Abstract
Respiratory syncytial virus (RSV) is the leading cause of lower respiratory tract infection in young children. The T cell response plays a critical role in facilitating clearance of an acute RSV infection, and memory T cell responses are vital for protection against secondary RSV exposures. Tissue resident memory (TRM) T cells have been identified as a subset of memory T cells that reside in non-lymphoid tissues and are critical for providing long-term immunity. There is currently limited information regarding the establishment and longevity of TRM T cell responses elicited following an acute RSV infection as well as their role in protection against repeated RSV infections. Here we examined the magnitude, phenotype, and protective capacity of TRM CD4 and CD8 T cells in the lungs of BALB/c mice following an acute RSV infection. TRM CD4 and CD8 T cells were established within the lungs and waned by 149 days following RSV infection. To determine the protective capacity of TRMs, FTY-720 administration was used to prevent trafficking of peripheral memory T cells into the lungs prior to challenge of RSV-immune mice with a recombinant influenza virus expressing either an RSV-derived CD4 or CD8 T cell epitope. We observed enhanced viral clearance in RSV-immune mice suggesting that TRM CD8 T cells can contribute to protection against a secondary RSV infection. Given the protective capacity of TRMs, future RSV vaccine candidates should focus on the generation of these cell populations within the lung to induce effective immunity against RSV infection.
Introduction
Respiratory syncytial virus (RSV) is a common cause of lower respiratory tract infections (LRTI) in children worldwide with an estimated 33.1 million cases per year leading to 3.2 million hospital admissions and 59,600 deaths (1). By two years of age, nearly all children have been infected with RSV at least once (2). Despite the high morbidity and mortality associated with RSV infection, there is currently no licensed RSV vaccine. In the 1960s, a formalin-inactivated RSV vaccine (FI-RSV) was produced, but a majority of patients vaccinated still experienced natural RSV infection. Of those infected, 80% required hospitalization, and two patients died secondary to their pulmonary disease with increased pulmonary eosinophils noted on autopsy (3). Further investigation has demonstrated that FI-RSV failed to generate neutralizing antibodies as well as RSV-specific memory CD8 T cells, the combination of which allowed for the development of a robust mixed Th1 and Th2 CD4 T cell memory response in the lung that resulted in mucus overproduction and airway hypersensitivity leading to vaccine-enhanced disease (4–7). The lack of CD8 T cell production is particularly noteworthy, as they have been shown to ameliorate the Th2 response and vaccine-enhanced disease (7, 8).
The T cell response to RSV infection plays a critical role in mediating both viral clearance and disease severity. After antibody depletion of either CD4 or CD8 T cells, mice infected with RSV demonstrated prolonged detectable viral titers and decreased manifestations of disease (9). A Th2-skewed CD4 T cell response has been implicated as particularly critical in mediating RSV-induced disease following acute infection of infants. Nasal washes from infants with RSV-confirmed lower respiratory tract infections exhibited increased IL-4/IFN-γ ratios compared to infants with upper respiratory tract infections (10). The role of CD8 T cells in protection and immunopathology has been demonstrated in irradiated mice, who were able to clear RSV following transfer of CD8 T cells; however, they also experienced increased weight loss and pulmonary disease (11). The importance of T cells for viral clearance has also been shown in humans, as children with immunodeficiencies that result in impaired T cell function exhibit prolonged viral shedding (12).
Memory CD4 T cells have been implicated in immunopathology following RSV infection. Previous work in our laboratory has demonstrated that the memory T cell effector response that develops following RSV challenge of FI-RSV-immunized mice consists of both Th1 and Th2 CD4 T cells. Th1 memory CD4 T cells mediate the induction of airway obstruction and weight loss, and Th2 memory CD4 T cells induce increased mucus production and airway hyperreactivity (5). Furthermore, when CD4 T cells were depleted in mice previously immunized with FI-RSV, there was a significant decrease in airway obstruction, weight loss, and airway resistance following RSV challenge (5). Memory CD4 T cells are also crucial in mediating viral clearance. In mice infected with RSV, adoptive transfer of in vitro-stimulated RSV-specific memory CD4 T cells led to reduced RSV titers. At the same time, transfer of these cells also led to enhanced weight loss and pulmonary disease. The effects of transferred CD4 T cells was specific to RSV-infected mice and was not seen in mice infected with influenza or naïve mice (13). Memory CD8 T cells have been demonstrated to provide protection against RSV. Mice infected with murine CMV expressing the RSV matrix (M) protein developed effector memory CD8 T cells that mediated enhanced viral clearance following RSV challenge (14). Additionally, work in our laboratory demonstrated that eliciting a high magnitude of RSV-specific memory CD8 T cells via a dendritic cell-Listeria monocytogenes prime-boost immunization regimen caused enhanced viral clearance at the expense of severe and fatal CD8 T cell-mediated immunopathology (15).
Tissue resident memory (TRM) T cells represent a recently identified subset of memory T cells that are retained in non-lymphoid tissues and contribute to protective immunity (16). TRMs are typically defined by the cell markers CD69 and CD103, with TRM CD4 T cells defined as CD69+CD103−, and TRM CD8 T cells defined as CD69+CD103+ (17). CD69 is an early marker of T cell activation and antagonizes sphingosine-1-phosphate receptor function to facilitate lymphocyte retention within tissues (18), and CD103 binds E-cadherin on epithelial cells (19). TRM CD4 and CD8 T cells have been identified in a variety of tissue types including the murine female genital tract and intestine (20–23). Additionally, TRM CD8 T cells have been demonstrated in the brain and skin of mice (24, 25). Both TRM CD4 and CD8 T cells have been identified in murine lungs following infection with influenza (17). TRM CD4 and CD8 T cells are also generated following influenza infection in mice and mediate protection against non-vaccine viral strains (26). In the setting of RSV infection, TRM CD8 T cells are established in the airways of African green monkeys and healthy human adults (27, 28). Adults infected with RSV also demonstrated decreased viral load and disease severity that was associated with increased TRM CD8 T cell populations (28). Similarly, TRM CD4 T cells were demonstrated in the airway following RSV infection in healthy adults without any correlations with disease severity or viral clearance (29). These studies showcase the presence of TRM CD4 and CD8 T cells following RSV infection in various models. However, there is a lack of exhaustive studies examining the kinetics and phenotypes of TRMs in the lungs following acute RSV infection. Furthermore, while there was a correlation with protection by TRM CD8 T cells in adults with RSV, direct experimental evidence of protection against RSV by TRMs is currently lacking.
We sought to establish the population kinetics of TRM CD4 and CD8 T cells following acute RSV infection to a late memory timepoint using a murine model. We demonstrated that both TRM CD4 and CD8 T cells are established in the lungs following acute RSV infection and wane with time by 5 months following infection. We also delineated the protective capacity of TRM CD4 and CD8 T cells in the lungs using FTY-720 administration prior to infection of RSV-immune mice with a recombinant influenza virus expressing RSV-derived CD4 and CD8 T cell epitopes. Our data demonstrate that TRM CD8 T cells in the lung contribute to protection and that protection is not mediated by circulating memory or effector cells. This has important implications in the development of an RSV vaccine. Vaccines capable of generating an RSV-specific memory T cell response, particularly a TRM CD8 T cell response in the lung, may offer a great opportunity for generating effective immunity to RSV.
