Abstract
Many skin manifestations of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection reflect activation of cutaneous and systemic immune responses involving effector pathways of both the innate and adaptive arms of the immune system. This article reviews evidence from the recent clinical and scientific literature that informs the current understanding of the consequences of coronavirus disease 2019 (COVID-19)–induced immune cell activation, as relevant to dermatology. Topics include the clinical consequences of autoantibody production in patients with COVID-19, immunologic evidence for chilblains as a manifestation of SARS-CoV-2 infection, and the relationship between type I interferons and COVID-19 disease severity.
Keywords: COVID-19, SARS-CoV-2, Immunology, Autoantibody, Type I interferon, Chilblains, Livedo
Key points
-
•
Both protective and autoreactive antibodies are produced during coronavirus disease 2019 (COVID-19) infection.
-
•
Autoreactive antibody (autoantibody) targets detected in patients with COVID-19 include phospholipids as well as proteins expressed in skin, connective tissue, and vasculature.
-
•
Autoantibodies likely contribute to the pathogenesis of multisystem inflammatory syndrome in children.
-
•
Viral nucleotide sensing by the innate immune system may provide a mechanistic basis for the chilblains–COVID-19 association.
-
•
Chilblains and severe COVID-19 lie at 2 ends of a type I interferon spectrum.
Introduction
Collaborative efforts among the international dermatology community have facilitated significant progress over the last year toward delineating the constellation of skin manifestations of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection.1 , 2 These cutaneous manifestations range from early viral exanthems to vasculopathy or vasculitis-associated–like skin lesions that typically appear weeks after systemic symptom onset (eg, livedo, acral cyanosis, chilblains, purpura).3, 4, 5, 6, 7, 8, 9, 10, 11 Like fever, malaise, cough, and other common signs of viral infection, skin manifestations of coronavirus disease 2019 (COVID-19) reflect activation of both localized and systemic immune responses to the pathogen. Similar to other viruses, SARS-CoV-2 triggers both innate and adaptive immune responses, including complement, antibody-mediated, T cell–mediated, and cytokine-mediated inflammatory pathways.12 This article discusses the ways in which dysregulation of the immune response contributes to COVID-19 pathology, with an emphasis on cutaneous manifestations of disease, as well as insights from genetic and autoimmune disease pathology. Elucidating the protective and pathologic features of the anti–SARS-CoV-2 immune response is directly relevant to clinical dermatologists because understanding mechanisms of disease is always an important first step toward optimizing skin-directed and systemic treatment.
Evidence of Intravascular Complement Activation in the Presence of Severe Acute Respiratory Syndrome Coronavirus-2 Proteins Highlights Contributions of Innate Immunity to Coronavirus Disease 2019–Associated Skin Conditions
Early clinical reports of critically ill patients with COVID-19 described skin findings suggestive of underlying vasculitis or vasculopathy, including livedo reticularis or racemosa, retiform purpura, and even acroischemia evolving to cyanosis and dry gangrene.4, 5, 6, 7, 8 , 13 These observations supported the hypothesis that COVID-19 infection induces a hypercoagulable state in skin as in other organs.14 When skin from patients with retiform purpura was biopsied, SARS-CoV-2 envelope and spike proteins were found deposited near vascular endothelial cells, along with striking amounts of complement.4 , 8 , 13 , 15 Detected components of the complement cascade included elements of the lectin pathway as well as the terminal effector molecules of the membrane attack complex, proteins C5b to C9. Detection of complement deposition in situ in skin was informative because it suggested that this fundamental component of the innate immune system could trigger an overly vigorous immune response to the perceived threat and act as an inciting event to drive changes that ultimately result in immune disorder and harm to the host. The complement cascade is capable of activating the coagulation cascade,16 and patients with COVID-19 with evidence of severe cutaneous vascular disorder also showed increased serum D-dimer levels and fibrin thrombi within cutaneous vessels.4, 5, 6, 7, 8 , 13 Therefore, detection of complement in these early case reports from critically ill patients highlighted the important possibility that the immune response to SARS-CoV-2 proteins could play a central role in initiating this cutaneous vascular disorder, and COVID-19–associated skin disorder generally.
Humoral Immune Responses
Severe acute respiratory syndrome coronavirus-2 infection leads to activation of a humoral immune response and protective antibody production
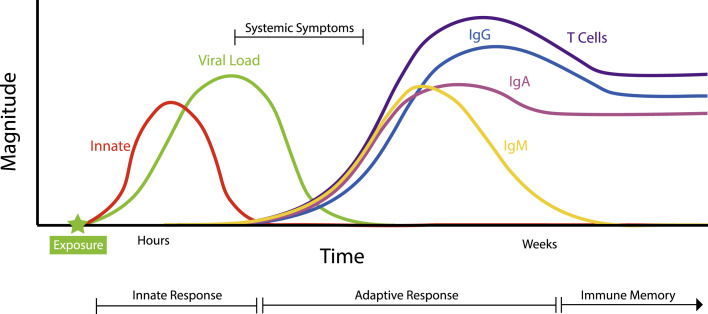
The immune response to SARS-CoV-2 features the same fundamental building blocks that are used by the immune system to respond to all pathogens (Fig. 1 ). Epithelial and mucosal surfaces form a physical and chemical barrier to invasion. Breach of these defenses leads to immediate activation of the innate arm of the immune system. The innate immune system is able to recognize common molecular patterns unique to pathogens, including viruses such as SARS-CoV-2, allowing rapid mobilization of defensive cells and molecules within hours of infection, such as activation of the complement cascade and production of type I interferons (IFNs), as discussed in this article. Often the actions of the innate immune system are sufficiently efficacious to dispel the threat to the host. However, a second essential function of the innate immune system is to instruct the adaptive immune system on the presence and nature of a pathogenic challenge. Although it takes weeks for an adaptive immune response by B and T lymphocytes to develop, these cells are capable of iteratively adapting their antigen receptor DNA sequences to evolve exquisitely specific capacities for recognition of individual pathogens. On recognition of pathogen-derived antigens, B and T lymphocytes target those pathogens with several effector mechanisms, one of which is the production of antibodies by B cell–derived plasma cells.17
Fig. 1.
