Abstract
Antibiotic-induced microbial imbalance, or dysbiosis, has systemic and long-lasting effects on the host and response to cancer therapies. However, the effects on tumor endothelial cells are largely unknown. Therefore, the goal of the current study was to generate matched B16-F10 melanoma associated endothelial cell lines isolated from mice with and without antibiotic-induced dysbiosis. After validating endothelial cell markers on a genomic and proteomic level, functional angiogenesis assays (i.e., migration and tube formation) also confirmed their vasculature origin. Subsequently, we found that tumor endothelial cells derived from dysbiotic mice (TEC-Dys) were more sensitive to ionizing radiotherapy in the range of clinically-relevant hypofractionated doses, as compared to tumor endothelial cells derived from orthobiotic mice (TEC-Ortho). In order to identify tumor vasculature-associated drug targets during dysbiosis, we used tandem mass tag mass spectroscopy and focused on the statistically significant cellular membrane proteins overexpressed in TEC-Dys. By these criteria c-Met was the most differentially expressed protein, which was validated histologically by comparing tumors with or without dysbiosis. Moreover, in vitro, c-Met inhibitors Foretinib, Crizotinib and Cabozantinib were significantly more effective against TEC-Dys than TEC-Ortho. In vivo, Foretinib inhibited tumor growth to a greater extent during dysbiosis as compared to orthobiotic conditions. Thus, we surmise that tumor response in dysbiotic patients may be greatly improved by targeting dysbiosis-induced pathways, such as c-Met, distinct from the many targets suppressed due to dysbiosis.
Keywords: dysbiosis, tumor microenvironment, endothelial cells, hypofractionated radiation, tandem mass tag mass spectroscopy, c-MET inhibitors, Foretinib
Introduction
Commensal bacteria reside in many parts of the human body, including the skin, airways, and gastro-intestinal tract (GI tract). It is now increasingly recognized that microbiota play diverse and crucial roles in the homeostasis of various processes. Microbial imbalance, or dysbiosis, causes cellular developmental changes, thereby increasing the risk of acquiring various diseases and accelerating their pathologies [1]. A common, significant influence on the microbiota is antibiotics (ABX). Antibiotics not only destroy pathogenic bacteria, but also affect beneficial commensal bacteria. This in turn induces long lasting consequences for the composition of the microbiome and thus the host. Pioneering epidemiological studies showed a direct correlation between antibiotic use and carcinogenesis [2], and tumor mouse models have subsequently validated this mechanistically [3,4]. Unfortunately, the overall use of antibiotics has increased by more than 30% in recent years according to the Centers of Disease Control and Prevention (CDC) [5]. Moreover, many cancer patients are prescribed antibiotics, as a prophylactic or to treat infection, a frequent complication during cancer progression and treatment.
We recently showed that antibiotic-induced dysbiosis suppresses stromal immune surveillance resulting in accelerated tumor progression in murine melanoma and lung carcinoma models [6]. It is increasingly recognized that the microbiome is integral in cancer susceptibility and progression both of which are, in part, mediated through changes in the tumor vasculature [7-10]. Thus, we and others hypothesized that this might also have therapeutic consequences. For instance, Zitvogel and colleagues have shown that patients with colon cancer only minimally respond to cyclophosphamide during dysbiosis [11]. Others have shown that the response to several immunotherapies is influenced by the bacterial abundance, composition and diversity of gut microbiome in patients [12]. In contrast, we wondered whether dysbiosis causes a differential sensibility of the tumor vasculature to cancer therapies, and if so, what strategies can be used to improve cancer treatment responses during dysbiosis.
Historically, cancer and cancer-associated stromal cell lines have had a very important role in the discovery and development of new cancer drugs. By using them in high-throughput cell-based profiling, several therapeutics were identified that have demonstrated therapeutic efficacy in the clinic [13], However, dysbiotic in vitro models for preclinical testing of cancer treatments are scarce, and tumor-derived endothelial cell lines derived under dysbiotic stress absent. To fulfill this need, we isolated matched endothelial cell lines derived from B16-F10 melanoma with and without antibiotic-induced dysbiotic pressure. After isolation and successful culturing, the endothelial lineage was confirmed by genomic and proteomic analysis of endothelial cell markers. Subsequently, morphological and functional read-outs, such as migration and tube formation assays also validated their vascular nature. Additionally, we tested the newly generated cell lines for their response to radiation using the clonogenic assay and we found that tumor endothelial cells derived during dysbiosis (TEC-Dys) were more sensitive to a hypofractionation-relevant dose (i.e. 6 Gy) as compared to endothelial cells derived during orthobiosis (TEC-Ortho). No statistical differences were seen for conventional 2 Gy radiation treatment regimens. Subsequently, tandem mass tag mass spectroscopy was used to identify possible tumor vasculature-associated drug targets during dysbiosis. We found that TEC-Dys had elevated c-Met expression and were indeed more sensitive to c-Met inhibitors Foretinib, Crizotinib and Cabozantinib than TEC-Ortho.
These matched tumor endothelial cell lines might have prognostic value as an initial screen for current and next generation anti-cancer treatment modalities taking into account dysbiosis induced changes of the tumor vasculature. Overall, a greater level of understanding of the changes in the distal tumor microenvironment due to a bacterial imbalance in the patient’s microbiome has the potential to influence clinical decisionmaking in the future. In the current report, we discuss the possible anti-cancer strategies our results suggest should be considered when treating dysbiotic patients with solid tumors.
Materials and Methods
Cell Lines and cell culture
B16-F10 (murine melanoma cell line; CRL-6475) and 2H11 (CRL-2163) were newly purchased at the start of the project from American Type Culture Collection (ATCC) and cultured according to the company’s instructions. All cell lines were cultured and maintained as previously described [14,15].
Mice and tumor mouse model
Mice (C57BL/6J; #0664) were purchased from Jackson Laboratory and allowed to acclimatize to local conditions for at least 1 week. Animals were provided water and standard chow ad libitum and were maintained on a 12-hour light/dark cycle. For tumor cell inoculation, a 100 μL solution of 2 x 105 of B16-F10 was deposited s.c. in the shaven right rear leg of the mice, as described previously [16], Dysbiosis was induced 2 weeks prior to the tumor inoculations by administering a cocktail of broad-spectrum antibiotics, i.e. ampicillin (250 mg/L), vancomycin (125 mg/L), neomycin (250 g/L), and metronidazole (250 mg/L) in their drinking water [17]. This was continued during tumor development. In the study involving Foretinib treatment (25 mg/kg; daily i.p.), mice were randomized once the mean size of tumors reached 75 mm3 (day 7), assuring treatment initiation in same size tumors. Control groups were given vehicle (2.5% DMSO, 0.5% methylcellulose in PBS). Tumor volume was determined by measuring the diameters of tumors with calipers and calculated by the equation: (a2 x b x π)/6, where a is the short axis and b is the long axis of the tumor. After euthanizing, tumors were excised and processed. Experiments were approved by the University of Arkansas for Medical Sciences institutional animal care and use committee (Protocol #3836).
Isolation and immortalization of tumoral ECs
Tumor ECs (TECs) were isolated as described earlier [18], albeit with some modifications. Namely, B16-F10 tumor cells were implanted in mice with or without orally administered antibiotics for 14 days (Fig. 1a). After 10 days same size tumors, approximately 750 mm3 were excised under sterile conditions. Subsequently they were mechanically dissociated with shears until pieces were < 1 mm3. This was followed by enzymatic dissociation with 1 mg ml−1 collagenase (Invitrogen #17101-015), 2.5 U ml−1 dispase (Invitrogen 17105-041) and 1 mg ml−1 DNaseI (Sigma #D-4527) for 30 mins with continuous agitation by a MACSmix tube rotator in a 37°C incubator. Subsequently the tissue suspensions were put on ice and cold EC medium was added. The samples were first sieved through a 70-μm cell strainer (BD Falcon #352350) fitted on a 50-ml tube on ice to remove undigested cell clumps and separate the single cells. This was then repeated through a 40-μm cell strainer (BD Falcon #352340). After lysing the red blood cells with lysis buffer (ACS #A1049201), cells were washed and collected by centrifugation at 300g for 5 min at 4°C.
Fig. 1. Generating tumor endothelial cell lines with and without dysbiotic stress.
