Abstract
Background
Inhalational exposures are increasingly recognized as contributing factors in interstitial lung disease (ILD). However, the characteristics of both exposures and exposed patients are not well understood. We hypothesized that domestic and occupational inhalational exposures would be common and associated with differences in demographics, clinical characteristics, and transplant-free survival in patients with all forms of ILD.
Research Question
What is the prevalence of inhalational exposures across all ILD diagnoses, and are these exposures associated with differences in demographics, clinical characteristics, and transplant-free survival?
Study Design and Methods
Patients from a tertiary ILD clinic underwent an interview designed to capture inhalational exposures including occupational, home, hobbies, and tobacco. Demographic and survival data were collected from the electronic medical record. Survival analysis was performed using Cox regression to compare exposed vs unexposed patients and adjusted for gender-age-physiology score and smoking.
Results
One hundred and fifty-six patients seen between May and October 2018 were analyzed. Patients had a wide variety of multidisciplinary diagnoses, with a minority of patients with hypersensitivity pneumonitis (14%). One hundred and one patients (65%) had potentially relevant inhalational exposures. More men than women had a history of any exposure (82% vs 51%; P < .001), occupational exposure (66% vs 14%, P < .001), and multiple exposures (56% vs 26%, P < .001), respectively. White race was associated with bird and hobby exposure. Patients with any exposure had worse transplant-free survival (unadjusted hazard ratio, 2.58; 95% CI, 1.13-5.92; P = .025), but this was not statistically significant after adjustment (hazard ratio, 1.82; 95% CI, 0.77-4.27; P = .17).
Interpretation
A standardized interview revealed most patients across all types of ILD had potentially relevant inhalational exposures. Exposures were markedly different based on demographics and were associated with worse transplant-free survival, but this survival difference was not significant after multivariable adjustment. Identification and avoidance of exposures represent actionable targets in ILD management.
Key Words: environmental health, interstitial lung disease, lung diseases, occupational disease
Abbreviations: ATS, American Thoracic Society; CTD, connective tissue disease; CTD-ILD, connective tissue disease-associated interstitial lung disease; EMR, electronic medical record; GAP, gender-age-physiology; HP, hypersensitivity pneumonitis; HRCT, high-resolution CT; ILD, interstitial lung disease; IPAF, interstitial pneumonia with autoimmune features; IPF, idiopathic pulmonary fibrosis; MDD, multidisciplinary diagnosis; PAF, population attributable fraction
FOR EDITORIAL COMMENT, SEE PAGE 19
Take-home Points.
Study Question: What is the prevalence of inhalational exposures across all interstitial lung disease (ILD) diagnoses, and are these exposures associated with differences in demographics, clinical characteristics, and transplant-free survival?
Results: In a tertiary care ILD clinic with a wide variety of ILD diagnoses, 65% of patients had potentially relevant inhalational exposures, which were associated with male sex and worse unadjusted transplant-free survival.
Interpretation: Inhalational exposures are common across all ILD subtypes, and their presence represents an actionable target in ILD management.
Interstitial lung disease (ILD) encompasses a diverse group of generally progressive, often fibrotic pulmonary disorders with an increasing incidence and mortality similar to some cancers.1 Whether these disorders are thought to be directly associated with environmental and occupational exposures differs based on ILD subtype. Pneumoconioses are a subset of ILDs that are known to result from exposure to certain workplace particulates (eg, silica, asbestos), with characteristic radiographic patterns of ILD.2,3 Similarly, hypersensitivity pneumonitis (HP) is thought to arise from inflammation and fibrosis in the setting of an inhaled antigen.4 However, some patients with other ILD subtypes that are not classically associated with specific inhalational dusts (eg, idiopathic pulmonary fibrosis [IPF]) also have a higher rate of occupational and organic dust exposures.5,6
The American Thoracic Society (ATS)7 released a document highlighting the burden of occupational causes in a spectrum of nonmalignant respiratory disorders. In this review, the pooled population attributable fraction (PAF) for occupational vapors, gas, dust, or fumes in IPF was 26%, and the PAF of occupational vapors, gas, dust, or fumes or HP was 19%. Not included in this analysis were exposures in the home (eg, mold, bird antigens), or connective tissue disease-associated interstitial lung disease (CTD-ILD), in which specific exposures have been related to certain connective tissue diseases (CTDs) but not to the autoimmune ILDs generally.8 The prevalence of these exposures throughout the spectrum of ILDs and clinical phenotypes arising from differential exposures remains unknown, and literature regarding the relationship between exposures and clinical outcomes is contradictory.9, 10, 11, 12
In this study, we systematically performed an extensive inhalational exposure evaluation in a tertiary care ILD center to elicit the prevalence and nature of inhalational exposures across a wide variety of ILD diagnoses. We hypothesized that in patients with ILD regardless of specific multidisciplinary diagnosis (MDD), inhalational exposures would be common, have differential prevalence by sex and race, and be associated with worse pulmonary function and survival compared with patients with ILD without exposures. We also hypothesized that inhalational exposures would be prevalent in CTD-ILD and interstitial pneumonia with autoimmune features (IPAF).
Methods
Study Design and Patient Selection
We analyzed consecutive patients seen in the ILD clinic from May to October 2018 of one provider (M. E. S.) with > 20 years of experience in the field of ILD. Patients were enrolled in the University of Chicago ILD Registry, a prospectively acquired cohort of patients with ILD approved by the University of Chicago institutional review board (protocol No. 14163-A), and all patients signed informed consent.
