Abstract
The demersal brown banded bamboo shark Chiloscyllium punctatum is a major component of sharks landed in Malaysia. However, little is known about their population structure and the effect of high fishing pressure on these weak swimming sharks. Both mitochondrial DNA control region (1072 bp) and NADH dehydrogenase subunit 2 (1044 bp) were used to elucidate the genetic structure and connectivity of C. punctatum among five major areas within the Sundaland region. Our findings revealed (i) strong genetic structure with little present day mixing between the major areas, (ii) high intra-population genetic diversity with unique haplotypes, (iii) significant correlation between genetic differentiation and geographical distance coupled with detectable presence of fine scale geographical barriers (i.e. the South China Sea), (iv) historical directional gene flow from the east coast of Peninsular Malaysia towards the west coast and Borneo, and (v) no detectable genetic differentiation along the coastline of east Peninsular Malaysia. Genetic patterns inferred from the mitochondrial DNA loci were consistent with the strong coastal shelf association in this species, the presence of contemporary barriers shaped by benthic features, and limited current-driven egg dispersal. Fine scale population structure of C. punctatum highlights the need to improve genetic understanding for fishery management and conservation of other small-sized sharks.
Subject terms: Population genetics, Haplotypes
Introduction
Sharks are generally highly vulnerable to overexploitation due to their life history strategies, such as late maturity and low fecundity1–5. Overexploitation has been shown to reduce genetic diversity and increase extinction risk especially for small populations6; therefore understanding the genetic structure and migration patterns or gene flow of shark species are essential to inform effective management and conservation plans. Earlier shark genetic studies had been largely focused on marine neritic shark species, that are charismatic, economically important and of conservation interest5, e.g. blacktip shark C. limbatus7, hammerhead shark Sphyrna lewini8, whale shark Rhincodon typus9 and great white shark Carcharodon carcharias10–15. Studies on small-sized benthic coastal sharks have increased in recent years in part due to their catch prominence and importance in coastal fisheries, both globally and in Southeast Asia, e.g. whitespotted bambooshark Chiloscyllium plagiosum16, nurse shark Ginglymostoma cirratum17, whitetip reef shark Triaenodon obesus18, leopard shark Triakis semifasciata19 and common smoothhound Mustelus mustelus20.
One such group of sharks is the longtail carpet shark or bamboo shark from the family Hemiscyllidae (order Orectolobiformes). The family Hemiscyllidae comprises two genera: Hemiscyllium Müller & Henle, 1838 (nine species) which is confined to the Australia-New Guinea region and Indonesia, and the Indo-Pacific genus of Chiloscyllium Müller & Henle, 1837 (eight species)21. These bottom-living sharks are known to be weak swimmers22 with great camouflage ability, allowing them to adapt to demersal habitat by hiding around crevices23. They live either solitarily or within a group and appear to be strongly territorial. They usually deposit egg cases on the sea floor (sheltered or deserted), with some cases attached to benthic marine plants23. Currently, their fecundity in the wild is still unknown, but studies on captive individuals suggested that they produce annually less than 30 egg cases that require a three-month incubation period24–27. To the best of our knowledge, no movement study on this animal exists. Dispersal is likely very restricted due to their oviparous nature28, thus rendering them highly vulnerable to localized exploitation and habitat destruction.
Presently, four species of Chiloscyllium are found in Malaysia: Indonesian bamboo shark C. hasseltii Bleeker, 1852; slender bamboo shark C. indicum (Gmelin, 1789); whitespotted bamboo shark C. plagiosum (Bennett, 1830); and brown banded bamboo shark C. punctatum Müller & Henle, 1838. A previous record of grey bamboo shark C. griseum Müller & Henle, 1838 in Malaysian waters was a misidentification of C. hasseltiiAhmad, personal communication. The brown banded bamboo shark C. punctatum has been classified as Near Threatened under the IUCN Red List but may be uplisted to Vulnerable in the near future in Southeast Asia due to high fishing pressure29. It is the top ten shark species landed by numbers in commercial fisheries across Malaysia30. This species makes up almost half of all shark individuals caught using bottom trawl fishing vessels in the west coast of Peninsular Malaysia31. Approximately 40% of C. punctatum landed in commercial fishing gears comprised of immature individuals, with higher percentage in the west than the east coasts of Peninsular Malaysia31,32,Lim, unpublished data. Among similar-sized sharks, C. punctatum has relatively lower commercial value, fetching an ex-vessel price of USD 0.20–0.60 per kg. Many artisanal fishers in Malaysia generally do not discard any of their non-trash fish catches; when they do, bamboo sharks are among those selectively discardedThen, unpublished data. No formal fishery assessment exists for this species (or other sharks) in Malaysia, and the effect of present fishing level on the animal is unknown.
One of the priorities for C. punctatum includes clarifying the population substructure due to concerns of population fragmentation29 likely due to being a weak swimmer and limited potential for dispersal. Genetic tools have been used to identify population vulnerability or risk of local extinction based on their genetic diversity and structure as well as connectivity among populations33–35. Intra-specific genetic studies on small-sized coastal sharks and rays with limited vagility showed that they display high genetic population differentiation across ocean basins due to environmental barriers (nurse shark Ginglymostoma cirratum17), vicariance and dispersal events (maskray Neotrygon kuhlii36), or a combination of both (small-spotted catshark Scyliorhinus canicula37). On the other hand, studies of these small coastal species along continuous continental coastlines either displayed clear genetic differentiation (leopard shark Triakis semifasciata19, short-tailed stingray Dasyatis brevicaudata38) or lack of genetic structure (milk shark Rhizoprionodon acutus39); reasons for these pattern variations included differences in habitat preferences, site fidelity, geographical distance, and physical barriers to movement. Despite growing concerns of shark exploitation in Malaysia as one of the top shark-fishing nation globally40, genetic studies of local shark populations, including C. punctatum are almost non-existent (see Dudgeon et al.41 for exception).
The genetic structure of any organism is influenced not only by the ecological processes but also the geological history42–46. Sundaland, a shelf within the biodiverse Indo-West Pacific region that extends from Thailand southwards primarily covering Malaysia and Indonesia, has undergone dramatic plate tectonic evolution forming episodic submergence and exposure of the shelf47,48. These series of events had affected distributions of local organisms (ranging from terrestrial to marine environment) in multiple ways due to different population dispersal patterns (e.g. Lim et al.45; Tan et al.46; Reid et al.49; Polgar et al.50; Leonard et al.51; Ma et al.52; Mason et al.53; Crandall et al.54). In relation to elasmobranch populations, the Sunda Shelf appeared to be acting as biogeographic barrier for wedgefish Rhynchobatus australiae55 but not for spottail shark Carcharhinus sorrah56. However finer-scale seascape features that may affect genetic structuring of sharks and rays within the Sundaland region had not been investigated previously.
The aim of the present study is therefore to assess the degree of genetic diversity and differentiation of C. punctatum in Malaysia, specifically along coastal Peninsular Malaysia and northern Malaysian Borneo coastline. Based on the biogeographic history of the Sundaland region, we hypothesized that there are at least two distinct local C. punctatum populations, with little genetic mixing between populations from either side of the peninsular (northern end of the Sunda Shelf barrier) and across the Karimata Strait that separates the peninsular from Borneo. Furthermore, we tested to see if observed population genetic differentiation can be explained by simple geographical distance or otherwise. In addition, we also assessed the gene flow directionality between distinctive populations to infer putative dispersal pattern. Knowledge of fine scale genetic structuring of C. punctatum provides the spatially relevant information for appropriate national fisheries management and conservation of this highly fished shark and likely of other sharks sharing similar life history strategies.
Results
A total of 2116 base pair sequences hereby named as ND2CR (concatenated between 1072 bp for CR and 1044 bp for ND2were successfully amplified for all 135 C. punctatum samples. A total of 70 unique haplotypes and 63 polymorphic sites were detected, in which 40 sites were found in the CR region and the rest were found in the ND2 region (Supplementary Tables S1 and S2). Most of the identified haplotypes (70%) was found only in a single individual, while the rest was represented by two to 17 individuals. In terms of genetic diversity, ND2 gene showed lower range (haplotype diversity: 0.58–0.71, nucleotide diversity: 0.0009–0.0026) compared to CR (haplotype diversity: 0.63–0.90, nucleotide diversity: 0.0021–0.0076) (Table 1). When the two markers were concatenated, the range of haplotype diversity increased (0.76–0.98) (Table 1).
Table 1.
Genetic diversity of Chiloscyllium punctatum according to area and markers. N = number of samples, k = number of polymorphic sites, Nha = number of haplotypes, ha = haplotype diversity, π = nucleotide diversity, D = Tajima’s D test, FS = Fu’s FS test.
| Area | N | k | Nha | ha | π | D | Fs |
|---|---|---|---|---|---|---|---|
| CR | |||||||
| West Peninsular | 30 | 13 | 13 | 0.89 ± 0.03 | 0.0028 ± 0.0017 | − 0.35 | − 4.38* |
| East Peninsular | 25 | 11 | 13 | 0.90 ± 0.04 | 0.0021 ± 0.0014 | − 0.73 | − 7.02* |
| Sarawak | 26 | 18 | 15 | 0.90 ± 0.05 | 0.0046 ± 0.0026 | 0.15 | − 4.34* |
| Western Sabah | 25 | 19 | 7 | 0.63 ± 0.10 | 0.0053 ± 0.0029 | 0.43 | 2.87 |
| Eastern Sabah | 29 | 20 | 10 | 0.87 ± 0.03 | 0.0076 ± 0.0040 | 2.02 | 2.28 |
| Total | 135 | 40 | 52 | 0.95 ± 0.01 | 0.0086 ± 0.0004 | ||
| ND2 | |||||||
| West Peninsular | 30 | 6 | 5 | 0.71 ± 0.05 | 0.0023 ± 0.0014 | 1.71 | 1.98 |
| East Peninsular | 25 | 5 | 6 | 0.68 ± 0.08 | 0.0009 ± 0.0007 | − 0.92 | − 2.23* |
| Sarawak | 26 | 6 | 6 | 0.63 ± 0.09 | 0.0018 ± 0.0012 | 0.58 | − 0.00 |
| Western Sabah | 25 | 10 | 7 | 0.70 ± 0.08 | 0.0026 ± 0.0016 | 0.03 | 0.13 |
| Eastern Sabah | 29 | 6 | 3 | 0.58 ± 0.05 | 0.0026 ± 0.0016 | 2.24 | 5.32 |
| Total | 135 | 23 | 19 | 0.85 ± 0.02 | 0.0041 ± 0.0002 | ||
| ND2CR | |||||||
| West Peninsular | 30 | 19 | 18 | 0.94 ± 0.02 | 0.0026 ± 0.0014 | 0.41 | − 6.17* |
| East Peninsular | 25 | 16 | 20 | 0.98 ± 0.02 | 0.0015 ± 0.0009 | − 0.88 | − 17.94* |
| Sarawak | 26 | 24 | 18 | 0.95 ± 0.03 | 0.0033 ± 0.0018 | 0.29 | − 5.85* |
| Western Sabah | 25 | 29 | 10 | 0.76 ± 0.08 | 0.0040 ± 0.0021 | 0.30 | 1.79 |
| Eastern Sabah | 29 | 26 | 11 | 0.89 ± 0.03 | 0.0051 ± 0.0027 | 2.24 | 2.86 |
| Total | 135 | 63 | 70 | 0.97 ± 0.01 | 0.0063 ± 0.0002 | ||
*Represent significant difference at p < 0.05.
