Abstract
Adrenocortical carcinoma (ACC) is a rare, aggressive malignancy with an annual incidence of ~1 case per million population. Differentiating between ACC and benign adrenocortical tumors can be challenging in patients who present with an incidentally discovered adrenal mass, due to the limited specificity of standard diagnostic imaging. Recently, urine steroid metabolite profiling has been prospectively validated as a novel diagnostic tool for the detection of malignancy with improved accuracy over current modalities. Surgery represents the only curative treatment for ACC, although local recurrence and metastases are common, even after a margin-negative resection is performed. Unlike other intra-abdominal cancers, the role of minimally invasive surgery and lymphadenectomy in ACC is controversial. Adjuvant therapy with the adrenolytic drug mitotane is used to reduce the risk of recurrence after surgery, although evidence supporting its efficacy is limited; it is also currently unclear whether all patients or a subset with the highest risk of recurrence should receive this treatment. Large-scale pan-genomic studies have yielded insights into the pathogenesis of ACC and have defined distinct molecular signatures associated with clinical outcomes that may be used to improve prognostication. For patients with advanced ACC, palliative combination chemotherapy with mitotane is the current standard of care; however, this is associated with poor response rates (RR). Knowledge from molecular profiling studies has been used to guide the development of novel targeted therapies; however, these have shown limited efficacy in early phase trials. As a result, there is an urgent unmet need for more effective therapies for patients with this devastating disease.
Keywords: adrenocortical carcinoma, adrenalectomy, mitotane, adrenal incidentaloma, diagnosis, treatment
Introduction
Adrenocortical carcinoma (ACC) is a rare endocrine malignancy that arises from the cortex of the adrenal gland and has an estimated worldwide incidence of ~1 case per million population per year.1 –3 Despite its rarity, ACC generally portends a poor prognosis as the majority of patients develop locally recurrent or metastatic disease, despite undergoing seemingly curative surgical resection. 4 A number of questions and controversies exist regarding aspects of diagnosis, medical treatment, and the surgical management of ACC. The rarity of this disease, and short duration of survival, has resulted in a paucity of prospective clinical trials. Therefore, current treatment recommendations are largely driven by consensus opinion based on retrospective data.5,6 While our understanding of the molecular mechanisms that underpin ACC development has greatly improved as a result of large-scale pan-genomic studies,7,8 the translation of this knowledge into effective clinical therapies for patients with advanced disease has been limited to date. In this review, we provide an overview of the current state of the art in the diagnosis and treatment of ACC, highlighting ongoing controversies and recent advances, as well as their potential effect on clinical practice.
Epidemiology
ACC is a rare malignancy that meets the criteria for ‘orphan’ disease designation in the European Union and in the United States (<50 cases per 100,000 population and <64 cases per 10,000 population, respectively). 9 Analysis of the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) database indicates an annual incidence of ACC of 0.72–1.02 per million in the United States.1,2 This is in accordance with a population-based study from the Netherlands, which reported an incidence of 1 case per million. 3 In contrast, the incidence of ACC is 10-fold higher in Southern Brazilian children, which is partly attributable to the high frequency of a specific founder tumor protein 53 (TP53) germline mutation in this population. 10 Although ACC can occur at any age, some studies have described a bimodal age distribution with an initial peak in incidence during childhood, followed by another in the fourth to fifth decade of life.11,12 Others have depicted only a single peak with a median age at diagnosis of 55–56 years.1 –3 Despite this, the observation that ACCs account for 1.3% of all pediatric cancers 13 versus ~0.02% of malignant tumors in adults,14,15 suggests a relatively higher incidence in childhood. Regardless of age, ACC shows a slight female predilection (ratio of affected females to males: 1.5–2.5:1).11,16 Epidemiological risk factors for ACC are not well understood, although recent studies have identified an increased incidence in male cigarette smokers.17 –19 In females, estrogen exposure has been implicated as a risk factor due to an increased incidence of ACC in users of the oral contraceptive pill, 19 and the anti-proliferative effects of estrogen inhibition on ACC cells in vitro. 20
Pathogenesis
Initial insights into the genes and signaling pathways involved in ACC tumorigenesis came from studies of familial diseases associated with ACC development, for which the causative germline alterations are well-defined. These include: Li-Fraumeni syndrome (caused by inactivating TP53 mutations); 21 Beckwith-Wiedemann syndrome [caused by genetic and epigenetic abnormalities of chromosome 11 (11p15.5)]; 22 multiple endocrine neoplasia (MEN) type 1 (caused by inactivating MEN1 mutations); 23 and Lynch syndrome [caused by inactivating mutations in the mismatch repair genes MutL homolog 1 (MLH1), MutS homolog 2 (MSH2), MutS homolog 6 (MSH6), and PSM1 homolog 1, mismatched repair system component (PMS2)]. 24 However, the vast majority of ACCs are sporadic; it is increasingly understood that they may be driven by a myriad of genetic and epigenetic aberrations. These include somatic DNA mutations, chromosomal aneuploidy, altered DNA methylation, and dysregulated microRNA (miRNA) expression. 25
Much of our current understanding of the landscape of molecular alterations in ACC is derived from two tour de force studies: one from the European Network for the Study of Adrenal Tumors (ENS@T) 7 and another from The Cancer Genome Atlas Study on ACC group (ACC-TCGA). 8 Both consortia utilized a range of multi-omics techniques, including DNA exome sequencing, mRNA expression profiling, miRNA profiling, DNA methylation analysis, and single nucleotide polymorphism (SNP) arrays that enabled in-depth pan-molecular characterization of a large number of primary ACCs for the first time. Although a detailed discussion of these findings is beyond the scope of this review and has been provided recently by others,25 –27 both studies showed that ACCs harbor somatic driver mutations that most frequently affect genes involved in Wnt/β-catenin signaling [Zinc and ring finger 3 (ZNRF3), Catenin Beta 1 (CTNNB1)]; cell cycle regulation [TP53, cyclin dependent kinase inhibitor 2A (CDKN2A), retinoblastoma protein 1 (RB1), cyclin-dependent kinase (CDK)]; and chromatin remodeling [MEN1, death domain associated protein (DAXX)].7,8 They also confirmed findings from earlier single platform studies28,29 which showed that overexpression of the insulin-like growth factor-2 (IGF2) occurs in 80–90% of ACCs, largely due to loss of heterozygosity at the IGF2 locus at chromosome 11p15 (i.e. loss of the imprinted maternal allele and duplication of the paternal allele).7,8 Importantly, these studies have enabled the classification of ACC into molecular subtypes with distinct biological signatures associated with good and poor prognosis, and have identified potentially druggable targets.
Clinical features
ACC generally presents in three forms: ~40–60% of patients present with symptoms and signs of hormone excess, ~30% present with nonspecific symptoms (e.g. abdominal pain and fullness due to tumor growth or constitutional symptoms of malignancy), and 20–30% are asymptomatic and diagnosed incidentally on cross-sectional imaging performed for other indications. 30 The most common syndrome of hormone excess in patients with functional ACC is Cushing’s syndrome, which occurs in ~45% of patients and may cause central weight gain, plethora, proximal muscle atrophy, diabetes mellitus, and easy bruising. A mixed clinical picture of Cushing’s and virilization due to concomitant cortisol and androgen hypersecretion may also be seen in 20–30% of patients.31,32 The latter phenotype is strongly suggestive of malignancy and is not seen in patients with benign adrenal adenomas. 30 While autonomous aldosterone secretion is rare in ACC, hypokalemia and hypertension may still occur secondary to glucocorticoid-induced mineralocorticoid receptor activation in patients with hypercortisolism. 30 Feminization in males due to excess estrogen production occurs in 1–5% of patients. 33 The effect of functional status on survival outcomes in patients with ACC is controversial. Although some series have shown hormone secretion to be an independent risk factor for poorer overall survival (OS) and recurrence-free survival (RFS),34 –37 these findings have not been consistently confirmed by others.38,39 A meta-analysis by Vanbrabant et al. 40 demonstrated an increased risk of mortality [relative risk (RR) 1.71] and recurrence (RR 1.43) in cortisol-secreting, but not androgen-secreting, ACC. However, it is unclear whether these findings are due to the deleterious effects of cortisol hypersecretion rather than true underlying differences in tumor biology and aggressiveness.
Diagnostic evaluation
Clinical workup of suspected ACC is centered around hormonal evaluation and cross-sectional imaging, with a final diagnosis confirmed by histopathological examination. The combined rarity of this malignancy and the lack of disease-specific symptoms can make ruling out a diagnosis of ACC challenging in a patient with an incidentally discovered adrenal mass, (an adrenal ‘incidentaloma’) particularly when imaging and biochemistry are inconclusive.
Biochemistry
Biochemical evidence of hormone excess is found in 60–70% of patients with ACC.41,42 Hormonal evaluation is useful in the workup of ACC for several reasons: (1) the presence of steroid hormone excess establishes the adrenocortical origin of the tumor and obviates the need for unnecessary investigations such as biopsies; (2) the specific pattern of hormones excess (e.g. co-secretion of cortisol and androgens) may further raise suspicion for malignancy; (3) the measurement of hormone levels may serve as a useful biomarker for recurrence during follow-up after surgery; (4) the need for postoperative hydrocortisone replacement in patients with cortisol-producing ACCs can be determined; and (5) the potential for intraoperative hypertensive crises due to undiagnosed pheochromocytoma is avoided.5,30 Specific biochemical tests, as recommended in current ENS@T/European Society of Endocrinology (ESE) guidelines, 5 are shown in Table 1.
Table 1.
Hormonal and biochemical workup in patients with suspected ACC.
| Category of hormonal excess | Recommended test(s) |
|---|---|
| Glucocorticoid excess | 1 mg dexamethasone suppression test |
| Basal ACTH (plasma) a | |
| Sex steroids and steroid precursors b | DHEA-S |
| 17-hydroxyprogesterone | |
| Androstenedione | |
| Testosterone (only in women) | |
| 17-beta-estradiol (only in men and postmenopausal women) | |
| 11-deoxycortisol | |
| Mineralocorticoid excess | Serum potassium |
| Aldosterone/renin ratio (only in patients with arterial hypertension and/or hypokalemia) | |
| Catecholamine excess (for exclusion of pheochromocytoma) | Free plasma-metanephrines or 24-h urinary fractionated metanephrines |
Table adapted from Fassnacht et al. 5
Can be omitted if hypercortisolism is excluded.
The most suitable set of precursors and sex hormones has not yet been established and local testing availability may dictate what tests are available.
ACTH, adrenocorticotropic hormone; DHEA-S, dehydroepiandrosterone sulfate.
