Abstract
A relationship between inhalational exposure to materials in the environment and development of interstitial lung disease (ILD) is long recognized. Hypersensitivity pneumonitis is an environmentally -induced diffuse parenchymal lung disease. In addition to hypersensitivity pneumonitis, domestic and occupational exposures have been shown to influence onset and progression of other ILDs, including idiopathic interstitial pneumonias such as idiopathic pulmonary fibrosis. A key component of the clinical evaluation of patients presenting with ILD includes elucidation of a complete exposure history, which may influence diagnostic classification of the ILD as well as its management. Currently, there is no standardized approach to environmental evaluation or remediation of potentially harmful exposures in home or workplace environments for patients with ILD. This review discusses evidence for environmental contributions to ILD pathogenesis and draws on asthma and occupational medicine literature to frame the potential utility of a professional evaluation for environmental factors contributing to the development and progression of ILD. Although several reports suggest benefits of environmental assessment for those with asthma or certain occupational exposures, lack of information about benefits in broader populations may limit application. Determining the feasibility, long-term outcomes, and cost-effectiveness of environmental evaluation and remediation in acute and chronic ILDs should be a focus of future research.
Key Words: environmental lung disease, hypersensitivity pneumonitis, pulmonary fibrosis
Abbreviations: HAA, high attenuation areas; HP, hypersensitivity pneumonitis; ILD, interstitial lung disease; IPF, idiopathic pulmonary fibrosis; JEM, job exposure matrix; NIOSH, National Institute for Occupational Safety and Health; PCR, polymerase chain reaction; VGDF, vapors, gas, dust, or fumes
Although hypersensitivity pneumonitis (HP) and pneumoconioses are archetypal environmental lung diseases, environmental exposures likely contribute to the pathogenesis of some idiopathic interstitial pneumonias, including idiopathic pulmonary fibrosis (IPF). HP occurs following inhalational exposure and immune-mediated sensitization to organic/protein antigen(s),1, 2, 3 and pneumoconiosis occurs following chronic exposure to respirable dust. A key component of the diagnostic evaluation for environmental lung disease includes elucidation of a compatible exposure history1, 2, 3, 4; without this information, the diagnosis may remain in question. Accurate identification of environmental exposures driving disease development may also influence outcomes. For example, an inciting antigen is not identified in up to 50% of patients with HP, and these individuals have shortened survival.5 Patients with IPF who are exposed to higher levels of particulate air pollutants have an increased risk for adverse events such as acute disease exacerbation and lung function decline.6,7 Unfortunately, there is no standardized approach to evaluation for, or remediation of, potentially harmful environmental exposures in home or workplace environments for patients with HP or other interstitial lung diseases (ILDs).
The current article summarizes the evidence for a pathologic role of various environmental factors in ILD, presents two complementary cases illustrating aspects of exposure identification and remediation, discusses evidence for use of a formal (ie, professional) evaluation for environmental factors contributing to ILD development and progression, and reviews practical considerations surrounding exposure identification and remediation.
ILD and the Environment
Two key reasons for query of environmental exposures among all patients with ILD include identification and removal of ongoing sources of lung injury and for diagnostic classification. The current paradigm of fibrotic ILD pathobiology involves recurrent injury to the lung epithelium, with consequent fibroblast activation and accumulation of collagen and other extracellular matrix components in the interstitium, in genetically susceptible individuals. Extrinsic exposures are increasingly recognized as a source of epithelial injury.8, 9, 10 Previously termed “extrinsic allergic alveolitis,” HP results from inhalation and sensitization to organic protein antigens (eg, environmental molds), with consequent infiltration of the lungs by lymphocytes, granuloma formation, and, in some cases, development of pulmonary fibrosis.3,11 Multiple studies have linked specific exposures or occupations with increased risk for having IPF, HP, and other ILDs (Table 1).12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25 An American Thoracic Society/European Respiratory Society statement estimated the occupational/environmental burden of IPF to be substantial, with an occupationally related population-attributable fraction of 26% (95% CI, 10-41) for vapors, gas, dust, or fumes (VGDF) exposures.26
Table 1.
Case-Control Studies Identify Exposures and Occupations Associated With Increased Risk for ILD
| Study | Disease | Specific Exposure; OR (95% CI) | Occupation/Task; OR or HR (95% CI) |
|---|---|---|---|
| Scott et al,23 1990a | IPF | Metal dust, 10.97 (2.3-52.4) Wood fires, 12.55 (1.40-114.0) Cows, 10.89 (1.24-96) |
... |
| Hubbard et al,17 1996 | IPF | Brass dust, 1.97 (1.10-3.52) Lead dust, 5.54 (1.63-18.8) Steel dust, 1.72 (1.09-2.70) Pine wood dust, 3.37 (1.14-9.96) |
... |
| Mullen et al,19 1998 | Fibrotic ILD | Mold/mildew, 16 (1.62-158) Silica, 11 (1.05-115) |
... |
| Baumgartner et al,13 2000b | IPF | Metal dust, 2.0 (1.0-4.0) Vegetable/animal dust, 4.7 (2.1-10.4) |
Raising livestock, 2.7 (1.3-5.5) Hairdressing, 4.4 (1.2-16.3) Raising birds, 4.7 (1.6-14.1) Stone cutting/polishing, 3.9 (1.2-10.4) |
| Miyake et al,18 2005a | IPF | Metal dust, 9.55 (1.68-181.12) | ... |
| Gustafson et al,15 2007 | IPF | Birch dust, 2.4 (1.18-4.92) Hardwood dust, 2.5 (1.06-5.89) |
... |
| Awadalla et al,12 2012b | IPF | Men Wood dust or preservatives, 2.71 (1.01-7.37) Birds, 3.49 (1.49-8.19) Cats, 6.38 (1.59-25.56) |
Men Chemical/petrochemical industry, 6.47 (1.66-25.1) Carpentry/woodworking, 2.56 (1.02-7.01) |
| Women Animal dust, 1.78 (1.01-3.13) Insecticides/pesticides, 8.68 (1.04-72.17) Birds, 3.86 (1.95-7.62) Cats, 8.24 (1.8-37.70) |
Women Farming, 3.34 (1.17-10.12) Raising birds, 1.82 (1.03-3.85) |
||
| García-Sancho et al,14 2011 | IPF | Dusts, smokes, gases, or chemicals, 2.8 (1.5-5.5) | |
| Paolocci et al,20 2018 | UIPc | Metal dust or fumes, 3.8 (1.2-12.2) Organic dust, 2.4 (1.3-4.3) |
Metal and steel industry, 4.8 (1.5-15.2) Farmers, vets, and gardeners, 2.73 (1.47-5.1) |
| Salisbury et al,21 2020 | FIP | Lead, 2.91 (1.05-8.05) Aluminum smelting, 14.88 (2.67-97.73) Mold, 3.83 (1.78-8.25) Birds, 3.37 (1.53-7.41) |
... |
| Abramson et al,22 2020 | IPFb | Asbestos, 1.37 (1.08-1.74) | |
| Trout and Harney,25 2002 | HP | Metal working fluid, 8.1 (1.0-65) | |
| Cramer et al,24 2016 | HP | ... | Pigeon breeding; HR, 14.36 (8.10-25.44) |
| Cramer et al,24 2016 | Non-HP ILD | ... | Pigeon breeding; HR, 1.33 (1.05-1.69) |
FIP = familial interstitial pneumonia; HP = hypersensitivity pneumonitis, ILD = interstitial lung disease; IPF = idiopathic pulmonary fibrosis; UIP = usual interstitial pneumonia.
