Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the etiologic agent of coronavirus disease 2019 (COVID-19), is a single-stranded RNA virus whose sequence is known. COVID-19 is associated with a heterogeneous clinical phenotype ranging from asymptomatic to fatal disease. It appears that access to nasopharyngeal respiratory epithelia expressing angiotensin-converting enzyme (ACE) 2, the receptor for SARS-CoV-2, is followed by viral replication in the pulmonary alveolar septal capillary bed. We have demonstrated in earlier studies that incomplete viral particles, termed pseudovirions, dock to deep subcutaneous and other vascular beds, potentially contributing to the prothrombotic state and systemic complement activation that characterizes severe and critical COVID-19. A variety of skin eruptions have been described in the setting of SARS-CoV-2 infection and more recently, after COVID-19 vaccination. The vaccines deliver a laboratory-synthesized mRNA that encodes a protein that is identical to the spike glycoprotein of SARS-CoV-2, allowing the production of immunogenic spike glycoprotein that will then elicit T cell and B cell adaptive immune responses. In this contribution, we review an array of cutaneous manifestations of COVID-19 that provide an opportunity to study critical pathophysiologic mechanisms that underlie all clinical facets of COVID-19, ranging from asymptomatic/mild to severe and critical COVID-19. We classify cutaneous COVID-19 according to underlying pathophysiologic principles. In this regard we propose three main pathways: (1) complement mediated thrombotic vascular injury syndromes deploying the alternative and mannan binding lectin pathways and resulting in the elaboration of cytokines like interleukin 6 from endothelium in the setting of severe and critical COVID-19 and (2) the robust T cell and type I interferon-driven inflammatory and (3) humoral-driven immune complex mediated vasculitic cutaneous reactions observed with mild and moderate COVID-19. Presented are novel data on cutaneous vaccine reactions that manifest a clinical and morphologic parallel with similar eruptions observed in patients with mild and moderate COVID-19 and in some cases represent systemic eczematoid hypersensitivity reactions to a putative vaccine-based antigen versus unmasking subclinical hypersensitivity due to immune enhancing effects of the vaccine. Finally, we demonstrate for the first time the localization of human synthesized spike glycoprotein after the COVID-19 vaccine to the cutaneous and subcutaneous vasculature confirming the ability of SARS-CoV-2 spike glycoprotein to bind endothelium in the absence of intact virus.
Key Words: SARS CoV-2, cutaneous manifestations of COVID-19 pseudovirions, pathophysiologic principles, complement pathway activation, Interferon and T cell driven responses, humoral immunity, vaccine reactions
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the etiologic agent of coronavirus disease 2019 (COVID-19). It is a single-stranded RNA (ribonucleic acid) virus1 and represents one of the seven β coronaviruses2 that causes human disease, although the natural reservoirs are the bat and the pangolin.3 The SARS-CoV-2 infection in humans is associated with a heterogeneous clinical phenotype4 that ranges from asymptomatic cases to mild disease to a severe and potentially fatal illness. It is this unpredictability in an individual's response to the virus that has caused so many of us to adopt a new pattern of daily interactive existence until herd immunity is reached.
Organ failure, particularly a progressive, therapy-resistant acute respiratory distress syndrome (ARDS)5 and acute renal insufficiency6 along with a hypercoagulable7 state portend the worst prognosis and qualify as critical COVID-19. SARS-CoV-2 is highly contagious, especially the more recently identified strains originating in England, Brazil, and South Africa.8 Its incubation period ranges from 2 to 14 days after spread via inhaled respiratory droplets or droplet nuclei and, less likely, through fomite contamination. The name given to the virus that causes COVID-19, SARS-CoV-2, emphasizes the structural similarity to the original SARS coronavirus that was responsible for the 2003 to 2004 pandemic. The earlier contagion was more virulent and less transmissible, but the new strain and other related mutant strains are more contagious but less virulent.
Common to the clinical presentation of SARS-CoV and SARS-CoV-2 are fever, dry cough, myalgias, headache, and diarrhea in most patients. In the setting of SARS-CoV-2, the majority of patients have a relatively mild, self-limited illness. Roughly 20% of patients have more serious disease that can progress to respiratory compromise necessitating supplemental oxygen either via nasal cannula or ventilator support, fulfilling criteria for severe and critical COVID-19 respectively.9 The pathophysiology that underlies the transition from moderate COVID-19 disease to critical and severe COVID-19 is complex, but a diminished type I interferon response in the earlier phase of the infection likely contributes to a more aggressive clinical course by permitting unchecked viral replication in infected cells.10, 11, 12 The excessive replication of virus in the lung contributes significantly to septal capillary compromise but also results in extrapulmonary pseudovirion dissemination with further extrapulmonary vascular sequelae.1313,14
We had the opportunity to examine the skin, lung, and various other organ systems including the brain, liver, kidney, and heart in a number of patients who had mild, moderate, severe, critical, and fatal COVID-19. A common theme that emerged is one of complement-mediated microvascular injury13 and a generalized procoagulant state in the absence of intact virus within the endothelium outside of the lung in patients with severe and critical COVID-19.
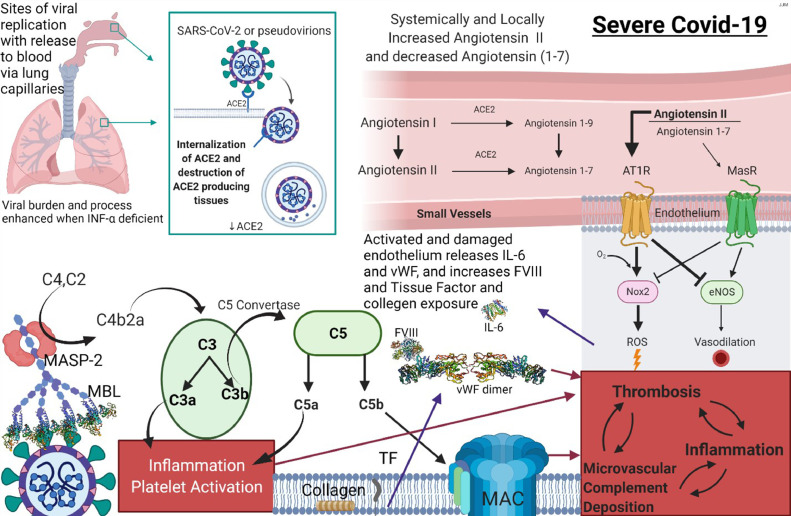
We first recognized and identified a pattern of tissue injury in the skin and lung, diagnostic of complement-mediated microvascular injury in patients with severe and critical COVID-19. Subsequently, we extended our studies to other organ systems including the heart, liver, kidney, brain, and placenta.14 , 15 We discovered localization of spike glycoprotein and other viral capsid proteins to microvessels, which expressed the receptor for spike glycoprotein, namely, angiotensin-converting enzyme 2 (ACE2). The greatest extent of ACE2 and viral capsid localization was in the septal capillaries of the lung, deeper dermal and subcutaneous capillaries, and venules of both diseased and normal-appearing skin as well as the microvessels of the brain. There was also evidence of complement pathway activation14 in these ACE2-positive microvascular beds expressing viral capsid protein based on the localization of C3d, C4d, mannan-binding lectin-associated serine protease-2 (MASP-2), and C5b-9 within the microvasculature.
Spike glycoprotein exhibits glycan moieties that are able to interact with mannan-binding lectin (MBL), leading to MBL pathway activation.16 , 17 In particular, the SARS-CoV-2 S spike glycoprotein exhibits surface oligomannose-type N234 and N709 that are largely accessible to α-1,2-mannosidases. Of the 22 sites on the S protein, 8 contain substantial populations of oligomannose-type glycans, hence rendering spike glycoprotein the perfect substrate for the activation of the MBL pathway. Our demonstration of microvascular endothelial cell localization of C4d and MASP-2 within the lung and skin procured from patients with critical and fatal COVID-19 provided confirmation of the role of MBL pathway activation and also prompted therapeutic interventions with eculizumab in critically ill patients with COVID-19 .18 Others have also highlighted the role of MBL activation in the pathogenesis of severe/critical COVID-19 including its role in the thrombosis and coagulopathy in critically ill patients with COVID-19.19 , 20 We further concluded that the hypercytokinemia was a direct sequela of elaboration of the various cytokines including tumor necrosis factor (TNF) α, MBL, caspase 3, and interleukin (IL) 6 from endothelium possibly attributed to MBL pathway activation.14 , 19 , 20 The addition of human MBL along with MBL-associated serine proteases complex or recombinant human MBL to cultures of human umbilical vein endothelial cells stimulated by bacterial lipopolysaccharide triggers enhanced secretion of select cytokines and chemokines including IL-8, IL-6, and monocyte chemoattractant protein-1 by endothelium indicative of an independent role of MBL activation in promoting an activated inflammatory phenotype in the endothelium.21 The end result of this state of complement activation and hypercytokinemia is one of endothelial cell injury and microvascular thrombosis.14 We postulated that the activation of the complement pathway and enhanced IL-6 expression on endothelium contributed to the generalized procoagulant state given the crosstalk between the complement and the coagulation pathways and the ability of endothelial-based IL-6 to promote platelet aggregation. There is no evidence of active viral replication in most microvascular beds despite the localization of viral capsid protein to the endothelium. Hence, endocytosis of viral protein as a pseudovirion is nonetheless capable of activating endothelium through the complement pathway and inducing the expression of certain cytokines such as IL-6 likely through MBL activation.14 , 22
A variety of skin eruptions have been described in the setting of SARS-CoV-2 infection and more recently, after the COVID-19 vaccine. Up to 20% of patients with active infection are said to manifest skin eruptions.23, 24, 25, 26, 27, 28, 29, 30, 31, 32 The clinicopathologic reaction patterns reported to date include a confluent erythematous maculopapular-morbilliform eruption33; exanthematous eruptions, urticarial reactions32 , 34; papulosquamous eruptions resembling pityriasis rosea (PR)35; varicella-like eruptions36; erythema multiforme-like reactions37, 38, 39; acute generalized exanthematous pustulosis40; vesicular eruptions41; and vascular injury patterns including livedo reticularis,42 thrombotic retiform purpura,27 , 43 acral purpuric nodules similar to idiopathic perniosis (chilblains)43, 44, 45, 46, 47, 48, 49, 50 and a unique acro-ischemic lesion occurring in patients with moderate COVID-19 heralded by lesions that mimic perniosis clinically but exhibit paucicellular ischemic alterations in association with dilated blood vessels devoid of thrombotic and/or vasculitic change.51
The main types of skin findings we encountered include those directly attributable to microvascular complement activation including thrombotic retiform purpura encountered in the severe and critical patient with COVID-19,13 , 27 , 43 , 52 and the subclinical microvascular complement deposition53 and subtle microvascular thrombosis observed in biopsies of normal deltoid skin in patients with severe and critical COVID-19. The latter findings in normal skin support the diagnosis of systemic complement pathway activation mirroring the microvascular complement pattern encountered in other catastrophic complement-mediated vascular injury syndromes such as atypical hemolytic uremic syndrome.53 , 54 We have observed cutaneous signs of the generalized procoagulant state55 operational in patients with significant COVID-19 represented by calciphylaxis and acute limb ischemia attributable to subcutaneous larger vessel arterial thrombosis.55 , 56 A hallmark of skin samples reflective of a procoagulant state and/or complement-mediated vascular injury is the lack of inflammation. In contrast, there are cases of cutaneous COVID-19 where significant T-cell enriched inflammation reflective of cellular immunity is observed. Others have also reported on similar eruptions in various case reports and series. Among these cases are interferon-driven COVID-19 perniosis43, 44, 45, 46, 47, 48, 49, 50 , 57 also referred to as “COVID toes,” a robust lymphocyte inflammatory lesion resembling histiocytoid Sweet syndrome,58 PR,59 and erythema multiforme.37, 38, 39 We have also encountered leukocytoclastic vasculitis temporally associated with COVID-19 where a different limb of antiviral immunity, namely a humoral immune response, likely is operative.60 Calcinosis cutis reflective of calcium phosphate metabolism in the setting of COVID-19–associated renal insufficiency was identified in one patient. Reactions temporally associated with COVID-19 vaccine administration are also presented, including Grover disease, urticaria, urticarial vasculitis, systemic eczematoid hypersensitivity reactions, morbilliform interface reactions, perniosis, and a patient with ulcerative colitis on mesalamine who developed a first onset of shingles one day after her first Pfizer vaccine dose. The clinical features, pathology, and pathophysiology of the main cutaneous reactions encountered in our clinical practices will be discussed. A categorical approach to cutaneous COVID-19 based on underlying pathogenetic mechanisms that are likely key in their evolution is presented.
Materials and Methods
Cutaneous cases of COVID-19 and COVID-19–vaccine reactions encountered from the database of Weill Cornell Medicine and Regional Medical Laboratory (Tulsa, Oklahoma) are reviewed. The database covers the time period since the beginning of the pandemic in March of 2020 to June of 2021. The clinical histories and light microscopic findings were reviewed. As part of the routine workup in each case, additional immunohistochemical studies for the expression of myxovirus resistance protein A(MXA) and for C3d, C4d, C5b-9 and mannan binding lectin serine protease (MASP) 2 were evaluated in some cases. In addition, molecular studies including the viral capsid protein, angiotensin converting enzyme 2 (ACE2) assessment, cytokine expression, and the SARS CoV-2 RNA were conducted as supplementary research tests in certain cases. The methodology has been previously reported.13 , 14 , 43 The study is covered under institutional review board protocol 20-02021524.
Cutaneous lesions attributable to complement-mediated microvascular injury due to spike glycoprotein activation of the complement pathway in the setting of a blunted interferon response
Thrombotic retiform purpura of critical COVID-19
Clinical features
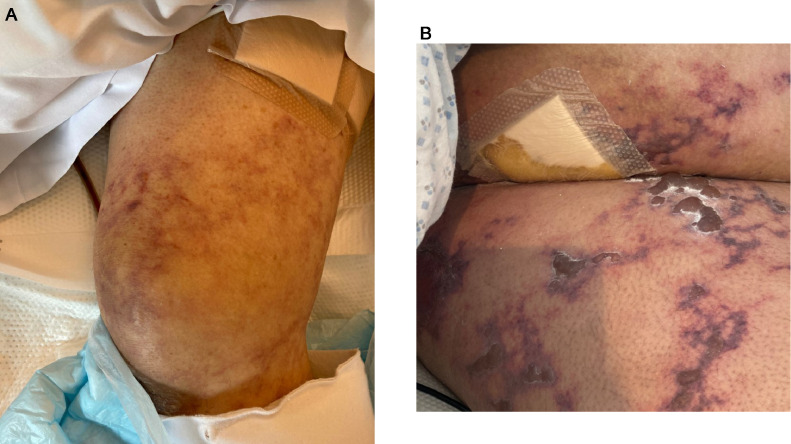
We encountered nine patients with cutaneous thrombotic microvascular disease who had progressed from moderate to critical COVID-19 (Table 1 ). Some of the cases have been reported on elsewhere.13 , 27 , 43 Each patient had acute respiratory failure and became ventilator dependent with polymerase chain reaction (PCR) proven SARS-CoV-2 infection. Typically, after a few days on ventilator support, patients developed retiform purpura primarily at acral sites (excluding two patients for whom the eruption was confined to the buttock and thigh areas, respectively). The retiform eruption was temporally associated with their worsening clinical features (Figures 1 , A and B). The patients were adults ranging in age from 32 years to 73 years, with no sex predilection. There were no known risk factors for the development of an acute thrombotic diathesis such as antiphospholipid antibodies, antibodies to heparin, warfarin administration, and sepsis; however, some patients were diabetic. Of the nine patients, four died from organ failure related to COVID-19
Table 1.
