Abstract
Idiopathic pulmonary fibrosis (IPF) is a progressive and fatal lung disease in which most patients die within 3 years of diagnosis. With an unknown etiology, IPF results in progressive fibrosis of the lung parenchyma, diminishing normal lung function, which results in respiratory failure, and eventually, death. While few therapies are available to reduce disease progression, patients continue to advance toward respiratory failure, leaving lung transplantation the only viable option for survival. As incidence and mortality rates steadily increase, the need for novel therapeutics is imperative. The receptor for advanced glycation endproducts (RAGE) is most highly expressed in the lungs and plays a significant role in a number of chronic lung diseases. RAGE has long been linked to IPF; however, confounding data from both human and experimental studies have left an incomplete and perplexing story. This review examines the present understanding of the role of RAGE in human and experimental models of IPF, drawing parallels to recent advances in RAGE biology. Moreover, this review discusses the role of RAGE in lung injury response, type 2 immunity, and cellular senescence, and how such mechanisms may relate to RAGE as both a biomarker of disease progression and potential therapeutic target in IPF.
The reviews of this paper are available via the supplemental material section.
Keywords: ILC2, interleukin-13, pulmonary fibrosis, RAGE, S100
Introduction
Idiopathic pulmonary fibrosis (IPF) is a progressive and universally fatal disease in which individuals usually die within 3 years of diagnosis due to progressive fibrosis of the pulmonary parenchyma. 1 Pathologically, IPF demonstrates a pattern of fibrosis referred to as usual interstitial pneumonia (UIP). UIP is recognized microscopically by the presence of temporally heterogeneous pulmonary fibrosis in which there are areas of mature fibrosis, immature fibrosis, and histologically normal alveolar parenchyma, sometimes in the same high-power field of view. The fibrosis is generally more severe in the lower lobes, lung bases and subpleural regions, but the disease progresses to involve more central areas with time. 2 This irreversible destruction of the normal lung architecture significantly reduces lung function, leading to respiratory failure. While there are known causes of pulmonary fibrosis that can lead to UIP such as collagen vascular disorders or asbestosis, most causes of UIP have no known cause and are diagnosed as IPF. 3
Although antifibrotic therapies such as nintedanib and pirfenidone have shown promise in slowing disease advancement, patients continue to progress toward respiratory failure, leaving lung transplantation the only other option to promote survival and avoid death.4,5 Therefore, there is a vital need for effective biomarkers to enhance early diagnosis and novel therapeutics to halt disease progression, improve quality of life and survival rates.
Tissue repair is an essential biological function in response to damage caused by endogenous or exogenous insults, in which damaged cells are removed, parenchymal space is repaired and structural cells reorganized to restore normal tissue function. 6 However, when dysregulated, this can lead to excessive remodeling and deposition of extracellular matrix components (e.g. fibronectin and collagens), resulting in fibrosis and disrupting normal tissue architecture, which in the lungs is critical to its primary function, gas exchange.7,8 The pathogenesis of pulmonary fibrosis is complicated and our understanding of it remains inadequate. It involves a number of complex mechanisms, including epithelial/endothelial injury, clotting response, innate and adaptive immunity, fibroblastic cell response, and tissue remodeling. However, full examination of these mechanisms is beyond the scope of this review and is discussed in detail elsewhere.9 –13 Herein, we will focus on the receptor for advanced glycation endproducts (RAGE) and its perplexing role in the pathogenesis of IPF.
RAGE is a member of the immunoglobulin superfamily of receptors and is a pattern-recognition receptor, first discovered for its ability to bind advanced glycation endproducts (AGEs), promoting inflammation and vascular complications in diabetes. 14 RAGE expression is highest in the lung, most notably in alveolar epithelial type I cells (AEC1)15,16 and is a central mediator of inflammation in lung disease. 17 RAGE is highly conserved across mammalian species exhibiting transcriptional variation throughout tissues.18,19 The RAGE protein exists as full-length membrane-bound RAGE (mRAGE), as well as a soluble form (sRAGE), which lacks the transmembrane and signaling domains, and functions as a decoy receptor. sRAGE can be produced endogenously by alternative splicing (esRAGE) or through proteolytic cleavage (cRAGE) by matrix metallopeptidase-9 (MMP9) or a disintegrin and metallopeptidase domain-10 (ADAM10).20 –23 RAGE is a promiscuous receptor, binding various ligands including, high-mobility group box-1 (HMGB1), S100 proteins, β-amyloid peptides and others. 17 RAGE-ligand binding can initiate feed-forward positive feedback, amplifying RAGE-signaling and contributing to the prominent role of RAGE during persistent inflammation.24,25 RAGE-activation induces a multitude of intracellular signaling pathways, contributing to various cellular processes including inflammation, migration and proliferation.26 –29 RAGE has long been linked to IPF; however, puzzling data from both human and experimental studies have left an incomplete and enigmatic story. This perspective review will explore the current understandings of the role of RAGE in IPF, proposing new potential mechanisms of RAGE signaling in fibrosis and how this may relate to RAGE as both a biomarker of disease progression and a potential therapeutic target.
RAGE expression in IPF
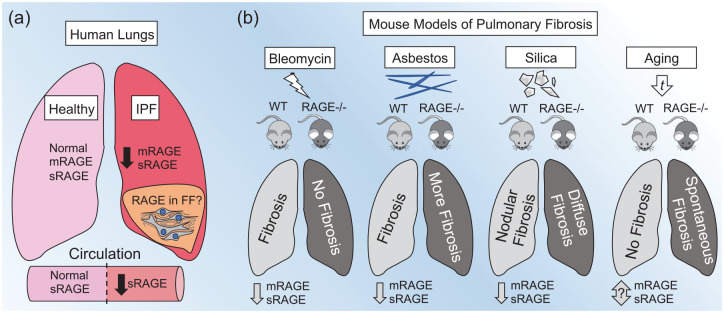
It has been well established that overall RAGE expression is significantly decreased in human IPF lungs, as well as mice with experimental pulmonary fibrosis (Figure 1). However, the cause and effect of this phenomenon remains a controversial subject of debate. Initially, Hanford et al. 16 demonstrated that both sRAGE and mRAGE were decreased in the lungs of mice after bleomycin-induced pulmonary fibrosis. Both RAGE protein and messenger ribonucleic acid (mRNA) expression were also decreased in asbestos and crystalline silica exposure models of pulmonary fibrosis;30,31 however, RAGE mRNA levels were not found to be affected in in a subsequent study in mice after bleomycin exposure 32 [Figure 1(b)]. Transcriptomic analyses of human lung tissues indicated that RAGE gene (AGER) transcripts were decreased in subjects with IPF.33,34 AGER expression was lower in the lungs of subjects who presented with more accelerated disease onset (based on time from symptom presentation to diagnosis), while the magnitude of downregulation in AGER transcripts was indistinguishable in both stable IPF and during acute exacerbations.33,34 This suggests that AGER levels may decrease more gradually prior to diagnosis, but remain stably decreased once the disease has progressed enough to be diagnosed. Moreover, multiple studies have shown both mRNA and protein levels of RAGE are significantly reduced in the whole-lung tissue of human subjects with IPF compared with healthy controls30,32 [Figure 1(a)]. Furthermore, in a comparative study of IPF and chronic obstructive pulmonary disease (COPD) lungs, full-length mRAGE and cRAGE were reduced in IPF and COPD lungs, whereas esRAGE was only decreased in IPF lungs. 35 As studies have shown that overall RAGE expression is decreased in IPF lungs, it is important to note that RAGE is most abundantly expressed in AEC1. 36 However, RAGE is expressed at various levels in a number of cell types in the lungs, including airway epithelia, airway smooth muscle cells, lung endothelia, fibroblasts or fibrocytes, alveolar macrophages, and neutrophils.37 –42 Two studies have suggested that RAGE and RAGE ligands were expressed in fibroblastic foci of lungs with UIP.43,44 However, these studies only demonstrated expression of RAGE in fibroblastic foci, and lacked comparison of RAGE expression in UIP compared with normal lung tissues. Another study showed that AGER mRNA levels were decreased in isolated alveolar epithelial type 2 cells (AEC2) cells but not fibroblasts from IPF lungs. 32 Additionally, in vitro stimulation of fibrocytes or fibroblasts with a RAGE ligand enhanced RAGE expression.40,45 Therefore, while data indicate that overall expression of RAGE in the lungs is decreased, this is likely due to loss of AEC1 cells which have the highest level of RAGE expression in the lung, while RAGE expression may persist in lung fibroblasts and fibroblastic foci [Figure 1(a)]. However, too few studies have been done to draw conclusions on the role of RAGE in fibroblastic foci. Therefore, more research is required to clarify the overall expression patterns of RAGE in IPF lungs compared with non-IPF lungs. Studies are needed to establish if there are consistent cell-specific expression patterns of RAGE and if they are associated with disease progression and associated with other biomarker expression patterns in IPF.
Figure 1.
RAGE expression in human IPF and in experimental mouse models of pulmonary fibrosis.
(a) In human lungs, overall expression of membrane receptor for advanced glycation endproducts RAGE (mRAGE) and soluble RAGE (sRAGE) is decreased in IPF compared with healthy lungs. In the lungs, RAGE expression is most abundant in the lung epithelium, most notably in the type 1 alveolar epithelial cells, which are diminished in IPF. However, it is suggested that RAGE expression may still be expressed in other cell types, specifically in fibrotic foci (FF). Systemically, sRAGE levels are reduced in subjects with IPF compared with healthy subjects. (b) In experimental models of IPF in mice, exposure to bleomycin, asbestos, or silica causes a decrease in whole-lung levels of both mRAGE and sRAGE. In the bleomycin model, RAGE-knockout (RAGE−/−) mice are protected from bleomycin-induced fibrosis as compared with wild-type (WT) mice. In the asbestos model, RAGE−/− mice had exacerbated fibrosis in response to asbestos exposure compared with WT mice. In the crystalline silica model, WT and RAGE−/− mice exhibited a similar magnitude of fibrosis, but RAGE−/− mice showed a more diffuse pattern of fibrosis as compared with the classical nodular fibrosis seen in WT mice. Extensive aging of mice results in the spontaneous development of features of fibrosis in RAGE−/− mice as compared with aged WT mice. The effects of aging on overall RAGE expression in WT mice remains to be investigated.
