Abstract
Acacia gum (AG) is a branched-polysaccharide gummy exudate that consists of arabinose and galactose. The traditional practice in African-Middle Eastern countries uses this gum as medicine. Traditional use of AG is to treat stomach disease, which can be a potential functional food. In this research, commercially available AG from Acacia senegal and Acacia seyal was investigated as the prebiotic. The experiment employed a pH-controlled in vitro colon model inoculated with human fecal microbiota to mimic the human colon. Fermentation samples at 0, 6, 12, and 24 h were brought for short-chain fatty acid (SCFA) analysis using high-performance liquid chromatography and bacterial enumeration via fluorescent in situ hybridization. Results showed that AG significantly promotes Bifidobacteria proliferation similar to fructo-oligosaccharides (FOS) while inhibiting the Clostridium histolyticum group, commonly associated with gut dysbiosis. Acetate, propionate, and butyrate showed a similar trend to FOS (p > 0.05). The AG shows potential against gut dysbiosis, as it promotes gut-probiotics, through modulation of microbial population and SCFA production, especially butyrate.
1. Introduction
Acacia gum (AG) is a soluble secretion found on the trunks and branches of Acacia senegal and Acacia seyal trees.1 The exudate is secreted as a result of subsequent injuries onto the bark of the trees. AG is among the hydrocolloids that have been exploited in various food and nonfood applications. Having exceptionally low viscosity (300 cP maximum in a 1% solution) made AG the best source of soluble dietary fiber because it can boost fiber levels in foods or beverages without modifying the final viscosity of food products.2 It is highly soluble in water even 30.0% gum arabic solutions have a lower viscosity than 1.00% xanthan gum and sodium carboxymethylcellulose at low shear rates.3,4 More than three-quarters of industrial applications of this hydrocolloid are for emulsification, encapsulation, coating, and confectionaries.5 It is made up of 95.0% long-chain complex polysaccharides, mainly from highly branched galactan polymers, side chains of galactose and/or arabinose, and rhamnose or glucuronic acid as termination residues.6 Looking at its carbohydrate-based structure and functions, AG is an excellent prospect to be commercialized as the prebiotic.
Prebiotics are a substrate that selectively nourished gut microbiota and in return bring health benefits to the host. Some strict criterions needed to be met before any substrates are known as the prebiotic. Numbers of tests have to be performed where the proposed substrate should be resistant to digestive activity from the mouth until the duodenal phase and fermentable by colonic microbes and selectively promote growth and metabolism of health beneficial probiotics such as Bifidobacterium and Lactobacillus.5 These bacteria are known to ferment established prebiotic compounds, i.e., fructo-oligosaccharides (FOS), galacto-oligosaccharides, and inulin to produce beneficial metabolites known as short-chain fatty acids (SCFAs) mainly acetate, propionate, and butyrate.7 These SCFAs may exert a direct effect on host health by lowering luminal pH as most enteric pathogens do not grow well in low pH. Butyrate especially contributes toward the energy requirement by epithelial cells, thus improving intestinal motility. There are currently no official dietary guidelines for prebiotics in healthy individuals describing ″adequate intake″ or ″recommended daily allowance.″ To provide a boost, most prebiotics for the gut require an oral dose of 3 grams per day or more. FOS in the daily diet should be about 5 grams, and this includes plant sources of prebiotics.8
Probiotics as defined by FDA and WHO (2002) are the “live microorganisms that when administered in adequate amounts confer a health benefit to the host”.9 For example, the mechanism of actions by probiotics includes the modulation of immune function, association with native gut microbiota, and interaction with the host epithelial tissue defence integrity and enzyme synthesis. Lactobacillus spp. and Bifidobacterium spp. are the most common probiotic bacteria that have been utilized by the food industry,10 but others such as Saccharomyces cerevisiae(boulardii), Enterococcus, Bacillus, and Escherichia are also applied. They are mainly saccharolytic metabolism that is capable of carbohydrate utilization. Therefore, a study on the importance of the species-specific among particular groups might uncover the health-promoting conditions.11
Colonic bacteria metabolized a range of carbohydrate-hydrolyzing enzymes in the production of several products, such as hydrogen, methane, carbon dioxide, SCFA (predominantly acetate, propionate, and butyrate), and lactate. This gut flora obtained its energy through the fermentation of food supplied from the upper gut.12 Several studies have claimed that dysbiosis, which is the imbalance and unhealthy condition of colon microbiota diversity, led to disorders such as obesity,13,14 metabolic complications, immunity dysregulation,15 changes in energy and hormone regulation, and even an irregular inflammatory mechanism.16 Dysbiosis that happened in the gut could affect normal host systems, most often to the metabolic and immune process, thus causing diseases such as inflammatory bowel disease (IBD),17,18 psoriatic arthritis,19 type 1 and type 2 diabetes,20,21 atopic eczema,22 coeliac disease,23,24 and arterial stiffness.25
Over the last few decades, investigation on AG for its health-promoting properties was studied and have shown positive effect like the regulation of autoimmune disease,15 reduced duodenal inflammation in mice,16 antiulcerogenicity on gastric mucosal injury in rats,26 methane gas mitigation in ruminants,27 and also the topical treatment of skin lesions of kwashiorkor children.28 This research aims to investigate the impact of commercially available AG as a prebiotic source through in vitro colon model study. Through in vitro approaches, this study investigates the digestibility of AG, changes in gut microbiota, and SCFA production under the influence of AG. The experiment includes in vitro digestion and fermentation of AG in a custom-build colon model, which mimics the distal colon of the human gastrointestinal system. This prebiotic study evaluates the potential of AG in a streamlined process from the in vitro digestion to fermentation using the colon model. Two species of AG (A. senegal and A. seyal) were tested and evaluated for their prebiotic potential. The finding from this research could help to further understand current food products, since our usual meal may have been incorporated with AG as a stabilizer ingredient, such as in candies, chewing gums, ice cream, salad-dressing, bread, cereals, and cola drinks.
2. Results and Discussion
2.1. Proximate Analysis of AG
The analysis in this research includes proximate analysis and digestibility of AG. The purpose of the proximate analysis of AG expressed the basic composition of both types of AG samples used. Two AG types were analyzed for proximate: total crude protein, moisture, fat, ash, and total carbohydrate content before advancing into the in vitro digestion to test for the human digestive resistance properties (Table 1).
Table 1. Proximate Analysis of AG1 and AG2 for the Content (%) of Total Crude Protein, Fat, Moisture, Ash, and Carbohydratea.
| component | AG1 | AG2 |
|---|---|---|
| protein | 1.86 ± 0.04 a | 0.85 ± 0.02 b |
| moisture | 11.95 ± 0.05 a | 10.40 ± 0.03 b |
| fat | 0.08 ± 0.02 a | 0.06 ± 0.03 a |
| ash | 3.21 ± 0.01 a | 3.50 ± 0.02 a |
| carbohydrate (by difference) | 82.92 a | 85.22 b |
All data are shown as mean ± SD, n = 3. Means with a lowercase letter represent significant nutrient composition in terms of percentage (%) between AG samples at a confidence level of 95.0%. (n = 3).
From the proximate results, the major nutrient component of AG is a carbohydrate with a significant portion, 83.0 and 85.2% for AG1 and AG2, respectively. The differences may be due to the substrate mixture and species difference. AG1 contains gum from Acacia senegal only, while AG2 contains the mixture of A. senegalandA. seyal. Initially, before the year 2011, production of A. seyal was only contributed to an average of 10.0% of all gum products in Sudan.29 During that period, these two species of AG were mixed during harvesting that made it impossible to justify precisely the exact composition balance of these two species in the market by the collectors7 because the availability of either species varied during the season. Nowadays, the staggering amount of coverage land area increased to more than 60.0% for A. seyal for the last decade. Therefore, based on the distribution, a corresponding relation that the composition of the AG2 sample contains presumably more than 50% of A. seyal species is provided. This explained the significant differences in the nutritional content of individual species.
Apart from this, individual species difference also explains the carbohydrate content. A. seyal contains higher arabinose and 4-O-methyl glucuronic acid contents that may contribute to these slight differences. On the other hand, A. senegal contains a higher protein content than A. seyal (1.86% > 0.850%). As reported in previous studies, A. senegal contains a higher proportion of nitrogenous material although the amino acid compositions suggested being similar between the species.3,30 Both species disperse naturally in the central belt of the low-rainfall grassland, where they exist in pure or mixed stands, in the clay plains in the East, and the sandy soils in the West.31
In addition, the moisture content is at ≈10.0–12.0% where AG1 is higher than AG2. The range of moisture content for A. senegal was 8.10–14.7%. According to Ibrahim et al.,32 it is at 13.5%. From the observation, AG2 is darker in color compared with AG1, which explained the hues of lightness portrayed by the moisture content of these two AG samples,33 which also indicates purity. After all, these substrates were deemed as premium grade, commercialized as healthy fiber substitutes.
The proximate analysis also shows the least significant nutrient of the fat content of less than 0.0826%. Both gums have the same composition except that AG1 has significantly higher protein and moisture contents. The observed distinction in the protein substance could be from the different contrasts between both species origin, geographic relativity where the gum was harvested, the soil profile, climatic conditions of the area, the age of the trees, any presence of pathogens, or a mix of all these components.34−36 The findings of Idris and Haddad (2011) are also confirmed, where A. senegal gum samples contain two times more protein substances when compared with the gum of A. seyal.37
Based on the findings, all the results correspond within the limit specified by Joint FAO/WHO Expert Committee on Food Additives (JECFA).38,39 The chemical composition of AG can change with its source, the age and place of the trees planted, rainfall intensity, and soil conditions.40 For example, A. senegal is best found grown in sandy soils, whereas A. seyal prefers natural clay soils. Therefore, the determination of the nutritional compounds mentioned could provide a meaningful idea of how these two substrates may exhibit distinct effects later in prebiotic evaluation.
2.2. Total Carbohydrate Content
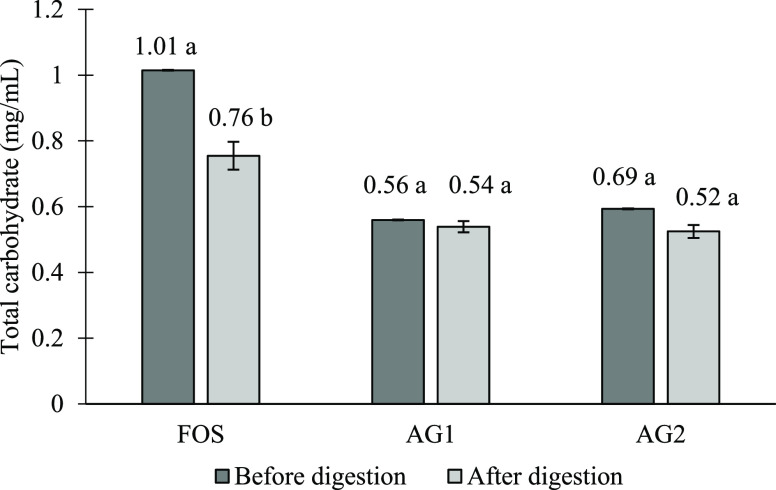
Figure 1 shows the changes in total carbohydrate content in AG after digestion in comparison to FOS. Following the digestion, both AG showed a small reduction of total carbohydrate content especially AG1. All the samples show losses of carbohydrate after digestion.
Figure 1.
Total carbohydrate content (mg/mL) of fructo-oligosaccharides and acacia gum. Means with a lowercase letter shows the significant comparison of samples before and after digestion at a confidence level of 95.0%. (n = 3) (Note: FOS, fructo-oligosaccharides; AG1, A. senegal; AG2, mix of A. senegal and A. seyal).
Based on the results, FOS with the highest carbohydrate content among the substrates afflicted the most reduction by 24.34% after digestion, whereas the carbohydrate content in AG1 and AG2 decreased slightly after the in vitro digestion process, by 3.63 and 11.59%, respectively. While it is unexpected to have a significant loss of carbohydrates in FOS after digestion, both FOS and AG consist of majorly soluble parts, which made usage of filtration recovery not as efficient, which explains the loss of carbohydrate portion during the filtration. The difference is that, in Mandalari et al. (2008), the research investigated the lipid content of almond seeds.41 Since almond is insoluble during the digestion process, the final recovery method using filter paper filtration was efficient. Thus, for a soluble substrate, the best recovery method is as described by Brodkorb et al., 2019,42 either using freeze-drying methods or dialysis membrane filtration.
AG is known to resist digestion in the small intestine (either by enzymatic hydrolysis or gastric acid) as based on multiple research studies12,43,44 and can only be degraded by microbial fermentation in the large intestine.45 As mentioned by Renard et al. (2014), arabinogalactan fraction, a high molecular weight gummy residue of AG, is resistant to hydrolysis in any pH conditions.35 The macromolecule of A. seyal was larger than that of A. senegal, average at 8.2 × 105 gmol–1 vs 6.8 × 105 gmol–1, respectively. In addition, the structure arrangement of A. seyal derivatives is believed to be more compact and less viscous (Rg: 15.2 nm; [η]: 16.5 mLg–1) than that of A. senegal (Rg: 28 nm; [η]: 22.8 mLg–1).30 In contrast, A. senegal is built with the highest degree of branching (78.2% vs 59.2%) with multiple branched galactopyranoses, shorter arabinosyl side branches, and more rhamnopyranoses located at the end on the chains. This could explain the better degree of resistance of AG1 compared to AG2, according to the percent of total carbohydrate loss (3.63% vs 11.6%).