(a) A timeline of the subsequent steps of generating tumor-derived endothelial cell lines. Week 0: Dysbiosis is initiated by administering a cocktail of broad-spectrum antibiotics, i.e. ampicillin (250 mg/L), neomycin (250 g/L), metronidazole (250 mg/L), and vancomycin (125 mg/L) in the drinking water of C57BL/6J. Week 2: 2 x 105 B16-F10 melanoma cells were injected s.c. in the right rear leg of the mice. Antibiotics were continued during tumor development. Week 4: same size tumors were excised and dissociated by mechanical shear and enzymatically digested to single-cell suspensions. Endothelial-specific magnetic beads targeting CD31 were added to the single-cell suspension to positive select and purify EC. Week 6: A second positive selection with CD31 magnetic beads was performed to select and purify EC. Week 8: TERT-GFP-puro plasmid is introduced. Week 12: EC positive for GFP and CD31 were sorted by FACS. Week 20: validation by endothelial cell specific molecular markers and angiogenic functional assays. (b) Representative brightfield image of TEC-Dys showing cobblestone morphology, stalk-forming alignment and filopodia-forming morphology. (c) Representative fluorescence image of TEC-Ortho and TEC-Dys cells, 7 days after puromycin selection. (d) Verification of TEC-Ortho and TEC-Dys immortalization by GFP expression. Scale bars = 20 μM.
Tumor endothelial cells were subsequently isolated by positive selection using CD31 magnetic microbeads (Miltenyi Biotec #130-097-418) according to the manufacturer’s instruction, and cultured in endothelial cell media (Lonza EGM-2 Bulletkit #CC-3162). After 2 weeks the microbeads-based CD31 positive selection was repeated and cells were subsequently cultured for another two weeks before immortalization.
The isolated endothelial cells were then immortalized by GFP-containing lentiviral transfection of telomerase reserve transcriptase (GenTarget #LVP1130-GP). Cells were selected by culturing them in endothelial media supplemented with puromycin for four weeks and subsequently sorted by flow cytomery (BD Aria) for CD31 and GFP double positive cells. The sorted cells were seeded as single cells in a 96-well plate and single colonies were selected for validation and characterization. All genomic, proteomic and functional assays were performed on cells < 10 passage number. The cells were tested up to passage 30 and maintained their viability and doubling capacity confirming their immortality.
Real-time quantitative reverse transcriptase PCR
Total RNA isolation, cDNA synthesis and real-time quantitative reverse transcriptase PCR (qRT-PCR) were carried out as described previously using SYBR Green PCR master mix (Applied Biosystems #43-091-55) [19]. The expression of each target gene was normalized to the expression of the control gene β-actin. Species-specific primers can be found in Supplementary Table S1.
Flow cytometry
In order to quantify cellular membrane protein expression on viable cells we employed fluorescence-activated cell sorting (FACS) analysis. Anti-mouse antibodies PECAM (CD31; clone MEC13.3), MART-1 (MelanA; clone A103), and GP100 (clone HMB45) were used and the analysis was based on pre-gating on single cells and fixable viability dye (FVD; eBioscience) negative cells. Positivity for the marker of interest was determined by using the fluorescence minus one (FMO) strategy. Samples were acquired by multiparameter flow cytometry on a LSR II flow cytometer (BD Biosciences) and analyzed by Flowjo software (Tree Star, Inc.) [15,20].
Migration
The migration or wound healing assay was performed as described earlier, although with some modifications [21,22]. Cells were grown overnight until confluence. When confluent, a wound was made in the well, using a blunt sterile plastic pipette tip. The well was carefully washed with phosphate buffered saline (PBS) and the medium was replaced with medium containing 0.5% FBS. The wound width was measured at four different predefined places. Photographs were made using an inverted photomicroscope (Olympus IX71).
Tube formation
The Matrigel tube formation was performed and analyzed as previously described, with minor modifications [21-23]. Namely, cells were added onto μ-slides Angiogenesis (Ibidi) and incubated at 37°C in 5% CO2 in medium containing 0.5% FBS. Endothelial cell tube formation was assessed with an inverted photomicroscope (Olympus IX71) and experiments were conducted in triplicates and repeated at least three times.
Clonogenic assay
Endothelial cells were cultured in DMEM with 10% fetal bovine serum. Cells in exponential growth phase were trypsinized, washed, counted, and seeded into 6-well plates in triplicate for all conditions. The plates were incubated overnight at 37 °C, and then irradiated at the indicated doses (vide infra), and incubated with fresh medium for 7 to 10 days in a 5% CO2 /95% air, 37 °C incubator. The resulting colonies were stained with crystal violet with methanol/acetic acid (10:1) and counted by hand, as described earlier [16]. Experiments were conducted in triplicates and repeated at least three times.
Irradiation
Irradiation was carried out in a CP 160 X-ray system (Faxitron X-ray Corporation Tucson, AZ, USA). The instrument may be operated at different peak kilovoltages, shelf height (solid state drives (SSD)), filtration thickness, and electrically operated turntable to ensure uniform dosing. Here, for all experiments shelf 6 (SSD 0 43.2 cm covering, 39 cm diameter field, and 0.8 mm Be/0.5 mm Cu filtration) was used with 150 kVp and 6 mA beam. Dosimetry was carried out using a pinpoint ion chamber (PTW N301013, ADCL calibrated for 225 kV) following the AAPM TG-61 protocol. Doses of 2 Gy and 6 Gy were applied at a radiation dose of 1.018 ± 0.10 Gy/min at 150 kV and 6.6 mA.
Proteomics
As we have described previously, purified proteins were reduced, alkylated, and digested using filter-aided sample preparation [24]. Tryptic peptides were labeled using a tandem mass tag 10-plex isobaric label reagent set (ThermoFisher #90309) following the manufacturer's instructions. Labeled peptides were separated into 36 fractions on a 100 × 1.0 mm Acquity BEH C18 column (Waters) using an UltiMate 3000 UHPLC system (ThermoFisher) with a 40-minute gradient from 99:1 to 60:40 buffer A:B ratio under basic pH conditions, and then consolidated into 12 nonadjacent superfractions (Buffer A = H2O with 10 mM ammonium hydroxide; Buffer B = acetonitrile with 10 mM ammonium hydroxide). Each superfraction was then further separated by reverse phase XSelect CSH C18 2.5 μm resin (Waters) on an in-line 120 × 0.075 mm column using an UltiMate 3000 RSLCnano system (ThermoFisher). Peptides were eluted using a 60-minute gradient from 98:2 to 67:33 buffer A:B ratio. Eluted peptides were ionized by electrospray (2.15 kV), followed by mass spectrometric analysis on an Orbitrap Fusion Lumos mass spectrometer (ThermoFisher) using MultiNotch MS3 parameters. MS data were acquired using the Fourier Transform Mass Spectrometry analyzer in top-speed (2.5-second cycle) mode at a resolution of 120,000 over a range of 375 to 1,500 m/z. Following collision-induced dissociation activation with normalized collision energy of 35.0, MS/MS data were acquired using the ion trap analyzer in centroid mode and normal mass range. Using synchronous precursor selection, up to 10 MS/MS precursors were selected for HCD activation with normalized collision energy of 65.0, followed by acquisition of MS3 reporter ion data using the Fourier Transform Mass Spectrometry analyzer in profile mode at a resolution of 50,000 over a range of 100 to 500 m/z.
Proteomics data analysis
A total of 5,409 proteins were identified, and TMT-10plex reporter ions quantified by searching a custom UniprotKB database [Mus musculus] using MaxQuant (version 1.6.2.10, Max Planck Institute) with a parent ion tolerance of 3 ppm, a fragment ion tolerance of 0.5 Da, and a reporter ion tolerance of 0.001 Da. Modifications searched included fixed modifications of carbamidomethyl on cysteine and TMT-10plex on lysine and N-terminal; as well as variable modifications of oxidation on methionine and acetylation on the N-terminal peptide. We first searched a contaminants database (262 entries) to identify common contaminating proteins followed by the main search. Protein identifications were accepted if they could be established with less than 1.0% false discovery and contained at least 2 identified peptides. Protein probabilities were assigned by the Protein Prophet algorithm.