IPF, idiopathic pulmonary fibrosis.
sRAGE as a biomarker of IPF disease progression
While analysis of whole-lung homogenates from both humans with IPF and mice with experimental IPF demonstrated a clear decrease in full-length RAGE, sRAGE has also been shown to be decreased.30,32 sRAGE levels were also found to be decreased in the bronchoalveolar lavage fluid (BALF) and plasma of IPF subjects compared with controls46,47 [Figure 1(a)].
A single nucleotide polymorphism (SNP) in the AGER gene, rs2070600, has been associated with natural variation of lung function in people of European ancestry.48,49 This SNP, which results in a glycine–serine amino acid substitution (G82S) in the ligand-binding domain of RAGE results in enhanced RAGE activity.50,51 In addition, rs2070600 has been associated with decreased systemic levels of sRAGE in COPD and severe neutrophilic asthma.52,53 Recently, Manichaikul and colleagues demonstrated that while there was no convincing association of rs2070600 with IPF risk, the minor allele A was associated with lower levels of plasma sRAGE in subjects with IPF and other interstitial lung diseases (ILDs). 54 Moreover, plasma sRAGE levels showed a strong association with disease severity (reduced forced vital capacity precentage), decreased survival, and increased transplantation rates. 54 A similar study also showed an association of decreased plasma sRAGE with the minor T allele, which correlated with acute exacerbation risk and decreased survival rates in IPF. 55 However, it remains unclear if decreased sRAGE levels associated with IPF progression act as a contributing pathological factor, or as a result of the loss of the AEC1 (the major source of sRAGE in the lungs) due to alveolar destruction. Nonetheless, accumulating evidence suggests that circulating sRAGE exhibits strong potential as a prognostic biomarker in IPF. 54
The puzzling role of RAGE in experimental models of IPF
While RAGE signaling is a major component of both homeostatic and pathogenic processes in the lungs, it has also been implicated in the development of fibrosis in the heart, liver, and renal system.56 –58 Although there is mounting evidence that RAGE plays a significant role in pulmonary fibrosis, inconsistent outcomes among different experimental models has been difficult to interpret. Initially, He et al. 59 demonstrated that RAGE-null mice were protected from bleomycin-induced injury and fibrosis, had better survival rates, and lacked production of profibrotic mediators [i.e. transforming growth-factor beta (TGFβ) and platelet-derived growth factor (PDGF)] as compared with wild-type (WT) mice. A later study confirmed these findings and added that mice heterozygous for RAGE also exhibited reduced fibrosis in response to bleomycin 60 [Figure 1(b)]. Conversely, Englert et al. 30 found that RAGE-null mice exhibited exacerbated pathology in response to asbestos-induced injury compared with WT mice, and that RAGE-null mice spontaneously developed fibrotic features with extensive aging [Figure 1(b)]. Alternatively, in response to crystalline silica-induced lung injury, WT and RAGE-null mice exhibited a similar magnitude of fibrosis; however, RAGE-null mice displayed a more diffuse pattern of fibrosis compared with the classic nodular fibrotic pattern seen in WT mice [Figure 1(b)]. In addition, RAGE-null mice exposed to silica had increased lymphocytes and decreased neutrophils and TGFβ levels in the BALF compared with WT mice. 31 Interestingly, intraperitoneal injection of WT mice with lung-derived mouse sRAGE had no effect on bleomycin or asbestos-induced fibrosis. 60 However, this effect may in part be explained by the short half-life of sRAGE in the mouse, where the only effective route of delivery of sRAGE to the lungs was by direct intratracheal instillation. 61 Notably, direct administration of mouse sRAGE intranasally had significant inhibitory effects on house dust-mite-induced asthma in mice. 62 Therefore, it is possible that direct administration of sRAGE or RAGE small-molecule antagonists to the lungs may have inhibitory or therapeutic effects in models of fibrosis, warranting future investigations.
It remains unclear why such differences were seen across different experimental models. However, results from cell and molecular studies offer some insight into the role of RAGE in cellular adhesion/re-epithelialization, and possibly promoting mesenchymal-cell activation. Firstly, as noted earlier, RAGE expression was decreased in AEC2 cells but not fibroblasts from IPF lungs compared with healthy donor lungs. 32 In vitro, genetic inhibition of RAGE had no effects on bleomycin or asbestos-induced cell death, but reduced epithelial cell adhesion, increased proliferation, migration, and wound-healing capacity.32,60 In addition, Zhai et al. 63 recently demonstrated that stimulation of alveolar epithelial cells with AGEs and HMGB1 promoted wound-healing capacity and proliferation in a RAGE-dependent manner. In line with this, previous studies demonstrated that RAGE on AEC1 cells binds to collagen and facilitates cell adhesion/spreading. 64 While there are inconsistencies in the role of RAGE in epithelial-cell adherence, it also promotes epithelial-cell proliferation, migration and wound healing, suggesting a complicated role in re-epithelialization.
Epithelial-to-mesenchymal transition (EMT) has been implicated as a key mechanism in the development of fibrosis, whereby cells lose epithelial characteristics and gain mesenchymal functions to promote fibrogenesis. 65 The role of RAGE in EMT is also controversial, based on mixed findings. For instance, the RAGE ligand HMGB1 induces EMT in mouse AEC2 cells from WT but not RAGE-null mice. 59 However, TGFβ is a potent inducer of EMT and reduces RAGE expression in epithelial cells in vitro.66,67 Conversely, AGE-RAGE signaling has been shown to inhibit TGFβ-induced EMT. 68 Hence, understanding the role of RAGE in EMT remains largely incomplete and likely depends on both initiating and inhibitory factors. Thus far, data suggest that RAGE may be a critical component of re-epithelialization in response to lung injury, while on the other hand, RAGE may also promote processes such as EMT. However, fibroblastic cells are major effector cell types in fibrogenesis, and the role of RAGE signaling in these cells remains poorly understood. The sections below provide new experimental concepts, which, upon investigation, may provide novel insights as to how RAGE signaling promotes fibrosis in the lungs, as well as other tissues.
New prospective mechanisms of RAGE in pulmonary fibrosis
RAGE-mediated regulation of type 2 inflammation
The role of inflammation in the pathogenesis of IPF has long been a subject of fierce debate. Theoretically, it has been thought that unknown insults and environmental exposures cause injury that causes repeated cycles of inflammation and improper repair and remodeling, leading to fibrogenesis. 69 However, lack of robust inflammation in subjects with IPF and ineffectiveness of corticosteroid treatment challenges this theory. 70 Conversely, various studies have demonstrated that inflammatory cell influx is present during exacerbation and is associated with worse disease outcomes.1,71 Moreover, pro-inflammatory and profibrotic cytokines, such as type 2 cytokines interleukin 4 (IL-4) and IL-13 are elevated in subjects with IPF, as are their receptors.72 –74 At mucosal barrier sites, type 2 immunity is a critical defense mechanism against parasitic infections. However, when dysregulated, it can lead to adverse immune responses which drive the development of not only allergic asthma and atopic dermatitis (AD) but also tissue fibrosis.12,75 During such reactions, type 2 cytokines (e.g. IL-4 and IL-13) are produced by various cells including T-helper 2 (Th2) cells, mast cells, and type 2 innate lymphoid cells (ILC2). 76
Of particular interest is IL-13, a pleiotropic type 2 cytokine which promotes inflammation, cell proliferation, and fibrogenesis. 77 In mice, induced over-expression of IL-13 promotes many features of asthma, including subepithelial fibrosis. 78 Transgenic over-expression of IL-13 in the lungs causes subepithelial and adventitial airway fibrosis via induction of TGFβ expression, MMP9-mediated activation of latent TGFβ and collagen deposition.79,80 In vitro, stimulation of pulmonary fibroblasts with rIL-13 signals via signal transducer and activator of transcription 6 (Stat6), inducing target gene expression (e.g. Postn, Col1a1, Pdgfaa, Ccl11, Egr1, Mmp9), TGFβ activation, fibroblast proliferation and collagen production81 –87 [Figure 2(b)]. In addition, IL-13 promotes the alternative polarization of macrophages to a profibrotic ‘M2’ phenotype, which are critical cellular mediators of fibrosis in various tissues. 9 Stimulation of macrophages with IL-13 also signals via Stat6, inducing gene expression of target genes, including Arg1, Relma, Pdgfaa, and Tgfb188,89 [Figure 2(c)]. The critical role of IL-13 in these processes has sparked keen interest in its contribution to lung fibrosis. Indeed, studies demonstrated increased levels of IL-13 in the BALF of subjects with IPF compared with subjects with non-specific interstitial pneumonia or healthy controls.72,90,91 In addition, expression levels of IL-13 receptor subunits are enhanced in fibroblasts from subjects with ILD.72,74 In fibroblasts, IL-13 induces the production of various profibrotic mediators, most notably, periostin, which has been associated with disease progression in IPF90,92,93 [Figure 2(b)]. Periostin is a matricellular protein produced by airway epithelial cells and fibroblasts which binds directly to collagen and cell-surface integrins and promotes collagen fibrillogenesis, cell proliferation, and activation of fibrogenic mediators.94,95 Periostin expression is increased in the lungs of subjects with fibrotic ILD compared with non-fibrotic pneumonias. 93 Moreover, systemic levels of periostin are increased in IPF, show an inverse correlation with lung function indices, and are predictive of pulmonary function decline and survival.93,96 Additionally, mechanistic studies in experimental models suggest that periostin mediates pulmonary fibrosis by promoting airway lung inflammation. 97 In macrophages, IL-13 induces expression of profibrotic mediators such as PDGF, and TGFβ. 98 In addition, IL-13 induces arginase-1, which promotes collagen production through L-arginine metabolism. 88 This is suggestive of local signaling of IL-13 and other profibrotic factors such as periostin, which likely coordinate imbalanced inflammation and tissue repair, and drive pulmonary fibroblast proliferation, activation, and matrix deposition in fibrotic foci [Figure 2(a–c)].
Figure 2.
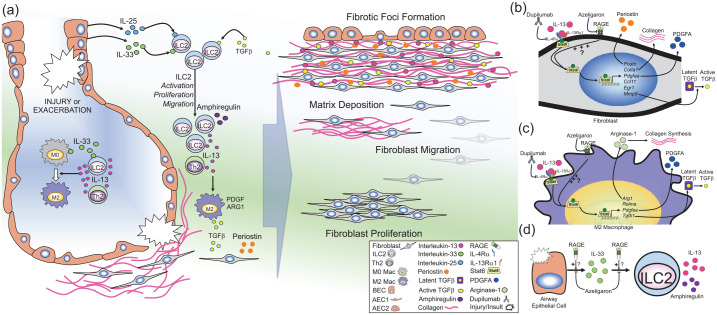
RAGE-mediated regulation of type 2 immunity.
