Abstract
Objective:
Determine risk of death or neurodevelopmental impairment (NDI) in infants with late-onset sepsis (LOS) versus late-onset, antibiotic-treated, blood culture-negative conditions (LOCNC).
Design:
Retrospective cohort study
Setting:
24 neonatal centers.
Patients:
Infants born 1/1/2006–12/31/2014, at 22–26 weeks gestation, with birth weight 401–1000 grams and surviving >7 days were included. Infants with early-onset sepsis, necrotizing enterocolitis, intestinal perforation, or both LOS and LOCNC were excluded.
Exposures:
LOS and LOCNC were defined as antibiotic administration for ≥5 days with and without a positive blood/CSF culture, respectively. Infants with these diagnoses were also compared to infants with neither condition.
Outcomes:
Death or NDI assessed at 18–26 months corrected age follow-up. Modified Poisson regression models were used to estimate relative risks adjusting for covariates occurring ≤7 days of age.
Results:
Of 7354 eligible infants, 3940 met inclusion criteria: 786 (20%) with LOS, 1601 (41%) with LOCNC, and 1553 (39%) with neither. Infants with LOS had higher adjusted relative risk [95% CI] for death/NDI (1.14 [1.05–1.25]) and death before follow-up (1.71 [1.44–2.03]) than those with LOCNC. Among survivors, risk for NDI did not differ between the two groups (0.99 [0.86–1.13]) but was higher for LOCNC infants (1.17 [1.04–1.31]) compared to unaffected infants.
Conclusions:
Infants with LOS had higher risk of death, but not NDI, compared to infants with LOCNC. Surviving infants with LOCNC had higher risk of NDI compared to unaffected infants. Improving outcomes for infants with LOCNC requires study of the underlying conditions and the potential impact of antibiotic exposure.
Keywords: Neonatology, Epidemiology, Intensive Care Medicine
INTRODUCTION
Preterm infants are diagnosed with late-onset sepsis (LOS), defined as positive blood or cerebrospinal fluid (CSF) culture obtained >72 hours of age, at rates varying from 10–30%.1, 2 Infants with culture-confirmed infections are at higher risk for abnormal neuroimaging findings and neurodevelopmental impairment (NDI) compared to uninfected infants.3–10 In analyses of infants born 1993–2001 with birth weight 401–1000 grams and admitted to hospitals in the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network (NRN), infants administered antibiotics ≥5 days in the absence of a positive blood culture also had a higher risk of NDI compared to uninfected infants.6
Although many studies refer to the concept of “culture-negative sepsis” there is uncertainty regarding this entity, including whether in some cases, infection is truly present.11 LOS and late-onset, blood-culture negative conditions (LOCNCs) share clinical presentations and by definition, both are managed with antibiotics. While LOS is a culture-based diagnosis,1, 8, 9, 12 LOCNC is variably defined as a combination of clinical signs suggesting sepsis with or without inflammatory markers, sterile blood cultures and the clinical team’s decision to administer variable durations of antibiotics.6, 7, 9, 10, 12, 13 LOCNC is frequently considered as the equivalent of ‘missed’ LOS. Studies often describe outcomes for preterm infants with LOS or LOCNC separately and compared with uninfected infants as reference; few studies directly compare outcomes of infants with LOS and LOCNC.14 Such a comparison would reveal differences between the two conditions, measuring not whether these conditions have an effect on outcomes but whether the effects differ in magnitude or character between the two diagnoses.
Our objective was to compare risk of death or NDI among infants with LOS compared to infants with LOCNC. Infants with LOS or LOCNC were also compared to unaffected infants.
METHODS
Setting:
This is a retrospective cohort study of infants with gestational age (GA) 22 0/7–26 6/7 weeks, birth weight (BW) 401–1000 grams, no major birth defect, born at NRN centers 1/1/2006–12/31/2014 and enrolled in the NRN registry of extremely preterm infants. The registry included clinical information prospectively collected during the birth hospitalization of infants. Details of the registry are noted in the Supplementary Appendix. Surviving infants were eligible for a follow-up developmental assessment. The institutional review board at each center approved participation in the registry and the follow-up study, with waiver of consent or written parental consent as required by individual sites.
Study definitions:
LOS was defined as isolation of a pathogen from blood or CSF obtained >72 hours of age and appropriate therapy for ≥5 days (≥7 days for CSF growth) or death before completed treatment. Cultures growing Bacillus, Micrococcus, and Corynebacterium were considered contaminants and were excluded. Polymicrobial cultures were counted as LOS cases if at least one species was a pathogen. Cultures growing coagulase-negative staphylococci (CoNS) were counted as LOS cases unless an additional contaminant organism was also isolated. Six infants whose only positive culture grew Bacillus species were excluded. LOCNC was defined as antibiotics administered for ≥5 days or death before completed treatment, without a positive blood culture obtained >72 hours of age. Brain injury was defined as cranial imaging with ≥1 of the following: severe (≥Grade 3) intraventricular hemorrhage (IVH),15 periventricular leukomalacia, porencephalic cyst, ventriculomegaly, or cerebellar hemorrhage.
Three exposure groups were identified: (1) LOS: infants with ≥1 episode of LOS and no episode of LOCNC; (2) LOCNC: infants with ≥1 episode of LOCNC and no LOS episode; (3) Unaffected: infants without either LOS or LOCNC. We excluded infants with conditions whose management overlaps with LOS or LOCNC including infants with both conditions, culture-confirmed early-onset sepsis, necrotizing enterocolitis16 and intestinal perforation.
Due to early mortality, many extremely preterm infants do not live long enough to suffer LOS/LOCNC.17 To decrease survival bias, we restricted analysis to infants surviving >7 days, which still allowed us to capture the majority of LOS cases (Supplementary Figure 1). We also excluded survivors missing neurodevelopmental assessment.
Outcomes:
The primary outcome was survival with NDI or death at >7 days age and before follow-up. Secondary outcomes were death and NDI assessed separately. Neurodevelopmental assessment: Surviving infants were assessed at 18–22 months (births before 7/1/2012) or 22–26 months (births on or after 7/1/2012) corrected age (CA). Neurodevelopmental outcomes assessed at <14 months or >30 months CA (2% of those assessed) were considered missing data. Assessment included a physical examination of the child and an interview with the primary caretaker to review clinical history. A neurologic examination and a developmental evaluation using the Bayley Scales of Infant and Toddler Development, Third Edition (Bayley-3) were administered by certified examiners.18 Motor function was classified using the gross motor function classification system (GMFCS) and cerebral palsy (CP) was classified using the GMFCS scores: mild (level 1), moderate (level 2–3), or severe (level 4–5).19 Bilateral blindness was defined as corrected vision <20/200 in both eyes. Hearing impairment was defined as permanent hearing loss with or without amplification. NDI was defined as ≥1 of the following: bilateral blindness, hearing impairment, GMFCS level ≥2 with or without CP, or a Bayley-3 cognitive composite score <85.
