Abstract
Purpose
Muscle relaxation following electrical automatic massage (EAM) has been found to reduce fatigue, depression, stress, anxiety, and pain in individuals with various conditions. However, the effects of EAM have not been extensively explored in patients with Alzheimer's disease (AD).
Materials and Methods
Here, we conducted a randomized controlled study to evaluate the effects of EAM on the cognitive and non-cognitive functions of patients with AD spectrum disorders.
Results
We found that EAM attenuated changes in attention-associated cognitive scores and subjective sleep quality relative to those in controls.
Conclusion
While further studies in a clinical setting are needed to support our findings, these encouraging results suggest that EAM may be an alternative therapy for the management of associated symptoms in AD (ClinicalTrials.gov ID: NCT03507192, 24/04/2018).
Keywords: Muscle relaxation, Alzheimer disease, attention, sleep quality
INTRODUCTION
Alzheimer's disease (AD), the most common cause of dementia in older adults, causes non-cognitive symptoms, including psychosis, depression, irritability, anxiety, and personality changes, in addition to cognitive symptoms. These non-cognitive symptoms have been associated with worse cognitive decline and accelerated disease progression.1 One of the most important non-cognitive symptoms is sleep disturbance, which is reported in 45% of AD patients.2 Previous studies have reported that sleep disturbance accelerates disease progression by increasing Aβ accumulation in the brain through decreased Aβ clearance.3 Therefore, evaluation and management of non-cognitive symptoms are important in treating AD patients.
Massage therapy leading to muscle relaxation is a non-pharmacological intervention that has been found to reduce fatigue, depression, stress, anxiety, and pain in individuals with various conditions.4 Previous studies have reported that massage therapy can improve sleep quality in subjects with sleep disturbance.5,6,7 Furthermore, a previous study has shown that electrical automatic massage (EAM), which automatically performs a full body massage, can improve sleep quality and relieve fatigue.8
To date, treatments for AD only provide symptomatic relief without altering the underlying disease pathology.9 Moreover, recent failures of clinical trials of anti-amyloid and tau therapies suggest that AD is a multifactorial disease.10 Therefore, multiple approaches are needed to treat AD and to improve quality of life in AD patients, potentially including non-pharmacological interventions.11 However, to the best of our knowledge, the effects of muscle relaxation following EAM have not yet been extensively examined in AD patients. Considering the beneficial effects of muscle relaxation on anxiety, depression, and sleep quality and the effects of non-cognitive symptoms on cognitive functions, we hypothesized that muscle relaxation would improve both cognitive and non-cognitive symptoms in patients with AD.
We conducted a randomized controlled study to evaluate the effects of EAM on cognitive and non-cognitive functions in patients with AD spectrum disorders. Furthermore, we evaluated the effects of muscle relaxation on cortical thickness and default mode network (DMN) changes using structural magnetic resonance imaging (MRI) and resting-state functional MRI (rs-fMRI).
MATERIALS AND METHODS
Study design and participants
This study was conducted as a 12-month, single-center, randomized controlled study of parallel groups of subjects with AD spectrum disorders. We prospectively recruited patients with amnestic mild cognitive impairments (aMCI) and early-stage AD who visited a memory clinic at Samsung Medical Center, Republic of Korea, from September 2017 to December 2019.
Subjects who met the following criteria were included: 1) age between 50 and 85 years, 2) diagnosed with either aMCI or early-stage AD, and 3) positive Aβ pathology (brain amyloid plaque load score ≥2) based on 18F-florbetaben positron emission tomography findings.12 Thus, all participants had AD spectrum disorders. Subjects with aMCI met Peterson's criteria,13 and those with early-stage AD satisfied the criteria of the National Institute of Neurological and Communicative Disorders and the Stroke and Alzheimer's Disease and Related Disorders Association,14 with a clinical dementia rating ranging from 0.5 to 1.
We excluded subjects 1) with severe white matter hyperintensities (WMH), which were defined as deep WMH ≥25 mm and periventricular WMH ≥10 mm; 2) who could not undergo MRI due to side effects related to contrast agents, claustrophobia, or an implanted device; and 3) with other medical or surgical diseases that caused dementia.
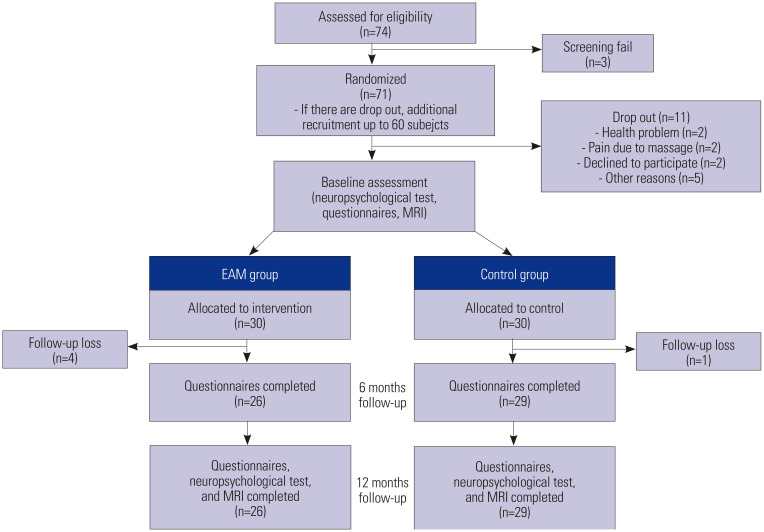
Since EAM studies have not been conducted in subjects with cognitive impairment, we calculated the sample size based on a previous intervention study, in which the difference in Pittsburgh Sleep Quality Index (PSQI) values between an intervention group and a control group was 2.13.7 Based on a statistical power of 0.8 to reject a null hypothesis at a significance of 0.05, we needed 24 subjects in each group. Considering a possible attrition rate of 25%, we set the target sample size at 60 (30 intervention and 30 control). Allocation was performed using six-block randomization. While both groups received routine medical care for cognitive impairment, the intervention group additionally received EAM for muscle relaxation. EAM was performed twice a day (every morning and evening) for 30 minutes over 12 months. Baseline neuropsychological tests [Seoul Neuropsychological Screening Battery (SNSB)15 and Mini-Mental State Examination (MMSE)]; questionnaires for non-cognitive symptoms, including sleep quality, anxiety, depression, and stress, and brain MRI were performed or both groups. While follow-up MMSE and questionnaires were performed every 6 months, follow-up SNSB and brain MRI were performed after 12 months. Five participants were lost to follow-up (Fig. 1). Since subjects lost during follow-up were unavailable for the final assessment, we performed a per protocol analysis. Although participants could not be blinded to their allocated treatment, between- group interactions were restricted to minimize bias. Although participants were unblinded and it would be difficult to guarantee that rater blindness was fully maintained due to several factors, such as participant disclosure, we tried to maintain rater blinding to attainable points. Nevertheless, the subjective questionnaires assessing non-cognitive symptoms might have been biased.
