Abstract
We investigated the structural characteristics and antioxidant activities of two types of neutral polysaccharides and two types of acidic polysaccharides from Stropharia rugosoannulata under different drying methods. Fresh S. rugosoannulata were processed with freeze-vacuum drying (FVD) and hot-air drying (HAD). Polysaccharides from the dried S. rugosoannulata (SRP) were purified using a DEAE-52 cellulose column to obtain two types of neutral SRPs (FSRP-1 and HSRP-1) and two types of acidic SRPs (FSRP-2 and HSRP-2). We found that drying can affect the structural characteristics and antioxidant activities of SRPs. Varied monosaccharide compositions were found in FSRP-1, FSRP-2, HSRP-1 and HSRP-2, and HAD-treated SRP had more glucose and less galactose. The (1 → 6)-α-D-Galp linkage was the primary chain in FSRP-1 and HSRP-1, whereas the (1 → 3)-β-D-Glcp was the backbone structure in FSRP-2 and HSRP-2. Our results thus suggest that hot air drying changed the β-configuration in polysaccharides. FSRP-1, FSRP-2, HSRP-1 and HSRP-2 had positive ferric ion reducing antioxidant power and scavenging activities on ABTS+ and hydroxyl radicals, whereas HSRP exhibited a stronger antioxidant activity than that of FSRP. Hot-air dried S. rugosoannulata could therefore be recommended as a suitable candidate for use in the preparation of antioxidant polysaccharides as functional foods.
Keywords: Stropharia rugosoannulata, Polysaccharides, Drying, Characterization, Antioxidant activity
Introduction
Stropharia rugosoannulata is a type of valuable edible mushroom that is recommended by the United Nations Food and Agriculture Organization (FAO) for cultivation in developing countries (Song et al. 2009). S. rugosoannulata is a grass-rotting edible mushroom that has been rapidly cultivated in China in recent years. S. rugosoannulata has high nutritional value and pharmacological activities (Yan et al. 2004). In addition, it has been reported that S. rugosoannulata confers medicinal benefits such as bacteriostatic activity, antitumor and antioxidant activity and reducing endoplasmic-reticulum (ER) stress (Wu et al. 2013, 2012; Luo et al. 2006). The functional activities of S. rugosoannulata are related to its chemical compounds including polysaccharides, steroids, flavone and lectins (Zhang et al. 2014; Wu et al. 2011; Yan et al. 2020). Among these compounds, polysaccharide is the primary bioactive component, and many types of bioactivity polysaccharides have been separated from S. rugosoannulata (Liu et al. 2020a).
However, fresh S. rugosoannulata mushrooms are highly perishable due to their high water content and high respiration rate. A drying process is often used to reduce the moisture content of S. rugosoannulata in order to extend its shelf life. Among various drying techniques, hot air drying is one of the most popular and frequently used drying methods for fruits and vegetables dehydration (Yu et al. 2020). With the sublimation of ice from frozen products, freeze-vacuum drying minimizes losses of flavor and nutritional composition and keeps the organoleptic properties of the initial fresh products due to the absence of liquid water and the low temperature used in the dehydration process, which hinder most of deterioration and microbiological reactions (Rezvankhah et al. 2020). So in current work, hot air drying was employed to dry S. rugosoannulata and compared with freeze dried samples. Freeze-vacuum and hot-air drying techniques are widely used to produce dried mushrooms, and it has been reported that the bioactivities of polysaccharides were influenced by drying methods (Ahmadi et al. 2019). For example, polysaccharides from Inonotus obliquus mushroom dried with the freeze drying method are reported to have better antioxidant ability (Ma et al. 2013). Moreover, Liu et al. (Liu et al. 2020b) reported that polysaccharides from freeze dried shiitake mushroom showed stronger antioxidant activities, and polysaccharides from hot-air dried mushrooms exhibited higher immunomodulatory activity. However, the effects of drying on the structural characteristics and antioxidant activities of polysaccharides from S. rugosoannulata are unknown.
The objective of this study was thus to investigate the impact of different drying methods on the structural properties and antioxidant activities of SRPs. The antioxidant activities of SRPs were determined by ferric ion reducing antioxidant power (FRAP) and scavenging capacities against ABTS+ and hydroxyl radicals.
Materials and methods
Dried S. rugosoannulata preparation
Fresh S. rugosoannulata mushrooms purchased from Chengdu (Sichuan Province, China) were separately dehydrated by freeze-vacuum drying and hot-air drying processes. One group of mushroom samples was treated with a freeze dryer (SCIENTZ-30ND, Ningbo Xinzhi Instrument Factory, Ningbo, China) for 48 h, while the other group of samples was prepared using a drying oven (Changzhou Yineng Instrument Factory, Changzhou, China) for 6 h at 50 °C. After the drying process, the two types of dried mushrooms had a moisture content of approximately 10% in wet basis.
Extraction and purification of SRPs
The dried mushrooms were treated with petroleum ether to remove any crude fat. 500 mL of distilled water was then added to 20 g of defatted sample for the extraction of polysaccharides using the microwave assisted extraction method (Liu et al. 2016). Next, the polysaccharide was precipitated with anhydrous ethanol and deproteinated by the Sevag method. Finally, the crude polysaccharide samples were obtained by lyophilization for 48 h.
The crude polysaccharide (200 mg) was dissolved in deionized water and subjected to a DEAE-52 column (3.6 × 20 cm) (Liu et al. 2020b). The column was then stepwise eluted with deionized water and a 0.3 M sodium chloride solution. According to the absorbance of 490 nm (DuBois et al. 1956), the first fraction eluted with distilled water was collected followed by collection of the second fraction that was eluted with 0.3 M NaCl. Finally, the obtained polysaccharides samples were dialyzed for 24 h and freeze-dried for further study.
Monosaccharide analysis
FSRP-1, FSRP-2, HSRP-1 and HSRP-2 were hydrolyzed for the measurement of monosaccharide compositions using our reported method (Liu et al. 2020a). Briefly, each sample (10 mg) was hydrolyzed for 4 h with 4.0 M trifluoroacetic acid at 120 °C. Next, 1-phenyl-3-methyl-5-pyrazolone (PMP) was added to react with the hydrolysate. The derivatives of monosaccharides were then determined by a high performance liquid chromatography system (HPLC, Agilent, United States) with a C18 column (SHISEIDO, 4.6 mm × 250 mm × 5 um) at a wavelength of 245 nm. Solvent A was phosphoric solution (0.1 M) with a pH value of 6.9, and solvent B was acetonitrile. The ratio of solvent A to B was 0.82: 0.18 with a flow rate of 1.0 mL/min.