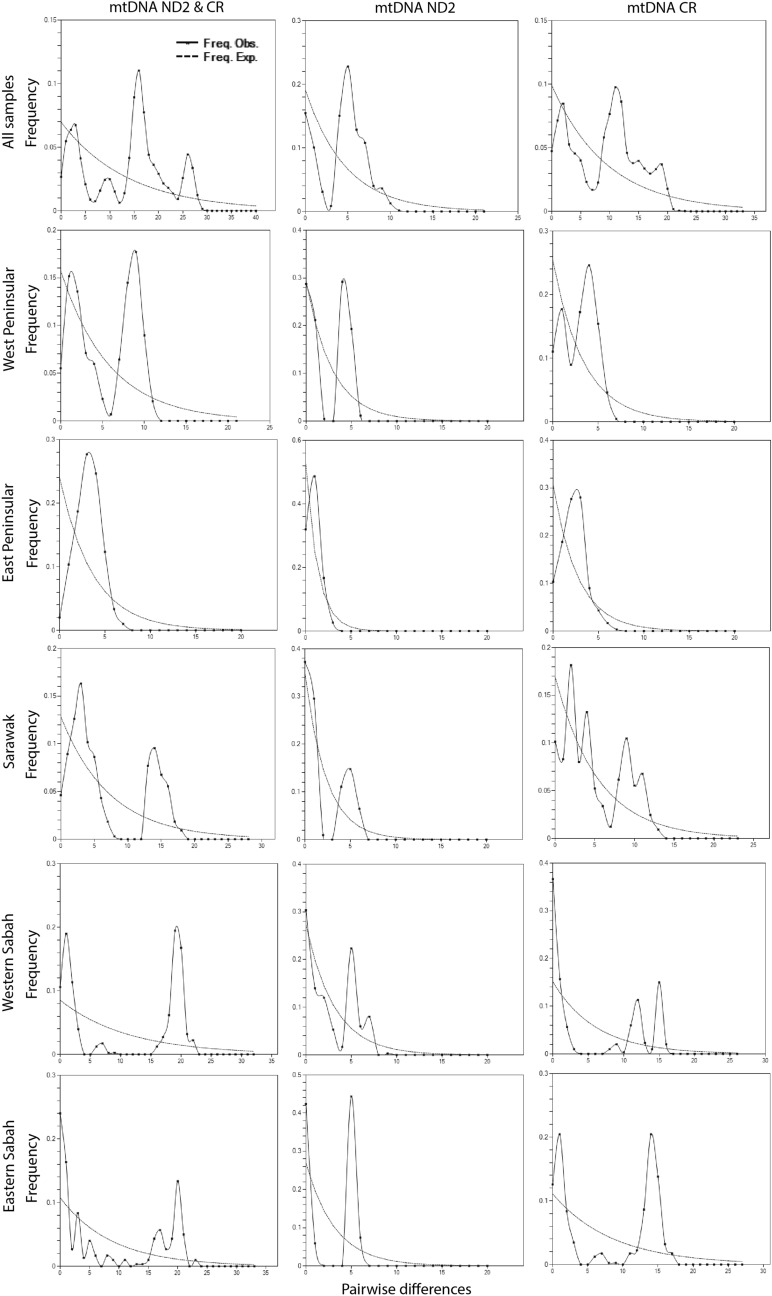
The Tajima’s D tests were all not significantly different from zero which suggested no evidence of departure from neutrality (Table 1). On the other hand, Fu's Fs which is more sensitive to recent population expansion57 suggested some evidence for population expansion with significantly negative values for both CR and concatenated markers for WP, EP and SR populations (Table 1). The mismatch distribution analysis for the individual and concatenated markers showed consistent results: across all sampling areas; only EP had smooth and unimodal distributions that closely fitted the expected distribution under the sudden expansion model (Fig. 1). The distribution for total samples and all other sampling areas demonstrated a multimodal distribution that is typically attributed to populations in demographic equilibrium.
Figure 1.
Historical demography of Chiloscyllium punctatum populations pair-wise mismatch distribution analyses inferred from individual and combined mtDNA ND2 and control region markers. The distributions are shown for total samples and all major study areas.
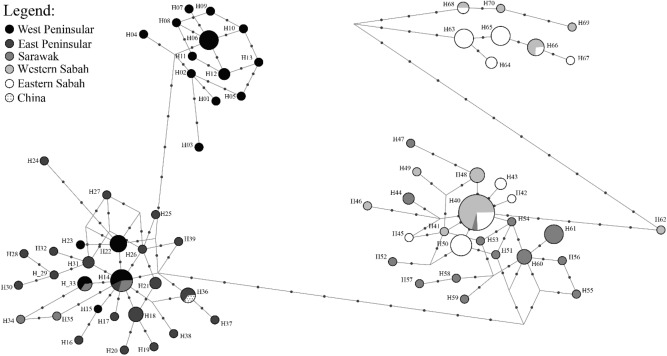
The haplotype network showed that most haplotypes were found almost exclusively within an area (Fig. 2). Only five haplotypes were found across multiple sampling areas, i.e. H14 was found in EP, WP and SR; H33 in EP and SR; H40 in SR, WS and ES; and both H66 and H68 in WS and ES (Supplementary Table S3). In general, four major haplotype clusters were found among the samples—one with haplotypes found only in WP, one with haplotypes found mostly in EP; one with mixed haplotypes from SR, WS and ES; and one with haplotypes from both WS and ES. The single sample obtained from GenBank (sampled in China) clustered together with haplotypes from EP. For samples from Sabah (WS and ES), four sub-clusters separated by a minimum of five mutations were observed. This includes one large cluster with nine haplotypes (seen in 18 individuals from WS, 14 from ES and one from SR) and three minor clusters each with five (three from WS and 14 from ES), three (three from WS and one from ES) and one (one from WS) haplotypes.
Figure 2.
Haplotype network for Chiloscyllium punctatum (n = 135). A sequence from an individual sampled from China from NCBI Genbank (JQ082337) is included for comparison. Circle size is proportional to number of samples sharing the haplotypes. Dots along the lines represent number of mutations between the connected haplotypes.
Overall, there was a weak but significant support for IBD between areas (r2 = 0.62, p < 0.05), with a broad range of pairwise genetic distances at all geographical distance concentrated mostly along the regression line (Fig. 3a). Pairwise comparison supported the high genetic differentiation (p < 0.05) found between all pairs of sampling areas, including weak significance between the two areas within Sabah (WS and ES) (Table 2). Results from the hierarchical AMOVA demonstrated 40–50% of genetic variation was attributed to differences among sampling areas (Table 3). When testing for genetic partitioning under various biogeographical barrier combinations, significant hierarchical partitioning of genetic variation at all levels for both markers was observed when only South China Sea was considered as a genetic barrier (the B2 only scenario). Under this scenario, 46.2% of genetic variation was partitioned among population groups, 15.73% among populations within group, and 38.07% within populations.
Figure 3.
Scatterplot of pairwise genetic distance and log10 of geographical distance (km) among (a) the five sampling areas of West Peninsular, East Peninsular, Sarawak, Western Sabah and Eastern Sabah, (b) among EP individuals.
Table 2.
Pairwise FST (lower diagonal) among sampling sites and respective p-values (upper diagonal) according to markers.
| West Peninsular | East Peninsular | Sarawak | Western Sabah | Eastern Sabah | |
|---|---|---|---|---|---|
| CR | |||||
| West Peninsular | – | *** | *** | *** | *** |
| East Peninsular | 0.3319 | – | *** | *** | *** |
| Sarawak | 0.5366 | 0.6110 | – | *** | *** |
| Western Sabah | 0.6465 | 0.7029 | 0.2218 | – | * |
| Eastern Sabah | 0.6111 | 0.6576 | 0.3763 | 0.1254 | – |
| ND2 | |||||
| West Peninsular | – | *** | *** | *** | *** |
| East Peninsular | 0.4658 | – | *** | *** | *** |
| Sarawak | 0.5819 | 0.6621 | – | *** | *** |
| Western Sabah | 0.6097 | 0.6836 | 0.1258 | – | * |
| Eastern Sabah | 0.6384 | 0.6975 | 0.3603 | 0.0920 | – |
| ND2CR | |||||
| West Peninsular | – | *** | *** | *** | *** |
| East Peninsular | 0.3934 | – | *** | *** | *** |
| Sarawak | 0.5554 | 0.6280 | – | *** | *** |
| Western Sabah | 0.6347 | 0.6980 | 0.1957 | – | * |
| Eastern Sabah | 0.6222 | 0.6700 | 0.3736 | 0.1164 | – |
* and *** represent statistical significance at p < 0.05 and p < 0.001, respectively.
