Abstract
Autosomal dominant polycystic kidney disease (ADPKD) and autosomal recessive polycystic kidney disease (ARPKD) are characterized by bilateral cystic kidney disease leading to progressive kidney function decline. These diseases also have distinct liver manifestations. The range of clinical presentation and severity of both ADPKD and ARPKD is much wider than was once recognized. Pediatric and adult nephrologists are likely to care for individuals with both diseases in their lifetimes. This article will review genetic, clinical, and imaging predictors of kidney and liver disease progression in ADPKD and ARPKD, and will briefly summarize pharmacologic therapies to prevent progression.
Keywords: Polycystic Kidney Disease, Stage 5 Chronic Kidney Disease, Progression, Children, Outcomes
Introduction
Polycystic kidney disease (PKD) is the most common hereditary form of kidney disease. There are two forms: autosomal dominant polycystic kidney disease (ADPKD), which typically manifests symptoms in adulthood, and autosomal recessive polycystic kidney disease (ARPKD), which typically manifests symptoms in childhood. In children, the overall prevalence of known PKD is estimated to be around 1 in 10,000 [1]. Both ADPKD and ARPKD are characterized by development of cysts resulting in kidney enlargement and progressive chronic kidney disease (CKD), which can ultimately lead to kidney failure. The mean age of developing kidney failure is in the 5th through 7th decades of life for those with ADPKD and in the 1st and 2nd decades of life for those with ARPKD [1-4]. Both diseases also have liver manifestations, characterized by polycystic liver disease in ADPKD, and congenital hepatic fibrosis in ARPKD. Both ADPKD and ARPKD can have wide phenotypic variability. In this review, we aim to present genetic, clinical, and imaging predictors of progression of ADPKD and ARPKD, and will briefly review pharmacologic therapies to prevent disease progression.
ADPKD
ADPKD occurs in in approximately 1 in 400 to 1 in 1,000 live births [1]. ADPKD is caused predominantly by mutations in one of two genes, PKD1 (~80-85% of cases) and PKD2 (~15% of cases), although several other genes have recently been implicated in a small percentage of patients. ADPKD is characterized by gradual expansion of bilateral kidney macrocysts, resulting in kidney enlargement and progressive CKD. Although the clinical manifestations of ADPKD occur mostly in adulthood, approximately 2-5% of cases are diagnosed in childhood, with presentation ranging from severe neonatal presentation resembling ARPKD to the incidental discovery of kidney cysts on imaging [5]. With growing recognition of clinical manifestations of ADPKD in childhood, pediatric nephrologists are increasingly becoming responsible for the care of patients with ADPKD.
Patients with ADPKD can also have liver cysts, with clinical expression that can range from no or few asymptomatic cysts to severe and debilitating polycystic liver disease requiring surgical intervention [6].
Predictors of kidney disease progression in ADPKD
Genetic predictors
ADPKD is most commonly caused by mutations in PKD1 or PKD2. Although the majority of ADPKD patients have a positive family history, 2-5% of patients may have de novo mutations [7]. Mutations in two additional genes, GANAB and DNAJB11, were recently also described in several pedigrees with atypical ADPKD phenotypes [8, 9].
PKD1 encodes polycystin-1 (PC1), a large glycoprotein, and PKD2 encodes polycystin-2 (PC2), a calcium-regulated cation channel [10]. PC1 and PC2 have been found to localize to the primary cilia (among other subcellular locations), where they interact with and are functionally dependent on one another [11].
The most notable genotypic predictor of ADPKD progression is the presence of PKD1 versus PKD2 mutations. The median age of kidney failure onset is 58 years for individuals with PKD1 mutations and 79 years for those with PKD2 mutations [12].
Patients with PKD1 mutations also have earlier onset of hypertension, with diagnosis occurring on average 10 years earlier than in those with PKD2 mutations [12].
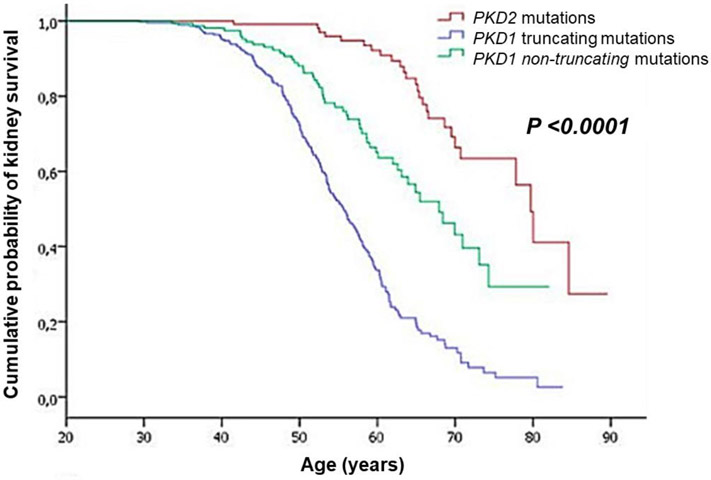
A large number of pathogenic variants exist for both PKD1 and PKD2, including missense, truncating, and intronic/splice-site mutations, and these mutations can occur anywhere along the lengths of the genes [12]. Patients with truncating PKD1 mutations have generally worse prognosis than those with non-truncating mutations [12, 13]. In one study, patients with truncating PKD1 mutations had a median age of kidney failure onset of 56 years, compared to 68 years for those with non-truncating PKD1 mutations (Fig. 1) [12]. Interestingly, among patients with truncating mutations, males had an earlier age of kidney failure onset than females (53 vs. 58 years), but this effect of sex was not observed in patients with non-truncating mutations [12]. The position of PKD1 mutations along the gene does not appear to affect kidney disease progression [12].
Figure 1.
Differences in kidney survival between individuals with PKD1 truncating mutations, PKD1 non-truncating mutations, and PKD2 mutations (figure reproduced from Cornec-Le Gall et al. [12], used with permission).
Occasionally, ADPKD can present prenatally or in infancy with a phenotype that resembles the classic presentation of ARPKD, a condition known as very early onset (VEO)-ADPKD. Some cases of VEO-ADPKD can be explained by coexistence of multiple PKD mutant alleles. Reported examples include coinheritance of an inactivating PKD1 allele from an affected parent and an incompletely penetrant or hypomorphic PKD1 allele from an unaffected parent [14-16], or coinheritance of two hypomorphic PKD1 alleles from unaffected or mildly affected parents [15, 17]. These findings suggest that the “dosage” of functional polycystin protein affects the severity of disease expression [15]. Co-inheritance of PKD1 or PKD2 mutations with other kidney cystic gene mutations such as HNF1B and PKHD1 is also associated with more severe ADPKD phenotypes that can resemble ARPKD [14]. In these cases, genetic testing using next-generation sequencing panels to test multiple cystic kidney disease genes can be helpful to verify the diagnosis and provide guidance for disease management in childhood.
The effect of other modifier genes on ADPKD progression has also been explored. For example, one study found that polymorphisms in the vascular endothelial growth factor (VEGF) promoter appeared to modify ADPKD risk in North Indian patients [18]. Consistent with this, another study found that higher serum VEGF levels correlated with more severe disease in children and young adults with ADPKD [19]. It is likely that other genes also have modifier effects on ADPKD severity, given the greater variability in disease expression seen between siblings compared to monozygotic twins [20].
Clinical predictors
Age at presentation
Studies comparing children with VEO-ADPKD (i.e. diagnosis in utero or at age < 18 months) to those with non-VEO ADPKD have found that children with VEO-ADPKD are more likely to develop hypertension, enlarged kidneys, decreased GFR, and kidney failure [21, 22]. There are conflicting data on whether outcomes differ between children diagnosed with ADPKD due to signs or symptoms compared to those diagnosed due to screening. One study reported lower GFR, higher kidney volumes, and higher prevalence of hypertension and gross hematuria in children diagnosed due to symptoms compared to those diagnosed by screening [22]. However, another study found similar kidney outcomes between those two groups [23].
Glomerular hyperfiltration
Although most children with ADPKD do not experience GFR decline until adulthood, many children have glomerular hyperfiltration early in their disease course [24]. In one longitudinal study, children with glomerular hyperfiltration at baseline (creatinine clearance > 140 mL/min/1.73 m2) had faster kidney growth and faster GFR decline than those without hyperfiltration [25]. The link between glomerular hyperfiltration and faster kidney growth may be due to intrarenal activation of the renin-angiotensin-aldosterone system (RAAS). Angiotensin II may cause hyperfiltration by increasing glomerular efferent arteriolar resistance [25], and may contribute to kidney cyst growth and GFR decline by upregulating cell proliferation, inflammation, and fibrosis [26]. Hyperfiltration may also be a precursor to the development of albuminuria [24, 27], a risk factor for ADPKD progression that is discussed further in the next section.
Urinary findings
Urinary abnormalities are sometimes the earliest presenting signs of ADPKD in children, and can be associated with worse outcomes. One study found decreased urinary concentrating capacity in almost 60% of children with ADPKD, and this was associated with a higher prevalence of hypertension and a greater number of kidney cysts [28]. Gross hematuria is a risk factor for faster CKD progression in adults with ADPKD [29, 30], and is associated with larger kidney volumes in children [22].
Children with ADPKD can also develop microalbuminuria and overt proteinuria [22, 23, 27]. In children and adults with ADPKD, the presence of proteinuria is associated with the development of hypertension and more severe kidney cystic disease [22, 31, 32]. Treatment of normotensive children with ADPKD with angiotensin converting enzyme inhibitors (ACEi) was shown to decrease microalbuminuria in a randomized clinical trial [33], but this trial did not demonstrate any benefit for kidney function decline or total kidney volume (TKV) growth compared to untreated normotensive children during the 5-year follow up period. However, since control of proteinuria decreases progression in other forms of CKD, a 2019 international consensus statement recommended screening for proteinuria and/or albuminuria (ideally with albumin: creatinine ratio, ACR) in children with ADPKD, and recommended treating with ACEi or angiotensin receptor blockers (ARBs) if proteinuria is present [34]. It should be noted, however, that a 2019 guideline committee in the United Kingdom did not make a recommendation on routine monitoring of urine ACR due to lack of consensus on the evidence [35].
Overall, given the relative ease of screening for urinary abnormalities, we believe it is reasonable to monitor urinalysis and urine protein: creatinine ratio or ACR in children with ADPKD. Given their generally favorable safety profiles and possible benefit to prevent ADPKD progression (discussed further in the next section), we feel ACEi or ARBs should be initiated in children with ADPKD if overt proteinuria (protein:creatinine ratio > 0.2 mg/mg) or severely increased albuminuria (albumin:creatinine ratio > 300 mg/g) are present. We also feel that ACEi or ARB therapy can be considered in children with ADPKD if microalbuminuria (now known as moderately increased albuminuria, ACR 30-300 mg/g), is present.
Blood pressure and cardiovascular
Hypertension is common in patients with ADPKD, and often precedes a decline in GFR [36]. About 35% of children [22, 37] and 80% of adults with ADPKD have hypertension, with a mean age of diagnosis of hypertension of around 39 years for patients with PKD1 mutations and around 49 years for those with PKD2 mutations [12]. Nocturnal hypertension is also very common in children with ADPKD: in an ambulatory blood pressure monitoring study of 310 children (mean age 11.5 years), 52% of the cohort lacked the physiologic nocturnal dip, and 18% had isolated nocturnal hypertension [37]. Hypertension is associated with higher kidney volumes and faster kidney function decline in children and adults with ADPKD [29, 33, 38].
Due to potential kidney protective effects, including improvements in glomerular hyperfiltration and proteinuria, ACEi and ARBs have generally been favored in patients with ADPKD [39-42]. The HALT-PKD study examined blood pressure targets and the effects of ACEi alone compared to ACEi/ARB dual therapy in hypertensive young adults (age 15-49 years) with early ADPKD [36]. This study found that strict blood pressure control (95/60 to 110/75 mmHg) was associated with slower kidney growth, improved left ventricular mass index (LVMI), and lower proteinuria compared to standard blood pressure control (120/70 to 130/80 mmHg) [36]. A randomized controlled trial in children with ADPKD did not demonstrate an effect of ACEi on kidney growth in patients with hypertension over a 5-year period, but did show potential benefit in preventing an increase in LVMI and loss of kidney function in patients with borderline hypertension (75th to 95th percentile) [33].
ADPKD is also associated with endothelial dysfunction, oxidative stress, and increased arterial stiffness [43], which is in part mediated through decreased nitric oxide bioavailability due to PC1 or PC2 deficiency [43]. Vascular dysfunction and oxidative stress can be detected even in children and young adults with ADPKD as alterations in vascular flow-mediated dilation and pulse wave velocity [44]. Due to its postulated role as an early an predictor for ADPKD progression, oxidative stress is the target of several candidate drugs currently being studied for ADPKD including pravastatin, curcumin, and metformin [43].
Other clinical factors
Other clinical factors that have been associated with kidney disease progression in adolescents and adults with ADPKD include male sex, higher body mass index, higher serum uric acid, higher sodium intake, lower serum HDL cholesterol [29, 45, 46]. Although there are no randomized clinical trials to show that lifestyle interventions can slow progression in children with ADPKD, expert recommendations include healthy lifestyle measures that are beneficial in the general population and individuals with CKD [47]. These include maintaining normal weight, limiting dietary salt intake, ensuring adequate water intake to prevent dehydration, and avoiding excessive protein intake [47].
Imaging predictors
Total kidney volume (TKV) and cyst volume increase progressively in adults and children with ADPKD, and increases in TKV generally precede a decline in GFR [33, 48, 49]. Long-term follow up of adult participants in the Consortium for Radiologic Imaging Studies of PKD (CRISP) has shown that baseline height-adjusted total kidney volume (htTKV) measured by magnetic resonance imaging (MRI) is strongly predictive of GFR decline over a 13-year period [50].
TKV can be measured by MRI, computed tomography, or ultrasound (US). MRI is the most accurate method, and is generally preferred in research contexts due to the lack of precision of US in detecting short-term disease progression [51, 52]. In children with early ADPKD, US can provide a reasonable estimate of TKV since the kidneys can usually fit within the US probe field of view [34]. However, the degree of measurement error by US increases with larger TKV [53]. In clinical contexts, US is particularly useful in non-cooperative children [34], and US measurements of htTKV and kidney length can be useful to help stratify risk of future CKD progression in adolescents and young adults [54]. In later sections, we will discuss the current use of htTKV in classification systems to predict risk of ADPKD progression to aid patient selection for clinical trials and pharmacologic therapy.