Working model of the typical immune response to SARS-CoV-2. The progression of the typical immune response to SARS-CoV-2 in a patient with nonsevere disease.12,17,18,20,22,82 The innate immune response is triggered immediately following viral exposure and acts to instruct the adaptive immune system. The adaptive immune response, consisting of lymphocyte activation and antibody production, takes several days to weeks to manifest. Although an increase in pathogen-specific immunoglobulin (Ig) M level usually precedes the appearance of other antibody isotypes, available data for patients with COVID-19 do not support a significantly earlier appearance of IgM compared with IgA and IgG.18,22 IgA is found in serum as well as in saliva and respiratory tract secretions, and the magnitude and timing of its appearance in these two fluids may differ (not depicted).22 T cells and B cells secreting IgG and IgA are maintained following infection, leading to immunologic memory.24,25
The fundamental elements of the immune response to SARS-CoV-2 have been reviewed elsewhere,12 but for the purposes of this review it is worth noting that the human adaptive immune system contains both T and B cells that can recognize the virus. Antibodies against SARS-CoV-2 viral components are detected in the serum and nasopharynx of infected patients.18, 19, 20, 21, 22 Multiple classes of antibodies called isotypes are produced in the course of SARS-CoV-2 infection, including immunoglobulin (Ig) M, IgA, and IgG.18 , 22 IgG is found in the bloodstream and in the extracellular spaces in tissues, and is the main isotype associated with disease protection. However, because SARS-CoV-2 is a respiratory virus that first encounters the immune system at mucosal surfaces, IgA also plays an important role. An initial large wave of IgA+ antibody-secreting B cells is in seen in the blood of patients, peaking between 10 and 15 days after symptom onset, followed by a wave of IgG+ antibody-secreting B cells that become dominant by day 22.22 IgA+ cells express receptors for homing to mucosal tissues such as the respiratory tract, where they secrete IgA in a dimeric form. Studies on patients with COVID-19 have shown that dimeric IgA antibodies from the respiratory tract were many-fold more potent at SARS-CoV-2 neutralization compared with IgG, and remained detectable in saliva for several months after infection.21 , 22
Although severe cases of COVID-19 tend to generate higher titers of antibodies, some patients develop antibody responses even with mild or asymptomatic disease,19 including skin-only presentations.23 Low-titer to moderate-titer antibody production after mild infection has been shown to be sufficient for neutralizing viral entry,19 and the antibodies generated seem to be long lived, having been measured up to 8 months following infection.24 , 25
The exquisite specificity of T and B lymphocytes is a powerful weapon in the arsenal of the immune system, but dysregulation of the adaptive response can lead these effector cells to become misguided, targeting host-derived self-antigens instead of foreign pathogen–derived antigens, in a process called autoreactivity. It is well established that viral infections can trigger autoreactive antibody production in susceptible individuals.26 This article discusses growing evidence that dysregulation of the humoral immune response is a hallmark of SARS-CoV-2 infection, and may contribute to skin manifestations of COVID-19–associated disease.
Coronavirus disease 2019 infection can trigger autoreactive antibody formation
Antiphospholipid antibodies
One mechanism by which autoantibody generation is thought to occur after infection is molecular mimicry, a naturally occurring phenomenon wherein T cells or antibody-producing B cells generated against pathogen-specific antigens also, by chance, bind host tissue because of structural similarity between pathogen-encoded proteins and host proteins.27 A well-defined example of molecular mimicry occurs in rheumatic fever, in which antibodies generated during Streptococcus pyogenes infection react not only against S pyogenes but also against normal cardiac proteins.28 Another group of autoantibodies thought to arise as a result of molecular mimicry are antiphospholipid antibodies; that is, antibodies that bind phospholipids and phospholipid-binding proteins present in cell membranes.29 Intriguingly, 2 case series reported detection of antiphospholipid autoantibodies in approximately half of critically ill patients with COVID-19.30 , 31 Other viral infections have been shown to increase the risk of developing antiphospholipid autoantibodies, and although in most cases these autoantibodies are present only transiently, persistent serum antiphospholipid antibodies have been reported in some patients and have been linked to thromboembolic events.27 The presence of antiphospholipid antibodies in patients with COVID-19 is of note when considering skin manifestations of SARS-CoV-2. The intense deposition of complement appreciable in cutaneous biopsies from patients with COVID-19 with retiform purpura is reminiscent of what can be seen in antiphospholipid syndrome (APS),29 a condition that can also manifest with retiform purpura and livedo reticularis.32 In APS, the binding of antiphospholipid autoantibodies to endothelial cell membranes can tip the vasculature toward a prothrombotic state.29
Given the association between postviral or APS-associated antiphospholipid antibody production and thromboembolus formulation, it can be hypothesized that antiphospholipid autoantibodies may also contribute to late-onset vascular skin findings in patients with COVID-19. The time period between COVID-19 symptom onset and presentation of respiratory distress and livedo8 roughly corresponds to the minimum window required for B-cell activation and antibody production. Furthermore, mouse models of APS also show deposition of C5b to C9 on the endothelial vasculature, as seen in patients with COVID-19, with the complement cascade in these mouse models playing a critical role in triggering the characteristic thromboses.29 When researchers injected purified IgG from patients with COVID-19 into mouse models of thrombosis, the immunoglobulin from patients with COVID-19 accelerated thrombus formation in vivo, at levels comparable with immunoglobulin from patients with catastrophic APS.31 Taken together, these data allow us to speculate that local immune stimulation, as shown by SARS-CoV-2 spike protein staining in the cutaneous vasculature,4 , 8 , 13 , 15 may synergize with antiphospholipid autoantibodies found in patients with severe COVID-19 to jointly trigger the complement cascade and stimulate thrombosis. This thrombosis may result in rashes associated with underlying vascular insults: livedo reticularis/racemosa, retiform purpura, and acroischemia.
