Abstract
Background
Atypical hemolytic uremic syndrome is a rare disease caused by complement dysregulation that can lead to progressive kidney damage or death if untreated. Owing to its rarity, the impact of atypical hemolytic uremic syndrome and available therapies (eculizumab and ravulizumab) on patients’ health-related quality of life is difficult to describe, but such data are required for an economic evaluation.
Objective
The objective of this study was to estimate utility values for atypical hemolytic uremic syndrome-related attributes in five countries for an economic evaluation.
Methods
Using discrete choice experiment surveys, key atypical hemolytic uremic syndrome-related attributes (life expectancy, administration frequency, risk of meningitis, need for hospitalization, and risk of kidney impairment) were evaluated in adult general population samples from Australia, Canada, the Netherlands, Sweden, and the UK. Survey choice sets were constructed using a published orthogonal array. A mixed-effects logit model estimated preference strength for each attribute. Utilities were estimated using marginal substitution rates between overall survival and other attributes, weighted against average life expectancy.
Results
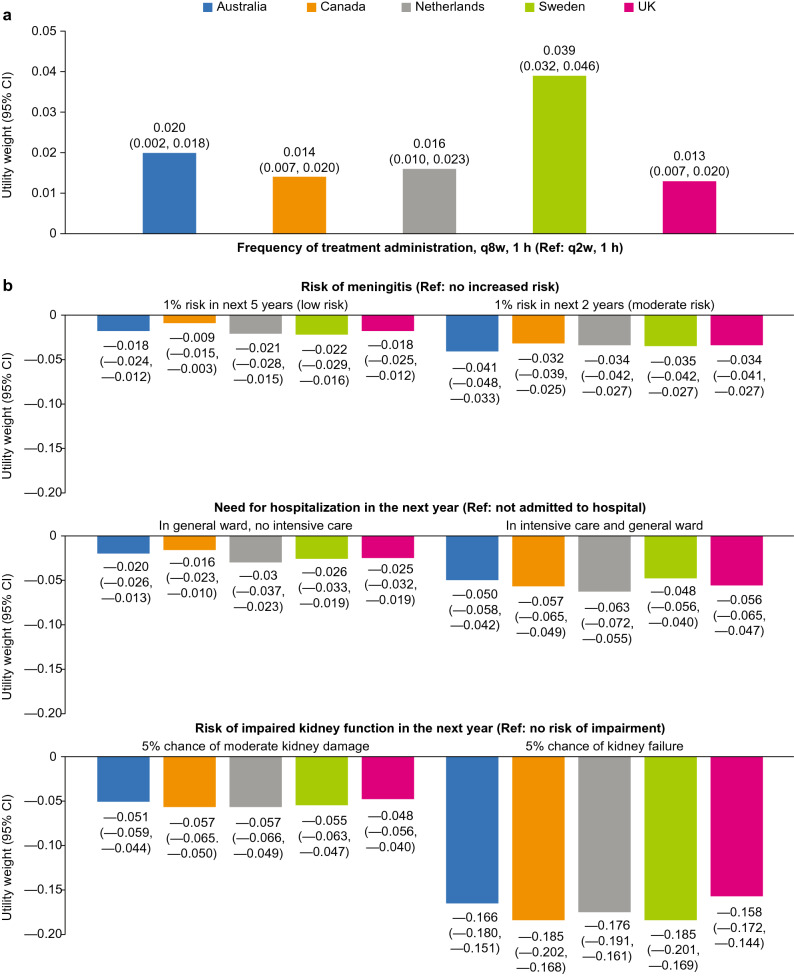
Across all countries (N = 2382), utility weights revealed a consistent pattern: participants were averse to the risk of kidney impairment (disutility/utility weight range: −0.185 to −0.158), risk of meningitis (−0.041 to −0.032), and the need for hospitalization (−0.063 to −0.048), but preferred 8-weekly vs 2-weekly infusions over 1 h (0.013–0.039).
Conclusions
Although all attributes played a role in determining treatment preferences, the largest drivers were life expectancy and risk of kidney impairment. Participants favored 8-weekly dosing (corresponding to ravulizumab administration frequency) vs 2-weekly dosing. The discrete choice experiment was designed such that estimated (dis)utility weights can be used in future cost-effectiveness models in atypical hemolytic uremic syndrome.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40273-021-01059-w.
Key Points for Decision Makers
| The measurement of utilities for cost-effectiveness modeling of orphan treatments is very challenging. |
| This study employs the discrete choice experiment approach to estimate health utility values for atypical hemolytic uremic syndrome-related attributes in different countries for economic modeling. |
| Among the atypical hemolytic uremic syndrome-related attributes explored in this study, the largest drivers of disutility were the risk of kidney impairment and the risk of meningitis; in addition, a less frequent dosing schedule was preferred. |
Introduction
Atypical hemolytic uremic syndrome (aHUS) is a rare disease of uncontrolled complement activation that causes progressive organ damage leading to severe morbidity or premature death [1]. Globally, depending on the region and age group, prevalence figures range from approximately two to nine per million population, while incidence ranges between 0.23 and 1.9 per million population annually [2].
Prior to the availability of terminal complement inhibitor therapies, outcomes in patients with aHUS were poor despite the use of plasma exchange/infusion, with patients experiencing impaired kidney function and progression to end-stage renal disease, resulting in the need for dialysis or kidney transplant [3, 4]. Approved in 2011 [5, 6], eculizumab, a humanized monoclonal antibody that blocks terminal complement activation at C5, has been shown to be effective in treating aHUS [7–11] and has become the standard of care for aHUS disease management, although the optimal duration of therapy is a key point of discussion in the literature [12, 13]. Eculizumab requires a standard treatment regimen of intravenous infusions every 2 weeks (q2w) in patients with a body weight of ≥ 10 kg [5, 6]. Ravulizumab, approved in the USA, Europe, and Japan for the treatment of aHUS [14–16], was engineered from eculizumab to leverage the clinical benefits and safety profile while decreasing drug clearance and reducing infusion frequency to every 8 weeks (q8w) [17–19]. The recently approved (USA and Europe) 100 mg/mL formulation also serves to reduce infusion time vs the 10-mg/mL formulation [14, 15]. Given its less frequent administration, ravulizumab has the potential to decrease the burden of treatment and the cost of managing aHUS, as well as improve the overall quality of life for patients and caregivers because of the reduced amount of time spent in treatment [20, 21].
