Abstract
Aim
The severe acute respiratory syndrome coronavirus 2 outbreak resulted in severe health impact with the loss of many lives across the world. Pulmonary parenchyma suffers the most from the brunt of the infection. However, evidence suggested a systemic involvement during the course of illness. Information on morphological changes of the liver is sparse in the literature. We aimed to evaluate the pathological findings in the liver by minimally invasive autopsies.
Methods
Postmortem core biopsies of the liver obtained from patients who succumbed to coronavirus disease 2019 disease were studied. Demographic findings, comorbidities, and relevant laboratory tests were collected. Detailed histopathological changes were assessed.
Results
Liver function tests were available in 40 cases, and it was deranged in 80% cases. A spectrum of histological changes was observed. Macrovesicular steatosis and nonspecific portal inflammation of mild degree were the common morphological changes. Features suggestive of vascular alteration were noted in more than half of the cases. These included increased portal vein branches, irregular luminal dilation, and herniation of portal veins into the periportal hepatocytes. In addition, we observed morphological changes attributed to terminal shock-related changes.
Conclusion
The present study results highlight that liver parenchyma changes may be related to multiple pathogenic mechanisms. The presence of vascular alteration in portal tracts suggests derangement of hepatic vasculature related to systemic hypercoagulable state induced by the viral infection. It remains to be established if the histological changes are related to direct viral insult or to the systemic response caused by the viral attack.
Keywords: autopsy, abnormal liver chemistries, COVID-19, liver pathology, SARS-CoV-2
Abbreviations: ACE2, Angiotensin-Converting Enzyme 2; ALT, Alanine Aminotransferase; ARDS, Adult Respiratory Distress syndrome; AST, Aspartate Aminotransferase; CBC, Complete Blood Count; CK-MB, Creatine Kinase-MB; COVID-19, Coronavirus Disease 2019; ISH, In situ Hybridization; LDH, Lactate Dehydrogenase; LFTs, Liver Function Tests; PCR, Polymerase Chain Reaction; RNA, Ribonucleic Acid; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2
Since the origin of the 2019 novel coronavirus from the Wuhan City of China, the entire world has faced the brunt of the pandemic caused by the virus. Until 1st April, 2021, nearly 130 million cases of coronavirus disease 2019 (COVID-19) have been reported across the globe, and 2.8 million deaths are attributed to this illness. India ranked third, after USA and Brazil, for the rising number of cases in this pandemic, with nearly 162 thousand deaths.1
The pathobiology of the illness has been recently begun to elucidate.2, 3, 4 Most of the cases have a mild clinical course. However, a small proportion of the patients develop pneumonia, and the clinical course progresses to an overt systemic illness to multiorgan dysfunction. Several studies reported that the lungs are severely affected by the brunt of the COVID-19. The classical lesion is the diffuse alveolar damage, the pathological correlate of the clinical picture of adult respiratory distress syndrome.5, 6, 7 Apart from the lung, involvement of extrapulmonary organs such as the liver, kidney, brain, and so on has also been documented.8 Approximately, 44% of these cases had deranged liver function tests (LFTs).9,10 However, studies describing the microscopic changes in the liver in these cases are limited. Hence, in the present study, we aimed to characterize the histopathological changes in the liver in COVID-19. Autopsy protocols have been modified to lessen the exposure, and tissues were obtained by performing minimally invasive autopsies.
