Abstract
Background
Ketamine appears to have a therapeutic role in certain mental disorders, most notably unipolar major depressive disorder. However, its efficacy in bipolar depression is less clear. This study aimed to assess the efficacy and tolerability of ketamine for bipolar depression.
Methods
We conducted a systematic review of experimental studies using ketamine for the treatment of bipolar depression. We searched PubMed, MEDLINE, Embase, PsycINFO, and the Cochrane Central Register for relevant studies published since each database’s inception. We synthesized evidence regarding efficacy (improvement in depression rating scores) and tolerability (adverse events, dissociation, dropouts) across studies.
Results
We identified 6 studies, with 135 participants (53% female; 44.7 years; standard deviation, 11.7 years). All studies used 0.5 mg/kg of add-on intravenous racemic ketamine, with the number of doses ranging from 1 to 6; all participants continued a mood-stabilizing agent. The overall proportion achieving a response (defined as those having a reduction in their baseline depression severity of at least 50%) was 61% for those receiving ketamine and 5% for those receiving a placebo. The overall response rates varied from 52% to 80% across studies. Ketamine was reasonably well tolerated; however, 2 participants (1 receiving ketamine and 1 receiving placebo) developed manic symptoms. Some participants developed significant dissociative symptoms at the 40-minute mark following ketamine infusion in 2 trials.
Conclusions
There is some preliminary evidence supporting use of intravenous racemic ketamine to treat adults with bipolar depression. There is a need for additional studies exploring longer-term outcomes and alterative formulations of ketamine.
Keywords: Bipolar disorder, depression, ketamine, systematic review
Introduction
Bipolar depression is a leading cause of disability globally, affecting nearly 1% of individuals worldwide (Ferrari et al., 2016). As in unipolar depression, treatment-resistant bipolar depression (TRBD) is widespread but remains understudied (Sachs, 1996; Gitlin, 2006; Sienaert et al., 2013). One definition of TRBD involves the failure to reach sustained symptomatic remission for 8 consecutive weeks after 2 different treatment trials, at adequate therapeutic doses, with at least 2 recommended monotherapy treatments or at least 1 monotherapy treatment and another combination treatment (Hidalgo-Mazzei et al., 2019).
Despite the importance of TRBD, only a small number of recognized treatment options are available (Hidalgo-Mazzei et al., 2019). A few trials have indicated a role for electroconvulsive therapy and repetitive transcranial magnetic stimulation (Schoeyen et al., 2015; Tavares et al., 2017). While recent network meta-analyses have shown consistent evidence for use of multiple pharmacotherapies in non-TRBD (Bahji et al., 2020a, 2020b, 2021a, 2021c), there is more limited evidence for use of medication-based treatments in TRBD.
Fortunately, there appears to be an emerging role for ketamine in managing unipolar depression (McIntyre et al., 2020; Bahji et al., 2021b, 2021c). Early ketamine studies demonstrated rapid, potent reductions in depressive symptoms following administration of a single sub-anesthetic dose of intravenous racemic ketamine (Berman et al., 2000; Zarate et al., 2006; Ionescu et al., 2015; Hu et al., 2016; Wilkinson et al., 2018). While these initial results were promising, effective means of maintaining the acute effects were actively sought (Phillips et al., 2019). To date, the use of other glutamatergic agents to prolong the acute antidepressant effects of ketamine has been mostly inconsistent, with some successful case reports and small open-label studies (Zarate et al., 2005; Mathew et al., 2010; Ibrahim et al., 2012; Caddy et al., 2015; McCloud et al., 2015). While repeat doses of intravenous racemic ketamine appear to sustain short-term antidepressant effects for individuals with unipolar depression (Murrough et al., 2013; Ghasemi et al., 2014; López-Díaz et al., 2017; Ionescu et al., 2019), it is unclear whether this holds for bipolar depression. Racemic ketamine can also rapidly reduce suicidal thoughts within 1 day and for up to 1 week in depressed patients with suicidal ideation (Reinstatler and Youssef, 2015; López-Díaz et al., 2017; Grunebaum et al., 2018; Wilkinson et al., 2018; Williams et al., 2019; Witt et al., 2020). While these findings are mostly limited to unipolar depression, some emerging studies point to the efficacy of ketamine for bipolar significant depression (Zarate et al., 2012; Grunebaum et al., 2017; Chen et al., 2019). Racemic ketamine has also led to many preclinical and biomarker discoveries (Zanos et al., 2016; Zanos and Gould, 2018), leading to new possibilities and safer alternatives for mitigating dissociation and reducing the propensity for misuse and diversion of ketamine (Newport et al., 2015; Burger et al., 2016; Lener et al., 2017).
Although clinical studies of ketamine for TRBD are now underway, the level of proof of efficacy remains low, and more RCTs are needed to explore efficacy and safety issues of ketamine (Corriger and Pickering, 2019). While previous reviews have explored ketamine’s utility in the treatment of TRBD, there is a need to update previous reviews given the recent increase in ketamine studies.
OBJECTIVE
We aimed to provide an updated synthesis of findings from studies examining the efficacy and safety of ketamine for bipolar depression.
