Abstract
The falsification of data related to c-kit+ cardiac progenitor cells (CPCs) by a Harvard laboratory has been a veritable tragedy. Does this fraud mean that CPCs are not beneficial in models of ischemic cardiomyopathy? At least 50 studies from 26 laboratories independent of the Harvard group have reported beneficial effects of CPCs in mice, rats, pigs, and cats. The mechanism of action remains unclear. Our group has shown that CPCs do not engraft in the diseased heart, do not differentiate into new cardiac myocytes, do not regenerate dead myocardium, and thus work via paracrine mechanisms. A casualty of the misconduct at Harvard has been the SCIPIO trial, a collaboration between the Harvard group and the group in Louisville. The retraction of the SCIPIO paper was caused exclusively by issues with data generated at Harvard, not those generated in Louisville. In the retraction notice, the Lancet editors stated: “Although we do not have any reservations about the clinical work in Louisville that used the preparations from Anversa’s laboratory in good faith, the lack of reliability regarding the laboratory work at Harvard means that we are now retracting this paper”. We must be careful not to dismiss all work on CPCs because of one laboratory’s misconduct. An unbiased review of the literature supports the therapeutic potential of CPCs for heart failure at the preclinical level.
Keywords: cardiac progenitor cell, c-kit, stem cells, cell therapy, chronic ischemic cardiomyopathy, myocardial infarction
Résumé:
La falsification de données liées aux cellules progénitrices cardiaques c-kit+ (CPC) par un laboratoire de Harvard a constitué une véritable tragédie. Cette fraude signifie-t-elle pour autant que les CPC ne contribuent pas aux avantages des modèles de cardiomyopathie ischémique? Au moins 50 études issues de 26 laboratoires indépendants du groupe de Harvard ont rapporté des bienfaits obtenus avec les CPC chez la souris, le rat, le porc et le chat. Le mode d’action demeure à clarifier. Notre groupe a montré que les CPC ne se greffent pas au cœur lésé, ne se différencient pas en nouveaux myocytes cardiaques, n’entraînent pas la régénérescence du myocarde mort, et fonctionnent donc par l’intermédiaire de modes d’action paracrines. L’essai SCIPIO, une collaboration entre le groupe de Harvard et le groupe de Louisville, a été victime des fautes à Harvard. La rétractation de l’article SCIPIO a été causée exclusivement par des problèmes issus des données produites à Harvard, pas de celles produites à Louisville. Dans l’acte de rétractation, les éditeurs du Lancet ont déclaré que bien qu’ils n’aient aucune réserve quant au travail clinique effectué à Louisville où des préparations provenant du laboratoire d’Anversa ont été utilisées de bonne foi, le manque de fiabilité du travail de laboratoire à Harvard implique qu’ils rétractent cet article. Nous devons prendre bien garde de ne pas disqualifier tout le travail sur les CPC en raison de fautes commises dans un seul laboratoire. Une synthèse non biaisée de la littérature appuie le potentiel thérapeutique des CPC pour l’insuffisance cardiaque aux stades précliniques. [Traduit par la Rédaction]
Keywords: cellules progénitrices cardiaques, c-kit, cellules souches, thérapie cellulaire, cardiomyopathie ischémique chronique, infarctus du myocarde
Introduction
The most tragic development in the field of cell-based therapies for heart disease has been the occurrence of scientific misconduct in one high-profile laboratory at Harvard Medical School (Oransky 2018). This laboratory did all the early work on c-kit+ cardiac progenitor cells (hereby referred to simply as CPCs) and published the initial papers describing them and characterizing their properties. Specifically, Beltrami et al. (2003) isolated a population of cells from the adult rat heart that expressed c-kit, a tyrosine kinase receptor known to be associated with cancer progression and maintenance of stem cells. They reported that these cells were self-renewing, exhibiting the ability to differentiate into smooth muscle cells, endothelial cells, and cardiomyocytes, both in vitro and in vivo, and that injection of these cells in infarcted rodent hearts resulted in regeneration of large swaths of dead myocardium (Beltrami et al. 2003). In subsequent years, numerous papers from the same laboratory appeared that supported the claim that these c-kit+ cells possessed the properties of genuine stem cells and were able to regenerate dead myocardium (Anversa et al. 2013; Leri et al. 2005). However, many of these papers were subsequently retracted when fraudulent data manipulation was discovered (Oransky 2018).
The effects of this tragedy have been devastating. The fraud perpetrated in that one laboratory has cast a negative light on the entire community of basic and clinical investigators working on cell-based therapies for heart disease, not only with CPCs but with other adult cell types, thereby inflicting immense damage to the entire field. Suspicion has been raised about other laboratories, other researchers, other cell types, and essentially all basic and clinical reports of therapeutic efficacy of cell-based therapies; this, in turn, has had a chilling effect on the funding of research projects and on the publication of manuscripts related to cell therapy. The scientific misconduct in one basic laboratory working on CPCs has even led some editorialists to advocate a moratorium on all clinical trials of cell therapy (regardless of the type of cell used), a position that is utterly irrational, as pointed out elsewhere (Bolli and Kahlon 2020).
The falsification of data related to CPCs by a prominent research group is a tragedy, but it should not lead to the conclusion that CPCs have no therapeutic potential. The purpose of this review is to examine this emotional issue objectively and to summarize current factual information pertaining to the properties of CPCs as a potential therapy for heart failure.
CPCs are effective in preclinical models of ischemic cardiomyopathy
The key question that we should ask ourselves is this: Does the fraud perpetrated in one high-profile laboratory mean that CPCs are not effective in improving cardiac function after myocardial infarction (MI) in animal models of ischemic cardiomyopathy? Based on the available data, the answer is “no”. In fact, a review of the literature demonstrates that there is extensive evidence from many other independent groups that administration of CPCs in preclinical models of ischemic cardiomyopathy (acute or old MI) is beneficial. As detailed in Table 1, at the time of this writing, at least 50 studies from 26 independent laboratories around the world have reported beneficial effects of CPCs in mice, rats, pigs, and cats (the 50 references are given in Table 1) (Alshammary et al. 2013; Avolio et al. 2015; Bao et al. 2017; Bhutani et al. 2018; Bolli et al. 2013; Cai et al. 2015; Carr et al. 2011; Dergilev et al. 2018; Duran et al. 2013; Ellison et al. 2013; Fischer et al. 2009; Hong et al. 2014; Kamata et al. 2014; Karantalis et al. 2015; Kazakov et al. 2015; Kulandavelu et al. 2016; Li et al. 2017; Li et al. 2011; Li et al. 2012; Li et al. 2018; Ma et al. 2018a, 2018b; Matsuda et al. 2014; Maxwell et al. 2019; Mohsin et al. 2012; Natsumeda et al. 2017; Oskouei et al. 2012; Puddighinu et al. 2018; Sharma et al. 2015; Simpson et al. 2012; Song et al. 2016; Taghavi et al. 2015; J.M. Tang et al. 2015; X.L. Tang et al. 2010, 2015, 2016, 2018; Tang et al. 2009; Tokita et al. 2016; Vahdat et al. 2015; Vicinanza et al. 2017; Wang et al. 2012; Wehman et al. 2017; Williams et al. 2013; Wysoczynski et al. 2016; Zakharova et al. 2014, 2015; Zhang et al. 2014; Zhang et al. 2016; Zhao et al. 2015). All of these investigations were performed in laboratories independent of Dr. Anversa’s group and used CPCs manufactured in their own facilities. These reports include at least 19 studies in mice, 23 in rats, seven in pigs, and one in cats; 12 of these studies were done in animal models of old MI (chronic ischemic heart failure), and seven were performed in large animal (swine) models (Table 1). Except for four studies in models of isoproterenol-induced cardiomyopathy (Taghavi et al. 2015), right ventricular injury by pulmonary artery banding (Maxwell et al. 2019; Wehman et al. 2017), and transverse aortic constriction (Kazakov et al. 2015), all of these studies consistently show that administration of CPCs improved cardiac function after an acute, subacute, or old MI, often in association with a reduction in scar size, which in aggregate provides robust preclinical evidence of efficacy (Table 1). One study (Ellison et al. 2013) used both a model of acute MI and isoproterenol-induced cardiomyopathy.
Table 1.