2.3. Total Reducing Sugar Content
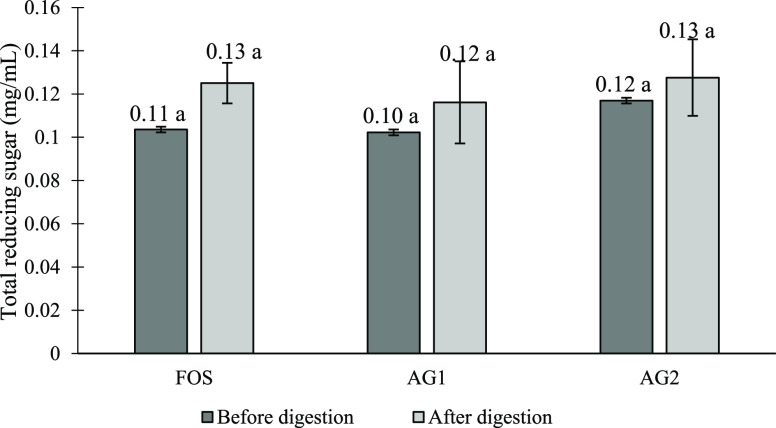
Figure 2 shows the total reducing sugar content for AG and FOS before and after digestion. All samples have a similar initial reducing sugar content and showed an increase in reducing sugar after digestion. An increment of about 20.7, 10.3, and 9.09% was shown for FOS, AG1, and AG2, respectively. Although there are changes in total reducing sugar, the increment for all samples was not significant. Some of the reducing sugar may have been released as a result of the acidic condition especially to the linear structure of FOS. The highest increase in reducing sugar was observed for FOS followed by AG1 and AG2 (20.7, 10.3, and 9.09%). In comparison to FOS and AG, the solubility differences among these two samples may affect the recovery amounts as mentioned above, shown in the phenol sulphuric assay of digested FOS.
Figure 2.
Total reducing sugar content (mg/mL) of fructo-oligosaccharides and acacia gum. Means with a lowercase letter shows the significant comparison of samples before and after digestion at a confidence level of 95.0%. (n = 3) (Note: FOS, fructo-oligosaccharides; AG1, A. senegal; AG2, mix of A. senegal and A. seyal).
The reducing power of the gum is calculated from arabinose-free reducing groups. Based on the previous finding, the reducing sugar content for A. senegal gum ranges between 0.160–0.440%,.1A. seyal gum has a higher arabinose level than A. senegal, as has been accounted previously. This may be because A. seyal possesses more and/or longer branches.34,36 The backbone of arabinogalactan macromolecules present in AG is made up of β 1,3-linked galactose residues; therefore, it is believed that a more compact structure of A. seyal contributes to an increasing arabinose content. On the other hand, the source of origin may not give any distinct sugar structures as noted by Gashua (2016) in the study comparing sugar structures of the A. senegal gums from Nigeria and Sudan.46
2.4. Degree of Polymerization of AG
Although the sugar content after digestion showed changes based on previous analyses, the percentage of hydrolysis of FOS (2.36%), AG1 (2.31%), and AG2 (2.32%) shows no significant difference. Hence, the calculated degree of polymerization (DP) of FOS is 10, while that of both AG samples is DP = 5. While referring to the high molecular weight of AG, the result of the DP value stated smaller than that for FOS. This may be due to the unsuitable experimental determination for the AG structure that is complex and not accessible for some reagents like the DNS assay. However, this may lead to the misinterpretation of results for AG. The value may only represent the changes of degraded parts at the free reducing side chain on the branch structure of AG during in vitro digestion.47
In addition, according to the work carried out by Anderson and Stoddart (1966a) and Anderson and McDougall (1987), AG that was subjected to prolonged (48 h) mild acid and low pH and from the sequential Smith-degradation could degrade the gum into several fractions.48,49 The sugars eliminated from the original gum macromolecules appear in either the insoluble or diffusate fractions. This, however, was only achieved at boiling temperature.
Therefore, based on the in vitro digestion, AG samples were resistant to human gut hydrolysis as no quantitative significant changes were present. The resistance indicates the unique and complex structure of AG to the absence of the human enzyme available to facilitate AG hydrolysis in the stomach. The undigested large molecules remained intact structurally until they reached the colon for fermentation to take place. In the present work, prebiotic evaluation was studied in a continuous flow, i.e., the combination of in vitro digestion and fermentation. This two-step continuous flow is a more accurate approach to determine the prebiotic potential, which mimics our gastrointestinal tract.
2.5. Prebiotic Evaluation
Previously the results stated that AG is not digested in the upper part of the human digestive system and therefore is transited to the large colon where microbial fermentation mostly takes place.50 As such, pH-controlled batch cultures were conducted using the in vitro colon model and FOS as a positive control. The experiment was conducted in four replicates. The changes in the population of gut bacteria against the time of fermentation (0, 6, 12, and 24 h) were evaluated so that a proper interpretation can be withdrawn to determine the promotion or inhibition of the particular species in healthy human gut microbiota. By evaluating colonic bacteria, we could study the effects of the AG to promote human health to exhibit its prebiotic potential.
2.6. Colonic Bacterial Enumeration
Table 2 shows the bacterial population growth according to its bacterial group. The fermentation of AG1 and AG2 shows an increase of Bifidobacterium spp. after 12 and 24 h compared with the numbers at 0 h. However, this is different from FOS where a significant increase of Bifidobacterium spp. was shown from 6 h until the end of fermentation. Although FOS shows higher Bifidobacterium spp. at 6 h compared with AG1 and AG2, the opposite was observed at 12 h of fermentation where Bifidobacterium spp. in FOS was outnumbered by the counts in AG2 and AG1 accordingly. Altogether, AG1, AG2, and FOS have significantly higher Bifidobacterium spp. at 24 h compared with the negative control.
Table 2. Mean Values of the Bacterial Population (log10 Cells/mL) in the Batch Culture Colon Model Fermentation at 0, 6, 12, and 24 h by Human Faecal Microbiotaa.
| probec | time (h) | mean ± SD | |||
|---|---|---|---|---|---|
| AG1 | AG2 | FOS | control | ||
| Bif164 | 0 | 7.94 ± 0.12 | 7.94 ± 0.12 | 7.94 ± 0.12 | 7.94 ± 0.12 |
| 6 | 8.28 ± 0.16 ab | 8.25 ± 0.21 ab | 8.47 ± 0.14 ab | 8.19 ± 0.09 bb | |
| 12 | 8.51 ± 0.04 ab | 8.54 ± 0.11 ab | 8.47 ± 0.12 ab | 8.41 ± 0.08 ab | |
| 24 | 8.59 ± 0.04 ab | 8.78 ± 0.10 ab | 8.67 ± 0.08 ab | 8.41 ± 0.06 bb | |
| Lab158 | 0 | 7.88 ± 0.23 | 7.88 ± 0.23 | 7.88 ± 0.23 | 7.88 ± 0.23 |
| 6 | 8.01 ± 0.36 a | 8.19 ± 0.23 ab | 8.14 ± 0.07 a | 7.96 ± 0.15 a | |
| 12 | 8.11 ± 0.24 abb | 8.14 ± 0.21 abb | 8.09 ± 0.21 a | 8.18 ± 0.20 b | |
| 24 | 8.08 ± 0.12 a | 8.13 ± 0.25 ab | 7.99 ± 0.27 a | 8.12 ± 0.28 a | |
| Chis150 | 0 | 7.22 ± 0.14 | 7.22 ± 0.14 | 7.22 ± 0.14 | 7.22 ± 0.14 |
| 6 | 7.46 ± 0.14 ab | 7.67 ± 0.21 bb | 7.34 ± 0.17 cb | 7.28 ± 0.17 d | |
| 12 | 7.36 ± 0.03 a | 7.39 ± 0.07 a | 7.15 ± 0.08 b | 7.24 ± 0.11 ab | |
| 24 | 7.18 ± 0.08 a | 7.20 ± 0.14 ab | 7.29 ± 0.15 ab | 7.32 ± 0.09 b | |
| Ato291 | 0 | 7.93 ± 0.14 | 7.93 ± 0.14 | 7.93 ± 0.14 | 7.93 ± 0.14 |
| 6 | 8.14 ± 0.25 a | 8.15 ± 0.25 a | 8.17 ± 0.15 ab | 8.07 ± 0.07 a | |
| 12 | 8.32 ± 0.14 ab | 8.35 ± 0.11 ab | 8.36 ± 0.02 abb | 8.00 ± 0.18 b | |
| 24 | 8.34 ± 0.06 abb | 8.38 ± 0.10 abb | 8.35 ± 0.14 ab | 8.16 ± 0.20 bb | |
| Erec482 | 0 | 7.81 ± 0.09 | 7.81 ± 0.09 | 7.81 ± 0.09 | 7.81 ± 0.09 |
| 6 | 7.51 ± 0.04 ab | 7.59 ± 0.03 bb | 7.55 ± 0.17 ab | 7.57 ± 0.05 abb | |
| 12 | 7.58 ± 0.10 a | 7.58 ± 0.13 a | 7.56 ± 0.03 ab | 7.53 ± 0.03 ab | |
| 24 | 7.55 ± 0.04 ab | 7.52 ± 0.10 ab | 7.54 ± 0.09 ab | 7.56 ± 0.05 ab | |
| Prop853 | 0 | 7.40 ± 0.15 | 7.40 ± 0.15 | 7.40 ± 0.15 | 7.40 ± 0.15 |
| 6 | 7.37 ± 0.06 a | 7.37 ± 0.16 a | 7.33 ± 0.19 a | 7.36 ± 0.17 a | |
| 12 | 7.31 ± 0.09 a | 7.36 ± 0.09 a | 7.32 ± 0.10 a | 7.48 ± 0.10 b | |
| 24 | 7.30 ± 0.13 abb | 7.27 ± 0.21 ab | 7.23 ± 0.11 ab | 7.40 ± 0.18 b | |
| Fpra655 | 0 | 7.61 ± 0.08 | 7.61 ± 0.08 | 7.61 ± 0.08 | 7.61 ± 0.08 |
| 6 | 7.63 ± 0.23 a | 7.59 ± 0.44 a | 7.65 ± 0.24 a | 7.69 ± 0.13 a | |
| 12 | 7.64 ± 0.30 a | 7.67 ± 0.13 a | 7.69 ± 0.23 a | 7.64 ± 0.05 a | |
| 24 | 7.67 ± 0.14 a | 7.69 ± 0.17 a | 7.64 ± 0.20 a | 7.50 ± 0.05 a | |
| Bac303 | 0 | 8.22 ± 0.19 | 8.22 ± 0.19 | 8.22 ± 0.19 | 8.22 ± 0.19 |
| 6 | 8.29 ± 0.07 a | 8.30 ± 0.03 a | 8.26 ± 0.14 a | 8.25 ± 0.11 a | |
| 12 | 8.36 ± 0.37 ab | 8.45 ± 0.21 abb | 8.51 ± 0.02 a | 8.35 ± 0.08 b | |
| 24 | 8.56 ± 0.02 ab | 8.56 ± 0.14 bb | 8.63 ± 0.06 bb | 8.30 ± 0.13 a | |
| Bac338 | 0 | 8.53 ± 0.21 | 8.53 ± 0.21 | 8.53 ± 0.21 | 8.53 ± 0.21 |
| 6 | 8.57 ± 0.09 a | 8.56 ± 0.16 a | 8.73 ± 0.15 ab | 8.56 ± 0.08 a | |
| 12 | 8.60 ± 0.12 a | 8.64 ± 0.09 ab | 8.76 ± 0.10 b | 8.52 ± 0.17 a | |
| 24 | 8.71 ± 0.09 ab | 8.70 ± 0.20 ab | 8.73 ± 0.10 ab | 8.50 ± 0.18 b | |
All data are shown as mean ± SD, n = 4. Means with a lowercase letter represent significant differences between substrates within the same sampling hour (p < 0.05).
Mean value represents significant differences from the 0 h value within the same treatment (p < 0.05).
Bif164, Bifidobacterium spp.; Lab158, Lactobacillus/Enterococcus; Chis150, Clostridium histolyticum; Ato291, Atopobium spp.; Erec482, Eubacterium rectale-Clostridium coccoides; Prop853, Clostridium cluster XI; Fpra655, Faecalibacterium prausnitzii; Bac303, most Bacteroidaceae and Prevotellaceae, some Porphyromonadaceae: Bac338, total bacteria.
Bifidobacterium spp. is considered an important reference to see the impact of tested prebiotics since immense work has been involving the use of species that belongs to the genera of this group and suggested a favorable impact in the large intestine.51,52Lactobacillus spp. population growth increased after 12 h compared with the numbers at 0 h, for AG1 and AG2, whereas counts of Lactobacillus spp. in FOS were not significant until the end of fermentation. Bifidobacterium spp. and Lactobacillus spp. have long been considered a positive microbial group producing antimicrobial metabolites, which might inhibit certain pathogen growth.53,54 The delay of bacterial growth and population could indicate slower fermentation of complex AG polysaccharides,12 while the simpler FOS chain is immediately fermented as early as 6 h. Their study assessment was done by comparing microbial change on agar plate culture between samples from different AG dosages and sucrose feed to volunteers.
Short chain molecules, such as FOS, are generally fermented faster than larger, longer chain molecules such as AG and partially hydrolyzed guar gum.55 The previous study on the consumption of FOS explained that ingestion of this prebiotic causes bloating and increased frequency of flatulence in the tested subjects,56 even so, Bruggencate et al. (2006)57 also speculated the adverse effects on the intestinal barrier functions based on their animal studies due to rapid fermentation. Gut fermentation that occurred slowly could reduce gastrointestinal discomfort, such as flatulence and bloating.11 Rapid response of fermentation would increase the rate of gas released and trapped inside the lumen.55 As reported in the work of Calame et al. (2008), all subjects tolerated the given high dose of AG up to 40 g/d for 4 weeks with no significant adverse symptoms.11 In the United States and European countries, AG has been recognized as safe (GRAS) and categorized as “acceptable daily intake” food with an approval food additive code, E414.58 Although FOS shows higher Bifidobacterium spp. at 6 h compared with AG1 and AG2, the opposite was observed at 12 h of fermentation where Bifidobacterium spp. in FOS was outnumbered by the counts in AG2 and AG1 accordingly. In this context, slower fermentation of AG would be a good relief for people who are easily feeling discomfort from the consumption of prebiotic FOS. Most of the dietary fibers including short-chain FOS were fermented in the proximal colon by the bacteria, while the exception to the long-chain FOS (inulin) is fermented in the distal colon.59 The relationship of FOS chain length and bowel transit time may suggest the outcome stated.
