”[tw] OR “video consults”[tw] OR “video consultation”[tw] OR “video consultations”[tw] OR “virtual visit”[tw] OR “virtual visits”[tw] OR “virtual consult”[tw] OR “virtual consults”[tw] OR “virtual consultation”[tw] OR “virtual consultations”[tw] OR “Zoom”[tw] OR “doximity”[tw] OR “Mdlive”[tw] OR “talkspace”[tw] OR “livehealth”[tw] OR “lemonaid”[tw] OR “teladoc”[tw] OR “doctor on demand”[tw] OR “amwell”[tiab] OR “k health”[tw] OR “khealth”[tw] OR “polycom”[tw] OR “healthtap”[tw] OR “first opinion”[tw] OR “simple contacts”[tw] OR “plushcare”[tw] OR “telemed”[tw] OR “express care virtual”[tw] OR “care virtual”[tw] OR “pingmd”[tw] OR “babylon health”[tw] OR “dialogue”[tw] OR “epic”[tw] OR “adappt”[tw] OR “virtual care”[tw] OR ((“phone based”[tw] OR “smartphone based”[tw] OR “telephone based”[tw] OR “video based”[tw] OR “web based”[tw] OR “internet”[tw] OR “online”[tw] OR “remote”[tw] OR “virtual”[tw]) AND (consult*[tw] OR “visit”[tw] OR “visits”[tw] OR appointment*[tw] OR “routine care”[tw] OR “care delivery”[tw] OR “primary care”[tw])))
#3 #1 AND #2
907
Embase Search Strategy
#1 (‘Alzheimer Disease’/de OR ‘cognitive defect’/de OR ‘dementia’/de OR ‘multiinfarct dementia’/de OR ‘frontotemporal dementia’/de OR ‘memory disorder’/de OR ‘ADRD’:ti,ab,kw OR ‘amentia’:ti,ab,kw OR ‘amentias’:ti,ab,kw OR ‘amnestic’:ti,ab,kw OR ‘amnestics’:ti,ab,kw OR ‘amnesia’:ti,ab,kw OR ‘amnesias’:ti,ab,kw OR ‘cognitive decline’:ti,ab,kw OR ‘cognitive defect’:ti,ab,kw OR ‘cognitive defects’:ti,ab,kw OR ‘cognition disorder’:ti,ab,kw OR ‘cognition disorders’:ti,ab,kw OR ‘cognitive disorder’:ti,ab,kw OR ‘cognitive disorders’:ti,ab,kw OR ‘cognitive dysfunction’:ti,ab,kw OR ‘cognitive dysfunctions’:ti,ab,kw OR ‘cognitive impairment’:ti,ab,kw OR ‘cognitive impairments’:ti,ab,kw OR ‘cognitive retention’:ti,ab,kw OR ‘dementia’:ti,ab,kw OR ‘dementias’:ti,ab,kw OR ‘demention’:ti,ab,kw OR ‘dementions’:ti,ab,kw OR ‘loss of memory’:ti,ab,kw OR ‘MCE’:ti,ab,kw OR ‘memory clinic’:ti,ab,kw OR ‘memory clinics’:ti,ab,kw OR ‘memory deficit’:ti,ab,kw OR ‘memory deficits’:ti,ab,kw OR ‘memory disorder’:ti,ab,kw OR ‘memory disorders’:ti,ab,kw OR ‘memory disorders’:ti,ab,kw OR ‘memory loss’:ti,ab,kw OR ‘mild cognitive impairment’:ti,ab,kw OR ‘pick disease’:ti,ab,kw OR ‘pick s disease’:ti,ab,kw OR ‘picks disease’:ti,ab,kw OR Alzheimer* :ti,ab)
#2 (‘telemedicine’/de OR ‘teleconsultation’/de OR ‘telerehabilitation’/de OR ‘videoconferencing’/de OR ‘distance counseling’:ti,ab,kw OR ‘e health’:ti,ab,kw OR ‘ehealth’:ti,ab,kw OR ‘internet based’:ti,ab,kw OR ‘m health’:ti,ab,kw OR ‘mHealth’:ti,ab,kw OR ‘mobile health’:ti,ab,kw OR ‘mobile visit’:ti,ab,kw OR ‘mobile visits’:ti,ab,kw OR ‘online meeting’:ti,ab,kw OR ‘online meetings’:ti,ab,kw OR ‘online consult’:ti,ab,kw OR ‘online consults’:ti,ab,kw OR ‘online consultation’:ti,ab,kw OR ‘online consultations’:ti,ab,kw OR ‘online therapy’:ti,ab,kw OR ‘online therapies’:ti,ab,kw OR ‘online visit’:ti,ab,kw OR ‘online visits’:ti,ab,kw OR ‘online appointment’:ti,ab,kw OR ‘online appointments’:ti,ab,kw OR ‘remote appointment’:ti,ab,kw OR ‘remote appointments’:ti,ab,kw OR ‘remote assessment’:ti,ab,kw OR ‘remote assessments’:ti,ab,kw OR ‘remote consult’:ti,ab,kw OR ‘remote consultation’:ti,ab,kw OR ‘remote consultations’:ti,ab,kw OR ‘remote consults’:ti,ab,kw OR ‘remote management’:ti,ab,kw OR ‘tele consultation’:ti,ab,kw OR ‘tele consultations’:ti,ab,kw OR ‘tele health’:ti,ab,kw OR ‘tele medicine’:ti,ab,kw OR ‘tele neurology’:ti,ab,kw OR ‘teleconsultation’:ti,ab,kw OR ‘teleconsultations’:ti,ab,kw OR ‘telehealth’:ti,ab,kw OR ‘telemedicine’:ti,ab,kw OR ‘teleneurology’:ti,ab,kw OR ‘telerehab’:ti,ab,kw OR ‘telerehabilitation’:ti,ab,kw OR ‘tele rehabilitation’:ti,ab,kw OR ‘teletherapy’:ti,ab,kw OR ‘teletherapies’:ti,ab,kw OR ‘tele therapy’:ti,ab,kw OR ‘tele therapies’:ti,ab,kw OR ‘videoconferencing’:ti,ab,kw OR ‘videoconference’:ti,ab,kw OR ‘video conference’:ti,ab,kw OR ‘video conferencing’:ti,ab,kw OR ‘videoconferences’:ti,ab,kw OR ‘video conferences’:ti,ab,kw OR ‘video consult’:ti,ab,kw OR ‘video consults’:ti,ab,kw OR ‘video consultation’:ti,ab,kw OR ‘video consultations’:ti,ab,kw OR ‘virtual visit’:ti,ab,kw OR ‘virtual visits’:ti,ab,kw OR ‘virtual consult’:ti,ab,kw OR ‘virtual consults’:ti,ab,kw OR ‘virtual consultation’:ti,ab,kw OR ‘virtual consultations’:ti,ab,kw OR ‘Zoom’:ti,ab,kw OR ‘doximity’:ti,ab,kw OR ‘Mdlive’:ti,ab,kw OR ‘talkspace’:ti,ab,kw OR ‘livehealth’:ti,ab,kw OR ‘lemonaid’:ti,ab,kw OR ‘teladoc’:ti,ab,kw OR ‘doctor on demand’:ti,ab,kw OR ‘amwell’:ti,ab OR ‘k health’:ti,ab,kw OR ‘khealth’:ti,ab,kw OR ‘polycom’:ti,ab,kw OR ‘healthtap’:ti,ab,kw OR ‘first opinion’:ti,ab,kw OR ‘simple contacts’:ti,ab,kw OR ‘plushcare’:ti,ab,kw OR ‘telemed’:ti,ab,kw OR ‘express care virtual’:ti,ab,kw OR ‘care virtual’:ti,ab,kw OR ‘pingmd’:ti,ab,kw OR ‘babylon health’:ti,ab,kw OR ‘dialogue’:ti,ab,kw OR ‘epic’:ti,ab,kw OR ‘adappt’:ti,ab,kw OR ‘virtual care’:ti,ab,kw OR ((‘phone based’:ti,ab,kw OR ‘smartphone based’:ti,ab,kw OR ‘telephone based’:ti,ab,kw OR ‘video based’:ti,ab,kw OR ‘web based’:ti,ab,kw OR ‘internet’:ti,ab,kw OR ‘online’:ti,ab,kw OR ‘remote’:ti,ab,kw OR ‘virtual’:ti,ab,kw) AND (consult*:ti,ab,kw OR ‘visit’:ti,ab,kw OR ‘visits’:ti,ab,kw OR appointment*:ti,ab,kw OR ‘routine care’:ti,ab,kw OR ‘care delivery’:ti,ab,kw OR ‘primary care’:ti,ab,kw)))
#3 #1 AND #2
1947
Cochrane Library Search Strategy
#1 ([mh “Alzheimer Disease”] OR [mh ^“cognitive dysfunction”] OR [mh ^“dementia”] OR [mh “dementia, multi-infarct”] OR [mh ^“dementia, vascular”] OR [mh ^“frontotemporal dementia”] OR [mh ^“frontotemporal lobar degeneration”] OR [mh ^“memory disorders”] OR [mh “pick disease of the brain”] OR “ADRD”:ti,ab,kw OR “amentia”:ti,ab,kw OR “amentias”:ti,ab,kw OR “amnestic”:ti,ab,kw OR “amnestics”:ti,ab,kw OR “amnesia”:ti,ab,kw OR “amnesias”:ti,ab,kw OR “cognitive decline”:ti,ab,kw OR “cognitive defect”:ti,ab,kw OR “cognitive defects”:ti,ab,kw OR “cognition disorder”:ti,ab,kw OR “cognition disorders”:ti,ab,kw OR “cognitive disorder”:ti,ab,kw OR “cognitive disorders”:ti,ab,kw OR “cognitive dysfunction”:ti,ab,kw OR “cognitive dysfunctions”:ti,ab,kw OR “cognitive impairment”:ti,ab,kw OR “cognitive impairments”:ti,ab,kw OR “cognitive retention”:ti,ab,kw OR “dementia”:ti,ab,kw OR “dementias”:ti,ab,kw OR “demention”:ti,ab,kw OR “dementions”:ti,ab,kw OR “loss of memory”:ti,ab,kw OR “MCI”:ti,ab,kw OR “memory clinic”:ti,ab,kw OR “memory clinics”:ti,ab,kw OR “memory deficit”:ti,ab,kw OR “memory deficits”:ti,ab,kw OR “memory disorder”:ti,ab,kw OR “memory disorders”:ti,ab,kw OR “memory disorders”:ti,ab,kw OR “memory loss”:ti,ab,kw OR “mild cognitive impairment”:ti,ab,kw OR “pick disease”:ti,ab,kw OR “pick s disease”:ti,ab,kw OR “picks disease”:ti,ab,kw OR Alzheimer* : ti,ab)