ORs are matched (by age, sex).
ORs are adjusted for age and cigarette smoking.
Case subjects had UIP on chest CT scanning; control subjects had no lung disease.
Further supporting a role of the environment in ILD pathobiology, features of occupational and domestic exposures have important prognostic implications. In IPF, people exposed to dusts, molds, or higher levels of air pollutants have earlier disease onset and worse outcomes.6,27, 28, 29 Patients with HP with a longer duration of antigen exposure have worse prognosis and less lung function recovery following antigen removal.30 Higher quantity of bird antigen detected in household dust samples has also been associated with faster decline in FVC and increased mortality among avian-associated HP.31 A subset of patients with HP do not have an identifiable antigen, and they experience shortened survival even after accounting for other disease severity measures such as the presence of fibrosis, presumably due to ongoing occult exposure(s).5,32, 33, 34, 35, 36
Diagnosis of specific ILDs is driven in part by disease morphology (ie, specific patterns or findings on high-resolution CT scanning and/or lung biopsy); however, existing guidelines for the diagnosis of IPF, HP, and others also require elucidation of a compatible exposure history (or lack thereof) for higher confidence diagnostic classification.3,37,38 HP diagnosis is intimately tied to identification of an inciting antigen, with lengthy lists of potential antigens and named subsyndromes (eg, mushroom worker’s lung, bagassosis).2,3,11 Some environmental risk factors, particularly exposure to organic dusts and birds, have been linked to increased risk for both HP and IPF.12,13,20,21,24 Identification of similar risk factors could be related to diagnostic uncertainty (eg, difficulty separating fibrotic HP from IPF morphologically) or variation around case definition in practice (eg, a bird-exposed patient with usual interstitial pneumonia on surgical lung biopsy specimen diagnosed with HP at one center and IPF at another).22,39,40 Unfortunately, little consensus exists to guide practical judgments about the significance or relevance of a given exposure in the diagnostic paradigm.
Clinical Case 1
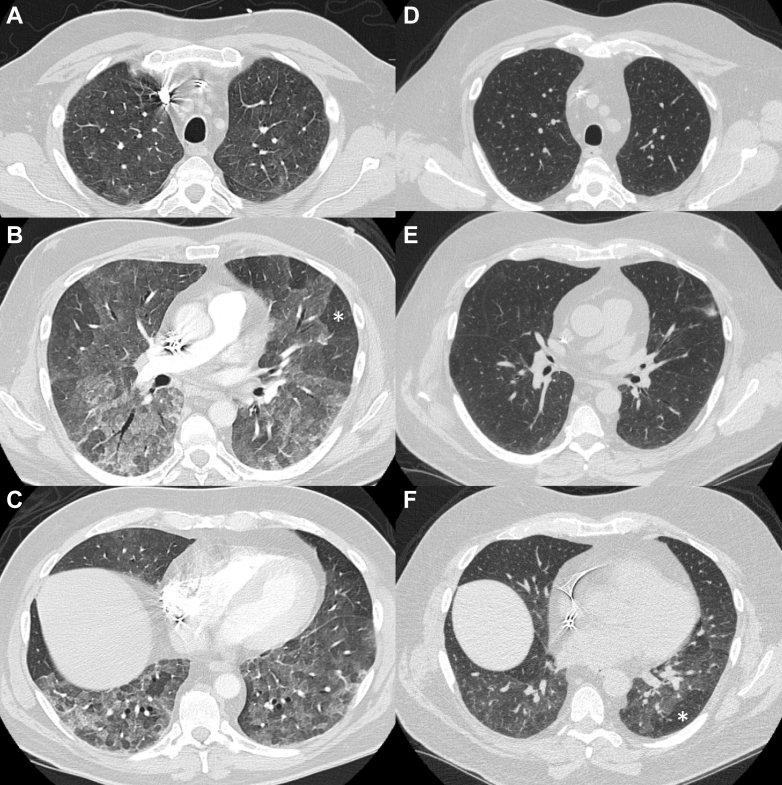
A 63-year-old man was admitted to another hospital with 1 week of dyspnea, cough, and hypoxemia. CT imaging of the chest (Figs 1A-1C) revealed patchy bilateral ground-glass opacities and air trapping, both diffusely distributed. Following exclusion of infection, corticosteroids were prescribed for nonspecific autoimmune ILD (antinuclear antibody was 1:80). Upon return home, the patient’s dyspnea and cough worsened despite corticosteroid treatment. Subsequent evaluation in an ILD clinic included a more thorough exposure history and disclosed new down feather comforter use a few weeks prior to hospitalization. Given typical CT features of nonfibrotic HP and a compatible exposure history, the patient was given a moderate-confidence diagnosis of HP, and antigen avoidance was advised.3 Elimination of feather products from the home resulted in sustained clinical improvement during corticosteroid tapering. Follow-up chest CT imaging showed improvement in radiologic abnormalities (Figs 1D-1E), although mild air trapping remained (Fig 1F).
Figure 1.
A-F, Chest imaging in Case 1 prior to and following antigen remediation and treatment. A-C, CT angiography chest images from the Case 1 patient at the time of ED presentation show diffusely distributed, bilateral, patchy ground-glass opacities. There are also some areas of relatively normal lung and some areas of hyperlucency (an example is indicated by the white asterisk) in the lower lobes. D-E, Inspiratory high-resolution CT images taken 3.5 months later show interval improvement following antigen avoidance and corticosteroid treatment. F, Expiratory CT images at 3.5 months show mild residual air trapping with hyperlucency (an example is indicated by the white asterisk).
Clinical Case 2
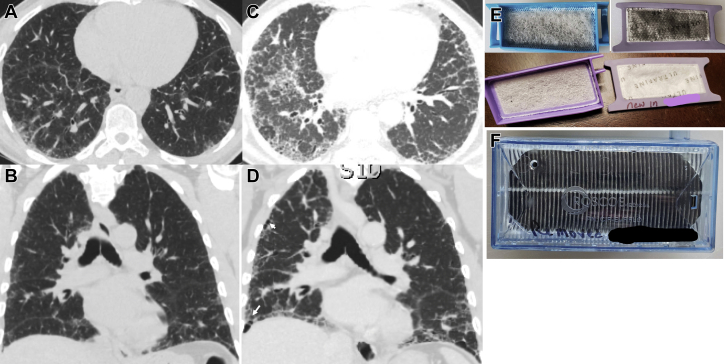
A 64-year-old man was diagnosed with IPF 4 years ago when chest CT imaging (Figs 2A-2B) and transbronchial lung cryobiopsy results showed compatible findings. The biopsy specimen showed alveolar parenchyma with temporally and spatially heterogeneous interstitial fibrosis with fibroblast foci, without granulomas, chronic inflammation, or airway-centeredness of the ILD. The patient had stable disease for approximately 3.5 years, at which time he developed worsening cough, dyspnea, and hypoxia, with significant decline in FVC. An updated chest CT scan (Figs 2C-2D) revealed progressive fibrosis without new abnormality. The patient had recently moved to a new home and noticed black discoloration of several home air filters, CPAP filters, and oxygen system filters (Figs 2E-2F). Given concern for possible mold exposure contributing to disease progression, the home was inspected. No mold was identified, but dust was identified in the air ducts, possibly sourced from refuse burning at a nearby construction site. Remediation was performed, but, unfortunately, disease progression continued.
Figure 2.