METHODS
Protocol and Registration
We registered this study with the Open Science Framework (https://osf.io/ksvnb/). We followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (Liberati et al., 2009).
Eligibility Criteria
We included randomized controlled trials and nonrandomized studies examining the use of ketamine in adults (aged 18 years or older) to treat bipolar depression. We considered studies examining any formulation of ketamine (e.g., intravenous racemic ketamine, intranasal enantiomeric S-ketamine [esketamine]) as a standalone treatment or in combination with psychotropic medications or psychotherapies. We excluded observational designs (i.e., surveys, cohort studies, case series, and case-control studies), reviews, post hoc and secondary analyses, commentaries, and clinical overviews. We also excluded studies pairing ketamine with a neurostimulation-based treatment. We only included studies reporting at least 1 outcome related to the efficacy or safety of ketamine, such as the response to treatment or adverse events. Finally, we excluded studies that did not separate participants with bipolar depression from those with unipolar depression (Berman et al., 2000).
Information Sources and Search
We searched MEDLINE, Embase, PsycINFO, the Cochrane Central Register of Controlled Clinical Trials (CENTRAL), and the Cochrane Database of Systematic Reviews via Ovid for studies published from inception to December 13, 2019. To identify ongoing or unpublished studies, we also searched ClinicalTrials.gov, the EU Clinical Trials Register, and the Australian and New Zealand Clinical Trials Registry using the keywords “ketamine” and “bipolar depression.” We also hand-searched reference lists of included studies and topical reviews for potentially relevant articles.
Study Selection
Two researchers (AB, GHV) independently examined titles and abstracts using the web-based systematic review program Covidence (Veritas Health Innovation, 2019). Relevant articles were obtained in full and assessed for inclusion independently by the 2 coauthors. Any disagreement between them was resolved via discussion to reach a consensus.
Data Collection Process and Data Items
Two co-authors (AB, GHV) extracted data via a pre-piloted, standardized data extraction tool in Microsoft Excel 2016. We pulled data on details of the populations, interventions, comparisons, outcomes of significance to the mental disorder, study methods, ketamine dose and route of administration, study withdrawals, and study withdrawals due to adverse events. Where data were missing, we contacted the authors for additional information. When authors reported multiple analyses (e.g., intention-to-treat or per-protocol analyses), we extracted the more conservative analysis, with a preference for intention-to-treat analyses.
Risk of Bias in Individual Studies
We assessed the risk of bias within individual trials using the Cochrane risk of bias tool for randomized controlled trials. Specifically, the bias tool assesses indicators of selection bias, performance bias, detection bias, attrition bias, and reporting bias (Higgins et al., 2011). The risk of bias assessments were completed independently by 2 authors (AB or GHV). Inter-researcher disagreements were resolved via discussion to reach a consensus.
Analytic Methods
While we intended to conduct a meta-analysis, we only identified a total of 6 studies, of which only 3 were randomized controlled trials. Instead, we present the results in tables and discuss the findings comprehensively in the text.
RESULTS
Study Selection
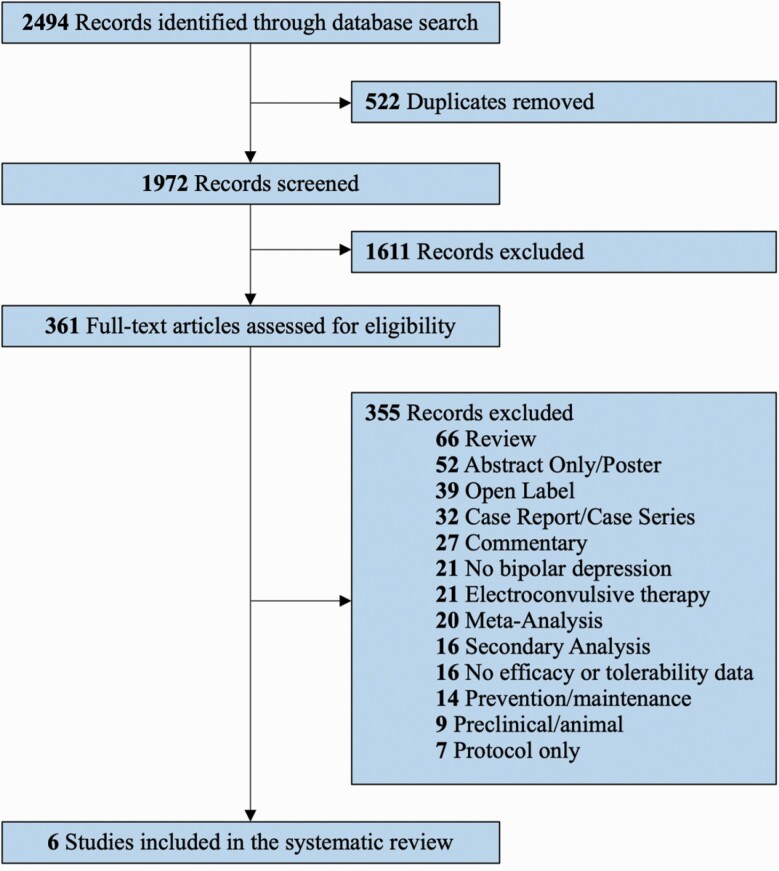
The search strategy identified a total of 2494 records (Figure 1). After removing duplicates, we screened the remaining 1972 unique articles by title and abstract. We then excluded 1611 irrelevant records, leaving 361 documents for a full-text review. After a full-text review, only 6 studies met the final inclusion criteria (Diazgranados et al., 2010; Zarate et al., 2012; Rybakowski et al., 2013; Permoda-Osip et al., 2014; Grunebaum et al., 2017; Zheng et al., 2020).