Using an already existing, ILD clinic-based dotphrase in the electronic medical record (EMR) (Fig 1), patients were assessed for inhalational exposures at home and work including mold, birds and down, lifetime occupational history when possible and occupational dust exposure, and hobbies known to be associated with ILD. A dotphrase is an addition to the note in the EMR that requires the provider to answer yes or no questions with the optional addition of free text. In Figure 1, free text denotes that the provider captured other exposures that fit in that category (eg, other occupations known to be associated with either IPF or HP).13,14 In an area separate from the dotphrase in the clinic note, family history of ILD or CTD and primary or secondhand smoking status were assessed narratively. The dotphrase as a general clinical tool has been previously found to be comparable with paper questionnaires.15
Figure 1.
Conceptual diagram of physician-administered electronic medical record dotphrase. Each blue box is a yes or no question, then prompting the user to check each of the white boxes with opportunity for free-texting additional options or more information.
Patients were then classified as having any inhalational exposure overall if they reported an inhalational exposure at work (occupational exposure, subsequently defined) or exposure at home, including mold in the home, birds or bird feathers in or around the home, or a hobby not already classified as related to mold or birds.
Occupational exposure was marked as present if the patient had a history of an occupation previously associated with ILD, including work with asbestos, silica, and steel, and any occupation known to be associated with ILD as retrospectively referenced on hplung.com.14 Patients were grouped into specific occupational categories of metal, organic, mold, agricultural, lumber, or silica as defined in a previous assessment of occupational exposures in patients with sarcoidosis.16 The groups of organic, mold, agricultural, and lumber were merged together for this analysis because they all involved exposure to organic antigens. Additionally, patients were classified as having an organic exposure at work if, despite not having a job fitting into the organic, mold, agricultural, and lumber categories, they noted substantial exposure to mold or bird antigens in their workplace. These patients were also noted as having an occupational exposure.
The category of home exposure was composed of exposure to mold, bird, or hobbies associated with ILD. A patient was defined to have mold exposure if mold was checked in the water category of the dotphrase (Fig 1) or any free text in the dotphrase denoted mold. Bird exposure was defined by a positive response to any of the listed means of exposure in the bird category of the dotphrase, which includes the regular use of down pillows. The hobby category included positive data from the dotphrase regarding hobbies not related to the patient's occupation and not involving mold or bird antigens. To assess the burden of overlapping exposures on the overall patient panel, a multiple exposure category was also created that included one or more exposures from work and/or home.
Data Collection
In addition to the aforementioned exposure data, demographic data, comorbid conditions, and laboratory data were collected from the EMR. MDD of ILD was performed in all patients. High-resolution CT (HRCT) scans were read by dedicated chest radiologists and findings were abstracted from their reports which systematically delineate all compartments of the chest and lung. The CT scan closest to the clinic date when the exposure history was taken was the first CT report analyzed, and any CT scans thereafter were also assessed for overall pattern, honeycombing, lymphadenopathy, coronary artery calcification, and emphysema. These radiologic outcomes were chosen based on their association with either severity of disease (honeycombing and lymphadenopathy) or association with inhalational exposure in other disease processes (coronary artery calcification and emphysema). Patients who met the European Respiratory Society/ATS international classification criteria of IPAF were designated as having IPAF at the time of MDD, and this designation is treated as an ILD subtype in our center and in this study.17 Pulmonary function testing at time of exposure history was abstracted from the medical record.
Statistical Analysis
Continuous variables were reported as means ± SDs, and categorical variables were reported as counts and percentages. Linear regression analyses were performed for continuous outcomes, and logistic regression analyses were performed for binary outcomes. Outcomes of interest for regression analyses were sex, race, and diagnosis, and presence of lymphadenopathy, honeycombing, coronary artery calcification, emphysema, usual interstitial pneumonia, FVC, diffusing capacity of carbon monoxide, and FEV1/FVC ratio at time the exposure history was taken. Regression analyses were adjusted for age, sex, and pack-years smoking, except for comparisons stratified by sex, which were adjusted for age and pack-years smoking. Statistical tests of our five main hypotheses examining the association of exposures with sex, race, diagnosis, HRCT scan, and lung function were performed using Bonferroni-adjusted P values. Because there were five main hypotheses, P < .01 was considered significant.
Survival analysis was performed using multivariable Cox proportional hazards analysis along with the log-rank test and plotted using the Kaplan-Meier survival estimator. The outcome was transplant-free survival, defined as the first clinic visit during the study period (May-October 2018) until death, lung transplantation, or the censor date of September 30, 2020, whichever came first. Both unadjusted hazard ratios and models adjusted for gender-age-physiology (GAP) score and pack-years smoking were analyzed.18 Statistical analysis was conducted using Stata (2017 release 15; StataCorp).
Results
One hundred and fifty-six distinct patients were seen and enrolled in the study. Characteristics of the entire cohort are displayed in Table 1. The mean age of the cohort at the time exposure history was taken was 64 ± 13 years, of whom 46% were men, 73% were white, and 44% had a tobacco history with mean pack-years of 24. Secondhand smoke exposure was reported in over one-half of the cohort. Twenty-nine percent of patients had a family history of CTD, whereas 12% of patients noted a family history of ILD. Sixty-six patients were diagnosed with CTD, the largest proportion of which had autoimmune myositis. CTD-ILD was the most common MDD, followed by IPF, HP, and IPAF.
Table 1.