Fig. 1. Flow chart of the study design. EAM, electrical automatic massage; MRI, magnetic resonance imaging.
Each participant was fully informed of the details of the experimental procedure, and all participants provided written consent to participate in the experiment. This study was registered with the Protocol Registration and Results System (ClinicalTrials.gov ID: NCT03507192, 24/04/2018). The research is reported in accordance with the CONSORT guidelines.
EAM
The intervention group received EAM chairs (Bodyfriend Inc., Seoul, Korea), which have been shown to induce muscle relaxation.16 It conducts a full body massage of the neck, shoulders, arms, hands, waist, hip, calf, and soles. Adherence to EAM was assessed using a daily log; all users were required to submit daily logs. All participants adhered to the EAM as instructed.
Neuropsychological tests
All participants underwent the second edition of the SNSB,15,17 which is a comprehensive neuropsychological test that assesses attention (digit span test, DST), language (the Korean version of the Boston Naming Test, K-BNT), visuospatial function (Rey-Osterrieth Complex Figure Test, RCFT), memory [immediate recall, 20-minute delayed recall, and recognition in the Seoul Verbal Learning Test-Elderly's version (SVLT-E) and RCFT], and frontal-executive function (Controlled Oral Word Association Test, COWAT), Stroop test, digit symbol coding (DSC), and the Korean Trail Making Test-Elderly version (K-TMT-E). Each cognitive score was normalized using reference scores obtained from the assessment of 1067 healthy Korean participants. General cognition was assessed using the Clinical Dementia Rating sum of boxes (CDR-SOB) scores (range 0–30) and Korean version of the MMSE (range 0–30).
Questionnaires
Non-cognitive symptoms were assessed using the following questionnaires.
PSQI
We used the Korean version of the PSQI (PSQI-K) to evaluate subjective sleep quality.18 The PSQI-K consists of 18 questions concerning sleep quality, sleep onset latency, sleep duration, sleep efficiency, sleep disturbance, use of sleeping medicine, and daytime dysfunction. The score for each question ranges from 0 to 3, with higher scores indicating worse sleep quality.
Geriatric Depression Scale (GDS)
We used the Korean version of the GDS (K-GDS) to identify depression in older patients,19 with higher scores indicating more severe depression.
Geriatric Anxiety Inventory (GAI)
We used the Korean version of the GAI (K-GAI) to measure anxiety among older patients. A high total score indicates a higher level of anxiety.20
Stress questionnaire
The Stress Questionnaire is a 9-item questionnaire used to identify stress in adults.21 The score of each question ranges between 1 and 5, with total scores ranging from 9 to 45. Higher scores indicate a higher degree of stress.
Acquisition of MR images and cortical thickness measurements
MR images were acquired using a 3.0-T MR scanner (Philips Healthcare, Eindhoven, Netherlands). T1-weighted anatomical MR images were obtained with a repetition time of 9.9 ms, an echo time of 4.6 ms, a flip angle of 8°, and a voxel size of 1.0×1.0×0.5 mm3. To obtain local cortical thickness measurements for each subject, all T1 volume scans were processed using the CIVET pipeline (version 2.1.0) developed at the Montreal Neurological Institute (MNI) for fully automated structural image analysis.22 Cortical thickness was calculated as the Euclidean distance between the linked vertices of the inner and outer surfaces. We extracted the regional cortical thickness value using automated anatomical labeling parcellation23 and calculated a composite value for the cingulate, frontal, parietal, temporal, and occipital cortices by averaging each constituent region. We calculated intracranial volume by measuring the total volume of the voxels within the brain mask using the FSL bet algorithm (FMRI software library package, http://www.fmrib.ox.ac.uk/fsl).24 To measure hippocampal volume, we used an automated hippocampus segmentation method using a graph cut algorithm combined with atlas-based segmentation and morphological opening.
Acquisition and preprocessing of resting-state functional MR images
T2*-weighted MR images were obtained using a gradient echo planar imaging pulse sequence with the following parameters: 1) repetition time=3 s, 2) echo time=35 ms, 3) flip angle=90°, 4) voxel size=1.72×1.72×4 mm3, 5) slice number=35, and 6) frame number=100. Subjects were instructed to remain awake with their eyes closed and not to focus on a specific thought during the scan. We performed the following preprocessing steps using Statistical Parametric Mapping software 12 (SPM, https://www.fil.ion.ucl.ac.uk/spm/) and custom code written on MATLAB (MathWorks 2014b) as previously described25: 1) slice-timing correction, 2) realignment with rigidbody transformation, 3) linear detrending, 4) regressing-out of nuisance variables (12 parameters for rigid-body head motion, signals averaged over deep white matter and lateral ventricles, and signals averaged over the whole brain), 5) normalization to MNI space, 6) spatial smoothing with a 6-mm full-width at half-maximum Gaussian kernel, and 7) temporal filtering (0.01–0.1 Hz). We removed the first five volumes for T1 equilibration. When we inspected all imaging data for motion artifacts, no subjects exhibited head motion exceeding 2 mm in any axis or 2° of rotation.