Health technology assessment bodies, such as Australia’s Pharmaceutical Benefits Advisory Committee, the Canadian Agency for Drugs and Technologies in Health, and the National Institute for Health and Care Excellence in the UK, evaluate the benefits of a new treatment against the existing treatment in terms of the incremental cost per quality-adjusted life-year. Quality-adjusted life-year estimation requires data on health-related quality of life, expressed as utility data [22]. Health technology assessment bodies typically state that they prefer utility data to be derived from patients who describe their health (in terms of generic dimensions of health), which is separately rated by the general public using preference data. The collection of utility values in rare diseases can be challenging because of limited patient numbers. In addition, a clinical trial may not capture the health-related quality of life (HRQoL) data on all important health states for the analysis.
The objectives of this study were to estimate utility values for aHUS-related attributes for use in cost-effectiveness analyses. In the study, descriptions of health were developed by the study team from different sources and then weighted by the public using a stated-preference discrete choice experiment (DCE) survey [23] to assess the value that general population samples from Australia, Canada, the Netherlands, Sweden, and the UK place on attributes of treatments and outcomes in aHUS. This approach is designed to provide a general public-derived valuation for aHUS-related attributes using choice-based methods [22].
Methods
Survey Design
Surveys started with screening questions (including age, sex, and geographic region) to determine the eligibility of potential participants and ensure representativeness of the sample. The next section contained sociodemographic questions (including education, employment status, ethnicity, and the presence of a long-term condition or rare disease) tailored to each study country, Australia, Canada, the Netherlands, Sweden, and the UK. These are countries that typically require cost-effectiveness analysis in the health technology assessment.
The selection of attributes was driven by the need to capture utility weights for specific aHUS-related events, outcomes, and treatment characteristics, which could then be incorporated into a cost-effectiveness model. The description of the attributes and levels for the DCE was based on clinical opinion and findings from a targeted literature search in aHUS and associated treatments. The attribute development process aimed to avoid conceptual overlap or association between attributes because this may lead to implausible hypothetical scenarios. The following five attributes were selected: (1) life expectancy; (2) frequency of treatment administration; (3) risk of meningitis as a potential side effect of treatment; (4) need for hospitalization as a result of the disease; and (5) risk of impaired kidney function. Risk of meningitis was included as it is a serious potential side effect of treatment with eculizumab or ravulizumab [11, 14, 24]. Individuals with aHUS are at risk of impaired kidney function, including kidney failure that requires the need for dialysis [3, 4]; therefore, this risk was included as an attribute. Three levels were selected for all attributes except life expectancy, for which six levels were selected to increase the sensitivity of this attribute by minimizing the distance between levels. Attributes and levels are summarized in Table 1. The terminal complement inhibitors approved for the treatment of aHUS, eculizumab and ravulizumab, are administered via intravenous infusion q2w and q8w, respectively. Administration q8w for ~3 h corresponds to a ravulizumab dose of 10 mg/mL (approved in the USA, Europe, and Japan) [14–16], and q8w for ~1 h corresponds to a dose of 100 mg/mL (approved in the USA and Europe) [14, 15]; thus frequency of administration was included as a treatment-related attribute.
Table 1.
Summary of attributes and levels included in the survey
| Attribute | Description | Level 0 | Level 1 | Level 2 | Level 3 | Level 4 | Level 5 |
|---|---|---|---|---|---|---|---|
| Life expectancy | Relative effectiveness of treatment in terms of reduced overall length of life | Life expectancy reduced by 10 years | Life expectancy reduced by 8 years | Life expectancy reduced by 6 years | Life expectancy reduced by 4 years | Life expectancy reduced by 2 years | Life expectancy not reduced |
| Frequency of treatment administration | Treatment schedules associated with eculizumab (10 mg/mL dose [Level 0]) and ravulizumab (10 mg/mL [Level 1] and 100 mg/mL [Level 2] doses) |
Every 2 weeks Delivered at home Treatment takes ~1 h |
Every 8 weeks Delivered at home Treatment takes ~3 h |
Every 8 weeks Delivered at home Treatment takes ~1 h |
– | – | – |
| Treatment risks | Differing levels of risk of meningitis, a potential side effect of treatment for aHUS | Over the next 2 years, 1 extra person in 100 (1%) will develop meningitis (moderate risk) | Over the next 5 years, 1 extra person in 100 (1%) will develop meningitis (low risk) | No increased risk of meningitis | – | – | – |
| Need for hospitalization | Levels of treatment effectiveness whereby, owing to the seriousness of the condition, participants could expect to be admitted to hospital to receive treatment |
You are admitted to hospital once in the next year You are in intensive care for 8 days, followed by 5 days in a general ward |
You are admitted to hospital once in the next year You are not in intensive care You have 7 days in a general ward |
You are not admitted to hospital in the next year | – | – | – |
| Risk of impaired kidney function | Risk of differing levels of impaired kidney function that could occur because of aHUS, even under treatment |
You have a 5% chance of kidney failure in the next year You would experience tiredness, headaches, nausea, and vomiting You would need dialysis at the hospital 3 times a week |
You have a 5% chance of moderate kidney damage in the next year You would experience tiredness, back pain, and poor sleep You would not need dialysis |
No risk of impaired kidney function in the next year You would not need dialysis |
– | – | – |
aHUS atypical hemolytic uremic syndrome
The DCE section of the survey started with an introduction to aHUS without naming the condition; the disease was anonymized to ‘XMX’ to prevent participants from researching the disease. Each attribute was then described; the description for treatment risks informed participants that patients with disease XMX would be vaccinated for meningitis before starting treatment. Attributes and defined levels were combined into sets of 36 paired-choice questions using a published orthogonal fractional factorial array [25] (example shown in the Electronic Supplementary Material [ESM]). Attributes in each paired choice were the same, but attribute levels were systematically varied from ‘choice a’ to’ choice b’ using the Street and Burgess’ shifting method (e.g., level 1 becomes level 2, level 2 becomes level 3, and level 3 becomes level 1) [26]. Choice questions were the same for each study country except Sweden, where the survey stated that the treatment is administered in hospital; all other countries’ surveys stated that it is administered at home. Thirty-six was the minimum required number of choices that retained the orthogonal properties of a full factorial design. Owing to the large number of choice questions, this part of the survey was divided into two blocks (A and B) and participants were randomized to one block. To check whether participants were fully engaged/giving logical answers, one choice question was reversed and repeated to check consistency in the response, and another ‘logic check’ question included a choice that was better in all aspects than the alternative. Surveys were administered in the language native to each study country.
