ABSTRACT
Background
The present study aimed to evaluate the effects of concomitant proton pump inhibitor (PPI) use on immune checkpoint inhibitor (ICI) efficacy among advanced cancer patients.
Methods and Materials
A systematic literature search of electronic database was performed to identify all potential reports. Then, meta-analyses were conducted to obtain pooled HRs with 95% CIs, which reveal the influence of PPI use on PFS and OS in patients receiving ICI treatment.
Results
A total of 7 studies with 3,647 advanced cancer patients fulfilled the inclusion criteria. The impact of PPI use was then evaluated on 3,340 patients for PFS and 3,647 patients for OS. Concomitant PPI use has a detrimental effect on the efficacy of ICIs that PPI use increased the risk of progression by 28% (HR = 1.28, 95% CI 1.17–1.40; I2 = 31.3%, Q test P = .21) when compared to those not receiving PPIs. Similarly, the meta-analysis showed that PPI use was also associated with shorter OS of advanced cancer patients receiving ICIs that PPI use increased risk of death by 39% (HR = 1.39, 95% CI 1.26–1.54; I2 = 36.5%, Q test P = .16). Sensitivity analysis showed that the pooled HRs were constant after excluding one study at a time, and no significant publication biases were detected.
Conclusion
The meta-analysis suggested that concomitant PPI use is significantly associated with low clinical benefit in ICI treatment, revealing a significantly reduced PFS and OS in advanced cancer patients receiving ICIs who are also exposed to PPI.
KEYWORDS: Immune checkpoint inhibitors, PD-1/PD-L1 inhibitor, CTLA-4 inhibitor, immunotherapy, proton pump inhibitor, progression-free survival, overall survival
Introduction
Immune checkpoint inhibitors (ICIs) have revolutionized the therapeutic management of solid tumors, further changing the treatment landscape of cancer with unprecedented effects on survival.1 The most studied immunotherapeutic drugs included cytotoxic t-lymphocyte antigen-4 (CTLA-4) inhibitors and programmed death-1/ligand-1 (PD-L1) inhibitors.2 By blocking the immune escape mechanisms of cancer, ICIs have fewer side effects and superior efficacy compared to traditional chemotherapy.3,4 Recently, immunotherapies have been proven effective in extending the life span of cancer patients. ICIs have also been approved to replace or complement chemotherapy with multiple indications, including non-small cell lung cancer (NSCLC) and melanoma.5–8 Since ICIs also have much higher efficacy among unselective patients, there is an unmet medical need to further understand the mechanisms and identify the biomarkers that are predictive of their response to ICIs. Currently, several factors have been considered to better predict ICIs efficacy, including PD-L1 expression, tumor mutation burden (TMB), and deficient mismatch repair(dMMR)/microsatellite instability-High (MSI-H), validating the use of ICIs by the Food and Drug Administration (FDA) in these indications.9–11
Beyond these classical predictive factors, intestinal microbiota has recently emerged as a potential predictor or modulator of response to ICIs. The composition and diversity of the intestinal microbiome are associated with inflammatory conditions and influence immune response, which has been an active area of interest in cancer immunotherapy.12,13 For example, Gopalakrishnan et al. reported a significant difference in the diversity and composition of gut microbiome among PD-1 inhibitor responders versus non-responders. Furthermore, the number of different species as well as their relative distribution was associated with the clinical benefits of ICIs.14 Previous studies have demonstrated that several concomitant medications during ICI treatment may affect their efficacy, such as antibiotics, which exert a significant influence on response to ICI by altering gut microbiota.14,15
Except for antibiotics, proton pump inhibitors (PPIs) have been associated with changes in the microbiome.16 Considering the hypothesized interaction between gut microbiota and ICI efficacy, several studies have examined the impact of PPI use on the survival of patients with cancer.17–25 Due to the small sample size and the conflicting nature of the findings among the different studies, there has been no consensus on the significance of concomitant PPI use with regard to the clinical benefit of ICIs treatment. In the present study, we systematically reviewed the literature regarding the association of PPI use with ICI efficacy and conducted a meta-analysis to determine the effects of PPI use on outcomes in advanced cancer patients receiving ICI treatment.
Methods and materials
Search strategy
A systematic literature search was conducted using a predetermined protocol based on guidelines set by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).26 A systematic review of the electronic databases, including PubMed, EMBASE and COCHRANE databases, was conducted for studies investigating the association of PPI use with ICI efficacy in advanced cancer patients (updated in December 2020). The comprehensive search strategy included the following Medical Subject Headings (MeSH) terms and keywords: “Immunotherapy,” “cancer,” “tumor,” “neoplasm,” “PD-1 inhibitor,” “programmed death receptor 1 inhibitor,” “PD-L1 inhibitor,” “programmed death-ligand 1 inhibitor,” “cytotoxic T lymphocyte antigen-4 inhibitor,” “CTLA-4 inhibitor,” “nivolumab,” “pembrolizumab,” “avelumab,” “durvalumab,” “atezolizumab,” and “ipilimumab.” The search was limited to studies in English, but there were no limitations on ethnicity or human subjects. The reference lists of relevant articles were also further reviewed. Finally, abstracts and presentations from all the major conference proceedings, including the American Society of Clinical Oncology (ASCO), European Society for Medical Oncology (ESMO), and American Association for Cancer Research (AACR) were also searched.
