Abstract
Moderate to severe pain occurs in many cancer patients during their clinical course and may stem from the primary pathology, metastasis, or as treatment side effects. Uncontrolled pain using conservative medical therapy can often lead to patient distress, loss of productivity, shorter life expectancy, longer hospital stays, and increase in healthcare utilization. Various publications shed light on strategies for conservative medical management for cancer pain and a few international publications have reviewed limited interventional data. Our multi-institutional working group was assembled to review and highlight the body of evidence that exists for opioid utilization for cancer pain, adjunct medication such as ketamine and methadone and interventional therapies. We discuss neurolysis via injections, neuromodulation including targeted drug delivery and spinal cord stimulation, vertebral tumor ablation and augmentation, radiotherapy and surgical techniques. In the United States, there is a significant variance in the interventional treatment of cancer pain based on fellowship training. As a first of its kind, this best practices and interventional guideline will offer evidenced-based recommendations for reducing pain and suffering associated with malignancy.
Keywords: cancer pain, neurolysis, ketamine, pain pump, intrathecal drug delivery, neuromodulation, radiofrequency, spinal cord stimulation, vertebral augmentation
Introduction
There were an estimated 1.8 million new cancer diagnoses and 606,520 cancer-related deaths in the United States in 2020. Moderate to severe pain occurs in approximately 35% of all cancer patients and 80% of the patients with advanced-stage cancer.1 While younger patients are more susceptible to acute pain exacerbations, all age groups reported pain to be the most significant impairment to their quality of life.2 The CONCERN study evaluated cancer patients presenting to the emergency department for acute symptom management, with 62.1% of the patients reporting pain as their primary problem.3 When presenting to palliative care, 68% of end-stage cancer patients reported pain related to their primary tumor, typically somatic in nature.4 Nearly three-quarters of pain therapies are directed toward either the primary tumor or accompanying metastases.4 Pain in this population is not limited to the underlying diagnosis and may stem from treatments and diagnostic procedures. Fifty-five percent of patients report significant pain from their anticancer treatment, including radiation, chemotherapy and hormone therapies.{ref 4} Common syndromes may present as mucositis, granulocyte colony-stimulating factors (G-CSFs) induced bone pain, radiation and chemotherapy-related dermatological pain, and diffuse musculoskeletal pain.5 Approximately one-third of patients have significant chronic pain after curative treatment, especially chronic post-operative pain leading to severe impairment of quality of life and daily activities.6 Cancer pain is associated with significantly increased emotional distress, with pain causing disability for active cancer patients on average of 12–20 days per month. Even after cancer survival, between 20% and 50% of the patients continue to experience pain and functional limitations years following treatment completion.7
A study evaluating the prevalence and characterization of pain in the oncologic patient population determined risk factors for increased pain as female gender, age over 65 years and advanced cancer stages.8
There are multiple studies that discuss the conservative medical management of cancer-related pain.9 The Royal College of Physicians National Clinical Programme for Palliative Care and American Society of Clinical Oncology summarized a review for pharmacologic treatment of cancer pain.10,11 In 2017, the World Health Organization (WHO) provided clinical guidelines for opioid administration, including when to initiate opioids, routes of administration, opioid rotation, and cessation. They also discussed adjuvant medications including antidepressant and anticonvulsants as well as bisphosphonates for bone metastasis. The European Association of Palliative Care (EAPC) published guidelines that specifically address the use of opioids including breakthrough pain for the treatment of cancer pain.12
There are multiple published articles that exist describing interventional pain management strategies for cancer pain; however, a formal guideline does not exist. Neurolytic blocks, intrathecal drug delivery systems (IDDS), vertebral augmentation, neuromodulation (including spinal cord stimulation (SCS) and dorsal root ganglion stimulation (DRG-S) for neuropathic pain, and radiotherapy have been described.13–17 Gulati et al18 and Pak et al16 have described various interventional techniques including sympathetic blocks, neuromodulation, and intrathecal drug delivery systems for various types of cancer. In a 2019 expert consensus on the management of breakthrough cancer pain in older patients, it was noted that despite discussion between various specialties and available literature, there was no agreement on how interventional techniques should be integrated into the therapeutic strategy.19 However, interventional treatments are effective in providing pain relief, reducing the burden of symptoms, minimizing opioid intake, and have a low complication rate.20 Best practice guidelines would be instrumental to bridge the gap between evidence and clinical practice. To our knowledge, there are no large studies planned or currently ongoing to define best practice guidelines for conservative and interventional management of cancer-related pain.
Currently, a significant variance in the interventional treatment of cancer pain exists due to patient volumes, potential affiliations with cancer institutions, and faculty expertise and training on cancer pain. A best practice guideline will offer evidence-based recommendations for utilizing advanced interventional therapies for cancer-related pain.
Methods
Development Process
The American Society of Pain and Neuroscience (ASPN) performed a needs-based assessment of the therapeutic efficacy and patient safety with interventional pain techniques for cancer-related pain, and determined an evidence-based best practice review was needed. A multidisciplinary panel of pain medicine specialists was selected to create a best practices guideline for conservative and interventional management of cancer-related pain. Selection was based on expertise, publications, research, clinical experience, practice setting, and diversity. Previous reviews have published guidelines on the management of cancer-associated pain; however, these were primarily focused on non-interventional therapies.21,22 Due to the recent expansion of interventional techniques in the management of cancer-associated pain, the panel reviewed the literature to create guidelines to help structure the management of cancer-associated pain through both interventional and non-interventional adjunct therapies. We aimed to provide an overview of commonly used opioids, adjuvant medications, radiotherapy and to conduct a systematic review of the interventions offered by pain physicians for cancer pain. A meta analysis was not conducted due to the heterogeneity in study types and clinical variances in the interventions performed. Best practice summary statements and evidence grading are provided for each section. Prior to development of the manuscript, authors’ financial relationships were disclosed and recusal was required for any relevant section. Final editing for bias was conducted by an author without any conflicts of interest (AM).
Literature Search Method
A librarian-assisted literature search was performed identifying publications relevant to the management of cancer-associated pain in all types of cancer. Searches were completed using, Ovid MEDLINE (1946–2021), EMBASE (1974–2021), and Cochrane Database of Systematic Reviews (2005–2020) in addition to reference section review of all literature search identified manuscripts. Formatting of identified manuscripts included meta-analyses, randomized controlled trials, prospective studies, retrospective studies, case series, reports of expert committee, and existing international guidelines, limited to the English language.
The following search terms were used: “cancer”, ‘'metastasis'’, “cancer pain”, “opioids”, “ketamine”, “methadone”, “radiotherapy”, “plexus blocks”, “neurolysis”, “epidural analgesia”, “celiac plexus”, “splanchnic plexus”, “superior hypogastric plexus”, “ganglion impar”, “external beam radiation”, “intrathecal analgesia”, “spinal cord stimulation”, “vertebral radiofrequency”, “vertebral augmentation”, “vertebroplasty”, “kyphoplasty”, “cingulotomy”, “myelotomy”, “DREZ-otomy”, “radiofrequency cordotomy”, and “resiniferotoxin”. The authors also conducted independent literature searches which were cross-referenced and compiled for analysis and consensus review.
Evaluation and Analysis of Evidence
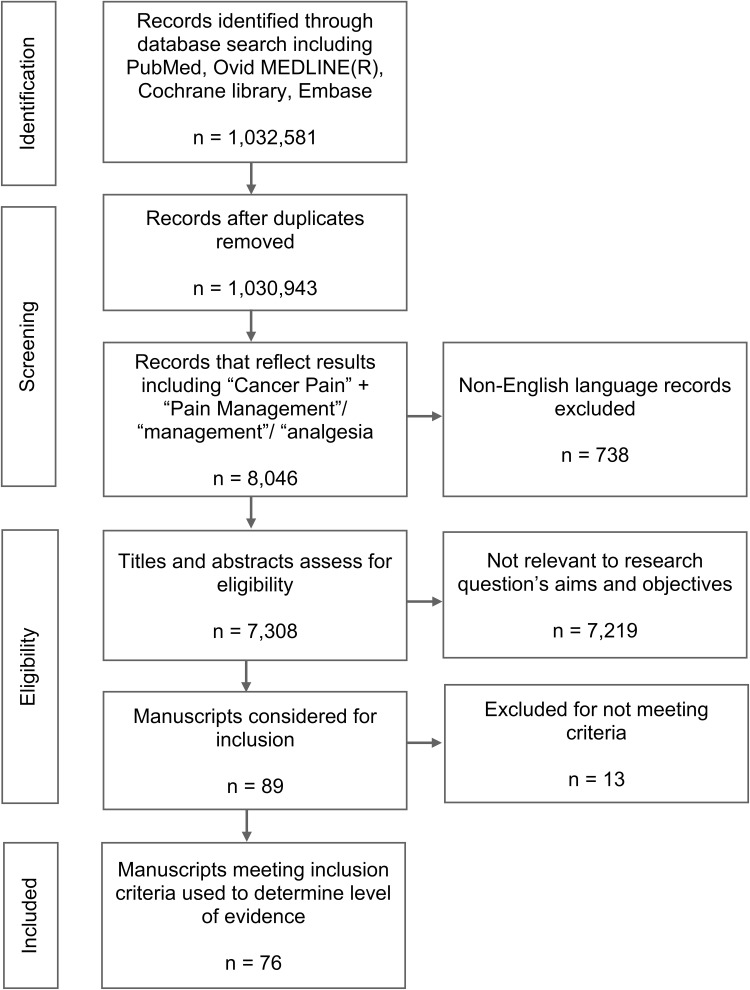
A total of 1,032,581 individual manuscripts were identified in the initial search. There was a total of 1638 duplicates and these were removed. The records were then filtered for “cancer pain” + “pain management” plus each key word resulting in 7308 articles. Two authors read the titles and abstracts and selected articles with a focus on interventional therapies for cancer pain. Criteria for exclusion at this stage were as follows: opioids only, acute postoperative pain in cancer patients and single patient case reports. A total of 7219 abstracts were excluded for these reasons. Eighty nine relevant abstracts were identified for review (Figure 1). Quality of evidence for each treatment class was graded from I–III as detailed in (Table 1) and a degree of recommendation was provided on a scale from A to D, or as insufficient (I), according to the United States Preventive Services Task Force (USPSTF) criteria (Table 2).23–25
Figure 1.
PRISMA diagram.
Table 1.
Evidence Levels
| Evidence Level | Study Type |
|---|---|
| I | At least one controlled and randomized clinical trial, properly designed |
| II-1 | Well-designed, controlled, nonrandomized clinical trials |
| II-2 | Cohort or case studies and well-designed controls, preferably multicenter |
| II-3 | Multiple series compared over time, with or without intervention, and surprising results in noncontrolled experiences |
| III | Clinical experiences-based opinions, descriptive studies, clinical observations, or reports of expert committees |
Note: Reprinted from Am J Prev Med, 20(3 Suppl), Harris RP, Helfand M, Woolf SH, et al, Current methods of the US Preventive Services Task Force: a review of the process, 21–35, 2001, with permission from Elsevier.23
Table 2.
United States Preventive Services Task Force Grading
| Degree of Recommendation | Meaning |
|---|---|
| A | Extremely recommendable (good evidence that the measure is effective, and benefits outweigh the harms) |
| B | Recommendable (at least, moderate evidence that the measure is effective, and benefits exceed harms) |
| C | Neither recommendable nor inadvisable (at least moderate evidence that the measure is effective, but benefits are similar to harms and a general recommendation cannot be justified) |
| D | Inadvisable (at least moderate evidence that the measure is ineffective or that the harms exceed the benefits) |
| I | Insufficient, low quality or contradictory evidence; the balance between benefit and harms cannot be determined |
Note: Reprinted from Am J Prev Med, 20(3 Suppl), Harris RP, Helfand M, Woolf SH, et al, Current methods of the US Preventive Services Task Force: a review of the process, 21–35, 2001, with permission from Elsevier.23
Data Evaluation and Discussion
Opioids for Cancer Pain
Prior to evaluating best practices for interventional methods of pain relief in the cancer patient it is valuable to review the current evidence for commonly used non-invasive options.
As pain can be moderate to severe in up to 50% of all individuals with cancer,9 opioids are commonly used in this patient population. The WHO developed a three step “Ladder” for treatment of cancer pain that recommends non-opioid medications first, followed by mild opioids, and lastly by strong opioids until the patient has achieved pain control.26 Adjuvant medications may be initiated at any step in the treatment process. Methadone has numerous clinical and pharmacological considerations differentiating it from other opioids and is addressed separately below.
Opioid agent selection should be individualized to account for the variance in pain presentations and co-existing medical comorbidities. Morphine and codeine should be avoided in advanced chronic kidney disease (CKD) and end-stage renal disease (ESRD); hydromorphone or oxycodone can be used with caution, while methadone and fentanyl appear to be safe to use.27 Similarly, liver function plays a key role in the activation, transformation, and metabolism of opioid medications. Opioid selection and dosing schedule should be carefully reviewed in patients with liver dysfunction. Methadone, meperidine, and codeine should be avoided in patients with liver dysfunction, while dose adjustments might be required with morphine, hydromorphone, oxycodone, and tramadol.28 Certain long-acting opioids with known immunosuppressive properties (ie, morphine, fentanyl, methadone) have been found to have increased incidence of infections compared to those without immunosuppressive properties (ie, oxycodone, oxymorphone, tramadol), and this must be carefully considered given the cancer-induced immunocompromised state in this patient population.29 Ultimately, the choice of opioid might be limited by the insurance coverage. Codeine, morphine, fentanyl, and methadone are the opioids listed on the WHO essential drug list and might be more accessible compared to some of the newer opioids, such as buprenorphine.30
A recent overview of Cochrane reviews concluded that while the quality of evidence on the use of opioids for cancer pain was low, data show that 19 of 20 patients who engage in opioid therapy for moderate to severe pain will have meaningful pain reduction within 14 days.9,31,32 In addition, they found that most patients will have adverse events, and 10–20% of cancer patients receiving opioid therapy may need to change treatment due to the severity of these side effects.9 While the most common side effects of opioids include constipation, nausea, and vomiting, other adverse effects include sedation, respiratory depression (which can be life threatening), urinary retention, pruritus, tolerance leading to loss of efficacy. In addition, dependence (mental and/or physical) leading to risk of withdrawal, hyperalgesia, and hypogonadism leading to decreased testosterone and osteopenia are reported.22 Given the potential for opioid use disorder, physicians must remain vigilant and perform careful evaluations and regular follow-up to address key safety concerns when maintaining patients on long-term opioid therapy, particularly given increased survival rates with certain types of cancer. Universal precautions can decrease the incidence of opioid misuse, and these include patient education, informed consent, a formal opioid agreement, utilization of prescription monitoring program, urine drug toxicology screening, utilizing screening tools such as the Opioid Risk Tool (ORT) and the Current Opioid Misuse Measure (COMM).33
Evidence Evaluation and Best Practice Statement
Opioids should be considered for moderate to severe cancer-related pain. I-A
Opioid agent selection should be individualized to account for the variance in pain presentations and co-existing medical comorbidities. III- B
Adjunct Medications
Methadone
Methadone is a racemic mixture of two enantiomers with applications to treat pain with nociceptive and neuropathic components. The L isomer (R-methadone) is a Mu/Delta receptor agonist while the D isomer (S-methadone) is a NMDA receptor antagonist and serotonin/norepinephrine reuptake inhibitor. Methadone has higher intrinsic activity but lower Mu receptor affinity than morphine allowing for pain relief while minimizing related side effects.34,35 The NMDA antagonism decreases excitatory neurotransmitter glutamate binding at the NMDA receptor site, leading to reduction of hyperalgesia, neuropathic pain, and depressed opioid receptor functionality.
