Abstract
The Women’s Health Initiative Randomized Controlled Dietary Modification Trial (WHIRCDMT) was designed to test whether the US Department of Agriculture’s 1977 Dietary Guidelines for Americans protects against coronary heart disease (CHD) and other chronic diseases. The only significant finding in the original 2006 WHIRCDMT publication was that postmenopausal women with CHD randomised to a low-fat ‘heart-healthy’ diet in 1993 were at 26% greater risk of developing additional CHD events compared with women with CHD eating the control diet. A 2017 WHIRCDMT publication includes data for an additional 5 years of follow-up. It finds that CHD risk in this subgroup of postmenopausal women had increased further to 47%–61%. The authors present three post-hoc rationalisations to explain why this finding is ‘inadmissible’: (1) only women in this subgroup were less likely to adhere to the prescribed dietary intervention; (2) their failure to follow the intervention diet increased their CHD risk; and (3) only these women were more likely to not have received cholesterol-lowering drugs. These rationalisations appear spurious. Rather these findings are better explained as a direct consequence of postmenopausal women with features of insulin resistance (IR) eating a low-fat high-carbohydrate diet for 13 years. All the worst clinical features of IR, including type 2 diabetes mellitus (T2DM) in some, can be ‘reversed’ by the prescription of a high-fat low-carbohydrate diet. The Women’s Health Study has recently reported that T2DM (10.71-fold increased risk) and other markers of IR including metabolic syndrome (6.09-fold increased risk) were the most powerful predictors of future CHD development in women; blood low-density lipoprotein-cholesterol concentration was a poor predictor (1.38-fold increased risk). These studies challenge the prescription of the low-fat high-carbohydrate heart-healthy diet, at least in postmenopausal women with IR, especially T2DM. According to the medical principle of ‘first do no harm’, this practice is now shown to be not evidence-based, making it scientifically unjustifiable, perhaps unethical.
Keywords: coronary artery disease, epidemiology, risk factors, diabetes mellitus
Introduction
The Women’s Health Initiative Randomized Controlled Dietary Modification Trial (WHIRCDMT)1 is one of the most expensive long-term dietary intervention trials yet undertaken. Beginning in 1993, the WHIRCDMT was designed to provide supporting evidence for a single dietary pattern, consistent with the US Department of Agriculture’s 1977 Dietary Guidelines for Americans (DGA), which encouraged North Americans to reduce their dietary, especially saturated, fat intake.2 This specific dietary intervention had yet to be evaluated with respect to its effects on weight gain and the development of coronary heart disease (CHD), cancer and type 2 diabetes mellitus (T2DM).
The goal of the intervention diet was to replace especially saturated fat intake with an increased intake of carbohydrates from grains, fruits and vegetables. The dietary intervention effectively lowered dietary fat intake and was associated with a reduction in blood cholesterol concentrations. Importantly the goal of the dietary intervention was not to replace dietary saturated fat intake with an increased intake of polyunsaturated fats. This particular intervention had been evaluated in two other trials, the final results of which were ultimately published in 20133 and 2016.4
During the WHIRCDMT, only postmenopausal women randomised to the intervention group received the programme: an ‘intensive behavioral modification program involved 18 group sessions in the first year and quarterly maintenance sessions thereafter, led by specially trained and certified nutritionists’ (p656).1 In addition, ‘group activities were supplemented during the intervention period by individual interviews…targeted-message campaigns, and personalized feedback on fat intake’ (p657).1 In contrast, ‘women in the comparison group received a copy of the DGA, as well as other health-related materials, but had no contact with the nutrition interventionists’ (p657).1
In this article I review two more recent publications from the WHIRCDMT5 6 which show that compared with postmenopausal women who continued to eat the more usual, higher-fat, supposedly heart-unhealthy control diet, postmenopausal women randomised to the intervention diet were at 47%–61% increased risk of developing additional CHD complications during a further 5 years of follow-up. The authors provide three post-hoc rationalisations to explain why their latest finding is ‘inadmissible’. I argue that none of these rationalisations is valid. I further propose that this iconic study definitively establishes that the prescription of the low-fat ‘heart-healthy’ diet to postmenopausal women with established CHD, because they are likely to be insulin-resistant, is scientifically unjustifiable and potentially unethical.
What are the true findings of the WHICRCDMT?
Findings of the original 2006 publication from the WHIRCDMT
As detailed previously,7 the original publication describing the first 8 years of the WHIRCDMT failed to identify even one statistically significant beneficial outcome of this dietary intervention. The low-fat dietary intervention failed to protect against invasive breast8 or colorectal9 cancer and produced only a marginal (0.4 kg) weight loss over the first 8 years of the trial.10 In contrast, strict adherence to low-fat and DGA diets was associated with increased risk of weight gain, whereas strict compliance with a higher-fat reduced-carbohydrate diet ‘was associated with a sharply lower risk of weight gain in adjusted models…’ (p1191).11 ‘Our findings therefore challenge prevailing dietary recommendations, suggesting instead that a low fat (diet) may promote rather than prevent weight gain after menopause’ (p1196).11
While the heart-healthy low-fat dietary intervention did not reduce the risk of developing T2DM during the first 8 years of the trial,12 already within the first year, women who began the experiment with T2DM showed significantly worsened (p<0.001) blood glucose control.13
An unexpected finding was that postmenopausal women prescribed cholesterol-lowering medications (statins) were at 49% increased risk of developing T2DM.14 A prior meta-analysis found only a 9% increased risk of T2DM associated with statin use.15
In contrast to these neutral or negative findings, the most important discovery in the 2006 report was essentially dismissed as unreliable: postmenopausal women who entered the trial with established CHD and who were randomised to the intervention diet in 1993 were at 26% increased risk of an adverse outcome compared with those women with CHD who continued eating their usual ‘high’-fat control diet. This was the sole outcome that reached statistical significance.
I have argued7 16 that this finding may not have been properly communicated.
Here I review the two most recent 20175 and 20196 publications from the WHIRCDMT which report additional findings from a further 5 years of follow-up of the population originally described in 2006.1 These publications are important because they provide an analysis of the effects of a more prolonged duration of exposure to the low-fat heart-healthy intervention diet.
Findings of the 2017 WHIRCDMT publication
The 2017 report describing the 13-year follow-up data for the WHIRCDMT5 introduced a novel subgroup analysis based on the health of the postmenopausal women on admission to the WHIRCDMT in 1993. Study participants were categorised into three subgroups based on their health status in 1993 when they entered the trial:
No CHD or hypertension (HTN).
HTN only.
Pre-existing CHD.
This categorisation allows for the identification of specific subgroups who may either benefit the most or be exposed to the greatest harm from the dietary intervention.
This subgroup analysis confirmed that the risk of developing additional CHD complications during the extended follow-up in the group of postmenopausal women with pre-existing CHD had increased from 26% in the first analysis1 to 47%–61% 5 years later (figure 1 and 2 in reference 5) if they were assigned to the intervention diet. Postmenopausal women with HTN in 1993 received neither overall benefit nor harm if they ate the intervention diet (figure 1 and 2 in reference5). Healthy postmenopausal women with neither CHD nor HTN in 1993 received some benefit in terms of a small reduction in CHD risk but at the cost of an increased risk of stroke (figure 1 and 2 in referenc5).