Preclinical studies of c-kit+ CPCs performed outside of Dr. Anversa’s laboratory.
| No. | Author and year | Species | Model | Route of injection | Outcome | Notes |
|---|---|---|---|---|---|---|
| 1 | Fischer et al. 2009 | Mouse | Acute MI | Intramyocardial | ↑function, ↓infarct size | |
| 2 | Tang et al. 2009 | Mouse | Acute MI | Intravenous | ↑function, ↓infarct size | |
| 3 | Tang et al. 2010 | Rat | Chronic HF (old MI) | Intracoronary | ↑function and structure | |
| 4 | Li et al. 2011 | Mouse | Subacute MI | Intracoronary | ↑function and viable tissue | |
| 5 | Carr et al. 2011 | Rat | Acute MI | Intramyocardial | ↑function | |
| 6 | Oskouei et al. 2012 | Mouse | Acute MI | Intramyocardial | ↑function, ↓scar | |
| 7 | Mohsin et al. 2012 | Mouse | Acute MI | Intramyocardial | ↑function, ↓infarct size | |
| 8 | Wang et al. 2012 | Mouse | Acute MI | Intramyocardial | ↑structure and function | |
| 9 | Li et al. 2012 | Mouse | Acute MI | Intramyocardial | ↑ function | |
| 10 | Simpson et al. 2012 | Rat | Acute MI | Intramyocardial | ↑function, ↓infarct size | |
| 11 | Bolli et al. 2013 | Swine | Chronic HF (old MI) | Intramyocardial | ↑function, ↓scar | |
| 12 | Duran et al. 2013 | Mouse | Acute MI | Intramyocardial | ↑function,* ↓infarct size | |
| 13 | Alshammary et al. 2013 | Rat | Acute MI | Cell sheet transplantation | ↑function, ↓fibrosis | |
| 14 | Ellison et al. 2013 | Rat | Acute MI and ISO-induced chronic HF | Intravenous | ↑function | |
| 15 | Williams et al. 2013 | Swine | Chronic HF (old MI) | Intramyocardial | ↑function, ↓scar | |
| 16 | Zhang et al. 2014 | Mouse | Acute MI | Intramyocardial | ↑function, ↓infarct size | |
| 17 | Hong et al. 2014 | Mouse | Subacute MI | Intracoronary | ↑function | |
| 18 | Matsuda et al. 2014 | Rat | Acute MI | Intramyocardial | ↑function, ↓fibrosis | |
| 19 | Zakharova et al. 2014 | Rat | Chronic HF (old MI) | Retrograde (coronary vein) | ↑function, ↓scar | |
| 20 | Kamata et al. 2014 | Swine | Chronic HF (old MI) | Cell sheet transplantation | ↑function, ↓fibrosis | |
| 21 | Zhao et al. 2015 | Mouse | Acute MI | Intramyocardial | ↑function, ↓infarct size | |
| 22 | Cai et al. 2015 | Mouse | Acute MI | Intramyocardial | ↑function, ↓scar | |
| 23 | Avolio et al. 2015 | Mouse | Acute MI | Intramyocardial | ↑function, ↓fibrosis | |
| 24 | J.M. Tang et al. 2015 | Rat | Acute MI | Intramyocardial | ↑function, ↓infarct size | |
| 25 | Vahdat et al. 2015 | Rat | Acute MI | Intramyocardial | ↓infarct size and fibrosis | |
| 26 | Sharma et al. 2015 | Rat | Acute MI | Intramyocardial | ↑function, ↓infarct size | |
| 27 | Zakharova et al. 2015 | Rat | Chronic HF (old MI) | Retrograde (coronary vein) | ↑function, ↓scar | |
| 28 | X.L. Tang et al. 2015 | Rat | Acute MI | Intracoronary | ↑function and structure | |
| 29 | Karantalis et al. 2015 | Swine | Chronic HF (old MI) | Intramyocardial | ↑function, ↓scar | |
| 30 | Taghavi et al. 2015 | Feline | ISO-induced chronic HF | Intracoronary | ↑function, ↓fibrosis | Not a model of ischemic heart disease |
| 31 | Kazakov et al. 2015 | Mouse | TAC model | Intravenous | ↓LV fibrosis | Not model of ischemic heart disease |
| 32 | Wysoczynski et al. 2016 | Mouse | Subacute MI | Echo-guided intraventricular | ↑function and viable tissue | |
| 33 | Song et al. 2016 | Rat | Acute MI | Unclear | ↑function, ↓infarct size | |
| 34 | Tang et al. 2016 | Rat | Acute MI | Intracoronary | ↑function and structure | |
| 35 | Tokita et al. 2016 | Rat | Chronic HF (old MI) | Echo-guided intraventricular | ↑function and structure | |
| 36 | Zhang et al. 2016 | Rat | Subacute MI | Intramyocardial | ↑function, ↓fibrosis | |
| 37 | Kulandavelu et al. 2016 | Swine | Chronic HF (old MI) | Intramyocardial | ↑function, ↓scar | |
| 38 | Li et al. 2017 | Rat | Acute MI | Intramyocardial | ↑function | |
| 39 | Vicinanza et al. 2017 | Rat | Acute MI | Intramyocardial | ↑function | |
| 40 | Bao et al. 2017 | Rat | Chronic HF (old MI) | Intramyocardial | ↑function | |
| 41 | Wehman et al. 2017 | Swine | Acute RV injury by PA binding | RV intramyocardial | ↑RV function, ↓fibrosis | Not a model of ischemic heart disease |
| 42 | Natsumeda et al. 2017 | Swine | Chronic HF (old MI) | Intramyocardial | ↑function, ↓scar | |
| 43 | Ma et al. 2018b | Mouse | Acute MI | Intramyocardial | ↑function | |
| 44 | Puddighinu et al. 2018 | Mouse | Acute MI | Intramyocardial | ↑function | |
| 45 | Ma et al. 2018a | Mouse | Acute MI | Intramyocardial | ↑function | |
| 46 | Li et al. 2018 | Mouse | Acute MI | Intramyocardial | ↑function, ↓fibrosis | |
| 47 | Dergilev et al. 2018 | Rat | Acute MI | Cell sheet transplantation | ↓infarct size and remodeling | |
| 48 | Bhutani et al. 2018 | Rat | Acute MI | Intramyocardial | ↑function, ↓remodeling | |
| 49 | Tang et al. 2018 | Rat | Chronic HF (old MI) | Echo-guided intraventricular | ↑function and structure | |
| 50 | Maxwell et al. 2019 | Rat | RV injury by PA banding model | Echo-guided RV intramyocardial | ↑RV functional parameters | Not a model of ischemic heart disease |
Note: MI, myocardial infarction; HF, heart failure; ISO, isoproterenol; PA, pulmonary artery; RV, right ventricle; TAC, transverse aortic constriction.
CPCs were found to be less effective than cortical bone stem cells, but nevertheless, CPCs produced beneficial effects on LV function.
We were unable to identify any “negative” preclinical studies involving CPCs, i.e., studies that reported no beneficial effects of these cells in animal models of ischemic cardiomyopathy. Among the 50 papers listed in Table 1, only one (Duran et al. 2013) could be construed as being partly “negative”. This was a study in which CPCs were compared with cortical bone stem cells (CBSCs) in a murine model of acute MI. Overall, CBSCs were found to be more effective than CPCs, but CPCs nevertheless produced positive signals. Specifically, the group treated with CPCs exhibited significantly reduced diastolic and systolic left ventricular (LV) volumes, attenuated thinning of the anterior LV wall, reduced scar size, and improvements in LV strain and LV strain rate in both the radial and longitudinal axes (Duran et al. 2013).
In summary, after excluding all papers from the laboratory of Dr. Anversa (whether retracted or not), a review of the available literature shows that there is little or no controversy as to whether CPCs are beneficial at the preclinical level in the setting of a recent or old MI. On the contrary, the results obtained by many independent groups in many different animal models have been remarkably consistent, probably more consistent than the results obtained by different investigators with other cell types or, for that matter, in other areas of scientific inquiry outside of cell therapy. In scientific research, it is rare to find a topic in which 50 papers agree and none disagrees. That one, two, or three laboratories may conduct flawed studies is possible; that 26 independent laboratories around the world may all conduct fallacious studies and that there is not even one truly “negative” paper on CPCs seems very unlikely. Thus, despite the recent retractions of several papers emanating from one high-profile laboratory, the most reasonable and objective conclusion of an unbiased observed is that, at the preclinical level, the available evidence strongly supports the therapeutic potential of CPCs for heart failure. This vast body of evidence provided a robust rationale for the ongoing CONCERT-HF clinical trial (Bolli et al. 2018).
CPCs do not engraft and do not differentiate into cardiac myocytes
In addition to the scientific misconduct scandal described above, another factor that has negatively affected the attitude of investigators toward CPCs is the realization that these cells do not possess the properties originally ascribed to them (Anversa et al. 2013; Beltrami et al. 2003; Leri et al. 2005): the attributes of bona fide stem cells and the ability to regenerate myocardium. As detailed below, our research group has played a major role in this shift in the understanding of CPCs (Bolli and Ghafghazi 2017; Bolli et al. 2013; Hong et al. 2013, 2014; Keith and Bolli 2015; Sanganalmath and Bolli 2013; X.L. Tang et al. 2010, 2015, 2016, 2018; Tokita et al. 2016; Wysoczynski et al. 2018). CPCs were initially claimed to be self-renewing, clonogenic, and multipotent, with the ability to differentiate into cardiomyocytes, smooth muscle cells, and endothelial cells, both in vitro and in vivo (Beltrami et al. 2003). The claim was also made that transplantation of CPCs in infarcted hearts results in remuscularization, i.e., regeneration of viable myocardium (Beltrami et al. 2003; Leri et al. 2005). However, subsequent work performed in our laboratory has shown that this is not the case.
The original claims that transplanted CPCs regenerate viable myocardium (Beltrami et al. 2003; Leri et al. 2005) motivated us to carefully investigate this phenomenon using rigorous protocols and quantitative measurements of cell engraftment and differentiation. Over the past 10 years, our group has published a series of papers examining the effects of CPCs in mouse, rat, and pig models of ischemic cardiomyopathy, both in the setting an acute MI and an old MI (Bolli et al. 2013; Hong et al. 2013, 2014; X.L. Tang et al. 2010, 2015, 2016, 2018; Tokita et al. 2016). In all of these papers, we reported that while CPCs do improve LV function, they do not engraft in the heart to a significant extent.