Study selection
The following inclusion criteria were used: 1) study testing the association of PPI use with PFS or OS of ICIs among advanced cancer patients and 2) data available on hazard ratios (HRs) for PFS or OS. Meanwhile, the following exclusion criteria were used: 1) without sufficiently published data or original data for calculating HRs with 95% CI; 2) reviews, case series, and case reports. The systematic review was not restricted to specific ICIs or cancer treatments. Two authors (QBD and JXD) compared the results, and any disagreements were resolved by consensus with the third-party authors (WY, LK, and ZYS).
Data extraction
The impact of concomitant PPI use on the efficacy of ICIs measured in terms of PFS and OS for advanced cancer patients was analyzed. The primary variables of interest were HRs with 95% CIs for PFS or OS. HRs with 95% CIs for PFS or OS were used to compare the PFS and OS of advanced cancer patients receiving ICIs based on exposure to PPI treatment compared with no PPI use. To adjust for the confounding factors such as other comedications, HRs from multivariate regression analysis were preferred to extract from each study. Secondary variables extracted from the studies included authors, publication year, country of origin, tumor type, ICI type, drug type, treatment line, sample size, and study endpoints. The Newcastle–Ottawa scoring system was used to evaluate the methodological quality of the included studies independently by two authors (QBD and JXD).
Statistical Analysis
The overall HRs with 95% CI for PFS or OS were used to compare the impact of concomitant PPI use on ICI treatment. The trial-specific ratios of HRs across each study were calculated based on the HRs reported in patients with or without PPI use.27–29 If the pooled HR ratio was <1, this indicated a greater treatment effect in patients with PPI use (beneficial effect of PPI use on ICIs treatment). If it was >1, this indicated a greater treatment effect in patients without PPI use (detrimental effect of PPI use on ICIs treatment). Heterogeneity of effects across studies was evaluated using the χ2-based Q test and quantified by using the I2 test.30,31 For the Q test, P < .10 represented statistically significant heterogeneity, and the I2 statistic represented the percentage of total variation between-study variation with a range of 0% to 100%. If there was no significant heterogeneity, a fixed model was used; otherwise, a random model was used. Funnel plots, Egger’s test, and Begg’s test were used to determine the possibility of publication bias. To evaluate the degree to which each study affected the overall HRs with 95% CI, sensitivity analysis was performed using the “one-study removed” method. All the statistical analyses were performed using a Comprehensive Meta-Analysis software version 3.0 (Biostat Inc., Englewood Cliffs, New Jersey, US). P-values of less than 0.05 were considered statistically significant.
Results
Study selection
A flowchart demonstrating how citations were identified and included or excluded from the study is shown in Figure 1. A total of 139 non-overlapping citations were identified and screened from the previously described electronic databases. After screening of abstracts or titles, leaving 24 studies for full-text review and eligibility assessment. After a detailed evaluation, 17 of these citations were excluded, and 7 citations met the inclusion criteria and were included in the present study.17–22,25 Agreement among reviewers regarding the eligibility of these articles was 100%.
Figure 1.
Flowchart showing article identification as well as inclusion and exclusion criteria
Characteristics of the included studies
All seven studies investigated the effects of PPI on ICI efficacy in patients with advanced metastatic cancer. A total of 3,647 advanced cancer patients were included. Three studies focused on NSCLC, one study focused on urothelial carcinoma (UC), and three studies focused on pan-cancer (i.e., NSCLC, melanoma, and renal cell carcinoma). One study was reported in Spain, one in China, one in Japan, two in Italy, and two were reported globally (OAK/POLPAR study, IMvigor210/IMvigor211 study). For immunotherapy types, two studies focused on PD-L1 inhibitor (atezolizumab), one focused on PD-1 inhibitor (Nivolumab), three focused on PD-1/L1 inhibitors or CTLA-4 inhibitor, while another study focused on PD-1/L1 inhibitors alone or combination with chemotherapy or targeted therapy. The study size range was 90 to 1,112 patients (Table 1). Immunotherapy use was first-line, second-line, and beyond treatment. The study endpoints of five studies were PFS or OS, while two studies mainly investigated OS. The quality of the four included studies was considered “good,” and another three were “moderate” based on the Newcastle–Ottawa scale system.
Table 1.