Table 3.
AMOVA analysis comparing the genetic variation grouped by various biogeographical barrier (B1, B2 and B3) among genetic markers.
| Source of variation | CR | ND2 | ND2CR | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DF | SS | VC | PV | DF | SS | VC | PV | DF | SS | VC | PV | ||||
| No group | |||||||||||||||
| Among populations | 5 | 307.992 | 2.714*** | 52.84 | ΦST = 0.528 | 5 | 148.056 | 1.309*** | 54.83 | ΦST = 0.548 | 5 | 461.087 | 4.068*** | 53.61 | ΦST = 0.536 |
| Within populations | 130 | 314.881 | 2.422 | 47.16 | 130 | 140.115 | 1.078 | 45.17 | 130 | 457.656 | 3.520 | 46.39 | |||
| Total | 135 | 622.873 | 5.136 | 135 | 288.171 | 2.386 | 135 | 918.743 | 7.588 | ||||||
| Four groups (B1, B2 and B3) (WP, EP, SR, WS-ES) | |||||||||||||||
| Among groups | 4 | 290.966 | 2.405n.s | 44.78 | ΦSC = 0.183 | 4 | 143.015 | 1.287n.s | 51.22 | ΦSC = 0.120 | 4 | 438.849 | 3.736n.s | 46.97 | ΦSC = 0.165 |
| Among populations within groups | 1 | 17.026 | 0.544* | 10.13 | ΦST = 0.549 | 1 | 5.041 | 0.148* | 5.87 | ΦST = 0.571 | 1 | 22.237 | 0.697* | 8.76 | ΦST = 0.557 |
| Within populations | 130 | 314.881 | 2.422*** | 45.10 | ΦCT = 0.448 | 130 | 140.115 | 1.078*** | 42.90 | ΦCT = 0.512 | 130 | 457.656 | 3.520*** | 44.26 | ΦCT = 0.470 |
| Total | 135 | 622.873 | 5.371 | 135 | 288.171 | 2.512 | 135 | 918.743 | 7.953 | ||||||
| Three groups (B2 and B3) (WP-EP, SR, WS-ES) | |||||||||||||||
| Among groups | 3 | 271.678 | 2.662* | 46.98 | ΦSC = 0.194 | 3 | 121.478 | 1.070n.s | 41.17 | ΦSC = 0.295 | 3 | 397.716 | 3.779n.s | 45.31 | ΦSC = 0.228 |
| Among populations within groups | 2 | 36.314 | 0.581*** | 10.26 | ΦST = 0.572 | 2 | 26.578 | 0.451*** | 17.36 | ΦST = 0.585 | 2 | 63.370 | 1.041*** | 12.48 | ΦST = 0.578 |
| Within populations | 130 | 314.881 | 2.422*** | 42.75 | ΦCT = 0.470 | 130 | 140.115 | 1.078*** | 41.47 | ΦCT = 0.412 | 130 | 457.656 | 3.520*** | 42.21 | ΦCT = 0.453 |
| Total | 135 | 622.873 | 5.666 | 135 | 288.171 | 2.599 | 135 | 918.743 | 8.340 | ||||||
| Three groups (B1 and B2) (WP, EP, SR-WS-ES) | |||||||||||||||
| Among groups | 3 | 242.383 | 2.205n.s | 38.23 | ΦSC = 0.320 | 3 | 128.071 | 1.366* | 49.15 | ΦSC = 0.237 | 3 | 374.596 | 3.612n.s | 41.88 | ΦSC = 0.298 |
| Among populations within groups | 2 | 65.608 | 1.142*** | 19.79 | ΦST = 0.580 | 2 | 19.985 | 0.335*** | 12.06 | ΦST = 0.612 | 2 | 86.491 | 1.493*** | 17.31 | ΦST = 0.592 |
| Within populations | 130 | 314.881 | 2.422*** | 41.98 | ΦCT = 0.382 | 130 | 140.115 | 1.078*** | 38.79 | ΦCT = 0.492 | 130 | 457.656 | 3.520*** | 40.82 | ΦCT = 0.419 |
| Total | 135 | 622.873 | 5.769 | 135 | 288.171 | 2.779 | 135 | 918.743 | 8.625 | ||||||
| Two groups (B2 only) (WP-EP, SR-WS-ES) | |||||||||||||||
| Among groups | 2 | 223.095 | 2.860* | 45.79 | ΦSC = 0.285 | 2 | 106.534 | 1.362n.s | 46.71 | ΦSC = 0.306 | 2 | 333.463 | 4.272** | 46.20 | ΦSC = 0.292 |
| Among populations within groups | 3 | 84.896 | 0.964*** | 15.44 | ΦST = 0.612 | 3 | 41.522 | 0.476*** | 16.32 | ΦST = 0.630 | 3 | 127.624 | 1.454*** | 15.73 | ΦST = 0.619 |
| Within populations | 130 | 314.881 | 2.422*** | 38.78 | ΦCT = 0.458 | 130 | 140.115 | 1.078*** | 36.97 | ΦCT = 0.467 | 130 | 457.656 | 3.520*** | 38.07 | ΦCT = 0.462 |
| Total | 135 | 622.873 | 6.247 | 135 | 288.171 | 2.915 | 135 | 918.743 | 9.247 | ||||||
* and *** represent statistical significance at p < 0.05 and p < 0.001, respectively.
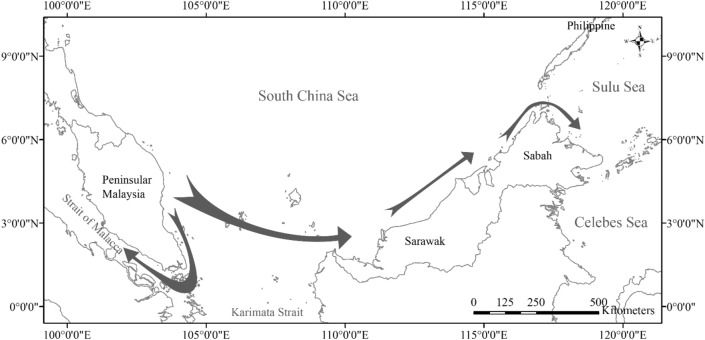
Migrate-n analyses with 10,000 steps and 50,000 steps reached convergence and were both in agreement on the direction of migration between pairs of sampling areas across all pairwise comparisons, with exception of the SR–WS pair (Table 4). An additional analysis using 100,000 steps concurred with the results using 50,000 steps for the SR–WS pair. The best models showed that gene flow occurred from EP to WP and from EP to SR. Specifically for C. punctatum sampled from Borneo, model results showed greatest support for gene flow directionality from SR to WS and from WS to ES (Table 4, Fig. 4, Supplementary Tables S4, S5).
Table 4.
Migrate-n model testing for ND2CR among the sampling area under 10,000 and 50,000 steps. The model with highest support is highlighted in bold.
| Area 1 | Area 2 | Model | 10,000 steps | 50,000 steps | ||||
|---|---|---|---|---|---|---|---|---|
| Bezier log marginal-likelihood | Model choice | Probability | Bezier log marginal-likelihood | Model choice | Probability | |||
| West Peninsular | East Peninsular | 1 ↔ 2 | − 3203.31 | 3 | 0.001 | − 3201.53 | 2 | 0.005 |
| 1 → 2 | − 3202.68 | 2 | 0.001 | − 3202.95 | 3 | 0.001 | ||
| 1 ← 2 | − 3195.81 | 1 | 0.998 | − 3196.33 | 1 | 0.993 | ||
| 1 ≠ 2 | − 3212.34 | 4 | 0.000 | − 3213.29 | 4 | 0.000 | ||
| East Peninsular | Sarawak | 1 ↔ 2 | − 3286.15 | 3 | 0.001 | − 3284.50 | 3 | 0.002 |
| 1 → 2 | − 3279.11 | 1 | 0.966 | − 3278.33 | 1 | 0.957 | ||
| 1 ← 2 | − 3282.49 | 2 | 0.033 | − 3281.97 | 2 | 0.025 | ||
| 1 ≠ 2 | − 3306.75 | 4 | 0.000 | − 3306.72 | 4 | 0.000 | ||
| Sarawak | Western Sabah | 1 ↔ 2 | − 3319.68 | 4 | 0.017 | − 3321.82 | 4 | 0.007 |
| 1 → 2 | − 3317.22 | 3 | 0.194 | − 3317.31 | 1 | 0.642 | ||
| 1 ← 2 | − 3316.12 | 1 | 0.582 | − 3318.12 | 2 | 0.286 | ||
| 1 ≠ 2 | − 3317.15 | 2 | 0.208 | − 3319.59 | 3 | 0.066 | ||
| Sarawak | Eastern Sabah | 1 ↔ 2 | − 3359.40 | 3 | 0.004 | − 3360.90 | 3 | 0.001 |
| 1 → 2 | − 3354.00 | 1 | 0.990 | − 3354.27 | 1 | 0.993 | ||
| 1 ← 2 | − 3359.15 | 2 | 0.006 | − 3359.38 | 2 | 0.006 | ||
| 1 ≠ 2 | − 3376.44 | 4 | 0.000 | − 3374.83 | 4 | 0.000 | ||
| Western Sabah | Eastern Sabah | 1 ↔ 2 | − 3152.67 | 4 | 0.041 | − 3151.58 | 2 | 0.259 |
| 1 → 2 | − 3149.85 | 1 | 0.684 | − 3150.61 | 1 | 0.684 | ||
| 1 ← 2 | − 3150.96 | 2 | 0.225 | − 3153.34 | 3 | 0.045 | ||
| 1 ≠ 2 | − 3152.46 | 3 | 0.050 | − 3156.54 | 4 | 0.002 | ||
Figure 4.
Migration route of Chiloscyllium punctatum in Malaysian waters. Direction of the arrows was based on Migrate-n model testing result. Width of arrow indicates the probability of the migration direction at 50,000 steps model (see Table 4 for actual values).