Materials and Methods
Mice
Female BALB/c mice between 6–8 weeks old were purchased from the National Cancer Institute (Frederick, MD). All experimental procedures were approved by the University of Iowa Animal Care and Use Committee under Animal Protocols #4101196 and #7041999. The experiments were performed under strict accordance to the Office of Laboratory Animal Welfare guidelines and the PHS Policy on Humane Care and Use of Laboratory Animals.
Viruses and infection
The A2 strain of RSV was a gift from Dr. Barney Graham (National Institutes of Health, Bethesda, MD). Mice were infected intranasally (i.n.) with SV, IAV-A/PR8/34 (IAV-PR8) or recombinant IAV strains following sedation with isoflurane. Recombinant IAV-M282 and IAV-F51 were provided by Dr. Ryan Langlois (University of Minnesota, Minneapolis, MN). The viruses were created using standard reverse genetics as previously described (30). They were rescued and grown in 10 day-old embryonated chicken eggs (Charles River). M282 and F51 epitopes were inserted into the mRNA at nucleotide position 186 encoding the neuraminidase stalk region, which has been previously demonstrated to be tolerant of such insertions (31).
FTY-720 Treatment
In the TRM protection studies, mice were treated with 1 mg/kg of FTY-720 (Cayman Chemical, Ann Arbor, MI) intraperitoneally (i.p.) once daily. A stock solution was prepared in DMSO and subsequently diluted to the desired concentration in endotoxin free water. Treatment lasted for 7 days beginning 3 days prior to recombinant influenza challenge and ending on day 4 post-infection (p.i.).
Flow cytometry analysis and tissue collection
Mice were injected intravenously (i.v.) with 1 ug CD45-APC (clone 30-F11) or CD45-FITC (clone 30-F11) antibody 3 minutes prior to euthanasia. Cells from the lungs were processed as previously described (32). Lungs were harvested and made into single-cell suspensions as previously described (33, 34). Cells from the lung were stained for extracellular surface molecules with antibodies specific to CD90.2 (clone 53–2.1), CD4 (clone GK1.5), CD8 (clone 53–6.7), CD49d (clone R1–2), CD11a (clone M17/4), CD103 (clone 2E7), CD69 (clone H1.2F3), CD62L (MEL-14), CD122 (5H4), and CXCR3 (clone CXCR3–173) for 30 minutes at 4°C and fixed with fix/lyse solution (eBioscience) for 10 minutes at room temperature. RSV-specific CD8 T cells were identified using a M282 tetramer, which is made in our laboratory. Stained cells were run on an LSRFortessa (BD Biosciences, San Jose, CA) and analyzed with FlowJo software (Tree Star, Ashland, OR).
Plaque assays for IAV
Whole lungs were harvested from mice, weighed, mechanically homogenized, and supernatants were stored at −80°C. For IAV plaque assays, MDCK (ATCC) cells in 6-well plates were washed three times with PBS. 1 mL of sterile Dulbecco’s modified Eagle’s medium was added afterwards. Plates were infected with 100 μL of serially diluted recombinant IAV-infected lung samples (10-fold dilutions) for 1 hour at 37°C and subsequently washed twice with PBS. Wells were overlaid with 2 mL of 1:1 mixture of 2X EMEM and 1.6% agarose containing 1 mg/mL TPCK-trypsin. After incubating at 37°C and 5% CO2 for 3 days, the agarose plugs were carefully removed. Monolayers were fixed with 2 mL 70% ethanol for 20 minutes at room temperature and subsequently stained with 1 mL of 1% crystal violet in methanol for 10 minutes at room temperature. Plates were washed with water, allowed to dry overnight, and plaques were counted the next morning.
Statistical analysis
Statistical analyses are detailed in each figure legend and were performed using Prism software (GraphPad Software, San Diego, CA). Data were evaluated using unpaired, two-tailed Student’s t-test between two groups with Holm-Sidak post-test analysis to determine if there was a statistical significance of at least α = 0.05.
Results
Kinetics and Persistence of RSV-specific CD4 and CD8 T Cells Following an Acute RSV Infection
Following an acute RSV infection, most virus-specific CD4 and CD8 effector T cells remain in the lung parenchyma for up to 30 days (35). However, it is not clear how long RSV-specific memory T cells remain in the lungs. We sought to assess the duration of virus-specific memory CD4 and CD8 T cells in the lungs following acute RSV infection. BALB/c mice were infected i.n. with RSV, and lungs were harvested on days 8, 10, 15, 30, 79, and 149 p.i. Importantly, mice were injected with an anti-CD45 antibody i.v. prior to euthanasia to distinguish cells in the lung parenchyma versus the pulmonary vasculature (32, 35, 36). RSV-specific CD4 T cells were identified using the surrogate activation marker approach CD49d+CD11ahi (37, 38) that identifies antigen-experienced CD4 T cells, and RSV-specific CD8 T cells were identified using an M282 tetramer. Following acute RSV infection, all CD4 T cell populations peaked within the lung parenchyma at day 8 p.i. and decreased in frequency up to 5 months following infection (Figure 1a). However, virus-specific CD49d+CD11ahi CD4 T cells were seen at higher frequencies than bulk CD4 T cells or CD49d−CD11alo non-activated CD4 T cells in the lung parenchyma, including at late memory timepoints (Figure 1a). Additionally, RSV-specific CD4 T cells were significantly increased in frequency and total number in the lung parenchyma compared to the vasculature (Figure 1b and 1c). While the total number of RSV-specific CD49d+CD11ahi CD4 T cells declined, the frequency was maintained up to 149 days p.i. (Figure 1b and 1c).
Figure 1. RSV-specific CD4 and CD8 T cells localize to the lungs.

BALB/c mice were infected with RSV i.n., and lungs were harvested at days 8, 10, 15, 30, 79, and 149 p.i. a) Frequency of CD4, CD49d−CD11alo, and CD49d+CD11ahi CD4 T cells in the lung parenchyma after RSV infection. b) Frequency and c) number of virus-specific CD49d+CD11ahi CD4 T cells in the lung parenchyma (IV−) and vasculature (IV+). d) Frequency of CD8, CD11alo, CD11ahi, and M282 tetramer positive CD8 T cells in the lung parenchyma after RSV infection. e) Frequency and f) number of IV− and IV+ CD11ahi CD8 T cells in the lung. g) Frequency and h) number of IV− and IV+ M282 tetramer+ CD8 T cells in the lung. Data are presented as mean ± SEM from two independent experiments that have been combined (n=3–4 mice per experiment). Statistical analysis was performed with Student’s t test, * p<0.05, ** p<0.01, *** p<0.001.
RSV-specific CD8 T cells were established in the lungs early following acute RSV infection and their frequency declined steadily over time (Figure 1d). M282 tetramer positive CD8 T cells were observed in the highest frequencies in the lung parenchyma compared to antigen-experienced CD11ahi, non-activated CD11alo, or bulk CD8 T cells (Figure 1d). The frequency of antigen-experienced CD11ahi CD8 T cells in the lungs was maintained at a higher level into memory as compared to the frequency maintained in the periphery (IV+), similar to what was observed with antigen-experienced CD49d+CD11ahi CD4 T cells (Figure 1e). Additionally, the number of antigen-experienced CD11ahi CD8 T cells in the lungs was significantly increased compared to the number in the periphery at early timepoints (p < 0.001) but decreased and showed no significant difference by day 30 p.i. (Figure 1f). M282-specific CD8 T cells were found almost exclusively in the lung tissue compared to the vasculature; however, there was a notable decrease in frequency at 149 days p.i. (Figure 1g and 1h). Thus, we demonstrate that RSV-specific CD4 and CD8 T cells are primarily found in the lung parenchyma following acute RSV infection, but their numbers gradually wane over time, which may contribute to the continued risk of reinfection with RSV observed in human adults (2, 39, 40).