Figure 1.
PRISMA flow diagram outlining the systematic review process. Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Characteristics of Studies, Participants, and Interventions
Table 1 provides an overview of the study characteristics. Three studies were randomized controlled trials (Diazgranados et al., 2010; Zarate et al., 2012; Grunebaum et al., 2017), while the other 3 were open-label, single-arm studies (Rybakowski et al., 2013; Permoda-Osip et al., 2014; Zheng et al., 2020). By country, most studies were from the United States (50%; k = 3) or Poland (33%; k = 2). There were 135 participants (53% female; 44.7 years; standard deviation, 11.7 years). Except for 1 study not reporting the diagnostic criteria for bipolar depression (BD; Permoda-Osip et al., 2014), the remaining 5 used Diagnostic and Statistical Manual of Mental Disorders fourth or fifth edition criteria. All 6 studies used add-on racemic ketamine at a dose of 0.5 mg/kg delivered intravenously; hence, all participants continued treatment with a primary mood-stabilizing agent throughout ketamine treatment. However, the number of doses varied across studies, with 3 single-dose studies (Rybakowski et al., 2013; Permoda-Osip et al., 2014; Grunebaum et al., 2017), 2 studies with 2 test doses (1 ketamine and 1 placebo) 2 weeks apart (Diazgranados et al., 2010; Zarate et al., 2012), and 1 study with 6 doses across 2 weeks (Zheng et al., 2020). Except for 1 study (Grunebaum et al., 2017), the remaining 5 involved TRBD, defined as an insufficient response to at least 1 (Rybakowski et al., 2013; Permoda-Osip et al., 2014) or 2 (Diazgranados et al., 2010; Zarate et al., 2012; Zheng et al., 2020) previous antidepressant trials. Two trials also required that participants have an inadequate response to prospective trials of lithium or valproate (Diazgranados et al., 2010; Zarate et al., 2012). Notably, most excluded those who had cooccurring general medical conditions, were pregnant or breastfeeding, or had comorbid psychosis or addiction.
Table 1.
Study characteristics (k = 6)
| Study | Design | Population | Intervention(s) | Primary Findings |
|---|---|---|---|---|
| Diazgranados et al., 2010 | Crossover RCT | TRBD (n = 17) | Racemic, adjunctive ketamine: 0.5 mg/ kg IV on 2 test days 2 weeks apart vs. placebo | 71% (vs. 6%) responded to ketamine (vs. placebo) during the trial on the MADRS. Dissociative symptoms occurred at the 40-minute mark. One participant in each group developed manic symptoms. |
| Zarate et al., 2012 | Crossover RCT | TRBD (n = 15) | Racemic, adjunctive ketamine: 0.5 mg/ kg IV on 2 test days 2 weeks apart vs. placebo | 79% (vs. 0%) responded to ketamine (vs. placebo) during the trial on the MADRS. Dissociative symptoms occurred at the 40-minute mark. |
| Rybakowski et al., 2013 | Open-label, single-arm trial | TRBD (n = 25) | Racemic, adjunctive ketamine: 0.5 mg/ kg IV, single dose | Using the HDRS, there were 13 ketamine responders and 8 remitters after 7 and 14 days, respectively. Serum NGF, NT3, NT4, and GDNF did not significantly change. |
| Permoda-Osip et al., 2014 | Open-label, single-arm trial | TRBD (n = 42) | Racemic, adjunctive ketamine: 0.5 mg/ kg IV, single dose | HDRS scores reduced significantly after 24 hours (from 22.6±5.1 hours to 15.6±7.4 hours), 7 days (to 13±7 days), and 14 days (to 11.8±7.8 days). |
| Grunebaum et al., 2017 | Parallel RCT | Non-TRBD (n = 16) | Racemic, adjunctive ketamine: 0.5 mg/ kg IV, single dose vs. midazolam 0.02 mg/kg IV | HDRS and SSI scores reduced by approximately 6 points each in the ketamine group, but the differences were not statistically significant. |
| Zheng et al., 2020 | Open-label, single-arm trial | TRBD (n = 19) | Racemic, adjunctive ketamine: 0.5 mg/ kg IV, 6 doses over 12 days | Rates of response and remission were 73.7% and 63.2% at the study end. There were no significant dissociative and psychotomimetic symptoms on the CADSS or BPRS. |
Abbreviations: BPRS, Brief Psychiatric Rating Scale; CADSS, Clinician-Administered Dissociative States Scale; GDNF, glial-derived neurotrophic factor; HDRS, Hamilton Depression Rating Scale; IV, intravenous; MADRS, Montgomery-Åsberg Depression Rating Scale; NGF, nerve growth factor; NTF3, neurotrophin-3; NTF4, neurotrophin-4; RCT, randomized controlled trial; SSI, Scale for Suicidal Ideation; TRBD, treatment-resistant bipolar depression.