Multisystem inflammatory syndrome in children
Another COVID-19 disease process that may result from a dysregulated immune response leading to autoantibody production is the rare multisystem inflammatory syndrome in children (MIS-C). MIS-C presents weeks after mild or asymptomatic infection with SARS-CoV-2 and is marked by high fever, increased levels of inflammatory markers, end-organ damage, and mucocutaneous findings.33, 34, 35, 36 This clinical presentation is similar to that seen in Kawasaki disease, a medium-sized vessel vasculitis that commonly presents in children and is thought to result from the production of autoantibodies in response to acute viral infection.37 Children with MIS-C show an altered immune profile compared with those with uncomplicated acute COVID-19 infection,34, 35, 36 and several lines of evidence support the hypothesis that, like Kawasaki disease, MIS-C may represent a postinfectious inflammatory episode driven by SARS-CoV-2. First, MIS-C presents 4 to 6 weeks after confirmed SARS-CoV-2 expression, and it responds well to immunomodulatory therapies such as intravenous immunoglobulin, suggesting the involvement of circulating factors produced during the adaptive immune response.34, 35, 36 , 38 Second, profiling of plasma from patients with MIS-C revealed the presence of autoantibodies that react to known autoantigens Jo-1 and La; to proteins involved in immune signaling pathways; and to factors expressed in normal endothelial, cardiac, and mucosal tissues.34 , 35 Strikingly, these autoantibody targets mirror many organ system manifestations of MIS-C disease.34 , 35 Children with MIS-C were shown to have increased numbers of proliferating antibody-secreting cells weeks after clearance of the virus, suggesting these cells may potentially be autoreactive, autoantibody-producing clones.36 Furthermore, circulating neutrophils and monocytes in affected children showed increased expression of CD64, an Fc receptor that can engage autoantibodies and immune complexes, triggering tissue injury.39 , 40 In addition, serum IgG from severely ill patients with MIS-C was shown to bind more tightly to cardiac endothelial cells in culture compared with serum from children with moderate MIS-C.36 Although the pathophysiology behind this disease is just beginning to be elucidated, it can be posited that the severe inflammation, end-organ damage, and mucocutaneous responses seen in MIS-C are a result of an autoantibody-directed or immune-complex–directed attack against the host.
Broad autoreactivity found in coronavirus disease 2019 patient sera
The broad spectrum of autoantibody tissue reactivity observed in case studies of children with MIS-C34 suggests that molecular mimicry is insufficient to explain the large number of autoreactive lymphocytes that target such a wide variety of host antigens in multiple tissues. A recent article describes efforts to profile autoantibody-antigen specificities in adults with acute COVID-19 disease, allowing an exploration of the hypothesis that misguided antibody production could contribute to the wide spectrum of clinical presentations seen not only in MIS-C but in acute SARS-CoV-2 infection as well.41 Remarkably, patients with COVID-19 show a dramatic increase in circulating autoantibodies compared with healthy controls, with the number of distinct autoantibody targets increasing with disease severity. For at least 10% of the autoreactivities identified, the autoreactive clones developed over the course of disease progression, implying that these 10% of autoreactivities are newly acquired, presumably as a result of COVID-19 infection. Many of these autoantibodies targeted various cells and tissues, including components of the immune system. The investigators were able to show that the autoantibodies corresponded to downstream alterations in their targeted immune signaling pathways or leukocyte frequencies. In some cases, there was dramatic depletion of autoantibody target cells, such as monocytes and B cells, which could contribute to impaired viral clearance.
Wang and colleagues41 also reported a notable number of acutely ill patients with COVID-19 with circulating autoantibodies whose target antigens mirror the variety of organ systems known to be involved in the disease course. For example, more than 20 autoantibodies were identified as reactive to proteins of the central nervous system, an interesting finding considering the population of severe patients with COVID-19 who develop neurologic symptoms.42 Of interest to the dermatology community, 26 of the identified autoantibodies were found to target the vasculature, connective tissue, and skin. Whether and how these autoantibodies contribute to the range of cutaneous findings of COVID-19 has yet to be established, but it seems plausible that they may augment the immune response that has been shown to be ongoing in the skin.
The breadth of targets across different patients is surprising and, as noted earlier, it suggests that self-reactive B-cell clones are unlikely to arise solely as a result of molecular mimicry. Instead it can be posited that the intense inflammatory milieu that has been described for COVID-19 is capable of disrupting fundamental tolerance checkpoints traditionally engaged by the adaptive immune system. Immunologists have described a phenomenon termed bystander activation in which an exaggerated immune response against a pathogenic challenge, such as a virus, leads to tissue damage and an increase in the exposure to self-antigens.43 These antigens can activate local autoreactive B and T cells, manifesting as autoimmunity.43 Autoreactivity may be an intrinsic part of the immunopathogenesis of COVID-19, but it remains to be seen whether this dysregulated host response is unique to SARS-CoV-2 or common to many other infections that have not been similarly investigated.