Furthermore, after 12 and 24 h, the counts for the Clostridium histolyticum group decreased in FOS and AG substrates when compared with 0 h, which confirmed the results of in vitro and in vivo feeding.11,12,60 This bacterial group is known to have an association in the inflammatory response and colon disease.61 Crohn’s disease and ulcerative colitis are known as chronic inflammatory disorders of the intestines.62 For negative control, there are no significant changes of C. histolyticum population. Although IBD may occur in any parts of the gastrointestinal tract, it most commonly happens in the distal part of the ileum and colon.63 Many researchers hypothesized that one of the causal contributions of IBD that trigger the immune response to the antigen over the mucosal wall was from this microbial pathogen.64
Based on Macfarlane et al.,65 along the proximal, transverse, and distal colon, the calculated pH values (taken from sudden death victims) of colonic contents are at 5.5, 6.2, and 6.8, respectively. The luminal pH in the distal colon is increased close to neutral pH, which suits and is preferable for pathogenic proliferation. The causal gradual increase of luminal pH was based on this theory. As food components travelled into the first part of the large colon (proximal), it may have undigested components, i.e., resistant starch, oligosaccharides, and arabinogalactan. High rates of fermentation take places and will subsequently reduce as the fecal transits into the transverse colon. By the time the fecal reached the distal part of the colon, low amounts of fermentable carbohydrates remained for vigorous microbial fermentation to occur.
Consequently, it is known that a large number of protein residues were fermented in the distal gut where saccharolytic fermentation is not as much due to the depletion of carbohydrate fermentation being further away from the stomach. High proteolytic fermentation produced several kinds of byproducts, such as ammonia, branched chain fatty acids, phenolic compounds, hydrogen sulfide and methanethiol, and nitrosamines and other biogenic amines, which are all putrefactive metabolites that may negatively affect the host.52,66−68 This is the important reason for the AG prebiotic to be slowly fermented so that a sufficient quantity of prebiotic reached the colon end; thus, it could inhibit the growth of pathogenic bacteria. By specifically promoting beneficial bacteria, the luminal pH of the colon can be lowered as a result making an undesired environment for pathogenic microbiota. Based on the present study, the fermentation of AG selectively promotes beneficial bacteria and inhibits the growth of harmful bacteria, which indirectly may ameliorate the diseases.
The counts of Bacteroides spp. were enhanced in fermentation for AG2 and FOS, all of which are significantly higher than the number in negative control at 24 h. In addition, it is worth noting that FOS has the highest number of Bacteroides spp. at 12 h. Bacteroides spp. is one of the largest groups residing in the healthy adult gut that involved in several metabolisms inside the colon.69−71 Bacteroides consist of beneficial and nonbeneficial members. Past articles have expressed the beneficial impact of members of this group toward gut health.72,73 Onderdonk and Garrett (2015) reviewed that some bowel infections and diseases like diarrhoea and cancer are contributed by these gram-negative bacteria.74
However, it is reported that the members of the subfamily in Bacteroides genera particularly species of Prevotella and Porphyromonas are symbiotic in the colon, which may boost immunity.74 The counts of Faecalibacterium prausnitzii were not significant in all substrates at all-time points. Apart from this, the total bacterial growth was statistically significant at 6 h for FOS and 24 h for AG2 compared with their respective baseline (0 h). In addition, a decreasing trend of total bacteria was observed in negative control after 12 h, however not statistically meaningful.
Bacterial counts for Atopobium spp. were increased from 6 h but only significantly intensified after 12 and 24 h fermentation for AG1 and AG2, whereas, Atopobium spp. was increased from 6 h until the end for FOS. The C. coccoides-Eubacterium rectale group was decreased in all substrates at 24 h of fermentation. In addition, significant decreases are also observed in Clostridium cluster XI population for AG1 and FOS substrates at the end of fermentation. Overall, the fermentation of both AG substrates happened to have a similar bacterial profile to FOS. While FOS had been profoundly studied as the prebiotic to regulate and selectively stimulate bacterial population in the colon,75−78 AG could have been exploited as the potential prebiotic as good as FOS. Moreover, the property of AG as high dietary fiber enables it to be a good additive ingredient.
As previously mentioned, there is a delay or some offset of time when fermentation of AG started. There are a couple of possible reasons that can be speculated: some of the bacteria were in their log phase long enough before the appropriate gene expression was done to an unfamiliar gum molecule and triggered the fermentation at 12 h compared to a shorter chain of FOS that took place sooner. Also, there are four distinct groups of Bifidobacteria in respect to their metabolism of the length of the prebiotic chain. Some strains are capable of utilizing fructose only, while others utilized both FOS and fructose but reducing their favor for the longer FOS. Some strains favor FOS but are not capable of metabolizing inulin or fructose, whereas the rest of them may metabolize any available substrates; FOS, inulin, or fructose.79
Different bacterial strains possess essential requirement in energy, carbon and nitrogen for their growth and metabolism. Ummadi and Curic-Bawden (2008)80 reviewed that the complex requirement of Lactobacillus depends on strain and species due to its properties of multiple auxotrophies. Similar to Bifidobacterium, carbohydrate sources are one of the growth requirements for Lactobacillus. Various studies reported that oligosaccharides are essential for Lactobacillus metabolism in the intestinal ecosystem.81−83
Furthermore, the oligosaccharide from the glycoprotein parts of AG may contribute to the prebiotic effect by the metabolism of Lactobacillus. Thus, it comes to a controversial proposition of another reason why fermentation is slower in AG. First, the likelihood of any Bifidobacterium and Lactobacillus to prefer metabolizing the complex branched chain of AG is questionable, thus suggesting that the delay was due to the cross-feeding, which only occurred after the first gum degradation by the primary degrader. Growth promotion of Bifidobacterium spp. was at 12 h because there are plenty of intermediate products resulting from the AG hydrolysis by other bacterial groups. This also explained that several bacteria were significantly proliferated earlier than Bifidobacterium spp. in AG fermentation, suggesting that those bacteria were the primary degraders to metabolize AG.
2.7. SCFA Production
The batch culture colon model fermentation allows for rapid analysis of the effects of AG as a substrate to the colonic microbes. As a result, SCFA production from the fermentation of AG can be monitored. Thus, batch culture systems enable an evaluation of how bacteria ferment a substrate in correspondence to the metabolites produced.84 The results from the current study indicated that AG substrates have selectively promoting effects similar to the known prebiotic FOS, as shown by the microbiota population and production of acetate, butyrate, and propionate. In practice, the in vitro method is very straightforward and reliable to predict what could not be achieved in vivo due to the inherent complexity of the process.85
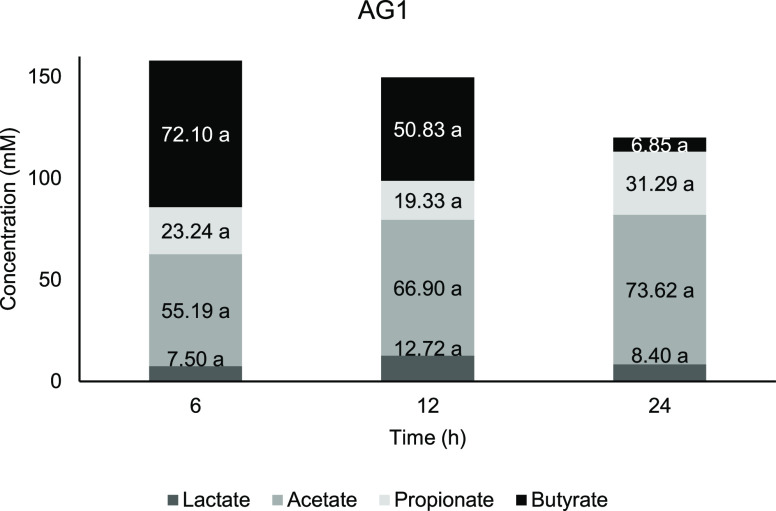
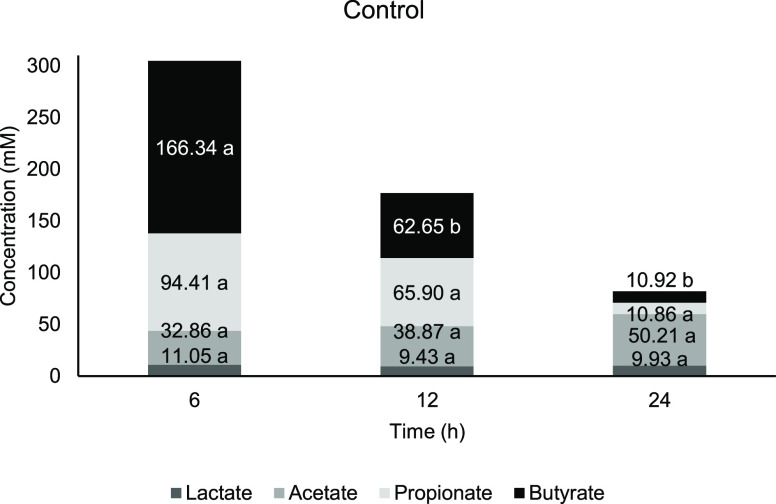
The organic acids evaluated for this study are lactate, acetate, propionate, and butyrate. The total SCFA in AG1 (Figure 3) and negative control (Figure 6) achieved its highest concentration at 6 h, respectively, whereas AG2 (Figure 5) and FOS (Figure 4) have the highest total SCFA at 12 h. FOS was observed to possess the highest total SCFA concentration after 24 h of fermentation compared to other treatments. It was previously suggested that consumption of arabinogalactan fraction (≈200 kDa) obtained from AG has greater SCFA production at least when it is administered in a longer period.86 Lactate and SCFA function to downregulated proinflammatory response in intestinal epithelial cells and myeloid cells and thus might contribute to colon health.87
Figure 3.
Concentration of short-chain fatty acids against time for AG1. Means with a lowercase letter shows the significant comparison between hours of each acids at a confidence level of 95.0%. (n = 4).
Figure 6.
Concentration of short-chain fatty acids against time for control. Means with a lowercase letter shows the significant comparison between hours of each acids at a confidence level of 95.0%. (n = 4).
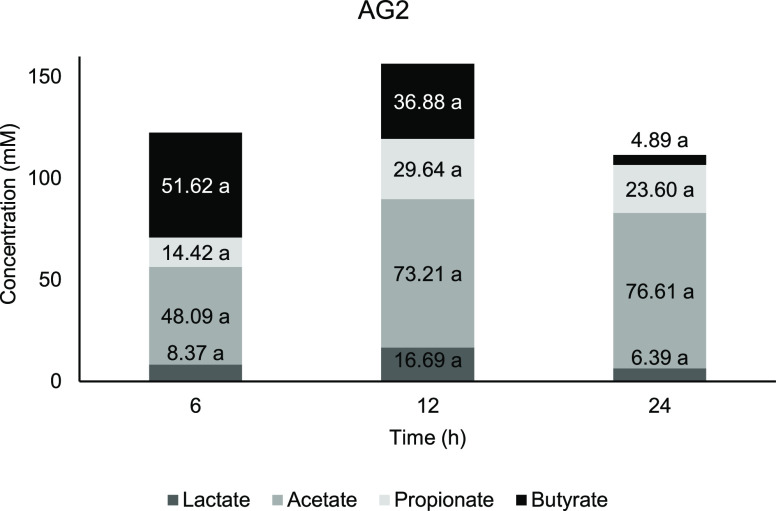
Figure 5.
Concentration of short-chain fatty acids against time for AG2. Means with a lowercase letter shows the significant comparison between hours of each acids at a confidence level of 95.0%. (n = 4).
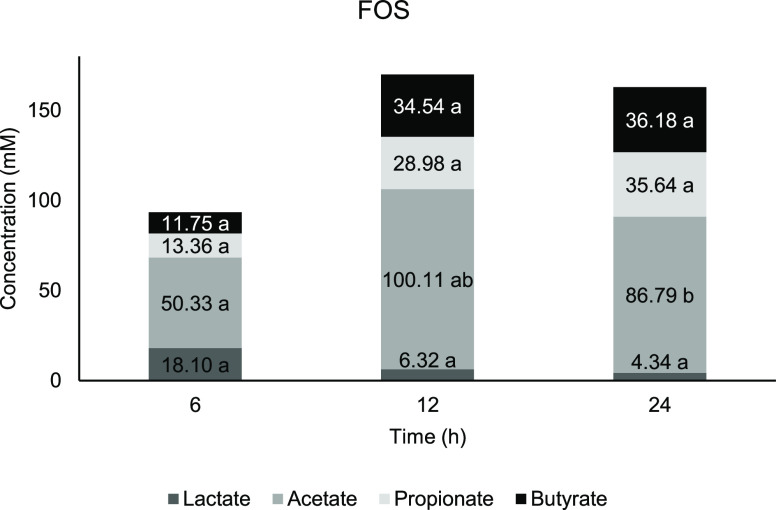
Figure 4.
Concentration of short-chain fatty acids against time for FOS. Means with a lowercase letter shows the significant comparison between hours of each acids at a confidence level of 95.0%. (n = 4).
From Figures 3 and 5, fermentation of AG1 and AG2 showed the highest concentration at 6 h before it started to reduce in 12 and 24 h of fermentation, whereas for FOS (Figure 4), both butyrate and acetate started at a lower concentration in the early part of fermentation and gradually increased until 24 h. There is no significant change occur at any time point for lactate content in AG1 and AG2 compared with 0 h. According to Ríos-Covián et al.,88 lactate, which is not an SCFA, is also produced during gut fermentation. In the current study, there is no significant change occuring at any time point for lactate content in AG1 and AG2 compared with 0 h. Lactate was immediately converted into other acids, which is the reason why it is rarely accumulated in the fermentation vessels. Lactate served as the precursor for cross-feeding by mixed culture of colonic bacteria, which transformed into particularly butyrate apart from acetate and propionate,89 while for the negative control, a significant lactate increment was observed at 12 h compared with 0 h, which signifies less microbial metabolism activity.