Accordingly these data indicate that the examined heart-healthy intervention diet substantially worsened outcomes in postmenopausal women with established CHD while providing only a marginal benefit for those who are the most healthy because they had neither CHD nor HTN when the trial began. While the authors of the 2017 publication5 acknowledge this increased risk of adverse CHD outcomes in those with prior CHD, this finding cannot be dismissed as simply due to chance.
The conclusion in the abstract also fails to mention any adverse outcomes for those with prior CHD eating the intervention diet for 13 years:
Conclusions: CVD risk in postmenopausal women appears to be sensitive to a change to a low-fat dietary pattern and among healthy women, including both CHD benefit and stroke risk (p35).5
As a result and as was the case in the 2006 report,1 7 these conclusions fail to emphasise that women with CHD at the start of the trial were at a substantially increased risk of additional cardiovascular events if they adopted the heart-healthy intervention diet.
Post-hoc rationalisations to explain why findings of harm are‘uninterpretable’
Rationalisation 1
Women in the dietary intervention group failed to comply with the required dietary change to a low-fat diet.
The main explanation offered by the authors to de-emphasise the importance of the statistically significant findings of potential harm of the intervention diet in those with prior CHD appears to be based on concerns regarding dietary compliance:
We concluded that the trial results for CHD were uninterpretable in the prior CVD subjects (i.e., the finding showing increased CHD risk in women with established CHD randomized to the “heart-healthy” intervention in 1993 – my addition). We were not able to rule out the possibility that dietary changes in the intervention group participants (my emphasis) could have contributed to their unfavorable CHD experience. Others have hypothesized an unfavorable CHD effect based on studies in other contexts.17–19 (p41)5
The logic of this post-hoc rationalisation seems to be the following: Women without CHD assigned to the heart-healthy low-fat dietary intervention in 1993 had adhered scrupulously to that diet. However, when randomised to the identical diet, another group of women, differing only because they started the trial with CHD, failed to comply with that same diet, producing results that are now ‘uninterpretable’ (p41).5
While the harms of the intervention diet were apparent in the original 2006 publication,1 7 questions about dietary compliance were not raised at that time. Rather the authors chose to dismiss the finding as most probably due to chance: ‘The intervention was associated with increased risk in the 3.4% of women with baseline CVD; this may be a chance observation, or rates in this small subset may be confounded by concurrent therapy or comorbid conditions’ (p663–664).1
This establishes that there was no hint in the original article1 that some women with prior CHD assigned to the heart-healthy intervention diet in 1993 had not complied with the experimental diet, reverting rather to their previous heart-unhealthy high-fat control diet and so increasing their risk for further CHD events (according to the original hypothesis being tested). The possibility that the heart-healthy intervention diet could be harmful rather than healthy was simply unimaginable when the WHIRCDMT was planned. The decades-long history of how this came about has been detailed by Teicholz,17 as also by Noakes and Sboros.18
However, the authors’ rationalisation is moot since the WHIRCDMT was designed and analysed as an intention-to-treat trial. As the authors describe the WHIRCDMT design: ‘Design: This randomized controlled trial was analyzed as intent to treat’ (p260).19
The intention-to-treat analysis is defined as the following: ‘A method for analyzing results in a prospective randomized study where all participants who are randomized are included in the statistical analysis and analyzed according to the group (to which) they were originally assigned, regardless of what treatment (if any) they received’.20
In summary, since the WHIRCDMT was designed as an intention-to-treat trial, this attempted post-hoc rationalisation is itself inadmissible.
Rationalisation 2
The existence of what the authors describe as ‘other contexts’, in particular the Estrogen Replacement and Atherosclerosis (ERA) trial of progression of coronary artery narrowing in postmenopausal women eating diets with different macronutrient compositions.
The authors submit an additional ‘other contexts’21–23 argument to support their post-hoc rationalisation. Of these three references, two refer to original data collected as part of the ERA trial.21 22 The third23 is a character reference for the heart-healthiness of the heart-healthy intervention diet.
The ERA trial21 found that coronary atherosclerosis did not progress in postmenopausal women who reported that they ate the most saturated fat during a 3-year observational trial to determine the effects of hormone replacement therapy on the progression of coronary atherosclerosis (arrow 1 in figure 1), whereas those women whose diets contained either more carbohydrates or more polyunsaturated fats (and therefore less saturated fat) showed progression of coronary atherosclerosis (arrows 2 and 3 in figure 1).
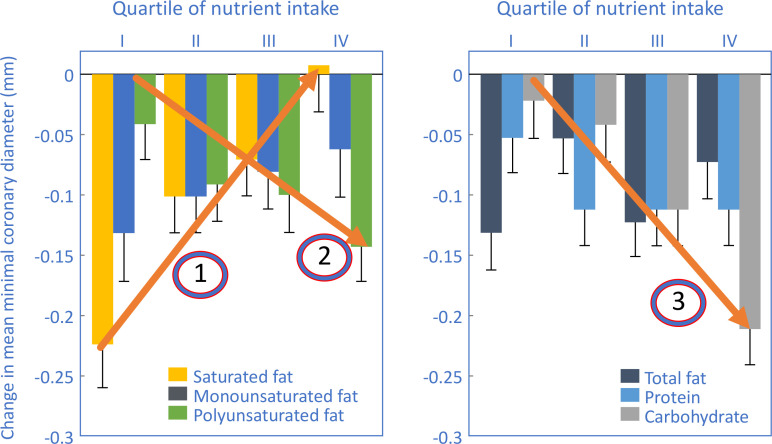
Figure 1.
Changes in mean minimal coronary artery diameter measured in postmenopausal women participating in the Estrogen Replacement and Atherosclerosis trial.21 Note that increased rates of coronary artery narrowing were associated with increasing intake of polyunsaturated fats (arrow 2) and carbohydrates (arrow 3). The highest intake of saturated fat was associated with a slight regression of coronary artery narrowing (arrow 1). Redrawn and reproduced from Mozaffarian et al21 with permission from the American Journal of Clinical Nutrition.
Figure 1 shows that the highest rates of progression of coronary artery narrowing occurred in postmenopausal women in the highest quartile of intake of either polyunsaturated fats (arrow 2) or carbohydrates (arrow 3). However, women in the highest quartile of saturated fat intake showed a modest regression of coronary artery narrowing (arrow 1).
Thus the findings of the ERA trial predict that those eating less fats and especially less saturated fats and more carbohydrates (and more polyunsaturated fats if they so chose) would experience a more rapid progression of coronary artery narrowing.
So, correctly interpreted, the results of the ERA trial indicate those postmenopausal women with prior CHD would be more likely to experience progression of their coronary artery narrowing when eating the intervention rather than the control diet. Importantly, this interpretation is consistent with and not discordant from the findings of the WHIRCDMT.
The logic of Prentice et al’s5 explanation must be that some women with prior CHD failed to comply with the heart-healthy intervention diet. Instead they reverted to their usual heart-unhealthy control diet containing too much dietary fat, especially saturated fat. This change, they must claim, would have caused CHD to progress in these women, whereas some other women in the same diet intervention group who faithfully followed the heart-healthy intervention diet presumably remained disease-free.