A-F, Chest imaging and filter discoloration in Case 2. Chest CT images from the Case 2 patient at the time of diagnosis of idiopathic pulmonary fibrosis and following progression of symptoms. A-B, Axial and coronal images taken at diagnosis show reticular fibrosis, with mild traction bronchiectasis, with peripheral and basilar predominance. C-D, Axial and coronal images taken following worsening of clinical symptoms, showing progression of mixed ground-glass and reticular fibrosis, and development of honeycombing (white arrows). E, Black discoloration of CPAP filters (top) compared with new filters (bottom). F, Oxygen system filters that were noted to be blackened during exchanges.
Methods for Environmental Inquiry
Despite robust evidence for association of various environmental exposures with ILD risk, scant evidence specific to ILD guides identification and management of these exposures. In practice, the initial evaluation consists of physician inquiry into potentially harmful exposures within the domestic and/or occupational environment, including exposure duration and timing of exposure relative to symptoms.1,2 According to a recent expert consensus (ascertained via Delphi methods), onset of symptoms around the time of an exposure and improvement of symptoms with removal from that exposure are key features in determining the clinical significance of a proposed exposure in the development of fibrotic HP.41 Consensus as to the minimum duration of exposure necessary for development of HP was not attained. Case 1 illustrates the importance of obtaining a thorough exposure history to achieve an accurate ILD diagnosis. It is also important to revisit exposure history over time, as patients with ILD may acquire new or unexpected exposures that contribute to worsening symptoms and/or progression of disease, as in Case 2. In practice, however, obtaining such a history can be difficult, with variation across providers. Standardized tools are needed to improve environmental assessment in ILD.
Exposure Assessment Based on Patient Self-Reporting
Physician inquiry into the plethora of potentially harmful environmental exposures associated with various ILDs can be time-consuming, making development of a standardized questionnaire (to facilitate patient self-reporting of exposures) appealing. In occupational asthma, validated questionnaires exist to elicit job site exposures among health care workers42,43 and for VGDF exposures.44,45 Development and validation of exposure questionnaires applicable to asthma involved collaboration among experts in occupational lung disease, epidemiology, industrial hygiene, and survey design.46 Following question development and selection, questionnaires were tested for language clarity and ease of administration, and then validated by comparison of agreement vs gold standards for exposure identification (eg, industrial hygienist evaluation and job exposure matrices [JEMs]).43
Environmental exposure questionnaires administered directly to patients have been used in determining environmental risk factors for the development of IPF in several case-control studies, although no single questionnaire has been validated.21,47 From a diagnostic standpoint, current HP diagnosis guidelines recognize that use of questionnaires may be beneficial in obtaining a thorough exposure history and suggest that exposure questionnaire development and validation should be a focus of future research.3 Recently, there has been progress in developing a questionnaire to determine significant exposures in fibrotic HP.41 Through review of the literature, a list of inciting exposures was created; it was then narrowed to key items by using the Delphi method to develop a questionnaire, which was then tested for timing and clarity in a small sample of patients.41 The questionnaire has not yet been validated against a gold standard.
Another tool available to clinicians is the website hpLung.com, which provides a searchable list of exposures linked with HP, along with a tally of the number of cases identified during a literature search.48 Although potentially useful, it is limited to HP-related exposures and is not itself peer reviewed. In general, diagnostically or prognostically relevant exposures likely vary based on geographic and cultural factors, and any tool based on self-report would need to be adapted.41,49, 50, 51, 52, 53
JEMs can be a useful alternative or adjunct to subject self-reporting of specific exposures for research purposes, linking specific exposures with risk of disease onset or progression.54 Construction of a JEM is typically completed by an expert (eg, industrial hygienist) who uses patient-reported information about job title, occupation, industry, and work tasks to assign likelihood of a particular exposure and the intensity of exposure (low, medium, or high) with each job.55,56 JEM have been developed for occupational COPD and asthma. Further studies have validated these JEM and revealed acceptable sensitivity and specificity.57,58 Currently, no ILD-specific JEM exists. Sack et al47 used a JEM developed for occupational VGDF assessment in COPD to determine environmental risk factors for the development of CT-identified high attenuation areas (HAA) of the lung in the Multi-Ethnic Study of Atherosclerosis (MESA) air and lung cohorts. In this study, higher JEM-based exposure scores were associated with a higher percentage of HAA on CT imaging, whereas self-reported exposures were only associated with HAA in those currently working. This study illustrates the need for tools more sensitive than self-reporting in ILD exposure assessment.
Direct Evaluation of the Home and Workplace
Following detailed physician interview, many patients do not have obvious exposures associated with specific ILDs or that are considered harmful. In some of these cases, there may be a role for environmental evaluation by a trained professional. There are three categories of qualified environmental assessment professionals: industrial hygienists, indoor environmental quality consultants, and environmental health professionals (Table 2).59, 60, 61 Because there is a paucity of data on professional environmental evaluation and remediation as a diagnostic or therapeutic tool applicable to ILD, the subsequent sections review evidence in the setting of domestic and occupational asthma and occupational lung disease (including HP) to describe the potential benefits and pitfalls of such an evaluation.
Table 2.
Professional Qualifications for Environmental Evaluation
| Profession | Education and Experience | Certification |
|---|---|---|
| Industrial hygienist | Bachelor’s degree 5 Years of experience | American Board of Industrial Hygiene |
| Indoor environmental quality consultant | Interval standardized examinations for maintenance of certification 8 Years of field experience | Council of Engineering and Scientific Specialty Boards |
| Environmental health professionals | Physicians, nurses, public health experts | Nonstandardized training in environmental investigation |
Exposure Identification: Lessons From Metalworking Fluid-Associated Lung Disease
Some of the most common traditional occupational sources of antigen among individuals with HP in both the United States and the United Kingdom have included farming and raising and/or handling birds.62,63 Over time, however, the rates of farming- and avian-associated occupational exposures have declined, with an increase in the rate of metalworking fluid-associated HP.64, 65, 66, 67 Metalworking fluids are lubricating fluids with varying ratios of oil and water that are used to reduce friction and prolong the life of machine equipment.66,67 In 1998, the National Institute for Occupational Safety and Health (NIOSH) set limits on aerosolized metalworking fluid to an average concentration of 0.4 mg/m3 of thoracic particulate mass over the course of a 40-h week or a total particulate mass of 0.5 mg/m3. These limits were set based on epidemiologic data revealing increased risk of development of asthma and decline in pulmonary function at or above these limits, as well as the technical feasibility of detecting such levels.66,68, 69, 70 Thoracic particulate mass, the aerosolized portion of materials able to reach the lower respiratory tract based on particle size, is measured by using a thoracic sampling device fitted with a 10 μm pore and filter that is placed on a worker or in workplace locations of interest.25 Metalworking fluids are then identified on the filter by using gravimetric or infrared measures. There is evidence that HP can occur below these set relative exposure limits. During an outbreak of HP at an aeronautical plant, the sampled mean total particulate mass was 0.15 mg/m3, with a range of 0.09 to 0.38 mg/m3 in all samples taken from source areas affecting 16 patients.71 A case series from an automotive plant also showed thoracic particulate mass below the NIOSH-recommended limits in 30 patients with respiratory illnesses (six with HP), although the measured levels were not published.72