The fermentation resulted in acetate concentration as the most abundant SCFAs after 24 h of fermentation, which agreed to the study by Louis et al.90 Acetate production for both AG treatments showed a significant increase from 6 h and further down until the end of fermentation compared to the baseline. All substrates on the other hand showed a significantly higher acetate concentration than the negative control throughout the fermentation period. The fermentation exhibits an increasing trend for acetate production in AG1 and AG2, while FOS has a sudden drop at 24 h after the initial elevation until 12 h. Some Bifidobacteria strains have been characterized in terms of acetate and lactate production.91−93Bifidobacteria may produce acetate and lactate when carbohydrates are available in excess.94
The FOS achieved its significant peak at 12 h with the highest amount of acetate compared to other treatments (Figure 4). The fermentation of FOS exhibits a sudden drop at 24 h after the initial elevation until 12 h. Acetate is produced by Bifidobacteria via the process of the fructose-6-phosphate phosphoketolase pathway and high acetate production commonly relating to the increased population of this group.95 Acetate is known for anti-inflammatory properties and combat against pathogens.96 Other than Bifidobacteria, approximately one-third of the total colonic bacteria, which includes some proteobacteria, are acetogenic, which synthesize through the Wood–Ljungdahl pathway.88,97−99 Additionally, Garrett and Onderdonk (2015) also reported that Bacteroidaceae spp., Prevotellaceae spp., and Porphyromonadaceae spp. produce acetate from the fermentation of carbohydrate.100
The quantity of propionate exhibits an increasing trend at 6, 12, and 24 h of fermentation compared to 0 h except for negative control with significant elevation at 6 h before it drastically drops further having the least amount of propionate at 24 h. As proposed by Hosseini et al., (2011), Clostridium cluster XI may able to produce propionate.101 However, in the present work, no significant change in Clostridium cluster XI population was observed for the tested substrates. Propionate may contribute directly to central appetite regulation; increasing satiety and regulating food intake by the host.102−105 According to studies, the producers of propionate are mainly the Bacteroides-Prevotella group as well as Clostridium within the colonic bacteria.106−110 Their metabolism pathway and SCFA production in the bacteria are largely unknown.
Two genome sequences belonging to methanogens have been described; however, their physiological importance is yet to be discovered. Generally, the relation would be involving microbial relationship common interaction known as competition and cross-feeding. This may be related to the significant increment of the Bacteroides group in fermentation for all substrates at 6, 12, and 24 h, which is significantly higher than the number in negative control at 24 h. The fermentation of AG provides sufficient nutrients for Bacteroides growth due to their capability in utilizing carbohydrate sources.88,99,100,111
Butyrate production shows a decreasing trend from 6 h of fermentation period for AG1 (Figure 3) and AG2 (Figure 5). It is reported that lactate and acetate are also being utilized by colonic bacteria and contribute to butyrate production as well.112 Based on previous studies, the main butyrogenic bacteria belong to the Firmicutes phylum, specifically in the Clostridial group of bacteria.113−115 Both AGs showed a similar butyrate trend with initial elevation at 6 h, which then reduced further down the fermentation period.
Meanwhile, butyrate concentration in FOS increased steadily with significant elevation at 12 h. While most of the butyrate (70.0%) ended up as the energy source by the epithelial cells, the host could have benefited from the butyrate abundance in the lumen. This commensal producer includes Clostridium spp., Eubacterium spp., Roseburia spp., and firmicutes such as Ruminococcus and Faecalibacterium.(116) At the same time, other bacteria will also take advantage of the available butyrate in the lumen, which may explain that it is not accumulated (the rate of butyrate produced is similar to the rate of butyrate utilized, reaching equilibrium). Negative control was observed with the highest amount of butyrate compared to other substrates at 6 and 12 h (Figure 6). The accumulation of the butyrate might indicate an imbalance or higher numbers of butyrate producers rather than the rate of where it is being utilized.
The acetate to propionate ratio was observed the highest, for negative control at 6 and 12 h compared with other substrates at the respective time points, while FOS has the highest ratio at 24 h of fermentation. AG is observed with a low acetate/propionate ratio. According to the studies by Sarbini et al.,117 a combination of acetate and propionate is reported to be involved in regulating cholesterol levels. Acetate plays an important role as the precursor of synthesizing cholesterol, whereas propionate inhibits it.118 Hence, it is proposed that a low ratio of acetate/propionate contributes to cholesterol management, thus decreasing the risk of obesity, hypertension, and even coronary disorders. Even so, some research emerged with the evidence of AG having these antiobesity properties.119,120
Studies on arabinogalactan fraction were suggested to confer a potential effect at the distal part of the colon, whereas FOS fermentation was vigorous while in the proximal colon.86 Furthermore, an increase in the total bacteria may be associated with the increase of Bacteroides and the Bifidobacteria, together with an increase in F. prausnitzii and also an increase in lactate, butyrate, and propionate at the same time a reduction of ammonia in the distal colon. The saccharolytic bacteria then underwent carbohydrate fermentation to produce SCFAs, which can confer positive effects to the host. Overall, the SCFA decreases the pH in the colon, which hampers pathogenic bacterial colonization and growth and activates the immune response of the host.
According to this study, several bacterial groups responded to AG fermentation either positively supported or negatively inhibited by the fermentation outcomes. The nondigestible AG was fermented by mixed fecal bacteria in anaerobic conditions to yield SCFAs and use for their proliferation.121 In the human body, most of these SCFAs produced usually were immediately absorbed from the lumen into the host peripheral for its energy, whereas in colon model fermentation, available SCFA will be transformed or utilized by the commensal bacteria. In this regard, these commensal bacteria suggested having a potential role in long-chain arabinoxylan fermentation toward the production of butyrate while also showing that B. longum grows well with known butyrate producers (Roseburia intestinalis, Eubacterium rectale, and Anaerostipes caccae).114 Together with the commensal bacteria, B. longum is a significant producer of intracellular thiamine and exopolysaccharides, which is also beneficial to other gut bacteria.122,123 Thus, this cooperation was very much beneficial for both parties in one way or the other.
Previous research aiming to identify the primary degraders of AG found several different bacterial species and has not been consistent. A study using porcine fecal inoculum with 2.00% of AG managed to isolate Bifidobacterium longum and Bacteroides ovatus, Bacteroides oris, and Bacteroides buccae.(124) Although the researchers concluded those results in human fecal, it turns out that presumptive measures led to their conclusions based only on the culture plating techniques but not molecular-based DNA sequencing. Therefore, the traditional identification of many uncultured species assigned to this genus may reflect current taxonomic limitations rather than a biological signal. Kishimoto et al. in 2006 have found that the predominant microbes responsible for AG fermentation contribute to the production of propionate, which is the Prevotella ruminicola-like bacterium.125 The study investigated pooled cecal inoculum of pig, showing that the isolated bacteria were the predominant species. This study also highlighted that the isolated bacteria were producing propionate, which is largely produced from the lactate conversion.126−128 However, this outcome was the work from the bacterial isolation from the enrichment culture of pig cecal slurry, where the anatomical and physiological differences of humans limit the comparison.
Degradation of energy-abundant complex carbohydrates provides opportunities not just for competition but also for mutualistic and cooperation by metabolic cross-feeding and by one or more bacterial species providing substrates to support the growth of other populations.129 Nondigestible carbohydrates, such as prebiotics, emerged with findings that their properties counteract diseases linked to obesity, including hyperglycemia, inflammation, and hepatic steatosis. While most of the discoveries were from animal studies, the effect was observed by the alterations in microbial compositions, which appear more complex than just by the single increase in Bifidobacterium numbers as most studies described. After all the healthy gut functions was the reflection of the whole composition of gut bacteria instead of one particular species.130
3. Conclusions
Current research increases the clarity for AG polysaccharides that could have been exploited as potential prebiotics as good as FOS. The fermentation shows selectivity of AG in promoting particular bacteria, which indirectly induce the luminal pH changes to inhibit pathogenic bacteria. Furthermore, slow fermentation of AG signifies suitability and good relief for people who suffer bloating on ingestion of FOS. In addition, AG2 (contained A. seyal) showed superior prebiotic effects to some extent compared to AG1. To better understand the implications of these results, however, more studies are needed to yield high consistency and accuracy of data using larger sample sizes especially in the study of pure A. seyal species. The property of AG as high dietary fiber enables it to be a good additive ingredient. This on the other hand raised arguments for researchers and food manufacturers to revisit food products that could take advantage of this knowledge on the food additive plus prebiotic potential sides of AG. However, it also raises the question of the possibility to isolate and identify first degrader or gum fermenting bacteria as mentioned in the discussion above. Further research would be appropriate to determine the causes or relationship between gut microbiota in prebiotic metabolism.
4. Materials and Methods
In this study, the substrate used was premium grade AG (Natural Prebiotic Sdn. Bhd., Malaysia), which is a water-soluble, free-flowing powder form. Two commercially available AG samples from the species of Acacia senegal (AG1) and the mixture of Acacia senegal and Acacia seyal (AG2) were investigated. This commercial product is available in the market, sold as dietary fiber drink. The control substrate used was FOS, Orafti HP derived from inulin (Beneo, Belgium).
4.1. Proximate Analysis of AG
Proximate analysis of crude protein content, fat content, ash content, moisture content, and carbohydrate content was carried out according to the 21st Edition AOAC methods.131 The protocol described from here onward was based on the AOAC methods.
4.2. Crude Protein Content
The Kjeldahl method was used to determine the total crude protein content in the AG. An amount of 0.5 g of AG was weighed into a digestion flask. An amount of 5 g of catalyst (with K2SO4 and CuSO4 at a ratio of 10:1) was added followed by 12 mL of concentrated sulphuric acid (H2SO4) and mixed by gently swirling the flask. The flask then heated with the digestion unit until a transparent slight blue solution developed. An amount of 75 mL of distilled water and 50 mL of 40.0% (w/v) sodium hydroxide (NaOH) were added afterward. Boric acid and the Kjeldahl flask were placed into a distiller (Kjeltec TM 211, Protein Analyser), and the distillation process was carried out until a greenish-blue solution was formed. Next, the boric acid was titrated with 0.1 N hydrochloric acid (HCl) until the solution turns red and the titration volume was recorded. The nitrogen content (%) in the sample was calculated, and the protein content (%) was obtained with the equation below:
| 1 |
| 2 |
4.3. Fat Content
The Soxhlet method was used in the determination of fat content in the sample. An amount of 2 g of AG sample was weighed and wrapped in filter paper prior to placing into an extraction thimble, which consists of pores that allowed the flow of hexane (C6H14) for the fat extraction process. The initial weight of the empty extraction cup was recorded, and 200 mL of hexane was placed into the extraction cup and fit into the Soxhlet extraction system (Soxtex system HT). The extraction process was carried out for 2 to 2.5 h. The final weight of the extraction cup that contains fat was then weighed and recorded. The fat content (%) was calculated by the equation as follow:
| 3 |
4.4. Ash Content
Ash content was determined by the method of “dry ashing”. An amount of 5 g of sample was weighed into a crucible with a lid prior to the overnight combustion process in the furnace (Nabertherm Model 205311) at 450 to 550 °C. The ash content (%) was calculated as below:
| 4 |
4.5. Moisture Content
The moisture content of AG was determined by the process of oven drying. An amount of 5 g of AG sample was weighed and spread in an aluminium container with a lid and dried in a drying oven (SM400, Memmert) at 105 °C for at least 16 h until a constant weight is obtained. The initial weight of the sample together with the aluminium container was recorded as well as the final weight after the drying process.
| 5 |
whereby W1 = Weight of the sample and container before oven drying; W2 = Weight of the sample and container after oven drying.
4.6. Total Carbohydrate Content
The total carbohydrate content was obtained by the different method of subtracting with the total protein content, fat content, moisture content, fiber content, and ash content. The carbohydrate content was obtained from the equation below:
| 6 |
4.7. In Vitro Digestion
In vitro digestion was performed as described in Mandalari et al. (2008) to simulate the upper gastrointestinal environment.41 Substrates (1.5 g) were suspended in 0.1 M NaCl solution (12.4 mL) at pH 7.0. The predigestion in the oral phase was initiated by adding heat-stable α-amylase into the suspension and incubating at 37 °C for 5 min. The amylase was prepared by adding 500 U/500 μL of the stock solution in NaH2PO4 buffer (20 mM).
The suspension was then further carried on in simulated gastric phase digestion. Generally, the suspension was added with 150 mM NaCl (12.4 mL) under the acidic condition at pH 2.5 readjusted using HCl. Pepsin and gastric lipase were then added with a final concentration of 146 U/mL and 0.56 mg/mL, respectively. This afforded a ratio of substrate to aqueous of 0.12 g/mL. Incubation was carried out in an orbital shaking incubator at 170 rpm, 37 °C for 2 h. Controls used were substrates in the saline solution (150 mmol/L) at pH 2.5 without the addition of any enzymes.
In vitro duodenal digestion was further applied to the digesta of gastric digestion. The duodenal digestion was performed at 37 °C, pH 6.5, and a shaking rate of 170 rpm for 1 h incubation. Based on the weight of the substrate used at the beginning of gastric digestion, the addition of NaOH, Bis-Tris solution, CaCl2, bile salts, and enzymes (150 mmol/L) brings the final ratio of substrate to aqueous at 0.11 g/mL. These include addition of sodium glycodeoxycholate (4.0 mmol/L), Bis-Tris buffer (0.73 mmol/L, pH 6.5), calcium chloride (11.7 mmol/L), pancreatic lipase (54 U/mL), trypsin (104 U/mL), colipase (3.2 mg/mL), and α-chymotrypsin (5.9 U/mL). The digesta from the duodenal digestion was then freeze-dried for further analyses.
4.8. Determination of Digestibility
The percentage of digestibility (hydrolysis) of AG was calculated based on the reducing sugar that liberated and the total sugar content of AG132 as follows:
| 7 |
where the reducing sugar released is the difference between the final reducing sugar and the initial reducing sugar content.
4.9. Carbohydrate Assay
Digestibility of AG samples was determined following the in vitro human α-amylase, gastric, and duodenal digestion compared with FOS, as control. The changes of total carbohydrate and total reducing sugar were analyzed for samples before digestion and after digestion.