Summary of Studies Included in the Meta-analysis
| Author | Year | Country | Histological type | ICIs type | Drug | Treatment line | Total No. of Patients | No. of patients with PPI (%) | Study Endpoint | PPI window respective to ICIs start |
|---|---|---|---|---|---|---|---|---|---|---|
| Iglesias‑Santamaría. A22 | 2020 | Spain | Pan-cancer (NSCLC, RCC, bladder cancer, melanoma, and head and neck cancer) | PD-1/L1 inhibitor CTLA-4 inhibitor |
Ipilimumab, Nivolumab, Pembrolizumab, Atezolizumab |
First, Second or beyond | 102 | 76 (74.5%) | PFS/OS | Within 4 weeks before or after ICIs initiation or any time later |
| Buti S, et al.17 | 2020 | Italy | Pan-cancer (NSCLC, melanoma, and RCC) | PD-1/L1 inhibitor CTLA-4 inhibitor |
NS | First, Second or beyond | 217 | 104 (47.9%) | OS | Within 30 days before ICIs initiation |
| Cortellini A, et al.19 | 2020 | Italy | Pan-cancer (NSCLC, melanoma, and RCC) | PD-1/L1 inhibitor | Pembrolizumab, Nivolumab, Atezolizumab | First, Second or beyond | 1012 | 491 (44.2%) | PFS/OS | Within the 30 days before ICIs initiation |
| Hopkins AM, et al.21 | 2020 | Worldwide (IMvigor210, IMvigor211) |
Urothelial carcinoma | PD-L1 inhibitor | Atezolizumab | First, second | 1360 | 471 (35.0%) | PFS/OS | Within a period of 30 days prior and 30 days after ICIs initiation |
| Chalabi M, et al.18 | 2020 | Worldwide (OAK/POPLAR) |
NSCLC | PD-L1 inhibitor | Atezolizumab | Second or beyond | 757 | 234 (30.9%) | PFS/OS | Within a period of 30 days prior and 30 days after ICIs initiation |
| Hakozaki T, et al.20 | 2019 | Japan | NSCLC | PD-1 inhibitor | Nivolumab | Second or beyond | 90 | 47 (52.2%) | OS | Within 2 months before and 1 month after ICIs initiation |
| Zhao, et al.25 | 2019 | China | NSCLC | PD-1 inhibitor/PD-1 inhibitor plus chemo or apatinib | Pembrolizumab, Nivolumab, Camrelizumab | First, Second or beyond | 109 | 40 (36.7%) | PFS/OS | Within 1 month before and 1 month after ICIs initiation |
PFS: progression-free survival; OS: overall survival; RCC: renal cell carcinoma; NSCLC: non-small cell lung cancer; PD-1/L1: programmed death-1/ligand-1; PPI: proton pump inhibitor; NS: not stated.
Effect of concomitant PPI use on ICI efficacy
Five studies investigated the influence of concomitant PPI use on PFS among advanced patients receiving ICI treatment. A total of 3,340 patients were included in the meta-analysis of the association of concomitant PPI use on PFS of ICIs treatment. Compared with patients without PPI use, the meta-analysis showed that PPI use could increase the risk of progression by 28%, and the pooled HR for PFS was 1.28 (95% CI 1.17–1.40) (Figure 2a). No statistically significant heterogeneity was observed across all five studies (I2 = 31.3%, Q test P = .21).
Figure 2.
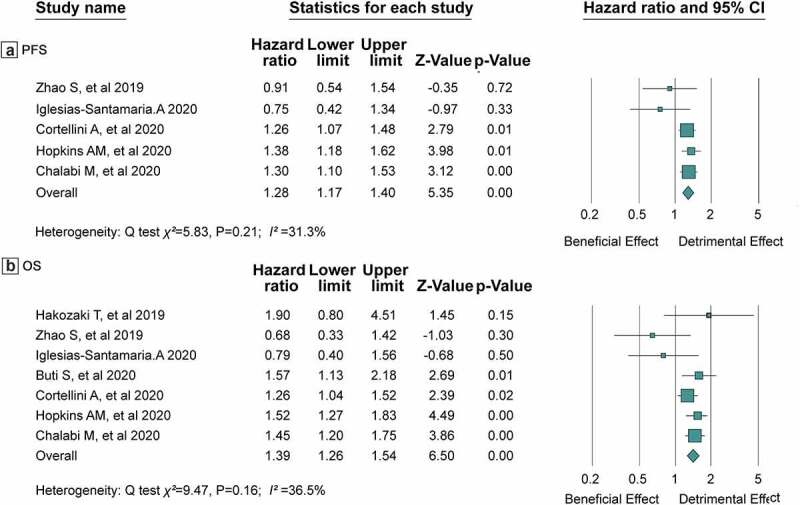
HRs of PFS(a) and OS(b) in patients receiving ICIs with concomitant PPIs use or not. HRs: Hazard Ratios; PFS: Progression-Free Survival; OS: Overall Survival
All seven studies including 3,647 advanced cancer patients described the effects of concomitant PPI use on OS among patients receiving ICI treatment. The meta-analysis showed that PPI use was also associated with shorter OS of advanced cancer patients receiving ICIs that PPI use increased the risk of death by 39% (HR = 1.39, 95% CI 1.26–1.54) (Figure 2b). Similarly, no statistically significant heterogeneity was observed across these studies (I2 = 36.5%, Q test P = .16).