Illustration of the prospective role of RAGE in regulating type 2 inflammation in pulmonary fibrosis. (a) Lung injury or insult caused by known or unknown factors can promote exacerbations or disease progression in IPF. Injury in the airways can cause the release of IL-25 and IL-33 from lung epithelial cells [e.g. bronchial epithelial cells (BECs) or alveolar epithelial cells (AECs)]. IL-33 can also be produced by lung macrophages. IL-25, IL-33 and transforming growth-factor beta (TGFβ) induce the activation, proliferation, and migration of type 2 innate lymphoid cells (ILC2s). ILC2s [as well as T-helper 2 (Th2) cells] produce large amounts of IL-13, while ILC2s also produce amphiregulin. IL-13 promotes the polarization of naïve macrophages (M0) to profibrotic (M2) macrophages which produce TGFβ, platelet-derived growth factor (PDGF), and arginase-1 (ARG1). In fibroblasts (or activated myofibroblasts), IL-13 stimulates production of periostin and TGFβ and promotes fibroblast activation. Together, the molecules promote fibroblast proliferation, migration, and matrix deposition, which eventually leads to the development of fibrotic foci in the interstitial space near regenerative airways. (b–d) Potential cellular mechanisms of RAGE signaling in development of fibrosis: (b) In fibroblasts, IL-13 binds to its cognate receptor complex which consists of IL-4Rα and IL-13Rα1, which induces phosphorylation (P) of Stat6, inducing nuclear translocation and subsequent expression of Stat6-target genes [e.g. periostin (Postn), collagen type 1A1 (Col1a1), PDGF-AA (Pdfgaa), chemokine C–C motif containing ligand-11 (Ccl11), early growth response-1 (Egr1), and matrix metalloproteinase-9 (Mmp9). This leads to production of periostin, collagen, PDGFA and MMP-9, which in turn can activate latent TGFβ. (c) In macrophages, stimulation with IL-13 causes polarization to the M2 phenotype, leading to Stat6-dependent expression of ARG-1 (Arg1) which promotes collagen production, resistin-like molecule alpha (Relma), Pdgfaa, and TGFβ-1 (Tgfb1). RAGE promotes IL-13/Stat6 signaling in airway epithelial cells. (b–c) Therefore, it is hypothesized that this RAGE-mediated mechanism may also contribute to IL-13/Stat6 signaling in fibroblasts and macrophages, promoting fibrogenesis in IPF, which could be inhibited by RAGE-specific small-molecule antagonist (azeligaron) or the monoclonal anti-IL-4Rα antibody (dupilumab). Use of such treatments have the potential to reduce disease progression in IPF. (d) Damage to airway epithelial cells can induce the release of IL-33, which in turn promotes the proliferation, migration, and activation of ILC2s, which in turn produce large amounts of IL-13 and amphiregulin. In models of asthma, RAGE promotes accumulation of IL-33 in the airways in response to allergens, and furthermore, RAGE also promotes ILC2 activation and accumulation in response to exogenous IL-33. Therefore, panel (d) suggests that RAGE may also promote IL-33/ILC2-signaling in IPF, which could be inhibited by RAGE-specific small-molecule antagonists (e.g. azeligaron).
IL, interleukin; IPF, idiopathic pulmonary fibrosis; RAGE, receptor for advanced glycation endproducts; Rα, receptor alpha; Stat6, signal transducer and activator of transcription 6.
ILC2s have emerged as a unique bridge between the innate and adaptive immune systems, functioning as tissue-resident immune sentinels. 77 Tissue damage and environmental stimuli cause the release of epithelial cytokines (e.g. IL-33, IL-25), which promote the local activation, proliferation, as well as recruitment of ILC2s to the lungs, leading to rapid and coordinated type 2 immune responses. 77 While various cells produce IL-13 (e.g. mast cells and Th2 cells), ILC2s are the most potent source of IL-13 in diseases such as allergic asthma 99 [Figure 2(a)]. Moreover, TGFβ is a critical mediator of ILC2 development, and regulates local ILC2 activity including elaboration of IL-13100,101 [Figure 2(a)]. Recent studies have demonstrated that ILC2s promote pulmonary fibrosis in experimental models of IPF and are increased in subjects with IPF.102 –105 Li and colleagues demonstrated that IL-33-mediated accumulation of ILC2s and M2 macrophages is a driving force in development of fibrosis in response to bleomycin. 104 In addition, IL-33 levels are shown to be increased in the BALF of subjects with IPF compared with healthy controls or other ILD. 106 Moreover, Hams et al. 105 demonstrated that IL-25-mediated activation of ILC2s promotes IL-13-driven lung fibrosis, and that both IL-25 and ILC2s were increased in the BALF of subjects with IPF. Additionally, ILC2s were increased in the circulation of subjects with systemic-sclerosis-related ILD. 103 These studies suggest that similar to asthma and AD, ILC2s are a major producer of IL-13 which is capable of driving pulmonary fibrosis [Figure 2(a)].
The intriguing role of IL-13-mediated processes in the development of pulmonary fibrosis prompted investigation of IL-13 inhibition in a humanized mouse model of IPF in severe combined immunodeficient mice. 90 Murray et al. 90 demonstrated that treatment with an anti-IL-13 antibody (tralokinumab) reduced lung fibrosis and restored markers of epithelialization in mice with established disease. Recent clinical trials have evaluated safety and efficacy of tralokinumab, a bi-specific anti-IL-4/IL-13 antibody (SAR156597), as well as an anti-IL-13Rα1 antibody (lebrikizumab) in IPF; however, none of the trials demonstrated efficacy in primary endpoints.107 –109 A recent experimental follow-up study has suggested that targeting cells expressing both IL-4/IL-13R subunits rather than IL-13 directly may be more effective in reducing fibrotic parameters in the humanized mouse model of IPF. 110 Similarly, in asthma, several clinical trials found that tralokinumab and lebrikizumab failed to meet primary endpoints in the treatment of moderate-to-severe asthma.111 –113 However, recent trials with dupilumab, which targets the shared IL-4/IL-13 receptor subunit (IL-4Rα), has shown strong efficacy in decreasing asthma exacerbations in subjects with severe eosinophilic asthma.114 –116 Dupilumab targets both the type 1 and type 2 IL-4R which are primarily present in immune cells and structural cells, respectively.117,118 IL-4Rα is able to target both IL-4 and IL-13 and block signaling in both structural and immune cells, both of which are key players in asthma and IPF. Therefore, it may be suitable to investigate the effectiveness of dupilumab in models of IPF, with hope that it may be effective in IPF, as seen with eosinophilic asthma (Figure 2).
Recent work from our laboratory and others has demonstrated that RAGE plays a central role in experimental models of type 2 inflammation-driven asthma.37,62,119 –121 RAGE was required at multiple steps in asthma progression, including release of IL-33 in response to allergens, as well as rIL-33 induced accumulation of ILC2s in the lungs 119 [Figure 2(d)]. Moreover, our latest findings revealed that RAGE is also a critical mediator of IL-13-induced signal transduction and consequent airway inflammation. 37 These studies demonstrated that RAGE is required for sustained IL-13-induced Stat6 activation and subsequent upregulation of Stat6-inducible genes (e.g. Clca1 and Ccl11), which drive mucus production and inflammation. 37 In addition, inhibition of RAGE blocks rIL-13 induced expression of periostin in primary human bronchial epithelial cells (unpublished findings). The critical role of RAGE in regulating IL-13 signaling either directly or through ILC2s in models of asthma suggests RAGE may also play a similar role in regulating IL-13-mediated lung fibrosis (Figure 2).
While whole-lung levels of RAGE are decreased in IPF lungs, it is most abundantly expressed in the type I alveolar epithelium, which is decimated in IPF. 122 However, it is possible that RAGE expression is still intact or even enhanced at local sites of active tissue repair or fibrosis. Studies have suggested that RAGE may be expressed in fibroblastic foci in the lungs of subjects with ILD, however more studies are needed to confirm this finding and determine if there is also active signaling.44,46 Moreover, although ILC2s are scarcely distributed in the lungs, local tissue-damage-induced release of epithelial cytokines (e.g. IL-33) causes them to produce copious amounts of IL-13.123,124 Therefore, although alveolar RAGE expression may be lost to injury and lack of adequate re-epithelialization, RAGE signaling may still promote focal inflammation and tissue repair in response to lung injury, leading to fibrotic foci development. Future investigations are needed to evaluate the effects of RAGE inhibition on epithelial initiator cytokines, ILC2s and IL-13 mediated effects in fibroblasts and macrophages in experimental models of pulmonary fibrosis [Figure 2(a–d)].
The calprotectin–RAGE axis in pulmonary fibrosis
In contrast to trends of sRAGE, various RAGE ligands are elevated in lungs and systemically in subjects with IPF, many of which have strong pro-inflammatory effects.44,125,126 The S100 calcium-binding proteins S100A8 and S100A9 are danger signals (or alarmins), which together can form a heterodimer referred to as calprotectin. S100A8/A9 signals through both TLR4 and RAGE and has pro-inflammatory and antimicrobial effects. 127 They are produced mainly by activated inflammatory cells and are elevated in severe neutrophilic asthma and cystic fibrosis (CF) exacerbations.128 –131
S100A8/A9 was shown to be elevated in the BALF of subjects with systemic-sclerosis-related ILD and correlated with the degree of fibrosis. 132 However, a number of studies have demonstrated increased levels of S100A9 in the BALF, but not systemically in subjects with IPF, suggesting it is produced and acts locally in the lungs133 –137 (Figure 3). Enhanced S100A9 levels in IPF correlated with severity of disease, as well as eosinophil and neutrophil numbers133,135,137 (Figure 3). Moreover, elevated S100A9 levels were able to distinguish between IPF and other ILD. 136
Figure 3.
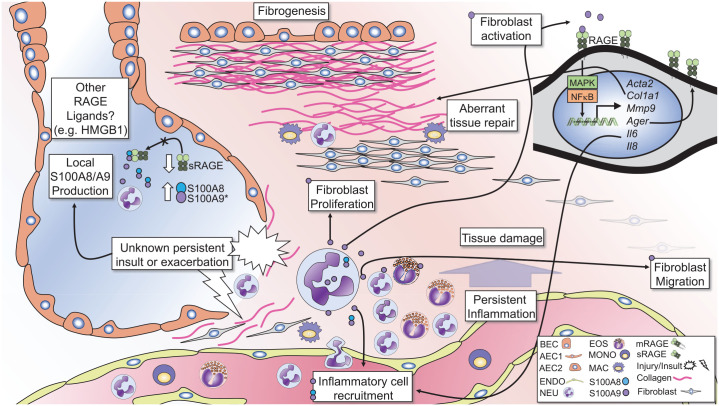
The calprotectin–RAGE axis in pulmonary fibrosis.