Chilblains as a Cutaneous Manifestation of Severe Acute Respiratory Syndrome Coronavirus-2
Evidence of an immune basis for severe acute respiratory syndrome coronavirus-2 association
Early in the COVID-19 pandemic, several case reports described children and adults with new-onset acral lesions closely resembling chilblains.44, 45, 46, 47 A definitive link between chilblains and COVID-19 has remained elusive because most patients in the gathered cohorts tested negative for serum antibodies against SARS-CoV-2, and many reported no respiratory or systemic symptoms of COVID-19 infection. This finding led some investigators to propose that these lesions are not caused by SARS-CoV-2 but instead represent an epiphenomenon.48, 49, 50, 51, 52 However, several studies provide histopathologic evidence that SARS-CoV-2 contributes to the pathogenesis of chilblains occurring during the pandemic. Two groups detected SARS-CoV-2 viral presence in skin biopsies from chilblains lesions. In a series of 7 pediatric cases, 1 group observed cytoplasmic granular positivity for the SARS-CoV-2 spike protein within vascular endothelial cells as well as in eccrine epithelial cells. Using electron microscopy, they were able to visualize spiked structures within the endothelial cells, which they interpreted as coronavirus particles.53 A second group detected SARS-CoV-2 RNA as well as viral envelope and spike proteins within biopsy tissue from chilblains lesions, with well-described reagent controls.13 In the latter case series, there was 1 patient with chilblains who tested negative for serum antibodies against SARS-CoV-2, but whose biopsy tissue contained detectable SARS-CoV-2 RNA and COVID-19 proteins, confirming presence of infection.13
These results highlight the potential for false-negative serum COVID-19 antibody testing in patients with true COVID-infected chilblains. Patients who present with chilblains but are otherwise asymptomatic are likely to evade polymerase chain reaction (PCR) and serology-based diagnostic testing for COVID-19 infection for multiple reasons. The authors hypothesize that a primary reason for the low sensitivity of COVID-19 PCR testing in patients with chilblains is one of test timing; the delayed onset of chilblains (ranging from 9 days to 1–2 months after onset of systemic symptoms, when present9, 10, 11) may occur after the window of nasopharyngeal viral particle shedding has closed. The low rate of COVID-19 seropositivity is more difficult to understand. Although many studies have reported cohorts with no seropositivity, 2 studies of 40 French and 19 Italian children detected serologic evidence of infection in 30% and 53% of children, respectively.9 , 11 One hypothesis to explain low rates of seropositivity posits that an initial, highly robust innate immune system response in these patients facilitates viral clearance such that little antigen remains to stimulate a robust adaptive immune response.11 Interestingly, IgA was revealed to be the most commonly detected antibody isotype in these groups, and several of the IgA+ patients were IgM and IgG negative. This finding is consistent with our unpublished case series of 7 adults in San Francisco, California, who developed chilblains during the first wave of infection; their blood was examined using an established enzyme-linked immunosorbent assay assay54 for the presence of anti–SARS-CoV-2 IgA. In our cohort, 1 patient was IgA+IgM+IgG− and another was IgA+IgG+IgM−; 3 others were borderline positive for IgA and negative for the other isotypes. The development of anti-COVID serum IgA reactivity may reflect a strong initial mucosal immune response in patients infected with SARS-CoV-2 who go on to develop chilblains lesions. This article is working under the assumption that many chilblains cases arising during the COVID-19 pandemic represent cutaneous pathology caused by SARS-CoV-2 infection.
Numerous studies have examined the histology of COVID-associated chilblains lesions, with a consensus emerging regarding the typical features. They are characterized by superficial and deep perivascular inflammation that extends in many cases to the eccrine glands.9 , 11 , 13 , 48, 49, 50, 51 , 53 , 55, 56, 57, 58, 59, 60 The inflammatory infiltrate is composed of mostly T cells, with a slight predominance of CD4+ rather than CD8+, and practically absent B cells. Interestingly, many studies have reported the presence of clusters of plasmacytoid dendritic cells (pDCs), cells of the innate immune system that are characterized by their strong production of the antiviral molecules, type I IFNs.12 , 55 , 57 , 58 Endothelial cells are often found to be edematous or damaged, and vessels in many cases feature intraluminal microthrombi.9 , 48 , 53 Although not noted in all cases, several reports highlight the findings of fibrinoid necrosis in the walls of the vessels and/or direct immunofluorescence staining for C3, IgM, and/or IgA.9 , 11 , 48 , 58
Familial chilblains pathogenesis provides clues to the inflammatory basis of coronavirus disease 2019–associated chilblains
Chilblains associated with COVID-19 is grossly and histologically similar to both idiopathic chilblains and familial chilblains, and this congruence may provide clues as to the pathophysiology of COVID-19–associated chilblains.13 , 55 Familial chilblains is an inherited condition known to flare in colder temperatures, similar to recurrent chilblains associated with SARS-CoV-2 infection.61 , 62 Familial chilblains is caused by specific mutations in intracellular exonucleases, which lead to inefficient nucleotide breakdown and thus intracellular nucleotide buildup.63 Innate immune receptors whose responsibility is to sense the presence of pathogen-derived nucleotides within the cell can also detect this pathologic increase in nucleotides, mistake it for a sign of viral infection, and trigger activation of the type I IFN system. Patients with familial chilblains show broad granular deposits of C3 and IgM along the basement membrane in cutaneous biopsies, as do mouse models of the disease, suggesting chilblain disorder involves not just the innate immune response but the adaptive immune response as well.63 , 64 Skin biopsies from familial chilblains have increased expression of type I IFN–induced genes,63 and the type I IFN response is central to the disease pathology, because genetic ablation of type I IFN signaling rescues mice from the development of autoimmunity.65 Several studies have proposed that COVID-19–associated chilblains may be the result of an overactive type I IFN response,13 , 55 , 57 , 59 , 60 , 66 a proposition that is supported by the presence of type I IFN–producing pDCs in COVID-19 chilblain biopsies, and by strong staining for the IFN-induced protein MxA in these biopsies.13 , 55
Type I Interferon Is Central to Coronavirus Disease 2019 Pathology
Increasing evidence suggests that activation of type I IFN pathways is essential for successful immune-mediated clearance of SARS-CoV-2. A critical arm of the immune response to many pathogens, the type I IFN family includes 3 secreted cytokines: IFN-α, IFN-β, and IFN-ω. These type I IFNs are produced toll-like receptor–mediated sensing of viral nucleotides. Type I IFNs are secreted and then sensed by other cells in the tissue environment, which respond by transcribing a large suite of antiviral genes. The system thus acts as an alarm, putting local cells on alert and bolstering the innate immune system in its initial response against viruses.17 However, when type I IFNs are activated inappropriately in response to self-antigens or the buildup of nucleic acids, this same pathway can contribute to the development of autoimmune disease, as described earlier for familial chilblains, and as seen in other autoimmune diseases, including lupus.67 Studies published in the summer of 2020 established that severe COVID-19 infections were correlated with low blood levels of type I IFN and low white blood cell expression of type I IFN–stimulated genes.68 , 69 Two complementary studies subsequently showed that deficiencies in the type I IFN pathway, either through inherited mutations or the development of autoantibodies, predispose patients to developing severe COVID-19. In the first, Zhang and colleagues70 show that 3.5% of patients with severe COVID-19 had inherited loss-of-function mutations in 8 genes involved in the pathway responsible for sensing viral nucleotides and leading to type I IFN production. In the accompanying study, Bastard and colleagues71 examined whether autoantibodies specific for elements of the type I IFN pathway might predispose to severe COVID-19. Astonishingly, the investigators found that, of 937 people tested with severe COVID-19, 135 (or 13.5%) had autoantibodies against type I IFN, versus 0% of patients with mild or asymptomatic COVID-19 and 0.3% of healthy controls, in agreement with subsequent publications.41 Three-fourths of these autoantibody-positive patients possessed autoantibodies capable of neutralizing downstream signaling of type I IFNs, which led to vastly reduced blood levels of type I IFN.