#2 ([mh ^”telemedicine”] OR [mh “telerehabilitation”] OR [mh “remote consultation”] OR [mh “videoconferencing”] OR “distance counseling”:ti,ab,kw OR “e health”:ti,ab,kw OR “ehealth”:ti,ab,kw OR “internet based”:ti,ab,kw OR “m health”:ti,ab,kw OR “mHealth”:ti,ab,kw OR “mobile health”:ti,ab,kw OR “mobile visit”:ti,ab,kw OR “mobile visits”:ti,ab,kw OR “online meeting”:ti,ab,kw OR “online meetings”:ti,ab,kw OR “online consult”:ti,ab,kw OR “online consults”:ti,ab,kw OR “online consultation”:ti,ab,kw OR “online consultations”:ti,ab,kw OR “online therapy”:ti,ab,kw OR “online therapies”:ti,ab,kw OR “online visit”:ti,ab,kw OR “online visits”:ti,ab,kw OR “online appointment”:ti,ab,kw OR “online appointments”:ti,ab,kw OR “remote appointment”:ti,ab,kw OR “remote appointments”:ti,ab,kw OR “remote assessment”:ti,ab,kw OR “remote assessments”:ti,ab,kw OR “remote consult”:ti,ab,kw OR “remote consultation”:ti,ab,kw OR “remote consultations”:ti,ab,kw OR “remote consults”:ti,ab,kw OR “remote management”:ti,ab,kw OR “tele consultation”:ti,ab,kw OR “tele consultations”:ti,ab,kw OR “tele health”:ti,ab,kw OR “tele medicine”:ti,ab,kw OR “tele neurology”:ti,ab,kw OR “teleconsultation”:ti,ab,kw OR “teleconsultations”:ti,ab,kw OR “telehealth”:ti,ab,kw OR “telemedicine”:ti,ab,kw OR “teleneurology”:ti,ab,kw OR “telerehab”:ti,ab,kw OR “telerehabilitation”:ti,ab,kw OR “tele rehabilitation”:ti,ab,kw OR “teletherapy”:ti,ab,kw OR “teletherapies”:ti,ab,kw OR “tele therapy”:ti,ab,kw OR “tele therapies”:ti,ab,kw OR “videoconferencing”:ti,ab,kw OR “videoconference”:ti,ab,kw OR “video conference”:ti,ab,kw OR “video conferencing”:ti,ab,kw OR “videoconferences”:ti,ab,kw OR “video conferences”:ti,ab,kw OR “video consult”:ti,ab,kw OR “video consults”:ti,ab,kw OR “video consultation”:ti,ab,kw OR “video consultations”:ti,ab,kw OR “virtual visit”:ti,ab,kw OR “virtual visits”:ti,ab,kw OR “virtual consult”:ti,ab,kw OR “virtual consults”:ti,ab,kw OR “virtual consultation”:ti,ab,kw OR “virtual consultations”:ti,ab,kw OR “Zoom”:ti,ab,kw OR “doximity”:ti,ab,kw OR “Mdlive”:ti,ab,kw OR “talkspace”:ti,ab,kw OR “livehealth”:ti,ab,kw OR “lemonaid”:ti,ab,kw OR “teladoc”:ti,ab,kw OR “doctor on demand”:ti,ab,kw OR “amwell”:ti,ab OR “k health”:ti,ab,kw OR “khealth”:ti,ab,kw OR “polycom”:ti,ab,kw OR “healthtap”:ti,ab,kw OR “first opinion”:ti,ab,kw OR “simple contacts”:ti,ab,kw OR “plushcare”:ti,ab,kw OR “telemed”:ti,ab,kw OR “express care virtual”:ti,ab,kw OR “care virtual”:ti,ab,kw OR “pingmd”:ti,ab,kw OR “babylon health”:ti,ab,kw OR “dialogue”:ti,ab,kw OR “epic”:ti,ab,kw OR “adappt”:ti,ab,kw OR “virtual care”:ti,ab,kw OR ((“phone based”:ti,ab,kw OR “smartphone based”:ti,ab,kw OR “telephone based”:ti,ab,kw OR “video based”:ti,ab,kw OR “web based”:ti,ab,kw OR “internet”:ti,ab,kw OR “online”:ti,ab,kw OR “remote”:ti,ab,kw OR “virtual”:ti,ab,kw) AND (consult*:ti,ab,kw OR “visit”:ti,ab,kw OR “visits”:ti,ab,kw OR appointment*:ti,ab,kw OR “routine care”:ti,ab,kw OR “care delivery”:ti,ab,kw OR “primary care”:ti,ab,kw)))
#3 #1 AND #2
527
PsycINFO Search Strategy
#1 (DE “Alzheimer’s Disease” OR DE “Cognitive Impairment” OR DE “dementia” OR DE “vascular dementia” OR DE “semantic dementia” OR DE “memory disorders” OR “picks disease” OR “ADRD” OR “amentia” OR “amentias” OR “amnestic” OR “amnestics” OR “amnesia” OR “amnesias” OR “cognitive decline” OR “cognitive defect” OR “cognitive defects” OR “cognition disorder” OR “cognition disorders” OR “cognitive disorder” OR “cognitive disorder” OR “cognitive dysfunction” OR “cognitive dysfunctions” OR “cognitive impairment” OR “cognitive impairments” OR “cognitive retention” OR “dementia” OR “dementias” OR “demention” OR “dementions” OR “loss of memory” OR “MCI” OR “memory clinic” OR “memory clinics” OR “memory deficit” OR “memory deficits” OR “memory disorder” OR “memory disorders” OR “memory disorders” OR “memory loss” OR “mild cognitive impairment” OR “pick disease” OR “pick s disease” OR “picks disease” OR Alzheimer*)
#2 (DE “telemedicine” OR DE “telerehabilitation” OR DE “online therapy” OR DE “videoconferencing” OR “distance counseling” OR “e health” OR “ehealth” OR “internet based” OR “m health” OR “mHealth” OR “mobile health” OR “mobile visit” OR “mobile visits” OR “online meeting” OR “online meetings” OR “online consult” OR “online consults” OR “online consultation” OR “online consultations” OR “online therapy” OR “online therapies” OR “online visit” OR “online visits” OR “online appointment” OR “online appointments” OR “remote appointment” OR “remote appointments” OR “remote assessment” OR “remote assessments” OR “remote consult” OR “remote consultation” OR “remote consultations” OR “remote consults” OR “remote management” OR “tele consultation” OR “tele consultations” OR “tele health” OR “tele medicine” OR “tele neurology” OR “teleconsultation” OR “teleconsultations” OR “telehealth” OR “telemedicine” OR “teleneurology” OR “telerehab” OR “telerehabilitation” OR “tele rehabilitation” OR “teletherapy” OR “teletherapies” OR “tele therapy” OR “tele therapies” OR “videoconferencing” OR “videoconference” OR “video conference” OR “video conferencing” OR “videoconferences” OR “video conferences” OR “video consult” OR “video consults” OR “video consultation” OR “video consultations” OR “virtual visit” OR “virtual visits” OR “virtual consult” OR “virtual consults” OR “virtual consultation” OR “virtual consultations” OR “Zoom” OR “doximity” OR “Mdlive” OR “talkspace” OR “livehealth” OR “lemonaid” OR “teladoc” OR “doctor on demand” OR “amwell” OR “k health” OR “khealth” OR “polycom” OR “healthtap” OR “first opinion” OR “simple contacts” OR “plushcare” OR “telemed” OR “express care virtual” OR “care virtual” OR “pingmd” OR “babylon health” OR “dialogue” OR “epic” OR “adappt” OR “virtual care” OR ((“phone based” OR “smartphone based” OR “telephone based” OR “video based” OR “web based” OR “internet” OR “online” OR “remote” OR “virtual”) AND (consult* OR “visit” OR “visits” OR appointment* OR “routine care” OR “care delivery” OR “primary care”)))
#3 #1 AND #2
739
CINAHL Plus Search Strategy