This explanation is only logical if the ERA study found that diets high in carbohydrates and low in saturated fats prevented progression of coronary artery narrowing.
However, the ERA study found the opposite (figure 1).
Rationalisation 3
Women with prior CHD randomised to the heart-healthy intervention diet in 1993 were less likely to be prescribed statin drugs during the trial and follow-up than were women in the respective control group. Since, according to this logic, statins reduce CHD events, CHD data from the intervention group are inadmissible.
The third rationalisation used to explain why follow-up data for postmenopausal women with CHD in 1993 randomised to the intervention diet are inadmissible is the claim that such women were less likely to be prescribed cholesterol-lowering statin drugs that, it is claimed, protect against future CHD events.
The claim is that the 13-year follow-up data of postmenopausal women with prior CHD randomised to the dietary intervention group were confounded by ‘postrandomization use of cholesterol-lowering medications’ (p35).5 The clear assumption is that statin use in women is associated with a significant reduction in CHD risk,24 a claim that is contested especially in women.25
Prentice et al’s5 explanation appears to be that postmenopausal women in the intervention group who failed to comply with the dietary advice and instead continued to consume higher levels of fat and saturated fat would be more likely to be prescribed and to comply with statin therapy. This assertion is however unsupported by baseline and follow-up data from the WHIRCDMT, which in fact demonstrate a higher level of statin use in women with prior CHD randomised to the dietary intervention (figure 2).
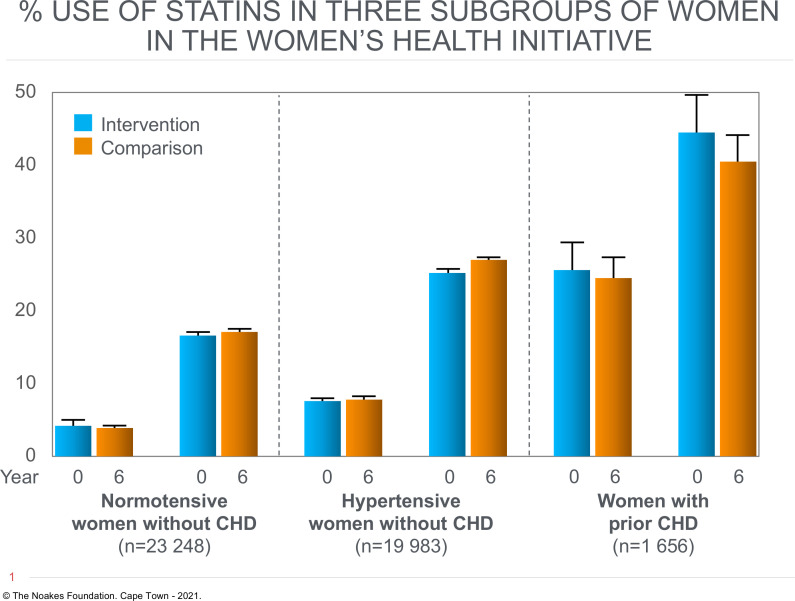
Figure 2.
Percentage of postmenopausal women in the three different subgroups who were using statin drugs at the start (year 0) and end (year 6) of WHIRCDMT. Figure drawn from data in figure 3 in Prentice et al.5 CHD, coronary heart disease; WHIRCDMT, Women’s Health Initiative Randomized Controlled Dietary Modification Trial.
Thus figure 2 shows that the subgroup of 1656 postmenopausal women with prior CHD in 1993 were ~15%–20% more likely to be prescribed statin drugs during the trial and follow-up than were postmenopausal women in the other two subgroups. However, the percentage of statin users in women with prior CHD was essentially the same at all times during the trial regardless of whether they were assigned to either the intervention or control diet (figure 2).
This finding that postmenopausal women with CHD in 1993 were equally likely to be taking statin drugs regardless of their dietary assignment seems to disprove the authors’ claim that ‘postrandomization use of cholesterol-lowering medications’ invalidates the findings of increased CHD events in women with prior CHD randomised to the low-fat heart-healthy intervention diet (p35).5
Post-hoc rationalisations should not be used to explain data that challenge a favoured hypothesis
These post-hoc rationalisations introduced by Prentice et al5 arise perhaps from an abiding, largely unchallenged certainty in the safety and efficacy of the low-fat heart-healthy diet.17 Thus, ‘the results from the WHI diet trial taken as a whole are consistent with our current understanding of the major dietary components that influence cardiovascular disease’ (p281).26 However, this statement is not supported by the extensive scientific evidence the authors have provided.
Rather the WHIRCDMT disproved the hypothesis it was designed to test. Once disproven, the tested hypothesis must be summarily rejected; it cannot be rescued by post-hoc rationalisations of convenience. As Stephen Hawking wrote, ‘if the observations don’t agree with the theory, one abandons the theory’ (p36).27
Findings of the 2019 WHIRCDMT publication
In their most recent6 2019 publication, the authors chose to report the results only for postmenopausal women who entered the trial without CHD in 1993. By excluding women for whom the diet has now been shown to be harmful, the authors were able to conclude that ‘reduction in dietary fat with corresponding increase in vegetables, fruit, and grains led to benefits related to breast cancer, CHD and diabetes, without adverse effects, among healthy post-menopausal US women’ (p1565).6
This statement fails to warn that this same diet produced measurable harm in unhealthy women with established CHD in 1993.
What are the current findings of the WHIRCDMT?
The reports of the findings of the WHIRCDMT could and perhaps should have emphasised the following:
Compared with the experience of women with CHD in 1993 who ate a diet with more fats, including saturated fats, postmenopausal women eating the heart-healthy low-fat intervention diet were at 47%–61% increased risk of developing additional CHD events during 13 years of follow-up.
This represented a substantial increase in adverse events for women in the intervention group, compared with the findings 5 years earlier, when the risk difference (26%) between the two study groups was substantially less.
This risk difference occurred even though only the intervention group had received the intensive behavioural modification programme led by especially trained and certified nutritionists during the first 8 years of the trial.
Postmenopausal women with HTN but without CHD in 1993 received neither benefit nor harm from eating the heart-healthy intervention diet for 13 years.
Postmenopausal women with neither HTN nor CHD in 1993 received some benefits from eating the heart-healthy intervention diet for 13 years but at the cost of an increased risk of stroke.
Percentage use of statins was equivalent in postmenopausal women with CHD in 1993 randomised to either the control or intervention diet. In both groups >40% of participants were prescribed statin drugs. The finding that risk of future CHD events was greater in the group receiving the low-fat dietary intervention, despite high rates of statin use, proves that statin use did not eliminate and may not have lessened the increased risk of future CHD events associated with eating the heart-healthy low-fat diet.
Postmenopausal women in either dietary intervention group who were prescribed statin drugs were at 49% increased risk of developing T2DM.
At the end of the first year of the trial, postmenopausal women with T2DM at the start of the trial in 1993 showed worsened glucose control if they were randomised to the low-fat dietary intervention.