This article has discussed how inflammation induced by SARS-CoV-2 may lead to the development of autoantibodies. However, Bastard and colleagues71 argue that the autoantibodies they detect against the type I IFN pathway in patients with COVID-19 may predate SARS-CoV-2 infection because they were detected at an early time point, within 1 to 2 weeks of symptom onset. In their study profiling broad autoreactivities, Wang and colleagues41 also noted that 50% of the autoantibodies detected by their platform were present within 10 days of symptom onset, suggesting that many of these antibodies could have been preexisting in patients. Thus it can be hypothesized that preexisting autoantibodies may predispose patients to developing severe COVID-19. Intriguingly, these autoantibodies and genetic mutations against the type I IFN pathway were clinically silent before COVID-19 diagnosis, meaning that patients in the studies from Bastard and colleagues71 and Zhang and colleagues70 did not report any previous life-threatening infections. This finding suggests that although alternative mechanisms may protect these individuals against other viral pathogens, a successful type I IFN response may lie at the crux of successful immune defense against SARS-CoV-2.72
Chilblains and severe coronavirus disease 2019 lie at 2 ends of a type I interferon spectrum
Viruses are well known to use immunoevasive strategies to promote survival within their hosts.73 SARS-CoV-2 uses many mechanisms to avoid triggering type I IFN responses.74 Together with the observation that patients with severe COVID-19 are more likely to have disruptions in their type I IFN pathway,70 , 71 this suggests that this axis is one of the major determinants of how an individual’s immune system interacts with SARS-CoV-2. There may exist a spectrum of possible type I IFN responses, with strong or even overactive IFN responses at one end, and severe IFN impairment from inherited mutations or preexisting autoantibodies at the other. An individual’s genetics and environment may dictate where they are on this spectrum, with severe COVID-19 disease as one outcome, and asymptomatic or mild SARS-CoV-2 infection as the polar opposite outcome. Chilblains may be one of the few clinically apparent signs of a robust type I IFN response. When blood cells from hospitalized, ambulatory, and chilblains-only patients with COVID-19 were stimulated to examine their ability to produce type I IFN, the patients with chilblains produced significantly more IFN-a than the other 2 groups, even when the patients were age matched.11 Type I IFNs have been shown directly to drive thrombotic microangiopathy in mouse models,75 and recombinant IFN given therapeutically to patients with multiple sclerosis has also been associated with thrombotic events in several patients.76 , 77 Thus SARS-CoV-2 infection may trigger a robust type I IFN response in young, genetically predisposed individuals that leads to the cutaneous disorder directly observed in COVID-19–associated chilblains.
Coronavirus Disease 2019 Longhaulers
Cutaneous disorder resulting from SARS-CoV-2 infection is intimately linked with the immune response to infection. Recent studies have underscored the role of the innate and adaptive immune systems in directing successful viral clearance but also the role these systems play in immune-mediated disorders.
With the epidemic ongoing, the number of patients with COVID-19 cared for by dermatologists will grow. Although most of these cutaneous manifestations are self-limited, there have been reports of so-called COVID-19 longhaulers, even in dermatology.23 These patients include some with persistent chilblains and some with alopecia.78 One commonly reported nondermatologic COVID longhauler symptom is postural tachycardia syndrome (POTS), in which patients develop an exaggerated orthostatic tachycardia. Recent research into POTS cases that predate the COVID-19 pandemic revealed that patients with this condition show autoantibodies against various receptors important in blood pressure regulation.79, 80, 81 Some of the persistent cutaneous manifestations of COVID-19 may be similarly mediated by self-directed immune responses, a hypothesis that would be consistent with the number of autoreactivities that have been described against immune targets, phospholipids, and molecules expressed in vascular, connective, and mucosal tissues, and skin.30 , 31 , 34 , 41 Further research is needed to examine the persistence and disease relevance of SARS-CoV-2–associated autoreactive T-cell and B-cell clones and the antibodies they produce.
Summary
The immune system is intricately linked with the increasing number and variety of skin findings being reported by dermatologists over the course of this pandemic. Significant opportunities remain for additional research into the epidemiologic and biological relationships between immune pathways activated or dysregulated by SARS-CoV-2 and cutaneous manifestations of COVID-19 infection. This mechanistic understanding has the potential to highlight therapeutic opportunities to restore immune homeostasis in the skin.
Clinics care points
-
•
It is possible to detect histopathologic evidence of SARS-CoV-2 infection in some patients who test negative for serum antibodies; consistent with Centers for Disease Control and Prevention guidelines, remember that a negative antibody test result cannot rule out infection in an individual patient.
-
•
Because patients with COVID-19 are at increased risk of carrying autoantibodies and the long-term clinical consequences of this are unknown, it may be prudent to include COVID-19 antibody testing in the work-up for patients who develop new autoimmune disease manifestations after infection.
-
•
The inverse relationship between type I interferons and COVID-19 disease severity suggests that therapeutically modulating this pathway may benefit patients with COVID-19 in the future.
Acknowledgments
The authors wish to acknowledge Dr Jason Cyster, PhD, for helpful discussions. In addition, we wish to thank the patients and Dr Michael Wilson, MD; Kanishka Koshal; and Dr Jason Cyster, PhD, for their support in serology testing of patients with COVID-19 chilblains. This work was supported by NIAID F30AI150061 (AEG), NIAMS K08AR074556(MSF), and the Dermatology Foundation (MSF).
Footnotes
Disclosure: The authors have no conflicts of interest to disclose.