Sensitivity analysis
The pooled HRs for PFS were not significantly different after excluding one study at a time in the sensitivity analysis, ranging from 1.24 (95% CI 1.11–1.38, after excluding Hopkin SM’s study) to 1.30 (95% CI 1.18–1.42, after excluding A. Iglesias-Santamaria’s study or Zhao S’s study) (Figure 3a). Moreover, the pooled HRs for OS also did not significantly change in the sensitivity analysis. The overall HRs ranged from 1.34 (95% CI, 1.19–1.51, after omitting Hopkin SM’s study) to 1.45 (95% CI 1.29–1.63, after omitting Cortellini A’s study) (Figure 3b).
Figure 3.

Sensitivity analysis for HRs of PFS (a) and OS (b)
Publication Bias
There was no evidence of publication bias for pooled HR for PFS analysis (Egger’s test: P = 1.00; Begg’s test: P = .45; Figure 4a) or pooled HR for OS (Egger’s test: P = .59, Begg’s test: P = .81; Figure 4b) across the studies. No significant publication bias was detected in other meta-analyses.
Figure 4.
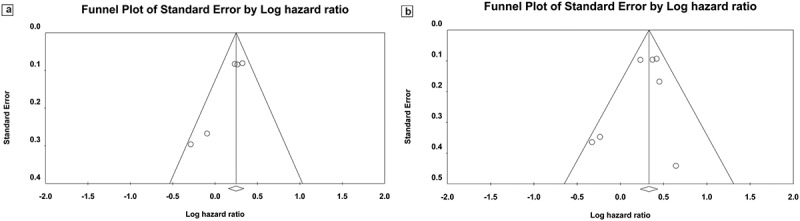
Publication bias. (a) Begg’s funnel plot of HR ratios of PFS; (b) Begg’s funnel plot of HR ratio of OS
Discussion
Accumulated evidence has demonstrated that the intestinal microbiota serve an important role in shaping systemic immune responses.12,13 Thus, further investigation has shown that concomitant medications with immune-modulatory properties through altering intestinal microbiota may affect the efficacy of ICIs among cancer patients such as antibiotics and PPIs.22,25 At present, few studies have reported the association between the PPI use and response to ICIs, but with conflicting results. Li C et al. conducted a meta-analysis including 1,167 cancer patients to investigate the impact of PPI use on the survival of cancer patients treated with ICI, suggesting concomitant ICI-PPI therapy does not appear to be significantly associated with ICI efficacy.32 However, the impact of PPI use on ICI efficacy remains poorly defined due to the small sample size. In the past year, the effect of PPI use on ICI efficacy has been investigated in several global large-scale clinical trials including IMvigor210/IMvigor211 trials. Thus, an updated systematic review and meta-analysis was required. In the present study, a systematic review was conducted to comprehensively summarize the current literature on the associations between PPI use and ICI efficacy.
The present analysis included seven studies, and the impact of PPI use was evaluated in 3,340 advanced cancer patients for PFS and 3,647 for OS. The meta-analysis showed that concomitant ICI use is associated with lower clinical benefit of ICIs with no significant between-study heterogeneity, regardless of PFS or OS. Pooled HRs suggested that PPI use increased the risk of progression by 28% and death by 39% among advanced cancer patients treated with ICIs, revealing significantly reduced clinical benefit of ICIs treatment. Among the included studies, data from OAK/POPLAR as well as IMvigor210/IMvigor211 study were consistent with the pooled results. Both studies demonstrated that concomitant PPI use influenced the efficacy and prognosis of NSCLC and UC patients who received ICI treatment.18,21 Conversely, some studies have not yielded a significant positive association between PPI use and ICI efficacy. For example, Routy et al. revealed shorter PFS and OS among NSCLC, UC, and RCC patients, but without significant statistical differences.24 Mukherjee et al. and Iglesias‑Santamaría’s studies also did not find statistically significant differences among patients with PPI.22,23 One possibility for this discrepancy is the sample size. Most studies observed shorter OS or PFS among patients with PPI use, but without statistically significant difference such as Routy et al., Mukherjee S et al., and A. Iglesias‑Santamaría’s studies.22–24 Three cohorts did not yield a significant association between PPI use and ICI efficacy, while each cohort only recruited around 100 patients.22,23,25 Thus, the present meta-analysis with larger sample sizes is required for elucidating this association.