Imaging
Computed tomography
Imaging forms the cornerstone of evaluation in patients with suspected ACC; the most commonly used first-line modality is typically an abdominal computed tomography (CT) scan. 43 CT features suggestive of an ACC rather than a benign adrenal mass include the presence of irregular borders, areas of necrosis, hemorrhage, and/or calcification [Figure 1(a)]. Invasion into surrounding structures, such as the inferior vena cava, may also be seen [Figure 1(b)]. 44 The risk of malignancy in an adrenal mass increases with size, with ACCs typically presenting with a median diameter of 10 cm. 31 The risk of ACC by size has been reported as 2%, 6%, and 25% for adrenal lesions of <4 cm, 4–6 cm, and >6 cm, respectively, leading to the recommendation in some consensus guidelines that adrenalectomy should be considered for lesions >4 cm.45,46 Calculation of tissue density as an approximation of intracellular lipid content on unenhanced CT, by measuring Hounsfield units (HU), is also used to distinguish between ACC and benign adrenocortical adenomas. Lipid-rich adenomas typically exhibit low attenuation on unenhanced CT and a HU threshold of ⩽10 is suggested as being indicative of a benign lesion in current ENS@T/ESE and European Society for Medical Oncology (ESMO) guidelines.33,47 In contrast, a HU of >10 is suggested as being indicative of malignancy. However, the distinction between benign and malignant adrenocortical tumors based on imaging is not always clear-cut, and the challenges surrounding this are discussed in detail elsewhere.33,48
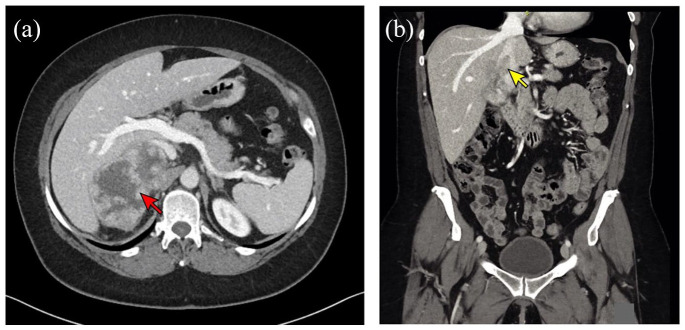
Figure 1.
CT imaging in a patient with advanced ACC. (a) Axial CT image showing a heterogeneously enhancing right adrenal mass (red arrow) measuring 9.9 × 6.0 × 8.0 cm with possible invasion into the liver. (b) Coronal CT image from the same patient showing tumor thrombus within the inferior vena cava (yellow arrow).
ACC, Adrenocortical carcinoma; CT, computed tomography.
Positron emission tomography-computed tomography
The utility of [ 18 F]-fluorodeoxyglucose–positron emission tomography (FDG-PET)/CT in the evaluation of ACC is not well defined. In patients with a history of extra-adrenal malignancy, this modality may be useful in distinguishing between benign lesions and metastases originating from cancers that have a propensity for spreading to the adrenals (e.g., liver, lung, lymph nodes, and bone). 49 However, in patients without known or suspected extra-adrenal malignancy and indeterminate CT findings, the role of FDG-PET/CT is less clear. In a study of 87 patients without known cancer, who underwent workup for adrenal lesions with indeterminate findings on washout CT, Guerin et al. 50 demonstrated that an FDG adrenal lesion/liver standardized uptake value (SUV) ratio of >1.5 detected malignant adrenal lesions with reasonable sensitivity and specificity (86.7% and 86.1%, respectively). In contrast, in a study of 106 ACC patients, FDG-PET/CT only provided additional information beyond contrast-enhanced CT in a minority (5%) of patients. 51 Other studies evaluating FDG-PET/CT for the assessment of adrenal masses are limited by the inclusion of small numbers of ACC patients and suboptimal reporting of test accuracy; 48 therefore, there is a lack of consensus regarding whether FDG-PET/CT regarding should be recommended for routine use in all patients with suspected ACC. 5 Furthermore, limitations of this modality include cost, additional radiation exposure, and the potential for false-positive findings, particularly in functional adenomas and nonmetastatic pheochromocytomas, which may exhibit FDG-avidity. 49
Metomidate (MTO) is a potent inhibitor of the adrenal enzymes CYP11B1 (11β-hydroxylase) and CYP11B2 (aldosterone synthase). Given its selectivity for these adrenal-specific targets, MTO labelled with [ 11 C] for PET imaging or [ 123 I] for single-photon emission computerized tomography (SPECT)/CT has been developed as an alternative tracer for functional adrenal imaging. 52 Evidence to date suggests that while MTO may be useful in distinguishing between cortical and noncortical adrenal lesions, its ability to differentiate benign from malignant adrenal tumors is limited. 52 Despite this, a subset of patients with metastatic ACC have been shown to demonstrate [ 123 I] MTO uptake, which has provided the rationale for trials of radionuclide-based systematic therapy in this subgroup (discussed below).53,54
Urine steroid metabolomics
Limitations in the diagnostic accuracy of the imaging tests described above are reflected in the low prevalence of malignancy (<10%) in contemporary series of patients undergoing adrenalectomy for non-functioning incidentalomas.55,56 Therefore, patients are commonly subjected to multiple radiological studies and unnecessary surgical resection of adrenal masses that are ultimately revealed to be benign. A technology that has recently come to the forefront as a means of improving current standard-of-care workup for ACC is urine steroid metabolomics, which utilizes mass spectrometry-based urinary steroid metabolite profiling in combination with machine-learning-based data analysis. 42 The rationale for this approach is based on the finding that ACCs are relatively inefficient steroid producers, due to the dysregulated expression of steroidogenic enzymes. 57 This results in the excessive secretion of a range of steroid hormone precursors, instead of the normal end products of steroid hormone biosynthesis. These precursors, while not routinely measured in blood tests, can be detected using gas or liquid chromatography/mass spectrometry analysis of 24-hour urine samples. Proof-of-concept for this approach was first demonstrated by Arlt et al., 41 who retrospectively compared 45 patients with ACC to 102 patients with benign adrenal adenomas, showing that the former had significantly greater urinary excretion of androgen precursor metabolites, metabolites of active androgens, deoxycorticosterone, and glucocorticoid precursors. Using a machine-learning-based algorithm, a malignant steroid ‘fingerprint’ was generated, which predicted ACC with 90% sensitivity and 90% specificity. 41
The recently published Evaluation of Urine Steroid Metabolomics in the Differential Diagnosis of Adrenocortical Tumours (EURINE-ACT) study prospectively validated the panel proposed by Arlt et al. 41 in an international cohort of 2017 patients, 98 of whom had ACC. 58 In this study, three tests (tumor diameter, imaging characteristics, and urine steroid metabolomics) were assessed for diagnostic accuracy, either separately or in combination. The combination of all three tests demonstrated the highest positive predictive value (76.5%) for diagnosing ACC in patients with the following results: tumor size >4 cm, CT attenuation >20 HU, and a high-risk urine steroid metabolomics profile. The negative predictive value of this triple testing strategy was high, (99.7%) highlighting its potential value in ruling out ACC and preventing a subset of patients from undergoing unnecessary investigations and surgery. The same group has also shown, in a preliminary study, that urine steroid metabolomics can be used as for the detection of ACC recurrence after surgery; however, prospective validation and comparison against the reference standard for recurrence detection (imaging) is awaited. 59
Despite the clear benefits of a non-invasive assay such as urine steroid metabolomics, the widespread implementation of this technology into routine clinical practice is currently limited by both the cost and availability of mass spectrometry equipment as well as the need for inter-laboratory cross-validation of assay results.
Pathology
While the clinical, biochemical, and radiological tests outlined above may raise suspicion for ACC, the final diagnosis is made on histological examination, which should be performed by an experienced endocrine pathologist. Macroscopically, ACCs tend to be large lobulated masses [Figure 2(a)], with heterogenous areas of hemorrhage and necrosis within a fibrous capsule. Their cut surface ranges from brown to orange to yellow depending upon intracellular lipid content [Figure 2(b)]. The only definitive criteria for malignancy are the presence of distant metastasis and/or loco-regional invasion; in the absence of these features, the diagnosis may be made using various histological multiparameter scoring systems. In practice, the system first proposed by Weiss in 1984 60 is most widely used. The Weiss score is based on nine microscopic criteria, each of which is weighted equally and given a score of 1 if present (Table 2). 60 A total score of ⩾3 is consistent with ACC, while a score of 0–2 indicates an adrenocortical adenoma. Some borderline lesions scoring 2, however, are still be considered suspicious and may be deemed as having ‘uncertain malignant potential’. 61 The reliability of the Weiss system has been challenged in these borderline cases and, in recent years, additional scoring systems have been developed. These are either simplified versions of the existing Weiss score 62 and/or incorporate immunohistochemical staining for reticulin 63 or Ki-67. 64 Each of these demonstrates similarly high performance, with sensitivities of ~100% and specificities of 90–99% for diagnosing ACC. 65 Nonetheless, the very existence of several competing scoring systems highlights the challenges and complexities faced in the pathological assessment of ACC and the fact that no individual system is ideal. In an effort to standardize the reporting process, the International Collaboration on Cancer Reporting (ICCR) have proposed a minimum reporting standard of 23 items that should be included in ACC pathology reports, based on the consensus of an international panel of expert adrenal pathologists. 66
Figure 2.
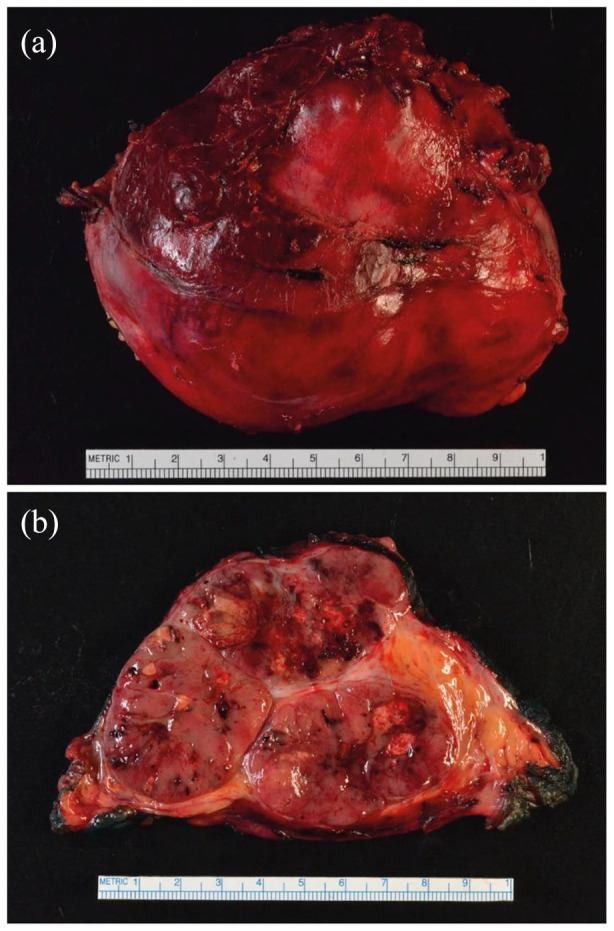
Gross pathology of a resected right-sided ACC. (a) Gross image showing a 10 cm brown mass with an attached rim of liver tissue that was resected en bloc. (b) Cut surface of the resected ACC showing a variegated appearance with focal areas of hemorrhage.
ACC, adrenocortical carcinoma.
Table 2.
Weiss criteria. 60
| Criteria | Score awarded a | |
|---|---|---|
| 0 | 1 | |
| Nuclear grade | I/II | III/IV |
| Mitosis | ⩽5 per 50 HPF | >5 per 50 HPF |
| Atypical mitoses | Absent | Present |
| Clear cell component | ⩽25% of tumor | >25% of tumor |
| Diffuse architecture | ⩽1/3 of tumor | >1/3 of tumor |
| Confluent necrosis | Absent | Present |
| Venous invasion | Absent | Present |
| Sinusoidal invasion | Absent | Present |
| Capsular invasion | Absent | Present |
A total score of >3 is suggestive of ACC.
uclear grading is based on the Fuhrman nuclear grading system used in renal cell carcinoma. 67
HPF, high power fields.