Efficacy of Intravenous, Racemic Ketamine for Bipolar Depression
Across all 6 studies, the proportion achieving a response (defined as those having a reduction in their baseline depression severity of at least 50%) was 61% for those receiving ketamine at some point during the trial (77/126). The overall response rate across studies varied from 52% (Rybakowski et al., 2013) to 80% (Zarate et al., 2012). For the 3 studies that involved control groups, the overall pooled response rate was only 5% (2/42). There were improvements in depression rating scores over time in all studies; however, in the 1 trial using a midazolam control in non-TRBD subjects, the difference was not statistically significant (Grunebaum et al., 2017). The efficacy of single-dose ketamine did not extend beyond the 2-week mark; however, the study that used 6 doses of ketamine over 2 weeks appeared to show longer-lasting efficacy.
Tolerability of Intravenous, Racemic Ketamine for Bipolar Depression
Across most of the included studies, participants tolerated ketamine treatment reasonably well. However, there were some significant adverse events. For example, 2 participants (1 receiving ketamine and 1 receiving placebo) developed manic symptoms (Diazgranados et al., 2010). In 2 trials, participants developed significant dissociative symptoms, primarily at the 40-minute mark following ketamine infusion (Diazgranados et al., 2010; Zarate et al., 2012). However, the remaining 4 trials did not note substantial dissociation or mania symptoms at any point during the study.
Study Quality and Risk of Bias
Three studies were double-blind, randomized, controlled trials with concealed allocation (Diazgranados et al., 2010; Zarate et al., 2012; Grunebaum et al., 2017). These studies were at very low risk of bias as per the Cochrane Risk of Bias Tool. The remaining 3 were all nonrandomized, open-label, single-arm studies, which lacked a control group and were more susceptible to participation bias.
Discussion
To our knowledge, this is the most recent systematic review that has explored the effectiveness and tolerability of ketamine for the treatment of BD. Overall, our findings—derived from 6 studies—indicate that ketamine appears to be an effective and relatively safe treatment for BD and TRBD.
All 6 studies in our review involved intravenous racemic ketamine at a dose of 0.5 mg/kg as an add-on treatment to primary mood-stabilizing medications. To that end, the rapid antidepressant effects of ketamine seen in individuals with TRBD appears to be predictive of a sustained outcome (Murrough et al., 2011, 2013; Atigari and Healy, 2013; Ionescu et al., 2014).
In a previous meta-analysis, there was no significant difference in the clinical response to intravenous ketamine between patients with unipolar major depression and bipolar depression (Bahji et al., 2021c). However, there are no available studies on intranasal esketamine for bipolar depression; hence, there are still unclear aspects concerning the role of ketamine in bipolar disorder. In contrast, several prior studies indicate a role for intravenous ketamine in treating bipolar depression (Ionescu et al., 2015; Bobo et al., 2016; Alberich et al., 2017; Kraus et al., 2017; López-Díaz et al., 2017; Gałuszko-Węgielnik et al., 2019). For very short-term use, the available data demonstrates a clear and consistent antidepressive effect of ketamine versus esketamine treatment in unipolar major depression, relative to various control conditions, beginning within hours of administration and lasting up to 7 days after a single dose (McGirr et al., 2015; Bahji et al., 2021c). However, we do not know whether this pattern is also present in cases of bipolar disorder, where we only have data for racemic ketamine. Hence, there is a need for head-to-head studies comparing ketamine to esketamine in bipolar disorder. Future studies could also measure blood levels of ketamine and norketamine with intravenous racemic ketamine and esketamine and determine whether the differences remain significant after controlling.
Regarding the side effect profiles, most studies indicate that ketamine is reasonably well tolerated for bipolar depression treatment. Two significant concerns involve the risk of dissociation and induction of mania or hypomania. However, in our review, most trials and most participants did not experience either of these adverse events. However, in 2 trials, participants developed significant dissociative symptoms, primarily at the 40-minute mark following ketamine infusion (Diazgranados et al., 2010; Zarate et al., 2012). However, the remaining 4 trials did not note substantial dissociation or mania symptoms at any point during the study. A related concern for ketamine in bipolar disorder involves the risk of switching to a manic or hypomanic episode. In 1 trial, 2 participants (1 receiving ketamine and 1 receiving placebo) developed manic symptoms (Diazgranados et al., 2010). While standard antidepressants can induce rapid cycling, it is unclear whether this can occur with ketamine, as trials are typically short. Still, mania switches with single-ketamine infusions or pulsed treatment (where repeated doses are spaced over several days or weeks) have had small sample sizes, which may be insufficiently powered to identify manic switching. However, there was insufficient evidence to support mania induction with a single subanesthetic dose of ketamine in 98 major depressed patients (Niciu et al., 2013).