The discrepancy in NIOSH limits and threshold of disease onset may be due to the fact that many cases of metalworking fluid-associated HP result from microbial contamination of the metal working fluid rather than direct sensitization to the particles themselves.25,64,73 Measurement of bacterial and fungal burden is tested separately during industrial evaluation when metalworking fluid-associated HP is suspected. Samples are taken as several milliliters of bulk fluid obtained from used metal-working sumps and other associated water-based industrial processes, with samples processed and plated for microbial growth.25,64,71,73,74 Polymerase chain reaction (PCR) amplification of Mycobacterium species has also been used.73 Maintaining bacterial concentrations < 106 CFUs/mL of metalworking fluid has been suggested, but regulatory agencies have cited insufficient data to set exposure limit standards.25,66 Microbial air samples have also been taken in some studies using an air sampler in areas of machine use, with samples plated for microbial growth71; however, there are no standards for tolerable microbial levels. Comparison with outdoor air samples may be helpful in determining potentially pathogenic species.71,75 When several microbes grow from bulk samples, antigen extracts were tested against sera of affected workers, measuring specific IgG titers.64,71,73,74,76 Elevated antibody titers to the same antigen in multiple symptomatic workers have been helpful in identifying the cause of disease, especially when titers are negative or significantly lower in control workers. When a specific antigenic source cannot be identified, qualitative remediation efforts aimed at reducing overall bacterial burden have been pursued.71,75 For example, one study focused on improvement in ventilation and reduction of aerosolized metalworking fluid and found significantly decreased bacterial concentration in sumps with these efforts, allowing 51% of workers with HP to return to work symptom free within 26 months of the initial outbreak.75
As illustrated in metalworking fluid-associated HP, serum testing for antigen-specific IgG antibodies may be helpful in identifying exposure to an antigen known to be associated with HP. However, extrapolation for use in an individual patient (rather than application among a group of coworkers with and without disease), and to situations in which likely culprit exposures are not clearly identified, is difficult.77 Elevated serum IgG levels to a particular antigen in an individual merely indicate exposure to that antigen at some point in time. Practically, serum IgG testing can be helpful when an exposure is not identified on history. For example, if a patient without self-reported exposure to mold has an elevated serum IgG to Penicillium species, environmental evaluation to assess for occult exposure to mold may be pursued.3 Furthermore, as illustrated with the evaluation of metalworking fluid, there are no well-established or validated exposure thresholds for disease development, which can make interpretation of environmental inspection challenging. Future study is needed to determine and quantify the level of an exposure that is potentially harmful, although this level may vary by individual based on genetics and other cofactors. Antigen avoidance with assessment for clinical improvement over time is proposed as an indirect way of determining the inciting environment. However, the optimal duration of exposure avoidance necessary to observe beneficial effects is unknown and could be particularly difficult among those with fibrotic ILD in which the benefit may be slowing of disease progression rather than objective improvement.1,2,78
Exposure Remediation: Lessons From Domestic Asthma
Home evaluation and remediation of triggers have recognized benefits for individuals with asthma. A systematic review documented decreased symptom burden, improved quality of life, and decreased health-care utilization in children and adolescents with asthma following home-based environmental assessments and interventions.79,80 Such interventions may also benefit adults with asthma, although the evidence is less robust.79,81, 82, 83, 84 Key studies are detailed in Table 3.85, 86, 87 In children with asthma that is related to household allergens, interventions to nonspecifically reduce allergens (eg, high-efficiency particulate air filter vacuums, education about tobacco smoke and pet avoidance), as well as those targeting specific allergens (eg, pests), reduced symptoms for up to 2 years. Remediation efforts reduced measured (using a thoracic particulate air sampler) household dust allergen and air pollutant levels concurrently with a reduction in asthma symptoms.88 In one study of children with asthma living in a home with visible mold, residents of the home underwent either education about home cleaning or professional home remediation efforts were provided.86 Fungal species in dust collected from inside and outside the home were measured by using PCR, and indoor mold was measured visually by an environmental professional. Professional remediation resulted in greater reduction in visible and indoor PCR-measured mold, without reducing mean indoor allergens (eg, dust mite, cockroach); children in the remediation group had significantly fewer days with symptoms and exacerbations. Of note, the investigators in each of these studies had knowledge of allergen testing to target environmental evaluation and remediation efforts. Individuals with ILD may require more in-depth environmental evaluation to determine exposures necessitating remediation.
Table 3.
Selected Randomized Controlled Trials Documenting Benefit of Home Interventions in Asthma
| Study | Population | Intervention | Outcomes |
|---|---|---|---|
| Morgan et al,87 2004 | Children with poorly controlled asthma and documented sensitivity to home allergens | Visual inspection and dust collection All participants receive allergen-impermeable bed covers and HEPA filtration vacuums Child-specific interventions included HEPA air filters and pest control measures |
Intervention reduced symptoms at 1- and 2-y follow-up time points |
| Evans et al,85 1999 | Children with moderate or severe asthma | Allergen-impermeable bed covers and education about minimizing exposure to tobacco smoke, pets, and pests Social worker-administered global asthma intervention based on the Asthma Risk Assessment Tool |
Intervention reduced symptom days and hospitalizations over a 2-y follow-up period |
| Kercsmar et al,86 2006 | Children with symptomatic asthma living in a home with visible mold | Control group education about home cleaning and asthma management Intervention group also received home repairs aimed at remediation of water infiltration and mold (eg, HVAC maintenance) |
Intervention reduced symptom days and exacerbations over 12 mo of follow-up |
HEPA = high-efficiency particulate air; HVAC = heating, ventilation, and air conditioning.
Professional Environmental Assessment in ILD
Few studies have assessed the use of home evaluation, sampling, and remediation as a diagnostic or therapeutic intervention in HP or other ILDs. One small pilot study focused on the feasibility of identifying a causative antigen in the home and/or workplace in 19 patients with HP using questionnaires, targeted environmental sampling of frequently visited locations, and testing of patient serum against antigens collected from environmental samples.89 Overall, only seven of 19 patients reacted against environmental samples; however, among the subset of eight subjects suspected to have an accessible culprit antigen following questionnaire administration, seven reacted against environmental samples. Most subjects without reactivity had cessation of ongoing antigen exposure or had completed some remediation efforts prior to study enrollment. Although small, this study suggests that environmental evaluation in HP may be most productive as a diagnostic intervention (to identify/confirm a causative antigen) when conducted early in the disease course and at the time of active exposure. Furthermore, when applied broadly, home evaluation may have limited benefit in antigen identification beyond that of taking a thorough clinical history or use of a standardized questionnaire.
The benefits of home evaluation and remediation as a therapeutic intervention are also not well delineated in ILDs. In one study evaluating an HP outbreak among workers in a contaminated building, investigators used walk-through evaluation, serum-specific IgG testing, and targeted microbial cultures to identify a contaminated heating, ventilation, and air conditioning system as the source of elevated serum titers to Aureobasidium pullulans among multiple affected workers. Remediation of the heating, ventilation, and air conditioning system resulted in improvement in pulmonary function for many of the affected workers, eliminated positive antibody test results, and led to cessation of new cases.90 Taken together with asthma literature, it is extrapolated that identification and remediation of mold in the homes of individuals with HP (and possibly other ILDs) could have similar benefits. Importantly, these studies focused on patients with acute to subacute symptoms. Short-term benefit of exposure avoidance may be less apparent in a patient with fibrotic ILD. For example, a small study of nine patients with HP and > 1 year of exposure to birds measured lung function prior to and following removal of the bird, and found that only four of the nine subjects experienced improvement of lung function despite negative results on follow-up antigen testing.91 As illustrated in Case 2, exposure mitigation may not halt fibrotic ILD progression. Rigorous research is needed to understand how to best measure exposure remediation in an environment as well as the effects of exposure remediation interventions on relevant ILD outcomes.