Calculation of individual DMN size
To calculate the individual DMN size, we first performed a voxel-wise seed-based analysis with the bilateral precuneus and posterior cingulate cortex as the seeds, which are known to act as hubs of the DMN.26 A DMN mask was created by thresholding individual correlation maps throughout the whole brain using a familywise error rate-corrected p<0.05 and a cluster extent of 15. We defined DMN size as the total number of voxels within the DMN mask divided by the total number of voxels within the whole brain gray matter.
Statistical analysis
The baseline demographic characteristics between the EAM and control groups were compared using an independent t-test or Mann-Whitney U test for continuous variables and chi-square test for categorical variables. Scores on baseline and follow-up neuropsychological tests and questionnaires were compared using the paired t-test or repeated-measures analysis of variance (rANOVA) for each group. We evaluated whether cognitive and non-cognitive function, regional cortical thickness, and DMN size differed between EAM intervention and control groups and whether they decreased over time using the linear mixed effect model expressed as follows: cognitive, non-cognitive function, or neuroimaging results (regional cortical thickness or DMN size)= β0+β1×group+β2×time+1|subject+covariates, where the subject was included as a random effect and baseline age, sex, and years of education were included as covariates. For the regional cortical thickness analysis, intracranial volume was additionally included as a covariate. To investigate whether EAM had an effect on longitudinal changes in cognitive and non-cognitive symptoms, regional cortical thickness, and DMN size over time, we used the following model: cognitive, non-cognitive function, or neuroimaging results (regional cortical thickness or DMN size)=β0+β1×group+β2×time+β3×group×time+1|subject+covariates. The effects of EAM on the longitudinal changes in each measure were tested with a time×group interaction term. For measures with 12-month follow-up, the terms for time were defined as 0 for baseline and 1 for 12-month follow-up. For measures with 6- and 12-month follow-up, the terms for time were defined as 0 for baseline, 1 for 6-month, and 2 for 12-month follow-up.
We applied a square-root or log transformation to highly skewed variables. We excluded variables with high skewedness or kurtosis after transformation from further analyses. A two-tailed p<0.05 was considered statistically significant. Statistical analyses were conducted with R 3.5.3 package (Vienna, Austria).
Ethics declarations
The study was conducted in accordance with the Declaration of Helsinki and reviewed and approved by the Public Institutional Review Board (No. 2017-05-135) committee designated by the Ministry of Health and Welfare, Korea.
RESULTS
Demographic features
The comparison of baseline demographic characteristics is presented in Table 1. Age, proportions of female, education level, proportions of apolipoprotein ε4 carriers, proportions of AD, and general cognitive ability did not significantly differ between the EAM intervention and control groups.
Table 1. Baseline Demographics of the EAM and Control Groups.
| EAM (n=26) | Control (n=29) | p value | ||
|---|---|---|---|---|
| Demographics | ||||
| Age (yr) | 69.4 (7.3) | 72.5 (8.4) | 0.152 | |
| Female | 19 (73.1) | 19 (65.5) | 0.545 | |
| Education (yr) | 10.4 (4.0) | 10.7 (4.6) | 0.772 | |
| APOE ε4 carrier | 14 (53.8) | 19 (65.5) | 0.378 | |
| aMCI/AD, no | 16/10 | 23/6 | 0.147 | |
| Neuropsychological tests | ||||
| MMSE | 22.9 (3.5) | 23.9 (3.8) | 0.299 | |
| CDR sum of boxes | 2.9 (1.7) | 2.4 (1.3) | 0.244 | |
| Digit span forward | 5.1 (1.3) | 5.6 (1.2) | 0.240 | |
| Digit span backward | 2.9 (1.1) | 3.6 (1.3) | 0.012 | |
| K-BNT | 39.4 (10.7) | 38.0 (11.0) | 0.544 | |
| RCFT copy | 24.5 (10.9) | 27.7 (7.6) | 0.362 | |
| SVLT immediate recall | 13.7 (3.7) | 12.9 (4.7) | 0.514 | |
| SVLT delayed recall | 1.2 (1.8) | 1.5 (1.9) | 0.631 | |
| SVLT recognition | 17.5 (3.1) | 17.0 (2.6) | 0.461 | |
| RCFT immediate recall | 4.0 (3.5) | 4.2 (3.5) | 0.748 | |
| RCFT delayed recall | 3.7 (4.2) | 3.5 (3.5) | 0.932 | |
| RCFT recognition | 16.8 (2.7) | 16.7 (2.8) | 0.993 | |
| COWAT animal | 11.0 (3.3) | 11.8 (4.2) | 0.446 | |
| COWAT supermarket | 11.4 (4.7) | 11.1 (5.2) | 0.804 | |
| COWAT phonemic | 16.2 (9.6) | 19.9 (10.1) | 0.174 | |
| Stroop color reading | 49.6 (30.1) | 58.5 (31.0) | 0.285 | |
| DSC | 34.0 (17.4) | 40.5 (17.9) | 0.230 | |
| K-TMT-E-B time | 125.8 (107.5) | 119.3 (95.2) | 0.989 | |
| PSQI-K | 3.8 (3.7) | 4.2 (3.4) | 0.459 | |
| K-GDS-30 | 8.6 (6.4) | 9.9 (5.9) | 0.428 | |
| K-GAI | 4.4 (4.5) | 5.2 (4.4) | 0.467 | |
| Stress inventory | 13.0 (5.6) | 12.7 (3.6) | 0.529 | |
AD, early-stage of Alzheimer's disease; aMCI, amnestic mild cognitive impairment; APOE ε4, apolipoprotein ε4; CDR, Clinical Dementia Rating; COWAT, Controlled Oral Word Association Test; DSC, Digit Symbol Coding; EAM, electrical automatic massage; K-BNT, Korean version of the Boston Naming Test; K-GAI, Korean version of the Geriatric Anxiety Inventory; K-GDS, Korean version of the Geriatric Depression Scale; K-TMT-E-B, Korean Trail Making Test-Elderly version part B; MMSE, Mini-Mental State Examination; PSQI-K, Korean version of the Pittsburgh Sleep Quality Index; RCFT, Rey-Osterrieth Complex Figure Test; SD, standard deviation; SVLT, Seoul Verbal Learning Test. Differences were analyzed using the independent t-test or chi-square test.