Nonetheless, there is a real necessity in our therapeutic armamentarium to discover and add more effective and safer treatments for patients with TRBD (Gao et al., 2016). Part of the challenge in elucidating the comparative performance of different formulations of ketamine may lie in the lack of a clear consensus on the mechanisms underlying ketamine’s therapeutic effects (Strasburger et al., 2017; Zanos and Gould, 2018). With the isolation of esketamine, there was also an option of providing much lower doses of ketamine and the opportunity to reduce the dose-dependent dissociative properties of ketamine (Correia-Melo et al., 2018). As esketamine was also available through an intranasal delivery system, it presented a substantially more practical option than intravenous racemic ketamine (Tibensky et al., 2016; Schatzberg, 2019). Ultimately, intranasal esketamine was approved by the US Food and Drug Administration on March 5, 2019, for use in TRBD (Kim et al., 2019); on December 19, 2019, Europe followed suit with approval of esketamine for the same indication (Wei et al., 2020).
Limitations
Although this review has several strengths, a few fundamental limitations deserve some expansion here. First, we were unable to conduct a meta-analysis given the low study yield. Despite this, we were still able to present the results across studies in a meaningful way. Second, there is always the possibility of publication bias, such that our review may not have identified negative studies (where ketamine did not improve depression scores). Third, while our review attempted to cover as much follow-up time as possible after ketamine treatment administration, there remains minimal information regarding longer-term follow-up beyond the 2-week mark. Hence, the results of our study are also limited to this treatment window. Fourth, participants in the presented trials are relatively unrepresentative of the real-world population with BD, given the studies’ strict exclusion criteria. Thus, the results of the trials may not represent the real-world efficacy of ketamine. Fifth, the high heterogeneity within the selected studies could have impacted our findings. For example, there are differences between patients with TRBD versus non-TRBD, between those who receive treatment as an inpatient versus at community clinics, and between studies where participants received single or multiple ketamine doses.
Conclusions
Ketamine represents an innovative, rapidly acting, experimental treatment for bipolar depression. This review found that preliminary evidence supports the use of intravenous racemic ketamine for the treatment of individuals with bipolar depression. At present, there are no studies that have used other formulations of ketamine, such as intranasal esketamine. To that end, the intravenous administration route presents a practical limitation. Future studies should explore the use of an intranasal formulation of esketamine for individuals with bipolar depression. While racemic ketamine has demonstrated significant short-term benefits in several clinical studies highlighted in this review, the long-term benefits remain insufficiently explored, which may contribute to the current lack of Food and Drug Administration approval for use in individuals with bipolar disorder. At present, the level of proof of efficacy remains low. More randomized controlled trials are needed to explore efficacy and safety issues for administering all forms of ketamine in the treatment of bipolar depression.
Acknowledgments
A.B. received research grants from the Matt Newell Endowment in Substance Use, the American Psychiatric Association, the Research in Addiction Medicine Scholars programs, and the Canadian Institutes of Health Research. However, the authors did not receive any specific funding for the present article.
Statement of Interest
C.A.Z. is listed as a coinventor on a patent for the use of (2R,6R)-HNK and other hydroxynorketamines in the treatment of depression. C.A.Z. is listed as a coinventor on a patent application for the use of (2R,6R)-HNK and (2S,6S)-HNK in the treatment of depression, anxiety, anhedonia, suicidal ideation, and post-traumatic stress disorder. C.A.Z. is listed as a coinventor on a patent application for the crystal forms and synthesis of (2R,6R)-hydroxynorketamine and (2S,6S)-hydroxynorketamine.
References
- Alberich S, Martínez-Cengotitabengoa M, López P, Zorrilla I, Núñez N, Vieta E, González-Pinto A (2017) Efficacy and safety of ketamine in bipolar depression: a systematic review. Rev Psiquiatr Salud Ment 10:104–112. [DOI] [PubMed] [Google Scholar]
- Atigari OV, Healy D (2013) Sustained antidepressant response to ketamine. BMJ Case Rep 2013:bcr2013200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahji A, Ermacora D, Stephenson C, Hawken E, Vazquez G (2020a) Effectiveness of pharmacotherapies for bipolar depression: a systematic review & network meta-analysis. Biol Psychiatry 87:S388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahji A, Ermacora D, Stephenson C, Hawken ER, Vazquez G (2020b) Comparative efficacy and tolerability of pharmacological treatments for the treatment of acute bipolar depression: a systematic review and network meta-analysis. J Affect Disord 269:154–184. [DOI] [PubMed] [Google Scholar]
