Abstract
Background:
Excessively high joint loading during dynamic movements may negatively influence articular cartilage health and contribute to the development of posttraumatic osteoarthritis after anterior cruciate ligament reconstruction (ACLR). Little is known regarding the link between aberrant jump-landing biomechanics and articular cartilage health after ACLR.
Purpose/Hypothesis:
The purpose of this study was to determine the associations between jump-landing biomechanics and tibiofemoral articular cartilage composition measured using T1ρ magnetic resonance imaging (MRI) relaxation times 12 months postoperatively. We hypothesized that individuals who demonstrate alterations in jump-landing biomechanics, commonly observed after ACLR, would have longer T1ρ MRI relaxation times (longer T1ρ relaxation times associated with less proteoglycan density).
Study Design:
Cross-sectional study; Level of evidence, 3.
Methods:
A total of 27 individuals with unilateral ACLR participated in this cross-sectional study. Jump-landing biomechanics (peak vertical ground-reaction force [vGRF], peak internal knee extension moment [KEM], peak internal knee adduction moment [KAM]) and T1ρ MRI were collected 12 months postoperatively. Mean T1ρ relaxation times for the entire weightbearing medial femoral condyle, lateral femoral condyle (global LFC), medial tibial condyle, and lateral tibial condyle (global LTC) were calculated bilaterally. Global regions of interest were further subsectioned into posterior, central, and anterior regions of interest. All T1ρ relaxation times in the ACLR limb were normalized to the uninjured contralateral limb. Linear regressions were used to determine associations between T1ρ relaxation times and biomechanics after accounting for meniscal/chondral injury.
Results:
Lower ACLR limb KEM was associated with longer T1ρ relaxation times for the global LTC (ΔR 2 = 0.24; P = .02), posterior LTC (ΔR 2 = 0.21; P = .03), and anterior LTC (ΔR 2 = 0.18; P = .04). Greater ACLR limb peak vGRF was associated with longer T1ρ relaxation times for the global LFC (ΔR 2 = 0.20; P = .02) and central LFC (ΔR 2 = 0.15; P = .05). Peak KAM was not associated with T1ρ outcomes.
Conclusion:
At 12 months postoperatively, lower peak KEM and greater peak vGRF during jump landing were related to longer T1ρ relaxation times, suggesting worse articular cartilage composition.
Keywords: ACL, biomechanics, imaging, magnetic resonance, motion analysis/kinesiology
Approximately one-third of individuals who sustain an anterior cruciate ligament (ACL) injury will develop radiographic posttraumatic osteoarthritis (PTOA) within 10 years of ACL reconstruction (ACLR). 25 Compositional changes to the articular cartilage occur within the first 12 months after ACL injury and ACLR, 24,48 which may contribute to PTOA onset. 7,40 T1ρ magnetic resonance imaging (MRI) is sensitive to proteoglycan density 6 and has been used to evaluate early in vivo compositional changes in the tibiofemoral articular cartilage after ACLR. 49 Individuals with ACLR also typically demonstrate aberrant joint biomechanics, which may alter forces imposed on the tibiofemoral articular cartilage. 4,14,31,32,51 Therefore, understanding the interplay between joint biomechanics and articular cartilage composition is critical to understanding the multifaceted early progression to PTOA.
Much of the initial research regarding the interplay between biomechanics and articular cartilage composition has focused on the influence of low-impact, cyclical loading such as walking gait. 20,36,46 Individuals who sustain ACL injuries are often young and physically active 13 and also engage in dynamic movements such as jump landing more commonly in their daily activities. Therefore, it is possible that alterations in the biomechanics of these commonly occurring dynamic movements (ie, jump landing) may be related to early changes in cartilage composition early after ACLR. Additionally, there is evidence to suggest that magnitudes of joint loading are task dependent, as individuals with an ACLR who demonstrate the greatest peak loading during walking do not necessarily demonstrate the greatest loading during jump landing 35 ; therefore, a relationship between jump-landing biomechanics and articular cartilage composition cannot be inferred from previous gait-related biomechanics research. Individuals with ACLR often land from a jump with lower internal knee extension moments (KEMs) on the ACLR limb 50 likely because of quadriceps inhibition, 29 which has been linked to an impaired capacity to attenuate force in the lower extremity. 30,50 Additionally, greater vertical ground-reaction force (vGRF) and internal knee adduction moment (KAM; the internal moment that resists dynamic knee valgus or compression of the lateral tibiofemoral compartment) are often implicated as risk factors for sustaining a second ACL injury. 15,31 Few studies have evaluated how KEM, vGRF, and KAM during jump landing are associated with early deleterious changes in articular cartilage composition that may be linked to the development of PTOA.
In uninjured individuals, lower KEM and greater KAM during a drop-landing task have been associated with longer medial femoral T1ρ MRI relaxation times. 44 Longer T1ρ relaxation times are interpreted as tissue consisting of lower proteoglycan density. 24 In the only study evaluating the association between jump-landing biomechanics and T1ρ MRI relaxation times in individuals after ACLR, lower peak vGRF and KEM in the reconstructed limb at 6 months postoperatively were associated with longer medial femoral T1ρ relaxation times between preoperative and 3-year postoperative time points. 42 While the association between less loading at 6 months after ACLR during jump landing and an increase in T1ρ relaxation times is counterintuitive, Shimizu et al 42 found in the same study that an increase in vGRF and KEM between 6 months and 3 years postoperatively was associated with an increase in T1ρ relaxation times from preinjury to 3 years after ACLR. The dynamic nature of the relationship between T1ρ relaxation times and jump-landing biomechanics suggests that further study is needed at an intermediate time point, such as 12 months postoperatively, when most individuals have returned to unrestricted physical activity. 2
Previous research has focused on the association between jump-landing biomechanics and T1ρ relaxation times in the medial tibiofemoral compartment, yet tissue of the lateral tibiofemoral compartment is commonly injured during ACL injury. T1ρ relaxation times are greater in the articular cartilage directly corresponding to areas of the lateral tibiofemoral compartment where traumatic bone bruising is evident. 16 As such, it is possible that aberrant loading of the knee during dynamic movements may negatively affect the articular cartilage of the lateral tibiofemoral compartment, which may be susceptible to further compositional changes because of traumatic bone bruising after ACLR. Additionally, worse patient-reported outcomes 12 months postoperatively are associated with longer T1ρ relaxation times of the lateral femoral condyle (LFC). Therefore, an evaluation of the association between jump-landing biomechanics and T1ρ relaxation times in both the lateral and medial tibiofemoral compartments after ACLR is needed in order to comprehensively understand how dynamic movements may be associated with deleterious joint tissue changes across the joint.
The purpose of this study was to determine associations between T1ρ MRI relaxation times (ACLR limb normalized to the uninjured limb) and peak vGRF, KEM, and KAM at 12 months after ACLR. We hypothesized that individuals with greater peak vGRF as well as lower KEM and greater KAM would demonstrate longer T1ρ relaxation times 12 months postoperatively.