Complement mediated COVID-19
| Case # | Sex | Age | Biopsy site | Diagnosis | Outcome | |
|---|---|---|---|---|---|---|
| A. Severe/Critical COVID-19–associated thrombotic retiform purpura | TRP 1 | M | 32 | Buttock | Thrombotic retiform purpura | Ventilator support, toculizumab and hydroxychloroquine recovered |
| TRP 2 | F | 66 | Right heel | Thrombotic retiform purpura | Deceased | |
| TRP 3 | F | 40 | Right lower arm | Thrombotic retiform purpura | Recovered | |
| TRP 4 | M | 73 | Right hand | Thrombotic retiform purpura | Deceased | |
| TRP 5 | M | 70 | Left foream | Thrombotic retiform purpura | Recovered | |
| TRP 6 | F | 36 | Right hand | Thrombotic retiform purpura | Recovered | |
| TRP 7 | M | 71 | Right hand | Thrombotic retiform purpura | Deceased | |
| TRP 8 | M | 80 | Right thigh | Thrombotic retiform purpura | Recovered | |
| TRP 9 | F | 22 | Right thigh | Thrombotic retiform purpura | Recovered | |
| B. Thrombotic lower limb ischemia | LL 10 | M | 56 | Leg left | Thrombotic retiform purpura | Recovering at home |
| LL 11 | F | 63 | Right leg | Thrombotic retiform purpura | Recovering at rehabilitation facility | |
| C. Calciphylaxis | CX 12 | F | 62 | Left thigh | Calciphylaxis/thrombotic retiform purpura | Deceased |
| CX 13 | M | 64 | Right leg | Calciphylaxis/thrombotic retiform purpura | Recovering; on peritoneal dialysis, | |
| D. Calcinosis cutis | CC 14 | M | 62 | Left arm | Calcinosis cutis with granulation tissue response | Recovering |
CC, Calcinosis Cutis; COVID-19, coronavirus disease 2019; CX, calciphylaxis; F, female; LL, lower limb; M, male; ; TRP, thrombotic retiform purpura.
Fig. 1.
Thrombotic retiform purpura (clinical features). (A), (B) Patients had progressed from moderate COVID-19 to critical COVID-19. Each patient had acute respiratory failure and was ventilator dependent. They had PCR-proven SARS-CoV-2 infection. Typically, after a few days on ventilator support, the patients developed retiform purpura that was invariably associated with their worsening clinical features. COVID-19, coronavirus disease 2019; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Light microscopic findings
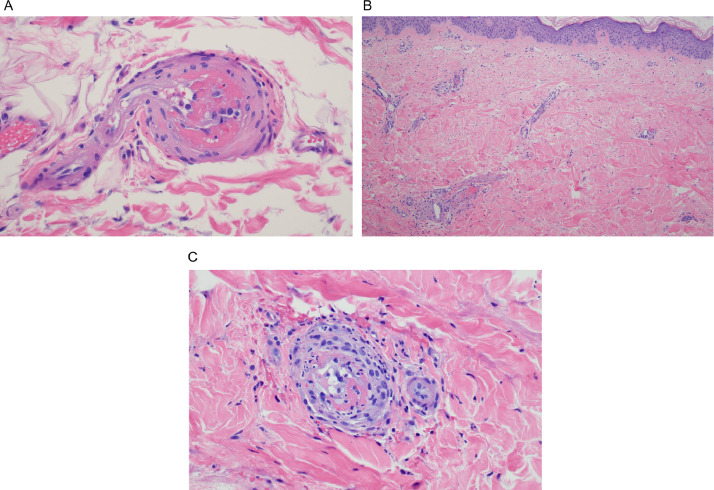
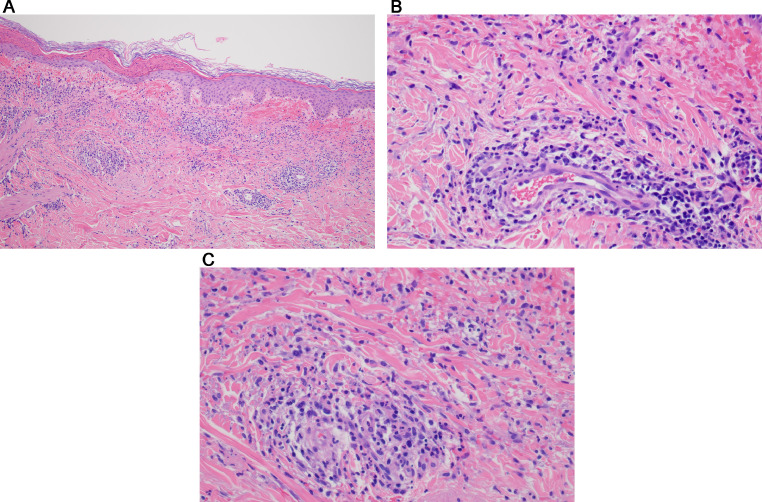
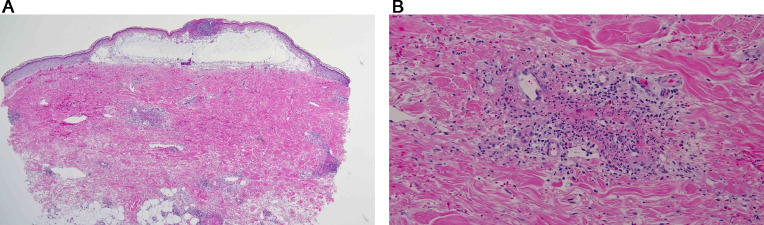
In all cases, the biopsy specimens showed a pauci-inflammatory thrombogenic vasculopathy affecting capillaries and venules, and in half of the cases a thrombotic diathesis was observed in arterioles and small arteries, a finding associated with stroke and death. In contrast with the striking degree of vascular thrombosis and endothelial cell injury there was a dearth of inflammatory cells, save for one case showing tissue neutrophilia (Figure 2a,b,c) that we attributed to secondary ischemic change. Degenerative endothelial cell changes with endothelial cell sloughing were more conspicuous in the deeper dermis and subcutaneous fat. Secondary ischemic necrosis of the eccrine coil was observed.
Fig. 2.
Thrombotic retiform purpura (light microscopic findings). In all cases of COVID-19–associated thrombotic retiform purpura, the biopsies show a relatively pauci-inflammatory thrombogenic vasculopathy affecting capillaries and venules. In half of the cases, a thrombotic diathesis is observed in arterioles and small arteries, a finding associated with stroke and death. Illustrated is a small artery exhibiting an occlusive thrombus. In most cases, at least in some of the sampled vessels, there is accompanying endothelial cell injury and variable mural fibrin deposition. The thrombotic diathesis reflects complement-mediated endothelial cell injury in concert with a generalized procoagulant state attributable to concomitant activation of the coagulation pathway reflecting the critical interplay between the complement pathway and coagulation pathway. (B) The biopsy specimen is from a patient whose clinical course is complicated by a stroke over and above respiratory failure. (hematoxylin and eosin, 400 ×). (B), (C) The patient was a woman aged 22 years with morbid obesity who developed critical COVID-19. (Clinical lesions are presented in Figure 1, B). (A) The lower power image (hematoxylin and eosin, 100 ×) shows a relatively pauci-inflammatory thrombogenic vasculopathy associated with livedoid superficial vascular ectasia. The combination of the occlusive thrombotic diathesis and resultant vascular ectasia leads to the distinctive clinical lesion of thrombotic retiform purpura. (C) The thrombotic diathesis was associated with signs of endothelial cell injury as revealed by proplastic endothelial cell alterations. A moderately dense vasocentric neutrophilic infiltrate was noted as well, although without significant mural fibrin deposition. The neutrophilic infiltrates likely reflect the sequelae of the proinflammatory effects of C5a, a component of complement pathway activation. COVID-19, coronavirus disease 2019.
Assessment of components of complement pathway activation on paraffin-embedded tissue
The vascular thrombosis was reflective of microvascular complement activation based on extensive deposition of complement components including C3d, C4d, and C5b-9.13 , 43 In addition, in certain cases there was very focal deposition of MASP-2, the activated form of MASP that triggers the remainder of the MBL complement cascade eventuating in the formation of C5b-9. Of all the complement stains available on paraffin-embedded tissue, however, MASP-2 is the least sensitive and hence exhibited the overall lowest level of detection. Also, there can be significant background staining contributing to the difficulty in its interpretation. C5b-9 is the end point of complement pathway activation and its presence could signify activation of any of the three complement pathways. C4d also is a sensitive marker expressed at significant levels in the cutaneous and subcutaneous microvessels in patients with severe and critical COVID-19. The C4d stain is a challenge to interpret owing to strong staining of elastic tissue in the dermis, but deposition was most prominent in the fat where there is little or no background elastic tissue positivity as is seen in the dermis. We know that C4d is formed with either classic complement pathway or MBL complement pathway activation. There is no reason to believe that classic complement pathway activation is the basis of the microvascular complement deposition especially because there is a compromised adaptive immune response in patients with severe and critical COVID-19. The prominent C4d and C5b-9 deposition with or without MASP2 in cutaneous microvessels in patients with severe and critical COVID-19 was held to be reflective of MBL and alternative pathway activation (Figures 3 , A and B). In particular, the alternative pathway will be triggered from injured cellular remnants that are formed when other complement pathways are activated.13 , 14 , 43 , 53
Fig. 3.
Complement studies in the setting of thrombotic retiform purpura. The vascular thrombosis is reflective of microvascular complement activation based on the extensive degree of deposition of components of complement activation including C3d, C4d, and C5b-9. C4d is also a very sensitive marker that is expressed at significant levels in the cutaneous and subcutaneous microvessels in patients with severe and critical COVID-19. (A) C4d is formed with either classic or mannan binding lectin pathway activation (diaminobenzidine [DAB]), 1000 ×). As already alluded to, both C4d and C5b-9 are present at significant levels within the microvessels of skin biopsy specimens in patients with severe and critical COVID-19, a finding well exemplified by this case. (B) C5b-9 is the effector mechanism of microvascular endothelial cell injury (DAB, 400 ×). COVID-19, coronavirus disease 2019.
SARS-CoV-2 immunohistochemistry for viral capsid proteins and ultrasensitive RNA in situ hybridization and microanatomic distribution of SARS-CoV-2 receptor ACE2
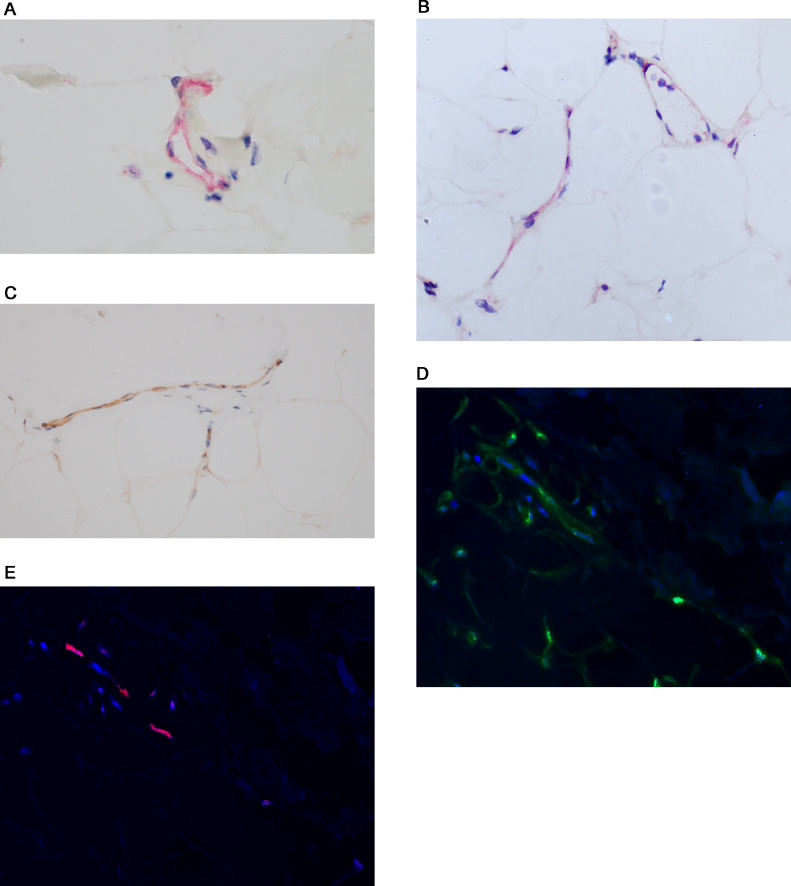
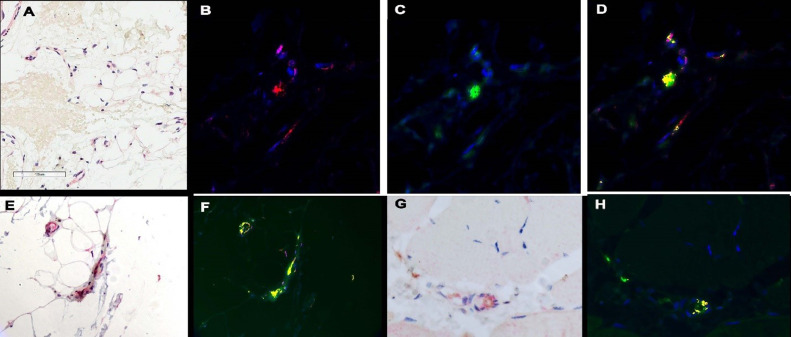
In all thrombotic retiform purpura cases, we showed localization of the SARS-CoV-2 capsid protein within the endothelium including spike glycoprotein, capsid membrane, and capsid envelope but without evidence of intact virus as determined through ultrasensitive RNA in situ hybridization using the RNAscope assay (Biotechne, Minneapolis, Minnesota) and ultrastructural studies (Figures 4 , A and B).13 , 14 , 43 , 53 The positive staining vessels were most apparent in the deeper dermis, adventitial dermis of the eccrine coil, and subcutaneous fat where a number of positive vessels was characteristically observed (Figures 4, A and B).The basis of the spike glycoprotein cell engagement with endothelium reflects the expression of the critical SARS-CoV-2 receptor, namely ACE2, by the endothelium of microvessels where it is most apparent in the deeper dermal and subcutaneous capillaries and venules (Figure 4, C). The viral capsid proteins binds to the endothelium of cutaneous vessels as a pseudovirion and not as an intact virus. The evidence for the latter point(i.e. the absence of intact virus), is based on the negative ultrasensitive RNA in situ hybridization studies for SARS-CoV-2, the lack of staining for nucleocapsid, and the absence of SARS-CoV-2 virions ultrastructurally. It is important to understand that the cutaneous endotheliopathy that underlies the ischemic dermopathy syndromes related to severe and critical COVID-19 is not reflective of a true infectious endothelialitis.
Fig. 4.
Thrombotic retiform purpura (SARS-CoV-2 viral capsid, cytokine, and furin immunohistochemical assessment). In each of thrombotic retiform purpura cases, localization of the SARS-CoV-2 capsid protein within the endothelium, including spike glycoprotein, capsid membrane, and capsid envelope, is observed but not intact virus. (A), (B) Spike glycoprotein endothelial cell localization within the deep dermal and subcutaneous microvessels in a patient who succumbed to COVID-19 (A, red chromogen, 1000 ×; B, red chromogen, 400 ×). At autopsy, the patient had classic thrombotic retiform purpura, which was biopsied. (C) In this case and other similar cases, the viral capsid protein localization is most apparent in the deeper dermal and subcutaneous microvessels and mirrors the distribution of ACE2 expression in microvessels of the skin and subcutaneous fat (DAB, 1000 ×). (D) The deep dermal and subcutaneous vessels that expressed spike glycoprotein (DAB, 1000 ×) also show (E) IL-6 (red chromogen, 1000 ×) and (G) TNF α expression (red chromogen, 1000 ×) whereby (E) endothelial cell colocalization of spike glycoprotein and IL-6 and spike glycoprotein and TNF α can be demonstrated using a nuance software that converts the red chromogen stain into a red signal (red chromogen, 1000 ×) and (D) the DAB stain into a green signal. (F), (H) Areas of colocalization appear yellow. In another case of thrombotic retiform purpura, there was very striking expression of furin within (I) endothelium in contrast with (J) its minimal expression in endothelium of biopsied normal deltoid skin in a healthy adult woman. ACE, angiotensin-converting enzyme; COVID-19, coronavirus disease 2019; DAB, diaminobenzidine; IL, interleukin; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TNF, tumor necrosis factor.