Data are presented as mean (SD) or n (%).
Effects of EAM on longitudinal cognitive function
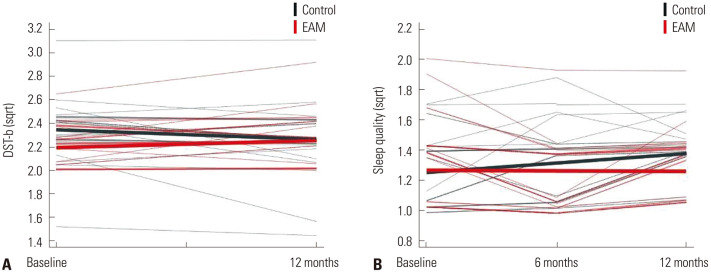
We compared baseline and follow-up neuropsychological test scores using the paired t-test or rANOVA for each group (Supplementary Table 1, only online). In the linear mixed effect model, there was no main effect for grouping (EAM and control), suggesting that cognitive function did not differ between the two groups. However, there was an effect for time in some cognitive tests, including the K-BNT, RCFT copy, immediate and delayed recall on the SVLT, recognition on the SVLT, immediate and delayed recall on the RCFT, COWAT, Stroop color reading test, DSC, and MMSE (Table 2). In addition, the DST-backward test showed a significant group-by-time interaction (t=2.274, p=0.027) (Table 2 and Fig. 2A).
Table 2. Results of Linear Mixed Effect Model Analysis of Neuropsychological Tests.
| Neuropsychological test | Group effect | Time effect | Group×time | |||
|---|---|---|---|---|---|---|
| β (95% CI) | p value | β (95% CI) | p value | β (95% CI) | p value | |
| Digit span forward | −0.55 (−1.23, 0.14) | 0.114 | −0.08 (−0.35, 0.20) | 0.584 | −0.21 (−0.73, 0.31) | 0.432 |
| Digit span backward | −0.08 (−0.22, 0.06) | 0.266 | −0.02 (−0.08, 0.05) | 0.557 | 0.14 (0.02, 0.25) | 0.027 |
| K-BNT | 0.98 (−4.36, 6.33) | 0.714 | −3.16 (−4.48, −1.85) | <0.001 | −0.72 (−3.18, 1.74) | 0.567 |
| RCFT copy | −0.33 (−1.06, 0.40) | 0.370 | −0.46 (−0.69, −0.22) | <0.001 | 0.13 (−0.32, 0.58) | 0.564 |
| SVLT immediate recall | 0.60 (−1.79, 3.00) | 0.615 | −0.89 (−1.64, −0.14) | 0.020 | −0.12 (−1.55, 1.31) | 0.868 |
| SVLT delayed recall | −0.04 (−0.26, 0.17) | 0.702 | −0.13 (−0.23, −0.03) | 0.010 | 0.05 (−0.14, 0.24) | 0.638 |
| SVLT recognition | 0.44 (−1.00, 1.88) | 0.543 | −0.91 (−1.56, −0.26) | 0.007 | −0.35 (−1.60, 0.90) | 0.588 |
| RCFT immediate recall | 0.01 (−0.34, 0.35) | 0.967 | −0.24 (−0.37, −0.11) | <0.001 | −0.01 (−0.26, 0.24) | 0.928 |
| RCFT delayed recall | 0.02 (−0.33, 0.38) | 0.892 | −0.28 (−0.42, −0.13) | <0.001 | −0.12 (−0.41, 0.17) | 0.415 |
| RCFT recognition | 0.29 (−1.09, 1.68) | 0.670 | −0.24 (−0.85, 0.37) | 0.434 | 0.08 (−1.09, 1.25) | 0.897 |
| COWAT animal | −0.34 (−2.28, 1.60) | 0.729 | −1.17 (−1.99, −0.36) | 0.006 | 0.98 (−0.58, 2.54) | 0.225 |
| COWAT supermarket | 0.50 (−1.73, 2.73) | 0.656 | −1.71 (−3.12, −0.30) | 0.018 | 1.41 (−1.33, 4.15) | 0.318 |
| COWAT phonemic | −1.80 (−6.51, 2.92) | 0.448 | −2.08 (−4.23, 0.06) | 0.057 | 3.37 (−0.70, 7.44) | 0.113 |
| Stroop color reading | −5.63 (−22.40, 11.15) | 0.504 | −6.35 (−11.19, −1.51) | 0.011 | 7.43 (−1.58, 16.44) | 0.113 |
| DSC | −4.90 (−14.42, 4.62) | 0.307 | −5.47 (−8.01, −2.93) | <0.001 | −0.46 (−5.22, 4.30) | 0.851 |
| K-TMT-E-B time | 32.15 (−21.75, 86.05) | 0.237 | 21.02 (−3.88, 45.92) | 0.095 | 4.02 (−44.15, 52.19) | 0.871 |
| MMSE | −0.83 (−2.73, 1.08) | 0.388 | −2.04 (−2.87, −1.22) | <0.001 | −0.32 (−1.96, 1.32) | 0.695 |
| CDR sum of boxes | 0.29 (−0.67, 1.25) | 0.542 | 1.05 (0.65, 1.46) | <0.001 | −0.29 (−1.07, 0.49) | 0.467 |
β, beta coefficient; CDR, Clinical Dementia Rating; CI, confidence interval; COWAT, Controlled Oral Word Association Test; DSC, Digit Symbol Coding; K-BNT, Korean version of the Boston Naming Test; K-TMT-E-B, Korean Trail Making Test-Elderly version part B; MMSE, Mini-Mental State Examination; RCFT, Rey-Osterrieth Complex Figure Test; SVLT, Seoul Verbal Learning Test.