Statistical analysis:
Statistical significance for unadjusted comparisons between infants was determined by χ2 test for categorical variables and student’s t test for continuous variables. Poisson regression models with robust variance estimators20 were used to assess risk of outcomes in the exposure groups while adjusting for the following pre-exposure covariates: maternal education, insurance, race/ethnicity, antenatal antibiotics, antenatal steroids, antepartum hemorrhage, infant GA, BW, sex, temperature at ≤60 minutes of birth, intubation at birth, maximum respiratory support ≤24 hours of age, enteral feeds started ≤3 days of birth, receipt of antibiotics for ≥5 days starting ≤72 hours of age, severe IVH diagnosed ≤7 days of birth and center. Maternal hypertension, chorioamnionitis, delivery mode and membrane rupture were not associated with death/NDI in univariate comparisons and not included in the models. Categorical variables with missing values for ≥1% of infants were entered in models with a level indicating missing. Risk of NDI associated with multiple episodes was assessed in a separate model that categorized exposure group as 0, 1, 2, or 3+ LOS or LOCNC episodes. Adjusted relative risks, 95% confidence intervals (CI), and p-values by the Wald χ2 test from these models were reported. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Study population:
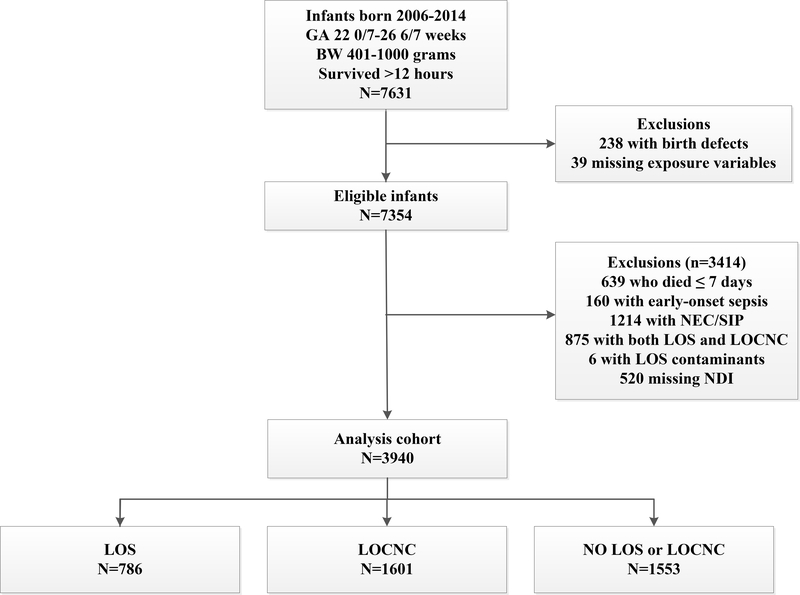
After exclusions, 3940 infants were included (Figure 1): 786 (20%) infants were diagnosed with LOS, 1601 (41%) with LOCNC, and 1553 (39%) were unaffected. Across study sites, the prevalence of infants diagnosed with LOS ranged from 8–37% and with LOCNC ranged from 23–63% (Supplementary Figure 2). All centers, except two, had a higher frequency of LOCNC diagnosis than LOS, and there was no relation between center-specific rates of LOS and LOCNC. Overall, 579 (15%) infants in the study cohort died before follow-up and 3881 (87%) surviving infants were evaluated for NDI. Follow-up visits were completed between October 2007 and August 2017.
Figure 1. Derivation of the study cohort.
BW, birth weight. GA, gestational age. LOS, late-onset sepsis. LOCNC, late-onset blood culture-negative condition. NEC, necrotizing enterocolitis. NDI, neurodevelopmental impairment. SIP, spontaneous intestinal perforation.
Microbiology:
LOS pathogens were isolated only from blood in 742 cases, only from CSF in 13 cases and from both in 31 infants (Supplementary Table 1). The most common organisms isolated from blood were CoNS (52%), Staphylococcus aureus (13%), and Escherichia coli (7%). Among 44 cases of meningitis, CoNS (17/44, 39%) was the most common organism. The median age of LOS diagnosis was 14 days (interquartile range 9–22) for the first episode and 29 days (interquartile range 18–41) for the second episode (Supplementary Figure 1). The age of onset was not recorded for LOCNC.
Clinical characteristics:
Most maternal characteristics did not differ between the three groups, although several infant characteristics differed, mainly between infants with LOS or LOCNC and unaffected infants (Table 1). Infant characteristics were not different between infants with LOS and LOCNC except infants with LOCNC were born at higher GA (p=0.03) and a greater proportion of them had received early (≤72 hours) antibiotics for ≥5 days without culture-confirmed infection (44% LOS vs. 57% LOCNC, p<0.001).
Table 1:
Maternal, Delivery, and Neonatal Characteristics
| n (column %) or mean (standard deviation)1 | LOS n=786 | LOCNC n=1601 | Unaffected n=1553 | p-value2 |
|---|---|---|---|---|
| Maternal and delivery characteristics | ||||
| Maternal education | 0.17 | |||
| < High school degree | 162 (22.9) | 298 (20.0) | 303 (21.0) | |
| High school degree | 223 (31.5) | 467 (31.4) | 412 (28.6) | |
| > High school degree | 322 (45.5) | 724 (48.6) | 725 (50.3) | |
| Unknown/missing | 79 | 112 | 113 | |
| Maternal medical insurance | 0.32 | |||
| Private | 298 (38.3) | 645 (40.6) | 640 (41.6) | |
| Public/self-pay/other | 480 (61.7) | 944 (59.4) | 900 (58.4) | |
| Maternal race/ethnicity | 0.10 | |||
| Black, non-Hispanic | 317 (40.5) | 710 (44.4) | 630 (40.8) | |
| White, non-Hispanic | 293 (37.4) | 612 (38.3) | 615 (39.8) | |
| Hispanic | 130 (16.6) | 210 (13.1) | 231 (15.0) | |
| Other | 43 (5.5) | 67 (4.2) | 69 (4.5) | |
| Maternal hypertension | 185 (23.5) | 386 (24.1) | 337 (21.7) | 0.27 |
| Antepartum hemorrhage | 163 (20.8) | 306 (19.1) | 328 (21.1) | 0.35 |
| Maternal clinical chorioamnionitis | 107 (13.6) | 281 (17.6) | 274 (17.7) | 0.03 |
| Maternal antibiotics during delivery admission | 568 (72.5) | 1,161 (72.7) | 1,172 (75.8) | 0.09 |
| Antenatal steroids | 697 (88.8) | 1,426 (89.3) | 1,379 (89.0) | 0.92 |
| Cesarean section | 502 (63.9) | 1,052 (65.8) | 1,027 (66.1) | 0.54 |
| Rupture of membranes | 0.02 | |||
| >18 hours | 179 (23.2) | 418 (26.5) | 442 (28.9) | |
| ≤ 18 hours | 591 (76.8) | 1,162 (73.5) | 1,090 (71.1) | |
| Unknown/missing | 16 | 21 | 21 | |
| Infant characteristics | ||||
| GA weeks, mean (SD) | 24.7 (1.0) | 24.8 (1.0) | 25.1 (1.0) | <0.001 |
| By GA week | <0.001 | |||
| 22 | 10 (1.3) | 16 (1.0) | 11 (0.7) | |
| 23 | 94 (12.0) | 152 (9.5) | 96 (6.2) | |
| 24 | 216 (27.5) | 441 (27.5) | 275 (17.7) | |
| 25 | 261 (33.2) | 509 (31.8) | 511 (32.9) | |
| 26 | 205 (26.1) | 483 (30.2) | 660 (42.5) | |
| BW grams, mean (SD) | 708 (135) | 717 (135) | 762 (137) | <0.001 |
| Male | 389 (49.6) | 827 (51.7) | 670 (43.2) | <0.001 |
| Endotracheal intubation at birth | 661 (84.1) | 1345 (84.0) | 1205 (77.6) | <0.001 |
| Infant temperature ≤60 minutes age | <0.001 | |||
| ≥ 96.5 F | 527 (72.6) | 1105 (74.2) | 1170 (79.8) | |
| < 96.5 F | 199 (27.4) | 385 (25.8) | 296 (20.2) | |
| Unknown/missing | 60 | 111 | 87 | |
| Highest respiratory support at 24 hours of age | <0.001 | |||
| High-frequency ventilation | 123 (15.7) | 275 (17.2) | 192 (12.4) | |
| Conventional ventilation | 459 (58.6) | 959 (59.9) | 804 (51.9) | |
| NSIMV | 41 (5.2) | 77 (4.8) | 96 (6.2) | |
| CPAP | 129 (16.5) | 255 (15.9) | 400 (25.8) | |
| Other/no support | 31 (4.0) | 34 (2.1) | 58 (3.7) | |
| Antibiotics ≥ 5 days started <72 hours of age for suspected early-onset sepsis3 | 346 (44.0) | 911 (56.9) | 684 (44.0) | <0.001 |
| Enteral feeds started within 3 days | 225 (28.7) | 522 (32.6) | 632 (40.7) | <0.001 |
BW, birth weight. CPAP, continuous positive airway pressure. GA, gestational age. LOS, late-onset sepsis. LOCNC, late-onset blood culture-negative condition. NSIMV, nasal synchronized intermittent mandatory ventilation.