Fourier-transform infrared spectra (FT-IR) analysis
FSRP-1, FSRP-2, HSRP-1 and HSRP-2 were mixed with potassium bromide powder to prepare pellets for the detection of FT-IR spectra using a Nicolet Nexus 470 spectrometer (Thermo Nicolet, United States). The spectra of SRPs were recorded in the range of 4000–400 cm−1 (Liu et al. 2016).
NMR spectra analysis
FSRP-1, FSRP-2, HSRP-1 and HSRP-2 were dissolved in D2O for the detection of 1H NMR and 13C NMR spectra using a Bruker Avance Neo NMR spectrometer (Bruker Corporation, United States) at 600 MHz (Liu et al. 2020b). The spectra data were recorded at 25 °C by standard Bruker software.
Antioxidant activity evaluation
ABTS+ radical scavenging capacity assay
The scavenging capacities of FSRP-1, FSRP-2, HSRP-1 and HSRP-2 on ABTS•+ were determined with our reported method (Liu et al. 2020b). The ABTS•+ solution (180 μL) was first mixed with various concentrations of sample solutions (20 μL). The mixed solution was kept at room temperature for 5 min and then the absorbance was assayed at 734 nm. The scavenging capacity of SRPs on ABTS•+ was measured using a scavenging rate (%) with the Eq. (1):
| 1 |
Here, the absorbance of the control (deionized water) is A0, the absorbance of the ABTS•+ sample is A1, and the absorbance of the blank sample (without ABTS•+) is A2.
Hydroxyl Radical Scavenging Capacity Assay
The scavenging capacities of FSRP-1, FSRP-2, HSRP-1 and HSRP-2 on hydroxyl radical were assayed using the method reported by Jiang et al. (2014). Various concentrations of the sample solution (50 μL) were mixed with FeSO4 (50 μL 9 mM), salicylic acid (50 μL 9 mM), and H2O2 (50 μL 20 mM). Next, the reaction was kept at 37 °C for 1 h and the absorbance was measured at 510 nm. The scavenging capacity of SRPs on hydroxyl radicals was determined as a scavenging rate (%) with the Eq. (2):
| 2 |
Here, the absorbance of the control (deionized water) is A0, the absorbance of sample and hydroxyl radical is A1, and the absorbance of the sample blank (without hydroxyl radical) is A2.
Ferric ion reducing antioxidant power (FRAP) analysis
The FRAP values of FSRP-1, FSRP-2, HSRP-1 and HSRP-2 were measured using a reported method (Benzie and Strain 1996). First, 10 mM of 2,4,6-Tris (2-pyridyl)-s-triazime (TPTZ) solution, 20 mM FeCl3 solution, and 300 mM acetate buffer (pH 3.6) were mixed at a volume ratio of 10:1:1 to prepare the FRAP working solution. Next, the FRAP working solution (180 μL) was mixed with 5 μL sample solution and incubated for 5 min at room temperature, and various concentrations of FeSO4 were used as the control group. The incubation solution was then determined at an absorbance of 593 nm. The FRAP values of the four types of SRPs were expressed as equivalent concentrations of FeSO4 (mM Fe2+/mg).
Statistical analysis
All analyses were performed in triplicate, and the results were given as mean ± standard deviation. The variance analysis was performed using one-way analysis of variance with an assay of differences (Duncan’s test, P < 0.05).
Results and discussion
Purification of SRPs
The crude polysaccharide from S. rugosoannulata was purified using a DEAE-52 cellulose column based on the acidic group levels in samples (Liu et al. 2016). As shown in Fig. 1, two main polysaccharide fractions from FVD treated mushrooms were separately collected with deionized water and 0.3 M of NaCl solution. Similar results were also reported in the purification of polysaccharides from four kinds of mushrooms (Yan et al. 2019). The neutral polysaccharide fraction eluted with distilled water was named FSRP-1. However, the acidic polysaccharide fraction eluted by 0.3 M NaCl solution was named FSRP-2 (Fan et al. 2012a). A similar result for HAD treated mushrooms is shown in Fig. 1. The two separated fractions named HSRP-1 and HSRP-2 were collected. Next, through concentration, dialysis and lyophilization, the four types of SRPs including FSRP-1, FSRP-2, HSRP-1 and HSRP-2 were obtained for further study.
Fig. 1.
