Abstract
Viral infections are one of the leading causes of human illness. Viruses take over host cell signaling cascades for their replication and infection. Calcium (Ca2+) is a versatile and ubiquitous second messenger that modulates plethora of cellular functions. In last two decades, a critical role of host cell Ca2+ signaling in modulating viral infections has emerged. Furthermore, recent literature clearly implicates a vital role for the organellar Ca2+ dynamics (influx and efflux across organelles) in regulating virus entry, replication and severity of the infection. Therefore, it is not surprising that a number of viral infections including current SARS-CoV-2 driven COVID-19 pandemic are associated with dysregulated Ca2+ homeostasis. The focus of this review is to first discuss the role of host cell Ca2+ signaling in viral entry, replication and egress. We further deliberate on emerging literature demonstrating hijacking of the host cell Ca2+ dynamics by viruses. In particular, a variety of viruses including SARS-CoV-2 modulate lysosomal and cytosolic Ca2+ signaling for host cell entry and replication. Moreover, we delve into the recent studies, which have demonstrated the potential of several FDA-approved drugs targeting Ca2+ handling machinery in inhibiting viral infections. Importantly, we discuss the prospective of targeting intracellular Ca2+ signaling for better management and treatment of viral pathogenesis including COVID-19. Finally, we highlight the key outstanding questions in the field that demand critical and timely attention.
Keywords: Virus, Calcium, STIM1/Orai1, TPC2, VGCC, COVID-19
1. Introduction
Viral infections are one of the most common causes of human illness worldwide (Burrell et al., 2017; Parvez and Parveen, 2017). Several viral infections are endemic in nature while some others can lead to pandemic diseases such as novel coronavirus disease (COVID-19). Viruses utilize host cell molecular machinery for driving their replication and dissemination. Therefore, virus-host cell interaction and subsequent modulation of host cell signaling pathways is a hallmark of viral pathogenesis (Rouse and Sehrawat, 2010). One of the most commonly dysregulated host cell signaling cascades during viral infections is cellular calcium (Ca2+) dynamics (Zhou et al., 2013). Ca2+ is a versatile second messenger that regulates a variety of cellular functions ranging from cell survival, proliferation, transcriptional regulation to apoptosis (Parys and Guse, 2019; Tanwar and Motiani, 2018). It is important to note that cells can neither synthesize nor metabolize Ca2+ and it is the complex interplay of Ca2+ handling machinery (Ca2+ channels, pumps, ATPases etc.) that regulates cytosolic and organellar Ca2+ homeostasis. In the last couple of decades, several new channels, uniporters, Ca2+ handling proteins etc. have been discovered. Especially, the proteins involved in a) maintaining mitochondrial Ca2+ dynamics b) mediating Ca2+ influx across plasma membrane (PM) in response to decrease in intracellular Ca2+ stores and c) modulating Ca2+ signaling in the endo-lysosomal compartments have gained significant attention in the last 10–15 years (Bagur and Hajnóczky, 2017; Parys and Guse, 2019).
Since Ca2+ signaling regulates plethora of cellular functions, it is not surprising that viruses hijack host cell Ca2+ signaling cascades for its own benefit. Indeed, a variety of viruses takeover host cell Ca2+ handling machinery for driving viral infection and pathogenesis. Moreover, viruses induce changes in cytosolic Ca2+ concentration to activate Ca2+ dependent enzymes for promoting their replication (Chen et al., 2019). In this review, we will provide a brief overview of the viral life cycle and cellular Ca2+ signaling. We will then discuss the literature demonstrating that viral proteins can interact and/or modulate activity of host cell Ca2+ handling machinery. Further, we will deliberate upon how viruses target organelle specific Ca2+ signaling for facilitating their entry and replication, which in turn determines viral pathogenesis. Importantly, we will prudently discuss the recent findings demonstrating the therapeutic potential of FDA-approved drugs targeting Ca2+ handling proteins in curtailing viral infections. Finally, we will provide future perspectives and highlight key outstanding questions in the field that demand critical and timely attention.
2. Viral life cycle
Viruses are microscopic obligatory intracellular parasites. Viruses live as a completely assembled virus particles known as virions (Gelderblom, 1996). The typical virion size is in the range of nanometers (Claverie, 2008; Carter, 2012). The virions are made up of two fundamental components: nucleic acid (single-stranded or double-stranded RNA or DNA) and a capsid protein coat. The capsid protects the viral genome from nucleases. Further, it helps in virion attachment to the specific receptors on the host cell during infection. The virions with an envelope are called enveloped viruses (eg. Human immunodeficiency viruses, Herpesviruses etc.), while those without an envelope are called naked viruses (eg. Papillomavirus, Parvovirus etc.).
For their replication and transmission, viruses must gain entrance into target cells and hijack the host cellular machinery. The various steps involved in the transmission of viruses inside cells are collectively referred as the “virus life cycle”. The virus life cycle can be divided into three stages i.e. entry, genome replication and exit.
2.1. Entry of the viral component
The viral entry into a host cell requires the identification of the viral receptor (present on host cell) by the virion. There are four stages of viral entry: attachment, penetration, cytoplasmic trafficking, and uncoating (Ryu, 2017).
2.1.1. Attachment
Virion binds to specific receptors on the surface of the host cell through one or more of its surface proteins. The virion-recognition of its receptor is highly specific.
2.1.2. Penetration
After attachment of the virus on host cell, the next step is penetration into the cytoplasm. The enveloped viruses use either of the following two pathways for penetrating into host cell: direct fusion or receptor-mediated endocytosis.
Direct fusion: It is a process in which two membranes (i.e., a viral envelope and a cell membrane) fuse together. The viral nucleocapsid is transmitted directly to the cytoplasm, leaving the viral envelope behind on the PM. For example: Retroviruses (Ryu, 2017).
Receptor-mediated endocytosis: Majority of the animal viruses enter the host cells through endocytosis pathway. Viruses can use different endocytosis mechanisms. The most common route is clathrin-mediated endocytosis. While some viruses utilize caveolin and lipid raft-dependent pathways (Liu, 2014).
2.1.3. Intracellular trafficking
After successful penetration, the virus particles need to get to an appropriate location within the cell for genome replication. This process is called intracellular trafficking (Ryu, 2017).
2.1.4. Uncoating
The uncoating is the removal of the capsid for releasing virus genome in the host cell. Depending on the virus, the uncoating can happen at one of the several sites. For example: at the cell surface, within cytoplasm, at the nuclear pore or inside the nucleus (Carter, 2012).
2.2. Viral replication
The replication strategy varies significantly between the different family of viruses (Liu, 2014). Regardless of the genomic composition and replication strategy, soon after infection the viral genome is expressed as functional messenger RNA (mRNA). This guides the host cell translational machinery to generate viral proteins.
2.3. Viral exit/egress
The viral exit from host cell can be classified into three steps: capsid assembly, release and maturation.
2.3.1. Capsid assembly
The capsid assembly can be divided into two steps: capsid assembly and genome packaging. These two mechanisms may occur sequentially or concurrently, depending on the virus.
2.3.2. Release
In the case of naked viruses, viral particles are released through cell lysis of the infected host cells. Therefore, post cell membrane disruption, no defined escape mechanism is required in such cases. While in the case of enveloped viruses, a mechanism in which the capsids are surrounded by a lipid bilayer takes place prior to their release (Ryu, 2017).
2.3.3. Maturation
Maturation is the last step of the viral particle assembly that occurs extracellularly after the release of viruses from host cells. Importantly, viral particles acquire infectivity through the maturation process.
In the following sections, we will deliberate upon the molecular choreography through which viruses perturb host cell Ca2+ homeostasis for supporting their life cycle. First of all, we will provide a brief overview of intracellular Ca2+ signaling and organellar Ca2+ dynamics. Subsequently, we will discuss the viral targeting of Ca2+ homeostasis in various cellular compartments individually.
3. Intracellular calcium signaling
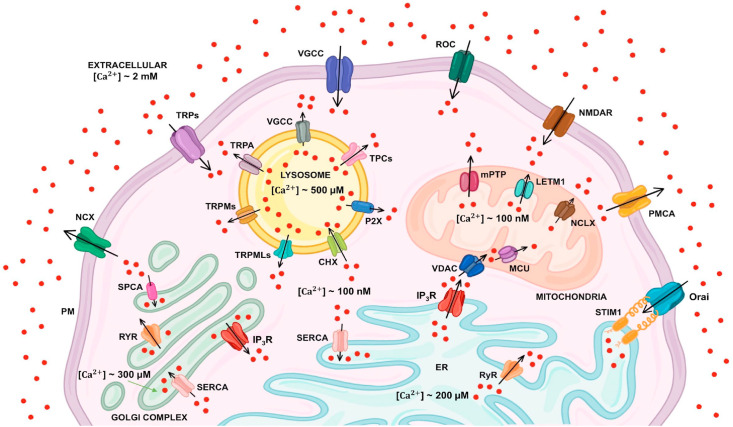
Ca2+ is a versatile second messenger involved in regulating wide array of cellular processes including proliferation, gene transcription, aerobic metabolism, muscle contraction, exocytosis and synaptic plasticity (Parys and Guse, 2019). Under resting conditions, cells maintain cytosolic Ca2+ concentrations (~100 nM) via efficient coordination between the extracellular milieu (Ca2+ concentrations ~2 mM) and intracellular Ca2+ stores (Ca2+ concentrations ~100–200 μM) (Bagur and Hajnóczky, 2017; Clapham, 2007). The intracellular Ca2+ homeostasis is maintained by various Ca2+ channels and pumps present on PM and intracellular organelles such as endoplasmic reticulum (ER), mitochondria, Golgi, and lysosomes. PM has several Ca2+-permeable channels and pumps such as voltage-gated calcium channels (VGCC), store-operated channels (SOC), receptor-operated channels (ROC) such as transient receptor potential channels (TRP), plasma membrane Ca2+ ATPase (PMCA) and the Na+/Ca2+ exchanger (NCX) (Bagur and Hajnóczky, 2017; Parys and Guse, 2019). The PM localized VGCC, SOC and ROC mediate Ca2+ entry from the extracellular milieu to cytosol. These PM resident channels are activated by the specific stimuli. The VGCCs are stimulated by membrane depolarization. These channels are found mostly in excitable cells (Atlas, 2014). The activation of these channels regulates various physiological functions such as excitation, contraction and relaxation of muscles, immune-protection and synaptic transmission among others (Catterall, 2011). Likewise, ROC are stimulated by external agonists, or intracellular messengers. TRP channels are major constituents of ROC and play an important role in decoding environmental stimuli. TRP channels are non-specific cationic channels that can mediate Ca2+ influx into the cells. The mammalian TRP channel superfamily is classified into six subfamilies based on sequence homology: TRPC (Canonical), TRPV (Vanilloid), TRPM (Melastatin), TRPA (Ankyrin), TRPML (Mucolipin), and TRPP (Polycystic) (Samanta et al., 2018). While SOC are stimulated by the depletion of ER Ca2+ stores (Smyth et al., 2010). The two key players responsible for SOC channels activation are the Orai channels on the PM and ER Ca2+ sensor STIM1 (stromal interaction molecule 1). The depletion of ER Ca2+ stores is sensed by STIM1 leading to Orai channels opening and subsequent Ca2+ entry (Smyth et al., 2010; Tanwar et al., 2020). On the other hand, PMCA and NCX mediate Ca2+ extrusion from cytosol to extracellular space.
Ca2+ is released from the internal stores via the IP3 receptor (IP3R) and the ryanodine receptors (RyR) present on ER (Stutzmann and Mattson, 2011). IP3R is activated by inositol-1,4,5-triphosphate (IP3) which leads to Ca2+ efflux from the stores. While the RyR releases Ca2+ from intracellular stores upon excitation and contraction in cardiac and skeletal muscles (Lanner et al., 2010). Ca2+ is pumped back into ER through sarcoplasmic/endoplasmic reticulum Ca2+ ATPase (SERCA). The Ca2+ released from ER can be further readily taken up by mitochondria especially at sites in close proximity to the ER, known as MAMs (mitochondria associated membranes) (Csordás et al., 2006; Rizzuto et al., 1998; Sharma et al., 2020; Vecellio Reane et al., 2020). At MAMs, Ca2+ can easily pass through outer mitochondrial membrane to reach inter-membrane space (IMS) via non-selective voltage-dependent anion channels (VDACs) (Madesh and Hajnóczky, 2001). From IMS, Ca2+ uptake into the mitochondrial matrix is mediated via a highly Ca2+ selective channel known as Mitochondrial Ca2+ Uniporter (MCU) (Baughman et al., 2011; De Stefani et al., 2011). Mitochondrial Ca2+ regulates Ca2+-dependent dehydrogenase enzymes, which are involved in tricarboxylic acid cycle (TCA) cycle thereby meeting cellular energy demand via ATP synthesis (Hajnóczky et al., 1995). However, Ca2+ overload within the mitochondrial matrix leads to apoptosis (Mammucari et al., 2018; Parks et al., 2018; Rasola and Bernardi, 2011; Tanwar et al., 2021). Ca2+ extrusion from mitochondrial matrix is majorly mediated through mitochondrial Ca2+ efflux proteins i.e. mitochondrial Na+/Ca2+/Li+ exchanger (NCLX) (Palty et al., 2010) and leucine zipper EF hand-containing transmembrane 1 (Letm1) (Jiang et al., 2009). Further, lysosomes can also regulate Ca2+ homeostasis by acting as Ca2+ stores (Patel and Docampo, 2010; Patel and Muallem, 2011). Ca2+ entry into endo-lysosomes is mediated by Ca2+/H+ exchangers (CHX) in an H+-dependent manner (Christensen et al., 2002; Melchionda et al., 2016). While, Ca2+ extrusion from the endo-lysosomes is mediated through the two-pore channel (TPCs) (Patel, 2015; Wang et al., 2012), TRPMLs (Cheng et al., 2010), TRPA1 and TRPM2 (Yang et al., 2018). The intracellular cues such as nicotinic acid adenine dinucleotide phosphate (NAADP) mobilizes Ca2+ from lysosomes through TPCs (Calcraft et al., 2009; Patel, 2015). In addition to ER and lysosomes, Golgi apparatus (GA) also serves as Ca2+ store (Dolman and Tepikin, 2006; Patel and Muallem, 2011). In membranes of GA SERCA, IP3Rs, RyRs and SPCA (secretory pathway calcium ATPase) are present. IP3 binding to IP3R releases Ca2+ from the Golgi. Ca2+ uptake in Golgi is mediated via SERCA and SPCA (Pizzo et al., 2011). The Ca2+ homeostasis within Golgi regulates cytosolic Ca2+ signaling. Taken together, Ca2+ dynamics within all these intracellular organelles (ER, mitochondria, lysosomes and Golgi) and the various PM Ca2+ channels crosstalk with each other for regulating cellular Ca2+ signaling (Raffaello et al., 2016) (Please refer Fig. 1 for the schematic illustration of the cellular Ca2+ handling toolkit). Importantly, dysregulation in the intracellular Ca2+ homeostasis is associated with several pathophysiological conditions including viral infections and pathogenesis. In the following sections, we will discuss the role of intracellular Ca2+ signaling in viral infections. Further, we will deliberate upon the molecular pathways adopted by viruses for hijacking host cell Ca2+ handling machinery to enhance viral pathogenesis.
Fig. 1.
Schematic representation of cellular Ca2+handling machinery. Ca2+ homeostasis is maintained by various Ca2+ channels and pumps present on plasma membrane (VGCC, SOC, ROC, PMCA, TRP, NCX etc.) and different intracellular compartments such as ER, mitochondria, Golgi, and lysosomes. The PM localized Ca2+ channels and pumps mediate Ca2+ entry (as shown by black arrows) from the extracellular milieu to cytosol. The IP3R and the RyR mediate the release of Ca2+ from ER and Golgi. SERCA pumps back Ca2+ into ER and Golgi from cytosol. The SPCA mediates Ca2+ entry into the Golgi from cytosol. VDACs and MCU mediate Ca2+ entry into the mitochondria while NCLX and Letm1 facilitate Ca2+ extrusion from mitochondrial matrix. The CHX on lysosomes mediates Ca2+ entry into the lysosomes while TPCs, TRPMLs, TRPMs along with others facilitate Ca2+ extrusion from the lysosomes. Ca2+ ions are shown as red dots. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Viral infections and perturbations in the Ca2+ signaling across PM
Host cell ion channels including Ca2+ channels are frequently targeted by viruses for their entry and replication (Charlton et al., 2020). In this section, we will discuss the molecular mechanisms through which viruses take over the PM Ca2+ handling machinery for their benefit.