The terms for time were defined as 0 for baseline and 1 for 12-month follow-up.
Fig. 2. Longitudinal changes in (A) digit span backward test scores and (B) subjective sleep quality in the EAM intervention and control groups. DST-b, digit span test-backward; EAM, electrical automatic massage; sqrt, square root transformation.
Effect of EAM on longitudinal non-cognitive symptoms
The baseline and follow-up questionnaires were compared in each group using rANOVA (Supplementary Table 2, only online). In the linear mixed effect model, there was no main effect for grouping (EAM and control), suggesting that non-cognitive function did not differ between the two groups. However, there was a main effect of time in some non-cognitive tests, including the K-GAI and stress inventory (Table 3). In addition, the PSQI-K revealed a significant group-by-time interaction (t=−2.179, p=0.032) (Table 3 and Fig. 2B).
Table 3. Results of the Linear Mixed Effect Model for Questionnaires.
| Questionnaire | Group effect | Time effect | Group×time | ||||
|---|---|---|---|---|---|---|---|
| β (95% CI) | p value | β (95% CI) | p value | β (95% CI) | p value | ||
| PSQI-K total score | −0.04 (−0.16, 0.08) | 0.468 | 0.01 (−0.02, 0.04) | 0.463 | 0.0007 (−0.05, 0.05) | 0.983 | |
| Sleep quality | −0.04 (−0.17, 0.09) | 0.523 | 0.03 (−0.003, 0.07) | 0.074 | −0.08 (−0.15, −0.001) | 0.032 | |
| Sleep latency | −0.33 (−0.81, 0.16) | 0.182 | −0.04 (−0.17, 0.10) | 0.577 | −0.05 (−0.30, 0.20) | 0.724 | |
| Sleep duration | 0.02 (−0.07, 0.10) | 0.694 | −0.01 (−0.04, 0.02) | 0.641 | 0.02 (−0.03, 0.07) | 0.513 | |
| Sleep disturbance | −0.01 (−0.23, 0.21) | 0.907 | −0.06 (−0.14, 0.02) | 0.168 | 0.04 (−0.11, 0.19) | 0.654 | |
| K-GDS-30 | −0.40 (−0.89, 0.08) | 0.103 | −0.10 (−0.22, 0.03) | 0.121 | −0.03 (−0.26, 0.20) | 0.831 | |
| K-GAI | −0.32 (−0.72, 0.07) | 0.107 | −0.19 (−0.30, −0.07) | 0.002 | −0.06 (−0.29, 0.17) | 0.615 | |
| Stress inventory | −0.03 (−0.08, 0.03) | 0.305 | −0.02 (−0.03, −0.001) | 0.038 | −0.01 (−0.04, 0.02) | 0.388 | |
β, beta coefficient; CI, confidence interval; K-GAI, Korean version of the Geriatric Anxiety Inventory; K-GDS, Korean version of the Geriatric Depression Scale; PSQI-K, Korean version of the Pittsburgh Sleep Quality Index.
The terms for time were defined as 0 for baseline, 1 for 6-month, and 2 for 12-month follow-up.
Effects of EAM on longitudinal changes in cortical thickness
The difference between baseline and follow-up cortical thickness was compared using the paired t-test for each group (Supplementary Table 3, only online). In the linear mixed effect model, there was no main effect of grouping, suggesting that cortical thickness and hippocampal volume did not differ between the two groups (EAM and control). However, there was a main effect for time on cortical thickness and hippocampal volume. There was no significant group-by-time interaction for cortical thickness or hippocampal volume (Table 4).
Table 4. Results of the Linear Mixed Effect Model for Regional Cortical Thickness and Hippocampal Volume.
| Cortical region | Group effect | Time effect | Group×time | |||
|---|---|---|---|---|---|---|
| β (95% CI) | p value | β (95% CI) | p value | β (95% CI) | p value | |
| Lt. cingulate thickness | −0.06 (−0.17, 0.04) | 0.233 | −0.03 (−0.06, −0.002) | 0.036 | 0.02 (−0.04, 0.07) | 0.601 |
| Rt. cingulate thickness | 0.01 (−0.08, 0.10) | 0.789 | −0.05 (−0.07, −0.02) | <0.001 | 0.01 (−0.03, 0.06) | 0.544 |
| Lt. frontal thickness | −0.04 (−0.11, 0.02) | 0.198 | −0.04 (−0.06, −0.02) | <0.001 | 0.001 (−0.04, 0.04) | 0.943 |
| Rt. frontal thickness | −0.02 (−0.08, 0.04) | 0.491 | −0.04 (−0.06, −0.02) | <0.001 | 0.01 (−0.02, 0.05) | 0.399 |
| Lt. parietal thickness | −0.04 (−0.14, 0.05) | 0.376 | −0.06 (−0.08, −0.03) | <0.001 | 0.01 (−0.04, 0.06) | 0.581 |
| Rt. parietal thickness | −0.01 (−0.10, 0.08) | 0.859 | −0.05 (−0.06, −0.03) | <0.001 | −0.005 (−0.04, 0.03) | 0.782 |
| Lt. temporal thickness | −0.05 (−0.14, 0.04) | 0.260 | −0.07 (−0.09, −0.04) | <0.001 | −0.000008 (−0.05, 0.05) | 0.999 |
| Rt. temporal thickness | −0.03 (−0.12, 0.06) | 0.532 | −0.06 (−0.08, −0.04) | <0.001 | 0.01 (−0.04, 0.05) | 0.664 |
| Lt. occipital thickness | −0.003 (−0.11, 0.10) | 0.954 | −0.07 (−0.10, −0.04) | <0.001 | 0.02 (−0.04, 0.08) | 0.546 |
| Rt. occipital thickness | 0.01 (−0.08, 0.11) | 0.778 | −0.05 (−0.07, −0.03) | <0.001 | 0.02 (−0.02, 0.07) | 0.309 |
| Lt. hippocampal volume | 42.72 (−275.9, 361.4) | 0.788 | −116.39 (−200.6, −32.2) | 0.008 | 43.30 (−126.0, 212.6) | 0.609 |
| Rt. hippocampal volume | −12.77 (−344.7, 319.2) | 0.939 | −111.22 (−201.3, −21.1) | 0.017 | 61.50 (−119.1, 242.1) | 0.496 |
β, beta coefficient; CI, confidence interval; Lt, left; Rt, right.