It should be noted that the Weiss score overdiagnoses malignancy in the oncocytic subtype of adrenocortical tumors. This is because oncocytic tumors inherently possess at least three features of the Weiss criteria (high nuclear polymorphism, <25% clear cells, and diffuse architecture), whether they are benign or malignant. 61 For this variant, a modified scoring system (the Lin-Weiss-Bisceglia system) 68 should be utilized. In contrast, the Weiss score underestimates the risk of malignancy in the even rarer myxoid variant of adrenal tumors. Although no specific scoring system exists for this subtype, the Helsinki score (which incorporates Ki-67) has been shown to outperform the Weiss score in this context. 65
Is there a role for preoperative adrenal biopsy?
Transcutaneous adrenal biopsy is not generally indicated in patients with ACC because the limited amount of tissue obtained from this procedure makes the differentiation between benign and malignant adrenal masses difficult. 69 Furthermore, complications may occur in 11% of patients, including hemorrhage, pneumothorax, pancreatitis, and the small risk of needle-track seeding due to violation of the tumor capsule. 70 Despite this, biopsy may be indicated in patients with metastatic ACC who are not surgical candidates and in whom a tissue diagnosis would help inform oncological management. Biopsy may also be useful in situations where the exclusion of suspected metastasis in patients with a known extra-adrenal primary malignancy would guide the choice of therapy (e.g. surgery for limited disease versus chemotherapy for metastatic disease). 5 Prior to biopsy being performed, biochemical exclusion of pheochromocytoma is necessary to avoid the possibility of potentially life-threatening catecholamine surge. 5
Staging and prognosis
Staging systems
Accurate staging of ACC plays a key role in treatment planning and prognostication. Although several staging systems have been described, the one proposed by the ENS@T group in 2009 is most widely used. 71 The ENS@T staging system is a modification of the original 2004 American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) tumor, node, metastasis (TNM) classification (Table 3), which was criticized for its inability to discriminate between the survival outcomes of patients with stage II and III disease. 71 Furthermore, it was realized that patients with tumor invasion into surrounding adipose tissue and positive lymph nodes, or those with invasion into adjacent organs fared significantly better than those with distant metastases, despite all of these patients being classified as stage IV disease according to the 2004 AJCC/UICC system.71,72 Instead, the ENS@T system defines stage I and stage II ACC as tumors confined to the adrenal gland, that measure ⩽5 cm and >5 cm, respectively; stage III denotes tumors that extend into surrounding adipose tissue or adjacent organs or involve locoregional lymph nodes; stage IV only includes tumors with established distant metastases. 71 The changes proposed by ENS@T were recently incorporated into the 2017 (8th edition) AJCC/UICC staging manual for ACC, 73 such that both staging systems are now virtually identical (Table 3). Other groups have attempted to further refine the discriminatory ability of the ENS@T staging system with the inclusion of additional clinicopathological factors such as age,74,75 Ki-67,75,76 lymphovascular invasion, 77 resection margin status, 75 number of tumor-involved organs, 75 and the presence of symptoms at diagnosis. 75 Of these factors, Ki-67 appears to be the single most powerful predictor of disease recurrence following resection, with the longest RFS seen in patients with a Ki-67 index of <10%. 78
Table 3.
Comparison of UICC/AJCC 2004 and ENS@T 2009/UICC/AJCC 2017 staging classifications for ACC.
| Stage | UICC/AJCC 2004 | ENS@T 2009 and UICC/AJCC 2017 |
|---|---|---|
| I | T1, N0, M0 | T1, N0, M0 |
| II | T2, N0, M0 | T2, N0, M0 |
| III | T3, N0, M0 T1-T2, N1, M0 |
T3-T4, N0, M0 T1-T4, N1, M0 |
| IV | T3, N1, M0 T4, N0-N1, M0 Any M1 |
Any M1 |
Tumors are classified as follows: T1, ⩽5 cm; T2, >5 cm tumor; T3, tumor infiltration into surrounding tissue; T4, tumor invasion into adjacent organs; N0, no positive lymph nodes; N1, positive lymph node(s); M0, no distant metastases; M1, presence of distant metastasis.
AJCC, American Joint Committee on Cancer; ENS@T, European Network for the Study of Adrenal Tumors; UICC, Union for International Cancer Control.
Prognosis
Although the overall prognosis of ACC is poor, survival can be heterogenous depending on the extent of disease. The majority of patients with ACC present with advanced disease (34% with stage III disease and 26% with stage IV disease) with the most common sites of metastatic spread being the liver, lungs, lymph nodes, and bone.5,30 Typically quoted 5-year survival rates (in European and North American cohorts) are 66–82% for stage I, 58–64% for stage II, 24–50% for stage III, and 0–17% for stage IV disease.3,71,72,79 A more recent Finnish series reported favorable 5-year survival rates of 100%, 93%, and 63% for stage I, II, and III disease, respectively. 32 This may reflect the increasing utilization of surgery and adjuvant therapy (discussed below) compared with historic studies. Despite this, 5-year survival remained dismal (11%) for patients with stage IV disease. 32
Targeted molecular classification as a future tool for individualized prognostication and management
As discussed above, in addition to providing insights into the pathogenesis of ACC, large-scale multi-omics studies from the ENS@T 7 and TCGA-ACC 8 groups have defined distinct subgroups of patients with clinically significant differences in survival based on genomic, epigenomic, and transcriptomic signatures. However, the requirement for prospective collection of fresh-frozen tissue and the costly and resource-intensive nature of pan-genomic bioinformatics analysis precludes its use in routine clinical practice. 80 As a method of overcoming these limitations, two recent studies have described how targeted molecular profiling (i.e. analysis of only a limited number of the most predictive biomarkers) could recapitulate the prognostic classification provided by the more comprehensive ENS@T and TCGA-ACC studies at a fraction of the required time and cost (Figure 3).81,82 Furthermore, in one of these studies, 81 targeted analysis was feasible using only formalin-fixed paraffin-embedded (FFPE) tissue, which is readily available in most clinical settings. Importantly, the combination of targeted molecular data with clinicopathological parameters in both studies was superior in predicting RFS compared to either component alone. In the future, increased utilization of targeted molecular analysis raises the possibility of individualized prognostication and precision medicine in patients with ACC, as has been implemented for other cancers. 83
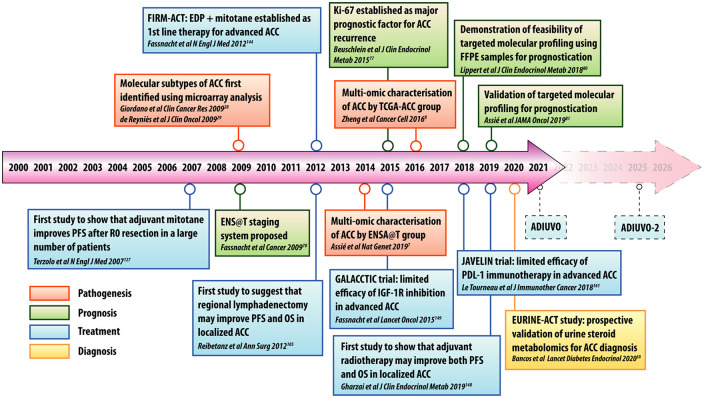
Figure 3.
Timeline of important studies in the pathogenesis, diagnosis, treatment, and prognostication of ACC over the past 20 years.
ACC, adrenocortical carcinoma; ADIUVO, Efficacy of Adjuvant Mitotane Treatment; EDP, etoposide, doxorubicin, and cisplatin; ENS@T, European Network for the Study of Adrenal Tumors; EURINE-ACT, Evaluation of Urine Steroid Metabolomics in the Differential Diagnosis of Adrenocortical Tumours; FFPE, formalin-fixed paraffin-embedded; FIRM-ACT, First International Randomized Trial in Locally Advanced and Metastatic ACC Treatment; GALACCTIC, A Study of OSI-906 in Patients with Locally Advanced or Metastatic Adrenocortical Carcinoma; JAVELIN, Avelumab in Metastatic or Locally Advanced Solid Tumors; OS, overall survival; PFS, progression-free survival; TCGA-ACC, The Cancer Genome Atlas ACC Study.