Methods
Study Design
All participants were recruited into this cross-sectional study within 14 days of ACL injury but before undergoing ACLR as part of a larger prospective longitudinal cohort study. Additionally, 1 of 3 participating orthopaedic surgeons confirmed the presence, or lack thereof, of a concomitant injury (ie, meniscal, chondral, or concomitant ligamentous injury). Jump-landing biomechanics and T1ρ MRI scans were assessed 12 months after ACLR (mean, 373 ± 18 days). Additionally, all participants were cleared for unrestricted physical activity at the time of jump-landing biomechanics testing and MRI acquisition. All participants provided informed consent to participate in the study and the university’s institutional review board approved all aspects of the study.
Participants
The current study included 27 individuals who were a part of a larger prospective study and were retained for both biomechanics and T1ρ MRI acquisition at 12 months after ACLR. We excluded individuals who were not between the ages of 18 and 35 years, were in need of a multiligamentous reconstruction, were previously diagnosed with any form of arthritis, had a previous history of an ACL injury to either limb, had a body mass index > 35 , or were pregnant or planned to become pregnant within 12 months of study enrollment. Participants completed the Knee injury and Osteoarthritis Outcome Score questionnaire during the 12-month follow-up in order to quantify self-perceived knee function for descriptive purposes. 41 We expected that the biomechanical outcomes examined in the current study (vGRF, KEM, and KAM) would demonstrate a moderate association (r = 0.5) with T1ρ relaxation times, as similar associations have been reported between jump-landing biomechanics and changes in T1ρ relaxation times early after ACLR. 42 Therefore, we estimated that we would need 26 participants to detect significant associations between jump-landing biomechanics and T1ρ relaxations times using a 2-tailed α level of .05 and 80% power (Version 3.1.9.2; G*Power). 11 We recruited 27 individuals for participation in this study.
ACLR
All participants underwent a similar ACLR procedure to that previously described. 37 Briefly, all participants underwent an arthroscopically assisted single-incision ACLR using a patellar tendon autograft performed by 1 of 3 participating orthopaedic surgeons. The autograft was harvested from the middle third of the patellar tendon using an anterior longitudinal incision. A target point on the lateral wall of the intercondylar notch of the femur was identified, and a femoral tunnel was drilled through an inframedial arthroscopic portal with the knee flexed to approximately 120°. While using a targeting guide, a pin was drilled and overreamed into the ACL footprint to create a tibial tunnel. Additionally, bone plugs of the graft were affixed to the femur and tibia using a metal interference screw. All participants were referred to a licensed physical therapist or athletic trainer for supervised, structured rehabilitation, which began during the first week after surgery and progressed over the following 6 months. Participants completed physical therapy at their clinic of choice and were cleared to return to unrestricted physical activity. Time to return to sport was not recorded.
Motion Analysis of Jump Landing
All participants completed the jump-landing protocol in their own athletic footwear from a box (standard height, 30 cm) positioned 50% of their height from the front edge of 2 floor-embedded force plates. 28 Participants were instructed to jump forward off the box to a double-leg landing with 1 foot on each force plate, followed by an immediate vertical jump for maximum height. A minimum of 3 practice trials was performed, and subsequent practice trials may have been conducted on an individual basis until investigators were comfortable that the participants understood how to properly perform the task. A minimum of 30 seconds separated each trial, yet participants were allowed as much rest time as needed between each jump-landing trial in order to ensure that the subsequent trials could be completed with maximal effort. Five successful jump-landing trials, during which the participant jumped from the box with both feet at the same time, landed on the force plates, and performed a subsequent vertical jump, were collected. In the event of an unsuccessful trial, a subsequent trial was collected and utilized for analysis.
Three-dimensional kinematic data were sampled at 120 Hz using a 10-camera motion capture system (Vicon; Nexus) and low-pass filtered at 10 Hz (fourth-order recursive Butterworth filter). 35 Kinetic data were sampled at 1200 Hz from 2 embedded force plates (FP406010; Bertec Corp) and low-pass filtered at 75 Hz (fourth-order recursive Butterworth filter). 3 Using a modified Helen Hayes marker set, 17 all participants were outfitted with 25 retroreflective markers as well as with a cluster of 3 additional markers secured over the sacrum. 35 A static trial was captured while the participant stood with arms positioned at 90° of shoulder abduction to estimate the location of the landmarks needed to calculate joint centers. Markers placed on the medial epicondyles and malleoli were removed during data collection to ensure that medial markers would not contact each other or influence the usual movements of the participants during the jump-landing trials. Knee and ankle joint centers were defined as the midpoint between the medial and lateral condyles and malleoli, respectively. The hip joint center was estimated from the coordinates of the L4-L5 and right and left anterior superior iliac spine markers using the Bell method. 5 Joint angles were defined based on the position of the distal segment relative to the proximal segment using the Euler method, 18 with the following planes of rotational motion: sagittal (y-axis), frontal (x-axis), and transverse (z-axis). Greater knee flexion and adduction were defined as positive values.
The loading phase of jump landing was defined as the first 100 milliseconds after ground contact (vGRF, >20 N), 35 which has previously been reported to be the time period during which the greatest amount of loading and ACL injury would most commonly occur. 19 All jump-landing biomechanics were extracted during the first 100 milliseconds after ground contact and averaged across the 5 trials for the jump-landing task. Additionally, while only jump-landing biomechanics for the ACLR limb were used in the primary analyses, data for the uninjured limb were collected to evaluate side-to-side differences for descriptive purposes. Peak vGRF was normalized to body weight (BW). Peak internal KEM and KAM were calculated using an inverse dynamics approach, normalized to the product of BW and height (BW × height) 26 and expressed as internal moments. 36
MRI Acquisition
The T1ρ MRI scans were acquired using either a Siemens Magnetom TIM Trio 3 T scanner using a 4-channel Siemens large flex coil (516 mm × 224 mm; Siemens; n = 17) or a Siemens Magnetom Prisma 3 T PowerPack scanner using a XR 80/200 gradient coil (60 cm × 213 cm; Siemens; n = 10). As previously reported, these 2 scanners demonstrate high interscanner reliability (intraclass correlation coefficient [ICC], >0.96; coefficient of variation range, 1.46%-5.02%). 36 Upon arrival to the imaging center, participants remained seated for 30 minutes to unload the knee articular cartilage. We used a T1ρ-prepared 3-dimensional fast low angle shot with a spin-lock power at 500 Hz, 5 different spin-lock durations (40, 30, 20, 10, 0 milliseconds), and a voxel size of 0.8 × 0.4 × 3 mm (field of view, 288 mm; slice thickness, 3.0 mm; repetition time, 9.2 milliseconds; echo time, 4.6 milliseconds; averaging, 1; bandwidth, 350 Hz; acquisition time range, 700-900 seconds [depending on the number of slices]; range of number of slices acquired, 28-36 slices; 160 × 320 matrix; gap, 0 mm; flip angle, 10°, echo-train duration time, 443 milliseconds; phase encode direction of anterior-posterior).
Before segmentation of the articular cartilage, an affine registration technique was utilized to register the 12-month ACLR limb image to the 12-month uninjured limb image using 3D Slicer software (Version 4.6.2). 12 After the affine registration, a nonrigid, deformable, voxel-by-voxel, intensity-based registration technique was applied to account for interlimb differences of specific tissues (eg, bone, cartilage) and ensure a more accurate alignment of the ACLR limb to the uninjured limb. 36 All registration procedures were initially performed on the 0-millisecond spin-lock time [TSL]) image and then subsequently applied to the relaxation map.