The first paper to challenge the concept that CPCs work by regenerating dead myocardium was published by Tang et al. (2010), seven years after the initial description of CPCs (Beltrami et al. 2003). In this study, syngeneic CPCs were infused intracoronarily in a rat model of chronic ischemic cardiomyopathy produced by an old (healed) MI. Specifically, a large MI was produced by a 2 h occlusion of the left anterior descending coronary artery; CPCs were administered 30 days later, when the healing process was completed and the infarct had been replaced by a stable scar. At 35 days after CPC infusion, LV function was significantly improved compared with controls; however, very few CPCs (or CPC-derived cells) remained in the heart that expressed markers of cardiogenic commitment such as sarcomeric proteins; furthermore, these cells were small and did not exhibit the typical morphology and sarcomeric structure of mature cardiac myocytes, raising the question as to whether they represented genuine differentiating myocytes (Fig. 1) (Tang et al. 2010). As all transplanted CPCs were labeled with EGFP, they or their progeny were identifiable by EGFP expression. We found EGFP+ cells only in 7 of 17 treated hearts, and in those 7 hearts, they accounted for ≈2.6% of the region at risk and ≈1.1% of the noninfarcted region (Tang et al. 2010). Clearly, the number of engrafted CPCs was insufficient to account for the improvement in LV function, implying that CPCs produced their beneficial effects by secreting mediators (such as cytokines, growth factors, microRNAs, bioactive lipids, etc.) that exerted favorable paracrine actions on the host heart.
Fig. 1.
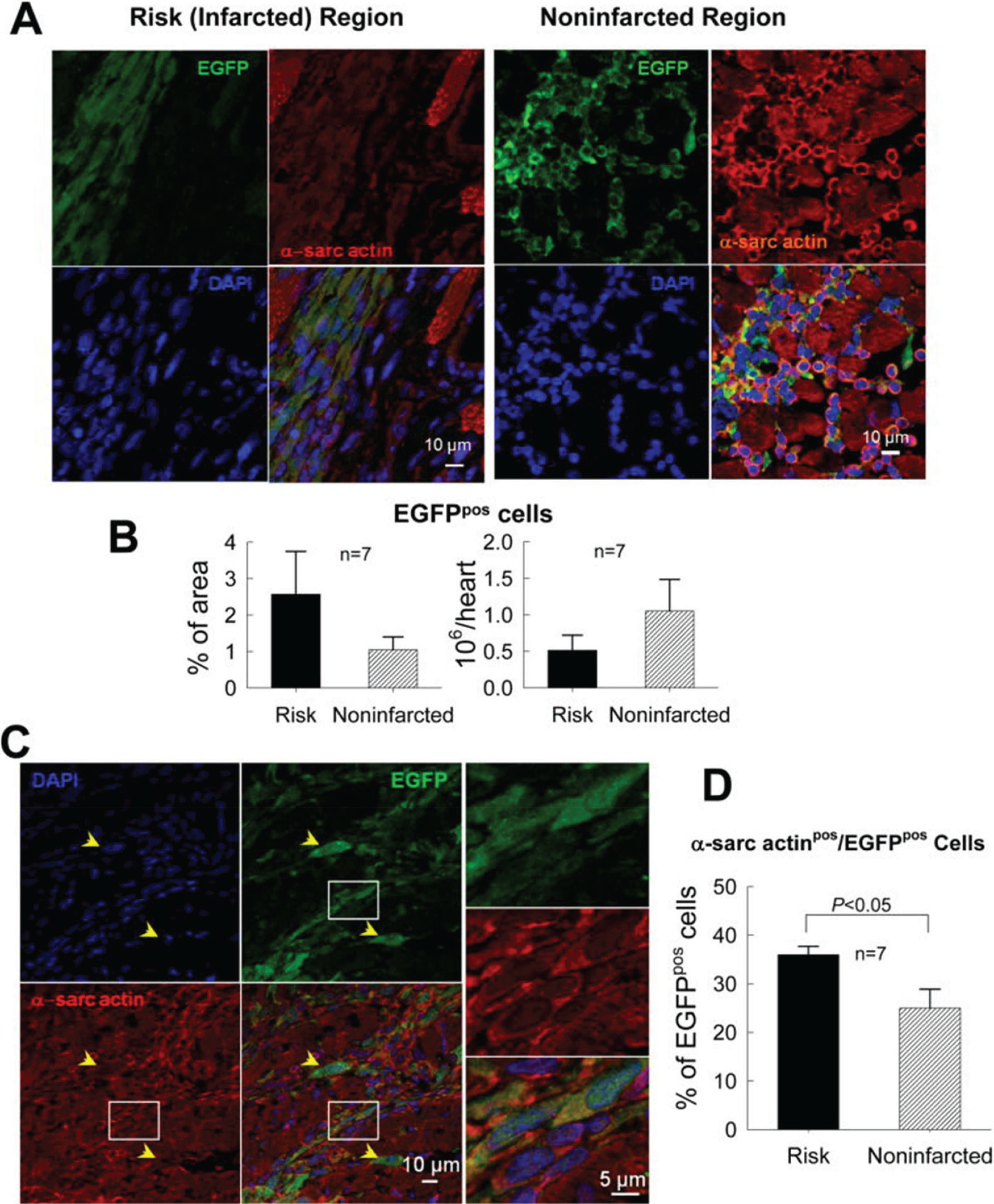
Myocardial content and differentiation of transplanted CPCs in a rat model of chronic ischemic cardiomyopathy (old MI). C-kit+ CPCs were labeled with EGFP and injected 30 days after MI. (A) Representative confocal microscopic images from a CPC-treated rat showing presence of transplanted CPCs in the risk (infarcted) and noninfarcted regions, as evinced from immunoreactivity for EGFP (green). Some EGFPpos cells also express α-sarcomeric actin (α-sarc actin; red). (B) Quantitation of EGFPpos cells in the risk and noninfarcted region, expressed as percent EGFPpos myocardial area and as total calculated number of EGFPpos cells per heart. The number of EGFPpos cells per heart was estimated by multiplying the number of EGFPpos cells per unit area by the estimated number of EGFPpos cells present through the thickness of each slice in which EGFPpos cells were found (estimated 100 cells per 2 mm thickness of slice). (C) Representative confocal microscopic images showing colocalization of EGFP and α-sarcomeric actin in several cells in the border zone (the area in the white box is magnified in the three panels on the right). Yellow arrowheads indicate EGFPpos cells that do not express α-sarcomeric actin. (D) Quantitative analysis of α-sarcomeric actinpos/EGFPpos cells. The figure illustrates the fact that very few CPCs (or CPC-derived cells) remained in the heart that expressed markers of cardiogenic commitment such as sarcomeric proteins; furthermore, these cells were small and did not exhibit the typical morphology and sarcomeric structure of mature cardiac myocytes. Data are means ± SEM. Reproduced with permission from Tang et al. (2010).
To our knowledge, the paper by Tang et al. (2010) was the first report that although CPCs improve LV function in an infarcted heart, they fail to engraft and do not differentiate into new cardiomyocytes. This paper is important because it challenged a major paradigm: when these findings were published in 2010, they were contrary to the preponderance of reports and to the then-widespread notion that CPCs improve LV function by regenerating dead myocardium. Therefore, to verify that our conclusions were correct, we designed studies to specifically measure the survival of CPCs after transplantation. We developed a highly sensitive PCR-based method that can precisely quantify the number of male transplanted cells in female recipients (Hong et al. 2013). Using this method, in 2014, we reported in a murine model of acute MI that only a miniscule fraction (<3%) of transplanted CPCs could be found in the heart 35 days after transplantation (<1500 cells/heart), yet LV function was significantly improved at this time (Fig. 2) (Hong et al. 2014). To ensure that our findings were not model- or species-dependent, we examined this issue in other models and species, including large animals. We found, and reported, similar results in rat and pig models of chronic (old) MI, where we observed a clear and consistent disassociation between persistent improvement in LV function and rapid disappearance of CPCs from the heart (Bolli et al. 2013; X.L. Tang et al. 2015, 2016, 2018; Tokita et al. 2016).
Fig. 2.