Pathophysiology of thrombotic retiform purpura and implications regarding disease pathogenesis operational in severe and critical COVID-19: The role of complement pathway activation
We hypothesized that the basis of the microvascular complement deposition is due in part to the engagement of spike glycoprotein with MBL based on extensive C4d and C5b-9 deposition, both representing components of MBL activation. One could argue that this complement expression pattern could also indicate classic complement pathway activation attributable to antibodies targeting the spike glycoprotein, but in our experience the typical granular deposition pattern for immunoglobin G in microvessels that would corroborate this hypothesis has not been observed.13 , 14 , 53 Furthermore at least in a few cases there was focal microvascular staining for MASP-2, a sign of MBL pathway activation.13 As already alluded to, the adaptive immune response in these patients is impaired owing to the blunted type I interferon signaling so classic complement pathway activation would be unlikely.61
Other authors studying the role of complement pathway activation in the pathogenesis of severe/critical COVID-19 have identified a subset of patients with strongly elevated MBL plasma levels correlated with thromboembolism and with high plasma D-dimer levels.19 , 62 Alternative complement pathway activation, however, is also contributory to vascular injury and in fact has been hypothesized to be the main pathway of complement pathway activation by some authors. They have suggested that the spike glycoprotein converts nonactivator surfaces into activator surfaces by preventing the inactivation of cell-surface alternative pathway of complement convertase.63 It has been thought that the SARS-CoV-2 spike protein (subunit 1 and 2), but not the nucleocapsid protein, directly activates the alternative complement pathway. The authors discovered that complement factor Bb, a serine protease that forms with alternative pathway activation to bind to C3b to form the alternative pathway convertase, is increased in the supernatant from spike protein–treated cells. C5 inhibition prevents the accumulation of C5b-9, but not of C3c, on cells. Addition of factor H, a complement regulator protein that controls the alternative pathway, mitigates the complement attack.63
Mechanisms underlying MBL and alternative pathway activation
One could further hypothesize that the release of cellular debris from MBL pathway activation is an independent activator of the alternative pathway. The mannose residues on the surface of a pathogen recognize certain sites on MBL, which will result in MASP-2 activation and trigger the remainder of the pathway to eventuate in the formation of C5b-9 with subsequent endothelial cell injury, defining a potential pathophysiologic construct to explain thrombotic complications attributable to MBL activation.64 , 65 MASP-2 staining as a sign of MBL pathway activation was observed in skin biopsies in some cases of thrombotic retiform purpura,13 but in general this particular stain has relatively low sensitivity compared with other complement stains such as C5b-9. SARS-CoV-2 associated spike glycoprotein is established to have specific sugar residues that are able to interact with MBL leading to MBL pathway activation.66 As already discussed in this contribution, however, others have suggested alternative and classic complement pathway activation as the basis of COVID-19–associated complement pathway activation. Regardless of the varied and potential mechanisms of complement pathway activation, C5b-9–mediated microvascular injury is the common end point of vascular injury. Components of complement pathway activation serve as a trigger to coagulation pathway contributing to the microvascular thrombosis. In addition, if one is to assume that MBL pathway activation is an important pathway of complement activation, there is synergistic activation of the alternative pathway. Hence, a significant component of the microvascular thrombosis in the skin and other organ systems could reflect systemic complement activation in concert with a generalized procoagulant state that underlies severe, critical and fatal COVID-19. Reflective of systemic complement activation is the presence of microvascular C5b-9 in apparently normal skin of the deltoid as will be discussed in the next section of this contribution.13 , 53
Thrombotic retiform purpura reflects complement pathway and coagulation pathway activation and is a hallmark of severe and critical COVID-19
Thrombotic retiform purpura does not occur in the setting of mild or moderate COVID-19 but rather is a cutaneous manifestation of severe and critical COVID-19. We propose that this distinctive eruption is a sequela of the excessive viral replication in the lung leading to the release of pseudovirions into the circulation as a fuel to complement pathway and coagulation pathway activation. Also, a blunted type I interferon response revealed by the lack of MXA staining in tissue sections in these patients is likely a key pathogenetic event leading to excessive viral replication in the lung.43 14
Thrombotic retiform purpura as a clue to the mechanisms of hypercytokinemia in the setting of severe and critical COVID-19
A characteristic feature of critical and severe COVID-19 is hypercytokinemia. Among the elevated cytokines are IL-6, TNF α, caspase 3, and interleukin β.67 Given the lack of inflammation encountered in biopsies of thrombotic retiform purpura and in the various organs studied in patients who die from COVID-19, it would seem unlikely that the hypercytokinemia is derived from T cells, B, cells or monocytes. We were able to show colocalization of endothelial-based viral pseudovirions including spike glycoprotein (Figure 4, D) with IL6 (Figures 4, E and F) and TNF α (Figures 4, G, and H) in biopsy specimens of COVID-19–associated thrombotic retiform purpura. We discovered high levels of IL-6 expression in the endothelium in cutaneous biopsies procured from lesions of thrombotic retiform purpura. A similar pattern of IL-6 upregulation was observed in microvessels of normal deltoid skin from patients with severe, critical, and fatal COVID-19. It has been demonstrated that IL-6 elaboration from endothelium can occur due to MBL activation.68 IL-6 elaboration from endothelium has a procoagulant effect. The mechanisms are complex and beyond the scope of this contribution. Among potential procoagulant IL-6 effects are platelet aggregation and activation.69 , 70
We are starting to examine furin expression in endothelium. Furin is the enzyme expressed by endothelium that cleaves the S1 and S2 subunits of spike glycoprotein and can facilitate the entry of virus into a cell. It is likely modulated by various local microenvironmental factors including anoxia but a genetic polymorphism in furin expression has also been postulated with higher levels leading to more severe disease because of its permissive effects on viral entry.71 We have examined two cases of thrombotic retiform purpura and have shown high levels of furin expression in endothelium in contradistinction to three biopsy specimens of normal skin in healthy adults with minimal furin expression. Additional studies examining furin expression in cases of severe and critical COVID-19 compared with other catastrophic complement syndromes such as atypical hemolytic uremic syndrome and normal skin samples are being conducted at the time of the completion of this contribution (Figures 4, I and J).
Normal deltoid skin biopsy in patients with severe and critical COVID-19 showing pseudovirion capsid protein microvascular localization and evidence of systemic complement pathway activation
Background
We have examined normal deltoid skin samples in patients who were significantly symptomatic with COVID-19, most of whom had severe, critical COVID-19 and/or succumbed to COVID-19. In a number of the cases there was subtle microscopic evidence of endothelial cell injury characterized by degenerative endothelial cell changes and focal vascular thrombosis. (Figure 5 , A).53 We discovered evidence of systemic complement pathway activation in all patients except two. In particular, within the deeper dermal and subcutaneous microvessels of apparently normal skin, there were significant deposits of C4d or C5b-9 compatible with systemic complement pathway activation (Figure 5, B).
Fig. 5.
Normal deltoid skin biopsy specimen in the setting of severe, critical, and fatal COVID-19 (light microscopic findings, complement studies, SARS-CoV-2 viral capsid, and cytokine immunohistochemical assessment). (A) In a number of the cases of apparently normal skin in patients with severe critical COVID-19 or fatal COVID-19. There is subtle microscopic evidence of endothelial cell injury characterized by degenerative endothelial cell changes and focal vascular thrombosis (hematoxylin and eosin, 400 ×). Within the deeper dermal and subcutaneous microvessels of apparently normal skin procured from the deltoid area, there are significant deposits of C4d or C5b-9. (B) Prominent microvascular deposits of C5b-9 (DAB, 400 ×). There is localization of viral capsid protein within the deeper and subcutaneous cutaneous microvasculature including spike glycoprotein, membrane, and envelope although with no evidence of viral replication as revealed by the lack of any SARS-CoV-2 RNA localization to endothelium. (C) Viral capsid membrane localization to endothelium (DAB, 1000 ×). In addition, the endothelial cells express various cytokines that are known to be elevated in COVID-19 including TNF α and IL-6. (D) In this image, there is striking localization of IL-6 to the endothelium of microvessels in a microanatomic distribution that mirrors complement and viral capsid protein expression (DAB, 400x). COVID-19, coronavirus disease 2019; DAB, diaminobenzidine; IL, interleukin; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TNF, tumor necrosis factor.
SARS-CoV-2 immunohistochemistry and ultrasensitive RNA in situ hybridization in biopsies of normal deltoid skin in the setting of severe and critical COVID-19
In addition, we observed colocalization of viral capsid protein including spike glycoprotein, membrane, and envelope protein in a subset of these complement-positive vessels emphasizing the role of viral capsid protein endothelial cell endocytosis as a key factor in microvascular complement pathway activation (Figure 5, C).13 , 14 , 43 , 53 Many positive staining vessels were observed similar to what was seen in the biopsy specimens procured from patients with thrombotic retiform purpura of severe and critical COVID-19 although quantitatively somewhat less. It was, however, much greater than the minimal microvascular localization of spike observed in skin biopsy specimens of vaccinated patients and in the T-cell–rich cutaneous reactions encountered in patients with mild COVID-19, best exemplified by COVID-19–associated perniosis.43
Evidence of hypercytokinemia is observed in microvessels of normal skin in the setting of severe COVID-19
The endothelial cells in the microvessels of the normal skin samples expressed various cytokines that are known to be elevated in COVID-19 including TNF α and IL-6, emphasizing the that the source of the hypercytokinemia is not one of T-cell or monocyte derivation but is derived from activated endothelial cells possibly through the effects of MBL pathway activation (Figure 5, D).14 We hypothesize that the basis of viral capsid localization to the deep dermal and subcutaneous vessels reflects higher levels of ACE2 expression in these vessels and have illustrated ACE2 complement and cytokine localization to microvessels of the deeper dermis and subcutaneous fat. Docking of viral proteins in normal skin and fat could serve as a fuel for both alternative complement pathway activation and coagulation pathway activation, given the known synergy and cross talk between the complement and coagulation pathways.14 , 53
Lower limb ischemia with cutaneous necrosis attributable to larger vessel thrombosis
Background
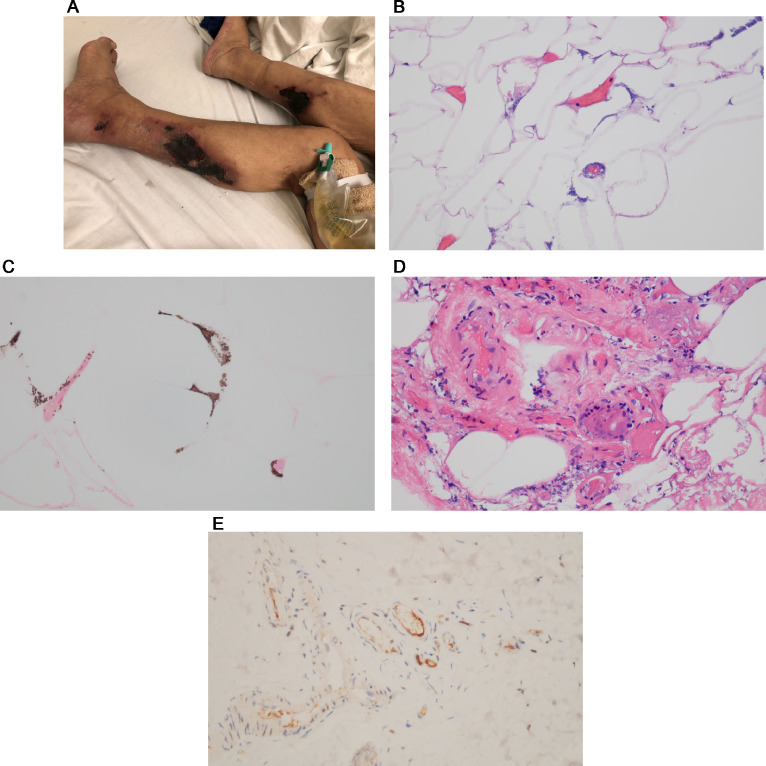
A procoagulant state contributes significantly to the morbidity and mortality of severe and critical COVID-19. Among the distinctive clinical features are excessively high D-dimer levels reflective of intravascular fibrin deposition and ischemic complications related to medium and larger vessel thrombosis exemplified by lower limb ischemia attributable to thrombotic arterial occlusion.72, 73, 74 We encountered two diabetic patients who developed occlusive thrombotic arterial disease resulting in below-knee amputation, one of whom developed thrombi in other larger arteries including the abdominal aorta and its branches in the setting of anticardiolipin antibodies of all isotypes that resolved after she had recovered from COVID-19 at day 48 of her hospitalization (Figure 6 , A).
Fig. 6.
Large vessel thrombosis reflective of the generalized procoagulant state operational in COVID-19. (A) Reflective to the striking procoagulant state observed in patients with severe and critical COVID-19 is the tendency toward multiorgan thrombosis including one characterized by arterial thrombotic occlusion of lower extremity vessels leading to lower limb ischemia. In the case illustrated, the patient had multiorgan larger vessel thrombosis in the setting of COVID-19 complicated by antiphospholipid antibodies. The development of larger vessel thrombosis led to lower extremity ischemia eventuating in the patient losing her leg below the knee. Illustrated is the explanted below-the-knee amputation specimen demonstrating ischemic gangrenous necrosis of the foot and distal leg. (B-G) Both amputated lower extremity specimens show extensive occlusion capillaries, venules, small arteries, and subcutaneous larger arteries by fibrin thrombi. (B) A component of endothelial cell injury and mural fibrin deposition is noted in capillaries and venules (hematoxylin and eosin, 400 ×), and (C) the subcutaneous large vessel arterial occlusion is unassociated with similar endothelial cell alterations and hence largely reflective of an underlying procoagulant state as opposed to a thrombotic diathesis triggered by endothelial cell injury (hematoxylin and eosin, 200 ×). (D) In the same vein, the vascular complement deposition is largely localized to the endothelium and in a subendothelial cell array within microvessels (DAB, 200 ×), and (E) significant complement deposition is not observed in the larger arteries within the subcutaneous fat (DAB, 200 ×). (F) SARS-CoV-2 viral envelope and membrane (red chromogen, 400 ×) proteins colocalized with TNF α and IL-6 shows (G) a similar pattern of vascular localization as that noted for complement (DAB, 400 ×) being localized to endothelium of microvessels but not to larger occluded arteries. RNA studies to detect intact virions are negative. COVID-19, coronavirus disease 2019; DAB, diaminobenzidine; IL, interleukin; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TNF, tumor necrosis factor.
Distribution of components of complement pathway activation and SARS-CoV-2 viral capsid protein in amputation specimens
Amputated lower extremity specimens showed extensive occlusion of small- and medium-sized arterial and venous vessels and capillaries by fibrin thrombi (Figures 6, B and C). There was deposition of C3d, C4d, and C5b-9 within dermal and subcutaneous microvessels (Figure 6, D). Colocalization studies showed SARS-CoV-2 viral capsid proteins, TNF α, and Il-6 in the same microvessels where there was complement deposition. Illustrated in Figure 6 (F) is the SARS-CoV-2 envelope and the SARS-CoV-2 membrane is illustrated in Figure 6 (G). A similar distribution of complement, viral capsid proteins and IL-6 was not observed in the larger thrombosed arteries. The larger vessel thrombosis was clearly reflective of a generalized procoagulant state and was not reflective of complement-mediated endothelial cell dysfunction that was evident in the microvessels. RNA studies to detect intact virions were negative.74
Pathophysiology of lower limb ischemia in the setting of severe/critical COVID-19 and its pathogenetic implications regarding the generalized procoagulant state of severe and critical COVID-19
The basis of the procoagulant state leading to this type of catastrophic complication in COVID-19 is multifactorial. We know that the complement pathway activation operational in significantly symptomatic patients with COVID-19 can trigger the coagulation pathway through the established synergy between complement pathway activation and coagulation pathway activation.13 , 14 , 43 , 74 Secondly, additional procoagulant factors such as cold agglutinins or anticardiolipin antibodies may emerge. Finally, there is diminished effectiveness of CD59, the regulator protein for C5b-9, attributable to its glycosylation in the setting of diabetes mellitus, leading to unchecked and overzealous C5b-9 deposition on endothelium, which we know plays a significant role in a number of thrombotic microangiopathies.75
Calciphylaxis as a complication of COVID-19 NOTE PLEASE HAVE THIS HEADING WITHOUT ITALICIZED TEXT!