Segmentation of the Tibiofemoral Articular Cartilage
After image registration, the articular cartilage of the weightbearing medial femoral condyle (MFC), LFC, medial tibial condyle (MTC), and lateral tibial condyle (LTC) was manually segmented using ITK-Snap software 54 on a T1ρ MRI scan acquired during the 0-millisecond TSL by 2 separate segmentors (S.J.P. and K.W.). The manual segmentation technique used in the current study has demonstrated strong reliability for all regions of interest (ROIs) (intrarater reliability [n = 8] ICC range, 0.80-0.97; intersegmentor reliability [n = 10] ICC range, 0.75-0.98). 34,36 Additionally, a fellowship-trained musculoskeletal radiologist (D.N.) confirmed the anatomic accuracy of the segmentations.
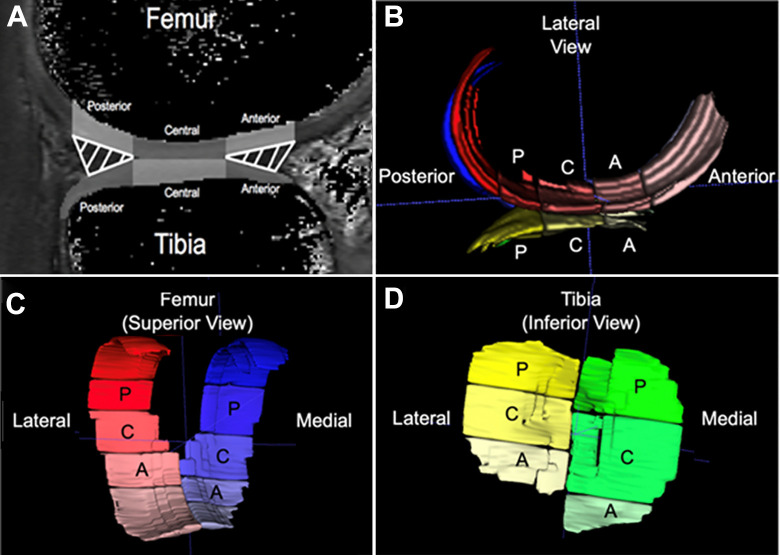
The mean T1ρ relaxation times of the weightbearing ROI of the medial and lateral femur and tibia (global ROI) were evaluated and used as a primary outcome. Furthermore, the global ROIs of the femoral and tibial condyles were subsectioned into 3 ROIs based on the location of the meniscus in the sagittal plane (Figure 1). 34,36 The 3 subsection ROIs represent loadbearing regions of the femoral condyle and included (1) the articular cartilage overlying the posterior horn of the meniscus (posterior MFC/LFC and MTC/LTC), (2) the central portion of the articular cartilage that lies between the anterior and posterior horns of the meniscus (central MFC/LFC and MTC/LTC), and (3) the articular cartilage corresponding with the anterior horn of the meniscus (anterior MFC/LFC and MTC/LTC) (Figure 1). The weightbearing measure for the femoral and tibial condyles, defined as the mean relaxation time for the 3 subsection ROIs (posterior, central, and anterior) averaged together, was used for analysis.
Figure 1.
(A) Weightbearing regions of interest (gray-shaded regions) were determined using the position of the meniscus in the sagittal plane: the articular cartilage overlying the anterior horn (anterior), between the anterior and posterior horns (central), and overlying the posterior horn (posterior) of the menisci. (B-D) Three-dimensional renderings of voxels determined to correspond to the segmented (B) femoral and tibial articular cartilage as seen from a lateral view in the sagittal plane, (C) femoral articular cartilage as seen from a superior view, and (D) tibial articular cartilage as seen from an inferior view. A, anterior; C, central; P, posterior.
T1ρ Relaxation Time Quantification
Voxel-by-voxel T1ρ relaxation maps were constructed from a 5-image sequence using a MATLAB program (MATLAB R2014b, 8.4.0; MathWorks) with the following equation:
where S corresponds to signal intensity, TSL is the duration of the TSL, S 0 is signal intensity when TSL equals 0, and T1ρ is the T1 relaxation time in the rotating frame. The previous segmentation image, performed on the 0-millisecond spin-lock image, was transposed over the T1ρ image to establish T1ρ relaxation times for each ROI. A mean of the T1ρ relaxation times for each ROI was calculated using ITK-SNAP software. 54 For analysis, we normalized T1ρ relaxation times for each ROI in the ACLR limb to the same ROI in the uninjured limb (ACLR limb/uninjured limb). 34,36,38 While it is possible that changes in T1ρ relaxation times may change over time in the uninjured limb, 33 in the absence of preinjury T1ρ data, the normalization procedure is used to assess the amount of change in tissue of the ACLR limb beyond that which would be expected in the uninjured limb. A T1ρ value >1.00 represents longer T1ρ relaxation times in the ACLR limb than the uninjured limb, which may be indicative of lower proteoglycan density in the ACLR limb. 34,36
Statistical Analysis
Means and standard deviations (SDs) were calculated for patient characteristics, jump-landing biomechanical outcome measures, and T1ρ outcome measures. Data distributions were assessed using the Shapiro-Wilk test for normality, and stem-and-leaf plots were utilized to visually inspect for potential outliers for all outcomes. Data points for any outcome that were >3 SDs from the mean were identified as outliers and removed before the final analysis.
Bonferroni-corrected dependent t tests were utilized to assess differences in T1ρ relaxation times (P ≤ [.05/4]) for each global ROI and subsectioned ROIs within the medial and lateral condyles of the femur and tibia, respectively. Similar Bonferroni-corrected dependent t tests were utilized to assess differences in biomechanical outcomes between the ACLR and uninjured limbs (P ≤ [.05/3]). Next, a total of 48 stepwise linear regression models were used to determine the variance in T1ρ relaxation times for all ROIs that were explained by peak vGRF, KEM, and KAM for the ACLR limb (predictor variables). Previous evidence has demonstrated that the presence of a meniscal injury 47 or a cartilage lesion 45 may influence T1ρ relaxation times after ACLR; therefore, a coded variable that represented the presence of a meniscal injury or cartilage lesion was entered into the linear regression models before the biomechanical variable of interest. The coded variable was entered based on the location of the ROI that was being examined in the given regression model (ie, medial meniscal or chondral injury coded variable entered for any association involving an ROI on the medial side, while the same was done for the lateral side). After accounting for the presence of a meniscal injury or cartilage lesion, we were able to determine the unique contribution of each predictor variable (vGRF, KEM, KAM) on the T1ρ relaxation times for each ROI by entering each biomechanical variable separately into its own regression model. The 2-tailed level of significance was set a priori for the regression analyses at P ≤ .05, and all analyses were performed using the Statistical Package for the Social Sciences (Version 25; IBM Corp).
Results
Participant Characteristics
All 27 individuals eligible for the study were retained for a 12-month follow-up visit (Table 1). Three participants (11%) sustained an isolated ACL injury, while 24 participants (89%) were evaluated with a secondary concomitant knee injury that occurred at the time of the ACL injury (chondral injury [n = 11; 41%], lateral meniscal injury [n = 19; 70%], medial meniscal injury [n = 8; 30%]).