Cohort Characteristics (N = 156)
| Characteristic | Value |
|---|---|
| Age, y | 64 ± 13 |
| Men | 71 (46) |
| Race | |
| White | 114 (73) |
| Black | 27 (17) |
| Hispanic | 9 (6) |
| Asian | 6 (4) |
| Ever smoked tobacco | 68 (44) |
| Pack-years if smoking | 24 ± 21 |
| Secondhand smoke exposure | 81 (52) |
| Family history of ILD | 19 (12) |
| Family history of CTD | 46 (29) |
| CTD diagnosis | 67 (43) |
| Autoimmune myositis | 25 (16) |
| Rheumatoid arthritis | 12 (8) |
| Mixed connective tissue disease | 12 (8) |
| Other | 18 (12) |
| Multidisciplinary diagnosis | |
| CTD-ILD | 66 (42) |
| IPF | 41 (26) |
| HP | 22 (14) |
| IPAF | 14 (9) |
| Unclassifiable | 4 (3) |
| COP | 3 (2) |
| CPFE | 3 (2) |
| PPFE | 2 (1) |
| Sarcoid | 1 (1) |
| Exposure | |
| Any | 101 (65) |
| Occupational | 59 (38) |
| Organic | 25 (16) |
| Metal | 21 (13) |
| Silica | 6 (4) |
| Domestic | 74 (47) |
| Mold | 39 (25) |
| Bird | 42 (27) |
| Hobby | 32 (21) |
| Gardening | 17 (11) |
| Woodworking | 13 (8) |
| Multiple (> 1) | 62 (40) |
Values are mean ± SD or No. (%). COP = cryptogenic organizing pneumonia; CPFE = combined pulmonary fibrosis emphysema; CTD = connective tissue disease; CTD-ILD = connective tissue disease-associated interstitial lung disease; HP = hypersensitivity pneumonitis; ILD = interstitial lung disease; IPAF = interstitial pneumonia with autoimmune features; IPF = idiopathic pulmonary fibrosis; PPFE = pleuroparenchymal fibroelastosis.
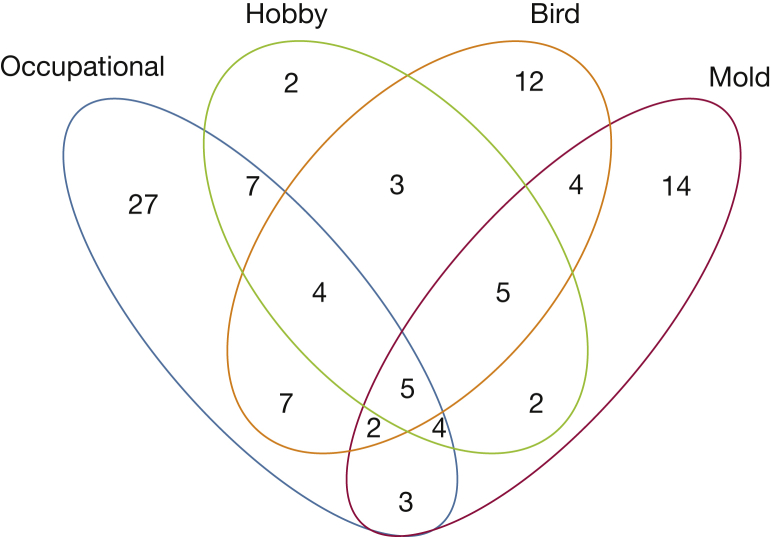
More men than women had any history of inhalational exposure (82% vs 51%, respectively; P < .001) (Table 2). A higher proportion of men had a history of an occupational exposure (66% vs 14%, respectively; P < .001). A substantial number of patients had overlapping exposures and are described in Figure 2. When stratifying exposures by race, no differences were seen in the prevalence of occupational exposures, but white race was associated with bird exposure (OR, 4.24; P = .01) and hobbies (OR, 3.58; P = .03) compared with other races. Exposures were common across all diagnoses; however, a diagnosis of HP was significantly associated with a higher prevalence of any inhalational exposure, mold exposure, and multiple exposures. When compared with patients with ILD without exposure, there were no significant differences in HRCT findings or lung function between exposed and unexposed groups (Table 3).
Table 2.