The tandem mass tag (TMT) samples consisted of five replicates of each cell line from 2 separate experiments (n=3 and n=2), both passages lower than 10. In order to identify the significantly differentiating proteins between TEC-Ortho and TEC-Dys, the TMT reporter ion intensity values were first normalized and statistical analysis performed. Protein TMT MS3 reporter ion intensity values were assessed for quality using our in-house ProteiNorm app, a user-friendly tool for a systematic evaluation of normalization methods, imputation of missing values and comparisons of different differential abundance methods [25]. Popular normalization methods were evaluated including log2 normalization (Log2), median normalization (Median), mean normalization (Mean), variance stabilizing normalization (VSN),[26] quantile normalization (Quantile) [27], cyclic loess normalization (Cyclic Loess) [28], global robust linear regression normalization (RLR),[29] and global intensity normalization (Global Intensity) [29]. The individual performance of each method was evaluated by comparing of the following metrices: total intensity, pooled intragroup coefficient of variation (PCV), pooled intragroup median absolute deviation (PMAD), pooled intragroup estimate of variance (PEV), intragroup correlation, sample correlation heatmap (Pearson), and log2-ratio distributions. The data was normalized using VSN since it had the smallest variance and highest correlation. The log2 VSN normalized MS3 reporter ion intensities were used to perform statistical analysis using linear models for microarray Data (Limma) with empirical Bayes (eBayes) smoothing to the standard errors [28]. Fold changes were calculated by dividing TEC-Dys from TEC-Ortho expressing samples. Proteins with an adjusted P value < 0.05 and a fold change > 2 were considered to be significant. P values were adjusted using the Benjamini-Hochberg (false discovery rate; FDR) procedure. Proteins deemed significant were superimposed on the Kyoto encyclopedia of genes and genomes (KEGG) pathways relevant to endothelial cell signaling.
Heatmap
A clustered heatmap was generated using the ComplexHeatmap Bioconductor package [30]. The log2 normalized protein intensities of the significant proteins was scaled using z-score and the samples and proteins were clustered using the Euclidean distance metric and the complete method.
Immunofluorescence
Similar size tumors were embedded in tissue freezing medium (Miles, Inc.), snap frozen in liquid nitrogen, and cut into 5 μm sections. Preparation and procedures, including morphometric analysis, for the frozen tumor sections were done as described earlier [22]. Briefly, sections were incubated in a 1:50 dilution with phycoerythrin-conjugated monoclonal antibody to mouse CD31 (clone MEC13.3) or a FITC-conjugated monoclonal c-Met antibody (eBioclone 7; eBioscience) to stain for microvessel density and c-MET, respectively.
Cell Viability Assay
The cell viability or drug cytotoxicity was determined using the CCK-8 assay (3-[4,5- dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide; Dojindo, Japan). The water-soluble tetrazolium salt WST-8 is reduced by dehydrogenase activities in cells to give a yellow-color formazan dye, which is soluble in the tissue culture media. The amount of the formazan dye, generated by the activities of dehydrogenases in cells, is directly proportional to the number of living cells. In brief, cells were seeded in 96-well tissue culture plates at a density of 2 × 103 cells/well. After 72 h drug exposure, cells were incubated with CCK-8 for 2 h at 37 °C. After incubation, the absorbance was measured at 450 nm by use of a microplate reader (Biotek Epoch). Wells with untreated cells or with drug-containing medium without cells were used as positive and negative controls, respectively. All drugs were purchased form SelleckChem (Foretinib #S1111; Crizotinib #S1068; Cabozantinib #S1119; and JNJ-38877605 #S1114). The effective dose (ED50) was determined as half the maximal inhibitory drug concentration based on non-linear, sigmoidal curve fitting using Prism 6.0 (Graphpad software) [22,31]. All measurements were performed in triplicate, and the experiments were conducted at least three times.
Statistical Analysis
Data are reported as mean +/− SEM unless otherwise stated and were analyzed by either an unpaired two-tailed t-test or a one-way analysis of variance (ANOVA) with a Tukey’s post hoc test for multiple comparisons using Prism 6.0, as indicated. P values <0.05 were considered statistically significant.
Results
Isolation and immortalization of tumoral ECs
In this study, we generated B16-F10 melanoma derived endothelial cell lines with and without antibiotic-induced dysbiosis in order to identify possible differential sensitivity against anti-cancer treatments. B16-F10 tumor cells were implanted in C57BL/6J mice pretreated with or without antibiotics for 14 days (Fig. 1a). After 10 days the tumors were excised and mechanically and enzymatically dissociated. The TECs were subsequently isolated by positive selection using endothelial cell marker CD31 magnetic microbeads. Endothelial cells derived from either control or ‘orthobiotic’ mice (TEC-Ortho) as well as from dysbiotic mice (TEC-Dys) showed typical endothelial cell phenotypes: early passages and low seeding showed cobblestone type appearance as well as stalk formation. After 6-10 days colony formation was noticeable with filopodia style structures (Fig. 1b). Subsequently the cells were immortalized by GFP-containing lentiviral transfection of telomerase reverse transcriptase. Microscopic immunofluorescence and flow cytometry verified the immortalization by GFP expression (Fig. 1c and d). GFP+ positive cells were sorted out and single colonies were generated and selected for further validation and characterization.
Characterization of endothelial cells under dysbiotic stress
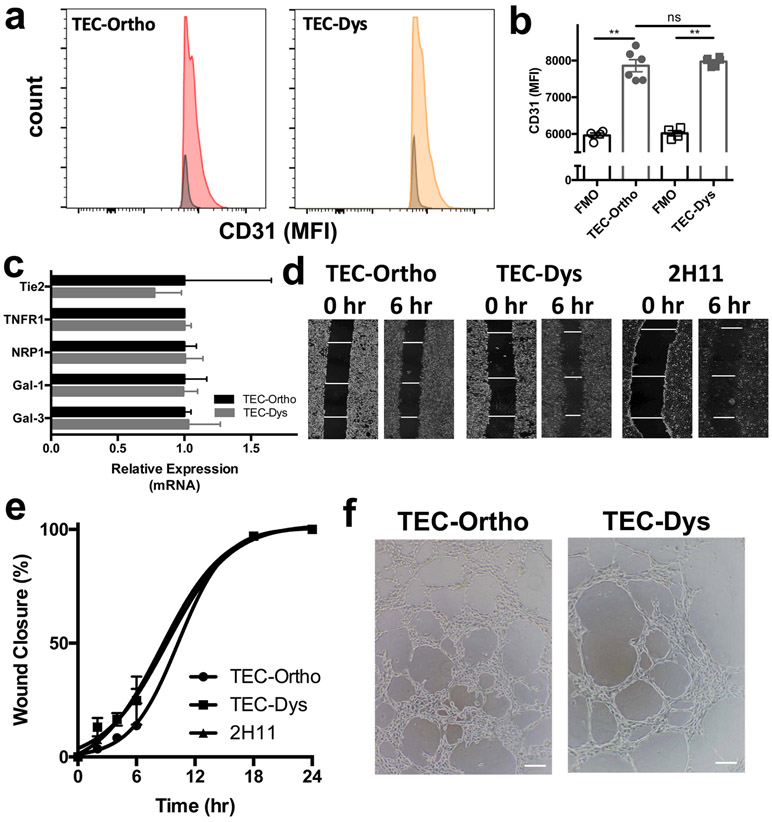
After we confirmed that the GFP+ cells expressed CD31 (Fig. 2a and b), we confirmed their endothelial cell origin further on a genomic level. Using common EC markers, we verified that the cells expressed receptor tyrosine kinase Tie2, TNF-R1, Neuropilin-1 (NRP-1), and galectin-1 (Gal-1) and galectin-3 (Gal-3). The relative expression level of these common EC markers did not significantly differ between TEC-Ortho and TEC-Dys (Fig. 2c and Supplemental Table 1 and Fig. 1). Similar to endothelial cell line 2H11, TEC-Ortho and TEC-Dys did not express melanoma markers GP100 or MART-1 (Supplemental Fig. 2).
Fig. 2. Validating endothelial cell genomics, proteomics and morphology of TEC-Ortho and TEC-Dys.
(a) Representative histogram indicating TEC-Ortho and TEC-Dys express endothelial cell marker CD31. (b) Quantification of CD31 on TEC-Ortho and TEC-Dys by flow cytometry. (c) Genomic validation of endothelial markers Tie-2, TNFR1, NRP1, Gal-1 and Gal-3 in TEC-Ortho and TEC-Dys cells by qRT-PCR analysis. (d) Representative pictures of wound closure for TEC-Ortho, TEC-Dys and 2H11 in time. (e) Quantification of wound closure on TEC-Ortho and TEC-Dys. (f) Representative pictures of TEC-Ortho and TEC-Dys displaying the ability to form vascular-like tubular structures.
Data presented as mean ± SEM. **P < 0.01 two-tailed t-test. ns = not significant. MFI = mean fluorescence intensity; FMO = fluorescence minus one. Magnification is 4x in the panel in (d) and 10x for the panel in (f).