Treatment
Complete surgical resection with negative margins (R0) is currently the only curative treatment for ACC and applies to patients with resectable stage I–III disease. Adjuvant therapies are used to decrease recurrence rates, which are reported to range from 40–70% even after R0 resection, depending on stage.4,84,85 For patients with unresectable stage III or metastatic (stage IV) disease, the goals of treatment are palliative rather than curative. An additional treatment challenge involves counteracting the deleterious effects of excess hormone secretion, which is associated with increased morbidity and impaired quality of life. For these reasons, and the fact that most patients present with advanced disease, it is strongly recommended that patients with ACC are managed by multidisciplinary teams at institutions with experience and expertise in treating this rare malignancy. 86 An overview of current emerging treatment options for patients with localized and advanced disease are provided below. The management of hormone excess in ACC is beyond the scope of this review, and has been extensively covered by Else et al. 30
Surgery
Because the only chance for cure in patients with ACC is afforded by complete resection, surgery may necessitate en bloc removal of not only the tumor, but also any adjacent involved organs, such as the ipsilateral kidney, pancreas, and/or diaphragm, as well as the periadrenal retroperitoneal fat, in order to achieve an R0 resection. 6 In the absence of direct invasion, however, there is no convincing evidence that routine en bloc resection of extra-adrenal organs is associated with superior oncological outcomes when negative margins can otherwise be achieved.87,88 Regardless of operative extent, it is crucial that surgery is performed meticulously to avoid intraoperative tumor rupture and spillage, which are associated with high recurrence rates and extremely poor survival. Extension of tumor thrombus into the inferior vena cava (T4 disease) is not a contraindication to surgery and may require cross-clamping of the retro-hepatic inferior vena cava and/or cardiopulmonary bypass. 89 However, this technique requires careful patient selection as long-term survival outcomes remain suboptimal in this subgroup. 90
The nuances and challenges of ACC surgery underscore the strong recommendations in current guidelines for these procedures to be performed at specialized centers by experienced adrenal surgeons.5,6 Indeed, a volume-outcome effect in adrenalectomy has been repeatedly described, with fewer complications reported when procedures are performed by expert surgeons at higher volume centers. 91 Whether this translates into improved oncological outcomes in ACC has not consistently been shown, however, with some studies suggesting longer time to recurrence 92 and better overall survival 93 when surgery is performed at high- versus low-volume centers, while others have failed to demonstrate any survival advantage. 94 Furthermore, the specific number of ACC surgeries that defines a ‘high-volume’ surgeon or center is unclear and varies widely in the literature from 4 to 20 cases per year.6,92,95
Choice of surgical approach
Despite the benefits afforded by minimally-invasive adrenalectomy in terms of reduced blood loss, improved cosmesis, and shorter recovery time, its role in ACC surgery remains controversial.96 –100 This is due to the concerns that achieving a curative margin-negative resection may be more challenging laparoscopically, particularly in cases where an infiltrative tumour obliterates dissection planes and precludes good exposure. 6 A thin tumour capsule (e.g. in cortisol-secreting ACCs) may also be more likely to rupture secondary to manipulation with laparoscopic instruments. In accordance with this, some reports have shown higher rates of ACC recurrence (including in port sites) and poorer disease-free survival with a laparoscopic approach.101,102 However, the literature is not unequivocal on this topic and, as additional experience has accumulated, comparable oncological outcomes with laparoscopic and open adrenalectomy for ACC have also been reported, particularly for smaller tumors without evidence of local invasion.103,104 The lack of consensus regarding the role of laparoscopy is reflected in differences between guidelines from the American Association of Endocrine Surgeons (AAES)/American Association of Clinical Endocrinologists (AACE) which recommend open resection for all cases of ACC (regardless of size) 105 versus newer ENS@T/ESE guidelines that suggest that laparoscopic resection is a reasonable option for localized tumors <6 cm in diameter. 6
The role of lymphadenectomy
Locoregional lymphadenectomy for therapeutic purposes is standard practice for many intra-abdominal malignancies; however, its role in ACC is poorly defined. The first study that attempted to address this topic retrospectively analyzed 283 patients from the German ACC Registry with stage I–III disease, 17% of whom underwent lymph node dissection (defined as the removal of ⩾5 lymph nodes) during curative-intent adrenalectomy. 106 On multivariable analysis, lymph node dissection was independently associated with a 35% lower risk of tumor recurrence and a 46% lower risk of disease-related death. 106 A subsequent retrospective US multi-center study evaluated 120 non-metastatic ACC patients, 27% of whom underwent lymphadenectomy (defined as a documented attempt to dissect regional lymph nodes in the operative note). 107 The authors found that lymphadenectomy was associated with a significantly higher 5-year OS rate of 76% compared to 59% in the group that had no lymph nodes excised. 107 However, multiple studies using the SEER registry and National Cancer Database (NCDB) have shown contradictory results, with either no effect, 108 worse survival,109 –112 or a marginal improvement in survival, 113 in patients who underwent lymphadenectomy. Reasons for these discrepancies include the fact that the inclusion criteria in these studies differ widely by stage, presence of metastases, multivisceral resection, and margin status. In addition, the lack of granularity intrinsic to datasets such as SEER and NCDB make it impossible to determine whether lymphadenectomy was performed intentionally, and which specific lymph node basins were removed. Collectively, these studies highlight the need for a prospective trial in which the a priori objective is to determine whether lymphadenectomy confers a survival benefit in ACC patients. A recent study that described radiological patterns of regional nodal recurrences in 56 ACC patients suggested that the definition of lymphadenectomy that is used in a future prospective trial should include removal of periadrenal, renal hilar, ipsilateral para-aortic, or paracaval nodes. 114
Surgery for locally recurrent or metastatic disease
Approximately 20–60% of reported recurrences in ACC patients are locoregional. 115 In these cases, reoperation may be indicated if an R0 resection is achievable. This is supported by data from a number of retrospective studies, which have shown improvements in symptoms and a median survival of >5 years in selected patients who undergo reoperation versus medical management alone.115 –119 Studies have also demonstrated the safety and efficacy of pulmonary 120 and hepatic metastatectomy, 121 with reported 5-year OS rates of ~25–50%. Overall, patients who derive the greatest benefit from reoperation appear to be those with a disease-free interval from initial resection to recurrence of >9–12 months.117,122,123 The decision to offer reoperation should weigh up the potential survival benefits against the risks of morbidity (12–55%), mortality 0–4%), and the high likelihood of further recurrence (~80%). 6
The role of metastasectomy in patients who have synchronous metastases at presentation is less clear, with studies showing that these patients have a poorer overall prognosis compared to those who develop metachronous metastases.124,125 Studies describing operative intervention in this group are lacking due to the paucity of cases and short duration of survival. A combined series of 27 patients from Mayo Clinic and the MD Anderson Cancer Center showed that those who underwent an R0 resection had significantly better median OS of 28.6 months versus 13.0 months when only an R2 resection was acheived. 126 A similar benefit was also reported in another US multi-center study of 26 patients with Stage IV disease (median survival of 19.0 months for R0 resection versus 5.5 months for R1/R2 resection). 125 Overall, these findings indicate that patients with metastatic ACC constitute a heterogenous group with distinct differences in tumor biology, prognosis, and degree of benefit derived from surgery.
Adjuvant therapy
Although surgical resection is technically feasible in most patients with stage I–III ACC, the majority of patients still succumb to disease, presumably due to occult micrometastases that are present at the time of initial presentation. As a result, adjuvant therapies may be used in an attempt to reduce rates of disease recurrence in patients who have a seemingly curative index operation.
Mitotane
Mitotane is a derivative of the insecticide dichlorodiphenyltrichloroethane (DDT) and exerts adrenolytic activity through mechanisms that are incompletely understood. One of these mechanisms includes endoplastic reticulum stress activation, which impairs steroidogenesis and induces apoptosis of ACC cells. 127 Impetus for the use of mitotane in the adjuvant setting arose from a 2007 retrospective study by Terzolo et al., 128 which analyzed 177 stage I–III ACC patients who were followed up for up to 10 years at referral centers in Italy and Germany (Figure 3). Adjuvant mitotane use was associated with significantly longer median recurrence-free survival of 42 months versus 10 and 25 months in the Italian and German control groups, respectively. Median OS was also better in the mitotane group at 110 months versus 52 and 67 months in the Italian and German cohorts, respsectively. 128 A subsequent description of this cohort, after almost 10 additional years of follow-up, showed a sustained survival benefit in the mitotane group. 129 A more recent study from this group of authors, in an independent sample of 152 patients, showed that adjuvant mitotane prolonged recurrence-free survival without improving overall survival, although better OS was seen in a subgroup of patients deemed to be at high risk of recurrence (elevated Ki-67 and stage III disease). 130
In contrast, studies from US centers have shown conflicting results regarding the benefits of adjuvant mitotane on survival outcomes.131 –133 The University of Michigan group observed that while adjuvant mitotane significantly improved RFS, no effect on OS was seen. 131 In a study from MD Anderson, patients who underwent index surgery at that institution had a recurrence rate of 50% after a median follow-up of 7.3 years, 132 which the authors noted was similar to the 49% 5-year RFS rate reported for patients who received adjuvant mitotane in the 2007 Italian/German study by Terzolo et al. 128 Because 90% of the MD Anderson cohort did not receive adjuvant mitotane, the authors attributed the comparable recurrence rates in their study to the quality and completeness of surgery performed at their institution, rather than to the use of adjuvant therapy. Similarly, in a 13-institution study from the US ACC group, no significant association between mitotane use and RFS or OS was seen on multivariable analysis. 133 It is important to note that all of the aforementioned studies are retrospective in nature and are fraught with selection bias and heterogeneity with regards to patient characteristics and treatment regiments. While recognizing that the evidence base is weak, current European guidelines recommended adjuvant mitotane for patients who are at high risk of recurrence (e.g. stage III disease, R1 resection, or Ki-67 > 10%).5,47 Whether or not adjuvant mitotane is beneficial in patients at low or moderate risk of recurrence (e.g. stage I–II disease, R0 resection, and Ki-67 < 10%) is unclear, and it is anticipated that the recently-completed Efficacy of Adjuvant Mitotane Treatment (ADIUVO) randomized controlled trial (RCT) will answer this question (Figure 3). Furthermore, the ongoing ADIUVO-2 RCT (due to complete in 2025) will determine whether the combination of cisplatin and etoposide with adjuvant mitotane is more effective than adjuvant mitotane alone in prolonging RFS and OS in patients with high-risk ACC (Figure 3).
Radiotherapy
Adjuvant radiotherapy is infrequently used in the management of ACC, with only 6 and 14% of patients reported as receiving this treatment modality in two separate NCDB studies covering the periods 1985–2005 134 and 2004–2013, 135 respectively. Reluctance to refer patients for radiotherapy likely reflects the widely held viewpoint that ACCs are radioresistant tumors, which is based on data from small pre-2000 series which failed to show any improvement in recurrence or overall survival with adjuvant radiation.136 –138 However, the results of recent studies that utilize modern radiotherapy techniques have challenged this notion.139 –141 For example, in a case-control study from the German ACC group, in which 14 patients with non-metastatic ACC who received adjuvant radiotherapy were matched to 14 patients that did not, local recurrence was observed in 14% of patients in the radiation group versus 79% of patients in the control group. 139 However, no significant differences were seen in terms of disease-free survival and OS. Findings consistent with these were reported by the University of Michigan group, in a case-control study of 20 patients who received adjuvant radiotherapy versus 20 matched controls who did not. 140 In this study, 5% of the patients who received radiation experienced a recurrence versus 60% of patients in the control group. Again, no significant differences in RFS or OS were found. The authors speculated that the lack of a detectable survival benefit was due to the study being underpowered and have recently published a larger series of 78 patients, half of whom received adjuvant radiation. 141 Interestingly, in addition to improved local recurrence rates, this study reported significantly better RFS and OS in the radiation group. It should be noted that there are no prospective data evaluating the benefit of adjuvant radiation, and the findings of retrospective studies should be carefully interpreted in light of the potential for referral and selection bias. Despite this, emerging evidence suggests that adjuvant radiotherapy in the contemporary era is probably more effective than previously thought.
Systemic therapy
The dearth of effective systemic therapies poses a significant challenge in the treatment of patients with metastatic ACC. For years, mitotane has remained the only drug approved by the United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the treatment of unresectable ACC. 142 For selected patients with low tumour burden and/or slower growth kinetics, mitotane monotherapy has been used, albeit with disappointing objective RR of 20–25%.143,144 Otherwise, standard of care palliative treatment is with etoposide, doxorubicin, and cisplatin, in combination with mitotane (EDP-M). Evidence in support of this regimen came from the FIRM-ACT (First International Randomized Trial in Locally Advanced and Metastatic ACC Treatment) study (Figure 3). 145 In this phase III RCT, the authors compared EDP-M (n = 151 patients) with another commonly used regimen at the time, streptozosin plus mitotane (SZ-M; n = 153), demonstrating superior efficacy in the EDP-M arm (23% RR) compared with the SZ-M arm (9% RR). 145 Progression-free survival (PFS) in the EDP-M arm was also significantly longer at 5 months versus 2 months in the SZ-M arm, and there was a suggestion of better OS in the former group (14.8 months for EDP-M versus 12 months for SZ-M), although this did not reach statistical significance, possibly due to the cross-over design of the trial. While this study was important in establishing the superiority of EDP-M for ACC patients with metastatic disease, it also highlighted that, despite gold-standard treatment, prognosis in this group of patients remains dismal.