To determine the genetic structure and dispersal pattern at a more local scale, we assessed the level of genetic differentiation, haplotype network and the presence of Isolation-By-Distance (IBD) among the a priori subpopulations along the coastline of EP. No significant genetic differentiation was found among subpopulations (p > 0.05) (Table 5). Congruent with this finding, the EP haplotype network showed no clear patterns of geographical segregation of haplotypes (Fig. 5). For instance, H18 was shared among EP2, EP3, and EP5; H25 from EP1 was one mutation away from H26 from EP5 (Supplementary Tables S1 and S2). Furthermore, IBD analysis showed no correlation between genetic and geographical distances for these subpopulations (r2 = 4.73 × 10–5, p > 0.05) (Fig. 3b).
Table 5.
Pairwise FST (lower diagonal) among subpopulations from the east coast of Peninsular Malaysia and respective significance p-values (upper diagonal) according to markers.
| EP1 | EP2 | EP3 | EP4 | EP5 | |
|---|---|---|---|---|---|
| CR | |||||
| EP1 | – | ns | ns | ns | ns |
| EP2 | 0.1518 | – | ns | ns | ns |
| EP3 | − 0.0934 | − 0.2035 | – | ns | ns |
| EP4 | − 0.0666 | − 0.0302 | − 0.0447 | – | ns |
| EP5 | − 0.0235 | − 0.0477 | − 0.0574 | − 0.0725 | – |
| ND2 | |||||
| EP1 | – | ns | ns | ns | ns |
| EP2 | − 0.0740 | – | ns | ns | ns |
| EP3 | − 0.0066 | − 0.1661 | – | ns | ns |
| EP4 | − 0.0589 | 0.0211 | 0.0463 | – | ns |
| EP5 | − 0.0936 | − 0.0199 | 0.0277 | − 0.1502 | – |
| ND2CR | |||||
| EP1 | – | ns | ns | ns | ns |
| EP2 | − 0.0185 | – | ns | ns | ns |
| EP3 | − 0.0621 | − 0.1897 | – | ns | ns |
| EP4 | − 0.0648 | − 0.0154 | − 0.0225 | – | ns |
| EP5 | − 0.0401 | − 0.0392 | − 0.0357 | − 0.0850 | – |
*represent significant difference at p < 0.05 after FDR correction.
Figure 5.
Haplotype network for the five subpopulations of Chiloscyllium punctatum from East Peninsular (n = 25). Dots along the line represent number of mutations between the connected haplotypes.
Discussion
Using mitochondrial DNA markers for the bamboo shark C. punctatum, we were able to show (i) strong genetic differentiation with little present day mixing between the five areas (populations) sampled, (ii) high intra-population genetic diversity with unique haplotypes, (iii) significant correlation between genetic differentiation and geographical distance coupled with detectable presence of fine scale geographical barriers (i.e. the South China Sea), (iv) historical directional gene flow from EP bifurcating towards WP and towards Borneo (from the western end of SR to Sabah), and (v) no detectable genetic differentiation along the EP coastline. The high genetic structure observed in C. punctatum within the central area of its distribution range is also consistent with their strong coastal association and life history strategies. We discuss these results in relation to biological and geographical barriers that shape the animal’s phylogeography and implications for fishery management of this small-sized shark.
Genetic diversity
The large number of unique mtDNA haplotypes in C. punctatum found within a small region had exceeded those reported for other elasmobranchs sampled across broader geographical range, e.g. 26 haplotypes in Negaprion brevirostris58, 39 in Carcharhinus sorrah56, and 12 in Ginglymostoma cirratum17. The exception is a report of 67 haplotypes found in C. plumbeus59. Analysis using cumulative haplotype curves suggested that there are many haplotypes remaining to be discovered for C. punctatum (Supplementary Figure S1). Compared to similar sized species of sharks60, haplotype diversity of C. punctatum is relatively high while the nucleotide diversity is comparable. High number of haplotypes seen in C. punctatum could be partially explained by the haplotype genealogy61 and the relationship between life history strategies and mutation rates62. Star-shaped haplotype genealogy and high haplotype diversity with low nucleotide diversity is a typical genetic signature of recent population expansion, especially when a new habitat became available61. Evidence from this study showed some support for selective directional population expansion for C. punctatum although the demographic history may not be shared similarly for all the populations in Malaysia (discussed further below).
The high haplotype diversity and directional population expansion detected in our C. punctatum samples underscores the need for careful fisheries management to maintain the natural diversity of this highly exploited species and the role of the Malaysian stock as a source population. To date, there is no study that directly assess the relationship between overfishing and genetic diversity in shark and ray species5. Overharvesting of marine fish species has been shown to result in reduced mean body size, faster growth rate, and earlier sexual maturity63, as well as lower genetic diversity, especially allelic richness64. On the other hand, the level of genetic diversity in fishes was surprisingly not found to be lower for threatened species relative to non-threatened ones65. Our findings thus provide an important baseline data for key genetic diversity metrics that can be used as indicators of population health and overall conservation status of C. punctatum.
Genetic structure and phylogeography
The lack of genetic structure along the coastline of EP, absence of IBD and sharing of haplotype H18 among EP subpopulations suggests high gene flow and that mixing of C. punctatum genetic information could occur as far as 400 km, the longest distance between two known catch locations of the trawl survey conducted. The coastwide genetic mixing can either be facilitated by adult movement or dispersal of egg cases. No mobility study had been conducted on C. punctatum or other congeners to date. However, a closely related species with similar behaviour, the nurse shark G. cirratum (total length, TL = 430 cm) is able to travel at least 541 km66 and genetic studies on the species indicated possibility of longer distance travel along a ~ 5000 km coastline17. Another continental shelf species, South African catshark Poroderma africanum (TL = 101 cm) could travel as far as 1964 km66.
Since coastal depth is probably one major restriction for adult C. punctatum movement, ancient and present day bathymetry maps of this region suggest that the species should be able to travel along the shallow waters of coastal EP connected to WP along the Johore Strait (also known as the Tebrau Strait, with maximum depth of 16.9 m) or Singapore Strait and through the Karimata Strait to the Borneo side67–69. However significant genetic differentiation and weak support for IBD in C. punctatum across the five sampling areas indicates that benthic seascape features may form substantial barriers for adult shark movement across EP-WP and EP-SR. The barrier between EP and WP could result from intensive anthropogenic activities at the Johor and Singapore Straits. These straits, though only approximately 50 km in length, are well known for major reclamation and busy shipping traffic since 181970. Furthermore, industrial activities within the Straits are likely to impact benthic habitat availability and deter adult movement between either coasts of Peninsular Malaysia. The Karimata Strait between EP and SR had been reported to have strong unpredictable currents71,72, thus potentially limiting adult movement across the Strait between EP and SR at the western end of Borneo. Study on movement and benthic habitat use for C. punctatum will be useful to corroborate the role of distance and bottom features that shape present day genetic structure.
The westward gene flow directionality observed from EP to WP and eastward flow from EP to Borneo (Fig. 4) could be partially explained by contemporary surface ocean currents. During the north-east monsoon, ocean current flows from EP towards WP and towards SR at about 0.3–0.5 m/s73; this would be most likely consistent with a pelagic stage dispersal. Egg cases for various elasmobranch species had been recorded to have holding abilities, adhesive fibre in the case of C. punctatum, to prevent drifting, exposure to predation or damage by wave and tidal action74–76. However, ROV video footage capturing movement of egg cases along with water currents lend support for the possibility of egg case dispersal as a mode of limited population connectivity across these areas77. The gene flow directionality implies that population in EP could act as a source for populations in Borneo and in WP.
Unexpectedly, significant genetic differentiation in C. punctatum could also be observed along the northern Borneo coastline of about 385 km. Detection of a biogeographical barrier between Sarawak and Sabah suggests that benthic seascape features coupled with differing water bodies (South China Sea and Sulu Sulawesi Sea) are important in shaping the genetic structure for C. punctatum of Borneo and possible other similar sharks. This physical delineation is sufficiently significant that Scoliodon macrorhynchos, a small sized spadenose shark, could be found along the coastline of the Sarawak Borneo but not of SabahThen, unpublished data. In relation to the weak genetic structuring observed between eastern and western Sabah, further investigation is warranted. Discussions with local fishers and other researchers suggest that sharks landed in Sabah, especially by trawlers, could have been caught from the waters of the Philippines (from the Sulu-Sulawesi waters) Thus, the genetically differentiated Sabah populations and their transboundary relationship with putative populations from the Philippines should be jointly considered.
The genetic differentiation and possible directional population expansion observed in this study could reflect historical vicariance events in the Sunda Shelf region. Other marine organisms in the Indo-Malaya archipelago showed genetic separation between the east and west peninsular populations. These range from smaller and less mobile species such as gastropods Nerita albicilla78 and Echinolittorina spp.49, echinoderm Diadema setosum54, small bony fishes (mudskipper Periophthalmus argentilineatus, and reef fish Centropyge loriculus)50,54, to larger and more mobile species like wedgefish Rhynchobatus australiae55. The congruency between our data and these past studies indicated the importance of the Sunda Shelf barrier in shaping the phylogeography of marine species in this region.
Overall, the genetic patterns and differentiation observed for C. punctatum inferred from the mitochondrial DNA loci would reflect signatures that occurred over historical time scale rather than during contemporary ecological time scale. However, an additional possibility to the strong genetic structure observed can be due to behavioural traits, for instance philopatry or site fidelity which has been shown for diverse species of sharks79; thus this cannot be ruled out for the strongly coastal-associated C. punctatum. The joint use of nuclear and mitochondrial markers will be important to discern whether the observed patterns of genetic structure in C. punctatum could be attributed to philopatric tendencies or due to the presence of biogeographic barriers, or both.