4.1. Voltage gated Ca2+ channels (VGCC)
VGCC are one of the most well studied viral targets. The availability of highly specific FDA-approved inhibitors of VGCC (e.g., verapamil and nifedipine) has further helped in evaluating their significance in viral pathogenesis. Epstein-Barr virus (EBV) causes a range of malignancies including Burkitt's lymphoma and nasopharyngeal carcinoma. EBV infection induces an increase in cytoplasmic Ca2+ in B lymphocytes due to augmented Ca2+ inflow from the extracellular space (Zhou et al., 2009). The pharmacological experiments using VGCC blockers (verapamil or diltiazem) showed that EBV-induced human B cell activation is inhibited either upon incubation in Ca2+-free medium or after treatment with VGCC blocking drugs (Dugas et al., 1989). On the contrary, no change in EBV-induced B cell activation was noted when the intracellular Ca2+ concentration was decreased with Ca2+ chelator BAPTA. This implicates a critical role for VGCC in EBV induced increase in intracellular Ca2+ required for B cells activation (Dugas et al., 1989).
Similarly, Nugent et al. reported that VGCC blocker Verapamil prevents influenza virus replication in canine kidney cells and in murine pulmonary macrophages (Nugent and Shanley, 1984). Recently, it was demonstrated that Influenza A virus (IAV) glycoprotein hemagglutinin (HA) binds to the Cav1.2 voltage-dependent Ca2+ channel (Fujioka et al., 2018). This leads to induction of intracellular Ca2+ oscillations and subsequent viral entry and replication. It was further reported that IAV entry is hampered by both pharmacological blocking and molecular knockdown of Cav1.2 (Fujioka et al., 2018). The small GTPase RhoA is reported to operate downstream of IAV induced Ca2+ response, which in turn regulates both clathrin-mediated and clathrin-independent endocytosis of virus. This signaling cascade is a key mechanism for regulating IAV internalization and infection (Fujioka et al., 2013).
HIV belongs to retroviruses family comprising of RNA as their genetic material (Karn, 2013). Two HIV-1 proteins namely the membrane glycoprotein gp120 and the transcriptional trans-activator Tat induce cytoplasmic Ca2+ elevation by modulating VGCC activity. These proteins have been shown to do so in both excitable (such as neuronal cells) and non-excitable cells (such as epithelial and immune cells) (Zhou et al., 2009). HIV-1 Tat interacts with L-type Ca2+ channels expressed by dendritic cells and inhibits cellular functions (Zocchi et al., 1998). Likewise, HIV-1 Tat targets L-type Ca2+ channels on NK cells, which leads to failure of NK cell-mediated cytotoxicity (Zocchi et al., 1998).
Flaviviruses include several pathogenic viruses such as Japanese encephalitis virus (JEV), Yellow Fever virus (YFV), Zika virus (ZIKV), West Nile virus (WNV), Dengue virus (DENV) etc. Excitingly, an unbiased screening of FDA-approved drugs for identifying the inhibitors of JEV infection led to identification of three VGCC blockers i.e. manidipine, cilnidipine, and benidipine hydrochloride as highly potent blockers of JEV infection (Wang et al., 2017). In the high-throughput screening assay, these three drugs robustly inhibited the viral (wild-type JEV strain AT31) infection, which was validated by a battery of subsequent experiments (Wang et al., 2017). Since flaviviruses are quite similar in structure and pathogenesis, authors first used JEV as a model system for the drug screening and later on validated antiviral activities of VGCC blockers in ZIKV, WNV, and DENV type 2 (DENV-2) (Wang et al., 2017). Mechanistically, these drugs most likely inhibit flavivirus genome replication as they imparted complete inhibition even when cells were treated with these drugs post viral infection. This suggests that Ca2+ influx mediated by VGCC is critical for flavivirus genome replication.
Likewise, VGCC were identified as a therapeutic target against New World arenavirus (NWA) infection (Lavanya et al., 2013). Lavanya et al. performed a high-throughput siRNA screening with pseudo-typed virus for identifying host cell proteins of high therapeutic value. Both pharmacological blocking and siRNA mediated silencing of VGCC subunits decreased viral entry into host cells. Moreover, the authors evaluated the role of VGCC in viral infection in vivo. They used the vaccine strain of the Junin virus for performing in vivo studies in mice. Importantly, an FDA-approved VGCC targeting drug, gabapentin robustly diminished the viral infection in vivo thereby corroborating an important role for VGCC in NWA entry and infection (Lavanya et al., 2013). Recently, same group reported that the haploinsufficiency of one of the chains of VGCC imparts protection to human cells and mice against NWA infections (Sarute and Ross, 2020). It was demonstrated that NWA target VGCC for host cell entry and mutation in α1s chain provides significant protection against vaccine strain of the Junin virus in vivo. Further, the heterozygous mice carrying this mutation showed higher efficacy of gabapentin in comparison to control littermates (Sarute and Ross, 2020). Taken together, above discussed studies elegantly demonstrate that VGCC are attractive therapeutic targets for stalling a variety of viral infections. With a battery of FDA-approved drugs targeting VGCC, it is an area that demands immediate and critical attention for fighting against viral infections.
4.2. Store operated Ca2+ channels (SOCC)
SOCC are stimulated by the loss of Ca2+ from ER, which is physiologically caused by activation of a variety of cell surface receptors (Chen et al., 2019; Prakriya and Lewis, 2015). Recently, Yao et al. reported that the Hepatitis b virus (HBV) non-structural protein X (HBx) increases intracellular Ca2+ by interacting with SOCC protein Orail (Yao et al., 2018). The authors performed confocal microscopy, co-immunoprecipitation and GST-pull-down assay for demonstrating that Orail C-terminus interacts with HBx (Yao et al., 2018). This interaction enhances store operated Ca2+ entry, thereby elevating cytosolic Ca2+ levels. It is important to note that HBx mediated modulation of intracellular Ca2+ dynamics is critical for HBV replication. Further, HBx regulates viral pathogenesis, host cell apoptosis, transcription and cell signaling (Bouchard and Navas-Martin, 2011). Consequences of HBx expression vary according to the cell type (Bouchard and Navas-Martin, 2011). Recently, a direct association between HBx mediated cytosolic Ca2+ elevation (via SOCC) and stimulation of HBV replication was reported (Casciano et al., 2017). It was demonstrated that expression and activity of SOCC components remain unaltered but Ca2+ entry via SOCC is prolonged. The persistent high cytosolic Ca2+ levels stimulate downstream effector proteins, which in turn creates favorable cellular environment for HBV replication.
In case of retroviruses, pX open reading frame I encoding two proteins i.e. p12I and p27I is necessary for developing persistent infection (Ding et al., 2002). The chemical inhibition experiments using SOCC inhibitor indicated that SOCC contribute to the p12I-mediated Nuclear Factor of Activated T-cells (NFAT) activation (Ding et al., 2002). The NFAT activation in turn leads to the replication of retroviruses (Ding et al., 2002). Similarly, rotavirus (RV) infection enhances cytosolic Ca2+ concentration, which is critical for virus replication (Chang-Graham et al., 2019). The pharmacological experiments demonstrated that both RV infection and RV nonstructural protein 4 (NSP4) induce a rise in cytoplasmic Ca2+ levels via SOCC (Díaz et al., 2012). Subsequently, Hyser et al. reported that in RV infected cells, STIM1 is constitutively active and STIM1 puncta are colocalized with the Orai1 (Hyser et al., 2013). The wild-type NSP4 was shown to activate STIM1, which results in Ca2+ influx. But an NSP4 viroporin mutant failed to cause STIM1 oligomerization and did not activate the SOC entry (SOCE) (Hyser et al., 2013). Importantly, STIM1 knockdown substantially decreased RV yield, suggesting that STIM1 plays a vital role in RV replication (Hyser et al., 2013). Recently, it was demonstrated that the inhibition of SOCE or Orai1 channel attenuates the cytosolic Ca2+ spikes critical for viral replication (Chang-Graham et al., 2019). Taken together, these studies establish an important role of SOCC mediated increase in cytosolic Ca2+ in RV replication.
Likewise, it was recently demonstrated that DENV infection in human hepatic cells leads to activation of SOC channels and rise in cytosolic Ca2+ levels via Orai1(Dionicio et al., 2018). It was observed that DENV infection induces ER Ca2+ store depletion followed by co-localization of STIM1 and Orai1 leading to Ca2+ influx via Orai1. Further, the treatment of infected cells with Ca2+ chelators (BAPTA-AM and EGTA) or SOC inhibitor (SKF96365) substantially reduced the viral titers, thereby emphasizing (Dionicio et al., 2018) that SOC mediated Ca2+ influx plays an important role in DENV replication. Similarly, Han et al. demonstrated that hemorrhagic fever viruses like the filoviruses (Ebola and Marburg) and arenaviruses (Lassa and Junín viruses) induce rise in cytosolic Ca2+ levels via STIM1/Orai1-mediated SOCE (Han et al., 2015). The authors silenced STIM1/Orai1 as well as pharmacologically blocked SOCE for demonstrating a critical role of this pathway in enhancing cellular Ca2+ levels upon filo and arenavirus infection. Importantly, STIM1 and Orai1 regulate viral budding and pathogenesis of Ebola, Marburg, Lassa and Junín viruses (Han et al., 2015) thereby suggesting that SOCE could be targeted for controlling the pathogenesis of hemorrhagic fever viruses. In summary, STIM1-Orai1 mediated SOCE plays a critical role in viral replication and budding in a battery of different viral families.
4.3. Receptor operated Ca2+ channels (ROCC)
The role of ROCC in viral infections remains poorly understood. The infection of HIV-1 coat protein gp120 in rat retinal ganglion cells and hippocampal neurons induces significant increase in the intracellular Ca2+ leading to cytotoxicity (Nath et al., 1995). In gpl20-mediated biological responses, glutamate is involved (Nath et al., 1995). The gp120 activity is blocked, at least in part, by NMDA-selective excitatory amino acid receptor antagonists, Ca2+ channel inhibitors (like dantrolene) and nitric oxide synthase inhibitors (Nath et al., 1995). Recent studies have reported that the two Tat protein variants, Tat 1–72 and Tat 1–86 induce rapid rise in intracellular Ca2+ in rat hippocampus organotypic slice cultures (Self et al., 2004). Importantly, NMDA receptor antagonist dizocilpine significantly attenuated changes in intracellular Ca2+ levels induced by Tat 1–72 or Tat 1–86. This suggests that targeting NMDA receptor could help in managing neurotoxic effects associated with HIV infection (Self et al., 2004). More recently, transient receptor potential vanilloid 4 (TRPV4) was reported to regulate replication of a variety of viruses. It was demonstrated that TRPV4 is frequently expressed after viral infection, exhibits some spontaneous activity, and induces intracellular Ca2+ signals (Doñate-Macián et al., 2018). TRPV4 binds and controls the functioning of the DEAD-box RNA helicase DDX3X. DDX3X is attacked by many RNA viruses. TRPV4-mediated Ca2+ influx leads to release of DDX3X from the channel and its subsequent nuclear translocation (Doñate-Macián et al., 2018). The molecular knockdown or pharmacological inhibition of TRPV4 reduces DDX3X-dependent functions such as translation and export of viral proteins (Doñate-Macián et al., 2018). Additionally, TRPV4 mediates nuclear aggregation of DDX3X in cells infected with Zika virus. Importantly, TRPV4 targeting decreases dengue, hepatitis C and Zika virus infectivity (Doñate-Macián et al., 2018).
4.4. Plasma membrane Ca2+ ATPase (PMCA) and sodium-calcium exchanger (NCX)
As major Ca2+ extruders, PMCA and NCX take Ca2+ out of the cell, thereby helping in maintaining cytosolic Ca2+ homeostasis. Viruses can directly or indirectly regulate the activity of PMCA and/or NCX for inducing changes in the cytosolic Ca2+ levels. For example, the ectopic expression of the HBx protein causes elevation of the cytosolic Ca2+ by modulating PMCA activity (Chami et al., 2003; Zhou et al., 2013). HBV infection did not modify the levels of PMCA-1, 2, 3, or 4 mRNAs suggesting that HBV does not influence PMCA expression at least at the mRNA level (Casciano et al., 2017). Mechanistically, it was demonstrated that HBx overexpression induces caspase-3-mediated cleavage of PMCA while blockage of caspase activity abolishes PMCA cleavage (Chami et al., 2003). Therefore, HBx regulates PMCA activity by inducing its cleavage. This in turn decreases Ca2+ efflux from the cytosol to the extracellular environment resulting in elevated cytoplasmic Ca2+ levels (Casciano et al., 2017). The rise in cytosolic Ca2+ concentration augments HBV replication and enhances the core assembly of HBV (Zhou et al., 2013). Similarly, RV infection dysregulates cellular Ca2+ homeostasis, which in turn helps in its replication and diarrheal pathogenesis (Chang-Graham et al., 2020). RV NSP4 is responsible for inducing perturbations in host cell Ca2+ dynamics (Sastri et al., 2016). Diaz et al. reported that RV infection or just NSP4 expression leads to reverse mode activation of NCX thereby increasing the cytosolic Ca2+ concentration. This suggests that RV targets NCX and stimulates its functioning in reverse mode, which in turn results in rise in cellular Ca2+ levels required for RV replication (Díaz et al., 2012).
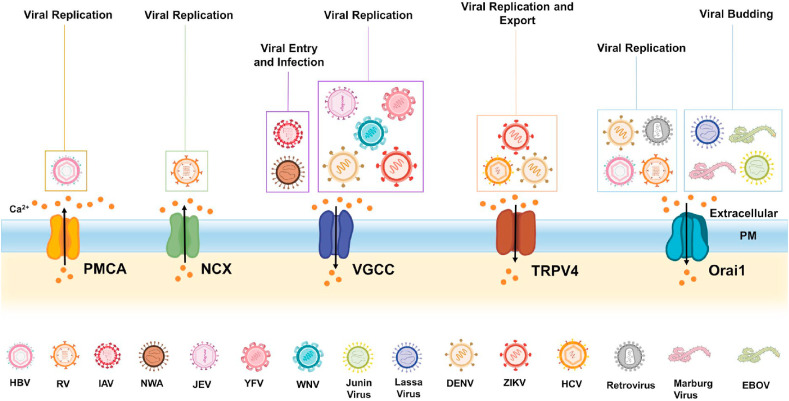
Taken together, above studies demonstrate that a variety of viruses hijack different types of PM Ca2+ handling proteins for the host cell entry and replication (please refer Fig. 2 for the diagrammatic illustration). Most of these viruses enhance cytosolic Ca2+ concentration for driving their replication. However, the molecular choreography connecting rise in cytosolic Ca2+ levels to augmented viral replication remains poorly understood. Certainly, it is critical to enrich this knowledge base so that it could be utilized in the future for developing therapeutic strategies in curtailing the replication of such viruses.
Fig. 2.
PM Ca2+handling toolkit and viral infections. A variety of viruses target PM Ca2+ handling machinery for increasing cytosolic Ca2+ levels and that in turn typically helps in driving viral replication and export. Viruses like JEV, DENV, ZIKV, YFV, WNV target VGCC for driving viral replication. Similarly, RV, HIV, HBV stimulate Orai1 mediated Ca2+ influx for enhancing replication. Whereas, ZIKV, HCV and DENV activate TRPV4 dependent signaling cascade for potentiating their replication and exit from host cells. Viruses also target PM Ca2+ efflux routes such as PMCA and NCX. For example, HBV and RV target PMCA and NCX respectively, for elevating cytosolic Ca2+ levels and this in turn aids in viral replication.
5. ER Ca2+ homeostasis and viral infections
Viruses exploit ER to promote various steps of their life cycle (Romero-Brey and Bartenschlager, 2016). A variety of viruses target host cell ER Ca2+ stores for driving their replication and enhancing viral infection. For example RV infection leads to elevation of cytosolic Ca2+ levels in the host cell because of increased efflux of Ca2+ from ER into cytosol followed by Ca2+ influx through PM (Hyser and Estes, 2015; Pérez et al., 1999; Tian et al., 1994). The virus encoded NSP4 protein has been reported to be responsible for the changes in Ca2+ dynamics (Hyser and Estes, 2015; Tian et al., 1994). NSP4 perturbs Ca2+ homeostasis by at least two mechanisms: viroporin function and endotoxin activity (Sastri et al., 2016). NSP4 is localized in the ER and functions as a viroporin that depletes ER Ca2+ stores. This leads to rise in cytosolic Ca2+ levels, which is important for replication of RV (Díaz et al., 2008; Hyser et al, 2010, 2013; Pham et al., 2017; Tian et al., 1995). Secondly, NSP4 possesses an enterotoxin domain (amino acids 114–135), which can induce chloride secretion from enterocytes due to PLC-dependent transient rise in cytosolic Ca2+(Dong et al., 1997; Morris et al., 1999). Recently, Chang-Graham et al. characterized RV modulated Ca2+ signaling by stably expressing genetically encoded Ca2+ indicators targeting cytosol and/or ER in cell lines and human intestinal enteroids (HIEs) (Chang-Graham et al., 2019). The virus induced increase in cytosolic Ca2+ exhibits spatial and temporal complexity. The authors measured alterations in cytosolic Ca2+ in single cells over a long time to understand if RV dysregulates Ca2+ levels during infection and its progression. They observed that release of Ca2+ from the ER is the prime cause of RV-induced cytosolic Ca2+ spikes. Importantly, modulation of Ca2+ signals by RV can direct the host cell either towards cell death or cell survival/proliferation. RV infection activates discrete, transient Ca2+ signaling events rather than a steady rise in cytosolic Ca2+ levels (Chang-Graham et al., 2019). This acts as a pro-survival signal by stimulating PI3K and calcineurin-dependent NFAT transcription factor. As Ca2+ levels increase drastically during the later stages of viral life cycle, cell damage and death occur (Crawford et al., 2012; Pérez et al., 1998; Zhu et al., 2017). In summary, RV targets ER Ca2+ signaling for promoting its replication and assembly.