According to the WHO analgesic ladder, strong opioids are considered the third step in treating adult cancer pain. Methadone is utilized when patients develop opioid tolerance, intolerance to side effects, or allergic reaction to other strong opioids such as morphine. Methadone is excreted through the feces showing benefit in patients with advanced CKD and ESRD. Patients having developed neuropathic pain from cancer treatment or compressive/radiation plexopathy may benefit from the dual nociceptive and neuropathic properties.
Methadone initiation dosing should be 2.5 mg TID in opioid naive patients with dose adjustments of 5 mg/day or less every 5–7 days. Opioid tolerant patients should be converted to an equianalgesic dose (1:2 to 1:20 conversion factor) with maximum starting dose of 30 mg/day and dose adjustment of 10 mg/day or less every 5–7 days.36 The analgesic half-life is 6–8 hours, so dosing every 6–8 hours is recommended.35,37,38 Common side effects include somnolence, nausea, constipation, and xerostomia, however rarer side effects have been described and include fatal arrhythmia, serotonin syndrome, respiratory depression, pruritus, diaphoresis, and neurotoxicity. Prior to initiation, cardiac arrhythmia risk should be assessed, and a baseline EKG obtained to evaluate for QT prolongation. A QTc between 450 and 500 ms puts the patient at a higher risk for development of torsades de pointes. When QTc >500 ms, alternative therapy should be considered. Follow-up EKG is recommended 2–4 weeks after methadone initiation.36 High gastric pH or proton pump inhibitor (PPI) use increases methadone absorption, while high urinary pH reduces methadone clearance leading to accumulation despite primarily fecal clearance.
According to a Cochrane review and secondary literature search, there is no evidence comparing methadone to placebo in the cancer population and there is weak evidence (small sample size, limited scope) with comparison to morphine. Studies demonstrate equipotent efficacy in treating mild pain when comparing methadone to morphine or transdermal fentanyl with comparable associated side effects.32,39
Evidence Evaluation and Best Practice Statement
Methadone should be considered when other opioids are ineffective, or additional NMDA or serotonin receptor modulation is desired. II-3 C
Dosing initiation is dependent on opioid tolerance with low introductory doses for naive patients. II-3 B
For opioid tolerant patients a conservative approach is recommended starting at 75–90% less than the calculated equianalgesic dose using 1:15 to 1:20 conversion factor. II-3 A
Ketamine
Ketamine exerts its effects on multiple receptors and pain pathways. The primary mechanism of action is through non-competitive NMDA receptor antagonism.40 The NMDA receptor has been demonstrated to be involved in the development of opioid tolerance. Ketamine has been shown in animal models to prevent fentanyl-induced hyperalgesia and subsequent acute morphine tolerance.40,41 Ketamine has been shown to activate the mu, delta and kappa opioid receptors and is not reliably reversed with naloxone.42 Ketamine activates descending inhibitory pathways and inhibits presynaptic neurons in the dorsal horn, thereby enhancing endogenous pain inhibition.42,43 Ketamine can be administered via multiple routes including intravenous (IV), intranasal, intramuscularly and oral. Oral bioavailability is 10–20% given first-pass metabolism.41
Ketamine related side effects are primarily dissociative in origin, including dysphoria, hallucinations, evoked nystagmus and altered perception. Other side effects include nausea, vomiting, elevated liver enzymes, hematuria, dysuria, hypertension and tachycardia.44 These side effects were rarely reported in the studies utilizing sub-anesthetic doses of ketamine for pain management. Utilizing lower doses of ketamine, careful titration, and concomitant benzodiazepines or alpha-2 agonists are helpful in mitigating these side effects should they occur.41,44 Prior to initiation, patients should be carefully evaluated for elevated cardiovascular and cerebrovascular risk factors. For patients undergoing an IV protocol, continuous hemodynamic monitoring is required. Patients should be counselled regarding residual psychotropic effects which may occur following infusions.
The use of ketamine in cancer pain remains limited to a few RCTs with varying results in the literature.45–48 The National Comprehensive Cancer Network and European Society for Medical Oncology guidelines on adult cancer pain management recommend considering oral or IV ketamine for refractory pain not responding to other analgesics, or adjuvants. It may also be considered in patients with central sensitization and for palliative treatment of neuropathic pain.49,50 A Cochrane review in 2017 found insufficient evidence to assess the benefits and harms of ketamine as an adjuvant to opioids for the relief of refractory cancer pain, which was a similar finding from the systematic review by Jonkman et al.51,52 There is currently an absence of conclusive evidence for ketamine as an adjuvant analgesic in cancer pain as the data remains mixed.
Evidence Evaluation and Best Practice Statement
Ketamine therapy for cancer pain should be considered on a case-by-case basis for refractory neuropathic, bone, and mucositis-related pain. II-1 B
Radiotherapy, Radioisotopes, and Bone-Modifying Agents for Metastasis
Metastatic bone disease is a significant source of morbidity and is a common manifestation of advanced cancer, particularly primary malignancies of the prostate, lung, and breast. The most common sites of metastases include the spine and sacrum, with patients often presenting with pain, pathologic fractures, and spinal cord compression.
External beam radiation therapy (EBRT) is perhaps the most common treatment modality for painful metastatic disease, with short fractionated regimens now being favored over more protracted schedules. A single treatment fraction of 8 Gray (Gy) has been demonstrated to provide equivalent pain relief as conventional 30 Gy treatments delivered in 10 treatment fractions.53 Stereotactic body radiation therapy (SBRT), which employs multiple beams of radiation to provide highly conformal treatment, may be preferred for patients with radio-resistant cancers (ie, melanoma) or those with oligometastatic disease.54
Radiopharmaceutical therapy is reserved for patients who have failed traditional radiotherapy or those with polymetastatic disease who are not candidates for palliative radiation. These agents are administered IV and selectively irradiate sites of metastatic bone involvement. The most commonly used radioisotopes include samarium-153 (153Sm), strontium-89 (89Sr), and radium-223 (223-Ra). Radium-223 is generally reserved for patients with multifocal disease from castration-resistant prostate cancer, while samarium-153 and strontium-89 may be used for other cancers. Most of the treatment efficacy data for radioisotope therapy is found in patients with metastatic prostate and breast cancer; therefore, its true clinical utility for other malignancies is unknown.55
Bisphosphonates and denosumab may also be used for painful metastatic disease. These agents inhibit osteoclast-mediated bone resorption, potentially reducing the risk of bone fracture and related bone pain. In a Cochrane review of patients with metastatic breast cancer, bisphosphonates were demonstrated to significantly improve pain scores.56 The analgesic potential of osteoclast inhibitors has been questioned for other cancers, particularly in patients with non-small cell lung cancer where a systematic review showed no high-level evidence that these agents reduce pain or improve quality of life metrics.57 The use of calcitonin to reduce metastatic bone pain is also controversial, with limited evidence supporting its use.58
Evidence Evaluation and Best Practice Statement
External beam radiation therapy with short, fractionated regimens are favored over conventional protracted schedules for painful metastatic bone disease. Stereotactic body radiation therapy may be preferred for radio-resistant cancers or oligometastatic disease. I-A
There is evidence for the use of osteoclast inhibitors, though it has not been found to be effective for some cancers, such as metastatic non-small cell lung cancer. Therefore, these agents should be used as an adjuvant treatment and considered on a case-by-case basis. II-1 B
Blocks and Neurolysis
Sympathetic blocks and neurolysis are often utilized for intractable visceral cancer-related pain. A variety of neural structures may be targeted based on the location of the primary malignancy or distant metastasis responsible for the pain presentation. Fluoroscopy, ultrasound, and computed tomography are commonly used to aid with procedural guidance and to ensure accurate needle placement near the target location. Diagnostic blockade helps identify likely pain generators and differentiate between visceral versus somatic origins of pain. The upper abdominal structures (including the gastrointestinal tract from the esophagus to transverse colon, pancreas, liver, spleen adrenals, ureters, and abdominal vessels) are supplied by the celiac plexus, while the lower abdominal and pelvic structures (including the bladder, prostate, testes, seminal vesicles, descending and sigmoid colon, uterus, ovaries, vaginal fundus, and rectum) are supplied by the superior hypogastric plexus.59 The ganglion impar supplies the perineum, distal rectum and anus, distal urethra, vulva, and distal third of the vagina.59
Celiac Plexus
The greater, lesser, and the least splanchnic nerves form the celiac plexus.60 The celiac plexus is located around the celiac artery and the superior mesenteric artery root. It is retroperitoneally located posterior to the stomach and pancreas, and anterior to the diaphragm. It is most commonly located and targeted at the level of the L1 vertebral body. However, its location may vary between the inferior aspect of the T12 vertebral body to the superior aspect of the L1 vertebral body.60 Various posterior percutaneous approaches for celiac plexus neurolysis have been described, including transaortic, antecrural, retrocrural, and splanchnic, based on the vertebral body level and final location of the needle tip.61,62 An anterior endoscopic transgastric approach can be performed by gastroenterologists,63–65 as well as percutaneously via ultrasound.66 Intraoperative approaches have been described during diagnostic laparoscopic staging67 or exploratory surgical resection/palliation.68 The celiac ganglion supplies efferent sympathetic tone to the gastrointestinal tract; thus, celiac plexus blockade may result in temporary orthostatic hypotension and diarrhea due to unopposed parasympathetic action. Other potential complications include retroperitoneal hematoma, local anesthetic toxicity, renal injury, and pneumothorax.69 The risk of potentially serious neurological complications is less than 1%, as reported in a survey of 2730 celiac neurolysis procedures in England and Wales; however, none were reported in the RCTs.70,71 Paraplegia can potentially result from direct trauma to the spinal cord or the artery of Adamkiewicz by the needle or the neurolytic agent.69 A case of irreversible paraplegia after endoscopic ultrasound-guided celiac plexus neurolysis has been reported due to concomitant embolic occlusion of the artery of Adamkiewicz leading to spinal cord ischemia.72 Fluoroscopic guided procedures offer the advantage of using live contrast flow and digital subtraction angiography, which can help identify vascular uptake.73
A systematic review conducted by Nagels et al concluded that percutaneous celiac plexus neurolysis significantly improves pain in patients with upper abdominal cancer with a concomitant decrease in opioid consumption and side effects.69 There are several studies evaluating the efficacy of celiac plexus neurolysis for abdominal cancer-related pain, which show better analgesia and reduced opioid consumption with the neurolysis compared to conventional medical management.70,74–76 Although an RCT by Wong et al70 did not find any difference in the quality of life (QOL), an RCT done by Kawamata found that celiac neurolysis prevented deterioration in QOL by the long-lasting analgesic effects.77 Furthermore, an RCT comparing celiac neurolysis combined with local tumor ablation versus celiac neurolysis alone demonstrated superior pain relief and better survival.78
An RCT done by Amr et al found that patients randomized to celiac neurolysis before Step 2 on the WHO analgesic ladder had better pain control and quality of life compared to those who were randomized to receive celiac neurolysis after they failed to achieve analgesia with Step 3 on WHO analgesic ladder.79 De Olivera et al studied the effects of neurolysis in patients taking less than 90 morphine milligram equivalents versus patients on greater than 90 morphine milligram equivalents) and found no difference in analgesic efficacy or QOL between the two groups.80 Intra-operative celiac plexus neurolysis (in patients with suspected pancreatic cancer who underwent exploratory laparotomy and found to have histologically proven, unresectable pancreatic cancer) was associated with better analgesia, elevated mood, reduced pain interference, as well as increased life expectancy, compared to saline placebo in an RCT by Staats et al.68,71 However, no difference in QOL or survival was shown in an RCT comparing the effects of early endoscopic celiac neurolysis done during diagnostic endoscopic ultrasound versus conventional medical management.81
Although several studies looking at different approaches to celiac neurolysis found no difference in results,61,82–84 an RCT done by Ozyalcin et al suggests that splanchnic neurolysis has superior results compared to celiac plexus neurolysis for pancreatic body and tail cancer.62 Dehydrated alcohol is the most commonly used agent and has been theorized to perform better for neurolytic procedures that must disrupt diffuse neuronal networks.85 Various volumes of neurolytic medications have been used for chemical celiac neurolysis ranging from 15 to 50 mL of 50–100% alcohol.75 An RCT done by Dolly et al to evaluate the efficacy of different volumes (20, 30, and 40 mL) of 70% alcohol found that pain relief, QOL, and reduction in opioids increased with increasing volume, with 40 mL being most effective of the three volumes studied. Recently, a few studies comparing endoscopic ultrasound guided celiac plexus radiofrequency ablation to alcohol neurolysis have demonstrated better outcomes with radiofrequency ablation.86,87
Superior Hypogastric Plexus
Plancarte et al first described the classical technique of superior hypogastric plexus (SHP) neurolysis with fluoroscopically guided bilateral needle insertion at the levels of L5 and S1. In 227 patients with pelvic pain from an oncologic source, 70–90% of the patients achieved significant pain relief.59 Most recently in 2019, Hou et al conducted a retrospective study on 46 patients who underwent an SHP block for pelvic pain related to malignancy, and they found a significant reduction in pain score, anxiety, and an increase in appetite.88 The largest retrospective study done to date was recently published. One hundred and eighty patients with malignant pelvic pain underwent SPH neurolysis via fluoroscopic technique and obtained a 48% VAS score decrease and 55% decrease in opioid consumption that persisted at 3 months.89 Mishra et al conducted the only randomized, controlled trial to date in 2013 in 50 patients with severe malignant pelvic pain randomized into either the interventional group (ultrasound-guided SPH neurolysis) or an opioid-only group. They found a greater than 50% reduction in VAS scores and opioid consumption that persisted at 2 and 3 months respectively in the interventional group compared to the opioid-only group.90 In 2020, a study combining SPH neurolysis with a pulsed radiofrequency ablation of the S2 and S3 nerve roots demonstrated moderately better pain control than SHP alone early in treatment but had equivalent results at 6 months.91
Two recent review articles stated that while there are many safe and efficient ways to block the superior hypogastric plexus with varying results, there have been minimal large, prospective, randomized studies proving efficacy.92 Mercadante et al in a 2015 systematic review and EAPC recommendations stated that given the current data, there is weak recommendation for using superior hypogastric plexus neurolysis for malignant pelvic pain.92 The newest data, as presented above, continue to highlight the potential benefits of superior hypogastric plexus neurolysis for malignant pelvic pain, yet the area still lacks large, prospective, randomized controlled trials.