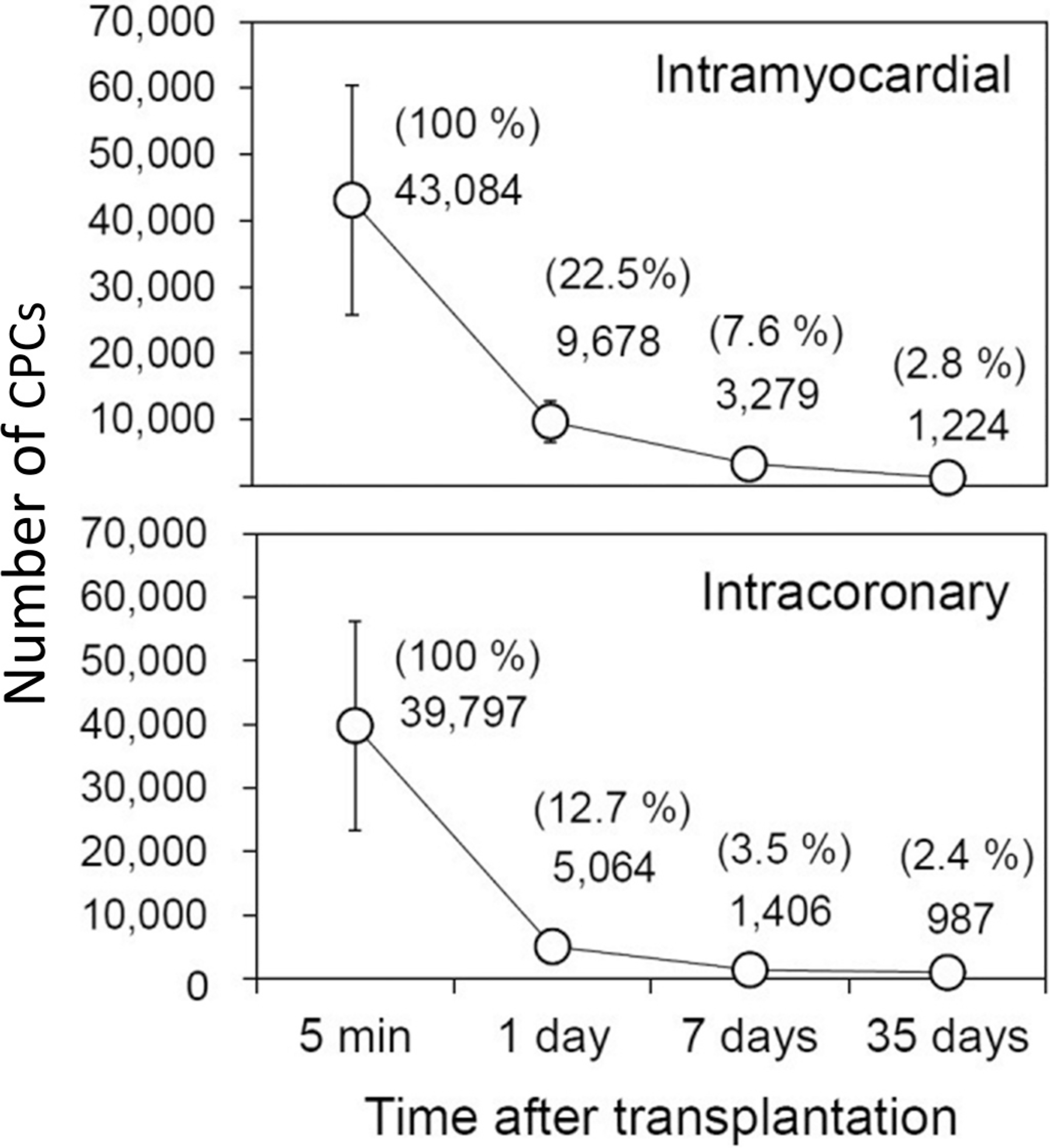
Comparison of CPC retention and engraftment after intramyocardial and intracoronary delivery in mice with acute MI. Female mice with acute MI were given 105 male c-kit+ CPCs by the intramyocardial (upper panel) or intracoronary (present study; lower panel) route. The CPC content in the heart was measured with a highly sensitive PCR-based method that can precisely quantify the number of male transplanted cells in female recipients by assessing a male gene. The absolute numbers of CPCs detected in the entire heart at indicated time points are shown. The number of cells found at each time point, expressed as a percentage of the cells found at 5 min after delivery, is shown in parentheses. Note that the number of CPCs retained in the heart drops precipitously after transplantation irrespective of the route used to inject them; only a miniscule fraction (<3%) of transplanted CPCs could be found in the heart 35 days after transplantation (<1500 cells per heart), yet LV function was improved at this time point. Data are means ± SEM. Reproduced from Hong et al. (2014).
In these studies (Bolli et al. 2013; Hong et al. 2014; X.L. Tang et al. 2010, 2015, 2018; Tokita et al. 2016), we followed animals for a relatively short period of time (~1 month after MI). To rule out the possibility that CPCs may differentiate into cardiomyocytes after a longer time span (several months) and to determine whether the salubrious functional effects of CPCs are sustained in time, we administered CPCs to rats with acute MI and examined the fate of CPCs as well as LV function 1 year later (Tang et al. 2016). LV function was still improved in treated animals at 1 year. Again, we found very few remaining CPCs (or their progeny) at that time; as was the case at 35 days after transplantation (Hong et al. 2014; X.L. Tang et al. 2010, 2015, 2018; Tokita et al. 2016), these cells did not exhibit the morphology and structure of mature cardiomyocytes (Tang et al. 2016) (Fig. 3). These data demonstrate that the functional improvement afforded by CPCs is long-lasting, but the cells fail to differentiate into mature cardiomyocytes even after 1 year.
Fig. 3.
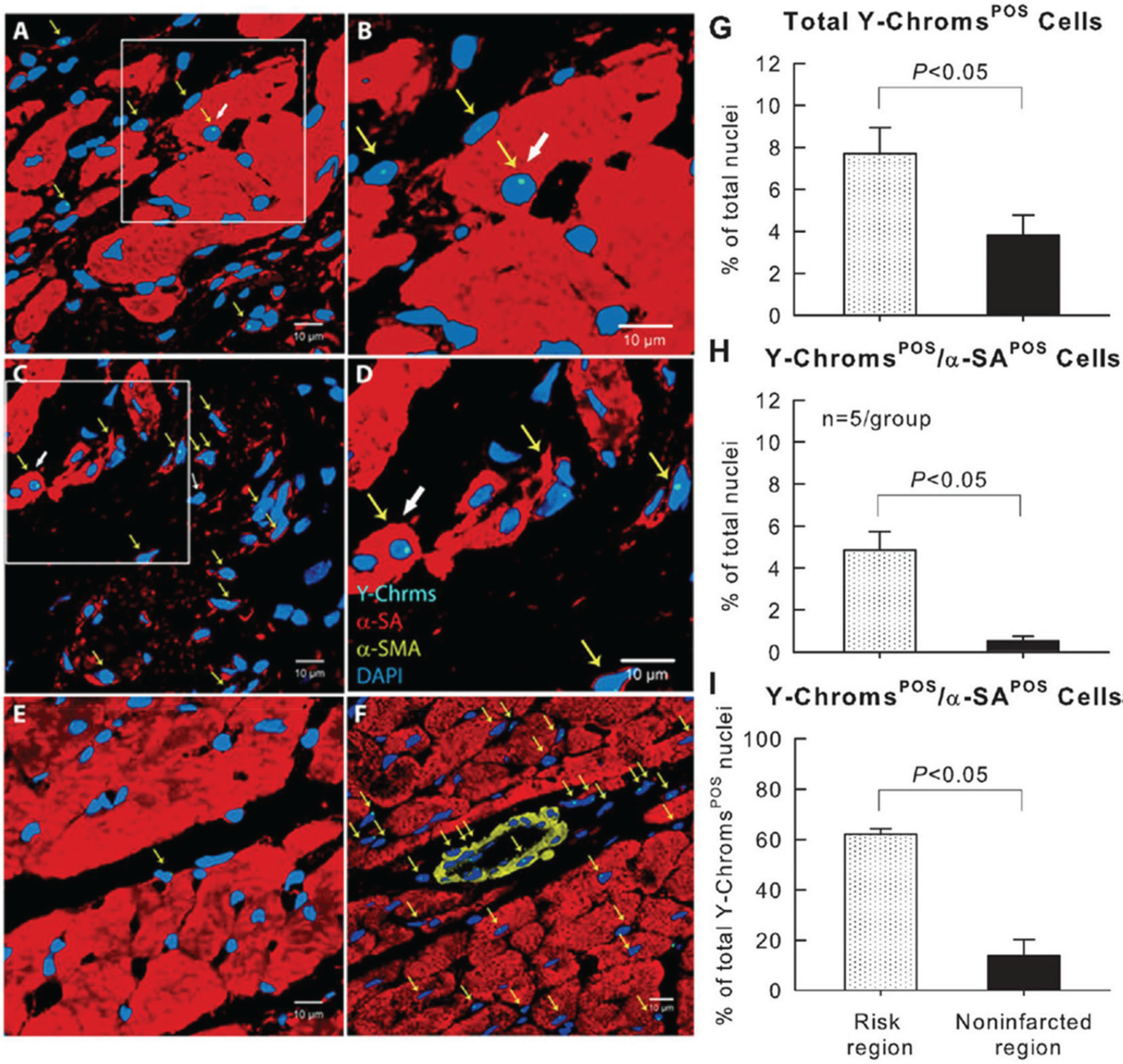
Detection of CPC retention and engraftment with fluorescence in situ hybridization (FISH) for detection of Y-chromosomes 1 year after CPC transplantation in rats. (A–E) Male c-kit+ CPCs were injected into female rats after acute MI and the hearts were harvested 1 year later. Positivity for Y-chromosomes in representative confocal microscopic images obtained from the border zone (A, B), scarred region (C, D), and noninfarcted region (E) identifies exogenous CPCs. Yellow arrows indicate all Y-chromosome positive cells, whereas white arrows indicate Y-chromosome cells with a myocyte-like appearance. (B) and (D) are higher magnification images of the boxes in (A) and (C), respectively. (F). Male heart tissue (positive control); Y-chromosome fluorescent signals are not present in all nuclei because some signals are out of the plane of focus used for the image. Y-chromosomes (Y-Chroms) are shown in cyanine blue, α-sarcomeric actin (α-SA) is shown in red, α-smooth muscle actin (α-SMC) is shown in yellow, and nuclei are stained with DAPI (blue). (G–I) Quantitative analysis of the number of Y-chromosome/α-SA double positive cells at 1 year after CPC transplantation. Note that very few CPCs (or their progeny) remained in the heart at 1 year after transplantation; as was the case at 35 days after transplantation (Hong et al. 2014; X.L. Tang et al. 2010, 2015, 2018; Tokita et al. 2016), these cells did not exhibit the morphology and structure of mature cardiomyocytes. Data are means ± SEM. The region at risk comprises both the border zones and the scarred region. Bars are 10 μm. Reproduced with permission from Tang et al. (2016).