Participants
The survey was administered to a general population sample (aged ≥ 18 years) resident in either Australia, Canada, the Netherlands, Sweden, or the UK. Participants were identified by a specialist recruitment agency from online recruitment panels in each country comprised of individuals from the general population with an interest in participating in research studies. Potential study participants were contacted by e-mail with a link to the survey and were screened for eligibility. Recruitment quotas were set for geographic region, age, and sex, to achieve a representative sample within two percentage points of the census data in each study country [27–31]. No formal sample size estimation was conducted; sample sizes reported in previous valuation research was used as a guide [32]. Eligible participants were given additional information about the study and provided online informed consent. Only those who consented were able to access and complete the survey.
Initially, a pilot survey (UK only; N = 418, results not shown) was conducted to assess: (1) whether attribute coefficients were in the expected direction (i.e., that participants would prefer to live longer, have treatment less frequently, have a lower risk of meningitis, not be admitted to hospital, and have a lower risk of impaired kidney function); (2) the appropriateness of the threshold for ‘speeding’ (based on participants taking ~20 s to complete the first choice question and 5–15 s for subsequent choice questions); and (3) whether participants were reading the questions carefully. Based on pilot study findings, it was decided that only participants who completed the main survey in > 2 min 45 s (estimated to be a reasonable minimal completion time and an optimal cut-off point to exclude respondents making multiple illogical responses) would be eligible for inclusion in the main study analyses. Additionally, as a quality-control measure, it was decided that participants would be excluded if their responses suggested that they did not understand the question or had not interpreted it incorrectly.
Statistical Analyses
Sociodemographic data were analyzed using descriptive statistics. Discrete choice data were analyzed using a mixed-effects logit regression model [33] to estimate preference strength for each attribute while accounting for preference heterogeneity among respondents. The model was estimated using the maximum simulated likelihood approach. The strength of preference associated with each attribute level was measured with respect to a reference level. For categorical variables, the least severe level was selected as the reference; for continuous variables, the model presents the odds of treatment selection for a 1-year reduction in life expectancy. An alternative-specific constant was added to the model to account for any bias in selecting the left or right option. This refers to a bias in which participants are more likely to choose either Treatment A (left column in the paired choice) or Treatment B (right column in the paired choice), after having accounted for the study attributes.
Marginal rates of substitution (MRS) indicate the extent to which participants were willing to trade years of life to avoid certain levels of other attributes. Marginal rates of substitution were estimated by taking a ratio of the coefficients for two attributes: the life expectancy coefficient for 1 year (obtained by entering life expectancy as a continuous variable into the models) and another treatment attribute. Utilities were calculated by dividing the MRS estimates for each attribute level by the remaining life expectancy for each country (calculated based on the proportion of men and women, and the average life expectancy of each, for each country) [34–38]; average remaining life expectancy for the UK, Australia, Canada, the Netherlands, and Sweden was calculated as 33.8, 39.3, 36.7, 38.2, and 36.3 years, respectively. The 95% confidence interval for the MRS and utility values were calculated in the same manner, using the 95% confidence intervals obtained from the mixed-effects models.
Because treatment in Sweden is administered in a hospital rather than at home, and because Quebec has a different health technology assessment body from the rest of Canada, interaction analyses were conducted to explore whether distance from a hospital or participant geographic location influenced preferences for the frequency of treatment administration for participants resident in Sweden or Canada, respectively (ESM). All data were analyzed in Stata Version 16.0.
Sensitivity Analyses
Sensitivity analyses were conducted to explore whether results from the main analyses differed from patient subgroups in which participants answering one logic question wrong and giving an illogical reason for this response were excluded, and in which participants from all countries who were older than the UK average life expectancy minus 10 years (i.e., age > 69 years for male individuals; age > 73 years for female individuals) were excluded. Older participants are likely to have fewer years of life remaining; thus, it was hypothesized that they may have different treatment preferences.
Results
Study Population
In total, a sample of 2382 members of the general population was analyzed in the study (Australia, n = 477; Canada, n = 471; the Netherlands, n = 481; Sweden, n = 476; UK, n = 477). Sample disposition can be seen in Fig. 1 and a summary of participant characteristics by country is presented in Table 2.
Fig. 1.
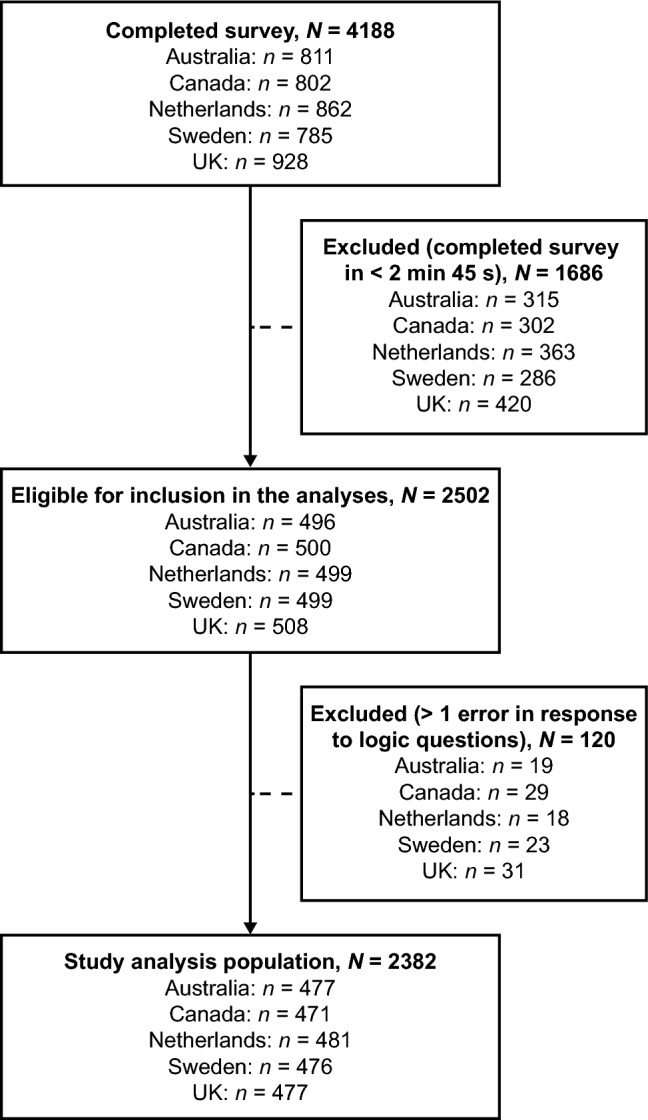
Sample disposition
Table 2.