Ganglion Impar Block
The ganglion impar or the ganglion of Walther is a solitary ganglion located on the anterior surface of the sacrum. It provides the nociceptive and sympathetic supply to the perineum, distal rectum, perianal region, distal urethra, vulva/scrotum, and the distal third of the vagina.93 The ganglion impar nerve block was first described by Plancarte et al in 1990, initially to treat sympathetic pain of malignant origin utilizing an anococcygeal approach under fluoroscopy.59 Since then, it has been largely used to treat many non-malignant forms of pain, especially intractable perineal pain and coccygodynia, but is also utilized in the management of intractable pain resulting from rectal, anal, vulvar or other perineal cancers.94
Ganglion impar block or neurolysis is most commonly performed using a percutaneous transcoccygeal approach with fluoroscopic guidance as it provides a shorter needle path and a more direct approach.95 Other anatomical approaches have also been described such as intercoccygeal joint approaches and paracoccygeal approaches if the sacrococcygeal joint or intercoccygeal joints are fused or there is an impediment to a direct approach.95 It is most commonly performed with fluoroscopic guidance but has been described with CT, and ultrasound guidance.96,97
Data regarding the efficacy of neurolytic ganglion impar block in a purely oncologic population is limited to case reports and case series; however, these have consistently shown improved pain with no significant adverse effects. Plancarte et al reported on 16 patients with advanced malignancies and significant pelvic pain, and found a 60% improvement in pain symptoms following neurolytic ganglion impar block.59 In 2008, Eker et al described a pain improvement of greater than 60% after completion of ganglion impar block in three patients diagnosed with malignant rectal neoplasia and perineal pain.98 Another study published in 2012 described six patients with pelvic or gastrointestinal carcinomas of advanced stages, whom obtained significant pain relief following ganglion impar block, which was sustained at 2 months.99
In addition, studies with mixed non-oncologic and oncologic populations undergoing ganglion impar neurolysis or block have reported good outcomes. Toshniwal et al conducted a prospective observational study, which included 16 patients with mixed chronic perineal pain of both malignant and benign origin. Patients underwent the trans-sacrococcygeal approach under fluoroscopic guidance, and achieved pain reductions of greater than 50% at the two-month follow-up period.100 In 2009, Agarwal-Kozlowski et al demonstrated a statistically significant reduction of pelvic pain in a retrospective review of 43 patients with mixed malignant and non-malignant refractory pelvic and perineal pain who underwent CT guided ganglion impar neurolysis. The authors reported a 75% decrease in VAS at 4-month follow-up.97
The complications of ganglion impar nerve block or neurolysis are infrequent, and include motor, sexual, bladder, bowel dysfunction, and perforation of the rectum.101 Ganglion impar nerve blocks and neurolysis, while lacking RCTs in perineal oncologic pain, has been shown to be effective and safe in helping with these pain conditions.
Evidence Evaluation and Best Practice Statement
Celiac plexus neurolysis should be performed for pancreatic cancer-related moderate-to-severe abdominal pain that is refractory to analgesics. I-A
Splanchnic neurolysis should be considered in patients with intractable abdominal cancer-related pain due to advanced body and tail located pancreatic cancer. I-B
Early neurolysis is associated with better outcomes. II3-B
Superior hypogastric plexus neurolysis should be considered in patients with intractable pelvic cancer-related pain. II-B
Ganglion impar neurolysis should be considered in patients with intractable perineal cancer-related pain. III-B
Epidural and Intrathecal Analgesia
Intrathecal analgesia via an implanted pump is indicated when cancer pain is severe despite adequate trials of conventional medication or when dose-limiting side effects are present. Randomized controlled trials,102,103 prospective studies15,104–106 and systematic reviews107 have demonstrated analgesic efficacy and side effect reduction. Cost-effectiveness has also been established in a large US payer database.13
Pharmacokinetics of Intrathecal Opioids
An important advantage of delivering opioids intrathecally is a reduction in opioid-related side effects.102 Targeted drug delivery allows the elimination or reduction of oral opioids in addition to dramatic reductions in serum opioid levels.104 In the swine model, intrathecal morphine and hydromorphone tend to enter the spinal cord rather than entering the systemic circulation because of their relative hydrophilic properties (with respect to fentanyl and other lipophilic opioids).108 This is further supported in the swine model which showed a relatively focal intrathecal spread of morphine restricted to 5–10 cm caudad and cephalad.109 Thus, in the clinical model, we expect morphine and hydromorphone infusions to act on the spinal cord near the location of the catheter tip. However, based on computer modelling data, dispersion of the opioid may be influenced by the flow rate and injectate velocity of the solution from the intrathecal pump.110 More importantly, dispersion of the opioid in cerebrospinal fluid (CSF) seems to be more related to the cardiac cycle rather than other physiologic systems (ie, respiration).111 Thus, catheter location, injectate velocity, cardiac cycle variables, and properties of the opioid are the most important variables in determining the kinetics and dispersion of an opioid within the CSF and intrathecal space.
Trial Procedure in Cancer Pain
Trialing practices are controversial and have questionable utility in the cancer pain setting with recent guidelines stating that trialing should be optional.112 There have been no prospective studies to support trialing in cancer pain and recent studies of IDD in cancer pain typically did not trial.104,105 A pump manufacturer database demonstrates that only 21.6% of cancer pain patients received a trial before implant.113
With level I evidence supporting the efficacy of IDDS, as well as compelling cost-effectiveness data, trialing should be considered discretionary based upon factors such clinician preference, patient population, referral patterns, and the payor environment. Selected payors still require a trial before approving coverage for an IDDS, but many will waive this in the setting of cancer pain (Table 3).
Table 3.
Advantages and Disadvantages of Intrathecal Trialing in Cancer Pain
| Disadvantages of Intrathecal Trialing | Advantages of Intrathecal Trialing |
|---|---|
| ● Delays definitive therapy in a population with limited life expectancy ● Many cancer patients are on anticoagulation; trialing requires an additional discontinuation of anticoagulation therapy and increased risk of thromboembolic events. ● Additional risk of trial ● Additional cost ● Inpatient trials are laborious |
● Payor authorization ● Assessment of efficacy in the reluctant patient ● Assess for tolerability |
In certain scenarios, trialing may have utility to help assess the individual patient’s response to the therapy, including analgesia, side effects, functional improvement, and appropriate starting doses after implantation.
Trials can be performed via single bolus injections, intermittent boluses with an indwelling catheter, and continuous infusions. No trialing methodology has been found to be superior for predicting long-term success.112,114 Therefore, the decision of which modality to utilize is often dependent on the provider’s practice setting and preferences. Single intrathecal bolus trials are usually done in an outpatient setting and are perhaps the most cost-effective method. Limitations include short duration of action and an inability to target particular dermatomes. Catheter trials, with placement in the intrathecal or epidural space, typically require an inpatient admission and allow for dose titration at a targeted dermatome. Multiple medications can also be trialed during the same hospitalization. This may be preferred for patients where opioid tolerability is a concern. It is important to point out that intrathecal trials have not been proven to better simulate a permanent system, though epidural infusions may overestimate starting intrathecal dosages.112,115 Additionally, patients undergoing indwelling catheter trials receive high flow and bolus drug delivery, which will result in more widespread dissemination of the injectate and will not effectively predict the very low flow therapy provided by an implantable system. Trialing practices for cancer pain syndromes remain diverse, and efforts are currently being undertaken to evaluate the necessity and best practices of trialing to establish more uniform practice habits.
Managing Chronic Opioid Therapy in the Setting of a Trial Procedure
In the non-cancer pain population, it has been suggested that tapering of systemic opioids prior to intrathecal pump implant may improve outcomes.112,116 However, in the patient with cancer-associated pain, it is impractical and unethical to withhold analgesics prior to initiating IDD. The literature supporting IDD for cancer pain does not discuss tapering systemic opioids prior to implantation.102,105,117 Recent studies have demonstrated the ability to safely and effectively discontinue systemic opioids in the perioperative period and transition exclusively to IDD.118
In a situation where systemic opioids are causing a concerning level of sedation hospital admission should be arranged for monitoring and consideration for interventional pain management options that may permit improved pain control with reduced systemic opioid doses. There may be instances when the patient is on extraordinarily high doses of opioids with concern for opiate-induced hyperalgesia in whom the intent of initiating IDD will be to reduce systemic opioid levels while introducing nonopioid analgesics such as ziconotide,119 local anesthetics, or clonidine.
Implant Considerations
Variable rate intrathecal pumps consist of a battery and integrated microelectronic circuits that control drug delivery. These pumps allow drug delivery rates to be changed as needed, and for the provision of patient-administered bolusing. The two most popular commercially available variable rate pumps are peristaltic continuous pumps (Synchromed II Pump, Medtronic Inc., Minneapolis, MN, USA) and valve-gated pumps (Prometra Intrathecal Drug Delivery System, Flowonix Medical Inc., Mount Olive, NJ, USA). Peristaltic pumps consist of a roller geared rotor system that delivers reservoir medications by a peristaltic sequence to an intrathecal catheter.116 A low-pressure reservoir and its rollers occlude the plastic tubing at multiple points along the catheter to control flow of fluid. The pump reservoir is called a metal bellows reservoir, which contains medications, and there is pressurized gas surrounding these bellows. The pressurized gas exerts pressure on the bellows, changing the volume depending on the volume of the drug present.
Valve-gated pumps use a positive-pressure design with two microvalves (inlet and outlet), a dosing chamber, and a flow-activated valve.120 Medications are delivered through a precision dosing system not susceptible to external factors like pressure or temperature. It is hypothesized that this positive pressure, valve-gated system reduces granuloma formation and prevents corrosion with combination therapy within the drug reservoir.
The location of the catheter tip for different cancer pain generators is often debated. The 2017 PACC guidelines recommend placing the tip around the spinal dermatome associated with the source of the pain.116 A recent publication provided level-specific bupivacaine dose recommendations based on the pain location (Table 4).121
Table 4.
Recommended Intrathecal Catheter Tip Location Based on Pain Location
| Pain Location | Vertebral Body Catheter Tip Location |
|---|---|
| Brachial plexus | C3-5 |
| Arm | C3-5 |
| Breast | T1-2 |
| Upper chest wall | T3-4 |
| Visceral abdomen | T5-6 |
| Lower chest wall | T6-7 |
| Abdominal wall | T6-7 |
| Pelvis | T9-12 |
| Leg | T10 |
| Sacral | Vertebral body at level of conus |
Note: Reproduced from Chen GH, Spiegel MA, Magram YC, et al. Evaluation of fixed intrathecal bupivacaine infusion doses in the oncologic population. Neuromodulation. 2020;23(7):984–990. © 2020 International Neuromodulation Society, with permission from John Wiley and Sons.121
Intrathecal Medications
The Food and Drug Administration (FDA) has approved three medications for intrathecal use: morphine, ziconotide, and baclofen. However, other medications are commonly and safely used within the intrathecal space such as fentanyl, sufentanil, hydromorphone, bupivacaine, and clonidine. The 2017 PACC guidelines recommend that off-label drugs should be tried after FDA-approved drugs are attempted and failed or are contraindicated.117 It is important to note that these guidelines were not specific for cancer-associated pain and many centers utilize a combination of opioid and local anesthetic often at a ratio of 1:2 or 1:3 to optimize analgesia for poorly controlled malignant pain.
Total Daily Dose of IT Medication
Starting intrathecal opioid doses may vary widely depending on the patient’s baseline opioid intake. Sindt et al suggest that the initial daily intrathecal dose following implantation of a permanent system be calculated using a ratio of 100:1 of the patient’s daily oral morphine equivalent dose prior to implant assuming all systemic opioids are discontinued.118 If appropriate, demand doses can also be programmed at 10% of the daily intrathecal dose for up to 24 additional hourly doses while the patient is admitted, which is then decreased to a maximum 12 daily activations upon discharge. The PACC guidelines recommend that the daily initiating intrathecal dose be 50% or less of the successful trial dose if an intrathecal trial was performed, with demand dosages of 5–20% of the total daily dose.116 Other factors that should be considered when determining initial intrathecal doses include risks of cardiopulmonary depression and whether the patient will be admitted to the hospital for observation. Based on clinical experience, a conversion of 10:1 can be considered for epidural trial to intrathecal dosing. Regardless, conservative dosing strategies are typically recommended.
Intrathecal Drug Delivery: Complications, Management, and Considerations
Pharmacological complications include cardiopulmonary depression, anaphylaxis, and meningitis with introduction of contaminated solution. While complication are rare, potential complications include catheter tip granuloma formation, bleeding complications, infection risks, and complications related to surgical management. Other adverse effects include opioid-induced hyperalgesia, hypotension, inflammatory mass, hypogonadotropic hypogonadism, immunologic compromise, headaches, seromas, hygromas, and bacterial meningitis. Risk factors that lead to higher complication rates include underlying psychological issues, obstructive sleep apnea, immunosuppression, smoking, diabetes mellitus, active infections, bleeding disorders, and anticoagulation therapy. The annual rate for IDDS complication requiring surgical intervention is 10.5%, with 35% being pump related and 65% catheter related.102,116,122
Cancer Pain–Specific IDD Considerations
Cancer patients are likely to undergo radiation and chemotherapy that may impact IDD. However, there are many factors to consider when ensuring patient safety, such as absolute neutrophil count greater than 500/μL and platelet counts greater than 80,000/uL when addressing candidacy for pump placement with concurrent chemotherapy.
A single-center retrospective review of 88 patients with IDDS receiving radiation therapy (0–18.0 Gy) demonstrated no incidence of IDDS malfunction related to RT.123 While there is limited evidence demonstrating adverse effects of radiation exposure on IDD systems, there are reports of battery drain or electric failure of the implanted device if the pump is directly within the field of radiation.102,116,122
Epidural or spinal metastases are not an absolute contraindication to placement of IDDS, but these lesions may affect device efficacy and increase complication rates of neuraxial treatments.
Intrathecal Catheter Tip Granuloma Formation
Intrathecal catheter tip granulomas can produce potentially irreversible neurologic sequelae. The prevalence of granuloma formation may be as high as 8%. Medications shown to cause catheter tip granulomas include morphine, hydromorphone, sufentanil, bupivacaine, and baclofen. Granuloma formation is more likely to occur with low flow rates and has been demonstrated to be dose and concentration dependent. Ziconotide has not been shown to cause intrathecal granuloma. MRI with and without contrast, CT myelogram, or a dye study into the catheter side-port can detect a granuloma typically by identifying a filling defect around the catheter tip.102,116,122,124,125
Bleeding Considerations
Intrathecal catheter and pump implant is defined as a high-risk procedure for potential serious neuraxial bleeding.126 The incidence of spinal hematoma associated with intrathecal therapy is not well defined.102,116,122,124,125 However, epidural hematoma has a reported incidence of 0.75% for percutaneous placement of SCS. In regards to the pump pocket site, we extrapolate from the cardiac device literature, where 2.2% of implantable cardioverter defibrillators demonstrated clinically significant pocket hematoma.102,116,122,124,125
Infection
Pocket and lumbar site infection, meningitis, and encephalitis are primary infection concerns with IDD. Factors associated with surgical site infections (SSI) include anemia, tobacco smoking, diabetes mellitus, cancer, malnutrition, obesity, cardiovascular disease, and immunosuppression. Cancer treatments, including corticosteroids and chemotherapy, may cause increased risk for SSI. The incidence of infections in IDD has been found to be 0.7–3.2% per year.102,116,122,124,125 A recent retrospective review in a large cancer center showed an infection incidence of 0.9% despite frequent concurrent cancer treatment and leukopenia.127 In other studies, the incidence of infection in the cancer patient population was 2.7–3.2%.128,129
Surgical and Technical Complications
Post-dural puncture headache (PDPH) occurs in 15.5% of the patients and CSF hygromas (pseudomeningocele) occur in 1.5%.102,116,122,124,125 Pocket site seroma is another possible complication. Seroma formation following IDD may be managed conservatively by using a pressure dressing and an abdominal binder for a number of weeks with serial follow-up to assess for resorption. If without improvement, sterile aspiration may be performed. Diagnostic glucose testing and fluid analysis can help differentiate between third space fluid due to seroma formation versus CSF accumulation. In rare cases, surgical evacuation and revision may be necessary for definitive treatment.102,116,122,124,125
Catheter and Pump Complications
The most common complications of IDD are catheter-related malfunctions. This includes catheter tip migration, kinking, occlusion, fracture, and loosening of connections. Studies have shown the incidence of catheter migration is 7.3%, catheter tear 6.4%, and catheter occlusion 1.8%.122 Pump-related complications such as motor stalls, over or under infusion, corrosion, and gear wear may occur to a lesser degree. The incidence of a motor stall has been reported to be 0.28% at 48 months and 0.69% at 84 months post-implantation.122 The risk of IDD over-infusion is 0.16%.122
An algorithmic approach to pump and catheter dysfunction as well as subsequent interrogation is demonstrated in Tables 5 and 6.