Methods
This study was performed in Western India at a tertiary care center from May 2020 to March 2021. In the ongoing COVID-19 pandemic, we have modified autopsy protocols and adopted minimally invasive autopsies in all COVID-19–positive cases where we can obtain consent from the relatives of the deceased. The diagnosis of COVID-19 was established by reverse transcriptase polymerase chain reaction testing of the samples obtained from nasal swab. A minimally invasive autopsy was performed in the form of core biopsies from the liver within an hour after death. A minimum of three cores of the liver tissue were sampled in each case. The tissue samples were immersed in neutral buffered formalin for a minimum of 48 h. The fixed tissue was then processed, and the slides prepared were analyzed by three pathologists (V.V., A.P., and P.A.E.). Additional stains (periodic acid-Schiff, Masson's trichrome, reticulin, and phosphotungustic acid hematoxylin) were ordered, wherever required. Semiquantitative scoring of several morphological findings (steatosis, cholestasis, portal inflammation) was performed in the liver parenchyma (0 = absent, 1 = mild, 2 = moderate, and 3 = prominent). Particular emphasis was done for the portal vascular alterations while interpreting the histological findings. The following vascular alterations were specifically searched in each case: portal vein herniation (defined by close opposition of the portal vein to periportal parenchyma), hypervascularised portal tracts (defined by multiple thin-walled vascular spaces in the portal tracts), periportal abnormal vessels (defined by one or more thin-walled vessels of a variable caliber lying in close contact with portal tracts but outside the portal tracts), phelbosclerosis, hepatic artery changes, and central vein alterations.11 In addition, the presence of fibrin thrombi in the portal vein, hepatic artery, sinusoids, and central vein was sought. Immunohistochemistry was performed for CD34 (Thermo Scientific, QBEnd/10, ready to use) and C4d (Cell Marque, polyclonal, 1:50). Medical records of these cases were reviewed. Following details were collected: age, sex, presenting complaints, comorbidities, duration symptom onset to death, duration of hospital stay, and results of laboratory investigations (including complete blood count, D-dimer, lactate dehydrogenase, creatine kinase-MB, LFTs). The study follows the ethical standards formulated in the Helsinki Declaration. Written informed consent was obtained from the close relative of all these deceased patients. The permission for this study was obtained from the institutional review board of the (certificate reference number AIIMS/IEC/2020–21/3058).
Data were summarized using descriptive statistics. The Fischer exact test was used to compare categorical variables. A P-value of less than 0.05 was considered statistically significant. All the computations were carried out using the Statistical Package for the Social Sciences for Windows (IBM SPSS Statistics for Windows, version 22.0., Armonk, NY: IBM Corp.2013).
Results
Study population and baseline demographics
In our COVID-19–dedicated center, we performed core biopsies in the deceased patients after getting proper informed consent from the closet relative. During this study period, core biopsies were performed in 50 cases. Of these, three cases turned to be COVID-19 negative, while in five cases, liver tissue could not be obtained, and one case had autolyzed liver tissue. Therefore, a total of 41 cases were included in the present study for further analysis.
The mean and median age of the cohort were 61.4 and 63 years, respectively (range: 28–83). The study population included 31 (75.6%) men and 10 (24.4%) women, with the male to female ratio of 3.1:1. The most common presenting complaints were breathlessness (38/41, 92.7%), fever (32/41, 78%), and cough (25/41, 60.9%). Uncommon presenting complaints include chest pain (2/41, 4.9%), seizure (1/41, 2.4%), and reduced urine output (1/41, 2.4%). Associated comorbidities were documented in 70.7% (29/41) cases. The most common comorbidities were diabetes mellitus (16/29, 55.2%), followed by hypertension (14/29, 48.3%) and coronary artery disease (6/29, 20.7%). The mean duration of symptom onset to the death of the patients was 13 days (median: 12 days; range: 4–35 days), while the mean duration of hospital course was nine days (median: 8 days; range: 2–31 days). On X-ray examination, bilateral lung opacities were documented in 73.2% (30/41) cases, whereas unilateral lung opacities were noted in 19.5% (8/41) cases. The laboratory results are summarized in Table 1. All patients were negative for hepatitis B virus and hepatitis C virus. LFTs were deranged in 80% (32/40) cases. At the time of admission, all cases have moderate (2 cases) or severe disease (39 cases). All these cases had received corticosteroids and anticoagulants. Mechanical ventilation was required in all cases except one, where the patient condition suddenly deteriorated before further management strategies could be started.
Table 1.
Summary of Laboratory Findings.