Next, we validated their vasculature nature by functional angiogenesis assays. Namely, the migration or wound healing assay as a measurement of the cell natural ability to migrate and reestablish a closed monolayer, as well as the tube formation to observe their capability of forming three dimensional vasculature-like structures. We found that TEC-Ortho and TEC-Dys, as well as classical endothelial cell line 2H11, returned to a closed monolayer within 18 hr (Fig. 2d and e). TEC-Ortho had an average closure rate of 5.6% / hr and fully closed at 17.7 hr, compared to a 7.6% / hr closure rate for TEC-Dys resulting in a complete closure at 13.2 hr. Non-tumor derived endothelial cells 2H11 had an average closure rate of 7.5% / hr and closed at 13.4 hr. This was not a function of statistical significant differences in doubling time as TEC-Ortho had an average doubling time of 15.6 hr ± 4.3 hr, TEC-Dys 15.0 hr ± 3.9 hr and 2H11 17.5 hr ± 4.7 hr.
Growing TEC-Ortho and TEC-Dys on Matrigel matrix showed their predisposition and ability to form vasculature-like structures, one of the hallmarks of angiogenesis (Fig. 2f). Morphometric analysis revealed TEC-Dys have a propensity to generate more segments and filopodia as compared to TEC-Ortho (Supplemental Table 2). The antibiotics did not directly influence TEC-Ortho, TEC-Dys or 2H11 viability (Supplemental Fig. 3).
Clonogenic ability and ionizing radiation sensitivity
Next we investigated the ability of TEC-Ortho and TEC-Dys to form colonies and their sensitivity to radiation (Fig. 3). We determined that the seeding efficiency of TEC-Ortho, TEC-Dys, as well as 2H11 did not significantly differ and was approximately 40-45% on average (Fig. 3a and b). A single conventional radiation dose of 2 Gy showed comparable survival fractions for TEC-Ortho (89.0% ± 1.8%), TEC-Dys (84.8 % ± 4.7 %) and 2H11 (93.1 ± 3.2 %; Fig. 3c and e). However, a radiation dose in the hypofractionated range was more effective against TEC-Dys than TEC-Ortho. Namely, 6 Gy reduced the survival fraction of TEC-Dys significantly more to 25.4 % ± 2.2 %, whereas TEC-Ortho survival fraction was 38.6 % ± 2.5 % and 2H11 was 49.1% ± 2.3% (Fig. 3d, e and f). This increased sensitivity of TEC-Dys to ionizing radiation could not be explained by differential ROS production (Supplemental Fig. 4).
Fig. 3. TEC-Dys are more sensitive to high-dose radiation than TEC-Ortho.

(a) Representative example of colony formation of TEC-Ortho and TEC-Dys cells.
(b) The seeding efficiency and clonogenic ability of TEC-Ortho and TEC-Dys does not significantly differ from each other or 2H11 cells. (c) Representative example of surviving fraction of TEC-Ortho and TEC-Dys colonies after 2 Gy or (d) 6 Gy. (e) Quantification of survival fraction of TEC-Ortho, TEC-Dys and 2H11 after different doses of radiation. (f) TEC-Dys are more sensitive to 6 Gy then TEC-Ortho.
Data presented as mean ± SEM, pooled from 3 experiments with triplicates. *P < 0.01, ***P < 0.001 one-way ANOVA with a Tukey’s post hoc test for multiple comparisons. ns = not significant.
Increased c-Met expression in the tumor stroma of dysbiotic versus orthobiotic tumors.
After confirming the EC origin on a genomic, proteomic, and functional level, we wanted to elucidate the proteomic difference between TEC-Ortho and TEC-Dys in order to identify potential drug targets on tumor vasculature during dysbiotic stress. A total of 5,409 proteins were identified by TMT, of which 61 were differentially expressed, as statistically defined by a FDR-adjusted P-values < 0.05 and fold change > 2 and < −2 (Fig. 4). We identified 27 proteins differentially overexpressed in TEC-Dys and 34 relatively suppressed (Fig. 4a). Since our interest was to identify potential drug targets during dysbiosis, we focused on the proteins significantly overexpressed in TEC-Dys (Fig. 4b), in particular expressed on the cellular membrane. As determined by TMT and validated by flow cytometry, C-Met fulfilled these criteria and was consistently overexpressed in TEC-Dys samples, as compared to TEC-Ortho samples (Fig. 4c and d). Based on the mean dispersion of protein abundance we also confirmed that these statistically significantly differences were not a function of arbitrary outlier values (Supplemental Fig. 5).
Fig. 4. TMT-MS3 reveals dynamic changes in protein abundance with increased c-MET expression in the tumor stroma of dysbiotic versus orthobiotic tumors.
(a) Heatmap of identified proteins with differentially expressed in TEC-Ortho and TEC-Dys based on their z-scores. (b) Volcano plot of all proteins identified. X-axis is the log2 fold change. Y-axis is –log10 of FDR-adjusted P-value. FDR-adjusted P-values < 0.05 and fold change > 2 are highlighted in red and fold change < −2 are highlighted in blue.(c) Expression levels of c-Met in TEC-Ortho and TEC-Dys, as assessed by TMT-MS3. (d) Extracellular c-Met expression levels in viable TEC-Ortho and TEC-Dys, as assessed by flow cytometry. (e) Representative examples of c-MET (red) and vasculature (CD31, green) in orthobiotic and dysbiotic B16-F10 tumors. (f) Quantification of c-MET and (g) CD31+ tumor endothelial cells in orthobiotic and dysbiotic B16-F10 tumors.
Panels a-c, data pooled from 2 experiments with and presented as means ± SEM in panel C. **P < 0.01, ***P < 0.001 two-tailed t-test. TMT, tandem mass tagging; MS3, triple-stage mass spectrometry. Panels f and g, data presented as mean ± SEM, pooled from 3 tumors and 15 random fields. **P < 0.01, ***P < 0.001 two-tailed t-test. ns = not significant. Magnification is 10x for the panels in (e).
To validate that c-Met expression is differentially overexpressed during dysbiosis in vivo, we performed immunofluorescent microscopy. Whereas tumors in orthobiotic mice did not express a lot of c-Met overall, the c-Met expression in the tumor stroma of B16-F10 tumors during dysbiotic stress was significantly higher (Fig. 4e). Namely, c-Met expression was almost an order of magnitude higher during dysbiotic conditions as compared to under orthotopic conditions, 1376.6 ± 163.5 pixels vs. 158.3 ± 41.5 pixels (Fig. 4f). The increase in c-Met expression was not due to changes in the amount of microvasculature. The tumor microvasculature as determined by CD31 positive endothelial cells was similar under dysbiotic conditions as compared to orthotopic conditions (2638.8 ± 473.2 pixels vs. 2601.5 ± 495.6 pixels; Fig. 4g).
TEC-Dys are more sensitive to c-Met inhibitors than orthobiotic endothelial cells.
After confirming that c-Met is differentially upregulated on the vasculature of tumors under dysbiotic stress, as compared to orthotopic conditions, we wanted to test a variety of different c-Met inhibitors in their ability to preferentially inhibit TEC-Dys viability as compared to TEC-Ortho (Fig. 5). Indeed, dysbiotic endothelial cells were more sensitive to Foretinib, Crizotinib, and Cabozantinib than orthobiotic cells (Fig. 5a-c). In terms of the ED50, TEC-Dys cell viability was reduced by 50% by 0.4 μM Foretinib, whereas TEC-Ortho required 0.7 μM. Similarly, for Cabozantinib, the ED50 of TEC-Dys was 3.5 μM, whereas TEC-Ortho ED50 was 4.8 μM (Fig. 5d). Thus, the greatest sensitivity increase of TEC-Dys was seen for Foretinib, with a relative sensitivity increase or ratio of 1.8 (Fig. 5d). The c-Met inhibitor JNJ-38877605 was relatively ineffective as no ED50 was reached for either cell line at the highest dose tested of 10 μM (Supplemental Fig. 6). Since not all c-Met inhibitors are specific and Foretinib and JNJ-38877605 also bind vascular endothelial cell receptor 2 (VEGF-R2) a receptor tyrosine kinase (RTK), we analyzed possible changes in RTK signaling pathways. By superimposing the proteomic signature of TEC-Ortho and TEC-Dys on KEGG pathways maps, we identified only significant increases in RTK category, both of which c-Met and VEGF-R2 are members, and protein chaperone / transcription factor heat shock protein 27 (HSP27) within the mitogen activated protein kinase (MAPK) pathway in TEC-Dys as compared to TEC-Ortho (Supplemental Fig. 7 and 8). Downstream signaling of c-Met can also occur through phosphatidylinositol 3-kinase (PI3K) and AKT, yet not statistically significant changes were observed within this pathway (Supplemental Fig. 9)
Fig. 5. TEC-Dys are more sensitive to c-Met inhibitors than TEC-Ortho.