TRM CD4 and CD8 T Cell Numbers Decline Over Time
We next sought to evaluate the kinetics of TRM CD4 and CD8 T cells following acute RSV infection. TRM CD4 T cells were classified as CD69+CD103−, and TRM CD8 T cells were defined as CD69+CD103+ (Figures 2a and 2b, respectively). The full gating strategy for TRM CD4 and CD8 T cells is outlined in Supplemental Figure 1. Following RSV infection, the frequency and total number of TRM CD4 T cells was significantly increased in the pulmonary tissue compared to the vasculature (Figure 2c and 2d). The frequency of TRM CD4 T cells remained relatively stable through 149 days p.i. (Figure 2c). However, the total number of TRM CD4 T cells declined over time (Figure 2d). In comparison to the number of total RSV-specific CD49d+CD11ahi CD4 T cells in the lung parenchyma, the number of TRM CD4 T cells in the lung tissue was decreased, although the difference was not statistically significant (Figure 2e). Both cell populations declined with time at a similar rate (Figure 2e).
Figure 2. CD4 and CD8 T cells numbers wane with time after RSV infection.
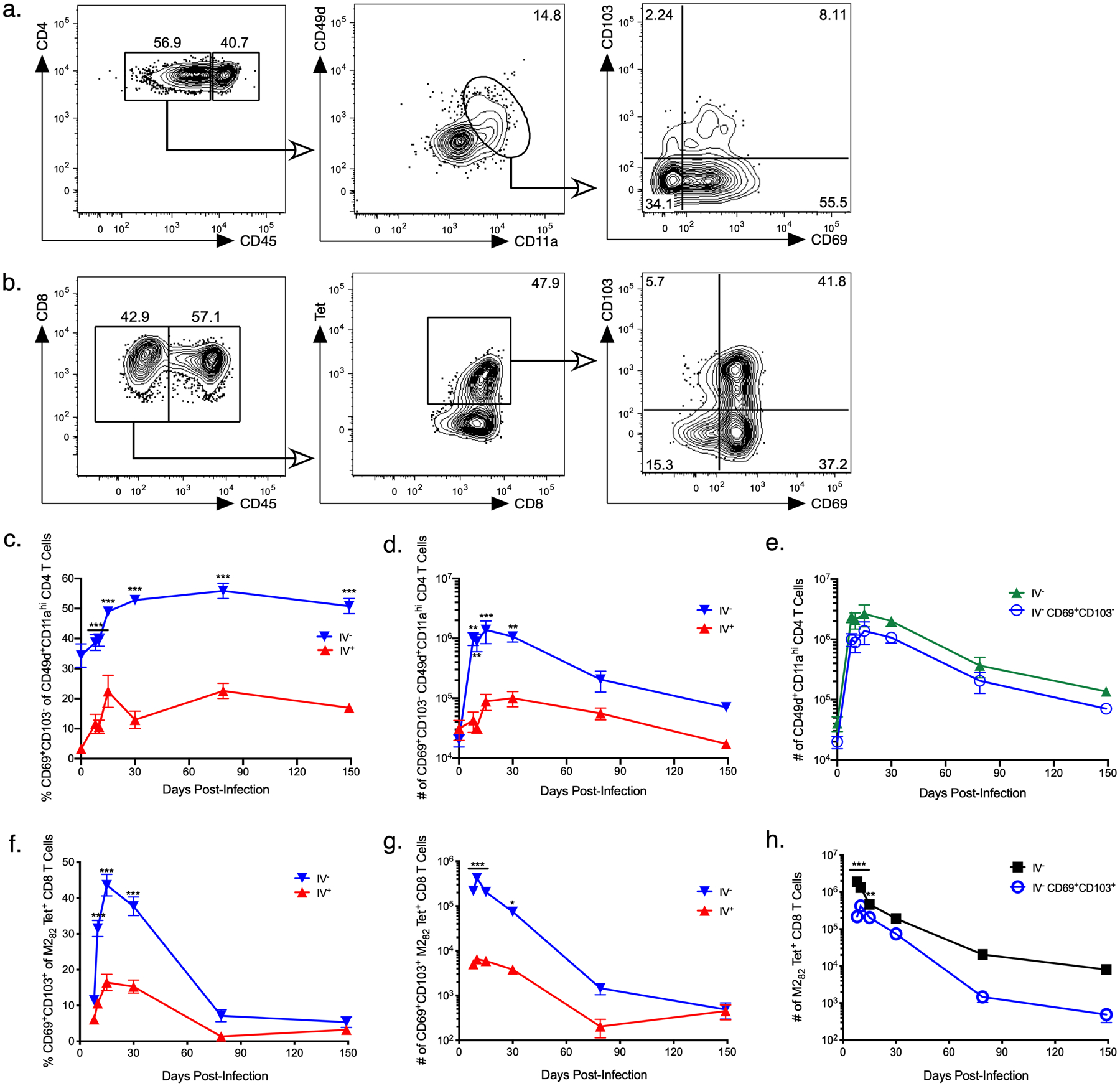
BALB/c mice were infected with RSV i.n., and lungs were harvested at days 8, 10, 15, 30, 79, and 149 p.i. Cells were analyzed by flow cytometry and gated on RSV-specific a) TRM CD4 T cells and b) TRM CD8 T cells in the lung parenchyma as shown. Representative staining panels are from day 30 p.i. c) Frequency and d) number of CD69+CD103− CD49d+CD11ahi CD4 T cells in the pulmonary parenchyma (IV−) and vasculature (IV+). e) Number of IV− CD69+CD103− CD49d+CD11ahi TRM and total CD49d+CD11ahi CD4 T cells in the lung. f) Frequency and g) number of IV− and IV+ CD69+CD103+ M282 tetramer+ CD8 T cells in the lung. h) Number of CD69+CD103+ M282 tetramer+ TRM and total M282 tetramer+ CD8 T cells in the lung. Data are presented as mean ± SEM from two independent experiments that have been combined (n=3–4 mice per experiment). Statistical analysis was performed with Student’s t test, * p<0.05, ** p <0.01, *** p<0.001.
As with TRM CD4 T cells, RSV-specific TRM CD8 T cells were significantly increased in both frequency and total number in the lung tissue compared to the vasculature at early timepoints following RSV infection (Figure 2f and 2g). Unlike TRM CD4 T cells, the frequency and total number of TRM CD8 T cells declined with time and showed no significant difference compared to that in the pulmonary vasculature at late time points (Figure 2f and 2g). M282-specific TRM CD8 T cells also exhibited significantly lower numbers in the lung tissue compared to all M282 tetramer+ CD8 T cells and showed a more rapid decline (Figure 2h). Therefore, acute RSV infection leads to the generation of RSV-specific TRM CD4 and CD8 T cells in the lung parenchyma. However, these populations appear to be transient and exhibit a more rapid decline as compared to the total number of virus-specific memory T cells identified using surrogate activation markers or tetramers.
