Abstract
Current clinical RAF inhibitors (RAFi) inhibit monomeric BRAF (mBRAF), but are less potent against dimeric BRAF (dBRAF). RAFi equipotent for mBRAF and dBRAF have been developed, but are predicted to have lower therapeutic index. Here we identify a third class of RAFi that selectively inhibits dBRAF over mBRAF. Molecular Dynamic simulations reveal restriction of the movement of the BRAF αC-helix as the basis of inhibitor selectivity. Combination of inhibitors based on their conformation selectivity (mBRAF- plus dBRAF-selective plus the most potent BRAF-MEK disruptor MEK inhibitor) promoted suppression of tumor growth in BRAF(V600E) therapy-resistant models. Strikingly, the triple combination showed no toxicities, whereas dBRAF-selective plus MEK inhibitor treatment caused weight loss in mice. Finally, the triple combination achieved durable response and improved clinical wellbeing in a stage IV colorectal cancer patient. Thus, exploiting allosteric properties of RAF and MEK inhibitors enables the design of effective and well-tolerated therapies for BRAF(V600E) tumors.
Keywords: BRAF, MEK, Inhibitors, Protein serine-threonine kinases, Colorectal cancer, Melanoma, Oncoprotein & tumor suppressor drug targets, Protein kinase & phosphatase drug targets, Reversal of drug resistance
Introduction
RAF kinases BRAF, CRAF and ARAF signal through their substrate MEK to activate MAPK signaling and promote cell proliferation and survival. Mutationally activated BRAF(V600E) kinase drives growth of about 8% of human tumors(1,2), most commonly melanomas and colorectal cancers (CRC), and three small-molecule RAF inhibitors (Vemurafenib (VEM), Dabrafenib (DAB) and Encorafenib (ENC)) are now FDA-approved drugs(3). Unlike most kinase inhibitors that bind and inhibit their target in all cells, these RAF inhibitors selectively inhibit the mutationally activated form of the protein (BRAF(V600E)) in tumors, but not BRAF(wild-type) in normal tissue. A model to explain this observation has been put forward by us and others, in which BRAF(wild-type) signals as an obligatory dimer, but BRAF(V600E) is able to signal as a monomer and these RAF inhibitors preferentially bind and inhibit monomeric over dimeric RAF(4–6). Subsequent studies revealed the structural basis of this property as negative allostery for inhibitor binding to the second protomer in the BRAF dimer(7,8). Nonetheless, while the inability of this class of RAF inhibitors to inhibit dimeric BRAF is the basis of their increased therapeutic index, it is also responsible for development of both acquired and adaptive drug resistance. In adaptive resistance, relief of negative feedback upon MAPK pathway inhibition results in rapid formation of RAF dimers and consequent resistance to RAF inhibitors(9,10). To address these limitations of the monomer-selective RAF inhibitors, RAF inhibitors that are equipotent inhibitors of RAF dimers and monomers have entered preclinical and clinical development(11,12). These drugs are predicted to overcome resistance due to RAF dimerization. However, as they are also potent inhibitors of dimeric BRAF(wild-type), they are also predicted to cause on-target toxicities in normal tissue at the high doses required for potent antitumor effect. This may account for the fact that none of these compounds has been yet successful in the clinic. Thus, despite significant progress, there is a pressing need to develop more effective therapeutic strategies for the majority of patients with BRAF(V600E) tumors.
A large number of MEK inhibitors have also entered preclinical and clinical development, and four of them (Trametinib, Selumetinib, Cobimetinib, and Binimetinib) are FDA-approved drugs. The impact of allosteric properties and on the antitumor effectiveness of MEK inhibitors in different cellular contexts, has also drawn a great deal of interest(13–15). However, which MEK inhibitors should be optimally used in combinatorial regimens with RAF inhibitors in BRAF(V600E) tumors remains elusive.
Here, we undertook a systematic approach to biochemically classify and characterize RAF and MEK inhibitors in preclinical or clinical development. We identified and characterized a new class of RAF inhibitors that are selective for dimeric RAF, and used them to gain new mechanistic insight into conformational states adopted by BRAF(V600E) in its native state. We further used this knowledge to design a more effective combinatorial strategy for BRAF(V600E) tumors, with the goal of maximally suppressing MAPK signaling in the tumor, while retaining a broad therapeutic index.
Results
A new class of RAF inhibitors are selective for dimeric over monomeric RAF
Protein kinases exist in an equilibrium of active and inactive states, where the switch between these states involves movements of two conserved structural motifs: the Asp-Phe-Gly (DFG)-motif and the αC-helix. Each of these motifs can adopt an “IN” or “OUT” conformations, where both motifs in the “IN” conformation (αC-OUT/DFG-IN) is associated with the catalytically active state. Small molecule inhibitors of kinase can be grouped by the kinase conformations they recognize(16). For example, the type-I kinase inhibitor gefitinib binds the ATP-binding site in the active αC-IN/DFG-IN state (“CIDI”), while the type I1/2 inhibitor vemurafenib and type II inhibitor sorafenib bind to the inactive αC-OUT/DFG-IN and αC-IN/DFG-OUT states, (“CODI” and “CIDO”, respectively)(17).
We previously characterized structurally and biochemically two classes of RAF inhibitors. One class of CODI inhibitors selectively inhibits monomeric compared to dimeric BRAF(V600E) (RAF monomer-selective) and includes the current clinical inhibitors VEM and DAB. The other class (including both CIDO and CIDI inhibitors) are potent inhibitors of both monomeric and dimeric BRAF(V600E), due to similar affinity for inhibitor binding to both RAF protomers(7,8). To identify RAF inhibitors with potentially novel properties, we used an approach that enabled us to determine the relative potency of each RAF inhibitor in inhibiting monomeric versus dimeric BRAF(V600E) in the endogenous setting(7,8): SKMEL239 Parental (PAR) (i.e. endogenously expressing full-length monomeric BRAF(V600E)) and C3, a clone of RAF inhibitor-resistant SKMEL239 cells that endogenously expresses a splice variant of constitutively dimerized BRAF(V600E)(5).
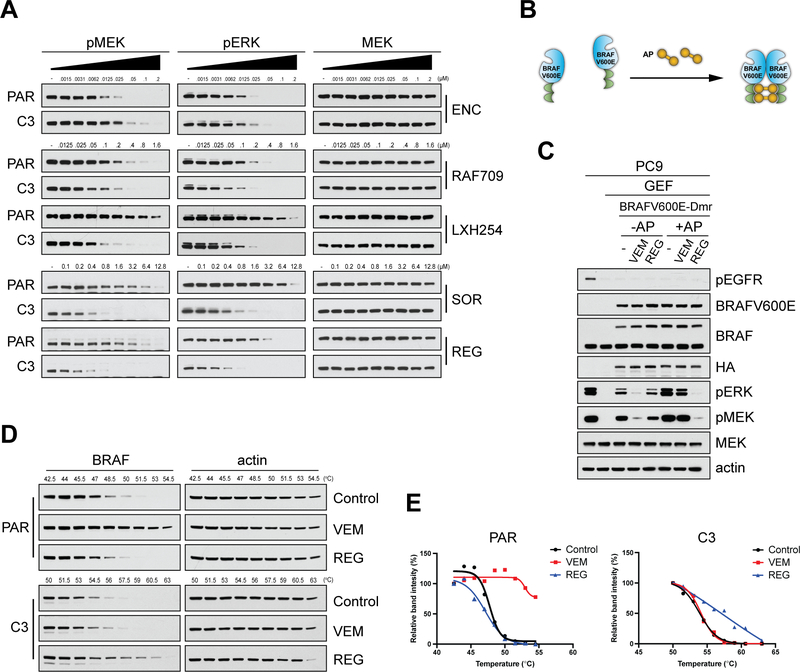
We first assayed the most recently FDA-approved RAF inhibitor ENC(18), which as expected, and similarly to the other clinical CODI RAF inhibitors, VEM(19) and DAB(20), showed more potent inhibition of MAPK signaling in PAR compared to C3 cells (Fig. 1A). Furthermore, other CIDO RAF inhibitors, such as AZ628, TAK632, LY3009120, BGB283, showed similar potency in the two settings, confirming their similar potency for BRAF(V600E) monomers and dimers(7,8,12). Surprisingly, we identified four CIDO RAF inhibitors (Sorafenib(21) (SOR), Regorafenib(22) (REG), RAF709(23) and LXH254(24)) that suppressed MAPK signaling more potently in C3 cells compared to PAR (Fig. 1A), suggesting that they might be more potent inhibitors of dimeric than monomeric BRAF(V600E). The increased potency of these compounds in suppressing MAPK signaling in cells expressing dimeric BRAF(V600E) was also confirmed in another pair of Parental (M397-P) and RAF inhibitor-resistant cells that express a different BRAF(V600E) splice variant (M397-R)(25) (Supplementary Fig. S1A).
Figure 1. Identification of RAF dimer-selective inhibitors.
(A) SKMEL239 Parental (PAR) cells and the resistant clone C3 were treated for 1 hr with increasing concentrations of the indicated RAF inhibitors, and cell lysates were immunoblotted for pMEK and pERK. (B) Schematic showing the Chemically Induced Dimerization (CID) system using a chemical ligand (AP). Ectopic expression of BRAFV600E engineered to dimerize (BRAFV600E-Dmr) enables AP ligand induced formation of BRAFV600E homodimers. (C) PC9 cells ectopically expressing BRAFV600E-Dmr were pre-treated for 1 hr with Gefitinib (GEF, 100 nM), followed by treatment with the AP ligand (100 nM/1 hr), then treated with Vemurafenib (VEM, 2 μΜ/1 hr) or Regorafenib (REG, 2 μΜ/1 hr). Cell lysates were immunoblotted with the indicated antibodies. (D) PAR or C3 cells were treated with VEM (4 μΜ and 2 μΜ, respectively), REG (4 μΜ or 2 μΜ, respectively) or DMSO (Control) for 2 hr followed by cellular thermal shift assay (CETSA) at the indicated increasing temperature points and the cell lysates were immunoblotted for BRAF and actin. (E) Graphs based on relative binding intensity (%) derived from Fig. 1D using ImageJ analysis. The graphs were plotted in GraphPad Prism using the Boltzmann sigmoid equation.
To assess whether these compounds inhibit more potently the dimeric compared to the monomeric form of BRAF(V600E) in an isogenic system, we used a chemically-induced dimerization (CID) system by ectopically expressing BRAF(V600E) engineered to dimerize (BRAFV600E-Dmr) upon treatment with the ligand AP20187 (AP) (Fig. 1B). To avoid the effects of baseline RAS-GTP levels, we ectopically expressed BRAFV600E-Dmr in PC9 cells, in which RAS signaling is under the control of mutant-EGFR, and it can thus be suppressed using the EGFR inhibitor Gefitinib (GEF)(7). Activated MAPK signaling in GEF-treated PC9 cells was inhibited by either VEM or DAB more potently in the absence than in the presence of AP, consistent with these RAF inhibitors being more potent inhibitors of monomeric than dimeric BRAF(V600E). In contrast, REG and LXH254 suppressed MAPK more potently in the presence than in the absence of AP, confirming that their increased potency towards the splice variants of BRAF(V600E) is a consequence of their increased potency towards dimeric compared to monomeric BRAF(V600E) (Fig. 1C and Supplementary Fig. S1B).
Cellular thermal shift assay (CETSA) enables monitoring changes in the thermal stability of proteins upon inhibitor engagement in intact cells(26,27). Treatment of PAR cells with VEM resulted in a shift in the protein’s melting temperature (Tm), indicating inhibitor binding to full-length BRAF(V600E) (i.e. BRAF(V600E) monomer), whereas treatment with REG did not affect the Tm of BRAF(V600E). In contrast, treatment of C3 cells with REG resulted in a shift in the Tm, indicating binding of inhibitor with the BRAF(V600E) splice variant (i.e. BRAF(V600E) dimer), whereas treatment with VEM did not affect the protein’s Tm (Fig. 1D and 1E). Together these experiments show that the two RAF inhibitors VEM and REG preferentially bind monomeric or dimeric BRAF(V600E) in cells, respectively.
Restriction of the movement of the αC helix of BRAF by inhibitor is the basis of the difference in selectivity between equipotent and RAF dimer-selective inhibitors
Examination of available crystal structures of the RAF dimer-selective inhibitors, sorafenib (PDB: 1UWH) and RAF709 (PDB: 5VAM), in complex with the catalytic domain of BRAF did not reveal any major differences in their mode of binding compared with other CIDO RAF inhibitors(7) that are equipotent for monomeric and dimeric BRAF(V600E), such as TAK632 (PDB:4KSP) or LY3009120 (PDB:5C9C). Thus, to identify the basis of the apparent biochemical difference between the two classes of compounds, we employed Molecular Dynamic (MD) simulations.