Importantly, we also tested the hypothesis that exogenous CPCs may activate endogenous (resident) CPCs which, in turn, may form new cardiomyocytes that are EGFP-negative. In this scenario, CPCs would still promote myocardial regeneration, albeit indirectly. Using BrdU and IdU labeling to detect new cells, we demonstrated that after administration of CPCs, the number of newly formed cardiomyocytes (regardless of their source) was negligible (<1% of nuclei); that is, transplantation of CPCs did not promote significant formation of new cardiomyocytes from either exogenous or endogenous (resident) cells (X.L. Tang et al. 2010, 2015, 2016, 2018; Tokita et al. 2016), even after multiple CPC doses (Fig. 4) or after a larger cell dose (Fig. 5). We therefore proposed that CPCs improve LV function not by forming new cardiomyocytes but by releasing mediators that modulate the contractile properties of the host heart in a favorable manner (Bolli et al. 2013; Hong et al. 2013, 2014; X.L. Tang et al. 2010, 2015, 2016, 2018; Tokita et al. 2016).
Fig. 4.
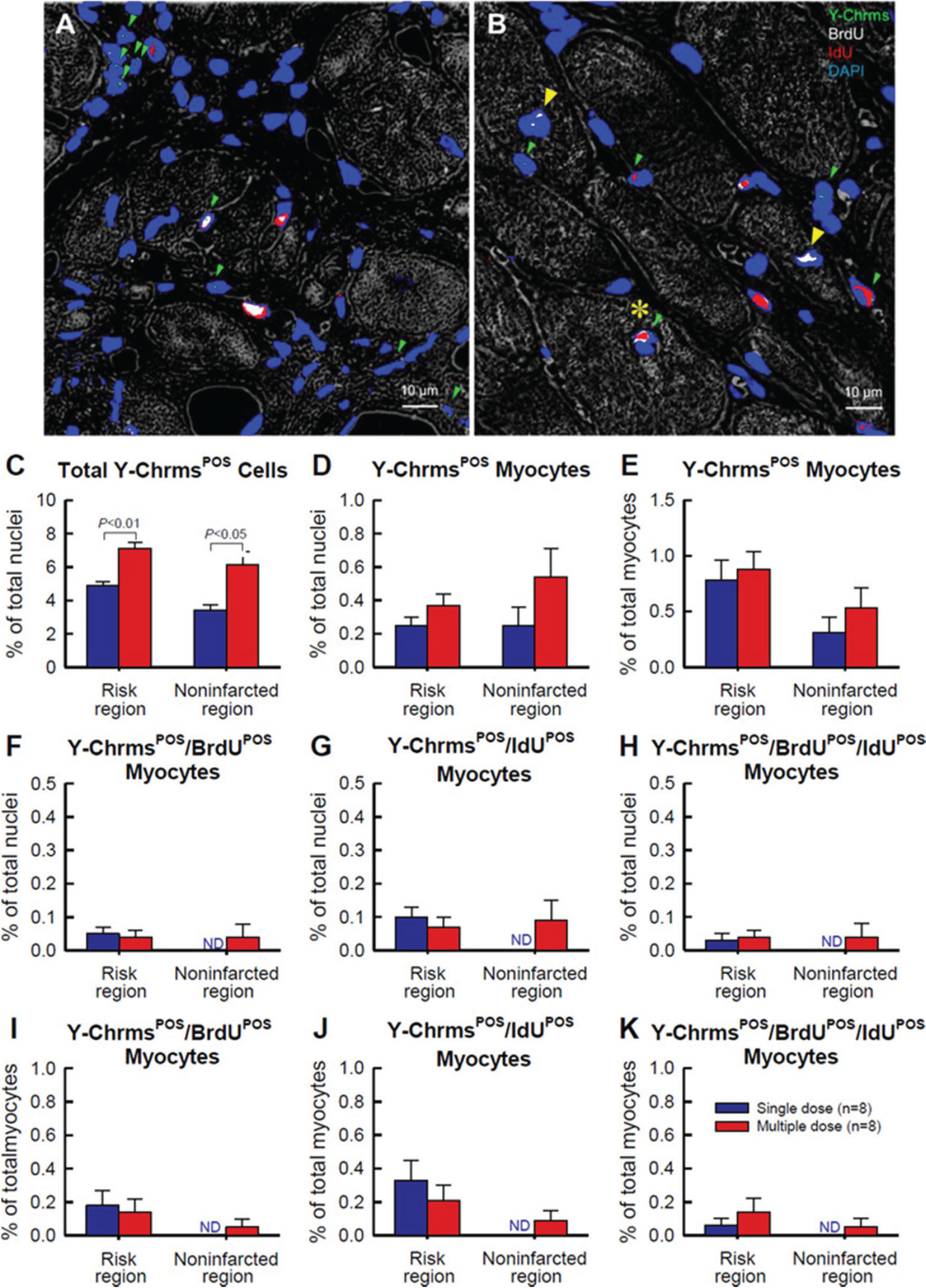
Detection of CPC retention and engraftment with fluorescence in situ hybridization (FISH) for detection of Y-chromosomes after multiple CPC administrations in rats with chronic ischemic cardiomyopathy. Male c-kit CPCs were injected into female rats with old MI 30 days after MI; three CPC injections were performed at 35-day intervals. Rats were given BrdU or IdU after CPC transplantation. (A–B) Representative confocal microscopic images acquired from the infarcted region (A) and border zone (B). Green arrowheads indicate Y-chromosome fluorescent signals (green/cyan) in nuclei. Note a cluster of five Y-chromosomePOS (Y-ChrmsPOS) nuclei in A (top left), suggesting Y-chromosomePOS cell division. Yellow arrowheads indicate BrdUPOS mature myocytes, whereas the yellow asterisk shows a Y-chromosomePOS/BrdUPOS/IdUPOS mature myocyte (B). BrdU is shown in white, IdU is shown in red, and nuclei are stained with DAPI in blue. Myocardial morphology was examined with the confocal transmitted light channel’s detector (ChD) in which the pseudocolor selected for the myocardial background in the ChD channel was gray white. (C–K) Quantitative analysis of the number of Y-chromosomePOS, BrdUPOS, and IdUPOS cells. The risk region comprises both the border zones and the infarcted region. Note that the number of newly formed (i.e., BrdUPOS and (or) IdUPOS) cardiomyocytes (regardless of their source, exogenous or endogenous) was negligible (<1% of nuclei); that is, transplantation of CPCs did not promote significant formation of new cardiomyocytes from either exogenous or endogenous (resident) cells, even after multiple CPC doses. Data are means ± SEM. Bar is 10 μm. Reproduced with permission from Tokita et al. (2016).
Fig. 5.
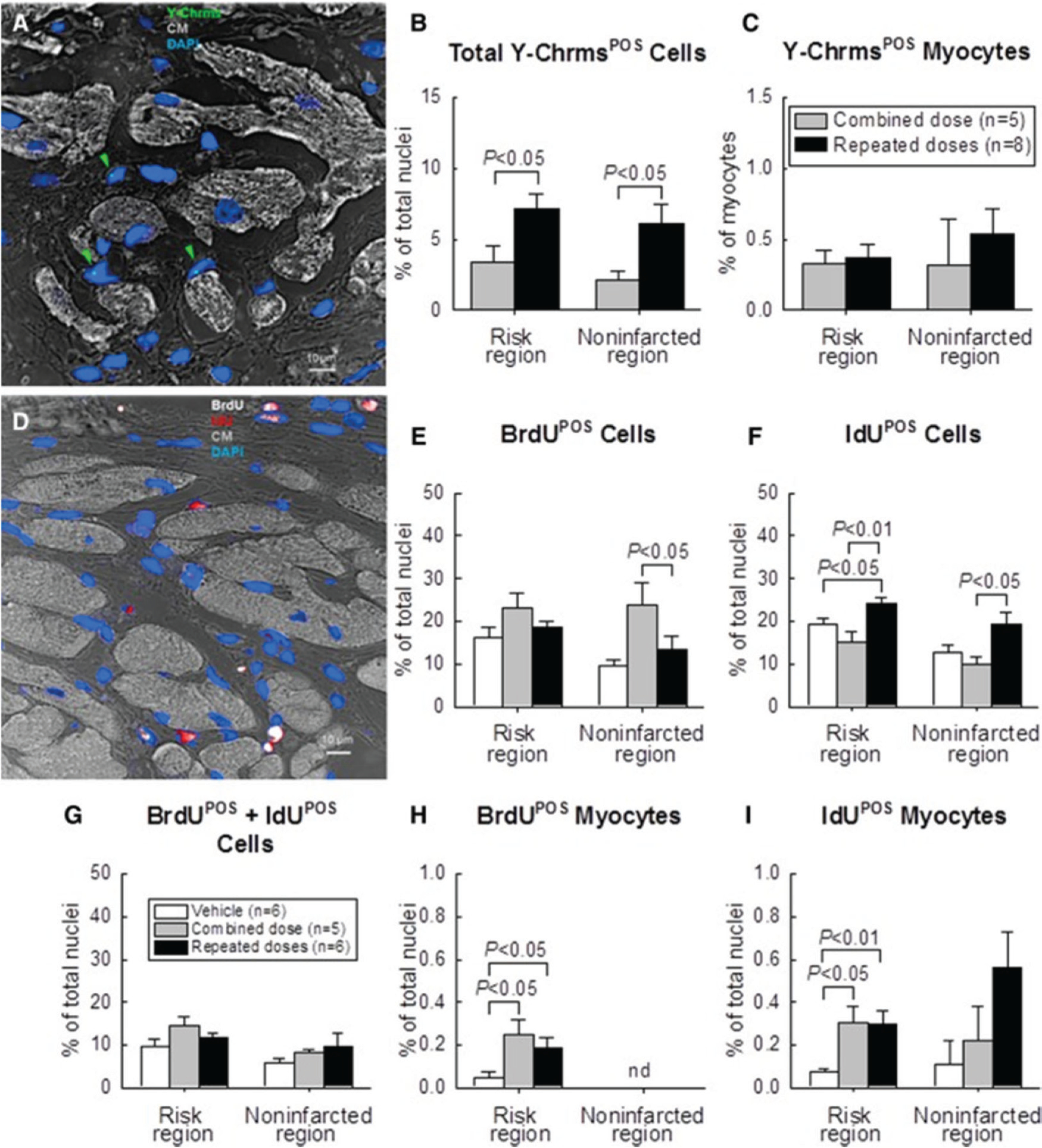
Detection of CPC retention and engraftment with fluorescence in situ hybridization (FISH) for detection of Y-chromosomes (Y-Chrms) after multiple CPC administrations in rats with chronic ischemic cardiomyopathy. Male c-kit CPCs were injected into female rats with old MI 30 days after MI; rats received either one combined dose or three CPC injections at 35-day intervals. Rats were given BrdU or IdU after CPC transplantation. (A) Representative confocal microscopic image acquired from the infarcted region. Green arrowheads indicate Y-chromosome fluorescent signals (green/cyan) in nuclei. Nuclei are stained with DAPI in blue. Cardiomyocyte (CM) morphology was examined with a confocal transmitted light channel’s detector (ChD) in which the pseudocolor selected for myocardial background in the ChD channel was gray white. (B) Quantitative analysis of the number of total Y-chromosomePOS cells and (C) Y-chromosomePOS matured myocytes at 105 days after CPC transplantation. The risk region comprises both the border zones and the infarcted region. (D) Representative confocal microscopic image acquired from the border zone. BrdU (given in the first 35 days after start of treatment) is shown in white, and IdU (given on days 70 to 105 after start of treatment) is shown in red. Nuclei are stained with DAPI. Cardiomyocyte (CM) morphology was examined with the confocal transmitted light ChD; the pseudocolor selected for myocardial background in the ChD channel was gray white. (E–G) Quantitative analysis of the number of BrdUPOS, IdUPOS, and BrdUPOS/IdUPOS nonmyocytes at 105 days after start of CPC administration. (H–J) Quantitative analysis of the number of BrdUPOS and IdUPOS myocytes at 105 days after start of CPC administration. (“Myocytes” were defined as α-sarcomeric actin positive cells, but these cells were small and did not resemble mature myocytes.) The risk region comprises both the border zones and the infarcted region. Note that the number of newly formed (i.e., BrdUPOS and (or) IdUPOS) cardiomyocytes (regardless of their source, exogenous or endogenous) was negligible (<1% of nuclei); that is, transplantation of CPCs did not promote significant formation of new cardiomyocytes from either exogenous or endogenous (resident) cells, even after multiple CPC doses. Data are means ± SEM. BrdU, 5-bromo-2′-deoxyuridine; DAPI, 4′,6-diamidino-2-phenylindole; IdU, 5-iodo-2′-deoxyuridine. Reproduced from Tang et al. (2018).