The present study did not elucidate causality between PPI use and ICI efficacy nor did it identify the underlying mechanisms of action involved. However, it contributes to the hypothesis of a potential link between PPI exposure and ICI efficacy. Most studies have promoted the vital role of gut microbiota in the association between PPI use and immunotherapy.12,13 In contrast to antibiotics, the mechanisms are indirect. PPI may alter the pH levels of the gut and change the number and types of bacteria that pass through the stomach through inhibition of gastric acid secretion.33 Notably, taxa enriched by the PPI-induced pH changes are also important for ICI response, and PPI use may affect the clinical outcome of ICIs. The above mechanisms are a potential explanation for this association, but the actual mechanisms remain unclear. Thus, basic research is also needed on the possible mechanisms of action of interactions between PPI use and decreased ICI efficacy.
Recently, ICIs have made remarkable achievements in the treatment of gastrointestinal cancer including colorectal cancer, gastric cancer, esophageal cancer, etc.34–36 Several studies have suggested the potential association of PPI use with the risk of gastrointestinal tumor via the regulation of the composition and diversity of gut microbiota.37,38 In the present study, no included study has focused on gastrointestinal tumor. Thus, it remains unclear whether the negativity of PPI use on ICIs efficacy is more pronounced in patients with gastrointestinal cancer, compared with other solid tumors. The impact of PPI use on gastrointestinal tumor patients receiving ICI treatment needs to be investigated in the future. In addition, the subgroup analysis stratified by tumor type could not be performed due to small sample size. Further research on the effect of individual ICIs in different cancer patients is required.
Accumulating evidence suggested that, in addition to the efficacy of ICIs, the occurrence of immune-related adverse events (irAEs) following ICI treatment may also be affected by intestinal microbiota.39–42 Dubin et al. conducted a prospective study of a cohort of melanoma patients receiving CTLA-4 inhibitor, found that the development of ICI-associated colitis could correlate with specific intestinal microflora.40 Patients in the non-colitis group had a higher abundance of Bacteroidetes. Meanwhile, Chaput et al. reported that the high abundance of Bacteroidetes was closely associated with a longer colitis-free therapy period, but poor prognosis. Conversely, patients with a high abundance of Faecalibacterium or Firmicutes were more likely to develop colitis while having better survival.39 This evidence demonstrated that specific intestinal microbiota could influence the susceptibility to colitis and therapeutic effect of ICIs, suggesting the existence of a delicate balance with respect to the tumor-killing versus colitis-inducing effects of ICI treatment.43 These findings suggest a new therapeutic microbiota-based paradigm for boosting the efficacy of ICIs or mitigating irAEs.41,42 However, only a few investigations concerning the association between PPI use and irAEs following ICI treatment has been reported until now. Hopkins AM et al.’s study based on the pooled analysis of IMvigor210/IMvigor211 trials demonstrated that no significant association between PPI use and the first occurrence of atezolizumab-induced AE was identified.21 However, due to the small sample size and nature of retrospective study, the potential mechanisms underlying these phenomena and accurate associations of PPI use with irAEs occurrence required to be extensively explored in future preclinical or clinical studies.
Although there was no significant heterogeneity across the included studies and no study solely responsible for the pooled effect in leave-one-out analysis, some confounding factors might affect the pooled conclusion. First, the PPI type and dose. The capacity for acid suppression among different types and doses of PPIs may lead to different alterations in the gut microbiome, potentially affecting the response to anti-PD-1/PD-L1 treatment. However, this information was not reported across these studies. The second is the ICI treatment regimen. Most studies reported efficacy of ICIs alone, while Zhou et al.’s study included patients treated with ICI alone, in combination with chemotherapy, or in combination with anti-VEGFR targeted therapy.25 After excluding this study, the sensitivity analysis showed that the pooled HR for PFS or OS remained consistent. Future studies should also further explore the effects of PPI use among patients treated with ICIs combined with chemotherapy during the first line, which is becoming the more commonly used method. In addition, the PPI window respective to ICIs start. Lurienne et al.’s study demonstrated that the influence of antibiotics on ICI immunotherapy among NSCLC patients depended on the time window of exposure, with stronger effects reported when the patients took antibiotics (−60 days to 60 days) around ICI initiation.44 Among these included studies, most PPI use was within 1–2 months before and 1 month after ICI initiation, but the subgroup analysis could not be conducted due to the inadequate information. Analysis confirmed that the association between PPI window respective to ICI initiation and ICI efficacy was also required. Thus, a larger prospective study adjusting for these confounding factors should be conducted to better understand the relationship between PPI use and ICI efficacy.
The recent meta-analysis of more than 3,000 patients yielded a positive association between PPI use and ICI efficacy without any significant heterogeneity across the included studies. Based on these results, in clinical practice, concomitant drugs could be held or replaced if suspected to have a detrimental interaction with response to ICIs. This is certainly the case for non-vital medications such as PPIs, which could be discontinued. Of course, the present study has several limitations. Because this topic has been discussed in recently, so the number of studies included was relatively small. Although there is no significant heterogeneity across those studies with more than 3,000 cases, but small sample size may affect the accuracy and reliability of the conclusions. Second, although we preferred HR with 95% CI from multivariate survival analysis, it was impossible to completely exclude the influence of confounding factors inherent (gut modulators such as diet, geography, or other concomitant medications) among these patients. Third, the most used study protocol was a retrospective design. Retrospective studies sometimes lack control over variables that can alter outcomes. Despite these limitations, this study provides a comprehensive summary of the current literature.