We performed 15-μs simulations of seven BRAF inhibitors, each bound to monomeric and dimeric BRAF, with three independent simulations for each condition (Table S1). For each inhibitor, simulations were initiated from the poses observed in co-crystal structures (when available) or from a docked pose modeled based on crystal structures of structurally similar compounds (Table S1). We analyzed ligand stability using the root-mean-square deviation (RMSD) of ligand heavy atoms with respect to the initial pose as an indication of binding strength to a receptor conformation.
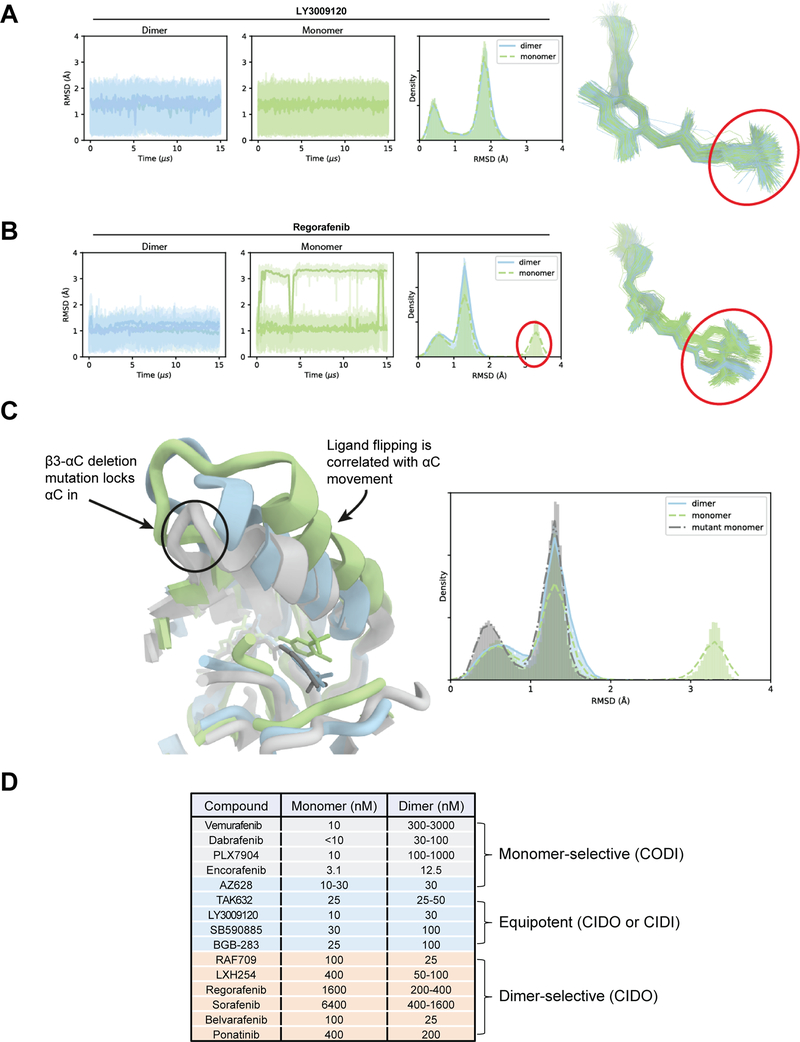
In these simulations, all seven ligands remained bound to dimeric BRAF with low RMSD compared to the initial pose, with the overall RMSD ranging from 0.5 Å to 2 Å. This was not the case for all ligands in the simulations with monomeric BRAF: For the equipotent inhibitors (TAK632, AZ628, and LY3009120), the RMSD range was comparable to the RMSD in the simulations with dimeric BRAF; for the dimer-selective compounds (REG, SOR, RAF709, and LXH254), we observed significantly larger ligand RMSD in at least two out of the three independent simulations (Supplementary Videos 1-4). To understand the structural basis for the differences in ligand stability among the monomeric BRAF simulations, we studied the dynamics of the protein structural elements that are involved in coordinating ligand binding in the specificity pocket (circled in red in Fig. 2A, 2B). We found that the αC-helix, which sits directly above the specificity pocket, sampled a variety of orientations in monomeric BRAF, both in its apo state and when bound to the dimer-selective BRAF inhibitors. In contrast, the motion of the αC-helix was restricted in the inhibitor-bound BRAF dimers (Supplementary Fig. S2A).
Figure 2. Restriction of the movement of the αC-helix of BRAF by inhibitor is the basis of the difference in selectivity between equipotent and dimer-selective RAF inhibitors.
(A) Simulation trajectories of LY3009120, an equipotent binder, bound to dimeric (blue) and monomeric (green) BRAF. Root-mean-square deviation (RMSD) of the heavy atoms of the circled portion of the ligand are plotted. The RMSD histograms represent aggregate values across all three replicates. (B) Simulation trajectories of Regorafenib, a dimer-preferring compound, bound to dimeric (blue) and monomeric (green) BRAF. In the monomeric case, the portion of the ligand that interacts with the specificity pocket is able to flip, producing the high RMSD conformation circled in red. (C) Overlay of simulated structure snapshots of Regorafenib bound to dimeric WT (blue), monomeric WT (green), and the monomeric β3-αC deletion mutant of BRAF (gray). The mutation restricts the motion of the αC helix, and thus Regorafenib. (D) Three different groups of RAF inhibitors based on their differential selectivity for monomeric or dimeric BRAF(V600E).
Based on these observations, we hypothesize that the stable engagement of the specificity pocket is correlated with dimer selectivity for CIDO (Type II) RAF inhibitors. These differential specificity pocket dynamics hold for all seven inhibitors studied retrospectively. To provide further evidence that ligand stability is correlated with the motion of αC helix, we also simulated REG, a compound we found experimentally to be RAF dimer-selective (Fig. 1A and 1C), with a β3-αC deletion mutant (PDB: 5HI2)(28). This mutation locks the αC-helix in a dimer-like “IN” state, which we hypothesized would maintain the low RMSD observed in the simulations of REG-bound monomeric BRAF(WT). As expected, REG was comparably stable in the monomeric and dimeric mutant constructs (Fig. 2C and Supplementary Video 5).
We further applied our simulation methodology to three additional compounds (Belvarafenib, BGB-283(29), and Ponatinib(30)) for which binding selectivity for the RAF dimer had not been determined experimentally, using the same computational protocol we used in the retrospective studies. In our simulations, Belvarafenib behaved similarly to the RAF dimer-selective compounds: It had a large RMSD in simulations with monomeric but not dimeric BRAF. BGB-283 showed comparable RMSD in simulations with the BRAF monomer and dimer, indicating equal potency toward each. The Ponatinib simulations, however, were less conclusive: On the one hand, Ponatinib had comparable RMSDs in monomer and dimer simulations, consistent with the behavior of the equipotent compounds. On the other hand, the BRAF αC helix remained highly dynamic in the monomeric simulation and was not stably engaged, behavior which was observed in simulations of the RAF dimer-selective compounds (Supplementary Fig. S2A and S2B). The dimer selectivity or equipotency of Ponatinib is thus unclear based on the MD simulations alone.
We then experimentally assessed the potencies of Belvarafenib, BGB-283 and Ponatinib in inhibiting monomeric versus dimeric BRAF(V600E) using both the PAR/C3 cell approach and the CID system. In accordance with our predictions based on the MD simulations, Belvarafenib was found to be a more potent inhibitor of dimeric RAF, whereas BGB-283 was not (Supplementary Fig. S2C and S2D). Ponatinib also showed selectivity towards dimeric RAF, consistent with the BRAF αC-helix remaining highly dynamic in the monomeric simulation when bound to the compound. We have also showed recently that Ponatinib stabilizes the αC-helix of BRAF in a unique position compared to other RAF inhibitors(31), which may account for its distinct features. The discrepancy between this finding and the observation that Ponatinib had comparable monomer and dimer RMSD in our 15-μs simulations suggests that more simulation time might be needed to reveal Ponatinib’s lower stability with monomeric BRAF (Supplementary Fig. S2A and S2B). A list of RAF inhibitors grouped according to their ex vivo potency in inhibiting monomeric or dimeric BRAF(V600E) is shown in Fig. 2D.
Transition of either BRAF(WT) or BRAF(V600E) to the dimeric state increases its interaction with MEK
The fact that certain RAF inhibitors preferentially bind the dimeric over the monomeric form of BRAF(V600E) indicates that the two forms are able to adopt different conformations that are not captured by the available crystallographic data. Thus, to gain insight into these BRAF conformations, we used the formation of the MEK-BRAF-CRAF signaling complex during growth factor-induced MAPK activation, to indirectly monitor the conformation of full-length BRAF as a function of its activation in the native state. As shown in Fig. 3A, MEK, BRAF and CRAF interact weakly at steady-state. EGF stimulation strongly promoted formation of the MEK-BRAF-CRAF complex within 5 min, followed by a gradual return to low basal levels of interaction after 30–60 min. The formation and subsequent disruption of the MEK-BRAF-CRAF signaling complex correlated well with activation and subsequent return to basal levels of MAPK signaling (Fig. 3B), suggesting that activated, dimeric BRAF interacts strongly with MEK, but inactive, monomeric BRAF adopts a conformation with low affinity for MEK (Fig. 3C). We further assessed the MEK-BRAF complex after inhibiting RAS activity in either RKO cells treated with the SHP2 inhibitor RMC-4550(32) (Fig. 3D) or in PC9 cells treated with the EGFR inhibitor GEF (Fig. 3E). In each case, drug treatment resulted in disruption of the MEK-BRAF complex, consistent with inactive, monomeric BRAF adopting a conformational state that interacts weakly with MEK.
Figure 3. Transition of either BRAF(WT) or BRAF(V600E) to the dimeric state increases its interaction with MEK.
(A-B) HeLa cells were treated with epidermal growth factor (EGF, 10 ng/mL) for the indicated time points. Cell lysates were subjected to immunoprecipitation with a MEK antibody followed by immunoblotting for BRAF, CRAF and ERK (A) or immunoblotted with the indicated antibodies (B). (C) Schematic showing the transition from an “inactive” signaling complex with weak interaction between BRAF and MEK to an “active” signaling complex with strong BRAF/MEK interaction upon treatment with EGF. (D) RKO cells were treated for 2 hr with the SHP2 inhibitor RMC-4550 (2 μΜ) to suppress endogenous RAS activity. Cell lysates were then either subjected to immunoprecipitation with a MEK antibody followed by immunoblotting for BRAF, or immunoblotted with the indicated antibodies. (E) PC9 cells were treated for 2 hr with Gefitinib (GEF, 0.5 μM) to suppress endogenous RAS activity. Cell lysates were then either subjected to immunoprecipitation with a MEK antibody followed by immunoblotting for BRAF, or immunoblotted with the indicated antibodies. (F) 293H cells ectopically expressing HA-tagged BRAFV600E-Dmr were treated with the AP ligand (100 nM/ 1 hr) and cell lysates were either subjected to immunoprecipitation with a MEK antibody followed by immunoblotting for HA or BRAFV600E, or immunoblotted with the indicated antibodies. (G) Schematic showing the Chemically Induced Dimerization (CID) system using a chemical ligand (AP). Ectopic expression of both BRAF and CRAF engineered to dimerize (BRAF-Dmr and CRAF-Dmr) enables AP ligand induced formation of both BRAF/CRAF homo- and heterodimers. (H) 293H cells ectopically co-expressing HA-tagged BRAF-Dmr and CRAF-Dmr were treated with the AP ligand (100 nM/ 1 hr). Cell lysates were either subjected to immunoprecipitation with a MEK antibody followed by immunoblotting for HA, or immunoblotted with the indicated antibodies. (I) Cells expressing full-length or splice variants of BRAF(V600E) (SKMEL239 Parental (PAR) and C3, M397-PAR and M397-R), or expressing full-length BRAF(V600E) or BRAF(V600E/DK), i.e. with a duplicate kinase domain ((SK28-PAR) and SK28-R, respectively) were either subjected to immunoprecipitation with a MEK antibody followed by immunoblotting for BRAFV600E, or immunoblotted with the indicated antibodies. (J) Schematic depicting different conformations adopted by monomeric and dimeric BRAF in different mutational contexts: BRAF(WT) transitions from monomeric and the catalytically inactive (1) to activated dimer bound to RAS in the membrane (2). BRAF(V600E) is a catalytically active monomer in the cytosol (3), whereas the splice variant of BRAF(V600E) is a cytosolic dimer (4). (1) and (3) are monomers and interact weakly with MEK, (2) and (4) are dimers and interact strongly with MEK.