Table 5.
IDDS Pump-Related Complications
| Causes of Pump Failure |
| Change in performance or failure of the catheter |
| ● Micro-fracture ● Pinhole leak ● Disconnection ● Breakage ● Migration ● Partial occlusion ● Tip fibrosis/granuloma ● Inflammatory mass |
| Unexpected battery depletion |
| Component or motor failure |
| Catheter access port failure |
Note: Reproduced with permission from Smith TJ, Staats PS, Deer T, et al. Randomized clinical trial of an implantable drug delivery system compared with comprehensive medical management for refractory cancer pain: impact on pain, drug-related toxicity, and survival. J Clin Oncol. 2002;20(19):4040–4049102 https://ascopubs.org/journal/jco.
Table 6.
Diagnostic Approach to IDDS Failure
| Initial evaluation, including patient history will often identify the source of the problem |
| Verification of pump contents, volume and pump setting is the critical initial step |
| Plain x-ray (PA and Lateral to visualize the entire catheter) |
| Serial x-ray or fluoroscopy to confirm that the pump roller is moving at the expected rate |
| Magnetic resonance imaging (MRI) study |
| Catheter access port aspiration |
| Nuclear medicine scan |
| Fluid collection assay |
Note: Reproduced with permission from Smith TJ, Staats PS, Deer T, et al. Randomized clinical trial of an implantable drug delivery system compared with comprehensive medical management for refractory cancer pain: impact on pain, drug-related toxicity, and survival. J Clin Oncol. 2002;20(19):4040–4049.102 https://ascopubs.org/journal/jco.
MRI Following IDDS Implantation
Magnetic resonance imaging is often necessary with ongoing cancer treatments or for other non-cancer-related issues. The two commonly utilized intrathecal pump systems (Medtronic SynchroMed II and Flowonix Prometra II) have MRI Conditional labelling.130 They are considered compatible with MRI when specific parameters are followed as specified by the device manufacturers. MRI safety profiles are found on each manufactures website and in online searchable databases. It is important to recognize that device malfunction can still occur during scanning and may pose a potential safety hazard for medication overdose or withdrawal.
The Medtronic SynchroMed II is labeled MRI conditional at 1.5 and 3.0 Tesla. Before MRI, the therapy should be discontinued, and restarted after the scan. If therapy is not discontinued, a “motor stall event” will occur with automatic restart within 30 minutes of the scan. It is recommended to interrogate the system within 24 hours after MRI to ensure the pump has restarted appropriately.131
The Prometra II pump is labelled MRI conditional at 1.5 Tesla and it requires pre-MRI discontinuation of therapy, emptying the medication from the pump reservoir, followed by a post-MRI interrogation, pump reservoir refill and re-initiation of therapy.132
Evidence Evaluation and Best Practice Statement
One multicenter, randomized controlled trial of programmable IDDS added to comprehensive medical management (CMM) versus CMM alone was analyzed, consisting of a six-month follow-up period.102 Evidence from this study suggested that the use of IDDS plus CMM reduces overall drug toxicity over a 12-week period when compared with CMM alone; however, no statistically significant difference was observed in pain scores. For the composite outcome of reduction in pain and drug toxicity, evidence favoured the use of IDDS.
A retrospective review of cancer patients compared the economic burden CMM to targeted drug delivery with CMM over a seven-year period. The IDDS plus CMM group had significant reduction in healthcare utilization due to intractable pain as reflected by fewer inpatient visits and shorter hospital stays, and overall cost savings. A total of 536 patients met criteria for review, 268 patients in TDD and CMM and 268 patients in CMM group. A mean total cost savings of $15,142 was found at 2 months and $64,498 at 12 months. The use of CMM alone was also associated with higher dose of opioid use at 12 months.13
In another study on cost-effectiveness, Brogan et al assessed patients with cancer-related chronic pain before pump placement and 4–6 weeks after pump implant.133 Patients were stratified into high-cost drug therapy (ie, parenteral drugs, high-dose opioids), and low-cost drug therapy (all others). With an average cost of pump placement estimated to be $35,601, economic evidence suggested that IDDS had the potential to be more cost-effective than high-cost oral therapy if administered for 7 months or longer.
Intrathecal drug delivery using an implantable pump should be strongly considered in patients with cancer-related pain that is not responding to, or who develop side effects from conventional medical management. I-A
Trialing before intrathecal pump implantation for cancer-related pain should be at the discretion of the physician and patient. It is not a requirement. III-C
Spinal Cord Stimulation
Indications for Use
The most common indications for SCS are complex regional pain syndrome (CRPS) Types I and II, post-laminectomy syndrome, chronic radiculopathy, intractable neuropathic pain, and visceral pain.134 While treatment of cancer-related pain is often focused on symptoms explicitly related to the primary malignant diagnosis, true supportive care and palliative care should include all treatments that may improve QOL.135 Cancer patients remain susceptible to all non-cancer-related pain pathologies. With the increase of MRI compatible technologies, we must ensure that patients are not excluded from evidence-based therapies because of their malignant diagnosis. This includes access to evidence-based use of neuromodulation for non-cancer-related syndromes.136
In addition, adverse effects of many cancer treatments are amenable to SCS. With five-year cancer survival rates for all cancer diagnoses increasing from 50.3% to 67% over the past 20 years, more patients are living with the chronic pain resulting from effective cancer treatments.137 Fifty-two percent of patients exposed to neurotoxic chemotherapeutic agents develop chemotherapy-induced peripheral neuropathy (CIPN) with median time to symptom development of 71 days.138 Many case reports demonstrate effective treatment of CIPN with SCS, but currently there are no RCTs demonstrating efficacy.139–141 Early bench research has shown the role of SCS in preventing CIPN. Sivanesan et al demonstrated that SCS may attenuate CIPN pain-related behavior in rats prior to treatment with paclitaxel.142 While more studies are needed, with few proven effective pharmaceutical treatments and continued improvements in technology, SCS is a viable treatment option for refractory CIPN.
Many patients with abdominal, gynecologic, and orthopedic cancer require surgical treatment which may result in nerve damage and subsequent neuropathic pain, post-surgical pain, or peripheral causalgia. A recent publication by Hagedorn et al reviewed the use of SCS for cancer-related pain, both cancer- and treatment-related pain.17 In general, the literature regarding SCS for the treatment of cancer-related pain is largely represented by case reports and small case series. In 2010, Yakovlev et al reported on 14 patients with lung cancer who underwent SCS for post-surgical pain. At 12 months post-implant, all patients had greater than 50% VAS pain reduction, and all patients had decreased or discontinued pain medications.143 In 2012, Yakovlev et al described 15 patients with treatment-related low back pain after undergoing treatments for metastatic colon cancer, anal cancer, or angiosarcoma of the sacrum. At 12 months post-implant, all patients had greater than 50% pain relief and 86% had decreased or discontinued pain medications. In the largest patient cohort to date, Shimoji et al published a retrospective review of 52 patients with carcinoma or sarcoma of the head/face, neck/upper extremities, trunk, or lower extremities. The authors reported that while the subjects obtained 80% pain relief initially, this decreased to 20% pain relief at 1 year.144
Extrapolating from non-malignant pain data, both SCS and DRG-S are effective for refractory neuropathic, postsurgical and peripheral causalgia-related pain.145–148 Also, many patients with primary spinal tumors or metastasis may require laminectomy, decompression, and fusion. Since approximately 30% of the patients develop pain worse than or equal to their pain prior to spine surgery, many of these patients may require treatment for failed back surgery syndrome (FBSS).149 With high-quality evidence for the treatment of this condition in both post-surgical lumbar and cervical radicular pain, SCS should be considered early in the treatment algorithm for these patients.150,151
Device Selection
Cancer patients may require screening MRI for progression of disease or evaluation of new symptoms. With regard to device selection, it is imperative to understand the MRI conditionality of the devices.130 In the non-cancer population, Desai et al found that 82–84% of SCS-implanted patients were expected to need at least one MRI within 5 years of implant. Within 10 years of implantation, 59–74% of all patients implanted were likely to require non-spine MRI.152 Additionally, consideration should be given to the degree of patient comfort in maintaining, charging, and programming the implantable pulse generator (IPG).
Evidence Evaluation and Best Practice Statement
Spinal cord stimulation may be considered in patients with refractory cancer pain. II-3-C
Spinal cord stimulation may be considered on a case-by-case basis for pain that is related to cancer treatment such as chemotherapy induced peripheral neuropathy. III-C
Vertebral Augmentation and Radiofrequency Ablation
In the United States, it is estimated that up to 30% of all newly diagnosed cancers displayed vertebral metastasis at presentation. Approximately 30–70% of cancer patients will present with back pain complaints related to metastasized spinal tumors often involving multiple levels. This is thought to be due to the high vascularity with anterograde spread and retrograde seeding through the valveless extradural Batson’s venous plexus. These tumors cause severe pain via several different mechanisms. Tumor growth within the vertebral body increases pressure on the endosteum triggering an inflammatory cascade. This inflammation, due to chemical mediators, activates nociceptive impulses via intraosseous basivertebral nerves. The vertebral body can also weaken with continued tumor growth resulting in painful pathologic compression fractures.
The clinical manifestations of spinal tumors include localized pain with or without radicular pattern, neurological deficits, and spinal deformities with some patients experiencing no symptoms at all. Appropriate management of spinal metastasis requires a multi-disciplinary approach assessing cancer staging, tumor involvement, spinal instability/deformities, neurological function, number of involved levels, radio- and chemo-sensitivity of the tumor, and patient prognosis.153
Vertebral augmentation should be considered for patients with severe pain secondary to pathological vertebral compression fractures.154 A multicenter, randomized controlled trial of kyphoplasty versus conservative management showed improved function and pain in cancer-related vertebral compression fractures.155,156 Systematic reviews have also demonstrated improvements in subsequent pain scores, opioid use, and pain-related disability.157,158 There is little data to suggest the superiority of either kyphoplasty versus vertebroplasty when treating malignant vertebral compression fractures; however, kyphoplasty has a lower risk of cement extravasation and results in greater kyphosis correction.159
The use of percutaneous radiofrequency ablation (RFA) with or without cement augmentation is indicated for treatment of back pain from spinal tumors and has proven to be a safe and effective palliative therapy for painful spinal metastasis.160 The application of RFA in osseous lesions was first described by Rosenthal et al in 1992.161 Radiofrequency ablation is a well-established percutaneous thermal energy that causes localized necrosis of targeted tissue. The mechanism of pain relief is believed to be due to destruction of the pain transmitting neural tissue and periosteal nociceptors. Levy et al reported data on 100 patients undergoing RFA with cementoplasty for painful spinal metastases in the prospective multicenter international OPuS One Study. There was significant improvement in pain (8.2 ± 1.7 at baseline to 3.5 ± 3.2 at 6 months), pain interference and QOL.162 The increasing number of prospective studies utilizing RFA with or without cement augmentation continue to demonstrate the safety and efficacy of this treatment with a more rapid rate of pain relief (0–2 weeks) compared to conventional fractionated radiotherapy (4–6) weeks.163 Current therapies and treatments involve a combination of analgesics, bisphosphonates, and radiotherapy with variable results and many patients experiencing inadequate pain relief. Conventional surgery is associated with high complication rates.164 Evidence supporting the efficacy of RFA includes retrospective and prospective series demonstrating it to be effective and safe in achieving short- to mid-term (from 1 week to 6 months) analgesia in patients affected by painful spinal metastasis.165 Vertebral RFA with or without cement augmentation allows for safe, effective, rapid pain reduction, and also enables quick initiation of adjuvant therapies.
Evidence Evaluation and Best Practice Statement
Vertebral augmentation should be strongly considered for patients with symptomatic vertebral compression fractures from spinal metastases. 1-A
Percutaneous radiofrequency ablation with or without cement augmentation is indicated for treatment of severe back pain from spinal tumors and has proven to be a safe and effective palliative therapy for painful spinal metastasis. II-2 B
Radiofrequency Lesioning and Nerve Blocks
Radiofrequency ablation and nerve blocks are techniques commonly used and most studied in the treatment of non-malignant pain. The former involves the application of heat generated from an electrical current to disrupt innervation from a painful anatomic site. In the cancer literature, there are case reports/series of pain relief efficacy in conditions arising from metastatic brachial plexus tumor, head and neck cancer, thoracic vertebral body metastases and glossopharyngeal neuralgia.166 The only RCT concluded superior efficacy of RFA lesioning over steroid treatment when applied to the dorsal root ganglion in patients experiencing axial thoracic pain from vertebral metastases.167 Nerve blocks using corticosteroids targeting the glossopharyngeal nerve in patients with tongue cancer can be considered if the pain is refractory to conventional medical therapy.168
Evidence Evaluation and Best Practice Statement
Consider radiofrequency lesioning of the dorsal root ganglion in the treatment of axial thoracic back pain from vertebral malignant metastases. I C
For cancer pain unresponsive to medical management, application of nerve blocks using corticosteroid or radiofrequency lesioning to a peripheral nerve can be considered. II-2 C
Surgical Options: CT Guided Radiofrequency Cordotomy, Myelotomy, DREZ-Otomy, and Cingulotomy
Surgical interventions in carefully selected patients remain a valid tool in the management of refractory cancer pain.169,170
Surgical cordotomy interrupts the spinothalamic tract and is effective in unilateral nociceptive pain related to direct tissue involvement. It was described by Kanpolat, who performed the procedure percutaneously under CT guidance at the C1-C2 level.171 Kanpolat also explains that cordotomy can be performed between the occiput and C1 for the lesion of the nucleus tractus to improve head and neck–related malignant pain.171,172 The procedure is offered to patients with life expectancy of more than 3 months, with no intracranial metastasis or suspicious high intracranial pressure, and intact autonomic nervous system. Procedure complications are mild and transient and may include dysesthesia, mirror pain, motor weakness, urinary retention, and ataxia. The procedure is performed by using RFA between 60°C and 80°C for 60 seconds under intraoperative sensory and motor monitoring. Alternatively, a double channel endoscopic technique has been described with similar efficacy and safety.173 A recent RCT assessed pain relief in patients with unilateral somatic pain undergoing CT guided cordotomy when compared to palliative care. Primary outcome was pain reduction of 33% or greater. A total of 85.7% of the patients in the cordotomy group achieved greater than 33% pain relief compared to zero patients in the palliative group. At 1 week, 77.8% of the patients randomized to palliative care opted to cross over to cordotomy and all achieved greater than 33% reduction in pain.174 According to different case series, the overall pain control was high with up to 80–100% reporting no pain at one month.175 In another report of 51 patients with cancer-related body or face pain, the initial pain reduction was 98% with 80% sustained pain relief at 6 months.176 A recent prospective observational case series has demonstrated both reduction in pain and opioid consumption post cordotomy.177 The incidence of new pain was variable and the underlying pathophysiology is still poorly understood, but progression of the disease and interruption of descending inhibitory pathways may play a role.
Myelotomy is used for abdominal or pelvic visceral pain by interrupting the dorsal columns. It can be performed either open or percutaneous.178 The open technique is performed under general anesthesia, where T3-T4 levels are approached for upper abdominal pain and T6-T8 for lower abdominal and pelvic pain. Laminectomy and dural opening are the initial steps. A 16-gauge angiocatheter is used to create a 0.5 mm lesion bilaterally from the midline with 5 mm depth.