The terms for time were defined as 0 for baseline and 1 for 12-month follow-up.
Effect of EAM on longitudinal changes in DMN size
In rs-fMRI analysis, there was no significant effect for time (t=0.935, p=0.343); furthermore, there was no interactive effect of EAM intervention on changes in DMN size (t=0.974, p=0.334).
DISCUSSION
We evaluated the effects of EAM on cognitive and non-cognitive functions in patients with AD spectrum disorders. The important finding of this study was that muscle relaxation following EAM attenuated changes in attention-associated cognitive scores (DST-backward score) and subjective sleep quality relative to that in controls. Our findings suggest that EAM may be an alternative therapy for the management of associated symptoms in AD.
As expected, a significant decrease was noted in most tested cognitive functions, and all brain regions showed a reduction in cortical thickness after 1 year. We did not find that EAM resulted in improvements in most cognitive functions or changes in cortical thickness. These negative results might be due to the fact that the participants in this study were in either the prodromal or early stages of AD, where there is obvious cognitive dysfunction with positive Aβ pathology. Since various pathogenic processes, such as Aβ and tau deposition and neurodegeneration, occur prior to overt cognitive impairment,27 EAM that provides symptomatic relief without ameliorating AD pathologies may have minimal effect. It may be more promising to apply EAM at an earlier stage, such as during the preclinical stage of AD.
Nevertheless, we found that EAM led to the attenuation of changes in attention relative to that in controls. In the early stage of AD pathogenesis, the frontal domain is relatively spared from pathogenic processes as AD involves temporo-parietal brain areas.28 We speculate that the effect of symptomatic therapies without altering AD pathologies might be evident in the cognitive domain that is relatively preserved at the early stages of AD. Furthermore, our findings are consistent with those of previous studies that showed a beneficial effect of muscle relaxation on attention in patients with attention-deficit hyperactivity disorders.29,30 Although the mechanisms of how muscle relaxation improves attention are not known, previous studies have demonstrated that it might be due to an increased level of serotonin.30,31,32
While most non-cognitive symptoms showed no improvement after EAM, we found that EAM led to the attenuation of declines in subjective sleep quality relative to that in controls. Sleep affects various cognitive functions, including attention and memory consolidation.33 Furthermore, sleep disturbances, such as insomnia, hypersomnia, and circadian rhythm disturbance, are common in AD2 and increase the risk of dementia.34,35 One mechanism explaining this association is that sleep disturbance inhibits metabolite clearance,36 precipitating the accumulation of Aβ in the frontal region of the brain.37 Furthermore, poor sleep quality has been reported to increase the risk of metabolic and cardiovascular diseases, which are known to be independent risk factors for AD.38 Previous studies have demonstrated the beneficial effects of muscle relaxation on sleep quality in subjects with insomnia.5,6,7,8 We demonstrated similar results of improvement in subjective sleep quality after massage therapy in subjects with AD spectrum disorders. While the biological mechanisms underlying the effects of muscle relaxation are not established, it is thought that the effects are a result of the activation of arterial and venous blood flow in the lymphatic system, stimulation of vagal activity, and reduction in cortisol levels.39
This study has some limitations. First, the effects of EAM on cognitive and non-cognitive symptoms were modest, especially since we did not correct for multiple comparisons, which might lead to concerns for type I error. However, considering that this was an exploratory study, a multiple comparison correction might have missed important associations in the early stages of the analysis. Nevertheless, further confirmatory investigations with a larger sample size will be necessary to confirm our results. Second, while DST-backward scores showed improvement, scores for DST-forward, which is also known to measure attention levels, did not show improvement. Previous studies dissociated DST-backward from DST-forward such that while both DST-forward and DST-backward evaluate short-term memory, DST-backward requires an additional attention-demanding transformation of digit sequence and thus measures levels of working memory.40,41 Third, non-cognitive functions were assessed using subjective questionnaires. Since participants cannot be blinded to their allocated treatment, we cannot guarantee that the subjective improvement in sleep quality was not due to a placebo effect. Since this is an exploratory study, exploring the possible effects of EAM on various phenotypes (cognitive symptoms, non-cognitive symptoms, brain structural changes, and brain functional changes), further confirmatory studies using objective measurements of sleep quality, such as polysomnography, are required to address this issue. In order to accurately assess the effect of EAM on various symptoms of AD patients, an ideal study design would include a positive control using a dummy massage device. However, given the lack of biomechanistic studies of EAM, sham massage devices may also cause non-specific effects in the control group.