For patients who experience disease progression despite EDP-M, there is limited evidence regarding the efficacy of non-first line chemotherapies. The two most commonly studied second-line agents include SZ-M and gemcitabine plus capecitabine, with or without mitotane.146,147 However, RRs with these regimens have been disappointing (<10%). A recent study investigated temozolomide as a second- or third-line treatment for metastatic ACC showing a RR of 20%, although this was short-lived and did not influence median OS, which remained poor at 7.2 months. 148
Newer treatments: targeted therapies, immunotherapy, and radiopharmaceuticals
Given the limited effectiveness of current treatments, the search for novel therapeutic strategies for advanced ACC is a current research priority. Findings from the comprehensive ENS@T 7 and TCGA-ACC 8 molecular profiling studies have provided an exhaustive road map of potential druggable targets. Unfortunately, however, this knowledge has not yet resulted in a treatment that demonstrates efficacy above what is currently used in the clinic. For example, the observation that IGF2 is overexpressed in the majority of ACCs and that inhibition of IGF2/IGF1R was effective in reducing tumor growth in pre-clinical models 149 led to the evaluation of lisitinib (a small molecule inhibitor of both IGF1R and the insulin receptor) in a phase III RCT, the GALACCTIC (A Study of OSI-906 in Patients with Locally Advanced or Metastatic Adrenocortical Carcinoma) trial (Figure 3). 150 Disappointingly, no differences in OS or RFS were seen compared to placebo. In addition, only 15.6% of patients in the lisitinib arm achieving disease control, with a median PFS survival of <2 months. Other targeted therapies that have been, or are currently undergoing, evaluation in phase I/II trials include epidermal growth factor receptor (EGFR) inhibitors, 151 vascular endothelial growth factor receptor (VEGFR) inhibitors, 152 fibroblast growth factor receptor (FGFR) inhibitors, 153 multityrosine kinase inhibitors (TKIs),154,155 and sterol-O-acetyltransferase (SOAT1) inhibitors. 156 Unfortunately, these compounds have shown limited efficacy, with temporary disease stabilization observed in best-case scenarios. Small molecule inhibitors that have been tested pre-clinically but have not yet been evaluated in early-phase trials include inhibitors of the Wnt/βcatenin signalling, 157 cyclin-dependent kinases, 158 Notch signalling, 81 and mitogen-activated protein kinase (MAPK)/ERK signalling. 159 Preclinical data supporting the rationale for their use in ACC have been reviewed recently by Altieri et al. 160
Immunotherapy has emerged as a standard pillar of treatment across a broad spectrum of solid tumors, 161 which was spurred enthusiasm for investigating this class of treatment in ACC. To date, four phase I/II clinical trials of immune checkpoint inhibitors in ACC have been published.162 –165 The largest of these, the Avelumib in Metastatic or Locally Advanced Solid Tumors (JAVELIN) trial, was a phase Ib study that evaluated the efficacy of avelumab [a programmed death-ligand 1 (PD-L1) antagonist] in 50 patients with metastatic ACC who had failed to respond to platinum-based chemotherapy (Figure 3). 162 Disappointingly, the overall RR was only 6% with a median PFS survival of 2.6 months. Potential explanations for the modest results seen in immunotherapy trials in ACC include an immunosuppressive action of locally secreted glucocorticoids, and deregulated Wnt/β-catenin signaling, leading to impaired immune cell infiltration. 166
As described above, a subset (~30%) of patients with metastatic ACC demonstrate significant uptake of MTO in both the primary tumor and distant metastases. 53 By replacing [ 123 I] with [ 131 I], MTO has been adapted for targeted radionuclide therapy. 167 In a preliminary study of 11 patients with advanced ACC who demonstrated [ 123 I] MTO avidity on diagnostic scans, a partial response to 1–3 cycles of [ 131 I] IMTO was achieved in 1 patient, with stable disease achieved in 5 patients. 167 Based on the observation that some ACCs may express somatostatin receptor (SSTRs), 168 peptide receptor radionuclide therapy (PRRT) using the radiolabeled somatostatin analogues [ 90 Lu]- and [177Y]-DOTATOC was recently reported in a preliminary study of 19 patients by Grisanti et al. 169 In this study, 2 patients were selected for PPRT based on the finding of strong uptake of [ 68 Ga]-DOTATOC on PET/CT, indicating high somatostatin receptor expression. Both patients experienced disease stabilization that lasted 4 months and 12 months, respectively. 169 As a result, radiopharmaceutical therapy may represent a potential salvage treatment option for a small subset of cases of metastatic ACC, although this requires validation in a larger number of patients.
Conclusions and future perspectives
ACC is a rare and devastating malignancy that entails a poor prognosis in the majority of cases. The past 20 years have seen the publication of a number of important studies that have provided insights into the pathogenesis of ACC and its diagnosis and treatment (Figure 3). In patients with adrenal incidentalomas, the diagnosis of ACC may be challenging due to the limited diagnostic accuracy of cross-sectional imaging. Implementation of urine steroid metabolomics into routine workup could improve detection rates and spare patients with benign masses from undergoing unnecessary surgery, although the cost-effectiveness of this technology remains to be demonstrated. Large-scale pan-genomic analyses have greatly improved our understanding of the pathogenesis of ACC and have paved the way for targeted molecular profiling in the clinic with the possibility of individualized prognostication to guide therapy in the future. Surgery remains the only curative treatment for ACC, although questions remain about the optimal surgical approach, the benefits and definition of regional lymphadenectomy, and the effects of surgeon volume on oncological outcomes. Despite seemingly curative resection, most patients experience disease recurrence and a better understanding of the factors that influence prognosis and response to adjuvant mitotane treatment are required. Results from ADIUVO and ADIUVO-2 will provide valuable insights into this topic. The role of postoperative radiation needs to be better defined in prospective studies, as emerging evidence suggests a survival benefit in selected patients. There is a clinically unmet need for improved treatments for advanced ACC, as current standard of care is associated with suboptimal survival. Trials of molecularly targeted therapy and immunotherapy have been disappointing to date, suggesting that combination therapies that are guided by molecular biomarkers may be more successful than a blanket ‘one-size-fits-all’ approach. Given the rarity of ACC, the success of future research efforts is contingent upon continued international collaborations between teams that recognize the urgent need to widen treatment options and improve outcomes for patients with this orphan disease.
Footnotes
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Financial disclosures: None
ORCID iD: Travis J. McKenzie  https://orcid.org/0000-0001-9140-6548
https://orcid.org/0000-0001-9140-6548
Contributor Information
Omair A. Shariq, Department of Surgery, Mayo Clinic, Rochester, MN, USA
Travis J. McKenzie, Department of Surgery, Mayo Clinic, 200 First Street S.W., Rochester, MN 55905, USA.
References
- 1. Sharma E, Dahal S, Sharma P, et al. The characteristics and trends in adrenocortical carcinoma: a United States population based study. J Clin Med Res 2018; 10: 636–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kebebew E, Reiff E, Duh QY, et al. Extent of disease at presentation and outcome for adrenocortical carcinoma: have we made progress? World J Surg 2006; 30: 872–878. [DOI] [PubMed] [Google Scholar]
- 3. Kerkhofs TM, Verhoeven RH, Van der Zwan JM, et al. Adrenocortical carcinoma: a population-based study on incidence and survival in the Netherlands since 1993. Eur J Cancer 2013; 49: 2579–2586. [DOI] [PubMed] [Google Scholar]
- 4. Glenn JA, Else T, Hughes DT, et al. Longitudinal patterns of recurrence in patients with adrenocortical carcinoma. Surgery 2019; 165: 186–195. [DOI] [PubMed] [Google Scholar]
- 5. Fassnacht M, Dekkers OM, Else T, et al. European Society of Endocrinology Clinical Practice Guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol 2018; 179: G1–G46. [DOI] [PubMed] [Google Scholar]
- 6. Gaujoux S, Mihai R. European Society of Endocrine Surgeons (ESES) and European Network for the Study of Adrenal Tumours (ENSAT) recommendations for the surgical management of adrenocortical carcinoma. Br J Surg 2017; 104: 358–376. [DOI] [PubMed] [Google Scholar]
- 7. Assié G, Letouzé E, Fassnacht M, et al. Integrated genomic characterization of adrenocortical carcinoma. Nat Genet 2014; 46: 607–612. [DOI] [PubMed] [Google Scholar]
- 8. Zheng S, Cherniack AD, Dewal N, et al. Comprehensive pan-genomic characterization of adrenocortical carcinoma. Cancer Cell 2016; 29: 723–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Richter T, Nestler-Parr S, Babela R, et al. Rare disease terminology and definitions-a systematic global review: report of the ISPOR rare disease special interest group. Value Health 2015; 18: 906–914. [DOI] [PubMed] [Google Scholar]
- 10. Ribeiro RC, Sandrini F, Figueiredo B, et al. An inherited p53 mutation that contributes in a tissue-specific manner to pediatric adrenal cortical carcinoma. Proc Natl Acad Sci U S A 2001; 98: 9330–9335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wooten MD, King DK. Adrenal cortical carcinoma. Epidemiology and treatment with mitotane and a review of the literature. Cancer 1993; 72: 3145–3155. [DOI] [PubMed] [Google Scholar]
- 12. Wajchenberg BL, Albergaria Pereira MA, Medonca BB, et al. Adrenocortical carcinoma: clinical and laboratory observations. Cancer 2000; 88: 711–736. [PubMed] [Google Scholar]
- 13. Sutter JA, Grimberg A. Adrenocortical tumors and hyperplasias in childhood–etiology, genetics, clinical presentation and therapy. Pediatr Endocrinol Rev 2006; 4: 32–39. [PMC free article] [PubMed] [Google Scholar]
- 14. Roman S. Adrenocortical carcinoma. Curr Opin Oncol 2006; 18: 36–42. [DOI] [PubMed] [Google Scholar]
- 15. Schteingart DE, Doherty GM, Gauger PG, et al. Management of patients with adrenal cancer: recommendations of an international consensus conference. Endocr Relat Cancer 2005; 12: 667–680. [DOI] [PubMed] [Google Scholar]
- 16. Michalkiewicz E, Sandrini R, Figueiredo B, et al. Clinical and outcome characteristics of children with adrenocortical tumors: a report from the international pediatric adrenocortical tumor registry. J Clin Oncol 2004; 22: 838–845. [DOI] [PubMed] [Google Scholar]
- 17. Habra MA, Sukkari MA, Hasan A, et al. Epidemiological risk factors for adrenocortical carcinoma: a hospital-based case-control study. Int J Cancer 2020; 146: 1836–1840. [DOI] [PubMed] [Google Scholar]