The diagram illustrates the potential role of S100A8/A9-RAGE signaling in inflammation and fibrogenesis in IPF. S100A8 and S100A9 are elevated in the lungs of subjects with pulmonary fibrosis and correlates with lung neutrophil (NEU) and eosinophil (EOS) numbers. Conversely, sRAGE levels are reduced in the lungs of subjects with IPF, decreasing the ability of sRAGE to inhibit S100A8/A9 signaling. Neutrophils are the primary source of S100A8/A9 and promote granulocytic inflammation. Persistent injury or exacerbations can lead to damage of alveolar epithelial cells (AECs), bronchial epithelial cells (BECs) and the surrounding parenchyma. These exacerbations lead to persistent inflammation causing damage to the lung architecture and subsequent aberrant tissue repair mechanisms, promoting fibrogenesis. S100A8/A9 and S100A9 alone can signal through RAGE to induce fibroblast migration, proliferation, and activation. In fibroblasts, S100A9 signals through RAGE to activate MAPK and NFkB pathways and induces expression of alpha smooth muscle actin (Acta2), Collagen 1A1 (Col1a1), matrix metalloproteinase-9 (Mmp9), and RAGE (Ager). S100A9 also induces expression of the pro-inflammatory cytokines IL-6 and IL-8, which promote inflammatory cell recruitment. Such signaling mechanisms may accompany persistent damage and concurrent inflammation, leading to proliferation and recruitment of fibroblasts to areas of tissue damage, promoting fibrosis in the lungs.
HMGB1, high mobility group box-1; IL, interleukin; IPF, idiopathic pulmonary fibrosis; RAGE, receptor for advanced glycation endproducts; sRAGE, soluble-form RAGE.
A growing body of evidence suggests that local production of S100A8 and S100A9 by inflammatory cells promotes lung inflammation in a feed-forward manner (Figure 3). Recently, a study found that S100A8/A9 drives neutrophil accumulation in the lungs in a mouse model of Mycobacterium tuberculosis infection. 138 Additionally, S100A8/A9 is associated with neutrophils and is a marker of exacerbation in CF, while S100A9 drives neutrophilic inflammation in experimental models of asthma.130,139,140 It is conceivable that S100A8/A9 may contribute to persistent inflammation or exacerbations in IPF, which can promote damage to the lung architecture, initiating progressive lung remodeling (Figure 3).
Experimental studies have demonstrated that S100A9 promotes fibrogenic processes in multiple organs.141 –143 Signaling through RAGE, recombinant (r)S100A9 induced migration and enhanced expression of RAGE, in human fibrocytes 40 and stimulation of fibroblasts with rS100A9-induced proliferation, and increased expression of collagen and α-smooth-muscle actin. 45 rS100A9 also enhanced expression of RAGE and pro-inflammatory cytokines (IL-6 and IL-8) in human lung fibroblasts 45 (Figure 3). Furthermore, S100A8/A9 induces expression of matrix metalloproteinase-9, which, as noted above, cleaves mRAGE to produce sRAGE and activates latent TGFβ, which is a driving force in lung fibrosis. 144
In addition to increased levels of S100A9 in the BALF, immunohistochemical examination of lung sections showed increased S100A9 expression in fibrotic foci. 133 It is also interesting to note that exposure to allergens or direct stimulation with rIL-13 induces production of S100A8/A9 in the lungs of mice.37,145 Active inflammation during stable disease progression or acute exacerbation may promote granulocytic influx, and in turn, further elaboration of inflammatory mediators like S100A8/A9 (Figure 3). This type of feed-forward inflammatory signaling promotes excessive inflammation and lung damage, followed by aberrant repair mechanisms and diminished lung functions in diseases such as pulmonary tuberculosis or CF. These findings suggest that local production S100A8 and/or S100A9 by accumulating immune cells can signal through RAGE on fibroblasts which may contribute to trafficking of fibroblasts to areas of aberrant remodeling, enhancing matrix deposition and fibrogenesis, consequently promoting disease progression (Figure 3).
Nuclear RAGE, cellular senescence, and fibrosis
While RAGE is conventionally known for its function as a pattern-recognition receptor localized at the plasma membrane, recent reports by Kumar et al. have identified a nuclear isoform of RAGE, which has a role in DNA-damage-repair regulation.146,147 Mechanisms that regulate cellular senescence contribute to age-related diseases and progressive fibrosis.148,149 It was shown that in well-aged (~40–50 weeks) RAGE-null mice, fibrotic areas of the lungs had signs of senescence demonstrated by the presence of senescence-associated beta galactosidase.146,147 These recent investigations demonstrated that RAGE can translocate to the nucleus where it is phosphorylated at Ser376 and Ser389 by the ataxia–telangiectasia-mutated kinase. In addition, phosphorylation recruits RAGE to areas of DNA double-strand breaks, where it enhances endonuclease activity, promoting DNA damage repair146,147 (Figure 4). Lack of adequate DNA-damage repair is thought to induce a senescence-associated secretory phenotype (SASP) in fibroblasts, which promotes secretion of pro-inflammatory (e.g. IL-6) and fibrogenic (e.g. TGFβ) mediators, which can drive progression of fibrosis 150 [Figure 4(a)]. It is suggested that lack of RAGE leads to increased cell senescence due to lack of adequate DNA-damage repair, promoting SASP and subsequent lung fibrosis in aged RAGE-null mice 146 (Figure 4). Moreover, data suggest that reconstitution of RAGE may be able to reverse cellular senescence, and in turn, restore lung function, to some degree, in aged mice. 146 Lastly, the authors expanded their studies to show that nuclear RAGE also plays a role in diabetic-associated kidney and lung fibrogenesis. 147 These exciting findings highlight a previously unknown role for RAGE in DNA repair, senescence, and suggest its potential role in regulation these processes may contribute to age-related fibrotic disease. It is noteworthy to mention that bleomycin is used as a chemotherapeutic agent, which induces DNA damage and premature cell senescence. 151 However, RAGE-null mice are completely protected from bleomycin-induced fibrosis.59,60 This presents an interesting paradox, that while both bleomycin-treatment and lack of RAGE expression over time (aging) induce DNA damage, cell senescence and fibrosis, non-aged RAGE-null mice do not develop fibrosis in response to bleomycin-induced injury. Age is a prominent risk factor for IPF in humans. 152 Experimentally, it has been shown that aged mice exhibit impaired resolution of fibrosis compared with younger mice. 153 This reduced ability to resolve fibrosis has been linked to age-related production of senescent and apoptosis-resistant myofibroblasts. 153 In addition, age-related increases in senescent AECs promote fibrosis in aged mice. 154 Likewise, studies have indicated epigenetic regulation of cellular senescence protects younger mice from fibrosis, while promoting fibrosis in aged mice. 155 It is possible that younger RAGE-null mice are resistant to or are able to recover from bleomycin-induced injury in a ‘one hit’ model preventing fibrosis. While on the other hand, continuous injury, or extensive DNA damage from aging without proper DNA-damage repair (due to lack of RAGE), may promote development of fibrosis, over time.
Figure 4.
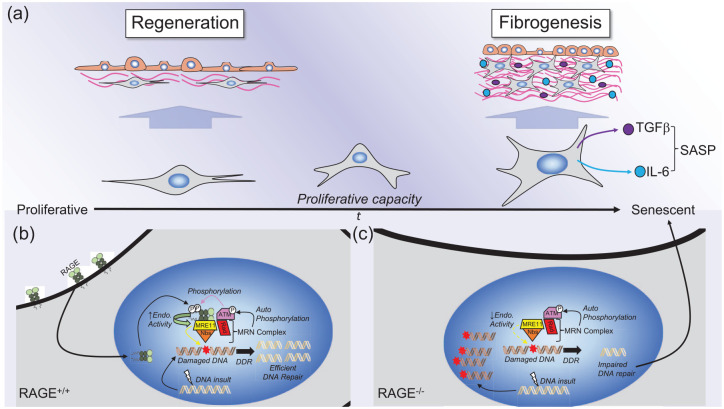
Nuclear RAGE, cellular senescence, and fibrosis.
The illustrations show the potential role of nuclear RAGE signaling in cellular senescence and associated fibrogenesis. (a) Over time (t) the proliferative capacity of cells (e.g. fibroblasts or myofibroblasts) is reduced, and cells eventually become senescent. Prior to aging, fibroblasts maintain their proliferative capacity, lack resistance to apoptosis, and exhibit a normal phenotype that promotes normal regeneration of damaged tissues. However, it is suggested that as the proliferative capacity of cells such as fibroblasts is reduced, and cells become more senescent with age, they can develop what is referred to as a senescence-associated secretory phenotype (SASP), whereby they become resistant to apoptosis, and produce profibrotic (e.g. TGFβ) and pro-inflammatory (e.g. IL-6) cytokines, which promote fibrogenesis. (b) In RAGE-expressing cells, RAGE is recruited to the nucleus to sites of DNA damage. Here, RAGE interacts with the MRN complex, which consists of the meiotic recombination 11 endonuclease (MRE11), Nijmegen breakage syndrome protein 1 (Nbs), Rad51 recombinase (Rad), and the ataxia–telangiectasia-mutated kinase (ATM). ATM phosphorylates RAGE, which, in turn, enhances endonuclease activity of MRE11, promoting efficient DNA-damage repair (DDR). In RAGE-deficient cells, RAGE is not recruited to the MRN complex, which, in turn, exhibits reduced endonuclease activity, leading to accumulation of damaged DNA. This impaired DNA damage leads to cellular senescence, promoting fibrogenesis. This hypothesis suggests that lack of RAGE over time can lead to inefficient DDR, causing senescence, and adoption of the SASP, promoting fibrogenesis with aging.
DNA, deoxyribonucleic acid; IL, interleukin; RAGE, receptor for advanced glycation endproducts; TGFβ, transforming growth-factor beta.