| Laboratory parameters | Reference range | Median | Interquartile range | Cases with deranged results |
|---|---|---|---|---|
| AST (n = 40) | 7–40 IU/L | 61 | 40–81 | 30 (75%) |
| ALT (n = 40) | 5–35 IU/L | 44.8 | 18–63 | 23 (57.5%) |
| ALP (n = 40) | 40–160 IU/L | 91 | 68–143 | 6 (15%) |
| TB (n=40) | 0.3–1 mg/dl | 0.58 | 0.5–0.77 | 3 (7.5%) |
| LDH (n = 18) | 140–280 IU/L | 529 | 447–667 | 18 (100%) |
| D-dimer (n = 33) | <0.5 ug/ml | 2.8 | 2.03–4.26 | 31 (93.9%) |
| PT (n = 30) | 12–16 s | 14.4 | 13.3–17.7 | 10 (33.3%) |
| APTT (n = 30) | 26–34 s | 33.8 | 29.4–42.3 | 15 (50%) |
| CK-MB (n = 16) | 5–25 IU/L | 27.5 | 23–33 | 10 (62.5%) |
| Sr creatinine (n = 32) | 0.6–1.2 mg/dl | 1.13 | 0.9–1.6 | 14 (43.7%) |
| Sr urea (n = 32) | 15–40 mg/dl | 41 | 27–63 | 16 (50%) |
AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; TB, total bilirubin; LDH, lactate dehydrogenase; PT, prothrombin time; APTT, activated partial thromboplastin time; CK-MB, creatine kinase-MB; Sr, serum.
Pathological findings
Portal tract changes
Portal inflammation was noted in 31.7% (13/41) cases (Figure 1a). The majority of these cases (84.6%) had a mild degree of portal inflammation, while only two cases (15.4%) had moderate inflammation. The inflammatory cells comprised lymphocytes and occasional macrophages. Only one case showed interface activity, which is of mild degree. None of the cases showed the presence of eosinophils and neutrophils. Only rare plasma cells are noted. However, none of those cases had significant infiltrate to consider autoimmune hepatitis. Lymphoid aggregates were absent. Cirrhosis was noted in one case. Porto-portal bridging fibrosis was documented in 3 cases, while fibrous expansion of portal tracts with fibrous septa was documented in 4 cases. No ductopenia was seen.
Figure 1.
(a) Portal tract expansion with mild to moderate degree of portal tract inflammation. (b) Microphotograph shows prominent macrovesicular steatosis. In addition, note the presence of neutrophilic lobular inflammation (encircled area). (c) Intracytoplasmic cholestasis Hematoxylin and eosin stain is used for (a–c); (a-b), 100 x magnification; (c), 400 x magnification.
Lobular changes
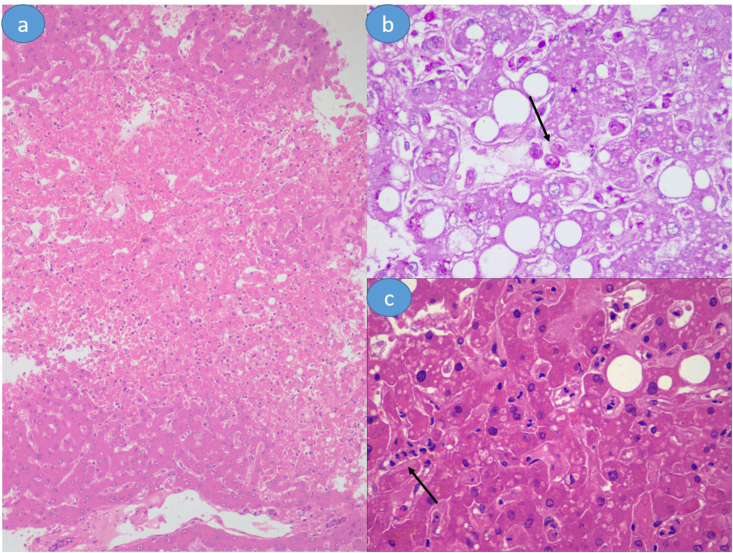
Steatosis was the common histological findings and was noted in 70.7% (29/41) cases (Figure 1b). Steatosis was macrovesicular. No true microvesicular steatosis was noted. The location of steatosis was centrizonal in 25 cases, while periportal in 4 cases. It was graded as mild (16 cases), moderate (11 cases), and severe (2 cases). Cholestasis was not significantly observed. It was noted in 41.4% (17/41) cases, where it was of a minimal to mild degree and mainly intracytoplasmic (Figure 1c) None of the cases had ductular cholestasis. Lobular inflammation was noted in 24.3% (10/41) cases. Of these cases, six cases had neutrophilic infiltrate (Figure 1a), while the remaining four cases had lymphocytic infiltrate. None of the cases had massive or submassive hepatic necrosis. Eight (19.5%) cases had occasional apoptotic hepatocytes without accompanying necroinflammation. Centrizonal congestion with ischemic hepatocytic loss was noted in 26.8% (11/41) cases (Figure 2a). Additional pathological findings, possibly related to the systemic response to viral infections, included sinusoidal congestion with reflux of neutrophils (34.1%, 14/34), Kupffer cell hyperplasia (21.9%, 9/14), and granuloma (2.4%, 1/41). In addition, Kupffer cell hemophagocytosis was noted in 4.8% (2/41) cases (Figures 2b–2c). In addition, pericellular fibrosis in zone 3 was noted in three cases, of which Mallory-hyaline bodies were noted in three cases and ballooning degeneration in two cases. These features were suggestive of alcoholic/nonalcoholic steatohepatitis.