We further examined the phenotypes of TRMs generated following RSV infection. CD62L, typically expressed at high levels on central memory cells, was expressed at low levels by both TRM CD4 (Figure 3a–c) and TRM CD8 (Figure 4a–c) T cells, which is consistent with previous descriptions of TRMs (16, 41). The frequency of TRM CD4 T cells that expressed either CXCR3 (Figure 3a and 3d) or CD122 (Figure 3a and 3f) was low throughout out all memory timepoints examined after day 30. However, the number of TRM CD4 T cells expressing either marker was significantly increased compared to circulating (IV+) CD49d+CD11ahi CD4 T cells at early timepoints (Figures 3e and 3g). CXCR3 expression was significantly increased on TRM CD8 T cells compared to circulating (IV+) M282 tetramer+ CD8 T cells through day 30 (Figure 4a). However, both the frequency and number of CXCR3+ TRM CD8 T cells decreased with time, correlating with the decline of TRM CD8 T cells in the lung (Figure 4d–e). The frequency of CD122-expressing TRM CD8 T cells and RSV-specific circulating (IV+) CD8 T cells was high on days 8–10 and then declined, remaining stable through day 149 (Figure 4a and 4f). The total number of TRM CD8 T cells expressing CD122 was significantly higher than circulating CD8 T cells at early timepoints (Figure 4g). This may represent the necessity of CD122 for TRM CD8 establishment and survival, which has been previously demonstrated in the skin of mice (42). Our data demonstrate that TRM CD4 and CD8 T cells generated after RSV infection have low expression levels of CD62L, consistent with general descriptions of TRMs. Expression of CD122 and CXCR3, however, is generally increased at acute timepoints and subsequently decreases over time.
Figure 3. Expression of phenotypic markers by TRM CD4 T cells.

a) Expression of CD62L, CXCR3, and CD122 by CD69+CD103− CD49d+CD11ahi CD4 T cells was compared to circulating (IV+) CD49d+CD11ahi CD4 T cells. Representative histograms from day 30 p.i. are shown. b) Frequency and c) number of CD62L+ cells in the lung. d) Frequency and e) number of CXCR3+ cells in the lung. f) Frequency and g) number of CD122+ cells in the lung. Data are represented as mean ± SEM from two independent experiments that have been combined (n=3–4 mice per experiment). Statistical analysis was performed with Student’s t test, * p<0.05, ** p<0.01, *** p<0.001.
Figure 4. Expression of phenotypic markers by CD8 T cells.
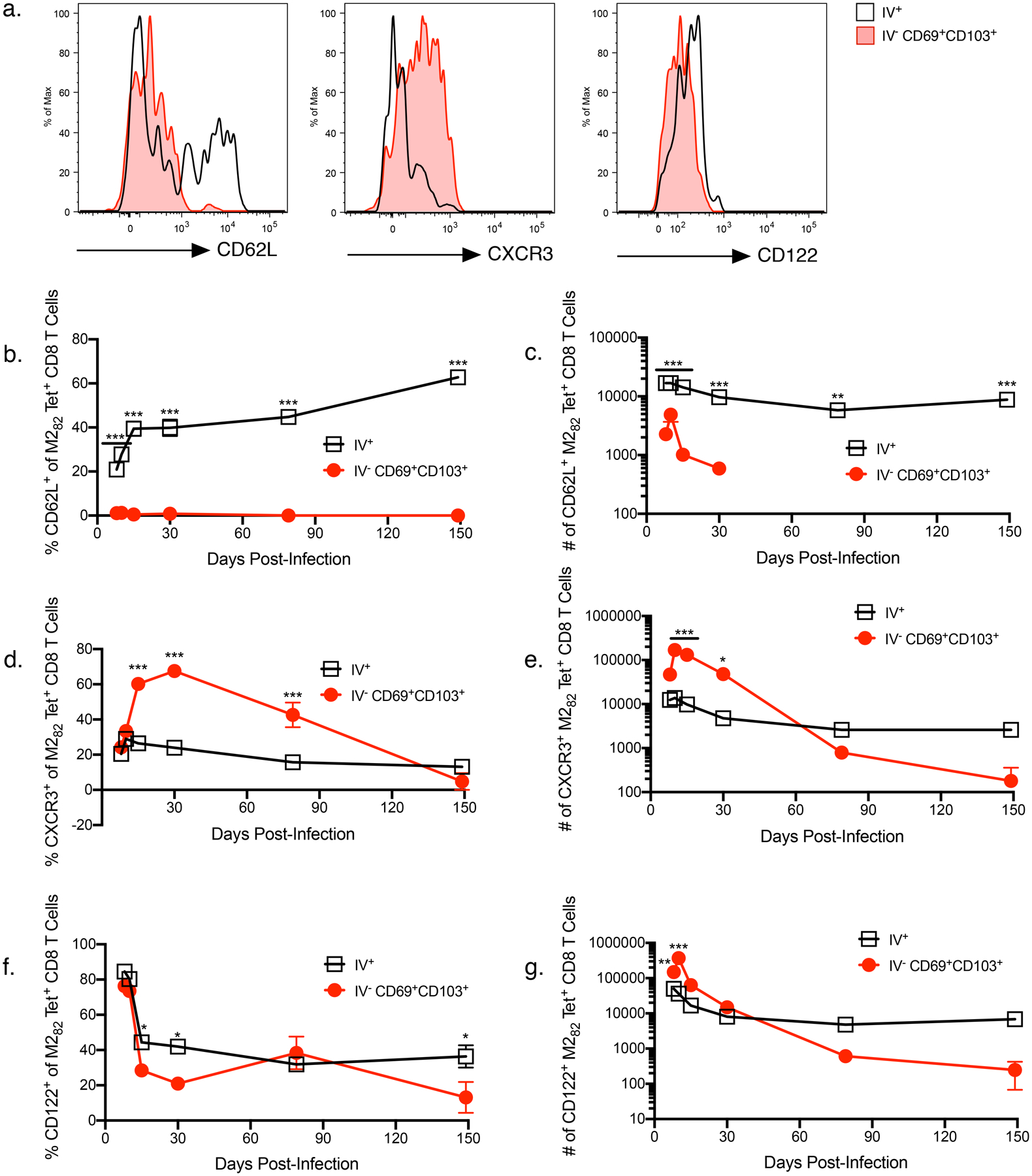
a) Expression of CD62L, CXCR3, and CD122 by CD69+CD103+ M282 tetramer+ CD8 T cells was compared to circulating (IV+) M282 tetramer+ CD8 T cells. Representative histograms from day 30 p.i. are shown. b) Frequency and c) number of CD62L+ cells in the lung. d) Frequency and e) number of CXCR3+ cells in the lung. f) Frequency and g) number of CD122+ cells in the lung. Data are presented as mean ± SEM from two independent experiments that have been combined (n=3–4 mice per experiment). Statistical analysis was performed with Student’s t test, * p<0.05, ** p<0.01, *** p<0.001.