Studies demonstrating that RAGE-null mice were protected from bleomycin-induced fibrosis were performed in younger mice. To our knowledge, no studies to date have evaluated the expression levels of RAGE in the lungs, over time. It is possible this reduced RAGE expression in aged mice could contribute to the increased susceptibility to, or loss of ability to, resolve fibrosis in response to injury in the lungs over time, leading to cellular senescence and fibrosis. Nonetheless, future research will be needed to determine the role of nuclear RAGE in the pathogenesis of IPF and other fibrotic diseases.
Future implications
It is noteworthy that while recent trials targeting type 2 cytokines in IPF have not yet shown efficacy in improving lung function, such therapies seem more likely to slow progression of disease, by reducing or preventing active fibrogenesis. This notion should be considered in determining the therapeutic potential of other drugs including dupilumab or RAGE antagonists in IPF, which could potentially be used in combination with the currently approved antifibrotics. With RAGE being most abundantly expressed in the alveolar epithelium, of which the primary function is gas exchange, it seems logical that alveolar damage and consequently, reduced RAGE expression, directly correlates with lung function decline in IPF. This suggests that loss of RAGE may be, in effect, a casualty of alveolar injury and loss of normal lung architecture in IPF. On the other hand, developmental studies indicate that RAGE contributes to alveolarization in the lungs, suggesting alternative mechanisms or genetic errors could causally lead to reduced RAGE expression, and in turn, aberrant re-epithelialization.148,149 Nonetheless, evidence suggests that decreased circulating levels of sRAGE have a strong correlation with decline in lung function and may serve as a critical biomarker of disease progression.54,156
The evidence of RAGE ligand signaling in fibroblasts and fibroblastic foci, as well as its role in regulating pro-fibrotic signaling (e.g. IL-13, S100A8/A9) suggest that RAGE may play a causal role in progressive fibrogenesis. RAGE small-molecule inhibitors are shown to be very safe in humans in clinical trials to treat Alzheimer’s disease, suggesting the possible use as a therapeutic. 157 It seems possible that while RAGE neutralization therapies may not necessarily be able to improve lung function, it may be able to inhibit active inflammation and fibrogenesis, thereby halting or slowing disease progression, and prevent acute exacerbations, leading to more long-term and stable disease. Unfortunately, findings from experimental mouse models of pulmonary fibrosis remain puzzling, as genetic ablation of RAGE has both protective and exacerbating effects in these models. Moreover, translation of mouse models to human disease remains a major burden to medical research in general. However, utilization of humanized mouse models of IPF may allow for determination of the capacity of human-safe RAGE inhibitors to improve lung function or slow progression and extend survival in mice with established disease, giving insight to its potential as a human therapeutic for IPF. 110
Interestingly, studies consistently found that genetic ablation of RAGE protects against bleomycin-induced injury in mice.30,59 Bleomycin is a major chemotherapeutic agent, and treatment dosing is limited by the development of pulmonary toxicity and fibrosis. 158 Use of RAGE antagonists may be very useful in enhancing the chemotherapeutic effects of bleomycin by allowing the use of higher doses while preventing bleomycin-induced injury and fibrosis. However, studies are needed to determine the ability of RAGE antagonists to inhibit bleomycin-induced fibrosis or therapeutic effects in established disease.
Lastly, the recent discovery of the function of RAGE in DNA-damage repair and cellular senescence is fascinating, and may suggest that loss of overall RAGE expression contributes to the progression of IPF.146,147 In light of this, it seems plausible that RAGE antagonists (which target the ligand-binding domain) may be able to block the effects of ligand binding without impeding the role of nuclear RAGE, which does not appear to be dependent on the ligand-binding domain. However, further research is required to fully elucidate the mechanisms of RAGE in DNA-damage repair, cell types involved, and how this may contribute to fibrosis in humanized models. Nonetheless, these are exciting findings that may present a new hope in not only halting disease progression, but also the possibility of remission in IPF.
Conclusion
Recent advances in the use of antifibrotics in treatment of IPF have had a significant impact on survival rates. 5 However, incidence and mortality do not wane, and continue to rise, with nearly 50% of patients dying within 5 years of diagnosis, necessitating the need for early detection and novel therapeutic approaches. 159 While the role of RAGE in IPF remains a perplexing and incomplete story, new advances in RAGE biology may provide insight into the confounding findings gathered thus far. Newly illuminated roles of RAGE in alarmin signaling, type 2 immunity, and cellular senescence may link RAGE to mechanisms involved in acute exacerbations and progression of fibrosis in IPF. Moreover, circulating sRAGE levels may present a strong prognostic biomarker and may be linked to genetic susceptibility, which is also present in other chronic lung disorders. While research is needed to fully elucidate the mechanisms by which RAGE contributes to lung injury and fibrogenesis, it remains an intriguing potential biomarker and therapeutic target in IPF.
Supplemental Material
Supplemental material, sj-pdf-1-tar-10.1177_17534666211016071 for The perplexing role of RAGE in pulmonary fibrosis: causality or casualty? by Timothy N. Perkins and Tim D. Oury in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-2-tar-10.1177_17534666211016071 for The perplexing role of RAGE in pulmonary fibrosis: causality or casualty? by Timothy N. Perkins and Tim D. Oury in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-3-tar-10.1177_17534666211016071 for The perplexing role of RAGE in pulmonary fibrosis: causality or casualty? by Timothy N. Perkins and Tim D. Oury in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-4-tar-10.1177_17534666211016071 for The perplexing role of RAGE in pulmonary fibrosis: causality or casualty? by Timothy N. Perkins and Tim D. Oury in Therapeutic Advances in Respiratory Disease
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: TNP is funded by an American Heart Association Postdoctoral Fellowship award: 19POST34370078. TDO is funded by a Cystic Fibrosis Foundation grant OURY19GO.
ORCID iD: Timothy N. Perkins  https://orcid.org/0000-0002-4719-4856
https://orcid.org/0000-0002-4719-4856
Supplemental material: The reviews of this paper are available via the supplemental material section.
Contributor Information
Timothy N. Perkins, Department of Pathology, University of Pittsburgh School of Medicine, 3550 Terrace Street, S-784 Scaife Hall, Pittsburgh, PA 15261, USA.
Tim D. Oury, Department of Pathology, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA
References
- 1. Kim DS, Collard HR, King TE, Jr. Classification and natural history of the idiopathic interstitial pneumonias. Proc Am Thorac Soc 2006; 3: 285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wolters PJ, Collard HR, Jones KD. Pathogenesis of idiopathic pulmonary fibrosis. Annu Rev Pathol 2014; 9: 157–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wilson MS, Wynn TA. Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunol 2009; 2: 103–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saito S, Alkhatib A, Kolls JK, et al. Pharmacotherapy and adjunctive treatment for idiopathic pulmonary fibrosis (IPF). J Thorac Dis 2019; 11: S1740–S1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Behr J, Prasse A, Wirtz H, et al. Survival and course of lung function in the presence or absence of antifibrotic treatment in patients with idiopathic pulmonary fibrosis: long-term results of the INSIGHTS-IPF registry. Eur Respir J 2020; 56: 1902279. [DOI] [PubMed] [Google Scholar]
- 6. Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med 2014; 6: 265sr6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest 2007; 117: 524–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wynn TA. Integrating mechanisms of pulmonary fibrosis. J Exp Med 2011; 208: 1339–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 2016; 44: 450–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med 2012; 18: 1028–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Snijder J, Peraza J, Padilla M, et al. Pulmonary fibrosis: a disease of alveolar collapse and collagen deposition. Expert Rev Respir Med 2019; 13: 615–619. [DOI] [PubMed] [Google Scholar]
- 12. Gieseck RL, III, Wilson MS, Wynn TA. Type 2 immunity in tissue repair and fibrosis. Nat Rev Immunol 2018; 18: 62–76. [DOI] [PubMed] [Google Scholar]
- 13. Galli SJ, Borregaard N, Wynn TA. Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat Immunol 2011; 12: 1035–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Neeper M, Schmidt AM, Brett J, et al. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem 1992; 267: 14998–15004. [PubMed] [Google Scholar]
- 15. Brett J, Schmidt AM, Yan SD, et al. Survey of the distribution of a newly characterized receptor for advanced glycation end products in tissues. Am J Pathol 1993; 143: 1699–1712. [PMC free article] [PubMed] [Google Scholar]
- 16. Hanford LE, Fattman CL, Shaefer LM, et al. Regulation of receptor for advanced glycation end products during bleomycin-induced lung injury. Am J Respir Cell Mol Biol 2003; 29: S77–S81. [PubMed] [Google Scholar]
- 17. Oczypok EA, Perkins TN, Oury TD. All the “RAGE” in lung disease: the receptor for advanced glycation endproducts (RAGE) is a major mediator of pulmonary inflammatory responses. Paediatr Respir Rev 2017; 23: 40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hudson BI, Carter AM, Harja E, et al. Identification, classification, and expression of RAGE gene splice variants. FASEB J 2008; 22: 1572–1580. [DOI] [PubMed] [Google Scholar]