Postmenopausal women who complied strictly with a personally chosen, reduced-fat, higher-carbohydrate diet showed a ‘sharply lower’ risk of weight gain during the trial.
The most important practical finding of the WHIRCDMT was that only those postmenopausal women who are the healthiest because they have neither CHD nor HTN can be reassured that eating the heart-healthy DGA intervention diet will not cause long-term cardiovascular harm and may instead provide some benefit.
Findings from the Women’s Heart Study
T2DM and other markers of insulin resistance as predictors of future CHD risk
The Women’s Heart Study (WHS), also established between 1992 and 1995 at Harvard Medical School, was designed as a clinical trial to evaluate the effects of vitamin E28 or low-dose aspirin29 30 on the risk of developing CHD or cancer in initially healthy women free from cardiovascular disease and cancer at baseline. These studies found no overall benefit for either intervention. A subsequent 21.4-year-long, prospective follow-up cohort study of 28 024 of these women31 has evaluated more than 50 clinical, lipid, inflammatory and metabolic risk factors and biomarkers for the subsequent development of CHD.
T2DM has long been considered by some32 to be a more important risk factor in the pathogenesis of CHD than elevated blood cholesterol concentrations,33 34 the latter being the hypothesis tested by the WHIRCDMT. The results of the 21.4-year-long, prospective follow-up WHS31 confirm that T2DM, not an elevated blood cholesterol concentration, is the key driver of future CHD development. figure 3 shows the most important results of this part of the WHS.
Figure 3.
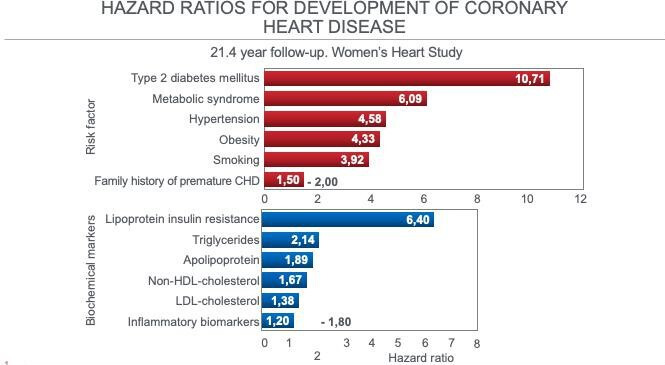
HR for the six most important risk factors and the six biochemical markers for the development of CHD in 28 024 postmenopausal women who were healthy on entry to the Women’s Heart Study. Drawn from data from Dugani et al.31 CHD, coronary heart disease; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Figure 3 shows that the strongest predictors of future CHD development in these postmenopausal women are all the classic clinical markers of insulin resistance (IR), most especially T2DM (10.7-fold increased risk), metabolic syndrome (6.09-fold increased risk), HTN (4.58-fold increased risk) and obesity (4.33-fold increased risk). All these markers of IR had greater predictive value for CHD than did smoking.
In addition the most important metabolic risk marker was the Lipoprotein Insulin Resistance (LPIR) score35 (6.40-fold increased risk). The LPIR score is based on lipoprotein subclass and size information measured with nuclear magnetic resonance (NMR) imaging. It has strong associations with multiple markers of IR and ‘may represent a simple means to identify individuals with IR’ (p422).35 The score is based on studies showing changes in lipoproteins in persons with IR. In particular, those with IR show the following characteristic NMR lipoprotein patterns35–38:
Greater number of the large subclass of very low-density lipoprotein (VLDL) particles.
Greater number of the small subclass of low-density lipoprotein (LDL) particles.
Lower number of the large subclass of high-density lipoprotein (HDL) particles.
In addition, mean VLDL particle sizes are generally larger and mean LDL and HDL particle sizes usually smaller in persons with IR36 37 or pre-diabetes.39
In contrast serum LDL-cholesterol concentration—the principal target of the low-fat heart-healthy intervention diet in the WHIRCDMT1 because the Women’s Health Initiative (WHI) principal investigators consider it to be the determinant of CHD risk33 34—was of little predictive value (1.38-fold increased risk) (figure 3).
Accordingly a low-fat diet, which may indeed lower blood LDL-cholesterol concentrations but at the cost of an increasing atherogenic dyslipidaemia,40–42 especially in those with IR,43–46 would be expected to worsen CHD outcomes, precisely as happened in the subgroup of postmenopausal women with prior CHD in the WHIRCDMT.
More evidence that abnormalities in carbohydrate rather than in fat metabolism drive coronary atherosclerosis comes from the Progression of Early Subclinical Atherosclerosis study,47 which found an association between haemoglobin A1c (HbA1c) values and subclinical atherosclerosis in persons without T2DM. The relationship was present at all HbA1c values, even at values below 5.5%, the level at which pre-diabetes is usually first diagnosed.
Indeed evidence disputing the traditional diet-heart and lipid hypotheses, now seemingly also disproven by the WHIRCDMT, continues to accumulate.48–59
Why did only the most healthy postmenopausal women benefit from eating the heart-healthy DGA diet in the WHIRCDMT?
A fundamental question is: Why did only the healthiest postmenopausal women in the WHIRCDMT receive some benefit from eating the heart-healthy intervention diet?
One possibility is that persons with higher levels of IR expressed clinically as metabolic syndrome, HTN or T2DM may be more likely to show reversal of some or all of the metabolic features of these conditions if they avoid the heart-healthy low-fat intervention diet.40–46 Historically the association between IR and ‘essential’ HTN,60–66 obesity,67–69 T2DM,60 endothelial dysfunction70 and CHD60 63 65 71–76 is well established. The clinical and metabolic characteristics of all these conditions improve and can be ‘reversed’ in some individuals when a low-carbohydrate, high-fat heart-unhealthy diet is eaten.43–46 77–92
A reasonable suggestion might be that the postmenopausal women in the WHIRCDMT who were not harmed by eating the low-fat high-carbohydrate intervention diet were insulin-sensitive in 1993 and remained so during the trial and follow-up. However, postmenopausal women with either HTN or CHD in 1993 likely had more advanced IR, placing them at risk of worsening IR if they continued to eat the low-fat high-carbohydrate diet.
Interestingly a 2015 study of the WHI population93 does not strongly support this interpretation, as this study found that, when corrected for blood HDL-cholesterol concentrations, markers of IR were not a significant predictor of future CHD risk in postmenopausal women free of T2DM in 1993.
However a low blood HDL-cholesterol concentration is a key marker of IR,60 so that correcting for this biomarker removes, in part, the influence of IR as a contributor to CHD risk.
However, IR in this population was associated with higher breast cancer incidence and all-cause mortality after breast cancer,94 as well as increased risk of cancer-specific and all-cause mortality.95
Evidence that replacing dietary saturated fat with polyunsaturated fats also causes harm
The significance of the Dugani et al31 study is to show, as Kraft first proposed,96 that T2DM/IR is the single most important risk factor, by far, for future development of CHD. However, as he argued, it is often missed because of inappropriate testing to detect either condition.97–99 While these new data are specific to postmenopausal women, it is reasonable to assume that they also apply to men (and women) of all ages. If correct, it follows that advising persons with IR to replace dietary saturated fat with heart-healthy carbohydrates from fruits, vegetables and grains will worsen blood glucose control especially in those with IR and T2DM43–46 and produce a proatherogenic dyslipidaemia,40–42 while increasing whole body inflammation and IR.42 These changes would be expected to lead inexorably to progression of CHD.