Establishing the Protective Capacity of RSV-specific TRM CD4 and CD8 T Cells
While previous studies have shown correlations with protection against RSV by TRMs, direct experimental evidence of the protective capacity of RSV-specific TRMs is currently lacking (43). To directly assess the protective capacity of RSV-specific TRMs, BALB/c mice were infected with RSV, and one month later, mice were challenged with either wild-type IAV (IAV-PR8) or a recombinant influenza virus expressing either the CD4 epitope F51–67 (IAV-F51) or the CD8 epitope M282–90 (IAV-M282) (Figure 5a). RSV naive mice were used as negative controls. Beginning 3 days prior to influenza challenge, mice were treated i.p. once daily with FTY-720, a sphingosine-1-phosphate receptor 1 agonist, to prevent trafficking of lymphocytes from secondary lymph organs into the lungs (14, 44). The effect of FTY-720 has been demonstrated in several studies. In mice infected with Bordatella pertussis, treatment with oral FTY-720 prevented egress of lymphocytes from lymphoid tissue to the lungs with only TRMs found in the lungs (41). Similarly, in mice immunized with a recombinant influenza A vaccine expressing Mycobacterium tuberculosis peptides, treatment with FTY-720 i.p. led to decreased circulating T cells but stable M. tuberculosis-specific TRM CD4 T cells in the lungs (45). FTY-720 treatment was continued throughout infection with recombinant influenza. Four days after influenza challenge, lungs were harvested, and plaque assays were performed to measure viral titers in the lungs. RSV-immune mice challenged with IAV-PR8 unexpectedly exhibited a significant (p<0.05) 1-log decrease in influenza viral titers in the lung compared to naive mice, suggesting that RSV-immune mice may exhibit enhanced protection against a subsequent influenza virus infection. Infection with either IAV-F51 or IAV-M282 exhibited significantly (p<0.05 and p<0.001, respectively) decreased influenza viral titers in the lung compared to naive mice not exposed to RSV (Figure 5b–d), with IAV-M282 demonstrating a greater decrease than was observed with the IAV-PR8 control. Thus, these data demonstrate that RSV-specific TRM CD8 T cells are directly protective when stimulated by their respective RSV-derived antigens.
Figure 5. TRM CD4 and CD8 T cells protect against RSV infection.
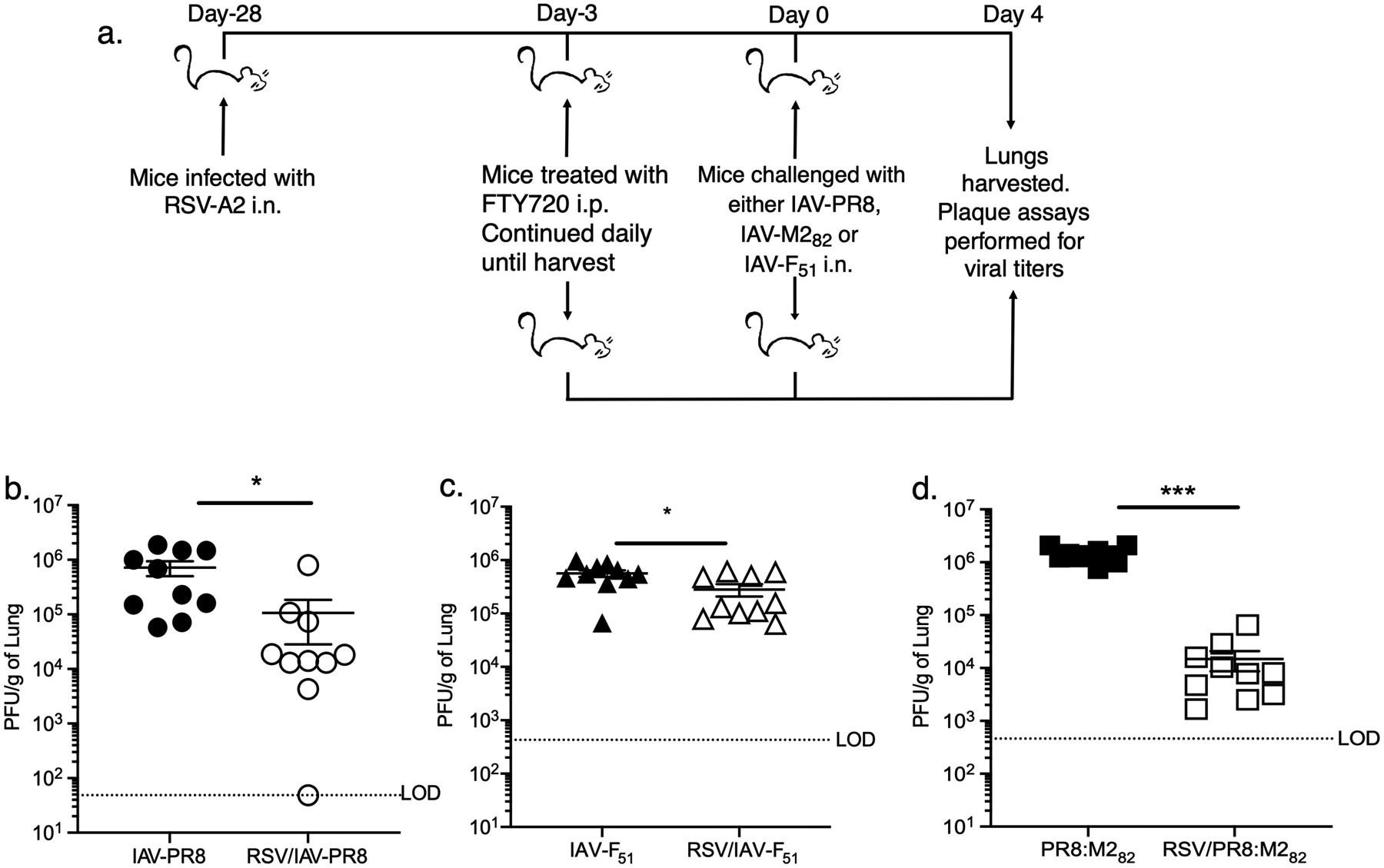
a) BALB/c mice were infected with RSV i.n. or left uninfected and then challenged with IAV expressing either the CD4 epitope F51–67 (IAV-F51) or the CD8 epitope M282–90 (IAV-M282) one month later. Mice were treated daily with FTY-720 i.p. for 7 days beginning 3 days prior to recombinant IAV challenge. Four days after IAV challenge, lungs were harvested, and plaque assays were performed. Viral titers of b) IAV-PR8, c) IAV-F51 and d) IAV-M282 in the lung are shown. The dotted line denotes the limit of detection (LOD) of the assay. Data are presented as mean ± SEM from two independent experiments that have been combined (n=5 mice per experiment). Statistical analysis was performed with Student’s t test, * p<0.05, ** p<0.01, *** p<0.001.
Discussion
T cells play a crucial role in the pathophysiology of acute RSV infection, contributing to both viral clearance and disease burden. TRMs have been demonstrated in multiple tissues, including the lungs following respiratory infections (46). Our data demonstrates that RSV-specific CD4 and CD8 T cells localize to the lungs and remain present at increased frequencies and numbers for at least 149 days p.i. This builds upon previous work in our laboratory demonstrating that RSV-specific T cells are found within the lung parenchyma up to 30 days after infection (35). Furthermore, our data demonstrate that TRM CD4 and CD8 T cells are established in the lungs following acute RSV infection but that the number of TRMs wanes with time. The development of TRMs following acute RSV infection has been demonstrated previously in African green monkeys. However, these cells were identified in the bronchial alveolar fluid at acute or short-term memory time points (27). Recently, both TRM CD4 and CD8 T cells were identified in the airways of adult humans experimentally infected with RSV (28, 29). Our study is able to expand upon this work to define the presence of TRMs within the pulmonary tissue and delineate the kinetics of TRMs after RSV infection.