The phenol-sulphuric acid assay was used in total carbohydrate determination, whereas the 3,5-dinitrosalicylic acid (DNS) assay was used for total reducing sugar quantification. The former was extensively used in the large range from measuring mono-, di-, oligo-, and polysaccharides due to its reliability and a straightforward operation.133,134 The concept employed the absorbance value (490 nm) of hydroxyl methyl furfural in hot acid. This dehydrated glucose develops different intensities of yellowish-brown color, which correspond to the carbohydrate content in the quantified sample,135,136 while in the DNS assay, it acted differently where this method tests for the presence of free carbonyl groups to specifically oxidized available reducing sugar in the samples, thus reduced the DNS reagent from yellow to orange-red intensity (3-amino,5-nitrosalicylic acid), and was measured at 540 nm absorbance wavelength.137
4.9.1. Determination of Reducing Sugar Content
Reducing sugar content in AG was determined by using the DNS method138 in which the DNS reacted with reducing sugars of AG broken down by the amylase. An amount of 1 mL of sample was added with 1 mL of DNS solution and 1 mL of water. The mixture was brought to a boil for 15 min and cooled in an ice water bath for 2 min followed by the addition of 9 mL of water. The absorbance value of the mixture was read at 540 nm (Epoch Microplate Spectrophotometer) and was compared to a glucose solution (1–10 mg/mL) standard curve to calculate the concentration of reducing sugar in the sample.
4.9.2. Determination of Total Sugar Content
The total sugar content was determined using a phenol–sulphuric acid method.135 The sample (2 mL) was mixed with 1 mL of phenol solution (5.00%) followed by the addition of concentrated sulphuric acid (5 mL).133 The mixture was well-mixed and incubated for 30 min at 30 °C. The absorbance was then measured at 490 nm.139
4.9.3. Determination of Carbohydrate Size
Determination of oligosaccharide size available in the tested samples was based on the DP. The DP of FOS and AG was calculated based on the amounts of total sugar and reducing sugar obtained from the previous assays. The following equation was used to calculate the DP:140
| 8 |
4.10. In vitro Fermentation
4.10.1. Fecal Sample Preparation
Fecal sample preparation used was as described in the study of Sarbini et al.129 Fresh fecal samples were obtained from four healthy male human volunteers added between 22–30 years old with a BMI of 19–23 kg/m2. All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted following the Declaration of Helsinki, and the protocol was approved by the Ethics Committee for Research involving Human Subjects of Universiti Putra Malaysia (JKEUPM-2020-117). The volunteers also required to have no history of gastrointestinal disorder, no intake of pro- or prebiotics for at least 1 month before the study and were not prescribed any antibiotics for 6 months. The fresh fecal samples were collected on-site and immediately used for fermentation within 15 min. The fecal samples were then diluted and homogenized with sterile phosphate-buffered saline (pH 7.3) at a ratio of 1:10 (w/v) using a stomacher (Seward) set at 120 rpm for 2 min.
4.10.2. Colon Model Fermentation
The following in vitro fermentation was conducted in the colon model as described by Sarbini et al.129 and Bajury et al.141 The colon model fermentation system was set up by using stirred batch culture vessels. Four independent batch culture fermentation was carried out using feces from four different donors. Each sterile vessel was then aseptically filled with 45 mL of sterile basal nutrient medium (BNM). The BNM was prepared with 2.0 g/L of peptone water, 2.0 g/L of yeast extract, 0.1 g/L of sodium chloride, 0.04 g/L of potassium dihydrogen phosphate, 0.01 g/L of magnesium sulphate heptahydrate, 0.01 g/L of calcium chloride hexahydrate, 2.0 g/L of sodium bicarbonate, 2.0 mL of tween 80, 0.05 g/L of hemin, 10 μL/L of vitamin K, 0.5 g/L of L-cysteine HCl, and 0.5 g/L of bile salts. The BNM was adjusted to pH 7.0 before the addition of 4 mL/L of 0.025% (w/v) resazurin solution before autoclaving. The BNM-containing vessels were then gassed overnight with oxygen-free nitrogen at a flow rate of 15 mL/min to obtain an anaerobic condition.
The next day, AG as the substrate (1.00%) was added into the vessels accordingly just before the addition of the fecal slurry. The vessels were then maintained at 37 °C and pH 6.8, using a circulating water bath (CW-05G, Lab Companion) and pH controllers (Fermac 260, Electrolab). Adjustment of pH was achieved by using 0.5 M NaOH and/or 0.5 M HCl. The vessels were stirred continuously using magnetic stirrers (UC151, 573,341 Stuart). Each vessel was further inoculated with 5 mL of a fresh fecal slurry prepared as described previously. Anaerobic conditions were maintained throughout the fermentation by sparging the vessels with oxygen-free nitrogen gas (15 mL/min). The fermentation vessels were left running for 24 h, and the samples were taken at 0, 6, 12, and 24 h. The samples were centrifuged in preparation for high-performance liquid chromatography (HPLC) analysis or prepared for bacterial enumeration by fluorescent in situ hybridization (FISH).
4.11. Prebiotic Evaluation
4.11.1. Microbial Analysis
The bacterial population in each sampling period was assessed by using the FISH method as described by Daims et al.142 Synthetic oligonucleotide probes targeting specific regions of the 16S rRNA molecule labeled with a fluorescent dye Cy3 were utilized for the enumeration of different bacterial groups (Bif164 for Bifidobacterium spp. (BIF), Lab158 for Lactobacillus/Enterococcus (LAB), Ato291 for Atopobium cluster (Atopobium, Coriobacterium, and Collinsella spp.) (ATO), Chis150 for Clostridium histolyticum group (CHIS), Fpra655 for Faecalibacterium prausnitzii (FPRA), Prop853 for Clostridium cluster XI (PROP), Erec482 for Eubacterium rectale – Clostridium coccoides group (EREC), Bac303 for Bacteroides-Prevotella group (BAC), and the EUB338 mixture consisting of EUB338I, EUB338II, and EUB338III for domain bacteria (Total)) (Table 3).
Table 3. 16S rRNA Oligonucleotide Probes Used in the Present Study.
| probe code | specificity | sequence (5′ to 3′) | temperature condition (°C) | ||
|---|---|---|---|---|---|
| hybridization | washing | ||||
| Chis150 | most of the bacteria in the Clostridium histolyticum (Clostridium clusters I and II) | TTATGCGGTATTAATCTYCCTTT | 50 | 50 | Franks et al.143 |
| Lab158 | Lactobacillus-Enterococcus group | GGTATTAGCAYCTGTTTCCA | 50 | 50 | Harmsen et al.144 |
| Erec482 | most of the bacteria in the Clostridium coccoides-Eubacterium rectale group (Clostridium clusters XIVa and XIVb) | GCTTCTTAGTCARGTACCG | 50 | 50 | Franks et al.143 |
| Bif164 | Bifidobacterium spp. | CATCCGGCATTACCACCC | 50 | 50 | Langendijk et al.145 |
| Bac303 | most Bacteroidaceae and Prevotellaceae, some Porphyromonadaceae | CCAATGTGGGGGACCTT | 46 | 48 | Manz et al.146 |
| Ato291 | Atopobium spp. | GGTCGGTCTCTCAACCC | 50 | 50 | Harmsen et al.147 |
| Prop853 | Clostridium cluster XI | ATTGCGTTAACTCCGGCAC | 50 | 50 | Walker et al.148 |
| Fpra655 | Faecalibacterium prausnitzii | CGCCTACCTCTGCACTAC | 58 | 58 | Hold et al.149 |
| EUB338a | GCTGCCTCCCGTAGGAGT | ||||
| EUB338IIa | total bacteria | GCAGCCACCCGTAGGTGT | 46 | 48 | Daims et al.142 |
| EUB338IIIa | GCTGCCACCCGTAGGTGT | ||||
These probes are used together in equimolar concentrations (all at 50 ng μL–1).
A sample (375 μL) obtained from each vessel at sampling time underwent fixation for 4 h in 1125 μL of 4% (w/v) paraformaldehyde at 4 °C. Samples were then centrifuged at 13000 × g for 5 min and then washed with 1 mL of filter-sterilized 1XPBS two times. The washed cells were re-suspended in 150 μL of filtered 1 × PBS; then, 150 μL of ethanol (99.9%) was added and stored at −20 °C before further analysis. Samples (10 μL) were appropriately diluted with 1 × PBS to obtain 30 to 100 fluorescent cell counts in each field of view. Then, 20 μL of the diluted sample was placed onto wells of Teflon poly-l-lysine-coated slide (Tekdon Inc., Florida, US). The slide was then dried using a slide dryer for 15 min at 46 °C.
After this, the dried slide was dehydrated in an alcohol series (50.0, 80.0, and 96.0% [v/v] ethanol) for 3 min each. Slides were returned to the slide dryer to let excess ethanol evaporate before the hybridization mixture was added. The hybridization mixture (50 μL, consisting of 5 μL probe and 45 μL of hybridization buffer) was added to each well, and slides were incubated in a hybridization oven (Grant-Boekel, Cambridge, UK) for 4 h. After hybridization, slides were soaked in 50 mL of washing buffer and warmed at the appropriate temperature for each probe for 15 min, in which the washing buffer was made up with 0.9 M NaCl, 0.02 M Tris/HCl (pH 8.0), and 0.005 M ethylenediaminetetraacetic acid solution (pH 8.0). Slides were then briefly dipped (2–3 s) in cold water and dried under a stream of compressed air. The polyvinyl alcohol mounting medium (5 μL), i.e., 1,4-diazabicyclo[2.2.2]octane (DABCO), was then added to each well. A coverslip (20 mm; thickness no. 1; VWR, Lutterworth, UK) was further applied. Slides were stored in the dark at 4 °C overnight before viewing. The bacterial cells were then observed and enumerated using an epifluorescence microscope (CX31; Olympus; Tokyo; Japan) equipped with a CX-RFL-2 reflected fluorescence attachment. For each well, 15 random different fields were viewed.129
4.11.2. Organic Acid Analysis
The fermentation sample from each sampling period was pipetted into a 2 mL microcentrifuge tube for centrifugation (Centrifuge-5804, Eppendorf) at 13,000 rpm for 10 min to obtain a clear supernatant. The supernatant was filtered through a 0.22 μm syringe filter unit (Millipore) into an HPLC vial (Agilent Technologies, Cheshire, UK).
A Prominence Series liquid chromatography instrument (Shimadzu Corp., Japan) with a reverse-phase ion-exclusion C12 column (Rezex ROA, Phenomenex) was used for the analysis of SCFAs. The analytes were read with a UV detector at 210 nm wavelength. The isocratic mobile phase used was 0.25 mM sulphuric acid (H2SO4). An amount of 15 μL of sample was injected into the heated column (40 °C) programmed to run in isocratic elution at a flow rate of 0.5 mL/min for 40 min. The peaks and response factor within the sample were calibrated and calculated using LC Solutions software (Shimadzu). The standard solution contained acetate, butyrate, propionate, and also lactate at a series concentration of 12.5, 25, 50, 75, 100, 125, 150, 175, and 200 mM.
4.12. Statistical Analysis
Data on nutritional content from the proximate analysis were presented as the mean value based on triplicate analyses, whereas mean values from the in vitro digestion of each sample were analyzed using the paired T-test comparing the difference between before and after digestion. All data of bacterial enumeration and quantification of organic acids were statistically analyzed according to repeated-measures ANOVA. Based on Tukey’s test, p ≤ 0.05 was considered statistically significant. The software used was Statistical Analysis System (SAS) version 9.4.
Acknowledgments
The authors would like to acknowledge staff at the Department of Crop Science, UPM Bintulu Campus, especially our laboratory assistants, Miss Siti Aziah Kushairi, Madam Siti Fatimah Razali, and Miss Georgina Sylvia Niwin for their assistance and cooperation throughout the research. The authors would like to express special acknowledgments to Miss Tan Hui Yan for her writing assistance in finalizing the manuscript.
Glossary
Abbreviations
- AG
Acacia gum
- SCFA
short-chain fatty acid
- FOS
fructo-oligosaccharide
- FISH
Fluorescent in situ hybridization
- HPLC
High-performance liquid chromatography
- IBD
Inflammatory bowel disease
This research was funded by the Ministry of Science, Technology and Innovation, (MOSTI) Malaysia, grant number 5450719.
The authors declare no competing financial interest.