The percutaneous technique is performed under CT guidance at the occiput-C1 level or at thoracic level using RFA at 70–80 °C for 60 seconds. Complication rate is low.178 In case series, myelotomy was found to be effective in reducing pain and opioid consumption.178
Dorsal root entry zone lesioning or DREZ-otomy is used for limited limb pain. The most common indication is Pancoast tumours, but its indication expands to lumbo-sacral plexopathy. The procedure is performed via an open technique with intradural approach. A 2–3 mm curved RFA needle is placed under somatosensory evoked potential monitoring.179 It can provide up to 80% pain relief.
Finally, at late stages of disease and pain control, and if all therapeutic options have failed, stereotactic anterior cingulotomy has shown to be effective in controlling pain.180,181 It carries cerebral risks, in addition to psychological side effects and cognitive impairment.182
Evidence Evaluation and Best Practice Statement
Cordotomy should be considered for uncontrolled unilateral nociceptive pain after failure of more conservative options. II-B
Myelotomy is used for infra-diaphragmatic visceral pain for pain control and decrease opioid consumption. III-C
DREZ-otomy is indicated for focal limb pain and in Pancoast tumors. III–Insufficient
Cingulotomy is indicated for late-stage and uncontrolled pain refractory to other therapies. III-C
Future Direction: Intrathecal Resiniferatoxin (RTX)
Resiniferatoxin (RTX) is derived from the Euphorbia Resinifera plant and is a potent agonist for transient receptor potential vanilloid 1 (TRPV1) expressed on Aδ and C nerve fibers. Receptor binding prolongs cation channel activation, leading to a large and rapid influx of intracellular calcium. This results in axonal destruction in TPRV1 expressing primary afferent spinal and dorsal root ganglion neurons while effectively sparing motor, proprioceptive and sensory cell bodies that do not express the TPRV1 receptor.183 Initial animal trials were conducted in a rat model,184 followed by a study in companion dogs afflicted with advanced bone cancer pain and showed favorable effects.185
The first human study of RTX was a single-center, Phase Ib, non-randomized, open-label, dose-escalation study to determine its safety and efficacy in patients with severe refractory pain due to advanced malignancy at or below the level of the chest.186 In all patients, intrathecal RTX was injected under propofol sedation to prevent the acute pain associated with excitotoxic destruction of TRPV1 neurons. Pre- and post-injection Numeric Rating Scale (NRS) scores, quality of life, quantitative thermal sensation, and safety data were observed. The lower doses of 3–13 µg demonstrated variable pain relief but permitted dose escalation with patients receiving up to 26 µg of RTX into the intrathecal space. Patients who received either 13 µg (n=3) or 26 µg (n=3) injections of RTX reported less pain and improved mobility. Numeric rating scale scores trended lower compared to pretreatment, although the NRS change was statistically insignificant at these doses. Thermal perception reduction was consistent with cell death of the TRPV1 neurons. There were no other sensory or motor changes post-treatment and no changes in EKG, MRI, or eye examination were noted.186
This paved the way for the currently ongoing multicenter, open-label, phase 1b trial to assess the safety and establish the maximally tolerated dose of epidural RTX injection for the treatment of intractable pain associated with cancer. Fourteen subjects with intractable cancer pain received a single epidural injection ranging from 0.4 to 35 µg.187 The primary outcome is to determine the dose limiting toxicity and the maximum tolerated dose. Secondary outcomes include NRS, Brief Pain Inventory-Short Form, and daily analgesic consumption, among others. Interim analysis presented at an annual conference in 2020 reported positive outcomes at doses of 8 µg with no dose limiting toxicity. The most common treatment-related adverse event was transient procedural pain described as a burning sensation in the lower extremities that subsided over several hours.187 Initial RTX human safety and efficacy data are promising in reducing the burden of cancer-associated intractable pain with minimal adverse effects. As of this publication, a multicenter, blinded, control, Phase 3 clinical is ongoing.
Evidence Evaluation and Best Practice Statement
RTX is still undergoing safety and efficacy trials. Grade for this treatment class is insufficient
Conclusion
This work provides a succinct clinical overview of conservative medical management for cancer-related pain including opioids, adjuvant mediations, and radiotherapy. We performed a detailed systematic review of all relevant literature for the interventional management of cancer and treatment-associated pain. Limitations of the current manuscript include being confined to the limitations of the available data and methodological differences between studies, including study design and heterogeneity of study populations. However, we have appropriately assessed the quality of evidence, and provided grading and recommendation levels using USPSTF criteria. Best practice statements with grading were made after critical evaluation of the literature (Table 7). The existing best practice and guidelines for therapies such as IDDS and116 SCS136 are not specific to cancer-associated pain. This paper bridges the gap between conservative medical management and recently published surgical neuroablative guidelines.170 We provide clear evidence-based guidance and recommendations on how interventional techniques should be integrated into the management of cancer-associated pain. When necessary, development of best practice statements in the setting of limited high-quality data is given based upon clinical expertise and scientific discourse between the authors.
Table 7.
Best Practices
| Therapy | Statement | Evidence Level | Grade |
|---|---|---|---|
| Opioids for cancer pain | Opioids should be considered for moderate to severe cancer-related pain. | I | A |
| Opioid agent selection should be individualized to account for the variance in pain presentations and co-existing medical comorbidities. | III | B | |
| Methadone | Methadone should be considered when other opioids are ineffective, or additional NMDA or serotonin receptor modulation is desired. | II-3 | C |
| Dosing initiation is dependent on opioid tolerance with low introductory doses for naïve patients. | II-3 | B | |
| For opioid tolerant patients a conservative approach is recommended starting at 75–90% less than the calculated equianalgesic dose using 1:15 to 1:20 conversion factor. | II-3 | A | |
| Ketamine | Ketamine therapy for cancer pain should be considered on a case-by-case basis for refractory neuropathic, bone, and mucositis-related pain. | II-1 | B |
| Radiotherapy, radioisotopes, and bone-modifying agents for metastasis | External beam radiation therapy with short, fractionated regimens are favored over conventional protracted schedules for painful metastatic bone disease. Stereotactic body radiation therapy may be preferred for radio-resistant cancers or oligometastatic disease. | I | A |
| There is evidence for the use of osteoclast inhibitors, though it has not been found to be effective for some cancers, such as metastatic non-small cell lung cancer. Therefore, these agents should be used as an adjuvant treatment and considered on a case-by-case basis | II-1 | B | |
| Blocks and neurolysis | Celiac plexus neurolysis should be performed for pancreatic cancer-related abdominal pain. | I | A |
| Splanchnic nerves neurolysis should be considered in patients with intractable abdominal cancer-related pain due to advanced body and tail located pancreatic CA. | I | B | |
| Early neurolysis is associated with better outcomes | II-3 | B | |
| Superior hypogastric plexus neurolysis should be considered in patients with intractable pelvic cancer-related pain. | II | B | |
| Ganglion impar neurolysis should be considered in patients with intractable perineal cancer-related pain. | III | B | |
| Targeted drug delivery | Intrathecal drug delivery using an implantable pump should be strongly considered in patients with cancer-related pain that is not responding to conventional medical management | I | A |
| Trialing before intrathecal pump implantation for cancer-related pain should be optional and at the discretion of the physician and patient. | III | C | |
| Spinal cord stimulation | Spinal cord stimulation may be considered in patients with refractory cancer pain. | II-3 | C |
| Spinal cord stimulation may be considered on a case-by-case basis for pain that is related to cancer treatment such as chemotherapy induced neuropathy. | III | C | |
| Vertebral augmentation and radiofrequency ablation | Vertebral augmentation should be strongly considered for patients with symptomatic vertebral compression fractures from spinal metastases. | I | A |
| Percutaneous radiofrequency ablation with or without cement augmentation is indicated for treatment of severe back pain from spinal tumors and has proven to be a safe and effective palliative therapy for painful spinal metastasis. | II-2 | B | |
| Radiofrequency lesioning and nerve blocks | Consider radiofrequency lesioning of the dorsal root ganglion in the treatment of axial thoracic back pain from vertebral malignant metastases. | I | C |
| For cancer pain unresponsive to medical management, application of nerve blocks using corticosteroid or radiofrequency lesioning to a peripheral nerve, brachial plexus can be considered. | II-2 | C | |
| Surgical options | Cordotomy should be considered for uncontrolled unilateral nociceptive pain after failure of more conservative options. | II | B |
| Myelotomy is used for infra-diaphragmatic visceral pain for pain control and decrease opioid consumption. | III | C | |
| DREZ-otomy is indicated for focal limb pain and in Pancoast tumors. | III | I | |
| Cingulotomy is indicated for late-stage and uncontrolled pain refractory to other therapies | III | C |
Acknowledgments
Thank you to Jessica Werhand B.S. for her contribution in the preparation of this manuscript.
Disclosure
MMA is a consultant for Abbott, Bioness, Vertos Medical. TD is a consultant for Abbott, Vertos, Axonics, Flowonix, SpineThera, Saluda Medical, Nalu, Medtronic, Nevro, SI Bone, Stimgenics, SPR Therapeutics, Cornerloc, Boston Scientific, PainTeq, Ethos, and Vertiflex. TD is a member of the advisory board for Abbott, Vertos, Flowonix, Nalu, SPR Therapeutics and Vertiflex. TD has stock options in Bioness, Vertiflex, Axonic, Vertos, SpineThera, Nalu, Cornerloc, PainTeq and SPR Therapeutics. TD has common stock in Saluda Medical. He is a research consultant for Abbott, Vertos, Mainstay Medical, Saluda Medical, SPR Therapeutics, Boston Scientific and Vertiflex. TD has a patent pending for the DRG paddle lead with Abbott. DS is a consultant for Flowonix, Medtronic, Merit, Abbott, Nevro, PainTeq, ans Vertos. JMH is a consultant for Abbott, Boston Scientific, and Nevro. SEB is a consultant for Medtronic. VS is a consultant for Releviate LLC. AG is a consultant for Medtronic, SPR therapeutics, Nalu Medical and serves on the Advisory Board for AIS HealthCare (advisory board) and Spark medical. NHS is a consultant for Abbott. JSW is a consultant for Abbott, Medtronic, Boston Scientific, Vertos, Saluda, PainTeq, Omnia Medical. Research Consultant for Abbott, Saluda. JHG is a consultant for Abbott and Stratus. JHG receives research support from SPR Therapeutics. AV is a consultant for CornerLoc, Vertos, Guidepoint Global. Research consultant for SPR. AEH is consultant for Abbott. AE is a consultant for Abbott, Boston Scientific, Medtronic, Nevro and Vertos Medical. KS is a consultant for Abbott, Boston Scientific, Flowonix, Nevro, Vertos Medical. JS is a consultant for Abbott labs, Executive leadership council for Vertos Medical, Shareholder and Medical Advisory Board for Celeri Health. SN is a consultant for Medtronic and AIS. The authors report no other conflicts of interest in this work.
References
- 1.Bruera E, Kim HN. Cancer pain. JAMA. 2003;290(18):2476–2479. doi: 10.1001/jama.290.18.2476 [DOI] [PubMed] [Google Scholar]
- 2.Kahan B. Cancer pain and current theory for pain control. Phys Med Rehabil Clin N Am. 2014;25(2):439–456. doi: 10.1016/j.pmr.2014.01.013 [DOI] [PubMed] [Google Scholar]
- 3.Caterino JM, et al. Analysis of diagnoses, symptoms, medications, and admissions among patients with cancer presenting to emergency departments. JAMA Netw Open. 2019;2(3):e190979. doi: 10.1001/jamanetworkopen.2019.0979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van den Beuken-van Everdingen MH, et al. Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. J Pain Symptom Manage. 2016;51(6):1070–1090.e9. [DOI] [PubMed] [Google Scholar]
- 5.Burton AW, Fine PG, Passik SD. Transformation of acute cancer pain to chronic cancer pain syndromes. J Support Oncol. 2012;10(3):89–95. doi: 10.1016/j.suponc.2011.08.004 [DOI] [PubMed] [Google Scholar]
- 6.Gallaway MS, Townsend JS, Shelby D, et al. Pain among cancer survivors. Prev Chronic Dis. 2020;17:E54. doi: 10.5888/pcd17.190367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown LF, et al. The association of depression and anxiety with health-related quality of life in cancer patients with depression and/or pain. Psychooncology. 2010;19(7):734–741. doi: 10.1002/pon.1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunha R, da Silva Oliveira AG, Carvalho TDP, et al. 1872P Pain in oncology: prevalence and characterization. Ann Oncol. 2020;31:S1067. doi: 10.1016/j.annonc.2020.08.1519 [DOI] [Google Scholar]
- 9.Wiffen PJ, et al. Opioids for cancer pain - an overview of Cochrane reviews. Cochrane Database Syst Rev. 2017;7(7):Cd012592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Brien T, Kane CM. Pain services and palliative medicine - an integrated approach to pain management in the cancer patient. Br J Pain. 2014;8(4):163–171. doi: 10.1177/2049463714548768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paice JA, Portenoy R, Lacchetti C, et al. Management of Chronic Pain in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34(27):3325–3345. doi: 10.1200/JCO.2016.68.5206 [DOI] [PubMed] [Google Scholar]
- 12.Caraceni A, Hanks G, Kaasa S, et al. Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol. 2012;13(2):e58–68. doi: 10.1016/S1470-2045(12)70040-2 [DOI] [PubMed] [Google Scholar]
- 13.Stearns LJ, Narang S, Albright RE, et al. Assessment of health care utilization and cost of targeted drug delivery and conventional medical management vs conventional medical management alone for patients with cancer-related pain. JAMA Netw Open. 2019;2(4):e191549. doi: 10.1001/jamanetworkopen.2019.1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deer TR, et al. Comprehensive consensus based guidelines on intrathecal drug delivery systems in the treatment of pain caused by cancer pain. Pain Physician. 2011;14(3):E283–312. doi: 10.36076/ppj.2011/14/E283 [DOI] [PubMed] [Google Scholar]
- 15.Stearns LM, Abd-Elsayed A, Perruchoud C, et al. Intrathecal drug delivery systems for cancer pain: an analysis of a prospective, multicenter product surveillance registry. Anesth Analg. 2020;130(2):289–297. doi: 10.1213/ANE.0000000000004425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pak DJ, Yong J, Shah K. Interventional Management of Chronic Visceral Pain Syndromes. Amsterdam: Elsevier; 2020. [Google Scholar]
- 17.Hagedorn JM, Pittelkow TP, Hunt CL, et al. <p>Current Perspectives on Spinal Cord Stimulation for the Treatment of Cancer Pain. J Pain Res. 2020;13:3295–3305. doi: 10.2147/JPR.S263857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gulati A, et al. Essentials of Interventional Cancer Pain Management. 1st. Cham, Switzerland: Springer; 2019. [Google Scholar]
- 19.Alarcón MDL, Estévez FV, Cabezón-Gutiérrez L, et al. Expert consensus on the management of breakthrough cancer pain in older patients. A Delphi study. J Geriatr Oncol. 2019;10(4):643–652. doi: 10.1016/j.jgo.2019.03.012 [DOI] [PubMed] [Google Scholar]
- 20.Hochberg U, et al. Interventional Pain Management for Cancer Pain: an Analysis of Outcomes and Predictors of Clinical Response. Pain Physician. 2020;23(5):E451–e460. doi: 10.36076/ppj.2020/23/E451 [DOI] [PubMed] [Google Scholar]
- 21.Gg F. Evidence Summary SMG-3 a Quality Initiative of the Program in Evidence-Based Care (PEBC). 2018. [Google Scholar]
- 22.Benyamin R, et al. Opioid complications and side effects. Pain Physician. 2008;11(2 Suppl):S105–20. doi: 10.36076/ppj.2008/11/S105 [DOI] [PubMed] [Google Scholar]
- 23.Harris RP, Helfand M, Woolf SH, et al. Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med. 2001;20(3 Suppl):21–35. doi: 10.1016/S0749-3797(01)00261-6 [DOI] [PubMed] [Google Scholar]
- 24.US Preventive Service Task Force Grade Definitions. 2018; Available from: https://www.uspreventiveservicestaskforce.org/uspstf/grade-definitions. Accessed June22, 2021.