Two other studies have also recently shown that replacing saturated fat with polyunsaturated fat according to the DGA dietary guidelines also worsens long-term outcomes.
The Recovered Minnesota Coronary Experiment (RMCE)3 found that persons randomised to the intervention diet which replaced saturated fat with the polyunsaturated fatty acid (PUFA), linoleic acid, were at 22% higher risk of death for each 30 mg/dL (0.78 mmol/L) reduction in blood cholesterol concentrations, an effect that was especially apparent in those over 65 years of age. The Recovered Sydney Diet Heart Study (RSDHS)4 also found that replacement of dietary saturated fat with linoleic acid was also associated with increased all-cause mortality and with increased deaths from both cardiovascular disease and CHD.
Importantly one criticism of the RMCE and the RSDHS is that neither controlled for the intake of trans fats considered to increase CHD risk.100 This criticism does not apply to the WHIRCDMT since the intervention increased the intake of carbohydrates, not of fats which could have been contaminated with trans fats.
In reviewing all the current evidence, Lawrence101 concludes that:
PUFAs are unstable to chemical oxidation and their oxidation products are harmful in a variety of ways. PUFAs also form powerful signaling agents that can initiate inflammation which can have dire health consequences…If saturated fats are replaced by carbohydrates in the diet, there would be no significant improvement in serum cholesterol, and it can result in a more atherogenic lipoprotein profile. When looking at much of the data in the context of known biochemical and physiological mechanisms, it appears that saturated fats are less harmful than the common alternatives.101
Dietary advice for persons with IR or T2DM
This set of findings from four different studies effectively ends the debate about which diet should be eaten to lower the risk of CHD, especially in those with IR.
The answer is that the prescribed diet must prevent the development of the clinical features of IR leading to T2DM. The two diets shown to achieve this are the restricted low-calorie diet developed by Lim et al102 and the ad libitum low-carbohydrate higher-healthy-fat ketogenic diet as reported by a number of research teams.79–83 86–88 90
According to the principle of first do no harm, it now becomes the ethical responsibility of all those managing persons with established CHD or at risk of its development because they have IR, especially if they have T2DM, not to prescribe the never-proven17 18 103 and now-disproven low-fat heart-healthy DGA diet.
Acknowledgments
The Cape Peninsula University of Technology contributes to the costs of the submission and publication of this article. The author covered all other expenses.
Footnotes
Twitter: @ProfTimNoakes
Contributors: TDN wrote the manuscript without assistance from anyone else.
Funding: The Cape Peninsula University of Technology contributes to the costs of the submission and publication of this article. TDN covered all other expenses.
Competing interests: TDN is the author of a number of books on low-carbohydrate diet, including The Real Meal Revolution, Super Food for Superchildren, Lore of Nutrition, The Banting Pocket Guide, Real Food on Trial and The Eat Right Revolution. TDN derives no personal income from the sale of these books. Instead all royalties are donated to the NGO The Noakes Foundation, of which TDN is the chairman. The money is used to fund the work of The Noakes Foundation, including the Eat Better South Africa Campaign.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
The publication makes use of data already published in the scientific literature.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Howard BV, Van Horn L, Hsia J, et al. Low-fat dietary pattern and risk of cardiovascular disease: the women's health Initiative randomized controlled dietary modification trial. JAMA 2006;295:655–66. 10.1001/jama.295.6.655 [DOI] [PubMed] [Google Scholar]
- 2.Select Committee on Nutrition and Human Needs of the United States Senate . Dietary goals for the United States. 2nd edn. Washington, DC: US Government Printing Office, 1977. [Google Scholar]
- 3.Ramsden CE, Zamora D, Leelarthaepin B, et al. Use of dietary linoleic acid for secondary prevention of coronary heart disease and death: evaluation of recovered data from the Sydney diet heart study and updated meta-analysis. BMJ 2013;346:e8707. 10.1136/bmj.e8707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramsden CE, Zamora D, Majchrzak-Hong S, et al. Re-evaluation of the traditional diet-heart hypothesis: analysis of recovered data from Minnesota coronary experiment (1968-73). BMJ 2016;353:i1246. 10.1136/bmj.i1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prentice RL, Aragaki AK, Van Horn L, et al. Low-fat dietary pattern and cardiovascular disease: results from the women's health initiative randomized controlled trial. Am J Clin Nutr 2017;106:35–43. 10.3945/ajcn.117.153270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prentice RL, Aragaki AK, Howard BV, et al. Low-fat dietary pattern among postmenopausal women influences long-term cancer, cardiovascular disease, and diabetes outcomes. J Nutr 2019;149:1565–74. 10.1093/jn/nxz107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noakes TD. The women's health Initiative randomized controlled dietary modification trial: an inconvenient finding and the diet-heart hypothesis. S Afr Med J 2013;103:824–5. 10.7196/samj.7343 [DOI] [PubMed] [Google Scholar]
- 8.Prentice RL, Caan B, Chlebowski RT, et al. Low–fat dietary pattern and risk of invasive breast cancer: the women's health Initiative randomized controlled dietary modification trial. JAMA 2006;295:629–42. 10.1001/jama.295.6.629 [DOI] [PubMed] [Google Scholar]
- 9.Beresford SAA, Johnson KC, Ritenbaugh C, et al. Low-fat dietary pattern and risk of colorectal cancer: the women's health Initiative randomized controlled dietary modification trial. JAMA 2006;295:643–54. 10.1001/jama.295.6.643 [DOI] [PubMed] [Google Scholar]
- 10.Howard BV, Manson JE, Stefanick ML, et al. Low-fat dietary pattern and weight change over 7 years: the women's health Initiative dietary modification trial. JAMA 2006;295:39–49. 10.1001/jama.295.1.39 [DOI] [PubMed] [Google Scholar]
- 11.Ford C, Chang S, Vitolins MZ, et al. Evaluation of diet pattern and weight gain in postmenopausal women enrolled in the women's health Initiative observational study. Br J Nutr 2017;117:1189–97. 10.1017/S0007114517000952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tinker LF, Bonds DE, Margolis KL, et al. Low-fat dietary pattern and risk of treated diabetes mellitus in postmenopausal women: the women's health Initiative randomized controlled dietary modification trial. Arch Intern Med 2008;168:1500–11. 10.1001/archinte.168.14.1500 [DOI] [PubMed] [Google Scholar]