Fisheries management implications
Chiloscyllium punctatum experiences high fishing pressure despite being caught primarily as non-target species (bycatch). In 2017 alone, we estimated that approximately 670,000 individuals were caught and landed at the four major shark landing states in Malaysia (namely Perak, Pahang, Sarawak, and Sabah), based on estimated landings of 750 metric tonnes of C. punctatum and average weight of C. punctatum landed in trawl fisheries of 1.12 kg31,80, Lim unpublished data. The national demersal resources were estimated to have decreased by 80% relative to assumed virgin (unexploited) levels during 1960s and this decrease was attributed to overfishing that had reportedly been ongoing for the past two decades81. As this species make up almost half of all sharks caught from trawlers, and almost half of the individuals landed were juveniles, impact of fishing pressure remains a great concern in the long run.
High level of genetic structuring in C. punctatum across Malaysian waters coupled with low levels of inter-region connectivity strongly suggests the need to consider separate fishery management stocks and conservation plans for the west coast of Peninsular, east coast of Peninsular and Borneo. These separate populations appeared to rely almost exclusively on self-recruitment (with FST mostly > 0.25 indicating low migration rate among areas)35,82; migrants would also likely find difficulty transmitting their genes locally given the territorial nature of the species. Localized population extinctions may be possible if present fishing pressure is not curbed.
Despite ongoing high fishing pressure on highly differentiated genetic stocks of C. punctatum, unique biological traits likely confer considerable resilience to this species. Chiloscyllium punctatum has shown the ability for long term sperm storage83, is extremely hardy and physiologically adapted to inhabiting environments that undergo cyclical hypoxic conditions (e.g., coral reef flats84). However, this is likely not the case for many other coastal-associated shark and ray species within the highly fished waters of Malaysia and other neighbouring countries. In addition to clarifying the genetic structuring of these exploited species, efforts to reduce present fishing effort should be prioritized. The proposed move towards a total ban of bottom trawl fishing within Malaysian waters in the next few years would substantially reduce fishing pressure on sharks and rays.
Conclusion
We present novel evidence of high genetic structure and diversity of C. punctatum within the Indo-Malaya Archipelago. Observed high genetic differentiation between the five sampled areas could be attributed to the Sunda Shelf formation acting as ancient barrier for the animals. This genetic pattern persists in the present day due to their strong coastal shelf association and likely limited adult movement, apart from limited directional gene flow between areas. Presence of contemporary barriers for population connectivity is shaped by both distance and benthic features and limited current-driven egg dispersal. Although present high fishing pressure do not appear to pose immediate concerns for C. punctatum, findings of strong genetic structure within the small geographical location considered raises the concern of the population health of other exploited small sized sharks in the area.
Materials and methods
Ethical statement
The studied shark species is not legally protected in Malaysia. All sharks were dead when encountered at the landing sites. Permission was obtained from the Department of Fisheries Malaysia (DOFM) for sampling onboard the demersal trawl survey in the east coast of Malaysia, in accordance with the DOFM sampling protocol. Collection permits and sampling protocol for samples in Sabah was approved by the Sabah Biodiversity Council [Access License Reference No: JKM/MBS.1000-2/2 JLD.9 (21–23) and Transfer License Reference No: JKM/MBS. 1000-2/3 JLD.4 (18)].
Sampling site
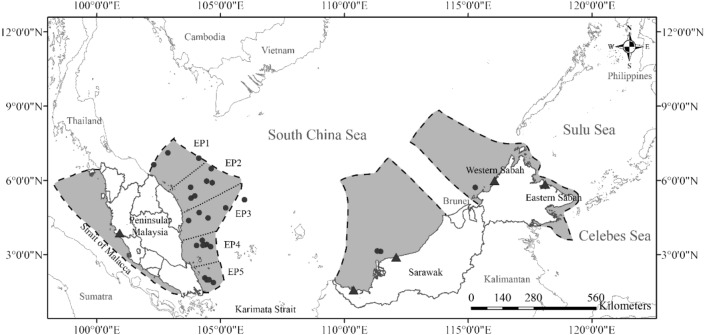
Malaysia is located in the centre of the Indo-West Pacific biogeographic region85 as well as the distribution range of C. punctatum86. It is surrounded by three major water bodies namely the Strait of Malacca, southern South China Sea and the Sulu-Celebes Sea (Fig. 6). Sampling was conducted at five major coastal areas: west coast of Peninsular Malaysia (WP), east coast of Peninsular Malaysia (EP), Sarawak (SR), western Sabah (WS) and eastern Sabah (ES) (Fig. 6). The Sabah samples were sub-divided into two areas representing different water bodies; WS faces the South China Sea while ES faces the Sulu-Celebes Sea. These sites were selected to examine the effect of biogeographical barriers to C. punctatum populations defined a priori: historical Sunda Shelf Barrier between WP and EP (B1), depth and distance barrier (South China Sea) between EP and SR (B2), and distance barrier between SR and Sabah (B3).
Figure 6.
Sampling sites for this study. Triangle = sample collected from landing sites, Circle = samples collected from Department of Fisheries demersal trawl survey, shaded area with dashed line margin denote the Malaysia Economic Exclusive Zone adapted from http://www.marineregions.org, dotted line = subpopulations separation line for East coast of Peninsular Malaysia samples.
The fin clips of 25–30 individuals per area were collected with a total of 135 individuals (Table 1). With the exception of EP, all other samples were obtained directly at fish landing sites during a 25-month ichthyofaunal survey from September 2015 to September 2017. These animals were caught via commercial fishing operations; the fishing vessels were mainly trawlers except in SR whereby some individuals were caught with gill nets. A maximum of two individuals per fishing vessel were sampled, to prevent over-sampling of maternally-linked individuals that may result in the reduction of potential haplotype diversity56,87,88. Samples from EP were obtained through a fisheries-independent demersal trawl survey within the Exclusive Economic Zone (EEZ) organised by the Department of Fisheries Malaysia from July 2015 to July 2016. This invaluable opportunity allowed us to examine finer-scale genetic structure and connectivity of C. punctatum along the 724 km-long EP coastline. The EP samples were further divided into five subpopulations (EP1 to EP5) along the coastline (Fig. 6). All tissue samples were preserved in absolute ethanol before subsequent molecular procedures.
Mitochondrial DNA extraction and analysis
The DNA of each sample was extracted using 10% Chelex resin incubated for 2 min at 60 °C, followed by 25 min at 103 °C (modified from Hyde et al.89). Two mitochondrial DNA (mtDNA) markers were employed to analyse the genetic diversity and structure of C. punctatum: Control region (CR) and NADH dehydrogenase subunit 2 (ND2). The primer sets used for the targeted regions were CR-F 5′ GAC CTT GTA AGT CGA AGA 3′ and CR-R 5′ TCT TAG CAT CTT CAG TGC 3′ for CR90, and Ilem-Mustelus 5'- AAG GAC CAC TTT GAT AGA GT -3' and Asn-Mustelus 5'- AAC GCT TAG CTG TTA ATT AA -3' for ND291. The PCR amplification was performed using a 20 µL reaction mix containing 2 µL of 10 × PCR buffer, 0.5 µL of dNTPs mixture (2.5 mM each), 1 µL of 10 pmol primer (both primers), 1.25 unit of Taq DNA polymerase (iNtRON Biotechnology, INC., Korea), 1 µL of 50 pg to 1.0 µg DNA templates, and molecular-grade water. The PCR cycles for both markers comprised of 2 min initial denaturation at 94 °C, followed by 30 cycles of 20 s at 94 °C, 20 s at 52 °C and 1 min at 72 °C, and subsequently a final extension of 5 min at 72 °C. All PCR products were examined using 1% agarose in TAE buffer prior to Sanger sequencing service at Apical Scientific Sdn Bhd (Selangor, Malaysia).
Data analysis
Sequences of the CR and ND2 regions were reviewed manually, aligned and edited using ClustalW92 implemented in Mega version 7 software93. All haplotype sequences used for the following analysis were submitted to Genbank database with accession numbers as in Supplementary Table S3. Cumulative haplotype curve using concatenated markers was constructed by randomising the sample order 999 times. The increment of new haplotypes against the number of sequences analysed was plotted using the PRIMER v6 software94. This is to observe the increment pattern of haplotype when new sequences was added in the analysis.
An additional sequence from NCBI Genbank (accession number: JQ082337; sampling location: China) that covered both CR and ND2 was included in the analysis to observe potential connection between samples in the South China Sea. Intra and inter-population genetic diversity by individual and concatenated markers (ND2CR) was characterized using DNASP v6 software95 with the following indices: the number of haplotypes (Nha), polymorphic sites (k) and, haplotype diversity (ha) and nucleotide diversity (π). Haplotype or gene diversity is the probability of getting two different alleles from random sampling while nucleotide diversity is the average differences in number of nucleotides per site among DNA sequences96. The NETWORK v5.0 software (http://www.fluxus-engineering.com was then used to construct the median-joining haplotype network to visualize the genetic connectivity among the populations.
AMOVA analysis was conducted in ARLEQUIN v3.5.2.297 with 10,000 permutations to identify the hierarchical partitioning of genetic variation under the influence of various combinations of biogeographical barriers stated earlier (B1, B2 and B3). The best model for each marker was selected using jModelTest2; since the best models identified were not implemented in ARLEQUIN, we used the next best available models, i.e., Tamura and Nei correction for CR, Kimura 2 Parameter for ND2 and Kimura 2 Parameter with Gamma 0.656 for ND2CR. Pairwise comparisons of population differentiation using FST values among (1) subpopulations in EP, and (2) all populations were determined and significant probability values were reported after correction for false discovery rate (FDR)98.
Isolation-by-distance (IBD) i.e., correlation between genetic distance and log (base 10) of geographical distances, between populations was calculated and their significance was tested using the Mantel test with 9999 randomizations. Genetic distance between populations was defined as (FST/(1 − FST))99. Pairwise geographical distance among populations was estimated as the mean value of the shortest straight-line distance between two points at the sea among the pairs adapting two turning point around southern tip of peninsular to reach WP (west point: 1.1767°N, 103.5197°E; east point: 1.3485°N, 104.3724°E) and one turning point at northern tip of Sabah to ES (7.1231°N, 116.9426°E). For EP, individual-based IBD analysis was performed given that individual catch locations were known and distances for some catch locations within a subpopulation were further than those between subpopulations; uncorrected p-distance calculated in PAUP* 4.0b10 software100. The IBD analysis was performed using Adegenet version 2.1.1 package in R version 3.5.1 software101.