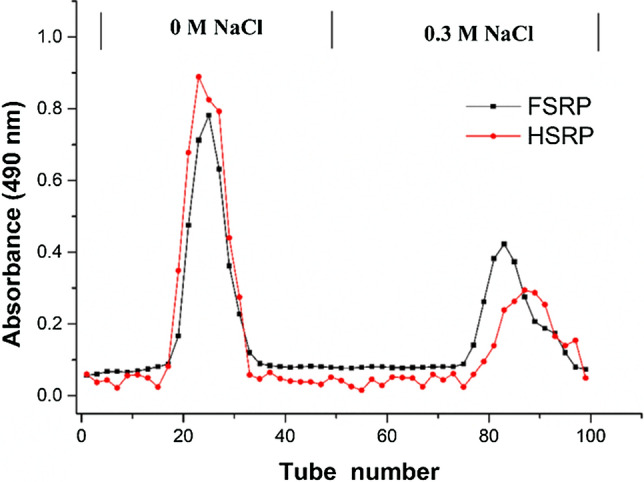
Elution profile of SRP on DEAE-52 chromatography column with gradient of NaCl solution (0 and 0.3 M)
Monosaccharide compositions analysis of SRPs
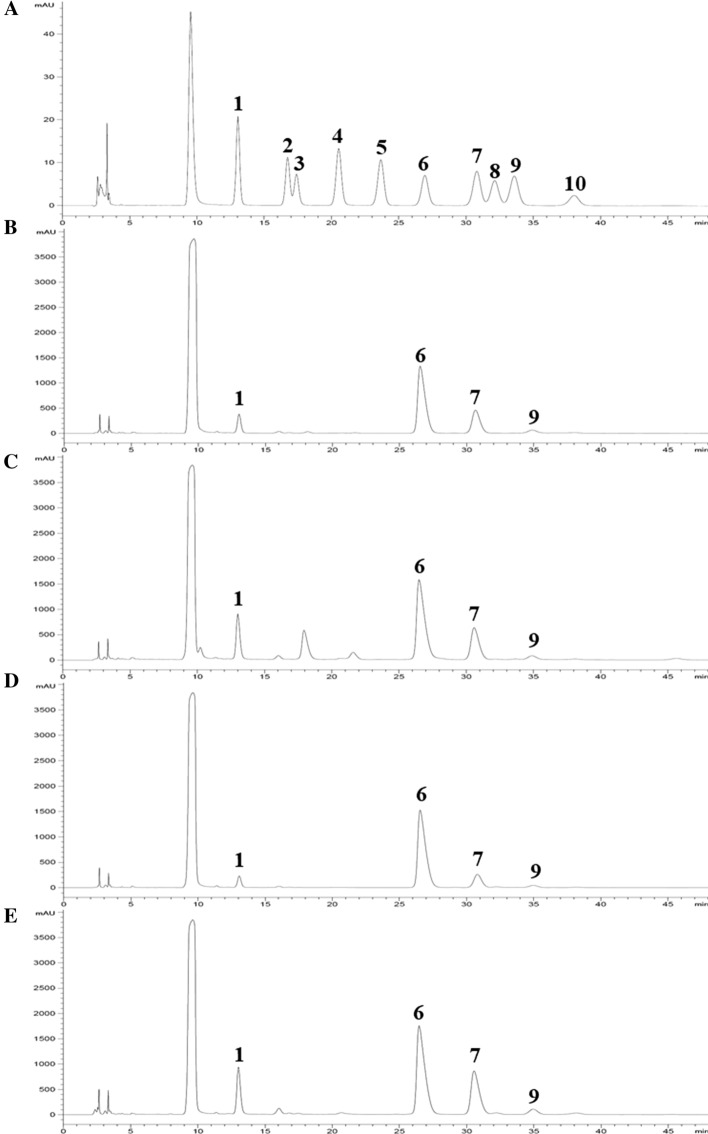
The HPLC profile of the standard monosaccharides and hydrolyzed FSRP-1, FSRP-2, HSRP-1 and HSRP-2 are shown in Fig. 2. Four kinds of monosaccharides including the mannose (tR = 13.072 min), glucose (tR = 26.546 min), galactose(tR = 30.811 min) and arabinose (tR = 34.985 min) were detected in the four types of SRPs. The results indicated that FSRP-1, FSRP-2, HSRP-1 and HSRP-2 were all heteropolysaccharides. Liu et al. (2020a) also determined that glucose, galactose and mannose were the primary monosaccharide components in the SRPs. In addition, the monosaccharide compositions of mushroom polysaccharides were mainly reported to be glucose, galactose and mannose (Morales et al. 2019).
Fig. 2.
HPLC chromatogram of standard monosaccharides (a) and hydrolyzed FSRP-1 (b), FSRP-2 (c), HSRP-1 (c) and HSRP-2 (e). Peak identities: Mannose (1), Ribose (2), Rhamnose (3), Glucuronic acid (4), Galacturonic acid (5), Glucose (6), Galactose (7), Xylose (8), Arabinose (9), and Fucose (10)
As shown in Table 1, the dominant monosaccharide of the four types of SRPs was all glucose. However, FSRP-1, FSRP-2, HSRP-1 and HSRP-2 had different molar ratios for the four kinds of monosaccharides. FSRP-1 and HSRP-1 (neutral polysaccharides) had molar ratios of 1:7.70:25.39:2.46 and 1:4.34:31.02:1.53, respectively.The molar ratios in FSRP-2 and HSRP-2 (acidic polysaccharides) were 1: 6.20:19.19:3.55 and 1:5.63:13.79: 0.37, respectively. A decrease in glucose and an increase in mannose levels were found in the acidic polysaccharides. Compared with the FVD treatment, higher levels of glucose and lower levels of galactose were found in the HAD treated SRPs. Similar results were also reported in bamboo polysaccharides (Chen et al. 2019). The degradation of galactose might be related to the higher temperature or oxygen content in the HAD process. Our results indicated that the monosaccharide ratios of polysaccharides were influenced by drying pretreatments. The monosaccharide compositions in other mushroom polysaccharides were also reported to be changed by drying methods. Ma et al. (2013) also reported that the treatments of freeze drying, hot air drying and vacuum drying changed the monosaccharide (rhamnose, arabinose, mannose, galactose and glucose) compositions of polysaccharides from Inonotus obliquus mushroom. Due to the different modes of water loss in S. rugosoannulata during FD (sublimation) and HD (evaporation) process, the bound water of the SRPs may be different (Huang et al. 2021a), which may affect the monosaccharide compositions.
Table 1.
Monosaccharide composition of polysaccharides from dried S. rugosoannulata
| Sugar components (molar ratio) | Polysaccharide samples | |||
|---|---|---|---|---|
| FSRP-1 | FSRP-2 | HSRP-1 | HSRP-2 | |
| Arabinose | 1 | 1 | 1 | 1 |
| Galactose | 7.70 | 6.20 | 4.34 | 5.63 |
| Glucose | 25.39 | 19.19 | 31.02 | 13.79 |
| Mannose | 2.46 | 3.55 | 1.53 | 2.37 |
FTIR spectroscopy measurements of SRPs
The infrared spectra of FSRP-1, FSRP-2, HSRP-1 and HSRP-2 are shown in Fig. 3. The absorption peaks at 3410 cm−1 were related to the hydroxyl groups in SRPs. The two peaks of 2925 cm−1 and 2360 cm−1 were due to the C-H stretching vibrations in SRPs (Chen and Huang 2019). The broad absorption bands with strong intensities at 1640 cm−1 were shown for C = O (Yang et al. 2020), and the weak peaks around 1540 cm−1 indicated that FSRP-1, FSRP-2, and HSRP-1 had combined proteins. The C-O stretching vibration of carboxyl groups was shown at about 1421 cm−1. The vibration of S = O shown at 1150 cm−1 was the presence of sulfates in FSRP-1, FSRP-2 and HSRP-1 (Wang et al. 2018). The two peaks around 1077 cm−1 and 1045 cm−1 were due to the galactose and glucose in the SRPs. This result was consistent with the monosaccharide compositions analysis (Table 1). Yang et al. (2018) also reported that the band at approximately 1070 cm−1 showed the presence of galactose. The diagnostic absorption peak at about 803 cm−1 would suggest that FSRP-2 and HSRP-1 had α-type glycosidic linkages of galactose (Shi et al. 2017). Based on the FT-IR analysis, the preliminary structures of SRPs were not changed by different drying methods. Similar results of polysaccharides from Hohenbuehelia serotina (Li et al. 2016) and Medicago sativa L. (Shang et al. 2021) were also reported.