Sample characteristics
| Characteristic | Australia | Canada | The Netherlands | Sweden | UK |
|---|---|---|---|---|---|
| n = 477 | n = 471 | n = 481 | n = 476 | n = 477 | |
| Age, mean (SD), years | 44.9 (16.2) | 47.4 (15.1) | 46.3 (16.9) | 50.0 (16.8) | 49.2 (15.6) |
| Age range, n (%), years | |||||
| 18–34 | 167 (35.0) | 118 (25.1) | 146 (30.4) | 112 (23.5) | 128 (26.8) |
| 35–54 | 181 (37.9) | 202 (42.9) | 172 (35.8) | 176 (37.0) | 175 (36.7) |
| ≥ 55 | 129 (27.0) | 151 (32.1) | 163 (33.9) | 188 (39.5) | 174 (36.5) |
| Sex, n (%) | |||||
| Male | 235 (49.3) | 239 (50.7) | 220 (45.7) | 224 (47.1) | 221 (46.3) |
| Female | 241 (50.5) | 232 (49.3) | 261 (54.3) | 249 (52.3) | 256 (53.7) |
| Other | 1 (0.2) | 0 (0) | 0 (0) | 3 (0.6) | 0 (0) |
| Educational level, n (%) | |||||
| No formal qualifications | 5 (1.1) | 7 (1.5) | 0 (0) | 5 (1.1) | 14 (2.9) |
| Left school at age 15 or 16 years | 55 (11.5) | 81 (17.2) | 6 (1.3) | 42 (8.8) | 119 (24.9)a |
| Left school at age 17 or 18 years | 123 (25.8) | 38 (8.1) | 191 (39.7) | 176 (37.0) | 148 (31.0)b |
| University degree or higher | 227 (47.6) | 335 (71.1) | 255 (53.0) | 204 (42.9)c | 187 (39.2) |
| Other | 66 (13.8) | 7 (1.5) | 29 (6.0) | 44 (9.2) | 9 (1.9) |
| Prefer not to answer | 1 (0.2) | 3 (0.6) | 0 (0) | 5 (1.1) | 0 (0) |
| Employment status, n (%) | |||||
| Full-time employment | 169 (35.4) | 226 (48.0) | 148 (30.8) | 151 (31.7) | 192 (40.3) |
| Part-time employment | 89 (18.7) | 41 (8.7) | 99 (20.6) | 44 (9.2) | 56 (11.7) |
| Self-employed | 29 (6.1) | 37 (7.9) | 26 (5.4) | 25 (5.3) | 26 (5.5) |
| Looking after family/home/caregiver | 27 (5.7) | 5 (1.1) | 31 (6.4) | 4 (0.8) | 35 (7.3) |
| Retired | 81 (17.0) | 99 (21.0) | 67 (13.9) | 129 (27.1) | 113 (23.7) |
| Seeking work, unemployed | 32 (6.7) | 24 (5.1) | 22 (4.6) | 44 (9.2) | 28 (5.9) |
| Not working, health problems | 19 (4.0) | 18 (3.8) | 42 (8.7) | 29 (6.1) | 23 (4.8) |
| In education/training | 25 (5.2) | 15 (3.2) | 36 (7.5) | 36 (7.6) | 1 (0.2) |
| Other | 4 (0.8) | 3 (0.6) | 8 (1.7) | 13 (2.7) | 2 (0.4) |
| Prefer not to answer | 2 (0.4) | 3 (0.6) | 2 (0.2) | 1 (0.2) | 0 (0) |
| Health status, n (%) | |||||
| Long-term condition requiring medication | 236 (49.5) | 202 (42.9) | 214 (44.5) | 209 (43.9) | 239 (50.1) |
| Diagnosis of rare disease | 40 (8.4) | 28 (5.9) | 39 (8.1) | 48 (10.1) | 28 (5.9) |
Percentages may not sum to 100% because of rounding
BTEC Business and Technology Education Council, GCSE General Certificate of Secondary Education, ONC Ordinary National Certificate, SD standard deviation
aO level/GCSE or equivalent (age 16 years)
bIncludes the categories ‘higher education below degree level’, ‘A level (age 18 years)’, and ‘further education certificate (ONC/BTEC)’
cCollege degree or higher
Overall, participants had a mean age of 47.6 years (mean range 44.9–50.0 years), with similar proportions of men (47.8%) and women (52.0%). In total, 46.2% of participants reported having a long-term condition requiring medication, with proportions similar across the sampled countries (range 42.9–50.1%), and 7.7% reported a diagnosis of a rare disease (range 5.9–10.1%).
Most quotas for geographic region, age, and sex were met (value within two percentage points of the country census data) within samples from Canada, the Netherlands, Sweden, and the UK with the following exceptions: in Canada, the 18–34 years of age group was slightly under-represented and the 35–54 years of age group was slightly over-represented; in the Netherlands, men and those in the 35–54 years of age group were slightly under-represented, and women were slightly over-represented; and in Sweden and the UK, men and those in the 18–34 years of age group were slightly under-represented. All quotas were met for Australia.
Estimation of Preference Strength
Results of the mixed-effect logit regression model are shown in Table 3. Participants from all countries showed a preference for choices that did not reduce life expectancy; for every year of life lost, treatments had a significantly lower odds of being chosen (odds ratios 0.582–0.631; all p < 0.001). In all countries, frequency of treatment administration had the smallest impact on treatment choice; however, treatments that were administered q8w for 1 h had a significantly higher odds of being chosen than q2w treatment for 1 h (odds ratios 1.273–1.946; all p < 0.001). Australia and Sweden were the only countries in which treatments that were administered q8w for 3 h had a significantly higher odds of being chosen than q2w treatment for 1 h (odds ratio [95% confidence interval] 1.193 [1.032–1.380], p = 0.017 and 1.511 [1.310–1.744], p < 0.001, respectively). In all countries, participants placed significant weight on the avoidance of treatments that increased the risk of meningitis, the need for hospitalization within the next year, or impaired kidney function within the next year. For all countries, the constant coefficient showed a statistically significant effect, indicating the presence of a right bias, which was not (fully) accounted for by the treatment attributes (Table 3). This means that participants were more likely to choose the treatment shown on the right (‘Treatment B’) over the one on the left (‘Treatment A’) in the paired choices.
Table 3.