Historical demographic expansion was inspected using Fu’s57 Fs and Tajima’s D tests102 of neutrality. Mismatch distribution analysis was subsequently conducted to assess demographic expansion using individual and combined markers in DnaSP v6.10.0396. The migration pattern of C. punctatum was determined by assessing the migration rate, population size and migration direction using the Bayesian Inference in Migrate-n v3.6.11103–105. The analysis was performed on both individual and concatenated markers (ND2CR). Migrate-n allows for the estimation of relative effective population size, θNe = (Neμ), and asymmetric gene flow, M (m/μ), among populations over longer periods of time (> 1000 years)106 with the assumption of constant population size, random mating within population, constant mutation rate, constant immigration rate and genetic materials can only exchange through migrants (no population divergence is allowed)1. Results from both pairwise testing and haplotype network were used to guide the selection of a priori population pairs for statistical testing and to reduce the number of models considered in Migrate-n. Five population pairs (WP-EP, EP-SR, SR-WS, SR-ES and WS-ES) were subjected to four migration models (1 ↔ 2: bi directional; 1 → 2: unidirectional from population 1 to 2; 1 ← 2: unidirectional from population 2 to 1; 1 ≠ 2: no gene flow) under 10,000 and 50,000 recorded Markov chain Monte Carlo (MCMC) steps; additional analyses using 100,000 steps were done when results did not agree between runs of 10,000 and 50,000 steps. Four-chain heating at temperatures of 1, 1.5, 3 and 1,000,000 was applied to increase the efficiency of the MCMC. Other analysis criteria followed the default settings Modelling protocol and convergence detection followed the guidelines by Beerli et al.107. Comparison of models was performed using the Bayes factors based on guidelines by Kass and Raftery108.
Supplementary Information
Acknowledgements
We thank the University Malaya Research Grant (UMRG) RP018C-16SUS, the University Malaya Research Fund Assistance (BKP) BK018-2015, WWF-Malaysia private fund PV049-2019 and the Top 100 Universities in The World Fund TU001-2018 for financial support of the study. We are grateful to the Department of Fisheries Malaysia and Fisheries Research Institute (FRI) Bintawa, Sarawak for the opportunity to participate in the fisheries-independent demersal trawl survey within the Malaysian Exclusive Economic Zone (EEZ). We thank the three anonymous reviewers for their comments that have significantly improved the manuscript.
Author contributions
K.C.L. and A.Y.T conceived the idea and designed the research methodology. K.C.L., K.H.L. and A.Y.T. collected the samples (tissue samples). K.C.L. work on the molecular analysis and data analysis. All authors participated in work conceptualization and result interpretations. All authors contributed critically to the drafts and gave final approval for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Amy Yee-Hui Then, Email: amy_then@um.edu.my.
Kar-Hoe Loh, Email: khloh@um.edu.my.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-94257-7.
References
- 1.Bertorelle G, Bruford MW, Hauffe HC, Rizzoli A, Vernesi C. Population Genetics for Animal Conservation (Conservation Biology) Cambridge University Press; 2009. [Google Scholar]
- 2.Allendorf FW, Luikart G, Aitken SN. Conservation and the Genetics of Populations. Wiley; 2013. [Google Scholar]
- 3.Dulvy NK, et al. Extinction risk and conservation of the world's sharks and rays. Elife. 2014;3:e00590. doi: 10.7554/eLife.00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sandoval-Castillo J, Beheregaray LB. Metapopulation structure informs conservation management in a heavily exploited coastal shark (Mustelus henlei) Mar. Ecol. Prog. Ser. 2015;533:191–203. doi: 10.3354/meps11395. [DOI] [Google Scholar]
- 5.Domingues RR, Hilsdorf AWS, Gadig OBF. The importance of considering genetic diversity in shark and ray conservation policies. Conserv. Genet. 2017;19(3):501–525. doi: 10.1007/s10592-017-1038-3. [DOI] [Google Scholar]
- 6.Allendorf FW, England PR, Luikart G, Ritchie PA, Ryman N. Genetic effects of harvest on wild animal populations. Trends Ecol. Evol. 2008;23(6):327–337. doi: 10.1016/j.tree.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Keeney DB, Heist EJ. Worldwide phylogeography of the blacktip shark (Carcharhinus limbatus) inferred from mitochondrial DNA reveals isolation of western Atlantic populations coupled with recent Pacific dispersal. Mol. Ecol. 2006;15:3669–3679. doi: 10.1111/j.1365-294X.2006.03036.x. [DOI] [PubMed] [Google Scholar]
- 8.Nance HA, Klimley P, Galván-Magaña F, Martínez-Ortíz J, Marko PB. Demographic processes underlying subtle patterns of population structure in the scalloped hammerhead shark, Sphyrnalewini. PLoS One. 2011;6(7):e21459. doi: 10.1371/journal.pone.0021459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt JV, et al. Low genetic differentiation across three major ocean populations of the whale shark, Rhincodontypus. PLoS One. 2009;4:e4988. doi: 10.1371/journal.pone.0004988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pardini AT, et al. Sex-biased dispersal of great white sharks. Nature. 2001;412:139–140. doi: 10.1038/35084125. [DOI] [PubMed] [Google Scholar]
- 11.Jorgensen SJ, et al. Philopatry and migration of Pacific white sharks. Proc. R. Soc. B. 2009;277:679–688. doi: 10.1098/rspb.2009.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blower DC, Pandolfi JM, Bruce BD, Gomez-Cabrera MD, Ovenden JR. Population genetics of Australian white sharks reveals fine-scale spatial structure, transoceanic dispersal events and low effective population sizes. Mar. Ecol. Prog. Ser. 2012;455:229–244. doi: 10.3354/meps09659. [DOI] [Google Scholar]
- 13.Oñate-González EC, Rocha-Olivares A, Saavedra-Sotelo N, Sosa-Nishizaki O. Mitochondrial genetic structure and matrilineal origin of white sharks, Carcharodon carcharias in the Northeastern Pacific: Implications for their conservation. J. Hered. 2015;106(4):347–354. doi: 10.1093/jhered/esv034. [DOI] [PubMed] [Google Scholar]
- 14.O’Leary SJ, et al. Genetic diversity of white sharks, Carcharodon carcharias, in the Northwest Atlantic and Southern Africa. J. Hered. 2015;106(3):258–265. doi: 10.1093/jhered/esv001. [DOI] [PubMed] [Google Scholar]
- 15.Andreotti S, et al. An integrated mark-recapture and genetic approach to estimate the population size of white sharks in South Africa. Mar. Ecol. Prog. Ser. 2016;552:241–253. doi: 10.3354/meps11744. [DOI] [Google Scholar]
- 16.Fu MN, Wang J, Ding SX, Du JY, Su YQ. Studies on the genetic structure and genetic subdivision of white spotted bamboo shark, Chiloscyllium plagiosum, by analyzing mitochondrial Cyt b genes. J. Trop. Oceanogr. 2010;29:86–91. [Google Scholar]
- 17.Karl SA, Castro ALF, Garla RC. Population genetics of the nurse shark (Ginglymostoma cirratum) in the western Atlantic. Mar. Biol. 2012;159:489–498. doi: 10.1007/s00227-011-1828-y. [DOI] [Google Scholar]
- 18.Whitney NM, Robbins WD, Schultz JK, Bowen BW, Holland KN. Oceanic dispersal in a sedentary reef shark (Triaenodon obesus): Genetic evidence for extensive connectivity without a pelagic larval stage. J. Biogeogr. 2012;39(6):1144–1156. doi: 10.1111/j.1365-2699.2011.02660.x. [DOI] [Google Scholar]
- 19.Barker AM, Nosal AP, Lewallen EA, Burton RS. Genetic structure of leopard shark (Triakis semifasciata) populations along the Pacific coast of North America. J. Exp. Mar. Biol. Ecol. 2015;472:151–157. doi: 10.1016/j.jembe.2015.06.020. [DOI] [Google Scholar]
- 20.Bitalo DN, Maduna SN, da Silva C, Roodt-Wilding R, Bester-van der Merwe AE. Differential gene flow patterns for two commercially exploited shark species, tope (Galeorhinus galeus) and common smoothhound (Mustelus mustelus) along the south–west coast of South Africa. Fish. Res. 2015;172:190–196. doi: 10.1016/j.fishres.2015.07.003. [DOI] [Google Scholar]
- 21.Fricke, R., Eschmeyer, W. N. & Van der Laan, R. (eds). Eschmeyer's Catalog of Fishes: Genera, Species, References. http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp (2020).
- 22.Wilga CAD, Lauder GV. In: Biology of Sharks and Their Relatives. Jeffrey CC, Musick JA, Heithaus MR, editors. CRC Press; 2004. pp. 139–164. [Google Scholar]
- 23.Compagno, L. J. V. Sharks of the world. An annotated and illustrated catalogue of shark species known to date. Volume 2 Bullhead, mackerel and carpet sharks (Heterodontiformes, Lamniformes and Orectolobiformes) (FAO, 2001).
- 24.Miki T. Spawning, hatching, and growth of the whitespotted bamboo shark, Chiloscyllium plagiosum. J. Jpn. Assoc. Zool. Aqua. 1994;36:10–19. [Google Scholar]
- 25.Masuda M, Teshima M. Reproduction of the whitespotted bamboo shark (Chiloscyllium plagiosum) in an aquarium. J. Jpn. Assoc. Zool. Aqua. 1994;36:20–23. [Google Scholar]
- 26.Jagadis I, Ignatius B. Captive breeding and rearing of grey bamboo shark, Chiloscyllium griseum (Müller 1839) Indian J. Fish. 2003;50:539–542. [Google Scholar]
- 27.Chen WK, Liu KM. Reproductive biology of white spotted bamboo shark Chiloscyllium plagiosum in the northern waters off Taiwan. Fish. Sci. 2006;72:1215–1224. doi: 10.1111/j.1444-2906.2006.01279.x. [DOI] [Google Scholar]
- 28.Allen GR, Erdmann MV, White WT, Fahmi, Dudgeon CL. Review of the bamboo shark genus Hemiscyllium (Orectolobiformes: Hemiscyllidae) J. Ocean Sci. Found. 2016;23:51–97. [Google Scholar]
- 29.Dudgeon, C. L., Bennett, M. B. & Kyne, P. M. Chiloscyllium punctatum. The IUCN Red List of Threatened Species 2016: e.T41872A68616745. 10.2305/IUCN.UK.2016-1.RLTS.T41872A68616745.en (2016). [DOI]
- 30.SEAFDEC. Report on the Study on Shark Production, Utilization and Management in the ASEAN Region 2003–2004 (Bangkok, 2006).