Fig. 3.
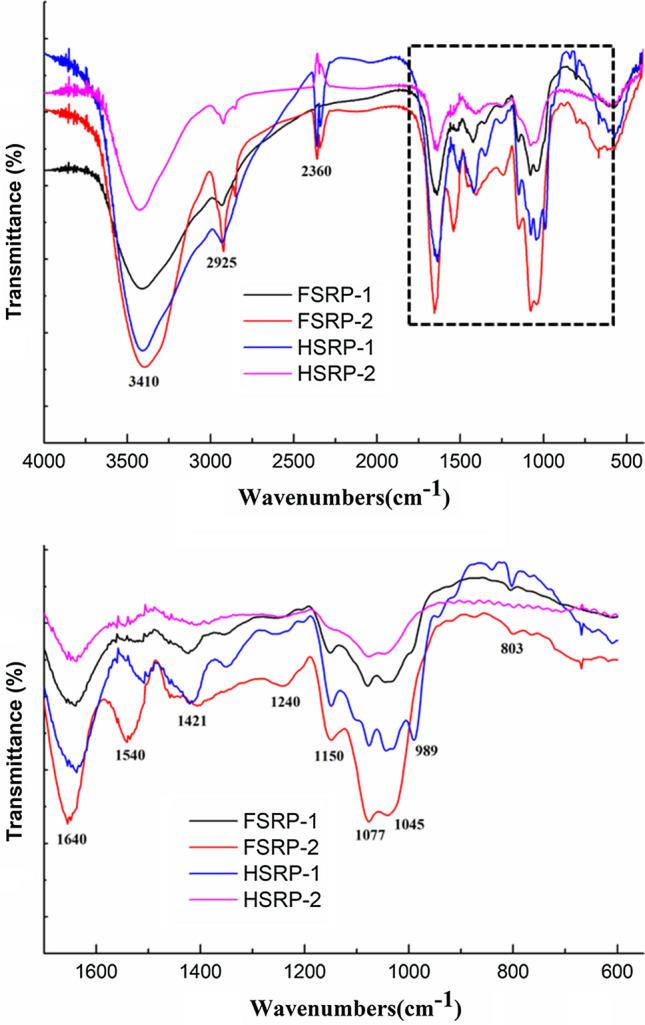
FT-IR spectrum of FSRP-1, FSRP-2, HSRP-1, and HSRP-2
NMR Spectroscopy Analysis of SRPs
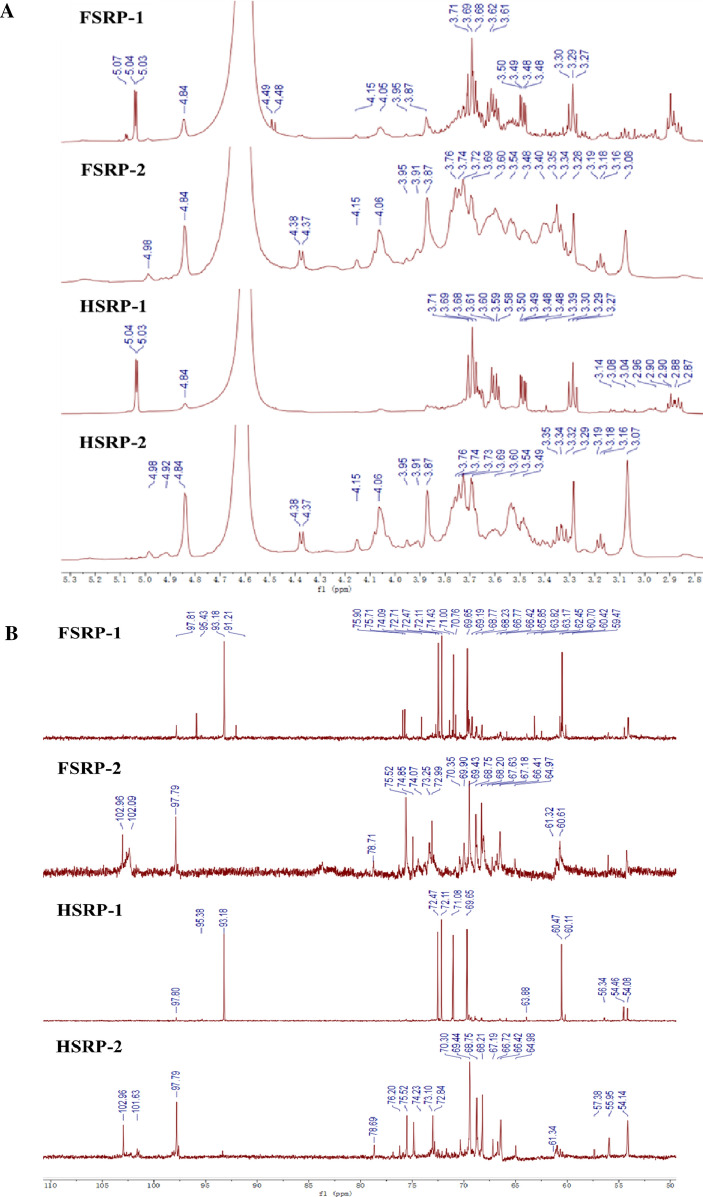
The NMR technique has been utilized in food systems to determine the chemical structure of polysaccharides (Yang and Yang 2020; Pizzoferrato et al. 2000). Figure 4 shows the 1H NMR and 13C NMR spectra of FSRP-1, FSRP-2, HSRP-1 and HSRP-2. The typical peak distributions of polysaccharides (δH3.4–5.4 ppm and δC 60–110 ppm) were found in SRPs (Wang et al. 2014). The strong signals in the δH5.04, 5.03 ppm and δC93.18 ppm regions of the FSRP-1 were due to the anomeric protons of α-D-Galp, whereas the β-D-Glcp generally occurred at the δ4.84, 4.49, and 4.48 ppm shifts. HSRP-1 was detected to have three chemical shifts of δ5.04, 5.03 and 4.84 ppm, but the signals at δ4.49 and 4.48 ppm were not determined, indicating that the HSRP-1 had lower levels of β-D-Glcp than that of the FSRP-1. Huang et al. (2021b) also used the corresponding areas in the NMR spectrum to determine the levels of β-D-glucose. The signals in the δC72.47, 72.11, 71.08, 69.65, and 60.47 ppm suggested that the primary chain in the both of neutral SRPs was (1 → 6)-α-D-Galp linkage (Maity et al. 2013). Moreover, FSRP-1 had side both chains of (1 → 6)-β-D-Glcp and (1 → 3)-β-D-Glcp, whereas HSRP-1 only had side chain of (1 → 3)-β-D-Glcp. After hot-air drying, the increased ratio of 1,3-link residues may be due to the fact that 1,3-links are more likely to formed during the high temperature drying process than 1,6-links (Gan et al. 2021). The α type of glycoside was also previously reported in mushroom polysaccharides (Tang et al. 2020). For FSRP-2, the strong signals at about δH4.84 ppm and δC102.96 ppm showed the β-D- Glcp, and the weak signal at δH4.98 ppm showed the α-D-Galp, whereas the β-D- Glcp was the dominant glycoside bond. Similar 1H NMR and 13C NMR chemical shifts were also determined in HSRP-2, while the signal at δ4.84 ppm was stronger than that of FSRP-2, indicating that the HSRP-2 had higher levels of β-D-Glcp. The signals in the δC75.52, 72.99, 69.43, 68.20, and 60.61 ppm suggest that the (1 → 3)-β-D-Glcp linkage was the primary chain in the acidic polysaccharides (FSRP-2 and HSRP-2), and both had side chains of (1 → 4)-α-D-Galp. In general, our results suggested that food drying pretreatments could chang the sugar configurations of SRPs. After the hot-air drying process, the neutral polysaccharides had a higher β-configuration level, whereas the acidic polysaccharides had a lower one. The results were in agreement with Gan et al. (2021), who reported that the drying process affected the configurations of longan polysaccharides.
Fig. 4.
1H NMR (a) and 13C NMR (b) spectra of FSRP-1, FSRP-2, HSRP-1, and HSRP-2
Effects of drying on antioxidant activities of SRPs
ABTS+ radical scavenging activity of SRPs