- 13.Shikany JM, Margolis KL, Pettinger M, et al. Effects of a low-fat dietary intervention on glucose, insulin, and insulin resistance in the women's health Initiative (WHI) dietary modification trial. Am J Clin Nutr 2011;94:75–85. 10.3945/ajcn.110.010843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Culver AL, Ockene IS, Balasubramanian R, et al. Statin use and risk of diabetes mellitus in postmenopausal women in the women's health Initiative. Arch Intern Med 2012;172:144–52. 10.1001/archinternmed.2011.625 [DOI] [PubMed] [Google Scholar]
- 15.Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet 2010;375:735–42. 10.1016/S0140-6736(09)61965-6 [DOI] [PubMed] [Google Scholar]
- 16.Noakes TD. WHIDMT: Rossouw and Howard blatantly miss the point. S Afr Med J 2014;104:262. 10.7196/SAMJ.8041 [DOI] [PubMed] [Google Scholar]
- 17.Teicholz N. The big fat surprise. Why butter, meat and cheese belong in a heathy diet. New York, NY: Simon and Schuster, 2014. [Google Scholar]
- 18.Noakes TD, Sboros M. Real food on trial. How the diet dictators tried to destroy a top scientist. UK: Columbus Publishing Ltd, 2019. [Google Scholar]
- 19.Assaf AR, Beresford SAA, Risica PM, et al. Low-fat dietary pattern intervention and health-related quality of life: the women's health Initiative randomized controlled dietary modification trial. J Acad Nutr Diet 2016;116:259–71. 10.1016/j.jand.2015.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCoy CE. Understanding the intention-to-treat principle in randomized controlled trials. West J Emerg Med 2017;18:1075–8. 10.5811/westjem.2017.8.35985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mozaffarian D, Rimm EB, Herrington DM. Dietary fats, carbohydrate, and progression of coronary atherosclerosis in postmenopausal women. Am J Clin Nutr 2004;80:1175–84. 10.1093/ajcn/80.5.1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mozaffarian D. Low-fat diet and cardiovascular disease. JAMA 2006;296:279–80. 10.1001/jama.296.3.279-b [DOI] [PubMed] [Google Scholar]
- 23.Anderson CAM, Appel LJ. Dietary modification and CVD prevention: a matter of fat. JAMA 2006;295:693–5. 10.1001/jama.295.6.693 [DOI] [PubMed] [Google Scholar]
- 24.Grundy SM. Should women be offered cholesterol lowering drugs to prevent cardiovascular disease? Yes. BMJ 2007;334:982. 10.1136/bmj.39202.399942.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kendrick M. Should women be offered cholesterol lowering drugs to prevent cardiovascular disease? no. BMJ 2007;334:983. 10.1136/bmj.39202.397488.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aberegg SK, Majure DT, Howard BV. Low-fat diet and cardiovascular disease. JAMA 2006;296:279–1. 10.1001/jama.296.3.280-a [DOI] [PubMed] [Google Scholar]
- 27.Hawking S. Black holes and baby universes and other essays. UK: Bantam Books London, 1993. [Google Scholar]
- 28.Lee I-M, Cook NR, Gaziano JM, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the women's health study: a randomized controlled trial. JAMA 2005;294:56–65. 10.1001/jama.294.1.56 [DOI] [PubMed] [Google Scholar]
- 29.Cook NR, Lee I-M, Gaziano JM, et al. Low-dose aspirin in the primary prevention of cancer: the women's health study: a randomized controlled trial. JAMA 2005;294:47–55. 10.1001/jama.294.1.47 [DOI] [PubMed] [Google Scholar]
- 30.Ridker PM, Cook NR, Lee I-M, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005;352:1293–305. 10.1056/NEJMoa050613 [DOI] [PubMed] [Google Scholar]
- 31.Dugani SB, Moorthy MV, Li C, et al. Association of lipid, inflammatory, and metabolic biomarkers with age at onset for incident coronary heart disease in women. JAMA Cardiol 2021;6:437–47. 10.1001/jamacardio.2020.7073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.University of Cape Town Faculty of Health Sciences centenary debate . Cholesterol is not an important risk factor for heart disease and dietary recommendations do more harm than good. S Afr J Clin Nutr 2012;2015:19–33. [Google Scholar]
- 33.Rossouw J. Serum cholesterol as a risk factor for coronary heart disease revisited. S Afr J Clin Nutr 2015;28:34–7. 10.1080/16070658.2015.11734523 [DOI] [Google Scholar]
- 34.Rossouw J. The diet-heart hypothesis, obesity and diabetes. S Afr J Clin Nutr 2015;28:38–43. 10.1080/16070658.2015.11734524 [DOI] [Google Scholar]
- 35.Shalaurova I, Connelly MA, Garvey WT, et al. Lipoprotein insulin resistance index: a lipoprotein particle-derived measure of insulin resistance. Metab Syndr Relat Disord 2014;12:422–9. 10.1089/met.2014.0050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garvey WT, Kwon S, Zheng D, et al. Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes 2003;52:453–62. 10.2337/diabetes.52.2.453 [DOI] [PubMed] [Google Scholar]
- 37.Goff DC, D'Agostino RB, Haffner SM, et al. Insulin resistance and adiposity influence lipoprotein size and subclass concentrations. results from the insulin resistance atherosclerosis study. Metabolism 2005;54:264–70. 10.1016/j.metabol.2004.09.002 [DOI] [PubMed] [Google Scholar]
- 38.Reaven GM, Chen YD, Jeppesen J, et al. Insulin resistance and hyperinsulinemia in individuals with small, dense low density lipoprotein particles. J Clin Invest 1993;92:141–6. 10.1172/JCI116541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Festa A, Williams K, Hanley AJG, et al. Nuclear magnetic resonance lipoprotein abnormalities in prediabetic subjects in the insulin resistance atherosclerosis study. Circulation 2005;111:3465–72. 10.1161/CIRCULATIONAHA.104.512079 [DOI] [PubMed] [Google Scholar]
- 40.Siri-Tarino PW, Sun Q, Hu FB, et al. Saturated fat, carbohydrate, and cardiovascular disease. Am J Clin Nutr 2010;91:502–9. 10.3945/ajcn.2008.26285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Volk BM, Kunces LJ, Freidenreich DJ, et al. Effects of step-wise increases in dietary carbohydrate on circulating saturated fatty acids and palmitoleic acid in adults with metabolic syndrome. PLoS One 2014;9:e113605. 10.1371/journal.pone.0113605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuipers RS, de Graaf DJ, Luxwolda MF, et al. Saturated fat, carbohydrates and cardiovascular disease. Neth J Med 2011;69:372–8. [PubMed] [Google Scholar]
- 43.Volek JS, Feinman RD. Carbohydrate restriction improves the features of metabolic syndrome. metabolic syndrome may be defined by the response to carbohydrate restriction. Nutr Metab 2005;2:31. 10.1186/1743-7075-2-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Volek JS, Fernandez ML, Feinman RD, et al. Dietary carbohydrate restriction induces a unique metabolic state positively affecting atherogenic dyslipidemia, fatty acid partitioning, and metabolic syndrome. Prog Lipid Res 2008;47:307–18. 10.1016/j.plipres.2008.02.003 [DOI] [PubMed] [Google Scholar]