Table 1.
Patient Characteristics (N = 27) a
| Variable | Value |
|---|---|
| Sex, male/female, n | 13/14 |
| Age, y | 22.11 ± 3.88 |
| Height, cm | 178.08 ± 11.06 |
| Weight, kg | 76.15 ± 13.17 |
| BMI | 23.88 ± 2.51 |
| Time between ACL injury and ACLR, d | 27.85 ± 13.68 |
| 12-mo postoperative KOOS | |
| Symptoms | 85.74 ± 10.50 |
| Pain | 91.87 ± 7.09 |
| Activities of Daily Living | 97.48 ± 3.81 |
| Sports | 84.81 ± 15.47 |
| Quality of Life | 76.25 ± 17.39 |
a Data are reported as mean ± SD unless otherwise indicated. ACL, anterior cruciate ligament; ACLR, ACL reconstruction; BMI, body mass index; KOOS, Knee injury and Osteoarthritis Outcome Score.
Mean T1ρ relaxation times were significantly longer in the ACLR limb compared with the uninjured limb for every ROI except for the global, central, and anterior LTC (P ≤ .0125 for all) (Table 2). Additionally, individuals demonstrated significantly lower peak vGRF and KEM as well as greater peak KAM in the ACLR limb compared with the uninjured limb (P ≤ .014 for all) (Table 3). Two outliers for peak KEM were detected (>3 SD from the mean) and were removed from the analyses involving these variables. No other outliers were detected, and all outcomes were normally distributed.
Table 2.
T1ρ Relaxation Times a
| T1ρ Relaxation Time, ms | |||||
|---|---|---|---|---|---|
| Region of Interest | ACLR Limb | Uninjured Limb | P Value | ILR (ACLR/Uninjured) | Cohen d |
| LFC | |||||
| Global | 53.5 ± 3.4 | 47.9 ± 3.7 | <.001 | 1.13 ± 0.09 | 1.58 |
| Posterior | 57.1 ± 5.8 | 53.2 ± 5.9 | .010 | 1.08 ± 0.14 | 0.66 |
| Central | 52.8 ± 3.8 | 47.1 ± 4.3 | <.001 | 1.13 ± 0.12 | 0.66 |
| Anterior | 50.8 ± 3.9 | 43.4 ± 3.2 | <.001 | 1.17 ± 0.10 | 2.07 |
| LTC | |||||
| Global | 48.7 ± 2.7 | 47.3 ± 3.7 | .036 | 1.04 ± 0.07 | 0.43 |
| Posterior | 50.9 ± 3.1 | 47.5 ± 3.8 | <.001 | 1.08 ± 0.09 | 0.98 |
| Central | 44.6 ± 3.1 | 43.1 ± 3.8 | .034 | 1.04 ± 0.09 | 0.43 |
| Anterior | 50.6 ± 3.9 | 51.2 ± 5.6 | .413 | 0.99 ± 0.08 | 0.12 |
| MFC | |||||
| Global | 54.5 ± 3.8 | 49.3 ± 2.5 | <.001 | 1.11 ± 0.07 | 1.62 |
| Posterior | 52.8 ± 4.5 | 50.3 ± 3.0 | .005 | 1.05 ± 0.08 | 0.65 |
| Central | 54.3 ± 4.6 | 47.8 ± 2.5 | <.001 | 1.14 ± 0.08 | 1.76 |
| Anterior | 56.5 ± 4.6 | 49.7 ± 3.4 | <.001 | 1.14 ± 0.08 | 1.68 |
| MTC | |||||
| Global | 48.8 ± 3.8 | 46.5 ± 3.9 | .001 | 1.05 ± 0.07 | 1.68 |
| Posterior | 47.5 ± 3.9 | 45.5 ± 4.0 | .011 | 1.05 ± 0.09 | 0.51 |
| Central | 47.1 ± 4.3 | 44.8 ± 4.9 | .002 | 1.06 ± 0.08 | 0.50 |
| Anterior | 51.9 ± 5.3 | 49.1 ± 3.9 | .006 | 1.06 ± 0.10 | 0.60 |
a Data are reported as mean ± SD. Boldface P values indicate a statistically significant difference between the ACLR and uninjured limbs (P ≤ [.05/4]). ACLR, anterior cruciate ligament reconstruction; ILR, interlimb ratio; LFC, lateral femoral condyle; LTC, lateral tibial condyle; MFC, medial femoral condyle; MTC, medial tibial condyle.
Table 3.
Jump-Landing Biomechanics a
| Variable | ACLR Limb | Uninjured Limb | P Value | Cohen d |
|---|---|---|---|---|
| Peak vGRF, BW | 2.24 ± 0.59 | 2.68 ± 0.69 | <.001 | 0.68 |
| Peak internal KEM, BW × height | 0.12 ± 0.04 | 0.17 ± 0.07 | <.001 | 0.88 |
| Peak internal KAM, BW × height | 0.02 ± 0.02 | 0.01 ± 0.01 | .014 | 0.63 |
a Data are reported as mean ± SD. Boldface P values indicate statistically significant difference between the ACLR and uninjured limbs (P ≤ [.05/3]). ACLR, anterior cruciate ligament reconstruction; BW, body weight; KAM, knee adduction moment; KEM, knee extension moment; vGRF, vertical ground-reaction force.
Association Between Jump-Landing Biomechanics and T1ρ Relaxation Times
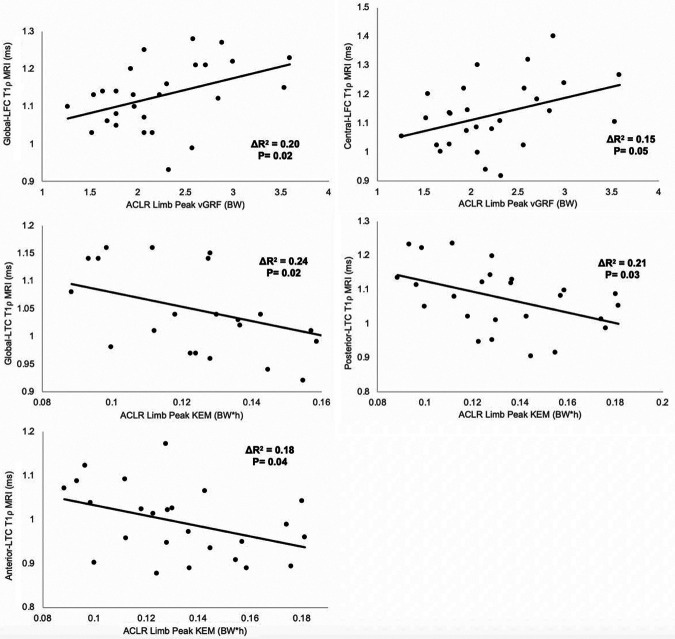
The association between jump-landing biomechanics and lateral compartment T1ρ relaxation times is shown in Table 4. After accounting for the presence of a meniscal injury or cartilage lesion, greater peak vGRF in the ACLR limb was associated with longer 12-month T1ρ relaxation times for the entire weightbearing region of the LFC (global LFC: ΔR 2 = 0.20; standardized β coefficient = 0.46; P = .02). More specifically, greater peak vGRF in the ACLR limb was associated with longer 12-month T1ρ relaxation times for the central ROI of the LFC (central LFC: ΔR 2 = 0.15; standardized β coefficient = 0.40; P = .05). Lower peak KEM for the ACLR limb was associated with longer 12-month T1ρ relaxation times for the entire weightbearing region of the LTC (global LTC: ΔR 2 = 0.24; standardized β coefficient = –0.49; P = .02) as well as the posterior ROI (posterior LTC: ΔR 2 = 0.21; standardized β coefficient = –0.45; P = .03) and anterior ROI (anterior LTC: ΔR 2 = 0.18; standardized β coefficient = –0.42; P = .04). There were no statistically significant associations between peak KAM and 12-month T1ρ relaxation times for any ROI of the LFC and LTC (Table 3). Jump-landing biomechanics were not significantly associated with 12-month T1ρ relaxation times for any ROI of the MFC or MTC (Table 5). Scatterplots of significant associations are presented in Figure 2.