TKV has now been formally qualified by both the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) as a prognostic enrichment biomarker to help identify patients at high risk of CKD progression for inclusion in clinical trials [55, 56]. Although TKV is also often used as a outcome measure in ADPKD clinical trials [57], it is not formally recognized as a qualified surrogate endpoint; therefore, TKV must be used in combination with clinical endpoints such as GFR decline or development of kidney failure for FDA approval [55, 58]. In pediatric clinical trials, however, the use of TKV as an endpoint may be essential, since GFR typically remains stable in childhood despite progressive increases in TKV [33, 49].
Additional novel MRI methods have been found to predict kidney function decline in patients with ADPKD, and may improve detection of disease progression in conjunction with TKV. For example, MRI image texture analysis was found to increase the performance of a model utilizing age, GFR, and htTKV to predict subsequent GFR decline in patients with early ADPKD [59]. Reduction in kidney blood flow (KBF) using MRI was found to correlate with increased TKV and preceded GFR decline in patients with early ADPKD, and baseline KBF was an independent predictor of future GFR decline [60]. However, these novel MRI methods are not yet routinely used in clinical practice.
Novel biomarkers
The serum kidney biomarker soluble urokinase plasminogen activator receptor (suPAR) was noted to be predictive of decline in GFR and incident kidney failure in young adult patients with ADPKD; this association was significant even after adjustment for htTKV and other traditional ADPKD risk factors such as age, sex, and presence of hypertension [61]. However, since suPAR is also associated with CKD progression regardless of underlying disorder [62], it is likely that it is not a specific marker for ADPKD.
Levels of multiple urinary biomarkers such as kidney injury molecule 1 (KIM-1), neutrophil gelatinase-associated lipocalin (NGAL), and β2-microglobulin were found to be elevated in patients with ADPKD compared to healthy controls, and were associated with other markers of ADPKD progression like GFR, KBF, and TKV [63]. Urinary proteomic biomarkers in ADPKD were found to have some overlap with those found in AKI, but Kistler et al. were able to develop a 142-peptide biomarker panel that had high sensitivity and specificity to distinguish ADPKD from other kidney and non-kidney diseases [64]. This proteomic biomarker panel also correlated with measures of severity like TKV and GFR decline, albeit only moderately [64].
The role of the innate immune system in ADPKD cyst formation has also been studied. In a mouse PKD model, the monocyte/macrophage marker CD14 was overexpressed by tubular epithelia in cystic kidneys and correlated with increased kidney volume; however, the correlation between urinary CD14 level and TKV change was only modest in a small cohort of adult patients with ADPKD [65].
Currently, these novel biomarkers have minimal clinical value as TKV and eGFR decline continue to be better correlated with disease progression in ADPKD. However, future studies may include these biomarkers to help better risk-stratify children for individualized disease progression.
Predictive models for kidney disease progression in ADPKD
Based on the clinical and imaging predictors of ADPKD progression outlined previously, predictive models have been developed to identify patients at highest risk of GFR decline. In research settings, these models can help enrich clinical trial recruitment for individuals who are most likely to show disease progression over a shorter period of time, thereby improving the efficiency of trials in identifying potentially promising drugs [55]. In clinical settings, these models can help provide prognostic information to patients and help identify individuals most likely to benefit from pharmacologic therapy such as tolvaptan [66].
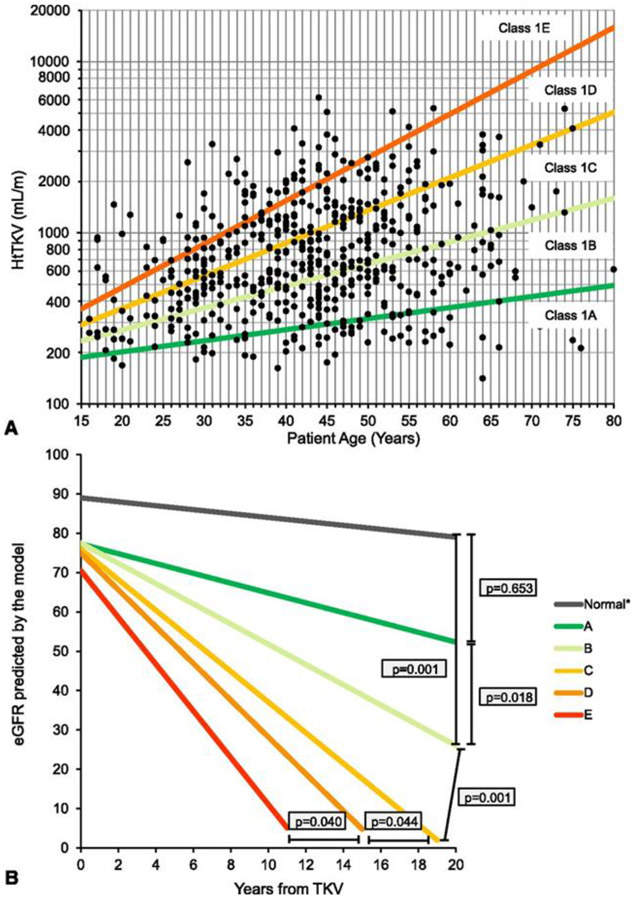
The Mayo Clinic classification model developed by Irazabal et al. [67, 68] uses htTKV and age to stratify patients aged 15-80 years with ADPKD into subclasses 1A through 1E with incrementally higher risk of kidney failure in each subclass. For example, in the original Mayo Clinic cohort (median age 44 years), patients in subclass 1A had a 10-year risk of kidney failure of 2.4%, compared to 66.9% for patients in subclass 1E (Fig. 2)[67].
Figure 2.
Mayo Clinic imaging classification to predict risk of GFR decline in patients with ADPKD. A. Subclassification of patients with typical (Class 1) ADPKD into subclasses 1A through 1E, based on baseline height-adjusted total kidney volume (HtTKV) for patient age; B. Predicted GFR slopes for males with ADPKD subclasses 1A through 1E (figure reproduced from Irazabal et al. [67], used with permission).
Cornec-Le Gall et al. [30] developed the PROPKD score using multivariate survival analysis to identify four factors that hasten ADPKD progression: male sex, development of hypertension at age < 35 years, first urologic event (hematuria, pain, or cyst infection) at age < 35 years, and mutation type (truncating PKD1 mutation > non-truncating PKD1 mutation > PKD2 mutation). The final PROPKD score ranges from 0 to 9 points, with patients in the low (0-3 points), intermediate (4-6 points), and high (7-9 points) risk categories having a median age of kidney failure of 71 years, 57 years, and 49 years, respectively [30].
Lastly, McEwan et al. [69] developed the ADPKD Outcomes Model (ADPKD-OM) using multivariable regression of patient-level clinical trial data to create a model including age, baseline TKV, and sex as factors to predict annual rates of GFR decline in adults [69].
There are currently no published predictive models for ADPKD progression in children, but this remains a critical research need in order to identify children at highest risk of CKD progression. Large international pediatric ADPKD cohort studies such as the ADPedKD initiative [70] will help provide additional insight and improved modeling of ADPKD progression in children. Based on the findings of observational studies outlined previously, we expect that a pediatric ADPKD risk prediction model may include factors such as PKD mutation type (PKD1 vs. PKD2, truncating vs. non-truncating), age at presentation (VEO- vs. non-VEO-ADPKD), htTKV, and presence of glomerular hyperfiltration, proteinuria, or hypertension.
Predictors of liver disease progression in ADPKD
Liver cysts are common in adults with ADPKD. One study found liver cysts by MRI in about 90% of patients over age 35 years [71]. In children, liver cysts are far less common and are rarely symptomatic [22]. Although many patients with liver cysts remain asymptomatic, some patients develop severe polycystic liver disease with symptomatic liver enlargement, cyst hemorrhage, rupture, or infection [6]. Female sex is associated with increased severity of ADPKD liver disease, likely due to estrogen sensitivity of liver cysts [6, 71]. Increased liver cysts are also seen in females with multiple pregnancies, again demonstrating the likely effect of estrogen on liver cyst development [6, 72]. Liver cyst volume and TKV have been found to be positively correlated in some studies [6, 71], but this correlation disappeared when adjusted for age, suggesting that it simply represents simultaneous progression of liver and kidney disease with age. In contrast to kidney involvement, genotype does not appear to be a significant predictor of the severity of polycystic liver disease in patients with ADPKD [73].
Pharmacologic agents to prevent ADPKD progression
Pathophysiologic mechanisms of cyst enlargement in ADPKD involve a number of biological pathways. The cyclic adenosine monophosphate (cAMP) pathway is upregulated in PKD cystic epithelia [74], leading to increased cell proliferation and fluid secretion [10]. cAMP activity can be downregulated by antagonism of arginine vasopressin (AVP) activity. The vasopressin V2 receptor antagonist tolvaptan has been shown to slow TKV growth and GFR decline, and is now FDA-approved for patients with ADPKD over 18 years of age [57]. Given the potential for side effects such as aquaresis and liver dysfunction [57], and the burden of potentially taking a medication for decades, tolvaptan is only recommended for adults at risk for rapid ADPKD progression [66]. Several groups have published recommendations to guide patient selection for tolvaptan therapy, based on factors including age, current eGFR, rate of eGFR decline, current TKV, and rate of TKV increase [66, 75]. The Mayo Clinic imaging classification model [67, 68] provides one tool to identify patients at highest risk of progression based on age and TKV; patients under the age of 55 years with early CKD stages (3A or earlier) who fall into Mayo subclasses 1C, 1D, or 1E are most likely to benefit from tolvaptan treatment [66]. A recent post-hoc analysis of the TEMPO 3:4 trials showed that tolvaptan can also be safe for young adults aged 18-24 years with ADPKD [76].
There are currently no data to support the efficacy or safety of tolvaptan in children and adolescents with ADPKD [34], but a multicenter clinical trial currently underway in Europe in children aged 12-17 years with ADPKD will help to address this issue (EudraCT number: 2016-000187-42; ClinicalTrials.gov identifier: NCT02964273) [77].
The use of increased water intake to suppress endogenous AVP release has also been studied. Although this intervention reduced kidney cyst progression in a PKD rat model [78], results in human studies have been inconclusive thus far [79]. Additional randomized clinical trials are now underway in adults with ADPKD [80, 81].
Somatostatin analogues such as octreotide or lanreotide also inhibit the cAMP pathway, and have been studied for both liver and kidney manifestations of ADPKD. A recent meta-analysis found no benefit for somatostatin analogues on kidney disease progression, but did find beneficial effects on liver disease progression [82]. Since severe ADPKD liver disease is exceedingly rare in children and pediatric experience is limited, this class of drugs is not recommended for children with ADPKD [34].
ADPKD progression is also associated with cell proliferation, inflammation, alterations in KBF [60, 83], endothelial dysfunction, and oxidative stress [43]. Several pharmacologic agents targeting these pathways are being studied in ADPKD, including statins, curcumin, metformin, and bardoxolone [43, 84-89]. In a randomized double-blind placebo-controlled trial in children and young adults with ADPKD aged 8-22 years, pravastatin was found to decrease the rate of TKV growth [90]. It is unclear whether statins provide benefit in older adults with ADPKD. A post-hoc analysis of adult participants in the HALT PKD study found no benefit of statins on ADPKD progression [91]; a randomized trial in adults is now underway (ClinicalTrials.gov identifier: NCT03273413) that will help to address this question more definitively. Clinical trials are also underway to evaluate curcumin in children and young adults with ADPKD aged 6-25 years (NCT02494141), metformin in adults with ADPKD (NCT02903511, NCT02656017, NCT03764605), and bardoxolone in adults with ADPKD (NCT03918447).
Pro-proliferative pathways that are involved in cyst enlargement in ADPKD include the mammalian target of rapamycin (mTOR) [92], and epidermal growth factor (EGF)/Src pathways. mTOR inhibitors such as sirolimus appeared promising to reduce ADPKD progression in mouse models [93]. However, in clinical trials in adults with ADPKD, sirolimus did not decrease kidney disease progression and was associated with unacceptable side effects [94, 95]. The multikinase EGF/Src inhibitor tesevatinib is currently undergoing clinical trials in adults with ADPKD (ClinicalTrials.gov Identifier: NCT03203642).
As mentioned previously, ACEi and ARBs may have beneficial effects on ADPKD progression, with some studies showing decreased proteinuria and slower GFR decline compared to calcium channel blockers [42] and diuretics [40] that were independent of the antihypertensive effects.
Finally, there is emerging evidence that ADPKD is associated with metabolic changes such as increased glycolysis, defective fatty acid oxidation, and altered mitochondrial function [96-98]. Multiple metabolic interventions have been shown to slow progression in ADPKD animal models, including caloric restriction, time-restricted feeding, ketogenic diet, protein restriction, the glycolysis inhibitor 2-deoxyglucose, and drugs such as metformin, statins, thiazolidinediones, and niacinamide (reviewed in [96, 98]). Clinical trials are currently underway in adults with ADPKD to study the effects of dietary interventions such as caloric restriction (ClinicalTrials.gov Identifier: NCT03342742), time-restricted feeding (NCT04534985), and/or ketogenic diet (NCT04472624), and drugs such as pioglitazone (NCT02697617) and niacinamide (NCT02558595) (as well as metformin and statins as discussed previously).
ARPKD
The incidence of ARPKD is estimated to be 1 in 26,500 live births [99]. The vast majority of ARPKD is caused by mutations in the PKHD1 gene. More recently, mutations in DZIP1L were reported in a very small subset of ARPKD pedigrees [100].
The classic description of ARPKD includes perinatal presentation with massively enlarged, echogenic kidneys, often accompanied by oligohydramnios and pulmonary hypoplasia. Since most patients with ARPKD are diagnosed in infancy and childhood, this disease is primarily managed by pediatric nephrologists. However, the clinical phenotype of ARPKD is extremely diverse. With wider availability of genetic testing, clinicians are now recognizing a wider spectrum of ARPKD phenotypes, including some patients who present in adolescence or even adulthood [101]. This recognition, along with improvements in medical management leading to improved survival of pediatric patients, have led to an increasing number of ARPKD patients being cared for by adult nephrologists. Although most patients have progressive CKD leading to kidney failure, the age at which kidney failure develops is highly variable.
Liver involvement in ARPKD may include biliary duct dilatation, congenital hepatic fibrosis (CHF), and portal hypertension. However, the clinical expression of liver disease also varies widely between patients. Although all patients have the developmental duct abnormality termed ductal plate malformation, some patients may remain asymptomatic while others may have severe consequences. Manifestations of severe liver disease include recurrent ascending cholangitis or refractory variceal bleeding, and ultimately may necessitate portosystemic shunt placement and/or liver transplantation [102].