We have also shown that RSV-specific TRM CD8 T cells can provide protective immunity. Previous studies have indicated that RSV-specific TRM CD8 T cells are correlated with decreased severity of RSV disease and decreased viral load in healthy adults undergoing an experimental RSV human challenge (28). Kinnear et al. transferred CD8 T cells from the airways of mice exposed to RSV to naïve mice, which led to decreased RSV disease severity and viral load (47). However, only 25% of the transferred CD8 T cells were TRMs. Thus, while TRMs may have contributed to protection, their protective effect could not be separated from that of the transferred effector CD8 T cells or T cells from secondary lymphoid organs. Using FTY-720, we were able to limit the immune response to T cells already present in the lung at the time of infection, the majority of which were TRMs. In addition, by using recombinant influenza viruses engineered to express either a CD4 or a CD8 T cell-specific RSV epitope, we were able to independently assess the protective capacity of TRM CD4 and CD8 T cells and demonstrate that TRM CD8 T cells can provide protection when stimulated with their respective RSV-derived antigens.
Alternative mechanisms may also have contributed to the protection observed following infection with the recombinant influenza strains, including non-specific protection by tissue-resident memory T cell present in the lung that have the capacity to make anti-viral cytokines in response to cytokine stimulation such as with IL-12 and IL-18 (48–50). In addition, non-specific protection could be conferred by the innate immune response. Mice infected with the influenza strain X-31 2 or 4 days prior to RSV challenge demonstrated significantly decreased RSV titers compared to unvaccinated mice; however, this effect was lost if mice were infected with X-31 14 or 28 days before RSV challenge. The protection observed was antibody-independent and conferred non-specifically by TLR3/TLR7-mediated innate immune responses (51). Similarly, immune cells and club cells that survived an initial influenza A virus infection non-specifically mediated protection against an influenza B virus challenge through alterations in the composition of cellular infiltration and inflammatory cytokine profiles; it is possible cells in the lung following the initial RSV infection in this study were similarly primed to protect against the secondary influenza virus challenge (52). Consistent with results in mice, infection with influenza virus or adenovirus has been documented to provide protection against subsequent febrile respiratory illnesses in humans (53). Thus, it is possible that memory T cells and innate immune responses may have contributed non-specifically to the TRM-mediated protection against IAV infection observed in this study.
We surprisingly observed that RSV-immune mice exhibited enhanced protection against a wild-type influenza virus infection, resulting in significantly reduced viral titers in the lung. This result made it difficult to determine if RSV-specific TRM CD4 T cells were capable of exhibiting protective immunity because the protection observed was similar between the wild-type influenza virus infection and the recombinant influenza strain expressing an RSV-derived CD4 T cell epitope. Despite this complication, we did observe a nearly 2 log reduction in viral titers in the lungs of RSV-immune mice challenged with a recombinant influenza strain expressing an RSV-derived CD8 T cell epitope suggesting that RSV-specific TRM CD8 T cells can exhibit protective functions.
Immunity to RSV has been demonstrated to wane with time with nearly half of children experiencing a second infection in the first 24 months of life (2). Recent studies have demonstrated that influenza virus-specific TRM CD8 T cells wane over time, which also correlated with a loss of heterosubtypic protection (54). Our protection experiments were performed at day 30 p.i., a time when we could readily detect RSV-specific TRM CD4 and CD8 T cells in the lung. Given that RSV-specific TRMs also wane by 149 days p.i., it is possible that the loss of long-term RSV immunity observed in humans is associated with declining TRMs in the lungs.
As TRMs have been demonstrated to provide protection against acute infection, future RSV vaccine designs should include the induction of this critical cell population. One challenge will be the long-term maintenance of lung TRMs given the temporary nature of these cells in the respiratory tract. Slütter et al. demonstrated that influenza virus-induced TRM CD8 T cells decreased in numbers secondary to apoptosis rather than migration to lymphoid tissue (54). In addition, TRM maintenance was dependent on circulating memory cells in an antigen-independent manner, but the ability of circulating memory CD8 T cells to form TRMs waned with time (54). Conversely, Takamura et al. demonstrated that TRM CD8 T cells are maintained independently from continual cell recruitment in niches called repair-associated memory deposits (RAMDs) located with the pulmonary interstitium (55). As RAMDs are transient in nature, this may explain the lack of longevity seen with TRMs in the lungs compared to other tissue types. Further delineation of the mechanisms of TRM establishment and maintenance are necessary to understand how this cell population can persist and continue to provide protective immunity.
In conclusion, our data demonstrate that RSV-specific TRM CD4 and CD8 T cells are established in the lungs and diminish with time following initial infection. Furthermore, TRM CD8 T cells exhibit the capacity to provide protective immunity. The generation of TRMs within the lungs is vital to developing protective immunity against RSV and should be a focus of on-going RSV vaccine development.