Results of the mixed-effects logit regression model of participant preference
| Attribute level | OR (95% CI) | ||||
|---|---|---|---|---|---|
| Australia, n = 477 | Canada, n = 471 | Netherlands, n = 481 | Sweden, n = 476 | UK, n = 477 | |
| Alternative-specific constant (Ref: treatment A [left column]) |
1.628 (1.487–1.783) p < 0.001 |
2.013 (1.812–2.236) p < 0.001 |
1.525 (1.393–1.670) p < 0.001 |
1.350 (1.237–1.472) p < 0.001 |
1.455 (1.326–1.595) p < 0.001 |
| Reduction in life expectancy, years |
0.628 (0.601–0.657) p < 0.001 |
0.587 (0.558–0.616) p < 0.001 |
0.631 (0.603–0.659) p < 0.001 |
0.624 (0.598–0.651) p < 0.001 |
0.582 (0.554–0.612) p < 0.001 |
| Frequency of treatment administration (Ref: every 2 weeks, 1 h) | |||||
| Every 8 weeks, 3 h |
1.193 (1.032–1.380) p = 0.017 |
0.966 (0.825–1.132) p = 0.668 |
1.042 (0.902–1.203) p = 0.580 |
1.511 (1.310–1.744) p < 0.001 |
1.132 (0.982–1.306) p = 0.088 |
| Every 8 weeks, 1 h |
1.450 (1.285–1.638) p < 0.001 |
1.305 (1.151–1.480) p < 0.001 |
1.336 (1.182–1.510) p < 0.001 |
1.946 (1.724–2.196) p < 0.001 |
1.273 (1.130–1.434) p < 0.001 |
| Risk of meningitis (Ref: no increased risk) | |||||
| 1% risk in next 5 years (low risk) |
0.716 (0.640–0.800) p < 0.001 |
0.835 (0.741–0.941) p = 0.003 |
0.688 (0.614–0.770) p < 0.001 |
0.682 (0.610–0.763) p < 0.001 |
0.716 (0.638–0.804) p < 0.001 |
| 1% risk in next 2 years (moderate risk) |
0.477 (0.417–0.545) p < 0.001 |
0.537 (0.468–0.615) p < 0.001 |
0.545 (0.476–0.623) p < 0.001 |
0.555 (0.491–0.626) p < 0.001 |
0.535 (0.470–0.609) p < 0.001 |
| Need for hospitalization in the next year (Ref: not admitted to hospital) | |||||
| In general ward, no intensive care |
0.696 (0.618–0.783) p < 0.001 |
0.724 (0.635–0.827) p < 0.001 |
0.587 (0.519–0.663) p < 0.001 |
0.639 (0.566–0.721) p < 0.001 |
0.627 (0.556–0.708) p < 0.001 |
| In intensive care and general ward |
0.403 (0.349–0.465) p < 0.001 |
0.330 (0.281–0.387) p < 0.001 |
0.327 (0.280–0.381) p < 0.001 |
0.441 (0.386–0.503) p < 0.001 |
0.359 (0.307–0.419) p < 0.001 |
| Risk of impaired kidney function in the next year (Ref: no risk of impaired kidney function) | |||||
| 5% chance of moderate kidney damage |
0.393 (0.343–0.450) p < 0.001 |
0.325 (0.279–0.379) p < 0.001 |
0.364 (0.314–0.422) p < 0.001 |
0.391 (0.340–0.449) p < 0.001 |
0.417 (0.362–0.480) p < 0.001 |
| 5% chance of kidney failure |
0.049 (0.037–0.064) p < 0.001 |
0.027 (0.019–0.037) p < 0.001 |
0.045 (0.034–0.059) p < 0.001 |
0.042 (0.032–0.055) p < 0.001 |
0.056 (0.043–0.072) p < 0.001 |
For categorical variables, an OR >1.0 indicates that the attribute level has a higher odds of being chosen by the individual over the Ref; an OR < 1.0 indicates that the attribute level has a lower odds of being chosen over the Ref. For life expectancy (continuous variable), an OR < 1.0 indicates that for every year of life lost, treatments had a lower odds of being chosen
Marginal rates of substitution were obtained by taking a ratio of the coefficients for two attributes, where the coefficient = ln(OR)
CI confidence interval, OR odds ratio, Ref reference level
In the interaction analyses, for Sweden, interaction terms were positive, suggesting that respondents who live further from a hospital have a stronger preference for less frequent treatment administration (ESM), while for Canada, the interaction terms were negative, suggesting that respondents who were not from Quebec had a stronger preference than those from Quebec for less frequent treatment administration (ESM).
Estimation of Utility
Marginal rates of substitution were estimated to indicate the number of years of life that an individual was willing to trade to avoid being in the state described by the attribute level. Utility increases and losses (disutility) derived from MRS estimates rescaled against remaining average life expectancy revealed a consistent pattern across the different countries. Participants had a significant preference for q8w vs q2w infusions over 1 h (utility scores: Australia 0.020; Canada 0.014; the Netherlands 0.016; Sweden 0.039; UK 0.013) (Fig. 2a) and were averse to the risk of meningitis, the need for hospitalization, and the risk of impaired kidney function (Fig. 2b). The disutility for a health state with a 5% chance of kidney failure in the next year ranged from −0.185 (Canada and Sweden) to −0.158 (UK). The disutility for a health state with a 5% chance of moderate kidney damage ranged from −0.057 to −0.048. The disutility associated with the risk of meningitis (vs no increased risk) ranged from −0.022 to −0.009 and −0.041 to −0.032 for low and moderate risk, respectively. The disutility associated with the need for hospitalization in the next year (vs no hospital admittance) ranged from −0.030 to −0.016 for the need for admission to a general ward and from −0.063 to −0.048 for the need to be admitted to intensive care followed by a general ward stay.
Fig. 2.
Estimate utilities (a) and disutilities (b) for differences in attribute levels. Utility weights were calculated by dividing the marginal rates of substitution estimates for each attribute level by the estimated remaining life expectancy per country. For example, in the UK, the marginal rates of substitution value for a 5% chance of kidney failure was 5.340 (assuming average life expectancy). The estimated remaining life expectancy of the UK sample was 33.8 years. The utility weight associated with a 5% chance of kidney failure is therefore estimated as 5.34/33.8 = −0.158 (negative figure indicates disutility). CI confidence interval, q2w every 2 weeks, q8w every 8 weeks, Ref reference level
Sensitivity Analyses
Associations were marginally stronger, but were unchanged overall compared with the main analysis, when participants who got one logic question wrong and gave an illogical reason for their response (Australia n = 9; Canada n = 11; the Netherlands n = 17; Sweden n = 13; UK n = 12; data not shown) and older participants (Australia n = 29; Canada n = 24; the Netherlands n = 39; Sweden n = 57; UK n = 47; data not shown) were excluded from the regression analyses.