The mechanism whereby CPCs (and other progenitor or stem cells, for that matter) improve the function of a diseased heart remains unclear and, in our view, represents one of the major challenges facing researchers interested in cardiac reparative therapies. A discussion of this issue is beyond the scope of the present review. Among the most plausible hypotheses put forth in recent years are paracrine actions that modulate cardiac contractility by reducing fibrosis, inflammation, or apoptosis and (or) by enhancing angiogenesis (Bolli and Ghafghazi 2017; Keith and Bolli 2015; Wysoczynski et al. 2018). However, to date, none of these mechanisms has been demonstrated to account for the beneficial effects of CPCs or other cell types on LV function after acute or old MI (Bolli and Ghafghazi 2017; Keith and Bolli 2015; Wysoczynski et al. 2018).
In summary, although CPCs are reproducibly effective in improving LV function after acute or old MI in various experimental models and species (vide supra), the mechanism for these effects is unclear. What is clear is that it is not the mechanism of action originally proposed, i.e., cardiac regeneration (Beltrami et al. 2003; Leri et al. 2005). Contrary to then-widespread views, we did not find CPCs to have the properties of bona fide stem cells in vitro, i.e., self-renewal and multi- or pluri-potency, or to regenerate new cardiac muscle in vivo. Over the past decade, we have repeatedly pointed out (Bolli and Ghafghazi 2017; Eschenhagen et al. 2017; Keith and Bolli 2015; Wysoczynski et al. 2018) that CPCs do not engraft in the diseased heart, do not differentiate into new cardiac myocytes, and do not regenerate dead myocardium. Instead, like all other cell types (including embryonic stem cells), they work by releasing as-yet-unknown factors that influence diseased myocardium in a favorable manner, possibly via angiogenic, anti-inflammatory, anti-fibrotic, and (or) anti-apoptotic effects (Bolli and Ghafghazi 2017; Keith and Bolli 2015; Wysoczynski et al. 2018) Thus, our work (Bolli and Ghafghazi 2017; Bolli et al. 2013; Eschenhagen et al. 2017; Hong et al. 2013, 2014; Keith and Bolli 2015; X.L. Tang et al. 2010, 2015, 2016, 2018; Tokita et al. 2016; Wysoczynski et al. 2018) has refuted a common misconception regarding the mechanism of action of CPCs.
Retraction of the SCIPIO paper
The scientific misconduct in the laboratory of Dr. Anversa also led to the retraction of the paper reporting the results of the SCIPIO trial, a Phase I study in which CPCs manufactured and characterized in Dr. Anversa’s laboratory in Boston were shipped to Louisville and infused intracoronarily in patients with chronic ischemic heart failure (Bolli et al. 2011). The SCIPIO paper consisted of two separate and independent parts: the phenotype of the CPCs used for treatment (in vitro results generated in Boston) and the effect of this treatment in patients (clinical results generated in Louisville). It is important for the scientific community to know exactly what was fraudulent and what was not in that paper. As pointed out elsewhere (Bolli and Kahlon 2020), the rationale for the decision by The Lancet to retract this paper is published and exculpates all of the clinical work with CPCs conducted at Louisville, being limited to the figures generated at Harvard that illustrate the in vitro phenotype of the cells (The Lancet Editors 2019). Specifically, the notice of retraction states: “...The results of these investigations persuade us that the laboratory work undertaken by Piero Anversa and colleagues at Harvard cannot be held to be reliable. Specifically, there are issues with the data presented in figures 2 and 3 and in supplemental figures 2 and 3. SCIPIO was a collaboration between Anversa’s laboratory in Boston, MA, USA, and Roberto Bolli’s team in Louisville, KY, USA. Anversa’s laboratory isolated, expanded, and characterised the c-kit positive cells, which were then shipped to Louisville, where they were administered to patients and all the clinical work was done. The Louisville team was not involved with the manufacturing and characterisation of c-kit positive cells. Although we do not have any reservations about the clinical work in Louisville that used the preparations from Anversa’s laboratory in good faith, the lack of reliability regarding the laboratory work at Harvard means that we are now retracting this paper.” (The Lancet Editors 2019).
Although the results of SCIPIO suggest that patients receiving the cell product benefited from treatment, and although the Lancet editors clearly indicate that the clinical results are not in question (The Lancet Editors 2019), the manipulation of the cell phenotype data at Harvard raises questions regarding the exact nature of the cells used, making it impossible to conclude that these benefits were imparted by genuine c-kit+ CPCs. The effects of CPCs in patients with ischemic heart failure are currently being examined in the ongoing CONCERT-HF trial (Bolli et al. 2018), a Phase II randomized, double-blind, multi-center study sponsored by the NIH CCTRN network and arguably one of the most rigorous cell therapy trials ever conducted. In this study, CPCs are manufactured at the University of Miami and shipped to the CCTRN clinical centers, where they are injected in patients. The results of CONCERT-HF should be available soon.2
Conclusions
The scientific misconduct in one laboratory at Harvard has been a tragedy not only because it has led to the publication of corrupted papers that have misled the community, but also because it has tainted all studies of CPCs done everywhere else in the world even though they have nothing to do with the scandal at Harvard. As a result, work on these cells has basically stopped. It is as though CPCs have become radioactive: everyone seems afraid of touching them. Review groups are reluctant to fund any research on CPCs, no matter how rigorous, and many journals are reluctant to publish papers dealing with CPCs just because they deal with CPCs. This is unfortunate, because CPCs do have therapeutic potential. We must be careful not to overreact to the scandal and dismiss all work on CPCs because of one laboratory’s misconduct. When the emotions of recent events are set aside, an unbiased review of the literature supports the position that despite the retractions and controversy, CPCs remain a viable therapeutic product at the preclinical level. At the clinical level, the answer to the question as to whether or not CPCs have a future in cardiac therapy will come from the ongoing CONCERT-HF trial, a highly rigorous test of the therapeutic potential of these cells in ischemic heart disease.
Acknowledgements
This work was supported by National Institutes of Health grants P01 HL078825 and UM1 HL113530.
Footnotes
This paper is part of a special issue of selected papers from the joint North American/European IACS 2019.
After this manuscript was accepted, the results of CONCERT-HF (NCT02501811) were reported (https://www.ahajournals.org/doi/10.1161/circ.142.suppl_3.14080). The results show that a single transendocardial administration of CPCs reduced major adverse cardiac events (i.e., death, hospitalization for heart failure, or exacerbation of heart failure) by ~80% vs. placebo. If these results are confirmed in a phase III trial, this would be a major advance in the management of heart failure.