The ABTS•+ scavenging abilities of FSRP-1, FSRP-2, HSRP-1 and HSRP-2 are shown in Fig. 5a. The scavenging rates of FSRP-1, FSRP-2, HSRP-1 and HSRP-2 reached 84.62%, 79.01%, 83.86.52%, and 99.70%, respectively, at a concentration of 5 mg/mL. The results indicated that the four types of polysaccharides had positive ABTS•+ scavenging abilities. Among the four types of SRPs, HSRP-2 had the highest ABTS•+ scavenging activity which might be due to its higher level of β-D-Glcp compared to that of the other SRPs. It has also been reported that the scavenging capacities of polysaccharides were affected by the glucoside bond type (Shi et al. 2013). The IC50 values of FSRP-1, FSRP-2, HSRP-1 and HSRP-2 were determined at 1.02, 1.38, 0.89 and 0.93 mg/mL, respectively. The results indicated that HAD treated samples had stronger scavenging abilities than the samples treated by FVD. We suggested that the scavenging activity of SRPs on ABTS•+ was affected by drying pretreatments, and the hot-air drying process would thus improve the ABTS•+ scavenging abilities of SRPs. However, Wu et al. (2014) reported that polysaccharides from Agaricus blazei Murrill obtained by FD method showed higher scavenging activity on ABTS radicals than that of HD method.
Fig. 5.
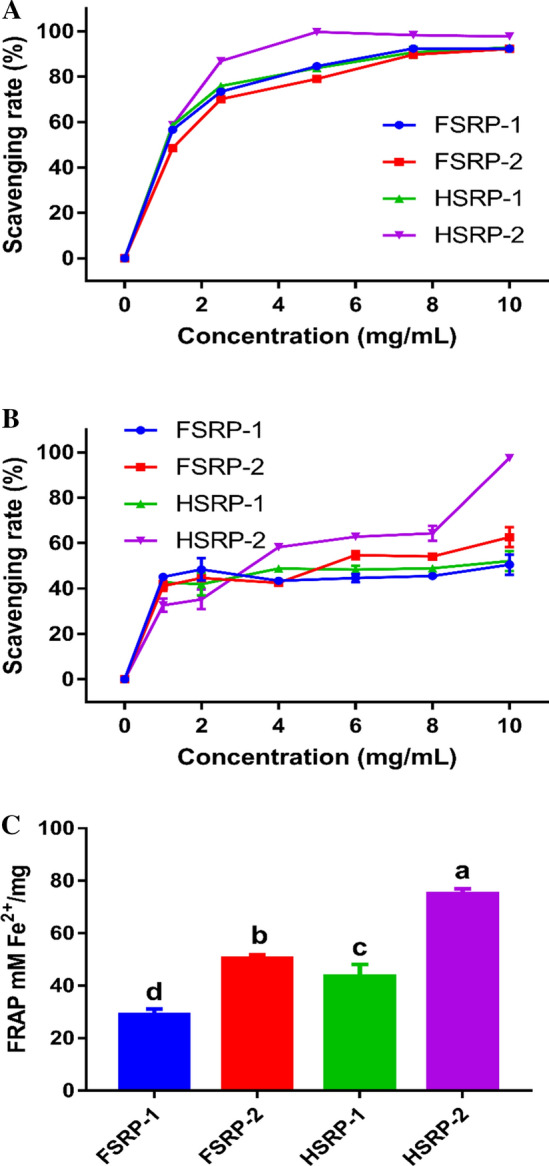
Antioxidant activity of FSRP-1, FSRP-2, HSRP-1, and HSRP-2. (a) ABTS+ radical scavenging capacity; (b) Hydroxyl radical scavenging capacity; (c) FRAP abilities, different letters (a, b, c and d) indicate significant differences (P < 0.05)
Hydroxyl radical scavenging activity of SRPs
The hydroxyl radicals scavenging activities of FSRP-1, FSRP-2, HSRP-1 and HSRP-2 are shown in Fig. 5b. Generally, FSRP-1, FSRP-2, HSRP-1 and HSRP-2 showed potential scavenging activities against hydroxyl radicals. The scavenging activities of FSRP-1, FSRP-2, HSRP-1 and HSRP-2 increased to 50.58%, 62.69%, 52.18% and 97.55%, respectively, when the sample concentration reached 10 mg/mL. This result was lower than that of polysaccharides from shiitake mushrooms (Wang et al. 2015), but higher than that of polysaccharides from Lepista nuda mushrooms (Shu et al. 2019). Moreover, the IC50 values of FSRP-1, FSRP-2, HSRP-1 and HSRP-2 were 9.85, 5.38, 9.35, and 2.87 mg/mL, respectively. The hydroxyl radicals scavenging activities of SRPs was in the order of: HSRP-2 > FSRP-2 > HSRP-1 ≈ FSRP-1. The results indicated that the neutral polysaccharides (HSRP-1 and FSRP-1) showed lower hydroxyl radicals scavenging activities that those of acidic polysaccharides (HSRP-2 and FSRP-2). Previous results also reported that the acidic polysaccharides from mushrooms had a higher antioxidant activity (Liu et al. 2016). Our results suggested that the scavenging capacity of acidic polysaccharides from S. rugosoannulata might be improved by hot-air drying pretreatment. However, the hydroxyl radical scavenging activity of polysaccharides from Ganoderma lucidum was reported to increase after freeze drying process (Fan et al. 2012b).