Figure 2.
(a) Centrizonal congestion with ischemic hepatocytic loss. (b) Kupffer cell hemophagocytosis (arrow). (c) Sinusoidal influx of neutrophils (arrow). Hematoxylin and eosin stain is used for (a) and (c); periodic acid-Schiff stain is used for (b); (a), 100 x magnification; (b-c), 400 x magnification.
Vascular changes
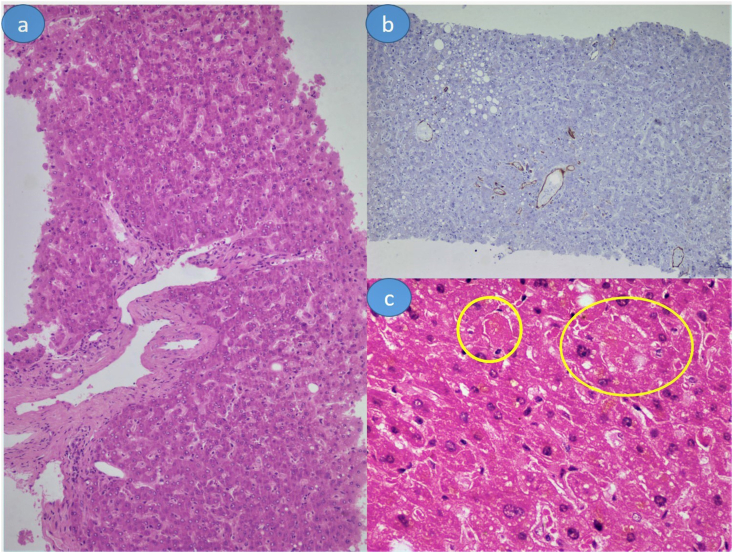
One of the common vascular alterations noted in the present study was increased portal vein structure within portal tracts, noted in 58.5% (24/41) cases (Figure 3a). The portal veins were markedly dilated in these portal tracts. In some portal tracts, herniation of portal veins into the periportal hepatocytes was also noted. CD34 highlights these abnormal portal venous channels herniating into periportal hepatocytes (Figure 3b). Two of the portal veins showed the presence of fresh fibrin thrombi. The hepatic artery did not reveal any specific changes. Central vein changes were noted in minority cases and included central vein thrombi, sclerosis, and central perivenulitis, each in one case. Sinusoidal microthrombi were noted in 12.2% (5/41) cases (Figure 3c).
Figure 3.
(a) Increased number of portal venous channels in portal tracts. Occasional ones are seen herniating into periportal hepatocytes. (b) CD34 highlights these abnormal vascular channels. (c) Sinusoidal microthrombi (encircled areas). Hematoxylin and eosin stain is used for (a) and (c); immunohistochemistry is used for (b); (a-b), 100 x magnification; (c), 400 x magnification.
C4d, a product of C4 activation involved in the classical complement pathway, was used to assess the role of complement in mediating liver injury. None of the cases showed C4d positivity. The individual histological findings were correlated with liver enzyme levels. However, none of the histological findings showed any association with aspartate aminotransferase or alanine aminotransferase levels (Table 2). Furthermore, no correlation was found between the presence of vascular changes (portal vein alterations and sinusoidal microthrombi) and abnormal coagulation profiles (prothrombin time and activated partial thromboplastin time).
Table 2.