Discussion
The DCE surveys presented in this study were designed to estimate utilities for use in cost-effectiveness modeling in aHUS where it is challenging to capture sufficient data with measures such as the EQ-5D. The study was designed to capture health utility data for key aHUS-related attributes (frequency of treatment administration, treatment risks, need for hospitalization, and impaired kidney function), which could be incorporated into an economic evaluation. The impact of these variables would be difficult to capture using a patient-directed prospective observational study in patients with aHUS. The DCE method of estimating utilities for cost-effectiveness analyses can be useful for healthcare decision makers in relation to rare diseases for which utility data are not available. In the current DCE, participants were presented with the prospect of a treatment for a disease, ‘XMX’, with attributes and levels tailored to those of aHUS-related events, outcomes, and treatment characteristics associated with eculizumab and ravulizumab. In aHUS, both eculizumab and ravulizumab have been shown to have a positive effect on patient outcomes through improving patient mortality and morbidity [11, 18], with ravulizumab having the advantage of a reduced infusion frequency, thus offering the potential to decrease treatment burden.
Similar patterns of results were seen across all countries. Not surprisingly, participants placed significant value on treatments that would help them live longer, avoid admittance to a hospital, were administered less frequently, had a lower risk of meningitis, and had a lower risk of impaired kidney function. Although all attributes played a role in determining treatment preferences, the risk of reduced life expectancy and the risk of impaired kidney function were the largest drivers of treatment choice within this analysis. The inclusion of the attribute that described years of life lost meant that we were able to estimate the extent to which participants were willing to trade years of life against gains in health or improvements in the health state, making the task comparable to a time trade-off exercise. There is an implicit assumption in the study that these events or attributes may affect a person’s health state and participants are being asked to make a judgment regarding the extent to which the health state is affected by their occurrence, and therefore how many years of life they may be willing to forego to avoid this deterioration in their health state. Participants were significantly less likely to choose a treatment for every year of life lost, or to choose a treatment that increased the risk of kidney failure by 5% in the next year. Renal impairment is recognized as a severe complication of aHUS and a substantial proportion of patients with aHUS progress to end-stage renal disease after the initial thrombotic microangiopathy manifestation [3, 4]. Eculizumab and ravulizumab have both been shown to improve renal function [7–10, 18].
Estimated disutilities should be interpreted as a reflection on the prospect of a year of life when a disease-related event or outcome is expected to happen (e.g., a year of life in which there was a risk of impaired kidney function). Across all countries, living with a risk of kidney failure had the most marked disutility (−0.185 to −0.158), which was comparable to that seen in a previous study of adult kidney transplant recipients [39]. One way to interpret this is that this disutility reflects the loss of HRQoL associated with a 5% risk of kidney failure. This could be attributed to this state causing symptoms that limit HRQoL and the possible impact on an individual’s ability to continue with their daily activities. Some patients with aHUS can expect relatively frequent hospital admissions and lengthy stays [40, 41]. The prospect of a year in which a patient can expect to end up in intensive care and on the general ward for a period was associated with a disutility of between −0.063 and −0.048. This specific attribute was designed to understand the prospect of a year of life during which such a serious hospital admission occurs, and thus the disutility can be applied for 12 months in any model. However, this may be considered double counting if a further disutility were to be applied for the period spent in hospital.
A lower frequency of treatment administration (i.e., q8w vs q2w) was associated with significant utility increases in the general population samples from all countries; this was most pronounced for Sweden. This is likely because of the need to travel to a hospital to receive treatment administration in Sweden, rather than being administered at home in the other countries. This is coupled with the finding from the interaction analyses for Sweden suggesting that respondents who live further from a hospital have a stronger preference for less frequent treatment administration. Across all countries, treatments administered q8w for 1 h (the dosing schedule associated with that of the ravulizumab 100 mg/mL formulation) were more likely to be chosen than those with the same duration of infusion but administered q2w, whereas Australia and Sweden were the only countries in which there was a significant preference for less frequent treatment regardless of administration time (i.e., q8w for 3 h also had a significantly higher odds of being chosen than treatment q2w for 1 h in these countries). The results from the DCE support the less frequent administration of ravulizumab as a favorable benefit.
A strength of this study is the implementation of various quality-control procedures including a minimum time limit to prevent participants from speeding through the questionnaire, as well as additional ‘logic’ questions to determine whether participants were paying attention/properly engaged. In the pilot, setting a minimum time limit considerably reduced the number of participants who made errors on the logic questions; as a result, this same limit was implemented in the main analyses. Another strength was the method of choice pairing, which retained the level balance, allowed all attribute levels to be different (in each choice), and has been shown to produce designs that are > 99% efficient [26]. This study shows strength as an alternative method for estimating utility weights outside of a clinical trial, when such data are unavailable.
One limitation of this study is the challenge associated with asking the general population to imagine how it feels to be in the states described by the different attribute levels. For example, they may not be able to fully appreciate what it means to have an infusion q2w for 1 h or q8w for 3 h—a difference that would be more relatable to individuals with diseases such as aHUS, for which treatment via an intravenous infusion may be required. Although the attribute descriptions were informed by key papers identified in the targeted literature review, a systematic review may have been more robust for identifying relevant studies. Additionally, while the attribute development process aimed to avoid conceptual overlap, it is acknowledged that renal impairment and meningococcal infection could require hospitalization and thus overlap may be perceived by some participants. It is possible that cognitive debrief interviews with members of the general population would have ensured that the questions were easily understood. Furthermore, despite large sample sizes, it is possible that this study may be under-powered to detect some associations. Future studies should consider published guidance on determining minimum sample size requirements to detect an effect in a DCE [42]. Although the findings are largely consistent across countries indicating a level of reliability to the results, repeating the survey would also add a greater level of certainty to the findings. Another limitation is that the DCE methodology means that the value placed on years of life/survival is effectively re-estimated for every new study. The estimates of disutility are very sensitive to the preference weights for survival in the DCE model. Therefore, any framing effects that influence the importance of the overall survival attribute could have a substantial impact on the estimated disutilities. In addition, the covariance between attributes was not accounted for in the calculation of the confidence intervals around the MRS and utility estimates, which may bias the precision of these estimates [43].