Predictors of kidney disease progression in ARPKD
Genetic predictors
ARPKD is most commonly caused by mutations in PKHD1, a very large gene that encodes a transmembrane protein called fibrocystin/polyductin that localizes to the primary cilium [103]. A large number of pathogenic variants in PKHD1 can cause ARPKD, including missense, truncating, and intronic/splice-site mutations. The most common mutation, c.107C>T (T36M), accounts for about 15 to 20% of mutant alleles and is sometimes called a “mutational hotspot” [104]. Aside from the T36M mutation, there is no other evidence of clustering mutations at specific sites in the PKHD1 gene. Rather, mutations are dispersed over the entire gene, and many mutations are unique to single pedigrees [3, 104]. This means the majority of patients with ARPKD are compound heterozygotes, making it very difficult to predict the clinical consequences of any particular mutation.
Most studies of genotype-phenotype correlations in ARPKD have therefore focused on broad categories of PKHD1 mutation types (i.e. missense vs. truncating). Biallelic truncating mutations have generally been associated with a severe phenotype, and the presence of at least one missense mutation was historically considered to be necessary for survival [3, 105]. However, more recent reports have described patients with biallelic truncating PKHD1 mutations who have survived the neonatal period [106, 107]. Individuals with missense mutations occurring in compound with a truncating mutation have been described as having a more moderate phenotype [3], and those with two missense mutations have been reported as having less severe phenotype [105]. However, there are many exceptions, and multiple individuals with homozygous missense mutations have had severe phenotypes and perinatal demise [104, 105]. In one cohort, phenotypic outcome was similar for those with only non-truncating PKHD1 mutations compared to those with at least one truncating mutation, and variability in kidney disease phenotype was not explained by mutation location [108].
Sibling studies support the hypothesis that there are other modifiers of the ARPKD phenotype, either with modifier genes or environmental factors [105]. Despite having the same mutations, approximately 20% of siblings have widely discordant phenotypes [3, 104, 109]. For example, in one family with the common T36M mutation and another missense mutation, one child had hypertension in infancy and required kidney transplantation at age 18 years, whereas her three siblings had no hypertension and normal to mildly decreased kidney function at ages 21-28 years [108].
Animal studies have provided additional data on genetic modifiers in ARPKD. For example, in the orthologous PCK rat model of ARPKD, crossing the mutant Pkhd1 allele into a different genetic background modified the kidney phenotype significantly [110]. Variants in other genes, such as Kif12, Atmin, and Kat2J, have also been shown to have disease-modifying effects in various ARPKD mouse models [111-113]. Co-inheritance of mutations in the ADPKD gene Pkd1 in mice with homozygous Pkhd1 mutations dramatically worsens the kidney cystic phenotype [114, 115]. This effect has also been observed in a human pedigree, in which co-inheritance of biallelic PKHD1 mutations with a mutation in the ADPKD gene PKD2 resulted in a significantly worse kidney phenotype [14]. It is therefore possible that other variants in PKD or ciliopathy genes could also modify the ARPKD phenotype.
More recently, mutations in another gene encoding a ciliary/basal body protein, DZIP1L, were described in some families with ARPKD who did not have evidence of PKHD1 mutations [100]. DZIP1L mutations appear to cause relatively moderate ARPKD phenotype, but further studies are needed to identify and characterize additional families with this mutation and elucidate genotype-phenotype correlations.
Although the diagnosis of ARPKD is generally made based on clinical features, genetic testing using next-generation sequencing panels including multiple cystic kidney disease genes can be useful to verify a diagnosis or ARPKD and rule out phenocopy disorders such as HNF1B-related disease, ciliopathies, or VEO-ADPKD [17, 116, 117].
Clinical predictors
Perinatal findings
A recent large European cohort study examined perinatal risk factors for requiring dialysis within the first year of life in children with ARPKD. Burgmaier et al. [106] showed that prenatal kidney enlargement, the presence of oligo- or anhydramnios, low Apgar score, and the need for assisted breathing or invasive ventilation increased risk of needing dialysis in first year of life [106]. Using these findings, they created a model based on prenatal symptoms to predict probabilities of needing dialysis within 12 months of life or kidney replacement therapy (KRT, i.e. dialysis or kidney transplantation) within 36 months of life [106]. Patients with no prenatal abnormalities had only a 1.5% probability of requiring dialysis by age 12 months, whereas those with oligo/anhydramnios, enlarged kidneys, and kidney cysts had a 32.3% probability of needing dialysis by 12 months (Table 1). Although this model provides an important framework to conceptualize risk of requiring KRT during infancy, it also illustrates the limitations of predicting clinical outcome based on prenatal imaging findings. This is evidenced by the fact that even among patients with the most severe findings on prenatal US, the majority did not require KRT by 36 months of life, and that some patients with normal prenatal imaging still required KRT in infancy. Clinicians must therefore approach prenatal counseling with caution and should advise families of the high degree of phenotypic variability in infants and children with ARPKD.
Table 1.
Model-based predicted probabilities for dialysis or kidney replacement therapy (KRT, i.e. dialysis or kidney transplantation) within 12 and 36 months after birth based on prenatal ultrasound (US) findings (created using data from Burgmaier et al. [106]).
| Prenatal US Findings | Probability of dialysis within 12 months of life |
Probability of KRT within 36 months of life |
|---|---|---|
| No prenatal abnormalities | 1.5% | 1.7% |
| Enlarged kidneys | 3.3% | 3.5% |
| Kidney cysts | 3.4% | 3.5% |
| Enlarged kidneys + kidney cysts | 7.1% | 7.6% |
| Oligo/anhydramnios | 8.7% | 10.3% |
| Oligo/anhydramnios + enlarged kidneys | 17.4% | 18.9% |
| Oligo/anhydramnios + kidney cysts | 17.8% | 20.7% |
| Oligo/anhydramnios + enlarged kidneys + kidney cysts | 32.3% | 34.8% |
Age at presentation
Overall survival and kidney survival are dependent on the age of ARPKD diagnosis. Patients diagnosed in the perinatal period have an estimated mortality rate of around 15--30%, with deaths caused primarily by respiratory compromise in the newborn period [3, 101, 118]. In a large North American cohort, patients who required mechanical ventilation had higher mortality rates [118]. In this cohort, 58% of deaths occurred before 30 days of life, and 94% of patients who died by age 5 years had a history of neonatal mechanical ventilation [118]. In patients who survive the first month of life, however, one-year survival is > 90% [3, 118]. In the North American cohort, overall survival at 5 years was around 75% [118]. In a large European cohort, overall survival at 20 years was around 80% [3]. In previous cohort studies, approximately 35% of patients who presented in the neonatal period progressed to kidney failure by 15 years of age [2, 108].
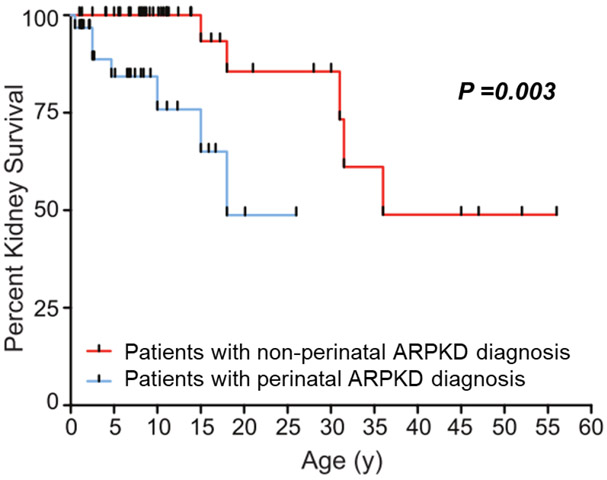
Several studies have compared rates of kidney failure based on age at diagnosis. In one retrospective chart review study of 65 patients diagnosed with ARPKD between 1961 and 2004, Adeva et al.[101] reported that the likelihood of being alive without kidney failure at 20 years after diagnosis was 36% in patients diagnosed at age < 1 year, 80% in those diagnosed at age 1-20 years, and 88% in those diagnosed at age > 20 years [101]. In an NIH cohort study of 73 patients from 63 families enrolled from 2003-2009, kidney survival at age 20 years was around 50% for patients diagnosed at < 30 days of life (perinatal group), and around 85% for those diagnosed at age > 30 days (non-perinatal group) (Fig. 3)[108]. In that study, the average age at kidney transplantation was 7.6 ± 6.8 years for the perinatal group and 26.3 ± 13.7 years for the non-perinatal group [108]. The more favorable outcomes in the NIH study compared to the analysis by Adeva et al. are likely due to over-representation of neonatal survivors in the NIH cohort, as well as advances in neonatal supportive care by era.
Figure 3.
Kidney survival in individuals diagnosed with ARPKD in the perinatal period (age < 30 days) compared to those diagnosed after the perinatal period (figure reproduced from Gunay-Aygun et al. [108], used with permission).
Urinary findings
Although proteinuria is associated with faster CKD progression in many pediatric kidney diseases [119], it is a relatively uncommon finding in patients with ARPKD, making it difficult to discern whether it influences CKD progression [120]. In one cohort, patients with lower glomerular filtration rate (GFR) were found to have lower urine osmolarity, but this is more likely to be a consequence of advancing CKD rather than a cause [108].
Blood pressure
Hypertension is common and may be severe in children with ARPKD. The overall prevalence of hypertension is around 80% [3, 120], and requires multi-drug therapy in about one third of patients [120]. Although the prevalence of hypertension increases with advancing age and worsening kidney function [2, 3, 118], it is unclear whether hypertension is simply a marker of disease severity or if it is responsible for hastening kidney function decline [118]. Although there are no ARPKD-specific studies to show that controlling hypertension can slow progression, strict blood pressure control is recommended due to its benefit in preserving kidney function in children with CKD from any cause [116, 121]. As discussed further in a later section, ACEi and ARBs are generally preferred in children with ARPKD.
Imaging predictors
Typical ultrasound (US) findings in patients with ARPKD include enlarged, echogenic kidneys with poor corticomedullary differentiation and innumerable microcysts derived from dilated collecting ducts. Several previous studies have examined whether imaging findings can predict kidney function decline in ARPKD. Although prenatal kidney enlargement is associated with increased risk for requiring KRT in infancy as discussed previously [106], children with ARPKD generally do not have ongoing kidney enlargement with disease progression. In one large cross-sectional cohort study, there was no correlation between kidney length on US and either age or serum creatinine [122]. In a more recent cross-sectional cohort, there was a weak inverse correlation between kidney volume on MRI and kidney function in children but not in adults with ARPKD [108]. Kidney size corrected for body surface area was actually lower in adults than in children in that cohort [108]. Similarly, a recent study of young adults with ARPKD who were not receiving KRT showed that their kidney lengths were only moderately above mean normal values [4]. Therefore, unlike in ADPKD, TKV is not a helpful predictor of CKD progression in ARPKD.
The extent and anatomical location of kidney imaging abnormalities has also been examined as a possible predictor of CKD progression. In one cohort, patients with only medullary abnormalities on MRI had higher GFR compared to those with both medullary and cortical involvement [108]. In addition, kidneys with only medullary changes were normal or only slightly enlarged, whereas those with medullary and cortical changes were larger in size [108].
Since TKV is not predictive for ARPKD progression, ongoing research efforts are attempting to develop more sensitive quantitative imaging methods to measure kidney disease progression in ARPKD, so that these may eventually serve as outcome measures in clinical trials. In one small study, diffuse tensor imaging, an MRI technique to characterize tissue microstructural abnormalities, was able to quantify differences in kidney parenchymal organization between patients with ARPKD and healthy controls [123]. In preclinical studies, T2 MRI was able to quantify kidney cystic burden to detect disease progression as well as the therapeutic effect of octreotide over a 2 month period in the PCK rat model of ARPKD [124]. Using high resolution quantitative MRI, an ARPKD mouse model was shown to have higher T1 and T2 relaxation times compared to wild-type mice; these findings were also seen in initial scans of a pediatric patient with ARPKD compared to a healthy volunteer using a novel rapid MR fingerprinting technique [125]. Larger longitudinal studies will be needed to evaluate the ability of these imaging methods to quantify or predict ARPKD progression in children with ARPKD.
Predictors of liver disease progression in ARPKD
Symptoms of liver disease in ARPKD can include ascending cholangitis due to bile stasis in dilated biliary ducts. Congenital hepatic fibrosis can lead to impairment of blood flow through intrahepatic portal vein branches, leading to portal hypertension. The best clinical predictors of the presence of portal hypertension are splenomegaly and thrombocytopenia due to hypersplenism [126]. Since hepatocellular function is not generally affected, transaminitis and impaired synthetic function are typically absent. In prior cohort studies of children and adults with ARPKD, patients had normal or only minimally abnormal liver enzymes, albumin, bilirubin, and prothrombin time [4, 126]. Some patients, particularly in the subgroup of patients presenting at older ages, can have liver-predominant disease with little to no kidney manifestations [101].
As with kidney involvement, genotype-phenotype correlations of liver disease severity have been difficult to discern. In one cohort, comparison of patients with missense versus truncating mutations revealed no significant differences in spleen size or biliary abnormalities [126].
There is no clear relationship between the severity of kidney and liver disease. Although some studies have found that portal hypertension is associated with lower GFR [3, 126], which may simply reflect progression of both kidney and liver disease with age [118, 126]. Another study found no significant correlations between development of systemic hypertension and portal hypertension, or between the age of diagnosis of CKD and portal hypertension [118].
Studies of imaging predictors of ARPKD liver disease progression have examined several elastography methods to measure liver stiffness as markers of congenital hepatic fibrosis and portal hypertension. The US-based elastography methods, transient elastography (Fibroscan®) and point shear wave US elastography, as well as MR elastography have been shown to detect higher liver stiffness in patients with ARPKD compared to healthy controls [127-129]. Patients with ARPKD who had signs of portal hypertension such as splenomegaly, thrombocytopenia, and esophageal varices had higher liver stiffness measurements than patients without signs of portal hypertension [127-129]. These elastographic methods may therefore be helpful to screen for liver involvement or stratify the risk of portal hypertension in children with ARPKD. With further validation, these methods may also become helpful outcome measures in future clinical trials of ARPKD therapies.
Pharmacologic agents to prevent ARPKD progression
There are currently no approved therapies to prevent ARPKD progression, but the effect of several biological pathways and pharmacologic agents has been studied, most of which overlap with those studied for ADPKD.