We next asked whether forced dimerization of BRAF, CRAF or BRAF(V600E) using CID would increase their interaction with MEK. In fact, forced dimerization of either BRAF(V600E) (Fig. 3F), or ectopically co-expressed BRAF and CRAF (Fig. 3G, 3H), markedly promoted their interaction with MEK. Finally, we examined the MEK-BRAF complex by co-immunoprecipitation experiments in additional settings in which BRAF(V600E) exists either as a monomer or a dimer, including the two cell line pairs expressing full-length or splice variants of BRAF(V600E) (SKMEL239 PAR and C3, M397-P and M397-R) and a pair of Parental and a RAF inhibitor-resistant cell line (SK28-PAR and SK28-R) that we generated after continuous treatment of BRAF(V600E)-expressing SKMEL28 melanoma cells to Vemurafenib. SK28-R expresses BRAF(V600E) with duplicated kinase domain (Supplementary Fig. S3), similar to a RAF inhibitor-resistant cell line reported previously(33). In each case, we detected much higher levels of MEK interacting with the dimeric compared to the monomeric state of BRAF(V600E) (Fig. 3I). Together these data indicate that monomeric and dimeric full-length BRAF(V600E) signal in different conformational states. Although both forms are highly catalytically active, monomeric full-length BRAF(V600E) appears to be in a conformation that resembles catalytically inactive, monomeric BRAF(WT) (i.e. with weak binding to MEK), whereas dimeric BRAF(V600E) appears to be in a conformation that resembles active dimeric BRAF(WT) (i.e. with strong binding to MEK) (Fig. 3J).
Selection of RAF inhibitors for use in combinatorial regimens based on their biochemical properties
Despite recent progress in the structural characterization of RAF-MEK complexes(34,35), how BRAF activation and dimerization affects its interaction with MEK remains elusive. In addition to our data presented here, it has been reported that the splice variant BRAF(V600E) [Δex2–8], which contains only the kinase domain, interacts strongly with MEK, but this interaction is reduced when introduced mutations force it to adopt the monomeric state (i.e. R509H or S729A)(36). These data suggest that the differential strength of the interaction between various BRAF states and MEK is at least in part determined by the BRAF kinase domain-MEK interactions. Comparisons between crystal structures of MEK bound to the active (PDB: 4MNE) and inactive (PDB: 6U2G) BRAF kinase domain did not reveal major differences at the enzyme-substrate interface, which is challenging to reconcile with the biochemistry data. Thus, to study the structural basis of the apparent biochemical difference between these states we employed MD simulations.
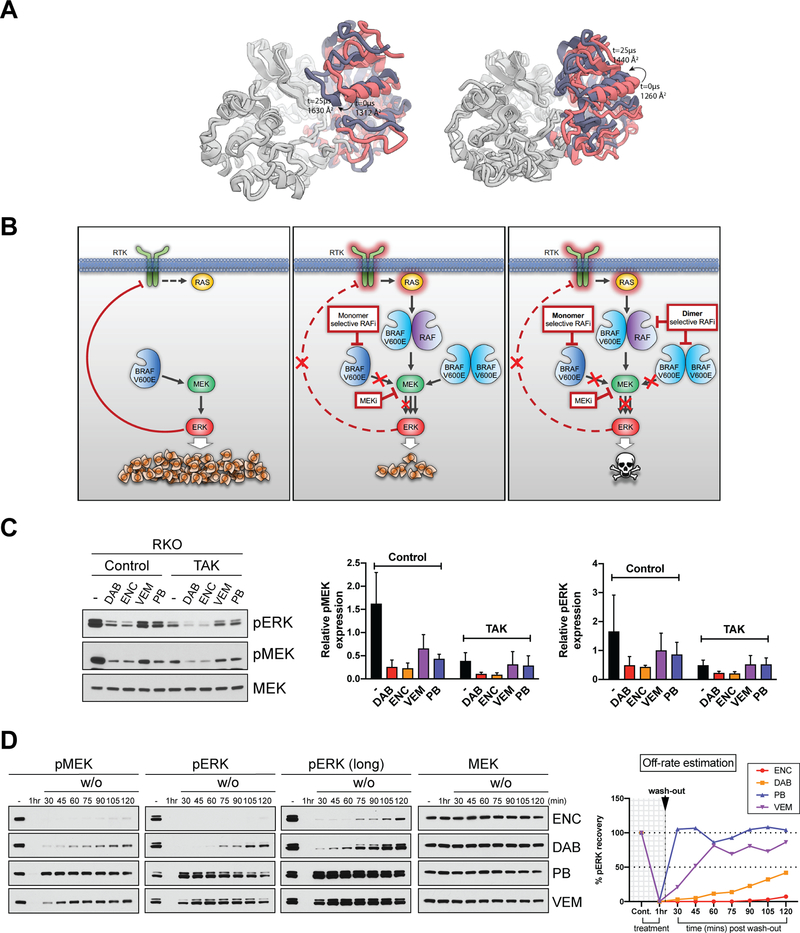
We performed 25-μs simulations initiated from crystal structures of MEK bound to active and inactive BRAF, with three independent simulations for each condition. In all three simulations initiated from the active, αC-IN conformation, the N-terminal region of the BRAF αC-helix came into contact with the N-lobe of MEK. Specifically, several residues in the BRAF β3-αC loop interact with the P-loop on MEK. The interactions between MEK and the C-lobe of BRAF remained intact, as seen in crystal structures, during the simulations. As a consequence, the overall interfacial area between the MEK and BRAF increases from the 1312 Å2 seen in the crystal structure to 1630 Å2. In contrast, the inactive conformation of BRAF had a mobile αC-helix, which did not engage MEK, and the overall interfacial surface area was around 1440 Å2 at the end of the simulations. (Fig. 4A and Supplementary Videos 6 and 7).
Figure 4. Selection of RAF inhibitors for use in combinatorial regimens based on their biochemical properties.
(A) Final structures from two 25-μs simulations of BRAF-MEK complexes, starting from active αC-IN (left) and αC-OUT (right) conformations. MEK is shown in gray, the starting BRAF structure is shown in pink, and the final frame of simulation is shown in purple. αC-IN BRAF, closely resembling the WT dimeric state, forms a larger interface with MEK, with key interactions between the αC helix of BRAF and the N-lobe of MEK. (B) Schematic model showing the proposed combinatorial targeting strategy for BRAF(V600E) tumors. At steady state, full-length monomeric BRAF(V600E) activates the MAPK pathway leading to cell proliferation and tumor growth, while activated ERK feedback-suppresses the upstream activation of receptor tyrosine kinases (RTK) (left). Addition of monomer-selective RAF inhibitors results in relief of negative feedback, RAS activation and induction of dimeric BRAF(V600E) (middle). Combining RAF dimer-selective with RAF monomer-selective inhibitors could overcome adaptive resistance in BRAF(V600E) tumors (right). (C) RKO cells treated for 48 hr with 0.8 μΜ of the indicated RAF inhibitors (Dabrafenib (DAB), Encorafenib (ENC), Vemurafenib (VEM), and PLX7904 (PB)) alone or with 0.6 μΜ of the same RAF inhibitors combined with 0.2 μΜ of TAK632 (TAK). Cell lysates were immunoblotted with the indicated antibodies and representative results of three repeats are shown (left). Quantitation of ERK (right) and MEK (middle) phosphorylation using ImageJ analysis. pERK and pMEK expression levels were normalized to MEK. (D) RKO cells were either treated for 1 hr with indicated RAF inhibitors, or treated for 1 hr, washed three times with PBS, supplemented with fresh inhibitor-free medium and collected at the indicated time points. The concentrations used were previously normalized to equipotently inhibit pERK in 1 hr: 100 nM ENC, 100 nM DAB, 200 nM PB and 600 nM VEM. Left: Cell lysates were immunoblotted with indicated antibodies. Right: Summary graph showing the percentage (%) of pMEK recovery over time (min) after the washout of the indicated RAF inhibitors, as an estimate of their off-rate. Graph was plotted based on relative expression levels of pMEK normalized to MEK after ImageJ analysis (Supplementary Fig. S4).
We next sought to exploit the properties of RAF inhibitors to rationally design a combinatorial strategy for BRAF(V600E) tumors. We showed previously that relief of negative feedback and induction of dimeric BRAF(V600E) promotes resistance to current clinical monomer-selective RAF inhibitors, such that combining with RAF inhibitors that potently inhibit dimeric RAF would be effective in blocking resistance in this context (Fig. 4B). However, since BRAF(WT) also signals as a dimer, such RAF inhibitors are predicted to have lower therapeutic index when used as single agents(7). We previously showed that the combination of VEM with an αC-IN RAF inhibitor (TAK632) was effective in melanoma and colorectal carcinoma (CRC) BRAF(V600E), but that the addition of VEM only marginally improved the effectiveness of TAK632 alone(7). To identify αC-OUT RAF inhibitors that would better synergize with αC-IN RAF inhibitors, we assayed the effect on MAPK signaling of four αC-OUT RAF inhibitors, (i.e. the three FDA-approved VEM, DAB, ENC and the “Paradox Breaker” PLX7904, PB(37)) in combination with the αC-IN RAF inhibitor TAK632(38) (TAK). As shown in Fig. 4C, addition of VEM or PB did not enhance the inhibitory effect of TAK on MAPK signaling. By contrast, the combination of either DAB or ENC with TAK showed significantly greater inhibition of MAPK signaling compared to either inhibitor alone (Fig. 4C). It is reported that TAK has a slow off-rate(11). Therefore, it was possible that the off-rate of αC-OUT RAF inhibitors might affect their synergy with TAK. We thus carried out washout experiments and monitored recovery of MAPK signaling as an estimate of the relative off-rate of the αC-OUT RAF inhibitors in cells. pERK quickly rebounded after VEM or PB washout. However, suppression of MAPK signaling persistent for much longer after washout of DAB or ENC (Fig. 4D and Supplementary Fig. S4), suggesting a slow rate of dissociation of the compound from BRAF(V600E). Thus, the ability of DAB and ENC to synergize with the αC-IN RAF inhibitor appears to be associated with their much slower off-rate compared to VEM and PB, resulting in prolonged association with monomeric BRAF(V600E) in cells.
Selection of MEK inhibitor for use in combinatorial regimens based on their biochemical properties
We next biochemically characterized a panel of candidate MEK inhibitors to test in triple combination, including the three MEK inhibitors, Trametinib (TRAM), Cobimetinib (COB) and Binimetinib (BIN) that have been approved in combination with RAF inhibitors for the treatment of BRAF(V600E) melanomas. Previous work using a smaller number of MEK inhibitors has shown that certain MEK inhibitors can overcome feedback-induced MEK activation either by disrupting the RAF-MEK complex or by promoting the MEK-RAF complex while preventing phosphorylation by RAF(13,39). To gain further insight into the biochemical properties of MEK inhibitors, we first compared potency and durability of pERK suppression by a panel of MEK inhibitors, using the same concentrations for each compound. Under these conditions, we found TRAM to most potently and durably suppress pERK across the compounds tested (Fig. 5A).
Figure 5. Selection of MEK inhibitors for use in combinatorial regimens based on their biochemical properties.
(A) Cell lysates from RAS-MUT (HCT116 KRAS(G13D)) or BRAF-MUT (A375 BRAF(V600E)) cells treated with the indicated concentrations of MEK inhibitors for 30 min or 24 hr were immunoblotted with the indicated antibodies and representative results of three repeats are shown (left). Graphs showing the percentage (%) of pERK inhibition in RAS MUT and BRAF MUT cells upon treatment with the indicated MEK inhibitors (right). Graphs were plotted based on pMEK and pERK relative expression levels normalized to MEK after ImageJ analysis (B) Top: Schematic showing the strategy followed to determine the direct allosteric effect of MEK inhibitors (BRAF/MEK complex formation or disruption). Middle: RAS mutant (RAS MUT, HCT116 KRAS(G13D)) cells or BRAF mutant (BRAF MUT, A375 BRAF(V600E)), cells were pre-treated with 2 μΜ of ERK inhibitor (ERKi) SCH772984 for 2 hr followed by treatment with the indicated concentrations of MEK inhibitors for 1 hr. Cell lysates were either subjected to immunoprecipitation with a MEK antibody followed by immunoblotting for BRAF and CRAF or immunoblotted with the indicated antibodies. Bottom: Graphs showing the percentage (%) of BRAF/MEK complex formation or disruption in RAS MUT and BRAF MUT cells upon subsequent treatment with ERKi and the indicated MEK inhibitors. Graphs were plotted based on pMEK and pERK relative expression levels normalized to MEK after ImageJ analysis. (C) Binding affinity of the indicated MEK inhibitors to purified MEK1. Kd values were determined using the KinomeScan binding assay. (D) RAS MUT (HCT116 KRAS(G13D)) cells pre-treated with 2 μΜ of ERKi for 2 hr followed by treatment with the indicated normalized concentrations of MEK inhibitors for 1 hr. Cell lysates were subjected to immunoprecipitation with a MEK antibody followed by immunoblotting for BRAF or immunoblotted with the indicated antibodies. (E) Overlay of docking binding pose for Trametinib (TRAM, navy-blue) and crystallographic binding poses for Selumetinib/Binimetinib (SEL/BIN, yellow), Cobimetinib (COB, dark purple), PD0325901 (PD901, grey), TAK-733 (cyan) and CH5126766 (CH766, salmon) with MEK1. SEL/BIN: LIG ID:3EW, PDB-ID:4U7Z; COB: LIG ID:EUI, PDB-ID:4AN2; PD901: LIG ID:4BM, PDB-ID:3EQG; TAK-733: LIG ID:IZG, PDB-ID:3PPL; CH766: LIG ID:CHU, PDB-ID:3WIG; and TRAM docked to 3WIG structure. (F) Table summarizing the relative biochemical properties of MEK inhibitors used in this study.