#1 (MH “Alzheimer’s Disease” OR MH “cognition disorders” OR MH “dementia” OR MH “dementia, multi-infarct” OR MH “dementia, vascular” OR MH “frontotemporal dementia” OR MH “frontotemporal lobar degeneration” OR MH “memory disorders” OR MH “pick disease of the brain” OR “ADRD” OR “amentia” OR “amentias” OR “amnestic” OR “amnestics” OR “amnesia” OR “amnesias” OR “cognitive decline” OR “cognitive defect” OR “cognitive defects” OR “cognition disorder” OR “cognition disorders” OR “cognitive disorder” OR “cognitive disorders” OR “cognitive dysfunction” OR “cognitive dysfunctions” OR “cognitive impairment” OR “cognitive impairments” OR “cognitive retention” OR “dementia” OR “dementias” OR “demention” OR “dementions” OR “loss of memory” OR “MCI” OR “memory clinic” OR “memory clinics” OR “memory deficit” OR “memory deficits” OR “memory disorder” OR “memory disorders” OR “memory disorders” OR “memory loss” OR “mild cognitive impairment” OR “pick disease” OR “pick s disease” OR “picks disease” OR Alzheimer*)
#2 (MH “telemedicine” OR MH “telerehabilitation” OR MH “telehealth” OR MH “remote consultation” OR MH “videoconferencing” OR “distance counseling” OR “e health” OR “ehealth” OR “internet based” OR “m health” OR “mHealth” OR “mobile health” OR “mobile visit” OR “mobile visits” OR “online meeting” OR “online meetings” OR “online consult” OR “online consults” OR “online consultation” OR “online consultations” OR “online therapy” OR “online therapies” OR “online visit” OR “online visits” OR “online appointment” OR “online appointments” OR “remote appointment” OR “remote appointments” OR “remote assessment” OR “remote assessments” OR “remote consult” OR “remote consultation” OR “remote consultations” OR “remote consults” OR “remote management” OR “tele consultation” OR “tele consultations” OR “tele health” OR “tele medicine” OR “tele neurology” OR “teleconsultation” OR “teleconsultations” OR “telehealth” OR “telemedicine” OR “teleneurology” OR “telerehab” OR “telerehabilitation” OR “tele rehabilitation” OR “teletherapy” OR “teletherapies” OR “tele therapy” OR “tele therapies” OR “videoconferencing” OR “videoconference” OR “video conference” OR “video conferencing” OR “videoconferences” OR “video conferences” OR “video consult” OR “video consults” OR “video consultation” OR “video consultations” OR “virtual visit” OR “virtual visits” OR “virtual consult” OR “virtual consults” OR “virtual consultation” OR “virtual consultations” OR “Zoom” OR “doximity” OR “Mdlive” OR “talkspace” OR “livehealth” OR “lemonaid” OR “teladoc” OR “doctor on demand” OR “amwell” OR “k health” OR “khealth” OR “polycom” OR “healthtap” OR “first opinion” OR “simple contacts” OR “plushcare” OR “telemed” OR “express care virtual” OR “care virtual” OR “pingmd” OR “babylon health” OR “dialogue” OR “epic” OR “adappt” OR “virtual care” OR ((“phone based” OR “smartphone based” OR “telephone based” OR “video based” OR “web based” OR “internet” OR “online” OR “remote” OR “virtual”) AND (consult* OR “visit” OR “visits” OR appointment* OR “routine care” OR “care delivery” OR “primary care”)))
#3 #1 AND #2
755
Scopus Search Strategy
#1 TITLE-ABS-KEY({Alzheimer Disease} OR {cognitive dysfunction} OR {dementia} OR {frontotemporal dementia} OR {frontotemporal lobar degeneration} OR {memory disorders} OR {pick disease of the brain} OR {ADRD} OR {amentia} OR {amentias} OR {amnestic} OR {amnestics} OR {amnesia} OR {amnesias} OR {cognitive decline} OR {cognitive defect} OR {cognitive defects} OR {cognition disorder} OR {cognition disorders} OR {cognitive disorder} OR {cognitive disorder} OR {cognitive dysfunction} OR {cognitive dysfunctions} OR {cognitive impairment} OR {cognitive impairments} OR {cognitive retention} OR {dementia} OR {dementias} OR {demention} OR {dementions} OR {loss of memory} OR {MCI} OR {memory clinic} OR {memory clinics} OR {memory deficit} OR {memory deficits} OR {memory disorder} OR {memory disorders} OR {memory disorders} OR {memory loss} OR {mild cognitive impairment} OR {pick disease} OR {pick s disease} OR {picks disease} OR Alzheimer*)
#2 TITLE-ABS-KEY({telemedicine} OR {telerehabilitation} OR {remote consultation} OR {videoconferencing} OR {distance counseling} OR {e health} OR {ehealth} OR {internet based} OR {m health} OR {mHealth} OR {mobile health} OR {mobile visit} OR {mobile visits} OR {online meeting} OR {online meetings} OR {online consult} OR {online consults} OR {online consultation} OR {online consultations} OR {online therapy} OR {online therapies} OR {online visit} OR {online visits} OR {online appointment} OR {online appointments} OR {remote appointment} OR {remote appointments} OR {remote assessment} OR {remote assessments} OR {remote consult} OR {remote consultation} OR {remote consultations} OR {remote consults} OR {remote management} OR {tele consultation} OR {tele consultations} OR {tele health} OR {tele medicine} OR {tele neurology} OR {teleconsultation} OR {teleconsultations} OR {telehealth} OR {telemedicine} OR {teleneurology} OR {telerehab} OR {telerehabilitation} OR {tele rehabilitation} OR {teletherapy} OR {tele therapy} OR {teletherapies} OR {tele therapies} OR {videoconferencing} OR {videoconference} OR {video conference} OR {video conferencing} OR {videoconferences} OR {video conferences} OR {video consult} OR {video consults} OR {video consultation} OR {video consultations} OR {virtual visit} OR {virtual visits} OR {virtual consult} OR {virtual consults} OR {virtual consultation} OR {virtual consultations} OR {Zoom} OR {doximity} OR {Mdlive} OR {talkspace} OR {livehealth} OR {lemonaid} OR {teladoc} OR {doctor on demand} OR {amwell} OR {k health} OR {khealth} OR {polycom} OR {healthtap} OR {first opinion} OR {simple contacts} OR {plushcare} OR {telemed} OR {express care virtual} OR {care virtual} OR {pingmd} OR {babylon health} OR {dialogue} OR {epic} OR {adappt} OR {virtual care})
#3 TITLE-ABS-KEY({phone based} OR {smartphone based} OR {telephone based} OR {video based} OR {web based} OR {internet} OR {online} OR {remote} OR {virtual}) AND TITLE-ABS-KEY(consult* OR {visit} OR {visits} OR appointment* OR {routine care} OR {care delivery} OR {primary care})
#4 (#1 AND (#2 OR #3))
1498
ClinicalTrials.gov Search Strategy
| Dementia AND Telemedicine | 21 |
| Alzheimer’s Disease AND Telemedicine | 12 |
| Memory Loss AND Telemedicine | 0 |
| Memory Disorders AND Telemedicine | 0 |
| Cognitive Dysfunction AND Telemedicine | 8 |
| TOTAL | 41 |
Appendix 3.
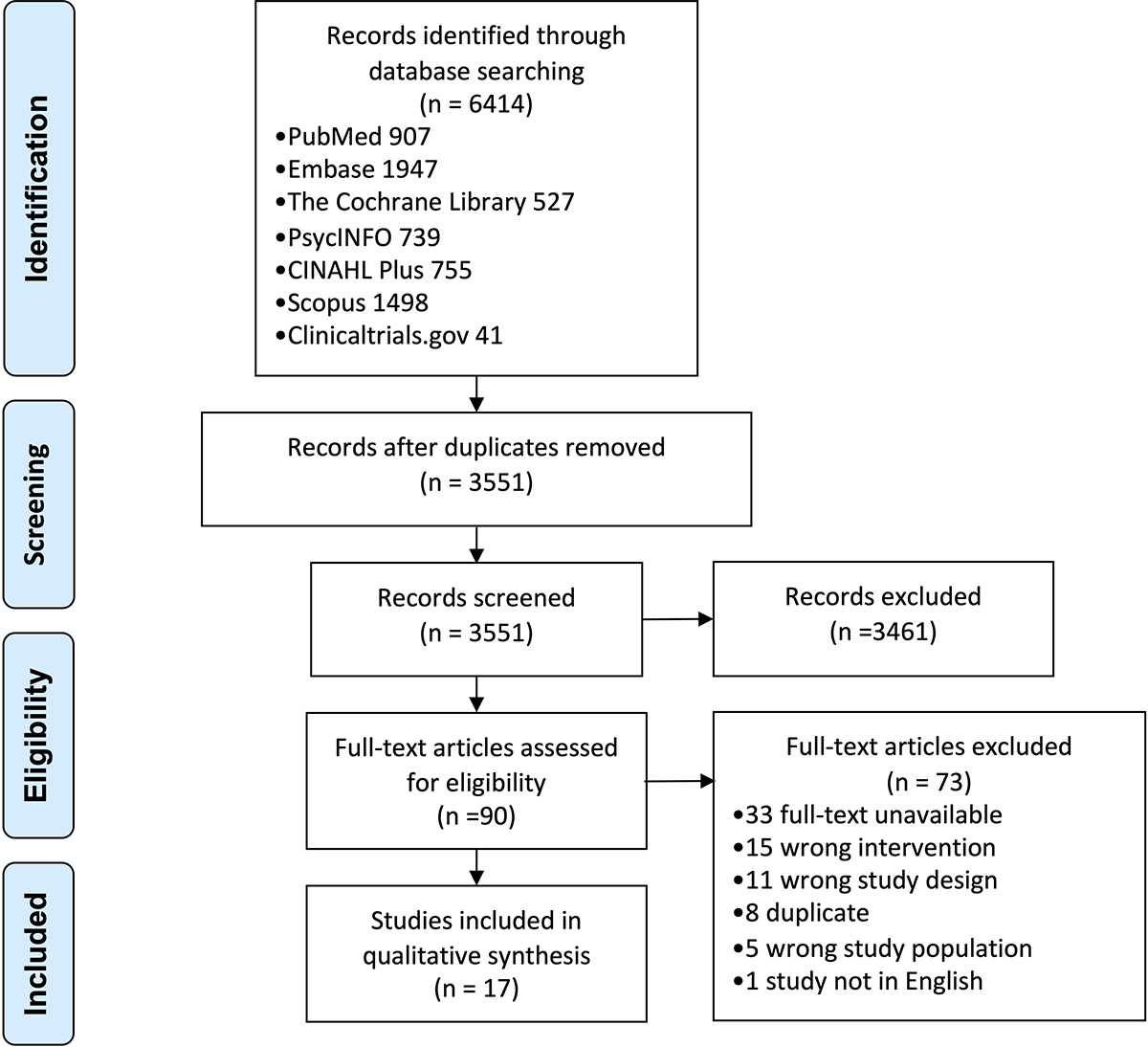
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram
Appendix 4.