Ferric ion reducing antioxidant power (FRAP) of SRPs
The ferric ion reducing antioxidant powers (FRAP) of the four types of SRPs are shown in Fig. 5c. FSRP-1, FSRP-2, HSRP-1 and HSRP-2 were determined with FRAP values of 28.96, 50.47, 43.66, and 75.12 mM Fe2+/mg, respectively. Our results indicated that the FRAP of the four types of SRPs decreased in the order of: HSRP-2 > FSRP-2 > FSRP-1 > FSRP-1 (P < 0.05). The results suggested that the FRAP activity of neutral polysaccharides was lower than that of the acid polysaccharides. Similar results wee also reported for Ganoderma lucidum polysaccharides (Shi et al. 2013). Compared with freeze-vacuum drying, the ferric ion reducing antioxidant power of SRPs was improved by the HD process.
Overall, FSRP-1, FSRP-2, HSRP-1 and HSRP-2 displayed potential antioxidant activities. HSRP-2, having a high β-D-Glcp level, showed the strongest antioxidant activity among the four types of polysaccharides. Our results indicated that hot-air drying process improved the antioxidant activities of polysaccharides from S. rugosoannulata, and acid polysaccharides would be the primary antioxidant polysaccharides. Similar results were also reported about polysaccharides from Taraxacum mongolicum (Li et al. 2021). The antioxidant activities changes of SRPs could be due to the varied enzyme activities in S. rugosoannulata during drying process, polysaccharides-related enzymes were activated by hot-air drying pretreatment (Chen et al. 2018).
Conclusion
In this study, four types of polysaccharides including two neutral polysaccharides (FSRP-1 and HSRP-1) and two acidic polysaccharides (FSRP-2 and HSRP-2) were purified from S. rugosoannulata treated by FVD and HAD. The monosaccharide composition, chain conformations, and antioxidant activities of polysaccharides from S. rugosoannulata were altered by food drying process. The neutral SRPs had a primary chain of (1 → 6)-α-D-Galp while the acidic SRPs had a primary chain of (1 → 3)-β-D-Glcp. Hot-air drying treatment enhanced the antioxidant activities of SRPs, especially the acidic SRPs. We would thus recommend preparing biological polysaccharides from dried S. rugosoannulata treated by the HAD process. However, the bioactive mechanism and structure–activity relationships of SRPs are envisaged by future in vivo experiments.
Acknowledgements
The authors greatly appreciate the support received from the Agricultural Science and Technology Innovation Center of Hubei Province (2016-620-000-001-044), the China Postdoctoral Science Foundation (2016T90701), and the Science and Technology Innovation Fund for College Students (2019314).
Authors contributions
QW: Methodology, Investigation, Data Curation. YZ: Methodology, Investigation, Data Curation. XF: Writing—Review & Editing. SAI: Writing—Review & Editing. WH: Data Curation, Resources. YL: Writing—Original Draft, Supervision, Resources.
Funding
This study was supported by grants from the Agricultural Science and Technology Innovation Center of Hubei Province (2016–620-000–001-044); the China Postdoctoral Science Foundation (2016T90701); the Science and Technology Innovation Fund for College Students (2019314).
Declaration
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qi Wang and Yalin Zhao have contributed equally to this work.