We next tested these same MEK inhibitors for their effect on the MEK-RAF complex. Formation or disruption of the RAF-MEK complex is the result of both RAF activation due to relief of negative feedback upon MEK/ERK inhibition, as well as a consequence of direct allosteric effects of inhibitor binding to MEK(40). To distinguish between the two effects, we first treated cells with an ERK inhibitor (SCH772984), which resulted in relief of negative feedback and formation of the RAF-MEK complex independently of inhibitor binding to MEK. Thus, we were able to determine the direct allosteric effect of inhibitor binding to MEK by subsequently treating with MEK inhibitor and determining the disruption or formation of the RAF-MEK complex by co-immunoprecipitation. We found that most MEK inhibitors disrupted the MEK-RAF complex to various degrees, with TRAM showing the most potent disruption of the complex among the MEK inhibitors tested (Fig. 5B and Supplementary Fig. S5A). In contrast to most MEK inhibitors, CH5126766 (CH766) promoted formation of the MEK-RAF complex, as previously reported(14).
All MEK inhibitors tested occupy the same allosteric site in MEK and bind in a similar fashion. To explain the differences in the potency of disrupting the MEK-RAF complex across inhibitors, we asked whether the relative potency of each inhibitor in disrupting the complex correlates with its affinity for binding MEK in vitro, and found that this was indeed the case (Fig. 5C and Supplementary Fig. S5B). In fact, after normalizing inhibitor concentrations based on the in vitro binding affinities, both COB and BIN potently disrupted the MEK-RAF complex, similar to TRAM (Fig. 5D), providing further evidence that despite the fact that most MEK inhibitors bind MEK in a similar structural fashion, the increased binding affinity of TRAM for MEK is the basis of the higher potency by which it disrupts the MEK-RAF complex.
To gain structural insight on the common features of most MEK inhibitors in disrupting the MEK-RAF complex, we overlaid the available atomic structures for MEK inhibitors used in this study. These MEK inhibitors bind the same allosteric site in MEK and interact with V211 and S212(13) (D+3 and D+4 positions from the DFG-motif) of the activation loop. This interaction usually involves the fluoro- or carbonyl- moiety of the inhibitors to form hydrogen-bonds with the backbone amide NH of V211 and S212, thereby stabilizing the local structure of the activation loop(41) (Fig. 5E). This conformation of the activation loop then positions the S212 hydroxyl side chain to form a hydrogen-bond with the Q214 backbone amide, thus promoting the formation of the α-helix in the G213-M219 segment of the activation loop by “capping” the N-terminal end of the α-helix (Fig. 5E). Furthermore, the S212 hydroxyl side chain forms a hydrogen bond with E114 in the αC-helix, locking MEK in the unique αC-helix-out/DFG-in (CODI) conformation(17). Docking of TRAM into atomic structures of MEK1 (PDB: 3PP1(42) and 3WIG(39)) revealed that the N-methylamide carbon of TRAM may have steric clashes with the side chains of L115 of αC-helix and V211 of the activation loop (Fig. 5E and Supplementary Fig. 5SC). These steric clashes are unique to TRAM as other MEK inhibitors do not interfere with L115 and V211. Thus, TRAM binds a local conformation of the αC-helix and activation loop different than other MEK-RAF “disruptor” inhibitors, which may account for its higher potency and durability in suppressing pERK in cells, compared to the other MEK inhibitors tested.
While most MEK inhibitors induce an activation loop conformation that impedes the formation of the MEK-RAF complex, CH766 has a unique chemical moiety that alters the activation loop conformation and may be responsible for promoting the formation of an unproductive MEK-RAF complex. The atomic structure of the MEK-CH766 complex (PDB: 3WIG(39)) indicates that the core scaffold of CH766 interacts with V211 and S212 via its carbonyl moiety (Supplementary Fig. S5D), in a similar fashion to the “MEK-RAF disruptors”. However, unique to this atomic structure is the sulfamoyl moiety of CH766, which extends toward the A220-T226 segment of the activation loop and interacts with N221 in an alternative conformation, not observed in other atomic structures of MEK1-inhibitor complexes (Fig. 5E and Supplementary Fig. S5D). This extended sulfamoyl NH interacts with the N221 side chain and stabilizes this unique local conformation of the activation loop that may promote the formation of the MEK-RAF complex, albeit in an alternative conformation that is not conducive for phosphorylation and results in “trapping” active RAF kinases in an unproductive MEK-RAF complex. To test this idea, we used CH4987655 (CH655), a MEK inhibitor that is structurally similar to CH766, but lacks the sulfamoyl moiety. Consistent with this model, treatment of cells with CH655 resulted in disruption (instead of promotion) of the RAF-MEK complex (Supplementary Fig. S5E) as well as failure to suppress MEK phosphorylation(39) (Supplementary Fig. S5F). The relative biochemical properties of MEK inhibitors are summarized in Fig. 5F. As TRAM showed the high potency in MEK binding, disruption of the RAF-MEK complex and in potently and durably suppressing pERK in cells, it was thus selected for subsequent studies in combination with RAF inhibitors.
The combination of a RAF monomer-selective with a RAF dimer-selective and a potent MEK-RAF “disruptor” MEK inhibitor potently and selectively suppresses MAPK signaling in BRAF(V600E) cells
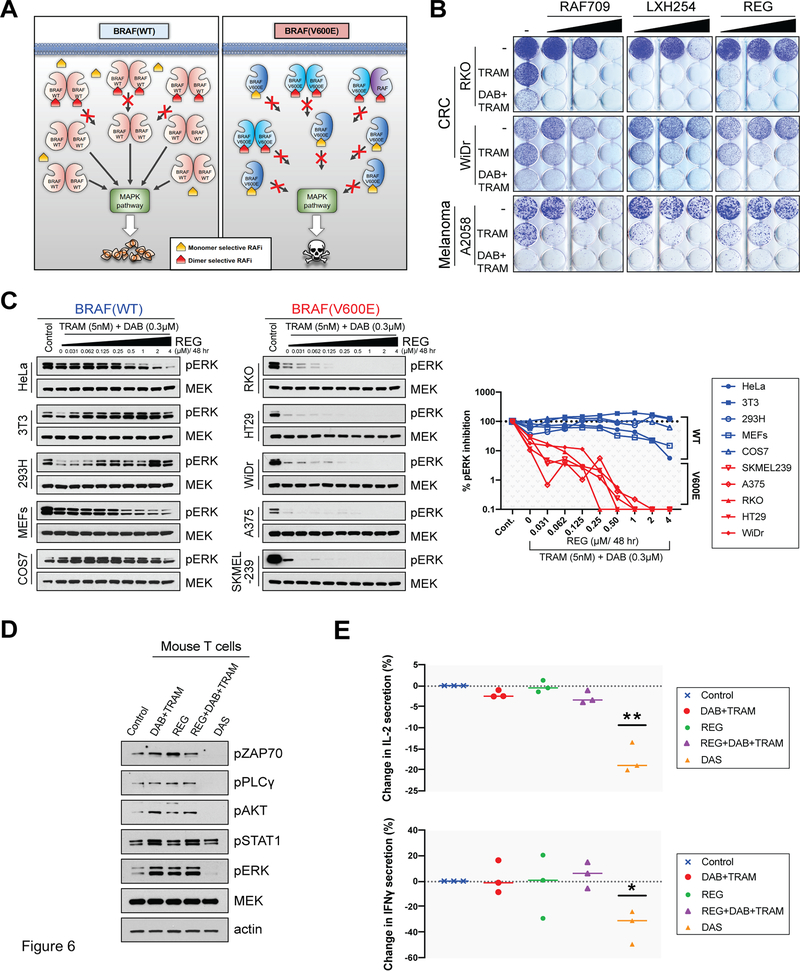
To both overcome adaptive resistance and retain a high therapeutic index when targeting BRAF(V600E) tumors, we utilized a strategy of combining a RAF monomer-selective inhibitor with a RAF dimer-selective RAF inhibitor and a MEK inhibitor (Fig. 4B). As MAPK signaling in normal tissue is driven by RAF dimers, but in BRAF(V600) tumors from both RAF monomers and dimers, this strategy includes the RAF dimer inhibitor at relatively lower concentrations to avoid toxicities, but sufficient to suppress the RAF dimers in the tumor (Fig. 6A). First, cell proliferation assays were carried out in the presence of various inhibitor combinations using BRAF(V600E) CRC cell lines (WiDr and RKO) and one BRAF(V600E) melanoma cell line (A2058) that are relatively insensitive to RAF monomer-selective inhibitors(7). We found that the triple combination of DAB+TRAM with a RAF dimer-selective inhibitor (either REG, LXH254 or RAF709) resulted in all cases in more potent suppression of colony formation, compared to TRAM alone, or combined DAB with TRAM (Fig. 6B) To obtain an estimate of the potential therapeutic index of the triple combination, we used cells wild-type for both BRAF and RAS (BRAF(WT)) as a surrogate for normal cells, and compared the effect of the triple combination on MAPK signaling in BRAF(WT) versus BRAF(V600E) cells. The triple combination REG+DAB+TRAM potently suppressed MAPK signaling in the BRAF(V600E) cell lines, compared to the BRAF(WT) cell lines, and in BRAF(WT) cells the presence of DAB reduced the inhibitory effect of REG+TRAM on MAPK signaling, suggesting a broad therapeutic window of the triple combination (Figure 6C, and Supplementary Fig. S6A, S6B). Further, potent suppression of the MAPK rebound by REG was not an indirect effect of RTK suppression, as both total and phosphorylated levels of EGFR were not affected by the presence of REG (Supplementary Fig. S6C).
Figure 6. The combination of a RAF monomer-selective with a RAF dimer-selective and a potent MEK-RAF “disruptor” MEK inhibitor potently and selectively suppresses MAPK signaling in BRAF(V600E) cells.
(A) Schematic model showing the differential effect of combining RAF monomer-selective and RAF dimer-selective inhibitors in normal versus tumor cells. In normal, BRAF(WT), cells the drug combination maintains MAPK signaling close to basal levels (left). In tumor, BRAF(V600E), cells the same amount of RAF monomer-selective and RAF dimer selective inhibitors synergize suppressing the MAPK pathway (right). (B) Crystal violet cell growth assays assessing the effect of increasing concentrations of Regorafenib (REG) (200, 500, and 1000 nM), LXH254 (100, 200, and 500 nM), and RAF709 (100, 200, and 500 nM), the double combination with Trametinib (TRAM) and the triple combination with TRAM and Dabrafenib (DAB) in the indicated colorectal cancer (CRC) or melanoma cell lines. The used concentrations were 200, 500, 1000 nM REG and LXH254, RAF709 100, 200, and 500 nM, TRAM 2 nM for RKO, 0.3 nM for WiDr, and 1 nM for A2058, and for DAB 300 nM for RKO, 50 nM for WiDr, and 150 nM for A2058. (C) The indicated BRAF(WT) and BRAF(V600E) cell lines were treated with increasing concentrations of REG for 48 hr in combination with TRAM and DAB and pERK inhibition was determined by immunoblotting (left). Summary graph showing the percentage (%) of pERK inhibition in the indicated BRAF(WT) and BRAF(V600E) cell lines (right). Graph was plotted based on relative expression levels of pERK normalized to MEK after ImageJ analysis (Supplementary Fig. S6A). (D-E) Activated mouse T cells treated with DAB (0.3μΜ) and TRAM (5nM), REG (1μM), the triple combination of DAB (0.3μΜ), TRAM (5nM) and REG (1μM) or with Dasatinib (DAS, 2 μΜ). (D) Cell lysates from activated mouse T cells treated for 48 hr with the indicated inhibitors were immunoblotted with the indicated antibodies. (E) The concentrations of IL-2 and IFN-γ were determined by ELISA in supernatants of activated mouse T cells treated with the indicated inhibitors for 24 hr (IL-2) or 48 hr (IFN-γ). Graphs showing the change (%) in IL-2 (top) or IFN-γ (bottom) concentrations. Data are represented as mean ± SEM (*, P < 0.05; **, P < 0.01; ***, P <0.001).