(a) TEC-Dys are more sensitive to Foretinib, (b) Crizotinib, and (c) Cabozantinib than TEC-Ortho. (d) Calculated ED50 based on non-linear, sigmoidal curve fitting. (e) Tumor growth inhibition is greater by Foretinib under dysbiotic conditions as compared to orthobiotic conditions. (f) Foretinib causes a greater reduction in CD31+ tumor endothelial cells, and (g) c-Met under dysbiotic conditions, as assessed by immunofluorescence.
Panels a-d, data presented as mean ± SEM, pooled from 3 experiments with triplicates. *P < 0.05 two-tailed t-test. Panel e, arrow indicates start of daily Foretinib treatment (25 mg/kg; i.p.; n=5 per group). Panels f and g, data presented as mean ± SEM, pooled from 4 tumors and at least 15 random fields. *P < 0.05, **P < 0.01 one-way ANOVA with a Tukey’s post hoc test for multiple comparisons. ns = not significant. FTB = Foretinib.
To validate that c-Met inhibition indeed had an increased effect during dysbiosis in vivo, we administered Foretinib to B16-F10 melanoma bearing mice with or without dysbiosis. Foretinib caused an approximately tumor growth inhibition of 35% ± 24% in orthobiotic mice. In dysbiotic mice, however, Foretinib caused an approximately tumor growth inhibition of 85% ± 10% (Fig. 5e). Tumor tissue analysis confirmed that dysbiotic conditions increased c-Met expression and that Foretinib caused greater inhibition of c-Met and tumor endothelial cells during dysbiosis, as compared to orthobiotic conditions (Fig. 5f and g)
Discussion
Our previous work showed that antibiotic-induced dysbiosis suppresses immune surveillance and accelerates tumor growth [6]. Here, we investigated whether antibiotic-induced dysbiosis causes a differential sensibility of the tumor vasculature to cancer treatments, with the overall intent to improve the success of cancer treatments during dysbiosis. This is in contrast to the various investigations currently ongoing, focusing on how dysbiosis decreases clinical success [11,12]. Thus, we generated matched tumor endothelial cell lines from tumors growing during orthobiotic and dysbiotic conditions. We found that the tumor vasculature generated under antibiotic-induced dysbiotic pressure were more sensitive to high and clinically-relevant ionizing radiation doses, as compared to the tumor vasculature during orthobiotic conditions. This is promising because novel methods in radiation planning and delivery currently promote hypofractionated techniques, where each fraction is prescribed at 6 Gy or higher. This includes stereotactic body radiation therapy (SBRT), which is currently being pursued in the clinic as a curative option for patients with unresectable solitary tumors, such as metastatic melanoma [32,33]. Therefore, our data suggest that these treatment strategies in the clinic may at least maintain their efficacy and response rate during dysbiosis. Ionizing radiation is also used in various combination strategies with immunotherapy, showing success in pre-clinical models and clinical trials under assumed orthobiotic conditions [34,35]. However, these combination treatments will likely be negatively influenced by a dysbiotic state, as many types of immunotherapies require a robust and healthy microbiome [36,11].
Since radiotherapy is combined with many other treatment modalities in the clinic, some of which are obstructed by dysbiosis, we pursued a precision medicine approach to identify tumor vasculature drug targets differentially expressed during dysbiosis. We identified that c-Met is differentially over expressed in tumor-associated endothelial cells during dysbiotic conditions, as compared to orthobiotic conditions. This was validated by c-Met-targeting therapeutics, which are FDA-approved or have entered clinical trials. Of the c-Met inhibitors tested, ATP-competitive inhibitor Foretinib displayed the greatest effect on TEC-Dys compared to the other c-Met inhibitors. This was in line with its reported cell free IC50 of 0.4 nM [37]. We tested another ATP-competitive inhibitor, JNJ-38877605, but this compound did not achieve an ED50 in the tested range (up to 10 μM) against either of the endothelial cell lines. The concentration needed to achieve an ED50 by Crizotinib and Cabozantinib on TEC-Ortho and TEC-Dys was greater than that for Foretinib, which correlates with their published higher IC50 [38,39]. Moreover, Foretinib showed the greatest increase in sensitivity between TEC-Ortho and TEC-Dys and inhibited tumor growth to a greater extent during dysbiosis. Thus, Foretinib in particular appears to be a promising therapeutic for patients with solid tumors unable to discontinue antibiotic treatment, or those who do not qualify for radiation or fecal microbiota transplants.
C-Met or hepatocyte growth factor receptor (HGFR) has been associated with treatment resistance and aberrant tumor blood vessel formation [40]. Specifically, it was found that c-Met is at the center of the process called endothelial to mesenchymal transition (Endo-MT). Endo-MT is characterized by EC expression of de novo fibroblast, stem cell-like, and/or smooth muscle cell markers, while retaining their key endothelial functions without cell fate transition [40]. This cell plasticity by vascular endothelial cells has been previously noted in embryogenesis [41], cardiac fibrosis [42], and in melanoma [43]. Here we found that antibiotic-induced dysbiosis results in c-Met positive endothelial cells, which are closely associated with and within the CD31 positive microvasculature. Although we did not observe any statistically significant differences in the amount of CD31 positive tumor vasculature between othobiotic and dysbiotic conditions, the amount of c-Met positive vasculature increases almost an order of magnitude. This would explain the accelerated tumor growth during dysbiotic conditions we previously documented [6]. The identification of c-Met is also promising in the context of personalized medicine, as the inherent interpersonal variability of the microbiome is great, due to various factors, including but not limited to genetic background, diet, environment, and medication use. Future studies are warranted to identify the exact influencers and bacterial variations responsible for these changes in the tumor microenvironment. While the microbiome field was initially hopeful to identify one particular bacterium responsible for the clinical effects seen, different multi-center investigations have identified a variety of bacteria to possibly fulfill that role [36,44]. This suggests that either certain mechanisms are shared among bacterial species or that the overall bacterial abundance, composition and diversity is of greater actual importance than a specific bacterial strain [45].
Over the last decade the field of immuno-oncology has gained tremendous popularity in part due to the clinical success of immune checkpoint inhibitors in a growing number of tumor types. Nonetheless, a significant proportion of the patients, up to 50% in melanoma, exhibit inherent or acquired resistance [46]. The efficacy of some cancer therapies has now been linked, at least in part, to the composition of gut microbiota, but the underlying mechanisms are poorly understood. Currently, very few examples are available on how the bacteria impact cancer directly, but the past decades of GI tract mucosal microbiome investigations has shown that the microbiome influences the host developmental processes and physiology [1]. Several paradigms are currently being explored by various groups: e.g. i) the direct involvement of bacteria in educating or priming the immune system in an potentially adjuvant-like manner; ii) the induction or suppression of cytokines due to acute or chronic bacterial inflammation; iii) the generated metabolites such as short-chain fatty acids by the microbiota, or iv) the involvement in the suppression or induction of Th1, Th2, Th17 cells or Tregs [47].
Here we expanded on how aberrant GI tract microbiota also influence the tumor stroma. Despite increased awareness and appreciation, many mechanisms remain unknown, particularly since each individual microbiome is a complex ecosystem with spatiotemporal dynamics. Moreover, these spatially isolated bionetworks interact with each other, and the cells of the host, including the heterogeneous tumor microenvironment. Furthermore, it is now increasingly recognized that every cancer type has its own inherent characteristic ecosystem with its intricate dynamic changes during carcinogenesis, progression and treatment response. Thus, the reductionist ideal of identifying one single instigator or effector is highly unlikely. Exploring the role(s) of the different microbiomes in cancer initiation, progression, and response to therapy will likely require a systems biology and multi-omics approach [47].
The matched tumor endothelial cell lines that we have generated under orthobiotic and dysbiotic conditions can now be used in additional two- and three-dimensional in vitro as well as in vivo cell-based models. Although the physiological relevance of the in vitro models, and the throughput of the in vivo models, remain limitations to these types of platforms, tumor and tumor-associated cell lines will continue to serve a pivotal role in the preclinical assessment of new or repurposed cancer therapeutics.
Supplementary Material
Supplemental Table 1, Primer sequences of endothelial cell markers.
Supplemental Fig. 1. Molecular marker authentication of TEC-Ortho and TEC-Dys validating endothelial origin.
Representative composite RT-PCR blot of TEC-Ortho and TEC-Dys expressing endothelial cell markers Tie-2, TNFR1, NRP1, Galectin-1 (Gal-1) and Galectin-3 (Gal-3).