Reported Exposures by Key Patient Characteristics
| Sex | Men (n = 71) | Women (n = 85) | OR (Men vs Women) | 95% CI | P Valuea |
|---|---|---|---|---|---|
| Any exposure | 58 (82) | 43 (51) | 4.37 | 1.99-9.60 | < .001b |
| Occupational exposure | 47 (66) | 12 (14) | 10.14 | 4.50-22.83 | < .001b |
| Mold | 12 (17) | 27 (32) | 0.43 | 0.19-0.98 | .05 |
| Bird | 22 (31) | 20 (24) | 1.45 | 0.67-3.12 | .29 |
| Hobby | 21 (30) | 11 (13) | 2.92 | 1.23-6.94 | .01b |
| Multiple exposures | 40 (56) | 22 (26) | 3.70 | 1.78-7.66 | < .001b |
| Race | White (n = 114) | Black (n = 27) | Hispanic (n = 9) | Asian (n = 6) | OR (White vs Nonwhite) | 95% CI | P Valuec |
|---|---|---|---|---|---|---|---|
| Any exposure | 78 (68) | 14 (52) | 5 (56) | 4 (67) | 1.44 | 0.67-3.11 | .35 |
| Occupational exposure | 45 (39) | 9 (33) | 2 (22) | 3 (50) | 0.87 | 0.35-2.16 | .68 |
| Mold | 30 (26) | 7 (26) | 1 (11) | 1 (17) | 1.52 | 0.63-3.63 | .34 |
| Bird | 38 (33) | 1 (4) | 3 (33) | 0 (0) | 4.24 | 1.38-13.00 | .01b |
| Hobby | 29 (25) | 3 (11) | 0 (0) | 0 (0) | 3.93 | 1.11-13.89 | .03 |
| Multiple exposures | 52 (46) | 6 (22) | 4 (44) | 0 (0) | 2.11 | 0.91-4.91 | .08 |
| Diagnosis | IPF (n = 41) | HP (n = 22) | CTD (n = 66) | IPAF (n = 14) | Other (n = 13) | OR (HP vs non-HP) | 95% CI | P Valuec |
|---|---|---|---|---|---|---|---|---|
| Any exposure | 34 (83) | 20 (91) | 30 (45) | 9 (64) | 8 (62) | 9.49 | 2.02-44.61 | .004b |
| Occupational exposure | 23 (56) | 10 (45) | 15 (23) | 7 (50) | 4 (31) | 2.91 | 0.94-9.03 | .06 |
| Mold | 10 (24) | 12 (55) | 11 (17) | 3 (21) | 3 (23) | 4.29 | 1.65-11.18 | .003b |
| Bird | 19 (46) | 10 (45) | 8 (12) | 2 (14) | 3 (23) | 2.75 | 1.05-7.18 | .04 |
| Hobby | 18 (44) | 8 (36) | 1 (2) | 4 (29) | 1 (8) | 3.35 | 1.17-9.54 | .02 |
| Multiple exposures | 26 (63) | 15 (68) | 9 (14) | 8 (57) | 4 (31) | 5.43 | 1.84-16.03 | .002b |
Values are No. (%) or as otherwise indicated. CTD = connective tissue disease; HP = hypersensitivity pneumonitis; IPAF = interstitial pneumonia with autoimmune features; IPF = idiopathic pulmonary fibrosis.
Adjusted for age and pack-years smoking.
Significant P value adjusting for multiple hypotheses.
Adjusted for age, sex, and pack-years smoking.
Figure 2.
Venn diagram of coexposures. Numbers are counts of patients with a history of each exposure.
Table 3.
HRCT Features and Baseline Pulmonary Function by Exposure
| Variable |
Any Exposure |
Occupational Exposure |
Multiple Exposures |
||||||
|---|---|---|---|---|---|---|---|---|---|
| HRCT Scan (n = 141) | Yes (n = 93) | No (n = 48) | P Valuea | Yes (n = 55) | No (n = 86) | P Valuea | Yes (n = 57) | No (n = 84) | P Valuea |
| Lymphadenopathy | 52 (56) | 25 (52) | .73 | 34 (62) | 43 (50) | .74 | 33 (58) | 44 (52) | .98 |
| Honeycombing | 58 (62) | 23 (48) | .23 | 34 (62) | 47 (55) | .61 | 40 (70) | 41 (49) | .05 |
| Coronary artery calcification | 72 (77) | 31 (65) | .86 | 45 (82) | 58 (68) | .91 | 43 (75) | 60 (71) | .13 |
| Emphysema | 14 (15) | 10 (21) | .30 | 10 (18) | 14 (16) | .72 | 7 (12) | 17 (20) | .13 |
| CT UIP | 46 (49) | 17 (35) | .54 | 29 (53) | 34 (40) | .24 | 31 (54) | 32 (38) | .47 |
| PFTs (n = 153) | Yes (n = 98) | No (n = 55) | P Valuea | Yes (n = 57) | No (n = 96) | P Valuea | Yes (n = 59) | No (n = 94) | P Valuea |
|---|---|---|---|---|---|---|---|---|---|
| FVC % predicted | 63 ± 19 | 67 ± 19 | .25 | 62 ± 18 | 67 ± 19 | .08 | 63 ± 17 | 66 ± 19 | .47 |
| Dlco % predicted | 55 ± 23 | 55 ± 23 | .81 | 54 ± 22 | 56 ± 24 | .82 | 54 ± 21 | 56 ± 24 | .86 |
| FEV1/FVC ratio, % | 84 ± 9 | 83 ± 10 | .32 | 83 ± 10 | 84 ± 9 | .69 | 83 ± 10 | 83 ± 9 | .40 |
Values are mean ± SD, No. (%), or as otherwise indicated. Dlco = diffusing capacity of carbon monoxide; HRCT = high-resolution CT; PFT = pulmonary function tests; UIP = usual interstitial pneumonia.
Adjusted for age, sex, and pack-years smoking.
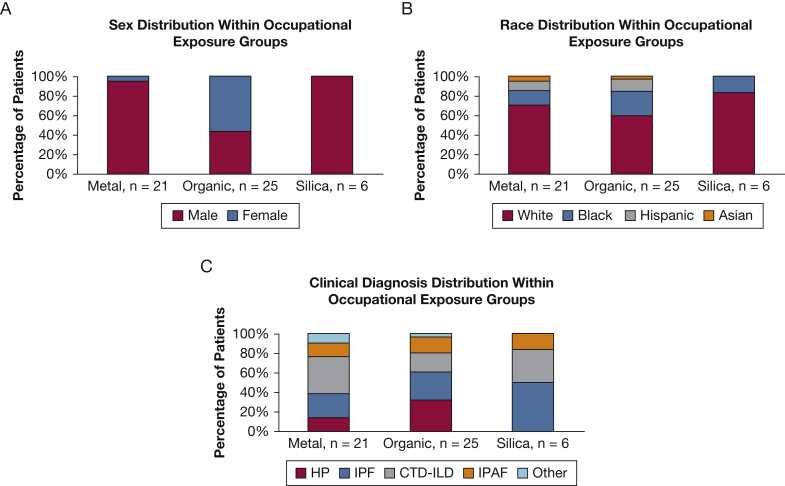
The most common occupational exposure categories were metal dust, organic material, and silica (Fig 3). Subjects in the metal and silica groups were predominantly men, whereas the organic group had a higher percentage of women. All three occupational categories consisted of a wide variety of ILD diagnoses; however, CTD was the most prevalent diagnosis in the metal working group, HP was the most common diagnosis in the organic group, and IPF was the most common diagnosis in the silica group (Fig 3, Table 4).