References
- Ahmadi S, Sheikh-Zeinoddin M, Soleimanian-Zad S, Alihosseini F, Yadav H. Effects of different drying methods on the physicochemical properties and antioxidant activities of isolated acorn polysaccharides. LWT Food Sci Technol. 2019;100:1–9. doi: 10.1016/j.lwt.2018.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzie IFF, Strain JJ. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Chen L, Huang G. The antioxidant activity of derivatized cushaw polysaccharides. Int J Biol Macromol. 2019;128:1–4. doi: 10.1016/j.ijbiomac.2019.01.091. [DOI] [PubMed] [Google Scholar]
- Chen L, Zhou Y, He Z, Liu Q, Lai S, Yang H. Effect of exogenous ATP on the postharvest properties and pectin degradation of mung bean sprouts (Vigna radiata) Food Chem. 2018;251:9–17. doi: 10.1016/j.foodchem.2018.01.061. [DOI] [PubMed] [Google Scholar]
- Chen G, Li C, Wang S, Mei X, Zhang H, Kan J. Characterization of physicochemical properties and antioxidant activity of polysaccharides from shoot residues of bamboo (Chimonobambusa quadrangularis): effect of drying procedures. Food Chem. 2019;292:281–293. doi: 10.1016/j.foodchem.2019.04.060. [DOI] [PubMed] [Google Scholar]
- DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28(3):350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- Fan L, Ding S, Ai L, Deng K. Antitumor and immunomodulatory activity of water-soluble polysaccharide from Inonotus obliquus. Carbohyd Polym. 2012;90(2):870–874. doi: 10.1016/j.carbpol.2012.06.013. [DOI] [PubMed] [Google Scholar]
- Fan L, Li J, Deng K, Ai L. Effects of drying methods on the antioxidant activities of polysaccharides extracted from Ganoderma lucidum. Carbohyd Polym. 2012;87(2):1849–1854. doi: 10.1016/j.carbpol.2011.10.018. [DOI] [Google Scholar]
- Gan T, Feng C, Lan H, Yang R, Zhang J, Li C, Li W. Comparison of the structure and immunomodulatory activity of polysaccharides from fresh and dried longan. J Funct Foods. 2021;76:104323. doi: 10.1016/j.jff.2020.104323. [DOI] [Google Scholar]
- Huang M, Zhao L, Yang H. Water loss and status in sponge cake: impact of Eucheuma as a flour replacement. J Food Sci. 2021;86(3):915–922. doi: 10.1111/1750-3841.15609. [DOI] [PubMed] [Google Scholar]
- Huang M, Zhao X, Mao Y, Chen L, Yang H. Metabolite release and rheological properties of sponge cake after in vitro digestion and the influence of a flour replacer rich in dietary fibre. Food Res Int. 2021;144:110355. doi: 10.1016/j.foodres.2021.110355. [DOI] [PubMed] [Google Scholar]
- Jiang S, Ma Y, Yan D. Antioxidant and antimicrobial properties of water soluble polysaccharide from Arachis hypogaea seeds. J Food Sci Technol. 2014;51(10):2839–2844. doi: 10.1007/s13197-012-0786-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wang L, Wang Y, Xiong Z. Effect of drying method on physicochemical properties and antioxidant activities of Hohenbuehelia serotina polysaccharides. Process Biochem. 2016;51(8):1100–1108. doi: 10.1016/j.procbio.2016.05.006. [DOI] [Google Scholar]
- Li F, Feng K-L, Yang J-C, He Y-S, Guo H, Wang S-P, Gan R-Y, Wu D-T. Polysaccharides from dandelion (Taraxacum mongolicum) leaves: Insights into innovative drying techniques on their structural characteristics and biological activities. Int J Biol Macromol. 2021;167:995–1005. doi: 10.1016/j.ijbiomac.2020.11.054. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhang B, Ibrahim SA, Gao S-S, Yang H, Huang W. Purification, characterization and antioxidant activity of polysaccharides from Flammulina velutipes residue. Carbohyd Polym. 2016;145:71–77. doi: 10.1016/j.carbpol.2016.03.020. [DOI] [PubMed] [Google Scholar]
- Liu Y, Hu C-F, Feng X, Cheng L, Ibrahim SA, Wang C-T, Huang W. Isolation, characterization and antioxidant of polysaccharides from Stropharia rugosoannulata. Int J Biol Macromol. 2020;155:883–889. doi: 10.1016/j.ijbiomac.2019.11.045. [DOI] [PubMed] [Google Scholar]
- Liu Y, Luo M, Liu F, Feng X, Ibrahim SA, Cheng L, Huang W. Effects of freeze drying and hot-air drying on the physicochemical properties and bioactivities of polysaccharides from Lentinula edodes. Int J Biol Macromol. 2020;145:476–483. doi: 10.1016/j.ijbiomac.2019.12.222. [DOI] [PubMed] [Google Scholar]
- Luo H, Li X, Li G, Pan Y, Zhang K. Acanthocytes of Stropharia rugosoannulata function as a nematode-attacking device. Appl Environ Microbiol. 2006;72(4):2982. doi: 10.1128/AEM.72.4.2982-2987.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Chen H, Zhu W, Wang Z. Effect of different drying methods on physicochemical properties and antioxidant activities of polysaccharides extracted from mushroom Inonotus obliquus. Food Res Int. 2013;50(2):633–640. doi: 10.1016/j.foodres.2011.05.005. [DOI] [Google Scholar]
- Maity S, Kar Mandal E, Maity K, Bhunia SK, Behera B, Maiti TK, Mallick P, Sikdar SR, Islam SS. Structural study of an immunoenhancing polysaccharide isolated from an edible hybrid mushroom of Pleurotus florida and Lentinula edodes. Bioactive Carbohydrates and Dietary Fibre. 2013;1(1):72–80. doi: 10.1016/j.bcdf.2013.01.003. [DOI] [Google Scholar]
- Morales D, Smiderle FR, Villalva M, Abreu H, Rico C, Santoyo S, Iacomini M, Soler-Rivas C. Testing the effect of combining innovative extraction technologies on the biological activities of obtained β-glucan-enriched fractions from Lentinula edodes. J Funct Foods. 2019;60:103446. doi: 10.1016/j.jff.2019.103446. [DOI] [Google Scholar]
- Pizzoferrato L, Manzi P, Bertocchi F, Fanelli C, Rotilio G, Paci M. Solid-State 13C CP MAS NMR spectroscopy of mushrooms gives directly the ratio between proteins and polysaccharides. J Agric Food Chem. 2000;48(11):5484–5488. doi: 10.1021/jf000448j. [DOI] [PubMed] [Google Scholar]