Supplemental Fig. 2. TEC-Dys and TEC-Ortho do not express melanoma markers.
(a) B16-F10 express GP100, whereas TEC-Dys do not.
(b) Quantification of GP100 and (c) MART-1 on B16-F10, TEC-Ortho, TEC-Dys and 2H11. Data presented as mean ± SEM (n = 5 per melanoma marker pooled from 2 representative experiments). **P < 0.01, **P < 0.001 two-sided t-test. FMO = fluorescence minus one.
Supplemental Fig. 3. Ampicillin, neomycin, metronidazole and vancomycin do not directly influence TEC-Ortho, TEC-Dys or 2H11.
The cell viability of Tec-Ortho, TEC-Dys and 2H11 after 72 hr being exposed to various concentrations of (a) Ampicillin, (b) Neomycin, (c) Metronidazole, and (d) Vancomycin. Data presented as means ± SD pooled from 2 experiments.
Supplemental Fig. 4. No differential in intracellular ROS production by TEC-Ortho and TEC-Dys during radiation as measured by dichlorofluorescein diacetate.
Cells were seeded in 96-well plates (Corning Costar #3603) at 5000 cells per well and allowed to adhere overnight. Medium was removed and cells were incubated for 1 hour with 1 μM dichlorofluorescein diacetate in PBS. Cells were then irradiated and the fluorescence (492 nm excitation, 515 nm emission) was measured. Background ROS was measured with the same incubation time and 0 Gy radiation and subtracted from radiation values.
Supplemental Fig. 5. Mean dispersion plot showing all proteins identified by TMT-MS3 between TEC-Ortho and TEC-Dys.
X-axis is the log2 intensity for each protein across all samples. Y-axis is log2 fold change of FDR-adjusted P-value. FDR-adjusted P-values < 0.05 and fold change > 2 are highlighted in red and fold change < −2 are highlighted in blue.
Supplemental Fig. 6. C-Met inhibitor JNJ-38877605 has no effect on TEC-Ortho and TEC-Dys.
The cell viability of Tec-Ortho and TEC-Dys after 72 hr being exposed to various concentrations of JNJ-38877605.
Data presented as means ± SEM, pooled from 3 experiments with triplicates.
Supplemental Fig. 7. Heat shock protein 27 (HSP27), part of the VEGF signaling pathway, is differentially expressed in TEC-Dys as compared to TEC-Ortho.
Based on TMT-MS3 analysis, proteins deemed significantly overexpressed in TEC-Dys were superimposed in blue on the Kyoto encyclopedia of genes and genomes (KEGG) VEGF pathway. HSP27 was identified within this pathway.
Supplemental Fig. 8. Heat shock protein 27 (HSP27), part of the receptor tyrosine kinase (RTK) and mitogen activated protein kinase (MAPK) signaling pathway is differentially expressed in TEC-Dys as compared to TEC-Ortho.
Based on TMT-MS3 analysis, proteins deemed significantly overexpressed in TEC-Dys were superimposed in blue on the Kyoto encyclopedia of genes and genomes (KEGG) MAPK pathway. HSP27 was identified within this pathway.
Supplemental Fig. 9. No significant proteomic changes within the phosphatidylinositol 3-kinase (PI3K-AKT) signaling pathway were detected in TEC-Dys as compared to TEC-Ortho.
Based on TMT-MS3 analysis, no proteins within the PI3K-AKT pathway were deemed significantly overexpressed in TEC-Dys. The PI3K-AKT signaling pathway is depicted based on the Kyoto encyclopedia of genes and genomes (KEGG).
Acknowledgments
The study was supported by P20GM103625: the Center for Microbial Pathogenesis and Host Inflammatory Responses grant through the NIH National Institute of General Medical Sciences Centers of Biomedical Research Excellence, as well as in part by a seed grant from the Vice Chancellor of Research & Innovation, and the Arkansas Biosciences Institute and the Winthrop P. Rockefeller Cancer Institute to R.P.M. Dings. The study was also supported in part by the Translational Research Institute (TRI), grant TL1 TR003109 through the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) to S.V. Jenkins. K.B. Vang and R.J. Griffin received support from NSF OIA-1457888 through the Center for Advanced Surface Engineering. J.W. Leung was supported by NIH (K22CA204354 and R35GM137798) and Arkansas Breast Cancer Research Program (AWD00054499 and AWD00053730). The proteomics and bioinformatics cores were supported by National Institutes of Health grant P20GM121293.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Naik S, Bouladoux N, Wilhelm C, Molloy MJ, Salcedo R, Kastenmuller W, Deming C, Quinones M, Koo L, Conlan S, Spencer S, Hall JA, Dzutsev A, Kong H, Campbell DJ, Trinchieri G, Segre JA, Belkaid Y (2012) Compartmentalized control of skin immunity by resident commensals. Science 337 (6098):1115–1119. doi: 10.1126/science.1225152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kilkkinen A, Rissanen H, Klaukka T, Pukkala E, Heliovaara M, Huovinen P, Mannisto S, Aromaa A, Knekt P (2008) Antibiotic use predicts an increased risk of cancer. Int J Cancer 123 (9):2152–2155. doi: 10.1002/ijc.23622 [DOI] [PubMed] [Google Scholar]
- 3.Cheng M, Qian L, Shen G, Bian G, Xu T, Xu W, Shen G, Hu S (2014) Microbiota Modulate Tumoral Immune Surveillance in Lung through a gammadeltaT17 Immune Cell-Dependent Mechanism. Cancer Res 74:4030–4041. doi: 10.1158/0008-5472.CAN-13-2462 [DOI] [PubMed] [Google Scholar]
- 4.Uribe-Herranz M, Bittinger K, Rafail S, Guedan S, Pierini S, Tanes C, Ganetsky A, Morgan MA, Gill S, Tanyi JL, Bushman FD, June CH, Facciabene A (2018) Gut microbiota modulates adoptive cell therapy via CD8alpha dendritic cells and IL-12. JCI Insight 3 (4). doi: 10.1172/jci.insight.94952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CDC (2017) Antibiotic use in the United States, 2017: Progress and Opportunities. US department of Health and human Services, Atlanta, GA [Google Scholar]
- 6.Jenkins SV, Robeson MS, Griffin RJ, Quick CM, Siegel ER, Cannon MJ, Vang KB, Dings RPM (2019) Gastrointestinal tract dysbiosis enhances distal tumor progression through suppression of leukocyte trafficking. Cancer Res 79 (23):5999–6009. doi: 10.1158/0008-5472.CAN-18-4108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Almeida RR, Vieira RS, Castoldi A, Terra FF, Melo ACL, Canesso MCC, Lemos L, Cipelli M, Rana N, Hiyane MI, Pearce EL, Martins FDS, Faria AMC, Camara NOS (2020) Host dysbiosis negatively impacts IL-9-producing T-cell differentiation and antitumour immunity. Br J Cancer 123 (4):534–541. doi: 10.1038/s41416-020-0915-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu N, Wang L, Li C, Ding C, Li C, Fan W, Cheng C, Gu B (2020) Microbiota dysbiosis in lung cancer: evidence of association and potential mechanisms. Transl Lung Cancer Res 9 (4):1554–1568. doi: 10.21037/tlcr-20-156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144 (5):646–674. doi: 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 10.Dudley AC (2012) Tumor endothelial cells. Cold Spring Harb Perspect Med 2 (3):a006536. doi: 10.1101/cshperspect.a006536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillere R, Hannani D, Enot DP, Pfirschke C, Engblom C, Pittet MJ, Schlitzer A, Ginhoux F, Apetoh L, Chachaty E, Woerther PL, Eberl G, Berard M, Ecobichon C, Clermont D, Bizet C, Gaboriau-Routhiau V, Cerf-Bensussan N, Opolon P, Yessaad N, Vivier E, Ryffel B, Elson CO, Dore J, Kroemer G, Lepage P, Boneca IG, Ghiringhelli F, Zitvogel L (2013) The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 342 (6161):971–976. doi: 10.1126/science.1240537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinato DJ, Howlett S, Ottaviani D, Urus H, Patel A, Mineo T, Brock C, Power D, Hatcher O, Falconer A, Ingle M, Brown A, Gujral D, Partridge S, Sarwar N, Gonzalez M, Bendle M, Lewanski C, Newsom-Davis T, Allara E, Bower M (2019) Association of Prior Antibiotic Treatment With Survival and Response to Immune Checkpoint Inhibitor Therapy in Patients With Cancer. JAMA Oncol. doi: 10.1001/jamaoncol.2019.2785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma SV, Haber DA, Settleman J (2010) Cell line-based platforms to evaluate the therapeutic efficacy of candidate anticancer agents. Nat Rev Cancer 10 (4):241–253. doi: 10.1038/nrc2820 [DOI] [PubMed] [Google Scholar]