References
- Mariod A. A.Gum Arabic: Structure, properties, application and economics; Academic Press, 2018. [Google Scholar]
- Kiiru S. N.; Mahungu S. M.; Omwamba M. Preparation and analysis of goat milk mozzarella cheese containing soluble fiber from Acacia senegal var. kerensis. Afr. J. Food Sci. 2018, 12, 46–53. 10.5897/AJFS2017.1652. [DOI] [Google Scholar]
- Williams P. A.; Phillips G. O.. Gum Arabic. In Handbook of Hydrocolloids (Second Edition); Phillips G. O., Williams P. A., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing, 2009; 252–273. 10.1533/9781845695873.252. [DOI] [Google Scholar]
- Masuelli M. A. Hydrodynamic properties of whole arabic gum. Am. J. Food Sci. Technol. 2013, 1, 60–66. 10.12691/ajfst-1-3-9. [DOI] [Google Scholar]
- Roberfroid M.; Gibson G. R.; Hoyles L.; McCartney A. L.; Rastall R.; Rowland I.; Wolvers D.; Watzl B.; Szajewska H.; Stahl B.; Guarner F.; Respondek F.; Whelan K.; Coxam V.; Davicco M.-J.; Léotoing L.; Wittrant Y.; Delzenne N. M.; Cani P. D.; Neyrinck A. M.; Meheust A. Prebiotic effects: Metabolic and health benefits. J. Geophys. Res. Oceans 2010, 104, S1–S63. 10.1017/S0007114510003363. [DOI] [PubMed] [Google Scholar]
- Nakov G.; Georgieva D.; Ivanova N.; Damyanova S.; Stamatovska V.; Necinova L.. Prebiotic effects of inulin and acacia gum. Food Environ. Saf. J.2016, 14 ( (2), ). [Google Scholar]
- Pourabedin M.; Guan L.; Zhao X. Xylo-oligosaccharides and virginiamycin differentially modulate gut microbial composition in chickens. Microbiome 2015, 3, 15. 10.1186/s40168-015-0079-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson G. R.; Hutkins R.; Sanders M. E.; Prescott S. L.; Reimer R. A.; Salminen S. J.; Scott K.; Stanton C.; Swanson K. S.; Cani P. D.; Verbeke K.; Reid G. Expert consensus document: the international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- FAO/WHO . Guidelines for the evaluation of probiotics in food. World Health Organisation (WHO), London, Ontario, Canada: 2002. [Google Scholar]
- Min M.; Bunt C. R.; Mason S. L.; Hussain M. A. Non-dairy probiotic food products: an emerging group of functional foods. Crit. Rev. Food Sci. Nutr. 2019, 59, 2626–2641. 10.1080/10408398.2018.1462760. [DOI] [PubMed] [Google Scholar]
- Calame W.; Weseler A. R.; Viebke C.; Flynn C.; Siemensma A. D. Gum arabic establishes prebiotic functionality in healthy human volunteers in a dose-dependent manner. J. Geophys. Res. Oceans 2008, 100, 1269–1275. 10.1017/S0007114508981447. [DOI] [PubMed] [Google Scholar]
- Cherbut C. Motor Effects of short-chain fatty acids and lactate in the gastrointestinal tract. Proc. Nutr. Soc. 2003, 62, 95–99. 10.1079/PNS2002213. [DOI] [PubMed] [Google Scholar]
- Turnbaugh P. J.; Hamady M.; Yatsunenko T.; Cantarel B. L.; Duncan A.; Ley R. E.; Sogin M. L.; Jones W. J.; Roe B. A.; Affourtit J. P.; Egholm M.; Henrissat B.; Heath A. C.; Knight R.; Gordon J. I. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X.; Zhao H.; Liu Z.; Sun X.; Zhang D.; Wang S.; Xu Y.; Zhang G.; Wang D. Modulation of gut microbiota by fucoxanthin during alleviation of obesity in high-fat diet-fed mice. J. Agric. Food Chem. 2020, 68, 5118–5128. 10.1021/acs.jafc.0c01467. [DOI] [PubMed] [Google Scholar]
- Kamal E.; Kaddam L. A.; Dahawi M.; Osman M.; Salih M. A.; Alagib A.; Saeed A. Gum arabic fibers decreased inflammatory markers and disease severity score among rheumatoid arthritis patients, phase II trial. Int. J. Rheumatol. 2018, 2018, e4197537 10.1155/2018/4197537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali B. H.; Al Za’abi M.; Al Suleimani Y.; Manoj P.; Ali H.; Ribeiro D. A.; Nemmar A. Gum arabic reduces inflammation, oxidative, and nitrosative stress in the gastrointestinal tract of mice with chronic kidney disease. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 1427–1436. 10.1007/s00210-020-01844-y. [DOI] [PubMed] [Google Scholar]
- Opstelten J. L.; Plassais J.; van Mil S. W. C.; Achouri E.; Pichaud M.; Siersema P. D.; Oldenburg B.; Cervino A. C. L. Gut microbial diversity is reduced in smokers with crohn’s disease. Inflamm. Bowel Dis. 2016, 22, 2070–2077. 10.1097/MIB.0000000000000875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao X.; Sun C.; Tang X.; Zhang X.; Han D.; Liang S.; Qu R.; Hui X.; Shan Y.; Hu L.; Fang H.; Zhang H.; Wu X.; Chen C. Anti-inflammatory and intestinal microbiota modulation properties of jinxiang garlic (Allium sativum l.) polysaccharides toward dextran sodium sulfate-induced colitis. J. Agric. Food Chem. 2020, 68, 12295–12309. 10.1021/acs.jafc.0c04773. [DOI] [PubMed] [Google Scholar]
- Scher J. U.; Ubeda C.; Artacho A.; Attur M.; Isaac S.; Reddy S. M.; Marmon S.; Neimann A.; Brusca S.; Patel T.; Manasson J.; Pamer E. G.; Littman D. R.; Abramson S. B. Decreased Bacterial diversity characterizes the altered gut microbiota in patients with psoriatic arthritis, resembling dysbiosis in inflammatory bowel disease. Arthritis Rheumatol. 2015, 67, 128–139. 10.1002/art.38892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Goffau M. C.; Luopajärvi K.; Knip M.; Ilonen J.; Ruohtula T.; Härkönen T.; Orivuori L.; Hakala S.; Welling G. W.; Harmsen H. J. Fecal microbiota composition differs between children with β-cell autoimmunity and those without. Diabetes 2013, 62, 1238–1244. 10.2337/db12-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth S. M.; Carson T.; Lowe J.; Ramaraj T.; Leff J. W.; Luo L.; Bell C. J.; Shah V. O. Composition, diversity and abundance of gut microbiome in prediabetes and type 2 diabetes. J. Diabetes Obes. 2015, 2, 108–114. 10.15436/2376-0949.15.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M.; Karlsson C.; Olsson C.; Adlerberth I.; Wold A. E.; Strachan D. P.; Martricardi P. M.; Åberg N.; Perkin M. R.; Tripodi S.; Coates A. R.; Hesselmar B.; Saalman R.; Molin G.; Ahrné S. Reduced diversity in the early fecal microbiota of infants with atopic eczema. J. Allergy Clin. Immunol. 2008, 121, 129–134. 10.1016/j.jaci.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Manichanh C.; Rigottier-Gois L.; Bonnaud E.; Gloux K.; Pelletier E.; Frangeul L.; Nalin R.; Jarrin C.; Chardon P.; Marteau P.; Roca J.; Dore J. reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut 2006, 55, 205–211. 10.1136/gut.2005.073817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schippa S.; Iebba V.; Barbato M.; Di Nardo G.; Totino V.; Checchi M. P.; Longhi C.; Maiella G.; Cucchiara S.; Conte M. P. A distinctive “microbial signature” in celiac pediatric patients. BMC Microbiol. 2010, 10, 175. 10.1186/1471-2180-10-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menni C.; Lin C.; Cecelja M.; Mangino M.; Matey-Hernandez M. L.; Keehn L.; Mohney R. P.; Steves C. J.; Spector T. D.; Kuo C.-F.; Chowienczyk P.; Valdes A. M. Gut microbial diversity is associated with lower arterial stiffness in women. Eur. Heart J. 2018, 39, 2390–2397. 10.1093/eurheartj/ehy226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha M. S.; El-Sherbiny E. M.; Osman H. F. Anti-ulcerogenic activity of gum arabic in gastric mucosal injury induced by ethanol in male albino rats. Appl. Physiol. Nutr. Metab. 2020, 45, 731–736. 10.1139/apnm-2018-0233. [DOI] [PubMed] [Google Scholar]
- Adejoro F. A.; Hassen A.; Thantsha M. S. Characterization of starch and gum arabic-maltodextrin microparticles encapsulating acacia tannin extract and evaluation of their potential use in ruminant nutrition. Asian-Australas J. Anim. Sci. 2019, 32, 977–987. 10.5713/ajas.18.0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali I. A. K. E.Use of acacia gum in the treatment of skin lesions of two children with Kwashiorkor. In Gum Arabic; Mariod A. A., Ed.; Academic Press, 2018; 221–228. 10.1016/B978-0-12-812002-6.00018-X. [DOI] [Google Scholar]
- Awad S. S.; Rabah A. A.; Ali H. I.; Mahmoud T. E.. Acacia seyal gums in Sudan: Ecology and economic contribution. In Gum Arabic; Mariod A. A., Ed.; Elsevier, 2018; 3–11. 10.1016/B978-0-12-812002-6.00001-4. [DOI] [Google Scholar]
- Lopez-Torrez L.; Nigen M.; Williams P.; Doco T.; Sanchez C. Acacia senegal vs. Acacia seyal gums – Part 1: Composition and structure of hyperbranched plant exudates. Food Hydrocolloids 2015, 51, 41–53. 10.1016/j.foodhyd.2015.04.019. [DOI] [Google Scholar]
- ElKhawad H. E.Gum arabic processing and marketing in the Sudan. PhD Thesis, MSc. thesis in Chemical Engineering, University of Khartoum, 2008. [Google Scholar]
- Ibrahim O.; Osman M. E.; Hassan E. A. Characterization and simple fractionation of Acacia senegal. J. Chem. Acta 2013, 2, 11–17. [Google Scholar]
- Altuntas E.; Erkol M. The effect of moisture content on colour characteristics of walnuts. Int. J. Food Eng. 2009, 5, 13. 10.2202/1556-3758.1577. [DOI] [Google Scholar]
- Al-Assaf S.; Phillips G. O.; Williams P. A. Studies on acacia exudate gums. Part I: The molecular weight of Acacia senegal gum exudate. Food Hydrocolloids 2005, 19, 647–660. 10.1016/j.foodhyd.2004.09.002. [DOI] [Google Scholar]
- Renard D.; Lavenant-Gourgeon L.; Lapp A.; Nigen M.; Sanchez C. Enzymatic hydrolysis studies of arabinogalactan-protein structure from acacia gum: The self-similarity hypothesis of assembly from a common building block. Carbohydr. Polym. 2014, 112, 648–661. 10.1016/j.carbpol.2014.06.041. [DOI] [PubMed] [Google Scholar]
- Siddig N. E.; Osman M. E.; Al-Assaf S.; Phillips G. O.; Williams P. A. Studies on acacia exudate gums, Part IV. Distribution of molecular components in Acacia seyal in relation to Acacia senegal. Food Hydrocolloids 2005, 19, 679–686. 10.1016/j.foodhyd.2004.09.005. [DOI] [Google Scholar]
- Idris O. H. M.; Haddad G. M.. Gum arabic’s (gum acacia’s) journey from tree to end user. In Gum Arabic; 2011; 3–17. 10.1039/9781849733106-00003. [DOI] [Google Scholar]
- Awad S. S.; Rabah A. A.; Ali H. I.; Mahmoud T. E.. Acacia seyal gums in Sudan: A review. University of Khartoum Engineering Journal; 2017. [Google Scholar]
- Food and Agriculture Organization (FAO) of the United Nations . Specifications for identity and purity of certain food additives: Emulsifiers, enzyme preparations, flavouring agents, food colours, thickening agents, miscellaneous food additives; Food and Nutrition Paper: Rome, Italy, 1990. [Google Scholar]
- Azzaoui K.; Hammouti B.; Lamhamdi A.; Mejdoubi E.; Berrabah M. The gum arabic in the southern region of Morocco. Mor. J. Chem. 2015, 3, 3–1. [Google Scholar]
- Mandalari G.; Nueno-Palop C.; Bisignano G.; Wickham M. S. J.; Narbad A. potential prebiotic properties of almond (Amygdalus Communis L.) Seeds. Appl. Environ. Microbiol. 2008, 74, 4264–4270. 10.1128/AEM.00739-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodkorb A.; Egger L.; Alminger M.; Alvito P.; Assunção R.; Ballance S.; Bohn T.; Bourlieu-Lacanal C.; Boutrou R.; Carrière F.; Clemente A.; Corredig M.; Dupont D.; Dufour C.; Edwards C.; Golding M.; Karakaya S.; Kirkhus B.; Le Feunteun S.; Lesmes U.; Macierzanka A.; Mackie A. R.; Martins C.; Marze S.; McClements D. J.; Ménard O.; Minekus M.; Portmann R.; Santos C. N.; Souchon I.; Singh R. P.; Vegarud G. E.; Wickham M. S. J.; Weitschies W.; Recio I. INFOGEST Static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. 10.1038/s41596-018-0119-1. [DOI] [PubMed] [Google Scholar]
- Ross A. H. M.; Eastwood M. A.; Brydon W. G.; Busuttil A.; McKay L. F.; Anderson D. M. W. A study of the effects of dietary gum arabic in the rat. J. Geophys. Res. Oceans 1984, 51, 47–56. 10.1079/BJN19840008. [DOI] [PubMed] [Google Scholar]
- Walter D. J.; Eastwood M. A.; Brydon W. G.; Elton R. A. Fermentation of wheat bran and gum arabic in rats fed on an elemental diet. J. Geophys. Res. Oceans 1988, 60, 225–232. 10.1079/BJN19880094. [DOI] [PubMed] [Google Scholar]
- Phillips A. O.; Phillips G. O. Biofunctional behaviour and health benefits of a specific gum arabic. Food Hydrocolloids 2011, 25, 165–169. 10.1016/j.foodhyd.2010.03.012. [DOI] [Google Scholar]
- Gashua I. B.An Investigation of the molecular structure, composition and biophysical properties of gum arabic. 2016.