References
- Alshammary S, Fukushima S, Miyagawa S, Matsuda T, Nishi H, Saito A, et al. 2013. Impact of cardiac stem cell sheet transplantation on myocardial infarction. Surg Today, 43(9): 970–976. doi: 10.1007/s00595-013-0528-2. [DOI] [PubMed] [Google Scholar]
- Anversa P, Kajstura J, Rota M, and Leri A 2013. Regenerating new heart with stem cells. J. Clin. Invest 123(1): 62–70. doi: 10.1172/JCI63068. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Avolio E, Meloni M, Spencer HL, Riu F, Katare R, Mangialardi G, et al. 2015. Combined intramyocardial delivery of human pericytes and cardiac stem cells additively improves the healing of mouse infarcted hearts through stimulation of vascular and muscular repair. Circ. Res 116(10): e81–e94. doi: 10.1161/circresaha.115.306146. [DOI] [PubMed] [Google Scholar]
- Bao L, Meng Q, Li Y, Deng S, Yu Z, Liu Z, et al. 2017. C-Kit positive cardiac stem cells and bone marrow-derived mesenchymal stem cells synergistically enhance angiogenesis and improve cardiac function after myocardial infarction in a paracrine manner. J. Card. Fail 23(5): 403–415. doi: 10.1016/j.cardfail.2017.03.002. [DOI] [PubMed] [Google Scholar]
- Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, et al. 2003. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell, 114(6): 763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- Bhutani S, Nachlas ALY, Brown ME, Pete T, Johnson CT, García AJ, and Davis ME 2018. Evaluation of hydrogels presenting extracellular matrix-derived adhesion peptides and encapsulating cardiac progenitor cells for cardiac repair. ACS Biomater. Sci. Eng 4(1): 200–210. doi: 10.1021/acsbiomaterials.7b00502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolli R, and Ghafghazi S 2017. Stem cells: Cell therapy for cardiac repair: what is needed to move forward? Nat. Rev. Cardiol 14(5): 257–258. doi: 10.1038/nrcardio.2017.38. [DOI] [PubMed] [Google Scholar]
- Bolli R, and Kahlon A 2020. Time to end the war on cell therapy. Eur. J. Heart Fail 22(5): 893–897. doi: 10.1002/ejhf.1767. [DOI] [PubMed] [Google Scholar]
- Bolli R, Chugh AR, D’Amario D, Loughran JH, Stoddard MF, Ikram S, et al. 2011. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet, 378 (9806): 1847–1857. doi: 10.1016/s0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bolli R, Tang XL, Sanganalmath SK, Rimoldi O, Mosna F, Abdel-Latif A, et al. 2013. Intracoronary delivery of autologous cardiac stem cells improves cardiac function in a porcine model of chronic ischemic cardiomyopathy. Circulation, 128(2): 122–131. doi: 10.1161/circulationaha.112.001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolli R, Hare JM, March KL, Pepine CJ, Willerson JT, Perin EC, et al. 2018. Rationale and design of the CONCERT-HF trial (Combination of Mesenchymal and c-kit(+) Cardiac Stem Cells As Regenerative Therapy for Heart Failure). Circ. Res 122(12): 1703–1715. doi: 10.1161/circresaha.118.312978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai C, Guo Y, Teng L, Nong Y, Tan M, Book MJ, et al. 2015. Preconditioning human cardiac stem cells with an HO-1 inducer exerts beneficial effects after cell transplantation in the infarcted murine heart. Stem Cells, 33(12): 3596–3607. doi: 10.1002/stem.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr CA, Stuckey DJ, Tan JJ, Tan SC, Gomes RS, Camelliti P, et al. 2011. Cardiosphere-derived cells improve function in the infarcted rat heart for at least 16 weeks — an MRI study. PLoS One, 6(10): e25669. doi: 10.1371/journal.pone.0025669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dergilev K, Tsokolaeva Z, Makarevich P, Beloglazova I, Zubkova E, Boldyreva M, et al. 2018. C-Kit cardiac progenitor cell based cell sheet improves vascularization and attenuates cardiac remodeling following myocardial infarction in rats. Biomed. Res. Int 2018: 3536854. doi: 10.1155/2018/3536854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran JM, Makarewich CA, Sharp TE, Starosta T, Zhu F, Hoffman NE, et al. 2013. Bone-derived stem cells repair the heart after myocardial infarction through transdifferentiation and paracrine signaling mechanisms. Circ. Res 113(5): 539–552. doi: 10.1161/circresaha.113.301202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison GM, Vicinanza C, Smith AJ, Aquila I, Leone A, Waring CD, et al. 2013. Adult c-kit(pos) cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair. Cell, 154(4): 827–842. doi: 10.1016/j.cell.2013.07.039. [DOI] [PubMed] [Google Scholar]
- Eschenhagen T, Bolli R, Braun T, Field LJ, Fleischmann BK, Frisén J, et al. 2017. Cardiomyocyte regeneration: A consensus statement. Circulation, 136(7): 680–686. doi: 10.1161/circulationaha.117.029343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer KM, Cottage CT, Wu W, Din S, Gude NA, Avitabile D, et al. 2009. Enhancement of myocardial regeneration through genetic engineering of cardiac progenitor cells expressing Pim-1 kinase. Circulation, 120(21): 2077–2087. doi: 10.1161/circulationaha.109.884403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong KU, Li QH, Guo Y, Patton NS, Moktar A, Bhatnagar A, and Bolli R 2013. A highly sensitive and accurate method to quantify absolute numbers of c-kit+ cardiac stem cells following transplantation in mice. Basic Res. Cardiol 108(3): 346. doi: 10.1007/s00395-013-0346-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong KU, Guo Y, Li QH, Cao P, Al-Maqtari T, Vajravelu BN, et al. 2014. C-kit+ cardiac stem cells alleviate post-myocardial infarction left ventricular dysfunction despite poor engraftment and negligible retention in the recipient heart. PLoS One, 9(5): e96725. doi: 10.1371/journal.pone.0096725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata S, Miyagawa S, Fukushima S, Nakatani S, Kawamoto A, Saito A, et al. 2014. Improvement of cardiac stem cell sheet therapy for chronic ischemic injury by adding endothelial progenitor cell transplantation: analysis of layer-specific regional cardiac function. Cell Transplant. 23(10): 1305–1319. doi:. [DOI] [PubMed] [Google Scholar]
- Karantalis V, Suncion-Loescher VY, Bagno L, Golpanian S, Wolf A, Sanina C, et al. 2015. Synergistic effects of combined cell therapy for chronic ischemic cardiomyopathy. J. Am. Coll. Cardiol 66(18): 1990–1999. doi: 10.1016/j.jacc.2015.08.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazakov A, Meier T, Werner C, Hall R, Klemmer B, Körbel C, et al. 2015. C-kit(+) resident cardiac stem cells improve left ventricular fibrosis in pressure overload. Stem Cell Res 15(3): 700–711. doi: 10.1016/j.scr.2015.10.017. [DOI] [PubMed] [Google Scholar]
- Keith MC, and Bolli R 2015. “String theory” of c-kit(pos) cardiac cells: a new paradigm regarding the nature of these cells that may reconcile apparently discrepant results. Circ. Res 116(7): 1216–1230. doi: 10.1161/circresaha.116.305557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulandavelu S, Karantalis V, Fritsch J, Hatzistergos KE, Loescher VY, McCall F, et al. 2016. Pim1 kinase overexpression enhances ckit(+) cardiac stem cell cardiac repair following myocardial infarction in swine. J. Am. Coll. Cardiol 68(22): 2454–2464. doi: 10.1016/j.jacc.2016.09.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leri A, Kajstura J, and Anversa P 2005. Cardiac stem cells and mechanisms of myocardial regeneration. Physiol. Rev 85(4): 1373–1416. doi: 10.1152/physrev.00013.2005. [DOI] [PubMed] [Google Scholar]
- Li C, Matsushita S, Li Z, Guan J, and Amano A 2017. c-kit positive cardiac outgrowth cells demonstrate better ability for cardiac recovery against ischemic myopathy. J. Stem Cell Res. Ther 7(10): 402. doi: 10.4172/2157-7633.1000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TS, Cheng K, Malliaras K, Smith RR, Zhang Y, Sun B, et al. 2012. Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. J. Am. Coll. Cardiol 59(10): 942–953. doi: 10.1016/j.jacc.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Guo Y, Ou Q, Chen N, Wu WJ, Yuan F, et al. 2011. Intracoronary administration of cardiac stem cells in mice: a new, improved technique for cell therapy in murine models. Basic Res. Cardiol 106(5): 849–864. doi: 10.1007/s00395-011-0180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Lu Y, Han R, Yue Q, Song X, Wang F, et al. 2018. Gremlin2 regulates the differentiation and function of cardiac progenitor cells via the notch signaling pathway. Cell Physiol. Biochem 47(2): 579–589. doi: 10.1159/000490012. [DOI] [PubMed] [Google Scholar]
- Ma W, Ding F, Wang X, Huang Q, Zhang L, Bi C, et al. 2018a. By targeting Atg7 MicroRNA-143 mediates oxidative stress-induced autophagy of c-Kit(+) mouse cardiac progenitor cells. EBioMedicine, 32: 182–191. doi: 10.1016/j.ebiom.2018.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, He F, Ding F, Zhang L, Huang Q, Bi C, et al. 2018b. Pre-treatment with melatonin enhances therapeutic efficacy of cardiac progenitor cells for myocardial infarction. Cell Physiol. Biochem 47(3): 1287–1298. doi: 10.1159/000490224. [DOI] [PubMed] [Google Scholar]
- Matsuda T, Miyagawa S, Fukushima S, Kitagawa-Sakakida S, Akimaru H, Horii-Komatsu M, et al. 2014. Human cardiac stem cells with reduced notch signaling show enhanced therapeutic potential in a rat acute infarction model. Circ J 78(1): 222–231. doi: 10.1253/circj.cj-13-0534. [DOI] [PubMed] [Google Scholar]
- Maxwell JT, Trac D, Shen M, Brown ME, Davis ME, Chao MS, et al. 2019. Electrical Stimulation of pediatric cardiac-derived c-kit(+) progenitor cells improves retention and cardiac function in right ventricular heart failure. Stem Cells, 37(12): 1528–1541. doi: 10.1002/stem.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohsin S, Khan M, Toko H, Bailey B, Cottage CT, Wallach K, et al. 2012. Human cardiac progenitor cells engineered with Pim-I kinase enhance myocardial repair. J. Am. Coll. Cardiol 60(14): 1278–1287. doi: 10.1016/j.jacc.2012.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natsumeda M, Florea V, Rieger AC, Tompkins BA, Banerjee MN, Golpanian S, et al. 2017. A combination of allogeneic stem cells promotes cardiac regeneration. J. Am. Coll. Cardiol 70(20): 2504–2515. doi: 10.1016/j.jacc.2017.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oransky I, 2018. Harvard and the Brigham recommend 31 retractions for cardiac stem cell work. Available from https://retractionwatch.com/2018/10/14/harvard-and-the-brigham-recommend-31-retractions-for-cardiac-stem-cell-work/ [accessed 14 October 2018].