Supplementary Material
Acknowledgments
Research in this publication was supported from funds from the Iowa Children’s Miracle Network (to MAL), from the Department of Microbiology at the University of Iowa (to SMV), and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Numbers R01 AI124093 (to SMV) and T32 AI007485 (MES). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Shi T, McAllister DA, O’Brien KL, Simoes EAF, Madhi SA, Gessner BD, Polack FP, Balsells E, Acacio S, Aguayo C, Alassani I, Ali A, Antonio M, Awasthi S, Awori JO, Azziz-Baumgartner E, Baggett HC, Baillie VL, Balmaseda A, Barahona A, Basnet S, Bassat Q, Basualdo W, Bigogo G, Bont L, Breiman RF, Brooks WA, Broor S, Bruce N, Bruden D, Buchy P, Campbell S, Carosone-Link P, Chadha M, Chipeta J, Chou M, Clara W, Cohen C, de Cuellar E, Dang DA, Dash-Yandag B, Deloria-Knoll M, Dherani M, Eap T, Ebruke BE, Echavarria M, de Freitas Lazaro Emediato CC, Fasce RA, Feikin DR, Feng L, Gentile A, Gordon A, Goswami D, Goyet S, Groome M, Halasa N, Hirve S, Homaira N, Howie SRC, Jara J, Jroundi I, Kartasasmita CB, Khuri-Bulos N, Kotloff KL, Krishnan A, Libster R, Lopez O, Lucero MG, Lucion F, Lupisan SP, Marcone DN, McCracken JP, Mejia M, Moisi JC, Montgomery JM, Moore DP, Moraleda C, Moyes J, Munywoki P, Mutyara K, Nicol MP, Nokes DJ, Nymadawa P, da Costa Oliveira MT, Oshitani H, Pandey N, Paranhos-Baccala G, Phillips LN, Picot VS, Rahman M, Rakoto-Andrianarivelo M, Rasmussen ZA, Rath BA, Robinson A, Romero C, Russomando G, Salimi V, Sawatwong P, Scheltema N, Schweiger B, Scott JAG, Seidenberg P, Shen K, Singleton R, Sotomayor V, Strand TA, Sutanto A, Sylla M, Tapia MD, Thamthitiwat S, Thomas ED, Tokarz R, Turner C, Venter M, Waicharoen S, Wang J, Watthanaworawit W, Yoshida LM, Yu H, Zar HJ, Campbell H, and Nair H. 2017. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. 390: 946–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glezen WP, Taber LH, Frank AL, and Kasel JA. 1986. Risk of primary infection and reinfection with respiratory syncytial virus. Am. J. Dis. Child 140: 543–546. [DOI] [PubMed] [Google Scholar]
- 3.Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, and Parrott RH. 1969. Respiratory Syncytial Virus Disease in Infants Despite Prior Administration of Antigenic Inactivated Vaccine. Am. J. Epidemiol 89: 422–434. [DOI] [PubMed] [Google Scholar]
- 4.Waris ME, Tsou C, Erdman DD, Zaki SR, and Anderson LJ. 1996. Respiratory synctial virus infection in BALB/c mice previously immunized with formalin-inactivated virus induces enhanced pulmonary inflammatory response with a predominant Th2-like cytokine pattern. J. Virol 70: 2852–2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knudson CJ, Hartwig SM, Meyerholz DK, and Varga SM. 2015. RSV vaccine-enhanced disease is orchestrated by the combined actions of distinct CD4 T cell subsets. PLoS Pathog. 11: e1004757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delgado MF, Coviello S, Monsalvo AC, Melendi GA, Hernandez JZ, Batalle JP, Diaz L, Trento A, Chang HY, Mitzner W, Ravetch J, Melero JA, Irusta PM, and Polack FP. 2009. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat. Med 15: 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olson MR, and Varga SM. 2007. CD8 T cells inhibit respiratory syncytial virus (RSV) vaccine-enhanced disease. J. Immunol 179: 5415–5424. [DOI] [PubMed] [Google Scholar]
- 8.Olson MR, Hartwig SM, and Varga SM. 2008. The number of respiratory syncytial virus (RSV)-specific memory CD8 T cells in the lung is critical for their ability to inhibit RSV vaccine-enhanced pulmonary eosinophilia. J. Immunol 181: 7958–7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graham BS, Bunton LA, Wright PF, and Karzon DT. 1991. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J. Clin. Invest 88: 1026–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Legg JP, Hussain IR, Warner JA, Johnston SL, and Warner JO. 2003. Type 1 and type 2 cytokine imbalance in acute respiratory syncytial virus bronchiolitis. Am. J. Respir. Crit. Care Med 168: 633–639. [DOI] [PubMed] [Google Scholar]
- 11.Cannon MJ, Openshaw PJ, and Askonas BA. 1988. Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. J. Exp. Med 168: 1163–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall CB, Powell KR, MacDonald NE, Gala CL, Menegus ME, Suffin SC, and Cohen HJ. 1986. Respiratory syncytial viral infection in children with compromised immune function. N. Engl. J. Med 315: 77–81. [DOI] [PubMed] [Google Scholar]
- 13.Alwan WH, Kozlowska WJ, and Openshaw PJ. 1994. Distinct types of lung disease caused by functional subsets of antiviral T cells. J. Exp. Med 179: 81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morabito KM, Ruckwardt TR, Redwood AJ, Moin SM, Price DA, and Graham BS. 2017. Intranasal administration of RSV antigen-expressing MCMV elicits robust tissue-resident effector and effector memory CD8+ T cells in the lung. Mucosal Immunol. 10: 545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt ME, Knudson CJ, Hartwig SM, Pewe LL, Meyerholz DK, Langlois RA, Harty JT, and Varga SM. 2018. Memory CD8 T cells mediate severe immunopathology following respiratory syncytial virus infection. PLoS Pathog. 14: e1006810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, and Carbone FR. 2009. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat. Immunol 10: 524–530. [DOI] [PubMed] [Google Scholar]
- 17.Turner DL, Bickham KL, Thome JJ, Kim CY, D’Ovidio F, Wherry EJ, and Farber DL. 2014. Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal Immunol. 7: 501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mackay LK, Braun A, Macleod BL, Collins N, Tebartz C, Bedoui S, Carbone FR, and Gebhardt T. 2015. Cutting edge: CD69 interference with sphingosine-1-phosphate receptor function regulates peripheral T cell retention. J. Immunol 194: 2059–2063. [DOI] [PubMed] [Google Scholar]
- 19.Cepek KL, Shaw SK, Parker CM, Russell GJ, Morrow JS, Rimm DL, and Brenner MB. 1994. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature. 372: 190–193. [DOI] [PubMed] [Google Scholar]
- 20.Iijima N, Linehan MM, Zamora M, Butkus D, Dunn R, Kehry MR, Laufer TM, and Iwasaki A. 2008. Dendritic cells and B cells maximize mucosal Th1 memory response to herpes simplex virus. J. Exp. Med 205: 3041–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shin H, and Iwasaki A. 2012. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature. 491: 463–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marzo AL, Vezys V, Williams K, Tough DF, and Lefrançois L. 2002. Tissue-level regulation of Th1 and Th2 primary and memory CD4 T cells in response to Listeria infection. J. Immunol 168: 4504–4510. [DOI] [PubMed] [Google Scholar]