Human cytomegalovirus (HCMV) encodes several genes (US21, UL37, US28) that can regulate host cell Ca2+ signaling (Dunn and Munger, 2020). US21 gene encodes a Ca2+ permeable channel, a viroporin, that depletes the ER Ca2+ stores (Luganini et al., 2018). This in turn decreases susceptibility of the cell to apoptosis. Likewise, UL37 is responsible for mobilizing Ca2+ from ER to cytosol thereby enhancing ATP production, which is used to meet energy demands of the virus (Sharon-Friling et al., 2006). On the contrary, US28 (a viral GPCR) activates caspase that promotes apoptosis (Gao and Murphy, 1994; Pleskoff et al., 2005). This aids in disease progression in certain specific cell types. Miller et al. reported that US28 triggers PLC-β signaling which leads to release of intracellular Ca2+ in smooth muscle and endothelial cells but not in glioblastoma cells (Miller et al., 2012). Further, UL146 encodes vCXCL1 (a viral chemokine), which is an agonist for human chemokine receptors (CXCR1 and CXCR2) and it induces intracellular Ca2+ release (Penfold et al., 1999; Wang et al., 2003). Additionally, interaction between HCMV and epidermal growth factor receptor (EGFR) is responsible for internalization of the virus. As a consequence, intracellular Ca2+ levels are increased (Wang et al., 2003) and that aids in viral dissemination through the host. In summary, HCMV gene products modulate ER Ca2+ signaling via multiple cascades eventually regulating host cell growth, apoptosis and inflammatory responses.
EBV, an oncogenic gamma-herpes virus infects normal resting B lymphocytes and leads to immortalization of the cells (Papp et al., 2020). EBV has the ability to immortalize primary naïve B lymphocytes, which results in various forms of lymphomas (Dellis et al., 2009). EBV infection leads to a rise in resting cytosolic Ca2+ levels (Chami et al., 2006; Scharenberg et al., 2007) by modulating the expression and activity of SERCA3 (Dellis et al., 2009). EBV protein, LMP-1 (latent membrane protein-1) brings about the down-regulation of SERCA3. This process eventually results in B cell immortalization associated with EBV infection.
Ca2+ signaling plays an important role in HIV-1 protein trafficking, release and viral replication. HIV-1 gp120 and Tat are responsible for IP3 generation (Ehrlich et al, 2011, 2014) whereas HIV Nef acts as an agonist for IP3R (Ehrlich et al., 2010; Manninen and Saksela, 2002). The activation of IP3R is required for efficient trafficking of HIV-1 Gag and for viral release (Ehrlich et al., 2010). Furthermore, the studies from Ehrlich et al. indicate that Ca2+ is required for Gag assembly, which is facilitated by IP3R mediated ER Ca2+ release. The authors demonstrated that Gag regulates both ER Ca2+ release and store refilling (Ehrlich et al., 2014). Interestingly, Gag stimulates IP3R and distributes them towards the cell periphery (Chalmers et al., 2006; Vermassen et al., 2004). Further, it is reported that functioning of PI(4,5)P2 as a PM anchor and PLC substrate are required for efficient HIV infection. Some of the ER released Ca2+ might keep activating PLC, which hydrolyses PI(4,5)P2 and provide a constant supply of IP3 and hence support continuous activation of IP3R (Rhee, 2001; Suh et al., 2008). Importantly, the increase in production of viral particles is observed upon ER Ca2+ release (Ehrlich et al., 2014). Similarly, HIV protein Nef plays an important role in AIDS pathogenesis (Manninen and Saksela, 2002). It acts as an agonist for IP3R and activates it directly (Ehrlich et al., 2010; Manninen and Saksela, 2002). This in turn induces ER Ca2+ release and enhances HIV replication (Manninen and Saksela, 2002).
The accumulation of HBV protein variants HBsAg and HBcAg in the ER (Hsieh et al., 2004; Lee et al, 2015a, 2015b) leads to release of ER Ca2+ into the cytosol and increases ROS production (Lee et al., 2015a). Moreover, ER Ca2+ stores are released upon activation by HCV (Wang et al., 2010). The released Ca2+ subsequently accumulates in the mitochondria and enhances ROS generation (Li et al., 2007; Piccoli et al., 2007; Sharma et al., 2020). The ability of HCV and HBV to deplete ER Ca2+ stores leads to hepato-carcinogenesis induction (Bartosch et al., 2009; Ivanov et al., 2017). Cheshenko et al. demonstrated that upon Herpes simplex virus (HSV) interaction with host cells, phosphoinositide-specific PLCγ is activated (Cheshenko et al., 2003). This triggers ER Ca2+ release downstream of PLCγ- pathway. It leads to activation of phosphorylation pathways, which aids in viral penetration and delivery of viral capsids to host cell nucleus. In a subsequent study, same group used confocal microscopy to demonstrate that viral penetration triggers rise in cellular Ca2+ via release of IP3 sensitive intracellular Ca2+ stores. Importantly, the abrogation of intracellular Ca2+ response prevented viral entry and infection. The inhibition of IP3R and chelation of intracellular Ca2+ inhibits viral infection. This suggests that IP3 induced Ca2+ release from intracellular stores plays an important in regulating HSV entry in the host cells (Cheshenko et al., 2007).
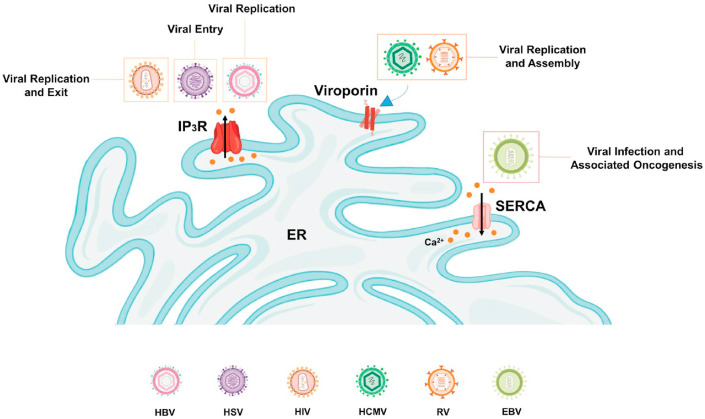
Taken together, above studies clearly exhibit that viral proteins target ER Ca2+ handling machinery for modulating ER and cytosolic Ca2+ levels. This in turn helps in viral entry, replication and exit thereby playing an important role in viral pathogenesis (Refer Fig. 3 for the pictorial summary). However, more mechanistic investigations are required to precisely decipher how changes in ER Ca2+ drive viral infections.
Fig. 3.
ER Ca2+homeostasis and viral infections. A number of viruses target ER Ca2+ handling toolkit, which in turn aids in all three stages of infection i.e. viral entry (eg. HSV), viral replication (eg. HIV and HBV) and viral exit (eg. HIV). Some viruses, such as RV and HCMV can induce ER Ca2+ efflux by forming viroporin in the ER membrane. This in turn helps in viral replication and assembly. While oncogenic EBV targets SERCA regulated ER Ca2+ homeostasis for driving viral infection and associated oncogenesis.
6. Mitochondrial Ca2+ dynamics and viral infections
Mitochondrial Ca2+ homeostasis is a critical regulator of host cell fate upon viral infection (Zhou et al., 2009). Mitochondria are the key targets for viruses as they are major player involved in regulating bioenergetics and apoptosis (Cavallari et al., 2018). Several studies have demonstrated that host cell mitochondrial Ca2+ signaling is altered during viral infections (Anand and Tikoo, 2013; Chaudhuri et al., 2021). Viruses modulate the Ca2+ flux across mitochondria to induce or prevent apoptosis (Panda et al., 2021). Host cells utilize apoptosis as an innate defense mechanism to control virus production and viral dissemination (Benedict et al., 2002). In contrast, viruses utilize an anti-apoptotic approach to evade host immune clearance. Interestingly, certain viruses induce apoptosis to aid viral dissemination (Zhou et al., 2009). Various types of viruses induce perturbations in mitochondrial Ca2+ signaling to aid persistent infection (Anand and Tikoo, 2013; Chaudhuri et al., 2021; Ohta and Nishiyama, 2011).
The HBx protein plays a critical role in replication of HBV and the development of liver cancer (Bouchard and Schneider, 2004). Several independent studies have reported localization of HBx to mitochondria (Clippinger and Bouchard, 2008; Henkler et al., 2001; Huh and Siddiqui, 2002; Takada et al., 1999). By utilizing aequorin-based recombinant probes, it was revealed that overexpression of HBx in HepG2 and HeLa cells leads to decrease in histamine induced mitochondrial Ca2+ uptake (Chami et al., 2003). Furthermore, the ectopic expression of HBx altered mitochondrial morphology i.e. round, fragmented and swollen mitochondria (Chami et al., 2003). Taken together, this study suggested that HBx induces apoptosis by modulating mitochondrial Ca2+ signaling. Several groups have reported a critical role for mitochondrial Permeability Transition Pore (mPTP) activity in HBV replication (Bouchard et al., 2001; Gearhart and Bouchard, 2010; McClain et al., 2007). An interesting report by Gearhart et al. implicated a role for mitochondrial Ca2+ signaling in replication of HBV (Gearhart and Bouchard, 2010). The authors demonstrated that HBx causes cell cycle arrest in G1 phase via mitochondria-dependent Ca2+ signaling. This in turn leads to stimulation of HBV replication (Gearhart and Bouchard, 2010). It was reported that mitochondrial Ca2+ dynamics regulates HBx activated HBV DNA replication (Bouchard et al., 2001). The treatment with cyclosporin A (CsA), a mPTP inhibitor reduces HBx activated HBV DNA replication (Bouchard et al., 2001). In an interesting study, Rahmani et al. described interaction between HBx and VDAC3 within mitochondrial membrane (Rahmani et al., 2000). The authors further reported altered mitochondrial membrane potential upon HBx expression in human hepatoma cells (Rahmani et al., 2000). Similarly, Yang et al. reported that HBx protein induces rise in cytosolic Ca2+ by modulating mitochondrial Ca2+ uptake (Yang and Bouchard, 2012). The authors demonstrated an increase in mitochondrial Ca2+ uptake in HBx-expressing cells. This increased mitochondrial Ca2+ uptake stimulates HBV replication (Yang and Bouchard, 2012).
The HCV infection is associated with various mitochondrial dysfunctions (Brault et al., 2013; Piccoli et al., 2006). Piccoli et al. revealed that expression of HCV protein leads to dysregulation of mitochondrial Ca2+ homeostasis in U2-OS human osteosarcoma cells (Piccoli et al., 2007). The rise in mitochondrial Ca2+ influx led to mitochondrial Ca2+ overload, which in turn results in altered bioenergetics, inhibition of complex I, decreased mitochondrial membrane potential, increased ROS and elevated nitro-oxidative stress. Importantly, these observations could be completely reversed upon inhibition of mitochondrial Ca2+ uptake by ruthenium red or Ru360 (Piccoli et al., 2007). Similarly, Gong et al. demonstrated the role of HCV nonstructural protein 5 A (NS5A) in altering mitochondrial Ca2+ homeostasis and inducing nuclear translocation of NF-κB and STAT-3 transcription factors (Gong et al., 2001). A decrease in NS5A-induced NF-κB activation was observed upon inhibition of mitochondrial Ca2+ uptake by ruthenium red (RR). Thus, suggesting a critical role for mitochondrial Ca2+ in regulating NS5A's ability to activate transcription factors (Gong et al., 2001). Likewise, Li et al. reported that HCV core protein enhances mitochondrial Ca2+ uptake and ROS generation by potentiating the activity of Ca2+ uniporter (Li et al., 2007). Further, the authors observed a rise in mitochondrial Ca2+ influx upon incubating mouse liver mitochondria with HCV core protein in vitro. This increased entry was inhibited by the mitochondrial Ca2+ uniporter inhibitor Ru360 but not with Na+/Ca2+ exchanger inhibitor or ROS scavengers. This suggests that the mitochondrial Ca2+ uniporter is one of the targets of HCV infection (Li et al., 2007). Interestingly, Quarato et al. reported that alisporivir (an analogue of mPTP inhibitor cyclosporine A and cyclophilin inhibitor) treatment gives protection against HCV-induced mitochondrial dysfunction (Quarato et al., 2012). The authors further demonstrated that alisporivir rescues HCV-induced increase in ROS production, depolarization of mitochondrial membrane potential and mitochondrial Ca2+ overload. Therefore, this study suggests a potential therapeutical value of alisporivir in managing HCV infections (Quarato et al., 2012).
A study by Moin et al. reported that hepatitis E virus open reading frame 3 (Orf3) protein provides protection to virus from mitochondrial depolarization and associated cell death (Moin et al., 2007). The authors reported that stable Orf3-expression leads to upregulation of VDAC. Further, siRNA mediated knockdown of Orf3 decreases VDAC expression. The authors showed a decrease in cell death in Orf3-expressing cells. This was associated with the reduction in OMM permeabilization via VDAC1 oligomerization and increase in Orf3 interaction with hexokinase I. This signaling cascade eventually prevents the release of cytochrome c and aids in cell survival, thereby allowing viral replication in these cells (Moin et al., 2007). Similarly, HCMV UL37 encodes a viral mitochondria-localized inhibitor of apoptosis (vMIA), which promotes cell survival by inhibiting a pro-apoptotic protein Bax (Arnoult et al., 2004; Goldmacher et al., 1999; Poncet et al., 2004). All the cited studies collectively recognize UL37 as a potent inhibitor of host cell apoptosis that in turn helps in continuous viral replication.
HIV viral proteins (Tat and gp120) induce mitochondrial impairment by perturbing processes such as mitochondrial dynamics, morphology and mitochondrial membrane potential (Rozzi et al., 2017). Kruman et al. reported that Tat induces apoptosis of cultured embryonic rat hippocampal neurons via caspase activation and mitochondrial Ca2+ overloading (Kruman II et al., 1998). The authors demonstrated that Tat induces rise in cytosolic Ca2+ leading to increase in mitochondrial Ca2+ uptake and generation of mitochondrial ROS. Furthermore, they showed protection against Tat-induced apoptosis with BAPTA-AM and ruthenium red (Kruman II et al., 1998) suggesting an important role for mitochondrial Ca2+ homeostasis in regulating host cell survival post HIV infection. Subsequent studies reported that Tat protein targets VDAC (Lecoeur et al., 2012). Lecoeur et al. demonstrated that Tat protein induces mitochondrial membrane permeabilization and inhibition of cytochrome c oxidase (COX) activity (Lecoeur et al., 2012). Importantly, it was shown that VDAC inhibition with 4,4′-diisothiocyanostilbene-2,2′-disulfonic acid (DIDS) leads to protection against Tat-induced mitochondrial membrane permeabilization (Lecoeur et al., 2012). Likewise, Brisac et al. demonstrated an important role for mitochondrial Ca2+ in poliovirus (PV) induced apoptosis of neuroblastoma cells (Brisac et al., 2010). The authors showed that PV infection leads to increase in cytosolic Ca2+. This in turn leads to rise in mitochondrial Ca2+ via VDACs and MCU. This increase in mitochondrial matrix Ca2+ concentration results in mitochondrial dysfunction and apoptosis in PV-infected cells (Brisac et al., 2010). It was further demonstrated that pretreatment of cells with ruthenium red or DIDS leads to reduction in cytochrome c release and PV-induced apoptosis (Brisac et al., 2010). Taken together, these studies suggest that HIV and PV target VDAC and MCU for inducing host cell apoptosis.
The group B coxsackieviruses (CVB) are ssRNA viruses that cause meningitis and myocarditis. The main pathogen leading to viral myocarditis is Coxsackievirus B3 (CVB3) (Wessler and Backert, 2011). Emerging data indicates that replication of CVB3 is correlated with apoptosis of the infected cardiac myocytes (Nie et al., 2020). In the CVB3-infected H9c2 cardiomyocytes, Ca2+ calmodulin-dependent kinase II (CaMKII) was activated by intracellular Ca2+ overload (Nie et al., 2020). Importantly, CVB3-induced CaMKII activation was reported to enhance mitochondrial Ca2+ uptake, activating mitochondrial-dependent apoptotic pathways. Further, CaMKII inhibition with a pharmacological inhibitor (KN-93) or its silencing by shRNA repressed cytochrome c release from mitochondria and abrogated CVB3-induced H9c2 apoptosis. While overexpression of the CaMKII activated mutant (CaMKII-T287D) increased CVB3-induced H9c2 apoptosis (Nie et al., 2020) thereby suggesting that mitochondrial Ca2+ dynamics play a critical role in CVB3 pathogenesis.