Studies in the PCK rat model of ARPKD found local upregulation of RAAS components in the liver and kidneys [130, 131]. The ARB telmisartan was found to improve congenital hepatic fibrosis but not kidney disease progression in PCK rats [132], but the effect of ACEi or ARBs on ARPKD progression has not been studied in humans. However, due to their favorable safety profile and possible benefit, ACEi or ARBs are generally preferred as first-line therapy for hypertension in patients with ARPKD [116].
Inhibition of the cAMP pathway has also been studied in ARPKD. In ARPKD animal models, decreasing AVP activity by increasing water intake [133], performing genetic crosses with AVP mutant animals [134], or administering AVP V2 receptor antagonists (e.g. tolvaptan, lixivaptan) [135-137] results in decreased kidney cyst formation and improved kidney function. Vasopressin also regulates biliary epithelial proliferation [138], and lixivaptan appeared to improve biliary duct dilatation in the PCK rat model [137]. The effects of increased water intake or V2 receptor antagonists have not yet been studied in humans with ARPKD. There are concerns, however, that potential adverse effects of V2 receptor antagonists may limit their utility in children in ARPKD. Aquaresis may cause difficulty with maintaining adequate fluid intake, particularly in infants or young children. The potential for liver toxicity with tolvaptan [57] is also a concern for children with ARPKD liver disease; it is possible, however, that newer more selective V2 receptor antagonists like lixivaptan may be less hepatotoxic and may even provide benefit [137]. Carefully controlled clinical trials will be needed to evaluate the safety and efficacy of V2 receptor antagonists in children with ARPKD.
The effect of dietary sodium restriction or epithelial sodium channel (ENaC) activity on ARPKD is unclear, with conflicting results in various studies in animal models and cell cultures. In the orthologous PCK rat and non-orthologous Tg737 mouse models of ARPKD, increased apical ENaC expression was observed in kidney tissues and increased ENaC activity was detected in cultured mutant collecting duct cells [139, 140]. These findings led to the hypothesis that ENaC inhibition may be helpful to treat ARPKD or associated hypertension. However, another group has shown impaired ENaC activity in cystic epithelia from the PCK rat, and observed increased cystogenesis with pharmacologic ENaC inhibition [141] or severe dietary salt restriction [142]. Further studies are therefore needed to determine the effects of ENaC inhibition and to determine the optimal level of dietary sodium intake in ARPKD.
Somatostatin analogues have also been studied in ARPKD, with both octreotide and pasireotide showing improvement in liver and kidney cystogenesis in the PCK rat model [124, 143]. There have not yet been any clinical trials of somatostatin analogues in patients with ARPKD.
Finally, ARPKD is associated with upregulation of the epidermal growth factor (EGF) and Src signaling pathways [144]. The multikinase inhibitor tesevatinib was found to improve kidney and liver progression in rodent models of ARPKD [145], and a Phase I trial of tesevatinib in children with ARPKD was recently completed [146].
Conclusion and future directions
Both ADPKD and ARPKD have highly variable clinical phenotypes, with a number of clinical, genetic, and imaging factors that can predict disease progression, as summarized in Table 2. Although a number of promising pharmacologic agents to slow disease progression are in development, the conduct of clinical trials is hindered by the relative rarity of both diseases and the lack of reliable surrogate endpoints for disease progression over the short term. Ongoing efforts to develop larger databases and cohort studies will facilitate collection of natural history data and recruitment of patients into clinical trials (e.g. ARegPKD [147], Hepatorenal Fibrocystic Diseases Core Center [99], and ADPedKD [70]). Use of large-scale electronic medical record data in a “learning health system” such as PEDSnet may also help accelerate PKD research [148]. Development of improved biomarkers to serve as surrogate endpoints of ADPKD and ARPKD progression will also be critical to the efficiency of clinical trials and the regulatory approval of novel agents [149].
Table 2.
Summary of predictors of kidney and liver disease progression in ADPKD and ARPKD, based on studies in adults (@), children (#), and/or animal models (&).
| ADPKD | ARPKD | |
|---|---|---|
| Predictors of kidney disease progression | ||
| Genetic predictors | PKD1 truncating > PKD1 non-truncating > PKD2 [1]@ Co-inheritance of other cystic gene mutations (e.g. HNF1B, PKHD1) [2]# Modifier genes (e.g. VEGF) [3]@ | Biallelic truncating PKHD1 mutations most severe, other mutation types variable [4, 5]# Co-inheritance of other cystic gene mutations (e.g. PKD1, PKD2) [2, 6, 7]#,& Genetic background / modifier genes [8-11]& |
| Clinical predictors | ||
| Perinatal findings | VEO-ADPKD (diagnosis in utero) [12]# | Prenatal oligo/anhydramnios, enlarged kidneys, and/or kidney cysts; postnatal low Apgar score or need for respiratory support [13]# |
| Age at presentation | VEO-ADPKD (diagnosis in utero or <18 months) [12]# | Perinatal presentation > non-perinatal presentation age [14-16] |
| Glomerular hyperfiltration | CrCl >140 mL/min/1.73m2[17]# | - |
| Urinary findings | Decreased urinary concentrating capacity [18]#, gross hematuria [19-21]@,#, albuminuria [21-23]@,# | lower urine osmolarity (likely non-specific) [15]# |
| Blood pressure & cardiovascular Vr | Hypertension [19, 24, 25]@,#, vascular dysfunction, oxidative stress [26, 27]&,@,# | Hypertension [4, 14, 28]# |
| Other | Male sex, higher BMI, higher serum uric acid, higher sodium intake, lower serum HDL [19, 29, 30]@ | - |
| Imaging predictors | Increased TKV (MRI or US) [31, 32]@ ; emerging imaging methods: MRI image texture analysis, KBF [33, 34]@ | Corticomedullary vs. medullary cystic changes [15]#,@; emerging imaging methods: DTI MRI, quantitative T1 and T2 MRI [35, 36]#,& |
| Novel biomarkers | Serum suPAR (likely non-specific) [37, 38]@, multiple urinary biomarkers [39-41]@ | - |
| Predictors of liver disease progression | ||
| Age | Adults > children [21, 42]@,# | Liver disease may be more prominent in patients presenting at older ages [16] |
| Sex | Female sex, multiple pregnancies [42-44]@ | - |
| Clinical & lab findings | - | Thrombocytopenia, splenomegaly [45] |
| Imaging | Liver elastography (US or MR) [46-48] | |
ADPKD, autosomal dominant polycystic kidney disease; ARPKD, autosomal recessive polycystic kidney disease; BP, blood pressure; CrCl, creatinine clearance; DTI, diffusion tensor imaging; KBF, kidney blood flow; MRI, magnetic resonance imaging; suPAR, soluble urokinase plasminogen activator receptor; TKV, total kidney volume; US, ultrasound; VEGF, vascular endothelial growth factor; VEO-ADPKD, very early onset ADPKD.
Key summary points.
ADPKD is characterized by the presence of multiple kidney macrocysts leading to progressive kidney enlargement and CKD, and liver involvement consisting of liver cysts.
ARPKD is characterized by enlarged, echogenic kidneys with diffuse microcysts,progressive CKD, and liver involvement that may include biliary duct dilatation, congenital hepatic fibrosis, and portal hypertension.
Both ADPKD and ARPKD have wide phenotypic variability.
Negative prognostic factors for kidney disease progression in ADPKD include the presence of a truncating PKD1 mutation, male sex, early age of diagnosis (particularly at < 18 months of age, known as very early onset ADPKD), gross hematuria, proteinuria, hypertension, and high total kidney volume.
Negative prognostic factors for kidney disease progression in ARPKD include perinatal diagnosis, presence of prenatal oligohydramnios, and presence of two truncating PKHD1 mutations.
Promising pharmacologic therapies are in the development pipeline, but improved methods to identify and recruit patients and improved surrogate endpoints are needed to accelerate clinical trials.
Multiple Choice Questions (answers follow the reference list).
- Which of the following is considered an FDA-qualified prognostic biomarker for clinical trials to help identify patients with ADPKD at high risk of progression?
- Urine albumin excretion
- Diastolic blood pressure load
- Total kidney volume
- Kidney cyst size
- Neutrophil gelatinase-associated lipocalin (NGAL)
- Which of the following clinical factors is associated with higher risk of progression to kidney failure in the PROPKD scoring algorithm for ADPKD?
- Female sex
- PKD2 mutation
- Proteinuria before age 35 years
- Gross hematuria before age 35 years
- Low urine osmolarity
- Which of the following prenatal ultrasound findings is most predictive of the need for dialysis within the first year of life in infants with ARPKD?
- Intrauterine growth retardation
- Enlarged kidneys
- Kidney cysts
- Oligohydramnios and echogenic kidneys
- Oligohydramnios, enlarged kidneys, and kidney cysts
- Which of the following clinical findings is NOT typical of ARPKD-associated liver disease?
- Transaminitis
- Portal hypertension
- Biliary duct dilatation
- Congenital hepatic fibrosis
- Esophageal varices
- Which of the following factors is associated with earlier onset of kidney failure in patients with ARPKD?
- Lack of liver involvement
- Younger age at diagnosis
- PKHD1 missense mutations
- Proteinuria
- High water intake
Multiple choice answers
1. c, 2. d, 3. e, 4. a, 5. b
Acknowledgments
Funding
The authors are supported by grants from the National Institutes of Diabetes and Digestive and Kidney Diseases, National Institutes of Health [grant numbers K23-DK109203 (Hartung) and T32-DK007785 (Benz)]
Footnotes
Conflicts of interest
None
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Bergmann C, Guay-Woodford LM, Harris PC, Horie S, Peters DJM, Torres VE (2018) Polycystic kidney disease. Nat Rev Dis Prim 4:50. 10.1038/s41572-018-0047-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roy S, Dillon MJ, Trompeter RS, Barratt TM (1997) Autosomal recessive polycystic kidney disease: Long-term outcome of neonatal survivors. Pediatr Nephrol 11:302–306. 10.1007/s004670050281 [DOI] [PubMed] [Google Scholar]
- 3.Bergmann C, Senderek J, Windelen E, Küpper F, Middeldorf I, Schneider F, Dornia C, Rudnik-Schöneborn S, Konrad M, Schmitt CP, Seeman T, Neuhaus TJ, Vester U, Kirfel J, Büttner R, Zerres K, Abel E, Ala-Mello S, Ausserer B, Bald M, Beetz R, Besbas N, Brandis M, Coulthard M, Dippel J, Garcia CD, Fischbach M, Foged N, Frishberg Y, Gellermann J, Gordjani N, Häffner K, Hennekam RC, Hoppe B, Hoyer P, John U, Kääriäinen H, Kemper MJ, Koivisto P, Krüger G, Kuwertz-Bröcking E, Lambert D, Lennert T, Li Volti S, Mache C, Matthijs G, Mehls O, Meiner V, Misselwitz J, Mononen T, Müller-Wiefel DE, Mustonen A, Özen S, Oliveira JP, Pirson Y, Querfeld U, Rascher W, Rudin C, Santos HG, Schröder M, Seyberth HW, Shalev S, Shohat M, Strehlau J, Vierimaa O, Völpel S, Wilson M, Zimmerhackl B (2005) Clinical consequences of PKHD1 mutations in 164 patients with autosomal-recessive polycystic kidney disease (ARPKD). Kidney Int 67:829–848. 10.1111/j.1523-1755.2005.00148.x [DOI] [PubMed] [Google Scholar]
- 4.Burgmaier K, Kilian S, Bammens B, Benzing T, Billing H, Büscher A, Galiano M, Grundmann F, Klaus G, Mekahli D, Michel-Calemard L, Milosevski-Lomic G, Ranchin B, Sauerstein K, Schaefer S, Shroff R, Sterenborg R, Verbeeck S, Weber LT, Wicher D, Wühl E, Dötsch J, Schaefer F, Liebau MC (2019) Clinical courses and complications of young adults with Autosomal Recessive Polycystic Kidney Disease (ARPKD). Sci Rep 9:7919. 10.1038/s41598-019-43488-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Rechter S, Breysem L, Mekahli D (2017) Is Autosomal Dominant Polycystic Kidney Disease Becoming a Pediatric Disorder? Front Pediatr 5:272. 10.3389/fped.2017.00272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hogan MC, Abebe K, Torres VE, Chapman AB, Bae KT, Tao C, Sun H, Perrone RD, Steinman TI, Braun W, Winklhofer FT, Miskulin DC, Rahbari-Oskoui F, Brosnahan G, Masoumi A, Karpov IO, Spillane S, Flessner M, Moore CG, Schrier RW (2014) Liver Involvement in Early Autosomal Dominant Polycystic Kidney Disease. Clin Gastroenterol Hepatol 13:155–164. 10.1016/j.cgh.2014.07.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergmann C (2015) ARPKD and early manifestations of ADPKD: the original polycystic kidney disease and phenocopies. Pediatr Nephrol 30:15–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornec-Le Gall E, Olson RJ, Besse W, Heyer CM, Gainullin VG, Smith JM, Audrézet M-P, Hopp K, Porath B, Shi B, Baheti S, Senum SR, Arroyo J, Madsen CD, Férec C, Joly D, Jouret F, Fikri-Benbrahim O, Charasse C, Coulibaly J-M, Yu AS, Khalili K, Pei Y, Somlo S, Le Meur Y, Torres VE, Genkyst Study Group, HALT Progression of Polycystic Kidney Disease Group, Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease, Harris PC (2018) Monoallelic Mutations to DNAJB11 Cause Atypical Autosomal-Dominant Polycystic Kidney Disease. Am J Hum Genet 102:832–844. 10.1016/j.ajhg.2018.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Porath B, Gainullin VG, Cornec-Le Gall E, Dillinger EK, Heyer CM, Hopp K, Edwards ME, Madsen CD, Mauritz SR, Banks CJ, Baheti S, Reddy B, Herrero JI, Bañales JM, Hogan MC, Tasic V, Watnick TJ, Chapman AB, Vigneau C, Lavainne F, Audrézet MP, Ferec C, Le Meur Y, Torres VE, Harris PC (2016) Mutations in GANAB, Encoding the Glucosidase IIα Subunit, Cause Autosomal-Dominant Polycystic Kidney and Liver Disease. Am J Hum Genet 98:1193–1207. 10.1016/j.ajhg.2016.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornec-Le Gall E, Alam A, Perrone RD (2019) Autosomal dominant polycystic kidney disease. Lancet 393:919–935. 10.1016/S0140-6736(18)32782-X [DOI] [PubMed] [Google Scholar]