Quality Assessment using Cochrane Risk of Bias Tool*
| Random sequence generation | Allocation concealment | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | |
|---|---|---|---|---|---|
| Azad et al. (2012)21 | NA | NA | NA | High | High |
| Barton et al. (2011)22 | NA | NA | NA | Low | High |
| Burton et al. (2018)29 | Low | Low | Unclear | Low | Low |
| Carotenuto et al. (2018)30 | Unclear | Unclear | High | Low | Low |
| Castanho et al. (2016)35 | NA | NA | NA | High | Low |
| Cheong et al. (2015)23 | NA | NA | NA | Low | High |
| Dang et al. (2018)24 | NA | NA | NA | High | High |
| Jelcic et al. (2014)36 | High | Unclear | Low | Low | Low |
| Laver et al. (2020)20 | Low | Low | Low | Low | Low |
| Lindauer et al. (2017)33 | Unclear | Unclear | High | Low | Low |
| Martin-Khan et al. (2012)31 | Low | Low | Low | Low | Low |
| Moo et al. (2020)34 | NA | NA | NA | High | High |
| Morgan et al. (2011)25 | Unclear | Unclear | Unclear | High | Low |
| Munro Cullum et al. (2014)26 | Low | Unclear | High | Low | Low |
| Parikh et al. (2013)32 | Unclear | Unclear | High | Low | Low |
| Powers et al. (2017)27 | NA | NA | NA | High | Low |
| Tso et al. (2016)28 | NA | NA | NA | High | High |
| High, No. (%)b | 5.9% (1/17) | 0% (0/17) | 23.5% (4/17) | 41.2% (7/17) | 35.3% (6/17) |
Blinding of participants and personnel, other sources of bias were not included in the assessment.
Percentage was calculated as the quotient of the number of “High” within a column and the total number of included citations.
Appendix 5.
Supporting judgment for quality assessment using Cochrane Risk of Bias Tool*
| Random sequence generation | Allocation concealment | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | |
|---|---|---|---|---|---|
| Azad et al. (2012)21 | Non-randomized | Non-randomized | Non-randomized, therefore no blinding | Surveys had a 51% response rate | Does not list pre-specified outcomes, descriptive study |
| Barton et al. (2011)22 | Non-randomized | Non-randomized | Non-randomized, therefore no blinding | Reports evaluation and recommendations for all 15 telemedicine visits | Does not list pre-specified outcomes, descriptive study |
| Burton et al. (2018)29 | Random assignment to in-person versus videoconferencing conditions occurred by random number generator | Random assignment to condition occurred before recruitment (conditions were determined before the study commenced and hidden in envelopes) | Blinding not mentioned | Pre-post measures and weekly measures reported for all patients. Missing data only involved care partners. | Reports all pre-specified outcomes |
| Carotenuto et al. (2018)30 | Patients were randomly recruited among outpatients followed by the Alzheimer and Neurodegenerative Diseases Unit. Does not describe random sequence generation process. | Does not describe allocation concealment | 2 blinded psychologists administered the MMSE and ADAS-Cog. Unclear how this was actually blinded since they were administered face-to-face and via videoconference. | No missing outcome data | Reports all pre-specified outcomes |
| Castanho et al. (2016)35 | Non-randomized | Non-randomized | Non-randomized, therefore no blinding | 8 participants were unable to participate in the telephone assessment due to changes in contact information, health problems, death or persistent difficulties to be reached within the time frame. The videoconference evaluation was stopped for one AD participant due to major difficulties in focusing attention and because the patient was unable to respond to the instrument questions. | Reports all pre-specified outcomes |
| Cheong et al. (2015)23 | Non-randomized | Non-randomized | Non-randomized, therefore no blinding | No missing outcome data | Does not list pre-specified outcomes, descriptive study |
| Dang et al. (2018)24 | Non-randomized | Non-randomized | Non-randomized, therefore no blinding | 27 out of 94 (29%) veterans responded. 11 out of 41 (27%) care partners responded to satisfaction outcomes. | Does not list pre-specified outcomes, descriptive study |
| Jelcic et al. (2014)36 | Patients were initially randomly assigned, but 2 patients subsequently switched treatment groups based on patient preference | Does not describe allocation concealment | All assessments conducted by neuropsychologist blinded to the treatment group to which each patient was allocated. Outcomes assessed at study entry and 3 months postintervention, so blinding of outcome assessments was feasible. | No missing outcome data | Reports all pre-specified outcomes |
| Laver et al. (2020)20 | The randomization sequence was generated by a statistician | Random sequence was transferred to sequentially numbered, opaque, sealed envelopes to conceal allocation from staff | Outcome assessments conducted by research assistant blinded to allocation. Outcomes assessed postintervention so blinding of outcome assessments was feasible. | No missing outcome data | Reports all pre-specified outcomes |
| Lindauer et al. (2017)33 | Participant dyads were randomized to receive either the inclinic battery first or the telemedicine visit first. Does not describe random sequence generation process. | Does not describe allocation concealment | Blinding not possible since neurocognitive tests administered via telemedicine | No missing outcome data | Reports all pre-specified outcomes |
| Martin-Khan et al. (2012)31 | Randomization using an electronic random number generator | Assignments were concealed in opaque, sealed envelopes | Each specialist physician was blinded to the findings of the other specialist physician with whom they were paired until a team case conference was held for the patient at the end of the clinic day. Main outcome was diagnosis agreement between in-person vs. telemedicine assessment. | No missing outcome data | Reports all pre-specified outcomes |
| Moo et al. (2020)34 | Non-randomized | Non-randomized | Non-randomized, therefore no blinding | Satisfaction survey had a 27.5% response rate | Does not list pre-specified outcomes, descriptive study |
| Morgan et al. (2011)25 | Patients are randomly assigned to in-person (standard care) or telehealth for the first follow-up, then alternating up to 1 year. Does not describe random sequence generation process. | Does not describe allocation concealment | Blinding not mentioned | Sample size at each follow-up varied due to pattern of missed appointments and requests for change. Satisfaction data presented only for a subgroup of dyads (n=28). Analysis excluded those who discontinued by 6 months, satisfaction with telehealth was lower for those who discontinued. | Reports all pre-specified outcomes |
| Munro Cullum et al. (2014)26 | Randomized to test condition using computer-generated random numbers | Does not describe allocation concealment | Blinding not possible since neurocognitive tests administered via telemedicine | No missing outcome data | Reports all pre-specified outcomes |
| Parikh et al. (2013)32 | Test forms and order of testing modality were randomly assigned and counterbalanced across subjects. Does not describe random sequence generation process. | Does not describe allocation concealment | Blinding not possible since neurocognitive tests administered via telemedicine | No missing outcome data | Reports all pre-specified outcomes |
| Powers et al. (2017)27 | Non-randomized | Non-randomized | Non-randomized, therefore no blinding | Satisfaction survey had a 36% response rate | Reports all pre-specified outcomes |
| Tso et al. (2016)28 | Non-randomized | Non-randomized | Non-randomized, therefore no blinding | Satisfaction survey had a 46% response rate | Does not list pre-specified outcomes, descriptive study |
Supporting judgment based on full-text manuscript for all included studies
Appendix 6.