- 25.Manchikanti L, et al. Assessment of methodologic quality of randomized trials of interventional techniques: development of an interventional pain management specific instrument. Pain Physician. 2014;17(3):E263–90. doi: 10.36076/ppj.2014/17/E263 [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization. WHO Guidelines for the Pharmacological and Radiotherapeutic Management of Cancer Pain in Adults and Adolescents. Geneva: World Health Organization; 2018. [PubMed] [Google Scholar]
- 27.Dean M. Opioids in renal failure and dialysis patients. J Pain Symptom Manage. 2004;28(5):497–504. doi: 10.1016/j.jpainsymman.2004.02.021 [DOI] [PubMed] [Google Scholar]
- 28.Soleimanpour H, Safari S, Shahsavari Nia K, et al. Opioid drugs in patients with liver disease: a systematic review. Hepat Mon. 2016;16(4):e32636. doi: 10.5812/hepatmon.32636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wiese AD, Griffin MR, Schaffner W, et al. Long-acting opioid use and the risk of serious infections: a retrospective cohort study. Clin Infect Dis. 2019;68(11):1862–1869. doi: 10.1093/cid/ciy809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.WHO Model lists of essential medicines; 2109. Available from: https://www.who.int/groups/expert-committee-on-selection-and-use-of-essential-medicines/essential-medicines-lists. Accessed June22, 2021.
- 31.Hadley G, et al. Transdermal fentanyl for cancer pain. Cochrane Database Syst Rev. 2013;2013(10):Cd010270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nicholson AB, et al. Methadone for cancer pain. Cochrane Database Syst Rev. 2017;2(2):Cd003971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hahn KL. Strategies to prevent opioid misuse, abuse, and diversion that may also reduce the associated costs. Am Health Drug Benefits. 2011;4(2):107–114. [PMC free article] [PubMed] [Google Scholar]
- 34.Davis MP, Walsh D. Methadone for relief of cancer pain: a review of pharmacokinetics, pharmacodynamics, drug interactions and protocols of administration. Support Care Cancer. 2001;9(2):73–83. doi: 10.1007/s005200000180 [DOI] [PubMed] [Google Scholar]
- 35.Eap CB, Buclin T, Baumann P. Interindividual variability of the clinical pharmacokinetics of methadone: implications for the treatment of opioid dependence. Clin Pharmacokinet. 2002;41(14):1153–1193. doi: 10.2165/00003088-200241140-00003 [DOI] [PubMed] [Google Scholar]
- 36.Chou R, et al. Methadone safety: a clinical practice guideline from the American Pain Society and College on Problems of Drug Dependence, in collaboration with the Heart Rhythm Society. J Pain. 2014;15(4):321–337. doi: 10.1016/j.jpain.2014.01.494 [DOI] [PubMed] [Google Scholar]
- 37.Anderson R, et al. Accuracy in equianalgesic dosing. conversion dilemmas. J Pain Symptom Manage. 2001;21(5):397–406. doi: 10.1016/S0885-3924(01)00271-8 [DOI] [PubMed] [Google Scholar]
- 38.Krames E, Peckham PH, Rezai AR. Neuromodulation: Comprehensive Textbook of Principles, Technologies, and Therapies. Second ed. London, United Kingdom: Academic Press is an imprint of Elsevier; 2018:1764. [Google Scholar]
- 39.Cherny N. Is oral methadone better than placebo or other oral/transdermal opioids in the management of pain? Palliat Med. 2011;25(5):488–493. doi: 10.1177/0269216310397687 [DOI] [PubMed] [Google Scholar]
- 40.Arendt-Nielsen L, Nielsen J, Petersen-Felix S, et al. Effect of racemic mixture and the (S+)-isomer of ketamine on temporal and spatial summation of pain. Br J Anaesth. 1996;77(5):625–631. doi: 10.1093/bja/77.5.625 [DOI] [PubMed] [Google Scholar]
- 41.Singh V, Gillespie TW, Harvey RD. Intranasal ketamine and its potential role in cancer-related pain. Pharmacotherapy. 2018;38(3):390–401. doi: 10.1002/phar.2090 [DOI] [PubMed] [Google Scholar]
- 42.White PF, Way WL, Trevor AJ. Ketamine--its pharmacology and therapeutic uses. Anesthesiology. 1982;56(2):119–136. doi: 10.1097/00000542-198202000-00007 [DOI] [PubMed] [Google Scholar]
- 43.Niesters M, Aarts L, Sarton E, et al. Influence of ketamine and morphine on descending pain modulation in chronic pain patients: a randomized placebo-controlled cross-over proof-of-concept study. Br J Anaesth. 2013;110(6):1010–1016. doi: 10.1093/bja/aes578 [DOI] [PubMed] [Google Scholar]
- 44.Fallon M, Giusti R, Aielli F, et al. Management of cancer pain in adult patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2018;29(Suppl 4):iv166–iv191. doi: 10.1093/annonc/mdy152 [DOI] [PubMed] [Google Scholar]
- 45.Lauretti GR, Lima ICPR, Reis MP, et al. Oral ketamine and transdermal nitroglycerin as analgesic adjuvants to oral morphine therapy for cancer pain management. Anesthesiology. 1999;90(6):1528–1533. doi: 10.1097/00000542-199906000-00005 [DOI] [PubMed] [Google Scholar]
- 46.Hardy J, Quinn S, Fazekas B, et al. Randomized, double-blind, placebo-controlled study to assess the efficacy and toxicity of subcutaneous ketamine in the management of cancer pain. J Clin Oncol. 2012;30(29):3611–3617. doi: 10.1200/JCO.2012.42.1081 [DOI] [PubMed] [Google Scholar]
- 47.Mercadante S, Arcuri E, Tirelli W, et al. Analgesic effect of intravenous ketamine in cancer patients on morphine therapy: a randomized, controlled, double-blind, crossover, double-dose study. J Pain Symptom Manage. 2000;20(4):246–252. doi: 10.1016/S0885-3924(00)00194-9 [DOI] [PubMed] [Google Scholar]
- 48.Yang CY, Wong C-S, Chang J-Y, et al. Intrathecal ketamine reduces morphine requirements in patients with terminal cancer pain. Can J Anaesth. 1996;43(4):379–383. doi: 10.1007/BF03011718 [DOI] [PubMed] [Google Scholar]
- 49.Fallon MT, Wilcock A, Kelly CA, et al. Oral ketamine vs placebo in patients with cancer-related neuropathic pain: a randomized clinical trial. JAMA Oncol. 2018;4(6):870–872. doi: 10.1001/jamaoncol.2018.0131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Swarm RA, Dans M. NCCN Frameworks for resource stratification of NCCN guidelines: adult cancer pain and palliative care. J Natl Compr Canc Netw. 2018;16(5S):628–631. doi: 10.6004/jnccn.2018.0044 [DOI] [PubMed] [Google Scholar]
- 51.Jonkman K, van de Donk T, Dahan A. Ketamine for cancer pain: what is the evidence? Curr Opin Support Palliat Care. 2017;11(2):88–92. doi: 10.1097/SPC.0000000000000262 [DOI] [PubMed] [Google Scholar]
- 52.Bell RF, Eccleston C, Kalso EA. Ketamine as an adjuvant to opioids for cancer pain. Cochrane Database Syst Rev. 2017;6:CD003351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hartsell WF, Scott CB, Bruner DW, et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst. 2005;97(11):798–804. doi: 10.1093/jnci/dji139 [DOI] [PubMed] [Google Scholar]
- 54.Nguyen QN, Chun SG, Chow E, et al. Single-fraction stereotactic vs conventional multifraction radiotherapy for pain relief in patients with predominantly nonspine bone metastases: a randomized Phase 2 Trial. JAMA Oncol. 2019;5(6):872–878. doi: 10.1001/jamaoncol.2019.0192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guidelines on Management of Pain in Cancer and/or Palliative Care; 2017. Available from: https://www.cancercareontario.ca/en/content/guidelines-management-pain-cancer-andor-palliative-care. Accessed June22, 2021.
- 56.O’Carrigan B, Wong MH, Willson ML, et al. Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst Rev. 2017;10(10):Cd003474. doi: 10.1002/14651858.CD003474.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hendriks LE, Hermans BCM, van den Beuken—van Everdingen MHJ, et al. Effect of bisphosphonates, denosumab, and radioisotopes on bone pain and quality of life in patients with non-small cell lung cancer and bone metastases: a systematic review. J Thorac Oncol. 2016;11(2):155–173. doi: 10.1016/j.jtho.2015.10.001 [DOI] [PubMed] [Google Scholar]
- 58.Martinez-Zapata MJ, et al. Calcitonin for metastatic bone pain. Cochrane Database Syst Rev. 2006;1(3):CD003223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Plancarte R, de Leon-casasola OA, El-Helaly M, et al. Neurolytic superior hypogastric plexus block for chronic pelvic pain associated with cancer. Reg Anesth. 1997;22(6):562–568. [PubMed] [Google Scholar]
- 60.Loukas M, Klaassen Z, Merbs W, et al. A review of the thoracic splanchnic nerves and celiac ganglia. Clin Anat. 2010;23(5):512–522. doi: 10.1002/ca.20964 [DOI] [PubMed] [Google Scholar]
- 61.Ischia S, Ischia A, Polati E, et al. Three posterior percutaneous celiac plexus block techniques. a prospective, randomized study in 61 patients with pancreatic cancer pain. Anesthesiology. 1992;76(4):534–540. doi: 10.1097/00000542-199204000-00008 [DOI] [PubMed] [Google Scholar]
- 62.Süleyman Ozyalçin N, et al. Efficacy of coeliac plexus and splanchnic nerve blockades in body and tail located pancreatic cancer pain. Eur J Pain. 2004;8(6):539–545. doi: 10.1016/j.ejpain.2004.01.001 [DOI] [PubMed] [Google Scholar]
- 63.Ardengh JC, Kemp R, Lima ER, et al. Tu1517 a prospective controlled study on the eus-cpn bilateral injection of 30 CC of alcohol in patients with pancreatic cancer pain. Gastrointest Endosc. 2012;75(4):AB432. doi: 10.1016/j.gie.2012.03.1163 [DOI] [Google Scholar]
- 64.Gunaratnam NT, et al. A prospective study of EUS-guided celiac plexus neurolysis for pancreatic cancer pain. Gastrointest Endosc. 2001;54(3):316–324. [DOI] [PubMed] [Google Scholar]
- 65.LeBlanc JK, et al. A prospective, randomized study of EUS-guided celiac plexus neurolysis for pancreatic cancer: one injection or two? Gastrointest Endosc. 2011;74(6):1300–1307. doi: 10.1016/j.gie.2011.07.073 [DOI] [PubMed] [Google Scholar]
- 66.Bhatnagar S, et al. Bedside ultrasound-guided celiac plexus neurolysis in upper abdominal cancer patients: a randomized, prospective study for comparison of percutaneous bilateral paramedian vs. unilateral paramedian needle-insertion technique. Pain Pract. 2014;14(2):E63–8. doi: 10.1111/papr.12107 [DOI] [PubMed] [Google Scholar]
- 67.Allen PJ, Manchikanti L, et al. Prospective evaluation of laparoscopic celiac plexus block in patients with unresectable pancreatic adenocarcinoma. Ann Surg Oncol. 2011;18(3):636–641. doi: 10.1245/s10434-010-1372-x [DOI] [PubMed] [Google Scholar]
- 68.Lillemoe KD, Cameron JL, Kaufman HS, et al. Chemical splanchnicectomy in patients with unresectable pancreatic cancer. A prospective randomized trial. Ann Surg. 1993;217(5):447–455. doi: 10.1097/00000658-199305010-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nagels W, et al. Celiac plexus neurolysis for abdominal cancer pain: a systematic review. Pain Med. 2013;14(8):1140–1163. doi: 10.1111/pme.12176 [DOI] [PubMed] [Google Scholar]
- 70.Wong GY, Schroeder DR, Carns PE, et al. Effect of neurolytic celiac plexus block on pain relief, quality of life, and survival in patients with unresectable pancreatic cancer: a randomized controlled trial. JAMA. 2004;291(9):1092–1099. doi: 10.1001/jama.291.9.1092 [DOI] [PubMed] [Google Scholar]
- 71.Staats PS, et al. The effects of alcohol celiac plexus block, pain, and mood on longevity in patients with unresectable pancreatic cancer: a double-blind, randomized, placebo-controlled study. Pain Med. 2001;2(1):28–34. [DOI] [PubMed] [Google Scholar]
- 72.Köker IH, Aralaşmak A, Ünver N, et al. Spinal cord ischemia after endoscopic ultrasound guided celiac plexus neurolysis: case report and review of the literature. Scand J Gastroenterol. 2017;52(10):1158–1161. doi: 10.1080/00365521.2017.1335771 [DOI] [PubMed] [Google Scholar]
- 73.Park K, Kim S. Digital subtraction angiography vs. real-time fluoroscopy for detection of intravascular injection during transforaminal epidural block. Yeungnam Univ J Med. 2019;36(2):109–114. doi: 10.12701/yujm.2019.00122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brown DL, Bulley CK, Quiel EL. Neurolytic celiac plexus block for pancreatic cancer pain. Anesth Analg. 1987;66(9):869–873. doi: 10.1213/00000539-198709000-00011 [DOI] [PubMed] [Google Scholar]
- 75.Eisenberg E, Carr DB, Chalmers TC. Neurolytic celiac plexus block for treatment of cancer pain: a meta-analysis. Anesth Analg. 1995;80(2):290–295. doi: 10.1097/00000539-199502000-00015 [DOI] [PubMed] [Google Scholar]
- 76.Dumitrescu A, Aggarwal A, Chye R. A retrospective case series of patients who have undergone coeliac plexus blocks for the purpose of alleviating pain due to intra-abdominal malignancy. Cancer Rep. 2020;3(5):e1265. doi: 10.1002/cnr2.1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kawamata M, Ishitani K, Ishikawa K, et al. Comparison between celiac plexus block and morphine treatment on quality of life in patients with pancreatic cancer pain. Pain. 1996;64(3):597–602. doi: 10.1016/0304-3959(95)00189-1 [DOI] [PubMed] [Google Scholar]
- 78.Facciorusso A, Di Maso M, Serviddio G, et al. Echoendoscopic ethanol ablation of tumor combined with celiac plexus neurolysis in patients with pancreatic adenocarcinoma. J Gastroenterol Hepatol. 2017;32(2):439–445. doi: 10.1111/jgh.13478 [DOI] [PubMed] [Google Scholar]
- 79.Amr YM, Makharita MY. Neurolytic sympathectomy in the management of cancer pain-time effect: a prospective, randomized multicenter study. J Pain Symptom Manage. 2014;48(5):944–56.e2. doi: 10.1016/j.jpainsymman.2014.01.015 [DOI] [PubMed] [Google Scholar]
- 80.de Oliveira R, Dos Reis MP, Prado WA. The effects of early or late neurolytic sympathetic plexus block on the management of abdominal or pelvic cancer pain. Pain. 2004;110(1–2):400–408. doi: 10.1016/j.pain.2004.04.023 [DOI] [PubMed] [Google Scholar]
- 81.Wyse JM, et al. Randomized, double-blind, controlled trial of early endoscopic ultrasound-guided celiac plexus neurolysis to prevent pain progression in patients with newly diagnosed, painful, inoperable pancreatic cancer. J Clin Oncol. 2011;29(26):3541–3546. doi: 10.1200/JCO.2010.32.2750 [DOI] [PubMed] [Google Scholar]
