In contrast to the mRNA vaccines, Ad26.COV2.S (Janssen COVID-19 vaccine) is single-shot replication-incompetent adenovirus-type-26 vectored vaccine encoding a stabilized variant of the SARS-CoV-2 S protein.1 Adenovirus vaccines have been widely used,2 efficiently infect host cells, and may be expected to elicit stronger Th1-biased cellular immunity than mRNA vaccines, which contributes to B cell differentiation and antibody production.3 For transplant recipients, given limited immunogenicity of mRNA vaccines,4 the Janssen vaccine platform might be a better choice. We sought to quantify the anti-spike antibody response to Janssen COVID-19 vaccination in transplant recipients and compare it to recipients of the mRNA series.
We leveraged our prospective cohort of transplant recipients without prior COVID-19 who underwent SARS-CoV-2 vaccination between 12/16/2020-3/27/2021.5 Serologic testing was undertaken on the Roche Elecsys anti-SARS-CoV-2 S enzyme immunoassay (range <0.4 to >250 U/mL [positive ≥0.8 U/mL]) which tests for antibodies against the receptor binding domain (RBD) of the spike protein at one month after COVID-19 vaccine (Janssen or mRNA).
We compared the percentage of participants with detectable anti-RBD antibody in the Janssen group (n=12) to the mRNA group (n=725) using Fisher’s exact test (Supplemental Table 1). We compared the two platforms using logistic regression adjusting for age (excluding those >70 in the mRNA group to match the Janssen group), sex, race, years since transplant (excluding those >20 years since transplant in the mRNA group to match the Janssen group) and immunosuppression. Two sensitivity analyses were undertaken weighting-by-the-odds, first weighting on age and years since transplant, and second weighting on anti-metabolite immunosuppression and years since transplant. We then used the Wilcoxon rank-sum test to compare non-negative anti-RBD titers of the Janssen group to those of the mRNA group (n=430). This study was approved by the Johns Hopkins institutional review board.
We studied 12 transplant recipients who underwent Janssen vaccine (Supplemental Table 2). At a median (IQR) 33 days (31-44) after vaccination, anti-RBD antibody was detectable in only 2/12 participants who received the Janssen vaccine compared to 430/725 who completed the mRNA vaccine series (17% versus 59%, p=0.005). Those who received the Janssen vaccine had lower odds (aOR 0.11 95%CI 0.02-0.53, p=0.006) of developing anti-RBD antibodies than those who completed the mRNA series. This association was similar in sensitivity analyses weighting by age and years since transplant (aOR 0.13 95%CI 0.03-0.61, p=0.01) and immunosuppression and years since transplant (aOR 0.17 95%CI 0.04-0.76, p=0.02). Median anti-RBD Ig titers in the Janssen group were significantly lower than the mRNA group (2.39 versus 106.9 U/mL) (p=0.047) (Figure 1).
Figure 1.
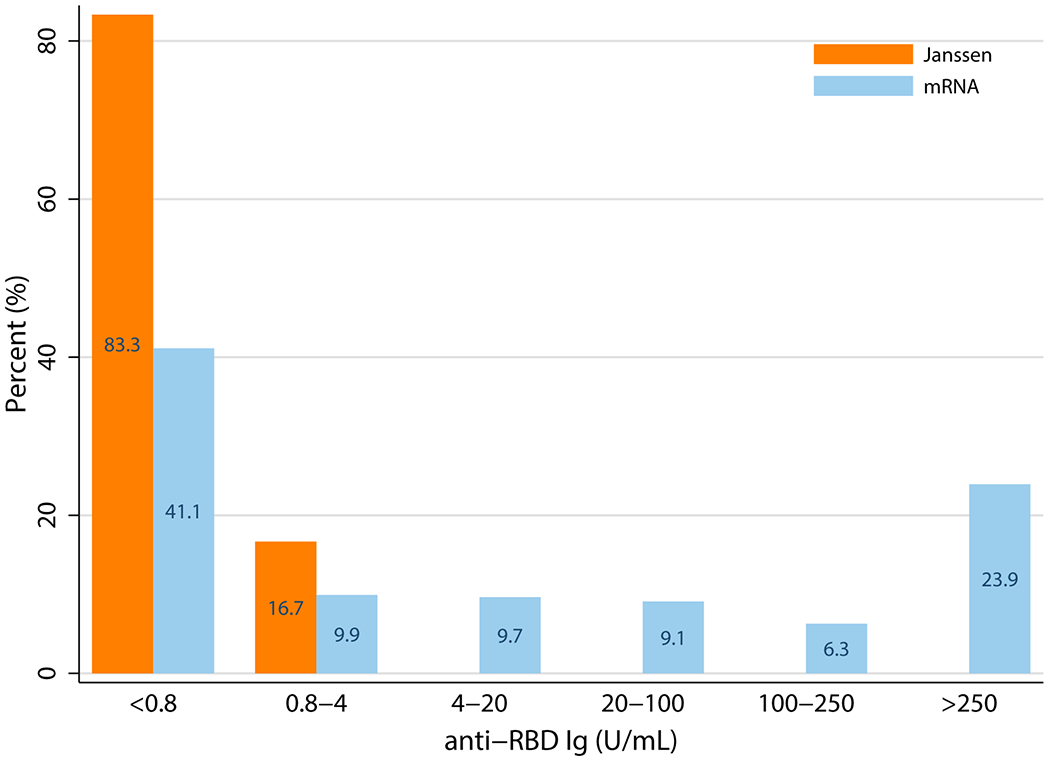
SARS-CoV-2 Anti-Receptor Binding Domain Antibody Titers Among Recipients of mRNA versus Janssen Vaccine.