- 11.Cai Y, Fedeles SV., Dong K, Anyatonwu G, Onoe T, Mitobe M, Gao JD, Okuhara D, Tian X, Gallagher AR, Tang Z, Xie X, Lalioti MD, Lee AH, Ehrlich BE, Somlo S (2014) Altered trafficking and stability of polycystins underlie polycystic kidney disease. J Clin Invest 124:5129–5144. 10.1172/JCI67273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cornec-Le Gall E, Audrézet M-P, Chen J-M, Hourmant M, Morin M-P, Perrichot R, Charasse C, Whebe B, Renaudineau E, Jousset P, Guillodo M-P, Grall-Jezequel A, Saliou P, Férec C, Le Meur Y (2013) Type of PKD1 Mutation Influences Renal Outcome in ADPKD. J Am Soc Nephrol 24:1006–1013. 10.1681/ASN.2012070650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kataoka H, Fukuoka H, Makabe S, Yoshida R, Teraoka A, Ushio Y, Akihisa T, Manabe S, Sato M, Mitobe M, Tsuchiya K, Nitta K, Mochizuki T (2020) Prediction of Renal Prognosis in Patients with Autosomal Dominant Polycystic Kidney Disease Using PKD1/PKD2 Mutations. J Clin Med 9:146. 10.3390/jcm9010146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergmann C, Von Bothmer J, Bru NO, Frank V, Fehrenbach H, Hampel T, Pape L, Buske A, Jonsson J, Sarioglu N, Ferreira JC, Becker JU, Cremer R, Hoefele J, Benz MR, Weber LT, Buettner R, Zerres K, von Bothmer J, Ortiz Bruchle N, Venghaus A, Santos A (2011) Mutations in multiple PKD genes may explain early and severe polycystic kidney disease. J Am Soc Nephrol 22:2047–2056. 10.1681/ASN.2010101080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossetti S, Kubly VJ, Consugar MB, Hopp K, Roy S, Horsley SW, Chauveau D, Rees L, Barratt TM, Van’t Hoff WG, Niaudet WP, Torres VE, Harris PC (2009) Incompletely penetrant PKD1 alleles suggest a role for gene dosage in cyst initiation in polycystic kidney disease. Kidney Int 75:848–855. 10.1038/ki.2008.686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Audrézet MP, Corbiere C, Lebbah S, Morinière V, Broux F, Louillet F, Fischbach M, Zaloszyc A, Cloarec S, Merieau E, Baudouin V, Deschênes G, Roussey G, Maestri S, Visconti C, Boyer O, Abel C, Lahoche A, Randrianaivo H, Bessenay L, Mekahli D, Ouertani I, Decramer S, Ryckenwaert A, Gall EC Le, Salomon R, Ferec C, Heidet L (2016) Comprehensive PKD1 and PKD2 mutation analysis in prenatal autosomal dominant polycystic kidney disease. J Am Soc Nephrol 27:722–729. 10.1681/ASN.2014101051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vujic M, Heyer CM, Ars E, Hopp K, Markoff A, Örndal C, Rudenhed B, Nasr SH, Torres VE, Torra R, Bogdanova N, Harris PC (2010) Incompletely penetrant PKD1 alleles mimic the renal manifestations of ARPKD. J Am Soc Nephrol 21:1097–1102. 10.1681/ASN.2009101070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pandita S, Maurya D, Ramachandran V, Verma J, Kohli S, Saxena R, Verma IC (2017) Vascular endothelial growth factor (VEGF) gene promoter polymorphisms and disease progression in North Indian cohort with autosomal dominant polycystic kidney disease. Int J Mol Cell Med 6:164–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reed B, Ph D, Masoumi A, Elhassan E, Mcfann K, Ph D, Cadnapaphornchai M, Maahs D, Snell-bergeon J, Ph D, Schrier RW (2011) Angiogenic growth factors correlate with disease severity in young patients with autosomal dominant polycystic kidney disease. Kidney Int 79:128–134. 10.1038/ki.2010.355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Persu A, Duyme M, Pirson Y, Lens XM, Messiaen T, Breuning MH, Chauveau D, Levy M, Grünfeld JP, Devuyst O (2004) Comparison between siblings and twins supports a role for modifier genes in ADPKD. Kidney Int 66:2132–2136. 10.1111/j.1523-1755.2004.66003.x [DOI] [PubMed] [Google Scholar]
- 21.Nowak KL, Cadnapaphornchai MA, Chonchol MB, Schrier RW, Gitomer B (2016) Long-Term Outcomes in Patients with Very-Early Onset Autosomal Dominant Polycystic Kidney Disease. Am J Nephrol 44:171–178. 10.1159/000448695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shamshirsaz A, Bekheirnia RM, Kamgar M, Johnson AM, McFann K, Cadnapaphornchai M, Haghighi NN, Schrier RW (2005) Autosomal-dominant polycystic kidney disease in infancy and childhood: Progression and outcome. Kidney Int 68:2218–2224. 10.1111/j.1523-1755.2005.00678.x [DOI] [PubMed] [Google Scholar]
- 23.Mekahli D, Woolf AS, Bockenhauer D (2010) Similar renal outcomes in children with ADPKD diagnosed by screening or presenting with symptoms. Pediatr Nephrol 25:2275–2282. 10.1007/s00467-010-1617-8 [DOI] [PubMed] [Google Scholar]
- 24.Wong H, Vivian L, Weiler G, Filler G (2004) Patients with autosomal dominant polycystic kidney disease hyperfiltrate early in their disease. Am J Kidney Dis 43:624–628 [DOI] [PubMed] [Google Scholar]
- 25.Helal I, Reed B, McFann K, Yan X-D, Fick-Brosnahan GM, Cadnapaphornchai M, Schrier RW (2011) Glomerular hyperfiltration and renal progression in children with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 6:2439–2443. 10.2215/CJN.01010211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schrier RW (2009) Renal volume, renin-angiotensin-aldosterone system, hypertension, and left ventricular hypertrophy in patients with autosomal dominant polycystic kidney disease. J Am Soc Nephrol 20:1888–1893 [DOI] [PubMed] [Google Scholar]
- 27.Selistre L, De Souza V, Ranchin B, Hadj-Aissa A, Cochat P, Dubourg L (2012) Early renal abnormalities in children with postnatally diagnosed autosomal dominant polycystic kidney disease. Pediatr Nephrol 27:1589–1593. 10.1007/s00467-012-2192-y [DOI] [PubMed] [Google Scholar]
- 28.Seeman T, Dušek J, Vondrák K, Bláhová K, Šimková E, Kreisinger J, Dvořák P, Kynčl M, Hříbal Z, Janda J (2004) Renal concentrating capacity is linked to blood pressure in children with autosomal dominant polycystic kidney disease. Physiol Res 53:629–634 [PubMed] [Google Scholar]
- 29.Gabow PA, Johnson AM, Kaehny WD, Kimberling WJ, Lezotte DC, Duley IT, Jones RH (1992) Factors affecting the progression of renal disease in autosomal-dominant polycystic kidney disease. Kidney Int 41:1311–1319. 10.1038/ki.1992.195 [DOI] [PubMed] [Google Scholar]
- 30.Cornec-Le Gall E, Audrézet M-P, Rousseau A, Hourmant M, Renaudineau E, Charasse C, Morin M-P, Moal M-C, Dantal J, Wehbe B, Perrichot R, Frouget T, Vigneau C, Potier J, Jousset P, Guillodo M-P, Siohan P, Terki N, Sawadogo T, Legrand D, Menoyo-Calonge V, Benarbia S, Besnier D, Longuet H, Férec C, Le Meur Y (2016) The PROPKD Score: A New Algorithm to Predict Renal Survival in Autosomal Dominant Polycystic Kidney Disease. J Am Soc Nephrol 27:942–951. 10.1681/ASN.2015010016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharp C, Johnson A, Gabow P (1998) Factors relating to urinary protein excretion in children with autosomal dominant polycystic kidney disease. J Am Soc Nephrol 9:1908–1914 [DOI] [PubMed] [Google Scholar]
- 32.Chapman AB, Johnson AM, Gabow PA, Schrier RW (1994) Overt proteinuria and microalbuminuria in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 5:1349–1354 [DOI] [PubMed] [Google Scholar]
- 33.Cadnapaphornchai MA, McFann K, Strain JD, Masoumi A, Schrier RW (2009) Prospective change in renal volume and function in children with ADPKD. Clin J Am Soc Nephrol 4:820–829. 10.2215/CJN.02810608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gimpel C, Bergmann C, Bockenhauer D, Breysem L, Cadnapaphornchai MA, Cetiner M, Dudley J, Emma F, Konrad M, Harris T, Harris PC, König J, Liebau MC, Marlais M, Mekahli D, Metcalfe AM, Oh J, Perrone RD, Sinha MD, Titieni A, Torra R, Weber S, Winyard PJD, Schaefer F (2019) International consensus statement on the diagnosis and management of autosomal dominant polycystic kidney disease in children and young people. Nat Rev Nephrol 15:713–726. 10.1038/s41581-019-0155-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dudley J, Winyard P, Marlais M, Cuthell O, Harris T, Chong J, Sayer J, Gale DP, Moore L, Turner K, Burrows S, Sandford R (2019) Clinical practice guideline monitoring children and young people with, or at risk of developing autosomal dominant polycystic kidney disease (ADPKD). BMC Nephrol 20:148. 10.1186/s12882-019-1285-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schrier RW, Abebe KZ, Perrone RD, Torres VE, Braun WE, Steinman TI, Winklhofer FT, Brosnahan G, Czarnecki PG, Hogan MC, Miskulin DC, Rahbari-Oskoui FF, Grantham JJ, Harris PC, Flessner MF, Bae KT, Moore CG, Chapman AB (2014) Blood pressure in early autosomal dominant polycystic kidney disease. N Engl J Med 371:2255–2266. 10.1056/NEJMoa1402685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Massella L, Mekahli D, Paripović D, Prikhodina L, Godefroid N, Niemirska A, Ağbaş A, Kalicka K, Jankauskiene A, Mizerska-Wasiak M, Afonso AC, Salomon R, Deschênes G, Ariceta G, Özçakar ZB, Teixeira A, Duzova A, Harambat J, Seeman T, Hrčková G, Lungu AC, Papizh S, Peco-Antic A, De Rechter S, Giordano U, Kirchner M, Lutz T, Schaefer F, Devuyst O, Wühl E, Emma F (2018) Prevalence of Hypertension in Children with Early-Stage ADPKD. Clin J Am Soc Nephrol 13:CJN.11401017. 10.2215/CJN.11401017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gabow PA, Chapman AB, Johnson AM, Tangel TJ, Duley IT, Kaehny WD, Manco-Johnson M, Schrier RW (1990) Renal structure and hypertension in autosomal dominant polycystic kidney disease. Kidney Int 38:1177–1180. 10.1038/ki.1990.330 [DOI] [PubMed] [Google Scholar]
- 39.van Dijk MA, Breuning MH, Duiser R, van Es LA, Westendorp RGJ (2003) No effect of enalapril on progression in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant 18:2314–2320. 10.1093/ndt/gfg417 [DOI] [PubMed] [Google Scholar]
- 40.Ecder T, Edelstein CL, Fick-Brosnahan GM, Johnson AM, Chapman AB, Gabow PA, Schrier RW (2001) Diuretics versus angiotensin-converting enzyme inhibitors in autosomal dominant polycystic kidney disease. Am J Nephrol 21:98–103. 10.1159/000046231 [DOI] [PubMed] [Google Scholar]
- 41.Jafar TH, Stark PC, Schmid CH, Strandgaard S, Kamper AL, Maschio G, Becicer G, Perrone RD, Levey AS (2005) The effect of angiotensin-converting-enzyme inhibitors on progression of advanced polycystic kidney disease. Kidney Int 67:265–271. 10.1111/j.1523-1755.2005.00077.x [DOI] [PubMed] [Google Scholar]
- 42.Nutahara K, Higashihara E, Horie S, Kamura K, Tsuchiya K, Mochizuki T, Hosoya T, Nakayama T, Yamamoto N, Higaki Y, Shimizu T (2005) Calcium channel blocker versus angiotensin II receptor blocker in autosomal dominant polycystic kidney disease. Nephron Clin Pract 99:18–23. 10.1159/000081790 [DOI] [PubMed] [Google Scholar]
- 43.Andries A, Daenen K, Jouret F, Bammens B, Mekahli D, Van Schepdael A (2019) Oxidative stress in autosomal dominant polycystic kidney disease: player and/or early predictor for disease progression? Pediatr Nephrol 34:993–1008 [DOI] [PubMed] [Google Scholar]
- 44.Nowak KL, Wang W, Farmer-Bailey H, Gitomer B, Malaczewski M, Klawitter J, Jovanovich A, Chonchol M (2018) Vascular dysfunction, oxidative stress, and inflammation in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 13:1493–1501. 10.2215/CJN.05850518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Torres VE, Grantham JJ, Chapman AB, Mrug M, Bae KT, King BF, Wetzel LH, Martin D, Lockhart ME, Bennett WM, Moxey-Mims M, Abebe KZ, Lin Y, Bost JE (2011) Potentially modifiable factors affecting the progression of autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 6:640–647. 10.2215/CJN.03250410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harris PC, Bae KT, Rossetti S, Torres VE, Grantham JJ, Chapman AB, Guay-Woodford LM, King BF, Wetzel LH, Baumgarten DA, Kenney PJ, Consugar M, Klahr S, Bennett WM, Meyers CM, Zhang Q, Thompson PA, Zhu F, Miller JP (2006) Cyst number but not the rate of cystic growth is associated with the mutated gene in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 17:3013–3019. 10.1681/ASN.2006080835 [DOI] [PubMed] [Google Scholar]