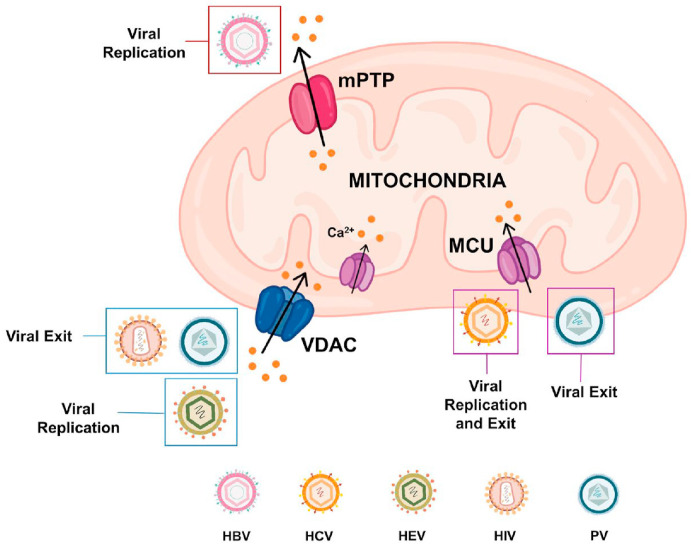
Collectively, above discussed studies establish that a number of different viruses perturb mitochondrial Ca2+ signaling either for enhancing their replication or for inducing host cell apoptosis which in turn aids in viral exit (Please refer to Fig. 4 for the diagrammatic summary). Recently, a high throughput screening of small molecules including NIH clinical collection drug library led to identification of highly specific drugs that can modulate mitochondrial Ca2+ dynamics (Arduino et al., 2017; Di Marco et al., 2020). Going forward, it would be interesting to evaluate the efficacy of these drugs in controlling viral infections associated with dysregulation of mitochondrial Ca2+ homeostasis.
Fig. 4.
Mitochondrial Ca2+dynamics and viral infections. Viruses act on mitochondrial Ca2+ dynamics for driving their replication and for inducing host cell apoptosis which in turn helps in viral exit. For example, HBV, HCV and HEV target mPTP, MCU and VDAC respectively for enhancing their replication. While HIV and PV stimulate VDAC and MCU activity thereby increasing mitochondrial Ca2+ concentration. The rise in mitochondrial Ca2+ levels leads to host cell apoptosis.
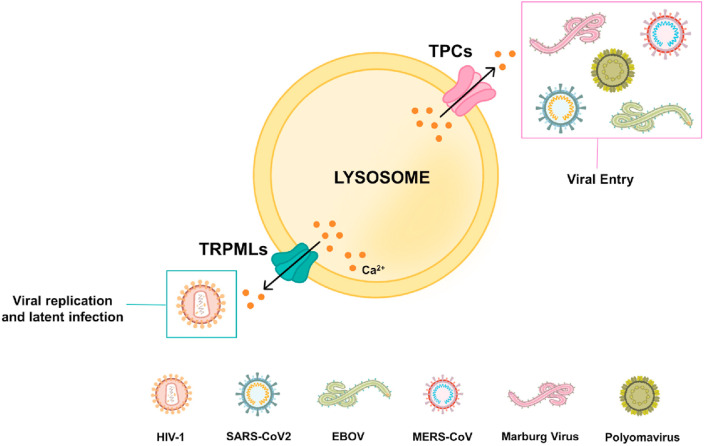
7. Lysosomal Ca2+ dynamics and viral entry
The lysosomal Ca2+ drives important cellular processes such as signal transduction, vesicular trafficking, autophagy, exocytosis etc. (Feng and Yang, 2016; Lloyd-Evans and Platt, 2011; Patel and Cai, 2015; Trivedi et al., 2020). Viruses target lysosomal Ca2+ handling machinery for entry into host cells. A fascinating study by Sakurai et al. reported an important role for endo-lysosomal TPCs in EBOV entry into host cells (Sakurai et al., 2015). The authors demonstrated that Ned19 (TPC inhibitor) and tetrandrine (NAADP antagonist) treatment reduces Ebola virus (EBOV) infection. A robust decrease in EBOV infection was observed in the mouse embryonic fibroblasts harvested from TPC1/2 knockout mice thereby confirming an essential role for TPCs in EBOV entry. Furthermore, tetrandrine treatment led to inhibition of Marburgvirus (distantly related filovirus) signifying a critical role of TPCs in filovirus infection (Sakurai et al., 2015). Independently, Simmons et al. reported that EBOV entry occurs through endolysosomes positive for two-pore Ca2+ channel 2 (TPC2) and Niemann–Pick C1 (NPC1) (Simmons et al., 2016). A recent study by Penny et al. identified FDA-approved blockers for TPC to inhibit viral entry into host cells (Penny et al., 2019). For identifying novel compounds targeting TPC, the authors virtually screened a library of around 1500 FDA-approved drugs by performing TPC2 structure-based virtual drug screening. It was observed that the drugs targeting dopamine and estrogen receptors bind to TPC pore. Further in vitro validation studies demonstrated that these drugs indeed inhibit endogenous NAADP-evoked Ca2+ release and TPC2 channel activity. Most importantly, these drugs led to a significant reduction in EBOV infection in HeLa cells. Taken together, these elegant studies established an important role for TPCs in EBOV entry and identified TPCs as an potential drug target against EBOV infection (Penny et al., 2019). More recently, Das et al. reported a role for Ca2+ in EBOV entry by utilizing single-molecule FRET (smFRET)-imaging (Das et al., 2020). These authors demonstrated that pH, Ca2+, and NPC1 receptor binding leads to conformational changes in EBOV envelope glycoprotein 2. This conformational change plays an important role in the fusion of virus with endosomal membrane, a critical step in the EBOV entry (Das et al., 2020).
Likewise, Gunaratne et al. reported a critical role of TPCs in Middle East Respiratory Syndrome coronavirus (MERS-CoV) infections (Gunaratne et al., 2018). The knockdown of TPCs but not that of lysosomal transient receptor potential mucolipin-1 (TRPML1) led to impairment of MERS-CoV translocation via endo-lysosomes (Gunaratne et al., 2018). Importantly, the inhibition of NAADP-evoked lysosomal Ca2+ release via bisbenzyl-isoquinoline alkaloids blocked MERS pseudovirions translocation (Gunaratne et al., 2018). This highlights the therapeutic significance of targeting TPCs in regulating MERS-CoV pathogenesis. Recently, TPC1/2 were reported to play a critical role in polyomavirus entry into the host cells (Dobson et al., 2020). Further, it was demonstrated that inhibition of TPC activity by tetrandrine abrogates Merkel cell polyomavirus and Simian virus 40 infection. In a timely and highly relevant study, Ou et al. demonstrated that human angiotensin converting enzyme 2 (hACE2) is the receptor for SARS-CoV-2, the virus responsible for current COVID-19 pandemic (Ou et al., 2020). The authors reported that SARS-CoV-2 enters cells primarily through endocytosis. Further, they showed that TPC2 but not TRPML1 plays a critical role in the SARS-CoV-2 entry. Importantly, Ou and colleagues demonstrated that inhibition of TPC2 by tetrandrine leads to decrease in SARS-CoV-2 pseudovirions entry (Ou et al., 2020). Therefore, further investigations focused on evaluating the efficacy of TPC targeting drugs as novel antiviral therapy are need of the hour.
Apart from TPCs, an important role for another endo-lysosomes localized Ca2+ channel TRPML1 is reported in HIV-1 infection (Khan et al., 2019). As discussed in previous sections, HIV-1 Tat protein is critical for HIV-1 replication and latent infection. The degradation of Tat protein depends on pH of host cell endo-lysosomal compartment. Khan et al. demonstrated that acidification of endo-lysosomes by inducing TRPML1 activation leads to Tat degradation (Khan et al., 2019). This suggests that TRPML1 could be a potential therapeutic candidate against latent HIV-1 infections (Khan et al., 2019).
Taken together, these studies highlight that lysosomal Ca2+ signaling is critical for viral entry. It is important to note that TPC2 but not TRPML1 is a promising target for EBOV, SARS-CoV-2 and MERS-CoV infections whereas TRPML1 plays a critical regulatory role in case of HIV-1 infections thereby suggesting viral specific targeting of lysosomal Ca2+ handling machinery (Please refer Fig. 5 for the diagrammatic summary). Therefore, these studies demonstrate that different viruses target specific endo-lysosomal Ca2+ channels for their entry into host cells. Notably, FDA-approved drugs targeting lysosomal Ca2+ dynamics can robustly decrease viral infections thereby suggesting that these drugs should be further evaluated in clinical trials for better management of viral diseases including current COVID-19 pandemic.
Fig. 5.
Lysosomal Ca2+dynamics and viral entry. A variety of viruses target lysosomal Ca2+ efflux channels for host cell entry via endocytic route. Viruses such as EBOV, Marburg virus, Polyoma virus and MERS-CoV target TPCs for facilitating receptor mediated entry into host cells. Importantly, TPCs also regulate SARS-CoV-2 entry into host cells. While TRPML1 regulates HIV-1 replication and latent infection.
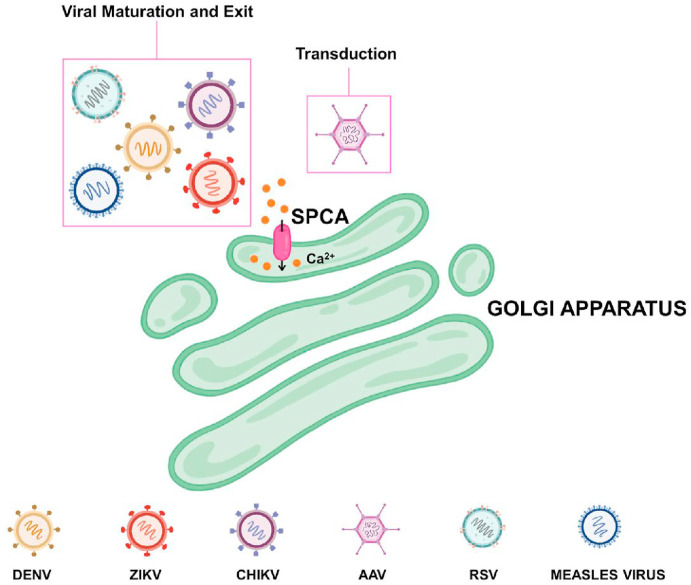
8. Disruption of the Golgi Ca2+ signaling and viral infections
GA plays a critical role in post-translational modification and sorting of lipids and proteins. Further, GA is actively involved in intracellular Ca2+ dynamics. It is equipped with Ca2+ release channels, Ca2+ pumps and Ca2+ binding proteins. This Ca2+ handling toolkit is involved in regulating the spatio-temporal complexity of the cellular Ca2+ signaling (Pizzo et al., 2011). The GA Ca2+ handling machinery includes IP3R, SERCA, RyR and SPCA1. SPCA1 is a critical regulator of GA Ca2+ content and plays an important role in the maintenance of GA ultrastructure and in the sorting of proteins to the PM.
The respiratory syncytial virus (RSV) is a RNA virus belonging to Paramyxoviridae family (Collins et al., 2013). Recently, Hoffmann et al. performed a genome-wide knockout screen in human haploid cells to identify host factors necessary for RSV infection (Hoffmann et al., 2017). Interestingly, SPCA1 was one of the most promising hits in this screening as it regulates processing of RSV glycoproteins and maturation of the virus (Hoffmann et al., 2017). Importantly, SPCA1 KO robustly decreased RSV infection and spread as viral glycoproteins critical for viral infectivity are not processed in absence of SPCA1. In addition to RSV, SPCA1 is required for infection of numerous other RNA viruses such as measles, dengue, zika, and chikungunya viruses (Hoffmann et al., 2017). Taken together, SPCA1 is emerging as a promising target for stalling spread of RNA viruses.
Parvoviruses such as adeno-associated viruses (AAVs) possess a single-stranded DNA genome (Madigan et al., 2020). AAV2 hijacks endosomal channels to migrate from the cell surface to the nucleus, including several endosome-trafficking proteins such as VPS29, VPS52, VPS53, VPS54, SPCA1 (Pillay et al., 2016). These proteins are engaged in the retrograde transport from endosomes to GA (Pillay et al., 2016). SPCA1 mediated GA Ca2+ homeostasis plays a critical role in AAV transduction (Madigan et al., 2020). The authors generated a SPCA1 CRISPR knockout (KO) line and demonstrated that AAV transduction is reduced upon SPCA1 KO (Madigan et al., 2020). Interestingly, SPCA1 KO does not inhibit AAV binding, its cellular uptake or nuclear entry, but SPCA1 KO cells exhibit scattered and punctuated trafficking (Madigan et al., 2020). Additionally, SPCA1 KO leads to significant inhibition of transcription of the vector genome. This SPCA1 KO phenotype and AAV transduction was rescued by the Ca2+ chelator BAPTA-AM, which decreases cytosolic Ca2+. Conversely, AAV transduction is inhibited by the Ca2+ ionophore ionomycin, which enhances cytosolic Ca2+ levels (Madigan et al., 2020). Collectively, these data suggest that SPCA1 mediated modulation of cytosolic Ca2+ concentration is a key regulator of AAV transduction. Collectively, the above-discussed studies shed light on the critical role of GA Ca2+ homeostasis and establish SPCA1 as an important regulator of viral infections (Also refer pictorial summary in Fig. 6 ).
Fig. 6.
Golgi Ca2+signaling and viral exit. Viruses frequently target Golgi Ca2+ signaling for facilitating their maturation and exit from the host cells. For example, DENV, ZIKV, CHIKV, RSV and Measles virus act on Golgi localized SPCA for enabling maturation of their glycoproteins and for their exit from the host cells. Further, SPCA regulated Golgi and cytosolic Ca2+ homeostasis plays a critical role in AAV transduction.
9. Targeting host cell Ca2+ signaling for curtailing viral pathogenesis
As discussed in above respective sections, viruses dysregulate intracellular Ca2+ homeostasis by targeting different cellular Ca2+ compartments (ER, PM, mitochondria, Golgi and lysosomes) for promoting viral pathogenesis. Since viruses interfere in the functioning of the Ca2+ handling machinery for its benefit, it is not surprising that host cell Ca2+ signaling toolkit has emerged as a promising target for clinical management of viral pathogenesis.
In a highly relevant study, Wang et al. screened FDA-approved drugs to target JEV (Wang et al., 2017). A high-throughput screening of FDA-approved drugs for inhibitors of JEV led to identification of 5 drugs, including 3 VGCCs blockers (manidipine, cilnidipine, and benidipine hydrochloride). Further, the authors demonstrated that Verapamil (another VGCCs inhibitor) treatment leads to dose dependent inhibition of JEV in human hepatocellular carcinoma (Huh-7) and African Green monkey kidney (Vero) cells. Moreover, VGCCs blockers inhibited replication of a variety of other flaviviruses such as ZIKV, DENV and WNV. Significantly, manidipine protects mice against JEV-induced lethality by reducing the viral load in the brain. This study clearly demonstrates clinical relevance VGCC inhibitors in targeting flavivirus infection (Wang et al., 2017). A similar study had earlier identified the FDA-approved VGCC blockers amiodarone and verapamil as potent inhibitors of filovirus entry in the host cells (Gehring et al., 2014). The experiments conducted in this study clearly demonstrated that these drugs act on host cells rather than the viruses directly (Gehring et al., 2014). Similarly, benidipine and nifedipine were demonstrated to curtail severe fever with thrombocytopenia syndrome virus (SFTSV) entry and replication (Li et al., 2019). Notably, both benidipine and nifedipine reduced SFTSV induced mortality in humanized mice model. Interestingly, authors performed a retrospective clinical study on the SFTSV patients data and suggested that nifedipine treatment significantly decreases the mortalities associated with SFTSV infection (Li et al., 2019). More recently, verapamil was reported to inhibit infection of polyomaviruses such as Simian virus 40 and Merkel cell polyomavirus (Dobson et al., 2020). Taken together, these studies highlight the potential benefit of targeting host cell VGCC for stalling viral infections.