The number of infants with unknown/missing information is shown for characteristics with information missing for ≥1% of infants. Otherwise, information was missing for (n) infants: maternal medical insurance (33); maternal race/ethnicity (13); maternal hypertension (4); antepartum hemorrhage (2); maternal clinical chorioamnionitis (7); maternal antibiotics (14); antenatal steroids (8); cesarean section delivery (1); male sex (3); highest respiratory support at 24 hours of age (7); enteral feeds within 3 days (4).
P-value by chi-square test (categorical variables) or T test (continuous variables).
Exact duration of antibiotic use was not collected.
In-hospital morbidities were also higher among infected versus unaffected infants (Table 2). Infants with LOS had higher frequency of brain injury than infants with LOCNC (29% vs. 24%, p=0.005) and lower survival at 36 weeks CA (77% LOS vs. 91% LOCNC, p<0.001). Among infants surviving to 36 weeks CA, bronchopulmonary dysplasia was more prevalent in infants with LOCNC (66%) than infants with LOS (60%), p=0.01.
Table 2:
In-hospital morbidities
| n (column %)1 | LOS n=786 | LOCNC n=1601 | Unaffected n=1553 | p-value2 |
|---|---|---|---|---|
| Infants with cranial imaging, N | 781 | 1597 | 1547 | |
| IVH any grade | 295 (37.9) | 525 (33.0) | 447 (28.9) | <0.001 |
| Severe IVH | 167 (21.5) | 262 (16.5) | 225 (14.6) | <0.001 |
| Severe IVH diagnosed within 7 days of birth | 48 (6.2) | 77 (4.8) | 89 (5.8) | 0.33 |
| Ventriculomegaly | 200 (25.6) | 334 (20.9) | 242 (15.6) | <0.001 |
| Cerebellar hemorrhage3 | 7/291 (2.4) | 19/717 (2.6) | 11/794 (1.4) | 0.20 |
| Periventricular leukomalacia | 39 (5.0) | 96 (6.0) | 54 (3.5) | 0.004 |
| Porencephalic cyst | 14 (1.8) | 45 (2.8) | 29 (1.9) | 0.13 |
| Brain injury4 | 228 (29.3) | 381 (23.9) | 290 (18.8) | <0.001 |
| Survived in-hospital at 28 d, N | 634 | 1490 | 1414 | |
| Had ROP exam | 609 | 1456 | 1390 | |
| ROP | 474 (77.8) | 1100 (75.5) | 883 (63.5) | <0.001 |
| ROP stage 3 or worse | 137 (22.5) | 319 (21.9) | 194 (14.0) | <0.001 |
| Survived to status5, N | 598 | 1449 | 1412 | |
| Had ROP assessed | 586 | 1414 | 1343 | |
| Severe ROP 6 | 76 (13.0) | 187 (13.2) | 87 (6.5) | <0.001 |
| Survived to 36 weeks PMA, N | 602 | 1459 | 1414 | |
| BPD 7 | 361 (60.2) | 957 (66.0) | 667 (47.5) | <0.001 |
BPD, bronchopulmonary dysplasia. IVH, intraventricular hemorrhage. LOS, late-onset sepsis. LOCNC, late-onset blood culture-negative condition. PMA, post-menstrual age. PVL, periventricular leukomalacia. ROP, retinopathy of prematurity.
Information was missing for (n) infants: IVH (9); PVL (1); porencephalic cyst (1); brain injury (6); BPD, (21).
P-value by chi-square test.
Cerebellar hemorrhage was collected beginning April 2011.
Brain injury was defined as one or more of the following findings on cranial imaging: severe IVH, PVL, ventriculomegaly, porencephalic cyst, or cerebellar hemorrhage.
Includes infants discharged home or transferred before 120 days or still in the hospital at 120 days.
ROP determined was considered severe if either eye met one of the following criteria: had ROP surgery, anti-VEGF injection, or retinal detachment from ROP.
BPD was defined as oxygen use at 36 weeks PMA.
Death and NDI:
LOS vs. LOCNC:
The adjusted relative risk for the primary composite outcome of death or NDI was significantly higher for infants with LOS compared to those with LOCNC, as was the risk of death alone before follow-up (Table 3). Among survivors assessed at follow-up, the proportion of infants with NDI was similar in the LOS (32%) and LOCNC (33%) groups and the infants had a comparable adjusted risk for NDI. Surviving infants with LOCNC had a higher risk for GMFCS level ≥2 than infants with LOS.
Table 3:
Mortality and neurodevelopmental outcomes
| LOS | LOCNC | Unaffected | Adjusted RR (95% CI) p-value1 | |||
|---|---|---|---|---|---|---|
| LOS vs. LOCNC | LOS vs. Unaffected | LOCNC vs. Unaffected | ||||
| Infants, n | 786 | 1601 | 1553 | |||
| Death/NDI, n (%) | 394 (50.1) | 662 (41.3) | 513 (33.0) | 1.14 (1.05–1.25) | 1.29 (1.17–1.42) | 1.13 (1.03–1.23) |
| 0.003 | <0.001 | 0.008 | ||||
| Death before follow-up, n (%) | 207 (26.3) | 206 (12.9) | 166 (10.7) | 1.71 (1.44–2.03) | 1.79 (1.48–2.16) | 1.04 (0.87–1.26) |
| <0.001 | <0.001 | 0.64 | ||||
| Survived and evaluated at 18–26 months follow-up, n | 579 | 1,395 | 1,387 | |||
| NDI, n (%) | 187 (32.3) | 456 (32.7) | 347 (25.0) | 0.99 (0.86–1.13) | 1.15 (0.99–1.34) | 1.17 (1.04–1.31) |
| 0.86 | 0.07 | 0.01 | ||||
| Components of NDI, n (%) | ||||||
| Bayley 3 cognitive composite score <85 | 173 (29.9) | 422 (30.4) | 327 (23.7) | 0.98 (0.85–1.13) | 1.12 (0.96–1.31) | 1.15 (1.01–1.30) |
| 0.80 | 0.14 | 0.03 | ||||
| GMFCS level ≥ 2 | 35 (6.1) | 130 (9.3) | 54 (3.9) | 0.61 (0.42–0.88) | 1.16 (0.77–1.76) | 1.91 (1.40–2.60) |
| 0.008 | 0.48 | <0.001 | ||||
| Bilateral blindness 2 | 7 (1.2) | 19 (1.4) | 7 (0.5) | 0.89 (0.37–2.10) | 2.40 (0.84–6.80) | 2.70 (1.14–6.41) |
| 1.0 | 0.14 | 0.03 | ||||
| Hearing impairment 2 | 16 (2.8) | 34 (2.4) | 21 (1.5) | 1.13 (0.63–2.04) | 1.83 (0.96–3.47) | 1.61 (0.94–2.76) |
| 0.64 | 0.07 | 0.10 | ||||
| Blindness or hearing impairment 3 | 23 (4.0) | 51 (3.7) | 26 (1.9) | 1.01 (0.62–1.66) | 1.83 (1.03–3.25) | 1.81 (1.12–2.92) |
| 0.96 | 0.04 | 0.01 | ||||
LOS, late-onset sepsis. LOCNC, late-onset blood culture-negative condition. NDI, neurodevelopmental impairment.