Finally, as therapies targeting components of MAPK signaling have been shown to affect tumor response to immunotherapy(43) we examined the effect of the triple combination on T cell signaling and function. Markers of TCR signaling, such as phosphorylated ZAP70 (pZAP70), pPLC1γ, pAKT and pERK, were at similar levels when primary mouse T cells were treated with DAB+TRAM, REG alone or REG+DAB+TRAM, whereas the Src and multikinase inhibitor Dasatinib (DAS) potently suppressed TCR signaling (Fig. 6D and Supplementary Fig. S6D). Further, treatment of activated mouse primary T cells with DAB+TRAM or REG+DAB+TRAM resulted in similar levels of secretion of IL-2 or IFNγ, whereas DAS suppressed both IL-2 and IFNγ secretion (Fig. 6E). To assess the effect of the triple combination on tumor killing by T cells, we used the Just eGFP Death Inducing (JEDI) T cells(44,45). JEDI T cells recognize the immunodominant epitope of GFP (GFP200–208) presented in MHC class I. JEDI cells were co-cultured with GFP or mCherry -expressing tumor cells at a ratio of 1:1 and killing was assessed by flow cytometry at day 3 after co-culture measuring the relative percentage of GFP+ and mCherry+ cells. Treatment with REG+DAB+TRAM resulted in tumor cell killing at levels comparable or higher to DAB+TRAM (Supplementary Fig. S6E). Together these data indicate that the triple combination will potently and durably suppress MAPK signaling in the tumor without negatively affecting tumor cell killing by T cells.
The combination of a RAF monomer-selective with a RAF dimer-selective and a potent MEK-RAF “disruptor” MEK inhibitor is effective and well tolerated in vivo
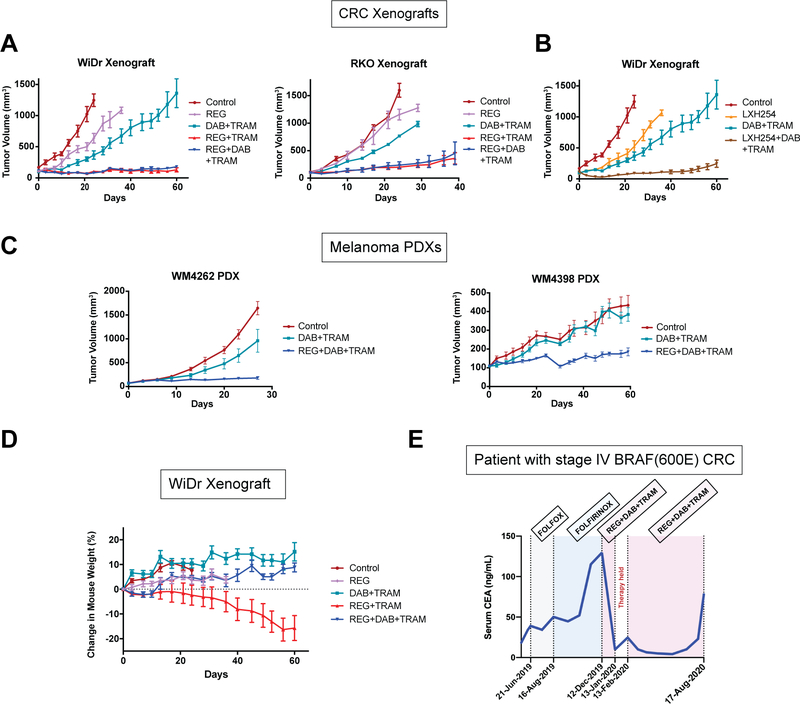
To assess the effectiveness of the combinatorial strategy in vivo, we first treated mice carrying xenograft BRAF(V600E) CRC tumor models (WiDr or RKO) with REG+DAB+TRAM, and compared the effect to the double combinations REG+TRAM or DAB+TRAM. In both xenografts, either control or DAB+TRAM treatment had only minimal effect. However, REG+DAB+TRAM or REG+TRAM combinations potently suppressed MAPK and downstream signaling as well as tumor growth (Fig. 7A, Supplementary Fig. S7A). Similar results were obtained using LXH254, instead of REG. As shown in Fig. 7B, the triple LXH254+DAB+TRAM combination potently suppressed growth of WiDr xenografts in vivo. We next assessed the in vivo effectiveness of the triple combination using two patient-derived xenograft (PDX) BRAF(V600E) melanoma models (WM4262 and WM4398) that are derived from tumors resistant to clinical RAF and MEK inhibitors(46). In both PDXs, control treatment and DAB+TRAM had minimal effect, while treatment with REG+DAB+TRAM potently suppressed tumor growth (Fig. 7C).
Figure 7. The combination of a RAF monomer-selective with a RAF dimer-selective and a potent MEK-RAF “disruptor” MEK inhibitor is effective and well tolerated in vivo.
. (A-B) WiDr or RKO cells were injected subcutaneously into the flanks of nude mice (10 million cells per injection). When tumors reached 100–150 mm3 in size, mice were randomized and treated with vehicle (Control), Regorafenib (REG, 30 mg/kg), Dabrafenib (DAB, 30 mg/kg)+Trametinib (TRAM, 0.25 mg/kg), REG (30 mg/kg)+TRAM (0.25 mg/kg) or REG (30 mg/kg)+DAB (30 mg/kg)+TRAM (0.25 mg/kg) and LXH254 (30 mg/kg) or LXH254 (30 mg/kg)+DAB (30 mg/kg)+TRAM (0.25 mg/kg) once daily for 40 (RKO xenograft) or 60 (WiDr xenograft) days. Graphs show mean tumor volumes (± SEM) vs time. (C) NSG mice were injected with the indicated PDX melanoma cells (WM4262 and WM4398). When the tumors were palpable, mice were randomized into either vehicle control, DAB (30 mg/kg)+TRAM (0.3 mg/kg) or REG (30 mg/kg)+DAB (30 mg/kg)+TRAM (0.3 mg/kg). Mice were fed chow containing the DAB+TRAM daily or treated with REG+DAB+TRAM 5 days on, 2 days off for 28 (WM4262 PDX) or 60 (WM4398 PDX) days. Graphs show mean tumor volumes (± SEM) vs time. (D) Body weight of mice bearing the WiDr xenograft and treated with the indicated inhibitors was measured every three days for 60 days. Graph shows the change in mouse weight (%). Data are represented as mean ± SEM. (E) Concentration of carcinoembryonic antigen (CEA) in the serum of a colorectal BRAF(V600E) patient treated with different regimens.
Limitations related to therapeutic index and potential added toxicities are major considerations when designing drug combinations. When treating BRAF-mutant tumors, the therapeutic index is expected to derive from the fact that two RAF inhibitors (i.e. a RAF monomer-selective and RAF dimer-selective) synergize in the tumor but exert opposite effects on MAPK signaling in BRAF(WT) cells. Thus, in normal cells, combining a RAF dimer-selective with a RAF monomer-selective inhibitor is expected to maintain MAPK signaling close to basal levels, consistent with our in vitro data shown in Fig. 6B and Supplementary Fig. S6B. Our in vivo observations were consistent with this concept. A gradual weight loss was observed in mice on the double combination REG+TRAM after the third week of treatment, suggesting accumulating toxicities caused by this treatment. Strikingly, mice receiving the triple combination REG+DAB+TRAM or LXH254+DAB+TRAM showed no evidence of weight loss or other apparent toxicities (Fig. 7D, Supplementary Fig. S7B, S7C). Together, these data show that by rationally designing combinations, addition of a compound targeting a conformational state of RAF could rescue from toxicities caused by another conformation-selective RAF inhibitor and, thus, increase the therapeutic index.
To assess this therapeutic strategy in the clinical setting, the combination of REG+DAB+TRAM was tested in a 53 year old male with stage IV sigmoid colon adenocarcinoma and peritoneal carcinomatosis whose disease had progressed on standard cytotoxic chemotherapies. Molecular analysis demonstrated the presence of a BRAF(V600E) mutation; the tumor was also microsatellite stable, RAS(wild-type) and Her-2 negative. The patient’s serum carcinoembryonic antigen (CEA) level at diagnosis was 18.3 ng/mL but increased to 39.1 ng/mL when he began therapy with FOLFOX. Due to a lack of serologic response and radiographic evidence of disease progression after 2.5 months of therapy, he was transitioned to FOLFIRINOX for 4 months. Although his disease appeared stable on imaging and he maintained a good functional status, his appetite remained poor, and he continued to report lower abdominal pain associated with an abrupt increase in CEA level to 115.2 ng/mL.
Given our preclinical data and the poor prognosis associated with BRAF(V600E), which compels the earlier use of combination targeted therapy, compassionate use off-label of REG+DAB+TRAM was initiated including DAB and TRAM at 150 mg PO BID and 2 mg PO QD, respectively, as used in melanoma(47). Given that REG has never been combined with DAB+TRAM, and based on the ReDOS trial(48), a starting REG dose of 40 mg PO QD for one week was selected with the plan to increase to 80 mg QD during week 2 and 120 mg QD during week 3 of therapy. Serum CEA at the start of treatment, approximately 4 weeks after his last dose of FOLFIRINOX, was 129.6 ng/mL (Fig. 7E). The first month on REG+DAB+TRAM was complicated by grade 2–3 cough, gingival bleeding and epistaxis, pyrexia, rigors and pancytopenia. The patient was hospitalized twice: first for thrombocytopenia and E. coli bacteremia, and second for pyrexia and rigors without a documented infection. Medication reconciliation revealed under-dosing of DAB and a doubling of the REG starting dose. Notably, his CEA level decreased significantly to 9.8 ng/mL and repeat imaging showed stable disease. However, during the 4 week interruption in therapy for toxicities, the patient’s CEA increased to 24.7 ng/mL. On February 13, 2020, he resumed modified dose TRAM 1.5 mg PO QD, DAB 100 mg PO BID and a flat dose of REG 40 mg PO QD, which has been well tolerated without further hospitalizations, the patient’s CEA dramatically decreased, his appetite has improved, and lower abdominal pain has decreased. The triple combination treatment continued for a total of almost 8 months, until August 17th, 2020, when an increase in CEA was observed, along with mild disease progression (Fig. 7E). Moreover, after experiencing weight loss on the prior regimen, the patient’s weight has remained stable during the triple combination regimen.
Discussion
Several lines of evidence indicate that adaptive drug resistance is frequently the result of incomplete inhibition of oncogenic signaling and to effectively overcome it, combinatorial targeting of multiple nodes of the pathway will be necessary (9,10,40,49–54). However, how to rationally design drug combinations with high effectiveness and minimal toxicities remains a major challenge. Here we identify and characterize a novel class of RAF inhibitors that selectively inhibits dimeric over monomeric RAF. The class includes a number of clinically relevant compounds, such as the FDA-approved multi-kinase inhibitor Regorafenib, as well as LHX254 and Belvarafenib (GDC-5773), that are currently in clinical trials. Although this class of compounds shows very similar structural and crystallographic properties to compounds equipotent for monomeric and dimeric RAF, MD simulations provide a structural explanation for their dimer selectivity: higher inhibitor stability in the dimer specificity pocket due to stabilization of the αC helix upon RAF dimerization. The selectivity of these compounds revealed a previously unknown difference in the conformation of the active site between dimeric and monomeric BRAF(V600E), and their affinity for MEK, thus uncovering a hitherto unappreciated complexity in the regulation of the BRAF(V600E) oncoprotein. A previous report indicated that BRAF(V600E) shows weaker interaction with MEK compared to RAS-activated wild-type BRAF, a phenotype attributed to lower affinity of highly phosphorylated MEK for BRAF(55). Another report showed that the splice variant of BRAF(V600E) interacts more strongly with MEK compared to full-length BRAF(V600E)(36). Two recent reports presented structural data using cryo-Electron Microscopy on BRAF(34,35). In Park et al., 2019(35), inactive and monomeric BRAF was studied in complex with MEK. However, based on our findings, in cells, BRAF in the inactive, monomeric conformation interacts much more weakly with MEK compared to dimeric BRAF. The discrepancies between structural and biochemical findings across different studies highlight the complexity of BRAF signaling, and the need for further biochemical and structural analysis to conclusively elucidate the regulation of wild-type and mutant BRAF in their native states, in different cellular contexts.
After characterizing a number of RAF and MEK inhibitors, we assessed the therapeutic strategy of the combination of a RAF monomer-selective, a RAF dimer-selective and a potent MEK-RAF “disruptor” MEK inhibitor to overcome adaptive resistance of BRAF(V600E) tumors. The triple combination was highly effective and well tolerated in multiple BRAF(V600E) tumor models in vivo, as well as in a patient with advanced colorectal cancer, suggesting that this may be a powerful therapeutic strategy for patients with BRAF(V600E) tumors. In the phase III BEACON trial the combination of a RAF inhibitor (Encorafenib) and an anti-EGFR antibody (Cetuximab) in patients with metastatic BRAF(V600E) CRC tumors showed a median Progression-Free survival (PFS) of 4.2 months and median overall survival (OS) of 8.4 months which represented an improvement over standard chemotherapy(56), resulting in approval of the combination for this indication by the FDA. Although case reports must be interpreted with caution, the response of our patient to REG+DAB+TRAM for almost 8 months compares favorably to prior studies of DAB+TRAM (median PFS 3.5 months)(57) and REG monotherapy (median PFS 1.9 months)(58) in BRAF(V600E) CRC. The combination also compares favorably to the BEACON trial results and to other combinations tested in this population such as irinotecan + cetuximab + vemurafenib in the SWOG 1406 study (median PFS 4.2 months)(59) and dabrafenib + trametinib + panitumumab (median PFS 4.2 months, median OS 9.1 months, median response duration 7.6 months)(60). This observation and the clear clinical benefit experienced by the patient provide anecdotal support for the clinical translation of the triplet regimen as a potentially active and tolerable option in patients with BRAF(V600E) mutant colorectal cancer. The close temporal association between the initiation, interruption and re-initiation of therapy, with changes in serum CEA levels and the subjective improvement experienced by the patient compellingly indicate the antineoplastic effect of this regimen, providing a basis for further evaluating this combination in a clinical trial.