Calciphylaxis is an ischemic dermopathy syndrome seen in select clinical settings, including renal insufficiency, other conditions associated with calcium phosphate abnormalities, and certain diseases such as inflammatory bowel disease, cirrhosis, and multiple myeloma. The cutaneous and subcutaneous ischemia is attributable to a subcutaneous obliterative calcific intimal arteriopathy and a thrombogenic microangiopathy targeting capillaries associated at times with calcific endothelial cell mummification. A procoagulant state is permissive to the bone-forming microenvironment of calciphylaxis in part through activation of osteopontin by thrombin.76 Not surprisingly, we have encountered two cases of this obliterative calcific thrombotic angiopathy in the setting of significant COVID-19. Pathogenetic synergies included the underlying procoagulant state and general state of endothelial cell dysfunction and the potential for renal insufficiency with its associated abnormalities in calcium phosphate metabolism. One of these cases has been reported elsewhere.28
COVID-19–associated calciphylaxis case vignettes and the distribution of viral capsid proteins and cytokines and ACE2 in the microvasculature of affected skin
The first patient was a man, aged 64 years, with end-stage renal disease. The patient had developed COVID-19 in early March of 2021. He was significantly symptomatic requiring supplemental oxygenation. During the third week of March, the patient developed progressive retiform plaques with lesions on the right fingers, back of the bilateral lower extremities, left ankle, and tip of the penis, which had increased in size since his hospitalization for COVID-19 (Figure 7 , A). The biopsy specimen showed a subcutaneous pauci-inflammatory thrombogenic vasculopathy accompanied by endothelial and mural calcification in microvessels. Many cutaneous and subcutaneous vessels had docked spike glycoprotein with a parallel staining pattern observed for IL-6, caspase 3, and TNF α, with the greatest extent of immunoreactivity observed for IL-6 and caspase 3. There was strong expression of ACE2 in dermal and subcutaneous microvessels mirroring the distribution of spike glycoprotein and cytokine microvascular expression.
Fig. 7.
Calciphylaxis in the setting of COVID-19 (clinical features). A man aged 64 years with history of end-stage kidney disease. The patient had severe COVID-19 in early March of 2021. While recovering from COVID-19 and on peritoneal dialysis, the patient developed retiform plaques consistent with calciphylaxis. (B–E) Calciphylaxis in the setting of COVID-19 (routine light microscopy, VON Kassa staining results, SARS-CoV-2 viral capsid immunohistochemical assessment). A skin biopsy specimen demonstrates classic features of calciphylaxis. (B) Over and above the calcific thrombotic diathesis in vessels of the subcutaneous fat (hematoxylin and eosin, 400 ×) and (C) confirmed on a Von Kassa stain (400 ×) there are (D) overlying skin changes of microvascular and arterial thrombosis(hematoxylin and eosin, 400 ×). Immunohistochemical assessment for the SARS-CoV-2 envelope protein and ACE2 reveals significant positivity in a few vessels in the deeper dermis and subcutaneous fat. (E) SARS-CoV-2 capsid envelope protein (DAB, 400 ×). ACE, angiotensin-converting enzyme; COVID-19, coronavirus disease 2019; DAB, diaminobenzidine; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
The second patient, previously reported,28 was a woman, aged 62 years, with end-stage renal disease secondary to focal segmental glomerulosclerosis and diabetes mellitus who suffered 3 weeks of cough and worsening atraumatic tender bilateral lower leg pain accompanied by purpuric plaques and was found to be COVID-19 positive via SARS-CoV-2 RT-PCR (reverse transcriptase polymerase chain reaction). A skin biopsy specimen demonstrated classic features of calciphylaxis (Figures 7, B and C).28 Over and above the calcific thrombotic diathesis in vessels of the subcutaneous fat (Figure 7, B), were overlying skin changes of microvascular and arterial thrombosis without calcification (Figure 7, D) reminiscent of thrombotic retiform purpura of severe and critical COVID-19. Immunohistochemical assessment for the SARS-CoV-2 envelope protein and ACE2 revealed significant positivity in a few vessels in the deeper dermis and subcutaneous fat (Figure 7, E).28
In both cases, there were extensive deposits of C5b-9 within the microvessels throughout the dermis and subcutaneous fat, corroborating a state of systemic complement activation typical of severe and critical COVID-19.
Calcinosis cutis secondary to COVID-19–associated chronic renal insufficiency
A man, aged 63 years, recovered from critical COVID-19 but developed chronic renal insufficiency and in the critical phase of the clinical course was found to have evidence of systemic complement pathway activation in a normal deltoid skin biopsy along with microvascular localization of capsid proteins and upregulation of certain cytokines in endothelium including caspase 3. During convalescence, with post-COVID–negative PCR and positive antibody testing, a second lesional skin biopsy specimen from an arm plaque demonstrated classic features of calcinosis cutis. There was no microvascular complement deposition, however, nor was there evidence of pseudovirion endocytosis or caspase 3 expression in endothelium. Renal insufficiency causing calcium phosphate abnormalities was believed causal of the metastatic calcification observed in this case.77
Robust interferon-driven and T-cell–immune cutaneous responses in association with COVID-19
SARS-CoV-2 elicits a robust T- and B-cell response attributable to the immunogenicity of single-stranded RNA, the nucleocapsid, and various capsid proteins such as envelope, membrane, and spike glycoprotein (Table 2)(Table 2). Certain distinctive cutaneous reactions in the skin reflect an interferon-driven T-cell response to the virus and are perhaps best and most commonly exemplified by COVID-associated perniosis/chilblains43 and the Kawasaki disease like multiorgan inflammatory syndrome; however, a broad spectrum of cutaneous reactions could be indicative of a cellular immune response to a virus such as the common morbilliform exanthem.78 , 79 When the T-cell–driven immune response is intense, the virus is eradicated quickly from the nasopharynx, and therefore PCR testing may be negative. An effective T-cell response may obviate the need for a humoral adaptive response and therefore, in this subset of patients with COVID-19, negative SARS-CoV-2 antibody results may be observed.
Table 2.
Interferon And T-Cell–Mediated Reactions
| Case # | Sex | Age | Biopsy | Diagnosis | Outcome | |
|---|---|---|---|---|---|---|
| A. Perniosis | PN 1 | M | 16 | Left dorsal foot | COVID-19–associated perniosis | Recovered |
| PN 2 | F | 65 | Right dorsal small metacarpophalangeal joint | COVID-19–associated perniosis | Recovered | |
| PN 3 | M | 15 | Left dorsal fourth toe | COVID-19–associated perniosis | Recovered | |
| PN 4 | F | 26 | Right fourth finger | COVID-19–associated perniosis | Recovered | |
| PN 5 | F | 48 | Right great toe | COVID-19–associated perniosis | ||
| B. Sweet Syndrome | SW 1 | M | 51 | Right thigh | Sweet syndrome with overlapping features of conventional Sweet syndrome and histiocytoid Sweet syndrome | Recovered |
| F | 41 | Left arm | Virally triggered pityriasis rosea. | |||
| TYPE 4 HR 1 | M | 38 | Left axilla | Virally triggered pityriasis rosea. | Recovered | |
| TYPE 4 HR 2 | F | 58 | Left medial breast | COVID-19–associated lymphocytic vasculitis as a manifestation of an effective robust T-cell and interferon-driven immune response to the virus (COVID-19 perniosis-like but at a truncal site). | Slowly recovering | |
| TYPE 4 HR 3 | M | 65 | Back | Subacute eczematous dermatitis | Recovered | |
| TYPE 4 HR 4 | F | 52 | Back | Subacute eczematous dermatitis | Recovered | |
| TYPE 4 HR 5 | M | 55 | Abdomen | Subacute eczematous dermatitis | Recovered | |
| TYPE 4 HR 6 | F | 59 | Left forearm | Acute generalized exanthematous pustulosis | Recovered |
COVID-19, coronavirus disease 2019; F, female; HR, hypersensitivity reaction; M, male; PN, perniosis; SW, Sweet syndrome.
Covid-19-associated perniosis
Background
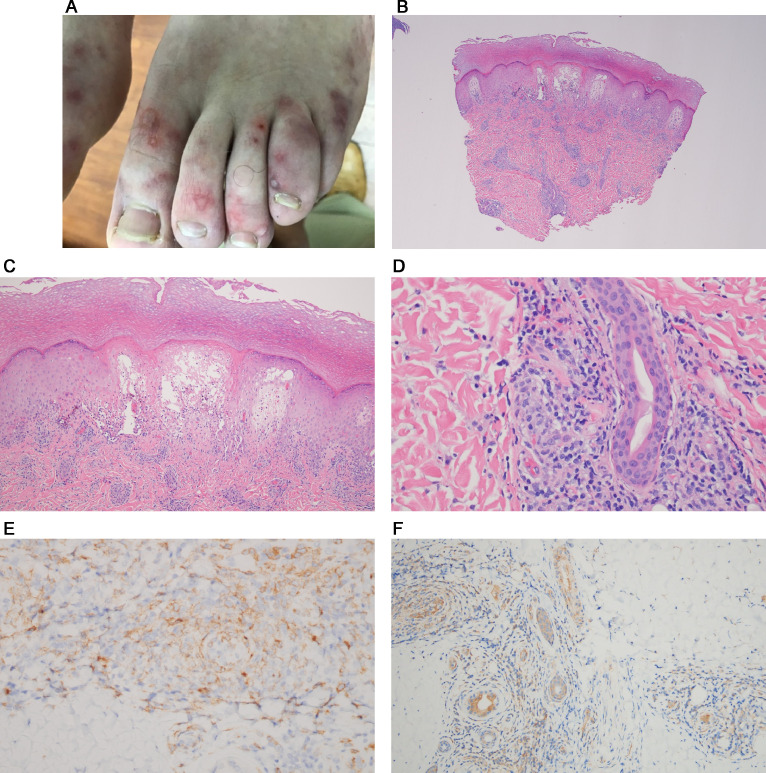
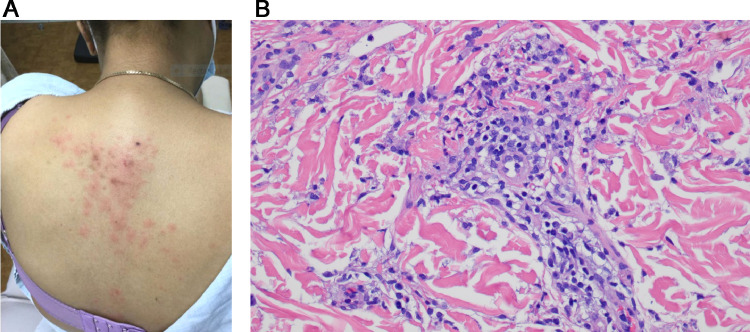
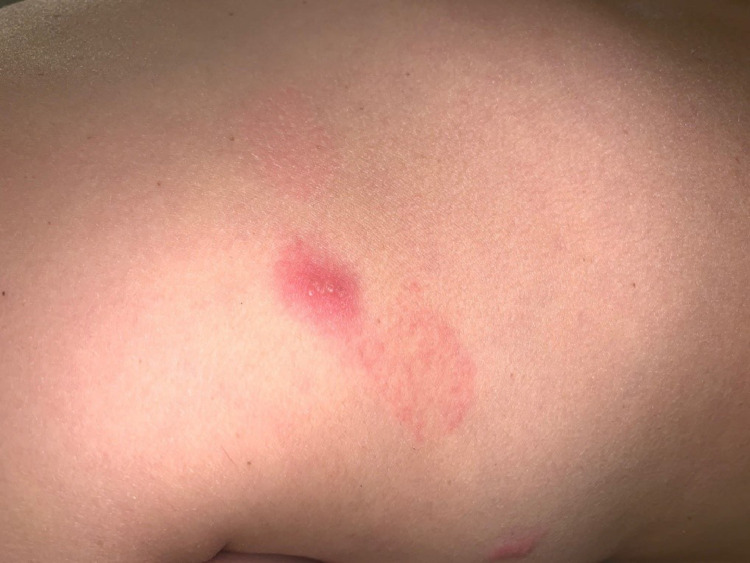
Numerous reports have been published of patients with SARS-CoV-2 infection presenting with pernio-like (ie, chilblains) lesions commonly referred to as “COVID toes.” These are red or purple papules that are often tender or itchy and may blister or ulcerate (Figures 8 , A and B). Primarily found in children, chilblains may be the only manifestation of COVID-19 in these patients who are typically negative for SARS-CoV-2 PCR and antibody testing for reasons already outlined and exhibit a significant degree of overlap with familial chilblains.43, 44, 45, 46, 47, 48, 49, 50 , 79, 80, 81, 82 We have encountered a number of COVID-19 perniosis cases with histologic samples procured from both children and adults ranging from 15 to 65 years of age. Some of these cases have been previously reported. There was no history of a similar eruption in these patients pre-COVID nor was there any history of Raynaud phenomenon as one might see with Idiopathic Perniosis . Their serologic autoimmune studies were negative.43
Fig. 8.
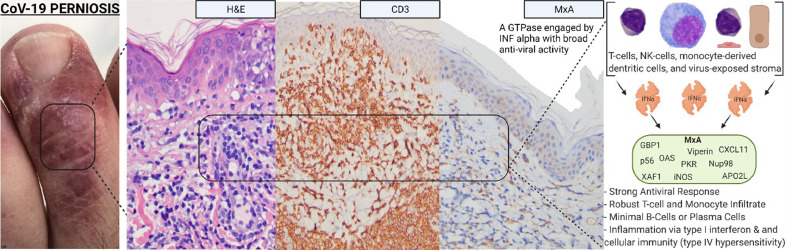
Skin eruptions associated with robust T-cell–driven and interferon-driven effective immune responses to COVID-19. (A) COVID-19 associated perniosis (clinical features). There have been numerous reports of patients with SARS-CoV-2 infection presenting with pernio-like (chilblains) lesions that popularly have been referred to as “COVID toes.” These are red or purple papules that are often tender or itchy and may blister or ulcerate (Reproduced with permission from Dr. Scott Sanders, New City, New York). (B) COVID-19–associated perniosis (routine light microscopy, phenotypic profile, interferon signaling profile). (D) Skin biopsies show a mononuclear cell dominant interface dermatitis with accentuation at tips of rete ridges, massive papillary dermal edema, and dense superficial and deep angiocentric lymphocytic and histiocytic infiltrates infiltrating blood vessels and eccrine ducts and eccrine coils defining a lymphocytic eccrine hidradenitis. (B), (C) One of the distinctive features of COVID-19–associated perniosis is the extent of epithelial necrosis apparently clinically and light microscopically (hematoxylin and eosin, 100 ×) . (D) In addition, within the reticular dermis while the infiltrates around the vessels are very brisk frank vasculitic destructive, changes are uncommon (hematoxylin and eosin, 400 ×). (E) Phenotypic studies demonstrate an infiltrate of CD3 + T cells, composing a mixture of CD4 and CD8 T cells and CD163+ CD68+ histiocytes exhibiting a mature monocyte derived dendritic cell phenotype as evidenced by immunoreactivity for CD14 (DAB, 400 ×). (F) All cases of COVID-19 perniosis manifest strong type I interferon signature revealed by intense MXA staining similar to the classic interferonopathy of idiopathic perniosis. MXA (human myxovirus resistance protein 1), the surrogate type I interferon marker available in paraffin embedded tissues, is strongly expressed defining this case of COVID-19 perniosis as a forme fruste interferonopathy (DAB, 200 ×). COVID-19, coronavirus disease 2019; DAB, diaminobenzidine; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Light microscopy and phenotypic analysis
A common light microscopic theme in all cases examined emerged being one of a mononuclear cell–dominant interface dermatitis with accentuation at tips of rete ridges, massive papillary dermal edema, and dense superficial and deep angiocentric lymphocytic and histiocytic infiltrates infiltrating blood vessels and adventitial dermis of the eccrine coil defining a lymphocytic eccrine hidradenitis (Figures 8, B and C). The histiocytes demonstrated reniform and serpiginous nuclei and contained intracytoplasmic cellular debris (Figure 8, D). Phenotypic studies demonstrated an infiltrate of CD3+ T cells, composing a mixture of CD4 and CD8 T cells and CD163+ CD68+ histiocytes exhibiting a mature monocyte-derived dendritic cell phenotype as evidenced by immunoreactivity for CD11c and CD14 (Figure 8, E). A cardinal hallmark of COVID-19–associated perniosis is the robust type I interferon response throughout a biopsy sample as revealed by the extent of MXA 1 staining in the epidermis, eccrine coil, endothelium, and inflammatory cells.43
Differential diagnosis
Differentiating COVID-19–associated perniosis from idiopathic perniosis
Although COVID-19–associated perniosis has many features in common with idiopathic perniosis, a differentiating feature is the presence of necrotizing vasculitic changes in reticular dermal-based blood vessels. In idiopathic perniosis fibrin deposition is largely localized to dermal papillae capillaries and typically does not involve reticular dermal-based blood vessels. All cases of COVID-19 perniosis manifest a strong type I interferon signature revealed by intense human MXA staining similar to the classic interferonopathy of idiopathic perniosis (Figure 8, F). MXA is the surrogate type I interferon marker detectable in paraffin-embedded tissues.43 Idiopathic/familial perniosis is associated with mutations in TREX1 and RNASEH2.80 , 82, 83, 84 Excessive interferon signaling is attributable to an accumulation of nucleases involved in removing nucleic acids that accumulate during apoptosis.