Study characteristics by participant telemedicine location
| # | Study | Design | Country | Random ization | Recruitment method | Sample size | Diagnosis | MMSE mean (SD, range) | Telemedicine encounter purpose and major findings | |
|---|---|---|---|---|---|---|---|---|---|---|
| Referral | Registry | |||||||||
| Home (n=3) | ||||||||||
| 1 | Laver et al. (2020)20 | RCT | Australia | T vs. P | X | 63 dyads 31 T, 32P | Dementia or probable dementia (MMSE < 24) | T - 18.58 (5.46), P - 20.69 (4.51) | Telerehabilitation; Both the telemedicine and in-person groups reported improvement in the caregiving mastery index and care partner’s perception of change | |
| 2 | Lindauer et al. (2017)33 | Pilot | US | T vs. P | X | 28 dyads 28 T&P | AD | Assessments (MoCA, CDR, RMBPC, GDS); All measures were found to be reliable with telemedicine | ||
| 3 | Moo et al. (2020)34 | Pilot | US | NR | X | 38 T,184P | Neurodegenera tive or vascular dementia | Routine care; Both in-person and telemedicine groups reported equivalent visit satisfaction | ||
| Adult Day Care Center, Nursing Home (n=2) | ||||||||||
| 4 | Castanho et al. (2016)35 | Pilot | Portugal | NR | X | 69 T&P&Ph | AD | Male - 24.1 (28), Female - 23.9 (0.94) | Cognitive assessment (TICSM-PT); High association between testing modalities (video-conference vs. telephone, and videoconference vs. in-person) | |
| 5 | Jelcic et al. (2014)36 | Pilot | Italy | T vs. P | X | 7 T, 20 P | Early AD (CDR 0.5–1, MMSE 26–30) | T - 23.7 (2.8), P - 24.9 (2.5) | Telerehabilitation; MMSE improved significantly in groups that received treatment through telemedicine or in-person | |
| Remote Clinic (n=8) | ||||||||||
| 6 | Azad et al. (2012)21 | Pilot | Canada | NR | X | 99 T | MCI, mild dementia (MMSE 20-24) | Routine care; High satisfaction rates among patients, physicians, case managers | ||
| 7 | Barton et al. (2011 )22 | Pilot | US | NR | X | 15 T | No prior dx (MMSE>12) | 22.8 (12-27) | Assessments (MMSE, GDS, Clock Drawing, BNT); Telemedicine visit allowed physician to diagnose dementia or MCI. | |
| 8 | Cheong et al. (2015)23 | Pilot | Korea | NR | X | 168 T, 259 P | AD, vascular dementia, other dementia | Not reported | Routine care; Lower age, lower CDR, and use of telemedicine were significant factors predicting long-term treatment | |
| 9 | Dang et al. (2018)24 | Pilot | US | NR | X | 94 T | No prior dx | 24.4 (5.7) | Routine care; 15/94 (16%) received a new diagnosis of dementia (Alzheimer’s, Vascular, or Lewy Body), and 20 (21%) received a new diagnosis of mild cognitive impairment (MCI) | |
| 10 | Morgan et al. (2011 )25 | Pilot | Canada | T vs. P | X | 82 T, 67 P | No prior dx | Routine care; Dyads reported high satisfaction with telemedicine and similar satisfaction to in-person appointments. Diagnoses included AD, MCI, dementia related to multiple etiologies, other dementias | ||
| 11 | Munro Cullum et al. (2014)26 | Pilot | US | T vs. P | X | 202 T&P | MCI, AD, normal cognition | T - 27.6 (3.10), P-27.6 (3.09) | Cognitive assessment (MMSE, HVLT-R, Digit Span forward and backward, BNT, Letter and Category Fluency, Clock Drawing); Telemedicine-based neuropsychological testing is a valid and reliable alternative | |
| 12 | Powers et al. (2017)27 | Pilot | US | NR | X | 95 T | No prior dx | Assessments (MoCA, ZBI, SLUMS, GDS); Telemedicine is a feasible means of providing interprofessional dementia evaluations and follow-up to rural residents. Diagnoses included dementia (75.8%), MCI (20%). | ||
| 13 | Tso et al. (2016)28 | Pilot | US | NR | X | 33 T | No prior dx | Routine care; Overall high satisfaction with the clinic, neurologist, and telemedicine system | ||
| Hospital, Academic Center (n=4) | ||||||||||
| 14 | Burton et al. (2018)29 | Pilot | Canada | T vs. P | X | 3 T, 3 P | Self-reported CI (MMSE 17–29) | 25.8 (4.5) | Telerehabilitation; Goal performance improved across both telehealth and in-person delivery | |
| 15 | Carotenuto et al. (2018)30 | Pilot | Italy | NR | X | 28 T&P | AD (MMSE 12–24) | 19.6 (3.0) | Cognitive assessment (MMSE ADAS-cog); No significant difference in scores for in-person vs. video-conference | |
| 16 | Martin-Khan et al. (2012)31 | Pilot | Australia | T vs. P | X | 102 T, 108 P | No prior dx (MMSE 9–30) | 23.9 (4.7) | Routine care; No significant differences found in interrater agreement or diagnoses (AD, vascular dementia, CI) between the telemedicine vs. in-person group. Diagnosed with AD, vascular dementia, CI. | |
| 17 | Parikh et al. (2013)32 | Pilot | US | T vs. P | X | 40 T&P | MCI, AD, normal cognition (MMSE 22–30) | 27.5 (3.4) | Cognitive assessment (MMSE, HVLT-R, Digit Span forward and backward, Oral Trail Making Test, BNT, Letter and Category Fluency, Clock Drawing); The telemedicine satisfaction rate was 98%, and roughly two-thirds of participants indicated no preference between in-person and telemedicine testing | |
AD – Alzheimer’s disease; ADAS-cog – Alzheimer's disease assessment scale-cognitive subscale; BNT –Boston Naming Test; CDR – clinical dementia rating; CI – cognitive impairment; HVLT-R – Hopkins Verbal Learning Test-Revised; MCI – mild cognitive impairment; MoCA – Montreal Cognitive Assessment; MMSE – mini-mental state exam; NR – no randomization; P – in-person; Ph – telephone; RCT – randomized controlled trial; RMPBC – Revised Memory and Behavioral Problems Checklist; SLUMS – Saint Louis University Mental Status Examination; T – telemedicine; TICSM-PT – Telephone Interview for Cognitive Status-Modified – Portuguese version; ZBI – Zarit Burden Interview
Appendix 7.
Ineligibility reasons and outcome measures of included studies.
| # | Study | Length of Intervention* | Ineligible/ declined | Ineligible due to tech | Outcome measures | Outcomes |
|---|---|---|---|---|---|---|
| Home (n=3) | ||||||
| 1 | Laver et al. (2020)20 | 4 months | NA | NA | Feasibility (caregiving mastery index) | Both groups reported improvements in the caregiving mastery index. |
| 2 | Lindauer et al. (2017)33 | 2 evaluations | 3 declined (poor health and time limitations) | 2 dyads ineligible due to technical difficulties | Feasibility (comparing the number of participants who attempted vs. completed the measures), test-retest reliability of all measures | Of the 28 dyads who completed the visits, 4 patients (14%) were unable to complete the telemedicine MoCA. Reliability was found to be good to excellent in all measures when used with telemedicine. |
| 3 | Moo et al. (2020)34 | 2 evaluations | 184 declined | 63 declined due to tech issues (not having a home computer (n=46), not being comfortable with computers (n=12), and computers being too old or lacking broadband service (n=5) | Feasibility/acceptability (participant willingness to participate, patient and care partner satisfaction) | 184 families declined to join telemedicine, 38 participated in telemedicine. Equivalent visit satisfaction was reported between in-person and telemedicine. |
| Adult Day Care Center, Nursing Home (n=2) | ||||||
| 4 | Castanho et al. (2016)35 | 3 evaluations | NA | NA | Acceptability (patient satisfaction), reliability/validity (correlation between testing modalities) | Participants’ acceptability of videoconference was satisfactory and on par with the acceptability of the telephone assessment. Correlation analyses showed high associations between the testing modalities: TICSM-PT VC and TICSM-PT telephone (r=0.885), TICSM-PT VC and MMSE face-to-face (r=0.801). |
| 5 | Jelcic et al. (2014)36 | 3 months | NA | NA | Feasibility (participant satisfaction) | 6/7 (86%) of patients undergoing telemedicine rated 10/10 for the satisfactory question item on general utility and appeal of exercises. All participants would advise the treatment to friends. |
| Remote Clinic (n=8) | ||||||
| 6 | Azad et al. (2012)21 | 1 evaluation | NA | NA | Feasibility (participant, provider, staff satisfaction) | Over 90% of physicians and patients indicated willingness to use telemedicine again. Physicians reported telemedicine provided enough information to assist in clinical decision-making (96%), and patients and case managers/geriatric assessors felt able to present the same information by video conferencing as in-person (92%). |
| 7 | Barton et al. (2011)22 | 1 evaluation | NA | NA | Feasibility (arriving at diagnosis, patient and provider satisfaction) | 12 patients were diagnosed with dementia, 2 with MCI, and 1 cognitively normal. Informal feedback from patients and providers indicated satisfaction with the evaluation and appreciation that the service could be provided locally. |
| 8 | Cheong et al. (2015)23 | 1 evaluation | 15 ineligible (did not return after initial evaluation) | NA | Evaluate the effectiveness of telemedicine for long-term follow-up of dementia patients in rural South Korea and identify the factors predicting long-term treatment | Lower age, lower CDR, and use of telemedicine were significant factors predicting long-term treatment. Mean treatment duration was significantly longer for the telemedicine group than for the clinical visit group (P < 0.001), with durations of 26.6 and 14.6 months, respectively. |
| 9 | Dang et al. (2018)24 | 1 evaluation | NA | NA | Feasibility/acceptability (arriving at a diagnosis, patient and care partnersatisfaction) | 15 patients were diagnosed with dementia and 20 were diagnosed with MCI. Patients and care partners expressed high satisfaction with the telemedicine and 90% of care partners indicated they would rather use telemedicine than travel to see the specialist in person. |
| 10 | Morgan et al. (2011)25 | alternating evaluations between in-person vs. telemedicine | 19 ineligible (admitted to nursing home (n=1), moved (n=2), missing data (n=1), discontinued (n=15) | NA | Feasibility/acceptability (participant and care partner satisfaction, distance saved) | On average, the distance saved by telemedicine was 213.4 km. Questionnaires showed similar satisfaction with telemedicine and in-person appointments, but telemedicine was rated as significantly more convenient. |
| 11 | Munro Cullum et al. (2014)26 | 2 evaluations | NA | NA | Reliability/validity (comparing scores in-person vs. videoconference) | Highly similar results across telemedicine and in-person conditions, with significant intraclass correlations between test scores. Findings remained consistent in subjects with or without cognitive impairment and in persons with MMSE scores as low as 15. |
| 12 | Powers et al. (2017)27 | 1 evaluation | 12 declined | NA | Feasibility (driving time and distance saved, participant and care partner satisfaction) | The estimated mean (SD) round trip distance saved in miles was 67.1 (39.7), and the estimated mean round trip on the road saved in minutes was 74.5 (43.2). Overall satisfaction score was 4.73/5. |
| 13 | Tso et al. (2016)28 | 1 evaluation | NA | NA | No explicit outcome measures stated | Overall satisfaction with the clinic was 4.84/5. |
| Hospital, Academic Center (n=4) | ||||||
| 14 | Burton et al. (2018)29 | 2 months | NA | NA | Feasibility/acceptability (comparison of cognitive rehabilitation delivered in-person vs. telemedicine) | 6/6 goals measured with the Canadian Occupational Performance Measure improved for those in the in-person group, and 7/9 goals improved for those in the telemedicine group. |
| 15 | Carotenuto et al. (2018)30 | 2 evaluations | NA | NA | Feasibility/reliability (comparing scores in-person vs. videoconference), acceptability (questionnaire on acceptance of videoconference for cognitive testing) | No differences in scores (MMSE, ADAS-cog) between videoconference and in-person for slight and moderate baseline MMSE impairment level. Patients in the severe baseline MMSE impairment level obtained lower scores on videoconference MMSE and higher scores on videoconference ADAS- cog. On the acceptance questionnaire, patients and care partners indicated they did not have concerns about privacy (4.8/5), and would like to repeat the experience (4.5/5). |
| 16 | Martin-Khan et al. (2012)31 | 1 evaluation | 65 declined | 1 ineligible due to technical problems | Reliability/validity (inter-rater reliability on the diagnosis of dementia) | No difference in levels of agreement for assessments conducted via telemedicine vs. in-person. |
| 17 | Parikh et al. (2013)32 | 2 evaluations | NA | NA | Acceptability (participant acceptability survey) | 98% participant satisfaction, with roughly two-thirds of indicating no preference between traditional face-to-face testing and examination by videoconference. |
For studies where length of intervention was not reported, number of interventions are reported.