- 14.Dings RP, Van Laar ES, Webber J, Zhang Y, Griffin RJ, Waters SJ, MacDonald JR, Mayo KH (2008) Ovarian tumor growth regression using a combination of vascular targeting agents anginex or topomimetic 0118 and the chemotherapeutic irofulven. Cancer Lett 265 (2):270–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dings RP, Vang KB, Castermans K, Popescu F, Zhang Y, Oude Egbrink MG, Mescher MF, Farrar MA, Griffioen AW, Mayo KH (2011) Enhancement of T-cell-mediated antitumor response: angiostatic adjuvant to immunotherapy against cancer. Clin Cancer Res 17 (10):3134–3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dings RP, Loren M, Heun H, McNiel E, Griffioen AW, Mayo KH, Griffin RJ (2007) Scheduling of radiation with angiogenesis inhibitors Anginex and Avastin improves therapeutic outcome via vessel normalization. Clin Cancer Res 13 (11):3395–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS, Iwasaki A (2011) Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci U S A 108 (13):5354–5359.doi: 10.1073/pnas.1019378108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Beijnum JR, Rousch M, Castermans K, van der Linden E, Griffioen AW (2008) Isolation of endothelial cells from fresh tissues. Nature protocols 3 (6):1085–1091 [DOI] [PubMed] [Google Scholar]
- 19.Thijssen VL, Brandwijk RJ, Dings RP, Griffioen AW (2004) Angiogenesis gene expression profiling in xenograft models to study cellular interactions. Exp Cell Res 299 (2):286–293 [DOI] [PubMed] [Google Scholar]
- 20.Jenkins S, Nima Z, Vang KB, Nedosekin D, Zharov VP, Griffin RJ, Biris AS, Dings RPM (2017) Triple negative breast cancer targeting and killing by EpCAM-directed, plasmonically active nanodrug systems Precision Oncology 1 (1):1–11. doi:doi: 10.1038/s41698-017-0030-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nowak-Sliwinska P, Alitalo K, Allen E, Anisimov A, Aplin AC, Auerbach R, Augustin HG, Bates DO, van Beijnum JR, Bender RHF, Bergers G, Bikfalvi A, Bischoff J, Bock BC, Brooks PC, Bussolino F, Cakir B, Carmeliet P, Castranova D, Cimpean AM, Cleaver O, Coukos G, Davis GE, De Palma M, Dimberg A, Dings RPM, Djonov V, Dudley AC, Dufton NP, Fendt SM, Ferrara N, Fruttiger M, Fukumura D, Ghesquiere B, Gong Y, Griffin RJ, Harris AL, Hughes CCW, Hultgren NW, Iruela-Arispe ML, Irving M, Jain RK, Kalluri R, Kalucka J, Kerbel RS, Kitajewski J, Klaassen I, Kleinmann HK, Koolwijk P, Kuczynski E, Kwak BR, Marien K, Melero-Martin JM, Munn LL, Nicosia RF, Noel A, Nurro J, Olsson AK, Petrova TV, Pietras K, Pili R, Pollard JW, Post MJ, Quax PHA, Rabinovich GA, Raica M, Randi AM, Ribatti D, Ruegg C, Schlingemann RO, Schulte-Merker S, Smith LEH, Song JW, Stacker SA, Stalin J, Stratman AN, Van de Velde M, van Hinsbergh VWM, Vermeulen PB, Waltenberger J, Weinstein BM, Xin H, Yetkin-Arik B, Yla-Herttuala S, Yoder MC, Griffioen AW (2018) Consensus guidelines for the use and interpretation of angiogenesis assays. Angiogenesis. doi: 10.1007/s10456-018-9613-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dings RP, Chen X, Hellebrekers DM, van Eijk LI, Zhang Y, Hoye TR, Griffioen AW, Mayo KH (2006) Design of nonpeptidic topomimetics of antiangiogenic proteins with antitumor activities. J Natl Cancer Inst 98 (13):932–936 [DOI] [PubMed] [Google Scholar]
- 23.Jenkins SV, Nedosekin DA, Shaulis BJ, Wang T, Jamshidi-Parsian A, Pollock ED, Chen J, Dings RPM, Griffin RJ (2019) Enhanced Photothermal Treatment Efficacy and Normal Tissue Protection via Vascular Targeted Gold Nanocages. Nanotheranostics 3 (2):145–155. doi: 10.7150/ntno.32395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shields BD, Koss B, Taylor EM, Storey AJ, West KL, Byrum SD, Mackintosh SG, Edmondson R, Mahmoud F, Shalin SC, Tackett AJ (2019) Loss of E-Cadherin Inhibits CD103 Antitumor Activity and Reduces Checkpoint Blockade Responsiveness in Melanoma. Cancer Res 79 (6):1113–1123. doi: 10.1158/0008-5472.CAN-18-1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graw S, Tang J, Zafar MK, Byrd AK, Bolden CT, Peterson EC, Byrum SD (2020) ProteiNorm - A user-friendly tool for normalization and analysis of TMT and label-free protein quantification. ACS Omega In Press. doi: 10.1021/acsomega.0c02564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huber W, von Heydebreck A, Sultmann H, Poustka A, Vingron M (2002) Variance stabilization applied to microarray data calibration and to the quantification of differential expression. Bioinformatics 18 Suppl 1:S96–104. doi: 10.1093/bioinformatics/18.suppl_1.s96 [DOI] [PubMed] [Google Scholar]
- 27.Bolstad B (2020) PreprocessCore: A collection of pre-processing functions., R package version 1.50.0 edn., [Google Scholar]
- 28.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK (2015) limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43 (7):e47. doi: 10.1093/nar/gkv007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chawade A, Alexandersson E, Levander F (2014) Normalyzer: a tool for rapid evaluation of normalization methods for omics data sets. J Proteome Res 13 (6):3114–3120. doi: 10.1021/pr401264n [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gu Z, Eils R, Schlesner M (2016) Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 32 (18):2847–2849. doi: 10.1093/bioinformatics/btw313 [DOI] [PubMed] [Google Scholar]
- 31.Dings RP, Miller MC, Nesmelova I, Astorgues-Xerri L, Kumar N, Serova M, Chen X, Raymond E, Hoye TR, Mayo KH (2012) Antitumor agent calixarene 0118 targets human galectin-1 as an allosteric inhibitor of carbohydrate binding. J Med Chem 55 (11):5121–5129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, Mulroy L, Lock M, Rodrigues GB, Yaremko BP, Schellenberg D, Ahmad B, Griffioen G, Senthi S, Swaminath A, Kopek N, Liu M, Moore K, Currie S, Bauman GS, Warner A, Senan S (2019) Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet 393 (10185):2051–2058. doi: 10.1016/S0140-6736(18)32487-5 [DOI] [PubMed] [Google Scholar]
- 33.Timmerman RD, Herman J, Cho LC (2014) Emergence of stereotactic body radiation therapy and its impact on current and future clinical practice. J Clin Oncol 32 (26):2847–2854. doi: 10.1200/JCO.2014.55.4675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnsrud AJ, Jenkins SV, Jamshidi-Parsian A, Quick CM, Galhardo EP, Dings RPM, Vang KB, Narayanasamy G, Makhoul I, Griffin RJ (2020) Evidence for Early Stage Anti-Tumor Immunity Elicited by Spatially Fractionated Radiotherapy-Immunotherapy Combinations. Radiation Research doi: 10.1667/RADE-20-00065.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, Cho BC, Bourhaba M, Quantin X, Tokito T, Mekhail T, Planchard D, Kim YC, Karapetis CS, Hiret S, Ostoros G, Kubota K, Gray JE, Paz-Ares L, de Castro Carpeno J, Wadsworth C, Melillo G, Jiang H, Huang Y, Dennis PA, Ozguroglu M, Investigators P (2017) Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 377 (20):1919–1929. doi: 10.1056/NEJMoa1709937 [DOI] [PubMed] [Google Scholar]