Histological Findings of the Liver in COVID-19 and Their Correlation With Elevated Liver Enzymes.
| Histological parameters | All cases (n = 41) | Normal AST or ALT N = 8 [I] | AST or ALT 1X-2X (n = 18) [II] | AST or ALT >2X (n = 14) [III] | P value I vs II + III |
P value I vs III |
|---|---|---|---|---|---|---|
| Macrovesicular steatosis | 29 (70.7%) | 8 (100%) | 13 (72.2%) | 8 (57.1%) | 0.080 | 0.051 |
| Mild | 16 (39%) | 3 (37.5%) | 8 (44.4%) | 5 (35.7%) | ||
| Moderate | 11 (26.8%) | 5 (62.5%) | 4 (22.2%) | 2 (14.3%) | ||
| Severe | 2 (4.8%) | 0 | 1 (5.5%) | 1 (7.1%) | ||
| Lobular necroinflammation | 10 (24.4%) | 0 | 5 (27.7%) | 5 (35.7%) | 0.165 | 0.115 |
| Rare individual apoptosis | 8∗ (19.5%) | 1 (12.5%) | 2 (11.1%) | 4 (28.6%) | 1.000 | 0.613 |
| Portal inflammation | 13 (31.7%) | 2 (25%) | 5 (27.7%) | 6 (42.8%) | 1.000 | 0.649 |
| Mild | 11 (26.8%) | 1 (12.5%) | 5 (27.7%) | 5 (35.7%) | ||
| Moderate | 2 (4.8%) | 1 (12.5%) | 0 | 1 (7.1%) | ||
| Severe | 0 | 0 | 0 | 0 | ||
| Fibrosis | 8$& | 2a (25%) | 1b (5.5%) | 5c (35.7%) | 0.649 | 1.000 |
| Centrilobular ischemic necrosis | 11 (26.8%) | 3 (37.5%) | 5 (27.7%) | 3 (21.4%) | 0.660 | 0.624 |
| Cholestasis (all mild) | 17∗ (41.5%) | 5 (62.5%) | 7 (38.9%) | 4 (28.6%) | 0.229 | 0.187 |
| Increased portal vascular channels | 24∗ (58.5%) | 4 (50%) | 10 (55.5%) | 9 (64.3%) | 0.702 | 0.661 |
| Sinusoidal microthrombi | 5∗ (12.2%) | 0 | 3 (16.6%) | 1 (7.1%) | 0.565 | 1.000 |
| Sinusoidal congestion with neutrophil influx | 14 (34.1%) | 1 (12.5%) | 8 (44.4%) | 5 (35.7%) | 0.221 | 0.351 |
| Kupffer cell prominence | 9∗ (21.9%) | 1 (12.5%) | 2# (11.1%) | 5# (35.7%) | 1.000 | 0.351 |
AST, aspartate aminotransferase; AST, alanine aminotransferase.
∗AST/ALT value not available in one case; # includes one case with Kupffer hemophagocytosis; $ includes one case of cirrhosis, three cases with stage 3 fibrosis, and four cases with stage 1 fibrosis (Ishak staging system); & excludes three cases of zone 3 pericellular fibrosis; a represents one case with stage 1 fibrosis and one case with stage 3 fibrosis; b represents one case with stage 3 fibrosis; c represents three cases with stage 1 fibrosis, one case with stage 3 fibrosis, and 1 case with cirrhosis.
Discussion
The present study highlights the morphological changes in the liver, which are documented in only a few studies.12,13 The main morphological changes observed in this study were macrovesicular steatosis, vascular alterations, and lobular necroinflammation. Portal inflammation and cholestasis were noted in a high proportion of cases; however, none of the cases had these changes of significant degree. Apart from these, centrizonal congestion with ischemic loss of hepatocytes was noted in one-fourth of cases. All these histological changes are attributed to multiple pathogenic mechanisms.