- Bahji A, Ermacora D, Stephenson C, Hawken ER, Vazquez G (2021a) Comparative efficacy and tolerability of adjunctive pharmacotherapies for acute bipolar depression: a systematic review and network meta-analysis. Can J Psychiatry 66(3):274-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahji A, Vazquez GH, Brietzke EM, Zarate CA (2021b) Chapter 5–Overview of ketamine for major depression: efficacy and effectiveness. In: Ketamine for treatment-resistant depression (Vazquez GH, Zarate CA, Brietzke EM, eds), pp 117–129. Cambridge, MA: Academic Press. [Google Scholar]
- Bahji A, Vazquez GH, Zarate CA Jr (2021c) Comparative efficacy of racemic ketamine and esketamine for depression: a systematic review and meta-analysis. J Affect Disord 278:542–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47:351–354. [DOI] [PubMed] [Google Scholar]
- Bobo WV, Vande Voort JL, Croarkin PE, Leung JG, Tye SJ, Frye MA (2016) Ketamine for treatment-resistant unipolar and bipolar major depression: critical review and implications for clinical practice. Depress Anxiety 33:698–710. [DOI] [PubMed] [Google Scholar]
- Burger J, Capobianco M, Lovern R, Boche B, Ross E, Darracq MA, McLay R (2016) A double-blinded, randomized, placebo-controlled sub-dissociative dose ketamine pilot study in the treatment of acute depression and suicidality in a military emergency department setting. Mil Med 181:1195–1199. [DOI] [PubMed] [Google Scholar]
- Caddy C, Amit BH, McCloud TL, Rendell JM, Furukawa TA, McShane R, Hawton K, Cipriani A (2015) Ketamine and other glutamate receptor modulators for depression in adults. Cochrane Database Syst Rev 2015;9:CD011612. [DOI] [PubMed] [Google Scholar]
- Chen MH, Cheng CM, Gueorguieva R, Lin WC, Li CT, Hong CJ, Tu PC, Bai YM, Tsai SJ, Krystal JH, Su TP (2019) Maintenance of antidepressant and antisuicidal effects by D-cycloserine among patients with treatment-resistant depression who responded to low-dose ketamine infusion: a double-blind randomized placebo-control study. Neuropsychopharmacology 44:2112–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia-Melo FS, Leal GC, Carvalho MS, Jesus-Nunes AP, Ferreira CBN, Vieira F, Magnavita G, Vale LAS, Mello RP, Nakahira C, Argolo FC, Cardoso T, Souza CDS, Fontes ATC, Ferreira MB, Araújo-de-Freitas L, Tuena MA, Echegaray MVF, Cavalcanti DE, Lucchese AC, et al. (2018) Comparative study of esketamine and racemic ketamine in treatment-resistant depression: protocol for a non-inferiority clinical trial. Medicine (Baltimore) 97:e12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corriger A, Pickering G (2019) Ketamine and depression: a narrative review. Drug Des Devel Ther 13:3051–3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, Machado-Vieira R, Manji HK, Zarate CA Jr (2010) A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry 67:793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari AJ, Stockings E, Khoo JP, Erskine HE, Degenhardt L, Vos T, Whiteford HA (2016) The prevalence and burden of bipolar disorder: findings from the Global Burden of Disease Study 2013. Bipolar Disord 18:440–450. [DOI] [PubMed] [Google Scholar]
- Gałuszko-Węgielnik M, Wiglusz MS, Słupski J, Szałach Ł, Włodarczk A, Górska N, Szarmach J, Jakuszkowiak-Wojten K, Wilkowska A, Cubała WJ (2019) Efficacy of ketamine in bipolar depression: focus on anhedonia. Psychiatr Danub 31:554–560. [PubMed] [Google Scholar]
- Gao M, Rejaei D, Liu H (2016) Ketamine use in current clinical practice. Acta Pharmacol Sin 37:865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemi M, Kazemi MH, Yoosefi A, Ghasemi A, Paragomi P, Amini H, Afzali MH (2014) Rapid antidepressant effects of repeated doses of ketamine compared with electroconvulsive therapy in hospitalized patients with major depressive disorder. Psychiatry Res 215:355–361. [DOI] [PubMed] [Google Scholar]
- Gitlin M (2006) Treatment-resistant bipolar disorder. Mol Psychiatry 11:227–240. [DOI] [PubMed] [Google Scholar]
- Grunebaum MF, Ellis SP, Keilp JG, Moitra VK, Cooper TB, Marver JE, Burke AK, Milak MS, Sublette ME, Oquendo MA, Mann JJ (2017) Ketamine versus midazolam in bipolar depression with suicidal thoughts: a pilot midazolam-controlled randomized clinical trial. Bipolar Disord 19:176–183. [DOI] [PubMed] [Google Scholar]
- Grunebaum MF, Galfalvy HC, Choo TH, Keilp JG, Moitra VK, Parris MS, Marver JE, Burke AK, Milak MS, Sublette ME, Oquendo MA, Mann JJ (2018) Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. Am J Psychiatry 175:327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo-Mazzei D, Berk M, Cipriani A, Cleare AJ, Florio AD, Dietch D, Geddes JR, Goodwin GM, Grunze H, Hayes JF, Jones I, Kasper S, Macritchie K, McAllister-Williams RH, Morriss R, Nayrouz S, Pappa S, Soares JC, Smith DJ, Suppes T, et al. (2019) Treatment-resistant and multi-therapy-resistant criteria for bipolar depression: consensus definition. Br J Psychiatry 214:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu YD, Xiang YT, Fang JX, Zu S, Sha S, Shi H, Ungvari GS, Correll CU, Chiu HF, Xue Y, Tian TF, Wu AS, Ma X, Wang G (2016) Single i.v. ketamine augmentation of newly initiated escitalopram for major depression: results from a randomized, placebo-controlled 4-week study. Psychol Med 46:623–635. [DOI] [PubMed] [Google Scholar]