Viral spike glycoprotein immunohistochemistry assessment in cutaneous lesions of COVID-19–associated perniosis
We studied the microanatomic distribution of viral protein expression in COVID-19–associated perniosis and observed no expression of SARS-CoV-2 protein in endothelium except one case where a single vessel exhibited docked spike glycoprotein as a pseudovirion. Only a few cells of probable monocytic lineage contained SARS-CoV-2 protein and RNA.
The literature is conflicting regarding direct infection of endothelium by the virus in the setting of COVID-19–associated perniosis and the localization of viral capsid protein to endothelium. One group at Yale University (New Haven, Connecticut) published two studies in which they were unable to find nucleocapsid protein in endothelium, which would corroborate our position that endothelial cell infection by viable SARS-CoV-2 has not occurred85; RT in situ PCR for spike glycoprotein and nucleocapsid protein were negative in all of their cases although three of the six cases had spike glycoprotein in endothelium.86 They suggested that the spike glycoprotein was endocytosed as a pseudovirion but could result in endothelial cell injury through its interaction with ACE2 and eventuate in lesions of perniosis. Another study demonstrated a uniform pattern of endothelial expression of SARS-CoV-2 in vessels in the superficial dermis, which defines a pattern we have never seen, including in cases of severe and critical COVID-19. In our experience, the pattern of microvascular staining for SARS-CoV-2 is a focal interrupted one with dominant localization of immunoreactivity within deeper vessels and subcutaneous fat. This uniform superficial microvascular staining pattern raises consideration in regard to background staining.87 In one study the authors claimed evidence of direct viral infection of endothelium in lesions of chilblains by observing intracellular structures reminiscent of the SARS-CoV-2 virion87; in one letter to the editor, the authors indicated that their illustrations were more likely of clathrin coated vesicles, a normal intracellular structural organelle and not viral proteins.88
COVID-19 perniosis as a form of secondary perniosis
COVID-19–associated perniosis-like lesions can be considered a form of secondary perniosis along with chilblain lupus of Hutchinson and perniosis in the setting of antiphospholipid antibody and cryopathy syndromes such as cold agglutinins and cryofibrinogens.89 The negative PCR nasopharyngeal studies in most cases of COVID-19 perniosis may reflect the efficacy of an interferon-driven monocyte and T-cell response to clear the virus but which could also have deleterious consequences if unleashed in a multiorgan inflammatory context best exemplified by the multiorgan inflammatory syndrome resembling Kawasaki disease seen in children infected with SARS-CoV-2.
Virally triggered Sweet syndrome
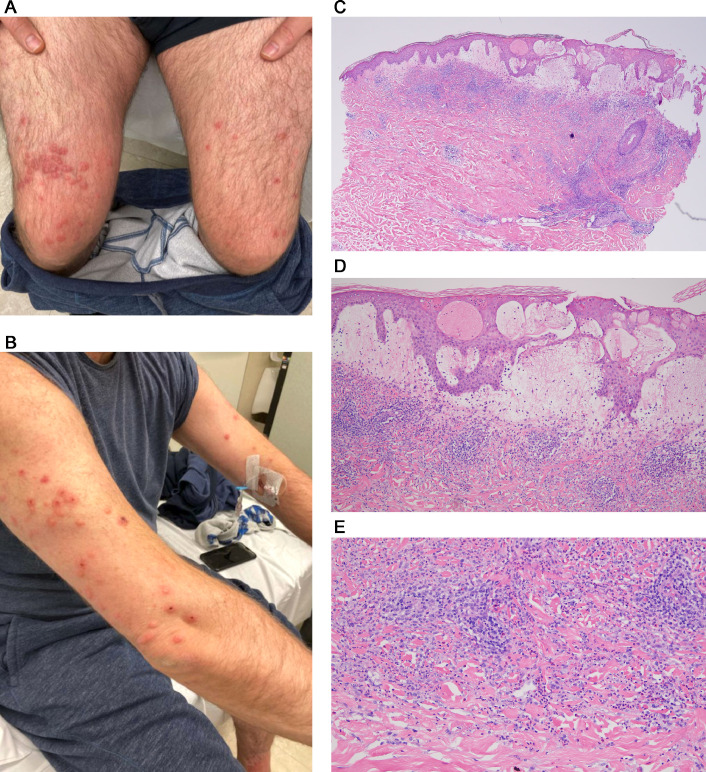
Sweet syndrome is a reactive T-cell–driven and monocyte-driven dermatosis observed in diverse clinical settings including hematologic dyscrasias, autoimmune disease, various infections, inflammatory bowel disease, and drug hypersensitivity. Microbial antigenic triggers include streptococcal sp, Staphylococcus aureus, yersinia sp, upper respiratory viral pathogens, and vaccinations that provoke a mononuclear cell-driven cytokine milieu associated with a secondary influx of neutrophils into the skin. The hallmarks clinically are infiltrative violaceous plaques accompanied by fever. Extracutaneous Sweet syndrome has been linked to underlying hematologic malignancy.80 , 90 We encountered a previously healthy man, aged 51 years, who presented with a papulovesicular eruption for 5 days in the setting of a recent diagnosis of COVID-19 (Figures 9 , A and B). The light microscopy showed classic features of Sweet syndrome, namely significant papillary dermal edema associated with interstitial neutrophilic and perivascular mononuclear cell infiltrates (Figures 9, C-F). The mononuclear cells exhibited a monocyte-derived dendritic cell phenotype as revealed by immunoreactivity for CD4, CD11c, CD14, and myeloperoxidase. Leukocytoclasia was noted. In areas, the dominant infiltrate was a histiocytic one, reflecting overlapping features of conventional Sweet syndrome with histiocytoid Sweet syndrome. Another reported case was that of a woman, aged 61years, who developed Sweet syndrome concurrent with moderately severe COVID-19. She had significant pulmonary disease radiographically along with the skin eruption, fever, and arthralgias.58 We surmise in these cases of COVID-19–associated Sweet syndrome that the virally driven type I interferon response preferentially recruits Th1 cells with a resultant cytokine microenvironment conducive to the influx of neutrophils and monocytes.
Fig. 9.
COVID-19–associated Sweet syndrome (clinical features, light microscopic findings, interferon signaling profile, and SARS-CoV-2 immunohistochemical assessment). (B) The patient was a previously healthy man aged 51 years who presented with a papulovesicular eruption accompanied by fever. (F) The biopsy specimen showed classic features of Sweet syndrome, namely significant papillary dermal edema associated with (C-F) interstitial neutrophilic and perivascular mononuclear cell infiltrates (C, hematoxylin and eosin, 100 ×; D-F, hematoxylin and eosin, 200x). Higher power magnification reveals that the dominant infiltrate is a histiocytic one confirmed phenotypically. (C–F) The overall morphology is most consistent with histiocytoid Sweet syndrome. (G) Upregulation in type I interferon signaling as revealed by MXA expression in endothelium, inflammatory cells and epithelial structures is observed (DAB, 200 ×). (H) Spike glycoprotein localized to rare microvessels is identified (red chromagen 400 ×). COVID-19, coronavirus disease 2019; DAB, diaminobenzidine; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Immunohistochemical assessment of type I interferon signaling and viral capsid protein expression
Upregulation in type I interferon signaling as revealed by MXA expression in endothelium, inflammatory cells and epithelial structures was observed (Figure 9, G). There were rare vessels positive for SARS-CoV-2 spike glycoprotein (Figure 9, H).
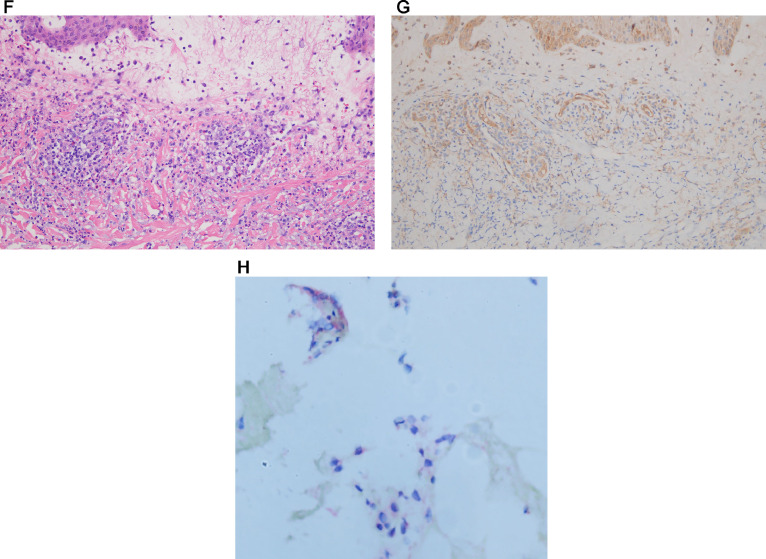
Additional T-cell–rich cutaneous eruptions (pityriasis rosea, eczematous reactions, lymphocytic purpuric vascular reaction, papular urticaria, and acute generalized exanthematous pustulosis)
The most commonly reported skin findings are similar to those encountered in typical viral exanthems such as maculopapular and morbilliform eruption and papulovesicular eruptions91 and are likely pathogenetically similar being reflective of a Gell and Coombs type IV immune reaction. The presentations clinically included eczematous dermatitis (three patients), PR (two patients), acute generalized exanthematous pustulosis (1 patient) (Figures 10 , A-C) and a pruritic papular eruption-mimicking papular urticaria (two patients). The most common pattern was a subacute eczematous invariably accompanied by a subtle cell-poor interface dermatitis consistent with a systemic eczematoid hypersensitivity reaction identified in four patients including two patients believed to have PR, acute generalized exanthematous pustulosis (one patient), a low-grade lymphocytic vasculitis (one patient), and papular urticaria (one patient) (Figures 11 , A and B). The eruptions were temporally associated with the diagnosis of acute COVID-19 with most developing the cutaneous eruption within 2 weeks of diagnosis. The eruptions were self-limited and lasted from 2 days to 5 months. A similar spectrum of T-cell–driven reactions have been described by others.36, 37, 38, 39, 40 In all cases, the patients were either asymptomatic or had mild COVID-19. In six of the cases we conducted spike glycoprotein, IL-6, and TNF α immunohistochemical stains. The results, similar to those seen with perniosis, showed only a few cells in the deeper dermis representing either endothelial cells or inflammatory cells expressing spike glycoprotein, IL-6 and TNF α.
Fig. 10.
COVID-19–associated acute generalized exanthematous pustulosis. (A) A woman aged 59 years presented with a widespread erythematous macular eruption and was subsequently found to be PCR positive for SARS-CoV-2 by nasopharyngeal swab (Reproduced with permission from Dr. S. Rougas, Oklahoma City, Oklahoma). (B) Small (1- to 3-mm diameter) pustules were superimposed on an erythematous base in a fashion consistent with acute generalized exanthematous pustulosis. (C) A discrete subcorneal and intra-epidermal pustule is present and corroborates the clinical diagnosis of acute generalized exanthematous pustulosis (hematoxylin and eosin, 100 ×). COVID-19, coronavirus disease 2019; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Fig. 11.
(A) A woman aged 52 years developed a widespread morbilliform eruption after testing positive for SARS-CoV-2 by nasophayryngeal PCR swab (Reproduced with permission from Dr. S. Rougas, Oklahoma City, Oklahoma). (B) There is a cell poor lymphocytic interface dermatitis with slight epidermal spongiosis and patchy parakeratosis in the absence of tissue eosinophilia. The histology resembles a viral exanthem. A morbilliform drug eruption is the differential diagnosis (hematoxylin and eosin, 400 ×). PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
One study in Spain examined more than 300 patients with COVID-19 via a questionnaire submitted to various clinicians who reported a variety of maculopapular eruptions including morbilliform eruptions and PR-like reactions along with erythema multiforme. Many of the patients had received drug therapy and therefore a drug reaction was possible. This contribution did not address histologic findings. It would appear that in most cases the patients did not undergo a biopsy.92
Role of humoral immunity in the pathogenesis of cutaneous lesions in the setting of COVID-19
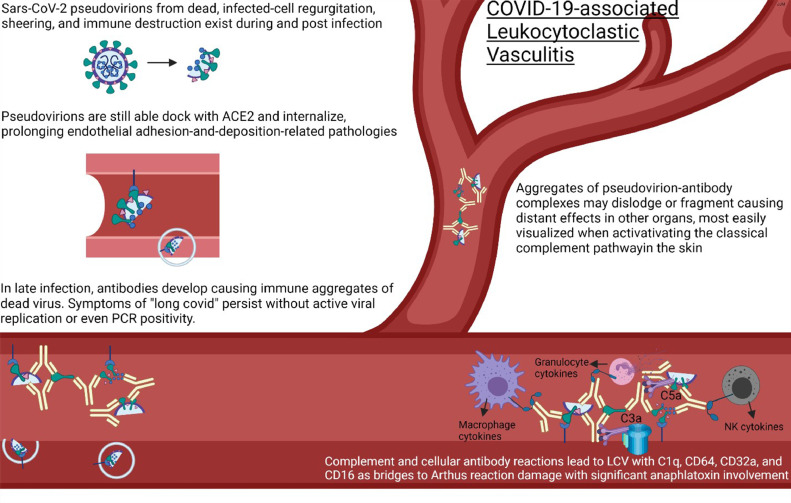
A humoral reaction to SARS-CoV-2 may be the basis of neutrophilic urticarial responses including urticarial vasculitis and leukocytoclastic vasculitis observed during the course of acute COVID-19(Table 1)(Table 3. In our experience, the patients developing this cutaneous complication have skin-limited vasculitis with COVID-19 disease of mild-to-moderate severity. We surmise that the etiologic basis is similar to other forms of cutaneous leukocytoclastic vasculitis, namely one reflective of an Arthus type III immune complex reaction. In the setting of SARS-CoV-2, the putative immune complexes likely compose antibody complexed to capsid proteins and other nonreplicative viral components released into the circulation. The circulating immune complexes would serve as a trigger for classic complement pathway activation leading to the release of neutrophil chemoattractants and vasopermeability factors with resultant vascular injury, inflammation, red cell extravasation, and vasocentric fibrin deposition.
COVID-19–associated leukocytoclastic vasculitis
We encountered four cases of leukocytoclastic vasculitis (LCV) where there was a temporal association between antecedent COVID-19 and where other LCV triggers could not be uncovered. These included one case of bullous LCV, one case of LCV associated with trace cryoglobulins, one case of urticarial vasculitis, and one case of a mixed lymphocytic vasculitis and LCV. There was an additional case in which the patient had been recovering from critical COVID-19 and which pseudomonas associated IgA vasculitis developed. There are anecdotal case reports of cutaneous LCV in the setting of COVID-19.