- 18. Yousaf A, Patterson J, Hobbs G, et al. Smoking is associated with adrenal adenomas and adrenocortical carcinomas: a nationwide multicenter analysis. Cancer Treat Res Commun 2020; 25: 100206. [DOI] [PubMed] [Google Scholar]
- 19. Hsing AW, Nam JM, Co Chien HT, et al. Risk factors for adrenal cancer: an exploratory study. Int J Cancer 1996; 65: 432–436. [DOI] [PubMed] [Google Scholar]
- 20. Sirianni R, Zolea F, Chimento A, et al. Targeting estrogen receptor-α reduces Adrenocortical Cancer (ACC) cell growth in vitro and in vivo: potential therapeutic role of Selective Estrogen Receptor Modulators (SERMs) for ACC treatment. J Clin Endocrinol Metab 2012; 97: E2238–E2250. [DOI] [PubMed] [Google Scholar]
- 21. Herrmann LJM, Heinze B, Fassnacht M, et al. TP53 germline mutations in adult patients with adrenocortical carcinoma. J Clin Endocrinol Metab 2012; 97: E476–E485. [DOI] [PubMed] [Google Scholar]
- 22. Hertel NT, Carlsen N, Kerndrup G, et al. Late relapse of adrenocortical carcinoma in Beckwith-Wiedemann syndrome. Clinical, endocrinological and genetic aspects. Acta Paediatr 2003; 92: 439–443. [DOI] [PubMed] [Google Scholar]
- 23. Gatta-Cherifi B, Chabre O, Murat A, et al. Adrenal involvement in MEN1. Analysis of 715 cases from the Groupe d’etude des Tumeurs Endocrines database. Eur J Endocrinol 2012; 166: 269–279. [DOI] [PubMed] [Google Scholar]
- 24. Raymond VM, Everett JN, Furtado LV, et al. Adrenocortical carcinoma is a lynch syndrome-associated cancer. J Clin Oncol 2013; 31: 3012–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pittaway JFH, Guasti L. Pathobiology and genetics of adrenocortical carcinoma. J Mol Endocrinol 2019; 62: R105–R119. [DOI] [PubMed] [Google Scholar]
- 26. Crona J, Beuschlein F. Adrenocortical carcinoma - towards genomics guided clinical care. Nat Rev Endocrinol 2019; 15: 548–560. [DOI] [PubMed] [Google Scholar]
- 27. Mohan DR, Lerario AM, Finco I, et al. New strategies for applying targeted therapies to adrenocortical carcinoma. Curr Opin Endocr Metab Res 2019; 8: 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Giordano TJ, Kuick R, Else T, et al. Molecular classification and prognostication of adrenocortical tumors by transcriptome profiling. Clin Cancer Res 2009; 15: 668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. de Reyniès A, Assié G, Rickman DS, et al. Gene expression profiling reveals a new classification of adrenocortical tumors and identifies molecular predictors of malignancy and survival. J Clin Oncol 2009; 27: 1108–1115. [DOI] [PubMed] [Google Scholar]
- 30. Else T, Kim AC, Sabolch A, et al. Adrenocortical carcinoma. Endocr Rev 2014; 35: 282–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Iñiguez-Ariza NM, Kohlenberg JD, Delivanis DA, et al. Clinical, biochemical, and radiological characteristics of a single-center retrospective cohort of 705 large adrenal tumors. Mayo Clin Proc Innov Qual Outcomes 2018; 2: 30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kostiainen I, Hakaste L, Kejo P, et al. Adrenocortical carcinoma: presentation and outcome of a contemporary patient series. Endocrine 2019; 65: 166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fassnacht M, Arlt W, Bancos I, et al. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol 2016; 175: G1–G34. [DOI] [PubMed] [Google Scholar]
- 34. Berruti A, Fassnacht M, Haak H, et al. Prognostic role of overt hypercortisolism in completely operated patients with adrenocortical cancer. Eur Urol 2014; 65: 832–838. [DOI] [PubMed] [Google Scholar]
- 35. Ayala-Ramirez M, Jasim S, Feng L, et al. Adrenocortical carcinoma: clinical outcomes and prognosis of 330 patients at a tertiary care center. Eur J Endocrinol 2013; 169: 891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sada A, Asaad M, Bews KA, et al. Comparison between functional and non-functional adrenocortical carcinoma. Surgery 2020; 167: 216–223. [DOI] [PubMed] [Google Scholar]
- 37. Margonis GA, Kim Y, Tran TB, et al. Outcomes after resection of cortisol-secreting adrenocortical carcinoma. Am J Surg 2016; 211: 1106–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Loncar Z, Djukic V, Zivaljevic V, et al. Survival and prognostic factors for adrenocortical carcinoma: a single institution experience. BMC Urol 2015; 15: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Scollo C, Russo M, Trovato MA, et al. Prognostic factors for adrenocortical carcinoma outcomes. Front Endocrinol (Lausanne) 2016; 7: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vanbrabant T, Fassnacht M, Assie G, et al. Influence of hormonal functional status on survival in adrenocortical carcinoma: systematic review and meta-analysis. Eur J Endocrinol 2018; 179: 429–436. [DOI] [PubMed] [Google Scholar]
- 41. Arlt W, Biehl M, Taylor AE, et al. Urine steroid metabolomics as a biomarker tool for detecting malignancy in adrenal tumors. J Clin Endocrinol Metab 2011; 96: 3775–3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bancos I, Arlt W. Steroid biomarkers and the diagnosis of adrenal cortical carcinoma. Curr Opin Endocr Metab Res 2019; 8: 167–173. [Google Scholar]
- 43. Young WF. Conventional imaging in adrenocortical carcinoma: update and perspectives. Horm Cancer 2011; 2: 341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Francis IR. Distinguishing benign from malignant adrenal masses. Cancer Imaging 2003; 3: 102–110. [Google Scholar]
- 45. NIH state-of-the-science statement on management of the clinically inapparent adrenal mass (“incidentaloma”). NIH Consens State Sci Statements 2002; 19: 1–25. [PubMed] [Google Scholar]
- 46. Kapoor A, Morris T, Rebello R. Guidelines for the management of the incidentally discovered adrenal mass. Can Urol Assoc J 2011; 5: 241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fassnacht M, Assie G, Baudin E, et al. Adrenocortical carcinomas and malignant phaeochromocytomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2020; 31: 1476–1490. [DOI] [PubMed] [Google Scholar]
- 48. Dinnes J, Bancos I, Ferrante di, Ruffano L, et al. Management of endocrine disease: imaging for the diagnosis of malignancy in incidentally discovered adrenal masses: a systematic review and meta-analysis. Eur J Endocrinol 2016; 175: R51–R64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dong A, Cui Y, Wang Y, et al. 18F-FDG PET/CT of adrenal lesions. AJR Am J Roentgenol 2014; 203: 245–252. [DOI] [PubMed] [Google Scholar]
- 50. Guerin C, Pattou F, Brunaud L, et al. Performance of 18F-FDG PET/CT in the characterization of adrenal masses in noncancer patients: a prospective study. J Clin Endocrinol Metab 2017; 102: 2465–2472. [DOI] [PubMed] [Google Scholar]
- 51. Takeuchi S, Balachandran A, Habra MA, et al. Impact of 18F-FDG PET/CT on the management of adrenocortical carcinoma: analysis of 106 patients. Eur J Nucl Med Mol Imaging 2014; 41: 2066–2073. [DOI] [PubMed] [Google Scholar]
- 52. Chen Cardenas SM, Santhanam P. 11C-metomidate PET in the diagnosis of adrenal masses and primary aldosteronism: a review of the literature. Endocrine 2020; 70: 479–487. [DOI] [PubMed] [Google Scholar]
- 53. Hahner S, Kreissl MC, Fassnacht M, et al. Functional characterization of adrenal lesions using [123I]IMTO-SPECT/CT. J Clin Endocrinol Metab 2013; 98: 1508–1518. [DOI] [PubMed] [Google Scholar]
- 54. Kreissl MC, Schirbel A, Fassnacht M, et al. [123I]Iodometomidate imaging in adrenocortical carcinoma. J Clin Endocrinol Metab 2013; 98: 2755–2764. [DOI] [PubMed] [Google Scholar]
- 55. Akbulut S, Erten O, Kahramangil B, et al. A critical analysis of computed tomography washout in lipid-poor adrenal incidentalomas. Ann Surg Oncol. Epub ahead of print 18 November 2020. DOI: 10.1245/s10434-020-09329-1. [DOI] [PubMed] [Google Scholar]
- 56. Musella M, Conzo G, Milone M, et al. Preoperative workup in the assessment of adrenal incidentalomas: outcome from 282 consecutive laparoscopic adrenalectomies. BMC Surg 2013; 13: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Uchida T, Nishimoto K, Fukumura Y, et al. Disorganized steroidogenesis in adrenocortical carcinoma, a case study. Endocr Pathol 2017; 28: 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bancos I, Taylor AE, Chortis V, et al. Urine steroid metabolomics for the differential diagnosis of adrenal incidentalomas in the EURINE-ACT study: a prospective test validation study. Lancet Diabetes Endocrinol 2020; 8: 773–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chortis V, Bancos I, Nijman T, et al. Urine steroid metabolomics as a novel tool for detection of recurrent adrenocortical carcinoma. J Clin Endocrinol Metab 2020; 105: e307–e318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Weiss LM. Comparative histologic study of 43 metastasizing and nonmetastasizing adrenocortical tumors. Am J Surg Pathol 1984; 8: 163–169. [DOI] [PubMed] [Google Scholar]
- 61. Erickson LA. Challenges in surgical pathology of adrenocortical tumours. Histopathology 2018; 72: 82–96. [DOI] [PubMed] [Google Scholar]
- 62. Aubert S, Wacrenier A, Leroy X, et al. Weiss system revisited: a clinicopathologic and immunohistochemical study of 49 adrenocortical tumors. Am J Surg Pathol 2002; 26: 1612–1619. [DOI] [PubMed] [Google Scholar]
- 63. Duregon E, Fassina A, Volante M, et al. The reticulin algorithm for adrenocortical tumor diagnosis: a multicentric validation study on 245 unpublished cases. Am J Surg Pathol 2013; 37: 1433–1440. [DOI] [PubMed] [Google Scholar]
- 64. Pennanen M, Heiskanen I, Sane T, et al. Helsinki score—a novel model for prediction of metastases in adrenocortical carcinomas. Human Pathol 2015; 46: 404–410. [DOI] [PubMed] [Google Scholar]
- 65. Duregon E, Cappellesso R, Maffeis V, et al. Validation of the prognostic role of the “Helsinki Score” in 225 cases of adrenocortical carcinoma. Hum Pathol 2017; 62: 1–7. [DOI] [PubMed] [Google Scholar]
- 66. Giordano TJ, Berney D, de Krijger RR, et al. Data set for reporting of carcinoma of the adrenal cortex: explanations and recommendations of the guidelines from the International Collaboration on Cancer Reporting. Human Pathol. Epub ahead of print 12 October 2020. DOI: 10.1016/j.humpath.2020.10.001. [DOI] [PubMed] [Google Scholar]
- 67. Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol 1982; 6: 655–664. [DOI] [PubMed] [Google Scholar]
- 68. Bisceglia M, Ludovico O, Di Mattia A, et al. Adrenocortical oncocytic tumors: report of 10 cases and review of the literature. Int J Surg Pathol 2004; 12: 231–243. [DOI] [PubMed] [Google Scholar]
- 69. Bancos I, Tamhane S, Shah M, et al. Diagnosis of endocrine disease: the diagnostic performance of adrenal biopsy: a systematic review and meta-analysis. Eur J Endocrinol 2016; 175: R65–R80. [DOI] [PubMed] [Google Scholar]