Relative risks (RR) and confidence intervals (CI) with adjustment for pre-exposure covariates. Pre-exposure covariates included study center, maternal education (< high school degree, high school degree, > high school degree, unknown/missing), maternal medical insurance (private, public/self-pay/other), maternal race/ethnicity (non-Hispanic black, non-Hispanic white, Hispanic, other), antepartum hemorrhage, antenatal antibiotics, antenatal steroids, GA (22, 23, 24, 25, 26), BW (continuous), male sex, endotracheal intubation, highest respiratory support at 24 hours (HFV, CV, NSIMV, CPAP, other/no support), enteral feeds started in the first 3 days, infant temperature within 60 minutes of birth (≥96.5, <96.5, unknown/missing), prolonged early antibiotics (antibiotics for 5 or more days started within 72 hours of birth), and severe IVH diagnosed within 7 days of birth. Statistical significance was determined by the Wald chi-square test.
Unadjusted relative risks are reported for blindness and for hearing impairment due to small numbers.
Due to small numbers, study center was not included in the model assessing this combined outcome and GA 22 and 23 weeks were combined.
LOS/LOCNC vs. unaffected infants:
Infants with either LOS or LOCNC were at significantly higher risk for death/NDI than unaffected infants (Table 3). Infants with LOS had a greater risk of death than unaffected infants but infants with LOCNC did not. While the adjusted risk estimates for NDI among survivors with LOS and LOCNC were higher than that for unaffected infants, the risk was statistically significant only for LOCNC infants. Differences in risk for components of NDI were found between those with LOCNC and unaffected infants but not between infants with LOS and unaffected infants.
Multiple episodes:
Among infants assessed at follow-up, more infants had repeated episodes of LOCNC (36%) than LOS (23%) (Table 4). Risk of NDI was increased for infants who had ≥2 episodes of LOCNC compared to those with one episode. While the proportion of infants with NDI increased from 31% to 35% to 42% among infants who had 1, 2 or ≥3 LOS episodes the adjusted risk of multiple episodes was not significantly different compared to one episode.
Table 4.
Neurodevelopmental impairment (NDI) by number of infection episodes
| Episodes | Infants, N | NDI, n (row %) | Comparisons | Adjusted RR1 (95% CI) | p-value |
|---|---|---|---|---|---|
| LOS, 1 | 446 | 139 (31.2) | |||
| 2 | 107 | 37 (34.6) | LOS 2 vs 1 episode | 1.06 (0.78–1.44) | 0.72 |
| 3+ | 26 | 11 (42.3) | LOS 3+ vs 1 episode | 1.10 (0.67–1.79) | 0.71 |
| LOCNC, 1 | 886 | 255 (28.8) | |||
| 2 | 330 | 131 (39.7) | LOCNC 2 vs 1 episode | 1.35 (1.15–1.59) | <0.001 |
| 3+ | 179 | 70 (39.1) | LOCNC 3+ vs 1 episode | 1.27 (1.02–1.56) | 0.03 |
LOS, late-onset sepsis. LOCNC, late-onset blood culture-negative condition. NDI, neurodevelopmental impairment.
Relative risks (RR) and confidence intervals (CI) adjusted for study center, maternal education (< high school degree, high school degree, > high school degree, unknown/missing), maternal medical insurance (private, public/self-pay/other), maternal race/ethnicity (non-Hispanic black, non-Hispanic white, Hispanic, other), antepartum hemorrhage, antenatal antibiotics, antenatal steroids, GA (22, 23, 24, 25, 26), BW (continuous), male sex, endotracheal intubation, highest respiratory support at 24 hours (HFV, CV, NSIMV, CPAP, other/no support), enteral feeds started in the first 3 days, infant temperature within 60 minutes of birth (≥96.5, <96.5, unknown/missing), prolonged early antibiotics (antibiotics for 5 or more days started within 72 hours of birth), and severe IVH diagnosed within 7 days of birth. Statistical significance was determined by the Wald chi-square test.
DISCUSSION
While infants with LOS and LOCNC did not differ in most clinical characteristics, those with LOS had higher risk of the combined outcome of death or NDI and of death alone before follow-up, when compared to either infants with LOCNC or to unaffected infants. Risk of death did not differ for infants with LOCNC and unaffected infants. The relationships between LOS, LOCNC and NDI among surviving infants were less straightforward. While brain injury was more common among infants with LOS than with LOCNC and in both groups approximately one-third of surviving infants had NDI compared to one-fourth of unaffected infants, the adjusted relative risk of NDI for infants with LOS compared to unaffected infants did not reach statistical significance. In contrast, the adjusted risk of NDI was greater for infants with LOCNC compared to those with no identified infection. The risk of NDI also increased significantly with repeated episodes of LOCNC, but not with LOS episodes. The smaller number of surviving infants with LOS (compared with LOCNC) may have contributed to these findings.
Few studies have specifically compared infants with LOS to infants with LOCNC.14 In doing so, we found a significantly increased risk of mortality among infants with LOS (Table 3). We eliminated from the analysis 875 infants (11.9% of eligible) with both LOS and LOCNC, to ensure a clear comparison. We defined LOCNC by administration of ≥5 days of antibiotics in the absence of blood or CSF-confirmed infection. This diagnosis can be due to non-systemic bacterial infection, missed bacterial infection, or to non-bacterial or non-infective conditions.21, 22 Etiologies such as urinary tract infection have a lower mortality risk than bacteremia and may have contributed to our findings. Frequently, however, LOCNC diagnoses reflect a concern for ‘false negative’ blood cultures.21, 22 Inadequate inoculant volume is reported as one driver of false negative cultures.23–25 The lower mortality observed among LOCNC infants (compared to infants with LOS) would not support the concern that LOCNC cases were predominantly due to false negatives from inadequate inoculant. A second concern is that of ‘low-level’ bacteremia.26 The vast majority of organisms between 1–10 CFU/mL can be detected reliably with one mL of blood.27, 28 Whether the majority of LOCNC cases occur due to low-level bacteremia and whether standard antibiotic regimens are effective or necessary in such cases requires further study.11 Finally, LOCNC may also simply reflect non-infectious causes of infant decompensation difficult to distinguish from bacterial sepsis using clinical judgment and laboratory markers – causes that would not respond to antibiotic therapy and may have a differential risk of mortality and morbidity.21, 22, 29
Bacteremia/meningitis in premature infants is known to be associated with white matter abnormalities5, 30 and NDI in early childhood6, 12, 31 that persists at school age.8–10, 32 However, the association of LOCNC and NDI is less clear. A prior NRN study found higher odds of NDI among infants with culture-confirmed infection and with LOCNC compared to uninfected infants.6 Culture-confirmed infection in that study, however, included early-onset sepsis and did not address infants with exclusive LOS versus LOCNC. Two other studies of infants born at <28 weeks gestation, in contrast, reported no difference in developmental outcomes at 10 years of age for infants with presumed infection compared to uninfected infants.10, 33 A similar lack of association has been reported by other investigators.9, 12 Some of the differences between studies may be from different definitions of presumed infection and inclusion of localized infection in the definition of culture-confirmed infection.9, 10 We found significantly increased risk for NDI with LOCNC that increased with multiple episodes (Table 3, 4). The mechanisms for neurologic injury attributed to LOCNC are likely related to its etiology. When attributable to bacteremia/meningitis not isolated in culture, or to localized bacterial infection, LOCNC-associated NDI may share pathogenesis with LOS. However, when viral infection or non-infectious causes of clinical decompensation lead to LOCNC diagnosis, injury may be attributed to a failure to provide appropriate therapies, as well as to dysbiosis from ineffective antibiotics.21, 22, 34–36
Our study is limited by the fact that dates of LOCNC episodes were not collected, and therefore we could not do an age-based comparison between the three groups. Specific information about the clinical conditions recorded as LOCNC, and clinical parameters associated with LOS and LOCNC that could define severity of illness were not collected. Thus, we cannot say with certainty whether LOCNC infants actually were infected with a bacterial/fungal pathogen the team failed to isolate; or if they were evaluated and treated with antibiotics for clinical instability that was non-infectious in origin, or due to a viral pathogen – and the reason for that instability led to neurodevelopmental consequences. Future studies that include data on type and severity of the clinical instability, for both LOS and LOCNC episodes, may be better able to distinguish specific outcome patterns to inform prevention and intervention strategies.