- 19. Kalea AZ, Reiniger N, Yang H, et al. Alternative splicing of the murine receptor for advanced glycation end-products (RAGE) gene. FASEB J 2009; 23: 1766–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hanford LE, Enghild JJ, Valnickova Z, et al. Purification and characterization of mouse soluble receptor for advanced glycation end products (sRAGE). J Biol Chem 2004; 279: 50019–50024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Malherbe P, Richards JG, Gaillard H, et al. CDNA cloning of a novel secreted isoform of the human receptor for advanced glycation end products and characterization of cells co-expressing cell-surface scavenger receptors and Swedish mutant amyloid precursor protein. Brain Res Mol Brain Res 1999; 71: 159–170. [DOI] [PubMed] [Google Scholar]
- 22. Yonekura H, Yamamoto Y, Sakurai S, et al. Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem J 2003; 370: 1097–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang L, Bukulin M, Kojro E, et al. Receptor for advanced glycation end products is subjected to protein ectodomain shedding by metalloproteinases. J Biol Chem 2008; 283: 35507–35516. [DOI] [PubMed] [Google Scholar]
- 24. Lopez-Diez R, Shekhtman A, Ramasamy R, et al. Cellular mechanisms and consequences of glycation in atherosclerosis and obesity. Biochim Biophys Acta 2016; 1862: 2244–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tobon-Velasco JC, Cuevas E, Torres-Ramos MA. Receptor for AGEs (RAGE) as mediator of NF-kB pathway activation in neuroinflammation and oxidative stress. CNS Neurol Disord Drug Targets 2014; 13: 1615–1626. [DOI] [PubMed] [Google Scholar]
- 26. Li J, Schmidt AM. Characterization and functional analysis of the promoter of RAGE, the receptor for advanced glycation end products. J Biol Chem 1997; 272: 16498–16506. [DOI] [PubMed] [Google Scholar]
- 27. Hudson BI, Kalea AZ, Del Mar Arriero M, et al. Interaction of the RAGE cytoplasmic domain with diaphanous-1 is required for ligand-stimulated cellular migration through activation of Rac1 and Cdc42. J Biol Chem 2008; 283: 34457–34468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brizzi MF, Dentelli P, Rosso A, et al. RAGE- and TGF-beta receptor-mediated signals converge on STAT5 and p21waf to control cell-cycle progression of mesangial cells: a possible role in the development and progression of diabetic nephropathy. FASEB J 2004; 18: 1249–1251. [DOI] [PubMed] [Google Scholar]
- 29. Fukami K, Ueda S, Yamagishi S, et al. AGEs activate mesangial TGF-beta-Smad signaling via an angiotensin II type I receptor interaction. Kidney Int 2004; 66: 2137–2147. [DOI] [PubMed] [Google Scholar]
- 30. Englert JM, Hanford LE, Kaminski N, et al. A role for the receptor for advanced glycation end products in idiopathic pulmonary fibrosis. Am J Pathol 2008; 172: 583–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ramsgaard L, Englert JM, Tobolewski J, et al. The role of the receptor for advanced glycation end-products in a murine model of silicosis. PLoS One 2010; 5: e9604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Queisser MA, Kouri FM, Konigshoff M, et al. Loss of RAGE in pulmonary fibrosis: molecular relations to functional changes in pulmonary cell types. Am J Respir Cell Mol Biol 2008; 39: 337–345. [DOI] [PubMed] [Google Scholar]
- 33. Selman M, Carrillo G, Estrada A, et al. Accelerated variant of idiopathic pulmonary fibrosis: clinical behavior and gene expression pattern. PLoS One 2007; 2: e482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Konishi K, Gibson KF, Lindell KO, et al. Gene expression profiles of acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2009; 180: 167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ohlmeier S, Mazur W, Salmenkivi K, et al. Proteomic studies on receptor for advanced glycation end product variants in idiopathic pulmonary fibrosis and chronic obstructive pulmonary disease. Proteomics Clin Appl 2010; 4: 97–105. [DOI] [PubMed] [Google Scholar]
- 36. Fehrenbach H, Kasper M, Tschernig T, et al. Receptor for advanced glycation endproducts (RAGE) exhibits highly differential cellular and subcellular localisation in rat and human lung. Cell Mol Biol (Noisy-le-grand) 1998; 44: 1147–1157. [PubMed] [Google Scholar]
- 37. Perkins TN, Oczypok EA, Dutz RE, et al. The receptor for advanced glycation endproducts is a critical mediator of type 2 cytokine signaling in the lungs. J Allergy Clin Immunol 2019; 144: 796–808.e12. [DOI] [PubMed] [Google Scholar]
- 38. Di Candia L, Gomez E, Venereau E, et al. HMGB1 is upregulated in the airways in asthma and potentiates airway smooth muscle contraction via TLR4. J Allergy Clin Immunol 2017; 140: 584–587.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Van Zoelen MA, Wieland CW, van der Windt GJ, et al. Receptor for advanced glycation end products is protective during murine tuberculosis. Mol Immunol 2012; 52: 183–189. [DOI] [PubMed] [Google Scholar]
- 40. Wang CH, Punde TH, Huang C-D, et al. Fibrocyte trafficking in patients with chronic obstructive asthma and during an acute asthma exacerbation. J Allergy Clin Immunol 2015; 135: 1154–1162.e1–e5. [DOI] [PubMed] [Google Scholar]
- 41. Robinson AB, Johnson KD, Bennion BG, et al. RAGE signaling by alveolar macrophages influences tobacco smoke-induced inflammation. Am J Physiol Lung Cell Mol Physiol 2012; 302: L1192–L1199. [DOI] [PubMed] [Google Scholar]
- 42. Huebener P, Pradere JP, Hernandez C, et al. The HMGB1/RAGE axis triggers neutrophil-mediated injury amplification following necrosis. J Clin Invest 2019; 130: 1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Inghilleri S, Morbini P, Campo I, et al. Factors influencing oxidative imbalance in pulmonary fibrosis: an immunohistochemical study. Pulm Med 2011; 2011: 421409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Morbini P, Villa C, Campo I, et al. The receptor for advanced glycation end products and its ligands: a new inflammatory pathway in lung disease? Mod Pathol 2006; 19: 1437–1445. [DOI] [PubMed] [Google Scholar]
- 45. Xu X, Chen H, Zhu X, et al. S100A9 promotes human lung fibroblast cells activation through receptor for advanced glycation end-product-mediated extracellular-regulated kinase 1/2, mitogen-activated protein-kinase and nuclear factor-kappaB-dependent pathways. Clin Exp Immunol 2013; 173: 523–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bargagli E, Penza F, Bianchi N, et al. Controversial role of RAGE in the pathogenesis of idiopathic pulmonary fibrosis. Respir Physiol Neurobiol 2009; 165: 119–120; author reply 21–22. [DOI] [PubMed] [Google Scholar]
- 47. Rosas IO, Richards TJ, Konishi K, et al. MMP1 and MMP7 as potential peripheral blood biomarkers in idiopathic pulmonary fibrosis. PLoS Med 2008; 5: e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Repapi E, Sayers I, Wain LV, et al. Genome-wide association study identifies five loci associated with lung function. Nat Genet 2010; 42: 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hancock DB, Eijgelsheim M, Wilk JB, et al. Meta-analyses of genome-wide association studies identify multiple loci associated with pulmonary function. Nat Genet 2010; 42: 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hofmann MA, Drury S, Hudson BI, et al. RAGE and arthritis: the G82S polymorphism amplifies the inflammatory response. Genes Immun 2002; 3: 123–135. [DOI] [PubMed] [Google Scholar]
- 51. Yan SF, Barile GR, D’Agati V, et al. The biology of RAGE and its ligands: uncovering mechanisms at the heart of diabetes and its complications. Curr Diab Rep 2007; 7: 146–153. [DOI] [PubMed] [Google Scholar]
- 52. Cheng DT, Kim DK, Cockayne DA, et al. Systemic soluble receptor for advanced glycation endproducts is a biomarker of emphysema and associated with AGER genetic variants in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013; 188: 948–957. [DOI] [PubMed] [Google Scholar]
- 53. Lyu Y, Zhao H, Ye Y, et al. Decreased soluble RAGE in neutrophilic asthma is correlated with disease severity and RAGE G82S variants. Mol Med Rep 2018; 17: 4131–4137. [DOI] [PubMed] [Google Scholar]
- 54. Manichaikul A, Sun L, Borczuk AC, et al. Plasma soluble receptor for advanced glycation end products in idiopathic pulmonary fibrosis. Ann Am Thorac Soc 2017; 14: 628–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yamaguchi K, Iwamoto H, Horimasu Y, et al. AGER gene polymorphisms and soluble receptor for advanced glycation end product in patients with idiopathic pulmonary fibrosis. Respirology 2017; 22: 965–971. [DOI] [PubMed] [Google Scholar]
- 56. Xia JR, Liu NF, Zhu NX. Specific siRNA targeting the receptor for advanced glycation end products inhibits experimental hepatic fibrosis in rats. Int J Mol Sci 2008; 9: 638–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Li JH, Wang W, Huang XR, et al. Advanced glycation end products induce tubular epithelial-myofibroblast transition through the RAGE-ERK1/2 MAP kinase signaling pathway. Am J Pathol 2004; 164: 1389–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yang PS, Lee SH, Park J, et al. Atrial tissue expression of receptor for advanced glycation end-products (RAGE) and atrial fibrosis in patients with mitral valve disease. Int J Cardiol 2016; 220: 1–6. [DOI] [PubMed] [Google Scholar]
- 59. He M, Kubo H, Ishizawa K, et al. The role of the receptor for advanced glycation end-products in lung fibrosis. Am J Physiol Lung Cell Mol Physiol 2007; 293: L1427–L1436. [DOI] [PubMed] [Google Scholar]
- 60. Englert JM, Kliment CR, Ramsgaard L, et al. Paradoxical function for the receptor for advanced glycation end products in mouse models of pulmonary fibrosis. Int J Clin Exp Pathol 2011; 4: 241–254. [PMC free article] [PubMed] [Google Scholar]
- 61. Milutinovic PS, Englert JM, Crum LT, et al. Clearance kinetics and matrix binding partners of the receptor for advanced glycation end products. PLoS One 2014; 9: e88259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Milutinovic PS, Alcorn JF, Englert JM, et al. The receptor for advanced glycation end products is a central mediator of asthma pathogenesis. Am J Pathol 2012; 181: 1215–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhai R, Blondonnet R, Ebrahimi E, et al. The receptor for advanced glycation end-products enhances lung epithelial wound repair: an in vitro study. Exp Cell Res 2020; 391: 112030. [DOI] [PubMed] [Google Scholar]
- 64. Demling N, Ehrhardt C, Kasper M, et al. Promotion of cell adherence and spreading: a novel function of RAGE, the highly selective differentiation marker of human alveolar epithelial type I cells. Cell Tissue Res 2006; 323: 475–488. [DOI] [PubMed] [Google Scholar]