Recently, Nathan et al. reported that Ca2+ interacts with EBOV fusion peptide (FP) and promotes the viral infection (Nathan et al., 2020). The authors demonstrated that Ca2+ promotes EBOV infection via direct interaction of Ca2+ with FP of EBOV. The glycoprotein residues D522 and E540 in the FP of EBOV are critical for Ca2+ interaction. By performing biophysical assays and spectroscopic studies, it was determined that interaction of Ca2+ with FP results in alteration in lipid ordering within host membrane. Further, by utilizing circular dichroism spectroscopy it was revealed that Ca2+ interaction with FP leads to conformational change (via promoting α-helical structure of the fusion peptide). This conformational change increases the membrane fusion and viral infectivity. Therefore, implicating the potential of FDA-approved Ca2+ interfering drugs in combating the viral infections (Jayaseelan and Paramasivam, 2020; Nathan et al., 2020). Indeed, a screening of around 2600 FDA-approved drugs and molecular probes identified a VGCC blocker Bepridil as one of the most potent inhibitor of EBOV entry in human cells in vitro (Johansen et al., 2015). Furthermore, in vivo experiments in mouse infection model of EBOV corroborated the efficacy of Bepridil in curtailing EBOV infection and associated pathogenesis. In a highly timely study relevant to the current COVID-19 pandemic, Vatansever et al. demonstrated that Bepridil inhibits SARS-CoV-2 infection in human cells (Vatansever et al., 2021). It was shown that Bepridil blocks both entry and infection of SARS-CoV-2 by modulating endo-lysosomal pH and by inhibiting key protease of SARS-CoV-2 respectively. Taken together, these studies elegantly demonstrate the potential clinical significance of FDA-approved VGCC inhibitor Bepridil in stalling SARS-CoV-2 and EBOV infections. Certainly, in current COVID-19 pandemic situation Bepridil must be further evaluated for its efficacy in controlling SARS-CoV-2 infection.
In an exciting study, Hyser lab recently demonstrated that rotaviral infection leads to a paracrine signaling resulting in generation of intercellular Ca2+ waves (ICWs) (Chang-Graham et al., 2020). Chang-Graham et al. showed that rotaviral infected cells secrete adenosine 5′-diphosphate (ADP) that activates P2Y1 purinergic receptors on neighboring uninfected cells, which in turn leads to rise in cellular Ca2+ concentration. The authors showed that P2Y1 knockout abrogates ICWs generation and viral replication. Importantly, using neonatal mice model, it was reported that P2Y1 inhibition significantly reduces severity of RV induced diarrhea in vivo (Chang-Graham et al., 2020). In the future, it would be pertinent to identify FDA-approved drug/s that can inhibit P2Y1 and evaluate their safety and efficacy in treating clinical rotaviral infections.
As discussed earlier, a large number of viruses attack mitochondrial Ca2+ signaling for either meeting higher energetic requirements or for inducing host cell apoptosis. Interestingly, Perocchi lab performed an orthogonal interspecies chemical screening in yeast mitochondria and human cells for identifying modulators of MCU (Arduino et al., 2017). Arduino et al. screened more than 600 small molecules belonging to NIH clinical collection library to eventually identify mitoxantrone (an FDA-approved drug) as a highly specific MCU inhibitor (Arduino et al., 2017). Similarly, a high throughput screening of around 120,000 compounds identified DS16570511 as a novel inhibitor of MCU activity in a variety of cells and beating rat heart (Kon et al., 2017). Subsequently, work from Madesh lab reported synthesis and characterization of Ru265 (Woods et al., 2019). The authors reported that Ru265 is highly specific inhibitor of MCU activity. Importantly, Woods et al. demonstrated that Ru265 is cell permeable and nominally toxic molecule that does not perturb cytosolic Ca2+ homeostasis and mitochondrial membrane potential. More recently, a high throughput screening of over 44,000 non-proprietary small molecules was performed for characterizing the inhibitors of mitochondrial Ca2+ uptake 1 (MICU1), which regulates mitochondrial Ca2+ influx (Di Marco et al., 2020). This arduous study identified 2 lead molecules that authors named as MCU-i4 and MCU-i11. Both of these molecules were able to efficiently and specifically inhibit MICU1 thereby decreasing mitochondrial Ca2+ concentration in in vitro and ex vivo assays (Di Marco et al., 2020; Nathan et al., 2020). Taken together, these recent studies have led to identification and characterization of highly specific small molecules that can target mitochondrial Ca2+ handling machinery. Considering the critical role of mitochondrial Ca2+ dynamics in viral infections, it would be interesting to evaluate the efficacy of these lead compounds in managing viral pathogenesis.
The emerging literature suggests that lysosomal Ca2+ dynamics, especially lysosomal Ca2+ efflux channel TPC2 plays a critical role in viral entry (please refer lysosomal Ca2+ signaling section for details). Excitingly, TPC2 inhibition is shown to stall entry of SARS-CoV-2, virus responsible for COVID-19 pandemic, as well (Ou et al., 2020). Recently, 1500 FDA-approved drugs were screened for inhibiting TPC2 activity (Penny et al., 2019). The authors identified and characterized two drugs that can inhibit endogenous NAADP-evoked lysosomal Ca2+ release and TPC2 channel activity. Significantly, these drugs lead to substantial reduction in EBOV infection in HeLa cells (Penny et al., 2019). In the future, it would be worth to investigate the potential of repurposing these FDA-approved drugs for clinical management of viral infections. Certainly, clinical trials for assessing the safety and efficacy of these drugs in restricting viral pathogenesis would be required to reveal the real potential of these drugs.
10. Future perspectives
In this review, we have discussed the dysregulation of host cell Ca2+ homeostasis during viral infections. Several viral proteins target specific cellular Ca2+ compartments (ER, PM, mitochondria, GA and lysosomes) for promoting viral pathogenesis. Viral proteins such as Tat and gp120 of HIV-1, HBx of HBV etc. modulate functioning of PM localized Ca2+ channels and pumps. Similarly, viral proteins like Gag and Nef of HIV, HBx of HBV etc. induce Ca2+ release from ER to cytosol (Please refer Table 1 for the detailed list of viral proteins, their host cell targets and the resulting effect on cellular Ca2+ homeostasis). Although these proteins have been reported to modulate functioning of host cell Ca2+ handling proteins, the detailed molecular choreography that connects viral infections to perturbations in Ca2+ homeostasis remains poorly understood. Likewise, how changes in cytosolic and/or organellar Ca2+ aid in viral life cycle progression and pathogenesis remains poorly understood. With the advent of highly specific genetically encoded Ca2+ sensing probes coupled with super resolution microscopy and systems biology approaches, the stage is set for dissecting out the intricate signaling cascades working at the intersection of viral infections and host cell Ca2+ dynamics.
Table 1.
The outcomes of viral interaction with host cell Ca2+ handling machinery.
| Site | S. No. | Virus | Viral Protein | Host target | Effect on host cell Ca2+ signaling | Relevance to viral pathogenesis | Reference |
|---|---|---|---|---|---|---|---|
| PM | 1 | IAV | Hemagglutinin (HA) | VGCC | Induces intracellular Ca2+ oscillation | Helps in viral entry and replication | Fujioka et al. (2018) |
| 2 | HIV-1 | Tat | VGCC | Elevation of intracellular Ca2+ | Host immune dysfunction | Zocchi et al. (1998) | |
| 3 | Flavivirus (JEV, ZIKV, DENV, YFV and WNV) | NS4B | VGCC | Increases cytosolic Ca2+ | Viral replication | Wang et al. (2017) | |
| 4 | NWA | Viral glycoproteins(GP) | VGCC | Increases cytosolic Ca2+ | Helps in viral entry and infection | (Lavanya et al., 2013; Sarute and Ross, 2020) | |
| 5 | HBV | HBx | Orai1 | Increases cytosolic Ca2+ | Essential for HBV replication | (Yao et al., 2018) | |
| 6 | HTLV-1 | p12I | SOCC | Elevation of cytoplasmic Ca2+ | Aids in viral replication | Ding et al. (2002) | |
| 7 | RV | NSP4 | SOCC | SOCE activation, increases cytoplasmic Ca2+ levels | Aid in viral replication | (Díaz et al., 2012; Hyser et al., 2013) | |
| 8 | DENV | Not known | Orai1 | Rise in cytosolic Ca2+ | Viral replication | Dionicio et al. (2018) | |
| 9 | Arenavirus and Filovirus | VP40 | Orai1 | Rise in cytosolic Ca2+ levels | Viral budding and spread | Han et al. (2015) | |
| 10 | HIV-1 | Gp120, Tat | NMDAR | Increases intracellular Ca2+ | Not known | (Nath et al., 1995; Self et al., 2004) | |
| 11 | HCV, DEVN, ZIKV | Not Known | TRPV4 | Rise in cytosolic Ca2+ | Viral replication and exit | Doñate-Macián et al. (2018) | |
| 12 | HBV | HBx | PMCA | Elevation of the cytosolic Ca2+ | Enhances HBV DNA replication and HBV core assembly | Zhou et al. (2013) | |
| 13 | RV | Not Known | NCX | Rise in cytosolic Ca2+ | RV replication | Díaz et al. (2012) | |
| ER | 1 | RV | NSP4 | Acts as ER Viroporin | Depletes ER Ca2+ and increase in cytosolic Ca2+ | Viral replication and assembly | (Díaz et al., 2008; Hyser et al., 2013; Pham et al., 2017) |
| 2 | HCMV | US21 | Acts as ER Viroporin | Depletes ER Ca2+ stores | Decreases cell susceptibility to apoptosis | Luganini et al. (2018) | |
| 3 | HCMV | UL37 | IP3R | Mobilizes Ca2+ from ER to cytosol | Enhanced ATP production to meet viral energy demands | Sharon-Friling et al. (2006) | |
| 4 | HCMV | US28 | Triggers PLC-β signaling | Release of intracellular Ca2+ | May have role in latent infection | Miller et al. (2012) | |
| 5 | HCMV | vCXCL1 | Agonist for human chemokines CXCR1 and CXCR2 | Intracellular Ca2+ release | Viral dissemination through the host | (Penfold et al., 1999; Wang et al., 2003) | |
| 6 | EBV | LMP-1 | SERCA3 | Increase in resting cytosolic Ca2+ levels | Development of latent, persistent infection and cell immortalization | Dellis et al. (2009) | |
| 7 | HIV | Gag | IP3R | Ca2+ release from ER | Viral budding, formation of VLPs | (Ehrlich et al, 2011, 2014; Ehrlich and Carter, 2012) | |
| 8 | HIV | Nef | IP3R | Ca2+ release from ER | AIDS pathogenesis | (Ehrlich et al., 2010; Manninen and Saksela, 2002) | |
| 9 | HBV | HBx | IP3R | Increase in mitochondrial Ca2+ uptake | Aid in viral replication | Yang and Bouchard (2012) | |
| 10 | HSV | Not defined | IP3R | Increases cytosolic Ca2+ | Viral penetration, delivery of viral capsids to host cell's nucleus. | (Cheshenko et al, 2003, 2007) | |
| Mitochondria | 1 | HBV | HBx | mPTP | Releases Ca2+ from mitochondria | Regulates HBV replication | Bouchard et al. (2001) |
| 2 | HCV | NS5A | MCU | Increased mitochondrial Ca2+ influx and nuclear translocation of transcription factors | Helps in viral pathogenesis | Gong et al. (2001) | |
| 3 | HCV | HCV polyprotein | MCU | Increase in mitochondrial Ca2+ influx | May induce apoptosis | Piccoli et al. (2007) | |
| 4 | HCV | Core protein | MCU | Increase in mitochondrial Ca2+ uptake | Increases ROS production and alters apoptosis | Li et al. (2007) | |
| 5 | HEV | Orf3 | VDAC1 | Upregulation of VDAC | Enhances viral replication | Moin et al. (2007) | |
| 6 | HIV-1 | Tat | MCU | Increase in mitochondrial Ca2+ uptake | Host cell apoptosis | (Kruman II et al., 1998) | |
| 7 | HIV-1 | Tat | VDAC | Rise in mitochondrial Ca2+ | Host cell apoptosis | Lecoeur et al. (2012) | |
| 8 | PV | Not known | VDAC and MCU | Increase mitochondrial Ca2+ levels | Apoptosis induction | Brisac et al. (2010) | |
| Lysosomes | 1 | EBOV and Marburgvirus | Not known | TPC2 | Ca2+ release from lysosomes | EBOV entry and infection, Marburgvirus infection |
(Penny et al., 2019; Sakurai et al., 2015; Simmons et al., 2016) |
| 2 | Polyomavirus | Not known | TPC1/2 | Ca2+ release from lysosomes | Viral entry | Dobson et al. (2020) | |
| 3 | MERS-CoV | Furin | TPCs | Ca2+ release from lysosomes | Viral entry | Gunaratne et al. (2018) | |
| 4 | SARS-CoV-2 | Not known | TPC2 | Ca2+ release from lysosomes | Viral entry | Ou et al. (2020) | |
| 5 | HIV-1 | Tat | TRPML1 | Ca2+ release from lysosomes | HIV-1 replication and latent infections | Khan et al. (2019) | |
| Golgi | 1 | DENV, ZIKV, CHIKV, RSV and Measles virus | Not known | SPCA1 | – | Viral maturation and exit | Hoffmann et al. (2017) |
| 2 | AAV | Not known | SPCA1 | Perturbs cytosolic Ca2+ | AAV transduction | (Madigan et al., 2020; Pillay et al., 2016) | |
As discussed in the lysosomal Ca2+ dynamics and viral entry section, a variety of viruses target lysosomal Ca2+ channels especially TPCs for entry into host cells. Going forward, it would be worth to examine the role of TPCs in non-viral infectious diseases caused by the pathogens that target endo-lysosomes for driving infections. Since FDA-approved drugs targeting TPCs have been recently identified, their further evaluation and efficacy in clinical settings is required.
The current COVID-19 pandemic has severely affected whole world with over 3 million deaths. Studies have reported that Ca2+ is required for the fusion of MERS-CoV, SARs-CoV and Rubella virus with the host cells suggesting that Ca2+ plays a critical role in these viral infections (Dubé et al., 2014; Lai et al., 2017; Straus et al., 2020b). Interestingly, based on structural homology, bioinformatics and metanalyses, it is suggested that Ca2+ could act as an important regulator of SARS-CoV-2 entry into host cells (Cashman, 2020a, 2020b). Further, TPCs have been recognized as targetable option for stalling SARS-CoV-2 entry into host cells (Filippini et al., 2020; Ou et al., 2020; Zhao et al., 2021) and TPC antagonist tetrandrine is hypothesized as a potential antiviral against SARS-CoV-2 (Heister and Poston, 2020). Indeed, there is an ongoing clinical trial that is evaluating efficacy of tetrandrine in COVID-19 treatment (https://clinicaltrials.gov/ct2/show/study/NCT04308317). Moreover, Bepridil a VGCC inhibitor is shown to inhibit SARS-CoV-2 infection in vitro (Vatansever et al., 2021). Likewise, FDA-approved VGCC blockers amlodipine and nifedipine have been shown to stall SARS-CoV-2 entry into host cells (Straus et al., 2020a). Taken together, these studies have highlighted a critical role of Ca2+ dynamics in COVID-19 infection. In light of current pandemic, further investigations on VGCC blockers and TPC inhibitors as potential antivirals against SARS-CoV-2 are urgently needed.
Further, as discussed earlier VGCC inhibitors have shown promising role in curtailing flavivirus and polyomavirus infections. It is important to note that VGCC inhibitors are routinely prescribed for managing hypertension and they are part of long term medication for hypertensive patients. Therefore, it would be relevant to conduct epidemiological surveys of the patients taking VGCC inhibitors for possible protection against flavivirus, SARS-CoV-2 and polyomavirus infections. Such surveys will most likely provide insightful information that may assist in designing future antiviral therapies.
In summary, several elegant studies have demonstrated that Ca2+ handling proteins are attractive therapeutic targets for stalling a variety of viral infections. With availability of both FDA-approved drugs and bio-active lead molecules targeting these proteins, it is an area that demands immediate and critical attention for managing viral infections. Certainly, preclinical safety and efficacy studies in relevant animal models would be needed before testing these drugs in clinical trials. In the current scenario wherein outbreaks of viral infections are frequently observed, it is actually need of the hour to investigate the clinical significance of targeting host cell Ca2+ signaling for curtailing viral pathogenesis.
Declaration of competing interest
Authors report no conflicts of interest.
Acknowledgments
This work was supported by the DBT/Wellcome Trust India Alliance Fellowship (IA/I/19/2/504651) awarded to Rajender K Motiani. Authors also acknowledge RCB core funding and Science & Engineering Research Board (SERB) Start-up Research Grant (SRG/2019/000495) to RKM. SS acknowledges his Junior Research Fellowship from CSIR, India and JT acknowledges her Senior Research Fellowship from CSIR, India.