In conclusion, this study evaluated the effect of concomitant PPI use on ICI efficacy in advanced cancer patients by systematically reviewing the relevant literature. The findings demonstrated that PPI use during ICI treatment initiation was correlated with decreased PFS and OS, which is hypothesized to decrease ICI efficacy among advanced cancer patients. The findings also highlight the need for larger prospective studies while adjusting for other confounding factors and evaluating patient survival and changes in intestinal microbiota affected by PPI use.
Acknowledgments
This work was supported by the Shanghai Sailing Program [grant number 17YF1425200, 2017]; Chinese National Natural Science Funding [grant number 81702249, 2017]; and Science and Technology Commission of Shanghai Municipality [grant number 17511103403, 2017]. The funder has no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of Interest
All authors declare no conflicts of interest.
References
- 1.Billan S, Kaidar-Person O, Gil Z.. Kaidar-Person O and Gil Z. Treatment after progression in the era of immunotherapy. Lancet Oncol. 2020;21(10):e463–9. doi: 10.1016/S1470-2045(20)30328-4. [DOI] [PubMed] [Google Scholar]
- 2.Postow MA, Callahan MK, Wolchok JD. Callahan MK and Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol. 2015;33(17):1974–1982. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, Castro G Jr., Srimuninnimit V, Laktionov KK, Bondarenko I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 4.Nosaki K, Saka H, Hosomi Y, Baas P, De Castro G Jr., Reck M, Wu YL, Brahmer JR, Felip E, Sawada T, et al. Kush DA and Lopes G. Safety and efficacy of pembrolizumab monotherapy in elderly patients with PD-L1-positive advanced non-small-cell lung cancer: pooled analysis from the KEYNOTE-010, KEYNOTE-024, and KEYNOTE-042 studies. Lung Cancer. 2019;135:188–195. doi: 10.1016/j.lungcan.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Gadgeel S, Rodriguez-Abreu D, Speranza G, Esteban E, Felip E, Domine M, Hui R, Hochmair MJ, Clingan P, Powell SF, et al. Pietanza MC and Garassino MC. Updated Analysis From KEYNOTE-189: pembrolizumab or Placebo Plus Pemetrexed and Platinum for Previously Untreated Metastatic Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol. 2020;38(14):1505–1517. doi: 10.1200/JCO.19.03136. [DOI] [PubMed] [Google Scholar]
- 6.Gettinger S, Horn L, Jackman D, Spigel D, Antonia S, Hellmann M, Powderly J, Heist R, Sequist LV, Smith DC, et al. Five-Year Follow-Up of Nivolumab in Previously Treated Advanced Non-Small-Cell Lung Cancer: results From the CA209-003 Study. J Clin Oncol. 2018;36(17):1675–1684. doi: 10.1200/JCO.2017.77.0412. [DOI] [PubMed] [Google Scholar]
- 7.Paz-Ares L, Vicente D, Tafreshi A, Robinson A, Soto Parra H, Mazieres J, Hermes B, Cicin I, Medgyasszay B, Rodriguez-Cid J, et al. Placebo-controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: protocol-specified final analysis of KEYNOTE-407. J Thorac Oncol. 2020;15(10):1657–1669. doi: 10.1016/j.jtho.2020.06.015. [DOI] [PubMed] [Google Scholar]
- 8.Keilholz U, Pa A, Dummer R, Robert C, Lorigan P, Van Akkooi A, Arance A, Cu B, Chiarion SV, Donia M, et al. Testori A and Michielin O. ESMO consensus conference recommendations on the management of metastatic melanoma: under the auspices of the ESMO Guidelines Committee. Ann Oncol. 2020;31(11):1435–1448. doi: 10.1016/j.annonc.2020.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, Chung HC, Kindler HL, Lopez-Martin JA, Miller WH Jr., et al. Norwood K and Bang YJ. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21(10):1353–1365. doi: 10.1016/S1470-2045(20)30445-9. [DOI] [PubMed] [Google Scholar]
- 10.Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, Geva R, Gottfried M, Penel N, Hansen AR, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase ii KEYNOTE-158 Study. J Clin Oncol. 2020;38:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al. Pietanza MC and Brahmer JR. Updated Analysis of KEYNOTE-024: pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J Clin Oncol. 2019;37(7):537–546. doi: 10.1200/JCO.18.00149. [DOI] [PubMed] [Google Scholar]