- Ibrahim L, Diazgranados N, Franco-Chaves J, Brutsche N, Henter ID, Kronstein P, Moaddel R, Wainer I, Luckenbaugh DA, Manji HK, Zarate CA Jr (2012) Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology 37:1526–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionescu DF, Luckenbaugh DA, Niciu MJ, Richards EM, Slonena EE, Vande Voort JL, Brutsche NE, Zarate CA Jr (2014) Effect of baseline anxious depression on initial and sustained antidepressant response to ketamine. J Clin Psychiatry 75:e932–e938. [DOI] [PubMed] [Google Scholar]
- Ionescu DF, Luckenbaugh DA, Niciu MJ, Richards EM, Zarate CA Jr (2015) A single infusion of ketamine improves depression scores in patients with anxious bipolar depression. Bipolar Disord 17:438–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionescu DF, Bentley KH, Eikermann M, Taylor N, Akeju O, Swee MB, Pavone KJ, Petrie SR, Dording C, Mischoulon D, Alpert JE, Brown EN, Baer L, Nock MK, Fava M, Cusin C (2019) Repeat-dose ketamine augmentation for treatment-resistant depression with chronic suicidal ideation: a randomized, double blind, placebo controlled trial. J Affect Disord 243:516–524. [DOI] [PubMed] [Google Scholar]
- Kim J, Farchione T, Potter A, Chen Q, Temple R (2019) Esketamine for treatment-resistant depression–first FDA-approved antidepressant in a new class. N Engl J Med 381:1–4. [DOI] [PubMed] [Google Scholar]
- Kraus C, Rabl U, Vanicek T, Carlberg L, Popovic A, Spies M, Bartova L, Gryglewski G, Papageorgiou K, Lanzenberger R, Willeit M, Winkler D, Rybakowski JK, Kasper S (2017) Administration of ketamine for unipolar and bipolar depression. Int J Psychiatry Clin Pract 21:2–12. [DOI] [PubMed] [Google Scholar]
- Lener MS, Kadriu B, Zarate CA Jr (2017) Ketamine and beyond: investigations into the potential of glutamatergic agents to treat depression. Drugs 77:381–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Díaz Á, Fernández-González JL, Luján-Jiménez JE, Galiano-Rus S, Gutiérrez-Rojas L (2017) Use of repeated intravenous ketamine therapy in treatment-resistant bipolar depression with suicidal behaviour: a case report from Spain. Ther Adv Psychopharmacol 7:137–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew SJ, Murrough JW, aan het Rot M, Collins KA, Reich DL, Charney DS (2010) Riluzole for relapse prevention following intravenous ketamine in treatment-resistant depression: a pilot randomized, placebo-controlled continuation trial. Int J Neuropsychopharmacol 13:71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloud TL, Caddy C, Jochim J, Rendell JM, Diamond PR, Shuttleworth C, Brett D, Amit BH, McShane R, Hamadi L, Hawton K, Cipriani A (2015) Ketamine and other glutamate receptor modulators for depression in bipolar disorder in adults. Cochrane Database Syst Rev 9: CD011611. [DOI] [PubMed] [Google Scholar]
- McGirr A, Berlim MT, Bond DJ, Fleck MP, Yatham LN, Lam RW (2015) A systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials of ketamine in the rapid treatment of major depressive episodes. Psychol Med 45:693–704. [DOI] [PubMed] [Google Scholar]
- McIntyre RS, Carvalho IP, Lui LMW, Majeed A, Masand PS, Gill H, Rodrigues NB, Lipsitz O, Coles AC, Lee Y, Tamura JK, Iacobucci M, Phan L, Nasri F, Singhal N, Wong ER, Subramaniapillai M, Mansur R, Ho R, Lam RW, et al. (2020) The effect of intravenous, intranasal, and oral ketamine in mood disorders: a meta-analysis. J Affect Disord 276:576–584. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Perez AM, Mathew SJ, Charney DS (2011) A case of sustained remission following an acute course of ketamine in treatment-resistant depression. J Clin Psychiatry 72:414–415. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, aan het Rot M, Collins KA, Mathew SJ, Charney DS, Iosifescu DV (2013) Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry 74:250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport DJ, Carpenter LL, McDonald WM, Potash JB, Tohen M, Nemeroff CB; APA Council of Research Task Force on Novel Biomarkers and Treatments (2015) Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry 172:950–966. [DOI] [PubMed] [Google Scholar]
- Niciu MJ, Luckenbaugh DA, Ionescu DF, Mathews DC, Richards EM, Zarate CA Jr (2013) Subanesthetic dose ketamine does not induce an affective switch in three independent samples of treatment-resistant major depression. Biol Psychiatry 74:e23–e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Permoda-Osip A, Skibińska M, Bartkowska-Sniatkowska A, Kliwicki S, Chłopocka-Woźniak M, Rybakowski JK (2014) Factors connected with efficacy of single ketamine infusion in bipolar depression. Psychiatr Pol 48:35–47. [PubMed] [Google Scholar]