Conclusions
From a DCE survey designed to capture health utility data for key aHUS-related attributes, it was found that although a reduced infusion burden played a positive role in determining treatment preferences in a general adult population, the largest drivers were life expectancy and the risk of impaired kidney function, which highlights the value of effectively treating aHUS. Participants favored treatment administration q8w vs q2w, which supports the less frequent administration of ravulizumab as a favorable benefit. Utility (or disutility) weights estimated for each aHUS-related attribute level in this study can be used in future cost-utility models in aHUS. This is a potentially useful approach for estimating utility weights in rare and ultra-rare conditions in which patient recruitment and collection of sufficient data to estimate the impact of disease and its treatment on patients’ HRQoL can be very challenging.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Medical writing support was provided by Jessica Donaldson-Jones, PhD of Oxford PharmaGenesis, Oxford, UK and was funded by Alexion Pharmaceuticals, Inc. The authors thank Natalia Piglowska (previously of Acaster Lloyd Consulting Ltd) for contributing to the DCE survey development and data analysis, and Karl-Johan Myren and Ioannis Tomazos of Alexion Pharmaceuticals, Inc. for their review of the first manuscript draft.
Declarations
Funding
Funding for this study was provided by Alexion Pharmaceuticals, Inc.
Conflicts of interest/Competing interests
Peter Chen, Katerina Anokhina, and Yan Wang are employees of, and may own stocks/options in, Alexion Pharmaceuticals, Inc. Kate Williams, Daniel Aggio, and Andrew J. Lloyd are employees of Acaster Lloyd Consulting Ltd.
Ethics approval
All study materials were submitted to the Western Institutional Review Board, a central institutional review board in the USA, for ethical review. The Western Institutional Review Board reviewed the documents and declared the study exempt from ethical review (tracking number: 2597751-44448981).
Code availability
Not applicable.
Consent to Participate
All participants consented to participation in the study.
Consent for Publication
All participants consented to their data being incorporated in the study's findings in an anonymized manner.
Author contributions
All authors contributed to the study conception and design as well as interpretation of the data. The DCE survey was developed by KW and AJL with input from all authors. The analyses were performed by KW, DA, and AJL. All co-authors have revised all manuscript drafts critically and have approved the final version of the manuscript. All co-authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work were appropriately addressed and resolved.
Availability of Data and Material
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Fakhouri F, Zuber J, Fremeaux-Bacchi V, Loirat C. Haemolytic uraemic syndrome. Lancet. 2017;390(10095):681–696. doi: 10.1016/S0140-6736(17)30062-4. [DOI] [PubMed] [Google Scholar]
- 2.Yan K, Desai K, Gullapalli L, Druyts E, Balijepalli C. Epidemiology of atypical hemolytic uremic syndrome: a systematic literature review. Clin Epidemiol. 2020;12:295–305. doi: 10.2147/CLEP.S245642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fremeaux-Bacchi V, Fakhouri F, Garnier A, Bienaimé F, Dragon-Durey M-A, Ngo S, et al. Genetics and outcome of atypical hemolytic uremic syndrome: a nationwide French series comparing children and adults. Clin J Am Soc Nephrol. 2013;8(4):554–562. doi: 10.2215/cjn.04760512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noris M, Caprioli J, Bresin E, Mossali C, Pianetti G, Gamba S, et al. Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin J Am Soc Nephrol. 2010;5(10):1844–1859. doi: 10.2215/cjn.02210310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Medicines Agency. Eculizumab (Soliris). 2021. https://www.ema.europa.eu/en/documents/product-information/soliris-epar-product-information_en.pdf. Accessed 1 Jun 2021.
- 6.US Food and Drug Administration. Eculizumab (Soliris). 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125166s434lbl.pdf. Accessed 1 Jun 2021.
- 7.Legendre CM, Licht C, Muus P, Greenbaum LA, Babu S, Bedrosian C, et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. New Engl J Med. 2013;368(23):2169–2181. doi: 10.1056/NEJMoa1208981. [DOI] [PubMed] [Google Scholar]
- 8.Licht C, Greenbaum LA, Muus P, Babu S, Bedrosian CL, Cohen DJ, et al. Efficacy and safety of eculizumab in atypical hemolytic uremic syndrome from 2-year extensions of phase 2 studies. Kidney Int. 2015;87(5):1061–1073. doi: 10.1038/ki.2014.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fakhouri F, Hourmant M, Campistol JM, Cataland SR, Espinosa M, Gaber AO, et al. Terminal complement inhibitor eculizumab in adult patients with atypical hemolytic uremic syndrome: a single-arm, open-label trial. Am J Kidney Dis. 2016;68(1):84–93. doi: 10.1053/j.ajkd.2015.12.034. [DOI] [PubMed] [Google Scholar]
- 10.Greenbaum LA, Fila M, Ardissino G, Al-Akash SI, Evans J, Henning P, et al. Eculizumab is a safe and effective treatment in pediatric patients with atypical hemolytic uremic syndrome. Kidney Int. 2016;89(3):701–711. doi: 10.1016/j.kint.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 11.Rondeau E, Cataland SR, Al-Dakkak I, Miller B, Webb NJA, Landau D. Eculizumab safety: five-year experience from the global atypical hemolytic uremic syndrome registry. Kidney Int Rep. 2019;4(11):1568–1576. doi: 10.1016/j.ekir.2019.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ariceta G. Optimal duration of treatment with eculizumab in atypical hemolytic uremic syndrome (aHUS): a question to be addressed in a scientific way. Pediatr Nephrol. 2019;34(5):943–949. doi: 10.1007/s00467-019-4192-7. [DOI] [PubMed] [Google Scholar]
- 13.Fakhouri F, Loirat C. Anticomplement treatment in atypical and typical hemolytic uremic syndrome. Semin Hematol. 2018;55(3):150–158. doi: 10.1053/j.seminhematol.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 14.US Food and Drug Administration. Ravulizumab (Ultomiris). 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761108s005lbl.pdf. Accessed 1 Jun 2021.
- 15.European Medicines Agency. Ravulizumab (Ultomiris). 2021. https://www.ema.europa.eu/en/documents/product-information/ultomiris-epar-product-information_en.pdf. Accessed 1 Jun 2021.
- 16.Alexion Pharmaceuticals Inc. Ultomiris® (ravulizumab) receives approval in Japan for atypical hemolytic uremic syndrome (aHUS) in adults and children. 2020. https://ir.alexion.com/news-releases/news-release-details/ultomirisr-ravulizumab-receives-approval-japan-atypical. Accessed 1 Jun 2021.