Table 4.
Association Between Jump-Landing Biomechanics and Lateral T1ρ Relaxation Times After Accounting for the Presence of Meniscal Tear and/or Cartilage Lesion a
| LFC, ΔR 2, β | LTC, ΔR 2, β | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Global | Posterior | Central | Anterior | Global | Posterior | Central | Anterior |
| Injured vGRF | 0.20, 0.46 | 0.12, 0.36 | 0.15, 0.40 | 0.06, 0.24 | 0.01, 0.08 | 0.04, 0.21 | 0.01, 0.05 | 0.01, –0.08 |
| P value | .02 | .08 | .05 | .24 | .70 | .30 | .79 | .69 |
| Injured KEM (n = 25) | 0.01, –0.03 | 0.01, –0.03 | 0.01, –0.04 | 0.01, –0.01 | 0.24, –0.49 | 0.21, –0.45 | 0.14, –0.37 | 0.18, –0.42 |
| P value | .88 | .89 | .87 | .99 | .02 | .03 | .07 | .04 |
| Injured KAM | 0.01, 0.01 | 0.01, –0.11 | 0.01, –0.03 | 0.05, 0.23 | 0.01, 0.09 | 0.01, –0.01 | 0.01, 0.04 | 0.04, 0.20 |
| P value | .95 | .58 | .90 | .25 | .66 | .98 | .84 | .32 |
a Boldface P values indicate statistical significance (P ≤ .05). KAM, peak internal knee adduction moment; KEM, peak internal knee extension moment; LFC, lateral femoral condyle; LTC, lateral tibial condyle; vGRF, vertical ground-reaction force.
Table 5.
Association Between Jump-Landing Biomechanics and Medial T1ρ Relaxation Times After Accounting for the Presence of Meniscal Tear and/or Cartilage Lesion a
| MFC, ΔR 2, β | MTC, ΔR 2, β | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Global | Posterior | Central | Anterior | Global | Posterior | Central | Anterior |
| Injured vGRF | 0.07, 0.26 | 0.09, 0.30 | 0.04, 0.20 | 0.02, 0.13 | 0.01, –0.04 | 0.06, –0.25 | 0.02, –0.13 | 0.01, –0.08 |
| P value | .20 | .13 | .33 | .52 | .84 | .23 | .52 | .69 |
| Injured KEM (n = 25) | 0.01, –0.07 | 0.02, 0.14 | 0.03, –0.16 | 0.03, –0.15 | 0.02, 0.15 | 0.01, –0.07 | 0.05, 0.21 | 0.03, 0.17 |
| P value | .76 | .51 | .46 | .48 | .49 | .75 | .32 | .42 |
| Injured KAM | 0.03, 0.19 | 0.01, –0.08 | 0.04, 0.21 | 0.11, 0.34 | 0.03, –0.17 | 0.01, –0.11 | 0.01, –0.08 | 0.04, –0.20 |
| P value | .38 | .69 | .31 | .10 | .41 | .60 | .72 | .33 |
a KAM, peak internal knee adduction moment; KEM, peak internal knee extension moment; MFC, medial femoral condyle; MTC, medial tibial condyle; vGRF, vertical ground-reaction force.
Figure 2.
Scatterplots of significant associations between variables of mechanical loading during jump landing and T1ρ relaxation times for specific regions of interest within the tibiofemoral joint. ACLR, anterior cruciate ligament reconstruction; BW, body weight; h, height; KEM, knee extension moment; LFC, lateral femoral condyle; LTC, lateral tibial condyle; MRI, magnetic resonance imaging; vGRF, vertical ground-reaction force.
Discussion
Consistent with our hypothesis, individuals with greater peak vGRF and lower peak KEM in the ACLR limb during jump landing demonstrated longer T1ρ MRI relaxation times in the LFC and LTC 12 months after ACLR. Peak KAM for the injured limb was not significantly associated with T1ρ relaxation times for any ROI in either knee compartment. To our knowledge, this study is the first to examine the relationship between biomechanical outcomes during a dynamic task (eg, jump landing) and T1ρ relaxation times in both the medial and the lateral tibiofemoral compartments. Additionally, this is the only study to evaluate the association between jump-landing biomechanics and articular cartilage composition at 12 months postoperatively, which is a time point when individuals are typically discharged from traditional physical therapy and are participating in unrestricted physical activity. These data suggest that greater peak vertical limb loading (ie, vGRF) during a jump landing, which may be influenced by inadequate KEM, is associated with more deleterious compositional changes in the articular cartilage of the lateral tibiofemoral compartment. Therefore, implementing treatment strategies to lower peak vGRF and maximize KEM during high-energy activities like jump landing may be beneficial for optimizing articular cartilage tissue health after ACLR.
Shimizu et al 42 demonstrated that medial tibiofemoral T1ρ relaxation times increased between a presurgical time point and 3 years postoperatively in individuals with lower peak KEM in the ACLR limb at 6 months postoperatively. Similarly, we found that lower KEM was associated with longer T1ρ relaxation times in the lateral tibiofemoral compartment at 12 months after ACLR. Together, these data suggest that lower KEM may affect articular cartilage composition in both medial and lateral tibiofemoral compartments at different postoperative time points. Contrary to our findings, Shimizu et al found that lower peak vGRF at 6 months postoperatively was associated with an increase in medial tibiofemoral articular cartilage T1ρ relaxation times from preinjury to 3 years postoperatively. Those authors also found that an increase in peak vGRF from 6 months to 3 years postoperatively was significantly associated with an increase in T1ρ relaxation times in the medial femoral compartment from preinjury to 3 years postoperatively. 42
The differences in findings between our study and that of Shimizu et al 42 may be because of the time frames in which jump-landing biomechanics were measured, as it is common within the first 6 months postoperatively for individuals to unload the ACLR limb. 43 It is possible that this early unloading of the ACLR may be because of neuromuscular insufficiencies (ie, muscle weakness) and factors related to psychological readiness (ie, fear avoidance), which may result in individuals’ shifting greater loads to the uninjured limb during dynamic tasks. Similar to the direction of the association in the current study, Shimizu et al reported that the greater loading between 6 months and 3 years postoperatively was associated with longer T1ρ relaxation times in the medial tibiofemoral compartment at 3 years postoperatively. It is possible that time after ACLR is a critical factor in the nature of the association between jump-landing biomechanics and T1ρ relaxation times. While greater loading was associated with longer T1ρ relaxation times in our study, peak loading variables were still less on the ACLR limb compared with the uninjured limb. Therefore, future efforts to improve impact load dissipation during jump landing may need to be directed bilaterally, as reduction of impact load only in the ACLR limb may further exacerbate interlimb loading asymmetries.