ADAS-Cog - Alzheimer’s Disease Assessment Scale-Cognitive Subscale; CDR – clinical dementia rating; MCI – mild cognitive impairment; MMSE – Mini Mental Status Exam; MoCA – Montreal Cognitive Assessment; SD – standard deviation; TICSM-PT - Telephone Interview for Cognitive Status-Modified – Portuguese version videoconference
Appendix 8.
Satisfaction rates and preference for telemedicine vs. in-person among included studies.
| # | Study | Response rate | Participant | Care Partner | Preference for telemedicine vs. in-person |
|---|---|---|---|---|---|
| Home (n=3) | |||||
| 1 | Laver et al. (2020)20 | Not reported | Participants reported moderate-to-high levels of satisfaction with the program although participants allocated to receive home visits appear to provide somewhat more favorable responses. | Not reported | Not reported |
| 2 | Lindauer et al. (2017)33 | Not reported | Participants indicated that they were satisfied with the effectiveness of the telemedicine platform for receiving care. | Not reported | Although there was uncertainty that telemedicine visits could completely replace the traditional face-face visit, many enjoyed the convenience of the telemedicine visit, as noted by one participant: “I would prefer to have it (the visit) as a telemedicine and not waste my time, energy, and resources. |
| 3 | Moo et al. (2020)34 | 61/222 (27.5%) participants | No significant difference in scores between in-person vs. telemedicine on the following items: visit disrupted routine, staff showed respect, staff listened/answered questions understandably. | Equivalent visit satisfaction was reported between in-person and video telemedicine. | |
| Adult Day Care Center, Nursing Home (n=2) | |||||
| 4 | Castanho et al. (2016)35 | Varying response rates for each question (2.8 to 88%) | The guided-informal discussion indicated that the participants' acceptability of videoconference administration was satisfactory and on par with the acceptability of the telephone assessment. | Not reported | Most participants did not indicate any preference between the videoconference and the telephone approach, if a computer was to be available, but most indicated they “enjoyed seeing the evaluator face-to-face.” |
| 5 | Jelcic et al. (2014)36 | Not reported | 6/7 (86%) of patients undergoing telemedicine rated 10/10 for the satisfactory question item on general utility and appeal of exercises. All participants would advise the treatment to friends. | Not reported | Two patients, initially enrolled in the telerehabilitation group, chose not be involved with computer technology and were shifted to in-person rehabilitation. |
| Remote Clinic (n=8) | |||||
| 6 | Azad et al. (2012)21 | 50/99 (51%) participants | 92% of the respondents were satisfied with the session, and 94% indicated that they would be willing to use video conferencing again. | Not reported | 92% of participants were able to present the same information they would have provided in-person through telemedicine, 88% were as confident about the doctor’s assessment through telemedicine as in-person assessment (88%), 30% were more anxious with telemedicine than in-person, 28% preferred to see their doctor in-person. |
| 7 | Barton et al. (2011)22 | Not reported | Informal feedback from patients indicated satisfaction with the evaluation and appreciation that the service could be provided locally. | Not reported | Not reported |
| 8 | Cheong et al. (2015)23 | Not reported | Not reported. | Not reported | |
| 9 | Dang et al. (2018)24 | 27/94 (29%) participants and 11/41 (27%) care partners | 88% indicated overall satisfaction, 77% would choose to have telemedicine visit again, 65% would recommend telemedicine to others, 65% would rather use telemedicine over in-person. | 91% indicated overall satisfaction, 73% would choose to have telemedicine visit again, 82% would recommend telemedicine to others, 91% would rather use telemedicine over in-person. | 65% of patients and 91% of care partners preferred to see the specialist through telemedicine rather than in-person. |
| 10 | Morgan et al. (2011 )25 | Varying response rates, 124/149 (83%) to 147/149 (99%) | All respondents would use telemedicine again, 99% would recommend. On a 4-point Likert scale (higher score, more satisfaction) means ranged from 3.05 (wait time for appointment) to 3.65 (how well telehealth staff answered questions about equipment). Most items were rated as “good” or “excellent.” Mean summary score (ranging from 12–48) was 42.1 (4.53). | Two patients preferred in-person visits. | |
| 11 | Munro Cullum et al. (2014)26 | Not reported | Not reported | Not reported | |
| 12 | Powers et al. (2017)27 | 21/58 (36%) patients and care partners | On a 5-point Likert scale (1=strongly disagree, 5=strongly agree), “It was easy to communicate with providers” 4.85, “There was enough technical assistance” 4.81, “Overall, I was satisfied with the experience” 4.73, and “I would use the teledementia clinic again” 4.58. | All patients and care partners who responded stated that they preferred not driving for their care. | |
| 13 | Tso et al. (2016)28 | 15/33 (46%) patients and/or their families | Overall satisfaction with the clinic was 4.84/5. General satisfaction with the neurologist was 4.88/5. Satisfaction with the telemedicine system was 4.65/5. 96% indicated a willingness to recommend telemedicine. | Although most patients still prefer seeing the doctor in-person, they overwhelmingly preferred the convenience of telemedicine over long-distance travel or lengthy wait-time. | |
| Hospital, Academic Center (n=4) | |||||
| 14 | Burton et al. (2018)29 | Not reported | Not reported | Participants commented that although they might have preferred to meet in-person, the telehealth sessions ran smoothly. | |
| 15 | Carotenuto et al. (2018)30 | Not reported | On a 5-point Likert scale (1=strongly disagree, 5=strongly agree), “Instructions are clear and understandable” 4.4 (1.3), “Data privacy is assured” 4.8 (0.6), “I saved my time” 4.0 (0.8), “I would like to repeat the experience” 4.5 (0.8), “I prefer the Web modality than coming to the hospital” 3.3 (1.5). | Preference for telemedicine vs. coming to the hospital was very high for both patients and care partners. | |
| 16 | Martin-Khan et al. (2012)31 | Not reported | Not reported | Not reported | |
| 17 | Parikh et al. (2013)32 | All participants responded | 98% expressed overall acceptability of videoconference-based assessment (2% were neutral). 100% reported testing instructions were easy to understand. 60% indicated no preference for testing format, 30% preferred in-person assessment, and 10% preferred videoconference. Approximately 50% indicated that they would prefer to drive no more than three hours to undergo face-to-face assessment. | Approximately two-thirds of individuals with or without cognitive impairment expressed no preference between telemedicine vs. in-person testing. | |
Appendix 9.