This is the first study to investigate the effects of muscle relaxation in subjects with AD spectrum disorders. This study revealed attenuation of changes in working memory and sleep quality in patients with AD spectrum disorders after receiving EAM, and EAM was well tolerated by patients with AD spectrum disorders. While further studies in a clinical setting are needed to support our findings, these encouraging results suggest that EAM may be an alternative therapy for the management of associated symptoms in AD.
ACKNOWLEDGEMENTS
This research was supported by the Bodyfriend, Seoul, Republic of Korea.
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Sang Won Seo.
- Data curation: Young Hee Jung and Sang Won Seo.
- Formal Analysis: Young Ju Kim, Hang-Rai Kim, and Young Hee Jung.
- Investigation: Young Ju Kim, Hang-Rai Kim, Young Hee Jung, and Sang Won Seo.
- Methodology: Young Ju Kim, Hang-Rai Kim, and Young Hee Jung.
- Project administration: Sang Won Seo.
- Resources: Young Hee Jung and Sang Won Seo.
- Software: Young Ju Kim, Hang-Rai Kim, and Yu Hyun Park.
- Supervision: Sang Won Seo.
- Validation: all authors.
- Visualization: Young Ju Kim and Yu Hyun Park.
- Writing—original draft: Young Ju Kim and Hang-Rai Kim.
- Writing—review & editing: Young Ju Kim, Hang-Rai Kim, and Sang Won Seo.
- Approval of final manuscript: all authors.
SUPPLEMENTARY MATERIALS
Results of Paired T-Test and Repeated Measures Analysis of Variance for Neuropsychological Tests
Results of Repeated Measures Analysis of Variance for Questionnaires
Results of Paired T-Test for Cortical Thickness and Hippocampal Volume
References
- 1.Eustace A, Coen R, Walsh C, Cunningham CJ, Walsh JB, Coakley D, et al. A longitudinal evaluation of behavioural and psychological symptoms of probable Alzheimer's disease. Int J Geriatr Psychiatry. 2002;17:968–973. doi: 10.1002/gps.736. [DOI] [PubMed] [Google Scholar]
- 2.Moran M, Lynch CA, Walsh C, Coen R, Coakley D, Lawlor BA. Sleep disturbance in mild to moderate Alzheimer's disease. Sleep Med. 2005;6:347–352. doi: 10.1016/j.sleep.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Ooms S, Overeem S, Besse K, Rikkert MO, Verbeek M, Claassen JA. Effect of 1 night of total sleep deprivation on cerebrospinal fluid β-amyloid 42 in healthy middle-aged men: a randomized clinical trial. JAMA Neurol. 2014;71:971–977. doi: 10.1001/jamaneurol.2014.1173. [DOI] [PubMed] [Google Scholar]
- 4.Goats GC. Massage--the scientific basis of an ancient art: part 2. Physiological and therapeutic effects. Br J Sports Med. 1994;28:153–156. doi: 10.1136/bjsm.28.3.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hachul H, Oliveira DS, Bittencourt LR, Andersen ML, Tufik S. The beneficial effects of massage therapy for insomnia in postmenopausal women. Sleep Sci. 2014;7:114–116. doi: 10.1016/j.slsci.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saeedi M, Ashktorab T, Saatchi K, Zayeri F, Amir Ali Akbari S. The effect of progressive muscle relaxation on sleep quality of patients undergoing hemodialysis. Iran J Crit Care Nurs. 2012;5:23–28. [Google Scholar]
- 7.Li CY, Chen SC, Li CY, Gau ML, Huang CM. Randomised controlled trial of the effectiveness of using foot reflexology to improve quality of sleep amongst Taiwanese postpartum women. Midwifery. 2011;27:181–186. doi: 10.1016/j.midw.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Choi SJ, Yun SH, Joo EY. Effects of electrical automatic massage of whole body at bedtime on sleep and fatigue. J Sleep Med. 2017;14:10–17. [Google Scholar]
- 9.Banik A, Brown RE, Bamburg J, Lahiri DK, Khurana D, Friedland RP, et al. Translation of pre-clinical studies into successful clinical trials for Alzheimer's disease: what are the roadblocks and how can they be overcome? J Alzheimers Dis. 2015;47:815–843. doi: 10.3233/JAD-150136. [DOI] [PubMed] [Google Scholar]
- 10.Hyman BT. Amyloid-dependent and amyloid-independent stages of Alzheimer disease. Arch Neurol. 2011;68:1062–1064. doi: 10.1001/archneurol.2011.70. [DOI] [PubMed] [Google Scholar]
- 11.Herholz SC, Herholz RS, Herholz K. Non-pharmacological interventions and neuroplasticity in early stage Alzheimer's disease. Expert Rev Neurother. 2013;13:1235–1245. doi: 10.1586/14737175.2013.845086. [DOI] [PubMed] [Google Scholar]
- 12.Seibyl J, Catafau AM, Barthel H, Ishii K, Rowe CC, Leverenz JB, et al. Impact of training method on the robustness of the visual assessment of 18F-florbetaben PET scans: results from a phase-3 study. J Nucl Med. 2016;57:900–906. doi: 10.2967/jnumed.115.161927. [DOI] [PubMed] [Google Scholar]
- 13.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 14.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang Y, Jahng S, Na DL. Seoul neuropsychological screening battery (SNSB-II) 2nd ed. Incheon: Human Brain Research & Consulting Co.; 2012. [Google Scholar]
- 16.Zullino DF, Krenz S, Frésard E, Cancela E, Khazaal Y. Local back massage with an automated massage chair: general muscle and psychophysiologic relaxing properties. J Altern Complement Med. 2005;11:1103–1106. doi: 10.1089/acm.2005.11.1103. [DOI] [PubMed] [Google Scholar]