- 47.Gimpel C, Bergmann C, Bockenhauer D, Breysem L, Cadnapaphornchai MA, Cetiner M, Dudley J, Emma F, Konrad M, Harris T, Harris PC, König J, Liebau MC, Marlais M, Mekahli D, Metcalfe AM, Oh J, Perrone RD, Sinha MD, Titieni A, Torra R, Weber S, Winyard PJD, Schaefer F (2019) International consensus statement on the diagnosis and management of autosomal dominant polycystic kidney disease in children and young people. Nat Rev Nephrol 15:713–726. 10.1038/s41581-019-0155-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chapman AB, Bost JE, Torres VE, Guay-Woodford L, Bae KT, Landsittel D, Li J, King BF, Martin D, Wetzel LH, Lockhart ME, Harris PC, Moxey-Mims M, Flessner M, Bennett WM, Grantham JJ (2012) Kidney volume and functional outcomes in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 7:479–486. 10.2215/CJN.09500911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cadnapaphornchai MA, Masoumi A, Strain JD, McFann K, Schrier RW (2011) Magnetic resonance imaging of kidney and cyst volume in children with ADPKD. Clin J Am Soc Nephrol 6:369–376. 10.2215/CJN.03780410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu ASL, Shen C, Landsittel DP, Harris PC, Torres VE, Mrug M, Bae KT, Grantham JJ, Rahbari-Oskoui FF, Flessner MF, Bennett WM, Chapman AB, Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) (2018) Baseline total kidney volume and the rate of kidney growth are associated with chronic kidney disease progression in Autosomal Dominant Polycystic Kidney Disease. Kidney Int 93:691–699. 10.1016/j.kint.2017.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’Neill WC, Robbin ML, Bae KT, Grantham JJ, Chapman AB, Guay-Woodford LM, Torres VE, King BF, Wetzel LH, Thompson PA, Miller JP (2005) Sonographic assessment of the severity and progression of autosomal dominant polycystic kidney disease: The Consortium of Renal Imaging Studies in Polycystic Kidney Disease (CRISP). Am J Kidney Dis 46:1058–1064. 10.1053/j.ajkd.2005.08.026 [DOI] [PubMed] [Google Scholar]
- 52.Chapman AB, Guay-Woodford LM, Grantham JJ, Torres VE, Bae KT, Baumgarten DA, Kenney PJ, King BF, Glockner JF, Wetzel LH, Brummer ME, O’Neill WC, Robbin ML, Bennett WM, Klahr S, Hirschman GH, Kimmel PL, Thompson PA, Miller JP (2003) Renal structure in early autosomal-dominant polycystic kidney disease (ADPKD): The Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) cohort. Kidney Int 64:1035–1045. 10.1046/j.1523-1755.2003.00185.x [DOI] [PubMed] [Google Scholar]
- 53.Breysem L, De Rechter S, De Keyzer F, Smet MH, Bammens B, Van Dyck M, Hofmans M, Oyen R, Levtchenko E, Mekahli D (2018) 3DUS as an alternative to MRI for measuring renal volume in children with autosomal dominant polycystic kidney disease. Pediatr Nephrol 33:827–835. 10.1007/s00467-017-3862-6 [DOI] [PubMed] [Google Scholar]
- 54.Bhutani H, Smith V, Rahbari-Oskoui F, Mittal A, Grantham JJ, Torres VE, Mrug M, Bae KT, Wu Z, Ge Y, Landslittel D, Gibbs P, O’Neill WC, Chapman AB, CRISP Investigators (2015) A comparison of ultrasound and magnetic resonance imaging shows that kidney length predicts chronic kidney disease in autosomal dominant polycystic kidney disease. Kidney Int 88:146–151. 10.1038/ki.2015.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perrone RD, Mouksassi MS, Romero K, Czerwiec FS, Chapman AB, Gitomer BY, Torres VE, Miskulin DC, Broadbent S, Marier JF (2017) Total Kidney Volume Is a Prognostic Biomarker of Renal Function Decline and Progression to End-Stage Renal Disease in Patients With Autosomal Dominant Polycystic Kidney Disease. Kidney Int Reports 2:442–450. 10.1016/j.ekir.2017.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.US Food and Drug Administration. Biomarker Qualification Program. https://www.fda.gov/drugs/cder-biomarker-qualification-program/list-qualified-biomarkers.Accessed 27 Apr 2020
- 57.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang J, Czerwiec FS (2012) Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 367:2407–2418. 10.1056/NEJMoa1205511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hartung EA (2016) Biomarkers and surrogate endpoints in kidney disease. Pediatr Nephrol 31:381–391. 10.1007/s00467-015-3104-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kline TL, Korfiatis P, Edwards ME, Bae KT, Yu A, Chapman AB, Mrug M, Grantham JJ, Landsittel D, Bennett WM, King BF, Harris PC, Torres VE, Erickson BJ (2017) Image texture features predict renal function decline in patients with autosomal dominant polycystic kidney disease. Kidney Int 92:1206–1216. 10.1016/j.kint.2017.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Torres VE, King BF, Chapman AB, Brummer ME, Bae KT, Glockner JF, Arya K, Risk D, Felmlee JP, Grantham JJ, Guay-Woodford LM, Bennett WM, Klahr S, Meyers CM, Zhang X, Thompson PA, Miller JP (2007) Magnetic resonance measurements of renal blood flow and disease progression in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 2:112–120. 10.2215/CJN.00910306 [DOI] [PubMed] [Google Scholar]
- 61.Hayek SS, Landsittel DP, Wei C, Zeier M, Yu ASL, Torres VE, Roth S, Pao CS, Reiser J (2019) Soluble Urokinase Plasminogen Activator Receptor and Decline in Kidney Function in Autosomal Dominant Polycystic Kidney Disease. J Am Soc Nephrol 30:1305–1313. 10.1681/ASN.2018121227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shuai T, Yan P, Xiong H, Huang Q, Zhu L, Yang K, Liu J (2019) Association between soluble urokinase-type plasminogen activator receptor levels and chronic kidney disease: A systematic review and meta-analysis. Biomed Res Int 2019:6927456. 10.1155/2019/6927456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meijer E, Boertien WE, Nauta FL, Bakker SJL, Van Oeveren W, Rook M, Van Der Jagt EJ, Van Goor H, Peters DJM, Navis G, De Jong PE, Gansevoort RT (2010) Association of urinary biomarkers with disease severity in patients with autosomal dominant polycystic kidney disease: A cross-sectional analysis. Am J Kidney Dis 56:883–895. 10.1053/j.ajkd.2010.06.023 [DOI] [PubMed] [Google Scholar]
- 64.Kistler AD, Serra AL, Siwy J, Poster D, Krauer F, Torres VE, Mrug M, Grantham JJ, Bae KT, Bost JE, Mullen W, Wüthrich RP, Mischak H, Chapman AB (2013) Urinary proteomic biomarkers for diagnosis and risk stratification of autosomal dominant polycystic kidney disease: a multicentric study. PLoS One 8:e53016. 10.1371/journal.pone.0053016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou J, Ouyang X, Cui X, Schoeb TR, Smythies LE, Johnson MR, Guay-Woodford LM, Chapman AB, Mrug M (2010) Renal CD14 expression correlates with the progression of cystic kidney disease. Kidney Int 78:550–560. 10.1038/ki.2010.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chebib FT, Perrone RD, Chapman AB, Dahl NK, Harris PC, Mrug M, Mustafa RA, Rastogi A, Watnick T, Yu ASL, Torres VE (2018) A Practical Guide for Treatment of Rapidly Progressive ADPKD with Tolvaptan. J Am Soc Nephrol 29:2458–2470. 10.1681/ASN.2018060590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Irazabal MV, Rangel LJ, Bergstralh EJ, Osborn SL, Harmon AJ, Sundsbak JL, Bae KT, Chapman AB, Grantham JJ, Mrug M, Hogan MC, El-Zoghby ZM, Harris PC, Erickson BJ, King BF, Torres VE (2015) Imaging Classification of Autosomal Dominant Polycystic Kidney Disease: A Simple Model for Selecting Patients for Clinical Trials. J Am Soc Nephrol 26:160–172. 10.1681/ASN.2013101138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Imaging classification of ADPKD: A simple model for selecting patients for clinical trials. https://www.mayo.edu/research/documents/pkd-center-adpkd-classification/doc-20094754
- 69.McEwan P, Bennett Wilton H, Ong ACM, Ørskov B, Sandford R, Scolari F, Cabrera MCV, Walz G, O’Reilly K, Robinson P (2018) A model to predict disease progression in patients with autosomal dominant polycystic kidney disease (ADPKD): The ADPKD Outcomes Model. BMC Nephrol 19:37. 10.1186/s12882-017-0804-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.De Rechter S, Bockenhauer D, Guay-Woodford LM, Liu I, Mallett AJ, Soliman NA, Sylvestre LC, Schaefer F, Liebau MC, Mekahli D, Adamczyk P, Akinci N, Alpay H, Ardelean C, Ayasreh N, Aydin Z, Bael A, Baudouin V, Bayrakci US, Bensman A, Bialkevich H, Biebuyck A, Boyer O, Bjanid O, Bryłka A, Çalışkan S, Cambier A, Camelio A, Carbone V, Charbit M, Chiodini B, Chirita A, Çiçek N, Cerkauskiene R, Collard L, Conceiçao M, Constantinescu I, Couderc A, Crapella B, Cvetkovic M, Dima B, Diomeda F, Docx M, Dolan N, Dossier C, Drozdz D, Drube J, Dunand O, Dusan P, Eid LA, Emma F, Espino Hernandez M, Fila M, Furlano M, Gafencu M, Ghuysen MS, Giani M, Giordano M, Girisgen I, Godefroid N, Godron-Dubrasquet A, Gojkovic I, Gonzalez E, Gökçe I, Groothoff JW, Guarino S, Guffens A, Hansen P, Harambat J, Haumann S, He G, Heidet L, Helmy R, Hemery F, Hooman N, llanas B, Jankauskiene A, Janssens P, Karamaria S, Kazyra I, Koenig J, Krid S, Krug P, Kwon V, La Manna A, Leroy V, Litwin M, Lombet J, Longo G, Lungu AC, Mallawaarachchi A, Marin A, Marzuillo P, Massella L, Mastrangelo A, McCarthy H, Miklaszewska M, Moczulska A, Montini G, Morawiec-Knysak A, Morin D, Murer L, Negru I, Nobili F, Obrycki L, Otoukesh H, Özcan S, Pape L, Papizh S, Parvex P, Pawlak-Bratkowska M, Prikhodina L, Prytula A, Quinlan C, Raes A, Ranchin B, Ranguelov N, Repeckiene R, Ronit C, Salomon R, Santagelo R, Saygılı SK, Schaefer S, Schreuder M, Schurmans T, Seeman T, Segers N, Sinha M, Snauwaert E, Spasojevic B, Stabouli S, Stoica C, Stroescu R, Szczepanik E, Szczepańska M, Taranta-Janusz K, Teixeira A, Thumfart J, Tkaczyk M, Torra R, Torres D, Tram N, Utsch B, Vande Walle J, Vieux R, Vitkevic R, Wilhelm-Bals A, Wühl E, Yildirim ZY, Yüksel S, Zachwieja K (2019) ADPedKD: A Global Online Platform on the Management of Children With ADPKD. Kidney Int Reports 4:1271–1284. 10.1016/j.ekir.2019.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bae KT, Zhu F, Chapman AB, Torres VE, Grantham JJ, Guay-Woodford LM, Baumgarten DA, King BF, Wetzel LH, Kenney PJ, Brummer ME, Bennett WM, Klahr S, Meyers CM, Zhang X, Thompson PA, Miller JP (2006) Magnetic resonance imaging evaluation of hepatic cysts in early autosomal-dominant polycystic kidney disease: the Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease cohort. Clin J Am Soc Nephrol 1:64–69. 10.2215/CJN.00080605 [DOI] [PubMed] [Google Scholar]
- 72.Gabow PA, Johnson AM, Kaehny WD, Manco-Johnson ML, Duley IT, Everson GT (1990) Risk factors for the development of hepatic cysts in autosomal dominant polycystic kidney disease. Hepatology 11:1033–1037. 10.1002/hep.1840110619 [DOI] [PubMed] [Google Scholar]
- 73.Chebib FT, Jung Y, Heyer CM, Irazabal MV, Hogan MC, Harris PC, Torres VE, El-Zoghby ZM (2016) Effect of genotype on the severity and volume progression of polycystic liver disease in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant 31:952–960. 10.1093/ndt/gfw008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yamaguchi T, Hempson SJ, Reif GA, Hedge A-M, Wallace DP (2006) Calcium restores a normal proliferation phenotype in human polycystic kidney disease epithelial cells. J Am Soc Nephrol 17:178–187. 10.1681/ASN.2005060645 [DOI] [PubMed] [Google Scholar]
- 75.Gansevoort RT, Arici M, Benzing T, Birn H, Capasso G, Covic A, Devuyst O, Drechsler C, Eckardt KU, Emma F, Knebelmann B, Le Meur Y, Massy ZA, Ong ACM, Ortiz A, Schaefer F, Torra R, Vanholder R, Więcek A, Zoccali C, Van Biesen W (2016) Recommendations for the use of tolvaptan in autosomal dominant polycystic kidney disease: A position statement on behalf of the ERA-EDTA Working Groups on Inherited Kidney Disorders and European Renal Best Practice. Nephrol Dial Transplant 31:337–348. 10.1093/ndt/gfv456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Raina R, Chakraborty R, DeCoy ME, Kline T (2020) Autosomal-dominant polycystic kidney disease: tolvaptan use in adolescents and young adults with rapid progression. Pediatr Res. 10.1038/s41390-020-0942-2 [DOI] [PubMed] [Google Scholar]