- 31.Department of Fisheries Malaysia. Malaysia National Plan of Action for the Conservation and Management of Shark (Putrajaya, 2006).
- 32.Arai T, Azri A. Diversity, occurrence and conservation of sharks in the southern South China Sea. PLoS One. 2019;14(3):e0213864. doi: 10.1371/journal.pone.0213864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phillips NM, Chaplin JA, Morgan DL, Peverell SC. Population genetic structure and genetic diversity of three critically endangered Pristis sawfishes in Australian waters. Mar. Biol. Res. 2011;158(4):903–915. doi: 10.1007/s00227-010-1617-z. [DOI] [Google Scholar]
- 34.Bitalo DN, Maduna SN, da Silva C, Roodt-Wilding R, Bester-van der Merwe AE. Differential gene flow patterns for two commercially exploited shark species, tope (Galeorhinus galeus) and common smoothhound (Mustelus mustelus) along the south-west coast of South Africa. Fish. Res. 2015;172:190–196. doi: 10.1016/j.fishres.2015.07.003. [DOI] [Google Scholar]
- 35.Vargas-Caro C, Bustamante C, Bennett MB, Ovenden JR. Towards sustainable fishery management for skates in South America: The genetic population structure of Zearaja chilensis and Dipturus trachyderma (Chondrichthyes, Rajiformes) in the south-east Pacific Ocean. PLoS One. 2017;12(2):e0172255. doi: 10.1371/journal.pone.0172255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puckridge M, Last PR, White WT, Andreakis N. Phylogeography of the Indo-West Pacific maskrays (Dasyatidae, Neotrygon): A complex example of chondrichthyan radiation in the Cenozoic. Ecol. Evol. 2013;3(2):217–232. doi: 10.1002/ece3.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kousteni V, Kasapidis P, Kotoulas G, Megalofonou P. Strong population genetic structure and contrasting histories for the small-spotted catshark (Scyliorhinus canicula) in the Mediterranean Sea. Heredity. 2015;114(3):333–343. doi: 10.1038/hdy.2014.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le Port A, Lavery S. Population structure and phylogeography of the short-tailed stingray, Dasyatis brevicaudata (Hutton 1875), in the Southern Hemisphere. J. Hered. 2012;103(2):174–185. doi: 10.1093/jhered/esr131. [DOI] [PubMed] [Google Scholar]
- 39.Ovenden JR, et al. Negligible evidence species for regional genetic population structure for two shark species Rhizoprionodon acutus (Rüppell, 1837) and Sphyrna lewini (Griffith & Smith, 1834) with contrasting biology. Mar. Biol. 2011;158:1497–1509. doi: 10.1007/s00227-011-1666-y. [DOI] [Google Scholar]
- 40.Okes, N. & Sant, G. An Overview of Major Shark Traders, Catchers and Species (Cambridge, 2019).
- 41.Dudgeon CL, Broderick D, Ovenden R. IUCN classification zones concord with, but underestimate, the population genetic structure of the zebra shark Stegostoma fasciatum in the Indo-West Pacific. Mol. Ecol. 2009;18:248–261. doi: 10.1111/j.1365-294X.2008.04025.x. [DOI] [PubMed] [Google Scholar]
- 42.DiBattista JD, Rocha LA, Craig MT, Feldheim KA, Bowen BW. Phylogeography of two closely related Indo-Pacific butterflyfishes reveals divergent evolutionary histories and discordant results from mtDNA and microsatellites. J. Hered. 2012;103(5):617–629. doi: 10.1093/jhered/ess056. [DOI] [PubMed] [Google Scholar]
- 43.Ludt WB, Bernal MA, Bowen BW, Rocha LA. Living in the past: Phylogeography and population histories of Indo-Pacific wrasses (genus Halichoeres) in shallow lagoons versus outer reef slopes. PLoS One. 2012;7(6):e38042. doi: 10.1371/journal.pone.0038042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schultz JK, et al. Global phylogeography and seascape genetics of the lemon sharks (genus Negaprion) Mol. Ecol. 2008;17:5336–5348. doi: 10.1111/j.1365-294X.2008.04000.x. [DOI] [PubMed] [Google Scholar]
- 45.Lim HC, Rahman MA, Lim SLH, Moyle RG, Sheldon FH. Revisiting Wallace's haunt: Coalescent simulations and comparative niche modeling reveal historical mechanisms that promoted avian population divergence in the Malay Archipelago. Evolution. 2010;65(2):321–334. doi: 10.1111/j.1558-5646.2010.01105.x. [DOI] [PubMed] [Google Scholar]
- 46.Tan MP, Jamsari AFJ, Siti Azizah MN. Phylogeographic pattern of the Striped Snakehead, Channa striata in Sundaland: Ancient river connectivity, geographical and anthropogenic signatures. PLoS One. 2012;7(12):e52089. doi: 10.1371/journal.pone.0052089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hall R. Cenozoic geological and plate tectonic evolution of SE Asia and the SW Pacific: Computer-based reconstructions, model and animations. J. Asian Earth Sci. 2002;20:353–431. doi: 10.1016/S1367-9120(01)00069-4. [DOI] [Google Scholar]
- 48.Sepkoski J. A compendium of fossil marine animal genera (Chondrichthyes entry) Bull. Am. Paleontol. 2002;364:560. [Google Scholar]
- 49.Reid DG, et al. Comparative phylogeography and species boundries in Echinolittorina snails in the central Indo-West Pacific. J. Biogeogr. 2006;33(6):990–1006. doi: 10.1111/j.1365-2699.2006.01469.x. [DOI] [Google Scholar]
- 50.Polgar G, et al. Phylogeography and demographic history of two widespread Indo-Pacific mudskippers (Gobiidae: Periophthalmus) Mol. Phylogenet. Evol. 2014;73:161–176. doi: 10.1016/j.ympev.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 51.Leonard JA, et al. Phylogeography of vertebrates on the Sunda Shelf: A multi-species comparison. J. Biogeogr. 2015;42(5):871–879. doi: 10.1111/jbi.12465. [DOI] [Google Scholar]
- 52.Ma KY, Van Herwerden L, Newman SJ, Berumen ML, Choat JH, Chu KH, de Mitcheson YS. Contrasting population genetic structure in three aggregating groupers (Percoidei: Epinephelidae) in the Indo-West Pacific: The importance of reproductive mode. BMC Evol. Biol. 2018;18(1):180. doi: 10.1186/s12862-018-1284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mason VC, Helgen KM, Murphy WJ. Comparative phylogeography of forest-dependent mammals reveals paleo-forest corridors throughout Sundaland. J. Hered. 2018;110(2):158–172. doi: 10.1093/jhered/esy046. [DOI] [PubMed] [Google Scholar]
- 54.Crandall ED, et al. The molecular biogeography of the Indo-Pacific: Testing hypotheses with multispecies genetic patterns. Glob. Ecol. Biogeogr. 2019;28:943–960. doi: 10.1111/geb.12905. [DOI] [Google Scholar]
- 55.Giles JL, Riginos C, Naylor GJ, Dharmadi, Ovenden JR. Genetic and phenotypic diversity in the wedgefish Rhynchobatus australiae, a threatened ray of high value in the shark fin trade. Mar. Ecol. Prog. Ser. 2016;548:165–180. doi: 10.3354/meps11617. [DOI] [Google Scholar]
- 56.Giles JL, et al. Extensive genetic population structure in the Indo-West Pacific spot-tail shark, Carcharhinus sorrah. Bull. Mar. Sci. 2014;90(1):427–454. doi: 10.5343/bms.2013.1009. [DOI] [Google Scholar]
- 57.Fu YX. Statistical tests of neutrality of mutations against population growth, hitchhiking, and background selection. Genetics. 1997;147:915–925. doi: 10.1093/genetics/147.2.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ashe JL, et al. Local population structure and context-dependent isolation by distance in a large coastal shark. Mar. Ecol. Prog. Ser. 2015;520:203–216. doi: 10.3354/meps11069. [DOI] [Google Scholar]
- 59.Portnoy DS, McDowell JR, Heist EJ, Musick JA, Graves JE. World phylogeography and male-mediated gene flow in the sandbar shark, Carcharhinus plumbeus. Mol. Ecol. 2010;19(10):1994–2010. doi: 10.1111/j.1365-294X.2010.04626.x. [DOI] [PubMed] [Google Scholar]
- 60.Domingues RR, Amorim AF, Hilsdorf AWS. Genetic identification of Carcharhinus sharks from the southwest Atlantic Ocean (Chondrichthyes: Carcharhiniformes) J. Appl. Ichthyol. 2013;29(4):738–742. doi: 10.1111/jai.12154. [DOI] [Google Scholar]
- 61.Hernández S, et al. Demographic history and the South Pacific dispersal barrier for school shark (Galeorhinus galeus) inferred by mitochondrial DNA and microsatellite DNA mark. Fish. Res. 2015;167:132–142. doi: 10.1016/j.fishres.2015.02.010. [DOI] [Google Scholar]
- 62.Britten RJ. Rates of DNA sequence evolution differ between taxonomic groups. Science. 1986;231(4744):1393–1398. doi: 10.1126/science.3082006. [DOI] [PubMed] [Google Scholar]
- 63.Belgrano A, Fowler CW. How fisheries affect evolution. Science. 2013;342(6163):1176–1177. doi: 10.1126/science.1245490. [DOI] [PubMed] [Google Scholar]
- 64.Pinsky ML, Palumbi SR. Meta-analysis reveals lower genetic diversity in overfished populations. Mol. Ecol. 2014;23(1):29–39. doi: 10.1111/mec.12509. [DOI] [PubMed] [Google Scholar]
- 65.Martinez AS, Willoughby JR, Christie MR. Genetic diversity in fishes is influenced by habitat type and life-history variation. Ecol. Evol. 2018;8(23):12022–12031. doi: 10.1002/ece3.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kohler NE, Turner PA. In: The Behavior and Sensory Biology of Elasmobranch Fishes: An Anthology in Memory of Donald Richard Nelson. Tricas TC, Gruber SH, editors. Springer; 2001. pp. 191–224. [Google Scholar]
- 67.Voris HK. Maps of Pleistocene sea levels in Southeast Asia: Shorelines, river systems and time durations. J. Biogeogr. 2000;27(5):1153–1167. doi: 10.1046/j.1365-2699.2000.00489.x. [DOI] [Google Scholar]
- 68.Lipa BJ, Barrick DE, Bourg J, Nyden BB. HF radar detection of tsunamis. J. Oceanogr. 2006;62:705–716. doi: 10.1007/s10872-006-0088-9. [DOI] [Google Scholar]
- 69.Lee WK, Zaharuddin NHA. Lagragian investigation on the compound effects of reclamation and proposed tidal barrage to the environmental flow. Jurnal Kejuruteraan. 2015;27:71–80. doi: 10.17576/jkukm-2015-27-10. [DOI] [Google Scholar]
- 70.Mohd Rusli, M. H. Straits of Malacca and Singapore: Pride of the Malay Archipelago, priceless maritime heritage of the world. Jurnal Hadhari Special Edition, 109–127 (2012).