The initial enthusiasm based on the objective clinical effectiveness of RAF and MEK inhibitors in BRAF(V600E) patients was subsequently tempered by the realization that for the majority of these patients, the antitumor effect of these drugs is temporary, frequently limited by various mechanisms of adaptive and acquired resistance. The discovery of a new class of RAF inhibitors described here, pave the way for the rational design of next generation combinatorial pharmacologic strategies with high efficacy and increased therapeutic index, for therapy of patients with BRAF(V600E) tumors.
Methods
Western blot and immunoprecipitation
Cells were washed with PBS and lysed on ice for 5 min in NP40 buffer (50mM Tris pH 7.5, 1% NP40, 150mM NaCl, 10% Glycerol 1mM EDTA) supplemented with protease and phosphatase inhibitors (Roche). Lysates were centrifuged at 15,000 rpm for 10 min and the protein concentration was quantified using BCA (Pierce). Proteins were separated by NuPAGE, 4–12% Bis Tris Gel (Novex) and immunoblotted and transferred to nitrocellulose membranes (GE Healthcare) according to standard protocols. Membranes were immunoblotted overnight with antibodies against pMEK1/2Ser217/221, MEK1 (61B12), pERK1/2Thr202/Tyr204 (D13.14.4E), ERK (137F5), pEGFRTyr1068 (D7A5), pBRAFSer445, pZAP70Tyr319, pPLCγ1Tyr783, pAKTSer473 (D9E), pSTAT1Tyr701 (58D6), EGFR (D38B1), and β-Actin (13E5) from Cell Signaling; BRAF from Santa Cruz Biotechnology; BRAFV600E from NewEast Biosciences; HA and DUSP6 from Abcam; CRAF from BD Transduction laboratories; and pCRAFSer338 from Millipore. Next day, membranes were probed with anti-rabbit IgG or anti-mouse IgG secondary antibody (Cell Signaling) and chemiluminescent signals were detected on X-ray films. All the western blot experiments were performed twice, unless stated otherwise, and representative results from each experiment are shown.
For immunoprecipitations, lysates were incubated with the indicated antibodies overnight at 4°C, followed by protein G agarose (Life technologies) for 1 hr at 4°C. Samples were washed three times with lysis buffer and sample buffer was added for subsequent immunoblot analysis.
Cell lines
RKO, HT29, WiDr, A375, A2058, HCT116, Calu-6 cell lines were purchased from the American Type Culture Collection (ATCC). 293H cells were purchased from Life Technologies. HeLa cells were kindly provided by Ramon Parsons and 3T3 cells were developed by Stuart Aaronson. MEFs, COS7, PC9, 239/C3, M397-P/-R, WM1382 and SKMEL28(SK28)/SKMEL28(SK28)-R cell lines have been described previously(7). All the cells used in the study were maintained in a humidified incubator at 37°C with 5% CO2, cultured in RPMI 1640 or DMEM supplemented with 10% FBS, 2 mM glutamine and 100 IU/ml penicillin and streptomycin and were passaged from 3 to 5 times. Cell lines were authenticated by LabCorp using short tandem repeat DNA profiling and were regularly tested negatively for mycoplasma using Venor™ GeM Mycoplasma Detection Kit (Sigma).
Compounds
CH4987655 and RMC-4550 were obtained from Medchem Express. PLX7904 was kindly provided by Plexxikon. Regorafenib was obtained from Selleckchem or Medchem Express. All other compounds were obtained from Selleckchem. Compounds were dissolved in DMSO to yield 10 mM stock. The ligand for chemically induced dimerization AP20187 (AP, Clontech) was dissolved in ethanol.
Cellular thermal shift assay
Cells were treated with 2 μM or 4 μM Vemurafenib or Regorafenib for 2 hr. Cells were resuspended in PBS containing protease inhibitor cocktail (Roche) and the cell suspensions were aliquoted into 100 μl PCR tubes. Samples were then heated individually at the indicated different temperature endpoints for 3 min using in the Veriti 96-well thermal cycler (ThermoFisher) and allowed to equilibrate to room temperature. The cell suspensions were freeze-thawed three times using liquid nitrogen and a thermal cycler at 25oC. The soluble fractions (lysates) were separated from the cell debris by centrifugation at 20,000 × g for 20 min at 4 °C. Supernatants were collected and analyzed by SDS-PAGE followed by Western blot analysis.
Crystal violet cell growth assays
Cells were plated in 6-well plates at a density of 1–10 × 103 cells per well. The next day, cells were treated with inhibitors as indicated in regular growth media for 10–14 days. Growth media with or without inhibitors was replaced every 3 days. Cells were fixed with 4% paraformaldehyde for 5 min and then stained with 0.5% crystal violet for 30 min. Cells were de-stained with tap water and air-dried.
Plasmids and transfections.
iDimerize inducible system (Clontech) was used for chemical induced dimerization. BRAFV600E, BRAF or CRAF were cloned at the N-terminus of the DmrB domains of the pHom-Mem1 vector using EcoRI and XbaI restriction sites. The vector was designed to express a hemagglutin (HA) epitope tag at the C-terminus of the created fusion proteins. Transfections were carried out using Lipofectamine 2000 (Life technologies).
Binding affinity studies
The dissociation constant (Kd) values for the binding between trametinib, cobimetinib, binimetinib or CH5126766 and MEK1 were determined using the KinomeScan binding assay(61). Binding studies were performed by Eurofins DiscoverX.
Murine primary T cell isolation and activation
CD4+ and CD8+ T cells were isolated from the spleens of C57BL/6 mice (Envigo laboratories) through negative selection, using the EasySep™ mouse T cell isolation kit (StemCell Technologies). The isolated T cells were activated for 48 hr using magnetic beads coupled with antibodies against CD3 and CD28 (Dynabeads® Mouse T-Activator CD3/CD28, ThermoFisher) and 20ng/ml recombinant IL-2 (Peprotech) according to manufacturer instructions in RPMI supplemented with 10% FBS, 100 U/ml penicillin/streptomycin, 2 mM L-glutamine, 1% non-essential amino acids, 1 mM sodium pyruvate 55 μM 2-mercaptoethanol and 20 mM HEPES.
Enzyme-linked immunosorbent assays
Activated murine primary T cells were plated in 96-well plates at a density of 1 × 105 cells per well and treated with different inhibitors or inhibitor combinations for 24 hr. Supernatants were collected and the concentrations of IFN-γ and IL-2 were determined using the Enzyme-linked immunosorbent assay (ELISA) MAX™ Standard Set Mouse IFN-γ (BioLegend) and the uncoated mouse IL-2 ELISA (ThermoFischer), respectively, according to manufacturer instructions.
T cell killing assay
CD8+ T cells were isolated from spleens of Jedi (Just eGFP death-inducing) mice(44). Splenic cells suspensions were obtained by mechanical disruption and filtering through 70-μm cell strainer. Red blood cells were lysed using RBC buffer (eBioscience), and CD8+ T cells were negatively selected using EasySep™ mouse CD8+ T cells isolation kit from Stem Cell Technologies, following manufacturer’s instructions. Cells were activated for 48 hr with Dynabeads™ Mouse T-Activator CD3/CD28 (ThermoFisher) and 20 ng/ml mouse recombinant IL-2 (Peprotech) in RPMI with 10% FBS, 100 U/ml penicillin/streptomycin, 2 mM L-glutamine, 1% non-essential amino acids, 1 mM sodium pyruvate 55 μM 2-mercaptoethanol and 20 mM HEPES. Target or bystander cells were generated by transducing MEL (mouse-derived erythroleukemia cell line) cells with a lentiviral vector expressing GFP or mCherry and then sorted to purity. A 50%:50% mix of GFP+ (104 target cells) and mCherry+ (104 bystander cells) MEL cells were plated in a 96-well plate (104 target cells per well) and activated T cells were added at different ratios along with different inhibitors or inhibitor combinations. Killing was assessed by flow cytometry at day 3 after co-culture measuring the relative percentage of GFP+ and mCherry+ cells.
Molecular docking
Docking of trametinib (TRAM) was performed with the Glide program of the Schrödinger suite (2019–2) (Schrödinger release 2019–2: LigPrep, Glide, Protein Preparation Wizard, Schrödinger, LLC, New York, NY 2019).
TRAM was prepared and tautomerized at pH 7.2 by LigPrep and MEK kinase domain structures (PDB: 3PP1 and 3WIG) were prepared by Protein Preparation Wizard. Ligand and protein were parameterized with OPLS3 force field(62). Protein grid generation has the following settings: (i) aromatic CH hydrogens and halogen atoms were treated as hydrogen-bond donors and acceptors, respectively, and (ii) van der Waals radius was softened (scaled to 0.8) for atoms with partial charge exceeding 0.25e. The standard Precision mode of Glide was used for molecular docking.
Molecular dynamics simulations
General Simulation Setup and Parameterization
Proteins and ions were parameterized with the Amber99SB*-ILDN force field(63,64) and ligands where parameterized using GAFF 2.0(65). The system was solvated with water parameterized with the TIP3P force field(66) and neutralized with a 100-mM NaCl buffer. Monomeric BRAF systems contained ~55,000 atoms in a cubic box of length 85 Å, and dimeric systems contained ~110,000 atoms with a cubic box of length 103 Å. BRAF-MEK systems contained ~121,000 atoms with a cubic box of length 107 Å.
Systems were each equilibrated on GPU Desmond using a mixed NVT/NPT schedule(67) and in https://www.deshawresearch.com/publications/Desmond-GPU%20Performance%20as%20of%20October%202015.pdf. All production simulations were run on Anton, a specialized machine for MD simulations(68). Simulations were performed in the NPT ensemble at 310 K using the Martyna-Tobias-Klein barostat(69). The simulation time step was 2.5 fs, using a modified r-RESPA integrator(70,71) and evaluating long range electrostatics every three-time steps. Electrostatics forces were calculated using the u-series method(72). A 9-Å cutoff was applied for the van der Waals calculations.
System Preparation
BRAF crystal structures from the Protein Data Bank were prepared for simulation using the Protein Preparation Wizard in Schrödinger Maestro (Schrödinger release 2019–2: Maestro, Schrödinger, LLC, New York, NY 2019). Unless otherwise noted, missing loops and termini were capped with ACE/NME capping groups.
Both protomers of a BRAF dimer contain a ligand molecule. The monomeric system was generated by deleting one copy of the dimeric system, and then solvating as previously described. Ligands were prepared and protonated using LigPrep, with EPIK at pH 7.0. Simulations of TAK632, AZ628, LY3009120, RAF709, and BGB283 were initiated from their respective crystal structure poses. Simulations of Ponatinib, LHX254, Sorafenib, Regorafenib, and Belvarafenib were initiated from docked poses, generated using Standard Precision Glide with default receptor grid generation parameters. For each ligand, three simulations of 15 μs were run for both the dimer and the monomer. Ligand placement methodology and starting crystal structures are summarized in Supplementary Table 1.
BRAF-MEK structures were prepared from PDB IDs 4MNE(55) and 6U2G(73) using the Protein Preparation Wizard. In the 6U2G structure, the unresolved activation loop of BRAF, from residue 602 to 610, was built using Schrödinger Prime(74,75). Both BRAF-MEK constructs were simulated for 15 μs.
Simulation Analysis
All simulations were visually inspected using the in-house visualization software Firefly. Ligands remained bound to the kinase throughout the simulations. For a given ligand, the chemical structure that interacted with the specificity pocket (as defined by residues 504, 505, 513, 567, and 572) exhibited larger dynamic fluctuation than the regions that interacted with the classic ATP binding pocket. To understand the differences between monomeric and dimeric BRAF ligand simulations, we measured the RMSD of the portion of the ligand that binds in the specificity pocket. These portions were manually selected, and correspond to the circled heavy atoms in Supplementary Table 1. The system was aligned on all ligand heavy atoms. For dimeric simulations, only the ligand bound to chain A was considered. RMSD data across the replicates was aggregated to produce the histograms in Fig. 2A and Supplementary Fig. S2A. The RMSD of the αC helix was quantified in a similar manner. The system was aligned on the alpha carbons of chain A residues 490 to 510, and the RMSD of this same selection was computed. These RMSD values were aggregated in the histograms in Supplementary Fig S2B.