References
- 1.Freeman E.E., McMahon D.E., Lipoff J.B., et al. The spectrum of COVID-19–associated dermatologic manifestations: An international registry of 716 patients from 31 countries. J Am Acad Dermatol. 2020;83(4):1118–1129. doi: 10.1016/j.jaad.2020.06.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jia J.L., Kamceva M., Rao S.A., et al. Cutaneous manifestations of COVID-19: A preliminary review. J Am Acad Dermatol. 2020;83(2):687–690. doi: 10.1016/j.jaad.2020.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naderi-Azad S., Vender R. Lessons From the First Wave of the Pandemic: Skin Features of COVID-19 can be Divided Into Inflammatory and Vascular Patterns. J Cutan Med Surg. 2020;7(1) doi: 10.1177/1203475420972343. 120347542097234-120347542097238. [DOI] [PubMed] [Google Scholar]
- 4.Droesch C., Do M.H., DeSancho M., et al. Livedoid and Purpuric Skin Eruptions Associated With Coagulopathy in Severe COVID-19. JAMA Dermatol. 2020;156(9):1–3. doi: 10.1001/jamadermatol.2020.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y., Cao W., Xiao M., et al. Clinical and coagulation characteristics in 7 patients with critical COVID-2019 pneumonia and acro-ischemia. Chin J Hematol. 2020;41(4):302–307. doi: 10.3760/cma.j.issn.0253-2727.2020.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y., Xiao M., Zhang Z., et al. Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19. N Engl J Med. 2020;382(17):1–3. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Del Giudice P., Boudoumi D., Le Guen B., et al. Catastrophic acute bilateral lower limbs necrosis associated with COVID-19 as a likely consequence of both vasculitis and coagulopathy. J Eur Acad Dermatol Venereol. 2020;34(11):e679–e680. doi: 10.1111/jdv.16763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magro C., Mulvey J.J., Berlin D., et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Translational Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hachem El M., Diociaiuti A., Concato C., et al. A clinical, histopathological and laboratory study of 19 consecutive Italian paediatric patients with chilblain-like lesions: lights and shadows on the relationship with COVID-19 infection. J Eur Acad Dermatol Venereol. 2020;34(11):2620–2629. doi: 10.1111/jdv.16682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandez-Nieto D., Jimenez-Cauhe J., Suarez-Valle A., et al. Characterization of acute acral skin lesions in nonhospitalized patients: A case series of 132 patients during the COVID-19 outbreak. J Am Acad Dermatol. 2020;83(1):e61–e63. doi: 10.1016/j.jaad.2020.04.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hubiche T., Cardot-Leccia N., Le Duff F., et al. Clinical, Laboratory, and Interferon-Alpha Response Characteristics of Patients With Chilblain-like Lesions During the COVID-19 Pandemic. JAMA Dermatol. 2020:1–12. doi: 10.1001/jamadermatol.2020.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sette A., Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021:1–31. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magro C.M., Mulvey J.J., Laurence J., et al. The differing pathophysiologies that underlie COVID-19-associated perniosis and thrombotic retiform purpura: a case series. Br J Dermatol. 2021;184(1):141–150. doi: 10.1111/bjd.19415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levi M., Thachil J., Iba T., et al. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7(6):e438–e440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magro C.M., Mulvey J., Kubiak J., et al. Severe COVID-19: A multifaceted viral vasculopathy syndrome. Ann Diagn Pathol. 2021;50:151645. doi: 10.1016/j.anndiagpath.2020.151645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foley J.H., Conway E.M. Cross Talk Pathways Between Coagulation and Inflammation. Circ Res. 2016;118(9):1392–1408. doi: 10.1161/CIRCRESAHA.116.306853. [DOI] [PubMed] [Google Scholar]
- 17.Murphy K., Travers P., Walport M., et al. 8th edition. Garland Science; New York, NY: 2012. Janeway's immunobiology. [Google Scholar]
- 18.Long Q.-X., Liu B.-Z., Deng H.-J., et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020:1–15. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 19.Robbiani D.F., Gaebler C., Muecksch F., et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020:1–23. doi: 10.1038/s41586-020-2456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodda L.B., Netland J., Shehata L., et al. Functional SARS-CoV-2-Specific Immune Memory Persists after Mild COVID-19. Cell. 2021;184(1):169–183.e17. doi: 10.1016/j.cell.2020.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z., Lorenzi J.C.C., Muecksch F., et al. Enhanced SARS-CoV-2 neutralization by dimeric IgA. Sci Translational Med. 2021;13(577):1–13. doi: 10.1126/scitranslmed.abf1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sterlin D., Mathian A., Miyara M., et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci Translational Med. 2021;13(577) doi: 10.1126/scitranslmed.abd2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMahon D.E., Gallman A.E., Hruza G.J., et al. Comment Long COVID in the skin: a registry analysis of COVID-19 dermatological duration. Lancet Infect Dis. 2021:1–2. doi: 10.1016/S1473-3099(20)30986-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dan J.M., Mateus J., Kato Y., et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021 doi: 10.1126/science.abf4063. eabf4063–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaebler C., Wang Z., Lorenzi J.C.C., et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021:1–33. doi: 10.1038/s41586-021-03207-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Novelli L., Motta F., De Santis M., et al. The JANUS of chronic inflammatory and autoimmune diseases onset during COVID-19 – A systematic review of the literature. J Autoimmun. 2021;117:102592. doi: 10.1016/j.jaut.2020.102592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mendoza-Pinto C., García-Carrasco M., Cervera R. Role of Infectious Diseases in the Antiphospholipid Syndrome (Including Its Catastrophic Variant) Curr Rheumatol Rep. 2018:1–7. doi: 10.1007/s11926-018-0773-x. [DOI] [PubMed] [Google Scholar]