COVID-19–associated leukocytoclastic vasculitis case summaries
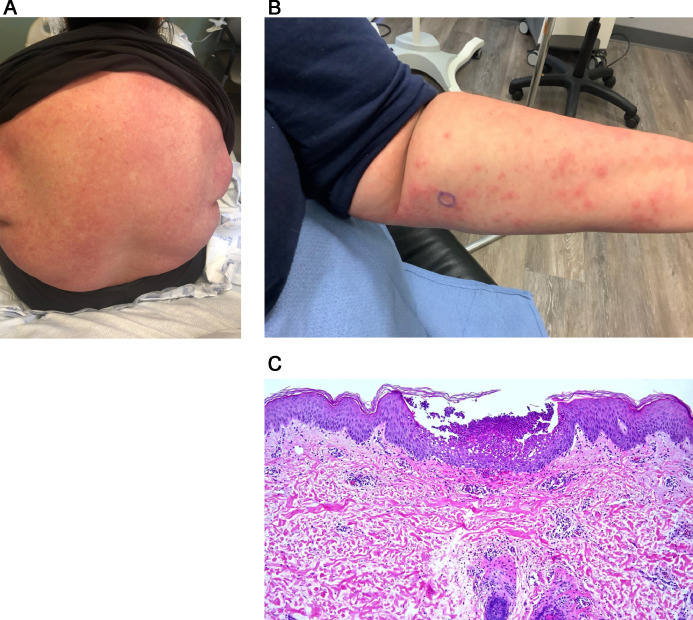
A woman from Dominica, aged 49 years, with a PCR-positive nasopharyngeal swab, developed palpable purpura several weeks later at which time she had positive COVID-19–associated antibodies. She also had evidence of trace cryoglobulinemia. A second patientwas a man, aged 72 years, with moderate COVID-19 who developed palpable purpura 2 weeks after his initial diagnosis. Both had ground glass pulmonary opacities, but neither was hypoxemic, neither required supplemental oxygen (Figures 12 , A-C) and therefore fulfilled criteria for moderate COVID-19. We observed two additional patients positive for COVID-19 who developed LCV after 2 weeks of mild COVID-19 symptoms.
Fig. 12.
Cutaneous eruptions reflective of an Arthus type III immune response to SARS-COV-2 signifying an effectual B-cell response. (A) The patient was a man aged 72 years who had lethargy, fever, and shortness of breath The patient also developed palpable purpura. The patient was found to be SARS-CoV-2 positive. A biopsy was performed of his skin eruption, which demonstrated a lymphocytic and neutrophil-enriched necrotizing vasculitis. The biopsy specimen showed a vasocentric lymphocytic and neutrophilic infiltrate that surrounds and permeates the vessel wall with evidence of vascular compromise characterized by mural fibrin deposition with attendant hemorrhage. (hematoxylin and eosin, 100 ×). (B), (C) The higher power magnification demonstrates an angiocentric and interstitial mixed infiltrate comprising lymphocytes, monocytes, and neutrophils. There is prominent endothelial cell swelling and there is prominent red cell extravasation corroborative of the diagnosis of a mixed leukocytoclastic and lymphocytic vasculitis (hematoxylin and eosin, 400 ×). SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Light microscopy
Skin biopsies demonstrated typical LCV changes in all four patients characterized by angiocentric neutrophilic infiltrates with leukocytoclasia and mural fibrin deposition (Figures 12, A and B; 13, A and B). One patient had classic IgA vasculitis triggered by pseudomonas infection in the setting of severe/critical COVID-19. One of the four cases had a significant degree of intervascular interstitial neutrophilia compatible with urticarial vasculitis. In another case, there was a conspicuous vasocentric lymphocytic component, therefore defining a hybrid leukocytoclastic and lymphocytic vasculitis albeit the dominant morphology was an LCV (Figures 12, A-C).
Complement studies and viral capsid protein immunohistochemical assessment in skin biopsies of COVID-19 associated leukocytoclastic vasculitis
Marked C4d and C5b-9 deposition within microvessels suggested classic complement and/or MBL pathway activation. It is likely that the basis of the vasculitis in the setting of COVID-19 is a combination of an Arthus type III immune complex reaction composing viral-related protein bound to antibody that is generated as part of the adaptive immune response triggering the classic complement pathway. In one case, virally triggered type III mixed cryoglobulinemia remains a possibility. These cases showed spike glycoprotein and other viral capsid protein localization to microvessels and/or to extravascular mononuclear cells. The microvascular spike glycoprotein was minimal compared with the extent of spike glycoprotein localization seen in the setting of thrombotic retiform purpura of severe and critical COVID-19.
Viral RNA assessment
Only one case expressed viral RNA, largely localized to rare mononuclear cells.
Pathophysiologic principles underlying COVID-19–associated leukocytoclastic vasculitis
In the case of IgA-associated vasculitis, we postulated that the trigger was pseudomonas septicemia. C5b-9–mediated endothelial cell injury could define a vascular cofactor that may lead to the preferential entrapment of immune complexes key to the development of postinfectious IgA vasculitis. The case showing a mixed leukocytoclastic and lymphocytic vasculitis demonstrated a strong upregulation of type I interferon in microvessels similar to COVID-19 perniosis. The enhanced type I interferon signaling could explain the component of lymphocytic vasculitis and may have contributed to the adaptive immune response to viral antigen.
Literature review of other cases of COVID-19 leukocytoclastic vasculitis
There are anecdotal reported cases of COVID-19–associated LCV in adults, ranging from 29 to 64 years of age. The LCV developed 1 week to 1 month after the diagnosis of COVID-19. Widespread palpable purpura involving the lower extremities, thigh, and trunk occurred in three of the patients, and the fourth patient had striking orbital edema with purpuric annular lesions involving the face, trunk, and upper extremities, consistent with urticarial vasculitis. Two of the patients had significant pulmonary involvement that would qualify as moderate COVID-19. The other two patients had no pulmonary symptoms but rather fever and malaise, symptoms of colitis in one, and loss of smell and loss of taste in the other, consistent with mild COVID-19. Molecular studies were conducted on the tissue samples in one of the cases. One of the four patients did not have a positive PCR result but had positive SARS-CoV-2–associated antibodies. The other three patients had positive nasopharyngeal swabs. The one case where nasopharyngeal swab studies were repeatedly negative had positive PCR studies to detect viral RNA in the skin biopsy specimen. One must be cautious when interpreting the PCR studies, specifically in regard to equating positive viral PCR results with evidence of active infection. Circulating free viral RNA released from nonviable cells or degenerated RNA containing viral fragments devoid of capsid proteins and hence not representing true replicative infection could give a positive result. The patient's vasculitic presentation occurred almost 1 month after his initial diagnosis of COVID-19.93, 94, 95, 96 In one case there was a delay of a few weeks between the initial presentation of COVID-19 and the development of urticarial vasculitis and as well the nasopharyngeal PCR studies went from a positive result at the time of the initial diagnosis of COVID-19 to a negative result when the patient presented with urticarial vasculitis.
We believe that LCV in the setting of COVID-19 represents a form of hypersensitivity to nonreplicative viral antigen. The delay of a few weeks between the onset of COVID-19 and the development of LCV reflects the role of the adaptive humoral immune response in its pathogenesis. Furthermore, the effective antibody response to various components of the virus would imply an adequate immune response likely effective in controlling viral replication hence explaining the relatively mild forms of COVID-19 seen in these patients.
Post vaccine biopsies in patients with no history of COVID-19 sorry I did not mean to delete the heading. please do not have it italicized though
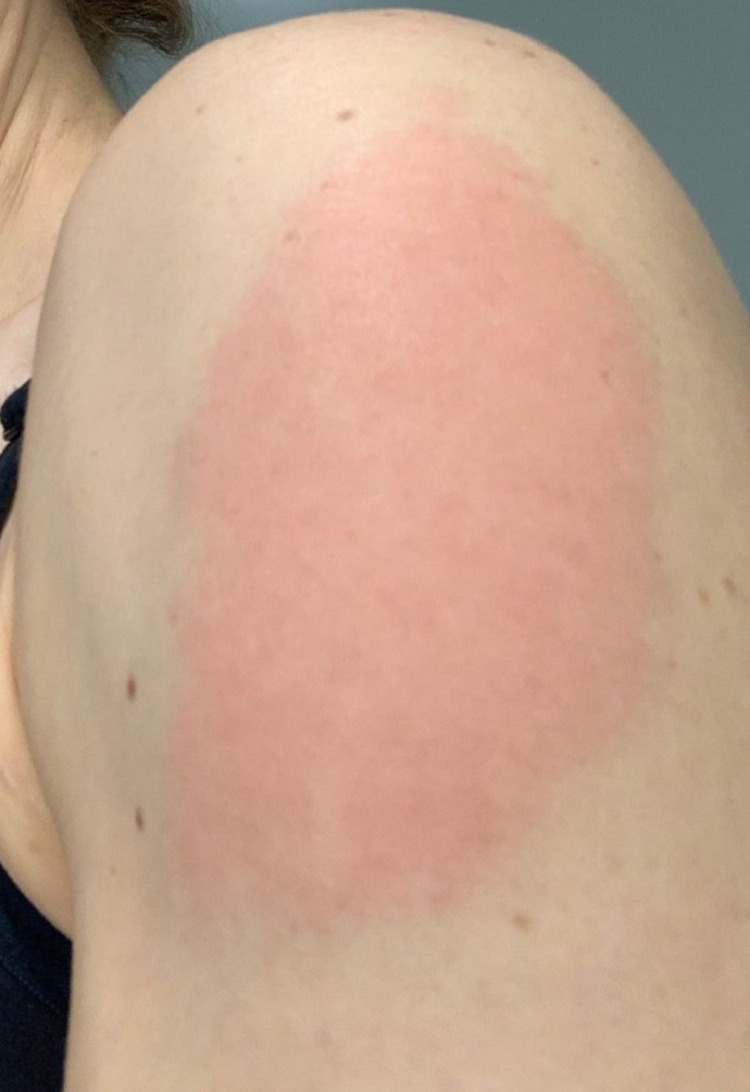
A number of vaccine candidates using a reverse genetic system primarily targeting the spike glycoprotein of SARS-CoV-2 have been developed with the earliest allowable age for vaccination being 12 years of age. At the time of writing, about 49.6% of the population of the United States of America has been fully vaccinated, having received 1 of the 3 currently available vaccines allowed for emergency use by the United States Food and Drug Administration, namely the Pfizer/BioNtech and Moderna lipid vehicle-based mRNA vaccines and the Johnson and Johnson viral vector vaccine. In January of 2021, we began to see cutaneous eruptions temporally associated with the administration of the vaccines(Table 4). We observed nine cases after the Moderna vaccine and 2 occurred after the Pfizer vaccine. In four cases, the vaccine administered was unknown. The reactions developed 1 day to 7 weeks after vaccination. In 9 cases, the eruption developed within 1 week after receiving either the first or second dose, including three cases in which the eruption developed within 48 hours after the vaccine. In five cases, the reaction was delayed, developing 9 days, 10 days, 3 weeks, 4 weeks, and 7 weeks after receiving the vaccine. Among the eruptions were localized erythema at the site of the vaccine, (2 cases) eczematous dermatitis (six cases), morbilliform hypersensitivity (one case), urticaria (one case), lymphocytic vasculitis (one case), Grover disease (one case), urticarial vasculitis (two cases), herpes zoster, and perniosis (one case) (Figures 14, A and B; 15, A; 16,A,B and C,A; 17). In all cases the clinical impression was congruous with the histologic findings, and in all cases the eruptions resolved either spontaneously or with topical or systemic steroid therapy (Figures 14, B; 15, B-D; 18; 19, A and B). In cases resembling eczema, the clue to the systemic nature of the hypersensitivity reaction was the concomitant interface dermatitis. Conversely, in the morbilliform reaction the dominant interface pattern is accompanied by subtle eczematous changes. In addition, in one case there were pustules noted clinically; however, there was no histologic documentation of a pustular diathesis. There were no accompanying significant systemic symptoms. One patient had significant peripheral blood eosinophilia. Similar eruptions were described by McMahon et al.97 and as well localized delayed hypersensitivity reactions at the site of the vaccine are reported similar to the cases reported in this series (Figure 20).97, 98, 99
Fig. 14.
(A) The patient is a woman aged 38 years who carries a diagnosis of lupus erythematosus. The patient had a history of limited discoid lupus erythematosus on the face that antedates her presentation by 12 years. The patient has been on plaquenil for a number of years. The patient had the COVID-19 vaccine on February 15, 2021. The next day the patient developed redness and swelling in the suprapubic area and a more full-blown eruption on the back and abdomen about a week later.(Case courtesy Dr. Henry Lee, New York, New York) (B) The biopsy specimen shows a cell poor interface dermatitis. A low-grade lymphocytic vasculitis was noted. In addition, there was a supervening interstitial granulomatous component. A diagnosis was made of a post-vaccine hypersensitivity reaction (hematoxylin and eosin, 400 ×). COVID-19, coronavirus disease 2019.
Fig. 20.
Shortly after the first dose of the Moderna vaccine, the patient developed an area of slight induration and erythema that spread circumferentially from the vaccine site (Reproduced with permission from Dr. Marc Grossman, White Plains, New York).
Viral spike glycoprotein immunohistochemistry assessment
The immunohistochemical stain to assess for spike glycoprotein was conducted in an attempt to document evidence of human myocyte synthesis and to establish the ability of spike glycoprotein to dock to ACE2 positive vessels as a pseudovirion, a hypothesis proffered as the basis of systemic complement activation in the setting of severe and critical COVID-19. We were able to document spike glycoprotein in the cutaneous microvasculature in all cases tested. In particular, there were occasional deep-seated vessels in the deep reticular dermis and fat that showed focal expression of spike glycoprotein in endothelium, which is not surprising because it reflects the preferential expression of ACE2 in deeper dermal and subcutaneous microvessels. There were only one rare positive staining vessels (Figure 21).
Fig. 21.
Post-vaccine detection of spike protein and host response in the skin's microvessels in the deep dermis and subcutaneous fat. (A) The strong expression of ACE2 in the microvessels of the subcutaneous fat in a person after the Moderna vaccine (fast red chromogen, 200 ×). Rare microvessels after vaccine showed (B) the spike protein (red chromagen) that co-expressed with (C) TNFα as seen in (D) the merged image (fluorescent yellow marks endothelial cells with spike protein and TNFα; 1000 ×). (E), (F) The co-expression of the viral membrane protein and TNFα in the microvessels of the leg in a case of person with severe COVID-19 whose hypercoagulable state led to a leg amputation (TNFα, DAB, viral membrane protein fluorescent red, with co-expression as fluorescent yellow, 1000 ×). (G), (H) A mouse model of COVID-19 where IV injection of the spike protein induces neurologic symptoms and endocytosis of the spike protein in the deep microvessels of the skin with concomitant TNFα expression (1000 ×, spike, red chromagen, TNFα DAB, with co-expression as fluorescent yellow). ACE, angiotensin-converting enzyme; COVID-19, coronavirus disease 2019; TNFα, tumor necrosis factor.
Cytokine assessment
A similar distribution was observed but without significant microvascular complement deposition for IL-6, caspase 3, and/or TNF α. (Figure 20). The overall amount of spike glycoprotein in the microvessels was much less than what we observed in the setting of thrombotic retiform purpura of severe and critical COVID-19.
Pathophysiologic principles underlying the post-vaccine reactions
It is our impression that the hypersensitivity reactions associated with the vaccine are mild, self limited, and primarily type IV delayed dermal hypersensitivity reactions. Vasculitic cases, however, represent a humorally mediated reaction. If one makes the assumption that the vehicle of delivery is largely inert, the newly synthesized foreign antigen—namely the spike glycoprotein, which is capable of eliciting a robust T-cell–driven and antibody-driven response—could be the trigger to these cases of post-vaccine–associated cutaneous hypersensitivity reflecting either a type IV immune response to small amounts of spike glycoprotein localized to cutaneous microvessels or an Arthus type III immune complex reaction composing spike glycoprotein bound to antibody. The fact that certain cases exhibit a morphology that resembles the cutaneous manifestations of mild COVID-19, such as perniosis and PR, perhaps provides some credence to this hypothesis. Vaccine constituents have been implicated in systemic allergic contact dermatitis whereby exogenous antigens such as neomycin, thimerosal, formaledehyde, and propylene glycol have been implicated. Polyethylene glycol moieties and polysorbate-80 have the potential to cause immediate hypersensitivity reactions, as well as delayed hypersensitivity reactions. Polyethylene glycol moieties have been implicated in the pathogenesis of the immediate and delayed hypersensitivity reactions associated with the Moderna and Pfizer vaccines.97
Despite microvascular localization of spike glycoprotein similar to what one observes in thrombotic retiform purpura of severe/critical COVID-19, significant microvascular sequelae do not appear to occur likely reflecting the exceptionally low burden of manufactured spike glycoprotein and the progressive neutralization of human myocyte synthesized spike by antibodies produced by the host.