References
- Anand S.K., Tikoo S.K. Viruses as modulators of mitochondrial functions. Adv. Virol. 2013:738794. doi: 10.1155/2013/738794. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arduino D.M., Wettmarshausen J., Vais H., Navas-Navarro P., Cheng Y., Leimpek A., Ma Z., Delrio-Lorenzo A., Giordano A., Garcia-Perez C., Médard G., Kuster B., García-Sancho J., Mokranjac D., Foskett J.K., Alonso M.T., Perocchi F. Systematic identification of MCU modulators by orthogonal interspecies chemical screening. Mol. Cell. 2017;67:711–723. doi: 10.1016/j.molcel.2017.07.019. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnoult D., Bartle L.M., Skaletskaya A., Poncet D., Zamzami N., Park P.U., Sharpe J., Youle R.J., Goldmacher V.S. Cytomegalovirus cell death suppressor vMIA blocks Bax- but not Bak-mediated apoptosis by binding and sequestering Bax at mitochondria. Proc. Natl. Acad. Sci. U.S.A. 2004;101:7988–7993. doi: 10.1073/pnas.0401897101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlas D. Voltage-gated calcium channels function as Ca2+-activated signaling receptors. Trends Biochem. Sci. 2014;39:45–52. doi: 10.1016/j.tibs.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Bagur R., Hajnóczky G. Intracellular Ca(2+) sensing: its role in calcium homeostasis and signaling. Mol. Cell. 2017;66:780–788. doi: 10.1016/j.molcel.2017.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartosch B., Thimme R., Blum H.E., Zoulim F. Hepatitis C virus-induced hepatocarcinogenesis. J. Hepatol. 2009 doi: 10.1016/j.jhep.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Baughman J.M., Perocchi F., Girgis H.S., Plovanich M., Belcher-Timme C.A., Sancak Y., Bao X.R., Strittmatter L., Goldberger O., Bogorad R.L., Koteliansky V., Mootha V.K. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011 doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict C.A., Norris P.S., Ware C.F. To kill or be killed: viral evasion of apoptosis. Nat. Immunol. 2002;3:1013–1018. doi: 10.1038/ni1102-1013. [DOI] [PubMed] [Google Scholar]
- Bouchard M.J., Navas-Martin S. Cancer Lett; 2011. Hepatitis B and C Virus Hepatocarcinogenesis: Lessons Learned and Future Challenges. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard M.J., Schneider R.J. The enigmatic X gene of hepatitis B virus. J. Virol. 2004;78:12725–12734. doi: 10.1128/jvi.78.23.12725-12734.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard M.J., Wang L.H., Schneider R.J. Calcium signaling by HBx protein in hepatitis B virus DNA replication. Science. 2001;294:2376–2378. doi: 10.1126/science.294.5550.2376. [DOI] [PubMed] [Google Scholar]
- Brault C., Levy P.L., Bartosch B. Hepatitis C virus-induced mitochondrial dysfunctions. Viruses. 2013;5:954–980. doi: 10.3390/v5030954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisac C., Téoulé F., Autret A., Pelletier I., Colbère-Garapin F., Brenner C., Lemaire C., Blondel B. Calcium flux between the endoplasmic reticulum and mitochondrion contributes to poliovirus-induced apoptosis. J. Virol. 2010;84:12226–12235. doi: 10.1128/jvi.00994-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrell C.J., Howard C.R., Murphy F.A. Fenner and White's Medical Virology. Elsevier; 2017. Epidemiology of viral infections; pp. 185–203. [DOI] [Google Scholar]
- Calcraft P.J., Ruas M., Pan Z., Cheng X., Arredouani A., Hao X., Tang J., Rietdorf K., Teboul L., Chuang K.T., Lin P., Xiao R., Wang C., Zhu Y., Lin Y., Wyatt C.N., Parrington J., Ma J., Evans A.M., Galione A., Zhu M.X. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature. 2009;459:596–600. doi: 10.1038/nature08030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter J. second ed. 2012. Virology: Principles and Applications.http://www.wiley.com/WileyCDA/WileyTitle/productCd-EHEP003007.html [WWW Document]. URL. [Google Scholar]
- Casciano J.C., Duchemin N.J., Lamontagne R.J., Steel L.F., Bouchard M.J. Hepatitis B virus modulates store-operated calcium entry to enhance viral replication in primary hepatocytes. PloS One. 2017;12 doi: 10.1371/journal.pone.0168328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashman D.P. Dominance of SARS-CoV-2 D614G variant explained by the requirement of COVID-19 for calcium; proximate therapeutic implication(S) for COVID-19. Clin. Immunol. Immunother. 2020;6:1–8. doi: 10.24966/ciit-8844/1000048. [DOI] [Google Scholar]
- Cashman D.P. Why the lower reported prevalence of asthma in patients diagnosed with COVID-19 validates repurposing EDTA solutions to prevent and manage treat COVID-19 disease. Med. Hypotheses. 2020;144 doi: 10.1016/j.mehy.2020.110027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W.A. Voltage-gated calcium channels. Cold Spring Harb Perspect Biol. 2011;3:a003947. doi: 10.1101/cshperspect.a003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallari I., Scattolin G., Silic-Benussi M., Raimondi V., D'Agostino D.M., Ciminale V. Mitochondrial proteins coded by human tumor viruses. Front. Microbiol. 2018;9:81. doi: 10.3389/fmicb.2018.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers M., Schell M.J., Thorn P. Agonist-evoked inositol trisphosphate receptor (IP3R) clustering is not dependent on changes in the structure of the endoplasmic reticulum. Biochem. J. 2006;394:57–66. doi: 10.1042/BJ20051130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chami M., Ferrari D., Nicotera P., Paterlini-Bréchot P., Rizzuto R. Caspase-dependent alterations of Ca2+ signaling in the induction of apoptosis by hepatitis B virus X protein. J. Biol. Chem. 2003;278:31745–31755. doi: 10.1074/jbc.M304202200. [DOI] [PubMed] [Google Scholar]
- Chami M., Oulès B., Paterlini-Bréchot P. Cytobiological consequences of calcium-signaling alterations induced by human viral proteins. Biochim. Biophys. Acta. 2006;1763:1344–1362. doi: 10.1016/j.bbamcr.2006.09.025. [DOI] [PubMed] [Google Scholar]
- Chang-Graham A.L., Perry J.L., Engevik M.A., Engevik K.A., Scribano F.J., Gebert J.T., Danhof H.A., Nelson J.C., Kellen J.S., Strtak A.C., Sastri N.P., Estes M.K., Britton R.A., Versalovic J., Hyser J.M. Rotavirus induces intercellular calcium waves through ADP signaling. Science. 2020;80:370. doi: 10.1126/science.abc3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang-Graham A.L., Perry J.L., Strtak A.C., Ramachandran N.K., Criglar J.M., Philip A.A., Patton J.T., Estes M.K., Hyser J.M. Rotavirus calcium dysregulation manifests as dynamic calcium signaling in the cytoplasm and endoplasmic reticulum. Sci. Rep. 2019;9:1–20. doi: 10.1038/s41598-019-46856-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton F.W., Pearson H.M., Hover S., Lippiat J.D., Fontana J., Barr J.N., Mankouri J. Ion channels as therapeutic targets for viral infections: further discoveries and future perspectives. Viruses. 2020;12:844. doi: 10.3390/v12080844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri R., Arora H., Seth P. Mitochondrial calcium signaling in the brain and its modulation by neurotropic viruses. Mitochondrion. 2021;59:8–16. doi: 10.1016/j.mito.2021.03.010. [DOI] [PubMed] [Google Scholar]
- Chen X., Cao R., Zhong W. Host calcium channels and pumps in viral infections. Cells. 2019;9:94. doi: 10.3390/cells9010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X., Shen D., Samie M., Xu H. Mucolipins: intracellular TRPML1-3 channels. FEBS Lett. 2010;584:2013–2021. doi: 10.1016/j.febslet.2009.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheshenko N., Del Rosario B., Woda C., Marcellino D., Satlin L.M., Herold B.C. Herpes simplex virus triggers activation of calcium-signaling pathways. J. Cell Biol. 2003;163:283–293. doi: 10.1083/jcb.200301084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheshenko N., Liu W., Satlin L.M., Herold B.C. Multiple receptor interactions trigger release of membrane and intracellular calcium stores critical for herpes simplex virus entry. Mol. Biol. Cell. 2007;18:3119–3130. doi: 10.1091/mbc.E07-01-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen K.A., Myers J.T., Swanson J.A. pH-dependent regulation of lysosomal calcium in macrophages. J. Cell Sci. 2002;115:599–607. doi: 10.1242/jcs.115.3.599. [DOI] [PubMed] [Google Scholar]
- Clapham D.E. Cell; 2007. Calcium signaling. [DOI] [Google Scholar]
- Claverie J.M. Encyclopedia of Virology. Elsevier Ltd; 2008. Mimivirus; pp. 311–319. [DOI] [Google Scholar]
- Clippinger A.J., Bouchard M.J. Hepatitis B virus HBx protein localizes to mitochondria in primary rat hepatocytes and modulates mitochondrial membrane potential. J. Virol. 2008;82:6798–6811. doi: 10.1128/jvi.00154-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P.L., Fearns R., Graham B.S. Current Topics in Microbiology and Immunology. NIH Public Access; 2013. Respiratory syncytial virus: virology, reverse genetics, and pathogenesis of disease; pp. 3–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford S.E., Hyser J.M., Utama B., Estes M.K. Autophagy hijacked through viroporin-activated calcium/calmodulin-dependent kinase kinase-β signaling is required for rotavirus replication. Proc. Natl. Acad. Sci. U.S.A. 2012;109:E3405–E3413. doi: 10.1073/pnas.1216539109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csordás G., Renken C., Várnai P., Walter L., Weaver D., Buttle K.F., Balla T., Mannella C.A., Hajnóczky G. Structural and functional features and significance of the physical linkage between ER and mitochondria. J. Cell Biol. 2006;174:915–921. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das D.K., Bulow U., Diehl W.E., Durham N.D., Senjobe F., Chandran K., Luban J., Munro J.B. Conformational changes in the Ebola virus membrane fusion machine induced by pH, Ca2+, and receptor binding. PLoS Biol. 2020;18 doi: 10.1371/journal.pbio.3000626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stefani D., Raffaello A., Teardo E., Szabò I., Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellis O., Arbabian A., Brouland J.P., Kovàcs T., Rowe M., Chomienne C., Joab I., Papp B. Modulation of b-cell endoplasmic reticulum calcium homeostasis by epstein-barr virus latent membrane protein-1. Mol. Canc. 2009;8:59. doi: 10.1186/1476-4598-8-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marco G., Vallese F., Jourde B., Bergsdorf C., Sturlese M., De Mario A., Techer-Etienne V., Haasen D., Oberhauser B., Schleeger S., Minetti G., Moro S., Rizzuto R., De Stefani D., Fornaro M., Mammucari C. A high-throughput screening identifies MICU1 targeting compounds. Cell Rep. 2020;30:2321–2331. doi: 10.1016/j.celrep.2020.01.081. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz Y., Chemello M.E., Peña F., Aristimuño O.C., Zambrano J.L., Rojas H., Bartoli F., Salazar L., Chwetzoff S., Sapin C., Trugnan G., Michelangeli F., Ruiz M.C. Expression of nonstructural rotavirus protein NSP4 mimics Ca2+ homeostasis changes induced by rotavirus infection in cultured cells. J. Virol. 2008;82:11331–11343. doi: 10.1128/jvi.00577-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz Y., Peña F., Aristimuño O.C., Matteo L., De Agrela M., Chemello M.E., Michelangeli F., Ruiz M.C. Dissecting the Ca 2+ entry pathways induced by rotavirus infection and NSP4-EGFP expression in Cos-7 cells. Virus Res. 2012;167:285–296. doi: 10.1016/j.virusres.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Ding W., Albrecht B., Kelley R.E., Muthusamy N., Kim S.-J., Altschuld R.A., Lairmore M.D. Human T-cell lymphotropic virus type 1 p12I expression increases cytoplasmic calcium to enhance the activation of nuclear factor of activated T cells. J. Virol. 2002;76:10374–10382. doi: 10.1128/jvi.76.20.10374-10382.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionicio C.L., Peña F., Constantino-Jonapa L.A., Vazquez C., Yocupicio-Monroy M., Rosales R., Zambrano J.L., Ruiz M.C., del Angel R.M., Ludert J.E. Dengue virus induced changes in Ca2+ homeostasis in human hepatic cells that favor the viral replicative cycle. Virus Res. 2018;245:17–28. doi: 10.1016/j.virusres.2017.11.029. [DOI] [PubMed] [Google Scholar]
- Dobson S.J., Mankouri J., Whitehouse A. Identification of potassium and calcium channel inhibitors as modulators of polyomavirus endosomal trafficking. Antivir. Res. 2020;179:104819. doi: 10.1016/j.antiviral.2020.104819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolman N.J., Tepikin A.V. Calcium gradients and the Golgi. Cell Calcium. 2006;40:505–512. doi: 10.1016/j.ceca.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Doñate-Macián P., Jungfleisch J., Pérez-Vilaró G., Rubio-Moscardo F., Perálvarez-Marín A., Diez J., Valverde M.A. The TRPV4 channel links calcium influx to DDX3X activity and viral infectivity. Nat. Commun. 2018;9:1–13. doi: 10.1038/s41467-018-04776-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y., Zeng C.Q.Y.Y., Ball J.M., Estes M.K., Morris A.P. The rotavirus enterotoxin NSP4 mobilizes intracellular calcium in human intestinal cells by stimulating phospholipase C-mediated inositol 1,4,5-trisphosphate production. Proc. Natl. Acad. Sci. U.S.A. 1997;94:3960–3965. doi: 10.1073/pnas.94.8.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubé M., Rey F.A., Kielian M. Rubella virus: first calcium-requiring viral fusion protein. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugas B., Mencia-Huerta J.-M., Braquet P., Galanaud P., Delfraissy J.-F. Extracellular but not intracellular calcium mobilization is required for epstein-barr virus-containing supernatant-induced b cell activation. Eur. J. Immunol. 1989;19:1867–1871. doi: 10.1002/eji.1830191017. [DOI] [PubMed] [Google Scholar]
- Dunn D.M., Munger J. Interplay between calcium and AMPK signaling in human cytomegalovirus infection. Front. Cell. Infect. Microbiol. 2020 doi: 10.3389/fcimb.2020.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich L.S., Carter C.A. HIV assembly and budding: Ca 2+ signaling and non-ESCRT proteins set the stage. Mol. Biol. Int. 2012:1–12. doi: 10.1155/2012/851670. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich L.S., Medina G.N., Carter C.A. ESCRT machinery potentiates HIV-1 utilization of the PI(4,5)P 2-PLC-IP3R-Ca2+ signaling cascade. J. Mol. Biol. 2011;413:347–358. doi: 10.1016/j.jmb.2011.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich L.S., Medina G.N., Khan M.B., Powell M.D., Mikoshiba K., Carter C.A. Activation of the inositol (1,4,5)-triphosphate calcium gate receptor is required for HIV-1 Gag release. J. Virol. 2010;84:6438–6451. doi: 10.1128/jvi.01588-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich L.S., Medina G.N., Photiadis S., Whittredge P.B., Watanabe S., Taraska J.W., Carter C.A. Tsg101 regulates PI(4,5)P2/Ca2+ signaling for HIV-1 Gag assembly. Front. Microbiol. 2014;5:234. doi: 10.3389/fmicb.2014.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X., Yang J. Lysosomal calcium in neurodegeneration. Messenger (Los Angel) 2016;5:56–66. doi: 10.1166/msr.2016.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini A., D'Amore A., Palombi F., Carpaneto A. Could the inhibition of endo-lysosomal two-pore channels (TPCs) by the natural flavonoid naringenin represent an option to fight SARS-CoV-2 infection? Front. Microbiol. 2020;11:970. doi: 10.3389/fmicb.2020.00970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka Y., Nishide S., Ose T., Suzuki T., Kato I., Fukuhara H., Fujioka M., Horiuchi K., Satoh A.O., Nepal P., Kashiwagi S., Wang J., Horiguchi M., Sato Y., Paudel S., Nanbo A., Miyazaki T., Hasegawa H., Maenaka K., Ohba Y. A sialylated voltage-dependent Ca 2+ channel binds hemagglutinin and mediates influenza A virus entry into mammalian cells. Cell Host Microbe. 2018;23:809–818. doi: 10.1016/j.chom.2018.04.015. e5. [DOI] [PubMed] [Google Scholar]