Figure 3.
A-C, Sex, race, and diagnosis of selected occupational types. A, Distribution of sex in the occupational groups of metal (n = 21); organic, including mold or bird exposure (n = 25); and silica (n = 6). Patients who had an alternative occupational exposure are not included. B, Distribution of race in the occupational groups of metal (n = 21); organic, including mold or bird exposure (n = 25); and silica (n = 6). C, Distribution of multidisciplinary diagnosis in the occupational groups of metal (n = 21), organic (n = 25), and silica (n = 6). CTD-ILD = connective tissue disease-associated interstitial lung disease; HP = hypersensitivity pneumonitis; IPAF = interstitial pneumonia with autoimmune features; IPF = idiopathic pulmonary fibrosis.
Table 4.
Clinical Characteristics of Selected Job Categories
| Metal Dust (n = 21) | Organic (n = 25) | Silica (n = 6) | |||
|---|---|---|---|---|---|
| Age, y | 63 ± 11 | Age, y | 64 ± 12 | Age, y | 63 ± 9 |
| White race | 15 (71) | White race | 15 (60) | White race | 5 (83) |
| Men | 20 (95) | Men | 11 (44) | Men | 6 (100) |
| Diagnosis | Diagnosis | Diagnosis | |||
| IPF | 5 (24) | IPF | 7 (28) | IPF | 3 (50) |
| HP | 3 (14) | HP | 8 (32) | HP | 0 (0) |
| CTD-ILD | 8 (38) | CTD-ILD | 5 (20) | CTD-ILD | 2 (33) |
| IPAF | 3 (14) | IPAF | 4 (16) | IPAF | 1 (17) |
| Other | 2 (10) | Other | 1 (4) | Other | 0 (0) |
| CT features | CT features | CT features | |||
| UIP | 9 (45) | UIP | 10 (40) | UIP | 2 (33) |
| Honeycombing | 14 (70) | Honeycombing | 17 (68) | Honeycombing | 6 (100) |
| Coronary calcification | 17 (85) | Coronary calcification | 21 (84) | Coronary calcification | 6 (100) |
| Lymphadenopathy | 11 (55) | Lymphadenopathy | 13 (52) | Lymphadenopathy | 4 (67) |
Values are mean ± SD or No. (%). CTD-ILD = connective tissue disease-associated interstitial lung disease; HP = hypersensitivity pneumonitis; IPAF = interstitial pneumonia with autoimmune features; IPF = idiopathic pulmonary fibrosis; UIP = usual interstitial pneumonia.
Twenty-five deaths and 10 lung transplants occurred before the censor date of September 30, 2020. Unadjusted transplant-free survival was significantly worse in patients with a history of inhalational exposure compared with those without (hazard ratio, 2.58; 95% CI, 1.13-5.92; P = .025) (Fig 4). Restricted mean survival time was 25.6 months in the exposed group and 26.9 months in the unexposed group. After adjustment for GAP score and pack-years smoking, this difference was no longer significant (hazard ratio, 1.82; 95% CI, 0.77-4.27; P = .17).
Figure 4.
Survival based on exposure history. Transplant-free survival categorized by inhalational exposure history. P = .025 unadjusted and P = .17 when adjusted for gender-age-physiology score and pack-years smoking.
Discussion
This comprehensive and systematic evaluation in patients with diverse forms of ILD demonstrated a high prevalence of potentially relevant inhalational exposures at home and at work regardless of ILD diagnosis. To our knowledge, this is the first study demonstrating that occupational exposures have a predominance of men in all-comers with non-pneumoconiosis-related ILD. Occupations well known for their association with ILD (eg, those involving metal work and silica) comprised a large proportion of patients exposed. White race was associated with hobbies known to cause ILD and significantly associated with bird exposure. Although we were unable to assess causation given the lack of control group, these findings suggest that further investigation regarding exposure-related etiologies across all ILDs is warranted.
The demographically and diagnostically diverse cohort was found to have a high exposure prevalence across disease subtypes, including autoimmune-related ILD. Although patients with HP were overall more likely to have a history of potentially relevant inhalational exposure, 45% of patients with CTD-ILD and 64% of patients with IPAF also had this exposure history. Although not generally recognized, previous studies have shown an increase in inhalational exposures in patients with autoimmune disease as a whole, and the Canadian Registry for Pulmonary Fibrosis found about one-half of patients with CTD-ILD had a history of organic exposure.19,20 Other studies have also identified associations between specific CTDs and exposures (eg, scleroderma in silica-exposed workers; rheumatoid arthritis in coal miners, brick layers, and concrete workers).8,19,21 However, to our knowledge, there are no previous studies that describe the prevalence of occupational exposure in patients with CTD-ILD.