- 36.Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman K, Wei SC, Cogdill AP, Zhao L, Hudgens CW, Hutchinson DS, Manzo T, Petaccia de Macedo M, Cotechini T, Kumar T, Chen WS, Reddy SM, Szczepaniak Sloane R, Galloway-Pena J, Jiang H, Chen PL, Shpall EJ, Rezvani K, Alousi AM, Chemaly RF, Shelburne S, Vence LM, Okhuysen PC, Jensen VB, Swennes AG, McAllister F, Marcelo Riquelme Sanchez E, Zhang Y, Le Chatelier E, Zitvogel L, Pons N, Austin-Breneman JL, Haydu LE, Burton EM, Gardner JM, Sirmans E, Hu J, Lazar AJ, Tsujikawa T, Diab A, Tawbi H, Glitza IC, Hwu WJ, Patel SP, Woodman SE, Amaria RN, Davies MA, Gershenwald JE, Hwu P, Lee JE, Zhang J, Coussens LM, Cooper ZA, Futreal PA, Daniel CR, Ajami NJ, Petrosino JF, Tetzlaff MT, Sharma P, Allison JP, Jenq RR, Wargo JA (2018) Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359 (6371):97–103. doi: 10.1126/science.aan4236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qian F, Engst S, Yamaguchi K, Yu P, Won KA, Mock L, Lou T, Tan J, Li C, Tam D, Lougheed J, Yakes FM, Bentzien F, Xu W, Zaks T, Wooster R, Greshock J, Joly AH (2009) Inhibition of tumor cell growth, invasion, and metastasis by EXEL-2880 (XL880, GSK1363089), a novel inhibitor of HGF and VEGF receptor tyrosine kinases. Cancer Res 69 (20):8009–8016. doi: 10.1158/0008-5472.CAN-08-4889 [DOI] [PubMed] [Google Scholar]
- 38.Zou HY, Li Q, Lee JH, Arango ME, McDonnell SR, Yamazaki S, Koudriakova TB, Alton G, Cui JJ, Kung PP, Nambu MD, Los G, Bender SL, Mroczkowski B, Christensen JG (2007) An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer Res 67 (9):4408–4417. doi: 10.1158/0008-5472.CAN-06-4443 [DOI] [PubMed] [Google Scholar]
- 39.You WK, Sennino B, Williamson CW, Falcon B, Hashizume H, Yao LC, Aftab DT, McDonald DM (2011) VEGF and c-Met blockade amplify angiogenesis inhibition in pancreatic islet cancer. Cancer Res 71 (14):4758–4768. doi: 10.1158/0008-5472.CAN-10-2527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang M, Liu T, Ma P, Mitteer RA Jr., Zhang Z, Kim HJ, Yeo E, Zhang D, Cai P, Li C, Zhang L, Zhao B, Roccograndi L, O'Rourke DM, Dahmane N, Gong Y, Koumenis C, Fan Y (2016) c-Met-mediated endothelial plasticity drives aberrant vascularization and chemoresistance in glioblastoma. J Clin Invest 126 (5):1801–1814. doi: 10.1172/JCI84876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kovacic JC, Mercader N, Torres M, Boehm M, Fuster V (2012) Epithelial-to-mesenchymal and endothelial-to-mesenchymal transition: from cardiovascular development to disease. Circulation 125 (14):1795–1808. doi: 10.1161/CIRCULATIONAHA.111.040352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R (2007) Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med 13 (8):952–961 [DOI] [PubMed] [Google Scholar]
- 43.Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R (2007) Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res 67 (21):10123–10128 [DOI] [PubMed] [Google Scholar]
- 44.Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML, Luke JJ, Gajewski TF (2018) The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 359 (6371):104–108. doi: 10.1126/science.aao3290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tanoue T, Morita S, Plichta DR, Skelly AN, Suda W, Sugiura Y, Narushima S, Vlamakis H, Motoo I, Sugita K, Shiota A, Takeshita K, Yasuma-Mitobe K, Riethmacher D, Kaisho T, Norman JM, Mucida D, Suematsu M, Yaguchi T, Bucci V, Inoue T, Kawakami Y, Olle B, Roberts B, Hattori M, Xavier RJ, Atarashi K, Honda K (2019) A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature 565 (7741):600–605. doi: 10.1038/s41586-019-0878-z [DOI] [PubMed] [Google Scholar]
- 46.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A (2017) Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 168 (4):707–723. doi: 10.1016/j.cell.2017.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xavier JB, Young VB, Skufca J, Ginty F, Testerman T, Pearson AT, Macklin P, Mitchell A, Shmulevich I, Xie L, Caporaso JG, Crandall KA, Simone NL, Godoy-Vitorino F, Griffin TJ, Whiteson KL, Gustafson HH, Slade DJ, Schmidt TM, Walther-Antonio MRS, Korem T, Webb-Robertson BM, Styczynski MP, Johnson WE, Jobin C, Ridlon JM, Koh AY, Yu M, Kelly L, Wargo JA (2020) The Cancer Microbiome: Distinguishing Direct and Indirect Effects Requires a Systemic View. Trends Cancer 6 (3):192–204. doi: 10.1016/j.trecan.2020.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1, Primer sequences of endothelial cell markers.
Supplemental Fig. 1. Molecular marker authentication of TEC-Ortho and TEC-Dys validating endothelial origin.
Representative composite RT-PCR blot of TEC-Ortho and TEC-Dys expressing endothelial cell markers Tie-2, TNFR1, NRP1, Galectin-1 (Gal-1) and Galectin-3 (Gal-3).
Supplemental Fig. 2. TEC-Dys and TEC-Ortho do not express melanoma markers.
(a) B16-F10 express GP100, whereas TEC-Dys do not.
(b) Quantification of GP100 and (c) MART-1 on B16-F10, TEC-Ortho, TEC-Dys and 2H11. Data presented as mean ± SEM (n = 5 per melanoma marker pooled from 2 representative experiments). **P < 0.01, **P < 0.001 two-sided t-test. FMO = fluorescence minus one.
Supplemental Fig. 3. Ampicillin, neomycin, metronidazole and vancomycin do not directly influence TEC-Ortho, TEC-Dys or 2H11.
The cell viability of Tec-Ortho, TEC-Dys and 2H11 after 72 hr being exposed to various concentrations of (a) Ampicillin, (b) Neomycin, (c) Metronidazole, and (d) Vancomycin. Data presented as means ± SD pooled from 2 experiments.
Supplemental Fig. 4. No differential in intracellular ROS production by TEC-Ortho and TEC-Dys during radiation as measured by dichlorofluorescein diacetate.
Cells were seeded in 96-well plates (Corning Costar #3603) at 5000 cells per well and allowed to adhere overnight. Medium was removed and cells were incubated for 1 hour with 1 μM dichlorofluorescein diacetate in PBS. Cells were then irradiated and the fluorescence (492 nm excitation, 515 nm emission) was measured. Background ROS was measured with the same incubation time and 0 Gy radiation and subtracted from radiation values.
Supplemental Fig. 5. Mean dispersion plot showing all proteins identified by TMT-MS3 between TEC-Ortho and TEC-Dys.
X-axis is the log2 intensity for each protein across all samples. Y-axis is log2 fold change of FDR-adjusted P-value. FDR-adjusted P-values < 0.05 and fold change > 2 are highlighted in red and fold change < −2 are highlighted in blue.
Supplemental Fig. 6. C-Met inhibitor JNJ-38877605 has no effect on TEC-Ortho and TEC-Dys.
The cell viability of Tec-Ortho and TEC-Dys after 72 hr being exposed to various concentrations of JNJ-38877605.
Data presented as means ± SEM, pooled from 3 experiments with triplicates.
Supplemental Fig. 7. Heat shock protein 27 (HSP27), part of the VEGF signaling pathway, is differentially expressed in TEC-Dys as compared to TEC-Ortho.
Based on TMT-MS3 analysis, proteins deemed significantly overexpressed in TEC-Dys were superimposed in blue on the Kyoto encyclopedia of genes and genomes (KEGG) VEGF pathway. HSP27 was identified within this pathway.
Supplemental Fig. 8. Heat shock protein 27 (HSP27), part of the receptor tyrosine kinase (RTK) and mitogen activated protein kinase (MAPK) signaling pathway is differentially expressed in TEC-Dys as compared to TEC-Ortho.
Based on TMT-MS3 analysis, proteins deemed significantly overexpressed in TEC-Dys were superimposed in blue on the Kyoto encyclopedia of genes and genomes (KEGG) MAPK pathway. HSP27 was identified within this pathway.
Supplemental Fig. 9. No significant proteomic changes within the phosphatidylinositol 3-kinase (PI3K-AKT) signaling pathway were detected in TEC-Dys as compared to TEC-Ortho.
Based on TMT-MS3 analysis, no proteins within the PI3K-AKT pathway were deemed significantly overexpressed in TEC-Dys. The PI3K-AKT signaling pathway is depicted based on the Kyoto encyclopedia of genes and genomes (KEGG).