Requirements and technological issues by participant location
| # | Study | Support Staff required | Care Partner required | Equipment requirements | Technological issues identified | ||||
|---|---|---|---|---|---|---|---|---|---|
| Set-up/Orientation | Real-time Support | Exam Assistance | Connection | Audio | Visual | ||||
| Home (n=3) | |||||||||
| 1 | Laver et al. (2020)20 | X | X | X |
Participant: laptop, tablet, smartphone Provided: tablet with software |
||||
| 2 | Lindauer et al. (2017)33 | X | X |
Participant: computer Provided: cameras, headphones, iPad |
|||||
| 3 | Moo et al. (2020)34 | X | X | X |
Participant: home computer with internet access Provided: videoconferencing software, webcam |
||||
| Adult Day Care Center, Nursing Home (n=2) | |||||||||
| 4 | Castanho et al. (2016)35 | X | X | Skype, telephone | X | X | |||
| 5 | Jelcic et al. (2014)36 | X | X | X | Skype | ||||
| Remote Clinic (n=8) | |||||||||
| 6 | Azad et al. (2012)21 | X | X | X | X | ||||
| 7 | Barton et al. (2011)22 | X | X | Tandberg teleconferencing system | |||||
| 8 | Cheong et al. (2015)23 | X | X | Tandberg teleconferencing system | |||||
| 9 | Dang et al. (2018)24 | X | X | Cisco Jabber Video TelePresence connected to TV | X | X | X | ||
| 10 | Morgan et al. (2011)25 | X | X | General camera for the interview, specialized high-quality camera for viewing patient’s writing | X | X | X | ||
| 11 | Munro Cullum et al. (2014)26 | X | Polycom, 26-inch monitor | ||||||
| 12 | Powers et al. (2017)27 | X | X | X | 24-inch monitor, camera, touch screen interface for the controls (TelePresence) and mobile video cart with dual 21.5-inch touchscreen monitors | X | |||
| 13 | Tso et al. (2016)28 | X | Camera with remote-controlled zooming and turning with view of the entire exam room. Two computer monitors provide simultaneous streaming of the neurologist and the visual tools for cognitive examination. | ||||||
| Hospital, Academic Center (n=4) | |||||||||
| 14 | Burton et al. (2018)29 | X | X | X | |||||
| 15 | Carotenuto et al. (2018)30 | X | X | Skype, remote control of the audiovisual system | X | ||||
| 16 | Martin-Khan et al. (2012)31 | X | Television screen, microphone | X | |||||
| 17 | Parikh et al. (2013)32 | Video camera, microphone, and 26-inch color monitor | |||||||
Appendix 10.
Patient level characteristics by participant location
| # | Study | Sensory health considerations | Comments regarding sensory impairment | |
|---|---|---|---|---|
| Excluded hearing impaired | Excluded vision impaired | |||
| Home (n=3) | ||||
| 1 | Laver et al. (2020)20 | |||
| 2 | Lindauer et al. (2017)33 | X | X | |
| 3 | Moo et al. (2020)34 | |||
| Adult Day Care Center, Nursing Home (n=2) | ||||
| 4 | Castanho et al. (2016)35 | 11.5% of participants indicated that videoconference had poor sound and visual quality. | ||
| 5 | Jelcic et al. (2014)36 | Hearing and vision impairment may contribute to distraction in older adults with minimal experience using technologies. | ||
| Remote Clinic (n=8) | ||||
| 6 | Azad et al. (2012)21 | Hearing loss was identified in 7 patients. | ||
| 7 | Barton et al. (2011)22 | X | X | Telemedicine technology is limited in patients with significant sensory and auditory problems. |
| 8 | Cheong et al. (2015)23 | |||
| 9 | Dang et al. (2018)24 | Hearing loss identified in 3 participants. A quarter of participants were not satisfied with sound quality. 93% reported no difficulty seeing the specialist through telemedicine. | ||
| 10 | Morgan et al. (2011 )25 | Coordinators observed that patients with hearing loss had difficulty following the conversation. Difficulty hearing was identified as a reason for dissatisfaction with telemedicine. | ||
| 11 | Munro Cullum et al. (2014)26 | X | X | There were no significant problems with audio or visual transmission. |
| 12 | Powers et al. (2017)27 | 2 patients had severe uncorrected hearing loss that it was difficult to proceed with the appointment. | ||
| 13 | Tso et al. (2016)28 | Many patients were initially reluctant to participate given their hearing loss, but this was a non-issue with high-fidelity videoconferencing equipment. | ||
| Hospital, Academic Center (n=4) | ||||
| 14 | Burton et al. (2018)29 | |||
| 15 | Carotenuto et al. (2018)30 | X | X | |
| 16 | Martin-Khan et al. (2012)31 | |||
| 17 | Pankh et al. (2013)32 | X | X | No respondents indicated any difficulty or dissatisfaction with seeing or hearing test stimuli. Given the potential challenges associated with examining subjects with significant sensory limitations, acceptability as well as validity of data among such individuals may be impacted. |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gately ME, Trudeau SA, Moo LR. In-Home Video Telehealth for Dementia Management: Implications for Rehabilitation. Curr Geriatr Reports. 2019;8(3):239–249. doi: 10.1007/s13670-019-00297-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calton B, Abedini N, Fratkin M. Telemedicine in the Time of Coronavirus. J Pain Symptom Manage. March 2020. doi: 10.1016/j.jpainsymman.2020.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams JL, Myers TL, Waddell EM, Spear KL, Schneider RB. Telemedicine: a Valuable Tool in Neurodegenerative Diseases. Curr Geriatr Reports. 2020;9(2):72–81. doi: 10.1007/s13670-020-00311-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Report 9 - Impact of non-pharmaceutical interventions (NPIs) to reduce COVID-19 mortality and healthcare demand | Faculty of Medicine | Imperial College; London. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stronge AJ, Rogers WA, Fisk AD. Human factors considerations in implementing telemedicine systems to accommodate older adults. J Telemed Telecare. 2007;13(1):1–3. doi: 10.1258/135763307779701158 [DOI] [PubMed] [Google Scholar]
- 6.Van Den Berg N, Schumann M, Kraft K, Hoffmann W. Telemedicine and telecare for older patients - A systematic review. Maturitas. 2012;73(2):94–114. doi: 10.1016/j.maturitas.2012.06.010 [DOI] [PubMed] [Google Scholar]
- 7.Lin FR, Thorpe R, Gordon-Salant S, Ferrucci L. Hearing loss prevalence and risk factors among older adults in the United States. journals Gerontol A, Biol Sci Med Sci. 2011;66(5):582–590. doi: 10.1093/gerona/glr002 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nirmalasari O, Mamo SK, Nieman CL, et al. Age-related hearing loss in older adults with cognitive impairment. Int Psychogeriatrics. 2017;29(1):115–121. doi: 10.1017/S1041610216001459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gold M, Lightfoot LA, Hnath-Chisolm T. Hearing loss in a memory disorders clinic. A specially vulnerable population. Arch Neurol. 1996;53(9):922–928. doi: 10.1001/archneur.1996.00550090134019 [DOI] [PubMed] [Google Scholar]
- 10.Jorgensen LE, Palmer C V., Pratt S, Erickson KI, Moncrieff D. The effect of decreased audibility on MMSE performance: A measure commonly used for diagnosing dementia. J Am AcadAudiol. 2016;27(4):311–323. doi: 10.3766/jaaa.15006 [DOI] [PubMed] [Google Scholar]
- 11.Dupuis K, Pichora-Fuller MK, Chasteen AL, Marchuk V, Singh G, Smith SL. Effects of hearing and vision impairments on the Montreal Cognitive Assessment. Aging, Neuropsychol Cogn 2015;22(4):413–437. doi: 10.1080/13825585.2014.968084 [DOI] [PubMed] [Google Scholar]
- 12.Mitzner TL, Savla J, Boot WR, et al. Technology Adoption by Older Adults: Findings From the PRISM Trial. Gerontologist. September 2018. doi: 10.1093/geront/gny113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowen M, Edgar D, Hancock B, et al. The Prevalence of Visual Impairment in People with Dementia (the PrOVIDe Study): A Cross-Sectional Study of People Aged 60–89 Years with Dementia and Qualitative Exploration of Individual, Carer and Professional Perspectives.; 2016. http://www.ncbi.nlm.nih.gov/pubmed/27489923. Accessed January 25, 2021. [PubMed]
- 14.Shang X, Zhu Z, Wang W, Ha J, He M. The association between vision impairment and incidence of dementia and cognitive impairment: a systematic review and meta-analysis. Ophthalmology. January 2021. doi: 10.1016/j.ophtha.2020.12.029 [DOI] [PubMed] [Google Scholar]
- 15.Swenor BK, Ramulu PY, Willis JR, Friedman D, Lin FR. Research letters: The prevalence of concurrent hearing and vision impairment in the United States. JAMA Intern Med. 2013;173(4):312–313. doi: 10.1001/jamainternmed.2013.1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–446. doi: 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoSMed. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JPT, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343(7829). doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laver K, Liu E, Clemson L, et al. Does Telehealth Delivery of a Dyadic Dementia Care Program Provide a Noninferior Alternative to Face-To-Face Delivery of the Same Program? A Randomized, Controlled Trial. Am J Geriatr Psychiatry. March 2020. doi: 10.1016/j.jagp.2020.02.009 [DOI] [PubMed] [Google Scholar]