We demonstrated significant associations between jump-landing biomechanics and T1ρ relaxation times in the lateral tibiofemoral compartment at 12 months postoperatively. After ACLR, compositional changes to articular cartilage can occur over time in both the medial and the lateral tibiofemoral compartments. 33 Additionally, previous research has demonstrated that individuals commonly sustain traumatic bone contusions to the LFT and LTC at the time of ACL injury. 16 As such, it is possible that the articular cartilage of the lateral tibiofemoral compartment may be at risk for alterations in articular cartilage composition that can influence long-term articular cartilage health and potential PTOA risk. Within the current study, 70% (n = 19) of participants sustained a concomitant lateral meniscal injury at the time of ACL injury. While we accounted for the presence of a meniscal injury during our analysis, it is possible that the number of individuals who sustained a concomitant injury to the lateral tibiofemoral compartment within our cohort may have affected our findings. Similarly, peak KAM was not significantly associated with T1ρ relaxation times for any ROI in the femur or tibia. It is possible that a high percentage of individuals with a concomitant lateral meniscal injury may have influenced our findings related to peak KAM as well. Future research should seek to evaluate whether increases in peak KAM during jump landing are associated with alterations in cartilage composition in individuals without a concomitant injury to the lateral tibiofemoral compartment.
The results of the current study indicate that greater peak vGRF and lower peak KEM during jump landing are significantly associated with longer T1ρ relaxation times for the LFC and LTC 12 months postoperatively. Previous research has demonstrated that feedback interventions, both internal and expert provided, have the capacity to reduce peak vGRF during jump-landing tasks. 8 –10 The findings of the current study may suggest that reducing peak vGRF during dynamic movements, such as jump landing, may be critical to the long-term preservation of articular cartilage health after ACLR. Feedback interventions may serve as a viable clinical intervention for eliciting these reductions in peak vGRF.
In addition, individuals have demonstrated quadriceps weakness as early as 6 months after ACLR, 21 –23,27 which has been shown to persist as long as 7 years postoperatively. 53 A reduction in quadriceps strength and function can directly influence the KEM during a jump-landing task, which may alter knee-joint motion and reduce the capacity for attenuating forces at the knee. 30,50 It is possible that underlying quadriceps dysfunction is a factor leading to the lower peak KEM and longer T1ρ relaxation times for the LTC in the ACLR limb. This hypothesis is consistent with previous research that demonstrated that less quadriceps strength was significantly associated with longer T1ρ relaxation times at 6 months postoperatively. 39 A reduction in peak KEM during a jump-landing task can cause individuals to land with a stiffened knee strategy, which may shift contact forces to portions of the articular cartilage that are unaccustomed to such loads. Together, these findings illustrate the importance of maximizing quadriceps strength throughout rehabilitation and return to activity after ACLR, as strength may be critical to promoting proper sagittal plane biomechanics during jump landing and maintaining long-term articular cartilage health postoperatively. However, further research is also needed to determine whether underlying issues such as pain and joint effusion as a result of early cartilage alterations may inhibit quadriceps strength and function, leading to alterations in jump-landing biomechanics commonly observed after ACLR.
The findings of the current study provide a novel insight into the association between jump-landing biomechanics and changes in articular cartilage composition 12 months postoperatively. However, there are limitations that need to be considered when interpreting these results. We chose to only evaluate individuals with unilateral ACLR; therefore, further research may be warranted to examine the relationship between mechanical loading during dynamic movements and articular cartilage health in individuals with multiple ACL injuries, as well as individuals with injuries to the contralateral limb. Furthermore, we did not evaluate the effects of a concomitant bone bruise on the T1ρ relaxation times for these individuals, and we did not account for differences in treatment strategies (ie, meniscectomy vs repair) for concomitant meniscal or chondral injuries, which may warrant further exploration. Future research should also evaluate associations between limb symmetry indices in both jump-landing outcomes and T1ρ relaxation times.
In addition, the current study evaluated outcome measures at 12 months postoperatively. Future longitudinal studies are needed to determine if this relationship between jump-landing biomechanics and T1ρ relaxation times may assist in predicting which individuals will go on to develop radiographic PTOA after ACLR. Similarly, because of the cross-sectional nature of the current study, we did not acquire baseline preinjury T1ρ MRI data for these individuals. As previous research has demonstrated that the injured and uninjured limbs undergo compositional changes after ACLR, 33 normalization of T1ρ MRI to the uninjured limb provided an estimate of differences in composition of the injured limb in addition to changes that may have occurred in the uninjured limb. For the current study, we did not evaluate associations between biomechanical and T1ρ MRI outcomes for the superficial and deep layers of the cartilage separately. Future research should assess whether the nature of these associations is dictated by the layer of cartilage that is being evaluated.
The statistically significant associations that were identified indicate that vGRF and KEM accounted for between 15% and 24% of the variance in T1ρ relaxation times of the lateral tibiofemoral compartment, suggesting that the majority of the variance in articular cartilage T1ρ relaxation times of the lateral tibiofemoral compartment is associated with variables other than the knee loading outcomes measured during jump landing. Future research should seek to determine the other variables associated with articular cartilage T1ρ relaxation times in the lateral tibiofemoral compartment 12 months after ACLR. As with previous research, 52 the longest TSL used for the current study during T1ρ MRI acquisition was 40 milliseconds, which is shorter than were our mean T1ρ relaxation times. Future studies with longer TSLs during acquisition may better estimate the T1ρ relaxation times of cartilage with relatively longer relaxation times.
The statistical approach used in the current study was consistent with that of other rigorous studies 1,36,42,44 using a similar sample size to evaluate a comparable number of associations between biomechanical outcomes and MRI outcomes. However, we acknowledge that performing a correction for multiple comparisons using a smaller sample size would potentially result in fewer associations presenting as statistically significant. Conversely, we note that utilizing a nonoperative correction for multiple comparisons with a small sample size may also increase the risk of an underpowered assessment of these associations and, therefore, an increased risk for type 2 error. Therefore, the findings from the current study should be considered hypothesis generating and justify the need for future studies to confirm these findings using larger sample sizes and more conservative statistical approaches.
Conclusion
This study demonstrated that greater ACLR limb peak vGRF and lower peak KEM during jump landing were associated with longer T1ρ relaxation times in the articular cartilage of the LFC and LTC at 12 months postoperatively. These findings provide evidence that jump-landing biomechanics early after ACLR are related to compositional changes within the tibiofemoral articular cartilage, which may be associated with tissue health and PTOA development. Interventions that seek to reduce peak vGRF and increase peak KEM during jump landing may be important for maintaining long-term articular cartilage health.