- 77.Schaefer F, Mekahli D, Emma F, Gilbert RD, Bockenhauer D, Cadnapaphornchai MA, Shi L, Dandurand A, Sikes K, Shoaf SE (2019) Tolvaptan use in children and adolescents with autosomal dominant polycystic kidney disease: rationale and design of a two-part, randomized, double-blind, placebo-controlled trial. Eur J Pediatr 178:1013–1021. 10.1007/s00431-019-03384-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sagar PS, Zhang J, Luciuk M, Mannix C, Wong ATY, Rangan GK (2019) Increased water intake reduces long-term renal and cardiovascular disease progression in experimental polycystic kidney disease. PLoS One 14:1–18. 10.1371/journal.pone.0209186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Higashihara E, Nutahara K, Tanbo M, Hara H, Miyazaki I, Kobayashi K, Nitatori T (2014) Does increased water intake prevent disease progression in autosomal dominant polycystic kidney disease? Nephrol Dial Transplant 29:1710–1719. 10.1093/ndt/gfu093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.El-Damanawi R, Lee M, Harris T, Mader LB, Bond S, Pavey H, Sandford RN, Wilkinson IB, Burrows A, Woznowski P, Ben-Shlomo Y, Karet Frankl FE, Hiemstra TF (2018) Randomised controlled trial of high versus ad libitum water intake in patients with autosomal dominant polycystic kidney disease: Rationale and design of the DRINK feasibility trial. BMJ Open 8:e022859. 10.1136/bmjopen-2018-022859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wong ATY, Mannix C, Grantham JJ, Allman-Farinelli M, Badve SV., Boudville N, Byth K, Chan J, Coulshed S, Edwards ME, Erickson BJ, Fernando M, Foster S, Haloob I, Harris DCH, Hawley CM, Hill J, Howard K, Howell M, Jiang SH, Johnson DW, Kline TL, Kumar K, Lee VW, Lonergan M, Mai J, McCloud P, Peduto A, Rangan A, Roger SD, Sud K, Torres V, Vliayuri E, Rangan GK, (2018) Randomised controlled trial to determine the efficacy and safety of prescribed water intake to prevent kidney failure due to autosomal dominant polycystic kidney disease (PREVENT-ADPKD). BMJ Open 8:e018794. 10.1136/bmjopen-2017-018794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Griffiths J, Mills MT, Ong ACM (2020) Long-acting somatostatin analogue treatments in autosomal dominant polycystic kidney disease and polycystic liver disease: A systematic review and meta-analysis. BMJ Open 10:e032620. 10.1136/bmjopen-2019-032620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Helal I, Reed B, Schrier RW (2012) Emergent early markers of renal progression in autosomal-dominant polycystic kidney disease patients: Implications for prevention and treatment. Am J Nephrol 36:162–167. 10.1159/000341263 [DOI] [PubMed] [Google Scholar]
- 84.Zafar I, Tao Y, Falk S, McFann K, Schrier RW, Edelstein CL (2007) Effect of statinand angiotensin-converting enzyme inhibition on structural and hemodynamic alterations in autosomal dominant polycystic kidney disease model. Am J Physiol - Ren Physiol 293:F854–859. 10.1152/ajprenal.00059.2007 [DOI] [PubMed] [Google Scholar]
- 85.Palaniswamy C, Selvaraj DR, Selvaraj T, Sukhija R (2010) Mechanisms underlying pleiotropic effects of statins. Am J Ther 17:75–78 [DOI] [PubMed] [Google Scholar]
- 86.Cadnapaphornchai MA (2017) Clinical Trials in Pediatric Autosomal Dominant Polycystic Kidney Disease. Front Pediatr 5:53. 10.3389/fped.2017.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Celentano S, Capolongo G, Pollastro RM (2019) Bardoxolone: a new potential therapeutic agent in the treatment of autosomal dominant polycystic kidney disease? G Ital Nefrol 36:2019-vol5 [PubMed] [Google Scholar]
- 88.Fujiki T, Ando F, Murakami K, Isobe K, Mori T, Susa K, Nomura N, Sohara E, Rai T, Uchida S (2019) Tolvaptan activates the Nrf2/HO-1 antioxidant pathway through PERK phosphorylation. Sci Rep 9:9245. 10.1038/s41598-019-45539-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Seliger SL, Abebe KZ, Hallows KR, Miskulin DC, Perrone RD, Watnick T, Bae KT (2018) A Randomized Clinical Trial of Metformin to Treat Autosomal Dominant Polycystic Kidney Disease. Am J Nephrol 47:352–360. 10.1159/000488807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cadnapaphornchai M a, George DM, McFann K, Wang W, Gitomer B, Strain JD, Schrier RW (2014) Effect of pravastatin on total kidney volume, left ventricular mass index, and microalbuminuria in pediatric autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 9:889–896. 10.2215/CJN.08350813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brosnahan GM, Abebe KZ, Rahbari-Oskoui FF, Patterson CG, Bae KT, Schrier RW, Braun WE, Chapman AB, Flessner MF, Harris PC, Perrone RD, Steinman TI, Torres VE (2017) Effect of Statin Therapy on the Progression of Autosomal Dominant Polycystic Kidney Disease. A Secondary Analysis of the HALT PKD Trials. Curr Hypertens Rev 13:109–120. 10.2174/1573402113666170427142815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shillingford JM, Murcia NS, Larson CH, Low SH, Hedgepeth R, Brown N, Flask CA, Novick AC, Goldfarb DA, Kramer-Zucker A, Walz G, Piontek KB, Germino GG, Weimbs T (2006) The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci U S A 103:5466–5471. 10.1073/pnas.0509694103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shillingford JM, Piontek KB, Germino GG, Weimbs T (2010) Rapamycin ameliorates PKD resulting from conditional inactivation of Pkd1. J Am Soc Nephrol 21:489–497. 10.1681/ASN.2009040421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ruggenenti P, Gentile G, Perico N, Perna A, Barcella L, Trillini M, Cortinovis M, Ferrer Siles CP, Reyes Loaeza JA, Aparicio MC, Fasolini G, Gaspari F, Martinetti D, Carrara F, Rubis N, Prandini S, Caroli A, Sharma K, Antiga L, Remuzzi A, Remuzzi G (2016) Effect of Sirolimus on Disease Progression in Patients with Autosomal Dominant Polycystic Kidney Disease and CKD Stages 3b-4. Clin J Am Soc Nephrol 11:785–794. 10.2215/CJN.09900915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Serra AL, Poster D, Kistler AD, Krauer F, Raina S, Young J, Rentsch KM, Spanaus KS, Senn O, Kristanto P, Scheffel H, Weishaupt D, Wüthrich RP (2010) Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med 363:820–829. 10.1056/NEJMoa0907419 [DOI] [PubMed] [Google Scholar]
- 96.Padovano V, Podrini C, Boletta A, Caplan MJ (2018) Metabolism and mitochondria in polycystic kidney disease research and therapy. Nat Rev Nephrol 14:678–687 [DOI] [PubMed] [Google Scholar]
- 97.Rowe I, Chiaravalli M, Mannella V, Ulisse V, Quilici G, Pema M, Song XW, Xu H, Mari S, Qian F, Pei Y, Musco G, Boletta A (2013) Defective glucose metabolism in polycystic kidney disease identifies a new therapeutic strategy. Nat Med 19:488–493. 10.1038/nm.3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nowak KL, Hopp K (2020) Metabolic Reprogramming in Autosomal Dominant Polycystic Kidney Disease: Evidence and Therapeutic Potential. Clin J Am Soc Nephrol 15:577–584. 10.2215/CJN.13291019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Alzarka B, Morizono H, Bollman JW, Kim D, Guay-Woodford LM (2017) Design and Implementation of the Hepatorenal Fibrocystic Disease Core Center Clinical Database: A Centralized Resource for Characterizing Autosomal Recessive Polycystic Kidney Disease and Other Hepatorenal Fibrocystic Diseases. Front Pediatr 5:80. 10.3389/fped.2017.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lu H, Galeano MCR, Ott E, Kaeslin G, Kausalya PJ, Kramer C, Ortiz-Brüchle N, Hilger N, Metzis V, Hiersche M, Tay SY, Tunningley R, Vij S, Courtney AD, Whittle B, Wühl E, Vester U, Hartleben B, Neuber S, Frank V, Little MH, Epting D, Papathanasiou P, Perkins AC, Wright GD, Hunziker W, Gee HY, Otto EA, Zerres K, Hildebrandt F, Roy S, Wicking C, Bergmann C (2017) Mutations in DZIP1L, which encodes a ciliary-transition-zone protein, cause autosomal recessive polycystic kidney disease. Nat Genet 49:1025–1034. 10.1038/ng.3871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Adeva M, El-Youssef M, Rossetti S, Kamath PS, Kubly V, Consugar MB, Milliner DM, King BF, Torres VE, Harris PC (2006) Clinical and molecular characterization defines a broadened spectrum of autosomal recessive polycystic kidney disease (ARPKD). Medicine (Baltimore) 85:1–21. 10.1097/01.md.0000200165.90373.9a [DOI] [PubMed] [Google Scholar]
- 102.Wehrman A, Kriegermeier A, Wen J (2017) Diagnosis and Management of Hepatobiliary Complications in Autosomal Recessive Polycystic Kidney Disease. Front Pediatr 5:124. 10.3389/fped.2017.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ward CJ, Hogan MC, Rossetti S, Walker D, Sneddon T, Wang X, Kubly V, Cunningham JM, Bacallao R, Ishibashi M, Milliner DS, Torres VE, Harris PC (2002) The gene mutated in autosomal recessive polycystic kidney disease encodes a large, receptor-like protein. Nat Genet 30:259–269. 10.1038/ng833 [DOI] [PubMed] [Google Scholar]
- 104.Bergmann C, Senderek J, Sedlacek B, Pegiazoglou I, Puglia P, Eggermann T, Rudnik-Schöneborn S, Furu L, Onuchic LF, De Baca M, Germino GG, Guay-Woodford L, Somlo S, Moser M, Büttner R, Zerres K (2003) Spectrum of Mutations in the Gene for Autosomal Recessive Polycystic Kidney Disease (ARPKD/PKHD1). J Am Soc Nephrol 14:76–89. 10.1136/jmg.2005.032318 [DOI] [PubMed] [Google Scholar]
- 105.Furu L, Onuchic LF, Gharavi A, Hou X, Esquivel EL, Nagasawa Y, Bergmann C, Senderek J, Avner E, Zerres K, Germino GG, Guay-Woodford LM, Somlo S (2003) Milder presentation of recessive polycystic kidney disease requires presence of amino acid substitution mutations. J Am Soc Nephrol 14:2004–2014 [DOI] [PubMed] [Google Scholar]
- 106.Burgmaier K, Kunzmann K, Ariceta G, Bergmann C, Buescher AK, Burgmaier M, Dursun I, Duzova A, Eid L, Erger F, Feldkoetter M, Galiano M, Geßner M, Goebel H, Gokce I, Haffner D, Hooman N, Hoppe B, Jankauskiene A, Klaus G, König J, Litwin M, Massella L, Mekahli D, Melek E, Mir S, Pape L, Prikhodina L, Ranchin B, Schild R, Seeman T, Sever L, Shroff R, Soliman NA, Stabouli S, Stanczyk M, Tabel Y, Taranta-Janusz K, Testa S, Thumfart J, Topaloglu R, Weber LT, Wicher D, Wühl E, Wygoda S, Yilmaz A, Zachwieja K, Zagozdzon I, Zerres K, Ranguelov N, Godefroid N, Collard L, Lombet J, Maquet J, Schalk G, Querfeld U, Beck BB, Benzing T, Buettner R, Grundmann F, Kurschat C, Benz K, Tzschoppe A, Buchholz B, Buescher R, Häffner K, Pohl M, Gross O, Krügel J, Stock J, Patzer L, Oh J, Bernhardt W, Doyon A, Vinke T, Sander A, Henn M, Derichs U, Beetz R, Jeck N, Lange-Sperandio B, Ponsel S, Kusser F, Uetz B, Benz M, Schmidt S, Huppertz-Kessler C, Kranz B, Titieni A, Wurm D, Leichter HE, Bald M, Billing H, Nabhan MM, Lara LE, Papachristou F, Emma F, Cerkauskiene R, Azukaitis K, Wasilewska A, Balasz-Chmielewska I, Miklaszewska M, Tkaczyk M, Sikora P, Zaniew M, Niemirska A, Antoniewicz J, Lesiak J, Afonso AC, Teixeira A, Milosevski-Lomic G, Paripović D, Peco-Antic A, Papizh S, Bayazit AK, Anarat A, Soylu A, Kavukcu S, Candan C, Caliskan S, Canpolat N, Emre S, Alpay H, Akinci N, Conkar S, Poyrazoglu HM, Dusunsel R, Dötsch J, Schaefer F, Liebau MC (2018) Risk Factors for Early Dialysis Dependency in Autosomal Recessive Polycystic Kidney Disease. J Pediatr 199:22–28.e6. 10.1016/j.jpeds.2018.03.052 [DOI] [PubMed] [Google Scholar]
- 107.Ebner K, Dafinger C, Ortiz-Bruechle N, Koerber F, Schermer B, Benzing T, Dötsch J, Zerres K, Weber LT, Beck BB, Liebau MC (2017) Challenges in establishing genotype–phenotype correlations in ARPKD: case report on a toddler with two severe PKHD1 mutations. Pediatr Nephrol 32:1269–1273. 10.1007/s00467-017-3648-x [DOI] [PubMed] [Google Scholar]
- 108.Gunay-Aygun M, Font-Montgomery E, Lukose L, Tuchman M, Graf J, Bryant JC, Kleta R, Garcia A, Edwards H, Piwnica-Worms K, Adams D, Bernardini I, Fischer RE, Krasnewich D, Oden N, Ling A, Quezado Z, Zak C, Daryanani KT, Turkbey B, Choyke P, Guay-Woodford LM, Gahl WA (2010) Correlation of kidney function, volume and imaging findings, and PKHD1 mutations in 73 patients with autosomal recessive polycystic kidney disease. Clin J Am Soc Nephrol 5:972–984. 10.2215/CJN.07141009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Deget F, Rudnik-Schaneborn S, Zerres K (1995) Course of autosomal recessive polycystic kidney disease (ARPKD) in siblings: a clinical comparison of 20 sibships. Clin Genet 47:248–253 [DOI] [PubMed] [Google Scholar]
- 110.O’Meara CC, Hoffman M, Sweeney WE Jr, Tsaih SW, Xiao B, Jacob HJ, Avner ED, Moreno C (2012) Role of genetic modifiers in an orthologous rat model of ARPKD. Physiol Genomics 44:741–753. 10.1152/physiolgenomics.00187.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Richards T, Modarage K, Dean C, McCarthy-Boxer A, Hilton H, Esapa C, Norman J, Wilson P, Goggolidou P (2019) Atmin modulates Pkhd1 expression and may mediate Autosomal Recessive Polycystic Kidney Disease (ARPKD) through altered non-canonical Wnt/Planar Cell Polarity (PCP) signalling. Biochim Biophys Acta Mol Basis Dis 1865:378–390. 10.1016/j.bbadis.2018.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mrug M, Li R, Cui X, Schoeb TR, Churchill GA, Guay-Woodford LM (2005) Kinesin family member 12 is a candidate polycystic kidney disease modifier in the cpk mouse. J Am Soc Nephrol 16:905–916. 10.1681/ASN.2004121083 [DOI] [PubMed] [Google Scholar]
- 113.Upadhya P, Churchill G, Birkenmeier EH, Barker JE, Frankel WN (1999) Genetic modifiers of polycystic kidney disease in intersubspecific KAT2J mutants. Genomics 58:129–137. 10.1006/geno.1999.5830 [DOI] [PubMed] [Google Scholar]