- Oskouei BN, Lamirault G, Joseph C, Treuer AV, Landa S, Da Silva J, et al. 2012. Increased potency of cardiac stem cells compared with bone marrow mesenchymal stem cells in cardiac repair. Stem Cells Transl. Med 1(2): 116–124. doi: 10.5966/sctm.2011-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puddighinu G, D’Amario D, Foglio E, Manchi M, Siracusano A, Pontemezzo E, et al. 2018. Molecular mechanisms of cardioprotective effects mediated by transplanted cardiac ckit(+) cells through the activation of an inflammatory hypoxia-dependent reparative response. Oncotarget, 9(1): 937–957. doi: 10.18632/oncotarget.22946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanganalmath SK, and Bolli R 2013. Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ. Res 113(6): 810–834. doi: 10.1161/circresaha.113.300219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Mishra R, Simpson D, Wehman B, Colletti EJ, Deshmukh S, et al. 2015. Cardiosphere-derived cells from pediatric end-stage heart failure patients have enhanced functional activity due to the heat shock response regulating the secretome. Stem Cells, 33(4): 1213–1229. doi: 10.1002/stem.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson DL, Mishra R, Sharma S, Goh SK, Deshmukh S, and Kaushal S 2012. A strong regenerative ability of cardiac stem cells derived from neonatal hearts. Circulation, 126(11 Suppl. 1): S46–S53. doi: 10.1161/circulationaha.111.084699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song F, Hua F, Li H, Zhou X, Yan L, Yang Q, et al. 2016. Cardiac stem cell transplantation with 2,3,5,40-tetrahydroxystilbene-2-O-β-d-glucoside improves cardiac function in rat myocardial infarction model. Life Sci 158: 37–45. doi: 10.1016/j.lfs.2016.06.011. [DOI] [PubMed] [Google Scholar]
- Taghavi S, Sharp TE 3rd., Duran JM, Makarewich CA, Berretta RM, Starosta T, et al. 2015. Autologous c-Kit+ mesenchymal stem cell injections provide superior therapeutic benefit as compared to c-Kit+ cardiac-derived stem cells in a feline model of isoproterenol-induced cardiomyopathy. Clin. Transl. Sci. 8(5): 425–431. doi: 10.1111/cts.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang JM, Luo B, Xiao JH, Lv YX, Li XL, Zhao JH, et al. 2015. VEGF-A promotes cardiac stem cell engraftment and myocardial repair in the infarcted heart. Int. J. Cardiol 183: 221–231. doi: 10.1016/j.ijcard.2015.01.050. [DOI] [PubMed] [Google Scholar]
- Tang XL, Rokosh G, Sanganalmath SK, Yuan F, Sato H, Mu J, et al. 2010. Intracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30-day-old infarction. Circulation, 121(2): 293–305. doi: 10.1161/circulationaha.109.871905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XL, Rokosh G, Sanganalmath SK, Tokita Y, Keith MC, Shirk G, et al. 2015. Effects of intracoronary infusion of escalating doses of cardiac stem cells in rats with acute myocardial infarction. Circ. Heart Fail 8(4): 757–765. doi: 10.1161/circheartfailure.115.002210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XL, Li Q, Rokosh G, Sanganalmath SK, Chen N, Ou Q, et al. 2016. Long-term outcome of administration of c-kit(POS) cardiac progenitor cells after acute myocardial infarction: transplanted cells do not become cardiomyocytes, but structural and functional improvement and proliferation of endogenous cells persist for at least one year. Circ. Res 118(7): 1091–1105. doi: 10.1161/circresaha.115.307647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XL, Nakamura S, Li Q, Wysoczynski M, Gumpert AM, Wu WJ, et al. 2018. Repeated administrations of cardiac progenitor cells are superior to a single administration of an equivalent cumulative dose. J. Am. Heart Assoc 7(4): e007400. doi: 10.1161/jaha.117.007400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YL, Zhu W, Cheng M, Chen L, Zhang J, Sun T, et al. 2009. Hypoxic preconditioning enhances the benefit of cardiac progenitor cell therapy for treatment of myocardial infarction by inducing CXCR4 expression. Circ. Res 104(10): 1209–1216. doi: 10.1161/circresaha.109.197723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Lancet Editors. 2019. Retraction — Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet, 393(10176): 1084. doi: 10.1016/s0140-6736(19)30542-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokita Y, Tang XL, Li Q, Wysoczynski M, Hong KU, Nakamura S, et al. 2016. Repeated administrations of cardiac progenitor cells are markedly more effective than a single administration: a new paradigm in cell therapy. Circ. Res 119(5): 635–651. doi: 10.1161/circresaha.116.308937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahdat S, Mousavi SA, Omrani G, Gholampour M, Sotoodehnejadnematalahi F, Ghazizadeh Z, et al. 2015. Cellular and molecular characterization of human cardiac stem cells reveals key features essential for their function and safety. Stem Cells Dev 24(12): 1390–1404. doi: 10.1089/scd.2014.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicinanza C, Aquila I, Scalise M, Cristiano F, Marino F, Cianflone E, et al. 2017. Adult cardiac stem cells are multipotent and robustly myogenic: c-kit expression is necessary but not sufficient for their identification. Cell Death Differ 24(12): 2101–2116. doi: 10.1038/cdd.2017.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Zhao X, Kuang C, Qian D, Wang H, Jiang H, et al. 2012. Overexpression of SDF-1a enhanced migration and engraftment of cardiac stem cells and reduced infarcted size via CXCR4/PI3K pathway. PLoS One, 7(9): e43922. doi: 10.1371/journal.pone.0043922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehman B, Pietris N, Bigham G, Siddiqui O, Mishra R, Li T, et al. 2017. Cardiac progenitor cells enhance neonatal right ventricular function after pulmonary artery banding. Ann. Thorac. Surg 104(6): 2045–2053. doi: 10.1016/j.athoracsur.2017.04.058. [DOI] [PubMed] [Google Scholar]
- Williams AR, Hatzistergos KE, Addicott B, McCall F, Carvalho D, Suncion V, et al. 2013. Enhanced effect of combining human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and to restore cardiac function after myocardial infarction. Circulation, 127(2): 213–223. doi: 10.1161/circulationaha.112.131110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysoczynski M, Dassanayaka S, Zafir A, Ghafghazi S, Long BW, Noble C, et al. 2016. A new method to stabilize c-kit expression in reparative cardiac mesenchymal cells. Front. Cell. Dev. Biol 4: 78. doi: 10.3389/fcell.2016.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysoczynski M, Khan A, and Bolli R 2018. New paradigms in cell therapy: repeated dosing, intravenous delivery, immunomodulatory actions, and new cell types. Circ. Res 123(2): 138–158. doi: 10.1161/circresaha.118.313251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharova L, Nural-Guvener H, Feehery L, Popovic S, Nimlos J, and Gaballa MA 2014. Retrograde coronary vein infusion of cardiac explantderived c-Kit+ cells improves function in ischemic heart failure. J. Heart Lung Transplant 33(6): 644–653. doi: 10.1016/j.healun.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Zakharova L, Nural-Guvener H, Feehery L, Popovic-Sljukic S, and Gaballa MA 2015. Transplantation of epigenetically modified adult cardiac c-Kit+ Cells retards remodeling and improves cardiac function in ischemic heart failure model. Stem Cells Transl. Med 4(9): 1086–1096. doi: 10.5966/sctm.2014-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LX, DeNicola M, Qin X, Du J, Ma J, Tina Zhao Y, et al. 2014. Specific inhibition of HDAC4 in cardiac progenitor cells enhances myocardial repairs. Am. J. Physiol. Cell. Physiol 307(4): C358–C372. doi: 10.1152/ajpcell.00187.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Yang J, Yan W, Li Y, Shen Z, and Asahara T 2016. Pretreatment of cardiac stem cells with exosomes derived from mesenchymal stem cells enhances myocardial repair. J. Am. Heart Assoc 5(1): e002856. doi: 10.1161/jaha.115.002856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YT, Du J, Chen Y, Tang Y, Qin G, Lv G, et al. 2015. Inhibition of Oct 3/4 mitigates the cardiac progenitor-derived myocardial repair in infarcted myocardium. Stem Cell Res Ther 6: 259. doi: 10.1186/s13287-015-0252-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