- Tischer C. A.; Gorin P. A. J.; Iacomini M. The free reducing oligosaccharides of gum arabic: Aids for structural assignments in the polysaccharide. Carbohydr. Polym. 2002, 47, 151–158. 10.1016/S0144-8617(01)00173-4. [DOI] [Google Scholar]
- Anderson D. M. W.; Stoddart J. F. Studies on uronic acid materials: Part XV. The use of molecular-sieve chromatography in studies on Acacia Senegal gum (gum arabic). Carbohydr. Res. 1966, 2, 104–114. 10.1016/S0008-6215(00)81474-3. [DOI] [Google Scholar]
- Anderson D. M. W.; McDougall F. J. Degradative studies of gum arabic (Acacia senegal (L.) willd.) with special reference to the fate of the amino acids present. Food Addit. Contam. 1987, 4, 247–255. 10.1080/02652038709373633. [DOI] [Google Scholar]
- Phillips G. O. Acacia gum (gum arabic): A nutritional fibre; metabolism and calorific value. Food Addit. Contam. 1998, 15, 251–264. 10.1080/02652039809374639. [DOI] [PubMed] [Google Scholar]
- Gibson G. R.; Wang X. Regulatory effects of Bifidobacteria on the growth of other colonic bacteria. J. Appl. Bacteriol. 1994, 77, 412–420. 10.1111/j.1365-2672.1994.tb03443.x. [DOI] [PubMed] [Google Scholar]
- Russell W. R.; Gratz S. W.; Duncan S. H.; Holtrop G.; Ince J.; Scobbie L.; Duncan G.; Johnstone A. M.; Lobley G. E.; Wallace R. J.; Duthie G. G.; Flint H. J. High-Protein, Reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. Am. J. Clin. Nutr. 2011, 93, 1062–1072. 10.3945/ajcn.110.002188. [DOI] [PubMed] [Google Scholar]
- Ouwehand A. C.; Tiihonen K.; Saarinen M.; Putaala H.; Rautonen N. Influence of a combination of Lactobacillus acidophilus NCFM and lactitol on healthy elderly: Intestinal and immune parameters. J. Geophys. Res. Oceans 2009, 101, 367–375. 10.1017/S0007114508003097. [DOI] [PubMed] [Google Scholar]
- Collado M. C.; Hernandez M.; Sanz Y. Production of bacteriocin-like inhibitory compounds by human fecal Bifidobacterium strains. J. Food Prot. 2005, 68, 1034–1040. 10.4315/0362-028X-68.5.1034. [DOI] [PubMed] [Google Scholar]
- Slavin J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. 10.3390/nu5041417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone-Dorshow T.; Levitt M. D. Gaseous response to ingestion of a poorly absorbed fructo-oligosaccharide sweetener. Am. J. Clin. Nutr. 1987, 46, 61–65. 10.1093/ajcn/46.1.61. [DOI] [PubMed] [Google Scholar]
- Ten Bruggencate S. J. M.; Bovee-Oudenhoven I. M. J.; Lettink-Wissink M. L. G.; Katan M. B.; van der Meer R. Dietary fructooligosaccharides affect intestinal barrier function in healthy men. J. Nutr. 2006, 136, 70–74. 10.1093/jn/136.1.70. [DOI] [PubMed] [Google Scholar]
- Food and Agriculture Organization (FAO) of the United Nations . Specifications for identity and purity of certain food additives. In Food and Nutrition Paper No. 49; FAO: Rome, Italy, 1990; 23–25. [Google Scholar]
- Meyer D.; Stasse-Wolthuis M. The bifidogenic effect of inulin and oligofructose and its consequences for gut health. Eur. J. Clin. Nutr. 2009, 63, 1277–1289. 10.1038/ejcn.2009.64. [DOI] [PubMed] [Google Scholar]
- Sun H.; Chen Y.; Cheng M.; Zhang X.; Zheng X.; Zhang Z. The modulatory effect of polyphenols from green tea, oolong tea and black tea on human intestinal microbiota in vitro. J. Food Sci. Technol. 2018, 55, 399–407. 10.1007/s13197-017-2951-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson G. R.; Roberfroid M.. Handbook of Prebiotics; CRC Press, 2008, 10.1201/9780849381829. [DOI] [Google Scholar]
- Kappelman M. D.; Rifas–Shiman S. L.; Kleinman K.; Ollendorf D.; Bousvaros A.; Grand R. J.; Finkelstein J. A. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United State. Clin. Gastroenterol. Hepatol. 2007, 5, 1424–1429. 10.1016/j.cgh.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Rubin D. C.; Shaker A.; Levin M. S. Chronic intestinal inflammation: Inflammatory bowel disease and colitis-associated colon cancer. Front. Immunol. 2012, 3, 107. 10.3389/fimmu.2012.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S.The effect of cereal cell wall fractions on fermentation by the human gut microbiota; with a focus upon health promoting effects. Ph.D., University of Reading, 2008. [Google Scholar]
- Macfarlane G. T.; Gibson G. R.; Cummings J. H. Comparison of fermentation reactions in different regions of the human colon. J. Appl. Bacteriol. 1992, 72, 57–64. 10.1111/j.1365-2672.1992.tb04882.x. [DOI] [PubMed] [Google Scholar]
- Cummings J. H.; Hill M. J.; Bone E. S.; Branch W. J.; Jenkins D. J. A. The effect of meat protein and dietary fiber on colonic function and metabolism. II. bacterial metabolites in feces and urine. Am. J. Clin. Nutr. 1979, 32, 2094–2101. 10.1093/ajcn/32.10.2094. [DOI] [PubMed] [Google Scholar]
- Magee E. A.; Richardson C. J.; Hughes R.; Cummings J. H. Contribution of dietary protein to sulfide production in the large intestine: An in vitro and a controlled feeding study in humans. Am. J. Clin. Nutr. 2000, 72, 1488–1494. 10.1093/ajcn/72.6.1488. [DOI] [PubMed] [Google Scholar]
- Cummings J. H.; Hill M. J.; Jivraj T.; Houston H.; Branch W. J.; Jenkins D. J. A. The effect of meat protein and dietary fiber on colonic function and metabolism I. Changes in bowel habit, bile acid excretion, and calcium absorption. Am. J. Clin. Nutr. 1979, 32, 2086–2093. 10.1093/ajcn/32.10.2086. [DOI] [PubMed] [Google Scholar]
- Nakamura J.; Kubota Y.; Miyaoka M.; Saitoh T.; Mizuno F.; Benno Y. Comparison of four microbial enzymes in clostridia and bacteroides isolated from human feces. Microbiol. Immunol. 2002, 46, 487–490. 10.1111/j.1348-0421.2002.tb02723.x. [DOI] [PubMed] [Google Scholar]
- Pool-Zobel B.; van Loo J.; Rowland I.; Roberfroid M. B. Experimental evidences on the potential of prebiotic fructans to reduce the risk of colon cancer. J. Geophys. Res. Oceans 2002, 87, S273–S281. 10.1079/BJN/2002548. [DOI] [PubMed] [Google Scholar]
- Walton G. E.The bacterial metabolism of dietary prebiotics and the potential for protection against colorectal cancer. Ph.D., University of Reading, 2006. [Google Scholar]
- Turnbaugh P. J.; Ley R. E.; Mahowald M. A.; Magrini V.; Mardis E. R.; Gordon J. I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- Ley R. E.; Turnbaugh P. J.; Klein S.; Gordon J. I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- Onderdonk A. B.; Garrett W. S.. Gas gangrene and other Clostridium-associated diseases. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases; Bennett J. E., Dolin R., Blaser M. J., Eds.; W.B. Saunders: Philadelphia, 2015; 2, 2768–2772. 10.1016/B978-1-4557-4801-3.00248-4. [DOI] [Google Scholar]
- Tuohy K. M.; Kolida S.; Lustenberger A. M.; Gibson G. R. The prebiotic effects of biscuits containing partially hydrolysed guar gum and fructo-oligosaccharides – a human volunteer study. J. Geophys. Res. Oceans 2001, 86, 341–348. 10.1079/BJN2001394. [DOI] [PubMed] [Google Scholar]
- Palframan R. J.; Gibson G. R.; Rastall R. A. Effect of pH and dose on the growth of gut bacteria on prebiotic carbohydrates in vitro. Anaerobe 2002, 8, 287–292. 10.1006/anae.2002.0434. [DOI] [PubMed] [Google Scholar]
- Hidalgo M.; Oruna-Concha M. J.; Kolida S.; Walton G. E.; Kallithraka S.; Spencer J. P. E.; Gibson G. R.; de Pascual-Teresa S. Metabolism of anthocyanins by human gut microflora and their influence on gut bacterial growth. J. Agric. Food Chem. 2012, 60, 3882–3890. 10.1021/jf3002153. [DOI] [PubMed] [Google Scholar]
- Cueva C.; Sánchez-Patán F.; Monagas M.; Walton G. E.; Gibson G. R.; Martín-Álvarez P. J.; Bartolomé B.; Moreno-Arribas M. V. In vitro fermentation of grape seed flavan-3-ol fractions by human faecal microbiota: Changes in microbial groups and phenolic metabolites. FEMS Microbiol. Ecol. 2013, 83, 792–805. 10.1111/1574-6941.12037. [DOI] [PubMed] [Google Scholar]
- Falony G.; Calmeyn T.; Leroy F.; De Vuyst L. Coculture fermentations of Bifidobacterium species and Bacteroides thetaiotaomicron reveal a mechanistic insight into the prebiotic effect of inulin-type fructans. Appl. Environ. Microbiol. 2009, 75, 2312–2319. 10.1128/AEM.02649-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ummadi M. S.; Curic-Bawden M.. Use of protein hydrolysates in industrial starter culture fermentations. In Protein Hydrolysates in Biotechnology; Pasupuleti V. K., Demain A. L., Eds.; Springer Netherlands: Dordrecht, 2008; 91–114. 10.1007/978-1-4020-6674-0_6. [DOI] [Google Scholar]
- Gänzle M. G.; Follador R. Metabolism of oligosaccharides and starch in Lactobacilli: A review. Front. Microbiol. 2012, 3, 340. 10.3389/fmicb.2012.00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock G. W.; Wilson C. M.; Loach D.; Cook G. M.; Eason J.; O’toole P. W.; Holtrop G.; Lawley B. Resource partitioning in relation to cohabitation of Lactobacillus species in the mouse forestomach. ISME J. 2012, 6, 927–938. 10.1038/ismej.2011.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter J. Ecological role of Lactobacilli in the gastrointestinal tract: Implications for fundamental and biomedical research. Appl. Environ. Microbiol. 2008, 74, 4985–4996. 10.1128/AEM.00753-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi Y.; Harada K.; Tokunaga M.; Ishihara N.; Okubo T.; Ogasawara Y.; Juneja L. R.; Fujisawa T. Faecal fermentation of partially hydrolyzed guar gum. J. Funct. Foods 2012, 4, 398–402. 10.1016/j.jff.2011.09.007. [DOI] [Google Scholar]
- Fuller M. F.In vitro Digestion for Pigs and Poultry; CAB International (CABI), 1991. [Google Scholar]
- Terpend K.; Possemiers S.; Daguet D.; Marzorati M. Arabinogalactan and fructo-oligosaccharides have a different fermentation profile in the Simulator of the Human Intestinal Microbial Ecosystem (SHIME®). Environ. Microbiol. Rep. 2013, 5, 595–603. 10.1111/1758-2229.12056. [DOI] [PubMed] [Google Scholar]
- Iraporda C.; Errea A.; Romanin D. E.; Cayet D.; Pereyra E.; Pignataro O.; Sirard J. C.; Garrote G. L.; Abraham A. G.; Rumbo M. Lactate and short chain fatty acids produced by microbial fermentation downregulate proinflammatory responses in intestinal epithelial cells and myeloid cells. Immunobiology 2015, 220, 1161–1169. 10.1016/j.imbio.2015.06.004. [DOI] [PubMed] [Google Scholar]
- Ríos-Covián D.; Ruas-Madiedo P.; Margolles A.; Gueimonde M.; de los Reyes-Gavilán C. G.; Salazar N. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 2016, 7, 185. 10.3389/fmicb.2016.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourriaud C.; Robins R. J.; Martin L.; Kozlowski F.; Tenailleau E.; Cherbut C.; Michel C. Lactate is mainly fermented to butyrate by human intestinal microfloras but inter-individual variation is evident. J. Appl. Microbiol. 2005, 99, 201–212. 10.1111/j.1365-2672.2005.02605.x. [DOI] [PubMed] [Google Scholar]
- Louis P.; Scott K. P.; Duncan S. H.; Flint H. J. Understanding the effects of diet on bacterial metabolism in the large intestine. J. Appl. Microbiol. 2007, 102, 1197–1208. 10.1111/j.1365-2672.2007.03322.x. [DOI] [PubMed] [Google Scholar]
- Amaretti A.; Bernardi T.; Tamburini E.; Zanoni S.; Lomma M.; Matteuzzi D.; Rossi M. Kinetics and metabolism of Bifidobacterium adolescentis MB 239 growing on glucose, galactose, lactose, and galactooligosaccharides. Appl. Environ. Microbiol. 2007, 73, 3637–3644. 10.1128/AEM.02914-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdin A. A.; Saeid E. M. An Experimental study on ulcerative colitis as a potential target for probiotic therapy by Lactobacillus acidophilus with or without “olsalazine.”. J. Crohn’s Colitis 2008, 2, 296–303. 10.1016/j.crohns.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Salazar N.; Binetti A.; Gueimonde M.; Alonso A.; Garrido P.; González del Rey C.; González C.; Ruas-Madiedo P.; de los Reyes-Gavilán C. G. Safety and intestinal microbiota modulation by the exopolysaccharide-producing strains Bifidobacterium animalis IPLA R1 and Bifidobacterium Longum IPLA E44 orally administered to wistar rats. Int. J. Food Microbiol. 2011, 144, 342–351. 10.1016/j.ijfoodmicro.2010.10.016. [DOI] [PubMed] [Google Scholar]
- Macfarlane S.; Macfarlane G. T. Regulation of short-chain fatty acid production. Proc. Nutr. Soc. 2003, 62, 67–72. 10.1079/PNS2002207. [DOI] [PubMed] [Google Scholar]
- Miller T. L.; Wolin M. J. Pathways of acetate, propionate, and butyrate formation by the human fecal microbial flora. Appl. Environ. Microbiol. 1996, 62, 1589–1592. 10.1128/AEM.62.5.1589-1592.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigottier-Gois L.; Rochet V.; Garrec N.; Suau A.; Doré J. Enumeration of Bacteroides species in human faeces by fluorescent in situ hybridisation combined with flow cytometry using 16s rRNA probes. Syst. Appl. Microbiol. 2003, 26, 110–118. 10.1078/072320203322337399. [DOI] [PubMed] [Google Scholar]
- Miller T. L.; Wolin M. J. Fermentations by saccharolytic intestinal bacteria. Am. J. Clin. Nutr. 1979, 32, 164–172. 10.1093/ajcn/32.1.164. [DOI] [PubMed] [Google Scholar]
- Louis P.; Hold G. L.; Flint H. J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014, 12, 661–672. 10.1038/nrmicro3344. [DOI] [PubMed] [Google Scholar]
- Flint H. J.; Duncan S. H.; Scott K. P.; Louis P. Links between diet, gut microbiota composition and gut metabolism. Proc. Nutr. Soc. 2015, 74, 13–22. 10.1017/S0029665114001463. [DOI] [PubMed] [Google Scholar]