- 17.Kang SH, Park YH, Lee D, Kim JP, Chin J, Ahn Y, et al. The cortical neuroanatomy related to specific neuropsychological deficits in Alzheimer's continuum. Dement Neurocogn Disord. 2019;18:77–95. doi: 10.12779/dnd.2019.18.3.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sohn SI, Kim DH, Lee MY, Cho YW. The reliability and validity of the Korean version of the Pittsburgh Sleep Quality Index. Sleep Breath. 2012;16:803–812. doi: 10.1007/s11325-011-0579-9. [DOI] [PubMed] [Google Scholar]
- 19.Jung IK, Kwak DI, Joe SH, Lee HS. A preliminary study on standardization of Korean form of geriatric depression scale (KGDS) J Korean Neuropsychiatr Assoc. 1998;37:340–351. [Google Scholar]
- 20.Kim J, Park MS, Oh DN. Reliability and validity of Korean geriatric anxiety inventory (K-GAI) J Muscle Joint Health. 2014;21:75–84. [Google Scholar]
- 21.Lee ES, Shin HC, Yang YJ, Cho JJ, Ahn KY, Kim SH. Development of the stress questionnaire for KNHANES: report of scientific study service. Seoul: Korea Centers for Disease Control and Prevention; 2010. [Google Scholar]
- 22.Zijdenbos AP, Forghani R, Evans AC. Automatic “pipeline” analysis of 3-D MRI data for clinical trials: application to multiple sclerosis. IEEE Trans Med Imaging. 2002;21:1280–1291. doi: 10.1109/TMI.2002.806283. [DOI] [PubMed] [Google Scholar]
- 23.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 24.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim HR, Lee P, Seo SW, Roh JH, Oh M, Oh JS, et al. Comparison of amyloid β and Tau spread models in Alzheimer's disease. Cereb Cortex. 2019;29:4291–4302. doi: 10.1093/cercor/bhy311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Utevsky AV, Smith DV, Huettel SA. Precuneus is a functional core of the default-mode network. J Neurosci. 2014;34:932–940. doi: 10.1523/JNEUROSCI.4227-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jack CR, Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.La Joie R, Landeau B, Perrotin A, Bejanin A, Egret S, Pélerin A, et al. Intrinsic connectivity identifies the hippocampus as a main crossroad between Alzheimer's and semantic dementia-targeted networks. Neuron. 2014;81:1417–1428. doi: 10.1016/j.neuron.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 29.Chen SC, Yu BY, Suen LK, Yu J, Ho FY, Yang JJ, et al. Massage therapy for the treatment of attention deficit/hyperactivity disorder (ADHD) in children and adolescents: a systematic review and meta-analysis. Complement Ther Med. 2019;42:389–399. doi: 10.1016/j.ctim.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 30.Maddigan B, Hodgson P, Heath S, Dick B, St John K, McWilliam-Burton T, et al. The effects of massage therapy & exercise therapy on children/adolescents with attention deficit hyperactivity disorder. Can Child Adolesc Psychiatr Rev. 2003;12:40–43. [PMC free article] [PubMed] [Google Scholar]
- 31.Field TM. Massage therapy effects. Am Psychol. 1998;53:1270–1281. doi: 10.1037//0003-066x.53.12.1270. [DOI] [PubMed] [Google Scholar]
- 32.Ironson G, Field T, Scafidi F, Hashimoto M, Kumar M, Kumar A, et al. Massage therapy is associated with enhancement of the immune system's cytotoxic capacity. Int J Neurosci. 1996;84:205–217. doi: 10.3109/00207459608987266. [DOI] [PubMed] [Google Scholar]
- 33.Karni A, Tanne D, Rubenstein BS, Askenasy JJ, Sagi D. Dependence on REM sleep of overnight improvement of a perceptual skill. Science. 1994;265:679–682. doi: 10.1126/science.8036518. [DOI] [PubMed] [Google Scholar]
- 34.Elwood PC, Bayer AJ, Fish M, Pickering J, Mitchell C, Gallacher JE. Sleep disturbance and daytime sleepiness predict vascular dementia. J Epidemiol Community Health. 2011;65:820–824. doi: 10.1136/jech.2009.100503. [DOI] [PubMed] [Google Scholar]
- 35.Sterniczuk R, Theou O, Rusak B, Rockwood K. Sleep disturbance is associated with incident dementia and mortality. Curr Alzheimer Res. 2013;10:767–775. doi: 10.2174/15672050113109990134. [DOI] [PubMed] [Google Scholar]
- 36.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Branger P, Arenaza-Urquijo EM, Tomadesso C, Mézenge F, André C, de Flores R, et al. Relationships between sleep quality and brain volume, metabolism, and amyloid deposition in late adulthood. Neurobiol Aging. 2016;41:107–114. doi: 10.1016/j.neurobiolaging.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 38.Landry GJ, Liu-Ambrose T. Buying time: a rationale for examining the use of circadian rhythm and sleep interventions to delay progression of mild cognitive impairment to Alzheimer's disease. Front Aging Neurosci. 2014;6:325. doi: 10.3389/fnagi.2014.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Field T. Massage therapy research review. Complement Ther Clin Pract. 2016;24:19–31. doi: 10.1016/j.ctcp.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alloway TP, Gathercole SE, Pickering SJ. Verbal and visuospatial short-term and working memory in children: are they separable. Child Dev. 2006;77:1698–1716. doi: 10.1111/j.1467-8624.2006.00968.x. [DOI] [PubMed] [Google Scholar]
- 41.St Clair-Thompson HL, Allen RJ. Are forward and backward recall the same? A dual-task study of digit recall. Mem Cognit. 2013;41:519–532. doi: 10.3758/s13421-012-0277-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Results of Paired T-Test and Repeated Measures Analysis of Variance for Neuropsychological Tests
Results of Repeated Measures Analysis of Variance for Questionnaires
Results of Paired T-Test for Cortical Thickness and Hippocampal Volume