- 21.Azad N, Amos S, Milne K, Power B. Telemedicine in a rural memory disorder clinic-remote management of patients with dementia. Can Geriatr J. 2012;15(4):96–100. doi: 10.5770/cgj.15.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barton C, Morris R, Rothlind J, Yaffe K. Video-telemedicine in a memory disorders clinic: Evaluation and management of rural elders with cognitive impairment. Telemed e-Health. 2011;17(10):789–793. doi: 10.1089/tmj.2011.0083 [DOI] [PubMed] [Google Scholar]
- 23.Cheong CK, Lim KH, Jang JW, Jhoo JH. The effect of telemedicine on the duration of treatment in dementia patients. J Telemed Telecare. 2015;21(4):214–218. doi: 10.1177/1357633X14566571 [DOI] [PubMed] [Google Scholar]
- 24.Dang S, Gomez-Orozco CA, van Zuilen MH, Levis S. Providing Dementia Consultations to Veterans Using Clinical Video Telehealth: Results from a Clinical Demonstration Project. Telemed e-Health. 2018;24(3):203–209. doi: 10.1089/tmj.2017.0089 [DOI] [PubMed] [Google Scholar]
- 25.Morgan DG, Crossley M, Kirk A, et al. Evaluation of telehealth for preclinic assessment and follow-up in an interprofessional rural and remote memory clinic. J Appl Gerontol. 2011;30(3):304–331. doi: 10.1177/0733464810366564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munro Cullum C, Hynan LS, Grosch M, Parikh M, Weiner MF. Teleneuropsychology: Evidence for video teleconference-based neuropsychological assessment. J Int NeuropsycholSoc. 2014;20(10):1028–1033. doi: 10.1017/S1355617714000873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Powers BB, Homer MC, Morone N, Edmonds N, Rossi MI. Creation of an Interprofessional Teledementia Clinic for Rural Veterans: Preliminary Data. J Am Geriatr Soc. 2017;65(5):1092–1099. doi: 10.1111/jgs.14839 [DOI] [PubMed] [Google Scholar]
- 28.Tso JV, Farinpour R, Chui HC, Liu CY. A multidisciplinary model of dementia care in an underserved retirement community, made possible by telemedicine. Front Neurol. 2016;7(DEC). doi: 10.3389/fneur.2016.00225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burton RL, O’Connell ME. Telehealth rehabilitation for cognitive impairment: Randomized controlled feasibility trial. J Med Internet Res. 2018;20(2). doi: 10.2196/resprot.9420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carotenuto A, Rea R, Traini E, Ricci G, Fasanaro AM, Amenta F. Cognitive assessment of patients with Alzheimer’s disease by telemedicine: Pilot study. J Med Internet Res. 2018;20(5). doi: 10.2196/mental.8097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin-Khan M, Flicker L, Wootton R, et al. The Diagnostic Accuracy of Telegeriatrics for the Diagnosis of Dementia via Video Conferencing. 2012. doi: 10.1016/j.jamda.2012.03.004 [DOI] [PubMed]
- 32.Parikh M, Grosch MC, Graham LL, et al. Consumer Acceptability of Brief Videoconference-based Neuropsychological Assessment in Older Individuals with and without Cognitive Impairment. Clin Neuropsychol. 2013;27(5):808–817. doi: 10.1080/13854046.2013.791723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lindauer A, Seelye A, Lyons B, et al. Dementia Care Comes Home: Patient and Caregiver Assessment via Telemedicine. Gerontologist. 2017;57(5):85–93. doi: 10.1093/geront/gnw206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moo LR, Gately ME, Jafri Z, Shirk SD. Home-Based Video Telemedicine for Dementia Management. Clin Gerontol. 2020;43(2):193–203. doi: 10.1080/07317115.2019.1655510 [DOI] [PubMed] [Google Scholar]
- 35.Castanho TC, Amorim L, Moreira PS, et al. Assessing Cognitive Function in Older Adults Using a Videoconference Approach. EBioMedicine. 2016;11:278–284. doi: 10.1016/j.ebiom.2016.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jelcic N, Agostini M, Meneghello F, et al. Feasibility and efficacy of cognitive telerehabilitation in early Alzheimer’s disease: A pilot study. Clin Interv Aging. 2014;9:1605–1611. doi: 10.2147/CIA.S68145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wosik J, Fudim M, Cameron B, et al. Telehealth Transformation: COVID-19 and the rise of Virtual Care. J Am Med Inform Assoc. April 2020. doi: 10.1093/jamia/ocaa067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kales HC, Gitlin LN, Lyketsos CG. Assessment and management of behavioral and psychological symptoms of dementia. BMJ. 2015;350(March):1–16. doi: 10.1136/bmj.h369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith M, Hall GR, Gerdner L, Buckwalter KC. Application of the progressively lowered stress threshold model across the continuum of care. Nurs Clin North Am. 2006;41(1):57–81. doi: 10.1016/j.cnur.2005.09.006 [DOI] [PubMed] [Google Scholar]
- 40.McKee M, Moran C, Zazove P. Overcoming Additional Barriers to Care for Deaf and Hard of Hearing Patients During COVID-19. JAMA Otolaryngol Neck Surg. July 2020. doi: 10.1001/jamaoto.2020.1705 [DOI] [PubMed] [Google Scholar]
- 41.Meyer CJ, Koh SS, Hill AJ, et al. Hear-Communicate-Remember: Feasibility of delivering an integrated intervention for family caregivers of people with dementia and hearing impairment via telehealth. Dementia. May 2019:147130121985070. doi: 10.1177/1471301219850703 [DOI] [PubMed] [Google Scholar]
- 42.Reed NS, Betz J, Kendig N, Korczak M, Lin FR. Personal sound amplification products vs a conventional hearing aid for speech understanding in noise. JAMA - J Am Med Assoc. 2017;318(1):89–90. doi: 10.1001/jama.2017.6905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mamo SK, Reed NS, Nieman CL, Oh ES, Lin FR. Personal Sound Amplifiers for Adults with Hearing Loss. Am J Med. 2016;129(3):245–250. doi: 10.1016/j.amjmed.2015.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams K, Pennathur P, Bossen A, Gloeckner A. Adapting telemonitoring technology use for older adults: A pilot study. Res Gerontol Nurs. 2016;9(1):17–23. doi: 10.3928/19404921-20150522-01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cohen G, Russo MJ, Campos JA, Allegri RF. Living with dementia: increased level of caregiver stress in times of COVID-19. Int Psychogeriatrics. July 2020:1–5. doi: 10.1017/s1041610220001593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boutoleau-Bretonniere C, Pouclet-Courtemanche H, Gillet A, et al. The Effects of Confinement on Neuropsychiatric Symptoms in Alzheimer’s Disease During the COVID-19 Crisis. J Alzheimers Dis. 2020;76(1):41–47. doi: 10.3233/JAD-200604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.yin Lai FH, hung Yan EW, ying Yu KK, Tsui WS, hoi Chan DT, Yee BK. The Protective Impact of Telemedicine on Persons With Dementia and Their Caregivers During the COVID-19 Pandemic. Am J Geriatr Psychiatry. 2020;28(11):1175–1184. doi: 10.1016/j.jagp.2020.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goodman-Casanova JM, Dura-Perez E, Guzman-Parra J, Cuesta-Vargas A, Mayoral-Cleries F. Telehealth home support during COVID-19 confinement for community-dwelling older adults with mild cognitive impairment or mild dementia: Survey study. J Med Internet Res. 2020;22(5):e19434. doi: 10.2196/19434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Capozzo R, Zoccolella S, Frisullo ME, et al. Telemedicine for Delivery of Care in Frontotemporal Lobar Degeneration during COVID-19 Pandemic: Results from Southern Italy. J Alzheimer’s Dis. 2020;76(2):481–489. doi: 10.3233/JAD-200589 [DOI] [PubMed] [Google Scholar]
- 50.Stewart S, O’Riley A, Edelstein B, Gould C. A Preliminary Comparison of Three Cognitive Screening Instruments in Long Term Care: The MMSE, SLUMS, and MoCA. Clin Gerontol. 2012;35(1):57–75. doi: 10.1080/07317115.2011.626515 [DOI] [Google Scholar]
- 51.Yang E, Chang SJ, Ryu H, Kim HJ, Jang SJ. Comparing Factors Associated With eHealth Literacy Between Young and Older Adults. J Gerontol Nurs. 2020;46(8):46–56. doi: 10.3928/00989134-20200707-02 [DOI] [PubMed] [Google Scholar]
- 52.Cimperman M, Brencic MM, Trkman P, Stanonik MDL. Older Adults’ perceptions of home telehealth services. Telemede-Health. 2013;19(10):786–790. doi: 10.1089/tmj.2012.0272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ellis RD, Allaire JC. Modeling computer interest in older adults: The role of age, education, computer knowledge, and computer anxiety. Hum Factors. 1999;41(3):345–355. doi: 10.1518/001872099779610996 [DOI] [PubMed] [Google Scholar]
- 54.Anderson M, Perrin A. Tech Adoption Climbs among Older Adults. 2017.
- 55.Babulal GM, Quiroz YT, Albensi BC, et al. Perspectives on ethnic and racial disparities in Alzheimer’s disease and related dementias: Update and areas of immediate need. Alzheimer’s Dement. 2019;15(2):292–312. doi: 10.1016/j.jalz.2018.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ilunga Tshiswaka D, Loggins Clay S, Chiu C-Y, Alston R, Lewis A. Assistive technology use by disability type and race: exploration of a population-based health survey. Disabil Rehabil Assist Technol. 2016;11(2):124–132. doi: 10.3109/17483107.2015.1090487 [DOI] [PubMed] [Google Scholar]
- 57.The Community Research for Assistive Technology Survey; 2005.
- 58.Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673–2734. doi: 10.1016/S0140-6736(17p1363-6 [DOI] [PubMed] [Google Scholar]