Footnotes
Final revision submitted December 8, 2020; accepted January 12, 2021.
One or more of the authors has declared the following potential conflict of interest or source of funding: This research was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (1R03AR066840-01A1), North Carolina Translational and Clinical Sciences (TRaCS) Institute, and National Athletic Trainers Association Research and Education Foundation (No. 14NewInv001). AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from the University of North Carolina at Chapel Hill (study No. 13-2385).
References
- 1. Amano K, Pedoia V, Su F, Souza RB, Li X, Ma CB. Persistent biomechanical alterations after ACL reconstruction are associated with early cartilage matrix changes detected by quantitative MR. Orthop J Sports Med. 2016;4(4):2325967116644421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ardern CL, Webster KE, Taylor NF, Feller JA. Return to sport following anterior cruciate ligament reconstruction surgery: a systematic review and meta-analysis of the state of play. Br J Sports Med. 2011;45(7):596–606. [DOI] [PubMed] [Google Scholar]
- 3. Blackburn JT, Pietrosimone BG, Harkey MS, Luc BA, Pamukoff DN. Comparison of three methods for identifying the heelstrike transient during walking gait. Med Eng Phys. 2016;38(6):581–585. [DOI] [PubMed] [Google Scholar]
- 4. Butler RJ, Minick KI, Ferber R, Underwood F. Gait mechanics after ACL reconstruction: implications for the early onset of knee osteoarthritis. Br J Sports Med. 2009;43(5):366–370. [DOI] [PubMed] [Google Scholar]
- 5. Cappozzo A, Catani F, Croce UD, Leardini A. Position and orientation in space of bones during movement: anatomical frame definition and determination. Clin Biomech (Bristol, Avon). 1995;10(4):171–178. [DOI] [PubMed] [Google Scholar]
- 6. Duvvuri U, Goldberg AD, Kranz JK, et al. Water magnetic relaxation dispersion in biological systems: the contribution of proton exchange and implications for the noninvasive detection of cartilage degradation. Proc Natl Acad Sci U S A. 2001;98(22):12479–12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Duvvuri U, Reddy R, Patel SD, Kaufman JH, Kneeland JB, Leigh JS. T1ρ-relaxation in articular cartilage: effects of enzymatic degradation. Magn Reson Med. 1997;38(6):863–867. [DOI] [PubMed] [Google Scholar]
- 8. Ericksen HM, Gribble PA, Pfile KR, Pietrosimone BG. Different modes of feedback and peak vertical ground reaction force during jump landing: a systematic review. J Athl Train. 2013;48(5):685–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ericksen HM, Lefevre C, Luc-Harkey BA, Thomas AC, Gribble PA, Pietrosimone B. Females decrease vertical ground reaction forces following 4-week jump-landing feedback intervention without negative affect on vertical jump performance. J Sport Rehabil. 2019;28(8):866–870. [DOI] [PubMed] [Google Scholar]
- 10. Ericksen HM, Thomas AC, Gribble PA, Armstrong C, Rice M, Pietrosimone B. Jump-landing biomechanics following a 4-week real-time feedback intervention and retention. Clin Biomech (Bristol, Avon). 2016;32:85–91. [DOI] [PubMed] [Google Scholar]
- 11. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. [DOI] [PubMed] [Google Scholar]
- 12. Fedorov A, Beichel R, Kalpathy-Cramer J, et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging. 2012;30(9):1323–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gianotti SM, Marshall SW, Hume PA, Bunt L. Incidence of anterior cruciate ligament injury and other knee ligament injuries: a national population-based study. J Sci Med Sport. 2009;12(6):622–627. [DOI] [PubMed] [Google Scholar]
- 14. Hall M, Stevermer CA, Gillette JC. Gait analysis post anterior cruciate ligament reconstruction: knee osteoarthritis perspective. Gait Posture. 2012;36(1):56–60. [DOI] [PubMed] [Google Scholar]
- 15. Hewett TE, Myer GD, Ford KR, et al. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. Am J Sports Med. 2005;33(4):492–501. [DOI] [PubMed] [Google Scholar]
- 16. Johnson DL, Urban WP, Jr, Caborn DN, Vanarthos WJ, Carlson CS. Articular cartilage changes seen with magnetic resonance imaging-detected bone bruises associated with acute anterior cruciate ligament rupture. Am J Sports Med. 1998;26(3):409–414. [DOI] [PubMed] [Google Scholar]
- 17. Kadaba MP, Ramakrishnan HK, Wootten ME. Measurement of lower extremity kinematics during level walking. J Orthop Res. 1990;8(3):383–392. [DOI] [PubMed] [Google Scholar]
- 18. Kidder SM, Abuzzahab FS, Jr, Harris GF, Johnson JE. A system for the analysis of foot and ankle kinematics during gait. IEEE Trans Rehabil Eng. 1996;4(1):25–32. [DOI] [PubMed] [Google Scholar]
- 19. Krosshaug T, Slauterbeck JR, Engebretsen L, Bahr R. Biomechanical analysis of anterior cruciate ligament injury mechanisms: three-dimensional motion reconstruction from video sequences. Scand J Med Sci Sports. 2007;17(5):508–519. [DOI] [PubMed] [Google Scholar]
- 20. Kumar D, Kothari A, Souza RB, Wu S, Benjamin Ma C, Li X. Frontal plane knee mechanics and medial cartilage MR relaxation times in individuals with ACL reconstruction: a pilot study. Knee. 2014;21(5):881–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lepley AS, Gribble PA, Thomas AC, Tevald MA, Sohn DH, Pietrosimone BG. Quadriceps neural alterations in anterior cruciate ligament reconstructed patients: a 6-month longitudinal investigation. Scand J Med Sci Sports. 2015;25(6):828–839. [DOI] [PubMed] [Google Scholar]
- 22. Lepley LK. Deficits in quadriceps strength and patient-oriented outcomes at return to activity after ACL reconstruction: a review of the current literature. Sports Health. 2015;7(3):231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lepley LK, Palmieri-Smith RM. Quadriceps strength, muscle activation failure, and patient-reported function at the time of return to activity in patients following anterior cruciate ligament reconstruction: a cross-sectional study. J Orthop Sports Phys Ther. 2015;45(12):1017–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li X, Kuo D, Theologis A, et al. Cartilage in anterior cruciate ligament-reconstructed knees: MR imaging T1ρ and T2—initial experience with 1-year follow-up. Radiology. 2011;258(2):505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Luc B, Gribble PA, Pietrosimone BG. Osteoarthritis prevalence following anterior cruciate ligament reconstruction: a systematic review and numbers-needed-to-treat analysis. J Athl Train. 2014;49(6):806–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moisio KC, Sumner DR, Shott S, Hurwitz DE. Normalization of joint moments during gait: a comparison of two techniques. J Biomech. 2003;36(4):599–603. [DOI] [PubMed] [Google Scholar]