- 70. Williams AR, Hammer GD, Else T. Transcutaneous biopsy of adrenocortical carcinoma is rarely helpful in diagnosis, potentially harmful, but does not affect patient outcome. Eur J Endocrinol 2014; 170: 829–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Fassnacht M, Johanssen S, Quinkler M, et al. Limited prognostic value of the 2004 international union against cancer staging classification for adrenocortical carcinoma: proposal for a revised TNM classification. Cancer 2009; 115: 243–250. [DOI] [PubMed] [Google Scholar]
- 72. Lughezzani G, Sun M, Perrotte P, et al. The European network for the study of adrenal tumors staging system is prognostically superior to the international union against cancer-staging system: a North American validation. Eur J Cancer 2010; 46: 713–719. [DOI] [PubMed] [Google Scholar]
- 73. Phan A, Grogran RH, Rohren E, et al. Adrenal cortical carcinoma. In: Amin MB, Edge S, Greene F, et al. (eds.) AJCC Cancer Staging Manual. 8th ed. New York: Springer, 2017. [Google Scholar]
- 74. Asare EA, Wang TS, Winchester DP, et al. A novel staging system for adrenocortical carcinoma better predicts survival in patients with stage I/II disease. Surgery 2014; 156: 1378–1386. [DOI] [PubMed] [Google Scholar]
- 75. Libé R, Borget I, Ronchi CL, et al. Prognostic factors in stage III–IV Adrenocortical Carcinomas (ACC): an European Network for the Study of Adrenal Tumor (ENSAT) study. Ann Oncol 2015; 26: 2119–2125. [DOI] [PubMed] [Google Scholar]
- 76. Miller BS, Gauger PG, Hammer GD, et al. Proposal for modification of the ENSAT staging system for adrenocortical carcinoma using tumor grade. Langenbecks Arch Surg 2010; 395: 955–961. [DOI] [PubMed] [Google Scholar]
- 77. Poorman CE, Ethun CG, Postlewait LM, et al. A novel T-stage classification system for adrenocortical carcinoma: proposal from the US Adrenocortical Carcinoma study group. Ann Surg Oncol 2018; 25: 520–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Beuschlein F, Weigel J, Saeger W, et al. Major prognostic role of Ki67 in localized adrenocortical carcinoma after complete resection. J Clin Endocrinol Metab 2015; 100: 841–849. [DOI] [PubMed] [Google Scholar]
- 79. Icard P, Goudet P, Charpenay C, et al. Adrenocortical carcinomas: surgical trends and results of a 253-patient series from the French Association of Endocrine Surgeons study group. World J Surg 2001; 25: 891–897. [DOI] [PubMed] [Google Scholar]
- 80. de Krijger RE, Bertherat J. 5th International ACC Symposium: classification of adrenocortical cancers from pathology to integrated genomics: real advances or lost in translation? Horm Cancer 2016; 7: 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lippert J, Appenzeller S, Liang R, et al. Targeted molecular analysis in adrenocortical carcinomas: a strategy toward improved personalized prognostication. J Clin Endocrinol Metab 2018; 103: 4511–4523. [DOI] [PubMed] [Google Scholar]
- 82. Assié G, Jouinot A, Fassnacht M, et al. Value of molecular classification for prognostic assessment of adrenocortical carcinoma. JAMA Oncol 2019; 5: 1440–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sparano JA, Gray RJ, Makower DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med 2018; 379: 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Margonis GA, Kim Y, Prescott JD, et al. Adrenocortical carcinoma: impact of surgical margin status on long-term outcomes. Ann Surg Oncol 2016; 23: 134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Amini N, Margonis GA, Kim Y, et al. Curative resection of adrenocortical carcinoma: rates and patterns of postoperative recurrence. Ann Surg Oncol 2016; 23: 126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Fassnacht M, Johanssen S, Fenske W, et al. Improved survival in patients with stage II adrenocortical carcinoma followed up prospectively by specialized centers. J Clin Endocrinol Metab 2010; 95: 4925–4932. [DOI] [PubMed] [Google Scholar]
- 87. Porpiglia F, Fiori C, Daffara FC, et al. Does nephrectomy during radical adrenalectomy for stage II adrenocortical cancer affect patient outcome? J Endocrinol Invest 2016; 39: 465–471. [DOI] [PubMed] [Google Scholar]
- 88. Marincola Smith P, Kiernan CM, Tran TB, et al. Role of additional organ resection in adrenocortical carcinoma: analysis of 167 patients from the U.S. adrenocortical carcinoma database. Ann Surg Oncol 2018; 25: 2308–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Gaujoux S, Weinandt M, Bonnet S, et al. Surgical treatment of adrenal carcinoma. J Visc Surg 2017; 154: 335–343. [DOI] [PubMed] [Google Scholar]
- 90. Laan DV, Thiels CA, Glasgow A, et al. Adrenocortical carcinoma with inferior vena cava tumor thrombus. Surgery 2017; 161: 240–248. [DOI] [PubMed] [Google Scholar]
- 91. Kazaure HS, Sosa JA. Volume-outcome relationship in adrenal surgery: a review of existing literature. Best Pract Res Clin Endocrinol Metab 2019; 33: 101296. [DOI] [PubMed] [Google Scholar]
- 92. Lombardi CP, Raffaelli M, Boniardi M, et al. Adrenocortical carcinoma: effect of hospital volume on patient outcome. Langenbecks Arch Surg 2012; 397: 201–207. [DOI] [PubMed] [Google Scholar]
- 93. Kerkhofs T, Verhoeven R, Bonjer H, et al. Surgery for adrenocortical carcinoma in the Netherlands: analysis of the national cancer registry data. Eur J Endocrinol 2013; 169: 83–89. [DOI] [PubMed] [Google Scholar]
- 94. Gratian L, Pura J, Dinan M, et al. Treatment patterns and outcomes for patients with adrenocortical carcinoma associated with hospital case volume in the United States. Ann Surg Oncol 2014; 21: 3509–3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Jurowich C, Fassnacht M, Kroiss M, et al. Is there a role for laparoscopic adrenalectomy in patients with suspected adrenocortical carcinoma? A critical appraisal of the literature. Horm Metab Res 2013; 45: 130–136. [DOI] [PubMed] [Google Scholar]
- 96. Brunt LM. The positive impact of laparoscopic adrenalectomy on complications of adrenal surgery. Surg Endosc 2002; 16: 252–257. [DOI] [PubMed] [Google Scholar]
- 97. Thompson GB, Grant CS, van Heerden JA, et al. Laparoscopic versus open posterior adrenalectomy: a case-control study of 100 patients. Surgery 1997; 122: 1132–1136. [DOI] [PubMed] [Google Scholar]
- 98. Elfenbein DM, Scarborough JE, Speicher PJ, et al. Comparison of laparoscopic versus open adrenalectomy: results from American College of Surgeons-National Surgery Quality Improvement Project. J Surg Res 2013; 184: 216–220. [DOI] [PubMed] [Google Scholar]
- 99. Lee J, El-Tamer M, Schifftner T, et al. Open and laparoscopic adrenalectomy: analysis of the National Surgical Quality Improvement Program. J Am Coll Surg 2008; 206: 953–959; discussion 959–961. [DOI] [PubMed] [Google Scholar]
- 100. Shariq OA, Bews KA, McKenna NP, et al. Is same-day discharge associated with increased 30-day postoperative complications and readmissions in patients undergoing laparoscopic adrenalectomy? Surgery. Epub ahead of print 30 September 2020. DOI: 10.1016/j.surg.2020.08.018. [DOI] [PubMed] [Google Scholar]
- 101. Gonzalez RJ, Shapiro S, Sarlis N, et al. Laparoscopic resection of adrenal cortical carcinoma: a cautionary note. Surgery 2005; 138: 1078–1085; discussion 1085–1076. [DOI] [PubMed] [Google Scholar]
- 102. Miller BS, Ammori JB, Gauger PG, et al. Laparoscopic resection is inappropriate in patients with known or suspected adrenocortical carcinoma. World J Surg 2010; 34: 1380–1385. [DOI] [PubMed] [Google Scholar]
- 103. Brix D, Allolio B, Fenske W, et al. Laparoscopic versus open adrenalectomy for adrenocortical carcinoma: surgical and oncologic outcome in 152 patients. Eur Urol 2010; 58: 609–615. [DOI] [PubMed] [Google Scholar]
- 104. Lombardi CP, Raffaelli M, De Crea C, et al. Open versus endoscopic adrenalectomy in the treatment of localized (stage I/II) adrenocortical carcinoma: results of a multiinstitutional Italian survey. Surgery 2012; 152: 1158–1164. [DOI] [PubMed] [Google Scholar]
- 105. Zeiger MA, Thompson GB, Duh QY, et al. The American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons medical guidelines for the management of adrenal incidentalomas. Endocr Pract 2009; 15(Suppl. 1): 1–20. [DOI] [PubMed] [Google Scholar]
- 106. Reibetanz J, Jurowich C, Erdogan I, et al. Impact of lymphadenectomy on the oncologic outcome of patients with adrenocortical carcinoma. Ann Surg 2012; 255: 363–369. [DOI] [PubMed] [Google Scholar]
- 107. Gerry JM, Tran TB, Postlewait LM, et al. Lymphadenectomy for adrenocortical carcinoma: is there a therapeutic benefit? Ann Surg Oncol 2016; 23(Suppl. 5): 708–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Saade N, Sadler C, Goldfarb M. Impact of regional lymph node dissection on disease specific survival in adrenal cortical carcinoma. Horm Metab Res 2015; 47: 820–825. [DOI] [PubMed] [Google Scholar]
- 109. Deschner BW, Stiles ZE, DeLozier OM, et al. Critical analysis of lymph node examination in patients undergoing curative-intent resection for adrenocortical carcinoma. J Surg Oncol 2020; 122: 1152–1162. [DOI] [PubMed] [Google Scholar]
- 110. Panjwani S, Moore MD, Gray KD, et al. The impact of nodal dissection on staging in adrenocortical carcinoma. Ann Surg Oncol 2017; 24: 3617–3623. [DOI] [PubMed] [Google Scholar]
- 111. Nilubol N, Patel D, Kebebew E. Does lymphadenectomy improve survival in patients with adrenocortical carcinoma? a population-based study. World J Surg 2016; 40: 697–705. [DOI] [PubMed] [Google Scholar]
- 112. Sada A, Glasgow AE, Lyden ML, et al. Positive lymph nodes in adrenocortical carcinoma: what does it mean? World J Surg. Epub ahead of print 9 October 2020. DOI: 10.1007/s00268-020-05801-x. [DOI] [PubMed] [Google Scholar]
- 113. Tran TB, Liou D, Menon VG, et al. Surgical management of advanced adrenocortical carcinoma: a 21-year population-based analysis. Am Surg 2013; 79: 1115–1118. [PubMed] [Google Scholar]
- 114. Reibetanz J, Rinn B, Kunz AS, et al. Patterns of lymph node recurrence in adrenocortical carcinoma: possible implications for primary surgical treatment. Ann Surg Oncol 2019; 26: 531–538. [DOI] [PubMed] [Google Scholar]
- 115. Bellantone R, Ferrante A, Boscherini M, et al. Role of reoperation in recurrence of adrenal cortical carcinoma: results from 188 cases collected in the Italian National Registry for Adrenal Cortical Carcinoma. Surgery 1997; 122: 1212–1218. [DOI] [PubMed] [Google Scholar]
- 116. Erdogan I, Deutschbein T, Jurowich C, et al. The role of surgery in the management of recurrent adrenocortical carcinoma. J Clin Endocrinol Metab 2013; 98: 181–191. [DOI] [PubMed] [Google Scholar]
- 117. Simon G, Pattou F, Mirallié E, et al. Surgery for recurrent adrenocortical carcinoma: a multicenter retrospective study. Surgery 2017; 161: 249–256. [DOI] [PubMed] [Google Scholar]
- 118. Schulick RD, Brennan MF. Long-term survival after complete resection and repeat resection in patients with adrenocortical carcinoma. Ann Surg Oncol 1999; 6: 719–726. [DOI] [PubMed] [Google Scholar]
- 119. Dy BM, Wise KB, Richards ML, et al. Operative intervention for recurrent adrenocortical cancer. Surgery 2013; 154: 1292–1299; discussion 1299. [DOI] [PubMed] [Google Scholar]