Interestingly, although the prevalence of occupational exposure in HP was higher when compared with the pooled prevalence of all other ILD diagnoses, these exposures were still found in over one-half of patients with IPF and one-quarter of patients with CTD-ILD. CTD-ILD was also the most common diagnosis among those working in the metal industry. The ATS statement7 on the occupational burden of nonmalignant respiratory disease calculated a PAF of 26% for IPF and 19% for HP. Because our study did not have a control group, we were unable to calculate the PAF, but by definition it would be lower than our raw prevalence and may be similar to these previously published data. Although the incidence of inhalational exposures as a whole in the general population or even in control groups of previous ILD studies is difficult to elicit, our incidence of occupational metal exposure of 13% is similar to the case groups of prior IPF studies and higher than the control group estimates of approximately 2%.22,23 These studies were in other locations than the United States, but the fact that some of our results are consistent with prior findings highlights the importance of further investigation regarding inhalational exposures and ILD incidence and outcomes, regardless of ILD subtype.
In our study, a history of exposure either at work or home was found across all ILD diagnoses; however, the contribution of these exposures to ILD development or progression cannot be demonstrated conclusively in all cases. Therefore, there may be added benefit to inclusion of professionals trained in occupational and environmental medicine or industrial hygiene in the multidisciplinary care of patients with ILD. Because this may not be possible, use of standardized exposure history questionnaires that trigger clinical decision support prompts to obtain additional information might allow broader but targeted exploration of potentially relevant exposure history and guide physicians in assessing relevance of the exposure with respect to disease contribution.24, 25, 26 Our use of a dotphrase to prompt routine evaluation of potentially relevant exposures clearly identified a significant amount of exposure data which may lend clarity to diagnoses and contribute to mitigation of ongoing exposures.
The Centers for Disease Control and Prevention has identified exposure assessment as one of the strategic objectives of the National Occupational Research Agenda for Respiratory Health26 in the evaluation of patients diagnosed with IPF to improve identification of a possible workplace contribution. By increasing recognition of potential occupational contributions to IPF and other ILDs, this knowledge may lead to improved exposure controls and prevention of disease. This is particularly important for IPF, for which no curative treatments exist and which accounts for almost one-third of lung transplantation performed in the United States.27
Our study found that more men overall had occupational exposures associated with ILD, and sex differences in occupational inhalational exposures have been described previously.16,28 Suggested mechanisms of the interaction between sex, occupation, and lung disease include both different job types in men and women and different tasks within the same job; for instance, men are more likely to have occupations that expose them to dust and also have greater dust exposure than women in the same occupation.28 Our study was not designed to ascertain differential exposures within the same job, but we did find that this higher proportion of men persisted over several different industries, including metal work and silica. Varying occupational-sex interactions have also been associated with sarcoidosis and systemic sclerosis.16,29 We found that there were more women than men who reported mold exposure in the home, and there was a larger proportion of women in the organic occupational exposure group than in other occupational subtypes. Future studies could elicit whether occupations featuring significant mold or bird exposure increase overall ILD risk in women outside of the typical associations with HP, similar to what has been described with disinfectants and COPD in nurses who were women.30 Therefore, further research is necessary to elucidate the complex relationship between sex, ILD, and exposures. In addition, we did not find specific radiologic abnormalities (eg, emphysema, lymphadenopathy) differentially associated with exposure. This could be addressed in future studies.
In the clinic cohort, white race was associated with an increased prevalence of both bird exposure in the home and hobbies associated with ILD, but no difference among races was seen in the prevalence of occupational or mold exposure. In a study of interstitial lung abnormalities and air pollution, the strongest association between pollutant exposure and progression of radiologic abnormalities was seen in non-Hispanic white patients.31 Additionally, our group has previously shown in a multicenter study that ILD in black patients may be associated with a survival benefit. Therefore, the overall incidence of clinically meaningful inhalational exposures and its association with race and ultimately outcome in ILD deserves further study.
Inhalational exposure was associated with worse survival in the cohort, but this difference did not remain significant after adjustment for GAP score and pack-years smoking. Previous studies regarding the relationship among exposures and ILD have focused on patients with IPF and have been contradictory. In a study of Korean patients with IPF, Lee et al11 found that patients with a dust-exposed occupation had worse survival than their reference group of homemakers and the unemployed. Contrarily, the De Sadeleer et al12 study of Belgian patients with IPF found that exposed patients had better survival than unexposed patients with IPF, but worse survival than patients with chronic HP. Our finding that worse survival in exposed patients did not maintain significance after adjustment for GAP score and pack-years smoking could reflect a relatively small number of events or that one of the adjustment variables (eg, lung function) is an intermediate step in the causal pathway between exposure and death or lung transplant. More work in larger studies could assess this exposure-survival relationship to identify an actionable target for prolonging the lives of patients with ILD by identification and mitigation of inhaled substances.
Our study has limitations, the first of which is the retrospective, self-reported nature of our data and the testing of multiple hypotheses, for which we have marked P values reaching significance after adjustment for multiple hypotheses in Table 2. Second, there was no control group in which to compare exposure prevalence; therefore, no conclusions about association between exposures and ILD incidence as a whole can be made. However, we were able to use a comparator group of nonexposed patients with ILD. There was also no information on intensity, frequency, or duration of exposure, which would be helpful to assess a potential dose-response relationship of cumulative exposure and severity of illness. Although knowledge of exposure could have contributed to a diagnosis of HP, therefore affecting the comparison of exposure among ILD subtypes, most (73%) patients with an MDD of HP also had an HP pattern on their chest CT scan, a pattern that was present in 3% of patients with non-HP. Additional unmeasured confounders of the relationship between exposure and survival could be present (eg, socioeconomic status). Despite these limitations, ascertainment of occupational history is not captured systematically across ILD diagnoses in clinical studies or registries, and remains a rudimentary and inconsistently obtained element of the ILD clinical evaluation.25 Therefore, this comprehensive assessment of patients with ILD as they are evaluated in clinic is a first step toward the recognition and codification of inhalational exposures as an essential element of ILD care across diagnoses.