- 45.Volek JS, Phinney SD, Forsythe CE, et al. Carbohydrate restriction has a more favorable impact on the metabolic syndrome than a low fat diet. Lipids 2009;44:297–309. 10.1007/s11745-008-3274-2 [DOI] [PubMed] [Google Scholar]
- 46.Hyde PN, Sapper TN, Crabtree CD, et al. Dietary carbohydrate restriction improves metabolic syndrome independent of weight loss. JCI Insight 2019;4:e128308. 10.1172/jci.insight.128308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rossello X, Raposeiras-Roubin S, Oliva B, et al. Glycated hemoglobin and subclinical atherosclerosis in people without diabetes. J Am Coll Cardiol 2021;77:2777–91. 10.1016/j.jacc.2021.03.335 [DOI] [PubMed] [Google Scholar]
- 48.Volek JS, Forsythe CE. The case for not restricting saturated fat on a low-carbohydrate diet. Nutr Metab 2005;2:21–2. 10.1186/1743-7075-2-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Demasi M, Lustig RH, Malhotra A. The cholesterol and calorie hypotheses are both dead – it is time to focus on the real culprit: insulin resistance. Clin Pharmacist 2017;9. 10.1211/PJ.2017.20203046 [DOI] [Google Scholar]
- 50.DuBroff R. Cholesterol paradox: a correlate does not a surrogate make. Evid Based Med 2017;22:15–19. 10.1136/ebmed-2016-110602 [DOI] [PubMed] [Google Scholar]
- 51.Ravnskov U, de Lorgeril M, Diamond DM, et al. LDL-C does not cause cardiovascular disease: a comprehensive review of the current literature. Expert Rev Clin Pharmacol 2018;11:959–70. 10.1080/17512433.2018.1519391 [DOI] [PubMed] [Google Scholar]
- 52.Ludwig DS, Willett WC, Volek JS, et al. Dietary fat: from foe to friend? Science 2018;362:764–70. 10.1126/science.aau2096 [DOI] [PubMed] [Google Scholar]
- 53.DuBroff R. A reappraisal of the lipid hypothesis. Am J Med 2018;131:993–7. 10.1016/j.amjmed.2018.04.027 [DOI] [PubMed] [Google Scholar]
- 54.Dehghan M, Mente A, Rangarajan S, et al. Association of dairy intake with cardiovascular disease and mortality in 21 countries from five continents (PURE): a prospective cohort study. Lancet 2018;392:2288–97. 10.1016/S0140-6736(18)31812-9 [DOI] [PubMed] [Google Scholar]
- 55.Astrup A, Geiker NRW, Magkos F. Effects of full-fat and fermented dairy products on cardiometabolic disease: food is more than the sum of its parts. Adv Nutr 2019;10:924S–30. 10.1093/advances/nmz069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Diamond DM, O'Neill BJ, Volek JS. Low carbohydrate diet: are concerns with saturated fat, lipids, and cardiovascular disease risk justified? Curr Opin Endocrinol Diabetes Obes 2020;27:291–300. 10.1097/MED.0000000000000568 [DOI] [PubMed] [Google Scholar]
- 57.Diamond DM, Alabdulgader AA, de Lorgeril M, et al. Dietary recommendations for familial hypercholesterolaemia: an evidence-free zone. BMJ Evid Based Med 2020:bmjebm-2020-111412. 10.1136/bmjebm-2020-111412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Astrup A, Magkos F, Bier DM, et al. Saturated fats and health: a reassessment and proposal for food-based recommendations. J Am Coll Cardiol 2020;76:844–57. 10.1016/j.jacc.2020.05.077 [DOI] [PubMed] [Google Scholar]
- 59.DuBroff R, de Lorgeril M. Fat or fiction: the diet-heart hypothesis. BMJ Evid Based Med 2021;26:3–7. 10.1136/bmjebm-2019-111180 [DOI] [PubMed] [Google Scholar]
- 60.Reaven GM, lecture B. Role of insulin resistance in human disease. Diabetes 1988;37:1595–607. 10.2337/diab.37.12.1595 [DOI] [PubMed] [Google Scholar]
- 61.Reaven GM, Hoffman BB. A role for insulin in the aetiology and course of hypertension? Lancet 1987;2:435–7. 10.1016/s0140-6736(87)90968-8 [DOI] [PubMed] [Google Scholar]
- 62.Ferrannini E, Buzzigoli G, Bonadonna R, et al. Insulin resistance in essential hypertension. N Engl J Med 1987;317:350–7. 10.1056/NEJM198708063170605 [DOI] [PubMed] [Google Scholar]
- 63.DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care 1991;14:173–94. 10.2337/diacare.14.3.173 [DOI] [PubMed] [Google Scholar]
- 64.Reaven GM, Lithell H, Landsberg L. Hypertension and associated metabolic abnormalities--the role of insulin resistance and the sympathoadrenal system. N Engl J Med 1996;334:374–81. 10.1056/NEJM199602083340607 [DOI] [PubMed] [Google Scholar]
- 65.Reaven GM. Insulin resistance/compensatory hyperinsulinemia, essential hypertension, and cardiovascular disease. J Clin Endocrinol Metab 2003;88:2399–403. 10.1210/jc.2003-030087 [DOI] [PubMed] [Google Scholar]
- 66.Xun P, Wu Y, He Q, He K, et al. Fasting insulin concentrations and incidence of hypertension, stroke, and coronary heart disease: a meta-analysis of prospective cohort studies. Am J Clin Nutr 2013;98:1543–54. 10.3945/ajcn.113.065565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Björntorp P. Adipose tissue distribution, plasma insulin, and cardiovascular disease. Diabete Metab 1987;13:381–5. [PubMed] [Google Scholar]
- 68.Wiebe N, Ye F, Crumley ET, et al. Temporal associations among body mass index, fasting insulin, and systemic inflammation: a systematic review and meta-analysis. JAMA Netw Open 2021;4:e211263. 10.1001/jamanetworkopen.2021.1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang AMY, Wellberg EA, Kopp JL, et al. Hyperinsulinemia in obesity, inflammation, and cancer. Diabetes Metab J 2021;45:285–311. 10.4093/dmj.2020.0250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Steinberg HO, Chaker H, Leaming R, et al. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest 1996;97:2601–10. 10.1172/JCI118709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yip J, Facchini FS, Reaven GM. Resistance to insulin-mediated glucose disposal as a predictor of cardiovascular disease. J Clin Endocrinol Metab 1998;83:2773–6. 10.1210/jcem.83.8.5005 [DOI] [PubMed] [Google Scholar]
- 72.Facchini FS, Hua N, Abbasi F, et al. Insulin resistance as a predictor of age-related diseases. J Clin Endocrinol Metab 2001;86:3574–8. 10.1210/jcem.86.8.7763 [DOI] [PubMed] [Google Scholar]
- 73.Eddy D, Schlessinger L, Kahn R, et al. Relationship of insulin resistance and related metabolic variables to coronary artery disease: a mathematical analysis. Diabetes Care 2009;32:361–6. 10.2337/dc08-0854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ginsberg HN. Insulin resistance and cardiovascular disease. J Clin Invest 2000;106:453–8. 10.1172/JCI10762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reaven G. Insulin resistance and coronary heart disease in nondiabetic individuals. Arterioscler Thromb Vasc Biol 2012;32:1754–9. 10.1161/ATVBAHA.111.241885 [DOI] [PubMed] [Google Scholar]
- 76.Ormazabal V, Nair S, Elfeky O, et al. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol 2018;17:122. 10.1186/s12933-018-0762-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Evans CE, Greenwood DC, Threapleton DE, et al. Glycemic index, glycemic load, and blood pressure: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr 2017;105:1176–90. 10.3945/ajcn.116.143685 [DOI] [PubMed] [Google Scholar]