Concluding remarks
COVID-19 presents many aspects in the skin, encompassing specific manifestations that correspond to the severity of the disease. In this regard, the broad categories of disease directly attributable to active infection can be segregated into those invariably seen in the setting of severe and critical COVID-19, namely cell-poor thrombotic retiform purpura and lower limb gangrene, versus the robust T-cell–driven and B-cell–driven cutaneous reactions seen in patients with mild or moderate COVID-19 that reflect an effectual immune response that facilitates viral eradication. The heterogeneous inflammatory reactions include perniosis, vasculitis with features of a lymphocyte driven and/or Arthus type III neutrophil-enriched pattern, PR, and erythema multiforme. Some cutaneous eruptions reflect metabolic consequences of kidney failure that develops in patients with severe and critical COVID-19. Finally, there are vaccine reactions that primarily reflect a type IV T-cell response. The antigenic epitope that elicits the response is unclear. Of interest is the localization of human manufactured spike glycoprotein to the same microanatomic sites observed in the setting of severe and critical COVID-19, namely, deeper dermal and subcutaneous microvessels. There is no reason to believe that active viral infection related to SARS-CoV-2 occurs in the skin, but clearly pseudovirions represented by viral capsid proteins can lodge in deeper dermal and subcutaneous vessels corresponding to microanatomic ACE2 expression and result in complement pathway activation and vascular thrombosis in patients with severe and critical COVID-19. That being said, pseudovirion localization to ACE2 positive does not always equate with microvascular sequelae best exemplified by post-vaccine COVID-19 skin biopsies.The pathophysiologic mechanisms that underlie the main cutaneous faces of COVID-19 are summarized in figures 22 through 24.
Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8, Fig. 9, Fig. 10, Fig. 11, Fig. 12, Fig. 13, Fig. 14, Fig. 15, Fig. 16, Fig. 17, Fig. 18, Fig. 19, Fig. 20, Fig. 21, Fig. 22, Fig. 23, Fig. 24
Fig. 13.
(A) The patient was a woman aged 55 years who developed mild COVID-19. Approximately 10 days later the patient developed a purpuric bullous eruption. There is massive papillary dermal edema with upper dermal hemorrhage visible from scanning magnification (hematoxylin and eosin, 100 ×). (B) There is leukocytoclastic vasculitis characterized by transmural neutrophil migration through vessels walls, showing fibrinoid necrosis, leukocytoclasia, and hemorrhage. The findings represents bullous leukocytoclastic vasculitis histopathologically (hematoxylin and eosin, 200 ×) (Reproduced with permission from Dr. Allison Hood, Oklahoma City, Oklahoma). COVID-19, coronavirus disease 2019.
Fig. 15.
(A) The patient is a woman aged 34 years with a pruritic eruption that is generalized over the body. It developed after her second COVID-19 Moderna vaccine on the February 14, 2021. After receiving the vaccine, the patient developed body aches and chills for about 2 days. On the third day she developed an eruption at the site of the vaccine and then it spread to the trunk, arms, and proximal thighs. The patient is healthy and does not take any medications (hematoxylin and eosin, 200 ×).(Case Courtesy Dr. Eva Kerby, New York, New York) (B), (C) The biopsy specimen shows a hybrid interface and eczematous dermatitis along with a superficial lymphocytic purpuric vascular reaction. Scattered eosinophils were also noted. The overall morphology is characteristic for a systemic type IV hypersensitivity reaction (hematoxylin and eosin, 400 ×). (D) The biopsy specimen exhibits spongiosis with lymphocytic exocytosis. A subtle interface dermatitis is identified. Focal parakeratosis with dyskeratosis is noted. There is a lymphocytic purpuric vascular reaction accompanied by a few eosinophils. Overall, the biopsy specimen is one that shows a spongiotic eczematous picture with a very subtle interface dermatitis compatible with a systemic type IV hypersensitivity (hematoxylin and eosin, 400 ×). COVID-19, coronavirus disease 2019.
Fig. 16.
(A) A man aged 66 years developed this urticarial reaction process after receiving the COVID-19 Moderna vaccine. (B), (C) The biopsy specimen demonstrates an inflammatory process within the dermis, exhibiting urticarial-like features. In particular, there is a mixed infiltrate that is interstitial and perivascular composed of lymphocytes, monocytes, and neutrophils along with a few eosinophils with concomitant dermal edema. Inflammatory cells migrate through the vessel wallwithout fibrin deposition, although nevertheless there is focal leukocytoclasia and red cell extravasation, a constellation of findings consistent with urticarial vasculitis(B, hematoxylin and eosin, 200 ×; B, hematoxylin and eosin, 400 ×) COVID-19, coronavirus disease 2019.
Fig. 17.
A woman aged 37 years suffered her first onset of shingles 1 day after her first dose of the Pfizer COVID-19 vaccine. No biopsy specimen was taken as the clinical picture was considered pathognomic. The eruption cleared after 7 days on valtrex. COVID-19, coronavirus disease 2019.(Case courtesy Erica Kumar)
Fig. 18.
A woman aged 48 years developed an urticarial tissue reaction days after receiving her first dose of the Moderna COVID-19 vaccine, characterized histologically by dermal edema with an interstitial mixed inflammatory infiltrate comprising lymphocytes, histiocytes, and interstitial mast cells. There was demodex colonization of a hair follicle structure with lymphocytic exocytosis into the follicular epithelium (hematoxylin and eosin, 200 ×) (Reproduced with permission from Dr. Miranda Smith, Tulsa, Oklahoma). COVID-19, coronavirus disease 2019.
Fig. 19.
(A) A woman aged 54 years developed tender purple-red plaques and nodules on her fingers 9 days after receiving a first dose of the Moderna vaccine. There is marked papillary dermal edema with a superficial and deep perivascular and peri-adnexal mononuclear cell infiltrate accompanied by a patchy lymphocytic interface injury pattern (hematoxylin and eosin, 100 ×) (Reproduced with permission from Dr. P. Safo, St Michael's Hospital, Steven's Point, Wisconsin). (B) There is deep perivascular and peri-adnexal mononuclear cell infiltrate accompanied by a transmural pattern of lymphocyte migration characteristic for perniosis (hematoxylin and eosin, 200 ×).
Fig. 22.
Pathophysiologic mechanisms underlying severe COVID-19 including the role of Mannan Binding lectin and alternative pathway complement-mediated microvascular injury and the blunted interferon response. COVID-19, coronavirus disease 2019.
Fig. 23.
Robust interferon driven and T-cell immune cutaneous responses in association with mild COVID-19. COVID-19, coronavirus disease 2019.
Fig. 24.
The role of humoral immunity and classic complement pathway activation in the pathogenesis of cutaneous lesions in the setting of mild and moderate COVID-19. COVID-19, coronavirus disease 2019.
The effectual interferon response will result in viral clearance and the relative absence of pseudovirions but can potentially elicit various forms of cutaneous type IV hypersensitivity. We have long recognized the skin as a critical window for understanding disease. Being our most superficial organ, the skin provides our easiest access for studying COVID-19 and providing an unparalleled opportunity for unraveling key pathophysiologic principles of COVID-19.
Table 3.
Humorally mediated immune reactions
| Case # | Sex | Age | Biopsy | Diagnosis | Outcome | |
|---|---|---|---|---|---|---|
| LCV | LCV 1 | W | 49 | N/A | Moderately severe leukocytoclastic vasculitis in association with significant complement activation likely representing an Arthus type III immune complex reaction but potentially with an underlying SARS-CoV-2–associated complement-driven microangiopathy. | Oxygen saturation while at the hospital remained at 100%. Gradually convalesced at home without any serious complications. |
| LCV 2 | M | 72 | Right thigh | COVID-19 associated low-grade lymphocytic vasculitis and leukocytoclastic vasculitis in the setting of a robust type I interferon immune response reflective of effective interferon-driven cellular and antibody-triggered immunity to SARS-CoV-2. | Recovered | |
| LCV 3 | M | 77 | Left calf; Right thigh | Iga-associated Vasculitis/infection-triggered Henoch-Schonlein Purpura. | Recovered | |
| LCV 4 | M | 59 | Left arm | Urticarial Vasculitis | Recovered | |
| LCV 5 | F | 55 | Right leg | Bullous LCV | Recovered |
COVID-19, coronavirus disease 2019; F, female; LCV, leukocytoclastic vasculitis; M, male; N/A,; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Table 4.
Post vaccine reactions
| Case # | Sex | Age | Biopsy | Diagnosis | Outcome |
|---|---|---|---|---|---|
| PV 1 | F | 38 | Superior back | Post-vaccine–associated cytotoxic interface dermatitis with low-grade lymphocytic vasculitis with an associated upregulated type I interferon microenvironment | Recovered |
| PV 2 | F | 90 | Left chest | Systemic eczematoid eosinophil-rich hypersensitivity reaction possibly representing an exuberant type IV immune response to the vaccine. | Recovered |
| PV 3 | F | 34 | Left abdomen | Post-vaccine eczematoid and delayed dermal hypersensitivity reaction. | Recovered |
| PV 4 | M | 66 | Right upper arm | Post-vaccine–associated urticarial vasculitis. | Recovered |
| PV 5 | M | 67 | Left back; right anterior thigh | Post-vaccine triggered morbilliform hypersensitivity reaction. | Recovered |
| PV 6 | F | 73 | Left thigh | Post-vaccine Urticarial vasculitis | Recovered |
| PV 7 | F | 48 | Chest | Post-vaccine urticaria | Recovered |
| PV 8 | F | 54 | Left finger | Post-vaccine–associated perniosis | Recovered |
| PV 9 | M | 66 | Right upper quadrant | Post-vaccine Grover disease | Recovered |
| PV 10 | M | 72 | Right arm | Subacute eczematous dermatitis | Recovered |
| PV 11 | F | 37 | Left chest | No biopsy | Recovered |
| PV 12 | F | 66 | Right thigh | Subacute eczematous dermatitis | Recovered |
| PV 13 | M | 86 | Back | Subacute eczematous dermatitis | Recovered |
F, female; M, male; PV, post vaccine.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 2.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levi M, Thachil J, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7:e438–e440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torres Acosta MA, Singer BD. Pathogenesis of COVID-19–induced ARDS: implications for an ageing population. Eur Respir J. 2020;56 doi: 10.1183/13993003.02049-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ronco C, Reis T, Husain-Syed F. Management of acute kidney injury in patients with COVID-19. Lancet Respir Med. 2020;8:738–742. doi: 10.1016/S2213-2600(20)30229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iba T, Levy JH, Levi M, Thachil J. Coagulopathy in COVID-19. J Thromb Haemost. 2020;18:2103–2109. doi: 10.1111/jth.14975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villoutreix BO, Calvez V, Marcelin AG, Khatib A-M. In Silico Investigation of the new UK (B.1.1.7) and South African (501Y.V2) SARS-CoV-2 variants with a focus at the ACE2-spike RBD interface. Int J Mol Sci. 2021;22:1695. doi: 10.3390/ijms22041695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grasselli G, Tonetti T, Protti A, et al. Pathophysiology of COVID-19–associated acute respiratory distress syndrome: a multicentre prospective observational study. Lancet Respir Med. 2020;8:1201–1208. doi: 10.1016/S2213-2600(20)30370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blanco-Melo D, Nilsson-Payant BE, Liu WC, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036–1045. doi: 10.1016/j.cell.2020.04.026. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopez L, Sang PC, Tian Y, Sang Y. Dysregulated interferon response underlying severe COVID-19. Viruses. 2020;12:1433. doi: 10.3390/v12121433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hadjadj J, Yatim N, Barnabei L, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magro CM, Mulvey J, Kubiak J, et al. Severe COVID-19: a multifaceted viral vasculopathy syndrome. Ann Diagn Pathol. 2021;50 doi: 10.1016/j.anndiagpath.2020.151645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mulvey JJ, Magro CM, Ma LX, Nuovo GJ, Baergen RN. Analysis of complement deposition and viral RNA in placentas of COVID-19 patients. Ann Diagn Pathol. 2020;46 doi: 10.1016/j.anndiagpath.2020.151530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watanabe Y, Allen JD, Wrapp D, McClellan JS, Crispin NM. Site-specific glycan analysis of the SARS-CoV-2 spike. Science. 2020;369:330–333. doi: 10.1126/science.abb9983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lokman SM, Rasheduzzaman M, Salauddin A, et al. Exploring the genomic and proteomic variations of SARS-CoV-2 spike glycoprotein: a computational biology approach. Infect Genet Evol. 2020;84 doi: 10.1016/j.meegid.2020.104389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laurence J, Mulvey JJ, Seshadri M, et al. Anti-complement C5 therapy with eculizumab in three cases of critical COVID-19. Clin Immunol. 2020;t219 doi: 10.1016/j.clim.2020.108555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eriksson O, Hultström M, Persson B, et al. Mannose-binding lectin is associated with thrombosis and coagulopathy in critically ill COVID-19 patients. Thromb Haemost. 2020;120:1720–1724. doi: 10.1055/s-0040-1715835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rambaldi A, Gritti G, Micò MC, et al. Endothelial injury and thrombotic microangiopathy in COVID-19: treatment with the lectin-pathway inhibitor narsoplimab. Immunobiology. 2020;225 doi: 10.1016/j.imbio.2020.152001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang HJ, Lee SM, Lee HH, et al. Mannose- binding lectin without the aid of its associated serine proteases alters lipopolysaccharide-mediated cytokine/chemokine secretion from human endothelial cells. Immunology. 2007;122:335–342. doi: 10.1111/j.1365-2567.2007.02644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hovhannesyan RA, Hovhannisyan IG. Platelet aggregation and interleukins indicators impacting the outcomes of ischemic stroke. J Stroke Cerebrovasc Dis. 2019;28:2038–2044. doi: 10.1016/j.jstrokecerebrovasdis.2019.02.023. [DOI] [PubMed] [Google Scholar]
- 23.Elmas ÖF, Freeman EE, McMahon DE, et al. The spectrum of COVID-19–associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol. 2020;83:1118–1129. doi: 10.1016/j.jaad.2020.06.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wollina U, Karadağ AS, Rowland-Payne C, Chiriac A, Lotti T. Cutaneous signs in COVID-19 patients: a review. Dermatol Ther. 2020;33:e13549. doi: 10.1111/dth.13549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao Q, Fang X, Pang Z, Zhang B, Liu H, Zhang F. COVID-19 and cutaneous manifestations: a systematic review. J Eur Acad Dermatol Venereol. 2020;34:2505–2510. doi: 10.1111/jdv.16778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manalo IF, Smith MK, Cheeley J, Jacobs R. A dermatologic manifestation of COVID-19: transient livedo reticularis. J Am Acad Dermatol. 2020;83:700. doi: 10.1016/j.jaad.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Droesch C, Do MH, DeSancho M, Lee E-J, Magro C, Harp J. Livedoid and purpuric skin eruptions associated with coagulopathy in severe COVID-19. JAMA Dermatol. 2020;156:1–3. doi: 10.1001/jamadermatol.2020.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rotman JA, Dean KE, Magro C, Nuovo G, Bartolotta RJ. Concomitant calciphylaxis and COVID-19 associated thrombotic retiform purpura. Skeletal Radiol. 2020;49:1879–1884. doi: 10.1007/s00256-020-03579-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatol Sci. 2020;98:75–81. doi: 10.1016/j.jdermsci.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henry D, Ackerman M, Sancelme E, Finon A, Esteve E. Urticarial eruption in COVID-19 infection. J Eur Acad Dermatol Venereol. 2020;34:e244–e245. doi: 10.1111/jdv.16472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang K, Wang Y, Zhang H, Zheng Q, Fang R, Sun Q. Cutaneous manifestations of the coronavirus disease 2019 (COVID-19): a brief review. Dermatol Ther. 2020;33:e13528. doi: 10.1111/dth.13528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:e212–e213. doi: 10.1111/jdv.16387. [DOI] [PubMed] [Google Scholar]