- 71.Susanto RD, et al. Observations of the Karimata Strait throughflow from December 2007 to November 2008. Acta Oceanol. Sin. 2013;32(5):1–6. doi: 10.1007/s13131-013-0307-3. [DOI] [Google Scholar]
- 72.Wei Z, et al. Tidal elevation, current, and energy flux in the area between the South China Sea and Java Sea. Ocean Sci. 2016;12(2):517–531. doi: 10.5194/os-12-517-2016. [DOI] [Google Scholar]
- 73.Wee AKS, et al. Oceanic currents, not land masses, maintain the genetic structure of the mangrove Rhizophoramucronata Lam. (Rhizophoraceae) in Southeast Asia. J. Biogeogr. 2014;41(5):954–964. doi: 10.1111/jbi.12263. [DOI] [Google Scholar]
- 74.Heiden TCK, Haines AN, Manire C, Lombardi J, Koob TJ. Structure and permeability of the egg capsule of the bonnethead shark, Sphyrna tiburo. J. Exp. Zool. A Comp. Exp. Biol. 2005;303(7):577–589. doi: 10.1002/jez.a.171. [DOI] [PubMed] [Google Scholar]
- 75.Ehrlich H. Biological Materials of Marine Origin: Vertebrates. Springer; 2015. [Google Scholar]
- 76.Gordon, C. The great eggcase hunt: Celebrating >100,000 records. The Shark Trust (2016).
- 77.Flammang BE, Ebert DA, Cailliet GM. Intraspecific and interspecific spatial distribution of three eastern North Pacific catshark species and their egg cases (Chondrichthyes: Scyliorhinidae) Breviora. 2011;525(1):1–18. doi: 10.3099/0006-9698-525.1.1. [DOI] [Google Scholar]
- 78.Benzie JAH. Genetic structure of coral reef organisms: Ghosts of dispersal past. Am. Zool. 1999;39:131–145. doi: 10.1093/icb/39.1.131. [DOI] [Google Scholar]
- 79.Crandall ED, Frey MA, Grosberg RK, Barber PH. Contrasting demographic history and phylogeographical patterns in two Indo-Pacific gastropods. Mol. Ecol. 2008;17(2):611–626. doi: 10.1111/j.1365-294X.2007.03600.x. [DOI] [PubMed] [Google Scholar]
- 80.Hueter RE, Heupel MR, Heist EJ, Keeney DB. Evidence of philopatry in sharks and implications for the management of shark fisheries. J. Northwest Atl. Fish. Sci. 2005;35:239–247. doi: 10.2960/J.v35.m493. [DOI] [Google Scholar]
- 81.Department of Fisheries Malaysia. Annual Fisheries Statistics, Department of Fisheries Malaysia. Ministry of Agriculture and Agro-based Industry Malaysia https://www.dof.gov.my/index.php/pages/view/3343 (2017).
- 82.Fisheries Research Institute (FRI). Fisheries Resources Survey in Malaysian Waters 2013–2016 Executive Summary (Pulau Pinang, 2017)
- 83.Ovenden JR, Kashiwagi T, Broderick D, Giles J, Salini J. The extent of population genetic subdivision differs among four co-distributed shark species in the Indo-Australian archipelago. BMC Evol. Biol. 2009;9:40. doi: 10.1186/1471-2148-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bernal MA, et al. Long-term sperm storage in the brownbanded bamboo shark Chiloscyllium punctatum. J. Fish Biol. 2015;86(3):1171–1176. doi: 10.1111/jfb.12606. [DOI] [PubMed] [Google Scholar]
- 85.Chapman CA, Harahush BK, Renshaw GMC. The physiological tolerance of the grey carpet shark (Chiloscyllium punctatum) and the epaulette shark (Hemiscyllium ocellatum) to anoxic exposure at three seasonal temperatures. Fish Physiol. Biochem. 2011;37(3):387–399. doi: 10.1007/s10695-010-9439-y. [DOI] [PubMed] [Google Scholar]
- 86.Hoeksema BW. In: Biogeography, Time, and Place: Distributions, Barriers, and Islands. Renema W, editor. Springer; 2007. pp. 117–178. [Google Scholar]
- 87.Galindo-Cardona A, Acevedo-Gonzalez JP, Rivera-Marchand B, Giray T. Genetic structure of the gentle Africanized honey bee population (gAHB) in Puerto Rico. BMC Genet. 2013;14:65. doi: 10.1186/1471-2156-14-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Verscheure S, Backeljau T, Desmyter S. Reviewing population studies for forensic purposes: Dog mitochondrial DNA. ZooKeys. 2013;365:381–411. doi: 10.3897/zookeys.365.5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hyde JR, et al. Shipboard identification of fish eggs and larvae by multiplex PCR, and a description of fertilized eggs of blue marlin, shortbill spearfish, and wahoo. Mar Ecol. Prog. Ser. 2005;286:269–277. doi: 10.3354/meps286269. [DOI] [Google Scholar]
- 90.Xie YJ, Su YQ, Weng ZH, Wang J, Wang ZY. Studies on mitochondrial DNA control region and cytochrome b gene sequences of white spotted bambooshark, Chiloscyllium plagiosum. Mar. Sci. 2008;32(12):35–41. [Google Scholar]
- 91.Naylor GJP, Ryburn JA, Fedrigo O, Lopez JA. In: Reproductive Biology and Phylogeny: Sharks, Skates, Stingrays, and Chimaeras. Hamlett WC, Jamieson BGM, editors. Science Publishers; 2005. pp. 1–25. [Google Scholar]
- 92.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22(22):4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kumar S, Stecher G, Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33(7):1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Clarke, K. R. & Gorley, R. N. PRIMER V6; user manual/tutorial. Plymouth: PRIMER-E (2006).
- 95.Rozas J, Ferrer-Mata A, Sánchez-DelBarrio JC, Guirao-Rico S, Librado P, Ramos-Onsins SE, Sánchez-Gracia A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017;34(12):3299–3302. doi: 10.1093/molbev/msx248. [DOI] [PubMed] [Google Scholar]
- 96.Nei M. Molecular Evolutionary Genetics. Columbia University Press; 1987. [Google Scholar]
- 97.Excoffier L, Lischer HE. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010;10(3):564–567. doi: 10.1111/j.1755-0998.2010.02847.x. [DOI] [PubMed] [Google Scholar]
- 98.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B. 1995;57:289–300. doi: 10.2307/2346101. [DOI] [Google Scholar]
- 99.Rousset F. Genetic differentiation and estimation of gene flow from F-statistics under isolation by distance. Genetics. 1997;145(4):1219–1228. doi: 10.1093/genetics/145.4.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Swofford, D. L. PAUP*: Phylogenetic analysis using parsimony (* and other methods). Version 4 (Sunderland, 2002).
- 101.R Core Team. R: A language and environment for statistical computing (Vienna, 2018).
- 102.Tajima F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics. 1989;123:585–595. doi: 10.1093/genetics/123.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Beerli P, Felsenstein J. Maximum likelihood estimation of a migration matrix and effective population sizes in n subpopulations by using a coalescent approach. Proc. Natl. Acad. Sci. U.S.A. 2001;98(8):4563–4568. doi: 10.1073/pnas.081068098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Beerli P. Comparison of Bayesian and maximum likelihood inference of population genetic parameters. Bioinformatics. 2006;22(3):341–345. doi: 10.1093/bioinformatics/bti803. [DOI] [PubMed] [Google Scholar]
- 105.Beerli P. In: Population Genetics for Animal Conservation, Conservation Biology. Bertorelle G, Bruford MW, Hauffe HC, Rizzoli A, Vernesi C, editors. Cambridge University Press; 2009. pp. 42–79. [Google Scholar]
- 106.Beerli, P. MIGRATE version 3.6.5: A maximum likelihood and Bayesian estimator of gene flow using the coalescent. http://popgen.scs.edu/migrate.html (2008).
- 107.Beerli P, Mashayekhi S, Sadeghi M, Khodaei M, Shaw K. Population genetic inference with MIGRATE. Curr. Protoc. Bioinform. 2019;68(1):e87. doi: 10.1002/cpbi.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kass RE, Raftery AE. Bayes factors. J. Am. Stat. Assoc. 1995;90(430):773–795. doi: 10.1080/01621459.1995.10476572. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.