- 78.Cucuzzella MT, Tondt T, Dockster NE. A low-carbohydrate survey: evidence for sustainable metabolic syndrome reversal. J Ins Resist 2017;2:a30. 10.4102/jir.v2i1.30 [DOI] [Google Scholar]
- 79.Hallberg SJ, McKenzie AL, Williams PT, et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: an open-label, non-randomized, controlled study. Diabetes Ther 2018;9:583–612. 10.1007/s13300-018-0373-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Athinarayanan SJ, Adams RN, Hallberg SJ, et al. Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: a 2-year non-randomized clinical trial. Front Endocrinol 2019;10:348. 10.3389/fendo.2019.00348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Walton CM, Perry K, Hart RH, et al. Improvement in glycemic and lipid profiles in type 2 diabetics with a 90-day ketogenic diet. J Diabetes Res 2019;2019:8681959. 10.1155/2019/8681959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Unwin DJ, Tobin SD, Murray SW, et al. Substantial and sustained improvements in blood pressure, weight and lipid profiles from a carbohydrate restricted diet: an observational study of insulin resistant patients in primary care. Int J Environ Res Public Health 2019;16:2680. 10.3390/ijerph16152680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Webster CC, Murphy TE, Larmuth KM, et al. Diet, diabetes status, and personal experiences of individuals with type 2 diabetes who self-selected and followed a low carbohydrate high fat diet. Diabetes Metab Syndr Obes 2019;12:2567–82. 10.2147/DMSO.S227090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Skytte MJ, Samkani A, Petersen AD, et al. A carbohydrate-reduced high-protein diet improves HbA1c and liver fat content in weight stable participants with type 2 diabetes: a randomised controlled trial. Diabetologia 2019;62:2066–78. 10.1007/s00125-019-4956-4 [DOI] [PubMed] [Google Scholar]
- 85.Choi YJ, Jeon S-M, Shin S. Impact of a ketogenic diet on metabolic parameters in patients with obesity or overweight and with or without type 2 diabetes: a meta-analysis of randomized controlled trials. Nutrients 2005;12:2005. 10.3390/nu12072005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Unwin D, Khalid AA, Unwin J, et al. Insights from a general practice service evaluation supporting a lower carbohydrate diet in patients with type 2 diabetes mellitus and prediabetes: a secondary analysis of routine clinic data including HbA1c, weight and prescribing over 6 years. BMJ Nutr Prev Health 2020;3:e000072. 10.1136/bmjnph-2020-000072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ahmed SR, Bellamkonda S, Zilbermint M, et al. Effects of the low carbohydrate, high fat diet on glycemic control and body weight in patients with type 2 diabetes: experience from a community-based cohort. BMJ Open Diabetes Res Care 2020;8:e000980. 10.1136/bmjdrc-2019-000980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McKenzie AL, Athinarayanan SJ, McCue JJ, et al. Type 2 diabetes prevention focused on normalization of glycemia: a two-year pilot study. Nutrients 2021;13:749. 10.3390/nu13030749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Moriconi E, Camajani E, Fabbri A, et al. Very-low-calorie ketogenic diet as a safe and valuable tool for long-term glycemic management in patients with obesity and type 2 diabetes. Nutrients 2021;13:758. 10.3390/nu13030758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Goldenberg JZ, Day A, Brinkworth GD, et al. Efficacy and safety of low and very low carbohydrate diets for type 2 diabetes remission: systematic review and meta-analysis of published and unpublished randomized trial data. BMJ 2021;372:m4743. 10.1136/bmj.m4743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Skytte MJ, Samkani A, Astrup A, et al. Effects of carbohydrate restriction on postprandial glucose metabolism, β-cell function, gut hormone secretion, and satiety in patients with Type 2 diabetes. Am J Physiol Endocrinol Metab 2021;320:E7–18. 10.1152/ajpendo.00165.2020 [DOI] [PubMed] [Google Scholar]
- 92.Lennerz BS, Koutnik AP, Azova S, et al. Carbohydrate restriction for diabetes: rediscovering centuries-old wisdom. J Clin Invest 2021;131:e142246. 10.1172/JCI142246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schmiegelow MD, Hedlin H, Stefanick ML, et al. Insulin resistance and risk of cardiovascular disease in postmenopausal women: a cohort study from the women's health Initiative. Circ Cardiovasc Qual Outcomes 2015;8:309–16. 10.1161/CIRCOUTCOMES.114.001563 [DOI] [PubMed] [Google Scholar]
- 94.Pan K, Chlebowski RT, Mortimer JE, et al. Insulin resistance and breast cancer incidence and mortality in postmenopausal women in the Women’s Health Initiative. Cancer 2020;126:3638–47. 10.1002/cncr.33002 [DOI] [PubMed] [Google Scholar]
- 95.Pan K, Nelson RA, Wactawski-Wende J. Insulin resistance and cancer-specific and all-cause mortality in post-menopausal women: The Women’s Health Initiative. J Natl Cancer Inst 2020;112:djz069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kraft JR. Diabetes epidemic and you. 2nd edn. Victoria, BC: Trafford, 2011. [Google Scholar]
- 97.Kraft JR. Detection of diabetes mellitus in situ (occult diabetes). Lab Med 1975;6:10–22. 10.1093/labmed/6.2.10 [DOI] [Google Scholar]
- 98.Crofts C, Schofield G, Zinn C, et al. Identifying hyperinsulinaemia in the absence of impaired glucose tolerance: an examination of the Kraft database. Diabetes Res Clin Pract 2016;118:50–7. 10.1016/j.diabres.2016.06.007 [DOI] [PubMed] [Google Scholar]
- 99.DiNicolantonio JJ, Bhutani J, OKeefe JH, et al. Postprandial insulin assay as the earliest biomarker for diagnosing pre-diabetes, type 2 diabetes and increased cardiovascular risk. Open Heart 2017;4:e000656. 10.1136/openhrt-2017-000656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mozaffarian D, Aro A, Willett WC. Health effects of trans-fatty acids: experimental and observational evidence. Eur J Clin Nutr 2009;63(Suppl 2):S5–21. 10.1038/sj.ejcn.1602973 [DOI] [PubMed] [Google Scholar]
- 101.Lawrence GD. Perspective: the saturated fat-unsaturated oil dilemma: relations of dietary fatty acids and serum cholesterol, atherosclerosis, inflammation, cancer, and all-cause mortality. Adv Nutr 2021;12:647–56. 10.1093/advances/nmab013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lim EL, Hollingsworth KG, Aribisala BS, et al. Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia 2011;54:2506–14. 10.1007/s00125-011-2204-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hite AH, Feinman RD, Guzman GE, et al. In the face of contradictory evidence: report of the dietary guidelines for Americans Committee. Nutrition 2010;26:915–24. 10.1016/j.nut.2010.08.012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The publication makes use of data already published in the scientific literature.