CONCLUSIONS
LOCNC is a commonly diagnosed condition, occurring twice as often in our study compared to LOS and with more frequent recurrences. Conservatively, this translated to ~2.8 times more antibiotic courses for LOCNC than for LOS. While less life-threatening than LOS, LOCNC was associated with worse neurological outcomes compared to unaffected infants. It remains unclear whether this injury is due to the etiology of decompensation or due to management decisions, including use of antibiotics. We use the term “late-onset culture-negative condition” rather than “late-onset culture-negative sepsis” to underscore the uncertainty in this diagnosis, and highlight the need for better diagnostic tools to evaluate sick newborns. Quality improvement efforts to reduce the incidence of LOS have successfully relied on interventions targeting LOS pathophysiology.1, 37 Poor understanding of both the etiology of LOCNC and of the impact of antibiotic exposures on brain development, presents a barrier to improving outcomes.11, 13, 34, 35 In the era of antibiotic stewardship, knowing the suspected adverse consequences of antibiotic misuse, a more accurate diagnosis would allow us to limit antibiotic usage to infants with clear need, and devise targeted interventions for other etiologies of LOCNC.
Supplementary Material
Supplementary Figure 1: Age of onset for first and second episode of culture confirmed LOS
LOS, late-onset sepsis. LOCNC, late-onset blood culture-negative condition. This histogram shows the proportion of first and second episode of LOS cases (Y- axis) occurring by days after birth (X-axis). LOS episodes were defined as episodes occurring after 72 hours of birth. The median age of LOS diagnosis was 14 days (interquartile range 9–22) for the first episode and 29 days (interquartile range 18–41) for the second episode. The density lines for the first and second episode of infection are shown as a solid blue line and dashed red line, respectively
Supplementary Figure 2: Proportion of infants diagnosed with LOS and LOCNC across participating study sites
LOS, late-onset sepsis. LOCNC, late-onset blood culture-negative condition. This bar graph shows the proportion of infants diagnosed with LOS or LOCNC (Y axis) per participating study site (X- axis). Data are arranged by increasing proportion of LOS cases and centers are named sequential to arrangement. Three centers that left the NRN in 2006 and contributed fewer than 10 infants each to the cohort were excluded from the figure.
What is already known on this topic:
Compared to unaffected infants, infants with late-onset sepsis and antibiotic-treated, blood culture-negative conditions have variably higher risks for death and neurodevelopmental impairment.
What this study adds:
Extremely preterm infants with late-onset sepsis had higher risk of death but similar risk of neurodevelopmental impairment, compared to infants with blood culture-negative conditions.
ACKNOWLEDGEMENTS
The National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Center for Research Resources, and the National Center for Advancing Translational Sciences provided grant support for the Neonatal Research Network’s Generic Database and Follow-up Studies through cooperative agreements. While NICHD staff had input into the study design, conduct, analysis, and manuscript drafting, the comments and views of the authors do not necessarily represent the views of NICHD, the National Institutes of Health, the Department of Health and Human Services, or the U.S. Government.
Data collected at participating sites of the NICHD Neonatal Research Network (NRN) were transmitted to RTI International, the data coordinating center (DCC) for the network, which stored, managed and analyzed the data for this study. RTI had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study. The following investigators, in addition to those listed as authors, participated in this study:
NRN Steering Committee Chairs: Alan H. Jobe, MD PhD, University of Cincinnati (2003–2006); Michael S. Caplan, MD, University of Chicago, Pritzker School of Medicine (2006–2011);
Richard A. Polin, MD, Division of Neonatology, College of Physicians and Surgeons, Columbia University, (2011-present).
Alpert Medical School of Brown University and Women & Infants Hospital of Rhode Island (UG1 HD27904) – Abbott R. Laptook, MD; Martin Keszler, MD; Betty R. Vohr, MD; Angelita M. Hensman, MS RNC-NIC; Barbara Alksninis, PNP; Kristin M. Basso, MaT BSN; Emily Little, BSN RN; Robert Burke, MD; Melinda Caskey, MD; Laurie Hoffman, MD; Katharine Johnson, MD; Mary Lenore Keszler, MD; Andrea M. Knoll; Theresa M. Leach, MEd CAES; Emilee Little, RN BSN; Elisabeth C. McGowan, MD; Elisa Vieira, RN BSN; Victoria E. Watson, MS CAS; Suzy Ventura.
Case Western Reserve University, Rainbow Babies & Children’s Hospital (UG1 HD21364, M01 RR80) – Michele C. Walsh, MD MS; Anna Maria Hibbs, MD MSCE; Deanne E. Wilson-Costello, MD; Nancy S. Newman, BA RN; Allison H. Payne, MD MS; Bonnie S. Siner, RN; Monika Bhola, MD; Gulgun Yalcinkaya, MD.
Children’s Mercy Hospital, University of Missouri Kansas City School of Medicine (UG1 HD68284) – William E. Truog, MD; Eugenia K. Pallotto, MD MSCE; Howard W. Kilbride MD; Cheri Gauldin, RN BS CCRC; Anne Holmes RN MSN MBA-HCM CCRC; Kathy Johnson RN, CCRC; Allison Scott, RNC-NIC BSN CCRC.
Cincinnati Children’s Hospital Medical Center, University Hospital, and Good Samaritan Hospital (UG1 HD27853, M01 RR8084) – Brenda B. Poindexter, MD MS; Kurt Schibler, MD; Edward F. Donovan, MD; Cathy Grisby, BSN CCRC; Barbara Alexander, RN; Kate Bridges, MD; Tanya E. Cahill, MD; Estelle E. Fischer, MHSA MBA; Stephanie Merhar, MD MS; Holly L. Mincey, RN BSN; Jody Hessling, RN; Teresa L. Gratton, PA; Lenora Jackson, CRC; Kristin Kirker, CRC; Greg Muthig, BS; Jean J. Steichen, MD; Stacey Tepe, BS; Kimberly Yolton, PhD.
Duke University School of Medicine, University Hospital, University of North Carolina, and Duke Regional Hospital (UG1 HD40492, UL1 TR1117, M01 RR30, UL1 TR1111) – Ronald N. Goldberg, MD; C. Michael Cotten, MD MHS; Ricki F. Goldstein, MD; Patricia L. Ashley, MD PhD; William F. Malcolm, MD; Kathy J. Auten, MSHS; Kimberley A. Fisher, PhD FNP-BC IBCLC; Sandra Grimes, RN BSN; Kathryn E. Gustafson, PhD; Melody B. Lohmeyer, RN MSN; Joanne Finkle, RN JD; Matthew M. Laughon, MD MPH; Carl L. Bose, MD; Janice Bernhardt, MS RN; Gennie Bose, RN; Cindy Clark, RN; Linda Manor, RPh; Diane Warner, MD MPH; Janice Wereszczak, NNP.
Emory University, Children’s Healthcare of Atlanta, Grady Memorial Hospital, and Emory University Hospital Midtown (UG1 HD27851, M01 RR39) – Barbara J. Stoll, MD; David P. Carlton, MD; Ira Adams-Chapman, MD; Ellen C. Hale, RN BS CCRC; Yvonne Loggins, RN BSN; Ann Blackwelder, RN MN; Lynn C. Wineski, RN MS; Maureen Mulligan LaRossa, RN; Sheena L. Carter, PhD.
Eunice Kennedy Shriver National Institute of Child Health and Human Development – Rosemary D. Higgins, MD; Stephanie Wilson Archer, MA.