Macrovesicular steatosis was a common morphological finding, and the same was documented in other studies as well. In the study by Lagana et al,12 75% of patients had macrovesicular steatosis, similar to the present study. A total of 39% of patients in the present cohort were diabetics, which could partly account for this proportion of macrovesicular steatosis observed. No direct association between diabetics and steatosis was observed in the present study. Although most of the specimen showed centrizonal steatosis, features of alcoholic/nonalcoholic steatohepatitis in the form of sinusoidal fibrosis, Mallory hyaline bodies, and/or ballooning degeneration were observed in only three cases. We could not obtain the history of alcohol intake or body mass index in every case to further investigate in this direction. In the present cohort, steroid was given to every patient as part of the treatment regimen. Steroid can lead to the development of steatosis.14 Apart from these factors, other insults are known to cause steatosis, including hypoxia, malnutrition, viral infections (hepatotropic and nonhepatotropic), and medication errors.15 Although all these factors have partly led to steatosis, COVID-19 illness might have contributed to certain cases. One study found the presence of steatosis, and a high neutrophil-lymphocyte ratio predicts the severity of the COVID-19 illness.16
Another important finding observed in the study was portal vein changes manifested as an increased number and herniation into periportal hepatocytes. This morphological change was also reported by Sonzogni et al13 in their 75% cases. Several of these vascular alterations are described in obliterative portal venopathy and hepatopulmonary syndrome17, 18, 19, 20 and hint toward the impaired circulation in COVID-19. This was further supported by CD34 expression in periportal hepatocytic sinusoids. Intrahepatic blood flow may be partially impaired by cardiac stress or thrombotic events in portal circulation or sinusoids, explaining these pathological findings. Earlier study has pointed that CD34 expression along the sinusoidal lining in nodular regenerative hyperplasia is a response to maintain the integrity of sinusoidal endothelial lining by perturbed perfusion.21 This CD34 expression can also be seen in chronic hepatitis and cirrhosis.22 Several cases had thrombotic events. Recent studies had documented the presence of microthrombi in the pulmonary circulation.5, 6, 7 Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection produced a hypercoagulable state, which is clinically evident from raised d-dimer levels seen in most of these patients.23 Sinusoidal microthrombi were also reported in other studies.12,13 Additional vascular changes described in the literature included portal arteriole hyperplasia, phlebosclerosis of the portal vein, and fibrinoid necrosis.12,13 However, we could not find significant phlebosclerosis of the portal vein wall or arteriole hyperplasia in any of our cases. The vascular alterations noted in these studies and our studies need to be further investigated.
Biliary epithelial cells express the angiotensin-converting enzyme 2 receptor.24 However, the interlobular bile ducts did not show any features of injury in the present study. This was supported by previous studies as well.12,13 However, functional impairment of these cells cannot be entirely excluded and requires further genomic or proteomic studies. In addition, portal inflammation was reported to be mild in these studies. Lagana et al12 documented portal inflammation in 50% cases; however, these were reported as minimal in the majority of their cases. Sonzogni et al13 also documented portal inflammation in two-third of their cohort, all having a mild degree of inflammation. In the present study, portal inflammation was reported in one-third of cases, and it was mild in all cases, except two. As steroid was administered in all cases, it could be possible that reduced density of inflammation was attributed to steroid administration and hence might not correlate with the viral load. Nevertheless, this degree of inflammatory response can also be explained as a nonspecific response to viral infection.
Apart from the hepatotropic viruses (such as hepatitis A virus, hepatitis B virus, and so on), the liver is reported to be affected by nonhepatotropic viruses such as herpes virus, adenovirus, parvovirus, and SARS-associated coronavirus.25 Hepatocytic damage manifested as lobular inflammation can occur due to the immune response against viral particles in both hepatotropic and nonhepatotropic viruses. In addition, loss of immune control can also be responsible for liver damage in certain viruses, including cytomegalovirus and parvovirus. In the present study, lobular necroinflammation was documented in one-fourth of cases. Two studies reported lobular necroinflammation in one-half cases and also used methods to demonstrate viral particles in the liver. Sonzogni et al13 used ribonucleic acid (RNA) in situ hybridization (ISH) technique to detect the presence of viral particles in liver parenchyma and found positive expression in 15 cases of 22 cases tested. In the study by Lagana et al,12 the authors reported detectable virus in liver tissue in 55% of cases tested using polymerase chain reaction (PCR). However, PCR positivity for viral RNA did not correlate with any histological parameters, including lobular inflammation. These findings provide evidence of possible replication of viral particles in hepatocytes. However, currently, it is not clear that targeting viral replication in hepatocytes may alter the clinical course of COVID-19 illness.