- 65. Willis BC, Borok Z. TGF-beta-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol 2007; 293: L525–L534. [DOI] [PubMed] [Google Scholar]
- 66. Ding H, Ji X, Chen R, et al. Antifibrotic properties of receptor for advanced glycation end products in idiopathic pulmonary fibrosis. Pulm Pharmacol Ther 2015; 35: 34–41. [DOI] [PubMed] [Google Scholar]
- 67. Buckley ST, Medina C, Kasper M, et al. Interplay between RAGE, CD44, and focal adhesion molecules in epithelial-mesenchymal transition of alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 2011; 300: L548–L559. [DOI] [PubMed] [Google Scholar]
- 68. Song JS, Kang CM, Park CK, et al. Inhibitory effect of receptor for advanced glycation end products (RAGE) on the TGF-beta-induced alveolar epithelial to mesenchymal transition. Exp Mol Med 2011; 43: 517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sack C, Raghu G. Idiopathic pulmonary fibrosis: unmasking cryptogenic environmental factors. Eur Respir J 2019; 53: 1801699. [DOI] [PubMed] [Google Scholar]
- 70. Bringardner BD, Baran CP, Eubank TD, et al. The role of inflammation in the pathogenesis of idiopathic pulmonary fibrosis. Antioxid Redox Signal 2008; 10: 287–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Parambil JG, Myers JL, Ryu JH. Histopathologic features and outcome of patients with acute exacerbation of idiopathic pulmonary fibrosis undergoing surgical lung biopsy. Chest 2005; 128: 3310–3315. [DOI] [PubMed] [Google Scholar]
- 72. Park SW, Ahn MH, Jang HK, et al. Interleukin-13 and its receptors in idiopathic interstitial pneumonia: clinical implications for lung function. J Korean Med Sci 2009; 24: 614–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Jakubzick C, Choi ES, Kunkel SL, et al. Augmented pulmonary IL-4 and IL-13 receptor subunit expression in idiopathic interstitial pneumonia. J Clin Pathol 2004; 57: 477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Jakubzick C, Choi ES, Carpenter KJ, et al. Human pulmonary fibroblasts exhibit altered interleukin-4 and interleukin-13 receptor subunit expression in idiopathic interstitial pneumonia. Am J Pathol 2004; 164: 1989–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Akdis CA, Arkwright PD, Bruggen M-C, et al. Type 2 immunity in the skin and lungs. Allergy 2020; 75: 1582–1605. [DOI] [PubMed] [Google Scholar]
- 76. Fahy JV. Type 2 inflammation in asthma–present in most, absent in many. Nat Rev Immunol 2015; 15: 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lloyd CM, Snelgrove RJ. Type 2 immunity: expanding our view. Sci Immunol 2018; 3: eaat1604. [DOI] [PubMed] [Google Scholar]
- 78. Zhu Z, Homer RJ, Wang Z, et al. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest 1999; 103: 779–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Elias JA, Zheng T, Lee CG, et al. Transgenic modeling of interleukin-13 in the lung. Chest 2003; 123: 339S–345S. [PubMed] [Google Scholar]
- 80. Lee CG, Homer RJ, Zhu Z, et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor β1. J Exp Med 2001; 194: 809–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ingram JL, Rice A, Geisenhoffer K, et al. Interleukin-13 stimulates the proliferation of lung myofibroblasts via a signal transducer and activator of transcription-6-dependent mechanism: a possible mechanism for the development of airway fibrosis in asthma. Chest 2003; 123: 422S–424S. [DOI] [PubMed] [Google Scholar]
- 82. Jakubzick C, Choi ES, Kunkel SL, et al. Impact of interleukin-13 responsiveness on the synthetic and proliferative properties of Th1- and Th2-type pulmonary granuloma fibroblasts. Am J Pathol 2003; 162: 1475–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Yoshihara T, Nanri Y, Nunomura S, et al. Periostin plays a critical role in the cell cycle in lung fibroblasts. Respir Res 2020; 21: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ingram JL, Antao-Menezes A, Mangum JB, et al. Opposing actions of Stat1 and Stat6 on IL-13-induced up-regulation of early growth response-1 and platelet-derived growth factor ligands in pulmonary fibroblasts. J Immunol 2006; 177: 4141–4148. [DOI] [PubMed] [Google Scholar]
- 85. Bhogal RK, Stoica CM, McGaha TL, et al. Molecular aspects of regulation of collagen gene expression in fibrosis. J Clin Immunol 2005; 25: 592–603. [DOI] [PubMed] [Google Scholar]
- 86. Wenzel SE, Trudeau JB, Barnes S, et al. TGF-beta and IL-13 synergistically increase eotaxin-1 production in human airway fibroblasts. J Immunol 2002; 169: 4613–4619. [DOI] [PubMed] [Google Scholar]
- 87. Cho SJ, Kang MJ, Homer RJ, et al. Role of early growth response-1 (Egr-1) in interleukin-13-induced inflammation and remodeling. J Biol Chem 2006; 281: 8161–8168. [DOI] [PubMed] [Google Scholar]
- 88. Wynn TA. Fibrotic disease and the TH1/TH2 paradigm. Nat Rev Immunol 2004; 4: 583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Raes G, De Baetselier P, Noel W, et al. Differential expression of FIZZ1 and Ym1 in alternatively versus classically activated macrophages. J Leukoc Biol 2002; 71: 597–602. [PubMed] [Google Scholar]
- 90. Murray LA, Zhang H, Oak SR, et al. Targeting interleukin-13 with tralokinumab attenuates lung fibrosis and epithelial damage in a humanized SCID idiopathic pulmonary fibrosis model. Am J Respir Cell Mol Biol 2014; 50: 985–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Hancock A, Armstrong L, Gama R, et al. Production of interleukin 13 by alveolar macrophages from normal and fibrotic lung. Am J Respir Cell Mol Biol 1998; 18: 60–65. [DOI] [PubMed] [Google Scholar]
- 92. Neighbors M, Cabanski CR, Ramalingam TR, et al. Prognostic and predictive biomarkers for patients with idiopathic pulmonary fibrosis treated with pirfenidone: post-hoc assessment of the CAPACITY and ASCEND trials. Lancet Respir Med 2018; 6: 615–626. [DOI] [PubMed] [Google Scholar]
- 93. Okamoto M, Hoshino T, Kitasato Y, et al. Periostin, a matrix protein, is a novel biomarker for idiopathic interstitial pneumonias. Eur Respir J 2011; 37: 1119–1127. [DOI] [PubMed] [Google Scholar]
- 94. Izuhara K, Conway SJ, Moore BB, et al. Roles of periostin in respiratory disorders. Am J Respir Crit Care Med 2016; 193: 949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kudo A. Periostin in fibrillogenesis for tissue regeneration: periostin actions inside and outside the cell. Cell Mol Life Sci 2011; 68: 3201–3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Okamoto M, Izuhara K, Ohta S, et al. Ability of periostin as a new biomarker of idiopathic pulmonary fibrosis. Adv Exp Med Biol 2019; 1132: 79–87. [DOI] [PubMed] [Google Scholar]
- 97. Uchida M, Shiraishi H, Ohta S, et al. Periostin, a matricellular protein, plays a role in the induction of chemokines in pulmonary fibrosis. Am J Respir Cell Mol Biol 2012; 46: 677–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Barron L, Wynn TA. Fibrosis is regulated by Th2 and Th17 responses and by dynamic interactions between fibroblasts and macrophages. Am J Physiol Gastrointest Liver Physiol 2011; 300: G723–G728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Klein Wolterink RG, Kleinjan A, van Nimwegen M, et al. Pulmonary innate lymphoid cells are major producers of IL-5 and IL-13 in murine models of allergic asthma. Eur J Immunol 2012; 42: 1106–1116. [DOI] [PubMed] [Google Scholar]
- 100. Wang L, Tang J, Yang X, et al. TGF-beta induces ST2 and programs ILC2 development. Nat Commun 2020; 11: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Denney L, Byrne AJ, Shea TJ, et al. Pulmonary epithelial cell-derived cytokine TGF-beta1 is a critical cofactor for enhanced innate lymphoid cell function. Immunity 2015; 43: 945–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zhao Y, De Los Santos FG, Wu Z, et al. An ST2-dependent role of bone marrow-derived group 2 innate lymphoid cells in pulmonary fibrosis. J Pathol 2018; 245: 399–409. [DOI] [PubMed] [Google Scholar]
- 103. Wohlfahrt T, Usherenko S, Englbrecht M, et al. Type 2 innate lymphoid cell counts are increased in patients with systemic sclerosis and correlate with the extent of fibrosis. Ann Rheum Dis 2016; 75: 623–626. [DOI] [PubMed] [Google Scholar]
- 104. Li D, Guabiraba R, Besnard AG, et al. IL-33 promotes ST2-dependent lung fibrosis by the induction of alternatively activated macrophages and innate lymphoid cells in mice. J Allergy Clin Immunol 2014; 134: 1422–1432.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Hams E, Armstrong ME, Barlow JL, et al. IL-25 and type 2 innate lymphoid cells induce pulmonary fibrosis. Proc Natl Acad Sci U S A 2014; 111: 367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lee JU, Chang HS, Lee HJ, et al. Upregulation of interleukin-33 and thymic stromal lymphopoietin levels in the lungs of idiopathic pulmonary fibrosis. BMC Pulm Med 2017; 17: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Parker JM, Glaspole IN, Lancaster LH, et al. A phase 2 randomized controlled study of tralokinumab in subjects with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2018; 197: 94–103. [DOI] [PubMed] [Google Scholar]
- 108. Raghu G, Richeldi L, Crestani B, et al. SAR156597 in idiopathic pulmonary fibrosis: a phase 2 placebo-controlled study (DRI11772). Eur Respir J 2018; 52: 1801130. [DOI] [PubMed] [Google Scholar]
- 109. Wijsenbeek MS, Kool M, Cottin V. Targeting interleukin-13 in idiopathic pulmonary fibrosis: from promising path to dead end. Eur Respir J 2018; 52: 1802111. [DOI] [PubMed] [Google Scholar]
- 110. Habiel DM, Espindola MS, Coelho AL, et al. Modeling idiopathic pulmonary fibrosis in humanized severe combined immunodeficient mice. Am J Pathol 2018; 188: 891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Russell RJ, Chachi L, FitzGerald JM, et al. Effect of tralokinumab, an interleukin-13 neutralising monoclonal antibody, on eosinophilic airway inflammation in uncontrolled moderate-to-severe asthma (MESOS): a multicentre, double-blind, randomised, placebo-controlled phase 2 trial. Lancet Respir Med 2018; 6: 499–510. [DOI] [PubMed] [Google Scholar]
- 112. Panettieri RA, Jr, Sjobring U, Peterffy A, et al. Tralokinumab for severe, uncontrolled asthma (STRATOS 1 and STRATOS 2): two randomised, double-blind, placebo-controlled, phase 3 clinical trials. Lancet Respir Med 2018; 6: 511–525. [DOI] [PubMed] [Google Scholar]