Other Practical Considerations in Exposure Identification and Remediation
Real-world considerations surrounding environmental evaluation and remediation include cost to the patient, both in terms of payment for professional services and interference with livelihood. For instance, a major remediation targeting asthma control averaged $8,358, whereas minor interventions averaged $447.92 Health and homeowner’s insurances in the United States generally do not cover environmental evaluation; the cost is typically borne by the patient and may be prohibitive. Exposures associated with HP, such as avian proteins, can linger in the environment following bird removal, and extensive cleaning of the home can be costly.93 When the inciting exposure is occupational, some patients may not have other income options. Studies of patients with farmer’s lung have found that a majority return to farming following the HP diagnosis due to economic and self-efficacy motives.94 In cases of avian antigen exposure, patients or family members may have a strong emotional attachment to the birds, and personal costs of rehoming the birds may outweigh a patient’s perceived potential benefit.95 To mitigate these costs, some opt for use of respiratory masks or air filtration systems to reduce exposures, although there is little evidence regarding effectiveness or to guide choice of protective modality.96, 97, 98 It is also important to note that although eliminating exposure to the inciting antigen is typically recommended, limited direct evidence for effectiveness of that practice exists, particularly in the setting of fibrotic disease.77,99
Future Directions
Exposures undoubtedly contribute to ILD pathogenesis beyond those classically considered environmental ILDs. Further research is needed to identify the best ways to determine the relevance of specific exposures in the diagnostic paradigm and to optimize remediation interventions for harmful exposures in ILD. Future studies should focus on the feasibility, long-term outcomes, and cost-effectiveness of various domestic remediation strategies in both nonfibrotic and fibrotic ILDs. Furthermore, deeper investigation into genetic factors, biomarkers, and environmental cofactors that may make an individual more susceptible to specific exposures is required, as illustrated in Case 2. Further investigation is also needed in determining whether the type or amount of an exposure alters clinical course or prognosis in HP and other ILDs.100
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: B. F. C. reports personal fees from Boehringer Ingelheim Pharmaceuticals, Inc., outside the submitted work. M. L. S. reports grants from the National Institutes of Health, during the conduct of the study; personal fees from Boehringer Ingelheim Pharmaceuticals, Inc.; and personal fees from Orinove, Inc., outside the submitted work. None declared (C. R. C.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Footnotes
FUNDING/SUPPORT: This study was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health [Grant K23HL141539].
References
- 1.Salisbury M.L., Myers J.L., Belloli E.A., Kazerooni E.A., Martinez F.J., Flaherty K.R. Diagnosis and treatment of fibrotic hypersensitivity pneumonia. Where we stand and where we need to go. Am J Respir Crit Care Med. 2017;196(6):690–699. doi: 10.1164/rccm.201608-1675PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vasakova M., Morell F., Walsh S., Leslie K., Raghu G. Hypersensitivity pneumonitis: perspectives in diagnosis and management. Am J Respir Crit Care Med. 2017;196(6):680–689. doi: 10.1164/rccm.201611-2201PP. [DOI] [PubMed] [Google Scholar]
- 3.Raghu G., Remy-Jardin M., Ryerson C.J. Diagnosis of hypersensitivity pneumonitis in adults. An official ATS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2020;202(3):e36–e69. doi: 10.1164/rccm.202005-2032ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morisset J., Johannson K.A., Jones K.D. Identification of diagnostic criteria for chronic hypersensitivity pneumonitis: an international modified Delphi survey. Am J Respir Crit Care Med. 2018;197(8):1036–1044. doi: 10.1164/rccm.201710-1986OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandez Perez E.R., Swigris J.J., Forssen A.V. Identifying an inciting antigen is associated with improved survival in patients with chronic hypersensitivity pneumonitis. Chest. 2013;144(5):1644–1651. doi: 10.1378/chest.12-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johannson K.A., Vittinghoff E., Lee K. Acute exacerbation of idiopathic pulmonary fibrosis associated with air pollution exposure. Eur Respir J. 2014;43(4):1124–1131. doi: 10.1183/09031936.00122213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winterbottom C.J., Shah R.J., Patterson K.C. Exposure to ambient particulate matter is associated with accelerated functional decline in idiopathic pulmonary fibrosis. Chest. 2018;153(5):1221–1228. doi: 10.1016/j.chest.2017.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baumgartner K.B., Samet J.M., Stidley C.A., Colby T.V., Waldron J.A. Cigarette smoking: a risk factor for idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1997;155(1):242–248. doi: 10.1164/ajrccm.155.1.9001319. [DOI] [PubMed] [Google Scholar]
- 9.Lederer D.J., Enright P.L., Kawut S.M. Cigarette smoking is associated with subclinical parenchymal lung disease: the Multi-Ethnic Study of Atherosclerosis (MESA)-Lung Study. Am J Respir Crit Care Med. 2009;180(5):407–414. doi: 10.1164/rccm.200812-1966OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lederer D.J., Martinez F.J. Idiopathic pulmonary fibrosis. N Engl J Med. 2018;378(19):1811–1823. doi: 10.1056/NEJMra1705751. [DOI] [PubMed] [Google Scholar]
- 11.Selman M., Pardo A., King T.E., Jr. Hypersensitivity pneumonitis: insights in diagnosis and pathobiology. Am J Respir Crit Care Med. 2012;186(4):314–324. doi: 10.1164/rccm.201203-0513CI. [DOI] [PubMed] [Google Scholar]
- 12.Awadalla N.J., Hegazy A., Elmetwally R.A., Wahby I. Occupational and environmental risk factors for idiopathic pulmonary fibrosis in Egypt: a multicenter case-control study. Int J Occup Environ Med. 2012;3(3):107–116. [PubMed] [Google Scholar]
- 13.Baumgartner K.B., Samet J.M., Coultas D.B. Occupational and environmental risk factors for idiopathic pulmonary fibrosis: a multicenter case-control study. Collaborating Centers. Am J Epidemiol. 2000;152(4):307–315. doi: 10.1093/aje/152.4.307. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Sancho C., Buendia-Roldan I., Fernandez-Plata M.R. Familial pulmonary fibrosis is the strongest risk factor for idiopathic pulmonary fibrosis. Respir Med. 2011;105(12):1902–1907. doi: 10.1016/j.rmed.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 15.Gustafson T., Dahlman-Hoglund A., Nilsson K., Strom K., Tornling G., Toren K. Occupational exposure and severe pulmonary fibrosis. Respir Med. 2007;101(10):2207–2212. doi: 10.1016/j.rmed.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 16.Hubbard R., Cooper M., Antoniak M. Risk of cryptogenic fibrosing alveolitis in metal workers. Lancet. 2000;355(9202):466–467. doi: 10.1016/S0140-6736(00)82017-6. [DOI] [PubMed] [Google Scholar]
- 17.Hubbard R., Lewis S., Richards K., Johnston I., Britton J. Occupational exposure to metal or wood dust and aetiology of cryptogenic fibrosing alveolitis. Lancet. 1996;347(8997):284–289. doi: 10.1016/s0140-6736(96)90465-1. [DOI] [PubMed] [Google Scholar]
- 18.Miyake Y., Sasaki S., Yokoyama T. Occupational and environmental factors and idiopathic pulmonary fibrosis in Japan. Ann Occup Hyg. 2005;49(3):259–265. doi: 10.1093/annhyg/meh090. [DOI] [PubMed] [Google Scholar]