- Rezvankhah A, Emam-Djomeh Z, Askari G. Encapsulation and delivery of bioactive compounds using spray and freeze-drying techniques: a review. Drying Technol. 2020;38(1–2):235–258. doi: 10.1080/07373937.2019.1653906. [DOI] [Google Scholar]
- Shang H, Cao Z, Zhang H, Guo Y, Zhao J, Wu H. Physicochemical characterization and in vitro biological activities of polysaccharides from alfalfa (Medicago sativa L.) as affected by different drying methods. Process Biochem. 2021;103:39–49. doi: 10.1016/j.procbio.2020.12.011. [DOI] [Google Scholar]
- Shi M, Zhang Z, Yang Y. Antioxidant and immunoregulatory activity of Ganoderma lucidum polysaccharide (GLP) Carbohyd Polym. 2013;95(1):200–206. doi: 10.1016/j.carbpol.2013.02.081. [DOI] [PubMed] [Google Scholar]
- Shi X-D, Nie S-P, Yin J-Y, Que Z-Q, Zhang L-J, Huang X-J. Polysaccharide from leaf skin of Aloe barbadensis Miller: Part I. Extraction, fractionation, physicochemical properties and structural characterization. Food Hydrocolloids. 2017;73:176–183. doi: 10.1016/j.foodhyd.2017.06.039. [DOI] [Google Scholar]
- Shu X, Zhang Y, Jia J, Ren X, Wang Y. Extraction, purification and properties of water-soluble polysaccharides from mushroom Lepista nuda. Int J Biol Macromol. 2019;128:858–869. doi: 10.1016/j.ijbiomac.2019.01.214. [DOI] [PubMed] [Google Scholar]
- Song Z, Jia L, Xu F, Meng F, Deng P, Fan K, Liu X. Characteristics of se-enriched mycelia by stropharia rugoso-annulata and its antioxidant activities in vivo. Biol Trace Elem Res. 2009;131(1):81–89. doi: 10.1007/s12011-009-8343-8. [DOI] [PubMed] [Google Scholar]
- Tang W, Liu C, Liu J, Hu L, Huang Y, Yuan L, Liu F, Pan S, Chen S, Bian S, Huang X, Yin J, Nie S. Purification of polysaccharide from Lentinus edodes water extract by membrane separation and its chemical composition and structure characterization. Food Hydrocolloids. 2020;105:105851. doi: 10.1016/j.foodhyd.2020.105851. [DOI] [Google Scholar]
- Wang K-p, Wang J, Li Q, Zhang Q-l, You R-x, Cheng Y, Luo L, Zhang Y. Structural differences and conformational characterization of five bioactive polysaccharides from Lentinus edodes. Food Res Int. 2014;62:223–232. doi: 10.1016/j.foodres.2014.02.047. [DOI] [Google Scholar]
- Wang J-H, Xu J-L, Zhang J-C, Liu Y, Sun H-J, Zha X. Physicochemical properties and antioxidant activities of polysaccharide from floral mushroom cultivated in Huangshan Mountain. Carbohyd Polym. 2015;131:240–247. doi: 10.1016/j.carbpol.2015.05.052. [DOI] [PubMed] [Google Scholar]
- Wang Y, Tian Y, Shao J, Shu X, Jia J, Ren X, Guan Y. Macrophage immunomodulatory activity of the polysaccharide isolated from Collybia radicata mushroom. Int J Biol Macromol. 2018;108:300–306. doi: 10.1016/j.ijbiomac.2017.12.025. [DOI] [PubMed] [Google Scholar]
- Wu J, Fushimi K, Tokuyama S, Ohno M, Miwa T, Koyama T, Yazawa K, Nagai K, Matsumoto T, Hirai H, Kawagishi H. Functional-food constituents in the fruiting bodies of Stropharia rugosoannulata. Biosci Biotechnol Biochem. 2011;75(8):1631–1634. doi: 10.1271/bbb.110308. [DOI] [PubMed] [Google Scholar]
- Wu J, Tokuyama S, Nagai K, Yasuda N, Noguchi K, Matsumoto T, Hirai H, Kawagishi H. Strophasterols A to D with an unprecedented steroid skeleton: from the mushroom Stropharia rugosoannulata. Angew Chem Int Ed. 2012;51(43):10820–10822. doi: 10.1002/anie.201205351. [DOI] [PubMed] [Google Scholar]
- Wu J, Suzuki T, Choi J-H, Yasuda N, Noguchi K, Hirai H, Kawagishi H. An unusual sterol from the mushroom Stropharia rugosoannulata. Tetrahedron Lett. 2013;54(36):4900–4902. doi: 10.1016/j.tetlet.2013.06.142. [DOI] [Google Scholar]
- Wu S, Li F, Jia S, Ren H, Gong G, Wang Y, Lv Z, liu Y, Drying effects on the antioxidant properties of polysaccharides obtained from Agaricus blazei Murrill. Carbohyd Polym. 2014;103:414–417. doi: 10.1016/j.carbpol.2013.11.075. [DOI] [PubMed] [Google Scholar]
- Yan P-S, Jiang J-H, Cui W-S. Characterization of protoplasts prepared from the edible fungus, Stropharia rugoso-annulata. World J Microbiol Biotechnol. 2004;20(2):173–177. doi: 10.1023/B:WIBI.0000021753.22257.27. [DOI] [Google Scholar]
- Yan J, Zhu L, Qu Y, Qu X, Mu M, Zhang M, Muneer G, Zhou Y, Sun L. Analyses of active antioxidant polysaccharides from four edible mushrooms. Int J Biol Macromol. 2019;123:945–956. doi: 10.1016/j.ijbiomac.2018.11.079. [DOI] [PubMed] [Google Scholar]
- Yan Q-X, Huang M-X, Sun P, Cheng S-x, Zhang Q, Dai H. Steroids, fatty acids and ceramide from the mushroom Stropharia rugosoannulata Farlow apud Murrill. Biochem Syst Ecol. 2020;88:103963. doi: 10.1016/j.bse.2019.103963. [DOI] [Google Scholar]
- Yang D, Yang H. The temperature dependent extraction of polysaccharides from eucheuma and the rheological synergistic effect in their mixtures with kappa carrageenan. LWT. 2020;129:109515. doi: 10.1016/j.lwt.2020.109515. [DOI] [Google Scholar]
- Yang Z, Yang H, Yang H. Effects of sucrose addition on the rheology and microstructure of κ-carrageenan gel. Food Hydrocolloids. 2018;75:164–173. doi: 10.1016/j.foodhyd.2017.08.032. [DOI] [Google Scholar]
- Yang D, Gao S, Yang H. Effects of sucrose addition on the rheology and structure of iota-carrageenan. Food Hydrocolloids. 2020;99:105317. doi: 10.1016/j.foodhyd.2019.105317. [DOI] [Google Scholar]
- Yu X-L, Zielinska M, Ju H-Y, Mujumdar AS, Duan X, Gao Z-J, Xiao H-W. Multistage relative humidity control strategy enhances energy and exergy efficiency of convective drying of carrot cubes. Int J Heat Mass Transf. 2020;149:119231. doi: 10.1016/j.ijheatmasstransfer.2019.119231. [DOI] [Google Scholar]
- Zhang W, Tian G, Geng X, Zhao Y, Ng BT, Zhao L, Wang H. Isolation and characterization of a novel lectin from the edible mushroom Stropharia rugosoannulata. Molecules. 2014;19(12):19880. doi: 10.3390/molecules191219880. [DOI] [PMC free article] [PubMed] [Google Scholar]