Indiana University, University Hospital, Methodist Hospital, Riley Hospital for Children, and Wishard Health Services (UG1 HD27856, M01 RR750) – Gregory M. Sokol, MD; Brenda B. Poindexter, MD MS; Anna M. Dusick, MD (deceased); Lu-Ann Papile, MD; Susan Gunn, NNP CCRC; Faithe Hamer, BS; Heidi M. Harmon, MD MS; Dianne E. Herron, RN CCRC; Abbey C. Hines, PsyD; Carolyn Lytle, MD MPH; Heike M. Minnich, PsyD HSPP; Lucy Smiley CCRC; Leslie Dawn Wilson, BSN CCRC.
McGovern Medical School at The University of Texas Health Science Center at Houston, Children’s Memorial Hermann Hospital, Memorial Hermann Southwest Hospital, and Lyndon Baines Johnson General Hospital/Harris County Hospital District (UG1 HD87229, U10 HD21373) – Kathleen A. Kennedy, MD MPH; Jon E. Tyson, MD MPH; Georgia E. McDavid, RN; Nora I. Alaniz, BS; Elizabeth Allain, MS; Julie Arldt-McAlister, RN BSN; Katrina Burson, RN BSN; Susan E. Dieterich, PhD; Allison G. Dempsey, PhD; Andrea F. Duncan, MD; Elizabeth Eason, MD; Patricia W. Evans, MD; Carmen Garcia, RN CCRP; Charles Green, PhD; Beverly Foley Harris, RN BSN; Margarita Jiminez, MD MPH; Janice John, CPNP; Patrick M. Jones, MD; M. Layne Lillie, RN BSN; Anna E. Lis, RN BSN; Carrie M. Mason, MA LPA; Karen Martin, RN; Sara C. Martin, RN BSN; Shannon McKee EdS; Brenda H. Morris, MD; Shawna Rodgers, RN BSN; Saba Siddiki, MD; Maegan C. Simmons, RN; Daniel Sperry, RN; Patti L. Pierce Tate, RCP; Sharon L. Wright, MT(ASCP).
Nationwide Children’s Hospital and the Ohio State University Medical Center (UG1 HD68278) – Pablo J. Sanchez, MD; Leif D. Nelin, MD; Sudarshan R. Jadcherla, MD; Patricia Luzader, RN; Christine A. Fortney, PhD RN; Gail E. Besner, MD; Nehal A. Parikh, MD.
RTI International (U10 HD36790) – Abhik Das, PhD; Dennis Wallace, PhD; Carla M. Bann, PhD; Marie G. Gantz, PhD; W. Kenneth Poole, PhD (deceased); Jamie E. Newman, PhD MPH; Jeanette O’Donnell Auman, BS; Margaret M. Crawford, BS; Jenna Gabrio, MPH; Carolyn M. Petrie Huitema, MS; Kristin M. Zaterka-Baxter, RN BSN.
Stanford University, Dominican Hospital, El Camino Hospital, and Lucile Packard Children’s Hospital (UG1 HD27880, M01 RR70) – Krisa P. Van Meurs, MD; David K. Stevenson, MD; Susan R. Hintz, MD MS Epi; Marian M. Adams, MD; M. Bethany Ball, BS CCRC; Barbara Bentley, PhD; Elizabeth Bruno, PhD; Maria Elena DeAnda, PhD; Anne M. DeBattista, RN PNP; Lynne C. Huffman, MD; Magdy Ismael, MD MPH; Jean G. Kohn, MD MPH; Casey Krueger, PhD; Andrew Palmquist, RN; Melinda S. Proud, RCP; Nicholas H. St. John, PhD; Hali Weiss, MD.
Tufts Medical Center, Floating Hospital for Children (U10 HD53119, M01 RR54) – Ivan D. Frantz III, MD; John M. Fiascone, MD; Brenda L. MacKinnon, RNC; Anne Furey, MPH; Ellen Nylen, RN BSN; Elisabeth C. McGowan, MD.
University of Alabama at Birmingham Health System and Children’s Hospital of Alabama (UG1 HD34216, M01 RR32) – Waldemar A. Carlo, MD; Namasivayam Ambalavanan, MD; Myriam Peralta-Carcelen, MD MPH; Monica V. Collins, RN BSN MaEd; Shirley S. Cosby, RN BSN; Fred J. Biasini, PhD; Kristen C. Johnston, MSN CRNP; Kathleen G. Nelson, MD; Cryshelle S. Patterson, PhD; Vivien A. Phillips, RN BSN; Sally Whitley, MA OTR-L FAOTA.
University of California - Los Angeles, Mattel Children’s Hospital, Santa Monica Hospital, Los Robles Hospital and Medical Center, and Olive View Medical Center (UG1 HD68270) – Uday Devaskar, MD; Meena Garg, MD; Isabell B. Purdy, PhD CPNP; Teresa Chanlaw, MPH; Rachel Geller, RN BSN.
University of California – San Diego Medical Center and Sharp Mary Birch Hospital for Women and Newborns (U10 HD40461) – Neil N. Finer, MD; Yvonne E. Vaucher, MD MPH; David Kaegi, MD; Maynard R. Rasmussen, MD; Kathy Arnell, RNC; Clarence Demetrio, RN; Martha G. Fuller, PhD RN MSN; Wade Rich, BSHS RRT.
University of Iowa and Mercy Medical Center (UG1 HD53109, M01 RR59) – Tarah T. Colaizy, MD MPH; Michael J. Acarregui, MD; Jane E. Brumbaugh, MD; Jonathan M. Klein, MD; John M. Dagle, MD; Diane L. Eastman, RN CPNP MA; Karen J. Johnson, RN BSN; Jacky R. Walker, RN; John A. Widness, MD; Dan L. Ellsbury, MD; Donia B. Campbell, RNC-NIC; Tracy L. Tud, RN.
University of Miami, Holtz Children’s Hospital (U10 HD21397, M01 RR16587) – Shahnaz Duara, MD; Charles R. Bauer, MD; Ruth Everett-Thomas, RN MSN; Sylvia Fajardo-Hiriart, MD; Arielle Rigaud, MD; Maria Calejo, MS; Silvia M. Frade Eguaras, MA; Michelle Harwood Berkowits, PhD; Andrea Garcia, MS; Helina Pierre, BA; Alexandra Stoerger, BA.
University of New Mexico Health Sciences Center (UG1 HD53089, M01 RR997) – Kristi L. Watterberg, MD; Jean R. Lowe, PhD; Janell F. Fuller, MD; Robin K. Ohls, MD; Conra Backstrom Lacy, RN; Andrea F. Duncan, MD MScr; Rebecca A. Thomson, RN BSN; Carol H. Hartenberger, MPH RN; Sandra Brown, RN BSN; Elizabeth Kuan, RN BSN.
University of Pennsylvania, Hospital of the University of Pennsylvania, Pennsylvania Hospital, and Children’s Hospital of Philadelphia (UG1 HD68244) – Barbara Schmidt, MD MSc; Haresh Kirpalani, MB MSc; Aasma S. Chaudhary, BS RRT; Soraya Abbasi, MD; Toni Mancini, RN BSN CCRC; Noah Cook, MD; Dara M. Cucinotta, RN; Judy C. Bernbaum, MD; Marsha Gerdes, PhD; Hallam Hurt, MD; Saritha Vangala, RN MSN.
University of Rochester Medical Center, Golisano Children’s Hospital, and the University at Buffalo Women’s and Children’s Hospital of Buffalo (UG1 HD68263, U10 HD40521, M01 RR44, UL1 TR42) – Carl T. D’Angio, MD; Dale L. Phelps, MD; Ronnie Guillet, MD PhD; Satyan Lakshminrusimha, MD; Michelle Andrews-Hartley, MD; Julie Babish Johnson, MSW; Kyle Binion, BS; Melissa Bowman, RN NP; Erica Burnell, RN; Cait Fallone, MA; Osman Farooq, MD; Stephanie Guilford, BS; Cassandra A. Horihan, MS; Julianne Hunn, BS; Diane Hust, MS RN CS; Rosemary L. Jensen; Emily Kushner, MA; Deanna Maffett, RN; Joan Merzbach, LMSW; Gary J. Myers, MD; Constance Orme; Diane Prinzing; Linda J. Reubens, RN CCRC; Anne Marie Reynolds, MD, MPH; Mary Rowan, RN; Michael G. Sacilowski, MAT CCRC; Ann Marie Scorsone, MS CCRC; Holly I.M. Wadkins, MA; Ashley Williams, MS Ed; Karen Wynn, RN; Kelley Yost, PhD; William Zorn, PhD; Lauren Zwetsch, RN MS PNP.