Additional findings noted in the present study included centrilobular congestion and ischemic hepatocytic loss, sinusoidal congestion with the influx of neutrophils, and Kupffer cell prominence. Passive venous congestion, often seen during terminal course of illness, seems to be the most plausible explanation for centrizonal congestion. Kupffer cell prominence and sinusoidal congestion with the influx of neutrophils, noted in a substantial proportion of cases, seems to be related to the cytokine-induced hyperinflammatory response induced by viral insult. Furthermore, in this study, no statistically significant association of deranged LFTs with any of the specific pathological findings was documented. This was supported by findings from Lagana et al12 in their cohort of 40 cases. This was not a surprising finding as earlier studies on hepatotropic viruses have also shown a lack of this association.26 Our study suffers from several limitations. Histological findings are described on core biopsies. In addition, orthogonal approaches such as immunohistochemistry, ISH, PCR, or electron microscopy were not used to demonstrate the presence of viral particles in these cases.
To summarize, hepatic pathological features in SARS-CoV-2 infections may be linked to a number of pathogenic mechanisms, including ischemic events, drugs, circulatory disturbances, and terminal shock-induced changes. However, the role of direct viral attack, although supported by previous studies using ISH and PCR studies, needs to be further investigated. Currently, ultrastructural demonstration of the viral particles is lacking in the current literature. The role of complement involvement in mediating the damage of liver parenchyma appears less likely; however, it needs further research as steroid was administered to all patients.
CRediT authorship contribution statement
Dr Vikarn Vishwajeet: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing - Original Draft, Visualization.
Dr Abhishek Purohit: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Writing - Original Draft, Visualization, Supervision.
Dr Deepak Kumar Sharma: Conceptualization, Investigation, Resources, Writing - Review & Editing, Supervision.
Dr Vijay Parag: Investigation, Resources, Writing - Original Draft, Writing - Review & Editing, Visualization.
Dr Swapnil Tripathi: Methodology, Validation, Formal analysis, Investigation, Resources, Writing - Original Draft.
Dr Tanuj Kanchan: Conceptualization, Methodology, Investigation, Writing - Original Draft, Visualization.
Dr Nikhil Kothari: Validation, Formal analysis, Investigation, Resources, Writing - Original Draft, Visualization, Supervision.
Dr Naveen Dutt: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Writing - Original Draft, Supervision.
Dr Prof. Poonam Abhay Elhence: Conceptualization, Methodology, Formal analysis, Investigation, Writing - Review & Editing, Supervision, Project administration.
Dr Prof. Pradeep Kumar Bhatia: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Writing - Review & Editing.
Dr Prof. Vijaya Lakshmi Nag: Conceptualization, Methodology, Supervision, Project administration.
Dr Prof. Mahendra Kumar Garg: Conceptualization, Methodology, Visualization, Writing - Review & Editing.
Dr Prof. Sanjeev Misra: Conceptualization, Methodology, Visualization, Supervision, Project administration.
Conflicts of interest
The authors have none to declare.
Funding
This research received no specific grant from any funding agency.
References
- 1.Coronavirus outbreak. Available at: https://www.worldometers.info/coronavirus/. Accessed 1 Apr. 2021.