- 27. Nawasreh Z, Logerstedt D, Cummer K, Axe MJ, Risberg MA, Snyder-Mackler L. Do patients failing return-to-activity criteria at 6 months after anterior cruciate ligament reconstruction continue demonstrating deficits at 2 years? Am J Sports Med. 2017;45(5):1037–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Padua DA, Marshall SW, Boling MC, Thigpen CA, Garrett WE, Jr, Beutler AI. The Landing Error Scoring System (LESS) is a valid and reliable clinical assessment tool of jump-landing biomechanics: the JUMP-ACL study. Am J Sports Med. 2009;37(10):1996–2002. [DOI] [PubMed] [Google Scholar]
- 29. Palmieri-Smith RM, Kreinbrink J, Ashton-Miller JA, Wojtys EM. Quadriceps inhibition induced by an experimental knee joint effusion affects knee joint mechanics during a single-legged drop landing. Am J Sports Med. 2007;35(8):1269–1275. [DOI] [PubMed] [Google Scholar]
- 30. Palmieri-Smith RM, Thomas AC. A neuromuscular mechanism of posttraumatic osteoarthritis associated with ACL injury. Exerc Sport Sci Rev. 2009;37(3):147–153. [DOI] [PubMed] [Google Scholar]
- 31. Paterno MV, Ford KR, Myer GD, Heyl R, Hewett TE. Limb asymmetries in landing and jumping 2 years following anterior cruciate ligament reconstruction. Clin J Sport Med. 2007;17(4):258–262. [DOI] [PubMed] [Google Scholar]
- 32. Paterno MV, Schmitt LC, Ford KR, et al. Biomechanical measures during landing and postural stability predict second anterior cruciate ligament injury after anterior cruciate ligament reconstruction and return to sport. Am J Sports Med. 2010;38(10):1968–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pedoia V, Su F, Amano K, et al. Analysis of the articular cartilage T1ρ and T2 relaxation times changes after ACL reconstruction in injured and contralateral knees and relationships with bone shape. J Orthop Res. 2017;35(3):707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pfeiffer S, Harkey MS, Stanley LE, et al. Associations between slower walking speed and T1ρ magnetic resonance imaging of femoral cartilage following anterior cruciate ligament reconstruction. Arthritis Care Res (Hoboken). 2018;70(8):1132–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pfeiffer SJ, Blackburn JT, Luc-Harkey B, et al. Peak knee biomechanics and limb symmetry following unilateral anterior cruciate ligament reconstruction: associations of walking gait and jump-landing outcomes. Clin Biomech (Bristol, Avon). 2018;53:79–85. [DOI] [PubMed] [Google Scholar]
- 36. Pfeiffer SJ, Spang J, Nissman D, et al. Gait mechanics and T1ρ MRI of tibiofemoral cartilage 6 months after ACL reconstruction. Med Sci Sports Exerc. 2019;51(4):630–639. [DOI] [PubMed] [Google Scholar]
- 37. Pietrosimone B, Loeser RF, Blackburn JT, et al. Biochemical markers of cartilage metabolism are associated with walking biomechanics 6-months following anterior cruciate ligament reconstruction. J Orthop Res. 2017;35(10):2288–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pietrosimone B, Nissman D, Padua DA, et al. Associations between cartilage proteoglycan density and patient outcomes 12 months following anterior cruciate ligament reconstruction. Knee. 2018;25(1):118–129. [DOI] [PubMed] [Google Scholar]
- 39. Pietrosimone B, Pfeiffer SJ, Harkey MS, et al. Quadriceps weakness associates with greater T1ρ relaxation time in the medial femoral articular cartilage 6 months following anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2019;27(8):2632–2642. [DOI] [PubMed] [Google Scholar]
- 40. Regatte RR, Akella SVS, Borthakur A, Kneeland JB, Reddy R. Proteoglycan depletion-induced changes in transverse relaxation maps of cartilage: comparison of T2 and T1ρ. Acad Radiol. 2002;9(12):1388–1394. [DOI] [PubMed] [Google Scholar]
- 41. Salavati M, Akhbari B, Mohammadi F, Mazaheri M, Khorrami M. Knee injury and Osteoarthritis Outcome Score (KOOS): reliability and validity in competitive athletes after anterior cruciate ligament reconstruction. Osteoarthritis Cartilage. 2011;19(4):406–410. [DOI] [PubMed] [Google Scholar]
- 42. Shimizu T, Samaan MA, Tanaka MS, et al. Abnormal biomechanics at 6 months are associated with cartilage degeneration at 3 years after anterior cruciate ligament reconstruction. Arthroscopy. 2019;35(2):511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sigward SM, Lin P, Pratt K. Knee loading asymmetries during gait and running in early rehabilitation following anterior cruciate ligament reconstruction: a longitudinal study. Clin Biomech (Bristol, Avon). 2016;32:249–254. [DOI] [PubMed] [Google Scholar]
- 44. Souza RB, Fang C, Luke A, Wu S, Li X, Majumdar S. Relationship between knee kinetics during jumping tasks and knee articular cartilage MRI T1ρ and T2 relaxation times. Clin Biomech (Bristol, Avon). 2012;27(4):403–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Souza RB, Feeley BT, Zarins ZA, Link TM, Li X, Majumdar S. T1ρ MRI relaxation in knee OA subjects with varying sizes of cartilage lesions. Knee. 2013;20(2):113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Teng HL, Wu D, Su F, et al. Gait characteristics associated with a greater increase in medial knee cartilage T1ρ and T2 relaxation times in patients undergoing anterior cruciate ligament reconstruction. Am J Sports Med. 2017;45(14):3262–3271. [DOI] [PubMed] [Google Scholar]
- 47. Theologis AA, Haughom B, Liang F, et al. Comparison of T1ρ relaxation times between ACL-reconstructed knees and contralateral uninjured knees. Knee Surg Sports Traumatol Arthrosc. 2014;22(2):298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Theologis AA, Kuo D, Cheng J, et al. Evaluation of bone bruises and associated cartilage in anterior cruciate ligament injured and reconstructed knees using quantitative T1ρ magnetic resonance imaging: 1-year cohort study. Arthroscopy. 2011;27(1):65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang A, Pedoia V, Su F, et al. MR T1ρ and T2 of meniscus after acute anterior cruciate ligament injuries. Osteoarthritis Cartilage. 2016;24(4):631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ward SH, Blackburn JT, Padua DA, et al. Quadriceps neuromuscular function and jump-landing sagittal-plane knee biomechanics after anterior cruciate ligament reconstruction. J Athl Train. 2018;53(2):135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Webster KE, McClelland JA, Palazzolo SE, Santamaria LJ, Feller JA. Gender differences in the knee adduction moment after anterior cruciate ligament reconstruction surgery. Br J Sports Med. 2012;46(5):355–359. [DOI] [PubMed] [Google Scholar]
- 52. Witschey WR, Borthakur A, Elliott MA, et al. T1ρ-prepared balanced gradient echo for rapid 3D T1ρ MRI. J Magn Reson Imaging. 2008;28(3):744–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yasuda K, Ohkoshi Y, Tanabe Y, Kaneda K. Muscle weakness after anterior cruciate ligament reconstruction using patellar and quadriceps tendons. Bull Hosp Jt Dis Orthop Inst. 1991;51(2):175–185. [PubMed] [Google Scholar]
- 54. Yushkevich PA, Piven J, Hazlett HC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31(3):1116–1128. [DOI] [PubMed] [Google Scholar]