- 28.Cunningham M.W. Rheumatic Fever, Autoimmunity, and Molecular Mimicry: The Streptococcal Connection. Int Rev Immunol. 2014;33(4):314–329. doi: 10.3109/08830185.2014.917411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaturvedi S., Brodsky R.A., McCrae K.R. Complement in the Pathophysiology of the Antiphospholipid Syndrome. Front Immunol. 2019;10:295–299. doi: 10.3389/fimmu.2019.00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao M., Zhang Y., Zhang S., et al. Antiphospholipid Antibodies in Critically Ill Patients With COVID-19. Arthritis Rheumatol. 2020;72(12):1998–2004. doi: 10.1002/art.41425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zuo Y. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci Translational Med. 2020;12(570):1–12. doi: 10.1126/scitranslmed.abd3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toubi E., Shoenfeld Y. Livedo Reticularis as a Criterion for Antiphospholipid Syndrome. Clinic Rev Allerg Immunol. 2007;32(2):138–144. doi: 10.1007/s12016-007-0004-0. [DOI] [PubMed] [Google Scholar]
- 33.Young T.K., Shaw K.S., Shah J.K., et al. Mucocutaneous Manifestations of Multisystem Inflammatory Syndrome in Children During the COVID-19 Pandemic. JAMA Dermatol. 2020:1–6. doi: 10.1001/jamadermatol.2020.4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gruber C.N., Patel R.S., Trachtman R., et al. Mapping Systemic Inflammation and Antibody Responses in Multisystem Inflammatory Syndrome in Children (MIS-C) Cell. 2020;183(4):982–995.e14. doi: 10.1016/j.cell.2020.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Consiglio C.R., Cotugno N., Sardh F., et al. The Immunology of Multisystem Inflammatory Syndrome in Children with COVID-19. Cell. 2020;183(4):968–981.e7. doi: 10.1016/j.cell.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramaswamy A., Brodsky N.N., Sumida T.S., et al. Immune dysregulation and autoreactivity correlate with disease severity in SARS-CoV-2-associated multisystem inflammatory syndrome in children. Immunity. 2021;54(5):1083–1095. doi: 10.1016/j.immuni.2021.04.003. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakurai Y. Autoimmune Aspects of Kawasaki Disease. J Investig Allergol Clin Immunol. 2019;29(4):251–261. doi: 10.18176/jiaci.0300. [DOI] [PubMed] [Google Scholar]
- 38.Kazatchkine M.D., Kaveri S.V. Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. N Engl J Med. 2001;345(10):1–9. doi: 10.1056/NEJMra993360. [DOI] [PubMed] [Google Scholar]
- 39.van der Poel C.E., Spaapen R.M., van de Winkel J.G.J., et al. Functional Characteristics of the High Affinity IgG Receptor, FcγRI. J Immunol. 2011;186(5):2699–2704. doi: 10.4049/jimmunol.1003526. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka M., Krutzik S.R., Sieling P.A., et al. Activation of FcγRI on Monocytes Triggers Differentiation into Immature Dendritic Cells That Induce Autoreactive T Cell Responses. J Immunol. 2009;183(4):2349–2355. doi: 10.4049/jimmunol.0801683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang E.Y., Mao T., Klein J., et al. Diverse Functional Autoantibodies in Patients with COVID-19. Nature. 2021 doi: 10.1038/s41586-021-03631-y. [DOI] [PubMed] [Google Scholar]
- 42.Mao L., Jin H., Wang M., et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–688. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Getts D.R., Chastain E.M.L., Terry R.L., et al. Virus infection, antiviral immunity, and autoimmunity. Immunol Rev. 2013;255(1):197–209. doi: 10.1111/imr.12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mazzotta F., Troccoli T. Acute acro-ischemia in the child at the time of COVID-19. Eur J Pediat Dermatol. 2020;30:71–74. [Google Scholar]
- 45.Romaní J., Baselga E., Mitjà O., et al. Chilblain and Acral Purpuric Lesions in Spain during Covid Confinement: Retrospective Analysis of 12 Cases. Actas Dermosifiliogr. 2020;111(5):426–429. doi: 10.1016/j.ad.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alramthan A., Aldaraji W. Two cases of COVID-19 presenting with a clinical picture resembling chilblains: first report from the Middle East. Clin Exp Dermatol. 2020;45(6):746–748. doi: 10.1111/ced.14243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galván Casas C., Català A., Carretero Hernández G., et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183(1):71–77. doi: 10.1111/bjd.19163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herman A., Peeters C., Verroken A., et al. Evaluation of Chilblains as a Manifestation of the COVID-19 Pandemic. JAMA Dermatol. 2020;156(9):998–1003. doi: 10.1001/jamadermatol.2020.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roca-Ginés J., Torres-Navarro I., Sánchez-Arráez J., et al. Assessment of Acute Acral Lesions in a Case Series of Children and Adolescents During the COVID-19 Pandemic. JAMA Dermatol. 2020;156(9):992–996. doi: 10.1001/jamadermatol.2020.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hébert V., Duval-Modeste A.-B., Joly P., et al. Lack of association between chilblains outbreak and severe acute respiratory syndrome coronavirus 2: Histologic and serologic findings from a new immunoassay. J Am Acad Dermatol. 2020;83(5):1434–1436. doi: 10.1016/j.jaad.2020.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Denina M., Pellegrino F., Morotti F., et al. All that glisters is not COVID: Low prevalence of seroconversion against SARS-CoV-2 in a pediatric cohort of patients with chilblain-like lesions. J Am Acad Dermatol. 2020;183(4):729–737. doi: 10.1016/j.jaad.2020.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stavert R. Evaluation of SARS-CoV-2 antibodies in 24 patients presenting with chilblains-like lesions during the COVID-19 pandemic. J Am Acad Dermatol. 2020;34(7):1–4. doi: 10.1016/j.jaad.2020.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Colmenero I., Santonja C., Alonso Riaño M., et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol. 2020;183(4):729–737. doi: 10.1111/bjd.19327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Whitman J.D., Hiatt J., Mowery C.T., et al. Evaluation of SARS-CoV-2 serology assays reveals a range of test performance. Nat Biotechnol. 2020:1–15. doi: 10.1038/s41587-020-0659-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Battesti G., Khalifa El J., Abdelhedi N., et al. New insights in COVID-19 associated chilblains: A comparative study with chilblain lupus erythematosus. J Am Acad Dermatol. 2020;83(4):1219–1222. doi: 10.1016/j.jaad.2020.06.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Masson A., Bouaziz J.-D., Sulimovic L., et al. Chilblains is a common cutaneous finding during the COVID-19 pandemic: A retrospective nationwide study from France. J Am Acad Dermatol. 2020;83(2):667–670. doi: 10.1016/j.jaad.2020.04.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sohier P., Matar S., Meritet J.-F., et al. Histopathological features of Chilblain-like lesions developing in the setting of the COVID-19 pandemic. Arch Pathol Lab Med. 2020:1–29. doi: 10.5858/arpa.2020-0613-SA. [DOI] [PubMed] [Google Scholar]