- 2.Ackermann M., Verleden S.E., Kuehnel M., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wichmann D., Sperhake J.P., Lütgehetmann M., et al. Autopsy findings and venous thromboembolism in patientswith COVID-19: a prospective cohort study. Ann Intern Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martines R.B., Ritter J.M., Matkovic E., et al. COVID-19 pathology working group. Pathology and pathogenesis of SARS-CoV-2 associated with fatal coronavirus disease, United States. Emerg Infect Dis. 2020;26:2005–2015. doi: 10.3201/eid2609.202095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu Z., Shi L., Wang Y., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menter T., Haslbauer J.D., Nienhold R., et al. Post-mortem examination of COVID19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77:198–209. doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bösmüller H., Traxler S., Bitzer M., et al. The evolution of pulmonary pathology in fatal COVID-19 disease:an autopsy study with clinical correlation. Virchows Arch. 2020;477:349–357. doi: 10.1007/s00428-020-02881-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta A., Madhavan M.V., Sehgal K., et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020 Jul;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Q., Zhao H., Liu L.G., et al. Pattern of liver injury in adult patients with COVID-19: a retrospective analysis of 105 patients. Mil Med Res. 2020;7:28. doi: 10.1186/s40779-020-00256-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guido M., Alves V.A.F., Balabaud C., et al. International Liver Pathology Study Group. Histology of portal vascular changes associated with idiopathic non-cirrhotic portal hypertension: nomenclature and definition. Histopathology. 2019;74:219–226. doi: 10.1111/his.13738. [DOI] [PubMed] [Google Scholar]
- 12.Lagana S.M., Kudose S., Iuga A.C., et al. Hepatic pathology in patients dying of COVID-19: a series of 40 cases including clinical, histologic, and virologic data. Mod Pathol. 2020;33:2147–2155. doi: 10.1038/s41379-020-00649-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sonzogni A., Previtali G., Seghezzi M., et al. Liver histopathology in severe COVID 19 respiratory failure is suggestive of vascular alterations. Liver Int. 2020;40:2110–2116. doi: 10.1111/liv.14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milroy C.M. Fatty liver and the forensic pathologist. Acad Forensic Pathol. 2018;8:296–310. doi: 10.1177/1925362118782061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ayyar V.S., Almon R.R., DuBois D.C., Sukumaran S., Qu J., Jusko W.J. Functional proteomic analysis of corticosteroid pharmacodynamics in rat liver: relationship to hepatic stress, signaling, energy regulation, and drug metabolism. J Proteom. 2017;160:84–105. doi: 10.1016/j.jprot.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Targher G., Mantovani A., Byrne C.D., et al. Detrimental effects of metabolic dysfunction-associated fatty liver disease and increased neutrophil-to-lymphocyte ratio on severity of COVID-19. Diabetes Metab. 2020;46:505–507. doi: 10.1016/j.diabet.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cazals-Hatem D., Hillaire S., Rudler M., et al. Obliterative portal venopathy: portal hypertension is not always present at diagnosis. J Hepatol. 2011;54:455–461. doi: 10.1016/j.jhep.2010.07.038. [DOI] [PubMed] [Google Scholar]
- 18.Sempoux C., Bioulac-Sage P. Vascular liver lesions: contemporary views on long-recognized entities. Virchows Arch. 2018;473:1–2. doi: 10.1007/s00428-018-2328-y. [DOI] [PubMed] [Google Scholar]
- 19.DeLeve L.D., Valla D.C., Garcia-Tsao G. Vascular disorders of the liver. Hepatology. 2009;49:1729–1764. doi: 10.1002/hep.22772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guido M., Sarcognato S., Sonzogni A., et al. Obliterative portal venopathy without portal hypertension: an underestimated condition. Liver Int. 2016;36:454–460. doi: 10.1111/liv.12936. [DOI] [PubMed] [Google Scholar]
- 21.Hartleb M., Gutkowski K., Milkiewicz P. Nodular regenerative hyperplasia: evolving concepts on underdiagnosed cause of portal hypertension. World J Gastroenterol. 2011;17:1400–1409. doi: 10.3748/wjg.v17.i11.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohmori S., Shiraki K., Sugimoto K., et al. Expression of CD34-positive sinusoidal endothelial cells in patients with HBV-associated chronic liver diseases. Int J Mol Med. 2004;14:179–184. [PubMed] [Google Scholar]
- 23.Abou-Ismail M.Y., Diamond A., Kapoor S., Arafah Y., Nayak L. The hypercoagulable state in COVID-19: incidence, pathophysiology, and management. Thromb Res. 2020;194:101–115. doi: 10.1016/j.thromres.2020.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salamanna F., Maglio M., Landini M.P., Fini M. Body localization of ACE-2: on the trail of the keyhole of SARS-CoV-2. Front Med. 2020;7:594495. doi: 10.3389/fmed.2020.594495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adams D.H., Hubscher S.G. Systemic viral infections and collateral damage in the liver. Am J Pathol. 2006;168:1057–1059. doi: 10.2353/ajpath.2006.051296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen M.H., Garcia R.T., Trinh H.N., et al. Histological disease in Asian-Americans with chronic hepatitis B, high hepatitis B virus DNA, and normal alanine aminotransferase levels. Am J Gastroenterol. 2009;104:2206–2213. doi: 10.1038/ajg.2009.248. [DOI] [PubMed] [Google Scholar]