- 19.Mullen J., Hodgson M.J., DeGraff C.A., Godar T. Case-control study of idiopathic pulmonary fibrosis and environmental exposures. J Occup Environ Med. 1998;40(4):363–367. doi: 10.1097/00043764-199804000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Paolocci G., Folletti I., Toren K. Occupational risk factors for idiopathic pulmonary fibrosis in Southern Europe: a case-control study. BMC Pulm Med. 2018;18(1):75. doi: 10.1186/s12890-018-0644-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salisbury M.L., Hewlett J.C., Ding G. Development and progression of radiologic abnormalities in individuals at risk for familial interstitial lung disease. Am J Respir Crit Care Med. 2020;201(10):1230–1239. doi: 10.1164/rccm.201909-1834OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abramson M.J., Murambadoro T., Alif S.M. Occupational and environmental risk factors for idiopathic pulmonary fibrosis in Australia: case-control study. Thorax. 2020;75(10):864–869. doi: 10.1136/thoraxjnl-2019-214478. [DOI] [PubMed] [Google Scholar]
- 23.Scott J., Johnston I., Britton J. What causes cryptogenic fibrosing alveolitis? A case-control study of environmental exposure to dust. BMJ. 1990;301(6759):1015–1017. doi: 10.1136/bmj.301.6759.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cramer C., Schlunssen V., Bendstrup E. Risk of hypersensitivity pneumonitis and interstitial lung diseases among pigeon breeders. Eur Respir J. 2016;48(3):818–825. doi: 10.1183/13993003.00376-2016. [DOI] [PubMed] [Google Scholar]
- 25.Trout D., Harney J. US Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health; Cincinnati, OH: 2002. Health Hazard Evaluation Report: HETA-2001-0303-2893, TRW Automotive, Mt. Vernon, Ohio. [Google Scholar]
- 26.Blanc P.D., Annesi-Maesano I., Balmes J.R. The occupational burden of nonmalignant respiratory diseases. An official American Thoracic Society and European Respiratory Society statement. Am J Respir Crit Care Med. 2019;199(11):1312–1334. doi: 10.1164/rccm.201904-0717ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee S.H., Kim D.S., Kim Y.W. Association between occupational dust exposure and prognosis of idiopathic pulmonary fibrosis: a Korean national survey. Chest. 2015;147(2):465–474. doi: 10.1378/chest.14-0994. [DOI] [PubMed] [Google Scholar]
- 28.De Sadeleer L.J., Verleden S.E., De Dycker E. Clinical behaviour of patients exposed to organic dust and diagnosed with idiopathic pulmonary fibrosis. Respirology. 2018;23(12):1160–1165. doi: 10.1111/resp.13342. [DOI] [PubMed] [Google Scholar]
- 29.Conti S., Harari S., Caminati A. The association between air pollution and the incidence of idiopathic pulmonary fibrosis in Northern Italy. Eur Respir J. 2018;51(1) doi: 10.1183/13993003.00397-2017. [DOI] [PubMed] [Google Scholar]
- 30.de Gracia J., Morell F., Bofill J.M., Curull V., Orriols R. Time of exposure as a prognostic factor in avian hypersensitivity pneumonitis. Respir Med. 1989;83(2):139–143. doi: 10.1016/s0954-6111(89)80230-6. [DOI] [PubMed] [Google Scholar]
- 31.Tsutsui T., Miyazaki Y., Kuramochi J., Uchida K., Eishi Y., Inase N. The amount of avian antigen in household dust predicts the prognosis of chronic bird-related hypersensitivity pneumonitis. Ann Am Thorac Soc. 2015;12(7):1013–1021. doi: 10.1513/AnnalsATS.201412-569OC. [DOI] [PubMed] [Google Scholar]
- 32.Churg A., Sin D.D., Everett D., Brown K., Cool C. Pathologic patterns and survival in chronic hypersensitivity pneumonitis. Am J Surg Pathol. 2009;33(12):1765–1770. doi: 10.1097/PAS.0b013e3181bb2538. [DOI] [PubMed] [Google Scholar]
- 33.Hanak V., Golbin J.M., Hartman T.E., Ryu J.H. High-resolution CT findings of parenchymal fibrosis correlate with prognosis in hypersensitivity pneumonitis. Chest. 2008;134(1):133–138. doi: 10.1378/chest.07-3005. [DOI] [PubMed] [Google Scholar]
- 34.Mooney J.J., Elicker B.M., Urbania T.H. Radiographic fibrosis score predicts survival in hypersensitivity pneumonitis. Chest. 2013;144(2):586–592. doi: 10.1378/chest.12-2623. [DOI] [PubMed] [Google Scholar]
- 35.Ohtani Y., Saiki S., Kitaichi M. Chronic bird fancier's lung: histopathological and clinical correlation. An application of the 2002 ATS/ERS consensus classification of the idiopathic interstitial pneumonias. Thorax. 2005;60(8):665–671. doi: 10.1136/thx.2004.027326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salisbury M.L., Gu T., Murray S. Hypersensitivity pneumonitis: radiologic phenotypes are associated with distinct survival time and pulmonary function trajectory. Chest. 2019;155(4):699–711. doi: 10.1016/j.chest.2018.08.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernandez Alvarez R., Martinez Gonzalez C., Quero Martinez A., Blanco Perez J.J., Carazo Fernandez L., Prieto Fernandez A. Guidelines for the diagnosis and monitoring of silicosis. Arch Bronconeumol. 2015;51(2):86–93. doi: 10.1016/j.arbres.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 38.Raghu G., Remy-Jardin M., Myers J.L. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198(5):e44–e68. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]
- 39.Morell F., Villar A., Montero M.A. Chronic hypersensitivity pneumonitis in patients diagnosed with idiopathic pulmonary fibrosis: a prospective case-cohort study. Lancet Respir Med. 2013;1(9):685–694. doi: 10.1016/S2213-2600(13)70191-7. [DOI] [PubMed] [Google Scholar]
- 40.Perez-Padilla R., Salas J., Chapela R. Mortality in Mexican patients with chronic pigeon breeder's lung compared with those with usual interstitial pneumonia. Am Rev Respir Dis. 1993;148(1):49–53. doi: 10.1164/ajrccm/148.1.49. [DOI] [PubMed] [Google Scholar]
- 41.Barnes H., Morisset J., Molyneaux P. A systematically derived exposure assessment instrument for chronic hypersensitivity pneumonitis. Chest. 2020;157(6):1506–1512. doi: 10.1016/j.chest.2019.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Delclos G.L., Arif A.A., Aday L. Validation of an asthma questionnaire for use in healthcare workers. Occup Environ Med. 2006;63(3):173–179. doi: 10.1136/oem.2005.021634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Delclos G.L., Gimeno D., Arif A.A., Benavides F.G., Zock J.P. Occupational exposures and asthma in health-care workers: comparison of self-reports with a workplace-specific job exposure matrix. Am J Epidemiol. 2009;169(5):581–587. doi: 10.1093/aje/kwn387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Vocht F., Zock J.P., Kromhout H. Comparison of self-reported occupational exposure with a job exposure matrix in an international community-based study on asthma. Am J Ind Med. 2005;47(5):434–442. doi: 10.1002/ajim.20154. [DOI] [PubMed] [Google Scholar]
- 45.Quinlan P.J., Earnest G., Eisner M.D. Performance of self-reported occupational exposure compared to a job-exposure matrix approach in asthma and chronic rhinitis. Occup Environ Med. 2009;66(3):154–160. doi: 10.1136/oem.2008.040022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Delclos G.L., Gimeno D., Arif A.A. Occupational risk factors and asthma among health care professionals. Am J Respir Crit Care Med. 2007;175(7):667–675. doi: 10.1164/rccm.200609-1331OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sack C.S., Doney B.C., Podolanczuk A.J. Occupational exposures and subclinical interstitial lung disease. The MESA (Multi-Ethnic Study of Atherosclerosis) Air and Lung Studies. Am J Respir Crit Care Med. 2017;196(8):1031–1039. doi: 10.1164/rccm.201612-2431OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu J., Barnes H., Johannson K. hpLung. https://www.hplung.com/ Published 2018. Accessed October 23, 2020.