- 12.Geva-Zatorsky N, Sefik E, Kua L, Pasman L, Tan TG, Ortiz-Lopez A, Yanortsang TB, Yang L, Jupp R, Mathis D. Benoist C and Kasper DL. Mining the human gut microbiota for immunomodulatory organisms. Cell. 2017;168(5):928–943 e911. doi: 10.1016/j.cell.2017.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schluter J, Peled JU, Taylor BP, Markey KA, Smith M, Taur Y, Niehus R, Staffas A, Dai A, Fontana E, et al. The gut microbiota is associated with immune cell dynamics in humans. Nature. 2020;588(7837):303–307. doi: 10.1038/s41586-020-2971-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman K, Wei SC, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359(6371):97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanoue T, Morita S, Plichta DR, Skelly AN, Suda W, Sugiura Y, Narushima S, Vlamakis H, Motoo I, Sugita K, et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature. 2019;565:600–605. doi: 10.1038/s41586-019-0878-z. [DOI] [PubMed] [Google Scholar]
- 16.Rogers MAM, Aronoff DM. The influence of non-steroidal anti-inflammatory drugs on the gut microbiome. Clin Microbiol Infect. 2016;22(2):178 e171–178 e179. doi: 10.1016/j.cmi.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buti S, Bersanelli M, Perrone F, Tiseo M, Tucci M, Adamo V, Stucci LS, Russo A, Tanda ET, Spagnolo F, et al. Ascierto PA and Cortellini A. Effect of concomitant medications with immune-modulatory properties on the outcomes of patients with advanced cancer treated with immune checkpoint inhibitors: development and validation of a novel prognostic index. Eur J Cancer. 2020;142:18–28. doi: 10.1016/j.ejca.2020.09.033. [DOI] [PubMed] [Google Scholar]
- 18.Chalabi M, Cardona A, Nagarkar DR, Dhawahir Scala A, Gandara DR, Rittmeyer A, Albert ML, Powles T, Kok M, Fg H, et al. Efficacy of chemotherapy and atezolizumab in patients with non-small-cell lung cancer receiving antibiotics and proton pump inhibitors: pooled post hoc analyses of the OAK and POPLAR trials. Ann Oncol. 2020;31(4):525–531. doi: 10.1016/j.annonc.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Cortellini A, Tucci M, Adamo V, Stucci LS, Russo A, Tanda ET, Spagnolo F, Rastelli F, Bisonni R, Santini D, et al. Ficorella C and Ascierto PA. Integrated analysis of concomitant medications and oncological outcomes from PD-1/PD-L1 checkpoint inhibitors in clinical practice. J Immunother Cancer. 2020;8:e001361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hakozaki T, Okuma Y, Omori M, Hosomi Y. Omori M and Hosomi Y. Impact of prior antibiotic use on the efficacy of nivolumab for non-small cell lung cancer. Oncol Lett. 2019;17(3):2946–2952. doi: 10.3892/ol.2019.9899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hopkins AM, Kichenadasse G, Karapetis CS, Rowland A, Sorich MJ. Rowland A and Sorich MJ. concomitant proton pump inhibitor use and survival in urothelial carcinoma treated with atezolizumab. Clin Cancer Res. 2020;26(20):5487–5493. doi: 10.1158/1078-0432.CCR-20-1876. [DOI] [PubMed] [Google Scholar]
- 22.Iglesias-Santamaria A. Impact of antibiotic use and other concomitant medications on the efficacy of immune checkpoint inhibitors in patients with advanced cancer. Clin Transl Oncol. 2020;22(9):1481–1490. doi: 10.1007/s12094-019-02282-w. [DOI] [PubMed] [Google Scholar]
- 23.Mukherjee S, Ibrahimi S, Khalid B, Roman D, Zhao D, Aljumaily R. Zhao D and Aljumaily R. Do proton pump inhibitors modulate the efficacy of anti-PD-1/PD-L1 therapy? A retrospective study. J Oncol Pharm Pract. 2019;25(3):762–764. doi: 10.1177/1078155218771152. [DOI] [PubMed] [Google Scholar]
- 24.Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, et al. Kroemer G and Zitvogel L. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 25.Zhao S, Gao G, Li W, Li X, Zhao C, Jiang T, Jia Y, He Y, Li A, Su C, et al. Chen X and Zhou C. Antibiotics are associated with attenuated efficacy of anti-PD-1/PD-L1 therapies in Chinese patients with advanced non-small cell lung cancer. Lung Cancer. 2019;130:10–17. doi: 10.1016/j.lungcan.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 26.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349(jan02 1):g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 27.Altman DG, Bland JM, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326(7382):219. doi: 10.1136/bmj.326.7382.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conforti F, Pala L, Bagnardi V, Viale G, De Pas T, Pagan E, Pennacchioli E, Cocorocchio E, Ferrucci PF, De Marinis F, et al. Sex-based heterogeneity in response to lung cancer immunotherapy: a systematic review and meta-analysis. J Natl Cancer Inst. 2019;111(8):772–781. doi: 10.1093/jnci/djz094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qin BD, Jiao XD, Liu J, Liu K, He X, Wu Y, Ling Y, Duan XP, Qin WX, Wang Z, et al. The effect of liver metastasis on efficacy of immunotherapy plus chemotherapy in advanced lung cancer. Crit Rev Oncol Hematol. 2020;147:102893. doi: 10.1016/j.critrevonc.2020.102893. [DOI] [PubMed] [Google Scholar]