- Garrett W. S.; Onderdonk A. B.. Bacteroides, Prevotella, Porphyromonas, and Fusobacterium species (and other medically important anaerobic gram-negative Bacilli). Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases 2010, 3111–3119. 10.1016/b978-0-443-06839-3.00247-2. [DOI] [Google Scholar]
- Hosseini E.; Grootaert C.; Verstraete W.; Van de Wiele T. Propionate as a health-promoting microbial metabolite in the human gut. Nutr. Rev. 2011, 69, 245–258. 10.1111/j.1753-4887.2011.00388.x. [DOI] [PubMed] [Google Scholar]
- Cherbut C.; Ferrier L.; Rozé C.; Anini Y.; Blottière H.; Lecannu G.; Galmiche J.-P. Short-chain fatty acids modify colonic motility through nerves and polypeptide yy release in the rat. Am. J. Phys.-Gastrointest. Liver Physiol. 1998, 275, G1415–G1422. 10.1152/ajpgi.1998.275.6.G1415. [DOI] [PubMed] [Google Scholar]
- Brown A. J.; Goldsworthy S. M.; Barnes A. A.; Eilert M. M.; Tcheang L.; Daniels D.; Muir A. I.; Wigglesworth M. J.; Kinghorn I.; Fraser N. J. The orphan g protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 2003, 278, 11312–11319. 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- Russell W. R.; Hoyles L.; Flint H. J.; Dumas M.-E. Colonic bacterial metabolites and human health. Curr. Opin. Microbiol. 2013, 16, 246–254. 10.1016/j.mib.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Chambers E. S.; Viardot A.; Psichas A.; Morrison D. J.; Murphy K. G.; Zac-Varghese S. E.; MacDougall K.; Preston T.; Tedford C.; Finlayson G. S. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut 2015, 64, 1744–1754. 10.1136/gutjnl-2014-307913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olano-Martin E.; Mountzouris K. C.; Gibson G. R.; Rastall R. A. In vitro fermentability of dextran, oligodextran and maltodextrin by human gut bacteria. J. Geophys. Res. Oceans 2000, 83, 247–255. 10.1017/S0007114500000325. [DOI] [PubMed] [Google Scholar]
- Salminen S.; Bouley C.; Boutron M.-C.; Cummings J. H.; Franck A.; Gibson G. R.; Isolauri E.; Moreau M.-C.; Roberfroid M.; Rowland I. Functional food science and gastrointestinal physiology and function. J. Geophys. Res. Oceans 1998, 80, S147–S171. 10.1079/BJN19980108. [DOI] [PubMed] [Google Scholar]
- Zigová J.; Šturdík E.; Vandák D.; Schlosser Š. Butyric acid production by Clostridium butyricum with integrated extraction and pertraction. Process Biochem. 1999, 34, 835–843. 10.1016/S0032-9592(99)00007-2. [DOI] [Google Scholar]
- Tulung B.; Rémésy C.; Demigné C. Specific effects of guar gum or gum arabic on adaptation of cecal digestion to high fiber diets in the rat. J. Nutr. 1987, 117, 1556–1561. 10.1093/jn/117.9.1556. [DOI] [PubMed] [Google Scholar]
- Frost G. S.; Walton G. E.; Swann J. R.; Psichas A.; Costabile A.; Johnson L. P.; Sponheimer M.; Gibson G. R.; Barraclough T. G. Impacts of plant-based foods in ancestral hominin diets on the metabolism and function of gut microbiota in vitro. MBio 2014, 5, e00853 10.1128/mBio.00853-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman K. A.; Bryant M. P. Peptides and other nitrogen sources for growth of Bacteroides ruminicola. J. Bacteriol. 1964, 88, 401–410. 10.1128/JB.88.2.401-410.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan S. H.; Louis P.; Flint H. J. Lactate-Utilizing Bacteria, Isolated from human feces, that produce butyrate as a major fermentation product. Appl. Environ. Microbiol. 2004, 70, 5810–5817. 10.1128/AEM.70.10.5810-5817.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis P.; Flint H. J. Diversity, Metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 2009, 294, 1–8. 10.1111/j.1574-6968.2009.01514.x. [DOI] [PubMed] [Google Scholar]
- Van den Abbeele P.; Belzer C.; Goossens M.; Kleerebezem M.; De Vos W. M.; Thas O.; De Weirdt R.; Kerckhof F.-M.; Van de Wiele T. Butyrate-producing Clostridium Cluster XIVa species specifically colonize mucins in an in vitro gut model. ISME J. 2013, 7, 949–961. 10.1038/ismej.2012.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vital M.; Howe A. C.; Tiedje J. M. Revealing the bacterial butyrate synthesis pathways by analyzing (meta)genomic data. MBio 2014, 5, e00889 10.1128/mBio.00889-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serpa J.; Caiado F.; Carvalho T.; Torre C.; Gonçalves L. G.; Casalou C.; Lamosa P.; Rodrigues M.; Zhu Z.; Lam E. W. F.; Dias S. butyrate-rich colonic microenvironment is a relevant selection factor for metabolically adapted tumor cells. J. Biol. Chem. 2010, 285, 39211–39223. 10.1074/jbc.M110.156026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbini S. R.; Kolida S.; Gibson G. R.; Rastall R. A. In vitro fermentation of commercial α-gluco-oligosaccharide by faecal microbiota from lean and obese human subjects. J. Geophys. Res. Oceans 2013, 109, 1980–1989. 10.1017/S0007114512004205. [DOI] [PubMed] [Google Scholar]
- Chen W.-J. L.; Anderson J. W.; Jennings D. Propionate may mediate the hypocholesterolemic effects of certain soluble plant fibers in cholesterol-fed rats. Exp. Biol. Med. 1984, 175, 215–218. 10.3181/00379727-175-41791. [DOI] [PubMed] [Google Scholar]
- Babiker R.; Merghani T. H.; Elmusharaf K.; Badi R. M.; Lang F.; Saeed A. M. Effects of gum arabic ingestion on body mass index and body fat percentage in healthy adult females: Two-arm randomized, placebo controlled, double-blind trial. Nutr. J. 2012, 11, 111. 10.1186/1475-2891-11-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calame W.; Thomassen F.; Hull S.; Viebke C.; Siemensma A. D. evaluation of satiety enhancement, including compensation, by blends of gum arabic. A methodological approach. Appetite 2011, 57, 358–364. 10.1016/j.appet.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Tremaroli V.; Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- LeBlanc J. G.; Chain F.; Martín R.; Bermúdez-Humarán L. G.; Courau S.; Langella P. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb. Cell Fact. 2017, 16, 79. 10.1186/s12934-017-0691-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar N.; Ruas-Madiedo P.; Kolida S.; Collins M.; Rastall R.; Gibson G.; de los Reyes-Gavilán C. G. Exopolysaccharides produced by Bifidobacterium longum IPLA E44 and Bifidobacterium animalis subsp. lactis IPLA R1 modify the composition and metabolic activity of human faecal microbiota in ph-controlled batch cultures. Int. J. Food Microbiol. 2009, 135, 260–267. 10.1016/j.ijfoodmicro.2009.08.017. [DOI] [PubMed] [Google Scholar]
- Wyatt G. M.; Bayliss C. E.; Holcroft J. D. A change in human faecal flora in response to inclusion of gum arabic in the diet. J. Geophys. Res. Oceans 1986, 55, 261–266. 10.1079/BJN19860033. [DOI] [PubMed] [Google Scholar]
- Kishimoto A.; Ushida K.; Phillips G. O.; Ogasawara T.; Sasaki Y. Identification of intestinal bacteria responsible for fermentation of gum arabic in pig model. Curr. Microbiol. 2006, 53, 173–177. 10.1007/s00284-005-0219-3. [DOI] [PubMed] [Google Scholar]
- Stevani J.; Grivet J. P.; Hannequart G.; Durand M. Glucose and lactate catabolism by bacteria of the pig large intestine and sheep rumen as assessed by 13C nuclear magnetic resonance. J. Appl. Bacteriol. 1991, 71, 524–530. 10.1111/j.1365-2672.1991.tb03827.x. [DOI] [PubMed] [Google Scholar]
- Hove H.; Mortensen P. B. Influence of intestinal inflammation (IBD) and small and large bowel length on faecal short-chain fatty acids and lactate. Dig. Dis. Sci. 1995, 40, 1372–1380. 10.1007/BF02065554. [DOI] [PubMed] [Google Scholar]
- Ushida K.; Hoshi S. 13 C-NMR studies on lactate metabolism in a porcine gut microbial ecosystem. Microb. Ecol. Health Dis. 2002, 14, 242–247. 10.1080/08910600310002136. [DOI] [Google Scholar]
- Sarbini S. R.; Rastall R. Prebiotics: metabolism, structure, and function. Funct. Food Rev. 2011, 3, 93–106. 10.2310/6180.2011.00004. [DOI] [Google Scholar]
- Trindade M. I.; Abratt V. R.; Reid S. J. Induction of sucrose utilization genes from Bifidobacterium lactis by sucrose and raffinose. Appl. Environ. Microbiol. 2003, 69, 24–32. 10.1128/AEM.69.1.24-32.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latimer G. W.Official Methods of Analysis of AOAC International Gaithersburg; The Scientific Association Dedicated to Analytical Excellence.: Rockville, MD, 2019. [Google Scholar]
- Korakli M.; Gänzle M. G.; Vogel R. F. Metabolism by Bifidobacteria and lactic acid bacteria of polysaccharides from wheat and rye, and exopolysaccharides produced by Lactobacillus sanfranciscensis. J. Appl. Microbiol. 2002, 92, 958–965. 10.1046/j.1365-2672.2002.01607.x. [DOI] [PubMed] [Google Scholar]
- Dubois M.; Gilles K. A.; Hamilton J. K.; Rebers P. A.; Smith F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. 10.1021/ac60111a017. [DOI] [Google Scholar]
- Agrawal N.; Minj D. K.; Rani K. Estimation of total carbohydrate present in dry fruits. IOSR J. Environ. Sci. Toxicol. Food Technol. 2015, 1, 24–27. [Google Scholar]
- Nielsen S. S.Total carbohydrate by phenol-sulfuric acid method. In Food Analysis Laboratory Manual; Nielsen S. S., Ed.; Food Science Text Series; Springer International Publishing: Cham, 2017; 137–141. 10.1007/978-3-319-44127-6_14. [DOI] [Google Scholar]
- Sadasivam S.; Manickam A. Phenol-sulphuric acid method for total carbohydrate. Biochem. Methods 2005, 47–53. 10.1007/978-1-4419-1463-7_6. [DOI] [Google Scholar]
- Pratt C. W.; Cornely K.. Essential Biochemistry, 3rd ed.; Wiley Hoboken: New Jersey, 2004. [Google Scholar]
- Robertson J. A.; Ryden P.; Louise Botham R.; Reading S.; Gibson G.; Ring S. G. Structural properties of diet-derived polysaccharides and their influence on butyrate production during fermentation. LWT - Food Sci. Technol. 2001, 34, 567–573. 10.1006/fstl.2001.0816. [DOI] [Google Scholar]
- Al-Sheraji S. H.; Ismail A.; Manap M. Y.; Mustafa S.; Yusof R. M.; Hassan F. A. Fermentation and non-digestibility of Mangifera Pajang fibrous pulp and its polysaccharides. J. Funct. Foods 2012, 4, 933–940. 10.1016/j.jff.2012.07.001. [DOI] [Google Scholar]
- Hayisama-ae W.; Kantachote D.; Bhongsuwan D.; Nokkaew U.; Chaiyasut C. A potential synbiotic beverage from fermented red seaweed (Gracilaria Fisheri) ssing Lactobacillus plantarum DW12. Int. Food Res. J. 2014, 21, 1789–1796. [Google Scholar]
- Bajury D. M.; Rawi M. H.; Sazali I. H.; Abdullah A.; Sarbini S. R. Prebiotic evaluation of red seaweed (Kappaphycus alvarezii) using in vitro colon model. Int. J. Food Sci. Nutr. 2017, 68, 821–828. 10.1080/09637486.2017.1309522. [DOI] [PubMed] [Google Scholar]
- Daims H.; Stoecker K.; Wagner M.. Fluorescence in situ hybridization for the detection of prokaryotes. In Molecular Microbial Ecology; Taylor & Francis, 2004; 208–228. [Google Scholar]
- Franks A. H.; Harmsen H. J. M.; Raangs G. C.; Jansen G. J.; Schut F.; Welling G. W. Variations of bacterial populations in human feces measured by fluorescent in situ hybridization with group-specific 16S rRNA-Targeted Oligonucleotide Probes. Appl. Environ. Microbiol. 1998, 64, 3336–3345. 10.1128/AEM.64.9.3336-3345.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmsen H. J. M.; Elfferich P.; Schut F.; Welling G. W. A 16S rRNA-targeted probe for detection of Lactobacilli and Enterococci in faecal samples by fluorescent in situ hybridization. Microb. Ecol. Health Dis. 1999, 11, 3–12. 10.1080/089106099435862. [DOI] [Google Scholar]
- Langendijk P. S.; Schut F.; Jansen G. J.; Raangs G. C.; Kamphuis G. R.; Wilkinson M. H.; Welling G. W. Quantitative fluorescence in situ hybridization of Bifidobacterium spp. with genus-specific 16S rRNA-targeted probes and its application in fecal samples. Appl. Environ. Microbiol. 1995, 61, 3069–3075. 10.1128/AEM.61.8.3069-3075.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manz W.; Amann R.; Ludwig W.; Vancanneyt M.; Schleifer K.-H. Application of a suite of 16S rRNA-specific oligonucleotide probes designed to investigate bacteria of the phylum Cytophaga-Flavobacter-Bacteroides in the natural environment. Microbiology 1996, 142, 1097–1106. 10.1099/13500872-142-5-1097. [DOI] [PubMed] [Google Scholar]
- Harmsen H. J. M.; Wildeboer-Veloo A. C. M.; Grijpstra J.; Knol J.; Degener J. E.; Welling G. W. Development of 16S rRNA-based probes for the Coriobacterium group and the Atopobium cluster and their application for enumeration of Coriobacteriaceae in human feces from volunteers of different age groups. Appl. Environ. Microbiol. 2000, 66, 4523–4527. 10.1128/AEM.66.10.4523-4527.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker A. W.; Duncan S. H.; Leitch E. C. M.; Child M. W.; Flint H. J. pH and peptide supply can radically alter bacterial populations and short-chain fatty acid ratios within microbial communities from the human colon. Appl. Environ. Microbiol. 2005, 71, 3692–3700. 10.1128/AEM.71.7.3692-3700.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hold G. L.; Schwiertz A.; Aminov R. I.; Blaut M.; Flint H. J. Oligonucleotide probes that detect quantitatively significant groups of butyrate-producing bacteria in human feces. Appl. Environ. Microbiol. 2003, 69, 4320–4324. 10.1128/AEM.69.7.4320-4324.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]