- 114.Olson RJ, Hopp K, Wells H, Smith JM, Furtado J, Constans MM, Escobar DL, Geurts AM, Torres VE, Harris PC (2019) Synergistic genetic interactions between pkhd1 and pkd1 result in an arpkd-like phenotype in murine models. J Am Soc Nephrol 30:2113–2127. 10.1681/ASN.2019020150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Garcia-Gonzalez MA, Menezes LF, Piontek KB, Kaimori J, Huso DL, Watnick T, Onuchic LF, Guay-Woodford LM, Germino GG (2007) Genetic interaction studies link autosomal dominant and recessive polycystic kidney disease in a common pathway. Hum Mol Genet 16:1940–1950. 10.1093/hmg/ddm141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Guay-Woodford LM, Bissler JJ, Braun MC, Bockenhauer D, Cadnapaphornchai MA, Dell KM, Kerecuk L, Liebau MC, Alonso-Peclet MH, Shneider B, Emre S, Heller T, Kamath BM, Murray KF, Moise K, Eichenwald EE, Evans J, Keller RL, Wilkins-Haug L, Bergmann C, Gunay-Aygun M, Hooper SR, Hardy KK, Hartung EA, Streisand R, Perrone R, Moxey-Mims M (2014) Consensus expert recommendations for the diagnosis and management of autosomal recessive polycystic kidney disease: report of an international conference. J Pediatr 165:611–617. 10.1016/j.jpeds.2014.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Szabó T, Orosz P, Balogh E, Jávorszky E, Máttyus I, Bereczki C, Maróti Z, Kalmár T, Szabó AJ, Reusz G, Várkonyi I, Marián E, Gombos É, Orosz O, Madar L, Balla G, Kappelmayer J, Tory K, Balogh I (2018) Comprehensive genetic testing in children with a clinical diagnosis of ARPKD identifies phenocopies. Pediatr Nephrol 33:1713–1721. 10.1007/s00467-018-3992-5 [DOI] [PubMed] [Google Scholar]
- 118.Guay-Woodford LM, Desmond RA (2003) Autosomal recessive polycystic kidney disease: the clinical experience in North America. Pediatrics 111:1072–1080 [DOI] [PubMed] [Google Scholar]
- 119.Furth SL, Pierce C, Hui WF, White CA, Wong CS, Schaefer F, Wühl E, Abraham AG, Warady BA, Samuels J, Furth S, Atkinson M, Wilson A, Quiroga A, Massengill S, Selewski D, Ferris M, Kogon A, Kaskel F, Lande M, Schwartz G, Saland J, Norwood V, Matoo T, Hidalgo G, Srivaths P, Carlson J, Langman C, Mendley S, John E, Upadhyay K, Seo-Mayer P, Patterson L, Parekh R, Robinson L, Weinstein A, Samsonov D, Kupferman J, Misurac J, Mongia A, Kiessling S, Sanchez-Kazi C, Dart A, Fathallah S, Claes D, Mitsnefes M, Blydt-Hansen T, Warady B, Greenbaum L, Flynn J, Wong C, Salusky I, Yadin O, Dell K, Jenkins R, Pan C, Ku E, Al-Uzri A, Jenkins R, Rodig N, Wong C, Davis K, Turman M, Bartosh S, Hastings C, Nayak A, Seikaly M, Benador N, Mak R, Wood E, Jenkins R, Lerner G, Barletta GM, Anarat A, Bakkaloglu A, Ozaltin F, Peco-Antic A, Querfeld U, Gellermann J, Sallay P, Drożdż D, Bonzel KE, Wingen AM, Żurowska A, Balasz I, Trivelli A, Perfumo F, Müller-Wiefel DE, Möller K, Offner G, Enke B, Wühl E, Hadtstein C, Mehls O, Schaefer F, Emre S, Caliskan S, Mir S, Wygoda S, Hohbach-Hohenfellner K, Jeck N, Klaus G, Ardissino G, Testa S, Montini G, Charbit M, Niaudet P, Caldas-Afonso A, Fernandes-Teixeira A, Dušek J, Matteucci MC, Picca S, Mastrostefano A, Wigger M, Berg UB, Celsi G, Fischbach M, Terzic J, Fydryk J, Urasinski T, Coppo R, Peruzzi L, Arbeiter K, Jankauskiené A, Grenda R, Litwin M, Janas R, Neuhaus TJ (2018) Estimating Time to ESRD in Children With CKD. Am J Kidney Dis 71:783–792. 10.1053/j.ajkd.2017.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dell KM, Matheson M, Hartung EA, Warady BA, Furth SL (2016) Kidney Disease Progression in Autosomal Recessive Polycystic Kidney Disease. J Pediatr 171:196–201.e1. 10.1016/j.jpeds.2015.12.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.ESCAPE Trial Group, Wühl E, Trivelli A, Picca S, Litwin M, Peco-Antic A, Zurowska A, Testa S, Jankauskiene A, Emre S, Caldas-Afonso A, Anarat A, Niaudet P, Mir S, Bakkaloglu A, Enke B, Montini G, Wingen A-M, Sallay P, Jeck N, Berg U, Caliskan S, Wygoda S, Hohbach-Hohenfellner K, Dusek J, Urasinski T, Arbeiter K, Neuhaus T, Gellermann J, Drozdz D, Fischbach M, Möller K, Wigger M, Peruzzi L, Mehls O, Schaefer F (2009) Strict blood-pressure control and progression of renal failure in children. N Engl J Med 361:1639–1650. 10.1056/NEJMoa0902066 [DOI] [PubMed] [Google Scholar]
- 122.Zerres K, Rudnik-Schoneborn S, Deget F, Holtkamp U, Brodehl J, Geisert J, Scharer K (1996) Autosomal recessive polycystic kidney disease in 115 children: clinical presentation, course and influence of gender. Arbeitsgemeinschaft fur Padiatrische, Nephrologie. Acta Paediatr 85:437–445 [DOI] [PubMed] [Google Scholar]
- 123.Serai SD, Otero HJ, Calle-Toro JS, Berman JI, Darge K, Hartung EA (2019) Diffusion tensor imaging of the kidney in healthy controls and in children and young adults with autosomal recessive polycystic kidney disease. Abdom Radiol 44:1867–1872. 10.1007/s00261-019-01933-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Erokwu BO, Anderson CE, Flask CA, Dell KM (2018) Quantitative magnetic resonance imaging assessments of autosomal recessive polycystic kidney disease progression and response to therapy in an animal model. Pediatr Res 83:1067–1074. 10.1038/pr.2018.24 [DOI] [PubMed] [Google Scholar]
- 125.MacAskill CJ, Erokwu BO, Markley M, Parsons A, Farr S, Zhang Y, Tran U, Chen Y, Anderson CE, Serai S, Hartung EA, Wessely O, Ma D, Dell KM, Flask CA (2020) Multi-parametric MRI of kidney disease progression for autosomal recessive polycystic kidney disease: mouse model and initial patient results. Pediatr Res. 10.1038/s41390-020-0883-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gunay-Aygun M, Font-Montgomery E, Lukose L, Tuchman Gerstein M, Piwnica-Worms K, Choyke P, Daryanani KT, Turkbey B, Fischer R, Bernardini I, Sincan M, Zhao X, Sandler NG, Roque A, Douek DC, Graf J, Huizing M, Bryant JC, Mohan P, Gahl WA, Heller T (2013) Characteristics of congenital hepatic fibrosis in a large cohort of patients with autosomal recessive polycystic kidney disease. Gastroenterology 144:112–121.e2. 10.1053/j.gastro.2012.09.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wicher D, Jankowska I, Lipiński P, Szymańska-Rożek P, Kmiotek J, Jańczyk W, Rubik J, Chrzanowska K, Socha P (2019) Transient Elastography for Detection of Liver Fibrosis in Children With Autosomal Recessive Polycystic Kidney Disease. Front Pediatr 6:422. 10.3389/fped.2018.00422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hartung EA, Wen J, Poznick L, Furth SL, Darge K (2019) Ultrasound Elastography to Quantify Liver Disease Severity in Autosomal Recessive Polycystic Kidney Disease. J Pediatr. 10.1016/j.jpeds.2019.01.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hartung EA, Calle-Toro JS, Lopera CM, Wen J, Carson RH, Dutt M, Howarth K, Furth SL, Darge K, Serai SD (2020) Magnetic resonance elastography to quantify liver disease severity in autosomal recessive polycystic kidney disease. Abdom Radiol. 10.1007/s00261-020-02694-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Goto M, Hoxha N, Osman R, Dell KM (2010) The renin-angiotensin system and hypertension in autosomal recessive polycystic kidney disease. Pediatr Nephrol 25:2449–2457. 10.1007/s00467-010-1621-z [DOI] [PubMed] [Google Scholar]
- 131.Goto M, Hoxha N, Osman R, Wen J, Wells RG, Dell KM (2010) Renin-angiotensin system activation in congenital hepatic fibrosis in the PCK rat model of autosomal recessive polycystic kidney disease. J Pediatr Gastroenterol Nutr 50:639–644. 10.1097/MPG.0b013e3181cc80e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yoshihara D, Kugita M, Sasaki M, Horie S, Nakanishi K, Abe T, Aukema HM, Yamaguchi T, Nagao S (2013) Telmisartan ameliorates fibrocystic liver disease in an orthologous rat model of human autosomal recessive polycystic kidney disease. PLoS One 8:e81480. 10.1371/journal.pone.0081480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nagao S, Nishii K, Katsuyama M, Kurahashi H, Marunouchi T, Takahashi H, Wallace DP (2006) Increased water intake decreases progression of polycystic kidney disease in the PCK rat. J Am Soc Nephrol 17:2220–2227. 10.1681/ASN.2006030251 [DOI] [PubMed] [Google Scholar]
- 134.Wang X, Wu Y, Ward CJ, Harris PC, Torres VE (2008) Vasopressin directly regulates cyst growth in polycystic kidney disease. J Am Soc Nephrol 19:102–108. 10.1681/ASN.2007060688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gattone VH, Wang X, Harris PC, Torres VE (2003) Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med 9:1323–1326. 10.1038/nm935 [DOI] [PubMed] [Google Scholar]
- 136.Wang X, Gattone V, Harris PC, Torres VE (2005) Effectiveness of vasopressin V2 receptor antagonists OPC-31260 and OPC-41061 on polycystic kidney disease development in the PCK rat. J Am Soc Nephrol 16:846–851. 10.1681/ASN.2004121090 [DOI] [PubMed] [Google Scholar]
- 137.Wang X, Constans MM, Chebib FT, Torres VE, Pellegrini L (2019) Effect of a Vasopressin V2 Receptor Antagonist on Polycystic Kidney Disease Development in a Rat Model. Am J Nephrol 49:487–493. 10.1159/000500667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mancinelli R, Franchitto A, Glaser S, Vetuschi A, Venter J, Sferra R, Pannarale L, Olivero F, Carpino G, Alpini G, Onori P, Gaudio E (2016) Vasopressin regulates the growth of the biliary epithelium in polycystic liver disease. Lab Investig 96:1147–1155. 10.1038/labinvest.2016.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kaimori J ya, Lin CC, Outeda P, Garcia-Gonzalez MA, Menezes LF, Hartung EA, Li A, Wu G, Fujita H, Sato Y, Nakanuma Y, Yamamoto S, Ichimaru N, Takahara S, Isaka Y, Watnick T, Onuchic LF, Guay-Woodford LM, Germino GG (2017) NEDD4-family E3 ligase dysfunction due to PKHD1/Pkhd1 defects suggests a mechanistic model for ARPKD pathobiology. Sci Rep 7:7733. 10.1038/s41598-017-08284-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Olteanu D, Yoder BK, Liu W, Croyle MJ, Welty EA, Rosborough K, Wyss JM, Bell PD, Guay-Woodford LM, Bevensee MO, Satlin LM, Schwiebert EM (2006) Heightened epithelial Na+ channel-mediated Na+ absorption in a murine polycystic kidney disease model epithelium lacking apical monocilia. Am J Physiol Physiol 290:C952–963. 10.1152/ajpcell.00339.2005 [DOI] [PubMed] [Google Scholar]
- 141.Pavlov TS, Levchenko V, Ilatovskaya DV., Palygin O, Staruschenko A (2015) Impaired epithelial Na + channel activity contributes to cystogenesis and development of autosomal recessive polycystic kidney disease in PCK rats. Pediatr Res 77:64–69. 10.1038/pr.2014.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ilatovskaya DV., Levchenko V, Pavlov TS, Isaeva E, Klemens CA, Johnson J, Liu P, Kriegel AJ, Staruschenko A (2019) Salt-deficient diet exacerbates cystogenesis in ARPKD via epithelial sodium channel (ENaC). EBioMedicine 40:663–674. 10.1016/j.ebiom.2019.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Masyuk TV, Radtke BN, Stroope AJ, Banales JM, Gradilone SA, Huang B, Masyuk AI, Hogan MC, Torres VE, Larusso NF (2013) Pasireotide is more effective than octreotide in reducing hepatorenal cystogenesis in rodents with polycystic kidney and liver diseases. Hepatology 58:409–421. 10.1002/hep.26140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sweeney WE, Avner ED (2014) Pathophysiology of childhood polycystic kidney diseases: new insights into disease-specific therapy. Pediatr Res 75:148–157. 10.1038/pr.2013.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sweeney WE, Frost P, Avner ED (2017) Tesevatinib ameliorates progression of polycystic kidney disease in rodent models of autosomal recessive polycystic kidney disease. World J Nephrol 6:188. 10.5527/wjn.v6.i4.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.A Safety, Pharmacokinetic, Single Ascending Dose Study of Tesevatinib in Pediatric Subjects With Autosomal Recessive Polycystic Kidney Disease (ARPKD). In: ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03096080 [Google Scholar]
- 147.Ebner K, Feldkoetter M, Ariceta G, Bergmann C, Buettner R, Doyon A, Duzova A, Goebel H, Haffner D, Hero B, Hoppe B, Illig T, Jankauskiene A, Klopp N, König J, Litwin M, Mekahli D, Ranchin B, Sander A, Testa S, Weber LT, Wicher D, Yuzbasioglu A, Zerres K, Dötsch J, Schaefer F, Liebau MC; ESCAPE Study Group; GPN Study Group (2015) Rationale, design and objectives of ARegPKD, a European ARPKD registry study. BMC Nephrol 16:22. 10.1186/s12882-015-0002-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Forrest CB, Margolis PA, Bailey LC, Marsolo K, Del Beccaro MA, Finkelstein JA, Milov DE, Vieland VJ, Wolf BA, Yu FB, Kahn MG (2014) PEDSnet: a National Pediatric Learning Health System. J Am Med Informatics Assoc 21:602–606. 10.1136/amiajnl-2014-002743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Smith KA, Thompson AM, Baron DA, Broadbent ST, Lundstrom GH, Perrone RD (2019) Addressing the Need for Clinical Trial End Points in Autosomal Dominant Polycystic Kidney Disease: A Report From the Polycystic Kidney Disease Outcomes Consortium (PKDOC). Am J Kidney Dis 73:533–541. 10.1053/j.ajkd.2018.11.001 [DOI] [PubMed] [Google Scholar]