- 82.Doi S, Yasuda I, Kawakami H, et al. Endoscopic ultrasound-guided celiac ganglia neurolysis vs. celiac plexus neurolysis: a randomized multicenter trial. Endoscopy. 2013;45(5):362–369. doi: 10.1055/s-0032-1326225 [DOI] [PubMed] [Google Scholar]
- 83.Lu F, Dong J, Tang Y, et al. Bilateral vs. unilateral endoscopic ultrasound-guided celiac plexus neurolysis for abdominal pain management in patients with pancreatic malignancy: a systematic review and meta-analysis. Supportive Care in Cancer. 2018;26(2):353–359. doi: 10.1007/s00520-017-3888-0 [DOI] [PubMed] [Google Scholar]
- 84.Yoon WJ, Oh Y, Yoo C, et al. EUS-guided versus percutaneous celiac neurolysis for the management of intractable pain due to unresectable pancreatic cancer: a randomized clinical trial. J Clin Med. 2020;9(6):1666. doi: 10.3390/jcm9061666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu M. Neural blockade in clinical anesthesia and management of pain. Anesth Analg. 1998;87(3):749. [Google Scholar]
- 86.Bang JY, et al. EUS-guided celiac ganglion radiofrequency ablation versus celiac plexus neurolysis for palliation of pain in pancreatic cancer: a randomized controlled trial (with videos). Gastrointest Endosc. 2019;89(1):58–66.e3. [DOI] [PubMed] [Google Scholar]
- 87.Amr SA, Reyad RM, Othman AH, et al. Comparison between radiofrequency ablation and chemical neurolysis of thoracic splanchnic nerves for the management of abdominal cancer pain, randomized trial. Eur J Pain. 2018;22(10):1782–1790. doi: 10.1002/ejp.1274 [DOI] [PubMed] [Google Scholar]
- 88.Hou S, et al. Efficacy of superior hypogastric plexus neurolysis for the treatment of cancer-related pelvic pain. Pain Med. 2020;21(6):1255–1262. doi: 10.1093/pm/pnz151 [DOI] [PubMed] [Google Scholar]
- 89.Rocha A, et al. Effectiveness of superior hypogastric plexus neurolysis for pelvic cancer pain. Pain Physician. 2020;23(2):203–208. [PubMed] [Google Scholar]
- 90.Mishra S, et al. Efficacy of the anterior ultrasound-guided superior hypogastric plexus neurolysis in pelvic cancer pain in advanced gynecological cancer patients. Pain Med. 2013;14(6):837–842. doi: 10.1111/pme.12106 [DOI] [PubMed] [Google Scholar]
- 91.Hetta DF, et al. Pulsed radiofrequency of the sacral roots improves the success rate of superior hypogastric plexus neurolysis in controlling pelvic and perineal cancer pain. Pain Physician. 2020;23(2):149–157. doi: 10.36076/ppj.2020/23/149 [DOI] [PubMed] [Google Scholar]
- 92.Mercadante S, et al. Sympathetic blocks for visceral cancer pain management: a systematic review and EAPC recommendations. Crit Rev Oncol Hematol. 2015;96(3):577–583. doi: 10.1016/j.critrevonc.2015.07.014 [DOI] [PubMed] [Google Scholar]
- 93.Sousa Correia J, et al. The efficacy of the ganglion impar block in perineal and pelvic cancer pain. Support Care Cancer. 2019;27(11):4327–4330. doi: 10.1007/s00520-019-04738-9 [DOI] [PubMed] [Google Scholar]
- 94.Sindt JE, Brogan SE. Interventional treatments of cancer pain. Anesthesiol Clin. 2016;34(2):317–339. doi: 10.1016/j.anclin.2016.01.004 [DOI] [PubMed] [Google Scholar]
- 95.Wemm K, Saberski L. Modified approach to block the ganglion impar (ganglion of Walther). Reg Anesth. 1995;20(6):544–545. [PubMed] [Google Scholar]
- 96.Lin CS, Cheng J-K, Hsu Y-W, et al. Ultrasound-guided ganglion impar block: a technical report. Pain Med. 2010;11(3):390–394. doi: 10.1111/j.1526-4637.2010.00797.x [DOI] [PubMed] [Google Scholar]
- 97.Agarwal-Kozlowski K, Lorke DE, Habermann CR, et al. CT-guided blocks and neuroablation of the ganglion impar (Walther) in perineal pain: anatomy, technique, safety, and efficacy. Clin J Pain. 2009;25(7):570–576. doi: 10.1097/AJP.0b013e3181a5f5c7 [DOI] [PubMed] [Google Scholar]
- 98.Eker HE, et al. Transsacrococcygeal Approach to Ganglion Impar for Pelvic Cancer Pain: A Report of 3 Cases, in Reg Anesth Pain Med. England; 2008: 381–382. [DOI] [PubMed] [Google Scholar]
- 99.Bhatnagar S, Khanna S, Roshni S, et al. Early ultrasound-guided neurolysis for pain management in gastrointestinal and pelvic malignancies: an observational study in a tertiary care center of urban India. Pain Pract. 2012;12(1):23–32. doi: 10.1111/j.1533-2500.2011.00467.x [DOI] [PubMed] [Google Scholar]
- 100.Toshniwal GR, Dureja GP, Prashanth SM. Transsacrococcygeal approach to ganglion impar block for management of chronic perineal pain: a prospective observational study. Pain Physician. 2007;10(5):661–666. doi: 10.36076/ppj.2007/10/661 [DOI] [PubMed] [Google Scholar]
- 101.Walters A, Muhleman M, Osiro S, et al. One is the loneliest number: a review of the ganglion impar and its relation to pelvic pain syndromes. Clin Anat. 2013;26(7):855–861. doi: 10.1002/ca.22193 [DOI] [PubMed] [Google Scholar]
- 102.Smith TJ, Staats PS, Deer T, et al. Randomized clinical trial of an implantable drug delivery system compared with comprehensive medical management for refractory cancer pain: impact on pain, drug-related toxicity, and survival. J Clin Oncol. 2002;20(19):4040–4049. doi: 10.1200/JCO.2002.02.118 [DOI] [PubMed] [Google Scholar]
- 103.Rauck RL, Wallace MS, Leong MS, et al. A randomized, double-blind, placebo-controlled study of intrathecal ziconotide in adults with severe chronic pain. J Pain Symptom Manage. 2006;31(5):393–406. doi: 10.1016/j.jpainsymman.2005.10.003 [DOI] [PubMed] [Google Scholar]
- 104.Brogan SE, Sindt JE, Jackman CM, et al. Prospective Association of Serum Opioid Levels and Clinical Outcomes in Patients With Cancer Pain Treated With Intrathecal Opioid Therapy. Anesth Analg. 2020;130(4):1035–1044. doi: 10.1213/ANE.0000000000004276 [DOI] [PubMed] [Google Scholar]
- 105.Carvajal G, Dupoiron D, Seegers V, et al. Intrathecal drug delivery systems for refractory pancreatic cancer pain: observational follow-up study over an 11-year period in a comprehensive cancer center. Anesth Analg. 2018;126(6):2038–2046. doi: 10.1213/ANE.0000000000002903 [DOI] [PubMed] [Google Scholar]
- 106.Zheng S, He L, Yang X, et al. Evaluation of intrathecal drug delivery system for intractable pain in advanced malignancies: a prospective cohort study. Medicine. 2017;96(11):e6354. doi: 10.1097/MD.0000000000006354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hayek SM, et al. Intrathecal therapy for cancer and non-cancer pain. Pain Physician. 2011;14(3):219–248. doi: 10.36076/ppj.2011/14/219 [DOI] [PubMed] [Google Scholar]
- 108.Ummenhofer WC, Arends R, Shen D, et al. Comparative spinal distribution and clearance kinetics of intrathecally administered morphine, fentanyl, alfentanil, and sufentanil. Anesthesiology. 2000;92(3):739–753. doi: 10.1097/00000542-200003000-00018 [DOI] [PubMed] [Google Scholar]
- 109.Flack SH, Anderson CM, Bernards C. Morphine distribution in the spinal cord after chronic infusion in pigs. Anesth Analg. 2011;112(2):460–464. doi: 10.1213/ANE.0b013e318203b7c0 [DOI] [PubMed] [Google Scholar]
- 110.Tangen KM, Hsu Y, Zhu DC, et al. CNS wide simulation of flow resistance and drug transport due to spinal microanatomy. J Biomech. 2015;48(10):2144–2154. doi: 10.1016/j.jbiomech.2015.02.018 [DOI] [PubMed] [Google Scholar]
- 111.Hsu Y, Hettiarachchi HDM, Zhu DC, et al. The frequency and magnitude of cerebrospinal fluid pulsations influence intrathecal drug distribution: key factors for interpatient variability. Anesth Analg. 2012;115(2):386–394. doi: 10.1213/ANE.0b013e3182536211 [DOI] [PubMed] [Google Scholar]
- 112.Deer TR, Hayek SM, Pope JE, et al. The Polyanalgesic Consensus Conference (PACC): recommendations for Trialing of Intrathecal Drug Delivery Infusion Therapy. Neuromodulation. 2017;20(2):133–154. doi: 10.1111/ner.12543 [DOI] [PubMed] [Google Scholar]
- 113.Medtronic. Product Surveillance Registry (PSR) Database. Minneapolis, MN; 2015. [Google Scholar]
- 114.Hamza M, Doleys DM, Saleh IA, et al. A prospective, randomized, single-blinded, head-to-head long-term outcome study, comparing intrathecal (IT) boluses with continuous infusion trialing techniques prior to implantation of drug delivery systems (DDS) for the treatment of severe intractable chronic nonmalignant pain. Neuromodulation. 2015;18(7):636–648. doi: 10.1111/ner.12342 [DOI] [PubMed] [Google Scholar]
- 115.Maniker AH, Krieger AJ, Adler RJ, et al. Epidural trial in implantation of intrathecal morphine infusion pumps. N J Med. 1991;88(11):797–801. [PubMed] [Google Scholar]
- 116.Deer TR, et al. The Polyanalgesic Consensus Conference (PACC): recommendations on Intrathecal Drug Infusion Systems Best Practices and Guidelines. Neuromodulation. 2017;20(2):96–132. [DOI] [PubMed] [Google Scholar]
- 117.Brogan SE, Winter NB, Okifuji A. Prospective observational study of patient-controlled intrathecal analgesia: impact on cancer-associated symptoms, breakthrough pain control, and patient satisfaction. Reg Anesth Pain Med. 2015;40(4):369–375. doi: 10.1097/AAP.0000000000000251 [DOI] [PubMed] [Google Scholar]
- 118.Sindt JE, Odell DW, Dalley AP, et al. Initiation of intrathecal drug delivery dramatically reduces systemic opioid use in patients with advanced cancer. Neuromodulation. 2020;23(7):978–983. doi: 10.1111/ner.13175 [DOI] [PubMed] [Google Scholar]
- 119.Staats PS, Yearwood T, Charapata SG, et al. Intrathecal ziconotide in the treatment of refractory pain in patients with cancer or AIDS: a randomized controlled trial. JAMA. 2004;291(1):63–70. doi: 10.1001/jama.291.1.63 [DOI] [PubMed] [Google Scholar]
- 120.Wilkes D. Programmable intrathecal pumps for the management of chronic pain: recommendations for improved efficiency. J Pain Res. 2014;7:571–577. doi: 10.2147/JPR.S46929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen GH, Spiegel MA, Magram YC, et al. Evaluation of fixed intrathecal bupivacaine infusion doses in the oncologic population. Neuromodulation. 2020;23(7):984–990. doi: 10.1111/ner.13161 [DOI] [PubMed] [Google Scholar]
- 122.Deer TR, Pope JE, Hayek SM, et al. The Polyanalgesic Consensus Conference (PACC): recommendations for Intrathecal Drug Delivery: guidance for Improving Safety and Mitigating Risks. Neuromodulation. 2017;20(2):155–176. doi: 10.1111/ner.12579 [DOI] [PubMed] [Google Scholar]
- 123.Odell DW, Albrechtsen RD, Sindt JE, et al. The effect of measured radiotherapy dose on intrathecal drug delivery system function. Neuromodulation. 2021. doi: 10.1111/ner.13372 [DOI] [PubMed] [Google Scholar]
- 124.Bhatia G, Lau ME, Gulur P, et al. Intrathecal Drug Delivery (ITDD) systems for cancer pain. F1000Res. 2013;2:96. doi: 10.12688/f1000research.2-96.v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shah R, Baqai-Stern A, Gulati A. Managing intrathecal drug delivery (ITDD) in cancer patients. Curr Pain Headache Rep. 2015;19(6):20. doi: 10.1007/s11916-015-0488-x [DOI] [PubMed] [Google Scholar]
- 126.Narouze S, Benzon HT, Provenzano D, et al. Interventional Spine and Pain Procedures in Patients on Antiplatelet and Anticoagulant Medications (Second Edition): guidelines From the American Society of Regional Anesthesia and Pain Medicine, the European Society of Regional Anaesthesia and Pain Therapy, the American Academy of Pain Medicine, the International Neuromodulation Society, the North American Neuromodulation Society, and the World Institute of Pain. Reg Anesth Pain Med. 2018;43(3):225–262. doi: 10.1097/AAP.0000000000000700 [DOI] [PubMed] [Google Scholar]
- 127.Sindt JE, Larsen SD, Dalley AP, et al. The rate of infectious complications after intrathecal drug delivery system implant for cancer-related pain is low despite frequent concurrent anticancer treatment or leukopenia. Anesth Analg. 2020;131(1):280–287. doi: 10.1213/ANE.0000000000004639 [DOI] [PubMed] [Google Scholar]
- 128.Engle MP, et al. Infectious complications related to intrathecal drug delivery system and spinal cord stimulator system implantations at a comprehensive cancer pain center. Pain Physician. 2013;16(3):251–257. doi: 10.36076/ppj.2013/16/251 [DOI] [PubMed] [Google Scholar]
- 129.Stearns L, et al. Intrathecal drug delivery systems for cancer pain: an analysis of a prospective, multicenter product surveillance registry. Anesth Analg. 2019;130:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sayed D, Chakravarthy K, Amirdelfan K, et al. A comprehensive practice guideline for magnetic resonance imaging compatibility in implanted neuromodulation devices. Neuromodulation. 2020;23(7):893–911. doi: 10.1111/ner.13233 [DOI] [PubMed] [Google Scholar]
- 131.Metronic. MRI Guidelines for Medtronic Implantable Infusion Systems; 2020. [Google Scholar]
- 132.Flowonix. Prometra® 20 mL (REF 11827), Prometra® II 20 mL (REF 13827) and Prometra® II 40 mL (REF 16827) Programmable Pumps Magnetic Resonance Imaging (MRI) Safety Information; 2020. [Google Scholar]
- 133.Brogan SE, Winter NB, Abiodun A, et al. A cost utilization analysis of intrathecal therapy for refractory cancer pain: identifying factors associated with cost benefit. Pain Med. 2013;14(4):478–486. doi: 10.1111/pme.12060 [DOI] [PubMed] [Google Scholar]
- 134.Mekhail NA, Mathews M, Nageeb F, et al. Retrospective review of 707 cases of spinal cord stimulation: indications and complications. Pain Pract. 2011;11(2):148–153. doi: 10.1111/j.1533-2500.2010.00407.x [DOI] [PubMed] [Google Scholar]
- 135.Hui D, Bruera E. Integrating palliative care into the trajectory of cancer care. Nat Rev Clin Oncol. 2016;13(3):159–171. doi: 10.1038/nrclinonc.2015.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Deer TR, Lamer TJ, Pope JE, et al. The Neurostimulation Appropriateness Consensus Committee (NACC) Safety Guidelines for the Reduction of Severe Neurological Injury. Neuromodulation. 2017;20(1):15–30. doi: 10.1111/ner.12564 [DOI] [PubMed] [Google Scholar]
- 137.Roser M, Ritchie H. Cancer; 2015. Available from: https://ourworldindata.org/cancer. Accessed June22, 2021.