In this case series of humoral response to the Janssen vaccine, only 2 of 12 participants mounted a detectable anti-RBD antibody response, which was significantly lower than what was seen among recipients of the mRNA vaccine series. Additionally, titers were significantly lower than those in the mRNA group.
These early results suggest that Janssen vaccine may result in even lower humoral immunity than mRNA vaccines in immunosuppressed transplant patients. The generalizability of these findings to other immunosuppressed populations merits further investigation.
Supplementary Material
FUNDING/GRANT/AWARD INFORMATION
This research was made possible with generous support of the Ben-Dov family. This work was supported by grant number F32DK124941 (Boyarsky), and K23DK115908 (Garonzik-Wang) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), K24AI144954 (Segev) from National Institute of Allergy and Infectious Diseases (NIAID), and by grant gSAN-201C0WW from the Transplantation and Immunology Research Network of the American Society of Transplantation (Werbel). The analyses described here are the responsibility of the authors alone and do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the US Government.
ABBREVIATIONS
- Ad26.COV2.S
adenovirus type 26 vectored vaccine (Janssen COVID-19 vaccine)
- Ig
immunoglobulin
- EIA
enzyme immunoassay
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- U/ml
units per milliliter
- RBD
receptor-binding domain
- COVID-19
coronavirus disease 2019
- mRNA
messenger ribonucleic acid
- IQR
interquartile range
- PCR
polymerase chain reaction
Footnotes
DISCLOSURE
D. L. Segev, MD PhD has the following financial disclosures: consulting and speaking honoraria from Sanofi, Novartis, CSL Behring, Jazz Pharmaceuticals, Veloxis, Mallinckrodt, Thermo Fisher Scientific. The remaining authors of this manuscript have no financial disclosures or conflicts of interest to disclose as described by Transplantation.
REFERENCES
- 1.Sadoff J, Le Gars M, Shukarev G, Heerwegh D, Truyers C, de Groot AM, et al. Interim Results of a Phase 1–2a Trial of Ad26.COV2.S Covid-19 Vaccine. New England Journal of Medicine. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Custers J, Kim D, Leyssen M, Gurwith M, Tomaka F, Robertson J, et al. Vaccines based on replication incompetent Ad26 viral vectors: Standardized template with key considerations for a risk/benefit assessment. Vaccine. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stephenson KE, Le Gars M, Sadoff J, de Groot AM, Heerwegh D, Truyers C, et al. Immunogenicity of the Ad26.COV2.S Vaccine for COVID-19. JAMA. 2021;325(15):1535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyarsky BJ, Werbel WA, Avery RK, Tobian AAR, Massie AB, Segev DL, et al. Antibody Response to 2-Dose SARS-CoV-2 mRNA Vaccine Series in Solid Organ Transplant Recipients. JAMA. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyarsky BJ, Werbel WA, Avery RK, Tobian AAR, Massie AB, Segev DL, et al. Immunogenicity of a Single Dose of SARS-CoV-2 Messenger RNA Vaccine in Solid Organ Transplant Recipients. JAMA. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