- 33.Marzano AV, Cassano N, Genovese G, Mostrasio C, Vena GA. Cutaneous manifestations in patients with COVID-19: a preliminary review of an emerging issue. Br J Dermatol. 2020;183:431–442. doi: 10.1111/bjd.19264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71–77. doi: 10.1111/bjd.19163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanchez A, Sanchez A, Sohier P, et al. Digitate papulosquamous eruption associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:819–820. doi: 10.1001/jamadermatol.2020.1704. [DOI] [PubMed] [Google Scholar]
- 36.Marzano AV, Genovese G, Fabbrocini G, et al. Varicella-like exanthem as a specific COVID-19–associated skin manifestation: Multicenter case series of 22 patients. J Am Acad Dermatol. 2020;83:280–285. doi: 10.1016/j.jaad.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janah H, Zinebi A, Elbenaye J. Atypical erythema multiforme palmar plaques lesions due to Sars-Cov-2. J Eur Acad Dermatol Venereol. 2020;34:e373–e375. doi: 10.1111/jdv.16623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jimenez-Cauhe J, Ortega-Quijano D, Carretero-Barrio I, et al. Erythema multiforme-like eruption in patients with COVID-19 infection: clinical and histological findings. Clin Exp Dermatol. 2020;45:892–895. doi: 10.1111/ced.14281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Torrelo A, Andina D, Santonja C, et al. Erythema multiforme-like lesions in children and COVID-19. Pediatr Dermatol. 2020;37:442–446. doi: 10.1111/pde.14246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robustelli Test E, Vezzoli P, Carugno A, et al. Acute generalized exanthematous pustulosis with erythema multiforme-like lesions in a COVID-19 woman. J Eur Acad Dermatol Venereol. 2020;34:e457–e459. doi: 10.1111/jdv.16613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Novak N, Peng W, Naegeli MC, et al. SARS-CoV-2, COVID-19, skin and immunology—what do we know so far? Allergy. 2021;76:698–713. doi: 10.1111/all.14498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verheyden M, Verheyden M, Grosber M, Velkeniers B. Relapsing symmetric livedo reticularis in a patient with COVID-19 infection. J Eur Acad Dermatol Venereol. 2020;34:e684–e686. doi: 10.1111/jdv.16773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Magro CM, Mulvey JJ, Laurence J, et al. The differing pathophysiologies that underlie COVID-19-associated perniosis and thrombotic retiform purpura: a case series. Br J Dermatol. 2021;184:141–150. doi: 10.1111/bjd.19415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sohier P, Matar S, Meritet JF, Laurent-Roussel S, Dupin N, Aractingi S. Histopathologic features of chilblain-like lesions developing in the setting of the coronavirus disease 2019 (COVID-19 pandemic) Arch Pathol Lab Med. 2021;145:137–144. doi: 10.5858/arpa.2020-0613-SA. [DOI] [PubMed] [Google Scholar]
- 45.Kolivras A, Kolivras A, Dehavay F, et al. Coronavirus (COVID-19) infection-induced chilblains: a case report with histopathologic findings. JAAD Case Rep. 2020;6:489–492. doi: 10.1016/j.jdcr.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.López-Robles J, de la Hera I, Pardo-Sánchez J, Ruiz-Martínez J, Cutillas-Marco E. Chilblain-like lesions: a case series of 41 patients during the COVID-19 pandemic. Clin Exp Dermatol. 2020;45:891–892. doi: 10.1111/ced.14275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kanitakis J, Lesort C, Danset M, Jullien D. Chilblain-like acral lesions during the COVID-19 pandemic (“COVID toes”): histologic, immunofluorescence, and immunohistochemical study of 17 cases. J Am Acad Dermatol. 2020;83:870–875. doi: 10.1016/j.jaad.2020.05.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andina D, Noguera-Morel L, Bascuas-Arribas M, et al. Chilblains in children in the setting of COVID-19 pandemic. Pediatr Dermatol. 2020;37:406–411. doi: 10.1111/pde.14215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Recalcati S, Barbagallo T, Frasin LA, et al. Acral cutaneous lesions in the time of COVID-19. J Eur Acad Dermatol Venereol. 2020;34:e346–e347. doi: 10.1111/jdv.16533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Piccolo V, Neri I, Filippeschi C, et al. Chilblain-like lesions during COVID-19 epidemic: a preliminary study on 63 patients. J Eur Acad Dermatol Venereol. 2020;34:e291–e293. doi: 10.1111/jdv.16526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fernandez-Nieto D, Jimenez-Cauhe J, Suarez-Valle A, et al. Characterization of acute acro-ischemic lesions in non-hospitalized patients: a case series of 132 patients during the COVID-19 outbreak. J Am Acad Dermatol. 2020 doi: 10.1016/j.jaad.2020.05.120. PMID: 32497704; PMCID: PMC7832803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Conway EM, Pryzdial ELG. Is the COVID-19 thrombotic catastrophe complement-connected? J Thromb Haemost. 2020;18:2812–2822. doi: 10.1111/jth.15050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Magro CM, Mulvey JJ, Laurence J, et al. Docked severe acute respiratory syndrome coronavirus 2 proteins within the cutaneous and subcutaneous microvasculature and their role in the pathogenesis of severe coronavirus disease 2019. Hum Pathol. 2020;106:106–116. doi: 10.1016/j.humpath.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Magro CM, Momtahen S, Mulvey JJ, Yassin AH, Kaplan RB, Laurence JC. Role of the skin biopsy in the diagnosis of atypical hemolytic uremic syndrome. Am J Dermatopathol. 2015;37:349–356. doi: 10.1097/DAD.0000000000000234. quiz 357-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iba T, Levy JH, Levi M, Connors JM, Thachil J. Coagulopathy of coronavirus disease 2019. Crit Care Med. 2020;48:1358–1364. doi: 10.1097/CCM.0000000000004458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Katneni UK, Alexaki A, Hunt RC, et al. Coagulopathy and thrombosis as a result of severe COVID-19 infection: a microvascular focus. Thromb Haemost. 2020;120:1668–1679. doi: 10.1055/s-0040-1715841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Massey PR, Jones KM. Going viral: a brief history of chilblain-like skin lesions (“COVID toes”) amidst the COVID-19 pandemic. Semin Oncol. 2020;47:330–334. doi: 10.1053/j.seminoncol.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taşkın B, Vural S, Altuğ E, Demirkesen C, et al. Coronavirus 19 presenting with atypical Sweet's syndrome. J Eur Acad Dermatol Venereol. 2020;34:e534–e535. doi: 10.1111/jdv.16662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Merhy R, Sarkis AS, Stephan F. Pityriasis rosea as a leading manifestation of COVID-19 infection. J Eur Acad Dermatol Venereol. 2021;35:e246–e247. doi: 10.1111/jdv.17052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Magro CM, Crowson AN. The cutaneous neutrophilic vascular injury syndromes: a review. Semin Diagn Pathol. 2001;18:47–58. [PubMed] [Google Scholar]
- 61.Sams Acharya D, Liu G, Gack MU. Dysregulation of type I interferon responses inCOVID-19. Nat Rev Immunol. 2020;20:397–398. doi: 10.1038/s41577-020-0346-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Java A, Apicelli AJ, Liszewski MK, et al. The complement system in COVID-19: friend and foe? JCI Insight. 2020;5 doi: 10.1172/jci.insight.140711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu J, Yuan X, Chen H, Chaturvedi S, Braunstein EM, Brodsky RA. Direct activation of the alternative complement pathway by SARS-CoV-2 spike proteins is blocked by factor D inhibition. Blood. 2020;136:2080–2089. doi: 10.1182/blood.2020008248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fumagalli S, De Simoni MG. Lectin complement pathway and its bloody interactions in brain ischemia. Stroke. 2016;47:3067–3073. doi: 10.1161/STROKEAHA.116.012407. [DOI] [PubMed] [Google Scholar]
- 65.Larsen JB, Hvas CL, Hvas AM. The lectin pathway in thrombotic conditions—a systematic review. Thromb Haemost. 2018;118:1141–1166. doi: 10.1055/s-0038-1654714. [DOI] [PubMed] [Google Scholar]
- 66.Zhou Y, Lu K, Pfefferle S, et al. A single asparagine-linked glycosylation site of the severe acute respiratory syndrome coronavirus spike glycoprotein facilitates inhibition by mannose-binding lectin through multiple mechanisms. J Virol. 2010;84:8753–8764. doi: 10.1128/JVI.00554-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Copaescu A, Smibert O, Gibson A, Phillips EJ, Trubiano JA. The role of IL-6 and other mediators in the cytokine storm associated with SARS-CoV-2 infection. J Allergy Clin Immunol. 2020;146:518–534. doi: 10.1016/j.jaci.2020.07.001. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jani PK, Kajdácsi E, Megyeri M, et al. MASP-1 induces a unique cytokine pattern in endothelial cells: a novel link between complement system and neutrophil granulocytes. PLoS One. 2014;9:e87104. doi: 10.1371/journal.pone.0087104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Acalovschi D, Wiest T, Hartmann M, et al. Multiple levels of regulation of the interleukin-6 system in stroke. Stroke. 2003;34:1864–1869. doi: 10.1161/01.STR.0000079815.38626.44. [DOI] [PubMed] [Google Scholar]
- 70.Beamer NB, Coull BM, Clark WM, Hazel JS, Silberger JR. Interleukin-6 and interleukin-1 receptor antagonist in acute stroke. Ann Neurol. 1995;37:800–805. doi: 10.1002/ana.410370614. [DOI] [PubMed] [Google Scholar]
- 71.Li Y, Schneider AM, Mehta A, et al. SARS-CoV-2 viremia is associated with distinct proteomic pathways and predicts COVID-19 outcomes. medRxiv. 2021:1.02.24.21252357. [DOI] [PMC free article] [PubMed]
- 72.Bellosta R, Luzzani L, Natalini G, et al. Acute limb ischemia in patients with COVID-19 pneumonia. J Vasc Surg. 2020;72:1864–1872. doi: 10.1016/j.jvs.2020.04.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Narvaneni S, Fasulo SM, Kumar V, et al. COVID-19 induced bilateral lower limb ischemia and visceral infarcts. Cureus. 2021;13:e12784. doi: 10.7759/cureus.12784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Showers CR, Nuovo GJ, Lakhanpal A, et al. A COVID-19 patient with complement-mediated coagulopathy and severe thrombosis. Pathobiology. 2021;88:28–36. doi: 10.1159/000512503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vasil KE, Magro CM. Cutaneous vascular deposition of C5b-9 and its role as a diagnostic adjunct in the setting of diabetes mellitus and porphyria cutanea tarda. J Am Acad Dermatol. 2007;56:96–104. doi: 10.1016/j.jaad.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 76.Magro CM, Simman R, Jackson S. Calciphylaxis: a review. J Am Col Certif Wound Spec. 2011;2:66–72. doi: 10.1016/j.jcws.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Raimer SS, Archer ME, Jorizzo JL. Metastatic calcinosis cutis. Cutis. 1983;32:463–465. 483. [PubMed] [Google Scholar]
- 78.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kuttiatt VS, Abraham PR, Menon RP, Vaidya PC, Rahi M. Coronavirus disease 2019 in children: clinical & epidemiological implications. Indian J Med Res. 2020;152:21–40. doi: 10.4103/ijmr.IJMR_977_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Peschke K, Friebe F, Zimmermann N, et al. Deregulated type I IFN response in TREX1-associated familial chilblain lupus. J Invest Dermatol. 2014;134:1456–1459. doi: 10.1038/jid.2013.496. [DOI] [PubMed] [Google Scholar]
- 81.De Masson A, Bouaziz JD, Sulimovic L, et al. Chilblains is a common cutaneous finding during the COVID-19 pandemic: a retrospective nationwide study from France. J Am Acad Dermatol. 2020;83:667–670. doi: 10.1016/j.jaad.2020.04.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fiehn C. Familial chilblain lupus—what can we learn from type I interferonopathies? Curr Rheumatol Rep. 2017;19:61. doi: 10.1007/s11926-017-0689-x. [DOI] [PubMed] [Google Scholar]
- 83.Volpi S, Picco P, Caorsi R, Candotti F, Gattorno NM. Type I interferonopathies in pediatric rheumatology. Pediatr Rheumatol Online J. 2016;14:35. doi: 10.1186/s12969-016-0094-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Crow YJ, Chase DS, Schmidt JL, et al. Characterization of human disease phenotypes associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR, and IFIH1. Am J Med Genet A. 2015;167A:296–312. doi: 10.1002/ajmg.a.36887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ko CJ, Harigopal M, Damsky W, et al. Perniosis during the COVID-19 pandemic: Negative anti-SARS-CoV-2 immunohistochemistry in six patients and comparison to perniosis before the emergence of SARS-CoV-2. J Cutan Pathol. 2020;47:997–1002. doi: 10.1111/cup.13830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ko CJ, Harigopal M, Gehlhausen JR, Bosenberg M, McNiff JM, Damsky W. Discordant anti-SARS-CoV-2 spike protein and RNA staining in cutaneous perniotic lesions suggests endothelial deposition of cleaved spike protein. J Cutan Pathol. 2021;48:47–52. doi: 10.1111/cup.13866. [DOI] [PubMed] [Google Scholar]
- 87.Colmenero I, Santonja C, Alonso-Riaño M, et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol. 2020;183:729–737. doi: 10.1111/bjd.19327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brealey JK, Miller SE. SARS-CoV-2 has not been detected directly by electron microscopy in the endothelium of chilblain lesions. Br J Dermatol. 2021;184:186. doi: 10.1111/bjd.19572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Crowson AN, Magro CM. Idiopathic perniosis and its mimics: a clinical and histological study of 38 cases. Hum Pathol. 1997;28:478–484. doi: 10.1016/s0046-8177(97)90038-1. [DOI] [PubMed] [Google Scholar]
- 90.Magro CM, De Moraes E, Burns F. Sweet's syndrome in the setting of CD34-positive acute myelogenous leukemia treated with granulocyte colony stimulating factor: evidence for a clonal neutrophilic dermatosis. J Cutan Pathol. 2001;28:90–96. doi: 10.1034/j.1600-0560.2001.280205.x. [DOI] [PubMed] [Google Scholar]
- 91.Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatol Sci. 2020;98:75–81. doi: 10.1016/j.jdermsci.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Català A, Galván-Casas C, Carretero-Hernández G, et al. Maculopapular eruptions associated to COVID-19: a subanalysis of the COVID-Piel study. Dermatol Ther. 2020;33:e14170. doi: 10.1111/dth.14170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gouveia PADC, Cipriano IC, de Melo MAZ, et al. Exuberant bullous vasculitis associated with SARS-CoV-2 infection. IDCases. 2021;23:e01047. doi: 10.1016/j.idcr.2021.e01047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Iraji F, Galehdari H, Siadat AH, Jazi SB. Cutaneous leukocytoclastic vasculitis secondary to COVID-19 infection: a case report. Clin Case Rep. 2020;9:830–834. doi: 10.1002/ccr3.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Camprodon Gómez M, González-Cruz C, Ferrer B, Barberá MJ. Leucocytoclastic vasculitis in a patient with COVID-19 with positive SARS-CoV-2 PCR in skin biopsy. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2020-238039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nasiri S, Dadkhahfar S, Abasifar H, Mortazavi N, Gheisari M. Urticarial vasculitis in a COVID-19 recovered patient. Int J Dermatol. 2020;59:1285–1286. doi: 10.1111/ijd.15112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. 2021;85:46–55. doi: 10.1016/j.jaad.2021.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Johnston MS, Galan A, Watsky KL, Little AJ. Delayed localized hypersensitivity reactions to the Moderna COVID-19 vaccine: a case series. JAMA Dermatol. 2021;157:716–720. doi: 10.1001/jamadermatol.2021.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kha C, Itkin A. New-onset chilblains in close temporal association to mRNA-1273 vaccination. JAAD Case Rep. 2021;12:12–14. doi: 10.1016/j.jdcr.2021.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]