- 30.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 31.Higgins JP, Thompson SG, Jj D, Dg A. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li C, Xia Z, Li A, Meng J. The effect of proton pump inhibitor uses on outcomes for cancer patients treated with immune checkpoint inhibitors: a meta-analysis. Ann Transl Med. 2020;8(24):1655. doi: 10.21037/atm-20-7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Imhann F, Bonder MJ, Vich Vila A, Fu J, Mujagic Z, Vork L, Tigchelaar EF, Jankipersadsing SA, Cenit MC, Harmsen HJ, et al. Weersma RK and Zhernakova A. Proton pump inhibitors affect the gut microbiome. Gut. 2016;65(5):740–748. doi: 10.1136/gutjnl-2015-310376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andre T, Amonkar M, Norquist JM, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt CJA, Smith D, Garcia-Carbonero R, et al. Health-related quality of life in patients with microsatellite instability-high or mismatch repair deficient metastatic colorectal cancer treated with first-line pembrolizumab versus chemotherapy (KEYNOTE-177): an open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22(5):665–677. doi: 10.1016/S1470-2045(21)00064-4. [DOI] [PubMed] [Google Scholar]
- 35.Kojima T, Shah MA, Muro K, Francois E, Adenis A, Hsu C-H, Doi T, Moriwaki T, Kim S-B, Lee S-H, et al. Randomized Phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol. 2020;38(35):4138–4148. doi: 10.1200/JCO.20.01888. [DOI] [PubMed] [Google Scholar]
- 36.Smyth EC, Gambardella V, Cervantes A, Fleitas T. Checkpoint inhibitors for gastroesophageal cancers: dissecting heterogeneity to better understand their role in first-line and adjuvant therapy. Ann Oncol. 2021;32(5):590–599. doi: 10.1016/j.annonc.2021.02.004. [DOI] [PubMed] [Google Scholar]
- 37.Brusselaers N, Lagergren J, Engstrand L. Duration of use of proton pump inhibitors and the risk of gastric and oesophageal cancer. Cancer Epidemiol. 2019;62:101585. doi: 10.1016/j.canep.2019.101585. [DOI] [PubMed] [Google Scholar]
- 38.Lei WY, Wang JH, Yi CH, Liu TT, Hung JS, Wong MW, Bair MJ, Vaezi MF, Wc O, Cl C. Association between use of proton pump inhibitors and colorectal cancer: a nationwide population-based study. Clin Res Hepatol Gastroenterol. 2021;45:101397. doi: 10.1016/j.clinre.2020.02.017. [DOI] [PubMed] [Google Scholar]
- 39.Chaput N, Lepage P, Coutzac C, Soularue E, Le Roux K, Monot C, Boselli L, Routier E, Cassard L, Collins M, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol. 2019;30(12):2012. doi: 10.1093/annonc/mdz224. [DOI] [PubMed] [Google Scholar]
- 40.Dubin K, Callahan MK, Ren B, Khanin R, Viale A, Ling L, No D, Gobourne A, Littmann E, Huttenhower C, et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat Commun. 2016;7(1):10391. doi: 10.1038/ncomms10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang F, Yin Q, Chen L, Mm D. Bifidobacterium can mitigate intestinal immunopathology in the context of CTLA-4 blockade. Proc Natl Acad Sci U S A. 2018;115(1):157–161. doi: 10.1073/pnas.1712901115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y, Wiesnoski DH, Helmink BA, Gopalakrishnan V, Choi K, DuPont HL, Jiang ZD, Abu-Sbeih H, Sanchez CA, Chang CC, et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat Med. 2018;24(12):1804–1808. doi: 10.1038/s41591-018-0238-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luo B, Zhang Y, Zhang C, Liu X, Shi C. Intestinal microbiota: a potential target for enhancing the antitumor efficacy and reducing the toxicity of immune checkpoint inhibitors. Cancer Lett. 2021;509:53–62. doi: 10.1016/j.canlet.2021.04.001. [DOI] [PubMed] [Google Scholar]
- 44.Lurienne L, Cervesi J, Duhalde L, De Gunzburg J, Andremont A, Zalcman G, Buffet R, Bandinelli PA. NSCLC immunotherapy efficacy and antibiotic use: a systematic review and meta-analysis. J Thorac Oncol. 2020;15(7):1147–1159. doi: 10.1016/j.jtho.2020.03.002. [DOI] [PubMed] [Google Scholar]