- 113. Hanania NA, Korenblat P, Chapman KR, et al. Efficacy and safety of lebrikizumab in patients with uncontrolled asthma (LAVOLTA I and LAVOLTA II): replicate, phase 3, randomised, double-blind, placebo-controlled trials. Lancet Respir Med 2016; 4: 781–796. [DOI] [PubMed] [Google Scholar]
- 114. Dupilumab (Dupixent) for asthma. JAMA 2019; 321: 1000–1001. [DOI] [PubMed] [Google Scholar]
- 115. Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med 2018; 378: 2486–2496. [DOI] [PubMed] [Google Scholar]
- 116. Rabe KF, Nair P, Brusselle G, et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med 2018; 378: 2475–2485. [DOI] [PubMed] [Google Scholar]
- 117. Kelly-Welch AE, Melo ME, Smith E, et al. Complex role of the IL-4 receptor alpha in a murine model of airway inflammation: expression of the IL-4 receptor alpha on nonlymphoid cells of bone marrow origin contributes to severity of inflammation. J Immunol 2004; 172: 4545–4555. [DOI] [PubMed] [Google Scholar]
- 118. Lowenthal JW, Castle BE, Christiansen J, et al. Expression of high affinity receptors for murine interleukin 4 (BSF-1) on hemopoietic and nonhemopoietic cells. J Immunol 1988; 140: 456–464. [PubMed] [Google Scholar]
- 119. Oczypok EA, Milutinovic PS, Alcorn JF, et al. Pulmonary receptor for advanced glycation end-products promotes asthma pathogenesis through IL-33 and accumulation of group 2 innate lymphoid cells. J Allergy Clin Immunol 2015; 136: 747–756.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Perkins TN, Oczypok EA, Milutinovic PS, et al. RAGE-dependent VCAM-1 expression in the lung endothelium mediates IL-33-induced allergic airway inflammation. Allergy 2019; 74: 89–99. [DOI] [PubMed] [Google Scholar]
- 121. Ullah MA, Loh Z, Gan WJ, et al. Receptor for advanced glycation end products and its ligand high-mobility group box-1 mediate allergic airway sensitization and airway inflammation. J Allergy Clin Immunol 2014; 134: 440–450. [DOI] [PubMed] [Google Scholar]
- 122. Harari S, Caminati A. IPF: new insight on pathogenesis and treatment. Allergy 2010; 65: 537–553. [DOI] [PubMed] [Google Scholar]
- 123. Starkey MR, McKenzie AN, Belz GT, et al. Pulmonary group 2 innate lymphoid cells: surprises and challenges. Mucosal Immunol 2019; 12: 299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Germain RN, Huang Y. ILC2s - resident lymphocytes pre-adapted to a specific tissue or migratory effectors that adapt to where they move? Curr Opin Immunol 2019; 56: 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Richards TJ, Kaminski N, Baribaud F, et al. Peripheral blood proteins predict mortality in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2012; 185: 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Yamaguchi K, Iwamoto H, Sakamoto S, et al. Serum high-mobility group box 1 is associated with the onset and severity of acute exacerbation of idiopathic pulmonary fibrosis. Respirology 2020; 25: 275–280. [DOI] [PubMed] [Google Scholar]
- 127. Foell D, Wittkowski H, Vogl T, et al. S100 proteins expressed in phagocytes: a novel group of damage-associated molecular pattern molecules. J Leukoc Biol 2007; 81: 28–37. [DOI] [PubMed] [Google Scholar]
- 128. Aoki T, Matsumoto Y, Hirata K, et al. Expression profiling of genes related to asthma exacerbations. Clin Exp Allergy 2009; 39: 213–221. [DOI] [PubMed] [Google Scholar]
- 129. Gray RD, MacGregor G, Noble D, et al. Sputum proteomics in inflammatory and suppurative respiratory diseases. Am J Respir Crit Care Med 2008; 178: 444–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Lee TH, Chang HS, Bae DJ, et al. Role of S100A9 in the development of neutrophilic inflammation in asthmatics and in a murine model. Clin Immunol 2017; 183: 158–166. [DOI] [PubMed] [Google Scholar]
- 131. Lee TH, Jang AS, Park JS, et al. Elevation of S100 calcium binding protein A9 in sputum of neutrophilic inflammation in severe uncontrolled asthma. Ann Allergy Asthma Immunol 2013; 111: 268–275.e1. [DOI] [PubMed] [Google Scholar]
- 132. Hesselstrand R, Wildt M, Bozovic G, et al. Biomarkers from bronchoalveolar lavage fluid in systemic sclerosis patients with interstitial lung disease relate to severity of lung fibrosis. Respir Med 2013; 107: 1079–1086. [DOI] [PubMed] [Google Scholar]
- 133. Bargagli E, Olivieri C, Cintorino M, et al. Calgranulin B (S100A9/MRP14): a key molecule in idiopathic pulmonary fibrosis? Inflammation 2011; 34: 85–91. [DOI] [PubMed] [Google Scholar]
- 134. Bargagli E, Olivieri C, Prasse A, et al. Calgranulin B (S100A9) levels in bronchoalveolar lavage fluid of patients with interstitial lung diseases. Inflammation 2008; 31: 351–354. [DOI] [PubMed] [Google Scholar]
- 135. Bennett D, Salvini M, Fui A, et al. Calgranulin B and KL-6 in bronchoalveolar lavage of patients with IPF and NSIP. Inflammation 2019; 42: 463–470. [DOI] [PubMed] [Google Scholar]
- 136. Hara A, Sakamoto N, Ishimatsu Y, et al. S100A9 in BALF is a candidate biomarker of idiopathic pulmonary fibrosis. Respir Med 2012; 106: 571–580. [DOI] [PubMed] [Google Scholar]
- 137. Korthagen NM, Nagtegaal MM, van Moorsel CH, et al. MRP14 is elevated in the bronchoalveolar lavage fluid of fibrosing interstitial lung diseases. Clin Exp Immunol 2010; 161: 342–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Scott NR, Swanson RV, Al-Hammadi N, et al. S100A8/A9 regulates CD11b expression and neutrophil recruitment during chronic tuberculosis. J Clin Invest 2020; 130: 3098–3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Muhlebach MS, Clancy JP, Heltshe SL, et al. Biomarkers for cystic fibrosis drug development. J Cyst Fibros 2016; 15: 714–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Gray RD, Imrie M, Boyd AC, et al. Sputum and serum calprotectin are useful biomarkers during CF exacerbation. J Cyst Fibros 2010; 9: 193–198. [DOI] [PubMed] [Google Scholar]
- 141. Zhong A, Xu W, Zhao J, et al. S100A8 and S100A9 are induced by decreased hydration in the epidermis and promote fibroblast activation and fibrosis in the dermis. Am J Pathol 2016; 186: 109–122. [DOI] [PubMed] [Google Scholar]
- 142. Zhang W, Lavine KJ, Epelman S, et al. Necrotic myocardial cells release damage-associated molecular patterns that provoke fibroblast activation in vitro and trigger myocardial inflammation and fibrosis in vivo. J Am Heart Assoc 2015; 4: e001993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Shibata F, Miyama K, Shinoda F, et al. Fibroblast growth-stimulating activity of S100A9 (MRP-14). Eur J Biochem 2004; 271: 2137–2143. [DOI] [PubMed] [Google Scholar]
- 144. Shi S, Yi JL. S100A8/A9 promotes MMP-9 expression in the fibroblasts from cardiac rupture after myocardial infarction by inducing macrophages secreting TNFalpha. Eur Rev Med Pharmacol Sci 2018; 22: 3925–3935. [DOI] [PubMed] [Google Scholar]
- 145. Palmer LD, Maloney KN, Boyd KL, et al. The innate immune protein S100A9 protects from Th2-mediated allergic airway inflammation. Am J Respir Cell Mol Biol 2019; 61: 459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Kumar V, Fleming T, Terjung S, et al. Homeostatic nuclear RAGE-ATM interaction is essential for efficient DNA repair. Nucleic Acids Res 2017; 45: 10595–10613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Kumar V, Agrawal R, Pandey A, et al. Compromised DNA repair is responsible for diabetes-associated fibrosis. EMBO J 2020; 39: e103477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Liu RM, Liu G. Cell senescence and fibrotic lung diseases. Exp Gerontol 2020; 132: 110836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Kato K, Logsdon NJ, Shin YJ, et al. Impaired myofibroblast dedifferentiation contributes to nonresolving fibrosis in aging. Am J Respir Cell Mol Biol 2020; 62: 633–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Rodier F, Coppe JP, Patil CK, et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol 2009; 11: 973–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Chen J, Stubbe J. Bleomycins: towards better therapeutics. Nat Rev Cancer 2005; 5: 102–112. [DOI] [PubMed] [Google Scholar]
- 152. Raghu G, Weycker D, Edelsberg J, et al. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2006; 174: 810–816. [DOI] [PubMed] [Google Scholar]
- 153. Hecker L, Logsdon NJ, Kurundkar D, et al. Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Sci Transl Med 2014; 6: 231ra47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Cui H, Ge J, Xie N, et al. miR-34a promotes fibrosis in aged lungs by inducing alveolarepithelial dysfunctions. Am J Physiol Lung Cell Mol Physiol 2017; 312: L415–L424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Cui H, Ge J, Xie N, et al. miR-34a inhibits lung fibrosis by inducing lung fibroblast senescence. Am J Respir Cell Mol Biol 2017; 56: 168–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Yamaguchi K, Iwamoto H, Mazur W, et al. Reduced endogenous secretory RAGE in blood and bronchoalveolar lavage fluid is associated with poor prognosis in idiopathic pulmonary fibrosis. Respir Res 2020; 21: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Deane R, Singh I, Sagare AP, et al. A multimodal RAGE-specific inhibitor reduces amyloid beta-mediated brain disorder in a mouse model of Alzheimer disease. J Clin Invest 2012; 122: 1377–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Comis RL. Bleomycin pulmonary toxicity: current status and future directions. Semin Oncol 1992; 19: 64–70. [PubMed] [Google Scholar]
- 159. Hutchinson JP, McKeever TM, Fogarty AW, et al. Increasing global mortality from idiopathic pulmonary fibrosis in the twenty-first century. Ann Am Thorac Soc 2014; 11: 1176–1185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tar-10.1177_17534666211016071 for The perplexing role of RAGE in pulmonary fibrosis: causality or casualty? by Timothy N. Perkins and Tim D. Oury in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-2-tar-10.1177_17534666211016071 for The perplexing role of RAGE in pulmonary fibrosis: causality or casualty? by Timothy N. Perkins and Tim D. Oury in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-3-tar-10.1177_17534666211016071 for The perplexing role of RAGE in pulmonary fibrosis: causality or casualty? by Timothy N. Perkins and Tim D. Oury in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-4-tar-10.1177_17534666211016071 for The perplexing role of RAGE in pulmonary fibrosis: causality or casualty? by Timothy N. Perkins and Tim D. Oury in Therapeutic Advances in Respiratory Disease