- 138.Shah A, Hoffman EM, Mauermann ML, et al. Incidence and disease burden of chemotherapy-induced peripheral neuropathy in a population-based cohort. J Neurol Neurosurg Psychiatry. 2018;89(6):636–641. doi: 10.1136/jnnp-2017-317215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Braun Filho JL, Braun LM. Spinal cord stimulation in the treatment of refractory painful polineuropathy induced by chemotherapy. Rev Bras Anestesiol. 2007;57(5):533–538. doi: 10.1590/S0034-70942007000500008 [DOI] [PubMed] [Google Scholar]
- 140.Cata JP, Cordella JV, Burton AW, et al. Spinal cord stimulation relieves chemotherapy-induced pain: a clinical case report. J Pain Symptom Manage. 2004;27(1):72–78. doi: 10.1016/j.jpainsymman.2003.05.007 [DOI] [PubMed] [Google Scholar]
- 141.Abd-Elsayed A, Schiavoni N, Sachdeva H. Efficacy of spinal cord stimulators in treating peripheral neuropathy: a case series. J Clin Anesth. 2016;28:74–77. doi: 10.1016/j.jclinane.2015.08.011 [DOI] [PubMed] [Google Scholar]
- 142.Sivanesan E, Stephens KE, Huang Q, et al. Spinal cord stimulation prevents paclitaxel-induced mechanical and cold hypersensitivity and modulates spinal gene expression in rats. Pain Rep. 2019;4(5):e785. doi: 10.1097/PR9.0000000000000785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yakovlev AE, Resch BE. Treatment of multifocal pain with spinal cord stimulation. Neuromodulation. 2012;15(3):210. doi: 10.1111/j.1525-1403.2012.00435.x [DOI] [PubMed] [Google Scholar]
- 144.Shimoji K, Hokari T, Kano T, et al. Management of intractable pain with percutaneous epidural spinal cord stimulation: differences in pain-relieving effects among diseases and sites of pain. Anesth Analg. 1993;77(1):110–116. doi: 10.1213/00000539-199307000-00022 [DOI] [PubMed] [Google Scholar]
- 145.Antony AB, Schultheis BC, Jolly SM, et al. Neuromodulation of the dorsal root ganglion for chronic postsurgical pain. Pain Med. 2019;20(Suppl 1):S41–s46. doi: 10.1093/pm/pnz072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hunter CW, Yang A. Dorsal root ganglion stimulation for chronic pelvic pain: a case series and technical report on a novel lead configuration. Neuromodulation. 2019;22(1):87–95. doi: 10.1111/ner.12801 [DOI] [PubMed] [Google Scholar]
- 147.Richter B, Novik Y, Bergman JJ, et al. The efficacy of BurstDR spinal cord stimulation for chronic abdominal pain: a clinical series. World Neurosurg. 2020;138:77–82. doi: 10.1016/j.wneu.2020.02.075 [DOI] [PubMed] [Google Scholar]
- 148.Kapural L, Nagem H, Tlucek H, et al. Spinal cord stimulation for chronic visceral abdominal pain. Pain Med. 2010;11(3):347–355. doi: 10.1111/j.1526-4637.2009.00785.x [DOI] [PubMed] [Google Scholar]
- 149.Skolasky RL, Wegener ST, Maggard AM, et al. The impact of reduction of pain after lumbar spine surgery: the relationship between changes in pain and physical function and disability. Spine. 2014;39(17):1426–1432. doi: 10.1097/BRS.0000000000000428 [DOI] [PubMed] [Google Scholar]
- 150.Kapural L, Yu C, Doust MW, et al. Novel 10-kHz high-frequency therapy (HF10 Therapy) is superior to traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: the SENZA-RCT randomized controlled trial. Anesthesiology. 2015;123(4):851–860. doi: 10.1097/ALN.0000000000000774 [DOI] [PubMed] [Google Scholar]
- 151.Deer T, Slavin KV, Amirdelfan K, et al. Success Using Neuromodulation With BURST (SUNBURST) Study: results from a prospective, randomized controlled trial using a novel burst waveform. Neuromodulation. 2018;21(1):56–66. doi: 10.1111/ner.12698 [DOI] [PubMed] [Google Scholar]
- 152.Desai MJ, Hargens LM, Breitenfeldt MD, et al. The rate of magnetic resonance imaging in patients with spinal cord stimulation. Spine. 2015;40(9):E531–7. doi: 10.1097/BRS.0000000000000805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Delank KS, Wendtner C, Eich HT, et al. The treatment of spinal metastases. Dtsch Arztebl Int. 2011;108(5):71–79. doi: 10.3238/arztebl.2011.0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chen KY, Ma HI, Chiang YH. Percutaneous transpedicular vertebroplasty with polymethyl methacrylate for pathological fracture of the spine. J Clin Neurosci. 2009;16(10):1300–1304. doi: 10.1016/j.jocn.2007.12.017 [DOI] [PubMed] [Google Scholar]
- 155.Berenson J, Pflugmacher R, Jarzem P, et al. Balloon kyphoplasty versus non-surgical fracture management for treatment of painful vertebral body compression fractures in patients with cancer: a multicentre, randomised controlled trial. Lancet Oncol. 2011;12(3):225–235. doi: 10.1016/S1470-2045(11)70008-0 [DOI] [PubMed] [Google Scholar]
- 156.Aghayev K, Papanastassiou ID, Vrionis F. Role of vertebral augmentation procedures in the management of vertebral compression fractures in cancer patients. Curr Opin Support Palliat Care. 2011;5(3):222–226. doi: 10.1097/SPC.0b013e328349652d [DOI] [PubMed] [Google Scholar]
- 157.Chew C, Craig L, Edwards R, et al. Safety and efficacy of percutaneous vertebroplasty in malignancy: a systematic review. Clin Radiol. 2011;66(1):63–72. doi: 10.1016/j.crad.2010.09.011 [DOI] [PubMed] [Google Scholar]
- 158.Alexander A, Rosenberg AE. Vertebral augmentation involving vertebroplasty or kyphoplasty for cancer-related vertebral compression fractures: a systematic review. Ont Health Technol Assess Ser. 2016;16(11):1–202. [PMC free article] [PubMed] [Google Scholar]
- 159.Köse KC, Cebesoy O, Akan B, et al. Functional results of vertebral augmentation techniques in pathological vertebral fractures of myelomatous patients. J Natl Med Assoc. 2006;98(10):1654–1658. [PMC free article] [PubMed] [Google Scholar]
- 160.Sayed D, et al. Spinal radiofrequency ablation combined with cement augmentation for painful spinal vertebral metastasis: a single-center prospective study. Pain Physician. 2019;22(5):E441–e449. doi: 10.36076/ppj/2019.22.E441 [DOI] [PubMed] [Google Scholar]
- 161.Rosenthal DI, Alexander A, Rosenberg AE, et al. Ablation of osteoid osteomas with a percutaneously placed electrode: a new procedure. Radiology. 1992;183(1):29–33. doi: 10.1148/radiology.183.1.1549690 [DOI] [PubMed] [Google Scholar]
- 162.Levy J, Hopkins T, Morris J, et al. Radiofrequency ablation for the palliative treatment of bone metastases: outcomes from the multicenter OsteoCool Tumor Ablation Post-Market Study (OPuS One Study) in 100 patients. J Vasc Interv Radiol. 2020;31(11):1745–1752. doi: 10.1016/j.jvir.2020.07.014 [DOI] [PubMed] [Google Scholar]
- 163.Cazzato RL, Garnon J, Caudrelier J, et al. Percutaneous radiofrequency ablation of painful spinal metastasis: a systematic literature assessment of analgesia and safety. Int J Hyperthermia. 2018;34(8):1272–1281. doi: 10.1080/02656736.2018.1425918 [DOI] [PubMed] [Google Scholar]
- 164.Zairi F, Vieillard MH, Assaker R. Spine metastases: are minimally invasive surgical techniques living up to the hype? CNS Oncol. 2015;4(4):257–264. doi: 10.2217/cns.15.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Khan MA, Deib G, Deldar B, et al. Efficacy and safety of percutaneous microwave ablation and cementoplasty in the treatment of painful spinal metastases and myeloma. AJNR Am J Neuroradiol. 2018;39(7):1376–1383. doi: 10.3174/ajnr.A5680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bharti N, et al. Pulsed radiofrequency ablation for the treatment of glossopharyngeal neuralgia secondary to oropharyngeal carcinoma. Pain Physician. 2018;21(3):295–302. doi: 10.36076/ppj.2018.3.295 [DOI] [PubMed] [Google Scholar]
- 167.Fanous SN, et al. Randomized controlled trials between dorsal root ganglion thermal radiofrequency, pulsed radiofrequency and steroids for the management of intractable metastatic back pain in thoracic vertebral body. Br J Pain. 2020;2049463720942538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Singh N, Singh S, Mishra N, et al. Comparison of extraoral and intraoral routes of glossopharyngeal nerve block for pain relief in patient with carcinoma tongue: a prospective randomized study. J Cancer Res Ther. 2020;16(3):534–538. doi: 10.4103/jcrt.JCRT_309_18 [DOI] [PubMed] [Google Scholar]
- 169.Hochberg U, Berger A, Atias M, et al. Tailoring of neurosurgical ablative procedures in the management of refractory cancer pain. Reg Anesth Pain Med. 2020;45(9):696–701. doi: 10.1136/rapm-2020-101566 [DOI] [PubMed] [Google Scholar]
- 170.Raslan AM, Ben-Haim S, Falowski SM, et al. Congress of neurological surgeons systematic review and evidence-based guideline on neuroablative procedures for patients with cancer pain. Neurosurgery. 2021;88(3):437–442. doi: 10.1093/neuros/nyaa527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kanpolat Y, Ugur HC, Ayten M, et al. Computed tomography-guided percutaneous cordotomy for intractable pain in malignancy. Neurosurgery. 2009;64(3 Suppl):ons187–93. doi: 10.1227/01.NEU.0000335645.67282.03 [DOI] [PubMed] [Google Scholar]
- 172.Kanpolat Y, Ozdemir M, Al-Beyati E. CT-guided percutaneous cordotomy for intractable pain in what is more than a disease: lung malignancies. Turk Neurosurg. 2013;23(1):81–87. doi: 10.5137/1019-5149.JTN.6980-12.0 [DOI] [PubMed] [Google Scholar]
- 173.Fonoff ET, Lopez WOC, de Oliveira YSA, et al. Microendoscopy-guided percutaneous cordotomy for intractable pain: case series of 24 patients. J Neurosurg. 2016;124(2):389–396. doi: 10.3171/2014.12.JNS141616 [DOI] [PubMed] [Google Scholar]
- 174.Viswanathan A, Vedantam A, Hess KR, et al. Minimally invasive cordotomy for refractory cancer pain: a randomized controlled trial. Oncologist. 2019;24(7):e590–e596. doi: 10.1634/theoncologist.2018-0570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bain E, Hugel H, Sharma M. Percutaneous cervical cordotomy for the management of pain from cancer: a prospective review of 45 cases. J Palliat Med. 2013;16(8):901–907. doi: 10.1089/jpm.2013.0027 [DOI] [PubMed] [Google Scholar]
- 176.Raslan AM. Percutaneous computed tomography-guided radiofrequency ablation of upper spinal cord pain pathways for cancer-related pain. Neurosurgery. 2008;62(3 Suppl 1):226–233. doi: 10.1227/01.neu.0000317397.16089.f5 [DOI] [PubMed] [Google Scholar]
- 177.Zomers PJW, et al. Percutaneous cervical cordotomy for the treatment of cancer pain: a prospective case series of 52 patients with a long-term follow-up. Pain Pract. 2020. [DOI] [PubMed] [Google Scholar]
- 178.Nauta HJ, Soukup VM, Fabian RH, et al. Punctate midline myelotomy for the relief of visceral cancer pain. J Neurosurg. 2000;92(2 Suppl):125–130. doi: 10.3171/spi.2000.92.2.0125 [DOI] [PubMed] [Google Scholar]
- 179.Gadgil N, Viswanathan A. DREZotomy in the treatment of cancer pain: a review. Stereotact Funct Neurosurg. 2012;90(6):356–360. doi: 10.1159/000341072 [DOI] [PubMed] [Google Scholar]
- 180.Yen CP, Kung SS, Su YF, et al. Stereotactic bilateral anterior cingulotomy for intractable pain. J Clin Neurosci. 2005;12(8):886–890. doi: 10.1016/j.jocn.2004.11.018 [DOI] [PubMed] [Google Scholar]
- 181.Strauss I, Berger A, Ben Moshe S, et al. Double anterior stereotactic cingulotomy for intractable oncological pain. Stereotact Funct Neurosurg. 2017;95(6):400–408. doi: 10.1159/000484613 [DOI] [PubMed] [Google Scholar]
- 182.Viswanathan A, Harsh V, Pereira EAC, et al. Cingulotomy for medically refractory cancer pain. Neurosurg Focus. 2013;35(3):E1. doi: 10.3171/2013.6.FOCUS13236 [DOI] [PubMed] [Google Scholar]
- 183.Karai L, Brown DC, Mannes AJ, et al. Deletion of vanilloid receptor 1-expressing primary afferent neurons for pain control. J Clin Invest. 2004;113(9):1344–1352. doi: 10.1172/JCI20449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lee MG, et al. The effect of epidural resiniferatoxin in the neuropathic pain rat model. Pain Physician. 2012;15(4):287–296. doi: 10.36076/ppj.2012/15/287 [DOI] [PubMed] [Google Scholar]
- 185.Brown DC, Agnello K, Iadarola MJ. Intrathecal resiniferatoxin in a dog model: efficacy in bone cancer pain. Pain. 2015;156(6):1018–1024. doi: 10.1097/j.pain.0000000000000115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Heiss JMD, A Phase I Study of the Intrathecal Administration of Resiniferatoxin for Treating Severe Refractory Pain Associated With Advanced Cancer; 2008, National Institutes of Health Clinical Center. Available from: https://clinicaltrials.gov/ct2/show/NCT00804154. Accessed June22, 2021. [Google Scholar]
- 187.Nedeljkovic S, A Multicenter, Open-Label, Phase 1b Study to Assess the Safety and Define the Maximally Tolerated Dose of Epidural Resiniferatoxin (RTX) Injection for Treatment of Intractable Pain Associated with Cancer; 2020. Sorrento Therapeutics, Inc. Available from: https://www.clinicaltrials.gov/ct2/show/NCT03226574. Accessed June22, 2021. [Google Scholar]