- 17.Sheridan D, Yu ZX, Zhang Y, Patel R, Sun F, Lasaro MA, et al. Design and preclinical characterization of ALXN1210: a novel anti-C5 antibody with extended duration of action. PLoS ONE. 2018;13(4):e0195909. doi: 10.1371/journal.pone.0195909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rondeau E, Scully M, Ariceta G, Barbour T, Cataland S, Heyne N, et al. The long-acting C5 inhibitor, ravulizumab, is effective and safe in adult patients with atypical hemolytic uremic syndrome naïve to complement inhibitor treatment. Kidney Int. 2020;97(6):1287–1296. doi: 10.1016/j.kint.2020.01.035. [DOI] [PubMed] [Google Scholar]
- 19.Greenbaum LA, Adams B, Cheong HI, Constantinescu ER, Denker AE, Dixon BP, et al. The long-acting complement inhibitor ravulizumab in children with atypical haemolytic uraemic syndrome (interim analysis) Pediatr Nephrol. 2019;34:1821–2260. doi: 10.1007/s00467-019-04325-4. [DOI] [Google Scholar]
- 20.Wang Y, Johnston K, Popoff E, Myren K-J, Cheung A, Faria C, et al. A US cost-minimization model comparing ravulizumab versus eculizumab for the treatment of atypical hemolytic uremic syndrome. J Med Econ. 2020;23:1503–1515. doi: 10.1080/13696998.2020.1831519. [DOI] [PubMed] [Google Scholar]
- 21.Levy A, Chen PGF, Tomazos I. Comparing productivity losses from treating atypical hemolytic uremic syndrome patients in the United States with eculizumab or ravulizumab in an infusion clinic or at home. Value Health. 2019;22:S841. doi: 10.1016/j.jval.2019.09.2337. [DOI] [Google Scholar]
- 22.Devlin NJ, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ. 2018;27(1):7–22. doi: 10.1002/hec.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson FR, Lancsar E, Marshall D, Kilambi V, Mühlbacher A, Regier DA, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR Conjoint Analysis Experimental Design Good Research Practices Task Force. Value Health. 2013;16(1):3–13. doi: 10.1016/j.jval.2012.08.2223. [DOI] [PubMed] [Google Scholar]
- 24.McNamara LA, Topaz N, Wang X, Hariri S, Fox L, MacNeil JR. High risk for invasive meningococcal disease among patients receiving eculizumab (Soliris) despite receipt of meningococcal vaccine. Morb Mortal Wkly Rep. 2017;66(27):734–737. doi: 10.15585/mmwr.mm6627e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sloane NJA. A library of orthogonal arrays. http://neilsloane.com/oadir/. Accessed 1 Jun 2021.
- 26.Street DJ, Burgess L. The construction of optimal stated choice experiments: theory and methods. Wiley series in probability and statistics. Hoboken: Wiley; 2007. [Google Scholar]
- 27.Statistics Canada. Census program. 2016. https://www12.statcan.gc.ca/census-recensement/index-eng.cfm?MM=1. Accessed 1 Jun 2021.
- 28.Australian Bureau of Statistics. Census. 2016. https://www.abs.gov.au/census. Accessed 1 Jun 2021.
- 29.Office for National Statistics. Census. 2011. https://www.ons.gov.uk/. Accessed 1 Jun 2021.
- 30.Centraal Bureau voor de Statistiek. StatLine. 2020. https://opendata.cbs.nl/statline/#/CBS/en/. Accessed 26 Nov 2020.
- 31.SCB Statistika Centralbyrån. Finding statistics. 2020. https://www.scb.se/en/finding-statistics/. Accessed 1 Jun 2021.
- 32.Dakin H, Abel L, Burns R, Yang Y. Review and critical appraisal of studies mapping from quality of life or clinical measures to EQ-5D: an online database and application of the MAPS statement. Health Qual Life Outcomes. 2018;16(1):31. doi: 10.1186/s12955-018-0857-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hole AR. Fitting mixed logit models by using maximum simulated likelihood. Stata J. 2007;7(3):388–401. doi: 10.1177/1536867X0700700306. [DOI] [Google Scholar]
- 34.Australian Government Actuary. Australian life tables 2015–17. 2019. https://aga.gov.au/publications/life-tables/australian-life-tables-2015-17. Accessed 1 Jun 2021.
- 35.Statistics Canada. Life expectancy at various ages, by population group and sex, Canada. 2020. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1310013401. Accessed 1 Jun 2021.
- 36.World Health Organization. Global Health Observatory data repository: life tables by country: Netherlands. 2016. https://apps.who.int/gho/data/?theme=main&vid=61160. Accessed 1 Jun 2021.
- 37.World Health Organization. Global Health Observatory data repository: life tables by country: Sweden. 2016. https://apps.who.int/gho/data/view.main.61600?lang=en. Accessed 26 Nov 2020.
- 38.Office for National Statistics. National life tables, UK: 2014 to 2016. 2017. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/bulletins/nationallifetablesunitedkingdom/2014to2016. Accessed 1 Jun 2021.
- 39.Neri L, McEwan P, Sennfält K, Baboolal K. Characterizing the relationship between health utility and renal function after kidney transplantation in UK and US: a cross-sectional study. Health Qual Life Outcomes. 2012;10:139. doi: 10.1186/1477-7525-10-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ryan M, Donato BMK, Irish W, Gasteyger C, L’Italien G, Laurence J. Economic impact of early-in-hospital diagnosis and initiation of eculizumab in atypical haemolytic uraemic syndrome. Pharmacoeconomics. 2020;38(3):307–313. doi: 10.1007/s40273-019-00862-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greenbaum LA, Licht C, Nikolaou V, Al-Dakkak I, Green J, Haas CS, et al. Functional assessment of fatigue and other patient-reported outcomes in patients enrolled in the Global aHUS Registry. Kidney Int Rep. 2020;5(8):1161–1171. doi: 10.1016/j.ekir.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Bekker-Grob EW, Donkers B, Jonker MF, Stolk EA. Sample size requirements for discrete-choice experiments in healthcare: a practical guide. Patient. 2015;8(5):373–384. doi: 10.1007/s40271-015-0118-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mott DJ, Chami N, Tervonen T. Reporting quality of marginal rates of substitution in discrete choice experiments that elicit patient preferences. Value Health. 2020;23(8):979–984. doi: 10.1016/j.jval.2020.04.1831. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.