- Phillips JL, Norris S, Talbot J, Birmingham M, Hatchard T, Ortiz A, Owoeye O, Batten LA, Blier P (2019) Single, repeated, and maintenance ketamine infusions for treatment-resistant depression: a randomized controlled trial. Am J Psychiatry 176:401–409. [DOI] [PubMed] [Google Scholar]
- Reinstatler L, Youssef NA (2015) Ketamine as a potential treatment for suicidal ideation: a systematic review of the literature. Drugs R D 15:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybakowski JK, Permoda-Osip A, Skibinska M, Adamski R, Bartkowska-Sniatkowska A (2013) Single ketamine infusion in bipolar depression resistant to antidepressants: are neurotrophins involved? Hum Psychopharmacol 28:87–90. [DOI] [PubMed] [Google Scholar]
- Sachs GS (1996) Treatment-resistant bipolar depression. Psychiatr Clin North Am 19:215–236. [DOI] [PubMed] [Google Scholar]
- Schatzberg AF (2019) A word to the wise about intranasal esketamine. Am J Psychiatry 176:422–424. [DOI] [PubMed] [Google Scholar]
- Schoeyen HK, Kessler U, Andreassen OA, Auestad BH, Bergsholm P, Malt UF, Morken G, Oedegaard KJ, Vaaler A (2015) Treatment-resistant bipolar depression: a randomized controlled trial of electroconvulsive therapy versus algorithm-based pharmacological treatment. Am J Psychiatry 172:41–51. [DOI] [PubMed] [Google Scholar]
- Sienaert P, Lambrichts L, Dols A, De Fruyt J (2013) Evidence-based treatment strategies for treatment-resistant bipolar depression: a systematic review. Bipolar Disord 15:61–69. [DOI] [PubMed] [Google Scholar]
- Strasburger SE, Bhimani PM, Kaabe JH, Krysiak JT, Nanchanatt DL, Nguyen TN, Pough KA, Prince TA, Ramsey NS, Savsani KH, Scandlen L, Cavaretta MJ, Raffa RB (2017) What is the mechanism of Ketamine’s rapid-onset antidepressant effect? A concise overview of the surprisingly large number of possibilities. J Clin Pharm Ther 42:147–154. [DOI] [PubMed] [Google Scholar]
- Tavares DF, Myczkowski ML, Alberto RL, Valiengo L, Rios RM, Gordon P, de Sampaio-Junior B, Klein I, Mansur CG, Marcolin MA, Lafer B, Moreno RA, Gattaz W, Daskalakis ZJ, Brunoni AR (2017) Treatment of bipolar depression with deep TMS: results from a double-blind, randomized, parallel group, sham-controlled clinical trial. Neuropsychopharmacology 42:2593–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibensky BN, de Léséleuc L, Perras C, Picheca L (2016) Esketamine for treatment-resistant depression. In: CADTH issues in emerging health technologies. Ottawa, Canada: Canadian Agency for Drugs and Technologies in Health. https://www.ncbi.nlm.nih.gov/books/NBK542712/ [PubMed] [Google Scholar]
- Veritas Health Innovation (2019) Covidence systematic review software. Melbourne, Australia: Veritas Health Innovation. [Google Scholar]
- Wei Y, Chang L, Hashimoto K (2020) A historical review of antidepressant effects of ketamine and its enantiomers. Pharmacol Biochem Behav 190:172870. [DOI] [PubMed] [Google Scholar]
- Wilkinson ST, Ballard ED, Bloch MH, Mathew SJ, Murrough JW, Feder A, Sos P, Wang G, Zarate CA Jr, Sanacora G (2018) The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry 175:150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams NR, Heifets BD, Bentzley BS, Blasey C, Sudheimer KD, Hawkins J, Lyons DM, Schatzberg AF (2019) Attenuation of antidepressant and antisuicidal effects of ketamine by opioid receptor antagonism. Mol Psychiatry 24:1779–1786. [DOI] [PubMed] [Google Scholar]
- Witt K, Potts J, Hubers A, Grunebaum MF, Murrough JW, Loo C, Cipriani A, Hawton K (2020) Ketamine for suicidal ideation in adults with psychiatric disorders: a systematic review and meta-analysis of treatment trials. Aust N Z J Psychiatry 54:29–45. [DOI] [PubMed] [Google Scholar]
- Zanos P, Gould TD (2018) Mechanisms of ketamine action as an antidepressant. Mol Psychiatry 23:801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KS, Fang Y, Huang XP, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CA Jr, Gould TD (2016) NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533:481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr, Quiroz JA, Singh JB, Denicoff KD, De Jesus G, Luckenbaugh DA, Charney DS, Manji HK (2005) An open-label trial of the glutamate-modulating agent riluzole in combination with lithium for the treatment of bipolar depression. Biol Psychiatry 57:430–432. [DOI] [PubMed] [Google Scholar]
- Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK (2006) A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63:856–864. [DOI] [PubMed] [Google Scholar]
- Zarate CA Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, Selter J, Marquardt CA, Liberty V, Luckenbaugh DA (2012) Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry 71:939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Zhou YL, Liu WJ, Wang CY, Zhan YN, Lan XF, Zhang B, Ning YP (2020) A preliminary study of adjunctive ketamine for treatment-resistant bipolar depression. J Affect Disord 275:38–43. [DOI] [PubMed] [Google Scholar]