As an additional metric to evaluate the αC helix dynamics, we report in Supplementary Table 2 the stability of the E501-K483 salt bridge (calculated by measuring the fraction of the trajectory in which the distance between the two residues was smaller than 3.5 Å).
For the BRAF-MEK dimer simulations, protein-protein contact areas were computed on the final frame of the simulation using the PDBe PISA server (Protein interfaces, surfaces and assemblies service (PISA) at the European Bioinformatics Institute. http://www.ebi.ac.uk/pdbe/prot_int/pistart.html(76). Reported interface areas are the per-protomer area, defined as the solvent-accessible surface area of the complex subtracted from the sum of the protomer areas, divided by two.
Animal experiments
CRC xenograft models
All animals were examined prior to the initiation of studies to ensure that they were healthy and acclimated to the laboratory environment. 5–7-week-old, female athymic Nude-Foxn1nu (Envigo laboratories) mice were used for animal experiments. All mouse experiments were approved by the Icahn School of Medicine at Mount Sinai Animal Care and Use Committee (protocol no. IACUC-2016–0066). Mice were maintained under specific pathogen-free conditions, and food and water were provided ad libitum.
RKO or WiDr cells were harvested on the day of use and injected subcutaneously in the left flank per mouse (10 × 106 per injection). After inoculation, mice were monitored daily, weighed every three days, and caliper measurements begun when tumors became visible. Tumor volume was calculated using the following formula: tumor volume = (D X d2)/2, in which D and d refer to the long and short tumor diameter, respectively. When tumors reached a size of 100–150 mm3, mice were randomized into 7 groups (n= 7/group) treated each with vehicle (5% DMSO, 0.5% hydroxypropyl methyl cellulose, 0.2% tween 80), regorafenib (30 mg/kg), LXH254 (30 mg/kg), Dabrafenib (30 mg/kg) and Trametinib (0.25 mg/kg), Regorafenib (30 mg/kg) and Trametinib (0.25 mg/kg), the triple combination of Regorafenib (30 mg/kg), Dabrafenib (30 mg/kg) and Trametinib (0.25 mg/kg) or the triple combination of LXH254 (30 mg/kg), Dabrafenib (30 mg/kg) and Trametinib (0.25 mg/kg), orally once a day, based on mean group body weight. The formulation for LXH254 was prepared after dilution with DI water of the microemulsion stock vehicle MEPC4 (45% cremophor RH40, 27% PEG400, 18% corn oil glycerides and 10% ethanol). The endpoint of the experiment for survival studies was considered a tumor volume of 1000 mm3 as per our approved protocol. Once the mice were sacrificed, tumors were used for further analysis.
Melanoma patient-derived xenograft (PDX) models
NSG (NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ) mice models were injected with single cell suspensions of the indicated PDX melanoma cells (WM4262 and WM4398). When the tumors were palpable, mice were randomized into 3 groups (n= 7/group) either vehicle control, Dabrafenib (30 mg/kg) and Trametinib (0.3 mg/kg) or the triple combination of Regorafenib (30 mg/kg) and Dabrafenib (30 mg/kg) and Trametinib (0.3 mg/kg). Mice were fed chow containing the Dabrafenib and Trametinib daily. Mice were treated with Regorafenib 5 days on, 2 days off for the duration of the therapy trial. All the animal experiments regarding the PDX models were approved by The Wistar Institute Institutional Animal Care and Use Committee(77).
Statistical Analysis
Data are presented as mean with SEM, as indicated in the figure legends. Statistical comparisons were performed using two-sided unpaired Student’s t test, using GraphPad Prism 9, unless otherwise specified (*, P < 0.05; **, P < 0.01; ***, P <0.001).
Supplementary Material
Significance.
This work identifies a new class of RAF inhibitors that are selective for dimeric over monomeric BRAF, and determines the basis of their selectivity. A rationally-designed combination of RAF and MEK inhibitors based on their conformation selectivity, achieved increased efficacy and high therapeutic index when used to target BRAF(V600E) tumors.
Acknowledgments
The authors thank Paul Maragakis for helpful discussions. P.I.P. is supported by TCI developmental awards, the NIH/NCI (R01CA204314, R01 CA240362 and R01CA238229), the Irma T. Hirschl Trust, the Manhasset Women’s Coalition against Breast Cancer, the Breast Cancer Alliance, the Melanoma Research Foundation and the Melanoma Research Alliance. M.H. is supported by NIH grants RO1 CA238237, U54 CA224070, PO1 CA114046 and the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation. B.D.B. was supported by funding from the Cancer Research Institute and NIH R01AT011326 and R33CA223947. S.A.A. would like to acknowledge funding from the Breast Cancer Research Foundation. T.A.A. is supported by grant T32CA078207, and Z.K. would like to acknowledge the 2017 Robin Chemers Neustein Postdoctoral Fellowship.
Footnotes
No competing financial interests.
References
- 1.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature 2002;417(6892):949–54 doi 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 2.Hodis E, Watson IR, Kryukov GV, Arold ST, Imielinski M, Theurillat JP, et al. A landscape of driver mutations in melanoma. Cell 2012;150(2):251–63 doi 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Subbiah V, Baik C, Kirkwood JM. Clinical Development of BRAF plus MEK Inhibitor Combinations. Trends Cancer 2020;6(9):797–810 doi 10.1016/j.trecan.2020.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature 2010;464(7287):427–30 doi 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poulikakos PI, Persaud Y, Janakiraman M, Kong X, Ng C, Moriceau G, et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E). Nature 2011;480(7377):387–90 doi 10.1038/nature10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freeman AK, Ritt DA, Morrison DK. Effects of Raf dimerization and its inhibition on normal and disease-associated Raf signaling. Molecular cell 2013;49(4):751–8 doi 10.1016/j.molcel.2012.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karoulia Z, Wu Y, Ahmed TA, Xin Q, Bollard J, Krepler C, et al. An Integrated Model of RAF Inhibitor Action Predicts Inhibitor Activity against Oncogenic BRAF Signaling. Cancer cell 2016;30(3):485–98 doi 10.1016/j.ccell.2016.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yao Z, Torres NM, Tao A, Gao Y, Luo L, Li Q, et al. BRAF Mutants Evade ERK-Dependent Feedback by Different Mechanisms that Determine Their Sensitivity to Pharmacologic Inhibition. Cancer cell 2015;28(3):370–83 doi 10.1016/j.ccell.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lito P, Pratilas CA, Joseph EW, Tadi M, Halilovic E, Zubrowski M, et al. Relief of profound feedback inhibition of mitogenic signaling by RAF inhibitors attenuates their activity in BRAFV600E melanomas. Cancer cell 2012;22(5):668–82 doi 10.1016/j.ccr.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corcoran RB, Ebi H, Turke AB, Coffee EM, Nishino M, Cogdill AP, et al. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer discovery 2012;2(3):227–35 doi 10.1158/2159-8290.CD-11-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakamura A, Arita T, Tsuchiya S, Donelan J, Chouitar J, Carideo E, et al. Antitumor activity of the selective pan-RAF inhibitor TAK-632 in BRAF inhibitor-resistant melanoma. Cancer research 2013;73(23):7043–55 doi 10.1158/0008-5472.CAN-13-1825. [DOI] [PubMed] [Google Scholar]
- 12.Peng SB, Henry JR, Kaufman MD, Lu WP, Smith BD, Vogeti S, et al. Inhibition of RAF Isoforms and Active Dimers by LY3009120 Leads to Anti-tumor Activities in RAS or BRAF Mutant Cancers. Cancer cell 2015;28(3):384–98 doi 10.1016/j.ccell.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Hatzivassiliou G, Haling JR, Chen H, Song K, Price S, Heald R, et al. Mechanism of MEK inhibition determines efficacy in mutant KRAS- versus BRAF-driven cancers. Nature 2013;501(7466):232–6 doi 10.1038/nature12441. [DOI] [PubMed] [Google Scholar]
- 14.Ishii N, Harada N, Joseph EW, Ohara K, Miura T, Sakamoto H, et al. Enhanced inhibition of ERK signaling by a novel allosteric MEK inhibitor, CH5126766, that suppresses feedback re-activation of RAF activity. Cancer research 2013;73(13):4050–60 doi 10.1158/0008-5472.CAN-12-3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lito P, Saborowski A, Yue J, Solomon M, Joseph E, Gadal S, et al. Disruption of CRAF-mediated MEK activation is required for effective MEK inhibition in KRAS mutant tumors. Cancer cell 2014;25(5):697–710 doi 10.1016/j.ccr.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J, Yang PL, Gray NS. Targeting cancer with small molecule kinase inhibitors. Nature reviews Cancer 2009;9(1):28–39 doi 10.1038/nrc2559. [DOI] [PubMed] [Google Scholar]
- 17.Ung PM, Rahman R, Schlessinger A. Redefining the Protein Kinase Conformational Space with Machine Learning. Cell Chem Biol 2018;25(7):916–-24 e2. doi 10.1016/j.chembiol.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dummer R, Ascierto PA, Gogas HJ, Arance A, Mandala M, Liszkay G, et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicentre, open-label, randomised phase 3 trial. The lancet oncology 2018;19(5):603–15 doi 10.1016/S1470-2045(18)30142-6. [DOI] [PubMed] [Google Scholar]
- 19.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. The New England journal of medicine 2011;364(26):2507–16 doi 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. The New England journal of medicine 2012;367(18):1694–703 doi 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer research 2004;64(19):7099–109 doi 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 22.Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schutz G, et al. Regorafenib (BAY 73–4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer 2011;129(1):245–55 doi 10.1002/ijc.25864. [DOI] [PubMed] [Google Scholar]
- 23.Shao W, Mishina YM, Feng Y, Caponigro G, Cooke VG, Rivera S, et al. Antitumor Properties of RAF709, a Highly Selective and Potent Inhibitor of RAF Kinase Dimers, in Tumors Driven by Mutant RAS or BRAF. Cancer research 2018;78(6):1537–48 doi 10.1158/0008-5472.CAN-17-2033. [DOI] [PubMed] [Google Scholar]
- 24.Ramurthy S, Taft BR, Aversa RJ, Barsanti PA, Burger MT, Lou Y, et al. Design and Discovery of N-(3-(2-(2-Hydroxyethoxy)-6-morpholinopyridin-4-yl)-4-methylphenyl)-2-(trifluorom ethyl)isonicotinamide, a Selective, Efficacious, and Well-Tolerated RAF Inhibitor Targeting RAS Mutant Cancers: The Path to the Clinic. Journal of medicinal chemistry 2020;63(5):2013–27 doi 10.1021/acs.jmedchem.9b00161. [DOI] [PubMed] [Google Scholar]
- 25.Moriceau G, Hugo W, Hong A, Shi H, Kong X, Yu CC, et al. Tunable-combinatorial mechanisms of acquired resistance limit the efficacy of BRAF/MEK cotargeting but result in melanoma drug addiction. Cancer cell 2015;27(2):240–56 doi 10.1016/j.ccell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez Molina D, Jafari R, Ignatushchenko M, Seki T, Larsson EA, Dan C, et al. Monitoring drug target engagement in cells and tissues using the cellular thermal shift assay. Science 2013;341(6141):84–7 doi 10.1126/science.1233606. [DOI] [PubMed] [Google Scholar]
- 27.Jafari R, Almqvist H, Axelsson H, Ignatushchenko M, Lundback T, Nordlund P, et al. The cellular thermal shift assay for evaluating drug target interactions in cells. Nature protocols 2014;9(9):2100–22 doi 10.1038/nprot.2014.138. [DOI] [PubMed] [Google Scholar]
- 28.Foster SA, Whalen DM, Ozen A, Wongchenko MJ, Yin J, Yen I, et al. Activation Mechanism of Oncogenic Deletion Mutations in BRAF, EGFR, and HER2. Cancer cell 2016;29(4):477–93 doi 10.1016/j.ccell.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 29.Tang Z, Yuan X, Du R, Cheung SH, Zhang G, Wei J, et al. BGB-283, a Novel RAF Kinase and EGFR Inhibitor, Displays Potent Antitumor Activity in BRAF-Mutated Colorectal Cancers. Molecular cancer therapeutics 2015;14(10):2187–97 doi 10.1158/1535-7163.MCT-15-0262. [DOI] [PubMed] [Google Scholar]