- 58.Kanitakis J., Lesort C., Danset M., et al. Chilblain-like acral lesions during the COVID-19 pandemic (“COVID toes”): Histologic, immunofluorescence, and immunohistochemical study of 17 cases. J Am Acad Dermatol. 2020;83(3):870–875. doi: 10.1016/j.jaad.2020.05.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cordoro K.M., Reynolds S.D., Wattier R., et al. Clustered cases of acral perniosis: Clinical features, histopathology, and relationship to COVID-19. Pediatr Dermatol. 2020;37(3):419–423. doi: 10.1111/pde.14227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kolivras A., Dehavay F., Delplace D., et al. Coronavirus (COVID-19) infection-induced chilblains: A case report with histopathologic findings. JAAD Case Rep. 2020;6(6):489–492. doi: 10.1016/j.jdcr.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Orcesi S., La Piana R., Fazzi E. Aicardi-Goutieres syndrome. Br Med Bull. 2009;89:183–201. doi: 10.1093/bmb/ldn049. [DOI] [PubMed] [Google Scholar]
- 62.Freeman E.E., McMahon D.E., Lipoff J.B., et al. Cold and COVID: Recurrent Pernio during the COVID-19 Pandemic. Br J Dermatol. 2021 doi: 10.1111/bjd.19894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peschke K., Friebe F., Zimmermann N., et al. Deregulated Type I IFN Response in TREX1-Associated Familial Chilblain. Lupus. 2010:1–4. doi: 10.1038/jid.2013.496. [DOI] [PubMed] [Google Scholar]
- 64.Günther C., Berndt N., Wolf C., et al. Familial Chilblain Lupus Due to a Novel Mutation in the Exonuclease III Domain of 3′ Repair Exonuclease 1 ( TREX1) JAMA Dermatol. 2015;151(4):426. doi: 10.1001/jamadermatol.2014.3438. [DOI] [PubMed] [Google Scholar]
- 65.Stetson D.B., Ko J.S., Heidmann T., et al. Trex1 Prevents Cell-Intrinsic Initiation of Autoimmunity. Cell. 2008;134(4):587–598. doi: 10.1016/j.cell.2008.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Damsky W., Peterson D., King B. When interferon tiptoes through COVID-19: Pernio-like lesions and their prognostic implications during SARS-CoV-2 infection. J Am Acad Dermatol. 2020;83(3):e269–e270. doi: 10.1016/j.jaad.2020.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crow M.K. Type I Interferon in the Pathogenesis of Lupus. J Immunol. 2014;192(12):5459–5468. doi: 10.4049/jimmunol.1002795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Trouillet-Assant S., Viel S., Gaymard A., et al. Type I IFN immunoprofiling in COVID-19 patients. J Allergy Clin Immunol. 2020;146(1):206–208.e2. doi: 10.1016/j.jaci.2020.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hadjadj J., Yatim N., Barnabei L., et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369(6504):718. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Q., Bastard P., Liu Z., et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370(6515) doi: 10.1126/science.abd4570. eabd4570–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bastard P., Rosen L.B., Zhang Q., et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370(6515) doi: 10.1126/science.abd4585. eabd4585–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Meffre E., Iwasaki A. Interferon deficiency can lead to severe COVID. Nature. 2020;587(7834):374–376. doi: 10.1038/d41586-020-03070-1. [DOI] [PubMed] [Google Scholar]
- 73.Ploegh H.L. Viral Strategies of Immune Evasion. Science. 1998;280(5361):248–253. doi: 10.1126/science.280.5361.248. [DOI] [PubMed] [Google Scholar]
- 74.Blanco-Melo D., Nilsson-Payant B.E., Liu W.-C., et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell. 2020;181(5):1036–1045.e9. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kavanagh D., McGlasson S., Jury A., et al. Type I interferon causes thrombotic microangiopathy by a dose-dependent toxic effect on the microvasculature. Blood. 2016;128(24):2824–2833. doi: 10.1182/blood-2016-05-715987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Larochelle C., Grand’maison F., Bernier G.P., et al. Thrombotic thrombocytopenic purpura-hemolytic uremic syndrome in relapsing-remitting multiple sclerosis patients on high-dose interferon β. Mult Scler. 2014;20(13):1783–1787. doi: 10.1177/1352458514523692. [DOI] [PubMed] [Google Scholar]
- 77.Hunt D., Kavanagh D., Drummond I., et al. Thrombotic Microangiopathy Associated with Interferon Beta. N Engl J Med. 2014;370(13):1268–1270. doi: 10.1056/NEJMc1316118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xiong Q., Xu M., Li J., et al. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin Microbiol Infect. 2021;27(1):89–95. doi: 10.1016/j.cmi.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li H., Yu X., Liles C., et al. Autoimmune basis for postural tachycardia syndrome. JAHA. 2014;3(1):e000755. doi: 10.1161/JAHA.113.000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fedorowski A., Li H., Yu X., et al. Antiadrenergic autoimmunity in postural tachycardia syndrome. EP Europace. 2016;19(7):1211–1219. doi: 10.1093/europace/euw154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yu X., Li H., Murphy T.A., et al. Angiotensin II Type 1 Receptor Autoantibodies in Postural Tachycardia Syndrome. JAHA. 2018;7(8):e008351. doi: 10.1161/JAHA.117.008351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu K.J., Corum J. Charting a Covid-19 Immune Response. The New York Times. 2020 https://www.nytimes.com/interactive/2020/10/05/science/charting-a-covid-immune-response.html Available at: [Google Scholar]