- Fujioka Y., Tsuda M., Nanbo A., Hattori T., Sasaki J., Sasaki T., Miyazaki T., Ohba Y. A Ca2+-dependent signalling circuit regulates influenza A virus internalization and infection. Nat. Commun. 2013;4:1–13. doi: 10.1038/ncomms3763. [DOI] [PubMed] [Google Scholar]
- Gao J.L., Murphy P.M. Human cytomegalovirus open reading frame US28 encodes a functional β chemokine receptor. J. Biol. Chem. 1994;269:28539–28542. doi: 10.1016/S0021-9258(19)61936-8. [DOI] [PubMed] [Google Scholar]
- Gearhart T.L., Bouchard M.J. Replication of the hepatitis B virus requires a calcium-dependent HBx-induced G1 phase arrest of hepatocytes. Virology. 2010;407:14–25. doi: 10.1016/j.virol.2010.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring G., Rohrmann K., Atenchong N., Mittler E., Becker S., Dahlmann F., Pöhlmann S., Vondran F.W.R., David S., Manns M.P., Ciesek S., von Hahn T. The clinically approved drugs amiodarone, dronedarone and verapamil inhibit filovirus cell entry. J. Antimicrob. Chemother. 2014;69:2123–2131. doi: 10.1093/jac/dku091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelderblom H.R. University of Texas Medical Branch at Galveston; 1996. Structure and Classification of Viruses, Medical Microbiology. [PubMed] [Google Scholar]
- Goldmacher V.S., Bartle L.M., Skaletskaya A., Dionne C.A., Kedersha N.L., Vater C.A., Han J.W., Lutz R.J., Watanabe S., Cahir McFarland E.D., Kieff E.D., Mocarski E.S., Chittenden T. A cytomegalovirus-encoded mitochondria-localized inhibitor of apoptosis structurally unrelated to Bcl-2. Proc. Natl. Acad. Sci. U.S.A. 1999;96:12536–12541. doi: 10.1073/pnas.96.22.12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong G., Waris G., Tanveer R., Siddiqui A. Human hepatitis C virus NS5A protein alters intracellular calcium levels, induces oxidative stress, and activates STAT-3 and NF-kappa B. Proc. Natl. Acad. Sci. U. S. A. 2001;98:9599–9604. doi: 10.1073/pnas.171311298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaratne G.S., Yang Y., Li F., Walseth T.F., Marchant J.S. NAADP-dependent Ca(2+) signaling regulates Middle East respiratory syndrome-coronavirus pseudovirus translocation through the endolysosomal system. Cell Calcium. 2018;75:30–41. doi: 10.1016/j.ceca.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajnóczky G., Robb-Gaspers L.D., Seitz M.B., Thomas A.P. Decoding of cytosolic calcium oscillations in the mitochondria. Cell. 1995;82:415–424. doi: 10.1016/0092-8674(95)90430-1. [DOI] [PubMed] [Google Scholar]
- Han Z., Madara J.J., Herbert A., Prugar L.I., Ruthel G., Lu J., Liu Y., Liu W., Liu X., Wrobel J.E., Reitz A.B., Dye J.M., Harty R.N., Freedman B.D. Calcium regulation of hemorrhagic fever virus budding: mechanistic implications for host-oriented therapeutic intervention. PLoS Pathog. 2015;11 doi: 10.1371/journal.ppat.1005220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heister P.M., Poston R.N. Pharmacological hypothesis: TPC2 antagonist tetrandrine as a potential therapeutic agent for COVID-19. Pharmacol. Res. Perspect. 2020;8 doi: 10.1002/prp2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkler F., Hoare J., Waseem N., Goldin R.D., McGarvey M.J., Koshy R., King I.A. Intracellular localization of the hepatitis B virus HBx protein. J. Gen. Virol. 2001;82:871–882. doi: 10.1099/0022-1317-82-4-871. [DOI] [PubMed] [Google Scholar]
- Hoffmann H.H., Schneider W.M., Blomen V.A., Scull M.A., Hovnanian A., Brummelkamp T.R., Rice C.M. Diverse viruses require the calcium transporter SPCA1 for maturation and spread. Cell Host Microbe. 2017;22:460–470. doi: 10.1016/j.chom.2017.09.002. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh Y.H., Su I.J., Wang H.C., Chang W.T.W.T.W., Lei H.Y., Lai M.D., Chang W.T.W.T.W., Huang W. Pre-S mutant surface antigens in chronic hepatitis B virus infection induce oxidative stress and DNA damage. Carcinogenesis. 2004;25:2023–2032. doi: 10.1093/carcin/bgh207. [DOI] [PubMed] [Google Scholar]
- Huh K.-W.W., Siddiqui A. Characterization of the mitochondrial association of hepatitis B virus X protein. HBx. Mitochondrion. 2002;1:349–359. doi: 10.1016/S1567-7249(01)00040-X. [DOI] [PubMed] [Google Scholar]
- Hyser J.M., Collinson-Pautz M.R., Utama B., Estes M.K. Rotavirus disrupts calcium homeostasis by NSP4 viroporin activity. mBio. 2010;1 doi: 10.1128/mBio.00265-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyser J.M., Estes M.K. Pathophysiological consequences of calcium-conducting viroporins. Annu. Rev. Virol. 2015 doi: 10.1146/annurev-virology-100114-054846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyser J.M., Utama B., Crawford S.E., Broughman J.R., Estes M.K. Activation of the endoplasmic reticulum calcium sensor STIM1 and store-operated calcium entry by rotavirus requires NSP4 viroporin activity. J. Virol. 2013;87:13579–13588. doi: 10.1128/jvi.02629-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov A.V., Valuev-Elliston V.T., Tyurina D.A., Ivanova O.N., Kochetkov S.N., Bartosch B., Isaguliants M.G. Oxidative stress, a trigger of hepatitis C and B virus-induced liver carcinogenesis. Oncotarget. 2017;8:3895–3932. doi: 10.18632/oncotarget.13904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaseelan V.P., Paramasivam A. Repurposing calcium channel blockers as antiviral drugs. J. Cell Commun. Signal. 2020;14:467–468. doi: 10.1007/s12079-020-00579-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D., Zhao L., Clapham D.E. Genome-wide RNAi screen identifies Letm1 as a mitochondrial Ca2+/H+ antiporter. Science. 2009;326:144–147. doi: 10.1126/science.1175145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen L.M., DeWald L.E., Shoemaker C.J., Hoffstrom B.G., Lear-Rooney C.M., Stossel A., Nelson E., Delos S.E., Simmons J.A., Grenier J.M., Pierce L.T., Pajouhesh H., Lehár J., Hensley L.E., Glass P.J., White J.M., Olinger G.G. A screen of approved drugs and molecular probes identifies therapeutics with anti-Ebola virus activity. Sci. Transl. Med. 2015;7 doi: 10.1126/scitranslmed.aaa5597. [DOI] [PubMed] [Google Scholar]
- Karn J. Brenner's Encyclopedia of Genetics. second ed. Elsevier Inc.; 2013. Retroviruses; pp. 211–215. [DOI] [Google Scholar]
- Khan N., Lakpa K.L., Halcrow P.W., Afghah Z., Miller N.M., Geiger J.D., Chen X. BK channels regulate extracellular Tat-mediated HIV-1 LTR transactivation. Sci. Rep. 2019;9:12285. doi: 10.1038/s41598-019-48777-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kon N., Murakoshi M., Isobe A., Kagechika K., Miyoshi N., Nagayama T. DS16570511 is a small-molecule inhibitor of the mitochondrial calcium uniporter. Cell Death Dis. 2017;3:1–7. doi: 10.1038/cddiscovery.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruman, Nath A., Mattson M.P., Kruman I.I., Nath A., Mattson M.P., II K., Nath A., Mattson M.P., Kruman, Nath A., Mattson M.P., Kruman I.I., Nath A., Mattson M.P. HIV-1 protein Tat induces apoptosis of hippocampal neurons by a mechanism involving caspase activation, calcium overload, and oxidative stress. Exp. Neurol. 1998;154:276–288. doi: 10.1006/exnr.1998.6958. [DOI] [PubMed] [Google Scholar]
- Lai A.L., Millet J.K., Daniel S., Freed J.H., Whittaker G.R. The SARS-CoV fusion peptide forms an extended bipartite fusion platform that perturbs membrane order in a calcium-dependent manner. J. Mol. Biol. 2017;429:3875–3892. doi: 10.1016/j.jmb.2017.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanner J.T., Georgiou D.K., Joshi A.D., Hamilton S.L. Ryanodine receptors: structure, expression, molecular details, and function in calcium release. Cold Spring Harb Perspect Biol. 2010;2:a003996. doi: 10.1101/cshperspect.a003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavanya M., Cuevas C.D., Thomas M., Cherry S., Ross S.R. siRNA screen for genes that affect Junín virus entry uncovers voltage-gated calcium channels as a therapeutic target. Sci. Transl. Med. 2013;5 doi: 10.1126/scitranslmed.3006827. 204ra131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecoeur H., Borgne-Sanchez A., Chaloin O., El-Khoury R., Brabant M., Langonné A., Porceddu M., Brière J.J., Buron N., Rebouillat D., Péchoux C., Deniaud A., Brenner C., Briand J.P., Muller S., Rustin P., Jacotot E. HIV-1 Tat protein directly induces mitochondrial membrane permeabilization and inactivates cytochrome c oxidase. Cell Death Dis. 2012;3:e282. doi: 10.1038/cddis.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Kim H., Lee S.A., Won Y.S., Kim H.I., Inn K.S., Kim B.J. Upregulation of endoplasmic reticulum stress and reactive oxygen species by naturally occurring mutations in hepatitis B virus core antigen. J. Gen. Virol. 2015;96:1850–1854. doi: 10.1099/vir.0.000134. [DOI] [PubMed] [Google Scholar]
- Lee, Lee S.-A.A., Kim H., Won Y.-S.S., Kim B.-J.J. Induction of endoplasmic reticulum-derived oxidative stress by an occult infection related S surface antigen variant. World J. Gastroenterol. 2015;21:6872–6883. doi: 10.3748/wjg.v21.i22.6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Zhang L.K., Li S.F., Zhang S.F., Wan W.W., Zhang Y.L., Xin Q.L., Dai K., Hu Y.Y., Wang Z.B., Zhu X.T., Fang Y.J., Cui N., Zhang P.H., Yuan C., Lu Q. Bin, Bai J.Y., Deng F., Xiao G.F., Liu W., Peng K. Calcium channel blockers reduce severe fever with thrombocytopenia syndrome virus (SFTSV) related fatality. Cell Res. 2019;29:739–753. doi: 10.1038/s41422-019-0214-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Boehning D.F., Qian T., Popov V.L., Weinman S.A. Hepatitis C virus core protein increases mitochondrial ROS production by stimulation of Ca2+ uniporter activity. Faseb. J. 2007;21:2474–2485. doi: 10.1096/fj.06-7345com. [DOI] [PubMed] [Google Scholar]
- Liu L. Fields virology. Clin. Infect. Dis. 2014;59 doi: 10.1093/cid/ciu346. 613–613, sixth ed. [DOI] [Google Scholar]
- Lloyd-Evans E., Platt F.M. Lysosomal Ca2+ homeostasis: role in pathogenesis of lysosomal storage diseases. Cell Calcium. 2011 doi: 10.1016/j.ceca.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Luganini A., Di Nardo G., Munaron L., Gilardi G., Pla A.F., Gribaudo G. Human cytomegalovirus US21 protein is a viroporin that modulates calcium homeostasis and protects cells against apoptosis. Proc. Natl. Acad. Sci. U.S.A. 2018;115:E12370–E12377. doi: 10.1073/pnas.1813183115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madesh M., Hajnóczky G. VDAC-dependent permeabilization of the outer mitochondrial membrane by superoxide induces rapid and massive cytochrome c release. J. Cell Biol. 2001;155:1003–1015. doi: 10.1083/jcb.200105057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madigan V.J., Berry G.E., Tyson T.O., Nardone-White D., Ark J., Elmore Z.C., Murlidharan G., Vincent H.A., Asokan A. The Golgi calcium ATPase pump plays an essential role in adeno-associated virus trafficking and transduction. J. Virol. 2020;94:1604–1624. doi: 10.1128/jvi.01604-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammucari C., Raffaello A., Vecellio Reane D., Gherardi G., De Mario A., Rizzuto R. Mitochondrial calcium uptake in organ physiology: from molecular mechanism to animal models. Pflügers Archiv. 2018;470:1165–1179. doi: 10.1007/s00424-018-2123-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manninen A., Saksela K. HIV-1 Nef interacts with inositol trisphosphate receptor to activate calcium signaling in T cells. J. Exp. Med. 2002;195:1023–1032. doi: 10.1084/jem.20012039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain S.L., Clippinger A.J., Lizzano R., Bouchard M.J. Hepatitis B virus replication is associated with an HBx-dependent mitochondrion-regulated increase in cytosolic calcium levels. J. Virol. 2007;81:12061–12065. doi: 10.1128/jvi.00740-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchionda M., Pittman J.K., Mayor R., Patel S. Ca2+/H+ exchange by acidic organelles regulates cell migration in vivo. J. Cell Biol. 2016;212:803–813. doi: 10.1083/jcb.201510019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W.E., Zagorski W.A., Brenneman J.D., Avery D., Miller J.L.C.C., O'Connor C.M. US28 is a potent activator of phospholipase C during HCMV infection of clinically relevant target cells. PloS One. 2012;7 doi: 10.1371/journal.pone.0050524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moin S.M., Panteva M., Jameel S. The hepatitis E virus Orf3 protein protects cells from mitochondrial depolarization and death. J. Biol. Chem. 2007;282:21124–21133. doi: 10.1074/jbc.M701696200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A.P., Scott J.K., Ball J.M., Zeng C.Q.Y.Y., O'Neal W.K., Estes M.K. NSP4 elicits age-dependent diarrhea and Ca2+-mediated I- influx into intestinal crypts of CF mice. Am. J. Physiol. Gastrointest. Liver Physiol. 1999;277 doi: 10.1152/ajpgi.1999.277.2.g431. [DOI] [PubMed] [Google Scholar]
- Nath A., Padua R.A., Geiger J.D. HIV-1 coat protein gp120-induced increases in levels of intrasynaptosomal calcium. Brain Res. 1995;678:200–206. doi: 10.1016/0006-8993(95)00185-S. [DOI] [PubMed] [Google Scholar]
- Nathan L., Lai A.L., Millet J.K., Straus M.R., Freed J.H., Whittaker G.R., Daniel S. Calcium ions directly interact with the Ebola virus fusion peptide to promote structure-function changes that enhance infection. ACS Infect. Dis. 2020;6:250–260. doi: 10.1021/acsinfecdis.9b00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie J., Ta N., Liu L., Shi G., Kang T., Zheng Z. Activation of CaMKII via ER-stress mediates coxsackievirus B3-induced cardiomyocyte apoptosis. Cell Biol. Int. 2020;44:488–498. doi: 10.1002/cbin.11249. [DOI] [PubMed] [Google Scholar]
- Nugent K.M., Shanley J.D. Verapamil inhibits influenza A virus replication. Arch. Virol. 1984;81:163–170. doi: 10.1007/BF01309305. [DOI] [PubMed] [Google Scholar]
- Ohta A., Nishiyama Y. Mitochondria and viruses. Mitochondrion. 2011;11:1–12. doi: 10.1016/j.mito.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., Guo L., Guo R., Chen T., Hu J., Xiang Z., Mu Z., Chen X., Chen J., Hu K., Jin Q., Wang J., Qian Z. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11:1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palty R., Silverman W.F., Hershfinkel M., Caporale T., Sensi S.L., Parnis J., Nolte C., Fishman D., Shoshan-Barmatz V., Herrmann S., Khananshvili D., Sekler I. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc. Natl. Acad. Sci. U. S. A. 2010;107:436–441. doi: 10.1073/pnas.0908099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S., Behera S., Alam M.F., Syed G.H. Endoplasmic reticulum & mitochondrial calcium homeostasis: the interplay with viruses. Mitochondrion. 2021;58:227–242. doi: 10.1016/j.mito.2021.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp B., Launay S., Gélébart P., Arbabian A., Enyedi A., Brouland J.P., Carosella E.D., Adle-Biassette H. Endoplasmic reticulum calcium pumps and tumor cell differentiation. Int. J. Mol. Sci. 2020 doi: 10.3390/ijms21093351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks R.J., Murphy E., Liu J.C. Mitochondrial permeability transition pore and calcium handling. Methods Mol. Biol. 2018;1782:187–196. doi: 10.1007/978-1-4939-7831-1_11. [DOI] [PubMed] [Google Scholar]
- Parvez M.K., Parveen S. Evolution and emergence of pathogenic viruses: past, present, and future. Intervirology. 2017;60:1–7. doi: 10.1159/000478729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parys J.B., Guse A.H. Full focus on calcium. Sci. Signal. 2019;12 doi: 10.1126/scisignal.aaz0961. [DOI] [PubMed] [Google Scholar]
- Patel S. Function and dysfunction of two-pore channels. Sci. Signal. 2015;8:re7. doi: 10.1126/scisignal.aab3314. [DOI] [PubMed] [Google Scholar]