Interpretation
Our study demonstrates that among a diverse group of patients presenting to an academic ILD clinic, potentially relevant domestic and occupational inhalational exposures are prevalent across multidisciplinary ILD diagnoses beyond the pneumoconioses and HP traditionally associated with such exposures. These historical elements can be captured by a systematic approach to data collection. We noted sex differences in exposures in the workplace with a strikingly higher proportion of men in exposed jobs. We also noted differential prevalence in bird and hobby exposures by race. Exposed patients had worse survival in this cohort; however, statistical significance was not maintained after multivariable adjustment. As more evidence accumulates regarding the identification of at-risk individuals for ILD, a thorough exposure history will be critical.32,33 Future investigations may benefit from even further comprehensive investigation with addition of an occupational medicine specialist to the multidisciplinary team.
Acknowledgments
Author contributions: C. T. L. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. C. T. L., A. A., J. H. C., I. B. V., R. J., S. M., R. V., and M. E. S. contributed to clinical data acquisition. C. T. L., A. A., R. V., and M. E. S. contributed to study design. C. T. L., A. A., and M. E. S. contributed to data analysis. C. T. L., A. A., J. H. C., I. B. V., R. J., S. M., R. V., S. E. H., and M. E. S. contributed to interpretation of results and manuscript preparation and review. All authors reviewed, revised, and approved the manuscript for submission.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: A. A. reports personal fees from Boehringer Ingelheim outside the submitted work. S. E. H. reports grant funding from CleanSpace Technology unrelated to the submitted work. M. E. S. reports grant funding from Boehringer Ingelheim, Galapagos, and Novartis and honoraria and consulting fees from Boehringer Ingelheim unrelated to the submitted work. None declared (C. T. L., J. H. C., I. B. V., R. J., S. M., R. V.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Footnotes
FUNDING/SUPPORT: This work was supported by the NHLBI [Grant T32 007605] and the NIH [Grant K23HL146942].
Supplementary Data
References
- 1.Hutchinson J., Fogarty A., Hubbard R., McKeever T. Global incidence and mortality of idiopathic pulmonary fibrosis: a systematic review. Eur Respir J. 2015;46(3):795–806. doi: 10.1183/09031936.00185114. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez Alvarez R., Martinez Gonzalez C., Quero Martinez A., Blanco Perez J.J., Carazo Fernandez L., Prieto Fernandez A. Guidelines for the diagnosis and monitoring of silicosis. Arch Bronconeumol. 2015;51(2):86–93. doi: 10.1016/j.arbres.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Diego Roza C., Cruz Carmona M.J., Fernandez Alvarez R. Recommendations for the diagnosis and management of asbestos-related pleural and pulmonary disease. Arch Bronconeumol. 2017;53(8):437–442. doi: 10.1016/j.arbres.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 4.Morisset J., Johannson K.A., Jones K.D. Identification of diagnostic criteria for chronic hypersensitivity pneumonitis: an international modified Delphi survey. Am J Respir Crit Care Med. 2018;197(8):1036–1044. doi: 10.1164/rccm.201710-1986OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baumgartner K.B., Samet J.M., Coultas D.B. Occupational and environmental risk factors for idiopathic pulmonary fibrosis: a multicenter case-control study. Am J Epidemiol. 2000;152(4):307–315. doi: 10.1093/aje/152.4.307. [DOI] [PubMed] [Google Scholar]
- 6.Awadalla N.J., Hegazy A., Elmetwally R.A., Wahby I. Occupational and environmental risk factors for idiopathic pulmonary fibrosis in Egypt: a multicenter case-control study. Int J Occup Environ Med. 2012;3(3):107–116. [PubMed] [Google Scholar]
- 7.Blanc P.D., Annesi-Maesano I., Balmes J.R. The occupational burden of nonmalignant respiratory diseases. An Official American Thoracic Society and European Respiratory Society Statement. Am J Respir Crit Care Med. 2019;199(11):1312–1334. doi: 10.1164/rccm.201904-0717ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rocha L.F., Luppino Assad A.P., Marangoni R.G., Del Rio A.P., Marques-Neto J.F., Sampaio-Barros P.D. Systemic sclerosis and silica exposure: a rare association in a large Brazilian cohort. Rheumatol Int. 2016;36(5):697–702. doi: 10.1007/s00296-015-3412-0. [DOI] [PubMed] [Google Scholar]
- 9.Winterbottom C.J., Shah R.J., Patterson K.C. Exposure to ambient particulate matter is associated with accelerated functional decline in idiopathic pulmonary fibrosis. Chest. 2018;153(5):1221–1228. doi: 10.1016/j.chest.2017.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johannson K.A., Vittinghoff E., Lee K. Acute exacerbation of idiopathic pulmonary fibrosis associated with air pollution exposure. Eur Respir J. 2014;43(4):1124–1131. doi: 10.1183/09031936.00122213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee S.H., Kim D.S., Kim Y.W. Association between occupational dust exposure and prognosis of idiopathic pulmonary fibrosis: a Korean national survey. Chest. 2015;147(2):465–474. doi: 10.1378/chest.14-0994. [DOI] [PubMed] [Google Scholar]
- 12.De Sadeleer L.J., Verleden S.E., De Dycker E. Clinical behaviour of patients exposed to organic dust and diagnosed with idiopathic pulmonary fibrosis. Respirology. 2018;23(12):1160–1165. doi: 10.1111/resp.13342. [DOI] [PubMed] [Google Scholar]
- 13.Trethewey S.P., Walters G.I. The role of occupational and environmental exposures in the pathogenesis of idiopathic pulmonary fibrosis: a narrative literature review. Medicina (Kaunas) 2018;54(6):108. doi: 10.3390/medicina54060108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.hpLung. www.hplung.com. Accessed March 1, 2020.