- 30.O’Hare T, Shakespeare WC, Zhu X, Eide CA, Rivera VM, Wang F, et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer cell 2009;16(5):401–12 doi 10.1016/j.ccr.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cotto-Rios XM, Agianian B, Gitego N, Zacharioudakis E, Giricz O, Wu Y, et al. Inhibitors of BRAF dimers using an allosteric site. Nature communications 2020;11(1):4370 doi 10.1038/s41467-020-18123-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nichols RJ, Haderk F, Stahlhut C, Schulze CJ, Hemmati G, Wildes D, et al. RAS nucleotide cycling underlies the SHP2 phosphatase dependence of mutant BRAF-, NF1- and RAS-driven cancers. Nat Cell Biol 2018;20(9):1064–73 doi 10.1038/s41556-018-0169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kemper K, Krijgsman O, Kong X, Cornelissen-Steijger P, Shahrabi A, Weeber F, et al. BRAF(V600E) Kinase Domain Duplication Identified in Therapy-Refractory Melanoma Patient-Derived Xenografts. Cell Rep 2016;16(1):263–77 doi 10.1016/j.celrep.2016.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kondo Y, Ognjenovic J, Banerjee S, Karandur D, Merk A, Kulhanek K, et al. Cryo-EM structure of a dimeric B-Raf:14–3-3 complex reveals asymmetry in the active sites of B-Raf kinases. Science 2019;366(6461):109–15 doi 10.1126/science.aay0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park E, Rawson S, Li K, Kim BW, Ficarro SB, Pino GG, et al. Architecture of autoinhibited and active BRAF-MEK1–14-3–3 complexes. Nature 2019;575(7783):545–50 doi 10.1038/s41586-019-1660-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vido MJ, Le K, Hartsough EJ, Aplin AE. BRAF Splice Variant Resistance to RAF Inhibitor Requires Enhanced MEK Association. Cell Rep 2018;25(6):1501–10 e3 doi 10.1016/j.celrep.2018.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang C, Spevak W, Zhang Y, Burton EA, Ma Y, Habets G, et al. RAF inhibitors that evade paradoxical MAPK pathway activation. Nature 2015;526(7574):583–6 doi 10.1038/nature14982. [DOI] [PubMed] [Google Scholar]
- 38.Okaniwa M, Hirose M, Arita T, Yabuki M, Nakamura A, Takagi T, et al. Discovery of a selective kinase inhibitor (TAK-632) targeting pan-RAF inhibition: design, synthesis, and biological evaluation of C-7-substituted 1,3-benzothiazole derivatives. Journal of medicinal chemistry 2013;56(16):6478–94 doi 10.1021/jm400778d. [DOI] [PubMed] [Google Scholar]
- 39.Lito P, Saborowski A, Yue J, Solomon M, Joseph E, Gadal S, et al. Disruption of CRAF-Mediated MEK Activation Is Required for Effective MEK Inhibition in KRAS Mutant Tumors. Cancer cell 2014. doi 10.1016/j.ccr.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Samatar AA, Poulikakos PI. Targeting RAS-ERK signalling in cancer: promises and challenges. Nature reviews Drug discovery 2014;13(12):928–42 doi 10.1038/nrd4281. [DOI] [PubMed] [Google Scholar]
- 41.Rahman R, Ung PM, Schlessinger A. KinaMetrix: a web resource to investigate kinase conformations and inhibitor space. Nucleic Acids Res 2019;47(D1):D361–D6 doi 10.1093/nar/gky916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dong Q, Dougan DR, Gong X, Halkowycz P, Jin B, Kanouni T, et al. Discovery of TAK-733, a potent and selective MEK allosteric site inhibitor for the treatment of cancer. Bioorganic & medicinal chemistry letters 2011;21(5):1315–9 doi 10.1016/j.bmcl.2011.01.071. [DOI] [PubMed] [Google Scholar]
- 43.Hugo W, Shi H, Sun L, Piva M, Song C, Kong X, et al. Non-genomic and Immune Evolution of Melanoma Acquiring MAPKi Resistance. Cell 2015;162(6):1271–85 doi 10.1016/j.cell.2015.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Agudo J, Ruzo A, Park ES, Sweeney R, Kana V, Wu M, et al. GFP-specific CD8 T cells enable targeted cell depletion and visualization of T-cell interactions. Nature biotechnology 2015;33(12):1287–92 doi 10.1038/nbt.3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wroblewska A, Dhainaut M, Ben-Zvi B, Rose SA, Park ES, Amir ED, et al. Protein Barcodes Enable High-Dimensional Single-Cell CRISPR Screens. Cell 2018;175(4):1141–55 e16 doi 10.1016/j.cell.2018.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krepler C, Sproesser K, Brafford P, Beqiri M, Garman B, Xiao M, et al. A Comprehensive Patient-Derived Xenograft Collection Representing the Heterogeneity of Melanoma. Cell Rep 2017;21(7):1953–67 doi 10.1016/j.celrep.2017.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. The New England journal of medicine 2014;371(20):1877–88 doi 10.1056/NEJMoa1406037. [DOI] [PubMed] [Google Scholar]
- 48.Bekaii-Saab TS, Ou FS, Ahn DH, Boland PM, Ciombor KK, Heying EN, et al. Regorafenib dose-optimisation in patients with refractory metastatic colorectal cancer (ReDOS): a randomised, multicentre, open-label, phase 2 study. The lancet oncology 2019;20(8):1070–82 doi 10.1016/S1470-2045(19)30272-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim PN, Cho H, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature 2010;467(7315):596–9 doi 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dardaei L, Wang HQ, Singh M, Fordjour P, Shaw KX, Yoda S, et al. SHP2 inhibition restores sensitivity in ALK-rearranged non-small-cell lung cancer resistant to ALK inhibitors. Nature medicine 2018;24(4):512–7 doi 10.1038/nm.4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun C, Bernards R. Feedback and redundancy in receptor tyrosine kinase signaling: relevance to cancer therapies. Trends Biochem Sci 2014;39(10):465–74 doi 10.1016/j.tibs.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 52.Prahallad A, Sun C, Huang S, Di Nicolantonio F, Salazar R, Zecchin D, et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature 2012;483(7387):100–3 doi 10.1038/nature10868. [DOI] [PubMed] [Google Scholar]
- 53.Prahallad A, Heynen GJ, Germano G, Willems SM, Evers B, Vecchione L, et al. PTPN11 Is a Central Node in Intrinsic and Acquired Resistance to Targeted Cancer Drugs. Cell Rep 2015;12(12):1978–85 doi 10.1016/j.celrep.2015.08.037. [DOI] [PubMed] [Google Scholar]
- 54.Ahmed TA, Adamopoulos C, Karoulia Z, Wu X, Sachidanandam R, Aaronson SA, et al. SHP2 Drives Adaptive Resistance to ERK Signaling Inhibition in Molecularly Defined Subsets of ERK-Dependent Tumors. Cell Rep 2019;26(1):65–78 e5 doi 10.1016/j.celrep.2018.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haling JR, Sudhamsu J, Yen I, Sideris S, Sandoval W, Phung W, et al. Structure of the BRAF-MEK complex reveals a kinase activity independent role for BRAF in MAPK signaling. Cancer cell 2014;26(3):402–13 doi 10.1016/j.ccr.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 56.Kopetz S, Grothey A, Yaeger R, Van Cutsem E, Desai J, Yoshino T, et al. Encorafenib, Binimetinib, and Cetuximab in BRAF V600E-Mutated Colorectal Cancer. The New England journal of medicine 2019;381(17):1632–43 doi 10.1056/NEJMoa1908075. [DOI] [PubMed] [Google Scholar]
- 57.Corcoran RB, Atreya CE, Falchook GS, Kwak EL, Ryan DP, Bendell JC, et al. Combined BRAF and MEK Inhibition With Dabrafenib and Trametinib in BRAF V600-Mutant Colorectal Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2015;33(34):4023–31 doi 10.1200/JCO.2015.63.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381(9863):303–12 doi 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 59.Kopetz S, Guthrie KA, Morris VK, Lenz HJ, Magliocco AM, Maru D, et al. Randomized Trial of Irinotecan and Cetuximab With or Without Vemurafenib in BRAF-Mutant Metastatic Colorectal Cancer (SWOG S1406). Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2020:JCO2001994 doi 10.1200/JCO.20.01994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Corcoran RB, Andre T, Atreya CE, Schellens JHM, Yoshino T, Bendell JC, et al. Combined BRAF, EGFR, and MEK Inhibition in Patients with BRAF(V600E)-Mutant Colorectal Cancer. Cancer discovery 2018;8(4):428–43 doi 10.1158/2159-8290.CD-17-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Davis MI, Hunt JP, Herrgard S, Ciceri P, Wodicka LM, Pallares G, et al. Comprehensive analysis of kinase inhibitor selectivity. Nature biotechnology 2011;29(11):1046–51 doi 10.1038/nbt.1990. [DOI] [PubMed] [Google Scholar]
- 62.Harder E, Damm W, Maple J, Wu C, Reboul M, Xiang JY, et al. OPLS3: A Force Field Providing Broad Coverage of Drug-like Small Molecules and Proteins. J Chem Theory Comput 2016;12(1):281–96 doi 10.1021/acs.jctc.5b00864. [DOI] [PubMed] [Google Scholar]
- 63.Lindorff-Larsen K, Piana S, Palmo K, Maragakis P, Klepeis JL, Dror RO, et al. Improved side-chain torsion potentials for the Amber ff99SB protein force field. Proteins 2010;78(8):1950–8 doi 10.1002/prot.22711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hornak V, Abel R, Okur A, Strockbine B, Roitberg A, Simmerling C. Comparison of multiple Amber force fields and development of improved protein backbone parameters. Proteins 2006;65(3):712–25 doi 10.1002/prot.21123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang J, Wolf RM, Caldwell JW, Kollman PA, Case DA. Development and testing of a general amber force field. J Comput Chem 2004;25(9):1157–74 doi 10.1002/jcc.20035. [DOI] [PubMed] [Google Scholar]
- 66.Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML. Comparison of simple potential functions for simulating liquid water. The Journal of Chemical Physics 1983;79(2):926–35 doi 10.1063/1.445869. [DOI] [Google Scholar]
- 67.Bowers KJ, Chow E, Xu H, Dror RO, Eastwood MP, Gregersen BA, et al. Scalable algorithms for molecular dynamics simulations on commodity clusters. 2006; New York, New York, USA. ACM Press. p 84-. [Google Scholar]
- 68.Shaw DE, Grossman JP, Bank JA, Batson B, Butts JA, Chao JC, et al. Anton 2: Raising the Bar for Performance and Programmability in a Special-Purpose Molecular Dynamics Supercomputer. 2014/01//. IEEE Computer Society. p 41–53. [Google Scholar]
- 69.Martyna GJ, Tobias DJ, Klein ML. Constant pressure molecular dynamics algorithms. The Journal of Chemical Physics 1994;101(5):4177–89 doi 10.1063/1.467468. [DOI] [Google Scholar]
- 70.Predescu C, Lippert RA, Eastwood MP, Ierardi D, Xu H, Jensen MØ, et al. Computationally efficient molecular dynamics integrators with improved sampling accuracy. Molecular Physics 2012;110(9–10):967–83 doi 10.1080/00268976.2012.681311. [DOI] [Google Scholar]
- 71.Tuckerman M, Berne BJ, Martyna GJ. Reversible multiple time scale molecular dynamics. The Journal of Chemical Physics 1992;97(3):1990–2001 doi 10.1063/1.463137. [DOI] [Google Scholar]
- 72.Predescu C, Lerer AK, Lippert RA, Towles B, Grossman JP, Dirks RM, et al. The u -series: A separable decomposition for electrostatics computation with improved accuracy. Journal of Chemical Physics 2020;152(8) doi 10.1063/1.5129393. [DOI] [PubMed] [Google Scholar]
- 73.Liau NPD, Wendorff TJ, Quinn JG, Steffek M, Phung W, Liu P, et al. Negative regulation of RAF kinase activity by ATP is overcome by 14–3-3-induced dimerization. Nature structural & molecular biology 2020;27(2):134–41 doi 10.1038/s41594-019-0365-0. [DOI] [PubMed] [Google Scholar]
- 74.Jacobson MP, Pincus DL, Rapp CS, Day TJ, Honig B, Shaw DE, et al. A hierarchical approach to all-atom protein loop prediction. Proteins 2004;55(2):351–67 doi 10.1002/prot.10613. [DOI] [PubMed] [Google Scholar]
- 75.Jacobson MP, Friesner RA, Xiang Z, Honig B. On the role of the crystal environment in determining protein side-chain conformations. J Mol Biol 2002;320(3):597–608 doi 10.1016/s0022-2836(02)00470-9. [DOI] [PubMed] [Google Scholar]
- 76.Krissinel E, Henrick K. Inference of macromolecular assemblies from crystalline state. J Mol Biol 2007;372(3):774–97 doi 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 77.Krepler C, Xiao M, Sproesser K, Brafford PA, Shannan B, Beqiri M, et al. Personalized Preclinical Trials in BRAF Inhibitor-Resistant Patient-Derived Xenograft Models Identify Second-Line Combination Therapies. Clinical cancer research : an official journal of the American Association for Cancer Research 2016;22(7):1592–602 doi 10.1158/1078-0432.CCR-15-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.