- Patel S., Cai X. Evolution of acidic Ca2+ stores and their resident Ca2+-permeable channels. Cell Calcium. 2015;57:222–230. doi: 10.1016/j.ceca.2014.12.005. [DOI] [PubMed] [Google Scholar]
- Patel S., Docampo R. Acidic calcium stores open for business: expanding the potential for intracellular Ca2+ signaling. Trends Cell Biol. 2010;20:277–286. doi: 10.1016/j.tcb.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S., Muallem S. Acidic Ca2+ stores come to the fore. Cell Calcium. 2011;50:109–112. doi: 10.1016/j.ceca.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Penfold M.E.T.T., Dairaghi D.J., Duke G.M., Saederup N., Mocarski E.S., Kemble G.W., Schall T.J. Cytomegalovirus encodes a potent α chemokine. Proc. Natl. Acad. Sci. U.S.A. 1999;96:9839–9844. doi: 10.1073/pnas.96.17.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny C.J., Vassileva K., Jha A., Yuan Y., Chee X., Yates E., Mazzon M., Kilpatrick B.S., Muallem S., Marsh M., Rahman T., Patel S. Mining of Ebola virus entry inhibitors identifies approved drugs as two-pore channel pore blockers. Biochim. Biophys. Acta Mol. Cell Res. 2019;1866:1151–1161. doi: 10.1016/j.bbamcr.2018.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez J.F., Chemello M.E., Liprandi F., Ruiz M.C., Michelangeli F. Oncosis in MA104 cells is induced by rotavirus infection through an increase in intracellular Ca2+ concentration. Virology. 1998;252:17–27. doi: 10.1006/viro.1998.9433. [DOI] [PubMed] [Google Scholar]
- Pérez J.F., Ruiz M.-C., Chemello M.E., Michelangeli F. Characterization of a membrane calcium pathway induced by rotavirus infection in cultured cells. J. Virol. 1999;73:2481–2490. doi: 10.1128/jvi.73.3.2481-2490.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham T., Perry J.L., Dosey T.L., Delcour A.H., Hyser J.M. The rotavirus NSP4 viroporin domain is a calcium-conducting ion channel. Sci. Rep. 2017;7 doi: 10.1038/srep43487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccoli C., Scrima R., D'Aprile A., Ripoli M., Lecce L., Boffoli D., Capitanio N. Mitochondrial dysfunction in hepatitis C virus infection. Biochim. Biophys. Acta. 2006;1757:1429–1437. doi: 10.1016/j.bbabio.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Piccoli C., Scrima R., Quarato G., D'Aprile A., Ripoli M., Lecce L., Boffoli D., Moradpour D., Capitanio N. Hepatitis C virus protein expression causes calcium-mediated mitochondrial bioenergetic dysfunction and nitro-oxidative stress. Hepatology. 2007;46:58–65. doi: 10.1002/hep.21679. [DOI] [PubMed] [Google Scholar]
- Pillay S., Meyer N.L., Puschnik A.S., Davulcu O., Diep J., Ishikawa Y., Jae L.T., Wosen J.E., Nagamine C.M., Chapman M.S., Carette J.E. An essential receptor for adeno-associated virus infection. Nature. 2016;530:108–112. doi: 10.1038/nature16465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzo P., Lissandron V., Capitanio P., Pozzan T. Ca2+ signalling in the Golgi apparatus. Cell Calcium. 2011 doi: 10.1016/j.ceca.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Pleskoff O., Casarosa P., Verneuil L., Ainoun F., Beisser P., Smit M., Leurs R., Schneider P., Michelson S., Ameisen J.C. The human cytomegalovirus-encoded chemokine receptor US28 induces caspase-dependent apoptosis. FEBS J. 2005;272:4163–4177. doi: 10.1111/j.1742-4658.2005.04829.x. [DOI] [PubMed] [Google Scholar]
- Poncet D., Larochette N., Pauleau A.L., Boya P., Jalil A.A., Cartron P.F., Vallette F., Schnebelen C., Bartle L.M., Skaletskaya A., Boutolleau D., Martinou J.C., Goldmacher V.S., Kroemer G., Zamzami N. An anti-apoptotic viral protein that recruits Bax to mitochondria. J. Biol. Chem. 2004;279:22605–22614. doi: 10.1074/jbc.M308408200. [DOI] [PubMed] [Google Scholar]
- Prakriya M., Lewis R.S. Store-operated calcium channels. Physiol. Rev. 2015;95:1383–1436. doi: 10.1152/physrev.00020.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarato G., D'Aprile A., Gavillet B., Vuagniaux G., Moradpour D., Capitanio N., Piccoli C. The cyclophilin inhibitor alisporivir prevents hepatitis C virus-mediated mitochondrial dysfunction. Hepatology. 2012;55:1333–1343. doi: 10.1002/hep.25514. [DOI] [PubMed] [Google Scholar]
- Raffaello A., Mammucari C., Gherardi G., Rizzuto R. Calcium at the center of cell signaling: interplay between endoplasmic reticulum, mitochondria, and lysosomes. Trends Biochem. Sci. 2016;41:1035–1049. doi: 10.1016/j.tibs.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmani Z., Huh K.W., Lasher R., Siddiqui A. Hepatitis B virus X protein colocalizes to mitochondria with a human voltage-dependent anion channel, HVDAC3, and alters its transmembrane potential. J. Virol. 2000;74:2840–2846. doi: 10.1128/jvi.74.6.2840-2846.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasola A., Bernardi P. Mitochondrial permeability transition in Ca(2+)-dependent apoptosis and necrosis. Cell Calcium. 2011;50:222–233. doi: 10.1016/j.ceca.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Rhee S.G. Regulation of phosphoinositide-specific phospholipase C. Annu. Rev. Biochem. 2001 doi: 10.1146/annurev.biochem.70.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R., Pinton P., Carrington W., Fay F.S., Fogarty K.E., Lifshitz L.M., Tuft R.A., Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280(80):1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- Romero-Brey I., Bartenschlager R. Endoplasmic reticulum: the favorite intracellular niche for viral replication and assembly. 2016. Viruses. [DOI] [PMC free article] [PubMed]
- Rouse B.T., Sehrawat S. Immunity and immunopathology to viruses: what decides the outcome? Nat. Rev. Immunol. 2010 doi: 10.1038/nri2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozzi S.J., Avdoshina V., Fields J.A., Trejo M., Ton H.T., Ahern G.P., Mocchetti I. Human immunodeficiency virus promotes mitochondrial toxicity. Neurotox. Res. 2017;32:723–733. doi: 10.1007/s12640-017-9776-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu W.-S. Molecular Virology of Human Pathogenic Viruses. Elsevier; 2017. Virus life cycle; pp. 31–45. [DOI] [Google Scholar]
- Sakurai Y., Kolokoltsov A.A., Chen C.C., Tidwell M.W., Bauta W.E., Klugbauer N., Grimm C., Wahl-Schott C., Biel M., Davey R.A. Two-pore channels control Ebola virus host cell entry and are drug targets for disease treatment. Science. 2015;347:995–998. doi: 10.1126/science.1258758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta A., Hughes T.E.T., Moiseenkova-Bell V.Y. Subcellular Biochemistry. Springer; New York: 2018. Transient receptor potential (TRP) channels; pp. 141–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarute N., Ross S.R. CACNA1S haploinsufficiency confers resistance to New World arenavirus infection. Proc. Natl. Acad. Sci. U.S.A. 2020;117 doi: 10.1073/pnas.1920551117. 19497–19506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastri N.P., Crawford S.E., Estes M.K. Viral Gastroenteritis: Molecular Epidemiology and Pathogenesis. Elsevier Inc.; 2016. Pleiotropic properties of rotavirus nonstructural protein 4 (NSP4) and their effects on viral replication and pathogenesis; pp. 145–174. [DOI] [Google Scholar]
- Scharenberg A.M., Humphries L.A., Rawlings D.J. Calcium signalling and cell-fate choice in B cells. Nat. Rev. Immunol. 2007 doi: 10.1038/nri2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self R.L., Mulholland P.J., Nath A., Harris B.R., Prendergast M.A. The human immunodeficiency virus type-1 transcription factor Tat produces elevations in intracellular Ca2+ that require function of an N-methyl-D-aspartate receptor polyamine-sensitive site. Brain Res. 2004;995:39–45. doi: 10.1016/j.brainres.2003.09.052. [DOI] [PubMed] [Google Scholar]
- Sharma N., Arora S., Saurav S., Motiani R.K. Pathophysiological significance of calcium signaling at mitochondria-associated endoplasmic reticulum membranes (MAMs) Curr. Opin. Physiol. 2020;17:234–242. doi: 10.1016/j.cophys.2020.08.012. [DOI] [Google Scholar]
- Sharon-Friling R., Goodhouse J., Colberg-Poley A.M., Shenk T. Human cytomegalovirus pUL37x1 induces the release of endoplasmic reticulum calcium stores. Proc. Natl. Acad. Sci. U.S.A. 2006;103 doi: 10.1073/pnas.0609353103. 19117–19122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons J.A., D'Souza R.S., Ruas M., Galione A., Casanova J.E., White J.M. Ebolavirus glycoprotein directs fusion through NPC1 + endolysosomes. J. Virol. 2016;90:605–610. doi: 10.1128/jvi.01828-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth J.T., Hwang S.Y., Tomita T., DeHaven W.I., Mercer J.C., Putney J.W. Activation and regulation of store-operated calcium entry. J. Cell Mol. Med. 2010;14:2337–2349. doi: 10.1111/j.1582-4934.2010.01168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus M.R., Bidon M., Tang T., Whittaker G.R., Daniel S. FDA approved calcium channel blockers inhibit SARS-CoV-2 infectivity in epithelial lung cells. 2020. bioRxiv. [DOI]
- Straus M.R., Tang T., Lai A.L., Flegel A., Bidon M., Freed J.H., Daniel S., Whittaker G.R. Ca 2+ ions promote fusion of Middle East respiratory syndrome coronavirus with host cells and increase infectivity. J. Virol. 2020;94:426–446. doi: 10.1128/jvi.00426-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutzmann G.E., Mattson M.P. Endoplasmic reticulum Ca(2+) handling in excitable cells in health and disease. Pharmacol. Rev. 2011;63:700–727. doi: 10.1124/pr.110.003814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh P.G., Park J. Il, Manzoli L., Cocco L., Peak J.C., Katan M., Fukami K., Kataoka T., Yun S., Sung H.R. Multiple roles of phosphoinositide-specific phospholipase C isozymes. J. Biochem. Mol. Biol. 2008;41:415–434. doi: 10.5483/bmbrep.2008.41.6.415. [DOI] [PubMed] [Google Scholar]
- Takada S., Shirakata Y., Kaneniwa N., Koike K. Association of hepatitis B virus X protein with mitochondria causes mitochondrial aggregation at the nuclear periphery, leading to cell death. Oncogene. 1999;18:6965–6973. doi: 10.1038/sj.onc.1203188. [DOI] [PubMed] [Google Scholar]
- Tanwar J., Arora S., Motiani R.K. Cell Calcium; 2020. Orai3: oncochannel with therapeutic potential. [DOI] [PubMed] [Google Scholar]
- Tanwar J., Motiani R.K. Cell Calcium; 2018. Role of SOCE architects STIM and Orai proteins in cell death. [DOI] [PubMed] [Google Scholar]
- Tanwar J., Singh J.B., Motiani R.K. Molecular machinery regulating mitochondrial calcium levels: the nuts and bolts of mitochondrial calcium dynamics. Mitochondrion. 2021;57:9–22. doi: 10.1016/j.mito.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian P., Estes M.K., Hu Y., Ball J.M., Zeng C.Q., Schilling W.P. The rotavirus nonstructural glycoprotein NSP4 mobilizes Ca2+ from the endoplasmic reticulum. J. Virol. 1995;69:5763–5772. doi: 10.1128/jvi.69.9.5763-5772.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian P., Hu Y., Schilling W.P., Lindsay D.A., Eiden J., Estes M.K. The nonstructural glycoprotein of rotavirus affects intracellular calcium levels. J. Virol. 1994;68:251–257. doi: 10.1128/jvi.68.1.251-257.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi P.C., Bartlett J.J., Pulinilkunnil T. Lysosomal biology and function: modern view of cellular debris bin. 2020. Cells. 9. [DOI] [PMC free article] [PubMed]
- Vatansever E.C., Yang K.S., Drelich A.K., Kratch K.C., Cho C.C., Kempaiah K.R., Hsu J.C., Mellott D.M., Xu S., Tseng C.T.K., Liu W.R. Bepridil is potent against SARS-CoV-2 in vitro. Proc. Natl. Acad. Sci. U.S.A. 2021;118 doi: 10.1073/pnas.2012201118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecellio Reane D., Rizzuto R., Raffaello A. The ER-mitochondria tether at the hub of Ca2+ signaling. Curr. Opin. Physiol. 2020;17:261–268. doi: 10.1016/j.cophys.2020.08.013. [DOI] [Google Scholar]
- Vermassen E., Parys J.B., Mauger J.P. Subcellular distribution of the inositol 1,4,5-trisphosphate receptors: functional relevance and molecular determinants. Biol. Cell. 2004 doi: 10.1016/j.biolcel.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Wang S., Liu Y., Guo J., Wang P., Zhang L., Xiao G., Wang W. Screening of FDA-approved drugs for inhibitors of Japanese encephalitis virus infection. J. Virol. 2017;91 doi: 10.1128/JVI.01055-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Campbell R.V., Yi M.K., Lemon S.M., Weinman S.A. Role of Hepatitis C virus core protein in viral-induced mitochondrial dysfunction. J. Viral Hepat. 2010;17:784–793. doi: 10.1111/j.1365-2893.2009.01238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Huong S.M., Chiu M.L., Raab-Traub N., Huang E.S. Epidermal growth factor receptor is a cellular receptor for human cytomegalovirus. Nature. 2003;424:456–461. doi: 10.1038/nature01818. [DOI] [PubMed] [Google Scholar]
- Wang X., Zhang X., Dong X.P., Samie M., Li X., Cheng X., Goschka A., Shen D., Zhou Y., Harlow J., Zhu M.X., Clapham D.E., Ren D., Xu H. TPC proteins are phosphoinositide- activated sodium-selective ion channels in endosomes and lysosomes. Cell. 2012;151:372–383. doi: 10.1016/j.cell.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessler S., Backert S. International Review of Cell and Molecular Biology. Elsevier Inc.; 2011. Abl family of tyrosine kinases and microbial pathogenesis; pp. 271–300. [DOI] [PubMed] [Google Scholar]
- Woods J.J., Nemani N., Shanmughapriya S., Kumar A., Zhang M., Nathan S.R., Thomas M., Carvalho E., Ramachandran K., Srikantan S., Stathopulos P.B., Wilson J.J., Madesh M. A selective and cell-permeable mitochondrial calcium uniporter (MCU) inhibitor preserves mitochondrial bioenergetics after hypoxia/reoxygenation injury. ACS Cent. Sci. 2019;5:153–166. doi: 10.1021/acscentsci.8b00773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B., Bouchard M.J. The hepatitis B virus X protein elevates cytosolic calcium signals by modulating mitochondrial calcium uptake. J. Virol. 2012;86:313–327. doi: 10.1128/jvi.06442-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Zhao X., Xu J., Shang W., Tong C. A novel fluorescent reporter detects plastic remodeling of mitochondria-ER contact sites. J. Cell Sci. 2018;131 doi: 10.1242/jcs.208686. [DOI] [PubMed] [Google Scholar]
- Yao J., hong, Liu Z., jian Yi, hua J., Wang J., Liu Y. nan. Hepatitis B virus X protein upregulates intracellular calcium signaling by binding C-terminal of Orail protein. Curr. Med. Sci. 2018;38:26–34. doi: 10.1007/s11596-018-1843-z. [DOI] [PubMed] [Google Scholar]
- Zhao Z., Qin P., Huang Y.W. Cell Calcium; 2021. Lysosomal ion channels involved in cellular entry and uncoating of enveloped viruses: implications for therapeutic strategies against SARS-CoV-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Frey T.K., Yang J.J. Viral calciomics: interplays between Ca2+ and virus. Cell Calcium. 2009;46:1–17. doi: 10.1016/j.ceca.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Xue S., Yang J.J. Encyclopedia of Metalloproteins. Springer; New York: 2013. Calcium and viruses; pp. 415–424. [DOI] [Google Scholar]
- Zhu S., Ding S., Wang P., Wei Z., Pan W., Palm N.W., Yang Y., Yu H., Li H.B., Wang G., Lei X., De Zoete M.R., Zhao J., Zheng Y., Chen H., Zhao Y., Jurado K.A., Feng N., Shan L., Kluger Y., Lu J., Abraham C., Fikrig E., Greenberg H.B., Flavell R.A. Nlrp9b inflammasome restricts rotavirus infection in intestinal epithelial cells. Nature. 2017;546:667–670. doi: 10.1038/nature22967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zocchi M.R., Rubartelli A., Morgavi P., Poggi A. HIV-1 tat inhibits human natural killer cell function by blocking L- type calcium channels. J. Immunol. 1998;161:2938–2943. [PubMed] [Google Scholar]