- 49.CHEST Foundation CHEST Interstitial and Diffuse Lung Disease Patient Questionnaire. https://foundation.chestnet.org/wp-content/uploads/2020/04/Interstitial-Diffuse-Lung-Disease-Patient-Questionnaire.pdf Published 2020. Accessed April 3, 2021.
- 50.Kurup V.P., Mantyjarvi R.A., Terho E.O., Ojanen T.H., Kalbfleisch J.H. Circulating IgG antibodies against fungal and actinomycete antigens in the sera of farmer's lung patients from different countries. Mycopathologia. 1987;98(2):91–99. doi: 10.1007/BF00437294. [DOI] [PubMed] [Google Scholar]
- 51.Reboux G., Reiman M., Roussel S. Impact of agricultural practices on microbiology of hay, silage and flour on Finnish and French farms. Ann Agric Environ Med. 2006;13(2):267–273. [PubMed] [Google Scholar]
- 52.Singh S., Collins B.F., Sharma B.B. Interstitial lung disease in India. Results of a prospective registry. Am J Respir Crit Care Med. 2017;195(6):801–813. doi: 10.1164/rccm.201607-1484OC. [DOI] [PubMed] [Google Scholar]
- 53.Yoshida K., Suga M., Nishiura Y. Occupational hypersensitivity pneumonitis in Japan: data on a nationwide epidemiological study. Occup Environ Med. 1995;52(9):570–574. doi: 10.1136/oem.52.9.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Teschke K., Olshan A.F., Daniels J.L. Occupational exposure assessment in case-control studies: opportunities for improvement. Occup Environ Med. 2002;59(9):575–593. doi: 10.1136/oem.59.9.575. discussion 594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Doney B., Hnizdo E., Graziani M. Occupational risk factors for COPD phenotypes in the Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. COPD. 2014;11(4):368–380. doi: 10.3109/15412555.2013.813448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kennedy S.M., Le Moual N., Choudat D., Kauffmann F. Development of an asthma specific job exposure matrix and its application in the epidemiological study of genetics and environment in asthma (EGEA) Occup Environ Med. 2000;57(9):635–641. doi: 10.1136/oem.57.9.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kurth L., Doney B., Weinmann S. Occupational exposures and chronic obstructive pulmonary disease (COPD): comparison of a COPD-specific job exposure matrix and expert-evaluated occupational exposures. Occup Environ Med. 2017;74(4):290–293. doi: 10.1136/oemed-2016-103753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suarthana E., Heederik D., Ghezzo H., Malo J.L., Kennedy S.M., Gautrin D. Risks for the development of outcomes related to occupational allergies: an application of the asthma-specific job exposure matrix compared with self-reports and investigator scores on job-training-related exposure. Occup Environ Med. 2009;66(4):256–263. doi: 10.1136/oem.2008.041962. [DOI] [PubMed] [Google Scholar]
- 59.American Board of Industrial Hygiene. www.abih.org/about-abih/qualifications Published 2020. Accessed April 3, 2021.
- 60.ACAC Council of Engineering and Scientific Specialty Boards. https://www.acac.org/find/CIEC-CIE.aspx Published 2019. Accessed April 3, 2021.
- 61.Storey E., Dangman K.H., Schenck P. University of Conneticut Health Center, Division of Occupational and Environmental Medicine, Center for Indoor Environments and Health; Farmington, CT: 2004. Guidance for Clinicians on the Recognition and Management of Health Effects Related to Mold Exposure and Moisture Indoors. [Google Scholar]
- 62.Barber C.M., Wiggans R.E., Carder M., Agius R. Epidemiology of occupational hypersensitivity pneumonitis; reports from the SWORD scheme in the UK from 1996 to 2015. Occup Environ Med. 2017;74(7):528–530. doi: 10.1136/oemed-2016-103838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Quirce S., Vandenplas O., Campo P. Occupational hypersensitivity pneumonitis: an EAACI position paper. Allergy. 2016;71(6):765–779. doi: 10.1111/all.12866. [DOI] [PubMed] [Google Scholar]
- 64.Bernstein D.I., Lummus Z.L., Santilli G., Siskosky J., Bernstein I.L. Machine operator’s lung. A hypersensitivity pneumonitis disorder associated with exposure to metalworking fluid aerosols. Chest. 1995;108(3):636–641. doi: 10.1378/chest.108.3.636. [DOI] [PubMed] [Google Scholar]
- 65.Burton C.M., Crook B., Scaife H., Evans G.S., Barber C.M. Systematic review of respiratory outbreaks associated with exposure to water-based metalworking fluids. Ann Occup Hyg. 2012;56(4):374–388. doi: 10.1093/annhyg/mer121. [DOI] [PubMed] [Google Scholar]
- 66.NIOSH . US Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health; Cincinnati, OH: 1998. Criteria for a Recommended Standard: Occupational Exposure to Metalworking Fluids. [Google Scholar]
- 67.NIOSH . US Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health; Cincinnati, OH: 1998. What You Need to Know About Occupational Exposure to Metalworking Fluids. [Google Scholar]
- 68.Greaves I.A., Eisen E.A., Smith T.J. Respiratory health of automobile workers exposed to metal-working fluid aerosols: respiratory symptoms. Am J Ind Med. 1997;32(5):450–459. doi: 10.1002/(sici)1097-0274(199711)32:5<450::aid-ajim4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 69.Hallock M.F., Smith T.J., Woskie S.R., Hammond S.K. Estimation of historical exposures to machining fluids in the automotive industry. Am J Ind Med. 1994;26(5):621–634. doi: 10.1002/ajim.4700260505. [DOI] [PubMed] [Google Scholar]
- 70.Kriebel D., Sama S.R., Woskie S. A field investigation of the acute respiratory effects of metal working fluids. I. Effects of aerosol exposures. Am J Ind Med. 1997;31(6):756–766. doi: 10.1002/(sici)1097-0274(199706)31:6<756::aid-ajim13>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 71.Hodgson M.J., Bracker A., Yang C. Hypersensitivity pneumonitis in a metal-working environment. Am J Ind Med. 2001;39(6):616–628. doi: 10.1002/ajim.1061. [DOI] [PubMed] [Google Scholar]
- 72.Zacharisen M.C., Kadambi A.R., Schlueter D.P. The spectrum of respiratory disease associated with exposure to metal working fluids. J Occup Environ Med. 1998;40(7):640–647. doi: 10.1097/00043764-199807000-00010. [DOI] [PubMed] [Google Scholar]
- 73.Tillie-Leblond I., Grenouillet F., Reboux G. Hypersensitivity pneumonitis and metalworking fluids contaminated by mycobacteria. Eur Respir J. 2011;37(3):640–647. doi: 10.1183/09031936.00195009. [DOI] [PubMed] [Google Scholar]
- 74.Trout D., Reh B., Weber A. US Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health; Cincinnati, OH: 1998. Health Hazard Evaluation Report: HETA-96-0156-2712, Ford Electronics and Refrigeration Corporation, Connersville, Indiana. [Google Scholar]