University of Texas Southwestern Medical Center at Dallas, Parkland Health & Hospital System, and Children’s Medical Center Dallas (UG1 HD40689, M01 RR633) – Myra H. Wyckoff, MD; Luc P. Brion, MD; Roy J. Heyne, MD; Walid A. Salhab, MD; Charles R. Rosenfeld, MD; Diana M. Vasil, MSN BSN RNC-NIC; Lijun Chen, PhD RN; Alicia Guzman; Gaynelle Hensley, RN; Melissa H. Leps, RN; Nancy A. Miller, RN; Janet S. Morgan, RN; Lara Pavageau, MD; Sally S. Adams, MS RN CPNP; Catherine Twell Boatman, MS CIMI; Elizabeth T. Heyne, MS MA PA-C PsyD; Linda A. Madden, RN CPNP; Lizette E. Lee, RN.
University of Utah Medical Center, Intermountain Medical Center, LDS Hospital, and Primary Children’s Medical Center (UG1 HD87226, U10 HD53124, M01 RR64, UL1 RR25764) – Roger G. Faix, MD; Bradley A. Yoder, MD; Karen A. Osborne, RN BSN CCRC; Cynthia Spencer, RNC BSN; Kimberlee Weaver-Lewis, RN MS; Shawna Baker, RN; Karie Bird, RN BSN; Jill Burnett, RNC BSN; Michael Steffen, MS CPM; Jennifer J. Jensen, RN BSN; Sarah Winter, MD; Karen Zanetti, RN.
Wake Forest University, Baptist Medical Center, Forsyth Medical Center, and Brenner Children’s Hospital (U10 HD40498, M01 RR7122) – T. Michael O’Shea, MD MPH; Robert G. Dillard, MD; Lisa K. Washburn, MD; Barbara G. Jackson, RN, BSN; Nancy Peters, RN; Korinne Chiu, MA; Deborah Evans Allred, MA LPA; Donald J. Goldstein, PhD; Raquel Halfond, MA; Carroll Peterson, MA; Ellen L. Waldrep, MS; Cherrie D. Welch, MD MPH; Melissa Whalen Morris, MA; Gail Wiley Hounshell, PhD.
Wayne State University, Hutzel Women’s Hospital and Children’s Hospital of Michigan (UG1 HD21385) – Seetha Shankaran, MD; Athina Pappas, MD; John Barks, MD; Rebecca Bara, RN BSN; Katherine Abramczyk; Prashant Agarwal, MD; Monika Bajaj, MD; Elizabeth Billian, RN MBA; Sanjay Chawla, MD; Mary Christensen, RT; Lilia C. De Jesus, MD; Debra Driscoll, RN BSN; Melissa February, MD; Laura A. Goldston, MA; Mary E. Johnson, RN BSN; Geraldine Muran, RN BSN; Girija Natarajan, MD; Jeannette E. Prentiss, MD; Beena G. Sood, MD MS; Stephanie A. Wiggins, MS; Diane White RT; Eunice Woldt, RN MSN.
Yale University, Yale-New Haven Children’s Hospital, and Bridgeport Hospital (U10 HD27871, ULTR142) – Richard A. Ehrenkranz, MD; Harris Jacobs, MD; Christine G. Butler, MD; Patricia Cervone, RN; Sheila Greisman, RN; Monica Konstantino, RN BSN; JoAnn Poulsen, RN; Janet Taft, RN BSN; Joanne Williams, RN BSN; Elaine Romano, MSN.
Funding: This work was conducted and supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) that provides grant support for the generic database and follow-up studies of the Neonatal Research Network. Please see appendix of acknowledgments for full funding information. SM is supported by a grant from the NICHD (K23HD088753).
Abbreviations
- BPD
Bronchopulmonary dysplasia
- BW
Birth weight
- CA
Corrected age
- CoNS
Coagulase-negative Staphylococci
- CP
Cerebral palsy
- CSF
Cerebrospinal fluid
- ELBW
Extremely low birth weight infant
- GA
Gestational age
- LOS
Late-onset sepsis
- LOCNC
Late-onset blood culture negative condition
- NEC
Necrotizing enterocolitis
- NICHD
Eunice Kennedy Shriver National Institute of Child Health and Human Development
- NRN
Neonatal Research Network
- PMA
Postmenstrual age
- PVL
Periventricular leukomalacia
- SIP
Spontaneous intestinal perforation
Footnotes
Potential Conflicts of Interest:
RGG has received support from industry for research services (https://dcri.org/about-us/conflict-of-interest/). The other authors have no conflicts of interest relevant to this article to disclose.
Financial Disclosure Statement for all authors. The authors have no financial relationships relevant to this article to disclose.
Disclosure of prior presentation of study data: Preliminary results from this study were presented at the Pediatric Academic Societies annual meeting in Toronto, Canada, May, 2018.
Data Sharing: Data reported in this paper may be requested through a data use agreement. Further details are available at https://neonatal.rti.org/index.cfm?fuseaction=DataRequest.Home.