- 23.Masopust D, Choo D, Vezys V, Wherry EJ, Duraiswamy J, Akondy R, Wang J, Casey KA, Barber DL, Kawamura KS, Fraser KA, Webby RJ, Brinkmann V, Butcher EC, Newell KA, and Ahmed R. 2010. Dynamic T cell migration program provides resident memory within intestinal epithelium. J. Exp. Med 207: 553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wakim LM, Woodward-Davis A, and Bevan MJ. 2010. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc. Natl. Acad. Sci. USA 107: 17872–17879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, and Kupper TS. 2012. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature. 483: 227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zens KD, Chen JK, and Farber DL. 2016. Vaccine-generated lung tissue-resident memory T cells provide heterosubtypic protection to influenza infection. JCI Insight. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li H, Callahan C, Citron M, Wen Z, Touch S, Monslow MA, Cox KS, DiStefano DJ, Vora KA, Bett A, and Espeseth A. 2017. Respiratory syncytial virus elicits enriched CD8+ T lymphocyte responses in lung compared with blood in African green monkeys. PLoS One. 12: e0187642–e0187642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jozwik A, Habibi MS, Paras A, Zhu J, Guvenel A, Dhariwal J, Almond M, Wong EH, Sykes A, and Maybeno M. 2015. RSV-specific airway resident memory CD8+ T cells and differential disease severity after experimental human infection. Nature Com. 6: 10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guvenel A, Jozwik A, Ascough S, Ung SK, Paterson S, Kalyan M, Gardener Z, Bergstrom E, Kar S, Habibi MS, Paras A, Zhu J, Park M, Dhariwal J, Almond M, Wong EHC, Sykes A, Del Rosario J, Trujillo-Torralbo M-B, Mallia P, Sidney J, Peters B, Kon OM, Sette A, Johnston SL, Openshaw PJ, and Chiu C. 2020. Epitope-specific airway-resident CD4+ T cell dynamics during experimental human RSV infection. J. Clin. Invest 130: 523–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoffmann E, Neumann G, Kawaoka Y, Hobom G, and Webster RG. 2000. A DNA transfection system for generation of influenza A virus from eight plasmids. Proc. Natl. Acad. Sci. USA 97: 6108–6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heaton NS, Sachs D, Chen C-J, Hai R, and Palese P. 2013. Genome-wide mutagenesis of influenza virus reveals unique plasticity of the hemagglutinin and NS1 proteins. Proc. Natl. Acad. Sci. USA 110: 20248–20253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson KG, Sung H, Skon CN, Lefrancois L, Deisinger A, Vezys V, and Masopust D. 2012. Cutting edge: intravascular staining redefines lung CD8 T cell responses. J. Immunol 189: 2702–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fulton RB, Meyerholz DK, and Varga SM. 2010. Foxp3+ CD4 regulatory T cells limit pulmonary immunopathology by modulating the CD8 T cell response during respiratory syncytial virus infection. J. Immunol 185: 2382–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiss KA, Christiaansen AF, Fulton RB, Meyerholz DK, and Varga SM. 2011. Multiple CD4+ T cell subsets produce immunomodulatory IL-10 during respiratory syncytial virus infection. J. Immunol 187: 3145–3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knudson CJ, Weiss KA, Hartwig SM, and Varga SM. 2014. The pulmonary localization of virus-specific T lymphocytes is governed by the tissue tropism of infection. J. Virol 88: 9010–9016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teijaro JR, Turner D, Pham Q, Wherry EJ, Lefrancois L, and Farber DL. 2011. Cutting edge: Tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J. Immunol 187: 5510–5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Christiaansen AF, Dixit UG, Coler RN, Marie Beckmann A, Reed SG, Winokur PL, Zimmerman MB, Varga SM, and Wilson ME. 2017. CD11a and CD49d enhance the detection of antigen-specific T cells following human vaccination. Vaccine. 35: 4255–4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McDermott DS, and Varga SM. 2011. Quantifying antigen-specific CD4 T cells during a viral infection: CD4 T cell responses are larger than we think. J. Immunol 187: 5568–5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henderson FW, Collier AM, Clyde WA Jr., and Denny FW. 1979. Respiratory-syncytial-virus infections, reinfections and immunity. A prospective, longitudinal study in young children. N. Engl. J. Med 300: 530–534. [DOI] [PubMed] [Google Scholar]
- 40.Johnson KM, Bloom HH, Mufson MA, and Chanock RM. 1962. Natural reinfection of adults by respiratory syncytial virus. Possible relation to mild upper respiratory disease. N. Engl. J. Med 267: 68–72. [DOI] [PubMed] [Google Scholar]
- 41.Wilk MM, Misiak A, McManus RM, Allen AC, Lynch MA, and Mills KHG. 2017. Lung CD4 Tissue-Resident Memory T Cells Mediate Adaptive Immunity Induced by Previous Infection of Mice with Bordetella pertussis. J. Immunol 199: 233–243. [DOI] [PubMed] [Google Scholar]
- 42.Mackay LK, Wynne-Jones E, Freestone D, Pellicci DG, Mielke LA, Newman DM, Braun A, Masson F, Kallies A, Belz GT, and Carbone FR. 2015. T-box Transcription Factors Combine with the Cytokines TGF-β and IL-15 to Control Tissue-Resident Memory T Cell Fate. Immunity. 43: 1101–1111. [DOI] [PubMed] [Google Scholar]
- 43.Retamal-Díaz A, Covián C, Pacheco GA, Castiglione-Matamala AT, Bueno SM, González PA, and Kalergis AM. 2019. Contribution of Resident Memory CD8(+) T Cells to Protective Immunity Against Respiratory Syncytial Virus and Their Impact on Vaccine Design. Pathogens. 8: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ledgerwood LG, Lal G, Zhang N, Garin A, Esses SJ, Ginhoux F, Merad M, Peche H, Lira SA, Ding Y, Yang Y, He X, Schuchman EH, Allende ML, Ochando JC, and Bromberg JS. 2008. The sphingosine 1-phosphate receptor 1 causes tissue retention by inhibiting the entry of peripheral tissue T lymphocytes into afferent lymphatics. Nat. Immunol 9: 42–53. [DOI] [PubMed] [Google Scholar]
- 45.Flórido M, Muflihah H, Lin LCW, Xia Y, Sierro F, Palendira M, Feng CG, Bertolino P, Stambas J, Triccas JA, and Britton WJ. 2018. Pulmonary immunization with a recombinant influenza A virus vaccine induces lung-resident CD4(+) memory T cells that are associated with protection against tuberculosis. Mucosal Immunol. 11: 1743–1752. [DOI] [PubMed] [Google Scholar]
- 46.Turner DL, and Farber DL. 2014. Mucosal resident memory CD4 T cells in protection and immunopathology. Front. Immunol 5: 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kinnear E, Lambert L, McDonald JU, Cheeseman HM, Caproni LJ, and Tregoning JS. 2018. Airway T cells protect against RSV infection in the absence of antibody. Mucosal Immunol. 11: 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berg RE, Cordes CJ, and Forman J. 2002. Contribution of CD8+ T cells to innate immunity: IFN-gamma secretion induced by IL-12 and IL-18. Eur. J. Immunol 32: 2807–2816. [DOI] [PubMed] [Google Scholar]
- 49.Berg RE, Crossley E, Murray S, and Forman J. 2003. Memory CD8+ T cells provide innate immune protection against Listeria monocytogenes in the absence of cognate antigen. J. Exp. Med 198: 1583–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kambayashi T, Assarsson E, Lukacher AE, Ljunggren HG, and Jensen PE. 2003. Memory CD8+ T cells provide an early source of IFN-gamma. J. Immunol 170: 2399–2408. [DOI] [PubMed] [Google Scholar]
- 51.Lee YN, Hwang HS, Kim MC, Lee YT, Lee JS, Moore ML, and Kang SM. 2015. Recombinant influenza virus expressing a fusion protein neutralizing epitope of respiratory syncytial virus (RSV) confers protection without vaccine-enhanced RSV disease. Antiviral. Res 115: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hamilton JR, Sachs D, Lim JK, Langlois RA, Palese P, and Heaton NS. 2016. Club cells surviving influenza A virus infection induce temporary nonspecific antiviral immunity. Proc. Natl. Acad. Sci. USA 113: 3861–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen IM, Loh JP, Chuah CXP, Gao QHC, Sun Y, Ng SH, Koh WV, Goh EH, Zhao X, Tambyah PA, Cook AR, Chng J, Pang J, Tan BH, and Lee VJ. 2019. Evidence for Cross-Protection Against Subsequent Febrile Respiratory Illness Episodes From Prior Infections by Different Viruses Among Singapore Military Recruits 2009–2014. J. Infect. Dis 219: 1913–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Slütter B, Van Braeckel-Budimir N, Abboud G, Varga SM, Salek-Ardakani S, and Harty JT. 2017. Dynamic equilibrium of lung Trm dictates waning immunity after Influenza A infection. Sci. Immunol 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takamura S, Yagi H, Hakata Y, Motozono C, McMaster SR, Masumoto T, Fujisawa M, Chikaishi T, Komeda J, Itoh J, Umemura M, Kyusai A, Tomura M, Nakayama T, Woodland DL, Kohlmeier JE, and Miyazawa M. 2016. Specific niches for lung-resident memory CD8+ T cells at the site of tissue regeneration enable CD69-independent maintenance. J. Exp. Med 213: 3057–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