- 15.Offerman S.R., Rauchwerger A.S., Nishijima D.K. Use of an electronic medical record "dotphrase" data template for a prospective head injury study. West J Emerg Med. 2013;14(2):109–113. doi: 10.5811/westjem.2012.11.13400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu H., Patel D., Welch A.M. Association between occupational exposures and sarcoidosis: an analysis from death certificates in the United States, 1988-1999. Chest. 2016;150(2):289–298. doi: 10.1016/j.chest.2016.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer A., Antoniou K.M., Brown K.K. An official European Respiratory Society/American Thoracic Society research statement: interstitial pneumonia with autoimmune features. Eur Respir J. 2015;46(4):976–987. doi: 10.1183/13993003.00150-2015. [DOI] [PubMed] [Google Scholar]
- 18.Ryerson C.J., Vittinghoff E., Ley B. Predicting survival across chronic interstitial lung disease: the ILD-GAP model. Chest. 2014;145(4):723–728. doi: 10.1378/chest.13-1474. [DOI] [PubMed] [Google Scholar]
- 19.Schmajuk G., Trupin L., Yelin E., Blanc P.D. Prevalence of arthritis and rheumatoid arthritis in coal mining counties of the United States. Arthritis Care Res. 2019;71(9):1209–1215. doi: 10.1002/acr.23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fisher J.H., Kolb M., Algamdi M. Baseline characteristics and comorbidities in the CAnadian REgistry for Pulmonary Fibrosis. BMC Pulm Med. 2019;19(1):223. doi: 10.1186/s12890-019-0986-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ilar A., Alfredsson L., Wiebert P., Klareskog L., Bengtsson C. Occupation and risk of developing rheumatoid arthritis: results from a population-based case-control study. Arthritis Care Res (Hoboken) 2018;70(4):499–509. doi: 10.1002/acr.23321. [DOI] [PubMed] [Google Scholar]
- 22.Miyake Y., Sasaki S., Yokoyama T. Occupational and environmental factors and idiopathic pulmonary fibrosis in Japan. Ann Occup Hyg. 2005;49(3):259–265. doi: 10.1093/annhyg/meh090. [DOI] [PubMed] [Google Scholar]
- 23.Paolocci G., Folletti I., Torén K. Occupational risk factors for idiopathic pulmonary fibrosis in Southern Europe: a case-control study. BMC Pulm Med. 2018;18(1):75. doi: 10.1186/s12890-018-0644-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention, The National Institute for Occupational Safety and Health (NIOSH) Electronic health records (EHRs) and patient work information. https://www.cdc.gov/niosh/topics/ehr/default.html Accessed March 1, 2020.
- 25.Kalchiem-Dekel O., Galvin J.R., Burke A.P., Atamas S.P., Todd N.W. Interstitial lung disease and pulmonary fibrosis: a practical approach for general medicine physicians with focus on the medical history. J Clin Med. 2018;7(12) doi: 10.3390/jcm7120476. :476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.NIOSH . 2019. National Occupational Research Agenda for Respiratory Health. https://www.cdc.gov/nora/councils/resp/pdfs/National_Occupational_Research_Agenda_for_Respiratory_Health_January_2019-508.pdf. Accessed March 1, 2020. [Google Scholar]
- 27.Yusen R.D., Edwards L.B., Dipchand A.I. The Registry of the International Society for Heart and Lung Transplantation: Thirty-third Adult Lung and Heart-Lung Transplant Report-2016; Focus Theme: Primary Diagnostic Indications for Transplant. J Heart Lung Transplant. 2016;35(10):1170–1184. doi: 10.1016/j.healun.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Eng A., 't Mannetje A., McLean D., Ellison-Loschmann L., Cheng S., Pearce N. Gender differences in occupational exposure patterns. Occup Environ Med. 2011;68(12):888–894. doi: 10.1136/oem.2010.064097. [DOI] [PubMed] [Google Scholar]
- 29.Marie I., Gehanno J.F., Bubenheim M. Prospective study to evaluate the association between systemic sclerosis and occupational exposure and review of the literature. Autoimmun Rev. 2014;13(2):151–156. doi: 10.1016/j.autrev.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Dumas O., Varraso R., Boggs K.M. Association of occupational exposure to disinfectants with incidence of chronic obstructive pulmonary disease among US female nurses. JAMA Netw Open. 2019;2(10) doi: 10.1001/jamanetworkopen.2019.13563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sack C., Vedal S., Sheppard L. Air pollution and subclinical interstitial lung disease: the Multi-Ethnic Study of Atherosclerosis (MESA) air-lung study. Eur Respir J. 2017;50(6) doi: 10.1183/13993003.00559-2017. :1700559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hunninghake G.M., Quesada-Arias L.D., Carmichael N.E. Interstitial lung disease in relatives of patients with pulmonary fibrosis. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.201908-1571OC. ;201(10):1240-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salisbury M.L., Hewlett J.C., Ding G. Development and progression of radiologic abnormalities in individuals at risk for familial ILD. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.201909-1834OC. ;201(10):1230-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.