ClinicalTrials.govID: Generic Database: NCT00063063
REFERENCES
- 1.Greenberg RG, Kandefer S, Do BT, et al. Late-onset sepsis in extremely premature infants: 2000–2011. The Pediatric infectious disease journal. 2017;36:774–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horbar JD, Edwards EM, Greenberg LT, et al. Variation in Performance of Neonatal Intensive Care Units in the United States. JAMA pediatrics. 2017;171:e164396. [DOI] [PubMed] [Google Scholar]
- 3.Glass TJA, Chau V, Grunau RE, et al. Multiple Postnatal Infections in Newborns Born Preterm Predict Delayed Maturation of Motor Pathways at Term-Equivalent Age with Poorer Motor Outcomes at 3 Years. The Journal of pediatrics. 2018;196:91–97.e1. [DOI] [PubMed] [Google Scholar]
- 4.Thompson DK, Chen J, Beare R, et al. Structural connectivity relates to perinatal factors and functional impairment at 7years in children born very preterm. NeuroImage (Orlando, Fla.). 2016;134:328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah DK, Doyle LW, Anderson PJ, et al. Adverse Neurodevelopment in Preterm Infants with Postnatal Sepsis or Necrotizing Enterocolitis is Mediated by White Matter Abnormalities on Magnetic Resonance Imaging at Term. The Journal of pediatrics. 2008;153:170–175.e1. [DOI] [PubMed] [Google Scholar]
- 6.Stoll BJ, Hansen NI, Adams-Chapman I, et al. Neurodevelopmental and Growth Impairment Among Extremely Low-Birth-Weight Infants With Neonatal Infection. JAMA : the journal of the American Medical Association. 2004;292:2357–2365. [DOI] [PubMed] [Google Scholar]
- 7.Savioli K, Rouse C, Susi A, Gorman G, Hisle-Gorman E. Suspected or known neonatal sepsis and neurodevelopmental delay by 5 years. Journal of perinatology. 2018;38:1573–1580. [DOI] [PubMed] [Google Scholar]
- 8.van der Ree M, Tanis JC, Van Braeckel, Koenraad NJA, Bos AF, Roze E Functional impairments at school age of preterm born children with late-onset sepsis. Early human development. 2011;87:821–826. [DOI] [PubMed] [Google Scholar]
- 9.Rand KM, Austin NC, Inder TE, Bora S, Woodward LJ. Neonatal Infection and Later Neurodevelopmental Risk in the Very Preterm Infant. The Journal of pediatrics. 2016;170:97–104. [DOI] [PubMed] [Google Scholar]
- 10.Bright HR, Babata K, Allred EN, et al. Neurocognitive Outcomes at 10 Years of Age in Extremely Preterm Newborns with Late-Onset Bacteremia. The Journal of pediatrics. 2017;187:43–49.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cantey JB, Wozniak PS, Pruszynski JE, Sánchez PJ. Reducing unnecessary antibiotic use in the neonatal intensive care unit (SCOUT): a prospective interrupted time-series study. The Lancet infectious diseases. 2016;16:1178–1184. [DOI] [PubMed] [Google Scholar]
- 12.Schlapbach LJ, Aebischer M, Adams M, et al. Impact of sepsis on neurodevelopmental outcome in a Swiss National Cohort of extremely premature infants. Pediatrics. 2011;128:348. [DOI] [PubMed] [Google Scholar]
- 13.Cantey JB, Wozniak PS, Sánchez PJ. Prospective surveillance of antibiotic use in the neonatal intensive care unit: results from the SCOUT study. The Pediatric infectious disease journal. 2015;34:267–272. [DOI] [PubMed] [Google Scholar]
- 14.Zonnenberg IA, van Dijk-Lokkart EM, van den Dungen FAM, Vermeulen RJ, van Weissenbruch MM. Neurodevelopmental outcome at 2 years of age in preterm infants with late-onset sepsis. Eur J Pediatr. 2019;178:673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papile L, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1,500 gm. The Journal of pediatrics. 1978;92:529–534. [DOI] [PubMed] [Google Scholar]
- 16.BELL MJ, TERNBERG JL, FEIGIN RD, et al. Neonatal Necrotizing Enterocolitis. Annals of surgery. 1978;187:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel RM, Kandefer S, Walsh MC, et al. Causes and Timing of Death in Extremely Premature Infants from 2000 through 2011. The New England journal of medicine. 2015;372:331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bayley N Manual for the Bayley Scales of Infant and Toddler Development. 3rd ed. San Antonio, TX: Hartcourt; 2006. [Google Scholar]
- 19.Palisano RJ, Avery L, Gorter JW, Galuppi B, McCoy SW. Stability of the Gross Motor Function Classification System, Manual Ability Classification System, and Communication Function Classification System. Developmental medicine and child neurology. 2018;60:1026–1032. [DOI] [PubMed] [Google Scholar]
- 20.Zou G A Modified Poisson Regression Approach to Prospective Studies with Binary Data. American journal of epidemiology. 2004;159:702–706. [DOI] [PubMed] [Google Scholar]
- 21.Piantino JH, Schreiber MD, Alexander K, Hageman J. Culture Negative Sepsis and Systemic Inflammatory Response Syndrome in Neonates. Neoreviews (Elk Grove Village, Ill.). 2013;14:e294–e305. [Google Scholar]
- 22.Ronchi A, Ouellette CP, Mejías A, et al. Detection of cytomegalovirus in saliva from infants undergoing sepsis evaluation in the neonatal intensive care unit: the VIRIoN-C study. Journal of perinatal medicine. 2018;47:90–98. [DOI] [PubMed] [Google Scholar]
- 23.Connell TG, Rele M, Cowley D, Buttery JP, Curtis N. How Reliable Is a Negative Blood Culture Result? Volume of Blood Submitted for Culture in Routine Practice in a Children’s Hospital. Pediatrics (Evanston). 2007;119:891–896. [DOI] [PubMed] [Google Scholar]
- 24.Neal PR, Kleiman MB, Reynolds JK, Allen SD, Lemons JA, Yu. PL Volume of blood submitted for culture from neonates. Journal of Clinical Microbiology. 1986;24:353–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simonsen KA, Anderson-Berry AL, Delair SF, Davies HD. Early-Onset Neonatal Sepsis. Clinical microbiology reviews. 2014;27:21–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kellogg J, Ferrentino F, Goodstein M, Liss J, Shapiro S, Bankert D. Frequency of low level bacteremia in infants from birth to two months of age. The Pediatric infectious disease journal. 1997;16:381–385. [DOI] [PubMed] [Google Scholar]
- 27.Lancaster DP, Friedman DF, Chiotos K, Sullivan KV. Blood Volume Required for Detection of Low Levels and Ultralow Levels of Organisms Responsible for Neonatal Bacteremia by Use of Bactec Peds Plus/F, Plus Aerobic/F Medium, and the BD Bactec FX System: an In Vitro Study. Journal of clinical microbiology. 2015;53:3609–3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schelonka RL, Chai MK, Yoder BA, Hensley D, Brockett RM, Ascher DP. Volume of blood required to detect common neonatal pathogens. The Journal of pediatrics. 1996;129:275–278. [DOI] [PubMed] [Google Scholar]
- 29.Ronchi A, Michelow IC, Chapin KC, et al. Viral Respiratory Tract Infections in the Neonatal Intensive Care Unit: The VIRIoN-I Study. The Journal of pediatrics. 2014;165:690–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leviton A, Gilles FH. An epidemiologic study of perinatal telencephalic leucoencephalopathy in an autopsy population. Journal of the neurological sciences. 1973;18:53–66. [DOI] [PubMed] [Google Scholar]
- 31.Bassler D, Stoll BJ, Schmidt B, et al. Using a Count of Neonatal Morbidities to Predict Poor Outcome in Extremely Low Birth Weight Infants: Added Role of Neonatal Infection. Pediatrics (Evanston). 2009;123:313–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitha A, Foix-L’Helias L, Arnaud C, et al. Neonatal Infection and 5-year Neurodevelopmental Outcome of Very Preterm Infants. Pediatrics (Evanston). 2013;132:e372–e380. [DOI] [PubMed] [Google Scholar]
- 33.Leviton A, O’Shea TM, Bednarek FJ, Allred EN, Fichorova RN, Dammann O. Systemic responses of preterm newborns with presumed or documented bacteraemia. Acta Paediatrica. 2012;101:355–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang AT, Choi JP, Kotzin JJ, et al. Endothelial TLR4 and the microbiome drive cerebral cavernous malformations. Nature (London). 2017;545:305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leclercq S, Mian FM, Stanisz AM, et al. Low-dose penicillin in early life induces long-term changes in murine gut microbiota, brain cytokines and behavior. Nature communications. 2017;8:15062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rozé J, Ancel P, Marchand-Martin L, et al. Assessment of Neonatal Intensive Care Unit Practices and Preterm Newborn Gut Microbiota and 2-Year Neurodevelopmental Outcomes. JAMA Netw Open. 2020;3:e2018119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sinha A, Murthy V, Nath P, Morris J, Millar M. Prevention of Late Onset Sepsis and Central-line Associated Blood Stream Infection in Preterm Infants. The Pediatric infectious disease journal. 2016;35:401–406. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Age of onset for first and second episode of culture confirmed LOS
LOS, late-onset sepsis. LOCNC, late-onset blood culture-negative condition. This histogram shows the proportion of first and second episode of LOS cases (Y- axis) occurring by days after birth (X-axis). LOS episodes were defined as episodes occurring after 72 hours of birth. The median age of LOS diagnosis was 14 days (interquartile range 9–22) for the first episode and 29 days (interquartile range 18–41) for the second episode. The density lines for the first and second episode of infection are shown as a solid blue line and dashed red line, respectively
Supplementary Figure 2: Proportion of infants diagnosed with LOS and LOCNC across participating study sites
LOS, late-onset sepsis. LOCNC, late-onset blood culture-negative condition. This bar graph shows the proportion of infants diagnosed with LOS or LOCNC (Y axis) per participating study site (X- axis). Data are arranged by increasing proportion of LOS cases and centers are named sequential to arrangement. Three centers that left the NRN in 2006 and contributed fewer than 10 infants each to the cohort were excluded from the figure.