- 75.Bracker A., Storey E., Yang C., Hodgson M.J. An outbreak of hypersensitivity pneumonitis at a metalworking plant: a longitudinal assessment of intervention effectiveness. Appl Occup Environ Hyg. 2003;18(2):96–108. doi: 10.1080/10473220301436. [DOI] [PubMed] [Google Scholar]
- 76.Trout D., Harney J. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health; Cincinnati, OH: 2002. Health Hazard Evaluation Report: HETA-2002-0155-2886, DaimlerChrysler Transmission Plant, Kokomo, Indiana. [Google Scholar]
- 77.Wuyts W., Sterclova M., Vasakova M. Pitfalls in diagnosis and management of hypersensitivity pneumonitis. Curr Opin Pulm Med. 2015;21(5):490–498. doi: 10.1097/MCP.0000000000000199. [DOI] [PubMed] [Google Scholar]
- 78.Tsutsui T., Miyazaki Y., Okamoto T. Antigen avoidance tests for diagnosis of chronic hypersensitivity pneumonitis. Respir Investig. 2015;53(5):217–224. doi: 10.1016/j.resinv.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 79.Crocker D.D., Kinyota S., Dumitru G.G. Effectiveness of home-based, multi-trigger, multicomponent interventions with an environmental focus for reducing asthma morbidity: a community guide systematic review. Am J Prev Med. 2011;41(2 suppl 1):S5–S32. doi: 10.1016/j.amepre.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 80.Task Force on Community Preventive S. Recommendations from the Task Force on Community Preventive Services to decrease asthma morbidity through home-based, multi-trigger, multicomponent interventions. Am J Prev Med. 2011;41(2 suppl 1):S1–S4. doi: 10.1016/j.amepre.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 81.Turcotte D.A., Woskie S., Gore R., Chaves E., Adejumo K.L. Asthma, COPD, and home environments: interventions with older adults. Ann Allergy Asthma Immunol. 2019;122(5):486–491. doi: 10.1016/j.anai.2019.02.026. [DOI] [PubMed] [Google Scholar]
- 82.Smith J.R., Mildenhall S., Noble M.J. The Coping with Asthma Study: a randomised controlled trial of a home based, nurse led psychoeducational intervention for adults at risk of adverse asthma outcomes. Thorax. 2005;60(12):1003–1011. doi: 10.1136/thx.2005.043877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brown M.D., Reeves M.J., Meyerson K., Korzeniewski S.J. Randomized trial of a comprehensive asthma education program after an emergency department visit. Ann Allergy Asthma Immunol. 2006;97(1):44–51. doi: 10.1016/S1081-1206(10)61368-3. [DOI] [PubMed] [Google Scholar]
- 84.Barton A., Basham M., Foy C., Buckingham K., Somerville M., Torbay Healthy Housing Group The Watcombe Housing Study: the short term effect of improving housing conditions on the health of residents. J Epidemiol Community Health. 2007;61(9):771–777. doi: 10.1136/jech.2006.048462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Evans R., III, Gergen P.J., Mitchell H. A randomized clinical trial to reduce asthma morbidity among inner-city children: results of the National Cooperative Inner-City Asthma Study. J Pediatr. 1999;135(3):332–338. doi: 10.1016/s0022-3476(99)70130-7. [DOI] [PubMed] [Google Scholar]
- 86.Kercsmar C.M., Dearborn D.G., Schluchter M. Reduction in asthma morbidity in children as a result of home remediation aimed at moisture sources. Environ Health Perspect. 2006;114(10):1574–1580. doi: 10.1289/ehp.8742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Morgan W.J., Crain E.F., Gruchalla R.S. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;351(11):1068–1080. doi: 10.1056/NEJMoa032097. [DOI] [PubMed] [Google Scholar]
- 88.Eggleston P.A., Butz A., Rand C. Home environmental intervention in inner-city asthma: a randomized controlled clinical trial. Ann Allergy Asthma Immunol. 2005;95(6):518–524. doi: 10.1016/S1081-1206(10)61012-5. [DOI] [PubMed] [Google Scholar]
- 89.Millerick-May M.L., Mulks M.H., Gerlach J. Hypersensitivity pneumonitis and antigen identification—an alternate approach. Respir Med. 2016;112:97–105. doi: 10.1016/j.rmed.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 90.Woodard E.D., Friedlander B., Lesher R.J., Font W., Kinsey R., Hearne F.T. Outbreak of hypersensitivity pneumonitis in an industrial setting. JAMA. 1988;259(13):1965–1969. [PubMed] [Google Scholar]
- 91.Allen D.H., Williams G.V., Woolcock A.J. Bird breeder's hypersensitivity pneumonitis: progress studies of lung function after cessation of exposure to the provoking antigen. Am Rev Respir Dis. 1976;114(3):555–566. doi: 10.1164/arrd.1976.114.3.555. [DOI] [PubMed] [Google Scholar]
- 92.Nurmagambetov T.A., Barnett S.B., Jacob V. Economic value of home-based, multi-trigger, multicomponent interventions with an environmental focus for reducing asthma morbidity a community guide systematic review. Am J Prev Med. 2011;41(2 suppl 1):S33–S47. doi: 10.1016/j.amepre.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 93.Craig T.J., Hershey J., Engler R.J., Davis W., Carpenter G.B., Salata K. Bird antigen persistence in the home environment after removal of the bird. Ann Allergy. 1992;69(6):510–512. [PubMed] [Google Scholar]
- 94.Monkare S., Haahtela T. Farmer's lung—a 5-year follow-up of eighty-six patients. Clin Allergy. 1987;17(2):143–151. doi: 10.1111/j.1365-2222.1987.tb02332.x. [DOI] [PubMed] [Google Scholar]
- 95.Hendrick D.J., Marshall R., Faux J.A., Krall J.M. Protective value of dust respirators in extrinsic allergic alveolitis: clinical assessment using inhalation provocation tests. Thorax. 1981;36(12):917–921. doi: 10.1136/thx.36.12.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Anderson K., Walker A., Boyd G. The long-term effect of a positive pressure respirator on the specific antibody response in pigeon breeders. Clin Exp Allergy. 1989;19(1):45–49. doi: 10.1111/j.1365-2222.1989.tb02342.x. [DOI] [PubMed] [Google Scholar]
- 97.Jacobs R.L., Andrews C.P., Jacobs F.O. Hypersensitivity pneumonitis treated with an electrostatic dust filter. Ann Intern Med. 1989;110(2):115–118. doi: 10.7326/0003-4819-110-2-115. [DOI] [PubMed] [Google Scholar]
- 98.Muller-Wening D., Repp H. Investigation on the protective value of breathing masks in farmer's lung using an inhalation provocation test. Chest. 1989;95(1):100–105. doi: 10.1378/chest.95.1.100. [DOI] [PubMed] [Google Scholar]
- 99.Cormier Y., Belanger J. Long-term physiologic outcome after acute farmer's lung. Chest. 1985;87(6):796–800. doi: 10.1378/chest.87.6.796. [DOI] [PubMed] [Google Scholar]
- 100.Adams T.N., Newton C.A., Glazer C.S. Role of antigen type in survival in chronic hypersensitivity pneumonitis. Lung. 2019;197(1):113–114. doi: 10.1007/s00408-018-0187-6. [DOI] [PMC free article] [PubMed] [Google Scholar]