- 120. op den Winkel J, Pfannschmidt J, Muley T, et al. Metastatic adrenocortical carcinoma: results of 56 pulmonary metastasectomies in 24 patients. Ann Thorac Surg 2011; 92: 1965–1970. [DOI] [PubMed] [Google Scholar]
- 121. Gaujoux S, Al-Ahmadie H, Allen PJ, et al. Resection of adrenocortical carcinoma liver metastasis: is it justified? Ann Surg Oncol 2012; 19: 2643–2651. [DOI] [PubMed] [Google Scholar]
- 122. Tran TB, Postlewait LM, Maithel SK, et al. Actual 10-year survivors following resection of adrenocortical carcinoma. J Surg Oncol 2016; 114: 971–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Ayabe RI, Narayan RR, Ruff SM, et al. Disease-free interval and tumor functional status can be used to select patients for resection/ablation of liver metastases from adrenocortical carcinoma: insights from a multi-institutional study. HPB (Oxford) 2020; 22: 169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Ettaieb MHT, Duker JC, Feelders RA, et al. Synchronous vs. metachronous metastases in adrenocortical carcinoma: an analysis of the dutch adrenal network. Horm Cancer 2016; 7: 336–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Prendergast KM, Smith PM, Tran TB, et al. Features of synchronous versus metachronous metastasectomy in adrenal cortical carcinoma: analysis from the US adrenocortical carcinoma database. Surgery 2020; 167: 352–357. [DOI] [PubMed] [Google Scholar]
- 126. Dy BM, Strajina V, Cayo AK, et al. Surgical resection of synchronously metastatic adrenocortical cancer. Ann Surg Oncol 2015; 22: 146–151. [DOI] [PubMed] [Google Scholar]
- 127. Sbiera S, Leich E, Liebisch G, et al. Mitotane inhibits sterol-O-acyl transferase 1 triggering lipid-mediated endoplasmic reticulum stress and apoptosis in adrenocortical carcinoma cells. Endocrinology 2015; 156: 3895–3908. [DOI] [PubMed] [Google Scholar]
- 128. Terzolo M, Angeli A, Fassnacht M, et al. Adjuvant mitotane treatment for adrenocortical carcinoma. N Engl J Med 2007; 356: 2372–2380. [DOI] [PubMed] [Google Scholar]
- 129. Berruti A, Grisanti S, Pulzer A, et al. Long-term outcomes of adjuvant mitotane therapy in patients with radically resected adrenocortical carcinoma. J Clin Endocrinol Metab 2017; 102: 1358–1365. [DOI] [PubMed] [Google Scholar]
- 130. Calabrese A, Basile V, Puglisi S, et al. Adjuvant mitotane therapy is beneficial in non-metastatic adrenocortical carcinoma at high risk of recurrence. Eur J Endocrinol 2019; 180: 387–396. [DOI] [PubMed] [Google Scholar]
- 131. Else T, Williams AR, Sabolch A, et al. Adjuvant therapies and patient and tumor characteristics associated with survival of adult patients with adrenocortical carcinoma. J Clin Endocrinol Metab 2014; 99: 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Grubbs EG, Callender GG, Xing Y, et al. Recurrence of adrenal cortical carcinoma following resection: surgery alone can achieve results equal to surgery plus mitotane. Ann Surg Oncol 2010; 17: 263–270. [DOI] [PubMed] [Google Scholar]
- 133. Postlewait LM, Ethun CG, Tran TB, et al. Outcomes of adjuvant mitotane after resection of adrenocortical carcinoma: a 13-institution study by the US adrenocortical carcinoma group. J Am Coll Surg 2016; 222: 480–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Bilimoria KY, Shen WT, Elaraj D, et al. Adrenocortical carcinoma in the United States: treatment utilization and prognostic factors. Cancer 2008; 113: 3130–3136. [DOI] [PubMed] [Google Scholar]
- 135. Nelson DW, Chang S-C, Bandera BC, et al. Adjuvant radiation is associated with improved survival for select patients with non-metastatic adrenocortical carcinoma. Ann Surg Oncol 2018; 25: 2060–2066. [DOI] [PubMed] [Google Scholar]
- 136. Pommier RF, Brennan MF. An eleven-year experience with adrenocortical carcinoma. Surgery 1992; 112: 963–970; discussion 970–961. [PubMed] [Google Scholar]
- 137. Markoe AM, Serber W, Micaily B, et al. Radiation therapy for adjunctive treatment of adrenal cortical carcinoma. Am J Clin Oncol 1991; 14: 170–174. [DOI] [PubMed] [Google Scholar]
- 138. Percarpio B, Knowlton AH. Radiation therapy of adrenal cortical carcinoma. Acta Radiol Ther Phys Biol 1976; 15: 288–292. [DOI] [PubMed] [Google Scholar]
- 139. Fassnacht M, Hahner S, Polat B, et al. Efficacy of adjuvant radiotherapy of the tumor bed on local recurrence of adrenocortical carcinoma. J Clin Endocrinol Metab 2006; 91: 4501–4504. [DOI] [PubMed] [Google Scholar]
- 140. Sabolch A, Else T, Griffith KA, et al. Adjuvant radiation therapy improves local control after surgical resection in patients with localized adrenocortical carcinoma. Int J Radiat Oncol Biol Phys 2015; 92: 252–259. [DOI] [PubMed] [Google Scholar]
- 141. Gharzai LA, Green MD, Griffith KA, et al. Adjuvant radiation improves recurrence-free survival and overall survival in adrenocortical carcinoma. J Clin Endocrinol Metab 2019; 104: 3743–3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Angeli A, Osella G, Ali A, et al. Adrenal incidentaloma: an overview of clinical and epidemiological data from the National Italian Study Group. Horm Res 1997; 47: 279–283. [DOI] [PubMed] [Google Scholar]
- 143. Megerle F, Herrmann W, Schloetelburg W, et al. Mitotane monotherapy in patients with advanced adrenocortical carcinoma. J Clin Endocrinol Metab 2018; 103: 1686–1695. [DOI] [PubMed] [Google Scholar]
- 144. Reidy-Lagunes DL, Lung B, Untch BR, et al. Complete responses to mitotane in metastatic adrenocortical carcinoma-a new look at an old drug. Oncologist 2017; 22: 1102–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Fassnacht M, Terzolo M, Allolio B, et al. Combination chemotherapy in advanced adrenocortical carcinoma. N Engl J Med 2012; 366: 2189–2197. [DOI] [PubMed] [Google Scholar]
- 146. Sperone P, Ferrero A, Daffara F, et al. Gemcitabine plus metronomic 5-fluorouracil or capecitabine as a second-/third-line chemotherapy in advanced adrenocortical carcinoma: a multicenter phase II study. Endocr Relat Cancer 2010; 17: 445–453. [DOI] [PubMed] [Google Scholar]
- 147. Henning JE, Deutschbein T, Altieri B, et al. Gemcitabine-based chemotherapy in adrenocortical carcinoma: a multicenter study of efficacy and predictive factors. J Clin Endocrinol Metab 2017; 102: 4323–4332. [DOI] [PubMed] [Google Scholar]
- 148. Cosentini D, Badalamenti G, Grisanti S, et al. Activity and safety of temozolomide in advanced adrenocortical carcinoma patients. Eur J Endocrinol 2019; 181: 681–689. [DOI] [PubMed] [Google Scholar]
- 149. Barlaskar FM, Spalding AC, Heaton JH, et al. Preclinical targeting of the type I insulin-like growth factor receptor in adrenocortical carcinoma. J Clin Endocrinol Metab 2009; 94: 204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Fassnacht M, Berruti A, Baudin E, et al. Linsitinib (OSI-906) versus placebo for patients with locally advanced or metastatic adrenocortical carcinoma: a double-blind, randomised, phase 3 study. Lancet Oncol 2015; 16: 426–435. [DOI] [PubMed] [Google Scholar]
- 151. Quinkler M, Hahner S, Wortmann S, et al. Treatment of advanced adrenocortical carcinoma with erlotinib plus gemcitabine. J Clin Endocrinol Metab 2008; 93: 2057–2062. [DOI] [PubMed] [Google Scholar]
- 152. O’Sullivan C, Edgerly M, Velarde M, et al. The VEGF inhibitor axitinib has limited effectiveness as a therapy for adrenocortical cancer. J Clin Endocrinol Metab 2014; 99: 1291–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Papadopoulos K, El-Rayes B, Tolcher A, et al. A phase 1 study of ARQ 087, an oral pan-FGFR inhibitor in patients with advanced solid tumours. Br J Cancer 2017; 117: 1592–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Berruti A, Sperone P, Ferrero A, et al. Phase II study of weekly paclitaxel and sorafenib as second/third-line therapy in patients with adrenocortical carcinoma. Eur J Endocrinol 2012; 166: 451–458. [DOI] [PubMed] [Google Scholar]
- 155. Kroiss M, Quinkler M, Johanssen S, et al. Sunitinib in refractory adrenocortical carcinoma: a phase II, single-arm, open-label trial. J Clin Endocrinol Metab 2012; 97: 3495–3503. [DOI] [PubMed] [Google Scholar]
- 156. Smith DC, Kroiss M, Kebebew E, et al. A phase 1 study of nevanimibe HCl, a novel adrenal-specific sterol O-acyltransferase 1 (SOAT1) inhibitor, in adrenocortical carcinoma. Invest New Drugs 2020; 38: 1421–1429. [DOI] [PubMed] [Google Scholar]
- 157. Leal LF, Bueno AC, Gomes DC, et al. Inhibition of the Tcf/beta-catenin complex increases apoptosis and impairs adrenocortical tumor cell proliferation and adrenal steroidogenesis. Oncotarget 2015; 6: 43016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Fiorentini C, Fragni M, Tiberio GA, et al. Palbociclib inhibits proliferation of human adrenocortical tumor cells. Endocrine 2018; 59: 213–217. [DOI] [PubMed] [Google Scholar]
- 159. Pereira SS, Monteiro MP, Costa MM, et al. MAPK/ERK pathway inhibition is a promising treatment target for adrenocortical tumors. J Cell Biochem 2019; 120: 894–906. [DOI] [PubMed] [Google Scholar]
- 160. Altieri B, Ronchi CL, Kroiss M, et al. Next-generation therapies for adrenocortical carcinoma. Best Pract Res Clin Endocrinol Metab 2020; 34: 101434. [DOI] [PubMed] [Google Scholar]
- 161. Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol 2020; 20: 651–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Le Tourneau C, Hoimes C, Zarwan C, et al. Avelumab in patients with previously treated metastatic adrenocortical carcinoma: phase 1b results from the JAVELIN solid tumor trial. J Immunother Cancer 2018; 6: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Carneiro BA, Konda B, Costa RB, et al. Nivolumab in metastatic adrenocortical carcinoma: results of a phase 2 trial. J Clin Endocrinol Metab 2019; 104: 6193–6200. [DOI] [PubMed] [Google Scholar]
- 164. Habra MA, Stephen B, Campbell M, et al. Phase II clinical trial of pembrolizumab efficacy and safety in advanced adrenocortical carcinoma. J Immunother Cancer 2019; 7: 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Raj N, Zheng Y, Kelly V, et al. PD-1 blockade in advanced adrenocortical carcinoma. J Clin Oncol 2020; 38: 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Cosentini D, Grisanti S, Dalla Volta A, et al. Immunotherapy failure in adrenocortical cancer: where next? Endocr Connect 2018; 7: E5–E8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Hahner S, Kreissl MC, Fassnacht M, et al. [131I]Iodometomidate for targeted radionuclide therapy of advanced adrenocortical carcinoma. J Clin Endocrinol Metab 2012; 97: 914–922. [DOI] [PubMed] [Google Scholar]
- 168. Mariniello B, Finco I, Sartorato P, et al. Somatostatin receptor expression in adrenocortical tumors and effect of a new somatostatin analog SOM230 on hormone secretion in vitro and in ex vivo adrenal cells. J Endocrinol Invest 2011; 34: e131–e138. [DOI] [PubMed] [Google Scholar]
- 169. Grisanti S, Filice A, Basile V, et al. Treatment with 90Y/177Lu-DOTATOC in patients with metastatic adrenocortical carcinoma expressing somatostatin receptors. J Clin Endocrinol Metab 2020; 105: e1–e5. [DOI] [PubMed] [Google Scholar]